Abstract
Introduction
Hyperbaric oxygen treatment (HBOT) has been suggested as an effective intervention to limit necrosis of ischaemic skin flaps after mastectomy. The purpose of this study was to evaluate outcomes of HBOT in the largest series of patients to date with mastectomy flap ischaemia.
Methods
A retrospective analysis was performed of 50 breasts requiring HBOT for mastectomy flap ischaemia. The severity of the ischaemia or necrosis was evaluated by four independent observers using the skin ischaemia necrosis (SKIN) score. Multivariate logistic regression analyses were used to assess associations between risk factors and re-operation.
Results
HBOT was started a median of 3 days (range 1–23) after surgery and continued for a median of 12 sessions (range 6–22). The breast SKIN surface area scores (n = 175 observations by the independent observers) improved in 34% (of observations) and the depth scores deteriorated in 42% (both P < 0.01). Both the surface area and depth scores were associated with the need for re-operation: higher scores, reflecting more severe necrosis of the mastectomy flap, were associated with increased need for re-operation. Twenty-nine breasts (58%) recovered without additional operation. Pre-operative radiotherapy (OR 7.2, 95% CI 1.4–37.3) and postoperative infection (OR 15.4, 95% CI 2.6–89.7) were risk factors for re-operation in multivariate analyses.
Conclusions
In this case series, the surface area of the breast affected by ischaemia decreased during HBOT, and most breasts (58%) did not undergo an additional operation. A randomised control trial is needed to confirm or refute the possibility that HBOT improves outcome in patients with mastectomy flap ischaemia.
Keywords: Necrosis, Nipple, Outcome, Radiotherapy, Skin, Surgery
Introduction
When patients with breast cancer who need a mastectomy opt for a breast reconstruction, a major benefit of an immediate reconstruction is the better aesthetic outcome compared to delayed reconstruction.[ 1] However, mastectomy with immediate reconstruction leads to a higher rate of postoperative complications and a greater need for reoperation.[ 2] Mastectomy flap ischaemia leading to necrosis is reported in 4.3% (n = 178/4,158),[ 3] 12% (n = 112/903),[ 4] and 14% of patients (n = 85/606)[ 5] who were followed prospectively after mastectomy and immediate reconstruction.
The following risk factors have been associated with mastectomy flap ischaemia and necrosis: age, body mass index (BMI) > 30 kg·m-2, larger cup size, previous or current smoking, hypertension, prior breast-reduction surgery, history of breast augmentation, previous radiation therapy, nipple-sparing mastectomy, time from incision to removal of specimen, mastectomy specimen weight (> 500 gram), one-stage breast reconstruction, use of an acellular dermal matrix, and the volume of operative tissue expander fill > 300 cm3.[ 1 , 5 - 7] These factors can lead to impaired perfusion of the mastectomy flap and result in skin necrosis.
Several classifications of severity of mastectomy flap necrosis have been described by Matsen et al. (mild, moderate, severe),[ 5] Frey et al. (minor, major)[ 8] and Lemaine et al. (depth, surface area).[ 9] The former scores are dependent on the time to healing and the type of intervention; the latter score parallels that of burn severity classification.
Current treatments of mastectomy flap ischaemia include wait-and-see[ 3 , 9] nitroglycerin ointment,[ 10 - 14] topical silver sulfadiazine,[ 1] topical dimethylsulfoxide,[ 15] oral or intravenous antibiotics,1 Dextran-40 infusion,[ 16] and tissue expander expansion of the well-perfused tissue to create sufficient tissue for excision of full-thickness necrosis and primary closure 4–6 weeks postoperatively.[ 3] Five to 67% of patients with mastectomy flap ischaemia require reoperation including debridement or removal of the tissue expander or implant.[ 1 , 3 , 11 , 12 , 14 , 17 , 18]
Hyperbaric oxygen treatment (HBOT) has been used to treat various ischaemic skin flaps and grafts.[ 19 - 27] Although some types of flap compromise can be addressed by re-exploration,[ 28] when there is no correctable mechanical cause of flap ischaemia, HBOT can be used to hyperoxygenate the flap and reduce oedema.[ 26 , 28 , 29] HBOT may prevent the progression of ischaemia to necrosis or limit the extent of necrosis. In a case series of 65 postoperatively compromised skin flaps treated with HBOT, 36 (55%) showed “complete healing” and 22 (34%) “marked improvement”.[ 26] Only five case reports[ 30 - 34] and a small case-control study[ 35] were identified reporting that HBOT can successfully prevent necrosis of ischaemic skin flaps after mastectomy.
In a recent review on the challenges and solutions for mastectomy skin flap necrosis, HBOT was mentioned as being successful in case reports. However, in that review the use of HBOT was not recommended due to lack of larger series to support its use.[ 13] Therefore, the purpose of this study was to evaluate outcomes of HBOT in patients with skin flap ischaemia following mastectomy. The primary outcome was the need for additional surgery following HBOT, while the secondary outcome was a decrease in tissue necrosis using the SKIN score.[ 9] We also sought risk factors for additional surgery despite HBOT.
Methods
In accordance with the Health Code of 2005 based on the Code of Good Conduct 1995, our institutional review board grants a universal waiver for retrospective chart reviews, such as this study. Patients signed informed consent forms to use photographs for clinical and research purposes. A retrospective chart review was performed of the patients with mastectomy flap ischaemia who were referred to the Da Vinci Clinic (Geldrop) for HBOT between January 2013 and January 2018. During this period 44 patients with compromised mastectomy flaps (50 breasts) were referred from five hospitals in the Netherlands, including Catharina Hospital (Eindhoven), Maxima Medical Centre (Veldhoven and Eindhoven), St. Anna Hospital (Geldrop), St. Jans Hospital (Weert), and University Medical Centre (Maastricht). It is not known if all patients with mastectomy flap ischaemia were referred for HBOT. Other hospitals in the area did not refer any patients. A retrospective analysis was performed. Medical records were reviewed from both the Da Vinci Clinic and the referring hospitals to gather information concerning patient demographics, operative details, HBOT and outcomes.
The Da Vinci Clinic has a multiplace hyperbaric chamber for 12 patients (IHC Hytech, Raamsdonkveer, The Netherlands) for HBOT. Patients were treated at 253 kPa (2.5 atmospheres absolute). At this pressure, 100% oxygen was breathed via a mask during four periods for a total of 85 minutes, interspersed by three 5-minute air breaks. Including compression and decompression time, the total duration of each session was 110 minutes. Patients underwent two sessions per day for the first three days, followed by one session per day until circulation in the mastectomy flap was restored or demarcation was achieved.
Relative contra-indications for HBOT are epilepsy, history of pneumothorax or pulmonary surgery, COPD with known bullae or requiring continuous normobaric oxygen, left ventricle ejection fraction < 20%, concomitant or recent treatment with cisplatinum, doxorubicin or bleomycin, previous middle ear reconstruction, or pregnancy.[ 36] None of the patients referred with mastectomy flap ischaemia had any relative contra-indications to HBOT.
To assess the decrease of tissue necrosis during HBOT, pre- and post-HBOT photographs were scored by the four authors independently using a previously validated system;[ 9] the SKIN (Skin Ischaemia Necrosis) score. Photographs were taken before the first session of HBOT and after completion of the course of HBOT, and scored in a random order. The SKIN score includes surface area and depth of ischaemia or necrosis of the mastectomy skin flap and nipple-areolar complex. The affected surface area was scored: 1 = 0%; 2 = 1–10%; 3 = 11–30%; and 4 = > 30%. The estimated affected depth was scored: A = none; B = colour change; C = partial thickness skin flap necrosis; and D = full thickness skin flap necrosis.
When the SKIN score improves, secondary surgery may be avoided or minimised.[ 9] The outcome of the mastectomy flap ischaemia treated with HBOT was collected from the medical records at the referring hospitals, and scored using the grades of Matsen et al., (mild, moderate, severe)[ 5] and Frey et al. (minor, major).[ 8]
STATISTICAL ANALYSIS
Analyses were performed per breast, not per patient. Statistical analyses were performed using SPSS version 22.0 software (SPSS inc., Illinois, USA) and with SAS version 9.2 (SAS Institute, North Carolina, USA). Differences were considered significant at a value of P < 0.05. Missing and inconsistent data were excluded. Descriptive statistics were reported as number and percentage of breasts, median with range, and counts of the SKIN scores. A binomial test was used for comparisons between pre-HBOT and post-HBOT SKIN scores. For assessment of inter-observer agreement for SKIN scores, Fleiss’ kappa was calculated. Cross tabulations were used to assess the association between post-HBOT measurements, SKIN scores and reoperation. No statistical tests were applied to investigate the significance here.
The Spearman test was used for evaluation of correlations between risk factors and the degree of necrosis as defined by Matsen et al.[ 5] Step-wise multivariate logistic regression analyses were used to assess associations between risk factors and re-operation.
Results
Between January 2013 and January 2018, 44 patients with 50 breasts with skin flap ischaemia after mastectomy were referred for HBOT. Patient demographics and comorbidity are presented in Table 1. Operative characteristics are presented in Table 2. Most underwent mastectomy for breast cancer (29 breasts; 58%), and most had an immediate breast reconstruction with a tissue expander (30 breasts; 60%) or breast implant (16 breasts; 32%). Two breasts were not immediately reconstructed after mastectomy because of ischaemic skin flaps peri-operatively. In addition to mastectomy flap ischaemia, some breasts had other postoperative complications, including infection, seroma, and haematoma (Table 3).
Table 1. Demographics and comorbidity (n = 44 patients). *Six of the 19 patients who underwent bilateral mastectomies had bilateral mastectomy flap ischaemia. ASA − American Society of Anesthetists classification. BMI − body mass index.
| Parameter | Median (range) |
| Age (years) | 52 (23−72) |
| BMI (kg·m-2) | 23 (18−31) |
| n% | |
| ASA > 2 | 5 (10) |
| Diabetes mellitus | 2 (5) |
| Hypertension | 3 (7) |
| Coagulation disorder | 5 (11) |
| Current smoker | 12 (27) |
| Use of immunosuppressant | 2 (5) |
| Preoperative chemotherapy | 12 (27) |
| Preoperative radiotherapy | 11 (25) |
| Previous breast augmentation | 4 (9) |
| Previous breast reduction | 3 (7) |
| Bilateral breast surgery | 19 (43)* |
Table 2. Operative characteristics (n = 50 breasts).
| Operative characteristics | n (%) |
| Nipple-sparing | 22 (44) |
| Indication for surgery | |
| - Ductal carcinoma in situ | 11 (22) |
| - Breast cancer | 29 (58) |
| - Prophylactic | 4 (8) |
| - BRCA mutation and breast cancer | 2 (4) |
| Operation | |
| - Tissue expander | |
| Primary | 16 (32) |
| Hammond | 13 (26) |
| Latissimus dorsi flap | 1 (2) |
| - Implant | |
| Primary | 6 (12) |
| Hammond | 4 (8) |
| Latissimus dorsi flap | 3 (6) |
| Acellular dermal matrix | 3 (6) |
| - Deep inferior epigastric artery perforator-flap | 1 (2) |
| - Reversed abdominoplasty | 1 (2) |
| - No reconstruction | 2 (4) |
Table 3. Outcome after mastectomy flap ischaemia and HBOT. * Matsen et al.[ 5], mild: no intervention needed, healing complete at eight weeks, moderate: office debridement, healing complete at eight weeks, severe: operating room debridement, implant loss, or healing not complete at eight weeks. **Frey et al.[ 8], minor: requiring only local wound care, major: requiring debridement either in the office or in the operating room.
| Postoperative outcome | n (%) |
| Infection | 12 (24) |
| Seroma | 9 (18) |
| Hematoma | 4 (8) |
| Reoperation | 21 (42) |
| - Removal of tissue expander or implant | 15 (30) |
| - Partial debridement of skin flap | 4 (8) |
| - Full-thickness skin graft | 1 (2) |
| - Latissimus dorsi-flap | 1 (2) |
| Matsen et al.* | |
| - Mild | 26 (52) |
| - Moderate | 3 (6) |
| - Severe | 21 (42) |
| Frey et al.** | |
| - Minor | 26 (52) |
| - Major | 24 (48) |
HBOT was started at a median of 3 (range 1–23) days following mastectomy. Patients underwent a median of 12 sessions of HBOT (range 6–22). The most common side effect of HBOT was problems equalising the ears: 10/44 patients (23%) used nasal decongestant spray and 4/44 patients (9%) needed myringotomy tubes. No central nervous system oxygen toxicity or visual changes were reported due to HBOT. No patients prematurely terminated the treatment.
OUTCOMES
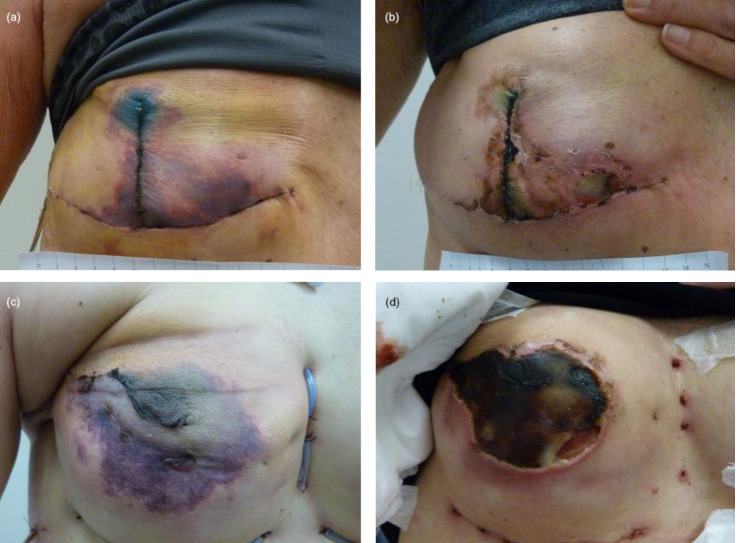
Most breasts recovered without reoperation (n = 29/50 (58%), Table 3 and Figure 1 A–B). Reoperation was required for 21/50 breasts (42%), including removal of the tissue expander or implant in 13/46 (28%), Figure 1 C–D), debridement of the skin flap in 4/50 (8%) or secondary reconstruction with a full-thickness skin graft (one patient) or latissimus dorsi flap (one patient).
Figure 1.
Breasts before and after the course of HBOT; (a) Breast on presentation for HBOT two days after Hammond mastectomy and immediate reconstruction with a tissue expander, and (b) after a course of 20 treatments with HBOT. Beneath the superficial necrosis, the breast reconstruction remained intact. No further surgery was necessary. (c) Breast on presentation for HBOT three days after nipple-sparing mastectomy and immediate reconstruction with an implant and (d) after course of 22 treatments with HBOT. The full thickness necrosis of the nipple areola complex and surrounding skin flap resulted in exposure of the implant, which needed to be removed
SKIN scores (surface area and depth) were given by four independent observers, with separate scores for the affected breast skin (n = 50) and the nipple areola complex (n = 22) at the start and end of the course of HBOT.
Pre- and post-HBOT breast SKIN scores were complete for 175 observations (Table 4). The changes between the pre- and post-HBOT surface area and affected depth scores showed a mix of improvement, no change, or deterioration. Overall, the surface area scores improved more often than they deteriorated (34% vs. 5%, P < 0.01), and the depth scores deteriorated more often than they improved (42% vs. 17%, P < 0.01). The inter-observer Kappa was low (0.213 and 0.282 respectively).
Table 4. Difference between pre- and post-HBOT breast SKIN score (n = 175 observations).
| Surface area | Depth | |
| Deteriorated | 8 (5%) | 74 (42%) |
| Unchanged | 107 (61%) | 72 (41%) |
| Improved | 60 (34%) | 29 (16–17%) |
| P-value | < 0.01 | < 0.01 |
| Interobserver Kappa (95% CI) | 0.213 (0.061−0.366) | 0.282 (0.157−0.407) |
Pre- and post-HBOT nipple-areolar complex SKIN scores were complete for 64 observations (Table 5). The changes between the pre- and post-HBOT surface area and affected depth scores also showed a mix of improvement, no change or deterioration. Overall, the change in surface area scores was not statistically significant (27% improvement vs. 17% deterioration, P = 0.13), and the depth scores deteriorated more often than they improved (50% vs. 6%, P < 0.01). The interobserver Kappa was low (0.138 and 0.073 respectively).
Table 5. Difference between pre- and post-HBOT nipple areola complex SKIN score (n = 64 observations).
| Surface area | Depth | |
| Deteriorated | 11 (17%) | 32 (50%) |
| Unchanged | 36 (56%) | 28 (44%) |
| Improved | 17 (27%) | 4 (6%) |
| P-value | 0.13 | < 0.01 |
| Interobserver Kappa (95% CI) | 0.138 (-0.037–0.313) | 0.073 (-1.156–0.302) |
Post-HBOT breast SKIN scores were available for 46 breasts (183 observations). Both the surface area and depth scores were associated with the need for re-operation: higher scores, reflecting more severe necrosis of the mastectomy flap, were associated with a more likely need for re-operation (Figure 2). The combined surface area and depth scores were categorized into three groups with differing prognosis (Table 6): good (5% re-operation), moderate (27% re-operation), and poor (67% re-operation). The interobserver Kappa was moderate (0.438, range 0.341–0.535).
Figure 2.
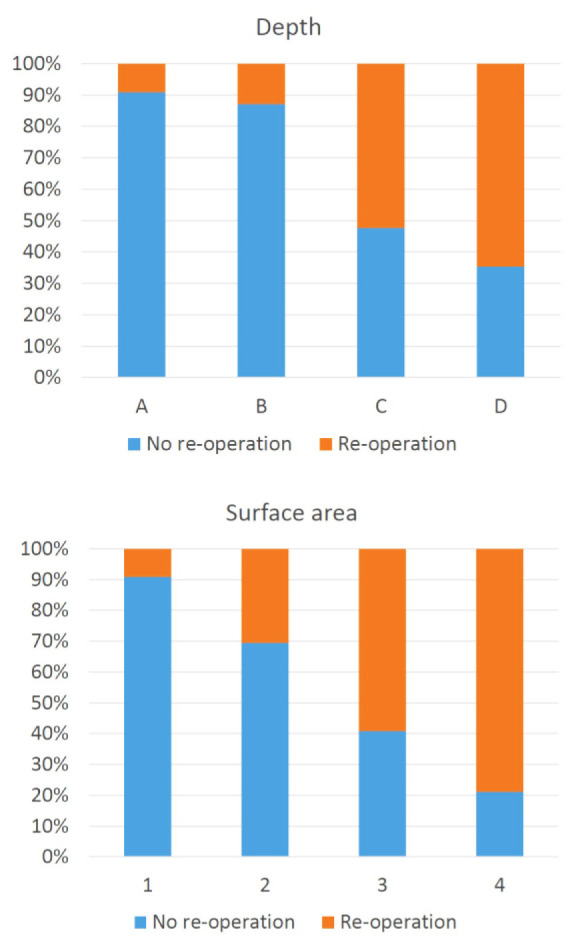
Association between the post-HBOT breast SKIN scores and re-operation (n = 183 observations). Affected surface area was scored: 1 − 0%; 2 − 1–10%; 3 − 11–30%; and 4 − > 30%. The estimated affected depth was scored: A − none; B − colour change; C − partial thickness skin flap necrosis; and D − full thickness skin flap necrosis
Table 6. Association between the post-HBOT breast SKIN scores and re-operation (n = 183 observations).
| Depth | Surface area | n | Re-operation | Prognosis |
| A | 1 | 61 | 5% | Good |
| B | 2 | |||
| B | 3 | 33 | 27% | Moderate |
| C | 2 | |||
| B | 4 | 89 | 67% | Poor |
| C | 3, 4 | |||
| D | 2, 3, 4 |
MULTIVARIATE ANALYSES
Associations between risk factors and necrosis were analysed by Spearman correlations, and between risk factors and re-operation by multivariate analyses (Table 7). The correlations were evaluated between risk factors and the degree of necrosis as graded by Matsen et al.5 Previous breast reduction (Spearman’s rho 0.3; P = 0.04), pre-operative radiotherapy (Spearman’s rho 0.3; P = 0.03), and infection (Spearman’s rho 0.4; P = 0.001) were significantly related to the degree of necrosis. Pre-operative radiotherapy (OR 7.2, 95% CI 1.4–37.3) and infection (OR 15.4, 95% CI 2.6–89.7) were risk factors for re-operation in multivariate analyses.
Table 7. Risk factors for necrosis and re-operation. *Matsen et al.,[ 5] mild: no intervention needed, healing complete at eight weeks, moderate: office debridement, healing complete at eight weeks, severe: operating room debridement, implant loss, or healing not complete at 8 weeks. Results in bold are statistically significant.
| Risk factor | Degree of necrosis* | Re-operation | ||
| Spearman’s rho | P-value | Odds’s ratio | 95% CI | |
| Age | 0.241 | 0.092 | ||
| BMI | 0.268 | 0.060 | ||
| Cup size | -0.056 | 0.697 | ||
| Previous or current smoking | -0.004 | 0.976 | ||
| Hypertension (n = 4) | 0.043 | 0.766 | ||
| Diabetes (n = 3) | -0.046 | 0.751 | ||
| Prior breast augmentation (n = 5) | -0.175 | 0.225 | ||
| Previous breast reduction (n = 3) | 0.286 | 0.044 | ||
| Previous radiation therapy (n = 11) | 0.302 | 0.033 | 7.2 | 1.4−37.3 |
| Previous chemotherapy (n = 14) | 0.153 | 0.288 | ||
| Infection (n = 12) | 0.443 | 0.001 | 15.4 | 2.6−89.7 |
| Nipple-sparing mastectomy (n = 22) | 0.011 | 0.939 | ||
| Hammond (n = 17) | -0.013 | 0.927 | ||
| Weight of mastectomy specimen | 0.173 | 0.229 | ||
| Use of an acellular dermal matrix (n = 3) | 0.029 | 0.843 | ||
| Number of days of delay to HBOT | -0.166 | 0.250 | ||
| Total number of sessions of HBOT | 0.130 | 0.367 | ||
Discussion
The aim of this study was to investigate the outcome of mastectomy flap ischaemia after HBOT in a series of 50 breasts. Our primary outcome was the need for additional surgery following HBOT, while our secondary outcome was a decrease in tissue necrosis using the SKIN score. We also sought risk factors for additional surgery despite HBOT.
HBOT improves oxygenation of poorly perfused tissue and reduces oedema, and may thereby prevent the progression of ischaemia to necrosis or limit the extent of necrosis of vascularly compromised skin grafts or flaps.[ 29 , 36] Postoperative skin flap ischaemia can progress to full thickness necrosis, resulting in wound dehiscence. In the case of mastectomy flap ischaemia and an immediate reconstruction with a tissue expander or implant, the exposed device must be removed, delaying further surgery and compromising the aesthetic outcome. Timely HBOT may sustain the ischaemic tissue until perfusion is restored, thereby preventing progression to necrosis or limiting the necrosis to partial thickness of the flap which can heal by secondary intention without additional surgery.[ 30 - 33]
In this study the SKIN score depth did deteriorate as the tissue demarcated, but the affected surface area decreased significantly with HBOT (see Figure 1 and Table 4). The SKIN score was developed to translate into groups with clinically meaningful differences: when the affected surface area decreases the likelihood of re-operation decreases9 (Figure 2 and Table 6). The inter-rater reliability for the change in SKIN score from before to after HBOT was low (Kappa 0.073–0.282); this was mostly due to differences in the pre-HBOT scores when the tissue colour is not clear. Once the tissue has demarcated post-HBOT, the interrater agreement was better. We used the post-HBOT scores to calculate the prognosis for reoperation (Kappa 0.438, range 0.341–0.535).
Only 21/50 (42%) of breasts with mastectomy flap ischaemia that were treated with HBOT underwent additional surgery (Table 3). In the literature, the need for further surgery in cases with mastectomy flap ischaemia ranges from 5–67% following treatments other than HBOT. The reoperation rate in this study with HBOT falls in the middle of that range. In a small study where mastectomy flap ischaemia was treated with a wait-and-see approach, 6/11 cases (55%) required debridement and coverage.[ 37] Another small study where all patients with mastectomy flap necrosis were treated with oral antibiotics, 10/15 patients (67%) required readmission with intravenous antibiotics, surgical debridement, and removal of their tissue expander.[ 1] In a series of nipple-sparing mastectomies, only 1/20 of nipples (5%) with necrosis required reoperation.[ 18] In another larger study where mastectomy flap necrosis was treated with a wait-and-see approach, 18/69 breasts (26%) required skin excision, debridement, or implant removal.[ 9] In a large series of nipple-sparing mastectomies, reoperation was required in 69/141 (49%) of ischaemic nipples.[ 38] In the largest study of 178 patients with mastectomy flap necrosis who were treated with expansion of the tissue expander, 120 (67%) healed spontaneously and 58 (33%) required surgical excision of the eschar or removal of the tissue expander.[ 3] It is not possible to compare the outcomes of these studies to our study since each uses a different definition of mastectomy flap ischaemia, leading to selection bias.
Until now only one large study had investigated the efficacy of HBOT in limiting necrosis of ischaemic skin flaps. The study reviewed the outcome of 65 compromised flaps in a heterogeneous population, including soft tissue injuries and osteomyelitis.[ 26] The treatment outcome was judged on the appearance of the flap. Following HBOT 55% had ‘no flap necrosis’, 34% had ‘minimal flap necrosis’, and 11% had ‘flap necrosis requiring a further covering procedure or extensive healing by secondary intention’. The authors concluded that 89% of compromised flaps were ‘salvaged’ by HBOT. Patients whose outcome were unsuccessful were older (60 vs. 48 years), had a longer delay to initiation of HBOT (20 vs. 5 days), a greater number of HBOT treatments (42 vs. 28 sessions), and a greater number of risk factors associated with poor wound healing (soft tissue infections, radiation therapy, peripheral vascular disease, and diabetes mellitus). Delay to HBOT and total number of HBOT treatments were not significant risk factors for reoperation in our study (Table 7).
In previous studies, various risk factors have been identified for necrosis; the common mechanism in all is impaired perfusion. In this study risk factors that were associated with necrosis and reoperation were previous breast reduction, preoperative radiotherapy, and infection (Table 7). These risk factors were also found to be significant in other studies. Prior surgical scars can compromise skin perfusion leading to a higher prevalence of necrosis in these patients.[ 39] Severe skin necrosis was 14 times more likely in previously irradiated patients.[ 37] Patients with mastectomy skin necrosis have a 15 times higher odds of developing an infection requiring intervention and an almost 16 times the odds of requiring their tissue expander to be prematurely removed.[ 1] Other risk factors that were also shown to be associated with necrosis and reoperation in other studies were not correlated in this study, perhaps due to small patient numbers in those subgroups.
Interestingly, in this study previous or current smoking was not correlated with the degree of necrosis nor the need for additional surgery. Other studies have shown that smoking was significantly associated with necrosis[ 37] and excision,[ 3] and reduced the effect of HBOT on compromised flaps.[ 25] In fact, in some HBOT centres, smoking is considered so detrimental to the effect of HBOT that the treatment was discontinued for patients who refused to refrain from smoking.[ 26]
The main limitation of this study is that there was no control group with mastectomy flap ischaemia who did not undergo HBOT. It is unknown what proportion would have resolved with a wait-and-see approach,[ 3 , 5 , 9] and what proportion would be re-operated. Another limitation is that the indication for reoperation was not clear: haematoma, seroma, infection, and flap necrosis could all be independent indications for reoperation.
Conclusions
Limiting necrosis is important to reduce morbidity and the costs of repetitive reoperation.[ 1 , 26] In this case series of patients with mastectomy flap ischaemia, the surface area of the breast affected by ischaemia decreased during HBOT, and most breasts (29/50, 58%) did not undergo an additional operation. A randomised controlled trial is needed to confirm or refute the possibility that HBOT improves outcome in patients with mastectomy flap ischaemia.
Footnotes
Acknowledgements
The authors thank Servaas Buijs of HealthCare Insights BV for reviewing the manuscript and performing the statistical analyses on the SKIN scores.
Conflict of interest and funding: nil
Contributor Information
Nicole E Spruijt, Da Vinci Clinic, Geldrop, the Netherlands.
Lisette T Hoekstra, Da Vinci Clinic, Geldrop, the Netherlands; Department of Plastic, Reconstructive and Hand Surgery, Maastricht UMC+, the Netherlands.
Johan Wilmink, Department of Plastic, Reconstructive and Hand Surgery, Maxima Medical Center Eindhoven, the Netherlands.
Maarten M Hoogbergen, Da Vinci Clinic, Geldrop, the Netherlands; Department of Plastic, Reconstructive and Hand Surgery, Catharina Hospital, Eindhoven, the Netherlands.
References
- Yalanis GC, Nag S, Georgek JR, Cooney CM, Manahan MA, Rosson GD, et al. Mastectomy weight and tissue expander volume predict necrosis and increased costs associated with breast reconstruction. Plast Reconstr Surg Glob Open. 2015;3(7):e450. doi: 10.1097/GOX.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen MA, Nickel KB, Fox IK, Margenthaler JA, Wallace AE, Fraser VJ. Comparison of wound complications after immediate, delayed, and secondary breast reconstruction procedures. JAMA Surg. 2017;152(9):e172338. doi: 10.1001/jamasurg.2017.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony AK, Mehrara BM, McCarthy CM, Zhong T, Kropf N, Disa JJ, et al. Salvage of tissue expander in the setting of mastectomy flap necrosis: A 13-year experience using timed excision with continued expansion. Plast Reconstr Surg. 2009;124:356–63. doi: 10.1097/PRS.0b013e3181aee9a3. [DOI] [PubMed] [Google Scholar]
- Hansen N, Espino S, Blough JT, Vu MM, Fine NA, Kim JYS. Evaluating mastectomy skin flap necrosis in the extended breast reconstruction risk assessment score for 1-year prediction of prosthetic reconstruction outcomes. J Am Coll Surg. 2018;227:96–104. doi: 10.1016/j.jamcollsurg.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Matsen CB, Mehrara B, Eaton A, Capko D, Berg A, Stempel M, et al. Skin flap necrosis after mastectomy with reconstruction: A prospective study. Ann Surg Oncol. 2016;23:257–64. doi: 10.1245/s10434-015-4709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta MN, Gerety PA, Serletti JM, Kovach SJ, Fischer JP. A systematic review and head-to-head meta-analysis of outcomes following direct-to-implant versus conventional two-stage implant reconstruction. Plast Reconstr Surg. 2015;136:1135–44. doi: 10.1097/PRS.0000000000001749. [DOI] [PubMed] [Google Scholar]
- Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: A meta-analysis. Ann Surg Oncol. 2016;23:600–10. doi: 10.1245/s10434-015-4873-9. [DOI] [PubMed] [Google Scholar]
- Frey JD, Alperovich M, Weichman KE, Wilson SC, Hazen A, Saadeh PB, et al. Breast reconstruction using contour fenestrated AlloDerm: Does improvement in design translate to improved outcomes? Plast Reconstr Surg Glob Open. 2015; 3 (9): e505. 10.1097/GOX.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaine V, Hoskin TL, Farley DR, Grant CS, Boughey JC, Torstenson TA, et al. Introducing the SKIN score: A validated scoring system to assess severity of mastectomy skin flap necrosis. Ann Surg Oncol. 2015;22:2925–32. doi: 10.1245/s10434-015-4409-3. [DOI] [PubMed] [Google Scholar]
- Sanniec K, Teotia S, Amirlak B. Management of tissue ischaemia in mastectomy skin flaps: Algorithm integrating SPY angiography and topical nitroglycerin. Plast Reconstr Surg Glob Open. 2016;4(10):e1075. doi: 10.1097/GOX.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalevitch P, Van Laeken N, Bahng S, Ho A, Bovill E, Lennox P, et al. Effects of nitroglycerin ointment on mastectomy flap necrosis in immediate breast reconstruction: a randomized controlled trial. Plast Reconstr Surg. 2015;135:1530–9. doi: 10.1097/PRS.0000000000001237. [DOI] [PubMed] [Google Scholar]
- Yun MH, Yoon ES, Lee BI, Park SH. The effect of low-dose nitroglycerin ointment on skin flap necrosis in breast reconstruction after skin-sparing or nipple-sparing mastectomy. Arch Plast Surg. 2017;44:509–15. doi: 10.5999/aps.2017.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Jeevaratnam JA, Agrawal A, Cutress RI. Mastectomy skin flap necrosis: Challenges and solutions. Breast Cancer (Dove Med Press). 2017;9:141–52. doi: 10.2147/bctt.s81712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin SY, Li DD, Vaca EE, Fine N. Nitroglycerin ointment for reducing the rate of mastectomy flap necrosis in immediate implant-based breast reconstruction. Plast Reconstr Surg. 2018;142:264e–70e. doi: 10.1097/prs.0000000000004633. [DOI] [PubMed] [Google Scholar]
- Rand-Luby L, Pommier RF, Williams ST, Woltering EA, Small KA. Fletcher WS. Improved outcome of surgical flaps treated with topical dimethylsulfoxide. Ann Surg. 1996;224:583–90. doi: 10.1097/00000658-199610000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz BD, Sulu B. Effects of dextran-40 on flap viability after modified radical mastectomy. Can J Plast Surg. 2013;21:83–6. doi: 10.1177/229255031302100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaine V, Hoskin TL, Boughey JC, Farley DR, Grant CS, Jacobson SR, et al. Abstract 99: Reducing unplanned reoperations for mastectomy skin flap necrosis – a multidisciplinary approach. Plast Reconstr Surg. 2014;133(3 Suppl):112. doi: 10.1097/01.prs.0000444923.68700.9e. [DOI] [PubMed] [Google Scholar]
- Carlson GW, Chu CK, Moyer HR, Duggal C, Losken A. Predictors of nipple ischaemia after nipple sparing mastectomy. Breast J. 2014;20:69–73. doi: 10.1111/tbj.12208. [DOI] [PubMed] [Google Scholar]
- McCrary BF. Hyperbaric oxygen (HBO2) treatment for a failing facial flap. Postgrad Med J. 2007;83(975):e1. doi: 10.1136/pgmj.2006.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR. Hyperbaric oxygen: Its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HI, Fitzmaurice M, Lefaivre JF, Vecchiolla T, Clarke D. An evidence-based appraisal of the use of hyperbaric oxygen on flaps and grafts. Plast Reconstr Surg. 2006;117(7 Suppl):175S–190S. doi: 10.1097/01.prs.0000222555.84962.86. [DOI] [PubMed] [Google Scholar]
- Kucur C, Durmus K, Uysal IO, Old M, Agrawal A, Arshad H, et al. Management of complications and compromised free flaps following major head and neck surgery. Eur Arch Otorhinolaryngol. 2016;273:209–13. doi: 10.1007/s00405-014-3489-1. [DOI] [PubMed] [Google Scholar]
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23–32. doi: 10.1089/wound.2016.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeik N, Porten BR, Isaacson E, Seong J, Klosterman DL, Garberich RF, et al. Hyperbaric oxygen treatment outcome for different indications from a single center. Ann Vasc Surg. 2015;29:206–14. doi: 10.1016/j.avsg.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Larson JV, Steensma EA, Flikkema RM, Norman EM. The application of hyperbaric oxygen therapy in the management of compromised flaps. Undersea Hyperb Med. 2013;40:499–504. [PubMed] [Google Scholar]
- Bowersox J, Strauss M, Hart G. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperbaric Med. 1986;1:141–9. [Google Scholar]
- Dauwe PB, Pulikkottil BJ, Lavery L, Stuzin JM, Rohrich RJ. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast Reconstr Surg. 2014;133:208e–215e. doi: 10.1097/01.prs.0000436849.79161.a4. [DOI] [PubMed] [Google Scholar]
- Baynosa RC, Zamboni WA. The effect of hyperbaric oxygen on compromised grafts and flaps. Undersea Hyperb Med. 2012;39:857–65. [PubMed] [Google Scholar]
- Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion, and transcutaneous oximetry. World J Surg. 2004;28:307–11. doi: 10.1007/s00268-003-7401-1. [DOI] [PubMed] [Google Scholar]
- Copeland-Halperin LR, Bruce SB, Mesbahi AN. Hyperbaric oxygen following bilateral skin-sparing mastectomies: A case report. Plast Reconstr Surg Glob Open. 2016;4(4):e680. doi: 10.1097/gox.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman R, Wise I, Friedman T, Heller L, Karni T. Skin-sparing mastectomy flap ischaemia salvage using urgent hyperbaric chamber oxygen therapy: A case report. Undersea Hyperb Med. 2014;41:145–7. [PubMed] [Google Scholar]
- Mermans JF, Tuinder S, von Meyenfeldt MF, van der Hulst RR. Hyperbaric oxygen treatment for skin flap necrosis after a mastectomy: A case study. Undersea Hyperb Med. 2012;39:719–23. [PubMed] [Google Scholar]
- Moffat AD, Weaver LK, Tettelbach WH. Compromised breast flap treated with leech therapy, hyperbaric oxygen, pentoxifylline and topical nitroglycerin: A case report. Undersea Hyperb Med. 2015;42:281–4. [PubMed] [Google Scholar]
- Alperovich M, Harmaty M, Chiu ES. Treatment of nipple-sparing mastectomy necrosis using hyperbaric oxygen therapy. Plast Reconstr Surg. 2015;135:1071e–1072e. doi: 10.1097/prs.0000000000001229. [DOI] [PubMed] [Google Scholar]
- Shuck J, O’Kelly N, Endara M, Nahabedian MY. A critical look at the effect of hyperbaric oxygen on the ischemic nipple following nipple sparing mastectomy and implant based reconstruction: A case series. Gland Surg. 2017;6:659–65. doi: 10.21037/gs.2017.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LK, editor. Undersea and Hyperbaric Medicine Society hyperbaric oxygen therapy indications. 13th ed. Palm Beach (FL): Best Publishing Company; 2014. [Google Scholar]
- Demiri E, Dionyssiou D, Sapountzis S, Pavlidis L, Natsiopoulos I, Miliaras S. Becker expander-based breast reconstruction following wise pattern skin-reducing mastectomy: Complication rates and risk factors. Aesthetic Plast Surg. 2017;41:304–11. doi: 10.1007/s00266-016-0732-8. [DOI] [PubMed] [Google Scholar]
- Ahn SJ, Woo TY, Lee DW, Lew DH, Song SY. Nipple-areolar complex ischaemia and necrosis in nipple-sparing mastectomy. Eur J Surg Oncol. 2018;44:1170–6. doi: 10.1016/j.ejso.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Dent BL, Cordeiro CN, Small K, Clemons JA, Kessler EG, Swistel A, et al. Nipple-sparing mastectomy via an inframammary fold incision with implant-based reconstruction in patients with prior cosmetic breast surgery. Aesthet Surg J. 2015;35:548–57. doi: 10.1093/asj/sju158. [DOI] [PubMed] [Google Scholar]