Graphical Abstract
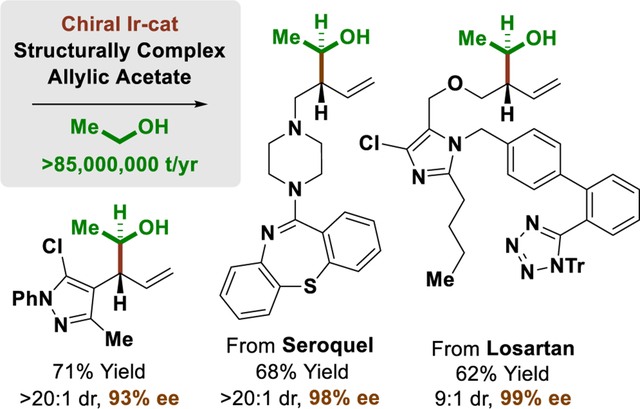
Maize to Medicine. With annual production at >85 million tons/year, ethanol is the world’s largest-volume renewable small molecule carbon source, yet its use as a C2-feedstock in enantioselective C-C coupling is unknown. Here, the first catalytic enantioselective C-C couplings of ethanol are demonstrated in reactions with structurally complex, nitrogen-rich allylic acetates incorporating the top 10 N-heterocycles found in FDA-approved drugs.
Keywords: Ethanol, Renewable, Enantioselective, Hydrogen Transfer, Iridium
A 2017 BASF survey reveals that only 13% of the 20.8 million tons of carbon feedstocks used to manufacture organic chemicals were derived from renewable sources, with the remainder generated from crude oil (76%), natural gas (10%) or coal (1%).[1] Ethanol is produced at a rate of >85 million tons/year worldwide,[2] making it the largest volume renewable small molecule feedstock. The vast majority of commercial ethanol is consumed as fuel. The goal of achieving sustainable, carbon-neutral routes to large-volume commodity chemicals using ethanol as a C2-feedstock is highly underdeveloped and merits greater attention.[3] The Lebedev ethanol-to-butadiene process,[4] and more recent industrial efforts to launch ethanol-to-polyethylene processes,[5] underscore the feasibility of ethanol-based chemical manufacture. In the realm of commodity and fine chemical synthesis, ethanol use is relegated to the preparation of achiral C2-compounds: ethyl halides, ethyl esters, diethyl ether, acetic acid, and ethyl amines.[2] Beyond ethanol-to-butanol[7,8] and other Guerbet-type reactions,[9] catalytic C-C couplings of ethanol are highly uncommon and largely limited to isolated examples in which racemic adducts are formed.[10,11] To our knowledge, catalytic enantioselective C-C couplings of ethanol are entirely absent from the chemical literature (Figure 1).[12] Here, in connection with our efforts to develop asymmetric alcohol-mediated carbonyl additions,[13] we report the first highly diastereo- and enantioselective conversions of ethanol to higher alcohols, which are achieved via catalytic C-C coupling with structurally complex nitrogen-rich allylic acetates, including those that incorporate the top 10 most frequently encountered N-heterocycles found in FDA-approved drugs.[14]
Figure 1.
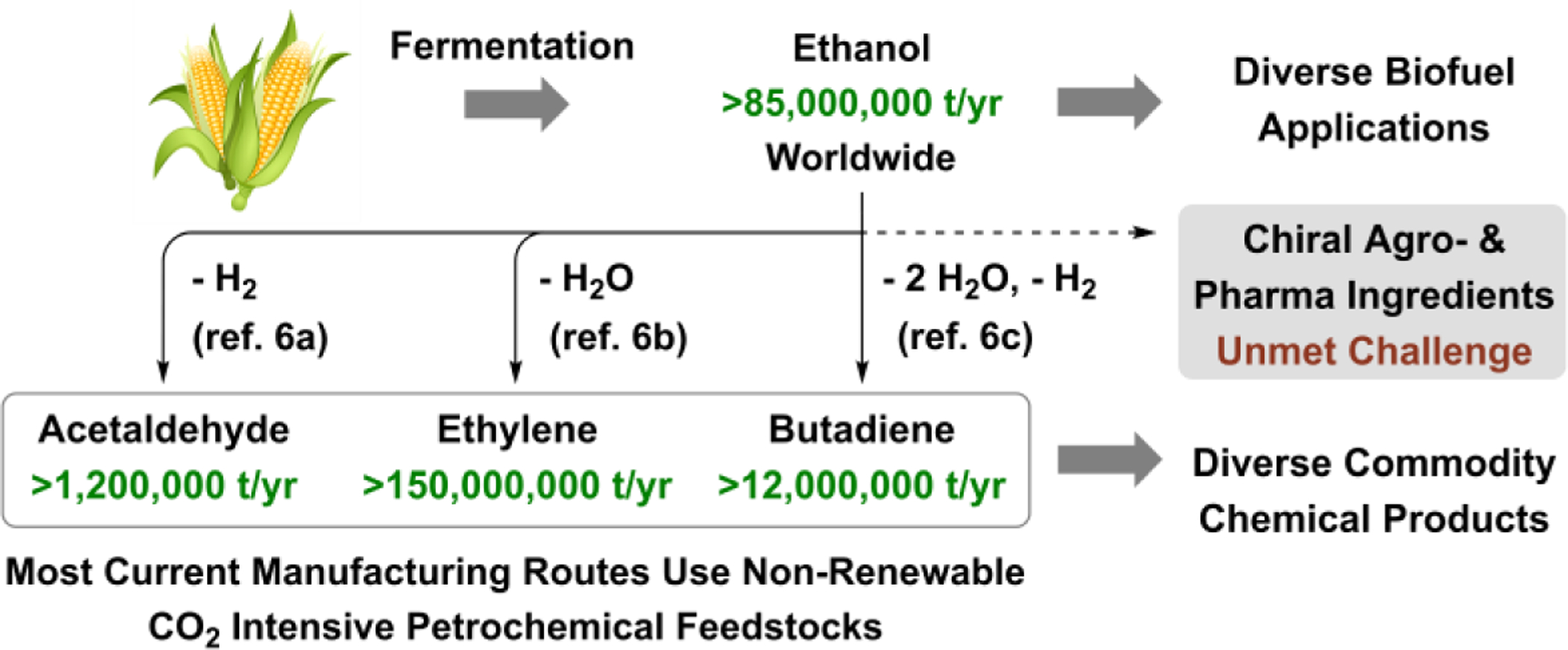
Opportunities for ethanol-based chemical manufacture.
Earlier studies from our laboratory on the enantioselective iridium-catalyzed C-C coupling of primary alcohols were conducted with excess allyl acetate using the alcohol as the limiting reagent.[15] To adapt this method for the catalytic conversion of ethanol to higher chiral non-racemic alcohols (Scheme 1), the more valuable and structurally complex allylic acetates were chosen as limiting reagents. This posed a major challenge as competing transfer hydrogenolysis, which occurs via protonation of the intervening π-allyliridium nucleophile, represents a major side reaction. Indeed, protonolytic cleavage of the π-allyliridium precatalyst is required for entry into the catalytic cycle, and prior work from our laboratory suggests carbonyl addition is the turn-over limiting event in the catalytic cycle.[15b] Upon evaluation of different axially chiral chelating phosphine ligands in the reaction of ethanol with allylic acetate 1a, it was found that optimal isolated yields of 2a were obtained using the π-allyliridium-C,O-benzoate modified by (S)-Cl,MeO-BIPHEP, (S)-Ir-V. This more electron-deficient ligand may retard the rate of π-allyl protonation, enabling higher conversion to 2a. Reactions catalyzed by (S)-Ir-V also displayed significantly higher enantioselectivity, which we attribute to enhanced Lewis acidity at iridium, which, in turn, may shorten Ir-O and Ir-C bonds in the transition structure for carbonyl addition to enhance asymmetric induction. Finally, it was reasoned that a less Lewis basic solvent, MTBE, would further facilitate carbonyl addition by promoting association of transient acetaldehyde with the allyliridium intermediate. A substantial increase in the isolated yields of adduct 2a was observed, which was further elevated by increasing reaction concentration.
Scheme 1.
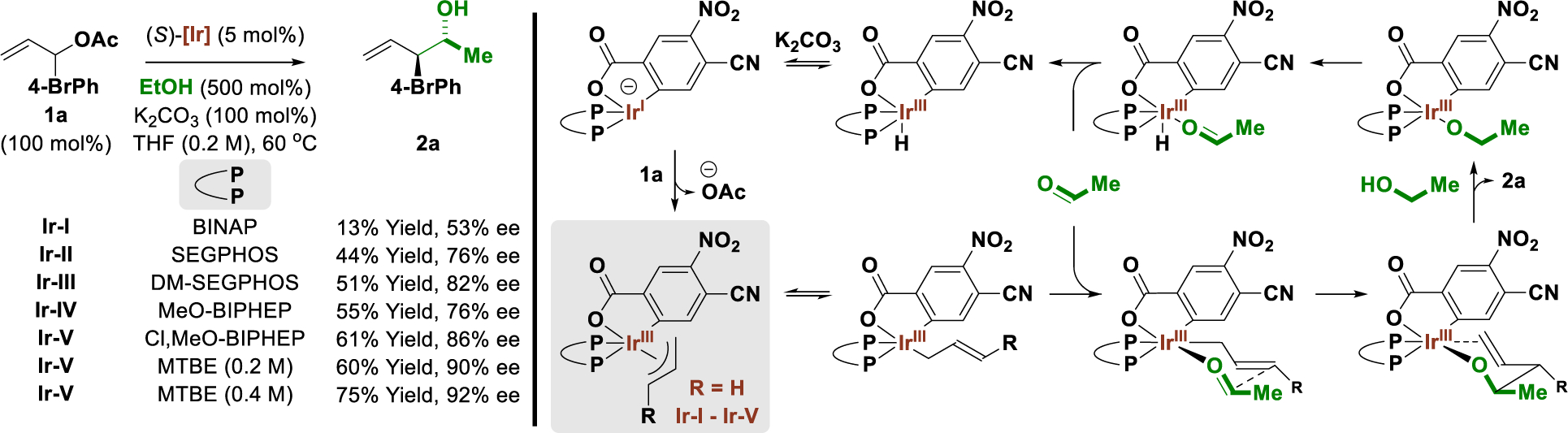
Selected optimization experiments in the enantioselective π-allyliridium-C,O-benzoate-catalyzed coupling of ethanol with allylic acetate 1a and general mechanism.a
aYields are of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
These optimized conditions were applied to the enantioselective π-allyliridium-C,O-benzoate-catalyzed coupling of ethanol with a structurally diverse array of allylic acetates (Table 1). As determined in a recent structural survey of U.S. FDA approved drugs, 59% of small molecule drugs incorporate N-heterocycles.[14] Hence, to highlight the potential utility of this method vis-à-vis drug discovery, allylic acetates 1a-1y that incorporate the 10 most frequently encountered N-heterocycles in FDA approved drugs (beyond β-lactams) were specifically chosen for evaluation as coupling partners. To our delight, the products of ethanol-mediated C-C coupling 2a-2y were formed in good yield with excellent levels of anti-diastereoselectivity and enantioselectivity. This includes allylic acetates substituted by aryl groups (2a, 2b, 2f-2k), heteroaryl groups (2c, 2d, 2l-2o), including ortho-,ortho-disubstituted heteroarene (2o), as well as alkyl (2s-2y) and cycloalkyl groups (2p-2r). Of particular significance, due to the high functional group tolerance of the catalyst, direct asymmetric (1-hydroxy)ethylation of allylic acetates derived from the FDA-approved drugs indomethacin (2w), losartan (2x) and seroquel (2y) can be achieved efficiently, establishing the utility of this method for late-stage functionalization of complex nitrogen-rich clinical candidates.[16] As illustrated by the conversion of chiral allylic acetate 1v to adducts 2v and iso-2v, which are derived from (+)-α-pinene, ethanol-mediated (1-hydroxy)ethylation proceeds with good levels of catalyst-directed diastereoselectivity. The absolute and relative stereochemical assignments of products 2a-2y were made in analogy to that observed for compound 2p, which was determined via single crystal X-ray diffraction. Finally, beyond allylic acetates, the vinyl epoxide 3a[17] and allenamide 3b[18] are competent pronucleophiles, as illustrated by the diastereo- and enantioselective formation of adducts 4a (eq. 1) and 4b (eq. 2), respectively.
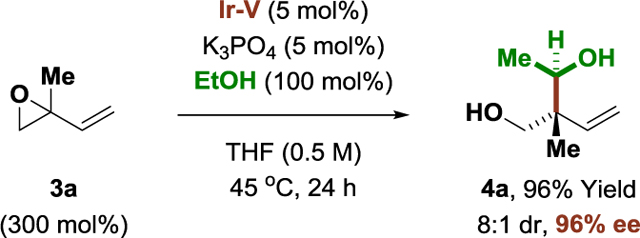 |
(eq. 1) |
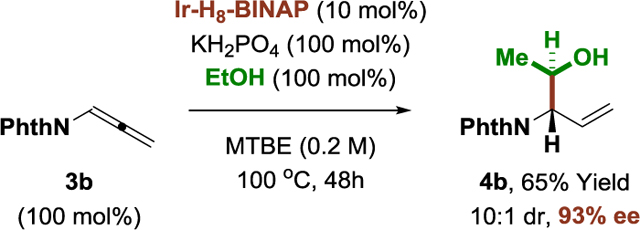 |
(eq. 2) |
Table 1.
Diastereo- and enantioselective π-allyliridium-C,O-benzoate-catalyzed coupling of ethanol with allylic acetates 1a-1y to form homoallylic alcohols 2a-2y.a
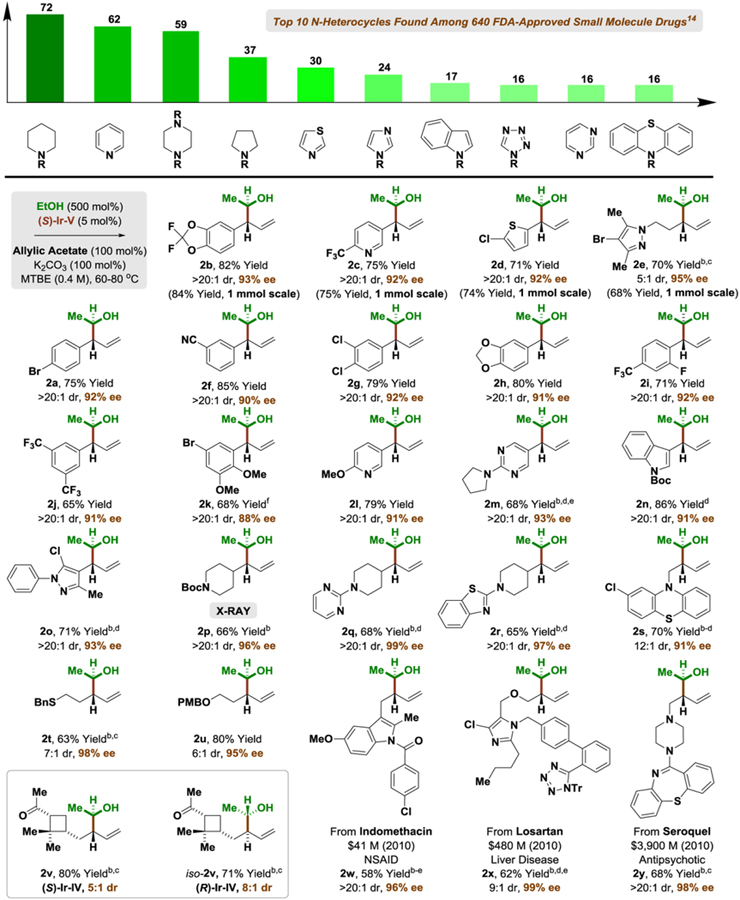
|
Yields are of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
Acetone (1.0 M),
(S)-Ir-IV,
EtOH (300 mol%),
K2CO3 (50 mol%),
K2CO3 (200 mol%).
To illustrate the utility of the present method vis-á-vis drug discovery, selected products of (1-hydroxy)ethylation 2b, 2c, 2e, 2n and 2o were converted to the N-Boc α-methylamines 5a-5f, respectively (Scheme 2). α-Methylphenethylamines, such as the parent “amphetamine,” are bioactive, often psychoactive,[19] substances that appear ubiquitously as substructures in clinical candidates. For example, the FDA-approved drug tamulosin[20a] and the clinical candidates talampanel,[20b] taranabant[20c] and cipargamin[20d] all incorporate α-methylphenethylamine substructures, yet act against diverse disease. Exposure of adducts 2b, 2c, 2e, 2n and 2o to diphenylphosphoryl azide under Mitsunobu conditions delivers the corresponding azides with inversion of stereochemistry.[21] Staudinger reduction[22] followed by treatment with di-tert-butyl dicarbonate in situ provided the N-Boc α-methylamines 5a-5f in good yield with no erosion of diastereomeric enrichment.
Scheme 2.
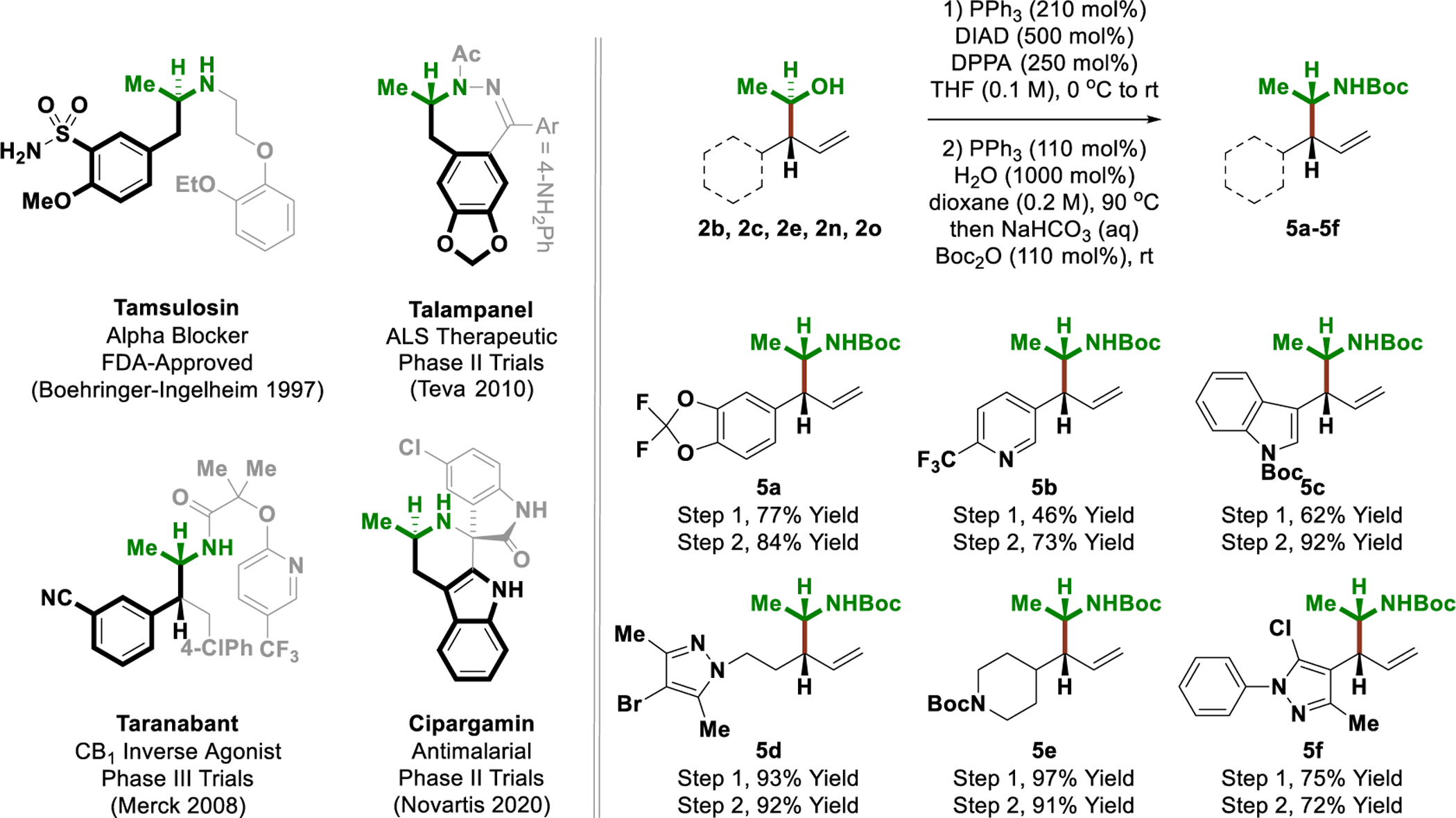
Representative phenethyl amines and conversion of ethanol adducts to phenethylamines 5a-5f.a
aYields are of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
In conclusion, we report the first catalytic enantioselective C-C couplings of ethanol, the world’s most abundant renewable small molecule carbon feedstock. Specifically, in a hydrogen auto-transfer process catalyzed by a cyclometallated π-allyliridium-C,O-benzoate complex modified by (S)-Cl,MeO-BIPHEP, ethanol dehydrogenation drives reductive C-O bond cleavage of allylic acetates to form allyliridium nucleophiles and acetaldehyde, which combine to deliver product of (1-hydroxy)ethylation. The broad scope of this method is demonstrated by couplings with structurally complex, nitrogen-rich allylic acetates that incorporate the top 10 N-heterocycles found in FDA-approved drugs. Conversion of selected adducts to α-methylphenethylamines is described, further highlighting applicability of this method to drug discovery. The present method requires no premetalated reagents and generates acetic acid as the sole stoichiometric byproduct. Given the increasing importance iridium-catalyzed dehydrogenation in process R&D,[23] it is the authors’ hope this work will inspire other “green” catalytic methods for the atom-efficient conversion of renewable feedstocks to value-added products.[24]
Supplementary Material
Acknowledgments
The Welch Foundation (F-0038) and the NIH (RO1-GM069445) are acknowledged for partial support of this research.
References
- [1].Statistic refers to consumer goods produced in Germany in 2017:; Schaub T, Chem. Eur. J 2021, 27, 1865–1869. [DOI] [PubMed] [Google Scholar]
- [2].For selected literature on ethanol production volumes and use as a C2 feedstock, see:; a) Kosaric N, Duvnjak Z, Farkas A, Sahm H, Bringer-Meyer S, Goebel O, Mayer D, Ullmann’s Encyclopedia of Industrial Chemistry, Vol. 13, Wiley-VCH Verlag GmbH & Co., Weinheim, 2011, pp. 333–403; [Google Scholar]; b) Renewable Fuels Association (RFA), “Annual World Fuel Ethanol Production (Mil. Gal.)” can be found at https://ethanolrfa.org/statistics/annual-ethanol-production/, 2021. [Google Scholar]
- [3].For selected reviews on sustainable chemical synthesis, see:; a) Petersen GR, Bozell JJ, Green Chem. 2010, 12, 539–554; [Google Scholar]; b) Varma RS, ACS Sustainable Chem. Eng 2016, 4, 5866–5878; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mika LT, Cséfalvay E, Németh Á, Chem Rev. 2018, 118, 505–613; [DOI] [PubMed] [Google Scholar]; d) Gunukula S, Pendse HP, DeSisto WJ, Wheeler MC, ACS Sustainable Chem. Eng 2018, 6, 5533–5539; [Google Scholar]; e) Gonzalez MA, Takkellapati S, Tadele K, Li T, Varma RS, ACS Sustainable Chem. Eng 2019, 7, 6744–6757; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Doerksen RS, Meyer CC, Krische MJ, Angew. Chem 2019, 131, 14193–14202; Angew. Chem. Int. Ed. 2019, 58, 14055–14064; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Dalton T, Faber T, Glorius F, ACS Cent. Sci 2021, 7, 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].For a historical perspective on the Lebedev ethanol-to-butadiene process, see:; Pomalaza G, Capron M, Ordomsky V, Dumeignil F, Catalysts 2016, 6, 203; 10.3390/catal6120203. [DOI] [Google Scholar]
- [5].For selected reviews on ethanol-to-polyethylene processes, see:; a) Collares-Queiroz FP, Queiroz AUB, Polym. Rev 2009, 49, 65–78; [Google Scholar]; b) Iles A, Martin AN, J. Clean. Prod 2013, 45, 38–49; [Google Scholar]; c) de Andrade Coutinho PL, Morita AT, Cassinelli LF, Morschbacker A, Do Carmo RW in Catalytic Process Development for Renewable Materials, (Eds.: Imhof P, van der Waal JC), Wiley-VCH Verlag GmbH & Co.: Weinheim, 2013, pp. 149–166. [Google Scholar]
- [6].For feedstock production volumes and synthesis from ethanol, see:; a) Eckert M, Fleischmann F, Jira R, Bolt HM, Golka K, Ullmann’s Encyclopedia of Industrial Chemistry, Vol. 1, Wiley-VCH Verlag GmbH & Co., Weinheim, 2006, pp. 191–207; [Google Scholar]; b) Zimmermann H, Walzl R, Ullmann’s Encyclopedia of Industrial Chemistry, Vol. 13, Wiley-VCH Verlag GmbH & Co., Weinheim, 2009, pp. 465–529; [Google Scholar]; c) Dahlmann M, Grub J, Loser E, Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co., Weinheim, 2011, pp. 1–24. [Google Scholar]
- [7].For selected examples of the catalytic conversion of ethanol-to-butanol, see:; a) Dowson GRM, Haddow MF, Lee J, Wingad RL, Wass DF, Angew. Chem 2013, 125, 9175–9178; Angew. Chem. Int. Ed. 2013, 52, 9005–9008; [DOI] [PubMed] [Google Scholar]; b) Chakraborty S, Piszel PE, Hayes CE, Baker RT, Jones WD, J. Am. Chem. Soc 2015, 137, 14264–14267; [DOI] [PubMed] [Google Scholar]; c) Xie Y, Ben-David Y, Shimon LJW, Milstein D, J. Am. Chem. Soc 2016, 138, 9077–9080. [DOI] [PubMed] [Google Scholar]
- [8].For a recent review of the catalytic conversion of ethanol-to-butanol, see:; Aitchison H, Wingad RL, Wass DF, ACS Catal. 2016, 6, 7125–7132. [Google Scholar]
- [9].For recent examples of the use of ethanol in Guerbet-type reactions, see:; a) Gawali SS, Pandia BK, Pal S, Gunanathan C, ACS Omega, 2019, 4, 10741–10754; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kobayashi M, Itoh S, Yoshimura K, Tsukamoto Y, Obora Y, J. Org. Chem 2020, 85, 11952–11958; [DOI] [PubMed] [Google Scholar]; c) Ng TW, Liao G, Lau KK, Pan H-J, Zhao Y, Angew. Chem 2020, 132, 11480–11485; Angew. Chem. Int. Ed. 2020, 59, 11384–11389. [DOI] [PubMed] [Google Scholar]
- [10].For isolated examples of the catalytic C-C couplings of ethanol to form racemic adducts beyond Guerbet-type reactions, see:; a) Shi L, Tu Y-Q, Wang M, Zhang F-M, Fan C-A, Zhao Y-M, J. Am. Chem. Soc 2005, 127, 10836–10837; [DOI] [PubMed] [Google Scholar]; b) Jiang Y-J, Tu Y-Q, Zhang E, Zhang S-Y, Cao K, Shi L, Adv. Synth. Catal 2008, 350, 522–556; [Google Scholar]; c) Obora Y, Hatanaka S, Ishii Y, Org. Lett 2009, 11, 3510–3513; [DOI] [PubMed] [Google Scholar]; d) Guo S.-r., Yuan Y.-q., Synlett 2015, 26, 1961–1968. [Google Scholar]
- [11].Han H, Krische MJ, Org. Lett 2010, 12, 2844–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].For an authoritative review on enantioselective hydrogen auto-transfer reactions, see:; Kwok T, Hoff O, Armstrong RJ, Donohoe TJ, Chem. Eur. J 2020, 26, 12912–12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].For recent reviews on alcohol-mediated carbonyl addition via metal-catalyzed hydrogen auto-transfer, see:; a) Ketcham JM, Shin I, Montgomery TP, Krische MJ, Angew. Chem 2014, 126, 9294–9302; Angew. Chem. Int. Ed. 2014, 53, 9142–9150; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ, Science 2016, 354, aah5133; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kim SW, Zhang W, Krische MJ, Acc. Chem. Res 2017, 50, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vitaku E, Smith DT, Njardarson JT, J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- [15].For selected examples of the enantioselective iridium-catalyzed C-C coupling of primary alcohols with allylic acetates, see:; a) Kim IS, Ngai M-Y, Krische MJ, J. Am. Chem. Soc 2008, 130, 6340–6341; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim IS, Ngai M-Y, Krische MJ, J. Am. Chem. Soc 2008, 130, 14891–14899; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lu Y, Kim IS, Hassan A, Del Valle DJ, Krische MJ, Angew. Chem 2009, 121, 5118–5121; Angew. Chem. Int. Ed. 2009, 48, 5018–5021; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hassan A, Lu Y, Krische MJ, Org. Lett 2009, 11, 3112–3115; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Schmitt DC, Dechert-Schmitt A-MR, Krische MJ, Org. Lett 2012, 14, 6302–6305; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Dechert-Schmitt A-MR, Schmitt DC, Krische MJ, Angew. Chem 2013, 125, 3277–3280; Angew. Chem. Int. Ed. 2013, 52, 3195–3198; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Shin I, Wang G, Krische MJ, Chem. Eur. J 2014, 20, 13382–13389; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Kim SW, Lee W, Krische MJ, Org. Lett 2017, 19, 1252–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].For a selected reviews on late-stage functionalization of complex small molecules, see:; a) Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW, Chem. Soc. Rev 2016, 45, 546–576; [DOI] [PubMed] [Google Scholar]; b) Shugrue CR, Miller SJ, Chem. Rev 2017, 117, 11894–11951; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Blakemore DC, Castro L, Churcher I, Rees DC, Thomas AW, Wilson DM, Wood A, Nat. Chem 2018, 10, 383–394; [DOI] [PubMed] [Google Scholar]; d) Dominguez-Huerta A, Dai X-J, Zhou F, Querard P, Qiu Z, Ung S, Liu W, Li J, Li C-J, Can. J. Chem 2019, 97, 67–85. [Google Scholar]
- [17].a) Feng J, Garza VJ, Krische MJ, J. Am. Chem. Soc 2014, 136, 8911–8914; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guo Y-A, Lee W, Krische MJ, Chem. Eur. J 2017, 23, 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spielmann K, Xiang M, Schwartz LA, Krische MJ, J. Am. Chem. Soc 2019, 141, 14136–14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].For a review on phenethylamines, see:; a) Aghajanian GK, Marek GJ, Neuropsychopharm. 1999, 21(2s), 16S–23S; [DOI] [PubMed] [Google Scholar]; b) Trachsel D, Drug Test. Anal 2012, 4, 577–590; [DOI] [PubMed] [Google Scholar]; c) King LA, Drug Test. Anal 2013, 6, 808–818; [DOI] [PubMed] [Google Scholar]; d) Inan F, Brunt TM, Contrucci RR, Hondebrink L, Franssen EJF, Ther. Drug Monit 2020, 42, 271–281. [DOI] [PubMed] [Google Scholar]
- [20].a) Lowe FC, Rev. Urol 2005, 7, S13–21; [PMC free article] [PubMed] [Google Scholar]; b) Iwamoto FM, Kreisl TN, Kim L, Duic JP, Butman JA, Albert PS, Fine HA, Cancer 2010, 116, 1776–1782; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Fong TM, Guan XM, Marsh DJ, Shen CP, Stribling DS, Rosko KM, Lao J, Yu H, Feng Y, Xiao JC, Van der Ploeg LHT, Goulet MT, Hagmann WK, Lin LS, Lanza TJ Jr., Jewell JP, Liu P, Shah SK, Qi H, Tong X, Wang J, Xu SS, Francis B, Strack AM, MacIntyre DE, Shearman LP, J. Pharmacol. Exp. Ther 2007, 321, 1013–1022; [DOI] [PubMed] [Google Scholar]; d) Bouwman SAM, Zoleko-Manego R, Renner KC, Schmitt EK, Mombo-Ngoma G, Grobusch MP, Travel Med. Infect. Dis 2020, 101765, 1–8. [DOI] [PubMed] [Google Scholar]
- [21].Lal B, Pramanik BN, Manhas MS, Bose AK, Tetrahedron Lett. 1977, 1977–1980. [Google Scholar]
- [22].For a review on the Staudinger reduction, see:; Gololobov YG, Zhmurova IN, Kasukhin LF, Tetrahedron 1981, 37, 437–472. [Google Scholar]
- [23].For notable examples, see:; a) Berliner MA, Dubant SPA, Makowski T, Ng K, Sitter B, Wager C, Zhang Y, Org. Process Res. Dev 2011, 15, 1052–1062; [Google Scholar]; b) Shimizu H, Maeda H, Nara H, Org. Process Res. Dev 2020, 24, 2772–2779. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


