There is a Blood Commentary on this article in this issue.
Key Points
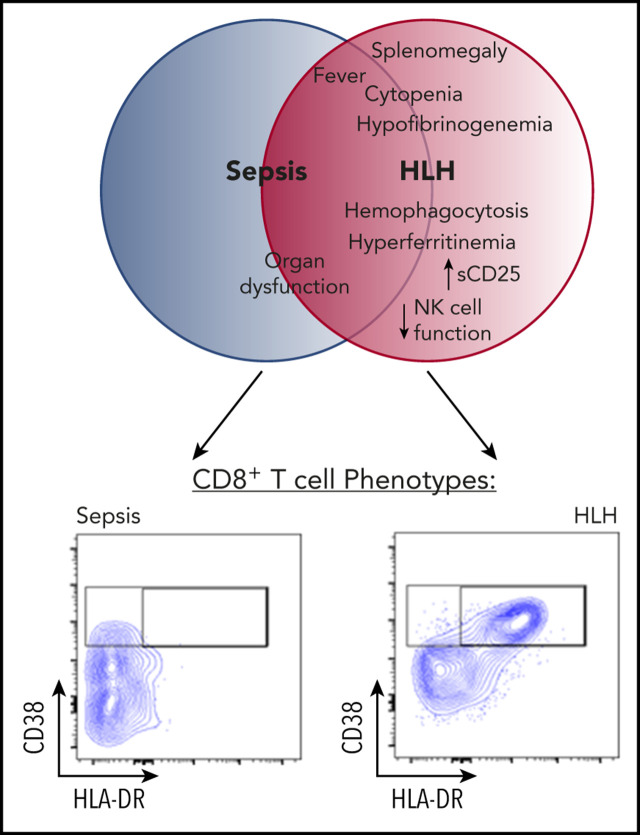
HLH and sepsis are readily distinguished by T-cell–activation profiles.
Increased frequency of CD38high/HLA-DR+ CD8+ T cells is an optimal diagnostic marker for identifying patients with active HLH.
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a fatal disorder of immune hyperactivation that has been described as a cytokine storm. Sepsis due to known or suspected infection has also been viewed as a cytokine storm. Although clinical similarities between these syndromes suggest similar immunopathology and may create diagnostic uncertainty, distinguishing them is critical as treatments are widely divergent. We examined T-cell profiles from children with either HLH or sepsis and found that HLH is characterized by acute T-cell activation, in clear contrast to sepsis. Activated T cells in patients with HLH were characterized as CD38high/HLA-DR+ effector cells, with activation of CD8+ T cells being most pronounced. Activated T cells were type 1 polarized, proliferative, and displayed evidence of recent and persistent activation. Circulating activated T cells appeared to be broadly characteristic of HLH, as they were seen in children with and without genetic lesions or identifiable infections and resolved with conventional treatment of HLH. Furthermore, we observed even greater activation and type 1 polarization in tissue-infiltrating T cells, described here for the first time in a series of patients with HLH. Finally, we observed that a threshold of >7% CD38high/HLA-DR+ cells among CD8+ T cells had strong positive and negative predictive value for distinguishing HLH from early sepsis or healthy controls. We conclude that the cytokine storm of HLH is marked by distinctive T-cell activation whereas early sepsis is not, and that these 2 syndromes can be readily distinguished by T-cell phenotypes.
Visual Abstract
Introduction
The term “cytokine storm” (CS) refers to a pathologic inflammatory state in which acute immune activation may be more harmful than helpful. Although this term assumes that soluble mediators (cytokines) play a critical role in pathology, evidence for this is limited in many clinical contexts. CS is recognized in a variety of conditions, including sepsis,1 severe viral infection (such as dengue virus2 and now severe acute respiratory syndrome coronavirus 23 infection), acute graft-versus-host disease,4 immune-activating therapies (where it is also called cytokine release syndrome),5-8 and a variety of intrinsically inflammatory conditions such as hemophagocytic lymphohistiocytosis (HLH).9 This array of conditions and the exquisitely adaptable nature of the immune response suggest that the term CS ambiguously refers to highly diverse forms of inflammation. Defining the distinctive aspects of immune activation in each instance of CS is likely to be important for tailoring more effective therapies for these conditions.
HLH is an immune-regulatory disorder characterized by episodes of acute immune activation, variably associated with infections and other triggers.10 Defects in the genes involved in lymphocyte cytotoxic function underlie many, but not all, cases.11 Irrespective of its etiology, patients with HLH exhibit excessive and harmful inflammation, which is widely viewed as a prototypical CS.12,13 Patients with HLH may present with a sepsis-like appearance confounding diagnosis. Even with increased awareness of this rare entity, some patients with HLH are not identified until after they are deceased.11 Indeed, the clinical similarities between patients with HLH and those with sepsis have led some to propose that the underlying inflammatory processes may be similar.14 Although experimental evidence suggests that HLH is driven by T-cell activation,15 immune profiling in patients with sepsis has mostly focused on monocytes, and studies examining T-cell profiles are generally sparse with mixed results.16-18 Although patients with HLH have been described to have increased T-cell activation,19,20 comparisons to sepsis or other highly inflamed states have not been described. To fill these gaps and to further differentiate the immune pathology underlying sepsis and HLH, we profiled T cells from patients with well-defined HLH or sepsis and found highly divergent phenotypes, allowing for ready discrimination of these disorders with particular focus on CD38, which is the marker that arguably has been shown to be most useful for identifying recently activated T cells in humans.21,22
Methods
Study subjects
Samples were obtained from patients with sepsis or HLH after written informed consent, utilizing protocols approved by the institutional review boards of Cincinnati Children’s Hospital Medical Center (CCHMC), Texas Children’s Hospital, Phoenix Children’s Hospital, The Hospital for Sick Children, Children’s National Hospital, UCSF Benioff Children's Hospital, and Lucile Packard Children’s Hospital Stanford. Some samples were obtained from subjects enrolled in the HIT-HLH trial (NCT01104025). Control samples were obtained from an institutional review board–approved protocol from deidentified children without known infections receiving blood draws as outpatients at CCHMC facilities. All HLH patients met the HLH-2004 diagnostic criteria.23 Patients with sepsis met the 2005 International Pediatric Sepsis Definitions Consensus Conference criteria for severe sepsis.24 Among patients with HLH, data presented are from pretreatment samples. Pretreatment of HLH was defined as <2 weeks of corticosteroid treatment and no doses of etoposide or other immune-suppressive agents directed at HLH. Patients with sepsis had samples drawn within 48 hours of the diagnosis of sepsis and did not receive corticosteroids prior to drawing research samples. Clinical details of the patients are outlined in Table 1 with additional information about mutations and age distribution in supplemental Figure 1 (available on the Blood Web site). No patients with malignancy or known rheumatologic diagnoses were included in this HLH cohort. Clinical data were measured at respective sites at the time of sample draw. Peripheral blood mononuclear cells were purified by Ficoll-Hypaque density grade centrifugation and either viably frozen or analyzed by flow cytometry within 24 hours of collection. Due to limited sample size, not all samples were analyzed for all markers; the N for specific analyses is listed in each figure legend.
Table 1.
Clinical details of the patients with HLH and sepsis in this study
| Clinical details | Values |
|---|---|
| Patients, N | |
| HLH | 43 |
| Sepsis | 19 |
| Median age, y | |
| HLH (minimum, maximum) | 2.6 (0.2-25) |
| Sepsis (minimum, maximum) | 3.0 (0.2-17.5) |
| HLH diagnosis features, % | |
| Fever | 86 |
| Splenomegaly | 95 |
| Bicytopenia | 71 |
| Hyperferritinemia | 88 |
| Hypofibrinogenemia | 65 |
| Hypertriglyceridemia | 57 |
| Elevated sCD25 | 94 |
| HLH-associated genetic lesion, N | |
| Yes | 26 |
| No | 17 |
| HLH: infection, N | |
| EBV | 10 |
| Other viruses | 8 |
| Other infections | 2 |
| No infection | 23 |
| Sepsis: infection, N | |
| Gram-positive bacteria | 3 |
| Gram-negative bacteria | 8 |
| Virus | 1 |
| No identified organism | 7 |
| Outcome | |
| HLH: live/dead | 30/13 |
| Sepsis: live/dead | 19/0 |
Antibodies and flow cytometry
Antibodies were obtained from BioLegend (San Diego, CA) except for anti-CD27, anti-CD5, and anti–granzyme B (BD Biosciences, San Diego, CA) and M620 Live/Dead (AAT Bioquest, Sunnyvale, CA). Our stain panels included: anti-CD3 (clone OKT3), anti-CD4 (clone RPAT4), anti-CD8 (clone RPAT-8), anti-CD38 (clone THB7), anti-HLA-DR (clone L243), anti-PD1 (clone EH12.2H7), anti-CXCR3 (clone G025H7), anti-CD95 (clone DX2), anti-CD5 (clone L17F12), anti-CD45RA (clone HI100), anti-CCR7 (clone G043H7), anti-CD27 (clone L128), and anti-CD28 (clone CD28.2). For cytokine production, cells were stimulated with phorbol myristate acetate/ionomycin for 5 hours in media supplemented with brefeldin A (Golgi Plug; BD Biosciences). Following stimulation, cells were stained for surface markers such CD3, CD4, CD8, and CD38. Cells were then fixed with 0.16% formaldehyde for 10 minutes and then permeabilized with FOXP3 Perm buffer and then incubated with antibodies against interferon γ (IFN-γ; clone B27), tumor necrosis factor α (TNF-α; clone MAb11), Ki-67 (clone Ki-67), granzyme B (clone GB11), and γ H2AX (clone 2F3). For staining, fresh samples (within 24 hours of blood draw) were used when possible, or those previously frozen were thawed from liquid nitrogen and rested for 2 to 4 hours in complete media. Parallel staining of fresh and frozen aliquots from the same patients revealed similar results, although the percentage of activated T cells was typically modestly higher in fresh samples. All samples were analyzed using Fortessa1 (BD Biosciences) and Flowjo software (TreeStar).
Establishing diagnostic cutoffs
Receiver operating curves (ROCs) were generated comparing patients with HLH, patients with sepsis, and healthy pediatric controls. We determined the optimal cutoff points, choosing the threshold with the highest specificity without compromising the sensitivity (the point closest to the upper left corner). Using the thresholds from the ROC curves, we created contingency tables for indicated markers to determine the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio (LR). The Wilson-Brown test was used to compute the 95% confidence intervals (CIs). GraphPad Prism 5.04 software (GraphPad Software Inc, La Jolla, CA), was used to generate both the ROC curves and contingency tables.
Statistical analysis
The results are presented as the mean plus or minus standard error of the mean with statistical significance determined by the 2-tailed Student t test, using either paired or unpaired (assuming equal variance) or 1-way analysis of variance with the Dunnett post hoc test, according to the data characteristics. Survival curves were assessed by the Wilcoxon-Gehan test for differences among groups. Significance was defined as P < .05. Statistical analysis was performed using GraphPad Prism 5.04 software (GraphPad Software Inc).
Results
Peripheral blood T-cell–activation status readily distinguishes patients with HLH and early sepsis
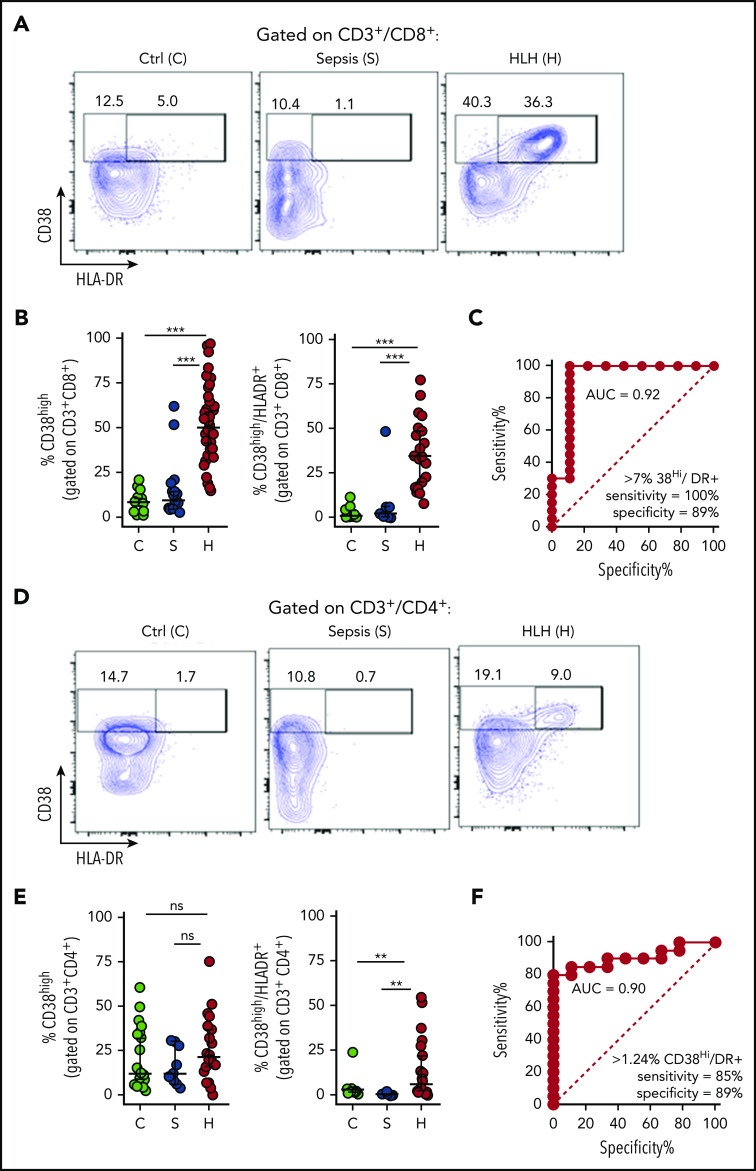
Mouse models have identified antigenic activation of T cells as a critical part of the pathophysiology of HLH but not of bacterial sepsis.15,25,26 In vivo antigen-activated human T cells have been shown to be best identified by a CD38 bright, HLA-DR+ phenotype, peaking 1 to 2 weeks after infection or vaccination.21,22 HLA-DR expression has been reported to be upregulated in patients with HLH.19 However, expression of CD38 has not been well described in patients with HLH, and neither marker has been well studied in sepsis. We analyzed peripheral blood T cells from children with HLH (N = 43) or sepsis (N = 19), whose clinical characteristics are summarized in Table 1, along with pediatric controls. Samples were obtained within 48 hours of onset of sepsis or prior to initiation of definitive treatment of HLH, respectively. We found that patients with HLH had distinctive, expanded populations of CD38high or CD38high/HLA-DR+ T cells, whereas most patients with sepsis did not (Figure 1; gating strategy shown in supplemental Figure 1). This difference was dramatic and ROC analysis revealed that the presence of these populations within either CD8+ or CD4+ T cells readily distinguished patients with HLH from those with early sepsis.
Figure 1.
Peripheral blood T-cell activation status readily distinguishes patients with HLH and sepsis. (A-B) Representative flow cytometric plots and cumulative data comparing the frequency of CD38high or CD38high/HLA-DR+ (double-positive) CD8+ T cells in pediatric controls (C), patients with sepsis (S), or patients with HLH (H). (C) ROC analysis of CD38high/HLA-DR+ (double-positive) CD8+ T cells, comparing HLH and sepsis. (D-E) Representative FACS plots and cumulative data comparing the frequency of CD38high or CD38high/HLA-DR+ (double-positive) CD4+ T cells in pediatric controls, patients with sepsis, or patients with HLH. (F) ROC analysis of CD38high/HLA-DR+ (double-positive) CD4+ T cells, comparing HLH and sepsis. Data are representative of 27 pediatric controls, 9 to 19 patients with sepsis and 19 to 43 patients with HLH. Error bars represent median with 95% CI. Differences between indicated groups were calculated using the unpaired Student t test. **P < .01; ***P < .001. Ctrl, control; ns, not significant.
Increased CD38high/HLA-DR+ CD8+ T cells is a robust marker of active HLH disease
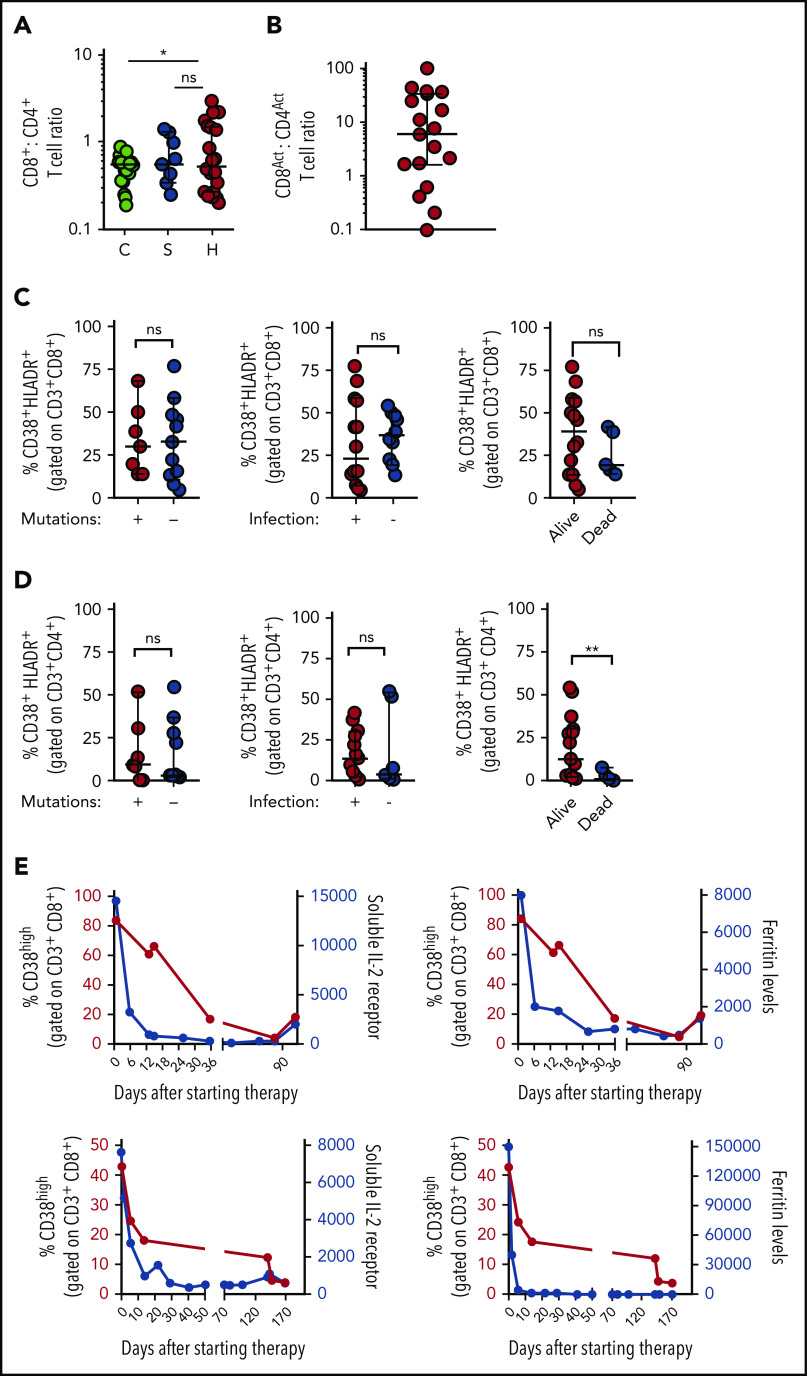
As activated CD8+ T cells appear uniquely important in animal models of HLH, and were more useful than CD4+ T cells for distinguishing HLH and sepsis, we analyzed what the presence of this population signified about individual patients with HLH. We found that although the CD8:CD4 ratio was only slightly elevated in patients with HLH, activated (CD38high/HLA-DR+) CD8+ T cells were approximately fivefold more prevalent than activated CD4+ T cells in most patients with HLH (Figure 2A-B). In contrast to a prior report that suggested primary and secondary HLH may be distinguished by the degree of T-cell activation,19 we found that there was no difference in the frequencies of CD38high/HLA-DR+ CD8+ and CD4+ T cells in HLH patients with and without identifiable mutations, infections, or mortality (Figure 2C-D). As Epstein-Barr virus (EBV) infection was the most common infection in the HLH cohort, we also analyzed these patients as a separate group and did not observe a difference (supplemental Figure 2). A similar pattern was observed when we analyzed the broader population of CD38high (single-positive) CD8+ and CD4+ T cells (supplemental Figure 2), suggesting that activated T cells are universally elevated in patients with HLH, although the frequency of activated T cells did not correlate well with other markers of HLH or patient age (supplemental Figure 2). Finally, we serially assessed the frequency of CD38high CD8+ T cells in 2 patients receiving treatment of HLH with etoposide and dexamethasone and found that the frequency of these cells decreased after therapy with similar kinetics as soluble CD25 (sCD25) and ferritin levels (Figure 2E). Thus, activated (CD38high/HLA-DR+) CD8+ T cells appear to be broadly characteristic of active HLH in all patients.
Figure 2.
Increased frequency of CD38high/HLA-DR+ CD8+ T cells is a robust marker of active HLH, irrespective of clinical context. (A) CD8:CD4 ratio is modestly elevated in patients with HLH, compared with controls, but not patients with sepsis. (B) Ratio of CD38high/HLA-DR+ CD8+ to CD38high/HLA-DR+ CD4+ T cells in individual patients with HLH. (C-D) Frequency of CD38high/HLA-DR+ CD8+ or CD4+ T cells in patients with HLH is plotted, comparing presence of HLH-associated mutations, identifiable infection at diagnosis, or subsequent survival. (E) Frequency of CD38high CD8+ T cells is plotted with either sCD25 or ferritin across time in 2 patients receiving etoposide and dexamethasone for HLH. Data are representative of 27 pediatric controls, 9 patients with sepsis, and 18 to 22 patients with HLH. Error bars represent median with 95% CI. Differences between indicated groups were calculated using the unpaired Student t test. *P < .05.
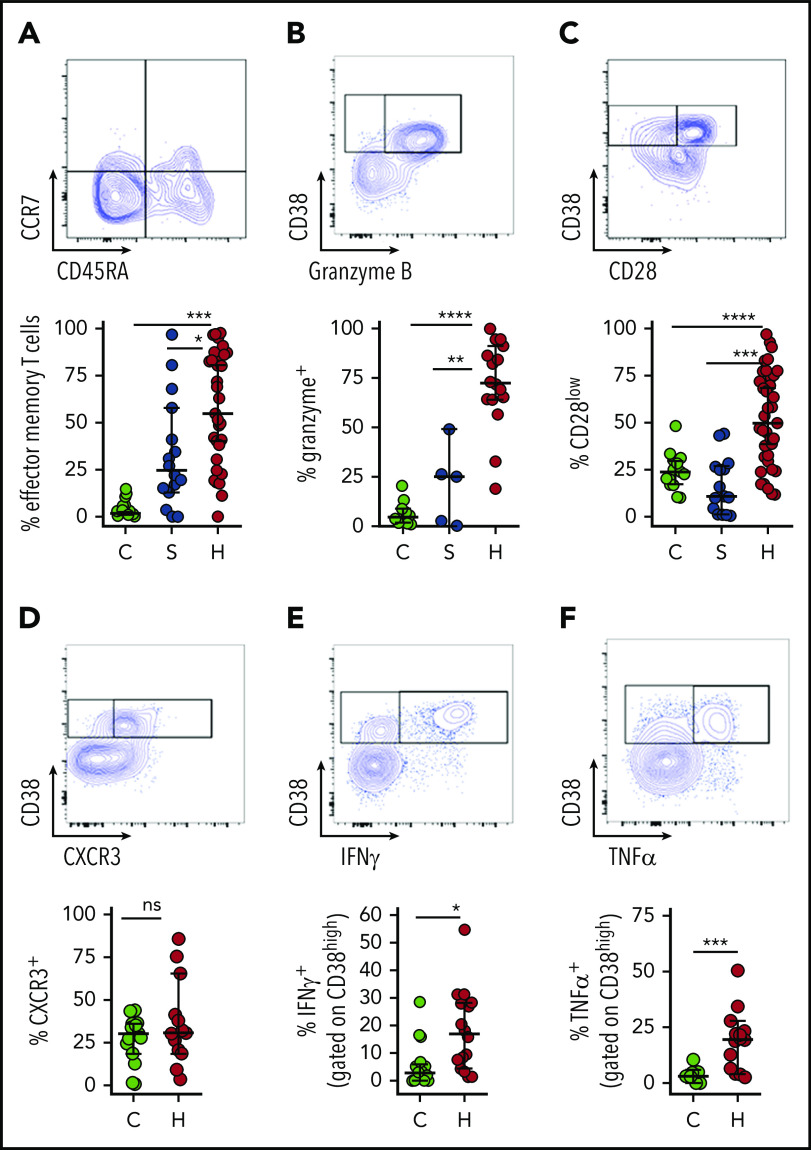
Activated CD8+ T cells in patients with HLH are predominantly effector memory T cells with Tc1/cytotoxic differentiation and display evidence of recent and persistent activation
To further characterize the activated T cells that are characteristic of HLH, we examined the differentiation and function of CD38high/HLA-DR+ CD8+ T cells. Notably, these cells are essentially absent from most healthy children or patients with sepsis; but, in order to have a comparison group for characterizing the phenotype of these cells, we gated on the top 10% of CD38-expressing CD8+ T cells from the latter 2 groups as they are presumably the most activated T cells in these individuals. Similar to a prior report,19 we found that activated CD8+ T cells in patients with HLH have a largely effector memory phenotype with differentiation toward cytotoxic function (Figure 3A-C). Alternatively, a portion of CD38high/HLA-DR+ CD8+ T cells displayed evidence of terminal differentiation, such as CD57 expression (supplemental Figure 3). We also observed that CD38high CD8+ T cells displayed type 1 polarization, expressed the cytotoxic T cell type 1 (Tc1) marker CXCR3 and secreted IFN-γ and TNF-α with stimulation (Figure 3D-F). A very similar phenotype was observed in CD38high/HLA-DR+ CD4+ T cells, but it was less robust (supplemental Figure 4). Thus, the phenotype and function of activated T cells in patients with HLH confirm prior findings from animal models of HLH.
Figure 3.
Activated CD8+ T cells in patients with HLH are predominantly effector memory T cells with Tc1 and cytotoxic differentiation. Representative flow cytometry plots from patients with HLH and composite data comparing controls and patients with sepsis or HLH, showing (A) frequency effector T-cell differentiation (CD45RA− CCR7−) among CD38high/HLA-DR+ CD8+ T cells. As CD38+/DR+ T cells are largely absent from healthy controls and patients with sepsis, frequency shown is derived from gating on the top 10% of CD38-expressing CD8+ T cells. (B-C) Expression of CD28 or granzyme B in CD38high/HLA-DR+ CD8+ T cells is shown as in panel A. (D) Representative FACS plots and composite data showing CXCR3 expression in CD8+ CD38high T cells of controls (top 10% of CD38 expressors) and patients with HLH. (E-F) Representative flow cytometry plots and composite data showing IFN-γ and TNF-α production among CD8+CD38high cells in indicated populations after phorbol myristate acetate/ionomycin stimulation. Data are representative of 12 to 27 pediatric controls, 5 to 16 patients with sepsis, and 13 to 37 patients with HLH. Error bars represent median with 95% CI. Differences between indicated groups were calculated using the unpaired Student t test. *P < .05; **P < .01; ***P < .001; ****P < .0001.
To corroborate our interpretation of CD38high/HLA-DR+ T cells as “highly activated” T cells, we assessed multiple other markers associated with T-cell activation. Similar to dengue virus infection or other contexts for T-cell activation,22,27 many of the cells with this phenotype expressed programmed cell death protein 1 (PD-1) and CD95 (Fas) (Figure 4A-B). A significant portion of this population was also positive for Ki67 and γH2AX, markers of DNA replication and damage, which signify recent proliferation of lymphocytes.28 γH2AX was detected prior to any treatment with etoposide. We also found that most activated CD8+ T cells in patients with HLH displayed downregulation of CD5, which is consistent with prior reports (Figure 4).29,30 Similar, although less pronounced, patterns were observed in CD38high/HLA-DR+ CD4+ T cells (supplemental Figure 4). However, none of these markers suggested T-cell activation in patients with sepsis (Figure 4).
Figure 4.
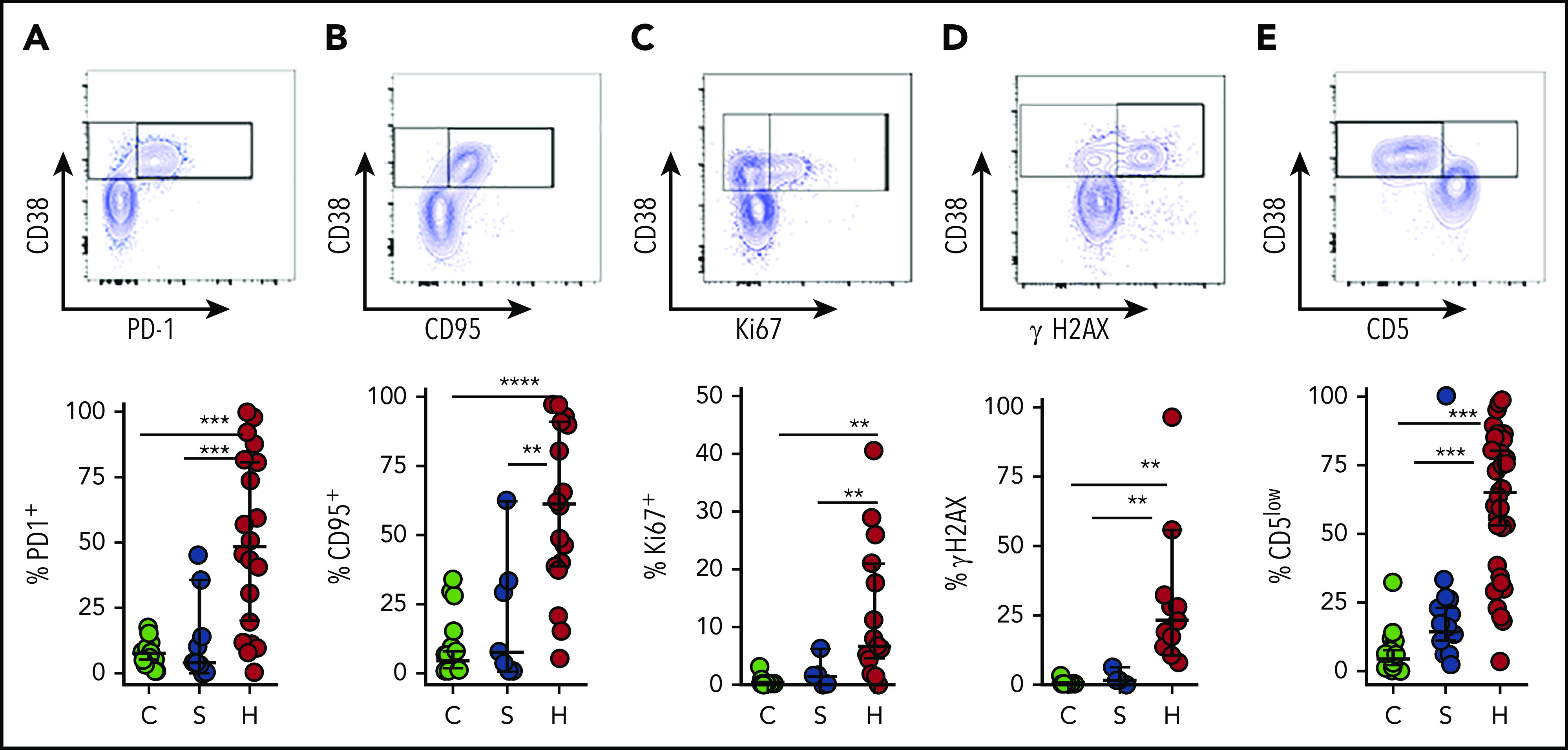
CD38high/HLA-DR+ CD8+ T cells in patients with HLH are proliferative and display evidence of recent and persistent activation. (A-E) Representative flow cytometry plots from patients with HLH and composite data comparing controls and patients with sepsis or HLH, showing the indicated markers of activation, proliferation, and persistent stimulation (top of each panel). For controls and patients with sepsis, frequency shown is derived from gating on the top 10% of CD38-expressing CD8+ T cells, as CD38+/DR+ T cells are largely absent in these samples (bottom of each panel). Data are representative of 18 to 27 pediatric controls, 5 to 18 patients with sepsis, and 11 to 36 patients with HLH. Error bars represent median with 95% CI. Differences between indicated groups were calculated using the unpaired Student t test. **P < .01; ***P < .001; ****P < .0001.
Tissue-infiltrating T cells in patients with HLH are highly activated, IFN-γ–producing T cells
Experimental evidence has suggested that T-cell activation in HLH occurs in response to antigen presentation and is especially prominent in lymphoid and other tissues.31,32 Clinically, injury to various tissues such as the bone marrow and brain suggests that these sites may be critical locations for T-cell activation. However, T-cell phenotypes have not been well studied in tissues of patients with HLH. We gathered samples, including bone marrow aspirates, cerebral spinal fluid (CSF), and lymph node biopsies from 10 different patients with HLH and analyzed T-cell phenotypes. In some cases, we were also able to analyze concurrently collected peripheral blood. This unique collection of samples revealed that T-cell activation was very high in all tissues, and, in most cases, was higher than what was seen in concurrent peripheral blood samples (Figure 5; supplemental Figure 5). In particular, T cells in CSF samples were strikingly activated: ∼80% of CD8+ T cells were CD38high/HLA-DR+. Furthermore, CD8+ T cells in the CSF or bone marrow were highly polarized toward production of IFN-γ, and more likely to produce this cytokine than T cells in the peripheral blood (Figure 5D). Of note, these CSF samples were obtained from patients with clinical evidence of HLH affecting the central nervous system, including seizures, focal neurologic deficient, and/or changes in brain magnetic resonance imaging. Thus, analysis of tissue-derived cells from patients with HLH demonstrates that HLH is a disease of T-cell hyperactivation in affected tissues and illustrates that analysis of peripheral blood lymphocytes may often underestimate the degree of T-cell activation in patients.
Figure 5.
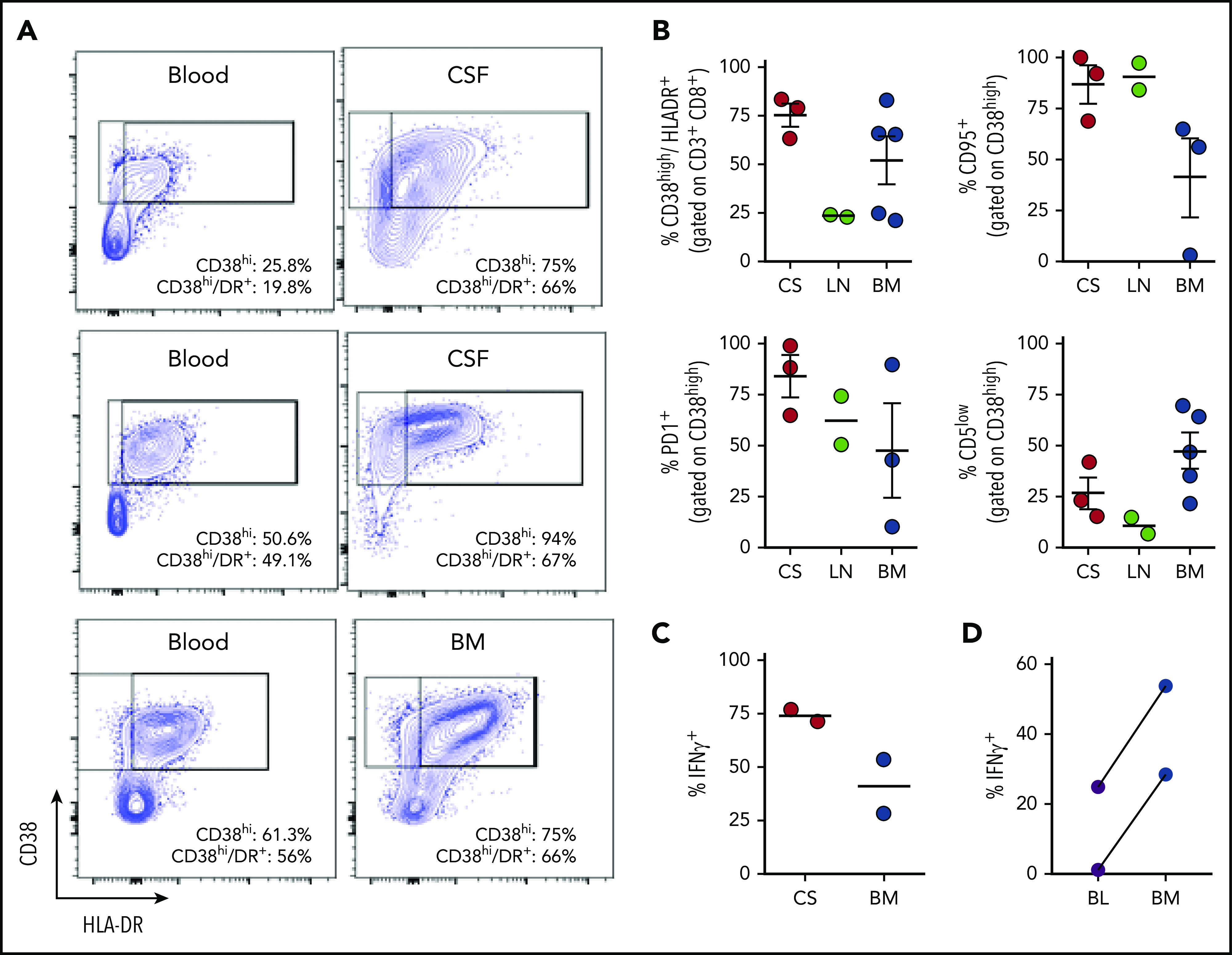
Tissue-infiltrating CD8+ T cells in patients with HLH are highly activated, IFN-γ–producing T cells. (A) Representative FACS plots of CD8+ T cells comparing blood and CSF or bone marrow in samples obtained concurrently from 3 different patients. (B) Cumulative data for the indicated markers in samples of CSF (CS), lymph node (LN), or bone marrow (BM) from 10 patients with HLH. CD38high/HLA-DR+ cells are displayed as a percentage of CD3+/CD8+ cells, the other markers are displayed as a percentage of CD8/CD38high/HLA-DR+ cells. (C) Representative FACS plots and cumulative data of intracellular cytokine staining for IFN-γ, as a percentage of CD3+/CD8+ cells in either CSF or bone marrow. (D) Comparison of IFN-γ staining of CD8+/CD38high T cells after stimulation of concurrent peripheral blood (BL) and bone marrow samples from 2 patients.
Frequency of CD38high/HLA-DR+ CD8+ T cells optimally differentiates HLH from sepsis and healthy controls
Our studies indicate that patients with HLH display a population of activated T cells (most prominently CD8+) that are largely absent in patients with sepsis or healthy pediatric controls. These activated T cells may be defined by the expression of CD38, HLA-DR, or both markers. We performed additional ROC analyses to assess the potential diagnostic value of each of these populations among either CD8+ or CD4+ T cells, comparing patients with HLH to those with sepsis or healthy pediatric controls (supplemental Figure 6). These analyses defined optimal thresholds for each population and demonstrated that the frequency of activated CD8+ T cells was generally better able to identify HLH compared with the frequency of activated CD4+ T cells. Among the different potential ways to identify activated T cells, our analyses showed that measurement of the CD8+ CD38high/HLA-DR+ population was the most diagnostically useful parameter, whether comparing HLH to sepsis or healthy controls (Table 2; supplemental Table 1). Remarkably, in our series, >7% CD38high/HLA-DR+ cells among CD8+ T cells differentiated HLH from sepsis with a PPV of 96%, an NPV of 100%, and an LR of 9 (Table 2). Together, these data strongly indicate that the presence of CD8+/CD38high/HLA-DR+ T cells is an excellent marker for the diagnosis of HLH.
Table 2.
T-cell–activation parameters distinguishing patients with HLH and sepsis
| Activation marker | Sensitivity | Specificity | PPV | NPV | LR |
|---|---|---|---|---|---|
| CD8+ CD38+ >22% | 0.93 (0.81-0.97) | 0.89 (0.68-98.1) | 0.95 (0.84-0.99) | 0.85 (0.64-0.95) | 8.8 |
| CD8+ HLA-DR+ >27% | 0.86 (0.66-0.95) | 0.88 (0.56-0.99) | 0.95 (0.76-1.0) | 0.72 (0.43-0.90) | 7.7 |
| CD8+ CD38+ HLA-DR+ >7% | 1.0 (0.85-1) | 0.89 (0.56-0.99) | 0.96 (0.79-1) | 1 (0.67-1) | 9 |
| CD4+ CD38+ >17.4% | 0.63 (0.42-0.80) | 0.66 (0.35-0.88) | 0.82 (0.59-0.94) | 0.43 (0.21-0.67) | 1.9 |
| CD4+ HLA-DR+ >2% | 0.95 (0.76-1.0) | 0.88 (0.56-0.99) | 0.95 (0.76-1) | 0.89 (0.56-0.99) | 8.5 |
| CD4+ CD38+ HLA-DR+ >1.2% | 0.85 (0.64-0.95) | 0.89 (0.56-0.99) | 0.94 (0.74-0.99) | 0.73 (0.43-0.90) | 7.6 |
Bold represents the parameter with the highest LR.
Discussion
HLH and sepsis are both syndromes of potentially overwhelming inflammation. Indeed, both HLH and sepsis may present with acute onset of shock and multisystem organ failure.33,34 Thus, it is not surprising that some have asked whether these syndromes may be related, or even on a single spectrum.14,35 However, data comparing immune profiles between patients with sepsis and HLH have not been described. The current results suggest that T-cell profiles are highly distinctive in these 2 disorders and they extend prior reports describing increased T-cell activation in patients with HLH.19,20,36,37
In this report, we compared the T-cell phenotypes of patients with HLH to those of patients with sepsis (early after each diagnosis) and found that, although HLH is characterized by expansion of activated T cells identified as CD38high/HLA-DR+, this population is largely absent in the peripheral blood during sepsis. Thus, although HLH and sepsis are 2 examples of CSs with sometimes overlapping clinical features, T-cell phenotypes readily distinguish them and have strong diagnostic utility. Although bacterial infections are most commonly associated with sepsis and viral infections with HLH, this difference does not underlie the dichotomy of T-cell profiles we observed as less than half of HLH patients had identifiable infections and the HLH phenotype was present regardless of infection. We further characterized this “HLH T-cell profile” and found that although both CD4+ and CD8+ T cells displayed increased activation, CD8+ T cells were the dominant population, echoing findings from animal models of HLH.25,38 CD38high/HLA-DR+ (or simply CD38high) CD8+ T cells were characteristic of active HLH, regardless of genotype or infection, further suggesting the diagnostic value of this cellular profile. This population of T cells displayed cytotoxic differentiation and increased IFN-γ secretion, consistent with the role that this cytokine has been shown to play in HLH.39 Although the profile of CD38high/HLA-DR+ has been shown to identify recently activated T cells after vaccination or various infections,21,22,40 we confirmed that these cells in patients with HLH were highly and persistently activated. Finally, we described, for the first time, the phenotype T cells infiltrating various tissues in a cohort of patients with HLH and found that they often appeared even more activated than circulating T cells, further suggesting a key role for acute T-cell activation in HLH pathogenesis.
Importantly, our report describes CD38 as a key marker for identifying T-cell activation in patients with HLH (Figure 1). Our report is also the first to describe an extended panel of activation markers and the IFN-γ production potential of these cells (Figures 3 and 4). Although reports (mainly describing EBV-HLH in east Asia) have described CD5 downregulation in HLH,41 here we confirm, in a large series of western patients, that CD5 downregulation is common, regardless of whether it is triggered by EBV (Figure 4). This observation is also informative for HLH pathophysiology, as CD5 downregulation is thought to be due to prolonged or chronic antigenic stimulation.42 Unlike a prior report,19 we did not observe different degrees of T-cell activation in patients with or without HLH-associated genetic mutations or infections. However, differences in methodology and the types of patients that were studied may explain this difference, as the prior report did not examine CD38 and also included a significant number of patients with underlying rheumatologic disorders. Finally, we provided an important and readily available diagnostic tool. In our series, a T-cell phenotype of >7% CD38high/HLA-DR+ cells among CD8+ T cells can distinguish patients with HLH from those with sepsis or who are healthy. Indeed, with an NPV of 100%, the absence of CD38high/HLA-DR+ cells among CD8+ T cells would be very strong evidence that a patient with uncertain diagnosis does not have HLH. This tool can specifically address the common and important problem of clarifying the diagnosis of patients with multiorgan failure in intensive care units, where time to appropriate diagnosis and initiation of appropriate therapy are critically important.
Three limitations of the current work are notable. First, although we include 19 patients with severe sepsis in our study, most with identifiable bacterial causes, this sample size is not sufficient to search for small subsets (eg, <10%) of sepsis patients that may have a more “HLH-like” physiology.43 Much larger cohorts will be needed to identify and study such patients. Second, our HLH cohort did not include patients with HLH secondary to rheumatologic conditions (macrophage activation syndrome) or malignancy, or adults with HLH. Additional studies will be needed to assess these potentially distinct populations. Third, although we identified a sharp threshold for distinguishing HLH and sepsis via T-cell–activation parameters, this research finding is not yet a clinically actionable assay. Due to limitations of our sample size and variations in flow cytometric techniques, additional studies will be needed to validate our findings and establish laboratory-specific thresholds, which may vary.
There is increasing awareness of the potential hazards of CSs, whether from catastrophic infections (sepsis, COVID-19, etc) or intrinsically dysregulated immune responses (HLH, immune-activating therapies, etc). As we seek to address these hazards with more rationally targeted therapies, defining the pathophysiology of specific CSs more precisely to improve diagnosis and better apply such therapies is essential. Future prospective studies will be needed to characterize immune activation, including expansion of CD38high/HLA-DR+ CD8+ T cells, across a broad range of hyperinflammatory conditions.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the families and patients participating in the HIT-HLH trial. The authors also thank the members of the M.B.J. laboratory for help with processing the peripheral blood mononuclear cells from patients and healthy control.
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant R34HL107801 (M.B.J.), Liam’s Lighthouse Foundation, and the Neumann family.
Footnotes
For original data, please e-mail the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Vandana Chaturvedi did the flow, analyzed the data, and wrote the manuscript; E.O. and Vijaya Chaturvedi processed samples and ran flow; A.Z.-L. helped with analysis of ROC curves and generation of contingency tables and writing; R.A.M., C.E.A., M.M.H., J.N.G., S.L., M.L.H., M.J., A.N., and M.B.J. provided the HLH patient samples; C.E.A., T.C.N., and J.R.G. provided HLH and sepsis samples; H.R.W. provided the sepsis patient samples and edited the manuscript; and M.B.J. analyzed the samples, wrote and edited the manuscript, and directed the research.
Conflict-of-interest disclosure: M.B.J., M.L.H., and C.E.A. have served as consultants for Sobi. M.L.H. serves on a data safety monitoring committee for Novimmune. The remaining authors declare no competing financial interests.
Correspondence: Michael B. Jordan, Cincinnati Children’s Hospital Medical Center/University of Cincinnati, 3333 Burnet Ave, ML7038, Cincinatti, OH 45229; e-mail: michael.jordan@cchmc.org.
REFERENCES
- 1.Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008;214(2):211-223. [DOI] [PubMed] [Google Scholar]
- 2.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532-543. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25(1 pt 2):1216-1217. [PubMed] [Google Scholar]
- 5.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018-1028. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apel A, Ofran Y, Wolach O, et al. Safety and efficacy of blinatumomab: a real world data. Ann Hematol. 2020;99(4):835-838. [DOI] [PubMed] [Google Scholar]
- 8.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy [published correction appears in Blood. 2016;128(10):1441]. Blood. 2013;121(26):5154-5157. [DOI] [PubMed] [Google Scholar]
- 9.Imashuku S, Teramura T, Morimoto A, Hibi S. Recent developments in the management of haemophagocytic lymphohistiocytosis. Expert Opin Pharmacother. 2001;2(9):1437-1448. [DOI] [PubMed] [Google Scholar]
- 10.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66(11):e27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovich AH. Hemophagocytic lymphohistiocytosis and related disorders. Curr Opin Allergy Clin Immunol. 2006;6(6):410-415. [DOI] [PubMed] [Google Scholar]
- 13.Canna SW, Behrens EM. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr Clin North Am. 2012;59(2):329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387-392. [DOI] [PubMed] [Google Scholar]
- 15.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735-743. [DOI] [PubMed] [Google Scholar]
- 16.Ditschkowski M, Kreuzfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229(2):246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Q, Shao WX, Wang QQ, Mao JH. An imbalance of T cell subgroups exists in children with sepsis. Microbes Infect. 2019;21(8-9):386-392. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Grande E, Cabrera CM, González B, Varela C, Urra JM. Enhanced HLA-DR expression on T-lymphocytes from patients in early stages of non-surgical sepsis. Med Clin (Barc). 2019;152(9):346-349. [DOI] [PubMed] [Google Scholar]
- 19.Ammann S, Lehmberg K, Zur Stadt U, et al. ; HLH study of the GPOH . Primary and secondary hemophagocytic lymphohistiocytosis have different patterns of T-cell activation, differentiation and repertoire. Eur J Immunol. 2017;47(2):364-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada T, Sakakibara Y, Nishimura R, et al. Down-regulation of CD5 expression on activated CD8+ T cells in familial hemophagocytic lymphohistiocytosis with perforin gene mutations. Hum Immunol. 2013;74(12):1579-1585. [DOI] [PubMed] [Google Scholar]
- 21.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710-722. [DOI] [PubMed] [Google Scholar]
- 22.Chandele A, Sewatanon J, Gunisetty S, et al. Characterization of human CD8 T cell responses in dengue virus-infected patients from India. J Virol. 2016;90(24):11259-11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis . International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2-8. [DOI] [PubMed] [Google Scholar]
- 25.Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011;118(3):618-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198-208. [DOI] [PubMed] [Google Scholar]
- 27.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153-167. [DOI] [PubMed] [Google Scholar]
- 28.McNally JP, Millen SH, Chaturvedi V, et al. Manipulating DNA damage-response signaling for the treatment of immune-mediated diseases. Proc Natl Acad Sci USA. 2017;114(24):E4782-E4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karandikar NJ, Kroft SH, Yegappan S, et al. Unusual immunophenotype of CD8+ T cells in familial hemophagocytic lymphohistiocytosis. Blood. 2004;104(7):2007-2009. [DOI] [PubMed] [Google Scholar]
- 30.Wada T, Kurokawa T, Toma T, et al. Immunophenotypic analysis of Epstein-Barr virus (EBV)-infected CD8(+) T cells in a patient with EBV-associated hemophagocytic lymphohistiocytosis. Eur J Haematol. 2007;79(1):72-75. [DOI] [PubMed] [Google Scholar]
- 31.Terrell CE, Jordan MB. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood. 2013;121(26):5184-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gather R, Aichele P, Goos N, et al. Trigger-dependent differences determine therapeutic outcome in murine primary hemophagocytic lymphohistiocytosis. Eur J Immunol. 2020;50(11):1770-1782. [DOI] [PubMed] [Google Scholar]
- 33.Kollipara V, Hussain S, Franco-Palacios D, Sofi U. A case series of endemic infections associated hemophagocytic lymphohistiocytosis (HLH) mimicking severe sepsis syndrome. Respir Med Case Rep. 2019;27:100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machowicz R, Janka G, Wiktor-Jedrzejczak W. Similar but not the same: Differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol. 2017;114:1-12. [DOI] [PubMed] [Google Scholar]
- 35.Castillo L. High elevated ferritin levels and the diagnosis of HLH/Sepsis/SIRS/MODS/MAS. Pediatr Blood Cancer. 2008;51(5):710. [DOI] [PubMed] [Google Scholar]
- 36.Lin MT, Chang HM, Huang CJ, et al. Massive expansion of EBV+ monoclonal T cells with CD5 down regulation in EBV-associated haemophagocytic lymphohistiocytosis. J Clin Pathol. 2007;60(1):101-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toga A, Wada T, Sakakibara Y, et al. Clinical significance of cloned expansion and CD5 down-regulation in Epstein-Barr virus (EBV)-infected CD8+ T lymphocytes in EBV-associated hemophagocytic lymphohistiocytosis. J Infect Dis. 2010;201(12):1923-1932. [DOI] [PubMed] [Google Scholar]
- 38.Terrell CE, Jordan MB. Mixed hematopoietic or T-cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice. Blood. 2013;122(15):2618-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382(19):1811-1822. [DOI] [PubMed] [Google Scholar]
- 40.Zidovec Lepej S, Vince A, Dakovic Rode O, Remenar A, Jeren T. Increased numbers of CD38 molecules on bright CD8+ T lymphocytes in infectious mononucleosis caused by Epstein-Barr virus infection. Clin Exp Immunol. 2003;133(3):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada T. Downregulation of CD5 and dysregulated CD8+ T-cell activation. Pediatr Int (Roma). 2018;60(9):776-780. [DOI] [PubMed] [Google Scholar]
- 42.Stamou P, de Jersey J, Carmignac D, Mamalaki C, Kioussis D, Stockinger B. Chronic exposure to low levels of antigen in the periphery causes reversible functional impairment correlating with changes in CD5 levels in monoclonal CD8 T cells. J Immunol. 2003;171(3):1278-1284. [DOI] [PubMed] [Google Scholar]
- 43.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44(2):275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.