Abstract
Background
The gut-brain axis, which mediates bidirectional communication between the gastrointestinal system and central nervous system (CNS), plays a fundamental role in multiple areas of physiology including regulating appetite, metabolism, and gastrointestinal function. The biology of the gut-brain axis is central to the efficacy of glucagon-like peptide-1 (GLP-1)-based therapies, which are now leading treatments for type 2 diabetes (T2DM) and obesity. This success and research to suggest a much broader role of gut-brain circuits in physiology and disease has led to increasing interest in targeting such circuits to discover new therapeutics. However, our current knowledge of this biology is limited, largely because the scientific tools have not been available to enable a detailed mechanistic understanding of gut-brain communication.
Scope of review
In this review, we provide an overview of the current understanding of how sensory information from the gastrointestinal system is communicated to the central nervous system, with an emphasis on circuits involved in regulating feeding and metabolism. We then describe how recent technologies are enabling a better understanding of this system at a molecular level and how this information is leading to novel insights into gut-brain communication. We also discuss current therapeutic approaches that leverage the gut-brain axis to treat diabetes, obesity, and related disorders and describe potential novel approaches that have been enabled by recent advances in the field.
Major conclusions
The gut-brain axis is intimately involved in regulating glucose homeostasis and appetite, and this system plays a key role in mediating the efficacy of therapeutics that have had a major impact on treating T2DM and obesity. Research into the gut-brain axis has historically largely focused on studying individual components in this system, but new technologies are now enabling a better understanding of how signals from these components are orchestrated to regulate metabolism. While this work reveals a complexity of signaling even greater than previously appreciated, new insights are already being leveraged to explore fundamentally new approaches to treating metabolic diseases.
Keywords: Gut-brain axis, Diabetes, Obesity, Gut peptides, Vagus
Highlights
-
•
The gut-brain axis plays an essential role in regulating metabolism and leading therapeutics for T2DM and obesity harness this machinery.
-
•
Multiple components of the gut-brain axis are involved in an integrated response to sensory information to maintain whole-body homeostasis.
-
•
A systems biology approach utilizing advanced technologies is enabling a detailed mechanistic understanding of gut-brain communication.
-
•
This understanding is leading to new approaches that may result in the next generation of therapeutics for metabolic diseases.
1. Introduction
The gut-brain axis plays an essential role in multiple aspects of physiology including regulating feeding and appetite, glucose homeostasis, and gut motility. This biology has been exploited to discover therapeutics for many diseases, including T2DM, obesity, and functional disorders of the gastrointestinal system. More recent research on the microbiome and other components of the gut-brain axis has suggested an even broader role for this system, with implications for discovering therapeutics for Parkinson's disease, autism, immune-related diseases, and mood disorders, among others [[1], [2], [3]].
The role of the gut-brain axis has been particularly well established in regulating appetite and metabolism. Indeed, gut hormones, most notably GLP-1, have been pursued as therapeutics in multiple drug discovery programs for diabetes and obesity over the past two decades. While some of the beneficial effects of GLP-1 and other gut hormones, most notably glucose control, may not involve gut-brain circuitry, the importance of this system in the regulation of feeding has been clearly established. Historically, obesity has been a particularly challenging area for the identification of safe and effective therapies. Indeed, the failure rate in this area, largely driven by modest efficacy and safety issues, has resulted in a de-prioritization of obesity research at multiple pharmaceutical companies. However, recent success with GLP-1-based injectable therapeutics and other approaches that leverage pharmacology of both GLP-1 and other gut hormones has suggested that clinically meaningful weight loss, in some cases exceeding 10%, can be safely achieved [4,5].
These advances, together with increasing evidence that the beneficial effects of bariatric surgery are mediated, at least in part, via an increase in the secretion of a number of endogenous gut hormones linked to glucose homeostasis and satiety, have motivated increasing research on the gut-brain axis and its role in regulating glucose control, feeding, and appetite [6,7]. This work, which has been highly enabled by new technologies, is resulting in a more detailed mechanistic understanding of gut-brain communication that is being exploited to identify new therapeutic approaches for obesity and related disorders.
2. Primary components of the gut-brain axis
Signaling between the gut and brain is bidirectional. Brain to gut communication, which is mediated by the autonomic (sympathetic and parasympathetic) nervous system and hypothalamic-pituitary-adrenal (HPA) axis, regulates several physiological responses including gastric motility and digestion [8,9]. This review will instead focus on communication of sensory information from the gut to the brain, which is mediated by both hormonal and neural circuits.
The gastrointestinal tract presents the largest body surface to the outside world and is considered a key sensory organ. It responds to a range of stimuli, including mechanical, nutrients, microbiome metabolites, pathogens, and toxins, and orchestrates a response critical for maintaining whole-body homeostasis. For example, following the ingestion of a meal, nutrients pass from the stomach to the duodenum and jejunum, producing chemo- and mechano-stimuli that the body has evolved to detect. Specialized chemosensory cells that line the intestinal epithelium, called enteroendocrine cells (EECs), are among the first sentinels engaged in the presence of nutrients. They secrete signaling peptides that both circulate in the blood and are detected by neighboring sensory cells, including vagal afferents, enteric nervous system (ENS) neurons, immune cells, and the brain [10,11]. These key peripheral components of the gut-brain axis are shown in Figure 1 and described in detail to follow.
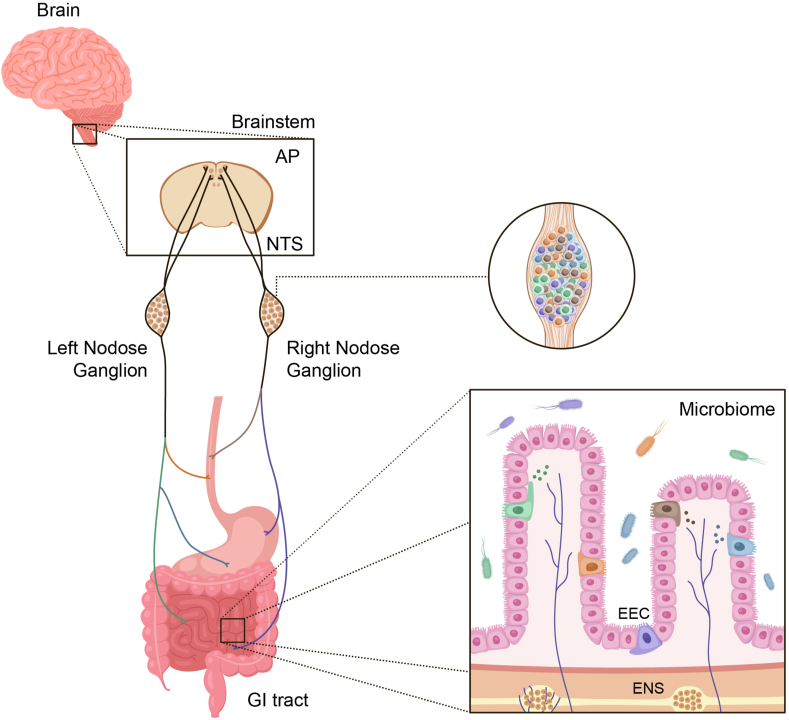
Figure 1.
Major components of the gut-brain axis. The gastrointestinal tract contains the largest surface area in the body exposed to the external environment. Composed of the mouth, pharynx, esophagus, stomach, small intestine (duodenum, jejunum, and ileum), and large intestine (appendix, cecum, colon, rectum, and anal canal), its surface area is 50-200x larger than the surface area of the skin [178]. There are multiple individual components, each containing highly specialized cells, that are responsible for decoding and communicating sensory information from the gut to the brain and other organs. These include enteroendocrine cells (EECs), which produce a variety of hormones involved in both endocrine and paracrine signaling, the enteric nervous system (ENS), gut microbiota, the vagus nerve (nodose ganglia) with specific cell types that innervate discrete regions of the gut, and the central nervous system (CNS).
2.1. Enteroendocrine cells (EECs)
EECs are specialized trans-epithelial cells that are present throughout the gut. They constitute fewer than 1% of the epithelial cells that line the intestines, but combined, they make the gut the largest endocrine organ in the body [12] and arguably one of the most functionally complex. Several unique EECs have been described, distinguished by their location along the gut and the hormones and neurotransmitters they secrete. EECs in the stomach are categorized into a few subsets that secrete the orexigenic hormone ghrelin, acid-modulating gastrin or histamine, and the general inhibitory hormone somatostatin that feedbacks onto other EECs to regulate secretion [13]. In the intestines, a different complement of EECs secrete anorexigenic hormones GLP-1, peptide YY (PYY), neurotensin (NTS), oxyntomodulin (OXM), and cholecystokinin (CCK), glucose-lowering incretins GLP-1 and glucose-dependent insulinotropic peptide (GIP), motility hormones motilin and serotonin, and a spectrum of other hormones that regulate digestion and gut maintenance, including secretin (SCT) and GLP-2. These hormones have overlapping functions and the relative roles of each are still being elucidated. Together they play a fundamental role in regulating energy homeostasis and physiology. This is illustrated by loss-of-function mutations in a key EEC transcription factor neurogenin 3 (Neurog3) in infants, which results in depleted EEC numbers and a failure to thrive with severe malabsorptive diarrhea [14].
2.2. Spatial differences between EECs
The small and large intestines can be viewed as having a spectrum of EECs that gradually change along its axis. The cells in proximal regions secrete higher levels of GIP, CCK, and motilin, whereas the cells in distal regions secrete more GLP-1, GLP-2, PYY, and NTS [15]. The nomenclature used to distinguish different subtypes is based on detecting individual hormones [16]. The most highly investigated EEC type to date is the L cell, which is found scattered along the entire intestine. While the L cell is a broad name to cover all GLP-1-secreting EECs, recent findings have shown there are many types of “L cells” depending on their spatial location along the gut and their developmental state as they migrate and mature along the crypt–villus axis, a process that takes 3–5 days [17].
Based on transcriptional analysis, duodenal L cells and colonic L cells are in fact distinct cell types, consistent with the different physiological functions of these gut regions. For example, proximal L cells are positioned and equipped with receptors that respond to the digested products of incoming carbohydrates (Sglt1 [18,19]), lipids (Ffar1 and Gpr119 [20,21]) and proteins (Casr and Pept1 [22]). When nutrients flood the duodenum and jejunum, these receptors increase intracellular cAMP and calcium levels in EECs, triggering vesicle docking and hormone secretion [23]. Conversely, a colonic L cell expresses a unique set of receptors (for example, Tlr4 and Avpr1b) that coordinate colon-centric functions in response to microbial products and fluid balance regulators [24,25]. Of note, the distal L cells are equipped with many nutrient receptors despite their apparent physiological redundancy [26].
The transcriptional differences between L cells as they transition along the crypt–villus axis are more subtle and highlighted by relative levels of hormone expression. L cells in the villus tip have higher expression levels of Pyy, Sct, and Nts compared to those in the crypts that primarily express Gcg [17]. The physiological relevance of this finding is unclear. When studied at the protein level, secretory vesicles in these cells can often contain a mixture of crypt and villus hormones, and selective secretion of vesicles containing primarily one hormone over another has not been demonstrated [27]. Furthermore, work in isolated segments of human intestines has shown that carbohydrate, fat, and protein infusions result in similar rather than different gut hormone secretions [28,29]. It is likely, therefore, that postprandial hormone secretion profiles are the result of exposure of stimuli to distinct gut segments that contain different combinations of hormones rather than selective secretion of hormones from individual cells.
There are many other overlapping EEC subsets beyond L cells and different EEC lineages are now being elucidated using single-cell sequencing as discussed in Section 3. Many of these are stimulated in unison in response to a meal or other environmental stimuli. One notable lineage that differs from the others is the ghrelin-secreting cell type found in the stomach, which is believed to be under neuronal control from signals that may originate from the brain [30,31]. Unlike other EECs, this cell type secretes signaling peptides between meals and ghrelin peaks in anticipation of a meal [32].
2.2.1. The sensory function of EECs
The cellular location of nutrient- and metabolite-sensitive GPCRs and channels expressed by EECs suggest that these cells require absorption of nutrients to engage their secretory machinery. For example, TGR5 is a receptor that senses bile acids and is located on the basolateral membrane, and thus EECs only secrete their complement of hormones when bile acids cross the gut epithelium [33,34]. Furthermore, glucose stimulates L cells and GIP, producing K cells in the proximal gut through the transporter SGLT1, which produces a depolarizing Na + current as glucose is physically transported from the lumen into the cell [18,19,35].
The complement of gut hormones detected in the systemic circulation over time is, therefore, a proxy for when and where certain nutrients and metabolites are crossing the gut epithelium. For example, in response to a meal, GIP is observed relatively quickly, consistent with the location of GIP-expressing EECs in the duodenum where nutrients are first absorbed [36]. Whereas after Roux-en-Y bariatric surgery, nutrients rapidly enter the jejunum instead of the duodenum and as a result, a different profile of hormones is seen following a meal, including high elevations of GLP-1, which corresponds to the higher density of L cells in this region [37]. The temporal nature of hormone secretion is likely important to the physiological response mediated by feeding centers in the brain as discussed to follow.
2.2.2. Gut hormones: signaling and function
The importance of endocrine vs paracrine signaling of gut hormones involved in satiety and glucose control is a subject of current debate. EECs secrete high concentrations of active hormones, including GLP-1, GLP-2, CCK, SCT, and NTS, and the receptors for these hormones reside on adjacent EECs, ENS, vagal, immune, and/or myofibroblasts [10,38,39], suggesting that many of the physiologic effects of these hormones are mediated via local signaling. This is also consistent with the observation that many gut hormones have short half-lives and are rapidly inactivated as they escape from the gut. For example, the plasma half-life of GLP-1 is 2–3 min and only 10–15% of GLP-1 is found in the systemic circulation in its intact form [40]. Nevertheless, receptors for GLP-1 and GIP are expressed on pancreatic beta cells as well as leaky areas of the brain such as the area postrema and areas of the hypothalamus that regulate food intake [39]. The signaling routes used by gut hormones are active research areas, and in Section 3, we highlight some of the most recent work being done using advanced techniques to elucidate these circuits.
Research on the relative importance of paracrine vs endocrine signaling of these hormones is highly relevant to drug discovery. Specifically, a better understanding of the degree to which the pharmacology of peripherally administered gut hormones reflects true physiology or not would inform the design of rational approaches to directly harness the endogenous machinery of the gut-brain axis. One such example involves directly activating gut EECs to stimulate the secretion of endogenous gut hormones that modulate metabolism (Section 5.2.3.2).
With the major focus being on the hormones themselves rather than EECs, the functions of EECs have mostly been implied from the results of studies that analyze the effects of individual hormones when injected at high levels. These studies have shown that many of the gut hormones that are co-expressed in the same lineage of EECs have overlapping functions. For example, the hormone GIP modulates glucose-dependent insulin secretion as does the hormone GLP-1 [36], and the hormone CCK reduces food intake, similar to the hormones GLP-1, OXM, NTS, and PYY [27]. CCK and SCT both elicit pancreatic secretions, while GLP-1, PYY, and CCK all reduce gastric emptying [41]. EECs are equipped to sense different stimuli in specific gut regions and may transmit different signals depending on the neurons that innervate different segments, but how these signals are integrated remains unclear. Experimental approaches that selectively stimulate different EEC populations along the length of the gut will enable a more comprehensive understanding of their functions, which may inform a more rational approach for the discovery of new therapeutics.
2.3. The enteric nervous system (ENS)
The ENS is the intrinsic nervous system of the gastrointestinal tract. Containing 200–400 million neurons and enteric glial cells, the ENS is embedded in the intestinal wall and extends along the entire gastrointestinal tract from the esophagus to the anus. It integrates sensory information and in turn modulates multiple aspects of gastrointestinal function including gut motility, fluid exchange between the gut and lumen, local blood flow to the intestinal mucosa, regulation of gastric and pancreatic acid secretion, immune function, and mucosal barrier function [42,43]. These functions are regulated through local autonomous neural circuits and communication with the central nervous system via the parasympathetic (e.g., vagal nerve) and sympathetic (e.g., prevertebral ganglia) nervous systems and spinal afferents.
Efforts to dissect the molecular mechanisms by which the ENS integrates and responds to sensory information are ongoing. The majority of enteric neurons fall into five historical classes based on their neurochemical code: intrinsic primary afferent neurons (IPANs), interneurons, viscerofugal neurons, secretomotor neurons, and motor neurons. The neurons of the ENS are organized in two main plexuses, the submucosal plexus and myenteric plexus [44]. These neurons communicate with each other and the other major components of the gut-brain axis [45]. This communication involves a number of neurotransmitters that are also expressed in the brain, including acetylcholine, dopamine, serotonin, and others. The ENS is also a major source of numerous neuropeptides, some of which have been shown to regulate immune function as well as basic intestinal physiology as demonstrated in the case of vasoactive intestinal peptide (VIP) and neuromedin U (NMU) [[46], [47], [48]]. A better understanding of the function of these neurotransmitters and neuropeptides in the ENS and gut-brain signaling in general is an area of active investigation.
The ENS is of increasing interest as a potential target to control glucose metabolism and food intake. Numerous receptors for hormones and/or neurotransmitters are expressed on enteric neurons, and several of these ligands are linked to metabolism. These include galanin, CCK, GLP-1, and ghrelin, although the list is likely to grow with recent high-resolution dissections of ENS subtype diversity and regional specification [39,[49], [50], [51], [52]]. Compared to EECs and gut hormones, it has been much more difficult to try to elucidate actions of the ENS by simply administering signaling peptides that they may secrete when activated. Recently, as described to follow, the results of gain- and loss-of-function studies of specific enteric neurons have provided compelling evidence for a role for at least some of these neurons in metabolism [52].
2.4. The vagus nerve
The vagus nerve is a composite nerve with both motor (efferent) and sensory (afferent) components. It plays a key role in the gut-brain axis by conveying sensory information from the gastrointestinal system and other major organs to the brain and directing effector functions via efferent pathways. Also called the tenth cranial nerve, it is the longest nerve of the autonomic nervous system in the human body, extending from its origin in the brainstem through the neck and thorax down to the gastrointestinal system. The vast majority (80–90%) of neurons that form the vagus nerve are afferents that communicate sensory information from multiple organs, including the gut, liver, heart, and lungs, to the brainstem. Within the brainstem, vagal afferents project primarily to the nucleus of the solitary tract (NST), but also to the area postrema and dorsal motor nucleus [53,54]. The NST in turn relays information to several regions of the CNS, regulating autonomic, neuroendocrine, and behavioral responses [53]. Clearly established roles for the vagus nerve include regulation of digestion, satiety, heart rate, respiration, vasomotor activity, and reflex actions such as coughing, swallowing, and vomiting [[55], [56], [57], [58]].
Vagal sensory neurons whose cell bodies reside in the nodose ganglia send their peripheral projections into different segments of the GI including the esophagus, stomach, and small intestine. Morphological analysis of the gut terminals distinguishes three subtypes, those that innervate the mucosal layer, myenteric layer, or have intraganglionic laminar endings (IGLEs) [59]. The IGLE terminals are thought to allow neurons to act as mechanosensors that detect gut distension. The other two subtypes are thought to be chemosensory and terminate in close proximity to EECs, mucosal immune cells, and neurons of the ENS [60]. Recent evidence suggests the existence of synaptic contacts between vagal afferents and a type of EEC that extends a cytoplasmic process called a neuropod at their basolateral side [11,61] although the nature of this synapse has yet to be confirmed. Thus, vagal afferents are poised to receive information through both paracrine signaling and synaptic transmission.
The vagus plays an integrative role in regulating appetite and, to some extent, glucose homeostasis [62]. For example, CCK has been well validated to have direct actions on the vagus to regulate appetite. When secreted from the proximal intestine, it acts on the CCKAR expressed by a subset of vagal afferents and causes the activation of NST neurons [63]. The downstream cells involved in this satiation circuit appear to include hypothalamic AGRP neurons [64,65]. Gastric emptying is another gut-brain circuit that has been shown to be modulated by CCK-responsive afferents [66].
Other hormones have been postulated to send orexigenic or anorexigenic signals to the brain via the vagus, which expresses multiple gut peptide receptors as discussed in more detail in Section 4. Signals that derive from mechanosensitive afferent activity also play a key role in regulating food intake [65]. How vagal neurons integrate mechanosensory and hormonal signaling to regulate feeding is incompletely understood.
2.5. Immune cells
As previously noted, the gastrointestinal tract is the largest organ exposed to external antigens and, as such, is extensively populated by the immune system. Indeed, the gut is the largest immune organ in the body, containing the majority of all immune cells [67]. The gut mucosal immune system is critically important for protecting the host from infectious pathogens but must also allow cohabitation of commensal microbiota in the gut and the entry of benign dietary antigens that are critical for life. This finely tuned balance between active immunity and immune tolerance is essential for maintaining intestinal homeostasis.
Regarding metabolism, recent findings have indicated that specific mucosal immune cell types can regulate gut peptides, such as GLP-1 or lipid absorption [48,68]. For example, intraepithelial lymphocytes sit adjacent to EECs and, in the mouse, express Glp1r [10]. These immune cells have been shown to gate the amount of GLP-1 available by regulating the number of EECs and eliminating GLP-1 as it is secreted [68]. Mice that lack these IELs are resistant to obesity, diabetes, and cardiovascular disease. Furthermore, other hemopoietic-derived cells express DPP4, the protease that inactivates a number of gut hormones including GLP-1 and GIP and thus may be responsible for cleavage of these hormones as they enter the circulation [69]. Patients with inflammatory bowel disease have recently been shown to have reduced expression of EEC markers, including GLP-1 and PYY in the colon, though it remains unclear whether this is immune mediated [70]. The potential role of the mucosal immune system in regulating metabolism is the subject of active research [71].
2.6. The gut microbiome
In recent years, it has become increasingly clear that the gut microbiome, the bacteria, fungi, protozoa, and viruses that populate the gastrointestinal tract, are involved in gut-brain signaling, and the concept of a microbiome–gut–brain axis is now emerging. Composed of trillions of microorganisms from thousands of species with unique microbial genes that outnumber human genes by more than 100-fold, these organisms produce a variety of metabolites that modulate other bacteria, bind to protein targets locally in the gut epithelium, and traffic across the intestinal barrier to influence the host [[72], [73], [74]].
The list of areas of physiology linked to the microbiome–gut–brain axis is rapidly growing, and there is increasing evidence that dysregulation of this system may be involved in metabolism [75]. Indeed, gut microbiota dysbiosis has been repeatedly observed in patients with these disorders, although a definitive causal relationship has not been established [76]. The ability of microbial products to interact with host cells involved in metabolism and the gut-brain axis is well documented. Metabolites including short-chain fatty acids (SCFA), bile acids, and lipopolysaccharides bind to and modulate L cells in the colon [27]. As a result, these microbial products can generate hormonal signals that may convey long-term dietary status and epithelial integrity to the brain [77]. The magnitude of metabolic effects these signals have on human physiology and whether they can cause disease remains unclear. Given that L cells secrete GLP-1, which modulates insulin and appetite, it could be postulated that altering the composition would impact metabolism, but a clinical study using antibiotics to change the composition did not find it led to a clinically relevant impact on any metabolic parameter studied [78]. However, fecal transplant studies in germ-free mice have shown that those inoculated with samples from obese donors gain more weight than those from lean or mouse donors [79].
Overall, the picture of how the microbiome influences the host is evolving and has led to a large investment in research in academia and industry to identify approaches to modulate the gut microbiome to treat a variety of different diseases. Rapid progress in this area is challenged by an incomplete census of the microorganisms that inhabit the gut and the difficulty in translating very clear effects seen in germ-free mice to the more complex system found in the human gut. In this regard, there is currently a major push to survey the microbiome at a molecular level in different disease states to gain a better understanding of the molecular mechanisms by which gut-brain circuits are triggered to enable a more rational approach to the discovery of new therapeutics.
3. Advanced technologies studying the gut-brain axis
3.1. Elucidating cell types
The majority of sensory cells along the gut-brain axis have historically been difficult to isolate and characterize. For example, sensing and processing ingested nutrients is the most fundamental function of the gut, yet our knowledge of the EECs that coordinate this response was poor for many decades because they are scattered in the epithelium and far outnumbered by absorptive and other secretory cells. Several methods have been employed to elucidate the vast heterogeneity of EECs.
The first experimental approach involved immunostaining fixed sections of the gut with antibodies against epitopes of hormones known to be elevated in postprandial states [80]. This resulted in a map of a dozen or so cell types characterized by a single hormone. The next technological advance involved creating transgenic mice that enabled the purification of primary cells based on a single molecular identity, that is, a hormone for each EEC type. Gip-expressing K cells [35], Cck-expressing I cells [81], Gcg-expressing L cells [18], and Sst-expressing D cells [82] were isolated in bulk and the transcriptomes were studied by microarray and qPCR. This work indicated that EECs are in fact plurihormonal, an observation that was solidified using immunohistochemistry and flow cytometry [26].
The unbiased transcriptomic approach made it possible to identify the expression of previously unknown secreted products, such as INSL5 in the colon [83] and drug targets expressed by different EEC populations [15]. Although a major step forward, the resolution was low and entire EEC lineages were poorly profiled due to the unavailability of transgenic mice for specific cell types.
The latest technological advance has been single-cell sequencing. This unbiased transcriptomic analysis allowed subtypes to be elucidated from all of the major endocrine lineages. Atlases have now been generated of EECs from mouse and human organoids [17], mouse small and large intestine [84], and human large intestine [85]. While this is a major advance, understanding the complete transcriptome is still limited by the rarity of EECs as many of these atlases consist of hundreds of cells split into over 20 types. Genetically modified human organoids have recently been developed to facilitate the purification of EECs but this is not yet possible with primary human cells [86]. Furthermore, in the mouse, while a comprehensive analysis of colon EECs has been produced using a Neurod1-Cre mouse line to isolate pure populations [87], the corresponding small intestine data remain unavailable. As a result, the full transcriptomic diversity of EECs in humans and mice is tantalizingly close but remains incomplete.
Nevertheless, it is now clear that there is a spectrum of endocrine cell types along the small and large intestines that split into ∼5 lineages. These lineages primarily express TPH1/SCT, GHRL/MLN, GCG/PYY/NTS/CCK/INSL5/SCT, GIP/CCK/GAST/SCT, or SST. There are exceptions with rare cells expressing hormones from two lineages, for example SST/GIP or TPH1/GCG. It remains unclear why these subsets are present; they may be transdifferentiating cells caught between two transcriptomic states or terminally differentiated subtypes.
Much effort has also been undertaken to profile the ENS and immune populations along the mouse and human gut [49,51,88]. Given these cell types also have a spatial axis, and the human gut is over 8 m long, it is difficult to know if all cell types have been adequately profiled. Regardless, a high-resolution understanding of the transcriptomic composition of the ENS will likely facilitate the use of new technologies that rely on knowing the molecular identities of cell types, which should enable the discovery of novel ENS biology and aid future therapeutic targeting of the neural circuits.
Conversely, the vagus has provided a ripe opportunity to perform single-cell sequencing, with all of the afferent nerve cell bodies bundled together in the nodose ganglia. Three recently published single-cell datasets found that there are between 18 and 27 cell types in the mouse, which express an array of gut hormone receptors [65,89,90].
The NST/area postrema (AP) region of the brainstem, which receives input from both the vagus and circulating gut hormones, has not yet been fully sequenced. Single-nuclear sequencing has recently been applied to elucidate cell types in various regions of the mouse and human brain, and these methods can also be applied to create an atlas of the NST/AP [91]. An atlas specifically of the mouse AP was recently published and it highlights unique populations that express the GLP-1 and amylin receptors [92]. Understanding these cell types is particularly relevant in the metabolism field, as many obesity therapeutics showing success in clinical trials, including GLP-1 and amylin, are known to engage satiety circuits originating from these neurons, and this new report highlights the specific neuron subsets that cause aversion in mice. GLP-1 agonists used in the clinic were shown to engage these AP neurons, and thus this signaling circuit could account for the commonly reported nausea and vomiting that is observed with GLP-1 agonists [39,[93], [94], [95]].
3.2. Circuit tracing
As previously described, single-cell sequencing enables an understanding of the molecular identity of all gut-brain cell types, which is foundational to mapping the circuitry involved in transmitting sensory information from the gut to the brain. One clear route of communication is endocrine, as secreted peptides from EECs are thought to target receptors in circumventricular organs of the brain. However, the importance of endocrine signaling in normal physiology has been questioned given the rapid inactivation of at least some of these peptides as they enter the systemic circulation [96]. The afferent terminals of the vagus nerve, in comparison, occupy an anatomically privileged position adjacent to EECs and express an array of hormone receptors.
Vagal afferents do not directly contact the lumen; therefore, it is likely that they are mainly second-order chemosensory neurons [97]. Based on this observation, it is tempting to analyze the transcriptomic data alone and hypothesize circuits between specific EECs and vagal neurons. For example, EECs secrete GLP-1 in response to nutrients; hence, one might predict that Glp1r-expressing vagal afferents are primarily stimulated by nutrients and innervate the mucosal layer of the mid to distal intestines, where GLP-1-producing EECs are most abundant. However, this does not appear to be the case: using a Glp1r-Cre mouse line combined with nodose ganglion-specific viral transduction, these vagal neurons were selectively labeled with either a fluorescent tracer to assess innervations or a genetically encoded calcium indicator to monitor activity in anesthetized mice. Studies with these animals found that Glp1r neurons primarily innervate the myenteric layer of the stomach, not the distal intestine, and respond mainly to stomach stretch, not nutrients [97]. One possible explanation is that gut hormones relay convergent state-dependent information on nutrients to the vagus, and so mechanosensory neurons are excited at lower distension levels when nutrients are abundant. A similar phenomenon has recently been described for CCK [65]. This calls into question the simplistic view that vagal neurons are either mechanosensors or chemosensors.
The analysis of Glp1r-expressing vagal neurons was the first in a sequence of papers that have started to illuminate the gut innervations of specific vagal populations using a combination of transgenic mice and viral-mediated tracing techniques [65,98]. For example, utilizing this approach, Bai et al. mapped five vagal populations that have chemosensory terminals and innervate various regions of the stomach and small intestine and two populations that primarily have mechanosensory IGLEs. They confirmed that one of these two populations expresses Glp1r and innervates the stomach while the other expresses Oxtr and innervates the intestines. Furthermore, they used dual-color retrograde tracing to show that individual vagal afferents do not terminate in multiple regions of the gut, suggesting that they deliver region-specific information [65].
A comprehensive cell-to-cell map of synapses between the vagus and NST/AP neurons is not yet available. However, an example of how such circuits can be mapped was provided by a recent study analyzing the link between peripheral and central GLP-1 [99]. This peptide is produced both by EECs in the gut and a specific population in the NST [100]. To determine whether gut GLP-1 stimulates the release of GLP-1 from the NST, rabies virus-mediated monosynaptic retrograde tracing from NST Gcg-expressing neurons was performed. They found that central GLP-1 neurons primarily synapsed onto the Oxtr-expressing, rather than Glp1r-expressing, vagal afferents. With other complimentary experiments, the authors concluded that peripheral and central GLP-1 cells are components of independent satiety circuits.
Circuit-tracing techniques have also begun to be applied to the ENS. Khomgrit et al. used peripherally restricted delivery of a fluorescent protein-expressing virus to redefine the traditional classification of IPANs [101]. In another recent study, local viral delivery was utilized to anatomically map neuropeptide-positive ENS populations in the distal gut, leading to the discovery of a glucoregulatory viscerofugal subtype that is functionally connected to the sympathetic celiac superior mesenteric ganglion (CG-SMG) [52]. Modern circuit-tracing techniques will likely aid research into how sensory vagal and ENS populations are coupled, a connection that has been previously described in the literature, with potential implications for satiety or metabolism in general [102].
3.3. Functional studies
The ability to genetically manipulate specific cell types for gain or loss of function has led to major advances in our understanding of the physiology mediated by gut-brain circuits. Loss-of-function experiments have historically relied on methods that affect multiple afferent and efferent vagal neurons, such as vagotomy, local anesthesia, and pharmacological agents such as capsaicin [103]. It is now possible to use transgenic animals expressing diphtheria toxin, tetanus toxin light chain (TTLC) or caspase 3, or local injections of the neurotoxin saporin conjugated to ligands to cause the loss of function of specific afferent neurons. To assess gain of function, optogenetics and chemogenetics can now be used, which provide superior specificity to previously used methods for cellular activation (pharmacology and electrical stimulation).
Furthermore, hundreds of elegant studies wherein gut hormones have been pharmacologically administered alone and in combination have suggested the physiological function of specific EECs, but studies validating this have been challenging. This is now possible using chemogenetics. The ability to activate specific populations of EECs can now be used to explore the physiology, rather than the pharmacology, of the hormones they produce, which is important as the two approaches likely engage different circuitry. We highlight a few examples of studies that have employed these techniques (Table 1) to gain new insights into circuits affecting metabolism (Figure 2).
Table 1.
Advanced technologies for studying gut-brain circuits. Key references relate to studies that have applied these technologies to study the gut-brain axis.
| Technique | Use | Advantages | Limitations | Key references |
|---|---|---|---|---|
| Sequencing | ||||
| Single-cell sequencing | Elucidate transcriptome of single cells | High resolution, possible to analyze cell states and trajectories, only requires a small amount of starting material, and can profile rare cells | Relatively expensive, bioinformatic pipelines are still evolving and often require tissue digestion that can perturb cells | EECs: [85,87] ENS: [49,51,101] Vagus: [65,89,90] NST/AP: [51] |
| Translating ribosome affinity purification | Profile the translated mRNA complement of a genetically defined cell population | Does not require tissue digestion, can analyze early response genes, and applicable to all cell types | Only useful for bulk sequencing | ENS: [52] Vagus: [52,182] |
| Circuit tracing | ||||
| Cholera-toxin beta subunit/wheat germ agglutinin-conjugated fluorophore | Tracing of neuronal innervations, transsynaptic labeling with WGA | Relatively rapid and possible to obtain regional information | Non-specific uptake by neurons | ENS: [52] Vagus: [65] |
| Adeno-associated virus (AAV) | Genetically guided, cell specific, tracing and delivery of genetic payload, and anterograde transsynaptic | Stable gene expression, non-toxic and many serotypes available with different tropisms | Long lead time, small vector capacity and few serotypes transduce gut-brain cell types | Vagus: [65,97,98] |
| Herpes simplex virus (HSV) | Genetically guided, cell or region specific, tracing Anterograde transsynaptic, polysynaptic, or monosynaptic options available | High gene expression and rapid propagation | High cytotoxicity limits length of experiment and unclear sequence of infected cells | Brain: [183,184] |
| Rabies virus (RV) | Genetically guided, cell specific, and monosynaptic retrograde tracing | Large vector capacity, high gene expression, and rapid propagation | Cytotoxicity limits length of experiment | Vagus: [99,108,185] |
| Pseudorabies virus (PRV) | Genetically guided, cell specific, polysynaptic retrograde tracing | High gene expression and rapid propagation | Cytotoxicity limits length of experiment | Brain: [186] |
| Modulating function | ||||
| Optogenetics | Genetically guided activation of specific cell types by expression of channelrhodopsin followed by illumination of cells with light | Time-locked cellular activation and specificity from both cell type-specific transduction of channelrhodopsin and implantation of light fiber | Supraphysiologic activation, requires implantation of light fiber: possible in the brain but problematic in the gut | EEC: [109] Vagus: [65,[97], [98], [99]] |
| Chemogenetics | Genetically guided activation of specific cell types by expression of DREADDs or PSAMS, followed administration of a specific ligand (CNO, Compound 21, or varenicline) | Cell-type specific activation, no requirement for implantations and ligand can be dosed in water bottle for prolonged activation. Ion channel guarantees depolarization or hyperpolarization | Supraphysiologic activation, off-target effects of ligand, and effects of G protein signaling may not always activate or inhibit | EEC: [104] ENS: [52] Vagus: [65,98,99] |
| Targeted recombination in active populations (TRAP) | Genetically guided labeling of neurons activated under specific conditions | Specific labeling of stimuli-responsive cells and not all cells within a population | Used in neurons that express early response genes, that is Fos or Arc | ENS: [52] NST: [108] |
| Tetanus toxin light chain (TTLC) | Genetically guided inhibition of synaptic signaling in selective neuronal populations | Cell type-specific silencing without the potential caveats of cell death | Variable transduction efficiency with virus, permanent, and may not prevent neuropeptide release | Vagus and NST: [108] |
| Saporin–ligand conjugates | Ablation of specific cell type expressing a receptor with a ligand amenable to saporin conjugation | Does not require transgenic lines and rapid | Limited to cell types with amenable ligands | Vagus: [98,187] |
| Diphtheria toxin receptor (DTR) | Genetically guided ablation of specific cell type by expression of diphtheria toxin receptor followed by administration of the toxin | Time-locked ablation of specific cells and high efficiency | Diphtheria toxin toxicity | EEC: [81] |
| Diphtheria toxin a chain | Genetically guided ablation of specific cell type by direct expression of diphtheria toxin | Time-locked ablation of specific cells and high efficiency | Slower ablation kinetics than DTR approach due to expression kinetics of the virus | Vagus: [188] |
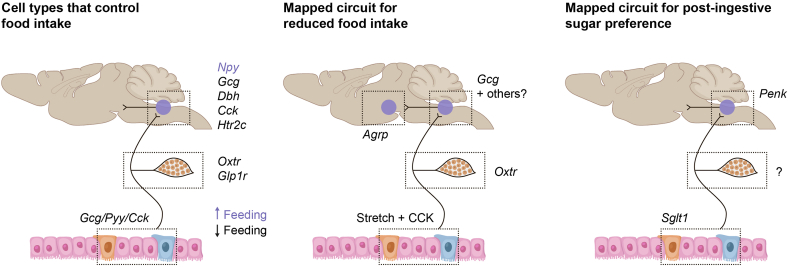
Figure 2.
Examples of gut-brain circuits uncovered using technological advances. Chemogenetic, optogenetic, and loss-of-function studies have elucidated individual cell types that can regulate feeding in the gut, vagus, and NST/AP [65,99,104,179,180]. One circuit that consists of cells in all three of these nodes integrates intestinal distension and CCK detection to suppress food intake [65,99]. Another circuit that originates in the gut has also been shown to be sufficient and necessary to signal post-ingestive sugar preference [108].
3.4. Examples of applying new technologies to study metabolic circuits
3.4.1. Selective gut modulation
A chemogenetic approach was used to explore the function of a specific population of GLP-1/PYY/INSL5 secreting EECs in the large intestine [104]. Activating this population resulted in better metabolic outcomes, including improved glucose tolerance, decreased food intake, and increased energy expenditure. Chemogenetic activation was then coupled with systemic inhibition of specific receptors, which led to findings that improved glucose tolerance was GLP-1 dependent, whereas the reduced food intake effect was PYY dependent. The precise circuits used by these hormones to improve the metabolic parameters remains unclear, as does the physiological relevance of the findings given that nutrient loads only reach the large intestine in exceptional situations. However, the role of distal GLP-1 in glucose handling is further supported by the work of Song et al. who found that the loss of the proglucagon gene from the distal gut resulted in decreased fasting GLP-1 levels and impaired glucose tolerance [105].
Similar techniques have recently been used to assess the role of the ENS in metabolism. Muller et al. analyzed the function of cocaine- and amphetamine-regulated transcript (CART)-expressing neurons by selective chemogenetic activation or diphtheria toxin-mediated ablation of these neurons [52]. They discovered that activating the CART populations in the ileum and colon increased glucose levels, whereas lower glucose was observed when this population was ablated. These neurons were subsequently found to be synaptically connected to the CG-SMG ganglions and modulated glucose via insulin secretion or hepatic gluconeogenesis. Further studies are needed to determine how this population of neurons is activated in normal physiology. This is a compelling example of how the tools used to elucidate a peripherally restricted circuit can enable the interrogation of other ENS subsets with unknown functions, work that will be facilitated by single-cell sequencing that has identified additional molecular subtypes of ENS neurons [49].
3.4.2. Selective vagal modulation
There has been substantial effort utilizing both chemogenetics and optogenetics to elucidate the functions of specific populations of vagal afferent neurons. Bai et al. have shown that specifically activating Glp1r-expressing afferents (stomach, IGLE+) reduced feeding by ∼50% while activating Oxtr-expressing neurons (small intestine, IGLE+) entirely suppressed feeding. Activating either cell types did not elicit aversive behavior, suggesting that their effects are mediated by a true satiety circuit. Consistent with this notion, both cell types were found to inhibit orexigenic hypothalamic AGRP neurons as measured by fiber photometry in awake and behaving animals. While activating Glp1r vagal afferents transiently inhibited hypothalamic AGRP neurons, more sustained inhibition was observed by activating the Oxtr population [65].
The complete cessation of feeding observed by activating Oxtr vagal neurons is consistent with a profound role of the vagus in modulating feeding. There is evidence to suggest that Oxtr-expressing neurons respond to mechanical stimuli and, in line with these findings, when hyperosmolar, non-nutritive solutions were delivered to the gut to produce large increases in fluid content in the middle intestine, mice ate significantly less food and the hypothalamic AGRP neurons were inhibited. This gut-brain circuit could underly some of the profound metabolic effects of bariatric surgery during which gastric contents rapidly empty into the intestines and cause distension. Further elucidating this circuit, including upstream central neurons, could result in new targets for treating obesity and related disorders.
3.4.3. Food preference
Most species have evolved dedicated brain circuits to seek and consume energy-rich foods, such as those high in sugar and lipids. Sugar sensing occurs in the periphery, with taste receptors located on the tongue providing an initial input. Agonizing these receptors with artificial sweeteners is sufficient to rapidly convey preference. When given the choice between glucose and artificial sweeteners, however, animals will ultimately prefer glucose [106]. This preference is not mediated by sweet taste receptors, as it is also observed in animals that lack these receptors [107]. Taken together, these observations suggest there is a second pathway that modulates post-ingestive sugar preference. The neural basis for this phenomenon was recently elucidated by Tan et al. who used a combination of targeted recombination in active populations (TRAP) to identify sugar-sensing neurons in the NST, selective chemogenetic activation or TTLC-mediated inhibition of sugar-sensing neurons in the NST, fiber photometry, and behavioral studies to demonstrate that a gut-brain circuit was sufficient and necessary to convey preference [108]. This circuit requires glucose sensing by the glucose transporter SGLT1 and vagal signaling to Penk-expressing neurons in the NST. Similar work has focused on the gut side of a vagal sugar circuit [109] and gut-brain circuits have also been proposed for lipid preference [110]. Further elucidating these circuits could allow the development of a new class of diet products that activate both the taste system and gut-brain axis to help moderate the strong drive to consume sugar and other high-energy foods.
3.4.4. Other metabolic traits
The vagus nerve has been studied for its effect on a variety of other metabolic phenotypes beyond food intake and post-ingestive preference. These include modulating energy expenditure, hepatic glucose production, and insulin secretion [2,69,111,112]. It is unclear if these are primarily modulated by gut-derived signals or afferents that project to the pancreas or liver, although there is evidence showing that exposing the proximal gut to lipids is sufficient to modulate some of the effects [113]. The approaches described herein should facilitate an understanding of vagal nerve physiology that can be harnessed to identify new approaches to these therapeutic areas.
4. Emerging view of the role of the gut-brain axis in regulating appetite and glucose
The role of the gut-brain axis in regulating appetite has been the subject of decades of research; however, the previously described technologies are now enabling a more comprehensive understanding of this circuitry. The emerging picture of the role of the gut-brain axis in feeding and meal termination follows.
First, in anticipation of food, EECs in the stomach secrete ghrelin, which modulates orexigenic signaling to the brain [31]. Some evidence suggests that ghrelin uses a vagal circuit to increase appetite; however, the latest mouse vagus single-cell sequencing data are notably absent of Ghsr expression [65,89,90], and functional studies with chemo- and optogenetics have not revealed an orexigenic vagal cell type [65]. Ghrelin is detected at high levels before meals and thus it is likely that the circulating form of ghrelin directly engages the hindbrain and ARC to promote appetite [31].
Once the meal is ingested, complex forms of fat, proteins, and carbohydrates enter the upper GI tract and undergo digestion to release the basic nutrient components. These rapidly enter the duodenum and are absorbed. Endocrine cells in the stomach that secrete ghrelin are inhibited, and studies have shown this requires entry of nutrients into the small intestine [114]. The gastric walls expand as the meal accumulates in the stomach, with the pyloric sphincter regulating nutrient entry into the small intestines. The expansion of the stomach has been shown to be detected by IGLE + vagal afferents, specifically the Glp1r + type that innervates the gastric myenteric layer [97]. These nerves have the potential to send the first satiation signals to the brainstem [65].
Nutrients flow from the stomach into the duodenum and are absorbed across the epithelium in sufficient concentrations to engage a variety of chemosensory receptors on EECs, including FFAR1, CaSR, and SGLT1 [27]. The combinatorial effect of engaging these receptors results in an increase in intracellular cAMP, calcium, and depolarization. The cells then secrete a number of hormones, including CCK, GIP, and SCT. All three hormones are co-localized in a subset of duodenal EECs in the villus tip [17]. CCK acts on vagal terminals to enforce contraction of the pyloric sphincter and reduce gastric emptying [66] while also engaging a vagal afferent subset defined by Oxtr expression [65]. These afferents also possess IGLE terminals and respond to small intestinal stretching, so they possibly integrate both chemo- and mechano-signals. This vagal subset synapses onto GLP-1-producing neurons in the NST as well as other neurons [99]. This circuit has been shown to ultimately reduce the activity of AGRP neurons in the hypothalamus, resulting in a diminished drive to consume additional food [64,65].
CCK also promotes pancreatic enzyme secretion and gall bladder emptying to aid digestion, although it is unlikely to be via a vagal-brain circuit, as recent functional work on gut-innervating vagal cells did not show any to induce gall bladder contractions when specifically activated [65]. SCT also promotes secretion of pancreatic digestive enzymes. Its role in brain signaling has yet to be fully elucidated, but Sctr was found by transcriptomics on a discrete vagal afferent type [89,90]. The exact function of this cell has yet to be determined.
GIP, which is also secreted from the duodenum, does not appear to signal to the brain through the vagus based on transcriptional analysis. Instead, its receptor is expressed by cells in several metabolic target organs, including pancreatic beta cells, the AP, and hypothalamus [115]. Upon secretion, it acts as an incretin to reduce circulating levels of ingested glucose by amplifying insulin secretion from beta cells that express the GIP receptor [36]. It also promotes adipocyte fat storage and could directly induce satiation through the hypothalamus, as recent work demonstrated that chemogenetic activation of Gipr-expressing neurons in this region resulted in reduced food intake [115].
As nutrients and bile acids pass through the duodenum and into the jejunum, multiple EECs containing GLP-1, PYY, and NTS as well as CCK, GIP, and SCT are activated. All of the receptors for these peptides, with the exception of GIP, are expressed by vagal afferents [65,89,90]. GLP, PYY, and CCK receptors have been detected in the ENS [49]. GLP-1, GIP, PYY, and NTS receptors are expressed in central feeding centers. How these hormones signal to the brain alone and in combination after a meal has yet to be fully determined, but when co-infused, they produce strong satiety effects [116].
GLP-1 is secreted from this region of the gut and is implicated in both satiety and insulin secretion. The stomach-projecting vagal afferent that express Glp1r may become sensitized to the distal intestinal absorption of nutrients via circulating GLP-1 [97]. Increased cAMP levels caused by GLP-1 receptor engagement could allow the neuron to activate at lower stomach distension levels and transmit satiety signals to the brainstem. This pathway has been shown by retrograde virus tracing to engage a circuit separate from the central GLP-1 circuit engaged by the CCK-sensitive afferents innervating the small intestine [99]. GLP-1 also acts as an incretin to amplify insulin secretion and reduces glucagon secretion to lower blood glucose either through direct actions on beta and delta cells or a neuronal circuit [39,69]. Beta cells also secrete amylin, a hormone with a receptor expressed in the AP, and can further induce satiety [117].
As nutrients continue to flow through the small intestine and into the ileum, GLP-1 and PYY levels work in combination as the “ileal break” to further contract the pylorus and prevent more food from entering the small intestine [118]. This further reduces glucose absorption and thus is a second mechanism by which GLP-1 modulates blood glucose levels [119]. Both hormones induce satiety and, in combination with CCK, NTS, and mechanosensitive vagal afferents, modulate meal termination.
As undigested nutrients, fiber, and bile acids from the meal pass into the large intestine, the microbiome communities that are best adapted to utilizing the available substrates are thought to thrive and outcompete neighboring microbes. They release metabolites such as short-chain fatty acids, indole, and secondary bile acids that interact with EECs to elicit additional hormone secretion [27].
5. Harnessing the gut-brain axis to treat metabolic diseases
There is a clearly unmet need for new agents that are safe, efficacious, and can be combined with current therapies to treat metabolic disorders. The obesity epidemic is widespread and will only worsen over the next decade [120]. It will be accompanied by increases in obesity-related complications, including T2DM, cardiovascular disease, NASH, osteoarthritis, and several types of cancers. The COVID-19 epidemic has further highlighted the importance of addressing this problem, as obesity, T2DM, and hypertension are major risk factors for severe illness and mortality [121].
Efforts to directly target the brain to reduce appetite to treat obesity have largely failed, as have approaches that directly increase energy expenditure, largely due to mechanism-based toxicities (Figure 3). However, there has been significant progress in exploiting gut-derived hormones to treat both T2DM and obesity (Figure 4). Indeed, GLP-1-based therapeutics that include GLP-1 analogs and DPP-4 inhibitors have dominated the therapeutic discovery landscape of T2DM for more than two decades, and recent clinical studies with GLP-1 analogs have shown that obesity is a tractable therapeutic area, that is, that clinically meaningful body weight loss can be achieved with an acceptable safety profile.
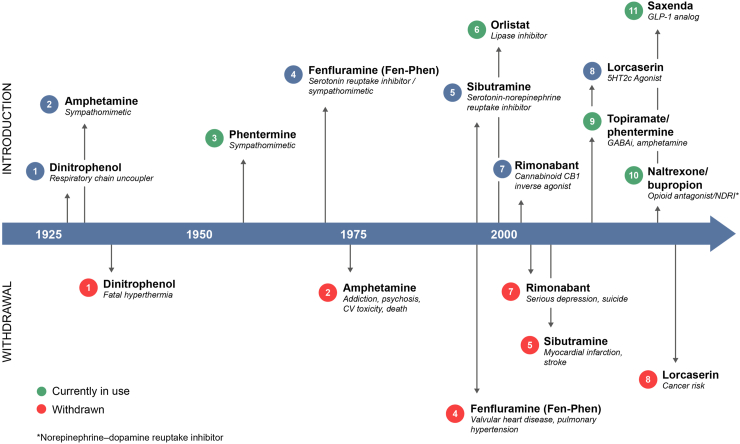
Figure 3.
History of obesity therapeutics. Historically, attempts to develop safe and effective therapeutics to treat obesity have been challenging [181]. The first obesity agent, dinitrophenol, a respiratory chain uncoupler that increases the metabolic rate, was withdrawn only a few years after it was introduced for safety reasons, including mortality. For several years thereafter, amphetamine derivatives such as amphetamine itself, phentermine, and fenfluramine dominated the landscape. Amphetamine and fenfluramine were later withdrawn for safety reasons, and FDA restrictions have been placed on the use of phentermine (limited to 12 weeks of use). Other efforts to directly target the brain, including monoamine re-uptake inhibitors (sibutramine), CB1R inverse agonists (rimonabant), 5HT2C agonists (lorcaserin), and combinations of central mechanisms (topiramate/phentermine and naltrexone/bupropion), have been withdrawn or have limited use. Orlistat, a lipase inhibitor, has also had limited success due to modest efficacy and tolerability issues. Despite the historical failures in this therapeutic area, more recent efforts with GLP-1 analogs have demonstrated that clinically meaningful weight loss, in some cases >10%, can be achieved with an acceptable safety profile.
Figure 4.
Evolution of T2DM and obesity therapeutics harnessing the gut-brain biology. For the last 15 years, the branded therapeutic market for T2DM has been dominated by GLP-1-based therapies, including DPP-4 inhibitors and GLP-1 analogs. While DPP-4 inhibitors are weight neutral (and thus not shown in this figure), GLP-1 analogs are associated with weight loss and thus have been explored to treat obesity. First-generation GLP-1 analogs are injectables that are dosed once daily or more frequently and are associated with relatively modest weight loss. Second-generation GLP-1 analogs, including once-weekly exenatide, semaglutide, dulaglutide, and oral semaglutide, which have been approved for T2DM have more convenient dosing regimens (1 weekly, oral) and high-dose semaglutide and dulaglutide have shown the potential for > 10% weight loss in clinical studies. All these analogs are associated with tolerability issues (nausea/vomiting). Next-generation approaches that are being investigated in the clinic include injectable agents that exploit dual pharmacology and oral small molecule GLP-1R agonists. Future approaches currently being explored include directly targeting gut EEC and vagal circuits with small molecule oral therapeutics.
Of note, these major advances in treatment have occurred despite a detailed mechanistic understanding of the biology of endogenously secreted GLP-1 and other gut hormones that are being pursued as therapeutics. It is also the case, as previously noted, that while the efficacy of GLP-1 analogs on weight loss is mediated by the gut-brain axis, this is likely not the case for controlling glucose. Nevertheless, the success of these approaches, together with the more intimate understanding of hormone signaling enabled by new technologies, is fueling numerous efforts to further harness gut-brain circuitry for therapeutic benefit.
5.1. Injectable gut peptide hormones as therapeutics
5.1.1. First-generation GLP-1-based therapies for T2DM and obesity
GLP-1 was the first gut-derived factor to be developed and approved as a therapeutic to treat T2DM. This hormone derived from the post-translational processing of proglucagon was discovered in the early 1980s (for a review of the history of GLP-1 therapeutics, see [40,122]). GLP-1 is one of two incretin hormones, the other being glucose-dependent insulinotropic peptide (GIP), which stimulate glucose-dependent insulin secretion from the pancreas [123]. GLP-1 also indirectly inhibits glucagon secretion, most likely by activating delta cells that express glp1r, and has been shown to inhibit food intake and promote weight loss in humans potentially via direct effects on GLP-1 receptors in the hindbrain, vagus, and hypothalamus [124]. GLP-1 analogs are also associated with delayed gastric emptying and modest blood pressure reductions [125].
Exenatide, a GLP-1-related peptide that was isolated from the Gila monster's saliva, was the first GLP-1R agonist to be approved for T2DM in 2005. Several GLP-1 analogs followed soon after, including liraglutide, lixisenatide, and albiglutide. All these agents require injection and are accompanied by nausea and vomiting, which are mechanism-based adverse effects of this class of therapies. Recent work has suggested that Glp1r-expressing neurons in the AP are the main mediator of this effect in mice [92]. These side effects can be mitigated in part with careful titration over weeks or months up to the therapeutic doses. Some GLP-1 analogs have been shown to improve cardiovascular outcomes in patients with T2DM [126].
First-generation GLP-1 analogs produce modest weight loss in patients with T2DM. For example, the maximal approved dose of liraglutide for T2DM (1.8 mg once daily) results in an approximately 2% placebo-corrected weight loss [127] in patients with T2DM. While this dose results in near-maximal glucose-lowering efficacy, higher doses have been shown to produce greater weight loss, with a once-daily injection of 3 mg leading to a 4.5% improvement over placebo in obese/overweight patients and a 3.7% improvement in obese/overweight patients with T2DM in a 1-year study [128]. Liraglutide 3 mg is the first FDA-approved GLP-1 analog indicated for treating chronic weight management and is currently the leading branded agent for treating obesity. The success of this therapeutic agent has demonstrated that patients and prescribers will value relatively modest efficacy if the safety profile is acceptable.
5.1.2. Second-generation GLP-1-based therapies
Building on the success of first-generation GLP-1 analogs, subsequent efforts have focused on improving efficacy and convenience [124,129]. A long-acting exenatide formulation (once-weekly exenatide) was approved by the FDA in 2012 [130]. A second once-weekly GLP-1 analog, dulaglutide, was approved for T2DM in 2014 [131]. Dulaglutide has been shown to reduce cardiovascular outcomes [132]. Semaglutide, a once-weekly GLP-1 analog, has been approved for T2DM at doses up to 1 mg [133]. While weight loss at this dose is relatively modest, a higher dose (2.4 mg once a week) is currently being investigated to treat obesity, and a cardiovascular outcome study is also ongoing. At the higher 2.4 mg dose, 12.4% placebo-corrected weight loss was observed in obese/overweight patients without T2DM [134]. Semaglutide is also being investigated to treat NASH. While tolerability remains an issue, high-dose semaglutide data demonstrated that >10% weight loss can be achieved with an agent that has an acceptable safety profile. This is a breakthrough in treating obesity.
An oral formulation of semaglutide has also been approved for T2DM. In head-to-head studies, HbA1c decreases were superior those achieved with the DPP-4 inhibitor sitagliptin [135] and non-inferior to liraglutide [136]. Modest weight loss (up to 2.3 kg) was achieved with the oral formulation in a monotherapy study in patients with T2DM [137,138]. Tolerability remains an issue, and the dosing regimen is relatively complex. It remains to be determined whether improved formulations can result in greater convenience and efficacy.
The collective data strongly suggest that the weight loss observed with GLP-1R agonists in humans is largely mediated by a reduction in food intake, although the molecular basis for the differential efficacy of different analogs on weight loss is not fully understood [125]. GLP-1R is expressed in the brain, particularly in areas involved in food intake, and in the periphery, including the gastrointestinal system, pancreatic islets, portal vein, and vagus nerve, but these receptors’ relative contribution to the pharmacology of various GLP-1 analogs remains unknown. Indeed, first- and second-generation GLP-1R agonists achieve high systemic bioavailability but are thought to engage central targets to varying degrees. To begin to address the importance of engaging central GLP-1 targets, brain penetration of both liraglutide and semaglutide has been explored in rats. The distribution of fluorescently labeled agonists suggest that neither cross the blood–brain barrier but instead have access to targets in the circumventricular organs and selected sites adjacent to ventricles. Neurons expressing Glp1r in the hindbrain and hypothalamus in particular have been explored for their role in modulating satiety [139]. While it has been shown that both semaglutide and liraglutide have access to neurons in these regions, albeit to various degrees, semaglutide has been demonstrated to have preferential access to two other regions of the brain, the septofimbrial nucleus and lateral septal nucleus, which have densely populated Glp1r-expressing neurons [139]. Moreover, semaglutide induces c-fos activation in brain areas expressing GLP1R and in regions that do not express the receptor, suggesting secondary indirect effects in regions implicated in meal termination [139]. It has yet to be determined whether signaling through these neurons is the major reason why semaglutide achieves greater weight loss vs liraglutide, and the results of future studies with GLP-1 receptor agonists that preferentially target these neurons may be enlightening. Of note, there are many other central GLP-1 receptors that reside beyond the blood–brain barrier that are unlikely to be engaged by currently available agonists. These are likely targets of GLP-1 released centrally from cells located in the NST.
5.1.3. Clinical studies with other gut peptides
As previously noted, a number of gut peptides are secreted from EECs in response to nutrients, and several have been pursued as potential therapeutics. OXM, another product of the proglucagon gene, is co-secreted with GLP-1 and has dual activities, activating both GLP-1 and glucagon receptors, although it is only a weak agonist of the latter. As such, this peptide has the potential to modulate glucose control and food intake via its effects on the GLP-1 receptor and energy expenditure via its effects on the glucagon receptor. Indeed, OXM has almost identical effects on food intake in Gcgr−/− mice [140]. Proof of concept (POC) for weight loss for OXM was achieved in a 4-week study wherein subcutaneous administration was shown to result in a 2.4% reduction in body weight [141]. As a consequence of this result, multiple GLP-1/glucagon co-agonists have been studied in the clinic as further described to follow.
CCK was one of the of the first satiety hormones to be isolated from the gut (review [142]) and has been shown to elicit satiety via vagal signaling pathways. Indeed, multiple studies have shown that infusing CCK in humans can lead to reduced food intake and appetite [143,144]. A small molecule CCK was also shown to be effective at reducing food intake in humans. However, only modest efficacy was observed due to dose-limiting tolerability issues including nausea and vomiting. Pancreatic and gallbladder adverse effects are also a concern with this mechanism [145].
PYY, another EEC-derived gut hormone, has been pursued to treat obesity. Infusions of the gut hormone fragment peptide YY3-36 (PYY) were shown to reduce 24-hour food intake in human subjects [146]. An intranasal formulation of PYY3-36 was tested in obese adults [147], but dose-limiting tolerability issues precluded further development.
5.2. Next-generation approaches
Despite the success of current therapies targeting gut hormone pathways, there is ample opportunity for improvement, particularly for treating obesity. Although impressive efficacy has been demonstrated with injectable agents, modes of administration, dose-limiting tolerability, and costs remain significant issues. New approaches that have the potential for efficacy comparable to or better than current agents, superior tolerability, more convenient administration, lower costs, and the potential for a dual T2DM/obesity indication would be highly valued by physicians and patients. Some of the most promising approaches are described to follow.
5.2.1. Dual pharmacology
As previously described, regulating glucose homeostasis and food intake involves integrating signals from multiple hormones that are released from EECs in response to postprandial nutrients. This has led to the hypothesis that activating complementary pathways modulated by these hormones may result in more robust and sustained glucose control and weight loss vs single-agent approaches, and multiple ongoing studies are exploring this concept.
As previously noted, OXM has pharmacological activity against the GLP-1 and glucagon receptors. Based on promising results from preclinical research [[148], [149], [150], [151]] and an initial POC study with co-administration of these peptides in humans [152,153], multiple programs have been initiated to develop a dual glucagon and GLP-1 agonist to treat T2DM and obesity and several are in clinical development [154]. Although weight loss and glycemic control were demonstrated in short clinical studies, results to date have suggested that achieving an optimal ratio between GLP-1 and glucagon signaling is critical to maximize efficacy on body weight. Investigation with these agents has been hampered by lack of a reliable biomarker showing glucagon receptor target engagement in clinical studies [155,156].
A novel dual agonist of the GLP-1 and GIP receptors, tirzepatide, is also being pursued and has recently entered phase III clinical studies to treat T2DM. In a phase 2b study, this agent caused dose-dependent reductions in HbA1c and body weight at 26 weeks (10–15%), with efficacy on HbA1c and body weight superior to dulaglutide and a comparable but significant incidence of gastrointestinal adverse events [4,157]. Phase III studies with tirzepatide are ongoing to support indications for T2DM and obesity and assess potential cardiovascular benefits. The contribution of GIP agonism to tirzepatide's overall profile is not completely understood.
Combinations of gut peptides are also being pursued. AM833 is an acylated long-acting analog of the human amylin hormone that is being developed as a combination therapy with the GLP-1 analog semaglutide. The sponsor recently reported 8% placebo-corrected weight loss with AM833 in a 26-week study. In a 20-week study, AM833 resulted in a 17.1% reduction from baseline body weight when combined with semaglutide compared to 9.5% for semaglutide alone (there was no placebo arm in this study). The potential additive anorectic effects of amylin and GLP-1 are believed to be mediated by activating distinct neuronal subpopulations in the hindbrain [93].
There is a strong rationale and multiple lines of evidence to support a combination of PYY and GLP-1 agonists. Co-administration of PYY3-36 and GLP-7-36 amide in obese human subjects elicited at least additive effects on food intake at doses that were tolerated [158,159]. Furthermore, a functional MRI study showed changes in brain activity following infusion of PYY3-36 and GLP-7-36 in fasted states that were similar to those observed following a meal in humans [160]. A PYY analog, NNC0165-1875, is currently being tested in phase I studies in monotherapy and in combination with semaglutide in overweight or obese subjects.
5.2.2. Oral small molecule GLP-1 agonists
The clinical landscape for T2DM and obesity is currently dominated by injectable gut peptides. However, oral agents for T2DM, including metformin, DPP-4 inhibitors, and SGLT2 inhibitors, are often preferred by physicians and patients because of the convenience of oral administration. Cost is also a potential issue with injectable therapies. As previously noted, an oral formulation of semaglutide has been approved for T2DM, and there are ongoing efforts to develop oral small molecule GLP-1 agonists. Weight loss was achieved in a small 4-week phase 1 study with PF-06882961, an oral GLP-1 agonist that is administered twice daily [161]. These data suggest, as with semaglutide and liraglutide, that doses needed for weight loss will be several-fold higher than those needed for maximal glucose control. As with all GLP-1 analogs, GI tolerability remains a potential issue with this approach.
5.2.3. Direct targeting of the gut-brain axis
With an increased understanding of the biology of the gut-brain axis, there is a potential to harness this knowledge to directly target this system using the approaches described as follows:
5.2.3.1. Direct targeting of the vagal nerve, NST, and area postrema
As previously described, the vagus is intimately involved in modulating feeding by integrating mechanosensory and hormonal signaling, and the previously described technologies enable an understanding of the specific neurons involved in this process. A notable example is the Oxtr-expressing vagal population, which is able to completely inhibit feeding when stimulated in transgenic mice using chemogenetics. The transcriptome of this cell type has been elucidated by single-cell sequencing of the nodose ganglion and thus it is now possible to identify targets that specifically stimulate this circuit. These targets have the potential to be more tractable than many previously studied obesity approaches that require directly engaging central neurons behind the blood–brain barrier. Indeed, because these targets may reside on vagal afferents, gut-restricted pharmacology could potentially be used to engage this circuit with limited systemic exposure.
Similarly, there is ongoing research to better understand the molecular identity of neurons in the NST and AP, regions that are also involved in hormonal signaling and have specialized capillaries more permeable than those of the blood–brain barrier. These neurons, which contain receptors for the satiety hormones GLP-1, amylin, and GDF15 and/or have been implicated in metabolism via loss or gain-of-function studies, are potential direct targets for discovering new therapeutics [93,117,162]. In addition, recent work elucidating the role of AP neurons in GLP-1-induced aversion could be harnessed to reduce the nausea and vomiting seen with GLP-1 receptor agonists, potentially via targeting inhibitory neurons [92]. Conversely, other targets expressed by these cell types could be avoided to reduce the likelihood of these side effects.
5.2.3.2. Modulation of endogenous satiety circuits: targeting the brain via the gut
The knowledge that multiple gut hormones are secreted in response to nutrients to modulate feeding, together with the promising results of clinical studies with dual pharmacology and combinations of gut hormones, have led to efforts to stimulate the release of multiple gut peptides simultaneously via selective activation of EECs that express these hormones. This approach is also supported by research exploring the mechanisms that mediate weight loss following bariatric surgery. Postprandial secretion of gut peptides is blunted in obese patients. However, following bariatric surgery, postprandial levels of multiple gut peptides are profoundly elevated [[163], [164], [165]], and this is believed to contribute, at least in part, to the profound weight loss and improved glucose control observed in these patients. The concept of “bariatric surgery in a pill” is of increasing interest and currently being explored.
To harness the endogenous machinery of the gut to elicit secretion of incretin and satiety hormones to levels observed after bariatric surgery, EECs that contain these hormones can be selectively modulated via receptors and transporters that reside on the luminal and/or basolateral surfaces of these cells. Transcriptomics can be used to inform the selection of the cell types, their corresponding targets, and the location of these targets along the gastrointestinal tract [15], which further informs the optimal strategy for achieving maximal target engagement. Using this approach, one can also contemplate the use of “gut-restricted” oral therapeutics, that is, small molecules with negligible systemic absorption. This approach has the potential to have a high degree of safety relative to systemically absorbed therapeutics and/or molecules that traverse the blood–brain barrier to directly modulate circuits in the brain.
There are several candidate targets for this approach. GPCRs involved in sensing nutrients such as proteins, lipids, and bile acids include CaSR, GPR40, GPR119, and TGR5. Transporters involved in sensing proteins and carbohydrates include SGLT1 and PEPT1. All have been shown to elicit the secretion of GLP-1 [166,167] in preclinical models and reside on EECs that contain a number of other satiety hormones (e.g., PYY, GIP, NTS, and CCK). While several of these targets have been studied extensively to treat T2DM in both preclinical and clinical studies [[168], [169], [170], [171], [172]], their potential as gut targets for treating both T2DM and obesity has not been thoroughly explored.
To achieve glucose control and weight loss efficacy comparable to that achieved with injectable therapies or bariatric surgery, available preclinical data suggest it will be necessary to engage multiple signaling pathways simultaneously by modulating more than one target.
Co-stimulation of TGR5 and GPR40 or of GPR119 and GPR40 in crypt cultures and in vivo leads to additive or synergistic effects on GLP-1 secretion [173,174]. An alternate combination strategy leverages recent findings on the mechanism of somatostatin (SST) inhibition of GLP-1 secretion: GLP-1 receptor is found on SST-expressing EECs, and the SST receptor 5 is localized on GLP-1-producing cells [175]. Evidence that this paracrine crosstalk can be leveraged using combination approaches is supported by the finding that super physiological endogenous GLP-1 levels can be achieved with combinations including an SSTR5 inhibitor [176].
Overall, the concept of triggering multiple endogenous satiety circuits simultaneously, analogous to what occurs after nutrient ingestion or during bariatric surgery, holds promise as a new therapeutic approach for both T2DM and obesity. This more physiological approach has the potential to achieve at least comparable efficacy and superior tolerability to what is observed with pharmacological administration of individual or combinations of gut hormones, although the latter remains to be demonstrated in the clinic.
5.3. Other areas of active exploration
New insights into the biology of the gut-brain axis are prompting additional areas of exploration to identify novel therapeutics. The increasing evidence that the microbiome may play a key role in regulating metabolism suggests that rational manipulation of this system could be therapeutically beneficial. Similarly, efforts to better understand signaling mediated by microbiome metabolites could reveal potential new targets on gut-brain components, including EECs and vagal neurons. Finally, as previously noted, there is research ongoing to identify molecularly defined subsets of ENS neurons and explore their potential role(s) in metabolism, which could identify additional novel therapeutic targets.
6. Future directions
Although gut-brain communication has been recognized for centuries, we have only recently begun to grasp the true complexity of this system. Single-cell sequencing enables us to build a comprehensive atlas of the specialized cell types that comprise the gut-brain axis, and this will likely be available for both murine and human systems in the next few years. The challenges that will remain are to contextualize this information with a better understanding of the circuitry of this system and how signaling from these cell types is integrated to mediate physiology. Using a systems biology approach that employs tools including viral tracing, imaging, and gain- and loss-of-function technologies to assess this biology, one can anticipate significant advances in this area over the next decade.
A second major area of future investigation is the extent to which gut-brain biology is conserved across species, and particularly mouse to human translation. A comparison of murine and human single-cell atlases of the gut-brain axis components will be informative in this regard. Efforts to answer this question are also facilitated using ex vivo systems, most notably organoids, to study signaling in the gut-brain axis. Both murine and human gut organoids are being used extensively to explore the conservation of cell types and biology, including hormone secretion. Technologies that enable gain- and loss-of-function studies in this system enable a mechanistic exploration of signaling pathways and paracrine circuits. These efforts are being expanded to build even more physiologically relevant models, including gut-ENS organoid systems [177]. Advances in human genetics and efforts to map trait-associated variants onto gut-brain circuits could also help inform the conversation of this biology between mice and humans.
All of this work will facilitate identifying new therapeutics in multiple diseases with high unmet need. Despite impressive progress identifying new therapeutics for T2DM and obesity, the disease burden in this space is only increasing. Clearly, new therapeutic approaches are needed that can safely achieve greater and sustained efficacy and/or be safely combined with existing therapies to achieve greater efficacy. The gut-brain axis is an untapped system that should enable the discovery of therapeutics, particularly for weight loss, that work in a fundamentally new way. Finally, while this review focused on the role of the gut-brain axis in metabolism, it is clear that this circuitry is fundamental to many other important physiologic processes, and it is likely that there are multiple other functions yet to be identified, suggesting that this will be a lucrative area for discovering new therapeutics in multiple disease areas for years to come.
Acknowledgments
We are grateful to Sebastian Poliak, Brett Lauring, Eric Parker, and Paul Muller for their thoughtful review of this manuscript.
Contributor Information
Paul Richards, Email: Paul@kallyope.com.
Nancy A. Thornberry, Email: Nancy@kallyope.com.
Shirly Pinto, Email: Shirly@kallyope.com.
Conflict of interest
Paul Richards, Nancy A. Thornberry, and Shirly Pinto are employees of Kallyope, Inc., a biotechnology company focused on identifying new therapeutic approaches targeting the gut-brain axis to treat metabolic diseases and other disorders.
References
- 1.Rietdijk C.D., Perez-Pardo P., Garssen J., van Wezel R.J.A., Kraneveld A.D. Exploring Braak's hypothesis of Parkinson's disease. Frontiers in Neurology. 2017;8(FEB) doi: 10.3389/fneur.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharon G., Cruz N.J., Kang D.W., Gandal M.J., Wang B., Kim Y.M. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618. doi: 10.1016/j.cell.2019.05.004. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster J.A., McVey Neufeld K.A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends in Neurosciences. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. The Lancet. 2018;392(10160):2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 5.O'Neil P.M., Birkenfeld A.L., McGowan B., Mosenzon O., Pedersen S.D., Wharton S. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. The Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 6.Schauer P.R., Bhatt D.L., Kirwan J.P., Wolski K., Aminian A., Brethauer S.A. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. New England Journal of Medicine. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svane M.S., Bojsen-Møller K.N., Madsbad S., Holst J.J. Updates in weight loss surgery and gastrointestinal peptides. Current Opinion in Endocrinology Diabetes and Obesity. 2015;22(1):21–28. doi: 10.1097/MED.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 8.Browning K.N., Travagli R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Comprehensive Physiology. 2014;4(4):1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stengel A., Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annual Review of Physiology. 2009;71(1):219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yusta B., Baggio L.L., Koehler J., Holland D., Cao X., Pinnell L.J. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537–2549. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 11.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408) doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlman H., Nilsson The gut as the largest endocrine organ in the body. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2001;12:S63–S68. doi: 10.1093/annonc/12.suppl_2.s63. Suppl 2(February 2001) [DOI] [PubMed] [Google Scholar]
- 13.Hunne B., Stebbing M.J., McQuade R.M., Furness J.B. Distributions and relationships of chemically defined enteroendocrine cells in the rat gastric mucosa. Cell and Tissue Research. 2019;378(1):33–48. doi: 10.1007/s00441-019-03029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Cortina G., Wu S.V., Tran R., Cho J.-H., Tsai M.-J. Mutant neurogenin-3 in congenital malabsorptive diarrhea. New England Journal of Medicine. 2006;355(3):270–280. doi: 10.1056/nejmoa054288. [DOI] [PubMed] [Google Scholar]
- 15.Roberts G.P., Larraufie P., Richards P., Kay R.G., Galvin S.G., Miedzybrodzka E.L. Comparison of human and murine enteroendocrine cells by transcriptomic and peptidomic profiling. Diabetes. 2019;68(5):1062–1072. doi: 10.2337/db18-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker D.J. Evolving concepts and translational relevance of enteroendocrine cell biology. Journal of Clinical Endocrinology & Metabolism. 2016;101(3):778–786. doi: 10.1210/jc.2015-3449. [DOI] [PubMed] [Google Scholar]
- 17.Beumer J., Artegiani B., Post Y., Reimann F., Gribble F., Nguyen T.N. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nature Cell Biology. 2018;20(8):909–916. doi: 10.1038/s41556-018-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabolism. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhre R.E., Frost C.R., Svendsen B., Holst J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes. 2015;64(2):370–382. doi: 10.2337/db14-0807. [DOI] [PubMed] [Google Scholar]
- 20.Lauffer L.M., Iakoubov R., Brubaker P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L cell. Diabetes. 2009;58(5):1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu V.B., Gribble F.M., Reimann F. Free fatty acid receptors in enteroendocrine cells. Endocrinology. 2018;159(7):2826–2835. doi: 10.1210/en.2018-00261. [DOI] [PubMed] [Google Scholar]
- 22.Diakogiannaki E., Pais R., Tolhurst G., Parker H.E., Horscroft J., Rauscher B. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56(12):2688–2696. doi: 10.1007/s00125-013-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psichas A., Reimann F., Gribble F.M. Gut chemosensing mechanisms. Journal of Clinical Investigation. 2015;125(3):908–917. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebrun L.J., Lenaerts K., Kiers D., Pais de Barros J.P., Le Guern N., Plesnik J. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Reports. 2017;21(5):1160–1168. doi: 10.1016/j.celrep.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Pais R., Rievaj J., Meek C., De Costa G., Jayamaha S., Alexander R.T. Role of enteroendocrine L cells in arginine vasopressin-mediated inhibition of colonic anion secretion. Journal of Physiology. 2016;594(17):4865–4878. doi: 10.1113/JP272053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habib A.M., Richards P., Cairns L.S., Rogers G.J., Bannon C.A.M., Parker H.E. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153(7):3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gribble F.M., Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nature Reviews Endocrinology. 2019;15(4):226–237. doi: 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- 28.Brennan I.M., Luscombe-Marsh N.D., Seimon R.V., Otto B., Horowitz M., Wishart J.M. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2012;303(1) doi: 10.1152/ajpgi.00478.2011. [DOI] [PubMed] [Google Scholar]
- 29.Little T.J., Doran S., Meyer J.H., Smout A.J.P.M., O'Donovan D.G., Wu K.L. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. American Journal of Physiology. Endocrinology and Metabolism. 2006;291(3):647–655. doi: 10.1152/ajpendo.00099.2006. [DOI] [PubMed] [Google Scholar]
- 30.Zhao T.J., Sakata I., Li R.L., Liang G., Richardson J.A., Brown M.S. Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J. Ghrelin. Molecular Metabolism. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 33.Kuhre R.E., Wewer Albrechtsen N.J., Larsen O., Jepsen S.L., Balk-Møller E., Andersen D.B. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Molecular Metabolism. 2018;11(March):84–95. doi: 10.1016/j.molmet.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brighton C.A., Rievaj J., Kuhre R.E., Glass L.L., Schoonjans K., Holst J.J. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156(11):3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker H.E., Habib A.M., Rogers G.J., Gribble F.M., Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52(2):289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holst J.J. The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism Clinical and Experimental. 2019;96:46–55. doi: 10.1016/j.metabol.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Jirapinyo P., Jin D.X., Qazi T., Mishra N., Thompson C.C. A meta-analysis of GLP-1 after roux-en-Y gastric bypass: impact of surgical technique and measurement strategy. Obesity Surgery. 2018;28(3):615–626. doi: 10.1007/s11695-017-2913-1. [DOI] [PubMed] [Google Scholar]
- 38.Yusta B., Matthews D., Koehler J.A., Pujadas G., Kaur K.D., Drucker D.J. Localization of glucagon-like peptide-2 receptor expression in the mouse. Endocrinology. 2019;160(8):1950–1963. doi: 10.1210/en.2019-00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards P., Parker H.E., Adriaenssens A.E., Hodgson J.M., Cork S.C., Trapp S. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holst J.J. The physiology of glucagon-like peptide 1. Physiological Reviews. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 41.Meek C.L., Lewis H.B., Reimann F., Gribble F.M., Park A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Furness J.B. The enteric nervous system and neurogastroenterology. Nature Reviews Gastroenterology & Hepatology. 2012;9(5):286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 43.Yoo B.B., Mazmanian S.K. The enteric network: interactions between the immune and nervous systems of the gut. Immunity. 2017;46(6):910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furness J.B. Types of neurons in the enteric nervous system. Journal of the Autonomic Nervous System. 2000;81(1–3):87–96. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 45.Powley T.L. Vagal input to the enteric nervous system. Gut. 2000;47(SUPPL. 4):30–32. doi: 10.1136/gut.47.suppl_4.iv30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klose C.S.N., Mahlakõiv T., Moeller J.B., Rankin L.C., Flamar A.-L., Kabata H. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549(7671):282–286. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallrapp A., Riesenfeld S.J., Burkett P.R., Abdulnour R.-E.E., Nyman J., Dionne D. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549(7672):351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talbot J., Hahn P., Kroehling L., Nguyen H., Li D., Littman D.R. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature. 2020;579(7800):575–580. doi: 10.1038/s41586-020-2039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drokhlyansky E., Smillie C.S., Van Wittenberghe N., Ericsson M., Griffin G.K., Eraslan G. The human and mouse enteric nervous system at single cell resolution. Cell. 2020;182(6):1606–1622. doi: 10.1016/j.cell.2020.08.003. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitazawa T., Kaiya H. Regulation of gastrointestinal motility by motilin and ghrelin in vertebrates. Frontiers in Endocrinology. 2019;10(MAY):1–17. doi: 10.3389/fendo.2019.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisel A., Hochgerner H., Lönnerberg P., Johnsson A., Memic F., van der Zwan J. Molecular architecture of the mouse nervous system. Cell. 2018;174(4):999–1014. doi: 10.1016/j.cell.2018.06.021. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller P.A., Matheis F., Schneeberger M., Kerner Z., Jové V., Mucida D. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science. 2020;6176(August):1–14. doi: 10.1126/science.abd6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berthoud H.R., Neuhuber W.L. Functional and chemical anatomy of the afferent vagal system. Autonomic Neuroscience: Basic & Clinical. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 54.Foley J.O., DuBois F.S. Quantitative studies of the vagus nerve in the cat. The Journal of Nervous and Mental Disease. 1937;86(5):587. doi: 10.1097/00005053-193711000-00019. [DOI] [Google Scholar]
- 55.Canning B.J., Mori N., Mazzone S.B. Vagal afferent nerves regulating the cough reflex. Respiratory Physiology & Neurobiology. 2006;152(3):223–242. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Tränkner D., Hahne N., Sugino K., Hoon M.A., Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(31):11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rüttimann E.B., Arnold M., Hillebrand J.J., Geary N., Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coleridge H.M., Coleridge J.C.G. Reflexes evoked from tracheobronchial tree and lungs. Comprehensive Physiology. 2011 doi: 10.1002/cphy.cp030212. [DOI] [Google Scholar]
- 59.Berthoud H.R., Blackshaw L.A., Brookes S.J.H., Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neuro-Gastroenterology and Motility. 2004;16(SUPPL. 1):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 60.Powley T.L., Spaulding R.A., Haglof S.A. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. The Journal of Comparative Neurology. 2011;519(4):644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O'Donnell T.A. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198. doi: 10.1016/j.cell.2017.05.034. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waise T.M.Z., Dranse H.J., Lam T.K.T. The metabolic role of vagal afferent innervation. Nature Reviews Gastroenterology & Hepatology. 2018;15(10):625–636. doi: 10.1038/s41575-018-0062-1. [DOI] [PubMed] [Google Scholar]
- 63.Zittel T.T., Glatzle J., Kreis M.E., Starlinger M., Eichner M., Raybould H.E. C-fos protein expression in the nucleus of the solitary tract correlates with cholecystokinin dose injected and food intake in rats. Brain Research. 1999;846(1):1–11. doi: 10.1016/S0006-8993(99)01842-9. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein N., Mcknight A.D., Carty J.R.E., Arnold M., Betley J.N., Alhadeff A.L. Short Article Hypothalamic detection of macronutrients via multiple gut-brain pathways Short Article Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metabolism. 2021:1–12. doi: 10.1016/j.cmet.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai L., Mesgarzadeh S., Ramesh K.S., Huey E.L., Liu Y., Gray L.A. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179(5):1129–1143. doi: 10.1016/j.cell.2019.10.031. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raybould H.E., Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1988;255(2) doi: 10.1152/ajpgi.1988.255.2.g242. [DOI] [PubMed] [Google Scholar]
- 67.Chassaing B., Kumar M., Baker M.T., Singh V., Vijay-Kumar M. Mammalian gut immunity. Biomedical Journal. 2014;37(5):246–258. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He S., Kahles F., Rattik S., Nairz M., McAlpine C.S., Anzai A. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. 2019;566(7742):115–119. doi: 10.1038/s41586-018-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Varin E.M., Mulvihill E.E., Baggio L.L., Koehler J.A., Cao X., Seeley R.J. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Reports. 2019;27(11):3371–3384. doi: 10.1016/j.celrep.2019.05.055. e3. [DOI] [PubMed] [Google Scholar]
- 70.Kanke M., Kennedy M.M., Connelly S., Schaner M., Shanahan M.T., Wolber E.A. Single cell analysis of colonic epithelium reveals unexpected shifts in cellular composition and molecular phenotype in treatment-naïve adult Crohn's disease. BioRxiv. 2021 doi: 10.1101/2021.01.13.426602. [DOI] [Google Scholar]
- 71.Boulangé C.L., Neves A.L., Chilloux J., Nicholson J.K., Dumas M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Medicine. 2016;8(1):1–12. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin J., Li R., Raes J., Arumugam M., Burgdorf S., Manichanh C. Europe PMC Funders Group Europe PMC Funders Author Manuscripts A human gut microbial gene catalog established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R. Current understanding of the human microbiome. Nature Medicine. 2018 doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almeida A., Mitchell A.L., Boland M., Forster S.C., Gloor G.B., Tarkowska A. A new genomic blueprint of the human gut microbiota. Nature. 2019;568(7753):499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singer-Englar T., Barlow G., Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Review of Gastroenterology & Hepatology. 2019;13(1):3–15. doi: 10.1080/17474124.2019.1543023. [DOI] [PubMed] [Google Scholar]
- 76.Martinez K.B., Leone V., Chang E.B. Western diets, gut dysbiosis, and metabolic diseases: are they linked? Gut Microbes. 2017;8(2):130–142. doi: 10.1080/19490976.2016.1270811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Worthington J.J., Reimann F., Gribble F.M. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunology. 2018;11(1):3–20. doi: 10.1038/mi.2017.73. [DOI] [PubMed] [Google Scholar]
- 78.Reijnders D., Goossens G.H., Hermes G.D.A., Neis E.P.J.G., van der Beek C.M., Most J. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metabolism. 2016;24(1):63–74. doi: 10.1016/j.cmet.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 80.Sjölund K., Sandén G., Håkanson R., Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85(5):1120–1130. doi: 10.1016/S0016-5085(83)80080-8. [DOI] [PubMed] [Google Scholar]
- 81.Egerod K.L., Engelstoft M.S., Grunddal K.V., Nøhr M.K., Secher A., Sakata I. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egerod K.L., Engelstoft M.S., Lund M.L., Grunddal K.V., Zhao M., Barir-Jensen D. Transcriptional and functional characterization of the G protein-coupled receptor repertoire of gastric somatostatin cells. Endocrinology. 2015;156(11):3909–3923. doi: 10.1210/EN.2015-1388. [DOI] [PubMed] [Google Scholar]
- 83.Grosse J., Heffron H., Burling K., Hossain M.A., Habib A.M., Rogers G.J. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(30):11133–11138. doi: 10.1073/pnas.1411413111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haber A.L., Biton M., Rogel N., Herbst R.H., Shekhar K., Smillie C. A single cell survey of the small intestinal epithelium. Nature. 2017;551(7680):333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smillie C.S., Biton M., Ordovas-Montanes J., Sullivan K.M., Burgin G., Graham D.B. Intra- and inter cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178(3):714–730. doi: 10.1016/j.cell.2019.06.029. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beumer J., Puschhof J., Bauzá-Martinez J., Martínez-Silgado A., Elmentaite R., James K.R. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell. 2020;181(6):1291–1306. doi: 10.1016/j.cell.2020.04.036. e19. [DOI] [PubMed] [Google Scholar]
- 87.Billing L.J., Larraufie P., Lewis J., Leiter A., Li J., Lam B. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice – identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Molecular Metabolism. 2019;29(September):158–169. doi: 10.1016/j.molmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu H., Ding J., Porter C.B.M., Wallrapp A., Tabaka M., Ma S. Transcriptional atlas of intestinal immune cells reveals that neuropeptide α-CGRP modulates group 2 innate lymphoid cell responses. Immunity. 2019;51(4):696–708. doi: 10.1016/j.immuni.2019.09.004. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prescott S.L., Umans B.D., Williams E.K., Brust R.D., Liberles S.D. An airway protection program revealed by sweeping genetic control of vagal afferents. Cell. 2020;181(3):574–589. doi: 10.1016/j.cell.2020.03.004. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kupari J., Häring M., Agirre E., Castelo-Branco G., Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Reports. 2019;27(8):2508–2523. doi: 10.1016/j.celrep.2019.04.096. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lake B.B., Codeluppi S., Yung Y.C., Gao D., Chun J., Kharchenko P.V. A comparative strategy for single-nucleus and single cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Scientific Reports. 2017;7(1):1–8. doi: 10.1038/s41598-017-04426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C., Kaye J.A., Cai Z., Wang Y., Prescott S.L., Liberles S.D. Area postrema cell types that mediate nausea-associated behaviors. Neuron. 2020:1–12. doi: 10.1016/j.neuron.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Züger D., Forster K., Lutz T.A., Riediger T. Amylin and GLP-1 target different populations of area postrema neurons that are both modulated by nutrient stimuli. Physiology & Behavior. 2013;112(113):61–69. doi: 10.1016/j.physbeh.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Hayes M.R., Skibicka K.P., Grill H.J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alhadeff A.L., Mergler B.D., Zimmer D.J., Turner C.A., Reiner D.J., Schmidt H.D. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology. 2017;42(7):1471–1479. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deacon C.F., Pridal L., Klarskov L., Olesen M., Holst J.J. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. American Journal of Physiology. Endocrinology and Metabolism. 1996;271(3 34–3):458–464. doi: 10.1152/ajpendo.1996.271.3.e458. [DOI] [PubMed] [Google Scholar]
- 97.Williams E.K.K., Chang R.B.B., Strochlic D.E.E., Umans B.D.D., Lowell B.B.B., Liberles S.D.D. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166(1):209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han W., Tellez L.A., Perkins M.H., Perez I.O., Qu T., Ferreira J. A neural circuit for gut-induced reward. Cell. 2018;175(3):665–678. doi: 10.1016/j.cell.2018.08.049. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brierley D.I., Holt M.K., Singh A., de Araujo A., Vergara M., Afaghani M.H. Central and peripheral GLP-1 systems independently and additively suppress eating. BioRxiv. 2020 doi: 10.1101/2020.08.03.234427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Llewellyn-Smith I.J., Reimann F., Gribble F.M., Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morarach K., Mikhailova A., Knoflach V., Memic F., Kumar R., Li W. Diversification of molecularly defined myenteric neuron classes revealed by single cell RNA-sequencing. BioRxiv. 2020 doi: 10.1101/2020.03.02.955757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perez-Burgos A., Mao Y.-K., Bienenstock J., Kunze W.A. The gut-brain axis rewired: adding a functional vagal nicotinic “sensory synapse.”. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2014;28(7):3064–3074. doi: 10.1096/fj.13-245282. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y.B., de Lartigue G., Page A.J. Dissecting the role of subtypes of gastrointestinal vagal afferents. Frontiers in Physiology. 2020;11(June) doi: 10.3389/fphys.2020.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lewis J.E., Miedzybrodzka E.L., Foreman R.E., Woodward O.R.M., Kay R.G., Goldspink D.A. Selective stimulation of colonic L cells improves metabolic outcomes in mice. Diabetologia. 2020;63(7):1396–1407. doi: 10.1007/s00125-020-05149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song Y., Koehler J.A., Baggio L.L., Powers A.C., Sandoval D.A., Drucker D.J. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metabolism. 2019;30(5):976–986. doi: 10.1016/j.cmet.2019.08.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sclafani A., Zukerman S., Ackroff K. Postoral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chemical Senses. 2015;40(4):245–258. doi: 10.1093/chemse/bjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sclafani A., Marambaud P., Ackroff K. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiology & Behavior. 2014;126(1):25–29. doi: 10.1016/j.physbeh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan H.E., Sisti A.C., Jin H., Vignovich M., Villavicencio M., Tsang K.S. The gut–brain axis mediates sugar preference. Nature. 2020;580(7804):511–516. doi: 10.1038/s41586-020-2199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buchanan K.L., Rupprecht L.E., Sahasrabudhe A., Kaelberer M.M., Klein M., Villalobos J. A gut sensor for sugar preference. BioRxiv. 2020 doi: 10.1101/2020.03.06.981365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hankir M.K., Seyfried F., Hintschich C.A., Diep T.A., Kleberg K., Kranz M. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metabolism. 2017;25(2):335–344. doi: 10.1016/j.cmet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 111.Morrison S.F., Madden C.J., Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metabolism. 2014;19(5):741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Breen D.M., Yue J.T.Y., Rasmussen B.A., Kokorovic A., Cheung G.W.C., Lam T.K.T. Duodenal PKC-δ and cholecystokinin signaling axis regulates glucose production. Diabetes. 2011;60(12):3148–3153. doi: 10.2337/db11-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwartz G.J. Roles for gut vagal sensory signals in determining energy availability and energy expenditure. Brain Research. 2018;1693(Pt B):151–153. doi: 10.1016/j.brainres.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams D.L., Cummings D.E., Grill H.J., Kaplan J.M. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144(7):2765–2767. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- 115.Adriaenssens A.E., Biggs E.K., Darwish T., Tadross J., Sukthankar T., Girish M. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metabolism. 2019;30(5):987–996. doi: 10.1016/j.cmet.2019.07.013. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tan T., Behary P., Tharakan G., Minnion J., Al-Najim W., Wewer Albrechtsen N.J. The effect of a subcutaneous infusion of GLP-1, OXM, and PYY on energy intake and expenditure in obese volunteers. Journal of Clinical Endocrinology & Metabolism. 2017;102(7):2364–2372. doi: 10.1210/jc.2017-00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lutz T.A., Mollet A., Rushing P.A., Riediger T., Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. International Journal of Obesity. 2001;25(7):1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 118.Spiller R.C., Trotman I.F., Higgins B.E., Ghatei M.A., Grimble G.K., Lee Y.C. The ileal brake - inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25(4):365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Willms B., Werner J., Holst J.J., Orskov C., Creutzfeldt W., Nauck M.A. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. Journal of Clinical Endocrinology & Metabolism. 1996;81(1):327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 120.Ward Z.J., Bleich S.N., Cradock A.L., Barrett J.L., Giles C.M., Flax C. Projected U.S. state-level prevalence of adult obesity and severe obesity. New England Journal of Medicine. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 121.Centers for Disease Control and Prevention . 2020. Coronavirus Disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- 122.Drucker D.J., Habener J.F., Holst J.J., Drucker D.J., Habener J.F., Holst J.J. Vol. 127. 2017. Development of the glucagon-like peptides Find the latest version : discovery , characterization , And clinical development of the glucagon-like peptides; pp. 4217–4227. (12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabolism. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Knudsen L.B., Lau J. The discovery and development of liraglutide and semaglutide. Frontiers in Endocrinology. 2019;10(APR) doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 126.Wilding J.P.H., Jacob S. Cardiovascular outcome trials in obesity: a review. Obesity Reviews. 2020:1–11. doi: 10.1111/obr.13112. [DOI] [PubMed] [Google Scholar]
- 127.Liraglutide - prescribing information. 2010. Https://Www.Novo-Pi.Com/Victoza.Pdf. [Google Scholar]
- 128.Saxenda - prescribing information. 2017. Https://Www.Novo-Pi.Com/Saxenda.Pdf. [Google Scholar]
- 129.Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectrum. 2017;30(3):202–210. doi: 10.2337/ds16-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bydureon - prescribing information. 2012. Http://www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2012/022200Orig1s000lbledt.Pdf. [Google Scholar]
- 131.Trulicity - prescribing information. 2014. Https://www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2017/125469s007s008lbl.Pdf. [Google Scholar]
- 132.Gerstein H.C., Colhoun H.M., Dagenais G.R., Diaz R., Lakshmanan M., Pais P. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 133.Ozempic - prescribing information. 2017. Https://Www.Novo- Pi.Com/Ozempic.Pdf
- 134.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021 doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 135.Rosenstock J., Allison D., Birkenfeld A.L., Blicher T.M., Deenadayalan S., Jacobsen J.B. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA - Journal of the American Medical Association. 2019;321(15):1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pratley R., Amod A., Hoff S.T., Kadowaki T., Lingvay I., Nauck M. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. The Lancet. 2019;394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 137.Aroda V.R., Rosenstock J., Terauchi Y., Altuntas Y., Lalic N.M., Villegas E.C.M. Pioneer 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 138.Rybelsus- prescribing information. 2017. Https://Www.Novo-Pi.Com/Rybelsus.Pdf. [Google Scholar]
- 139.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Rønne J., Alanentalo T., Baquero A.F. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6) doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baggio L.L., Huang Q., Brown T.J., Drucker D.J. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 141.Wynne K., Park A.J., Small C.J., Patterson M., Ellis S.M., Murphy K.G. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects. Diabetes. 2005;54(8):2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 142.Morley J.E. Neuropeptide regulation of appetite and weight. Endocrine Reviews. 1987;8(3):256–287. doi: 10.1210/edrv-8-3-256. [DOI] [PubMed] [Google Scholar]
- 143.Lieverse R.J., Jansen J.B.M.J., Masclee A.A.M., Lamers C.B.H.W. Satiety effects of a physiological dose of cholecystokinin in humans. Gut. 1995;36(2):176–179. doi: 10.1136/gut.36.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith P., D P., Xavier F. C-terminal decreases octapeptide food intake of cholecystokinin. American Journal of Clinical Nutrition. 1981;(February):154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- 145.Jordan J., Greenway F., Leiter L., Li Z., Jacobson P., Murphy K. Stimulation of cholecystokinin-A receptors with GI181771X does not cause weight loss in overweight or obese patients. Clinical Pharmacology & Therapeutics. 2008;83(2):281–287. doi: 10.1038/sj.clpt.6100272. [DOI] [PubMed] [Google Scholar]
- 146.Batterham R.L., Cohen M.A., Ellis S.M., Le Roux C.W., Withers D.J., Frost G.S. Inhibition of food intake in obese subjects by peptide YY3-36. New England Journal of Medicine. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 147.Gantz I., Erondu N., Mallick M., Musser B., Krishna R., Tanaka W.K. Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. Journal of Clinical Endocrinology & Metabolism. 2007;92(5):1754–1757. doi: 10.1210/jc.2006-1806. [DOI] [PubMed] [Google Scholar]
- 148.Pocai A., Carrington P.E., Adams J.R., Wright M., Eiermann G., Zhu L. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Day J.W., Ottaway N., Patterson J.T., Gelfanov V., Smiley D., Gidda J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nature Chemical Biology. 2009;5(10):749–757. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 150.Elvert R., Bossart M., Herling A.W., Weiss T., Zhang B., Kannt A. Team players or opponents: coadministration of selective glucagon and GLP-1 receptor agonists in obese diabetic monkeys. Endocrinology. 2018;159(8):3105–3119. doi: 10.1210/en.2018-00399. [DOI] [PubMed] [Google Scholar]
- 151.Henderson S.J., Konkar A., Hornigold D.C., Trevaskis J.L., Jackson R., Fritsch Fredin M. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes, Obesity and Metabolism. 2016;18(12):1176–1190. doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tan T.M., Field B.C.T., McCullough K.A., Troke R.C., Chambers E.S., Salem V. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131–1138. doi: 10.2337/db12-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cegla J., Troke R.C., Jones B., Tharakan G., Kenkre J., McCullough K.A. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63(11):3711–3720. doi: 10.2337/db14-0242. [DOI] [PubMed] [Google Scholar]
- 154.Sánchez-Garrido M.A., Brandt S.J., Clemmensen C., Müller T.D., DiMarchi R.D., Tschöp M.H. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60(10):1851–1861. doi: 10.1007/s00125-017-4354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tillner J., Posch M.G., Wagner F., Teichert L., Hijazi Y., Einig C. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes, Obesity and Metabolism. 2019;21(1):120–128. doi: 10.1111/dom.13494. [DOI] [PubMed] [Google Scholar]
- 156.Ambery P., Parker V.E., Stumvoll M., Posch M.G., Heise T., Plum-Moerschel L. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. The Lancet. 2018;391(10140):2607–2618. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
- 157.Frias J.P., Nauck M.A., Van J., Benson C., Bray R., Cui X. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different do. Diabetes, Obesity and Metabolism. 2020;22(6):938–946. doi: 10.1111/dom.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Neary N.M., Small C.J., Druce M.R., Park A.J., Ellis S.M., Semjonous N.M. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology. 2005;146(12):5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 159.Schmidt J.B., Gregersen N.T., Pedersen S.D., Arentoft J.L., Ritz C., Schwartz T.W. Effects of PYY 3–36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(11):E1248–E1256. doi: 10.1152/ajpendo.00569.2013. [DOI] [PubMed] [Google Scholar]
- 160.De Silva A., Salem V., Long C.J., Makwana A., Newbould R.D., Rabiner E.A. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metabolism. 2011;14(5):700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aditi Saxena, Gorman Donal, Kristin Chidsey, Buckeridge Clare, A.M.K., A.B. Oral small molecule GLP-1R agonist PF-06882961 robustly reduces plasma glucose and body weight after 28 Days in adults with T2DM. Diabetes. 2020;69(Supplement 1) [Google Scholar]
- 162.Yang L., Chang C.C., Sun Z., Madsen D., Zhu H., Padkjær S.B. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nature Medicine. 2017;23(10):1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 163.Moffett R.C., Docherty N.G., le Roux C.W. The altered enteroendocrine reportoire following roux-en-Y-gastric bypass as an effector of weight loss and improved glycaemic control. Appetite. 2021;156(August 2020):104807. doi: 10.1016/j.appet.2020.104807. [DOI] [PubMed] [Google Scholar]
- 164.Svane M.S., Bojsen-Møller K.N., Martinussen C., Dirksen C., Madsen J.L., Reitelseder S. Postprandial nutrient handling and gastrointestinal hormone secretion after roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2019;156(6):1627–1641. doi: 10.1053/j.gastro.2019.01.262. e1. [DOI] [PubMed] [Google Scholar]
- 165.Laferrère B. Bariatric surgery and obesity: influence on the incretins. International Journal of Obesity Supplements. 2016;6(S1):S32–S36. doi: 10.1038/ijosup.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reimann F., Gribble F.M. G protein-coupled receptors as new therapeutic targets for type 2 diabetes. Diabetologia. 2016;59(2):229–233. doi: 10.1007/s00125-015-3825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Engelstoft M.S., Egerod K.L., Holst B., Schwartz T.W. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metabolism. 2008;8(6):447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 168.Hansen H.S., Rosenkilde M.M., Holst J.J., Schwartz T.W. GPR119 as a fat sensor. Trends in Pharmacological Sciences. 2012;33(7):374–381. doi: 10.1016/j.tips.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 169.Shah U., Kowalski T.J. 2010. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders; pp. 415–448. [DOI] [PubMed] [Google Scholar]
- 170.Pols T.W.H., Noriega L.G., Nomura M., Auwerx J., Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Digestive Diseases. 2011;29(1):37–44. doi: 10.1159/000324126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burant C.F. Activation of GPR40 as a therapeutic target for the treatment of type 2 diabetes. Diabetes Care. 2013;36(Supplement_2):S175–S179. doi: 10.2337/dcS13-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mancini A.D., Poitout V. GPR40 agonists for the treatment of type 2 diabetes: life after ‘TAKing’ a hit. Diabetes, Obesity and Metabolism. 2015;17(7):622–629. doi: 10.1111/dom.12442. [DOI] [PubMed] [Google Scholar]
- 173.Hauge M., Ekberg J.P., Engelstoft M.S., Timshel P., Madsen A.N., Schwartz T.W. Gq and Gs signaling acting in synergy to control GLP-1 secretion. Molecular and Cellular Endocrinology. 2017;449:64–73. doi: 10.1016/j.mce.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 174.Goldspink D.A., Lu V.B., Billing L.J., Larraufie P., Tolhurst G., Gribble F.M. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Molecular Metabolism. 2018;7:90–101. doi: 10.1016/j.molmet.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jepsen S.L., Grunddal K.V., Albrechtsen N.J.W., Engelstoft M.S., Gabe M.B.N., Jensen E.P. Paracrine crosstalk between intestinal L- and D cells controls secretion of glucagon-like peptide-1 in mice. American Journal of Physiology. Endocrinology and Metabolism. 2019 doi: 10.1152/AJPENDO.00239.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Briere D.A., Bueno A.B., Gunn E.J., Michael M.D., Sloop K.W. Mechanisms to elevate endogenous GLP-1 beyond injectable GLP-1 analogs and metabolic surgery. Diabetes. 2018;67(2):309–320. doi: 10.2337/db17-0607. [DOI] [PubMed] [Google Scholar]
- 177.Workman M.J., Mahe M.M., Trisno S., Poling H.M., Watson C.L., Sundaram N. Engineered human pluripotent-stem cell-derived intestinal tissues with a functional enteric nervous system. Nature Medicine. 2017;23(1):49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Furness J.B., Rivera L.R., Cho H.J., Bravo D.M., Callaghan B. The gut as a sensory organ. Nature Reviews Gastroenterology & Hepatology. 2013 doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 179.Roman C.W., Sloat S.R., Palmiter R.D. A tale of two circuits: CCKNTS neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience. 2017;358:316–324. doi: 10.1016/j.neuroscience.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.D'Agostino G., Lyons D., Cristiano C., Lettieri M., Olarte-Sanchez C., Burke L.K. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metabolism. 2018;28(4):619–630. doi: 10.1016/j.cmet.2018.07.017. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Haslam D. Weight management in obesity - past and present. International Journal of Clinical Practice. 2016;70(3):206–217. doi: 10.1111/ijcp.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Muller P.A., Schneeberger M., Matheis F., Wang P., Kerner Z., Ilanges A. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature. 2020;583(7816):441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lo L., Anderson D.J. A cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72(6):938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zeng W.B., Jiang H.F., Gang Y.D., Song Y.G., Shen Z.Z., Yang H. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Molecular Neurodegeneration. 2017;12(1):1–17. doi: 10.1186/s13024-017-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen J., Cheng M., Wang L., Zhang L., Xu D., Cao P. A vagal-NTS neural pathway that stimulates feeding. Current Biology. 2020:1–13. doi: 10.1016/j.cub.2020.07.084. [DOI] [PubMed] [Google Scholar]
- 186.DeFalco J., Tomishima M., Liu H., Zhao C., Cai X., Marth J.D. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 187.Diepenbroek C., Quinn D., Stephens R., Zollinger B., Anderson S., Pan A. Validation and characterization of a novel method for selective vagal deafferentation of the gut. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2017;313(4):G342–G352. doi: 10.1152/ajpgi.00095.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Udit S., Burton M., Rutkowski J.M., Lee S., Bookout A.L., Scherer P.E. Nav1.8 neurons are involved in limiting acute phase responses to dietary fat. Molecular Metabolism. 2017;6(10):1081–1091. doi: 10.1016/j.molmet.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]