Key Points
Question
What are the outcomes in newborn infants of mothers testing positive for SARS-CoV-2 in pregnancy?
Findings
In this nationwide, prospective cohort study that included 88 159 infants from Sweden, SARS-CoV-2 infection in pregnancy was significantly associated with higher risk of any neonatal respiratory disorder (2.8% vs 2.0%; odds ratio, 1.42) and some other neonatal morbidities, but not neonatal mortality (0.30% vs 0.12%; odds ratio, 2.55).
Meaning
Maternal SARS-CoV-2 infection in pregnancy was significantly associated with small increases in the absolute risk of respiratory disorders and some other neonatal morbidities.
Abstract
Importance
The outcomes of newborn infants of women testing positive for SARS-CoV-2 in pregnancy is unclear.
Objective
To evaluate neonatal outcomes in relation to maternal SARS-CoV-2 test positivity in pregnancy.
Design, Setting, and Participants
Nationwide, prospective cohort study based on linkage of the Swedish Pregnancy Register, the Neonatal Quality Register, and the Register for Communicable Diseases. Ninety-two percent of all live births in Sweden between March 11, 2020, and January 31, 2021, were investigated for neonatal outcomes by March 8, 2021. Infants with malformations were excluded. Infants of women who tested positive for SARS-CoV-2 were matched, directly and using propensity scores, on maternal characteristics with up to 4 comparator infants.
Exposures
Maternal test positivity for SARS-CoV-2 in pregnancy.
Main Outcomes and Measures
In-hospital mortality; neonatal resuscitation; admission for neonatal care; respiratory, circulatory, neurologic, infectious, gastrointestinal, metabolic, and hematologic disorders and their treatments; length of hospital stay; breastfeeding; and infant test positivity for SARS-CoV-2.
Results
Of 88 159 infants (49.0% girls), 2323 (1.6%) were delivered by mothers who tested positive for SARS-CoV-2. The mean gestational age of infants of SARS-CoV-2–positive mothers was 39.2 (SD, 2.2) weeks vs 39.6 (SD, 1.8) weeks for comparator infants, and the proportions of preterm infants (gestational age <37 weeks) were 205/2323 (8.8%) among infants of SARS-CoV-2–positive mothers and 4719/85 836 (5.5%) among comparator infants. After matching on maternal characteristics, maternal SARS-CoV-2 test positivity was significantly associated with admission for neonatal care (11.7% vs 8.4%; odds ratio [OR], 1.47; 95% CI, 1.26-1.70) and with neonatal morbidities such as respiratory distress syndrome (1.2% vs 0.5%; OR, 2.40; 95% CI, 1.50-3.84), any neonatal respiratory disorder (2.8% vs 2.0%; OR, 1.42; 95% CI, 1.07-1.90), and hyperbilirubinemia (3.6% vs 2.5%; OR, 1.47; 95% CI, 1.13-1.90). Mortality (0.30% vs 0.12%; OR, 2.55; 95% CI, 0.99-6.57), breastfeeding rates at discharge (94.4% vs 95.1%; OR, 0.84; 95% CI, 0.67-1.05), and length of stay in neonatal care (median, 6 days in both groups; difference, 0 days; 95% CI, −2 to 7 days) did not differ significantly between the groups. Twenty-one infants (0.90%) of SARS-CoV-2–positive mothers tested positive for SARS-CoV-2 in the neonatal period; 12 did not have neonatal morbidity, 9 had diagnoses with unclear relation to SARS-CoV-2, and none had congenital pneumonia.
Conclusions and Relevance
In a nationwide cohort of infants in Sweden, maternal SARS-CoV-2 infection in pregnancy was significantly associated with small increases in some neonatal morbidities. Given the small numbers of events for many of the outcomes and the large number of statistical comparisons, the findings should be interpreted as exploratory.
This cohort study uses Swedish national registry data to investigate associations between maternal SARS-CoV-2 test positivity in pregnancy and neonatal outcomes, including mortality and need for resuscitation, between March 2020 and January 2021.
Introduction
Since the start of the COVID-19 pandemic, there has been concern about how to protect vulnerable persons from SARS-CoV-2. In the past, newborn infants experienced increased mortality in pandemics,1 and infections are the most common cause of mortality in children younger than 5 years. These reasons have most likely been important drivers for guidelines—especially in countries affected early by COVID-19—recommending mother-infant separation at birth in case the mother tested positive for SARS-CoV-2 on delivery.2,3,4 In other guidelines, nonseparation of mother-infant dyads and breastfeeding have been promoted.2,3,4 While recommendations on management of newborn infants of mothers with COVID-19 vary, they are all hampered by uncertainty and associated with potentially severe adverse effects from interfering too much or too little with mother-infant interactions after birth.2,4,5
Systematic reviews have begun to shed light on maternal and pregnancy outcomes, but knowledge on neonatal outcomes following maternal COVID-19 in pregnancy is still based on case series and center experiences.3,6,7,8,9,10,11,12 Because of potential selection biases, lack of denominators, lack of contemporary comparators, and limited and often unspecific symptoms and poorly defined outcome measures reported, there are insufficient data to draw solid conclusions about neonatal complications after maternal COVID-19 in pregnancy.9,13 A comprehensive assessment would help to build a more robust evidence base for clinical practice. To test the hypothesis that maternal SARS-CoV-2 positivity in pregnancy may be associated with adverse neonatal outcomes, a Swedish nationwide, prospective cohort study was conducted.
Methods
All pregnant women in antenatal care and all parents of infants admitted for neonatal care received information regarding processing of personal data by the Swedish national quality registers, with a right to opt out and have all personal information removed. The study was approved by the Swedish Ethical Review Authority, which waived patient informed consent.
Participants and Setting
In Sweden, participation in the prenatal care program is almost universal, and prenatal, delivery, and neonatal care is tax funded. The Swedish COVID-19 strategy has been described elsewhere.14 The first pregnant woman testing positive for SARS-CoV-2 in Sweden gave birth on March 11, 2020. A national recommendation on perinatal management of SARS-CoV-2 was issued on March 17. As in other countries, the rigor of development and the evidence base of this guideline was of low quality.2 After an update on April 5, the main features of the guideline for pregnant women testing positive for SARS-CoV-2 and their infants were (1) mode of delivery according to standard obstetric assessment; (2) nonseparation of mothers and infants unless any was unwell; (3) all infants to be tested (polymerase chain reaction [PCR] on nasopharyngeal swab) within 12 to 24 hours after birth, and for infants admitted for neonatal care, PCR tests repeated at 48 and 96 hours; (4) breastfeeding and expressed breast milk allowed given strict hygiene routines; (5) antiviral drugs not recommended to be given to infants; and (6) standard criteria for hospital discharge. Primary separation at birth of SARS-CoV-2–positive mothers and their infants was indicated only if an infant needed resuscitation followed by neonatal admission or if a condition diagnosed antenatally was known to necessitate admission to a neonatal unit (eg, preterm birth). Secondary separation was done if an infant developed a need for neonatal care after birth. SARS-CoV-2–positive mothers were not allowed in neonatal units, and if an infant was admitted after having had postnatal contact with the mother, the infant was considered incubated with SARS-CoV-2 and isolated until the infant had a negative SARS-CoV-2 test result.
On February 1, 2020, SARS-CoV-2 was included in the Swedish Communicable Diseases Act, making it mandatory to report all laboratory-confirmed PCR cases within 24 hours to the Public Health Agency’s national register for communicable diseases, the Swedish Register for Communicable Diseases (SmiNet). The National Board of Health and Welfare also issued instructions to assign SARS-CoV-2–positive patients with the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code U07.1 followed by a code for manifestation (eg, ICD-10 code P23 for neonatal pneumonia).14 All ICD codes were captured in population-based health and quality registers.
Study Cohort and Data Sources
The study cohort consisted of all live-born infants delivered by women captured in the Swedish Pregnancy Register between March 11, 2020, and January 31, 2021, and investigated for neonatal outcomes by March 8, 2021. Infants with malformations as defined by a Q code in ICD-10 were excluded, with the exception of patent ductus arteriosus in infants younger than 37 weeks of gestational age (eTable 1 in the Supplement).
The inception of this study was on March 25, 2020. Using the unique personal identity numbers for mothers and infants, data were linked between the Swedish Pregnancy Register, the Swedish Neonatal Quality Register, and SmiNet. All 3 registers prospectively extracted standardized and predefined data on a daily basis. The Swedish Pregnancy Register provided information on each individual pregnancy and birth outcomes for 92% of all pregnant women in Sweden; incompleteness is attributed to 4 hospitals not reporting to the register.15 The Swedish Neonatal Quality Register provided detailed information on procedures and diagnoses for all infants admitted for neonatal care.16 SmiNet included dates of first positive result on a PCR test for SARS-CoV-2 in mothers and infants.
Exposures
The exposure was SARS-CoV-2 test positivity in mothers from conception to 1 week after birth. In the beginning of the study period, only pregnant women admitted for hospital care with symptoms of COVID-19 were tested. In June 2020, a general testing strategy was implemented including outpatient testing and contact tracing.14 Some but not all hospitals tested all women admitted for delivery.
Covariates
Maternal, pregnancy, and birth characteristics were collected for descriptive purposes and to create cohorts matched on maternal characteristics for associations between SARS-CoV-2 in pregnancy and neonatal outcomes.8,9,10,11,17,18,19 Small for gestational age was defined as a birth-weight z score more than 2 SDs below the mean in a Swedish reference for normal fetal growth.20
Because near-term infants with gestational ages of 35 weeks and 0 days to 36 weeks and 6 days were not routinely admitted for neonatal care, gestational age was categorized into very preterm (gestational age less than 32 weeks 0 days); moderately preterm (gestational age of 32 weeks 0 days to 34 weeks 6 days); near-term or term (gestational age of 35 weeks 0 days to 41 weeks 6 days); and postterm (gestational age of 42 weeks 0 days or more).
Outcomes
This study was exploratory in design, and no primary outcome was predefined. Some of the neonatal outcomes described herein have previously been described in association with maternal SARS-CoV-2 in pregnancy6,7,8,9,10,12,13,18,21,22,23,24,25,26 whereas others have not. Altogether, the neonatal outcomes, as defined herein or in eTable 1 in the Supplement, included (1) neonatal resuscitation (assisted ventilation [ie, mask ventilation or use of continuous positive airway pressure {CPAP}] or intubation at birth); (2) admission for neonatal care; (3) neurologic conditions (hypoxic-ischemic encephalopathy grade 2-3,27 neonatal convulsions, and severe brain injury in very preterm infants, defined as intraventricular hemorrhage grade 3-428 or cystic periventricular leukomalacia29); (4) respiratory disorders (respiratory distress syndrome, transient tachypnea of the newborn, and meconium aspiration syndrome) and their treatments (CPAP, mechanical ventilation, surfactant administration, postnatal corticosteroids for severe lung disease, and need for supplemental oxygen at 28 days of postnatal age); (5) circulatory problems (persistent pulmonary hypertension of the newborn and patent ductus arteriosus [treated pharmacologically or by surgery]); (6) infections (neonatal pneumonia and blood culture–proven sepsis) and antibiotic therapy; (7) gastrointestinal disease (necrotizing enterocolitis in very preterm infants, surgically treated); (8) metabolic and hematologic problems (hypoglycemia [defined as plasma glucose level <2.6 mmol/L {<47 mg/dL} more than 3 hours after birth], treated hyperbilirubinemia, and blood transfusion); (9) in-hospital mortality; (10) length of hospital stay; and (11) breastfeeding at discharge. A composite outcome of respiratory distress syndrome, transient tachypnea of the newborn, meconium aspiration syndrome, or neonatal pneumonia was also defined as any respiratory disorder. In addition, infant test positivity for SARS-CoV-2 in the early neonatal (0-6 days), late neonatal (7-28 days), and postneonatal periods were included as outcomes.
Statistical Analysis
To assess any association between SARS-CoV-2 in pregnancy and neonatal outcomes, each exposed infant was matched with up to 4 comparator infants of mothers without a positive SARS-CoV-2 test result on parity, multiple pregnancy, health care region, and a propensity score (estimated using logistic regression) that included maternal age, early pregnancy body mass index, educational level, country of birth, smoking status, living with partner, and prepregnancy comorbidity (a composite including hypertension, cardiovascular disease, diabetes, lung disease, and kidney disease). In the propensity score model, the dependent variable was maternal SARS-CoV-2 test result. In the propensity score matching, the nearest neighbor was sampled, allowing a maximum caliper width of 0.2 of the pooled standard deviation of the logit of the propensity score. For variables included in the matching algorithm (maternal age, parity, early pregnancy body mass index, educational level, birth country, and smoking), missing information was replaced using a missing indicator category.
Standardized differences between the 2 groups were calculated before and after matching.30 Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals conditioned on each matching set.
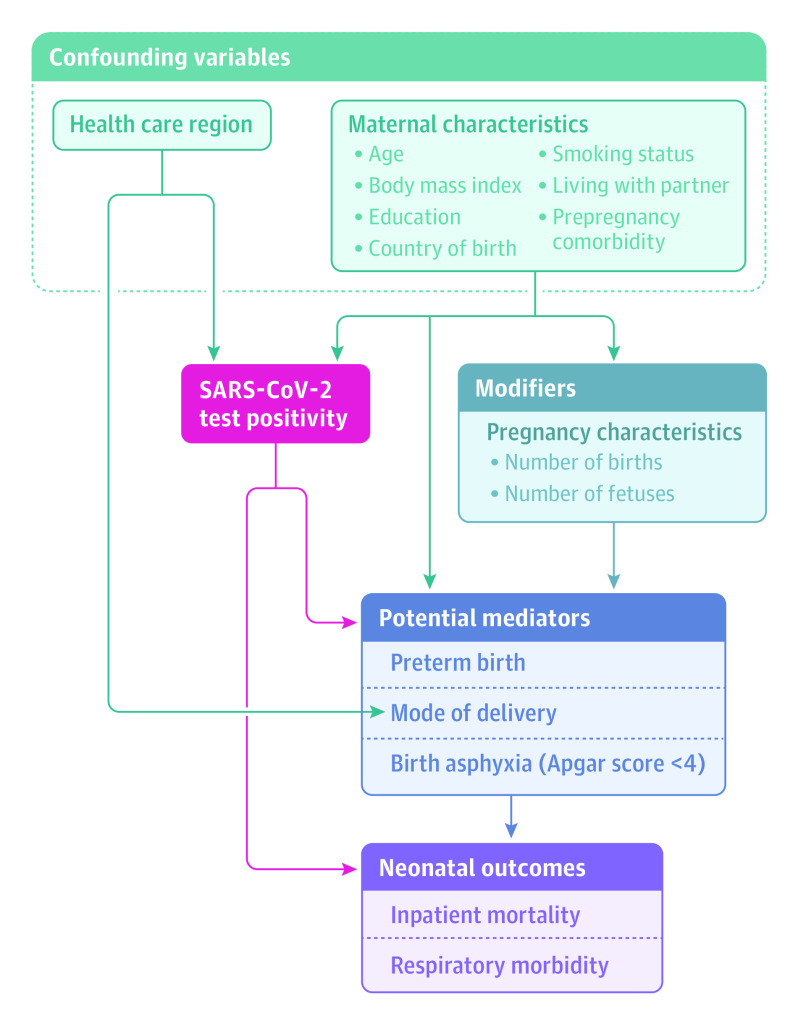
To provide a deeper understanding of mediating pathways among maternal exposure to SARS-CoV-2, pregnancy and birth events, and neonatal outcomes, a mediation analysis was performed. The exposure was maternal SARS-CoV-2 in pregnancy, and the relations between confounding and mediator variables were identified, as shown in Figure 1. Three potential mediators were tested: preterm birth (gestational age <37 weeks), mode of delivery (cesarean delivery), and Apgar score less than 4 at 5 minutes. The neonatal outcome, chosen on the basis of the most prevalent symptoms and conditions reported in newborn infants with positive SARS-CoV-2 test results,12 was any respiratory disorder.
Figure 1. Conceptual Model of Relationships Between Maternal SARS-CoV-2 in Pregnancy, Birth Characteristics, and Neonatal Outcomes.
Based on Etminan et al.31
Data were analyzed using SAS software, version 9.4 (SAS Institute Inc). Mediation analyses were performed using CAUSALMED procedures, and median differences were estimated using quantile regression with QUANTREG procedures in SAS. A standardized mean difference of less than −0.10 or more than 0.10 was considered to indicate significant group differences or imbalance in matching. For 95% CIs, those that did not include 1 for ORs and those that did not include 0 for risk or median differences were considered statistically significant. A sensitivity analysis with a narrowed exposure window from conception to birth was also performed. Because of the potential for type I error due to multiple comparisons, the findings of this study should be interpreted as exploratory.
Results
A total of 2905 infants (3.2%) were excluded because of malformations. Among 88 159 newborn infants included (49.0% girls), 2323 (1.6%) were delivered by 2286 SARS-CoV-2–positive mothers. Among SARS-CoV-2–positive mothers, the median time from a positive test result to delivery was 36 (interquartile range, 5-85) days. Among the 2323 infants, 642 (28%) had mothers testing positive at the time of delivery (ie, from 2 weeks before to 2 days after delivery), and 68 infants (2.9%) had mothers who tested positive within 1 week after delivery.
Maternal, Pregnancy, and Infant Characteristics
Maternal age, parity, prepregnancy comorbidity, maternal education, proportions living with a partner, smoking in pregnancy, multiple pregnancy, gestational diabetes, type of delivery onset, and mode of delivery were not significantly different between SARS-CoV-2–positive mothers and mothers without a positive SARS-CoV-2 test result. In contrast, non-Nordic country of birth, high body mass index, antenatal corticosteroid treatment for preterm birth, and delivery at a hospital with a neonatal intensive care unit were significantly more common in SARS-CoV-2–positive mothers than in mothers without a positive SARS-CoV-2 test result. The distribution of health care region also differed significantly between the 2 groups. Infant sex and proportions with Apgar scores less than 7 or less than 4 at 5 minutes were not significantly different in the 2 groups. Gestational age and birth weight were significantly lower in infants born to SARS-CoV-2–positive mothers than in comparator infants (Table).
Table. Maternal, Pregnancy, and Birth Characteristics by SARS-CoV-2 Test Positivity in Pregnant Women.
| Characteristics | Maternal SARS-CoV-2 test status | ||||
|---|---|---|---|---|---|
| Positive test result | No positive test result (full cohort) | Standardized differencea | No positive test result (matched cohort)b | Standardized differencea | |
| No. of mothersc | 2286 | 84 719 | 9137 | ||
| No. of infantsc | 2323 | 85 836 | 9275 | ||
| Maternal age at delivery, y | |||||
| Mean (SD) | 31.4 (5.0) | 31.4 (4.9) | 0.003 | 31.5 (4.9) | −0.009 |
| Median (IQR) [range] | 31.3 (28.0-34.8) [17.3 to 49.8] | 31.3 (28.2-34.7) [14.1 to 59.9] | 31.3 (28.1-34.8) [16.6 to 52.0] | ||
| No. (%) | |||||
| 13-24 | 224 (9.6) | 7672 (8.9) | 0.024 | 865 (9.3) | 0.011 |
| 25-29 | 689 (29.7) | 25 926 (30.2) | −0.012 | 2734 (29.5) | 0.004 |
| 30-34 | 855 (36.8) | 32 343 (37.7) | −0.018 | 3437 (37.1) | −0.005 |
| ≥35 | 555 (23.9) | 19 886 (23.2) | 0.017 | 2239 (24.1) | −0.006 |
| Missing data | 0 | 9 | −0.014 | 0 | 0 |
| Parity, No. (%) | |||||
| Nulliparous | 1001 (43.1) | 37 058 (43.2) | −0.002 | 3995 (43.1) | 0.000 |
| Multiparous | 1321 (56.9) | 48 740 (56.8) | 0.002 | 5278 (56.9) | −0.001 |
| Missing data | 1 | 38 | −0.001 | 2 | 0.012 |
| Singletons, No. (%) | 2247 (96.7) | 83 532 (97.3) | −0.035 | 8988 (96.9) | −0.010 |
| Multiple pregnancy, No. (%) | 76 (3.3) | 2304 (2.7) | 0.035 | 287 (3.1) | 0.010 |
| Prepregnancy comorbidity, No. (%)d | 231 (9.9) | 8581 (10.0) | −0.002 | 850 (9.2) | 0.027 |
| Gestational diabetes, No. (%) | 163 (7.0) | 4331 (5.0) | 0.083 | 592 (6.4) | 0.025 |
| Body mass indexe | |||||
| Mean (SD) | 26.0 (5.2) | 25.3 (5.0) | 0.132 | 25.9 (5.3) | 0.020 |
| Median (IQR) [range] | 24.9 (22.3-28.9) [14.5 to 55.7] | 24.2 (21.8-27.8) [13.2 to 69.8] | 24.8 (22.1-28.7) [14.7 to 54.6] | ||
| No. (%) | |||||
| <18.5 | 36 (1.6) | 1949 (2.4) | −0.053 | 151 (1.7) | −0.006 |
| 18.5 to <25 | 1092 (48.7) | 44 481 (54.1) | −0.096 | 4433 (49.6) | −0.016 |
| 25 to <30 | 661 (29.5) | 22 558 (27.5) | 0.049 | 2615 (29.2) | 0.006 |
| 30 to <35 | 309 (13.8) | 8998 (10.9) | 0.087 | 1155 (12.9) | 0.025 |
| ≥35 | 144 (6.4) | 4190 (5.1) | 0.058 | 591 (6.6) | −0.007 |
| Missing data | 81 | 3660 | −0.040 | 330 | −0.004 |
| Educational level, No. (%), y | |||||
| ≤9 | 207 (10.8) | 5616 (7.9) | 0.089 | 761 (10.0) | 0.025 |
| 10-12 | 656 (34.2) | 25 090 (35.3) | −0.022 | 2632 (34.5) | −0.003 |
| >12 | 1053 (55.0) | 40 287 (56.7) | −0.032 | 4241 (55.6) | −0.008 |
| Missing data | 407 | 14 843 | 0.006 | 1641 | −0.005 |
| Smoking status, No. (%) | |||||
| Nonsmoker | 2201 (97.6) | 79 820 (96.3) | 0.073 | 8887 (97.9) | −0.050 |
| Smoker | 55 (2.4) | 3048 (3.7) | −0.070 | 195 (2.1) | 0.018 |
| Missing data | 67 | 2968 | −0.033 | 193 | 0.052 |
| Birth country, No. (%) | |||||
| Nordic | 1320 (62.6) | 56 127 (71.9) | −0.176 | 5345 (63.6) | −0.016 |
| Non-Nordic Europe | 152 (7.2) | 5546 (7.1) | 0.003 | 591 (7.0) | 0.007 |
| Middle East/Africa | 536 (25.4) | 12 429 (15.9) | 0.221 | 2142 (25.5) | 0.000 |
| Other | 99 (4.7) | 3977 (5.1) | −0.018 | 332 (3.9) | 0.035 |
| Missing data | 216 | 7757 | 0.009 | 865 | −0.001 |
| Living with partner, No. (%) | |||||
| Yes | 2101 (91.8) | 77 263 (91.7) | 0.015 | 8412 (92.0) | −0.009 |
| No | 187 (8.2) | 6980 (8.3) | −0.003 | 736 (8.0) | 0.004 |
| Missing data | 35 | 1593 | −0.027 | 127 | 0.012 |
| Health care region, No. (%) | |||||
| North | 117 (5.0) | 5536 (6.4) | −0.061 | 460 (5.0) | 0.004 |
| Middle | 378 (16.3) | 12 516 (14.6) | 0.047 | 1512 (16.3) | −0.001 |
| East | 850 (36.6) | 24 176 (28.2) | 0.181 | 3393 (36.6) | 0.000 |
| West | 468 (20.1) | 19 473 (22.7) | −0.062 | 1872 (20.2) | −0.001 |
| Southeast | 231 (9.9) | 9802 (11.4) | −0.048 | 924 (10.0) | −0.001 |
| South | 279 (12.0) | 14 332 (16.7) | −0.134 | 1114 (12.0) | 0.000 |
| Delivery hospital, No. (%) | |||||
| Hospital with full neonatal intensive care unit | 838 (36.1) | 24 778 (28.9) | 0.154 | 2724 (29.4) | 0.143 |
| Other unit | 1485 (63.9) | 61 058 (71.1) | −0.154 | 6551 (70.6) | −0.143 |
| Antenatal corticosteroids administered because of preterm birth, No. (%) | 58 (2.5) | 1061 (1.2) | 0.193 | 104 (1.1) | 0.103 |
| Onset of delivery, No. (%) | |||||
| Spontaneous | 1490 (64.1) | 55 662 (64.8) | −0.015 | 6033 (65.0) | −0.019 |
| Induction of labor | 563 (24.2) | 21 857 (25.5) | −0.028 | 2258 (24.3) | −0.003 |
| Cesarean delivery | 270 (11.6) | 8317 (9.7) | 0.063 | 984 (10.6) | 0.032 |
| Mode of delivery, No. (%) | |||||
| Vaginal birth | 1828 (78.7) | 70 386 (82.0) | −0.083 | 7525 (81.1) | −0.061 |
| Cesarean delivery | 495 (21.3) | 15 450 (18.0) | 0.083 | 1750 (18.9) | 0.061 |
| Time from date of positive maternal SARS-CoV-2 test result to birth, d | |||||
| Mean (SD) | 56.3 (62.6) | ||||
| Median (IQR) [range] | 36 (5-85) [−7 to 284] | ||||
| Infant sex, No. (%) | |||||
| Male | 1157 (49.8) | 43 839 (51.1) | −0.025 | 4776 (51.5) | −0.034 |
| Female | 1166 (50.2) | 41 997 (48.9) | 0.025 | 4499 (48.5) | 0.034 |
| Gestational age | |||||
| Mean (SD), d | 274.7 (15.1) | 277.0 (12.6) | −0.162 | 277.1 (12.6) | −0.170 |
| Median (IQR) [range], d | 278 (270-284) [157 to 296] | 279 (272-285) [154 to 305] | 279 (272-285) [159 to 302] | ||
| No. (%), wk | |||||
| 22 to <32 | 38 (1.6) | 675 (0.8) | 0.078 | 66 (0.7) | 0.086 |
| 32 to <35 | 90 (3.9) | 1742 (2.0) | 0.109 | 177 (1.9) | 0.118 |
| 35 to <42 | 2194 (94.4) | 82 052 (95.6) | −0.053 | 8830 (95.2) | −0.034 |
| ≥42 | 39 (1.7) | 2042 (2.4) | −0.050 | 268 (2.9) | −0.081 |
| Apgar score at 5 min, No. (%) | |||||
| <7 | 48 (2.1) | 1300 (1.5) | 0.042 | 134 (1.5) | 0.047 |
| <4 | 9 (0.4) | 248 (0.3) | 0.017 | 29 (0.3) | 0.013 |
| Missing data | 32 | 601 | 0.067 | 52 | 0.083 |
| Birth weight, g | |||||
| Mean (SD) | 3436 (628) | 3502 (559) | −0.111 | 3495 (558) | −0.099 |
| Median (IQR) [range] | 3485 (3100-3826) [540 to 5860] | 3522 (3185-3855) [360 to 6040] | 3525 (3190-3840) [505 to 5800] | ||
| No. (%) | |||||
| Large for gestational age | 84 (3.7) | 3144 (3.7) | −0.002 | 328 (3.5) | 0.004 |
| Small for gestational age | 54 (2.4) | 1864 (2.2) | 0.010 | 246 (2.7) | −0.021 |
| Missing data | 52 | 799 | 0.105 | 31 | 0.170 |
Abbreviation: IQR, interquartile range.
Standardized differences were calculated according to Austin.30
Ten exposed infants (all twins) were not possible to match in a 1:4 ratio.
Numbers of mothers and infants differ because of multiple births.
Prepregnancy comorbidity included diabetes, hypertension, cardiovascular disease, kidney disease, and lung disease.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Matching
After matching, the groups were well balanced on matching factors except for significantly higher proportions of exposed infants having antenatal corticosteroid treatment for preterm birth, being born preterm, and being delivered at university hospitals than in the comparator group (Table).
Neonatal Outcomes
In the unmatched cohort, 7 (0.30%) of 2323 infants of SARS-CoV-2–positive mothers died and 96 (0.11%) of 85 836 infants of mothers without a positive SARS-CoV-2 test result died. The clinical characteristics of the 7 SARS-CoV-2–exposed infants who died are presented in eTable 2 in the Supplement. In addition, 65 (2.8%) of the 2323 infants of SARS-CoV-2–positive mothers and 1518 (1.8%) of the 85 836 infants of mothers without a positive SARS-CoV-2 test result had a diagnosis of a noninfectious respiratory disorder, and 3 (0.13%) of the SARS-CoV-2–exposed infants vs 85 (0.10%) of the control infants had a neonatal diagnosis of pneumonia, none of which was attributed to neonatal COVID-19 (Figure 2 and Figure 3).
Figure 2. Neonatal Outcomes (Resuscitation at Birth, Admission to Neonatal Unit, Admission Hypothermia, Neurologic Disorders, and Respiratory Disorders and Treatments) Among Infants Born in Sweden (March 11, 2020–January 31, 2021) by Maternal SARS-CoV-2 Test Status in Pregnancy (Assessed as of March 8, 2021).
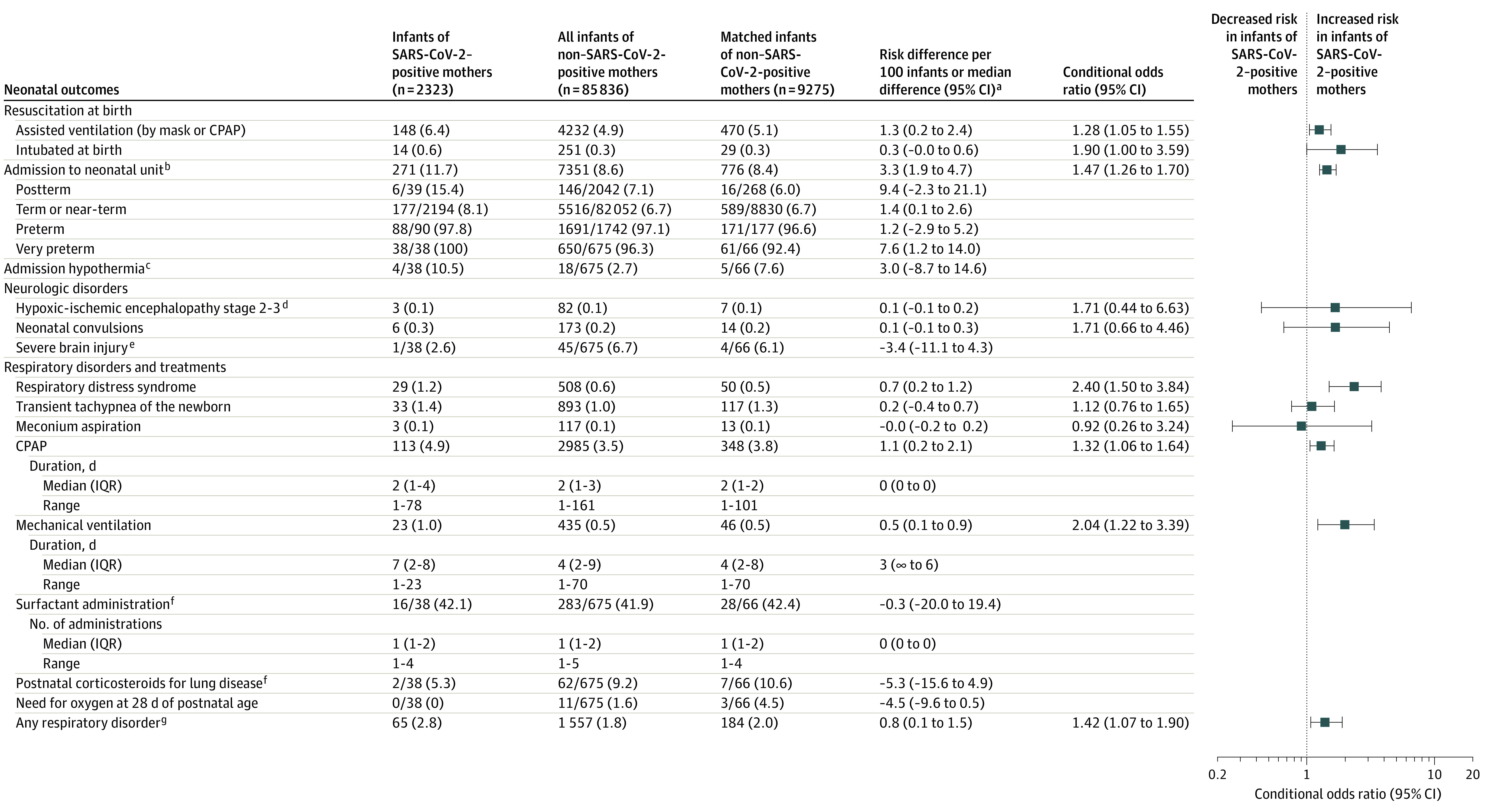
Data are No. (%) unless otherwise indicated. CPAP indicates continuous positive airway pressure.
aBetween infants of SARS-CoV-2–positive mothers vs matched infants of mothers without a positive test result.
bAdmission to a unit with capacity to treat and care for ill or preterm infants. Very preterm indicates <32 weeks; preterm, <35 weeks; near-term, 35 to 36 weeks; term, 37 to 41 weeks; and postterm, ≥42 weeks.
cDefined as <35.5 °C in infants <32 weeks of gestational age.
dStage 2 to 3 is moderate to severe encephalopathy.27
eDefined as intraventricular hemorrhage grade 3 to 4 (grade 4 is most severe, with hemorrhage from the ventricles extending into the surrounding brain) or cystic periventricular leukomalacia (last stage of white-matter brain injury with cystic scaring) among infants <32 weeks of gestational age.
fIn infants <32 weeks of gestational age.
gRespiratory distress syndrome, transient tachypnea of the newborn, meconium aspiration, or pneumonia.
Figure 3. Neonatal Outcomes (Infections; Circulatory, Metabolic, Hematologic, and Gastrointestinal Problems; In-Hospital Mortality; Length of Hospital Stay; Breastfeeding; and Infant SARS-CoV-2 Test Status) Among Infants Born in Sweden (March 11, 2020–January 31, 2021) by Maternal SARS-CoV-2 Test Status in Pregnancy.
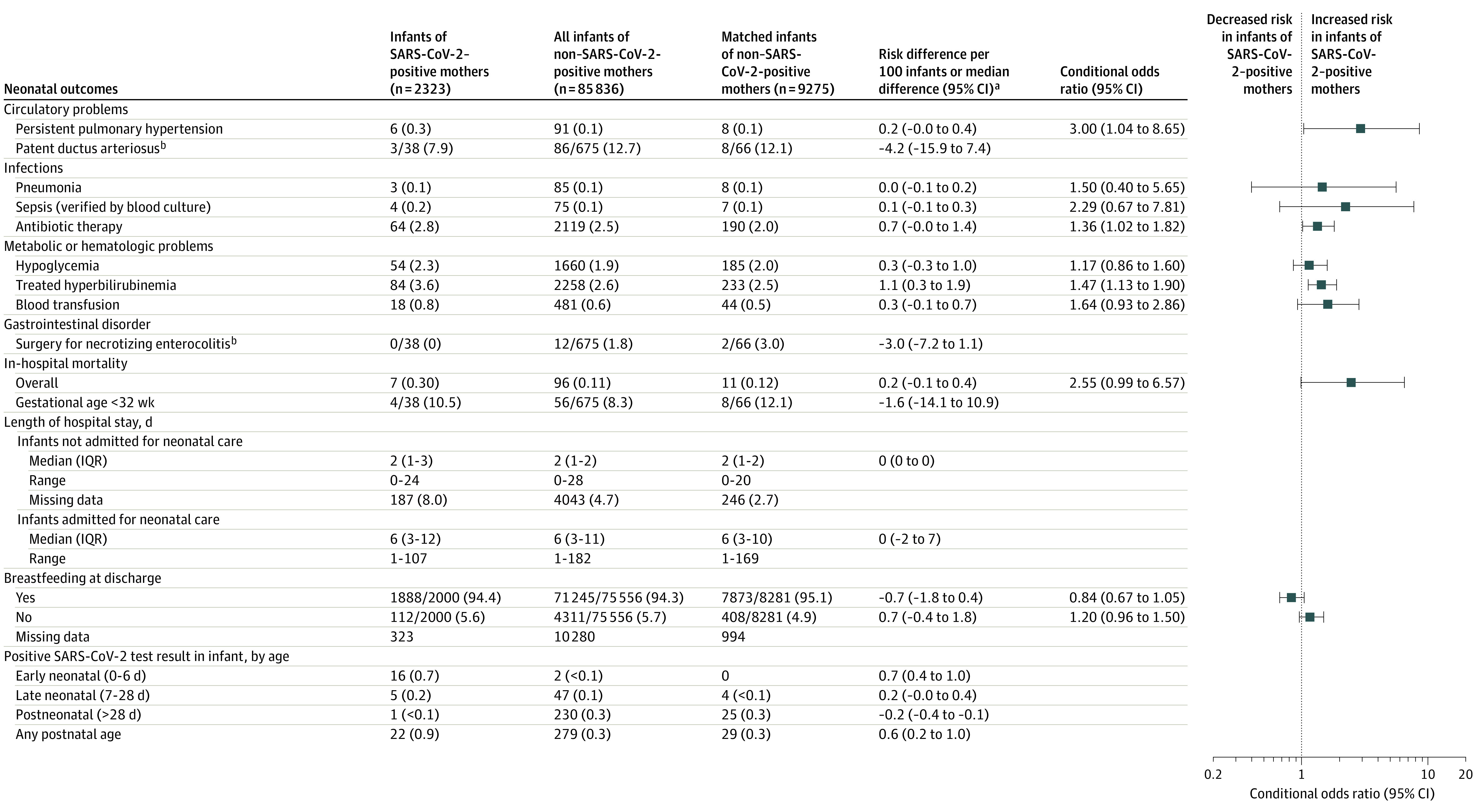
Data are No. (%) unless otherwise indicated. IQR indicates interquartile range. Neonatal outcomes were assessed as of March 8, 2021.
aRisk differences or median differences between infants of SARS-CoV-2–positive mothers vs matched infants of mothers without a positive test result.
bIn infants <32 weeks of gestational age.
After matching infants by maternal characteristics, some neonatal outcomes (procedures and morbidities) were significantly more common in infants of SARS-CoV-2–positive women (n = 2323) than in infants of comparator women (n = 9275): assisted ventilation at birth (with mask or CPAP; 6.4% vs 5.1%; OR, 1.28; 95% CI, 1.05-1.55), intubation at birth (0.6% vs 0.3%; OR, 1.90; 95% CI, 1.002-3.59), admission for neonatal care (11.7% vs 8.4%; OR, 1.47; 95% CI, 1.26-1.70), respiratory distress syndrome (1.2% vs 0.5%; OR, 2.40; 95% CI, 1.50-3.84), use of CPAP (4.9% vs 3.8%; OR, 1.32; 95% CI, 1.06-1.64), mechanical ventilation (1.6% vs 0.5%; OR, 3.51; 95% CI, 1.85-6.65), any respiratory disorder (2.8% vs 2.0%; OR, 1.42; 95% CI, 1.07-1.90), persistent pulmonary hypertension (0.3% vs 0.1%; OR, 3.00; 95% CI, 1.04-8.65), antibiotic therapy (2.8% vs 2.0%; OR, 1.36; 95% CI, 1.02-1.82), and hyperbilirubinemia (3.6% vs 2.5%; OR, 1.47; 95% CI, 1.13-1.90). Mortality (0.30% vs 0.12%; OR, 2.55; 95% CI, 0.99-6.57), breastfeeding rates at discharge (94.4% vs 95.1%; OR, 0.84; 95% CI, 0.67-1.05), and length of stay in neonatal care (median, 6 days in both groups; difference, 0 days; 95% CI, −2 to 7 days) did not differ significantly between the groups. Hypoxic-ischemic encephalopathy, transient tachypnea, meconium aspiration, pneumonia, sepsis, and hypoglycemia also did not differ significantly between the 2 groups (Figure 2 and Figure 3).
In mediation analysis, preterm birth (gestational age <37 weeks; 205 [8.8%] of 2323 SARS-CoV-2–exposed infants; 518 [5.6%] of 9275 comparator infants matched on maternal characteristics) was identified to mediate 89.3% of the estimated association between maternal SARS-CoV-2 test positivity and any neonatal respiratory disorder. Adding cesarean delivery and an Apgar score less than 4 at 5 minutes as potential mediators diluted the mediation of preterm birth. There was no statistically significant direct (infectious) association between maternal SARS-CoV-2 test positivity and any neonatal respiratory disorder (eTable 3 in the Supplement).
Twenty-one (0.90%) of 2323 infants of SARS-CoV-2–positive mothers tested positive for SARS-CoV-2 in the neonatal period. Of these, 12 did not have any neonatal morbidity, 9 had diagnoses with unclear relation to SARS-CoV-2, and none had congenital pneumonia. The proportion of infants testing positive for SARS-CoV-2 among infants born to mothers who tested positive at delivery was 17 (2.7%) of 642. In the comparator group of infants of mothers without a positive SARS-CoV-2 test result in pregnancy up to 1 week after birth, 279 (0.33%) of 85 836 infants tested positive for SARS-CoV-2, 230 of them (82%) after the neonatal period (0-28 days after birth). Characteristics of all infants testing positive for SARS-CoV-2 in the neonatal period are presented in eTable 4 in the Supplement.
The sensitivity analysis restricting the exposure window for test positivity among pregnant women from conception to delivery did not alter the results and interpretation in any significant way (eTable 5 in the Supplement).
Discussion
In a nationwide cohort of infants in Sweden, maternal SARS-CoV-2 test positivity was significantly associated with increased risks of some neonatal morbidities. Preterm birth was identified as a mediator of neonatal respiratory disorders.
Previously, viral RNA has been detected in the placenta32 and fetal membranes,33 but intrauterine transmission of SARS-CoV-2 from mother to fetus seems to be rare.12 Postnatal viral transmission has been described to occur.12,13,21,26,34,35,36 One case series reported most infants of SARS-CoV-2–positive mothers to be asymptomatic, but a smaller proportion experienced neonatal morbidity, and 6 of 450 died.13 However, because none of the fatal cases were SARS-CoV-2 positive, death due to other causes could not be excluded.
A review of 77 studies mainly from China, the US, and Europe reported that 2 (0.5%) of 427 infants of COVID-19–positive mothers died, which was a higher mortality rate than in historical controls (0.1%).10 The difference in mortality rates found herein was smaller, and no deceased infant was considered to have had COVID-19 infection as currently defined by the World Health Organization.37 In addition, considering that maternal SARS-CoV-2 test positivity in pregnancy occurred 18 to 74 days before delivery for 5 of the 7 deceased infants and that none of the mothers had been admitted for intensive care during pregnancy or at delivery, a potentially causal relationship (direct or indirect) between SARS-CoV-2 and neonatal mortality appears to be very weak.
In 176 cases of neonatal SARS-CoV-2 from different parts of the world, selected according to diagnostic criteria based on PCR tests,38 various but nonspecific symptoms were reported in 55%.12 In a subgroup analysis, mother-infant co-care was reported to increase the risk of neonatal infection.12 Increased admission rates for neonatal care among infants born to mothers infected with SARS-CoV-2 have also been reported previously in the United Kingdom,26 from Turkey,3 and in systematic reviews of case series from China, the US, and elsewhere.9,10,12,13 The present study confirms an increased admission rate in infants of SARS-CoV-2–positive women. Given that all preterm infants less than 35 weeks of gestational age in both groups were admitted for neonatal care (except for infants who died after birth but before neonatal admission), the higher admission rates in infants of SARS-CoV-2–positive women were confined to near-term or term infants. Based on the findings presented herein, the excess admissions in near-term or term infants of SARS-CoV-2–positive women most likely reflect widened indications for observation and isolation in neonatal units,18 maternal illness after delivery, or recommendations in guidelines.3
This study could neither confirm nor rule out an association between maternal SARS-CoV-2 test positivity and increased fetal distress during delivery or birth asphyxia.8,39 Point estimates for ORs regarding asphyxia-related outcomes in near-term and term infants, such as hypoxic-ischemic encephalopathy grade 2 to 3 and convulsions, were above 1. This may indicate an increased vulnerability in some SARS-CoV-2–exposed fetuses and infants to asphyxia-related complications, possibly mediated by placental insufficiency or maternal fever superimposed on a hypoxic-ischemic event during delivery.40 There was no information on maternal temperature during delivery in this study. To detect a statistically significant difference (with an OR of 1.7 as estimated herein and with a statistical power of 80%) between SARS-CoV-2–exposed and unexposed infants in the rare outcome of hypoxic-ischemic encephalopathy grade 2 or 3, approximately 33 000 infants in the exposed group would be needed. This means sampling for more than 13 years in a country such as Sweden, provided that the rates of SARS-CoV-2 in pregnancy would stay the same as in March 2020–January 2021, which is highly unlikely.
Preterm birth has been reported to be a major risk among pregnant women who test positive for SARS-CoV-2.3,7,8,11,26,39,41 This study found support for preterm birth being an important mediator of the association between maternal SARS-CoV-2 and increased neonatal respiratory morbidity, whereas no statistically significant direct, infectious association with maternal SARS-CoV-2 could be demonstrated. The mediator of preterm birth may explain other associations between maternal SARS-CoV-2 and neonatal outcomes as well, such as more frequent need for assisted ventilation (at birth and later), a higher proportion of infants with hyperbilirubinemia, and more frequent use of antibiotics among SARS-CoV-2–exposed infants.
The follow-up time for SARS-CoV-2 test positivity among participants in this study was at least 1 month. All SARS-CoV-2–exposed infants underwent SARS-CoV-2 PCR testing at least once (those admitted for neonatal care were tested 3 times), and a majority tested negative for SARS-CoV-2 in the neonatal period. While a low transmission rate is in accordance with previous reports,3,8,21,26 interpretations vary. Given that mothers and their infants were kept together, the results presented herein suggest that the risk of viral transmission from mothers to their newborns and older infants is low, and should it occur, infants are not severely affected. These findings suggest that routine use of interventions such as mother-infant separation12 and stopping breastfeeding may not be necessary. An additional message of this study is that SARS-CoV-2 test positivity in mothers did not prolong hospital stay for families.
Strengths of this study include its population-based, nationwide design including a large number of infants and the direct and propensity score matching procedure followed by formal mediation analyses to minimize bias and confounding. Matching and mediation analyses were based on maternal and perinatal characteristics known to affect clinically important outcomes such as admission rates for neonatal care, neonatal morbidity and mortality, and breastfeeding. A wide range of neonatal outcomes that have been discussed as a matter of concern in connection to SARS-CoV-2 were explored.12 The follow-up time for SARS-CoV-2 test positivity in infants reached at most 9 months. In addition, the possibility of daily data capture online in all 3 national registers and using unique personal identity numbers for linkage enabled a minimum of delay in reporting this study’s results.
Limitations
This study had a number of limitations. First, numbers for some neonatal outcomes were small and the magnitude of the absolute risks was low. Second, imbalanced testing between health care regions and hospitals as well as regional variations may have occurred, attributed to clusters of COVID-19 infection. However, all analyses adjusted for health care region. Third, some mothers of matched comparators could have been infected since COVID-19 infection in pregnancy may be asymptomatic.11 Furthermore, women with near-term or term infants admitted for neonatal care could have been more likely to be tested, which may lead to an overestimation of risks associated with SARS-CoV-2 test positivity in near-term and term infants. Fourth, although surveillance bias could not be excluded, there were no recommendations in the Swedish guidelines for COVID-19 in pregnancy to test women with pregnancy complications differently than other pregnant women, ie, on suspicion of COVID-19 infection. Fifth, there was no information on severity of illness among SARS-CoV-2–positive women before or during delivery. Sixth, all newborn infants of SARS-CoV-2–positive mothers had 1 to 3 SARS-CoV-2 PCR tests after birth, but testing in the unexposed population was performed only in infants with suspected symptomatic infection, introducing detection bias. Seventh, neonatal tests on the day of birth may have been contaminated by maternal viral DNA, resulting in falsely high test positivity among SARS-CoV-2–exposed infants. On the other hand, some SARS-CoV-2–positive women may have become negative by the time of delivery, suggesting that their infants were unexposed to postnatal viral transmission. Eighth, although test positivity could be traced throughout infancy, clinical outcomes were limited to the neonatal period. Ninth, given population differences and that general precautionary measures and specific recommendations for SARS-CoV-2–positive mothers and their infants are country-specific,2,4 external validity of the findings may be limited.
Conclusions
In a nationwide cohort of infants in Sweden, maternal SARS-CoV-2 infection in pregnancy was significantly associated with small increases in some neonatal morbidities. Given the small numbers of events for many of the outcomes and the large number of statistical comparisons, the findings should be interpreted as exploratory.
eTable 1. ICD-10 Codes Used for Outcomes Not Defined in Text and Exclusion Criteria
eTable 2. Characteristics of Live Born Infants Experiencing In-Hospital Neonatal Mortality (n=7, Infants Delivered by Mothers Testing Positive for SARS-CoV-2 in Pregnancy Only)
eTable 3. Mediation Analysis for Maternal SARS-CoV-2 Test-Status in Pregnancy and the Risk of Any Neonatal Respiratory Disease
eTable 4. Characteristics of Infants Born in Sweden (March 11, 2020 – Jan 31, 2021) and Testing Positive for SARS-CoV-2 in the Neonatal Period (0-28 Days After Birth)
eTable 5. Neonatal Outcomes of Infants Born in Sweden (March 11, 2020 – Jan 31, 2021) by SARS-CoV-2 Test-Status of Mother in Pregnancy, Restricting the Exposure Window for Test-Positivity Among Pregnant Women From Conception to Delivery
References
- 1.Morens DM, Taubenberger JK, Harvey HA, Memoli MJ. The 1918 influenza pandemic: lessons for 2009 and the future. Crit Care Med. 2010;38(4)(suppl):e10-e20. doi: 10.1097/CCM.0b013e3181ceb25b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo KT, Oei JL, De Luca D, et al. Review of guidelines and recommendations from 17 countries highlights the challenges that clinicians face caring for neonates born to mothers with COVID-19. Acta Paediatr. 2020;109(11):2192-2207. doi: 10.1111/apa.15495 [DOI] [PubMed] [Google Scholar]
- 3.Oncel MY, Akin IM, Kanburoglu MK, et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish Neonatal Society. Eur J Pediatr. 2020;180(3):733-742. doi: 10.1007/s00431-020-03767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavizzari A, Klingenberg C, Profit J, et al. ; International Neonatal COVID-19 Consortium . International comparison of guidelines for managing neonates at the early phase of the SARS-CoV-2 pandemic. Pediatr Res. Published online June 15, 2020. doi: 10.1038/s41390-020-0976-5 [DOI] [PubMed] [Google Scholar]
- 5.Poon LC, Yang H, Kapur A, et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynaecol Obstet. 2020;149(3):273-286. doi: 10.1002/ijgo.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessami K, Homayoon N, Hashemi A, Vafaei H, Kasraeian M, Asadi N. COVID-19 and maternal, fetal and neonatal mortality: a systematic review. J Matern Fetal Neonatal Med. Published online August 16, 2020. doi: 10.1080/14767058.2020.1806817 [DOI] [PubMed] [Google Scholar]
- 7.Yoon SH, Kang JM, Ahn JG. Clinical outcomes of 201 neonates born to mothers with COVID-19: a systematic review. Eur Rev Med Pharmacol Sci. 2020;24(14):7804-7815. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 2020;39(6):469-477. doi: 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15-27. doi: 10.1002/uog.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allotey J, Stallings E, Bonet M, et al. ; PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11(1):5164. doi: 10.1038/s41467-020-18982-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettirosso E, Giles M, Cole S, Rees M. COVID-19 and pregnancy: a review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60(5):640-659. doi: 10.1111/ajo.13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludvigsson JF. The first eight months of Sweden’s COVID-19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020;109(12):2459-2471. doi: 10.1111/apa.15582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephansson O, Petersson K, Björk C, Conner P, Wikström AK. The Swedish Pregnancy Register—for quality of care improvement and research. Acta Obstet Gynecol Scand. 2018;97(4):466-476. doi: 10.1111/aogs.13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman M, Källén K, Wahlström E, Håkansson S; SNQ Collaboration . The Swedish Neonatal Quality Register—contents, completeness and validity. Acta Paediatr. 2019;108(8):1411-1418. doi: 10.1111/apa.14823 [DOI] [PubMed] [Google Scholar]
- 17.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823-829. doi: 10.1111/aogs.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Dong L, Ming L, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. BMC Pregnancy Childbirth. 2020;20(1):394. doi: 10.1186/s12884-020-03026-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843-848. doi: 10.1111/j.1651-2227.1996.tb14164.x [DOI] [PubMed] [Google Scholar]
- 21.Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2021;175(2):157-167. doi: 10.1001/jamapediatrics.2020.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Gao J, Wei Y, et al. Managing preterm infants born to COVID-19 mothers: evidence from a retrospective cohort study in Wuhan, China. Neonatology. 2020;117(5):592-598. doi: 10.1159/000509141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyle MH, Glassman ME, Khan A, et al. A review of newborn outcomes during the COVID-19 pandemic. Semin Perinatol. 2020;44(7):151286. doi: 10.1016/j.semperi.2020.151286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323(13):1313-1314. doi: 10.1001/jama.2020.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722-725. doi: 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight M, Bunch K, Vousden N, et al. ; UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696-705. doi: 10.1001/archneur.1976.00500100030012 [DOI] [PubMed] [Google Scholar]
- 28.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi: 10.1016/S0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 29.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1-6. doi: 10.1016/S0166-4328(05)80189-5 [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etminan M, Collins GS, Mansournia MA. Using causal diagrams to improve the design and interpretation of medical research. Chest. 2020;158(1S):S21-S28. doi: 10.1016/j.chest.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 32.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penfield CA, Brubaker SG, Limaye MA, et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020;2(3):100133. doi: 10.1016/j.ajogmf.2020.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groß R, Conzelmann C, Müller JA, et al. Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020;395(10239):1757-1758. doi: 10.1016/S0140-6736(20)31181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam PCK, Ly KM, Kernich ML, et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;72(1):128-130. doi: 10.1093/cid/ciaa673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambers C, Krogstad P, Bertrand K, et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA. 2020;324(13):1347-1348. doi: 10.1001/jama.2020.15580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . Definition and Categorization of the Timing of Mother-to-Child Transmission of SARS-CoV-2. Published February 7, 2021. Accessed April 19, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1
- 38.Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565-568. doi: 10.1111/aogs.13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111.e1-111.e14. doi: 10.1016/j.ajog.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laptook AR, Corbett RJ. The effects of temperature on hypoxic-ischemic brain injury. Clin Perinatol. 2002;29(4):623-649. doi: 10.1016/S0095-5108(02)00057-X [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Liu C, Dong L, et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. 2020;127(9):1109-1115. doi: 10.1111/1471-0528.16276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 Codes Used for Outcomes Not Defined in Text and Exclusion Criteria
eTable 2. Characteristics of Live Born Infants Experiencing In-Hospital Neonatal Mortality (n=7, Infants Delivered by Mothers Testing Positive for SARS-CoV-2 in Pregnancy Only)
eTable 3. Mediation Analysis for Maternal SARS-CoV-2 Test-Status in Pregnancy and the Risk of Any Neonatal Respiratory Disease
eTable 4. Characteristics of Infants Born in Sweden (March 11, 2020 – Jan 31, 2021) and Testing Positive for SARS-CoV-2 in the Neonatal Period (0-28 Days After Birth)
eTable 5. Neonatal Outcomes of Infants Born in Sweden (March 11, 2020 – Jan 31, 2021) by SARS-CoV-2 Test-Status of Mother in Pregnancy, Restricting the Exposure Window for Test-Positivity Among Pregnant Women From Conception to Delivery