Abstract
Background
The word ‘pandemic’ conjures dystopian images of bodies stacked in the streets and societies on the brink of collapse. Despite this frightening picture, denialism and noncompliance with public health measures are common in the historical record, for example during the 1918 Influenza pandemic or the 2015 Ebola epidemic. The unique characteristics of SARS-CoV-2—its high basic reproduction number (R0), time-limited natural immunity and considerable potential for asymptomatic spread—exacerbate the public health repercussions of noncompliance with interventions (such as vaccines and masks) to limit disease transmission. Our work explores the rationality and impact of noncompliance with measures aimed at limiting the spread of SARS-CoV-2.
Methods
In this work, we used game theory to explore when noncompliance confers a perceived benefit to individuals. We then used epidemiological modeling to predict the impact of noncompliance on control of SARS-CoV-2, demonstrating that the presence of a noncompliant subpopulation prevents suppression of disease spread.
Results
Our modeling demonstrates that noncompliance is a Nash equilibrium under a broad set of conditions and that the existence of a noncompliant population can result in extensive endemic disease in the long-term after a return to pre-pandemic social and economic activity. Endemic disease poses a threat for both compliant and noncompliant individuals; all community members are protected if complete suppression is achieved, which is only possible with a high degree of compliance. For interventions that are highly effective at preventing disease spread, however, the consequences of noncompliance are borne disproportionately by noncompliant individuals.
Conclusions
In sum, our work demonstrates the limits of free-market approaches to compliance with disease control measures during a pandemic. The act of noncompliance with disease intervention measures creates a negative externality, rendering suppression of SARS-CoV-2 spread ineffective. Our work underscores the importance of developing effective strategies for prophylaxis through public health measures aimed at complete suppression and the need to focus on compliance at a population level.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-10829-2.
Keywords: COVID-19, Game theory, Infectious disease control, Epidemiological modeling, SARS-CoV-2, Vaccine hesitancy
Background
As we enter the unfamiliar territory of the worst global pandemic in a century, the worldwide emergence of noncompliance with public health measures aimed at limiting the spread of SARS-CoV-2 is not as surprising as it may seem at first blush [1, 2]. During the 1918 Influenza pandemic, for example, resistance to public health measures aimed at reducing the spread of disease manifested at the individual level, leading to violence [3] and stiff punishments for “mask slackers” [4, 5]. Anti-mask protesters led large demonstrations [6], and city councils questioned the value of mask ordinances [7, 8] with emotionally charged language: “under no circumstances will I be muzzled like a hydrophobic dog” [9]. The phrasing may be dated, but the sentiment echoes precisely across a century [10].
For COVID-19, a number of features of the disease facilitate noncompliance with disease control measures such as masking and vaccination. Hospitalization and death happen away from the public eye, and our changing understanding of the mechanism of transmission, the risk of mortality and the long-term consequences of the disease have favored the spread of misinformation. The spread of confusion and misinformation has been a common feature for other novel pathogen-induced pandemics such as Ebola [1, 11, 12] and the 1918 Flu [13]. While the existence of pandemic denialism was easy to anticipate [14], the unique characteristics of COVID-19 amplify its effect. Studies suggest that asymptomatic or presymptomatic patients account for up to 40% of SARS-CoV-2 transmission [15], severely limiting the utility of more traditional and intuitive disease control measures such as symptomatic isolation [16]. The high reproductive number (R0) of SARS-CoV-2 (the average number of individuals who contract a contagious disease from one infected individual, which was reported to be 5.7 in the early days of the pandemic in Wuhan [17]) creates the potential for explosive growth in situations where the virus has not been completely eradicated, as has been demonstrated by a massive second wave in many European countries [18, 19]. Making matters worse, estimates for natural immunity as a consequence of SARS-CoV-2 infection range from 6 to 24 months [20–22], creating the potential for multiple waves of disease in the short term.
Thus, the unique characteristics of COVID-19 raise the possibility that noncompliance with public health measures may create conditions that make disease control in the short term impossible or prevent any return to pre-pandemic lifestyles in the long run. With this in mind, we asked three questions: First, in the specific case of COVID-19, are there circumstances that lead to a perceived benefit to noncompliance with public health measures for a substantial portion of the population? Second, what is the impact of noncompliance on the attainability of suppression of SARS-CoV-2 spread? Third, what is the magnitude of the negative externality (a cost incurred by them that is not of their choosing) created for the compliant population as a result of noncompliance of others?
We approached the first question from the perspective of game theory, which has previously been applied to decision-making around vaccine uptake [23]. Our approach involved building a mathematical model of the strategic interaction between compliers and non-compliers for a given (nonpharmaceutical or biomedical) intervention aimed at controlling SARS-CoV-2 spread. The approach weighs the perceived cost of complying with an intervention against the perceived benefit to determine under what conditions individuals acting in their own self-interest will choose to comply. A number of studies have previously examined noncompliance with measures to control SARS-CoV-2 spread through a social-sciences lens, exploring social and psychological risk factors associated with this behavior. These studies, from a range of different countries, have linked noncompliance to Dark Triad traits (i.e., Machiavellianism, Psychopathy Factor 1, and narcissistic rivalry [24]), antisocial behaviors [25], higher levels of impulsivity [26] and a prior record of delinquent behaviors [27]. A positive, rather than normative, framing of the question involves exploring the set of conditions for which the perceived benefit of noncompliance to the individual is simply greater than the perceived benefit of compliance. This allows us to examine the problem of compliance from the limited perspective of individuals optimizing for their own benefit without accounting for the common good, particularly relevant in the context of arguments based on personal liberty being used as a justification for noncompliance [28].
For the next two questions, we used a Susceptible-Exposed-Infected-Recovered-Susceptible (SEIRS) epidemiological modeling framework with a duration of immunity ranging from 6 to 24 months to explore the range of levels of compliance and intervention efficacy required for disease suppression. Our intent in this study was to explore a possible link between the free optimization of individuals’ outcomes as a result of noncompliance, the externalities generated by those choices, and the implications for epidemic control in the short and long term.
Methods
Game theory modeling of compliance with interventions aimed at limiting SARS-CoV-2 spread
For the purposes of this work, we defined an “intervention” as being a public health measure that reduces the transmission of SARS-CoV-2. This may be a nonpharmaceutical intervention, such as masks, or a biomedical intervention, such as a vaccine. Compliance with an intervention is defined as a binary choice. An individual can choose whether or not to comply with an intervention based on the perceived costs and benefits of the intervention. We modeled this choice using a game theoretic framework, which compares the perceived cost of compliance (reduction of quality of life resulting from the intervention) in relation to perceived cost of infection (risk-weighted morbidity/mortality burden) to the individual. Individuals derive a benefit or cost (i.e., a payoff) from interactions with other individuals in the population, who can also either be compliers or noncompliers.
We sought to determine the conditions under which noncompliance is the Nash equilibrium, or optimal behavior strategy for individuals seeking to maximize their own payoff. In a Nash equilibrium, the expected payoff to noncompliers is higher than the payoff to compliers when interacting with any other individual in the population [29].
For this two-strategy “game”, the payoffs to compliers and noncompliers are given in Table 1, where q is the cost of the intervention, αi is the fraction of infected individuals of type i, and mi is the perceived cost of infection for type i individuals, where i can either be u (noncompliers) or v (compliers). The cost mi is the perceived risk of a negative health outcome given exposure to an infected individual. Other parameter definitions are given in Table 2. As in the SEIRS model, the efficacy of the intervention in protecting the individual from getting infected (b) is assumed equal to the efficacy in preventing transmission (c) (i.e. b = c).
Table 1.
Payoff matrix for compliers/noncompliers
| Noncompliant interaction partner | Compliant interaction partner | |
|---|---|---|
| Noncomplier payoff | -αumu | -αvmuc |
| Complier payoff | -q - αumvb | -q - αvmvbc |
αi: fraction of infected individuals of type i, mi: the perceived risk of a negative health outcome given exposure to an infected individual, where i can either be u (noncompliers) or v (compliers), q: the perceived cost of the intervention. All other parameter definitions are given in Table 2
Table 2.
Model parameters for SEIRS model
| Parameter | Symbol | Value | Source |
|---|---|---|---|
| Latency period | 1/ α | 3 days | [30] |
| Reproductive number | R0 | 5.7 individuals | [17] |
| Infectious period | 1/ γ | 10 days | [31] |
| Natural immunity duration | 1/ δ | 18 months | [32] |
| Infection fatality rate | σ | 0.68% | [33] |
| Population birth rate | μ | 1% annually | [34] |
| Population death rate | λ | 0.9% annually | [35] |
| Fraction compliant | f | Variable | |
| Protective efficacy | 1-b | Variable | |
| Transmission reduction | 1-c | Variable |
All parameters defining the ODE-based SEIRS model. In this analysis, the fraction compliant, protective efficacy against infection, and reduction in transmission are treated as independent variables
Noncompliance is a Nash equilibrium if and only if both of the following conditions are met:
Or, equivalently
Since noncompliers are much more likely to be infected than compliers, αu > cαv. Therefore, meeting the first condition alone (noncompliers receive a greater payoff than compliers when interacting with other noncompliers) is sufficient for noncompliance to be a Nash equilibrium.
SEIRS model of SARS-CoV-2 spread
To support predictions of short- and long-term outcomes for the COVID-19 pandemic, we built an SEIRS ordinary differential equations (ODE) model to account for disease spread, waning immunity in the recovered population, and the acceptance of a vaccine or non-pharmaceutical intervention (NPI) in a fraction of the population. The model consists of two parallel sets of SEIR compartments representing the vaccinated or NPI-compliant (“compliant”) and unvaccinated or NPI-noncompliant (“noncompliant”) populations. The compliant population has a reduced risk of infection which is conferred by the vaccine or NPI (“protective efficacy”). The compliant population may also have a reduced risk of transmission to others upon infection resulting from physiological or behavioral changes (“transmission reduction.”) All compartments were assumed to be well-mixed, meaning that compliant and noncompliant individuals are in contact within and between groups. Vaccination or NPI compliance-based reductions in susceptibility, transmissibility, or contact rate were assumed to be time-invariant, reflecting the most optimistic case for disease control. Similarly, individuals do not move between the compliant and noncompliant compartments. Model equations are summarized below:
Where S represents the susceptible population, E the exposed population, I the infectious population, and R the recovered population. Subscript v represents the vaccinated or compliant sub-population, while subscript u represents the unvaccinated or noncompliant sub-population. Model parameters are summarized in Table 2.
According to the CDC, the R0 for SARS-CoV-2 under pre-pandemic social and economic conditions is estimated to be approximately 5.7 [17]. For the purpose of this study, an R0 of 5.7 is used to represent epidemiological conditions under a theoretical full return to pre-pandemic activity. The contact rate β is derived from the relationship between R0 and the infectious period:
In this “normal” scenario, disease reduction interventions reduce the compliant population’s infection rate by the factor b, which represents the intervention’s protective efficacy, and the compliant population’s transmission rate by the factor c, representing the intervention’s reduction in transmissibility. For simplicity, the reduction of transmission was assumed to be equivalent to the protective efficacy (reduction of susceptibility) of each intervention. This is an optimistic assumption; in some cases, an intervention may provide little or no reduction in transmission in compliant infected individuals.
The model’s initial conditions are set to approximate current United States disease prevalence and seroprevalence (as of September 2020) [36]:
Our model lacks a seasonal component for SARS-CoV-2 transmission, as such associations have been conjectured [37] but not proven, and it also assumes a 18-month duration of natural immunity, as an optimistic estimate based on the duration of antibody responses currently reported [20–22]. The disease-preventing interventions and return to normalcy (which would correspond to a return to the pre-pandemic R0 of 5.7) are assumed to occur at the beginning of the simulation interval.
Compliance sweeps
To gauge the impact of NPI or vaccine compliance on population outcomes, we varied the compliant fraction under a series of simulated vaccine or NPI deployment schemes with varying degrees of protective efficacy. The model allows tracking of outcomes for the population as a whole and for the compliant and noncompliant sub-populations.
Results
Structural incentives for noncompliance with interventions aimed at controlling SARS-CoV-2 spread
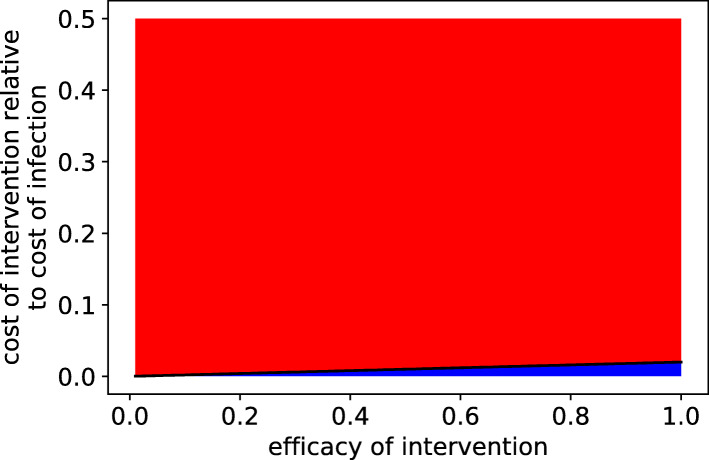
In Fig. 1, we modeled the decision to comply with public health measures in terms of its perceived short-term impact to individuals. In game theory, a Nash equilibrium is a strategy which has a higher payoff for the individual than all other possible strategies (“no regrets”) [29]. Individuals using a strategy that is a Nash equilibrium are unable to improve their outcome by switching strategies. Strikingly, for a large region of parameter space in this model, noncompliance is a Nash equilibrium. Even so, one can make the case that, using realistic estimates for risk of infection and risk of adverse outcomes given infection, compliance would still be a rational choice for the vast majority of the population. For example, for an intervention that is 50% effective at reducing the risk of infection, when 2% of individuals are infected, compliance is a Nash equilibrium at a 1% relative cost (ratio of the loss of quality of life associated with the intervention over the cost of infection in terms of risk of mortality, morbidity, and disability). While the decision to comply is determined by the perceived cost of infection and the perceived cost of intervention, the actual costs may be very different. The cost of wearing a mask, for example, is likely to be much less than the risk-weighted cost of death or disability due to COVID-19 (see Tables S1, S2 for a more detailed analysis).
Fig. 1.
Noncompliance is a Nash equilibrium when infection rates are low or prevention is costly or ineffective. Intervention efficacy and intervention cost conditions for which noncompliance is a Nash equilibrium (red) or not a Nash equilibrium (blue) if the disease is present in 2% of individuals in the population. Intervention cost relative to infection cost is defined as the ratio of intervention cost to risk-weighted infection cost
Failure to suppress SARS-CoV-2 spread results in waves of transmission
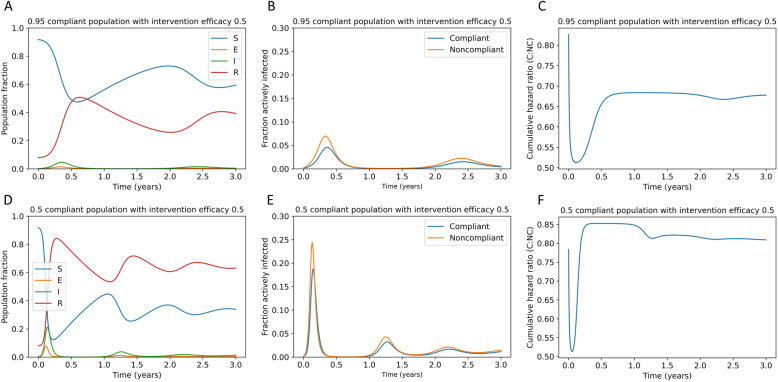
As shown in Fig. 2, insufficient reduction in SARS-CoV-2 transmission allows the disease to persist upon a rapid return to pre-pandemic activity and spread in multiple waves over time. The model does not account for changes in behavior or environmental factors over time, so these oscillations in transmission are caused by a predator-prey dynamic within the SEIRS system rather than triggered by external factors. This dynamic is driven by the time-variant availability of susceptible hosts as immunity wanes in individuals who have recovered from COVID-19. Panels 2a-c represent a high compliance (95%) scenario with a 50% effective intervention. The efficacy of an intervention describes the fraction of possible transmission events it prevents. In this case, the oscillations and variability in risk for the compliant population are relatively small because the intervention serves to dampen the oscillations in transmission rate. However, in panels 2d-f, representing a low compliance (50%) scenario with a 50% effective intervention, the oscillatory pattern is much more pronounced and risk to the compliant population is variable over time (Fig. 2f). Additionally, the cumulative risk to the compliant population relative to the noncompliant population is higher when more of the population is noncompliant (Fig. 2c, f).
Fig. 2.
Failure to eradicate SARS-CoV-2 results in waves of disease upon rapid return to pre-pandemic activity. Panels a and d represent the fraction of the population, including both compliant and noncompliant individuals, that is susceptible, exposed, infectious, and recovered populations over time after a return to pre-pandemic conditions under (a-c) 95% compliance or (d-f) 50% compliance with a 50% effective intervention. Panels b and e demonstrate the fraction of compliant and noncompliant individuals who are infected over time. Panels c and f demonstrate the cumulative hazard ratio for infection in noncompliant (NC) versus compliant (C) individuals
Near-term suppression of SARS-CoV-2 spread requires a high degree of compliance
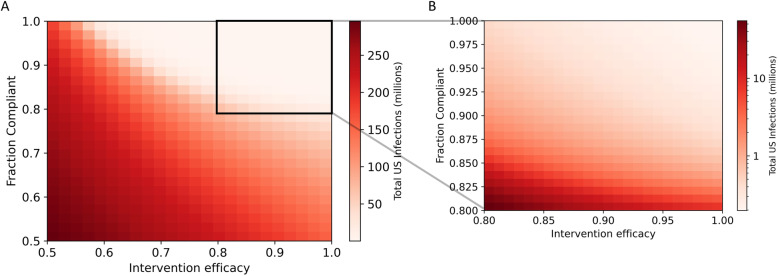
In the short term, to suppress SARS-CoV-2 transmission while returning to pre-pandemic social and economic activity, an intervention with a high degree of efficacy and compliance is required (Fig. 3). Although effective suppression can be achieved with an intervention with as low as 65% efficacy, at least 80% compliance is required for even the most effective interventions. The predicted number of cases in the next year span three orders of magnitude, from less than one million cases to hundreds of millions of cases, depending on the effectiveness and the degree of compliance with transmission reduction interventions.
Fig. 3.
Short-term suppression of COVID-19 requires a high degree of compliance with a highly effective measure. Total US SARS-CoV-2 infections in the next year under interventions with varying efficacy and compliance are shown in panel (a). Panel b shows the black box on panel (a) expanded. Total US infections are displayed on a log scale in panel (b)
If SARS-CoV-2 becomes endemic, steady-state yearly spread depends on population compliance
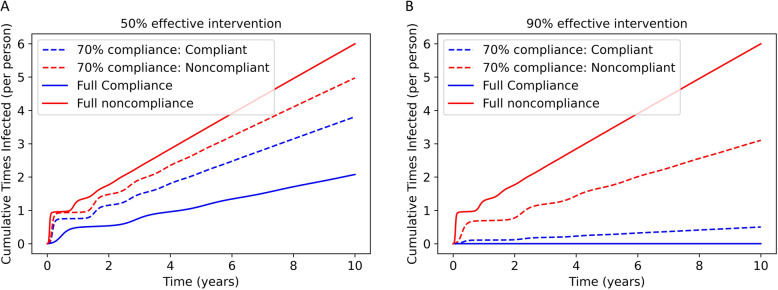
If immunity to SARS-CoV-2 by natural infection is not life-long, as suggested by many studies [20–22], and if effective interventions are not undertaken at a large scale, the virus will become endemic. As shown in Fig. 4, this means that in the long term, SARS-CoV-2 will reach a steady-state prevalence in the population. For a 50% effective intervention, the disease will become endemic even if the entire population complies with the intervention. As expected, the benefit of compliance for an individual is smaller for a 50% effective intervention (Fig. 4a) relative to a 90% effective intervention (Fig. 4b). The full compliance scenario for the 90% effective intervention is an example of disease suppression.
Fig. 4.
Steady-state individual risk is impacted by individual and population compliance. Cumulative average number of times infected (including reinfections) per individual under a 50% (a) or 90% (b) effective intervention. Three scenarios are simulated: full noncompliance, full compliance, and 70% compliance (with outcomes for compliant and noncompliant individuals shown)
Failure to suppress SARS-CoV-2 spread in the long-term results in persistent high disease burden
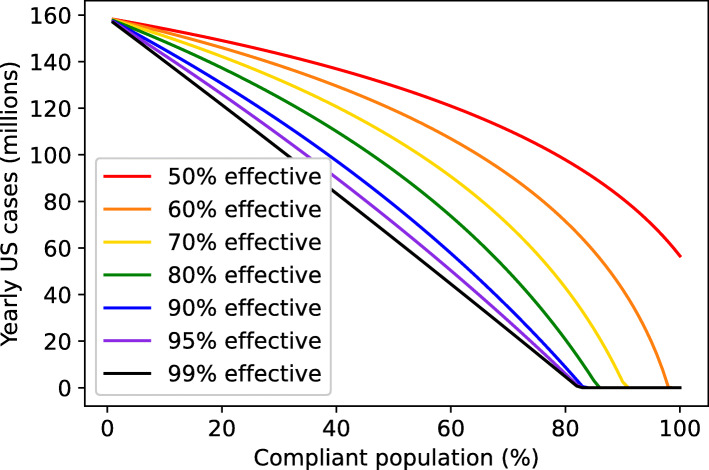
If complete suppression of disease is not achieved, a high annual disease burden persists indefinitely in most scenarios (Fig. 5). The marginal cost in terms of yearly cases for failures to suppress disease is highest for near-success cases and is steeply dependent on the degree of compliance (Fig. S1, see Supplementary Figures). This suggests that the best strategic objective for a stable return to pre-pandemic activity is complete suppression of SARS-CoV-2 spread.
Fig. 5.
Population-level impact of interventions is highly dependent on compliance. Yearly US cases at steady-state under interventions with varying degrees of efficacy and compliance
Complete suppression of SARS-CoV-2 spread requires high compliance and at least 60% efficacy
In Fig. 5, the steady-state yearly caseload of COVID-19 is plotted against the fraction of the population complying with a variety of theoretical interventions. For interventions with greater than 60% efficacy, complete suppression can be achieved if compliance is above a certain high threshold, depending on the intervention’s efficacy. For example, with an intervention with 70% efficacy, complete suppression can be achieved with at least 92% compliance. Increasingly effective interventions reduce the compliance threshold for complete suppression and reduce the yearly caseload in endemic scenarios. However, the impact of progressive improvements in efficacy shrinks as 100% efficacy is approached. Even for a 99% effective vaccine, greater than 80% compliance is required to achieve complete suppression of SARS-CoV-2. This suggests that a high degree of intervention efficacy cannot compensate for the epidemiological impact of a large noncompliant population.
Additionally, we note that the duration of immunity does not impact these compliance thresholds for achieving complete suppression (Fig. S3, see Supplementary Figures). However, the duration of immunity does impact the expected yearly disease burden. To further demonstrate this point, Figs. 2, 3, 4, 5, and S2 were reimplemented in the additional materials assuming a shorter (6-month) or longer (36-month) duration of immunity (Figs. S5-S14).
If complete suppression is not achieved, compliant populations remain at risk without a highly effective vaccine
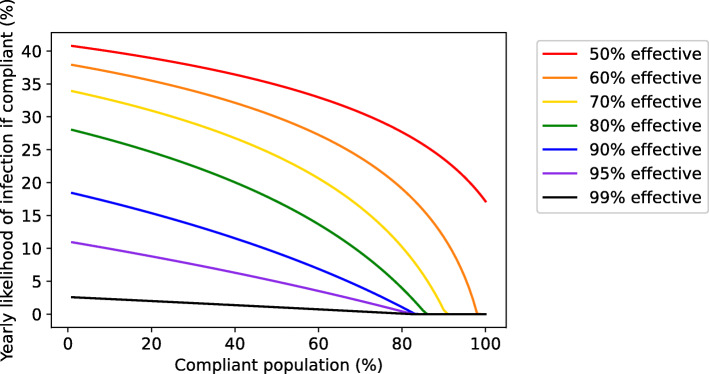
Although improvements in vaccine or intervention efficacy face diminishing returns on the population level and cannot fully compensate for poor compliance, compliant individuals stand to gain from even small improvements in efficacy (Fig. 6). This means that although the compliance threshold required for complete suppression may not shift substantially as efficacy improves, the incentive for individuals to comply or seek vaccination on a voluntary basis will increase as the efficacy increases.
Fig. 6.
More effective interventions provide greater benefit to compliant individuals if disease spread persists. Reduction in yearly likelihood of infection for compliant individuals as a function of the overall fraction of the population in compliance and the efficacy of the intervention
Discussion
Our work supports the case that noncompliance is embedded in human nature, as individuals optimizing their own self-interest can justify their actions in terms of their own perceived cost-benefit.
Individuals may perceive noncompliance as favorable for a number of reasons [38, 39]. They may view their own risk of being infected as lower than average (the optimism bias [40], which has been documented as a risk factor in predicting noncompliance for SARS-CoV-2 spread mitigation measures [41]), or they may view their own risk of adverse outcomes as a result of infection as being lower than average [27]. Globally, the public health messaging around noncompliance has focused on the low risk of death for younger individuals [42–44] and has invoked the imagery of “shielding” highly vulnerable populations from the disease [45] as an altruistic motive [46]. To the extent that many countries in the Americas and Western Europe at present are facing uncontrolled disease spread, it is likely that invoking altruism may not be the most effective means of disease control. Underestimating the risk of infection may also lead to individuals believing that noncompliance is the better choice.
The interplay between risk perception and compliance is complex, and fear may also play a paradoxical role in noncompliance. A number of studies have demonstrated a link between emotions and cognitive assessment of risk. In particular, high levels of fear coupled with a low sense of efficacy may create a defensive response in individuals who then proceed to dismiss the risk (“we’re all going to die anyway”) [47]. Studies have also shown that psychological affect plays a role in risk perception in individuals who are less comfortable and/or experienced interpreting probability [48, 49].
Regardless of the underlying causes, a Nash equilibrium of noncompliance creates a Tragedy of the Commons situation, where individuals acting according to their own self-interest create outcomes that are suboptimal for the common good by spoiling the shared resource through their collective actions. The term Tragedy of the Commons dates back to an influential article written over 50 years ago [50], which in turn was inspired by a nineteenth-century essay describing grazing practices of farmers. Tragedy of the Commons situations are indeed common in the fields of economics, politics, environmental policy and sociology. What makes Tragedy of the Commons situations particularly intractable is that it usually only takes a small proportion of individuals optimizing for their own self-interest to create devastating externalities for the rest of the population. This behavior underscores the limitations of the laissez-faire, individualistic approach to disease control during a pandemic. A laissez-faire approach is often said to lead to the best outcomes for the population overall as part of utilitarian economic theory [51], as put forward by John Stuart Mill. However such an approach actually violates the standard originally laid out by Mill by which a person’s liberty may be restricted: “The only purpose for which power can rightfully be exercised over any member of a civilized community, against his will, is to prevent harm to others” [52].
Given the ubiquity of the problem, some public policy solutions can be found that have close analogies to successful interventions in other spheres of human activity. First, public health messaging that seeks to alter the Nash equilibrium at an individual level are worth exploring. In individualistic societies, this may be accomplished by de-emphasizing altruism and focusing on the individual cost-benefit. One way this may be achieved is by emphasizing the long-term morbidity costs (such as cryptic heart, lung, brain and kidney damage) as have been documented to occur in even asymptomatic COVID-19 patients in an age-independent manner [53–55]. An additional approach is to provide an accurate and current picture of the risk of contracting the disease. Second, public health policy that creates costs for noncompliers may serve to shift some of the externalities back on to the originator (as was the case with mask ordinances during the 1918 Flu [4] and fines imposed for noncompliance with measures aimed at limiting SARS-CoV-2 spread in some countries [56, 57]). Third, public health interventions should engage at the level of the community. Public health and communications experts could test a number of different messages that underscore the downside of negative externality-creating behavior at a societal level. Some of these approaches have been used previously in the context of vaccine acceptance [58]. It is worth noting that our analysis points out a potential mechanism for the high levels of compliance observed in countries such as South Korea [23], with strong societal norms and a positive view of restrictions aimed at limiting SARS-CoV-2 spread such as mask-wearing [59, 60]. In these cultures, the prevailing cultural beliefs may serve to lower the cost of the intervention. In this context, we note that there is a modest association (Fig. S4, see Supplementary Figures) between societies with strong societal norms (“tight cultures” [61]) and the total case count per million at this point in the pandemic (p = 0.04).
From the perspective of biomedical interventions, our work points out that interventions with a high degree of protective efficacy are required for complete suppression of SARS-CoV-2, making this disease particularly challenging to control. Highly effective interventions have the dual effect of making the creation of negative externalities less beneficial for the noncompliant population (Fig. 4), and also increasing the benefit to the compliant population (Fig. 6). Notably, highly effective interventions provide more wiggle room for public policy, as the threshold level of compliance required for the complete suppression of SARS-CoV-2 spread drops from approximately 95% for a minimally effective intervention to approximately 80% for highly effective interventions. Another path to disease suppression lies in implementing passive interventions that reduce the R0, such as improving ventilation. Such passive interventions, not being subject to the problem of individual noncompliance, can serve to lower the bar for compliance for any given intervention to achieve complete suppression (Fig. S2, see Supplementary Figures).
Our work has a number of key limitations. First, we assume that the impact of biomedical and nonpharmaceutical interventions is not variable over time. In practice, changes in SARS-CoV-2’s transmissibility or response to interventions, such as seasonal fluctuations and genewration of new viral variants, will affect the long-term trajectory of the pandemic and are not accounted for in our model. Additionally, many factors may cause individuals to change their compliance behavior over time, which we also did not incorporate into our model. For example, in some settings, “pandemic fatigue” may drive increased noncompliance with nonpharmaceutical interventions over time [62], and relaxations of individual caution and public health guidelines may follow improvements in regional transmission, creating reactive variability in intervention effectiveness. Although biomedical interventions such as vaccines are less susceptible to variability in day-to-day decision-making, immunity to SARS-CoV-2 is expected to wane over a period of months [20–22], which can be expected to impact the duration of vaccine protection. Challenges in vaccine distribution and compliance may compound this waning immunity, reducing the apparent effectiveness of vaccines at the individual and population scale. Additionally, we assumed that compliant individuals have a reduced risk of transmission upon infection, equal to their reduction in risk of infection. This indirect benefit is challenging to measure in clinical trials, and preclinical studies show that some vaccine candidates are capable of reducing nasal viral load (and by implication, risk of transmission) in vaccinated animals [63, 64], while others are not [65, 66]. As additional data becomes available on evolution of SARS-CoV-2 and on the efficacy of interventions aimed at preventing SARS-CoV-2 spread, further studies on the impact of intervention noncompliance could provide more accurate predictions.
Taken together, our work demonstrates that noncompliance with measures to control SARS-CoV-2 spread is at once easy to justify on an individual level and leads to devastating public health consequences. Even under optimistic assumptions about the transmission benefit and durability of preventive interventions, noncompliance presents a significant obstacle to SARS-CoV-2 suppression. Three key messages are worth keeping in mind. First, the importance of focusing on complete suppression as a desirable end goal for SARS-CoV-2 (Fig. 5) and as a prerequisite for a return to a pre-pandemic lifestyle. SARS-CoV-2 is highly transmissible and can be expected to circulate at high rates if it becomes endemic. Second, the need for public policy to focus on compliance as a key prerequisite for both short-term suppression (Fig. 3) and long-term complete suppression (Fig. 5) of SARS-CoV-2 spread, and to seek ways to alter the space where compliance is the Nash equilibrium by increasing the cost of noncompliance. Finally, the need to focus on highly effective interventions from a biomedical perspective and to view partially effective prophylactics as contributors to the solution rather than the solution in its entirety.
It is our hope that this work draws the attention of the biomedical community to how high the bar is actually set for us to return to normalcy, and to public policymakers to highlight the need for concerted action that is focused on complete suppression of SARS-CoV-2 spread.
Conclusions
In this study, we analyzed the public health impact of noncompliance with nonpharmaceutical and biomedical interventions (such as masks and vaccines) designed to limit SARS-CoV-2 spread. Using a game-theoretic framework, we demonstrated that noncompliance can be rationalized in terms of benefit to the individual. We further demonstrated, using SEIRS model-based analyses that this noncompliance is a significant impediment to suppression of SARS-CoV-2 spread. The compliance threshold for achieving complete suppression of SARS-CoV-2 spread is a function of the intervention’s efficacy. Even for completely effective interventions, complete suppression requires a minimum of approximately 80% compliance. These findings demonstrate that a successful SARS-CoV-2 suppression strategy must involve highly effective interventions for which broad compliance can be achieved. Interventions that provide benefit to individuals in compliance may inspire greater uptake by shifting the externality onto noncompliant individuals.
Supplementary Information
Additional file 1: Table S1. COVID-19 Death Risk Assessment for South Dakota in August 2020. Table S2. COVID-19 Death Risk Assessment for South Dakota in November 2020. Figure S1. The marginal cost of noncompliance is highest as complete suppression is approached. Millions of additional yearly US cases per 1% reduction in compliance, as a function of the fraction of noncompliant individuals in the population for an intervention that is (A) 50% effective, (B) 70% effective, (C) 90% effective, or (D) 95% effective. Figure S2. Attainability of complete suppression depends on R0 and effectiveness of the intervention. Yearly US cases as a function of compliance for hypothetical scenarios in which R0 = 2, 3, 4 or 5.7. Figure S3. Duration of natural immunity impacts yearly disease burden but not the compliance threshold for complete suppression. Yearly predicted US cases at steady-state under a range of assumptions about the duration of natural immunity. Figure S4. Association between loose-tight score and COVID-19 cases per capita [7]. There is a weak association with loose-tight cultures and total case count observed based on reported case counts as of October. Figure S5. Complement to Fig. 2, with simulated 6-month duration of natural immunity. Panels A and D represent the fraction of the population, including both compliant and noncompliant individuals, that is susceptible, exposed, infectious, and recovered populations over time after a return to pre-pandemic conditions under (A-C) 95% compliance or (D-F) 50% compliance with a 50% effective intervention. Panels B and E demonstrate the fraction of compliant and noncompliant individuals who are infected over time. Panels C and F demonstrate the cumulative hazard ratio for infection in noncompliant versus compliant individuals. Figure S6. Complement to Fig. 3, with simulated 6-month duration of natural immunity. Total US COVID-19 infections in the next year under interventions with varying efficacy and compliance. Black box on panel A delineates region expanded in panel B. Total US infections in panel B are displayed on a log scale. Figure S7. Complement to Fig. 4, with simulated 6-month duration of natural immunity. Cumulative infections per individual under a 50% or 90% effective intervention. Three scenarios are simulated: full noncompliance, full compliance, and 70% compliance (with outcomes for compliant and noncompliant individuals shown). Figure S8. Complement to Fig. 5, with simulated 6-month duration of natural immunity. Yearly US cases at steady-state under interventions with varying degrees of efficacy and compliance. Figure S9. Complement to Figure S2, with simulated 6-month duration of natural immunity. Yearly US cases as a function of compliance for hypothetical scenarios in which R0 = 2, 3, 4 or 5.7. Figure S10. Complement to Fig. 2, with simulated 36-month duration of natural immunity. Panels A and D represent the fraction of the population, including both compliant and noncompliant individuals, that is susceptible, exposed, infectious, and recovered populations over time after a return to pre-pandemic conditions under (A-C) 95% compliance or (D-F) 50% compliance with a 50% effective intervention. Panels B and E demonstrate the fraction of compliant and noncompliant individuals who are infected over time. Panels C and F demonstrate the cumulative hazard ratio for infection in noncompliant versus compliant individuals. Figure S11. Complement to Fig. 3, with simulated 36-month duration of natural immunity. Total US COVID-19 infections in the next year under interventions with varying efficacy and compliance. Black box on panel A shows region expanded in panel B. Panel B is displayed on a log scale. Figure S12. Complement to Fig. 4, with simulated 36-month duration of natural immunity. Cumulative average number of times infected (including reinfections) per individual under a 50% (A) or 90% (B) effective intervention. Three scenarios are simulated: full noncompliance, full compliance, and 70% compliance (with outcomes for compliant and noncompliant individuals shown). Figure S13. Complement to Fig. 5, with simulated 36-month duration of natural immunity. Yearly US cases at steady-state under interventions with varying degrees of efficacy and compliance. Figure S14. Complement to Figure S2, with simulated 36-month duration of natural immunity. Yearly US cases as a function of compliance for hypothetical scenarios in which R0 = 2, 3, 4 or 5.7.
Acknowledgements
The authors would like to acknowledge Aparna Ramesh of Farmer Mac for suggesting the concept of a Nash equilibrium for noncompliance and Dr. Ankur Vora of the Bill & Melinda Gates Foundation for comments and input in the early modeling work described in this paper.
Abbreviations
- R0
Basic reproduction number
- SEIRS
Susceptible-Exposed-Infected-Recovered-Susceptible
- ODE
Ordinary differential equations
- NPI
Nonpharmaceutical intervention
Authors’ contributions
MS provided epidemiological modeling and related figures. DVE provided game theoretic modeling and related analysis. MS, DVE, and AC contributed substantially to manuscript writing. KJ, SR, JF, DW, RN, and NH read, revised, and approved the final manuscript. The author(s) read and approved the final manuscript.
Funding
No funding was received to support this research.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Please contact AC for simulation code.
Declarations
Ethics approval and consent to participate
Not applicable. No human subjects, human tissues, or human data were involved in the execution of this study.
Consent for publication
Not applicable.
Competing interests
AC, MS are employees of and shareholders in Fractal Therapeutics. DVE, DW and RN are advisors to and shareholders of Fractal Therapeutics. KJ, SR, JF, and NH have no conflicts of interest to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Masumbuko Claude K, Underschultz J, Hawkes MT. Social resistance drives persistent transmission of Ebola virus disease in eastern Democratic Republic of Congo: a mixed-methods study. PLoS One. 2019;14(9):e0223104. doi: 10.1371/journal.pone.0223104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balinska M, Rizzo C. Behavioural responses to influenza pandemics. PLoS Curr. 2009;1. 10.1371/currents.RRN1037. [DOI] [PMC free article] [PubMed]
- 3.Three shot in row over “Flu” Mask. In: Influenza encyclopedia. University of Michigan Center for the History of Medicine. 1918. http://hdl.handle.net/2027/spo.4710flu.0009.174. Accessed 25 Nov 2020.
- 4.Masking order is to be rigidly enforced. In: Influenza encyclopedia. University of Michigan Center for the History of Medicine. 1918. http://hdl.handle.net/2027/spo.0940flu.0010.490. Accessed 25 Nov 2020.
- 5.Flu mask or jail is choice In S. F. Today. In: Influenza encyclopedia. University of Michigan Center for the History of Medicine. 1919. http://hdl.handle.net/2027/spo.4220flu.0009.224. Accessed 25 Nov 2020.
- 6.New cases of influenza at low record. In: Influenza encyclopedia. University of Michigan Center for the History of Medicine. 1919. http://hdl.handle.net/2027/spo.4220flu.0009.224. Accessed 25 Nov 2020.
- 7.Dr. Beatty makes plain his position on mask question. In: Influenza encyclopedia. University of Michigan Center for the History of Medicine. 1918. http://hdl.handle.net/2027/spo.9830flu.0009.389. Accessed 25 Nov 2020.
- 8.Flu masking ordinance is turned down. In: influenza encyclopedia. University of Michigan Center for the History of Medicine 1919. http://hdl.handle.net/2027/spo.3360flu.0007.633. Accessed 25 Nov 2020.
- 9.Decline in “Flu” cases expected. In: Influenza encyclopedia. University of Michigan Center for the History of Medicine. 1919. http://hdl.handle.net/2027/spo.2510flu.0008.152. Accessed 25 Nov 2020.
- 10.“I will not be muzzled like a mad dog”: Face mask debate turns fierce in St. Lucie County. CBS 12. https://cbs12.com/news/local/st-lucie-county-to-discuss-countywide-mask-mandate-friday. Accessed 25 Nov 2020.
- 11.Disinformation and disease: social media and the ebola epidemic in the Democratic Republic of the Congo. Council on Foreign Relations. https://www.cfr.org/blog/disinformation-and-disease-social-media-and-ebola-epidemic-democratic-republic-congo. Accessed 25 Nov 2020.
- 12.Sell TK, Hosangadi D, Trotochaud M. Misinformation and the US Ebola communication crisis: analyzing the veracity and content of social media messages related to a fear-inducing infectious disease outbreak. BMC Public Health. 2020;20(1):550. doi: 10.1186/s12889-020-08697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobler S, Mack A, Mahmoud A, Lemon S. The threat of pandemic influenza: are we ready?: workshop summary. Washington, D.C: National Academies Press; 2005. [PubMed] [Google Scholar]
- 14.Gates B. The next epidemic — lessons from Ebola. N Engl J Med. 2015;372(15):1381–1384. doi: 10.1056/NEJMp1502918. [DOI] [PubMed] [Google Scholar]
- 15.He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. 2020. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KE, Stoddard M, Nolan RP, White DE, Hochberg N, Chakravarty A. This time is different: model-based evaluation of the implications of SARS-CoV-2 infection kinetics for disease control. medRxiv [Preprint] 2020. Available from: 10.1101/2020.08.19.20177550.
- 17.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suter P, Pargger H. Strong second COVID-19 wave calls for a second look at ICU triage guidelines. Swiss Med Wkly. 2020;150. 10.4414/smw.2020.20407. [DOI] [PubMed]
- 19.How hard is the coronavirus second wave hitting in Europe? | Euronews. https://www.euronews.com/2020/11/13/europe-s-second-wave-of-coronavirus-here-s-what-s-happening-across-the-continent. Accessed 25 Nov 2020.
- 20.Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, Mills R, Teng E, Kamruzzaman M, Garcia-Beltran WF, Astudillo M, Yang D, Miller TE, Oliver E, Fischinger S, Atyeo C, Iafrate AJ, Calderwood SB, Lauer SA, Yu J, Li Z, Feldman J, Hauser BM, Caradonna TM, Branda JA, Turbett SE, LaRocque RC, Mellon G, Barouch DH, Schmidt AG, Azman AS, Alter G, Ryan ET, Harris JB, Charles RC. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature. 2021;591:639-44. 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed]
- 22.Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5:eabe5511. [DOI] [PMC free article] [PubMed]
- 23.Bauch CT, Earn DJD. Vaccination and the theory of games. Proc Natl Acad Sci U S A. 2004;101(36):13391–13394. doi: 10.1073/pnas.0403823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zajenkowski M, Jonason PK, Leniarska M, Kozakiewicz Z. Who complies with the restrictions to reduce the spread of COVID-19?: personality and perceptions of the COVID-19 situation. Pers Individ Dif. 2020;166:110199. doi: 10.1016/j.paid.2020.110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miguel FK, Machado GM, Pianowski G, de Carvalho L. F. Compliance with containment measures to the COVID-19 pandemic over time: do antisocial traits matter? Pers Individ Dif. 2021;168:110346. doi: 10.1016/j.paid.2020.110346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuiper ME, de Bruijn AL, Folmer CR, Olthuis E, Brownlee M, Kooistra EB, et al. The intelligent lockdown: Compliance with COVID-19 mitigation measures in the Netherlands. PsyArXiv. 2020. 10.31234/osf.io/5wdb3.
- 27.Nivette A, Ribeaud D, Murray A, Steinhoff A, Bechtiger L, Hepp U, Shanahan L, Eisner M. Non-compliance with COVID-19-related public health measures among young adults in Switzerland: insights from a longitudinal cohort study. Soc Sci Med. 2021;268:113370. doi: 10.1016/j.socscimed.2020.113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behind the mask: Anti-mask and pro-mask attitudes in North America. Cascade Institute. https://cascadeinstitute.org/isc-brief/behind-the-mask-anti-mask-and-pro-mask-attitudes-in-north-america/. Accessed 25 Nov 2020.
- 29.Nash JF. Equilibrium points in n-person games. PNAS. 1950;36(1):48–49. doi: 10.1073/pnas.36.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocklöv J, Sjödin H, Wilder-Smith A. COVID-19 outbreak on the diamond princess cruise ship: estimating the epidemic potential and effectiveness of public health countermeasures. J Travel Med. 2020;27(3). 10.1093/jtm/taaa030. [DOI] [PMC free article] [PubMed]
- 32.Iyer AS, Jones FK, Nodoushania A, Kelly M, Becker M, Slater D, et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv [Preprint] 2020. Available from: 10.1101/2020.07.18.20155374.
- 33.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin J, Hamilton B, Osterman M, Driscoll A. Births: final data for 2018. Nat Vital Stat Rep. 2021;68:47. https://www.cdc.gov/nchs/data/nvsr/nvsr70/nvsr70-02-508.pdf. [PubMed]
- 35.Underlying Cause of Death, 1999-2018 Results. Centers for Disease Control and Prevention, National Center for Health Statistics 2020. https://wonder.cdc.gov/controller/datarequest/D76;jsessionid=82F1194E27F069D727A618AD3BC5. Accessed 25 Nov 2020.
- 36.Bajema KL, Wiegand RE, Cuffe K, Patel SV, Iachan R, Lim T, Lee A, Moyse D, Havers FP, Harding L, Fry AM, Hall AJ, Martin K, Biel M, Deng Y, Meyer WA, III, Mathur M, Kyle T, Gundlapalli AV, Thornburg NJ, Petersen LR, Edens C. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Intern Med. 2020;181(4):450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Understanding Non-Compliance During COVID-19: A Human Factors Perspective on Public Health Messaging. 2020. https://www.exponent.com/~/media/knowledge/thought-leadership/2020/08/understanding-non-compliance-during-covid/tl103_understanding-noncompliance-during-covid19%2D%2Da-human-factors-perspective-on-public-health-messaging.pdf. Accessed 25 Nov 2020.
- 39.The Masks We Wear (and Don’t Wear). Psychology Today. https://www.psychologytoday.com/blog/presence-mind/202006/the-masks-we-wear-and-don-t-wear. Accessed 25 Nov 2020.
- 40.Sharot T. The optimism bias. Curr Biol. 2011;21(23):R941–R945. doi: 10.1016/j.cub.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Wise T, Zbozinek TD, Michelini G, Hagan CC, Mobbs D. Changes in risk perception and self-reported protective behaviour during the first week of the COVID-19 pandemic in the United States. R Soc Open Sci. 2020;7(9):200742. doi: 10.1098/rsos.200742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html. Accessed 25 Nov 2020.
- 43.Coronavirus disease (COVID-19) Advice for the Public. World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed 25 Nov 2020.
- 44.Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890. doi: 10.1016/j.envres.2020.109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. Public Health England Department of Health & Social Care. https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19. Accessed 17 Dec 2020.
- 46.Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity. Lancet. 2020. 10.1016/S0140-6736(20)30918-1. [DOI] [PMC free article] [PubMed]
- 47.Bavel JJV, Baicker K, Boggio PS, Capraro V, Cichocka A, Cikara M, Crockett MJ, Crum AJ, Douglas KM, Druckman JN, Drury J, Dube O, Ellemers N, Finkel EJ, Fowler JH, Gelfand M, Han S, Haslam SA, Jetten J, Kitayama S, Mobbs D, Napper LE, Packer DJ, Pennycook G, Peters E, Petty RE, Rand DG, Reicher SD, Schnall S, Shariff A, Skitka LJ, Smith SS, Sunstein CR, Tabri N, Tucker JA, Linden S, Lange P, Weeden KA, Wohl MJA, Zaki J, Zion SR, Willer R. Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav. 2020;4(5):460–471. doi: 10.1038/s41562-020-0884-z. [DOI] [PubMed] [Google Scholar]
- 48.Peters E, Västfjäll D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychol Sci. 2006;17(5):407–413. doi: 10.1111/j.1467-9280.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 49.Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. SSRN scholarly paper. Rochester: Social Science Research Network; 2006. [DOI] [PubMed] [Google Scholar]
- 50.Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. doi: 10.1126/science.162.3859.1243. [DOI] [PubMed] [Google Scholar]
- 51.Fairchild A, Gostin L, Bayer R. Vexing, veiled, and inequitable: social distancing and the “rights” divide in the age of COVID-19. Am J Bioeth. 2020;20(7):55–61. doi: 10.1080/15265161.2020.1764142. [DOI] [PubMed] [Google Scholar]
- 52.Mill JS. On liberty and other essays. Oxford: Oxford Paperbacks; 2008.
- 53.Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2020;14(3):541-55. [DOI] [PMC free article] [PubMed]
- 54.Barber C. COVID-19 can wreck your heart, even if you Haven’t had any symptoms. Sci Am https://www.scientificamerican.com/article/covid-19-can-wreck-your-heart-even-if-you-havent-had-any-symptoms/. Accessed 26 Nov 2020.
- 55.Chang MC, Lee W, Hur J, Park D. Chest computed tomography findings in asymptomatic patients with COVID-19. Respiration. 2020;99:1–7. [DOI] [PMC free article] [PubMed]
- 56.Chae SH, Park HJ. Effectiveness of penalties for lockdown violations during the COVID-19 pandemic in Germany. Am J Public Health. 2020;110(12):1844–1849. doi: 10.2105/AJPH.2020.305903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mykhalovskiy E, Kazatchkine C, Foreman-Mackey A, McClelland A, Peck R, Hastings C, Elliott R. Human rights, public health and COVID-19 in Canada. Can J Public Health. 2020;111(6):975–979. doi: 10.17269/s41997-020-00408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisman DN, Laupland KB. The sounds of silence: public goods, externalities, and the value of infectious disease control programs. Can J Infect Dis Med Microbiol. 2009;20(2):39–41. doi: 10.1155/2009/946012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dighe A, Cattarino L, Cuomo-Dannenburg G, Skarp J, Imai N, Bhatia S, Gaythorpe KAM, Ainslie KEC, Baguelin M, Bhatt S, Boonyasiri A, Brazeau NF, Cooper LV, Coupland H, Cucunuba Z, Dorigatti I, Eales OD, van Elsland SL, FitzJohn RG, Green WD, Haw DJ, Hinsley W, Knock E, Laydon DJ, Mellan T, Mishra S, Nedjati-Gilani G, Nouvellet P, Pons-Salort M, Thompson HA, Unwin HJT, Verity R, Vollmer MAC, Walters CE, Watson OJ, Whittaker C, Whittles LK, Ghani AC, Donnelly CA, Ferguson NM, Riley S. Response to COVID-19 in South Korea and implications for lifting stringent interventions. BMC Med. 2020;18(1):321. doi: 10.1186/s12916-020-01791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Kim B, Lee J. Social distancing and outdoor physical activity during the COVID-19 outbreak in South Korea: implications for physical distancing strategies. Asia Pac J Public Health. 2020;32(6-7):360–362. doi: 10.1177/1010539520940929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gelfand MJ, Raver JL, Nishii L, Leslie LM, Lun J, Lim BC, Duan L, Almaliach A, Ang S, Arnadottir J, Aycan Z, Boehnke K, Boski P, Cabecinhas R, Chan D, Chhokar J, D'Amato A, Ferrer M, Fischlmayr IC, Fischer R, Fulop M, Georgas J, Kashima ES, Kashima Y, Kim K, Lempereur A, Marquez P, Othman R, Overlaet B, Panagiotopoulou P, Peltzer K, Perez-Florizno LR, Ponomarenko L, Realo A, Schei V, Schmitt M, Smith PB, Soomro N, Szabo E, Taveesin N, Toyama M, van de Vliert E, Vohra N, Ward C, Yamaguchi S. Differences between tight and loose cultures: a 33-nation study. Science. 2011;332(6033):1100–1104. doi: 10.1126/science.1197754. [DOI] [PubMed] [Google Scholar]
- 62.Pandemic Fatigue: Reinvigorating the public to prevent COVID-19. World Health Organization 2020. https://apps.who.int/iris/bitstream/handle/10665/335820/WHO-EURO-2020-1160-40906-55390-eng.pdf?sequence=3&isAllowed=y. Accessed 25 Nov 2020.
- 63.Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O’Connell S, Bock KW, Minai M, Nagata BM, Andersen H, Martinez DR, Noe AT, Douek N, Donaldson MM, Nji NN, Alvarado GS, Edwards DK, Flebbe DR, Lamb E, Doria-Rose NA, Lin BC, Louder MK, O’Dell S, Schmidt SD, Phung E, Chang LA, Yap C, Todd JPM, Pessaint L, van Ry A, Browne S, Greenhouse J, Putman-Taylor T, Strasbaugh A, Campbell TA, Cook A, Dodson A, Steingrebe K, Shi W, Zhang Y, Abiona OM, Wang L, Pegu A, Yang ES, Leung K, Zhou T, Teng IT, Widge A, Gordon I, Novik L, Gillespie RA, Loomis RJ, Moliva JI, Stewart-Jones G, Himansu S, Kong WP, Nason MC, Morabito KM, Ruckwardt TJ, Ledgerwood JE, Gaudinski MR, Kwong PD, Mascola JR, Carfi A, Lewis MG, Baric RS, McDermott A, Moore IN, Sullivan NJ, Roederer M, Seder RA, Graham BS. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383(16):1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A, Martinez DR, Loos C, Atyeo C, Fischinger S, Burke JS, Slein MD, Chen Y, Zuiani A, Lelis FJN, Travers M, Habibi S, Pessaint L, van Ry A, Blade K, Brown R, Cook A, Finneyfrock B, Dodson A, Teow E, Velasco J, Zahn R, Wegmann F, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kirilova M, Kordana N, Lin Z, Maxfield LF, Nampanya F, Nityanandam R, Ventura JD, Wan H, Cai Y, Chen B, Schmidt AG, Wesemann DR, Baric RS, Alter G, Andersen H, Lewis MG, Barouch DH. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sia SF, Yan L-M, Chin AW, Fung K, Choy K-T, Wong AY, et al. Pathogenesis and transmission of SARS-CoV-2 in golden Syrian hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020. 10.1101/2020.05.13.093195.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. COVID-19 Death Risk Assessment for South Dakota in August 2020. Table S2. COVID-19 Death Risk Assessment for South Dakota in November 2020. Figure S1. The marginal cost of noncompliance is highest as complete suppression is approached. Millions of additional yearly US cases per 1% reduction in compliance, as a function of the fraction of noncompliant individuals in the population for an intervention that is (A) 50% effective, (B) 70% effective, (C) 90% effective, or (D) 95% effective. Figure S2. Attainability of complete suppression depends on R0 and effectiveness of the intervention. Yearly US cases as a function of compliance for hypothetical scenarios in which R0 = 2, 3, 4 or 5.7. Figure S3. Duration of natural immunity impacts yearly disease burden but not the compliance threshold for complete suppression. Yearly predicted US cases at steady-state under a range of assumptions about the duration of natural immunity. Figure S4. Association between loose-tight score and COVID-19 cases per capita [7]. There is a weak association with loose-tight cultures and total case count observed based on reported case counts as of October. Figure S5. Complement to Fig. 2, with simulated 6-month duration of natural immunity. Panels A and D represent the fraction of the population, including both compliant and noncompliant individuals, that is susceptible, exposed, infectious, and recovered populations over time after a return to pre-pandemic conditions under (A-C) 95% compliance or (D-F) 50% compliance with a 50% effective intervention. Panels B and E demonstrate the fraction of compliant and noncompliant individuals who are infected over time. Panels C and F demonstrate the cumulative hazard ratio for infection in noncompliant versus compliant individuals. Figure S6. Complement to Fig. 3, with simulated 6-month duration of natural immunity. Total US COVID-19 infections in the next year under interventions with varying efficacy and compliance. Black box on panel A delineates region expanded in panel B. Total US infections in panel B are displayed on a log scale. Figure S7. Complement to Fig. 4, with simulated 6-month duration of natural immunity. Cumulative infections per individual under a 50% or 90% effective intervention. Three scenarios are simulated: full noncompliance, full compliance, and 70% compliance (with outcomes for compliant and noncompliant individuals shown). Figure S8. Complement to Fig. 5, with simulated 6-month duration of natural immunity. Yearly US cases at steady-state under interventions with varying degrees of efficacy and compliance. Figure S9. Complement to Figure S2, with simulated 6-month duration of natural immunity. Yearly US cases as a function of compliance for hypothetical scenarios in which R0 = 2, 3, 4 or 5.7. Figure S10. Complement to Fig. 2, with simulated 36-month duration of natural immunity. Panels A and D represent the fraction of the population, including both compliant and noncompliant individuals, that is susceptible, exposed, infectious, and recovered populations over time after a return to pre-pandemic conditions under (A-C) 95% compliance or (D-F) 50% compliance with a 50% effective intervention. Panels B and E demonstrate the fraction of compliant and noncompliant individuals who are infected over time. Panels C and F demonstrate the cumulative hazard ratio for infection in noncompliant versus compliant individuals. Figure S11. Complement to Fig. 3, with simulated 36-month duration of natural immunity. Total US COVID-19 infections in the next year under interventions with varying efficacy and compliance. Black box on panel A shows region expanded in panel B. Panel B is displayed on a log scale. Figure S12. Complement to Fig. 4, with simulated 36-month duration of natural immunity. Cumulative average number of times infected (including reinfections) per individual under a 50% (A) or 90% (B) effective intervention. Three scenarios are simulated: full noncompliance, full compliance, and 70% compliance (with outcomes for compliant and noncompliant individuals shown). Figure S13. Complement to Fig. 5, with simulated 36-month duration of natural immunity. Yearly US cases at steady-state under interventions with varying degrees of efficacy and compliance. Figure S14. Complement to Figure S2, with simulated 36-month duration of natural immunity. Yearly US cases as a function of compliance for hypothetical scenarios in which R0 = 2, 3, 4 or 5.7.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Please contact AC for simulation code.