Abstract
Nanocarrier-based delivery systems can be used to increase the safety and efficacy of active ingredients in medical, veterinary, or agricultural applications, particularly when such ingredients are unstable, sparingly soluble, or cause off-target effects. In this review, we highlight the diversity of nanocarrier materials and their key advantages compared to free active ingredients. We discuss current trends based on peer-reviewed research articles, patent applications, clinical trials, and the nanocarrier formulations already approved by regulatory bodies. Although most nanocarriers have been engineered to combat cancer, the number of formulations developed for other purposes is growing rapidly, especially those for the treatment of infectious diseases and parasites affecting humans, livestock, and companion animals. The regulation and prohibition of many pesticides have also fueled research to develop targeted pesticide delivery systems based on nanocarriers, which maximize efficacy while minimizing the environmental impact of agrochemicals.
Keywords: nanocarrier, nanomedicine, veterinary medicine, drug delivery, gene delivery, clinical trials, cancer, precision farming, pest management, agrochemical delivery
Graphical Abstract
Over the past 30 years, nanoparticle engineering has led to the development of delivery systems for active ingredients with medical, veterinary, and agricultural applications. The increasing cost of research and development combined with the growing number of competitive manufacturing entities, short patent cycles, and the tightening regulatory guidelines for active ingredients have made it difficult to bring additional formulations from the bench to the market.1,2 Furthermore, the efficacy of many drugs is limited by their low solubility and/or stability as well as off-target effects following systemic delivery. For example, cancer therapy is often unsuccessful due to the toxicity of cancer drugs toward healthy cells and/or the development of resistant cells overexpressing efflux transporters and multidrug-resistance proteins.3,4 The resulting low bioavailability of the active ingredient in the tumor requires the administration of larger doses to ensure the drug concentration stays within the therapeutic window, which in turn increases off-target toxicity. Nanocarriers can address this challenge by delivering active ingredients via the enhanced permeability and retention (EPR) effect, a well-established phenomenon based on the combination of leaky vasculature and poor lymphatic drainage at the tumor site.5 The EPR effect is complex and often heterogeneous within the tumor microenvironment and only increases the tumor homing of nanoparticles by 2-fold compared to normal tissue;6 nanoparticles can also be functionalized with targeting ligands, aptamers, antibodies, or antibody fragments to promote their binding to receptors overexpressed on tumor cells or in the surrounding extracellular matrix.7,8 As reviewed elsewhere, two other translocation mechanisms of nanocarriers from the systemic circulation into the tumor microenvironment are being investigated, namely the interendothelial and transendothelial pathways.9,10 The entrapment of active ingredients in nanocarriers also reduces the clearance rate via renal elimination and phagocytosis, which increases the active ingredient circulation time and therefore its therapeutic longevity.
The medical and veterinary applications of nanocarriers are analogous, but only experimental veterinary applications have been reported.11 Most research in veterinary drug delivery has focused on diseases in animals that can be translated to humans. However, the importance of animal welfare per se is increasingly important to consumers, and nanocarriers that improve the efficacy and safety of active ingredients are demanded in the context of companion animals such as cats, dogs, and horses as well as farm animals such cattle, sheep, swine, and poultry.12 Pet owners consider companion animals as an extension of the family and are willing to pay their bills, including the high cost of cancer treatment, with the cost of veterinary care in the USA therefore rising from $7 billion in 2001 to $19 billion in 2019.13 This increase most likely reflects a combination of inflation, high drug costs, better treatment options (with higher survival rates), and an increased willingness to care for pets. In contrast, the food industry works with low profit margins and would only treat animals suffering from temporary and low-risk diseases, such as infections.14 Veterinary nanocarriers must therefore combine low costs with the release of active ingredients for sustained periods to minimize the frequency of animal handling and improve therapeutic efficacy. For example, animals are often subject to bacterial infections, and a nanomedicine approach could achieve the targeted delivery of drugs to pathogens, killing them on demand. This avoids the unnecessary use of antibiotics, which can encourage the emergence of resistant strains.
The controlled delivery of agrochemicals and nutrients to plants is conceptually similar to drug delivery in humans and animals. However, agricultural delivery takes place in an open field, with variable weather and geographic features and no specific transport pathway to the target, in contrast to the closed and regulated nature of the bloodstream. Nanocarriers can be administered via the foliage, where they are taken up passively through stomata and any wounds, or can be transported through the soil and taken up via the roots.15 Among the agrochemicals that can be delivered using nanoparticles, pesticides are particularly suitable candidates because they are effective at very low doses (grams per acre) but are difficult to apply in such small amounts due to their non-uniform distribution in the field.16 To compensate, the active ingredient can be diluted within a mixture of liquid or solid diluents. However, the active ingredient is often unstable, sparingly soluble, and binds with high affinity to soil particles, thus reducing its efficacy against target pests and increasing the amount required to achieve an effective dose.17 In an analogous manner to the off-target effects caused by systemic drugs, the persistence of large quantities of pesticides in the environment is toxic to other species and contaminates the soil and groundwater, leading to health problems in domestic animals and humans, including cancer and infertility.18,19 Governments have therefore started to prohibit many pesticides or strictly regulate their use. In one strategy, the active ingredient is enveloped in organic or inorganic coatings (microencapsulation) for protection against photolysis or biodegradation, allowing the controlled release of the ingredient.20 But even microencapsulation is limited by the poor chemical and thermal stability of the capsules, and degradation promotes the acidification of soil, which can impair its fertility. As discussed in more detail below, these drawbacks can be addressed by the next generation of nanocarriers based on polymers, lipids and other materials.
The definition of a nanomaterial is not yet harmonized, but the International Organization for Standardization (ISO) defines nanoparticles as objects with dimensions of 1–100 nm, because the physicochemical properties of the material at this scale differ from the bulk material. Unfortunately, this ISO definition excludes most nanomaterials that are relevant in the medical, veterinary, and agricultural sectors. A less stringent definition would include any materials with at least one dimension in the range of 1–100 nm, thus including nanowires and nanotubes.21 In this review, we consider nanocarriers with at least one dimension smaller than 1000 nm and their use for the targeted delivery of active ingredients (drugs or pesticides) to achieve greater efficacy. The review focuses on the translation of nanocarriers from the bench to the market in the medical, veterinary, and agricultural industries in order to describe the current landscape and potential future directions for active ingredient delivery systems. Specifically, we discuss research articles (retrieved from PubMed and the Web of Science), patents (retrieved from Orbit Express using Questel software), clinical trials (listed in the clinicaltrials.gov database), and commercially available nanocarrier formulations approved by (1) the US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA) for medical use; (2) the American Veterinary Medical Association (AVMA) for use in animals, i.e., products listed in the Approved Animal Drug Products (Green Book) and/or AVMA Animal Health Studies database; and (3) the US Environmental Protection Agency (EPA), i.e., products listed in the National Pesticide Information Center database. Nanoparticles have also been used for diagnosis, drug discovery, gene delivery, immunotherapy, photothermal/photodynamic therapy, and biosensor applications, which are reviewed elsewhere.22–27
FROM IDEA TO COMMERCIAL PRODUCT
Trends in Active Ingredient Delivery.
The term “nanotechnology” was coined in 1974 by the Japanese scientist Norio Taniguchi,28 but the worldwide proliferation of nanotechnology started in the 1990s and has thus far led to the publication of more than 60,000 research articles in the pharmaceutical and environmental sciences. More than 93% of these articles relate to medical research, whereas nanoparticles in agriculture and veterinary research emerged later in the 2010s and represent only ~4% and ~3% of the publications, respectively (Figure 1a). Even so, the growing political and consumer interest in global food security and environmental protection is likely to drive additional research in the use of nanocarriers in agriculture and veterinary research in the future. Nanoparticle-based innovations also account for more than 150,000 patents (Figure 1b). Since the Bayh–Dole (Patent and Trademark Law Amendments) Act was ratified in the United States in 1980, allowing small businesses, nonprofit institutions, and universities to own inventions created via research funded by the federal government, the majority of these patents have been filed by universities. The University of California is the largest patent holder in this field, with more than 1200. Interestingly, 16% of all nanotechnology patents (~24,000) are held by the pharmaceutical sector, highlighting the growing interest in nanoparticles for drug delivery, diagnosis, and imaging.
Figure 1.

Timeline and corresponding distribution of: (a) peer-reviewed publications involving nanoparticles in the medical, veterinary, and agricultural fields; (b) patent applications involving nanoparticles; (c) clinical trials using nanocarriers based on different materials; and (d) market approved nanocarrier formulations. See Methods section for more information on the data compilation and analysis.
Nanocarriers for medical and veterinary applications are regulated by the FDA in the United States and the EMA in Europe. Since 1990, the FDA and EMA have approved 21 nanocarriers (Table 1), and more candidates are undergoing preclinical and clinical testing (Table 2). Most of these nanocarriers are administered orally or intravenously, and some transdermally. The materials used in these formulations include polymers, micelles, liposomes, proteins, and viruses. Most clinical trials (48%) involve micellar formulations, whereas viruses account for only 1% (Figure 1c). In contrast, most approved nanocarriers are based on liposomes (47%), followed by viral (19%), micellar (14%), polymeric (10%), and proteinaceous (10%) formulations (Figure 1d). Most of these nanocarriers have demonstrated lower toxicity rather than improved efficacy compared to the active ingredient alone.29 Accordingly, nanocarriers may not survive clinical trials because they do not achieve greater efficacy and because the reduction in toxicity might be achieved using an already-approved nanocarrier formulation.
Table 1.
FDA and EMA Approved Medical Nanocarriers
| Liposomal nanocarriers | ||||
|---|---|---|---|---|
| Drug name | Active ingredient | Nanoparticle size | Specific treatment | Year of approval |
| (company) | ||||
| Ambisome (Gilead Sciences) | amphotericin B | 80–120 nm | fungal infection | 1997 [US] |
| Curosurf (Dey Laboratories) | poractant α | 50–1000 nm | respiratory distress syndrome | 1998 [US] |
| DaunoXome (Galen) | daunorubicin | 35–65 nm | Kaposi’s sarcoma | 1996 [US] Discontinued in 2016 |
| Doxil (Janssen) | doxorubicin | 50–100 nm | Kaposi’s sarcoma | 1995 [US] - 1996 [EU] |
| breast cancer | 1996 [EU] | |||
| ovarian cancer | 2005 [US] - 1996 [EU] | |||
| myeloma | 2008 [US] - 1996 [EU] | |||
| Marqibo (Onco TCS) | vincristine | 100 nm | acute lymphoblastic leukemia | 2012 [US] |
| Myocet (Teva UK) | doxorubicin | 150–250 nm | metastatic breast cancer | 2000 [EU] |
| Onpattro (Ainylam Pharmaceuticals) | transthyretin-directed small interfering RNA | 80 nm | hereditary transthyretin-mediated amyloidosis | 2018 [US] - 2018 [EU] |
| Onivyde | irinotecan | 90–130 nm | pancreatic cancer | 2015 [US] - 2016 [EU] |
| (Merrimack) | ||||
| Visudyne | verteporfin | 150–300 nm | macular degeneration | 2000 [US] - 2000 [EU] |
| (Novartis) | ||||
| Vyxeos (Jazz Pharmaceuticals) | daunorubicin and cytarabine | 100–200 nm | acute myeloid leukemia | 2017 [US] - 2018 [EU] |
| Polymeric nanocarriers | ||||
| Drug name | Active ingredient | Nanoparticle size | Specific treatment | Year of approval |
| (company) | ||||
| Eligard | leuprolide acetate | N.A. | prostate cancer | 2002 [US] |
| (Tolmar) | in PLGA | |||
| Welchol | colesevelam hydrochloride | N.A. | type 2 diabetes | 2000 [US] |
| (Daiichi-Sankyo) | in allylamine polymer | |||
| Micellar nanocarriers | ||||
| Drug name | Active ingredient | Nanoparticle size | Specific treatment | Year of approval |
| (company) | ||||
| Estrasorb (Novavax) | 17β-estradiol | 20–80 nm | menopausal therapy | 2003 [US] |
| Taxol (Bristol Myers Squibb) | paclitaxel | 20–80 nm | ovarian cancer | 1992 [US] |
| breast cancer | 1994 [US] | |||
| Kaposi’s sarcoma | 1997 [US] | |||
| non-small-cell lung cancer | 1998 [US] | |||
| Taxotere (Sanofi-Aventis) | docetaxel | 20–80 nm | breast cancer | 1996 [US] - 1995 [EU] |
| non-small-cell lung cancer | 1999 [US] - 1995 [EU] | |||
| prostate cancer | 2004 [US] - 1995 [EU] | |||
| gastric cancer | 2006 [US] - 1995 [EU] | |||
| head and neck cancer | 2006 [US] - 1995 [EU] | |||
| Proteinaceous nanocarriers | ||||
| Drug name | Active ingredient | Nanoparticle size | Specific treatment | Year of approval |
| (company) | ||||
| Abraxane (Celgene) | albumin-bound paclitaxel | 130 nm | breast cancer | 2005 [US] - 2008 [EU] |
| non-small-cell lung cancer | 2012 [US] - 2005 [EU] | |||
| pancreatic cancer | 2013 [US] - 2008 [EU] | |||
| Ontak (Eisai) | diphtheria toxin fragments + human interleukin-2 | 58 kDa | cutaneous T-cell lymphoma | 1999 [US] Discontinued in 2014 |
| Viral nanocarriers | ||||
| Drug name | Active ingredient | Nanoparticle size | Specific treatment | Year of approval |
| (company) | ||||
| Glybera (UniQure) | AAV1 vector containing lipoprotein lipase gene | 22 nm | lipoprotein lipase deficiency | 2012 [EU] Discontinued in 2017 |
| Imlygic (Amgen) | Herpes simplex type I | 110–200 nm | melanoma | 2015 [US] - 2015 [EU] |
| Luxtuma (Spark Therapeutics) | AAV2 vector containing RPE65 cDNA | 22 nm | RPE65 mutation associated retinal dystrophy | 2017 [US] - 2018 [EU] |
| Zolgensma (Novartis) | AAV9 vector containing SMN1 gene | 22 nm | spinal muscular atrophy | 2019 [US] |
Table 2.
Medical Nanocarriers in Clinical Trials
| Liposomal nanocarriers | ||||||
|---|---|---|---|---|---|---|
| Active ingredient | Nanoparticle | Specific treatment | Start | Phase | Status | ClinicalTrials.gov ID |
| alprostadil | liposome | cardiovascular diseases | 2010 | I | completed [2010] | NCT02889822 |
| (irinotecan HCl:floxuridine) | CPX-1 | colorectal neoplasms | 2006 | II | completed [2008] | NCT00361842 |
| gemcitabine | FF-10832 | solid tumors | 2018 | I | recruiting | NCT03440450 |
| alendronate | liposome | coronary artery stenosis | 2008 | II | completed [2015] | NCT00739466 |
| annamycin | liposome | acute lymphocytic myelogenous leukemia | 2007 | I | terminated [2009] | NCT00430443 |
| myeloid leukemia | 2018 | I/II | recruiting | NCT03315039 | ||
| 2018 | I/II | recruiting | NCT03388749 | |||
| cyclosporine A | liposome | bronchiolitis obliterans | 2019 | III | recruiting | NCT03657342 |
| 2019 | III | recruiting | NCT03656926 | |||
| 2012 | I/II | completed [2015] | NCT01650545 | |||
| adenosylcobalamin | HL-009 | mild to moderate atopic dermatitis | 2012 | II | completed [2013] | NCT01568489 |
| latanoprost | liposome | ocular hypertension | 2013 | I/II | completed [2013] | NCT01987323 |
| meglumine antimoniate and paromomycin | liposome | cutaneous leishmaniasis | 2011 | I | completed [2012] | NCT01050777 |
| BoNT-A (botulinum toxin A) | liposome | interstitial cystitis | 2014 | IV | completed [2017] | NCT02247557 |
| overactive bladder | 2010 | II | completed [2013] | NCT01167257 | ||
| docetaxel | LE-DT | pancreatic cancer | 2010 | II | completed [2011] | NCT01186731 |
| prostate cancer | 2010 | II | withdrawn [2011] | NCT01188408 | ||
| mitoxantrone hydrochloride | liposome | metastatic breast cancer | 2015 | II | recruiting | NCT02596373 |
| neoplasms | 2011 | I | completed [2013] | NCT02043756 | ||
| Relapsed-refractory diffuse large B cell lymphoma and peripheral T/NK cell lymphoma | 2015 | II | recruiting | NCT02597387 | ||
| T cell lymphoma | 2015 | II | terminated [2019] | NCT02597153 | ||
| T cell and NK/T cell lymphoma | 2018 | II | recruiting | NCT03776279 | ||
| rhenium | liposome | glioblastoma/astrocytoma | 2015 | I/II | recruiting | NCT01906385 |
| SN-38 | liposome | colorectal cancer | 2006 | II | completed [2010] | NCT00311610 |
| lung cancer | 2016 | II | withdrawn [2016] | NCT00104754 | ||
| neoplasms | 2002 | I | completed [2010] | NCT00046540 | ||
| Polymeric nanocarriers | ||||||
| Active ingredient | Nanoparticle | Specific treatment | Start | Phase | Status | ClinicalTrials.gov ID |
| chlorhexidine gluconate | chitosan nanoparticles | intra canal antiseptic | 2018 | N.A | active | NCT03588351 |
| docetaxel | CriPec | solid tumor | 2015 | I | completed [2018] | NCT02442531 |
| platinum resistant ovarian cancer | 2018 | II | recruiting | NCT03742713 | ||
| rapamycin | eRapa | prostate cancer | 2018 | I | recruiting | NCT03618355 |
| cetuximab | ethylcellulose polymer coated with somatostatin analogue | colon cancer | 2018 | I | recruiting | NCT03774680 |
| N-acetylcysteine | hydroxyl dendrimer (OP-101) | inflammation | 2018 | I | completed [2018] | NCT03500627 |
| curcumin + doxorubicin | IMX-110 | solid tumors | 2017 | I/II | recruiting | NCT03382340 |
| paclitaxel | polyethylozaxoline (PEOX) | solid tumors | 2018 | I | recruiting | NCT03537690 |
| 188Re | poly-l-lysine dendrimer (ImDendrim) | liver cancer | 2017 | N.A | recruiting | NCT03255343 |
| docetaxel | PSMA-targeted PLA (BIND-014) | prostate cancer | 2013 | II | completed [2016] | NCT01812746 |
| non-small-cell lung cancer | 2013 | II | completed [2016] | NCT01792479 | ||
| metastatic cancer | 2011 | I | completed [2016] | NCT01300533 | ||
| cervical cancer squamous cell carcinoma of the head and neck |
2015 | II | terminated [2016] | NCT02479178 | ||
| Micellar nanocarriers | ||||||
| Active ingredient | Nanoparticle | Specific treatment | Start | Phase | Status | ClinicalTrials.gov ID |
| paclitaxel | micelle (NK105) | breast cancer | 2012 | III | completed [2017] | NCT01644890 |
| NK105 + cisplatin | non-small-cell lung cancer | 2016 | III | active | NCT02667743 | |
| Genexol PM | non-small-cell lung cancer | 2009 | III | completed [2017] | NCT01023347 | |
| hepatocellular carcinoma | 2017 | II | recruiting | NCT03008512 | ||
| hepatocellular carcinoma | 2017 | II | recruiting | NCT03008512 | ||
| urothelial cancer | 2011 | II | completed [2011] | NCT01426126 | ||
| Genexol PM + carboplatin | ovarian cancer | 2011 | II | completed [2017] | NCT01276548 | |
| Genexol PM + gemcitabine | pancreatic cancer | 2016 | II | recruiting | NCT02739633 | |
| Genexol PM + gemcitabine | pancreatic cancer | 2009 | I | completed [2017] | NCT00882973 | |
| antioxidant-rich multivitamin supplement (AquADEKs) | Micelle | cystic fibrosis | 2009 | completed [2012] | NCT01018303 | |
| cisplatin | micelle (NC-6004) + pembrolizumab | head and neck cancer | 2018 | II | not recruiting | NCT03771820 |
| micelle (NC-6004) + 5-FU + cetuximab | head and neck cancer | 2017 | I | active | NCT03109158 | |
| micelle (NC-6004) + gemcitabine | non-small-cell lung cancer | 2014 | I | recruiting | NCT02240238 | |
| curcumin | micelle | metabolic syndrome | 2018 | N.A | recruiting | NCT03534024 |
| paclitaxel | micelle | psoriasis | 2000 | II | completed [2008] | NCT00006276 |
| Paxceed | rheumatoid arthritis | 2003 | II | completed [2008] | NCT00055133 | |
| SN-38 | poly(l-glutamic acid)-PEG (NK012) | solid tumors | 2007 | I | completed [2013] | NCT00542958 |
| triple-negative breast cancer | 2009 | II | completed [2015] | NCT00951054 | ||
| small-cell lung cancer | 2009 | II | completed [2013] | NCT00951613 | ||
| poly(l-glutamic acid)-PEG (NK012) + 5-FU | colorectal cancer | 2010 | I | completed [2014] | NCT01238939 | |
| poly(l-glutamic acid)-PEG (NK012) + carboplatin | triple-negative breast cancer | 2010 | I | completed [2014] | NCT01238952 | |
| Proteinaceous nanocarriers | ||||||
| Active ingredient | Nanoparticle | Specific treatment | Start | Phase | Status | ClinicalTrials.gov ID |
| dodecanol alkyl ester of bendamustine (RXDX-107) | albumin nanoparticle | solid tumors | 2015 | I | terminated [2018] | NCT02548390 |
| rapamycin | albumin nanoparticles (ABI-009) | mTOR-mutated cancer | 2016 | I | active | NCT02646319 |
| epilepsy intractable | 2018 | I | recruiting | NCT03646240 | ||
| glioblastoma | 2018 | II | recruiting | NCT03463265 | ||
| neuroendocrine tumors | 2018 | II | recruiting | NCT03670030 | ||
| malignant PEComa | 2015 | II | active | NCT02494570 | ||
| leigh syndrome | 2018 | II | not recruiting | NCT03747328 | ||
| bladder cancer | 2013 | I | recruiting | NCT02009332 | ||
| ABI-009 + folfox + bevacizumab | colorectal cancer | 2018 | I | recruiting | NCT03439462 | |
| ABI-009 + nivolumab | advanced sarcoma | 2017 | I | recruiting | NCT03190174 | |
| ABI-009 + pazopanib | soft tissue sarcoma | 2018 | I | not recruiting | NCT03660930 | |
| ABI-009 + pazopanib | soft tissue sarcomas | 2018 | I | active | NCT03660930 | |
| ABI-009 + pomalidomide + dexamethasone | myeloma | 2018 | I | not recruiting | NCT03657420 | |
| ABI-009 + temozolomide + irinotecan | recurrent or refractory solid tumors | 2016 | I | recruiting | NCT02975882 | |
The regulation of nanocarriers for agricultural applications is not yet harmonized because a clear definition of agricultural nanocarriers has not yet been agreed, which makes it difficult to determine how many products are already commercialized. Such products are overseen by the EPA in the United States and the European Commission in Europe. In 2011, the EPA approved a nanopesticide, but this nanosilver-based product was restricted to its use as an antimicrobial agent in clothing, not for agricultural applications. In contrast, an agricultural product consisting of tobacco mild green mosaic virus (TMGMV) was approved by the EPA in 2007 as a herbicide in the state of Florida for the treatment of tropical soda apple, an invasive weed.30,31 No commercialized agricultural nanoformulations for pesticide or fertilizer delivery are yet branded as nanocarriers. This is most likely a marketing strategy to deal with the unclear regulation of agricultural nanocarriers while ensuring public acceptance. However, 42 microencapsulated products (including microscale and nanoscale carriers) have been approved by the EPA (Table 3). Although most of these formulations are used as herbicides or insecticides, a growing body of literature has demonstrated the usefulness of nanocarriers based on clay, chitosan, silica, or zeolites for the delivery of fertilizers, as discussed elsewhere.32
Table 3.
EPA Approved Pesticide Nanocarriers
| Product name (Company) | Active ingredient | Specific treatment | Year of approval | Registration no. |
|---|---|---|---|---|
| Poridon (Neogen Corporation) | permethrin + piperonyl butoxide | insecticide, miticide | 1985 | 72726–1 |
| Sump Buddy WT antimicrobial (Dow Chemical) | 2,2-dibromo-3-nitrilopropionamide | algaecide, bacteriostatic, fungicide, microbicide | 1989 | 464–624 |
| Ezject (EZ-Ject) | glyphosate-isopropylammonium | herbicide | 1989 | 83220–1 |
| Whitmire PT 275 Dur-O-Cap (BASF) | chlorpyrifos | insecticide, miticide | 1993 | 499–367 |
| NoMate TPW MEC (Scentry Biologicals) | (E)-4-tridecen-l-yl acetate | biochemical pesticide | 1994 | 26638–28 |
| ReJeX-IT AG-36 (Avian Enterprises) | methyl antranilate | repellent | 1994 | 91897–3 |
| Command (FMC) | clomazone | herbicide | 1995 | 279–3150 |
| Sump Buddy MWF (Dow Chemical) | 2,2-dibromo-3-nitrilopropionamide | microbicide | 1996 | 464–632 |
| Optashield CS (BASF) | cyfluthrin | insecticide | 1998 | 499–477 |
| For-Mite (Mann Lake) | formic acid | miticide | 1999 | 61671–3 |
| Strategy (Loveland Products) | clomazone + ethalfluralin | herbicide | 2001 | 34704–836 |
| CheckMate DBM-F (Suttera) | (Z)-11-hexadecenal | biochemical pesticide | 2002 | 56336–35 |
| Evercide Esfenvalerate CS (McLaughlin Gormley King Company) | esfenvalerate | insecticide | 2005 | 1021–1815 |
| Ricemax (Riceco) | clomazone + propanil | herbicide | 2006 | 71085–25 |
| Apiguard (Vita Bee Health) | thymol | insecticide, miticide | 2006 | 79671–1 |
| Casoron CS (Macdermid Agricultural Solutions) | dichlobenil | herbicide | 2007 | 400–541 |
| LX417 lambda-Cyhalothrin (BASF) | lambda-cyhalothrin | insecticide | 2007 | 499–535 |
| MGK F-2926(P) (McLaughlin Gormley King Company) | cyphenothrin | insecticide, miticide | 2008 | 1021–1873 |
| TC 251B (BASF) | permethrin | insecticide | 2008 | 499–528 |
| CSI Chlorpyrifos (Control Solutions) | chlorpyrifos | insecticide | 2009 | 53883–264 |
| Declare (FMC Corporation) | gamma-cyhalothrin | insecticide | 2009 | 279–3571 |
| Instinct (Dow Agrosciences) | nitrapyrin | fertilizer | 2009 | 62719–583 |
| Mon 63410 Herbicide (Monsanto) | acetochlor | herbicide | 2010 | 524–591 |
| Warrior II (Syngenta) | lambda-cyhalothrin | insecticide, miticide | 2010 | 100–1112 |
| MGK Formula 2964 (McLaughlin Gormley King Company) | esfenvalerate + piperonyl butoxide + prallethrin | insecticide | 2011 | 1021–2574 |
| GAT Permethrin 25 CS (FMC) | permethrin | insecticide | 2011 | 279–9573 |
| CSI Permethrin 25 CS (Control Solutions) | permethrin | insecticide | 2011 | 53883–282 |
| Trinexapac-ethyl 1 ME (Syngenta) | trinexapac-ethyl | fungicide, plant growth regulator | 2011 | 100–1401 |
| Permatek 100 Encaps (Lonza) | bifentrin | insecticide | 2013 | 72616–8 |
| Chlorpyrifos 42 CS (FMC) | chlorpyrifos | insecticide | 2013 | 279–9574 |
| Deer-Ban (TR Labs) | coyote urine | repellent | 2013 | 91132–1 |
| Aqua-Tec (Silversan) | silver | algicide | 2013 | 91025–1 |
| Lambdastar Urban Cap (Farmhannong America) | lambda-cyhalothrin | insecticide | 2014 | 71532–33 |
| Force CS Insecticide (Syngenta) | tefluthrin | insecticide | 2014 | 100–1253 |
| Trupick 0.7 (Decco US Post-harvest) | 1-methylcyclopropene | pesticide | 2016 | 2792–79 |
| Clomazone 360 CS (Sipcam Agro USA) | clomazone | herbicide | 2016 | 60063–58 |
| EH-1594 Herbicide (PBI/Gordon Corporation) | 2,4-D, 2-ethylhexyl ester + dicamba + mecoprop-P + sulfentrazone | herbicide | 2017 | 2217–1018 |
| AiM-A Abamectin (Smartvet USA) | abamectin | insecticide | 2017 | 88050–3 |
| Oxon Clomazone 360 CS (Oxon Italia) | clomazone | herbicide | 2017 | 35915–25 |
| Fendona CS II (BASF) | alpha-cypermethrin | insecticide | 2018 | 7969–425 |
| Fenvastar Ecocap (Farmhannong America) | esfenvalerate | insecticide | 2018 | 71532-28-91026 |
| RightLine Pyraprop Mec (Rightline) | pyraclostrobin | fungicide | 2018 | 93051–2 |
Ingredients Delivered by Nanocarriers.
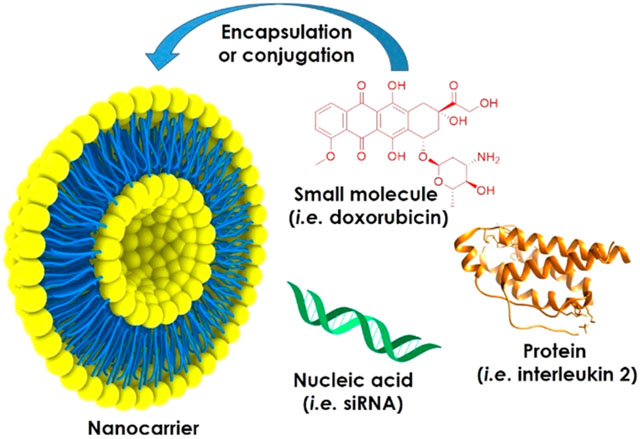
The cargos delivered by nanocarriers include small molecules, peptides and proteins, nucleic acids, or combinations of the above. Small molecules are low-molecular-weight organic compounds with beneficial biological activities, such as cancer drugs, antibiotics, fertilizers, and pesticides. Relevant examples in clinical and veterinary medicine include the antimetabolites paclitaxel and vincristine, the DNA intercalator doxorubicin, and the antibiotic amphotericin B.33–36 When delivered systemically, hydrophobic small molecules are rapidly metabolized and eliminated, narrowing their therapeutic window. Large doses are therefore required for therapeutic efficacy, which in the case of cancer drugs can lead to off-target effects such as cardiotoxicity and nephrotoxicity.37,38 Similarly, only a small fraction of pesticides and fertilizers applied to fields ever reach their target, due to leaching, evaporation, photolysis, chemical hydrolysis, and biodegradation. To feed the growing world population, today’s yields must increase by 60–100%, and this must be achieved in part by more effective treatment methods to eliminate pests and by increasing the efficiency of fertilizers.32
Peptide and protein pharmaceuticals may act as receptor agonists, essentially fulfilling the functions of endogenous molecules, whereas others act as antagonists. Neuroprotective proteins such as nerve growth factor and brain-derived neurotrophic factor are examples of agonists. They may help to combat Alzheimer’s disease and Parkinson’s disease, although they remain at the preclinical development stage.39 The blood–brain barrier (BBB) remains a major hurdle to deliver these proteins to the central nervous system, and nanocarriers have been engineered to deliver proteinaceous active ingredients across the BBB.40 Small peptides and proteins are often unstable in vivo due to the presence of proteases and may also be removed by renal filtration, reducing their bioavailability. Nanocarriers can also overcome this challenge. For example, the antimicrobial peptide HPA3PHis was delivered using aptamer-targeted gold nanoparticles, which led to the complete inhibition of Vibrio vulnificus colonization in infected mice.41 As well as stabilizing peptide and protein drugs with nanocarriers, multivalent display can be used as a strategy to enhance therapeutic efficacy, as demonstrated by the delivery of TNF-related apoptosis-inducing ligand (TRAIL) using liposomal and plant viral nanoparticle formulations.42,43 Monoclonal antibodies (mAbs) are a large class of protein drugs that often act as antagonists. For example, Herceptin is a mAb approved by the FDA for the treatment of HER2+ breast, gastric, and esophageal cancers by blocking HER2 receptor signaling.44 Herceptin is one of 59 therapeutic mAbs that have been approved since 1992, when mAb formulations were introduced.45 Four antibody–drug conjugates (ADCs) have also been approved, with another 22 currently undergoing clinical trials.46 Antibody–nanoparticle conjugates can be targeted to specific cells using the properties of nanocarrier, the antibody, or both. Similarly, the target specificity of the antibody can be combined with the cargo-loading capacity of nanoparticles, which has proven effective in many research studies but has yet to be deployed successfully in the clinic.47 Nanoparticle-mediated antibody delivery is particularly useful when the target is intracellular.48 For example, a liposomal nanocarrier was designed to display CD44-specific antibodies on the surface in order to target CD44+ cells and to carry a second IL6R-specific antibody as a cargo, which was taken up by the target cells to inhibit the intracellular IL6R-Stat3 signaling pathway in mice with triple-negative breast cancer. The treated mice showed a significant reduction in metastatic events.49
Like antibodies, peptides and proteins can also be used as targeting ligands to direct nanoparticles to disease sites. Such ligands displayed on nanoparticles promote cell binding, internalization, and endosomal escape, allowing the nanoparticles to accumulate and release their active ingredient within target cells while sparing healthy tissue from damage.50 However, actively targeted nanocarriers developed for the treatment of cancer have yet to progress beyond clinical trials.51 Targeted nanoparticles are more complex than their passive counterparts, which makes them more difficult to produce according to good manufacturing practice (GMP) and significantly increases the cost of therapy. Furthermore, it is unclear whether active targeting improves therapeutic efficacy. A meta-analysis of the literature over the past 10 years has shown that, on average, 0.9% of each dose of active nanocarriers reaches its target, compared to 0.6% for the passive nanocarriers.52
Finally, nucleic acids can be used as active agents in medical, veterinary, and agricultural applications, particularly DNA, microRNA (miRNA), and short interfering RNA (siRNA).32 Gene therapy in humans and domestic animals involves the delivery of DNA to the nucleus in order to augment or repair malfunctioning genes, whereas gene transfer to crops can introduce additional functionalities, such as resistance to pests and diseases, or pesticide tolerance.20,45 MicroRNA is noncoding RNA ~20 nucleotides in length that regulates endogenous genes, and the delivery of miRNA to the cytoplasm of target cells can be used to suppress the expression of disease-causing genes.53 The delivery of noncoding double-stranded dsRNA or siRNA derived from it promotes the assembly of a protein complex that binds complementary mRNA, leading to its cleavage and the targeted suppression of gene expression. The systemic delivery of miRNA and siRNA is generally ineffective because such molecules are rapidly degraded and cleared and often trigger an innate immune response, the exact nature of which is sequence dependent. Furthermore, miRNA and siRNA are hydrophilic and cannot penetrate the hydrophobic cell membrane to reach the cytoplasm. In the cytoplasm, they are rapidly degraded by nucleases, and multiple doses are therefore needed to suppress gene expression enough for a therapeutic effect. The drawbacks of conventional nucleic acid therapies can be addressed by encapsulating them in nanocarriers (Supporting Tables 1 and 2), and five such carriers have already reached the market (Table 1).
Genetic engineering has facilitated significant advances in human and veterinary medicine and has also helped to improve the yield of crops by enhancing pest and disease resistance and abiotic stress tolerance.54 Most genetically engineered plants are produced by transformation using the soil bacterium Agrobacterium tumefaciens, which introduces DNA via a type IV secretion system, or by biolistic delivery systems, which introduce DNA by physically penetrating the cell wall using high-velocity metal particles.55,56 Nanocarrier-based delivery systems would need to find an alternative strategy to pass through the cell wall, and current research is focusing on the size, charge, and surface properties of metallic, liposomal, silicon-based, and polymeric nanocarriers to enable cell wall penetration.54
The limitations of small molecules, peptides, proteins, and nucleic acids can be overcome by using nanocarriers to achieve three key goals: (1) enhance the aqueous solubility and therefore bioavailability of active ingredients; (2) increase the stability of active ingredients by inhibiting their degradation in vivo or in the environment, effectively increasing their half-life; and (3) promote the accumulation of the active ingredient at the target site. If all three goals are achieved, the dose of active ingredient required for efficacy is greatly reduced, thus limiting overall costs and avoiding off-target effects. These goals can be achieved using nanocarriers made from a wide range of materials (Table 2), which are discussed in more detail below.
Liposomal Nanocarriers.
Liposomes are spherical vesicles comprising one or more concentric lipid bilayers with an aqueous core.57 These amphiphilic structures are well-suited to entrap both lipophilic and hydrophilic compounds, making them attractive nanocarriers for a diverse range of active ingredients. Hydrophobic ingredients can be inserted into the lipid bilayer or sequestered in the core, whereas hydrophilic ingredients can be encapsulated in the core. The lipid bilayer is usually composed of phospholipids and sterols such as cholesterol, the latter controlling membrane permeability and fluidity.
In the medical and veterinary fields, conventional liposomal nanocarriers can reduce the off-target effects of active ingredients by modifying their pharmacokinetic properties and biodistribution, promoting their accumulation at the target site and avoiding nontarget tissues.58 Most liposomal nanocarriers deliver their cargo by fusing with the plasma membrane of the target cell, causing the active ingredient to be deposited in the cytoplasm. For example, Myocet is a conventional liposomal carrier approved by the EMA for the delivery of doxorubicin to metastatic breast cancer cells.59 Commercially available liposomal nanocarriers range from 30 to 1000 nm in diameter, which makes them the largest nanocarriers used in the clinic (Table 1). The physicochemical properties of liposomes are determined by the lipid composition, sterol concentration, surface charge, and nanoparticle size.60 Increasing the concentration of unsaturated phospholipids (e.g., phosphatidylcholine) ensures that the lipid bilayer is permeable, whereas saturated phospholipids (e.g., dipalmitoylphosphatidylcholine) make the barrier impermeable. By controlling the lipid composition and length of the fatty acid chains, liposomal nanocarriers can be engineered to respond to temperature and/or pH, allowing the controlled release of the active ingredient under physiological conditions that are specific to the disease site. The cellular uptake of liposomes can be also tuned by the lipid composition, which influences the overall surface charge.61,62 Neutral liposomes tend to remain in circulation longer and do not readily interact with cells, promoting the release of active ingredients in the extracellular space. This strategy is used by DaunoXome (for the delivery of daunorubicin to Kaposi’s sarcoma), Marqibo (for the delivery of vincristine to lymphoblastic leukemia), and Onivyde (for the delivery of irinotecan to pancreatic cancer cells).63–65 Whereas positively charged liposomes readily interact with the negative charge on the cell surface via electrostatic forces, neutral liposomes are prone to faster clearance. However, conventional liposomes of all types are rapidly eliminated from the bloodstream due to opsonization and uptake by Kupffer cells in the liver and spleen, which limits their bioavailability.66 This has been addressed by conjugating hydrophilic polymers to the liposome surface, such as polyethylene glycol (PEG) in the case of Doxil.67 Depending on the liposome formulation, PEGylation has been shown to increase the half-life of the active ingredient from 2 to 24 h in rodents and up to 45 h in humans, resulting in a 4–16-fold higher concentration at the target site.68 However, PEGylation can inhibit the interaction between liposomes and the cell surface, preventing fusion and uptake. Furthermore, passive targeting is limited by the heterogeneity of the EPR effect both within the tumor environment and when comparing different types of cancer. Ligand-targeted liposomes have therefore been engineered to promote site-specific binding. Low-molecular-weight molecules such as folate, as well as peptides and monoclonal antibodies or their fragments, have been incorporated onto the surface of liposomes to achieve targeted delivery.69,70 Liposomes also have the versatility to deliver multiple active ingredients simultaneously at a suitable molar ratio to maximize their synergistic interactions. The liposomal nanocarrier Vyxeos is thus far the only approved formulation that delivers more than one active ingredient, namely cytarabine and daunorubicin (5:1 molar ratio).70
Liposome nanocarriers reached the market in 1995, and since then 10 further products have been approved by the FDA/EMA for clinical use, mostly for combination cancer therapy (Table 1). Exceptionally, AmBisome (carrying amphotericin B) is indicated for fungal infections,71 Curosurf (carrying poractant α) is indicated for respiratory distress syndrome, and Visudyne (carrying verteporfin) is indicated for macular degeneration.72 The most recent addition is Onpattro, an siRNA delivery formulation approved in 2018 by the FDA.73 Onpattro encapsulates a transthyretin-directed siRNA for the treatment of amyloidosis.
Additional liposomal nanocarriers are undergoing clinical trials against a wide range of diseases, including ocular and topical applications (Table 2). Although many of the recent formulations are cancer therapies, the landscape of current trials highlights the potential of nanomedicine across the field. For example, liposomal alprostadil is a potent vasodilator that increases peripheral blood flow, inhibits platelet aggregation, and induces bronchodilation (NCT02889822); liposomal cyclosporine A is also being investigated as an inhaled delivery formulation for the treatment of bronchiolitis obliterans syndrome.74 Another example targeting cardiovascular disease consists of sodium alendronate encapsulated in liposomes of distearoylphosphatidylglycerol, 1,2-distearoyl-sn-glycero-3-phosphocholine, and cholesterol. Phase II testing is underway for the treatment of de novo stenotic lesions in native coronary arteries in patients undergoing percutaneous coronary intervention with implantation of a bare metal stent (NCT00739466). Furthermore, a topical gel nanoliposome formulation of vitamin B12 (adenosylcobalamin) is undergoing clinical trials to treat moderate atopic dermatitis (HL-009; NCT01568489), and liposomal latanoprostis being tested as a means to lower intraocular pressure in patients with open-angle glaucoma and ocular hypertension.75 Liposomal meglumine antimoniate and liposomal paromomycin are being investigated for the treatment of anthroponotic cutaneous leishmaniasis caused by Leishmania tropica in both humans and dogs.76
Additional liposomal nanocarriers are also undergoing veterinary trials (Table 4).77,78 In 2006, a phase I trial of doxorubicin encapsulated into temperature-sensitive liposomes was carried out in companion dogs suffering from spontaneous tumors.79 Injection of the nanocarrier followed by the induction of tumor hyperthermia caused 100% of the drug cargo to be released within 20 s at 41.3 °C. Of the 21 dogs enrolled in the study, 18 showed a decrease in tumor volume, including 12 with a decrease of more than 50%. Liposomal doxorubicin in combination with palliative radiotherapy improved the clinical outcome of cats with soft tissue sarcomas.80 Clodronate encapsulated in liposomes was able to eliminate malignant histocytosis in dogs.81 Liposome-encapsulated amphotericin B demonstrated high efficacy in dogs infected with the blastomyces fungus, while reducing the adverse effects often associated with amphotericin B.82
Table 4.
Nanocarriers for Veterinary Applications
| Approved veterinary nanocarriers | ||||
|---|---|---|---|---|
| Drug name (company) | Active ingredient | Nanoparticle | Specific treatment | Year of approval |
| Imrestor (Elanco US) | PEGylated granulocyte colony stimulating factor (pegbovigrastim) | 30 kDa protein + PEG | inflammation in the breast tissue of cows | 2016 [US] |
| Paccal Vet-Cal (Oasmia Pharmaceutical) | paclitaxel | retinoic acid (XR-17) micelle | canine mast cell tumors | 2017 discontinued |
| Veterinary nanocarriers undergoing clinical trials | ||||
| Active ingredient | Nanoparticle | Specific treatment | Start | Animal health studies ID/ref. number |
| amphotericin B | liposome | dogs injected with blastomyces fungus | 1996 | ref. 114 |
| cisplatin | hyaluronate nanoparticles | canine brain tumors | 2013 | AAHSD000024 |
| 2017 | AAHSD004339 | |||
| clodronate | liposome | canine histocytosis | 2010 | ref. 113 |
| DNA | cationic liposome-DNA complexes + losartan | canine metastatic osteosarcoma | 2016 | AAHSD004241 |
| doxorubicin | liposome | canine spontaneous tumors | 2006 | ref. 111 |
| liposome + radiotherapy | canine soft tissue sarcoma | 2010 | ref. 112 | |
| interleukin-12 | GEN-1 | canine brain tumors | 2016 | AAHSD000445 |
| paclitaxel | CTI 52010 | canine solid tumors | 2010 | AAHSD000021 |
| SOD1 gene silencing | adeno-associated virus | canine degenerative myelopathy | 2016 | AAHSD004063 |
| temozolomide | PLGA | canine brain tumors | 2015 | AAHSD000385 |
| vitamin E | micelle | equine oxidative stress during prolonged aerobic exercise | 2013 | ref. 74 |
Liposomes have also been used to facilitate the absorption of hydrophobic active ingredients via the cuticles of plants and insects.20,83 However, because they are so expensive to produce, agricultural applications are likely to be restricted for the foreseeable future. For example, Doxil costs $1313 for 5 mg (despite being on the market for two decades and the fact that doxorubicin costs only $8.5 for 5 mg), whereas newer formulations are even more expensive, such as Marquibo ($15,747 per 5 mg kit) and Onpattro ($9500 per vial, typical annual cost $345,000). Even so, a few studies have investigated the liposomal delivery of pesticides such as entofenprox.84 An alternative and less expensive solution may be the use of liposomes comprising plant-derived lipids (or alternative nanocarrier systems). This was proposed for the delivery of Fe and Mg to tomato plants, and 33% of the encapsulated metal was able to penetrate leaves and enter plant cells compared to 1% of the free active ingredient.85
Polymeric Nanocarriers.
Polymeric biomaterials are easy to produce at low cost and have therefore been developed and tested as inert shells to promote the accumulation and controlled release of active ingredients at a given target site. Natural polymers have been derived from chitosan, sodium alginate, collagen, heparin, and silk, whereas many different synthetic polymers have been tested, including, polyacrylate (PAL), PEG, polycaprolactone (PCL), polylactic acid (PLA), polyglycolic acid (PGA), polylactic-co-glycolic acid (PLGA), polyesters, and polyurethanes. Natural and synthetic polymers can be biocompatible and biodegradable, and their physicochemical properties (e.g., size, morphology, porosity, surface charge, surface chemistry, and hydrophilicity) are inherently flexible and can be tuned to control mechanical and physiological behavior.86
For clinical and veterinary applications, the nanocarrier shell must comprise linear or branched polymers with a molecular weight in the range 0.4–40 kDa to increase the circulation time of the active ingredient while ultimately ensuring renal elimination.87 PLGA is particularly promising as a nanocarrier material because the PLA-to-PGA ratio can be adjusted to control the rate of degradation, and thus the release rate of the active ingredient. Using this concept, Eligard was approved by the FDA in 2002 to deliver leuprolide acetate to prostate cancer cells (Table 1).88 PLGA nanocarriers are also being tested in the veterinary field for the delivery of Temozolomide to canine brain tumors (Table 4). The only other polymeric nanocarrier approved by the FDA is Welchol, an allylamine polymer that encapsulates colesevelam hydrochloride to lower the levels of sugar and low-density lipoproteins (LDL) circulating in adults suffering from type 2 diabetes and high cholesterol.89 Although the development pipeline for polymeric drug-delivery systems is moving rapidly, there is a puzzling lack of approvals. A possible explanation is that most polymeric drug delivery systems do not improve efficacy but rather enhance safety and thus do not achieve significant improvements over liposomal formulations or the free drug at a lower dose.
Dendrimers are a special class of highly branched polymeric nanocarriers with organized tree-like structures and a low polydispersity, ranging in size from 5 to 500 kDa.90–92 They comprise a central core that radiates a series of repeated branching units (generations), terminating with chemical groups available for functionalization. The active ingredient can be encapsulated in the core micelle via hydrophobic/electrostatic interactions or conjugated to the surface. The greater the number of branches, the more reactive terminal groups can be coupled with the active ingredient. Branches exposed on the surface can also be functionalized to increase tissue specificity.93 Most of the dendrimers used as nanocarriers are synthesized from hydrophilic polyamidoamine (PAMAM) or polypropylene imine units, which are not recognized by the immune system. Poly-l-lysine dendrimers are positively charged and are therefore ideal for the delivery of nucleic acids. Other dendrimers are being developed from PEG, polyglycerol, polyglycerol-co-succinic acid, poly-2,2-bis(hydroxymethyl)propionic acid, melamine, and triazine.93 Only two dendrimer-based nanocarriers are currently undergoing clinical trials (Table 2). One (OP-101) consists of N-acetyl cysteine covalently coupled to a metabolically stable inactive hydroxyl dendrimer and has been administered to healthy volunteers to determine its safety, tolerability, and pharmacokinetics. The other (imdendrim) is a poly-l-lysine dendrimer that encapsulates nitro-imidazole-methyl-1,2,3-triazol-methyl-di-[2-pycolyl]amine bound to a rhenium isotope (188Re) and is currently under investigation for the treatment of liver cancer.
Many other polymeric nanocarriers are undergoing clinical trials (Table 2). CriPec is a polymeric nanocarrier, 30–100 nm in diameter and of uncertain composition (Crystal Therapeutics does not disclose the details), which encapsulates docetaxel and is shielded by PEG. It is being tested for the treatment of solid tumors and platinum-resistant ovarian cancer. PEOX is a branched polymer shell composed of polyethyloxazoline, encapsulating paclitaxel for the treatment of solid tumors. PEOX circumvents the need to solubilize paclitaxel with Kolliphor EL (formerly Cremophor EL), a toxic solvent that requires the coadministration of antihistamines to prevent an immune response.34 IMX-110 is a polymeric nanoshell encapsulating curcumin and doxorubicin for the treatment of solid tumors. Following its release in the tumor environment, curcumin targets and inhibits the activation of the transcription factors STAT3 and NF-κB, which prevents apoptotic tumor resistance and enhances the efficacy of doxorubicin.94 Another example is eRapa, the protein kinase inhibitor rapamycin encapsulated in poly(methyl methacrylate), which is undergoing clinical testing for the treatment of prostate cancer.95 A nanocarrier for the delivery of cisplatin to canine brain tumors has been developed using hyaluronic acid, a linear polymer of alternating d-glucuronic acid and N-acetyl-d-glucosamine residues. Hyaluronic acid is a component of the extracellular matrix and is degraded by hyaluronidase, an enzyme overexpressed in the glioma microenvironment.96 Therefore, the nanocarrier accumulates in the tumor environment, where its degradation causes the local release of cisplatin to minimize systemic toxicity.
One formulation that did not progress beyond clinical trials is BIND-014, a PLA-PEG copolymer displaying a ligand targeting the extracellular domain of the prostate specific membrane antigen. Early studies in rats indicated that BIND-014 could delay tumor growth by preferentially delivering docetaxel to prostate cancer xenografts, limiting the accumulation of this drug in the liver and bone marrow.97 BIND-014 was applied in several clinical trials for the treatment of prostate cancer, nonsmall-cell lung cancer, cervical cancer, and head and neck cancer.98 Unfortunately, the trials did not demonstrate sufficient efficacy, with an objective response of only 10% in the head and neck cancer cohort. Pfizer acquired BIND Therapeutics for $40 million in 2016, but no further clinical trials have been reported.
Various synthetic and natural biodegradable polymers have also been synthesized for the delivery of agrochemicals. The formulations have been prepared using emulsion or double emulsion strategies as well as layer-by-layer deposition, nanoprecipitation, and solvent evaporation.20,99 Such formulations include nanospheres (where the active ingredient is uniformly distributed throughout the polymer matrix) and nanocapsules (where the active ingredient is encapsulated in the liquid inner core). These nanocarriers have been prepared from PEG, PCL, PAL, chitosan, or sodium alginate20 and have been used to deliver diverse pesticides, including ametryn,100 atrazine,100 acephate,101 emanectin benzoate,102 garlic essential oil,103 imidacloprid,104,105 lansiumamide B,106 methomyl,107 paraquat,108 and simazine.100 However, these formulations are currently at an early developmental stage and are still being tested in vitro as well as in field trials.
Polymers have also been used to prepare other forms of active ingredient delivery system, such as hydrogels,20,109,110 polymer–drug conjugates,111 and seed coatings.112 Hydrogels are cross-linked hydrophilic polymers with a high water retention capacity. A reservoir of the active ingredient may be present at the core of the hydrogel, or it may be uniformly dispersed. The controlled release of the active ingredient is achieved by regulating the physical properties of the hydrogel matrix, such as its porosity and swelling capacity. Environmental stimuli such as temperature, pH, ionic strength, and enzyme activity are often use to control the rate of polymer degradation to achieve the slow and sustained release of the active ingredient. Examples include the FDA-approved intracanalicular implant Dextenza, a dexamethasone-loaded PEG hydrogel for the treatment of ocular pain following ophthalmic surgery113 as well as hydrogel compositions containing dextran, PAL, propylene glycol, hyaluronic acid, or carboxymethyl cellulose.114
Although not technically a nanocarrier application, active ingredients are often conjugated to PEG in a process known as PEGylation, which increases the hydrophilicity and hydrodynamic radius of small-molecule drugs and proteins, thus improving their solubility, masking them from the immune system, slowing their renal clearance, and increasing their circulation half-life while retaining their bioactivity.115 Adagen, a PEGylated adenosine deaminase reached the clinic in 1990 to treat severe combined immunodeficiency disease. Since then, 16 more PEGylated drugs have been approved by the FDA/EMA, and the majority are indicated for cancer, hepatitis C, or hemophilia (Supporting Tables 3 and 5).111,116 To our knowledge, only one PEGylated drug has been approved for veterinary applications. This is Imrestor, a PEGylated granulocyte colony-stimulating factor, which was approved in 2016 to increase the number of circulating neutrophils in cows and thus prevent breast tissue inflammation (Table 2).117 Although PEGylated drugs have been successfully translated to the clinic, a growing body of literature has highlighted the increased presence of PEG-specific antibodies in the general population due to the extensive use of PEG in cosmetic and pharmaceutical products, correlating with the declining therapeutic efficacy of PEGylated active ingredients.118 This issue is being addressed by the development of alternative polymer-drug conjugates (Supporting Table 5).
In the agricultural industry, polymeric seed coatings are used to control pests and diseases that would otherwise inhibit germination and growth.112,119 Coating seeds increases their viability, reduces the risk of the active ingredient leaching into the environment, and minimizes off-target toxicity to other organisms compared to free pesticides. More than 180 coating formulations have been reported, including chitosan, polyvinyl acetate (latex), poly(vinyl alcohol), PEG, ethyl cellulose, and methyl cellulose.112 On the market, the majority of seed coating technologies have been developed by Bayer Crop Science, BASF, Corteva, Monsanto, Syngenta, Incotec/Croda, and Germains.
Micellar Nanocarriers.
Micelles are composed of amphiphilic surfactant molecules that spontaneously aggregate into spherical vesicles in an aqueous environment. This phenomenon is only possible if the quantity of the surfactant molecules is greater than the critical micelle concentration. The core of the micelle is hydrophobic and can sequester hydrophobic active ingredients. The size of the micelle and therefore the amount of active ingredient that can be loaded in its core is dependent on the molecular size, geometry, and polarity of the surfactant.45 The small size of polymeric micelles (20–80 nm) reduces their recognition by scavenging phagocytic and interendothelial cells located in the liver and spleen, respectively, and therefore increases the bioavailability of the active ingredient.120,121 Most micelles are made of block copolymers with alternating hydrophilic and hydrophobic segments, and the ratio of drug molecules to the block copolymers determines their properties. Micelles are often composed of PEG, PLA, PCL, polypropylene oxide, poly-l-lysine, or combinations of the above. Estrasorb was approved by the FDA in 2003 as a topical lotion and consists of micelles designed for the transdermal delivery of 17β-estradiol to the blood for the treatment of menopausal-related vasomotor symptoms.122 This administration route evades first-pass metabolism, achieving stable levels of 17β-estradiol in the serum for 14 days. Furthermore, paclitaxel and docetaxel are commercially available formulated as micellar nanocarriers, thus avoiding the use of Kolliphor EL as a solvent.123,124
Various micellar nanocarriers are currently undergoing clinical trials (Table 2). For example, NK012 is a micellar polyglutamate-PEG formulation covalently bound to the antineoplastic topoisomerase inhibitor SN-38 via an ester bond. SN-38 is slowly released from NK012 by the hydrolysis of the ester bond under physiological conditions, which increases the SN-38 half-life to 210 h. NK012 is undergoing clinical trials for the treatment of solid tumors, triple-negative breast cancer, colorectal cancer, and small-cell lung cancer.125 Similarly, the NK105 micelle is being investigated for the delivery of paclitaxel to breast cancer, gastric cancer, and nonsmall-cell lung cancer. NK105 polymers consist of PEG as the hydrophilic segment and modified polyaspartate as the hydrophobic segment.126 NK105 demonstrated efficacy in patients with advanced gastric cancer that failed to respond to chemotherapy. Genexol-PM is a micellar nanocarrier consisting of mPEG-block-d,l-PLA for the delivery of paclitaxel for the treatment of nonsmall-cell lung cancer, hepatocellular carcinoma, urothelial cancer, ovarian cancer, and pancreatic cancer.127 Genexol-PM was shown to behave similarly to the FDA/EMA-approved nanocarrier Abraxane and has been approved for the treatment of metastatic breast cancer and advanced nonsmall-cell lung cancer in South Korea. NC-6004 is being investigated for the delivery of cisplatin to head and neck cancer as well as nonsmall-cell lung cancer. NC-6004 demonstrated a significant reduction in cisplatin-induced neurotoxicity and nephrotoxicity (again, the nanocarrier enhances safety not efficacy). Micelles are also being investigated for the treatment of cystic fibrosis, metabolic syndrome, psoriasis, and rheumatoid arthritis.128
In veterinary medicine, a randomized trial was initiated in 2013 to investigate the safety and efficacy of micellar paclitaxel (Paccal Vet-CA1) for the treatment of dogs with grade II or III mast cell tumors (Table 4).129 The micelle consisted of a surfactant derivative of retinoic acid (XR-17). Dogs treated with micellar paclitaxel showed a 3-fold higher treatment response compared to a control group receiving the standard-of-care drug lomustine. However, the FDA conditional approval of Paccal Vet-CA1 was withdrawn in 2017 by the manufacturer Oasmia Pharmaceutical AB to allow them time to study lower doses in order to reduce adverse effects such as neutropenia, hepatopathy, anorexia, and diarrhea. In a different application, a micellar vitamin E has been tested as an antioxidant in race horses undergoing prolonged aerobic exercise to prevent exercise-induced oxidative lesions and maintained the general oxidative status to a healthy level for horses undergoing intensive training.130
Micelles have also been developed as promising nanocarriers for the encapsulation of pesticides, helping to prevent adsorption to soil particles. Examples include the micellar encapsulation of azadirachtin,131 carbendazim,132 carbofuran,133 imidacloprid,134,135 rotenone,136 thiamethoxam,137 and thiram.138 These formulations are still undergoing development and have been tested in vitro and in the field.
Inorganic Nanocarriers.
Inorganic nanocarriers include natural and synthetic materials based on silica, clay, and metals such as silver, gold, titanium, iron, copper, and zinc. These nanocarriers are physiologically compatible, resistant to microbial degradation, and environmentally friendly, which makes them suitable for medical, veterinary, and agricultural applications. Even so, their use as nanocarriers has been somewhat overshadowed by their success in other medical applications. In particular, metallic nanoparticles have been developed as theranostic and photothermal reagents and for the treatment of iron deficiency.24,139,140 In 1974, the FDA approved iron dextran (INFeD) for the treatment of iron deficiency. Eight more formulations have since been approved by the FDA or EMA (Supporting Table 5). We do not consider these formulations as nanocarriers because the treatment modalities rely entirely on the nanoparticle itself without a cargo of active ingredients. However, metallic nanocarriers have recently been proposed in which the active ingredient is attached to the surface by physical absorption, electrostatic interactions, or conjugation.141 In particular, gold nanoparticles allow the conjugation of many biological ligands, including DNA and siRNA.142 Thus far, only one clinical trial has been carried out using metallic nanocarriers, namely spherical nucleic acid gold nanoparticles (NU-0129) for the delivery of siRNA to patients with glioblastoma or gliosarcoma (Supporting Table 1). More advanced metallic nanocarriers are under development, including particles that can respond to external triggers, such as light, magnetic fields, and hyperthermia to release their cargo in a controlled manner. For example, gold and silver nanoparticles have been conjugated to various cancer drugs.143–146
Mesoporous silica nanocarriers (MSNs) have been investigated extensively because they are stable particles with a high payload capacity due their porous structure, they have a tunable pore diameter (2–50 nm), and surface modifications can impart additional functionalities such as targeted delivery.147 MSNs have already been tested in the laboratory to deliver cancer drugs such as doxorubicin and camptothecin, antibiotics such as erythromycin and vancomycin, and anti-inflammatories such as ibuprofen and naproxen, with high loading rates of up to 600 mg of cargo per gram of silica.147 This loading capacity of up to 60% far exceeds that of liposomal and polymeric nanocarriers. For example, the liposomal formulation Doxil and the polymeric formulation Eligard achieve loading capacities of 31% and 27%, respectively. However, some silica nanoparticle formulations have been shown to cause hemolysis due to strong interactions between silanol groups on the carrier and phospholipids in the erythrocyte plasma membrane.148 Another concern is their persistence in vivo due to the absence of renal clearance. These issues could be addressed by modifying the surface chemistry or applying coatings.
In an agricultural context, silica is already highly abundant in soil, and such particles could therefore be engineered for the controlled release of active ingredients without the carrier itself causing environmental harm. For example, MSNs have been used to deliver the insecticide chlorfenapyr over a period of 20 weeks, which doubled the insecticidal activity in field tests.149 The fungicide metalaxyl was also loaded into MSNs, allowing its slow release in soil and water for a period of 30 days.150 Similarly, nanocarriers based on naturally occurring aluminum silicates have been formed into phyllosilicate sheets for the intercalation of antibiotics and herbicides, allowing sustained delivery.151,152 Also in the context of delivery of antiviral nucleic acid therapeutics, sustained delivery of the therapeutic is desired. For example, application of small interfering RNAs onto leaves provided protection against plant viral infection for ~6 days; the therapeutic window was greatly enhanced achieving antiviral activity for ~20 days by making use of layered double hydroxide clay nanosheets.153 Several metallic nanoparticles have demonstrated antimicrobial properties, and the EPA has already approved silver nanoparticles for use as an antimicrobial agent in clothing, but not yet for the delivery of active ingredients.142 Finally, carbon nanotubes are also being investigated for medical and agricultural uses because their shape and surface chemistry confer advantageous properties, although their toxicity remains a translational barrier. We recommend the following reviews for further information found in refs 154–156.
Proteinaceous Nanocarriers.
Over the course of evolution, nature has yielded a variety of biomaterials with great structural complexity that remains difficult to emulate. The analysis of such complexity requires the appropriate molecular methods, and for this reason, the development of proteinaceous nanocarriers has lagged behind that of the simpler liposomal, polymeric, and micellar structures.157,158 The production of proteinaceous nanocarriers has also required the development of tools for the expression of recombinant proteins and strategies for creation or diversity, such as directed evolution, genome editing, and synthetic biology. These tools have allowed the production of hierarchically organized proteinaceous structures, including albumin nanoparticles, heat shock protein cages, vault proteins, and ferritins.159 These comprise repeated protein subunits forming highly organized nanostructures that are identical in size and chemical composition. Although synthetic nanoparticles can also be assembled into complex structures, the sophistication and monodispersity that can be achieved with proteins has yet to be replicated. Proteinaceous nanoparticles have been used as biocatalysts for the synthesis of materials, but are also useful for the delivery of active ingredients in medicine and agriculture.158
Early on, proteinaceous nanocarriers were developed to mimic the properties of plasma proteins, thus increasing circulation times and reducing systemic side effects. In 2005, the FDA approved the proteinaceous nanoshell Abraxane, consisting of albumin-bound paclitaxel for the treatment of breast cancer. The conjugation of paclitaxel to albumin stabilized the drug even in the absence of Kolliphor EL and enhanced the uptake of the active ingredient compared to the Kolliphor EL formulation.34 Given the safety and efficacy of drugs conjugated to albumin, two other albumin nanocarriers are undergoing clinical trials (Table 2). One is an albumin conjugate of the protein kinase inhibitor rapamycin (ABI-009) indicated for colorectal cancer, bladder cancer, glioblastoma, sarcoma, and myeloma.160 The other is an albumin conjugate of docetaxel (ABI-008) indicated for the treatment of prostate cancer. Albumin has a long circulation half-life due to its interaction with the recycling Fc receptor. It is beneficial for the delivery of small molecules that are unstable or have low solubility in blood as well as proteins and peptides that are rapidly cleared from the circulation. Small molecules can be chemically fused to albumin and administered as conjugate, and strategies to target small-molecule drug cargoes to albumin in vivo have also been developed.161
Heat shock protein cages, vault proteins, and ferritins have also been investigated for the delivery of active ingredients, although no clinical trials have been reported thus far. Heat shock proteins are chaperones that promote the folding of newly synthesized proteins and the refolding of denatured ones, which means they are naturally stable and possess channels and cavities for the sequestration of cargo.159 There are five families of heat shock proteins: Hsp100, Hsp90, Hsp70, and Hsp60 (which are named for the molecular mass of the proteins in kDa) and the small heat shock protein (sHsp) family, ranging in size from 12 to 43 kDa. Heat shock proteins assemble into large complexes that vary in size and shape (including rings and spheres), and they can be engineered to carry and deliver active ingredients such as doxorubicin.162,163 Vault nanoparticles are barrel-like ribonucleoproteins found in many eukaryotes. They are 41 × 73 nm in size and resemble the vault of a gothic cathedral. Their precise biological function remains unknown, although they are thought to play a role in nuclear transport, immunity, and defense against toxins.164,165 Several proteins have been encapsulated in vault nanocarriers, including the lymphoid chemokines CCL19 and CCL21, the New York esophageal squamous cell carcinoma 1 (NY-ESO-1) antigen, the precursor of adenovirus protein VI (pVI), the major outer membrane protein of Chlamydia trachomatis, and the egg storage protein ovalbumin.165 Vault Pharma is one company specializing in the development of these structures. Finally, ferritin is an iron-storage protein with 24 subunits that self-assemble into a spherical cage structure 12 nm in diameter with a molecular mass of 450 kDa.166 Each ferritin complex can sequester up to 4500 Fe2+ ions and convert them to Fe3+ to prevent oxidative stress in the cytosol, nucleus, and mitochondria. Ferritin has been investigated as an imaging reagent and vaccine platform as well as a nanocarrier.166 It has already been used to deliver cisplatin,167 doxorubicin,168 and curcumin,169 and the contrast agents gadolinium170 and Mn(II).171
There are few examples of proteinaceous nanocarriers used in agriculture, but nanocarriers based on maize storage proteins (zeins) are being tested for the delivery of pesticides that protect soybean crops from defoliator parasites.172
The number of proteinaceous nanocarriers reaching the market will continue to grow as we learn more from nature and expand our bioengineering tools and processing capabilities, including the use of genome editing and directed evolution.173 Furthermore, rather than harnessing protein complexes from nature, advances in de novo protein design will allow us to select customized proteins with shapes that may be difficult to obtain via the directed evolution of natural proteins.174 Modular building concepts have been established to achieve the defined folding and programmed assembly of proteins into complex architectures.175,176 Accordingly, some synthetically designed protein-based nanoparticles have entered translational development. For example, the start-up company Tychon Bioscience is developing prosthetic antigen receptors that modulate protein dimerization to produce self-assembling nanoscale ring structures for applications in cancer immunotherapy.
Virus-based Nanocarriers.
Viruses have evolved to deliver their genetic payload to host cells and can therefore be regarded as nature’s nanocarrier systems. The structure of a virus capsid is genetically programmed so replication yields millions of identical particles, a level of monodispersity that cannot yet be achieved with synthetic nanoparticles. Viruses are proteinaceous structures and are therefore similar to the protein cages discussed above in terms of biocompatibility. The capsids are highly symmetrical structures that come in various shapes and sizes, and they are amenable to both chemical and genetic modification to impart additional functionalities, including the encapsulation or conjugation of active ingredients177
Given the natural function of viruses, it is unsurprising that one of the early applications of virus-based nanocarriers was the delivery of nucleic acids. Mammalian viruses such as Adeno-associated virus (AAV) are established as gene delivery vectors.178 The AAV-based gene therapy vector (Glybera) was approved by the EMA in 2012 for the treatment of lipoprotein lipase deficiency, but was not approved by the FDA, and UniQure subsequently announced its withdrawal from the European market in 2017 following the treatment of only 31 patients.179 The FDA has since approved two AAV-based vectors, namely Luxturna in 2017 for the treatment of patients with RPE65 mutation-associated retinal dystrophy, and Zolgensma in 2019 for the treatment of young infants with spinal muscular atrophy. One of the major drawbacks of AAV therapies is their high cost. Luxturna treatment is estimated to cost $850,000, whereas Zolgensma was priced at $2.125 million by Novartis as a one-time cure. In addition to AAV nanocarriers (ssDNA, 4-kb inserts), other mammalian viruses have been developed for gene delivery including adenoviruses (dsDNA, 7.5-kb inserts), herpesviruses (dsDNA, >30 kb inserts), and lentiviruses (ssRNA, 8 kb inserts) and are undergoing clinical trials (Supporting Table 2), whereas retroviruses (ssRNA, 8 kb inserts) and alphaviruses (ssRNA, 6–8 kb inserts) remain at the preclinical development stage.178,180,181
As an alternative to mammalian viruses, several plant viruses and bacteriophages have also been repurposed as nanocarriers or vaccines because they are noninfectious to humans and can be manufactured on a large scale as viral nanoparticles (VNPs, which retain the virus genome) or virus-like particles (VLPs, which are empty shells devoid of nucleic acid). For example, based on the immunostimulatory nature of VNPs/VLPs, several have been developed as in situ cancer vaccines, including Cowpea mosaic virus (CPMV),182,183 bacteriophage M13,184 Potato virus X (PVX),185 Tobacco mosaic virus (TMV),186 and Papaya mosaic virus.187 The CPMV system has already demonstrated efficacy in canine trials.188–190 The development of plant viruses or bacteriophages as vaccine candidates for infectious diseases, autoimmune disorders, and cancer has been extensively reviewed.191–193 One of the key applications of VNP/VLP vaccine candidates is the display of heterologous epitopes.194–196 This protects the epitope from degradation, ensures delivery to antigen-presenting cells, provides an inbuilt adjuvant, and also generates cross-stimulatory virus-based antigens to boost humoral and cellular immunity.15–17 Several VNPs/VLPs presenting heterologous epitopes have been tested in human clinical trials197 and veterinary tests.198
The use of VNPs and VLPs as nanocarriers for active ingredients can be achieved by passive infusion through pores in the capsid, encapsulation during assembly, as well as chemical conjugation and/or genetic fusion of active ingredients to the outer or inner surfaces. For example, doxorubicin has been conjugated to TMV,199 infused into Cucumber mosaic virus (CMV)200 and Red clover necrotic mosaic virus (RCNMV),201 and passively complexed with PVX.202 Virus-based nanocarriers have also been used to deliver bortezomib,203 cisplatin,204,205 5-fluorouracil,206 hygromycin,207 mitoxantrone,208,209 phenanthriplatin,210 and paclitaxel.211 Examples of protein delivery include TRAIL,42 TPA,212 and Herceptin.213,214 Filamentous phages have been developed to deliver antibiotics such as chloramphenicol and neomycin to prevent the growth of Escherichia coli, Streptococcus pyogenes, and Staphylococcus aureus.215,216 Finally, siRNA has been delivered using bacteriophage MS2217 and Cowpea chlorotic mosaic virus (CCMV),218 and mRNA for the in situ expression of green fluorescent protein (GFP) has been successfully encapsulated into CCMV and TMV, followed by the release of the mRNA cargo into the cytosol of mammalian cells and its subsequent translation.219,220
Plant VNPs have also been proposed as pesticide carriers because they are already part of the natural soil ecosystem and are harmless to humans and domestic animals.221,222 Compared to synthetic nanocarriers, plant viruses are highly mobile in soil and can deliver pesticides to the roots, where many pests are concentrated. TMGMV was approved by the EPA in 2007 as a bioherbicide for the treatment of the invasive tropical soda apple weed in the state of Florida.30 Since then, RCNMV has been proposed for the delivery of abamectin to crops.221 The higher stability and superior soil mobility of abamectin encapsulated in RCNMV increased the efficacy of the nematicide in tomato seedlings infested with root knot nematodes (Meloidogyne hapla) compared to the free chemical. Similarly, TMGMV loaded with the anthelmintic drug crystal violet was highly toxic toward the nematode Caenorhabditis elegans in vitro.222 Rational design and size and shape engineering in plant virus-based carriers may enable multilevel targeting of different soil zones.223
CONCLUSIONS
Nanocarriers have revolutionized the medical, veterinary, and agricultural sectors through their ability to deliver active ingredients in a targeted and controlled manner to appropriate sites, such as cancer cells or plant roots, thereby maximizing efficacy while minimizing off-target effects. Based on the current landscape of research articles, patents, clinical trials, and approved nanocarriers, we have revealed a growth trend which predicts that more formulations will be commercially available over time, allowing the targeted delivery of small molecules, peptides, and proteins as well as nucleic acids to combat pests, pathogens, and diseases in animals and plants. We observed a shift away from the development of nanocarriers for small-molecule reagents and toward the delivery of peptides, proteins, and nucleic acids. Advances in bioengineering have also encouraged the development of bioinspired nanocarriers that are friendly to the environment.
Several challenges must be addressed to streamline the translation of nanocarriers from the bench to the market. In medicine, nanocarriers could greatly benefit from the incorporation of targeting ligands, aptamers, antibodies, or antibody fragments to promote their binding to receptors overexpressed on target cells or in the surrounding extracellular matrix. Additional targeted nanocarriers must undergo clinical trials before we can conclude that active targeting achieves greater therapeutic efficacy. In parallel, the development of veterinary nanocarriers has intensified with the growing public interest in animal welfare and food safety and security. Companion animals with cancer have benefited the most from nanocarriers, whereas livestock require low-cost and prolonged treatments, such as the delivery of antibiotics. The future of veterinary nanocarriers will depend on our ability to manufacture products at a relatively low cost. Finally, precision agriculture is required to meet the growing demand for food. Nanocarriers provide the opportunity to increase crop yields in an environmentally friendly manner by delivering fertilizers and pesticides directly to plants while minimizing leaching. However, the translation of nanocarriers for agricultural applications is restricted by the lack of well-structured regulations for commercial approval. More research is therefore required to determine the fate and toxicity of nanocarriers applied in the field.
METHODS
All patent searches were carried out with Orbit Express using Questel software. Initially, the key word “nanoparticle” was used with a time frame starting from 1990 to the present. The total number of patent families for this search was grouped and plotted according to the year of publication and the application field based on World Intellectual Property Organization (WIPO) patent classifications. Approved clinical drugs were extracted and compiled from the Food and Drug Administration (FDA) and European Medicines Agency (EMA) databases. Nanocarriers undergoing clinical trials were mined from the ClinicalTrials.gov database, excluding any trials whose clinical status was unknown. Approved veterinary nanocarriers were mined from the Approved Animal Drug Products (Green Book) on the FDA website, whereas nanocarriers undergoing veterinary trials were compiled from the American Veterinary Medical Association (AVMA) Animal Health Studies database. Federally approved nanoencapsulated pesticides are listed on the National Pesticide Information Center (NPIC) database (NPRO). Peer-reviewed publications were searched using PubMed and the Web of Science database using keywords: nanoparticle, medicine, agriculture, and veterinary.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the following grants to N.F.S.: CAREER DMR 1841848 from the National Science Foundation and R01 CA202814, R01 HL137674, R01CA224605, U01CA218292 from the National Institute of Health and 128319-RSG-15-144-01-CDD from the American Cancer Society. O.A.O.-R. acknowledges the UC MEXUS-CONACYT Postdoctoral Fellowship 2019-2020 number FE-19-58. We would like to thank David Wirth (UC San Diego) for helping with Figure 2 to show the liposome, micelle, and albumin nanoparticles.
Figure 2.
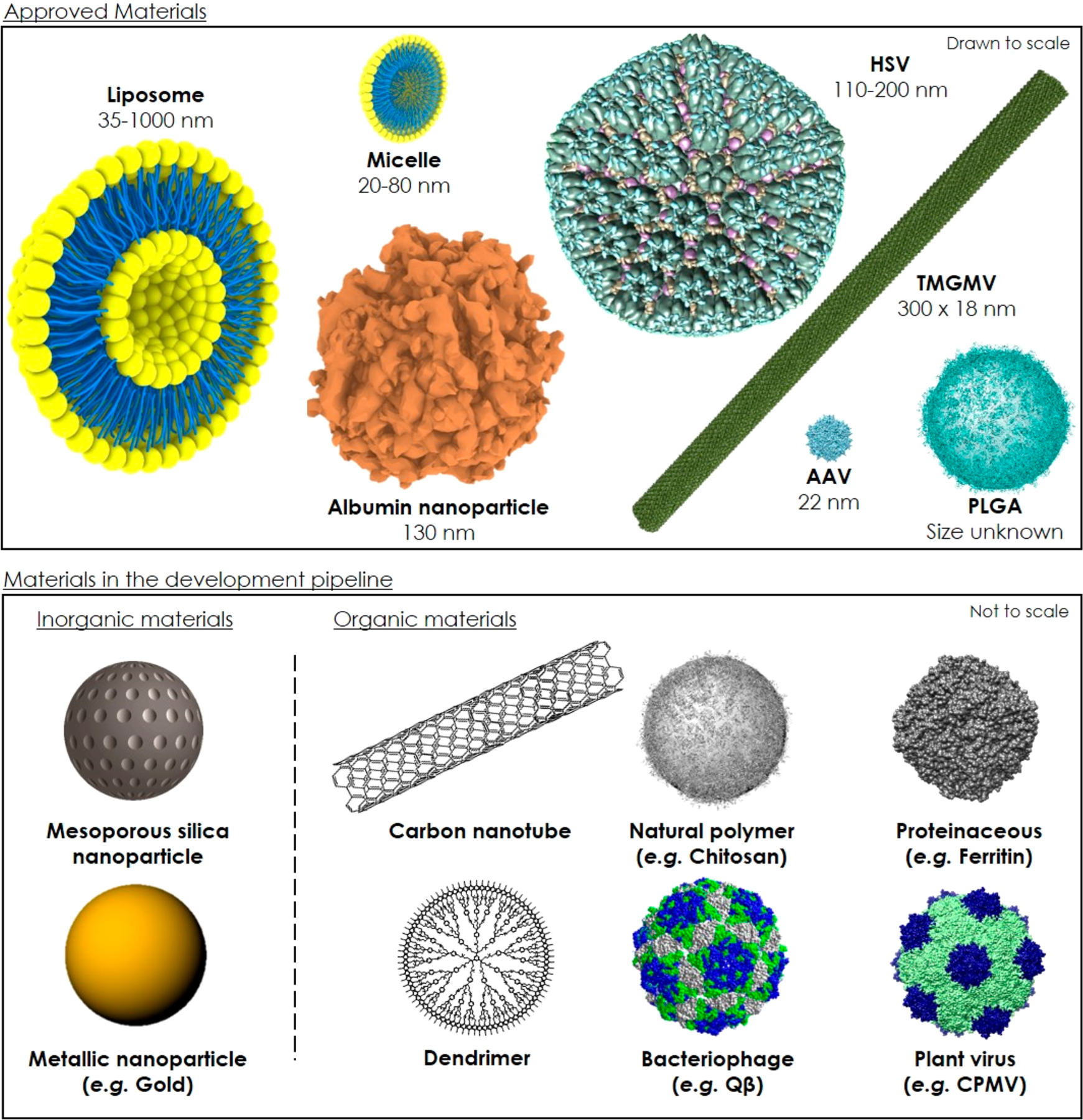
Size and structure of nanocarriers (approved and in development). Adeno-associated virus (AAV, PDB ID: 1LP3), Herpes simplex virus (HSV, PDB ID: 5ZAP), Tobacco mild green mosaic virus TMGMV (PDB ID: 1VTM), ferritin (PDB: 6BP7), bacteriophage Qβ (PDB ID: 5KIP), and Cowpea mosaic virus (CPMV, PDB ID: 1NY7) were reconstructed using the UCSF Chimera software. The albumin, micelle, liposome, PLGA, chitosan polymer, mesoporous silica, and gold nanocarriers were rendered using Rhinoceros 3D v5.0. The carbon nanotube and dendrimer were drawn using ChemDraw software.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.0c00173.
Tables summarizing gene therapy nanocarriers, viral nanocarriers, and polymer-protein conjugates undergoing clinical trials; and FDA/EMA approved polymer-protein conjugates and inorganic nanoparticles (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acsnano.0c00173
VOCABULARY
Active ingredient, a pharmaceutical or agriculture compound that confers activity against a specific abnormal condition; nanomaterial, a material with a least one dimension smaller than 1000 nm; nanocarrier, a nanomaterial used as a transport module for the delivery of an active ingredient; precision farming, site specific crop management relying on observing, measuring, and responding to field variability in crops in order to maximize yields while preserving resources; targeted drug delivery, engineered nanotechnologies promoting the accumulation of active ingredients to specific diseased sites to minimize dosage and administration frequency and off-target side effects.
Contributor Information
Paul L. Chariou, Department of Bioengineering, University of California San Diego, La Jolla, California 92039, United States.
Oscar A. Ortega-Rivera, Department of NanoEngineering, University of California San Diego, La Jolla, California 92039, United States.
Nicole F. Steinmetz, Department of Bioengineering, Department of NanoEngineering, Department of Radiology, Moores Cancer Center, and Center for Nano-ImmunoEngineering, University of California San Diego, La Jolla, California 92039, United States.
REFERENCES
- (1).Eder J; Herrling PL Trends in Modern Drug Discovery. In New Approaches to Drug Discovery; Nielsch U, Fuhrmann U, Jaroch S, Eds.; Springer International Publishing: Cham, 2016; pp 3–22. [Google Scholar]
- (2).Hogan G; Tangney M The Who, What, and Why of Drug Discovery and Development. Trends Pharmacol. Sci 2018, 39, 848–852. [DOI] [PubMed] [Google Scholar]
- (3).Holohan C; Van Schaeybroeck S; Longley DB; Johnston PG Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [DOI] [PubMed] [Google Scholar]
- (4).Mansoori B; Mohammadi A; Davudian S; Shirjang S; Baradaran B The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull 2017, 7, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Maeda H; Tsukigawa K; Fang J A Retrospective 30 Years after Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy—Problems, Solutions, and Prospects. Micro-circulation 2016, 23, 173–182. [DOI] [PubMed] [Google Scholar]
- (6).Nakamura Y; Mochida A; Choyke PL; Kobayashi H Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bazak R; Houri M; El Achy S; Kamel S; Refaat T Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol 2015, 141, 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Muhamad N; Plengsuriyakarn T; Na-Bangchang K Application of Active Targeting Nanoparticle Delivery System for Chemotherapeutic Drugs and Traditional/Herbal Medicines in Cancer Therapy: A Systematic Review. Int. J. Nanomed 2018, 13, 3921–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Barua S; Mitragotri S Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Moghimi SM; Simberg D Nanoparticle Transport Pathways into Tumors. J. Nanopart. Res 2018, 20, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Witchey-Lakshmanan LC; Li Y Controlled Drug Delivery and the Companion Animal. In Controlled Release Veterinary Drug Delivery: Biological and Pharmaceutical Consideration; Rathbone MJ, Gurny R, Eds; Elsevier Science: Amsterdam, 2000; 249–267. [Google Scholar]
- (12).McDowell A; Rathbone MJ Veterinary Drug Delivery. J. Pharm. BioAllied Sci 2014, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).American Pet Products Association:Pet Industry Market Size. https://www.americanpetproducts.org/press_industrytrends.asp (accessed 2019/02/12).
- (14).Sekhon BS Nanotechnology in Agri-Food Production: An Overview. Nanotechnol., Sci. Appl 2014, 7, 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schwab F; Zhai G; Kern M; Turner A; Schnoor JL; Wiesner MR Barriers, Pathways and Processes for Uptake, Translocation and Accumulation of Nanomaterials in Plants – Critical Review. Nanotoxicology 2016, 3, 257–278. [DOI] [PubMed] [Google Scholar]
- (16).Tsuji K Microencapsulation of Pesticides and Their Improved Handling Safety. J. Microencapsulation 2001, 18, 137–147. [DOI] [PubMed] [Google Scholar]
- (17).Lamberth C; Jeanmart S; Luksch T; Plant A Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [DOI] [PubMed] [Google Scholar]
- (18).Nicolopoulou-Stamati P; Maipas S; Kotampasi C; Stamatis P; Hens L Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Damalas CA; Eleftherohorinos IG Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Nuruzzaman Md.; Rahman MM; Liu Y; Naidu R Nanoencapsulation, Nano-Guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem 2016, 64, 1447–1483. [DOI] [PubMed] [Google Scholar]
- (21).Boverhof DR; Bramante CM; Butala JH; Clancy SF; Lafranconi M; West J; Gordon SC Comparative Assessment of Nanomaterial Definitions and Safety Evaluation Considerations. Regul. Toxicol. Pharmacol 2015, 73, 137–150. [DOI] [PubMed] [Google Scholar]
- (22).Chen J; Guo Z; Tian H; Chen X Production and Clinical Development of Nanoparticles for Gene Delivery. Mol. Ther.–Methods Clin. Dev 2016, 3, 16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Velpurisiva P; Gad A; Piel B; Jadia R; Rai P Nanoparticle Design Strategies for Effective Cancer Immunotherapy. J. Biomed. (Syd) 2017, 2, 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gun’ko YK Nanoparticles in Bioimaging. Nanomaterials 2016, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Jain KK The Role of Nanobiotechnology in Drug Discovery. Drug Discovery Today 2005, 10, 1435–1442. [DOI] [PubMed] [Google Scholar]
- (26).Jaque D; Martínez Maestro L; del Rosal B; Haro-Gonzalez P; Benayas A; Plaza JL; Martín Rodríguez E; García Solé J Nanoparticles for Photothermal Therapies. Nanoscale 2014, 6, 9494–9530. [DOI] [PubMed] [Google Scholar]
- (27).Agostinis P; Berg K; Cengel KA; Foster TH; Girotti AW; Gollnick SO; Hahn SM; Hamblin MR; Juzeniene A; Kessel D; Korbelik M; Moan J; Mroz P; Nowis D; Piette J; Wilson BC; Golab J Photodynamic Therapy of Cancer: An Update. Ca-Cancer J. Clin 2011, 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Taniguchi N On the Basic Concept of Nano-Technology. Proceedings from the Proceedings of International Conference of the Japan Society for Precision Engineering; Japan Society for Precision Engineering: Tokyo, 1974; p 245. [Google Scholar]
- (29).Caster JM; Patel AN; Zhang T; Wang A Investigational Nanomedicines in 2016: A Review of Nanotherapeutics Currently Undergoing Clinical Trials. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol 2017, 9, e1416. [DOI] [PubMed] [Google Scholar]
- (30).Charudattan R; Hiebert E A Plant Virus as a Bioherbicide for Tropical Soda Apple, Solanum Viarum. Outlooks Pest Manage. 2007, 18, 167–171. [Google Scholar]
- (31).Charudattan R; Pettersen MS; Hiebert E Use of Tobacco Mild Green Mosaic Virus (TMGMV) Mediated Lethal Hypersensitive Response (HR) as a Novel Method of Weed Control. US7494955B2, February 24, 2009. [Google Scholar]
- (32).Shang Y; Hasan Md. K.; Ahammed GJ; Li M; Yin H; Zhou J Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zhao N; C Woodle M; Mixson AJ Advances in Delivery Systems for Doxorubicin. J. Nanomed. Nanotechnol 2018, 09, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ma P; Mumper RJ Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J. Nanomed. Nanotechnol 2013, 4, 1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lee C-T; Huang Y-W; Yang C-H; Huang K-S Drug Delivery Systems and Combination Therapy by Using Vinca Alkaloids. Curr. Top. Med. Chem 2015, 15, 1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Zaioncz S; Khalil N; Mainardes R Exploring the Role of Nanoparticles in Amphotericin B Delivery. Curr. Pharm. Des 2017, 23, 509–521. [DOI] [PubMed] [Google Scholar]
- (37).Lameire N Nephrotoxicity of Recent Anti-Cancer Agents. Clin. Kidney J 2014, 7, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Varricchi G; Ameri P; Cadeddu C; Ghigo A; Madonna R; Marone G; Mercurio V; Monte I; Novo G; Parrella P; Pirozzi F; Pecorato A; Spallarossa P; Zito C; Mercuro G; Pagliaro P; Tocchetti CG Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol 2018, 9, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Van Bulck M; Sierra-Magro A; Alarcon-Gil J; Perez-Castillo A; Morales-Garcia JA Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci 2019, 20, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Teleanu DM; Chircov C; Grumezescu AM; Volceanov A; Teleanu RI Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Lee B; Park J; Ryu M; Kim S; Joo M; Yeom J-H; Kim S; Park Y; Lee K; Bae J Antimicrobial Peptide-Loaded Gold Nanoparticle-DNA Aptamer Conjugates as Highly Effective Antibacterial Therapeutics against Vibrio Vulnificus. Sci. Rep 2017, 7, 13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Le DHT; Commandeur U; Steinmetz NF Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy. ACS Nano 2019, 13, 2501–2510. [DOI] [PubMed] [Google Scholar]
- (43).Mitchell MJ; Wayne E; Rana K; Schaffer CB; King MR TRAIL-Coated Leukocytes That Kill Cancer Cells in the Circulation. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).McKeage K; Perry CM Trastuzumab. Drugs 2002, 62, 209–243. [DOI] [PubMed] [Google Scholar]
- (45).Singh S; Kumar NK; Dwiwedi P; Charan J; Kaur R; Sidhu P; Chugh VK Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol 2018, 13, 85–99. [DOI] [PubMed] [Google Scholar]
- (46).Birrer MJ; Moore KN; Betella I; Bates RC Antibody-Drug Conjugate-Based Therapeutics: State of the Science. JNCI J. Natl. Cancer Inst 2019, 111, 538–549. [DOI] [PubMed] [Google Scholar]
- (47).Carter T; Mulholland P; Chester K Antibody-Targeted Nanoparticles for Cancer Treatment. Immunotherapy 2016, 8, 941–958. [DOI] [PubMed] [Google Scholar]
- (48).Slastnikova TA; Ulasov AV; Rosenkranz AA; Sobolev AS Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol 2018, 9, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Guo C; Chen Y; Gao W; Chang A; Ye Y; Shen W; Luo Y; Yang S; Sun P; Xiang R; Li N Liposomal Nanoparticles Carrying Anti-IL6R Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models. Theranostics 2017, 7, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Yoo J; Park C; Yi G; Lee D; Koo H Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Rosenblum D; Joshi N; Tao W; Karp JM; Peer D Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun 2018, 9, 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WCW Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater 2016, 1, 16014. [Google Scholar]
- (53).Chaudhary V; Jangra S; Yadav NR Nanotechnology Based Approaches for Detection and Delivery of MicroRNA in Healthcare and Crop Protection. J. Nanobiotechnol 2018, 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Cunningham FJ; Goh NS; Demirer GS; Matos JL; Landry MP Nanoparticle-Mediated Delivery towards Advancing Plant Genetic Engineering. Trends Biotechnol. 2018, 36, 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hwang H-H; Yu M; Lai E-M Agrobacterium-Mediated Plant Transformation: Biology and Applications. Arabidopsis Book 2017, 15, e0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ismagul A; Yang N; Maltseva E; Iskakova G; Mazonka I; Skiba Y; Bi H; Eliby S; Jatayev S; Shavrukov Y; Borisjuk N; Langridge P A Biolistic Method for High-Throughput Production of Transgenic Wheat Plants with Single Gene Insertions. BMC Plant Biol. 2018, 18, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Bangham AD; Standish MM; Watkins JC Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol 1965, 13, 238–252. [DOI] [PubMed] [Google Scholar]
- (58).Bulbake U; Doppalapudi S; Kommineni N; Khan W Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Swenson CE; Perkins WR; Roberts P; Janoff AS Liposome Technology and the Development of Myocet (Liposomal Doxorubicin Citrate). Breast 2001, 10, 1–7.14965549 [Google Scholar]
- (60).Signorell RD; Luciani P; Brambilla D; Leroux J-C Pharmacokinetics of Lipid-Drug Conjugates Loaded into Liposomes. Eur. J. Pharm. Biopharm 2018, 128, 188–199. [DOI] [PubMed] [Google Scholar]
- (61).Fröhlich E The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed 2012, 7, 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Krasnici S; Werner A; Eichhorn ME; Schmitt-Sody M; Pahernik SA; Sauer B; Schulze B; Teifel M; Michaelis U; Naujoks K; Dellian M Effect of the Surface Charge of Liposomes on Their Uptake by Angiogenic Tumor Vessels. Int. J. Cancer 2003, 105, 561–567. [DOI] [PubMed] [Google Scholar]
- (63).Piccaluga P; Visani G; Martinelli G; Isidori A; Malagola M; Rondoni M; Baccarani M; Tura S Liposomal Daunorubicin (DaunoXome) for Treatment of Relapsed Meningeal Acute Myeloid Leukemia. Leukemia 2002, 16, 1880–1881. [DOI] [PubMed] [Google Scholar]
- (64).Silverman JA; Deitcher SR Marqibo® (Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol 2013, 71, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Lamb YN; Scott LJ Liposomal Irinotecan: A Review in Metastatic Pancreatic Adenocarcinoma. Drugs 2017, 77, 785–792. [DOI] [PubMed] [Google Scholar]
- (66).Sercombe L; Veerati T; Moheimani F; Wu SY; Sood AK; Hua S Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol 2015, 6, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Duggan ST; Keating GM Pegylated Liposomal Doxorubicin: A Review of Its Use in Metastatic Breast Cancer, Ovarian Cancer, Multiple Myeloma and AIDS-Related Kaposi’s Sarcoma. Drugs 2011, 71, 2531–2558. [DOI] [PubMed] [Google Scholar]
- (68).Gabizon A; Catane R; Uziely B; Kaufman B; Safra T; Cohen R; Martin F; Barenholz Y Prolonged Circulation Time and Enhanced Accumulation in Malignant Exudates of Doxorubicin Encapsulated in Polyethylene-Glycol Coated Liposomes. Cancer Res. 1994, 54, 987–992. [PubMed] [Google Scholar]
- (69).Deshpande PP; Biswas S; Torchilin VP Current Trends in the Use of Liposomes for Tumor Targeting. Nanomedicine 2013, 8, 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Blair HA Daunorubicin/Cytarabine Liposome: A Review in Acute Myeloid Leukaemia. Drugs 2018, 78, 1903–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Stone NR; Bicanic T; Salim R; Hope W Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Fenton C; Perry CM Verteporfin: A Review of Its Use in the Management of Subfoveal Choroidal Neovascularisation. Drugs Aging 2006, 23, 421–425. [DOI] [PubMed] [Google Scholar]
- (73).Weng Y; Xiao H; Zhang J; Liang X-J; Huang Y RNAi Therapeutic and Its Innovative Biotechnological Evolution. Biotechnol. Adv 2019, 37, 801–825. [DOI] [PubMed] [Google Scholar]
- (74).Behr J; Zimmermann G; Baumgartner R; Leuchte H; Neurohr C; Brand P; Herpich C; Sommerer K; Seitz J; Menges G; Tillmanns S; Keller M Lung Deposition of a Liposomal Cyclosporine A Inhalation Solution in Patients after Lung Transplantation. J. Aerosol Med. Pulm. Drug Delivery 2009, 22, 121–130. [DOI] [PubMed] [Google Scholar]
- (75).Venkatraman S; Natarajan; Ang M; Darwitan; Wong. Nanomedicine for Glaucoma: Liposomes Provide Sustained Release of Latanoprost in the Eye . Int. J. Nanomed 2012, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).da Silva SM; Amorim IFG; Ribeiro RR; Azevedo EG; Demicheli C; Melo MN; Tafuri WL; Gontijo NF; Michalick MSM; Frézard F Efficacy of Combined Therapy with Liposome-Encapsulated Meglumine Antimoniate and Allopurinol in Treatment of Canine Visceral Leishmaniasis. Antimicrob. Agents Chemother 2012, 56, 2858–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Bai D-P; Lin X-Y; Huang Y-F; Zhang X-F Theranostics Aspects of Various Nanoparticles in Veterinary Medicine. Int. J. Mol. Sci 2018, 19, 3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Sadozai H; Saeidi D Recent Developments in Liposome-Based Veterinary Therapeutics. ISRN Vet. Sci 2013, 2013, 167521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Hauck ML; LaRue SM; Petros WP; Poulson JM; Yu D; Spasojevic I; Pruitt AF; Klein A; Case B; Thrall DE; Needham D; Dewhirst MW Phase I Trial of Doxorubicin-Containing Low Temperature Sensitive Liposomes in Spontaneous Canine Tumors. Clin. Cancer Res 2006, 12, 4004–4010. [DOI] [PubMed] [Google Scholar]
- (80).Kleiter M; Tichy A; Willmann M; Pagitz M; Wolfesberger B Concomitant Liposomal Doxorubicin and Daily Palliative Radiotherapy in Advanced Feline Soft Tissue Sarcomas. Vet. Radiol. Ultrasound 2010, 51, 349–355. [DOI] [PubMed] [Google Scholar]
- (81).Hafeman S; London C; Elmslie R; Dow S Evaluation of Liposomal Clodronate for Treatment of Malignant Histiocytosis in Dogs. Cancer Immunol. Immunother 2010, 59, 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Krawiec DR; McKiernan BC; Twardock AR; Swenson CE; Itkin RJ; Johnson LR; Kurowsky LK; Marks CA Use of an Amphotericin B Lipid Complex for Treatment of Blastomycosis in Dogs. J. Am. Vet. Med. Assoc 1996, 209, 2073–2075. [PubMed] [Google Scholar]
- (83).Taylor TM; Weiss J; Davidson PM; Bruce BD Liposomal Nanocapsules in Food Science and Agriculture. Crit. Rev. Food Sci. Nutr 2005, 45, 587–605. [DOI] [PubMed] [Google Scholar]
- (84).Hwang IC; Kim TH; Bang SH; Kim KS; Kwon HR; Seo MJ; Youn YN; Park HJ; Yasunaga C; Yu YM Insecticidal Effect of Controlled Release Formulations of Etofenprox Based on Nano–Bio Technique. J. Fac. Agric., Kyushu Univ 2011, 56, 33–40. [Google Scholar]
- (85).Karny A; Zinger A; Kajal A; Shainsky-Roitman J; Schroeder A Therapeutic Nanoparticles Penetrate Leaves and Deliver Nutrients to Agricultural Crops. Sci. Rep 2018, 8, 7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Priya James H; John R; Alex A; Anoop KR Smart Polymers for the Controlled Delivery of Drugs – a Concise Overview. Acta Pharm. Sin. B 2014, 4, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Knop K; Hoogenboom R; Fischer D; Schubert US Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem., Int. Ed 2010, 49, 6288–6308. [DOI] [PubMed] [Google Scholar]
- (88).Sartor O Eligard: Leuprolide Acetate in a Novel Sustained-Release Delivery System. Urology 2003, 61, 25–31. [DOI] [PubMed] [Google Scholar]
- (89).Avitabile N; Banka A; Fonseca VA Safety Evaluation of Colesevelam Therapy to Achieve Glycemic and Lipid Goals in Type 2 Diabetes. Expert Opin. Drug Saf. 2011, 10, 305–310. [DOI] [PubMed] [Google Scholar]
- (90).Madaan K; Kumar S; Poonia N; Lather V; Pandita D Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. BioAllied Sci 2014, 6, 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Palmerston Mendes L; Pan J; Torchilin VP Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Kannan RM; Nance E; Kannan S; Tomalia DA Emerging Concepts in Dendrimer-Based Nanomedicine: From Design Principles to Clinical Applications. J. Intern. Med 2014, 276, 579–617. [DOI] [PubMed] [Google Scholar]
- (93).Kesharwani P; Iyer AK Recent Advances in Dendrimer-Based Nanovectors for Tumor-Targeted Drug and Gene Delivery. Drug Discovery Today 2015, 20, 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Wilken R; Veena MS; Wang MB; Srivatsan ES Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2011, 10 (12), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Hasty P; Livi CB; Dodds SG; Jones D; Strong R; Javors M; Fischer KE; Sloane L; Murthy K; Hubbard G; Sun L; Hurez V; Curiel TJ; Sharp ZD ERapa Restores a Normal Life Span in a FAP Mouse Model. Cancer Prev. Res 2014, 7, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Jeong Y; Kim S; Jin S; Ryu H; Jin Y; Jung T; Kim I; Jung S Cisplatin-incorporated Hyaluronic Acid Nanoparticles Based on Ion-complex Formation. J. Pharm. Sci 2008, 97, 1268–1276. [DOI] [PubMed] [Google Scholar]
- (97).Hrkach J; Von Hoff D; Ali MM; Andrianova E; Auer J; Campbell T; De Witt D; Figa M; Figueiredo M; Horhota A; Low s.; McDonnell K; Peeke E; Retnarajan B; Sabnis A; Schnipper E; Song JJ; Song YH; Summa J; Tompsett D; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmaco-logical Profile. Sci. Transl. Med 2012, 4, 128–139. [DOI] [PubMed] [Google Scholar]
- (98).Autio KA; Dreicer R; Anderson J; Garcia JA; Alva A; Hart LL; Milowsky MI; Posadas E; Ryan CJ; Graf RP; Dittamore R; Schreiber NA; Summa JM; Youssoufian H; Morris MJ; Scher HI Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients with Metastatic Sastration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Perlatti B; Luísa de Souza Bergo P; das Graças Fernandes da Silva MF; Fernandes JB; Forim MR Polymeric Nanoparticle-Based Insecticides: A Controlled Release Purpose for Agrochemicals. In Insecticides: Development of Safer and More Effective Technologies; Trdan D, Ed.; IntechOpen, 2013; pp 523–550. (accessed 2020/02/13). [Google Scholar]
- (100).Grillo R; Pereira dos Santos NZ; Maruyama CR; Rosa AH; de Lima R; Fraceto LF Poly(ε-Caprolactone)Nanocapsules as Carrier Systems for Herbicides: Physico-Chemical Characterization and Genotoxicity Evaluation. J. Hazard. Mater 2012, 231–232, 1–9. [DOI] [PubMed] [Google Scholar]
- (101).Pradhan S; Roy I; Lodh G; Patra P; Choudhury SR; Samanta A; Goswami A Entomotoxicity and Biosafety Assessment of PEGylated Acephate Nanoparticles: A Biologically Safe Alternative to Neurotoxic Pesticides. J. Environ. Sci. Health, Part B 2013, 48, 559–569. [DOI] [PubMed] [Google Scholar]
- (102).Shang Q; Shi Y; Zhang Y; Zheng T; Shi H Pesticide-Conjugated Polyacrylate Nanoparticles: Novel Opportunities for Improving the Photostability of Emamectin Benzoate. Polym. Adv. Technol 2013, 24, 137–143. [Google Scholar]
- (103).Yang F-L; Li X-G; Zhu F; Lei C-L Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium Castaneum. J. Agric. Food Chem 2009, 57, 10156–10162. [DOI] [PubMed] [Google Scholar]
- (104).Kumar S; Bhanjana G; Sharma A; Sidhu MC; Dilbaghi N Synthesis, Characterization and on Field Evaluation of Pesticide Loaded Sodium Alginate Nanoparticles. Carbohydr. Polym 2014, 101, 1061–1067. [DOI] [PubMed] [Google Scholar]
- (105).Guan H; Chi D; Yu J; Li X A Novel Photodegradable Insecticide: Preparation, Characterization and Properties Evaluation of Nano-Imidacloprid. Pestic. Biochem. Physiol 2008, 92, 83–91. [Google Scholar]
- (106).Yin Y; Guo Q; Han Y; Wang L; Wan S Preparation, Characterization and Nematicidal Activity of Lansiumamide B Nano-Capsules. J. Integr. Agric 2012, 11, 1151–1158. [Google Scholar]
- (107).Yin Y; Xu S; Chang D; Zheng H; Li J; Liu X; Xu P; Xiong F One-Pot Synthesis of Biopolymeric Hollow Nanospheres by Photocrosslinking. Chem. Commun 2009, 46, 8222–8224. [DOI] [PubMed] [Google Scholar]
- (108).Silva M. dos S.; Cocenza DS; Grillo R; Melo N. F. S. de; Tonello PS; Oliveira L. C. de; Cassimiro DL; Rosa AH; Fraceto LF Paraquat-Loaded Alginate/Chitosan Nanoparticles: Preparation, Characterization and Soil Sorption Studies. J. Hazard. Mater 2011, 190, 366–374. [DOI] [PubMed] [Google Scholar]
- (109).Li J; Mooney DJ Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater 2016, 1, 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Hoare TR; Kohane DS Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar]
- (111).Ekladious I; Colson YL; Grinstaff MW Polymer–Drug Conjugate Therapeutics: Advances, Insights and Prospects. Nat. Rev. Drug Discovery 2019, 18, 273–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Pedrini S; Merritt DJ; Stevens J; Dixon K Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [DOI] [PubMed] [Google Scholar]
- (113).Blizzard C; Desai A; Driscoll A Pharmacokinetic Studies of Sustained-Release Depot of Dexamethasone in Beagle Dogs. J. Ocul. Pharmacol. Ther 2016, 32, 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Narayanaswamy R; Torchilin VP Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Greenwald RB; Choe YH; McGuire J; Conover CD Effective Drug Delivery by PEGylated Drug Conjugates. Adv. Drug Delivery Rev 2003, 55, 217–250. [DOI] [PubMed] [Google Scholar]
- (116).Alconcel SNS; Baas AS; Maynard HD FDA-Approved Poly(Ethylene Glycol)–Protein Conjugate Drugs. Polym. Chem 2011, 2, 1442–1448. [Google Scholar]
- (117).Zinicola M; Korzec H; Teixeira AGV; Ganda EK; Bringhenti L; Tomazi ACCH; Gilbert RO; Bicalho RC Effects of Pegbovigrastim Administration on Periparturient Diseases, Milk Production, and Reproductive Performance of Holstein Cows. J. Dairy Sci 2018, 101, 11199–11217. [DOI] [PubMed] [Google Scholar]
- (118).Zhang P; Sun F; Liu S; Jiang S Anti-PEG Antibodies in the Clinic: Current Issues and beyond PEGylation. J. Controlled Release 2016, 244, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Sharma KK; Singh US; Sharma P; Kumar A; Sharma L Seed Treatments for Sustainable Agriculture-A Review. J. Appl. Nat. Sci 2015, 7, 521–539. [Google Scholar]
- (120).Croy SR; Kwon GS Polymeric Micelles for Drug Delivery. Curr. Pharm. Des 2006, 12, 4669–4684. [DOI] [PubMed] [Google Scholar]
- (121).Lu Y; Park K Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm 2013, 453, 198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Simon JA Estradiol in Micellar Nanoparticles: The Efficacy and Safety of a Novel Transdermal Drug-Delivery Technology in the Management of Moderate to Severe Vasomotor Symptoms. Menopause 2006, 13, 222–231. [DOI] [PubMed] [Google Scholar]
- (123).Hart M; Acott S Physical and Chemical Stability of Taxotere (Docetaxel) One-Vial (20 Mg/Ml) Infusion Solution Following Refrigerated Storage. ecancer 2010, 4, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Scripture C; Szebeni J; Loos WJ; Figg W II; Sparreboom A Comparative In Vitro Properties and Clinical Pharmacokinetics of Paclitaxel Following the Administration of Taxol and Paxene. Cancer Biol. Ther 2005, 4, 555–560. [DOI] [PubMed] [Google Scholar]
- (125).Hamaguchi T; Tsuji A; Yamaguchi K; Takeda K; Uetake H; Esaki T; Amagai K; Sakai D; Baba H; Kimura M; Matsumura Y; Tsukamoto T A Phase II Study of NK012, a Polymeric Micelle Formulation of SN-38, in Unresectable, Metastatic or Recurrent Colorectal Cancer Patients. Cancer Chemother. Pharmacol 2018, 82, 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Hamaguchi T; Matsumura Y; Suzuki M; Shimizu K; Goda R; Nakamura I; Nakatomi I; Yokoyama M; Kataoka K; Kakizoe T NK105, a Paclitaxel-Incorporating Micellar Nanoparticle Formulation, Can Extend In Vivo Antitumour Activity and Reduce the Neurotoxicity of Paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Kim D-W; Kim S-Y; Kim H-K; Kim S-W; Shin SW; Kim JS; Park K; Lee MY; Heo DS Multicenter Phase II Trial of Genexol-PM, a Novel Cremophor-Free, Polymeric Micelle Formulation of Paclitaxel, with Cisplatin in Patients with Advanced Non-Small-Cell Lung Cancer. Ann. Oncol 2007, 18, 2009–2014. [DOI] [PubMed] [Google Scholar]
- (128).Plummer R; Wilson RH; Calvert H; Boddy AV; Griffin M; Sludden J; Tilby MJ; Eatock M; Pearson DG; Ottley CJ; Matsumura Y; Kataoka K; Nishiya T A Phase I Clinical Study of Cisplatin-Incorporated Polymeric Micelles (NC-6004) in Patients with Solid Tumours. Br. J. Cancer 2011, 104, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Vail DM; von Euler H; Rusk AW; Barber L; Clifford C; Elmslie R; Fulton L; Hirschberger J; Klein M; London C; Matano M; McNiel EA; Morris JS; Northup N; Phillips B; Polton G; Post G; Rosenberg M; Ruslander D; Sahora A; et al. A Randomized Trial Investigating the Efficacy and Safety of Water Soluble Micellar Paclitaxel (Paccal Vet) for Treatment of Non-resectable Grade 2 or 3 Mast Cell Tumors in Dogs. J. Vet. Intern. Med 2012, 26, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Rey AI; Segura J; Arandilla E; López-Bote CJ Short- and Long-Term Effect of Oral Administration of Micellized Natural Vitamin E (D-α-Tocopherol) on Oxidative Status in Race Horses under Intense Training1. J. Anim. Sci 2013, 91, 1277–1284. [DOI] [PubMed] [Google Scholar]
- (131).Feng B-H; Peng L-F Synthesis and Characterization of Carboxymethyl Chitosan Carrying Ricinoleic Functions as an Emulsifier for Azadirachtin. Carbohydr. Polym 2012, 88, 576–582. [Google Scholar]
- (132).Koli P; Singh BB; Shakil NA; Kumar J; Kamil D Development of Controlled Release Nanoformulations of Carbendazim Employing Amphiphilic Polymers and Their Bioefficacy Evaluation against Rhizoctonia Solani. J. Environ. Sci. Health, Part B 2015, 9, 674–681. [DOI] [PubMed] [Google Scholar]
- (133).Shakil NA; Singh MK; Pandey A; Kumar J; Pankaj; Parmar VS; Singh MK; Pandey RP; Watterson AC Development of Poly(Ethylene Glycol) Based Amphiphilic Copolymers for Controlled Release Delivery of Carbofuran. J. Macromol. Sci., Part A: Pure Appl.Chem 2010, 47, 241–247. [Google Scholar]
- (134).Adak T; Kumar J; Shakil NA; Walia S Development of Controlled Release Formulations of Imidacloprid Employing Novel Nano-Ranged Amphiphilic Polymers. J. Environ. Sci. Health, Part B 2012, 47, 217–225. [DOI] [PubMed] [Google Scholar]
- (135).Adak T; Kumar J; Dey D; Shakil NA; Walia S Residue and Bio-Efficacy Evaluation of Controlled Release Formulations of Imidacloprid against Pests in Soybean (G Lycine Max). J. Environ. Sci. Health, Part B 2012, 47, 226–231. [DOI] [PubMed] [Google Scholar]
- (136).Lao S-B; Zhang Z-X; Xu H-H; Jiang G-B Novel Amphiphilic Chitosan Derivatives: Synthesis, Characterization and Micellar Solubilization of Rotenone. Carbohydr. Polym 2010, 82, 1136–1142. [Google Scholar]
- (137).Sarkar DJ; Kumar J; Shakil NA; Walia S Release Kinetics of Controlled Release Formulations of Thiamethoxam Employing Nano-Ranged Amphiphilic PEG and Diacid Based Block Polymers in Soil. J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng 2012, 47, 1701–1712. [DOI] [PubMed] [Google Scholar]
- (138).Kaushik P; Shakil NA; Kumar J; Singh MK; Singh MK; Yadav SK Development of Controlled Release Formulations of Thiram Employing Amphiphilic Polymers and Their Bioefficacy Evaluation in Seed Quality Enhancement Studies. J. Environ. Sci. Health, Part B 2013, 48, 677–685. [DOI] [PubMed] [Google Scholar]
- (139).Pinto A; Pocard M Photodynamic Therapy and Photothermal Therapy for the Treatment of Peritoneal Metastasis: A Systematic Review. Pleura Peritoneum 2018, 3, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Anselmo AC; Mitragotri S A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Lin G; Mi P; Chu C; Zhang J; Liu G Inorganic Nanocarriers Overcoming Multidrug Resistance for Cancer Theranostics. Adv. Sci 2016, 3, 1600134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Mei W; Wu Q Applications of Metal Nanoparticles in Medicine/Metal Nanoparticles as Anticancer Agents. In Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences; Thota S, Crans DC, Eds.; Wiley: Weinheim, 2017; pp 169–190. [Google Scholar]
- (143).Chen Y-H; Tsai C-Y; Huang P-Y; Chang M-Y; Cheng P-C; Chou C-H; Chen D-H; Wang C-R; Shiau A-L; Wu C-L Methotrexate Conjugated to Gold Nanoparticles Inhibits Tumor Growth in a Syngeneic Lung Tumor Model. Mol. Pharmaceutics 2007, 4, 713–722. [DOI] [PubMed] [Google Scholar]
- (144).Wang F; Wang Y-C; Dou S; Xiong M-H; Sun T-M; Wang J Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [DOI] [PubMed] [Google Scholar]
- (145).Brown SD; Nativo P; Smith J-A; Stirling D; Edwards PR; Venugopal B; Flint DJ; Plumb JA; Graham D; Wheate NJ Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc 2010, 132, 4678–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Duraipandy N; Lakra R; Vinjimur SK; Samanta D; K PS; Kiran MS Caging of Plumbagin on Silver Nanoparticles Imparts Selectivity and Sensitivity to Plumbagin for Targeted Cancer Cell Apoptosis. Metallomics 2014, 6, 2025–2033. [DOI] [PubMed] [Google Scholar]
- (147).Bharti C; Nagaich U; Pal AK; Gulati N Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig 2015, 5, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Kettiger H; Québatte G; Perrone B; Huwyler J Interactions between Silica Nanoparticles and Phospholipid Membranes. Biochim. Biophys. Acta, Biomembr 2016, 1858, 2163–2170. [DOI] [PubMed] [Google Scholar]
- (149).Song M-R; Cui S-M; Gao F; Liu Y-R; Fan C-L; Lei T-Q; Liu D-C Dispersible Silica Nanoparticles as Carrier for Enhanced Bioactivity of Chlorfenapyr. J. Pestic. Sci 2012, 37, 258–260. [Google Scholar]
- (150).Wanyika H Sustained Release of Fungicide Metalaxyl by Mesoporous Silica Nanospheres. J. Nanopart. Res 2013, 15, 1831. [Google Scholar]
- (151).Park M; Lee C-I; Seo YJ; Woo SR; Shin D; Choi J Hybridization of the Natural Antibiotic, Cinnamic Acid, with Layered Double Hydroxides (LDH) as Green Pesticide. Environ. Sci. Pollut. Res 2010, 17, 203–209. [DOI] [PubMed] [Google Scholar]
- (152).Zobir bin Hussein M; Hj Yahaya A; Zainal Z; Hee Kian L Nanocomposite-Based Controlled Release Formulation of an Herbicide, 2,4-Dichlorophenoxyacetate Incapsulated in Zinc–Aluminium-Layered Double Hydroxide. Sci. Technol. Adv. Mater 2005, 6, 956–962. [Google Scholar]
- (153).Mitter N; Worrall EA; Robinson KE; Li P; Jain RG; Taochy C; Fletcher SJ; Carroll BJ; Lu GQ; Xu ZP Clay Nanosheets for Topical Delivery of RNAi for Sustained Protection against Plant Viruses. Nat. Plants 2017, 3, 16207. [DOI] [PubMed] [Google Scholar]
- (154).Elhissi AMA; Ahmed W; Hassan IU; Dhanak VR Carbon Nanotubes in Cancer Therapy and Drug Delivery. J. Drug Delivery 2012, 2012, 837327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Guo Q; Shen X; Li Y; Xu S Carbon Nanotubes-Based Drug Delivery to Cancer and Brain. Curr. Med. Sci 2017, 37, 635–641. [DOI] [PubMed] [Google Scholar]
- (156).Mahajan S; Patharkar A; Kuche K; Maheshwari R; Deb PK; Kalia K; Tekade RK Functionalized Carbon Nanotubes as Emerging Delivery System for the Treatment of Cancer. Int. J. Pharm 2018, 548, 540–558. [DOI] [PubMed] [Google Scholar]
- (157).Matthews B Tools for Protein Science. Protein Sci. Publ. Protein Soc 2018, 27, 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Niemeyer CM Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science. Angew. Chem., Int. Ed 2001, 40, 4128–4158. [DOI] [PubMed] [Google Scholar]
- (159).Rong J; Niu Z; Lee LA; Wang Q Chemistry and Materials Development of Protein-Based Nanoparticles. In Comprehensive Nanoscience and Nanotechnogy; Andrews DL, Scholes GD, Wiederrecht GP, Eds.; Elsevier: Amsterdam, 2011; pp 153–174. [Google Scholar]
- (160).Hawkins M; Soon-Shiong P; Desai N Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Delivery Rev 2008, 60, 876–885. [DOI] [PubMed] [Google Scholar]
- (161).Larsen MT; Kuhlmann M; Hvam ML; Howard KA Albumin-Based Drug Delivery: Harnessing Nature to Cure Disease. Mol. Cell. Ther 2016, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Sauvage F; Messaoudi S; Fattal E; Barratt G; Vergnaud-Gauduchon J Heat Shock Proteins and Cancer: How Can Nanomedicine Be Harnessed? J. Controlled Release 2017, 248, 133–143. [DOI] [PubMed] [Google Scholar]
- (163).Flenniken ML; Liepold LO; Crowley BE; Willits DA; Young MJ; Douglas T Selective Attachment and Release of a Chemotherapeutic Agent from the Interior of a Protein Cage Architecture. Chem. Commun 2005, 4, 447–449. [DOI] [PubMed] [Google Scholar]
- (164).Rother M; Nussbaumer MG; Renggli K; Bruns N Protein Cages and Synthetic Polymers: A Fruitful Symbiosis for Drug Delivery Applications, Bionanotechnology and Materials Science. Chem. Soc. Rev 2016, 45, 6213–6249. [DOI] [PubMed] [Google Scholar]
- (165).Muñoz-Juan A; Carreño A; Mendoza R; Corchero JL Latest Advances in the Development of Eukaryotic Vaults as Targeted Drug Delivery Systems. Pharmaceutics 2019, 11, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Truffi M; Fiandra L; Sorrentino L; Monieri M; Corsi F; Mazzucchelli S Ferritin Nanocages: A Biological Platform for Drug Delivery, Imaging and Theranostics in Cancer. Pharmacol. Res 2016, 107, 57–65. [DOI] [PubMed] [Google Scholar]
- (167).Yang Z; Wang X; Diao H; Zhang J; Li H; Sun H; Guo Z Encapsulation of Platinum Anticancer Drugs by Apoferritin. Chem. Commun 2007, 33, 3453–3455. [DOI] [PubMed] [Google Scholar]
- (168).Zhen Z; Tang W; Chen H; Lin X; Todd T; Wang G; Cowger T; Chen X; Xie J RGD-Modified Apoferritin Nanoparticles for Efficient Drug Delivery to Tumors. ACS Nano 2013, 7, 4830–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Chen L; Bai G; Yang S; Yang R; Zhao G; Xu C; Leung W Encapsulation of Curcumin in Recombinant Human H-Chain Ferritin Increases Its Water-Solubility and Stability. Food Res. Int 2014, 62, 1147–1153. [Google Scholar]
- (170).Sánchez P; Valero E; Gálvez N; Domínguez-Vera JM; Marinone M; Poletti G; Corti M; Lascialfari A MRI Relaxation Properties of Water-Soluble Apoferritin-Encapsulated Gadolinium Oxide-Hydroxide Nanoparticles. Dalton Trans. 2009, 5, 800–804. [DOI] [PubMed] [Google Scholar]
- (171).Kálmán FK; Geninatti-Crich S; Aime S Reduction/Dissolution of a β-MnOOH Nanophase in the Ferritin Cavity to Yield a Highly Sensitive, Biologically Compatible Magnetic Resonance Imaging Agent. Angew. Chem., Int. Ed 2010, 49, 612–615. [DOI] [PubMed] [Google Scholar]
- (172).Ristroph KD; Astete CE; Bodoki E; Sabliov CM Zein Nanoparticles Uptake by Hydroponically Grown Soybean Plants. Environ. Sci. Technol 2017, 51, 14065–14071. [DOI] [PubMed] [Google Scholar]
- (173).Adli M The CRISPR Tool Kit for Genome Editing and Beyond. Nat. Commun 2018, 9, 1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Chevalier A; Silva D-A; Rocklin GJ; Hicks DR; Vergara R; Murapa P; Bernard SM; Zhang L; Lam K-H; Yao G; Bahl CD; Miyashita SI; Goreshnik I; Fuller JT; Koday MT; Jenkins CM; Colvin T; Carter L; Bohn A; Bryan CM; et al. Massively Parallel de Novo Protein Design for Targeted Therapeutics. Nature 2017, 550, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Ljubetič A; Lapenta F; Gradišar H; Drobnak I; Aupič J; Strmšek Ž; Lainšček D; Hafner-Bratkovič I; Majerle A; Krivec N; Bencina M; Pisanski T; Velickovic TC; Round A; Carazo JM; Melero R; Jerala R Design of Coiled-Coil Protein-Origami Cages That Self-Assemble In Vitro and In Vivo. Nat. Biotechnol 2017, 35, 1094. [DOI] [PubMed] [Google Scholar]
- (176).Modica JA; Lin Y; Mrksich M Synthesis of Cyclic Megamolecules. J. Am. Chem. Soc 2018, 140, 6391–6399. [DOI] [PubMed] [Google Scholar]
- (177).Wen AM; Steinmetz NF Design of Virus-Based Nanomaterials for Medicine, Biotechnology, and Energy. Chem. Soc. Rev 2016, 45, 4074–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Naso MF; Tomkowicz B; Perry WL; Strohl WR Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Ylä-Herttuala S Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol. Ther 2012, 20, 1831–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Keeler AM; Flotte TR Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol 2019, 6, 601–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Lundstrom K Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Lizotte PH; Wen AM; Sheen MR; Fields J; Rojanasopondist P; Steinmetz NF; Fiering S In Situ Vaccination with Cowpea Mosaic Virus Nanoparticles Suppresses Metastatic Cancer. Nat. Nanotechnol 2016, 11, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Kerstetter-Fogle A; Shukla S; Wang C; Beiss V; Harris PLR; Sloan AE; Steinmetz NF Plant Virus-Like Particle In Situ Vaccine for Intracranial Glioma Immunotherapy. Cancers 2019, 11, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Eriksson F; Tsagozis P; Lundberg K; Parsa R; Mangsbo SM; Persson MAA; Harris RA; Pisa P Tumor-Specific Bacteriophages Induce Tumor Destruction through Activation of Tumor-Associated Macrophages. J. Immunol 2009, 182, 3105–3111. [DOI] [PubMed] [Google Scholar]
- (185).Lee KL; Murray AA; Le DHT; Sheen MR; Shukla S; Commandeur U; Fiering S; Steinmetz NF Combination of Plant Virus Nanoparticle-Based In Situ Vaccination with Chemotherapy Potentiates Antitumor Response. Nano Lett. 2017, 17, 4019–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (186).Murray AA; Wang C; Fiering S; Steinmetz NF In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol. Pharmaceutics 2018, 15, 3700–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Lebel M-È; Chartrand K; Tarrab E; Savard P; Leclerc D; Lamarre A Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Lett. 2016, 16, 1826–1832. [DOI] [PubMed] [Google Scholar]
- (188).Hoopes PJ; Wagner RJ; Duval K; Kang K; Gladstone DJ; Moodie KL; Crary-Burney M; Ariaspulido H; Veliz FA; Steinmetz NF; Fiering SN Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Mol. Pharmaceutics 2018, 15, 3717–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Hoopes PJ; Mazur CM; Osterberg B; Song A; Gladstone DJ; Steinmetz NF; Veliz FA; Bursey AA; Wagner RJ; Fiering SN Effect of Intra-Tumoral Magnetic Nanoparticle Hyperthermia and Viral Nanoparticle Immunogenicity on Primary and Metastatic Cancer. Proceedings from the SPIE BiOS , 2017, San Francisco, CA, January 28–February 2, 2017; SPIE: Bellingham, WA, 2017; Vol. 10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Hoopes PJ; Moodie KL; Petryk AA; Petryk JD; Sechrist S; Gladstone DJ; Steinmetz NF; Veliz FA; Bursey AA; Wagner RJ; Rajan A; Dugat D; Crary-Burney M; Fiering SN Hypo-Fractionated Radiation, Magnetic Nanoparticle Hyperthermia and a Viral Immunotherapy Treatment of Spontaneous Canine Cancer. Proceedings from the SPIE BiOS, 2017, San Francisco, CA, January 28–February 2, 2017; SPIE: Bellingham, WA, 2017; Vol. 10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Donaldson B; Lateef Z; Walker GF; Young SL; Ward VK Virus-Like Particle Vaccines: Immunology and Formulation for Clinical Translation. Expert Rev. Vaccines 2018, 17, 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Balke I; Zeltins A Use of Plant Viruses and Virus-Like Particles for the Creation of Novel Vaccines. Adv. Drug Delivery Rev 2019, 145, 119–129. [DOI] [PubMed] [Google Scholar]
- (193).Crisci E; Bárcena J; Montoya M Virus-Like Particle-Based Vaccines for Animal Viral Infections. Inmunologia 2013, 32, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Mohsen MO; Zha L; Cabral-Miranda G; Bachmann MF Major Findings and Recent Advances in Virus–Like Particle (VLP)-Based Vaccines. Semin. Immunol 2017, 34, 123–132. [DOI] [PubMed] [Google Scholar]
- (195).Mohsen MO; Speiser DE; Knuth A; Bachmann MF Virus-Like Particles for Vaccination against Cancer. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol 2020, 12, No. e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Lee KL; Twyman RM; Fiering S; Steinmetz NF Virus-Based Nanoparticles as Platform Technologies for Modern Vaccines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2016, 8, 554–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Huang X; Wang X; Zhang J; Xia N; Zhao Q Escherichia Coli-Derived Virus-Like Particles in Vaccine Development. npj Vaccines 2017, 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Fettelschoss-Gabriel A; Fettelschoss V; Thoms F; Giese C; Daniel M; Olomski F; Kamarachev J; Birkmann K; Bühler M; Kummer M; Zeltins A; Marti E; Kundig TM; Bachmann MF Treating Insect-Bite Hypersensitivity in Horses with Active Vaccination against IL-5. J. Allergy Clin. Immunol 2018, 142, 1194–1205. [DOI] [PubMed] [Google Scholar]
- (199).Bruckman MA; Czapar AE; VanMeter A; Randolph LN; Steinmetz NF Tobacco Mosaic Virus-Based Protein Nanoparticles and Nanorods for Chemotherapy Delivery Targeting Breast Cancer. J. Controlled Release 2016, 231, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Zeng Q; Wen H; Wen Q; Chen X; Wang Y; Xuan W; Liang J; Wan S Cucumber Mosaic Virus as Drug Delivery Vehicle for Doxorubicin. Biomaterials 2013, 34, 4632–4642. [DOI] [PubMed] [Google Scholar]
- (201).Cao J; Guenther RH; Sit TL; Opperman CH; Lommel SA; Willoughby JA Loading and Release Mechanism of Red Clover Necrotic Mosaic Virus Derived Plant Viral Nanoparticles for Drug Delivery of Doxorubicin. Small 2014, 10, 5126–5136. [DOI] [PubMed] [Google Scholar]
- (202).Le DHT; Lee KL; Shukla S; Commandeur U; Steinmetz NF Potato Virus X, a Filamentous Plant Viral Nanoparticle for Doxorubicin Delivery in Cancer Therapy. Nanoscale 2017, 9, 2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Min J; Moon H; Yang HJ; Shin H-H; Hong SY; Kang S Development of P22 Viral Capsid Nanocomposites as Anti-Cancer Drug, Bortezomib (BTZ), Delivery Nanoplatforms. Macromol. Biosci 2014, 14, 557–564. [DOI] [PubMed] [Google Scholar]
- (204).Franke CE; Czapar AE; Patel RB; Steinmetz NF Tobacco Mosaic Virus-Delivered Cisplatin Restores Efficacy in Platinum-Resistant Ovarian Cancer Cells. Mol. Pharmaceutics 2018, 15, 2922–2931. [DOI] [PubMed] [Google Scholar]
- (205).Liu X; Liu B; Gao S; Wang Z; Tian Y; Wu M; Jiang S; Niu Z Glyco-Decorated Tobacco Mosaic Virus as a Vector for Cisplatin Delivery. J. Mater. Chem. B 2017, 5, 2078–2085. [DOI] [PubMed] [Google Scholar]
- (206).Ashley CE; Carnes EC; Phillips GK; Durfee PN; Buley MD; Lino CA; Padilla DP; Phillips B; Carter MB; Willman CL; Brinker CJ; Caldeira J; Chackerian B; Wharton W; Peabody DS Cell-Specific Delivery of Diverse Cargos by Bacteriophage MS2 Virus-Like Particles. ACS Nano 2011, 5, 5729–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Bar H; Yacoby I; Benhar I Killing Cancer Cells by Targeted Drug-Carrying Phage Nanomedicines. BMC Biotechnol. 2008, 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Lam P; Lin RD; Steinmetz NF Delivery of Mitoxantrone Using a Plant Virus-Based Nanoparticle for the Treatment of Glioblastomas. J. Mater. Chem. B 2018, 6, 5888–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Lin RD; Steinmetz NF Tobacco Mosaic Virus Delivery of Mitoxantrone for Cancer Therapy. Nanoscale 2018, 10, 16307–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Czapar AE; Zheng Y-R; Riddell IA; Shukla S; Awuah SG; Lippard SJ; Steinmetz NF Tobacco Mosaic Virus Delivery of Phenanthriplatin for Cancer Therapy. ACS Nano 2016, 10, 4119–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Wu W; Hsiao SC; Carrico ZM; Francis MB Genome-Free Viral Capsids as Multivalent Carriers for Taxol Delivery. Angew. Chem., Int. Ed 2009, 48, 9493–9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Pitek AS; Wang Y; Gulati S; Gao H; Stewart PL; Simon DI; Steinmetz NF Elongated Plant Virus-Based Nanoparticles for Enhanced Delivery of Thrombolytic Therapies. Mol. Pharmaceutics 2017, 14, 3815–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Esfandiari N; Arzanani MK; Soleimani M; Kohi-Habibi M; Svendsen WE A New Application of Plant Virus Nanoparticles as Drug Delivery in Breast Cancer. Tumor Biol. 2016, 37, 1229–1236. [DOI] [PubMed] [Google Scholar]
- (214).Rothwell WT; Bell P; Richman LK; Limberis MP; Tretiakova AP; Li M; Wilson JM Intrathecal Viral Vector Delivery of Trastuzumab Prevents or Inhibits Tumor Growth of Human HER2-Positive Xenografts in Mice. Cancer Res. 2018, 78, 6171–6182. [DOI] [PubMed] [Google Scholar]
- (215).Yacoby I; Bar H; Benhar I Targeted Drug-Carrying Bacteriophages as Antibacterial Nanomedicines. Antimicrob. Agents Chemother 2007, 51, 2156–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Yacoby I; Shamis M; Bar H; Shabat D; Benhar I Targeting Antibacterial Agents by Using Drug-Carrying Filamentous Bacteriophages. Antimicrob. Agents Chemother 2006, 50, 2087–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Galaway FA; Stockley PG MS2 Virus-Like Particles: A Robust, Semisynthetic Targeted Drug Delivery Platform. Mol. Pharmaceutics 2013, 10, 59–68. [DOI] [PubMed] [Google Scholar]
- (218).Lam P; Steinmetz NF Delivery of SiRNA Therapeutics Using Cowpea Chlorotic Mottle Virus-Like Particles. Biomater. Sci 2019, 7, 3138–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Azizgolshani O; Garmann RF; Cadena-Nava R; Knobler CM; Gelbart WM Reconstituted Plant Viral Capsids Can Release Genes to Mammalian Cells. Virology 2013, 441, 12–17. [DOI] [PubMed] [Google Scholar]
- (220).Zhou Y; Maharaj PD; Mallajosyula JK; McCormick AA; Kearney CM In Planta Production of Flock House Virus Transencapsidated RNA and Its Potential Use as a Vaccine. Mol. Biotechnol 2015, 57, 325–336. [DOI] [PubMed] [Google Scholar]
- (221).Cao J; Guenther RH; Sit TL; Lommel SA; Opperman CH; Willoughby JA Development of Abamectin Loaded Plant Virus Nanoparticles for Efficacious Plant Parasitic Nematode Control. ACS Appl. Mater. Interfaces 2015, 7, 9546–9553. [DOI] [PubMed] [Google Scholar]
- (222).Chariou PL; Steinmetz NF Delivery of Pesticides to Plant Parasitic Nematodes Using Tobacco Mild Green Mosaic Virus as a Nanocarrier. ACS Nano 2017, 11, 4719–4730. [DOI] [PubMed] [Google Scholar]
- (223).Chariou PL; Dogan AB; Welsh AG; Saidel GM; Baskaran H; Steinmetz NF Soil Mobility of Synthetic and Virus-Based Model Nanopesticides. Nat. Nanotechnol 2019, 14, 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


