Abstract
Lycorine hydrochloride (LH) is an active ingredient sourced from the medicinal herb Lycoris radiata. Previous studies have suggested that LH exerts tumor suppression activity in several human cancers. However, the anti-cancer effect of LH in melanoma and the potential molecular mechanisms still need to be further studied. p21Cip1/WAF1, unlike its traditional cyclin-dependent kinase (CDK) inhibitor role, is believed to act as an oncogene under certain cellular conditions. In this research, an increased expression of p21Cip1/WAF1 was found in human melanoma tissues and positively related to the tumor invasion depth. High level of p21Cip1/WAF1 was found to correlate with bad outcomes of melanoma patients by Kaplan-Meier survival analysis. Functional experiments demonstrated that the proliferation, migration and invasion ability of A375 and MV3 melanoma cells was powerfully inhibited by LH through inducing S phase cell cycle arrest and regulating epithelial-mesenchymal transition (EMT). In NOD/SCID mice model, LH effectively inhibited the xenograft tumor growth and lung metastasis of A375 cells. Further research revealed that LH reduced p21Cip1/WAF1 protein by accelerating its ubiquitination. Importantly, the LH-induced suppression of cell proliferation and metastasis was rescued by p21Cip1/WAF1 overexpression, both in vitro an in vivo. Taken together, LH, which suppresses the proliferation and metastasis of melanoma cells via down-regulating p21Cip1/WAF1, is expected to be developed as an effective medicine for melanoma therapy.
Keywords: Melanoma, lycorine hydrochloride, p21Cip1/WAF1, cell proliferation, migration, invasion
Introduction
Melanoma has always been the most malignant form of cutaneous tumor, which makes up the largest proportion of mortality related to skin cancer [1]. What’s worse, the morbidity of melanoma is increasing worldwide, especially in fair-skinned populations. For the American male and female population respectively, the incidence of melanoma ranked fifth and sixth among all kinds of tumors [1]. By 2030, the annual treatment costs for newly diagnosed melanoma in American are estimated to be three times that of 2011 [2]. In China, the age-standardized prevalence of melanoma was more than doubled from 1990 to 2017 [3]. Surgical excision is the preferred therapeutic strategy for early-stage melanoma, which can significantly prolong the survival of patients [4]. However, advanced melanoma still has a poor prognosis and remains a challenge for treating physicians [5]. Among melanoma patients with three or more metastatic areas, the one-year survival rate is less than 5% [6]. Although significant progress has been made on immunotherapies and targeted therapies for advanced melanoma in recent years, a considerable proportion of patients still have no remarkable therapeutic response or experience various side effects [7,8]. Thus, developing new drugs with excellent effectiveness and high bio-safety for melanoma therapy is still an urgent mission.
LH is a natural alkaloid derived from the medicinal plant Lycoris radiata. Accumulated evidences demonstrated that lycorine possesses diverse bio-activities, including anti-inflammation [9], anti-viral [10], anti-fungal [11], especially anti-tumor [12-17]. Previous studies have shown that lycorine suppresses cell proliferation of ovarian carcinoma and osteosarcoma by mediating cell cycle arrest [12,13]. Lycorine promotes autophagy and apoptosis of hepatocellular carcinoma cells by inactivating TCRP1/Akt/mTOR axis [14]. In breast cancer mice model, lycorine could potently inhibit tumor growth and pulmonary metastasis without apparent toxicity [15]. Additionally, lycorine dramatically enhanced the tumor-suppressive effect of vemurafenib on colorectal carcinoma in mice [17]. Meanwhile, what should be emphasized that lycorine exhibits ideal biosafety [12,14,15,17].
p21Cip1/WAF1 (hereafter referred as p21), encoded by CDKN1A, is an important member of CDK inhibitors. It can block the cell cycle transition by specifically interacting with a series of CDK-cyclin complexes and inhibiting their activity [18,19]. In addition, p21 promotes apoptosis and senescence [18,19]. Based on the above mechanisms, p21 has been widely known as a tumor suppressor. However, mounting proofs demonstrate that p21 also acts as an oncogenic factor by promoting cell cycle progression [20], inhibiting apoptosis [21], and favoring migration [22]. A growing number of researches have proved that the p21 expression is elevated in various human cancers which promotes tumor progression and suggests an unfavorable prognosis [23-27]. Regarding the dual role of p21 as either a suppressor or a promoter in cancers, further research is needed to identify its utility as an indicator of prognosis and a molecular target of therapeutic in individual carcinoma entities. Our current study proves that LH exerts anti-tumor activity in melanoma cells through a p21 dependent way. Therefore, p21 may serve as a tumor promoter in melanoma, and LH is considered as a potential anti-melanoma agent.
Materials and methods
Cell culture and LH treatment
A375, MV3 human melanoma cell lines and 293FT human embryonic kidney cell line were acquired from the American Type Culture Collection (ATCC, USA). MV3 cells were cultured with RPMI-1640 (Gibco, USA) and A375 cells were cultured with DMEM (Gibco). The mediums were all added FBS (Gibco) to its final concentration of 10% and penicillin-streptomycin (P/S, Gibco) to its final concentration of 1%. Culturing of 293FT cells was according to a previous description [28]. A 37°C incubator with 5% CO2 was used for cells maintaining. LH (molecular formula: C16H17NO4•HCl, purity ≥ 99%) was bought from Must Bio-technology (Chengdu, China) and prepared into an initial solution of 100 mM with dimethyl sulfoxide (DMSO, Sigma-Aldrich, USA). For cell treatment, LH was diluted with culture medium to specified concentrations and incubated for different time. The cell morphology changes induced by LH were observed by an optics microscope (Nikon, Japan).
Cell viability assay
Cells in amount of 1×103/well were placed into 96-well plates and cultured for 8 h. Subsequently, 200 μL medium containing different concentrations of LH replace the original for further culturing, DMSO was used as control. After 1, 3, 5 and 7 days of culturing, the wells were dripped with MTT (Beyotime, China) solution to meet a working concentration of 0.5 µg/mL. The supernatant was aspirated away 2 h later, add 200 μL of DMSO to each well. Optical density of the dissolved formazan at 560 nm was quantified by a microplate reader (Thermo Fisher, USA).
Edu staining assay
2×104/well cells were added into a 24-well plate followed by an 8 h culture, then treated with 20 μM LH or DMSO for 48 h. Edu staining was performed with BeyoClick™ EdU Cell Proliferation Kit (Beyotime) following manufacturer’s instructions. Cells stained with EdU were photographed under fluorescence microscope (Olympus, Japan).
Flow cytometry assay (FCA)
The influence of LH on cell cycle progression was evaluated via FCA. After 20 μM LH or DMSO treatment for 48 h, cells were collected up. Following the fixation with 75% ethanol and washed thrice, use propidium iodide solution (PI, Sigma-Aldrich) to stain the cells at room-temperature for half an hour. Afterwards, the FCA was performed by virtue of BD Accuri C6 flow cytometer (BD, USA), and data was analyzed with FlowJo 7.6.1 (FlowJo LLC, USA).
Soft agar assay
In order to analyze whether the colony formation capacities of melanoma cells were influenced by LH treatment. The 2× medium containing 20% serum, 2% P/S was first mixed with an equal volume of 1.2% agarose (Sigma-Aldrich), then 1.5 mL/well was loaded into six-well plates to generate base agar. Afterwards, 1 mL complete medium containing 1×103 cells and 0.3% agarose was added as top agar. Both the base and top agar were containing 20 μM LH, DMSO was used as control. The colonies were photographed after 3 weeks culture, and colony numbers were recorded after MTT staining.
Wound-healing assay
Linear wounds were carved with a 200 μL pipette tip on the full confluent cell layer. After washing three times, the cells were cultured with FBS-free medium containing 20 μM LH or isometric DMSO. The wounds were imaged at 0, 24, 48 and 72 h and the closure indexes were calculated according to a previous report [29].
Migration and invasion transwell assay
Transwell assay was performed by virtue of transwell chambers having 8-μm sized pores (Corning, China). For cell migration and invasion assay respectively, the upper chambers were without or with Matrigel (BD) pre-coated. Cells were suspended in FBS-free medium containing 20 μM LH or isometric DMSO at a concentration of 5×105/mL. Then, the mixture was loaded into each upper chamber with volume of 200 µL. The bottom chambers were loaded with 500 µL complete medium. After incubation for 16 to 24 h, cells remaining in the upper chambers were carefully erased followed by crystal violet solution (Beyotime) staining. The stained cells were observed under microscope (Nikon) and photos were taken.
Vector construction and infection
For the over-expression of p21 in melanoma cells, full-length human p21 cDNA (NCBI accession number: NM_000389.5) was cloned into pCDH-CMV-MCS-EF1-GFP+Puro lentiviral vector (Unibio, China). Lentiviral particles harboring p21 were packaged by Lipofectamine 2000 (Thermo Fisher, USA) and transfected into 293FT cells to generate viruses, empty vector was used as control. Afterwards, A375 and MV3 cells were infected with lentivirus with the assistance of 6 μg/mL polybrene (Invitrogen). After 48 h, the cells that failed to infect were eliminated using 4 μg/mL puromycin (Sigma-Aldrich). Finally, stable transfected cell lines were obtained.
Quantitative real-time PCR (qRT-PCR)
RNA was isolated from cells by Trizol reagent (Invitrogen). GoScript™ Reverse Transcriptase (Promega, USA) was used to synthesis first-strand cDNA. Mixed samples and SYBRTM Green PCR Master Mix (Takara, Japan) were reacted on LightCycler 96 real-time PCR instrument (Roche, USA). The primer sequences of p21 and house-keeping gene GAPDH were used as below: p21 (forward: 5’-CGATGGAACTTCGACTTTGTCA-3’, reverse: 5’-GCACAAGGGTACAAGACAGTG-3’); GAPDH (forward: 5’-ACAACTTTGGTATCGTGGAAGG-3’, reverse: 5’-GCCATCACGCCACAGTTTC-3’). 2-ΔΔCt method was used to analyze relative gene expression. Detailed steps were according to a previous description [30].
Western blotting
In order to extract total proteins, the cells were lysed using ice-cold RIPA buffer (Beyotime) containing protease inhibitor. Nuclear protein and Cytoplasmic Protein Extraction Kit (Beyotime) to extract proteins from the nucleus or cytoplasm. The detailed steps of western blotting were referred to a previous study [31]. The immunoblots were visualized by Super ECL prime (US Everbright, China) and scanned using a ChemiScope system (Clinx Science, China). Antibodies used are listed as follows: α-Tubulin (1:1,000, Cell Signaling Technology, CST), CDK1 (1:1000, Proteintech, China), CDK2 (1:2000, Proteintech), Cyclin E1 (1:1000, CST), E-cadherin (1:1,000, CST), Histone H3 (1:2,000, CST), N-cadherin (1:1,000, CST), p21 (1:1000, Proteintech), Vimentin (1:1,000, CST), HRP-conjugated goat anti-rabbit IgG (1:1000, Beyotime).
Protein turnover assay
A375 cells were pretreated with 20 μM LH or DMSO for 16 h and further exposed to 10 μM protein synthesis inhibitor cycloheximide (CHX, Sigma-Aldrich). After CHX incubation for 0, 1, 2 and 4 h, the cells were lysed and the p21 protein in each sample was detected by western blotting.
Protein ubiquitylation assay
293FT cells transiently transfected with HA-UB were pretreated with 20 μM LH or DMSO for 16 h, then further exposed to 20 μM MG132 for another 8 h. Total proteins from the cell lysates were quantified and equal amount of each sample was overnight incubated with p21 antibody (1:50, Proteintech) at 4°C. Then the immuno-complexes were captured by protein A + G agarose (Beyotime), followed by centrifugation to collect the beads. After washed 5×5 min in ice-cold PBS, the beads were re-suspended in 1× loading buffer thus heating at 100°C for 15 min. Then, immunoblotting was performed and HA antibody (1:1,000, CST) was used to detect the ubiquitinated p21.
Tumor xenograft assay
Animal experiments were authorized and supervised by the Institutional Animal Care and Use Committees of the Southwest University. To find out whether LH inhibits tumor growth in vivo, 12 NOD/SCID mice (HFK Bioscience, China) were subcutaneously injected with 1×106 A375 cells on the left groin to generate xenograft mice model. From the 10th day on, the mice with xenografts were randomized into two groups (6 in each group), followed by intraperitoneal injection of LH (30 mg/kg/day) or equal volume DMSO for 25 times. In order to figure out the status of p21 in tumor growth inhibition induced by LH, we randomly divided 18 mice into 3 groups (6 in each group). The vector-overexpressing A375 cells (1×106/locus) were subcutaneously injected on the left groin of mice in group I and II. Equal amount of p21-overexpressing A375 cells were injected in group III. For group I, DMSO was intraperitoneally injected every day as control, and the other two groups were injected with LH (30 mg/kg/day) for 25 times. The mice were weighed per 5 days and recorded. A digital electronic vernier caliper was used to measure the width and length of the tumors per 5 days, and the formula “Volume = length × width2 × π/6” was utilized to calculate the tumor volumes. Finally, the developed tumors were excised and weighed after euthanized the mice.
Immunohistochemistry (IHC) staining
Paraffin-embedded tissue sections from 14 cutaneous melanoma patients and 10 benign nevi patients were collected from the Third Hospital of Hebei Medical University. Usage of samples of human origin was strictly followed the Declaration of Helsinki and obtained informed consent. Xenograft tumors excised from the mice were fixed, dehydrated, paraffin imbedded and sliced into sections of 4 μm thick. The IHC staining assay was conducted according to the method reported previously [32]. p21 antibody (1:400, Proteintech) was used for staining of clinical sections, the p21 positivity was scored as follows: 0 (less than 25% positive), 1 (25% to 50% positive), 2 (50% to 75% positive) and 3 (more than 75% positive). p21 (1:400, Proteintech) and Ki67 (1:400, CST) antibodies were used for staining of xenograft tumor sections. Invasion depth of the 14 cutaneous melanomas were measured by Image J software, and Pearson’s correlation analysis of GraphPad Prism 6 was utilized to analyze the relationship between tumor invasion depth and p21 positive index.
In vivo metastasis assay
Animal model of melanoma metastasis in vivo was generated using NOD/SCID mice. Briefly, 8 mice were injected with 5×105 A375 cells into the tail veins once a day for 3 days. The mice were randomized into two groups (4 in each group) on the fourth day, followed by intraperitoneal injection of LH (30 mg/kg/day) or equal volume DMSO for 40 times. In order to figure out the status of p21 in metastasis inhibition induced by LH, 12 mice were randomly divided into 3 groups (4 in each group). The mice in group I and II were injected with 5×105 vector-overexpressing A375 cells once a day for 3 days. Equal amount of p21-overexpressing A375 cells were injected in group III. For group I, DMSO was intraperitoneally injected every day as control, and the other two groups were injected with LH (30 mg/kg/day) for 40 times. At last, the mice were euthanized and lung metastatic tumors were observed after H&E staining.
Oncomine database and Kaplan-Meier survival analysis
The differential expression of CDKN1A gene in melanoma and benign nevi was analyzed on Oncomine database (https://www.oncomine.org/resource/login.html), data was considered as statistically different if P-value<0.05. The association of CDKN1A gene with clinical outcomes of melanoma patients was analyzed with online tool Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia. cancer-pku.cn). Overall survival (OS) and disease-free survival (DFS) were plotted by Kaplan-Meier survival analysis. Group cutoff was set as Cutoff-High (%) = 33% and Cutoff-Low (%) = 67%, the confidence interval was 95%, data was considered as statistically different if log-rank P-value<0.05.
Statistical analysis
Data are shown as mean ± SD. Statistical analysis and graphic charts generation were performed by software GraphPad Prism 6. The difference between two sample means were evaluated using two-tailed unpaired Student’s t-test. Comparison among multiple groups was done with One-way ANOVA. A significant statistical difference was determined when P-value<0.05.
Results
LH treatment inhibits the proliferation of melanoma cells
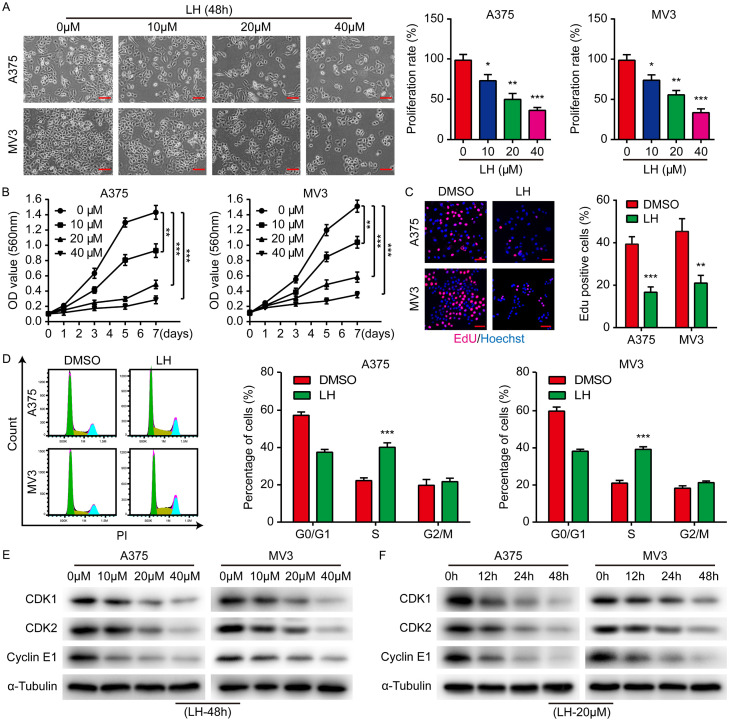
A375 and MV3 melanoma cells were treated with 0, 10, 20 and 40 μM LH for 48 h. We observed that the cell proliferation rate decreased with the increasing LH dose (Figure 1A). MTT assays were further carried out on the cells treated with 0, 10, 20, and 40 μM LH respectively. As expected, the viability curves of A375 and MV3 cells exhibited a significant decline with a dose-dependent pattern (Figure 1B). In addition, significant decreases in the EdU positive rates of both A375 and MV3 cells after LH treatment were observed (Figure 1C). To uncover the mechanisms underlying the anti-proliferation ability of LH, cell cycle was further analyzed, which indicated that the percentage of cells in S-phase was markedly elevated after 20 μM LH treatment for 48 h (Figure 1D). In addition, the expression of CDK1, CDK2 and Cyclin E1 in LH treated cells was dose- and time-dependently down regulated (Figure 1E, 1F). Thus, the anti-proliferative effect of LH against melanoma cells could be achieved mainly through inducing S-phase cell cycle arrest.
Figure 1.
Anti-proliferative effect of LH on melanoma cells. A. A375 and MV3 cells were treated with LH (0, 10, 20 and 40 μM) for 48 h and proliferation of the cells was observed. Cell percentage in 0 μM group is regarded as 100%. Scale bar, 100 μm. B. Under LH (0, 10, 20, and 40 μM) treatment, cell viability was measured by MTT assay on days 1, 3, 5 and 7. C. Percentage of Edu positive cells after LH (20 μM) treatment for 48 h. Scale bar, 50 μm. D. Cell cycle distribution of the cells after LH (20 μM) treatment for 48 h. E, F. The protein levels of CDK1, CDK2 and Cyclin E1 in LH treated cells were measured by western blotting. Mean ± SD; *P<0.05, **P<0.01, ***P<0.001.
The migration and invasion of melanoma cells were inhibited by LH treatment
Considering that the malignant behavior of melanoma is mainly attributed to its high metastatic capacity, the migration and invasion of LH-treated cells were evaluated. It is shown that the wound healing in LH treated cells was significantly delayed (Figure 2A). Transwell assays showed that the cellular migration and invasion was hindered by LH (Figure 2B, 2C). Hence, we further explored the changes in EMT-related proteins. Epithelial marker E-cadherin protein level was elevated by LH treatment in A375 and MV3 cells, while mesenchymal markers N-cadherin and Vimentin were decreased (Figure 2D, 2E). In brief, LH regulates the expression of EMT-related proteins and thereby inhibits the mobility of melanoma cells.
Figure 2.
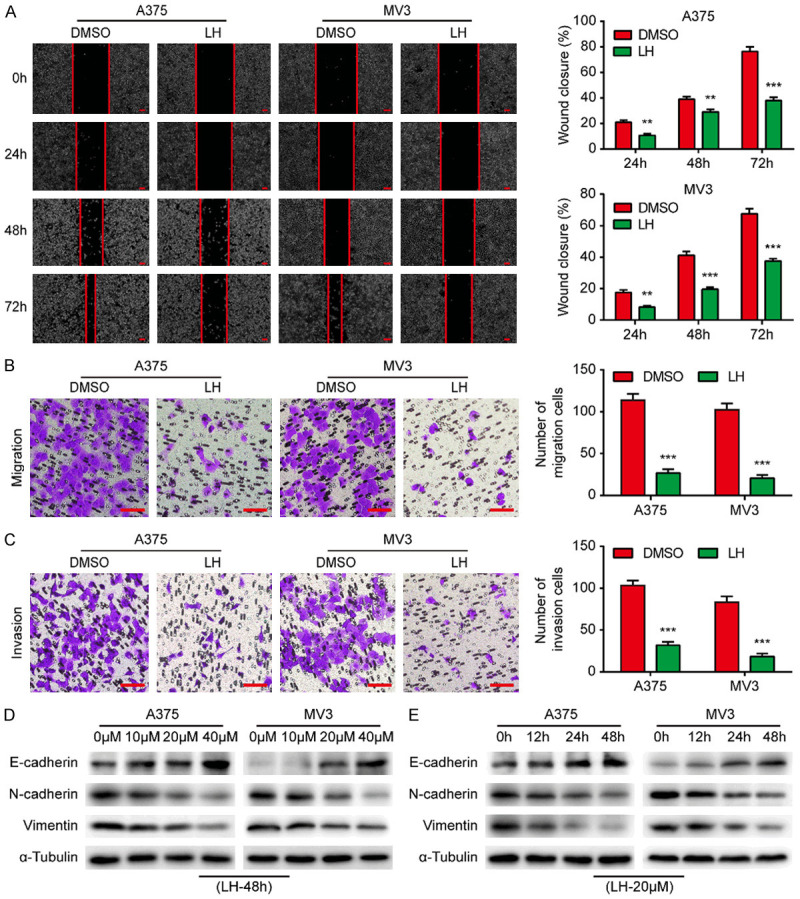
LH inhibits the mobility of melanoma cells. A. The wounds on cell layers under the incubation of 20 μM LH or DMSO were observed at different times (0, 24, 48, 72 h) and the closure rates were calculated. Scale bar, 100 μm. B, C. Migration and invasion of the cells under LH (20 μM) or DMSO treatment. Scale bar, 100 μm. D, E. The protein levels of E-cadherin, N-cadherin and Vimentin in LH treated cells were detected by western blotting. Mean ± SD; **P<0.01, ***P<0.001.
LH possesses anti-tumorigenic and anti-metastatic activity against melanoma in mice model
The effect of LH on tumorigenicity of melanoma cells in vitro was firstly investigated by soft agar assay. The findings revealed that the cells treated with LH generated smaller and fewer colonies in comparation with the control group (Figure 3A). An A375 xenograft mice model was subsequently established to verify whether LH can inhibit tumor growth in vivo. It was found that tumor size and weight of the mice administered with LH were smaller than those from the DMSO treated mice (Figure 3B, 3C). It is worth mentioning that the weight of the mice was not significantly affected by LH treatment (Figure 3D). The expression of Ki67, an indicator of cell proliferation activity, was significantly reduced in LH-treated tumors according to IHC staining assay (Figure 3E). Moreover, fewer metastases were found in the lungs of A375 metastasized mice under LH treatment (Figure 3F). In conclusion, LH can effectively suppress the melanoma growth and metastasis in vivo.
Figure 3.
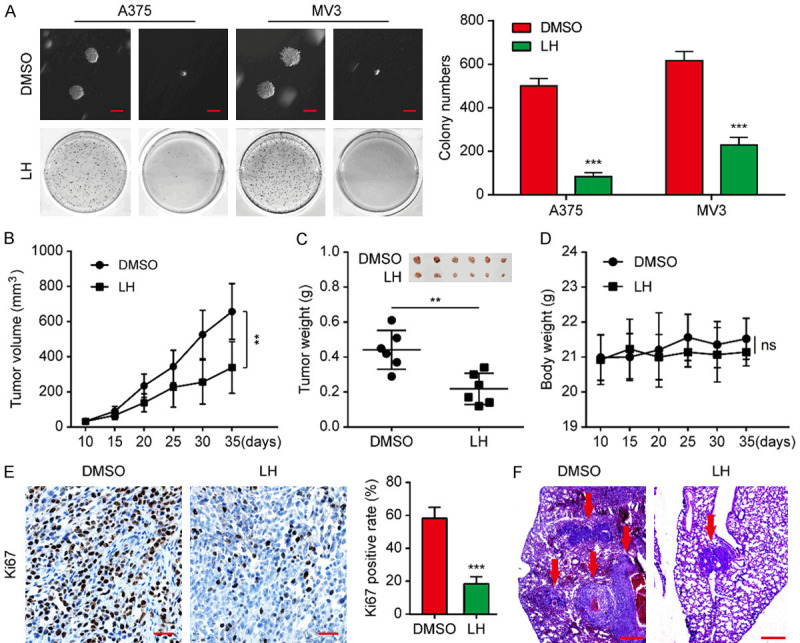
LH suppresses melanoma growth and lung metastasis in NOD/SCID mice. A. After 3 weeks incubation with LH (20 μM) or DMSO, colonies formed by A375 and MV3 cells were imaged and counted. Scale bar, 1 mm. B. Under the treatment of LH (30 mg/kg/day) or DMSO, volume of A375 xenograft tumors was calculated every 5 days. C. After 25 days treatment with LH (30 mg/kg/day) or DMSO, the tumors were excised from the mice and weighed to analyze. D. Weight monitoring curve of the mice. E. Expression of Ki-67 in the xenograft tumors from LH or DMSO treated mice model. IHC; Scale bar, 100 μm. F. Tumors in the lung of mice that metastasized with A375 cells were observed after LH (30 mg/kg/day for 40 days) or DMSO treatment. H&E; Scale bar, 1 mm. Mean ± SD; **P<0.01, ***P<0.001; ns, not significant.
p21 overexpression indicates poor prognosis of melanoma and LH promotes ubiquitination degradation of p21
In the present study, a decreased p21 expression was observed in melanoma cells after LH treatment (Figure 4A, 4B), which was inconsistent with its general function of blocking cell cycle progression. In addition, lower expression of p21 in LH treated A375 xenograft tumors was observed compared with DMSO group (Figure 4C). Based on the fact that p21 promotes tumor development in certain cancers [23-27], we speculated that p21 might play similar roles in melanoma. Hence, the expression of p21 in 14 cutaneous melanoma samples was analyzed by IHC, with 10 benign nevi (benign lesion of melanocytes) as control. It was found that the positive index of p21 in cutaneous melanoma was dramatically higher than in benign nevi (Figure 4D), and positively related to the tumor invasion depth (R = 0.407, P = 0.014) (Figure S1). The clinical significance of p21 in melanoma was further studied with the help of online database. Compared with benign nevi, data from Oncomine database showed that CDKN1A is highly expressed in melanoma (Figure 4E), which was consistent with our IHC results. Kaplan-Meier survival plots indicated that the CDKN1A levels were significantly related with unfavorable OS and DFS of melanoma patients (log-rank P = 0.029 and 0.024 respectively) (Figure 4F).
Figure 4.
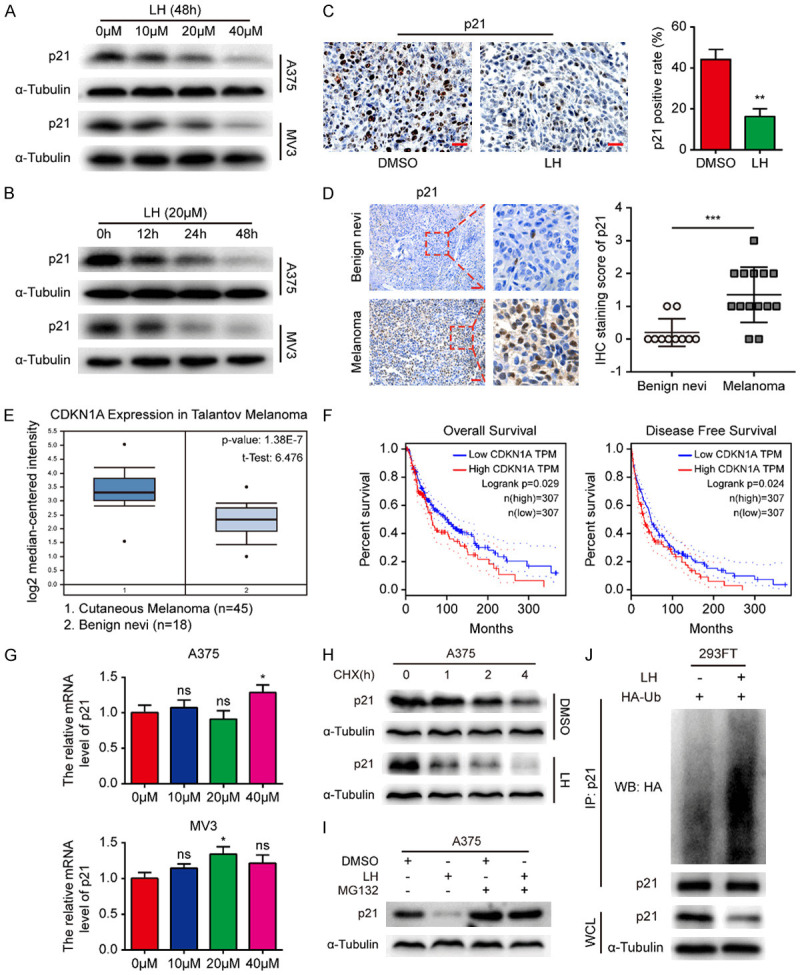
High p21 expression indicates poor prognosis of melanoma and LH promotes ubiquitination degradation of p21. A, B. Expression of p21 in A375 and MV3 cells was detected by western blotting after LH treatment. C. Expression of p21 in the xenograft tumors from LH or DMSO treated mice model. IHC; Scale bar, 100 μm. D. Expression of p21 in human cutaneous melanoma (n = 14) was detected by IHC staining, benign nevi (n = 10) was as control. Scale bar, 200 μm. E. The CDKN1A level in cutaneous melanoma tissues (n = 45) compared with benign nevi tissues (n = 18) from Oncomine database. F. The association of CDKN1A gene with clinical outcomes (OS and DFS) of melanoma patients were analyzed through GEPIA database. G. The effect of LH on the expression of p21 mRNA in melanoma cells. H. The LH and DMSO pre-treated A375 cells were incubated with 10 μM CHX and collected at 0, 1, 2 and 4 h, cell lysates were immunoblotted with anti-p21 antibody. I. Cells were cultured in the absence or presence condition of 20 μM LH, the p21 protein level was detected after 20 μM MG132 treatment. J. The ubiquitination of p21 was detected in LH treated 293-FT cells. Mean ± SD; *P<0.05, **P<0.01, ***P<0.001; ns, not significant.
Considering the previous studies suggested that the oncogenic role of p21 could depend largely on its cytoplasmic localization [20-22], the expression of p21 in A375 nucleus and cytoplasm was further studied. The results showed that p21 was decreased both in nucleus and cytoplasm of the cells treated by LH (Figure S2). In order to clarify the mechanism of LH down-regulates p21 in melanoma cells, qRT-PCR was performed to investigate whether LH affected the transcription of p21. However, the p21 mRNA level in melanoma cells was barely changed after LH treatment (Figure 4G). Therefore, we speculated that LH affected the post-transcriptional modification of p21. As expected, the degradation rate of p21 was accelerated by LH (Figure 4H), while the degradation was blocked by 20 μM proteasomal inhibitor MG132 (Figure 4I). These findings suggested that p21 may undergo ubiquitin-mediated degradation. Ubiquitination experiment was further carried out and found that the ubiquitination level of p21 was increased significantly after LH treatment (Figure 4J). These results indicated that LH promotes p21 protein degradation via ubiquitin-proteasome pathway.
Overexpressing p21 partially attenuates the anti-proliferative effect of LH on melanoma cells
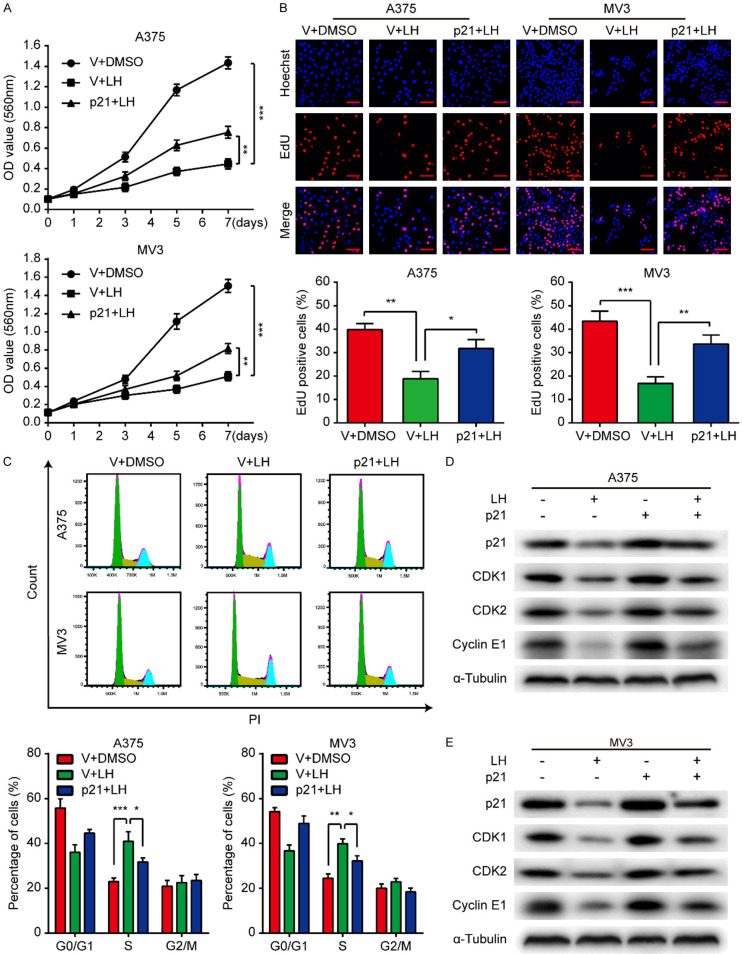
We established stable A375 and MV3 cell lines overexpressing p21 to clarify the status of p21 in the LH-induced cell proliferation inhibition. The results of MTT and EdU assays showed that the LH-induced proliferation inhibition was partially reversed after p21 was up-regulated (Figure 5A, 5B). Moreover, overexpression of p21 reduced the cell cycle arrest in S-phase which caused by LH (Figure 5C). Meanwhile, western blotting assays demonstrated that the LH-induced decrease of CDK1, CDK2, and Cyclin E1 were partially rescued after p21 was up-regulated (Figure 5D, 5E). Based on these data, p21 may be a target for LH to inhibit cell proliferation.
Figure 5.
Overexpressing p21 attenuates the anti-proliferative effect of LH. A. Viability of p21/vector-overexpressing A375 and MV3 cells under the treatment of 20 μM LH was measured by MTT assay. B. EdU staining of the p21/vector-overexpressing cells after LH (20 μM) treatment for 48 h. Scale bar, 50 μm. C. Cell cycle distribution of the p21/vector-overexpressing cells after LH (20 μM) treatment for 48 h. D, E. After being treated with LH (20 μM) or DMSO for 48 h, the protein levels of p21, CDK1, CDK2 and Cyclin E1 in p21/vector-overexpressing cells were detected by western blotting. Mean ± SD; *P<0.05, **P<0.01, ***P<0.001.
Overexpressing p21 partially counteracts the anti-migration/invasion effect of LH on melanoma cells
Transwell assays were conducted using p21/vector-overexpressing A375 and MV3 cells to explore the mechanisms in LH-induced migration and invasion inhibition. We noticed p21 overexpression could attenuate the inhibitory effect of LH on cell migration and invasion (Figure 6A, 6B). Additionally, the increased E-cadherin, as well as the decreased N-cadherin and Vimentin, in the cells after LH treatment were partially reversed by p21 overexpression (Figure 6C, 6D). The above findings suggested that LH may suppress melanoma cell migration and invasion through down-regulating p21.
Figure 6.
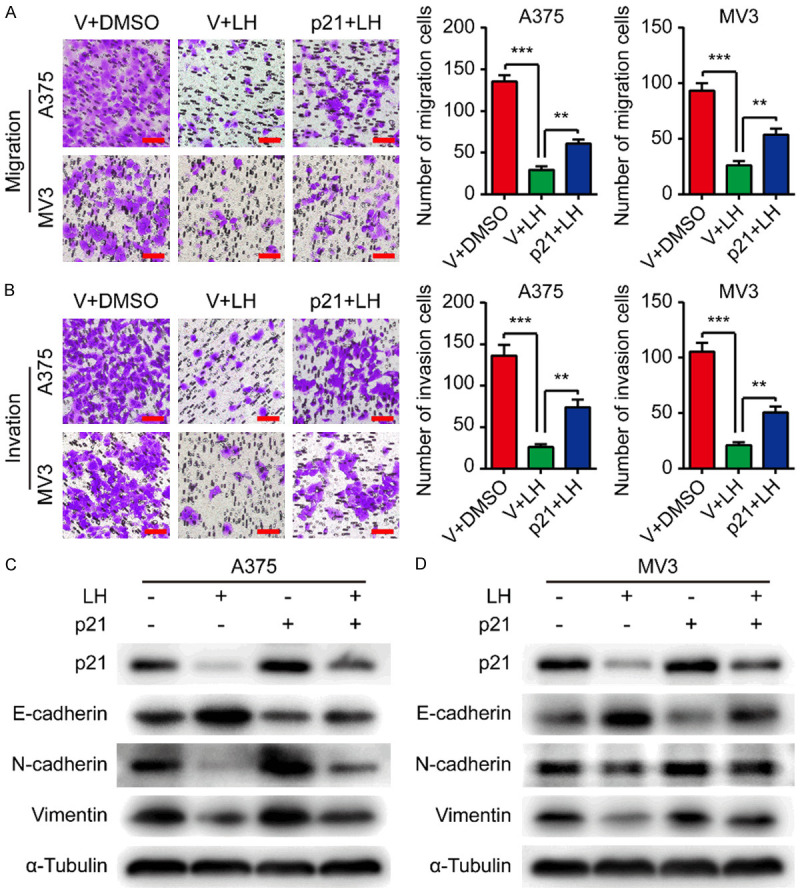
Overexpressing p21 partially counteracts the anti-migration/invasion effect of LH. A, B. Migration and invasion of p21/vector-overexpressing cells under LH (20 μM) or DMSO treatment. Scale bar, 100 μm. C, D. p21, E-cadherin, N-cadherin, and Vimentin proteins in p21/vector-overexpressing cells after LH (20 μM) or DMSO treatment for 48 h. Mean ± SD; **P<0.01, ***P<0.001.
Overexpressing p21 partially offsets the inhibitory effects of LH on tumor growth and metastasis of melanoma cells
We herein investigated the tumorigenesis of p21-overexpressing cells after LH treatment. The results of soft agar assay showed that under the treatment of LH, the colonies generated by A375 and MV3 cells overexpressing p21 were larger than the control group, both in volume and quantity (Figure 7A). In addition, subcutaneous xenograft tumors were generated with p21/vector-overexpressing A375 cells on NOD/SCID mice. After treatment with LH at 30 mg/kg/day for 25 days, the mice xenografted with p21-overexpressing A375 cells developed tumors larger in volume and weight compared with vector-overexpressing group (Figure 7B-D). At the same time, the decreased expression of Ki67 in LH treated tumors was partly rescued by p21 overexpression (Figure 7E). Moreover, p21-overexpressing A375 cells formed more metastases in lungs of the mice under LH treatment, compared with the vector-overexpressing cells (Figure 7F). Taken together, LH may inhibit tumorigenicity and metastasis of melanoma cells via down-regulating p21.
Figure 7.
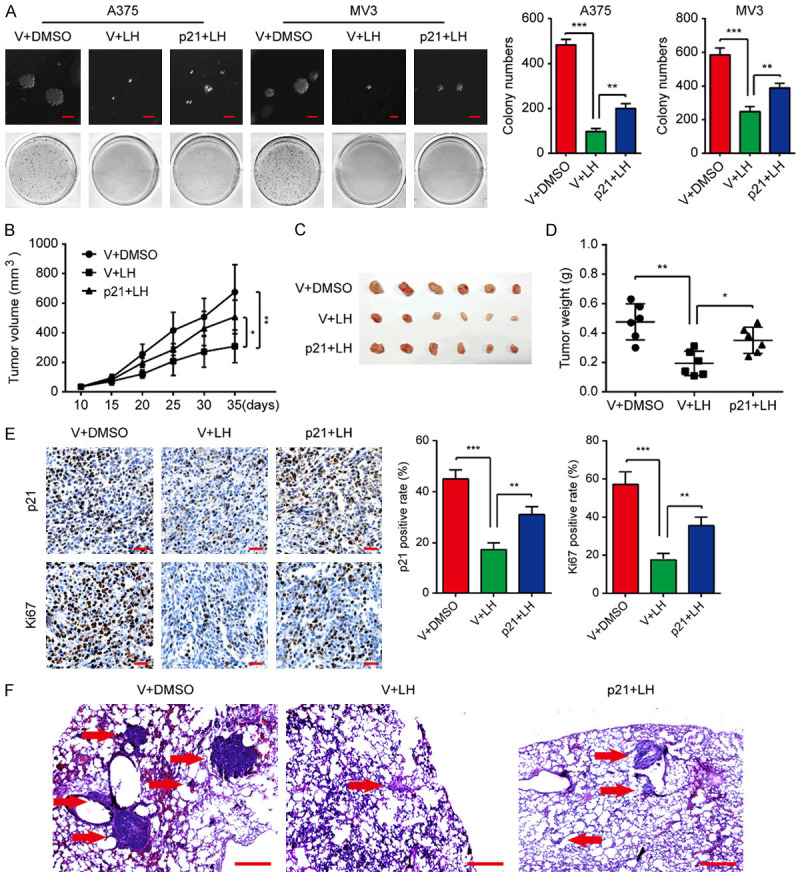
Overexpressing p21 attenuates the LH-induced suppression on tumor growth and lung metastasis of melanoma cells. (A) After 3 weeks incubation with DMSO or 20 μM LH, colonies formed by p21/vector-overexpressing cells were imaged and counted. Scale bar, 1 mm. (B) Tumor volume of p21/vector-overexpressing A375 xenograft tumors in mice under LH (30 mg/kg/day for 25 days) or DMSO treatment was measured. (C) Tumors were excised from the mice after 25 days treatment. (D) Weight of the tumors in (C) was measured. (E) IHC staining for p21 and Ki67 was performed in the xenograft tumors. Scale bar, 100 μm. (F) Tumors in the lung of mice that metastasized with p21/vector-overexpressed A375 cells were observed after LH (30 mg/kg/day for 40 days) or DMSO treatment. H&E; Scale bar, 1 mm. Mean ± SD; *P<0.05, **P<0.01, ***P<0.001.
Discussion
Organic compounds from natural sources have a long history of being applied to treat diseases, including cancer. In the past few decades, many kinds of plant-derived ingredients with anti-tumor activity have been discovered, and some of them have been approved by the USFDA [33,34]. Lycorine is an active alkaloid obtained from the medical herb Lycoris radiata, which possesses prominent anti-cancer activity for several cancers [12-17]. In a previous research, LH can inhibit the vasculogenic mimicry (VM) of melanoma cells [16]. VM is a process where invasive tumor cells undergoing trans-differentiation under ischemia and hypoxia to obtain endothelial cell behavior, forming capillary-like tubes with blood supply function [35]. Tumor progression and aggressive growth can be promoted by this structure. In the present study, we found that the cell proliferation, migration and invasion of A375 and MV3 melanoma cells were inhibited by LH through regulating cell cycle progression and EMT. In NOD/SCID mice model, tumorigenesis and lung metastasis of melanoma cells was significantly inhibited by LH. Therefore, LH may inhibit melanoma by diverse mechanisms. What’s more, we surprisingly found that p21, a classical CDK inhibitor which generally inhibits cellular processes, was down-regulated after LH treatment. This seemingly paradoxical phenomenon aroused our interest and led us to further research.
As a CDK inhibitor and a representative Cip/Kip family member, p21 plays an important role in cell cycle regulation [36]. The p21 protein specifically binds to and inactivates the Cyclin A/E-CDK2 complexes at G1/S cell cycle check-point, which leads to G1 phase cell cycle arrest [37,38]. Additionally, p21 attenuates the transcription activity of E2F complex through interacting directly with it, causing cell growth suppression [39,40]. Furthermore, p21 binds to PCNA in competition with PCNA-reliant DNA polymerase, thereby hindering its catalytic function, leading to inhibition of DNA duplication and enabling DNA repair [41]. Primarily owing to these properties, p21 has long been considered as a tumor-suppressor protein. However, there are accumulating evidences suggesting p21 can act as an oncogene, which promotes cellular proliferation [20], inhibits apoptosis [21] and facilitates migration [22]. Clinically, overexpression of p21 has been found in amount of cancers and related to tumor progression as well as negative prognosis, including prostate cancer [24], glioma [25], breast cancer [26] and squamous cell carcinoma of the esophagus [27].
In this study, data from the Oncomine database showed that CDKN1A gene is highly expressed in melanoma compared with benign nevi. Survival analysis from the GEPIA database showed that high CDKN1A expression is positively correlated with poor outcomes of melanoma patients. Immunohistochemical pathology analysis between 14 cases of melanoma and 10 cases of benign nevi indicated a significantly higher p21 protein expression in melanoma. It is noteworthy that the invasion thickness among primary melanomas is positively correlated with the expression index of p21, which is consistent with a previous research [42]. In addition, a follow-up survival study based on 60 patients with primary malignant melanoma revealed that the elevated p21 protein expression predicted a poor prognosis [43]. The above evidences illustrate that p21 is highly expressed both at mRNA and protein level in melanoma, which is not conducive to the prognosis of patients. This reminds us that p21 may have an oncogenic role in melanoma. Therefore, interfering with p21 transcriptionally or post-transcriptionally could be an alternative strategy for melanoma treatment. According to our data, protein level of p21 in melanoma cells was significantly decreased after LH treatment while the p21 mRNA was barely changed. The following ubiquitination experiment further confirmed that LH promoted the ubiquitination degradation of p21 protein. We subsequently overexpressed p21 in melanoma cells, as a consequence, the inhibitory effect of LH on cell proliferation, migration and invasion was partially attenuated. Similarly, p21 overexpressing also hindered the inhibitory effect of LH on tumor growth and lung metastasis of melanoma cells in mice. Taken together, LH promotes the degradation of p21 in melanoma cells through the ubiquitin-proteasome pathway, thereby exerting an anti-cancer effect.
Referring to previous studies, the mechanisms by which p21 promotes tumor development has been partially revealed. In cytoplasm, p21 could work as an adaptor for the assembly of cyclins and CDKs, promoting the formation of active complexes without inhibiting their kinase activity, thus enhancing cell proliferation via inducing cell cycle progression [44-46]. Besides, cytoplasmic p21 exert the functions of anti-apoptosis and promoting survival through inhibiting several caspases (pro-caspase-3, caspase-8&10) and apoptotic effectors (apoptosis signal-regulating kinase 1 and stress-activated protein kinase) [41,47]. Moreover, cytoplasmic p21 acts as a Rho-kinase (ROCK) inhibitor to compromise the ROCK/LIMK/cofilin pathway and promote cell motility, thereby promoting metastasis and invasion [20,22]. In this study, p21 proteins were down-regulated in both nucleus and cytoplasm of LH treated melanoma cells. Although nuclear-localized p21 is always regarded as a tumor suppressor, it does not seem to be absolute. For instance, p21 was found to interact with smad3 and acetyltransferase p/CAF in the nucleus and enhance the DNA binding and transcription activity of smad3 on several pro-invasive genes, such as MMP9, in breast cancer cells [48]. Whereas silencing p21 gene blocked the tumor invasion in xenograft mouse model [48]. Considering that smad3 can also transcriptionally regulate E-cadherin, N-cadherin and Vimentin gene expression [49-51], we speculated that p21 may modulate the expression of these factors in a similar way. For further confirmation, more follow-up studies are indispensable.
In conclusion, this study proved that LH inhibits the proliferation, migration and invasion of melanoma cells in vitro and in vivo. More than that, LH post-transcriptionally regulates the expression of p21 by promoting its ubiquitination-mediated degradation, and the suppressive effects of LH on melanoma cells could be relieved by p21 overexpression. Therefore, pharmacologically down-regulating p21 with LH could be developed as a therapeutic strategy against melanoma in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81872071, 81672502); the Natural Science Foundation of Chongqing (cstc2019jcyj-zdxmX0033); the Graduate Research Innovation Project of Chongqing (CYB18105, CYS18124 and CYS20137). We thank Kui Zhang, Jianbing Hou, Erhu Zhao, Zhen Dong and Liqun Yang for technical support and helpful comments.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Gershenwald JE, Guy GP Jr. Stemming the rising incidence of melanoma: calling prevention to action. J Natl Cancer Inst. 2016;108:djv381. doi: 10.1093/jnci/djv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guy GP, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591–596. [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Wang Y, Wang L, Yin P, Lin Y, Zhou M. Burden of melanoma in China, 1990-2017: findings from the 2017 global burden of disease study. Int J Cancer. 2020;147:692–701. doi: 10.1002/ijc.32764. [DOI] [PubMed] [Google Scholar]
- 4.Ross MI, Gershenwald JE. Evidence-based treatment of early-stage melanoma. J Surg Oncol. 2011;104:341–353. doi: 10.1002/jso.21962. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill CH, Scoggins CR. Melanoma. J Surg Oncol. 2019;120:873–881. doi: 10.1002/jso.25604. [DOI] [PubMed] [Google Scholar]
- 6.Oh E, Hong J, Yun CO. Regulatory T cells induce metastasis by increasing Tgf-beta and enhancing the epithelial-mesenchymal tansition. Cells. 2019;8:1387. doi: 10.3390/cells8111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 8.Luther C, Swami U, Zhang J, Milhem M, Zakharia Y. Advanced stage melanoma therapies: detailing the present and exploring the future. Crit Rev Oncol Hematol. 2019;133:99–111. doi: 10.1016/j.critrevonc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kang J, Zhang Y, Cao X, Fan J, Li G, Wang Q, Diao Y, Zhao Z, Luo L, Yin Z. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264.7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int Immunopharmacol. 2012;12:249–256. doi: 10.1016/j.intimp.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Yang Y, Xu Y, Ma C, Qin C, Zhang L. Lycorine reduces mortality of human enterovirus 71-infected mice by inhibiting virus replication. Virol J. 2011;8:483. doi: 10.1186/1743-422X-8-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang LF, Liu X, Sui YJ, Ma ZM, Feng XC, Wang F, Ma TH. Lycorine hydrochloride inhibits the virulence traits of Candida albicans. Biomed Res Int. 2019;2019:1851740. doi: 10.1155/2019/1851740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Z, Yu D, Fu S, Zhang G, Pan Y, Bao M, Tu J, Shang B, Guo P, Yang P, Zhou Q. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol Lett. 2013;218:174–185. doi: 10.1016/j.toxlet.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Wang S, Shi D, Zhong B, Huang X, Shi C, Shao Z. Lycorine exerts antitumor activity against osteosarcoma cells in vitro and in vivo xenograft model through the JAK2/STAT3 pathway. Onco Targets Ther. 2019;12:5377–5388. doi: 10.2147/OTT.S202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu HY, Qiu YL, Pang X, Wu JLS, Yin SS, Han LF, Zhang Y, Jin CY, Gao XM, Hu WW, Wang T. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma. Mol Cancer Ther. 2017;16:2711–2723. doi: 10.1158/1535-7163.MCT-17-0498. [DOI] [PubMed] [Google Scholar]
- 15.Ying XX, Huang AL, Xing YJ, Lan LP, Yi ZF, He PQ. Lycorine inhibits breast cancer growth and metastasis via inducing apoptosis and blocking Src/FAK-involved pathway. Sci China Life Sci. 2017;60:417–428. doi: 10.1007/s11427-016-0368-y. [DOI] [PubMed] [Google Scholar]
- 16.Liu RF, Cao ZF, Tu J, Pan YY, Shang BX, Zhang GC, Bao MM, Zhang SS, Yang P, Zhou QS. Lycorine hydrochloride inhibits metastatic melanoma cell-dominant vasculogenic mimicry. Pigment Cell Melanoma Res. 2012;25:630–638. doi: 10.1111/j.1755-148X.2012.01036.x. [DOI] [PubMed] [Google Scholar]
- 17.Hu M, Yu ZM, Mei PY, Li JX, Luo D, Zhang HM, Zhou MF, Liang FX, Chen R. Lycorine induces autophagy-associated apoptosis by targeting MEK2 and enhances vemurafenib activity in colorectal cancer. Aging (Albany NY) 2020;12:138–155. doi: 10.18632/aging.102606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamloo B, Usluer S. p21 in cancer research. Cancers (Basel) 2019;11:1178. doi: 10.3390/cancers11081178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parveen A, Akash MSH, Rehman K, Kyunn WW. Dual role of p21 in the progression of cancer and its treatment. Crit Rev Eukaryot Gene Expr. 2016;26:49–62. doi: 10.1615/CritRevEukaryotGeneExpr.v26.i1.60. [DOI] [PubMed] [Google Scholar]
- 20.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–855. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 21.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 22.Lee S, Helfman DM. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem. 2004;279:1885–1891. doi: 10.1074/jbc.M306968200. [DOI] [PubMed] [Google Scholar]
- 23.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaltomaa S, Lipponen P, Eskelinen M, Ala-Opas M, Kosma VM. Prognostic value and expression of p21(waf1/cip1) protein in prostate cancer. Prostate. 1999;39:8–15. doi: 10.1002/(sici)1097-0045(19990401)39:1<8::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Korkolopoulou P, Kouzelis K, Christodoulou P, Papanikolaou A, Thomas-Tsagli E. Expression of retinoblastoma gene product and p21 (WAF1/Cip1) protein in gliomas: correlations with proliferation markers, p53 expression and survival. Acta Neuropathol. 1998;95:617–624. doi: 10.1007/s004010050848. [DOI] [PubMed] [Google Scholar]
- 26.Winters ZE, Hunt NC, Bradburn MJ, Royds JA, Turley H, Harris AL, Norbury CJ. Subcellular localisation of cyclin B, Cdc2 and p21(WAF1/CIP1) in breast cancer: association with prognosis. Eur J Cancer. 2001;37:2405–2412. doi: 10.1016/s0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 27.Sarbia M, Gabbert HE. Modern pathology: prognostic parameters in squamous cell carcinoma of the esophagus. Recent Results Cancer Res. 2000;155:15–27. doi: 10.1007/978-3-642-59600-1_2. [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Zhao E, Zhu Q, Ji J, Wei Z, Xu B, Cui H. Tubeimoside-1 inhibits glioblastoma growth, migration, and invasion via inducing ubiquitylation of MET. Cells. 2019;8:774. doi: 10.3390/cells8080774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H, Dong Z, Wang X, Bai L, Lei Q, Yang J, Li L, Li Q, Liu L, Zhang Y, Ji Y, Guo L, Liu Y, Cui H. Dehydrocorydaline inhibits cell proliferation, migration and invasion via suppressing MEK1/2-ERK1/2 cascade in melanoma. Onco Targets Ther. 2019;12:5163–5175. doi: 10.2147/OTT.S183558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, Fu G, Pan GZ, Li CY, Shen L, Hu RJ, Zhu SQ, Chen YB, Cui HJ. Demethylzeylasteral inhibits glioma growth by regulating the miR-30e-5p/MYBL2 axis. Cell Death Dis. 2018;9:1035. doi: 10.1038/s41419-018-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu F, Zhou J. CircAGFG1 promotes cervical cancer progression via miR-370-3p/RAF1 signaling. BMC Cancer. 2019;19:1067. doi: 10.1186/s12885-019-6269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J, Deng Q, Zhou J, Zou J, Zhang Y, Tan P, Zhang W, Cui H. CSN6 controls the proliferation and metastasis of glioblastoma by CHIP-mediated degradation of EGFR. Oncogene. 2017;36:1134–1144. doi: 10.1038/onc.2016.280. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant based natural products. Curr Med Chem. 2001;8:1467–1486. doi: 10.2174/0929867013372094. [DOI] [PubMed] [Google Scholar]
- 34.Seca AML, Pinto DCGA. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int J Mol Sci. 2018;19:263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181:1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. P21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 37.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 38.Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. P21 (WAF1/Cip1) is a negative transcriptional regulator of Wnt4 expression downstream of notch1 activation. Genes Dev. 2005;19:1485–1495. doi: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst) 2016;42:63–71. doi: 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704:12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Sparrow LE, Eldon MJ, English DR, Heenan PJ. p16 and p21(WAF1) protein expression in melanocytic tumors by immunohistochemistry. Am J Dermatopathol. 1998;20:255–261. doi: 10.1097/00000372-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Alonso SR, Ortiz P, Pollan M, Perez-Gomez B, Sanchez L, Acuna MJ, Pajares R, Martinez-Tello FJ, Hortelano CM, Piris MA, Rodriguez-Peralto JL. Progression in cutaneous malignant melanoma is associated with distinct expression profiles - a tissue microarray-based study. Am J Pathol. 2004;164:193–203. doi: 10.1016/s0002-9440(10)63110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 45.Besson A, Yong VW. Involvement of p21(Waf1/Cip1) in protein kinase C alpha-induced cell cycle progression. Mol Cell Biol. 2000;20:4580–4590. doi: 10.1128/mcb.20.13.4580-4590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss RH, Joo A, Randour C. p21(Waf1/Cip1) is an assembly factor required for platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem. 2000;275:10285–10290. doi: 10.1074/jbc.275.14.10285. [DOI] [PubMed] [Google Scholar]
- 47.Kreis NN, Louwen F, Yuan J. Less understood issues: p21(Cip1) in mitosis and its therapeutic potential. Oncogene. 2015;34:1758–1767. doi: 10.1038/onc.2014.133. [DOI] [PubMed] [Google Scholar]
- 48.Dai M, Al-Odaini AA, Arakelian A, Rabbani SA, Ali S, Lebrun JJ. A novel function for p21Cip1 and acetyltransferase p/CAF as critical transcriptional regulators of TGF beta-mediated breast cancer cell migration and invasion. Breast Cancer Res. 2012;14:R127. doi: 10.1186/bcr3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang HP, Wang LQ, Zhao J, Chen YB, Lei Z, Liu X, Xia W, Guo LL, Zhang HT. TGF-beta-activated SMAD3/4 complex transcriptionally upregulates N-cadherin expression in non-small cell lung cancer. Lung Cancer. 2015;87:249–257. doi: 10.1016/j.lungcan.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Wu YZ, Zhang XP, Salmon M, Lin X, Zehner ZE. TGF beta 1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members. Biochim Biophys Acta. 2007;1773:427–439. doi: 10.1016/j.bbamcr.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.