Abstract
Purpose:
This systematic review and meta-analysis evaluated the effectiveness of diabetes self-management education (DSME) in reducing glycosylated hemoglobin (A1C) levels in adult Latinos with type 2 diabetes (T2DM).
Methods:
Five databases were searched for DSME randomized controlled trials or quasi-experimental trials published between January 1997 and March 2019. A random effects model was utilized to calculate combined effect sizes. Subgroup analyses were performed to explore possible sources of heterogeneity between studies.
Results:
Twenty-three unique studies met criteria for this systematic review and of these, 18 were included in the meta-analysis. Pooled estimate effect of DSME on A1C from the random effect model was −0.240 (95% confidence interval = −0.345, −0.135, p < 0.001). There was moderate heterogeneity (Cochrane Q=30.977, P=0.020, I^2 = 45.121) between the studies. Subgroup analyses demonstrated greater A1C reductions in studies with intervention duration ≤6 months, initial A1C baseline values >8.0 [69 mmol/mol], and team-based approach.
Conclusions:
Meta-analysis results showed that culturally tailored DSME interventions significantly reduce AIC in Latinos with T2DM despite the heterogeneity across the studies.
Implications:
The heterogeneity in the study methodologies reinforce the need for additional studies to better understand DSME interventions to reduce disparities in Latino adults with T2DM.
Keywords: Meta-analysis, Latino adults, Disparities, Type 2 diabetes, Diabetes self-management education
1. Introduction
Diabetes mellitus is a complex multisystem disease that accounts for over 20% of health care spending in the United States [1]. Type 2 diabetes mellitus (T2DM) accounts for about 95% of all diagnosed cases and is expected to increase by 1.7 million new cases each year and reach to an incidence ratio of one in three adults by 2050 [1]. Although the incidence of T2DM is slowing the prevalence continues to escalate in minority and socioeconomically disadvantaged groups [1,2]. Poor glycemic control is most prevalent in minority populations, those living in poverty, and with low educational levels [3]. The co-occurrence between T2DM, hypertension, and hypercholesterolemia increased over two-fold between 1999 and 2012 [4]. Uncontrolled diabetes led to increased risks for heart disease, stroke, blindness and amputations [5,6]. Latinos were twice as likely to be diagnosed with T2DM, less likely to meet glycosylated hemoglobin (A1C) targets for glycemic control, which resulted in a higher incidence of end-stage renal disease and increased rates of morbidity and mortality compared to non-Hispanic White counterparts [5,7].
There is a consensus that less than optimal T2DM control escalates preventable complications [4,8]. Effective interventions must integrate individuals, families, health care providers, and health system determinants to improve health outcomes. Disease control depends on the individual’s engagement in healthy lifestyle behaviors and participation in disease management aside from the numerous options of pharmacologic therapies available for diabetes care [6,9]. Disease management requires a collaborative partnership between the individual, family, support personnel and the health care provider [3,10]. The basis for T2DM self-management education (DSME) is to provide an accessible program to improve knowledge, skill, and ability necessary for self-care that addresses health beliefs, cultural needs, emotional concerns, family support, financial status and health literacy, and numeracy [6,11,12]. The American Diabetes Association (ADA) recommends DSME and T2DM self-management support (DSMS) at four critical times: (1) diagnosis, (2) annually, (3) when complicating factors affect self-management, and (4) when any transition in care occur [6,13]. The Diabetes Education and Self-Management for Ongoing and Newly Diagnosed study reported that structured education improved beliefs and attitudes for T2DM that positively influenced behavior and lifestyle modifications and sustained motivation [9]. Despite the benefits of diabetes education, only 6.8% of patients received DSME within 12 months of being newly diagnosed with T2DM in an insured population [9,12] and findings from the Behavioral Risk Factor Surveillance Survey suggest that only half of the eligible respondents received DSME [14].
Diabetes self-management education encourages behaviors that promote healthy decision-making. The DSME curriculum varies but topics include nutrition, benefits of exercise, medication adherence, foot care, glucose monitoring, and smoking cessation [6,11,13]. The mode DSME delivery varies from individual, group or a combination of both. The DSME program is provided in a wide range of venues including, but not limited to community centers, home, clinical office, through social media, or a combination of settings. In addition, the interventionist that provides education for T2DM is diverse, ranging from a licensed health provider (i.e., certified diabetes educator, dietician, pharmacist, nurse or physician), a community health worker, peer worker/leader, or other trained lay educators. Furthermore, education can be provided by a solo interventionist or by an interdisciplinary team. Regardless of the diversification of DSME programs, the educational components target behaviors to integrate healthy lifestyles to improve health outcomes.
The literature is replete on the effectiveness of DSME in various ethnic backgrounds with either type 1 diabetes or T2DM. An appraisal of systematic reviews and meta-analyses literature was conducted to assess the effects of DSME in adults with T2DM and three studies are summarized. In the first study, Norris, Lau, Smith, Schmid and Engelgau [15] included 31 published studies that were conducted between 1980 through 1999. The meta-analysis demonstrated a moderate decline in A1C levels in the intervention group by 0.76% (95% CI 0.34, 1.18) compared to the control group, 0.26% (95% CI 0.05–0.48) at four months of follow-up. They estimated an average of 23.6 contact hours between the participant and the interventionist are needed to achieve a 1% reduction in A1C. In the second study, the systematic review by Chrvala, Sherr and Lipman [16] included 120 articles from 1997 to 2013 and was restricted to randomized controlled trial (RCT) published in the English language. The overall A1C reduction for participants randomized to DSME was 0.74% (SD 0.63). Interventions that were ≥ 10 contact hours resulted in a slightly greater reduction in mean A1C of −1.01% (p = 0.04) compared to ≤10 h (−0.96%). However, the authors did not delineate or examine racial and/or ethnic differences, thus making it difficult to make inferences related to health disparities [16]. The third study was a systematic review and meta-analysis by Ferguson, Swan and Smaldone [17]. The authors evaluated the effectiveness of DSME in conjunction with primary care in adult Latinos with T2DM in 13 studies and utilized 11 of those studies in the meta-analysis. The authors found a pooled A1C reduction of −0.25% (95% CI −0.42, −0.07) at six months following DSME intervention. However, four of the eleven studies included in the meta-analysis were not exclusively Latino and the pre and post A1C results were not reported based on ethnicity.
Although these studies demonstrate DSME effectiveness, results are difficult to interpret because of the heterogeneity in the characteristics of these studies, such as DSME program elements and methodologies, duration, ethnicity, and psychosocial issues. There is a paucity of research that examines the impact of tailored interventions among culturally diverse groups and it is unclear whether less than optimal T2DM outcomes in Latinos can be explained by poorly designed interventions, lack of culturally competent interventions, inadequate access, the clinician attitude toward T2DM management, structural/environmental factors, social determinants, health literacy or self-efficacy [18–21]. The primary objective of this systematic review and meta-analysis is to evaluate the evidence of the effectiveness of DSME interventions on glycemic control and research gaps in the adult Latino population with T2DM across all settings.
2. Methods
2.1. Search strategy
The PICO (population, intervention, comparison and outcome) approach was used to guide study selection and appraisal of studies. The key research question was identified to guide the review: In Latino adults with T2DM, does DSME improve glycemic control? The search was limited to studies published in peer-reviewed journals between January 1997 to March 2019 and without language restrictions. The start date of January 1997 was selected because the Balanced Budget Act of 1997 approved 10 h of DSME allowance through Medicare benefits [16].
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were used to conduct and report our systematic review and meta-analysis [22]. A systematic search was conducted of Medline, Cumulative Index to Nursing and Allied Health Literature, PsycINFO, Cochrane Library and Web of Science using the following Medical Subject Headings (MeSH) terms: diabetes, type 2 diabetes mellitus, self-management education, self-care, A1C, Latino, Latina, Hispanic, Spanish-speaking or Mexican American. These terms were combined using Boolean operators, truncation and search strategies specific for each database to identify potential studies. References of the retained studies were manually searched for other eligible studies.
2.2. Eligibility
To focus on the aim of this systematic review, the inclusion and exclusion criteria resulted directly from the PICO question. Studies meeting the following inclusion criteria were included in the review: a) RCT and quasi-experimental studies and pilot or feasibility studies with a matched control group with no active DSME component (i.e., usual care or wait-list); b) the study participants were exclusively adult Latinos with T2DM; and c) usual care included standard primary care or minimal educational intervention. The inclusion of quasi-experimental studies was consistent with the Cochrane Consumers and Communication Review Group standards evaluating complex interventions [23].
Although there is no standard DSME format, educational topics included components to improve participants’ knowledge, skills and ability to achieve self-management activities that can positively affect glycemic control [6,13]. Because A1C was the clinical outcome of focus, each study that was included in the review had to report baseline and endpoint A1C values. There were no limitations related to the length of the DSME intervention, study duration, or type of healthcare provider to include as many studies as possible for this review.
Pilot studies without a comparison group, single cohort feasibility studies, or program evaluations were excluded. Studies that did not include T2DM as the primary diagnoses were excluded such as type 1 diabetes, asthma or arthritis. Study enrollees may be from any Latin country or of Latino descent, and bilingual or monolingual Spanish speaking. Studies that had a mixture of minority groups, even though most subjects were Latino, were excluded if the study did not include a Latino subgroup analysis that included pre and post A1C.
2.3. Search and extraction
Two authors [JAH, LSE] screened all abstracts identified in the initial search and excluded studies that violated the inclusion criteria. All authors screened full-text studies and differences were resolved by consensus. A data extraction tool was utilized to collect author, year of publication, study design, baseline and final sample size, duration and overall retention. There were two sets ([24,25] and [26,27]) of articles that reported on the same study and were placed together on the extraction sheet to prevent duplication of data. Following the data extraction, the corresponding data was reviewed by all authors for accuracy.
2.4. Quality assessment criteria
The Cochrane risk of bias tool [28] was utilized to assess the methodological quality of studies in this review. The instrument uses specific criteria for scoring as low, unclear or high risk across seven categories: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessor, incomplete outcome data, selective outcome reporting, and a category of “other bias,” which includes other areas of concern that is not covered in the previous six categories.
2.5. Quantitative analysis approach
Twenty-three studies were assessed for inclusion in the meta-analysis (see Table 1). Standard deviations were calculated for studies where a change in A1C was presented with confidence intervals or standard errors. A random-effects model was utilized to estimate the effect size of DSME on A1C due to the moderate heterogeneity between the studies [29]. Cohen described effect size (d) by expressing an observed mean difference in standard deviation units with the following guidelines: d ≤ 20 as small, d ≈ 50 as medium and d > 80 as large [30]. Data were analyzed using the Comprehensive Meta-Analysis, version 3.1 software (Biostat, Inc) [29].
Table 1.
Characteristics of eligible studies for systematic review and meta-analysis.
| Author Study Design | Baseline age mean (years) | Baseline sample size | Est CT (hours) | Mode Provider | Attrition rate (%) | Baseline A1C (mmol/mol) | Final A1C (mmol/mol) | Intervention Duration | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG ± SD | CG ± SD | IG ± SD | CG ± SD | Time of A1C measure | ||||
| Aponte et al., 2017 [49] | 58.8 | 58.6 | 60 | 60 | 32 | C | 16 | 9.5 (80) ± 1.1 | 9.4 (79) ± 1.3 | 8.6 (70) ± 1.5 | 9.1 (76) ± 1.3 | 9 months |
| RCT | Team | 9 months | ||||||||||
| Ayala et al., 2015 [50] | 56.7 | 55.9 | 149 | 155 | NR | I | 13 | 8.7 (72) ± 1.6 | 8.7 (72) ± 1.5 | 8.3 (67) ± 1.9 | 8.7 (72) ± 2.0 | 12 months |
| RCT | Single | 12 months | ||||||||||
| Babamoto et al., 2009 [54] | 51 | 50 | 75 | 54 | 10 | I | 39 | 8.6 (70) | 9.5 (80) | 7.2(55)1 | 7.4 (55)1 | 6 months |
| RCT | Single | 6 months | ||||||||||
| Baig et al., 2015 [45] | 51.7 | 55.7 | 50 | 50 | 12 | G | 16 | 8.2 (66) ± 1.8 | 7.8 (62) ± 2.1 | 7.9 (63)2 ± 1.83 | 7.8(62)2 ± 1.83 | 8 weeks |
| RCT Pilot | Single | 6 months | ||||||||||
| Brown et al., 2002 [51] | 54.7 | 53.2 | 126 | 125 | 52 | G | 9 | 11.8 (105) ± 3.0 | 11.8 (105) ± 3.0 | 10.9 (96) ± 2.6 | 11.6 (103) ± 2.9 | 12 months |
| RCT | Team | 12 months | ||||||||||
| Castejon et al., 2013 [35] | 54 | 55 | NR | NR | 13.5 | C | NR | 8.3 (67) ± 0.4“ | 8.2 (66) ± 0.4“ | 7.3(56)1 ± 1.35 | 8.0(64)1 ± 1.05 | 3 months |
| RCT Pilot | Single | 3 months | ||||||||||
| Gallegos et al., 2006 [33] | 52.0 | 49.5 | 29 | 28 | 29 | C | 20 | 10.4 (90) ± 2.6 | 9.4 (79) ± 1.5 | 8.0 (64) ± 1.46 | 9.8 (84) ± 1.36 | 12 months |
| Quasi Exp. | Team | 12 months | ||||||||||
| Garcia et al., 2015 [36] | 50 | 49.1 | 39 | 33 | 8 | I | 36 | 8.6 (70) ± 0.04 | 8.6 (70) ± 0.04 | 7.9(63) ± 1.65 | 8.5 (69) ± 1.55 | 6 months |
| RCT | Single | 6 months | ||||||||||
| Lorig et al., 2008 [53] | 52.9 | 52.8 | 219 | 198 | 15 | G | 16 | 7.4 (55) ± 2.0 | 7.4 (55) ± 1.9 | 7.0 (53)2 ± 1.4 | 7.3 (56)2 ± 1.6 | 6 months |
| RCT | Single | 6 months | ||||||||||
| Lujan et al., 2007 [43] | 58 | 58 | 75 | 75 | 16 | C | 6 | 8.2 (66) ± 2.2 | 7.7 (61) ± 1.5 | 7.8 (62) ± 1.9 | 8.0 (64) ± 1.8 | 6 months |
| RCT | Single | 6 months | ||||||||||
| Osborn et al., 2010 [38] | 56.9 | 58.4 | 48 | 43 | 1.5 | G | 30 | 7.8 (62) ± 1.4 | 7.5 (58) ± 1.6 | 7.3 (56) ± 1.3 | 7.2 (55) ± 1.5 | 90 minutes |
| RCT | Single | 3 months | ||||||||||
| Palmas et al., 2014 [46] | 57.1 | 58.1 | 178 | 177 | NR | C | 16 | 8.8 (73) ± 1.7 | 8.6 (70) ± 1.6 | 8.4 (68) ± 1.6 | 8.5 (69) ± 1.5 | 12 months |
| RCT | Single | 12 months | ||||||||||
| Peña-Purcell et al., 2011 [34] | 59.4 | 63.9 | 74 | 65 | 10 | G | NR | 7.9 (63) | 7.4 (57) | 7.1 (54)6 | 7.4 (57)6 | 3 months |
| Quasi Exp. | Team | 3 months | ||||||||||
| Perez-Escamilla et al., 2015 [48] | 55.4 | 57.3 | 105 | 106 | 17 | I | 30 | 9.6 (81) ± 1.85 | 9.6 (81) ± 1.85 | 8.9 (73) ± 2.03 | 9.4 (78) ± 1.43 | 12 months |
| RTC | Single | 12 months | ||||||||||
| Philis-Tsimikas et al., 2011 [44] | 52.2 | 49.2 | 104 | 103 | 32 | G | 25 | 10.5 (91) ± 1.7 | 10.3 (89) ± 1.7 | 9.1 (76) ± 2.0 | 9.7 (83) ± 2.3 | 10 months |
| RCT | Single | 10 months | ||||||||||
| Rosal et al., 2005 [37] | 62.7 | 62.4 | 15 | 10 | 31.5 | C | 8 | 7.7 (61) ± 1.2 | 9.3 (78) ± 1.8 | 6.9(52)2 ± 2.15 | 9.2 (77)2 ± 2.75 | 6 months |
| RCT | Team | 6 months | ||||||||||
| Rosal et al. 2011 [24] | NR | NR | 124 | 128 | 38 | C | 7 | 8.9 (74) ± 1.8 | 9.1 (76) ± 2.0 | 8.4 (68)2 ± 1.73 | 8.9 (74)2 ± 2.53 | 12 months |
| Wang et al., 2014 [25] | Team | 12 months | ||||||||||
| RCT | ||||||||||||
| Rothschild et al., 2014 [39] | 53.7 | 53.6 | 73 | 71 | 36 | I | 16 | 8.4 (74) ± 1.23 | 8.2 (66) ± 1.63 | 7.6 (60) ± l.l3 | 8.3 (67) ± 1.23 | 24 months |
| RCT | Single | 24 months | ||||||||||
| Tang et al., 2014 [41] | 50.2 | 48.4 | 60 | 56 | >24 | C | 41 | 8.2 (66) ± 2.2 | 7.8 (62) ± 1.7 | 7.6 (60)2 ± 1.23 | 7.5 (58)2 ± 1.23 | 18 months |
| RCT | Team | 18 months | ||||||||||
| Toobert et al., 2011 [40] | 58.7 | 55.6 | 142 | 138 | >100 | C | NR | 8.4 (68) ± 1.9 | 8.2 (66) ± 1.7 | 8.3 (67) ± 1.9 | 8.3 (67) ± 1.6 | 12 months |
| RCT | Team | 12 months | ||||||||||
| Vincent et al., 2007 [26] | 56.7 | 55.3 | 10 | 10 | 16 | C | 15 | 6.6 (49) ± 0.9 | 6.7 (50) ± 1.2 | 6.1 (43)6 ± 0.5 | 6.8 (51)6 ± 1.3 | 3 months |
| Vincent, 2009 [27] | Team | 3 months | ||||||||||
| Pilot | ||||||||||||
| Welch et al., 2011 [47] | 54.4 | 57.5 | 25 | 21 | 7 | I | 15 | 9.0 (75) ± 1.2 | 8.5 (69) ± 1.0 | 7.4 (57) ± 1.4 | 7.9 (63) ± 1.4 | 12 months |
| RCT | Team | 12 months | ||||||||||
| Welch et al., 2015 [52] | 54.8 | 55.2 | 199 | 200 | 3 | I | 12 | 8.9 (74) ± 1.4 | 9.0 (75) ± 1.5 | 8.4(68) ± 1.35 | 9.2 (77) ± 1.35 | 6 months |
| RCT | Team | 6 months | ||||||||||
A1C = glycosylated hemoglobin; C = combination of individual and group education; CG = control group; Est CT = Estimated contact time; G = group education; I = individual education; IG = intervention group; NR = not reported; Quasi Exp. = quasi experimental; RCT = randomized controlled trial; SD = standard deviation
RCT without adequate data and not included in the meta-analysis.
Final A1C calculated based on the change in A1C.
Standard deviation calculated based on the confidence interval.
Standard error as reported in the study.
Standard deviation calculated based on the standard error.
Non-RCT study not included in the meta-analysis.
Heterogeneity describes variability among studies which occurs when the treatment effect estimates vary [31]. Some of this variation occurs by chance, but statistical heterogeneity refers to variation beyond chance. Since this meta-analysis included studies of varying participant demographics, DSME curriculum, study duration, and baseline A1C values, heterogeneity was analyzed. Heterogeneity was appraised using Cochrane Q and I^2 statistics. The I^2 denotes the approximate proportion of total variability in point estimates that can be associated with heterogeneity [31]. The following values were utilized to determine heterogeneity: low as 25% or I^2 = 25, moderate as 50% or I^2 = 50 and high as 75% or I^2 = 75 [31].
Subgroup analyses were conducted to explore possible sources of heterogeneity between studies and based on findings from previous meta-analyses. The intervention duration (≤ 6 months and > 6 months), initial A1C of the intervention group (≤ 8.5% [69 and mmol/mol] >8.5%), interventionist (solo or team approach)and mode of DSME delivery (individual, group or combination approach) were examined. To decrease the false positives and maintain power, the p-value thresholds were adjusted and a 99% confidence interval was used in subgroup analysis calculations [32].
3. Results
3.1. General description of studies
A three-step approach was utilized to determine eligibility for inclusion and a flow diagram for study selection is depicted in Fig. 1. The search of the five databases generated 607 citations. A review of three systematic reviews produced three articles that were not in the original online search. After deleting duplicates, 383 articles remained. Each article abstract was further reviewed and 307 were discarded because they did not meet review criteria. A total of 76 studies were fully assessed for eligibility and 51 were excluded. Twenty-five articles were identified and retained for inclusion, but two studies [24,26] in the systematic review had another published study report on the same data, thus, leaving 23 distinct studies that provided enough information for pooled estimates of A1C in this meta-analysis. Two of the 23 studies were quasi-experimental with a control group [33,34], three were pilot RCTs [35–37], one was a feasibility study [26] and the remaining 17 studies were RCTs. All studies were conducted in the US except for one study, which was conducted in Mexico [33]. The shortest intervention duration was 90 min [38] and the longest was 24 months [39].
Fig. 1.
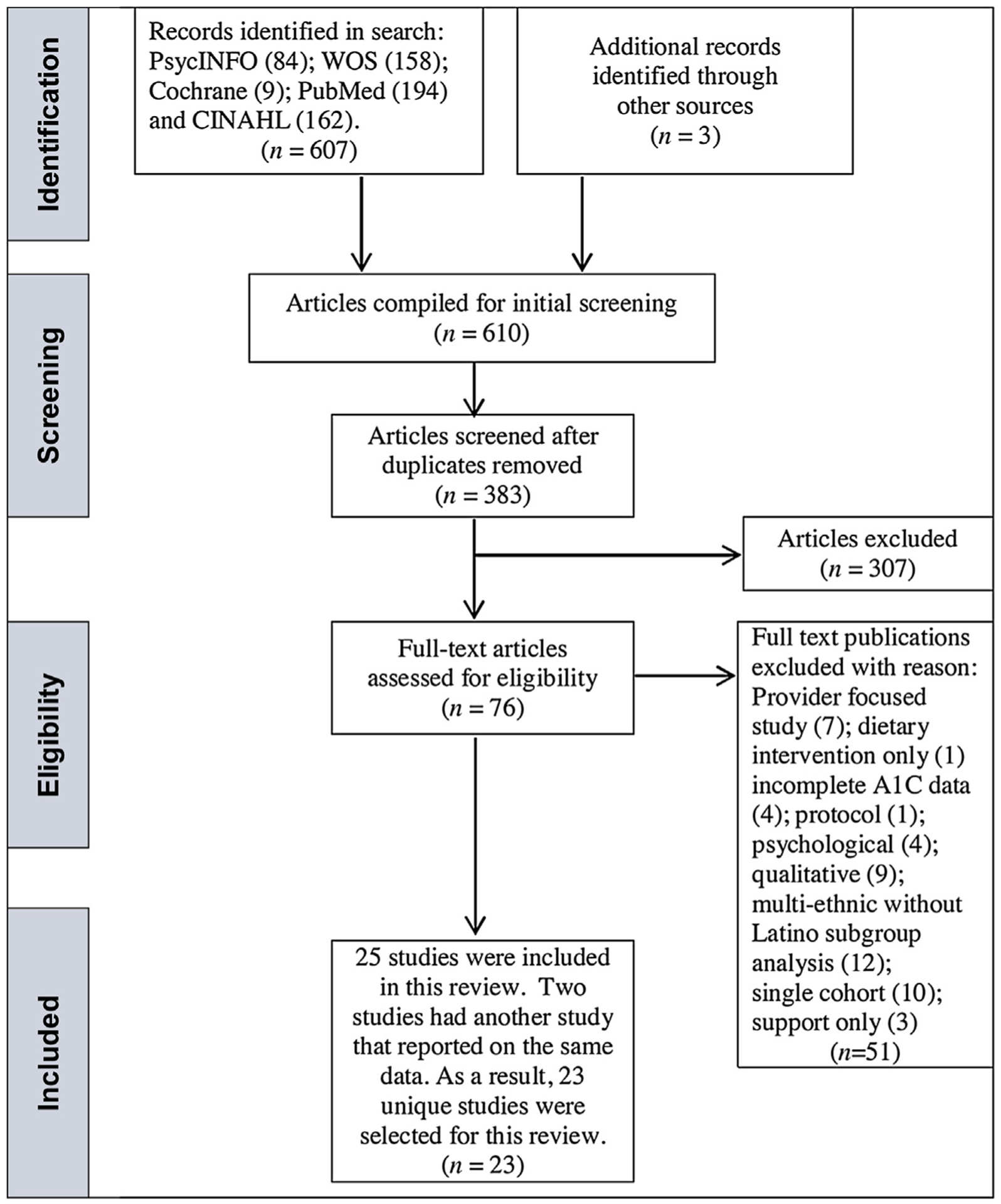
Flow diagram for study selection Study selection according to PRISMA [22].
Studies included in this review involved a combined total of 3969 Latino adult participants with T2DM. One study reported a total baseline sample size without differentiating between the control and intervention arms [35]. As a result, this study was not retained for the meta-analysis. One study did not specify gender distribution [33], one was exclusively female [40] while the remaining studies ranged from 59% [41] to 83% [37] female. Seven studies were exclusively Mexican [26,33,36,39,42–44] one exclusively Puerto Rican [38] and the remaining studies were a mixture of various Latino subcultures. All studies had a mixture of Spanish monolingual and bilingual participants, provided bilingual staff, and incorporated cultural practices specific for the Latino subculture, i.e., food choices specific to the subculture.
3.2. Risk evaluation
Only 18 studies with complete A1C data were included in the meta-analysis. The summary of the risk evaluation of the retained studies for the meta-analysis is provided in Fig. 2. Due to the nature of the intervention, blinding of participants and personnel was somewhat challenging; however, two studies documented health provider blinding [24,41] and one study blinded the research assistant [38]. Five studies utilized enhanced care, which boosts attention to the control group and may have attenuated between-group data [39,41,45–47]. Outcome assessors were blinded in seven studies [24,37,39–41,48,49]. Attrition rates varied from 6% [43] to over 40% [41]. Most of the studies minimized risk for incomplete outcome data through an attrition rate of less than 20% [24,37,39,43,45–47,49–53] or by use of intent-to-treat analysis [36,39–41,45,46,48–50,52]. Common reasons for dropouts were similar across studies and included lack of transportation, child-care, time, illness, and/or lack of interest.
Fig. 2.
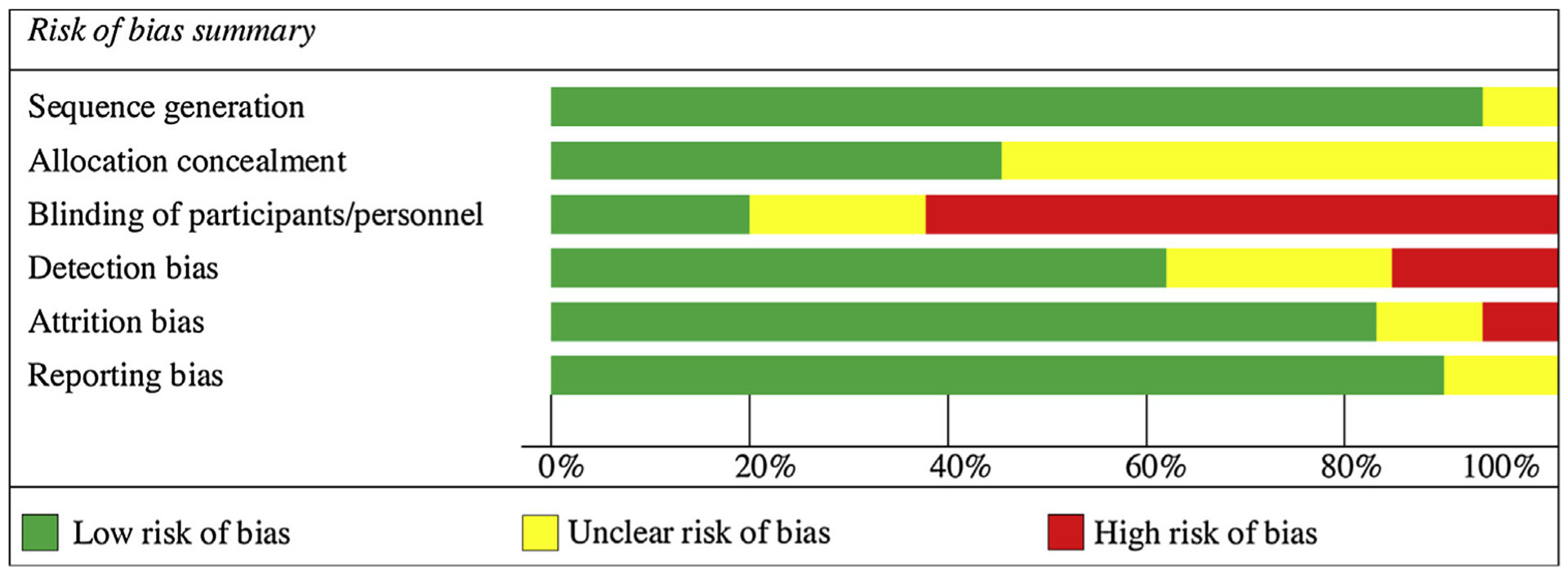
Risk of bias summary.
3.3. Effects on A1C
A meta-analysis of the effect on A1C was carried out by using data from a final sample size of 3540 participants in 18 RCT studies. Five studies were excluded: two quasi-experimental studies [33,34], one feasibility study [26] and two RCT [35,54] that lacked adequate data. Fig. 3 provides the forest plot details assuming a 0.5 (moderate) correlation between change in A1C in the control and intervention arms [31]. The standard error of the effect is plotted on the vertical axis with a reversed scale that places larger studies towards the top [55]. The pooled estimate effect on A1C using a random effect model was −0.240% (95% confidence interval [CI] = −0.345, −0.135, p < 0.001). Since the pooled estimate does not cross the line of no effect or zero and the probability value was p < 0.05, the difference between the intervention and control group is statistically significant [56]. Similarly, the Z-value for testing the null hypothesis is −4.477 with a corresponding p < 0.001, thus we reject the null that DSME has no impact on A1C.
Fig. 3.
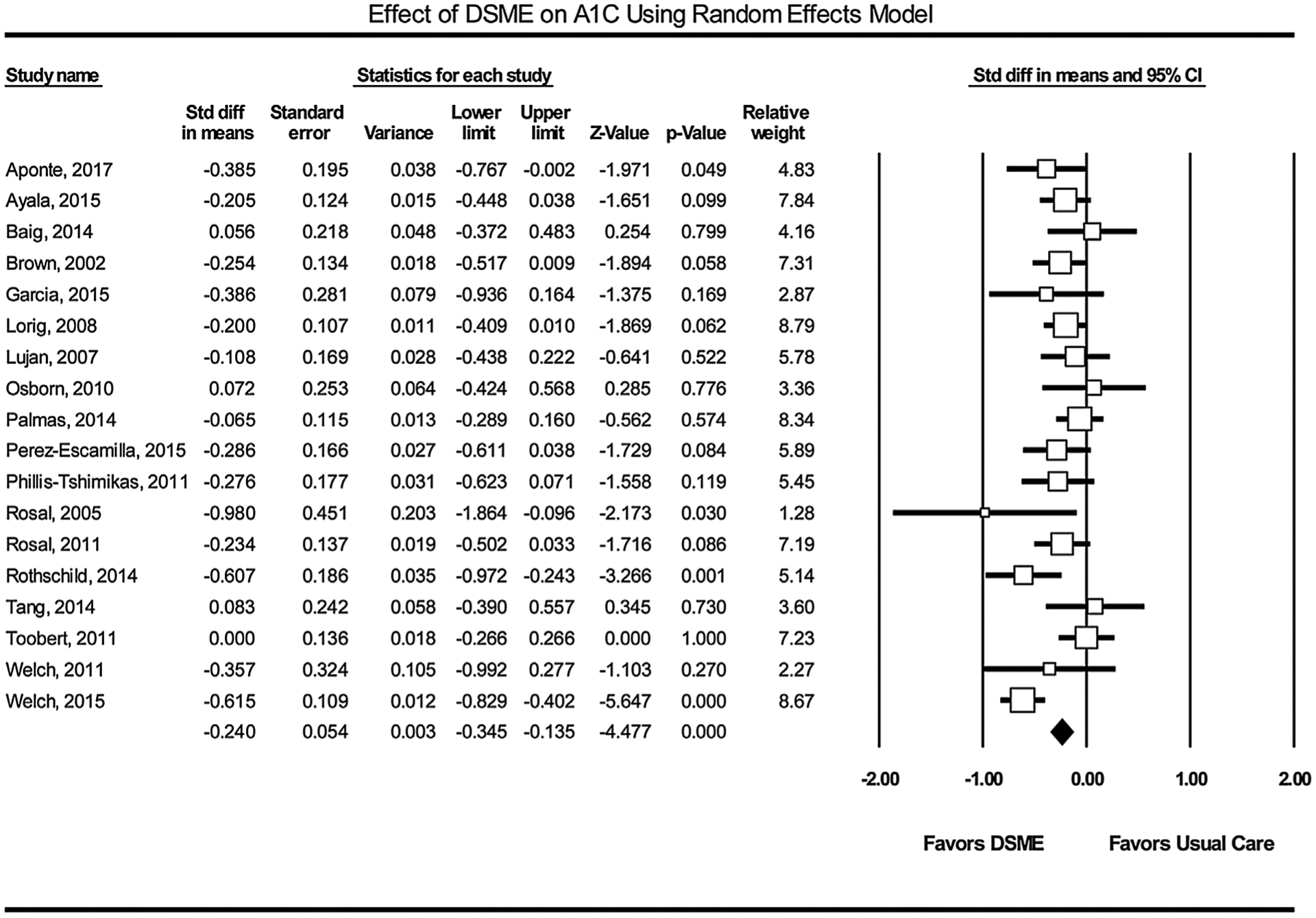
The forest plot of the mean difference of A1C reduction in study participants. Squares represent effect sizes of individual studies with extended lines denoting 95% confidence interval. Sizes of squares indicate the weight of each study based on the sample size using random effects analysis. The diamond represents the estimated pooled effect size. A ratio of estimates below zero indicates the beneficial treatment effect of DSME.
3.4. Heterogeneity
Generally, the larger sample size studies have greater relative weight and narrower CI. The Q-statistic provides a test of the null hypothesis that all studies in the analysis share a common effect size. Our findings showed moderate heterogeneity (Cochrane Q = 30.977, p = 0.020) between the 18 studies, thus we reject the null hypothesis that the true effect size is identical in all studies. The inverse variance index value (I^2 = 45.121) also indicated the presence of moderate variations between the studies that were not due to chance. About 45% of the variance in observed effects reflects variance in true effects rather than sampling error. A visual inspection of the forest plot also suggests heterogeneity, since whiskers from all studies in the meta-analysis graph do not overlap [56]. Sensitivity analysis indicated that the pooled standard difference in the means was not statistically significant, no matter which study was excluded from the analysis.
3.5. Publication bias
Publication bias was evaluated using Egger’s regression intercept where the treatment effect is captured by the slope of the regression line (B1) while bias is captured by the intercept (B0) and an intercept of zero indicates no bias [57]. The larger the deviation from zero or a p value < 0.05, signifies asymmetry and publication bias [31]. In this study the intercept (B0) was −0.094 (95% CI = −2.195, 2.007), p = 0.463, indicating no bias. Fig. 4 represents the funnel plot of the 18 studies. The funnel plot measures the study size on the vertical axis as a function of effect size on the horizontal axis [55,58]. The funnel plot has scattered points on the left of the means. Various factors cause asymmetry: heterogeneity, reporting bias, language bias, or chance [55,58]. Smaller studies are more likely to be published if they have larger than average effects which makes them more likely to meet statistical significance criterion. This asymmetry is further investigated through subgroup analysis.
Fig. 4.
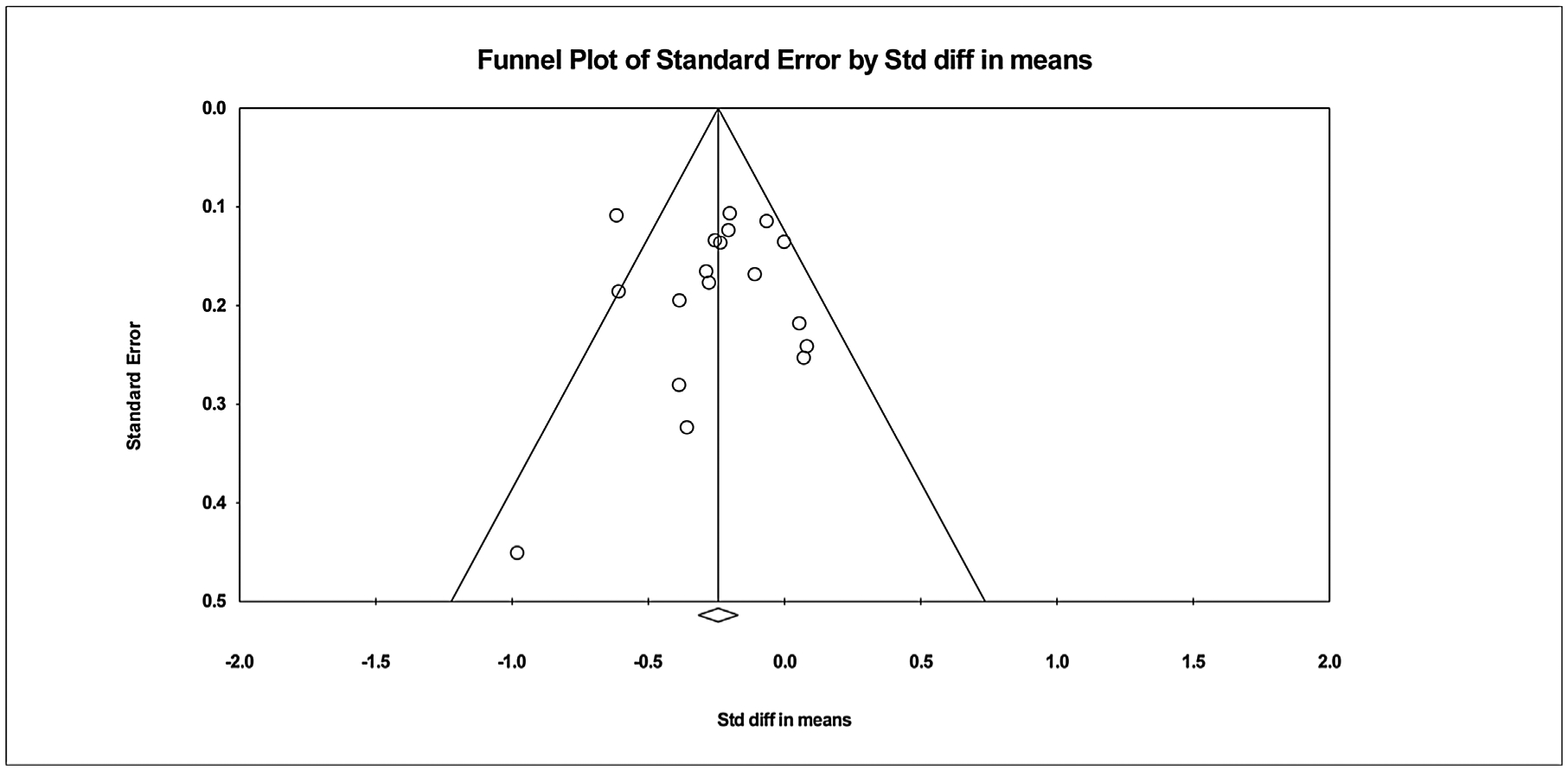
Potential for publication bias. The funnel plot displays the treatment effects of DSME on A1C estimated from the individual studies against the measure of study size.
3.6. Subgroup analysis
The duration of the DSME intervention, initial A1C levels, interventionist and mode of DMSE delivery were further investigated based on previous meta-analysis results [15,16]. Subgroup analysis is summarized in Table 2.
Table 2.
Subgroup Analyses.
| Variable | No. of studies | Mean A1C Reduction | 99% Confidence Level | Cochrane Q; P Value | I^2 |
|---|---|---|---|---|---|
| Duration of intervention | |||||
| ≤6 months | 7 | −0.274 | −0.510, −0.039 | 17.713 | 66.126 |
| 0.007 | |||||
| >6 months | 11 | −0.221 | −0.394, −0.048 | 11.533 | 13.295 |
| .317 | |||||
| Initial A1C | |||||
| ≥8.5 | 9 | −0.219 | −0.673, 0.235 | 4.145 | 51.744 |
| .126 | |||||
| > 8.5 | 9 | −0.236 | −0.446, −0.026 | 27.220 | 63.262 |
| .002 | |||||
| Interventionist | |||||
| Solo interventionist | 10 | −0.199 | −0.379, −0.018 | 9.885 | 8.954 |
| 0.360 | |||||
| Team approach | 8 | −0.295 | −0.505, −0.085 | 18.573 | 62.310 |
| 0.010 | |||||
| Mode of DSME delivery | |||||
| Individual | 6 | −0.422 | −0.618, −0.227 | 7.935 | 36.988 |
| 0.160 | |||||
| Group | 5 | −0.172 | −0.379, 0.035 | 2.788 | 0.000 |
| 0.594 | |||||
| Combination | 7 | −0.137 | −0.319, 0.044 | 7.878 | 23.837 |
| 0.247 |
Duration.
The duration of the DSME intervention was divided into two groups (≤ 6 months and > 6 months) due to the variation across studies (90 min to 24 months). The 6-month results were utilized from the 18-month study by Lorig et al. [53] because the DSME program was provided in this interval and the remaining study focused on telephonic supportive care. The greatest reduction in A1C was in studies that were ≤ 6 months (−0.274% [99% CI p = −0.510, −0.039], = 0.007).
Initial A1C.
To maintain power in the subgroup analysis, initial A1C values were divided into two groups: ≤ 8.5% [69 mmol/mol], (n = 9) and > 8.5% (n = 9). Studies with initial A1C values higher than 8.5% [69 mmol/mol] reported greater A1C reductions (−0.236%, [99% CI = −0.446, −0.026], p = 0.002). Additionally, all studies (n = 9) with A1C > 8.5% demonstrated statistically significant change from baseline in the intervention group.
Interventionist.
The interventionist subgroup differentiated between a solo interventionist (n = 10) and a team approach (n = 8). There were no limitations on the type of provider (e.g., licensed or non-licensed staff). Participants assigned to a team approach experienced greater A1C reductions (−0.295%, [99% CI = 0.505, −0.085], p = 0.010).
Mode of DSME.
Subgroup analysis of the mode of DSME delivery resulted in three categories: individual education (n = 6), group approach (n = 5) or a combination of both (n = 7). The Cochrane Q decreased, and the p-value increased in all subgroups suggesting greater consistency within this subgroup [31]. Participants assigned to individual programs experienced greater A1C reduction (−0.422%, [99% CI = −0.618, −0.227], p = 0.160).
4. Discussion and conclusion
4.1. Summary of evidence
Type 2 diabetes is a public health epidemic that requires effective interventions that span across age, ethnicities, and socioeconomic levels. Accessible DSME is recommended as a component of clinical practice to improve behavioral and metabolic factors [13]. Diabetes self-management education provides knowledge and skills to enhance behavioral changes that improve glycemic control and are comparable to the effects of some T2DM pharmaceutical agents. This study focused on DSME effectiveness in a population of adult Latinos.
This meta-analysis included 18 RCT studies, each of which compared adult Latinos with T2DM who were randomly assigned to either DSME or usual care. The outcome was A1C and the effect size was the standard difference in the means. A random-effects model was employed for this analysis. The primary goal of the meta-analysis was to compare subgroups of studies, i.e., to see if the standard mean difference is higher in the intervention group as compared to usual care. The conclusions apply to the studies in this analysis but can be used to guide future studies since the pooled results demonstrate the effectiveness of DSME in this sample population of Latinos. The standardized difference in the means was −0.240%. On average, people assigned to DSME experienced 0.24 standard deviations lower in A1C value compared to those in usual care.
The duration of the DSME intervention was analyzed as a variance. Contact hours could not be used as a subgroup analysis due to the lack of enough studies reporting this variable in each subgroup, i.e., ≤ 10 contact hours (n = 4) versus > 10 contact hours (n = 12). Therefore, duration of DSME intervention was evaluated by the length of time; studies that were less than six months demonstrated the most improvement, but the heterogeneity in this group was moderate. Removing the 90-minute intervention outlier [38] did not achieve homogeneity (i.e., I^2 = 0) nor have a statistically significant effect on the results (Cochrane Q = 15.311, p = 0.009, I^2 = 67.343; data not shown), thus confirming the difficulty of identifying the most beneficial intervention duration. Previous meta-analyses also found inconsistencies in reporting the duration of DSME intervention across selected studies, i.e., contact hours versus frequency of visits (i.e., weekly or monthly) [15–17]. These findings confirm the complexity of patient education. In addition, the context of the target population must be considered during the program development in order to implement appropriate and effective educational programs [59]. Adult learning theory purports the effect of new information diminishes after about 6 months and old behaviors can relapse within one year [10,60]. Integrating ongoing support to the educational format may boost and maintain self-management behaviors that improve glycemic control.
Initial A1C values were divided into two groups to maintain power for subgroup analysis. Results of this study are similar to a previous meta-analysis [15–17]. Although the greatest reduction in A1C was observed in studies with initial A1C levels greater than 8.5% (69 mmol/mol), the I^2 was over 50 in both subgroups indicating moderate heterogeneity differences in true effect sizes. Studies with relatively low A1C baselines are apt to show a less significant reduction, also known as the “floor effect” [26,46]. On the other hand, five studies in the systematic review with A1C greater than 8.0% [69 mmol/mol] experienced over 1% reduction [33,35,44,47,54]. A one percent reduction in A1C translates to a 35% decrease in microvascular complications (diabetic nephropathy, neuropathy and retinopathy) based on the United Kingdom Prospective Diabetes study [61]. Although A1C levels decreased across studies, most participants did not achieve target levels by the end of the research timeline based on ADA and American Association of Clinical Endocrinologists (AACE) guidelines. The ADA recommends an A1C target less than 7.0% for non-pregnant adults with T2DM, but this number can be more stringent (<6.5%) or less stringent (<8.0%) based on age, co-morbidities, disability, life expectancy, or risk for hypoglycemia [13]. The AACE recommends a target less than or equal to 6.5% for healthy individuals and suggests flexibility for those who are less healthy [62]. The ADA recommends ongoing self-management support to sustain healthy behaviors that may be hindered by a change in context [6]. However, reimbursement for DSME, not self-management support, is limited to Medicare recipients. Thus, health care systems must find other financial means to cover ongoing education and support to non-Medicare patients.
Should the interventionalist be a solo provider or work in a team to deliver DSME? In studies for this meta-analysis, the team included a healthcare professional (i.e., registered nurse or registered dietician) paired with a non-professional (i.e., community health worker or peer educator). A team approach (n = 8) was more efficacious than a solo interventionalist (n = 10) which supported the findings in the study by Ferguson et al. [17] and Chrvala et al. [16]. In Project Dulce, researchers paired a nurse case management with a peer educator and found a significant lowering of A1C from 12.0% [108 mmol/mol] to 8.3% [67 mmol/mol] (p < 0.0001) in the interval of one year [63]. Coupling the clinical expertise of licensed professionals with non-medical professionals acknowledges the strength of this dyad to provide education and support to improve health outcomes in high-risk populations.
The mode of intervention delivery varied across studies and added a level of complexity to identify the most effective mode to provide DSME. Unlike the previous meta-analysis [16], the results from this study favored individual education. However, two [36,47] of six studies provided a licensed professional interventionalist and participants in the intervention group experienced 1.6% improvement compared to usual care [47]. The heterogeneity in this subgroup limits the conclusion of the best mode of delivery.
Integration of culturally tailored interventions for Latinos must be considered cautiously. Based on the 2010 census, the US Census Bureau reported that the Mexican, Puerto Rican and Cuban ethnicities comprised 75.5% of the Latino population followed by Central American (7.9%), South American (5.5%), Dominican (2.8%), Spaniard (1.3%) and other Latino (6.8%) and are concentrated in various regions across the US [64]. Each subculture has unique traditions, food preferences and dialects making it difficult to generalize the findings to the entire Latino population. Whittemore [65] conducted an integrative review of 11 studies to investigate culturally competent DSME interventions in Latino adults with T2DM. Cultural strategies included bilingual staff with common identity and incorporation of cultural beliefs and traditions. The author concluded that these interventions were efficacious as measured by clinical, behavioral and knowledge outcomes. Further studies must identify Latino subcultures to determine unique interventions that positively effect glycemic control across this diverse population.
4.2. Limitations
This systematic review utilized PRISMA guidelines to identify studies that were exclusively Latino participants and there is a limited number of RCTs meeting this criterion. Studies that included Latinos as a subset of a larger racial/ethnic profile were excluded from this review if the subgroup A1C data was not available. Only peer-reviewed and published articles were included; the grey literature, any unpublished work or dissertations were not evaluated. Some MeSH terms may have been overlooked in our initial search which could conceal pertinent studies.
Other limiting factors considered for this study were the inclusion of an unbiased sample of relevant studies, the effect of smaller studies in the pooled effect size, the rigor of each study and the biases in each study. The meta-analysis focused on the effect of DSME on the primary outcome of A1C and other factors were not considered such as specificity of the interventionist (i.e., nursing, dietician, community health worker, or other lay people) or the setting of the intervention (i.e., clinic, community or home). Although a subgroup analysis was performed, the number of studies in each subset limits generalizability to the Latino population. Further research is needed to examine the inclusion of articles that could be a source of information to further this knowledge.
The A1C value is an accepted standard to measure glycemic control in the previous 12 weeks [66], but the interval between the time the intervention ended to the time when the A1C was measured varied across studies. Some studies used A1C values that were extracted from medical records if it was within one month of the start of the study.
4.3. Conclusion
Comprehensive care for individuals with T2DM includes interventions that reinforce behavioral changes that improve glycemic control. Despite the limited number of RCT’s that focus on DSME in the Latino population, our study suggests that culturally tailored DSME programs reduce A1C levels in adult Latinos. The heterogeneity in the study methodologies reinforces the need for additional studies that identify common cultural interventions and timelines that would maximize health outcomes in this vulnerable population.
4.4. Practice & research implication
Although DSME programs promote skills, knowledge, self-care and decision-making that improve clinical and behavioral outcomes, an ideal DSME program protocol for Latinos may not be realistic. Factors such as the heterogeneity of the Latino population, contextual circumstances of each participant and community resources impact outcome results. Nevertheless, primary care providers must afford their patients opportunities to engage in DSME programs to improve health outcomes. Finally, researchers are in a unique position to initiate discourse among stakeholders to identify structural barriers that impede access and prescribe reasonable and cost-effective proposals to improve access and health outcomes for vulnerable populations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- [1].CDC. Centers for Disease Control and Prevention, (2019). [cited 2019 3/18/2019]; Available from: https://www.cdc.gov/features/hispanichealth/index.html.
- [2].Beckles GL, Chou CF, Diabetes - United States, 2006 and 2010, MMWR Suppl. 62 (3) (2013) 99–104. [PubMed] [Google Scholar]
- [3].Liburd LC, et al. , Intervening on the social determinants of cardiovascular disease and diabetes, Am. J. Prev. Med 29 (5 Suppl 1) (2005) 18–24. [DOI] [PubMed] [Google Scholar]
- [4].Benjamin EJ, et al. , Heart disease and stroke Statistics-2018 update: a report from the American Heart Association, Circulation 137 (12) (2018) e67–e492. [DOI] [PubMed] [Google Scholar]
- [5].Chandler RF, Monnat SM, Racial/Ethnic differences in use of health care services for diabetes management, Health Educ. Behav (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beck J, et al. , National standards for diabetes self-management education and support, Diabetes Educ. 44 (1) (2017) 35–50 2018. [DOI] [PubMed] [Google Scholar]
- [7].Laiteerapong N, et al. , Revisiting disparities in quality of care among US adults with diabetes in the era of individualized care, NHANES 2007–2010, Med. Care 53 (1) (2015) 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chamberlain JJ, et al. , Cardiovascular disease and risk management: review of the American diabetes association standards of medical care in diabetes 2018, Ann. Intern. Med 168 (9) (2018) 640–650. [DOI] [PubMed] [Google Scholar]
- [9].Khunti K, et al. , Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care, Br. Med. J 344 (2012) e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marrero DG, et al. , Twenty-first century behavioral medicine: a context for empowering clinicians and patients with diabetes: a consensus report, Diabetes Care 36 (2) (2013) 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Powers MA, et al. , Diabetes self-management education and support in type 2 diabetes, Diabetes Educ. 43 (1) (2017) 40–53. [DOI] [PubMed] [Google Scholar]
- [12].Li R, et al. , Diabetes self-management education and training among privately insured persons with newly diagnosed diabetes–United States, 2011–2012, MMWR Morb. Mortal. Wkly. Rep 63 (46) (2014) 1045–1049. [PMC free article] [PubMed] [Google Scholar]
- [13].ADA, Standards of medical care in diabetes—2019 abridged for primary care providers, Clin. Diabetes 37 (1) (2019) 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen R, et al. , US trends in receipt of appropriate diabetes clinical and self-care from 2001 to 2010 and racial/ethnic disparities in care, Diabetes Educ. 40 (6) (2014) 756–766. [DOI] [PubMed] [Google Scholar]
- [15].Norris SL, et al. , Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control, Diabetes Care 25 (7) (2002) 1159–1171. [DOI] [PubMed] [Google Scholar]
- [16].Chrvala CA, Sherr D, Lipman RD, Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control, Patient Educ. Couns 99 (6) (2016) 926–943. [DOI] [PubMed] [Google Scholar]
- [17].Ferguson S, Swan M, Smaldone A, Does diabetes self-management education in conjunction with primary care improve glycemic control in Hispanic patients? A systematic review and meta-analysis, Diabetes Educ 41 (4) (2015) 472–484. [DOI] [PubMed] [Google Scholar]
- [18].Carbone ET, et al. , Diabetes self-management: perspectives of Latino patients and their health care providers, Patient Educ. Couns 66 (2) (2007) 202–210. [DOI] [PubMed] [Google Scholar]
- [19].Mansyur CL, et al. , Social factors and barriers to self-care adherence in Hispanic men and women with diabetes, Patient Educ. Couns 98 (6) (2015) 805–810. [DOI] [PubMed] [Google Scholar]
- [20].Sarkar U, et al. , Preferences for self-management support: findings from a survey of diabetes patients in safety-net health systems, Patient Educ. Couns 70 (1) (2008) 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hildebrand JA, et al. , Facilitators and barriers to research participation: perspectives of Latinos with type 2 diabetes, Eur. J. Cardiovasc. Nurs 17 (8) (2018) 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liberati A, et al. , The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration, Ann. Intern. Med 151 (4) (2009) W-65–W-94. [DOI] [PubMed] [Google Scholar]
- [23].Cunningham AT, et al. , The effect of diabetes self-management education on HbA1c and quality of life in African-Americans: a systematic review and meta-analysis, BMC Health Serv. Res 18 (1) (2018) 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosal MC, et al. , Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income Latinos, Diabetes Care 34 (4) (2011) 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang ML, et al. , Who benefits from diabetes self-management interventions? The influence of depression in the Latinos en Control trial, Ann. Behav. Med 48 (2) (2014) 256–264. [DOI] [PubMed] [Google Scholar]
- [26].Vincent D, Pasvogel A, Barrera L, A feasibility study of a culturally tailored diabetes intervention for Mexican Americans, Biol. Res. Nurs 9 (2) (2007) 130–141. [DOI] [PubMed] [Google Scholar]
- [27].Vincent D, Culturally tailored education to promote lifestyle change in Mexican Americans with type 2 diabetes, J. Am. Acad. Nurse Pract 21 (9) (2009) 520–527. [DOI] [PubMed] [Google Scholar]
- [28].Higgins J, Green S (Eds.), Cochrane Handbook for Systematic Reviews of Interventions, The Cochrane Collaboration, 2011 Vol. Version 5.1.0 [updated March 2011]. 2011. [Google Scholar]
- [29].Borenstein M, et al. , A basic introduction to fixed-effect and random-effects models for meta-analysis, Res. Synth. Methods 1 (2) (2010) 97–111. [DOI] [PubMed] [Google Scholar]
- [30].Witte RS, Witte JS, Statistics, Tenth ed., John Wiley & Sons., New Jersey, 2013. [Google Scholar]
- [31].Israel H, Richter RR, A guide to understanding meta-analysis, J. Orthop. Sports Phys. Ther 41 (7) (2011) 496–504. [DOI] [PubMed] [Google Scholar]
- [32].Borenstein M, Higgins JP, Meta-analysis and subgroups, Prev. Sci 14 (2) (2013) 134–143. [DOI] [PubMed] [Google Scholar]
- [33].Gallegos EC, Ovalle-Berúmen F, Gomez-Meza MV, Metabolic control of adults with type 2 diabetes mellitus through education and counseling, J. Nurs. Scholarsh 38 (4) (2006) 344–351. [DOI] [PubMed] [Google Scholar]
- [34].Peña-Purcell NC, Boggess MM, Jimenez N, An empowerment-based diabetes self-management education program for hispanic/latinos: a quasi-experimental pilot study, Diabetes Educ. 37 (6) (2011) 770–779. [DOI] [PubMed] [Google Scholar]
- [35].Castejón AM, et al. , A community-based pilot study of a diabetes pharmacist intervention in Latinos: impact on weight and hemoglobin A1c, J. Health Care Poor Underserved 24 (4) (2013) 48–60. [DOI] [PubMed] [Google Scholar]
- [36].García AA, et al. , Home-based diabetes symptom self-management education for Mexican Americans with type 2 diabetes, Health Educ. Res 30 (3) (2015) 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosal MC, et al. , Diabetes self-management among low-income Spanish-speaking patients: a pilot study, Ann. Behav. Med 29 (3) (2005) 225–235. [DOI] [PubMed] [Google Scholar]
- [38].Osborn CY, et al. , A brief culturally tailored intervention for Puerto Ricans with type 2 diabetes, Health Educ. Behav 37 (6) (2010) 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rothschild SK, et al. , Mexican american trial of community health workers: a randomized controlled trial of a community health worker intervention for Mexican Americans with type 2 diabetes mellitus, Am. J. Public Health 104 (8) (2014) 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Toobert DJ, et al. , Outcomes from a multiple risk factor diabetes self-management trial for Latinas: ! Viva Bien!, Ann. Behav. Med 41 (3) (2011) 310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tang TS, et al. , Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial, Diabetes Care 37 (6) (2014) 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brown SA, et al. , Dosage effects of diabetes self-management education for Mexican Americans: the starr county border health initiative, Diabetes Care 28 (3) (2005) 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lujan J, Ostwald SK, Ortiz M, Promotora diabetes intervention for Mexican Americans, Diabetes Educ. 33 (4) (2007) 660–670. [DOI] [PubMed] [Google Scholar]
- [44].Philis-Tsimikas A, et al. , Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a project dulce promotora randomized trial, Diabetes Care 34 (9) (2011) 1926–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baig AA, et al. , Picture good health: a church-based self-management intervention among latino adults with diabetes, J. Gen. Intern. Med 30 (10) (2015) 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Palmas W, et al. , Results of the northern Manhattan diabetes community outreach project: a randomized trial studying a community health worker intervention to improve diabetes care in Hispanic adults, Diabetes Care 37 (4) (2014) 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Welch G, et al. , Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center, Diabetes Educ. 37 (5) (2011) 680–688. [DOI] [PubMed] [Google Scholar]
- [48].Perez-Escamilla R, et al. , Impact of a community health workers-led structured program on blood glucose control among latinos with type 2 diabetes: the DIALBEST trial, Diabetes Care 38 (2) (2015) 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aponte J, et al. , Health effectiveness of community health workers as a diabetes self-management intervention, Diab. Vasc. Dis. Res 14 (4) (2017) 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ayala GX, et al. , Puentes hacia una mejor vida (bridges to a Better Life): outcome of a diabetes control peer support intervention, Ann. Fam. Med 13 (2015) S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Brown SA, et al. , Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative, Diabetes Care 25 (2) (2002) 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Welch G, et al. , An internet-based diabetes management platform improves team care and outcomes in an urban Latino population, Diabetes Care 38 (4) (2015) 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lorig K, et al. , Spanish diabetes self-management with and without automated telephone reinforcement two randomized trials, Diabetes Care 31 (3) (2008) 408–414. [DOI] [PubMed] [Google Scholar]
- [54].Babamoto KS, et al. , Improving diabetes care and health measures among hispanics using community health workers results from a randomized controlled trial, Health Educ. Behav 36 (1) (2009) 113–126. [DOI] [PubMed] [Google Scholar]
- [55].Sterne JAC, et al. , Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials, Br. Med. J (2011) 343. [DOI] [PubMed] [Google Scholar]
- [56].Ried K, Interpreting and understanding meta-analysis graphs–a practical guide, Aust. Fam. Physician 35 (8) (2006) 635–638. [PubMed] [Google Scholar]
- [57].Egger M, et al. , Bias in meta-analysis detected by a simple, graphical test, Br. Med. J 315 (7109) (1997) 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Egger M, et al. , How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study, Health Technol. Assess 7 (1) (2003) 1–76. [PubMed] [Google Scholar]
- [59].Snoek F, Visser A, Improving quality of life in diabetes: how effective is education? Patient Educ. Couns 51 (1) (2003) 1–3. [DOI] [PubMed] [Google Scholar]
- [60].Bouton ME, Why behavior change is difficult to sustain, Prev. Med 68 (2014) 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].UKPDS, United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group, Ann. Intern. Med 128 (3) (1998) 165–175. [DOI] [PubMed] [Google Scholar]
- [62].Garber AJ, et al. , Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2018 executive summary, Endocr. Pract 24 (1) (2018) 91–120. [DOI] [PubMed] [Google Scholar]
- [63].Philis-Tsimikas A, et al. , Improvement in Diabetes Care of Underinsured Patients Enrolled in Project Dulce. A community-based, culturally appropriate, nurse case management and peer education diabetes care model, Diabetes Care 27 (1) (2004) 110–115. [DOI] [PubMed] [Google Scholar]
- [64].United States Census Bureau. (2019) 7/31/2018]; Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf.
- [65].Whittemore R, Culturally competent interventions for Hispanic adults with type 2 diabetes: a systematic review, J. Transcult. Nurs 18 (2) (2007) 157–166. [DOI] [PubMed] [Google Scholar]
- [66].Radin MS, Pitfalls in hemoglobin A1c measurement: when results may be misleading, J. Gen. Intern. Med 29 (2) (2014) 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]


