Abstract
Background:
Control of hypertension delays progression of pediatric chronic kidney disease (CKD), yet few data are available regarding what office blood pressure (BP) levels may slow progression.
Methods:
Longitudinal BP data from children in the Chronic Kidney Disease in Children cohort study who had hypertension or an auscultatory BP ≥90th percentile enrolled were studied. BP categories were defined as the maximum systolic or diastolic BP percentile (<50th, 50th to 75th, 75th to 90th and ≥90th percentile) with time-updated classifications corresponding to annual study visits. The primary outcome was time to kidney replacement therapy or a 30% decline in estimated glomerular filtration rate. Cox proportional hazard models described the effect of each BP category compared to BP ≥90th percentile.
Results:
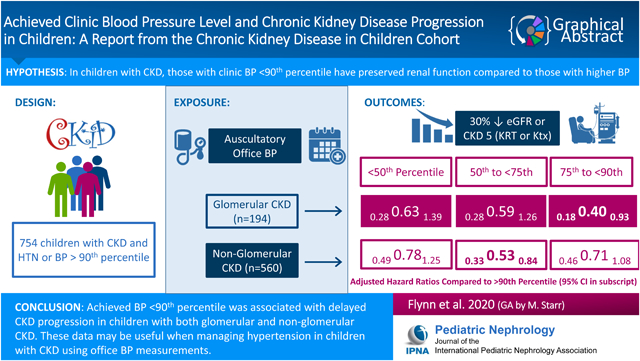
754 participants (median age 9.9 years at study entry) met inclusion criteria; 65% were male and 26% had glomerular CKD. Any BP <90th percentile was associated with a decreased risk of progression for those with glomerular CKD (hazard Ratio (HR), 0.63; 95% CI, 0.28–1.39 (<50th); HR, 0.59; 95% CI, 0.28–1.26 (50th–75th); HR, 0.40; 95% CI, 0.18–0.93 (75th–90th). Similar results were found for those with non-glomerular CKD: any BP < 90th percentile was associated with decreased risk of progression (HR, 0.78; 90% CI, 0.49–1.25 (<50th); HR, 0.53; 95% CI, 0.33–0.84 (50th–75th); HR, 0.71; 95% CI, 0.46–1.08 (75th–90th).
Conclusions:
Achieved BP <90th percentile was associated with slower CKD progression in children with glomerular or non-glomerular CKD. These data provide guidance for management of children with CKD in the office setting.
Keywords: hypertension, chronic kidney disease, glomerular filtration rate, children, adolescents, cohort study
Graphical Abstract
Introduction
There is a growing body of evidence that hypertension is a major contributor to progression in pediatric patients with established chronic kidney disease (CKD) [1]. In a study designed to test the effects of a low-protein versus conventional diet on CKD progression in children, hypertension (defined as systolic blood pressure (BP) > 120 mmHg) and proteinuria (24-hour urine protein > 50 mg/kg) were independently associated with glomerular filtration rate (GFR) decline [2]. Early prospective data from the Chronic Kidney Disease in Children (CKiD) study demonstrated that the annualized GFR decline was faster among patients with an abnormal ambulatory BP compared to those with normal ambulatory BP (ABP), though the relationship was not statistically significant [3]. In another analysis, CKiD participants with a baseline clinic BP < 50th percentile were found to have a significantly slower rate of progression compared to those with higher baseline BP [4]. More recent publications from CKiD have confirmed that hypertension accelerates progression of CKD, both in the overall CKiD cohort and in a sub-analysis of children with non-glomerular forms of CKD [5, 6].
These data, as well as adult observational and interventional studies of CKD [7–9], suggest that BP reduction is a critical intervention to slow the progression of CKD. Most notably, the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial has provided strong evidence for the crucial role of BP management when treating patients with CKD [10]. This trial demonstrated that children who received intensified ABP control (24-hr mean arterial pressure (MAP) < 50th percentile) were less likely to reach the primary end point (50% decline in estimated GFR or progression to stage 5 chronic kidney disease (CKD 5)) after 5 years than those who received conventional ABP control (24-hr MAP between 50th and 90th percentiles). What is not known, however, is what clinic BP level should be targeted during longitudinal follow-up to achieve renoprotection in children with CKD. This is an important question since the measurement of ABP does not readily translate to clinic BP, which is more commonly measured. The aim of the present study, therefore, was to utilize the longitudinal assessment of clinic BP among participants in the CKiD cohort study to determine the association between achieved clinic BP levels and GFR decline. We hypothesized that children with clinic BP < 50th percentile would have preserved kidney function compared to children with clinic BP > 75th and 90th percentiles.
Methods
Study population
The CKiD study is an observational cohort of CKD in children conducted at 56 centers in North America to characterize the natural and treated history of CKD. Inclusion criteria include age 1–16 years and estimated GFR of 30–90 ml/min per 1.73 m2, calculated using the bedside CKiD study formula [11]. Clinical data were collected at annual study visits and included measures of clinic BP, kidney health (GFR) and medical/general health. Kidney replacement therapy (dialysis or transplant) were collected by medical records or self-report. The CKiD study protocol adheres to the Declaration of Helsinki and has been reviewed and approved by the institutional review boards of each participating center. All participants and/or guardians provided written informed consent or assent according to local requirements. The full details of the CKiD study protocol, including exclusion criteria, have been described previously [12].
The primary purpose of this analysis was to estimate the risk of CKD progression associated with BP percentile categories among those with a history of hypertension or elevated blood pressure. Therefore, this analysis was restricted to CKiD participants with a previous diagnosis of hypertension reported at entry into CKiD or who had an elevated BP (systolic or diastolic BP ≥ 90th percentile) measured at least once during follow-up. Since the target population was children with history of hypertension, participants with no previous diagnosis of hypertension and no visits with BP ≥ 90th percentile were excluded from the analysis. Subsequently, we excluded from the analysis all visits prior to the first visit with a measured BP ≥ 90th percentile among participants with no previous diagnosis of hypertension. Also excluded from the study population were person-visits where age ≥ 18, as blood pressure percentiles were not calculated for adults.
Exposure
As previously described [13], a trained examiner obtained three resting BP measurements approximately 30 sections apart using an aneroid sphygmomanometer at each annual CKiD study visit. The mean of the three measurements comprised the clinic BP value for each participant. BP categories were defined as the maximum systolic or diastolic BP percentile for age and sex according to the 2017 American Academy of Pediatrics Clinical Practice Guideline [14] (< 50th, 50th to < 75th, 75th to < 90th and ≥ 90th percentile) and were allowed to vary over time, according to BP measurements at each annual study visit. Since the pediatric BP percentiles are not applicable to adults, this study population was restricted to person-visits with age less than 18 years.
Outcome and censoring
The outcome was a composite event defined as the first occurrence of kidney replacement therapy (dialysis or kidney transplant) or a 30% GFR decline from the first visit at which hypertension or elevated BP (i.e., analytical baseline) was detected. Censoring approaches were based on the inherent short-term variability in exposure (i.e., BP) status. For participants who were not observed to have an event, censoring occurred at their last visit. For consecutive visits that occurred more than 1.5 years apart, censoring occurred at 1.5 years after the earlier visit in order to retain the data from the visit while avoiding extrapolating their exposure beyond 1.5 years. Participants with events occurring more than 1.5 years after their last visit free of the event were censored at their last event-free visit, since BP levels leading up to the event were not known.
Stratification and covariates
Participant diagnoses in the CKiD cohort have been broadly classified as either glomerular or non-glomerular [13, 15], which have different pathology and progression patterns. In particular, children with glomerular diagnoses are more likely to have proteinuria, are commonly treated with inhibitors of the renin-aldosterone-angiotensin system and tend to progress faster than children with non-glomerular CKD. Thus, all analyses were stratified by diagnosis, broadly defined as glomerular or non-glomerular in etiology. Potential confounders of the BP and kidney disease progression relationship included race, defined as African American or non-African American, socioeconomic status variables defined as annual household income (≤ $36,000, $36,001 to 75,000, > $75,000) and maternal education (college degree or more), age (on a continuous scale), and CKD-related variables defined as log-transformed GFR and nephrotic range proteinuria (urine protein to creatinine ratio > 2), where all were measured at entry.
Statistical analysis
Clinical and demographic characteristics of this sub-cohort of CKiD participants were stratified by CKD diagnosis, as well as BP percentile categories at baseline. Non-parametric Kaplan Meier functions described the distribution of composite outcome timing across BP categories. Since blood pressure was allowed to vary across visits, we estimated cumulative survival for each blood pressure category using the counting process formulation [16]. Participants entered a risk set at t0 (time since origin that a given BP was measured) and exited at t1, which was defined as either the time of the composite event, time of transition to another BP category without the event, or time of transition out of the study without the event. Cox proportional hazard models described the putative reduction in risk associated with BP < 50th percentile, BP 50th – < 75th percentile, 75th – < 90th percentile compared to ≥ 90th percentile as the reference in both unadjusted and adjusted models.
Since those who entered the study with a previous diagnosis of hypertension had prevalent exposure and the time since hypertension diagnosis (i.e., duration) was not known, we performed a sensitivity analysis restricted to participants who had incident hypertension. In this group, there was a more homogenous time since incident hypertension and data were available for characteristics at the time of hypertension onset. This sensitivity analysis was conducted only among those with non-glomerular CKD. The subgroup with glomerular CKD had an insufficient sample size for meaningful inference.
Hazard ratios are presented with 2-sided 95% confidence intervals, where intervals not containing the null value 1 were considered statistically significant. Analyses were performed using SAS version 9.4 and R version 3.6.1.
Results
Of the 1093 participants enrolled in CKiD, a total of 754 (194 and 560 children with glomerular and non-glomerular CKD, respectively) met the inclusion criteria for this analysis. Inclusion criteria was not met for 339 participants: 261 had no previous diagnosis or onset of hypertension, 55 had only 1 study visit, 16 had missing BP at all study visits, and 7 had the event > 1.5 years after the first study visit. Participants contributed an average of 3.5 and 4.3 person-visits (679 and 2403 total) over 2.7 and 3.6 years (517 and 2008 total) to the analysis for those with glomerular and non-glomerular disease, respectively. Of the 754 participants who met inclusion criteria, median age at entry was 9.9 years, 65% were male, 26% had glomerular CKD, and median duration of CKD at entry was 6.8 years. Tables 1 and 2 describe the baseline characteristics of participants with glomerular and non-glomerular diagnoses, respectively. Among participants with glomerular CKD, 57% (n = 110) had BP ≥ 90th percentile. Of the 73% (n = 142) who entered with a previous diagnosis of hypertension, 41% (n = 58) had BP ≥ 90th percentile. Those with BP ≥ 90th percentile at baseline were shorter, less likely to use antihypertensive medications, more likely to have nephrotic range proteinuria, and had lower GFR. Among participants with non-glomerular CKD, 72% (n = 403) had BP ≥ 90th percentile. Of the 51% (n = 285) who entered with a previous diagnosis of hypertension, 45% (n = 128) had BP ≥ 90th percentile. Those with BP ≥ 90th percentile at baseline were younger and less likely to use antihypertensive medications.
Table 1.
Demographic and clinical characteristics of the study population with glomerular CKD diagnosis by baseline blood pressure percentile. Median (25th percentile, 75th percentile) or N (%).
| BP percentile <50 (N = 31) | BP percentile 50 to <75 (N = 28) | BP percentile 75 to <90 (N = 24) | BP percentile 90+ with prior HTN (N = 58) | BP percentile 90+ with no prior HTN (N = 52) | Overall (N = 194) | |
|---|---|---|---|---|---|---|
| Age (years) | 14.2 [12.3, 15.5] | 13.9 [10.2, 15.9] | 14.3 [12.1, 15.3] | 13.6 [8.6, 15.9] | 12.7 [9.5, 15.5] | 13.6 [10.2, 15.8] |
| Male | 16 (51.6) | 16 (57.1) | 13 (54.2) | 33 (56.9) | 26 (50.0) | 104 (53.6) |
| Black Race | 5 (16.1) | 5 (17.9) | 12 (50.0) | 22 (37.9) | 18 (34.6) | 63 (32.5) |
| Hispanic Ethnicity | 3 (9.7) | 4 (14.3) | 4 (16.7) | 7 (12.1) | 12 (23.1) | 30 (15.5) |
| Height Percentile | 43.4 [16.9, 74.2] | 52.3 [25.4, 82.4] | 38.3 [22.9, 62.1] | 37.4 [10.4, 78.3] | 31.8 [13.1, 68.7] | 40.1 [14.5, 76.2] |
| Weight Percentile | 60.9 [33.5, 91.8] | 85.9 [56.2, 98.5] | 79.1 [38.8, 96.7] | 84.4 [56.6, 98.3] | 67.4 [30.5, 94.2] | 76.4 [45.1, 96.6] |
| BMI | ||||||
| <5th percentile | 2 (6.5) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 3 (5.9) | 6 (3.1) |
| 5–<85th percentile | 15 (48.4) | 14 (50.0) | 13 (54.2) | 20 (34.5) | 27 (52.9) | 89 (46.1) |
| 85th-<95th percentile | 11 (35.5) | 3 (10.7) | 4 (16.7) | 16 (27.6) | 8 (15.7) | 43 (22.3) |
| ≥95 percentile | 3 (9.7) | 11 (39.3) | 7 (29.2) | 21 (36.2) | 13 (25.5) | 55 (28.5) |
| Use of Antihypertensive | ||||||
| None | 2 (6.5) | 1 (3.6) | 1 (4.2) | 1 (1.7) | 9 (19.6) | 14 (7.4) |
| Other antihypertensive | 1 (3.2) | 2 (7.1) | 4 (16.7) | 14 (24.1) | 5 (10.9) | 26 (13.8) |
| ACE-inhibitor/ARB | 28 (90.3) | 25 (89.3) | 19 (79.2) | 43 (74.1) | 32 (69.6) | 148 (78.7) |
| CKD Duration (years) | 3.0 [1.5, 5.7] | 3.8 [1.7, 6.0] | 2.0 [1.0, 6.7] | 3.3 [1.2, 5.8] | 3.8 [1.6, 7.7] | 3.4 [1.4, 6.4] |
| eGFR, (ml/min|1.73m2) | 63.3 [48.2, 76.0] | 63.3 [42.5, 84.4] | 57.1 [46.2, 78.0] | 53.4 [35.3, 67.2] | 56.5 [42.2, 67.3] | 58.7 [42.3, 73.0] |
| Urine P:C ratio | 0.4 [0.2, 0.7] | 0.5 [0.1, 1.2] | 0.6 [0.1, 2.5] | 1.7 [0.4, 4.7] | 1.5 [0.6, 4.8] | 0.9 [0.2, 3.1] |
| P:C ratio >2.0 | 2 (6.5) | 4 (14.3) | 6 (26.1) | 26 (46.4) | 18 (42.9) | 57 (31.5) |
| Household Income | ||||||
| $0-$36000/year | 10 (33.3) | 15 (53.6) | 15 (62.5) | 25 (45.5) | 16 (36.4) | 81 (44.5) |
| $36001-$75000/year | 10 (33.3) | 7 (25.0) | 6 (25.0) | 15 (27.3) | 15 (34.1) | 53 (29.1) |
| $75001/year + | 10 (33.3) | 6 (21.4) | 3 (12.5) | 15 (27.3) | 13 (29.5) | 48 (26.4) |
| Maternal education college or more | 18 (62.1) | 18 (64.3) | 16 (66.7) | 43 (75.4) | 27 (65.9) | 123 (68.3) |
| Eventsb | 7 | 8 | 10 | 31 | 26 | 83 |
N = 1 missing BP percentile at baseline
Initiation of kidney replacement therapy (dialysis or kidney transplant), or 30% decline in estimated glomerular filtration rate ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; P:C protein:creatinine
Table 2.
Demographic and clinical characteristics of the study population with non-glomerular CKD diagnosis by baseline blood pressure percentile. Median (25th percentile, 75th percentile) or N (%).
| BP percentile <50 (N = 35) | BP percentile 50 to <75 (N = 40) | BP percentile 75 to <90 (N = 66) | BP percentile 90+ with prior HTN (N = 128) | BP percentile 90+ with no prior HTN (N = 275) | Overalla (N = 560) | |
|---|---|---|---|---|---|---|
| Age (years) | 12.5 [9.0, 14.9] | 11.0 [8.4, 12.9] | 10.4 [6.6, 13.3] | 8.7 [4.6, 12.8] | 7.4 [4.3, 11.6] | 8.6 [4.8, 12.6] |
| Male | 20 (57.1) | 20 (50.0) | 42 (63.6) | 82 (64.1) | 208 (75.6) | 383 (68.4) |
| Black Race | 5 (14.3) | 6 (15.0) | 16 (24.2) | 27 (21.1) | 53 (19.3) | 109 (19.5) |
| Hispanic Ethnicity | 2 (5.7) | 1 (2.5) | 8 (12.1) | 25 (19.5) | 40 (14.6) | 77 (13.8) |
| Height Percentile | 24.2 [7.6, 42.3] | 15.4 [7.1, 49.3] | 23.4 [7.7, 51.0] | 24.4 [7.5, 49.7] | 29.0 [8.4, 58.7] | 25.1 [7.7, 51.4] |
| Weight Percentile | 46.1 [19.2, 81.5] | 41.6 [11.0, 76.0] | 38.7 [24.3, 83.0] | 51.1 [23.8, 81.2] | 43.0 [18.2, 76.5] | 43.2 [17.9, 79.7] |
| BMI | ||||||
| <5th percentile | 2 (5.7) | 1 (2.6) | 2 (3.0) | 3 (2.4) | 15 (5.8) | 23 (4.3) |
| 5–<85th percentile | 21 (60.0) | 28 (71.8) | 44 (66.7) | 75 (61.0) | 171 (65.8) | 346 (64.8) |
| 85th-<95th percentile | 6 (17.1) | 6 (15.4) | 6 (9.1) | 17 (13.8) | 37 (14.2) | 74 (13.9) |
| ≥95 perentile | 6 (17.1) | 4 (10.3) | 14 (21.2) | 28 (22.8) | 37 (14.2) | 91 (17.0) |
| Use of Antihypertensive | ||||||
| None | 1 (2.9) | 8 (20.0) | 9 (13.6) | 16 (12.5) | 157 (74.1) | 197 (39.6) |
| Other antihypertensive | 3 (8.6) | 4 (10.0) | 13 (19.7) | 33 (25.8) | 17 (8.0) | 77 (15.5) |
| ACE-inhibitor/ARB | 31 (88.6) | 28 (70.0) | 44 (66.7) | 79 (61.7) | 38 (17.9) | 223 (44.9) |
| CKD Duration (years) | 12.3 [9.0, 14.9] | 11.0 [8.4, 12.9] | 9.4 [4.8, 13.3] | 8.0 [4.2, 11.0] | 7.2 [4.2, 10.9] | 8.1 [4.5, 12.1] |
| eGFR, (ml/min|1.73m2) | 44.3 [34.4, 50.1] | 49.8 [34.5, 64.5] | 54.6 [37.9, 63.1] | 49.1 [39.5, 66.8] | 52.3 [37.7, 62.4] | 50.3 [37.4, 62.9] |
| Urine P:C ratio | 0.2 [0.1, 0.5] | 0.2 [0.1, 0.4] | 0.3 [0.1, 1.1] | 0.2 [0.1, 0.7] | 0.4 [0.2, 0.9] | 0.3 [0.1, 0.8] |
| P:C ratio >2.0 | 3 (8.8) | 2 (5.3) | 8 (12.3) | 11 (9.1) | 15 (7.7) | 41 (8.8) |
| Household Income | ||||||
| $0-$36000/year | 16 (45.7) | 18 (46.2) | 27 (42.2) | 54 (43.5) | 65 (34.6) | 184 (39.5) |
| $36001-$75000/year | 8 (22.9) | 10 (25.6) | 17 (26.6) | 39 (31.5) | 62 (33.0) | 141 (30.3) |
| $75001/year + | 11 (31.4) | 11 (28.2) | 20 (31.2) | 31 (25.0) | 61 (32.4) | 141 (30.3) |
| Maternal education college or more | 22 (64.7) | 28 (70.0) | 43 (65.2) | 78 (62.4) | 115 (63.2) | 294 (63.5) |
| Eventsb | 10 | 9 | 28 | 53 | 89 | 194 |
N = 16 missing BP percentile at baseline
Initiation of kidney replacement therapy (dialysis or kidney transplant), or 30% decline in estimated glomerular filtration rate ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; P:C protein:creatinine
Figure 1 depicts the nonparametric Kaplan-Meier survival functions and corresponding risk sets of the four time-updated blood pressure groups stratified by those with glomerular or non-glomerular diagnoses (Figure 1a and 1b, respectively). For both diagnoses, the distribution of participants in each risk set is skewed towards the highest BP category at study entry and becomes more balanced as time passes. Size of the risk set is allowed to increase over time, as the exposure is time-varying and individuals can change exposure group across study visits. Participants with blood pressure ≥ 90th at any time after the baseline visit had the highest risk of CKD progression among participants with both glomerular and non-glomerular CKD. Among those with a glomerular diagnosis, the 75th percentile of cumulative survival was 0.9 years, 4.8 years, 3.1 years, and 2.8 years for those with BP percentile > 90th, 75th to 90th, 50th to 75th and > 50th, respectively. For non-glomerular participants, the 75th percentile of cumulative survival was 2.9 years, 3.7 years, 6.6 years, and 3.2 years for those with BP percentile > 90th, 75th to 90th, 50th to 75th and > 50th, respectively.
Figure 1.
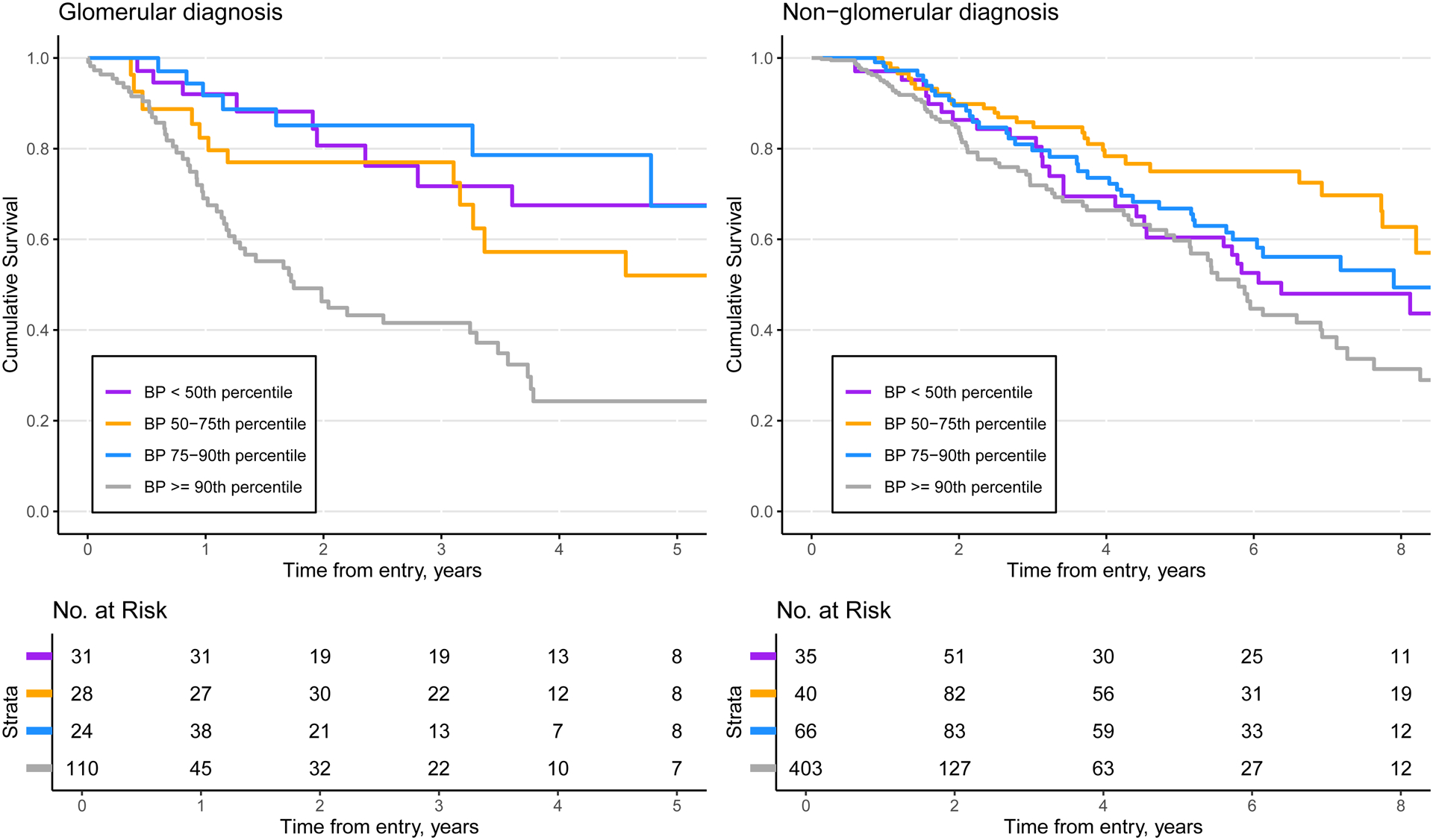
Time to kidney replacement therapy or 30% decline in GFR by CKD diagnosis (n = 679 person-visits from n = 194 participants. (G) and n = 2403 person-visits from n = 560 participants (NG))
The hazard ratios (HRs) from both unadjusted and adjusted Cox proportional hazard models are listed in Table 3 and illustrated in Figure 2. In participants with glomerular CKD, any BP < 90th percentile was associated with a decreased risk of disease progression (HR, 0.27; 95% CI, 0.13–0.55 (< 50th); HR, 0.40; 95% CI, 0.21–0.75 (50th to 75th); HR, 0.24; 95% CI, 0.11–0.53 (75th to 90th)). After adjusting for race, income, maternal education, baseline proteinuria and baseline GFR, similar results were found (HR, 0.63; 95% CI, 0.28–1.39 (< 50th); HR, 0.59; 95% CI, 0.28–1.26 (50th–75th); HR, 0.40; 95% CI, 0.18–0.93 (75th–90th)). Participants with non-glomerular CKD whose BP < 90th percentile were at a decreased hazard of the composite event (HR, 0.83; 90% CI, 0.54–1.26 (< 50th); HR, 0.49; 95% CI, 0.32–0.76 (50th–75th); HR, 0.62; 95% CI, 0.42–0.91 (75th–90th). The association remained after adjustment for race, income, maternal education, baseline proteinuria and baseline GFR (HR, 0.78; 90% CI, 0.49–1.25 (< 50th); HR, 0.53; 95% CI, 0.33–0.84 (50th–75th); HR, 0.71; 95% CI, 0.46–1.08 (75th–90th). For both glomerular and non-glomerular participants, there was not a clear dose response between the exposure and outcome. For those with a glomerular diagnosis, the lowest risk was seen in those with BP 75th to 90th percentile. For those with a non-glomerular diagnosis, the lowest risk was seen in those with BP 50th to 75th percentile. There were no significant differences in pairwise comparisons between BP categories less than the 90th percentile within each diagnosis. For example, among those with a glomerular diagnosis, the risk of the composite outcome was higher for those with BP < 50th relative to BP 50th to 75th percentile, although this was not statistically significant (HR, 1.57; 95%CI, 0.56–4.38). Full results of all pairwise comparisons are provided in Supplemental Table 1.
Table 3.
Unadjusted and adjusted (race, income, maternal education, baseline proteinuria, baseline GFR) hazard ratios (95% CI) of kidney replacement therapy or 30% decline in GFR, by diagnosis.
| Glomerular diagnosis | Non-glomerular diagnosis | |||
|---|---|---|---|---|
| Blood Pressure Percentile | Unadjusted N = 194 Person-visits = 679 |
Adjusted N = 175 Person-visits = 628 |
Unadjusted N = 560 Person-visits = 2403 |
Adjusted N = 451 Person-visits = 2010 |
| <50th | 0.27 (0.13, 0.55) | 0.63 (0.28, 1.39) | 0.82 (0.54, 1.26) | 0.78 (0.49, 1.25) |
| 50th to <75th | 0.40 (0.21, 0.75) | 0.59 (0.28, 1.26) | 0.49 (0.32, 0.76) | 0.53 (0.33, 0.84) |
| 75th to <90th | 0.24 (0.11, 0.53) | 0.40 (0.18, 0.93) | 0.62 (0.42, 0.91) | 0.71 (0.46, 1.08) |
| ≥90th | 1.00 | 1.00 | 1.00 | 1.00 |
Figure 2.
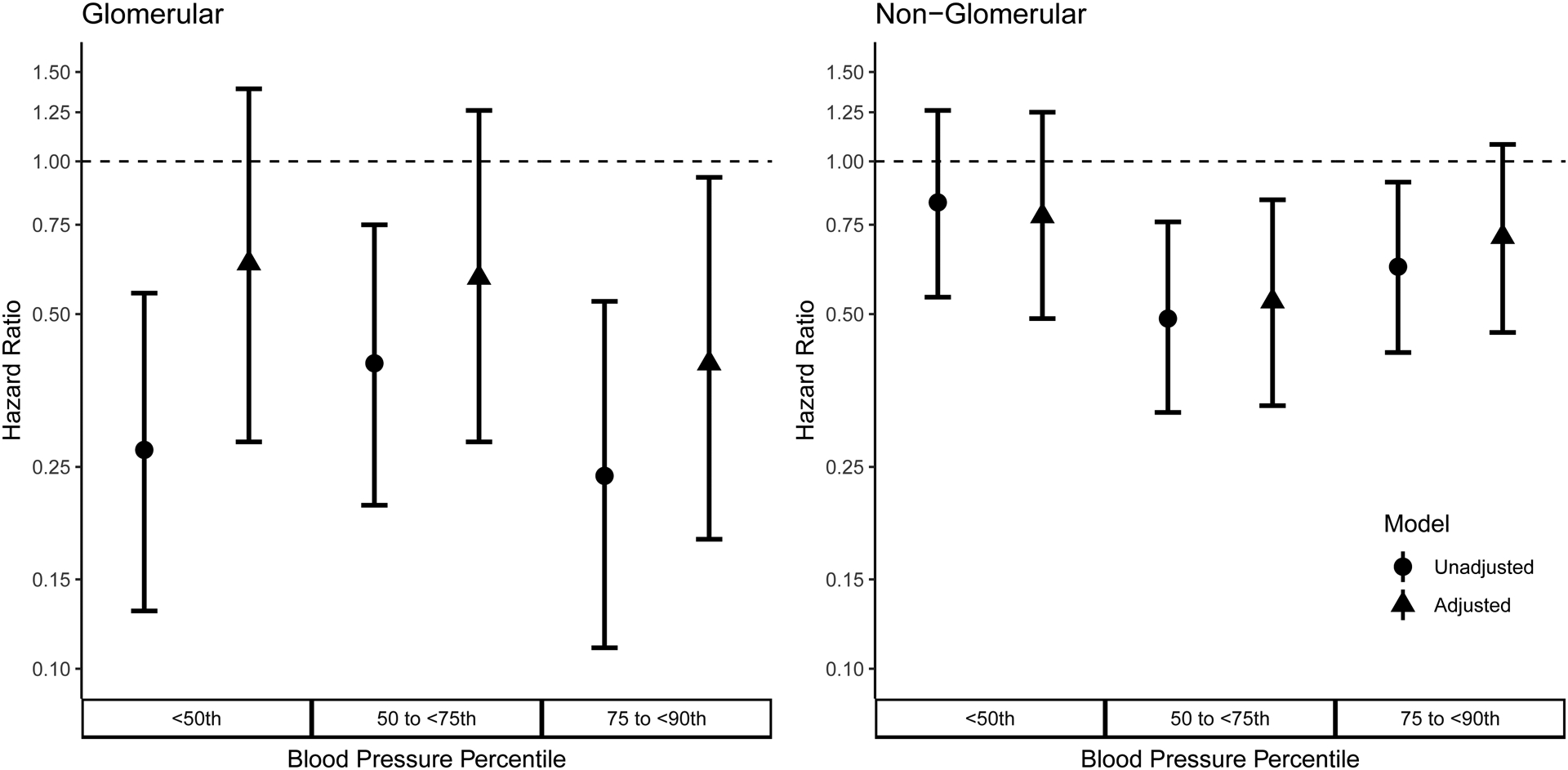
Unadjusted and adjusted hazard ratios and 95% confidence intervals comparing the risk of stage 5 chronic kidney disease or a 30% decline in GFR for different blood pressure percentile categories with blood pressure ≥ 90th percentile as the reference. Adjustment models included race (black race vs. non-black race), income, maternal education, baseline age, baseline proteinuria and baseline GFR as covariates. Circles and triangles represent the estimated hazard ratios relative to BP >90th percentile for unadjusted and adjusted models, respectively. Whiskers depict the 95% confidence intervals of the hazard ratio estimates.
As a sensitivity analysis, we restricted to those with a non-glomerular diagnosis, SBP or DBP ≥ 90th percentile and no previous diagnosis of hypertension. In this subpopulation, where we only looked at visits immediately after onset of elevated blood pressure, we saw similar results to the full analysis. A summary of the analysis and its results are presented in the supplemental material.
Discussion
This study demonstrates that achievement of clinic BP below the 90th percentile in hypertensive children with CKD is associated with slowed progression of CKD. More importantly, we also show that among children with glomerular CKD, a clinic BP between 75th and 90th percentile was associated with the lowest risk of disease progression, while for those with non-glomerular forms of CKD, achieving a clinic BP between the 50th and 75th percentiles may offer an additional advantage in terms of slowing CKD progression. This is notable given that most children with CKD have non-glomerular diagnoses [10] and are usually felt not to be at significant risk for hypertension.
Hypertension is a well-established risk factor for progression of CKD in both adults [7] and children [2, 17]. Data from the North American Pediatric Renal Transplant Cooperative Study registry showed that the estimated GFR declined faster in children with CKD who were hypertensive at baseline compared to those who were normotensive at baseline [17]. In a prospective multicenter study designed to assess the effects of a low-protein diet on kidney function and growth in children with CKD, it was found that although a low-protein diet did not reduce the rate of decline in creatinine clearance, systolic BP > 120 mm Hg was an independent predictor of an increased rate of kidney function decline [2]. Many studies in adults have suggested that aggressive BP control is renoprotective. In the Modification of Diet in Renal Disease (MDRD) study, for example, participants randomized to the lower BP goal (defined as mean arterial pressure [MAP] of ≤ 92 mm Hg for those ≤ 60 years of age, or ≤ 98 mm Hg for those ≥ 61 years of age) had slower rates of progression of CKD than did subjects randomized to the “usual” BP goal [8]. On the basis of the findings in the MDRD study, among others, recent consensus guidelines have recommended strict BP control in adults with proteinuric kidney disease as a method of slowing the progression of CKD [18, 19].
In children with CKD, the primary evidence for the role of aggressive BP control in slowing progression of CKD comes from the ESCAPE trial, which randomized children with stage 2–4 CKD to either intensive or usual BP control based upon 24-hour ambulatory BP monitoring [10]. Fewer children randomized to intensive BP control, defined as 24-hour mean arterial pressure < 50th percentile, had either a 50% decline in estimated GFR or progression to CKD 5 than children randomized to usual BP control, defined as 24-hour mean arterial pressure between the 50th and 95th percentiles. Given these results, both the recent European and American guidelines for management of hypertension in children have recommended that children with CKD should be treated based on 24-hr ambulatory BP monitoring to BP targets similar to those in the ESCAPE trial [14, 20].
However, 24-hour ambulatory BP monitoring has notable drawbacks, including limited availability, poor patient tolerability [21] and lack of insurance reimbursement – all of which make it difficult to obtain repeated studies at the frequency recommended in current guidelines. Thus, despite the evidence base for 24-hour ambulatory BP-guided treatment, many clinicians rely upon clinic (sometimes referred to as office or casual) BP measurement to guide management of hypertension in children with CKD. While lower (< 90th percentile) clinic BP targets have been recommended for children with CKD in all recent consensus guidelines [14, 18, 20], these recommendations are largely based upon expert opinion, as there are no available data on clinic BP targets and progression of CKD in children. We recognize that 24-hour ambulatory measurement is currently the gold standard for BP monitoring in children with CKD. The current analyses address the important role of clinic BP in the management of hypertension in this population.
The results of this analysis, based upon longitudinal follow-up of participants in the CKID study, begin to fill in this knowledge gap. We show that CKD progression in children with mild to moderate CKD is slower among those who achieved clinic BP below the 90th percentile, and that in children with non-glomerular forms of CKD, BP between the 50th and 75th percentiles is associated with the slowest rate of CKD progression. While there were no statistically significant differences between BP categories that were less than the 90th percentile, this dataset was likely underpowered to detect further differences. These data may prove useful to clinicians who manage hypertension in pediatric CKD based upon clinic BP measurements, and support recommendations of consensus organizations that clinic BP < 90th percentile should be targeted in children with CKD [14, 20].
In this analysis, the clinically relevant exposure metric is achieved BP percentile rather than antihypertensive therapy use. Most of the antihypertensive therapy use in this cohort was ACEi/ARBs, but data were not available for dosing or intensity of therapy. In addition, we note there is likely heterogeneity in prescribing practices, adherence and patient response to therapy, thus the observed BP percentile offers a useful clinical parameter.
Our findings have certain limitations. These are observational data that reflect the usual care at academic centers in North America, and thus may not be generalizable to all children with CKD, but are expected to be applicable to children with CKD and a diagnosis of hypertension. Given that the study is observational in nature, there was no pre-specified treatment protocol, and we do not know how the local site physicians responded to the participants’ BPs. Indeed, we have previously shown that several years after demonstrating poor control of BP in this cohort at baseline, BP control still remains sub-optimal [22]; reasons for this are unclear and may reflect patient or provider factors that prevent achievement of lower BP targets. For those entering the present analysis with a history of hypertension, data were not available on duration of hypertension prior to study observation, which is a limitation. Since exposure (BP percentile) was considered time-varying, these participants entered the study at their first observed BP category. However, this was consistent with the assumptions and interpretation of the HR for a time-varying exposure. Specifically, HRs for a given exposure level are interpreted as the risk associated for a person who remains in that category, but individuals are allowed to vary over time and their exposure status can change according to longitudinal updated data. It is important to note the interpretation of these hazard ratios as summary estimates of risk are for groups of hypothetical participants who persist in each category. Analytically, each individual’s BP exposure was updated at each visit, and this corresponded to appropriate methodologic assumptions for modeling time-varying exposures [23]. It is possible, and perhaps likely, that children with chronically high BP may not fully reduce risk with effective treatment. Future studies should investigate the risks associated with burden of chronic hypertension.
We also note that this study population included both those with prevalent and those with incident hypertension. Ideally, we would have preferred to have had only participants with incident hypertension included in our study population to address this question since we do not have data on the duration or severity of hypertension prior to study enrollment. Unfortunately, because of the high prevalence of hypertension in this population, for sufficient numbers and to maximize data use, we used a combination of both incident and prevalent participants. For the sensitivity analysis, we had adequate data to investigate those with incident hypertension and non-glomerular diagnoses, but did not for those with incident hypertension and glomerular diagnoses.
Additionally, we recognize that unmeasured factors related to blood pressure control could explain some of the hazard ratio associations observed. Using the E-value methodology, an unmeasured confounder that was associated with the exposure and the outcome with HR = 2.47 would nullify the significant effect observed among non-glomerular participants with BP 50th to 75th percentile [24]. Confounding due to unmeasured socioeconomic status factors outside of income and maternal education, or unmeasured data on what BP physicians targeted after onset of hypertension and why, may explain some of these effects. In addition, only data from annual visits were used and it is likely that heterogeneity of BP control between visits would modify the associations observed, although it is unclear in what direction that would be. Nonetheless, the study design restricted to individuals with a previous diagnosis of hypertension or evidence of uncontrolled high blood pressure ensured reasonably comparable individuals at baseline to explore the relationship between level of control and CKD progression [25]. Since most clinical decisions are most conveniently made using office blood pressure measurements, future research should continue to explore and describe these relationships. Lastly, we note that these are observational data and should not be interpreted as causal effects of treatment (as an intervention) or BP percentiles (as a biomarker). Our goal is to describe the phenomenon observed between achieved BP and CKD progression to help understand how hypertension management using clinic BP may affect CKD progression. These observations could potentially serve as the foundation for future randomized clinical trials of hypertension management in pediatric CKD.
At the same time, these data have notable strengths. Auscultatory BP measurement in the CKiD study is standardized across all participating centers [12, 13] and is consistent with current guidelines for BP measurement in children [14, 20]. We have also incorporated the most recently published normative data for pediatric BP, thus making our findings immediately applicable to patient management in the clinic setting. The analysis was restricted to children with a history of hypertension or elevated BP, which is again applicable to targeting particular BP levels in hypertensive children with CKD.
Conclusions
As shown in previous publications from the CKiD cohort, hypertension is an important modifiable factor affecting the rate of progression of pediatric CKD [5, 6]. Clinicians treating children with CKD require guidance on optimal BP levels that may reduce the contribution of hypertension to CKD progression. We now provide observational evidence that clinic BP below the 90th percentile is associated with slower progression in children with CKD, and that BP between the 50th and 75th percentiles may confer additional benefit to children with non-glomerular diagnoses, which constitute the majority of pediatric CKD. Ideally these findings should be confirmed prospectively.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek K. Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD website is located at https://statepi.jhsph.edu/ckid.
The authors thank Michelle C. Starr, MD, MPH for her help with creation of the visual abstract.
Declarations
Sources of funding
The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK066143, U01 DK066174, U24 DK082194, U24 DK066116).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
Joseph Flynn reports grants from the American Heart Association, personal fees from Silvergate Phamaceuticals, Ultragenyx, Springer, and Up To Date outside the published work. No other conflicts of interest to report.
Ethics approvals
The CKiD protocol has been approved by the Institutional Review Boards/Ethics Committees of the participating centers. All participants/their parents provided written informed consent/assent according to local requirements.
Consent to publication
All authors take responsibility for the published work and give their consent for publication.
Availability of Data
Data that support the findings of this study are available from the corresponding author on reasonable request. The CKiD cohort study additionally provides comprehensive publicly available data through the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository (https://www.niddkrepository.org/home/).
References:
- 1.VanDeVoorde RG, Mitsnefes MM (2011) Hypertension and CKD. Adv Chronic Kidney Dis 18:355–361 [DOI] [PubMed] [Google Scholar]
- 2.Wingen AM, Fabian-Bach C, Schaefer F, Mehls O (1997) Randomized multi-centre study of a low-protein diet on the progression of chronic renal failure in children. Lancet 349:1117–1123. [DOI] [PubMed] [Google Scholar]
- 3.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S; Chronic Kidney Disease in Children Study Group (2012) Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furth S, Flynn J, Pierce C, et al. (2012) Lower systolic BP associated with slower CKD progression in the CKiD Study (abstract). J Am Soc Nephrol 21:551A [Google Scholar]
- 5.Warady BA, Abraham A, Schwartz G, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S (2015) Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kid Dis 65:878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, Moxey-Mims MM, Warady BA, Furth SL, Wong CS (2015) Progression of pediatric chronic kidney disease of non-glomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 10:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J (1996) Blood pressure and end-stage renal disease in men. N Engl J Med 334:13–18 [DOI] [PubMed] [Google Scholar]
- 8.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL (1995) Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 123:754–762 [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group (2010) Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ESCAPE Trial Group Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361:1639–1650 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A (2012) Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA (2006) Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1:1006–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA; Chronic Kidney Disease in Children Study Group (2008) Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study. Hypertension 52:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM; SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904. [DOI] [PubMed] [Google Scholar]
- 15.Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ; CKiD Investigators (2009) Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol 4:812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen PK, Gill RD (1982) Cox’s regression model for counting processes: a large sample study. Ann Stat 10:1100–1120 [Google Scholar]
- 17.Mitsnefes M, Ho P-L, McEnery PT (2003) Hypertension and progression of chronic renal insufficiency in children: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTICS). J Am Soc Nephrol 14:2618–2622 [DOI] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group (2012) KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kid Int Suppl 2:337–414 [Google Scholar]
- 19.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 20.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wühl E, Zanchetti A (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34:1887–1920 [DOI] [PubMed] [Google Scholar]
- 21.Hamdani G, Flynn JT, Daniels S, Falkner B, Hanevold C, Ingelfinger J, Lande MB, Martin LJ, Meyers KE, Mitsnefes M, Rosner B, Samuels J, Urbina EM (2019) Ambulatory blood pressure monitoring tolerability and blood pressure status in adolescents: the SHIP AHOY study. Blood Press Monit 24:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barletta G-M, Pierce C, Mitsnefes M, Samuels J, Warady BA, Furth S, Flynn J (2018) Is blood pressure improving in children with chronic kidney disease? A period analysis. Hypertension 71:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Smith AR, Mariani LH, Nair V, Zee J (2019) Methods for assessing longitudinal biomarkers of time-to-event outcomes in CKD: a simulation study. Clin J Am Soc Nephrol 14:1315–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanderWeele TJ, Ding P (2017) Sensitivity analysis in observational research: introducing the e-value. Ann Int Med 167:268–274 [DOI] [PubMed] [Google Scholar]
- 25.Hernán MA, Robins JM (2016) Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


