Abstract
Introduction:
Recent advances in immuno-oncology and bioengineering have rekindled the interest in monoclonal antibody (mAb)-based immunotherapies for malignancies. Crucial for their success is the identification of tumor antigens (TAs) that can serve as targets. B7-H3, a member of the B7 ligand family, represents such a TA. Although its exact functions and receptor(s) remain unclear, B7-H3 has predominantly a pro-tumorigenic effect mainly by suppressing the anti-tumor functions of T-cells.
Areas covered:
Initially we present a historical perspective on TA-specific antibodies for diagnosis and treatment of malignancies. Following a description of the TA requirements to be an attractive antibody-based immunotherapy target, we show that B7-H3 fulfills these criteria. We discuss its structure and functions. In a review and pooled analysis, we describe the limited B7-H3 expression in normal tissues and estimate B7-H3 expression frequency in tumors, tumor-associated vasculature and cancer initiating cells (CICs). Lastly, we discuss the association of B7-H3 expression in tumors with poor prognosis.
Expert opinion:
B7-H3 is an attractive target for mAb-based cancer immunotherapy. B7-H3-targeting strategies are expected to be highly effective and – importantly – safe. To fully exploit the diagnostic and therapeutic potential of B7-H3, its expression in pre-malignant lesions, serum, metastases, and CICs requires further investigation.
Keywords: B7-H3, immunotherapy, monoclonal antibody, tumor antigen
1. INTRODUCTION
Over the years there has been an increasing interest in the use of monoclonal antibodies (mAbs) for the treatment of malignant diseases. Critical for the development and successful application of mAb-based immunotherapies is the identification of suitable tumor antigens (TAs) which can serve as targets for these therapies. In this review, after firstly reflecting on a historical perspective on the use of TA-specific antibodies for the diagnosis and treatment of malignancies, we present the requirements for a TA to be an attractive target of antibody-based immunotherapy. We then discuss the TA B7-Homolog 3 (B7-H3), a promising tumor antigen which fulfills the characteristics of an attractive TA target. We focus on its structure and function, its expression modulation and the different B7-H3-targeting mAb-based strategies that are being developed for the treatment of malignant diseases. We also summarize the data on B7-H3 expression in non-malignant tissues and calculate the cumulative B7-H3 expression frequency across all malignancies and by cancer type. Lastly, we evaluate the B7-H3 distribution (membranous or cytoplasmic) in cancer cells, as well as tumor-associated vasculature (TAV) B7-H3 expression frequency for each cancer type. The provided information will contribute to optimize the selection of patients and cancer types for treatment with B7-H3 targeting immunotherapeutic strategies.
2. HISTORICAL PERSPECTIVE
Tumor immunologists realized long ago that malignant transformation of cells may be associated not only with changes in their morphology, proliferation, growth requirements and tumorigenic potential, but also with the induction or upregulation of cytoplasmic and/or membrane-bound molecules. Molecules which are selectively, although non-specifically expressed by malignant cells were referred to as TAs. The possibility that these molecules could be used as diagnostic biomarkers and/or therapeutic targets stimulated the generation or isolation of probes to detect them. As a result, there was a major effort to isolate TA-specific antibodies present in the blood of patients with malignant disease and/or in eliciting them in animals utilizing cancer cells or TAs with different degrees of purification as immunogens. Some of these approaches were successful and generated antibodies to TAs like α-fetoprotein and carcinoembryonic antigen, which were used to develop and implement clinically relevant diagnostic assays [1, 2]. In addition, some of the TA-specific antibodies were used to design therapeutic strategies for the treatment of malignant diseases. The antibodies were utilized either as i) naked antibodies to activate the complement system or antibody-dependent cellular cytotoxicity (ADCC), ii) conjugated to radioisotopes for imaging and/or elimination of tumors [3], or iii) as carriers of chemotherapeutic agents or toxins for therapeutic purposes (antibody-drug conjugates, ADC). In general, the latter strategies had a low, if any success mostly because of the poor characteristics of the TA-specific antibodies isolated from patients with malignancies or elicited in animals. The latter included the low specificity and high heterogeneity of the antibody populations, their low association constant and the presence of contaminating antibodies in the antibody preparations, as well as the practical difficulties to produce large amounts of antibodies with reproducible and standardized characteristics.
All these limitations were overcome when the hybridoma methodology was developed and immunization of mice yielded many TA-specific mAb-secreting hybridomas [4]. The high degree of specificity and homogeneity of these reagents, their reproducible characteristics and their availability in unlimited amount, as well as the possibility to develop standardized tests and strategies with these reagents generated a significant amount of optimism among tumor immunologists and clinical oncologists. It was generally felt that the solution of the diagnosis and cure of cancer was within reach and a large number of clinical studies was performed. Their implementation was facilitated by the less stringent administrative requirements to translate methodology and results from the bench and/or animal models to a clinical setting, as well by the funding made available by investors who realized the high commercial value of biotechnology. Some clinical benefits were obtained, however, antibody-based immunotherapy was not as successful as expected. As a result, a high degree of skepticism about the clinical usefulness of TA-specific mAbs for the treatment of cancers, replaced the initial optimism in the scientific community. Therefore, TA-specific mAbs lost their popularity in the scientific community also because the major progress made in the identification of T-cell-defined TAs revived tumor immunologists’ general belief that T-cells and not antibodies play the major role in the defense against cancer.
This scenario changed at the end of the 1980’s when EGFR-, HER2- and CD20-specific mAbs were introduced for the treatment of various types of solid and hematological cancers [5]. The antibodies were successfully utilized routinely in clinical settings. However, these therapies were not considered immunotherapies per se by many. mAb-based immunotherapies occupied the spotlight again with the introduction of checkpoint-specific mAbs. The impressive clinical responses observed in a large proportion of patients with many types of malignant diseases, has restored tumor immunologists’ and clinical oncologists’ confidence in the ability of the immune system to control cancer cell growth and in the value of mAbs as reagents for the treatment of malignant diseases. These results have reinforced the use of antibodies in a clinical setting, as well as their use for the diagnosis and treatment of malignant diseases. An additional factor contributing to the use of TA-specific mAbs has been the major progress made in cell engineering methodology. The latter has facilitated the use of strategies which combine antibody specificity with the cytolytic activity of effector cells to specifically eliminate malignant cells. Therefore, the current environment is a fertile ground for the development and characterization of TA-specific mAbs, as well as for their utilization to design strategies for the treatment of malignant diseases.
3. REQUIREMENTS FOR A TUMOR ANTIGEN TO BE AN ATTRACTIVE TARGET OF ANTIBODY-BASED IMMUNOTHERAPY
Critical for the development of novel effective mAb-based immunotherapies is the identification of suitable TAs which can serve as targets for these therapeutic strategies. Indeed, an ideal TA needs to be highly expressed with low, if any heterogeneity on cancer cells, since an association has been found between targeted TA expression level and efficacy of antibody-based immunotherapy, and cancer cells with low targeted TA expression level tend to generate escape variants under selective pressure. It is generally accepted that targeted TAs need to be expressed on the membrane of cancer cells in order to be accessible to antibodies. However, there is growing evidence that also some cytoplasmic molecules can be expressed on the cell membrane and become accessible to recognition by corresponding antibodies. The latter can be divided into 2 groups: 1) intracellular TAs, such as HER2, which following degradation by the proteasome into short peptides, are presented by major histocompatibility complex (MHC) class I on the cell surface and can be targeted by T-cell receptor mimic antibodies [6, 7]; and 2) intracellular TAs, such as glucose-regulated protein 78 (Grp78), Grp94 and phosphatase of regenerating liver 3 (PRL-3) which migrate to cell membrane independent of MHC class I antigens. It is not known at present if they migrate without a carrier, and if they migrate as a whole molecule or as a fragment [8–10].
Furthermore, targeted TA expression on normal tissues has to be restricted, preferably at levels below that required for effector mechanism activation, in order to minimize “on-target, off-tumor” toxicity. Lastly, a TA needs to be expressed not only on differentiated cancer cells, but also on cancer initiating cells (CICs). The latter, although they represent only a small fraction of a cancer cell population, according to the cancer stem cell theory [11, 12], need to be eradicated for a therapy to be effective, since they are the major driving force behind metastatic spread and disease recurrence. An additional characteristic of an attractive TA is its expression on other components of the tumor microenvironment (TME) such as tumor stroma and TAV. As a result, TA targeting immunotherapies would disrupt the supportive tissue of the tumor and inhibit neoangiogenesis, thus contributing to the elimination of cancer cells, even those that do not express or display a low targeted TA expression level. From a practical perspective, expression of a TA with high frequency in multiple, if not all (pan-cancer TA) cancer types, as well as homogeneous high expression on multiple metastases present in a cancer patient, would allow repurposing of already designed effector mechanisms to target multiple cancer types. Additional desirable characteristics of an attractive TA include a crucial role in the biology and/or survival of cancer cells, to minimize the generation of escape variants and low or lack of susceptibility of the targeted TA to downregulation by chemotherapeutic agents, radiotherapy and biotherapy, to avoid that the use of these therapeutic agents reduces the susceptibility of malignant cells to the antitumor activity of the antibody-based immunotherapy used.
Since the development of the hybridoma methodology to produce mAbs about 40 years ago, many TAs which have a higher expression on cancer cells than on normal cells have been identified. However, none are specific for cancer cells, except for those mutated molecules that are expressed by malignant cells, but not by normal cells. An example is EGRFvIII which is expressed uniquely by cancer cells carrying the respective genetic mutations in the EGFR gene, such as glioblastoma cells [13]. However, these mutations are present only in a small fraction of patients with particular cancer types.
As will be described below, B7-H3 fulfills all the above requirements. This explains why B7-H3 is a popular target of antibody-based immunotherapy for the treatment of malignant diseases in both the scientific and the commercial community.
4. B7-H3 STRUCTURE AND FUNCTION
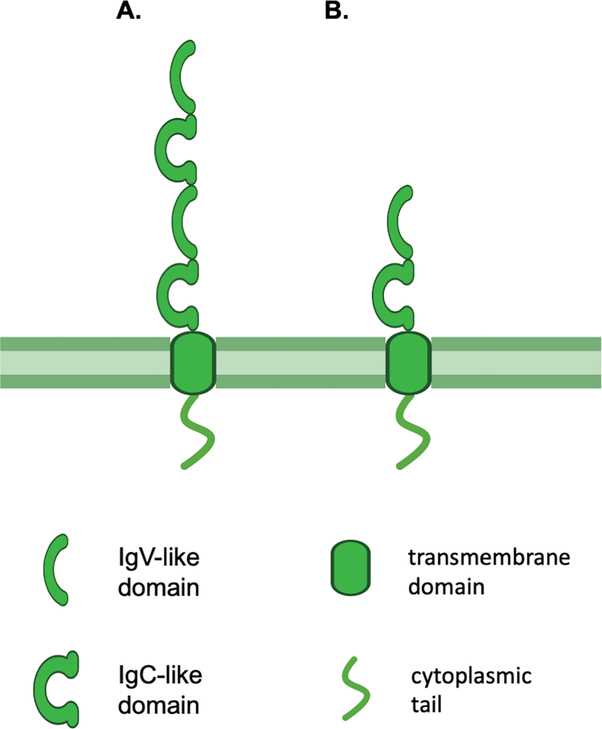
B7-H3 (also known as CD276), is a type I transmembrane protein belonging to the B7 immune co-stimulatory and co-inhibitory family. It maintains its structure, most of its aminoacid sequence and its functional properties through phylogenetic evolution [14]. Its exact biological function(s) remain(s) unclear, however, it seems to act as both a co-stimulatory and/or a co-inhibitory molecule depending on the involved immune cells and on the microenvironment conditions [15, 16]. In humans it is encoded by chromosome 15q24 and consists of an extracellular domain, a transmembrane domain and a short intracellular tail with no known signaling motif. B7-H3 exists in two isoforms: 2IgB7-H3 which comprises a single pair of immunoglobulin variable (IgV)-like and immunoglobulin constant (IgC)-like extracellular domains, and 4IgB7-H3 (protein moiety ~45–66 kDa, glycosylated isoform ~100 kDa [17]) which comprises two identical pairs of IgV-like and IgC-like extracellular domains due to exon duplication [18–20]. The latter is the predominant isoform on human cells (Fig. 1). An isoform present in serum has also been described[21]. B7-H3 is expressed in many normal and malignant tissues at the mRNA level, however it is subject to post-transcriptional regulation by miRNAs, leading to limited expression in normal tissues at the protein level [22, 23]. Therefore, mRNA expression cannot be used as a marker of B7-H3 expression at the protein level.
Figure 1. B7-H3 structure.
B7-H3 is a type I transmembrane protein composed of an extracellular, a transmembrane and a short intracellular domain. Human B7-H3 exists in two isoforms as determined by its extracellular domain: 4IgB7-H3 which comprises two pairs of IgV-like and IgC-like domains (A), and 2IgB7-H3 which comprises a single pair of IgV-like and IgC-like domains (B). Murine B7-H3 comprises a single pair of IgV-like and IgC-like domains. IgV: immunoglobulin variable; IgC: immunoglobulin constant.
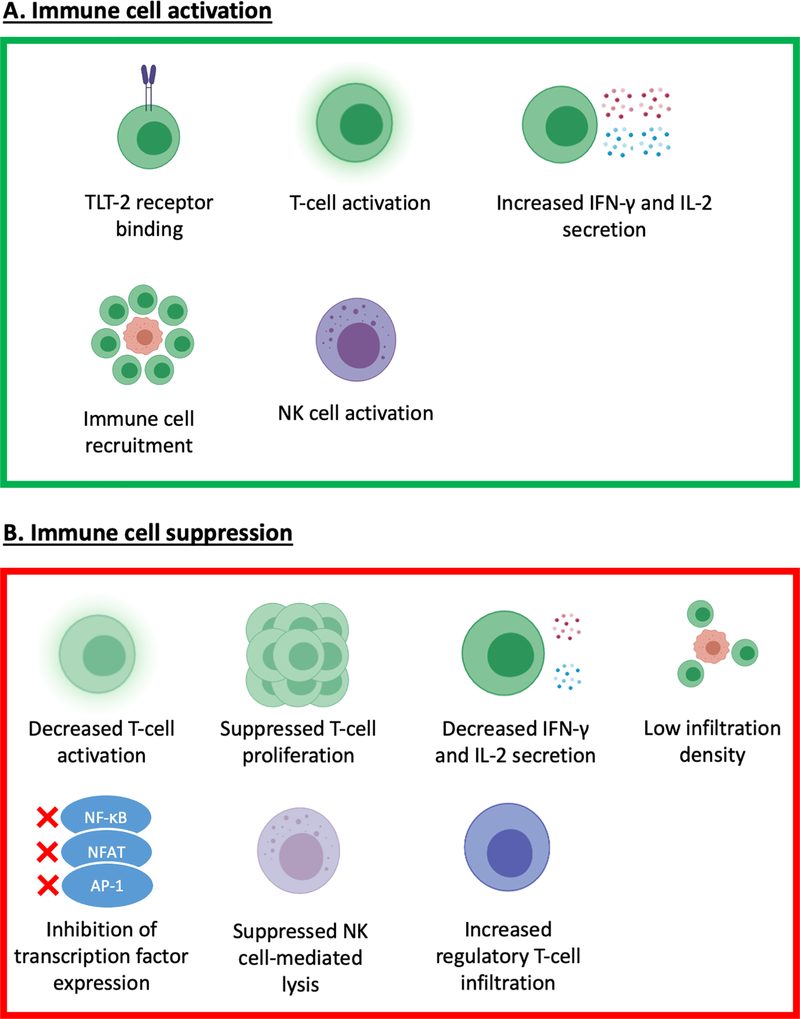
The exact function(s) and receptor(s) of B7-H3 have not been elucidated yet. In non-malignant tissues B7-H3 appears to play an important role in adaptive immunity via regulation of T-cell function. Although early studies supported the notion that B7-H3 acts as a co-stimulatory molecule necessary for the activation of T-cells [14], B7-H3 has more recently been shown to have a predominantly inhibitory role in adaptive immunity, suppressing T-cell activation, proliferation and release of effector cytokines, mainly interferon-γ and interleukin-2 [24]. The role of B7-H3 in TME appears to be more complex. In mice B7-H3 seems to have an anti-tumor function mediated by CD8+ T-cell and NK-cell activation [25, 26], potentially via binding to the Trem-like transcript 2 (TLT-2) receptor on CD8+ T-cells [27]. Surprisingly, in humans only three studies have shown a correlation between high B7-H3 expression and improved prognosis [28–30], as will be discussed below. In contrast, there is overwhelming evidence portraying B7-H3 as a promoter of tumorigenesis. Indeed, a large body of preclinical and clinical evidence indicates that B7-H3 suppresses the TA-specific immune response by multiple mechanisms including decreased immune cell tumor infiltration density, suppressed NK cell-mediated tumor cell lysis, increased regulatory T cell infiltration, decreased release of effector cytokines, and inhibition of the expression of major transcriptional factors [24, 31–37]; the mentioned mechanisms lead to aggressive cancer biology and metastatic potential, and ultimately poor prognosis [21, 36–38] (Fig. 2). Finally, B7-H3 appears to promote cancer progression through non-immunological functions as well, such as by increasing cancer cell migratory, invasive and metastatic potential, by enhancing chemoresistance, and by promoting pro-tumorigenic cancer cell metabolic profiles [17, 39–44].
Figure 2. Schematic representation of the potential mechanisms underlying the immunostimulatory and immunoinhibitory effects mediated by B7-H3.
B7-H3 has been shown to have both immunostimulatory (A) and immunoinhibitory (B). In mice, B7-H3 has been shown to bind to the CD8+ T-cell Trem-like Transcript 2 (TLT-2) receptor leading to their activation. However, such interaction has not been found with human B7-H3. B7-H3 has also been shown to play a role in the recruitment and activation of T-cells, and the subsequent release of cytokines such as IFN-γ and IL-2, as well as the activation of NK cells. On the other hand, B7-H3 has been shown to reduce T-cell activation, proliferation and cytokine release, and has been associated with low tumor-infiltrating T-cell density. Additionally, B7-H3 may inhibit the activation of effector cells by reducing the expression of major transcriptional factors such as Activator Protein-1 (AP-1), Nuclear Factor of Activated T cells (NFAT), and Nuclear Factor Kappa B (NF-κB). Lastly, B7-H3 has been shown to suppress NK cell-mediated lysis of tumor cells and has been associated with increased tumor infiltration by regulatory T cells.
5. MODULATION OF B7-H3 EXPRESSION ON MALIGNANT CELLS
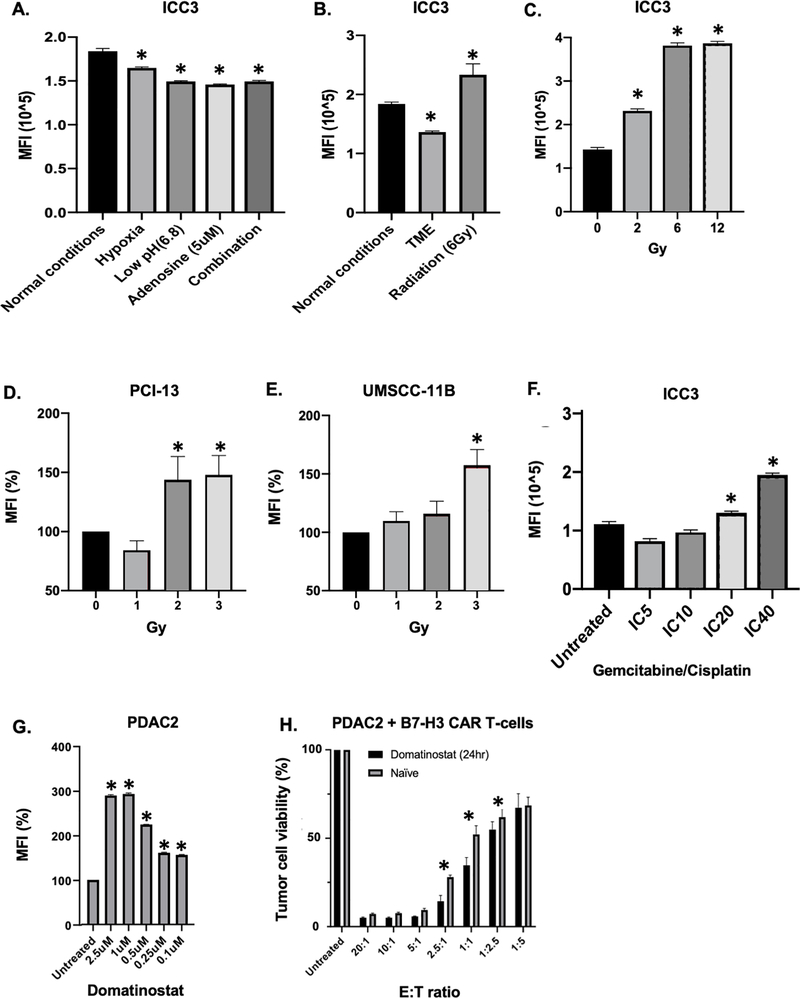
B7-H3 expression is downregulated when cancer cells are incubated under immunosuppressive tumor microenvironment (TME)-like conditions; conversely, it can be upregulated on cancer cells treated with radiotherapy or chemotherapeutic agents. Indeed, we have found that B7-H3 expression is downregulated on intrahepatic cholangiocarcinoma (ICC) cells incubated in vitro under TME-like conditions, namely hypoxia (1% O2), low pH (6.8) and high adenosine concentration (5μM) (Fig. 3A); this change however can be counteracted by radiation (Fig. 3B). Radiation can upregulate B7-H3 expression also on cells incubated in vitro in a neutral pH medium with a low adenosine concentration under normoxic conditions, as we have found in experiments performed with head and neck squamous cell carcinoma (HNSCC) and ICC cell lines (Fig. 3C–E). In addition, B7-H3 expression is upregulated in vitro by chemotherapy, specifically by gemcitabine and cisplatin on ICC cells, as well as by histone deacetylase inhibitors, specifically by domatinostat on pancreatic ductal adenocarcinoma (PDAC) cells (Fig. 3F–G). The above findings may have clinical implications since counteracting TME-induced B7-H3 downregulation may enhance the therapeutic efficacy of B7-H3-targeting antibody-based strategies. This possibility is corroborated by the results of our experiments which have shown that B7-H3 upregulation by domatinostat on PDAC cells enhances their in vitro susceptibility to the anti-tumor activity of B7-H3-specific chimeric antigen receptor (CAR) T-cells (Fig. 3H).
Figure 3. Modulation of B7-H3 expression on tumor cells in vitro by tumor microenvironment-like conditions, radiation and chemotherapy.
A: Intrahepatic cholangiocarcinoma ICC3 cells were cultured under different tumor microenvironment (TME)-like conditions for 6 days and subsequently B7-H3 expression was analyzed with flow cytometry using monoclonal antibody (mAb) 376.96. B: ICC3 cells were cultured either under normal conditions (normoxia, neutral pH, without adenosine) or under TME-like conditions (1% O2, pH 6.8, adenosine 5uM) for 6 days. An aliquot of cells was cultured under TME-like conditions for 3 days. These cells were then irradiated (6Gy) and kept in culture for an additional 3 days. All the cells were then analyzed for B7-H3 expression with flow cytometry using mAb 376.96. C: ICC3 cells were irradiated with different radiation doses and after 48hrs B7-H3 expression was analyzed with flow cytometry. D, E: Head and neck squamous cell carcinoma cells PCI-13 (D) and UMSCC-11B (E) were exposed to 3 fractionated doses of 2Gy, 3Gy or 4Gy radiation, with a 48hr interval between each dose. B7-H3 expression was then assessed by flow cytometry using mAb 376.96 48hrs after the last radiotherapy administration. F: Following treatment with gemcitabine and cisplatin for 72hrs, B7-H3 expression was assessed on ICC3 cells with flow cytometry using mAb 376.96. G: Following treatment with domatinostat (0.1–2.5uM) for 72hrs, B7-H3 expression was assessed on pancreatic ductal adenocarcinoma PDAC2 cells with flow cytometry using mAb 376.96. H: PDAC-2 cells were pre-treated with 1uM of domatinostat for 24hrs prior to co-culture with B7-H3 CAR T-cells. The control group (naïve) was cultured without domatinostat for 24hrs. Co-culture with B7-H3 CAR T-cells was performed for 3 days. At the end of the incubation period, viability of PDAC2 cells was assessed with an MTT assay. MFI: mean fluorescence intensity; CAR: chimeric antigen receptor; E:T: effector to target ratio. *p<0.05.
6. B7-H3 EXPRESSION IN NORMAL, PRE-MALIGNANT AND MALIGNANT TISSUES
6.1. Restricted B7-H3 expression in normal tissues
Immunohistochemical (IHC) analyses with monoclonal and polyclonal antibodies of a large panel of normal tissues has demonstrated low or barely detectable B7-H3 expression in only a few tissues. Indeed, Modak et al., staining normal tissues with the mAb 8H9, demonstrated only heterogeneous, non-specific cytoplasmic staining in normal stomach, liver, pancreas and adrenal cortex; the staining intensity was diminished when the whole IgG of mAb 8H9 was replaced with its F(ab’)2 fragments, suggesting that the staining was, at least in part, non-specific, as it was caused by the interaction of the Fc part of the mAb with normal epithelial cells [45]. Roth et al., stained approximately 30 tissue types with a polyclonal goat anti-human B7-H3-specific antibody (R&D Systems). They detected staining only of Kupffer cells and adrenal glands [46]. Additionally, in our own experience, staining with the B7-H3-specific mAb 376.96, of all normal human samples present in tissue micro-arrays commercially available from US Biomax (Derwood, MD, USA) resulted in only weak cytoplasmic staining of salivary gland acinar cells, gastric epithelial cells, and adrenal gland cells [47]. Similarly, in our previous unpublished studies we observed only weak membranous baso-lateral staining in stomach, gall bladder, prostate, cervix and endometrium.
It is also worth mentioning that peri-tumoral non-malignant tissues, including but not limited to esophagus, breast, lung, liver, pancreas, kidney, colon/rectum, and ovary were not stained or only weakly stained; the staining intensity was significantly lower than that of the corresponding malignant cells [16, 33, 43, 48–69]. Of note, Li et al. [70] using a B7-H3-specific rabbit polyclonal antibody preparation (clone 80831, Proteintech) reported staining of renal tubules. However, this result was not confirmed by other investigators [46, 51, 61], who all used a B7-H3-specific goat polyclonal antibody preparation (AF1027, R&D Systems). They detected no staining of renal parenchyma. The conflicting results may reflect differences in the fine specificity and/or association constants of the antibodies used and/or, although less likely, in the sensitivity of the IHC methodology used. Additionally, strong staining of peritumoral liver sinusoid endothelial cells and resident Kupffer cells, but no staining of hepatic cells was reported by Sun et al. using a rabbit anti-human 4Ig-B7-H3 [64]. The latter staining pattern was also described by Kang et al. [43], Wang et al. [65], and Cheng et al. [71] who used different B7-H3-specific mAbs. The validity of the results obtained by IHC is supported by the low toxicity rates caused by the administration of B7-H3-targeting therapies to patients. In an early open-label single-arm imaging study in patients with B7-H3-positive tumors, using murine 131I labeled mAb 8H9 (omburtamab, Y-mAbs), moderate hepatic uptake of 8H9 was observed (NCT00582608), but no hepatotoxicity was reported. Additionally, since the whole antibody was used, it is not known if that was specific uptake mediated by the antigen-antibody interaction or a background uptake mediated by the Fc portion of the antibody. Similarly, in a Phase I clinical trial the fully humanized B7-H3-targeting mAb enoblituzumab (MGA271, MacroGenics) was well-tolerated with no dose-limiting toxicity (NCT01391143) [72]. Furthermore, a trial assessing the bispecific antibody obrindatamab (MGD009, MacroGenics), a humanized CD3xB7-H3 dual affinity re-targeting (DART) platform protein, only uncomplicated short-lived reversible transaminase elevations were reported [73, 74].
6.2. B7-H3 expression in pre-malignant and benign lesions
Limited information is available regarding B7-H3 expression in pre-malignant and/or dysplastic lesions. Analysis of B7-H3 expression in nevi with malignant potential demonstrated staining in 20 of the 40 (50%) lesions tested [75]. However, the stage of progression to malignancy at which B7-H3 becomes detectable on cells remains unknown. This piece of information could be particularly useful for cancers that arise from distinct pre-malignant lesions such as cervical cancer, and could guide the development of early diagnosis and/or prevention strategies.
Information is also scarce on B7-H3 expression on benign lesions. In previous unpublished studies, we detected no staining with the B7-H3-specific mAb 376.96 among the 4 gynecomastia, 3 mastitis and 6 fibroadenoma lesions tested, but we detected staining in only 1 of the 3 ductal papillomatosis lesions tested.
6.3. Broad B7-H3 expression in primary malignant tumors
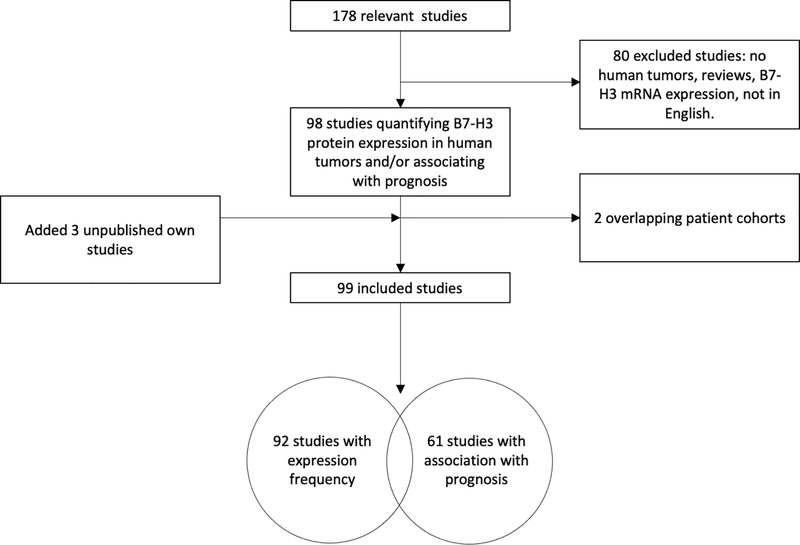
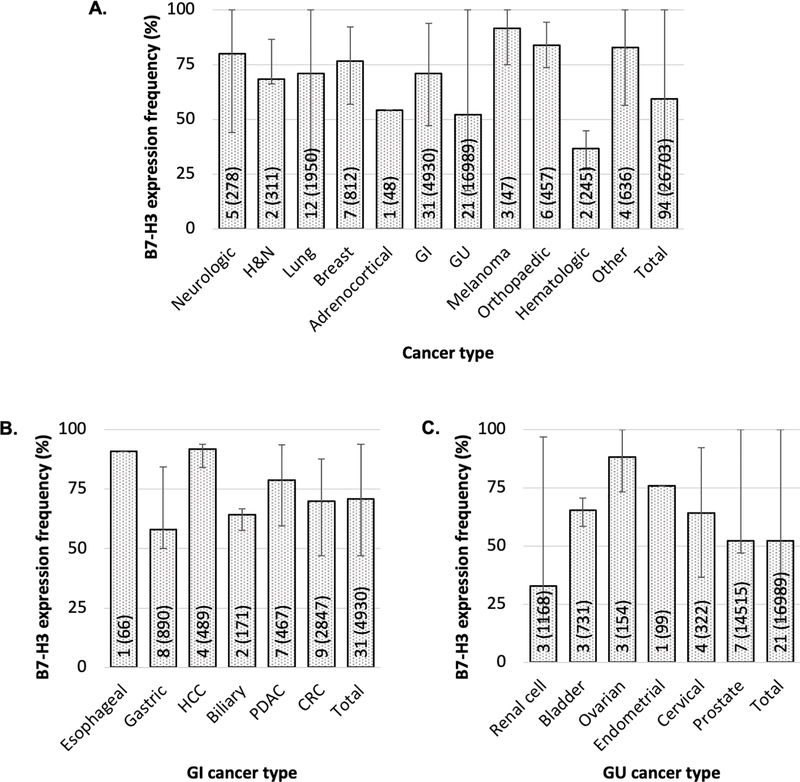
In order to evaluate B7-H3 expression at the protein level in primary tumors and its clinical significance, we performed a review of the literature and a pooled data analysis. The resulting unpublished information is summarized as follows. Of the 178 relevant studies identified via PubMed, Embase and a relevant paper bibliography list search, 80 were excluded because they i) did not quantify B7-H3 expression frequency, ii) described B7-H3 expression on cell lines or animal models rather than human cancer specimens, iii) described B7-H3 expression on tumor-associated non-malignant cells only, iv) were review/meta-analyses, v) described B7-H3 mRNA rather than protein expression, and/or vi) were not published in English. Two additional studies were excluded because of overlapping patient cohorts. Furthermore, we included data from 3 of our unpublished studies on chondrosarcoma (1 study) and chordoma (2 studies). Of the 99 [28–31, 33–37, 39, 43, 45, 46, 49–58, 60–68, 71, 76–123] [124–130] included studies, 92 [28–31, 33–37, 39, 43, 45, 46, 49–57, 60–68, 71, 76–80, 82–105, 108–129, 131] reported frequency of tumor samples with positive B7-H3 protein expression on cancer cells and 61 [28–30, 34, 35, 37, 43, 46, 49, 50, 52–56, 62–64, 66–68, 71, 76, 78, 80, 82–93, 96, 98, 99, 102, 103, 106, 108, 109, 111–114, 116–118, 120–124, 126–130, 132] associated B7-H3 expression with prognosis (Fig. 4). Among the former, two studies [45, 51] included >1 type of malignancy, thus increasing the number of individual studies per cancer type to 94. The number of B7-H3 positive and negative specimens were extracted from the former group of studies, pooled for each cancer type and the cumulative frequency of B7-H3 expression for each cancer type was calculated. The 94 studies quantifying B7-H3 expression frequency on malignant tumors comprised 21 types of malignancies including a total of 26,703 patients. (Fig. 5A). Each cancer type was analyzed in a median of 4 studies (interquartile range 2–7), with lung cancer including the largest number of studies (12 studies) and prostate including the largest number of cancer specimens (N=14,515).
Figure 4. Study selection diagram.
Figure 5. Frequency of B7-H3 expression by cancer type.
B7-H3 expression was assessed in surgically resected primary tumors by immunohistochemical staining with B7-H3-specific antibodies. Bar graphs represent the average of the frequency of B7-H3 expression in each cancer type yielded from a pooled data analysis. The number of included studies (number of cases) for each cancer type is presented within each bar. Error bars represent the range of B7-H3 expression frequency reported. Data are presented for all cancer types (A), as well as for gastrointestinal (B) and genitourinary (C) cacners. H&N: head and neck; GI: gastrointestinal; GU: genitourinary; HCC: hepatocellular carcinoma; PDAC: pancreatic ductal adenocarcinoma; CRC: colorectal cancer. The cancer types labeled as “other” include small round blue cell tumors of childhood, pediatric brain and solid tumors, Ewing’s family of tumors and renal angiomyolipoma. B7-H3 expression frequency was statistically significantly different among all cancer types (p<0.001), among GI cancers (p<0.001) and among GU cancers (p<0.001). P values derived from χ2 test.
The frequency of B7-H3 expression ranged from 33.0% in renal cell carcinoma to 91.8% in hepatocellular carcinoma. Of note, renal cell carcinoma and hematologic malignancies exhibited by far the lowest frequency of B7-H3 expression (33.0% and 36.7%, respectively, chi-square p-value < 0.001), while for all other solid cancer types the expression frequency was at least 52.3% (prostate cancer). Among gastrointestinal cancers, hepatocellular carcinoma exhibited the highest (91.8%) and gastric cancer the lowest (58.0%) B7-H3 expression frequency (Fig. 5B). Among genitourinary malignancies, renal cell carcinoma had the lowest B7-H3 expression frequency, as previously mentioned, while ovarian cancer had the highest (88.3%) (Fig. 5C). When all cancers were included, the cumulative frequency of B7-H3 positivity was 59.5% (15,877/26,703). As depicted in Fig. 5, there is significant variation in the frequency of B7-H3 expression within the same cancer type among different studies. This variation may reflect differences in i) study patient population, ii) disease stage, iii) fine specificity of the B7-H3-specific antibodies used, and/or iv) sensitivity of the method utilized to analyze B7-H3 expression.
6.4. Cellular localization of B7-H3 expression in cancer cells
B7-H3 is a transmembrane protein with 2 or 4 extracellular domains and only a short intracellular tail. Thus, it is expected that B7-H3 expressing cancer cells will be targeted by mAb-mediated immunotherapeutic effector mechanisms. Almost none of the studies provided information on the expression frequency in each cellular compartment (membrane vs. cytoplasm). Additionally, there was heterogeneity on the criteria for B7-H3 expression positivity: most studies did not mention localization in the criteria used for positivity or considered positive the presence of staining in either the membrane or cytoplasm, while others evaluated only membranous staining. Nevertheless, no study reported cytoplasmic staining of malignant cells in the absence of membranous staining.
6.5. B7-H3 expression in TAV
In addition to high expression on cancer cells, B7-H3 also demonstrates high expression on stromal fibroblasts and TAV in the tumor microenvironment. This expression pattern may allow for B7-H3-targeting therapies to eliminate even those cancer cells with no detectable B7-H3 expression. Indeed, B7-H3 is highly expressed on TAV [34–36, 56, 61, 64, 89, 112, 133–135] even in cancer types with low B7-H3 expression on cancer cells, such as renal cell carcinoma [56, 61, 112].
B7-H3 TAV expression frequency has been quantified in only a limited number of cancer types. It has been reported to range from 86% to 98% in hepatocellular carcinoma [64], colorectal cancer (CRC) [89], renal cell carcinoma [56, 61], and melanoma [133], with ovarian cancer being an outlier with a frequency of 44% [68].
6.6. B7-H3 expression on cancer initiating cells (CICs)
CICs represent the most tumorigenic and treatment resistant subpopulation of cancer cells. They form spheres in vitro, and express high levels of stemness genes [136]. Several markers have been used for their identification in various types of cancer, including CD133, CD44 and high activity of aldehyde dehydrogenase (ALDH)-1A1. The lack of standardization in the methodology to identify CICs is contributing to the difficulty of interpreting data related to marker expression on CICs. As already mentioned, to be effective, a therapy must eradicate both differentiated cells and CICs, since, according to the cancer stem cell theory, the latter play a major role in disease recurrence and metastatic spread [11, 12]. B7-H3 is expressed on CICs isolated from ovarian [119] and glioblastoma [60] cells. Immunotargeting of B7-H3 expressed on CICs with antibody-based strategies resulted in inhibition of tumor growth both in vitro and in vivo. In our own experience, B7-H3 is also expressed on CICs isolated from HNSCC, triple-negative breast cancer (TNBC) and PDAC cell lines [21].
6.7. B7-H3 expression in metastatic lesions
High expression in metastases is an attractive feature of B7-H3 since its immunotargeting could be effectively applied to patients with advanced disease. However, scant information is available about B7-H3 expression in metastatic lesions. In melanoma, 30 (97%) of the 31 metastatic lesions analyzed stained positive for B7-H3 expression [62, 105]. Sentinel lymph nodes with malignant cell infiltration resected from breast cancer patients demonstrated high B7-H3 expression in all the 24 samples tested [48]. Similar findings were obtained in metastatic prostate cancer [79] and fibrolamellar hepatocellular cancer [83]. Interestingly, preliminary evidence suggests that in breast cancer, metastatic lesions are frequently positive for B7-H3 expression, even if autologous primary tumors are B7-H3-negative (unpublished data). Investigating B7-H3 expression in metastatic lesions in future studies will not only determine eligibility of metastatic lesions for B7-H3-targeting, but will also elucidate the role of B7-H3in malignancy progression and the stage in cancer progression at which B7-H3 starts being expressed.
6.8. Association of B7-H3 expression in primary tumors with tumor infiltrating lymphocyte (TIL) density
As shown in studies in esophageal cancer [52], non-small cell lung cancer (NSCLC) [31], CRC [33], cervical [34] and endometrial cancer [35], B7-H3 expression on cancer cells negatively correlated with the extent of tumor-infiltrating T-lymphocytes both in tumor nests and in tumor stroma. The underlying mechanism may be mediated by immunoglobulin-like transcript 4 overexpression on cancer cells. This phenotypic change causes an increased expression of the co-inhibitory molecule B7-H3, mediated by the activation of the PI3K/AKT/mTOR signaling pathway [38]. B7-H3 expression has also been correlated with activated regulatory T (Treg, FoxP3+) cell infiltration and poor survival in NSCLC [37].
6.9. Association of B7-H3 expression in primary tumors with prognosis
As previously mentioned, of the 99 included studies, 61 associated B7-H3 expression in tumors with prognosis. In all types of malignancies analyzed, except gastric cancer, 41 of the 61 studies (67.2%) demonstrated an association of positive/high B7-H3 expression with poor prognosis in the form of overall, disease-free and/or progression-free survival. Seventeen of the 61 studies (27.9%) found no correlation between B7-H3 expression and prognosis, however, this could be attributed to the small cohorts included (median N=101). Only 3 studies (4.9%), one on gastric cancer, one on PDAC and one on acute myeloid leukemia, demonstrated an association of positive/high B7-H3 expression with improved prognosis [28–30]. Moreover, in CRC, 3 of the 6 published studies showed an association of B7-H3 expression with poor prognosis, while the remaining 3, including the largest included study comprising 939 participants, found no association between B7-H3 expression and survival. Lastly, in all the cancer types analyzed, more than 80% of the included studies reported an association of positive/high B7-H3 expression with poor pathologic characteristics such as larger tumors, lymph node metastasis, advanced stage, poor grade of differentiation and vascular invasion.
7. B7-H3 TARGETING STRATEGIES
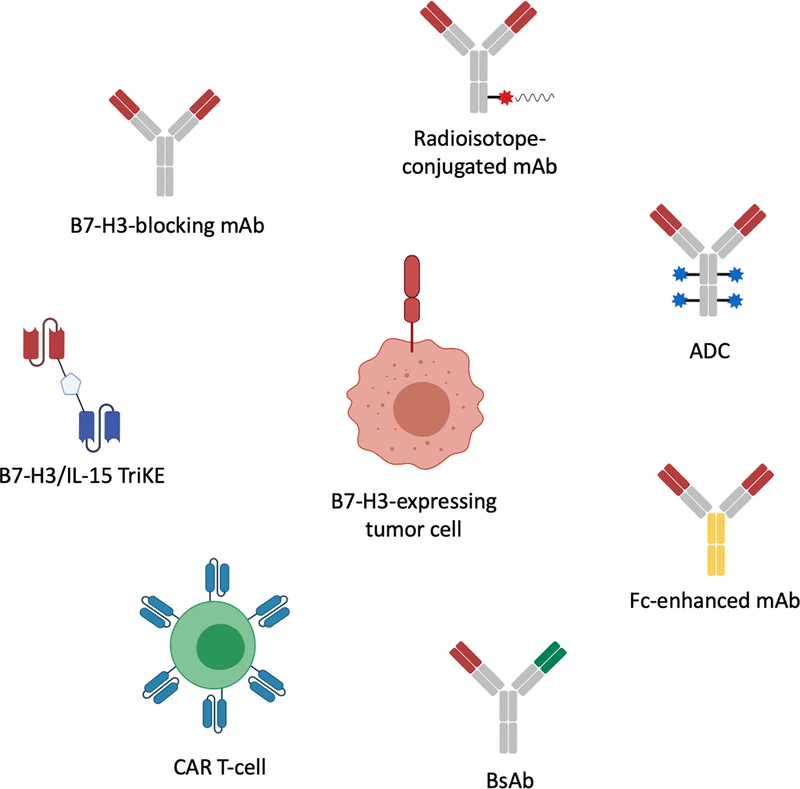
As described above, B7-H3 represents an attractive target for mAb-based immunotherapeutic strategies. As a result, a number of B7-H3-targeting immunotherapeutic strategies utilizing multiple effector mechanisms have been developed (Fig. 6). Several strategies have been translated to clinical trials (7.1), while others are still in pre-clinical development (7.2).
Figure 6. B7-H3-targeting monoclonal antibody-based immunotherapeutic strategies.
ADC: antibody-drug conjugate; mAb: monoclonal antibody; CAR: chimeric antigen receptor; IL-15: interleukin 15; TriKE: trispecific killer engager.
7.1. Therapeutic strategies tested in clinical trials
More than 20 clinical trials have or are currently testing the safety and efficacy of B7-H3-targeting mAb-based immunotherapeutic strategies [21]. B7-H3-targeting radioimmunotherapies have been developed to selectively localize radioisotopes to tumors. To further reduce potential toxicity risk, compartmental administration of B7-H3-specific mAb conjugated radioisotopes has been tested. Specifically, murine 131I labeled mAb 8H9 (omburtamab, Y-mAbs) has been administered to patients with metastatic central nervous system neuroblastoma (NCT00089245) [137] and intraperitoneally to patients with desmoplastic small round cell tumors (NCT01099644) [138] and was well tolerated. Similarly, 124I labeled mAb 8H9 has been tested in diffuse pontine glioma (NCT01502917) where convection-enhanced brainstem delivery resulted in negligible systemic exposure and no toxicity [139].
B7-H3-specific mAbs have also been conjugated with chemotherapeutic drugs. For instance, MGC018 (humanized B7-H3 mAb with a cleavable linker-duocarmycin payload, MacroGenics) which delivers duocarmycin to tumors is currently being tested for elimination of B7-H3-expressing solid tumors in a Phase I/II trial (NCT03729596). Additionally, DS-7300a (Daiichi Sankyo), an ADC consisting of a B7-H3-specific mAb conjugated to four topoisomerase I inhibitor particles [140] is currently being tested in a Phase I/II trial (NCT04145622).
Another B7-H3-targeting immunotherapeutic strategy is represented by Fc-enhanced mAbs. In that therapeutic category, enoblituzumab (MGA271, MacroGenics), a fully humanized mAb bearing an Fc domain engineered to enhance its anti-tumor function by increasing its binding to the activating receptor CD16A and reducing that to the inhibitory receptor CD32B [141], was found in two Phase I trials (NCT01391143, NCT02475213) to be well-tolerated and safe [72, 142]. Results from a Phase I trial evaluating enoblituzumab combined with ipilimumab in non-small cell lung cancer and melanoma (NCT02381314), as well as from a Phase II trial assessing neoadjuvant enoblituzumab in prostate cancer (NCT02923180) are pending.
Bispecific antibodies (BsAbs) utilizing a B7-H3 mAb scFv linked to an anti-CD3 mAb scFv to recruit and activate T-cells against cancer cells [143] represent an additional B7-H3 targeting modality. To date, only obrindatamab (MGD009, MacroGenics), a humanized CD3xB7-H3 BsAb has been tested in advanced B7-H3-expressing tumors (NCT02628535), causing only uncomplicated short-lived hepatic adverse events, likely due to cytokine release syndrome secondary to increased T-cell activation.
Lastly, B7-H3-targeting chimeric antigen receptor (CAR) T-cells have been generated and are currently being evaluated in 2 trials targeting glioblastoma (NCT04077866) and pediatric glioma (NCT04185038).
7.2. Therapeutic strategies in early development
A number of B7-H3-targeting mAb-based strategies are currently being developed in preclinical studies. First, based on the success of checkpoint molecule blocking, B7-H3 blocking mAbs have been tested in mice. Although CD8+ T and NK-cell tumor infiltration density increased and tumor growth was reduced [16, 144, 145], the limited information on the B7-H3 receptor(s) and the lack of a human B7-H3-specific blocking mAb, hinder currently the translation of these findings to the clinical setting.
Progress is also being made in the field of B7-H3-targeting radioimmunotherapy: the B7-H3-specific mAb 376.96 conjugated with 212Pb, a source of α-particles, has shown promising results in ovarian cancer and PDAC in mice [146, 147]. Furthermore, lutetium-77 labeled mAb 8H9 has been conjugated with the chelator diethylenetriamine pentaacetate to facilitate rapid radioactivity clearance [148].
Novel B7-H3-targeting Fc-enhanced mAbs as well as BsAbs are also being developed. For instance, a novel Fc enhanced bispecific anti-B7-H3 mAb/PD-1 fusion protein showed promising results in a breast cancer mouse model [149], while a B7-H3xCD3 BsAb created by coupling an anti-human B7-H3 mAb with an anti-CD3 mAb showed potent cytotoxicity toward hematological malignant cells in vitro [150].
A highly promising B7-H3-targeting strategy is represented by tri-specific killer engagers (TriKEs). TriKEs form an antigen-specific immunological synapse between cancer cells and NK cells, which subsequently leads to NK-cell-mediated cancer cell lysis [151]. TriKEs are composed of either 3 scFvs of antibodies with different specificity or 2 scFvs (CD16-specific and TA-specific) and a cytokine, such as IL-15. Vallera et al. have generated a B7-H3/IL-15 TriKE using the scFv of the B7-H3-specific mAb 376.96. This strategy has demonstrated significant tumor burden decrease in vitro and in mice against head and neck squamous cell carcinoma, PDAC and ovarian cancer [152, 153]. The same group has generated a 2nd generation TriKE with human IL-15 as a modified crosslinker between an anti-B7-H3 scFv and a humanized camelid anti-CD16 single domain antibody. The latter allows improved function of the IL15 moiety, inducing robust and specific NK cell proliferation and potent elimination of ovarian cancer cells in vitro and in mice [154].
Lastly, B7-H3 CAR T-cells have demonstrated potent in vitro anti-tumor activity against multiple cancer types [47, 60, 100, 131, 155, 156]. However, their efficacy remains limited in mice, likely due to the negative impact of tumor escape mechanisms on cancer cell - CAR T-cell interactions [157]. Although preclinical studies suggest an acceptable safety profile, these results should be interpreted with caution because no information is available about the cross-reactivity of the human B7-H3-specific mAbs used with the endogenous mouse B7-H3 expressed by murine normal tissues.
8. CONCLUSION
In conclusion, B7-H3 is a highly attractive target for mAb-based immunotherapeutic strategies. This is important since for the successful development of mAb-based strategies, the identification of attractive TAs is crucial. Although the exact functions of B7-H3, as well as its receptor, remain to be elucidated, the presented literature review and pooled analysis convincingly show that B7-H3 is highly expressed on all the types of cancers tested, but has a limited distribution on normal tissues, thus representing an effective safe target. Furthermore, it is expressed on TAV, even in cancer types with the lowest frequency of B7-H3 expression on cancer cells, as well as on CIC, the subpopulation of cancer cells that need to be eliminated for a therapy to be effective. Lastly, B7-H3 expression is associated with poor prognosis in the vast majority of the studies analyzing this association. Several B7-H3-targeting mAb-based immunotherapeutic strategies have been developed and tested in clinical trials, while a number of promising modalities are currently being developed and are being tested in preclinical models.
9. EXPERT OPINION
As the excitement for mAb-based immunotherapeutic approaches for malignant diseases is being reinvigorated, identification of TAs which meet the criteria required to be used as targets is not only timely but also necessary, in order to reinforce the use of mAb-based therapies in clinical settings. We are currently in a unique situation to pursue further development of such strategies, given firstly the realization and confidence that TA-targeting mAb-based therapies harbor promising, yet unexplored potential, and secondly the unprecedented progress in cell bioengineering, which allows the development of novel and reliable reagents. In this landscape of TA-targeting mAb-based immunotherapy, a detailed, critical evaluation of the expression and functional properties of B7-H3 is highly timely and clinically relevant.
The evidence presented in this comprehensive review convincingly shows that B7-H3 is expressed with high frequency across multiple types of malignant tissues. Even in cancer types with the lowest B7-H3 expression frequency, B7-H3 is still highly expressed in TAV. The expression patterns of B7-H3 in malignant tissues provides widespread applicability of B7-H3-targeting strategies. Indeed, B7-H3-targeting immunotherapeutic strategies have been tested in a plethora of cancer types including neuroblastoma, glioma, HNSCC, NSCLC, TNBC, PDAC, ovarian cancer, urothelial cancer, prostate cancer and melanoma, to name a few. The widespread applicability of B7-H3-targeting strategies is also manifested by the multiple effector mechanisms tested: blocking, radioisotope-conjugated, drug-conjugated, bi-specific, tri-specific, and Fc-enhanced B7-H3 mAbs, as well as B7-H3-specific CAR T-cells have all been utilized.
The use of B7-H3 as a target is further supported by its expression on CICs, since their eradication is a requirement for an anti-tumor therapy to be effective. Furthermore, the high B7-H3 expression on TAV provides an additional argument for targeting it, given that this will disrupt neoangiogenesis and be effective even against cells or tumors with low B7-H3 expression. Lastly, the membranous location of B7-H3 expression on cancer cells, provides accessibility to anti-tumor effector mechanisms.
In keeping with the Hippocratean moto “first, do no harm”, B7-H3 targeting has been shown to be safe. As we demonstrate in this review, B7-H3 has only limited and weak expression in non-malignant as well as in juxta-tumoral tissues. Thus, “on-target, off-tumor” toxicity is not anticipated. Indeed, minimal toxicity has been observed in clinical trials evaluating B7-H3 targeting strategies. To further minimize the probability of adverse effects by decreasing systemic exposure, investigators have also tested local administration of B7-H3-targeting effector mechanisms such as intrathecal administration for central nervous system tumors [137].
Despite all the encouraging aforementioned data, there are still areas requiring further investigation. First, the evidence about B7-H3 expression in metastases is scant. This area has to be further developed, since information about B7-H3 expression pattern in metastases in different anatomic sites is crucial to optimize the design of B7-H3-targeting therapies. Other areas which need development include i) characterization of the relationship between malignant cell transformation stage and appearance of B7-H3 expression in many types of cancer, to assess its value as a diagnostic marker of malignant transformation and as a target of antibody-based prevention strategies, ii) evaluation of B7-H3 expression level in patients’ sera, as a soluble molecule and/or as an exosome-bound moiety, for its value as a marker for diagnosing and for monitoring the clinical course of malignant diseases, iii) assessment of the ability of B7-H3 targeting to mediate elimination of CICs; if successful, it may significantly impact the treatment of malignant diseases given the postulated role of these cells in metastatic spread and disease recurrence, iv) development and implementation of strategies to counteract the tumor escape mechanisms which limit the in vivo efficacy of B7-H3-specific CAR T-cells, and v) identification of the B7-H3 receptor; this information will greatly contribute to the functional characterization of this molecule and will facilitate the generation of blocking mAbs. The latter might have a major impact on the treatment of malignant diseases expressing this molecule.
In conclusion, B7-H3 represents an attractive target for mAb-based immunotherapy. Its attractiveness stems from its expression patterns on malignant and normal cells, its presence on CICs, and the safety profile demonstrated by targeting it thus far. In an era where TA-targeting mAb-based therapy of malignant diseases is not only promising, but also technically feasible, further examining B7-H3 characteristics and developing B7-H3-targeting strategies is timely and warranted.
ARTICLE HIGHLIGHTS.
Recent successes with the use of mAbs for the treatment of malignancies, as well as advances in bioengineering have stimulated interest in identifying TAs to serve as targets for mAb-based immunotherapies.
A TA which represents an attractive target is highly expressed on multiple types of cancers with limited heterogeneity, but has limited distribution in normal tissues, is expressed on CICs, is expressed on TAV, and plays a role in tumor biology. B7-H3 fulfills all the above criteria.
The biological function and receptor(s) of B7-H3 remain unclear; it can act either as a co-stimulatory or a co-inhibitory molecule depending on the involved immune cells and/or on the microenvironment. However, its main pro-tumorigenic function seems to be via inhibition of the anti-tumor activity of T-cells.
B7-H3 exhibits limited distribution on normal tissues as corroborated by the lack of major toxicity of B7-H3-targeting strategies in clinical trials. In a pooled analysis of 94 studies we found that B7-H3 is highly expressed in all cancer types analyzed with a cumulative frequency of B7-H3 positivity of 60%. B7-H3 is primarily expressed on the cancer cell membrane. B7-H3 is also highly expressed on TAV, as well as on CICs.
In a systematic review including 61 studies we found that B7-H3 is associated with poor prognosis in more than two thirds of the studies analyzed, while only 3 studies demonstrated an association of positive/high B7-H3 expression with better prognosis.
Future research should focus on the identification of the B7-H3 receptor, the characterization of the relationship between malignant cell transformation and appearance of B7-H3 expression, the analysis of B7-H3 expression on metastases, the evaluation of the correlation between B7-H3 serum levels and clinical characteristics and response to therapy, the assessment of the ability of B7-H3 targeting to mediate elimination of CICs, and the development of B7-H3 blocking mAbs.
Funding
This work was supported by the National Institutes of Health grant RO1DE028172, by the National Cancer Institute grants RO3CA223886 and RO3CA231766, and by the Department of Defense grants W81XWH-16-1-0500 and W81XWH-20-1-0315 (BC190615).
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
ABBREVIATION LIST
- ADC
antibody-drug conjugate
- ADCC
antibody-dependent cellular cytotoxicity
- ALDH
aldehyde dehydrogenase
- CAR
chimeric antigen receptor
- CIC
cancer initiating cell
- CRC
colorectal cancer
- DART
dual affinity re-targeting
- Grp78
glucose-regulated protein 78
- Grp94
glucose-regulated protein 94
- HNSCC
head and neck squamous cell carcinoma
- ICC
intrahepatic cholangiocarcinoma
- IHC
immunohistochemistry
- IgC
immunoglobulin constant region
- IgV
immunoglobulin variable region
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NSCLC
non-small cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- PRL-3
phosphatase of regenerating liver 3
- TA
tumor antigen
- TAV
tumor-associated vasculature
- TIL
tumor infiltrating lymphocyte
- TLT-2
Trem-like transcript 2
- TME
tumor microenvironment
- TNBC
triple negative breast cancer
- Treg
regulatory T
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Contributor Information
Theodoros Michelakos, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 55 Fruit Street, Jackson 9, Boston, MA 02114, USA.
Filippos Kontos, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 55 Fruit Street, Jackson 9, Boston, MA 02114, USA.
Omar Barakat, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 55 Fruit Street, Jackson 9, Boston, MA 02114, USA.
Luke Maggs, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 55 Fruit Street, Jackson 9, Boston, MA 02114, USA.
Joseph H. Schwab, Department of Orthopaedic Surgery, Massachusetts General Hospital/Harvard Medical School, 55 Fruit Street, Suite 3800, Boston, MA 02114, USA.
Cristina R. Ferrone, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 15 Parkman Street, Boston, MA 02114, USA.
Soldano Ferrone, Department of Surgery, Massachusetts General Hospital/Harvard Medical School, 55 Fruit Street, Jackson 9, Boston, MA 02114, USA.
REFERENCES
- 1.Tatarinov YS. Detection of embryospecific alpha-globulin in serum of patients with primary liver cancer. 1st All-Union Biochem Congress Abstract Book. Moscow-Leningrad; 1963. [Google Scholar]
- 2.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965. September 1;122(3):467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg DM, Sharkey RM. Radioactive antibodies: a historical review of selective targeting and treatment of cancer. Hosp Pract (1995) 2010;38(3):82–93. [DOI] [PubMed] [Google Scholar]
- 4.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975. August 7;256(5517):495–7. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Z, Yan L. Next generation of antibody therapy for cancer. Chin J Cancer 2011. May;30(5):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trenevska I, Li D, Banham AH. Therapeutic Antibodies against Intracellular Tumor Antigens. Front Immunol 2017;8:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krokhotin A, Du H, Hirabayashi K, et al. Computationally Guided Design of Single-Chain Variable Fragment Improves Specificity of Chimeric Antigen Receptors. Mol Ther Oncolytics 2019. December 20;15:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J 2011. March 1;434(2):181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo K, Li J, Tang JP, et al. Targeting intracellular oncoproteins with antibody therapy or vaccination. Sci Transl Med 2011. September 7;3(99):99ra85. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang X, Ferrone CR, et al. Intracellular antigens as targets for antibody based immunotherapy of malignant diseases. Mol Oncol 2015. December;9(10):1982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006. October 1;66(19):9339–44. [DOI] [PubMed] [Google Scholar]
- 12.Visvader JE. Cells of origin in cancer. Nature 2011. January 20;469(7330):314–22. [DOI] [PubMed] [Google Scholar]
- 13.An Z, Aksoy O, Zheng T, et al. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene 2018. March;37(12):1561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2001. March;2(3):269–74. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos JR, Purvis IJ, Labak CM, et al. B7-H3 role in the immune landscape of cancer. Am J Clin Exp Immunol 2017;6(4):66–75. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YH, Martin-Orozco N, Zheng P, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res 2017. August;27(8):1034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets 2008. August;8(5):404–13. [DOI] [PubMed] [Google Scholar]
- 18.Steinberger P, Majdic O, Derdak SV, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol 2004. February 15;172(4):2352–9. [DOI] [PubMed] [Google Scholar]
- 19.Sun M, Richards S, Prasad DV, et al. Characterization of mouse and human B7-H3 genes. J Immunol 2002. June 15;168(12):6294–7. [DOI] [PubMed] [Google Scholar]
- 20.Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res 2016. July 15;22(14):3425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontos F, Michelakos T, Kurokawa T, et al. B7-H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res 2020. October 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res 2009. August 1;69(15):6275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Zhao Y, Xu M, et al. Serum miR-1301–3p, miR-335–5p, miR-28–5p, and their target B7-H3 may serve as novel biomarkers for colorectal cancer. J BUON 2019. May-June;24(3):1120–27. [PubMed] [Google Scholar]
- 24.Prasad DV, Nguyen T, Li Z, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol 2004. August 15;173(4):2500–6. [DOI] [PubMed] [Google Scholar]
- 25.Sun X, Vale M, Leung E, et al. Mouse B7-H3 induces antitumor immunity. Gene Ther 2003. September;10(20):1728–34. [DOI] [PubMed] [Google Scholar]
- 26.Luo L, Chapoval AI, Flies DB, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol 2004. November 1;173(9):5445–50. [DOI] [PubMed] [Google Scholar]
- 27.Hashiguchi M, Kobori H, Ritprajak P, et al. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A 2008. July 29;105(30):10495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loos M, Hedderich DM, Ottenhausen M, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009. December 26;9:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu CP, Jiang JT, Tan M, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 2006. January 21;12(3):457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guery T, Roumier C, Berthon C, et al. B7-H3 protein expression in acute myeloid leukemia. Cancer Med 2015. December;4(12):1879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Wang Y, Zhao J, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006. August;53(2):143–51. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Chen J, Xu B, et al. B7-H3 expression associates with tumor invasion and patient’s poor survival in human esophageal cancer. Am J Transl Res 2015;7(12):2646–60. [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Chen LJ, Zhang GB, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother 2010. August;59(8):1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brustmann H, Igaz M, Eder C, Brunner A. Epithelial and tumor-associated endothelial expression of B7-H3 in cervical carcinoma: relation with CD8+ intraepithelial lymphocytes, FIGO stage, and phosphohistone H3 (PHH3) reactivity. Int J Gynecol Pathol 2015. March;34(2):187–95. [DOI] [PubMed] [Google Scholar]
- 35.Brunner A, Hinterholzer S, Riss P, et al. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol 2012. January;124(1):105–11. [DOI] [PubMed] [Google Scholar]
- 36.Lemke D, Pfenning PN, Sahm F, et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res 2012. January 1;18(1):105–17. [DOI] [PubMed] [Google Scholar]
- 37.Jin Y, Zhang P, Li J, et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol 2015;8(11):13987–95. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang P, Yu S, Li H, et al. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett 2015. August 4;589(17):2248–56. [DOI] [PubMed] [Google Scholar]
- 39.Yuan H, Wei X, Zhang G, et al. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol 2011. September;186(3):1093–9. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Zhang T, Zou S, et al. B7H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med Rep 2015. October;12(4):5455–60. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Tekle C, Chen YW, et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther 2011. June;10(6):960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T, Jiang B, Zou ST, et al. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol 2015. February 14;21(6):1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang FB, Wang L, Jia HC, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nunes-Xavier CE, Karlsen KF, Tekle C, et al. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget 2016. February 9;7(6):6891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modak S, Kramer K, Gultekin SH, et al. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001. May 15;61(10):4048–54. [PubMed] [Google Scholar]
- 46.Roth TJ, Sheinin Y, Lohse CM, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res 2007. August 15;67(16):7893–900. [DOI] [PubMed] [Google Scholar]
- 47.Du H, Hirabayashi K, Ahn S, et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019. February 11;35(2):221–37 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arigami T, Narita N, Mizuno R, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg 2010. December;252(6):1044–51. [DOI] [PubMed] [Google Scholar]
- 49.Bin Z, Guangbo Z, Yan G, et al. Overexpression of B7-H3 in CD133+ colorectal cancer cells is associated with cancer progression and survival in human patients. J Surg Res 2014. May 15;188(2):396–403. [DOI] [PubMed] [Google Scholar]
- 50.Boland JM, Kwon ED, Harrington SM, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013. March;14(2):157–63. [DOI] [PubMed] [Google Scholar]
- 51.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell co-regulatory molecule expression in renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Urology 2009. December;74(6):1359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao Y, Li W, Chen K, et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget 2015. February 20;6(5):3452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen L, Xie Q, Wang Z, et al. Assessment of combined expression of B7-H3 and B7-H4 as prognostic marker in esophageal cancer patients. Oncotarget 2016. November 22;7(47):77237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y, Sun J, Zhao H, et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther 2014;7:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Cong F, Yu H, Gao X. Expression of CD24 and B7-H3 in breast cancer and the clinical significance. Oncol Lett 2017. December;14(6):7185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res 2008. August 15;14(16):5150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai W, Shen G, Qiu J, et al. Aberrant expression of B7-H3 in gastric adenocarcinoma promotes cancer cell metastasis. Oncol Rep 2014. November;32(5):2086–92. [DOI] [PubMed] [Google Scholar]
- 58.Hu J, Jiang C, Zheng M, et al. Overexpression of B7-H3 as an opportunity for targeted therapy in head and neck cancers. Am J Transl Res 2019;11(8):5183–96. [PMC free article] [PubMed] [Google Scholar]
- 59.Liu CL, Zang XX, Huang H, et al. The expression of B7-H3 and B7-H4 in human gallbladder carcinoma and their clinical implications. Eur Rev Med Pharmacol Sci 2016. November;20(21):4466–73. [PubMed] [Google Scholar]
- 60.Nehama D, Di Ianni N, Musio S, et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine 2019. September;47:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin X, Zhang H, Ye D, et al. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther 2013;6:1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quandt D, Fiedler E, Boettcher D, et al. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res 2011. May 15;17(10):3100–11. [DOI] [PubMed] [Google Scholar]
- 63.Maeda N, Yoshimura K, Yamamoto S, et al. Expression of B7-H3, a potential factor of tumor immune evasion in combination with the number of regulatory T cells, affects against recurrence-free survival in breast cancer patients. Ann Surg Oncol 2014. December;21 Suppl 4:S546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun TW, Gao Q, Qiu SJ, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother 2012. November;61(11):2171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F, Wang G, Liu T, et al. B7-H3 was highly expressed in human primary hepatocellular carcinoma and promoted tumor progression. Cancer Invest 2014. July;32(6):262–71. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Zhang Q, Chen W, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One 2013;8(8):e70689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H, Chen X, Tao M, et al. B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Lett 2016. March;11(3):1841–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zang X, Sullivan PS, Soslow RA, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol 2010. August;23(8):1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X, Li DC, Zhu XG, et al. B7-H3 overexpression in pancreatic cancer promotes tumor progression. Int J Mol Med 2013. February;31(2):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M, Zhang G, Zhang X, et al. Overexpression of B7-H3 in CD14+ monocytes is associated with renal cell carcinoma progression. Med Oncol 2014. December;31(12):349. [DOI] [PubMed] [Google Scholar]
- 71.Cheng R, Chen Y, Zhou H, et al. B7-H3 expression and its correlation with clinicopathologic features, angiogenesis, and prognosis in intrahepatic cholangiocarcinoma. APMIS 2018. May;126(5):396–402. [DOI] [PubMed] [Google Scholar]
- 72.Powderly J, Cote G, Flaherty K, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer 2015;3(Suppl 2):O8-O8. [Google Scholar]
- 73.MacroGenics. MacroGenics Announces Partial Clinical Hold on MGD009 Phase 1 Studies. 2018. [cited 2020 February 19]; Available from: http://ir.macrogenics.com/news-releases/news-release-details/macrogenics-announces-partial-clinical-hold-mgd009-phase-1 [Google Scholar]
- 74.MacroGenics. MacroGenics Announces Removal of Partial Clinical Hold on MGD009 Program by FDA. 2019. [cited 2020 February 19]; Available from: http://ir.macrogenics.com/news-releases/news-release-details/macrogenics-announces-removal-partial-clinical-hold-mgd009 [Google Scholar]
- 75.Natali P, Bigotti A, Cavalieri R, et al. Distribution of a cross-species melanoma-associated antigen in normal and neoplastic human tissues. J Invest Dermatol 1985. October;85(4):340–6. [DOI] [PubMed] [Google Scholar]
- 76.Altan M, Pelekanou V, Schalper KA, et al. B7-H3 Expression in NSCLC and Its Association with B7-H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clin Cancer Res 2017. September 1;23(17):5202–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arigami T, Uenosono Y, Hirata M, et al. B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci 2011. May;102(5):1019–24. [DOI] [PubMed] [Google Scholar]
- 78.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 2008. August 1;14(15):4800–8. [DOI] [PubMed] [Google Scholar]
- 79.Chavin G, Sheinin Y, Crispen PL, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res 2009. March 15;15(6):2174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gregorio A, Corrias MV, Castriconi R, et al. Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008. July;53(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo L, Liu Z, Zhang Y, et al. Association of increased B7 protein expression by infiltrating immune cells with progression of gastric carcinogenesis. Medicine (Baltimore) 2019. February;98(8):e14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han S, Shi X, Liu L, et al. Roles of B7-H3 in Cervical Cancer and Its Prognostic Value. J Cancer 2018;9(15):2612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim AK, Gani F, Layman AJ, et al. Multiple Immune-Suppressive Mechanisms in Fibrolamellar Carcinoma. Cancer Immunol Res 2019. May;7(5):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu Y, Lv X, Wu Y, et al. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology 2015. May;20(4):187–95. [DOI] [PubMed] [Google Scholar]
- 85.Huang C, Zhou L, Chang X, et al. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: Associations with patient outcome and clinical significance. Oncol Rep 2016. April;35(4):2183–90. [DOI] [PubMed] [Google Scholar]
- 86.Inamura K, Takazawa Y, Inoue Y, et al. Tumor B7-H3 (CD276) Expression and Survival in Pancreatic Cancer. J Clin Med 2018. July 10;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inamura K, Yokouchi Y, Kobayashi M, et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer 2017. January;103:44–51. [DOI] [PubMed] [Google Scholar]
- 88.Ingebrigtsen VA, Boye K, Nesland JM, et al. B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer 2014. August 20;14:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingebrigtsen VA, Boye K, Tekle C, et al. B7-H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer 2012. December 1;131(11):2528–36. [DOI] [PubMed] [Google Scholar]
- 90.Jiang B, Zhang T, Liu F, et al. The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget 2016. May 31;7(22):31755–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katayama A, Takahara M, Kishibe K, et al. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int J Oncol 2011. May;38(5):1219–26. [DOI] [PubMed] [Google Scholar]
- 92.Kim GE, Kim NI, Park MH, Lee JS. B7-H3 and B7-H4 expression in phyllodes tumors of the breast detected by RNA in situ hybridization and immunohistochemistry: Association with clinicopathological features and T-cell infiltration. Tumour Biol 2018. November;40(11):1010428318815032. [DOI] [PubMed] [Google Scholar]
- 93.Li D, Wang J, Zhou J, et al. B7-H3 combats apoptosis induced by chemotherapy by delivering signals to pancreatic cancer cells. Oncotarget 2017. September 26;8(43):74856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Yang X, Wu Y, et al. B7-H3 promotes gastric cancer cell migration and invasion. Oncotarget 2017. September 22;8(42):71725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Yang X, Yao P, et al. B7-H3 increases the radioresistance of gastric cancer cells through regulating baseline levels of cell autophagy. Am J Transl Res 2019;11(7):4438–49. [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Zhang J, Han S, et al. B7-H3 promotes the proliferation, migration and invasiveness of cervical cancer cells and is an indicator of poor prognosis. Oncol Rep 2017. August;38(2):1043–50. [DOI] [PubMed] [Google Scholar]
- 97.Liu C, Liu J, Wang J, et al. B7-H3 expression in ductal and lobular breast cancer and its association with IL-10. Mol Med Rep 2013. January;7(1):134–8. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y, Vlatkovic L, Saeter T, et al. Is the clinical malignant phenotype of prostate cancer a result of a highly proliferative immune-evasive B7-H3-expressing cell population? Int J Urol 2012. August;19(8):749–56. [DOI] [PubMed] [Google Scholar]
- 99.Luo D, Xiao H, Dong J, et al. B7-H3 regulates lipid metabolism of lung cancer through SREBP1-mediated expression of FASN. Biochem Biophys Res Commun 2017. January 22;482(4):1246–51. [DOI] [PubMed] [Google Scholar]
- 100.Majzner RG, Theruvath JL, Nellan A, et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res 2019. April 15;25(8):2560–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parker AS, Heckman MG, Sheinin Y, et al. Evaluation of B7-H3 expression as a biomarker of biochemical recurrence after salvage radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys 2011. April 1;79(5):1343–9. [DOI] [PubMed] [Google Scholar]
- 102.Parra ER, Villalobos P, Zhang J, et al. Immunohistochemical and Image Analysis-Based Study Shows That Several Immune Checkpoints are Co-expressed in Non-Small Cell Lung Carcinoma Tumors. J Thorac Oncol 2018. June;13(6):779–91. [DOI] [PubMed] [Google Scholar]
- 103.Schoenfeld A, Nielsen GP, Hornicek F, et al. CSPG4 and B7-H3 as prognostic biomarkers in chordoma. Journal of Clinical Oncology 2010;28(15_suppl):10050–50. [Google Scholar]
- 104.Scilla KA, Zandberg DP, Bentzen SM, et al. Case-control study of PD-1, PD-L1 and B7-H3 expression in lung cancer patients with and without human immunodeficiency virus (HIV) infection. Lung Cancer 2018. September;123:87–90. [DOI] [PubMed] [Google Scholar]
- 105.Tekle C, Nygren MK, Chen YW, et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer 2012. May 15;130(10):2282–90. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Chong KK, Nakamura Y, et al. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J Invest Dermatol 2013. August;133(8):2050–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang L, Cao NN, Wang S, et al. Roles of coinhibitory molecules B7-H3 and B7-H4 in esophageal squamous cell carcinoma. Tumour Biol 2016. March;37(3):2961–71. [DOI] [PubMed] [Google Scholar]
- 108.Wu J, Wang F, Liu X, et al. Correlation of IDH1 and B7H3 expression with prognosis of CRC patients. Eur J Surg Oncol 2018. August;44(8):1254–60. [DOI] [PubMed] [Google Scholar]
- 109.Xu L, Ding X, Tan H, Qian J. Correlation between B7-H3 expression and matrix metalloproteinases 2 expression in pancreatic cancer. Cancer Cell Int 2013. August 16;13(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu YH, Zhang GB, Wang JM, Hu HC. B7-H3 and CD133 expression in non-small cell lung cancer and correlation with clinicopathologic factors and prognosis. Saudi Med J 2010. September;31(9):980–6. [PubMed] [Google Scholar]
- 111.Xu ZL, Zhang Y, Wang L, et al. B7H3 promotes malignant progression of muscleinvasive bladder cancer. Oncol Rep 2018. November;40(5):2722–33. [DOI] [PubMed] [Google Scholar]
- 112.Xylinas E, Robinson BD, Kluth LA, et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol 2014. January;40(1):121–7. [DOI] [PubMed] [Google Scholar]
- 113.Yamato I, Sho M, Nomi T, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer 2009. November 17;101(10):1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zang X, Thompson RH, Al-Ahmadie HA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A 2007. December 4;104(49):19458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J, Liu L, Han S, et al. B7-H3 is related to tumor progression in ovarian cancer. Oncol Rep 2017. October;38(4):2426–34. [DOI] [PubMed] [Google Scholar]
- 116.Zhang T, Wang F, Wu JY, et al. Clinical correlation of B7-H3 and B3GALT4 with the prognosis of colorectal cancer. World J Gastroenterol 2018. August 21;24(31):3538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng Y, Liao N, Wu Y, et al. High expression of B7H2 or B7H3 is associated with poor prognosis in hepatocellular carcinoma. Mol Med Rep 2019. May;19(5):4315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou Z, Luther N, Ibrahim GM, et al. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neurooncol 2013. February;111(3):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fauci JM, Sabbatino F, Wang Y, et al. Monoclonal antibody-based immunotherapy of ovarian cancer: targeting ovarian cancer cells with the B7-H3-specific mAb 376.96. Gynecol Oncol 2014. January;132(1):203–10. [DOI] [PubMed] [Google Scholar]
- 120.Kim NI, Park MH, Kweon SS, Lee JS. B7-H3 and B7-H4 Expression in Breast Cancer and Their Association with Clinicopathological Variables and T Cell Infiltration. Pathobiology 2020;87(3):179–92. [DOI] [PubMed] [Google Scholar]
- 121.Maachani UB, Tosi U, Pisapia DJ, et al. B7-H3 as a Prognostic Biomarker and Therapeutic Target in Pediatric central nervous system Tumors. Transl Oncol 2020. February;13(2):365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu Z, Zhao ZX, Cheng P, et al. B7-H3 immune checkpoint expression is a poor prognostic factor in colorectal carcinoma. Mod Pathol 2020. June 8. [DOI] [PubMed] [Google Scholar]
- 123.Zhang C, Chen Y, Li F, et al. B7-H3 is spliced by SRSF3 in colorectal cancer. Cancer Immunol Immunother 2020. July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhan S, Liu Z, Zhang M, et al. Overexpression of B7-H3 in α-SMA-Positive Fibroblasts Is Associated With Cancer Progression and Survival in Gastric Adenocarcinomas. Front Oncol 2019;9:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li D, Xiang S, Shen J, et al. Comprehensive understanding of B7 family in gastric cancer: expression profile, association with clinicopathological parameters and downstream targets. Int J Biol Sci 2020;16(4):568–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zong L, Zhang Q, Zhou Y, et al. Expression and Significance of Immune Checkpoints in Clear Cell Carcinoma of the Uterine Cervix. J Immunol Res 2020;2020:1283632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saeednejad Zanjani L, Madjd Z, Axcrona U, et al. Cytoplasmic expression of B7-H3 and membranous EpCAM expression are associated with higher grade and survival outcomes in patients with clear cell renal cell carcinoma. Ann Diagn Pathol 2020. June;46:151483. [DOI] [PubMed] [Google Scholar]
- 128.Liang J, Liu Z, Pei T, et al. Clinicopathological and Prognostic Characteristics of CD276 (B7-H3) Expression in Adrenocortical Carcinoma. Dis Markers 2020;2020:5354825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bonk S, Tasdelen P, Kluth M, et al. High B7-H3 expression is linked to increased risk of prostate cancer progression. Pathol Int 2020. August 10. [DOI] [PubMed] [Google Scholar]
- 130.Gao Y, Fang P, Li WJ, et al. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol Immunol 2020. January;117:20–28. [DOI] [PubMed] [Google Scholar]
- 131.Tang X, Zhao S, Zhang Y, et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol Ther Oncolytics 2019. September 27;14:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen JT, Chen CH, Ku KL, et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc Natl Acad Sci U S A 2015. October 20;112(42):13057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aung PP, Parra ER, Barua S, et al. B7-H3 Expression in Merkel Cell Carcinoma-Associated Endothelial Cells Correlates with Locally Aggressive Primary Tumor Features and Increased Vascular Density. Clin Cancer Res 2019. June 1;25(11):3455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bachawal SV, Jensen KC, Wilson KE, et al. Breast Cancer Detection by B7-H3-Targeted Ultrasound Molecular Imaging. Cancer Res 2015. June 15;75(12):2501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kraan J, van den Broek P, Verhoef C, et al. Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br J Cancer 2014. July 8;111(1):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang M, Xiao J, Shen M, et al. Isolation and characterization of tumorigenic extrahepatic cholangiocarcinoma cells with stem cell-like properties. Int J Cancer 2011. January 1;128(1):72–81. [DOI] [PubMed] [Google Scholar]
- 137.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol 2010. May;97(3):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Modak S, Carrasquillo J, LaQuaglia M, et al. Abstract CT006: Intraperitoneal radioimmunotherapy for desmoplastic small round cell tumor: Results of a phase I study (NCT01099644). Cancer Research 2018;78(13 Supplement):CT006–CT06. [Google Scholar]
- 139.Souweidane MM, Kramer K, Pandit-Taskar N, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol 2018. August;19(8):1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daiichi-Sanky. Daiichi Sankyo Initiates Clinical Trial with its 4DXd Antibody Drug Conjugate, DS-7300, in Collaboration with Sarah Cannon Research Institute. 2019. [cited 2020 03.05.2020]; Available from: https://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/007073.html
- 141.Loo D, Alderson RF, Chen FZ, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res 2012. July 15;18(14):3834–45. [DOI] [PubMed] [Google Scholar]
- 142.Charu Aggarwal AJ, Ferris Robert, Antonia Scott, Rahma Osama, Tolcher Anthony, Cohen Roger B., Lou Yanyan, Hauke Ralph, Vogelzang Nicholas, Zandberg Dan, Kalebasty Arash, Atkinson Victoria, Adjei Alex, Seetharam Mahesh, Birnbaum Ariel, Weickhardt Andrew, Ganju Vinod, Bondarenko Riva, Peng Linda, Wu Tony, Currence Scott, Baughman Jan, Bonvini Ezio, Goldberg Stacie, Wigginton Jon, Lakhani Nehal. A Phase 1, Open-Label, Dose Escalation Study of Enoblituzumab in Combination with Pembrolizumab in Patients with Select Solid Tumors. SITC. Washington, D.C. 2018. [Google Scholar]
- 143.Weidle UH, Kontermann RE, Brinkmann U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin Oncol 2014. October;41(5):653–60. [DOI] [PubMed] [Google Scholar]
- 144.Lu H, Shi T, Wang M, et al. B7-H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology 2020;9(1):1748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cai D, Li J, Liu D, et al. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol 2020. March;17(3):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kasten BB, Arend RC, Katre AA, et al. B7-H3-targeted (212)Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl Med Biol 2017. April;47:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kasten BB, Gangrade A, Kim H, et al. (212)Pb-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nucl Med Biol 2018. March;58:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.CommissionSecuritiesandExchange. Y-mAbs Therapeutics, Inc. 2018. [cited; Available from: https://www.sec.gov/Archives/edgar/data/1722964/000104746918005786/a2236551zs-1.htm [Google Scholar]
- 149.Xu Y, Xiao Y, Luo C, et al. Blocking PD-1/PD-L1 by an ADCC enhanced anti-B7-H3/PD-1 fusion protein engages immune activation and cytotoxicity. Int Immunopharmacol 2020. July;84:106584. [DOI] [PubMed] [Google Scholar]
- 150.Sun X, Yu Y, Ma L, et al. T cell cytotoxicity toward hematologic malignancy via B7-H3 targeting. Invest New Drugs 2020. June;38(3):722–32. [DOI] [PubMed] [Google Scholar]
- 151.Davis ZB, Vallera DA, Miller JS, Felices M. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol 2017. June;31:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Inc GB. GT BIOPHARMA ANNOUNCES POSTIVE RESULTS FOR TWO NEXT GENERATION TRIKES IN SOLID TUMORS. 2018. 09/13/2018 [cited 2020 03/06]; Available from: https://www.gtbiopharma.com/news-media/press-releases/detail/157/gt-biopharma-announces-postive-results-for-two-next
- 153.Kontos F, Kurokawa T, Vallera DA, et al. IL-15/B7-H3 TriKEs-Based Immunotherapy for Pancreatic Ductal Adenocarcinoma. Journal of the American College of Surgeons 2019;229(4):S176. [Google Scholar]
- 154.Vallera DA, Ferrone S, Kodal B, et al. NK-Cell-Mediated Targeting of Various Solid Tumors Using a B7-H3 Tri-Specific Killer Engager In Vitro and In Vivo. Cancers (Basel) 2020. September 18;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Z, Jiang C, Liu Z, et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol Ther Oncolytics 2020. June 26;17:180–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng M, Yu L, Hu J, et al. Efficacy of B7-H3-Redirected BiTE and CAR-T Immunotherapies Against Extranodal Nasal Natural Killer/T Cell Lymphoma. Transl Oncol 2020. May;13(5):100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]