Abstract
Objectives:
To determine patients who have undergone MRI with Gadolinium Based Contrast Agents (GBCAs) and meet the proposed diagnostic criteria for Gadolinium Deposition Disease (GDD): 1. The effectiveness of chelation therapy (CT) with intravenous Ca-DTPA in removing retained gadolinium (Gd) and factors affecting the amount removed; 2. The frequency of CT-induced Flare, i.e., GDD diagnostic symptom worsening, and factors affecting Flare intensity; 3. Whether, as reported in a separate cohort, GDD patients’ serum cytokine levels differ significantly from those in healthy normal controls and change significantly in response to CT; 4. Whether urine Gd, Flare reaction, and serum cytokine findings in GDD patients are mimicked in non-ill patients described as having Gadolinium Storage Condition (GSC).
Materials and Methods:
Twenty-one GDD subjects and three GSC subjects underwent CT. Patients provided pre- and post-CT 24-hour urine samples for Gd content determination along with pre- and 24-hour post-CT serum samples for cytokine analysis. Patients rated potential Flare 24 hours after CT. Pre- and post-CT 24-hour urine Gd analyses and Luminex serum cytokine assays were performed blind to patients’ GDD and GSC status and all other data except age and gender. Serum cytokine levels in a healthy normal control group of age- and sex-matched subjects drawn from Stanford influenza vaccination studies were measured once, contemporaneously with those of GDD and GSC patients, using the same Luminex assay.
Results:
Urine Gd amounts increased post-CT by four times or more after 87% of the 30 CT sessions. The most important factors appeared to be the time since the last GBCA dose and the cumulative dose received. Urine Gd amounts for GDD and GSC patients fell in the same ranges. All GDD patients, and no GSC patient, reported a Flare 24 hours post-CT. Linear regression found that Flare intensity was significantly predicted by a model including pre- and post-CT Gd amounts and the number of GBCA-enhanced MRIs. Post-CT, multiple cytokines showed strong positive relationships with GDD patients’ Flare intensity in multivariable models. Pre-CT serum levels of 12 cytokines were significantly different in GDD patients compared to healthy Flu vaccine controls. The small number of GSC patients precluded analogous statistical testing. Post CT, GDD patients’ serum levels of 20 cytokines were significantly decreased, and 2 cytokines significantly increased. These cytokines did not exhibit the same change pattern in the 3 GSC patients. The small number of GSC patients precluded statistical comparisons of GSC to GDD patients’ results.
Conclusions:
In this preliminary study, 24-hour urine Gd content increased markedly and similarly in GDD and GSC patients following Ca-DTPA CT. Post-CT Flare reaction developed only in GDD patients. The current study is the second finding significantly different serum cytokine levels in GDD patients compared to healthy normal controls. These differences and the difference between GDD and GSC patients’ Flare and cytokine responses to CT suggest some inflammatory, immunologic, or other physiological differences in patients with GDD. Further research into the treatment and physiological underpinnings of GDD is warranted.
Keywords: Gadolinium, Gadolinium Deposition Disease, Cytokines, Chelation, MRI
Introduction
Gadolinium Deposition Disease (GDD) is a newly described disorder that has not achieved wide recognition in the medical community1,2. Gadolinium (Gd) toxicity has, however, been broadly recognized to occur in individuals with advanced renal failure and manifests in Nephrogenic Systemic Fibrosis (NSF)3. This disease process has been identified almost exclusively secondary to less stable linear Gadolinium Based Contrast Agents (GBCAs)4. Acute hypersensitivity reaction is another well recognized adverse effect resulting from many medications, including GBCAs. This can occur independently of renal function and is observed with all GBCAs5.
In individuals with normal renal function, data regarding Gd retention are limited. Studies have reported Gd retention without related symptoms in brain, bone, skin, and cerebral spinal fluid1,6. An animal study reported gadolinium retention in peripheral nerves after GBCA administration and associated hyperalgesia7, a finding consistent with GDD patients’ common complaint of persistent burning pain.
Several papers describe the GDD symptom complex8–10, but no study has incorporated blinded comparison with a symptom-free, GBCA-exposed control group. This has been one primary roadblock to recognizing the disorder as a valid disease entity.
In vitro studies,11,12 have shown cytokine elaboration by peripheral blood mononuclear cells after exposure to several GBCAs. Cytokine release is one of the immune system’s principal defense mechanisms against foreign antigens. These studies have laid the groundwork for investigating cytokine changes as disease mechanisms, not only for NSF but also for GDD. The only biological study of patients with GDD found significantly elevated serum cytokine levels compared to levels in controls but lacked data on the patients’ cytokine levels before receiving the symptom-associated GBCA13.
Determining the presence of heavy metals via urine collection is a common tool14–16 and has been utilized for Gd. In patients with onset of specified symptoms of GDD after a GBCA-enhanced MRI, the presence of Gd in urine pre-CT and its increase post-CT have been considered evidence of Gd toxicity9. A Flare reaction (GDD symptom worsening) has been reported to occur in a little less than half of GDD patients immediately or within a few days after CT17. In our clinical experience, it has been a major concern of GDD sufferers contemplating CT.
The present study had several aims: To determine in patients who have undergone MRI with Gadolinium Based Contrast Agents (GBCAs) and meet the proposed diagnostic criteria for Gadolinium Deposition Disease (GDD): 1. The effectiveness of chelation therapy (CT) with intravenous Ca-DTPA in removing retained gadolinium (Gd) and factors affecting the amount removed; 2. The frequency of CT-induced Flare reactions, i.e., worsening of symptoms included in the GDD diagnostic criteria, and factors affecting Flare intensity; 3. whether, as reported in a separate cohort, GDD patients’ serum levels of a range of cytokines differ significantly from those in healthy normal controls, and whether these levels change significantly in response to CT; 4. To determine whether urine Gd, Flare reaction, and serum cytokine findings in GDD patients are mimicked in non-ill patients described as having Gadolinium Storage Condition (GSC).
Materials and Methods
Patients
Study patients were enrolled from March 2019 to January 2020 at private medical clinics in Chapel Hill, NC. They constituted consecutive, study-eligible patients seeking CT for removal from their bodies of retained Gd. All patients, both those with GDD and GSC, signed an Informed Consent to use their data approved by the Stanford University Medical Center IRB. No study patient had provided data used in other publications. Those with GDD (n= 15 females, 6 males, age range 21 – 66 years, mean 47 ± 11 years) were undergoing off-label, clinical treatment, and testing for removal of Gd from their bodies, including urine Gd and Flare reaction measurements, as described elsewhere17. Briefly, 2.5 ml (1g/5L) Ca-DTPA was administered intravenously as a 1-minute push concurrent with a 1 L normal saline drip, followed 50–60 minutes later by hand injection of 2.5 ml. All study measurements were obtained before administration 24 hours later of Zn-DTPA. On examination and interview by the treating MD (RCS), all GDD patients met the GDD diagnostic criteria specified in an FDA-approved GDD treatment protocol (Semelka and Jay, Investigational New Drug Application, 2016). These criteria are the presence of:
Onset within 30 days of administration of a GBCA of ≥ 3 of 8 symptoms: cognitive disturbance, extremity pain, arthralgia, chest wall pain, skin pain, headache, skin induration, and skin hyperpigmentation,
The symptoms are new to the patient, i.e., do not reflect any pre-existent disease or symptoms observed before GBCA administration,
The patients had normal or near-normal renal function at the time of the GBCA administration.
Indications for the GBCA-enhanced MRI preceding GDD onset included: low back pain (7), post-motor vehicle accident neck/back trauma (2), hip pain (1), pituitary visualization (2), headache/evaluation for stroke (4), pelvic pain (1), abdominal exam (2), and breast exam (2). Subjects without GDD symptoms had undergone an MRI for prostate exam (1), headache (1), or abdominal exam (1).
Study exclusion criteria were age less than 18 years, suffering from hemochromatosis or Wilson’s disease, or a medical condition or taking a medication known to strongly influence serum cytokine levels, e.g., an infectious disease, cancer, rheumatoid arthritis, or an autoimmune disorder.
Two GDD patients had well-treated Hashimoto’s thyroiditis. An exception to the exclusion criteria was made for one GDD patient, a physician who was taking mycophenolate mofetil, which may reduce in vitro production of the cytokines IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α, TNF-β, and GM-CSF18.
The patients began Ca-DTPA (diethylenetriaminepentaacetic acid) CT from one month to five years after their last exposure to a GBCA (Table 1).
Table 1.
Summary of patient characteristics1
| Patient ID | Visit | Age | Sex | Urine Gd Pre-CT | Urine Gd Post-CT | Flare Severity | Control | Batch | Years since GBCA | Years Since Onset | # MRIs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P01 | V1 | 59 | M | 0.5 | 23 | 0 | yes | 1 | 4 | 0 | 15 |
| P01 | V2 | 59 | M | 0.5 | 31 | 0 | yes | 1 | 4.3 | 0 | 15 |
| P02 | V1 | 48 | F | 0.7 | 34 | 8 | no | 1 | 2 | 10 | 17 |
| P02 | V2 | 48 | F | 0.4 | 14 | 5 | no | 1 | 2.3 | 10 | 17 |
| P03 | V1 | 28 | F | 0 | 5.6 | 5 | no | 1 | 0.3 | 0.3 | 2 |
| P03 | V2 | 28 | F | 0.3 | 5.1 | 4 | no | 1 | 0.6 | 0.6 | 2 |
| P04 | V1 | 44 | F | 0.2 | 4.7 | 5 | no | 1 | 1 | 1 | 3 |
| P04 | V2 | 44 | F | 0 | 5.1 | 4 | no | 2 | 1.3 | 1 | 3 |
| P05 | V1 | 55 | F | 0.4 | 25 | 7 | no | 1 | 2 | 7 | 7 |
| P05 | V2 | 55 | F | 0.4 | 28 | 7 | no | 2 | 2.3 | 7.3 | 13 |
| P07 | V1 | 66 | M | 0.2 | 17 | 4 | no | 1 | 2 | 7 | 3 |
| P07 | V2 | 66 | M | 2 | 10 | 3 | no | 2 | 2.3 | 7.3 | 3 |
| P09 | V1 | 59 | F | 0.9 | 29 | 7 | no | 1 | 0.3 | 4 | 5 |
| P09 | V2 | 59 | F | 0.3 | 20 | 7 | no | 2 | 0.6 | 4.3 | 5 |
| P10 | V1 | 49 | M | 0.7 | 25 | 6 | no | 1 | 0.3 | 0.5 | 2 |
| P11 | V1 | 21 | F | 0.3 | 12 | 7 | no | 1 | 5 | 13 | 15 |
| P12 | V1 | 60 | F | 0.5 | 8.7 | 9 | no | 1 | 0.3 | 0.3 | 1* |
| P13 | V1 | 44 | F | 0.5 | 31 | 7 | no | 1 | 0.2 | 1 | 7 |
| P14 | V1 | 36 | M | 72 | 68 | 5 | no | 1 | 0.1 | 2 | 15 |
| P15 | V1 | 44 | F | 0.4 | 9.1 | 6 | no | 1 | 0.8 | 7 | 2 |
| P16 | V1 | 59 | M | 0 | 5.7 | 5 | no | 1 | 4 | 14 | 2 |
| P17 | V1 | 66 | M | 0 | 15 | 0 | yes | 1 | 0.5 | 0 | 2 |
| P18 | V1 | 57 | F | 1.4 | 4.9 | 8 | no | 1 | 0.2 | 1 | 4 |
| P19 | V1 | 32 | F | 10 | 12 | 5 | no | 1 | 0.2 | 0.3 | 2* |
| P20 | V1 | 56 | M | 0 | 3.6 | 0 | yes | 2 | 11 | 0 | 1 |
| P21 | V1 | 61 | F | 0 | 2.2 | 5 | no | 2 | 1.5 | 1.8 | 5 |
| P22 | V1 | 47 | M | 0 | 4.6 | 4 | no | 2 | 2.5 | 3.5 | 3 |
| P23 | V1 | 44 | F | 0.3 | ? | 8 | no | 2 | 0.8 | 2.4 | 6 |
| P24 | V1 | 48 | F | 4.9 | 12 | 7 | no | 2 | 0.3 | 0.3 | 2* |
| P25 | V1 | 50 | M | 0.6 | 4.7 | 5 | no | 2 | 0.2 | 0.4 | 6* |
| P26 | V1 | 36 | F | 0.1 | 0.7 | 4 | no | 2 | 0.3 | 0.3 | 1* |
Patient ID numbers are discontinuous between PO5 and P07. Visit = first or fifth Ca-DTPA chelation therapy session. Gd = gadolinium. Urine Gd = 24-hour urine Gd content. Flare severity = the patient’s Flare rating 24 hours after the visit. Control = Gadolinium Storage Condition patient. Batch refers to the first and the second Luminex Cytokine immunoassay. GBCA = Gadolinium Based Contrast Agent. Years Since GBCA = number of years since receiving the most recently administered GBCA dose. Years Since Onset = number of years since GDD symptom onset. # MRIs = number of GBCA MRIs the patient had received.
= received only a macrocyclic GBCA.
Three asymptomatic male patients (age range 56 – 66 years, mean 60 ± 4 years) with Gadolinium Storage Condition (GSC)19 were concerned about Gd retention after their GBCA injections and requested CT. They began DTPA CT treatment at the study clinics from six months to 11 years after their last GBCA exposure (Table 1). No GSC patient had previous DTPA CT treatment.
Six GDD patients and one GSC patient provided cytokine, urine Gd, and Flare data twice, three months apart, at their first and fifth CT sessions, time points regularly used to evaluate pre- and post-CT 24-hr urine Gd amounts. The assessments were used clinically to determine CT effectiveness and to help determine the advisability of further CT. The second GSC patient data was a check on the reproducibility of measurements.
The Luminex serum cytokine results for the healthy age- and sex-matched control group (N=35) were drawn from influenza vaccination studies at Stanford University Medical Center20 and measured once, contemporaneously with GDD and GSC patients, using the same Luminex assay. Potential subjects for these vaccination studies were excluded for the presence of acute illness, a history of autoimmune disease, cancer (other than non-melanoma skin cancer), clinically significant liver disease, moderate to severe renal disease, diabetes mellitus requiring insulin, other major chronic illness, receipt of blood products in the prior 6 months, and current pregnancy or breastfeeding, but not for a history of cardiovascular disease. Further description is provided elsewhere21. No healthy control group subject was suffering from a medical condition known to influence serum cytokine levels strongly.
Urine Gd measurement
24-hr urine for Gd was obtained the day before CT and during approximately 24-hours post-Ca-DTPA CT. Urine samples were sent to Doctor’s Data, Inc., St Charles, IL (htpps://www.doctorsdata.com/contact; info@doctorsdaa.com), which determined Gd amounts using inductively coupled plasma mass spectrometry. The Doctor’s Data, Inc. norm = ≤ 0.6 μg/24 hours for women, and ≤ 1.0 for men, based on unprovoked urine samples from individuals (336 females and 204 males) asked to refrain from a GBCA-enhanced MRI for at least 48 hours before starting urine collection. The norms represent the 95th percentiles in this sample. DDI’s approach to establishing reference ranges and quality control measure is consistent with other laboratories. (See: https://doctorsdata.com/licensing). Two physicians were blinded as to whether results were from GDD or GSC patients independently examined urine Gd reports to investigate whether potential differences between pre- and post-CT results and between GDD and GSC patients’ results could be accurately distinguished.
Cytokine Analysis
At the start of CT, 8 ml of blood was drawn, serum separated in standard fashion, and the serum tubes packed on dry ice for overnight shipping to the Stanford Human Immune Monitoring Center laboratory. The post-Ca-DTPA CT serum sample was acquired the next day at approximately the same time of day. All samples were drawn between 10 AM and 11:00 AM to avoid circadian effects on cytokine levels. Serum sample tube labels encoded patient identity and whether the sample was related to the first or fifth CT. However, laboratory staff was blind to whether samples were pre- or post-CT, and whether from GDD or GSC patients.
Luminex Cytokine Immunoassay: This assay was performed by the Stanford University Human Immune Monitoring Center (Palo Alto, CA, https://iti.stanford.edu/himc.html yaelhr@stanford.edu;). Seventy-six cytokines were measured in GDD patients and GSC controls. Kits were purchased from EMD Millipore Corporation (Burlington, MA) and used according to the manufacturer’s recommendations with modifications as follows. The assay included 3 panels (Supplementary Table 1). Panel 1 was Milliplex HCYTMAG60PMX41BK with IL-18 and IL-22 added to generate a 43 plex panel. Panel 2 was Milliplex HCP2MAG62KPX23BK, with CXCL9 added to generate a 24 plex panel. Panel 3 included the Milliplex HSP1MAG-63K with Resistin, Leptin, and HGF added to generate a 9 plex panel. The assay setup was as recommended by the vendor. Briefly, samples were mixed with antibody-linked magnetic beads on a 96-well plate and incubated overnight at 4°C with shaking. Cold temperature and room temperature incubation steps were performed on an orbital shaker at 500–600 rpm. Plates were washed twice with wash buffer in a Biotek ELx405 washer. Following a one-hour incubation at room temperature with biotinylated detection antibody, streptavidin-PE was added for 30 minutes with shaking. Plates were washed as above, and phosphate-buffered saline (PBS) was added to wells for reading in the Luminex FlexMap3D Instrument (Luminex Corp., Austin, TX) with a lower bound of 50 beads per sample per cytokine. Each sample was measured in duplicate. Custom Assay Chex control beads were purchased from Radix Biosolutions, Georgetown, Texas, and were added to all wells. Duplicate wells of the samples on the same plates had mean CV values of 3–6% depending on the plate.
GDD patient and GSC control samples were run in two batches, with healthy Flu vaccine controls run in a third batch, all using the same lot of Luminex kits. We compared the inter-batch variation of a shared duplicate control sample between batches and found reproducibility within 20% CV for all cytokines to check for batch effects. Therefore, no correction for batch effects was made in the downstream analyses.
All patients received intravenous Ca-DTPA as the chelating agent, utilizing the technique previously reported17. 24-hour urine samples, post-CT serum samples, and Flare ratings were obtained before beginning the reported technique’s Zn-DTPA CT session, which began approximately 24 hours after the Ca-DTPA CT session ended. The nurse or physician adjusted the rate of standard saline administration. GSC patients received Ca-DTPA as a single 1-minute duration bolus push of the 5 ml aliquot of Ca-DTPA followed by rapid i.v. drip of 50 ml of normal saline. They received single-dose administration for increased throughput, despite the increased possibility of eliciting a Flare. GDD patients’ split dose was intended in part to mitigate the Flare reaction.
Flare determination
A flare was defined as the exacerbation in the 24 hours after Ca-DTPA CT of pre-existent GDD symptoms or onset of new but related to pre-existent symptoms (e.g., pain in a bone, but one not previously painful). As is standard for GDD patients, patients rated their Flare reaction from 0 (absent) to 10 (extremely severe). Flare ratings were made approximately 24 hours after Ca-DTPA CT. For the three days pre-CT and before the post-Ca-DTPA CT blood draw, no patient took medication to diminish a Flare. Current prescribed medications were not discontinued before or after CT, but none, except mycophenolate mofetil, had known substantial effects on cytokines.
Statistical analysis
Because of the small number of GSC patients, the statistical analysis focused on the GDD patient data. At a high level, comparisons were made between measurements taken pre- and post-CT for these GDD patients and between the pre-CT cytokine measurements and healthy Flu vaccine controls. Descriptions of specific analyses follow. (1) The p-value for the chi-squared test of reported Flare by GDD and GSC groups was estimated using a Monte-Carlo simulation with 2000 replicates to account for the small number of GSC patients. (2) To estimate post-CT Gd levels, a multivariable linear regression model was fit using pre-CT Gd, the number of MRIs, and Flare. Age, sex, years since most-recent GBCA receipts, and years since GDD symptom onset did not improve the model, as tested by ANOVA. Multiple visits (i.e., multiple pre- to post-CT cycles) from the same patient were treated independently. (2) Cytokine data for GDD patient pre-CT samples (n=21) were compared to healthy “Flu” controls (described above, n=35) using a two-sided Student’s t-test. (4) Cytokine data were compared between time points and GDD and GSC patients using a linear mixed-effects model. The models’ estimated change between pre- and post-CT time points, allowing the change to differ between GDD and GSC patients, with intercept differing by patient-visit. We report p-values associated with the pre- to post-CT change for GDD patients only and calibrate the estimated change as a percentage of the Flu control range. (5) To estimate post-CT cytokine levels, a full regression linear regression model (post-chelation cytokine ~ pre-chelation cytokine + post-chelation Gd + Flare + Age + Sex + number of MRIs + years since most recent GBCA + years since GDD symptom onset) was fit for GDD patient data. The backward selection was used to eliminate terms other than pre-chelation cytokine. Separate models were created for different cytokines. Across all analyses, no corrections were made for multiple comparisons due to this work’s exploratory nature. P-values < 0.05 were considered of interest in this exploratory study. R version 3.5.1 was used for all statistical analyses. Median fluorescence intensity (MFI) of duplicate wells was used to compare cytokine levels, rather than calculated concentration, based on Breen et al.22.
Results
Table 1 presents the patient ID number, the Ca-DTPA CT visit (whether first [V1} or fifth [V2] CT visit), and data regarding GDD and GSC patient demographics, 24-hour urine Gd content pre- and post-CT, the patient’s Flare rating 24 hours after the visit, the number of years since receiving the most recently administered GBCA dose and since GDD symptom onset, and the number of GBCA-enhanced MRIs the patient had received. Note: patient ID numbers are discontinuous between P05 and P07.
24-hour Urine Gd
Pre-CT 24-hour Gd amounts varied from < 0.1 to 4.9 mcg (plus an outlier of 72 mcg). Six of 12 GDD patients, ill for ≤ 2 years before blood draw, and 2 of 9 GDD patients, ill for > 2 years, had 24-hour Gd amounts above the Doctor’s Data, Inc. laboratory norm. In the urine samples collected for 24-hours after CT, 19 of 21 GDD patients and 2 of the 3 GSC patients (and 87% of 30 post-CT visit samples) had urine Gd amounts from 4 times to more than 20 times greater than the laboratory norm (Table 1), evidencing substantial Gd retention. Five patients had received only a macrocyclic GBCA (Table 1). The most important factors appeared to be the time since the last GBCA injection and cumulative Gd dose received from all GBCA-enhanced MRIs. GDD sufferers and GSC patients exhibited no appreciable difference in pre- and post-CT urine Gd levels. Neither pre- nor post-CT urine Gd amounts permitted distinction between GDD and GSC patients.
Flare
All GDD patients reported a Flare within 24 hours post-CT, and many experienced the Flare within minutes of Ca-DTPA injection. No GSC patient reported a Flare (Table 1). Mean amounts of Gd excreted post-CT were considerably higher (19.1 mcg) in GDD patients with Flare ratings of ≥ 6 (n=10) than in those with Flare ratings of ≤ 5 (6.3 mcg, n=9). (One patient’s post-CT Gd amount was unknown, and one patient was an outlier, with urine Gd of 68 mcg [Flare rating 5] as a result of having CT one month after the last GBCA injection, and additionally, having had >15 GBCA-enhanced MRIs.)
Linear regression revealed that the Flare intensity was not significantly associated with post-CT urine levels of Gd alone but was significantly associated when pre-CT urine Gd was added to the model (Figure 1). The model was further improved by adding the number of GBCA-enhanced MRIs (r2=0.69) but not by adding age, gender, time since GDD onset, or time since last MRI.
Figure 1.
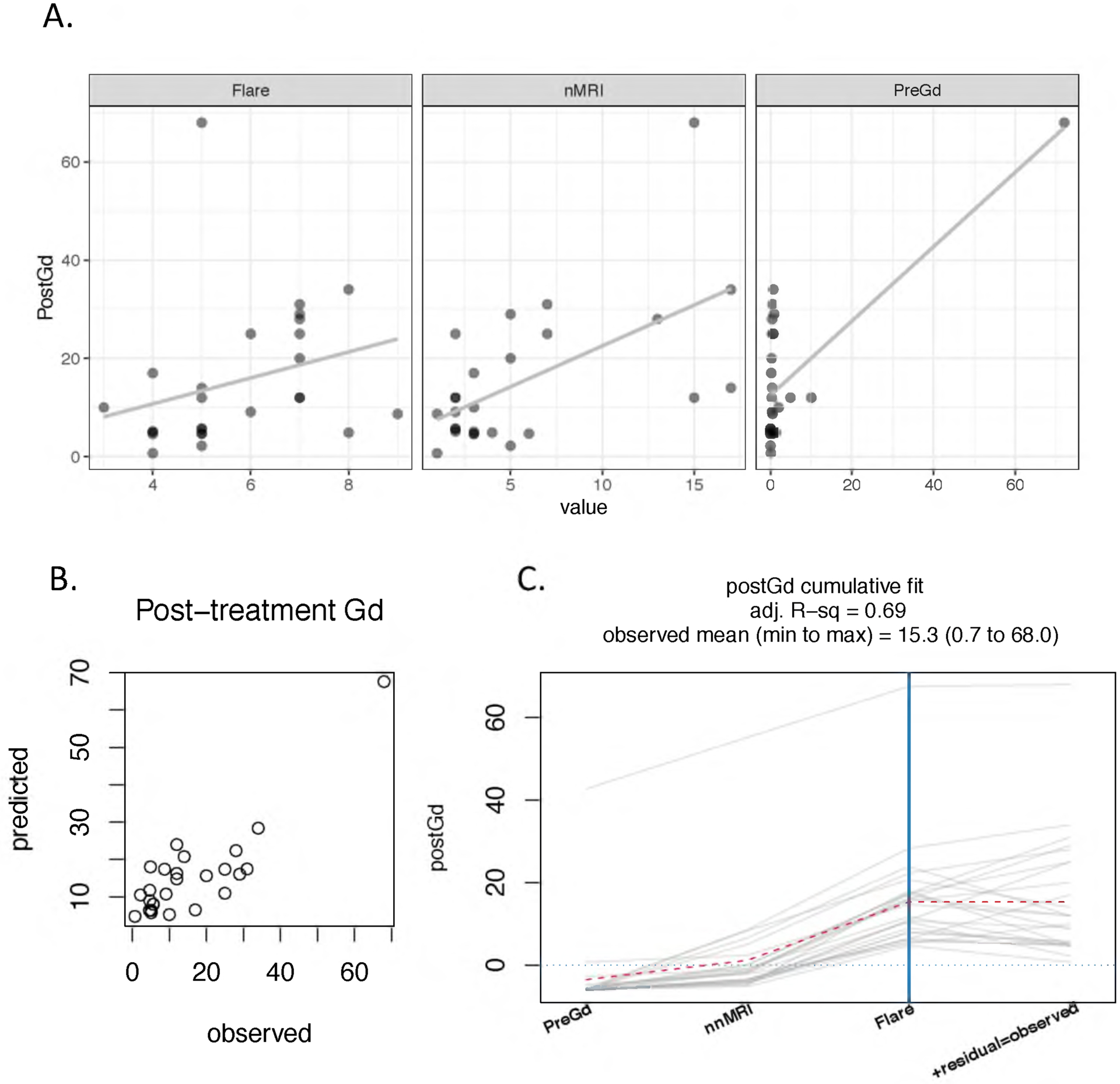
While the correlation between post-CT Gd and Flare was not significant, a linear model that included pre-CT Gd and the number of MRIs levels was significant. (A) Relationships between post-CT Gd and the three predictors (Flare, severity scale 0 to 10; nMRI, number of MRIs; preCtGd, μg/24 hours). The solid line is the estimated linear fit. (B) Relationship between the predicted and observed post-treatment Gd. (C) The adjusted R-squared of the multivariable model (Post-CT Gd ~ Flare + Pre-CT Gd + nnMRI) is 0.69. The cumulative fit graph displays the estimated response (post-CT Gd) by accumulating the contribution of one predictor at a time. A dotted red “trend line” connects the mean cumulative value at each predictor addition. Each gray path represents one patient-visit (or pre-post cycle), with 6 GDD patients having two visits. The intersection of the patient paths and the blue vertical line represents the estimated response for each patient, while the paths’ final points indicate the observed responses. The predictors are ordered by mean contribution to the fit, from lowest to highest. The intercept is not shown. As Flare severity increases from 5 to 7, predicted post-CT Gd increases by approximately 5, just over 7% of the observed range for post-CT Gd.
Cytokines
Cytokines were significantly different in pre-CT GDD patients vs. healthy controls.
Table 2 lists the 12 cytokines whose serum levels were significantly different in the GDD patients than the healthy Flu vaccine controls. Their distributions are shown in Figure 2. GDD patients’ levels were not related to gender or to time since the last MRI. Levels were significantly higher for six cytokines. Among these six, three were also significantly elevated in the subset of 12 GDD patients ill for ≤ 2 years (CCL15, CCL27, and TRAIL), and one (GROA) was nearly so at p = 0.0536.
Table 2.
Cytokines with a significant difference between GDD patients and healthy Flu vaccine controls1
| Cytokine | P-value, GDD vs. Control | GDD Mean | GDD SD | Control Mean | Control SD | Difference in Means as Pct of Control Range | P-value, Recent onset vs. Control | Recent Onset Mean | Recent Onset SD |
|---|---|---|---|---|---|---|---|---|---|
| MIP1D | 0.000001 | 2803.6 | 1467.3 | 1023.9 | 517.5 | 72.8 | 0.001497 | 3009.2 | 1853.7 |
| SCD40L | 0.000001 | 1547.0 | 1389.4 | 3539.1 | 1495.6 | −29.3 | 0.000031 | 1644.5 | 1116.4 |
| CTACK | 0.000007 | 6287.9 | 1386.1 | 4636.5 | 1149.3 | 33.5 | 0.000001 | 6755.6 | 1046.3 |
| TRAIL | 0.001790 | 202.2 | 47.8 | 158.3 | 57.8 | 15.7 | 0.026655 | 197.1 | 50.1 |
| GCSF | 0.001936 | 230.2 | 66.2 | 180.0 | 49.8 | 25.5 | 0.107031 | 219.4 | 80.8 |
| PAI1 | 0.004547 | 25576.6 | 4070.5 | 28709.6 | 4229.3 | −15.8 | 0.051418 | 26026.1 | 4110.5 |
| EGF | 0.004884 | 257.4 | 368.0 | 511.9 | 291.5 | −24.2 | 0.000006 | 211.2 | 119.9 |
| GROA | 0.009568 | 6612.9 | 5920.8 | 3289.8 | 2341.9 | 34.7 | 0.053633 | 7107.3 | 6629.4 |
| EOTAXIN2 | 0.019041 | 3295.5 | 2926.4 | 1786.6 | 1453.4 | 22.2 | 0.172307 | 2844.0 | 2615.0 |
| HGF | 0.037928 | 28.8 | 10.9 | 34.5 | 9.6 | −14.1 | 0.000149 | 25.9 | 4.7 |
| IFNA2 | 0.042243 | 24.4 | 5.9 | 28.4 | 9.2 | −10.6 | 0.154038 | 24.9 | 6.7 |
| IL12P70 | 0.042394 | 25.7 | 6.6 | 31.6 | 14.8 | −10.3 | 0.142343 | 26.8 | 7.5 |
Columns include cytokine, the p-value for the comparison between GDD patients and controls, the mean levels and standard deviations for both GDD patients and controls, the difference in means between GDD patients and controls expressed as a percentage of the control range, the p-value of a comparison of recent onset GDD patients (≤ 2years) to the controls, and the mean levels and standard deviations for the recent onset GDD patients. P-values are from Student’s t-test.
Serum samples.
Figure 2.
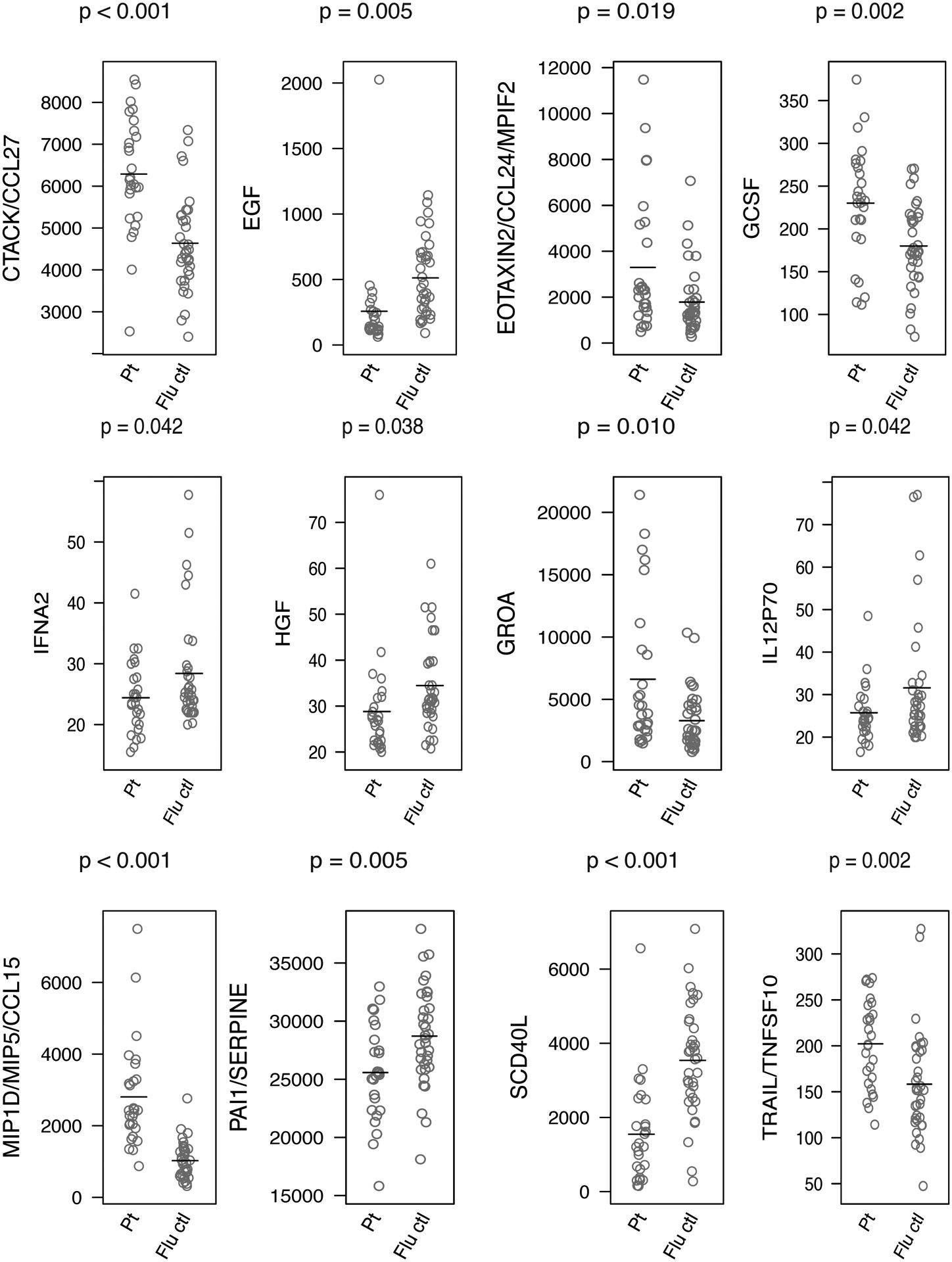
Cytokines with significant difference in GDD patients vs. age- and sex-matched healthy controls, based on t-test (ordered alphabetically).
Among the six cytokines whose levels were significantly lower in the GDD patients, a similar split was seen in the GDD patients ill for ≤ 2 years: three cytokines were significantly lower (SCD40L, EGF, and HGF), and one (SERPINE) nearly so at p = 0.0514.
The small number of GSC patients, (3) prevented statistical testing of their pre-CT cytokine levels versus the normal Flu controls.
Cytokines significantly changed pre- to post-CT.
Table 3 shows the 22 cytokines (of 76 measured) whose serum levels were significantly changed in GDD patients 24 hours after CT. Of the 22 cytokines, 20 were reduced post-CT; only two (SFAS and G-CSF) were increased. This changing pattern was not seen in the group of 3 GSC patients (see Supplementary figure 1). None of these 22 cytokines in the GSC patients were significantly decreased post-CT, and only one (CTACK) was significantly increased.
Table 3.
Cytokines significantly different (p < 0.05) in GDD patients between pre- and post-CT1
| Cytokine | GDD pre-CT mean | GDD pre-CT SD | GDD post-CT mean | GDD post-CT SD | Change, post - pre | p-value, change | Direction |
|---|---|---|---|---|---|---|---|
| IL-4 | 71.1 | 131.5 | 65.7 | 128.9 | −5.4 | 0.0003 | down |
| ICAM-1 | 6678.0 | 2657.7 | 6408.4 | 2601.4 | −269.6 | 0.0004 | down |
| IL-16 | 831.9 | 1426.6 | 779.6 | 1426.2 | −52.2 | 0.0026 | down |
| SFAS | 264.8 | 99.4 | 286.9 | 95.9 | 22.1 | 0.0043 | up |
| MCP3 | 196.2 | 414.7 | 191.0 | 414.3 | −5.2 | 0.0049 | down |
| TPO | 70.8 | 79.9 | 64.7 | 77.7 | −6.0 | 0.0051 | down |
| Resistin | 9289.8 | 2993.5 | 8344.0 | 2190.1 | −945.7 | 0.0078 | down |
| Eotaxin | 3295.5 | 2926.4 | 2903.4 | 2607.2 | −392.0 | 0.0128 | down |
| Leptin | 1301.3 | 2008.3 | 1045.1 | 1676.7 | −256.1 | 0.0163 | down |
| TSLP | 95.0 | 134.4 | 88.5 | 129.6 | −6.5 | 0.0170 | down |
| FGF2 | 25.4 | 7.3 | 23.6 | 8.0 | −1.8 | 0.0225 | down |
| GROA | 6612.9 | 5920.8 | 4633.5 | 5699.6 | −1979.4 | 0.0240 | down |
| ENA78 | 1853.9 | 2484.9 | 1486.2 | 2429.2 | −367.7 | 0.0254 | down |
| IL-33 | 54.9 | 75.9 | 51.4 | 73.7 | −3.5 | 0.0258 | down |
| TGFA | 51.9 | 39.7 | 45.2 | 32.8 | −6.6 | 0.0300 | down |
| TNFB | 192.8 | 415.0 | 183.2 | 402.0 | −9.6 | 0.0308 | down |
| RANTES | 9388.6 | 2718.1 | 8767.4 | 2543.8 | −621.3 | 0.0330 | down |
| CTACK | 6287.9 | 1386.1 | 5894.1 | 1340.5 | −393.8 | 0.0358 | down |
| IL-20 | 80.1 | 35.6 | 77.2 | 31.9 | −2.9 | 0.0372 | down |
| IL-22 | 36.0 | 30.0 | 34.7 | 29.0 | −1.3 | 0.0405 | down |
| G-CSF | 230.2 | 66.2 | 236.4 | 67.3 | 6.3 | 0.0456 | up |
| IL-1RA | 84.0 | 150.8 | 80.8 | 146.8 | −3.3 | 0.0459 | down |
Columns include cytokine, mean and SD for pre-CT levels, mean and SD for post-CT levels, Change, the p-value for the change between pre- and post-CT. and the direction of this change (up or down). All data is for GDD patients. P-values are from the mixed-effects model. Representative relationships are illustrated in Figure 3.
Figure 3 displays examples of cytokines with significant CT-related changes. Leptin, which was significantly decreased after CT in GDD patients, showed no significant change in GSC patients. The largest decreases were seen in the GDD patients who had the highest pre-CT serum levels. A similar pattern was seen for TGFA (Figure 3A). On the other hand, SICAM-1 appeared to show a small but relatively consistent decrease in GDD patients, but not in GSC patients (Figure 3B). Finally, TNFRSF6, one of only two cytokines that increased after CT in GDD patients, showed a similar trend for GSC patients (Figure 3C).
Figure 3.
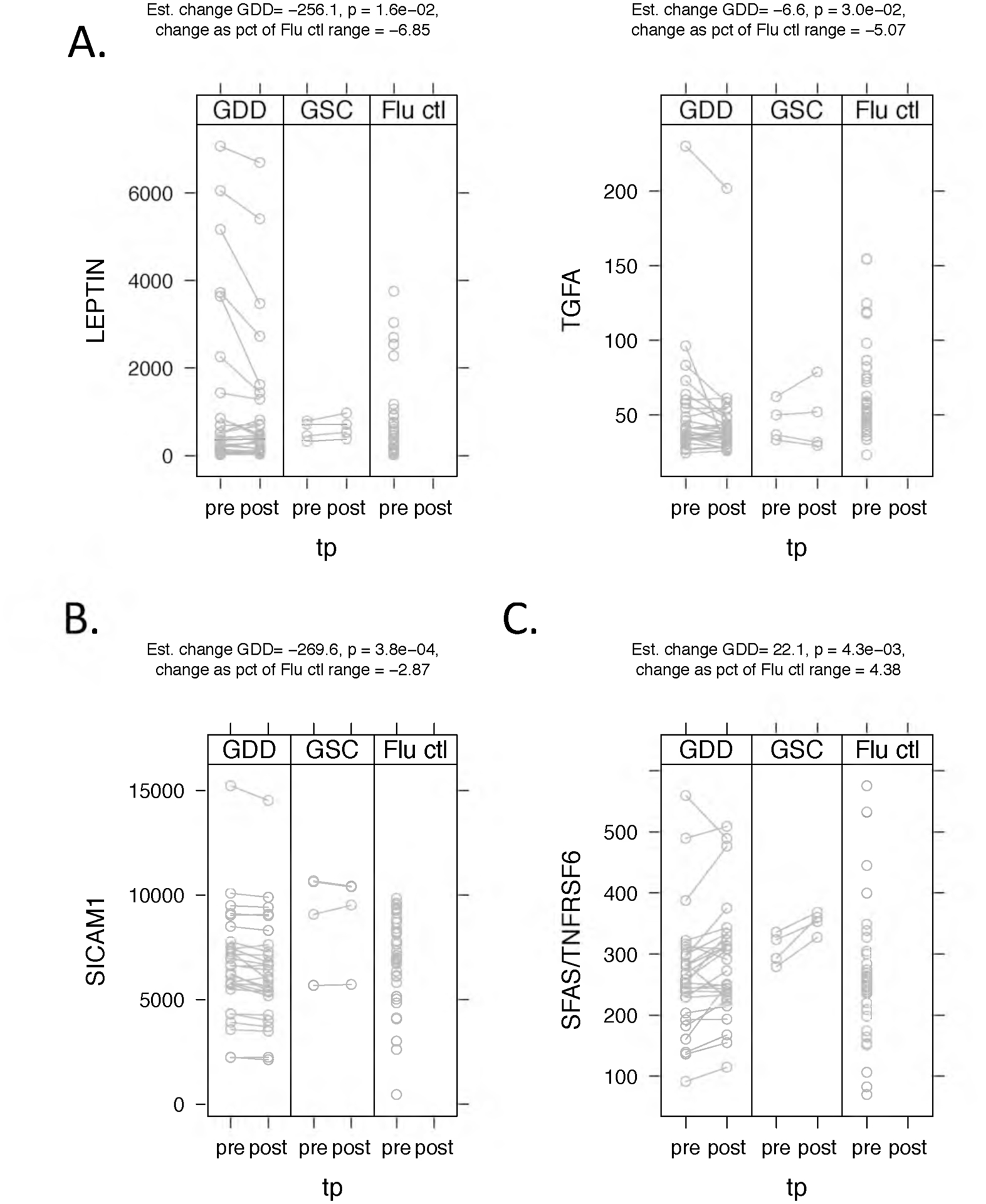
Examples of significant cytokine changes with chelation therapy. P-values are for the change between pre- and post-chelation therapy in GDD patients. (A) Two cytokines, Leptin and TGFA, appear to show a greater decrease with chelation therapy in patients with the highest levels. (B) By contrast, ICAM-1 appears to show a small but relatively consistent decrease across GDD patients. GSC patients show no significant trends with chelation therapy for any of these cytokines. (C) One of only the two cytokines that increased with CCT, SFAS/TNFRSF6, showed a similar change in GDD patients and GSC controls.
Cytokine relationships to Flare ratings and urine Gd amounts.
Multiple regression analysis examined cytokines’ relationships, post-CT urine Gd amount, Flare intensity, age, sex, time since GBCA exposure, and time since onset of GDD. The findings included relationships of cytokines with Flare intensity and urine Gd. For two cytokines, IL-18 and IL-12p40, the regression models showed a strong positive relationship of cytokine serum levels with Flare intensity (Figure 4). Flare intensity had the largest interquartile range (IQR) effect size of any term in the IL-18 model and the third-highest effect in the IL-12p70 model. The IQR effect size is the estimated difference in outcome associated with a change in a predictor from the first quartile to the third quartile, a span that includes 50% of the data values.
Figure 4.
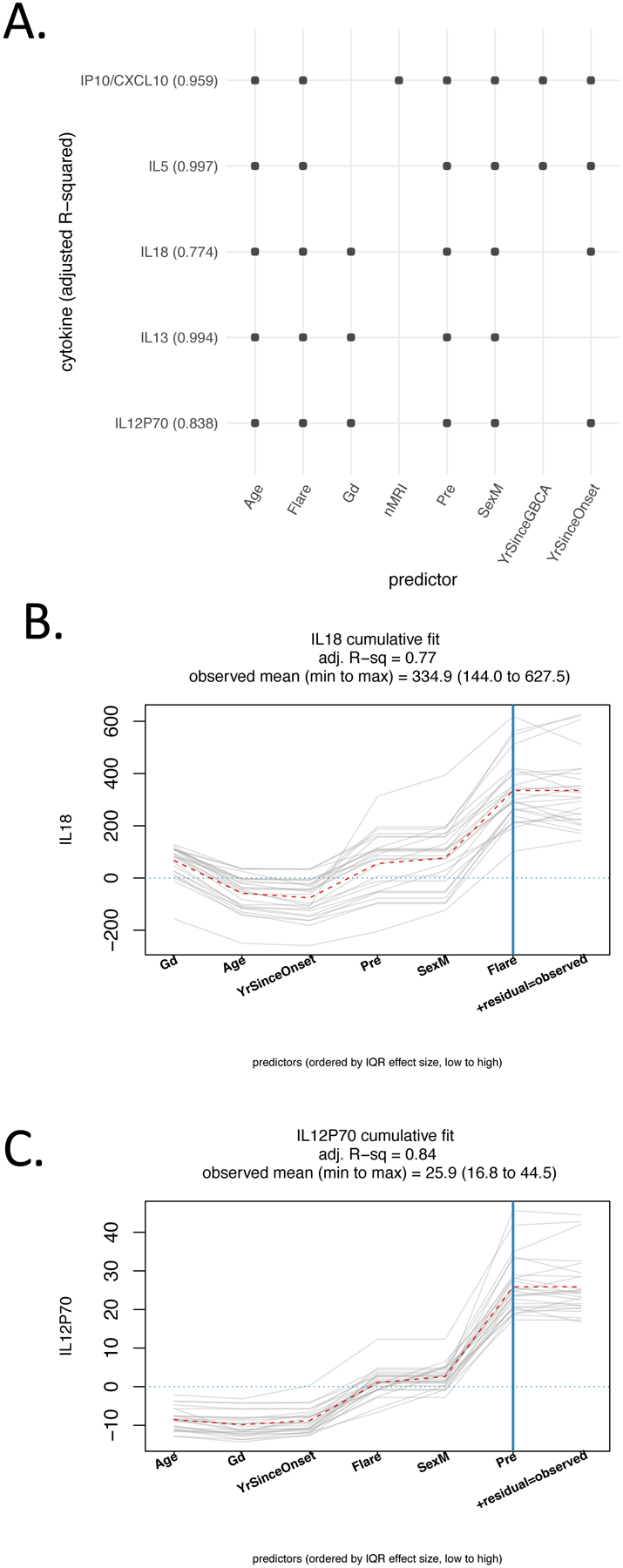
Regression modeling of post-chelation therapy cytokines and clinical factors. For each cytokine, a regression model (post-CT cytokine ~ pre-CT cytokine + post-CT Gd + Flare + Age + Sex + number of MRIs + years since most recent GBCA + years since GDD symptom onset) was fit for GDD patient data. Backward selection was used to eliminate terms other than pre-chelation cytokine. Results are shown for those cytokines with p< 0.01 for the Flare term, and a positive association between post-treatment cytokine and Flare. (A) For each cytokine, included predictors are indicated with a small circle. Adjusted R-squared values are in parentheses. All models include Flare and pre-CT cytokine by design. All models retained Age and Sex as predictors, while post-CT Gd, number of MRIs, years since most recent GBCA, and years since GDD symptom onset were eliminated from some models (B, C). The graphs show the cumulative model fit by adding the contribution of one predictor at a time. Gray lines are individual patients; the dotted red line indicates the mean cumulative value at each predictor. For IL18, Flare had the largest interquartile range (IQR) effect size, approximately 19% of the observed range. The IQR effect size is the estimated difference in outcome associated with a change in a predictor from the first quartile to the third quartile, which includes 50% of the data values. For IL12P70, Flare has an effect size of approximately 12% of the observed range. Gd=post-CT Gd, Pre=pre-CT cytokine, SexM=predictor for male patients, years since most recent GBCA, YrSinceOnset=years since GDD symptom onset.
We further examined whether there were significant correlations of Flare intensity with the difference between pre- and post-CT cytokine levels (Supplementary Figure 2). While there were positive trends, no cytokine level difference had a significant relationship, suggesting that the multivariable models were better at capturing relationships with Flare.
Discussion
In the current study, 24-hour Gd urine content pre- and post-CT were easily distinguished since post-CT amounts were multiple times higher. The post-CT urine levels confirm long-term retention of Gd in GDD patients and patients with no related symptoms (GSC patients); the levels also confirm Gd’s accessibility to DTPA CT even many years after the administration of a GBCA, despite the expected long-term washout, especially with linear GBCA agents23,24. A critical observation of our study was that DTPA induced mobilization of Gd after GBCA-enhanced MRI in patients exposed only to one macrocyclic GBCA, confirming observations in a separate cohort17. In contrast, using a rat model, Boyken J et al. only found mobilization of the linear agent gadodiamide but not of the macrocyclic agent gadobutrol25. In what forms Gd has been retained and is removed have not, however, been established. The clinical significance of Gd retention in patients without GDD symptoms is unknown.
The kidneys’ urine production exhibits diurnal variation, and the importance of diurnal variation in serum creatinine has been emphasized26. Until the effect of diurnal variation is better understood, deriving Gd’s urine measure from the entire 24-hour period seems prudent. As in the present study, utilizing similar start and end times for each collection may be of value, particularly because the degree of retention varies over time for different GBCAs27.
Although post-CT urine Gd content may not distinguish GDD patients from those free of the condition, it provides useful information regarding the likely effectiveness of further CT therapy in GDD. If pre- and post-CT urine Gd amounts do not differ substantially, additional CT seems unlikely to be helpful. DTPA CT has been reported to reduce GDD symptoms, albeit in an open-label, non-randomized study17.
All GDD patients reported a Flare reaction of moderate or greater intensity in the first 24 hours post-CT, suggesting the need to find a preventive treatment, a need recognized for the acute hypersensitivity reaction (AHR) to GBCAs5. A Flare was reported in approximately 50% of GDD patients in a prior study17, and in our clinical practice, is universal. That no Flare symptoms occurred in the three GSC patients is consistent with their not having experienced GDD symptoms after their MRI GBCA exposure(s). The absence of Flare suggests that some inflammatory response, immunological, or other physiological difference exists between the GDD and GSC patients.
The intensity of the Flare reaction was related to the amount of Gd in the pre- and post-CT urine sample and the number of GBCA exposures, suggesting that the related pathophysiology was exacerbated as more Gd, or Gd carrier molecule, was extracted from tissues and into the vascular system. Research into the mechanisms of rheumatoid arthritis spontaneous flares28 raises the possibility that the CT-induced Flare in GDD may reflect the migration of activated immune cells from the circulation to the tissue sites in which DTPA is extracting Gd. In rheumatoid arthritis, pre-inflammatory mesenchymal (PRIME) cells migrated from the circulation to the synovium to produce inflammation. Perhaps similarly, the post-CT fall in cytokine levels in GDD patients reflects the migration of PRIME cells into tissues. The rheumatoid flare finding also supports our opinion that GDD Flare is immunologic.
GDD appears to have features of both an AHR and NSF. As with an AHR to GBCA exposure, a Flare reaction in GDD can arise rapidly after Ca-DTPA injection. Also, both GDD and an AHR can arise after administering all molecular structures of GBCA (linear and macrocyclic, ionic, and nonionic). In contrast, the vast majority of NSF cases arise following nonionic linear GBCAs. The clinical features and persistence of GDD and NSF17 are more similar than either disorder is to an AHR.
The current study is the second to find significantly different serum cytokine levels in GDD patients than in healthy normal controls. However, only one elevated cytokine, GROA, is common to the two studies13. Physicians who are skeptical of the existence of GDD2,29 may assert that the finding of elevated levels of different cytokines in the current study as compared to a prior study13 indicates that the cytokine findings in both studies are due simply to chance. While this is possible, many other explanations are more probable. First, two studies with only modest sample sizes are unlikely to generate identical cytokine results. Second, the cytokine distributions of patients and controls in both studies were heterogeneous and overlapping, even for cytokines having significantly different mean serum levels. This is not unlike what is seen for cytokines in other disorders; cytokine findings in different individuals with the same disorder often vary30–35. Third, the frequency of various GDD symptoms in the two studies differs. For example, all GDD patients in the current study had bone pain and burning skin pain compared to 63% and 58%, respectively, in the prior study13. Fourth, the lengths of time ill and patients’ fasting status at blood draw were not identical in the two study groups. Fifth, in vitro studies,11,12 have found different GBCAs evoke different patterns of cytokine production. The distribution of single and multiple GBCAs administered to the two studies’ patients differs, along with the GBCA order and timing. Unfortunately, the number of GDD patients receiving only one GBCA was too small to provide definitive cytokine results for any given agent. Sixth, some of the significant cytokines in the present study were not included in the 62-plex Luminex kit used in the prior study; and, one significant cytokine in the earlier study, IL-31, is not in the current study’s Luminex kit. Moreover, the two studies utilized healthy normal Flu vaccine serum sets. Seventh, in the GDD patients who had been ill for ≤ 2 years, p-values for the comparison of serum cytokine levels that were elevated compared to the levels in Flu vaccine controls range, for the five most significant cytokines, from p = 0.01 to 0.004 in the earlier study, and from p = 0.0015 to 0.000001 in the present study. The p-values in the two studies for the shared cytokine, GROA, were p = 0.006 and 0.0096. These probability levels argue that chance is not the explanation of the GDD patients’ higher serum cytokine levels.
Whether the physiological functions of the cytokines whose pre-CT serum levels differed in GDD patients vs. normal controls play a role in causing GDD symptoms deserves investigation. CCL15 and CCL27, whose levels were elevated compared to controls, are chemokines that bring inflammation-related cells into areas of inflammation36. TRAIL, the third elevated cytokine, prevents the progression of autoimmune diseases37. The levels of three cytokines were lower in GDD patients compared to controls. SCD40L reduces immunosuppression, so lower levels indicate that less immunosuppression is occurring38. EGF stimulates cellular proliferation, differentiation, and survival in many tissues39, including the brain40. HGF stimulates angiogenesis and tissue regeneration41. Leptin, which was significantly decreased after CT in GDD patients, but unchanged in GSC controls, is associated with pain and fatigue levels in fibromyalgia42,43.
This study’s strengths include blinded judgment regarding pre- or post-CT status of urine Gd results, the inclusion of a comparison group of patients with retained Gd but no GDD symptoms, standardized collection of data regarding the Flare reaction, the standardized time of serum collection before and after CT, absence of medical diagnoses or concomitant medications that could affect serum cytokine levels and blinded determination of cytokine serum levels. The study’s primary limitations include the small GDD patient sample size, the small number of GSC comparison patients, and the absence of detailed data regarding every patients’ GBCAs. Thus, the study must be considered preliminary. Further investigation, however, is warranted.
Future research might look for differences between cytokine results of in vitro studies and studies in vivo after GBCA exposure; cytokine changes in larger samples of GDD subjects and GBCA-exposed, asymptomatic GSC subjects; and potential treatments for GDD and for reducing the Flare reaction. Studies of other mechanisms possibly underlying GDD symptoms, including Gd’s interference with the function of Ca2+ -dependent enzymes44,45, and with mitochondrial energy production,46,47 would also be of great interest.
In summary, our preliminary study showed the following: i) GDD sufferers and GSC patients exhibited no appreciable difference in pre- and post-CT urine Gd levels; ii) Gd retention was observed in patients receiving only a macrocyclic GBCA, confirming observations in an earlier study; iii) all GDD sufferers, but no GSC patients, experienced Flare reactions to CT; iv) cytokine levels differed between GDD patients, and normal Flu vaccine controls pre- and post-CT, raising the possibility of inflammatory, immunological, or other physiological difference in GDD patients; and, v) the decrease in levels of some cytokines post-CT in GDD raises the possibility that immune cell transit from the circulation to tissue sites accompanies Gd extraction.
Supplementary Material
Acknowledgments:
Supported by grant 2U19AI057229 from NIH and contributions from The Avy L. and Roberta L. Miller Foundation.
Footnotes
Conflicts of interest: None
References
- 1.Tedeschi E, Caranci F, Giordano F, et al. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017;122(8):589–600. [DOI] [PubMed] [Google Scholar]
- 2.Harvey HB, Gowda V, Cheng G. Gadolinium deposition disease: A new risk management threat. J Am Coll Radiol. 2020;17(4):546–550. [DOI] [PubMed] [Google Scholar]
- 3.Woolen SA, Shankar PR, Gagnier JJ, et al. Risk of Nephrogenic Systemic Fibrosis in Patients With Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent: A Systematic Review and Meta-analysis. JAMA Intern. Med 2020;180(2):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BJ, Laumann AE, Nardone B, et al. Advancing pharmacovigilance through academic-legal collaboration: the case of gadolinium-based contrast agents and nephrogenic systemic fibrosis-a Research on Adverse Drug Events and Reports (RADAR) report. Br. J. Radiol 2014;87(1042):20140307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince MR. A Breakthrough in Gadolinum-based Contrast Agent Hypersensitivity Reactions. Radiology. 2020;296(2):322–323. [DOI] [PubMed] [Google Scholar]
- 6.Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of Gadolinium in Human Cerebrospinal Fluid after Gadobutrol-enhanced MR Imaging: A Prospective Observational Cohort Study. Radiology. 2018;288(2):416–423. [DOI] [PubMed] [Google Scholar]
- 7.Alkhunizi SM, Fakhoury M, Abou-Kheir W, et al. Gadolinium retention in the central and peripheral nervous system: implications for pain, cognition, and neurogenesis. Radiology. 2020:192645. [DOI] [PubMed] [Google Scholar]
- 8.Semelka RC, Ramalho J, Vakharia A, et al. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magn. Reson. Imaging 2016;34(10):1383–1390. [DOI] [PubMed] [Google Scholar]
- 9.Ramalho J, Ramalho M, Jay M, et al. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016;34(10):1394–1398. [DOI] [PubMed] [Google Scholar]
- 10.Burke LMB, Ramalho M, AlObaidy M, et al. Self-reported gadolinium toxicity: A survey of patients with chronic symptoms. Magn. Reson. Imaging 2016;34(8):1078–1080. [DOI] [PubMed] [Google Scholar]
- 11.Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wermuth PJ, Jimenez SA. Induction of a type I interferon signature in normal human monocytes by gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Clin. Exp. Immunol 2014;175(1):113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maecker HT, Wang W, Rosenberg-Hasson Y, et al. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol. Bras 2020;53(5):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alegría-Torres JA, Pérez-Rodríguez RY, García-Torres L, et al. Exposure to arsenic and lead in children from Salamanca México, effects on telomeric lengthening and mitochondrial DNA. Environ. Sci. Pollut. Res. Int 2020;27(6):6420–6428. [DOI] [PubMed] [Google Scholar]
- 15.Waseem A, Arshad J. A review of Human Biomonitoring studies of trace elements in Pakistan. Chemosphere. 2016;163:153–176. [DOI] [PubMed] [Google Scholar]
- 16.S Herath HMA, Kawakami T, Nagasawa S, et al. Arsenic, cadmium, lead, and chromium in well water, rice, and human urine in Sri Lanka in relation to chronic kidney disease of unknown etiology. J Water Health. 2018;16(2):212–222. [DOI] [PubMed] [Google Scholar]
- 17.Semelka RC, Ramalho M, Jay M, et al. Intravenous Calcium-/Zinc-Diethylene Triamine Penta-Acetic Acid in Patients With Presumed Gadolinium Deposition Disease: A Preliminary Report on 25 Patients. Invest Radiol. 2018;53(6):373–379. [DOI] [PubMed] [Google Scholar]
- 18.Nagy SE, Andersson JP, Andersson UG. Effect of mycophenolate mofetil (RS-61443) on cytokine production: inhibition of superantigen-induced cytokines. Immunopharmacology. 1993;26(1):11–20. [DOI] [PubMed] [Google Scholar]
- 19.Semelka RC, Ramalho M, AlObaidy M, et al. Gadolinium in humans: A family of disorders. AJR Am. J. Roentgenol 2016;207(2):229–233. [DOI] [PubMed] [Google Scholar]
- 20.Tomic A, Tomic I, Dekker CL, et al. The FluPRINT dataset, a multidimensional analysis of the influenza vaccine imprint on the immune system. Sci. Data 2019;6(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breen EJ, Tan W, Khan A. The Statistical Value of Raw Fluorescence Signal in Luminex xMAP Based Multiplex Immunoassays. Sci. Rep 2016;6:26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behzadi AH, Farooq Z, Zhao Y, et al. Dentate Nucleus Signal Intensity Decrease on T1-weighted MR Images after Switching from Gadopentetate Dimeglumine to Gadobutrol. Radiology. 2018;287(3):816–823. [DOI] [PubMed] [Google Scholar]
- 24.Frenzel T, Apte C, Jost G, et al. Quantification and Assessment of the Chemical Form of Residual Gadolinium in the Brain After Repeated Administration of Gadolinium-Based Contrast Agents: Comparative Study in Rats. Invest Radiol. 2017;52(7):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyken J, Frenzel T, Lohrke J, et al. Impact of treatment with chelating agents depends on the stability of administered gbcas: A comparative study in rats. Invest Radiol. 2019;54(2):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newhouse JH, Kho D, Rao QA, et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am. J. Roentgenol 2008;191(2):376–382. [DOI] [PubMed] [Google Scholar]
- 27.Lancelot E Revisiting the Pharmacokinetic Profiles of Gadolinium-Based Contrast Agents: Differences in Long-Term Biodistribution and Excretion. Invest Radiol. 2016;51(11):691–700. [DOI] [PubMed] [Google Scholar]
- 28.Orange DE, Yao V, Sawicka K, et al. RNA identification of PRIME cells predicting rheumatoid arthritis flares. N. Engl. J. Med 2020;383(3):218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents - what is the evidence for “gadolinium deposition disease” and the use of chelation therapy? Clin. Toxicol 2020;58(3):151–160. [DOI] [PubMed] [Google Scholar]
- 30.Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc. Natl. Acad. Sci. USA 2017;114(34):E7150–E7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson JG, Simpson LJ, Ferreira A-M, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima N, Siebert JC, Maecker H, et al. Cytokine expression in Treponema pallidum infection. J. Transl. Med 2019;17(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomares C, Holmes TH, Estran R, et al. Cytokine profiles in patients with toxoplasmic lymphadenitis in the setting of pregnancy. Cytokine. 2016;90:14–20. [DOI] [PubMed] [Google Scholar]
- 34.DiCarlo J, Agarwal-Hashmi R, Shah A, et al. Cytokine and chemokine patterns across 100 days after hematopoietic stem cell transplantation in children. Biol. Blood Marrow Transplant 2014;20(3):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuznetsova T, Haddad F, Knez J, et al. Cytokines profile in hypertensive patients with left ventricular remodeling and dysfunction. J Am Soc Hypertens. 2015;9(12):975–84.e3. [DOI] [PubMed] [Google Scholar]
- 36.Blatt NL, Khaiboullin TI, Lombardi VC, et al. The Skin-Brain Connection Hypothesis, Bringing Together CCL27-Mediated T-Cell Activation in the Skin and Neural Cell Damage in the Adult Brain. Front. Immunol 2016;7:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer U, Voloshanenko O, Willen D, et al. TRAIL: a multifunctional cytokine. Front. Biosci 2007;12:3813–3824. [DOI] [PubMed] [Google Scholar]
- 38.Schlom J, Jochems C, Gulley JL, et al. The role of soluble CD40L in immunosuppression. Oncoimmunology. 2013;2(1):e22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbst RS. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys 2004;59(2 Suppl):21–26. [DOI] [PubMed] [Google Scholar]
- 40.Wong RWC, Guillaud L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 2004;15(2–3):147–156. [DOI] [PubMed] [Google Scholar]
- 41.Fukushima T, Uchiyama S, Tanaka H, et al. Hepatocyte Growth Factor Activator: A Proteinase Linking Tissue Injury with Repair. Int. J. Mol. Sci 2018;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younger J, Kapphahn K, Brennan K, et al. Association of Leptin with Body Pain in Women. J. Womens Health (Larchmt) 2016;25(7):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stringer EA, Baker KS, Carroll IR, et al. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. J. Transl. Med 2013;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Layne KA, Dargan PI, Archer JRH, et al. Gadolinium deposition and the potential for toxicological sequelae - A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol 2018;84(11):2522–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29(3):365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bower DV, Richter JK, von Tengg-Kobligk H, et al. Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent. Invest Radiol. 2019;54(8):453–463. [DOI] [PubMed] [Google Scholar]
- 47.Feng X, Xia Q, Yuan L, et al. Impaired mitochondrial function and oxidative stress in rat cortical neurons: implications for gadolinium-induced neurotoxicity. Neurotoxicology. 2010;31(4):391–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


