Abstract
Purpose
The International Myopia Institute (IMI) Yearly Digest highlights new research considered to be of importance since the publication of the first series of IMI white papers.
Methods
A literature search was conducted for articles on myopia between 2019 and mid-2020 to inform definitions and classifications, experimental models, genetics, interventions, clinical trials, and clinical management. Conference abstracts from key meetings in the same period were also considered.
Results
One thousand articles on myopia have been published between 2019 and mid-2020. Key advances include the use of the definition of premyopia in studies currently under way to test interventions in myopia, new definitions in the field of pathologic myopia, the role of new pharmacologic treatments in experimental models such as intraocular pressure–lowering latanoprost, a large meta-analysis of refractive error identifying 336 new genetic loci, new clinical interventions such as the defocus incorporated multisegment spectacles and combination therapy with low-dose atropine and orthokeratology (OK), normative standards in refractive error, the ethical dilemma of a placebo control group when myopia control treatments are established, reporting the physical metric of myopia reduction versus a percentage reduction, comparison of the risk of pediatric OK wear with risk of vision impairment in myopia, the justification of preventing myopic and axial length increase versus quality of life, and future vision loss.
Conclusions
Large amounts of research in myopia have been published since the IMI 2019 white papers were released. The yearly digest serves to highlight the latest research and advances in myopia.
Keywords: myopia, classification, definitions, high myopia, pathologic myopia, genetics, emmetropization, interventions, atropine, contact lenses, spectacles, orthokeratology, management guidelines, clinical trials, axial length, cycloplegia
The International Myopia Institute (IMI) consensus group was founded in 2015 by the late Professor Brien Holden (BHVI, Sydney) following the joint World Health Organization (WHO) and BHVI Meeting on Myopia held in Sydney. The IMI was formed to facilitate the sharing of evidence-based findings related to the worldwide significant increase of myopia with practitioners, researchers, and policy makers. The initial impetus for the IMI was to develop consensus definitions of myopia, high myopia, and pathologic myopia, as well as recommendations on treatment strategies to prevent myopia onset and to slow myopia progression, especially in low-income settings, and to promote basic and clinical research on myopia.1
The subsequent International Myopia Conference Meeting (IMC) in 2015 further highlighted the need for a consensus group and led to the collaboration between the IMC and IMI, which resulted in the formation of the initial seven task forces that produced the first series of IMI white papers on myopia. Wolffsohn et al.1 details the history of the IMI. This was a truly global collaborative effort, and today the IMI has over 130 experts involved in 13 taskforces. The first white papers were published in Investigative Ophthalmology and Visual Science (IOVS) in 2019, a process chaired by Professors Earl Smith, James Wolffsohn, and Serge Resnikoff and facilitated by Dr. Monica Jong.
Professor Serge Resnikoff accepted the role of chair of IMI in 2018, and since the publication of the first IMI white papers, IMI has pursued its mission to disseminate evidence-based information to advance research, education, and myopia management to prevent future myopia-related vision loss and blindness. Recent key achievements include the IMI definitions2 being referenced at the ICD-11 Revision Technical meeting in 2019, the IMI white papers being referenced in the WHO World Report on Vision, clinical summaries derived from the IMI white papers being translated into 12 languages, dedicated sessions being included at key practitioner and scientific meetings, and five new 2021 IMI white papers published in this special issue of IOVS. Raising awareness for myopia and high myopia as a significant public health issue is an ongoing process involving the collective efforts of researchers, clinicians, industry, policy makers, and various groups that work with children. More voices calling for collective action are necessary to move this area forward and ensure the latest evidence-based practice.
A search of PubMed using the term “myopia” from 2019 to mid-2020 alone yielded almost 1000 peer-reviewed articles. In other words, almost 1000 articles have been published since the first series of IMI white papers (2019). This presents a daunting challenge in trying to keep up with the latest information. The yearly digest is a simple and convenient way for clinicians and researchers to access the recent highlights in myopia. The yearly digests are organized around six of the original seven IMI white papers. The taskforce members producing the digests were involved in the original IMI white papers and have curated between 3 and 10 of the most potentially impactful articles published in their area from 2019 to the mid-2020, with personal insights. Various online databases were searched from 2019 up to mid-2020, and notable conference presentations that featured myopia such as the IMC 2019, Tokyo Medical and Dental University, and the ARVO 2020 virtual meeting were included. The Yearly Digest updates readers in the key advances in myopia in the following sections until the next series of these IMI white papers are published:
-
•
Defining and classifying myopia
-
•
Experimental models of emmetropization and myopia
-
•
Genetics of myopia
-
•
Interventions for controlling myopia onset and progression
-
•
Clinical myopia control trials and instrumentation
-
•
Clinical management guidelines for myopia
IMI Digest 2021—Defining and Classifying Myopia
The IMI “Defining and Classifying Myopia” white paper proposed definitions for myopia, high myopia, and pathologic myopia based on statistical analysis of thresholds used in the literature and clinical relevance (Table 1 and Table 2).2 Previously, over 400 definitions had been used in the literature, and many different cutoffs for myopia and high myopia had been suggested. This caused some confusion for differentiating various grades of myopia, in particular for the delineation of high myopia and pathologic myopia.2,3 Standardizing definitions will ease evidence-based management of myopia and improve comparability of research outcomes. Since the publication of the IMI white paper on the myopia definitions, several studies have been published and selected here for commentary. These studies further highlight the need for standardization of the myopia definitions and show that definitions may be selected based on the relevant population and what is being evaluated as an outcome. In addition to defining thresholds for myopia and pathologic myopia as a structural and not a refractive concept, a range of other terms were defined such as premyopia, secondary myopia, myopic traction maculopathy (MTM), and myopia-associated glaucoma-like optic neuropathy (MAGON) (further discussed in the IMI 2021 white paper: “IMI Pathologic Myopia”4). These terms are starting to become more widely used and are further discussed below.
Table 1.
Summary of Proposed General and Quantitative Thresholds for Myopia Adapted From IMI Defining and Classifying Myopia White Paper2
| Term | Definition |
|---|---|
| Qualitative definitions | |
| Myopia | A refractive error in which rays of light entering the eye parallel to the optic axis are brought to a focus in front of the retina when ocular accommodation is relaxed. This usually results from the eyeball being too long from front to back but can be caused by an overly curved cornea or a lens with increased optical power, or both. It is also called near sightedness. |
| Axial myopia | A myopic refractive state primarily resulting from a greater than normal axial length. |
| Refractive myopia | A myopic refractive state that can be attributed to changes in the structure or location of the image forming structures of the eye (i.e., the cornea and lens). |
| Secondary myopia | A myopic refractive state for which a single, specific cause (e.g., drug, corneal disease, or systemic clinical syndrome) can be identified that is not a recognized population risk factor for myopia development. |
| Quantitative definitions | |
| Myopia | A condition in which the spherical equivalent refractive error of an eye is ≤ –0.50 D when ocular accommodation is relaxed. |
| Low myopia | A condition in which the spherical equivalent refractive error of an eye is ≤ –0.50 D and > –6.00 D when ocular accommodation is relaxed. |
| High myopia | A condition in which the spherical equivalent refractive error of an eye is ≤ –6.00 D when ocular accommodation is relaxed. |
| Premyopia | A refractive state of an eye of ≤ +0.75 D and > –0.50 D in children where a combination of baseline refraction, age, and other quantifiable risk factors provide a sufficient likelihood of the future development of myopia to merit preventative interventions. |
Table 2.
| Term | Definition |
|---|---|
| Descriptive definitions | |
| Pathologic myopia | Excessive axial elongation associated with myopia that leads to structural changes in the posterior segment of the eye (including posterior staphyloma, myopic maculopathy, and high myopia-associated optic neuropathy) and that can lead to loss of best-corrected visual acuity. |
| Myopic macular degeneration (MMD) | A vision-threatening condition occurring in people with myopia, usually high myopia that comprises diffuse or patchy macular atrophy with or without lacquer cracks, macular Bruch´s membrane defects, choroidal neovascularization, and Fuchs spot. |
| Diagnostic subdivisions of MMD | |
| Myopic maculopathy | Category 0: no myopic retinal degenerative lesion. |
| Category 1: tessellated fundus. | |
| Category 2: diffuse chorioretinal atrophy. | |
| Category 3: patchy chorioretinal atrophy. | |
| Category 4: macular atrophy. | |
| “Plus” features: lacquer cracks, myopic choroidal neovascularization, and Fuchs spot. | |
| Presumed myopic macular degeneration | A person who has vision impairment and vision acuity that is not improved by pinhole, which cannot be attributed to other causes, and |
| The direct ophthalmoscopy records a supplementary lens > ‒5.00 D and shows changes such as “patchy atrophy” in the retina or | |
| The direct ophthalmoscopy records a supplementary lens > ‒10.00 D. | |
| Specific clinical conditions characteristic of pathologic myopia | |
| Myopic traction maculopathy (MTM) | A combination of macular retinoschisis, lamellar macula hole, and/or foveal RD (FRD) in highly myopic eyes attributable to traction forces arising from adherent vitreous cortex, epiretinal membrane, internal limiting membrane, retinal vessels, and posterior staphyloma. |
| Myopia-associated glaucoma-like optic neuropathy | Optic neuropathy characterized by a loss of neuroretinal rim and enlargement of the optic cup, occurring in highly myopic eyes with a secondary macrodisc or parapapillary delta zone at a normal intraocular pressure. |
Myopia Thresholds
In relation to the standardization of refractive thresholds, several articles have explicitly cited the proposed standards.5,6 The need for standardization of definitions is highlighted by the continued publication of papers with varying definitions for high myopia.7 For example, a recent refractive surgery study defined a threshold for high myopia at –9.00 D.8 This paper, like many others, also highlighted the issue of the inconsistent use of the mathematical symbols for “less than” in relation to myopia, in that extremely high myopia was described as ≥ –9.00 D, but the context indicates that this was intended to mean more myopic than –9 D, rather than a refractive error ≥ –9.00 D. Another recent paper defined high myopia as “as the presence of a highly negative refractive error (>−6.00 to −8.00 D),” again using the mathematical symbol “>” to mean “more myopic” when mathematically, it should be written as “<” to indicate a more negative value and more myopia.9 The IMI definitions and classifications white paper2 proposed that mathematical symbols should be used in a strict mathematical sense for consistency and that words be used where potential ambiguity arises (e.g., “more than 6.00 D of myopia” or “more myopic2 than –6.00 D”).
Achieving complete consistency in reporting refractive errors is unlikely to be achieved in the short term. One suggestion in the IMI white paper2 for reporting standards for myopia studies was that when different thresholds are used to better suit the research question, a sensitivity analysis should be performed at the chosen and standardized thresholds (i.e., spherical equivalent refraction ≤ –0.5 for myopia and ≤ –6.0 D for high myopia). This would be invaluable when it comes to comparing studies or performing meta-analyses. The value of this approach has been demonstrated in a paper published prior to the IMI report10 and supported in a paper published in 2020.11 The cutoff values for myopia have a strong effect on the estimated prevalence of myopia and high myopia in population-based studies. For example, in the study by Parssinen and Kauppinen,12 defining high myopia as a spherical equivalent refraction < –6.00 D in the right eye or by a spherical equivalent of ≤ –6.00 D or ≤ –5.00 D in either eye was associated with a myopia prevalence of 24%, 32% and 52%, respectively.
The recent publication of normative standards for refractive errors is also a step forward. San-Diez et al.13 reported axial length growth curves for Chinese children for estimating the risk of myopia, based on age, gender, the axial length/corneal radius ratio, and spherical refractive error. The growth curves were based on a data set from 12,554 children aged 5 to 16 years from Wuhan, China. Compared with the axial length growth curves for European children of the same age,13b the Chinese children had significantly longer axial lengths. San-Diez and colleagues13 used a threshold of −5.00 D, but this study was initiated prior to the publication of the IMI white paper.2 The definition of centile-based standards to refraction offers a rational basis for an age-adjusted definition of high myopia in children and adolescents, in whom the development of high myopia in adulthood is likely or very likely. Refraction reference curves in European children have also been published but were noncycloplegic.14 More longitudinal studies will be needed to validate these approaches.
In the IMI definitions white paper,2 the proposed definition of myopia did not stipulate cycloplegia as a requirement but included the caveat “when ocular accommodation is relaxed” (Table 1). This was intentional to avoid potentially invalidating many epidemiologic studies in adults. Cycloplegia remains the gold standard in studies of refractive error in children,15 but in some settings, for example, school screenings, cycloplegia can be impractical, but the data are still useful (covered in detail in the “IMI Clinical Myopia Control Trials and Instrumentation” white paper).16 From a functional point of view, the various degrees of myopia may be defined by the uncorrected visual acuity of the respective refractive error. Introducing unaided distance visual acuity criteria could be useful to avoid overdiagnosing myopia due to instrument myopia when cycloplegia is not used.17
Other Definitions: Premyopia and Secondary Myopia
The concept of premyopia has emerged over the past few years, and the IMI proposed a practical definition of this concept (Table 1). Several clinical trials, notably the ATOM3 study from Singapore (NCT03140358, https://clinicaltrials.gov/ct2/show/NCT03140358), are currently recruiting premyopes to test whether atropine can delay or prevent myopia onset. Premyopia is now also being discussed in the context of a comprehensive approach to myopia progression management.18 Secondary myopia is a potentially useful concept but remains rarely used in this field, despite being a widely adopted term in other conditions (e.g., glaucoma). It has appeared recently in several publications as an exclusion criterion19,20 and in a review on the perennial “nature versus nurture” question.21
Pathologic Myopia
In relation to terminology and definitions, the area that remains in greatest flux is the concept of pathologic myopic. New classifications are still being proposed, and it is likely to be some time until a clear consensus emerges in this area.9 Rather than a refractive definition, the concept of pathologic myopia was defined in purely structural terms as a set of complications that arise from high myopia with increasing age.2 The understanding of structural complications and their visual implications continues to evolve.22 Several recent papers provide excellent data on the risk of such complications and the impact on vision.23,24 Although high myopia–related ocular complications are recognized to increase with age, particularly after age 50 years, these complications are now also being recognized in highly myopic children.25 The continual refinement of optical coherence tomography (OCT) will undoubtedly contribute to improving the understanding of pathologic myopia, and one specific OCT-based definition—myopic traction maculopathy (MTM), which is becoming more widely used. The clinical characteristics of MTM are becoming well defined,26 and a staging system has now been proposed.27 The impact of high myopia on optic nerve structure and function, as encapsulated in the term myopia associated glaucomatous optic neuropathy (MAGON) is also a topic of current research and interest.28 For a detailed discussion, please refer to the IMI 2021 white paper “IMI Pathologic Myopia.”4
Conclusion
Standardization of myopia thresholds and definitions is improving, but much work remains to be done on this topic. Meta-analyses are now a very important aspect of myopia research, and use of consistent standards will enhance the potential of this powerful statistical approach. The field of pathologic myopia is rapidly evolving, and new classifications are likely to emerge over the coming years, led by both growing clinical interest in the topic and advances in imaging technology.
IMI Digest 2021—Experimental Models of Emmetropization and Myopia
The discovery of the phenomenon of form-deprivation myopia in 1977 ushered in the modern era of animal research on refractive development.30 The 2019 IMI white paper on experimental models of emmetropization and myopia31 reviewed the significant progress that was achieved via research involving laboratory animals over the following 40+ years. Since then and through 2020, research involving animal models has continued to expand and to provide new and critical insights into factors that influence ocular growth and refractive development and that contribute to the genesis of common refractive errors like myopia. This digest highlights some of the high-interest, high-impact papers that have been published during the intervening period. The highlighted papers were selected based on a survey of the authors of the 2019 IMI experimental models paper.32 The accompanying reference list includes papers that were published since submission of the experimental models paper, specifically 3 more recent reviews,32–34 3 papers that involved nonhuman primates,35–37 3 that employed tree shrews,38–40 14 that used chickens,41–54 19 that involved guinea pigs55–72 and 22 that utilized mice.73–94 While the chicken continues to be a mainstay in experimental myopia research, the number of publications using guinea pigs and mice has increased, particularly in the latter, to take advantage of the genetic manipulations that are possible in the mouse.
Intraocular Pressure and Myopia Progression
El-Nimri NW, Wildsoet CF. Effects of topical latanoprost on intraocular pressure and myopia progression in young guinea pigs. Invest Ophthalmol Vis Sci. 2018;59:2644–2651.66
Intraocular pressure (IOP) has been hypothesized to be a contributing factor to myopia progression, primarily because the biomechanically weaker scleras in myopic eyes would be more susceptible to the stretching influence of IOP. If this were true, treatment strategies that lower IOP should slow axial myopic progression. In this respect, clinical trials using the β-blocker, timolol, the only ocular hypotensive drug to be clinically tested, have yielded inconsistent results. Similarly, timolol failed to reduce the degree of form-deprivation myopia in chickens, possibly because β-blockers have minimal effects on IOP at night when myopic growth appears to dominate.
El-Nimri and Wildsoet66 demonstrated that topical latanoprost, a prostaglandin analogue, was effective in reducing IOP over 24 hours in guinea pigs, and more importantly, this commonly used ocular hypotensive drug was effective in blocking the axial myopia produced by form deprivation. In addition, they observed that latanoprost normalized the diurnal IOP rhythms in deprived eyes and that the observed reductions in myopic axial elongation were correlated with the magnitude of the IOP reductions, supporting a biomechanical explanation for the myopia control effects. This investigation is in agreement with a recent study by Liu et al.,95 who showed that the α-adrenoreceptor agonist, brimonidine, which is a different class of ocular hypotensive from latanoprost, was also effective in reducing IOP and defocus-induced myopia in guinea pigs.
At present, alternative IOP-independent explanations for the actions of these drugs cannot be ruled out. Further investigations into underlying site and mechanisms of action for the myopia control effects for both latanoprost and brimonidine are needed. Nevertheless, the results of these studies are exciting because they indicate that well-tolerated, topically administered, ocular hypotensive drugs may provide a qualitatively new line of myopia control therapy.
The Role of ON and OFF Channels in Refractive Development
Wang M, Aleman A, Schaeffel F. Probing the potency of artificial dynamic ON or OFF stimuli to inhibit myopia development. Invest Ophthalmol Vis Sci. 2019;60:2599–2611.54
The ON and OFF channels originate at the sign-inverting and sign-conserving synapses between the photoreceptors and the ON and OFF bipolar cells, respectively. These channels remain largely separate through the retina, the lateral geniculate nucleus, and the early stages of cortical processing. Evidence associated with genetic mutations, pharmaceutical interventions, and manipulations of the temporal luminance profiles of ambient lighting indicates that selective interruptions/adaptations of the ON and OFF retinal channels can have qualitatively different effects on normal emmetropization and vision-induced alterations in refractive development.
In this paper, the most recent in a series of papers on this topic from the Schaeffel lab, the authors investigated the effects of dynamic ON or OFF visual stimuli on choroidal thickness (CT), a predictive indicator of the direction of refractive development. In addition, they investigated the associated changes in retinal dopamine release, a key element in the signal cascade that regulates ocular growth that is dependent on ON-pathway activity. The primary findings were that in both humans and chickens, ON stimuli produced choroidal thickening, whereas OFF stimuli caused choroidal thinning. In chickens, ON stimulation also elevated dopamine release when compared to OFF stimulation. An unexpected finding in chickens was that over longer treatment periods, both ON and OFF stimulation were associated with increased myopia in response to imposed hyperopic defocus, even though relative to OFF stimulation, ON stimulation increased dopamine, a known inhibitor of myopia.
This study is noteworthy because the results show that dynamic ON and OFF local luminance stimuli have qualitatively similar effects on CT in humans and chickens. The fact that dopamine release also varied in a bidirectional manner supports the hypothesis that dopamine is involved in the CT changes produced by ON and OFF stimuli. Significantly, the direction of changes in CT produced by these dynamic luminance stimuli agreed with previous findings from the Schaeffel lab obtained in participants viewing texts of different contrast polarities. It will be important to determine the time course for the effects of these dynamic ON and OFF stimuli because it may be possible to manipulate signals in the ON or OFF pathways in ways that are not dependent on retinal focus but that can selectively alter axial elongation rates and the progression of myopia.
Circadian Clocks and Refractive Development
Stone, RA, McGlinn AM, Chakraborty R, et al. Altered ocular parameters from circadian clock gene disruptions. PLoS One. 2019;14(6):e0217111.87
Many ocular processes show diurnal oscillations that are relevant to ocular growth and refractive development. In growing eyes, axial length and choroidal thickness exhibit circadian rhythms that are altered by visual conditions that are known to interfere with normal refractive development. In animals with experimentally induced myopia, diurnal rhythms in retinal dopamine turnover and the expression of circadian rhythm–related genes are altered. The fact that manipulations of the intensity and diurnal cycle of ambient lighting, key factors in coordinating diurnal rhythms, also alter refractive development suggests that ocular circadian rhythms play a role in emmetropization and the development of common refractive errors.
In this intriguing paper, the authors investigated the effects of disrupting clock genes on optical development in 2 very phylogenetically different species, the mouse and the fly (Drosophila melanogaster). In comparison to littermate control mice (Bmal1fl/fl), retinal specific knockouts for Bmal1, an essential component of the circadian clock, exhibited myopic refractive errors that, like common myopia in children, were associated with increases in vitreous chamber depth. In Drosophila, knockouts of either the cycle or period genes in the circadian clock resulted in an elongation of the fluid-filled pseudocones of the ommatidia, an optical component considered analogous to the vitreous chamber in the camera eyes of vertebrates. Thus, it appears that circadian clocks influence the pathways that regulate ocular development in both of these widely separated species.
It is known that the basic molecular mechanisms for circadian rhythms are conserved from Drosophila to mammals. This study demonstrates that at least some aspects of these mechanisms that play a role in regulating optical development have also been conserved across species and that the maintenance of normal ocular diurnal rhythms is fundamental to normal emmetropization. This study provides further evidence that genetic and environmental factors that influence diurnal rhythms can produce alterations in optical development of the eye that are similar in nature to those associated with common refractive errors like myopia. These findings support the idea that it may be possible to manipulate ambient lighting and societal behaviors that potentially impact circadian rhythms in ways that reduce the burden of myopia.
Contribution of Cone Pathway Signaling in Form-Deprivation Myopia
Chakraborty R, Yang V, Park HN, et al. Lack of cone mediated retinal function increases susceptibility to form-deprivation myopia in mice. Exp Eye Res. 2019;180:226–230.73
Elegant experiments in animal models using partial diffusers have demonstrated that refractive eye growth depends on detection of visual input by the retina.96,97 However, the retinal signaling pathways that control refractive development and myopic eye growth remain elusive. Rod and cone photoreceptor pathways are fundamental to light detection across a large range of luminance. While there is experimental evidence that both rods and cones contribute to normal refractive development,98,99 cones have been thought to be more influential due to their roles in high acuity and color vision.
Chakraborty et al.73 set out to investigate the role of cone photoreceptor signaling in normal refractive development and form deprivation myopia by using a mouse model with a gene mutation in Gnat2, the α-subunit of cone transducin. The Gnat2–/– mice have loss of cone function with normal rod function. The authors found that the loss of cone function did not influence normal refractive development in mice. However, the Gnat2–/– mice were more susceptible to form deprivation myopia, showing a ∼65% increase in myopic shift compared to wild-type control mice after 3 weeks of goggling.
These results suggest that the retinal signaling for normal emmetropization and the response to form deprivation may not be the same. Cones were not essential for normal refractive development but significantly influenced the susceptibility to form deprivation. Interestingly, these authors have previously reported on the response of a mouse with loss of rod function, Gnat1–/–, which showed opposite effects.98b The absence of rod signaling results in a “flat” refractive development curve and unresponsiveness to form-deprivation myopia. Thus, rods and cones appear to play different roles in refractive eye growth: cones may modulate the response to form deprivation, while rods may be required to respond to form deprivation.
Importantly, these experiments take advantage of the mouse model of myopia in which genetic and environmental factors can be altered. The ability to genetically modify specific ocular cell types or pathways of interest is unique to the mouse model and provides an opportunity to examine how a given mutation alters normal refractive development across age as well as the response to myogenic stimuli. Such an approach is expected to offer new insights into the mechanisms controlling refractive eye growth and eventually to lead to new treatment targets.
Genes, Environment, and Interactions Drive Refractive Development
Tkatchenko TV, Shah RL, Nagasaki T, Tkatchenko AV. Analysis of genetic networks regulating refractive eye development in collaborative cross progenitor strain mice reveals new genes and pathways underlying human myopia. BMC Med Genomics. 2019;12:113.91
The increase in the prevalence of myopia is attributed to both environmental and genetic factors as well as the interplay between them. While the global rise of myopia is happening too rapidly to be solely caused by genetic factors, it is well documented that genetics play a role in the risk of developing hyperopia and myopia. As Dr. Judith Stern famously said, “Genetics loads the gun but the environment pulls the trigger,”99b succinctly illustrating the idea that the interactions between genes and the environment are responsible for driving the progression of many complex diseases, such as hyperopia and myopia.
In this novel experiment, Tkatchenko et al.91 evaluated how genetic backgrounds of different mouse strains interact with the visual environment to affect refractive error. By tracking the refractive errors of 8 genetically different mouse strains, they uncovered differences in refractive development between strains placed in normal and myopigenic environments. To further understand why some mouse strains are more susceptible to myopia than others, Tkatchenko et al.91 performed RNA sequencing on the retinas from these strains to evaluate differential gene expression. In doing so, they identified thousands of genes and multiple pathways responsible for normal refractive development in mice, many of which overlap with findings in human studies. Intriguingly, they found that while many genes were associated with both normal refractive development and myopia susceptibility, they tended to act in opposite directions.
The integration of both phenotypic measures and transcriptomic data in this study provides a powerful approach to understanding the molecular underpinnings of refractive error development. Additionally, these findings are likely relevant for human myopia, as many of the identified biological pathways and genes are conserved across species. Overall, this study identified well-defined retinal signaling pathways that may be responsible for driving ocular growth in response to the visual environment, which could guide approaches in drug development and treatment (also see the recent review by Tkatchenko and Tkatchenko33).
IMI Digest 2021—Genetics of Myopia
Introduction
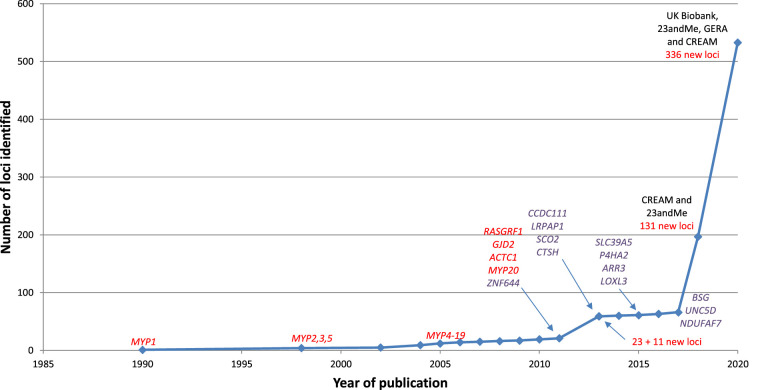
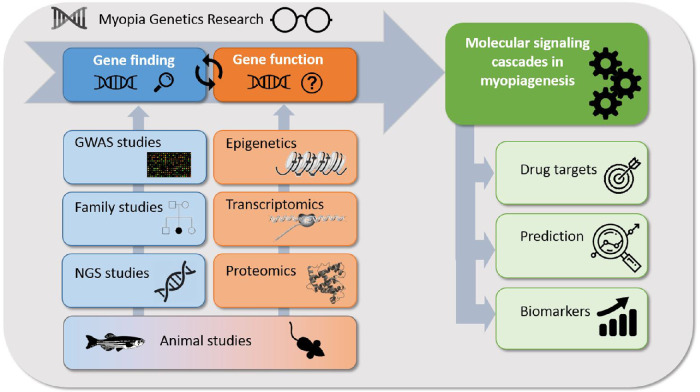
Myopia is a complex disorder in which both genetic influences and environmental factors play a role. Since the first genome-wide association study (GWAS) in 2010, the number of loci associated with refractive error has increased dramatically due to larger samples sizes. Recently, over 500 loci have been associated with refractive error (Fig. 1). The genes residing within these loci are involved in a variety of pathways, including light-processing pathways, retinal cell physiology, glutamate receptor signaling, circadian rhythm regulation, dopamine pathway, and extracellular matrix organization. Expression analyses of identified genes have implicated a role for almost all ocular cell types in the pathogenesis of myopia. While more and more loci have been associated with myopia, the exact mechanisms by which they confer susceptibility to this trait are still largely unknown. Furthermore, most of the heritability of refractive error (defined as the proportion of interindividual variation in the trait contributed by genetics) remains unexplained. Some of the missing heritability might be explained by gene–gene and gene–environment interaction, but a significant fraction of causally related genes for refractive error has yet to be discovered. There have been some key updates to the field of myopia genetics since the publication of the IMI white paper on myopia genetics.100 The PubMed database was searched using various MeSH terms (e.g., “myopia/genetics,” “refractive errors/genetics,” “genetic predisposition to disease”) to identify articles published between February 2019 and April 2020. Most of these articles focused on gene-discovery in (pathologic) myopia101–104 the genetic overlap with myopia-related complications,105,106,126 epigenetics,78,85,107,108 transcriptomics,109–113 proteomics113,114,120 and the interaction between environmental factors and genetics.115,116 The key advances are highlighted below in Figure 2.
Figure 1.
Historic overview of myopia gene finding from 1990 to 2020. Genes identified using whole exome sequencing (WES) are marked in purple. Other loci (linkage studies, GWAS) are marked in red. The cohorts used in the GWAS studies are indicated in black.
Figure 2.
Overview of myopia genetics research.
Commentary
Gene Finding
Perhaps the most important breakthrough in the past 12 months was the publication by Hysi et al.101 of the largest GWAS meta-analysis of refractive error to date, which included over 500,000 participants of European ancestry. This study combined data from the UK Biobank, the consumer genomics company 23andMe Inc., and the Genetic Epidemiology Research on Adult Health and Aging study for discovery, along with data from the CREAM consortium for replication. The authors also carried out a meta-analysis including all studies; this meta-analysis identified 449 loci, of which 336 represented new genetic loci.
This work confirmed that refractive error is genetically an extremely heterogeneous disorder involving many different processes, genes, and ocular tissues. Among the newly identified genes are genes regulating circadian rhythm, genes with known roles in corneal dystrophies, cataract and retinal dystrophies, genes in the Wnt signaling pathway, and genes with prominent effects on skin, hair, and eye pigmentation. Two major sets of mechanisms were proposed by the authors: first, those affecting ocular structure, development, and physiology, including IOP, and second, central nervous system–related genes, such as those with effects on retinal signaling pathways.
In addition to gene discovery, the authors assessed whether the newly identified genetic loci could be used to detect individuals at risk of developing high myopia who could possibly benefit from intervention (e.g., at-risk individuals could be targeted for early intervention with atropine eye drops or dual-focus lenses designed to slow myopia progression).117 A polygenic risk score (PRS) for refractive error had an area under the receiver operating characteristics curve (AUC) of 0.75 for predicting high myopia (defined as spherical equivalent [SER] < –5.00 D), which compares favorably with previous methods for predicting myopia based on cycloplegic refraction in young children.118 Another genetic study similarly derived a PRS for refractive error that attained an AUC of 0.75 for predicting high myopia and found that children with a PRS in the top 10% were at 6-fold higher risk of high myopia than those in the remaining 90%.119 This latter study was based on a much smaller sample than Hysi et al.,111 suggesting that there is hope for even greater accuracy in genetic prediction of myopia in the future. The genetic loci associated with myopia have relatively small effect sizes, so identifying more loci will not necessarily improve prediction of common myopia. Nevertheless, discovering even greater numbers of genes associated with refractive error development by enlarging GWAS sample sizes and improving imputation quality and usage of alternative genetic techniques will help to further elucidate the pathogenesis of myopia.
Family Studies
One alternative technique to identify genes, and highly penetrant rare variants in particular, is to use family-based linkage studies, since any one particular causal rare variant is more likely to be observed in multiple affected individuals within a highly aggregated family than to be observed in a sample of unrelated affected individuals. Linkage analysis takes advantage of long haplotypes shared by related affected individuals and has good power to detect high-penetrance variants in large pedigrees with multiple affected individuals. Two new studies have used this approach in families from 2 founder populations in the United States, the Pennsylvania Amish102 and an orthodox Ashkenazi Jewish community.103 In both studies, pedigrees with a strong family history of common myopia were enrolled, and exome-based microarray genotyping was performed. Participants underwent extensive eye examinations; myopia was defined as a mean spherical equivalent ≤ –1 D. An autosomal dominant model with a rare disease allele was assumed in the 2-point parametric linkage analyses. In the Amish families, genome-wide significant linkages to myopia were identified at 12q15 and 8q21.3 across all Amish families, centered on the genes PTPRB and CNGB3. PTPRB (a protein tyrosine phosphatase) has not been previously linked to eye disease, although other protein tyrosine phosphatases have been implicated in myopia. CNGB3 is expressed in cones, and a genetic defect in this gene underlies achromatopsia. Further, 3 genome-wide significant linked variants were also found within a single Amish family. These variants were all located in the gene SLC618, which would be novel for eye disease. In the Ashkenazi families, genome-wide significant linkage signals, not previously associated with eye disease, were observed on 7q36.1 and 8q24, centered on the genes SSPO and WISP1. This data set also replicated a previously published linkage on 1p36.1. Since linkage peaks are broad, the genes with the strongest evidence for linkage above may not be causal. Genome-wide sequencing of the most informative families in these data will help identify the causal genes and variants.
Epigenetics
Individuals who develop myopia early in life have a higher likelihood of progressing to high myopia. This may be caused by myopia genes with expression and effect early in life. Another explanation of this effect might be related to epigenetic changes due to influences in utero. Furthermore, epigenetic changes might influence myopia development in childhood or even between generations. Only a few studies have focused on epigenetic changes—those possibly influenced by early environmental factors—in myopia development.
An epigenome-wide association study by Seow et al.85 aimed to find CpG methylation sites in umbilical cord tissue associated with early onset myopia. A study population of 519 Malay, Indian, and Chinese children with available umbilical cord blood and SER at 3 years was included for analysis (29 cases [SER < –0.50 D] and 490 nonmyopic controls). Epigenetic signals were evaluated at 160,418 separate loci after adjusting for ethnicity, sex, gestational age, cellular composition, and batch effects. Five CpG probes (cg21880079, cg14066632, cg03155767, cg17154092, cg26299044) showed evidence of association with myopia; all were hypomethylated in myopia cases compared to controls (2.81% to 4.49% methylation change in cases compared to controls). Adjusting for parental myopia and smoking did not materially change the results. One of the 5 sites was located in a known myopia locus (MYP10) and 4 of the probes could be annotated to genes (ARL1, FGB, PQLC1, KRT12). ARL1, PQLC1, and KRT12 showed expression in both fetal and adult human ocular tissue, whereas the FGB gene showed significant scleral expression in ocular mouse tissue. The authors considered that all 4 genes might be involved in corneal epithelium development and membrane transport. Umbilical cord blood was used to investigate the methylation profile. Therefore, these results may help in predicting early onset myopia but may not fully reflect biological changes in ocular tissue. The authors, however, suggest that the biological changes seen in early onset myopia in very young children are already reflected in umbilical cord tissue at birth. In addition, differentially methylated CpG sites may be used as biomarkers to predict high myopia in children. Replication in a larger data set and examination of the correlation between umbilical cord tissue methylation and eye tissue are warranted to strengthen the evidence.
A second epigenetic study by Williams et al.107 examined DNA methylation at approximately 450,000 CpG sites across the genome of 921 children of European ancestry from the United Kingdom (ALSPAC study), using cord blood samples collected at birth and peripheral blood samples collected at age 7 and 15 years. The capacity of these 450,000 epigenetic probes to predict myopia at age 7 and 15 years was assessed using 10-fold cross-validation. The AUC for predicting myopia at age 7 years was significantly better than at age 15 years (P = 0.001), with an AUC in the range of 0.60 to 0.64. Detailed analysis of 9 preselected genes (APLP2, RASGRF1, GJD2, ZMAT4, LAMA2, RBFOX1, TSPAN10, DRD1, CASC15) previously implicated as being imprinted or involved in gene–environment interactions were tested for enriched CpG sites whose methylation level was associated with myopia at age 7 or 15 years. This revealed an association of myopia at age 7 years with DNA methylation at CpG site cg13403566 near RASGRF1 (uncorrected P = 6.4 × 10−5; Bonferroni-corrected P = 0.025). The authors demonstrate stronger links of early epigenetic marks with myopia at age 7 years rather than age 15 years in the context of an intriguing observation that the prevalence of myopia at age 7 years (but not 15 years) was lower if the paternal grandmother had smoked in pregnancy. This association was primarily found among grandsons compared to granddaughters. Smoking is one of the best-known environmental exposures affecting epigenetic profiles, and several examples of adverse or adaptive “transgenerational” inheritance carried by epigenetic marks have been documented in humans and animal models.107
Vishweswaraiah et al.108 studied epigenetic differences in 18 children aged 4 to 12 years with a high degree of myopia (SER ≤ –6 D) and 18 healthy controls from 1 Polish center. The authors identified 1541 CpG sites in 1745 unique genes with a 2-fold or higher differential methylation in the high-myopia cases compared to controls. Methylation was assessed using the EPIC array (with some 850,000 CpG) probes from peripheral blood. The study of more extreme cases may be a powerful approach, but on the other hand, the small sample size means that firm conclusions cannot be made and replication in much larger samples is needed. Epigenetic mechanisms can also be explored using animal models, which enable use of the relevant tissues.
Liang et al.78 successfully used this strategy; their study was based on the hypothesis that HOXA9 plays a role in myopia development similarly to other established homeobox genes associated with myopia (PAX6 and MEIS1). Moreover, HOXA9 is known to transcriptionally activate the well-known myopia-associated transforming growth factor β (TGF-β) signaling cascade. In the Growing Up in Singapore Towards Healthy Outcomes birth cohort study, 8 preschool children represented refractive error outliers (SER < –2 D), and 7 of them had hypomethylation at HOXA9, suggesting that overexpression of HOXA9 could be a risk factor for early onset myopia. The researchers then performed animal studies, measuring HOXA9 RNA levels in the retina of form deprivation myopia (FDM) mice (n = 9) by real-time polymerase chain reaction (PCR). These levels in the retina of myopic eyes were significantly higher (P = 0.029, paired t-test) than of the uncovered fellow eye. Lastly, the cellular studies based on murine retinal pigment epithelium (RPE) cells demonstrated that an increase of HOXA9 could increase expression in several myopia-associated genes, including TGF-β, FGF2, IGF1R, and MMP2. The authors concluded that since HOXA9 is a transcription factor, it may directly or indirectly affect expression of myopia-associated genes. Albeit an elegant approach, there remains the need for replication, a larger sample size, analyses on human eye–related tissue, and bulk testing of multiple genes.
Transcriptomics (MicroRNAs)
Regulatory mechanisms have been implicated in myopiagenesis.121 While there are many regulatory elements, such as enhancer RNAs, long noncoding RNAs, and microRNAs (miRNAs), most studies focused on miRNAs for their potential therapeutic role or as a clinical biomarker. Several studies have performed miRNA next-generation sequencing and quantitative PCR (qPCR) on human aqueous humor derived at onset of cataract surgery or refractive surgery between highly myopic versus nonmyopic eyes. Zhu et al. detected differential expression between 249 mature miRNAs and 17 novel miRNAs in myopic eyes (age range 19–67 years) compared to control eyes (age range 55–89 years). The authors postulated that the TNF, MAPK, PI3K-Akt, and HIF-1 signaling pathways might be regulated by these miRNAs. A subset of miRNAs was confirmed by qPCR (hsa(homo sapiens)-let-7i-5p, hsa-miR-127-3p, and hsa-miR-98-5p).109 Chen et al.110 focused on exosomal miRNA profiles in aqueous humor and their role in myopia development (n = 16 patients; 8 myopia (age range 57–71 years) and 8 control (age range 57–87 years). These exosomal miRNAs are likely involved in the pathogenesis of various eye diseases. The researchers found that the numbers and sizes of exosomes were not significantly different between the myopia and control group. The individual exosomes of the same group were pooled to purify RNA. Unexpectedly, the myopia group contained 2.78-fold more RNA than that in the control group: 15 miRNAs were myopia specific, and 4 miRNAs were absent in the myopia group. Six well-known myopia-associated genes (CHRM2, CNGB3, VEGFA, ADORA2A, IGF1 and LUM) were identified as potential targets for 5 myopia-specific miRNAs (hsa-miR-582-3p, hsa-miR-17-5p, hsa-miR-885-3p, hsa-miR-19b-3p, and hsa-miR-450b-5p).
Two reports on the novel topic of miRNA expression in low-dose atropine treatment (0.003%) against myopia development were published.111,112 Both studies focused on gaining more insight into the molecular mechanism of atropine on myopia treatment and defending the safety of low-dose atropine treatment, using either human scleral fibroblasts112 or human corneal epithelial cells.111 Hsiao et al.112 identified slight changes in scleral gene expression after 0.003% atropine treatment, supporting the safety of low-dose atropine treatment. This study revealed the association of hsa-miR-2682-5p-KCNJ5 and hsa-miR-2682-5p-PRLR with scleral growth repression and circadian rhythm. Chang et al.111 analyzed the messenger RNA (mRNA) and miRNA expression profiles between atropine-treated and control corneal epithelial cells. They found that low-dose (0.003%) atropine was associated with dysregulation of certain genes. Several bioinformatics tools predicted that this dysregulation might suppress the apoptosis of the corneal epithelial cells, potentially through Ras and protein kinase A signaling pathways. Hsa-miR-651-3p-EPHA7, hsa-miR-3148-TMEM108, and hsa-miR-874-5p-TBX6 were validated as possible miRNA regulators of mRNA dysregulation (i.e., miRNA–mRNA interaction) involved in corneal epithelial cells treated with 0.003%. Although the findings of these studies give more insight into the molecular mechanisms of atropine treatment, the results should be replicated using larger studies and similar human eye–related tissues. Moreover, studies should include the entire range of doses in atropine treatment, since there is a clear dose–effect relationship between atropine and axial elongation.122,123
Proteomics
One important question is how differences in genetic background, miRNA expression, and methylation translate to changes in protein expression and how this relates to the development of myopia and pathologic myopia. Currently, most studies focusing on proteomics followed a candidate gene approach using vitreous humor. Wei et al.113 investigated differences in protein expression in vitreous humor of pathologic myopia (myopes with retinal detachment [RD], macular hole, epiretinal membrane, or retinoschisis) compared to healthy eyes (SER >−6.00 D and axial length < 26.5 mm without any chorioretinal degeneration) and discovered differences in levels of 2 antioxidative proteins (PGDS, GPX3). Ding et al.120 used vitreous humor of patients with vitreomacular interface disease as a control group and discovered differences in expression of CTGF and HGF in these patients compared to high-myopia patients. A candidate gene study from Peng et al.114 found elevated levels of DKK1, involved in the canonical Wnt/β-catenin pathway, in the vitreous humor of pathologic myopia eyes. These proteins could be potential drug targets for myopia prevention treatments.
Animal Models
Previous work has demonstrated an overlap between myopia susceptibility in humans and animal models42,104 Regarding myopia pathways across species and identification of major pathways, Tkatchenko et al.91 examined normal refractive error development and susceptibility to form-deprivation myopia using RNA sequencing in 8 distinct mouse strains. An extensive review of this publication can be found in this IMI 2021 yearly digest section on experimental models. In short, the gene sets controlling baseline refractive development and those regulating susceptibility to myopia overlapped, but these 2 processes appeared to be controlled by largely distinct sets of genes. The authors suggest that regulation of emmetropization may be different from pathways that modulate environmental influences, such as optical defocus, and only a subset of the genes controls both processes. An alternative explanation, derived from a chick study, suggests that genes controlling normal variation in eye size are distinct from genes conferring susceptibility to form-deprivation myopia.125 Furthermore, Tkatchenko et al.91 performed a genome-wide gene-based association analysis in the CREAM consortium and UK Biobank human cohorts to identify mouse genes and pathways associated with myopia in humans. They identified that 985 differentially expressed mouse genes were associated with myopia in humans, of which 847 were newly reported associations and need validation in humans. However, the authors did not clarify if such a large number of genes could have been found simply by chance. Nevertheless, investigating the consistency of findings from animal studies and human genetic studies is a promising approach for elucidating the causal mechanisms of the trait.
Shared Genetic Background Between Myopia and Other Eye Diseases
Myopia is well known to be associated with various complications such as RD, or primary open angle glaucoma (POAG). It was unsurprising that several genetic loci associated with refractive error overlapped with significant RD risk loci.105 A more interesting aspect was the nature of those loci: BMP3 and ZC3H11B were among the replicated RD risk loci, suggesting that myopia-related pathways, such as eye elongation control, may be more pertinent to RD risk than others. The authors also remarked that PRSS56, which strongly associated with high myopia, did not appear to impact RD. Together with GWAS for other myopia-associated conditions and traits, RD GWAS will help to better understand myopia heterogeneity and associated loci function. Risk of detachment seemed to increase steadily with increasing polygenic myopia risk score (PRS) population quintile—a risk score the authors derived from a small fraction (n = 71) of significant variants from the previous CREAM refractive error GWAS121—both for clinically ascertained rhegmatogenous RD and for a less well-defined set of retinal detachments from the UK Biobank.
The study by Han et al.106 examined the RD–myopia link in a formal Mendelian randomization (MR) framework, which can provide evidence for causality. MR studies can be thought of as “natural RCTs,” with the randomization coming from the random assortment of alleles at conception. The MR design can help avoid problems such as confounding and provide genetic support for a causal link. By using genetic data from the UK Biobank, Han et al.106 showed that there is likely to be a causal link between myopia and RD, with each 6-D decrease in refractive error leading to a 7.2-fold increase in RD risk. By leveraging our understanding of myopia genetics, knowledge about RD pathogenesis is also increased, enabling the possible identification of potential targets for prevention of RD in general and RD in myopes.
The clinical association between myopia and POAG has been studied extensively. However, it was unclear whether these 2 complex conditions share a common genetic background. Iglesias et al.126 aimed to quantify the degree of genetic overlap between myopia and POAG (and POAG endophenotypes) in an Australian/New Zealand (the Australian & New Zealand Registry of Advanced Glaucoma study) and Dutch population-based cohort (the Rotterdam Study). PRSs were derived and the genetic correlation between myopia and POAG, IOP, vertical cup to disc ratio, cup area, disc area, and retinal nerve fiber layer was assessed using a technique known as linkage disequilibrium score regression analysis. Surprisingly, genetic predisposition to myopia did not predict POAG status in either population. In fact, evidence of a shared genetic architecture was only found between myopia and optic disk area, which is in line with previously found associations between refractive error and optic disk size.124,127,128 Nevertheless, concluding that there is no clear genetic association between myopia and POAG, the authors were careful to point out that the relatively small sample size and the challenge of diagnosing POAG in myopic eyes were limiting factors. Interestingly, MR in the largest GWAS to date, described above, showed that higher IOP causes more negative spherical equivalent (more myopia) in the general population, and this might explain the previously suggested relationship between myopia and glaucoma.101
Breaking Developments
The results from the largest GWAS meta-analysis to date for refractive error confirmed that this approach continues to yield new discoveries as sample sizes increase.101 As well as providing opportunities to better understand the mechanisms regulating myopia development via the discovery of novel genes and pathways, these studies also offer potential new targets for treatment of myopia and myopia progression.
The widespread availability of omics data enables new discoveries in the field of myopia genetics.78,85,107–114,120 As discussed, 3 human epigenetic myopia studies were published in the past year.85,107,108 Epigenetics might allow greater understanding of how the environment causes myopia but may require prospective longitudinal studies to investigate changes of methylation signatures during life. In healthy volunteers, we are usually limited to data from blood. Epigenetic signals can be tissue specific; therefore, studies should ideally be performed on samples of retina, choroid, and sclera. Tissue samples taken during surgery for other conditions may be a possible source. However, in the absence of such samples, the studies above demonstrate a role for surrogate tissues in the identification of biomarkers.
Finally, another significant development was the joint analysis of data from animal models and human studies,91 which highlights the possibility of strengthening the evidence and gaining new insights on novel pathways by combining these data. In addition to finding new myopia loci, future research should focus on understanding the molecular signaling cascade leading to myopia. Defining particularly the crucial steps in myopia pathogenesis will help in discovering new potential molecules for targeted intervention.
Conclusions
With increasing sample size of studies and new genetic analysis techniques such as whole-genome sequencing and gene–environment interaction analysis, we will find more genetic loci in the future to explain the missing heritability for refractive error. Genetic vulnerability to myopic complications such as myopic macular degeneration or RD as well as genetic susceptibility to a positive response to treatment should be the focus of future research as these have immediate clinical impact. Furthermore, examining prenatal and intergenerational influences on myopia development, such as in the previously described epigenetic studies, is a first step to explain how the environment has an impact at an early stage in life. Methylation analysis and RNA sequencing data are valuable approaches, but the limited availability of ocular tissue restricts widespread use in humans at this moment.
For now, the great challenge of our field is to unravel the pathophysiologic pathways from the current known genetic loci. Next steps should be aimed at understanding how these myopia genes contribute to certain biological processes, how they interact together, and how they are influenced by environmental factors, such as lifestyle. Subsequently, when more insight is gained in the function of these genes, they may be a starting point for new treatment options and prevention strategies.
IMI Digest 2021—Interventions for Controlling Myopia Onset and Progression
In a survey conducted in 2018 to 2019, eye care practitioners in many countries were found to be concerned about the problem of myopia, yet most were not actively intervening to slow myopia. Unfortunately, while 80% of practitioners agreed that single-vision (SV) spectacles or undercorrection was not effective in slowing myopia, some 4 years later, approximately 64% continued to prescribe SV refractive corrections, citing increased cost and inadequate information as the reasons.130 Nonetheless, the search for myopia control treatment has continued since the intervention white paper in 2019,131 with studies over the intervening period largely focused on understanding and improving the efficacy of treatments. In this update, we summarize recent advances for myopia control in relation to specially designed spectacle and contact lens devices; topical atropine, which remains the only pharmacologic treatment option; and some novel intervention strategies. This update is largely limited to already completed clinical trials.
Specially Designed Multiple Segment Focal Spectacles
There is just 1 new study to report. In a 3-year prospective, randomized, multicenter clinical trial, 600 myopic schoolchildren (age: 6 to 12 years, myopia: –1.00 to –4.00 D) were randomized to wear either a novel multifocal lens design (defocus-incorporated multisegment [DIMS]) or SV lenses.132 The DIMS lens has a central distance optical zone, with an annular peripheral zone incorporating multiple small segments of positive power (∼ 1.03 mm in diameter, +3.50 D), with the goal of imposing myopic defocus on local peripheral retinal regions. At the end of the first 2 years of this trial, myopia progression in the DIMS lens-wearing group was significantly reduced relative to that of the SV lens group; myopia increased by −0.38 ± 0.06 vs. −0.85 ± 0.08 D (expressed as spherical equivalent refraction [SER]), and axial lengths increased by 0.21±0.02 vs. 0.56 ± 0.02 mm, respectively. These results represent improved efficacy over previously tested multifocal spectacle lens designs. This multisegment spectacle lens design is currently also being trialed in northern China, although no results are yet available.133
Specially Designed Multifocal Contact Lenses
Three recent trials not only confirmed that it is feasible to slow myopia with optical interventions, but like the specially designed multisegment spectacle lens study, these trials also reported substantially greater slowing of myopia than in most previous trials.132,134,135
One of these trials, a 3-year, multicenter, double-masked, randomized clinical trial, involved the MiSight 1 day (ScottsVille, NY) contact lens, which was compared with SV contact lens. Both lenses were prescribed on a daily wear, daily disposable basis.134 Participants comprised 144 children, aged between 8 and 12 years, with low to moderate myopia (–0.75 to –4.00 D). Over the 3-year trial period, myopia progressed by −0.51 ± 0.64 vs. −1.24 ± 0.61 D (expressed as SER) and axial lengths increased by 0.30 ± 0.27 D vs. 0.62 ± 0.30 mm, representing a greater than 50% reduction in progression with MiSight by both indices.
The second randomized clinical trial involved 508 children, assigned to wear 1 of 4 test contact lenses; 2 were based on an extended depth of focus principle, and 2 were novel multifocal designs that imposed myopic defocus on both central and peripheral retinal regions. SV daily disposable lenses were included as the control reference.135 As in the case of the MiSight lens trial, both changes in refractive errors and axial lengths over 2 years were significantly lower in the groups wearing test lenses, ranging from −0.78 to −0.87 D (SER) and 0.41 to 0.46 mm compared to −1.15 D (95% confidence interval [CI], –0.99 to –1.30) and 0.60 mm (95% CI, 0.53 to 0.66 mm) in the SV lens group. Interestingly, the rates of progression across the 4 test contact lenses were not significantly different, and improved efficacy was also linked to better wearer compliance in the case of the test lenses.
The third trial was a 3-year, double-masked clinical trial of 294 children with myopia randomized to wear soft center-distance multifocal lenses with either a medium or high add (+1.50 vs. +2.50 D) or an SV soft contact lens.136 The choice of multifocal contact lens design aimed to provide clear central vision while simultaneously focusing some light in front of the retina to slow eye growth. The 3-year adjusted myopia progression (SER) was –1.05 D with the SV contact lenses compared to –0.60 and –0.89 D with the high and medium add multifocal lenses, respectively. The slowed myopia progression apparent in the refractive error data is mirrored in the smaller axial length increases in the high and medium add groups over the same period, of 0.42 and 0.58 mm, respectively, compared to 0.66 mm for the SV group. Nonetheless, differences in progression between the multifocal and SV lens groups reached statistical significance only for the high add group. Also, unlike other reports,135 longer wearing times were not found to enhance the treatment effect.
Orthokeratology
Research seeking to both understand the mechanism underlying the myopia control effect of orthokeratology (OK) and improve its effectiveness is also ongoing. In one such retrospective longitudinal study of 103 subjects,137 both total higher-order aberration root mean square and spherical aberrations were found to be significantly and positively correlated with axial length and negatively correlated with axial elongation. Further studies are warranted to follow up on the possibility that spherical aberration plays a key role in the OK myopia control effect, as suggested by these data.
Although not specifically addressing the issue of myopia control efficacy in relation to OK, one other OK study worthy of mention monitored 66 children (6 to < 16 years) wearing SV spectacles for 7 months before switching them to OK for another 7 months.138 All children displaying rapid axial elongation were found to benefit from OK, with younger children (< 9 years), who tended to show more rapid axial elongation compared to older children, benefiting most. On the basis of these results, the authors suggested monitoring progression for a period of time (3–6 months) before intervening with OK, to ensure that the benefits from the intervention outweighed the risks of serious adverse events, given that OK is also a costly, time-consuming intervention option.
Topical Atropine
In relation to topical atropine for myopia control, important new findings since the 2019 white paper relate to (1) concentrations and (2) combination therapies. Late 2019 saw the publication of results of phase 2 of the Low-Concentration Atropine for Myopia Progression (LAMP) study.139 Phase 1 involved daily treatment of children (4 to 12 years, at least 1.00 D myopia) with 0.01%, 0.025%, or 0.05% atropine, with a placebo treatment also included. In phase 2, 383 of the 438 participants in phase 1 continued their originally assigned treatments, with the exception of the placebo group, which was switched to 0.05% atropine. Over the 2-year trial period, increases in myopia for the 0.05%, 0.025%, and 0.01% concentrations were 0.55 ± 0.86 D, 0.85 ± 0.73 D, and 1.12 ± 0.85 D, respectively, and increases in axial lengths, 0.39 ± 0.35 mm, 0.50 ± 0.33 mm, and 0.59 ± 0.38 mm, respectively. Myopia progression (based on both indices) in the placebo group decreased with the switch to 0.05% atropine for the second year of the trial. For both the 0.05% and 0.025% concentrations, treatment effects were comparable across both years. The overall treatment effect with the 0.01% concentration lagged behind the 0.025% and 0.05% concentrations, which were also both well tolerated. Note that both this study and the well-known ATOM series of clinical trials involved East Asian sites. As atropine is known to bind avidly to melanin, with pigmented ocular tissues serving as a local reservoir, it will be of interest to compare these published data with results from other ongoing clinical trials of low-concentration atropine, such as The Childhood Atropine for Myopia Progression in the UK study (CHAMP)147b and the Myopia Outcome Study of Atropine in Children (MOSAIC).148 Both of these trials involve multiple sites distributed throughout United States and Europe, with much higher representations of Caucasian children, who typically have less heavily pigmented ocular tissues than East Asian children.
Results from a retrospective study and early findings from a clinical trial combining topical 0.01% atropine with OK lenses were also published in 2019. The underlying premise of combination therapies is that overall treatment efficacy can be improved, assuming that complementary rather than shared mechanisms underlie each of the treatment effects. In one retrospective study,140 60 children undergoing OK were found to benefit from the addition of nightly 0.01% atropine in the second year; axial elongation dropped from 0.46 ± 0.16 mm in the first year to 0.14 ± 0.14 mm, irrespective of age and refractive error, although those showing the fastest progression in year 1 appeared to benefit most. However, it should be noted that those who refused atropine treatment were not included in the analysis as controls; instead, the authors used a group of historical controls (n = 29) whose axial elongation was significantly different from their test group (0.35 ± 0.11 mm) after 1 year of OK lens wear (P = 0.0013). Nonetheless, these results are consistent with results from an earlier, smaller-scale study141 and a closely related 2-year clinical trial involving 59 myopic children (6 to 11 years, –1.00 to –4.00 D).142 In the latter study, the children were fitted with OK lenses, 29 of whom also used 1 drop of 0.01% atropine nightly prior to insertion of their lenses. Significantly less axial elongation was recorded in those using the combination therapy (0.07 ± 0.16 vs. 0.16 ± 0.15 mm; P = 0.03) at the end of 12 months. Lens performance was also reported to be unaffected with 0.01% atropine use in this study. Recommendations on the value of combining topical atropine with therapies involving multifocal manipulations must await further data, although the apparent enhancement of the effect of imposed myopic defocus on the peripheral retinal induced component of multifocal ERG recording by topical 0.1% atropine is intriguing.143
Environmental Influences and Novel Treatments
While spending more time outdoors has been shown to have therapeutic benefit, as reported in the early intervention white paper, the mechanism underlying this protective effect remains to be resolved. While there are no new studies to report here, the ongoing interest in novel treatments for myopia and on refractive error development more generally can be assessed indirectly through filed patents (NCT03538002 and NCT03623074; clinicaltrials.gov); also, there are ongoing trials related to environmental and optical interventions, although no published clinical data are currently available.
IMI Digest 2021—Clinical Myopia Control Trials and Instrumentation
The 2019 IMI—Clinical Myopia Control Trials and Instrumentation report reviewed the prospective evidence base from existing myopia control trials of at least 1 year in duration along with the supporting academic literature. The review yielded recommendations on the design of future clinical trials for determining the effectiveness of treatments at slowing myopia progression and the impact of these treatments on patients. Since the publication of the first series of the IMI white papers to the end of 2019, 2 papers have been published on spectacle interventions,133,144 3 on soft contact lens trials (1 being an extension of an existing cohort),134,135,145 1 orthokeratology study,146 4 on the effect of administering atropine,147–150 and 2 trials of combined atropine and soft contact lenses150 or OK.151
Participant Inclusion
These trials have all used cycloplegic refraction with participant selection criteria of a maximum astigmatism from –0.75 D to –2.50 D (generally higher for atropine studies), maximum anisometropia of 1.00 to 1.50 D (although not reported in several studies), minimum distance visual acuity from 20/20 to 20/25, a minimum age from 6 to 8 years (although 1 study included participants as low as 4 years of age), and a maximum age typically between 11 and 13 years (although 2 studies recruited children up to 15–16 years of age) (Table 3). Recruiting patients with high astigmatism could lead to them having a very different optical environment (such as an increased depth of focus),152 making it more difficult to evaluate the myopia intervention. The progression of childhood myopia slows with age, so including 15- to 16-year-old children in a trial that lasts several years will reduce the apparent effectiveness of the intervention when considering the actual reduction in eye growth in millimeters or increase in myopia measured in diopters. It should be noted that if reporting treatment effects as a percentage reduction, enrolling older children could yield a falsely higher increased apparent efficacy, as discussed later. Enrolling older children also adds additional complexities such as the necessity of exclusion criteria such as “negative pregnancy test for females with childbearing potential,”148 as was done in one such trial. In addition to the previously recommended participant selection criteria,16 several studies also limited ethnic diversity,132,152 the maximum amount of myopia (either due to optical correction availability or increased risk of pathology), birth term/weight,134 and specifically excluded connective tissue disorders such as Marfan and other more general systemic pathology.135,152 One study required at least 1 parent to be myopic.144 Two studies required a threshold amount of myopia progression over the past year as an inclusion criterion,149,150 despite the previously identified issues with such an entry criterion including the variability of subjective refraction, determining progression based on just 2 data points, and the lack of standardization of the refraction when extracting retrospective data from a chart review. None of these differences in recently published studies would appear to warrant revision to the previously recommended participant selection criteria of:
Table 3.
Selection Criteria in Recent Myopia Control Clinical Trials
| Author, Year | Intervention | SER, Min to Max (D) | Cycloplegia | Ast Limit (D) | Aniso Limit (D) | VA Min | Age, Min to Max, y |
|---|---|---|---|---|---|---|---|
| Kanda et al., 2018144 | Spectacle (novel plus design) | –4.50 to –1.50 | Y | 1.50 | 1.50 | 20/20 | 6 to 12 |
| Lam et al., 2020132 | Spectacle (novel plus design) | –5.00 to –1.00 | Y | 1.50 | 1.50 | 20/20 | 8 to 13 |
| Li et al., 2019146 | OK | –4.00 to –1.00 (sphere) | N? | 1.50 | NR | 20/20 | 8 to 15 |
| Ruiz-Pomeda et al., 2018145 | SCL (concentric bifocal) | –4.00 to –0.75 | Y | 1.00 | 1.00 | 20/25 | 8 to 12 |
| Chamberlain et al., 2019134 | SCL (concentric bifocal) | –4.00 to –0.75 | Y | 0.75 | 1.00 | 20/25 | 8 to 12 |
| Sankaridurg et al., 2019135 | SCL (multifocal and novel design) | –3.50 to –0.75 | Y | 0.75 | NR | 20/30 | 7 to 13 |
| Walline et al., 2020136 | SCL (multifocal +1.50 and +2.50 add) | –5.00 to –0.75(sphere) | Y | 1.00 | 2.00 | 20/25 | 7 to 11 |
| Azuara-Blanco et al., 2019147b | Atropine (0.01%) | –10.00 to –0.50 | Y | 2.00 | NR | 20/32 | 6 to 12 |
| Joachimsen et al., 2019147 | Atropine (0.01%) | NR | NR | NR | NR | NR | 6 to 17 |
| McCrann et al., 2019148 | Atropine (0.01%) | –1.00 or more myopic (least myopic meridian must be –0.50 or more myopic) | Y | 2.50 | NR | 20/32 | 6 to 16 |
| Yam et al., 2019149 | Atropine (0.01%, 0.025%, 0.05%) | –1.00 or more myopic refraction | Y | 2.50 | NR | NR | 4 to 12 |
| Huang et al., 2019150 | SCL (multifocal) plus atropine (0.01%) | –5.00 to –0.75 sphere | Y | 1.00 | 2.00 | 20/25 | 7 to 11 |
| Tan et al., 2019151 | OK plus atropine (0.01%) | –4.00 to –1.00 | Y | 2.50 | 1.00 | 20/25 | 6 to 11 |
Aniso, anisometropia; Ast, astigmatism; N, no; NR, not reported; SCL, soft contact lens; VA, visual acuity; Y, yes; ?, unclear.
Inclusions
| Refractive error | Cyclopleged spherical or SER myopia of at least –0.75 D |
| Astigmatism | ≤1.00 D |
| Anisometropia | ≤1.50 D |
| Age | 6–12 years |
| Visual acuity | 20/20 minimum |
Exclusions
| History | Previous rigid lens wear or myopia control treatment |
| Ocular disease | Any (other than myopia) |
| Binocular vision | Anomaly |
| Systemic disease | Those that may affect vision, vision development, or the treatment modality |
| Medications | Those that may affect pupil size, accommodation, or have an impact on the ocular surface |
If these criteria are not followed, the apparent efficacy of a treatment may be underestimated and it may be more difficult to compare approaches across studies.
Study Design
Most of these studies have followed their cohort for 2 years (Table 4), with an additional year to examine whether there was any rebound in 2 of the atropine studies and in the second year in one of the spectacle studies. All studies have shown a reduced effectiveness of treatments in the second year, demonstrating why more than 1 year of follow-up is needed to assess the long-term efficacy of the treatment. The extrapolation of a 1-year treatment effect to multiple years (an approach taken by many myopia calculators, for example) can lead to incorrect conclusions. Hence, the prior IMI recommendation of a 3-year minimum length (plus an additional year of no treatment to examine any rebound effect) of a clinical trial assessing the efficacy of a treatment for myopia control is still upheld. All new studies, except one on orthokeratology, appropriately applied masking and randomization; however, stratification of subjects is rare. Appropriate controls have been selected for the control group, although Tan et al.151 did not include a placebo drop in their orthokeratology alone control group, which could have identified those patients in the treatment group who used atropine drops in addition to orthokeratology.
Table 4.
Control Group, Randomization, and Masking of Recent Myopia Control Trials
| Author, Year | Intervention | Control | Rando-mization | Strati-fication | Masking | Initial Study Length, y | Rebound Assessment |
|---|---|---|---|---|---|---|---|
| Kanda et al., 2018144 | Spectacle (novel plus design) | Spectacle (SV) | Y | N | Y | 2 | N |
| Lam et al., 2020132 | Spectacle (novel plus design) | Spectacle (SV) | Y | N | Y | 2 | N |
| Li et al., 2019146 | OK | Spectacle (SV) | N | N | N | 1 | Y (1 month) |
| Ruiz-Pomeda et al., 2018145 | SCL (concentric bifocal) | Spectacle(SV) | Y | Y | N | 2 | N |
| Chamberlain et al., 2019134 | SCL (concentric bifocal) | SCL (SV) | Y | Y | Y | 3* | Y (planned)† |
| Sankaridurg et al., 2019135 | SCL (multifocal and novel design) | SCL (SV) | Y | N | Y | 2 | N |
| Walline et al., 2020136 | SCL (multifocal +1.50 and +2.50 add) | SCL (SV) | Y | Y | Y | 3* | Y (planned)† |
| Azuara-Blanco et al., 2019147b | Atropine (0.01%) | Placebo | Y | N | Y | 2 | N |
| Joachimsen et al., 2019147 | Atropine (0.01%) | None | N | N | N | 1 | N |
| McCrann et al., 2019148 | Atropine (0.01%) | Placebo | Y | Y | Y | 3 | Y (study year 3) |
| Yam et al., 2019149 | Atropine (0.01%, 0.025%, 0.05%) | Placebo (year 1 only) | Y | Y | Y | 3 | Y (study year 3) |
| Huang et al., 2019150 | SCL (multifocal) plus atropine (0.01%) | Historical (SCL SV and SCL multifocal) | N | N | N | 3 | N |
| Tan et al., 2019151 | OK plus atropine (0.01%) | OK only | Y | N | Y | 2 | N |
SV, single vision.
*Followed for an additional period after ending randomization.
† Based on communication with authors.
One ethical dilemma that is now being discussed is the appropriateness of including a control group in studies. If the treatment is well enough established to slow or prevent myopia progression, is it ethical to randomly assign subjects to an ineffective sham/control group given their likelihood to develop myopia or have myopic progression? The decision to include a control group rests on whether treatments with a similar risk profile are well enough established to be recommended (as previously indicated by the IMI clinical trials report)16 or not (as indicated for all therapies except for atropine by a recent Cochrane systematic review153 and as is advocated by some professional bodies).154 Another consideration is whether there should be triggers within the rebound examination year to reinstate treatment if myopia progression seems to accelerate after discontinuing the active treatment. This issue is problematic as eye growth varies by season throughout the year and thus is not linear; therefore, determining whether there has been an acceleration in eye growth is difficult without a full year's observation. There is also the issue as to whether good comparative control data on myopia progression in children already exist in the academic literature that can serve as a suitable historical control for any cohort of children recruited to study a new intervention. Progression differs by age, ethnicity, and season and has been hypothesized to vary depending on environmental factors such as outdoor time or light levels16; therefore, it is difficult to argue that a historical control can be expected to adequately represent, without bias, the progression one would expect with a randomized control group. Hence, our assessment is that, at present, an appropriately selected concurrent control group is still ethical for myopia control trials, along with an additional full year of no treatment in the treated group, to assess any rebound effect.
Outcome Measures
The outcomes of myopia progression clinical trials can still be classified as primary (refractive error and/or axial length), secondary (patient-reported outcomes and treatment compliance), and exploratory (peripheral refraction, accommodative changes, ocular alignment, pupil size, outdoor activity/lighting levels, anterior and posterior segment imaging, and tissue biomechanics).16 However, visual disturbances (reported subjectively such as halos and glare or measured objectively such as with halometry) and patient-reported outcomes have been further specified in the minimum data set for different modalities of treatment (Table 5).
Table 5.
Expected Minimum Data Set for Each Treatment Modality
| Treatment Modality | Distance Visual Acuity | Near Visual Acuity | Contrast Sensitivity | Pupil Size | Cycloplegic Refraction | Axial Length | Amplitude of Accommodation | Visual Disturbances | Lens Centration | Wearing Time | Instillation Compliance | Patient-Reported Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectacles | X | X | X | X | X | X | X | X | X | X | ||
| Soft multifocal contact lenses | X | X | X | X | X | X | X | X | X | X | X | |
| Orthokeratology | X | X | X | X | X | X | X | X | X | X | X | |
| Pharmaceuticals | X | X | X | X | X | X | X | X | X | X |
It has been recently questioned at relevant conferences whether the slowing of myopia progression should be reported as a percentage, as has been traditionally done, or as an absolute/cumulative reduction in myopia progression (in diopters) or slowed axial elongation (in millimeters) between a treatment and control group. This issue has arisen due to the reduction in treatment efficacy over time during a trial, which is presumed to be due to the increasing age of the children. Another issue with percentages is that they depend on the rate of progression in the control group; the same absolute effect size when reported as a percentage will appear larger or smaller depending on whether there is slower or faster myopia progression in the control group. It has also recently been suggested that perhaps only a maximum amount of myopia can be prevented by a treatment, regardless of treatment duration. Reporting the overall slowing of myopia progression or axial growth between the treatment and control group seems to be a sensible suggestion; therefore, we would advocate the reporting of both a percentage and absolute amount of reduced myopia progression/axial elongation in future clinical trial reports.
Instrumentation
New instrumentation relevant to myopia progression trials includes exploratory outcome devices to objectively monitor working distance155 and light levels to assess key environmental factors. A new aberrometer that utilizes pyramidal optics (as opposed to Hartmannn–Shack aberrometers) for measuring ocular aberrations has been developed and validated on model eyes156 and in humans157 as repeatable and accurate. Choroidal OCT and angiography have been used to explore differences in structure and function of this tissue, particularly in high myopes.158,159
Conclusion
Clinical trials for myopia control must be carefully designed to inform clinical practice and to better understand the mechanism of action of treatments to allow further refinements. Some recently published studies still do not include fundamental elements, such as masking and randomization, a simultaneous control group, and clearly defined enrollment criteria to minimize bias and the potential to report inaccurate conclusions. The original 2019 IMI white paper16 and this update can be used by researchers and industry to inform their clinical trial design. This information can also be used by clinicians and regulatory bodies to interpret the strength of evidence provided by published trials and to compare risks and benefits between treatments.
IMI Digest 2021—Clinical Management Guidelines for Myopia
The intent of this Yearly Digest is to highlight significant additions to the clinical management of myopia since the publication of the of the 2019 IMI white paper on clinical management guidelines.160 This review takes into account publications on new interventions and key understandings of the myopia management landscape, which have influenced how science is translated into practice.
Several notable randomized controlled studies on myopia control interventions have been published since early 2019. While examining the detail of these intervention studies is not the remit of the Yearly Digest, the impact of these studies on clinician awareness of myopia control options and how this has influenced clinical management must be noted. First, 2 new contact lens studies marked a departure from traditional multifocal contact lens designs, typically prescribed for presbyopia, to myopia control-specific designs. The MiSight 3-year134 and extended depth of focus 2-year135 randomized controlled contact lens studies presented robust evidence of efficacy to slow both axial and refractive myopia progression. Second, the Defocus Incorporated Multiple Segments (DIMS) spectacle lens 2-year study132 revealed perhaps the strongest efficacy results of all spectacle lens interventions, arguably similar to that of contact lens designs. As well, the Bifocal Lenses in Nearsighted Kids group demonstrated a significant slowing of axial length and refractive error with the CooperVisionBiofinity +2.50 D add but not the +1.50 D add lenses in a 3-year randomized trial136 (discussed in Interventions for controlling myopia onset and progression). Finally, the LAMP 1-year study149 provided a control group comparison that importantly highlighted the concentration-dependent success of atropine intervention, which had been unclear from previous studies. The absolute differences in refractive and axial length outcomes between treatment and control groups in these studies are provided in Table 6. The sum total of these studies provides clarity in intervention evidence and, in combination with increasing availability of the optical treatments, leads to improved prescribing options for childhood myopia.
Table 6.
Summary of Results for Recently Published Myopia Control Intervention Studies
| Absolute Difference in Progression Between Treatment and Control Groups | |||
|---|---|---|---|
| Intervention Study | Duration, y | Refractive (D) | Axial Length (mm) |
| MiSight contact lens134 | 3 | 0.66 | 0.28 |
| Extended depth of focus contact lens135 (4 designs tested) | 2 | 0.27 to 0.37 (across 4 test designs) | 0.14 to 0.19 (across 4 test designs) |
| Biofinity +2.50 contact lens136 | 3 | 0.45 | 0.23 |
| DIMS spectacle lens132 | 2 | 0.44 | 0.34 |
| LAMP atropine149 | |||
| 0.01% | 1 | 0.22 | 0.05 |
| 0.025% | 0.35 | 0.12 | |
| 0.05% | 0.54 | 0.21 | |
Differences between adjusted means are presented as detailed in each paper.
As clinicians have also begun combining atropine with optical interventions, 2 randomized controlled trials are currently under way. Recently reported 2-year data on combining atropine 0.01% with orthokeratology showed no benefit for children who had more than 3 D and less than 6 D of myopia at baseline, but 0.11 mm less total axial elongation in myopes of 1 to 3 D at baseline.161 There was no correlation between age and efficacy of the combined treatment.161 Baseline data for bifocal soft contact lenses combined with 0.01% atropine have also been presented.149
The remit of the Clinical Management Guidelines report was to canvas all aspects of patient identification, communication, clinical tests, and guidelines for management. Four newly published papers describe the current landscape of myopia control research and practice, as well as key depictions of the risk-to-benefit balance to assist clinicians in understanding and communicating the imperative for myopia management.
Myopia Control 2020: Where are We and Where are We Heading? Bullimore MA, Richdale K. Ophthalmic and Physiological Optics 2020;40:254–27018
This review highlighted key outcomes of recent peer-reviewed literature. Original spectacle lens studies employing progressive addition and traditional flat-top bifocals reported no clinically meaningful results, limiting their uptake by clinicians; however, novel spectacle designs such as those developed by Hong Kong Polytechnic University (DIMS) and SightGlassVison are reporting myopia control results approaching that of contact lens designs. Likewise, symptoms and rebound associated with 1% atropine were not considered clinically acceptable, and 0.01% showed little efficacy in controlling axial elongation. The recent LAMP study demonstrated excellent tolerability and efficacy with 0.05% atropine and, based on current research, it is arguably the optimal concentration with which to initiate treatment. Advances in these modalities provide safe and effective treatment options for children not ready for or interested in contact lenses. Children as young as 7 to 8 years of age can successfully wear contact lenses. The longest myopia control data available to date, a single orthokeratology study, demonstrate a myopia control effect of 0.45 mm over 7 years of wear. The 2 recent soft contact lens trials show excellent 2- and 3-year efficacy in controlling progression (Table 6). Approval for myopia control varies broadly by country and may influence practitioner and parental decisions on treatment.
There is no valid progression-based criterion for initiation of myopia control. Age is the single most important factor in predicting progression—with younger children typically progressing more quickly—but ethnicity, sex, and parental history of myopia also influence progression. The vast majority of children with myopia will progress, and thus parents should be educated about treatment and management options as soon as a child develops myopia. The only caveat to treating all children with myopia is that some may be considered to have “pathologic” myopia, such as those developing myopia before the age of 5 years, or in association with a systemic condition. There is no research to suggest that standard myopia control treatments will be effective in these cases. Parents should also be educated to anticipate that myopia control treatment should be continued until myopia stabilizes, which is expected to be in the later teens or early 20s. Future research is aimed at maintaining high safety standards while further improving efficacy of single and combination treatments.
International Survey of Contact Lens Fitting for Myopia Control in Children; Efron N, Morgan PB, Woods CA et al. Contact Lens and Anterior Eye 2020;43:4–8162
Childhood myopia requires correction as well as control; contact lens interventions show the best propensity for achieving both as a monotherapy. Uptake of contact lens fitting for myopia control (MC) in children was evaluated in a serial survey. The proportion of pediatric contact lens fits for MC increased from 0.2% of all fits in 2011 to 6.8% in 2018. This trend is mostly attributable to increase in rigid lens fitting for MC, particularly since 2016. Rigid lens fits for myopia control have increased from only a few percent of all rigid lens fits in 2011 to over 20% from 2016 to 2018, now representing over half of all MC fits but only 12% of all non-MC fits.
Variation by country in the proportion of pediatric contact lens (CL) fits for MC ranged from over 20% of all fits in Austria, Germany, and Hong Kong to no fits in the Czech Republic, Japan, South Korea, and Puerto Rico. Across the 31 countries surveyed, children fit for MC are on average younger (mean 13 years) compared to non-MC fits (mean 15 years); 84% wear their lenses 7 days per week compared to 56% of non-MC fit children.
This survey indicates that despite increases in education and research, clinical application of contact lenses for childhood myopia control is still low. Moreover, the average age for MC fits in this survey is higher than ideal, given that intervention at a younger age (before age 12) is indicated to achieve the greatest myopia control effects.160 The authors cited international differences in the extent of CL fitting for MC as due to specific lens availability, along with practitioner training, attitudes, and confidence. Overall low uptake was described as likely due to lack of on-label products for most of the survey period and in most parts of the world, uncertainty about efficacy, and lack of awareness among parents of MC benefits. As these data were truncated prior to publication of the IMI Clinical Management Guidelines, it is hoped that future prescribing surveys will indicate a continued upward trend in myopia control contact lens fits to children, along with a downward trend in mean age.
Childhood and Lifetime Risk Comparison of Myopia Control With Contact Lenses; Gifford KL. Contact Lens and Anterior Eye 2020;43:26–32163
Barriers to pediatric contact lens prescribing can include concerns about safety. To address this, the short-term risk of pediatric contact lens wear for myopia control was compared to the lifetime risk of myopia-associated pathology. This analysis determined that the comparative risk of microbial keratitis (MK) across a lifetime of any type of contact lens wear, when presumed to commence at age 8 for myopia control, is less than the lifetime risk of vision impairment with myopia more than 6 D or axial length more than 26 mm. Childhood contact lens wear risks were calculated over 10 years of wear—from age 8 to 17 inclusive. When added to these childhood risks, a lifetime of contact lens wear was modeled by calculation of 47 years of adult contact lens wear (age 18 to 65 years inclusive) plus 10 years of no contact lens wear (from 66 to 75 years inclusive). Peer-reviewed data on infection risk in various contact lens wear modalities were compared to published cumulative risk of vision impairment by age 75.
The findings indicated that 10 years of pediatric overnight orthokeratology wear—the “riskiest” type of myopia control contact lens wear in childhood—presents a 1 in 66 likelihood of MK, compared to a 1 in 33 lifetime risk of vision impairment with myopia of 0.5 to 3.0 D. The “riskiest” way to wear contact lenses over a lifetime was modeled to be 10 years of childhood reusable soft contact lens wear on a daily schedule with 47 years of adult soft contact lens extended wear, presenting a 1 in 7.7 lifetime risk of MK. When compared to lifetime risks presented by myopia to an individual, the risk of vision impairment is 1 in 3.9 for eyes 26 to 28 mm long and 1 in 5 for eyes with 6 to 10 D of myopia. Myopia under 3 D and axial lengths less than 26 mm make a lifetime of orthokeratology or reusable soft contact lens wear more risky by comparison, while a lifetime of daily disposable wear presents a 1 in 76 risk of MK, which is less than any of the calculated myopia risks.
These findings provide scientific basis for clinicians to be proactive in recommending myopia control contact lenses to younger children, as both the short-term safety profile and long-term preventative eye health benefits are evident.
Myopia Control: Why Each Diopter Matters; Bullimore MA, Brennan NA. Optometry and Vision Science 2019;96:463–465164
While keeping myopia below 6 D and axial length less than 26 mm through myopia control intervention is a key goal, the value of the smaller goal of 1 D of control is perhaps the strongest clinical message to emerge since publication of the IMI Clinical Management Guidelines. Three arguments for the advantages of 1 D lower myopia are presented. First, less visual disability while uncorrected and the relationship to quality of life were explored. As stated by the authors, “Corrected or not, greater refractive error produces greater disability and dependence on whatever mode of correction [is] used.”
Second, improved availability and better visual outcomes for future surgical myopia correction were described. Laser in situ keratomileusis has been demonstrated to reduce best-corrected low-contrast acuity in higher myopes, and higher ablations can increase risk of postoperative ectasia. Finally, this important analysis demonstrated that across the range of refractions, each 1 D less of final myopia translates to 40% less lifetime risk of developing myopic maculopathy, which is the most common and serious sight-threatening complication of myopia. This was compared to the Age-Related Eye Disease Study for age-related macular degeneration (ARMD), which found a 25% risk reduction in progression to advanced ARMD through years of administration of antioxidants plus zinc. When viewed similarly as a preventative eye health treatment, and that most myopia control interventions pose low relative risk, reducing an individual's final level of myopia by 1 D presents a clear public health imperative for reducing future visual impairment for millions of myopes around the world. The authors note that while research continues on treatment options and their absolute efficacy, and long-term data are lacking, it is reasonable to speculate that currently available myopia control interventions could provide a cumulative treatment effect of 1 D. This simple perspective on what is a complex field provides the clinical “why” for proactive myopia management.
Acknowledgments
Supported by the International Myopia Institute. The publication costs of the International Myopia Institute reports were supported by donations from the Brien Holden Vision Institute, Carl Zeiss Vision, CooperVision, Essilor, and Alcon.
IMI Digest 2021—Experimental Models of Emmetropization and Myopia. This work was supported by National Institutes of Health Grants R01 EY-03611 (ELS), F31 HD-097918 (DCT), T32 ES-012870 (DCT), and R01 EY-016435 (MTP); Department of Veterans Affairs Rehabilitation R&D Service Senior Research Career Scientist Award IK6 RX-003134 (MTP); and funds from the Brien Holden Vision Institute and the UH Foundation.
IMI Digest 2021—Genetics. JEBW and AMM were supported by the Intramural Research Program of the National Institutes of Health and CREAM consortium.
SM was supported by Australian National Health and Medical Research Council Grants 1150144 and 1116360. VV was supported by the Netherlands Organization for Scientific Research (NWO); Grant 91617076. CCWK was supported by Netherlands Organization for Scientific Research (NWO); Grant 91815655 and European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme Grant 648268.
Disclosure: M. Jong, None; J.B. Jonas, European patent application 16 720 043.5 (P), US patent application US 2019 0085065 (P); J.S. Wolffsohn, British Contact Lens Association (C), University of Houston (C), Visioncare Research & CooperVision (C), Portable Aberrometer Japanese Application 2010-220857 (P), Contrast Sensitivity Chart PAT 2012-044/P.O. No: 90580852 UK Patent Application No. 1300844.6 (P), Johnson & Johnson (R); D.A. Berntsen, National Institutes of Health/National Eye Institute (F), Bausch + Lomb (F), Visioneering Technologies (C); P. Cho, JnJ Vision Care (C), Euclid Systems Corporation (C & S), Menicon Japan Pauline Cho also has (S); D. Clarkson-Townsend, None; D.I. Flitcroft, None; K.L. Gifford, Alcon, Coopervision, Essilor, Hoya, Johnson & Johnson Vision Care, Menicon, Nevakar, Oculus, Visioneering Technologies (C); A.E.G. Haarman, None; C.C.W. Klaver, None; M.T. Pardue, None; K. Richdale, Paragon (F), Alcon (F), Euclid (F), Novartis (F), Paragon (C), Novartis (C), CooperVision (C); P. Sankaridurg, BHVI (E), coinventor on multiple patents related to myopia (P), Alcon (R), SEED (R), Mark Ennovy (R), Carl Zeiss Vision (R); V.J.M. Verhoeven, None; C.F. Wildsoet, None; J.E. Bailey-Wilson, None; J.A. Guggenheim, None; C.J. Hammond, None; J. Kaprio, None; S. Macgregor, None; D.A. Mackey, None; A.M. Musolf, None; M.S. Tedja, None; V. Vitart, None; E.L. Smith III, optical treatment strategies for myopia (P), Nevakar (C), SightGlass Vision (C), Treehouse Eyes (C), Essilor of America (C), Acucela (C)
References
- 1. Wolffsohn JS, Flitcroft DI, Gifford KL, et al.. IMI—myopia control reports overview and introduction. Invest Ophthalmol Vis Sci. 2019; 60: M1–M19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flitcroft DI, He M, Jonas JB, et al.. IMI—defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019; 60: M20–M30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 4. Ohno-Matsui K, Wu P-C, Yamashiro K, et al.. IMI pathologic myopia. Invest Ophthalmol Vis Sci. 2021; 62(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yotsukura E, Torii H, Inokuchi M, et al.. Current prevalence of myopia and association of myopia with environmental factors among schoolchildren in Japan. JAMA Ophthalmol. 2019; 137(11): 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hopf S, Korb C, Nickels S, et al.. Prevalence of myopic maculopathy in the German population: results from the Gutenberg health study. Br J Ophthalmol. 2020; 104: 1254–1259. [DOI] [PubMed] [Google Scholar]
- 7. Zheng F, Wong CW, Sabanayagam C, et al.. Prevalence, risk factors and impact of posterior staphyloma diagnosed from wide-field optical coherence tomography in Singapore adults with high myopia. Acta Ophthalmol. 2021; 99(2): e144–e153 [DOI] [PubMed] [Google Scholar]
- 8. Han T, Shang J, Zhou X, Xu Y, Ang M, Zhou X. Refractive outcomes comparing small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for high myopia. J Cataract Refract Surg. 2020; 46: 419–427. [DOI] [PubMed] [Google Scholar]
- 9. Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, Garcia-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019; 69: 80–115. [DOI] [PubMed] [Google Scholar]
- 10. Cumberland PM, Bountziouka V, Rahi JS. Impact of varying the definition of myopia on estimates of prevalence and associations with risk factors: time for an approach that serves research, practice and policy. Br J Ophthalmol. 2018; 102: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020; 20: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parssinen O, Kauppinen M. Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019; 97: 510–518. [DOI] [PubMed] [Google Scholar]
- 13. Sanz Diez P, Yang LH, Lu MX, Wahl S, Ohlendorf A. Growth curves of myopia-related parameters to clinically monitor the refractive development in Chinese schoolchildren. Graefes Arch Clin Exp Ophthalmol. 2019; 257(5): 1045–1053, doi: 10.1007/s00417-019-04290-6. Epub 2019 Mar 23. PMID: 30903312. [DOI] [PubMed] [Google Scholar]
- 13b. Tideman JWL, Polling JR, Vingerling JR, et al.. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018; 96: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Truckenbrod C, Meigen C, Brandt M, et al.. Reference curves for refraction in a German cohort of healthy children and adolescents. PLoS One. 2020; 15: e0230291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun YY, et al.. Cycloplegic refraction by 1% cyclopentolate in young adults: Is it the gold standard? the Anyang University Students Eye Study (AUSES). Br J Ophthalmol. 2019; 103: 654–658. [DOI] [PubMed] [Google Scholar]
- 16. Wolffsohn JS, Kollbaum PS, Berntsen DA, et al.. IMI—clinical myopia control trials and instrumentation report. Invest Ophthalmol Vis Sci. 2019; 60: M132–M160. [DOI] [PubMed] [Google Scholar]
- 17. Jiang D, Zhang D, Zhang Y, et al.. The trend of myopia rate in 61 350 children and adolescents: a cross-sectional research in Ningbo, Zhejiang. Acta Ophthalmol. 2020; 98: e525–e526. [DOI] [PubMed] [Google Scholar]
- 18. Bullimore MA, Richdale K. Myopia control 2020: where are we and where are we heading? Ophthalmic Physiol Opt. 2020; 40: 254–270. [DOI] [PubMed] [Google Scholar]
- 19. Lee JTL, Guo X, Li Z, Jong M, Sankaridurg P, He M. Progression and longitudinal biometric changes in highly myopic eyes. Invest Ophthalmol Vis Sci. 2020; 61: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Y, Xiao O, Guo X, et al.. Methodology of the ZOC-BHVI High Myopia Cohort Study: the onset and progression of myopic pathologies and associated risk factors in highly myopic Chinese. Ophthalmic Epidemiol. 2018; 25: 31–38. [DOI] [PubMed] [Google Scholar]
- 21. Morgan IG, Rose KA. Myopia: is the nature-nurture debate finally over? Clin Exp Optom. 2019; 102: 3–17. [DOI] [PubMed] [Google Scholar]
- 22. Jonas JB, Ohno-Matsui K, Panda-Jonas S. Myopia: anatomic changes and consequences for its etiology. Asia Pac J Ophthalmol (Phila). 2019; 8: 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leveziel N, Marillet S, Dufour Q, et al.. Prevalence of macular complications related to myopia—results of a multicenter evaluation of myopic patients in eye clinics in France. Acta Ophthalmol. 2020; 98: e245–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020; 61: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka N, Shinohara K, Yokoi T, et al.. Posterior staphylomas and scleral curvature in highly myopic children and adolescents investigated by ultra-widefield optical coherence tomography. PLoS One. 2019; 14(6): e0218107, doi: 10.1371/journal.pone.0218107. PMID: 31181108; PMCID: PMC6557512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsumura S, et al.. Characteristics of myopic traction maculopathy in myopic Singaporean adults. Br J Ophthalmol. 2020, doi: 10.1136/bjophthalmol-2020-316182. [DOI] [PubMed] [Google Scholar]
- 27. Parolini B, Palmieri M, Finzi A, et al.. The new Myopic Traction Maculopathy Staging System. Eur J Ophthalmol; 2020;8: 1120672120930590, doi: 10.1177/1120672120930590. Epub ahead of print. PMID: 32506945. [DOI] [PubMed] [Google Scholar]
- 28. Jonas JB, Wang YX, Dong L, Panda-Jonas S. High myopia and glaucoma-like optic neuropathy. Asia Pac J Ophthalmol (Phila). 2020; 9: 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohno-Matsui K, Kawasaki R, Jonas JB, et al.. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015; 159: 877–883.e877. [DOI] [PubMed] [Google Scholar]
- 30. Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977; 266: 66–68. [DOI] [PubMed] [Google Scholar]
- 31. Troilo D, Smith EL, III, Nickla DL, et al.. IMI—report on experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci. 2019; 60: M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rucker F. Monochromatic and white light and the regulation of eye growth. Exp Eye Res. 2019; 184: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tkatchenko TV, Tkatchenko AV. Pharmacogenomic approach to antimyopia drug development: pathways lead the way. Trends Pharmacol Sci. 2019; 40: 833–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chakraborty R, Ostrin LA, Benavente-Perez A, Verkicharla PK. Optical mechanisms regulating emmetropisation and refractive errors: evidence from animal models. Clin Exp Optom. 2020; 103: 55–67. [DOI] [PubMed] [Google Scholar]
- 35. Benavente-Perez A, Nour A, Troilo D. Short interruptions of imposed hyperopic defocus earlier in treatment are more effective at preventing myopia development. Sci Rep. 2019; 9: 11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y, Liu C, Sun M, et al.. Ocular safety evaluation of blue light scleral cross-linking in vivo in rhesus macaques. Graefes Arch Clin Exp Ophthalmol. 2019; 257: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 37. Whatham AR, Lunn D, Judge SJ. Effects of monocular atropinization on refractive error and eye growth in infant New World monkeys. Invest Ophthalmol Vis Sci. 2019; 60: 2623–2630. [DOI] [PubMed] [Google Scholar]
- 38. Gawne TJ, Ward AH, Norton TT. Juvenile tree shrews do not maintain emmetropia in narrow-band blue light. Optom Vis Sci. 2018; 95: 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera during recovery from minus-lens wear and during plus-lens wear. Mol Vis. 2019; 25: 311–328. [PMC free article] [PubMed] [Google Scholar]
- 40. Sajdak BS, Salmon AE, Cava JA, et al.. Noninvasive imaging of the tree shrew eye: wavefront analysis and retinal imaging with correlative histology. Exp Eye Res. 2019; 185: 107683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. El-Nimri NW, Zhang H, Wildsoet CF. The effect of part-time wear of 2-zone concentric bifocal spectacle lenses on refractive error development & eye growth in young chicks. Exp Eye Res. 2019; 180: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang Y, Kee CS, Hocking PM, et al.. A genome-wide association study for susceptibility to visual experience-induced myopia. Invest Ophthalmol Vis Sci. 2019; 60: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang BS, Lam TC, Cheung JK, Li KK, Kee CS. Data on corneal proteome and differentially expressed corneal proteins in highly myopic chicks using a data independent quantification approach. Data Brief. 2019; 26: 104478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kang BS, Wang LK, Zheng YP, Guggenheim JA, Stell WK, Kee CS. High myopia induced by form deprivation is associated with altered corneal biomechanical properties in chicks. PLoS One. 2018; 13: e0207189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kato M, Sato K, Habuta M, et al.. Localization of the ultraviolet-sensor Opn5m and its effect on myopia-related gene expression in the late-embryonic chick eye. BiochemBiophys Rep. 2019; 19: 100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin G, Taylor C, Rucker F. Effect of duration, and temporal modulation, of monochromatic light on emmetropization in chicks. Vision Res. 2020; 166: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu H, Schaeffel F, Trier K, Feldkaemper M. Effects of 7-methylxanthine on deprivation myopia and retinal dopamine release in chickens. Ophthalmic Res. 2020; 63: 347–357. [DOI] [PubMed] [Google Scholar]
- 48. Nickla DL, Schroedl F. Effects of autonomic denervations on the rhythms in axial length and choroidal thickness in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019; 205: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nickla DL, Jordan K, Yang J, Singh P. Effects of time-of-day on inhibition of lens-induced myopia by quinpirole, pirenzepine and atropine in chicks. Exp Eye Res. 2019; 181: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rucker F, Henriksen M, Yanase T, Taylor C. The role of temporal contrast and blue light in emmetropization. Vision Res. 2018; 151: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Swiatczak B, Feldkaemper M, Schaeffel F. Changes in fundus reflectivity during myopia development in chickens. Biomed Opt Express. 2019; 10: 1822–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Swiatczak B, Feldkaemper M, Schraermeyer U, Schaeffel F. Demyelination and shrinkage of axons in the retinal nerve fiber layer in chickens developing deprivation myopia. Exp Eye Res. 2019; 188: 107783. [DOI] [PubMed] [Google Scholar]
- 53. Thomson K, Karouta C, Morgan I, Kelly T, Ashby R. Effectiveness and safety of topical levodopa in a chick model of myopia. Sci Rep. 2019; 9: 18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang M, Aleman AC, Schaeffel F. Probing the potency of artificial dynamic ON or OFF stimuli to inhibit myopia development. Invest Ophthalmol Vis Sci. 2019; 60: 2599–2611. [DOI] [PubMed] [Google Scholar]
- 55. Chen W, Zhang H, Zhang Y, Wang Q, Wang Y, Li ZW. Relationship between aquaporin-1 protein expression and choroidal thickness during the recovery of form-deprivation myopia in guinea pigs. Curr Eye Res. 2020; 45: 705–712. [DOI] [PubMed] [Google Scholar]
- 56. Ding M, Guo D, Wu J, et al.. Effects of glucocorticoid on the eye development in guinea pigs. Steroids. 2018; 139: 1–9. [DOI] [PubMed] [Google Scholar]
- 57. Dong L, Shi XH, Kang YK, Wei WB, Wang YX, Jonas JB. Ocular size and shape in lens-induced Myopization in young Guinea pigs. BMC ophthalmology. 2019; 19: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dong L, Shi XH, Kang YK, Wei WB, Wang YX, Jonas JB. Ocular size and shape in lens-induced myopization in young guinea pigs. BMC Ophthalmol. 2019; 19: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dong L, Shi XH, Kang YK, et al. Bruch's Membrane Thickness and Retinal Pigment Epithelium Cell Density in Experimental Axial Elongation. Scientific reports. 2019; 9: 6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59b. El-Nimri NW, Yao M, Huerta A, Hoang M, Wildsoet CF. Effect of chronic topical latanoprost on the sclera and lamina cribrosa of form-deprived myopic guinea pigs. Exp Eye Res. 2019; 186: 107740. [DOI] [PubMed] [Google Scholar]
- 60. Dong L, Shi XH, Kang YK, et al. Amphiregulin and ocular axial length. Acta ophthalmologica 2019; 97: e460–e470. [DOI] [PubMed] [Google Scholar]
- 60b. Hoang QV, Rohrbach D, McFadden SA, Mamou J. Regional changes in the elastic properties of myopic guinea pig sclera. Exp Eye Res. 2019; 186: 107739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang L, Garcia MB, Hammond D, Dahanayake D, Wildsoet CF. Strain-dependent differences in sensitivity to myopia-inducing stimuli in guinea pigs and role of choroid. Invest Ophthalmol Vis Sci. 2019; 60: 1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li RQ, Lan WZ, Li XN, et al.. Effects of the long wavelength-filtered continuous spectrum on natural refractive development in juvenile guinea pigs. Int J Ophthalmol. 2019; 12: 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pan M, Jiao S, Reinach PS, et al.. Opposing effects of PPARalpha agonism and antagonism on refractive development and form deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2018; 59: 5803–5815. [DOI] [PubMed] [Google Scholar]
- 64. Shan SW, Tse DY, Zuo B, et al.. Data on differentially expressed proteins in retinal emmetropization process in guinea pig using integrated SWATH-based and targeted-based proteomics. Data Brief. 2018; 21: 1750–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun Y, Zhao N, Liu W, et al.. Study of vesicular monoamine transporter 2 in myopic retina using [(18)F]FP-(+)-DTBZ. Mol Imaging Biol. 2018; 20: 771–779. [DOI] [PubMed] [Google Scholar]
- 66. El-Nimri NW, Wildsoet CF. Effects of topical latanoprost on intraocular pressure and myopia progression in young guinea pigs. Invest Ophthalmol Vis Sci. 2018; 59: 2644–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tao Y, Li XL, Sun LY, Wei YH, Yu XT, Wang H. Effect of green flickering light on myopia development and expression of M1 muscarinic acetylcholine receptor in guinea pigs. Int J Ophthalmol. 2018; 11: 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tian T, Zou L, Wu S, Liu H, Liu R. Wavelength defocus and temporal sensitivity affect refractive development in guinea pigs. Invest Ophthalmol Vis Sci. 2019; 60: 2173–2180. [DOI] [PubMed] [Google Scholar]
- 69. Yang GY, Liu FY, Li X, Zhu QR, Chen BJ, Liu LQ. Decreased expression of gap junction delta-2 (GJD2) messenger RNA and connexin 36 protein in form-deprivation myopia of guinea pigs. Chin Med J (Engl). 2019; 132: 1700–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhan X, Zhu ZC, Sun SQ, Wen YC. Dynamic changes of activator protein 1 and collagen I expression in the sclera of myopia guinea pigs. Int J Ophthalmol. 2019; 12: 1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang L, Qu X. The effects of high lighting on the development of form-deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2019; 60: 4319–4327. [DOI] [PubMed] [Google Scholar]
- 72. Zhang S, Zhang G, Zhou X, et al.. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Invest Ophthalmol Vis Sci. 2019; 60: 3074–3083. [DOI] [PubMed] [Google Scholar]
- 73. Chakraborty R, Yang V, Park HN, et al.. Lack of cone mediated retinal function increases susceptibility to form-deprivation myopia in mice. Exp Eye Res. 2019; 180: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gong X, Wu XH, Liu AL, et al.. Optic nerve crush modulates refractive development of the C57BL/6 mouse by changing multiple ocular dimensions. Brain Res. 2020; 1726: 146537. [DOI] [PubMed] [Google Scholar]
- 75. Gong Y, He X, Li Q, et al.. SCF/SCFR signaling plays an important role in the early morphogenesis and neurogenesis of human embryonic neural retina. Development. 2019; 146(20): dev174409. [DOI] [PubMed] [Google Scholar]
- 76. Driver SGW, Jackson MR, Richter K, et al.. Biallelic variants in EFEMP1 in a man with a pronounced connective tissue phenotype. Eur J Hum Genet. 2020; 28: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hsi E, Wang YS, Huang CW, Yu ML, Juo SH, Liang CL. Genome-wide DNA hypermethylation and homocysteine increase a risk for myopia. Int J Ophthalmol. 2019; 12: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liang CL, Hsu PY, Ngo CS, Seow WJ, et al.. HOXA9 is a novel myopia risk gene. BMC Ophthalmol. 2019 Jan 23; 19(1): 28, doi: 10.1186/s12886-019-1038-9. PMID: 30674274; PMCID: PMC6343304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jiang X, Kurihara T, Ikeda SI, et al.. Inducement and evaluation of a murine model of experimental myopia. J Vis Exp. 2019. [DOI] [PubMed] [Google Scholar]
- 80. Li Q, Lei F, Tang Y, et al.. Megalin mediates plasma membrane to mitochondria cross-talk and regulates mitochondrial metabolism. Cell Mol Life Sci. 2018; 75: 4021–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mori K, Kurihara T, Jiang X, et al.. Effects of hyperoxia on the refraction in murine neonatal and adult models. Int J Mol Sci. 2019; 20(23): 6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mori K, Kurihara T, Miyauchi M, et al.. Oral crocetin administration suppressed refractive shift and axial elongation in a murine model of lens-induced myopia. Sci Rep. 2019; 9: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nebert DW, Liu Z. SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside. Hum Genomics. 2019; 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pan F. Defocused image changes signaling of ganglion cells in the mouse retina. Cells. 2019; 8(7): 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seow WJ, Ngo CS, Pan H, et al.. In-utero epigenetic factors are associated with early-onset myopia in young children. PLoS One. 2019; 14: e0214791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Singh H, Sahajpal NS, Singh H, et al.. Pre-clinical and cellular toxicity evaluation of 7-methylxanthine: an investigational drug for the treatment of myopia. Drug ChemToxicol. 2019 Jul 12;1–10, doi: 10.1080/01480545.2019.1635615. Epub ahead of print. PMID: 31298043. [DOI] [PubMed] [Google Scholar]
- 87. Stone RA, McGlinn AM, Chakraborty R, et al.. Altered ocular parameters from circadian clock gene disruptions. PLoS One. 2019; 14: e0217111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Storm T, Burgoyne T, Dunaief JL, Christensen EI, Futter C, Nielsen R. Selective ablation of megalin in the retinal pigment epithelium results in megaophthalmos, macromelanosome formation and severe retina degeneration. Invest Ophthalmol Vis Sci. 2019; 60: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Szczerkowska KI, Petrezselyova S, Lindovsky J, et al.. Myopia disease mouse models: a missense point mutation (S673G) and a protein-truncating mutation of the Zfp644 mimic human disease phenotype. Cell Biosci. 2019; 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tanaka Y, Kurihara T, Hagiwara Y, et al.. Ocular-component-specific miRNA expression in a murine model of lens-induced myopia. Int J Mol Sci. 2019; 20(15): 3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tkatchenko TV, Shah RL, Nagasaki T, Tkatchenko AV. Analysis of genetic networks regulating refractive eye development in collaborative cross progenitor strain mice reveals new genes and pathways underlying human myopia. BMC Med Genomics. 2019; 12: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vutipongsatorn K, Nagaoka N, Yokoi T, et al.. Correlations between experimental myopia models and human pathologic myopia. Retina. 2019; 39: 621–635. [DOI] [PubMed] [Google Scholar]
- 93. Wang LZ, Syn N, Li S, et al.. The penetration and distribution of topical atropine in animal ocular tissues. Acta Ophthalmol. 2019; 97: e238–e247. [DOI] [PubMed] [Google Scholar]
- 94. Zhou YF, Shi LJ, Yao J, et al.. Microarray analysis of circRNA expression pattern in corneal neovascularization. Cornea. 2019; 38: 1443–1449. [DOI] [PubMed] [Google Scholar]
- 95. Liu Y, Wang Y, Lv H, Jiang X, Zhang M, Li X. Alpha-adrenergic agonist brimonidine control of experimentally induced myopia in guinea pigs: a pilot study. Mol Vis. 2017; 23: 785–798. [PMC free article] [PubMed] [Google Scholar]
- 96. Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987; 237: 73–77. [DOI] [PubMed] [Google Scholar]
- 97. Smith EL, III, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009; 50: 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chakraborty R, Pardue MT. Molecular and Biochemical Aspects of the Retina on Refraction. Progress in molecular biology and translational science. 2015; 134: 249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98b. Park H, Jabbar SB, Tan CC, et al.. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014; 55: 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Crewther DP. The role of photoreceptors in the control of refractive state. Progress in retinal and eye research. 2000; 19: 421–457. [DOI] [PubMed] [Google Scholar]
- 99b. Ramos RG, Olden K. Gene-environment interactions in the development of complex disease phenotypes. Int J Environ Res Public Health. 2008; 5: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tedja MS, Haarman AEG, Meester-Smoor MA, et al.. IMI—myopia genetics report. Invest Ophthalmol Vis Sci. 2019; 60: M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hysi PG, Choquet H, Khawaja AP, et al.. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat Genet. 2020; 52: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Musolf AM, Simpson CL, Alexander TA, et al.. Genome-wide scans of myopia in Pennsylvania Amish families reveal significant linkage to 12q15, 8q21.3 and 5p15.33. Hum Genet. 2019; 138: 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Simpson CL, Musolf AM, Li Q, et al.. Exome genotyping and linkage analysis identifies two novel linked regions and replicates two others for myopia in Ashkenazi Jewish families. BMC Med Genet. 2019; 20: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tkatchenko AV, Tkatchenko TV, Guggenheim JA, et al.. APLP2 regulates refractive error and myopia development in mice and humans. PLoS Genet. 2015; 11: e1005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boutin TS, Charteris DG, Chandra A, et al.. Insights into the genetic basis of retinal detachment. Hum Mol Genet. 2020; 29: 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Han X, Ong JS, An J, et al.. Association of myopia and intraocular pressure with retinal detachment in European descent participants of the UK Biobank cohort: a Mendelian randomization study. JAMA Ophthalmol. 2020; 138: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Williams C, Suderman M, Guggenheim JA, et al.. Grandmothers' smoking in pregnancy is associated with a reduced prevalence of early-onset myopia. Sci Rep. 2019; 9: 15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vishweswaraiah S, Swierkowska J, Ratnamala U, et al.. Epigenetically dysregulated genes and pathways implicated in the pathogenesis of non-syndromic high myopia. Sci Rep. 2019; 9: 4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhu Y, Li W, Zhu D, Zhou J. microRNA profiling in the aqueous humor of highly myopic eyes using next generation sequencing. Exp Eye Res. 2020; 195: 108034. [DOI] [PubMed] [Google Scholar]
- 110. Chen CF, Hua K, Woung LC, et al.. Expression profiling of exosomal miRNAs derived from the aqueous humor of myopia patients. Tohoku J Exp Med. 2019; 249: 213–221. [DOI] [PubMed] [Google Scholar]
- 111. Chang WA, Hsiao YT, Lin HC, Jian SF, Chen YJ, Kuo PL. Deduction of novel genes potentially involved in the effects of very low dose atropine (0.003%) treatment on corneal epithelial cells using next-generation sequencing and bioinformatics approaches. Medicina (Kaunas). 2019; 55(9): 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hsiao YT, Chang WA, Kuo MT, et al.. Systematic analysis of transcriptomic profile of the effects of low dose atropine treatment on scleral fibroblasts using next-generation sequencing and bioinformatics. Int J Med Sci. 2019; 16: 1652–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wei Q, Zhang T, Fan J, et al.. Pathological myopia-induced antioxidative proteins in the vitreous humor. Ann Transl Med. 2020; 8: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Peng M, Wei Y, Zhang Z, et al.. Increased levels of DKK1 in vitreous fluid of patients with pathological myopia and the correlation between DKK1 levels and axial length. Curr Eye Res. 2020; 45: 104–110. [DOI] [PubMed] [Google Scholar]
- 115. Enthoven CA, Tideman JWL, Polling JR, et al.. Interaction between lifestyle and genetic susceptibility in myopia: the Generation R study. Eur J Epidemiol. 2019; 34: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pozarickij A, Williams C, Hysi PG, Guggenheim JA, Eye UKB, Vision C. Quantile regression analysis reveals widespread evidence for gene-environment or gene-gene interactions in myopia development. Commun Biol. 2019; 2: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wildsoet CF, Chia A, Cho P, et al.. IMI—interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019; 60: M106–M131. [DOI] [PubMed] [Google Scholar]
- 118. Zadnik K, Sinnott LT, Cotter SA, et al.. Prediction of juvenile-onset myopia. JAMA Ophthalmol. 2015; 133: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ghorbani Mojarrad N, Plotnikov D, Williams C, et al.. Association Between Polygenic Risk Score and Risk of Myopia. JAMA Ophthalmol. 2019 Oct 31; 138(1): 7–13, doi: 10.1001/jamaophthalmol.2019.4421. Epub ahead of print. PMID: 31670792; PMCID: PMC6824229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ding X, Zhang R, Zhang S, Zhuang H, Xu G.. Differential expression of connective tissue growth factor and hepatocyte growth factor in the vitreous of patients with high myopia versus vitreomacular interface disease. BMC Ophthalmol. 2019; 19: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tedja MS, Wojciechowski R, Hysi PG, et al.. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet. 2018; 50: 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chua WH, Balakrishnan V, Chan YH, et al.. Atropine for the treatment of childhood myopia. Ophthalmology. 2006; 113: 2285–2291. [DOI] [PubMed] [Google Scholar]
- 123. Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989; 21: 180–182, 187. [PubMed] [Google Scholar]
- 124. Seider MI, Lee RY, Wang D, Pekmezci M, Porco TC, Lin SC. Optic disk size variability between African, Asian, white, Hispanic, and Filipino Americans using Heidelberg retinal tomography. J Glaucoma. 2009; 18: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen YP, Prashar A, Erichsen JT, To CH, Hocking PM, Guggenheim JA. Heritability of ocular component dimensions in chickens: genetic variants controlling susceptibility to experimentally induced myopia and pretreatment eye size are distinct. Invest Ophthalmol Vis Sci. 2011; 52: 4012–4020. [DOI] [PubMed] [Google Scholar]
- 126. Iglesias AI, Ong JS, Khawaja AP, et al.. Determining possible shared genetic architecture between myopia and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2019; 60: 3142–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Leung CK, Cheng AC, Chong KK, et al.. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2007; 48: 3178–3183. [DOI] [PubMed] [Google Scholar]
- 128. Jonas JB. Optic disk size correlated with refractive error. Am J Ophthalmol. 2005; 139: 346–348. [DOI] [PubMed] [Google Scholar]
- 130. Wolffsohn JS, Calossi A, Cho P, et al.. Global trends in myopia management attitudes and strategies in clinical practice—2019 update. Cont Lens Anterior Eye. 2020; 43: 9–17. [DOI] [PubMed] [Google Scholar]
- 131. Wildsoet CF, Chia A, Cho P, et al.. IMI—Interventions Myopia Institute: interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019; 60: M106–M131. [DOI] [PubMed] [Google Scholar]
- 132. Lam CSY, Tang WC, Tse DY, et al.. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020; 104(3): 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li Y, Fu Y, Wang K, Liu Z, Shi X, Zhao M. Evaluating the myopia progression control efficacy of defocus incorporated multiple segments (DIMS) lenses and Apollo progressive addition spectacle lenses (PALs) in 6- to 12-year-old children: study protocol for a prospective, multicenter, randomized controlled trial. Trials. 2020; 21: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019; 96: 556–567. [DOI] [PubMed] [Google Scholar]
- 135. Sankaridurg P, Bakaraju RC, Naduvilath T, et al.. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt. 2019; 39: 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Walline JJ, Walker MK, Mutti DO, et al.. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020; 324: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lau JK, Vincent SJ, Cheung SW, Cho P. The influence of orthokeratology compression factor on ocular higher-order aberrations. Clin Exp Optom. 2020; 103: 123–128. [DOI] [PubMed] [Google Scholar]
- 138. Cheung SW, Boost MV, Cho P. Pre-treatment observation of axial elongation for evidence-based selection of children in Hong Kong for myopia control. Cont Lens Anterior Eye. 2019; 42: 392–398. [DOI] [PubMed] [Google Scholar]
- 139. Yam JC, Li FF, Zhang X, et al.. Two-year clinical trial of the Low-Concentration Atropine for Myopia Progression (LAMP) study: phase 2 report. Ophthalmology. 2020; 127: 910–919. [DOI] [PubMed] [Google Scholar]
- 140. Chen Z, Huang S, Zhou J, Xiaomei Q, Zhou X, Xue F. Adjunctive effect of orthokeratology and low dose atropine on axial elongation in fast-progressing myopic children: a preliminary retrospective study. Cont Lens Anterior Eye. 2019; 42: 439–442. [DOI] [PubMed] [Google Scholar]
- 141. Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kakehashi A. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol. 2018; 62: 544–553. [DOI] [PubMed] [Google Scholar]
- 142. Tan Q, Ng AL, Choy BN, Cheng GP, Woo VC, Cho P. One-year results of 0.01% atropine with orthokeratology (AOK) study: a randomised clinical trial. Ophthalmic Physiol Opt. 2020; 40: 557–566. [DOI] [PubMed] [Google Scholar]
- 143. Khanal S, Turnbull PRK, Lee N, Phillips JR. The effect of atropine on human global flash mfERG responses to retinal defocus. Invest Ophthalmol Vis Sci. 2019; 60: 218–225. [DOI] [PubMed] [Google Scholar]
- 144. Kanda H, Oshika T, Hiraoka T, et al.. Effect of spectacle lenses designed to reduce relative peripheral hyperopia on myopia progression in Japanese children: a 2-year multicenter randomized controlled trial. Jpn J Ophthalmol. 2018; 62: 537–543. [DOI] [PubMed] [Google Scholar]
- 145. Ruiz-Pomeda A, Perez-Sanchez B, Valls I, Prieto-Garrido FL, Gutierrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain (MASS): a 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018; 256: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 146. Li Z, Hu Y, Cui D, Long W, He M, Yang X. Change in subfovealchoroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. 2019; 97: e454–e459. [DOI] [PubMed] [Google Scholar]
- 147. Joachimsen L, Bohringer D, Gross NJ, et al.. A pilot study on the efficacy and safety of 0.01% atropine in German schoolchildren with progressive myopia. Ophthalmol Ther. 2019; 8: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147b. Azuara-Blanco A, Logan N, Strang N, et al.. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: a multicentre placebo-controlled randomised trial in the UK (CHAMP-UK)-study protocol. Br J Ophthalmol. 2020; 104(7): 950–955, doi: 10.1136/bjophthalmol-2019-314819. Epub 2019 Oct 25. PMID: 31653669. [DOI] [PubMed] [Google Scholar]
- 148. McCrann S, Flitcroft I, Strang NC, et al.. Myopia Outcome Study of Atropine in Children (MOSAIC): an investigator-led, double-masked, placebo-controlled, randomised clinical trial protocol. HRB Open Res. 2019; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yam JC, Jiang Y, Tang SM, et al.. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019; 126: 113–124. [DOI] [PubMed] [Google Scholar]
- 150. Huang J, Mutti DO, Jones-Jordan LA, Walline JJ. Bifocal & atropine in myopia study: baseline data and methods. Optom Vis Sci. 2019; 96: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tan Q, Ng AL, Cheng GP, Woo VC, Cho P. Combined atropine with orthokeratology for myopia control: study design and preliminary results. Curr Eye Res. 2019; 44: 671–678. [DOI] [PubMed] [Google Scholar]
- 152. Aguila-Carrasco AJ, Read SA, Montes-Mico R, Iskander DR. The effect of aberrations on objectively assessed image quality and depth of focus. J Vis. 2017; 17: 2. [DOI] [PubMed] [Google Scholar]
- 153. Walline JJ, Lindsley KB, Vedula SS, et al.. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020; 1: CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. College of Optometrists Myopia Management. https://www.college-optometrists.org/the-college/policy/myopia-management.html. Published 2020. Accessed March 7, 2020. [Google Scholar]
- 155. Williams R, Bakshi S, Ostrin EJ, Ostrin LA. Continuous objective assessment of near work. Sci Rep. 2019; 9: 6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Singh NK, Jaskulski M, Ramasubramanian V, et al.. Validation of a clinical aberrometer using pyramidal wavefront sensing. Optom Vis Sci. 2019; 96: 733–744. [DOI] [PubMed] [Google Scholar]
- 157. Plaza-Puche AB, Salerno LC, Versaci F, Romero D, Alio JL. Clinical evaluation of the repeatability of ocular aberrometry obtained with a new pyramid wavefront sensor. Eur J Ophthalmol. 2019; 29: 585–592. [DOI] [PubMed] [Google Scholar]
- 158. Devarajan K, Sim R, Chua J, et al.. Optical coherence tomography angiography for the assessment of choroidal vasculature in high myopia. Br J Ophthalmol. 2020; 104: 917–923. [DOI] [PubMed] [Google Scholar]
- 159. Ye J, Wang M, Shen M, et al.. Deep retinal capillary plexus decreasing correlated with the outer retinal layer alteration and visual acuity impairment in pathological myopia. Invest Ophthalmol Vis Sci. 2020; 61: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gifford KL, Richdale K, Kang P, et al.. IMI—clinical management guidelines report. Invest Ophthalmol Vis Sci. 2019; 60: M184–M203. [DOI] [PubMed] [Google Scholar]
- 161. Kinoshita N, Konno Y, Hamada N, et al.. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020; 10: 12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Efron N, Morgan PB, Woods CA, Santodomingo-Rubido J, Nichols JJ; International Contact Lens Prescribing Survey Consortium. International survey of contact lens fitting for myopia control in children. Cont Lens Anterior Eye. 2020; 43: 4–8. [DOI] [PubMed] [Google Scholar]
- 163. Gifford KL. Childhood and lifetime risk comparison of myopia control with contact lenses. Cont Lens Anterior Eye. 2020; 43: 26–32. [DOI] [PubMed] [Google Scholar]
- 164. Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019; 96: 463–465. [DOI] [PubMed] [Google Scholar]