Abstract
Background
Poor self-care skills and personal hygiene resulted from limitations in learning and understanding, put intellectually disabled individuals at greater risk for intestinal parasitic infections (IPIs). Despite several regional reports in Iran, the overall burden on IPIs among intellectually disabled individuals is poorly understood. Hence, the present study aimed to estimate the pooled prevalence of IPIs among intellectually disabled individuals in Iran.
Methods
Using the PRISMA guidelines, we conducted a systematic review and meta-analysis of data retrieved from seven electronic databases (PubMed, Web of Science, Scopus, Embase, and ProQuest for English articles, as well as SID and Magiran for Persian) from their inception up to December 2020. Pooled prevalence was estimated using a random-effects model with a 95% confidence interval (CI) and depicted as a forest plot, while heterogeneity was evaluated using Cochran’s Q-test.
Results
Exactly 1263 of the 3004 intellectually disabled individuals examined by 14 studies across 10 provinces of Iran were positive for IPIs. Overall pooled prevalence estimate was 41% (95% CI 29–53%) with a range of 21% (95% CI 10–32%) to 68% (95% CI 55–80%) across sub-groups. Entamoeba coli (16.2%; 95% CI 10.3–22%), Blastocystis spp. (12.2%; 95% CI 7.2–17.2%), and Giardia duodenalis (11.9%; 95% CI 7.4–16.3%) were the most prevalent protozoan species. In terms of helminthic agents, the most prevalent species were Enterobius vermicularis (11.3%; 95% CI 6.3–16.3%) followed by Strongyloides stercoralis (10.9%; 95% CI 5.0–16.9%) and Hymenolepis nana (2.8%; 95% CI 0.4–5.2%)
Conclusion
IPIs are highly prevalent among intellectually disabled individuals in Iran. Improving the health status and implementing infectious disease prevention strategies in rehabilitation centers, health promotion interventions to improve personal hygiene of intellectually disabled individuals, as well as utilize sensitive diagnostic methods besides routine stool examination techniques, and treatment of infected individuals will help in the control of these infections among intellectually disabled individuals.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13099-021-00424-6.
Keywords: Intestinal parasites, Intellectual disability, Mental retardation, Systematic review, Meta-analysis, Iran
Background
Intestinal parasitic infections (IPIs) are among the leading causes of global health problems, especially among the deprived communities where poor personal hygiene, environmental sanitation, socio-economic, demographic, and health-related behaviors have contributed to a notable prevalence. Giardia duodenalis, Entamoeba histolytica, Cryptosporidium spp., and Ascaris lumbricoides play significant roles in this scenario. However, parasites such as Enterobius vermicularis and Strongyloides stercoralis are still ignored [1, 2].
In the case of IPIs, the vulnerabilities of certain groups of people have been highlighted, such as children, immunocompromised patients, and intellectually disabled individuals. Among these, less apparent has been the plight of people with intellectual disability, who have a range of vulnerabilities that include health problems, mental disorders, and social disadvantage [3]. Intellectual disability, formerly defined as mental retardation, is a cluster of disorders characterized by low intelligence and associated limitations in adaptive behavior [4]. Most people with such disabilities cannot be trained for proper health behaviors and are prone to get the infection. Due to limitations in learning and understanding, poor self-care skills, poor personal hygiene, and pica habits, intellectually disabled individuals are at higher risk of IPIs. In addition, people with intellectual disabilities are mostly kept in crowded places such as rehabilitation centers or care homes for a long time, where puts them at a greater risk for IPIs [5, 6].
In a resource-limited country like Iran, with about 80 million people, cost-effectiveness in the control of IPIs is essential to ensure efficient allocation of resources and achievement of high impact. Hence, investigation and providing epidemiological information on IPIs among intellectually disabled individuals, as a high-risk population, could help to design a targeted and cost-effective control program. Despite several regional reports in Iran, the overall burden on IPIs among intellectually disabled individuals is poorly understood. Hence, the present systematic review and meta-analysis, which is the first of its kind, aimed to estimate the pooled prevalence of IPIs among intellectually disabled individuals in Iran. This will serve as a guide for targeted control and ensure cost-effective control of IPIs in Iran.
Methods
The present study followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guideline published by Moher et al. [7], and the inclusion of data for quantitative synthesis was entered, based on the PRISMA checklist (Additional file 1). The infection of intellectually disabled individuals with intestinal parasites in Iran was the outcome of interest.
Search strategy
Published studies searched in five international databases (PubMed, Web of Science, Scopus, Embase, and ProQuest) and two national databases (SID and Magiran) from their inception up to December 2020 (Additional file 2). Furthermore, the Google Scholar database was used for proofing the search. Relevant articles were found using the following search terms: (‘intestinal parasites’ or ‘parasitic intestinal disease’ or ‘intestinal protozoa’ or ‘intestinal helminths’ or ‘soil-transmitted helminth’) and (‘intellectually disabled’ or ‘mentally disabled’ or ‘mentally retarded’ or ‘rehabilitation center’) and (‘Iran’). Bibliographic lists of the relevant studies were searched to find other associated articles that were not found through database searching. All searched articles were imported to EndNote X8 software (Thompson Reuter, CA, USA) for management.
Eligibility criteria
Eligibility for the inclusion of each study was based on the following conditions: (a) it was carried out in Iran, (b) it was reported IPIs among intellectually disabled individuals, (c) it was published in English or Persian, (d) it was a cross-sectional study, (e) sample size and the number of positive cases were clearly stated, (f) it was published in a peer-reviewed journal, and (g) parasites were identified at least to the genus level. Studies that did not meet these inclusion criteria were excluded.
Study selection and data extraction
After removing duplicated articles, the studies screened through title and abstract for relevance. Then, a full-text review to determine the presence of the inclusion requirements. To ensure data validation and increase the likelihood of detecting errors, literature search, screening of articles, article selection for eligibility, and data extraction were performed by two authors independently. Also, discrepancies were removed following discussion with the third reviewer. Data pulled out from each included study, where the author names, the year the study was carried out, the year of the publication, sample size, number of positive cases, study location (region/province), type of the reported intestinal parasites, and method of diagnosis.
Quality assessment
Quality assessment of the included studies was carried out using the Joanna Briggs Institute (JBI) critical appraisal instrument for studies reporting prevalence data [8] (Additional file 3). This tool comprised nine items with four options include ‘yes’, ‘no’, ‘unclear’, and ‘not applicable’. The ‘yes’ answers were used to calculate the final score of each article. Answers to the mentioned questions for individual studies were respectively assigned scores of 0 or 1 for ‘yes’ or ‘no’ answers, while ‘unclear’ or ‘not applicable’ were used when a study does not clearly answer the question or when the question was not applicable for the study. For study inclusion in the quantitative synthesis, a minimum quality assessment score of 6 (66.7%), which means answering ‘yes’ to at least six of the nine questions on the checklist was required.
Data synthesis and statistical analysis
Preliminary analyses, including summations, subtractions, divisions, multiplications, and estimation of percentages, were carried out using Microsoft Excel (Microsoft® Office 2013). Statistical and meta-analyses were conducted using Stata® statistical software version 14 (Stata Corp, College Station, TX, USA).
Pooling, sub-group, and heterogeneity analyses
Pooled prevalence and their 95% confidence interval (CI) were estimated by the random-effects model and presented as a forest plot [9]. Sub-group analysis was carried out based on the geographical regions, study period (year/s of conduction), sample size, diagnostic methods, risk of bias, and type of the reported intestinal parasites (protozoa/helminth).
Heterogeneity, which is the measure of variability between studies analyzed, was evaluated using Cochran’s Q-test, while percentage variation in prevalence estimate due to heterogeneity, was quantified using the inverse variance (I2) statistic. I2 values of 0, 25, 50, and 75% were considered as ‘no’, ‘low’, ‘medium’, and ‘high’ heterogeneities, respectively [10].
Publication bias, sensitivity, and meta-regression analyses
Publication bias (across-study bias) was examined by funnel plots, while the statistical significance was assessed by the Egger’s regression asymmetry test [11] and Begg’s rank correlation methods [12], respectively. A sensitivity analysis was carried out using a random-effect model through step-by-step omitting of a single study to evaluate the robustness of the pooled prevalence estimate [13]. Moreover, meta-regression was carried out by considering the publication year and sample size to detect the potential source of heterogeneity.
Results
Literature search and eligible studies
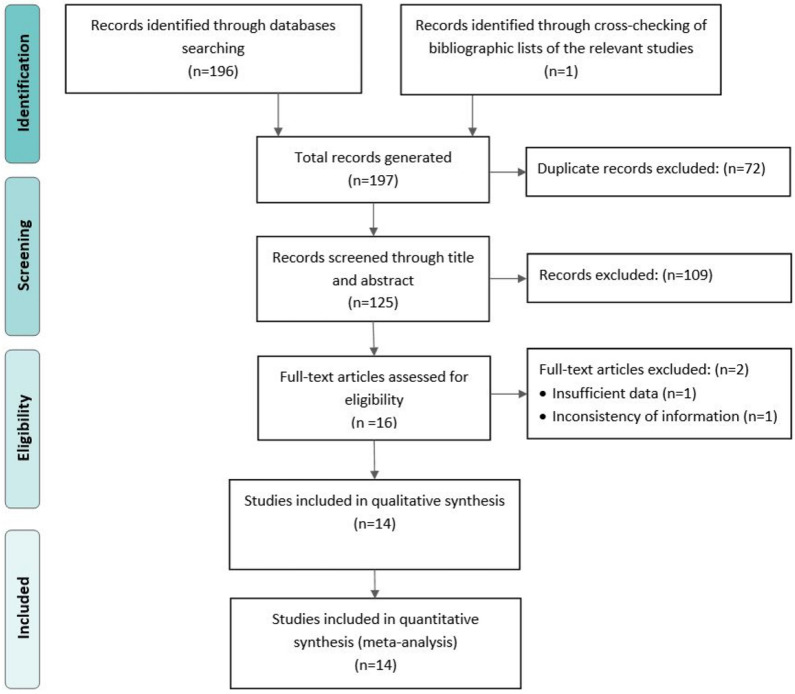
The procedure for the selection of eligible studies is presented in Fig. 1. Of the 197 studies identified, 196 were retrieved through the search of databases and one from the lists of eligible article references. Seventy-two studies were excluded because of duplication. One hundred-twenty-five studies were subjected to title and abstract review, where 109 studies were excluded. Full text of 16 remains studies assessed, and two studies excluded because of the inconsistency in the information (n = 1) and insufficient data on sample sizes and the number of cases (n = 1). Finally, 14 studies were subjected to quantitative synthesis.
Fig. 1.
PRISMA flowchart diagram for the selection process of eligible studies
Characteristics of the eligible studies
Table 1 shows the characteristics of the eligible studies. Fourteen studies examined 3004 intellectually disabled individuals for IPIs in Iran and reported prevalence rates ranging between 5.2 and 79%. Studies were conducted between 1991 and 2017 and published between 1994 and 2019. Three studies utilized the Graham test for E. vermicularis diagnosis. Also, three studies utilized agar plate culture for S. stercoralis and hookworm diagnosis. Seven studies had a sample size of less than 200, while only three studies had a sample size of more than 300. None of the studies assessed for quality by the JBI critical appraisal instrument was excluded for lack of merit. Quality scores ranged between 6 and 8 (66.67–88.89%) of a total of 9 scores (Table 1 and Additional file 4).
Table 1.
List and characteristics of the 14 eligible studies for IPIs among intellectually disabled individuals in Iran, 2020
| No. | Authors (Reference) | Year of study | Publication year | Province | Region | Sample size | No. of positive | Prev. (%) | Diagnostic method | QAS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rouhani et al. [31] | 1991/1992 | 1994 | Tehran | Center | 330 | 196 | 59.4 | D-F-G | 7 |
| 2 | Mahyar et al. [32] | 1998 | 2000 | Qazvin | Center | 258 | 146 | 56.6 | D-F | 7 |
| 3 | Mousavian et al. [33] | 2004 | 2006 | Tehran | Center | 175 | 113 | 64.6 | D-F-G | 8 |
| 4 | Sharif et al. [34] | 2008 | 2010 | Mazandaran | North | 362 | 95 | 26.2 | D-F-S | 7 |
| 5 | Hazrati Tappeh et al. [35] | 2007/2008 | 2010 | Urmia | North-west | 225 | 46 | 20.4 | D-F | 8 |
| 6 | Davari et al. [36] | 2011 | 2011 | Ardabil | North-west | 216 | 95 | 44.0 | D-F-S | 7 |
| 7 | Rasti et al. [37] | 2006/2007 | 2012 | Isfahan | Center | 243 | 192 | 79.0 | D-F-G | 7 |
| 8 | Shokri et al. [29] | 2010 | 2012 | Hormozgan | South | 133 | 64 | 48.1 | D-F-S | 6 |
| 9 | Soosaraei et al. [38] | 2009 | 2014 | Golestan | North | 196 | 24 | 12.2 | D-F-S | 7 |
| 10 | Anvari et al. [39] | 2014 | 2015 | Yazd | Center | 129 | 55 | 42.6 | D-F-S | 6 |
| 11 | Ahmadi et al. [40] | 2013/2014 | 2015 | Mazandaran | North | 341 | 112 | 32.8 | D-F-S-C | 7 |
| 12 | Soleimani et al. [41] | 2015 | 2016 | Mazandaran | North | 97 | 5 | 5.2 | D-F | 7 |
| 13 | Saeidnia et al. [42] | 2013 | 2016 | Gilan | North | 173 | 51 | 29.5 | D-F-S-C | 8 |
| 14 | Mohammadi et al. [43] | 2016/2017 | 2019 | Hormozgan | South | 126 | 69 | 54.8 | D-F-S-C | 7 |
D: Direct wet-mount; F: Formalin-ether; S: Staining (Trichrome and Ziehl–Neelsen); G: Graham test; C: Agar plate culture; Prev.: Prevalence; QAS: Quality assessment score
Pooling, sub-group, and heterogeneity analyses
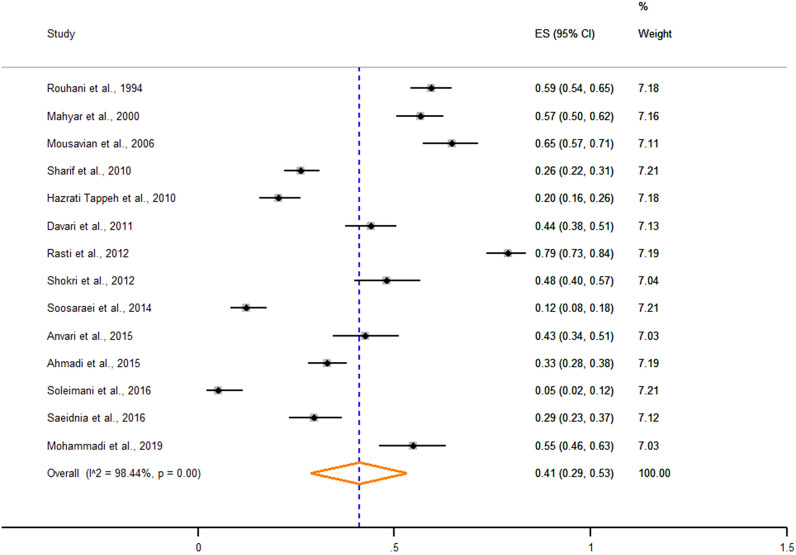
Fourteen studies reported 1263 positive cases of IPIs among 3004 intellectually disabled individuals examined from ten provinces of Iran. The overall pooled prevalence of the IPIs among intellectually disabled individuals was estimated at 41% (95% CI 29–53%) (Fig. 2). Pooled prevalence estimates for subgroups, including geographical region, study period, sample size, diagnostic method, and risk of bias, are presented in Table 2. The highest prevalence estimates were recorded in center regions (61%; 95% CI 49–72%). In terms of the diagnostic method, studies that implemented the Graham test, besides direct wet-mount and concentration methods, recorded the highest pooled prevalence estimate (68%; 95% CI 55–80%). Also, high heterogeneity was observed in the results of the included studies (Q = 833.9, I2 = 98.4%, and p < 0.001).
Fig. 2.
Forest plot showing the pooled prevalence of IPIs among intellectually disabled individuals in Iran, 2020
Table 2.
Pooled prevalence estimates for IPIs among intellectually disabled individuals stratified according to sub-groups in Iran, 2020
| Variables | No. of studies | Pooled prevalence estimates | Heterogeneity | |||
|---|---|---|---|---|---|---|
| Sample size | Positives | Prevalence (95% CI) | I2 (%) | Q-p | ||
| Region | ||||||
| Center | 5 | 1135 | 596 | 61 (49–72) | 93.9 | < 0.001 |
| North | 5 | 1169 | 430 | 21 (10–32) | 95.7 | < 0.001 |
| North-east | 2 | 441 | 117 | 30 (25–34) | – | – |
| South | 2 | 259 | 120 | 51 (45–57) | – | – |
| Study period | ||||||
| 1991–2000 | 2 | 588 | 342 | 58 (54–62) | – | – |
| 2001–2010 | 6 | 1334 | 605 | 42 (19–64) | 98.9 | < 0.001 |
| 2011–2020 | 6 | 1082 | 316 | 35 (19–50) | 97.1 | < 0.001 |
| Sample size | ||||||
| < 200 | 7 | 1029 | 403 | 37 (19–54) | 98.4 | < 0.001 |
| 200–300 | 4 | 942 | 479 | 50 (24–76) | 99.1 | < 0.001 |
| 300 < | 3 | 1033 | 403 | 39 (20–59) | 98.8 | – |
| Diagnostic method | ||||||
| D-F | 3 | 580 | 197 | 27 (0–56) | – | – |
| D-F-G | 3 | 748 | 501 | 68 (55–80) | – | – |
| D-F-S | 5 | 1036 | 333 | 34 (21–48) | 96.0 | < 0.001 |
| D-F-S-C | 3 | 640 | 232 | 39 (26–52) | – | – |
| Risk of bias | ||||||
| Low | 9 | 2123 | 884 | 42 (27–56) | 98.3 | < 0.001 |
| Medium | 5 | 881 | 379 | 40 (16–64) | 98.7 | < 0.001 |
| Overall | 14 | 3004 | 1263 | 41 (29–53) | 99.8 | < 0.001 |
D: Direct wet-mount; F: Formalin-ether; S: Staining (Trichrome and Ziehl–Neelsen); G: Graham test, C: Agar plate culture; CI: Confidence interval; I2: Inverse variance index; Q-p: Cochran’s p-value
Intestinal parasites species-specific pooled prevalence
For intestinal protozoa, Entamoeba coli (16.2%; 95% CI 10.3–22%), Blastocystis spp. (12.2%; 95% CI 7.2–17.2%), and G. duodenalis (11.9%; 95% CI 7.4–16.3%) were the most prevalent species. In terms of helminthic agents, the most prevalent species were E. vermicularis (11.3%; 95% CI 6.3–16.3%) followed by S. stercoralis (10.9%; 95% CI 5.0–16.9%) and Hymenolepis nana (2.8%; 95% CI 0.4–5.2%). Species-specific pooled prevalence estimates for IPIs among intellectually disabled individuals in Iran are embedded in Table 3.
Table 3.
Species-specific pooled prevalence estimates for IPIs among intellectually disabled individuals in Iran, 2020
| Parasite | No. of studies | Pooled prevalence estimates | Heterogeneity | |||
|---|---|---|---|---|---|---|
| Sample size | Positives | Prevalence (95% CI) | I2 (%) | p | ||
| Protozoa | ||||||
| Entamoeba coli | 13 | 2829 | 495 | 16.2 (10.3–22.0) | 96.4 | < 0.001 |
| Blastocystis spp. | 10 | 1900 | 248 | 12.2 (7.2–17.2) | 94.8 | < 0.001 |
| Giardia duodenalis | 12 | 2566 | 329 | 11.9 (7.4–16.3) | 94.4 | < 0.001 |
| Iodamoeba butschlii | 8 | 1868 | 84 | 3.9 (2.2–5.6) | 76.1 | < 0.001 |
| Chilomastix mesnili | 7 | 1734 | 84 | 3.6 (1.5–5.7) | 92.2 | < 0.001 |
| Endolimax nana | 6 | 1370 | 51 | 2.9 (0.9–5.0) | 88.0 | < 0.001 |
| Entamoeba histolytica/dispar | 7 | 1830 | 69 | 2.6 (1.0–4.2) | 89.3 | < 0.001 |
| Dientamoeba fragilis | 1 | 243 | 4 | – | – | – |
| Entamoeba hartmanni | 1 | 243 | 58 | – | – | – |
| Helminths | ||||||
| Enterobius vermicularis | 8 | 1686 | 234 | 11.3 (6.3–16.3) | 97.5 | < 0.001 |
| Strongyloides stercoralis | 5 | 1103 | 112 | 10.9 (5.0–16.9) | 95.6 | < 0.001 |
| Hymenolepis nana | 4 | 847 | 33 | 2.8 (0.4–5.2) | 83.7 | < 0.001 |
| Trichuris trichiura | 4 | 984 | 24 | 2.0 (0.7–3.2) | 52.2 | 0.09 |
| Ascaris lumbricoides | 2 | 588 | 7 | 1.2 (0.3–2.1) | – | – |
| Dicrocoelium dendriticum | 2 | 599 | 28 | 0.9 (0.1–1.6) | – | – |
| Taenia spp. | 2 | 671 | 3 | 0.4 (0.0–0.9) | – | – |
| Trichostrongylus spp. | 2 | 599 | 3 | 0.4 (0.1–0.9) | – | – |
| Hookworms | 1 | 330 | 1 | – | – | – |
CI: Confidence interval; I2: Inverse variance index
Publication bias, sensitivity, and meta-regression analyses
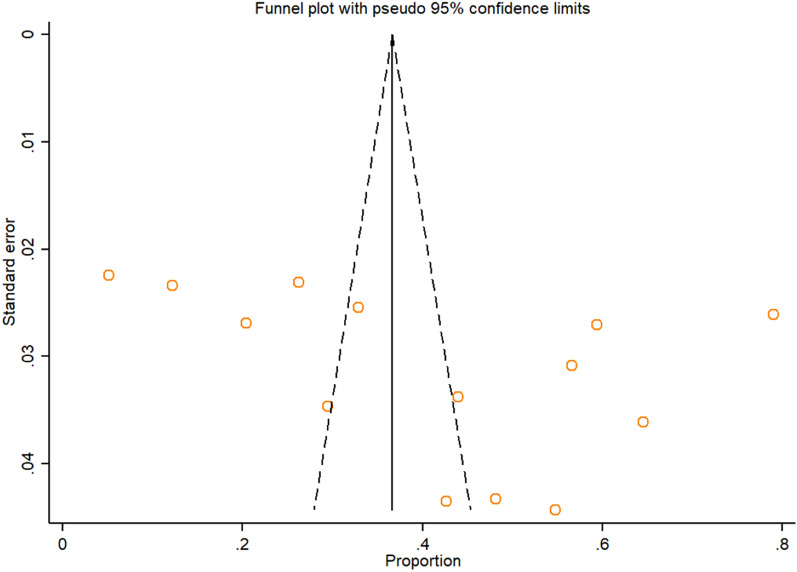
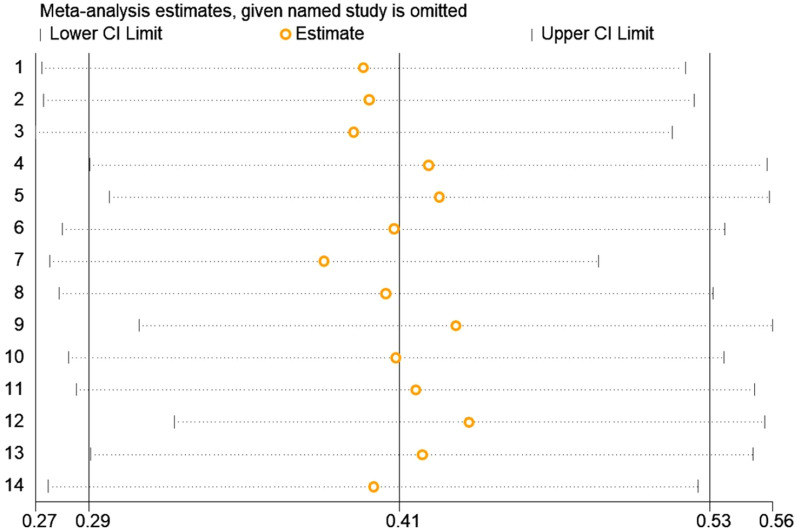
Symmetrical funnel plot visual inspection (Fig. 3) showed absence of publication bias, which was statistically verified by Begg’s test (p = 0.112) and Egger’s test (bias coefficient (B) = 15.79 (95% CI − 3.98–35.56; p = 0.107). Sensitivity analysis was carried out by excluding each study step-by-step from the meta-analysis and comparing the point prevalence estimates before and after removing a single study. Removing a single study did not alter the pooled prevalence estimate considerably, with sensitivity analysis ranging from 38% (when study No.7 was removed) to 44% (when study No.12 was removed) (Fig. 4). A univariate meta-regression between the infection prevalence and the year of publication and the sample size showed no statistically significant correlations (p > 0.05) (Table 4).
Fig. 3.
Funnel plot displaying the prevalence estimate of IPIs among intellectually disabled individuals in Iran, 2020
Fig. 4.
Sensitivity analysis of included studies to determine the pooled prevalence estimate of IPIs among intellectually disabled individuals in Iran, 2020 (1 to 14: The included studies, in the order embedded in Table 1)
Table 4.
Univariate meta-regression of factors related to the heterogeneity of IPIs among intellectually disabled individuals in Iran, 2020
| Variables | Coefficient | p-value |
|---|---|---|
| Year of publication | − 0.0129388 | 0.143 |
| Sample size | 0.0003108 | 0.672 |
Discussion
An adequate understanding of the nationwide burden of IPIs and the widespread species, particularly in high-risk populations is essential for targeted and cost-effective prevention and control. Thus, the current systematic review and meta-analysis is the first of its kind in Iran that provides comprehensive useful epidemiological information regarding IPIs among intellectually disabled individuals that may serve as a guide for disease control in Iran.
We observed an overall pooled prevalence of 41% among intellectually disabled individuals which is more than 38% and 19% for Iranian school children [14] and food handlers [15], respectively. The population density in rehabilitation centers and care homes, close contact, poor personal hygiene due to limitation in learning, lack of health facilities, as well as lack of sufficient knowledge of the authorities managing rehabilitation centers on intestinal parasites transmission modes may explain the high prevalence of IPIs among intellectually disabled individuals in Iran. On the other hand, as intellectually disabled individuals have difficulties in communications, and it is not easy for them to express their gastrointestinal discomforts and complaints, many IPIs harm them chronically.
Though there are no available systematic reviews and meta-analyses conducted on related topics in Iran and elsewhere, the pooled prevalence of IPIs among intellectually disabled individuals in the present study is higher than the primary studies conducted in Tanzania (12.4%) and Italy (23%) [16, 17]. However, the pooled prevalence estimate of IPIs among intellectually disabled individuals in the present study is lower than the primary study conducted in Egypt (43.5%) [18]. These variations may be attributable to differences in the levels of hygiene and sanitation and health education among different rehabilitation centers as well as the specificity and sensitivity of the diagnostic methods employed by the individual studies.
Rehabilitation center staff and their families are at risk of infection due to their direct/indirect contact with intellectually disabled individuals. In this case, Barazesh et al. [19] reported a significant prevalence (34%) of IPIs among the staff of a rehabilitation center in the north-east of Iran. Regarding the high prevalence of IPIs among intellectually disabled individuals in the present study, it is crucial for informing the staff about transmission modes of intestinal parasites.
Sub-group analysis based on the study period revealed a 23% decline in the prevalence of IPIs among intellectually disabled individuals during the past three decades in Iran. Although due to the implementation of a non-targeted control strategy in Iran, the prevalence of IPIs has a dramatic decrease in children [14], these infections remain a challenging public health problem in intellectually disabled individuals.
E. coli, Blastocystis spp., and G. duodenalis, were the most predominant protozoan species reported during the period under review. These species are the most commonly reported in Iran in almost all studies on IPIs among different populations [2, 20]. The majority of individuals infected with these species are asymptomatic and excrete numerous cysts that remain viable for a long time in the environment. It seems the healthy carrier, an environmentally resistant cyst, which enhances the chance of these species to infect the new host, as well as a great potential to transmit as a water/foodborne infection [15, 21, 22], could explain the high prevalence of these species compared with other protozoan species.
E. vermicularis and S. stercoralis following H. nana were the most predominant helminth records among intellectually disabled individuals in Iran. Institutions for intellectually disabled individuals have been reported to be hyper-endemic for several helminths, such as S. stercoralis and E. vermicularis (24). The high prevalence of these species could be attributable to the fact that these species can easily be spread in crowded places, such as rehabilitation centers and their dormitories where the person-to-person is the main mode of intestinal parasites transmission due to close contact. On the other hand, autoinfection, as a hallmark of enterobiasis, strongyloidiasis, and hymenolepiasis, could cause protracted infection in intellectually disabled individuals [23–25]. Although the prevalence of human helminthic diseases has plunged in recent decades throughout Iran, some of them, particularly those with direct fecal–oral transmissions, such as Enterobius and Hymenolepis, remain common [26]. The high prevalence of these parasites among intellectually disabled individuals should not be considered a sudden infection and seems to be due to the previous, chronic, accumulated, and untreated infections.
In the present study, the highest prevalence estimates were recorded in central regions. Urbanization and subsequently more tendency to keeping intellectually disabled individuals in rehabilitation centers and their crowded dormitories may explain the high prevalence of IPIs in the central region of Iran. However, S. stercoralis, as a soil-transmitted helminth, was mostly reported from the northern (Mazandaran and Gilan) and southern (Khuzestan, Bushehr, and Hormozgan) regions [27–29], which are considered the humid areas in Iran.
The higher pooled prevalence estimates in the studies which employed the Graham test and agar plate culture besides routine stool examinations reflect the fact that implementation of these methods together enhanced the sensitivity of intestinal parasites detection [30]. Since the routine stool examination methods such as direct and concentration techniques, have low sensitivity for detection of E. vermicularis and S. stercoralis and due to the high prevalence of these two nematodes in the rehabilitation centers, additional tests such as the Graham test for E. vermicularis and agar plate culture and serological methods for S. stercoralis should be considered in the periodic medical check-ups of intellectually disabled individuals.
As the public health implications of our findings, the present epidemiological information could be a springboard for more investigation of IPIs among intellectually disabled individuals and subsequently designing a targeted control program to reduce the burden of IPIs in Iran. For this purpose, further direct interventions are suggested as follows: (a) health promotion interventions to improve personal hygiene among intellectually disabled individuals, besides implementing infectious disease prevention strategies in rehabilitation centers, (b) informing staff about how intestinal parasites are transmitted among intellectually disabled individuals and between them and the staff, (c) periodic check-ups of intellectually disabled individuals and rehabilitation centers staffs for IPIs, (d) regarding the high prevalence of E. vermicularis and S. stercoralis in rehabilitation centers, considering sensitive diagnostic methods such as Graham test, and serological and stool culture methods, and (e) allocate sufficient funds by health policymakers to prevent and control IPIs in rehabilitation centers. Also, we suggest more studies using sensitive diagnostic methods in rehabilitation centers of different provinces such as Khorasan, Sistan and Baluchistan, and Kerman provinces.
Limitations
Though this study provided valuable epidemiological information on the prevalence of IPIs among intellectually disabled individuals in Iran, which will be useful in disease control, it is not devoid of limitations. We could not include some potentially relevant studies which would have added to the understanding of IPIs among intellectually disabled individuals due to insufficiency of data. Some regions (east, north-east, and south-east) were not represented in the analysis because no study was published from these regions. Another setback is the study revealed a high heterogeneity among studies, which may be due to variations in sample populations, region, and diagnostic methods employed by the various studies.
Conclusion
IPIs are highly prevalent among intellectually disabled individuals in Iran with an overall pooled prevalence estimate of 41%. However, there was a 23% decline in the pooled prevalence over 26 years, but IPIs in this high-risk population remain substantial. In addition to E. coli, G. duodenalis, and Blastocystis spp. as prevalent protozoan species, the prevalence of E. vermicularis and S. stercoralis were significant in intellectually disabled individuals. Besides taking into account the presented epidemiological information, rehabilitation centers authorities should adopt a responsible approach, including improving the health status of rehabilitation centers through implementing infectious disease prevention strategies and providing sanitary facilities. Health education and awareness about intestinal parasites transmission routes in rehabilitation centers, utilizing sensitive diagnostic methods besides routine stool examination techniques, as well as treatment of infected individuals, will help in the control of IPIs among intellectually disabled individuals.
Supplementary Information
Additional file 3. JBI Critical Appraisal Checklist for Studies Reporting Prevalence Data.
Additional file 4. Quality assessment scores for eligible studies.
Acknowledgements
Not applicable.
Abbreviations
- IPIs
Intestinal parasitic infections
- PRISMA
Preferred reporting items for systematic reviews and meta-analysis
- SID
Scientific Information Database
- JBI
Joanna Briggs Institute
- CI
Confidence interval
- I2
Inverse variance
Authors' contributions
MJAA: involved in designing, conducting a literature search, analysis, interpretations, and writing of the manuscript. MM: participated in designing, interpretations, and revision of the manuscript. SM and AT: participated in designing, interpretations, and drafting the manuscript. BS and RS: participated in literature search and data extraction. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites–should we use a holistic approach? Int J Infect Dis. 2010;14(9):e732–738. doi: 10.1016/j.ijid.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Mohebali M, Keshavarz H, Abbaszadeh Afshar MJ, Hanafi-Bojd AA, Hassanpour GH. Spatial distribution of common pathogenic human intestinal protozoa in Iran: a systematic review article. Iran J Public Health. 2021;50(1):1–5. doi: 10.18502/ijph.v50i1.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtenay K, Perera B. COVID-19 and people with intellectual disability. Impacts of a pandemic. Ir J Psychol Med. 2020;37(3):1–21. doi: 10.1017/ipm.2020.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carulla LS, Reed GM, Vaez-Azizi LM, Cooper S-A, Leal RM, Bertelli M, Adnams C, Cooray S, Deb S, Dirani LA. Intellectual developmental disorders: towards a new name, definition and framework for “mental retardation/intellectual disability” in ICD-11. World Psychiatry. 2011;10(3):175. doi: 10.1002/j.2051-5545.2011.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fentahun AA, Asrat A, Bitew A, Mulat S. Intestinal parasitic infections and associated factors among mentally disabled and non-disabled primary school students, Bahir Dar, Amhara regional state, Ethiopia, 2018: a comparative cross-sectional study. BMC Infect Dis. 2019;19(1):549. doi: 10.1186/s12879-019-4165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAlpine C, Singh NN. Pica in institutionalized mentally retarded persons. J Ment Defic Res. 1986;30(2):171–178. doi: 10.1111/j.1365-2788.1986.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 9.Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods. 1998;3(4):486. [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 13.Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247–262. doi: 10.1093/biostatistics/1.3.247. [DOI] [PubMed] [Google Scholar]
- 14.Daryani A, Hosseini-Teshnizi S, Hosseini S-A, Ahmadpour E, Sarvi S, Amouei A, Mizani A, Gholami S, Sharif M. Intestinal parasitic infections in Iranian preschool and school children: a systematic review and meta-analysis. Acta trop. 2017;169:69–83. doi: 10.1016/j.actatropica.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Teimouri A, Keshavarz H, Mohtasebi S, Goudarzi F, Mikaeili F, Borjian A, Allahmoradi M, Yimam Y, Abbaszadeh Afshar MJ. Intestinal parasites among food handlers in Iran: a systematic review and meta-analysis. Food Microbiol. 2020;95:103703. doi: 10.1016/j.fm.2020.103703. [DOI] [PubMed] [Google Scholar]
- 16.Nyundo AA, Munisi DZ, Gesase AP. Prevalence and correlates of intestinal parasites among patients admitted to Mirembe National Mental Health Hospital, Dodoma. Tanzania. J Parasitol Res. 2017;2017:5651717. doi: 10.1155/2017/5651717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatti S, Lopes R, Cevini C, Ijaoba B, Bruno A, Bernuzzi A, De Lio P, Monco A, Scaglia M. Intestinal parasitic infections in an institution for the mentally retarded. Ann Trop Med PH. 2000;94(5):453–460. doi: 10.1080/00034983.2000.11813564. [DOI] [PubMed] [Google Scholar]
- 18.Shehata AI, Hassanein F. Intestinal parasitic infections among mentally handicapped individuals in Alexandria. Egypt Ann parasitol. 2015;61(4):275–281. doi: 10.17420/ap6104.19. [DOI] [PubMed] [Google Scholar]
- 19.Barazesh A, Hazrati Tappeh K, Mohammadzadeh H, Khashave S. The study of prevalence of intestinal parasitic infections in the personnel of private and governmental rehabilitation centers of Urmia. Nurs Midwifery J. 2007;5(3):101–106. [Google Scholar]
- 20.Abbaszadeh Afshar MJ, Mehni MB, Rezaeian M, Mohebali M, Baigi V, Amiri S, Amirshekari MB, Hamidinia R, Samimi M. Prevalence and associated risk factors of human intestinal parasitic infections: a population-based study in the southeast of Kerman province, southeastern Iran. BMC Infect Dis. 2020;20(1):12. doi: 10.1186/s12879-019-4730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cernikova L, Faso C, Hehl AB. Five facts about Giardia lamblia. PLoS Pathog. 2018;14(9):e1007250. doi: 10.1371/journal.ppat.1007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepczyńska M, Białkowska J, Dzika E, Piskorz-Ogórek K, Korycińska J. Blastocystis: how do specific diets and human gut microbiota affect its development and pathogenicity? Eur J Clin Microbiol Infect Dis. 2017;36(9):1531–1540. doi: 10.1007/s10096-017-2965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page W, Judd JA, Bradbury RS. The unique life cycle of Strongyloides stercoralis and implications for public health action. Trop Med Infect Dis. 2018;3(2):53. doi: 10.3390/tropicalmed3020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goudarzi F, Mohtasebi S, Teimouri A, Yimam Y, Heydarian P, Salehi Sangani G, Abbaszadeh Afshar MJ. A systematic review and meta-analysis of Hymenolepis nana in human and rodent hosts in Iran: a remaining public health concern. Comp Immunol Microbiol Infect Dis. 2021;74:101580. doi: 10.1016/j.cimid.2020.101580. [DOI] [PubMed] [Google Scholar]
- 25.Kang WH, Jee SC. Enterobius vermicularis (Pinworm) infection. N Engl J Med. 2019;381(1):e1. doi: 10.1056/NEJMicm1811156. [DOI] [PubMed] [Google Scholar]
- 26.Rokni M. The present status of human helminthic diseases in Iran. Ann Trop Med PH. 2008;102(4):283–295. doi: 10.1179/136485908X300805. [DOI] [PubMed] [Google Scholar]
- 27.Sharifdini M, Kia EB, Ashrafi K, Hosseini M, Mirhendi H, Mohebali M, Kamranrashani B. An analysis of clinical characteristics of Strongyloides stercoralis in 70 indigenous patients in Iran. Iran J Parasitol. 2014;9(2):155. [PMC free article] [PubMed] [Google Scholar]
- 28.Ashrafi K, Tahbaz A, Rahmati B. Strongyloides stercoralis: The most prevalent parasitic cause of eosinophilia in Gilan province, northern Iran. Iran J Parasitol. 2010;5(3):40. [PMC free article] [PubMed] [Google Scholar]
- 29.Shokri A, Sarasiabi KS, Teshnizi SH, Mahmoodi H. Prevalence of Strongyloides stercoralis and other intestinal parasitic infections among mentally retarded residents in central institution of southern Iran. Asian Pac J Trop Biomed. 2012;2(2):88–91. doi: 10.1016/S2221-1691(11)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia LS, Arrowood M, Kokoskin E, Paltridge GP, Pillai DR, Procop GW, Ryan N, Shimizu RY, Visvesvara G. Laboratory diagnosis of parasites from the gastrointestinal tract. Clinic Microbiol Rev. 2018;31(1):e00025–e117. doi: 10.1128/CMR.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouhani S, Zahiri N, Alimohamadi Z. Intestinal parasites in rehabilitation centers of Tehran. Iran Res Med. 1994;18(1):20–28. [Google Scholar]
- 32.Mahyar A, Daneshi M, Saghafi H, Rezai M. Intestinal parasitic in mentally retarded children of Qazvin. J Qazvin Uni Med Sci. 2000;4(2):64–70. [Google Scholar]
- 33.Mousaviani Z. Contamination with Oxyure and Giardia in children of kindergartens and welfare centers in Tehran. Avicenna J Nurs Midwifery Care. 2006;14(1):40–50. [Google Scholar]
- 34.Sharif M, Daryani A, Asgarian F, Nasrolahei M. Intestinal parasitic infections among intellectual disability children in rehabilitation centers of northern Iran. Res Dev Disabil. 2010;31(4):924–928. doi: 10.1016/j.ridd.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Hazrati Tappeh K, Mohammadzadeh H, Rahim RN, Barazesh A, Khashaveh S, Taherkhani H. Prevalence of intestinal parasitic infections among mentally disabled children and adults of Urmia. Iran Iran J Parasitol. 2010;5(2):60. [PMC free article] [PubMed] [Google Scholar]
- 36.Davari A, Akhlaghi L, Memar A, Namazi M, Hadighi R, Tabatabaee F, Tarihi S. Frequency of intestinal parasites on mental disabilities in rehabilitation centers in Ardabil city at 2011. J Sabzevar Uni Med Sci. 2013;20(1):101–108. [Google Scholar]
- 37.Rasti S, Arbabi M, Hooshyar H. High prevalence of Entamoeba histolytica/Entamoeba dispar and Enterobius vermicularis among elderly and mentally retarded residence in Golabchi center, Kashan, Iran, 2006–2007. Jundishapur J Microbiol. 2012;5(4):585–589. [Google Scholar]
- 38.Soosaraie M, Pagheh A, Gholami S. Prevalence of intestinal parasitic infections in rehabilitation centers in Golestan province. Iran Med Lab J. 2014;8(1):42–47. [Google Scholar]
- 39.Anvari TM, Fattahi BA, Jafari A, Ghafouzadeh M. Prevalence of intestinal parasites among mentally disabled in welfare boarding center of Taft city. Toloo-e Behdasht. 2015;14(1):54–62. [Google Scholar]
- 40.Ahmadi M, Beigom Kia E, Rezaeian M, Hosseini M, Kamranrashani B, Tarighi F. Prevalence of Strongyloides stercoralis and other intestinal parasites in rehabilitation centers in Mazandaran province, northern Iran. J Mazandaran Uni Med Sci. 2015;25(130):1–7. [Google Scholar]
- 41.Soleymani E, Davoodi L, Azami D. The prevalence of intestinal parasitic infections among the mentally retarded patients in Lamook Rehabilitation Center of Qaemshahr, Mazandaran province, 2015. Tabari Biomed Stu Res J. 2016;2(1):1–5. [Google Scholar]
- 42.Saeidinia A, Tavakoli I, Naghipour MR, Rahmati B, Lahiji HG, Salkhori O, Ashrafi K. Prevalence of Strongyloides stercoralis and other intestinal parasites among institutionalized mentally disabled individuals in Rasht, northern Iran. Iran J Parasitol. 2016;11(4):527. [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadi-Meskin V, Hamedi Y, Heydari-Hengami M, Eftekhar E, Shamseddin J, Sharifi-Sarasiabi K. Intestinal parasitic infections in mental retardation center of Bandar Abbas, southern Iran. Iran J Parasitol. 2019;14(2):318. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 3. JBI Critical Appraisal Checklist for Studies Reporting Prevalence Data.
Additional file 4. Quality assessment scores for eligible studies.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional files