Abstract
Severe sepsis is an important cause of mortality and morbidity in critically ill children. Meropenem is a broad spectrum antibiotic commonly used to treat sepsis. Current meropenem dosage recommendations for children on continuous renal replacement therapy are extrapolated from pharmacokinetics (PK) studies done in adults. Our study aims to determine the optimal dosing in critically ill septic children receiving CRRT. A prospective single-center PK study was performed in 9 ICU children on CRRT. Meropenem concentrations were measured from blood and effluent fluid samples. A population PK model was developed using nonlinear mixed effects modeling software (NONMEM®). Monte Carlo simulations were performed. The PK/pharmacodynamic (PD) target aimed for plasma concentrations above minimum inhibitory concentration (MIC) of 4 mg/L for 100% of dosing interval (100%ƒT>MIC). A two-compartment model best characterized meropenem PK. Mean (range) clearance and elimination half-life was 0.091 L/hr/kg (0.04–0.157) and 3.9 hr (2.1–7.5) respectively. Dosing of 40mg/kg/dose q12h over 30-mins achieved PK/PD target in only 32% while 20mg/kg q8h over 4-hour or 40mg/kg q8h over 2-hour achieved 100%ƒT>MIC target for at least 90% of simulated patients.
Keywords: Meropenem, pharmacokinetics, pediatrics, critical care, renal replacement therapy
INTRODUCTION
Severe sepsis is an important cause of morbidity and mortality in critically ill children.1,2 Early appropriate antimicrobial therapy is crucial.3–5 Acute kidney injury during sepsis requiring continuous renal replacement therapy (CRRT) is not uncommon.6,7 Critically ill children on CRRT often have altered pharmacokinetics (PK) due to drug extraction by the CRRT circuit and the underlying critical illness.8,9 Drug extraction by CRRT depends on drug characteristics, the pore size of the dailysis filter membranes, the type of CRRT modality employed and the changes in blood or dialysate flow rate.10,11,12 Fluid overload also affects drug disposition,10,13
Meropenem, a commonly used carbapenem for sepsis treatment, is effective for highly resistant pathogens including extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae and Pseudomonas aeruginosa.14 It exhibits primarily time-dependent antimicrobial activity, and the pharmacokinetic-pharmacodynamic (PK/PD) index that best predicts the in-vivo antimicrobial activity is the fraction of time of the dosing interval during which the free serum concentration of meropenem remains above the minimum inhibitory concentration (MIC) of the pathogen (ƒT>MIC).15,16 Traditionally a PK/PD target of percentage time above MIC of ≥40% of the dosing interval (40%ƒT>MIC) is defined as the primary threshold.17 For critically ill patients with severe gram-negative sepsis, recent studies have proposed PK/PD target of ƒT>MIC >100% for meropenem, using susceptibility breakpoint of MIC of 4 mg/L.10,18–21
Meropenem is primarily eliminated by the kidneys. Its small molecular size, small volume of distribution (V) and insignificant protein binding (<2%) predisposes it to extensive clearance by CRRT.9,22,18,20,23 Current meropenem dosage recommendations for paediatric patients on CRRT are extrapolated from PK studies done in adult CRRT patients.6,24 This is a lack of meropenem population PK (PopPK) modelling study that includes critically ill children on CRRT who are less than five years of age.
Our study aims to (i) evaluate the pharmacokinetics of intravenous meropenem in critically ill children receiving meropenem for the treatment of presumed or proven sepsis while on CRRT and (ii) determine the optimal meropenem dosing regimens in critically ill children on CRRT using PopPK modelling and dosing simulations.
METHODS
This study is an open-label was performed at a 16-bed PICU at KK Women’s and Children’s Hospital on patients who received intravenous meropenem for proven or presumed sepsis and supported with either CVVH or CVVHDF for acute renal failure (ARF). This trial was approved by the institutional review boards for Duke (Protocol ID: Pro00084313) and KK Women’s and Children’s Hospital (CIRB Ref No: 2011/538/E) with written consent prior to enrollment.
Study Design
This was an open-label study that enrolled children supported with CRRT and prescribed meropenem by their treating physician. Meropenem dosing was determined by the treating physician based on institutional guidelines of 20mg/kg/dose or 40mg/kg/dose dosed q12h over a 30-minute infusion for children on CRRT. According to the hospital protocol, IV meropenem (Meronem™, 500mg powder) was administered as 30-minute infusions at a maximum concentration of 50mg/ml. CRRT was performed using the Prismaflex™ for continuous venovenous hemofiltration (CVVH) or hemodiafiltration (CVVHDF) with either a 0.2m2 polyarylethersulfone filter (PAES, HF 20, Gambro) for patient weight ≤10kg, or 0.6m2 and 0.9m2 polyacrylonitrile filter (AN69, Gambro, Deerfield, IL) for patient weight 11 to 30kg and >30kg, respectively. The CRRT program prescribed Hemosol BO (Baxter) for replacement and dialysate fluids except for the patients who were on regional citrate anticoagulation when Prismocal (Gambro) was used instead. All replacement fluids were administered pre-filter. Patients’ actual body weight on admission was used for PK calculations. Demographic, clinical, laboratory and CRRT parameters were collected. Hematology and biochemistry laboratory data were obtained for the tests done within 24 hours of the sampling.
Sample Collection and Analysis
Arterial blood samples from indwelling catheters in the patients and effluent fluid samples from the dialysis machine were collected at specified time points: prior to infusion, at the end of infusion, 1, 2, 4, 8 and 12 hours post infusion. The total volume of urine and effluent were also measured. The supernatant plasma and effluent samples were frozen at −7°C. Meropenem concentrations in plasma and the effluent were quantified using a commercial, validated high-performance liquid chromatography-tandem mass spectrometry bioanalytical assay.22 Accuracy and precision were assessed using 4 determinations at theoretical levels of 0.050, 0.100, 0.500, 1.0, 5.0, 10.0, 50.0 and 100.0 mcg/mL within the Food and Drug Administration (FDA) bioanalytical assay validation criteria (e.g. ± 15%). The lower limit of quantification for meropenem was 0.050 mcg/L. Because meropenem has neglible protein binding25, total and unbound concentration were assumed to be equivalent.
Pharmacokinetic Analysis
A PopPK model was developed based on the measured meropenem concentrations in plasma using nonlinear mixed effects modeling software (NONMEM, version 7.2). First-order conditional estimation method with interaction was used for all model runs. Run management was performed using Pirana (version 2.8.1). Visual predictive checks and bootstrap methods were performed with Perl-speaks-NONMEM (version 3.6.2).26 Data manipulation and visualization were performed using Stata software (version 13.1, College Station, TX), R (version 3.4.1, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 0.97.551, RStudio, Boston, MA, US). Based on visual inspection of the PK data and a review of the primary literature,18–20,23,27–31 both one and two compartment models were evaluated using the ADVAN2 TRANS2 and ADVAN4 TRANS4 subroutines, respectively. CRRT clearance model using effluent and plasma concentrations, and CRRT clearance model using effluent concentrations only were also evaluated using ADVAN6 TRANS1. In addition, two CRRT clearance models were also evaluated using ADVAN6 TRANS1 (Supplementary Figure S1).
After the base structural model was identified, covariate analysis was performed using a forward inclusion and backward elimination approach to evaluate the statistical significance of the following covariates: postnatal age, total bilirubin, serum creatinine, albumin, dialysate rate, total ultrafiltration rate, CRRT type, sex, and extracorporeal membrane oxygenation (ECMO) on clearance. Actual body weight (WT) was assumed to be a significant covariate for both clearance and volume of distribution parameters and was included in the base model prior to assessment of other covariates.
A decrease in the OFV with p value < 0.01 and an increase in the OFV with p value < 0.001 were accepted as statistically significant in the forward inclusion and backward elimination steps, respectively, to obtain the final PK irreducible model.
The PK analysis dataset was generated and formatted by merging clinical database data (dosing, demographics, laboratory data) with raw concentration values received from the central laboratory (OpAns).
During the popPK model-building process, successful minimization, diagnostic plots, plausibility, and precision of parameter estimates, as well as objective function and shrinkage values, were used to assess model appropriateness. Diagnostic plots generated included individual predictions and population predictions (PREDs) vs observations, conditional weighted residuals vs PRED and time.
Parameter precision for the final popPK model was evaluated using non-parametric bootstrapping (1000 replicates) to generate the 95% CIs for parameter estimates. Visual predictive checks were performed using the final model to generate 1000 Monte Carlo simulation replicates per time point of meropenem exposure. Simulated results were compared at the participant level with those observed in the study by calculating and plotting the percentile of each observed concentration in relation to its 1000 simulated observations derived from the final model.32
Extracorporeal Analysis
Area under the curve to the last sample collection time (AUCτ) for both plasma (AUCτpl) and effluent (AUCτuf) was determined for each patient using non-compartmental PK analysis in Phoenix WinNonlin (version 6.3, Pharsight Corporation).
The following PK parameters were derived:
- Sieving/saturation coefficient (Sc/Sa) for CVVH/CVVHDF:
where AUCτuf is the exposure of meropenem in the ultrafiltrate and AUCτpl is the exposure of drug in the plasma of simultaneously collected patients’ blood specimens. - Clearance by CVVH/CVVHDF:
where CLHF is the hemofilter clearance and Qf is the ultrafiltration rate for CVVH and ultrafiltrate plus the dialysate flow rates for CVVHDF. - Fraction of total CL contributed by CRRT (FractEC):
Extracorporeal clearance was defined as being significant if the FractEC was greater than 0.333.
Probability of target attainment (PTA)
We used the PK-Sim® (version 7.2; Open Systems Pharmacology Suite, open-systems-pharmacology.com) population generator to generate a population of virtual children (n=1000) for simulations. For each simulated individual, we used the final NONMEM-generated PK model to generate empirical Bayes estimates (EBEs) for clearance and volume of distribution and used Monte Carlo simulations to simulate concentration-time curves at steady state for each dose. Dosing regimens simulated and evaluated included 10mg/kg q8h, 20mg/kg q8h, 40mg/kg q8h (maximum 6grams per day), 20mg/kg q12h, and 40mg/kg q12h infused over 30mins, 2hours and 4hours. We then determined the PTA for the PK/PD targets of 40%ƒT>MIC and 100%ƒT>MIC for various MICs (0.5–32mg/L). The PK/PD target was set to achieve meropenem plasma concentrations above MIC of 4 mg/L for 100% of the dosing interval (100%ƒT>MIC), with the assumption that >98% of the drug is unbound. Optimal meropenem dosing was selected when greater than 90% of simulated children achieved the PK/PD target.
RESULTS
Nine patients were enrolled in the study. Their demographic and clinical data are summarized in Table 1. None of the enrolled patients had chronic or end-stage renal failure prior to PICU admission. All patients were were anuric or had neglible urine output at the time of enrolment. All were critically ill with 4 patients on ECMO support and the rest either with multi-organ failure or on inotropic support. The underlying diseases were a mix of postoperative congenital heart disease, hemato-oncology disease and pulmonary disease. The mean duration of CRRT and the mean number of doses of meropenem given before the dose sampling were 5.9 days (2 – 18) and 3.9 doses (1 – 8), respectively.
TABLE 1.
Clinical Parameters of Study Population
| ID | Gender | Race | Age (years) | Weight (kg) | Day of CRRT | No. of prior doses of meropenem | Underlying Illness | Positive Microbiolgy Results | Urine output (ml/kg/h) | Mode of Renal Replacement | Other concurrent extracorporeal therapy | Dialysis/Replacement Fluid | Concurrent medications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | Myanmese | 0.1 | 3.3 | 6 | 8 | Critical pertussis, MOF | NPA: Pertussis | 0 | CVVHDF | VA ECMO | Hemosol BO | Erythromycin, Amphotericin B, Rocuronium, Midazolam, Fentanyl, Omeprazole |
| 2 | Male | Chinese | 0.3 | 6 | 3 | 3 | Myocarditis, acute renal and liver failure | NPA: Influenza A | 0 | CVVH | VA ECMO | Hemosol BO | Ampicillin, Neomycin, Rocuronium, Midazolam, Fentanyl, Ranitidine, Oseltamivir, Dopamine, Mirlinone, Hydrocortisone |
| 3 | Female | Chinese | 0.1 | 2.6 | 6 | 2 | Postoperative complex cyanotic heart disease, prematurity, severe capillary leak | 0 | CVVH | Nil | Hemosol BO | Vancomycin, Fluconazole, Morphine, Dopamine, Epinephrine, Norepinephrine, Heparin | |
| 4 | Male | Chinese | 16 | 52.7 | 2 | 1 | Relapsed suprasellar germinoma post stem cell transplant | Blood culture: E. Coli (ESBL), Candida krusei ETT culture: A. baumanii | <0.1 | CVVHDF | Nil | PrismOCal | Acyclovir, Voriconazle, Ambisome, Caspofungin, Morphine, Midazolam, Omeprazole, Dopamine, Norepinephrine |
| 5 | Male | Chinese | 9.6 | 41.5 | 2 | 5 | Relapsed ALL post stem cell transplant, CMV pneumonitis | Blood: CMV PCR + NPA: Parainfluenza 1 | 0 | CVVH | Nil | Hemosol BO | Cotrimoxazole, Ciclosporin, Voriconazole, Ambisome, Cas pofungin, Acetaminophen, Midazolam, Omeprazole, Dopamine, Norepinephrine |
| 6 | Female | Malay | 13.8 | 29 | 18 | 2 | Pulmonary hemosiderosis with ARDS | BAL culture: S. maltophilia, A. baumanii | 0 | CVVHDF | VV ECMO | Hemosol BO | Ambisome, Anidulafungin, Morphine, Midazolam, Epinephrine, Tranexamic acid, Heparin |
| 7 | Female | Chinese | 1.8 | 10.3 | 6 | 4 | Streptococcus pneumonia with HUS | Blood culture: S. pneumoniae ETT culture: A. baumanii | 0 | CVVHDF | Nil | PrismOCal | Levofloxacin, Fluconazole, Fentanyl, Midazolam, Acetaminophen |
| 8 | Female | Japanese | 4.9 | 19 | 2 | 7 | EBV related HLH, MOF | Stool culture: E. coli (ESBL) | <0.1 | CVVH | Nil | PrismOCal | Doxycycline, Ambisome, Morphine, Midazolam, Acetaminophen, Dexamethasone, Tranexamic acid |
| 9 | Male | Malay | 18.9 | 56.3 | 8 | 3 | Postoperative Fontan | ETT culture: E. cloacae, A. baumanii | 0 | CVVHDF | VA ECMO | Hemosol BO | Morphine, Midazolam, Omeprazole, Epinephrine, Milrinone, Amiodarone, Sodium valproate, Heparin, Trenaxemic acid, Hydrocortisone |
BAL: Bronchoalveolar lavage; ETT: endotracheal tube; NPA: nasopharyngeal aspirate; EBV: Ebstein Barr virus; CMV: Cytomegalovirus; ESBL: Extended spectrum beta=lactamase; MOF: Multiorgan failure; ARDS: Acute respiratory distress syndrome; ALL: Acute lymphoblastic leukemia; HLH: Hemophagocytic lymphohistiocytosis; HUS: Hemolytic uremic syndrome; VA ECMO: Veno-arterial extra-corporeal membrane oxygenation; VV ECMO: Veno-venous extra-corporeal membrane oxygenation
A total of 53 plasma and 38 dialysate samples contributed to the development of the meropenem PopPK model. A 2-compartment model provided the overall best fit for the data (Supplementary Figure S2).
The mean (range) of combined hemofiltration and dialysate corrected to 1.73m2 body surface area (BSA), the mean serum albumin level and mean total bilirubin levels were 2689.5ml/h (940.2–4201.4), 29.9 g/L (21–52) and 77.2 umol/L (18–219), respectively (Supplementary Table S3). We included postnatal age, total bilirubin, serum creatinine, albumin, dialysate rate, total hemofiltration rate, CRRT type, gender and extracorporeal membrane oxygenation (ECMO) on clearance but none were retained as statistically significant in the final population PK irreducible model (Supplementary S4) except for the allometric relationship with total WT with fixed estimates of 0.75 for clearance and 1.0 for volume of distribution. The goodness-of-fits plots are shown in Figure 1.
Figure 1.
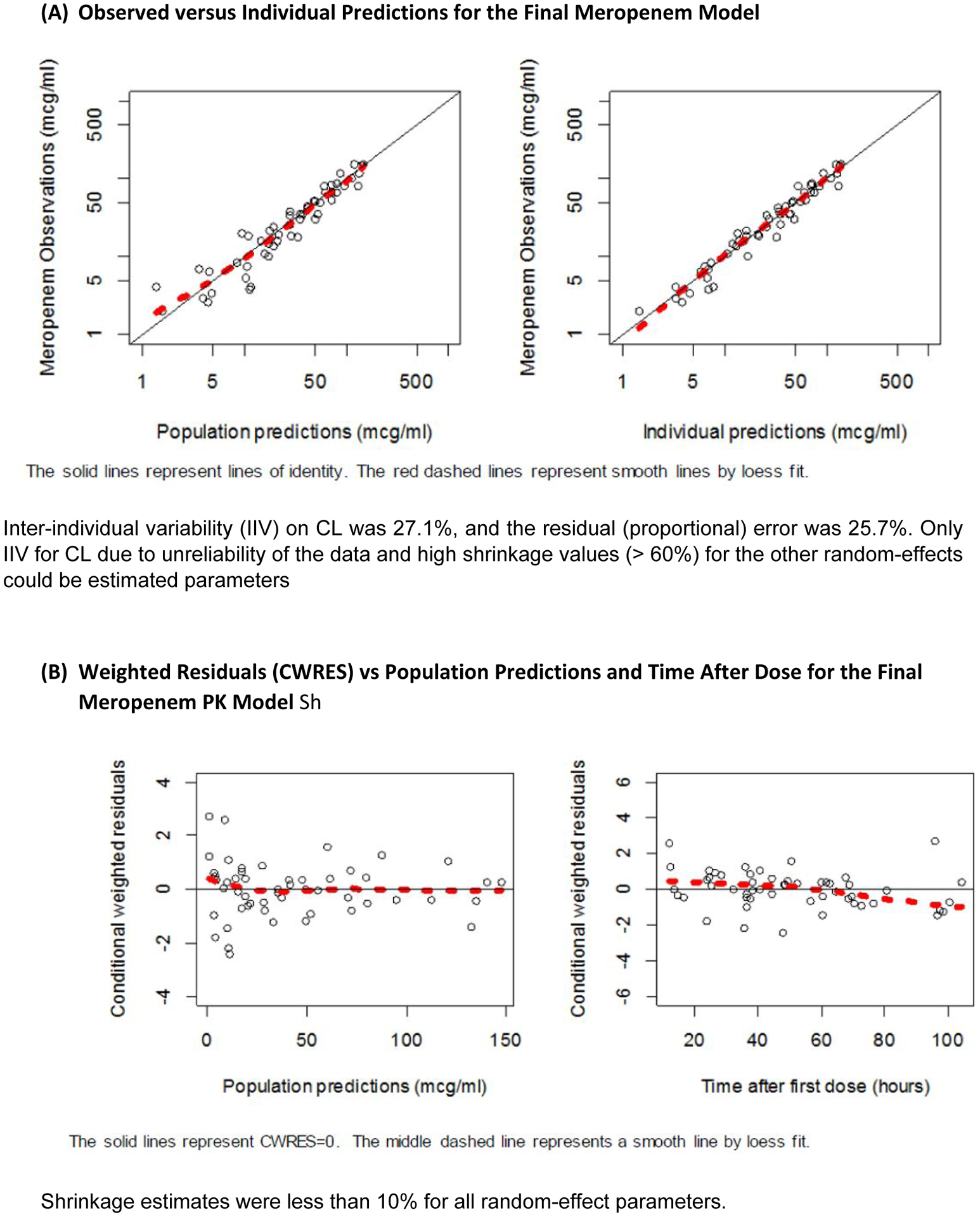
Goodness-of-fits plots for the construction model.
The model was evaluated using a 1000-set bootstrap analysis. The median of bootstrap parameter estimates were within 10% of population estimates from the original data set for all parameters (Supplementary Table S5). The prediction corrected visual predictive check revealed a reasonable fit between the observed and predicted meropenem concentrations with only 5.7% (3/53) of observed concentrations outside of the 90% prediction interval (Supplementary Figure S6). Median (range) weight adjusted CL from the EBEs obtained from the final model was 0.096 L/hr/kg (0.040–0.157). Summaries for other EBE PK parameters are shown in Table 2. The median (range) AUCτp and AUCτeff were 196.6 (39.6–515.8) and 160.8 (42.0–575.8) respectively. Median (range) Sc/Sa was 0.958 (0.737–1.1) and mean FractEC was 0.816 (0.457–1.3).
TABLE 2.
Indiv idual Empirical Baye s Post-hoc Parameter Estimates and Extracorporeal Parameters
| ID | Meropenem dose per body weight (mg/kg) | Dosing interval | CL (L/hr/kg) | V (L/kg) | Vp (L/kg) | Q (L/kg/hr) | T½ (hrs) | CL (L/hr) | AUCt(p) (mg*h/L) | AUCt(eff) (mg*h/L) | Sc/Sa | QE (L/h) | CLCRRT (QE*Sc) (L/h) | FractEC (CLCRRT/CL) (L/hr) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39.4 | Q12H | 0.157 | 0.194 | 0.209 | 0.277 | 2.1 | 0.519 | 39.6 | 42 | 1.1 | 0.525 | 0.557 | 1.1 |
| 2 | 20 | Q12H | 0.097 | 0.2 | 0.217 | 0.233 | 3.2 | 0.581 | 104.3 | 97.5 | 0.935 | 0.612 | 0.572 | 0.983 |
| 3 | 38.5 | Q12H | 0.102 | 0.194 | 0.209 | 0.294 | 3 | 0.265 | 0.099 | |||||
| 4 | 19 | Q12H | 0.04 | 0.194 | 0.209 | 0.139 | 7.5 | 2.1 | 81.7 | 70.9 | 0.867 | 3.3 | 2.9 | 1.3 |
| 5 | 24.1 | Q12H | 0.058 | 0.193 | 0.21 | 0.147 | 5.4 | 2.4 | 267.1 | 302.2 | 1.1 | 1.9 | 2.1 | 0.885 |
| 6 | 20 | Q12H | 0.096 | 0.193 | 0.21 | 0.162 | 3.5 | 2.8 | 155.9 | 114.9 | 0.737 | 1.8 | 1.3 | 0.476 |
| 7 | 39.8 | Q12H | 0.096 | 0.194 | 0.214 | 0.204 | 3.3 | 0.989 | 515.8 | 575.8 | 1.1 | 0.423 | 0.472 | 0.477 |
| 8 | 42.1 | Q12H | 0.082 | 0.195 | 0.211 | 0.179 | 3.9 | 1.6 | 280.2 | 274.8 | 0.981 | 1.2 | 1.2 | 0.747 |
| 9 | 35.5 | Q12H | 0.095 | 0.194 | 0.21 | 0.137 | 3.6 | 5.3 | 237.2 | 206.6 | 0.871 | 2.8 | 2.4 | 0.457 |
| MEAN | 29.8 | 0.091 | 0.194 | 0.211 | 0.197 | 3.9 | 1.8 | 210.2 | 210.6 | 0.962 | 1.4 | 1.4 | 0.806 | |
| SD | 9.5 | 0.032 | 0.002 | 0.002 | 0.056 | 1.6 | 1.6 | 152.1 | 175.6 | 0.137 | 1.1 | 0.93 | 0.326 |
CL=plasma clearance;MAX=maximum; MIN=minimum; Q= I nter-compartmental clearance;T ½ = half-life;
Vc= Volume of distribution in central compartment; Vp= Volume of distribution in peripheral compartment
AUCt (eff)=Area under the curve from the last dose to last measurable concentration for effluent; AUCt(p=Area under the curve from the last dose to last measurable concentration for plasma; CL=plasma clearance; CL CRRT=continuous renal replacement therapy clearance;
FractEC=Fraction of contribution of CRRT CL to total CL; QE=effluent flow rate; SC/SA= sieving/saturation coefficient. †Patient with ID #3 had only 1 PK sample.
Based on simulations, meropenem regimens of 20mg/kg/dose q8h over 4hours or 40mg/kg/dose q8H over 2hours achieved the target attainment of ⩾90% patients achieving concentration above MIC of 4mg/L for 100% of the dosing interval (100% ƒT>MIC) (Table 3, Supplementary Figure S7).
TABLE 3.
Target Attainment Rates for T>MIC of 40% and T>MIC of 100% Using Different Dosing Regimens:
| 30 minutes infusion | 2 hours infusion | 4 hours infusion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | 20mg/kg q12H | 40mg/kg q12H | 10mg/kg q8H | 20mg/kg q8H | 40mg/kg q8H | 20mg/kg q12H | 40mg/kg q12H | 10mg/kg q8H | 20mg/kg q8H | 40mg/kg q8H | 20mg/kg q12H | 40mgrkg q12H | 10mg/kg q8H | 20mg/kg q8H | 40mg/kg q8H |
| 0.5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 4 | 98 | 100 | 99 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 8 | 89 | 98 | 95 | 99 | 100 | 97 | 100 | 99 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 16 | 56 | 89 | 56 | 95 | 99 | 79 | 97 | 88 | 99 | 100 | 99 | 100 | 100 | 100 | 100 |
| 32 | 11 | 56 | 3 | 56 | 95 | 26 | 79 | 12 | 88 | 99 | 72 | 99 | 91 | 100 | 100 |
| 30 minutes infusion | 2 hours infusion | 4 hours infusion | |||||||||||||
| MIC | 20mg/kg q12H | 40mg/kg q12H | 10mg/kg q8H | 20mg/kg q8H | 40mg/kg q8H | 20mg/kg q12H | 40mg/kg q12H | 10mg/kg q8H | 20mg/kg q8H | 40mg/kg q8H | 20mg/kg q12H | 40mg/kg q12H | 10mg/kg q8H | 20mg/kg q8H | 40mg/kg q8H |
| 0.5 | 67 | 81 | 92 | 96 | 98 | 76 | 87 | 96 | 98 | 99 | 87 | 94 | 99 | 100 | 100 |
| 1 | 51 | 67 | 82 | 92 | 96 | 60 | 76 | 90 | 96 | 98 | 74 | 87 | 97 | 99 | 100 |
| 2 | 32 | 51 | 60 | 82 | 92 | 39 | 60 | 73 | 90 | 96 | 53 | 74 | 90 | 97 | 99 |
| 4 | 13 | 32 | 31 | 60 | 82 | 21 | 39 | 45 | 73 | 90 | 31 | 53 | 69 | 90 | 97 |
| 8 | 3 | 13 | 9 | 31 | 59 | 4 | 21 | 14 | 45 | 73 | 10 | 31 | 30 | 69 | 90 |
| 16 | 0 | 3 | 1 | 9 | 31 | 0 | 4 | 1 | 14 | 45 | 0 | 10 | 3 | 30 | 68 |
| 32 | 0 | 0 | 0 | 1 | 8 | 0 | 0 | 0 | 1 | 13 | 0 | 0 | 0 | 3 | 29 |
MIC=Minimum Inhibitory Concentration (mg/L); q12h= every 12hours; q8h= every 8hours
Red box indicate the optimal target attainment rates (>90%) for 100% ƒT>MIC= 4
DISCUSSION
Optimal meropenem dosage regimen in critically ill patients with acute renal failure on CRRT is lacking especially in young children. Inadequate dosing to achieve the desirable PK targets can lead to treatment failure and emergence of antibiotic resistance. Our results show that longer infusions of meropenem in this group of patients are required to achieve adequate PK targets.
We demonstrated that a 2-compartment model provided the overall best fit for our meropenem PK data which is consistent with other meropenem PK studies in patients on CRRT.18–20,27–30 The mean Sc/Sa of approximately 1 meant that meropenem was freely filtered by the hemofilter/dialysis membrane. This was consistent with the values reported.18–20 The population estimates of CL in children on CRRT normalized to 70 kg (median [range] of 4.1 L/hour [2.6 – 6.3]) differed substantially from the CL estimates of 14.1 L/hour based on the FDA label for children with normal renal function.25 This was likely due to low or negligible renal clearance of meropenem in these patients and the CRRT clearance of meropenem was lower compared to normal renal clearance.34 The mean meropenem CRRT clearance of 80% exceeded the suggested 30% threshold required for dosing adjustment while on CRRT.33
Of note, we included a very sick group of patients in PICU with almost half of our study population on ECMO support. ECMO can alter PK by increasing volume of distribution through adsorption and hemodilution.35 We explored ECMO as a covariate in our analysis but in our final irreducible model, ECMO was not retained as a covariate.
Recent evidence suggests improved outcome when the percentage of T>MIC is 100% of the dosing interval (100% ƒT>MIC) for critically ill or neutropenic patients on meropenem.36 Based on this surrogate endpoint for efficacy, current meropenem dosing recommendations from FDA label may be sub-therapeutic in critically ill children on CRRT. A population PK simulation in 9 critically ill children with normal renal function also suggested that current dosing recommendation was inadequate and a meropenem dosage regimen of 120 to 160mg/kg/day as continuous infusion was necessary to achieve 80% ƒT>MIC (MIC ≤ 2mg/L).29 However, continuous infusions can be a challenge in critically children when there is often competing needs for limited venous access and when constituted meropenem at room temperature is only stable for 4 hours. Other studies had suggested a PK target based on meropenem concentration >4X MIC for 75% to 100% of the time was required in ill patients.37,38 In our study, only one patient had a blood culture positive for E.coli (ESBL) but there was no data on MIC available. During the study period, the institution’s antibiogram showed a 100% sensitivity to meropenem for Enterobacteriaceae and 80–100% sensitivity to meropenem for Pseudomonas aeruginosa using a susceptibility breakpoint of 2mg/L for both. The Clinical and Laboratory Standards Institute (CLSI) MIC breakpoints for Pseudomonas aeruginosa and Enterobacteriaceae are 2 mg/L and 1 mg/L, respectively.39 With this, we based our PTA on the PK/PD target to achieve 100% ƒT>MIC (MIC=4 mg/L as breakpoint) in ≥90% of the critically ill children on CRRT. Our simulations suggest either a 20mg/kg/dose q8h over 4-hour infusion or a 40-mg/kg/dose q8h over 2-hour infusion is required. The dosing regimen differed from an earlier study by Nehus et al20 that suggested that meropenem dosing of 20mg/kg q12h over 30mins was adequate to achieve the same targets.20 One explanation could be that our study involved younger children and younger children may require more frequent dosing to achieve the same therapeutic target compared to older children.19
As the sensitivity patterns of Enterobacteriaceae and Pseudomonas aeruginosa to meropenem may differ in different settings, and PK targets also differ based on site and severity of infection, we also express the target attainment rates for T>MIC of 40% and T>MIC of 100% using different dosing regimen and different MIC breakpoints (Table 3).
Meropenem is generally well tolerated but its serum concentration has been shown to correlate with acute neurotoxicity and nephrotoxicity in adults, with trough thresholds of 64mg/L and 44mg/L, respectively.40 We did not simulate trough levels for the dosing regimen of 20mg/kg/dose q8h over 4-hour infusion or a 40-mg/kg/dose q8h over 2-hour infusion. However, a study demonstrated a low proportion of simulated troughs exceeding toxic levels using a meropenem regimen of 2g/kg q8h (equivalent to 40mg/kg for a 50-kg individual) over 2- or 4-hour infusions in adults with hematological malignancies including those with creatinine clearance ≤ 50ml/min/1.73m2.41 With the median population estimates of CL in children on CRRT normalized to 1.73 m2 in our study to be 60.0 ml/min (range 41.2 – 91.3), we postulate that our dosing regimen to be at low risk of attaining toxic trough levels.There are a few limitations to this investigation. Firstly, the study was conducted in a single centre with a small sample size (n = 9). The patients had heterogeneous underlying diseases. This could affect the reliability and generalizability of the data and is a possible reason why no covariates were retained in the final model. Secondly, we have assumed a low protein binding of 2% for meropenem. A study has recently shown a wide variability of protein binding for meropenem and the measurement of total meropenem level in our study could have overestimated the unbound fraction.42 Thirdly, as dialysis clearance is influenced by the physicochemical properties of the dialysis filter, caution must be exercised when extrapolating these results to other types of dialysis membranes.
In conclusion, our findings support the use of longer meropenem infusions in critically ill patients. Due to the limitations of this study, larger prospective clinical studies are needed to validate this dosing regimen.
Supplementary Material
ACKNOWLEDGEMENT
We thank the nursing staff in the Children’s ICU, KK Women’s and Children’s Hospital, Singapore for their assistance collecting the blood, urine and dialysate samples.
DISCLOSURES
Yoke Hwee Chan was awarded research grant from the Khoo Clinical Scholars Pilot Award (Duke-NUS-KCSP/2012/0007). Kevin Watt receives support for research from the Pediatric Critical Care and Trauma Scientist Development Program (5K12HD047349) and the National Institute of Child Health and Human Development (1K23HD075891, R01HD097775). Michael Cohen-Wolkowiez receives support for research from the National Institutes of Health (1R01HD076676 and 1K24AI143971), National Institute of Allergy and Infectious Diseases (HHSN272201500006I and HHSN272201300017I), NICHD (HHSN275201000003I), U.S. Food and Drug Administration (5U18-FD006298), and industry for drug development in adults and children.
All other authors has no financial disclosure to declare.
Footnotes
DATA AVAILABILITY STATEMENT:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Niederman MS. Use of broad-spectrum antimicrobials for the treatment of pneumonia in seriously ill patients: maximizing clinical outcomes and minimizing selection of resistant organisms. Clin Infect Dis. January 15 2006;42 Suppl 2:S72–81. [DOI] [PubMed] [Google Scholar]
- 2.Biban P, Gaffuri M, Spaggiari S, Zaglia F, Serra A, Santuz P. Early recognition and management of septic shock in children. Pediatr Rep. January 2 2012;4(1):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. February 2010;55(2):316–325. [DOI] [PubMed] [Google Scholar]
- 4.Seyler L, Cotton F, Taccone FS, et al. Recommended beta-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care. 2011;15(3):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varghese JM, Roberts JA, Lipman J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit Care Clin. January 2011;27(1):19–34. [DOI] [PubMed] [Google Scholar]
- 6.Thalhammer F, Horl WH. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin Pharmacokinet. October 2000;39(4):271–279. [DOI] [PubMed] [Google Scholar]
- 7.Taccone FS, Laterre PF, Dugernier T, et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14(4):R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical Pharmacology Studies in Critically Ill Children. Pharm Res. January 2017;34(1):7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. September 15 2008;47 Suppl 1:S32–40. [DOI] [PubMed] [Google Scholar]
- 10.Pea F, Viale P, Cojutti P, Furlanut M. Dosing nomograms for attaining optimum concentrations of meropenem by continuous infusion in critically ill patients with severe gram-negative infections: a pharmacokinetics/pharmacodynamics-based approach. Antimicrob Agents Chemother. December 2012;56(12):6343–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl. November 1999(72):S24–28. [PubMed] [Google Scholar]
- 12.Wong WT, Choi G, Gomersall CD, Lipman J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: the eternal doubt. Curr Opin Pharmacol. October 2015;24:68–78. [DOI] [PubMed] [Google Scholar]
- 13.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. January 1998;26(1):1–10; quiz 11–12. [DOI] [PubMed] [Google Scholar]
- 14.Pea F, Viale P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock--does the dose matter? Crit Care. 2009;13(3):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinder M, Bellomo R, Lipman J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care. April 2002;30(2):134–144. [DOI] [PubMed] [Google Scholar]
- 16.Hassan E, Ober JD. Predicted and measured aminoglycoside pharmacokinetic parameters in critically ill patients. Antimicrob Agents Chemother. November 1987;31(11):1855–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis JM, Kuti JL, Nicolau DP. Use of Monte Carlo simulation to assess the pharmacodynamics of beta-lactams against Pseudomonas aeruginosa infections in children: a report from the OPTAMA program. Clin Ther. November 2005;27(11):1820–1830. [DOI] [PubMed] [Google Scholar]
- 18.Isla A, Rodriguez-Gascon A, Troconiz IF, et al. Population pharmacokinetics of meropenem in critically ill patients undergoing continuous renal replacement therapy. Clin Pharmacokinet. 2008;47(3):173–180. [DOI] [PubMed] [Google Scholar]
- 19.Nehus EJ, Mouksassi S, Vinks AA, Goldstein S. Meropenem in children receiving continuous renal replacement therapy: clinical trial simulations using realistic covariates. J Clin Pharmacol. December 2014;54(12):1421–1428. [DOI] [PubMed] [Google Scholar]
- 20.Nehus EJ, Mizuno T, Cox S, Goldstein SL, Vinks AA. Pharmacokinetics of meropenem in children receiving continuous renal replacement therapy: Validation of clinical trial simulations. J Clin Pharmacol. March 2016;56(3):291–297. [DOI] [PubMed] [Google Scholar]
- 21.JA Roberts SP MA, Matteo Bassetti, De Waele Jan J., Dimopoulos G, Kaukonen Kirsi-Maija, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A,Starr T, Wallis SC and Lipman J. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current β-Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clinical Infectious Diseases 2014;58(8):1072–1083. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration, U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Veterinary Medicine. FDA Bioanalytical Method Validation Guidance for Industry. May 2018.
- 23.Goldstein SL, Murry DJ, May S, Aleksic A, Sowinski KM, Blaney S. Meropenem pharmacokinetics in children and adolescents receiving hemodialysis. Pediatr Nephrol. December 2001;16(12):1015–1018. [DOI] [PubMed] [Google Scholar]
- 24.Cies JJ, Moore WS 2nd, Conley SB, et al. Pharmacokinetics of Continuous Infusion Meropenem With Concurrent Extracorporeal Life Support and Continuous Renal Replacement Therapy: A Case Report. J Pediatr Pharmacol Ther. Jan-Feb 2016;21(1):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Meropenem FDA Label. Accessed Jan 2018.
- 26.Lindbom LRJ, Jonsson EN. Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Computer methods and programs in biomedicine. August 2004; 75(2):85–94. [DOI] [PubMed] [Google Scholar]
- 27.Blumer JL, Reed MD, Kearns GL, et al. Sequential, single-dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother. August 1995;39(8):1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J. September 2008;27(9):794–799. [DOI] [PubMed] [Google Scholar]
- 29.Cies JJ, Moore WS 2nd, Enache A, Chopra A. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Meropenem in Critically Ill Young Children. J Pediatr Pharmacol Ther. Jul-Aug 2017;22(4):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol. January 2006;46(1):69–75. [DOI] [PubMed] [Google Scholar]
- 31.Ikawa K, Morikawa N, Ikeda K, Miki M, Kobayashi M. Population pharmacokinetics and pharmacodynamics of meropenem in Japanese pediatric patients. J Infect Chemother. April 2010;16(2):139–143. [DOI] [PubMed] [Google Scholar]
- 32.Wang DD, Zhang S. Standardized visual predictive check versus visual predictive check for model evaluation. J Clin Pharmacol. January 2012;52(1):39–54. [DOI] [PubMed] [Google Scholar]
- 33.Roberts DM, Liu X, Roberts JA, et al. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit Care. March 13 2015;19:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valtonen M, Tiula E, Backman JT, Neuvonen PJ. Elimination of meropenem during continuous veno-venous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother. May 2000;45(5):701–704. [DOI] [PubMed] [Google Scholar]
- 35.Sherwin JHT, Watt K. Pharmacokinetics and Dosing of Anti-Infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin Ther. 2016;38(9):1976–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. April 2008;31(4):345–351. [DOI] [PubMed] [Google Scholar]
- 37.Mouton JW VA. Is continuous infusion of b-lactam antibiotics worthwhile? Efficacy and pharmacokinetic considerations. J Antimicrob Chemother. 1996;38(1):5–15. [DOI] [PubMed] [Google Scholar]
- 38.Abdul-Aziz MH LJ, Akova M et al. Is prolonged infusion of piperacillin/tasobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J Antimicrob Chemother. 2016;71(1):196–207. [DOI] [PubMed] [Google Scholar]
- 39.Humphries RM AA, Hindler JA. Understanding and Addressing CLSI Breakpoint Revisions: a Primer for Clinical Laboratories. J Clin Microbiol. 2019;57(6):e00203–00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imani SBH, Marriott D, Gentili S, Sandaradura I. Too much of a good thing: a retrospective study of β-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72(10):2891–2897. [DOI] [PubMed] [Google Scholar]
- 41.Contegean AJL, Benaboud S et al. A meropenem pharmacokinetics model inpatients with haematological malignancies. J Antimicrob Chemother. 2020;75(10):2960–2968. [DOI] [PubMed] [Google Scholar]
- 42.Al-Shaer MH AW, Graham E, Peloquin CA. Meropenem, Cefepime, and Piperacillin Protein Binding in Patient Samples. Ther Drug Monit. 2020;42(1):129–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


