Abstract
Background
A systematic review and meta-analysis was conducted to assess breast cancer (BC) outcomes among patients with early-stage hormone receptor positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) BC, receiving adjuvant endocrine therapy.
Methods
Randomized controlled trials (RCTs) and real-world evidence (RWE) studies were identified using Ovid MEDLINE®, Embase, and Evidence-Based Medicine Reviews. Clinical and methodological similarities including alignment of outcome definitions with standardized definitions for efficacy endpoints criteria were assessed to evaluate feasibility of conducting a meta-analysis. Where feasible, 5-year probabilities of BC recurrence or death were estimated using a Bayesian hierarchical arm-based model.
Results
Of 21 included studies, 8 RCTs and 4 RWE studies reported outcome data of interest. There was heterogeneity in outcome reporting, as well as variation in recurrence risk amongst studies with aligned reporting. Of the 12 studies, 10 were considered for inclusion in a meta-analysis of BC recurrence or death. Only a subgroup analysis of node-positive patients (3 studies; n = 7307) was deemed feasible. The 5-year probability of BC recurrence or death was 17.2% (95% credible interval: 14.6%–20.3%).
Conclusion
Although studies reporting recurrence outcomes were limited, there remains a high risk of BC recurrence, especially among node-positive patients. Approximately 1 in 6 women with node-positive HR+/HER2- early-stage BC receiving endocrine therapy experience recurrence or death within 5-years of initiating treatment, suggesting a need for novel treatments for this population.
Keywords: Early breast cancer, Adjuvant endocrine therapy, Recurrence risk, Recurrence-free survival, Systematic literature review, Meta-analysis
Highlights
-
•
Recurrence data from RCTs and RWE studies in HR+/HER2- early BC is limited.
-
•
Recurrence rates for patients receiving endocrine therapy (ET) were summarized.
-
•
Substantial outcome and clinical heterogeneity between studies was identified.
-
•
An estimated 1 in 6 patients with node-positive BC may relapse within 5-years.
-
•
Unmet need exists for therapies to further reduce recurrence in high-risk early BC.
1. Introduction
Within breast cancer (BC), the main molecular subtypes are characterized by key tumor markers that include the hormone receptors (HR) (estrogen [ER] and progesterone receptors [PR]) and human epidermal growth factor receptor-2 (HER2) [1]. Among the four distinct subtypes of BC (HR+/HER2-, HR+/HER2+, HR-/HER2+ and triple-negative [TNBC]), HR+/HER2- disease represents the most common invasive cancer subtype in women, accounting for 70% of all BC cases [1,2]. Further, more than 90% of primary diagnoses in HR+/HER2- BC occur in non-metastatic early stages (stages I-III) [3].
In recent years, several studies have concluded that disease prognosis, treatment options, and the response to BC therapies vary based on disease subtype [4]. For example, cytotoxic chemotherapy is widely used in the treatment of TNBC while the combination of a HER2-targeted monoclonal antibody with chemotherapy is used in the treatment of HER2+ BC [5]. In HR + patients, endogenous hormones interact with hormone receptors on the cancer cells to further augment proliferation. Considering the main goals of early BC therapy, current clinical practice guidelines recommend the use of endocrine therapy (ET), such as tamoxifen and aromatase inhibitors (AI), in the adjuvant setting to reduce the risk of disease recurrence and death [6,7]. Despite the effectiveness of standard ET, as many as 41% of women diagnosed with HR+ early-stage BC will experience distant (or metastatic) recurrence, with risk varying by tumor characteristics [8]. In particular, greater nodal involvement is a key determinant of AJCC cancer stage [9], and has been associated with an increased risk of disease recurrence in HR+ breast cancer [8,10]. Metastatic BC remains an incurable disease with poor prognosis and a substantial negative impact on quality of life, highlighting the limitations of current therapies and further reducing the risk of recurrence [3].
While clinical trials are often designed to investigate HR+ disease, patients with any HER2 status are often enrolled, as endocrine therapies used in patients with HER2-disease are also used in patients with HER2+ disease [11]. This prevents a full understanding of prognosis and treatment efficacy in HR+/HER2- disease. Although subgroup analyses examining subtype-specific recurrence rates may be reported, such subgroups are often small and result in imprecise estimates of risk. To our knowledge, there are no published systematic literature reviews (SLRs) that have examined adjuvant ETs in women with HR+/HER2- early BC. In this study, we sought to summarize the current literature surrounding the impact of adjuvant ETs on recurrence or death in women with HR+/HER2- early BC and, if feasible, conduct a meta-analysis (including subgroups based on nodal status), synthesizing data around expected recurrence rates in the contemporary era of AIs, where heterogeneous ET regimens are considered holistically.
2. Methods
2.1. Study selection and data synthesis
Two systematic searches of published literature were conducted on July 24, 2019 to identify eligible randomized controlled trials (RCTs) and observational or real-world evidence (RWE) studies reporting any recurrence outcomes (e.g., recurrence-free survival [RFS], disease-free survival [DFS], recurrence events) for adult patients with HR+/HER2- early BC receiving adjuvant ETs. Ovid MEDLINE®, MEDLINE® In-Process, Embase, and Evidence-Based Medicine Reviews (including the Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials [CENTRAL]) were searched, restricting to articles published in the prior 15 years to reflect contemporary clinical practice, including the widespread approval for the most common AIs (i.e., letrozole, anastrozole, exemestane). The literature searches were conducted by an information specialist and peer-reviewed using the Peer Review of Electronic Search Strategies Guideline [12]. Recent scientific congresses and relevant systematic reviews or meta-analysis articles were also reviewed. Citation titles and abstracts identified in the literature searches were screened for relevance then further evaluated in full-text form based on the same selection criteria. Literature searches, study selection, data extraction, and quality assessments were performed by duplicate independent reviewers (where a third reviewer resolved any discrepancies), according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement. [13] The review protocol was registered with the International Prospective Register of Systematic Reviews (CRD161470). The full search strategy, eligibility criteria, and list of excluded articles are available in the Supplementary.
For studies meeting eligibility, data relating to trial design and methodology, details of interventions, patient eligibility criteria, reported baseline characteristics, and recurrence outcome measures were extracted. Quality of each RCT was assessed using the National Institute for Health and Care Excellence (NICE) Quality Appraisal checklist for quantitative intervention studies, whereas the quality of each RWE study was assessed using the Newcastle-Ottawa Scale [14,15].
2.2. Assessing the feasibility of a meta-analysis
The validity of any results generated through meta-analysis is dependent on the evidence meeting the exchangeability assumption [16]. Under this assumption, all interventions studied could have been included as comparators in a clinical trial; thus, all treatments under study are truly competing interventions. Failure to meet this assumption can result in biased estimates of effect. To ascertain whether this assumption was met, included studies reporting common outcomes (defined herein as either local recurrence events, distant recurrence events, any recurrence events, or RFS) were examined to assess their clinical and methodological similarities. Studies reporting the incidence of recurrence events were grouped based on the location of recurrence (i.e., local, distant, or any location). A distinct feasibility assessment was conducted for each incident event by location. Where outcomes were reported as a composite (e.g., DFS, RFS), consistency across studies was measured and compared to standardized definitions for efficacy endpoints (STEEP)-defined criteria for both the invasive disease-free survival (iDFS) outcome and the RFS outcome, which excludes secondary cancers (i.e., contralateral BC or non-BC) [17]. Only studies reporting composite outcomes aligning with STEEP-iDFS or STEEP-RFS definitions were considered when evaluating meta-analysis feasibility. Studies were excluded if outcome data of interest was only available in graphical form and required digitization.
In addition to an assessment of trial-specific outcome definitions, a rigorous qualitative assessment of between-trial heterogeneity for the following elements was conducted: study design (e.g., RCT, retrospective, prospective, enrolment periods, follow-up), study eligibility criteria (e.g., prior therapy restrictions), baseline patient characteristics (e.g., age, menopausal status, nodal status, tumor status, hormone receptor status), intervention, and comparators (e.g., treatments and their corresponding regimens) [18]. All details related to the assessment of clinical heterogeneity were built upon existing recommendations [[19], [20], [21]]. Subgroups defined by nodal status (node-positive and node-negative) were considered.
2.3. Statistical analysis
A Bayesian hierarchical arm-based meta-analysis was performed using the methods outlined by the NICE Decision Support Unit Technical Support Documents [22]. Bayesian methods were selected for the base case analysis owing to increased clinical interpretability, and consideration of uncertainty in the data [22,23]. The base case analysis was conducted using a random-effects model with uninformative (or vague) priors for the overall treatment effects and common heterogeneity standard deviation. Each model was run with 4 separate chains and 40,000 iterations with 40,000 burn-in iterations. Absolute probabilities and 95% credible intervals (CrIs) were calculated. All Bayesian analyses were performed using Just Another Gibbs Sampler (JAGS) version 4.3.0, and R Statistical Software version 3.6.1. Sensitivity analyses were conducted to assess the robustness of the base case analysis. To assess the influence of study design on results, sensitivity analyses were conducted where RWE studies were down weighed 50% compared with RCT studies [24,25]. Additional sensitivity analyses included using fixed-effect models and a direct (i.e., frequentist) meta-analysis for all outcomes, where statistical heterogeneity tests were performed using I2. The Comprehensive Meta-Analysis software was used to perform all direct meta-analyses [26].
3. Results
3.1. Study selection and characteristics
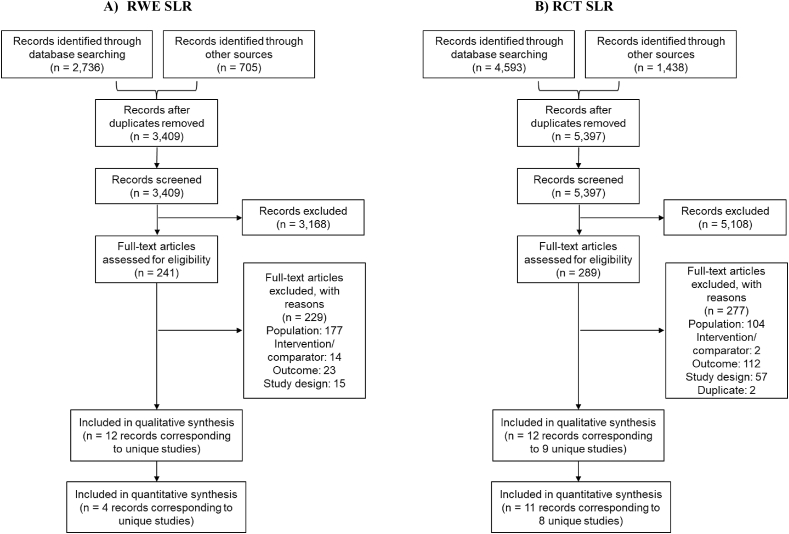
A total of 5397 RCTs and 3409 RWE studies published between 2011 and 2019 were identified. Of these, 12 RCTs corresponding to 9 unique trials and 12 RWE studies were considered for full-text review. In total, 9 RCTs and 12 RWE studies were identified that fulfilled the inclusion criteria (Fig. 1). Finally, 8 RCTs and 4 RWE studies were included for meta-analysis feasibility assessment after screening for the reporting of relevant recurrence outcomes of interest (Fig. 1). Two studies provided poor reporting of recurrence data of interest and thus were subsequently excluded from the compilation of recurrence outcomes; however, study and patient baseline characteristics information from these studies were compiled [27,28].
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram summarizing the process for the identification of the eligible studies.
Abbreviations: PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT = randomized controlled trials; RWE = real-world evidence; SLR = systematic literature review.
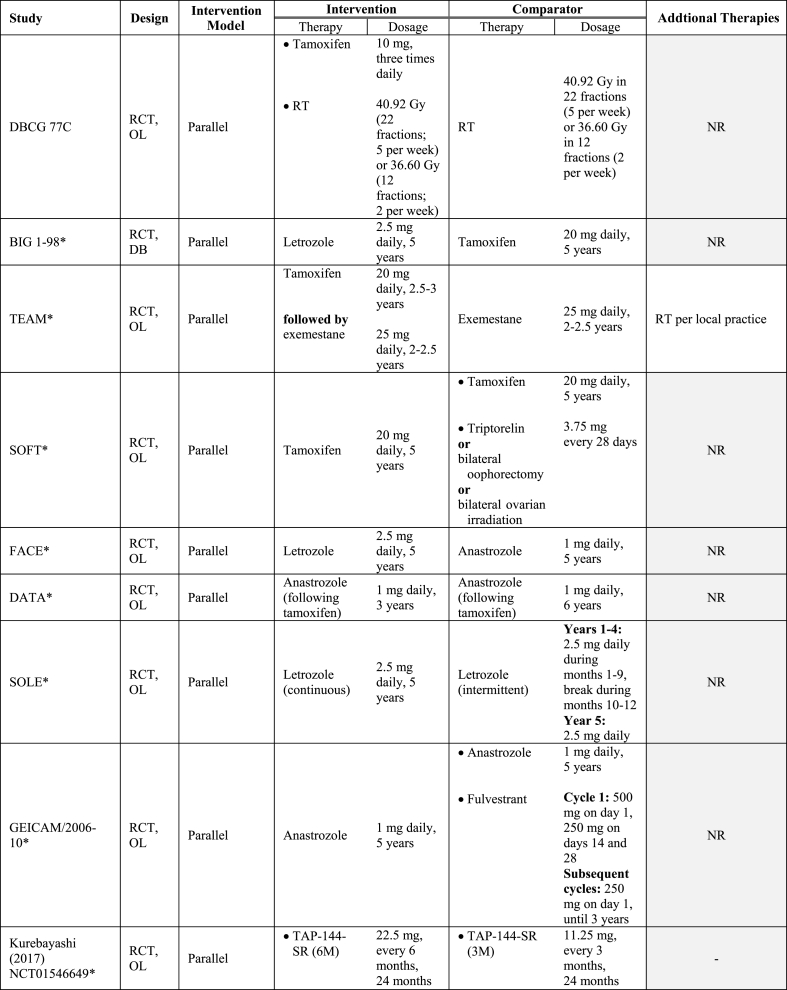
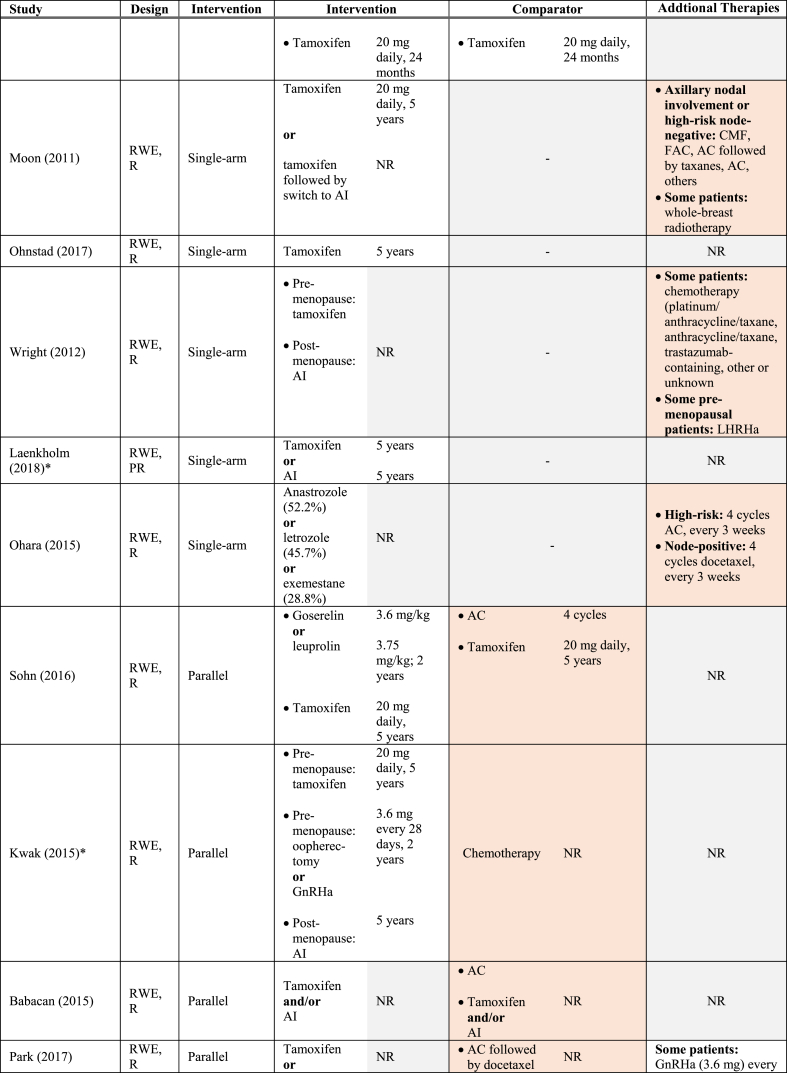
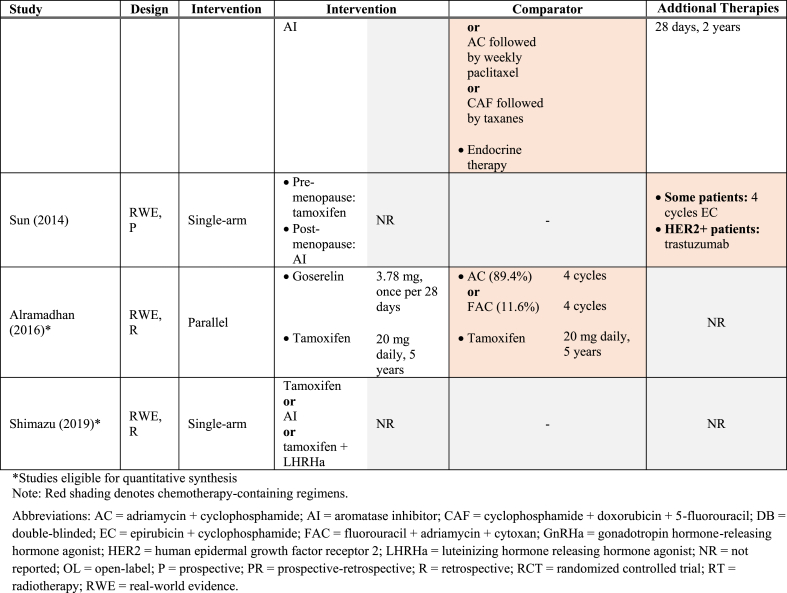
Table 1 summarizes the key characteristics of the included studies. Overall, data were reported from 34,582 women with HR+/HER2- early-stage BC enrolled (or identified) from 1977 through 2015. All RCTs were phase III studies. Most RWE studies were retrospective with one prospective study [29] and one bidirectional (prospective-retrospective) study included [30]. Median follow-up varied between 4 and 10 years, considering recurrence outcomes [28,[30], [31], [32], [33], [34]]. While all RCTs were conducted across multiple continents [[35], [36], [37], [38], [39], [40]], two were based in Europe, [[32], [41], [42]] and one in Japan [31,33]. Conversely, the majority of RWE studies were conducted in Asia [27,29,34,[43], [44], [45], [46], [47], [48]]. Nine of the 12 included RWE studies were single-arm investigations of ET [29,30,34,[44], [45], [46], [47], [48], [49]]. The remaining RCTs compared ET to a combination of ET and chemotherapy [27,28,43]. Collectively, AIs such as letrozole were administered in 48% of all studies [30,[35], [36], [37],[39], [40], [41], [42],44,46,47], and tamoxifen in 52% [30,32,[36], [37], [38],[43], [44], [45],[47], [48], [49]]. Ovarian function suppression [38,44], fulvestrant [41,42,50], luteinizing hormone-releasing hormone agonist (LHRHa) [31,33], and gonadotropin hormone-releasing hormone agonist (GnHRHa) were also investigated [43,48]. See Table 2 for the interventions evaluated in the included RCT and RWE studies.
Table 1.
Study design elements amongst studies included in the SLR.
| Study | Study Design |
Enrolment Dates | Country/Region | Patients (n) | Median Follow-up (years) | Recurrence Descriptors Reported for HR+/HER2- eBC Patients | |||
|---|---|---|---|---|---|---|---|---|---|
| RCT |
RWE |
||||||||
| Phase | Blinding | Data Source | Design | ||||||
| DBCG 77C | III | Open-label | Parallel | – | 1977–1982 | Denmark | 1716 | Recurrence: 10 Survival: 30 |
BCRR |
| BIG 1–98a | III | Double-blind | Parallel | – | 1998–2003 | International | 2923 | 8.1 | DFS |
| TEAMa | III | Open-label | Parallel | – | 2001–2006 | Europe | 6120 | 9.8 | DFS |
| SOFTa | III | Open-label | Parallel | – | 2003–2011 | United States, Canada, Switzerland |
3066 | 5.6 | DFS |
| FACEa | IIIb | Open-label | Parallel | – | 2005–2008 | International | 4172 | 5.4 | DFS |
| DATAa | III | Open-label | Parallel | – | 2006–2009 | Netherlands | 1860 | 4.2 | DFS |
| SOLEa | III | Open-label | Parallel | – | 2007–2012 | International | 4884 | 5.0 | DFS |
| GEICAM/2006–10a | III | Open-label | Parallel | – | 2008–2010 | Spain | 870 | 6.4 | DFS, LR, DR, any R, TTR |
| Kurebayashi (2017)a NCT01546649 |
III |
Open-label |
Parallel |
– |
2012–2014 |
Japan |
167 |
1.8 |
DFS, DDFS |
| Moon (2011) | – | Retrospective | 1994–2004 | Korea | 819 | 6.4 | RFS | ||
| Ohnstad (2017) | – | Retrospective | 1995–1998 | Norway | 653 | DDFS: 7.1 | DDFS | ||
| Wright (2012) | – | Retrospective | 1999–2009 | United States | 582 | 3.7 | PFS | ||
| Laenkholm (2018)a | – | Prospective-retrospective | 2000–2003 | Denmark | 2558 | 9.2 | DR, TTR | ||
| Ohara (2015) | – | Retrospective | 2002–2012 | Japan | 184 | 3.8 | RFI | ||
| Sohn (2016) | – | Retrospective | 2003–2008 | Korea | 994 | 7.4 | RFS | ||
| Kwak (2015)a | – | Retrospective | 2003–2011 | Korea | 242 | 5.1 | DFS | ||
| Babacan (2015) | – | Retrospective | 2003–2014 | Turkey | 634 | NR | PFS/DFSb | ||
| Park (2017) | – | Retrospective | 2004–2013 | Korea | 851 | 4.3 | DFS | ||
| Sun (2014) | – | Prospective | 2008–2010 | China | 541 | 4.4 | RFS | ||
| Alramadhan (2016)a | – | Retrospective | 2008–2013 | Korea | 406 | 4.3 | DFS, LR, DR, any R | ||
| Shimazu (2019)a | – | Retrospective | 2008–2015 | Japan | 340 | 5 | DFS, DR | ||
Abbreviations: BCRR = breast cancer recurrence rate; DDFS = distant disease-free survival; DFS = disease-free survival; DR = distant recurrence; eBC = early breast cancer; HER2: human epidermal growth factor receptor 2; HR = hormone receptor; LR = local recurrence; PFS = progression-free survival; R = recurrence; RFS = recurrence-free survival; RCT = randomized controlled trial; RFI = recurrence-free interval; RFS = recurrence-free survival; RWE = real-world evidence; TTR = time to recurrence.
Studies eligible for quantitative synthesis.
Babacan et al. (2015) study used the terms PFS and DFS interchangeably and did not provide outcome definitions.
Table 2.
Endocrine therapies investigated amongst studies included in the SLR.
Individual trials used different endpoints for recurrence. DFS was reported in all included RCTs, whereas only 5 RWE studies reported this outcome [27,28,43,44,47] (the remainder reported PFS [34], RFS [29,48], or DDFS [49]). Several studies also examined the incidence of recurrence events, often by location [30,32,37,[41], [42], [43],47].
The included RCTs were all well-conducted, and the risk of bias was low to moderate based on internal and external validity scores (Supplementary Table 2). For the included RWE studies, Newcastle-Ottawa Scale quality scores ranged from 5 to 8 points out of a maximum of 9 points (Supplementary Table 3).
3.2. Recurrence outcomes
3.2.1. Composite outcomes (disease- and recurrence-free survival)
DFS reported in 3 RWE studies ranged from 76.2% [44] to 98.9% [43] with follow-up periods ranging from 5 to 6 years (Table 3) [43,44,47]. Where provided, DFS definitions differed in the inclusion of all-cause death [43,44]. In 8 RCTs, DFS was reported in HR+/HER2- patients for timepoints ranging from 1.8 years (reported as 96 weeks) [31,33] to 10 years [36] (Table 4), and provided DFS definitions were broadly aligned. Kurebayashi et al. reported the most short-term DFS, at 1.8 years, to be approximately 97% (95% CI: 93.6%–100%) for both LHRHa regimens [31,33]. Five-year DFS was reported in 5 studies [35,[38], [39], [40], [41],50] ranging from 79.7% (95% CI: 76.2%–83.3%) [40] to 91% (95% CI: 88.2%–93.9%) [41,50]. Ten-year DFS was reported for patients with HR+/HER2- disease in the TEAM trial at approximately 67% [36].
Table 3.
Disease-free survival in RWE studies.
| First Author (year) | Follow-up | Event-free (%) |
|---|---|---|
| Kwak (2015) | 5 years | ET arm: 76.2 |
| Alramadhan (2016) | 5 years | ET arm: 98.9 |
| Shimazu (2019) | 6 years | osN0: 92.4 pN0: 87.0 |
Abbreviations: ET = endocrine therapy; osN0 = negative sentinel lymph nodes assessed by one-step nucleic acid amplification; pN0 = negative sentinel lymph node assessed by pathology; RWE = real-world evidence.
Table 4.
Disease-free survival in RCTs.
| First Author (year) or NCT record number [trial name] | Intergroup Difference |
Intervention |
Comparator |
||||
|---|---|---|---|---|---|---|---|
| Effect Estimate | 95% CI | p-value | Treatment | Proportion | Treatment | Proportion (95% CI) | |
| 96-week (2 year) DFS | |||||||
| Kurebayashi 2017; NCT01546649 |
Difference: 1.2% | −5.2 - 7.8 | NR | TAP-144-SR (6 month depot) |
97.3% (95% CI: 93.6–100.0) | TAP-144-SR (3 month depot) | 97.5% (95% CI: 94.1–100.0) |
| 5-year DFS | |||||||
| Ruiz-Borrego (2019); NCT00543127 [GEICAM/2006–10] |
NR | NR | NR | Anastrozole + fulvestrant | 91.0% (95% CI: 88.2–93.9) | Anastrozole | 90.8% (95% CI: 88.0–93.6) |
| Smith (2017) [FACE] | HR: 0.96 | 0.82–1.13 | NR | Letrozole | 84.7% (95% CI: 82.9–86.3) | Anastrozole | 83.4% (95% CI: 81.6–85.1) |
| Tjan-Heijnen (2017)a [DATA] | HR: 0.79 | 0.61–1.03 | NR | Anastrozole (6 years) |
83.2% (95% CI: 79.7–86.7) | Anastrozole (3 years) | 79.7% (95% CI: 76.2–83.3) |
| Colleoni (2018) [SOLE] | HR: 1.12 | 0.94–1.33 | NR | Intermittent letrozole | 85.0% | Continuous letrozole | 86.6% |
| Francis (2015) [SOFT] | HR: 0.88 | 0.69–1.13 | NR | Tamoxifen + OFS | 86.3%b (95% CI: NR) | Tamoxifen | 85.3%b (95% CI: NR) |
| 7-year DFS | |||||||
| Ruiz-Borrego (2019); NCT00543127 [GEICAM/2006–10] |
HR: 0.84 | 0.58–1.22 | 0.352 | Anastrozole + fulvestrant | 86.9% (95% CI: 83.3–90.6) | Anastrozole | 83.3 (95% CI: 79.2–87.5) |
| 8-year DFS | |||||||
| Filho (2015) [BIG 1–98] | HR for ILC: 0.48 | 0.31–0.74 | NR | Letrozole | 82% (95% CI: NR) | Tamoxifen | 66% (95% CI: NR) |
| HR for IDC: 0.80 | 0.68–0.94 | 82% (95% CI: NR) | 75% (95% CI: NR) | ||||
| 10-year DFS | |||||||
| Derks (2017) [TEAM] | NR | NR | NR | Tamoxifen → exemestane | 67% (95% CI: 66–69) | Exemestane | 68% (95% CI: 66–70) |
Abbreviations: CI = confidence interval; DFS = disease-free survival; HR = hazard ratio; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; NR = not reported; OFS = ovarian function suppression; RCT = randomized controlled trial.
Study included patients who were disease-free after 3 years, as such the DFS outcome was termed 5-year “adapted” DFS.
Reported in-text as proportion experiencing DFS events; subtracted from 100%.
Four RWE studies reported RFS or RFI, ranging from 88.4% to 96.5% [29] for follow-up periods ranging from 4 to 11 years (Table 5) [29,45,46,48]. Outcome definitions were not aligned across these studies – RFS events were defined as either distant relapse [29], locoregional and distant recurrences [45], or recurrences and death [48]. Ohara et al. defined RFI as the time to cancer recurrence [46].
Table 5.
Recurrence-free survival and recurrence-free interval in RWE studies.
| First Author (year) | Outcome | Follow-up | Event-free (%) |
|---|---|---|---|
| Moon (2011) | RFS | 10 years | 88.6 |
| Sun (2014) | RFS | 5 years | Luminal A: 96.5a Luminal B: 88.4a |
| Ohara (2015) | RFI | 3.8 years | 91.3 |
| Sohn (2016) | RFS | 10.9 years | ET arm: 90.5 |
Abbreviations: ET = endocrine therapy; RFI = recurrence-free interval; RFS = recurrence-free survival; RWE = real-world evidence.
Values obtained by digitizing available Kaplan-Meier curves.
A single RWE study reported PFS, defined as the time to locoregional recurrence, distant metastasis, or death [34]. Outcome information for the overall patient cohort was unavailable. Instead, HR+/HER2- patient data were stratified based on patient traits such as race [34]. At approximately 12 years, black patients had worse outcomes compared to other races, and these differences were statistically significant in the analyses of post-menopausal patients (39.9% versus 73.5%) and PR+ patients (27.5% versus 63%) [34].
Outcomes termed time to recurrence (TTR) and breast cancer recurrence rate (BCRR) were also identified in RCTs, both of which considered only recurrence events [32,41]. At 5 years, TTR ranged from 92.7% to 94% between treatment arms in the GEICAM/2006-10 study (Table 6) [41]. 10-year BCRR reported by Knoop et al. in the DBCG 77C trial was much lower, ranging from 40.8% to 57.0% in patients receiving ET, where a significant benefit of tamoxifen in addition to radiotherapy was reported [32]. The 10-year BCRR rates in DCBG 77C were appreciably high, which is consistent with the high-risk profile of recruited participants [32].
Table 6.
Other recurrence outcomes in RCTs.
| First Author (year) [trial name] | Intergroup Difference |
Intervention |
Comparator |
||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-value | Treatment | Proportion | Treatment | Proportion | |
| TTR at 5 years | |||||||
| Ruiz-Borrego (2019) [GEICAM/2006–10] | NR | NR | 0.406 | Anastrozole | 92.7% | Anastrozole + fulvestrant | 94.0% |
| BCRR at 10 years | |||||||
| Knoop (2014) [DBCG 77C] | LA: 0.66 | 0.53–0.84 | NR | Tamoxifen + radiotherapy | 40.8% | Radiotherapy alone | 55.1% |
| LB: 0.54 | 0.39–0.74 | NR | 57.0% | 69.1% | |||
Abbreviations: BCRR = breast cancer recurrence rate; CI = confidence interval; LA = luminal A; LB = luminal B/HER2-; NR = not reported; RCT = randomized controlled trial.
3.2.2. Location-specific outcomes (locoregional, distant, and any site recurrence)
Six RWE studies reported location-specific recurrence outcomes (Table 7) [30,43,[46], [47], [48], [49]]. Two of these studies reported DDFS [49] or distant relapse-free survival (DRFS) [47]. However, the provided definitions considered only distant recurrence. For follow-up periods of less than 6 years, more than 94% of patients were free from distant recurrence [43,46,48], and more than 96% of patients were free from local recurrence [46,48]. Shimazu et al. (2019) reported 6-year DRFS exceeding 90% in their assessment of node-negative patients [47]. Laenkholm et al. (2018) reported a sequential increase in the incidence of distant recurrence at 10 years based on the number of lymph nodes involved (86% for patients with 2 lymph nodes, 77.3% for patients with 3 affected lymph nodes) [30]. Ohnstad et al. (2017) reported DDFS stratified by Prosigna-determined risk of recurrence (ROR) groups, where DDFS at 8 years was 74.3% for high-risk patients as defined by ROR grouping [49].
Table 7.
Distant and local recurrence outcomes in RWE studies.
| First Author (year) | Outcome Term | Follow-up | Proportion of Patients |
|---|---|---|---|
| Distant recurrence | |||
| Ohara (2015) | Distant recurrence (free of) | ∼4 years | 94.0% |
| Alramadhan (2016) | Distant recurrence (free of) | ∼4 years | ET arm: 99.5% |
| Sohn (2016) | Distant metastasis (free of) | 5 years | 96.7% |
| Ohnstad (2017) | DDFSa | 8 years |
ROR group Low-risk: 87.8% Intermediate risk: 77.7% High-risk: 74.3% |
| Laenkholm (2018) | Distant recurrence (free of) | 10 years |
Lymph nodes involved 0 lymph nodes: 89.5% 1 lymph node: 87.9% 2 lymph nodes: 86.0% 3 lymph nodes: 77.3% |
| Shimazu (2019) | DRFS | 6 years | osN0: 99.5% pN0: 90.1% |
| Local recurrence | |||
| Ohara (2015) | Local recurrence (free of) | ∼4 years | 97.3% |
| Sohn (2016) | Locoregional recurrence (free of) | 5 years | 96.5% |
Abbreviations: DDFS = distant disease-free survival; DRFS = distant relapse-free survival; ET = endocrine therapy; osN0 = negative sentinel lymph nodes assessed by one-step nucleic acid amplification; pN0 = negative sentinel lymph node assessed by pathology; ROR = risk of recurrence.
Values were collected by digitizing available Kaplan-Meier curves.
A single RCT reported a location-specific recurrence outcome: Kurebayashi et al. defined DDFS events as distant recurrence, second primary cancer, and death. Over a short-term follow-up of 1.8 years, DDFS for participants treated with LHRHa regimens was approximately 99% (Table 8). [31,33].
Table 8.
Distant disease-free survival at 1.8 years (Kurebayashi et al., 2017).
| First Author (year) or NCT record number [trial name] | Intergroup Difference |
Intervention |
Comparator |
||||
|---|---|---|---|---|---|---|---|
| Difference | 95% CI | p-value | Treatment | Proportion | Treatment | Proportion | |
| Kurebayashi 2017; NCT01546649 | −0.3% | −4.0 – 3.4 | NR | TAP-144-SR (6 month depot) | 98.5% (95% CI: 95.7–100.0) | TAP-144-SR (3 month depot) | 98.8% (95% CI: 96.4–100.0) |
Note: timepoint was more specifically reported by authors as 96 weeks and has been converted to years.
Abbreviations: CI = confidence interval; NR = not reported.
3.3. Meta-analysis
Study characteristics and reported recurrence outcomes, respectively, of the subset of 12 studies included in the meta-analysis assessment [30,31,33,[35], [36], [37], [38], [39], [40], [41], [42], [43], [44],47,50] are summarized in Table 1, Table 9. For the included RCTs, 5 of the 8 trials fulfilled all STEEP-defined iDFS criteria [31,33,35,37,38,40], whereas the remaining 3 trials did not report whether their definitions considered a second primary or non-breast cancer [36,39,41,42]. The definitions of DFS as reported in 3 of the included RWE studies [43,44,48] did not meet all STEEP-defined iDFS criteria (Table 10). Specifically, Alramadhan et al. did not report whether non-BC deaths, death from unknown causes, or secondary cancers (contralateral BC and non-BC) were part of their DFS criteria [43]. Definitions of DFS reported by Shimazu et al. and Kwak et al. met many of the STEEP iDFS criteria; however, the inclusion of secondary cancers in their definitions was unclear [44,47]. All included RCTs and RWE studies reported efficacy endpoints that were consistent with criteria meeting the STEEP-defined RFS definition, except one [43]. As such, 10 studies (8 RCTs and 2 RWE studies) reported outcomes that were considered similar and consistent with STEEP-defined RFS to be included in a potential meta-analysis [31,33,[35], [36], [37], [38], [39], [40], [41], [42],44,47]. Incidence of recurrence events were consistently defined (i.e., local recurrences, distant recurrences, and/or any location recurrences) among the included studies. The feasibility assessment presented herein pertain to studies aligning with the STEEP-defined RFS outcome. A meta-analysis of recurrence events was not determined to be feasible.
Table 9.
Recurrence outcomes reported in studies eligible for quantitative synthesis.
| Study | Study Design | Recurrence Descriptors Reported for HR+/HER2- eBC Patients |
|||||
|---|---|---|---|---|---|---|---|
| DFS | DDFS | LR | DR | Any R | Other | ||
| BIG 1-98 | RCT | X | |||||
| TEAM | RCT | X | |||||
| SOFT | RCT | X | |||||
| FACE | RCT | X | |||||
| DATA | RCT | X | |||||
| SOLE | RCT | X | |||||
| GEICAM/2006-10 | RCT | X | X | X | X | TTR | |
| Kurebayashi (2017) NCT01546649 | RCT | X | X | ||||
| Laenkholm (2018) | RWE | X | TTR | ||||
| Kwak (2015) | RWE | X | |||||
| Alramadhan (2016) | RWE | X | X | X | X | ||
| Shimazu (2019) | RWE | X | X | ||||
Abbreviations: R = recurrence; DDFS = distant disease-free survival; DFS = disease-free survival; DR = distant recurrence; eBC = early breast cancer; HER2 = human epidermal growth factor receptor 2; HR = hormone receptor; RCT = randomized controlled trial; RWE = real-world evidence; TTR = time to recurrence.
Table 10.
Assessment of recurrence outcome definition comparability using the STEEP system.
| Study Design | STEEP Outcome/Studya | Invasive Ipsilateral Breast Tumor Recurrence | Local/Regional Invasive Recurrence | Distant Recurrence | Death from Breast Cancer | Death from Non-Breast Cancer Cause | Death from Unknown Cause | Invasive Contra-lateral Breast Cancer | Second Primary Invasive Cancer (non-breast) |
|---|---|---|---|---|---|---|---|---|---|
| – | STEEP: iDFS | X | X | X | X | X | X | X | X |
| – | STEEP: RFS | X | X | X | X | X | X | ||
| RCT | BIG 1-98 | X | X | X | X | X | X | X | X |
| RCT | TEAM | X | X | X | X | X | X | X | |
| RCT | SOFT | X | X | X | X | X | X | X | X |
| RCT | FACE | X | X | X | X | X | X | X | |
| RCT | DATA | X | X | X | X | X | X | X | X |
| RCT | SOLE | X | X | X | X | X | X | X | X |
| RCT | GEICAM/2006-10 | X | X | X | X | X | X | X | |
| RCT | Kurebayashi (2017) NCT01546649 |
X | X | X | X | X | X | X | X |
| RWE | Kwak (2015) | X | X | X | X | X | X | X | |
| RWE | Alramadhan (2016) | X | X | X | X | ||||
| RWE | Shimazu (2019) | X | X | X | X | X | X |
Abbreviations: iDFS = invasive disease-free survival; RCT = randomized controlled trial; RFS = recurrence-free survival; RWE = real-world evidence; STEEP = standardized definitions for efficacy end points.
Obtained from study by Hudis et al. [17].
A detailed assessment of the comparability of selected baseline demographic characteristics (e.g., age, menopausal status), disease-related characteristics (e.g., nodal status, tumor size), and study eligibility criteria across the studies reporting RFS, as defined by STEEP, are presented as Supplementary. Clinical heterogeneity amongst the studies was deemed substantial, and heterogeneity was noted for traits that were considered plausible effect modifiers. Given the marked between-trial differences in patient characteristics (e.g., nodal status), eligibility criteria, and study characteristics among the included studies, it was determined that a meta-analysis combining all 8 RCTs and 2 RWE studies to determine the risk of recurrence or death (RFS as defined by STEEP) or for any location-specific event were not feasible.
The feasibility of subgroup analyses defined by nodal status was assessed for inclusion in a meta-analysis of recurrence or death (RFS as defined by STEEP). We determined that a subgroup defined as HR+/HER2- early-stage BC patients receiving adjuvant ET with ≥ 99% node-positive status was feasible to meta-analyze. A total of 3 studies (2 RCTs [35,39] and 1 RWE study [44]) reporting RFS were considered in this evidence network. Patient characteristics were similar across all 3 studies (Table 11). No subgroups evaluating the incidence of recurrence events were determined to be feasible (see Supplementary).
Table 11.
Comparability of patient baseline characteristics amongst studies reporting RFS for node-positive patients (subgroup).
| Parameter | SOLE | FACE | Kwak (2015) |
|---|---|---|---|
| Study design | RCT | RCT | RWE |
| Age (median) | LC: 60c LI: 60c |
L: 62c A:62c |
48.5a |
| Post-menopausal (%) | LC: 77c LI: 76c |
L: 100 A: 100 |
51.5 |
| T1 (%) | LC: 47c LI: 58c |
L: 47c,b A: 45.5c,b |
51.5 |
| Stage 1 (%) | NR | 0 | 25.2 |
| ER+ (%) | LC: 98c LI: 97.9c |
L: 98.4c A: 98.9c |
93.2 |
| PR+ (%) | LC: 80c LI: 78c |
L: 79.8c A: 79.4c |
81.6 |
Abbreviations: A = anastrozole; ER = estrogen receptor; L = letrozole; LC = continuous letrozole; LI = intermittent letrozole; NR = not reported; PR = progesterone receptor; RCT = randomized controlled trial; RFS = recurrence-free survival; RWE = real-world evidence.
Mean age.
Includes T1 and T0 patients.
Full cohort (not exclusively HR+/HER2-).
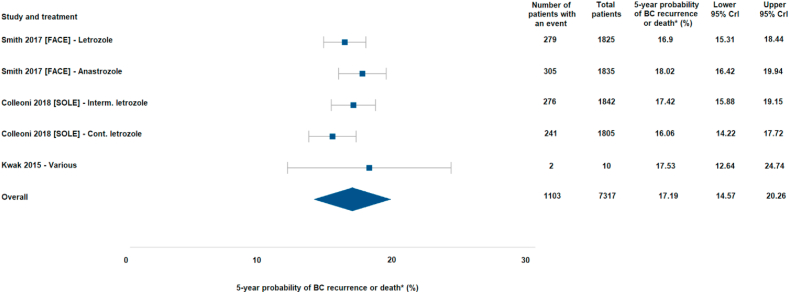
In the base case analysis using a Bayesian framework (Fig. 2), the pooled 5-year probability of recurrence or death in patients with node-positive HR+/HER2- early-stage BC receiving adjuvant ETs was 17.2% (95% CrI: 14.6%–20.3%). Results of sensitivity analyses (e.g., frequentist framework, fixed-effect model) confirmed the robustness of the base case analysis: pooled 5-year probabilities of recurrence or death ranged from 15.1% to 17.2% (Supplementary Figure 2). The impact of downweighing or excluding the single RWE study in sensitivity analyses had a minimal impact on the overall estimates. Additionally, an exploratory analysis was conducted for completeness, including more broadly defined patient populations (ie., not restricted to patients with node-positive status; see Supplementary). In addition to large dispersion in risk across the studies included therein, the between trial heterogeneity (indicated as standard deviation) was approximately five times greater as compared with the node-positive subgroup.
Fig. 2.
Forest plot of the base-case meta-analysis of RFS events in HR+/HER2- node-positive patients from RCT and RWE.
Abbreviations: BC = breast cancer; CrI = credible interval. ∗Mean probability of patients experiencing a recurrence event was based on recurrence-free survival (RFS) events as defined by STEEP (any breast cancer recurrence or all-cause death).
4. Discussion
We conducted a comprehensive literature review to understand recurrence outcomes in patients with HR+/HER2- disease receiving adjuvant ET. Where outcome data were deemed comparable, considerable variation was noted in the rate of disease recurrence among patients with HR+/HER2- BC, likely reflecting clinical heterogeneity, which was explored in our meta-analysis feasibility assessment. Indeed, only one subgroup meta-analysis was deemed appropriate to conduct. We found that approximately 1 in 6 patients with node-positive disease, will experience recurrence within 5 years of initiating endocrine therapy. To our knowledge, this is the first review to summarize the literature for patients with HR+/HER2- BC. Existing literature reviews and meta-analyses often narrow the study population to HR+ BC, including patients with HER2+ disease, which likely confounds disease recurrence estimates. These findings contrast broad perceptions that the vast majority of recurrences in HR+/HER2- disease occur later (i.e., more than 5 years after diagnosis) [[51], [52], [53]]. Furthermore, these results suggest a substantial impact of high-risk features on patient prognosis, particularly node-positive disease.
This study also evaluated author-reported recurrence outcome definitions to ensure the appropriateness of combining and analyzing different studies. It was concluded that a combination of results across several studies reporting similar outcomes (either RFS or recurrence events) was not feasible. However, cross-trial heterogeneity was largely resolved upon isolation of a more narrowly defined subgroup of node-positive patients. In the meta-analysis of node-positive HR+/HER2- patients with low clinical heterogeneity, the probability of recurrence or death was 17.2% over 5 years. These findings indicate that the risk of recurrence or death in HR+/HER2- patients is greater than expected when patients have high-risk features such as node-positive status.
Although patients with key risk features such as node-positive status are seldom investigated exclusively in clinical trials, the results of published studies investigating risk factors for recurrence in HR+ disease are aligned with our findings [8,10]. In a longer-term assessment, Pan et al. assessed 88 trials of early-stage ER+ BC patients who received ET and found that the risk of distant recurrence in the first 5 years was closely related to number of positive nodes (6% in women without positive nodes and 22% in women with 4–9 positive nodes) [8]. Colleoni et al. noted that difference in recurrence risk according to nodal status was the highest in the first 5 years [10]. This work should be considered complimentary to the meta-analyses conducted by Pan et al., which were more broad and focused on numerous disease-related traits [8]. In contrast, our analyses considered the impact of heterogeneity by focusing on more narrowly defined subgroups in order to yield more precision in the effect estimates (ie., we considered nodal status within the HR+/HER2- population, and treatments that focused on endocrine therapy alone).
The existence of high-risk groups within the HR+/HER2- subtype indicates an unmet need in the treatment landscape for the prevention of recurrence. Several phase III studies evaluating the efficacy of cyclin-dependent kinase 4/6 inhibitors in the adjuvant setting for non-metastatic BC are ongoing [[54], [55], [56], [57]]. In the context of the present study, the results of high-quality trial data considering high-risk patients as a compliment to data considering broader cohorts will allow clinicians to tailor treatments to the unique risk profiles of individual patients.
A strength of this review was its comprehensive search strategy, rigorously developed in collaboration with an experienced information specialist. Furthermore, we adhered to the PRISMA method to ensure complete and transparent reporting of studies. Combining clinically heterogeneous studies introduces the potential to bias pooled effect estimates, leading to inaccurate estimates of BC recurrence risk. Similarly, a comprehensive evaluation of cross-trial heterogeneity was critical to mitigating potential sources of bias.
This study should also be considered in the context of several limitations. Despite our efforts to minimize cross-trial imbalances by examining subgroups, baseline patient characteristics were often not reported for the target population. Therefore, it is possible that imbalances between subpopulations persisted, which may have led to underestimation of cross-trial heterogeneity. However, this was difficult to confirm without additional information that was not reported. Furthermore, our focus on patients with HR+/HER2- early BC limited the number of studies identified for this review. However, excluding other BC subtypes ensured identification of the existing evidence in addition to gaps in the literature related to real-world effectiveness and RCT data in these patients. The lack of studies evaluating recurrence in this subtype highlights a need for the investigation of risk factors involved in recurrence as well as potential targets for interventions to further reduce recurrence and potential metastases after diagnosis of HR+/HER2- early BC.
5. Conclusion
Although this study identified a dearth of evidence regarding HR+/HER2- BC recurrence in both RCT and real-world settings, a synthesis of published studies was feasible in order to ascertain the probability of recurrence or death in this population. Together with prior literature, our results indicate an unmet need to further reduce recurrence risk early in the treatment of patients with high-risk non-metastatic disease. Additional randomized and real-world studies investigating the risk of recurrence in HR+/HER2- early BC patients are needed to improve our understanding of this clinically heterogeneous disease.
Funding
Sponsorship for this study was provided by Pfizer Inc.
Declarations of competing interest
EMS, AOR, AS, CC, and IAS have disclosed that they are employees of EVERSANA, who were paid consultants to Pfizer Inc in connection with the development of this manuscript. CC has disclosed that he is also a shareholder of EVERSANA. JC and EHL are employed by Pfizer Inc. EHL has disclosed that he is also a shareholder of Pfizer Inc.
Acknowledgements
The authors thank Rana Qadeer for his analytical insights and Ashfaqul Azraf for his editorial assistance. The authors also thank Catarina Aniceto da Silva, Sheryl Fogarty, and Becky Skidmore for assistance with the literature review.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.02.009.
Role of Funder
The funders had a role in study design and editorial assistance. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions
All authors made substantive intellectual contributions to this study to qualify as authors. All authors participated in study design through drafting or approval of the protocol. EMS, AOR, and IAS contributed to the literature search. EMS and AOR worked on data collection. EMS, AOR, AS, CC, and IAS analyzed and interpreted the data. EMS and IAS wrote the manuscript draft. EL and JC assisted with manuscript revisions. All authors reviewed and approved the final version of the manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Howlader N., Altekruse S.F., Li C.I., Chen V.W., Clarke C.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D. Breast cancer statistics, 2019. CA. Canc J Clinic. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.Waks A.G., Winer E.P. Breast cancer treatment: a review. Jama. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 4.Yang S.X., Polley E.C. Systemic treatment and radiotherapy, breast cancer subtypes, and survival after long-term clinical follow-up. Breast Canc Res Treat. 2019;175(2):287–295. doi: 10.1007/s10549-019-05142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeferlin L.A., E Chalfant C., Park M.A. Challenges in the treatment of triple negative and HER2-overexpressing breast cancer. J Surgery Sci. 2013;1(1):3–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Balic M., Thomssen C., Würstlein R., Gnant M., Harbeck N. St. Gallen/vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019;14(2):103–110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao R.D., Cobleigh M.A. Adjuvant endocrine therapy for breast cancer. Oncology (Williston Park) 2012;26(6):541–547. 550, 552 passim. [PubMed] [Google Scholar]
- 8.Pan H., Gray R., Braybrooke J., Davies C., Taylor C. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliano A.E., Edge S.B., Hortobagyi G.N. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 10.Colleoni M., Sun Z., Price K.N., Karlsson P., Forbes J.F. Annual hazard rates of recurrence for breast cancer during 24 Years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol : Off J Am Soc Clinic Oncol. 2016;34(9):927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 12.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V. PRESS peer review of electronic search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. e1000097-e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence Methods for the development of NICE public health guidance (third ed.) 2012. https://www.nice.org.uk/process/pmg4/chapter/about-this-document Available online at: September 26. [PubMed]
- 15.Wells G., Shea B., O’Connell D., Peterson j, Welch V. University of Ottawa; 2000. The newcastle–ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available online at: [Google Scholar]
- 16.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 17.Hudis C.A., Barlow W.E., Costantino J.P., Gray R.J., Pritchard K.I. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol : Off J Am Soc Clinic Oncol. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 18.Cope S., Zhang J., Saletan S., Smiechowski B., Jansen J.P. A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med. 2014;12 doi: 10.1186/1741-7015-12-93. 93-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoaglin D.C., Hawkins N., Jansen J.P., Scott D.A., Itzler R. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2. Value Health. 2011;14(4):429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Jansen J.P., Fleurence R., Devine B., Itzler R., Barrett A. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Jansen J.P., Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013;11(1):159. doi: 10.1186/1741-7015-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias S., Welton N.J., Sutton A.J., Ades A.E. NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. April 2014. http://www.nicedsu.org.uk Last updated: Available online at: [PubMed]
- 23.Hong H., Carlin B.P., Shamliyan T.A., Wyman J.F., Ramakrishnan R. Comparing bayesian and frequentist approaches for multiple outcome mixed treatment comparisons. Med Decis Making. 2013;33(5):702–714. doi: 10.1177/0272989X13481110. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins D., Bujkiewicz S., Martina R., Dequen-O’Byrne P., Abrams K. 2018. Methods for the inclusion of real world evidence in network meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz S., Adams R., Walsh C. Incorporating data from various trial designs into a mixed treatment comparison model. Stat Med. 2013;32(17):2935–2949. doi: 10.1002/sim.5764. [DOI] [PubMed] [Google Scholar]
- 26.Bax L., Yu L.-M., Ikeda N., Tsuruta H., Moons K.G.M. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6 doi: 10.1186/1471-2288-6-50. 50-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S., Lee S.K., Paik H.J., Ryu J.M., Kim I. Adjuvant endocrine therapy alone in patients with node-positive, luminal A type breast cancer. Medicine (United States) 2017;96(22) doi: 10.1097/MD.0000000000006777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babacan T., Buyukhatipoglu H., Balakan O., Kertmen N., Suner A. Chemotherapy might not be beneficial in lymph node-negative, hormone-positive, and HER2-negative breast cancer patients: a long-term retrospective analysis. 2015;20(2):479. [PubMed] [Google Scholar]
- 29.Sun Y., Nie G., Wei Z., Lv Z., Liu X. Luminal breast cancer classification according to proliferative indices: clinicopathological characteristics and short-term survival analysis. Med Oncol. 2014;31(7):55. doi: 10.1007/s12032-014-0055-z. [DOI] [PubMed] [Google Scholar]
- 30.Laenkholm A.V., Jensen M.B., Eriksen J.O., Rasmussen B.B., Knoop A.S. PAM50 risk of recurrence score predicts 10-year distant recurrence in a comprehensive Danish cohort of postmenopausal women allocated to 5 years of endocrine therapy for hormone receptor-positive early breast cancer. J Clin Oncol. 2018;36(8):735. doi: 10.1200/JCO.2017.74.6586. [DOI] [PubMed] [Google Scholar]
- 31.National Library of Medicine (U.S.) A phase 3 comparative study of TAP-144-SR(6M) in postoperative and hormone therapy-naïve patients with premenopausal breast cancer. Identifier NCT01546649. 2015. https://clinicaltrials.gov/ct2/show/record/NCT01546649 vailable online at: (2012, February 28, November 26)
- 32.Knoop A.S., Laenkholm A.V., Jensen M.B., Nielsen K.V., Andersen J. Estrogen receptor, Progesterone receptor, HER2 status and Ki67 index and responsiveness to adjuvant tamoxifen in postmenopausal high-risk breast cancer patients enrolled in the DBCG 77C trial. Eur J Canc. 2014;50(8):1412. doi: 10.1016/j.ejca.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Kurebayashi J., Toyama T., Sumino S., Miyajima E., Fujimoto T. Efficacy and safety of leuprorelin acetate 6-month depot, TAP-144-SR (6M), in combination with tamoxifen in postoperative, premenopausal patients with hormone receptor-positive breast cancer: a phase III, randomized, open-label, parallel-group comparative study. Breast Cancer. 2017;24(1):161–170. doi: 10.1007/s12282-016-0691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright J.L., Reis I.M., Zhao W., Panoff J.E., Takita C. Racial disparity in estrogen receptor positive breast cancer patients receiving trimodality therapy. Breast. 2012;21(3):276. doi: 10.1016/j.breast.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Colleoni M., Luo W., Karlsson P., Chirgwin J., Aebi S. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(1):127. doi: 10.1016/S1470-2045(17)30715-5. [DOI] [PubMed] [Google Scholar]
- 36.Derks M.G.M., Blok E.J., Seynaeve C., Nortier J.W.R., Kranenbarg E.M. Adjuvant tamoxifen and exemestane in women with postmenopausal early breast cancer (TEAM): 10-year follow-up of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(9):1211–1220. doi: 10.1016/S1470-2045(17)30419-9. [DOI] [PubMed] [Google Scholar]
- 37.Filho O.M., Giobbie-Hurder A., Mallon E., Gusterson B., Viale G. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. J Clin Oncol. 2015;33(25):2772. doi: 10.1200/JCO.2015.60.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis P.A., Regan M.M., Fleming G.F., Lang I., Ciruelos E. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith I., Yardley D., Burris H., Boer R.D., Amadori D. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized phase III femara versus anastrozole clinical evaluation (FACE) trial. J Clin Oncol. 2017;35(10):1041. doi: 10.1200/JCO.2016.69.2871. [DOI] [PubMed] [Google Scholar]
- 40.Tjan-Heijnen V.C.G., IEGv Hellemond, Peer P.G.M., Swinkels A.C.P., Smorenburg C.H. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 2017;18(11):1502. doi: 10.1016/S1470-2045(17)30600-9. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Borrego AG-Z M., Bermejo MR B., Cruz JMB-C J., Cirauqui AR-L B., Alba NM-J E. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2-) early breast cancer (EBC): results from the GEICAM/2006-10 study. Breast Canc Res Treat. 2019;177(1):115. doi: 10.1007/s10549-019-05296-8. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Borrego M., Jimenez M.M., Ruiz A., Lluch A., Ramos M. Phase III evaluating the addition of fulvestrant (F) to anastrozol (A) as adjuvant therapy in postmenopausal women with hormone receptor positive HER2 negative (HR1/HER2-) early breast cancer (EBC): results from the GEICAM/2006-10 study. Ann Oncol Conf. 2017;(Supplement 5):v43. doi: 10.1007/s10549-019-05296-8. [DOI] [PubMed] [Google Scholar]
- 43.Alramadhan M., Ryu J.M., Rayzah M., Nam S.J., Kim S.W. Goserelin plus tamoxifen compared to chemotherapy followed by tamoxifen in premenopausal patients with early stage-, lymph node-negative breast cancer of luminal A subtype. Breast. 2016;30:111. doi: 10.1016/j.breast.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Kwak H.Y., Chae B.J., Eom Y.H., Hong Y.R., Seo J.B. Is adjuvant chemotherapy omissible in women with T1-2 stage, node-positive, luminal a type breast cancer? J Chemother. 2015;27(5):290. doi: 10.1179/1973947815Y.0000000015. [DOI] [PubMed] [Google Scholar]
- 45.Moon Y.W., Park S., Sohn J.H., Kang D.R., Koo J.S. Clinical significance of progesterone receptor and HER2 status in estrogen receptor-positive, operable breast cancer with adjuvant tamoxifen. J Canc Res Clin Oncol. 2011;137(7):1123. doi: 10.1007/s00432-011-0976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohara M., Akimoto E., Noma M., Matsuura K., Doi M. Prognostic impact of progesterone receptor status combined with body mass index in breast cancer patients treated with adjuvant aromatase inhibitor. Oncol Lett. 2015;10(5):3286. doi: 10.3892/ol.2015.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimazu K., Miyake T., Okuno J., Naoi Y., Tanei T. One-step nucleic acid amplification can identify sentinel node-negative breast cancer patients with excellent prognosis. Anticancer Res. 2019;39(3):1447. doi: 10.21873/anticanres.13261. [DOI] [PubMed] [Google Scholar]
- 48.Sohn G., Ahn S.H., Kim H.J., Son B.H., Lee J.W. Survival outcome of combined GnRH agonist and tamoxifen is comparable to that of sequential adriamycin and cyclophosphamide chemotherapy plus tamoxifen in premenopausal patients with lymph-node-negative, hormone-responsive, HER2-negative, T1-T2 breast cancer. Canc Res Treat. 2016;48(4):1351. doi: 10.4143/crt.2015.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohnstad H.O., Borgen E., Falk R.S., Lien T.G., Aaserud M. Prognostic value of PAM50 and risk of recurrence score in patients with early-stage breast cancer with long-term follow-up. Breast Canc Res. 2017;19(1):120. doi: 10.1186/s13058-017-0911-9. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Library of Medicine (U.S.) Fulvestrant (Faslodex) + Anastrozole (Arimidex) vs Anastrozole. Identifier NCT00543127. 2019. https://clinicaltrials.gov/ct2/show/record/NCT00543127 Available online at: 2007, October 11, April 5.
- 51.Macias M.N. Mixed results for profiling in predicting late recurrence in ER+ breast cancer - Tools may, however, help select patients for extended treatment. 2019. https://www.medpagetoday.com/reading-room/asco/breast-cancer/80812 Updated July 2, 2019. Available online at: Last updated: July 2, 2019.
- 52.Richman J., Dowsett M. Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat Rev Clin Oncol. 2019;16(5):296–311. doi: 10.1038/s41571-018-0145-5. [DOI] [PubMed] [Google Scholar]
- 53.Wangchinda P., Ithimakin S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J Surg Oncol. 2016;14(1):223. doi: 10.1186/s12957-016-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sledge G.W., Jr., Toi M., Neven P., Sohn J., Inoue K. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Im S.-A., Lu Y.-S., Bardia A., Harbeck N., Colleoni M. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 56.National Library of Medicine (U.S.) A Study of palbociclib in addition to standard endocrine treatment in hormone receptor positive Her2 normal patients with residual disease after neoadjuvant chemotherapy and surgery (PENELOPE-B). Identifier NCT01864746. 2021. https://clinicaltrials.gov/ct2/show/record/NCT01864746 Available online at: 2013, May 14, February 2.
- 57.Mayer E., DeMichele A., Gnant M., Barry W., Pfeiler G. Abstract OT3-05-08: PALLAS: PALbociclib CoLlaborative Adjuvant Study: a randomized phase 3 trial of palbociclib with standard adjuvant endocrine therapy versus standard adjuvant endocrine therapy alone for HR+/HER2- early breast cancer. Canc Res. 2018;78(4 Supplement) OT3-05-08. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.