Abstract
Introduction
Acute kidney injury (AKI) in coronavirus infection disease (COVID-19) is associated with disease severity. We aimed to evaluate risk factors associated with AKI beyond COVID-19 severity.
Methods
A retrospective observational study of COVID-19 patients admitted to a tertiary hospital in Singapore. Logistic regression was used to evaluate associations between risk factors and AKI (based on Kidney Disease Improving Global Outcomes criteria). Dominance analysis was performed to evaluate the relative importance of individual factors.
Results
Seven hundred seven patients were included. Median age was 46 years (interquartile range [IQR]: 29–57) and 57% were male with few comorbidities (93%, Charlson Comorbidity Index [CCI] <1). AKI occurred in 57 patients (8.1%); 39 were in AKI stage 1 (68%), 9 in stage 2 (16%), and 9 in stage 3 (16%). Older age (adjusted odds ratio [aOR] 1.04; 95% confidence interval [CI]: 1.01–1.07), baseline use of angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB) (aOR 2.86; 95% CI: 1.20–6.83), exposure to vancomycin (aOR 5.84; 95% CI: 2.10–16.19), use of nonsteroidal anti-inflammatory drugs (NSAIDs) (aOR 3.04; 95% CI: 1.15–8.05), and severe COVID-19 with hypoxia (aOR 13.94; 95% CI: 6.07–31.98) were associated with AKI in the multivariable logistic regression model. The 3 highest ranked predictors were severe COVID-19 with hypoxia, vancomycin exposure, and age, accounting for 79.6% of the predicted variance (41.6, 23.1, and 14.9%, respectively) on dominance analysis.
Conclusion
Severe COVID-19 is independently associated with increased risk of AKI beyond premorbid conditions and age. Appropriate avoidance of vancomycin and NSAIDs are potentially modifiable means to prevent AKI in patients with COVID-19.
Keywords: Acute renal failure, Chronic kidney disease, Creatinine
Introduction
Since, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) was reported in late December 2019, our understanding of coronavirus infection disease (COVID-19) has evolved. COVID-19 affects the respiratory system predominantly, leading to acute respiratory distress syndrome (ARDS) [1], but it is increasingly recognized to have systemic involvements such as acute kidney injury (AKI), thrombotic events leading to stroke, acute myocardial infarction, and pulmonary embolism [2, 3]. Systemic involvement further adds to the challenges of treating COVID-19 patients with poor outcomes [2, 3, 4].
AKI is of particular interest as it is associated with poorer outcomes in general population and COVID-19 [2, 5]. The incidence of AKI in COVID-19 patients ranges from 0.5 to 36.6% [2, 6, 7, 8, 9, 10, 11] in different study populations and using different case definitions of AKI. However, few studies have examined risk factors associated with the development of AKI in COVID-19 patients [11]. In a meta-analysis of 79 research articles on AKI and COVID-19, only 8 had investigated primarily the risk factors for AKI in COVID-19 patients, whereas most studies confined to describing the incidence and prognosis [12]. The elucidation of risk factors leading to AKI is important for physicians to better manage their patients, given the diverse presentation and clinical course of COVID-19 patients [6] from asymptomatic to ARDS.
The primary aim of this study was to elucidate risk factors associated with the development of AKI among COVID-19 patients. The secondary aim was to describe the healthcare impact of AKI in COVID-19 patients such as duration of hospitalization, need for intensive care unit (ICU) support, need for renal replacement therapy (RRT), and mortality.
Materials and Methods
This was a retrospective observational study of COVID-19 patients admitted to the National Center of Infectious Disease (NCID) in Singapore between 23rd January and 15th April, 2020. Approval for this study with a waiver of informed consent from participants was obtained from the Singapore Ministry of Health under the Infectious Disease Act (Singapore). Demographic parameters, baseline comorbidities and medications, patient symptomatology, blood and radiological investigations, and outcomes such as hypoxia requiring supplementary oxygen and intubation, need for ICU, length of stay in hospital, and death were collected and entered into a centralized database and extracted for analysis.
The primary outcome of AKI was defined as any increase in serum Cr by ≥26.5 µmol/L within 48 h or a change in serum Cr by ≥ of 1.5 times of baseline within 1 week, based on Kidney Disease Improving Global Outcomes guidelines [13]. The urinary output criteria were not applied as most patients were well and universal strict urinary output charting was impractical during an outbreak situation. Additionally, many patients in this cohort were relatively well and repeated blood test may not always be necessary clinically. All patients with no serum Cr recorded were excluded. For patients with a single serum Cr, they were identified as not having AKI if their Cr was within normal limits of our local laboratory standard (<75 µmol/L for females and <105 µmol/L for males); however, those beyond the normal laboratory limits were excluded as absence of AKI or preexisting CKD could not be excluded. The stage of AKI was determined using the peak serum Cr level after AKI detection, with increases of 1.5–1.9, 2.0–2.9, and ≥3 times baseline or requiring RRT being defined as AKI stage 1, 2, and 3, respectively [13].
Baseline characteristics of COVID-19 patients with and without AKI were described in frequency and percentage for categorical variables. Continuous variables with non-normal distributions were reported as medians (interquartile range [IQR]) and mean (standard deviation [SD]) for continuous variables with normal distribution. Differences in baseline characteristics between patients with and without AKI was analyzed by the χ2 test and Fisher's exact test as appropriate. The independent Student's t test was used to evaluate difference in continuous variables that were normally distributed, while the Mann-Whitney U test was performed for those with non-normal distribution.
Crude odds ratio and adjusted odds ratio (aOR) as well as their 95% confidence interval (CI) were estimated using univariable and multivariable logistic regression analyzes, respectively. Multivariable logistic regression analysis was carried out to identify factors independently associated with AKI. The variables for the multivariable model were selected through stepwise use of Akaike's information criterion. Older age and comorbidities such as hypertension and diabetes mellitus (DM) were previously reported to be associated with AKI [11]. Therefore, age and Charlson Comorbidity Index (CCI) as a measure of comorbidity disease burden was added to the multivariable model as variables selected a priori. Multicollinearity was checked using the variance inflation factor for the predictor variables included in the final multivariable model. We generated the receiver operating characteristic curve to assess the predictive performance of the final multivariable logistic regression model, and calculated the corresponding area under the curve.
Dominance analysis was used to assess the relative importance of each independent variable in the multivariable model, which took into consideration both its unique contribution and its contribution when combined with other predictors [14, 15, 16]. This approach uses changes in model fit statistics (i.e., pseudo-R2 values from logistic regression models) of all possible combinations of predictors, and relative importance is determined by pairwise comparisons of all predictors in the model. The dominance of one predictor over another at the general level is achieved if its average conditional contribution is greater than that of the latter across all model sizes that involve the predictor. To reflect the proportional average additional contribution of each variable, the general dominance value is rescaled by dividing the general dominance value based on the McFadden R2 ascribed to individual variable by the total R2 of the full multivariable model, which is known as standardized dominance statistics.
We then confined the study population to COVID-19 patients with at least 2 serum Cr results and repeated the multivariable logistic regression analysis using the same approach and dominance analysis to assess the impact on our findings as a part of sensitivity analysis. P values <0.05 were considered statistically significant. Statistical analyzes were performed with Stata version 14.1 (Stata Corporation, College Station, TX, USA) and R version 3.6.2 (R foundation for Statistical Computing, Vienna, Austria).
Results
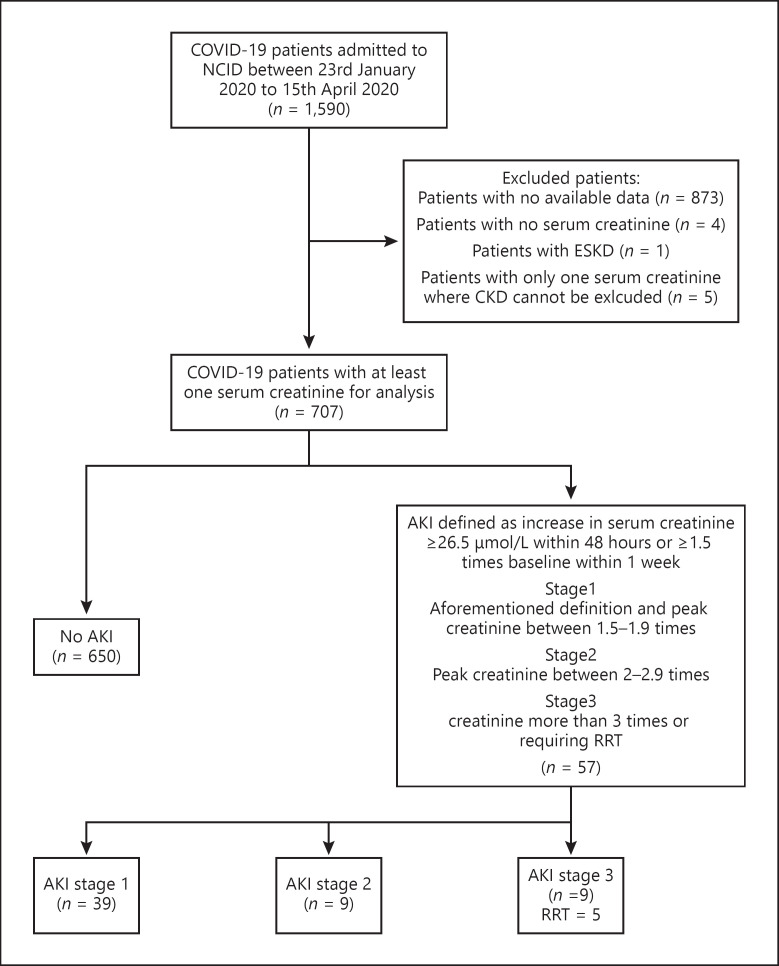
A total of 1590 COVID-19 patients were admitted to NCID between 23rd January and 15th April, 2020. Data on 717 patients was available and of this cohort, 707 were included in this analysis; four excluded due to no recorded serum Cr during hospitalization, one excluded due to preexisting end-stage kidney disease status, and five had only one serum Cr level above the normal laboratory limits, where AKI or underlying CKD status could not be determined (shown in Fig. 1). AKI occurred in 8.1% (n = 57) of all the patients and most were mild. Thirty-nine patients were peaked at AKI stage 1 (68%), 9 in stage 2 (16%), and another 9 patients in stage 3 (16%). Five patients (9%) required RRT.
Fig. 1.
Flow chart of study population of COVID-19 patients admitted to the National Center for Infectious Diseases, Singapore. Eight hundred seventy-three patients excluded as there were no available data collected. An additional four excluded as there was no record of serum Cr, one had preexisting end-stage kidney disease and was on regular hemodialysis, and five had single serum Cr level, which was above the upper limit of laboratory range in the hospital where CKD cannot be excluded.
The median age of the cohort was 46 years (IQR: 29–57) and 57% were male (n = 405). Most patients had few comorbidities (shown in Table 1); only 12% had DM, 19% hypertension, 5% with cardiovascular disease, and 1% with the history of CKD. The majority of patients in this cohort have low comorbid burden and 94% of the patients had CCI ≤ 1.
Table 1.
Baseline characteristics and outcomes of study cohort of COVID-19 patients by AKI status
| Variable | Overall (n = 707) | No AKI (n = 650) | AKI (n = 57) | p valuea |
|---|---|---|---|---|
| Duration from symptom onset to admission, days (IQR) | 5 (3–8) | 5 (3–8) | 6 (3–8) | 0.2911 |
| Symptoms, n (%) | ||||
| Fever | 488 (69) | 437 (67) | 51 (89) | <0.005 |
| Cough | 465 (66) | 428 (66) | 37 (65) | 0.887 |
| Dyspnea | 80 (11) | 63 (10) | 17 (30) | <0.005 |
| Sore throat | 295 (42) | 282 (43) | 13 (23) | 0.003 |
| Rhinorrhea | 223 (32) | 216 (33) | 7 (12) | 0.001 |
| Diarrhea | 130 (18) | 117 (18) | 13 (23) | 0.369 |
| Baseline demographics and comorbidities | ||||
| Age, years (IQR) | 46 (29–57) | 43 (29–56) | 62 (57–71) | <0.005 |
| Female sex, n (%) | 302 (43) | 284 (44) | 18 (32) | 0.076 |
| Ethnicity, n (%) | ||||
| Chinese | 398 (56) | 360 (55) | 38 (67) | |
| Malay | 79 (11) | 71 (11) | 8 (14) | 0.153 |
| Indian | 82 (12) | 77 (12) | 5 (9) | |
| Others | 148 (21) | 142 (22) | 6 (11) | |
| DM, n (%) | 82 (12) | 60 (9) | 22 (39) | <0.005 |
| Hypertension, n (%) | 137 (19) | 103 (16) | 34 (60) | <0.005 |
| Dyslipidemia, n (%) | 155 (22) | 122 (19) | 33 (58) | <0.005 |
| Cardiovascular disease,bn (%) | 35 (5) | 29 (4) | 7 (12) | 0.010 |
| COPD or asthma, n (%) | 24 (3) | 20 (3) | 4 (7) | 0.120 |
| Malignancy, n (%) | 15 (2) | 11 (2) | 4 (7) | 0.027 |
| Liver disease, n (%) | 5 (1) | 4 (1) | 1 (2) | 0.344 |
| Renal disease, n (%) | 5 (1) | 0(0) | 5 (9) | <0.005 |
| CCI score, n (%) | ||||
| 0 | 553 (78) | 530 (82) | 23 (40) | |
| 1 | 116 (16) | 92 (14) | 24 (42) | |
| 2 | 15 (2) | 11 (2) | 4 (7) | |
| 3 | 17 (2) | 16 (2) | 1 (2) | <0.005 |
| 4 | 3 (0) | 1 (0) | 2 (4) | |
| 6 | 1 (0) | 0 (0) | 1 (2) | |
| 7 | 1 (0) | 0 (0) | 1 (2) | |
| 8 | 1 (0) | 0 (0) | 1 (2) | |
| Medications | ||||
| Baseline ACE-I/ARB use prior to hospitalization, n (%) | 87 (12) | 59 (9) | 28 (49) | <0.005 |
| Baseline statin use, n (%) | 151(21) | 120 (18) | 31 (54) | <0.005 |
| In-hospital NSAIDs use, n (%) | 77 (11) | 61 (9) | 16 (28) | <0.005 |
| In hospital antibiotics use, n (%) | 154 (22) | 108 (17) | 46 (81) | <0.005 |
| Any antibiotics | 10 (1) | 4 (1) | 6 (11) | <0.005 |
| Aminoglycoside vancomycin | 34 (5) | 9 (1) | 25 (44) | <0.005 |
| Piperacillin-tazobactam | 37 (5) | 13 (2) | 24 (42) | <0.005 |
| Laboratory investigations at admission | ||||
| eGFR ±SD | 116±38 | 119±36 | 75±41 | <0.005 |
| Serum Cr, µmol/L ±SD | 72±23 | 69±16 | 101±53 | <0.005 |
| Serum urea, mmol/L ±SD, n = 600 | 3.8±1.8 | 3.6±1.2 | 6.4±4.3 | <0.005 |
| Hemoglobin, g/dL ±SD | 14.1±1.5 | 14.1±1.5 | 13.6±2.0 | 0.0075 |
| Platelets, 109/L ±SD | 214±72 | 214±70 | 206±90 | 0.4063 |
| Leukocyte count, 109/L ±SD | 5.3±2.1 | 5.2±1.9 | 6.3±3.2 | 0.0001 |
| Lymphocyte count, 109/L (IQR) | 1.31 (0.96–1.72) | 1.34 (0.99–1.78) | 0.95 (0.61–1.23) | <0.005 |
| Neutrophil count, 109/L ±SD | 3.2±1.8 | 3.0±1.6 | 4.7±3.2 | <0.005 |
| CRP, mg/L (IQR) | 5.4 (1.6–15.9) | 4.3 (1.4–12.9) | 50.5(19–109.6) | <0.005 |
| LDH, U/L (IQR) | 402 (341–500) | 394 (337–479) | 576 (425–703) | <0.005 |
| Radiological investigations | ||||
| Ever abnormal chest radiograph, n (%) | 290 (60) | 234 (36) | 55 (96) | <0.005 |
| Contrasted CT-scan exposure, n (%) | 21 (3) | 12 (2) | 9 (16) | <0.005 |
| Outcomes | ||||
| Length of stay, days (IQR) | 7 (4–13) | 7 (4–12) | 17 (12–26) | <0.005 |
| Hypoxia requiring oxygen supplementation, n (%) | 90 (13) | 45 (7) | 45(79) | <0.005 |
| ICU, n (%) | 46(7) | 9(1) | 37 (65) | <0.005 |
| RRT, n (%) | 5 (1) | 0 (0) | 5 (9) | <0.005 |
| Intubation, n (%) | 25 (4) | 3 (0) | 22 (38) | <0.005 |
| In-hospital mortality, n (%) | 12 (2) | 5 (1) | 7 (12) | <0.005 |
Sample size, n = 707, except where indicated. ACE-I, angiotensin-converting enzymes inhibitors; ARB, angiotensin receptor blocker AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, Computed tomography; eGFR, estimated glomerular filtration rate using CKD-EPI, equation; LDH, lactate dehydrogenase; ICU, intensive care unit; IQR, interquartile range; NSAIDs, nonsteroidal anti-inflammatory drugs; RRT, renal replacement therapy; SD, standard deviation; DM, diabetes mellitus; CCI, Charles Comorbidity Index.
p value for comparison between patients with AKI, and patients without AKI, χ2 test or Fisher's exact test was performed for categorical variables as appropriate. Independent student t test was performed for continuous variables described in mean ± SD, and Mann-Whitney U test was performed for continuous variables described in median (IQR).
Cardiovascular diseases refer to ischemic heart disease, congestive cardiac history or cerebrovascular disease.
Patients who developed AKI were older (62 vs. 43 years old, p < 0.005) and a higher proportion had DM (39 vs. 9%, p < 0.005), hypertension (60 vs. 16%, p < 0.005), dyslipidemia (58 vs. 19%, p < 0.005) cardiovascular disease (12 vs. 4%, p < 0.005), and CKD (9 vs. 0%, p < 0.005). Patients with AKI were also more likely to be on angiotensin-converting enzyme inhibitor (ACE-I), angiotensin receptor blocker (ARB), and statins prior to hospitalization. They were more likely to have received nonsteroidal anti-inflammatory drugs (NSAIDs) and potentially nephrotoxic antibiotics such as aminoglycosides and vancomycin during hospitalization. Majority (90%) of the NSAIDs used in this cohort were selective cyclooxygenase-2 inhibitor (COX-2). Most of the use of vancomycin in this cohort was empirical and only 5 of the 34 patients exposed had clinically relevant bacteremia.
COVID-19 patients with AKI were more likely to have more severe disease. They were more likely to present with lower lymphocyte counts, higher C-reactive protein, lactate dehydrogenase levels, and more likely to have abnormal chest radiography during their hospitalization. Consequently, COVID-19 patients with AKI were more likely to have prolonged hospitalization with median of 17 days compared with 7 days (shown in Table 1). A higher proportion of patients who developed AKI had hypoxia (defined as requiring supplementary oxygen) (79 vs. 7%, p < 0.005), required ICU (65 vs. 1%, p < 0.005), and were intubated (38 vs. 0%, p < 0.005). A total of 12% of patients with AKI in this cohort died compared with 1% in those without AKI. Among the 5 patients who required RRT, four passed away and only one had fully recovered with no residual CKD.
Among the potential predictors, univariable logistic regression suggested that older age, CCI, baseline use of ACE-I or ARBs, baseline use of statins, exposure to each of the 3 antibiotics, aminoglycoside, piperacillin/tazobactam and vancomycin, use of NSAIDs in hospital, exposure to contrasted scan, and hypoxia were associated with the risk of developing AKI (shown in Table 2). In the final multivariable logistic regression model, the following predictors remained statistically significant: older age (aOR 1.04; 05% CI: 1.01–1.07), baseline use of ACE-I or ARB (aOR 2.86; 95% CI: 1.20–6.83), exposure to vancomycin (aOR 5.84; 95% CI: 2.10–16.19), use of NSAIDs in hospital (aOR 3.04; 95% CI: 1.15–8.05), and hypoxia (aOR 13.94; 95% CI: 6.07–31.98) (shown in Table 2). The variance inflation factor, for factors included in the multivariable logistic regression ranged from 1.06 (use of NSAIDs in hospital) to 1.36 (CCI). The area under the curve of the multivariable model containing the five statistically significant predictors and CCI was 0.949 (95% CI 0.922–0.975). When dominance analysis was performed, the 3 highest ranked predictors among the 6 variables in the multivariable model were hypoxia, vancomycin exposure, and age, accounting for 79.6% of the predicted variance (41.6, 23.1, and 14.9%, respectively) (shown in Table 3).
Table 2.
Crude and aORs for developing AKI among COVID-19 patients (whole cohort)
| Variable | Univariable model | Multivariable model† | ||||
| cOR | 95% CI | p value | aOR | 95% CI | p value | |
| Age | 1.08 | 1.06–1.10 | <0.0005 | 1.04 | 1.01–1.07 | 0.011 |
| Female sex | 0.59 | 0.33–1.06 | 0.079 | |||
| Ethnic group | 0.172 | |||||
| Chinese | 1.00 | Referent | ||||
| Malay | 1.07 | 0.48–2.38 | 0.874 | |||
| Indian | 0.62 | 0.23–1.61 | 0.323 | |||
| Others | 0.40 | 0.17–0.97 | 0.042 | |||
| CCI | 2.16 | 1.66–2.80 | <0.0005 | 1.22 | 0.83–1.79 | 0.304 |
| Baseline use of ACE-I or ARB | 9.67 | 5.39–17.35 | <0.0005 | 2.86 | 1.20–6.83 | 0.018 |
| Baseline use of statins | 5.27 | 3.02–9.20 | <0.0005 | |||
| Aminoglycoside exposure | 19.00 | 5.19–69.50 | <0.0005 | |||
| Piperacillin/Tazobactam exposure | 35.64 | 16.66–76.22 | <0.0005 | |||
| Vancomycin exposure | 55.64 | 24.01–128.96 | <0.0005 | 5.84 | 2.10–16.19 | 0.001 |
| In-hospital NSAIDs use | 3.77 | 2.00–7.11 | <0.0005 | 3.04 | 1.15–8.05 | 0.025 |
| Exposure to contrasted scan | 9.97 | 4.00–24.83 | <0.0005 | |||
| Hypoxia | 50.42 | 24.91–102.05 | <0.0005 | 13.94 | 6.07–31.98 | <0.0005 |
aOR, adjusted odds ratio; cOR, crude odds ratio; CI, confidence interval; CCI, Charlson Comorbidity Index; ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; NSAIDs, nonsteroidal anti-inflammatory drugs; AKI, acute kidney injury.
Adjusted for age, CCI, baseline use of ACE, inhibitors or ARBs, vancomycin exposure, use of NSAIDs, in hospital and hypoxia.
Table 3.
Relative importance of predictors for developing AKI among COVID-19 patients (whole cohort)
| Variable | McFadden's R2 | Standardized dominance statistics§ | Rank |
| Age | 0.076 | 0.149 | 3 |
| CCI | 0.031 | 0.061 | 5 |
| Baseline use of ACE-I or ARB | 0.051 | 0.100 | 4 |
| Vancomycin exposure | 0.118 | 0.231 | 2 |
| In-hospital NSAIDs use | 0.022 | 0.043 | 6 |
| Hypoxia | 0.212 | 0.416 | 1 |
CCI, Charlson Comorbidity Index; ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; NSAIDs, nonsteroidal anti-inflammatory drugs; AKI, acute kidney injury.
Standardized weight was the share of general dominance value from McFadden R2, which adds to 1 across the variables.
The baseline characteristics of the subgroup of 357 patients with at least 2 serum Cr results are shown in online suppl. Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/0005514064). The median age of this subgroup was 54 (IQR 38–62) and they had similarly low comorbidity disease burden with 93% of all patients in this subgroup having CCI ≤ 1. On multivariable logistic regression analysis, as a part of sensitivity analysis, older age (aOR 1.03 [95% CI 1.00–1.06]), baseline use of ACE-I or ARB (aOR 2.61 [95% CI 1.09–6.23]), use of vancomycin (aOR 5.37 [95% CI 1.94–14.85]), and hypoxia (aOR 8.67 [3.78–19.92]) remained as independent factors associated with AKI in this subgroup of patients (online suppl. Table 2). However, use of NSAIDs was no longer statistically associated with AKI. In dominance analysis of this subgroup, the order of relative importance of each factor remained unchanged with hypoxia being most important followed by vancomycin and age accounting for 40.5, 26.0, and 13.1% of predicted variance, respectively (online suppl. Table 3).
Discussion and Conclusion
Acute kidney injury occurred in 8.1% of the whole cohort. This incidence is within the range reported in previous studies from 0.5 to 36.6% [2, 6, 7, 8, 9, 10, 11]. Besides the differences in definitions for AKI used in different studies, the low incidence in this study was likely due to a younger population with fewer comorbidities. The median age in this cohort was 46 years compared with 64 years in the largest study examining AKI to date in New York city [11]. In the US study, the incidence of AKI was 36.6% [11], more than four times higher than our cohort. It is important to understand the cohort examined in our study was from the early phases of the outbreak in Singapore where all COVID-19 patients were admitted. Therefore, many patients had mild illness and were relatively young and fit, which is different from studies, where more severe cases were recruited [11, 12]. However, AKI was not uncommon even in our cohort and among patients who developed AKI in this study, significant healthcare burden was observed with increased need for ICU, prolonged hospitalization, and higher risk of death. Therefore, identifying predisposing factors to development of AKI in COVID-19 patients was of paramount importance. In our study, hypoxia (surrogate for disease severity) was by far the most important factor predicting AKI. Baseline comorbidities and age are unmodifiable and effective treatment of COVID-19 to alter disease severity is still currently limited. However, the association between vancomycin, NSAIDs, and ACE-I/ARB exposure with AKI in this study present potentially modifiable factors, which deserves further evaluation.
The pathophysiology of AKI in COVID-19 patients is not completely understood but is likely multifactorial [17]. The development of AKI was more commonly seen in patients with severe COVID-19 patients in our cohort, which corroborated previous reports where severe AKI occurred in 65.5% of ventilated patients compared with 6.7% of non-ventilated patients [11]. Critically ill patients with COVID-19 may be at increased risk of AKI due to hypoperfusion of kidneys as a result of circulatory shock secondary to hypovolemia, sepsis or cardiogenic shock from underlying cardiovascular disease, or new cardiac event. Pro-inflammatory and proapoptotic consequence of the lung inflammation secondary to severe SARS-COV-2 infection may directly lead to AKI [17]. Direct SARS-CoV-2 immune response dysregulation leading to cytokine related renal tubular cell injury was postulated [10]. Patients with immune response dysregulation may present with lymphopenia, which was commonly observed in our cohort with AKI. Last, microemboli and thrombi as a result of a hypercoagulable state in COVID-19 patients have been proposed as a possible cause of AKI [18]. However, the relationship between severe COVID-19 and AKI is likely bidirectional. The lung-kidney organ cross talk in the critically ill is well established in the non-COVID-19 literature [19]. Patients with AKI frequently present with derangements in the fluid balance and acid-base homeostasis, which compound the severity of ARDS in COVID-19 patients. Therefore, the strong association observed between COVID-19 and AKI in this study is likely the result of this intimate interplay of cause and effect between AKI and lung injury arising from COVID-19.
Older COVID-19 patients are particularly vulnerable to severe COVID-19 disease and death [7]. Similar to the study from New York [11], older patients in this cohort were at increased odds of developing AKI. Microanatomical changes in the kidneys associated with aging has been previously described in both autopsy and living donor biopsy samples [20]. Features of nephrosclerosis observed include glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis of small vessels, which leads to ischemia and formation of globally sclerotic glomeruli [20]. Therefore, elderly patients may have fewer numbers of functioning nephrons but compensatory hypertrophy of remaining functioning ones often masks manifestations of CKD. These changes reduce the functional reserves in the elderly, predisposing them to the development of AKI during COVID-19 as observed in this study.
Use of ACE-I and ARB has been a subject of much debate in COVID-19. Concerns of increased risk of COVID-19 ARDS with ACE-I and ARB have been raised in 2 studies [21, 22]. It was further argued that high affinity of SARS-CoV2 to human angiotensin-converting enzyme 2 receptors [23] may play a role in increasing susceptibility of patients with upregulation due to long term use of ACE-I or ARB. On the other hand, ACE-I and ARB are also indicated for use in comorbid conditions such as hypertension, DM, and cardiovascular disease, which are also factors associated with poorer outcome [1]. Therefore, the association between ACE-I/ARB and development of severe COVID-19 were no longer observed when potential confounders were included [24]. ACE-I and ARB are not nephrotoxic agents per se. The main effect of these agents on the kidneys is intrarenal efferent arteriole vasodilatation, which reduces glomerular filtration pressure [25]. In situations where intact angiotensin-aldosterone axis is important in maintaining renal glomerular filtration such as hypovolemia, the presence of ACE inhibition, or blockade may predispose patients to AKI as seen in our study. However, some patients treated with ACE-I and ARB may have concomitant proteinuric renal disease secondary to hypertension or DM that are in itself a risk factor for AKI. Unfortunately, there was no available urinalysis data in this study on proteinuria to allow further evaluation of this hypothesis.
While much interest has been focused on evaluating how novel SARS-CoV2 may directly infect and affect renal tissues, this study has also highlighted the importance of considering other causes of nephrotoxicity such as NSAIDs and vancomycin. Nephrotoxicity related to vancomycin was historically related to impurities included during the preparation of the antibiotic [26]. Improved fermentation methods have improved the purity of vancomycin and toxicity has reduced significantly. However, vancomycin induced renal toxicity is still reported in 10–20% of patients [27]. The pathophysiology is unclear but oxidative stress in proximal renal tubular cells was shown in animals receiving vancomycin [28]. Other than direct vancomycin toxicity, it is important to consider if COVID-19 patients receiving vancomycin are concurrently bacteremic, which could increase the risk of AKI. In this cohort, only a minority of patients on vancomycin were bacteremic; this highlights the challenge of managing COVID-19 patients with progressive pneumonia, where secondary bacterial infection cannot be excluded. Where appropriate use of antibiotics cannot be avoided, care should be taken to reduce the risk of vancomycin induced nephrotoxicity such as shortening duration of exposure, maintaining a conservative therapeutic level, and avoidance of concomitant nephrotoxic agents [28].
There are many potential renal effects that NSAIDs have; but in the setting of acute illness, the hemodynamic effects as a result of inhibition of prostaglandin leading to reduced prostaglandin-mediated afferent arteriole vasodilatation is pertinent [29]. In a meta-analysis of five studies and a total of 28,992 patients, AKI risk in users of individual traditional NSAIDs was 1.58–2.11 times higher than non-users [30]. Selective COX-2 inhibitors were more commonly used in this study and are generally safer than traditional NSAIDs [31], but COX-2 is inducible in the kidney in response to inflammation and has been demonstrated to play a role in regulation of renal hemodynamics especially with salt and volume depletion [32]. It is prudent to consider the safety of either selective or nonselective cyclooxygenase inhibition in COVID-19 patients given the increased odds of AKI seen in this study.
The strength of this study is the comprehensive evaluation of clinically relevant factors and important outcome using internationally determined definition of AKI. Although the association of severity of disease and incidence of AKI has been reported previously [12], the use of dominance analysis in this study allowed us to frame the relative importance of this factor with greater clarity. The studies from Asia investigating risk factors of AKI have largely been from China and this study provides some insight from the perspective of a different Asian country.
We acknowledge several limitations in this study. As 350 (49.5%) patients did not have repeated serum creatinine test during their hospitalization, sensitivity analysis was undertaken to investigate the impact of patients included with single record of creatinine in our study, and the findings remained materially unchanged. Due to the observational design of our study, we could only determine associations rather than cause-effect relationships. In addition, ascertainment bias may arise if patients with mild AKI were discharged early. We also could not rule out the potential for residual confounding by unmeasured factors related to AKI. The study population was confined to a single center and the patients were relatively young with no or few comorbidities; therefore, the findings may not be generalizable to all individuals with COVID-19. On the other hand, NCID sees the largest number of COVID-19 patients in Singapore and therefore provides a reasonable indication of AKI incidence among those hospitalized in Singapore. Last, there were specific data which were not available such as the urine output, pre-hospitalization serum creatinine, and urinalysis during hospitalization. In conclusion, this study demonstrated that severe COVID-19 is associated with increased risk of AKI. Until the discovery of better therapeutic agents for COVID-19, the appropriate avoidance of vancomycin and NSAIDs are important aspects in prevention of AKI in patients with COVID-19.
Statement of Ethics
Consent to participate: approval for this study with a waiver of informed consent from participants was obtained from the Singapore Ministry of Health under the Infectious Disease Act (Singapore).
Conflict of Interest Statement
Yong Pey See, Xi Yan Ooi, Wan Limm Looi, Chi Peng Chan, Li Wei Ang, See Cheng Yeo, and David Chien Lye have nothing to disclose. Barnaby Edwards Young reports personal fees from Sanofi and personal fees from Roche outside of submitted work.
Funding Sources
The authors did not receive support from any organization for the submitted work.
Author Contributions
Yong Pey See, Barnaby Edward Young, See Cheng Yeo, Xi Yan Ooi, and David Chien Lye conceptualized and designed the study. Yong Pey See, Barnaby Edward Young, Xi Yan Ooi, Wan Limm Looi, and Chi Peng Chan performed data collection and entry. Yong Pey See and Li Wei Ang analyzed the data. Yong Pey See and Xi Yan Ooi prepared the tables and figures. Yong Pey See, Xi Yan Ooi, Li Wei Ang, See Cheng Yeo, and David Chien Lye drafted and revised the paper. All authors approved the final version of the manuscript.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323((13)):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97((5)):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–50. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020 Aug 18;95((7)):e910–20. doi: 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 5.Silver SA, Harel Z, McArthur E, Nash DM, Acedillo R, Kitchlu A, et al. Causes of death after a hospitalization with AKI. J Am Soc Nephrol. 2018;29((3)):1001–10. doi: 10.1681/ASN.2017080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323((11)):1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395((10223)):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98((1)):209–18. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lirong L, Xiang W, Jiangwen R. Risk factors and prognosis of COVID-19 induced acute kidney injury: a meta-analysis. BMJ Open. 2020;10:e042573. doi: 10.1136/bmjopen-2020-042573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khwaja A. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:8–12. [Google Scholar]
- 14.Budescu DV. Dominance analysis: a new approach to the problem of relative importance of predictors in multiple regression. Psychol Bull. 1993;114((3)):542–51. [Google Scholar]
- 15.Azen R, Budescu DV. The dominance analysis approach for comparing predictors in multiple regression. Psychol Methods. 2003;8((2)):129–48. doi: 10.1037/1082-989x.8.2.129. [DOI] [PubMed] [Google Scholar]
- 16.Budescu DV, Azen R. Beyond global measures of relative importance: some insights from dominance analysis. Organ Res Methods. 2004;7((3)):341–50. [Google Scholar]
- 17.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8((7)):738–42. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020 Apr 23;382((17)):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically Ill patient. Am J Respir Crit Care Med. 2016 Aug 15;194((4)):402–14. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 20.Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23((1)):19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madeddu P. Rapid response: ACE-inhibitors may facilitate COVID-19 related respiratory distress syndrome besides increasing the risk of infections. BMJ. 2020;368:m810. [Google Scholar]
- 22.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8((4)):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581((7807)):221–4. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. 2020;15((8)):e0238281. doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navis G, Faber HJ, de Zeeuw D, de Jong PE. ACE inhibitors and the kidney. A risk-benefit assessment. Drug Saf. 1996;15((3)):200–11. doi: 10.2165/00002018-199615030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Bailie GR, Neal D. Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol Adverse Drug Exp. 1988 Sep-Oct;3((5)):376–86. doi: 10.1007/BF03259891. [DOI] [PubMed] [Google Scholar]
- 27.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68((9)):1243–55. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 28.Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, et al. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology. 2005 Nov 15;215((3)):227–33. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Oates JA, FitzGerald GA, Branch RA, Jackson EK, Knapp HR, Roberts LJ., 2nd Clinical implications of prostaglandin and thromboxane A2 formation (2) N Engl J Med. 1988 Sep 22;319((12)):761–7. doi: 10.1056/NEJM198809223191206. [DOI] [PubMed] [Google Scholar]
- 30.Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26((4)):285–91. doi: 10.1016/j.ejim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trials. JAMA. 2006 Oct 4;296((13)):1619–32. doi: 10.1001/jama.296.13.jrv60015. [DOI] [PubMed] [Google Scholar]
- 32.Harris RC, Breyer MD. Update on cyclooxygenase-2 inhibitors. Clin J Am Soc Nephrol. 2006;1((2)):236–45. doi: 10.2215/CJN.00890805. [DOI] [PubMed] [Google Scholar]