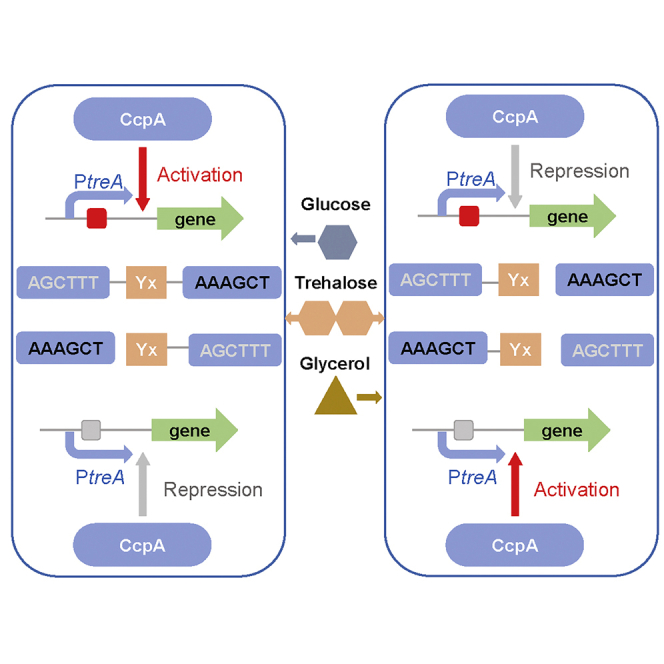
Summary
Bacillus licheniformis is widely used to produce various valuable products, such as food enzymes, industrial chemicals, and biocides. The carbon catabolite regulation process in the utilization of raw materials is crucial to maximizing the efficiency of this microbial cell factory. The current understanding of the molecular mechanism of this regulation is based on limited motif patterns in protein-DNA recognition, where the typical catabolite-responsive element (CRE) motif is “TGWNANCGNTNWCA”. Here, CRETre is identified and characterized as a new CRE. It consists of two palindrome arms of 6 nucleotides (AGCTTT/AAAGCT) and an intermediate spacer. CRETre is involved in bidirectional regulation in a glucose stress environment. When AGCTTT appears in the 5′ end, the regulatory element exhibits a carbon catabolite activation effect, while AAAGCT in the 5′ end corresponds to carbon catabolite repression. Further investigation indicated a wide occurrence of CRETre in the genome of B. licheniformis.
Subject areas: Microbiology, Microbial Metabolism, Structural Biology
Graphical abstract
Highlights
-
•
A novel CcpA binding site CRETre was identified and characterized
-
•
CRETre consists of two palindrome arms of 6 nucleotides (AGCTTT/AAAGCT)
-
•
CRETre is involved in a bidirectional regulation under the glucose stress
Microbiology; Microbial Metabolism; Structural Biology
Introduction
To cope with various environments, bacteria have developed a sophisticated carbon source utilization mechanism, which is mainly characterized by hierarchical uptake and metabolism of mixed carbon sources (Jörg and Hillen, 1999; Sonenshein, 2007). Usually, this mechanism avoids a simultaneous utilization of all available carbohydrates in order to save cell energy. Two regulatory phenotypes, carbon catabolite activation (CCA) and carbon catabolite repression (CCR), are widely observed in this bacterial process (Görke and Stülke, 2008). Previous studies found that carbon catabolite protein A (CcpA) played a vital role in the regulation of both catabolism and anabolism (Yoshida et al., 2001; Xiao and Xu, 2007). To carry out its regulation, CcpA or the complex CcpA-Hpr-Ser46-P (a combination of CcpA and the Ser46 phosphorylated form of a histidine-containing phosphocarrier protein [HPr]) needs to bind a cis-acting element—catabolite-responsive element (CRE)—either in the region upstream of the transcription start site or in the coding region (Schumacher et al., 2011). To date, a classic CRE motif “TGWNANCGNTNWCA” (where W stands for A or T and N for any base) (Weickert and Chambliss, 1990) has been identified and characterized. Researchers generally agreed that CRE sites were highly degenerated pseudo-palindromes and that the lack of stringent sequence conservation provides CcpA with a high degree of regulatory flexibility. Examples include “RRGAAAANGTTTTCWW” in Clostridium difficile (Antunes et al., 2012), TGTTATATAACA in Clostridium acetobutylicum (Willenborg et al., 2014), four CcpA binding sites (GAAGTTTTAAG; ATTTTTTGT; TATGAAAAATTTTAAAAAGTGGA; AGGCTTATCATAG) in Streptococcus mutans (Kim and Burne, 2017), and “TTTTYHWDHHWWTTTY” (Y represents base C or T, H represents base A, C, or T, W represents base A or T, and D represents base A, G, or T) in Streptococcus suis (Zhang et al., 2018). However, more differentiated CRE patterns are needed to explain how CcpA can bind such a diverse set of operator sites.
Bacillus licheniformis has been used widely as a microbial cell factory for enzymes and chemical production (Shi et al., 2019). It has exhibited obvious selective utilization of mixed carbon sources in industrial fermentation (Li et al., 2019). Various oligosaccharides, in particular, significantly affect cell growth and metabolism (Patricia et al., 2014). Much has been explained about the central role of CcpA in the coordinated regulation of catabolism and anabolism using the classic CRE motif to ensure optimal cell growth under varying environmental conditions (Fujita, 2014). However, a large number of CcpA target genes, revealed by microarray or RNA sequencing, do not contain the classical CRE motif (Ruud et al., 2015; Lin et al., 2013b). In this context, the specific molecular mechanism of CcpA target gene recognition remains elusive.
Another problem that must be considered is that CcpA is involved in both CCR and CCA, even under the same stress from glucose or other oligosaccharides. For example, some Bacillus exhibited a preference for malic acid over glucose (Bruckner and Titgemeyer, 2002; Asai et al., 2000). Transcriptome information showed that genes of the malic acid catabolism pathway were activated in the presence of this organic acid, while those within the glucose catabolism pathway were repressed (Kleijn et al., 2010). Glucose can also suppress the catabolism of other phosphotransferase system (PTS) carbohydrates, such as xylose and mannitol (Li et al., 2018; Xiao et al., 2020). The pleiotropic role of CcpA is related in part to its protein structure (Loll et al., 2007), while the target DNA site is also an important basis of its function. The current research on CcpA focuses on its CCR effect, the molecular mechanism of which has been explained using the classical CRE motif. However, little is known about the CCA mechanism and the related target DNA sites.
Trehalose is a disaccharide consisting of two glucose moieties. It also acts as a stress protectant in various bacteria (Okabe et al., 2020). The trehalose operon is reported to regulate the transportation of trehalose and the interconversion between this disaccharide and glucose (Eastmond and Graham, 2003); thus, in the past decade, it has attracted increasing research interest on the adaptability of microorganisms. Previous results proved that the trehalose operon could interact with CcpA in response to glucose or trehalose, where a classical CRE motif was identified in the trehalose promoter (Gosseringer et al., 1997). In this study, the structure of the trehalose operon of B. licheniformis was elucidated. A new CcpA binding site in the treR gene, CRETre, was identified and characterized as a new CRE. The new motif presents completely different structural characteristics than those of the classical motif. In this study, the relationship between its structure and the CcpA-mediated CCR/CCA effect was investigated. The results provide a novel model for the regulation of the CcpA protein in B. licheniformis.
Results
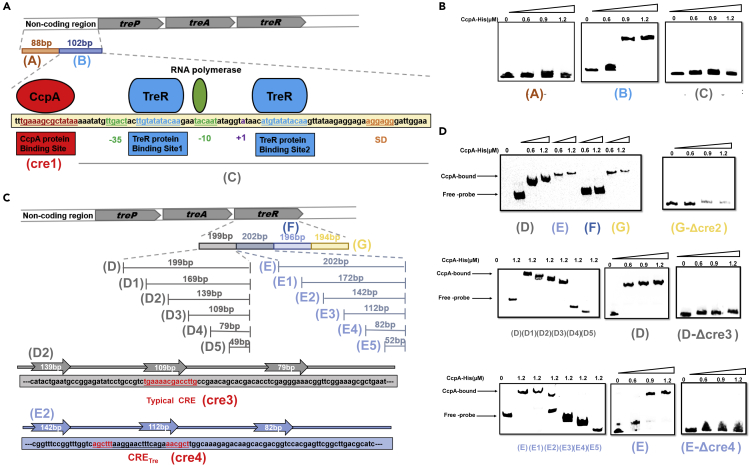
Discovery of a new CcpA protein binding site in the B. licheniformis trehalose operon
The trehalose operon consists of a promoter and three structural genes, treP (encoding EIIBCTre), treA (encoding trehalose 6-phosphate hydrolase), and treR (encoding the regulatory protein) (Schöck and Dahl, 1996). Two transcription elements (the PtreA promoter and the repressor protein TreR) were identified in the trehalose operon. PtreA is responsible for the transcription of the structural genes, treA and treP, and the TreR protein is capable of turning the promoter “on” or “off”. Several potential CRE sites were located by searching using a typical CRE motif in PtreA and treR. A sequence that is highly similar to the typical CRE motif (with only base pair 13 inconsistent), “TGAAAGCGCTATAA” (cre1) was found downstream of the −35 region in PtreA. Next, to identify other possible CcpA binding sites, 200-bp PtreA was divided into two fragments, an 88-bp fragment in the 5′ end (A) and a 102-bp fragment in the 3′ end (B) (Figure 1A). Additionally, the cre1 was deleted from fragment B, resulting in a fragment C. The above three fragments were subjected to electrophoretic mobility shift assay (EMSA) to investigate their affinity with CcpA. The results are shown in Figure 1B. CcpA did not recognize or bind to fragments A and C. However, it clearly showed that the shift of fragment B was blocked. This result indicated that there is only one CcpA binding site in PtreA. Notably, Hpr (Ser-P) binds to CcpA, thereby inducing the binding of the complex to the cre site (Warner and Lolkema, 2003). Hence, the function of Hpr (Ser-P) was tested for CcpA binding in vitro. As shown in Figure S1, there were the same results for CcpA binding in the lane regardless of whether Hpr (Ser-P) was added. Hence, in the next experiment, the Hpr (Ser-P) was not added in the lane for the EMSA experiment.
Figure 1.
Identification of CcpA binding sites in trehalose operon
(A) The non-coding region of the trehalose operon was divided into two fragments ([fragment A] and [fragment B]). The CcpA binding region is shown in red, and the TreR binding region is shown in blue. The −10 region and −35 region are shown in green. The transcription start site is shown in purple. The SD sequence is shown in orange.
(B) EMSA of CcpA protein binding to three fragments (fragment A, fragment B, and fragment C) labeled with 5′ biotin.
(C) The region of treR gene was divided into four fragments (fragment D, fragment E, fragment F, and fragment G). The fragment D of the treR gene was further divided into six fragments (fragment D, fragment D1, fragment D2, fragment D3, fragment D4, and fragment D5). The fragment E of the treR gene was further divided into six fragments (fragment E, fragment E1, fragment E2, fragment E3, fragment E4, and fragment E5). The CcpA binding site in fragment D or fragment E is shown in red.
(D) EMSA of CcpA protein binding to four fragments (fragment D, fragment E, fragment F, and fragment G) labeled with 5′ biotin, six fragments labeled with 5′ biotin (fragment D, fragment D1, fragment D2, fragment D3, fragment D4, and fragment D5) that were derived from fragment D, and six fragments labeled with 5′ biotin (fragment E, fragment E1, fragment E2, fragment E3, fragment E4, and fragment E5) that were derived from fragment E.
Then, the sequence of treR was also searched for the typical CRE site. A sequence “AGACACCGCTTGGA” (cre2) was found between 664 and 677 bp from the initial codon. It had two bases (1 and 13) that did not fit with the typical CRE motif. Next, to identify other possible CcpA binding sites, treR was divided into four fragments, a 199-bp fragment in the 5′ end (D), a 202-bp fragment in the 5′ end (E), a 196-bp fragment in the 3′ end (F), and a 194-bp fragment (G) in the 3′ end (Figure 1C). The results are shown in Figure 1D. A DNA band shift was observed for fragments D, E, and G, suggesting that there exist CcpA binding sites in these fragments. Hence, it is necessary to verify the specific CcpA binding site in these fragments. The cre2 was first deleted from fragment G, resulting in a G-Δcre2 fragment. The EMSA result showed no affinity between this fragment and CcpA, indicating that cre2 was the sole binding site. After that, fragment D was gradually truncated at intervals of 30-bp bases from the 3′ end, thus resulting in the following fragments: 169-bp fragment (D1), 139-bp fragment (D2), 109-bp fragment (D3), 79-bp fragment (D4), and 49-bp fragment (D5) (Figure 1D). A significantly shifted band was found in fragments D1, D2, and D3. In contrast, the band was completely eliminated when fragment D4 or D5 was used, indicating that the binding site was located between fragments D4 and D5. The truncated 30 base fragment was scanned for possible CRE structure, and a sequence “TGAAAACGACCTTG” (cre3) was found. In the conservative region, there were three bases (10, 13,14) that did not fit with the typical CRE motif. Then, the cre3 was deleted from fragment D, resulting in a D-Δcre3 fragment. As predicted, the EMSA results showed that CcpA could no longer bind to fragment D-Δcre3 (Figure 1D).
Next, fragment E, another CcpA-binding fragment, was scanned for possible CRE structure. However, no classical CRE site was found. Then, the same method was carried out as described above, generating the following fragments: 172-bp fragment (E1), 142-bp fragment (E2), 112-bp fragment (E3), 82-bp fragment (E4), and 52-bp fragment (E5) (Figure 1C). The EMSA results showed that the CcpA protein lost its affinity for fragment E3, while it maintained its affinity for fragment E2, indicating that the binding site was located between these two fragments. Additionally, a 26-bp pseudopalindrome sequence (AGCTTT-aaggaactttcaga-AACGCT) (cre4) was found in this region. To validate its function, the cre4 was deleted from fragment E, resulting in an E-Δcre4 fragment. The EMSA results showed no affinity between CcpA and fragment E-Δcre4, indicating that the pseudo-palindromic sequence (cre4) may be the binding site for the CcpA protein. Based on fragment E, two fragments, fragments E6 and E7, were artificially generated. These fragments contain cre4-1 (AGCGTT-aaggaactttcaga-AACGCT) and cre4-2 (AGCTTT-aaggaactttcaga-AAAGCT) (Figure S2). The results showed that fragment E7 has a significantly shifted band, while fragment E6 does not, indicating that cre4-2 is the novel CcpA binding site.
Taken together, the motif of the newly found CRE, CRETre, is “AGCTTT-Yx-AAAGCT” (Y stands for any base, and X stands for the number of bases), which consists of a symmetrical region and intermediate spacer region. Compared with previously reported CRE sites in bacteria (Bacillus, Lactobacillus, and Staphylococcus), the novel CcpA binding site exhibits a high “AT” content feature.
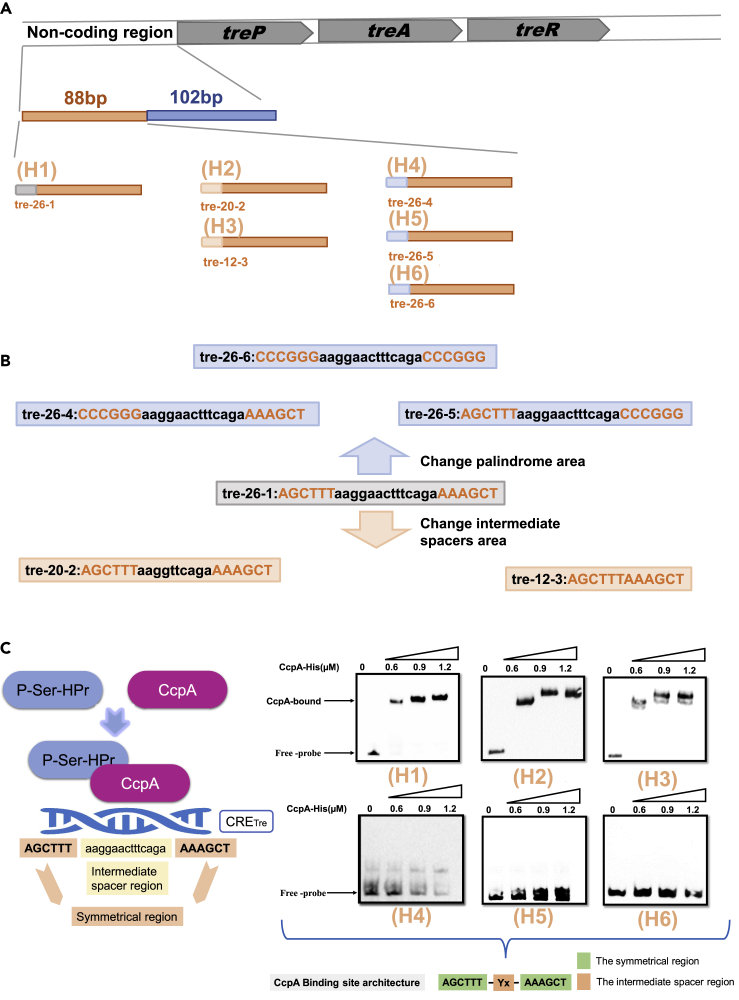
Characterization of the CcpA binding motif CRETre
CRETre contains two parts, a 12-bp symmetrical region (AGCTTT/AAAGCT) and an intermediate spacer region. The effect of the nucleotide structure on the function remains elusive. Considering the nanoaffinity of CcpA for fragment A, the following fragments were constructed to determine the role of the two parts. First, the role of the intermediate spacer region was confirmed. To this end, three intermediate spacer fragments with different lengths, tre-26-1, tre-20-2, and tre-12-3, were constructed (Figure 2B). They were inserted into fragment A to obtain H1, H2, and H3, respectively (Figure 2A). All three constructed fragments showed shifted bands (Figure 2C), indicating that the truncated intermediate spacer region retained binding affinity with CcpA. Next, the role of the symmetrical region was confirmed. Three fragments, tre-26-4, tre-26-5, and tre-26-6, were constructed. These fragments contained artificially changed symmetrical regions, as shown in Figure 2B. They were also inserted into fragment A, yielding H4, H5, and H6, respectively. When the above three hybrid fragments were subjected to EMSA with CcpA, no shifted band was observed (Figure 2C). These results indicate that the symmetrical region is crucial for CcpA binding.
Figure 2.
Influence of the 12-bp symmetrical region and the intermediate spacer region with CRETre (AGCTTT-Yx-AAAGCT) on CcpA protein regulation
(A) Construction of recombination fragments (fragment H1, fragment H2, fragment H3, fragment H4, fragment H5, and fragment H6) harboring the CRETre with different intermediate spacers length or different 12-bp symmetrical region.
(B) Two fragments that change the intermediate spacer region and three fragments that change the 12-bp symmetrical region are derived from tre-26-1 fragment, the intermediate spacer region with black and the 12-bp symmetrical region with red.
(C) EMSA of CcpA protein binding to six fragments (fragment H1, fragment H2, fragment H3, fragment H4, fragment H5, and fragment H6) that carrying different CRETre sites.
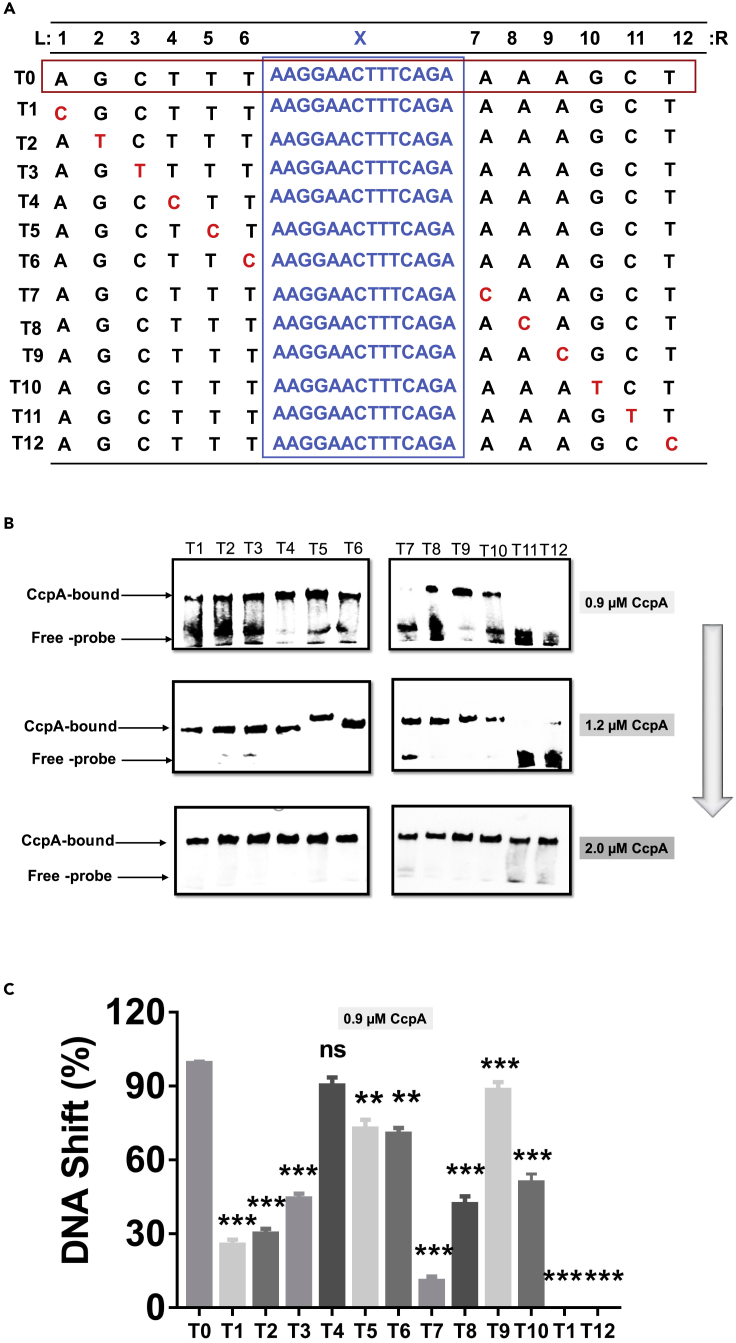
The above result indicates that the two inverted 6-bp repetitions are important for the binding of CcpA-CRETre. Therefore, the question of whether each base is essential was explored. Every signal nucleotide within the 6-bp palindromes in fragment H1 was separately mutated, resulting in 12 derivative probes (T1, T2, T3, T4, T5, T6, T7, T8, T9, T10, T11, and T12) (Figure 3A). When 1.2 μM CcpA was used to incubate, the probes T1, T2, T3, T4, T5, T6, T7, T8, T9, and T10 exhibited high affinity for CcpA, while T11 and T12 showed significantly decreased affinity for CcpA. Next, the concentration of CcpA in the lane was gradually reduced to put the probes in a semi-binding state. When using 0.9 μM CcpA in the lane, a high affinity for CcpA was shown in T4, T5, T6, T9, and T10, indicating lower conservation for these five positions. In contrast, a low affinity for CcpA was shown in T1, T2, T3, T7, T8, T11, and T12, indicating that these seven positions are crucial for CcpA binding (Figures 3B and 3C). A further mutation in the symmetrical region showed that the affinity of probes for CcpA was weakened or disappeared, indicating that the symmetrical region in CRETre was not allowed to be nonmatched in more positions (Figure S3).
Figure 3.
Influence of single point mutation of the 12-bp symmetrical region in CRETre for CcpA protein binding
(A) Single point mutation of the 12-bp symmetrical region of CRETre.
(B) EMSA of CcpA protein for 12 mutants (5′ biotin), 12 mutants (T1, T2, T3, T4, T5, T6, T7, T8, T9, T10, T11, and T12) derived from CRETre, with concentrations of 0.9 μM–2.0 μM of CcpA protein.
(C) The ratio of protein-bound probe/total probe for 12 mutants that derived from CRETre at the 0.9 μM CcpA protein was shown. Statistical significance was determined by Student's t-test (∗P ≤ 0.05; ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001).
Inversion of symmetric regions leads to the transition from CCA to CCR
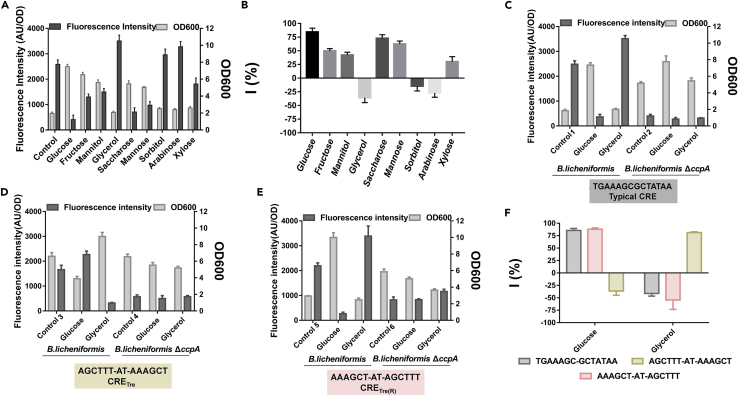
Next, pBLTE was constructed with a reporter protein eGFP controlled by PtreA to screen different carbon sources for significant effectors corresponding to either CCR or CCA. Glucose, fructose, mannitol, glycerol, sucrose, mannose, sorbitol, arabinose, xylose, and trehalose were added at concentrations of 1.5%. The results showed that glucose, fructose, mannitol, sucrose, mannose, and xylose had negative effects on eGFP production. Of these carbohydrates, glucose exhibited the greatest inhibition (Figure 4A). Glycerol, sorbitol, and arabinose had a promoting effect, and glycerol obtained the highest fluorescence intensity (Figure 4A). The formula I = (FI1-FI2)/FI1×100%, where FI1 represents the fluorescence intensity when only trehalose was added and FI2 represents the fluorescence intensity when trehalose and a specific carbohydrate were both added, was used to define the CCR/CCA effect. Therefore, Iglucose was 84.35% and Iglycerol was −35.72% (Figure 4B). As a result, glucose and glycerol were chosen as significant effectors for CCR and CCA, respectively.
Figure 4.
Characterization of the CcpA regulation in vivo for trehalose-inducible system
(A) The fluorescence intensity and OD 600 were measured for the strain carrying pBLTE plasmid under ten conditions (control: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% fructose, 1.5% trehalose + 1.5% mannitol, 1.5% trehalose + 1.5% glycerol, 1.5% trehalose + 1.5% glycerol, 1.5% trehalose + 1.5% saccharose, 1.5% trehalose + 1.5% mannose, 1.5% trehalose + 1.5% sorbitol, 1.5% trehalose + 1.5% arabinose, 1.5% trehalose + 1.5% xylose). Data are shown as means ± SD, n = 3.
(B) Quantify the CCR/CCA effect with the formula I=(FI1-FI2)/FI1×100% due to different carbohydrates (FI1 represents the fluorescence intensity when only trehalose is added. FI2 represents the fluorescence intensity when trehalose is added and another carbohydrate is also added. Glucose, fructose, mannitol, glycerol, sucrose, mannose, sorbitol, arabinose, and xylose added at a concentration of 1.5% while adding 1.5% trehalose. Data are shown as means ± SD, n = 3.
(C) The fluorescence intensity and OD 600 were measured for trehalose promoter in both the strain BlspTE (control 1: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% glycerol) and CcpA-deletion strain BlspTE1 (control 2: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% glycerol). Data are shown as means ± SD, n = 3.
(D) The fluorescence intensity and OD 600 were measured for trehalose promoter whose CRE site was replaced by CRETre site in both the strain BlspT1E (control 3: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% glycerol) and CcpA-deletion strain BlspT1E1 (control 4: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% glycerol). Data are shown as means ± SD, n = 3.
(E) The fluorescence intensity and OD 600 were measured for trehalose promoter whose CRE site was replaced by CRETre(R) site in both the strain BlspT2E (control 5: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% glycerol) and CcpA-deletion strain BlspT2E1 (control 6: 1.5% trehalose, 1.5% trehalose + 1.5% glucose, 1.5% trehalose + 1.5% glycerol). Data are shown as means ± SD, n = 3.
(F) Compared three CRE sites by using formula I=(FI1-FI2)/FI1×100% while extra adding glucose or glycerol. Data are shown as means ± SD, n = 3.
Then, BlspT1E and BlspT2E were constructed with a reporter protein eGFP controlled by PtreA-1 and PtreA-2 to explore the relationship of the sequence of CRE with CCR/CCA. CRETre (AGCTTT-AT-AAAGCT) was used to replace the original CRE site (TGAAAGCGCTATAA) in PtreA, generating PtreA-1. Meanwhile, the symmetric region in CRETre was inversed to generate CRETre(R) (AAAGCT-AT-AGCTTT). This synthetic CRE was used to replace the original CRE site in PtreA, generating PtreA-2. Two plasmids, pBLT1E and pBLT2E, which were carrying PtreA-1 and PtreA-2, were transformed to yield BlspT1E and BlspT2E. The above two plasmids were also transformed into Bacillus licheniformis CA, pBLT1E, and pBLT2E. Six strains, BlspTE, BlspTE1, BlspT1E, BlspT1E1, BlspT2E, and BlspT2E1 (Table S1), were cultured under different induction conditions: (1) 1.5% trehalose, (2) 1.5% trehalose +1.5% glucose, and (3) 1.5% trehalose +1.5% glycerol. For strains BlspTE and BlspT2E, the fluorescence intensity decreased when glucose was present (Figures 4C and 4E). Compared to the set without glucose, the fluorescence intensities decreased by 85.41% and 87.91%, indicating that CRE and CRETre(R) corresponded to the CCR effect mediated by glucose. In contrast, for strain BlspT1E, fluorescence intensity increased by 36.36% compared to that of the set without glucose, indicating that CRETre corresponded to the CCA effect in the presence of glucose (Figures 4D and 4F). We next investigated the effect of glycerol. For strains BlspTE and BlspT2E, the fluorescence intensity increased (Figures 4C and 4E). Compared to the set without glycerol, fluorescence intensities increased by 41.27% and 54.41%, respectively, indicating that CRE and CRETre(R) responded to the CCA effect caused by glycerol. In contrast, for strain BlspT1E, the fluorescence intensity decreased by 81.51% compared to the set without glycerol (Figures 4D and 4F), indicating that CRETre corresponded to the CCR effect in the presence of glycerol. These results suggest that CRE and the synthetic CRETre are opposite in regulatory direction (activation or inhibition). When the CcpA protein was deleted, the CCA effect or the CCR effect in the strains BlspTE1, BlspT1E1, and BlspT2E1 was weakened or even disappeared (Figures 4C–4E). This result also indicates that the CCA effect and the CCR effect in B. licheniformis are mainly mediated by the CcpA protein. Therefore, we can conclude that the fine-tuning of CcpA-mediated regulation may be modulated by the sequence of the CRE and the availability of the extracellular carbon source.
Characterization of the CcpA binding motif CRETre(R)
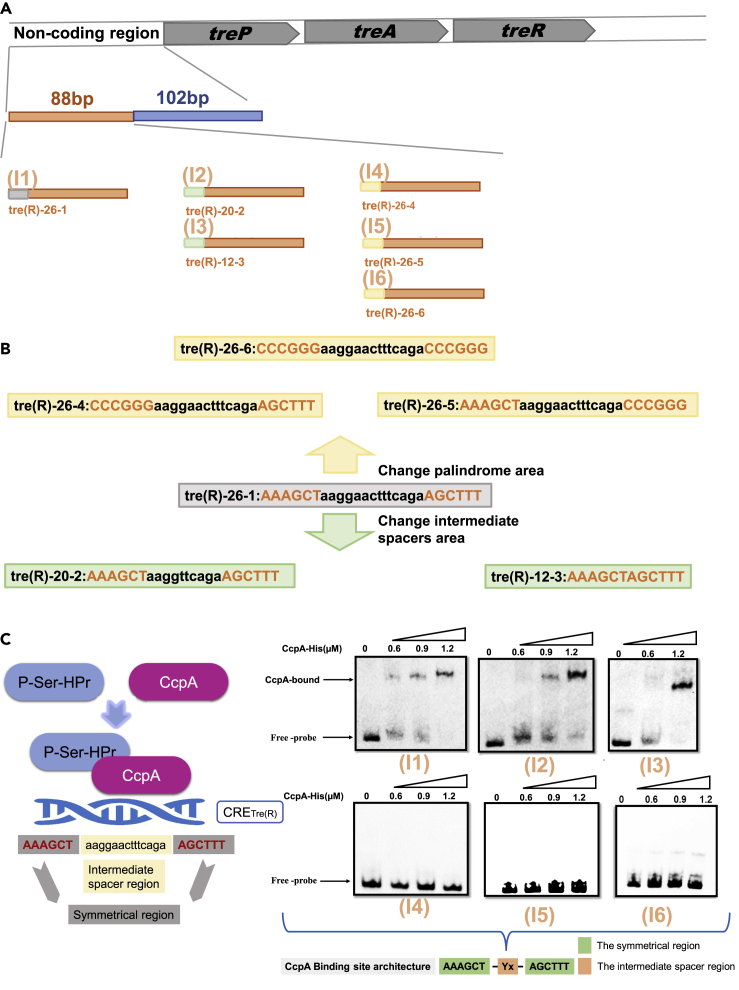
The above results show that CRETre and CRETre(R) exhibit opposite directions in CcpA-mediated regulatory in vivo. It is necessary to explore whether this difference is due to a varied affinity of CcpA. First, the role of the intermediate spacer region was confirmed. Three fragments, I1, I2, and I3, were constructed by changing the length of the intermediate spacer, as shown in Figures 5A and 5B. The EMSA results show that CcpA can still bind to all of them (Figure 5C), even to the one with the intermediate spacer completely removed. This result suggests that CcpA-CRETre(R) interaction is independent of the intermediate spacer. Next, the role of 6-bp palindromes was investigated. The palindromic sequence in the 5′ end “AAAGCT” was replaced by “CCCGGG” to yield I4. In the 3′ end, “AGCTTT” was replaced by “CCCGGG” to yield I5. For I6, both palindromic sequences were replaced by “CCCGGG” and “CCCGGG”, respectively (Figures 5A and 5B). The EMSA results showed that CcpA could no longer bind to I4, I5, or I6 (Figure 5C), indicating that these palindromic regions were crucial for CcpA binding. Thus, it can be hypothesized that the motif of CRETre(R) is “AAAGCT-Yx-AGCTTT” (Y stands for any base, and X stands for the number of bases).
Figure 5.
Influence of the 12-bp symmetrical region and the intermediate spacer region with CRETre(R) (AAAGCT-Yx-AGCTTT) on CcpA protein regulation
(A) Construction of recombination fragments (fragment I1, fragment I2, fragment I3, fragment I4, fragment I5, and fragment I6) harboring the CRETre(R) with different intermediate spacer length or different 12-bp symmetrical region.
(B) Two fragments that change the intermediate spacer region and three fragments that change the 12-bp symmetrical region are derived from tre(R)-26-1 fragment, the intermediate spacer region with black and the 12-bp symmetrical region with red.
(C) EMSA of CcpA protein binding to six fragments (fragment I1, fragment I2, fragment I3, fragment I4, fragment I5, and fragment I6) that carry different CRETre(R) sites.
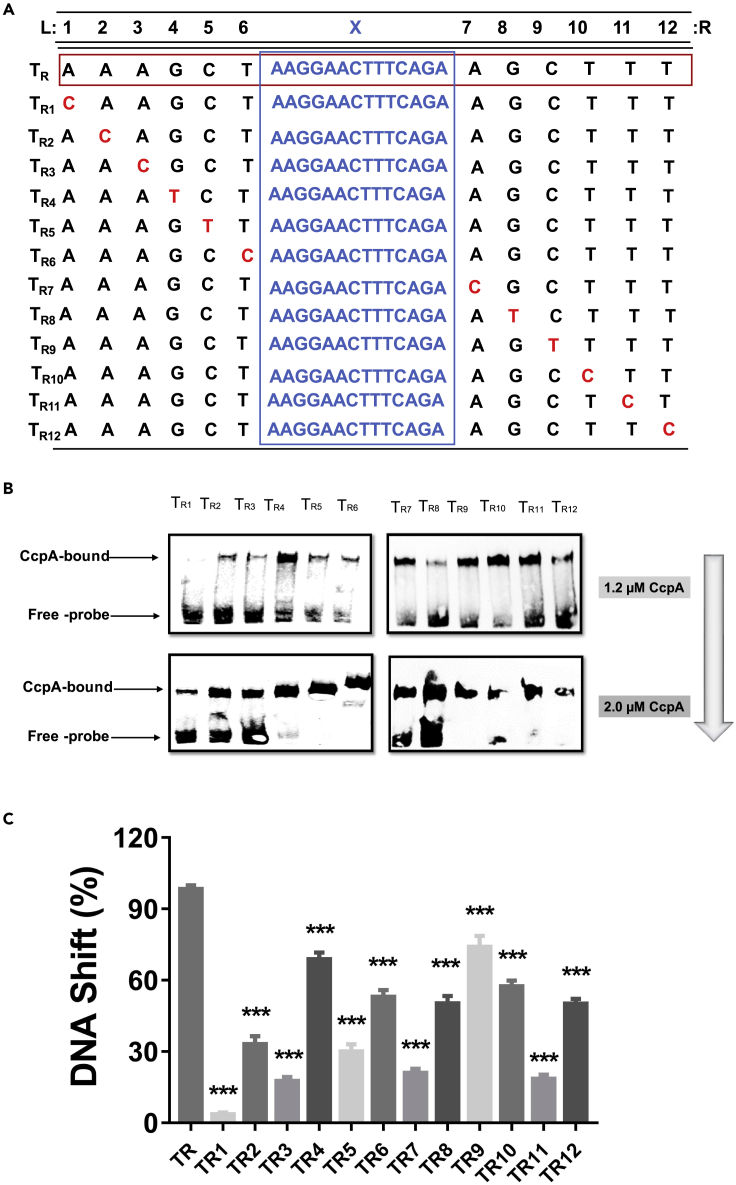
Then, the different roles of the specific bases within the palindromic regions were characterized. For this purpose, 12 mutants (TR1, TR2, TR3, TR4, TR5, TR6, TR7, TR8, TR9, TR10, TR11, and TR12) (Figure 6A) were constructed based on fragment I1. Under a CcpA protein concentration of 1.2 μM, TR4, TR6, TR8, TR9, TR10, and TR12 still exhibited high affinity for CcpA, suggesting that these positions could tolerate substantial degeneracy. On the other hand, binding affinity was strongly reduced for mutants TR1, TR2, TR3, TR5, TR7, and TR11. The high conservatism of these positions suggests their important functional role (Figures 6B and 6C). Further mutations within the symmetric region show that the affinity of these probes for CcpA protein could be also diminished or even eliminated (Figure S4). These results indicate that the symmetric region could not tolerate more non-match bases.
Figure 6.
Influence of single point mutation of the 12-bp symmetrical region in CRETre(R) for CcpA protein binding
(A) Single point mutation of the 12-bp symmetrical region of CRETre(R).
(B) EMSA of CcpA protein for 12 mutants (5′ biotin), 12 mutants (TR1, TR2, TR3, TR4, TR5, TR6, TR7, TR8, TR9, TR10, TR11, and TR12) derived from CRETre(R), with concentrations of 1.2 μM–2.0 μM of CcpA protein.
(C) The ratio of protein-bound probe/total probe for 12 mutants that derived from CRETre(R) at the 1.2 μM CcpA protein were shown. Statistical significance was determined by Student's t-test (∗P ≤ 0.05; ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001).
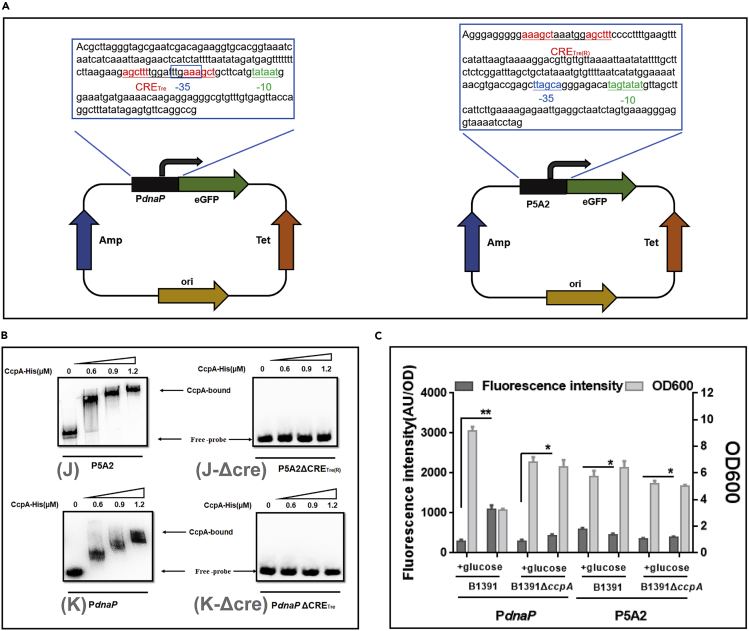
Wide occurrence of the novel binding site CRETre and CRETre(R) in B. licheniformis
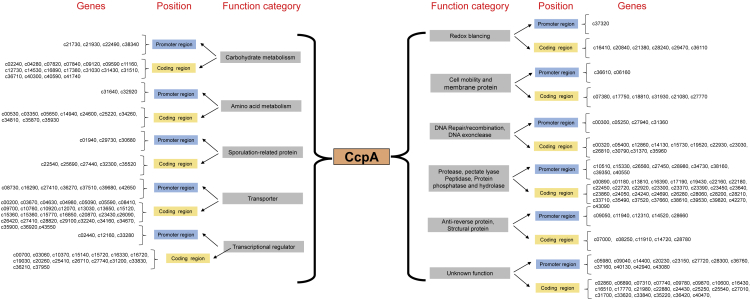
To further confirm the newly found motif, two genes containing CRETre or CRETre(R)-like sequence were cloned. The former (AGCTTT-tggatttg-AAAGCT) was located in the 5′ non-coding regions of the gene dnaP (GenBank: NM_00300) (J) and the latter (AAAGCT-aaatgg-AGCTTT) was in gene 5A2 (GenBank: NM_09050) (K). As the EMSA results showed that CcpA protein can bind to J or K, the CRE-like sequence in both fragments was deleted, yielding J-Δcre and K-Δcre. As expected, CcpA could no longer bind to J-Δcre or K-Δcre (Figure 7B), suggesting the existence of CRETre and CRETre(R). The strains pBLDE and pBLAE, which carried J and K as transcription initiation elements, were also constructed (Figure 7A). Both ensured the functional expression of eGFP. However, when the strains were subjected to cultivation under glucose stress (30 g/L), the fluorescence intensity of BlspDE increased by 273.98% and that of BlspAE decreased by 22.64%. These results further proved that CRETre is a CCA-responsive element under glucose stress, while CRETre is a CCR-responsive element (Figure 7C). Furthermore, the B.licheniformis genome was selected for CRETre and CRETre(R), and 279 possible sequences were found distributed in 264 genes (Figure 8, Table S3). From the function of genes, these genes are mainly related to the following aspects: (1) carbohydrate metabolism, (2) amino acid metabolism, (3) sporulation formation, (4) bacterial migration, (5) transcription factors, (6) transporters, (7) DNA repair, recombination, (8) proteases, peptidases, hydrolases, (9) redox balance, and (10) unknown.
Figure 7.
Exploring the property of CRETre in Pdanp, the CRETre(R) in P5A2
(A) The sequences of the PdnaP and P5A2 from Bacillus licheniformis are shown, and the CRETre or CRETre(R) is shown in red, −10 region is shown in green, and −35 region is shown in blue.
(B) EMSA of CcpA protein binding to two fragments (PdnaP, P5A2) labeled with 5′ biotin, while not binding to two fragments (PdnaPΔCRETre, P5A2ΔCRETre(R)) labeled with 5′ biotin.
(C) The OD 600 and the fluorescence intensity were measured in both the Bacillus licheniformis CICIM B1391 and CcpA-deletion strain when using PdnaP or P5A2 as the expression element. Data are shown as means ± SD, n = 3. Statistical significance was determined by Student's t-test (∗P ≤ 0.05; ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001).
Figure 8.
Searching Bacillus licheniformis genome for CRETre and CRETre(R)
Four degenerate CRETre sites were found in the xylose operon and mannose operon by alignment. The further EMSA results proved that CcpA can bind to xylB∗410 and xylB∗1384, while the other two, xylR∗775 and xylA∗249, had no affinity for CcpA. xylR∗775 and xylA∗249 both had a signal base mutation in the 5′ end (“T”→“C”), which could explain why these CRETre-like sequences had no interaction with CcpA (Figure S5). Two degenerate CRETre sites were found in the mannose operon, and the EMSA result showed that the CcpA protein can bind to both of the fragments (Figure S5).
Discussion
CcpA participates in the regulation of central carbon and nitrogen metabolism (Yoshida et al., 2001; Halsey et al., 2017), the formation of biofilms (Römling and Galperin, 2015), the formation of spores (Voltersen et al., 2018), physiological processes (Charbonnier et al., 2017), DNA replication (Jolly et al., 2020), and the CCR effect. The interaction between this protein and target genes is the key molecular basis in the above process. In the current study, a new CcpA protein binding site in the treR gene, “AGCTTT-Yx-AAAGCT”, called CRETre, was identified. It is composed of two palindromic sequences and variable spacers. It has been proven that the DNA-binding domain contains a helix-turn-helix motif that makes base-specific contacts in the major groove of the DNA (Schumacher et al., 2004). In previous research, structure elucidation revealed that the CcpA-CRE complex varies in bending angle according to different CRE sequences (Schumacher et al., 1994). Compared to classical CRE sites with only highly conserved structures, the flexible structure of CRETre provides new functionalities for CcpA-mediated regulation. A similar motif for protein recognition sites has been reported in E. coli. The transcription factor HipB can recognize a motif consisting of a spacer sequence and a palindromic sequence. The crystal structure revealed that the protein can bind to the motif by extruding the spacer (Schumacher et al., 2015; Lin et al., 2013a). However, the functional properties of the spacer sequence remained unknown. This study confirms that although the palindromic sequence was the indispensable element of the recognition site, modification in the variable spacers was able to modulate the interaction between CRETre and CcpA. This result provides new insight into the mechanism by which the regulator interacts with the target genes, suggesting that the spacer sequence within this motif may serve as a target for the fine-tuning of the regulation.
The CcpA protein can bind to different target sites, resulting in either a CCR effect or a CCA effect. However, the relationship between the target sequence feature and the direction of CcpA-mediated regulation is poorly understood. This study reported an interesting new motif, CRETre, which is involved in a bidirectional regulation in the glucose stress environment. Previous studies mainly focused on substrates corresponding to CCR or CCA. For example, glucose-related CCR effects have been investigated extensively. The CCR effect or CCA effect is also caused by the different priority utilization levels of the carbon source. When a rapid-acting carbon source (glucose) is present in a fermentation system, the utilization priority of the rapid-acting carbon source is higher than that of the inducer, causing the inducer (another sugar or alcohol)-related genes to be inhibited by the CcpA protein and subjecting the expression system to the CCR effect (Jankovic and Brückner, 2007; Moreno et al., 2010). When a delayed carbon source is present in a fermentation system, the inducer (sugar or alcohol) is the fast-acting carbon source, and the related metabolic utilization genes involved are activated by the CcpA protein, thereby causing the expression system to be subjected to the CCA effect (Yu et al., 2017). Sugar/alcohol-inducible promoters usually contain a classical CRE site in the core region (Heravi et al., 2011; Ren et al., 2010), which makes the regulation of the CcpA protein particularly important for the promoter. It is worth mentioning that the mutation of CcpA causes different effects for target genes (Hueck et al., 2010). Our study indicated that the transition between CCR and CCA is made possible by rearrangement of the nucleotide sequence of CRETre. Because of its indispensable role in the CcpA-mediated CCR effect, the classical CRE can be likened to a molecular switch that turns the regulation on and off. The newly found CRETre exhibits competence in this two-way molecular switch, guiding a regulation with either CCR or CCA effect. We also used both constitutive and inducible promoters as examples to further explore the influence of CRETre or CRETre(R) on transcription. The results were consistent with those in the trehalose operon. This CRE-engineering strategy has great potential in synthetic biology, particularly for genetic element development.
From the distribution of CRETre and CRETre(R) in B. licheniformis, it was found that the genes present in CRETre and CRETre(R) are relevant to important biological activities in bacteria, such as DNA mismatch and repair genes, sporulation genes, cell movement and division genes, sugar alcohol transporters, and ion transporters, which are responsible for the PTS system. It is worth mentioning that CRETre and CRETre(R) are also present in toxin-encoding genes and promoters, such as subtilisin (GenBank: NM_12310) and autolysin inhibitor (GenBank: NM_14520). This fact indicates that the CcpA protein still has some unknown functions, and it shows the importance of CRETre and CRETre(R) as cis-acting elements. CRETre and CRETre(R) have opposite functional properties, and there is also some symmetry in their sequences. The result of a single base mutation on the two 6-bp palindromic sequences of CRETre and CRETre(R) indicates that a single base mutation is allowed, but further mutations after a single base mutation result in the loss of CcpA protein binding ability, which indicates that CRETre and CRETre(R) allow some changes for two 6-bp palindromic sequences, but this change will affect the binding of the CcpA protein to the site. CRETre and CRETre(R) provide two effective pathways for the regulation of the CcpA protein in both function and sequence.
In conclusion, in addition to more research on carbon metabolism via the CcpA protein, further research is needed on other aspects of its function. The discovery and function of the CRETre and CRETre(R) sites further clarify the regulatory network of CcpA proteins. It is conceivable that the functions and sites play important roles in the CcpA protein-mediated regulatory network.
Limitation of the study
Here, we show the CCA/CCR effect with the newly CcpA binding site under different carbon sources. Notably, there also exists unknown CRE site in B.licheniformis. Further studies would mine the potential CRE sites in B.licheniformis.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Prof. Guiyang Shi, gyshi@jiangnan.edu.cn.
Material availability
All tables and figures are included in the text and supplemental information.
Data and code availability
The published article contains all data generated or analyzed.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2020YFA0907700, 2018YFA0900300, 2018YFA0900504 and 2016YFD0401404), the National Natural Foundation of China (31401674), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018-22), and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions.
Author contributions
F.X., Y.L., and G.S. designed the study, carried out the experiments, analyzed data, and wrote the paper. Y.Z. and H.W. carried out the experiments. L.Z. and Z.D. analyzed data. Z.G. and S.X. designed the study.
Declaration of interests
The authors declare that they have no conflicts of interest.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102400.
Contributor Information
Youran Li, Email: liyouran@jiangnan.edu.cn.
Guiyang Shi, Email: gyshi@jiangnan.edu.cn.
Supplemental information
References
- Antunes A., Camiade E., Monot M., Courtois E., Barbut F., Sernova N.V., Rodionov D.A., Martin-Verstraete I., Dupuy B. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 2012;40:10701–10718. doi: 10.1093/nar/gks864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai K., Baik S.H., Kasahara Y., Moriya S., Ogasawara N. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology. 2000;146:263–271. doi: 10.1099/00221287-146-2-263. [DOI] [PubMed] [Google Scholar]
- Bruckner R., Titgemeyer F. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 2002;209:141–148. doi: 10.1111/j.1574-6968.2002.tb11123.x. [DOI] [PubMed] [Google Scholar]
- Charbonnier T., LeCoq D., McGovern S., Calabre M., Delumeau O., Aymerich S., Jules M. Molecular and physiological logics of the pyruvate-induced response of a novel transporter in Bacillus subtilis. mBio. 2017;8:e0097617. doi: 10.1128/mBio.00976-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J., Graham I.A. Trehalose metabolism: a regulatory role for trehalose-6-phosphate? Curr. Opin. Plant Biol. 2003;6:231–235. doi: 10.1016/s1369-5266(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2014;3:245–259. doi: 10.1271/bbb.80479. [DOI] [PubMed] [Google Scholar]
- Görke B., Stülke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- Gosseringer R., Küster E., Galinier A., Deutscher J., Hillen W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J. Mol. Biol. 1997;266:665–676. doi: 10.1006/jmbi.1996.0820. [DOI] [PubMed] [Google Scholar]
- Halsey C.R., Lei S., Wax J.K., Lehman M.K., Nuxoll A.S., Steinke L., Sadykov M., Powers R., Fey P.D. Amino acid catabolism in Staphylococcus aureus and the function of carbon catabolite repression. mBio. 2017;8:e0143416. doi: 10.1128/mBio.01434-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heravi K.M., Wenzel M., Altenbuchner J. Regulation of mtl operon promoter of Bacillus subtilis: requirements of its use in expression vectors. Microb. Cell Fact. 2011;10:83. doi: 10.1186/1475-2859-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueck C.J., Kraus A., Schmiedel D., Hillen W. Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol. Microbiol. 2010;16:855–864. doi: 10.1111/j.1365-2958.1995.tb02313.x. [DOI] [PubMed] [Google Scholar]
- Jankovic I., Brückner R. Carbon catabolite repression of sucrose utilization in Staphylococcus xylosus: catabolite control protein CcpA ensures glucose preference and autoregulatory limitation of sucrose utilization. J. Mol. Microb. Biotech. 2007;12:114–120. doi: 10.1159/000096466. [DOI] [PubMed] [Google Scholar]
- Jolly S.M., Gainetdino I., Jouravleva K., Zhang H., Strittmatter L., Bailey S.M., Hendricks G.M., Dhabaria A., Ueberheide B., Zamore P.D. Thermus thermophilus argonaute functions in the completion of DNA replication. Cell. 2020;182:1545–1559. doi: 10.1016/j.cell.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörg S., Hillen W. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 1999;2:195–201. doi: 10.1016/S1369-5274(99)80034-4. [DOI] [PubMed] [Google Scholar]
- Kim J.N., Burne R.A. CcpA and CodY coordinate acetate metabolism in Streptococcus mutans. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.03274-16. e03274–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn R.J., Buescher J.M., Chat L.L., Jules M., Aymerich S., Saue U. Metabolic fluxes during strong carbon catabolite repression by malate in Bacillus subtilis. J. Biol. Chem. 2010;285:1587–1596. doi: 10.1074/jbc.M109.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.R., Jin K., Zhang L., Ding Z.Y., Gu Z.H., Shi G.Y. Development of an inducible secretory expression system in Bacillus licheniformis based on an engineered xylose operon. J. Agric. Food Chem. 2018;66:9456–9464. doi: 10.1021/acs.jafc.8b02857. [DOI] [PubMed] [Google Scholar]
- Li Y.R., Liu X., Zhang L., Ding Z., Shi G.Y. Transcriptional changes in the xylose operon in Bacillus licheniformis and their use in fermentation optimization. Int. J. Mol. Sci. 2019;20:4615. doi: 10.3390/ijms20184615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.Y., Awano N., Masuda H., Park J.H., Inouye M. Transcriptional repressor HipB regulates the multiple promoters in Escherichia coli. J. Mol. Microbiol. Biotechnol. 2013;23:440–447. doi: 10.1159/000354311. [DOI] [PubMed] [Google Scholar]
- Lin Z., Chul C.S., Danko C.G., Adam S., Stanhope M.J., Burne R.A. Gene regulation by CcpA and catabolite repression explored by RNA-seq in Streptococcus mutans. PLoS One. 2013;8:e60465. doi: 10.1371/journal.pone.0060465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll B., Alings C., Kowalczyk M., Chieduch A., Bardowski J., Saenger W., Biesiadka J. Structure of the transcription regulator CcpA from Lactococcus lactis. Acta Crystallogr. D. 2007;63:431–436. doi: 10.1107/S0907444907000546. [DOI] [PubMed] [Google Scholar]
- Moreno M.S., Schneider B.L., Maile R.R., Weyler W., Saier M.H. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol. Microbiol. 2010;39:1366–1381. doi: 10.1111/j.1365-2958.2001.02328.x. [DOI] [PubMed] [Google Scholar]
- Okabe S., Shafdar A.A., Kobayashi K., Zhang L., oshiki M. Glycogen metabolism of the anammox bacterium “Candidatus Brocadia sinica”. ISME J. 2020 doi: 10.1038/s41396-020-00850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricia F.R., Ipsita R., Mark O., Tajalli K. Proteomics analysis of Bacillus licheniformis in response to oligosaccharides elicitors. Enzyme Microb. Tech. 2014;61:61–66. doi: 10.1016/j.enzmictec.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Ren C., Gu Y., Hu S., Wu Y., Wang P., Yang Y.L., Yang C., Yang S., Jiang W.H. Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab. Eng. 2010;12:446–454. doi: 10.1016/j.ymben.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Römling U., Galperin M.Y. Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol. 2015;23:545–557. doi: 10.1016/j.tim.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruud D.O.W., Gerald S., Kuipers O.P. Probing the regulatory effects of specific mutations in three major binding domains of the pleiotropic regulator CcpA of Bacillus subtilis. Front. Microbiol. 2015;6:1051. doi: 10.3389/fmicb.2015.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöck F., Dahl M.K. Expression of the tre operon of Bcillus subtilis168 is regulated by the repressor TreR. J. Biotechnol. 1996;178:4576–4581. doi: 10.1128/jb.178.15.4576-4581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M.A., Choi K.Y., Zalkin H., Brennan R.G. Crystal structure of LacI member PurR bound to DNA: minor groove binding by alpha helices. Science. 1994;266:763–770. doi: 10.1126/science.7973627. [DOI] [PubMed] [Google Scholar]
- Schumacher M.A., Allen G.S., Diel M., Seidel G., Hillen W., Brennan R.G. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell. 2004;118:731–741. doi: 10.1016/j.cell.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Schumacher M.A., Sprehe M., Bartholomae M., Hillen W., Brennan R.G. Structures of carbon catabolite protein A-(Hpr-Ser46-P) bound to diverse catabolite response element sites reveal the basis for high-affinity binding to degenerate DNA operators. Nucleic Acids Res. 2011;39:2931–2942. doi: 10.1093/nar/gkq1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M.A., Balani P., Min J.K., Chinnam N.B., Hansen S., Vulic M., Lewis K., Brennan R.G. HipBA-promoter structures reveal the basis of heritable multidrug tolerance. Nature. 2015;524:59–64. doi: 10.1038/nature14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhan Y.Y., Zhou M.L., He M., Wang Q., Li X., Wen Z.Y., Chen S.W. High-level production of short branched-chain fatty acids from waste materials by genetically modified Bacillus licheniformis. Bioresour. Technol. 2019;271:325–331. doi: 10.1016/j.biortech.2018.08.134. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 2007;5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- Voltersen V., Blango M.G., Herrmann S., Schmidt F., Heinekamp T., Strassburger M., Krüger T., Bacher P., Lother J., Weiss E. Proteome analysis reveals the conidial surface protein CcpA essential for virulence of the pathogenic fungus Aspergillus fumigatus. mBio. 2018;9 doi: 10.1128/mBio.01557-18. e01557–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J.B., Lolkema J.S. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 2003;67:475–490. doi: 10.1128/MMBR.67.4.475-490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert M.J., Chambliss G.H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. U S A. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg J., DeGreeff A., Jarek M., Valentin-Weigand P., Goethe R. The CcpA regulon of Streptococcus suis reveals novel insights into the regulation of the streptococcal central carbon metabolism by binding of CcpA to two distinct binding motifs. Mol. Microbiol. 2014;92:61–83. doi: 10.1111/mmi.12537. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Xu P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007;33:127–140. doi: 10.1080/10408410701364604. [DOI] [PubMed] [Google Scholar]
- Xiao F.X., Li Y.R., Zhang Y.P., Wang H.,R., Zhang L., Ding Z.Y., Gu Z.H., Xu S., Shi G.Y. Construction of a novel sugar alcohol-inducible expression system in Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2020;104:5409–5425. doi: 10.1007/s00253-020-10618-8. [DOI] [PubMed] [Google Scholar]
- Yoshida K.I., Kobayashi K., Miwa Y., Kang C.M., Matsunaga M., Yamaguchi H., Tojo S., Yamamoto M., Nishi R., Ogasawara N. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 2001;29:683–692. doi: 10.1093/nar/29.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Chen Z., Ye H., Liu P., Li Z., Wang Y. Effect of glucose on poly-γ-glutamic acid metabolism in Bacillus licheniformis. Microb. Cell Fact. 2017;16:22. doi: 10.1186/s12934-017-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y.Q., Yang Y.P., Jiang W.H., Gu Y. A novel dual-cre motif enables two-way autoregulation of CcpA in Clostridium acetobutylicum. Appl. Environ. Microbiol. 2018;84:e00114–e00118. doi: 10.1128/AEM.00114-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article contains all data generated or analyzed.