Abstract
Bone-tissue defects affect millions of people worldwide. Despite being common treatment approaches, autologous and allogeneic bone grafting have not achieved the ideal therapeutic effect. This has prompted researchers to explore novel bone-regeneration methods. In recent decades, the development of bone tissue engineering (BTE) scaffolds has been leading the forefront of this field. As researchers have provided deep insights into bone physiology and the bone-healing mechanism, various biomimicking and bioinspired BTE scaffolds have been reported. Now it is necessary to review the progress of natural bone physiology and bone healing mechanism, which will provide more valuable enlightenments for researchers in this field. This work details the physiological microenvironment of the natural bone tissue, bone-healing process, and various biomolecules involved therein. Next, according to the bone physiological microenvironment and the delivery of bioactive factors based on the bone-healing mechanism, it elaborates the biomimetic design of a scaffold, highlighting the designing of BTE scaffolds according to bone biology and providing the rationale for designing next-generation BTE scaffolds that conform to natural bone healing and regeneration.
Keywords: Bone tissue engineering, Scaffold, Bone biology, Cytokine, Bone regeneration
Graphical abstract
Bone-tissue engineering has become a promising treatment strategy for large bone defects. This work first introduces the advanced knowledge of bone biology, including the physiological microenvironment and healing process. Based on this concept, it further details the current biomimetic and bioactive bone-tissue engineering scaffolds promoting the healing process. Finally, it provides the future perspective in this field.
Highlights
-
•
Elaborate the advanced knowledge of bone physiological microenvironment and healing process.
-
•
Summary the biomolecules involved in the natural bone healing process which could be applied to BTE materials and scaffolds.
-
•
Detail the current biomimetic and bioinspired scaffolds based on the bone physiological microenvironment.
-
•
Review the delivery of bioactive factors based on the bone healing mechanism.
-
•
Discuss the current limitations that still need to be solved, and the feasible improvement and outlooks are proposed.
1. Introduction
Bone is a highly vascularized and dynamic natural composite that is constantly remodeled throughout an individual's lifespan. It has excellent mechanical properties and fracture toughness, which can provide sufficient load-bearing capacity for locomotion, while also acting as a casing to protect delicate internal organs [1]. In addition to these structural functions, bone tissue also functions as an endocrine organ, playing an important role in global minerals (especially Ca and P ions) and nutrient homeostasis [2]. In contrast to other tissues and organs, bone tissue usually has better self-healing ability, as the damaged part can regain its original structure and mechanical strength without leaving fibrotic scars [3]. However, when the range of bone defects exceeds the critical-size defect (CSD), the bone defects cannot heal by themselves and require reasonable clinical intervention [1,4]. Large-sized bone defects can be caused by factors such as trauma, developmental deformity, tumor resection, and infection, which are common issues in clinical treatment. According to an epidemiological study, in 2010–2025, the incidence of fractures in Europe increased at an annual rate of 28%, with an additional 25% economic burden, drawing extensive attention from bone repair medicine research [5].
Current clinical approaches for treating large-sized bone defects mainly include autologous bone grafts and allogeneic bone grafts. Autologous bone grafts are considered the gold standard for bone-tissue repair and regeneration because the autogenous bone has good osteoinductivity, osteoconductivity, and osseointegration properties, which can form a coordinated structure and ensure mechanical strength at the bone-defect site. However, allogeneic bone transplantation still has inevitable drawbacks, such as donor site neurovascular injury, inflammation, infection, limited donor bone transplantation, and high costs [6,7]. To address these shortcomings, allogeneic bone grafts are widely used because of their accessibility. However, allograft bone transplantation has problems such as poor osseointegration, immune rejection, and blood disease transmission [8,9]. Confronted with this situation, researchers began to explore artificial alternatives to natural bone-derived grafts. Recent advances in BTE have allowed researchers to use multiple methods to combine cells, biomaterials, and biological factors to create artificial tissues for repairing bone defects [10]. This method has the advantages of high modifiability, low risk of infectivity, and excellent biocompatibility, and does not result in obvious complications [11,12].
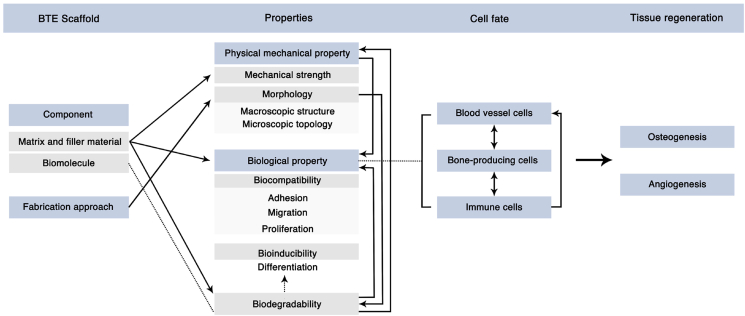
The design of scaffolds is the core of BTE, because, when implanted into the bone defect area, the scaffold provides the cells with a substrate for adhesion, proliferation, migration, and differentiation. Such regulation of bone-tissue cells (including osteoblast cell lines, angioblast cell lines, immune cells, etc.) will direct the cell's fate and determine the ultimate bone repair and regeneration effect [[13], [14], [15], [16]]. In natural bone tissues, the biological behavior of bone-tissue cells is regulated by extracellular matrix (ECM) interactions. Organic components, inorganic components, and soluble bioactive factors in the ECM are crucial in regulating the cell's fate [17,18]. Thus, it is reasonable, and feasible, to promote cellular function and bone regeneration by mimicking the ECM of natural bone tissue [19].
In addition, the purpose of the application of BTE scaffolds is to promote bone defect repair. Researchers must realize that the ECM in the defect site is not completely consistent with the physiological environment, and that it is related to the destruction and reconstruction of the structure and function of bone tissues, involving a cascade of biological events [[20], [21], [22], [23]]. Therefore, when designing BTE scaffolds, researchers should first gain a deep understanding of this special biological environment, from which they can draw inspiration to create scaffolds that promote the natural bone-healing process. Understandably, cognition of the physiological environment of bone tissue and the mechanism of bone healing is the cornerstone of a well-designed scaffold. An in-depth understanding of bone biology will also provide feasible ideas for the development of next-generation BTE scaffolds.
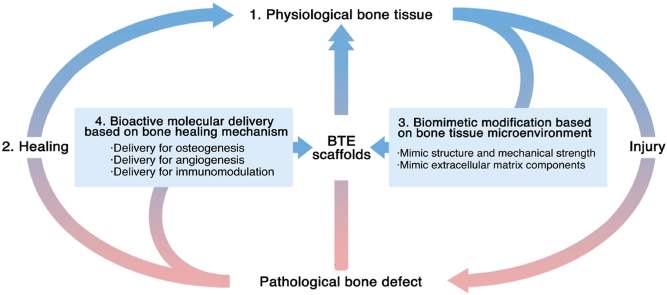
In this review, we first recapitulate the hierarchical structure of the natural bone tissue and focus on the bone microenvironment that provides biochemical, physical, and mechanical signals for bone tissue cells. We then introduce the bone development and healing process, and summarize the underlying bioactive factors. Based on this, we present an exhaustive overview of current biomimetic and bioinspired scaffolds based on the bone physiological microenvironment and the delivery of bioactive factors based on the bone-healing mechanism. We not only focus on the recent progress in the field of BTE but also discuss the current limitations and problems that still need to be solved. Accordingly, we propose feasible improvement measures. At the end of this review, we conclude with an insight into the future perspectives for designing next-generation scaffolds. This review uniquely incorporates the current knowledge of the natural bone microenvironment and the bone-healing mechanism into the design of promising BTE scaffolds, which will provide cues for researchers and clinicians in the field to design more clinically valuable BTE scaffolds.
1. Natural bone microenvironment: components and its functions
The mature lamellar bone has a hierarchical and anisotropic structure. The natural bone tissue with this structure can be divided into multiple consecutive research levels. In 1988, Weiner and Wagner first divided the natural bone tissue into seven hierarchical levels. Since then, classification methods with four to nine hierarchical levels have been proposed [24,25]. These methods aim to better explain the bone structure from the whole-bone scale to macroscale and nanoscale [25]. Researchers in different fields focus on different hierarchical levels. In the field of BTE, the scaffold structure of bone tissue and the ECM microenvironment with biological functions have drawn research interest. This section provides a detailed overview of the bone support structure (macroscopic view) and bone-tissue microenvironment (microscopic view), in an attempt to provide ideas for designing BTE scaffolds.
1.1. Mechanical scaffold of natural bone tissue
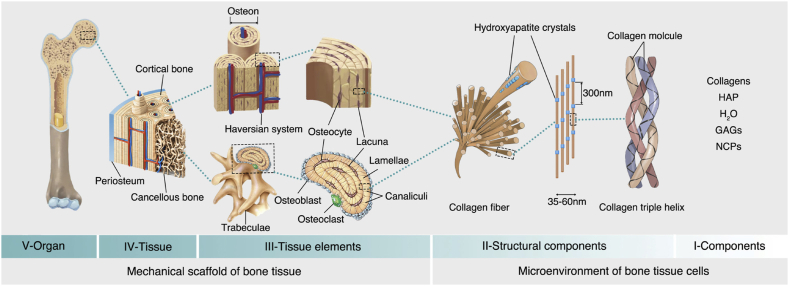
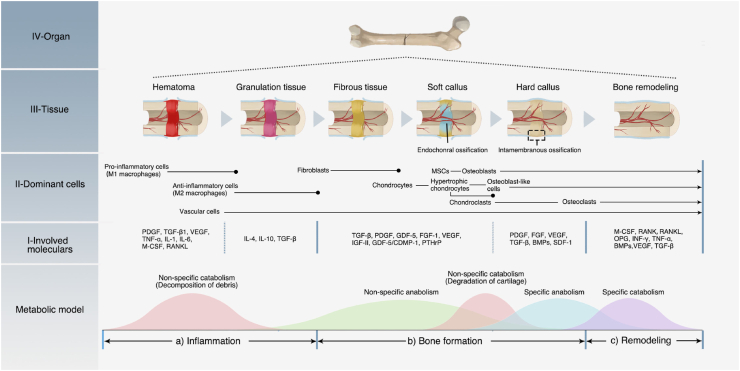
The structure that supports the lamellar bone is primarily composed of a dense cortical bone and a spongy cancellous bone. The cortical bone has a high mechanical strength and encomposses the cancellous bone on the periphery, which has a stabilizing and supporting effect. In contrast, the cancellous bone has a low mechanical strength and is a light and porous structure that provides a suitable space for bone metabolism and hematopoietic function, besides facilitating the transmission and support of multidirectional forces during bodily movement [28]. A table in the review by Wang et al. summarized the mechanical properties of natural bone tissue [29]. The cortical bone and the cancellous bone are inconsistently distributed among different anatomical parts [30], and there is a gentle transition between the two structures [27]. On the micron level, the cortical bone is composed of osteons arranged along the long axis of the bone. An osteon, which is a functional unit of bone tissue, is composed of the lamellar bone arranged in a concentric circle. Nerve fibers and blood vessels pass through the bone to form a Haversian system. As for the cancellous bone, anisotropically arranged rod-like trabecular bones form a honeycomb-like network. The space between the trabecular bones is filled by blood vessels and bone marrow, which is where hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) are found [31]. Such natural bone scaffold structures with strong mechanical properties and porosity form a good template for BTE scaffold design. The biomimetic application of the scaffold structure will be elaborated in the next section. The hierarchy of the bone structure is presented in Fig. 1.
Fig. 1.
Bone hierarchical structure. The V, IV, and III levels construct the mechanical support structure for bone tissues, and the II and I levels construct the microenvironment for the bone tissue cells. These structures and components provide a biomimetic template for BTE scaffolds. HAP: hydroxyapatite; GAGs: glycosaminoglycans; NCPs: non-collagenous proteins. This Fig. is adapted from Refs. [[26], [27], [28], [29]].
1.2. Extracellular microenvironment of bone-tissue cells
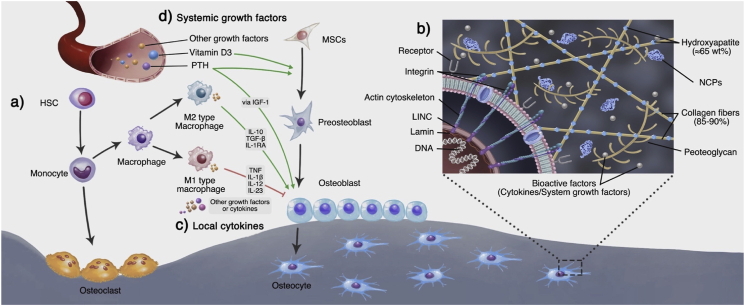
Cortical bone and cancellous bone provide a supportive and protective space for the growth of bone-tissue cells. These cells are mainly composed of osteoblastic and vascular niche, including HSCs and MSCs [31], and are found in the bone marrow. Between them, HSCs are mainly responsible for the formation of blood vessels and immune cells besides contributing to the formation of osteoclasts [32]. MSCs are primarily responsible for the formation of mesenchymal lineage cells, including osteoblasts, chondroblasts, adipocytes, and other stromal cells (Fig. 2a). These cells are essential for the maintenance of the physiological homeostasis of bone tissue and for the regeneration of bone defects [31]. MSCs build the ECM, and in turn are regulated by the ECM. They interact with the ECM through a variety of protein receptors on the latter's surface, such as integrin, selectin, and immunoglobulin. The binding of cell receptors and multiple ECM ligands not only affects cell adhesion to the ECM but also transmits various signals to the cells [33,34]. As a highly dynamic and complex network, the ECM mainly regulates the biological behavior of cells by the following mechanisms: 1) Provide cells with biochemical signals; 2) Provide cells with physical and mechanical signals [28]. The following subsections provide a detailed overview of the composition and function of the bone extracellular microenvironment from these two aspects.
Fig. 2.
Bone-tissue cells and extracellular matrix. a) In the bone marrow, HSCs and MSCs adjacent to the blood vessels can differentiate into osteoclasts and osteoblasts, which are directly involved in bone formation and resorption. b1) Osteoblasts can secrete the ECM and participate in its mineralization. They are gradually wrapped in new bone and differentiate into bone cells. In this process, they continuously receive biological signals from the components and biomolecules of the ECM. b2) The cells can also receive mechanical signals through sensors like integrins on the cell membrane. The integrin can adhere to the collagen of the ECM to form focal adhesions. The focal adhesions anchor the actin cytoskeleton, which links the LINC complex at the nuclear membrane. The LINC complexes interact with the lamins in the nuclear membrane, which finally transmits the mechanical signals to the nucleus and regulates the gene expression. c) In addition to osteoblasts and osteoclast cell lines that directly are involved in bone remodeling, the blood vessel growth brings endothelial cell lines. HSCs can also differentiate into the immune cell line. These cells communicate through cytokines and maintain the homeostasis of bone tissue. For example, as the most widely studied immune cells, macrophages can regulate osteoblast functions via inflammation-related cytokines. Generally, M1-type macrophages mainly inhibit osteogenesis, while M2-type macrophages mainly promote osteogenesis; d) Apart from the local regulation, bone homeostasis is also regulated by other organs through growth factors in the endocrine system.
1.2.1. Extracellular matrix provides biochemical signals to cells
The main component of the ECM is collagen fibers (85–90%), which are periodically arranged by collagen fibrils with a diameter of approximately 35–60 nm and a length of 1 μm. Collagen fibrils are formed by the self-assembly of unique triple helical biomolecules [28,35] (Fig. 1). Hydroxyapatite crystals with superior anisotropic mechanical properties are mineralized in the interstices of these collagen fibers in a controlled biomineralization process, which involves more than 200 different acid proteins constituting the main inorganic component of bone tissue (approximately 65 wt %) [[36], [37], [38]].
As a component for strengthening the mechanical properties of collagen fibers, hydroxyapatite can increase the collagen matrix tensile modulus by four times and induce superior energy dissipation and fracture resistance properties in bone tissue [38]. In addition to collagen fibers, the ECM contains a considerable quantity of non-collagenous proteins (NCPs), which include osteocalcin, osteonectin, osteopontin, adhesion proteins (e.g., fibronectin and vitronectin), and proteoglycans (e.g., versican, decorin, and hyaluronan) [34] (Fig. 2b1). They are not only involved in the construction of the ECM, such as the collagen mineralization process, but also participate in the regulation of the cell fate of bone tissue [[39], [40], [41]]. A summary of ECM proteins, including the type, location, biological function, ligation site, and application can be found in the review by Lopes et al. [28]. We will introduce the application of these biochemical signals in BTE scaffolds in Section 4.
In addition to direct interaction with cells, the ECM can also serve as a platform to deliver soluble biomolecules secreted from various cells, including cytokines (e.g., growth factors and immunomodulatory factors) and hormones, thereby regulating the biological behavior of cells in the microenvironment (Fig. 2c). These soluble biomolecules are an important medium for communication between cells and play an important role in the homeostasis of the cell microenvironment [42]. As for cytokines, growth factors are a type of cytokines that can induce the proliferation and differentiation of pluripotent cells, which mainly include platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), insulin-like growth factor (IGF), and growth and differentiation factor (GDF); they play an important regulatory role in tissue regeneration [42]. For example, TGF-β participates in the control of bone-tissue homeostasis and remodeling, and the widely concerned cytokine bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily.
It is well known that BMP-2, -4, -5, -6, and -7 can bind with type I and type II serine-threonine kinase receptors and activate the Smad and MAPK pathways, inducing significant osteogenic effects [43]. In another example, VEGF is a key growth factor that has been widely studied. It can mediate the formation of blood vessels and play a regulatory role in vascular development and bone remodeling [44,45]. Other growth factors also play a crucial role in the regulation of bone-tissue homeostasis, detailed summaries of their functions have been provided by recent reviews [42,46]. In addition to the growth factors involved in osteogenesis and angiogenesis, there are cytokines such as apoptosis and osteoclastogenesis that are involved in catabolism.
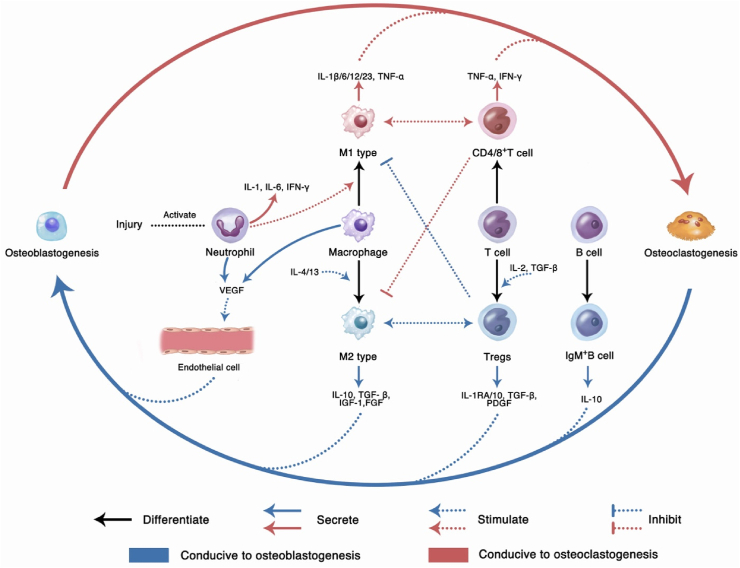
In this dynamic balance between osteoblastogenesis and osteoclastogenesis, immune cell lines derived from HSCs in bone tissues, including T cells, monocytes, and macrophages, play a key role. For example, M1-and M2-type macrophages can affect the balance by secreting inflammation-related cytokines [47]. M1 macrophages are regulated by the local microenvironment and can be activated by Toll-like receptor (TLR) ligands and interferon-gamma (IFN-γ). They mainly secrete pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL‐1β), IL‐12, and IL‐23, which can promote inflammation and inhibit the synthesis of ECM. Meanwhile, M1 can also secrete IL‐6 and IL-11 to activate glycoprotein 130, promoting the osteogenic differentiation of MSCs [48]. M2 macrophages can be activated by IL-4 and IL-13 and mainly secrete anti-inflammatory cytokines, including IL-10, TGF-β, and IL-1RA, which can inhibit inflammation and promote osteogenesis, angiogenesis, and other anabolism [47,49,50].
These cytokines are both the components of bone-tissue microenvironment and the regulators of bone-tissue homeostasis and repair. Although they have their own unique biological effects, cytokines coordinate with multiple cells to perform bone-tissue homeostasis. In Section 3, we will elaborate on how these cytokines take effect in an orderly manner in bone development and defect repair. In addition, the delivery of cytokines through scaffolds to manipulate the bone repair effect is an area of interest in the field of BTE [51]. In Section 5, we will discuss the rational application of cytokines in BTE scaffolds.
Another type of biomolecule involved in the regulation of bone-tissue homeostasis, hormones are different from the local regulatory cytokines. Hormones are generally secreted by system endocrine organs, meanwhile, the expression and release of the local and system biomolecules are coordinated. The major hormones involved in the regulation of bone-tissue homeostasis include parathyroid hormone (PTH), calcitonin, and vitamin D3 [1,25(OH)2 vitamin D3]. (Fig. 2d). They mainly respond to changes in Ca levels in the blood circulation and participate in the regulation of systemic bone-tissue homeostasis [46]. Although this part is not the focus of this review, the understanding of these systemic biomolecules can lay a reliable foundation for the treatment of bone defects accompanied by endocrine disorders. Siddique et al. [33] presented an exhaustive overview of how the systemic factors regulate the physiological bone remodeling.
1.2.2. Extracellular matrix provides physical and mechanical signals to cells
In addition to providing biochemical background, the ECM also provides physical and mechanical cues for cells, including endogenous and exogenous stresses [52]. The normal mechanical environment is important for maintaining the differentiation potential of stem cells and regulating their differentiation direction [53]. Endogenous stress mainly comes from the physical and mechanical properties of the ECM, including the topological structure and substrate stiffness. Among them, the matrix stiffness is a widely reported form of endogenous stress, which can regulate the stem cell differentiation independently of protein tethering and matrix porosity [54]. At different microenvironmental matrix stiffnesses, the expression level of the nucleoskeletal protein lamin-A/C in the stem cell nucleus is exhibited in a logarithmic dose-dependent manner, which in turn regulates the differentiation fate of stem cells such as the adipogenic differentiation in the soft matrix and the osteogenic differentiation in the hard matrix [[55], [56], [57], [58]] (Fig. 2b2).
Topology is another important source of endogenous stress, which can regulate the osteogenic and osteoclastic effects of bone tissue [52,59]. In this regulatory mechanism, the Yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) of the Hippo pathway are essential sensors and mediators of mechanical cues, which respond to the topological and stiffness signal of the ECM. The distribution of YAP in the cells is biphasic and can be transferred from the cytoplasm to the nucleus as the mechanical signal increases, thereby affecting the differentiation of MSCs [60,61]. The degree of mineralization of collagen and the size of hydroxyapatite crystals directly affect the stiffness of the ECM and the morphology of the cell contact surface. They are important sources of endogenous stress and can affect the adhesion, morphology, migration, and differentiation of MSCs [[61], [62], [63], [64]].
Several studies have confirmed that the topological structure formed by hydroxyapatite can provide geometrical cues for the in vitro osteogenic differentiation of MSCs [61,[65], [66], [67], [68], [69]]. In fact, the trabecular structure of the natural cancellous bone undergoes topological changes with physiological or pathological alterations, such as the conversion between plates and rods, or even gets disconnected [70], which means that the endogenous stress of the ECM is dynamic. In the future, deepening the understanding of the ECM topology and geometrical cues can lay a foundation for BTE on which researchers can produce scaffold structures that mimic the cellular microenvironment at the nanometer level.
The exogenous stress is another type of mechanical signal. Various bodily movements can produce mechanical and physical signals on the bone tissue, which can be transmitted to the cells via the ECM. Meanwhile, the tissue fluid in the ECM produces hydrostatic and shear stresses that act directly on the cells. These normal exogenous stresses play an important role in facilitating bone formation and maintaining bone-tissue health [71,72]. As early as 1982, Wolff proposed that bone tissue grows and remodels in response to the mechanical environment throughout its life [73].
In 1987, Frost proposed the mechanostat theory, demonstrating that the physiological stress ranges between and 300 and 1500 microstrains. Stresses above 1500–3000 microstrains would contribute to osteogenesis, whereas stresses below 100–300 would cause bone resorption [74]. However, cells in the bone tissue do not directly respond to the bone stress, as described by Frost, they receive microstructural stresses from the lacunae and microcracks in the ECM. These stresses can be amplified at the cellular level and may regulate the osteogenic differentiation of cells [75]. In addition, when bone tissue bears mechanical stress, it presents as compressive stress on one side and tensile on the other. Scientists in general agree that cyclic tensile stress is related to the osteogenic effect of MSCs, while sustained compressive stress is related to the osteoclast effect [57,[76], [77], [78], [79], [80]]. This knowledge has been applied to the clinical practice of orthodontic tooth movement and distraction osteogenesis.
Fluid shear stress is another type of exogenous stress from the ECM. Compared with most soft tissues, it has a greater impact on bone-tissue cells [81]. The interstitial fluid distributed in the ECM provides nutrients to cells and remove metabolic waste. During bodily movement, the flow rate of interstitial fluid in bone tissue changes due to changes in the surrounding blood pressure and mechanical load [82]. When bone tissue undergoes deformation due to mechanical load, the interstitial fluid flows from the compressive stress area to the tensile stress area through the channels where bone cells live (e.g., canaliculi), and generates fluid shear stress (0.8–3 Pa) [83]. Several in vitro studies demonstrated that the osteoblast cell lines in bone tissue, including MSCs, osteoblasts, and osteocytes, respond to the mechanical stimulation of fluid stress and regulate osteogenic differentiation in a dose-dependent manner [72,84,85].
A recent study demonstrated that fluid stress was also involved in osteoclast differentiation. Fluid shear stress with low stimulus amplitudes can activate Piezo1 and sarcoplasmic/endoplasmic Ca2+ reticulum ATPase 2 (SERCA2), reduce extracellular adenosine triphosphate (ATP), and inhibit osteoclastogenesis and bone resorption, whereas a high stimulus stress can induce hematopoietic progenitor cells to differentiate into osteoclasts [86]. In addition, fluid shear stress is also involved in the regulation of the ECM morphology, the angiogenic differentiation of stem cells, and angiogenesis [83,87], and could be an important exogenous stress signal in the microenvironment of bone-tissue cells.
However, the roles of perfusion shear stress and in vivo interstitial flow are still unclear, since the channel geometries in bone tissue are constantly remodeling. Moreover, the lack of knowledge on the tube wall properties has led to difficulties in in vivo research [81]. Therefore, it is unrealistic to directly apply fluid shear stress to BTE in vivo. In recent years, in vitro finite element analysis and computational fluid dynamics analysis have brought new ideas for the applications of fluid shear stress to the BTE scaffold design. Based on micro-CT scans and finite element models, Hendrikson et al. found that the fluid shear stress in BTE scaffolds was mainly affected by the pore shape and size of the scaffold, whereas the mechanical strain distribution was affected by the design of the columnar support structure. These parameters indirectly affect the osteochondral cell differentiation [88,89]. Meanwhile, Ali et al. found that the surface roughness of a scaffold affected the internal fluid shear stress [90]. These studies indicated that controlling the scaffold structure could regulate the fluid shear stress on cells. They also highlighted the importance of gaining a comprehensive understanding of the bone-tissue microenvironment, on the account of the change in one parameter in the scaffold design, such as surface topology, will have a multi-biomechanical effect on the cells in the scaffold, including endogenous stress and fluid shear stress.
In the next section, we shall focus on the dynamic balance of bone tissue, including its physiological homeostasis and the healing process of bone defects, to provide more comprehensive guidelines for the BTE scaffold design.
2. Natural bone-healing process and mechanism
The postnatal bone tissue has a strong ability to maintain homeostasis and is constantly rebuilding in the dynamic balance of bone resorption and regeneration. Once a bone defect has occurred, the bone may begin to heal itself. Although the healing process of bone defects is different from the process of bone development, their osteogenesis forms are similar, e.g., intramembranous bone formation and endochondral ossification [91]. Providing insight into the bone development mechanisms and bone defect healing process is the basis for designing BTE scaffolds. In this section, we first outline the process of embryonic bone morphogenesis, and then elaborate on the bone-healing process initiated from the cellular cascade.
2.1. Intramembranous bone formation and endochondral ossification
Intramembranous bone formation commonly occurs in the development of flat bones such as the craniofacial bones and the clavicle. It is the process by which stem cells directly differentiate into osteoblasts to form new bones [92]. Adult bone stem cells generally are believed to originate from the bone marrow mesenchyme. Recently, Debnath et al. discovered a periosteal stem cell that exhibits multipotency and self-renewal ability, playing a key role in intramembranous bone formation. Inhibiting the osteogenic differentiation of such stem cells can cause selective impairments in the cortical bone structure and defects in fracture healing [93].
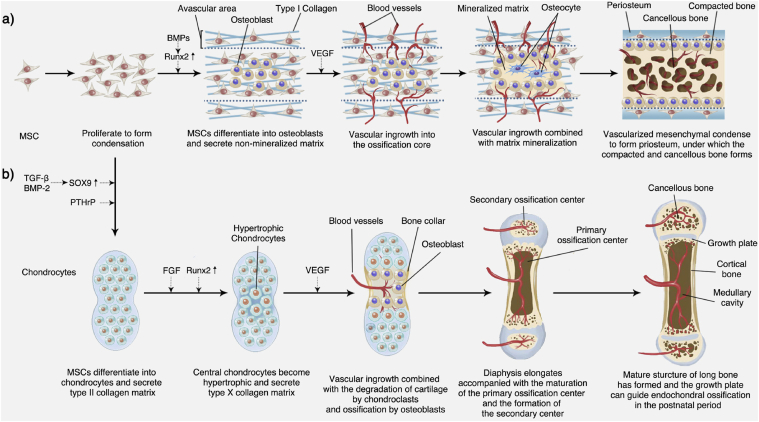
Similar to endochondral ossification, intramembranous ossification initiates from the proliferation of pluripotent stem cells, which will further migrate into clusters to form condensation. As the stem cells proliferate, the condensation expand outward. The expanding condensation are the center of osteogenic differentiation and participate in the formation of early bone morphology. The MSCs around the condensation area are loosely arranged to form a thin avascular area, and the stem cells inside condensations are induced by cytokines, such as BMPs, which upregulate the expression of Runx2 to differentiate into osteoblasts. Differentiated and mature osteoblasts can secrete non-mineralized type I-collagen-rich osteoids to the surrounding area. Before osteoid mineralization is affected by angiogenesis cytokines (e.g., VEGF), small bore capillaries move into this avascular layer and then invade the condensation approaching the ossification core [94].
Subsequently, accompanied by vascular invasion, the ECM continuously forms and mineralizes. This vascularization process in intramembranous ossification was described in detail by Percival et al. [92]. Finally, the osteoblasts are encapsulated in the mineralized ECM and differentiate into osteocytes. The vascularized mesenchymal condensed around the mineralized matrix transforms into the periosteum, and the trabecular bone under the periosteum is compacted to form a compact bone. The ossification core is highly vascularized to form cancellous bone and a narrow cavity [28]. A schematic representation of the intramembranous bone formation process is depicted in Fig. 3a.
Fig. 3.
Embryonic bone development. a) Intramembranous bone formation; b) Endochondral ossification.
Endochondral ossification commonly occurs in the development of long bones and is initiated by the condensation of stem cells. However, unlike intramembranous ossification, these stem cells first differentiate into chondrocytes. In the early stage of endochondral osteogenesis, stem cells are induced by cytokines (e.g., TGF-β and BMP-2), upregulating the expression of SOX9 to condense and differentiate into chondrocytes [95,96]. Chondrocytes in the condensation area are stimulated by the parathyroid hormone-related protein (PTHrP) secreted by the perichondrium to continuously proliferate and secrete type II collagen [97]. As the cartilage matrix continues to expand, the chondrocytes in the central area are affected by cytokines (e.g., FGF), stop proliferation, and become hypertrophic [98,99].
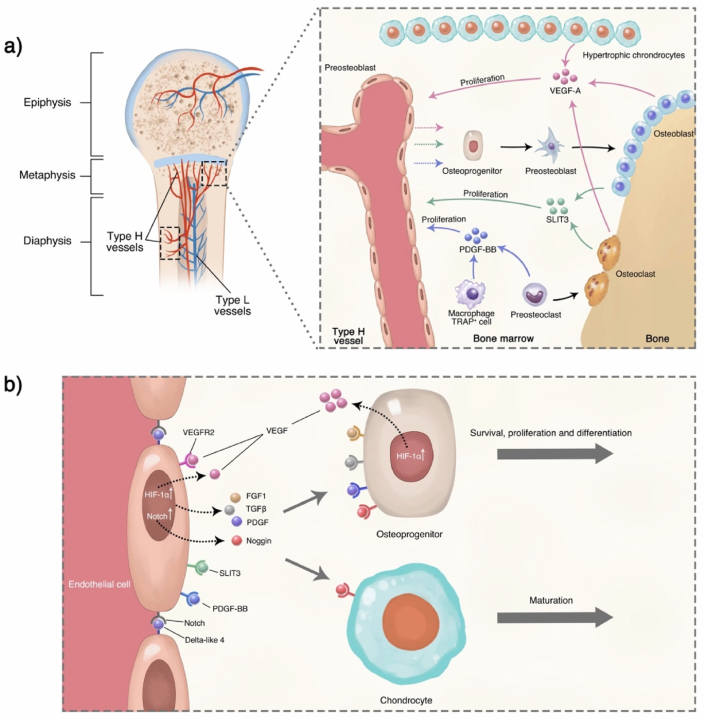
Hypertrophic chondrocytes can secrete cartilage matrix mainly composed of type X collagen and release CaP granules, which become cores for the growth of apatite microcrystals [91]. Perichondrial cells at the edge of the condensation area are induced by hypertrophic chondrocytes to differentiate into osteoblasts and then form the bone collar. Meanwhile, hypertrophic chondrocytes also secrete cytokines, such as VEGF [44], which induce chondroclasts to participate in the blood vessel and osteoblast invasion. Recently, Romeo et al. identified a non-bone-resorbing osteoclast subtype termed vessel-associated osteoclast (VAO) that is dispensable for cartilage resorption and the anastomoses of type-H vessels. During this process, H-type endothelial cells at the bone/cartilage interface support VAOs through receptor activator of nuclear factor-kappa B ligand (RANKL)-RANK signaling [100]. The role of H vessels in bone regeneration will be elaborated in Section 5.2.
After passing through the perichondrial membrane, the peripheral vascular endothelial cells enter the cartilage matrix area and form a highly vascularized ossification center. With the apoptosis of hypertrophic chondrocytes, the cartilage matrix forms the primary spongiosa. In the primary spongiosa, the hypertrophic chondrocytes located at the “borderland” between cartilage and (non-cartilage) osteogenic tissues undergo further differentiation into osteoblast-like cells, and together with osteoblasts secrete type I collagen to form the cancellous bone matrix [101]. Osteoblasts in the bone collar area form the cortical bone. Both ends of long bones undergo hypertrophy of chondrocytes, vascular invasion, and osteogenic activity to form secondary ossification centers. The area between the primary and secondary ossification centers is transformed into a growth plate, which is a reservoir for storing chondrocytes and can continue to guide the growth of long bones via endochondral ossification in the postnatal period. Although it is not the focus of this review, endochondral osteogenesis is an important form of bone regeneration. Kronenberg provided a detailed summary of this process and the underlying molecular mechanism [98]. A schematic representation of the endochondral ossification process is illustrated in Fig. 3b.
In the next section, we shall elaborate on the bone-healing process involved in intramembranous and endochondral ossifications, as well as its potential cytokine regulation mechanism.
2.2. Process and mechanism of bone healing
Maintaining the structure and function of the skeletal system is the basis of bodily movement, and it also has an important protective effect on the fragile internal organs. Once it has been pathologically damaged (especially due to trauma), the damaged part of the bone tissue activates a cellular cascade to participate in an orderly healing process, greatly reconstructing the bone structure and function without leaving scars [102]. This regeneration process recapitulates multiple morphogenesis events of embryonic bone development, as described above. However, bone repair is usually induced by pathological trauma, and some of its aspects are distinct from natural bone development, including the obvious inflammation stage and the long-term reconstruction process. In the following paragraph, we shall provide a detailed overview of the bone-healing process, including three stages: 1) inflammation, 2) bone formation, 3) remodeling, and the potential cytokine regulation mechanisms are also discussed.
2.2.1. Inflammatory phase
When a bone tissue is injured, the blood vessels inside the bone defect disrupt. The blood enters the bone defect area, forming a hematoma. Fibrin in the hematoma coagulates to form a provisional matrix, which contains components from the blood, bone tissue, and surrounding tissues. Inside the defect, macrophages and their recruited immune cells (granulocytes, lymphocytes, monocytes, etc.) mediate inflammatory reactions, playing the most crucial role in this stage. They secrete a range of cytokines spatiotemporally to trigger inflammation. During this process, the inflammatory cytokines mainly play a role in recruiting and regulating cells involved in the healing process. They also 1) recruit other inflammatory cells through a positive feedback loop to amplify the inflammatory response, clean up bone-tissue fragments, and fight infection; 2) recruit endothelial cells to participate in the formation of new blood vessels and invasion; 3) recruit and promote fibroblast proliferation, promote fibrosis of the fibrin clot, and improve stability; and, 4) recruit multipotent MSCs and regulate their proliferation and differentiation [103,104].
In the early stage of inflammation, various components of the hematoma participate in the healing regulation. Platelets in the hematoma degranulate and release PDGF and TGF-β1. PDGF promotes the proliferation of MSCs, fibroblasts, osteoblasts, and endothelial cells [105], and TGF-β1 recruits MSCs [106]. Stimulated by the ischemic and hypoxic environment of the hematoma, as well as the macrophage colony stimulating factor (M-CSF) and RANKL secreted by the osteoblast cell line, macrophages polarize to M1 type [107,108]. M1 type macrophages can secrete a variety of pro-inflammatory cytokines, including TNF-α, IL-1, IL-6, etc., recruiting inflammatory and repair-related cells (e.g., fibroblasts, MSCs, and osteoprogenitor cells), and release angiogenic factors to promote angiogenesis [[109], [110], [111]]. In addition, the hypoxic environment can induce the up-regulation of the transcription factor hypoxia-induced factor-α (HIFα) in osteoblasts and endothelial cells, which can indirectly promote the release of angiogenic factors, including angiopoietin-1 and VEGF, to enhance the revascularization process [112]. When new blood vessels invade, various cellular components respond to chemokines and accompany the blood vessels to enter the bone defect area. Subsequently, the fibrin matrix transforms into granulation tissue. With revascularization and cell recruitment, the hypoxic–ischemic extracellular environment is relieved. Macrophages transform from M1-type polarization to M2 type [113], and secrete anti-inflammatory cytokines, including IL-4, IL-10, and TGF-β. Gradually, the inflammatory reaction is weakened, the catabolism transforms into anabolic metabolism, and the bone healing enters the next stage. This process is depicted in Fig. 4a.
Fig. 4.
Process and mechanism of bone healing. a) Inflammation phase; b) Bone formation phase; c) Remodeling phase. Bone healing is a dynamic and continuous process accompanied by an alternating metabolic model. In each phase, different cells and cytokines play the dominant roles.
2.2.2. Bone formation phase
When the inflammatory phase is completed, bone tissue gradually enhances the anabolic and repair processes [103]. In this phase, the anabolism is dominated by fibroblasts, chondrocytes, and osteoblasts. The cellular components involved in bone regeneration in the granulation tissue include fibroblasts, osteoprogenitor cells, and MSCs. Osteoprogenitor cells and MSCs are stimulated by cytokines and hypoxic environments to proliferate, concentrate, and differentiate. Fibroblasts proliferate and secrete a fibrous matrix, gradually replacing the granulation tissue with a fibrous tissue of enhanced mechanical strength. In the process of gradual maturation of the connective tissue, type III collagen becomes the main component of the ECM [114], providing a template for subsequent bone regeneration. During the bone-healing process, four specific repair environments are formed according to the location of the bone defect, including the cancellous bone area, cortical bone area, periosteum area, and surrounding soft tissue area. The cancellous and inter-cortical bone areas are repaired by endochondral ossification, while the subperiosteal area and the adjacent soft tissue areas are repaired by intramembranous ossification [115].
In the cancellous and inter-cortical bone areas, MSCs recruited from the bone marrow, periosteum, adjacent soft tissues, and peripheral circulation [114] aggregate and proliferate. They further differentiate into chondrocytes and secrete a semi-rigid avascular cartilage matrix, gradually transforming the fiber-rich granulation tissue gradually into a fibrocartilage- and hyaline cartilage-rich soft callus. The early soft callus is mainly composed of type II collagen and proteoglycan core biomarkers, which are gradually replaced by type X collagen as the callus matures. When chondrocytes secrete a matrix to the ECM and induce mineralization, they also release VEGF to promote new blood vessel ingrowth and cooperate with BMPs to enhance the bone-healing effect [116,117]. During vascular invasion, endothelial cells, osteoblasts, and chondrocytes accompanying new blood vessels secrete matrix metalloproteinases (MMPs) to degrade the cartilage matrix. Upon completion of the revascularization, mature hypertrophic chondrocytes undergo apoptosis or differentiate into osteoblast-like cells, which, together with osteoblasts, secrete type I collagen and participate in the ECM mineralization. Finally, the soft callus is transformed into a hard callus with the disordered trabecular bone [114]. This process recapitulates endochondral ossification during embryonic bone development.
During the establishment and maturation of the soft callus, growth factors (e.g., TGF-β, PDGF, GDF-5, FGF-1, and IGF-II) and hormones (e.g., PTHrP) are involved in the recruitment and proliferation of fibroblasts and MSCs. They also play an important role in inducing the MSC differentiation into osteoblasts or chondrocytes [114]. Among them, TGFs can recruit MSCs, pre-osteoblasts, chondrocytes, and osteoblasts. TGF-β2 and TGF-β3 reach peak expression during cartilage formation and participate in chondrogenesis and endochondral ossification [118,119]. As mentioned above, BMPs are also members of the TGF-β family. They are essential cytokines that initiate intramembranous and endochondral ossification, and induce healing cascades and possibly callus formation. BMPs not only promote bone formation but also stimulate the production of VEGF-A, which in turn activates endothelial cell angiogenesis [120]. In addition, the GDF-5/cartilage-derived morphogenetic protein-1 (CDMP-1) of the BMP family plays a regulatory role in promoting endochondral ossification [121].
Bone defects in the subperiosteal and adjacent soft tissue areas are repaired by intramembranous osteogenesis. The periosteum, as the outer surface of bone tissue, consists of two distinct layers. The outer layer is a fibrous layer composed of fibroblasts, collagen, and elastin fibers. The inner layer is a cambium layer, which is a rich source of undifferentiated pluripotential mesenchymal cells, differentiated osteoprogenitor cells, osteoblasts, and fibroblasts [122]. Once the periosteum is injured, the cells of the cambium layer proliferate, in response to inflammation, thickening the periosteum. This is called the periosteum reaction. After 7–10 days of bone injury, the repaired periosteum initially forms and stabilizes the bone defect.
The cells involved in intramembranous bone formation mainly include osteoprogenitor cells present in the periosteum and MSCs recruited from the periosteum, bone marrow, adjacent soft tissues, and peripheral circulation. Undifferentiated MSCs release BMPs to promote angiogenesis, chemotaxis, mitogenesis, and induce cell differentiation into osteoblasts and chondroblasts [123]. With angiogenesis and osteoblast secretion and mineralization of the ECM, a hard callus is formed directly under the periosteum. Meanwhile, the vascularized matrix outside the hard callus condenses to repair the periosteum. This process recapitulates the embryonic bone development process of intramembranous ossification, as described above. The repair of the periosteal area is of great significance for stabilizing and protecting the internal bone defect area.
A series of cytokines are involved in the healing process of the subperiosteal bone, including PDGF, FGF, VEGF, TGF-β, BMPs, and stromal cell-derived factor-1 (SDF-1). Among them, SDF-1, TGF-β, and BMPs directly participate in the recruitment of MSCs and induce their osteogenic differentiation. FGF is involved in the proliferation of periosteal cells and bone progenitor cells, and promotes callus and bone formation [113,122,124].
Soft callus formation is a nonspecific anabolic process, whereas hard callus formation is a specific anabolic process (Fig. 4b). After this healing phase, the healing area can regain sufficient mechanical properties to support low-intensity functional exercise. To further restore the normal functional structure and mechanical strength, the healing of bone tissue enters the next stage.
2.2.3. Bone remodeling phase
Bone remodeling is the last phase of bone healing, and is also known as the secondary bone formation, which includes the reconstruction of the hard callus into the lamellar bone and the change of configuration of the cancellous bone trabeculae. As the new bone gradually supports the functional movements, the irregular woven bone responds to mechanical stimulation and remodels through an orderly bone resorption and osteogenesis process [109]. Thus, the crosstalk between osteoblasts and osteoclasts plays an important role in this phase. Mature osteoblasts can balance bone formation and bone resorption by secreting M-CSF and the RANKL [125]. M-CSF and RANKL can bind to c-FMS and RANK on the surface of HSCs, respectively, to promote the differentiation of HSCs into osteoclast cell lines [126]. The OPG secreted by osteoblasts can competitively bind to RANKL, thereby inhibiting the differentiation effect of osteoclasts. Meanwhile, osteoclasts can release extracellular vesicles containing RANK on their surface to bind RANKL on the surface of osteoblasts, which triggers the production of proteins such as Runx2 to promote bone formation [127]. This crosstalk is presented in Fig. S1. In addition to this specific regulatory mechanism, other cytokines are also involved in the regulation of this phase, including interleukins, INF-γ, TNF-α, BMPs, VEGF, and TGF-β. The cytokines involved in the different phases and their functions are summarized in Table 1. From the table, we derive the following knowledge: 1) bone repair is a repair process led by a variety of cells that coordinate their functions through cytokines; 2) the bone-healing process is a continuous process, which can be roughly divided into multiple phases. These phases overlap with each other and are mediated by a variety of cytokines, which can have synergistic or antagonistic effects. In general, these cytokines can be divided into two types that participate in anabolism and catabolism; 3) the same cytokine can take effect in one or more healing phases, and these effects can be similar or opposite. Thus, in the process of bone healing, cytokines form a complex yet orderly network.
Table 1.
Cytokines involved in bone healing process.
| Cytokine | Phase | Expression | Main source/location | Function |
|---|---|---|---|---|
| TNF-α | Inflammatory phase | Increase and reach peak in 24h (Mouse) [128] | M1 type macrophages; Recruited immune cells | Induced by platelet hyperreactivity, recruit megakaryocytes and induce inflammation [129]; Recruit MSCs and enhance its proliferation and differentiation, but inhibit bone matrix synthesis at the injury site [[130], [131], [132]] |
| Bone formation phase | Decrease [128] | M1 type macrophages; Recruited immune cells | Promote angiogenesis, and MMPs production in endochondral bone [133] | |
| Remodeling phase | Increase again on day 24 and 28 (Mouse) [128] | Macrophages | Promote osteoclast migration and differentiation [134,135] | |
| IL-1 | Inflammatory phase | Increase and reach peak in 24h (Mouse) [128] | M1 type macrophages; Recruited immune cells | Promote catabolism and the degradation of proteoglycan [136] Enhance the chemotactic effect of immune cells migration and is a central role in responses to infections [137,138] |
| Remodeling phase | Increase again on day 24 and 28 (Mouse) [128] | M1 type macrophages; Recruited immune cells |
Promote osteoclast differentiation via RANKL and M-CSF [139,140] | |
| IL-4 | Inflammatory phase | Increase | M2 type macrophages | Inhibit inflammation and bone resorption [141]; Recruit and activate osteoblasts [142]; Inhibit osteogenic differentiation [143] |
| Remodeling phase | Increase | M2 type macrophages | Inhibit osteoclast differentiation and bone resorption via the RANKL/RANK/OPG system [144] | |
| IL-6 | Inflammatory phase; Remodeling phase |
Increase in 72h (Rat) [145] | M1 type macrophages; Monocytes; Other recruited immune cells |
Promote osteoblast differentiation [146,147]; Enhance the production of VEGF to promote angiogenesis [143]; Induce osteoclast formation via gp130 [148,149] |
| IL-10 | Bone formation phase | Increase | M2 type macrophages; B-cell | Suppress the expression of TNF-α and IFN-γ to inhibit inflammation and facilitate bone formation [150] |
| INF-γ | Inflammatory phase; Remodeling phase |
Increase | T lymphocyte; NK cell | Present positive or negative effect in bone formation depending on the experimental model and conditions used: Mediate macrophage polarization to M1 releasing pro-inflammatory cytokine [151]; Inhibit osteogenesis and bone formation [[152], [153], [154]]; Inhibit osteoclastogenesis and bone resorption [[155], [156], [157]] |
| BMPs | Inflammatory phase | Increase and BMP-2 reach peak in 24h (Mouse) [118] | Osteoprogenitor cells; Osteoblasts | BMP-2 initiate the repair cascade [118]; BMP-2, 6, and 9 induce MSCs differentiation to osteoblasts [158]; Promotes differentiation of MSCs into chondrocytes and osteoblasts; |
| Bone formation phase | BMP-3, 4, 5,6,7 and 8 increase (Mouse) [118] | Osteoprogenitor cells; Chondrocytes; Osteoblasts | BMP-3, 4, 7, and 8 specifically participate in osteogenic stage, while BMP-5 and 6 engage in intramembranous and endochondral ossification [118,159]; | |
| Remodeling phase | BMP-3, BMP-4, BMP-7, and BMP-8 Increase |
Osteoblasts; Osteoclast |
BMP-2 and 4 are observed in osteoclast-like cells, as well as prerequisites for osteoclast development [[160], [161], [162]]; BMP2 enhances osteoclast differentiation [163,164] |
|
| TGF-β | Inflammatory phase; | Increase | Platelets; Bone extracellular matrix |
Recruit inflammatory cells and MSCs [165,166]; Repress the differentiation of MSCs and chondrocyte, as well as relieve catabolism [[167], [168], [169], [170]] |
| Bone formation phase | TGF-β2 and TGF-β3 reach peak on day 7 (Mouse) [118] | Bone extracellular matrix | Promotes osteogenesis and chondrogenesis [119,[171], [172], [173]]; | |
| Remodeling phase | Increase | Bone extracellular matrix | Recruit MSCs to participate in bone remodeling [106,166]; Induce the bone resorption effect of osteoclast [172,174]; Recruit and promote the differentiation of osteoblast precursors [175]. |
|
| PDGF | Inflammatory phase; Bone formation phase |
Increase | Platelets; Macrophages; Osteoblasts | Promotes migration, proliferation, angiogenesis and osteogenic differentiation of MSCs [[176], [177], [178]] |
| FGF | Inflammatory phase; Bone formation phase |
Increase | Macrophages; Mesenchymal cells; Chondrocytes; Osteoblasts | Serve as a mitogen for mesenchymal cells [179]; Promote differentiation of osteoblast and chondrocytes [180,181]; Repress the degradation of cartilage in inflammations [182]; |
| VEGF | Inflammatory phase | Increase | Endothelial progenitor cells; Mesenchymal cells; Chondrocytes; Osteoblasts | Promote macrophage recruitment and angiogenesis [183] |
| Bone formation phase | Increase | Hypertrophic chondrocytes; Osteoblasts | Recruit blood vessels and osteoclasts and promote cartilage resorption [183] Indirectly promote bone formation via BMPs [184,185] |
|
| Remodeling phase | Increase | Osteoblasts | Stimulate osteoclast formation [183] | |
| IGF-1 | Bone formation phase | Increase | Chondrocytes; MSCs |
Enhance the synthesis of cartilage matrix [159,186]; Promote MSCs differentiation into osteoblast and enhance bone formation [187,188] |
| GDF-5/CDMP-1 (Homologues of BMP family) | Bone formation phase | Increase | MSCs; Chondrocytes; Hypertrophic chondrocytes | Stimulates the expression of cartilage anabolic Sox-9 to enhance chondrogenesis [189]; Promote osteogenic differentiation and angiogenic activity of stromal cells [190]; Promote angiogenesis and indirectly enhance osteogenesis [191]; Promotion of mesenchymal cell recruitment and chondrocyte differentiation [121,192] |
| SDF-1 | Bone formation phase | Increase | Stromal cell | Recruit stem cells and encourage osteogenic differentiation and production of bone [[193], [194], [195]] |
| M-CSF | Inflammatory phase | Increase and reach the first peak on day 3 (Mouse) [128] | Immune cells; Stromal cells; Osteoblast lineage | Promote macrophage polarization into M1 type releasing pro-inflammatory cytokines [108]; Promote osteoclast survival, activation and differentiation, as well as induce bone resorption [126,[196], [197], [198]]; |
| Late bone formation phase and remodeling phase | Increase and reach the second peak on day 14 (Mouse) [128] | |||
| RANKL | Inflammatory phase | Increase and reach the first peak on day 3 (Mouse) [128] | Stromal cells; Osteoblast lineage | Promote macrophage polarization into M1 type releasing pro-inflammatory cytokines [107]; Promote osteoclast differentiation and bone resorption [126]; |
| Late bone formation phase and remodeling phase | Increase and reach the second peak on day 14 (Mouse) [128] | Stromal cells; Osteoblast lineage | Promote osteoclast differentiation and bone remodeling [125,126]; | |
| OPG | Inflammatory phase | Increase and reach the first peak in 24h (Mouse) [128] | Stromal cells; Osteoblast lineage | Negatively regulate bone resorption by osteoblast [199] |
| Bone formation phase | Increase and reach the second peak on day 7 (Mouse) [128] | Participate in the long-term anabolism of bone healing [200]; | ||
| Remodeling phase | Increased | Inhibit osteoclastogenesis via competing with RANK for RANKL [201] |
Current research on these cytokines mainly focuses on exploring their independent functions. The crosstalk between different cytokines, however, remains elusive, and should be discussed in future research. Today, the application of cytokines has become an important means to promote bone regeneration. However, first, it is necessary to scrutinize their role in natural bone healing to avoid negatively influencing the healing process. For example, Stegen et al. recently proposed that prolonged HIF-1α signaling in chondrocytes can lead to skeletal dysplasia by interfering with cellular bioenergetics and biosynthesis, which may limit proliferation and curtail collagen synthesis. This effect may contribute to other ECM-related diseases such as cancer and fibrosis [202]. Therefore, the bone-healing regulatory network will remain the focus of bone-regeneration research.
In addition, although the healing process can restore the pre-injury cellular composition, structure, and biomechanical function of damaged skeletal organs, approximately 10% of fractures do not heal normally, especially for large-volume defects exceeding the critical bone defect, which requires reasonable clinical intervention [103]. The application of BTE scaffolds has become a promising approach to solve this problem. In the following sections, we shall review the biomimetic design of a BTE scaffold based on the bone physiological microenvironment and the delivery of bioactive factors/cytokines based on the bone-healing pattern, which will provide comprehensive ideas for developing novel BTE scaffolds.
2.3. Biomimetic modification of scaffold based on bone-tissue microenvironment
The design of scaffolds is key to BTE. This design should be based on the natural tissue environment as that would be conducive to promoting natural healing and regeneration. In this section, we exhaustively review the current biomimetic BTE scaffolds according to the natural bone-tissue microenvironment described above.
2.4. Biomimicking structure and mechanical strength
At the bone defect site, the mechanical structure of bone tissue is damaged, which weakens the bone's structural strength and functions. Therefore, in the process of constructing the BTE scaffold, the first consideration should be to restore the bone morphology and mechanical structure. In this section, we review the biomimetic design of the BTE scaffold structure from both macroscopic and microscopic views.
2.4.1. Macroscopic morphological structure and mechanical properties
The primary role of tissue engineering scaffolds is to provide a platform for cells involved in bone regeneration. This platform should be conducive to blood vessel invasion, cell migration, proliferation, differentiation, and communication. The natural cancellous bone structure rich in blood vessels is an ideal template for BTE scaffolds. Therefore, biomimicry of the porous cancellous bone structure is a reasonable idea for the design of BTE scaffolds.
The parameters of the biomimetic porous structure include porosity, surface area, pore size, and connectivity [203]. Pore size has an important effect on bone repair. Large pores reduce the scaffold's surface area/volume ratio and limit the cell's proliferation space, while small pores are not conducive to cell migration and communication. Pore size ranging between 100 and 1000 μm is desirable for cellular growth, blood flux, and mechanical resistance [204]; incidentally, the pore size of current scaffolds is mainly in this range. At present, the optimal pore size of BTE scaffolds is still inconclusive, since the change in experimental conditions (e.g., scaffold materials and bone defect site) can yield different results. However, there is a tendency that small pore size (approximately 100 μm) may contribute to early cell attachment in vitro, while large pore size (400–600 μm) may be conducive to in vivo vascularization and osteogenesis [[205], [206], [207], [208]].
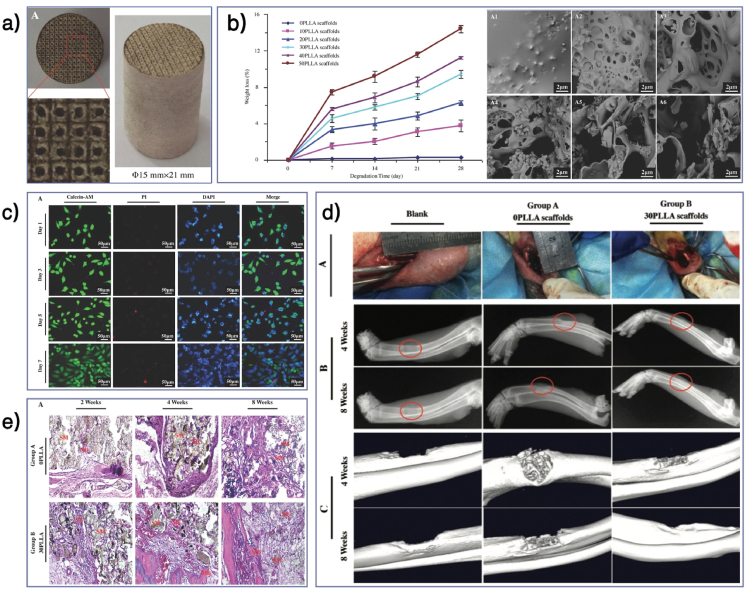
In addition to pore size, high porosity and connectivity are also critical parameters and are related to the scaffold manufacturing process. For example, additive manufacturing (AM; e.g., 3D printing) has enabled the flexible control of the morphology of scaffold materials with high precision [209,210]. Recently, Gloria et al. first reported the use of blends of poly (ε-caprolactone) (PCL) and poly (ester amide) (PEA) for the fabrication of 3D additive manufactured scaffolds. This method could control both scaffolds’ mechanical properties and morphology. Besides, the addition of PEA improved the hydrophilicity of scaffold which could further facilitate the adhesion and proliferation of human mesenchymal stem cells (hMSCs) [211]. Zhang et al. summarized the current manufacturing approaches, including the structure control and material selection of 3D-printed scaffolds [212]. In addition, mixing additional components (e.g., synthetic polymers) with the matrix material or changing the particle properties of the matrix material can also enhance porosity and connectivity [[213], [214], [215]]. High porosity and connectivity contribute to the ingrowth of blood vessels, osteogenesis, and the uniform degradation of scaffolds, albeit at the cost of weakened mechanical properties. Therefore, strengthening of mechanical properties should be another consideration.
The first step to strengthening the mechanical properties of a scaffold is to choose the right matrix material. Compared with hydrogels, synthetic polymers usually have superior mechanical properties and plasticity. Mixing reinforcing filler, such as hydroxyapatite and iron particle, can help scaffolds achieve the strength of the natural cancellous bone (compressive modulus: 10–50 MPa, elastic modulus: 0.01–3.0 GPa) [216,217]. For instance, Santis et al. fabricated a morphologically controlled scaffold with a fully interconnected pore network via 3D fibre deposition technique. Thought the addition of iron oxide (Fe3O4) or iron-doped hydroxyapatite (FeHA) nanoparticles, the PCL matrix scaffolds gained with enhanced mechanical strength and magnetic property. It's also confirmed that this PCL/Fe3O4 and PCL/FeHA nanocomposite scaffolds could promote the adhesion and spread of hMSCs [218]. Recently, the use of high-strength nanomaterials to reinforce BTE scaffolds has become a novel strategy. Shuai et al. introduced a co-dispersing Graphene oxide (GO)-Ag nanosystem in biodegradable polymer (poly l-lactic acid (PLLA)/polyglycolic acid (PGA)) scaffolds via selective laser sintering (SLS). GO is a high-strength (~130 GPa) and modulus (>0.5–1 TPa) material. It's confirmed that the co-dispersing of GO and Ag could synergistically enhance the compressive strength and modulus of the scaffolds by 102 and 82% respectively and introduce a desirable antibacterial property to the scaffolds [219]. Feng et al. embedded nanodiamond particles into molybdenum disulfide nanosheets to construct a high strength nanosystem. It's verified that this nanosystem could increase the tensile and compressive strength of the poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) bone scaffold by 94% and 52% respectively, and the main strengthening mechanisms were crack deflection, crack bridging, crack pinning, and pulling out [220].
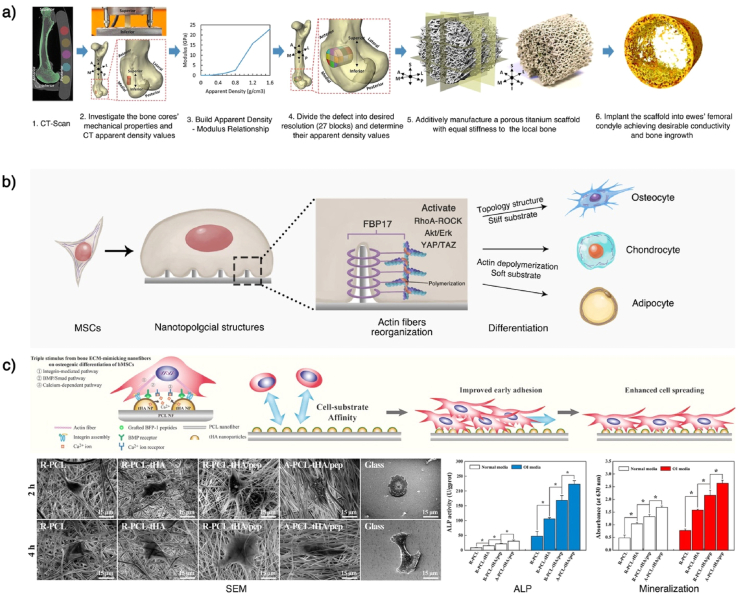
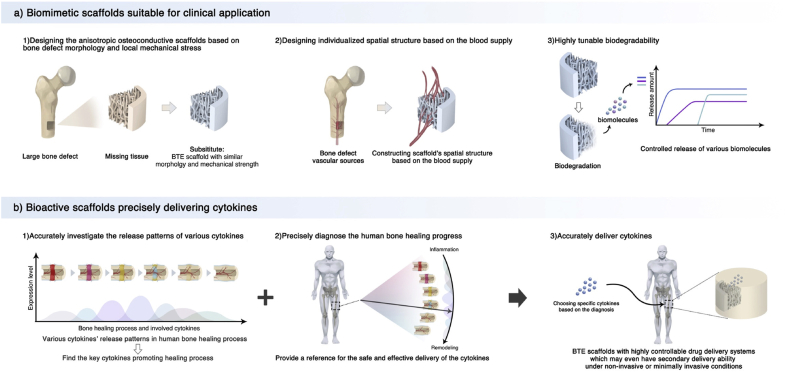
Hydrogels have better porosity and connectivity but limited mechanical properties. Although the mechanical properties can be improved by adding fillers such as hydroxyapatite and high-strength polymer, it is still challenging to achieve the strength of the natural cancellous bone [221,222]. In addition, these mechanical strengthening methods are isotropic, whereas the natural bone tissue has anisotropic mechanical properties. Designing an anisotropic scaffold that has coordinated conductivity with the surrounding bone is challenging. Recently, Ghouse et al. provided an approach to determine local bone mechanical strengths and developed a locally stiffness-matched scaffold for bone regeneration via AM. Six weeks after implanting the scaffold into ewes' femoral condyle, they observed a bone ingrowth of 10.73 ± 2.97%, which proved the desirable osteoconductivity of the scaffolds [223] (Fig. 5a). In the future, a combination of computer simulation analysis and AM technology should enhance the scaffolds’ morphology and mechanical properties closer to those of the natural bone tissues.
Fig. 5.
Mimic bone's mechanical and morphological structure. a) Mimicking the mechanical strength of local bone tissues. Combining a CT-scan and a mechanical test of natural bone can help establish a relationship between imageological apparent density and bone modulus, which can be used to predict the local bone mechanical strength. After predicting the local bone's mechanical strength, a stiffness-matched scaffold with desirable bone conductivity can be constructed using AM. b) Underlying regulation mechanism of scaffolds' topology structures. The surface topology can cause cell membrane curvature. The curvature can activate curvature-sensing protein FBP17, which will further induce F-actin polymerization and actin reorganization in whole-cell. This cytoskeletal system change can activate the RhoA-ROCK, Akt/Erk, and YAP/TAZ effectors of the Hippo pathway, affecting the stem cells' differentiation. The ordered topology pattern and the stiff substrate can induce osteogenic differentiation, while actin depolymerization and the soft substrate can induce chondro/adipogenic differentiation. c) Mimicking the ECM structure by nanofiber scaffolds. Topology structure nanofibers can be constructed using electrospinning and can be used to develop porous scaffolds. Gao et al. modified nanofiber scaffolds with calcium phosphate (HA), to further mimic the bone ECM and release cytokines (BMP-7) and enhance the osteogenesis effects. This scaffold was proved to promote the in vitro osteogenic differentiation of hMSCs and enhance in vivo bone formation. This Fig. was adopted from Refs. [[223], [224], [225]].
2.4.2. Physical–mechanical signals on microscopic surface
The natural bone tissue has a hierarchical structure. In addition to the macroscopic supporting structure, morphological biomimicry can be performed at the microscopic level. Currently, the modification of a nanotopological structure on the surface of a scaffold has been extensively investigated. In the natural bone tissue, the physiological remodeling and pathological changes affect the topological morphology of the trabecular bone and the arrangement of hydroxyapatite nanocrystals (thickness: 2–5 nm; length: 20–80 nm) at the nanometer scale [70,203]. These nanotopological changes will further affect the biological functions of the osteoblast or osteoclast cell line on the surface.
Several studies have reported that the manufacturing of two-dimensional topological structures with different shapes and arrangements can provide mechanical signals for cells, thereby affecting cell adhesion, proliferation, migration, and differentiation in vitro [[226], [227], [228], [229]]. These studies provide more accurate knowledge of the effects of physical and mechanical signals on the cell fate. Recent studies have shown that the topology could induce curvature formation in the cell membrane, which is recognized by the intracellular curvature-sensing protein FBP17, and induce the whole-cell reorganization of actin fibers [224]. Changes in the cytoskeletal system can activate RhoA-ROCK, Akt/Erk, and YAP/TAZ effectors of the Hippo pathway, which will further affect the stem cell fate (Fig. 5b) [230,231].
As mentioned above, the mechanical microenvironment in the natural bone tissue is complex and dynamic. Therefore, it is challenging to prepare a three-dimensional degradable scaffold with biomimetic surface topology. At present, the topological modification of three-dimensional scaffolds is mainly divided into two categories: 1) Modification of the topology structure on the surface of the formed scaffold. For example, the surface modification of nHA has been widely used in BTE scaffolds. 2) Directly using the topology structure to construct the scaffold. In recent years, researchers have used electrospinning technology to prepare polymeric nanofibrous scaffolds with high surface area/volume ratios, tunable components, and well-retained topographies. This nanofiber-based porous structure is also a promising platform for further topological modification and biomimicry [232]. For example, Gao et al. integrated a calcium phosphate mineral (HA) and a bioactive molecule (BMP-7) into a highly ordered fiber topography PCL scaffold to mimic the bone ECM. After in vivo implantation, the ordered PCL-HA/BMP-7 composite nanofibers significantly promoted lamellar-like bone formation in a rat calvarial critical-sized defect (Fig. 5c) [225]. Moreover, Xu et al. designed a core-shell or island-like topography with bioactive chitosan (CS) on a PLA electrospun fiber surface and demonstrated its ability to improve the cell biocompatibility of PLA, as well as the osteogenesis of preosteoblast cells [233]. This versatile technology has applied to multiple advanced biomedical applications. Recently, Ding et al. provided a comprehensive overview of the recent progress and potential developments of electrospun polymer matrices and their application as biomaterials [234]. Besides, Zhang et al. summarized the several properties of polymer fibers and detailed their applications in bone, cartilage, and osteochondral tissue engineering comprehensively, which could provide reliable suggestions for the development of polymer fiber scaffolds in bone tissue engineering [235].
Note that, after implantation into the bone defect, the BTE scaffold does not only generate endogenous stress, but also changes the local exogenous stresses such as fluid shear stress. In addition, the modification of the surface topology will inevitably change the exogenous stresses. The stress environment of natural bone is complex and difficult to simulate. In addition, current BTE scaffolds are biodegradable, and the structure of their surface topology can only provide early mechanical stimulation signals. During the degradation process, the surface topography changes constantly [236]. Khetan et al. demonstrated that the degradation of HA hydrogels led to a change in cellular tension, which could further affect the osteogenesis or adipogenesis of hMSCs [237]. Thus, when designing the surface structure of a BTE scaffold, we should recognize its regulatory effect on the cell fate. Meanwhile, we cannot simply rely on these physical–mechanical signals due to their instability. In addition, there are many types of cells involved in bone repair. The regulation of different cells by these signals is still unclear; excessive transmission of mechanical signals may hinder the bone-healing process.
The process of bone-tissue healing involves a complex biological signal network, and physical–mechanical stimulation such as surface topology is only a part of it. When constructing the physical–mechanical structure of scaffolds, we should start by providing a proper platform for cell migration and proliferation. Regarding the regulation of the cell's biological functions, researchers should further focus on the biochemical signals among ECM–cell and cell–cell. In the next section, we shall elaborate on the biomimicry of the ECM components to provide an appropriate biochemical signal to the cells inside the BTE scaffold.
2.5. Biomimicking extracellular matrix components
As described in Section 2.2.1, the component of the natural bone ECM can be divided into organic and inorganic components. The organic component is mainly collagen and a small amount of NCP, while the inorganic part is mainly hydroxyapatite. They constitute the ECM structure and possess certain biological functions. This section reviews how the current BTE scaffold mimics these three main components to promote the bone-healing process.
2.5.1. Collagen
As the primary component of bone-tissue ECM, collagen can be applied to BTE as a matrix component of scaffolds. Twenty-nine different types of collagen have been identified thus far, and collagen types I, II, III, V, and IX are known to form fibers. Among them, type I collagen secreted by osteoblasts constitutes the main organic component of natural bone tissue [238], becoming a desirable biomimetic material for the BTE scaffold. Collagen-based scaffolds have the advantages of high biocompatibility, high porosity, hydrophilicity, low antigenicity, and biodegradability [239]. However, they also have some shortcomings, such as 1) poor mechanical properties, easy deformability under applied force; 2) high biodegradability, which may degrade too quickly to match the normal tissue-healing process; and, 3) a native structure that is easily disrupted by specific fabrication techniques such as chemical crosslinking and physical treatments (e.g., UV irradiation) [240,241]. Therefore, in the design of collagen-based scaffolds, researchers should improve the fabrication technology or modify the collagen-based matrix with other materials to maintain the biological properties of collagen and make up for these drawbacks.
The morphology and structure of collagen-based materials are significantly affected by the fabrication approach. Current fabrication techniques include freeze-drying, phase separation, electrospinning, gas foaming, and 3D-bioprinting [240,241]. The collagen-based materials formed by the first several methods have relatively smaller pores. As mentioned above, the pore size of a BTE scaffold is critical for cell migration, new blood vessels, and bone formation during bone healing. In recent years, 3D-bioprinting has become a reliable method for the precise control of the collagen-based scaffold structure [[242], [243], [244]]. Lee et al. developed a three-dimensional collagen scaffold consisting of multilayered nanofibrous collagen using a 3D-printing process and Pluronic F-127 (PF-127), which is a nontoxic and thermoreversible polymer. By selecting the appropriate processing parameters, such as the concentration of PF-127, weight of collagen, and printing stage temperature, researchers could successfully control the pore geometry of the three-dimensional scaffold [244]. Therefore, we can generally include the design of collagen scaffolds in two major aspects: fabrication approaches and material selection.
For material selection, the components of collagen-based scaffolds determine the physical–mechanical properties and biological properties of the scaffold. In the fabrication of collagen-based scaffolds, the use of necessary components such as collagen, photoinitiators, and crosslinking agents can form a basic matrix that can be further modified by other materials. For example, the addition of high-strength fillers (e.g., bioinert polymer materials, CaPs, etc.) can enhance the mechanical properties and plasticity of the scaffold or biomimic the ECM inorganic components [240]. Note that collagen-based materials can also be used as a platform for the controlled release of biomolecules, such as cytokines [245], osteogenic agents [246,247] and miRNA [248]. An exhaustive introduction of the delivery of bioactive molecules is presented in Section 5.
In addition to being a matrix component of BTE scaffolds, collagen can be used for surface modification. In recent years, surface modification has made up for the shortcomings of synthetic polymer materials (e.g., aliphatic polyester scaffolds), which have excellent morphological and mechanical properties but are bioinert. Among these, the surface coating of collagen can be a promising strategy for maintaining the scaffolds’ mechanical properties and promoting cell proliferation and osteogenesis [247,249]. For example, Martin et al. recently developed 3D-printed poly (lactic acid) (PLA) scaffolds and coated them with collagen, minocycline, and citrate-hydroxyapatite nanoparticles to enhance its antimicrobial and osteogenic effects. Collagen improved the biocompatibility of the scaffold. It also served as a sustained-release matrix for minocycline and a template for hydroxyapatite to enhance the osteogenesis effects [243]. The key steps and considerations for designing biomimetic collagen-based scaffolds are summarized in Fig. S2.
From the above, it can be seen that CaP, like hydroxyapatite, has been widely used in collagen-based scaffolds as a high-strength biomaterial. In the next section, we specifically discuss the application of CaPs in BTE scaffold.
2.5.2. Inorganic component
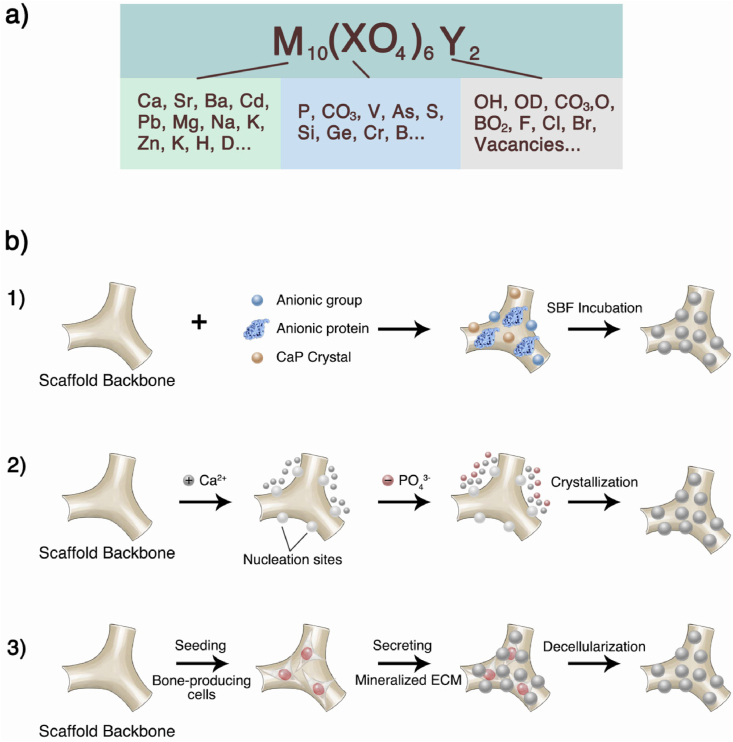
As mentioned earlier, natural bone is highly mineralized, with a composition of approximately 65 wt% mineral phase, achieved by intrafibrillar mineralization [36]. These mineral components are mainly composed of the CaP family, which can be a desirable biomimetic template. As a biomaterial, CaP shows desirable biocompatibility, osteoconductivity, and osteoinductivity when mimicking the inorganic components of natural bone tissue [250]. Osteoconductivity reflects the biomaterials can provide a physical structure for bone growth on the surface and across, which is a passive process involved in cell adhesion, proliferation, migration [251]. And osteoinductivity indicates that the biomaterials are able to stimulate the differentiation of bone precursor cells into preosteoblasts which reflects an active process [252]. In term of CaP materials, these biological properties are derived from the inorganic chemical groups’ composition and its release. In the following paragraphs, we elaborate on the properties, selection, and application of inorganic materials for BTE from this perspective.
HA (Ca10(PO4)6(OH)2) is the main inorganic component of natural bone and the most stable CaP under physiological conditions (including temperature, pH, and body fluid composition). Therefore, it has become a widely used material for BTE [253]. Numerous members of the CaP family can be transformed into one another under specific conditions. For example, at physiological pH, amorphous CaP is converted to octacalcium phosphate (OCP), which can further evolve into carbonate hydroxyapatite. At a lower pH, this intermediate phase appears to be dehydrated dicalcium phosphate (DCPD) [254]. These phosphates, such as HA, are mainly composed of Ca and P groups.
Shih et al. demonstrated that the phosphate released by the biodegradable CaP scaffold can be acquired by stem cells, facilitating the conversion of adenosine diphosphate (ADP) to adenosine triphosphate (ATP) inside cells. The elevated concentration of ATP can further activate the intracellular adenosine signaling pathways through autocrine and paracrine mechanisms, activating the expression of bone-related transcription factors such as osteocalcin and osteopontin, as well as enhancing osteogenic differentiation [255]. The release rate of phosphate is largely determined by the chemical composition of CaP, and the Ca/P ratio is an important indicator for evaluating the solubility and acidity of CaP. The higher the Ca/P ratio, the lower the acidity and solubility of CaP. The Ca/P ratio of HA is 1.67, which shows lower acidity and solubility, consistent with the low degradation of HA as a BTE material. It is also one of the main reasons why HA cannot be used alone to fabricate a desirable BTE scaffold [250].
β-TCP (Ca3(PO4)2) is a synthetic CaP whose Ca/P ratio is close to 1.50. Therefore, compared with HA, its degradability is higher in BTE applications. Thus, the Ca/P ratio is a critical parameter in applying CaP materials, which also laid the foundation for the combined application of multiple CaPs. A table containing various CaPs and their Ca/P ratios is provided by Vallet-Regí et al. [37]. Currently, the most widely used CaP mixture is biphasic calcium phosphate (BCP) ceramics formed by mixing HA and β-TCP. Daculsi confirmed that mixing HA and TCP with different mass ratios could control the Ca/P ratio of BCP and its degradation rate [256]. This study showed that BCP materials have biological effects similar to those of natural bone tissues both in vitro and in vivo. As the TCP/HA mass ratio increased, the rate of BCP absorption also increased because of the change in the mixture Ca/P ratio. In recent years, there have been many studies on the development of CaP mixtures for BTE [257,258], and understanding the chemical composition and biological properties of CaPs is the basis for these studies.
In fact, the chemical element composition of HA in natural bone is not fixed and can be generally expressed as M10 (XO4)6Y2, where M represents the partial substitution of Ca2+ by cations (e.g., Sr2+, Mg2+, Zn2+, Na+, K+, F+), and X and Y represent the anionic substitutions (e.g., HPO42−, CO32−, F−) (Fig. 6a). The younger, less crystalline tissue can develop and grow faster while storing other elements that the body needs during its growth [37]. These substitutions play a fundamental role in the crystal structure and dissolution behavior of biological apatite. For example, silicates can enhance the mechanical strength and biological effects of CaPs, which are important properties for porous scaffolds [259]. F− substitution can reduce the solubility of CaPs, while carbonate and strontium can increase the solubility, which is beneficial to implant absorption [260]. It has also been suggested that the incorporation of ions such as Zn2+, Sr2+, Li+, and Mn2+ into CaPs could increase osteogenesis, and the incorporation of Mg2+, Cu2+, and Co2+ may enhance neovascularization [261]. There have been reviews discussing the biological effects of inorganic substances with different chemical groups [37,261].
Fig. 6.
Modify scaffolds with natural bone inorganic components. a) Chemical composition of HA in natural bone. M represents the cationic substitutions, while X and Y represent the anionic substitutions. These chemical groups are formed to meet the body's needs during growth and play essential roles in maintaining bone homeostasis and direct the bone cells' functions. b) Approaches for modifying crystallization on BTE scaffolds' surface. 1) Introduce nucleation sites on the surface followed by incubation in simulated body fluid; 2) Alternative exposure to Ca2+ and PO43− solutions; 3) Seeding osteogenic lineages on scaffold surface to secrete mineralized ECM followed by decellularization. This Fig. was adopted from Refs. [37,262].
In addition to HA, natural bone also contains other inorganic substances with different biological properties due to the presence of new chemical groups. For example, whitlockite (WH) is the second-most abundant inorganic component in the living bone, with an estimated 20 wt%. based on the amount of Mg [250]. Although the content of WH(Ca9Mg(HPO4) (PO4)6) in natural bone tissue is not high, compared with HA, WH can continuously release Mg2+ ions, which has been shown to play an important role in bone fracture healing. Zhang et al. showed that Mg2+ could increase neuronal calcitonin gene-related polypeptide-α (CGRP) in both the peripheral cortex of the femur and the ipsilateral dorsal root ganglia. CGRP further activates the osteogenic differentiation of periosteum‐derived stem cells [263].
Recently, Jang et al. demonstrated that, compared with HA, WH-modified scaffolds exhibit an enhanced bone-specific differentiation ability, and compared with HA and β-TCP, WH implants could induce similar or better bone regeneration in vivo, with an intermediate resorption rate [264]. In addition, the research group also combined the application of WH and HA into BTE scaffolds, which was confirmed to facilitate osteogenic differentiation and prohibit osteoclastic activity. Finally, WH could transform into HA with enhanced mechanical strength [265]. Other non-phosphate inorganic materials can also be used in BTE, such as bioglass, which is an artificially synthesized oxide. It has satisfactory bone integration capability, the mechanism of which is still the interaction between SiO2 and calcium phosphate ions to form an amorphous calcium phosphate layer, which then crystallizes into a hydroxyapatite crystal [250]. Therefore, in-depth exploration of the inorganic composition, its biological properties, and the development mechanism of the natural bone tissue is the basis for the selection and modification of inorganic biomaterials for scaffolds.
Currently, there are various approaches to applying inorganic components into BTE scaffolds, mainly including serving as a scaffold matrix and modifying the scaffold surface. As the matrix material of the BTE scaffolds, inorganic biomaterials can provide superior mechanical properties and osteoconductivity and can be used as a sustained-release system for bioactive ingredients. Inorganic materials such as HA, β-TCP, and BCP can be directly sintered to construct the morphology of scaffolds [[266], [267], [268]]. However, owing to the high sintering temperature, this fabrication approach limits the combined application with other biomaterials, such as protein-based materials.
In recent years, inorganic biomaterials have been used as particle fillers in natural or synthetic polymer material matrices [269,270]. This method maintains the biological properties of CaP and facilitates the use of various fabrication techniques such as lyophilization, salt leaching, gas-forming, 3D-printing, etc., forming scaffolds with various morphologies and biological functions [250,262]. However, simply mixing CaP fillers with other materials can easily cause CaP to be covered and undermine its excellent biocompatibility and osteoconductivity. To address this issue, the corporation CaP with biodegradable materials could be a satisfactory solution. For instance, Shuai et al. developed a (glycolic acid) (PGA) -PLLA- HA BTE scaffold via laser 3D printing. The incorporation of HA could enhance the bioactivity and osteoconductivity of the scaffolds. And the blend of biodegradable polymer PGA could make the scaffold exhibit tunable biodegradability which facilitated the exposure of HA during the degradation process. It's confirmed that this scaffold displayed desirable cytocompatibility in vitro and excellent bone repair capacity in vivo [271]. Feng et al. incorporated biodegradable polymer PLLA into Polyetheretherketone (PEEK)/β-TCP scaffold to facilitate the exposure of β-TCP during scaffold degradation. This method maintained the excellent mechanical strength of PEEK and prevented β-TCP from being wrapped inside during melting/solidifying processing by SLS. The results showed that the degradation of PLLA provided numerous caverns enabling direct contact between β-TCP and body fluid. Consequently, the scaffolds display the satisfactory cytocompatibility and osteoconductivity in vitro as well as bone repair effect in vivo (Fig. 7) [272]. At present, there are many kinds of biodegradable polymer materials. In addition to the above, poly (lactic-co-glycolic acid) (PLGA) is another widely used biodegradable polymer in BTE. Recently, Zhao et al. comprehensively reviewed the application of this polymer for bone regeneration [273].
Fig. 7.
Combine CaP with degradable polymer materials for bone tissue engineering. a) PEEK/β-TCP-PLLA scaffold via SLS. b) Degradation behaviors of the scaffolds with 0–50 wt% of PLLA content after PBS immersion, including Weight loss curve and SEM micrographs. c) Cell viability on 30 wt% PLLA scaffold after culture for different time intervals. d) Bone regeneration ability of the scaffolds. e) Histological analysis of new bone formation by H&E staining [272].
In addition to serving as a scaffold matrix component, CaP can also be used to modify the scaffold surface to promote biological properties, especially for synthetic polymer scaffolds that are usually highly plastic but biologically inert [274]. There are three main methods for surface crystallization modification: 1) introducing nucleation sites (e.g., CaP crystals and anionic group) on the scaffold surface followed by incubation in the simulated body fluid (SBF); 2) chemical deposition by alternative exposure to Ca2+ and PO43− solutions; and 3) seeding osteogenic lineages onto the scaffold surface to secrete mineralized ECM followed by decellularization [262]. These three methods are presented in Fig. 6b. Notably, the research on surface modification focuses not only on the inorganic biomaterial's chemical composition but also on the surface microstructure, such as crystal size, morphology, and roughness, which could also affect bone formation. Recently, Xiao et al. reviewed the effects of CaP surface structure on osteogenesis and its mechanism [261].
2.5.3. Insoluble non-collagen protein
In addition to 90% collagen, there are 5% NCPs among the organic components of natural bone. Multiple types of insoluble NCPs form a meshwork of structural proteins, including adhesive proteins (e.g., fibronectin and vitronectin) and proteoglycans (e.g., versican, decorin, and glycosaminoglycans). These NCPs play a vital role in bone homeostasis and ECM–cell interactions [275]. Modification of the scaffold with NCPs can mimic specific biological properties of the natural bone ECM, especially for increasing the binding sites, which can 1) directly interact with the cells and affect their biological functions, including adhesion, proliferation, and migration; and, 2) promote the binding of other ECM components, such as collagen, calcium phosphate, and cytokines, indirectly affecting the biological functions of cells. Therefore, before applying the insoluble NCPs to BTE scaffolds, we should understand the crosstalk between these proteins and cells.
Cells can communicate with the extracellular environment via a variety of proteins on their surface, including integrins, selectins, and immunoglobulins. These proteins can transmit the ECM biochemical and physical–mechanical signals to the cell, resulting in specific cellular biological effects [28]. Among them, integrins are widely reported heterodimeric transmembrane proteins that can respond to ECM signals and mediate cell adhesion. Each integrin consists of α and β subunits. Currently, eight β and 18 α integrin subunits have been reported. Various combinations of these subunits bind different ECM ligands and mediate multiple biological effects, including adhesion, proliferation, and differentiation, and even mechanical sensing [[275], [276], [277], [278]]. Therefore, using specific ECM proteins (ligations) to modify the BTE scaffolds is a promising approach to enhance the scaffolds’ biological effects via a biomimetic method [279]. Currently, several reviews have detailed the composition and function of ECM protein components in the bone tissue, as well as the relationship between these proteins and the integrins [28,275,280], which can be used as a reference for the selection of NCPs in BTE. In addition, Coutu et al. analyzed the distribution of ECM proteins in whole mouse femurs through multicolor three-dimensional imaging. This study focused on the spatial distribution characteristics and function of ECM proteins in the bone tissue [281], which provided insights for mimicking the ECM components of specific bone structures (e.g., cortical and cancellous bone).
Currently, fibronectin, laminin, and vitronectin are the widely used soluble NCPs in BTE scaffolds. Their application forms include full-length natural proteins, recombinant fragments, and ECM-derived peptides. Full-length natural proteins should be extracted and purified from the bone tissue, which has the limitations of high cost, batch-to-batch variation, and immunogenicity. These limitations have spurred the development of recombinant fragments and ECM-derived peptides, which are the minimal functional sequence of the parent protein [275]. For example, RGD and PHSRN sequences are responsible for the integrin-binding of fibronectin [282]. The recombinant fragment FNIII7-10 encompasses the RGD site in the 10th type III repeat and PHSRN synergy site in the 9th type III repeat [283]. Agarwal et al. coated FNIII7-10 on stainless steel screws and then implanted them into healthy and osteoporotic rats, which resulted in enhanced osteointegration and higher mechanical fixation at one and three months compared with uncoated implants [284].
They hypothesized that the FNIII7-10 coating initiates pro-healing responses at the implant surface, resulting in enhanced implant–bone integration, which may also be applied to the surface modification of the BTE scaffold. Besides fibronectin-derived proteins, there are several types of recombinant fragments and ECM-derived peptides, which are detailed in the review by Shekaran et al. [275]. Furthermore, the modified form of insoluble NCP in the scaffold can also be mixed into the hydrogel-based scaffolds to enhance and modify their biological properties, except for the surface properties [[285], [286], [287], [288], [289]]. Recently, Trujillo et al. developed a full-length FN-based three-dimensional hydrogel scaffold that had controlled stiffness and degradability, so that it could effectively load and release the growth factors (BMP-2 and VEGF). It achieved the desirable osteogenesis and angiogenesis effects in vivo [290]. In addition, a few researchers have directly used decellularized bone scaffolds that imitated the ECM structure and composition of natural bone tissue to a great extent and exhibited satisfactory biological effects [291,292].
As mentioned above, the bone-healing process is orderly and complex. While modifying scaffolds with biological functions, it is necessary to consider the influence of these additional biological signals on the natural bone-healing process. A decade ago, Siebers et al. proposed critical considerations for integrin-related protein modification, including 1) the surface modification proteins will be enzymatically degraded, denatured, undergo conformational and configurational changes, and will even be replaced by other proteins in vivo or culture medium; and 2) the in vivo application of integrin–ligand-modified biomaterials may yield results different from the in vitro studies because the cell on the surface of in vivo implants commonly transform from immune cells to bone formation cells. The protein on the implant surface is already changed during this process [293]. Present BTE materials are generally biocompatible and degradable, and the biological functions of integrins have also been investigated. However, those two considerations should still be considered seriously in the design and application of scaffolds. In future research, deepening the understanding of bone homeostasis, the interaction of cells–ECM during bone healing, and in vivo studies should form the basis for the development of next-generation insoluble NCP biomimetic scaffolds.
When designing a BTE scaffold, besides constructing a relatively fixed morphology and component based on the bone ECM, the delivery of soluble cytokines is also an important way to modify the scaffold's biological properties. Such modification should be based on the cytokine release pattern in the bone-healing process. We summarized and explained this part in previous sections. Below we provide a detailed overview of its application in BTE, and further provide clues and cautions for the design of next-generation scaffolds.
3. Bioactive molecular delivery based on bone-healing mechanism
As mentioned in Section 3, a variety of bioactive molecules are involved in the bone physiological homeostasis and healing process. These molecules are secreted by cells, and this process is affected by ECM–cell and cell–cell interactions. Since these molecules can effectively participate in and regulate the bone-healing process, researchers can load them into scaffolds to modify their biological functions and promote the healing process. However, unlike the release of cytokines in the natural bone tissue, BTE scaffold materials commonly serve as carriers and reservoirs. The delivery of biomolecules is the result of scaffold degradation, including surface degradation and bulk degradation. Recently, Yang et al. summarized the crosslinking and degradation process of BTE polymer scaffolds, as well as the factors influencing the degradation process [294]. In this section, we provide a detailed overview of the current applications of BTE scaffolds to deliver biomolecules and propose our considerations from the perspective of the bone-healing process and mechanism.
3.1. Biomolecular delivery for osteogenesis
The main purpose of BTE treatment is to promote bone regeneration in the bone defect sites. As mentioned earlier, in the process of bone healing, intramembranous and endochondral osteogeneses led by chondrocytes/osteocytes are the key processes of bone regeneration. In this nonspecific anabolic stage, a variety of cytokines participate in and promote osteogenesis. Therefore, exploring the role of these osteogenesis-related cytokines in the bone-healing process and applying them in BTE has been an important and mainstream idea in the field for over 10 years.
When using BTE scaffolds to deliver biomolecules, it is necessary to design the scaffold as a drug release system. Moreover, the biological functions of these biomolecules and crosstalk among cells in the bone-healing process are the basis for the design of BTE scaffolds. In Section 3.2, we summarized the osteogenic cytokines in the bone-healing process. They all have the potential to promote osteogenesis at the defect site, but appropriate delivery is the key to achieving the underlying effects. When designing BTE scaffolds that deliver biomolecules, two main factors must be considered carefully: 1) cytokine types; and 2) delivery timing, dose, and duration. These selections should also be based on the bone-healing process and mechanism.
As mentioned above, several biomolecules are involved in cell communication and regulation of the bone-healing processes. The application of specific cytokines can interfere with the healing process of bone defects. Let us take BMP-2 as an example. At the cellular level, it can trigger a healing cascade, induce MSCs to differentiate into osteoblasts or chondroblasts, and promote osteoblast proliferation [295,296]. Accordingly, it has been frequently incorporated into scaffolds for applications ranging from spinal fusion [297,298] to dental and craniofacial regeneration [[299], [300], [301]]. However, a few studies have found that the direct delivery of cytokines to bone defects could cause individual differences in repair effects. The treatment results of each of these studies are different, or even opposite. For instance, in early published human studies, rhBMP-2 solution-soaked collagen scaffolds were implanted into the bone tissue to stimulate osteogenic effects, which yielded varying results in patients, ranging from obvious bone formation to no bone formation with fibrous tissue [302].
In addition, through a comparative review of FDA documents and subsequent publications, Carragee et al. estimated that the rhBMP-2 used for spinal fusion therapy might cause 10–50% of adverse events, including radiculitis, ectopic bone formation, osteolysis, and even life-threatening events depending on the approach [303]. The variations in the effectiveness and safety of these experimental results may be attributed to the release of cytokines that did not meet the physiological needs for bone regeneration. As discussed earlier, in the natural bone-healing process, cytokines take effects in a specific spatiotemporal manner, and have a specific localization and orchestration to guide an orderly repair. If this release process is mostly changed, it may lead to undesirable bone-healing effects or even harmful results. Thus, a well-designed sustained-release and controlled-release system based on the bone-healing mechanism is currently necessary for the BTE scaffolds to deliver biomolecules. This design has come to the forefront of the field of BTE in recent years, and it is also a way to develop next-generation BTE scaffolds.
For the delivery of a single cytokine, it is necessary to deliver the cytokine based on the release pattern in the bone-healing process. Let us take BMP as an example. Several BMPs are present in the late stages of bone healing, and some studies have also confirmed that delayed BMP delivery may result in a better bone-regeneration effect [[304], [305], [306]]. In particular, Betz et al. investigated whether the BMP-2 administration timing influenced the mineralization within the critical-sized femoral bone defects in a rabbit. They found that delaying the administration of BMP-2 until days 5 and 10 enabled greater bone formation with enhanced mineralization and mechanical strength [304]. Notably, this administration timing is also consistent with the bone formation phase of natural bone healing summarized above.
PDGF is another example of a cytokine. As mentioned above, its release peak is located in the early stage of bone healing (initial days to a few weeks depending on the specific condition), which can recruit MSCs and other osteoblastic precursors to the bone-healing site [307]. Therefore, the application of PDGF in the initial stage of bone healing can reasonably and effectively promote bone regeneration. Ranly et al. confirmed that the cartilage and bone formation effects of PDGF acted in a temporal and dose-dependent manner. Delayed delivery or high dose could lead to decreased bone formation [308]. The current sustained/controlled release of a single cytokine is relatively mature. Thus, in the design of the next-generation osteogenic factor-delivery BTE scaffolds, researchers should mainly consider the cytokine release pattern in the bone-healing process, and then modify the BTE scaffolds as a similar spatiotemporal delivery system that could achieve an enhanced bone-regeneration effect.
Each stage of the bone-healing process involves a variety of cytokines. At present, the controlled-release technology allows scaffolds to deliver two or more cytokines at a time, which not only provides researchers with more approaches for designing multifunctional scaffolds but also requires them to pay more attention to the design rationalities, including the administration sequence, timing, dose, duration, and the interaction relationship of different cytokines. First, we need to consider the types of delivered cytokines. Currently, dual delivery is the most common form of co-delivery, especially the use of cytokines that play a synergistic effect during bone regeneration, such as BMP and VEGF [309].
Second, it is necessary to design release sequences. Researchers can modify the encapsulation relationship between the BTE scaffold and cytokine to release the two cytokines in an orderly manner that complies with the natural process and achieves enhanced regeneration effects [[310], [311], [312], [313]]. For example, Barati et al. developed a dual-factor sequential releasing scaffold, which possessed a hierarchical structure with two delivery systems that could release VEGF in 10 days and BMP-2 in 21 days. They confirmed that, compared with the direct addition of dual cytokines, delivering the cytokines in a spatiotemporal manner enhanced the osteogenic differentiation of human MSCs and the vasculogenic differentiation of endothelial colony-forming cells. These co-cultured cells could further couple the mineralization and vascularization in a paracrine fashion [313]. Recently, Tang et al. developed a dual modular chitosan-based scaffold. They incorporated rhBMP-2 and VEGF into differentiated delivery systems (mesoporous bioactive glass and GelMA hydrogel) inside the hierarchical scaffold, resulting in well-orchestrated and enhanced osteogenesis and angiogenesis effects in vitro and in vivo. This result re-emphasizes the importance of sequencing the delivery pattern in multi-cytokine delivery [314].
In addition, the release kinetics of cytokines is another factor to consider, which includes the released dose, rate, and duration that are related to the material and the manufacturing approach. Several scholars have outlined this aspect. For example, Bayer et al. in their review specifically discussed the fabrication and modification of BTE scaffolds with the ability to release cytokines sequentially [315]. Additionally, a review by Samorezov et al. described the current strategies for delivering growth factors in a spatiotemporal manner, which might contribute to effectively mimicking the signals present in bone development and healing [316]. These reports all implied the important value of research on scaffolds with controlled-release capabilities. Recently, Cui et al. developed an electroactive BTE scaffold that could control release hBMP-4 gene via electrical stimulation. They constructed a doxycycline-triggered gene expression carrier by introducing an hBMP-4 gene fragment into a non-viral artificial restructuring plasmid vector (pSTAR) to form the pSTAR-hBMP-4 plasmid (phBMP-4). Then, they corporated the phBMP-4 into an electroactive scaffold which is fabricated by combining a triblock copolymer of poly (l-lactic acid)-block-aniline pentamer-block-poly (l-lactic acid) (PLA-AP) with PLGA/HA. Regulating the presence of doxycycline (Dox) and electrical stimulation could strictly control the release of hBMP-4 gene in the composite scaffold. It's confirmed that the scaffold could improve cell proliferation and osteogenesis differentiation in vitro, and effectively promote bone healing in a rabbit radial defect model (Fig. 8) [317].
Fig. 8.
Construction of controlled release BTE scaffold. a) Construction scheme of an electroactive BTE scaffold with ability to control release and expression of hBMP-4 and its underlying mechanism. The addition of doxycycline could activate the expression of hBMP4, and the electrical stimulation could accelerate the release of hBMP4. b) SEM morphology of the scaffolds which maintained uniform porous structure during construction. c) Dox dose-dependent hBMP4 expression. (c1-c2) Detection of hBMP4 expression by RT-qPCR and ELISA methods. (c3-6) Dox regulation on expression of four osteogenesis-related genes. d) In vivo bone repair ability of the scaffolds [317].
In view of these studies, we can see that the development of bioactive BTE scaffolds is based on deepening the understanding of bone-healing mechanisms, which can help researchers design novel scaffolds that comply with and promote the healing process. However, it must be noted that the pursuit of the completely consistent with the natural healing process would be time- and cost-prohibitive because the cytokine regulation of natural bone results from the dynamic crosstalk between cell–cell and cell–ECM. As the cytokine delivery mode of the BTE scaffold is predesigned, it is difficult to continuously respond to the regulatory network during the bone healing process. Therefore, we recommend not only pursuing the biomimetic and controlled release of biomolecules, but also exploring continuously the biomolecules that play a key role in the bone-regeneration process. Ultimately, researchers can apply these essential biomolecules to regulate the healing process in a simple, safe, and controllable manner.
In addition, compared with the delivery technology of other biomolecules, the controlled-release technology for osteogenic factors is a more extensively investigated field. As BTE researchers further focus on vascularization and immune regulation, the osteogenic factor-delivery methods can still provide reliable references. In the next section, we provide a detailed overview of the application of BTE scaffolds for angiogenesis in the bone defect areas, which has attracted substantial attention in recent years.
3.2. Biomolecular delivery for angiogenesis
Bone tissue is rich in blood supply and Bone marrow is the only permanent hematopoietic organ in humans [318]. Therefore, normal blood supply is extremely important for the homeostasis of bone tissue. Insufficient blood supply caused by trauma, etc. could reduce the activity of bone cells, and further lead to bone destruction, eventually resulting in osteonecrosis. Recently, Zhu et al. discussed this bone disease and comprehensively detailed how to apply bone tissue engineering methods to solve this problem [319]. In recent years, promoting bone regeneration by enhancing revascularization has become a focal point in BTE. Recently, Rather et al. summarized the characteristics of BTE scaffolds with dual effects of osteogenesis and angiogenesis. They divided the scaffolds into four categories according to osteoinductive/conductive and angioinductive/conductive properties (Fig. 9a) [252]. Among them, conductivity commonly refers to the scaffold's biocompatibility, which can provide an appropriate niche for cell attachment, proliferation, migration, and differentiation. Previously, we described the design of a biocompatible scaffold by mimicking the component and morphology of natural bone. In this section, we focus on the application of BTE scaffolds to deliver the angioinductive factors properly based on the bone-healing mechanism. We considered four essential points: 1) the degradation properties of scaffolds; 2) the delivery timing and duration of angioinductive factors; 3) the spatial arrangement of angioinductive factors; and 4) the types of angioinductive factors.
Fig. 9.
Scaffolds for angiogenesis a) The category of angiogenesis scaffolds for BTE. 1) Scaffolds that can deliver osteoinductive and angioinductive molecules; 2) Scaffolds that deliver molecules with both osteoinductive and angioinductive abilities; 3) Scaffolds conducive for osteogenesis to deliver angioinductive molecules; 4) Scaffolds conductive for osteogenesis and angiogenesis without delivering inductive molecules. b) The approach for constructing scaffolds that support vascularization. The top-down approach represents the use of naturally derived polymer, which has inherent cell binding and degradation motif to construct scaffolds, while the bottom-up approach refers to the modification of synthetic polymer with growth factors, binding, and degrading protein sequences to form biodegradable angiogenesis scaffolds. c) Kolesky et al. used 3D bioprinting to construct a hydrogel matrix with a pre-designed vascular network. The procedure is as follows. 1) 3D-print a vascular network containing thermoreversible polymer pluronic and thrombin. Introduce cell components via printing cell-laden inks that contain gelatin, fibrinogen, and cells. 2) Cover ECM material containing gelatin, fibrinogen, cells, thrombin, and transglutaminase. Thrombin can cause the polymerization of fibrinogen into fibrin outside the vascular network and inside the ECM. Besides, transglutaminase further diffuses to crosslink gelatin and fibrin; 3) After casting, lower the temperature to liquefy pluronic and evacuate the vascular network. 4) Perfuse vascular ECs into the network to achieve vascularization. This Fig. was adopted from Refs. [252,320,321].
First, the angioinductive scaffolds need reasonable vascular growth space, as determined by the porosity, connectivity, and degradability of the scaffolds. During bone embryonic development and healing, cells such as vascular epithelium cells and osteoclast cells secrete MMPs to guide the degradation and remodeling of the matrix, which provides space for vascular invasion and bone regeneration. This process is led by the interaction between the surface receptors of vascular endothelial cells and the proteins and immunoglobulin superfamily molecules in the ECM [[322], [323], [324]]. Thus, to comply with this natural angiogenesis mode, BTE scaffolds must have desirable biodegradability. Recently, Ngo et al. divided the approaches for constructing these scaffolds into two categories: top-down and bottom-up vascularization approaches. The top-down approach refers to the application of nature-derived materials, such as collagen, gelatin, and fibrin, which already contain cell binding and degradation motif to instruct the desired cell behavior.
However, owing to the limitations of high cost and batch-to-batch variability, researchers can also use synthetic materials, such as PEG and glycosaminoglycans (e.g., hyaluronic acid). Despite lacking any inherent biological functionality, these materials can be outfitted with bioactive modalities such as growth factors, binding, and degradative protein sequences to mimic the aspects of native tissues, which is called the bottom-up approach (Fig. 9b) [321,325]. After recognizing the biodegradable matrix materials suitable for vascular invasion, researchers can combine them with other BTE materials as needed [252]. For example, Tsao et al. combined an MMP-degradable electrospun gelatin patch (vascular ingrowth matrix) with PLGA porous silica nanoparticles, which performed the sustained release of VEGF and PDGF. This scaffold was proven to promote localized neovascularization and reduce tissue cytotoxicity in vivo [326]. Apart from serving as the matrix components of scaffolds, these degradable materials can be used as delivery systems for angioinductive factors. Generally, the delivery mode of factors is determined by the scaffold degradation pattern, which can be modified via the regulation of the scaffold component, morphology, and drug encapsulation approaches, as mentioned previously [294,315,316].
In terms of the delivery timing and duration of the angioinductive factors, in natural bone healing, angiogenesis runs through most of the healing process (except for early inflammatory reactions). However, angiogenesis is the result of the regulation of multiple angiogenic factors, and researchers must deliver them properly based on the natural release pattern. For example, the most widely used angiogenic factor, VEGF, can directly stimulate the proliferation and migration of vascular endothelial cells [327]. However, it has an inhibitory effect on the biological functions of pericytes (e.g., vascular smooth muscle cells). Continued expression of VEGF could lead to vessel destabilization and leakage [328]. This result indicates that VEGF is an effective angiogenic factor, and its administration mode should comply with specific rules and limits.
Researchers noticed that the expression of VEGF is upregulated in the early stage of bone healing, which is earlier than the expression peak of the osteogenic factor BMP-2. Thus, they preferentially delivered VEGF first in the dual-cytokine delivery and obtained enhanced angiogenic osteogenesis effects [329,330]. In addition, a recent systematic review concluded that there was still a lack of clinical trials or human application related to the use of VEGF. The results of existing in vivo studies reveal that the best release time is within the first three weeks, and the best effect is before eight weeks [327]. Thus, although the angiogenic effect occurs nearly throughout the bone-healing process, the application of an angioinductive factor still needs to be based on its specific release pattern. Meanwhile, increasing exploration efforts via clinical trials should provide a reliable basis for the application of angioinductive factors in the future.
In addition to the delivery timing and duration of angioinductive factors, spatial arrangement is also crucial. In large-volume bone defects, especially defects exceeding the critical range, consistent ischemia and hypoxia in the center of the bone defect are the main problems leading to nonunion bone healing [331,332]. To address this problem, researchers are paying attention to the delivery of angiogenic factors in a spatially specific manner. For example, Park et al. applied the 3D-printing technology to construct a pre-vascularized scaffold that can spatiotemporally promote the repair of large-volume bone defects. They applied collagen to the sustained release of BMP-2 in the peripheral zone, and applied alginate/gelatin to the burst release of VEGF in the central zone, which is a hypoxic area. Twenty-eight days after implantation in the back of the mouse, newly formed microvessels were observed in the hypoxic area, and larger bone regeneration was found in the entire scaffold [333].
In the field of vascular engineering, Kolesky et al. used 3D bioprinting and a sacrificial material (Pluronic F127) to construct a pre-designed vascular network and encapsulated it within a hydrogel matrix. After the scaffold was cast, Pluronic F127 was removed, which left a perfusion chamber for intravascular flow (Fig. 9c) [320,334]. This method of constructing space-specific structures has not been reported in the field of BTE, which can be an effective method for the spatial delivery of angioinductive factors in future applications. Thus, accelerating revascularization in the ischemic area of the bone defect is a promising approach to repair large-volume bone defects. However, given that there are few vascular sources that remain around the bone defects, regulating the spatial arrangement of angioinductive factors to accelerate the vascular ingrowth into hypoxic areas is an idea worthy of attention.
Choosing the type of angioinductive factors depends on the cognition of the angiogenic target of the bone-tissue vascular endothelial cells, and their interaction with the osteoblast cell lines. In fact, the morphology and function of blood vessels in the natural bone tissue are also spatially specific. In recent years, researchers have found that blood vessels in actively osteogenic bone regions, such as the metaphysis and endosteum, are columnar and strongly express CD31/Pecam1 and Endomucin (Emcn). Therefore, these blood vessels and ECs have been classified as type H (CD31high Emcnhigh).
The majority of MSCs and osteoprogenitor cells of the bone tissue are located around the type-H blood vessels, including Runx2+ (82.63 ± 1.8%), collagen 1α+ (74 ± 3.3%), and Osterix+ cells (70 ± 1.9%) [335]. Meanwhile, the main constituent of type-H blood vessels, endothelial cells (ECs), can orchestrate the self-renewal and differentiation of mesenchymal stem and progenitor cells via the angiocrine (e.g., FGFs, PDGFs, and BMPs) and juxtacrine (e.g., notch signaling). This process is not only involved in the regulation of bone development and homeostasis, but also plays an important role in bone defect regeneration [[335], [336], [337], [338], [339]].
In contrast, sinusoidal capillaries distributed in the bone marrow area have low expression of CD31 and Emcn; thus, they are classified as type L (CD31low Emcnlow). There are no osteogenic-related stem cells around this type of blood vessel [335]. Therefore, type-H vascular ECs in the metaphysis and endosteum can be a potential target for promoting bone regeneration. At present, the factors reportedly involved in coupling type-H vessel formation and osteogenesis mainly include PDGF-BB, SLIT3, HIF-α, notch, and VEGFA. Their influence on the type-H vessels’ biological function and their interaction with osteogenesis are summarized in Table 2 and Fig. 10. From these results, we can see that type-H ECs and osteoblast cell lines are closely linked by multiple factors that can be used in next-generation angioinductive scaffolds. In the future, researchers should further explore the biomolecules that play a vital role in this angiogenesis–osteogenesis interaction and apply them in BTE. In the next chapter, we further discuss another critical factor in bone healing—immunomodulation—which is the initiator of the bone-healing process, as well as the participant throughout the process. The current application of immunomodulatory factors in BTE is also detailed.
Table 2.
Factors involved in coupling type H vessel formation and osteogenesis.
| Factors | Property | Main source/location | Effect on type H vessel | Coupling effect on osteogenesis | Reference |
|---|---|---|---|---|---|
| PDGF-BB | Growth factor | Macrophage/non-resorbing osteoclast lineage cells | Induce type H vessel formation during bone modeling and remodeling | Promote the migration and differentiation of MSCs and endothelial progenitor cells; Stimulate bone formation in the ovariectomy induced osteoporotic mouse model |
[340] |
| Macrophage-lineage TRAP+ cells | Recruit periosteum-derived cells to the periosteal surface and direct its differentiation which contribute to cortical bone formation | [341] | |||
| VEGF-A | Growth factor | Chondrocytes; Osteoclasts; Osteoblasts | Promote type H vessel formation; However, persistent VEGFA signaling can impair PDGF-BB effects on pericytes leading to leaky blood vessels | Couple angiogenesis to enhance osteogenesis; Direct the differentiation of MSCs into osteoblast; However, high concentration of VEGF recruit osteoclast resulting in bone loss |
[[342], [343], [344], [345]] |
| SLIT3 | SLIT ligand (protein) | Osteoblast | Promote type H vessel formation | Mediate osteogenesis by regulating production of type H vessel | [346] |
| Osteoclast | Promote osteoblast migration and proliferation via activating β-catenin; Suppress osteoclast differentiation and bone resorption in an autocrine manner |
[347] | |||
| HIF-1α | Transcription factor | Osteoprogenitors; Osteoblast; Endothelial cell |
Activation of HIF-1α signaling in ECs led to an increased formation of type H vessels | Activation of HIF-1α promote the formation of trabecular bone and increase the numbers of Runx2+ and Osterix+ osteoprogenitors; The expression of HIF-1α in ECs decreases with age, accompanied by a decrease in type H vessels and bone mass; Promote bone regeneration in a VEGF dependent manner |
[335,348] |
| Notch (Delta-like 4) | Receptor (ligand) | Endothelial cell | Promote type H vessel formation and EC Noggin (Notch signaling activator) secretion | Promote proliferation and inhibit differentiation of mesenchymal progenitors; Promote proliferation and differentiation of osteoblasts; Activation of endothelial-cell-specific Notch signaling increase Runx2+ osteoprogenitors and osteoblastic differentiation, as well as promote chondrocyte maturation, bone growth and the formation of trabeculae |
[335,342,349,350] |
Fig. 10.
Mechanisms coupling angiogenesis and osteogenesis. a) Type-H vessels are mainly located at the metaphysis and endosteum, which are rich in osteoprogenitors and MSCs. Several factors have been proved to proliferate type-H ECs, including PDGF-BB secreted by preosteoclasts and TRAP+ macrophages (blue arrows), SLIT3 secreted by osteoblasts and osteoclasts (green arrows), and VEGF released by osteoblasts, osteoclasts and chondrocytes (pink arrows). The formation of type-H ECs will further facilitate osteogenesis by bone-producing cells (dotted arrow). b) The influence of type-H ECs on osteogenesis cells. Blood fluid stress can activate notch/Dll4 signaling, which can further induce the release of Noggin. Noggin promotes the proliferation and differentiation of osteoprogenitors and the maturation of chondrocytes. In a hypoxic environment, HIF-1α is upregulated in ECs and osteoprogenitors, which can trigger the expression of VEGF and promote vessel growth. Besides, the activation of HIF-1α facilitates the self-renewal and proliferation of osteoprogenitors. Compared to type L, type-H ECs have a strong expression of multiple cytokines, including FGF1, TGFβ, and PDGF, which play essential roles in the osteoprogenitors' survival, proliferation, and differentiation.
3.3. Biomolecular delivery for immunomodulation
Promotion of the osteogenic effect of osteoblast cell lines and the angiogenic effect of vascular ECs has been the focus of BTE for many years. However, as mentioned earlier, this nonspecific anabolism involved in osteogenesis and angiogenesis is only a part of the healing process. The immune response triggered by immune cells dominates the nonspecific catabolism in the inflammation phase and participates in the regulation of catabolism/anabolism in the bone formation and remodeling phases, playing a vital role in the healing process. Therefore, the immunomodulatory effect of bone healing has become a field of concern in recent years, and the design of immunomodulatory BTE scaffolds has become a novel approach to promote bone regeneration [47]. Unlike scaffolds that directly promote osteogenesis and angiogenesis, the immunomodulatory scaffolds generally indirectly affect the healing process by regulating the functions of immune cells. Thus, giving insight into the biological functions of various immune cells, the crosstalk between immune cells and their interaction with bone repair-related cells is the basis and key to designing such scaffolds.
Immune cells involved in bone healing mainly include polymorphonuclear cells (neutrophils, eosinophils, and basophils), lymphocytes (NK cells, γδT cells, innate lymphoid cells, T and B lymphocytes), and mononuclear phagocyte cells (monocytes and macrophages) [294]. These immune cells can be activated by ECM changes or regulated by other cells. Their primary function is to perform specific immune functions. For example, neutrophils are usually recruited soon after the injury and are responsible for wound detection and removal of dead cells and debris [351]. They can also secrete various antimicrobial substances to hinder pathogen growth and release chemokines to recruit more neutrophils and other immune cells such as macrophages to the injury sites [352].
In addition to performing direct immune functions, cytokines secreted by immune cells can directly or indirectly affect bone regeneration. For example, macrophages, which are responsible for the phagocytosis of unwanted debris and the recruitment of other cells, can secrete various cytokines to regulate inflammation, as well as control the differentiation of MSCs and the function of osteoblasts, thereby playing an important role in tissue remodeling and bone turnover [353]. In turn, cells involved in bone repair, such as MSCs, also regulate the biological activity of immune cells. For example, IL1RA secreted by MSCs can control the polarization of macrophages toward the M2 phenotype and inhibit B-cell differentiation in vivo [354]. Therefore, bone immune regulation is a complex network directed by immune cells, bone-tissue cells, and the extracellular environment. Several reviews discussed the role of these immune cells in bone healing [47,294,355,356]; however, the exact crosstalk pattern between immune cells and other cells involved in bone regeneration has yet to be elucidated.
At present, macrophages are the most widely reported immune cells in BTE. Like other inflammatory cells, two types of macrophages (M1 and M2 types) play an important role in bone regeneration and profoundly affect the regeneration site's microenvironment via cytokine secretion. As mentioned earlier, M2-type macrophages can secrete anti-inflammatory factors (e.g., IL-4 and 10) and growth factors (e.g., PDGF and VEGF), participating in the anabolism of bone regeneration. Thus, promoting M2-type macrophage polarization has become a common immunomodulation idea in BTE.
Loi et al. co-cultured polarized murine macrophages and preosteoblastic MC3T3 cells to investigate the effect of macrophage polarization on the MC3T3-E1's osteogenic ability. They found that modulating macrophages into the M2 phenotype by IL‐4 increased the osteogenic effects of osteoblasts [357]. Recently, Qian et al. fabricated silk fibroin (SF)-functionalized electrospun PCL fibers using layer-by-layer assembly and decorated SF with IL-4, resulting in macrophage polarization to M2 at the early stage and a mitigated foreign body reaction at the late-stage in a murine subcutaneous model [358]. However, pro-inflammatory cells in the inflammation phase are also of great significance. Excessive suppression of the inflammatory response may lead to undesirable results.
Vi et al. confirmed that the depletion of macrophages in transgenic mice led to early skeletal growth retardation as they found progressive osteoporosis. Besides, they found that the ablation of macrophages resulted in a 60% reduction in the number of BMSCs and hindered the cells from differentiating into osteoblasts [359]. In turn, the introduction of pro-inflammatory factors at the proper time could aid in promoting bone regeneration. Guihard et al. found that oncostatin M (OSM), a pro-inflammatory cytokine of the IL-6 family, produced by monocytes and M1 type macrophages, could induce osteoblast differentiation and matrix mineralization of human MSCs and inhibit adipogenesis [147]. This result indicated that the inflammation phase is of great significance to normal bone healing.
When applying these immunomodulatory factors, we should follow the spatiotemporal bone-healing process and promote healing without affecting the orderly progress. For example, Spiller et al. used BTE scaffolds to deliver pro-inflammatory and anti-inflammatory factors in an orderly fashion to achieve satisfactory bone repair effects. They loaded INF-γ and IL4 on the scaffold using two different approaches, enabling it to achieve short-term release of INF-γ and sustained release of IL4 to promote the sequential polarization of M1-and M2-type macrophages, resulting in enhanced angiogenesis and healing [360]. This process complies with the natural bone immunoregulatory process, which provides a feasible guideline for applying immunomodulatory in BTE.
In addition to macrophages, there are other immune cells involved in bone regeneration and can be the target for immunomodulatory effects. For example, CD4+ regulatory T cells (Tregs)—a distinct subpopulation of T cells—are critical regulators of the immune system that prevent autoimmunity and maintain transplantation tolerance by suppressing the activation and proliferation of other T cells [361]. Tregs as inflammation inhibitors regulate the differentiation of macrophages into M2 macrophages and play a synergistic role with B cells in bone immunological homeostasis [[362], [363], [364]]. Recently, Liu et al. developed a PLLA nanofibrous spongy microsphere, an injectable scaffold that could burst release the Treg recruitment cytokines (IL-2/TGF-β) and controlled release the Treg marker miR-10a. This scaffold was proven to induce Treg enrichment, expansion, and Treg-mediated immune therapy against bone loss in a mouse periodontitis model [365].
Another research group fabricated a macroporous hydrogel-based scaffold that permitted cellular infiltration and released BMP-2 to facilitate the recruitment of host stromal cells and their osteogenic differentiation. They confirmed that the differentiated stromal cells produced delta-like ligand-4 (DLL-4), which could promote Treg generation and further mitigate the inflammatory response against allogeneic implantation [366]. This result also indicated that the delivery of BMP-2 could have dual effects, including directly inducing bone regeneration and indirectly regulating Treg immunomodulation, which provides a new strategy for BTE application.
According to research thus far, immune cells are important participants in bone tissue homeostasis and regeneration. They communicate and regulate the bone-healing process through immunological cytokines. Thus, delivering an immune cytokine can activate specific immune cell functions, which in turn can affect bone regeneration (Fig. 11). However, caution should be exercised when choosing the type of regulated immune cells, cytokines, and their administrative patterns. Because an immunomodulatory factor could have multiple effects, and in each bone-healing phase the immune cell that plays the dominant role could be different. Moreover, the communication mechanism between immune cells is still unclear, and excessive immunological intervention may delay the natural bone-healing process. Thus, an in-depth investigation into the immune mechanism of bone healing is the basis for designing immunoregulatory BTE scaffolds. This design should also follow the natural bone spatiotemporal healing process, to obtain reliable regeneration outcomes.
Fig. 11.
Crosstalk among immune cells and its effect on osteogenesis. During the bone homeostasis and healing process, immune cells can regulate the balance between osteoblastogenesis and osteoclastogenesis. They communicate and cooperate via cytokines [294,355], which can be used as biomolecules in BTE immunomodulatory scaffolds.
In addition to the delivery of immunoregulatory cytokines, we should also consider the interaction between the scaffold's inherent properties and the host immune system. Recently, the construction of biomimetic scaffolds with excellent immunomodulatory properties has become a focal point in BTE. For instance, Mahon et al. developed an immunomodulatory scaffold containing bone-mimicking nanosized hydroxyapatite particles. They confirmed that, compared with commercially available micron-sized hydroxyapatite, the nanosized hydroxyapatite could preferentially polarize human macrophages toward the M2 phenotype, and specifically enhance the production of the anti-inflammatory cytokine (IL-10), possessing a greater potential for bone tissue repair and regeneration [367].
Furthermore, Jin et al. fabricated an immunomodulatory nanointerface that could recruit host MSCs and promote endogenous bone regeneration by inducing macrophage polarization through IL-4 [368]. In fact, the biological, chemical, and physical properties of the scaffold material all have immune effects to a certain extent. Exploration in this field can provide ideas for material selection, morphological construction, and surface modification of scaffold materials. This, however, is not the focus of this review. The reviews by Nair et al. and Lee et al. provided detailed insights in that field [47,369].
4. Conclusions and future perspectives
This review reveals that BTE development closely follows the cognition of the bone's physiological environment and healing process. The structure of natural bone tissue is complex. In the future, researchers in the field will continue to focus on developing materials that mimic the hierarchical and anisotropic bone tissue structure since an osteoconductive structure is conducive for bone regeneration and vascular ingrowth, and can also serve as a desirable template for further bioactive modification.
Notably, the scaffold component is the basis for emulating natural structures. Natural bone components are orderly and complex, formed by embryonic development, during which the organic and inorganic components coordinate and assemble to form the bone ECM. At present, the continued update of research on bone compositions and functions and the continuous emergence of highly plastic and biodegradable materials have provided researchers with a framework for selecting scaffold components. Besides, with the emergence of new manufacturing approaches (e.g., AM) and the combined use of various traditional fabrication technologies, researchers can flexibly combine the framework and the method, taking natural bone tissue as a template to develop more promising biomimetic scaffolds for BTE.
However, it should be noted that the goal of designing biomimetic BTE scaffolds is not to achieve complete consistency with the natural bone tissue, because the close imitation of this complex structure and composition is extremely challenging and unnecessary. The healing of bone defects and the physiological remodeling of natural bone tissue are dynamic processes, and bone tissue has superior regeneration ability. Therefore, the design of a biomimetic BTE scaffold should take natural bone tissue as a template to facilitate the natural healing process, which is also the original intention of applying biodegradable materials.
At present, the basic bone-healing process and related cytokines have been revealed by several studies, which provide references for research into the delivery of biomolecules via BTE scaffolds. The current investigation on cytokines is mainly focused on the biological function of a specific cytokine or cytokine family, and the crosstalk network of cytokines between bone-tissue cells has not been fully understood. Under such circumstances, when designing BTE scaffolds to deliver cytokines, we should beware that the regulation of natural cytokines in the bone-healing process is phased, dynamic, and coordinated, and then cautiously apply them without interfering with the natural bone-healing process. In addition, with a deeper understanding of the bone cells’ crosstalk during the healing process, researchers are not only focusing on the direct regulation of osteoblast cell lines, but also are shifting their attention to the angiogenesis cell line and immune cells. By regulating the biological effects of these cells, they can create scaffolds that comply with the natural healing process, indirectly and effectively promoting bone regeneration. In the future, researchers should explore the major factors that regulate the bone-healing process and apply them to construct more valuable and reliable BTE scaffolds.
Note that the biomimetic and biomolecule delivery designs discussed in Sections 3, 4 are not independent. Both should be considered when developing BTE scaffolds that meet the bone's physiological needs. This design consideration is presented in Fig. 12.
Fig. 12.
Considerations for constructing BTE scaffolds. Biomimetic and biomolecule delivery designs are not independent. An integral design consideration is needed. The biomaterials of scaffolds largely affect the mechanical strength, the basic biological property, and the biodegradability of scaffolds, while the fabrication approaches commonly determine the morphology of scaffolds. The physical–mechanical property (both mechanical strength and morphology) further influence the biological properties. The biomolecules inside the scaffolds are delivered via scaffold degradation, which could greatly affect the cell fate, particularly differentiation. This property of degradation is consistent with the biomolecules' delivery pattern, which could be affected by the scaffolds' matrix material and morphology. We should also beware that scaffold degradation must be accompanied by physical–mechanical and biological changes. The combined biological properties will influence the cell fate, including vascular cells, bone-producing cell lines, and immune cells. These cells will also interact with each other and determine the final tissue regeneration effects.
The advancement of BTE will rely on the progress of materials engineering and the exploration of bone biology; the two fields are complementary. The exploration of bone biology will provide ideas for constructing BTE scaffolds with specific biological functions, and the process of materials engineering will be the basis for realizing those ideas. For next-generation BTE scaffolds, researchers should focus on both frontier exploration and clinical applications. Apparently, biomimetic scaffolds suitable for clinical applications will be the research direction of next-generation scaffold materials. The direction will also include 1) designing anisotropic osteoconductive materials based on bone defect morphology and local mechanical stress; 2) designing individualized spatial structure based on blood supply; and 3) highly tunable biodegradability. Precision will be the research direction for biomolecule delivery in next-generation BTE scaffolds. The research direction will include 1) accurately exploring the release patterns of various cytokines, especially the release peak, in the human bone-healing process; 2) precisely diagnosing the human bone-healing phases, to provide a reference for the safe and effective delivery of cytokines; and, 3)accurately delivering and releasing cytokines through BTE scaffolds with highly controllable drug delivery systems, which may even have a secondary delivery ability under non-invasive or minimally invasive conditions, based on this review. The outlook for this part is presented in Fig. 13.
Fig. 13.
Outlook for next-generation BTE scaffold.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81771048, 81870743), Research and Develop Program, West China Hospital of Stomatology Sichuan University (RD-03-202012), and Sichuan Science and Technology Program (2020YFS0170).
We would like to express our gratitude to Ziyue Yin (Central Academy of Fine Arts, China) and Zijie Yin (Communication University of China) who made tremendous contribution to figures painting.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2021.03.043.
Contributor Information
Jun Liu, Email: junliu@scu.edu.cn.
Yunbing Wang, Email: yunbing.wang@scu.edu.cn.
Zhihe Zhao, Email: zhzhao@scu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Stevens M.M. Biomaterials for bone tissue engineering. Mater. Today. 2008;11(5):18–25. [Google Scholar]
- 2.DiGirolamo D.J., Clemens T.L., Kousteni S. The skeleton as an endocrine organ. Nat. Rev. Rheumatol. 2012;8(11):674–683. doi: 10.1038/nrrheum.2012.157. [DOI] [PubMed] [Google Scholar]
- 3.Loebel C., Burdick J.A. Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell. 2018;22(3):325–339. doi: 10.1016/j.stem.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Defects B. 2011. Standard Guide for Pre-clinical in Vivo Evaluation in Critical Size Segmental Bone Defects. [Google Scholar]
- 5.Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European union: medical management, epidemiology and economic burden. A report prepared in collaboration with the international osteoporosis foundation (IOF) and the European federation of pharmaceutical industry associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar G., Narayan B. Morbidity at bone graft donor sites. Classic Papers in Orthopaedics. 2014:503–505. [Google Scholar]
- 7.John T.A.S., Vaccaro A.R., Sah A.P., Schaefer M., Hilibrand A. Physical and monetary costs associated with autogenous bone graft harvesting. Am. J. Orthoped. 2003;32(1):18–23. [PubMed] [Google Scholar]
- 8.Finkemeier C.G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Donati D., Di Bella C., Col angeli Marco, Bianchi G., Mercuri M. The use of massive bone allografts in bone tumor surgery of the limb. Curr. Orthop. 2005;19:393–399. [Google Scholar]
- 10.Lanza Robert. fourth ed. 2014. Principles of Tissue Engineering. xxix. [Google Scholar]
- 11.Henkel J., Woodruff M.A., Epari D.R., Steck R., Glatt V., Dickinson I.C., Choong P.F., Schuetz M.A., Hutmacher D.W. Bone regeneration based on tissue engineering conceptions - a 21st century perspective. Bone Res. 2013;1(3):216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehy E.J., Kelly D.J., O'Brien F.J. Biomaterial-based endochondral bone regeneration: a shift from traditional tissue engineering paradigms to developmentally inspired strategies. Mater Today Bio. 2019;3 doi: 10.1016/j.mtbio.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richbourg N.R., Peppas N.A., Sikavitsas V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J Tissue Eng Regen Med. 2019;13(8):1275–1293. doi: 10.1002/term.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C., Ashok D., Nisbet D.R., Gautam V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials. 2019;219:119366. doi: 10.1016/j.biomaterials.2019.119366. [DOI] [PubMed] [Google Scholar]
- 15.Chan G., Mooney D.J. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26(7):382–392. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Murphy W.L., McDevitt T.C., Engler A.J. Materials as stem cell regulators. Nat. Mater. 2014;13(6):547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C, Ashok D, Nisbet DR, Gautam V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials. 2019 doi: 10.1016/j.biomaterials.2019.119366. [DOI] [PubMed] [Google Scholar]
- 20.Goodman S.B., Pajarinen J., Yao Z., Lin T. Inflammation and bone repair: from particle disease to tissue regeneration. Front Bioeng Biotechnol. 2019;7:230. doi: 10.3389/fbioe.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankenson K.D., Gagne K., Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015;94:3–12. doi: 10.1016/j.addr.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Loi F., Cordova L.A., Pajarinen J., Lin T.H., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibon E., Lu L., Goodman S.B. Aging, inflammation, stem cells, and bone healing. Stem Cell Res. Ther. 2016;7:44. doi: 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currey J.D. The structure and mechanics of bone. J. Mater. Sci. 2012;47(1):41–54. [Google Scholar]
- 25.Reznikov N., Shahar R., Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10(9):3815–3826. doi: 10.1016/j.actbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Wegst U.G.K., Bai H., Saiz E., Tomsia A.P., Ritchie R.O. Bioinspired structural materials. Nat. Mater. 2015;14(1):23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 27.Reznikov N., Shahar R., Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10(9):3815–3826. doi: 10.1016/j.actbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Lopes D., Martins-Cruz C., Oliveira M.B., Mano J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240–275. doi: 10.1016/j.biomaterials.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Xu S., Zhou S., Xu W., Leary M., Choong P., Qian M., Brandt M., Xie Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review. Biomaterials. 2016;83:127–141. doi: 10.1016/j.biomaterials.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3(Supplement 3):S131. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Y., Li L. The stem cell niches in bone. J. Clin. Invest. 2006;116(5):1195. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gori J.L., Butler J.M., Chan Y.-Y., Chandrasekaran D., Poulos M.G., Ginsberg M., Nolan D.J., Elemento O., Wood B.L., Adair J.E., Rafii S., Kiem H.-P. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J. Clin. Invest. 2015;125(3):1243–1254. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shekaran A., García A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. 2011;96(1):261–272. doi: 10.1002/jbm.a.32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidalgo-Bastida L.A., Cartmell S.H. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng. B Rev. 2010;16(4):405–412. doi: 10.1089/ten.TEB.2009.0714. [DOI] [PubMed] [Google Scholar]
- 35.Hassenkam T., Fantner G.E., Cutroni J.A., Weaver J.C., Morse D.E., Hansma P.K. High-resolution AFM imaging of intact and fractured trabecular bone. Bone. 2004;35(1):4–10. doi: 10.1016/j.bone.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Olszta M.J., Cheng X., Jee S.S., Kumar R., Kim Y.-Y., Kaufman M.J., Douglas E.P., Gower L.B. Bone structure and formation: a new perspective. Mater. Sci. Eng. R Rep. 2007;58(3):77–116. [Google Scholar]
- 37.Vallet-Regí M., González-Calbet J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004;32(1):1–31. [Google Scholar]
- 38.Nair A.K., Gautieri A., Chang S.W., Buehler M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013;4:1724. doi: 10.1038/ncomms2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He G., Dahl T., Veis A., George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003;2(8):552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 40.Sroga G.E., Karim L., Colón W., Vashishth D. Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and proteomics methodology. Mol. Cell. Proteomics. 2011;10(9) doi: 10.1074/mcp.M110.006718. M110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sroga G.E., Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr. Osteoporos. Rep. 2012;10(2):141–150. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toosi S., Behravan J. BioFactors; Oxford, England): 2019. Osteogenesis and Bone Remodeling: A Focus on Growth Factors and Bioactive Peptides. [DOI] [PubMed] [Google Scholar]
- 43.Katagiri T., Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8(6) doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu K., Olsen B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest. 2016;126(2):509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu K., Olsen B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiqui J.A., Partridge N.C. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda, Md. 2016;31(3):233–245. doi: 10.1152/physiol.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J., Byun H., Madhurakkat Perikamana S.K., Lee S., Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv Healthc Mater. 2019;8(4) doi: 10.1002/adhm.201801106. [DOI] [PubMed] [Google Scholar]
- 48.Johnson R.W., Brennan H.J., Vrahnas C., Poulton I.J., McGregor N.E., Standal T., Walker E.C., Koh T.-T., Nguyen H., Walsh N.C., Forwood M.R., Martin T.J., Sims N.A. The primary function of gp130 signaling in osteoblasts is to maintain bone formation and strength, rather than promote osteoclast formation. J. Bone Miner. Res. 2014;29(6):1492–1505. doi: 10.1002/jbmr.2159. [DOI] [PubMed] [Google Scholar]
- 49.Horwood N.J. Macrophage polarization and bone formation: a review. Clin. Rev. Allergy Immunol. 2016;51(1):79–86. doi: 10.1007/s12016-015-8519-2. [DOI] [PubMed] [Google Scholar]
- 50.Zhou D., Huang C., Lin Z., Zhan S., Kong L., Fang C., Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014;26(2):192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Haumer A., Bourgine P.E., Occhetta P., Born G., Tasso R., Martin I. Delivery of cellular factors to regulate bone healing. Adv. Drug Deliv. Rev. 2018;129:285–294. doi: 10.1016/j.addr.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Kelly D.J., Jacobs C.R. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90(1):75–85. doi: 10.1002/bdrc.20173. [DOI] [PubMed] [Google Scholar]
- 53.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18(12):728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen J.H., Vincent L.G., Fuhrmann A., Choi Y.S., Hribar K.C., Taylor-Weiner H., Chen S., Engler A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014;13(10):979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C.D.P., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.-W., Tewari M., Rehfeldt F., Speicher D.W., Discher D.E. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149) doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith L.R., Cho S., Discher D.E. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology. 2018;33(1):16–25. doi: 10.1152/physiol.00026.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L., Jiang F., Qiao Y., Li H., Wei Z., Huang T., Lan J., Xia Y., Li J. Nucleoskeletal stiffness regulates stem cell migration and differentiation through lamin A/C. J. Cell. Physiol. 2018;233(7):5112–5118. doi: 10.1002/jcp.26336. [DOI] [PubMed] [Google Scholar]
- 58.Hadden W.J., Young J.L., Holle A.W., McFetridge M.L., Kim D.Y., Wijesinghe P., Taylor-Weiner H., Wen J.H., Lee A.R., Bieback K., Vo B.N., Sampson D.D., Kennedy B.F., Spatz J.P., Engler A.J., Choi Y.S. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl. Acad. Sci. U. S. A. 2017;114(22):5647–5652. doi: 10.1073/pnas.1618239114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang M.P., Subbiah R., Kim I.G., Lee K.E., Park J., Kim S.H., Park K. Approximating bone ECM: crosslinking directs individual and coupled osteoblast/osteoclast behavior. Biomaterials. 2016;103:22–32. doi: 10.1016/j.biomaterials.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 60.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 61.Yang W., Han W., He W., Li J., Wang J., Feng H., Qian Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells, Materials science & engineering. C, Materials for biological applications. 2016;60:45–53. doi: 10.1016/j.msec.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 62.A.A. Baig, J.L. Fox, R.A. Young, Z. Wang, J. Hsu, W.I. Higuchi, A. Chhettry, H. Zhuang, M. Otsuka, Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters, Calcif. Tissue Int. 64(5) 437-449. [DOI] [PubMed]
- 63.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang C.H., Chen M.H., Young T.H., Jeng J.H., Chen Y.J. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 2009;108(6):1263–1273. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- 65.D'Elia N.L., Mathieu C., Hoemann C.D., Laiuppa J.A., Santillan G.E., Messina P.V. Bone-repair properties of biodegradable hydroxyapatite nano-rod superstructures. Nanoscale. 2015;7(44):18751–18762. doi: 10.1039/c5nr04850h. [DOI] [PubMed] [Google Scholar]
- 66.Xia L., Lin K., Jiang X., Fang B., Xu Y., Liu J., Zeng D., Zhang M., Zhang X., Chang J., Zhang Z. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials. 2014;35(30):8514–8527. doi: 10.1016/j.biomaterials.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 67.Li S., Yu W., Zhang W., Zhang G., Yu L., Lu E. Evaluation of highly carbonated hydroxyapatite bioceramic implant coatings with hierarchical micro-/nanorod topography optimized for osseointegration. Int. J. Nanomed. 2018;13:3643–3659. doi: 10.2147/IJN.S159989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J., Wang M., Chen F., Wei Y., Chen X., Zhou Y., Yang X., Zhu X., Tu C., Zhang X. Nano-hydroxyapatite coating promotes porous calcium phosphate ceramic-induced osteogenesis via BMP/Smad signaling pathway. Int. J. Nanomed. 2019;14:7987–8000. doi: 10.2147/IJN.S216182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao C., Wang X., Gao L., Jing L., Zhou Q., Chang J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018;73:509–521. doi: 10.1016/j.actbio.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 70.Wehrli F.W. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J. Magn. Reson. Imag. 2007;25(2):390–409. doi: 10.1002/jmri.20807. [DOI] [PubMed] [Google Scholar]
- 71.Chen J.-H., Liu C., You L., Simmons C.A. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. J. Biomech. 2010;43(1):108–118. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 72.Wittkowske C., Reilly G.C., Lacroix D., Perrault C.M. In Vitro bone cell models: impact of fluid shear stress on bone formation. Front Bioeng Biotechnol. 2016;4:87. doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolff Julius. 1987. The Law of Bone Remodelling. [Google Scholar]
- 74.Frost H.M. Bone "mass" and the "mechanostat": a proposal. Anat. Rec. 1987;219(1):1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 75.Bonivtch A.R., Bonewald L.F., Nicolella D.P. Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J. Biomech. 2007;40(10):2199–2206. doi: 10.1016/j.jbiomech.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang B., Liu Y., Zheng D., Shan S., Wang C., Gao Y., Wang J., Xie Y., Zhang Y., Li Q. The effects of mechanical stretch on the biological characteristics of human adipose-derived stem cells. J. Cell Mol. Med. 2019;23(6):4244–4255. doi: 10.1111/jcmm.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X., Yan J., He F., Zhong D., Yang H., Pei M., Luo Z.P. Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic. Biol. Med. 2018;126:187–201. doi: 10.1016/j.freeradbiomed.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X., Liu Y., Ding W., Shi J., Li S., Liu Y., Wu M., Wang H. Mechanical stretch-induced osteogenic differentiation of human jaw bone marrow mesenchymal stem cells (hJBMMSCs) via inhibition of the NF-kappaB pathway. Cell Death Dis. 2018;9(2):207. doi: 10.1038/s41419-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.C.H. Huang, M.H. Chen, T.H. Young, J.H. Jeng, Y.J. Chen, Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells, J. Cell. Biochem. 108(6) 1263-1273. [DOI] [PubMed]
- 80.Burger E.H., Klein-Nulend J., Veldhuijzen J.P. Mechanical stress and osteogenesis in vitro. J. Bone Miner. Res. 1992;7(Suppl 2):S397–S401. doi: 10.1002/jbmr.5650071406. [DOI] [PubMed] [Google Scholar]
- 81.W. Claudia, G.C. Reilly, L. Damien, C.M. Perrault, In Vitro bone cell models: impact of fluid shear stress on bone formation, Frontiers in Bioengineering & Biotechnology 4. [DOI] [PMC free article] [PubMed]
- 82.Burger E.H., Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. Faseb Journal Official Publication of the Federation of American Societies for Experimental Biology. 1999;13(Suppl):S101. 8. [PubMed] [Google Scholar]
- 83.McCoy R.J., O'Brien F.J. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review, Tissue engineering. Part B, Reviews. 2010;16(6):587–601. doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- 84.Dallas S.L., Prideaux M., Bonewald L.F. The osteocyte: an endocrine cell and more. Endocr. Rev. 2013;34(5):658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim K.M., Choi Y.J., Hwang J.H., Kim A.R., Cho H.J., Hwang E.S., Park J.Y., Lee S.H., Hong J.H. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bratengeier C., Liszka A., Hoffman J., Bakker A.D., Fahlgren A. High shear stress amplitude in combination with prolonged stimulus duration determine induction of osteoclast formation by hematopoietic progenitor cells. Faseb. J. 2020;34(3):3755–3772. doi: 10.1096/fj.201901458R. [DOI] [PubMed] [Google Scholar]
- 87.Patwari P., Lee R.T. Mechanical control of tissue morphogenesis. Circ. Res. 2008;103(3):234–243. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hendrikson W.J., Deegan A.J., Yang Y., van Blitterswijk C.A., Verdonschot N., Moroni L., Rouwkema J. Influence of additive manufactured scaffold architecture on the distribution of surface strains and fluid flow shear stresses and expected osteochondral cell differentiation. Frontiers in bioengineering and biotechnology. 2017;5:6. doi: 10.3389/fbioe.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritton S.P., Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ali D., Sen S. Computational fluid dynamics study of the effects of surface roughness on permeability and fluid flow-induced wall shear stress in scaffolds. Ann. Biomed. Eng. 2018;46(12):2023–2035. doi: 10.1007/s10439-018-2101-z. [DOI] [PubMed] [Google Scholar]
- 91.Cameron J.A., Milner D.J., Lee J.S., Cheng J., Jasiuk I.M. Employing the biology of successful fracture repair to heal critical size bone defects. Curr. Top. Microbiol. Immunol. 2012;367:113–132. doi: 10.1007/82_2012_291. [DOI] [PubMed] [Google Scholar]
- 92.Percival C.J., Richtsmeier J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dynam. 2013;242(8):909–922. doi: 10.1002/dvdy.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Debnath S., Yallowitz A.R., McCormick J., Lalani S., Zhang T., Xu R., Li N., Liu Y., Yang Y.S., Eiseman M., Shim J.-H., Hameed M., Healey J.H., Bostrom M.P., Landau D.A., Greenblatt M.B. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duan X., Bradbury S.R., Olsen B.R., Berendsen A.D. VEGF stimulates intramembranous bone formation during craniofacial skeletal development. Matrix Biol. 2016;52–54:127–140. doi: 10.1016/j.matbio.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pizette S., Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev. Biol. 2000;219(2):237–249. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- 96.Akiyama H., Chaboissier M.C., Martin J.F., Schedl A., de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lanske B., Karaplis A.C., Lee K., Luz A., Vortkamp A., Pirro A., Karperien M., Defize L.H., Ho C., Mulligan R.C., Abou-Samra A.B., Juppner H., Segre G.V., Kronenberg H.M. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273(5275):663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 98.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 99.Minina E., Kreschel C., Naski M.C., Ornitz D.M., Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell. 2002;3(3):439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 100.Romeo S.G., Alawi K.M., Rodrigues J., Singh A., Kusumbe A.P., Ramasamy S.K. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 2019;21(4):430–441. doi: 10.1038/s41556-019-0304-7. [DOI] [PubMed] [Google Scholar]
- 101.Bianco P., Cancedda F.D., Riminucci M., Cancedda R. Bone formation via cartilage models: the "borderline" chondrocyte. Matrix Biol. 1998;17(3):185–192. doi: 10.1016/s0945-053x(98)90057-9. [DOI] [PubMed] [Google Scholar]
- 102.Taichman R.S. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105(7):2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 103.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loi F., Córdova L.A., Pajarinen J., Lin T.-h., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malizos K.N., Papatheodorou L.K. The healing potential of the periosteum: molecular aspects. Injury. 2005;36(3):S13–S19. doi: 10.1016/j.injury.2005.07.030. Supplement. [DOI] [PubMed] [Google Scholar]
- 106.Crane J.L., Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J. Clin. Invest. 2014;124(2):466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang R., Wang X., Zhou Y., Xiao Y. RANKL-induced M1 macrophages are involved in bone formation. Bone research. 2017;5:17019. doi: 10.1038/boneres.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sierra-Filardi E., Nieto C., Domínguez-Soto A., Barroso R., Sánchez-Mateos P., Puig-Kroger A., López-Bravo M., Joven J., Ardavín C., Rodríguez-Fernández J.L., Sánchez-Torres C., Mellado M., Corbí A.L. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014;192(8):3858–3867. doi: 10.4049/jimmunol.1302821. [DOI] [PubMed] [Google Scholar]
- 109.Schindeler A., McDonald M.M., Bokko P., Little D.G. Bone remodeling during fracture repair: the cellular picture. Semin. Cell Dev. Biol. 2008;19(5):459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 110.Michalski M.N., McCauley L.K. Macrophages and skeletal health. Pharmacol. Ther. 2017;174:43–54. doi: 10.1016/j.pharmthera.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knighton D.R., Hunt T.K., Scheuenstuhl H., Halliday B.J., Werb Z., Banda M.J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 112.Ai-Aql Z.S., Alagl A.S., Graves D.T., Gerstenfeld L.C., Einhorn T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008;87(2):107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bahney C.S., Zondervan R.L., Allison P., Theologis A., Ashley J.W., Ahn J., Miclau T., Marcucio R.S., Hankenson K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019;37(1):35–50. doi: 10.1002/jor.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schindeler A., McDonald M.M., Bokko P., Little D.G. Bone remodeling during fracture repair: the cellular picture. Semin. Cell Dev. Biol. 2008;19(5):459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 115.Phillips A.M. Overview of the fracture healing cascade. Injury. 2005;36(Suppl 3):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 116.Peng H., Wright V., Usas A., Gearhart B., Shen H.C., Cummins J., Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Invest. 2002;110(6):751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Street J., Bao M., deGuzman L., Bunting S., Peale F.V., Jr., Ferrara N., Steinmetz H., Hoeffel J., Cleland J.L., Daugherty A., van Bruggen N., Redmond H.P., Carano R.A., Filvaroff E.H. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. U. S. A. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cho T.-J., Gerstenfeld L.C., Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J. Bone Miner. Res. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 119.Joyce M.E., Roberts A.B., Sporn M.B., Bolander M.E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J. Cell Biol. 1990;110(6):2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deckers M.M., van Bezooijen R.L., van der Horst G., Hoogendam J., van Der Bent C., Papapoulos S.E., Löwik C.W. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 121.Tsumaki N., Tanaka K., Arikawa-Hirasawa E., Nakase T., Kimura T., Thomas J.T., Ochi T., Luyten F.P., Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J. Cell Biol. 1999;144(1):161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang T., Zhang X., Bikle D.D. Osteogenic differentiation of periosteal cells during fracture healing. J. Cell. Physiol. 2017;232(5):913–921. doi: 10.1002/jcp.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malizos K.N., Papatheodorou L.K. The healing potential of the periosteum molecular aspects. Injury. 2005;36(Suppl 3):S13–S19. doi: 10.1016/j.injury.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 124.Du X., Xie Y., Xian C.J., Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J. Cell. Physiol. 2012;227(12):3731–3743. doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 125.Ikebuchi Y., Aoki S., Honma M., Hayashi M., Sugamori Y., Khan M., Kariya Y., Kato G., Tabata Y., Penninger J.M., Udagawa N., Aoki K., Suzuki H. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561(7722):195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 126.Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 127.Zaidi M., Cardozo C.P. Receptor becomes a ligand to control bone remodelling. Nature. 2018;561(7722):180–181. doi: 10.1038/d41586-018-05960-x. [DOI] [PubMed] [Google Scholar]
- 128.Kon T., Cho T.-J., Aizawa T., Yamazaki M., Nooh N., Graves D., Gerstenfeld L.C., Einhorn T.A. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001;16(6):1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 129.Davizon-Castillo P., McMahon B., Aguila S., Bark D., Ashworth K., Allawzi A., Campbell R.A., Montenont E., Nemkov T., D'Alessandro A., Clendenen N., Shih L., Sanders N.A., Higa K., Cox A., Padilla-Romo Z., Hernandez G., Wartchow E., Trahan G.D., Nozik-Grayck E., Jones K., Pietras E.M., DeGregori J., Rondina M.T., Di Paola J. TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134(9):727–740. doi: 10.1182/blood.2019000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou F.H., Foster B.K., Zhou X.-F., Cowin A.J., Xian C.J. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J. Bone Miner. Res. 2006;21(7):1075–1088. doi: 10.1359/jbmr.060410. [DOI] [PubMed] [Google Scholar]
- 131.Fu X., Han B., Cai S., Lei Y., Sun T., Sheng Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen. 2009;17(2):185–191. doi: 10.1111/j.1524-475X.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 132.Lu Z., Wang G., Dunstan C.R., Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cell. Dev. 2012;21(13):2420–2429. doi: 10.1089/scd.2011.0589. [DOI] [PubMed] [Google Scholar]
- 133.Lehmann W., Edgar C.M., Wang K., Cho T.J., Barnes G.L., Kakar S., Graves D.T., Rueger J.M., Gerstenfeld L.C., Einhorn T.A. Tumor necrosis factor alpha (TNF-α) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36(2):300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Kindle L., Rothe L., Kriss M., Osdoby P., Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J. Bone Miner. Res. 2006;21(2):193–206. doi: 10.1359/JBMR.051027. [DOI] [PubMed] [Google Scholar]
- 135.Kobayashi K., Takahashi N., Jimi E., Udagawa N., Takami M., Kotake S., Nakagawa N., Kinosaki M., Yamaguchi K., Shima N., Yasuda H., Morinaga T., Higashio K., Martin T.J., Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pettipher E.R., Higgs G.A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc. Natl. Acad. Sci. U. S. A. 1986;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miossec P., Cavender D., Ziff M. Interleukin 1 derived from human endothelial cells enhances the binding and chemotactic step of T lymphocyte emigration. Clin. Exp. Immunol. 1988;73(2):250–254. [PMC free article] [PubMed] [Google Scholar]
- 138.Ueda Y., Cain D.W., Kuraoka M., Kondo M., Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J. Immunol. 2009;182(10):6477–6484. doi: 10.4049/jimmunol.0803961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maruotti N., Corrado A., Rotondo C., Cantatore F.P. Janus kinase inhibitors role in bone remodeling. J. Cell. Physiol. 2020;235(3):1915–1920. doi: 10.1002/jcp.29149. [DOI] [PubMed] [Google Scholar]
- 140.Ma T., Miyanishi K., Suen A., Epstein N.J., Tomita T., Smith R.L., Goodman S.B. Human interleukin-1-induced murine osteoclastogenesis is dependent on RANKL, but independent of TNF-alpha. Cytokine. 2004;26(3):138–144. doi: 10.1016/j.cyto.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Watanabe K., Tanaka Y., Morimoto I., Yahata K., Zeki K., Fujihira T., Yamashita U., Eto S. Interleukin-4 as a potent inhibitor of bone resorption. Biochem. Biophys. Res. Commun. 1990;172(3):1035–1041. doi: 10.1016/0006-291x(90)91550-c. [DOI] [PubMed] [Google Scholar]
- 142.Lind M., Deleuran B., Yssel H., Fink-Eriksen E., Thestrup-Pedersen K. IL-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts. Cytokine. 1995;7(1):78–82. doi: 10.1006/cyto.1995.1010. [DOI] [PubMed] [Google Scholar]
- 143.Bastidas-Coral A.P., Hogervorst J.M.A., Forouzanfar T., Kleverlaan C.J., Koolwijk P., Klein-Nulend J., Bakker A.D. IL-6 counteracts the inhibitory effect of IL-4 on osteogenic differentiation of human adipose stem cells. J. Cell. Physiol. 2019;234(11):20520–20532. doi: 10.1002/jcp.28652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Palmqvist P., Lundberg P., Persson E., Johansson A., Lundgren I., Lie A., Conaway H.H., Lerner U.H. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J. Biol. Chem. 2006;281(5):2414–2429. doi: 10.1074/jbc.M510160200. [DOI] [PubMed] [Google Scholar]
- 145.Einhorn T.A., Majeska R.J., Rush E.B., Levine P.M., Horowitz M.C. The expression of cytokine activity by fracture callus. J. Bone Miner. Res. 1995;10(8):1272–1281. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 146.Bellido T., Borba V.Z., Roberson P., Manolagas S.C. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138(9):3666–3676. doi: 10.1210/endo.138.9.5364. [DOI] [PubMed] [Google Scholar]
- 147.Guihard P., Danger Y., Brounais B., David E., Brion R., Delecrin J., Richards C.D., Chevalier S., Rédini F., Heymann D., Gascan H., Blanchard F. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cell. 2012;30(4):762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 148.Tamura T., Udagawa N., Takahashi N., Miyaura C., Tanaka S., Yamada Y., Koishihara Y., Ohsugi Y., Kumaki K., Taga T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. U. S. A. 1993;90(24):11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang X., Ricciardi B.F., Hernandez-Soria A., Shi Y., Pleshko Camacho N., Bostrom M.P.G. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41(6):928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun G., Wang Y., Ti Y., Wang J., Zhao J., Qian H. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clin. Exp. Pharmacol. Physiol. 2017;44(4):455–462. doi: 10.1111/1440-1681.12719. [DOI] [PubMed] [Google Scholar]
- 151.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.-L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Y., Wang L., Kikuiri T., Akiyama K., Chen C., Xu X., Yang R., Chen W., Wang S., Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011;17(12):1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reinke S., Geissler S., Taylor W.R., Schmidt-Bleek K., Juelke K., Schwachmeyer V., Dahne M., Hartwig T., Akyüz L., Meisel C., Unterwalder N., Singh N.B., Reinke P., Haas N.P., Volk H.-D., Duda G.N. Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 2013;5(177) doi: 10.1126/scitranslmed.3004754. 177ra36. [DOI] [PubMed] [Google Scholar]
- 154.Kasama T., Isozaki T., Odai T., Matsunawa M., Wakabayashi K., Takeuchi H.T., Matsukura S., Adachi M., Tezuka M., Kobayashi K. Expression of angiopoietin-1 in osteoblasts and its inhibition by tumor necrosis factor-alpha and interferon-gamma. Transl. Res. 2007;149(5):265–273. doi: 10.1016/j.trsl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 155.Gowen M., Nedwin G.E., Mundy G.R. Preferential inhibition of cytokine-stimulated bone resorption by recombinant interferon gamma. J. Bone Miner. Res. 1986;1(5):469–474. doi: 10.1002/jbmr.5650010511. [DOI] [PubMed] [Google Scholar]
- 156.Ji J.-D., Park-Min K.-H., Shen Z., Fajardo R.J., Goldring S.R., McHugh K.P., Ivashkiv L.B. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J. Immunol. 2009;183(11):7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mermut S., Bengi A.O., Akin E., Kürkçü M., Karaçay S. Effects of interferon-gamma on bone remodeling during experimental tooth movement. Angle Orthod. 2007;77(1):135–141. doi: 10.2319/122105-451R.1. [DOI] [PubMed] [Google Scholar]
- 158.Cheng H., Jiang W., Phillips F.M., Haydon R.C., Peng Y., Zhou L., Luu H.H., An N., Breyer B., Vanichakarn P., Szatkowski J.P., Park J.Y., He T.-C. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 159.Loeser R.F., Pacione C.A., Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48(8):2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 160.Onishi T., Ishidou Y., Nagamine T., Yone K., Imamura T., Kato M., Sampath T.K., ten Dijke P., Sakou T. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22(6):605–612. doi: 10.1016/s8756-3282(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 161.Abe E., Yamamoto M., Taguchi Y., Lecka-Czernik B., O'Brien C.A., Economides A.N., Stahl N., Jilka R.L., Manolagas S.C. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J. Bone Miner. Res. 2000;15(4):663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 162.Okamoto M., Murai J., Yoshikawa H., Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J. Bone Miner. Res. 2006;21(7):1022–1033. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 163.Itoh K., Udagawa N., Katagiri T., Iemura S., Ueno N., Yasuda H., Higashio K., Quinn J.M., Gillespie M.T., Martin T.J., Suda T., Takahashi N. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142(8):3656–3662. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 164.Tachi K., Takami M., Zhao B., Mochizuki A., Yamada A., Miyamoto Y., Inoue T., Baba K., Kamijo R. Bone morphogenetic protein 2 enhances mouse osteoclast differentiation via increased levels of receptor activator of NF-κB ligand expression in osteoblasts. Cell Tissue Res. 2010;342(2):213–220. doi: 10.1007/s00441-010-1052-y. [DOI] [PubMed] [Google Scholar]
- 165.Ehnert S., Baur J., Schmitt A., Neumaier M., Lucke M., Dooley S., Vester H., Wildemann B., Stöckle U., Nussler A.K. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PloS One. 2010;5(11) doi: 10.1371/journal.pone.0014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T.R., Peng X., Hu J., Feng X., Van Hul W., Wan M., Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Serra R., Johnson M., Filvaroff E.H., LaBorde J., Sheehan D.M., Derynck R., Moses H.L. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J. Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang X., Chen L., Xu X., Li C., Huang C., Deng C.X. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van Beuningen H.M., van der Kraan P.M., Arntz O.J., van den Berg W.B. Protection from interleukin 1 induced destruction of articular cartilage by transforming growth factor beta: studies in anatomically intact cartilage in vitro and in vivo. Ann. Rheum. Dis. 1993;52(3):185–191. doi: 10.1136/ard.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alliston T., Choy L., Ducy P., Karsenty G., Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20(9):2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yoshioka Y., Ono M., Maeda A., Kilts T.M., Hara E.S., Khattab H., Ueda J., Aoyama E., Oohashi T., Takigawa M., Young M.F., Kuboki T. CCN4/WISP-1 positively regulates chondrogenesis by controlling TGF-β3 function. Bone. 2016;83:162–170. doi: 10.1016/j.bone.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ganguly S.S., Daft P.G., Cao J., Meng X., Zhong Z.A., Vander Ark A., Meadows A., Madaj Z., Williams B., Li X. Loss of myeloid-specific TGF-β signaling decreases CTHRC1 to downregulate bFGF and the development of H1993-induced osteolytic bone lesions. Cancers. 2018;10(12) doi: 10.3390/cancers10120463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Steinbrech D.S., Mehrara B.J., Rowe N.M., Dudziak M.E., Luchs J.S., Saadeh P.B., Gittes G.K., Longaker M.T. Gene expression of TGF-beta, TGF-beta receptor, and extracellular matrix proteins during membranous bone healing in rats. Plast. Reconstr. Surg. 2000;105(6):2028–2038. doi: 10.1097/00006534-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 174.Weivoda M.M., Ruan M., Pederson L., Hachfeld C., Davey R.A., Zajac J.D., Westendorf J.J., Khosla S., Oursler M.J. Osteoclast TGF-β receptor signaling induces Wnt1 secretion and couples bone resorption to bone formation. J. Bone Miner. Res. 2016;31(1):76–85. doi: 10.1002/jbmr.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ota K., Quint P., Ruan M., Pederson L., Westendorf J.J., Khosla S., Oursler M.J. TGF-β induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology. 2013;154(10):3745–3752. doi: 10.1210/en.2013-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andersen R.K., Zaher W., Larsen K.H., Ditzel N., Drews K., Wruck W., Adjaye J., Abdallah B.M., Kassem M. Association between in vivo bone formation and ex vivo migratory capacity of human bone marrow stromal cells. Stem Cell Res. Ther. 2015;6:196. doi: 10.1186/s13287-015-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Caverzasio J., Biver E., Thouverey C. Predominant role of PDGF receptor transactivation in Wnt3a-induced osteoblastic cell proliferation. J. Bone Miner. Res. 2013;28(2):260–270. doi: 10.1002/jbmr.1748. [DOI] [PubMed] [Google Scholar]
- 178.Caplan A.I., Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J. Orthop. Res. : official publication of the Orthopaedic Research Society. 2011;29(12):1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 179.Martin I., Muraglia A., Campanile G., Cancedda R., Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138(10):4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- 180.Lu X., Su N., Yang J., Huang W., Li C., Zhao L., He Q., Du X., Shen Y., Chen B., Chen L. Fibroblast growth factor receptor 1 regulates the differentiation and activation of osteoclasts through Erk1/2 pathway. Biochem. Biophys. Res. Commun. 2009;390(3):494–499. doi: 10.1016/j.bbrc.2009.09.123. [DOI] [PubMed] [Google Scholar]
- 181.Minina E., Kreschel C., Naski M.C., Ornitz D.M., Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell. 2002;3(3):439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 182.Chia S.-L., Sawaji Y., Burleigh A., McLean C., Inglis J., Saklatvala J., Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60(7):2019–2027. doi: 10.1002/art.24654. [DOI] [PubMed] [Google Scholar]
- 183.Hu K., Olsen B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest. 2016;126(2):509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peng H., Wright V., Usas A., Gearhart B., Shen H.-C., Cummins J., Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Invest. 2002;110(6):751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Peng H., Usas A., Olshanski A., Ho A.M., Gearhart B., Cooper G.M., Huard J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J. Bone Miner. Res. 2005;20(11):2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 186.Madry H., Kaul G., Cucchiarini M., Stein U., Zurakowski D., Remberger K., Menger M.D., Kohn D., Trippel S.B. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12(15):1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 187.Granero-Moltó F., Myers T.J., Weis J.A., Longobardi L., Li T., Yan Y., Case N., Rubin J., Spagnoli A. Mesenchymal stem cells expressing insulin-like growth factor-I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: analyses of MSCIGF autocrine and paracrine regenerative effects. Stem Cell. 2011;29(10):1537–1548. doi: 10.1002/stem.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Reible B., Schmidmaier G., Moghaddam A., Westhauser F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Enochson L., Stenberg J., Brittberg M., Lindahl A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis Cartilage. 2014;22(4):566–577. doi: 10.1016/j.joca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 190.Zeng Q., Li X., Beck G., Balian G., Shen F.H. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40(2):374–381. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 191.Kleinschmidt K., Wagner-Ecker M., Bartek B., Holschbach J., Richter W. Superior angiogenic potential of GDF-5 and GDF-5(V453/V456) compared with BMP-2 in a rabbit long-bone defect model. J Bone Joint Surg Am. 2014;96(20):1699–1707. doi: 10.2106/JBJS.M.01462. [DOI] [PubMed] [Google Scholar]
- 192.Chang S.C., Hoang B., Thomas J.T., Vukicevic S., Luyten F.P., Ryba N.J., Kozak C.A., Reddi A.H., Moos M. Cartilage-derived morphogenetic proteins New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J. Biol. Chem. 1994;269(45):28227–28234. [PubMed] [Google Scholar]
- 193.Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E., Capla J.M., Galiano R.D., Levine J.P., Gurtner G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 194.Ho C.-Y., Sanghani A., Hua J., Coathup M., Kalia P., Blunn G. Mesenchymal stem cells with increased stromal cell-derived factor 1 expression enhanced fracture healing. Tissue Eng. 2015;21(3–4):594–602. doi: 10.1089/ten.tea.2013.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Toupadakis C.A., Wong A., Genetos D.C., Chung D.-J., Murugesh D., Anderson M.J., Loots G.G., Christiansen B.A., Kapatkin A.S., Yellowley C.E. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J. Orthop. Res. : official publication of the Orthopaedic Research Society. 2012;30(11):1853–1859. doi: 10.1002/jor.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Glantschnig H., Fisher J.E., Wesolowski G., Rodan G.A., Reszka A.A., M-CSF TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10(10):1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 197.De Vries T.J., Schoenmaker T., Aerts D., Grevers L.C., Souza P.P.C., Nazmi K., van de Wiel M., Ylstra B., Lent P.L.V., Leenen P.J.M., Everts V. M-CSF priming of osteoclast precursors can cause osteoclastogenesis-insensitivity, which can be prevented and overcome on bone. J. Cell. Physiol. 2015;230(1):210–225. doi: 10.1002/jcp.24702. [DOI] [PubMed] [Google Scholar]
- 198.D'Amelio P., Roato I., D'Amico L., Veneziano L., Suman E., Sassi F., Bisignano G., Ferracini R., Gargiulo G., Castoldi F., Pescarmona G.P., Isaia G.C. Bone and bone marrow pro-osteoclastogenic cytokines are up-regulated in osteoporosis fragility fractures. Osteoporos. Int. 2011;22(11):2869–2877. doi: 10.1007/s00198-010-1496-7. [DOI] [PubMed] [Google Scholar]
- 199.Kon T., Cho T.J., Aizawa T., Yamazaki M., Nooh N., Graves D., Gerstenfeld L.C., Einhorn T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001;16(6):1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 200.Cassuto J., Folestad A., Göthlin J., Malchau H., Kärrholm J. The key role of proinflammatory cytokines, matrix proteins, RANKL/OPG and Wnt/β-catenin in bone healing of hip arthroplasty patients. Bone. 2018;107:66–77. doi: 10.1016/j.bone.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 201.Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y.X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W.J. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 202.Stegen S., Laperre K., Eelen G., Rinaldi G., Fraisl P., Torrekens S., Van Looveren R., Loopmans S., Bultynck G., Vinckier S., Meersman F., Maxwell P.H., Rai J., Weis M., Eyre D.R., Ghesquière B., Fendt S.M., Carmeliet P., Carmeliet G. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565(7740):511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen X., Fan H., Deng X., Wu L., Yi T., Gu L., Zhou C., Fan Y., Zhang X. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials. 2018;8(11):960. doi: 10.3390/nano8110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vallet-Regí M., Ruiz-Hernández E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv. Mater. 2011;23(44):5177–5218. doi: 10.1002/adma.201101586. [DOI] [PubMed] [Google Scholar]
- 205.Murphy C.M., Haugh M.G., O'Brien F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3):461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 206.Taniguchi N., Fujibayashi S., Takemoto M., Sasaki K., Otsuki B., Nakamura T., Matsushita T., Kokubo T., Matsuda S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment, Materials science & engineering. C, Materials for biological applications. 2016;59:690–701. doi: 10.1016/j.msec.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 207.Cheng M.-q., Wahafu T., Jiang G.-f., Liu W., Qiao Y.-q., Peng X.-c., Cheng T., Zhang X.-l., He G., Liu X.-y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016;6:24134. doi: 10.1038/srep24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen Z., Yan X., Yin S., Liu L., Liu X., Zhao G., Ma W., Qi W., Ren Z., Liao H., Liu M., Cai D., Fang H. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater Sci Eng C Mater Biol Appl. 2020;106 doi: 10.1016/j.msec.2019.110289. [DOI] [PubMed] [Google Scholar]
- 209.Du Y., Liu H., Yang Q., Wang S., Wang J., Ma J., Noh I., Mikos A.G., Zhang S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017;137:37–48. doi: 10.1016/j.biomaterials.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Freeman F.E., Browe D.C., Nulty J., Von Euw S., Grayson W.L., Kelly D.J. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cell. Mater. 2019;38:168–187. doi: 10.22203/eCM.v038a12. [DOI] [PubMed] [Google Scholar]
- 211.Gloria A., Frydman B., Lamas M.L., Serra A.C., Martorelli M., Coelho J.F.J., Fonseca A.C., Domingos M. The influence of poly(ester amide) on the structural and functional features of 3D additive manufactured poly(ε-caprolactone) scaffolds. Mater Sci Eng C Mater Biol Appl. 2019;98:994–1004. doi: 10.1016/j.msec.2019.01.063. [DOI] [PubMed] [Google Scholar]
- 212.Zhang L., Yang G., Johnson B.N., Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33. doi: 10.1016/j.actbio.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 213.Gleeson J.P., Plunkett N.A., O'Brien F.J. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur. Cell. Mater. 2010;20:218–230. doi: 10.22203/ecm.v020a18. [DOI] [PubMed] [Google Scholar]
- 214.Bhaskar B., Owen R., Bahmaee H., Wally Z., Sreenivasa Rao P., Reilly G.C. Composite porous scaffold of PEG/PLA support improved bone matrix deposition in vitro compared to PLA-only scaffolds. J. Biomed. Mater. Res. 2018;106(5):1334–1340. doi: 10.1002/jbm.a.36336. [DOI] [PubMed] [Google Scholar]
- 215.Zhang J., Xiao D., He X., Shi F., Luo P., Zhi W., Duan K., Weng J. A novel porous bioceramic scaffold by accumulating hydroxyapatite spheres for large bone tissue engineering. III: characterization of porous structure, Materials science & engineering. C, Materials for biological applications. 2018;89:223–229. doi: 10.1016/j.msec.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 216.Li Y., Liao C., Tjong S.C. Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomaterials. 2019;9(4) doi: 10.3390/nano9040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bittner S.M., Smith B.T., Diaz-Gomez L., Hudgins C.D., Melchiorri A.J., Scott D.W., Fisher J.P., Mikos A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019;90:37–48. doi: 10.1016/j.actbio.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.De Santis R., Gloria A., Russo T., D'Amora U., Zeppetelli S., Tampieri A., Herrmannsdörfer T., Ambrosio L. A route toward the development of 3D magnetic scaffolds with tailored mechanical and morphological properties for hard tissue regeneration: preliminary study. Virtual Phys. Prototyp. 2011;6(4):189–195. [Google Scholar]
- 219.Shuai C., Guo W., Wu P., Yang W., Hu S., Xia Y., Feng P. A graphene oxide-Ag co-dispersing nanosystem: dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018;347:322–333. [Google Scholar]
- 220.Feng P., Kong Y., Yu L., Li Y., Gao C., Peng S., Pan H., Zhao Z., Shuai C. Molybdenum disulfide nanosheets embedded with nanodiamond particles: co-dispersion nanostructures as reinforcements for polymer scaffolds. Appl Mater Today. 2019;17:216–226. [Google Scholar]
- 221.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M., Zhao X., Cui X., Ruan C., Liu W. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv. Sci. 2019;6(15) doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Deng Y., Sun A.X., Overholt K.J., Yu G.Z., Fritch M.R., Alexander P.G., Shen H., Tuan R.S., Lin H. Enhancing chondrogenesis and mechanical strength retention in physiologically relevant hydrogels with incorporation of hyaluronic acid and direct loading of TGF-β. Acta Biomater. 2019;83:167–176. doi: 10.1016/j.actbio.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ghouse S., Reznikov N., Boughton O.R., Babu S., Geoffrey Ng K.C., Blunn G., Cobb J.P., Stevens M.M., Jeffers J.R.T. The design and in vivo testing of a locally stiffness-matched porous scaffold. Appl Mater Today. 2019;15:377–388. doi: 10.1016/j.apmt.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lou H.-Y., Zhao W., Li X., Duan L., Powers A., Akamatsu M., Santoro F., McGuire A.F., Cui Y., Drubin D.G., Cui B. Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl. Acad. Sci. U. S. A. 2019;116(46):23143–23151. doi: 10.1073/pnas.1910166116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gao X., Song J., Zhang Y., Xu X., Zhang S., Ji P., Wei S. Bioinspired design of polycaprolactone composite nanofibers as artificial bone extracellular matrix for bone regeneration application. ACS Appl. Mater. Interfaces. 2016;8(41):27594–27610. doi: 10.1021/acsami.6b10417. [DOI] [PubMed] [Google Scholar]
- 226.Wan Y., Wang Y., Liu Z., Qu X., Han B., Bei J., Wang S. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(L-lactide) Biomaterials. 2005;26(21):4453–4459. doi: 10.1016/j.biomaterials.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 227.Te Boekhorst V., Preziosi L., Friedl P. Plasticity of cell migration in vivo and in silico. Annu. Rev. Cell Dev. Biol. 2016;32:491–526. doi: 10.1146/annurev-cellbio-111315-125201. [DOI] [PubMed] [Google Scholar]
- 228.Gui N., Xu W., Myers D.E., Shukla R., Tang H.P., Qian M. The effect of ordered and partially ordered surface topography on bone cell responses: a review. Biomater Sci. 2018;6(2):250–264. doi: 10.1039/c7bm01016h. [DOI] [PubMed] [Google Scholar]
- 229.Bobbert F.S.L., Zadpoor A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B. 2017;5(31):6175–6192. doi: 10.1039/c7tb00741h. [DOI] [PubMed] [Google Scholar]
- 230.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 231.Saidova A.A., Vorobjev I.A. Lineage commitment, signaling pathways, and the cytoskeleton systems in mesenchymal stem cells. Tissue Eng. B Rev. 2020;26(1):13–25. doi: 10.1089/ten.TEB.2019.0250. [DOI] [PubMed] [Google Scholar]
- 232.Wang S., Hu F., Li J., Zhang S., Shen M., Huang M., Shi X. Design of electrospun nanofibrous mats for osteogenic differentiation of mesenchymal stem cells. Nanomedicine. 2018;14(7):2505–2520. doi: 10.1016/j.nano.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 233.Xu T., Yang H., Yang D., Yu Z.Z. Polylactic acid nanofiber scaffold decorated with chitosan islandlike topography for bone tissue engineering. ACS Appl. Mater. Interfaces. 2017;9(25):21094–21104. doi: 10.1021/acsami.7b01176. [DOI] [PubMed] [Google Scholar]
- 234.Ding J., Zhang J., Li J., Li D., Xiao C., Xiao H., Yang H., Zhuang X., Chen X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019;90:1–34. [Google Scholar]
- 235.Zhang Y., Liu X., Zeng L., Zhang J., Zuo J., Zou J., Ding J., Chen X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019;29(36) [Google Scholar]
- 236.Raghunath J., Georgiou G., Armitage D., Nazhat S.N., Sales K.M., Butler P.E., Seifalian A.M. Degradation studies on biodegradable nanocomposite based on polycaprolactone/polycarbonate (80:20%) polyhedral oligomeric silsesquioxane. J. Biomed. Mater. Res. 2009;91(3):834–844. doi: 10.1002/jbm.a.32335. [DOI] [PubMed] [Google Scholar]
- 237.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gelse K., Pöschl E., Aigner T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 239.Glowacki J., Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 240.Preethi Soundarya S., Haritha Menon A., Viji Chandran S., Selvamurugan N. Bone tissue engineering: scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018;119:1228–1239. doi: 10.1016/j.ijbiomac.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 241.Ferreira A.M., Gentile P., Chiono V., Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191–3200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 242.Li Q., Lei X., Wang X., Cai Z., Lyu P., Zhang G. Hydroxyapatite/collagen three-dimensional printed scaffolds and their osteogenic effects on human bone marrow-derived mesenchymal stem cells. Tissue Eng. 2019;25(17–18):1261–1271. doi: 10.1089/ten.TEA.2018.0201. [DOI] [PubMed] [Google Scholar]
- 243.Martin V., Ribeiro I.A., Alves M.M., Gonçalves L., Claudio R.A., Grenho L., Fernandes M.H., Gomes P., Santos C.F., Bettencourt A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2019;101:15–26. doi: 10.1016/j.msec.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 244.Lee J., Kim G. Three-dimensional hierarchical nanofibrous collagen scaffold fabricated using fibrillated collagen and pluronic F-127 for regenerating bone tissue. ACS Appl. Mater. Interfaces. 2018;10(42):35801–35811. doi: 10.1021/acsami.8b14088. [DOI] [PubMed] [Google Scholar]
- 245.Durham E.L., Howie R.N., Hall S., Larson N., Oakes B., Houck R., Grey Z., Steed M., LaRue A.C., Muise-Helmericks R., Cray J. Optimizing bone wound healing using BMP2 with absorbable collagen sponge and Talymed nanofiber scaffold. J. Transl. Med. 2018;16(1):321. doi: 10.1186/s12967-018-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang M.-L., Cheng J., Xiao Y.-C., Yin R.-F., Feng X. Raloxifene microsphere-embedded collagen/chitosan/β-tricalcium phosphate scaffold for effective bone tissue engineering. Int. J. Pharm. 2017;518(1):80–85. doi: 10.1016/j.ijpharm.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 247.Maliha S.G., Lopez C.D., Coelho P.G., Witek L., Cox M., Meskin A., Rusi S., Torroni A., Cronstein B.N., Flores R.L. Plast Reconstr Surg; 2019. Bone tissue engineering in the growing calvarium using dipyridamole-coated 3D printed bioceramic scaffolds: construct optimization and effects to cranial suture patency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Castaño I.M., Raftery R.M., Chen G., Cavanagh B., Quinn B., Duffy G.P., O’Brien F.J., Curtin C.M. Rapid bone repair with the recruitment of CD206(+)M2-like macrophages using non-viral scaffold-mediated miR-133a inhibition of host cells. Acta Biomater. 2020;109:267–279. doi: 10.1016/j.actbio.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 249.Kim H., Yang G.H., Kim G. Three-dimensional gelatin/PVA scaffold with nanofibrillated collagen surface for applications in hard-tissue regeneration. Int. J. Biol. Macromol. 2019;135:21–28. doi: 10.1016/j.ijbiomac.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 250.Kim H.D., Amirthalingam S., Kim S.L., Lee S.S., Rangasamy J., Hwang N.S. Biomimetic materials and fabrication approaches for bone tissue engineering. Advanced Healthcare Materials. 2017;6(23) doi: 10.1002/adhm.201700612. [DOI] [PubMed] [Google Scholar]
- 251.Navarro M., Michiardi A., Castaño O., Planell J.A. Biomaterials in orthopaedics. J. R. Soc. Interface. 2008;5(27):1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rather H.A., Jhala D., Vasita R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C. 2019;103 doi: 10.1016/j.msec.2019.109761. [DOI] [PubMed] [Google Scholar]
- 253.Kim H., Camata R.P., Vohra Y.K., Lacefield W.R. Control of phase composition in hydroxyapatite/tetracalcium phosphate biphasic thin coatings for biomedical applications. J. Mater. Sci. Mater. Med. 2005;16(10):961–966. doi: 10.1007/s10856-005-4430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mann S., Webb J., Williams R.J.P. Federal Republic of Germany; New York, NY, USA: 1989. Biomineralization : Chemical and Biochemical Perspectives, VCH, Weinheim. [Google Scholar]
- 255.Shih Y.R., Hwang Y., Phadke A., Kang H., Hwang N.S., Caro E.J., Nguyen S., Siu M., Theodorakis E.A., Gianneschi N.C., Vecchio K.S., Chien S., Lee O.K., Varghese S. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. U. S. A. 2014;111(3):990–995. doi: 10.1073/pnas.1321717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials. 1998;19(16):1473–1478. doi: 10.1016/s0142-9612(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 257.Oryan A., Alidadi S., Bigham-Sadegh A. Dicalcium phosphate anhydrous: an appropriate bioceramic in regeneration of critical-sized radial bone defects in rats. Calcif. Tissue Int. 2017;101(5):530–544. doi: 10.1007/s00223-017-0309-9. [DOI] [PubMed] [Google Scholar]
- 258.Bouler J.M., Pilet P., Gauthier O., Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: a review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 259.Götz W., Tobiasch E., Witzleben S., Schulze M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics. 2019;11(3) doi: 10.3390/pharmaceutics11030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yao F., LeGeros J.P., LeGeros R.Z. Simultaneous incorporation of carbonate and fluoride in synthetic apatites: effect on crystallographic and physico-chemical properties. Acta Biomater. 2009;5(6):2169–2177. doi: 10.1016/j.actbio.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 261.Xiao D., Zhang J., Zhang C., Barbieri D., Yuan H., Moroni L., Feng G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020;106:22–33. doi: 10.1016/j.actbio.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 262.Li J., Baker B.A., Mou X., Ren N., Qiu J., Boughton R.I., Liu H. Biopolymer/calcium phosphate scaffolds for bone tissue engineering. Advanced Healthcare Materials. 2014;3(4):469–484. doi: 10.1002/adhm.201300562. [DOI] [PubMed] [Google Scholar]
- 263.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., Chen S., Feng J.Q., Chow D.H., Xie X., Zheng L., Huang L., Huang S., Leung K., Lu N., Zhao L., Li H., Zhao D., Guo X., Chan K., Witte F., Chan H.C., Zheng Y., Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jang H.L., Zheng G.B., Park J., Kim H.D., Baek H.R., Lee H.K., Lee K., Han H.N., Lee C.K., Hwang N.S., Lee J.H., Nam K.T. In Vitro and in vivo evaluation of whitlockite biocompatibility: comparative study with hydroxyapatite and β-tricalcium phosphate. Adv Healthc Mater. 2016;5(1):128–136. doi: 10.1002/adhm.201400824. [DOI] [PubMed] [Google Scholar]
- 265.Kim H.D., Jang H.L., Ahn H.Y., Lee H.K., Park J., Lee E.S., Lee E.A., Jeong Y.H., Kim D.G., Nam K.T., Hwang N.S. Biomimetic whitlockite inorganic nanoparticles-mediated in situ remodeling and rapid bone regeneration. Biomaterials. 2017;112:31–43. doi: 10.1016/j.biomaterials.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 266.Bouler J.M., Pilet P., Gauthier O., Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: a review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 267.Sarkar N., Bose S. Liposome-encapsulated curcumin-loaded 3D printed scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces. 2019;11(19):17184–17192. doi: 10.1021/acsami.9b01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zeng H., Pathak J.L., Shi Y., Ran J., Liang L., Yan Q., Wu T., Fan Q., Li M., Bai Y. Indirect selective laser sintering-printed microporous biphasic calcium phosphate scaffold promotes endogenous bone regeneration via activation of ERK1/2 signaling. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab78ed. [DOI] [PubMed] [Google Scholar]
- 269.Zhao C., Qazvini N.T., Sadati M., Zeng Z., Huang S., De La Lastra A.L., Zhang L., Feng Y., Liu W., Huang B., Zhang B., Dai Z., Shen Y., Wang X., Luo W., Liu B., Lei Y., Ye Z., Zhao L., Cao D., Yang L., Chen X., Athiviraham A., Lee M.J., Wolf J.M., Reid R.R., Tirrell M., Huang W., de Pablo J.J., He T.C. A pH-triggered, self-assembled, and bioprintable hybrid hydrogel scaffold for mesenchymal stem cell based bone tissue engineering. ACS Appl. Mater. Interfaces. 2019;11(9):8749–8762. doi: 10.1021/acsami.8b19094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Touri M., Moztarzadeh F., Osman N.A.A., Dehghan M.M., Mozafari M. 3D-printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Mater Sci Eng C Mater Biol Appl. 2018;84:236–242. doi: 10.1016/j.msec.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 271.Shuai C., Yang W., Feng P., Peng S., Pan H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioactive Materials. 2021;6(2):490–502. doi: 10.1016/j.bioactmat.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Feng P., Wu P., Gao C., Yang Y., Guo W., Yang W., Shuai C. A multimaterial scaffold with tunable properties: toward bone tissue repair. Advanced Science. 2018;5(6) doi: 10.1002/advs.201700817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhao D., Zhu T., Li J., Cui L., Zhang Z., Zhuang X., Ding J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioactive Materials. 2021;6(2):346–360. doi: 10.1016/j.bioactmat.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kang Z., Zhang X., Chen Y., Akram M.Y., Nie J., Zhu X. Preparation of polymer/calcium phosphate porous composite as bone tissue scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 2):1125–1131. doi: 10.1016/j.msec.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 275.Shekaran A., García A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. 2011;96A(1):261–272. doi: 10.1002/jbm.a.32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 277.Happe C.L., Engler A.J. Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ. Res. 2016;118(2):296–310. doi: 10.1161/CIRCRESAHA.115.305139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.S. Mathews, R. Bhonde, P.K. Gupta, S. Totey, Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells, Differentiation 84(2) 185---192. [DOI] [PubMed]
- 279.Marie P.J. Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 2013;9(5):288–295. doi: 10.1038/nrendo.2013.4. [DOI] [PubMed] [Google Scholar]
- 280.Siebers M.C., ter Brugge P.J., Walboomers X.F., Jansen J.A. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review, Biomaterials. 2005;26(2):137–146. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 281.Coutu D.L., Kokkaliaris K.D., Kunz L., Schroeder T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat. Biotechnol. 2017;35(12):1202–1210. doi: 10.1038/nbt.4006. [DOI] [PubMed] [Google Scholar]
- 282.Aota S., Nomizu M., Yamada K.M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994;269(40):24756–24761. [PubMed] [Google Scholar]
- 283.Leahy D.J., Aukhil I., Erickson H.P. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84(1):155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 284.Agarwal R., González-García C., Torstrick B., Guldberg R.E., Salmerón-Sánchez M., García A.J. Simple coating with fibronectin fragment enhances stainless steel screw osseointegration in healthy and osteoporotic rats. Biomaterials. 2015;63:137–145. doi: 10.1016/j.biomaterials.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sato Y., Yamamoto K., Horiguchi S., Tahara Y., Nakai K., Kotani S.I., Oseko F., Pezzotti G., Yamamoto T., Kishida T., Kanamura N., Akiyoshi K., Mazda O. Nanogel tectonic porous 3D scaffold for direct reprogramming fibroblasts into osteoblasts and bone regeneration. Sci. Rep. 2018;8(1):15824. doi: 10.1038/s41598-018-33892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Francisco A.T., Hwang P.Y., Jeong C.G., Jing L., Chen J., Setton L.A. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014;10(3):1102–1111. doi: 10.1016/j.actbio.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yang F., Williams C.G., Wang D.-a., Lee H., Manson P.N., Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30):5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 288.Pataquiva-Mateus A.Y., Wu H.C., Lucchesi C., Ferraz M.P., Monteiro F.J., Spector M. Supplementation of collagen scaffolds with SPARC to facilitate mineralization. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100B(3):862–870. doi: 10.1002/jbm.b.32650. [DOI] [PubMed] [Google Scholar]
- 289.Lin Y., Huang S., Zou R., Gao X., Ruan J., Weir M.D., Reynolds M.A., Qin W., Chang X., Fu H., Xu H.H.K. Calcium phosphate cement scaffold with stem cell co-culture and prevascularization for dental and craniofacial bone tissue engineering. Dent. Mater. 2019;35(7):1031–1041. doi: 10.1016/j.dental.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 290.Trujillo S., Gonzalez-Garcia C., Rico P., Reid A., Windmill J., Dalby M.J., Salmeron-Sanchez M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials. 2020;252 doi: 10.1016/j.biomaterials.2020.120104. [DOI] [PubMed] [Google Scholar]
- 291.Bianco J.E.R., Rosa R.G., Congrains-Castillo A., Joazeiro P.P., Waldman S.D., Weber J.F., Saad S.T.O. Characterization of a novel decellularized bone marrow scaffold as an inductive environment for hematopoietic stem cells. Biomater Sci. 2019;7(4):1516–1528. doi: 10.1039/c8bm01503a. [DOI] [PubMed] [Google Scholar]
- 292.Kargar-Abarghouei E., Vojdani Z., Hassanpour A., Alaee S., Talaei-Khozani T. Characterization, recellularization, and transplantation of rat decellularized testis scaffold with bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018;9(1):324. doi: 10.1186/s13287-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Siebers M.C., ter Brugge P.J., Walboomers X.F., Jansen J.A. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review, Biomaterials. 2005;26(2):137–146. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 294.Yang D., Xiao J., Wang B., Li L., Kong X., Liao J. The immune reaction and degradation fate of scaffold in cartilage/bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;104 doi: 10.1016/j.msec.2019.109927. [DOI] [PubMed] [Google Scholar]
- 295.Sampath T.K., Maliakal J.C., Hauschka P.V., Jones W.K., Sasak H., Tucker R.F., White K.H., Coughlin J.E., Tucker M.M., Pang R.H. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J. Biol. Chem. 1992;267(28):20352–20362. [PubMed] [Google Scholar]
- 296.Cho T.J., Gerstenfeld L.C., Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J. Bone Miner. Res. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 297.Cho J.H., Lee J.H., Yeom J.S., Chang B.S., Yang J.J., Koo K.H., Hwang C.J., Lee K.B., Kim H.J., Lee C.K., Kim H., Suk K.S., Nam W.D., Han J. Efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: an open, active-controlled, randomized, multicenter trial. Spine J. 2017;17(12):1866–1874. doi: 10.1016/j.spinee.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 298.Coughlan M., Davies M., Mostert A.K., Nanda D., Willems P.C., Rosenberg G., Ferch R. A prospective, randomized, multicenter study comparing silicated calcium phosphate versus BMP-2 synthetic bone graft in posterolateral instrumented lumbar fusion for degenerative spinal disorders. Spine (Phila Pa 1976) 2018;43(15):E860–e868. doi: 10.1097/BRS.0000000000002678. [DOI] [PubMed] [Google Scholar]
- 299.Jung J., Yoo H.Y., Kim G.T., Lee J.W., Lee Y.A., Kim D.Y., Kwon Y.D. Short-term teriparatide and recombinant human bone morphogenetic protein-2 for regenerative approach to medication-related osteonecrosis of the jaw: a preliminary study. J. Bone Miner. Res. 2017;32(12):2445–2452. doi: 10.1002/jbmr.3237. [DOI] [PubMed] [Google Scholar]
- 300.Kim H.J., Chung J.H., Shin S.Y., Shin S.I., Kye S.B., Kim N.K., Kwon T.G., Paeng J.Y., Kim J.W., Oh O.H., Kook M.S., Yang H.J., Hwang S.J. Efficacy of rhBMP-2/hydroxyapatite on sinus floor augmentation: a multicenter, randomized controlled clinical trial. J. Dent. Res. 2015;94(9 Suppl) doi: 10.1177/0022034515594573. 158s-65s. [DOI] [PubMed] [Google Scholar]
- 301.Jo D.W., Cho Y.D., Seol Y.J., Lee Y.M., Lee H.J., Kim Y.K. A randomized controlled clinical trial evaluating efficacy and adverse events of different types of recombinant human bone morphogenetic protein-2 delivery systems for alveolar ridge preservation. Clin. Oral Implants Res. 2019;30(5):396–409. doi: 10.1111/clr.13423. [DOI] [PubMed] [Google Scholar]
- 302.Groeneveld E., Burger E. Bone morphogenetic proteins in human bone regeneration. 2000;142(1):9. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 303.Carragee E.J., Hurwitz E.L., Weiner B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 304.Betz O.B., Betz V.M., Nazarian A., Egermann M., Gerstenfeld L.C., Einhorn T.A., Vrahas M.S., Bouxsein M.L., Evans C.H. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14(13):1039–1044. doi: 10.1038/sj.gt.3302956. [DOI] [PubMed] [Google Scholar]
- 305.Wilson C.G., Martín-Saavedra F.M., Vilaboa N., Franceschi R.T. Advanced BMP gene therapies for temporal and spatial control of bone regeneration. J. Dent. Res. 2013;92(5):409–417. doi: 10.1177/0022034513483771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Becker S.T., Bolte H., Schünemann K., Seitz H., Bara J.J., Beck-Broichsitter B.E., Russo P.A., Wiltfang J., Warnke P.H. Endocultivation: the influence of delayed vs. simultaneous application of BMP-2 onto individually formed hydroxyapatite matrices for heterotopic bone induction. Int. J. Oral Maxillofac. Surg. 2012;41(9):1153–1160. doi: 10.1016/j.ijom.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 307.Caplan A.I., Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011;29(12):1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 308.Ranly D.M., McMillan J., Keller T., Lohmann C.H., Meunch T., Cochran D.L., Schwartz Z., Boyan B.D. Platelet-derived growth factor inhibits demineralized bone matrix-induced intramuscular cartilage and bone formation. A study of immunocompromised mice. J Bone Joint Surg Am. 2005;87(9):2052–2064. doi: 10.2106/JBJS.D.02752. [DOI] [PubMed] [Google Scholar]
- 309.Huang Y.C., Kaigler D., Rice K.G., Krebsbach P.H., Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J. Bone Miner. Res. 2005;20(5):848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 310.Kempen D.H.R., Lu L., Heijink A., Hefferan T.E., Creemers L.B., Maran A., Yaszemski M.J., Dhert W.J.A. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 311.Bao X., Zhu L., Huang X., Tang D., He D., Shi J., Xu G. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017;7(1):7814. doi: 10.1038/s41598-017-08412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Dou D.D., Zhou G., Liu H.W., Zhang J., Liu M.L., Xiao X.F., Fei J.J., Guan X.L., Fan Y.B. Sequential releasing of VEGF and BMP-2 in hydroxyapatite collagen scaffolds for bone tissue engineering: design and characterization. Int. J. Biol. Macromol. 2019;123:622–628. doi: 10.1016/j.ijbiomac.2018.11.099. [DOI] [PubMed] [Google Scholar]
- 313.Barati D., Shariati S.R.P., Moeinzadeh S., Melero-Martin J.M., Khademhosseini A., Jabbari E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Contr. Release. 2016;223:126–136. doi: 10.1016/j.jconrel.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tang W., Yu Y., Wang J., Liu H., Pan H., Wang G., Liu C. Enhancement and orchestration of osteogenesis and angiogenesis by a dual-modular design of growth factors delivery scaffolds and 26SCS decoration. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119645. [DOI] [PubMed] [Google Scholar]
- 315.Bayer E.A., Gottardi R., Fedorchak M.V., Little S.R. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J. Contr. Release. 2015;219:129–140. doi: 10.1016/j.jconrel.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 316.Samorezov J.E., Alsberg E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv. Drug Deliv. Rev. 2015;84:45–67. doi: 10.1016/j.addr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Cui L., Zhang J., Zou J., Yang X., Guo H., Tian H., Zhang P., Wang Y., Zhang N., Zhuang X., Li Z., Ding J., Chen X. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials. 2020;230:119617. doi: 10.1016/j.biomaterials.2019.119617. [DOI] [PubMed] [Google Scholar]
- 318.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P.G., Riminucci M., Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 319.Zhu T., Cui Y., Zhang M., Zhao D., Liu G., Ding J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioactive Materials. 2020;5(3):584–601. doi: 10.1016/j.bioactmat.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kolesky D.B., Homan K.A., Skylar-Scott M.A., Lewis J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. U. S. A. 2016;113(12):3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ngo M.T., Harley B.A.C. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120207. 120207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ortega N., Behonick D.J., Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14(2):86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Avraamides C.J., Garmy-Susini B., Varner J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Canc. 2008;8(8):604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sivaraj K.K., Adams R.H. Blood vessel formation and function in bone. Development. 2016;143(15):2706–2715. doi: 10.1242/dev.136861. [DOI] [PubMed] [Google Scholar]
- 325.Brown A., He H., Trumper E., Valdez J., Hammond P., Griffith L.G. Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments. Biomaterials. 2020;243:119921. doi: 10.1016/j.biomaterials.2020.119921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Tsao C.J., Pandolfi L., Wang X., Minardi S., Lupo C., Evangelopoulos M., Hendrickson T., Shi A., Storci G., Taraballi F., Tasciotti E. Electrospun patch functionalized with nanoparticles allows for spatiotemporal release of VEGF and PDGF-BB promoting in vivo neovascularization. ACS Appl. Mater. Interfaces. 2018;10(51):44344–44353. doi: 10.1021/acsami.8b19975. [DOI] [PubMed] [Google Scholar]
- 327.Dreyer C.H., Kjaergaard K., Ding M., Qin L. Vascular endothelial growth factor for in vivo bone formation: a systematic review. J Orthop Translat. 2020;24:46–57. doi: 10.1016/j.jot.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Greenberg J.I., Shields D.J., Barillas S.G., Acevedo L.M., Murphy E., Huang J., Scheppke L., Stockmann C., Johnson R.S., Angle N., Cheresh D.A. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wang Q., Zhang Y., Li B., Chen L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin-nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B. 2017;5(33):6963–6972. doi: 10.1039/c7tb00949f. [DOI] [PubMed] [Google Scholar]
- 330.Kempen D.H., Lu L., Heijink A., Hefferan T.E., Creemers L.B., Maran A., Yaszemski M.J., Dhert W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 331.Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng. 2008;14(8):1331–1340. doi: 10.1089/ten.tea.2007.0231. [DOI] [PubMed] [Google Scholar]
- 332.Becquart P., Cambon-Binder A., Monfoulet L.E., Bourguignon M., Vandamme K., Bensidhoum M., Petite H., Logeart-Avramoglou D. Ischemia is the prime but not the only cause of human multipotent stromal cell death in tissue-engineered constructs in vivo. Tissue Eng. 2012;18(19–20):2084–2094. doi: 10.1089/ten.TEA.2011.0690. [DOI] [PubMed] [Google Scholar]
- 333.Park J.Y., Shim J.H., Choi S.A., Jang J., Kim M., Lee S.H., Cho D.W. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J. Mater. Chem. B. 2015;3(27):5415–5425. doi: 10.1039/c5tb00637f. [DOI] [PubMed] [Google Scholar]
- 334.Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 335.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rafii S., Butler J.M., Ding B.S. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ramasamy S.K., Kusumbe A.P., Adams R.H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25(3):148–157. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ramasamy S.K., Kusumbe A.P., Itkin T., Gur-Cohen S., Lapidot T., Adams R.H. Regulation of hematopoiesis and osteogenesis by blood vessel-derived signals. Annu. Rev. Cell Dev. Biol. 2016;32:649–675. doi: 10.1146/annurev-cellbio-111315-124936. [DOI] [PubMed] [Google Scholar]
- 339.Peng Y., Wu S., Li Y., Crane J.L. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10(1):426–436. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Xie H., Cui Z., Wang L., Xia Z., Hu Y., Xian L., Li C., Xie L., Crane J., Wan M., Zhen G., Bian Q., Yu B., Chang W., Qiu T., Pickarski M., Duong L.T., Windle J.J., Luo X., Liao E., Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014;20(11):1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Gao B., Deng R., Chai Y., Chen H., Hu B., Wang X., Zhu S., Cao Y., Ni S., Wan M., Yang L., Luo Z., Cao X. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J. Clin. Invest. 2019;129(6):2578–2594. doi: 10.1172/JCI98857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ramasamy S.K., Kusumbe A.P., Wang L., Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Greenberg J.I., Shields D.J., Barillas S.G., Acevedo L.M., Murphy E., Huang J., Scheppke L., Stockmann C., Johnson R.S., Angle N., Cheresh D.A. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yang Q., McHugh K.P., Patntirapong S., Gu X., Wunderlich L., Hauschka P.V. VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and beta3-integrin. Matrix Biol. 2008;27(7):589–599. doi: 10.1016/j.matbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 345.Liu Y., Berendsen A.D., Jia S., Lotinun S., Baron R., Ferrara N., Olsen B.R. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Invest. 2012;122(9):3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xu R., Yallowitz A., Qin A., Wu Z., Shin D.Y., Kim J.M., Debnath S., Ji G., Bostrom M.P., Yang X., Zhang C., Dong H., Kermani P., Lalani S., Li N., Liu Y., Poulos M.G., Wach A., Zhang Y., Inoue K., Di Lorenzo A., Zhao B., Butler J.M., Shim J.H., Glimcher L.H., Greenblatt M.B. Targeting skeletal endothelium to ameliorate bone loss. Nat. Med. 2018;24(6):823–833. doi: 10.1038/s41591-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kim B.J., Lee Y.S., Lee S.Y., Baek W.Y., Choi Y.J., Moon S.A., Lee S.H., Kim J.E., Chang E.J., Kim E.Y., Yoon J., Kim S.W., Ryu S.H., Lee S.K., Lorenzo J.A., Ahn S.H., Kim H., Lee K.U., Kim G.S., Koh J.M. Osteoclast-secreted SLIT3 coordinates bone resorption and formation. J. Clin. Invest. 2018;128(4):1429–1441. doi: 10.1172/JCI91086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wan C., Gilbert S.R., Wang Y., Cao X., Shen X., Ramaswamy G., Jacobsen K.A., Alaql Z.S., Eberhardt A.W., Gerstenfeld L.C., Einhorn T.A., Deng L., Clemens T.L. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. U. S. A. 2008;105(2):686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hilton M.J., Tu X., Wu X., Bai S., Zhao H., Kobayashi T., Kronenberg H.M., Teitelbaum S.L., Ross F.P., Kopan R., Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat. Med. 2008;14(3):306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Engin F., Yao Z., Yang T., Zhou G., Bertin T., Jiang M.M., Chen Y., Wang L., Zheng H., Sutton R.E., Boyce B.F., Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat. Med. 2008;14(3):299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 352.Soehnlein O., Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 353.Koh T.J., DiPietro L.A. Inflammation and wound healing: the role of the macrophage. Expet Rev. Mol. Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Luz-Crawford P., Djouad F., Toupet K., Bony C., Franquesa M., Hoogduijn M.J., Jorgensen C., Noël D. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cell. 2016;34(2):483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 355.Ono T., Takayanagi H. Osteoimmunology in bone fracture healing. Curr. Osteoporos. Rep. 2017;15(4):367–375. doi: 10.1007/s11914-017-0381-0. [DOI] [PubMed] [Google Scholar]
- 356.Julier Z., Park A.J., Briquez P.S., Martino M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 357.Loi F., Córdova L.A., Zhang R., Pajarinen J., Lin T.H., Goodman S.B., Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016;7:15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Qian Y., Li L., Song Y., Dong L., Chen P., Li X., Cai K., Germershaus O., Yang L., Fan Y. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials. 2018;164:22–37. doi: 10.1016/j.biomaterials.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 359.Vi L., Baht G.S., Whetstone H., Ng A., Wei Q., Poon R., Mylvaganam S., Grynpas M., Alman B.A. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J. Bone Miner. Res. 2015;30(6):1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 360.Spiller K.L., Nassiri S., Witherel C.E., Anfang R.R., Ng J., Nakazawa K.R., Yu T., Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sakaguchi S., Sakaguchi N., Shimizu J., Yamazaki S., Sakihama T., Itoh M., Kuniyasu Y., Nomura T., Toda M., Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 362.Li Y., Toraldo G., Li A., Yang X., Zhang H., Qian W.P., Weitzmann M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Lei H., Schmidt-Bleek K., Dienelt A., Reinke P., Volk H.D. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front. Pharmacol. 2015;6:184. doi: 10.3389/fphar.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Pierini A., Nishikii H., Baker J., Kimura T., Kwon H.S., Pan Y., Chen Y., Alvarez M., Strober W., Velardi A., Shizuru J.A., Wu J.Y., Chiba S., Negrin R.S. Foxp3(+) regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat. Commun. 2017;8:15068. doi: 10.1038/ncomms15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Liu Z., Chen X., Zhang Z., Zhang X., Saunders L., Zhou Y., Ma P.X. Nanofibrous spongy microspheres to distinctly release miRNA and growth factors to enrich regulatory T cells and rescue periodontal bone loss. ACS Nano. 2018;12(10):9785–9799. doi: 10.1021/acsnano.7b08976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Shah N.J., Mao A.S., Shih T.Y., Kerr M.D., Sharda A., Raimondo T.M., Weaver J.C., Vrbanac V.D., Deruaz M., Tager A.M., Mooney D.J., Scadden D.T. An injectable bone marrow-like scaffold enhances T cell immunity after hematopoietic stem cell transplantation. Nat. Biotechnol. 2019;37(3):293–302. doi: 10.1038/s41587-019-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mahon O.R., Browe D.C., Gonzalez-Fernandez T., Pitacco P., Whelan I.T., Von Euw S., Hobbs C., Nicolosi V., Cunningham K.T., Mills K.H.G., Kelly D.J., Dunne A. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials. 2020;239:119833. doi: 10.1016/j.biomaterials.2020.119833. [DOI] [PubMed] [Google Scholar]
- 368.Jin S.S., He D.Q., Luo D., Wang Y., Yu M., Guan B., Fu Y., Li Z.X., Zhang T., Zhou Y.H., Wang C.Y., Liu Y. A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano. 2019;13(6):6581–6595. doi: 10.1021/acsnano.9b00489. [DOI] [PubMed] [Google Scholar]
- 369.Nair A., Tang L. Influence of scaffold design on host immune and stem cell responses. Semin. Immunol. 2017;29:62–71. doi: 10.1016/j.smim.2017.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.