The object of this study was to determine the diagnostic performance of four commercially available IgM tests in the diagnosis of measles virus (MeV) primary infection and cases with a serological profile indicating reinfection. Sera from 187 patients with MeV primary infection, 30 patients with suspected reinfection (after vaccine failure), and 153 patients with rash-like symptoms after exclusion of MeV infection were retested with four IgM tests.
KEYWORDS: IgM, MeV, antibodies, immunoassay, measles, reinfection
ABSTRACT
The object of this study was to determine the diagnostic performance of four commercially available IgM tests in the diagnosis of measles virus (MeV) primary infection and cases with a serological profile indicating reinfection. Sera from 187 patients with MeV primary infection, 30 patients with suspected reinfection (after vaccine failure), and 153 patients with rash-like symptoms after exclusion of MeV infection were retested with four IgM tests. MeV infection was verified by reverse transcriptase PCR (RT-PCR), and primary and suspected reinfections were differentiated by IgG avidity and neutralization assays. All IgM assays displayed significant agreement (Cohen’s κ, ≥0.604; all P < 0.001) and a higher diagnostic accuracy in primary infection than in suspected reinfection (indicated by high IgG avidity and significantly higher anti-MeV-IgG and neutralizing titers). In the overall cohort, the areas under the curve (AUC) were comparable among all tests, ranging from 0.875 to 0.931, with ranges increasing to 0.911 to 0.930 in the primary infection and decreasing to 0.765 to 0.940 in the setting of high anti-MeV-IgG avidity, and all tests displayed high specificity (81.1 to 92.2%). Of note, IgM tests with the highest diagnostic accuracy had discriminatory abilities not significantly different than PCR from serum. Although reinfections pose a challenge for IgM testing, IgM assays remain a cornerstone in the diagnosis of MeV infections. Especially in samples with a serological profile indicating reinfections, IgM tests displayed an equal or even superior diagnostic ability compared to PCR from serum.
INTRODUCTION
According to the European Centre of Disease Prevention and Control (ECDC), 13,460 cases of measles virus (MeV) infections occurred in European countries between 1st December 2018 and 30 November 2019, most of them in France, Romania, Italy, Poland, and Bulgaria, resulting in a total of 10 deaths (1). In view of these recent outbreaks causing high morbidity and even mortality, the maintenance of sufficiently high vaccination coverage and precise MeV surveillance remains the cornerstone for MeV elimination (2). Currently, this is of special importance since vaccine coverage rates have continuously not been sufficient to eliminate the virus, and suspension of measles vaccination campaigns due to the ongoing severe acute respiratory syndrome coronavirus 2 pandemic with the potential of declining MeV vaccination coverages has been reported for more than 20 countries (3–5).
High-quality laboratory testing is crucial for a rapid and concise diagnosis and containment of MeV infections, especially in countries approaching MeV elimination (6). The sequential production of virus-specific IgM and IgG antibodies (as a correlate of the host’s humoral immune response against the virus) can be used for the laboratory confirmation of MeV infections, supplemented by direct molecular assays assessing viral RNA (7, 8).
Thus, serological testing is being most widely used since it is a fast, inexpensive, and reliable method, which can be performed in a high-throughput manner (9). With regard to test performance, previous studies comparing IgM tests—with reverse transcriptase PCR (RT-PCR)-based assays as a reference—reported sensitivities of 89.9 to 98.8% and a specificity of 92.5 to 97.9% (10–12).
However, patient cohorts have been comparatively small, and to the best of our knowledge, no study of IgM tests has specifically investigated the difficulties in cases with a serological profile indicating MeV reinfection (breakthrough infection) after vaccine failure. Such reinfections may occur when baseline levels of protecting antibodies are low (after an incomplete vaccination series or an inefficient vaccination response), triggering a booster reaction of memory B- and T-cells, previously primed during primary infection with the vaccine-derived virus (13, 14).
Correspondingly, in the context of diagnostics, a fast and strong increase of virus-specific IgG and neutralization titers may indicate reinfection (15). However, since smaller amounts of naive B-cells producing IgM are activated in the course of a booster response, the question arises of how the diagnostic accuracy of IgM assays may be affected (16–19). Thus, we comprehensively analyzed the performance of four commercially available MeV IgM tests in the diagnosis of MeV primary infection and suspected reinfection.
MATERIALS AND METHODS
Samples, patients, and group definitions.
The study included serum samples acquired from 370 patients and sent to the Center for Virology of the Medical University of Vienna between 1 January 2015 and 1 July 2019 for comprehensive MeV testing. Samples were obtained and sent by primary practitioners, pediatricians, or hospitals either when MeV infection was clinically suspected (presentation of typical symptoms, e.g., maculopapular rash in a patient) or after a prior MeV-specific IgM positive test result requiring verification.
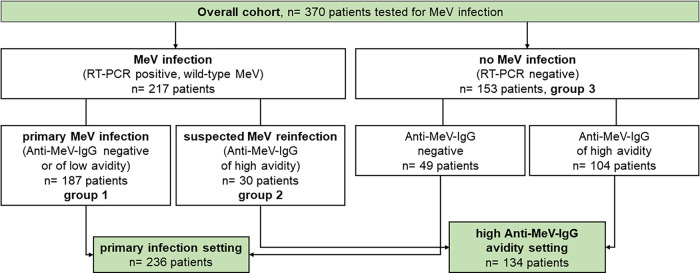
As shown in Fig. 1, the following samples were included in this study: (i) samples from patients with confirmed MeV infection, defined by positive MeV-specific RT-PCR (genotyped as wild-type MeV) from any material (serum, oral fluid, throat swab, or urine; n = 217; one sample per patient), and (ii) samples from patients which were tested for measles-like symptoms or after a prior positive MeV-specific IgM test result, but MeV infection was finally excluded (n = 153; one sample per patient). Exclusion of MeV infection was defined by negative MeV-specific RT-PCR results from all submitted clinical materials plus complete absence of IgG antibodies or presence of IgG antibodies with high avidity.
FIG 1.
Patient flowchart and explanation of settings being analyzed.
Samples with confirmed MeV infection could be further subdivided into patients with primary MeV infection (group 1, defined by positive MeV RNA of wild-type virus together with undetectable IgG antibodies or IgG antibodies with low avidity; n = 187) and suspected reinfection (group 2, defined by positive MeV RNA of wild-type virus in the presence of IgG antibodies with high-avidity; n = 30). Thus, samples from these two groups comprised laboratory-confirmed cases by the WHO criteria (laboratory confirmation by IgM detection and/or a positive RT-PCR result for virus-specific RNA) (7). The 153 patients without MeV infection served as controls and were termed group 3. Importantly, the following patients were excluded from the study to avoid misclassification: (i) patients receiving postexposure vaccination or postexposure prophylaxis, (i) patients with recent vaccine-type MeV infection (as identified by genotyping), (iii) patients with borderline avidity indices, and (iv) patients from whom insufficient serum material was available to perform all four anti-MeV-IgM immunoassays. When consecutive samples were available from the same patient, only the earliest serum sample was included this study.
Anti MeV-IgM immunoassays.
Four commercially available MeV ELISAs were used to comparatively assess anti-MeV IgM antibodies, test A (anti-measles virus IgM ELISA [Euroimmun, Lübeck, Germany]), test B (Platelia measles IgM ELISA [Bio-Rad, Hercules, CA, USA]), test C (Serion ELISA classic measles virus IgM [Virion/Serion, Würzburg, Germany]), and test D (Liaison measles IgM chemiluminescent immunoassay [CLIA; DiaSorin, Saluggia, Italy]). All tests were performed according to the manufacturer’s instructions and interpreted with the following cutoffs provided by the manufacturers: test A ratio: <0.80 negative, 0.80 to 1.10 borderline, and >1.10 positive; test B ratio: <0.80 negative, 0.80 to 1.20 borderline, and >1.20 positive; test C ratio: <10.0 negative, 10.0 to 15.0 borderline, and >15.0 positive; test D ratio: <0.90 negative, 0.90 to 1.10 borderline, and >1.10 positive.
Further information on laboratory methods (including the neutralization assay, RT-PCR, and genotyping) and the ethical statement are presented in the supplemental material.
Data analysis and statistical methods.
In a first step, the diagnostic accuracy of each evaluated anti-MeV-IgM test was evaluated in the overall cohort by comparing patients with primary MeV infection (group 1) and suspected reinfection (group 2) with the control group, who did not display any evidence for MeV infection (group 3). Then, the diagnostic accuracy of IgM assays was assessed in two real-life scenarios, as shown in Fig. 1. In a “primary infection setting,” all patients from group 1 were compared to corresponding anti-MeV-IgG negative samples from the RT-PCR negative-control group 3 (n = 49 out of 153). In a “high anti-MeV-IgG avidity setting,” all patients from group 2 with suspected reinfection (MeV RNA positive in the presence of anti-MeV-IgG antibodies with high avidity) were compared to corresponding anti-MeV-IgG positive samples with high avidity from the RT-PCR negative-control group 3 (n = 104 out of 153).
To assess the diagnostic accuracies, receiver operating characteristic (ROC) analyses calculating the area under the curve (AUC) were performed, and 95% confidence intervals (95% CI) were included to quantify uncertainty. In addition, the DeLong test was used to compare the diagnostic performance (AUC) among the four anti-MeV-IgM tests A to D. To assess the agreement of the anti-MeV-IgM tests, Cohen’s kappa coefficient was used. Comparisons of IgM titers were performed using the Mann-Whitney U test.
Statistical analyses were performed using IBM Statistics 25 (SPSS, Inc., USA) and Prism 8.3.1 (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables were reported as median and interquartile range (IQR), and categorical variables were reported as numbers (n) and proportions (%) of patients. A P value of <0.05 was considered statistically significant.
RESULTS
Patient characterization.
Out of 217 patients with RT-PCR-confirmed wild-type MeV infection, 187 patients (82.4%) displayed serological evidence for primary infection (group 1), and 30 patients displayed evidence for suspected reinfection (17.6%, group 2). A total of 153 patients with clinical suspicion of MeV infection or a prior positive screening IgM result in an external laboratory, in which MeV RT-PCR finally tested negative in serum (and all additionally submitted materials), served as controls (group 3; Fig. 1).
Importantly, patients from group 2 (suspected reinfection) displayed significantly higher levels of total anti-MeV-IgG antibodies (2,881 [IQR, 1,359 to 4,920] versus 121 [64 to 249] IU/liter; P < 0.001), higher avidity indices (80.5% [70.3 to 90.0%] versus 15.9% [12.8 to 20.7%]; P < 0.001) and higher neutralizing antibody titers (480 [150 to 1,280] versus 35 [20 to 60]; P < 0.001) than samples from group 1 (primary infection; see Table S1 in the supplemental material). Detailed results from RT-PCR analyses are presented in Table S2. Of note, all patients from group 1 and 2 had positive RT-PCR (wild-type MeV) from at least one material type, while all patients from group 3 displayed negative RT-PCR results from all materials submitted. Of 16 patients with negative RT-PCR from serum, 2 were RT-PCR positive in urine, 6 in oral fluid/throat swap, and 8 in both materials. Although serological results clearly indicated primary infection, 12 patients of group 1 (6.4%) reported a previous vaccination, indicating the possibility of primary vaccine failure. In group 2, positive anamnesis of previous vaccinations was available for 20 patients (66.6%), while no vaccination data were available for the other 10 patients. Previous vaccinations were confirmed through health records.
Detection rates of IgM tests.
The highest rates of positive test results for MeV primary infection (group 1) and suspected reinfection (group 2) were found with IgM test B (164 patients of group 1, 87.7%; 24 patients of group 2, 80.0%), with the highest negative rate in controls (139 patients of group 3, 90.8%; Table 1). However, 16 acutely infected patients of group 1 and 3 patients of group 2 displayed negative results in all 4 IgM tests. Additionally, 12 controls of group 3 tested positive in at least 3 of 4 performed IgM assays despite negative RT-PCR from serum.
TABLE 1.
Test results of four commercially available Anti-MeV-IgM immunoassays
| Group | Measurement | No. (%) for: |
|||
|---|---|---|---|---|---|
| Test A | Test B | Test C | Test D | ||
| Primary infection, group 1 (n = 187) | Negative | 43 (23.0) | 18 (9.6) | 22 (14.4) | 27 (14.4) |
| Borderline | 8 (4.3) | 5 (2.7) | 3 (1.6) | 5 (2.7) | |
| Positive | 136 (72.7) | 164 (87.7) | 162 (86.6) | 155 (82.9) | |
| Suspected reinfection, group 2 (n = 30) | Negative | 14 (46.7) | 3 (10.0) | 7 (23.3) | 14 (46.7) |
| Borderline | 1 (3.3) | 3 (10.0) | 1 (3.3) | 2 (6.7) | |
| Positive | 15 (50.0) | 24 (80.0) | 22 (73.3) | 14 (46.7) | |
| Control, group 3 (n = 153) | Negative | 124 (81.0) | 139 (90.8) | 132 (86.3) | 124 (81.0) |
| Borderline | 13 (8.5) | 2 (1.3) | 7 (4.6) | ||
| Positive | 16 (10.4) | 12 (7.8) | 14 (9.2) | 29 (19.0) | |
Agreement among the tests.
Overall, there was substantial agreement among all 4 IgM tests performed (all P < 0.001), being statistically strongest in the overall cohort among tests B and C (Cohen’s κ, 0.834; P < 0.001) and weakest among Test A and D (Cohen’s κ = 0.604, P < 0.001; Table S3). Importantly, agreement was higher in the primary infection setting and was reduced among all combinations in the high anti-MeV-IgG avidity setting.
Diagnostic performance.
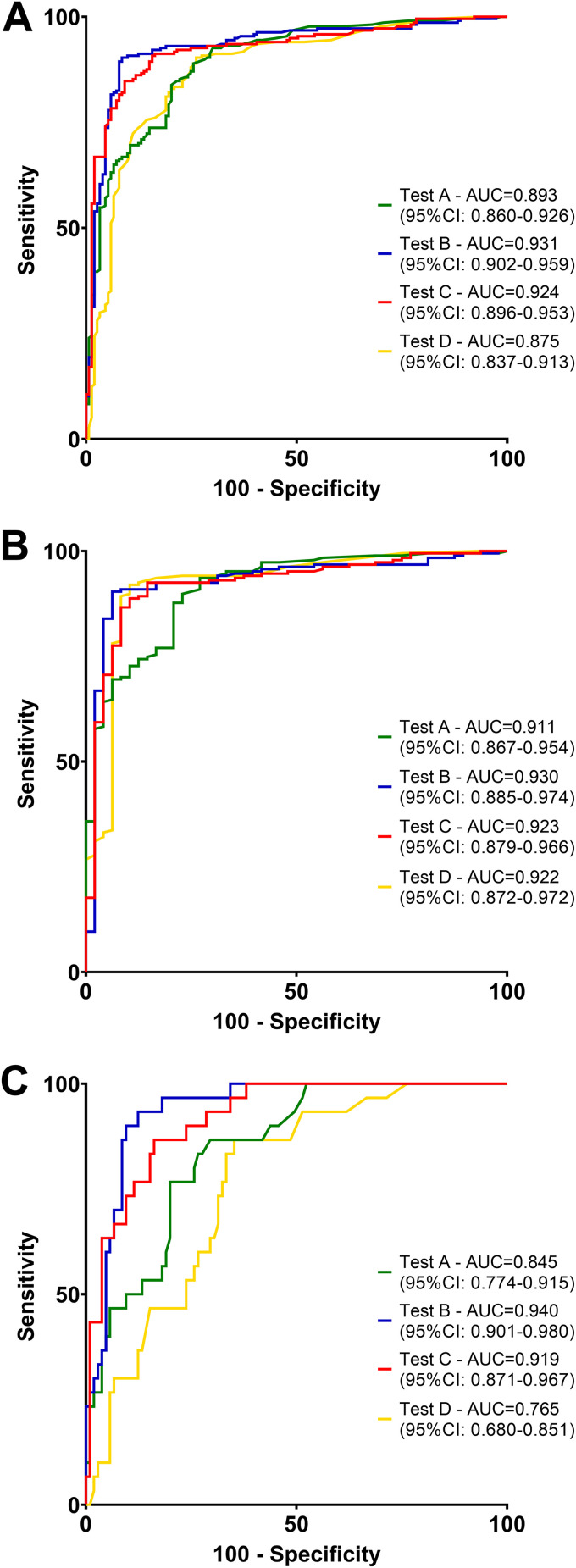
To assess the diagnostic accuracy, we performed ROC analyses for each test to diagnose acute MeV infections using positivity in RT-PCR from any material as reference (see Materials and Methods). Importantly, all tests were highly accurate in diagnosing MeV infection, with an AUC of 0.893 (95% CI, 0.860 to 0.926) for test A, 0.931 (95% CI, 0.902 to 0.959) for test B, 0.924 (95% CI, 0.896 to 0.953) for test C, and 0.875 (95% CI, 0.837 to 0.913) for test D in the overall cohort, being significantly different between test B versus D (P = 0.021) and C versus D (P = 0.042).
Correspondingly, the AUC was comparable among all four tests in the primary infection setting (test A, 0.911 [95% CI, 0.867 to 0.954]; test B, 0.930 [95% CI, 0.885 to 0.974]; test C, 0.923 [95% CI, 0.879 to 0.966]; test D, 0.922 [95% CI, 0.872 to 0.972]). In the high anti-MeV-IgG avidity setting, the AUC was 0.845 (95% CI, 0.774 to 0.915) for test A, 0.940 (95% CI, 0.901 to 0.980) for test B, 0.919 (95% CI, 0.871 to 0.967) for test C, and 0.765 (95% CI, 0.680 to 0.851) for test D, being significantly different between A versus B (P = 0.020), B versus D (P < 0.001), and C versus D (P = 0.002) (Fig. 2, Fig. S1 to S3).
FIG 2.
(A to C) Receiver operator characteristic (ROC) analysis of IgM tests to diagnose acute MeV infections (A) in the overall cohort, (B) in the primary infection setting, and (C) in the suspected reinfection (high anti-MeV-IgG avidity) setting. Differences among the area under the curve using the DeLong test were as follows: overall cohort (A): test A versus test B, P = 0.093; test A versus test C, P = 0.164; test A versus test D, P = 0.4733; test B versus test C, P = 0.756; test B versus test D, P = 0.021; test C versus test D, P = 0.042. Primary infection setting (B): test A versus test B, P = 0.550; test A versus test C, P = 0.699; test A versus test D, P = 0.743; test B versus test C, P = 0.831; test B versus test D, 0.P = 817; test C versus test D, P = 0.974. Reinfection setting (C): test A versus test B, P = 0.020; test A versus test C, P = 0.067; test A versus test D, P = 0.162; test B versus test C, P = 0.508; test B versus test D, P < 0.001; test C versus test D, P = 0.002.
Furthermore, we evaluated the diagnostic indices (sensitivity and specificity) of each test in each setting and found a high specificity for all tests in the overall cohort (81.1 to 92.2%, Table 2). Notably, sensitivity (72.3 to 87.2%) and specificity (89.6 to 93.8%) were higher in the primary infection setting and slightly lower in the setting of high anti-MeV-IgG avidity (sensitivity, 48.3 to 82.8%; specificity, 76.2 to 91.4%). In general, tests B and C displayed the highest sensitivity and specificity in the overall cohort and the high anti-MeV-IgG avidity setting, while all tests were similar in the primary infection setting. Finally, sensitivity did not significantly differ between RT-PCR from serum and tests B and C in any setting. Importantly, diagnostic performance of the assay results did not significantly change when borderline results were excluded from analyses (Table S4).
TABLE 2.
Sensitivity and specificitya of IgM tests to diagnose acute MeV infections in the overall cohort, in the primary infection setting, and in the high Anti-MeV-IgG avidity setting
| Group | Measurement | Result (range) for (%): |
||||
|---|---|---|---|---|---|---|
| Test A | Test B | Test C | Test D | PCR (serum)b | ||
| Overall cohort (n = 370) | Sensitivity | 69.6 (63.2–75.3) | 86.6 (81.5–90.5) | 84.8 (79.4–89.0) | 78.3 (72.4–83.3) | 93.1 (88.9–95.8) |
| Specificity | 89.5 (83.7–93.5) | 92.2 (86.8–95.5) | 90.9 (85.2–94.5) | 81.1 (74.1–86.5) | ||
| Primary infection setting (n = 236) | Sensitivity | 72.3 (65.6–78.2) | 87.2 (81.7–91.3) | 86.2 (80.5–90.4) | 83.0 (77.0–87.7) | 94.7 (90.4–97.1) |
| Specificity | 89.6 (77.8–95.5) | 93.8 (83.2–97.9) | 91.7 (80.5–96.7) | 91.7 (80.5–96.7) | ||
| High anti-MeV-IgG avidity setting (n = 134) | Sensitivity | 51.7 (34.4–68.6) | 82.8 (65.5–92.4) | 75.9 (57.9–87.8) | 48.3 (31.4–65.6) | 82.8 (65.5–92.4) |
| Specificity | 89.5 (82.2–94.1) | 91.4 (84.5–95.4) | 90.5 (83.4–94.7) | 76.2 (67.2–83.3) | ||
Diagnostic indices were calculated grading “borderline” results as “negative” using the cutoffs according to the manufacturer.
Diagnostic indices were calculated using PCR from any material as positive reference.
Test performance in relation to onset of symptoms and serological infection stages.
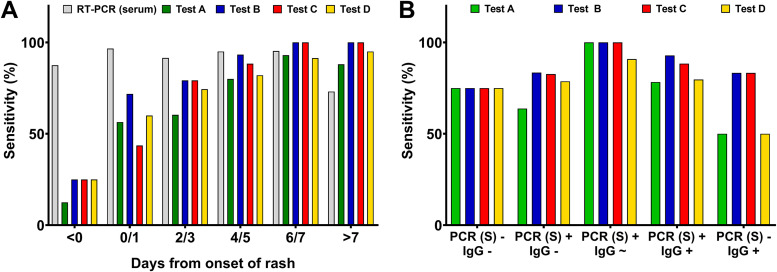
Next, we investigated the positive detection rates (sensitivity) of the four IgM immunoassays during the course of infection. Interestingly, sensitivity was <30% in all tests before onset of rash, increasing to 43.6 to 71.8% at day 0 or 1 postonset and being comparable to serum RT-PCR at day 6 or 7 (91.4 to 100% versus 95.3%; Fig. 3A). Of note, sensitivity was higher than serum PCR from day 7 postonset of rash (88.0 to 100% versus 73.1%). In an analysis comparing sensitivities among serological stages, positive detection rates peaked in all tests (90.9 to 100%) when MeV IgG became initially detectable and tested borderline (Fig. 3B). The sensitivity subsequently declined after Ig class switching when IgG assays provided positive results, irrespective of concurrent RT-PCR results from serum (IgG positive and PCR positive, 78.3 to 92.8%; IgG positive and RT-PCR negative, 50.0 to 83.3%).
FIG 3.
Sensitivity of IgM tests (A) at different days post onset of rash and (B) at different serological stages.
Finally, we aimed to analyze the longitudinal increase of anti-MeV-IgM antibodies measured by each test. Since the semiquantitative anti-MeV-IgM tests use different cutoffs, a ratio was computed for such a comparison. The ratio was calculated for each test by dividing the measured anti-IgM antibody values by the respective cutoff of the test. This analysis showed that values detected by tests B and C increased earlier (median ratio, 3.61 to 4.59 on day 0/1; increasing to 8.54 to 13.63 after day 7; Fig. S4). In contrast, values of tests A and D remained lower, reaching a median ratio of 2.67 for test A and 3.36 for test D after day 7.
Combination of tests.
We then analyzed whether a combination of two IgM assays increased the diagnostic accuracy compared to using one test alone (Table S5). Increased equalized diagnostic indices were particularly observed for the combinations of test A or D with test B or C. However, no combinations achieved a significantly improved diagnostic ability compared to the ability of test B or C alone, despite a combination of these two yielding the highest diagnostic values.
Importantly, double-positive test results in any two tests achieved a high specificity (overall cohort, 92.8 to 95.4%; primary infection setting, 93.8%; high anti-MeV-IgG avidity setting, 92.4 to 96.2%) among all combinations, while one positive test result among two combined tests generally increased sensitivity.
Performance in serum samples with negative PCR.
Finally, we specifically analyzed 16 patients (7.4%) with a negative RT-PCR result from serum despite a positive RT-PCR result from any other material (verifying the infection). IgM tests were positive in up to 75.0% of patients with confirmed MeV infection, being highest for tests B and C in the primary infection (70.0%) as well as in the reinfection setting (83.5%; Table S6).
DISCUSSION
IgM testing remains a cornerstone of MeV diagnostic in clinical virology. In our comprehensive, comparative study, we demonstrate the excellent clinical applicability of four commercially available IgM tests to diagnose MeV infections in a real-life cohort of symptomatic patients with suspected MeV infection. Importantly, we demonstrate data from one of the largest cohorts of MeV infections not only being comprehensively analyzed, but also in relation to RT-PCR as the reference method (not only from serum, but also from oral fluid, throat swab, or urine). Furthermore, we here provide additional data to address the problem of serological diagnosis in samples with a serological profile indicating possible reinfection (after vaccination breakthrough).
False-positive test results using IgM and IgG ELISAs or CLIAs have been reported due to binding of nonspecific IgM antibodies caused by other viral infections, such as human parvovirus B19 (B19V), rubella, or human herpesvirus, or interference by rheumatoid factor (20). In general, our study demonstrates comparable false-positive rates in all evaluated assays (most frequently in test D, followed by tests A, C, and B). Notably, out of the 153 controls without MeV infection, only 6 (3.9%) displayed IgM antibodies against Epstein-Barr virus (EBV) and only 5 (3.3%) against B19V, indicating that cross-reactivity due to EBV and B19V infections was relatively uncommon in our control group (data not shown). Such a low EBV and B19V prevalence in controls could contribute to the high specificity we observed for the evaluated IgM tests. It should thus be considered that the specificity data we provide refer to our selected control group (individuals with measles-like symptoms or with a previous IgM-positive screening test). In other cohorts, e.g., including more individuals with confirmed EBV infections, specificity may differ.
To assess the assays’ diagnostic performance, we used a primary infection setting (comparing confirmed primary infections to those individuals from the control group who did not display any detectable MeV-specific IgG antibodies) and a high anti-MeV-IgG avidity setting (comparing a serological profile indicating possible reinfection with those patients from the control group with anti-MeV-IgG antibodies of high avidity). Of note, sensitivity was higher in the primary infection setting, confirming that high IgM antibody levels reflect the initial contact of naive B-cells with a pathogen (16–19). IgM positivity in suspected reinfection cases, however, also yielded high diagnostic significance. The lower sensitivity we observed for all four evaluated assays in the setting when anti-MeV-IgG antibodies with high avidity were present may be caused by a lower number of activated naive B-cells due to the booster response of memory cells, limiting viral replication earlier than in primary infection and thereby causing a delayed stimulation of naive cells (6, 21, 22). However, tests B and C were equally able to identify suspected MeV reinfection as RT-PCR from serum, probably due to lower levels of MeV RNA in blood from reinfected patients.
This observation suggests a promising role of IgM testing not only in primary MeV infection but also in cases with suspected reinfection. However, our data indicate that the detection rates of MeV-specific IgM antibodies depend not only on the applied immunoassay but also on the interval postinfection (or since the symptom onset). The sensitivity of RT-PCR also depends on the time point postinfection but strongly on the sample material (23). Notably, a previous study with serologically confirmed MeV cases demonstrated that in 65% of the patients, the diagnosis was confirmed by at least one RT-PCR positive sample, with higher detection rates in throat swabs than in serum samples (8). Notably, RT-PCR from serum less frequently yielded positive test results as the infection progressed (and IgG antibody responses developed) (8). Similarly, in our study, 12 patients with negative RT-PCR from serum (group 3) unanimously tested positive in ≥3 IgM tests, proposing the possibility of false-negative results from serum RT-PCR (due to low viral RNAemia or preanalytic limitations) and correct identification as MeV cases by IgM serology only, which would have resulted in even higher specificities (6). Throat swab, oral fluid, or urine samples, which would have been required for ultimate verification, were unfortunately not available for these patients.
Similar to these patients and in accordance with the previous study, we identified the considerable number of 16 samples with negative RT-PCR from serum despite positive RT-PCR from any other material, being 10/187 patients (5.3%) in primary infections and 6/30 (20.0%) in suspected reinfection cases (8). Our data thus indicate a certain weakness of RT-PCR diagnosis when it is only performed in serum samples, especially in patients with possible reinfection. However, all four IgM ELISAs correctly identified between 50 and 83.5% of these cases as MeV infections, highlighting the additional benefit of serological IgM testing in MeV diagnosis. Finally, our data provide evidence that only the combination of specific immunoassays might increase the overall sensitivity, while combining multiple IgM tests might result in an increase of specificity.
While the sensitivity of IgM testing clearly differs between primary infection and reinfection, time post-onset of symptoms poses an additional factor affecting the accuracy of IgM test results (24, 25). Usually, sensitivities of IgM tests have been reported to be lower in samples collected within 3 days of rash onset and highest between 6 and 14 days after symptom onset, reaching sensitivities of 94 to 100% until day 30 (10, 26–28). Specifically, 16 patients of group 1 and 3 patients of group 2 tested negative in all IgM tests, probably due to a very early sampling post-onset of symptoms. Our data of four different assays confirm that the sensitivity of IgM testing gradually increases over time, being comparable to RT-PCR from day 4/5-post onset of symptoms.
The results of this study are certainly limited by the fact that RT-PCR from serum was performed in every patient and control individual, but additional sample materials were not available in every patient. This might impose a certain degree of false-negative samples in the control group (group 3), as noted above, and limit the specificity of IgM tests in our cohort.
Nevertheless, we provide comprehensive and comparative data indicating that IgM testing provides excellent accuracy for diagnosis of MeV cases, not only for primary infection, but also in samples with a serological profile indicating the possibility of reinfection after vaccination breakthrough. When interpreted properly and combined with RT-PCR-based assays, the evaluated commercial assays provided a highly accurate stratification of suspected MeV cases and even showed a diagnostic performance comparable to RT-PCR from serum in cases with high anti-MeV-IgG avidity.
Supplementary Material
ACKNOWLEDGMENTS
We state that we do not have a commercial or other association that might pose a conflict of interest.
We thank Jutta Hutecek for technical support.
ELISA/CLIA plates were given to the authors free of charge by Virion and DiaSorin. No other funding was received for this study.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.European Centre for Disease Prevention and Control. 2020. Monthly measles and rubella monitoring report: January 2020. ECDC, Stockholm, Sweden. [Google Scholar]
- 2.O’Connor P, Jankovic D, Muscat M, Ben-Mamou M, Reef S, Papania M, Singh S, Kaloumenos T, Butler R, Datta S. 2017. Measles and rubella elimination in the WHO Region for Europe: progress and challenges. Clin Microbiol Infect 23:504–510. 10.1016/j.cmi.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, Shen AK. 2020. Decline in child vaccination coverage during the COVID-19 pandemic: Michigan Care Improvement Registry, May 2016–May 2020. MMWR Morb Mortal Wkly Rep 69:630–631. 10.15585/mmwr.mm6920e1. [DOI] [PubMed] [Google Scholar]
- 4.Roberts L. 2020. Why measles deaths are surging—and coronavirus could make it worse. Nature 580:446–447. 10.1038/d41586-020-01011-6. [DOI] [PubMed] [Google Scholar]
- 5.Carias C, Pawaskar M, Nyaku M, Conway JH, Roberts CS, Finelli L, Chen YT. 2021. Potential impact of COVID-19 pandemic on vaccination coverage in children: a case study of measles-containing vaccine administration in the United States (US). Vaccine 39:1201–1204. 10.1016/j.vaccine.2020.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubschen JM, Bork SM, Brown KE, Mankertz A, Santibanez S, Ben Mamou M, Mulders MN, Muller CP. 2017. Challenges of measles and rubella laboratory diagnostic in the era of elimination. Clin Microbiol Infect 23:511–515. 10.1016/j.cmi.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 7.WHO. 2018. Manual for the laboratory diagnosis of measles and rubella virus infection, 3rd ed. World Health Organization (WHO), Geneva, Switzerland. [Google Scholar]
- 8.Riddell MA, Chibo D, Kelly HA, Catton MG, Birch CJ. 2001. Investigation of optimal specimen type and sampling time for detection of measles virus RNA during a measles epidemic. J Clin Microbiol 39:375–376. 10.1128/JCM.39.1.375-376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss WJ. 2017. Measles. Lancet 390:2490–2502. 10.1016/S0140-6736(17)31463-0. [DOI] [PubMed] [Google Scholar]
- 10.Sampedro A, Rodriguez-Granger J, Gomez C, Lara A, Gutierrez J, Otero A. 2013. Comparative evaluation of a new chemiluminiscent assay and an ELISA for the detection of IgM against measles. J Clin Lab Anal 27:477–480. 10.1002/jcla.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanz JC, Mosquera M, Ramos B, Ramirez R, de Ory F, Echevarria JE. 2010. Assessment of RNA amplification by multiplex RT-PCR and IgM detection by indirect and capture ELISAs for the diagnosis of measles and rubella. APMIS 118:203–209. 10.1111/j.1600-0463.2009.02581.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Camarasa C, Lara-Oya A, Cobo F, Sampedro-Martinez A, Rodriguez-Granger J, Gutierrez-Fernandez J, Navarro-Mari JM. 2016. Comparison of two chemiluminescent immunoassays in the detection of measles IgM antibodies. J Virol Methods 237:38–39. 10.1016/j.jviromet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Bernasconi NL, Traggiai E, Lanzavecchia A. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199–2202. 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 14.Stiasny K, Holzmann H, Heinz FX. 2009. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine 27:7021–7026. 10.1016/j.vaccine.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 15.Sowers SB, Rota JS, Hickman CJ, Mercader S, Redd S, McNall RJ, Williams N, McGrew M, Walls ML, Rota PA, Bellini WJ. 2016. High concentrations of measles neutralizing antibodies and high-avidity measles IgG accurately identify measles reinfection cases. Clin Vaccine Immunol 23:707–716. 10.1128/CVI.00268-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A 97:13263–13268. 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabel F, Mohanan D, Bessa J, Link A, Fettelschoss A, Saudan P, Kündig TM, Bachmann MF. 2014. Viral particles drive rapid differentiation of memory B cells into secondary plasma cells producing increased levels of antibodies. J Immunol 192:5499–5508. 10.4049/jimmunol.1400065. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. 2009. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood 114:4998–5002. 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. 2003. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol 170:686–694. 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 20.Woods CR. 2013. False-positive results for immunoglobulin M serologic results: explanations and examples. J Pediatric Infect Dis Soc 2:87–90. 10.1093/jpids/pis133. [DOI] [PubMed] [Google Scholar]
- 21.Bolotin S, Lim G, Dang V, Crowcroft N, Gubbay J, Mazzulli T, Schabas R. 2017. The utility of measles and rubella IgM serology in an elimination setting, Ontario, Canada, 2009–2014. PLoS One 12:e0181172. 10.1371/journal.pone.0181172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semmler G, Griebler H, Aberle SW, Stiasny K, Richter L, Holzmann H, Weseslindtner L. 2020. Elevated CXCL10 serum levels in Measles virus primary infection and reinfection correlate with the serological stage and hospitalization status. J Infect Dis 222:2030–2034. 10.1093/infdis/jiaa326. [DOI] [PubMed] [Google Scholar]
- 23.Rota PA, Brown KE, Hübschen JM, Muller CP, Icenogle J, Chen MH, Bankamp B, Kessler JR, Brown DW, Bellini WJ, Featherstone D. 2011. Improving global virologic surveillance for measles and rubella. J Infect Dis 204(Suppl 1):S506–S513. 10.1093/infdis/jir117. [DOI] [PubMed] [Google Scholar]
- 24.Michel Y, Saloum K, Tournier C, Quinet B, Lassel L, Pérignon A, Grimprel E, Carbajal R, Vabret A, Freymuth F, Garbarg-Chenon A, Schnuriger A. 2013. Rapid molecular diagnosis of measles virus infection in an epidemic setting. J Med Virol 85:723–730. 10.1002/jmv.23515. [DOI] [PubMed] [Google Scholar]
- 25.Cui A, Mao N, Wang H, Xu S, Zhu Z, Ji Y, Ren L, Gao L, Zhang Y, Xu W. 2018. Importance of real-time RT-PCR to supplement the laboratory diagnosis in the measles elimination program in China. PLoS One 13:e0208161. 10.1371/journal.pone.0208161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helfand RF, Heath JL, Anderson LJ, Maes EF, Guris D, Bellini WJ. 1997. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis 175:195–199. 10.1093/infdis/175.1.195. [DOI] [PubMed] [Google Scholar]
- 27.Ratnam S, Tipples G, Head C, Fauvel M, Fearon M, Ward BJ. 2000. Performance of indirect immunoglobulin M (IgM) serology tests and IgM capture assays for laboratory diagnosis of measles. J Clin Microbiol 38:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozanne G, d’Halewyn MA. 1992. Performance and reliability of the Enzygnost measles enzyme-linked immuno-sorbent assay for detection of measles virus-specific immunoglobulin M antibody during a large measles epidemic. J Clin Microbiol 30:564–569. 10.1128/JCM.30.3.564-569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.