Abstract
Mosquitoes transmit pathogens that kill >700,000 people annually. These insects use body heat to locate and feed on warm-blooded hosts, but the molecular basis of such behavior is unknown. Here we identify ionotropic receptor IR21a, a receptor conserved throughout insects, as a key mediator of heat seeking in the malaria vector Anopheles gambiae. Although Ir21a mediates heat avoidance in Drosophila, we find it drives heat seeking and heat-stimulated blood feeding in Anopheles. At a cellular level, Ir21a is essential for the detection of cooling, suggesting that mosquito heat seeking relies on cooling-mediated repulsion. Our data indicate that the evolution of blood feeding in Anopheles involved repurposing an ancestral thermoreceptor from non-blood-feeding Diptera.
Main Text:
Insect-borne diseases kill over 700,000 people annually, with >400,000 deaths resulting from malaria, a disease caused by protozoan Plasmodium spp. parasites that are transmitted by blood-feeding anopheline mosquitoes (1). Host seeking by mosquitoes and other pathogen-spreading insects relies on the detection of host-associated cues including carbon dioxide (CO2), odors, and body heat (2–5). Receptors for CO2 and host odors have been characterized in mosquitoes (6-9), but receptors that promote heat-seeking and heat-induced blood feeding have remained elusive (4, 10–12). As vector mosquitoes are descendants of non-blood-feeding ancestors (13), it remains unknown whether the emergence of heat-seeking and warming-induced blood feeding in mosquitoes involved the generation of novel thermoreceptors or the repurposing of existing thermoreceptors.
To date, mosquito orthologs of two Drosophila warmth receptors, TRPA1 (14) and GR28b (15), have been tested as candidate heat-seeking receptors in the yellow fever mosquito Aedes aegypti (10–12). However, neither is required for heat seeking in Aedes (12). Rather, TRPA1 promotes heat avoidance in both Aedes and Drosophila (12, 14). Although efforts have focused on warm receptors, insects also possess cooling-activated receptors, which should be equally capable of supporting heat seeking through cooling-mediated repulsion. In Drosophila, cooling detection is mediated by IR21a, IR25a and IR93a (16–18), three members of the ionotropic receptor (IR) family, a group of invertebrate-specific sensory receptors related to ionotropic glutamate receptors (19). IR21a is specifically required for cooling detection in the fly and can confer cooling sensitivity when ectopically expressed (16, 18), while IR25a and IR93a are more broadly acting co-receptors that support cooling detection and other IR-dependent sensory modalities (17, 19, 20). At the behavioral level in Drosophila, IR21a, IR25a and IR93a help the fly achieve optimal body temperatures by supporting avoidance of excessively cool and warm temperatures (16, 18). Beyond Drosophila, IR21a, Ir25a and IR93a are each widely conserved from Diptera (like flies and mosquitoes) to Isoptera (termites) (19), raising the possibility that their thermosensory functions may also be conserved. Using Anopheles gambiae, a major vector of malaria in sub-Saharan Africa, we first tested whether IR21a is required for detecting cooling in mosquitoes and subsequently whether it can drive heat attraction and heat-stimulated blood feeding.
Two mutant alleles of An. gambiae Ir21a were generated using CRISPR-Cas9 (see methods). Ir21a+7bp contains a 7bp insertion, introducing a frameshift positioned to disrupt IR21a’s translation starting within the second of IR21a’s three transmembrane domains; a lesion predicted to generate a non-functional receptor (Fig. 1A). In Ir21aEYFP, a disruption cassette containing a Yellow Fluorescent Protein (EYFP) marker was inserted into IR21a’s fourth exon; a lesion also predicted to create a non-functional receptor (Fig. 1B). Both mutants lacked detectable IR21a protein expression (Fig. 1C-E, Fig. S1), consistent with their acting as Ir21a null mutations.
Fig. 1. IR21a is expressed in the antennal tip.
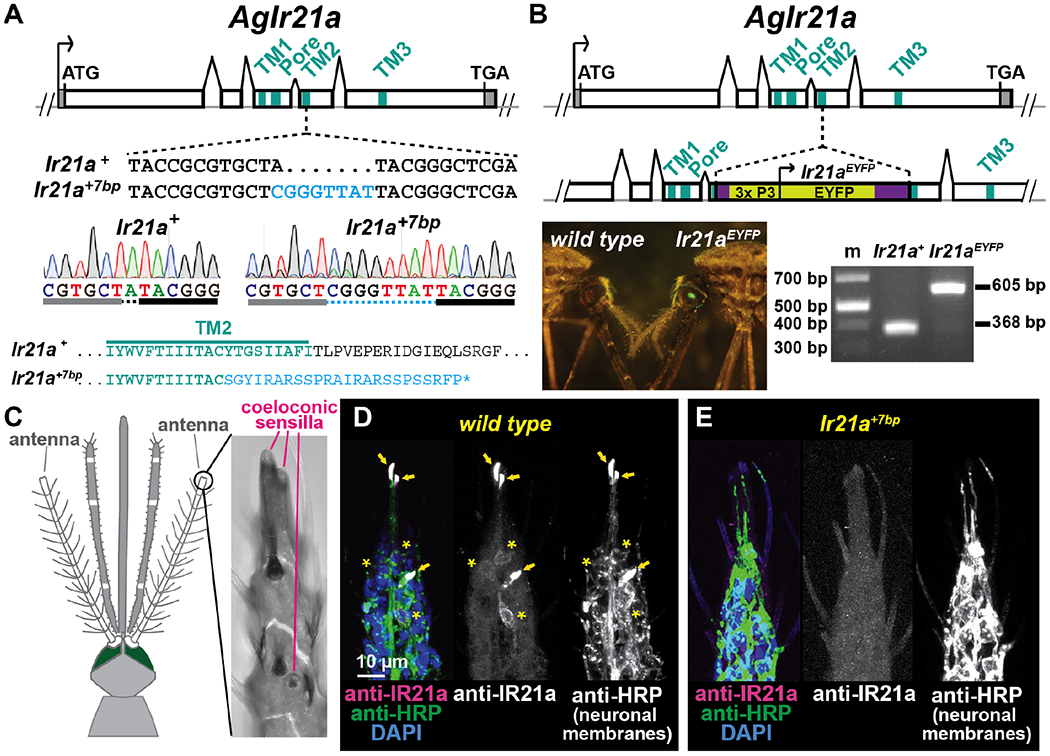
(A) Upper, Ir21a locus. Middle, Ir21a+7bp mutations (blue). Lower, virtual translations, with 450 C-terminal amino acids of wild-type IR21a replaced by 24 novel amino acids (blue) and premature stop in Ir21a+7bp. (B) Targeted integration generating Ir21aEYFP. Lower: Combined bright-field/fluorescent illumination (left) and molecular genotyping (right) of Ir21a+ and Ir21aEYFP homozygotes. m, markers. (C ) Mosquito anterior (drawing based on ref. (27)). Inset, flagellomere 13. D,E, wild type (D) and Ir21a+7bp (E) female immunostaining, flagellomere 13. wild type, n=13 animals stained, Ir21a+7bp n=7. IR21a-expressing cell bodies (asterisks), sensory endings (arrows). Anti-HRP labels neuronal membrane proteins. DAPI labels nuclei.
Genome-wide analyses of An. gambiae sensory tissues suggest that Ir21a RNA is specifically expressed in the antenna (21). To visualize IR21a protein expression and localization with cellular resolution, anti-IR21a antisera were generated. The antenna’s most distal segment (flagellomere 13) contains three coeloconic sensilla that house sensitive thermoreceptors (22–24) (Fig. 1C). In females, IR21a expression was detected in three sensory neurons in flagellomere 13, one innervating each of the coeloconic sensilla (Fig. 1D). Consistent with a role in thermosensory transduction, IR21a protein strongly localized to the sensory ending of each of these neurons (Fig. 1D). IR21a immunostaining was absent in Ir21a mutants, confirming antisera specificity (Fig. 1E, Fig. S1A). The male antennal tip also contains thermoreceptors (23), and IR21a expression was detected in sensory endings there as well (Fig. S1B).
Extracellular recordings were performed from the IR21a-positive coeloconic sensilla at the antennal tip (Fig. 2A). In the wild type, the activity of the Cooling Cell, a thermosensory neuron stimulated by cooling and inhibited by warming, was readily detected (Fig. 2B). On rare occasions of exceptional signal to noise, a smaller amplitude spike was also detected, corresponding to a Heating Cell, activated by warming and inhibited by cooling (Fig. S2). Cooling Cell responses were highly thermosensitive: an ~0.5°C drop from ~30°C increased spiking by ~40%, and an ~0.5°C drop from ~37°C increased spiking by ~80% (Fig. 2C). Response adaptation initiated rapidly, followed by a slower decline to baseline (Fig. 2C; Fig. S3). Heating inhibited spiking, in a similarly transient manner (Fig. 2C). Importantly, Cooling Cells remained highly active at warm temperatures (e.g., 37°C) and were neither more active nor more thermosensitive at colder temperatures (Fig. 2C, D). Thus, while often referred to as Cold Cells in the classical literature, cooling and not cold is their activating stimulus. In addition, while often referred to as “phasic-tonic” receptors, their responses to temperature shifts adapted fully, albeit slowly, requiring sustained observation (>20 sec) to fully appreciate (Fig. S3). Therefore, although they fire robustly at constant temperature, Cooling Cells are phasic thermoreceptors. Interestingly, their rate of baseline firing was relatively temperature-insensitive (Q10 ~1.6, reflecting a slight increase with warmth), enabling the cell to respond to small temperature fluctuations over a wide range of absolute temperatures. (Physiologically similar Cooling and Heating Cells have been described in Aedes aegypti (22, 24) and Drosophila melanogaster (18).) Taken together, these data indicate that Cooling Cells are phasic thermoreceptors that respond to temperature change rather than absolute temperature, and that they are capable of responding to abrupt changes in temperature over the wide range of absolute temperatures relevant for host-seeking (Fig. 2C, D).
Fig. 2: Ir21a is required for thermosensing.
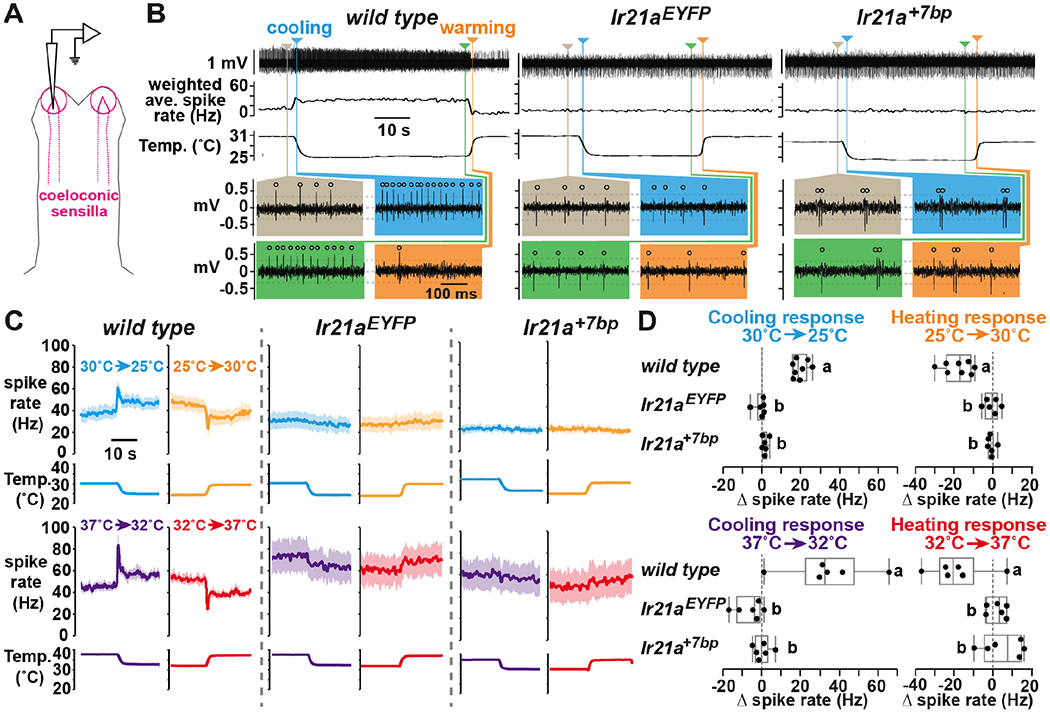
(A) Recording electrode insertion site. (B) Representative recordings, indicated regions displayed on expanded time scale. Circles, spikes. Weighted average spike rate, instantaneous spike frequency smoothed using 1s triangular window. Dotted lines, spike thresholds. (C) Peri-stimulus time histograms (average +/− SEM). wild type, n=8 animals tested for 30°C/25°C; others, n=6. One heating/cooling trial per animal. (D) Cooling response = (average Hz 0.2s-0.7s after cooling onset) – (average Hz 5s-10s pre-cooling). Heating response = (average Hz 0.5s-1.5s after heating onset) – (average Hz 5s-10s pre-heating). Letters, distinct groups (Tukey HSD, alpha=0.01, except 32°C➔37°C, alpha=0.05). Shapiro-Wilk and ANOVA test values are provided in statistics section of methods.
Cooling Cell thermosensitivity was eliminated in An. gambiae Ir21a mutants. The large amplitude spike detected in Ir21a mutants was neither activated by cooling nor inhibited by warming (Fig. 2B-D). Rather, its activity increased slightly upon warming, with a Q10 under 2, average for a biological process. Thus, Ir21a is essential for thermosensing by Cooling Cells in the mosquito, demonstrating that An. gambiae IR21a’s molecular function is conserved with its Drosophila ortholog (18).
In female mosquitoes, heat seeking is part of a multi-modal host-seeking program activated upon exposure to CO2, with body heat serving as an important cue close to the host (within ~10 to 15 cm) (3, 8, 12). To assess heat seeking, female mosquitoes were provided a 20 sec puff of 4% CO2 and exposed to two targets, a control target at ambient temperature (~26°C) and a heated target at ~37°C (Fig 3A, B; Fig. S4A). Wild type mosquitoes exhibited robust heat seeking, with 43 +/− 3% of CO2-activated mosquitoes landing on the 37°C target (average +/− SEM) (Fig. 3C, F, Movie S1, Fig. S4B). The loss of Ir21a greatly reduced this behavior, with only 15 +/− 4% of Ir21aEYFP mutants and 14 +/− 4% of Ir21a+7bp mutants landing on the 37°C target (Fig. 3D-F, Movie S2, Fig. S4B). In all cases, the control target was largely ignored, confirming temperature’s importance in the assay (Fig. 3C-F). While heat seeking was greatly reduced in Ir21a mutants, it was not entirely eliminated (Fig. 3D-F). This residual activity likely reflects signaling from other as yet uncharacterized thermosensors. However, the strong reduction of heat seeking in Ir21a mutants (Fig. 3G) identifies this receptor as a major driver of mosquito attraction to warmth.
Fig. 3: Ir21a mediates heat-seeking.
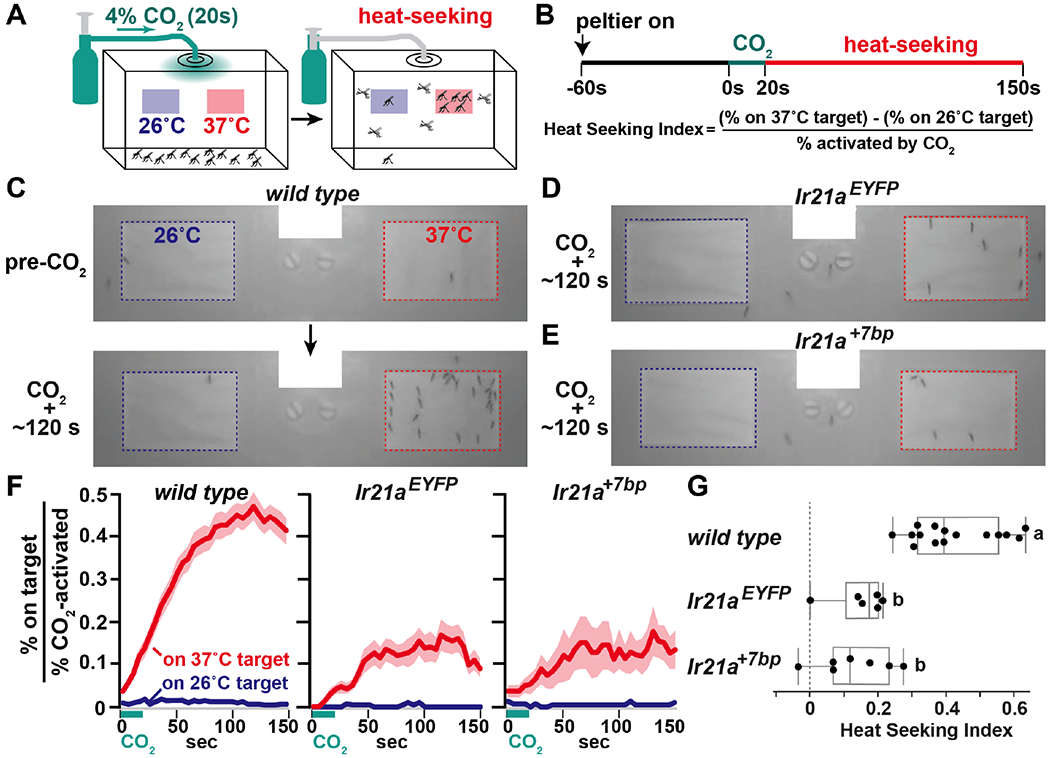
(A) Heat-seeking assay. Assay box is 28 cm deep, 40 cm long and 16 cm tall. (B) Upper, stimulus sequence. Lower, Heat Seeking Index. (C) Representative images of 26°C (blue) and 37°C (red) targets before (upper) and ~120 s after (lower) CO2 pulse initiation in wild type. (D-E) ~120 s after CO2 pulse initiation in Ir21aEYFP (D) and Ir21a+7bp (E). (F) Landing on 37°C and 26°C targets as percent of mosquitoes taking flight. Average +/− SEM. wild type n=15 independent groups, Ir21aEYFP n=6, Ir21a+7bp n=7. 42–52 females/group. (G) Heat seeking index (average from 105s to 135s). Letters denote distinct categories (Steel-Dwass test, p<0.01). Shapiro-Wilk, Kruskal-Wallis and Steel-Dwass test values are provided in statistics section of methods.
To test the specificity of the Ir21a mutant behavioral deficit for heat seeking, we examined their ability to perform an activation-dependent behavior less reliant on thermosensation. While body heat is a powerful short-range cue, a multi-modal combination of longer-range chemosensory and visual cues mediates initial approach (3), suggesting that such behavior should be largely unaffected by a specific thermosensory deficit. To assess approach, mosquitoes were activated by five human breaths and presented a human hand, positioned on a platform to prevent physical contact with the mosquitoes, but otherwise providing host-associated cues (Fig. 4A). Hand approach was strong in wild type, with 57 +/− 2% (average +/− SEM) of mosquitoes landing on the surface beneath the hand (Fig. 4B, C). Hand-associated cues were critical, as hand withdrawal prompted rapid dispersal (Fig. 4B). Ir21aEYFP mutants remained robustly responsive, exhibiting maximum levels of approach (55 +/− 2%) similar to wild type (Fig. 4B, C). Careful examination of response kinetics revealed that, compared to wild type, their initial accumulation rate decreased by ~25% (Fig. 4B, D), and their dispersal rate upon hand removal increased by ~40% (Fig. 4B, E), potentially reflecting subtle contributions of the warmth gradient created by the presence of the hand (Fig. S4C) to the avidity of host approach. Similar results were obtained for Ir21a+7bp (Fig. S4D-G). Overall, these data demonstrate that the loss of Ir21a does not broadly disrupt orientation toward sensory cues and argue against the presence of global behavioral deficits in the mutants. These results are consistent with prior work indicating that the disruption of single sensory modalities is insufficient to completely eliminate host approach (2, 3, 8, 9).
Fig. 4: Ir21a promotes warmth-stimulated blood feeding.
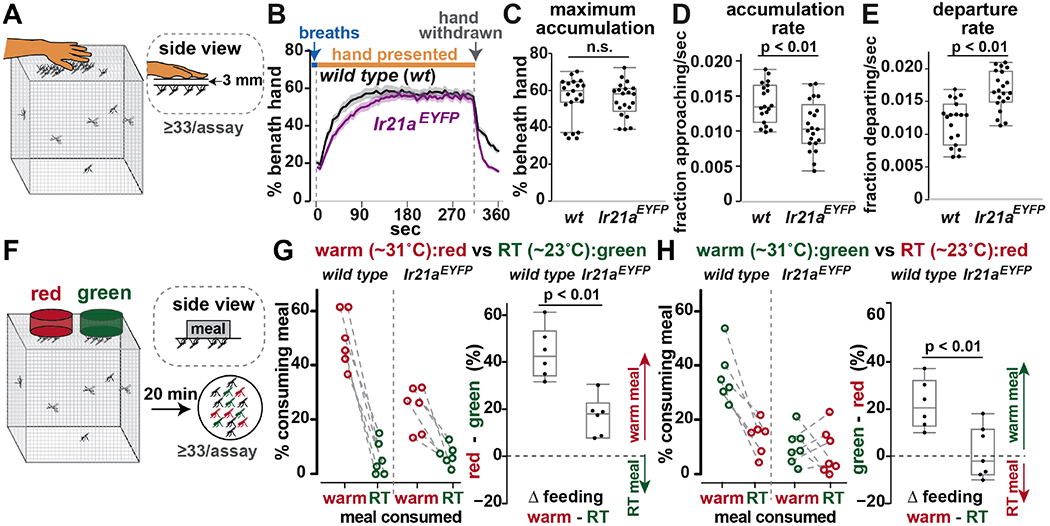
(A-E) Host approach. Mosquitoes were activated by five breaths, then presented a hand. (B) Mosquitoes landed (accumulated) on cage roof below hand. Average +/− SEM. 33–75 females/assay. wild type, n=19 independent groups; Ir21aEYFP , n=21. (C) Average accumulation, 180s to 300s. (D) Accumulation rate=[{(accumulation at 45s) – (accumulation pre-hand exposure)}/{(maximum accumulation) – (accumulation pre-hand exposure)}]/45s. (E) Departure rate=[1 – {(accumulation at 340s)/(accumulation at 300s)}]/40s. (F-H) Blood-feeding. Two meals (one dyed green) placed on cage. (G) RT meal dyed. (H) Warm meal dyed. Dotted lines link pairs. 33-75 females/assay. n=6 independent groups/genotype, except H, Ir21aEYFP n=7. p values indicate t-test results. The green tracking dye partially reduced relative consumption of the meals to which it was added. Cages are 17.5 cm-sided cubes. Shapiro-Wilk test values are provided in statistics section of methods.
Heat strongly stimulates mosquito blood feeding (8, 9). To assess the effect of warmth on blood feeding, artificial membrane feeders (25) were used to present human blood meals at different temperatures (Fig. 4F). One meal was held at room temperature (RT, ~23°C) and the other warmed to ~31°C, a temperature similar to the surface temperatures (~29°C-33°C) of human torsos and extremities in a 23-24°C room (26). In each trial, green food coloring was added to one meal so that the consumption of warm versus RT food could be distinguished (Fig. S5). Each class of trial was assessed independently (RT meal colored in Fig. 4G, Fig. S4H; warm meal colored in Fig. 4H, Fig. S4I), and each yielded similar results. In wild type, elevated temperature robustly promoted feeding, as reflected in the greater percentages consuming warm over RT meals (Fig. 4G,H; Fig. S4H,I). In both Ir21aEYFP (Fig. 4G,H) and Ir21+7bp (Fig. S4,H,I), this preference for warm blood was significantly reduced. Thus, similar to heat-seeking, warmth-promoted blood feeding was reduced in the absence of Ir21a.
These data identify IR21a as a key mediator of heat-seeking behavior in An. gambiae mosquitoes. Although a cooling-activated receptor driving heat seeking is superficially counterintuitive, repulsion from cooling would yield a similar behavioral outcome as attraction to warming. Furthermore, Cooling Cells are bi-directional and are not only activated by cooling, but also inhibited by heating (Fig. 2D,E); each phase of the response could modulate downstream circuits to control behavior. Ultimately, the detection of temperature change by the Cooling Cells is critical, but just one step in heat seeking, a response that involves the processing of multiple sensory inputs to generate a coherent response. Identifying a key molecular receptor for heat seeking provides a starting point for a deeper understanding of this complex behavior and its contribution to the multi-modal process that culminates in mosquito blood feeding.
The conservation of IR21a’s thermosensory function between Drosophila (18) and Anopheles (Fig. 2), whose last common ancestor lived ~250 million years ago, suggests thermosensing is an ancestral function of IR21a. As this ancestor predates the evolution of blood-feeding (13), its IR21a would have regulated other behaviors, such as thermoregulation. Thus, our findings indicate that the evolution of blood feeding in An. gambiae mosquitoes involved repurposing an ancestral thermoreceptor to facilitate host seeking. Alterations in the connectivity or function of downstream circuits would likely have been crucial in this behavioral shift. Given the conservation of IR21a as well as IR25a and IR93a (IR21a’s co-receptors in Drosophila) across insects (19), these IRs may be used in heat seeking not only by other mosquitoes, but across a range of hematophagous insect taxa.
In addition to Ir21a’s role in heat seeking, IR21a’s expression in the antennae of An. gambiae males suggests it continues to serve additional thermosensory functions. It will be interesting to assess whether IR21a mediates thermal preference in male and possibly female mosquitoes, and the extent to which thermal preference and heat-seeking circuits overlap. It is also worth noting that not all thermoreceptors appear to have been repurposed, as the TRPA1 warmth receptor has a similar role in flies and mosquitoes, mediating heat avoidance in both (12). At a practical level, exploiting and manipulating the sensory systems of vector insects offers an avenue for disease control strategies.
Supplementary Material
Acknowledgments:
We thank Andrew Hammond, Rob Harrell, Tony Nolan, Susan McIver, Andrea Crisanti, Eric Marois, Ben White and Leslie Vosshall for reagents and advice, Rebecca Albuquerque for assistance with data analysis, Rachel Gerber for assistance with mosquito husbandry and behavioral assays, and Amy Lee, Eve Marder, Michael Rosbash, Piali Sengupta, and Crystal Zhu for comments on the manuscript.
Funding:
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (F31 AI133945.) to C.G., the National Institute of Neurological Disorders and Stroke (2T32NS007292-31) to W.J.L., the National Institute of General Medicine (F32 GM113318) to B.G, the Swiss National Science Foundation (P2FRP3_168480) to L.v.G, a Faculty Research Scholar Award by the Howard Hughes Medical Institute (HHMI) and the Bill & Melinda Gates Foundation (BMGF) to F.C. (Grant ID: OPP1158190), the National Science Foundation (IOS 1557781) to P.A.G., and the National Institute of Allergy and Infectious Diseases (R01 AI122802 and R21 AI140018) to P.A.G. and F.C.
Footnotes
Competing interests:
A.L.S. is a co-inventor on patent WO2015105928A1 (WIPO PCT; pending; inventors: K. Esvelt and A.L. Smidler) “Rna-guided gene drives”. Patent involves spreading desirable traits genetically through mosquito populations using Cas9-based gene drives. IR21a could potentially be used as a target for such a gene drive. P.A.G. is a co-inventor on patent WO2017196861A1 (WIPO PCT; pending; inventors: Z. Knecht, P.Garrity, Lina Ni) "Methods for modulating insect hygro- and/or thermosensation". This patent proposes using members of the Ionotropic Receptor family as targets for strategies to disrupt hygro- and thermosensation in insects.
Data and materials availability:
The datasets generated during and analyzed during the current study are available at DRYAD, https://datadryad.org/stash
(https://doi.org/10.5061/dryad.pzgmsbcg3 ). All reasonable additional requests for data and materials will be fulfilled.
References and Notes:
- 1.World, Health, Organization, A global brief on vector-borne diseases. WHO/DCO/WHD/2014.1 (2014). [Google Scholar]
- 2.Brown AWA, The attraction of mosquitoes to hosts. Journal of the American Medical Association 196, 159–162 (1966).5952113 [Google Scholar]
- 3.Carde RT, Multi-Cue Integration: How Female Mosquitoes Locate a Human Host. Current biology : CB 25, R793–795 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Lazzari CR, The thermal sense of blood-sucking insects: why physics matters. Curr Opin Insect Sci 34, 112–116 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Howlett FM, The influence of temperature upon the biting of mosquitoes. Parasitology 3, 479– 484 (1910). [Google Scholar]
- 6.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR, Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, et al. , orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB, Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raji JI, Melo N, Castillo JS, Gonzalez S, Saldana V, et al. , Aedes aegypti Mosquitoes Detect Acidic Volatiles Found in Human Odor Using the IR8a Pathway. Current biology : CB 29, 1253–1262 e1257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G, Qiu YT, Lu T, Kwon HW, Pitts RJ, et al. , Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur J Neurosci 30, 967–974 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maekawa E, Aonuma H, Nelson B, Yoshimura A, Tokunaga F, et al. , The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasit Vectors 4, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corfas RA, Vosshall LB, The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. eLife 10.7554/eLife.11750 (2015); [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimaldi D, Engel MS, Evolution of the insects. (Cambridge University Press, Hong Kong, 2005). [Google Scholar]
- 14.Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, et al. , Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481, 76–80 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, et al. , A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580–584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni L, Klein M, Svec KV, Budelli G, Chang EC, et al. , The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. eLife 10.7554/eLife.13254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knecht ZA, Silbering A, Cruz J, Yang L, Croset V, et al. , Ionotropic Receptor-dependent moist and dry cells control hygrosensation in Drosophila. eLife 10.7554/eLife.26654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budelli G, Ni L, Berciu C, van Giesen L, Knecht ZA, et al. , Ionotropic Receptors Specify the Morphogenesis of Phasic Sensors Controlling Rapid Thermal Preference in Drosophila. Neuron 101, 738–747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rytz R, Croset V, Benton R, Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol 43, 888–897 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Enjin A, Zaharieva EE, Frank DD, Mansourian S, Suh GS, et al. , Humidity sensing in Drosophila. Current biology : CB 26, 1352–1358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ, Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics 12, 271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis EE, Sokolove PG, Temperature responses of antennal receptors of the mosquito, Aedes aegypti. J. Comp. Physiol. 96, 223–236 (1975). [Google Scholar]
- 23.McIver SB, Sensilla mosquitoes (Diptera: Culicidae). J Med Entomol 19, 489–535 (1982). [DOI] [PubMed] [Google Scholar]
- 24.Gingl E, Hinterwirth A, Tichy H, Sensory Representation of Temperature in Mosquito Warm and Cold Cells. Journal of Neurophysiology 94, 176–185 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove JB, Wood RJ, Petric D, Evans DT, Abbott RH, A convenient mosquito membrane feeding system. Journal of the American Mosquito Control Association 10, 434–436 (1994). [PubMed] [Google Scholar]
- 26.Zaproudina N, Varmavuo V, Airaksinen O, Narhi M, Reproducibility of infrared thermography measurements in healthy individuals. Physiological measurement 29, 515–524 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Littig KS, Stovanovich CJ, in Pictorial keys to arthropods, reptiles, birds and mammals of public health significance. Communicable Disease Center, U.S. Department of Health, Education and Welfare, Public Health Service, Atlanta, Georgia, (1966), pp. 134. [Google Scholar]
- 28.Volohonsky G, Terenzi O, Soichot J, Naujoks DA, Nolan T, et al. , Tools for Anopheles gambiae Transgenesis. Genes, Genomes, Genetics (Bethesda) 5, 1151–1163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, et al. , CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol 34, 78–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diao F, Ironfield H, Luan H, Diao F, Shropshire WC, et al. , Plug-and-play genetic access to drosophila cell types using exchangeable exon cassettes. Cell Rep 10, 1410–1421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werling K, Shaw WR, Itoe MA, Westervelt KA, Marcenac P, et al. , Steroid Hormone Function Controls Non-competitive Plasmodium Development in Anopheles. Cell 177, 315–325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrino M, Nakagawa T, Vosshall LB, Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J Vis Exp, 10.3791/1725 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


