Abstract
Trehalose-6-phosphate synthase (TPS) is significant in the growth, development and stress resistance of plants. We identified the cucumber TPS family and its physicochemical properties, domains, gene structures, evolutionary relationships, gene locations, cis-acting elements, conserved motifs, and expression patterns using bioinformatics. Our results uncovered seven CsTPS genes in the cucumber genome and named CsTPS1–CsTPS7 according to their locations in the chromosomes. Seven CsTPS genes were randomly distributed in six cucumber chromosomes. Domain analysis showed that the TPS and TPP domains exist in all CsTPSs, and an additional hydrolase-3 domain exist in CsTPS3, CsTPS5 and CsTPS6. Phylogenetic analysis showed that TPS proteins from Arabidopsis, rice, soybean, and cucumber were divided into two subfamilies (Class I and Class II) and they were further divided into seven subgroups. TPS proteins from Arabidopsis and cucumber were grouped together, suggesting a close evolutionary relationship. Gene structure analysis indicated that most Class I genes contained 16–17 introns, while Class II genes (except CsTPS7) had two introns. Motif analysis showed that Class II genes had 10 complete conserved motifs, while Class I genes lacked motif 8 and motif 9. Furthermore, CsTPS genes possessed numerous cis-acting elements related to stress, hormone, and light response in the promoter regions. GO analysis indicated multiple functions for the CsTPS proteins. Expression analysis of CsTPS genes in different tissues found that they were expressed in roots, stems and leaves, with the highest expression levels in roots. The expression analysis of CsTPSs under different treatments showed that CsTPS genes may participate in the response to abiotic stress, plant hormones and sugar treatments.
Keywords: Cucumber TPS, Domains, Gene structures, Evolutionary relationships, Conserved motifs, Expression patterns
Introduction
The growth and development of plants in agriculture are often affected by various adverse conditions, including submergence, drought, low or high temperatures, and saline and alkaline soils (Zhu, 2016). These adverse conditions may dehydrate plants, which reduces the photosynthesis rate (Baninasab, 2010), promotes the production of reactive oxygen species (ROS), and damages the cell membrane (Deinlein et al., 2014). Plants have produced a series of physiological mechanisms over time to protect themselves from adversity. For example, under stress conditions, plants express stress-related genes to produce stress proteins, including the heat shock protein (HSP), low temperature-induced protein, osmoregulatory protein, and the pathogenesis-related protein (PR) (Hightower, 1991). Plants may also accumulate osmotic adjustment substances (Ashraf & Foolad, 2007) under unfavorable conditions, including inorganic ions (Na+, K+ and Cl-), proline, betaine, abscisic acid (Kim, 2012) and sugars (sucrose, fructose and trehalose) to protect the integrity of the membrane structure.
Trehalose (α-D-glucopyranosyl-1, 1-α-D-glucopyranoside) (Lunn et al., 2014) is a non-reducing disaccharide composed of two molecules of glucose (Elbein et al., 2003). In higher plants, trehalose is synthesized through the catalysis of two enzymes: trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase (TPP). TPS first catalyzes UDP-glucose and glucose-6-phosphate to produce trehalose-6-phosphate (T6P) and UDP. Then, TPP dephosphorylates trehalose-6-phosphate to produce trehalose (Goddijn & Van Dun, 1999). Trehalose is widely found in plants and plays a specific role in plant growth, development, and resistance to stress. Compost treated with trehalose has been used to cultivate quinoa plants to increase their growth and yield (Abdallah et al., 2020), and soaking rice seeds with trehalose may relieve salt stress (Abdallah, Abdelgawad & El-bassiouny, 2016). Trehalose may form Cd-Trehalose chelate with cadmium (Cd) to alleviate the damage of cadmium stress in rice (Wang et al., 2020). The expression of the TPS gene and the accumulation of trehalose has been shown to increase when wheat are exposed to drought (El-Bashiti et al., 2005).
TPS plays a vital role in trehalose metabolism and stress resistance in plants (Yang et al., 2012). Previous studies have shown that light quality may affect the growth and phase transition by influencing the TPS1-T6P signaling pathway in tomatoes (Chen & Lou, 2017). The overexpression of the TPS1 gene in rice and potato enhanced their stress resistance (Li et al., 2011; Kondrák et al., 2011). The overexpression of the TPS11 gene in wheat improved cold resistance in Arabidopsis (Liu et al., 2019b); TPS1 played an important role in the embryogenesis, post-embryonic growth, and development in Arabidopsis (Fichtner et al., 2020). Studies have shown that TPS affected development and metabolic processes by altering T6P levels (Lunn et al., 2014)
T6P is an intermediary in trehalose biosynthesis with a vital role in plant growth and development (Yadav et al., 2014; Zhang et al., 2015). T6P serves as a sugar-signaling molecule in Arabidopsis that coordinates the hypocotyl elongation mediated by high temperature and the availability of endogenous sugar (Geonhee et al., 2019). The accumulation of T6P inhibited the growth of Arabidopsis seedlings mediated by trehalose (Schluepmann et al., 2003). The T6P signaling pathway played an important role in the flowering of the Arabidopsis leaf and stem meristems (Wahl et al., 2013).
T6P was also involved in regulating the use and distribution of sucrose, coordinating source-sink relationships, the efficient use of carbohydrates (Schluepmann et al., 2003), and improving crop yield (Paul, Watson & Griffiths, 2020). Research on cucumber fruit has shown that there was a strong correlation between T6P and sucrose (Zhang et al., 2015). The Tre6P:sucrose ratio could maintain sucrose levels within a range that is appropriate for the cell type and developmental stage of plants (Yadav et al., 2014). Varying T6P levels and sugar signaling through chemical intervention significantly impacted crop yield and resilience (Griffiths et al., 2016; Smeekens, 2017).
The overexpression of rice TPP genes in maize ears under well-watered or drought conditions reduced the level of T6P, increased the level of sucrose, and improved yield (Nuccio et al., 2015). A study of Arabidopsis indicated that many growth and developmental defects were due to T6P rather than trehalose (Schluepmann et al., 2003).
Cucumber (Cucumis sativus L.) is a widely cultivated crop with high nutritional value. Its growth and development are easily affected by adverse condition, especially salt stress (Miao et al., 2020). We sought to identify members of the TPS family in cucumber using bioinformatics methods. We analyzed the gene structure and location, motif distribution and composition, evolutionary relationship and expression patterns. We hope that our work supports future functional research of the TPS family in cucumber plants.
Materials and Methods
Genome-wide identification and bioinformatics analysis
We downloaded the whole genome in the gff, cds, pep and FASTA file format from the EnsemblPlants- Cucumber genome database (ASM407v2) (http://plants.ensembl.org/index.html) (Li et al., 2019b). The TPS (Glyco-transf-20, PF00982) and TPP (Trehalose_PPase, PF02358) domains’ hidden Markov Models (HMM) were downloaded from the Pfam database (http://pfam.xfam.org) (Liu et al., 2019a). HMMsearch software and the TPS and TPP’s HMMs were used to search all possible TPS candidate sequences containing typical TPS and TPP domains under a Linux system (Chen et al., 2019). Pfam (Liu et al., 2019a) and NCBI-CDD (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) (Yan, Li & Zhao, 2019) databases were used to manually confirm that the candidate sequences had complete TPS and TPP domains. The remaining genes were subsequently identified as members of the cucumber TPS family and were named according to their location on the cucumber’s chromosome.
The length of open reading frames (ORFs) of cucumber TPS genes was predicted using the NCBI-ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/) website (Xie et al., 2015). The physicochemical properties and subcellular locations of the cucumber TPS proteins were forecast using Protparam (https://web.expasy.org/protparam/) (Zhang et al., 2019) and the Cell-PLoc2.0 (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) website, respectively.
The cucumber TPS proteins’ secondary structures were determined using the PRABI (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html) website (Xu et al., 2016).
Phylogenetic analysis
The phylogenetic tree, containing seven cucumber, 11 rice (Zang et al., 2011), 11 Arabidopsis (Yang et al., 2012) and 20 soybean (Xie, Wang & Huang, 2014) TPS protein sequences, was constructed based on multiple sequence alignments using Fasttree software and the maximum likelihood method. The bootstrap replication value was set as 1,000 and the other parameters remained constant. We used the evolview (https://evolgenius.info//evolview-v2/#login) website to improve the appearance of the evolutionary tree. The TPS protein sequences of Arabidopsis, rice, and soybean were downloaded from TAIR (https://www.arabidopsis.org/) (Song et al., 2019), the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/) (Zang et al., 2011), and the Phytozome (https://phytozome.jgi.doe.gov) (Yue et al., 2019) database, respectively (Supplemental File 1).
Gene structure, chromosomal location and cis-acting element analysis
The GSDS2.0 (http://gsds.gao-lab.org/index.php) (Li et al., 2019a) website was used to analyze the gene structure of cucumber TPS genes and to plot the exon-intron diagram. We used the TBtools software to combine the evolutionary tree with the gene structure diagram and Mapchart software was applied to visualize the location of the genes on the chromosomes. The 2kb sequences in the cucumber TPS genes’ upstream region were screened as promoter sequences using Tbtools software. We used plantCARE (Song et al., 2019) (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to investigate the cis-acting elements in promoter regions to study the roles of genes in stress and hormone responses.
Conserved motifs analysis
We searched the MEME (http://meme-suite.org/) (Liu et al., 2019c) website for conserved motifs of cucumber TPS proteins. The maximum retrieval value for the motif was set to 10 and the other parameters were set to default. InterProScan software was used to annotate the retrieved motifs.
GO annotation
We used the EggNOG mapper software (http://eggnog-mapper.embl.de/) to perform the gene ontology analysis. Cucumber TPS protein sequences were uploaded and Arabidopsis TPSs were used as the reference. GO analysis was categorized as: molecular function (MF), biological process (BP), and cellular component (CC).
Plant materials, cultivation conditions and treatments
Cucumber seeds (C. sativus L. “Xin Chun 4”) were germinated and grown in culture dishes with wet filter paper. Seedlings were transferred into hydroponic boxes once the cotyledon fully unfolded. The boxes contained Yamazaki cucumber nutrient solution and were placed in plant incubators at 25 °C, a light intensity of 200 μmol.m−2s−1, and a photoperiod of 14 h light/10 h dark (Niu et al., 2019). The nutrient solution was replaced every two days in order to maintain an adequate level of nutrients. Stress treatments were carried out when seedlings were at the two-leaf stage. Seedlings were grown in a 1/2 nutrient solution containing 8% (w/v) PEG, 50 mM NaCl, 1 μM IAA, 8% (w/v) H2O2, 50 mM sucrose and 50 mM mannitol, respectively, for drought, salt, IAA, H2O2, sucrose and mannitol treatments. The concentrations of these reagents were determined by a preliminary experiment. Whole seedlings were frozen with liquid nitrogen after treatment at 0, 6, 12 and 24 h, and were stored at −80 °C (Zhu et al., 2019). The roots, stems, and leaves of untreated seedlings were collected at the two-leaf stage and were stored at −80 °C to investigate the expression of cucumber TPS genes in the different tissues. Each treatment was performed with three biological replicates and each sample was collected from five cucumber seedlings.
RNA extraction, reverse transcription and quantitative real-time PCR
The total RNA from different tissues and whole seedlings under different treatments were extracted using the MiniBEST Plant RNA Extraction Kit (TaKaRa, Dalian, China). The RNA concentration and purity were determined using the NaNo drop 1,000 spectrophotometer and agarose gel electrophoresis (Xie et al., 2018). The FastQuant first strand cDNA synthesis kit (TIANGEN, Beijing, China) was used for the synthesis of cDNA following the manufacturer’s protocol. The SuperReal PreMix Plus kit (TIANGEN, Beijing, China) and a Roche LightCycler instrument were used for qRT-PCR. The reaction system of qRT-PCR was as follows: 10 μL 2×SuperReal PreMix Plus, 0.6 μL 10 μM forward primers, 0.6 μL 10 μM reverse primers, 2 μL cDNA and 6.8 μL RNase-free ddH2O. The qRT-PCR procedure was as follow: 95 °C for 15 min and 40 cycles of 95 °C for 10 s and 60 °C for 20 s. CsActin was used as an internal reference gene (Zhou et al., 2017). The primers of the cucumber TPS genes and CsActin for qRT-PCR were designed and synthesized using Sangon Biotech online software (Table 1). Three technical replicates were performed for each reaction.
Table 1. Primer sequence for qRT-PCR.
| Gene name | Primer sequence (5′ to 3′) | |
|---|---|---|
| CsTPS1 | F: AAGTGGTGCTGTCAGGGTAAATCC | R: GCCCAGTAAGCAACATCGTGAGAG |
| CsTPS2 | F: AGCGTTGGTGGTTTAGTCAGTGC | R: TGCTTTCTCTAGGGCTCTCTGTCC |
| CsTPS3 | F: TGGGCTCGGAGAAGATGTGGAAG | R: GTCGGGACGCACTTGAATCGG |
| CsTPS4 | F: ACCCTTCCATCCCGATCAGAGC | R: TCCTTGGTCCTCAACTCCTTCTGG |
|
CsTPS5 CsTPS6 CsTPS7 CsActin |
F: AAGCCAAGGAATTGCTGGACCATC F: CTGTCATGCCGCAAACTTCAATCG F: AGACGGTGTTGCTTGGTGTTGATG F: TGGACTCTGGTGATGGTGTTA |
R: TGCGACCAACCCTTTGCTTACTC R: AAACTTTCACGCCCTCTTCCACTG R: ACAGCCTTCCCTTGCCACTTTG R: CAATGAGGGATGGCTGGAAAA |
Statistical analysis
The relative expression of the genes was calculated using the 2−ΔΔCt method (Han et al., 2020). The expression of the CsTPS genes in the roots was used as a calibration sample to calculate their relative expressions in the stem and leaf (Zhang et al., 2014). The expression of CsTPS genes in untreated seedlings (0 h) was used as a calibration sample to calculate their relative expression at 6, 12 and 24 h. We adopted Duncan’s (p < 0.05) method for significance analysis.
Results
Genome-wide identification of TPS family in cucumber
Seven TPS sequences were identified in the cucumber genome using bioinformatics methods. The Pfam and NCBI-CDD databases were used for domain analysis to further prove the reliability of these candidate sequences. Our results indicated that all seven sequences had a typical TPS domain and belonged to the cucumber TPS family. The cucumber TPS genes identified were named CsTPS1-CsTPS7 according to their location in the cucumber chromosome (Table 2).
Table 2. Fundamental information of CsTPS genes.
| Gene | Gene ID | Gene locus | ORF(bp) | Amino acid | Molecular weight/KDa |
pI | TPS domain location | TPP domain location | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| CsTPS1 | Csa_1G005560 | Chr1 | 2568 | 855 | 97.14 | 6.34 | 53-540 | 589-824 | Chloroplast. Vacuole. |
| CsTPS2 | Csa_1G467060 | Chr1 | 2604 | 867 | 97.87 | 7.02 | 21-492 | 537-765 | Chloroplast. Vacuole. |
| CsTPS3 | Csa_3G009420 | Chr3 | 2595 | 864 | 97.46 | 5.73 | 62-548 | 597-830 | Cytoplasm. Vacuole. |
| CsTPS4 | Csa_4G622880 | Chr4 | 2787 | 928 | 105.15 | 6.24 | 94-561 | 606-834 | Cytoplasm. Vacuole. Chloroplast. |
| CsTPS5 | Csa_5G602180 | Chr5 | 2583 | 860 | 97.23 | 6.25 | 58-545 | 594-829 | Cytoplasm. Vacuole. |
| CsTPS6 | Csa_6G520240 | Chr6 | 2553 | 850 | 96.31 | 5.50 | 59-544 | 593-828 | Cytoplasm. Vacuole. |
| CsTPS7 | Csa_7G049190 | Chr7 | 2916 | 971 | 110.33 | 6.85 | 174-659 | 708-943 | Chloroplast. Vacuole. |
Domain analysis revealed that all cucumber TPS proteins contained a typical TPS (Glyco_transf_20; Pfam: PF00982) domain in the N-terminal and a TPP (Trehalose_PPase; Pfam: PF02358) domain in the C-terminal (Table 2). CsTPS3, CsTPS5, and CsTPS6 proteins all contained an additional Hydrolase-3 (Pfam: PF08282) domain (Supplemental File 2).
Physical and chemical property analysis revealed that the length of the open reading frame of the CsTPS genes was between 2,553 bp (CsTPS6) and 2,916 bp (CsTPS7); the length of cucumber TPS proteins was between 850 (CsTPS6) and 971 (CsTPS7) amino acids; and their molecular weight was between 96.31 KDa (CsTPS6) and 110.33 KDa (CsTPS7). The isoelectric point (pI) ranged from 5.50 (CsTPS6) to 7.02 (CsTPS2). CsTPS2 was the only alkalescence (pI > 7) in the cucumber TPS proteins and the rest were acidic (pI < 7) (Table 2).
The subcellular localization prediction indicated that cucumber TPS genes were mainly distributed in the vacuole, chloroplast, and cytoplasm (Table 2).
The analysis of the secondary structure showed that cucumber TPS proteins were composed of an α-helix, random coil, extended strand and a β-turn (Table 3).
Table 3. The secondary structures of CsTPS proteins.
| Protein | Alpha helix (%) | Beta turn (%) | Random coil (%) | Extended strand (%) |
|---|---|---|---|---|
| CsTPS1 CsTPS2 CsTPS3 CsTPS4 CsTPS5 CsTPS6 CsTPS7 |
42.81 42.68 43.63 43.97 41.98 43.06 39.03 |
4.21 5.19 5.44 5.39 5.35 5.18 5.46 |
35.79 35.87 34.26 37.07 35.23 34.82 36.05 |
17.19 16.26 16.67 13.58 17.44 16.94 19.46 |
Phylogeny and gene structure analysis of CsTPSs
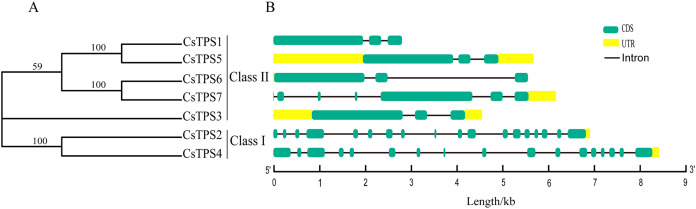
Seven cucumber TPS proteins were divided into two subfamilies, Class I and Class II, based on the results of previous studies (Lunn, 2007; Mu et al., 2016). Among the seven CsTPS proteins, CsTPS2 and CsTPS4 were classified as Class I and the remaining five proteins were classified as Class II (Fig. 1A). In addition, the exon-intron diagram revealed that two members (CsTPS2 and CsTPS4) of Class I possessed 17 and 16 introns, respectively. In Class II, only CsTPS7 had six introns, while the other members all contained two introns (Fig. 1B). We inferred that two subfamilies went through functional differentiation in the course of evolution (Du et al., 2017).
Figure 1. Phylogenetic relationships and gene structures of CsTPSs.
(A) The evolutionary tree was built based on the full-length cucumber TPS protein sequences using Fasttree software. (B) The exon-intron diagram of cucumber TPS genes was mapped using GSDS2.0.
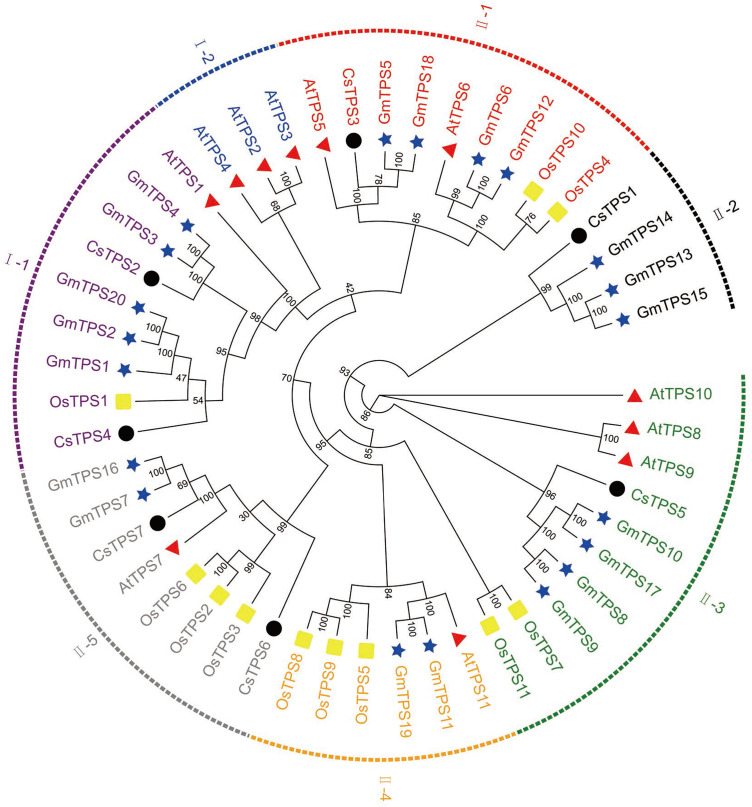
We aligned the full-length protein sequences of seven cucumber TPS proteins, 11 Arabidopsis TPS proteins (Yang et al., 2012), 11 rice TPS proteins (Zang et al., 2011) and 20 soybean TPS proteins (Xie, Wang & Huang, 2014) to establish a maximum likelihood phylogenetic tree (Fig. 2) to further investigate the evolutionary relationships of TPS family in various species. As previously described, the TPSs in cucumber, Arabidopsis, rice, and soybean may be differentiated into two subfamilies: Class I and Class II. The two subfamilies were further divided into seven subgroups: I-1, I-2, II-1, II-2, II-3, II-4 and II-5, based on the phylogenetic relationship with high bootstrap support (Xie et al., 2015). Subgroup I-1 contained nine members originating from Arabidopsis (1), rice (1), cucumber (2) and soybean (5), respectively. I-2 contained three members which all originated from Arabidopsis. II-1 contained nine members which were derived from Arabidopsis (2), rice (2), cucumber (1), and soybean (4), respectively. II-2 contained four members which derived from cucumber (1) and soybean (3), respectively. II-3 contained ten members which originated from Arabidopsis (3), rice (2), cucumber (1), and soybean (4), respectively. II-4 contained six members which originated from Arabidopsis (1), rice (3), and soybean (2), respectively. II-5 contained eight members which derived from Arabidopsis (1), rice (3), cucumber (2), and soybean (2), respectively. With the exception of I-2, II-2 and II-4, the other subgroups contained at least one member of four species. Our results showed that some of the TPS proteins of cucumber and soybean were divided into the same subgroup, indicating that they were closely related. In addition, previous studies have shown that most of TPS genes in the Class I had 16 introns, while TPS genes in the Class II possessed 2 introns (Yang et al., 2012). When combined with the analysis of the cucumber TPS gene structure, we can speculate that the genes of the same group were close to each other during the evolution process, while the genes of different groups were far away from each other during the evolution process (Xie et al., 2015).
Figure 2. Evolutionary relationships of TPS family in various species.
A phylogenetic tree containing seven cucumber, 11 rice (Os), 11 Arabidopsis (At), and 20 soybean (Gm) TPS proteins was constructed using the maximum likelihood method (Cheng et al., 2018). The seven subgroups are colored differently. The four differently-colored shapes represent TPS proteins from four species. The black circle, yellow rectangle, red triangle, and blue star represent cucumber, rice, Arabidopsis, and soybean TPS proteins, respectively.
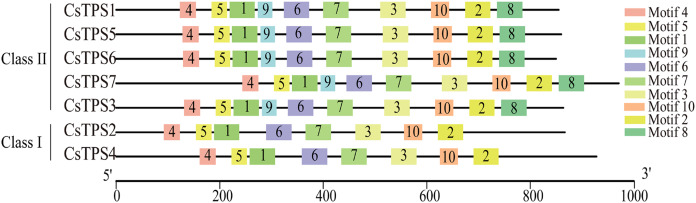
Conserved motifs of cucumber TPS proteins
We used the MEME online website to study the characteristic regions of cucumber TPS proteins. We searched 10 conserved motifs in cucumber TPS proteins (Fig. 3). The lengths of these conserved motifs were between 29 and 50 amino acids and the sequence information of these 10 conserved motifs is listed in Table 4. Cucumber TPS proteins classified into the same subfamily in the evolutionary tree possessed a similar or identical motif composition (Ou et al., 2018). For instance, members of Class II possessed all 10 conserved motifs, while members (CsTPS2 and CsTPS4) of Class I lacked motifs 8 and 9. Motifs 4, 5, 1, 9, 6, 7 and 3 together constituted the TPS domain and motifs 10, 2 and 8 constituted the TPP domain, according to annotation. These results further supported the reliability of the phylogenetic classification of cucumber TPS proteins and indicated that the two subfamilies may have produced a functional difference during evolution (Xie et al., 2018).
Figure 3. The motif composition and distribution of cucumber TPS proteins.
Colored boxes represent different conserved motifs.
Table 4. Details of the 10 conserved motifs of cucumber TPS proteins.
| Motif | Width (aa) | Motif Sequence | Annotation |
|---|---|---|---|
| Motif 1 | 50 | GFFLHSPFPSSEIYRTLPVRDEJLRALLNADLIGFHTFDYARHFLSCCSR | Glyco-transf-20 |
| Motif 2 | 50 | WIQIAEPVMKLYTEATDGSHIETKESALVWHYQDADPDFGSCQAKELLDH | Trehalose_PPase |
| Motif 3 | 50 | KQLRHEKHYRYVSTHDVAYWSRSFLQDLERACRDHYRRRCWGIGFGLGFR | — |
| Motif 4 | 32 | FKCIPTFLPPEJLKQFYHGFCKQHLWPLFHYM | Glyco-transf-20 |
| Motif 5 | 31 | VVEVINPEDDYVWIHDYHLMVLPTFLRKRFN | Glyco-transf-20 |
| Motif 6 | 50 | FKGKKVJLGVDDLDIFKGINLKLLAFEQLLRQHPKWRGKAVLVQIANPAR | Glyco-transf-20 |
| Motif 7 | 50 | PGYEPIVLJDRPVPFHERIAYYAIAECCJVTAVRDGMNLVPYEYVVCRQG | Glyco-transf-20 |
| Motif 8 | 50 | KSGQHIVEVKPQGVSKGLVAEKILSSMAESGKLPDFVLCIGDDRSDEDMF | Trehalose_PPase |
| Motif 9 | 29 | YQSKRGYIGLEYYGRTVGIKILPVGIHMG | — |
| Motif 10 | 36 | EVISILNTLCDDPKNTVFIVSGRGRSSLGDWFGPCE | Trehalose_PPase |
Chromosomal location of cucumber TPS genes
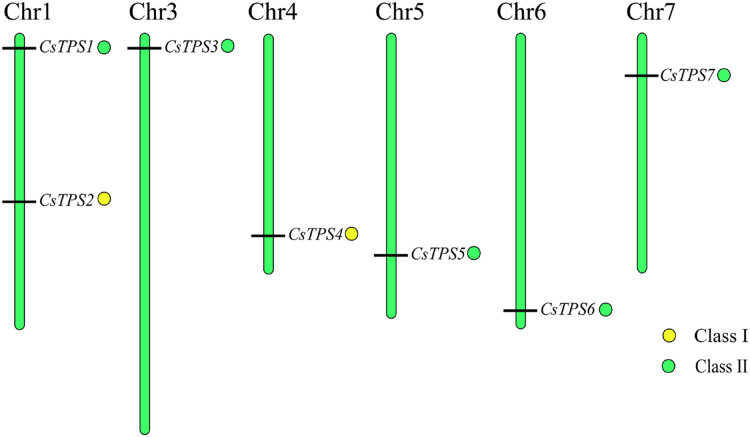
We used Mapchart software to analyze the location of CsTPS genes on cucumber chromosomes. The results showed that seven CsTPS genes were randomly located on six cucumber chromosomes (Fig. 4). Two genes (CsTPS1 and CsTPS2) were located on chromosome 1, while only single genes existed on the other chromosomes. The majority of the CsTPS genes were located on the proximate or distal ends of the cucumber chromosomes (Zhao et al., 2018).
Figure 4. TPS gene locations in cucumber chromosomes.
The chromosomes are represented by green bars.
Cis-acting element analysis of cucumber TPS genes
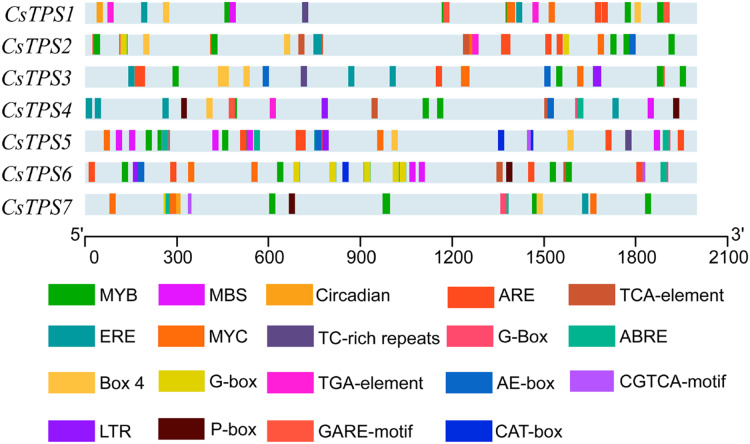
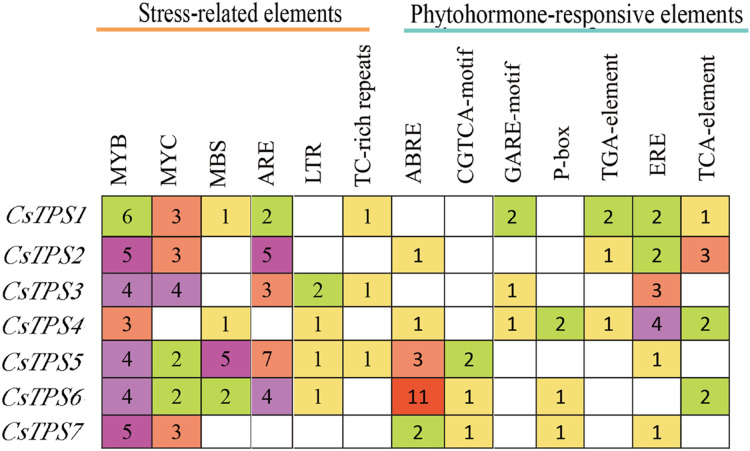
A total of 72 types of elements were found in the promoter regions of the CsTPS genes. All CsTPS genes contained CAAT and TATA boxes in the promoter regions, which were core and common promoter elements (Zhou et al., 2017) (Supplemental File 3). The cis-acting elements were identified and categorized as stress-related elements and plant hormone-responsive elements (Zhu et al., 2019). Stress-related elements, including MYB (stress response element), MYC (the recognition site of cold-resistant element), and ARE (anaerobic induction element) were mainly found in promoter regions of most CsTPS genes (Zhou et al., 2017). Some plant hormone-responsive elements, including ERE (ethylene-responsive element), ABRE (abscisic acid responsive element), TCA-element (salicylic acid-responsive element), and TGA-element (auxin-responsive element) (Han et al., 2020) were also found in most CsTPS genes (Figs. 5 and 6). Light responsive elements including Box4, AE-box, G-Box and GATA-motif were widely found in promoter regions of cucumber TPS genes (Fig. 5 and Supplemental File 3). Our results indicated that TPS genes played a significant role in stress, hormone and light response (Zhao et al., 2018).
Figure 5. The distribution of cis-elements in cucumber TPS genes.
Colored rectangles represent different cis-acting elements.
Figure 6. The number of Cis-acting elements in cucumber TPS genes.
GO annotation of cucumber TPS proteins
We found that a majority of cucumber TPS proteins participated in UDP-glycosyltransferase activity, hydrolase activity, phosphatase activity, catalytic activity, and alpha-trehalose-phosphate synthase activity (Supplemental File 4). CsTPS2 and CsTPS4 were involved in transferase activity and phosphoric ester hydrolase activity. Cellular component analysis showed that most CsTPS proteins were located on the cytoplasm and cytosol. However, CsTPS2 and CsTPS4 were also located intracellularly. Biological process analysis indicated that most CsTPS proteins were involved in various biological processes, including metabolism, biosynthesis, cellular processes, and development. Some CsTPS proteins also participated in the stress response, signal transduction, post-embryonic development, and seed, fruit and reproductive system development.
Expression analysis of cucumber TPS genes in different tissues
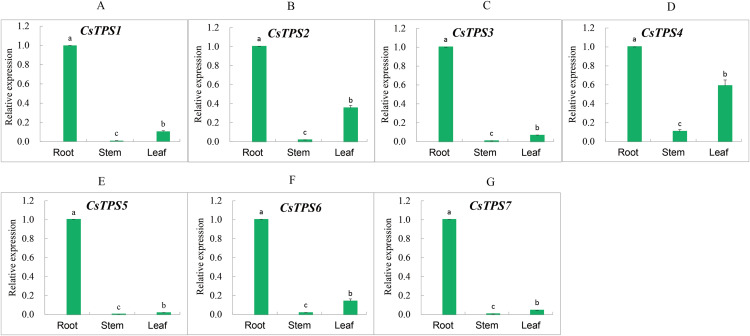
We measured the expression of CsTPS genes in root, stem and leaf using qRT-PCR to determine the expression specificity of cucumber TPS genes in different tissues. The expression of CsTPS genes was detected in the root, stem and leaf (Fig. 7) and their expressions were high, moderate, and low in the root, leaf and stem, respectively. Our results indicated that CsTPS genes may play a specific role in the growth of cucumber seedlings.
Figure 7. Expression levels of CsTPS genes in root, stem and leaf.
The expression patterns of CsTPS1–CsTPS7 in different tissues are shown in A–G, respectively. Error bars represent the standard error of three replicates. The relative expression of each gene in different tissues is expressed as mean ± SE (n = 3). Bars with different lowercase letters were significantly different by Duncan’s multiple range tests (p < 0.05).
Expression analysis of cucumber TPS genes under different treatments
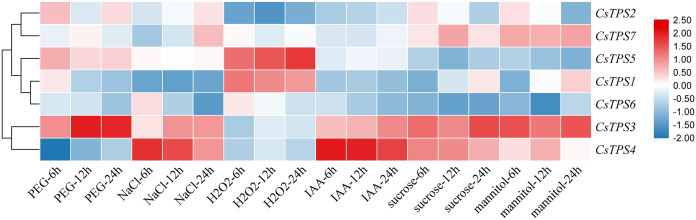
We conducted qRT-PCR experiments and drew a cluster heatmap to determine the expression patterns of CsTPS genes under various treatments (Fig. 8). Our results indicated different patterns for the expression of CsTPS genes with various treatments. Under PEG, the expression of CsTPS3 was up-regulated significantly and peaked at 24 h and CsTPS2 and CsTPS5 were up-regulated slightly. However, CsTPS1, CsTPS4, CsTPS6 and CsTPS7 were down-regulated and reached their lowest expression levels at 24 h. The expression of CsTPS3 and CsTPS4 was up-regulated significantly with NaCl treatment and reached the highest expression level at 24 h; the expression of CsTPS2 and CsTPS7 also increased. However, CsTPS1 and CsTPS6 were down-regulated with the NaCl treatment and reached the lowest expression level at 24 h. Under H2O2 treatment, CsTPS1 and CsTPS5 were activated while others genes were inhibited (CsTPS2, CsTPS3, CsTPS4 and CsTPS6) or did not show obvious trends (CsTPS7). Under mannitol treatment, CsTPS3 and CsTPS7 were up-regulated with the highest expression level at 24 h and CsTPS1 and CsTPS4 were slightly induced. Conversely, the mannitol treatment caused a large decline in the expression of CsTPS2, CsTPS5 and CsTPS6. Under IAA treatment, CsTPS3 and CsTPS4 were up-regulated significantly, whereas others genes showed no clear trends. CsTPS3 and CsTPS4 showed a strong expression under sucrose treatment, whereas CsTPS1 and CsTPS7 were induced slightly by sucrose. CsTPS2, CsTPS5 and CsTPS6 were inhibited by sucrose.
Figure 8. Expression levels of CsTPS genes under PEG, NaCl, H2O2, IAA, mannitol and sucrose treatments.
Seedlings were treated with 8% (w/v) PEG, 50 mM NaCl, 1 μM IAA, 8% (w/v) H2O2, 50 mM sucrose and 50 mM mannitol. The color scale represents fold changes normalized by log2 transformed data. Red represents up- regulated genes and blue represents down-regulated genes.
These results showed that CsTPS genes may be involved in stress, hormone, and sugar responses.
Discussion
Trehalose-6-phosphate synthase (TPS) is vital for the stress response and trehalose metabolism. Genes encoding TPS have been identified in many plants in the form of a gene family (Avonce et al., 2004). Previous studies have found 11, 11, 11, 8, 20, 13, 12, 10, 9 and 53 TPS genes in Arabidopsis (Yang et al., 2012), rice (Zang et al., 2011), pepper (Wei et al., 2016), potato (Xu et al., 2017), soybean (Xie, Wang & Huang, 2014), apple (Du et al., 2017), winter wheat (Xie et al., 2015), tomato (Chen & Lou, 2017), sugarcane (Hu et al., 2020), and cotton (Mu et al., 2016), respectively. However, the TPS gene family in cucumber has not been well-studied. We identified seven TPS genes in the cucumber genome, which were randomly located in six chromosomes of cucumber. Differences in the genome size of different species may cause variations in the number of members of the TPS family (Xie et al., 2018).
Cucumber TPS genes were divided into two subfamilies: Class I (CsTPS2 and CsTPS4) and Class II, which was consistent with the classification in Arabidopsis (Yang et al., 2012), rice (Zang et al., 2011) and pepper (Wei et al., 2016). We found that four (AtTPS1-AtTPS4), one (OsTPS1) and three (GaTPS1-GaTPS3) TPS genes belonged to Class I in Arabidopsis, rice and pepper, respectively. Class I genes in our study had 16–17 introns, while Class II genes had two introns, with the exception of CsTPS7. Motif analysis showed that Class II genes possessed all 10 conserved motifs, whereas Class I genes lacked motif 8 and 9 in cucumber. These results were supported by results from earlier studies in Arabidopsis (Yang et al., 2012), rice (Zang et al., 2011) and cotton (Mu et al., 2016). Class I genes possessed 16 introns in Arabidopsis and rice, whereas Class II genes had two introns. Class I genes in cotton had more introns than Class II genes and lacked motif 8 (Mu et al., 2016). According to a previous study, three mechanisms (exon/intron gain/loss, exonization/pseudoexonization and insertion/deletion) may cause the difference in gene structure (Xu et al., 2012). These studies illustrated that the two subfamilies may have experienced functional differentiation during evolution (Xie et al., 2018). Domain analysis indicated that all cucumber TPS genes possessed a TPS domain at the N-terminal and a TPP domain at the C-terminal, which was consistent with results from earlier studies in Arabidopsis (Yang et al., 2012), pepper (Wei et al., 2016) and apple (Du et al., 2017). CsTPS3, CsTPS5 and CsTPS6 proteins all contained a Hydrolase-3 (Pfam: PF08282) domain. However, some TPS genes lacked either a TPS domain or a TPP domain; GrTPS6, GhTPS4 and GhTPS9 genes in cotton lacked a TPP domain (Mu et al., 2016) and the loss of the domain may be the result of evolution. Evolutionary analysis showed that 49 TPS proteins from Arabidopsis, rice, cucumber and soybean were divided into two subfamilies and they were further classified into seven subgroups, similar to the classifications from a previous study (Xie et al., 2015). The CsTPS proteins were grouped together with at least one TPS protein from other species in the evolutionary tree, indicating that TPS proteins from different species had similar functions (Zhou et al., 2017).
Cis-acting elements were involved in the regulation of gene expression. Certain transcription factors have been shown to be activated and combined with cis-acting elements to activate the expression of stress-related genes when plants were exposed to adverse conditions (Hadiarto & Tran, 2011). We found that some elements related to stress (MBS, LTR, ARE), hormones (ABRE, ERE, TCA-element), and light response (AE-box, Box 4, TCT-motif) existed widely in promoter regions of most cucumber TPS genes. These elements consistently appeared in potato TPS genes (Xu et al., 2017), indicating that TPS genes may be involved in the stress, hormone, and light responses. The overexpression of TPS genes in Arabidopsis, rice, and potato improved their stress tolerance (Avonce et al., 2004; Li et al., 2011; Kondrák et al., 2011). Our study showed that CsTPS3 was significantly induced by drought stress, which was consistent with a study in Arabidopsis (Avonce et al., 2004). CsTPS3 and CsTPS4 showed strong expression under salt stress, which was in agreement with the results of OsTPS1 (Li et al., 2011). CsTPS1 and CsTPS5 showed strong expression under oxidative stress. CsTPS3 and CsTPS7 were clearly induced by osmotic stress by mannitol, which coincides with the results from cotton and watermelon (Mu et al., 2016). CsTPS3 was induced significantly by drought, salt and osmotic stresses, indicating that CsTPS3 may be more sensitive to various abiotic stress than other CsTPS genes. Plant hormones play an important role in signal transduction, plant growth, and development. We found that CsTPS3 and CsTPS4 were up-regulated by IAA, while other CsTPS genes were down-regulated by IAA, which were similar to the results for potato (Xu et al., 2017). GO analysis indicated that CsTPS proteins may participate in the response to stress, which further supported our results.
Studies have shown that AtTPS1 participated in embryonic development and vegetative growth through the ABA mechanism and sugar metabolism (Gómez et al., 2010). AtTPS1 played an essential role in regulating sugar signaling (Avonce et al., 2004) and Arabidopsis TPSs could be repressed or induced by sugar. We found that CsTPS3 and CsTPS4 were significantly induced by sucrose, while CsTPS2, CsTPS5 and CsTPS6 were repressed by sucrose. CsTPS3 was highly sensitive to abiotic stress (except oxidative stress), hormone, and sucrose treatments. In contrast, abiotic stress, hormone, and sucrose treatments caused a large decline in the expression of CsTPS6. The expression analysis of CsTPS genes in various tissues showed that CsTPS genes were expressed in the root, stem, and leaf and had the highest expression levels in the root. The expression of most TPS genes in cotton was induced by low temperature, salt and drought, while their expression patterns were different (Mu et al., 2016). In winter wheat, TaTPS1 and TaTPS3 expression was up-regulated under a freeze treatment (−20 °C) (Xie et al., 2015). In potato, the expression and patterns of StTPS genes were regulated by different stresses (salt, heat and osmotic) and hormones (IAA, ABA and GA3) (Xu et al., 2017). In maize, the expression of ZmTPS genes was induced under salt and low temperature stresses (Jiang et al., 2010). In tomato, the expression of SlTPS1 was inhibited by red and blue light (Chen & Lou, 2017). These results indicate that TPS genes may play critical roles in responding to stress, hormones, and light.
Trehalose may be involved in plant stress resistance including salt, cold, drought, and heavy metal. TPS is a key enzyme of trehalose metabolism with an essential role in plant stress resistance. Related studies have shown that the overexpression of TPS genes improved the tolerance of plants under unfavorable conditions (Li et al., 2011; Kondrák et al., 2011; Liu et al., 2019b). Only one or two genes classified as Class I encoded active trehalose-6-phosphate synthase (TPS) in most species. In rice, only proteins encoded by OsTPS1 had TPS activity and all TPS proteins had no TPP activity (Zang et al., 2011). In Arabidopsis, only AtTPS1 (encoded by Class I genes) had TPS activity but no TPP activity, while TPS proteins encoded by Class II genes and remaining Class I genes had neither TPS nor TPP activity (Yang et al., 2012). In maize, ZmTPS1 possessed TPS activity (Jiang et al., 2010). We believe that CsTPS2 and CsTPS4 have TPS activity, but this requires further experimental support. Related studies have indicated that the accumulation of harmful mutations and changes in protein conformation may lead to the loss of TPS activity (Yang et al., 2012; Vandesteene et al., 2010). Previous studies have shown that the trehalose pathway was essential in regulating the use and distribution of sucrose, coordinating source-sink relation, the effective utilization of carbohydrates (Schluepmann et al., 2003), and improving crop yield (Paul, Watson & Griffiths, 2020). Studies in cucumber fruit showed that there was a strong correlation between T6P and sucrose (Zhang et al., 2015). The role of the trehalose pathway in reproductive growth and sugar metabolism should be the focus of future research.
Conclusion
We identified seven TPS genes in the cucumber genome and analyzed their physicochemical properties, gene structures, domains, conserved motifs, evolutionary relationships, gene locations, cis-elements, GO analysis and expression patterns. Our results demonstrated that cucumber TPS genes play an important role in the response to stresses, sucrose, and phytohormones. Our study provides reference information for future studies of the mechanisms of TPS proteins on the growth, development, stress-resistance and trehalose pathway in cucumber.
Supplemental Information
Funding Statement
This work was supported by the National Key Research and Development Program (2018YFD1000800); the National Natural Science Foundation of China (Nos. 31860568, 31560563 and 31160398); the Research Fund of Higher Education of Gansu, China (No. 2018C-14); the Post-Doctoral Foundation of China (Nos. 20100470887 and 2012T50828) and the Natural Science Foundation of Gansu Province, China (Nos. 1606RJZA073 and 1606RJZA077). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Yuanyuan Dan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yuan Niu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Chunlei Wang analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Mei Yan performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Weibiao Liao conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.
References
- Abdallah, Abdelgawad & El-bassiouny (2016).Abdallah MM, Abdelgawad ZA, El-bassiouny HMS. Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. South African Journal of Botany. 2016;103(3–4):275–282. doi: 10.1016/j.sajb.2015.09.019. [DOI] [Google Scholar]
- Abdallah et al. (2020).Abdallah MM, El Sebai TN, Ramadan AAE, El-Bassiouny HMS. Physiological and biochemical role of proline, trehalose, and compost on enhancing salinity tolerance of quinoa plant. Bulletin of the National Research Centre. 2020;44(1):96. doi: 10.1186/s42269-020-00354-4. [DOI] [Google Scholar]
- Ashraf & Foolad (2007).Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany. 2007;59(2):206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- Avonce et al. (2004).Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G. The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiology. 2004;136(3):3649–3659. doi: 10.1104/pp.104.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baninasab (2010).Baninasab B. Induction of drought tolerance by salicylic acid in seedlings of cucumber (Cucumis sativus L.) Journal of Horticultural Science and Biotechnology. 2010;85(3):191–196. doi: 10.1080/14620316.2010.11512653. [DOI] [Google Scholar]
- Chen et al. (2019).Chen Q, Chen QJ, Sun GQ, Zheng K, Yao ZP, Han YH, Wang LP, Duan YJ, Yu DQ, Qu YY. Genome-wide identification of Cyclophilin gene family in cotton and expression analysis of the fibre development in Gossypium barbadense. International Journal of Molecular Sciences. 2019;20(2):349. doi: 10.3390/ijms20020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen & Lou (2017).Chen ZX, Lou J. Identification and expression of the trehalose-6-phosphate synthase gene family members in tomato exposed to different light spectra. Archives of Biological Sciences. 2017;69(1):93–101. doi: 10.2298/ABS160325082C. [DOI] [Google Scholar]
- Cheng et al. (2018).Cheng C, Wang Y, Chai FM, Li SH, Xin HP. Genome-wide identification and characterization of the 14–3-3 family in Vitis vinifera L. during berry development and cold- and heat-stress response. BMC Genomics. 2018;19(1):579. doi: 10.1186/s12864-018-4955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinlein et al. (2014).Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI. Plant salt-tolerance mechanisms. Trends in Plant Science. 2014;19(6):371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du et al. (2017).Du LS, Qi SY, Ma JJ, Xing LB, Fan S, Zhang SW, Li YM, Zhang D, Han MY. Identification of TPS family members in apple (Malus x domestica Borkh.) and the effect of sucrose sprays on TPS expression and floral induction. Plant Physiology and Biochemistry. 2017;120:10–23. doi: 10.1016/j.plaphy.2017.09.015. [DOI] [PubMed] [Google Scholar]
- El-Bashiti et al. (2005).El-Bashiti T, Hamamci H, Oktem HA, Yucel M. Biochemical analysis of trehalose and its metabolizing enzymes in wheat under abiotic stress conditions. Plant Science. 2005;169(1):47–54. doi: 10.1016/j.plantsci.2005.02.024. [DOI] [Google Scholar]
- Elbein et al. (2003).Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13(4):17–27. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- Fichtner et al. (2020).Fichtner F, Olas JJ, Feil R, Watanabe M, Krause U, Hoefgen R, Stitt M, Lunn JE. Functional features of TREHALOSE-6-PHOSPHATE SYNTHASE1, an essential enzyme in Arabidopsis. The Plant Cell. 2020;32(6):1949–1972. doi: 10.1105/tpc.19.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geonhee et al. (2019).Geonhee H, Sara K, Jae-Yong C, Inyup P, Jeong K, Eunkyoo O. Trehalose-6-phosphate signaling regulates thermoresponsive hypocotyl growth in Arabidopsis thaliana. EMBO reports. 2019;20(10):e47828. doi: 10.15252/embr.201947828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddijn & Van Dun (1999).Goddijn OJ, Van Dun K. Trehalose metabolism in plants. Trends in Plant Science. 1999;4(8):315–319. doi: 10.1016/S1360-1385(99)01446-6. [DOI] [PubMed] [Google Scholar]
- Griffiths et al. (2016).Griffiths CA, Sagar R, Geng YQ, Primavesi LF, Patel MK, Passarelli MK, Gilmore IS, Steven RT, Bunch J, Paul MJ, Davis BG. Chemical intervention in plant sugar signalling increases yield and resilience. Nature. 2016;540(7634):574–578. doi: 10.1038/nature20591. [DOI] [PubMed] [Google Scholar]
- Gómez et al. (2010).Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA. AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. The Plant Journal. 2010;64:1–13. doi: 10.1111/j.1365-313X.2010.04312.x. [DOI] [PubMed] [Google Scholar]
- Hadiarto & Tran (2011).Hadiarto T, Tran LS. Progress studies of drought-responsive genes in rice. Plant Cell Reports. 2011;30(3):297–310. doi: 10.1007/s00299-010-0956-z. [DOI] [PubMed] [Google Scholar]
- Han et al. (2020).Han LM, Hua WP, Cao XY, Yan JA, Chen C, Wang ZZ. Genome-wide identification and expression analysis of the superoxide dismutase (SOD) gene family in Salvia miltiorrhiza. Gene. 2020;144603(2):144603. doi: 10.1016/j.gene.2020.144603. [DOI] [PubMed] [Google Scholar]
- Hightower (1991).Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2020).Hu X, Wu ZD, Luo ZY, Burner DM, Pan YB, Wu CW. Genome-wide analysis of the trehalose-6-phosphate synthase (TPS) gene family and expression profiling of ScTPS genes in sugarcane. Agronomy. 2020;10(7):969. doi: 10.3390/agronomy10070969. [DOI] [Google Scholar]
- Jiang et al. (2010).Jiang W, Fu FL, Zhang SZ, Wu L, Li WC. Cloning and characterization of functional trehalose-6-phosphate synthase gene in Maize. Journal of Plant Biology. 2010;53(2):134–141. doi: 10.1007/s12374-010-9098-7. [DOI] [Google Scholar]
- Kim (2012).Kim TH. Plant stress surveillance monitored by ABA and disease signaling interactions. Molecules and Cells. 2012;33(1):1–7. doi: 10.1007/s10059-012-2299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrák et al. (2011).Kondrák M, Marincs F, Kalapos B, Juhász Z, Bánfalvi Z. Transcriptome analysis of potato leaves expressing the trehalose-6-phosphate synthase 1 gene of yeast. PLOS ONE. 2011;6(8):e23466. doi: 10.1371/journal.pone.0023466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019a).Li HY, Xu GZ, Yang C, Yang L, Liang ZH. Genome-wide identification and expression analysis of HKT transcription factor under salt stress in nine plant species. Ecotoxicology and Environmental Safety. 2019a;171:435–442. doi: 10.1016/j.ecoenv.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Li et al. (2011).Li HW, Zang BS, Deng XW, Wang XP. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta. 2011;234(5):1007–1018. doi: 10.1007/s00425-011-1458-0. [DOI] [PubMed] [Google Scholar]
- Li et al. (2019b).Li JM, Zhang MH, Sun J, Mao XR, Wang J, Wang JG, Liu HL, Zheng HL, Zhen Z, Zhao HW, Zou DT. Genome-wide characterization and identification of trihelix transcription factor and expression profiling in response to abiotic stresses in rice (Oryza sativa L.) International Journal of Molecular Sciences. 2019b;20(2):251. doi: 10.3390/ijms20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2019a).Liu MY, Fu QK, Ma ZT, Sun WJ, Huang L, Wu Q, Tang ZZ, Bu TL. Genome-wide investigation of the MADS gene family and dehulling genes in tartary buckwheat (Fagopyrum tataricum) Planta. 2019a;249(5):1301–1318. doi: 10.1007/s00425-019-03089-3. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2019b).Liu X, Fu LS, Qin P, Sun YL, Liu J, Wang X. Overexpression of the wheat trehalose 6-phosphate synthase 11 gene enhances cold tolerance in Arabidopsis thaliana. Gene. 2019b;710:210–217. doi: 10.1016/j.gene.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2019c).Liu Y, Wen HS, Qi X, Zhang XY, Zhang KQ, Fan HY, Tian Y, Hu YB, Li Y. Genome-wide identification of the Na+/H+ exchanger gene family in Lateolabrax maculatus and its involvement in salinity regulation. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2019c;29(1–6):286–298. doi: 10.1016/j.cbd.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Lunn (2007).Lunn JE. Gene families and evolution of trehalose metabolism in plants. Functional Plant Biology. 2007;34(6):550–563. doi: 10.1071/FP06315. [DOI] [PubMed] [Google Scholar]
- Lunn et al. (2014).Lunn JE, Delorge I, Figueroa CM, Dijck PV, Stitt M. Trehalose metabolism in plants. Plant Journal. 2014;79(4):544–567. doi: 10.1111/tpj.12509. [DOI] [PubMed] [Google Scholar]
- Miao et al. (2020).Miao Y, Luo X, Gao X, Wang W, Li B, Hou L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Scientia Horticulturae. 2020;272:109577. doi: 10.1016/j.scienta.2020.109577. [DOI] [Google Scholar]
- Mu et al. (2016).Mu M, Lu XK, Wang JJ, Wang DL, Yin ZJ, Wang S, Fan WL, Ye WW. Genome-wide identification and analysis of the stress-resistance function of the TPS (Trehalose-6-Phosphate Synthase) gene family in cotton. BMC Genetics. 2016;17(1):54. doi: 10.1186/s12863-016-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu et al. (2019).Niu LJ, Yu JH, Liao WB, Xie JM, Yu J, Xiao XM, Hu LL, Wu Y. Proteomic investigation of S-nitrosylated proteins during NO-induced adventitious rooting of cucumber. International Journal of Molecular Sciences. 2019;20(21):5363. doi: 10.3390/ijms20215363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio et al. (2015).Nuccio ML, Wu J, Mowers R, Zhou HP, Meghji M, Primavesi LF, Paul MJ, Chen X, Gao Y, Haque E, Basu SS, Lagrimini LM. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nature Biotechnology. 2015;33(8):862–869. doi: 10.1038/nbt.3277. [DOI] [PubMed] [Google Scholar]
- Ou et al. (2018).Ou WJ, Mao X, Huang C, Tie WW, Yan Y, Ding ZH, Wu CL, Xia ZQ, Wang WQ, Zhou SY, Li KM, Hu W. Genome-wide identification and expression analysis of the KUP family under abiotic stress in Cassava (Manihot esculenta Crantz) Frontiers in Physiology. 2018;9:17. doi: 10.3389/fphys.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, Watson & Griffiths (2020).Paul MJ, Watson A, Griffiths CA. Trehalose 6-phosphate signalling and impact on crop yield. Biochemical Society Transactions. 2020;48(5):2127–2137. doi: 10.1042/BST20200286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann et al. (2003).Schluepmann H, Pellny T, Van Dijken A, Smeekens S, Paul M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6849–6854. doi: 10.1073/pnas.1132018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens (2017).Smeekens S. Drought resistance: spraying for yield. Nature Plants. 2017;3(3):17–23. doi: 10.1038/nplants.2017.23. [DOI] [PubMed] [Google Scholar]
- Song et al. (2019).Song ZP, Pan FL, Lou XP, Wang DB, Yang C, Zhang BQ, Zhang HY. Genome-wide identification and characterization of Hsp70 gene family in Nicotiana tabacum. Molecular Biology Reports. 2019;46(2):1941–1954. doi: 10.1007/s11033-019-04644-7. [DOI] [PubMed] [Google Scholar]
- Vandesteene et al. (2010).Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F. A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Molecular Plant. 2010;3(2):406–419. doi: 10.1093/mp/ssp114. [DOI] [PubMed] [Google Scholar]
- Wahl et al. (2013).Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmidet M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science. 2013;339(6120):704–707. doi: 10.1126/science.1230406. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang K, Li FJ, Gao ML, Huang YC, Song ZG. Mechanisms of trehalose-mediated mitigation of Cd toxicity in rice seedlings. Journal of Cleaner Production. 2020;267(2020):121982. doi: 10.1016/j.jclepro.2020.121982. [DOI] [Google Scholar]
- Wei et al. (2016).Wei BQ, Wang LL, Zhang R, Chen LZ, Zhang SL, Zhang JN. Identification of CaTPS gene family and expression analysis of CaTPS1 in hot pepper. Acta Horticulturae Sinica. 2016;43(8):1504–1512. doi: 10.16420/j.issn.0513-353x.2016-0207. [DOI] [Google Scholar]
- Xie et al. (2018).Xie T, Chen CJ, Li CH, Liu JR, Liu CY, He YH. Genome-wide investigation of WRKY gene family in pineapple: evolution and expression profiles during development and stress. BMC Genomics. 2018;19(1):490. doi: 10.1186/s12864-018-4880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2015).Xie DW, Wang XN, Fu LS, Sun J, Zheng W, Li ZF. Identification of the trehalose-6-phosphate synthase gene family in winter wheat and expression analysis under conditions of freezing stress. Journal of Genetics. 2015;94(1):55–65. doi: 10.1007/s12041-015-0495-z. [DOI] [PubMed] [Google Scholar]
- Xie, Wang & Huang (2014).Xie L, Wang ZX, Huang B. Genome-wide identification classification and expression of TPS family genes in soybean. Chinese Journal of Oil Crop Sciences. 2014;36(2):160–167. doi: 10.7505/j.issn.1007-9084.2014.02.004. [DOI] [Google Scholar]
- Xu et al. (2012).Xu GX, Guo C, Shan HY, Kong HZ. Divergence of duplicate genes in exon-intron structure. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(4):1187–1192. doi: 10.1073/pnas.1109047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu YC, Wang YJ, Mattson N, Yang L, Jin QJ. Genome-wide analysis of the Solanum tuberosum (potato) trehalose-6-phosphate synthase (TPS) gene family: evolution and differential expression during development and stress. BMC Genomics. 2017;18(1):926. doi: 10.1186/s12864-017-4298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2016).Xu JN, Xing SS, Cui HR, Chen XS, Wang XY. Genome-wide identification and characterization of the apple (Malus domestica) HECT ubiquitin-protein ligase family and expression analysis of their responsiveness to abiotic stresses. Molecular Genetics and Genomics. 2016;291(2):635–646. doi: 10.1007/s00438-015-1129-0. [DOI] [PubMed] [Google Scholar]
- Yadav et al. (2014).Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, Lunn JE. The sucrose–trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. Journal of Experimental Botany. 2014;65(4):1051–1068. doi: 10.1093/jxb/ert457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Li & Zhao (2019).Yan F, Li HZ, Zhao P. Genome-wide identification and transcriptional expression of the PAL gene family in common Walnut (Juglans Regia L.) Genes. 2019;10(1):46. doi: 10.3390/genes10010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2012).Yang HL, Liu YJ, Wang CL, Zeng QY. Molecular evolution of trehalose-6-phosphate synthase (TPS) gene family in Populus, Arabidopsis and rice. PLOS ONE. 2012;7(8):e42438. doi: 10.1371/journal.pone.0042438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue et al. (2019).Yue H, Chang X, Zhi YQ, Wang L, Xing GW, Song WN, Nie XJ. Evolution and identification of the WRKY gene family in Quinoa (Chenopodium quinoa) Genes. 2019;10(2):131. doi: 10.3390/genes10020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang et al. (2011).Zang BS, Li HW, Li WJ, Deng XW, Wang XP. Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Molecular Biology. 2011;76(6):507–522. doi: 10.1007/s11103-011-9781-1. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang ZP, Deng Y, Song X, Miao M. Trehalose-6-phosphate and SNF1-related protein kinase 1 are involved in the first-fruit inhibition of cucumber. Journal of Plant Physiology. 2015;177:110–120. doi: 10.1016/j.jplph.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang W, Wang SY, Yu FW, Tang J, Yu L, Wang H, Li JB. Genome-wide identification and expression profiling of sugar transporter protein (STP) family genes in cabbage (Brassica oleracea var. capitata L.) reveals their involvement in clubroot disease responses. Genes. 2019;10(1):71. doi: 10.3390/genes10010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang LS, Wu B, Zhao DG, Li CL, Shao FJ, Lu SF. Genome-wide analysis and molecular dissection of the SPL gene family in Salvia miltiorrhiza [J] Journal of Integrative Plant Biology. 2014;56(1):38–50. doi: 10.1111/jipb.12111. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2018).Zhao P, Wang DD, Wang RQ, Kong NN, Zhang C, Yang CH, Wu WT, Ma HL, Chen Q. Genome-wide analysis of the potato Hsp20 gene family: identification, genomic organization and expression profiles in response to heat stress. BMC Genomics. 2018;19(1):61. doi: 10.1186/s12864-018-4443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2017).Zhou Y, Hu LF, Wu H, Jiang LW, Liu SQ, Pesole G. Genome-wide identification and transcriptional expression analysis of cucumber superoxide dismutase (SOD) family in response to various abiotic stresses. International Journal of Genomics. 2017;2017:1–14. doi: 10.1155/2017/7243973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu (2016).Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167(2):313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2019).Zhu YX, Yang L, Liu N, Yang J, Zhou XK, Xia YC, He Y, He YQ, Gong HJ, Ma DF, Yin JL. Genome-wide identification, structure characterization, and expression pattern profiling of aquaporin gene family in cucumber. BMC Plant Biology. 2019;19(1):345. doi: 10.1186/s12870-019-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental File.