Abstract
Background
Poor retention of participants in randomised trials can lead to missing outcome data which can introduce bias and reduce study power, affecting the generalisability, validity and reliability of results. Many strategies are used to improve retention but few have been formally evaluated.
Objectives
To quantify the effect of strategies to improve retention of participants in randomised trials and to investigate if the effect varied by trial setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Scopus, PsycINFO, CINAHL, Web of Science Core Collection (SCI‐expanded, SSCI, CPSI‐S, CPCI‐SSH and ESCI) either directly with a specified search strategy or indirectly through the ORRCA database. We also searched the SWAT repository to identify ongoing or recently completed retention trials. We did our most recent searches in January 2020.
Selection criteria
We included eligible randomised or quasi‐randomised trials of evaluations of strategies to increase retention that were embedded in 'host' randomised trials from all disease areas and healthcare settings. We excluded studies aiming to increase treatment compliance.
Data collection and analysis
We extracted data on: the retention strategy being evaluated; location of study; host trial setting; method of randomisation; numbers and proportions in each intervention and comparator group. We used a risk difference (RD) and 95% confidence interval (CI) to estimate the effectiveness of the strategies to improve retention. We assessed heterogeneity between trials. We applied GRADE to determine the certainty of the evidence within each comparison.
Main results
We identified 70 eligible papers that reported data from 81 retention trials. We included 69 studies with more than 100,000 participants in the final meta‐analyses, of which 67 studies evaluated interventions aimed at trial participants and two evaluated interventions aimed at trial staff involved in retention. All studies were in health care and most aimed to improve postal questionnaire response. Interventions were categorised into broad comparison groups: Data collection; Participants; Sites and site staff; Central study management; and Study design.
These intervention groups consisted of 52 comparisons, none of which were supported by high‐certainty evidence as determined by GRADE assessment. There were four comparisons presenting moderate‐certainty evidence, three supporting retention (self‐sampling kits, monetary reward together with reminder or prenotification and giving a pen at recruitment) and one reducing retention (inclusion of a diary with usual follow‐up compared to usual follow‐up alone). Of the remaining studies, 20 presented GRADE low‐certainty evidence and 28 presented very low‐certainty evidence.
Our findings do provide a priority list for future replication studies, especially with regard to comparisons that currently rely on a single study.
Authors' conclusions
Most of the interventions we identified aimed to improve retention in the form of postal questionnaire response. There were few evaluations of ways to improve participants returning to trial sites for trial follow‐up. None of the comparisons are supported by high‐certainty evidence. Comparisons in the review where the evidence certainty could be improved with the addition of well‐done studies should be the focus for future evaluations.
Plain language summary
Strategies that might help to encourage people to continue to participate in a randomised trial (a type of scientific study)
Why is this review important?
Randomised trials are a type of scientific study typically used to test new healthcare treatments. In a randomised trial, people who agree to take part are randomly (by chance) put into one of two or more treatment groups and then studied for a period of time. The research team try to keep in touch with them to collect information about how they are doing. This 'follow up' can last from days to years depending on the trial, but the longer the trial lasts, the more difficult it can be. This might be because people are too busy to reply, are unable to come to a clinic, or just do not want to participate any longer. Keeping people in a trial is called 'retention'. If retention is poor, it can make the trial results less certain but most trials do not get data from all the people who started out in the trial.
The information gathered during follow‐up, sometimes called data, helps the trial team to determine which of the treatments being tested works the best. Often this information is collected directly from patients by asking them to complete a questionnaire or by asking them to come back for a clinic visit.
There are many ways to collect data from people in trials. These include using letters, the internet, telephone calls, text messaging, face‐to‐face meetings or the return of medical test kits. Research teams use different methods to try to collect data and it's important to know which strategies are effective and worthwhile, which is why we did this review to compare the success of different strategies.
How did we identify and evaluate the evidence?
We searched scientific databases for studies that compared strategies that research teams use to improve trial retention against each other or against not using such a strategy.We looked for studies that included participants from any age, gender, ethnic, language or geographic group. We then compared the results of the studies, and summarised the evidence that we had found. Finally, we rated our confidence in this evidence, based on factors such as the methods used in the studies and their size, and the consistency of findings across studies.
What did we find?
We identified 70 relevant articles, which reported 81 retention studies involving more than 100,000 participants, that had investigated different ways of trying to encourage randomised trial participants to provide data and stay in the trial. We organised these into broad comparison groups but, unfortunately, we are not able to say with confidence that any of the results we found is a true effect and not caused by other factors, such as flaws with the design of the studies. As such, the effect of ways to encourage people to stay involved in trials is still not clear and more research is needed to see if these retention methods really do work.
How certain is the evidence and how up‐to‐date is this review?
The strategies we identified were tested in randomised trials run in many different disease areas and settings but, in some cases, were tested in only one trial. None of the comparisons we made provided high quality evidence and more studies are needed to help provide more confidence for the results we did find. The evidence in this Cochrane Review is current to January 2020.
Summary of findings
Background
Randomised trials are considered the gold standard for evaluating the effectiveness and efficacy of interventions. Poor retention (or high attrition) in randomised trials has serious consequences for the validity, reliability and usability of their results. Missing data, resulting from poor retention, are of particular concern if the data that are not missing are not at random. In other words, if there is a difference in the amount of missing data between the trial arms or amongst people who are more unwell. However, even if data are missing at random, this is also a potential problem because it will weaken the power of the trial and mean that more participants are needed to achieve a satisfactory sample size. It has been proposed that loss of less than 5% is not problematic but that more than 20% is a serious threat to validity, with anything in between also requiring attention (Fewtrell 2008; Schulz 2002). Recent work suggests that up to 50% of all trials have loss to follow‐up of more than 11% (Walters 2016).
Missing data from loss to follow‐up can be dealt with statistically by various methods including, for example, imputing values based on assumptions about the missing data to give a conservative estimate of the treatment effect (methods such as maximum likelihood estimation routines or multiple imputation). However, the risk of bias still remains when trials do not collect adequate data to give accurate estimates (Hollis 1999). Loss to follow‐up from randomised trials can sometimes go unreported and using different, but plausible, assumptions about outcomes for participants lost to follow‐up can change the results of randomised trials (Walsh 2014). However, rather than adjusting for missingness in the analysis of a trial, and inflating the sample size during recruitment, it seems much more sensible to mitigate the problem of poor retention by designing and evaluating approaches and strategies to maximise data collection. Not knowing how best to retain people in trials means trials will take longer (and cost more) and may expose additional patients to unnecessary risk or forgo the opportunity for others to receive effective treatments. Evidence for effective retention strategies would enable trial teams to include strategies in their trial which are likely to maximise trial design, efficiency and reduce research waste.
This is a substantially revised update of the first full version of this Cochrane Methodology Review (Brueton 2013). The scope of this review is restricted to interventions that are designed to maximise data collection from trial participants once they have been recruited and randomised. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines define non‐retention as instances in which participants are prematurely ’off‐study’ (i.e. consent withdrawn or lost to follow‐up), and therefore outcome data cannot be obtained (Chan 2013). However, participants can still be 'on‐study' but not provide outcome data. Trial non‐retention is distinct from non‐adherence to the trial intervention, which refers to the degree to which the behaviour of trial participants corresponds to the intervention assigned to them. There are Studies Witihin A Trial (SWAT) for this (Bensaaud 2020), but it is not within the scope of this review.
Description of the methods being investigated
Strategies to improve trial retention include those designed to generate maximum data return or compliance and follow‐up procedures that aim to collect data from participants (e.g. weight measurements, blood tests). These strategies can include how outcomes are collected (e.g. postal or telephone); who collects outcomes (e.g. participant‐reported or routine data), when outcomes are collected and also consider, where outcomes are collected (e.g. postal questionnaire or clinic visits).
Objectives
To quantify the effects of strategies for improving retention of participants in randomised trials. A secondary objective is to investigate if the effects vary by trial setting.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised trials of interventions to improve retention of participants in randomised trials (hereafter referred to as retention trials).
Strategies to improve retention are designed to have an impact after participants are randomised to one of the intervention groups of the host and the retention trial, however, they could be delivered at any point (including at the time of recruitment, for example by modifying the information that focuses on retention that is presented to potential participants). Participants in the host trials cover a range of groups and can include (but not be limited to): patients, public, healthcare professionals, etc, and likewise the retention trials might include a range of designs such as individually‐randomised, cluster‐randomised, etc. We excluded trials of strategies that were intended to increase recruitment only, because these are covered by a complementary Cochrane Methodology Review (Treweek 2018). We excluded cohort studies with embedded randomised retention trials, which are the subject of a separate systematic review (Booker 2011).
When referring to embedded trials, we mean randomised trials of retention interventions (e.g. monetary incentives to improve response to postal questionnaires) that are set within a clinical trial (e.g. drug treatment for stroke). Clinical trials that embed retention trials are sometimes referred to as the host trial. Embedded trials are also sometimes referred to as Studies Within A Trial or SWATs. As per guidance by Treweek 2018, ‘a SWAT is a self‐contained research study that has been embedded within a host trial with the aim of evaluating or exploring alternative ways of delivering or organising a particular trial process’.
Types of data
We included retention trials within the context of a host randomised trial with participants from any age, gender, ethnic, language and geographic groups. We included unpublished and published participant retention data from randomised trials addressing health care (including all disciplines and disease areas) and non‐healthcare (education, social sciences) topics. We also included trials set in the community that were healthcare‐related. However, whilst the setting could be non‐health care, the outcomes being measured in the host randomised trial were required to be clinical‐ or health‐related. The retention trials were embedded in real trials (host trials) and not hypothetical trials.
Types of methods
Any intervention that aimed to improve retention of participants to a randomised trial. We considered any strategy aimed at increasing retention, whether it was directed towards the clinician, researcher or participant. The retention trials included at least one randomised comparison of two or more strategies to improve retention, or compared one or more strategies with usual study procedures. We also included trials with any combination of strategies to increase retention. Strategies could include any of the following:
data collection (e.g. shorter length of follow‐up or variation in follow‐up visit frequency);
participant strategies (e.g. monetary incentives, non‐monetary incentives, reminders, behavioural strategies, etc);
sites and site staff (e.g. monitoring approaches);
central study management (e.g. patient and public involvement);
study design (e.g. blinding and treatment preference).
For trials that simultaneously evaluated more than one intervention, unless designed as a factorial trial, or interaction effects were accounted for in the analysis, interventions had to be separated by at least six months to be considered eligible for the review. The reason for this was to account for contamination effects from carry over of previous intervention effects.
Types of outcome measures
Primary outcomes
The proportion of participants retained at the primary analysis point as defined in each individual retention trial is our primary outcome. If the primary outcome was not predefined in a retention trial, we took the first time point reported for analysis. In most cases, this was final response. If retention at a number of time points was reported and no clear time point for the primary outcome for the retention trial was stated, we took data for the nearest time point to the intervention in the retention trial analyses. For studies that reported data captured 'without additional chasing' (i.e. no further standard follow‐up processes such as telephone calls were included before data collection), this was selected as the primary analysis point for data to be included in this review. For studies that delivered an intervention at trial recruitment, we took the total number of participants in the intervention trial as the number who consented as the denominator and the number retained as the numerator. All decisions about primary outcome timing were based on the retention trial publication and discussion within our team; we did not check study protocols or contact authors for clarification.
Secondary outcomes
This update includes no secondary outcomes. This is a change from the previous version of the review (Brueton 2013) which stated "Retention of participants at secondary analysis points" as a secondary outcome. However, because this is rarely reported, we decided to no longer include it as a secondary outcome.
Search methods for identification of studies
We used the Online Resource for Recruitment research in Clinical triAls (ORRCA, www.orrca.org.uk) database to search for studies that had been published up to the end of December 2017. As the scope of this update had changed from that of the original review (Brueton 2013), we re‐ran the full search from database inception rather than only for the period required for the update (which would have been 2013 to 2020). The ORRCA database provides a comprehensive online database of published research (empirical and non‐empirical) about recruitment and/or retention to clinical research. ORRCA is populated from an extensive systematic search of the Cochrane Library, MEDLINE (Ovid), SCOPUS, CINAHL, psycINFO, and SCI‐EXPANDED and SSCI (via ISI Web of Science). The search strategy used to populate ORRCA was based on the original Cochrane Review of strategies to improve trial retention (Brueton 2013), but updated and extended to ensure capture of all relevant studies in this area (see below and Appendices for details). Eligible articles are categorised on the ORRCA database according to research methods and host study characteristics. We searched the ORRCA retention database in April 2020 to identify randomised evaluations of retention strategies that were nested within randomised trials (including factorial, cluster and cross‐over trials), patient preference studies, registries or where the host study type was unknown.
The search strategy used to develop ORRCA aimed to identify published research addressing retention challenges in healthcare and social science settings involving any method of follow‐up. At the time of updating this review, ORRCA only captured studies published until January 2018. Therefore we also ran the search strategy across all platforms described above, to capture studies published between January 2018 and January 2020.
Electronic searches
Each search comprised a filter to identify randomised trials plus free‐text terms and database subject headings relating to reducing loss to follow‐up or increasing retention (Appendix 1). Electronic databases that we searched included the following.
Cochrane Central Register of Controlled Trials (CENTRAL) (to January 2020)
MEDLINE (OVID) (1950 to January 2020) (Appendix 1)
CINAHL (Cumulative Index to Nursing and Allied Health; 1981 to January 2020) (Appendix 1)
PsycINFO (1806 to January 2020) (Appendix 1)
SCOPUS (to January 2020)
Web of Science Core Collection (SCI‐expanded, SSCI, CPSI‐S, CPCI‐SSH and ESCI) (1900 to January 2020)
Searching other resources
We also searched the SWAT repository (SWAT) to identify retention trials that were unpublished or ongoing.
Data collection and analysis
Selection of studies
All review authors were involved in the screening of titles and abstracts retrieved by the searches (in batches of 600) using a predesigned study eligibility screening form. A random 10% of each batch and all potentially eligible titles and abstracts were double screened by one of the review team (KG). We obtained full‐text papers for all potentially eligible studies for inclusion. All review authors were involved in independently assessing full‐text articles to determine if they fulfilled the inclusion criteria, with two review authors allocated to each full‐text article. We contacted study authors for electronic copies of papers that we could not access through library sources. We were able to obtain copies of all the potentially eligible papers, or abstracts, that we wanted to screen. When necessary, we sought information from the original investigators for potentially eligible trials where we wished to clarify eligibility. We resolved disagreements by discussion with a third review author (MAM or KG).
Data extraction and management
All review authors were involved in independently extracting data from included studies using a prespecified data extraction form, with two review authors allocated to each study. A third review author (MAM) checked the extractions for inconsistencies and any discrepancies were resolved by discussion with another review author (KG). Data extracted for the host trial were: design, location, setting, population, intervention, and comparator. For the embedded retention trial, we extracted data on: randomised or quasi‐randomised; design; aim; definition of retention used; retention period; the source of the retention trial sample (e.g. all host participants, participants lost to follow‐up, etc), and participant details. The retention strategy details extracted included: type, theoretically based; description; frequency and timing; mode of delivery; co‐interventions; economic information; resource requirements, numbers and proportions of participants in the intervention and comparator groups of the retention trial.
Assessment of risk of bias in included studies
All included studies (from previous version of review (n = 32) and this update (n = 39)) were assessed independently by two review authors (KG and MAM or ST) for risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2008a), with any disagreements being resolved by a third member of the review team (ST or MAM). Information on risk of bias for all included studies is presented in the Characteristics of included studies table. When assessing studies on 'Blinding of participants and/or personal', we determined that if study authors noted that participants/personnel were not able to be blinded but that they were not given explicit knowledge of the retention trial and/or there was no way staff could use this knowledge to influence the objective outcome of retention, we determined these to be of low risk of bias for this domain. Likewise, when assessing 'Blinding of outcome assessment' we made a judgement as to whether the lack of blinding of outcome assessors would impact on their assessment of the objective outcome of retention. For the majority assessed, we considered studies to be low risk of bias on this domain. If studies were scored as low risk of bis on any one element, or unclear on any one element, this was the corresponding overall risk of bias rating.
We applied GRADE to all comparisons, including when only one study was available for a comparison (Guyatt 2008). For meta‐analyses, GRADE assessment data for the relevant meta‐analyses are provided in the relevant 'Summary of findings' table.
For single studies, we used the rules applied in the Cochrane recruitment review (Treweek 2018), with all studies initially assigned a high GRADE rating of certainty, with the following rules then applied to determine the overall rating.
Study limitations: downgrade all studies at high risk of bias by two levels; downgrade all studies at uncertain risk of bias by one level.
Inconsistency: assume no serious inconsistency.
Indirectness: assume no serious indirectness (all studies provided direct retention data).
Imprecision: downgrade all single studies by one level because of the sparsity of data; downgrade by a further level if the confidence interval is wide and includes a risk difference of zero.
Reporting bias: assume no serious reporting bias.
We provided an informative statement with each GRADE assessment following the guidance in GRADE Guideline 26 (Santesso 2020). This uses both the GRADE assessment and the effect size to produce an informative statement. We used the following rules regarding effect size.
Large effect: 10% or over
Moderate effect: 5% to 9%
Small important effect: 2% to 4%
Trivial, small unimportant effect or no effect: 0% to‐ 1%
We applied the same rules to effect size, regardless of whether the effect was an increase or a decrease in retention.
As per the Cochrane recruitment review (Treweek 2018), we did not exclude studies that were assessed to be at high risk of bias. However, where a high risk of bias study is the only study in a comparison, we do not describe them in the Results or Discussion sections due to the low confidence we have in their findings. We encourage more rigorous evaluations of these interventions but would discourage interpretation about their effects on retention from existing evaluations. The exception to this is if the data from high risk of bias studies could be included in a meta‐analysis alongside data from other studies and where a cumulative judgement on the certainty of the body of evidence using GRADE (as described above) could then be done.
Measures of the effect of the methods
We calculated risk difference (RD) and 95% confidence intervals (CIs) for retention to determine the effect of strategies on this outcome.
Unit of analysis issues
For retention trials that randomised individuals and clusters, the unit of analysis was the participant. For cluster‐randomised trials that ignored clustering in the analysis, we inflated the standard errors (SEs) to avoid over precise estimates of effect as follows (Higgins 2008b).
We calculated the RD, 95% CI and SE based on participants in the usual way (i.e. ignoring clustering).
This SE was then inflated using the design effect to get an adjusted SE: adjusted SE = SE X√ design effect. With the design effect calculated as follows: design effect = 1 + (M ‐ 1) Intra‐cluster coefficient (ICC) where M = mean cluster size, ICC = the intracluster correlation coefficient.
Where published ICCs were not available, we used the mean ICC from appropriate external estimates for Land 2007. This was the mean of estimates for the return of EuroQol questionnaires (ICC = 0.054) from a source recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4) (Higgins 2008b) and www.abdn.ac.uk/hsru/documents/iccs-web.xls (last accessed 24 November 2020).
We entered the effect estimate and the new updated SE into Review Manager 5 using the generic inverse variance (RevMan 2012).
Where the number of participants randomised was not clearly stated in the included study report, we contacted the study authors for this information.
Dealing with missing data
For unpublished studies, we contacted study authors for data for the'R risk of bias' assessment, numbers randomised to each group and numbers retained in each group at the primary endpoint.
Assessment of heterogeneity
We agreed the presence of heterogeneity of the intervention effect where the Chi2 statistic has a significance level of 0.10 (representing a 10% chance of a Type I error). This figure was chosen as it counterbalances the relatively low power of the test. We also used the I² test (Higgins 2003). It represents the total variation across studies and is unlike the Chi2 test in that it is independent from the number of studies. Instead theI² is based on treatment effect. Heterogeneity was also explored through subgroup analyses.
Assessment of reporting biases
We would have assessed reporting bias using tests for funnel plot asymmetry if sufficient data were available (Egger 1997; Sterne 2008).
Data synthesis
We grouped included trials based on the type of intervention under investigation with groups directly informed by the ORRCA retention domains (https://www.orrca.org.uk/Uploads/ORRCA_Retention_Domains.pdf). We added a further domain within the 'Participants' domain to allow separate consideration of prompts and reminders targeting retention. This classification resulted in five broad categories with intervention functions grouped within them.
-
Data collection (Category A), interventions include:
questionnaire design;
data collection frequency/timing;
data collection location and method.
-
Participants (Category B), interventions include:
reminders ‐ intention to be received after a retention time point is reached;
prompts ‐ intention to be received before a retention time point is reached;
monetary incentives and rewards‐ includes both incentives (i.e. not conditional on behaviour) and rewards (i.e. is conditional on behaviour);
non‐monetary incentives;
maintaining participant engagement;
behavioural intervention.
-
Sites and site staff (Category C), interventions include:
prompt;
monitoring visits.
-
Central Study Management (Category D), interventions include:
patient public involvement.
-
Study design (Category E), interventions include:
impact of recruitment;
blinding and treatment preference.
We present the results as RD, pooled using a random‐effects model for all meta‐analyses with more than one included study,and with associated CIs where sufficient data were available. If heterogeneity was detected and could not be explained by subgroup or sensitivity analyses, we did not pool results.
For factorial trials, the data for different categories of interventions were included as separate trial comparisons. For multiple retention trials conducted within the same host trial that were not designed to allow for interaction effects between interventions (i.e. did not stratify at randomisation or account for interaction effects in analysis), we pre‐specified the requirement for interventions to be delivered at least six months apart in order to minimise the potential for any intervention interaction effects. In order to minimise interaction effects, we chose not to include data in the meta‐analyses from trials that had evaluated interventions within six months of each other that had not accounted for the interaction effects in the analysis. This resulted in three studies and data from a further four studies (with multiple evaluations) being omitted from our analyses.
Subgroup analysis and investigation of heterogeneity
We planned to explore the following factors in subgroup analyses assuming enough studies were identified within each comparison.
Type of design used to evaluate the retention strategy (randomised versus quasi‐randomised)
Setting of the host trial (e.g. primary versus secondary care, healthcare setting versus non‐healthcare setting)
Disease area of the host trial (e.g. oncology versus ante‐natal)
Duration of follow‐up (e.g. short versus long term)
Value of monetary incentive (e.g. £5 versus £10 etc)
Sensitivity analysis
To assess the robustness of the results, we planned sensitivity analyses that excluded quasi‐randomised retention trials.
Results
Description of studies
The studies are described in the Characteristics of included studies, Characteristics of studies awaiting classification, Characteristics of ongoing studies, and Characteristics of excluded studies tables. We identified 18,756 abstracts, titles and other records and sought the full text for 150 records to confirm eligibility. In total, 70 papers (reporting data from 81 retention trials) were considered eligible for inclusion (Figure 1). The studies were conducted in eight countries with two multi‐national studies. The majority of studies (n = 53) were conducted in the UK followed by the USA (n = 10) (Table 17). Of these 70 papers, 68 evaluated interventions targeting trial participants and two evaluated interventions targeting individuals involved in trial retention. A total of 101,689 participants were included across the retention trials, which included all participants originally randomised to the retention trial. Included retention trials were conducted in a broad spectrum of clinical conditions across a range of different settings including primary care, secondary care, and community settings. However, similar to the previous version of this review (Brueton 2013), the included studies were predominantly composed of studies evaluating interventions to improve questionnaire return (n = 70) rather than clinic attendance (n = 2).
1.
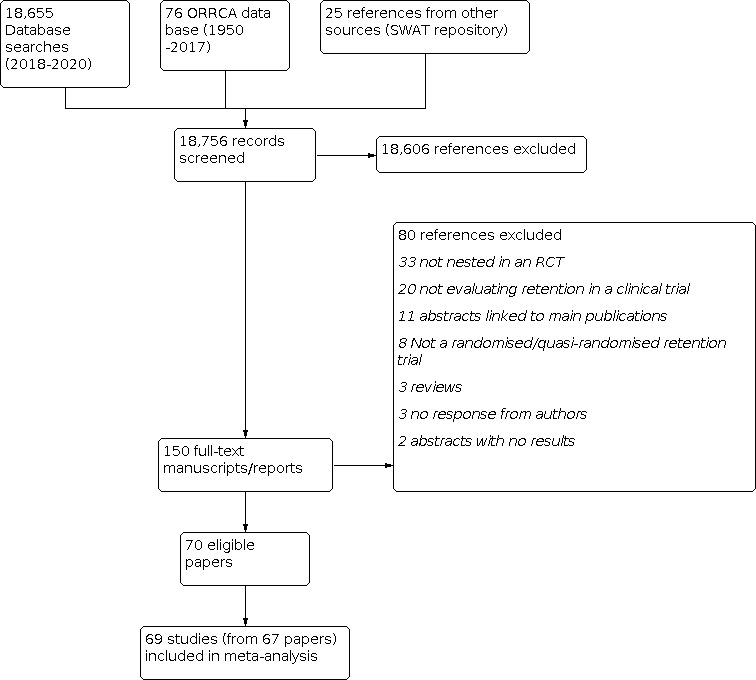
1 Included studies flow diagram.
1. Countries where the included studies took place.
| Country | Number of studies |
| Australia | 1 |
| Canada | 2 |
| Denmark | 1 |
| France | 1 |
| Norway | 1 |
| UK | 53 |
| USA | 10 |
| Multinational | 2 (one involving UK and Ireland and one involving the USA and Canada). |
The majority of the included trials (42 host trials) included a single retention trial. Some of the included studies reported multiple retention trials (i.e. tested more than one intervention) within one publication (non‐factorial) such as Dinglas 2015 (two retention trials), Edwards 2016 (three retention trials), Goulao 2020 (four retention trials), and Keding 2016 (three retention trials). Other retention trials were reported separately but embedded within the same host trial. These included trials by Avenell 2004 and MacLennan 2014 in the RECORD fracture prevention trial; Cockayne 2017 and Rodgers 2019 in the REFORM trial; Khadjesari 2011 and McCambridge 2011 in the Down your Drink Trial; Bailey 2013 in a feasibility study for the Sex unzipped website; McColl 2003 ‐ Trial 1 and McColl 2003 ‐ Trial 2 in the COGENT trial; Mitchell 2011, Mitchell 2012, and Bell 2016 in the SCOOP trial; Cochrane 2020, James 2020 and Whiteside 2019 in the OTIS trial; and Mitchell 2020a and Mitchell 2020b in the KReBS trial.
There was too much variability and not enough depth (i.e. meaningful replication) in the data set to allow us to conduct any of our planned subgroup analyses.
Two studies (Letley 2000 and Sutherland 1996) are awaiting classification. We were unable to include them due to a lack of information on the number of participants randomised to each arm (Letley 2000), or whether the feasibility they report ahead of the full trial was also randomised (Sutherland 1996).
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3. Authors of trials included in the meta‐analysis reported their studies as either randomised (n = 70) or quasi‐randomised (n = 2). One study included both randomised and quasi‐randomised retention trials (Edwards 2016). The overall risk of bias was considered low for 14 studies, unclear for 50 studies and high for eight studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effect of methods
We only report comparisons including studies (single studies or overall comparisons) at low or unclear risk of bias in these results. The list of all 52 comparisons, including those of high risk of bias, is included in Table 18, Table 19, Table 20, Table 21 and Table 22. The categorisation of interventions into categories, based on the ORRCA retention domains, was not always clear and was largely informed by the original study authors' intention as described or implied within their report.
2. Data collection (Category A).
| Sub‐domains | Study ID | Intervention | Control |
| 1A. Questionnaire design: Questionnaire length | |||
| Dorman 1997 | New questionnaire (Shorter version) |
Standard questionnaire | |
| Edwards 2004 | New questionnaire (Shorter version) |
Standard questionnaire | |
| Subar 2001 | New questionnaire (Shorter version) |
Standard questionnaire | |
| 2A. Questionnaire design: Addition of a diary to usual follow‐up | |||
| Griffin 2019 | Diaries follow‐up | Postal questionnaires follow‐up | |
| Marques2013 | Resource use log to prospectively record their use of health services | No resource use log | |
| 3A. Questionnaire design: Question order, condition first vs generic first question | |||
| McColl 2003 | Condition‐specific measures of quality of life preceded generic instruments | Questionnaires in a reverse order | |
| 4A. Data collection frequency and timing: Timing of questionnaire delivery | |||
| Renfroe 2002 | Timing of postal questionnaire, cover letter signatory, express | Regular mail, non‐monetary incentive | |
| 5A. Data Collection Location and Method: Postal follow‐up vs clinic follow‐up | |||
| Greig 2017 | Postal follow‐up | Clinic follow‐up | |
| 6A. Data Collection Location and Method: Telephone follow‐up vs postal questionnaire | |||
| Couper 2007 | Telephone follow‐up | Postal questionnaire | |
| Marsh 1999 (Postal trial) | Postal follow‐up with an incentive | Postal follow‐up without incentive | |
| Marsh 1999 (Telephone trail) | Telephone follow‐up with an incentive | Telephone follow‐up without incentive | |
| 7A. Data Collection Location and Method: First class vs second class outward mailing | |||
| Sharp 2006 | First‐class post | Second class | |
| 8A. Data Collection Location and Method: Return postage | |||
| Sharp 2006 | Preaddressed second class stamped envelope | Business reply envelope | |
| Kenton 2007 | 'high priority' stamp to the mailing | Business format mailing | |
| Dinglas 2015 (Mail trial) | Personalised postal follow‐up | Generic postal follow‐up | |
| 9A. Data Collection Location and Method: Use of self‐sampling kits | |||
| Tranberg 2018 | Received a modified second reminder, a leaflet, and a self‐sampling kit. | received the same material as those in the directly mailed group but received no kit | |
3. Participants (Category B).
| Sub‐domains | Study ID | Intervention | Control |
| 10B. Reminders: electronic reminder vs usual follow‐up | |||
| Ashby 2011 | Additional electronic reminder in follow‐up | Usual follow‐up | |
| Starr 2015 (Email reminder) | Email reminder | Postal email reminder | |
| Starr 2015 (SMS text pre‐notification) | Prenotification reminder | Usual follow‐up | |
| 11B. Reminders: action oriented electronic reminder vs standard electronic reminder | |||
| Edwards 2016 (photo trial) | The personalised photo on the letter | Usual letter | |
| Edwards 2016 (pre‐call trial) | Active reminder | Usual reminder | |
| 12B. Reminders: personalised reminder vs non‐personalised reminder | |||
| Nakash2007 | Calendar | Usual follow‐up | |
| Bradshaw 2020 | Intervention group 1 received an SMS message the day before the email with the link to the questionnaire. | No SMS | |
| 13B. Reminders: telephone reminder vs usual follow‐up | |||
| Severi 2011 | Telephone call reminder | Usual follow‐up | |
| 14B. Reminders: telephone reminder vs postal reminder | |||
| Tai 1997 | Telephone reminder | Postal reminder | |
| 15B. Prompts: electronic prompt vs no prompt | |||
| Bradshaw 2020 | Intervention group 1 received an SMS message and a further £10 high‐street shopping voucher sent by post before the 24 months visit. Intervention group 2 received a further £10 high‐street shopping voucher given at the visit. |
No voucher | |
| Clark 2015 | Received an SMS or e‐mail to return a study questionnaire | Received no electronic prompt to returns a study questionnaire | |
| Keding 2016 | Text message prompt Prompt Reminder |
Usual follow‐up Reminder Usual follow‐up |
|
| Man 2011 | Electronic reminder | No reminder | |
| Starr 2015 (Email reminder) | Email reminder | Postal email reminder | |
| Starr 2015 (SMS text pre‐notification) | Prenotification reminder | Usual follow‐up | |
| 16B. Prompts: telephone prompt vs usual follow‐up | |||
| Edwards 2016 (Email trial) | Addition of an email as prompt | Usual Follow‐up | |
| MacLennan 2014 | Received a telephone call from the trial office ahead of the reminder questionnaire in addition to the usual reminder schedule | Received the usual reminder schedule only | |
| 17B. Prompts: Prenotification card vs no card | |||
| Treweek 2020a | Pre‐notification card sent around 1 month before | No pre‐notification card | |
| 18B. Prompts: sticker vs no sticker | |||
| Goulao 2020 | Received a logo sticker on questionnaire envelopes | Received no sticker | |
| 19B. Prompts: personalised prompt vs no prompt | |||
| Cochrane 2020 | Personalised reminder | Non‐personalised reminder | |
| Mitchell 2020 | Personalised text message | No personalised text message | |
| Nakash 2007 | Calendar | Usual follow‐up | |
| 20B. Monetary incentives: addition of monetary incentives vs usual follow‐up | |||
| Bauer 2004 | Interventions group 1 received an incentive of US$10 Interventions group 2 received an incentive of US$2 |
Received no incentive | |
| Gates 2009 | £5 gift voucher | Received no gift voucher | |
| Kenyon 2005 | Monetary incentive (£5 voucher) | No incentive | |
| 21B. Monetary incentives: addition of monetary incentives to all trial arms | |||
| Bauer 2004 | Interventions group 1 received an incentive of US$10 Interventions group 2 received an incentive of US$2 |
Received no incentive | |
| Bradshaw 2020 | Intervention group 1 received an SMS message and a further £10 high‐street shopping voucher sent by post before the 24‐month visit. Intervention group 2 received a further £10 high‐street shopping voucher given at the visit. |
No voucher | |
| 22B. Monetary incentives: addition of monetary incentives vs addition of monetary reward | |||
| Bradshaw 2020 | Intervention group 1 received an SMS message and a further £10 high‐street shopping voucher sent by post before the 24 months visit. Intervention group 2 received a further £10 high‐street shopping voucher given at the visit. |
No voucher | |
| Cook 2020 | £20 gift voucher given to study at the end of the recruitment visit | A conditional offer of monetary incentive | |
| Dorling 2020 | Received the first paper letter to parents included a promise of an incentive (£15 gift voucher redeemable at some shops) after receipt of a completed form. | Received the first paper letter to parents would enclose the incentive (£15 gift voucher redeemable at high‐street shops) before the receipt of a completed form | |
| Young 2020 | Addition of monetary incentive (£5 multistore voucher) | An offer of incentive (i.e. Conditional vs unconditional £5 multistore voucher) | |
| 23B. Monetary incentives: addition of monetary reward vs usual follow‐up | |||
| Marsh 1999 (Clinic trial) | Clinic visit with an incentive (£2 voucher) | Clinic visit without incentive | |
| Marsh 1999 (Postal trial) | Postal follow‐up with incentive (£2 voucher) | Postal follow‐up without incentive | |
| Marsh 1999 (Telephone trail) | Telephone follow‐up with incentive (£2 voucher) | Telephone follow‐up without incentive | |
| Watson 2017 |
Intervention group 1 received unconditional (£5 gift voucher) at 12 but not 24 months. Intervention group 2 received unconditional (£5 gift voucher) at 12 and 24 months. Intervention group 3 received unconditional (£5 gift voucher) at 24 but not 12 months. |
No voucher | |
| Arundel 2019 | An offer of conditional monetary incentive (£10 cash reliant on providing in addition to the £10 gift voucher routinely provided) | Usual follow‐up (£10 gift voucher routinely provided) | |
| 24B. Monetary incentives: addition of monetary rewards to all trial arms | |||
| Hardy 2016 | An offer of conditional monetary incentive (£10 gift voucher) | Later offer of conditional monetary incentive (£10 gift voucher) | |
| 25B. Monetary incentives: addition of monetary incentives vs lottery | |||
| Kenton 2007 | Monetary incentive (CAN$2 coin mailed with the questionnaire or draw for a CAN$50 gift certificate upon questionnaire receipt) | Lottery | |
| 26B. Monetary incentives: lottery vs usual follow‐up | |||
| No incentive | |||
| 27B. Monetary incentives: addition of lottery to both trial arms | |||
| Henderson 2010 | An offer of winning voucher (winning 1 of 25 £20 shopping vouchers or winning one £500 shopping voucher) | No incentive | |
| 28B. Non‐monetary incentives: addition of pen vs usual follow‐up | |||
| Bell 2016 | Addition of a pen | No pen | |
| Cunningham‐Burley 2020 | Branded pen with their questionnaire | No pen | |
| James 2020 | Pen with trial invitation pack | No pen | |
| Mitchell 2020b | Addition of a pen | No pen | |
| Sharp 2006 | Pen | No pen | |
| 29B. Non‐monetary incentives: addition of societal benefit message vs usual follow‐up | |||
| Severi 2011 | Fridge magnet and benefit to society message | Usual follow‐up | |
| 30B. Non‐monetary incentives: certificate of appreciation vs usual follow‐up | |||
| Renfroe 2002 | Timing of postal questionnaire, cover letter signatory, express mail | Regular mail and non‐monetary incentive. | |
| 31B. Maintaining participant engagement: newsletter vs usual follow‐up | |||
| Goulao 2020 | Received a tested a theoretically informed newsletter sent before the questionnaire | Received no newsletter | |
| MARMOTH trial | Newsletter one month before the 24‐month paper follow‐up questionnaire | No newsletter | |
| Mitchell 2012 | Invitation mailing packs with a white envelope | Invitation mailing packs with a brown envelope | |
| Rodgers 2019 | Newsletter + handwritten posit it notes Newsletter + printed posit it notes Newsletter only Handwritten posit it notes only Printed posit it note only |
Usual follow‐up | |
| 32B. Maintaining participant engagement: offer of receiving trial results vs usual follow‐up | |||
| Cockayne 2005 | Offered the result of the trial in a questionnaire | No offer of knowing the results | |
| 33B. Maintaining participant engagement: cover letter including a social incentive vs standard cover letter | |||
| James 2020 |
Intervention group 1 received a branded pen and a standard cover letter. Intervention group 2 received a branded pen and a social incentive cover letter. Intervention group 3 received no pen and a social incentive cover letter. |
Control group received no pen, standard cover letter. | |
| 34B. Maintaining participant engagement: personalised cover letter vs usual cover letter | |||
| Edwards 2016 (Email trial) | Addition of an email as prompt | Usual follow‐up | |
| Edwards 2016 (photo trial) | Personalised photo on the letter | Usual letter | |
| Edwards 2016 (pre‐call trial) | Active reminder | Usual reminder | |
| 35B. Maintaining participant engagement: varying signatory on cover letter | |||
| Renfroe 2002 | Timing of postal questionnaire, cover letter signatory, express mail | Regular mail and non‐monetary incentive. | |
| 36B. Maintaining participant engagement: addition of a deadline vs usual follow‐up | |||
| Gatellari 2004 | Cover letter advising return within 1‐week | Standard cover letter | |
| 37B. Maintaining participant engagement: addition of an estimate of time to complete vs no addition | |||
| Marson 2007 | Cover letter though post with the questionnaire that included an estimate of the length of time that it may take to complete | Standard cover letter with no indication of length of time required | |
| 38B. Maintaining participant engagement: brown vs white envelope | |||
| Mitchell 2011 | Invitation mailing packs with a white envelope | Invitation mailing packs with a brown envelope | |
| 39B. Maintaining participant engagement: post‐it notes vs usual follow‐up | |||
| Lewis 2017 | Addition of a post‐it note | Usual follow‐up | |
| Rodgers 2019 | Newsletter + handwritten posit it notes Newsletter + printed posit it notes Newsletter only Handwritten posit it notes only Printed posit it note only |
Usual follow‐up | |
| Tilbrook 2015 | Addition of a post‐it note | Usual follow‐up | |
| 40B. Maintaining participant engagement: inclusion of trial newspaper article vs usual follow‐up | |||
| Salvesen 1992 | Newspaper article | Usual follow‐up | |
| 41B. Maintaining participant engagement: frequency of telephone contact | |||
| Glassman 2020 | Received telephone calls at baseline, six months, and at annual visits after that (annual contact) | Received a call at baseline only (baseline contact) | |
| 42B. Maintaining participant engagement: request for collateral (concomitant) | |||
| Cunningham 2004 |
Intervention group1 were asked to provide a collateral. Intervention group2 asked to provide collateral and told that there was a 50% chance that the collateral would be contacted. All those respondents asked for collateral were told that the collateral would receive a CAN$20 payment for a brief telephone interview. |
Not asked to provide a collateral | |
| 43B. Behavioural interventions: theory informed cover letter vs usual cover letter | |||
| AMBER trial | Received a tested a theoretically informed letter sent with the questionnaire | Received a standard letter | |
| Goulao 2020 | Theory informed letter to follow‐up | Usual letter follow‐up | |
| Goulao 2020 (replication) | Theory informed letter to follow‐up | Usual letter follow‐up | |
| OPAL trial | Received a tested a theoretically informed letter sent with the questionnaire | Received a standard letter | |
| 44B. Behavioural interventions: motivational interviewing vs usual follow‐up | |||
| Bean 2018 | Theory informed to follow‐up | Usual follow‐up | |
4. Sites and site staff (Category C).
| Sub‐domains | Study ID | Intervention | Control |
| 45C. Prompts: site prompts for upcoming assessments vs usual follow‐up | |||
| Land 2007 | Received a monthly reminder to sites listing participants who were due to have a measure in the next three months | Received no reminder | |
| 46C. Monitoring visits: on‐site monitoring vs no visit | |||
| Lienard 2006 | Centres received a systematic on‐site visit (Visited group) | Did not receive a systematic on‐site visit (Non‐visited group) | |
5. Central Study Management (Category D).
| Sub‐domains | Study ID | Intervention | Control |
| 47D. Patient Public Involvement: peer‐led follow‐up strategy vs usual follow‐up | |||
| Fouad 2014 | Peer‐led strategy | Usual follow‐up | |
6. Study design (Category E).
| Sub‐domains | Study ID | Intervention | Control |
| 48E. Impact of recruitment: video‐enhanced patient information vs standard information | |||
| Brubaker 2019 | Change to information provided at recruitment | Standard information | |
| 49E. Impact of recruitment: optimised information vs standard information | |||
| Cockayne 2017 | Optimised patient information | Standard patient information | |
| Guarino 2006 | Change to information provided at recruitment | Standard information | |
| 50E. Impact of recruitment: addition of optimised information to both arms | |||
| Cockayne 2017 | Optimised patient information | Standard patient information | |
| 51E. Impact of recruitment: pen vs no pen | |||
| Whiteside 2019 | Non‐monetary incentive | Usual practice (at recruitment) | |
| 52E. Blinding and treatment preference: open vs blind trial design | |||
| Avenell 2004 | Open design | Blinded design | |
'Summary of findings' tables were produced for all interventions where more than one study evaluated effectiveness. This provided 16 in total: Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15 and Table 16.
Summary of findings 1. Questionnaire design: short vs usual questionnaire.
| Short questionnaire compared with long questionnaire for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: short questionnaire Comparison: usual questionnaire | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Short questionnaire | Usual questionnaire | ||||
|
Retention [follow‐up] |
As measured | ||||
| Lowa | RR 1.01 (0.89 to 1.14) | 3252 (3) | ⊕⊝⊝⊝ very low | ||
| 25 per 100 | 25 per 100 (22 to 29 ) | ||||
| Mediuma | |||||
| 50 per 100 | 51 per 100 (45 to 57) | ||||
| Higha | |||||
| 80 per 100 | 81 per 100 (71 to 91) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of a short questionnaire (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 2. Questionnaire design: addition of diary to usual follow‐up vs usual follow‐up.
| Addition of diary to usual follow‐up compared with usual follow‐up for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: diary Comparison: no diary | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Diary | No diary | ||||
|
Retention [follow‐up] |
Lowa | RR 0.97 (0.96 to 0.98) | 9906 (2) | ⊕⊕⊕⊝ moderate | |
| 25 per 100 | 24 per 100 (24 to 25) | ||||
| Mediuma | |||||
| 50 per 100 | 49 per 100 (48 to 49) | ||||
| Higha | |||||
| 80 per 100 | 78 per 100 (77 to 78) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of not including a diary (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| aWe selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 3. Data collection location and method: telephone follow‐up vs postal questionnaire.
| Telephone follow‐up compared with postal questionnaire for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: telephone follow‐up Comparison: postal questionnaire | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Telephone follow‐up | Postal questionnaire | ||||
|
Retention [follow‐up] |
Lowa | RR 1.04 (0.94 to 1.17) | 1006 (2) | ⊕⊝⊝⊝ very low | |
| 25 per 100 | 26 per 100 (24 to 29) | ||||
| Mediuma | |||||
| 50 per 100 | 52 per 100 (47 to 59) | ||||
| Higha | |||||
| 80 per 100 | 83 per 100 (75 to 94) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of telephone follow‐up (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 4. Data collection location and method: return postage.
| Return postage compared with control intervention for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: various return postage strategies Comparison: control intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Various return postage strategies (such as free post versus second class stamp; high priority mail stamp versus usual postage; and personal form) | Standard return postage | ||||
|
Retention [follow‐up] |
Lowa | RR 1.06 (0.99 to 1.15) | 1543 (3) | ⊕⊕⊝⊝ low | |
| 25 per 100 | 27 per 100 (25 to 29) | ||||
| Mediuma | |||||
| 50 per 100 | 53 per 100 (50 to 58) | ||||
| Higha | |||||
| 80 per 100 | 85 per 100 (79 to 92) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 5. Reminders: electronic reminder vs usual follow‐up.
| Electronic reminder compared with usual follow‐up for trial retention | ||||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: electronic reminder Comparison: usual follow‐up | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Electonic reminder | Usual follow‐up | |||||
|
Retention [follow‐up] |
Lowa | RR 1.01 (0.95 to 1.09) | 790 (3) | ⊕⊕⊝⊝ low | ||
| 25 per 100 | 25 per 100 (24 to 27) | |||||
| Mediuma | ||||||
| 50 per 100 | 51 per 100 (48 to 55) | |||||
| Higha | ||||||
| 80 per 100 | 81 per 100 (76 to 87) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of an electronic reminder (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | ||||||
Summary of findings 6. Prompts: Electronic prompt vs no prompt.
| Electronic prompt compared with no prompt for trial retention | ||||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: electronic prompt Comparison: no prompt | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Electronic prompt | No prompt | |||||
|
Retention [follow‐up] |
Lowa | RR 1.03 (0.98 to 1.08) | 2897 (5) | ⊕⊝⊝⊝ very low | ||
| 25 per 100 | 26 per 100 (25 to 27) | |||||
| Mediuma | ||||||
| 50 per 100 | 52 per 100 (49 to 54) | |||||
| Higha | ||||||
| 80 per 100 | 82 per 100 (78 to 86) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of electronic prompts (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | ||||||
Summary of findings 7. Prompts: Telephone prompt vs usual follow‐up.
| Telephone prompt compared with usual follow‐up for trial retention | ||||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: telephone prompt Comparison: usual follow‐up | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Telephone prompt | Usual follow‐up | |||||
|
Retention [follow‐up] |
Lowa | RR 1.02 (0.85 to 1.22) | 943 (2) | ⊕⊝⊝⊝ very low | ||
| 25 per 100 | 26 per 100 (21 to 31) | |||||
| Mediuma | ||||||
| 50 per 100 | 51 per 100 (43 to 61) | |||||
| Higha | ||||||
| 80 per 100 | 82 per 100 (68 to 98) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of telephone prompts (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | ||||||
Summary of findings 8. Prompts: personalised prompt vs usual follow‐up.
| Personalised prompt compared with usual follow‐up for trial retention | ||||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: personalised prompt Comparison: usual follow‐up | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Personalised prompt | Usual follow‐up | |||||
|
Retention [follow‐up] |
Lowa | RR 0.97 (0.89 to 1.07) | 701 (2) | ⊕⊕⊝⊝ low | ||
| 25 per 100 | 24 per 100 (22 to 27) | |||||
| Mediuma | ||||||
| 50 per 100 | 49 per 100 (45 to 54) | |||||
| Higha | ||||||
| 80 per 100 | 78 per 100 (71 to 86) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of personalised prompts (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | ||||||
Summary of findings 9. Monetary incentives: addition of monetary incentives vs usual follow‐up.
| Addition of monetary incentives compared with usual follow‐up for trial retention | ||||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: monetary incentives Comparison: usual follow‐up | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Monetary incentives | Usual follow‐up | |||||
|
Retention [follow‐up] |
Lowa | RR 1.20 (1.06 to 1.36) | 3166 (3) | ⊕⊕⊝⊝ low | ||
| 25 per 100 | 30 per 100 (27 to 34) | |||||
| Mediuma | ||||||
| 50 per 100 | 60 per 100 (53 to 68) | |||||
| Higha | ||||||
| 80 per 100 | 96 per 100 (85 to [109) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of monetary incentives (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | ||||||
Summary of findings 10. Monetary incentives: addition of monetary incentives vs addition of a monetary reward.
| Addition of monetary incentives compared with addition of a monetary reward for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: monetary incentive Comparison: monetary reward | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Monetary incentive | Monetary reward | ||||
|
Retention [follow‐up] |
Lowa | RR 1.00 (0.91 to 1.09) | 3765 (4) | ⊕⊝⊝⊝ very low | |
| 25 per 100 | 25 per 100 (23 to 28) | ||||
| Mediuma | |||||
| 50 per 100 | 50 per 100 (46 to 55) | ||||
| Higha | |||||
| 80 per 100 | 80 per 100 (73 to 87) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 11. Monetary incentives: addition of monetary reward vs usual follow‐up.
| Addition of monetary reward compared with usual follow‐up for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: monetary reward Comparison: usual follow‐up | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Monetary reward | Usual follow‐up | ||||
|
Retention [follow‐up] |
Lowa | RR 1.02 (0.96 to 1.09) | 1159 (3) | ⊕⊝⊝⊝ very low | |
| 25 per 100 | 26 per 100 (24 to 27) | ||||
| Mediuma | |||||
| 50 per 100 | 51 per 100 (48 to 55) | ||||
| Higha | |||||
| 80 per 100 | 82 per 100 (77 to 87) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of monetary reward (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 12. Non‐monetary incentives: addition of pen vs usual follow‐up.
| Pen compared with no pen for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: pen Comparison: no pen | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Pen | No pen | ||||
|
Retention [follow‐up] |
Lowa | RR 1.02 (1.00 to 1.05) | 13013 (5) | ⊕⊕⊝⊝ low | |
| 25 per 100 | 26 per 100 (25 to 26) | ||||
| Mediuma | |||||
| 50 per 100 | 51 per 100 50 to 53) | ||||
| Higha | |||||
| 80 per 100 | 82 per 100 (80 to 84) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 13. Maintaining participant engagement: newsletter vs usual follow‐up.
| Newsletter compared with usual follow‐up for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: newsletter Comparison: usual follow‐up | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Newsletter | Usual follow‐up | ||||
|
Retention [follow‐up] |
Lowa | RR 0.99 (0.95 to 1.04) | 5622 (4) | ⊕⊝⊝⊝ very low | |
| 25 per 100 | 25 per 100 (24 to 26) | ||||
| Mediuma | |||||
| 50 per 100 | 50 per 100 (48 to 52) | ||||
| Higha | |||||
| 80 per 100 | 79 per 100 (76 to 83) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 14. Maintaining participant engagement: post‐it note vs usual follow‐up.
| Post‐it note compared with usual follow‐up for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: post‐it note Comparison: usual follow‐up | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Post‐it note | Usual follow‐up | ||||
|
Retention [follow‐up] |
Lowa | RR 1.00 (0.99 to 1.01) | 4698 (3) | ⊕⊕⊝⊝ low | |
| 25 per 100 | 25 per 100 (25 to 25) | ||||
| Mediuma | |||||
| 50 per 100 | 50 per 100 (50 to 51) | ||||
| Higha | |||||
| 80 per 100 | 80 per 100 (79 to 81) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of a post‐it note (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 15. Behavioural interventions: theory informed cover letter vs usual cover letter.
| Theory informed cover letter compared with usual cover letter for trial retention | |||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: theory informed cover letter Comparison: usual cover letter | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Theory informed cover letter | Usual cover letter | ||||
|
Retention [follow‐up] |
Lowa | RR 1.05 (0.98 to 1.12) | 3343 (4) | ⊕⊝⊝⊝ very low | |
| 25 per 100 | 26 per 100 (25 to 28) | ||||
| Mediuma | |||||
| 50 per 100 | 53 per 100 (49 to 56) | ||||
| Higha | |||||
| 80 per 100 | 84 per 100 (78 to 90) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect of a theory informed cover letter (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | |||||
Summary of findings 16. Impact of recruitment: optimised information vs standard information.
| Addition of optimised information compared with standard information for trial retention | ||||||
|
Patient or population: trial participants being followed up for data collection Settings: any setting Intervention: optimised patient information leaflet (PIL) Comparison: standard PIL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Optimised PIL | Standard PIL | |||||
|
Retention [follow‐up] |
Lowa | RR 0.96 (0.85 to 1.09) | 1285 (2) | ⊕⊝⊝⊝ very low | ||
| 25 per 100 | 24 per 100 (21 to 27) | |||||
| Mediuma | ||||||
| 50 per 100 | 48 per 100 (43 to 55) | |||||
| Higha | ||||||
| 80 per 100 | 77 per 100 (68 to 87) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| a We selected low, medium and high illustrative retention levels of 25%, 50% and 80% based on prior experience with trial retention and evidence from the literature. For example, it has been previously stated that it is common for up to 20% trial participants to drop out before the trial finishes (Walsh 2015), as such we set the upper limit of good retention as 80%. The other extreme of 25% was informed by evidence that some trials (largely internet based) can have retention as low as 10% to 25% (Murray 2009). The mid point of 50% was a judgement made by the review team and was deemed appropriate given the evidence for the other parameters. | ||||||
Data Collection ‐ Category A (Table 18)
Fourteen studies across nine comparisons focused on aspects of data collection to improve retention. The results from studies, or comparisons, that were low or unclear risk of bias are presented below and included 35,215 participants. We have not presented data for comparisons where only a single high risk of bias study was available for any of the compairsons across categories.
Questionnaire design
The evidence is very uncertain about the effect of a short questionnaire compared to the usual trial questionnaire: risk difference (RD) = 0% (95% confidence interval (CI) ‐8% to 8%); GRADE: very low; (Analysis 1.1, Table 1). This result is based on three studies, n = 3252: Edwards 2004 (head injury), Subar 2001 (cancer screening) and Dorman 1997 (stroke).
Addition of a diary to usual follow‐up compared to usual follow‐up alone probably reduces retention slightly: RD = ‐3% (95% CI ‐4% to ‐2%); GRADE: moderate; (Analysis 2.1, Table 2). This result is based on two studies, n =9906: Griffin 2019 (falls prevention) and Marques 2013 (hip/knee replacement).
1.1. Analysis.

Comparison 1: A ‐ Questionnaire Design: Short vs usual questionnaire, Outcome 1: Retention
2.1. Analysis.

Comparison 2: A ‐ Questionnaire Design: Addition of diary to usual follow up vs usual follow‐up, Outcome 1: Retention
Data collection frequency/timing
The evidence is very uncertain about the effect of a final questionnaire sent at trial close out compared to last study visit: Renfroe 2002 (arrhythmia): RD = 7% (95% CI ‐1% to 14%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 479; wide CI crossing RD = 0) (Analysis 4.1).
4.1. Analysis.

Comparison 4: A ‐ Data Collection Frequency and Timing: Timing of questionnaire delivery, Outcome 1: Retention
Data collection location and method
The evidence is very uncertain about the effect of postal follow‐up compared to clinic follow‐up on retention: Greig 2017 (nail‐bed injury): RD = 16% (95% CI ‐8% to 40%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 60; wide CI crossing RD = 0) (Analysis 5.1).
The evidence is very uncertain about the effect of telephone follow‐up compared to postal follow‐up on retention: RD = 2% (95% CI ‐4% to 9%); GRADE: very low; (Analysis 6.1, Table 3). This result is based on two studies, n = 1006: Couper 2007 (obesity) and Marsh 1999 (injury prevention).
First class postage for outward mail compared to second class postage may increase retention slightly: Sharp 2006 (cervical screening): RD = 2% (95% CI ‐4% to 8%); GRADE low (‐1 level: study limitations– unclear risk of bias; ‐1 level: imprecision‐single study, n = 930) (Analysis 7.1)
Various strategies compared to usual practice for return postage, such as free post versus second class stamp; high priority mail stamp versus usual postage; and personal form may increase retention slightly: RD = 4% (95% CI ‐0% to 9%); GRADE: low; (Analysis 8.1, Table 4). This result is based on three studies, n = 1543: Sharp 2006 (cervical screening), Dorman 1997 (stroke) and Dinglas 2015 (acute lung injury)
The use of self‐sampling kits (directly mailed or an invitation to order) probably increases retention: Tranberg 2018 (cervical screening) split across several subgroups: RD = 9% (95% CI 4% to 13%); GRADE moderate (‐1 level: imprecision‐single study, n = 19,582) (Analysis 9.1).
5.1. Analysis.

Comparison 5: A ‐ Data Collection Location and Method: Postal follow‐up vs clinic follow‐up, Outcome 1: Retention
6.1. Analysis.

Comparison 6: A ‐ Data Collection Location and Method: Telephone follow‐up vs postal questionnaire, Outcome 1: Retention
7.1. Analysis.

Comparison 7: A ‐ Data Collection Location and Method: First class vs second class outward mailing, Outcome 1: Retention
8.1. Analysis.

Comparison 8: A ‐ Data Collection Location and Method: Return postage, Outcome 1: Retention
9.1. Analysis.

Comparison 9: A ‐ Data Collection Location and Method: Use of self‐sampling kits, Outcome 1: Retention
Participants ‐ Category B (Table 19)
The domain for interventions focusing on participants contained the largest number of interventions (35 comparisons) and studies (n = 49) and included 57,033 participants are presented below.
Reminders
Electronic reminders compared to usual follow‐up may result in little or no difference to retention RD = 1% (95% CI ‐4% to 6%); GRADE: low; (Analysis 10.1, Table 5). This result is based on three studies, n = 790: Ashby 2011 (migraine), Keding 2016 (depression),and Starr 2015 (ureteric stones).
The evidence is very uncertain about the effect on retention of action oriented electronic reminders (e.g. 'ACTION REQUIRED' in email subject line) compared to a standard electronic reminder: Edwards 2016 (depression with cardiovascular disease): RD = ‐4% (95% CI ‐10% to 3%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 231; wide CI crossing RD = 0) (Analysis 11.1).
The evidence is very uncertain about the effect on retention of a personalised versus a non‐personalised reminder: Nakash 2007 (severe ankle sprains): RD = ‐1% (95% CI ‐11% to 8%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 298; wide CI crossing RD = 0) (Analysis 12.1).
Telephone reminders compared to usual follow‐up may result in little or no difference to retention: Severi 2011 (smoking cessation): RD = ‐1% (95% CI ‐18% to 15%); GRADE low (‐2 levels: imprecision‐single study, n = 127; wide CI crossing RD = 0) (Analysis 13.1).
Telephone reminders compared to postal reminders, may result in a large increase in retention: Tai 1997, (asthma and/or diabetes): RD = ‐19% (95% CI ‐33% to ‐5%); GRADE low (‐1 level: study limitations– unclear risk of bias; ‐1 levels: imprecision‐single study, n = 148) (Analysis 14.1).
10.1. Analysis.

Comparison 10: B ‐ Reminders: Electronic reminder vs usual follow‐up, Outcome 1: Retention
11.1. Analysis.

Comparison 11: B ‐ Reminders: Action oriented electronic reminder vs standard electronic reminder, Outcome 1: Retention
12.1. Analysis.

Comparison 12: B ‐ Reminders: Personalised reminder vs non‐personalised reminder, Outcome 1: Retention
13.1. Analysis.

Comparison 13: B ‐ Reminders: Telephone reminder vs usual follow‐up, Outcome 1: Retention
14.1. Analysis.

Comparison 14: B ‐ Reminders: Telephone reminder vs postal reminder, Outcome 1: Retention
Prompts
The evidence is very uncertain about the effect on retention of electronic prompts compared to no prompt: RD = 2% (95% CI ‐1% to 6%); GRADE: very low; (Analysis 15.1, Table 6). This result is based on five studies, n = 2897: Bradshaw 2020 (eczema), Clark 2015 (chronic obstructive pulmonary disease), Keding 2016 (depression), Man 2011 (low back pain), and Starr 2015 (ureteric stones).
The evidence is very uncertain about the effect on retention of telephone prompts compared to usual follow‐up: RD = 1% (95% CI ‐10% to 12%); GRADE: very low; (Analysis 16.1, Table 7). This result is based on two studies, n = 943: Edwards 2016 (depression with cardiovascular disease) and MacLennan 2014 (fracture prevention).
Prenotification cards compared to no card may increase retention slightly: Treweek 2020a, n = 558 (breast cancer prevention): RD = 3% (95% CI ‐3% to 10%); GRADE low (‐2 levels: imprecision‐single study; wide CI crossing RD = 0) (Analysis 17.1).
Use of a sticker on envelope compared to no sticker may result in little or no difference to retention: Goulao 2020. (dentistry): RD = 1% (95% CI ‐7% to 10%); GRADE low (‐2 levels: imprecision‐single study, n = 517; wide CI crossing RD = 0) (Analysis 18.1).
Personalised prompts compared to usual follow‐up may reduce retention slightly: RD = ‐2% (95% CI ‐9% to 5%); GRADE: low; (Analysis 19.1, Table 8). This result is based on two studies, n = 701: Cochrane 2020 (falls prevention) with low risk of bias and Nakash 2007 (severe ankle sprains) with unclear risk of bias.
Electronic prompts compared to electronic reminders seemed to favour electronic reminders may increase retention slightly: Sarathy 2020 (frozen shoulder): RD 2% (95% CI ‐6% to 9%) GRADE low (‐2 levels: imprecision‐single study, n = 269; wide CI crossing RD = 0) (Analysis 20.1)
15.1. Analysis.

Comparison 15: B ‐ Prompts: Electronic prompt vs no prompt, Outcome 1: Retention
16.1. Analysis.

Comparison 16: B ‐ Prompts: Telephone prompt vs usual follow‐up, Outcome 1: Retention
17.1. Analysis.

Comparison 17: B ‐ Prompts: Prenotification card vs no card, Outcome 1: Retention
18.1. Analysis.

Comparison 18: B ‐ Prompts: Sticker vs no sticker, Outcome 1: Retention
19.1. Analysis.

Comparison 19: B ‐ Prompts: Personalised prompt vs usual follow‐up, Outcome 1: Retention
20.1. Analysis.

Comparison 20: B ‐ Prompts: Electronic prompts vs electronic reminders, Outcome 1: Retention
Monetary incentives and rewards
Monetary incentives compared to no incentive may increase retention: RD = 7% (95% CI 4% to 11%); GRADE: low; (Analysis 21.1, Table 9). This result is based on three studies, n = 3166: Bauer 2004 (smoking cessation), Gates 2009 (acute whiplash) both with high risk of bias, and Kenyon 2005 (neonatal) with unclear risk of bias. A sensitivity analysis was conducted that excluded the quasi‐randomised trial (Gates 2009). This showed a similar effect in that it may increase retention: RD 9%, 95% CI 2% to 16%; but the certainty in the certainty in the evidence is GRADE low (Analysis 21.2)
Addition of monetary incentives to all trial arms may favour the higher value incentive to increase retention: Bauer 2004 (smoking cessation): RD = 10% (95% CI 3% to 23%); GRADE: low (‐2 levels: imprecision‐single study, n = 200 ; wide CI crossing RD = 0) ( Analysis 21.1)
The evidence is very uncertain about the effect on retention of the addition of a monetary incentive (unconditional) versus addition of a monetary reward (conditional): RD = ‐0% (95% CI ‐7% to 6%); GRADE: very low; (Analysis 23.1, Table 10 ). This result is based on four studies, n = 3765: Bradshaw 2020 (eczema), Dorling 2020 (infant feeding), Cook 2020 (influenza), Young 2020 (lung cancer screening) .
The evidence is very uncertain about the effect of the addition of a monetary reward compared to usual follow‐up on retention: RD = 2% (95% CI ‐3% to 6%); GRADE: very low; (Analysis 24.1, Table 11). This result is based on three studies, n = 1159: Marsh 1999 (injury prevention), Watson 2017 (haemorrhoids) and focus on return of postal questionnaires and found no effect: RD = 0% (95% CI ‐6% to 7%). The third study, Arundel 2019 (smoking cessation), shows an effect on attendance at follow‐up visits. A sensitivity analysis excluding the quasi‐randomised trial (Marsh 1999) showed a similar effect on retention with sustained uncertainty in the evidence: RD 1%, 95% CI ‐4% to 6%: GRADE: very low (Analysis 24.2).
Addition of a monetary reward to both trial arms delivered either with the prenotification or with the reminder letter, probably increases retention: Hardy 2016 (labour): RD = 9% (95% CI 3% to 15%); GRADE moderate (‐1 level: imprecision‐single study, n = 1018) (Analysis 25.1).
The evidence is very uncertain about the effect on retention of the addition of a monetary incentive compared to inclusion in a lottery: Kenton 2007, (postnatal depression): RD = 2% (95% CI ‐9% to 12%);GRADE very low (‐1 level: study limitations– unclear risk of bias;‐2 levels: imprecision‐single study, n = 281; wide CI crossing RD = 0) (Analysis 26.1).
The evidence is very uncertain about the effect on retention of inclusion in a lottery compared to usual follow‐up: Henderson 2010 (sexual health): RD = ‐1% (95% CI ‐3% to 2%); GRADE very low (‐1 level: study limitations– unclear risk of bias;‐2 levels: imprecision‐single study, n = 4206; wide CI crossing RD = 0) (Analysis 27.1).
The evidence is very uncertain about the effect on retention of inclusion in a high‐ versus low‐value lottery: Henderson 2010 (sexual health): RD = 2% (95% CI ‐1% to 6%); GRADE very low (‐1 level: study limitations– unclear risk of bias;‐2 levels: imprecision‐single study, n = 2758; wide CI crossing RD = 0) (Analysis 28.1).
21.1. Analysis.

Comparison 21: B ‐ Monetary incentives: Addition of monetary incentives vs usual follow‐up, Outcome 1: Retention
21.2. Analysis.

Comparison 21: B ‐ Monetary incentives: Addition of monetary incentives vs usual follow‐up, Outcome 2: Retention‐ sensitivity anlysis removing quasi‐RCTs
23.1. Analysis.

Comparison 23: B ‐ Monetary incentives: Addition of monetary incentives vs addition of monetary reward, Outcome 1: Retention
24.1. Analysis.

Comparison 24: B‐ Monetary incentives: Addition of monetary reward vs usual follow‐up, Outcome 1: Retention
24.2. Analysis.

Comparison 24: B‐ Monetary incentives: Addition of monetary reward vs usual follow‐up, Outcome 2: Retention ‐ sensitivity analysis removing quasi‐RCTs
25.1. Analysis.

Comparison 25: B ‐ Monetary incentives: Addition of monetary rewards to all trial arms, Outcome 1: Retention
26.1. Analysis.

Comparison 26: B ‐ Monetary incentives: Addition of monetary incentives vs lottery, Outcome 1: Retention
27.1. Analysis.

Comparison 27: B ‐ Monetary incentives: Lottery vs usual follow‐up, Outcome 1: Retention
28.1. Analysis.

Comparison 28: B ‐ Monetary incentives: Addition of lottery to both trial arms, Outcome 1: Retention
Non‐monetary incentives
Addition of a pen compared to no pen may increase retention slightly: RD = 2% (95% CI 0% to 4%); GRADE: low; (Analysis 29.1, Table 12 ). This result is based on five studies, n = 13,013: Mitchell 2020a (knee replacement), James 2020 (falls prevention), Bell 2016 (fracture prevention), Cunningham‐Burley 2020 (falls prevention), and Sharp 2006 (cervical screening).
Inclusion of a societal benefit message compared to usual follow‐up may result in little or no difference to retention: Severi 2011 (smoking cessation): RD = ‐0% (95% CI ‐4% to 4%); GRADE low (‐2 levels: imprecision‐single study, n = 1950; wide CI crossing RD = 0) (Analysis 30.1).
The evidence is very uncertain about the effect on retention of providing a certificate of appreciation compared to no certificate: Renfroe 2002 (arrhythmia): RD = ‐5% (95% CI ‐13% to 3%); GRADE very low (‐1 level: study limitations– unclear risk of bias;‐2 levels: imprecision‐single study, n = 479; wide CI crossing RD = 0) (Analysis 31.1).
29.1. Analysis.

Comparison 29: B ‐ Non‐monetary incentives: Addition of pen vs usual follow‐up, Outcome 1: Retention
30.1. Analysis.

Comparison 30: B ‐ Non‐monetary incentives: Addition of societal benefit message vs usual follow‐up, Outcome 1: Retention
31.1. Analysis.

Comparison 31: B ‐ Non‐monetary incentives: Certificate of appreciation vs usual follow‐up, Outcome 1: Retention
Maintaining participant engagement
The evidence is very uncertain about the effect on retention of including a newsletter compared to no newsletter: RD = ‐0% (95% CI ‐4% to 3%); GRADE: very low; (Analysis 32.1, Table 13). This result is based on four studies, n = 5622: Goulao 2020 (dentistry), Mitchell 2012 (fracture prevention), Rodgers 2019 (fall prevention), and MamMOTH 2020 (chronic pain).
The offer of recieving the results of the resutls of the trial compared to no offer may result in little to no difference to retention based on very uncertian evidence: Cockayne 2005 (fracture prevention): RD = ‐2% (95% CI ‐5% to 2%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 1038; wide CI crossing RD = 0) (Analysis 33.1).
Including a social incentive (e.g. personalised table of questionnaire response to date to evidence previous responses noted and valued) in the cover letter compared to the standard cover letter may result in little or no difference to retention James 2020 (falls prevention): RD = ‐1% (95% CI ‐4% to 2%); GRADE low (‐2 levels: imprecision‐single study, n = 755; wide CI crossing RD=) (Analysis 34.1).
The evidence is very uncertain about the effect on retention of varying the signatory on cover letters: Renfroe 2002 (arrhythmia): RD = 2% (95% CI ‐6% to 10%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 479; wide CI crossing RD = 0) (Analysis 35.1).
The evidence is very uncertain about the effect on retention of including a deadline for completion versus no deadline, Gattellari 2004 (prostate cancer): RD = 4% (95% CI ‐5% to 12%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 246; wide CI crossing RD = 0) (Analysis 36.1).
The evidence is very uncertain about the effect on retention of adding an estimate of time to completion versus no addition: Marson 2007 (epilepsy): RD = 1% (95% CI ‐2% to 4%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 1815; wide CI crossing RD = 0) (Analysis 37.1).
The evidence is very uncertain about the effect on retention of comparing brown to white envelopes: Mitchell 2011 (fracture prevention): RD = 2% (95% CI ‐1% to 5%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 1119; wide CI crossing RD = 0) (Analysis 38.1).
Addition of a post‐it note compared to no post‐it note or alternative post‐it notes may result in little or no difference to retention: RD = 0% (95% CI ‐1% to 1%); GRADE low; (Analysis 39.1, Table 14). This result is based on eight trials from three studies, n = 4698: Rodgers 2019 (fall prevention), Lewis 2017 (depression), and Tilbrook 2015 (neck pain).
Inclusion of a newspaper article about the trial compared to no article may increase retention: Salvesen 1992 (pregnancy): RD = 8% (95% CI 1% to 15%); GRADE low (‐1 level: study limitations– unclear risk of bias; ‐1 level: imprecision‐single study, n = 716) (Analysis 40.1).
Frequency of telephone contact comparing only at baseline to annual contact to contact only at baseline may increase retention: Glassman 2020 (diabetic retinopathy): RD = 8% (95% CI 1% to 15%); GRADE low (‐1 level: study limitations– unclear risk of bias; ‐1 level: imprecision‐single study, n = 305) (Analysis 41.1).
The evidence is very uncertain about the effect on retention of a request for a collateral (i.e. a contact person) compared to no request or request with 50% chance of contact: Cunningham 2004 (alcohol consumption): RD = 7% (95% CI ‐1% to 16%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 408; wide CI crossing RD = 0) (Analysis 42.1).
32.1. Analysis.

Comparison 32: B ‐ Maintaining participant engagement: Newsletter vs usual follow‐up, Outcome 1: Retention
33.1. Analysis.

Comparison 33: B ‐ Maintaining participant engagement: Offer of receiving trial results vs usual follow‐up, Outcome 1: Retention
34.1. Analysis.

Comparison 34: B‐ Maintaining Participant Engagement: Cover letter including a social incentive vs standard cover letter, Outcome 1: Retention
35.1. Analysis.

Comparison 35: B ‐ Maintaining participant engagement: Varying signatory on cover letter, Outcome 1: Retention
36.1. Analysis.

Comparison 36: B ‐ Maintaining participant engagement: Addition of a deadline vs usual follow‐up, Outcome 1: Retention
37.1. Analysis.

Comparison 37: B ‐ Maintaining participant engagement: Addition of an estimate of time to complete vs no addition, Outcome 1: Retention
38.1. Analysis.

Comparison 38: B. Maintaining participant engagement: Brown vs white envelope, Outcome 1: Retention
39.1. Analysis.

Comparison 39: B ‐ Maintaining participant engagement: Post‐it note vs usual follow‐up, Outcome 1: Retention
40.1. Analysis.

Comparison 40: B ‐ Maintaining participant engagement: Inclusion of trial newspaper article vs usual follow‐up, Outcome 1: Retention
41.1. Analysis.

Comparison 41: B. Maintaining participant engagement: Frequency of telephone contact, Outcome 1: Retention
42.1. Analysis.

Comparison 42: B ‐ Maintaining participant engagement: Request for collateral (concomitant), Outcome 1: Retention
Behavioural interventions
The evidence is very uncertain about the effect on retention of a theory informed cover letter compared to a usual cover letter: RD = 3% (95% CI ‐2% to 8%); GRADE very low; (Analysis 43.1, Table 15). This result is based on four trials, n = 3343 from three studies: Goulao 2020, Goulao 2020 (replication of SWAT #2) (both dentistry), OPAL 2020 (urinary incontinence), and AMBER 2020 (multiple sclerosis).
The evidence is very uncertain about the effect on retention of motivational interviewing compared to usual follow‐up: Bean 2019 (childhood obesity): RD = 0% (95% CI ‐17% to 17%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 128; wide CI crossing RD = 0) (Analysis 44.1).
43.1. Analysis.

Comparison 43: B ‐ Behavioural interventions: Theory informed cover letter vs usual cover letter, Outcome 1: Retention
44.1. Analysis.

Comparison 44: B ‐ Behavioural interventions: Motivational interviewing vs usual follow‐up, Outcome 1: Retention
Sites and site staff ‐ Category C (Table 20)
Two studies assessed interventions, grouped into two comparisons, aimed at trial sites. One, a cluster‐randomised trial but reporting data on questionnaires returned (Land 2007) and the other an individually‐randomised trial reporting submission of case report forms by sites (Lienard 2006).
Prompts
The evidence is very uncertain about the effect on retention of prompts targeting sites for upcoming assessment compared to no prompts: Land 2007 (breast cancer prevention): RD = ‐3% (95% CI ‐13% to 7%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study; wide CI crossing RD = 0) (Analysis 45.1).
45.1. Analysis.

Comparison 45: C ‐ Prompts: Site prompts for upcoming assessments vs usual follow‐up, Outcome 1: Retention
Monitoring visits
The evidence is very uncertain about the effect on retention of on site monitoring compared to no visits: Lienard 2006 (breast cancer treatment): RD = ‐5% (95% CI ‐20% to 10%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 69; wide CI crossing RD = 0) (Analysis 46.1).
46.1. Analysis.

Comparison 46: C ‐ Monitoring visits: On‐site monitoring vs no visits, Outcome 1: Retention
Central Study Management ‐ Category D (Table 21)
Only one study (Fouad 2014) assessed the effect of a central study management intervention on retention. This study was judged to be at unclear risk of bias and involved 632 participants.
Patient Public Involvement
A peer‐led follow‐up strategy compared to usual follow‐up may result in a large increase in retention: Fouad 2014 (cervical cancer): RD = 22% (95% CI 14% to 30%); GRADE low (‐1 level: study limitations– unclear risk of bias; ‐1 levels: imprecision‐single study, n = 632) (Analysis 47.1).
47.1. Analysis.

Comparison 47: D ‐ Patient Public Involvement: Peer‐led follow‐up strategy vs usual follow‐up, Outcome 1: Retention
Study design ‐ Category E (Table 22)
Five studies across two comparisons evaluated five interventions targeting aspects of study design. These comparisons included 2160 participants.
Impact of recruitment
The evidence is very uncertain about the effect on retention of video‐enhanced patient information compared to standard information: Brubaker 2019 (urinary incontinence): RD = 3% (95% CI ‐5% to 12%); GRADE very low (‐1 level: study limitations– unclear risk of bias; ‐2 levels: imprecision‐single study, n = 285; wide CI crossing RD = 0) (Analysis 48.1).
The evidence is very uncertain about the effect on retention of optimised patient information compared to standard patient information: RD = ‐3% (95% CI ‐13% to 7%); GRADE: very low; (Analysis 49.1, Table 16). This result is based two studies, n = 1285: Cockayne 2017 (falls prevention) and Guarino 2006 (Gulf War Syndrome).
The addition of optimised information, either as bespoke or template formats, may increase retention: Cockayne 2017 (falls prevention): RD = 6% (95% CI ‐7% to 20%); GRADE low (‐2 levels: imprecision‐single study, n = 131; wide CI crossing RD = 0) (Analysis 50.1).
Giving a pen at recruitment compared to no pen probably increases retention: Whiteside 2019 (falls prevention): RD = 20% (95% CI 7% to 32%); GRADE moderate (‐1 level: imprecision‐single study, n = 92) (Analysis 51.1).
48.1. Analysis.

Comparison 48: E ‐ Impact of recruitment: Video‐enhanced patient information vs standard information, Outcome 1: Retention
49.1. Analysis.

Comparison 49: E ‐ Impact of recruitment: Optimised information vs standard information, Outcome 1: Retention
50.1. Analysis.

Comparison 50: E ‐ Impact of recruitment: Addition of optimised information to both arms, Outcome 1: Retention
51.1. Analysis.

Comparison 51: E ‐ Impact of recruitment: Pen vs no pen, Outcome 1: Retention
Blinding and treatment preference
Randomising participants at recruitment to an open rather than a blinded trial may result in a large increase in retention: Avenell 2004 (fracture prevention): RD = 13% (95% CI 4% to 22%); GRADE low (‐1 level: study limitations– unclear risk of bias; ‐1 level: imprecision‐single study, n = 367) (Analysis 52.1).
52.1. Analysis.

Comparison 52: E ‐ Blinding and treatment preference: Open vs blind trial design, Outcome 1: Retention
Discussion
Principal findings
As it stands, there is nothing in the evidence base with high‐certainty evidence (as determined by GRADE) that can be applied to trials to improve trial retention. Four interventions have moderate‐certainty evidence, although one of them (adding a diary to usual follow‐up) probably reduces retention. Given the frequency with which diaries are used in trials, this is a useful piece of information. The bulk of all retention intervention evidence is rated as low or very low certainty using GRADE. Whilst there are replications of a few interventions, most (33 of 51 comparisons) have been evaluated in a single study. However, what is more encouraging is that of the 68 studies identified, 33 of those have been published in the last five years, indicating a ground swell in efforts for evaluations of interventions to improve trial retention. Now, the focus needs to be on more joined up rigorous evaluations of existing or priority interventions to industrialise the evidence generation in this area.
The previous version of this Cochrane Review identified monetary incentives as an effective strategy for improving return of postal questionnaires (Brueton 2013). Whilst the overall findings remained the same in this update (addition of a monetary incentive compared to usual follow‐up) with an improvement in retention (RD: 7% (95% CI 4% to 11%), the overall certainty in the evidence was assessed as low, largely due to two of the three studies in this comparison being assessed as high risk of bias. Therefore, further replications of the evaluation of monetary incentive compared to no monetary incentive are still required in well‐designed studies. Also, mirroring the previous version of this Cochrane Review (Brueton 2013), this update predominantly identified studies that aimed to improve questionnaire return with a very small proportion (3%, n = 2) of all included studies targeting clinic attendance. There may be several reasons for this large evidence gap. Firstly, many trials that collect patient‐reported outcome data do so through self‐completion of questionnaires which can be administered remotely. It may also be perceived that return of a questionnaire is an easier behaviour to target and change than attending a clinic follow‐up visit. However, as face‐to‐face visits are central to many, perhaps most, trials there is a critical need to identify effective ways to enhance clinic follow‐up. One suggestion could be to identify high‐quality evidence for interventions that have been shown to improve clinic attendance at clinical care appointments and evaluate them for use in a trial setting e.g. electronic text notifications (Robotham 2016). Examining systematic reviews of factors known to affect retention (e.g. Skea 2019), such as the compatibility of trial processes with participants’ capabilities, would provide targets for well‐designed future interventions.
Some comparisons with low‐certainty evidence should be considered for future replication studies to help improve the overall certainty in the evidence (see Implications for methodological research). For example: return postage strategies (Analysis 8.1); addition of a pen versus no pen (Analysis 29.1); and electronic reminders compared to usual follow‐up (Analysis 10.1). There is also merit in conducting replication of interventions evaluated in single studies, especially where the single studies have moderate‐certainty evidence and potentially large overall effect sizes, such as: giving a pen at recruitment (Analysis 51.1), and addition of monetary rewards to both trial arms (Analysis 25.1).
Many of these comparisons and single‐study evaluations offer improvements in the region of 1% to 7%, the latter of which we would consider a moderate effect size. However, one of the included studies (GRADE low) which evaluated a peer‐led intervention to improve retention in a cervical screening trial found an overall improvement of 22% (Analysis 47.1), which we would consider a large effect size. This should also be a focus for future evaluation. A single additional study could improve the GRADE assessment to moderate if the results were consistent with the existing study. Although these patient and public involvement (PPI) interventions are likely to be more costly to develop and implement, if the gains seen are replicable, the benefit of a large effect may outweigh the costs. This is especially true where poor retention is dealt with by inflating the recruitment target to compensate for future expected loss‐to‐follow‐up; given that recruitment is both difficult and expensive. Future evaluations and development of interventions should also consider how to meaningfully include patient and public partners in these embedded evaluations of retention interventions. Ensuring the interventions are embedded in trial participants’ accounts of barriers to retention will be key to developing interventions that target what actually matters to trial participants.
PPI interventions and their impact on trial retention was identified as one of the Top 10 research questions from a James Lind Priority Setting Partnership exercise to identify the unanswered questions for trial retention (Brunsdon 2019). Many of the evaluations in this update fit within some of those questions but they tend to cluster around question 4 "What are the best ways to encourage trial participants to compete the tasks (e.g. attend follow‐up visits, complete questionnaires) required by the trial?". There are still very few evaluations of how to encourage participants to attend clinic visits and the same can be said for interventions targeting trial staff involved with retention.
We are hopeful that through key initiatives such as the PROMETHEUS (PROMoting THE USe of SWATs) project that collaborative efforts in this area will ensure that high‐quality evaluations of retention interventions are conducted in a timely manner (PROMETHEUS). More so, specific support from funders such as the National Institute for Health Research Health Technology Assessment Programme (UK) and the Health Research Board (Ireland) to provide funding for embedding these evaluations within ongoing trials should encourage trial teams to ensure they develop the evidence base whilst delivering their trials. Future evaluations (of any retention intervention) should also consider economic evaluation. Some trials included in this review did consider cost, with some authors hypothesising what the overall cost reduction could be for the strategy as a whole and others providing more clear examples of cost‐effectiveness by demonstrating cost per additional person retained. For example, Gates 2009 demonstrated that their use of monetary incentives cost £67.29 for every questionnaire returned. Similarly, Cunningham‐Burley 2020 demonstrated the cost of using a pen to retain an extra participant was £10.56. These two examples show the potential difference in costs of implementing retention interventions (both for evaluation and in practice) and, therefore, cost is an important outcome for evaluators to include in future comparisons so as to provide trial teams with the information they need to make a decision on what will work from an effectiveness and an economic perspective.
Finally, we welcome notifications for missed or newly published studies that require inclusion in future updates of this review.
Authors' conclusions
Implication for systematic reviews and evaluations of healthcare.
Trialists should consider including well‐designed evaluations of strategies to increase retention in randomised trials. A focus on replication of existing interventions for which additional high‐quality evidence is required should be the priority rather than uncoordinated scattergun approaches to identify improvements.
Implication for methodological research.
There are key recommendations for methodological research in this area.
Prioritisation: this is important both in terms of replication of existing studies and development and evaluation of interventions for priority questions. With regard to replication of existing studies, we believe there are three categories consisting of eight interventions in total that could immediately benefit from further research. These are presented below in order of priority. Decisions about whether further evaluation is required have been guided by Treweek 2020b, but are largely based on the fact that none of these comparisons have high GRADE certainty in the evidence.
Category A – interventions with multiple existing evaluations that currently provide low‐certainty evidence but rigorous replication could move the evidence up to moderate or high certainty. These are presented in order of effect size from highest (7%) to lowest (1%), which also corresponds with the predicted cost of each.
1. Monetary incentive compared to no incentive.
2. Return postage strategies (e.g. such as free post versus second class stamp; high‐priority mail stamp versus usual postage; and personal form) compared to usual practice for return postage.
3. Addition of a pen versus no pen.
4. Electronic reminders compared to usual follow‐up.
Two other interventions were identified that fulfilled this criterion, but which may have a detrimental effect on retention and have therefore not been prioritised for further evaluations at this stage: Personalised prompts compared to no prompt, and inclusion of a diary in addition to normal follow‐up.
Category B – interventions with multiple existing evaluations but which together currently provide low‐ or very low‐certainty evidence and which are nevertheless likely to be in routine use.
5. Post‐it note compared to no post‐it note or alternative post‐it notes (e.g. printed versus handwritten).
6. Newsletter compared to no newsletter.
Category C ‐ interventions currently with a single evaluation but which provide moderate‐certainty evidence and large potential effect sizes (RD 20% and 9%, respectively), are relatively easy to implement and may be cheap to implement.
7. Giving a pen at recruitment.
8. Addition of monetary rewards to both trial arms.
One further intervention (use of self‐sampling kits (directly mailed or an invitation to order)) also had moderate‐certainty evidence of benefit for retention but given it would only be of relevance for a small sub‐set of trials, it was judged to be of lower priority than the other two.
In addition to these replications, the questions identified in the PRioRiTY II project (which aimed to identify the unanswered questions for trial retention research) should act as a guiding framework to enable research teams to develop interventions to improve retention for which there is community buy‐in and desire for evidence (Brunsdon 2019). For example, questions in the Top 10 do consider impacts on trial staff, yet only two of the 68 studies included in this review evaluated strategies targeting staff. Likewise, retention behaviours that go beyond participants returning a trial questionnaire, such as attending a follow‐up clinic, also warrant evaluation.
Design: well‐designed retention trials that are considered from the design stage of the host trial are also key. Ensuring appropriate detail in terms of process and intervention are critical for timely replication. As a minimum, SWATs should have a publicly available outline registered on the SWAT repository (SWAT).
Reporting: retention trials were often poorly reported, without CONSORT flow diagrams, clear primary outcomes, sample size, and sociodemographic composition. In the context of this review, this meant that we had to contact authors for unreported data that we needed for robust meta‐analyses. Trialists writing their reports should adhere to CONSORT guidelines for trial reporting (Moher 2010), which would facilitate the synthesis of results in future methodology reviews. Guidance for reporting embedded recruitment trials have been developed and these could also serve as a set of guiding principles when reporting embedded retention trials (Madurasinghe 2016). Furthermore, clarity on the intention of interventions with regard to their proposed mechanism of action and reporting on all key aspects (for example using the TiDier framework (Hoffman 2014)) is important for future replication and, when available, implementation of effective strategies.
Context: linked to prioritisation, it is important to consider which areas in terms of trial populations and contexts need more attention. The majority of the trials included in this review included white adults in non‐emergency settings who were able to consent for themselves in high‐income countries. Research to understand whether the findings from interventions shown to be effective in this population translate into other settings (e.g. in people from ethnic backgrounds, paediatric trials, emergency care trials, and trials in adults who lack capacity and other underserved groups).This additional contextual information is essential for making informed decisions about whether further replication studies are required (Treweek 2020b).
Collaboration: in order to generate evidence on what interventions do, or do not improve retention, there needs to be coordinated collaboration to enable key questions to be answered in a timely fashion. This is of critical importance. The current approach is more scattergun than targeted. Ongoing efforts such as the PROMETHEUS project and Trial Forge aim to offer support (both in terms of advice but also tools such as text for ethics applications etc) for trial teams conducting SWATs and encourage parallel evaluations of interventions across a range of trials. Funders also have a role to play and whilst recent times have seen a shift to funds being available to support SWATs (especially in the UK and Ireland), a move to commissioned calls to evaluate priority SWAT interventions might accelerate the evidence generation for priority topics.
What's new
| Date | Event | Description |
|---|---|---|
| 8 April 2021 | Amended | Minor correction made to the title of the plain language summary |
History
Protocol first published: Issue 2, 2011 Review first published: Issue 12, 2013
| Date | Event | Description |
|---|---|---|
| 22 January 2021 | New citation required and conclusions have changed | Based on the revisions made during this update, the overall findings of the review and its conclusions have changed substantially. |
| 22 January 2021 | New search has been performed | Review updated. Significant amendments during update include the following.
|
Acknowledgements
We thank Jayne Tierney, Sally Stenning, Seeromanie Harding, Sarah Meredith, and Irwin Nazareth for their contributions to earlier versions of this review. We also thank all authors of included published studies who provided additional or unreported data and Principal investigators for data on studies in progress or completed and unpublished.
This update was funded by a National Institute for Health Research (NIHR) Incentive Award Scheme 2019 Reference 130660. The Health Services Research Unit, University of Aberdeen receives core funding from the Chief Scientist Office of the Scottish Government Health Directorates. The views expressed in this review are those of the authors and do not necessarily reflect those of the NIHR, the Department of Health and Social Care or these other funders.
Appendices
Appendix 1. Search Strategy
Medline (Ovid)
1. ((minimi* or prevent* or lessen* or decreas* or reduc*) adj2 (attrition or drop*‐out* or dropout* or withdr*w* or missing data)).ab,ti.
2. ((increas* or encourag* or maximi* or promot* or improv*) adj2 (retention or follow‐up or followup or completion or data collection or data return)).ab,ti.
3. ((strateg* or intervention* or method* or technique*) adj3 (retention or attrition or drop*‐out* or dropout* or follow‐up or followup)).ab,ti.
4. Complian* adj2 (follow‐up or followup).ab,ti.
5. ((loss or lost) adj2 (follow‐up or followup)).ab,ti.
6. ((difficult* or problem* or challeng* or success* or feasibl*) adj3 (retain* or retention)).ab,ti.
7. (retention adj2 rate*).ab,ti.
8. (attrition adj2 rate*).ab,ti.
9. ((Dropout* or Drop‐out*) adj2 rate*).ab,ti
10. (Completion adj2 rate*).ab,ti.
11. ((Follow‐up or followup) adj2 rate*).ab,ti.
12. (Incomplete adj2 (follow‐up or followup)).ab,ti
13. (questionnaire* adj3 (response* adj2 method*)).ab,ti.
14. (questionnaire* adj3 (response adj2 technique*)).ab,ti.
15. (questionnaire response rate*).ab,ti.
16. ((Strateg* or increas* or encourag* or maximi* or promot* or improv* or influenc* or success*) adj2 (questionnaire* adj3 response*)).ab,ti.
17. ((incentiv* or reminder*) adj3 (retention or retain or respon*e*)).ab,ti.
18. retention adj4 training.ab,ti
19. Trial site adj2 (retention or retain*). ab,ti.
20. Exp "Lost to Follow‐Up"/
21. Exp Patient Dropouts/
22. (Patient retention or Dropout* or Drop*‐out* or attrition).kw
23. ((survey* or questionnaire*) AND (respon*e* or return* or rate*)).ti
24. OR(1‐23)
25. Randomized controlled trial.pt
26. Controlled clinical trial.pt
27. Randomi*ed.tw
28. Placebo.tw
29. Clinical trials as topic.sh
30. Randomly.tw
31. Trial*.tw
32. Or/25‐31
33. 24 AND 32
34. Exp animals/not humans.sh
35. 33 not 34
36. Limit to comment, editorial, news and letter
37. 35 not 36
38. limit 37 to (English language and yr="2018 ‐2019")
Scopus
1. TITLE‐ABS‐KEY ((minimi* or prevent* or lessen* or decreas* or reduc*) w/2 (attrition or drop*‐out* or dropout* or withdr*w* or “missing data”))
2. TITLE‐ABS‐KEY((increas* or encourag* or maximi* or promot* or improv*) w/2 (retention or follow‐up or followup or completion or “data collection” or “data return”))
3. TITLE‐ABS‐KEY ((strateg* or intervention* or method* or technique*) w/3 (retention or attrition or drop*‐out* or dropout* or follow‐up or followup))
4. TITLE‐ABS‐KEY (Complian* w/2 (follow‐up or followup))
5. TITLE‐ABS‐KEY ((loss or lost) w/2 (follow‐up or followup))
6. TITLE‐ABS‐KEY ((difficult* or problem* or challeng* or success* or feasibl*) w/3 (retain* or retention))
7. TITLE‐ABS‐KEY (retention w/2 rate*)
8. TITLE‐ABS‐KEY (attrition w/2 rate*)
9. TITLE‐ABS‐KEY ((Dropout* or Drop‐out*) w/2 rate*)
10. TITLE‐ABS‐KEY (Completion w/2 rate*)
11. TITLE‐ABS‐KEY ((Follow‐up or followup) w/2 rate*)
12. TITLE‐ABS‐KEY (Incomplete w/2 (follow‐up or followup))
13. TITLE‐ABS‐KEY (questionnaire* w/3 (response* w/2 method*))
14. TITLE‐ABS‐KEY (questionnaire* w/3 (response w/2 technique*))
15. TITLE‐ABS‐KEY (“questionnaire response rate*”)
16. TITLE‐ABS‐KEY ((Strateg* or increas* or encourag* or maximi* or promot* or improv* or influenc* or success*) w/2 (questionnaire* w/3 response*))
17. TITLE‐ABS‐KEY ((incentiv* or reminder*) w/3 (retention or retain or respon*e*))
18. TITLE‐ABS‐KEY (retention w/4 training)
19. TITLE‐ABS‐KEY (“Trial site” w/2 (retention or retain*))
20. KEY (“Patient retention” or Dropout* or Drop*‐out* or attrition)
21. TITLE ((survey* or questionnaire*) AND (respon*e* or return* or rate*))
22. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
23. #22 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
24. #23 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
25. TITLE‐ABS‐KEY((clinic* w/1 trial*) OR (randomi* w/1 control*) OR (randomi* w/2 trial*) OR (random* w/1 assign*) OR (random* w/1 allocat*) OR (control* w/1 clinic*) OR (control* w/1 trial) OR placebo* OR (Quantitat* w/1 Stud*) OR (control* w/1 stud*) OR (randomi* w/1 stud*) OR (singl* w/1 blind*) or (singl* w/1 mask*) OR (doubl* w/1 blind*) OR (doubl* w/1 mask*) OR (tripl* w/1 blind*) OR (tripl* w/1 mask*) OR (trebl* w/1 blind*) OR (trebl* w/1 mask*))
26. #24 AND #25
27. INDEXTERMS (animals OR nonhuman)
28. #26 AND NOT #27
29. LANGUAGE(English)
30. #28 AND #29
31. DOCTYPE (ed OR le OR no OR pr)
32. #30 AND NOT #31
33. PUBYEAR > 2017 AND PUBYEAR < 2020
34. #32 AND #33
Web of Science (CCSSI and SSCI)
1. TS= ((minimi* or prevent* or lessen* or decreas* or reduc*) near/2 (attrition or drop*‐out* or dropout* or withdr$w* or “missing data”))
2. TS=((increas* or encourag* or maximi* or promot* or improv* ) near/2 (retention or follow‐up or followup or completion or “data collection” or “data return”))
3. TS= ((strateg* or intervention* or method* or technique*) near/3 (retention or attrition or drop*‐out* or dropout* or follow‐up or followup))
4. TS= (Complian* near/2 (follow‐up or followup))
5. TS= ((loss or lost) near/2 (follow‐up or followup))
6. TS= ((difficult* or problem* or challeng* or success* or feasibl*) near/3 (retain* or retention))
7. TS= (retention near/2 rate*)
8. TS= (attrition near/2 rate*)
9. TS= ((Dropout* or Drop‐out*) near/2 rate*)
10. TS= (Completion near/2 rate*)
11. TS= ((Follow‐up or followup) near/2 rate*)
12. TS= (Incomplete near/2 (follow‐up or followup))
13. TS= (questionnaire* near/3 (response* near/2 method*))
14. TS= (questionnaire* near/3 (response near/2 technique*))
15. TS= (“questionnaire response rate*”)
16. TS= ((Strateg* or increas* or encourag* or maximi* or promot* or improv* or influenc* or success*) near/2 (questionnaire* near/3 response*))
17. TS= ((incentiv* or reminder*) near/3 (retention or retain or respon$e*))
18. TS= (retention near/4 training)
19. TS= (“Trial site” near/2 (retention or retain*))
20. TS=(“Patient retention” or Dropout* or Drop*‐out* or attrition)
21. TI= ((survey* or questionnaire*) AND (respon$e* or return* or rate*))
22. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
23. TS=((clinic* near/1 trial*) OR (randomi* near/1 control*) OR (randomi* near/2 trial*) OR (random* near/1 assign*) OR (random* near/1 allocat*) OR (control* near/1 clinic*) OR (control* near/1 trial) OR placebo* OR (Quantitat* near/1 Stud*) OR (control* near/1 stud*) OR (randomi* near/1 stud*) OR (singl* near/1 blind*) or (singl* near/1 mask*) OR (doubl* near/1 blind*) OR (doubl* near/1 mask*) OR (tripl* near/1 blind*) OR (tripl* near/1 mask*) OR (trebl* near/1 blind*) OR (trebl* near/1 mask*))
24. #22 and #23
25. Refined by: DOCUMENT TYPES: ( ARTICLE OR REVIEW )
PsycInfo (Ovid)
1. ((minimi* or prevent* or lessen* or decreas* or reduc*) adj2 (attrition or drop*‐out* or dropout* or withdr*w* or missing data)).ab,ti.
2. ((increas* or encourag* or maximi* or promot* or improv*) adj2 (retention or follow‐up or followup or completion or data collection or data return)).ab,ti.
3. ((strateg* or intervention* or method* or technique*) adj3 (retention or attrition or drop*‐out* or dropout* or follow‐up or followup)).ab,ti.
4. (Complian* adj2 (follow‐up or followup)).ab,ti.
5. ((loss or lost) adj2 (follow‐up or followup)).ab,ti.
6. ((difficult* or problem* or challeng* or success* or feasibl*) adj3 (retain* or retention)).ab,ti.
7. (retention adj2 rate*).ab,ti.
8. (attrition adj2 rate*).ab,ti.
9. ((Dropout* or Drop‐out*) adj2 rate*).ab,ti.
10. (Completion adj2 rate*).ab,ti.
11. ((Follow‐up or followup) adj2 rate*).ab,ti.
12. (Incomplete adj2 (follow‐up or followup)).ab,ti.
13. (questionnaire* adj3 (response* adj2 method*)).ab,ti.
14. (questionnaire* adj3 (response adj2 technique*)).ab,ti.
15. questionnaire response rate*.ab,ti.
16. ((Strateg* or increas* or encourag* or maximi* or promot* or improv* or influenc* or success*) adj2 (questionnaire* adj3 response*)).ab,ti.
17. ((incentiv* or reminder*) adj3 (retention or retain or respon*e*)).ab,ti.
18. (retention adj4 training).ab,ti.
19. (Trial site adj2 (retention or retain*)).ab,ti.
20. exp Experimental Attrition/
21. exp Dropouts/ or exp Potential Dropouts/
22. ("patient retention" or dropout or drop*‐out* or attrition).id.
23. ((survey* or questionnaire*) and (respon*e* or return* or rate*)).ti.
24. or/1‐23
25. Double‐blind.tw.
26. "random* assigned".tw.
27. control.tw.
28. or/25‐27
29. 24 and 28
30. limit 29 to animal
31. 29 not 30
32. limit 31 to (english language and yr="2018 ‐2019")
CINHAL Plus (EBSCO)
1. TX ((minimi* or prevent* or lessen* or decreas* or reduc*) n2 (attrition or drop*‐out* or dropout* or withdr#w* or “missing data”))
2. TX((increas* or encourag* or maximi* or promot* or improv*) n2 (retention or follow‐up or followup or completion or “data collection” or “data return”))
3. TX ((strateg* or intervention* or method* or technique*) n3 (retention or attrition or drop*‐out* or dropout* or follow‐up or followup))
4. TX (Complian* n2 (follow‐up or followup))
5. TX ((loss or lost) n2 (follow‐up or followup))
6. TX ((difficult* or problem* or challeng* or success* or feasibl*) n3 (retain* or retention))
7. TX (retention n2 rate*)
8. TX (attrition n2 rate*)
9. TX (Dropout* or Drop‐out*) n2 rate*
10. TX Completion n2 rate*
11. TX ((Follow‐up or followup) n2 rate*)
12. TX (Incomplete n2 (follow‐up or followup))
13. TX (questionnaire* n3 (response* n2 method*))
14. TX (questionnaire* n3 (response n2 technique*))
15. TX (“questionnaire response rate*”)
16. TX ((Strateg* or increas* or encourag* or maximi* or promot* or improv* or influenc* or success*) n2 (questionnaire* n3 response*))
17. TX ((incentiv* or reminder*) n3 (retention or retain or respon#e*))
18. TX (retention n4 training)
19. TX (“Trial site” n2 (retention or retain*))
20. TX (“Patient retention” or Dropout* or Drop*‐out* or attrition)
21. TI ((survey* or questionnaire*) AND (respon#e* or return* or rate*))
22. OR(1‐21)
23. PT Clinical trial
24. MH “treatment outcomes”
25. TX randomi#ed
26. S23 or S24 or S25
27. S22 and S26 Limiters Published Date: 20180101‐20191231
Cochrane library
1. ((minimi* or prevent* or lessen* or decreas* or reduc*) near/2 (attrition or “drop*‐out*” or dropout* or withdr*w* or “missing data”)):ab,ti
2. ((increas* or encourag* or maximi* or promot* or improv*) near/2 (retention or “follow‐up” or followup or completion or “data collection” or “data return”)):ab,ti
3. ((strateg* or intervention* or method* or technique*) near/3 (retention or attrition or “drop*‐out*” or dropout* or “follow‐up” or followup)):ab,ti
4. Complian* near/2 (“follow‐up” or followup):ab,ti
5. ((loss or lost) near/2 (“follow‐up” or followup)):ab,ti
6. ((difficult* or problem* or challeng* or success* or feasibl*) near/3 (retain* or retention)):ab,ti
7. (retention near/2 rate*):ab,ti
8. (attrition near/2 rate*):ab,ti
9. ((Dropout* or “Drop‐out*”) near/2 rate*):ab,ti
10. (Completion near/2 rate*):ab,ti
11. ((“Follow‐up” or followup) near/2 rate*):ab,ti
12. (Incomplete near/2 (“follow‐up” or followup)):ab,ti
13. (questionnaire* near/3 (response* near/2 method*)):ab,ti
14. (questionnaire* near/3 (response near/2 technique*)):ab,ti
15. (“questionnaire response rate*”):ab,ti
16. ((Strateg* or increas* or encourag* or maximi* or promot* or improv* or influenc* or success*) near/2 (questionnaire* near/3 response*)):ab,ti
17. ((incentiv* or reminder*) near/3 (retention or retain or respon*e*)):ab,ti
18. retention near/4 training:ab,ti
19. “Trial site” near/2 (retention or retain*):ab,ti
20. Exp "Lost to Follow‐Up"/
21. Exp Patient Dropouts/
22. (“Patient retention” or Dropout* or “Drop*‐out*” or attrition):kw
23. ((survey* or questionnaire*) AND (respon*e* or return* or rate*)):ti
24. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
25. Randomized controlled trial:pt
26. Controlled clinical trial:pt
27. Randomi*ed:ab,ti,kw
28. Placebo:ab,ti,kw
29. Randomly:ab,ti,kw
30. Trial*:ab,ti,kw
31. #25 OR #26 OR #27 OR #28 OR #29 OR #30
32. #24 AND #31 with Cochrane Library publication date Between Jan 2018 and Dec 2019, in Cochrane Reviews, Trials
Appendix 2. Reference lists of reviews and other publications searched
Edwards P, Roberts I, Sandercock P, Frost C. Follow‐up by mail in clinical trials: does questionnaire length matter? Control Clinical Trials 2004;25:31‐52.
Edwards PJ, Roberts IG, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I et al. Methods to increase response rates to postal and electronic questionnaires. Cochrane Database of Systematic Reviews 2009, Issue 3 Art No: MR000008 2009.Edwards 2009
Appendix 3. Characteristics of host trials
| STUDY ID | Clinical area main trial | Condition | Host trial | Participants | Overall characteristic |
| AMBER trial | Treatment | Multiple sclerosis | Abdominal Massage for Bowel Dysfunction Effectiveness Research (AMBER) trial | ≥18 years participants, with a diagnosis of multiple sclerosis (in a stable phase, i.e. no multiple sclerosis relapse for 3 months) | Multi‐centred patient‐randomised superiority trial comparing an experimental strategy of once daily abdominal massage for 6 weeks against a control strategy of no massage in people with multiple sclerosis who have stated that their constipation is troublesome. |
| Arundel 2019 | Smoking Cessation | Smoking | Smoking Cessation Intervention for Severe Mental Ill Health Trial (SCIMITAR+) | ≥18 years participants, with a documented diagnosis of bipolar disorder, schizophrenia or schizoaffective disorder who smoke were included in this study. | The intervention group were assigned a mental health professional trained to deliver smoking‐cessation interventions who worked with the participant and participant's GP or mental health specialist to provide an individually tailored smoking‐cessation service. The control group received usual care (following NICE guidelines for smoking cessation) |
| Ashby 2011 | Treatment | Migraine | No name provided for the host trial. | 18‐65 years with a self‐reported diagnosis of migraine for at least 12 months, with no evidence of any other significant co‐existing pathology and experienced two or more migraine‐like attacks (or four or more headache days) in the previous 4‐week period were included in this study. | The intervention consisted of a diet based on eliminating foods to which participants exhibited IgG antibodies vs eliminating the same number of foods from the diet but not those for which the participant exhibited IgG antibodies. |
| Avenell 2004 | Prevention | Fractures | UK RECORD trial | Participants ≥70 years old that had an osteoporotic fracture within the last ten years identified from the hospital notes and seen either in a fracture clinic or on an orthopaedic ward were included in this study. | Randomised double‐blind placebo‐controlled, factorial design, evaluation of oral calcium (1g/day) or vitamin D (800 IU/20 Mg) supplementation in the secondary prevention of osteoporotic fractures. |
| Bailey 2013 | Prevention | Sexual health | “Sexunzipped” website | Youth 16‐20 years recruited online. | Theory‐based website that aims to give young people the tools to make informed decisions about their sexual well‐being. |
| Bauer 2004 | Smoking Cessation | Smoking | Community Intervention Trial for Smoking Cessation (COMMIT) study |
A cohort of smokers in each community and administered a detailed telephone‐based questionnaire about their tobacco use in 1988 and 1993 were included in this study. | The design was a matched pair, randomised, control trial, which involved 11 pairs of small‐ to medium‐sized communities in the USA and Canada. Each pair of communities contained one intervention site and one control site. The study was conducted as a community‐level intervention to help smokers achieve and maintain cessation. Within each community, the smoking behaviours and habits of the population were monitored over five years. |
| Bean 2018 | Treatment | Childhood obesity | Nourishing Our Understanding of Role‐modelling to Improve Support and Health (NOURISH+) study | Parents/caregivers were eligible if parent age ≥18 years; child age 5–11 years; the child had overweight, or obesity and the child primarily reside in the caregiver's home were included in this study. | The intervention group received a culturally tailored parent‐based treatment for parents of children with overweight or obesity issues. Participants received eight sessions of group‐based parent‐based treatments (6 core group sessions with two adjunctive experiential sessions (a group cooking and an individual dietician visit)). This treatment was targeted at parents as the agent of change of their children with overweight or obesity issues. The control group received an educational control intervention. |
| Bell 2016 | Prevention | Fracture | Women for Prevention of Fracture Trial (SCOOP) | 70‐85‐year old women at risk of fracture were included in this study. | Those in the intervention group received a 10‐year fracture risk assessment calculated using a WHO risk algorithm computed from baseline questionnaire data and bone mineral density values measured via a Dual‐energy X‐ray absorptiometry scan in selected participants. For women in the control group, fracture risk was not calculated, and participants continued to receive usual care. |
| Bradshaw 2020 | Diagnosis | Eczema | Barrier Enhancement for Eczema Prevention (BEEP) randomised trial | Infants at high risk of developing atopic eczema and with no more than 21 days old at the point of randomisation, the mother must be aged at least 16 years, and the consenting adult must be able to understand English were included in this study. | This trial investigated the effect of applying emollient for 12 and 24 months from birth on the development of eczema in high‐risk infants. Both groups received standard skincare advice. The intervention group receives additional advice to apply emollient daily for the first year of life and are supplied with emollient free of charge. |
| Brubaker 2019 | Treatment | Urinary incontinence | Extended Operations and Pelvic Muscle Training in the Management of Apical Support Loss (E‐OPTIMAL) study | Women undergoing vaginal apical prolapse repair with a mid‐urethral sling for stress urinary incontinence were included in this study. | Multi‐centre randomised to (1) perioperative behavioural therapy with pelvic floor muscle training vs usual care and (2) surgical intervention or (3) usual care. The extended version was designed to lengthen the follow‐up of these women and compare 5‐ year success and complication rates. |
| Clark 2015 | Smoking Cessation | Smoking in participants with chronic obstructive pulmonary disease | Determining the Optimal approach to identify individuals with Chronic obstructive pulmonary disease (DOC) study | Smokers aged ≥35 years undertaking lung function tests and symptom‐based questionnaires were included in this study. | DOC is a case‐finding study for chronic obstructive pulmonary disease and a randomised trial of the impact of case‐finding on smoking cessation. The intervention group received lung function tests and symptom‐based questionnaires. The control group was in a 6‐month waitlist for tests and symptom questionnaires. |
| Cochrane 2020 | Prevention | Risk of falling | Occupational Therapist Intervention Study (OTIS) | Participants ≥65 years, community‐dwelling, currently able to walk 10 feet (with a walking aid if needed) were included in this study. | Participants were randomised to either home environmental assessment and modification, led by an occupational therapist or usual care from GP or other healthcare professional. |
| Cockayne 2005 | Prevention | Fracture prevention | No name provided for the Host trial | Community‐dwelling women aged ≥70 years, living in the York and Cumbria area were included in this study. | The intervention group received daily oral supplementation of 1,000 mg of calcium with 800 IU vitamin D3 with a patient information leaflet on dietary calcium intake and falls prevention. The control group received the patient information leaflet only. |
| Cockayne 2017 | Prevention | Risk of falls | REducing Falls with ORthoses and a Multifaceted podiatry intervention (REFORM) study | Participants ≥65 years from routine podiatry clinics in the UK and Ireland to the REFORM cohort were included in this study. Participants had one fall in the past 12 months: or one fall in the past 24 months requiring hospital attention were included in this study. | Podiatry intervention for the prevention of falls in older people. Participants were randomised to receive routine podiatry care, and a falls prevention leaflet or routine podiatry care, a falls prevention leaflet, and a multifaceted podiatry intervention. |
| Cook 2020 | Treatment | Influenza | Antivirals for influenza‐Like Illness, An rCt of Clinical and Cost ‐effectiveness in primary CarE (ALIC4E) Trial | Participants ≥1 year presenting with influenza‐like illness, with symptom duration ≤72 hours in primary care over three consecutive periods of confirmed high influenza incidence were included in this study. | European multinational, multi‐centre, open‐labelled, non‐industry funded, pragmatic, adaptive‐platform, RCT. Control group received the best usual primary care, and the intervention group received the best usual primary care plus treatment with oseltamivir for five days. |
| Couper 2007 | Treatment | Obesity | No name provided for the host trial. | Adult participants with overweight and obesity (BMI 27 to 40 kg/m2) members from four regions of Kaiser Permanente's integrated healthcare delivery system were included in this study. | Participants were randomly assigned to one of two Web‐based treatments for weight management: the expert system materials or information‐only materials. |
| Cunningham 2004 | Treatment | Alcohol Consumption | No name provided for the host trial. | Participants were recruited through a random digit dialling telephone survey conducted by the Centre for Addiction and Mental Health were included in this study. | Participants were randomly assigned to one of four conditions in a two‐by‐two factorial design: no‐intervention control group, personalised feedback only, self‐help book only and both personalised feedback and self‐help book. |
| Cunningham‐Burley 2020 | Prevention | Risk of falls | The Stopping Slips among Healthcare Workers (SSHeW) trial | NHS staff, aged ≥18 years, who adhere to a dress code policy and work in a clinical, catering, or general hospital environment were included in this study. | This trial evaluated the effectiveness of slip‐resistant footwear to reduce slips in NHS staff. |
| Dinglas 2015 | Treatment | Acute lung injury survivors | ARDS Network Long Term Outcomes Study (ALTOS) |
Participants with an acute lung injury survivor, recruited from 41 hospital sites at 12 centres across the US were included in this study. | Participants were enrolled in randomised trials of novel interventional therapies initial trophic vs full enteral feeding. |
| Dorman 1997 | Prevention | Stroke | International Stroke Trial | Patients who have a clinical diagnosis of acute ischaemic stroke, with onset within the previous 48 hours and no clear indication for, or clear contraindication to, treatment with aspirin or subcutaneous heparin were included in this study. | This was an international trial involving around 36 countries. The intervention group received early administration of aspirin or heparin or both. The analysis was structured as immediate heparin (low or medium dose) vs avoiding heparin, and immediate aspirin vs avoiding aspirin. |
| Edwards 2004 | Treatment | Head injury | CRASH Trial | All head‐injured adults who were observed while in the hospital to have GCS of 14 or less (out of a maximum score of 15), and who were within eight hours of the injury were included in this study. | This was a multi‐centre RCT evaluating the efficacy and safety of a high dose of corticosteroid infusion after head injury. |
| Edwards 2016 | Treatment | Depression & cardiovascular health | The Healthlines Study | Participants with depression aged between 40 and 74 years old recruited from 42 general practices in or near Bristol, Sheffield and Southampton were included in this study. | Two linked, parallel RCTs of patients with depression and raised risk of cardiovascular disease who were allocated to a telehealth intervention plus usual care or usual care alone. |
| Fouad 2014 | Diagnosis | Cancer | ASCUS‐LSIL Triage Study (ALTS) | Women residing in Jefferson County, Alabama were included in this study. | This was a multi‐centre clinical trial to evaluate the optimal clinical management of low‐grade cervical cytologic abnormalities. ALTS participants were randomised to three management strategies: 1) immediate colposcopy; 2) human papillomavirus (HPV) DNA testing, which triaged to colposcopy only participants with oncogenic HPV type; and 3) conservative management followed with serial Pap smears and colposcopy if Pap smear progressed to high grade. |
| Gattellari 2004 | Diagnosis | Cancer | No name provided for the host trial. | Men recruited from local general practices, aged 40–70 years, fluent in English and who had not been diagnosed with prostate cancer were included in this study. | Participants were randomised to receive a 32‐page evidence‐based booklet contained content previously identified by experts as essential to informed screening or conventional information about prostate cancer screening (pamphlet had been published by the Australian government). |
| Gates 2009 | Treatment | Acute whiplash injuries | Managing Injuries of the Neck Trial (MINT) study | Patients presenting with acute whiplash injuries, in which eligible patients were identified in emergency departments were included in this study. | Cluster randomised trial of advice interventions given in emergency departments. Participants were then followed up by postal questionnaires sent from the study office, 4, 8 and 12 months after their injury. The intervention group received advice (psycho‐educational intervention, including Whiplash book advice/active management advice) and the control group received usual care advice. |
| Glassman 2020 | Treatment | Diabetic retinopathy | Diabetic Retinopathy Clinical Research Retina Network Protocol S randomised trial | Participants ≥18 years with at least one eye with Proliferative diabetic retinopathy were included in this study. | Participants were randomly assigned to receive either laser treatment or injections of a drug (ranibizumab) into the study eye. |
| Goulao 2020 | Prevention | Bleeding on probing | IQuaD trial & INTERVAL trial | Adults with good oral health who are regular attendees to the United Kingdom's National Health System primary care dental services were included in this study. |
Intervention measuring if providing no scale and polish or 12‐month was compared with the standard 6‐month scale and polish. Personalized (intervention) vs standard oral hygiene advice (control) was also compared. |
| Greig 2017 | Treatment | Nail‐bed injury | The Nail bed INJury Analysis (NINJA RCT) study | Participants <16 years with acute nail bed injury within the last 48 hrs and requiring surgery were included in this study. | Children were randomised for the surgeon to either replace or discard the nail plate after nail‐bed repair. |
| Griffin 2009 | Prevention | Falls | the Prevention of Falls Injury Trial (Pre‐FIT) | Community‐dwelling adults aged ≥70 years from 63 practices across England were included in this study. |
Cluster‐randomised pragmatic design to test alternative falls prevention interventions (to receive one of three fall prevention interventions) of advice, exercise, and multifactorial assessment, on outcomes of falls and fractures. |
| Guarino 2006 | Treatment | War illnesses | No name provided for the Host trial | American Gulf War veterans were included in this study. | 2 x 2 factorial randomised clinical trial of exercise and cognitive behavioural therapy for the treatment of Gulf War veterans' illnesses. |
| Hardy 2016 | Treatment | Women in labour | Birth in Upright Maternal Positionvslying down position, in women with a low‐dose Epidural, in the Second stage of labour (BUMPES) trial | Women ≥16 years, having their first child, who was admitted to a labour ward, ≥37 weeks gestation and with low dose epidural in situ were included in this study. | This was a multi‐centre RCT investigating the effect of maternal position during the late stages of labour in women with an epidural. The intervention group were upright during the second stage of labour. The control group were Laying down during the second stage of labour. |
| Henderson 2010 | Prevention | unsafe sexual behaviours among youth. | The Sexual Health and Relationships: Safe, Happy and Responsible (SHARE) intervention | Young people aged 14 to 20, starting in school and following them into the community were included in this study. Pupils were from 47 non‐Catholic state schools within 24 km of the main cities in Tayside and Lothian regions in Scotland, UK. | This was a five‐day teacher training programme plus a 20‐session pack: 10 sessions in the third year of secondary school (at 13‐14 years) and 10 in the fourth year (at 14‐15 years). In the 12 control schools, sex education for third and fourth years varied from seven to 12 lessons in total and was primarily devoted to the provision of information and discussion. |
| James 2020 | Prevention | Risk of falling | Occupational Therapist Intervention Study (OTIS) |
Participants ≥ 65 years, community‐dwelling, currently able to walk 10 feet (with a walking aid if needed) were included in this study. | Participants were randomised to either home environmental assessment and modification, led by an occupational therapist or usual care from GP or other healthcare professional. |
| Dorling 2020 | Treatment | Very preterm or very low‐birthweight infants | Speed of Increasing milk Feeds Trial (SIFT) | Infants born at <32 weeks' gestation or who had a birth weight of <1500 g, who were receiving <30 ml/kg/day of milk recruited from 78 UK and Republic of Ireland neonatal units were included in this study. | This was a multi‐centre, two‐arm, parallel‐group, RCT in very preterm or very low‐birthweight infants. When advancing feed volumes, participants were randomised to receive daily increments in feed volume of 30 ml/kg or 18 ml/kg. |
| Keding 2016 | Treatment | Depression | Acupuncture and Depression (ACUDep) trial | Patients with depression from Yorkshire and northern England were included in this study. | Participants were randomised to receive either acupuncture, counselling, or usual care. |
| Kenton 2007 | Prevention | Postnatal depression | Postpartum Depression Peer Support Trial. | Women were recruited from seven large health regions and their corresponding public health departments across Ontario, Canada were included in this study. | Study evaluating the effect of telephone‐based peer (mother to mother) support on preventing postnatal depression among women identified as high risk within the first two weeks postpartum. Participants support initiated within 48‐72 hours of randomisation, provided by a volunteer recruited from the community who had previously experienced and recovered from self‐reported postnatal depression and attended a four‐hour training session. |
| Kenyon 2005 | Prevention | Neonatal outcomes | The MRC ORACLE Children Study (MOCS) | Participants were seven‐year‐old children whose mothers joined the MRC ORACLE Trial (Broad‐spectrum antibiotics for spontaneous preterm labour) | The original trial evaluated the use of antibiotics to improve neonatal outcome after preterm labour or preterm rupture of the membranes. Women were randomly assigned to one of four possible treatments: 325 mg co‐amoxiclav (250 mg amoxicillin and 125 mg clavulanic acid) plus 250 mg erythromycin; co‐amoxiclav plus erythromycin placebo; erythromycin plus co‐amoxiclav placebo; or co‐amoxiclav placebo plus erythromycin placebo. |
| Khadjesari 2011 | Prevention | Alcohol consumption | Down your Drink randomised controlled trial (DYD‐RCT) |
Participants were people who came across DownYourDrink while browsing the web were included in this study. Eligible participants were people drinking potentially unhealthy levels of alcohol who were also willing to consider changing their behaviour. | A two‐arm individually RCT for people with hazardous alcohol consumption. It was conducted in three phases: pilot, main trial and main trial extension. The intervention website was a theoretically informed programme based on brief intervention and psychological treatment principles. The control website used a similar graphical design and style to present simple, text‐based information about the harms caused by excess alcohol consumption. |
| Land 2007 | Treatment | Cancer | The Study of Raloxifene and Tamoxifen (STAR). | Postmenopausal women were included in this study. | This study compared raloxifene vs tamoxifen for the prevention of breast cancer in high‐risk postmenopausal women. |
| Lewis 2017 | Treatment | Depression | Collaborative Care in Screen‐Positive Elders (CASPER) trials |
Participants ≥65 years were included in this study. | This multi‐centred RCT was looking at the effectiveness and cost‐effectiveness of a form of collaborative care with behavioural activation in patients identified with above‐threshold depressive symptoms. |
| Lienard 2006 | Treatment | Cancer | Randomised clinical trial for patients with node positive breast cancer (AERO‐B2000) |
Women with breast cancer were included in this study. | The trial compared six courses of FEC 100 (5‐fluorouracil, epirubicin and cyclophosphamide) with four courses of FEC 100 followed by four courses of Taxol. |
| MacLennan 2014 | Prevention | Fracture | The RECORD trial (Randomised Evaluation of Calcium and/OR vitamin D) | People ≥70 years or who had a previous fracture were included in this study. | Participants were randomly allocated to four equal groups and assigned two tablets with meals daily consisting of 800 IU vitamin D3, 1000 mg calcium (given as carbonate), vitamin D3 (800 IU) combined with calcium (1000 mg), or placebo. |
| Man 2011 | Treatment | Low back pain | Yoga for chronic Low Back Pain RCT | Adults aged 18–65; that presented to their GP with low back pain in the previous 18 months; with a score of 4 or more on the Roland & Morris Disability Questionnaire; physically mobile were included in this study. | Participants randomly allocated to receive yoga or usual care to treat lower back pain. |
| MAmMOTH 2020 | Prevention | Chronic widespread pain | The Maintaining Musculoskeletal Health (MAmMOTH) Study | Participants ≥25 years registered with participating general practices in the study areas (NHS Grampian, NHS Highland, and NHS Greater Glasgow and Clyde) were included in this study. | Patients were randomised to either Cognitive Behavioural Therapy delivered by telephone or usual care. Those receiving telephone CBT received a treatment manual, had an initial assessment with a trained CBT therapist, and six weekly treatment sessions as well as two follow‐up sessions 3‐ and 6‐ months later. |
| Marques 2013 | Treatment | Joint pain | Arthroplasty Pain Experience Study (APEX) trial |
Participants needing a total hip replacement and total knee replacement were included in this study. | This trial studied if using local wound infiltration in addition to the standard anaesthetic regimen significantly reduces joint pain at 1 year after total hip replacement and total knee replacement. The intervention group received local wound infiltration in addition to the standard anaesthetic regimen, and the control group received a standard anaesthetic regimen. |
| Marsh 1999 | Prevention | Injury | Trial of injury prevention in primary care | Children aged 3‐12 months registered with participating practices were included in this study. | Intervention groups received safety advice at child health surveillance consultations, provision of low‐cost safety equipment to families receiving means‐tested state benefits, home safety checks, and first aid training and the control group received standard care. |
| Marson 2007 | Treatment | Epilepsy | A RCT examining the longer‐term outcomes of standard vs new antiepileptic drugs. The SANAD trial | Patients with an adequately documented history of two or more clinically definite unprovoked epileptic seizures within the last year for whom treatment with a single antiepileptic drug represented the optimal therapeutic option were included in this study. | Intervention tested the effect of carbamazepine vs gabapentin vs lamotrigine vs oxcarbazepine vs topiramate or valproate vs lamotriginevstopiramate. |
| McCambridge 2011 | Prevention | Alcohol consumption | Down your Drink randomised controlled trial (DYD‐RCT) |
Participants were people who came across DownYourDrink while browsing the web were included in this study. Eligible participants were people drinking potentially unhealthy levels of alcohol who were also willing to consider changing their behaviour. | A two‐arm individually RCT for people with hazardous alcohol consumption. It was conducted in three phases: pilot, main trial and main trial extension. The intervention website was a theoretically informed programme based on brief intervention and psychological treatment principles. The control website used a similar graphical design and style to present simple, text‐based information about the harms caused by excess alcohol consumption. |
| McColl 2003 | Treatment | Asthma or angina | No name provided for the Host trial | Participants ≥18 years with asthma or angina managed in 62 family practices in northeast England were included in this study. | GP practices were participating in a RCT of computerised guidelines for primary care management of asthma or angina. |
| Mitchell 2011 & Mitchell 2012 | Prevention | Osteoporotic fracture | SCreening of Older women for Osteoporotic fracture Prevention (SCOOP) | Women between 70 and 85 years old were included in this study. | The trial was a two‐armed pragmatic RCT of screening vs usual care. |
| Mitchell 2020 & Mitchell 2020b | Treatment | Knee Replacement | Knee Replacement Bandaging Study (KReBS) randomised controlled trial. | Participants needing a knee replacement from hospital NHS trust sites were included in this study. | Evaluated the effectiveness of a two‐layer compression bandage compared with a standard wool and crepe bandage applied post‐operatively on patient‐reported outcomes in total knee replacement patients. |
| Nakash 2007 | Treatment | Severe ankle sprains | the Collaborative Ankle Support Trial (CAST) | All people who attended accident and emergency with a grade II or III sprain of the ankle, ≥16 years were included in this study. | Participants were randomised to receive one of four different types of ankle support: Tubigrip or plaster cast or Aircast splint or Bledsoe Boot |
| OPAL trial | Treatment | Uriinary incontinence | OPAL trial | Adult women newly referred with stress or mixed urinary incontinence. | This study compared the effectiveness and cost‐effectiveness of pelvic floor muscle training versus biofeedback‐mediated pelvic floor muscle training or women with stress urinary incontinence or mixed urinary incontinence. |
| Renfroe 2002 | Treatment | Cardiac patients | The AntiarrhythmicsvsImplantale Defibrillators (AVID) Trial | Patients with recent ventricular fibrillation, ventricular tachycardia with symptomatic with a left ventricular ejection fraction <40% were included in this study. | This was a multi‐centre randomised trial comparing survival in patients with malignant arrhythmias treated with antiarrhythmic drugs to survival in patients receiving implantable cardioverter‐defibrillators. |
| Rodgers 2019 | Prevention | Risk of falls | REducing Falls with ORthoses and a Multifaceted podiatry intervention (REFORM) study | Participants ≥65 years from routine podiatry clinics in the UK and Ireland to the REFORM cohort. Participants had one fall in the past 12 months: or one fall in the past 24 months requiring hospital attention were included in this study. | Podiatry intervention for the prevention of falls in older people. Participants were randomised to receive routine podiatry care, and a falls prevention leaflet or routine podiatry care, a falls prevention leaflet, and a multifaceted podiatry intervention. |
| Salvesen 1992 | Prevention | Pregnancy | Two randomised trials of routine ultrasonography in pregnancy | Pregnant women in the Trondheim area (Norway) were included in this study. | Participants were randomly selected for ultrasound examination at the 19th and 32nd weeks of pregnancy in addition to routine antenatal care. |
| Sarathy 2020 | Treatment | frozen shoulder | UK Frozen Shoulder Trial (UK FROST) | Patients aged ≥18 years with the primary frozen shoulder were recruited in hospitals and were included in this study. | Participants were randomised to either early structured physiotherapy including steroid injection, manipulation under anaesthesia, or arthroscopic capsular release with manipulation under anaesthesia. |
| Severi 2011 | Smoking Cessation | Smoking | Txt2stop | Participants ≥16 years who were daily smoker; willing to quit in the next month; owned a mobile phone and resident in the UK were included in this study. | Evaluate the effect of mobile phone‐based smoking cessation support on smoking rates at six months after enrolment. Intervention group They received five text messages a day for the first five weeks and then three a week for the next 26 weeks. Control group participants received fortnightly, simple, short; text messages related to the importance of trial participation. |
| Sharp 2006 | Prevention | Abnormal Cervical smears | Management of Borderline and Other Low‐grade Abnormal smears (TOMBOLA) trial. | Women aged 20‐59 with low‐grade abnormal cervical smear living in Tayside, Grampian or Nottingham were included in this study. | Management policy in the community linked to low grade abnormal cervical smears Participants in the intervention group was randomised to a colposcopy. In contrast, participants in the control group received a six‐monthly smear. |
| Starr 2015 | Treatment | ureteric stones |
Symptomatic ureteric stones in hospitalised adults (SUSPEND) trial |
Participants with ureteric colic aged 18–65 years with one stone of 10 mm or less (at the largest dimension) in either ureter identified on CT KUB were included in this study. | Participants were randomised to receive a self‐administered tamsulosin 400 μg OR nifedipine 30 mg or placebo. |
| Subar 2001 | Prevention | Cancer | The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial | Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer patients. Males and females aged 55‐74 years were included in this study. | Trial of screening procedures for prostate, lung, colon, and ovarian cancer. This trial measured whether screening with flexible sigmoidoscopy can reduce mortality from colorectal cancer. Also, it measured if screening with chest X‐ray can reduce mortality from lung cancer or, whether screening men with digital rectal examination plus serum prostate‐specific antigen can reduce mortality from prostate cancer. In women, it measured whether screening through transvaginal ultrasound can reduce mortality from ovarian cancer. |
| Tai 1997 | Treatment | Asthma and diabetes | No name provided for the Host trial | Patients with asthma and/or diabetes recruited from 6 general practices in London were included in this study. | Participants were randomised to Receive different structured computerised prompts to help the management of asthma and diabetes. |
| Tilbrook 2015 | Treatment | neck pain | Alexander Technique Lessons, Acupuncture Sessions (ATLAS) trial | Patients who have been diagnosed with neck pain in primary care, who have continued to experience neck pain for at least three months were included in this study. | Participants were randomised to Alexander Technique lessons, acupuncture, or usual care alone. |
| Tranberg 2018 | Diagnosis | Cancer | Cervical HOme‐based CancEr screening (CHOiCE) study | Women aged 30‐64 years who are living in the Central Denmark Region and who have not participated in cervical cancer screening after an invitation and one reminder were included in this study. | This was a randomised, controlled, effectiveness population‐based trial, nested in the Danish organised cervical cancer screening program conducted in the Central Denmark Region. The cervical cytology specimen was mailed to the local department of pathology for analysis. If no cervical cytology is registered, up to two reminders will be sent at 3 and 6 months after the initial invitation. |
| Treweek 2020A | Prevention | Cancer | ActWELL trial | Women were invited to take part in ActWELL when they attended a routine mammography appointment at one of four Scottish National Health Service Breast Screening. | This trial evaluates whether women who receive two, face‐to‐face lifestyle change sessions from volunteer coaches followed by up to nine telephone calls over a year, compared to no counselling, improve a range of lifestyle outcomes including weight and physical activity. |
| Watson 2017 | Treatment | haemorrhoids | either Traditional Hemorrhoidectomy or Stapled haemorrhoidopexy for haemorrhoidal disease (eTHoS) Study | Patients aged ≥ 18 years, with haemorrhoids of grades II–IV recruited in 32 UK NHS hospitals. were included in this study. | This study evaluated the clinical effectiveness of stapled haemorrhoidopexy compared to excisional (or traditional) haemorrhoidectomy in the treatment of II–IV‐grade haemorrhoids. |
| Whiteside 2019 | Prevention | Risk of falling | Occupational Therapist Intervention Study (OTIS) | Participants ≥65 years, community‐dwelling, currently able to walk 10 feet (with a walking aid if needed) were included in this study. | Participants were randomised to either home environmental assessment and modification, led by an occupational therapist or usual care from GP or other healthcare professional. |
| Young 2019 | Prevention | Cancer | Lung cancer screening trial (ECLS) | Participants ≥50 years who were current or former cigarette or tobacco smokers with at least 20 pack‐years, or with a history of smoking of fewer than 20 pack‐years plus immediate family history (mother, father, sibling, child) of lung cancer and living in Tayside or Greater Glasgow were included in this study. | Participants were randomised to receive a blood test for lung cancer + CT scanning if blood test positive or standard NHS care (i.e. person notices symptoms and attends GP). |
Data and analyses
Comparison 1. A ‐ Questionnaire Design: Short vs usual questionnaire.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Retention | 3 | 3252 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.08, 0.08] |
Comparison 2. A ‐ Questionnaire Design: Addition of diary to usual follow up vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Retention | 2 | 9906 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.04, ‐0.02] |
Comparison 3. A ‐ Questionnaire Design: Question order, condition first vs generic first questions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Retention | 2 | 9435 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
3.1. Analysis.

Comparison 3: A ‐ Questionnaire Design: Question order, condition first vs generic first questions, Outcome 1: Retention
Comparison 4. A ‐ Data Collection Frequency and Timing: Timing of questionnaire delivery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Retention | 1 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.01, 0.14] |
Comparison 5. A ‐ Data Collection Location and Method: Postal follow‐up vs clinic follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Retention | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.16 [‐0.08, 0.40] |
Comparison 6. A ‐ Data Collection Location and Method: Telephone follow‐up vs postal questionnaire.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Retention | 2 | 1006 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 6.2 Retention ‐ sensitivity analysis removing quasi‐RCTs | 1 | 672 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.04, 0.11] |
6.2. Analysis.

Comparison 6: A ‐ Data Collection Location and Method: Telephone follow‐up vs postal questionnaire, Outcome 2: Retention ‐ sensitivity analysis removing quasi‐RCTs
Comparison 7. A ‐ Data Collection Location and Method: First class vs second class outward mailing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Retention | 1 | 930 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
Comparison 8. A ‐ Data Collection Location and Method: Return postage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Retention | 3 | 1543 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.00, 0.09] |
| 8.1.1 Freepost vs second class stamp | 1 | 930 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| 8.1.2 High priority mail stamp vs usual postage | 1 | 281 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.13] |
| 8.1.3 Personal format mailing vs business format mailing | 1 | 332 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.02, 0.19] |
Comparison 9. A ‐ Data Collection Location and Method: Use of self‐sampling kits.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Retention | 1 | 19582 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.04, 0.13] |
| 9.1.1 Directly mailed self‐sampling kit vs invitation to order salf‐sampling kit | 1 | 6529 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.05, 0.09] |
| 9.1.2 Directly mailed self‐sampling kit vs usual follow up | 1 | 6527 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [0.11, 0.15] |
| 9.1.3 Invitation to order self‐sampling vs usual follow up | 1 | 6526 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.04, 0.08] |
Comparison 10. B ‐ Reminders: Electronic reminder vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Retention | 3 | 790 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
Comparison 11. B ‐ Reminders: Action oriented electronic reminder vs standard electronic reminder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Retention | 1 | 231 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.10, 0.03] |
Comparison 12. B ‐ Reminders: Personalised reminder vs non‐personalised reminder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Retention | 1 | 298 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.11, 0.08] |
Comparison 13. B ‐ Reminders: Telephone reminder vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Retention | 1 | 127 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.18, 0.15] |
Comparison 14. B ‐ Reminders: Telephone reminder vs postal reminder.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Retention | 1 | 148 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
Comparison 15. B ‐ Prompts: Electronic prompt vs no prompt.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Retention | 5 | 2897 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.06] |
Comparison 16. B ‐ Prompts: Telephone prompt vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Retention | 2 | 943 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
Comparison 17. B ‐ Prompts: Prenotification card vs no card.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Retention | 1 | 558 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.03, 0.10] |
Comparison 18. B ‐ Prompts: Sticker vs no sticker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Retention | 1 | 517 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.07, 0.10] |
Comparison 19. B ‐ Prompts: Personalised prompt vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Retention | 2 | 701 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
Comparison 20. B ‐ Prompts: Electronic prompts vs electronic reminders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Retention | 1 | 269 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.09] |
Comparison 21. B ‐ Monetary incentives: Addition of monetary incentives vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Retention | 3 | 3166 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.04, 0.11] |
| 21.2 Retention‐ sensitivity anlysis removing quasi‐RCTs | 2 | 1022 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.16] |
Comparison 22. B ‐ Monetary incentives: Addition of monetary incentives to all trial arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Retention | 1 | 200 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [‐0.03, 0.23] |
22.1. Analysis.

Comparison 22: B ‐ Monetary incentives: Addition of monetary incentives to all trial arms, Outcome 1: Retention
Comparison 23. B ‐ Monetary incentives: Addition of monetary incentives vs addition of monetary reward.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Retention | 4 | 3765 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.07, 0.06] |
Comparison 24. B‐ Monetary incentives: Addition of monetary reward vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Retention | 3 | 1159 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.03, 0.06] |
| 24.1.1 Return of postal questionnaires | 2 | 725 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.07] |
| 24.1.2 Attendance at follow up visits | 1 | 434 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 24.2 Retention ‐ sensitivity analysis removing quasi‐RCTs | 2 | 955 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 24.2.1 Return of postal questionnaires | 1 | 521 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.09, 0.06] |
| 24.2.2 Attendance at follow up visits | 1 | 434 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
Comparison 25. B ‐ Monetary incentives: Addition of monetary rewards to all trial arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Retention | 1 | 1018 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.15] |
Comparison 26. B ‐ Monetary incentives: Addition of monetary incentives vs lottery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 Retention | 1 | 281 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.09, 0.12] |
Comparison 27. B ‐ Monetary incentives: Lottery vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 27.1 Retention | 1 | 4206 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.02] |
Comparison 28. B ‐ Monetary incentives: Addition of lottery to both trial arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 28.1 Retention | 1 | 2758 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.01, 0.06] |
Comparison 29. B ‐ Non‐monetary incentives: Addition of pen vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 29.1 Retention | 5 | 13013 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.04] |
Comparison 30. B ‐ Non‐monetary incentives: Addition of societal benefit message vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 30.1 Retention | 1 | 1950 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.04, 0.04] |
Comparison 31. B ‐ Non‐monetary incentives: Certificate of appreciation vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 31.1 Retention | 1 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.03] |
Comparison 32. B ‐ Maintaining participant engagement: Newsletter vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 32.1 Retention | 4 | 5622 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.03] |
Comparison 33. B ‐ Maintaining participant engagement: Offer of receiving trial results vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 33.1 Retention | 1 | 1038 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.02] |
Comparison 34. B‐ Maintaining Participant Engagement: Cover letter including a social incentive vs standard cover letter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 34.1 Retention | 1 | 755 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
Comparison 35. B ‐ Maintaining participant engagement: Varying signatory on cover letter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 35.1 Retention | 1 | 479 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.10] |
Comparison 36. B ‐ Maintaining participant engagement: Addition of a deadline vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 36.1 Retention | 1 | 246 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.05, 0.12] |
Comparison 37. B ‐ Maintaining participant engagement: Addition of an estimate of time to complete vs no addition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 37.1 Retention | 1 | 1815 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
Comparison 38. B. Maintaining participant engagement: Brown vs white envelope.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 38.1 Retention | 1 | 1119 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
Comparison 39. B ‐ Maintaining participant engagement: Post‐it note vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 39.1 Retention | 3 | 4698 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 39.1.1 Printed post‐it note vs no post‐it note | 2 | 1165 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 39.1.2 Handwritten post‐it note vs no post‐it note | 2 | 1051 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 39.1.3 Printed post‐it note vs handwritten post‐it note | 1 | 546 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.03] |
| 39.1.4 Post‐it note vs no post‐it note | 3 | 1936 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
Comparison 40. B ‐ Maintaining participant engagement: Inclusion of trial newspaper article vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 40.1 Retention | 1 | 716 | Risk Difference (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.15] |
Comparison 41. B. Maintaining participant engagement: Frequency of telephone contact.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 41.1 Retention | 1 | 305 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.05, 0.17] |
Comparison 42. B ‐ Maintaining participant engagement: Request for collateral (concomitant).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 42.1 Retention | 1 | 408 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.01, 0.16] |
| 42.1.1 Request for a collateral vs no request | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | 0.12 [‐0.04, 0.28] |
| 42.1.2 Request for a collateral with 50% chance of use vs no request | 1 | 152 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.09, 0.21] |
| 42.1.3 Request for a collateral vs Request for a collateral with 50% chance of use | 1 | 156 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.07, 0.18] |
Comparison 43. B ‐ Behavioural interventions: Theory informed cover letter vs usual cover letter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 43.1 Retention | 4 | 3343 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
Comparison 44. B ‐ Behavioural interventions: Motivational interviewing vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 44.1 Retention | 1 | 128 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.17, 0.17] |
Comparison 45. C ‐ Prompts: Site prompts for upcoming assessments vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 45.1 Retention | 1 | Risk Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
Comparison 46. C ‐ Monitoring visits: On‐site monitoring vs no visits.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 46.1 Retention | 1 | 69 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.20, 0.10] |
Comparison 47. D ‐ Patient Public Involvement: Peer‐led follow‐up strategy vs usual follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 47.1 Retention | 1 | 632 | Risk Difference (M‐H, Fixed, 95% CI) | 0.22 [0.14, 0.30] |
Comparison 48. E ‐ Impact of recruitment: Video‐enhanced patient information vs standard information.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 48.1 Retention | 1 | 285 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.05, 0.12] |
Comparison 49. E ‐ Impact of recruitment: Optimised information vs standard information.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 49.1 Retention | 2 | 1285 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.13, 0.07] |
Comparison 50. E ‐ Impact of recruitment: Addition of optimised information to both arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 50.1 Retention | 1 | 131 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.07, 0.20] |
Comparison 51. E ‐ Impact of recruitment: Pen vs no pen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 51.1 Retention | 1 | 92 | Risk Difference (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.32] |
Comparison 52. E ‐ Blinding and treatment preference: Open vs blind trial design.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 52.1 Retention | 1 | 367 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMBER 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care settings. Not all participants in the host trial that took part in this embedded trial. Only those who had yet to complete the study at the time of the SWAT set up. Total n = 64, age NR; sex NR. |
|
| Comparisons |
Intervention group received a tested a theoretically informed letter sent with the questionnaire Control group received a standard letter |
|
| Outcomes | Questionnaires returned | |
| Notes | 6 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | No information of the nested RCT was provided. |
| Adequate sequence generation? | No | According to the authors, the randomisation list was generated and was not concealed. |
| Blinding of participants and personnel? | Unclear | Participants were unaware if they were receiving a standard or theory‐based cover letter but may have noticed from earlier letters that it had a different “tone” |
| Blinding of outcome assessment? | Unclear | No information of the nested RCT was provided. |
| Incomplete outcome data addressed? All outcomes | Unclear | No concerns raised. |
| Free of selective outcome reporting? | Unclear | No concerns raised. |
| Other sources of bias | No | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Arundel 2019.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, 21 NHS Trusts and 16 primary care settings. All host trial participants, as a source of the retention trial sample. Total n = 434; age NR; sex NR |
|
| Comparisons |
Intervention group were contacted by the research team to arrange their follow‐up appointment. They were advised of the potential of receiving £10 cash reliant on providing a carbon monoxide breath test in addition to the £10 gift voucher routinely provided all other pre‐planned retention strategies within host trial. Control group received a £10 gift voucher routinely provided and all other pre‐planned retention strategies within host trial. |
|
| Outcomes | Proportion of participants completing a test. | |
| Notes | Retention period: 6‐month follow‐up and the response time was two months after initial contact. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Participants were allocated with a 2:1 allocation ratio. |
| Adequate sequence generation? | Yes | An independent statistician carried out simple randomisation using random numbers. |
| Blinding of participants and personnel? | No | It was not possible to blind research staff to the participant's allocation. |
| Blinding of outcome assessment? | No | It was not possible to blind research staff to the participant's allocation. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | No | Unclear |
Ashby 2011.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, community setting. All host trial participants, as a source of the retention trial sample. Host trial participants who provided an e‐mail address or SMS number were included. Total n = 178; mean age 46.7 (SD 10.7) years; 87% females |
|
| Comparisons |
Intervention group received an SMS text message, e‐mail message, or both. Control group received no message. |
|
| Outcomes | Questionnaires returned (questionnaire response rate). | |
| Notes | Retention period: 1 month. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | An independent data manager was responsible for generating the allocation sequence and assigning participants into intervention and control groups. |
| Adequate sequence generation? | Unclear | Randomly‐generated numbers were used to list all participants by ID number. No clarification of how the numbers were generated. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | All data accounted for. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Avenell 2004.
| Study characteristics | ||
| Methods | RCT, individuals randomised | |
| Data | UK, secondary care setting. Participants were those approached about the host trial and willing to receive further information about taking part. Total n = 180; mean age 77 (SD 5) years; 82.8% females |
|
| Comparisons |
Intervention group received full information provided to eligible people, who were approached by a nurse and informed they would know their treatment allocation Control group received conventional trial methods, comprising randomised blinded, placebo‐controlled. |
|
| Outcomes | Participants remaining in the study (i.e. not withdrawn). | |
| Notes | Retention period: 12 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The study nurse then used a pre‐programmed laptop computer to generate random allocation to either the open‐trial design or the blinded, placebo‐controlled trial design in a 1: 2 ratio. |
| Adequate sequence generation? | Yes | Computer used to generate random allocation to either the open‐trial design or the blinded. |
| Blinding of participants and personnel? | Unclear | Participant unblinded but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Bailey 2013.
| Study characteristics | ||
| Methods | R 2x2 factorial RCT, individuals randomised | |
| Data | UK, community setting (online) Host trial respondents who agreed to be contacted. However, 15 were randomly selected from each of the 20 original US communities to participate. Total n= 1030; range age 18‐20 years; sex NR |
|
| Comparisons |
Interventions group 1 were allocated to a postal request for a urine sample for genital chlamydia testing and receipt for £10 shopping voucher Interventions group 2 Interventions group 1 were allocated to a postal request for a urine sample for genital chlamydia testing and receipt for £20 shopping voucher Control group no request for a sample nor vouchers |
|
| Outcomes | Completion of sexual health survey and return of kits | |
| Notes | Retetnion period: 3 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | At recruitment, all participants were randomised in a factorial (2x2) design to either the intervention or control website and to receive or not receive a urine sample kit for chlamydia testing at follow‐up. In addition, the final 902 participants were randomised after recruitment to a £10 or £20 voucher to complete follow‐up as requested. |
| Adequate sequence generation? | Yes | The first two randomisations were performed using an automated computer algorithm and the third was performed off‐site by random permutation of participant identifiers. Participants were automatically allocated by computer to control or intervention after submitting baseline data. |
| Blinding of participants and personnel? | Unclear | Not reported in the paper. |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Bauer 2004.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | US, multi‐centre community setting. Host trial respondents that agreed to be contacted. However, 15 were randomly selected from each of the 20 original US communities to participate. Total n = 300; range age 38‐77 years; 58.3% female; 65%; White non‐Hispanic; 41% reported 13‐15 years of education; 40% had an income >US$60,000; 60.3% were married. |
|
| Comparisons |
Interventions group 1 received an incentive of US$10 Interventions group 2 received an incentive of US$2 Control group received no incentive (US$0) |
|
| Outcomes | Return of DNA kits and questionnaire. | |
| Notes | Retention period: unclear. DNA kit and questionnaire to be returned within 24 hours of completing. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | No | Among host trial respondents that agreed to be contacted, 15 randomly selected from each of the 20 originals—no allocation concealment. |
| Adequate sequence generation? | No | Among host trial respondents that agreed to be contacted, 15 randomly selected from each of the 20 originals. No sequence was generated. |
| Blinding of participants and personnel? | Unclear | Not reported in the paper. |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. |
| Incomplete outcome data addressed? All outcomes | Yes | Clearly stated in the paper and missing values accounted for. |
| Free of selective outcome reporting? | No | Missing data. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | No | High |
Bean 2019.
| Study characteristics | ||
| Methods | Parallel RCT, Individuals randomised | |
| Data | US, primary care setting. Screened, eligible participants were randomised at telephone screening to participate in either retention intervention or the main host trial. Total n = 64; mean age 40.7 (SD 10.2); 90.6% females; 51.6% were Black or African American; 42.2% were White or Caucasian; 34.4% reported having some college degree, and 32.8% reported having a college degree; 21.9% had an income US$≥75,000. |
|
| Comparisons |
Intervention group received two brief pre‐treatment motivational interviewing. Control group received the host trial intervention or control, both with no motivational interviewing component |
|
| Outcomes | Enhance retention beyond baseline or to increase treatment attendance | |
| Notes | Retention period: baseline, post‐test, and 4‐months follow‐up assessment | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | Random number generator developed by the study biostatistician |
| Blinding of participants and personnel? | Yes | Trained, blinded staff measured parent and child height and weight using a stadiometer and digital bariatric scale, respectively. Trained, blinded ratters coded randomly selected 20‐minute segments of each session; all sessions were double rated by two independent ratters. |
| Blinding of outcome assessment? | Unclear | Trained, blinded ratters coded randomly selected 20‐minute segments of each session; all sessions were double rated by two independent ratters. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Bell 2016.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, primary care setting. All host trial participants, as a source of the retention trial sample. Host trial participants from 5 centres were followed up using postal questionnaires at the fifth year of follow‐up. Total n = 7,655; range age 70‐85 years; 100% females. |
|
| Comparisons |
Intervention group received trial‐branded pen with the 60‐month follow‐up questionnaire. Control group 60‐month follow‐up questionnaire alone. |
|
| Outcomes | Questionnaire return. | |
| Notes | Retention period: 60 months follow‐up. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | A computer‐randomisation package was used to allocate all eligible participants |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Bradshaw 2020.
| Study characteristics | ||
| Methods | Parallel factorial RCT, individuals randomised | |
| Data | UK, mainly secondary care centres with a smaller number of primary care centres. All host trial participants, as a source of the retention trial sample. Total n = 1394; age NR; sex NR |
|
| Comparisons |
Intervention groups 1 received an SMS message (text message) was sent the day before the e‐mail with the link to the questionnaire. Intervention groups 2 received a further £10 high‐street shopping voucher sent by post to parents at around 22 months before the 24‐month visit. Intervention groups 3 received a further £10 high‐street shopping voucher given at the visit. Control group received no SMS or voucher. |
|
| Outcomes | Collection of outcome data | |
| Notes | Retention periods: 3‐, 6‐, 12‐, 18‐ and 24‐months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Using an allocation schedule created by the Nottingham Clinical Trials Unit |
| Adequate sequence generation? | Yes | Research nurses randomised participants to the BEEP host trial by accessing an online system provided by the co‐ordinating centre. The second randomisation was then automatically performed for the SWAT to each of the retention strategies (1:1). |
| Blinding of participants and personnel? | No | Participants were informed in the host trial information sheet about the SWAT for SMS notification for questionnaires and timing of the voucher for the 24‐month visit but were not informed at the time of the randomisation of their allocated groups for the SWAT. Research nurses were not blinded |
| Blinding of outcome assessment? | Yes | The Trial Management Team and the research nurses were not blinded to the allocations for the SWAT. The sequence of allocations for the SWAT was concealed from the statisticians until the database was locked in the host trial. However, objective outcome, participants blind (did not know there was a study) staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | No | No |
Brubaker 2019.
| Study characteristics | ||
| Methods | RCT, individuals randomised. | |
| Data | US, multi‐centre setting. Participants who completed 24‐month follow‐up outcomes were included. Total n= 305, mean age 57.4 (SD 10.8) years; 100% females; 83.9% White. |
|
| Comparisons |
Intervention group received a standardised video followed by the usual consent process. Control group received the usual consent process alone. |
|
| Outcomes | Follow‐up data collection. | |
| Notes | Retention period: 36‐, 48‐ and 60‐months (a window of 3 months on each side of the visit was allowed for data collection). | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | The study co‐ordinator was not masked to the enrolment intervention |
| Adequate sequence generation? | Unclear | Not discussed in the paper. |
| Blinding of participants and personnel? | Unclear | Only mentions that the study co‐ordinator was not masked to the enrolment intervention. However, unclear for participants or other personnel. |
| Blinding of outcome assessment? | Yes | Evaluators of outcome assessments were masked to surgical, behavioural therapy with pelvic floor muscle training and enrolment process randomisations |
| Incomplete outcome data addressed? All outcomes | Yes | The detail provided on lost and withdrawn participants |
| Free of selective outcome reporting? | Yes | They present and describe additional outcomes as 'exploratory'. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Clark 2015.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, primary care setting. Those participants taking part in the host trial who provided a mobile phone number and/or an electronic mail address were randomised. Total n = 437; mean age 50.4 (SD 9.4) years; 46.2% females. |
|
| Comparisons |
Intervention group received an SMS or e‐mail to return the study questionnaire. Control group received the usual no electronic prompt to return the study questionnaire. |
|
| Outcomes | Questionnaires returned. | |
| Notes | Retention period: 2‐6 months (depending on site) after randomisation. Response period was up to 2 months after the follow‐up questionnaire was sent. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Participants were securely randomised to either receiving an electronic prompt or not by the data manager at the York Trials Unit. Simple randomisation between the two groups was undertaken without any blocking or stratification. |
| Adequate sequence generation? | Unclear | The method used to randomise participants is not stated in the paper. There is an imbalance between the numbers receiving e‐mails and SMS text messages see consort diagram. |
| Blinding of participants and personnel? | Yes | The data manager was unaware of any baseline characteristics of participants before randomisation |
| Blinding of outcome assessment? | Yes | Not discussed in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | Everything accounted for. |
| Free of selective outcome reporting? | Yes | Author report on all expected outcomes stated in the aims of the paper. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Cochrane 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, community setting. Host trial participants who agreed to receive text communication during participation, provided a mobile number, and were due to receive their four‐month post‐randomisation postal questionnaire were included. Total n = 283; mean age 77.3 (SD 5.9) years; 64% female. |
|
| Comparisons |
Intervention group1 received a personalised text message four days after their four‐month questionnaire was posted. Intervention group 2 received the usual standard text alone. |
|
| Outcomes | Questionnaires returned. | |
| Notes | Retention period: 4‐months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Allocations were generated by the host trial statistician, before being shared with the data management staff responsible for the setup of the text messaging system. |
| Adequate sequence generation? | Yes | Randomised (1:1) using randomly varying blocks of four and six, stratified by host trial group allocation. |
| Blinding of participants and personnel? | Yes | Participants were not aware of their involvement within this SWAT, only to the OTIS trial group allocation. But data entry staff were blinded. |
| Blinding of outcome assessment? | Yes | Objective outcome, participants (do not know there is a study) data entry staff blind, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Cockayne 2005.
| Study characteristics | ||
| Methods | Parallel RCT, clusters randomised | |
| Data | UK, community setting. Participants who were due to be sent a final follow‐up questionnaire as part of the host trial. Total n = 1038; mean age 76.4 (SD 4.6); 100% females. |
|
| Comparisons |
Intervention group participants were offered the results of the trial in a postal questionnaire. Control group participants did not receive the offer of knowing the results of the trial. |
|
| Outcomes | Return of final follow‐up questionnaire. | |
| Notes | Retention period: Three weeks. Those participants nor returning questionnaires within three weeks were sent up to two reminder letters, questionnaires and business reply envelopes, three and six weeks after the initial mailing. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | An independent researcher from the York Trials Unit randomised eligible women in a 3:1 ratio in favour of offering the results of the trial, by computer |
| Blinding of participants and personnel? | Yes | Not clear if participants were blinded to effect of embedded trial intervention on retention but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Statistical analysis was not undertaken blind to group allocation. However, objective outcome, participants blind (did not know there is a study) staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Cockayne 2017.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK and Ireland, in either primary or secondary care settings. Participants who could be randomised as there was sufficient capacity in the clinics to see them. Total n = 193; mean age 78.1 (6.8) years; 56.5% females. |
|
| Comparisons |
Intervention group 1 received an optimised version of the participant information sheet and invitation letter developed through bespoke user testing. Intervention group 2 received an optimised template‐developed participant information sheet and the original invitation letter. Control group received the control participant information sheet for the host trial and control invitation letter. |
|
| Outcomes | Proportion of participants retained in the trial post‐randomisation | |
| Notes | Retention period: 3 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Participants were then sent the allocated invitation pack by members of the research team based at the University of York. |
| Adequate sequence generation? | Yes | An independent data manager, who was not involved in the recruitment of participants, generated the allocation sequence for the embedded methodology trial electronically |
| Blinding of participants and personnel? | Yes | The researchers, participants and podiatrists were blind to the allocation. |
| Blinding of outcome assessment? | Yes | The researchers, participants and podiatrists were blind to the allocation. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Cook 2020.
| Study characteristics | ||
| Methods | Parallel RCT, cluster randomisation | |
| Data | UK, primary care setting. Host trial was a multinational trial. Only UK sites were included. 42 out of the 43 participant GP surgeries were included. Total n = 345; median age of intervention group 26 (IQR 12,46) years and control group median 36 (IQR 23,53) years; 60.4% females. |
|
| Comparisons |
Intervention group received a £20 gift voucher given to study at the end of the recruitment visit. Control group were informed that a £20 gift voucher would be given upon the return of the trial symptom diary, and voucher then sent to those who returned a diary by the trial team. |
|
| Outcomes | Proportion of participants returning a symptom diary. | |
| Notes | Retention period: 14 days. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not discussed in the paper. |
| Adequate sequence generation? | Yes | The trial sites were the unit of randomisation. The sites were cluster‐randomised to keep the process as straight forward as possible for the recruiters. Randomisation was performed in two waves (before the start of seasons 2 and 3) using computer‐generated random numbers carried by one of the investigators. |
| Blinding of participants and personnel? | Yes | GP practices were not blinded to their allocation due to the necessity of them either distributing the incentives initially or not. Participants were unaware that there was a SWAT taking place as it was thought this might influence whether or not they returned their diary.Unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not discussed in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | Whether the Symptom diary was returned, when it was received and how many pages were completed. There was no imputation of outcome data for sites where no‐one was recruited, or where a participant did not return their Symptom diary. |
| Free of selective outcome reporting? | Yes | All data reported for pre‐specified objectives. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Couper 2007.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | US, community setting (online intervention). A sample of host trial participants that did not return their 12‐month questionnaire (from the 3 US regions) who approved the follow‐up study. Sampling involved a subset of participants who did not return any questionnaires as this was the largest group. Total n = 700; age NR; sex NR. |
|
| Comparisons |
Intervention group received a telephone follow‐up. Control group received mail follow‐up a questionnaire with $US5 incentive enclosed. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 3‐, 6‐ and 12‐months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not discussed in the paper. |
| Adequate sequence generation? | Unclear | Three hundred of the nonrespondents being randomly assigned to telephone and 400, to mail. The disproportionate allocation reflects the cost differential |
| Blinding of participants and personnel? | Unclear | Not discussed in the paper. Unblinded staff may have potential for influence due to phone call. |
| Blinding of outcome assessment? | Yes | Not discussed in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | Reports reasons why not all analysed in each arm (duplicates and those subsequently found to be ineligible). |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Cunningham 2004.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | Canada, community setting. Participants who provided address to participate in the 6‐month follow‐up. Total n = 204, age NR, sex NR. |
|
| Comparisons |
Intervention group1 were asked to provide a collateral. Intervention group2 asked to provide collateral and told that there was a 50% chance that the collateral would be contacted. All those respondents asked for collateral were told that the collateral would receive a CAN$20 payment for a brief telephone interview. Control group was not asked to provide a collateral. |
|
| Outcomes | Proportion of respondents who returned the survey at follow‐up. | |
| Notes | Retention period: 6 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not discussed in the paper. |
| Adequate sequence generation? | Unclear | Not discussed in the paper. |
| Blinding of participants and personnel? | Unclear | Not discussed in the paper. |
| Blinding of outcome assessment? | Unclear | Not discussed in the paper. |
| Incomplete outcome data addressed? All outcomes | Yes | Outcomes seem to be clearly reported. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Cunningham‐Burley 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care setting. Participants in the host trial who were due to be sent their 14‐week postal questionnaire. Total n = 1466, mean age 43.0 (SD 11.3) years, sex 86.1%. |
|
| Comparisons |
Intervention group received a branded pen with their questionnaire. Control group did not receive a pen. |
|
| Outcomes | Proportion of participants who return the questionnaire. | |
| Notes | Retention period: 14‐week questionnaire | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not discussed in the paper |
| Adequate sequence generation? | Yes | Participants were allocated to either the intervention (pen) or control (no pen) group using simple randomisation in a 1:1 ratio. The allocation sequence was generated by the host trial statistician, who was not involved in sending out the questionnaires. |
| Blinding of participants and personnel? | Yes | Participants were not aware of their involvement in this SWAT, but due to the nature of the intervention participants and study team members could not be blinded to group allocation.Unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not discussed in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Dinglas 2015.
| Study characteristics | ||
| Methods | Two RCTs, individuals randomised. | |
| Data | US, secondary care setting. 1) Trial 1 (mail trial): participants who had been enrolled before the introduction of this survey were sequentially randomised. Total n = 332; mean age 48.8 (SD 15.0) years; 52% females; 20% of ethnic minorities (i.e. African American, Asian, American Indian and Alaskan Native), 24% unemployed; 46% retired or disabled. 2) Trial 2 (Phone trial): non‐responders from the prior mail and those excluded from mail trial due to lack of a correct mailing address were eligible. Total n = 171; mean age 46.0 (SD 14.7) years; 51% females; 26% of ethnic minorities (i.e. African American, Asian, American Indian and Alaskan Native), 26% unemployed; 43% retired or disabled. |
|
| Comparisons | 1) Trial 1 (Mail trial) participants were randomised to mailed letters every two weeks until the survey was completed. Intervention group 1 received a "personal format letter" in which their mailing address and the return address were handwritten, and a traditional stamp was stamped using the envelope. Intervention group 2 received a "business format letter" in which the addresses were typed, and a commercial stamp‐machine affixed the postage. 2) Trial 2 (Phone trial): started 20 days after the end of the mail trial. These telephone calls were made once weekly by the same caller, for up to 4 weeks, until the participant was reached by telephone or the participant called back and completed the survey. Intervention group receive a personalised voice message. Control group received generic voice message. |
|
| Outcomes | Participant to complete the insurance survey. | |
| Notes | Retention period: 1) Trial 1 (mail trial): unclear. However, every two weeks until the survey was completed, or the participant was sent a total of 4 mailings. Trial 2 (Phone trial): unclear. However, once weekly by the same caller, for up to 4 weeks |
|
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not discussed in the paper. |
| Adequate sequence generation? | Yes | In both the mail and the phone trials, randomisation was performed by a statistician using computer‐generated random numbers with an allocation ratio of 1:1. |
| Blinding of participants and personnel? | Yes | Given the nature of this study design, outcome assessment was not blinded, but participants were blinded. Unclear about the personnel. unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Given the nature of this study design, outcome assessment was not blinded. However, objective outcome, participants blinded (did not know there was a study) telephone contact was only those participants who received the scripted message, not those who were spoken to, staff have no/very limited plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Dorling 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK and Ireland Republic, secondary care. Participants from the host trial who were due to be sent a questionnaire at the age of 24 months. Total n= 799; mean age 30.9 (SD 6.2) years; 100% females. |
|
| Comparisons |
Intervention group 1 received the first paper letter to parents included a promise of an incentive (£15 gift voucher redeemable at some shops) after receipt of a completed form. Intervention group 2 received the first paper letter to parents would enclose the incentive (£15 gift voucher redeemable at high‐street shops) before the receipt of a completed form. |
|
| Outcomes | Rate of questionnaire return. | |
| Notes | Retention period: 24 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Infants from multiple births were allocated to the same incentive group. Vouchers were allocated per questionnaire, so parents of multiple births received a voucher for each infant. |
| Adequate sequence generation? | Yes | Participants were randomised in a 1:1 allocation ratio by permuted block randomisation (using variable block sizes) and stratified by original SIFT allocation. |
| Blinding of participants and personnel? | Yes | The SIFT office staff at the NPEU CTU were aware of participant allocation owing to the nature of the interventions and the practicalities involved in sending out the letters and the vouchers. Not clear if participants were blinded to effect of embedded trial intervention on retention but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Dorman 1997.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, secondary care setting. Multinational host trial. Only UK sites took part in this trial. Participants who had been entered by UK centres who were not known to be dead. Total n = 2253, age NR, sex NR. |
|
| Comparisons |
Intervention group 1 received a short questionnaire: EuroQol questionnaire (six separate questions and a visual analogue scale) sent by post. Intervention group 2 received a long questionnaire: SF36 (34 separate questions) sent by post. |
|
| Outcomes | Questionnaire response rate | |
| Notes | Retention period: unclear. Follow‐up time point not specified. Authors mention as quote:"response to first mailing or response after two mailings". | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not discussed in the paper. |
| Adequate sequence generation? | Yes | Authors response "generated by a computer". |
| Blinding of participants and personnel? | Yes | Authors reported, quote: "there was no blinding for either study staff or participants." Unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Authors reported, quote: "there was no blinding for either study staff or participants". No clarification on outcome assessment. However, objective outcome, participants blind (did not know there was a study) staff had no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | Everyone is included in the denominator as well as a compilation of data |
| Free of selective outcome reporting? | Yes | All defined outcomes reported |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Edwards 2004.
| Study characteristics | ||
| Methods | RCT, unclear if individuals or clusters randomised. | |
| Data | UK, setting unclear. Data retrieved from a review. Data on included participants not available. Total n = 99, age NR; sex NR |
|
| Comparisons |
Intervention group received a one‐page questionnaire that contained seven questions. Intervention group 2 received three‐page questionnaire contained 16 questions with space provided for comments. |
|
| Outcomes | Questionnaire return. | |
| Notes | Retention period: Unclear. The publication says that reminders sent after 1‐ and after 2‐ months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Random allocation: central computer |
| Adequate sequence generation? | Unclear | No information provided. |
| Blinding of participants and personnel? | Unclear | No information provided. |
| Blinding of outcome assessment? | Unclear | No information provided. |
| Incomplete outcome data addressed? All outcomes | Unclear | No information provided. |
| Free of selective outcome reporting? | Unclear | No information provided. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Edwards 2016.
| Study characteristics | ||
| Methods | Trial 1: Quasi randomised, Trial 2 and 3 parallel RCTs | |
| Data | UK, community setting Trial 1 was introduced six months into the trial, so did not include all host trial participants. Total n = 190, mean age 50.1 (SD 13.5) years; 70% females. Trial 2 included participants from the Bristol study centre who were due to complete the 12‐month. Total n = 251, mean age 49.6 (SD 13.3) years; 69.7% females Trial 3 involved 'approx. half' of participants at Sheffield and Southampton. Total n = 231, mean age 49.2 (SD 11.8) years; 64% females |
|
| Comparisons | This reference contains three trials within: Trial 1. Advance notification through pre‐calling trial Intervention group received an advance notification telephone call (i.e. a pre‐call) from a researcher one to three days ahead of being sent the questionnaire. Control group received e‐mailed or were posted the questionnaire without a telephone call. Trial 2. Research team study photo Intervention group to receive a cover letter with a colour photo of the Bristol research team. Control group to receive the standard, black and white cover letter without a photo. Trial 3. Action‐oriented e‐mail reminder subject line Intervention group receive either the intervention reminder e‐mail subject line. Control group the standard reminder e‐mail subject line. |
|
| Outcomes | Follow‐up questionnaire response | |
| Notes | Retention period: Trial 1: 8‐month follow‐up Trial 2 and 3: 12‐month follow‐up. |
|
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Trial 1: No Trial 2 and 3: Yes For Trial 1 it is possible to foresee who will get what. |
| Adequate sequence generation? | Unclear | Trial 1: No Trial 2 and 3: Yes Trial 1 is unclear because they ordered host trial randomisation date and for SWAT just alternated host trial participants to SWAT int or control. It depends on what the randomisation system used in the host trial is doing re—minimisation, stratification. Plausible I think that initial randomisation in host trial could be a bit different, and perhaps something different about people who sign up at the start of the trial, or potential at sites before others. |
| Blinding of participants and personnel? | Unclear | Trial 1: unclear Trial 2 and 3: Yes Not clear if the researcher making phone call knew what the SWAT trial was about, which could influence what was said, and therefore what was done by participant. |
| Blinding of outcome assessment? | Yes | Trial 1‐3: Yes. No concerns raised in any of the studies. |
| Incomplete outcome data addressed? All outcomes | Yes | Trial 1‐3: Yes. No concerns raised in any of the studies. |
| Free of selective outcome reporting? | Yes | Trial 1‐3: Yes. No concerns raised in any of the studies. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Fouad 2014.
| Study characteristics | ||
| Methods | RCT, cluster randomised | |
| Data | US, community setting. Participants from the host trial residing two low‐income communities in Jefferson County, Alabama, matched according to population demographics. Total n = 632; mean age 27.1 (SD NR); 100% females; 90.8% Afro American; 53.0% with Highschool degree or less. |
|
| Comparisons |
Intervention group received Community Health Advisor supported retention activities (four types of communication and support), in addition to the reminder calls, cards, and retention incentives offered by the ALTS trial (US$20 at each visit and US$100 and a gift bag at final visit). Control group received only the reminder calls, cards, and retention incentives offered by the ALTS trial. |
|
| Outcomes | Retention in trial for four follow‐up visits | |
| Notes | Retention period: Four follow‐up visits (time unclear). | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | No details provided in the paper. |
| Adequate sequence generation? | Unclear | No details provided in the paper. |
| Blinding of participants and personnel? | Unclear | No details provided in the paper. |
| Blinding of outcome assessment? | Unclear | No details provided in the paper. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Gates 2009.
| Study characteristics | ||
| Methods | Quasi‐randomisation, individuals randomised | |
| Data | UK, secondary care setting. The sample included all host trial participants were being sent a follow‐up questionnaire at 4 months or 8 months. Total n = 2144; mean age 36.9 (SD 13.3) years; 56% females |
|
| Comparisons |
Intervention group received a £5 gift voucher, redeemable at a range of shops with their questionnaire, and a covering letter including a sentence explaining that the voucher is to thank participants for their time and effort. Control group received no gift voucher, and a standard covering letter |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 4 and 8 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | No | There was lack of concealment of allocations before randomisation. |
| Adequate sequence generation? | No | Allocation to trial arms was according to whether a specific digit of the participant's trial number was odd or even. |
| Blinding of participants and personnel? | Yes | Trial office staff were unblinded. Unclear about other personnel or participants. Not clear if participants were blinded to effect of embedded trial intervention on retention but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Trial office staff were unblinded. However, objective outcome, participants blind (did not know there is a study) staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | No | There is a difference between the CONSORT diagram and Table 2 regarding the number of non‐responders. |
| Free of selective outcome reporting? | Unclear | Concerns raised due to inconsistency of outcome reporting (see above). |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | No | Low |
Gattellari 2004.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | Australia, primary care setting. All host trial participants lost to follow‐up, as the source of the retention trial sample. Total n = 246; mean age NR, 0% females |
|
| Comparisons |
Intervention group received a cover letter with their follow‐up questionnaire that advised them to return their questionnaire within 1 week—a reminder phone call scheduled 11 days after letter 18 days after initial mail.The not of the follow‐up questionnaire, and an extra remainder Control group received standard covering letter with no deadline. |
|
| Outcomes | Proportion of participants who returned the follow‐up questionnaire. | |
| Notes | Retention period: unclear. Participants were asked to 1 week + 18 days (non‐responders were scheduled to receive a reminder letter 18 days after the initial mailout). | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Information about randomisation was sealed in sequentially‐ordered opaque envelopes. |
| Adequate sequence generation? | Yes | The randomisation sequence was generated by a computer program using block randomisation. Block size was varied to obscure randomisation sequence. |
| Blinding of participants and personnel? | Yes | No details provided in the paper. Unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | No details provided in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | No details provided in the paper. |
| Free of selective outcome reporting? | Unclear | No details provided in the paper. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Glassman 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | USA, secondary care setting. All participants from the host trial. Total n = 305; median age from intervention group 53 (44–60) years, and in the control group 51 (45–59) years; 44.2% females; 52.4% Non‐Hispanic White; 25.5% Hispanic or Latino; 15% Non‐Hispanic Black or African American. |
|
| Comparisons |
Intervention group received telephone calls at baseline, six months, and at annual visits after that (annual contact). Control group received a call at baseline only (baseline contact). |
|
| Outcomes | Visit completion rates. | |
| Notes | Retention period: 24‐,36‐, 48‐ and 60‐months visits | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not described in the paper |
| Adequate sequence generation? | Yes | Randomisation was stratified by treatment group and performed using study website using computer‐generated random numbers |
| Blinding of participants and personnel? | Unclear | Not described in the paper and telephone calls from staff may have potential to unblind participants or affect outcome. |
| Blinding of outcome assessment? | Yes | Not described in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | The trial clearly reported why participants randomised where not included in the main analysis – withdrawn with reasons and death but this is at 5 years only |
| Free of selective outcome reporting? | Yes | All defined outcomes reported |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Goulao 2020.
| Study characteristics | ||
| Methods | Parallel RCTs, individual randomisation. | |
| Data | UK, primary care setting (dental practices). All participants from both host trials. Trial 1‐3: Total n = 1877; host trial participants were on average, 48 (SD 16) years, 65% females. |
|
| Comparisons | Trial 1: Intervention group received a logo sticker on questionnaire envelopes Control group received no sticker Trial 2: Intervention group received a tested theoretically informed letter sent with the questionnaire Control group received a standard letter Trial 3: Intervention group received a tested theoretically informed newsletter sent before the questionnaire Control group received no newsletter |
|
| Outcomes | Questionnaire return | |
| Notes | Retention periods: Trial 1: 12 months Trial 2: 12 months or 24 months Trial 3: unclear |
|
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Trial 1: computer‐generated by an independent statistician Trial 2 and 3: the centralised computerised system automatically through simple randomisation |
| Adequate sequence generation? | Yes | Trial 1: simple randomisation via an automated, central randomisation service in a 1:1 participant randomised 2‐arm parallel trial. Trial 2: were randomised via an automated, central randomisation service in a 1:1 participant randomised 2‐arm parallel trial Trial 3: were randomised via an automated, central randomisation service in a 1:1 participant |
| Blinding of participants and personnel? | Yes | Not described in the paper. Unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not described in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Goulao 2020 (replication of SWAT #2).
| Study characteristics | ||
| Methods | Parallel RCTs, individual randomisation. | |
| Data | UK, primary care setting (dental practices). All participants from both host trials. Total n = 2372; participants on average 48 (SD 15) years, 60% were female. |
|
| Comparisons |
Intervention group received a tested theoretically informed letter sent with the questionnaire. Control group received a standard letter. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention periods: replication trial: 24 months follow‐up | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported. |
| Adequate sequence generation? | Unclear | Not reported |
| Blinding of participants and personnel? | Yes | Not described in the paper. Unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not described in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Greig 2017.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care. All host trial participants were included in this trial. Total n = 60; mean age 5.8 (SD 3.5); 48.3% females. |
|
| Comparisons |
Intervention group 1 had a postal follow‐up. Intervention group 2 had a clinic follow‐up. |
|
| Outcomes | Participants with a valid response. | |
| Notes | Retention period: 4 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Not reported in the paper. |
| Blinding of participants and personnel? | Yes | Not clear if participants were blinded to effect of embedded trial intervention on retention but unblinding not a likely impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Griffin 2019.
| Study characteristics | ||
| Methods | RCT, individuals randomised | |
| Data | UK, primary care setting. All host trials participants included. Total n = 9375; mean age 77.9 (SD 5.8) years; 52.4% females; 61% married. |
|
| Comparisons | Participants were randomised allocated participants to receive prospective monthly falls diaries for one simultaneous 4‐month period: · Intervention group 1 received falls diaries from randomisation to 4 months follow‐up. · Intervention group 2 received falls diaries from 5 and 8 months. · Intervention group 3 received falls diaries from or between 9 and 12 months Furthermore, all trial participants received a postal questionnaire at baseline and at 4‐, 8‐, 12‐, and 18‐months post‐randomisation to evaluate data retrospectively. |
|
| Outcomes | Number of participants who provided falls data on a full set of diaries and on the questionnaire for the corresponding period | |
| Notes | Retention period: up to 4 months. Data also available for 5‐8 months and 9‐12 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | This nested trial design used a separate randomisation strategy to allocate trial participants. However, allocation concealment unclear. |
| Adequate sequence generation? | Unclear | This nested trial mentions that the design used a different randomisation strategy to allocate trial participants. However, not reported the sequence generation. The number of people in the control arm was higher compared at the intervention arm. |
| Blinding of participants and personnel? | Yes | Not reported in the paper.Unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Guarino 2006.
| Study characteristics | ||
| Methods | Parallel RCT, cluster randomised | |
| Data | USA, secondary care setting. All host trial participants included. Total n = 1092; mean age 40.6 (SD 8.7) years; 14.8% females; 53.8% white non‐Hispanic; 24.3% were Black, non‐Hispanic; Mean education years 14.0 (SD 1.9) years; 81% were employed. |
|
| Comparisons |
Intervention group received a consent form that was revised by a consumer focus group. The changes involved revising treatment and eligibility descriptions, specifying participants would receive renumeration for three follow‐up visits but not treatment session, eliminating the enumeration for the risks of exercise. Control group received the original consent form with no modifications |
|
| Outcomes | Attendance at follow‐up visit/ collection of primary outcomes. | |
| Notes | Retention period: 3‐, 6‐ and 12‐months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | Participating centres were randomised to either the participant‐ or investigator developed consent document in a 1:1 ratio. |
| Blinding of participants and personnel? | Yes | IRB and sites were only shown the consent form they were randomised to. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | It states missing data were excluded and not imputed, but it does not expand on this or highlight where there were missing data. |
| Free of selective outcome reporting? | Unclear | There is a lack of clarity around the definitions of 'retention' Adherence. At times this refers to visit attendance at other times primary outcome data |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Hardy 2016.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, secondary care setting. Participants from the host trial who consented 1‐year follow‐up. Total n = 1018; mean age 29.1 (SD 5.5) years; 100% women; 17.9% were firm the most deprived SES category and 13.3% were from the least deprived SES category; 83% were White. |
|
| Comparisons |
Intervention group 1 received an incentive cover letter sent with the first mailout of the questionnaire containing details of a promise of a £10 gift voucher (redeemable at some shops) on the return of a completed questionnaire. The covering letter included a sentence explaining that the voucher was to thank participants for their time and effort. All reminder letters included a sentence about the incentive. Intervention group 2 received a cover letter sent at first mailout did not mention the incentive. If the questionnaire was not returned, all reminder letters detailed the promise of a £10 gift voucher on the return of a completed questionnaire. |
|
| Outcomes | Return of questionnaires | |
| Notes | Retention period: 12‐months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The allocation was by computer random number generation and stratified by host trial allocation and by the centre. |
| Adequate sequence generation? | Yes | The randomisation schedule was generated by the National Perinatal Epidemiology Unit Clinical Trials Unit and sent to the host trial office at the Comprehensive Clinical Trials Unit at University College London via a secure web‐link. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Henderson 2010.
| Study characteristics | ||
| Methods | Parallel RCT, clusters randomised | |
| Data | UK. Schools and then community setting. All host trial participants, as a source of the retention trial sample. Total n = 4134; range age 13‐20 years old; Sex NR. |
|
| Comparisons |
Intervention group 1 had a chance of winning 1 of 25 £20 shopping vouchers. Intervention group 2 had a chance of winning one £500 shopping voucher. Control group received no incentive. |
|
| Outcomes | Increased response rate. | |
| Notes | Retention period: unclear. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Just mentions quote: "randomly assigned groups clustered" by school" |
| Blinding of participants and personnel? | Yes | Mostly handled through post/web and asking for an interview required the participant to do something before anyone could influence the decision to ask for an interview. |
| Blinding of outcome assessment? | Yes | No concerns raised. |
| Incomplete outcome data addressed? All outcomes | Yes | For Wave 3 considered in this review. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
James 2020.
| Study characteristics | ||
| Methods | 2x2 factorial RCT, individuals randomised. | |
| Data | UK, community setting. Participants from the host trial who were due to be sent a questionnaire at 12 months. Total n = 779; mean age 79.7 (SD 6.2) years; 63.9% females. |
|
| Comparisons |
Intervention group 1 received a branded pen and a standard cover letter. Intervention group 2 received a branded pen and a social incentive cover letter. Intervention group 3 received no pen and a social incentive cover letter. Control group received no pen, standard cover letter. All participants received an unconditional £5 note with the questionnaire. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 12 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The allocation sequence was generated by the host trial statistician, who was not involved with the sending of the questionnaires, |
| Adequate sequence generation? | Yes | The participants were randomised in a single block in a 1:1:1:1 ratio |
| Blinding of participants and personnel? | Yes | Participants were blind to their participation. Research administrators and research team members posting the questionnaire packs were not blind to the intervention; Not clear if participants were blinded to effect of embedded trial intervention on retention but unblinding likely not impact objective outcome. |
| Blinding of outcome assessment? | Yes | However, administrators who recorded the outcome data were blind to allocation |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Keding 2016.
| Study characteristics | ||
| Methods | Factorial RCT, individuals randomised. | |
| Data | UK, primary care setting. All host trial participants who consented to SMS notifications. Total n = 523; mean age 41.0 (SD 11.5) years; 76% females; 41% were full time workers. |
|
| Comparisons | Three sequential trials: Trial 1 (3‐month follow‐up): Intervention group received pre‐notification text. Control group received no text. Trial 2 (6‐month follow‐up): Intervention group received a pre‐notification text Control group received a post notification text Trial 3 (9‐month follow‐up): Intervention group received a post notification reminder text Control group did not receive a text |
|
| Outcomes | Proportion of participants who returned a valid questionnaire to the trial team. | |
| Notes | Retention period: 3‐, 6‐ and 9‐months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Not reported in the paper. |
| Blinding of participants and personnel? | Yes | independent randomisation and that participants were blind to the trial hypothesis. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Kenton 2007.
| Study characteristics | ||
| Methods | Factorial RCT, individuals randomised | |
| Data | Canada, community setting. All host trial participants in the intervention arm as a source of the retention trial sample. Total n = 281; age NR; sex NR. |
|
| Comparisons | Participants were randomised to: a) Receiving a monetary incentive alone (CAN$2 coin mailed with the questionnaire). b) Receiving a monetary incentive and 'high priority' stamp to the mailing envelope. c) Receiving lottery alone (draw for a CAN$50 gift certificate upon questionnaire receipt). Or a lottery and 'high priority' stamp to the mailing envelope. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: unclear | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the abstract. |
| Adequate sequence generation? | Unclear | Not reported in the abstract. |
| Blinding of participants and personnel? | Yes | Not reported in the abstract but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the abstract. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the abstract. |
| Free of selective outcome reporting? | Unclear | Not reported in the abstract. |
| Other sources of bias | Unclear | Abstract poorly reported. |
| Overall Risk of Bias | Unclear | Unclear |
Kenyon 2005.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care setting. All host trial participants (parents) who consented to the follow‐up study but did not return the questionnaire. Total n = 722; age NR; sex NR |
|
| Comparisons |
Intervention group received a £5 voucher (redeemable at some shops) with their mailed questionnaire, Control group received no voucher. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 84 months after the original trial. The retention time was six weeks after an initial questionnaire sent in the follow‐up study. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | Randomly assigned by computer |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Khadjesari 2011.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care setting. All host trial participants (parents) who consented to the follow‐up study but did not return the questionnaire. Total n = 722; age NR; sex NR |
|
| Comparisons |
Intervention group received a £5 voucher (redeemable at some shops) with their mailed questionnaire, Control group received no voucher. |
|
| Outcomes | Questionnaire return. | |
| Notes | Retention period: 84 months after the original trial. The retention time was six weeks after an initial questionnaire sent in the follow‐up study. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | Randomly assigned by computer |
| Blinding of participants and personnel? | Unclear | Not reported in the paper. |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. |
| Incomplete outcome data addressed? All outcomes | Unclear | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Land 2007.
| Study characteristics | ||
| Methods | Parallel RCT, cluster randomised. | |
| Data | USA, multi‐centre cancer clinical trials. Not clear which participants were included in the nested RCT. Total n = 3104; 41.7% of the participants had between 50‐59 years old; 100% females; and 87.7% were White. |
|
| Comparisons |
Intervention group received a monthly reminder to sites listing participants who were due to have a measure in the next three months. Control group received no reminder. |
|
| Outcomes | The receipt of an expected form for a given institution, participant, and assessment time point. A form was considered expected if the participant had survived past the scheduled time point. | |
| Notes | Retention period: unclear | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Not reported in the paper. |
| Blinding of participants and personnel? | Yes | Protocol reported as "double‐blind" |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. Site assessed objective outcome |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Lewis 2017.
| Study characteristics | ||
| Methods | RCT, individuals randomised. | |
| Data | UK, primary care setting. All host trial participants, as a source of the retention trial sample. Participants who were due to be sent a follow‐up questionnaire for the host trial were included. Total n = 611; mean age 74.0 (SD 6.5) years; 59.5% female. |
|
| Comparisons |
Intervention group received a questionnaire with a printed Post‐it® note. Control group questionnaires without a note. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 4 months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The personnel who added the Post‐it® notes to questionnaires were different to those who had participant contact to ensure allocation concealment. |
| Adequate sequence generation? | Yes | Participant allocation was carried out by simple computerised randomisation using an SQL function through the trial management database by the York Trials Unit. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Participants who reached four months follow‐up before commencement recruitment, as well as participants who asked to be withdrawn from the CASPER trials or did not want to receive a questionnaire at this time point were excluded. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Lienard 2006.
| Study characteristics | ||
| Methods | RCT, clusters randomised. | |
| Data | France, secondary care setting. The number of case report forms completed by sites was included. 69 centres, one participant (35/68 in the Visited group, 34/67 in the Non‐visited group). Total n = 66; age NR; sex NR. |
|
| Comparisons |
Intervention group centres received a systematic on‐site visit (Visited group) Control group did not receive a systematic on‐site visit (Non‐visited group) |
|
| Outcomes | Quantity of data spontaneously reported. | |
| Notes | Retention period: unclear. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | Randomly allocated by the coordinating office to either the Visited or Non‐visited group, by a minimisation technique, to ensure a balance between groups concerning centre type and location. |
| Blinding of participants and personnel? | Yes | Sites blinded. |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. Site objective assessment |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
MacLennan 2014.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, secondary care setting. Non‐responders to annual questionnaires. Total n = 753; mean age 77 (SD 6.0) years; 85.1% female. |
|
| Comparisons |
Intervention group received a telephone call from the trial office ahead of the reminder questionnaire in addition to the usual reminder schedule. Control group received the usual reminder schedule only. |
|
| Outcomes | Response rates to the reminder questionnaire. | |
| Notes | Retention period: 12 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Eligible participants were stratified by their host trial allocation and randomised using a computerised central allocation process at the Trial Office. |
| Adequate sequence generation? | Unclear | Not reported in the paper. |
| Blinding of participants and personnel? | Unclear | Not reported in the paper but as staff delivered telephone call is some potential for unblinding and impact on outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
MamMOTH 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, primary care setting. All host trial participants were included, however, those who had withdrawn from the main trial before the time of the 24‐month follow‐up, or were deceased, were not. Total n = 1001; mean age 59 (SD 14.3) years; 58.8% females. |
|
| Comparisons |
Intervention group received a newsletter one month before the 24‐month paper follow‐up questionnaire was due to be sent. The newsletter included details of the host trial and the participants involved. Comparator group did not receive a newsletter. They just received their final 24‐month follow‐up questionnaire as usual. |
|
| Outcomes | Follow‐up data provided by participants. | |
| Notes | Retention period: 24 months The MAmMOTH study was funded by Arthritis Research UK (now Versus Arthritis), Grant number 20748 awarded to University of Aberdeen (CI Professor GJ Macfarlane. |
|
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The randomisation was carried out by CHaRT after recruitment to the Main Trial was complete. |
| Adequate sequence generation? | Yes | Randomisation was carried out by CHaRT, after recruitment was complete, to ensure the two groups were balanced for Main Trial Treatment allocation, and for centre (i.e. the GP practice the participant was registered at). |
| Blinding of participants and personnel? | Yes | Neither the participants nor the trial team were blinded to the allocation of receipt of a newsletter or not. Unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Assessment of the outcome was not blinded. However, objective outcome, participants blind (don’t know there is a study) staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | No | Because this was an intention to treat analysis, some of those allocated to the newsletter did not receive the intervention, but were still included in the main analysis in the intervention group, as were those allocated to no newsletter included in the comparator group. |
| Free of selective outcome reporting? | Yes | Only one outcome was examined – retention defined as the return of 24‐month follow‐up data. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | No | High |
Man 2011.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, primary care setting. Participants from the host trial who provided an electronic mail address and/or mobile phone. Total n = 125; mean age 46 (SD 11); 74.4% females. |
|
| Comparisons |
Intervention group received an electronic reminder Control group did not receive a reminder. |
|
| Outcomes | Questionnaire returned | |
| Notes | Retention period: 6 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | An independent data manager generated a computerised sequence to randomly allocate participants regardless of yoga host trial treatment allocation. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Marques 2013.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care setting. All pilot host trial participants were included. Total n = 85; range age 26 to 92 years; 64% females; 31% were single; 8% were Non‐White; 33% had higher education; 19% were working, |
|
| Comparisons |
Intervention group received a resource use log at baseline were participants could prospectively record their use of health services and expenses by using tick boxes and open questions. Control group did not receive a resource use log. |
|
| Outcomes | Diary return rate | |
| Notes | Retention period: 3 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Not reported in the paper. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Marsh 1999.
| Study characteristics | ||
| Methods | Quasi randomised, clusters randomised | |
| Data | UK, a multi‐centre trial recruiting participants from hospital outpatient clinics. Participants included parents of children aged 3–12 months registered with the practices participating in the main trial. Total n = 434; age NR; sex NR |
|
| Comparisons |
Intervention group 1 received postal administration with financial incentive (£2 voucher to spend in a local children's store) once the completed diary had been received or postal group without financial incentive Intervention group 2 received telephone administration with financial incentive (£2 voucher to spend in a local children's store) once the completed diary had been received or telephone group without financial incentive Control group were selected from four practices and their matched control practices were selected for the clinic visits |
|
| Outcomes | Return of diaries | |
| Notes | Retention period: Unclear | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | No | Quasi‐randomised.Allocation to trial arms was according to the order the participant appeared in an existing list |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | No | High |
Marson 2007.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care setting. It is unclear if all participants from host trial were involved. Total n = 1815; age NR; sex NR |
|
| Comparisons |
Intervention group received a cover letter though post with the questionnaire that included an estimate of the length of time that it may take to complete. Control group received standard cover letter with no indication of length of time required. |
|
| Outcomes | Return of questionnaire. | |
| Notes | Retention period: baseline assessment | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the monograph. |
| Adequate sequence generation? | Unclear | Not reported in the monograph. |
| Blinding of participants and personnel? | Yes | Not reported in the monograph but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the monograph. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the monograph. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Free of selective outcome reporting? | Unclear | Not reported in the monograph. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
McCambridge 2011.
| Study characteristics | ||
| Methods | RCT, individuals randomised | |
| Data | UK, community setting. The numbers of participants in the present trial slightly exceed those in the parent trial as some participants completed the first randomisation to secondary outcome questionnaire and did not complete the subsequent randomisation to parent trial study condition. Total n = 8285 (4957 in trial 1 and 3328 in trial 2), mean age 37.8 (SD 10.8), 57% female. |
|
| Comparisons | All participants were sent e‐mail requests for follow‐up data: Trial 1: in the pilot phase after 1 and 3 months Trial 2: in the main trial phase after 3 and 12 months |
|
| Outcomes | Proportion of participants who responded | |
| Notes | Retention period: Trial 1: 1 and 3 months Trial 2: 3 and 12 months |
|
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Randomisation could not be subverted, therefore, by the trial team, and allocation was fully concealed. |
| Adequate sequence generation? | Yes | Randomisation was performed by a computer‐generated randomisation procedure |
| Blinding of participants and personnel? | Unclear | Participants were blinded to the conduct of this trial. However, unclear about personnel. |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
McColl 2003 ‐ Trial 1.
| Study characteristics | ||
| Methods | RCT, clusters randomised | |
| Data | UK. Primary care setting. All host trial participants, as a source of the retention trial sample. Total n = 6576; mean age 59.6 (SD 13.7) years, 50.8% females; 66% were married; 60% were unemployed; 54.2% had no formal education qualifications. |
|
| Comparisons | Participants were randomised to two different versions of a self‐response questionnaire: Intervention group 1, condition‐specific measures of quality of life preceded generic instruments Intervention group 2, the relative position of the questionnaire was reversed. |
|
| Outcomes | Response rates on the questionnaire | |
| Notes | Retention period: 9 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not described only that participants were randomly assigned to each group. |
| Adequate sequence generation? | No | A random sample of 80 participants per condition per practice was selected. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | No | High |
McColl 2003 ‐ Trial 2.
| Study characteristics | ||
| Methods | RCT, clusters randomised | |
| Data | UK. Primary care setting. All host trial participants, as a source of the retention trial sample. Total n = 6576; mean age 59.6 (SD 13.7) years, 50.8% females; 66% were married; 60% were unemployed; 54.2% had no formal education qualifications. |
|
| Comparisons | Participants were randomised to two different versions of a self‐response questionnaire: Intervention group 1, condition‐specific measures of quality of life preceded generic instruments Intervention group 2, the relative position of the questionnaire was reversed. |
|
| Outcomes | Response rates on the questionnaire run | |
| Notes | Retention period: 9 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not described only that participants were randomly assigned to each group. |
| Adequate sequence generation? | No | A random sample of 80 participants per condition per practice was selected. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | No | High |
Mitchell 2011.
| Study characteristics | ||
| Methods | RCT, individual randomisation | |
| Data | UK, primary care setting. All host trial participants, as a source of the retention trial sample. Total n = 2803; range age from 70 to 85 years old; 100% females |
|
| Comparisons |
Intervention group 1 received an invitation mailing packs with a white envelope. Intervention group 2 received an invitation mailing packs with a brown envelope. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: this trial was done in the first phases of the host trial. This comprise the return of the invitation pack 14 days after the original questionnaire was sent | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | These packs were alternately arranged (brown, white, brown, white, etc.). In each GP practice, an alphabetical (by surname) list of all eligible participants was produced by the practice. Participants were sent a brown or white envelope depending on the colour in the sequence. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Mitchell 2012.
| Study characteristics | ||
| Methods | Parallel RCT, Individuals randomised | |
| Data | UK, primary care setting. The sample size was arbitrary in that it was limited to the numbers of host trial participants recruited at the two sites. Total n = 2704; Range age from 70 to 85 years old; 100% females. |
|
| Comparisons |
Intervention group received a newsletter approximately 6 weeks before the follow‐up questionnaire Control group did not receive a newsletter. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 24 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The randomisation was undertaken by the York data manager who randomised to two equally sized groups in one single block allocation (the block was the size of all the potential participants). |
| Adequate sequence generation? | Yes | A computer program randomly divided the total numbers of participants into two equally. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Mitchell 2020a.
| Study characteristics | ||
| Methods | Parallel RCT, Individuals randomised | |
| Data | UK, secondary care setting. Participants from the host trial who provided they had opted in to receiving SMS messages and were not deceased or withdrawn from follow‐up before being due to be sent their 12‐month postal questionnaire Total n = 1465; mean age 66.8 (8.5); 54.0% females. |
|
| Comparisons |
Intervention group received a personalised text message four days after their 12‐month questionnaire was sent. Control group received a non‐personalised text message. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 12 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Participants were randomised into the embeded trial using simple randomisation in a 1:1 allocation ratio. The allocation schedule was generated by a researcher at the Trials Unit not involved in the recruitment or follow‐up of participants. |
| Adequate sequence generation? | Yes | Participants were randomised into the embeded trial using simple randomisation in a 1:1 allocation ratio. The allocation schedule was generated by a researcher at the Trials Unit not involved in the recruitment or follow‐up of participants. |
| Blinding of participants and personnel? | Yes | Participants were not informed of their explicit participation in the embeded trial, but due to the nature of the intervention could not be blinded to whether the text was personalised or non‐personalised. Unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | It was not possible to blind research staff to SWAT allocation. However, objective outcome, participants blinded (did not know there was a study), staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Mitchell 2020b.
| Study characteristics | ||
| Methods | Parallel RCT, Individuals randomised | |
| Data | UK, secondary care setting. All participants from the host trial being due to be sent their 12‐month postal questionnaire Total n = 2306; mean age 69.0 (8.9); 55.2% females. |
|
| Comparisons |
Intervention group addition of a pen Control group did not receive a pen. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 12 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Two batches, using a 1:1 allocation ratio, in a single large block the size of the batch |
| Adequate sequence generation? | Yes | Generated by a statistician at York Trials Unit using Stata v15 |
| Blinding of participants and personnel? | Yes | Participants were not informed of their explicit participation in the SWAT, but due to the nature of the intervention could not be blinded. Researchers were not blinded as well. Unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | It was not possible to blind research staff to SWAT allocation.However, objective outcome, participants blinded (don’t know there is a study), staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Nakash 2007.
| Study characteristics | ||
| Methods | RCT, individuals randomised | |
| Data | UK, secondary care setting. Only certain centres were included in this nested trial. Total n = 298, age range 29.5 (SD 10.5), sex 40.9%; 24% were professional |
|
| Comparisons |
Intervention group received a trial calendar. Control group did not receive na calendar. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 1‐, 3‐ and 9‐months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Allocation concealment was ensured by using a remote computer‐generated randomisation system that was independently administered and quality controlled. |
| Adequate sequence generation? | Yes | A computer‐generated random sequence was used to allocate host trial participants to either the 'Calendar' or 'No Calendar' group. |
| Blinding of participants and personnel? | Unclear | Due to the nature of the trial, blinding of the participant and those administering the intervention to treatment allocation was not possible. Some communication between trial office and participants. |
| Blinding of outcome assessment? | Yes | Personnel responsible for data inputting and outcome assessment were, however, blind to treatment allocation However, objective outcome, participants blind (did not know there was a study) staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
OPAL 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, secondary care settings. not ALL participants in the trial that took part in the trial. Only those due to be receiving their 12‐ or 24‐month questionnaire at the time the trial was running. |
|
| Comparisons |
Intervention group received a tested theoretically informed letter sent with the questionnaire Control group received a standard letter |
|
| Outcomes | Questionnaires returned | |
| Notes | 12 or 24 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | No information of the nested RCT was provided. |
| Adequate sequence generation? | Yes | The database would automatically produce the appropriate letter for each woman |
| Blinding of participants and personnel? | Yes | Women were unaware if they were receiving a standard or theory‐based cover letter but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | No information of the nested RCT was provided.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Renfroe 2002.
| Study characteristics | ||
| Methods | 2x2x2x2 factorial RCT, individuals randomised. | |
| Data | USa and Canada All surviving participants were involved in this nested trial. Total n = 640; Mean age 63.6 (SD 10.0) years old; 11.4% females; 63.9%were high school graduates. |
|
| Comparisons | 1) Trial 1 measured mode of delivery. Surveys were sent to participants by either overnight express mail in vs regular mail delivered by the postal service in the other half 2) Trial 2 measured enclosure of a certificate of appreciation. Some participants received packets including a certificate of appreciation, while the others did not. 3) Trial 3 measured the effect of timing of the delivery. Some participants received the survey within 2–3 weeks after the last hot trial follow‐up visit versus after the trial closed out in the other half (1–4 months after the last follow‐up visit). 4) Trial 4 measured the effect of a signature on the cover letter thanking them for their participation. The trial coordinator signed half the letters, and the principal investigator signed the other half. Participants were randomly assigned to received one of 16 combinations of these four factors. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: unclear, authors state as "end of the study". | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in this abstract. |
| Adequate sequence generation? | Yes | Patients were initially randomised (1:1) into one of two arms. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Rodgers 2019.
| Study characteristics | ||
| Methods | Factorial RCT, individuals randomised | |
| Data | UK, primary care setting. Participants in the host trial who were due to be sent their 12‐month follow‐up questionnaire who had not withdrawn or requested not to be sent the 12‐month questionnaire. Total n = 826; mean age 77.5 (SD 7.0) years old; 61.6% females. |
|
| Comparisons | Participants were assigned to one of the following six groups: a) trial update newsletter plus handwritten Post‐it® note applied to the questionnaire b) newsletter plus printed Post‐it®; c) newsletter only d) handwritten Post‐it® note only e) printed Post‐it ® note only f) none |
|
| Outcomes | Return of questionnaire | |
| Notes | Retention period: 12 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | An independent data manager who was not involved in the recruitment of participants generated the allocation sequence. |
| Adequate sequence generation? | Yes | Generated the allocation sequence by computer and allocated participants in a 1:1:1:1:1:1 ratio. |
| Blinding of participants and personnel? | Yes | Not reported in the paper but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Salvesen 1992.
| Study characteristics | ||
| Methods | RCT, individuals randomised | |
| Data | Norway, primary care setting. Participants received an accompanying newspaper article with a description of the study, a postal questionnaire and a postage‐paid. Non‐responders of this first nested trial were included. Total n = 716; age NR; 100% female. |
|
| Comparisons |
Intervention group received a newspaper article with a description of the study. A photocopy of the article was randomly allocated with a letter of reminder, and the response was monitored for 30 days. Control group no newspaper article sent. |
|
| Outcomes | Questionnaire return. | |
| Notes | Retention period: unclear | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the letter to the editor. |
| Adequate sequence generation? | Unclear | Not reported in the letter to the editor. |
| Blinding of participants and personnel? | Yes | Not reported in the letter to the editor but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the letter to the editor.However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the letter to the editor. |
| Free of selective outcome reporting? | Unclear | Not reported in the letter to the editor. |
| Other sources of bias | Unclear | Letter to the editor with few detailed provided. |
| Overall Risk of Bias | Unclear | Unclear |
Sarathy 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, secondary care All host trial participants, as a source of the retention trial sample, were included. Total n = 269; mean age 53.5 (SD 7.6) years; 65% females |
|
| Comparisons |
Intervention group received text messages as pre‐notification on the day of the questionnaire mailout. Control group post notification four days after the questionnaire mailout. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period: 3 months post‐randomisation into the host trial | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | A statistician at YTU generated the allocation sequence and the assignment of participants to either SMS group. |
| Adequate sequence generation? | Yes | Randomisation was achieved using computer‐generated random permuted blocks with a 1:1 ratio |
| Blinding of participants and personnel? | Yes | Participants did not know they were taking part in the SWAT and were therefore blinded. However unclear about personnel. However unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | The analysis was undertaken on an intention‐to‐treat basis by a statistician blind to group allocation. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Severi 2011.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, community setting. For trial 1 participants who enrolled between 1 March 2009 and 1 June 2009 and had provided postal addresses were eligible. For trial 2 host trial participants >6 weeks overdue for cotinine sample follow‐up were eligible. Trial 1: Total n = 1950; age NR; 45.3% females Trial 2: Total n = 127; age NR; 47.2% females |
|
| Comparisons | Trial 1 aimed to evaluate the effect on the trial follow‐up of written information regarding the benefits of participation to society. Intervention group received written information on a refrigerator magnet by post between 16 and 20 weeks after randomisation into the host trial followed by a mobile phone text message three days after the host trial postal follow‐up questionnaire was sent. Control group received a text message reminding participant follow‐up. Trial 2 aimed to evaluate the effect on the trial follow‐up of a telephone call from a senior female clinician and researcher Intervention group received a telephone call from senior female clinician and researcher inviting the participant to complete follow‐up. Control group received standard host trial procedures. |
|
| Outcomes | Trial 1: Completed follow‐up questionnaires Trial 2: Completed cotinine sample follow‐up |
|
| Notes | Retention periods: Trial 1: 30 weeks from randomisation. Trial 2: unclear |
|
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | In both studies, the allocation of the participants to the intervention or control group was concealed from the investigators. |
| Adequate sequence generation? | Yes | Trial 1: The participants were allocated to intervention or control through minimisation (using Minim software). Trial 2: This was a single‐blind controlled trial, with those recording and assessing outcomes blind to the intervention. |
| Blinding of participants and personnel? | Yes | Not possible to blind participants. The allocation was concealed from investigators |
| Blinding of outcome assessment? | Yes | Both studies were a single‐blind controlled trial, with those recording and assessing outcomes blind to the intervention. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised |
| Overall Risk of Bias | Yes | Low |
Sharp 2006.
| Study characteristics | ||
| Methods | 2 x 2 x 2 factorial parallel RCT, Individuals randomised | |
| Data | UK, secondary care setting. Participants due to receive a host trial questionnaire were involved. Total n = 930; mean age 34 (SD 10.4); 100% females; 96% White. |
|
| Comparisons | Three trials were evaluated in this study: Trial 1: Intervention group received a TOMBOLA‐branded pen Control group received no pen Trial 2: Intervention group questionnaire was dispatched by first class post Control group questionnaire was dispatched by second class Trial 3: Intervention group received an enclosed pre‐addressed return envelope on which there was a second‐class postage stamp Control group received Freepost business‐reply envelope. This generated eight intervention groups: 1. standard (i.e. no pen, second class dispatch, Freepost return envelope) 2. pen 3. pen and first‐class dispatch 4. first‐class dispatch 5. stamp on the return envelope 6. stamp on the return envelope and pen 7. stamp on the return envelope and first‐class dispatch stamp on the return envelope, pen and first‐class dispatch |
|
| Outcomes | Questionnaire response rate | |
| Notes | Retention period: 12‐, 18‐, 24‐ and 30‐months questionnaires | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Yes | Computer randomised by two authors using random numbers, to one of the eight intervention groups |
| Blinding of participants and personnel? | Yes | Not reported in the paper. Not clear if participants were blinded to effect of embedded trial intervention on retention and unblinding but likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Staff not blind but they had no influence on a participant's decision to reply |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised |
| Overall Risk of Bias | Unclear | Unclear |
Starr 2015.
| Study characteristics | ||
| Methods | 2×2 partial factorial RCT, individuals randomised. | |
| Data | UK, secondary care setting. Participants who were newly randomly assigned to the host trial had not reached the 4‐week time point and were willing to supply a mobile phone number, or an e‐mail address were included. Total n = 418; mean age 41 (SD 11.1) years; 20% females. |
|
| Comparisons | Two trials were included in this study: Trial 1: Intervention group received an SMS text message pre‐notification of the delivery of the initial 4‐ and 12‐week questionnaires. Control group received no message. Trial 2 (for participants who did not respond to the initial 4‐ or 12‐week questionnaire): Intervention group received an e‐mail which included a link to complete the questionnaire online or was invited to return the paper copy if they wished. Control group received their reminder by post with a further copy of the questionnaire. |
|
| Outcomes | Questionnaire return. | |
| Notes | Retention period: 1‐ or 3‐ months questionnaire | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | A computer‐generated system that was concealed and remote from the users. |
| Adequate sequence generation? | Yes | Participants were randomly allocated to the intervention on a 1:1 basis, |
| Blinding of participants and personnel? | Yes | Due to the nature of the intervention, it was not possible to blind the participants or trial office staff to allocation. Unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | The researchers remained blinded. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised |
| Overall Risk of Bias | Yes | Low |
Subar 2001.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | USA, secondary care setting. Three out of 10 screening centres were included in this trial. Total n = 900; age of participants >50 years old, 51% were females. |
|
| Comparisons |
Intervention group 1 received by mail the Diet History Questionnaire (including frequency of intake for 114 individual food items, five questions about the proportions, four summary questions, and nine questions on the use of vitamin and mineral supplement) accompanied by a cover letter and a postage‐paid return envelope. Intervention group 2 received by mail a 36‐page machine readable food frequency questionnaire, accompanied by a cover letter and a postage‐paid return envelope. For participants who did not return their questionnaires within three weeks, up to five telephone calls were made by staff at each centre. |
|
| Outcomes | Questionnaires returned | |
| Notes | Retention period: 36 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Not reported in the paper. |
| Blinding of participants and personnel? | Unclear | Not reported in the paper. |
| Blinding of outcome assessment? | Unclear | Not reported in the paper. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Unclear | No concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Tai 1997.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, primary care setting. All host trial participants lost to follow‐up who had a telephone. Total n = 148, median age 43.7; sex NR |
|
| Comparisons |
Intervention group 1 received a telephone reminder after non‐response to 1st reminder. Intervention group 2 were sent a new set of questionnaires with the reminder letter and sent after non‐response to 1st reminder. |
|
| Outcomes | Questionnaires returned | |
| Notes | Retention period: unclear | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear | Not reported in the paper. |
| Adequate sequence generation? | Unclear | Just says randomised |
| Blinding of participants and personnel? | Unclear | Does not mention blinding and it is not clear if the researchers making calls were also part of the main trial team. In principle, they could have influenced the outcome depending on their knowledge of allocation. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Unclear | Not reported in the paper. |
| Free of selective outcome reporting? | Unclear | Not reported in the paper. |
| Other sources of bias | Unclear | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Tilbrook 2015.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | UK, primary care setting. Participants who were due to receive either their 6‐month follow‐up questionnaire or who had not fully or partially withdrawn from the host trial were included. Total n = 499; mean age 53 (SD 13.7); 68.5% female. |
|
| Comparisons |
Intervention group received a signed Post‐it® note with handwritten text, in black ink (by four researchers), signed with the first name of the person whose name was on the cover letter accompanying the questionnaire in addition to the 'standard' contact(sent an SMS message 7 days before they were due to receive the questionnaire encouraging them to return the questionnaire). Control group received standard contact procedures |
|
| Outcomes | Questionnaire response | |
| Notes | Retention period: 6 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Conducted by one of the York Trials Unit's data managers so allocation concealment was achieved. |
| Adequate sequence generation? | Yes | The randomisation sequence was generated by computer. |
| Blinding of participants and personnel? | Yes | Researchers signed the post it. but unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | The response rate was determined by York Trials Unit data clerks who were not aware to which group the participants belonged. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Tranberg 2018.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised | |
| Data | Denmark, community setting. Participants that were due to receive the host trial second remainder. Total n = 9791; range age 30‐64 years; 100% females. |
|
| Comparisons |
Intervention group 1 (directly mailed group) received a modified second reminder, a leaflet, and a self‐sampling kit. Intervention group 2 (opt‐in group) received the same material as those in the directly mailed group but received no kit. Additionally, the leaflet for this group held information describing how to order the kit by e‐mail, text message, phone, or via a study webpage. Control group received a standard second reminder that informed them about the current test opportunity. |
|
| Outcomes | Participation rate (by returning a self‐sample or attending regular cytology screening). | |
| Notes | Retention period: 6 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The randomisation list was produced by an independent programmer who was not otherwise involved in the trial |
| Adequate sequence generation? | Yes | Web‐based computer randomisation in RedCap was used to allocate eligible participants to the three groups of the trial at a 1:1:1 ratio by the method of individual randomisation with randomly varying block sizes of 3, 6, and 9. |
| Blinding of participants and personnel? | Unclear | The women were unaware of the randomisation, but blinding of the participants and study staff was impossible due to the nature of the interventions. Unblinding not likely to impact objective outcome. Phone contact in intervention could have influenced outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Treweek 2020a.
| Study characteristics | ||
| Methods | Parallel RCT, clusters randomised | |
| Data | UK, secondary care setting. All host trial participants were considered in this trial. Total n = 560; age NR; 100% females |
|
| Comparisons |
Intervention group received a pre‐notification card sent around 1 month before the face‐to‐face primary outcome measurement visit. Control group received no pre‐notification card. |
|
| Outcomes | Proportion attending the primary outcome measurement visit. | |
| Notes | Retention period:12 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | The list was then passed to the data manager at Tayside Clinical Trials Unit to implement. |
| Adequate sequence generation? | Yes | Two‐arm, parallel randomised with a 1:1 allocation ratio, stratified by centre. One of the authors prepared a central randomisation list using. |
| Blinding of participants and personnel? | Yes | All trial team members were blind to host trial allocation. Primary outcome visits were organised, done and recorded by research nurses, who had no knowledge of the SWAT or host trial allocation. However, unclear about participants. However, unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | All trial team members were blind to host trial allocation. Primary outcome visits were organised, done and recorded by research nurses, who had no knowledge of the SWAT or host trial allocation. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Watson 2017.
| Study characteristics | ||
| Methods | 2x2 factorial RCT, individuals randomised. | |
| Data | UK, secondary care setting. Participants the host trial who had yet to receive their 12‐and 24‐month follow‐up questionnaires. Total n = 521; age ranged from 38 to 61 years; 49.8% females. |
|
| Comparisons |
Intervention group 1 received unconditional £5 high‐street gift voucher at 12 but not 24 months. Intervention group 2 received unconditional £5 high‐street gift voucher at 12 and 24 months. Intervention group 3 received unconditional £5 high‐street gift voucher at 24 but not 12 months. Control group did not receive a voucher. |
|
| Outcomes | Questionnaire return | |
| Notes | Retention period:12 and 24 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Not reported in the monograph. |
| Adequate sequence generation? | Unclear | Simple randomisation (1:1:1:1) was completed to allocate the host trial participants to one of the four groups |
| Blinding of participants and personnel? | Yes | Not reported in the monograph.However, unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the monograph. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Unclear | Unclear |
Whiteside 2019.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, community setting Participants from two of the UK‐based GP practices involved in the host trial. Total n = 1943; age NR; sex NR. |
|
| Comparisons |
Intervention group received a pen with trial invitation pack. Control group did not receive a pen in their invitation pack. |
|
| Outcomes | Participant retention and return of screening form | |
| Notes | Retention period: remaining in trial at 3 months | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | Generation of the allocation sequence was undertaken by the host trial statistician, who was not involved with the production of the invitation packs, using Stata version 13. |
| Adequate sequence generation? | Yes | A 2:1 allocation ratio was used, in favour of the no pen arm. |
| Blinding of participants and personnel? | Yes | Not reported in the paper. Staff may not have been blinded, but they had no influence on decision by the participant to respond. Also unblinding not likely to impact objective outcome |
| Blinding of outcome assessment? | Yes | Not reported in the paper. However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No further concerns raised. |
| Overall Risk of Bias | Yes | Low |
Young 2020.
| Study characteristics | ||
| Methods | Parallel RCT, individuals randomised. | |
| Data | UK, community setting Trial participants who received a positive blood test result were included in this nested study. Total n = 1079; age >50 years; 50.4% females. |
|
| Comparisons |
Intervention group 1 received an unconditional £5 multistore voucher Intervention group 2 received a conditional (on completion of the questionnaire) £5 multistore voucher. |
|
| Outcomes | Questionnaire response rate. | |
| Notes | Retention period:1‐, 3‐, 6‐ and 12‐months. | |
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Allocation concealment? | Yes | SWAT randomisation was conducted independently by a specialist unit. Individuals were stratified by host trial group (control arm, positive test, and negative test) and |
| Adequate sequence generation? | Yes | ordered randomly on computer‐generated lists. |
| Blinding of participants and personnel? | Yes | Participants were not informed about the different conditions for receiving vouchers. Researchers mailing questionnaires, vouchers, and making telephone reminder calls were not blinded to condition. Unblinding not likely to impact objective outcome. |
| Blinding of outcome assessment? | Yes | Not reported in the paper However, objective outcome, staff have no plausible additional opportunity to influence postal response rate once questionnaires sent. |
| Incomplete outcome data addressed? All outcomes | Yes | No concerns raised. The analysis does ignore some data, but everything is presented so possible to redo, and everything is accounted for even if not used. |
| Free of selective outcome reporting? | Yes | No concerns raised. |
| Other sources of bias | Yes | No concerns raised. |
| Overall Risk of Bias | Yes | Low |
IQR: interquartile range; NR: not reported; RCT: randomised controlled trial; SD: standard deviation; SWAT: Studies Witihin A Trial;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abboah‐Offei 2020 | Not nested in an RCT |
| Alexander 2008 | Not nested in an RCT |
| Arnevik 2009 | [Excluded in the original review] This retention trial was not embedded in a randomised trial. |
| Arundel 2017 | Not evaluating retention |
| Atherton 2010 | [Excluded in the original review] Comparison of Internet vs. postal questionnaires not randomised. |
| Aysola 2018 | Not nested in an RCT |
| Barry 1996 | [Excluded in the original review] Retention trial compared distribution of scores for participants completing different questionnaire versions. Author confirmed retention/questionnaire return was not an outcome measure. |
| Bednarek 2008 | [Excluded in the original review] Retention trial outcome is continuation of treatment. |
| Bisla 2019 | Not nested in an RCT |
| Bowen 2000 | [Included in the original review, excluded in the update]. Study looking at reducing the number of people who stop taking the study drug, not evaluating retention in the trial. |
| Bromley 2019 | Not a randomised/quasi‐randomised retention trial |
| Chaffin 2009 | Not evaluating retention in a clinical trial |
| Chee 2019 | Not nested in an RCT |
| Cheung 2019 | Not nested in an RCT |
| Cox 2003 | [Excluded in the original review] Retention trial outcome treatment compliance |
| Cox 2006 | Not evaluating retention |
| Cox 2008 | Not evaluating retention |
| Day 1998 | [Excluded in the original review] Retention trial measured adherence to treatment. Authors do not have retention data. |
| Diaz 2001 | Not evaluating retention |
| Eaker 2004 | [Excluded in the original review] Retention trial embedded in a cohort. |
| Edelstein 2005 | [Excluded in the original review] Retention study is not a randomised trial. Incentives not randomised. Author confirmed these were not instituted to help with retention but with adherence to pill taking and life style modification requirements. |
| Edwards 2013 | Not evaluating retention |
| Farabee 2016 | RCT not embedded in a host trial |
| Ford 2006 | Measuring adaherence not retention |
| Galaragga 2017 | It is not an embedded trial of a retention intervention |
| Gaurino 2006 | Not targeting at trial retention |
| Grabowski 1995 | [Excluded in the original review] Substudy aim is retention in treatment comparing different follow‐up schedules for addiction treatment trial. |
| Haines 2019 | Not a randomised/quasi‐randomised retention trial |
| Hall 1975 | [Excluded in the original review] Not a randomised/quasi‐randomised retention trial |
| Hall 1978 | [Excluded in the original review] Not a randomised/quasi‐randomised retention trial |
| Henderson 2019 | Not a randomised/quasi‐randomised retention trial |
| Hoang 2014 | Not nested in an RCT |
| Hoffman 1998 | [Excluded in the original review] Retention trial embedded in a blood bank cohort |
| Hopkins 1983 | [Excluded in the original review] Retention trial embedded in a survey |
| Hughes 1989 | [Included in the original review, excluded in the update] Not a randomised/quasi‐randomised retention trial. |
| Hunter 2018 | Not evaluating retention |
| Iglesias 2000 | [Excluded in the original review] Retention trial embedded in a cohort of general practitioner practice participants. |
| Iglesias 2001 | [Excluded in the original review] Retention trial embedded in the recruitment phase of the host trial. |
| Johnson 2004 | [Excluded in the original review] Retention study not embedded in a randomised trial. |
| Juraskova 2014 | Not targeting at trial retention |
| Karras‐Jean Gilles 2019 | Not nested in an RCT |
| Katz 2001 | [Excluded in the original review] Retention study is not a randomised trial. Authors confirmed the effectiveness of gift incentives was not evaluated in a substudy for the Pride in Parenting trial. |
| Kim 2020 | Not nested in an RCT |
| Kiwanuka 2018 | Not nested in an RCT |
| Krammer 1986 | Not nested in an RCT |
| Kuhlmann 2017 | Not a randomised/quasi‐randomised retention trial |
| Lannin 2013 | Not evaluating retention |
| Leidy 2000 | [Excluded in the original review] Retention study appears to be a randomised trial but no response from authors to establish if retention was an outcome. For the substudy, trial sites randomised to 1 of 2 orders of administration of quality of life questionnaires. Response rates not reported. Missing data, internal consistency reliability, mean score values, relationship between the 2 measures evaluated. |
| Leigh Brown 1997 | [Included in the original review, excluded in the update] Authors confirmed that this is not a randomised/quasi‐randomised retention trial. |
| Leighton 2018 | Not nested in an RCT |
| Litchfield 2005 | Not evaluating retention |
| Malden 2019 | Not nested in an RCT |
| McAuley 1994 | [Excluded in the original review] Retention study is not a randomised trial. There is a single randomisation stratified by classes in the morning and early evening. No response from authors regarding randomisation to class times. |
| McBee 2009 | [Excluded in the original review] Retention study not a randomised trial. Authors confirm strategies to improve retention were not evaluated in an Age‐Related Eye Disease Study 2 (AREDS2) substudy. |
| Munoz 2017 | Not nested in an RCT |
| Murray 2019 | Not nested in an RCT |
| Murray 2020 | Not nested in an RCT |
| Nicholas 2013 | Not a randomised/quasi‐randomised retention trial |
| Nielson 2018 | Not nested in an RCT |
| Novak 2019 | Not a randomised/quasi‐randomised retention trial |
| Nuzzolese 2020 | Review paper |
| Parker 2019 | Review paper |
| Paul 2011 | Not evaluating retention |
| Phiri 2019 | Not nested in an RCT |
| Pieper 2018 | Not evaluating retention |
| Poling 2006 | [Excluded in the original review] Substudy aim is about diagnostic compliance. 4‐arm trial comparing contingency management with or without active bupropion and voucher control with or without active bupropion. Here contingency management and voucher control are aimed at getting information on the disease condition/response to treatment for the primary outcome of the host trial i.e. negative urine sample for cocaine and opioids. Contingency management and voucher control are not related to retention in the host trial but related to diagnostic compliance. |
| Price 2019 | Not a randomised/quasi‐randomised retention trial |
| Puffer 2004 | [Excluded in the original review] Retention RCT was embedded in a survey. Authors confirmed that the 2 x 2 factorial study testing four different questionnaire designs was embedded in a survey. |
| Rhoades 1998 | [Excluded in the original review] Substudy retention in treatment. 2 x 2 trial of dose and visit frequency of attending a clinic either 2 or 5 days per week. Primary outcome was retention in treatment for all randomizations. Similar to Grabowski 1995 trial. |
| Roberts 2000 | [Excluded in the original review] Retention trial embedded in a survey about menopause services. |
| Rodgers 2019a | Not evaluating retention |
| Rodgers 2019b | Not evaluating retention |
| Rolfson 2011 | Not nested in an RCT |
| Sano 2013 | Not nested in an RCT |
| Schmitz 2005 | [Excluded in the original review] Substudy about compliance to treatment and pill taking behaviour rather than trial retention. |
| Shulman 2019 | Not nested in an RCT |
| Smeeth 2001ab | [Excluded in the original review] Substudy about response to baseline assessment. |
| Smith 2015 | Not evaluating retention |
| Stoner 1998 | [Excluded in the original review] Retention study was not a randomised trial. Host study was a cluster‐randomised trial. Effectiveness of vouchers not evaluated in a substudy. |
| Svoboda 2001 | [Included in the original review, excluded in the update] Unclear if nested in an RCT. Authors contacted, and no reponse was received. |
| Tariq 2019 | Not nested in an RCT |
| Tassopoulos 2007 | [Excluded in the original review] Not a retention randomised trial. |
| Trevena 2006 | Not targeting at trial retention |
| von Allmen 2019 | Not a randomised/quasi‐randomised retention trial |
| Wagstaff 2019 | Not a randomised/quasi‐randomised retention trial |
| Wensing 2005 | [Included in the original review, excluded in the update] Unclear if nested in an RCT. Authors contacted, and were not able to confirm that the parent study is a cluster‐RCT. |
| Weston 2017 | Not nested in an RCT |
| Wood 2015 | Not nested in an RCT |
| Wood 2017 | Not nested in an RCT |
| Wu 1997 | [Excluded in the original review] Substudy designed to evaluate whether scores are different using 3 modes of questionnaire administration, rather than retention. |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Letley 2000.
| Methods | RCT, individuals randomised |
| Data | UK, primary care setting. Unclear from the abstract who was included in each arm of the embedded trial. Total n = 181; Mean age 74.0 (SD 6.5) years; 59.5% female. |
| Comparisons |
Intervention group received a 23‐page self‐complete questionnaire and SF‐36 Control group questionnaires in a reverse order |
| Outcomes | Questionnaire return |
| Notes | Retention period: 4 months |
Sutherland 1996.
| Methods | To be confirmed ‐ reports a feasibility evaluation for a breast cancer prevention trial but it has not been possible to confirm whether the feasibility phase was also randomised. |
| Data | Canada Total n = 226; Mean age 44 years; 100% female. |
| Comparisons |
Intervention group used the Total Design Method to inform provision of follow‐up questionnaire (included white envelope with hospital logo and commemorative stamp, hand‐typed, hand‐signed letter, etc). Control group used standard method used in follow‐up (included brown envelope with return address, computer‐printed label, no signature, etc). |
| Outcomes | Questionnaire return |
| Notes | Retention period: 70 days |
RCT: randomised controlled trial; SD: standard deviation; SF‐36: Short Form 36.
Characteristics of ongoing studies [ordered by study ID]
SWAT #100.
| Study name | Patient and family co‐developed participant information to improve recruitment rates, retention, and patient understanding of a randomised trial |
| Methods | To examine if participant information co‐developed by patients and their families can lead to greater recruitment rates, retention, and participant understanding of the study in comparison to standard participation information leaflets in the Rehabilitation Strategies following Oesophagogastric and Hepatopancreaticobiliary Cancer (ReStOre II) trial. |
| Data | |
| Comparisons | Intervention 1: Patient and family co‐developed participant information; Intervention 2: Standard participant information |
| Outcomes | Primary: Recruitment rate; Secondary: Retention rate; Trial Understanding (Decision Making Questionnaire) |
| Starting date | |
| Contact information | oneilll8@tcd.ie |
| Notes |
SWAT #105.
| Study name | Effects of a patient‐designed‐and‐informed participant information sheet versus a standard, researcher‐designed information sheet on recruitment to a randomised trial |
| Methods | To examine the effects of a (patient) PPI‐designed‐and‐informed participant information sheet (PIS) in comparison with a standard, researcher‐designed information sheet on recruitment to the trial, rate of consent and relationship with participant retention, and understanding regarding the two PIS. |
| Data | |
| Comparisons | Intervention 1: standard, researcher‐designed PIS intervention; 2: PPI‐designed‐and‐informed PIS |
| Outcomes | Primary: recruitment. Secondary: understanding, retention and likeability |
| Starting date | |
| Contact information | sinead.hynes@nuigalway.ie |
| Notes |
SWAT #107.
| Study name | Effects of a multi‐trial programmable animation platform on the efficiency and success of pre‐screening and subsequent recruitment to a randomised trial. |
| Methods | To use a mixed‐methods sequential explanatory design to develop and test a novel approach of using a programmable multimedia animation to improve the success of pre‐screening and enhance recruitment to randomised trial. |
| Data | |
| Comparisons | Intervention 1: audiovisual programmable animation. Intervention 2: control |
| Outcomes | Primary: 1. Host trial recruitment: Proportion of screened participants who meet the eligibility criteria who consent to participate in the host trial. 2. Self‐reported visual analogue scale (VAS) of participants' confidence in their ability to make the right decision regarding trial participation independently of the clinician’s recommendation (assessed following the consent process for the host trial). Secondary: 1. Pre‐screening success. Proportion of pre‐screened participants who agree to proceed at that point. 2. Self‐reported assessment on VAS of adequacy of understanding regarding clinical trials (after the consent process for the host trial). 3. Effectiveness of the animation as measured using visualization effectiveness scales proposed by Few et al[5] and measured on the post‐consent questionnaire. 4. Proportion of participants recruited to the host trial who are retained in that trial (assessable to the end of the funding for the SWAT). |
| Starting date | |
| Contact information | f.shiely@ucc.ie |
| Notes |
SWAT #109.
| Study name | The effectiveness of a text message reminder which participants can respond to, compared with a ‘no reply’ text message on questionnaire response rates |
| Methods | To evaluate the effectiveness on completion of follow‐up postal questionnaires of sending a two‐way text message reminder compared with a standard one‐way text message with no option to reply. |
| Data | |
| Comparisons | Intervention 1: “Two way” text messages sent at the same time as host trial participants are expected to receive their postal follow‐up questionnaire. The text message will encourage them to text back if they have any queries. Intervention 2: “One way” text message sent at the same time as host trial participants are expected to receive their postal follow‐up questionnaire. Participants will not be able to reply to this message. |
| Outcomes | Primary: proportion of questionnaires completed at the 3‐month follow‐up. Secondary: ~ Time to questionnaire return (number of days between the questionnaire being mailed to participants and it being recorded as returned). ~ Proportion of patients requiring at least one return reminder notice (a letter at 2 and 4 weeks and a telephone call at 6 weeks if the questionnaire is not returned). ~ If possible, qualitative methods will be used to interrogate the text message responses sent by participants to explore topics and reasons for contacting the trial team. ~ If possible, a descriptive exploration will be done of whether text message topics sent by participants were associated with response rates to questionnaires. |
| Starting date | |
| Contact information | adwoa.parker@york.ac.uk; prometheus‐group@york.ac.uk |
| Notes |
SWAT#110.
| Study name | Printing the primary outcomE on Pink PapER versus standard paper to increase participant engagement to postal questionnaires (PEPPER) |
| Methods | To evaluate the effects of printing the primary outcome measure on pink paper versus on white paper in a questionnaire collecting the primary outcome measure in a randomised trial. |
| Data | |
| Comparisons | Intervention 1: Primary Outcome PROM printed on pink paper in the 6‐month follow‐up questionnaire Intervention 2: Primary Outcome PROM printed on white paper in the 6‐month follow‐up questionnaire |
| Outcomes | Primary: proportion of participants in each group who complete the host trial's primary outcome measure. Secondary: proportion of participants reminded to fill in the questionnaire; proportion of other questions in the questionnaire completed; overall return rate of the questionnaire. |
| Starting date | |
| Contact information | alexander.ooms@ndorms.ox.ac.uk |
| Notes |
SWAT#112.
| Study name | Effects on recruitment of a personalised compared with a standard study invitation letter |
| Methods | To evaluate the effects of a personalised letter including the parent’s name and address compared with a standard, non‐personalised letter on recruitment to a prospective study. |
| Data | |
| Comparisons | Intervention 1: personalised invitation letter, including the parent’s name and address. The wording of this invitation letter has been designed in consultation with the parent research partners’ group for the host trial. Intervention 2: standard invitation letter, not including the parent’s name and address. |
| Outcomes | Primary: proportion of participants agreeing to join the host trial in each SWAT intervention group. Secondary: proportion of parents in each group who express an interest in participating; proportion of parents in each group who opt out; proportion of parents in each group who complete the reasons for non‐participation questionnaire; proportion of parents in each group who complete the eligibility interview; proportion of parents in each group who complete the baseline assessment; proportion of parents in each group retained at (a) 12‐weeks and (b) 6‐months follow‐up; proportion of parents in each group who require a telephone reminder at (a) recruitment; (b) posttreatment (12 weeks); and (c) 6‐months follow‐up. |
| Starting date | |
| Contact information | louise‐von.essen@kbh.uu.se |
| Notes |
SWAT #119.
| Study name | Effects on retention of giving trial participants a thank you card following each study visit |
| Methods | To evaluate the effects of giving trial participants a thank you card following each study visit, compared with not giving them a thank you card. |
| Data | |
| Comparisons | Intervention 1: a thank you card is sent to trial participants at 4.5 and 9 months after randomisation. The host trial includes routine weekly clinical follow‐up assessments (if the participant’s wound is yet to heal) and participants in this SWAT group will also be sent questionnaires for the next outcome assessment time point (at months 6 and 12) when due. Intervention 2: Standard practice for the host trial (i.e.no thank you card). The host trial includes routine weekly clinical follow‐up assessments (if the participant’s wound is yet to heal) and participants in this SWAT group will receive no further contact until the next outcome assessment time point (at months 6 and 12). |
| Outcomes | Primary: questionnaire response rate, defined as the proportion of participants in each group who complete and return the questionnaire at the 6‐month follow‐up visit. Secondary: 1) Completeness of response (percentage of questions completed) at 6 months. 2) Whether a reminder notice is required (number of participants requiring a reminder mailing divided by the number of participants who were sent a questionnaire) at 6 months. 3) Cost of SWAT intervention per participant retained at 6 months. 4) Completeness of response, whether a reminder notice is required, and cost per participant retained at 12 months. |
| Starting date | |
| Contact information | catherine.arundel@york.ac.uk |
| Notes |
SWAT #121.
| Study name | What are the effects on retention and follow‐up of courtesy telephone calls versus postcards to trial participants following enrolment? |
| Methods | To evaluate the effect on response rates to subsequent follow‐up questionnaires of making a courtesy introductory telephone call to newly recruited participants in a randomised trial compared with a written card with equivalent information. |
| Data | |
| Comparisons | Intervention 1: a courtesy introductory telephone call [within two weeks] of being randomised into ARTISAN. This telephone call will include the following content: a) thanks for taking part in the ARTISAN trial; b) reminder about how valuable their contribution is; c) reminder that they will be contacted by post at six weeks, and then at 3, 6 and 12 months post randomisation, and that these contacts are just as important as their first visit; d) information about when the trial results are expected; e) reminder that they can contact the ARTISAN team if they have any queries. Intervention 2: a postcard‐sized written card, with similar content as above, signed by the Chief Investigator and Trial Manager posted in an envelope to participants’ homes within one week of being randomised. |
| Outcomes | Primary: the primary outcome is the questionnaire response rate at six months. This is defined as the proportion of participants who return the questionnaire by post at the 6‐month time point within the response window. Secondary: 1. Time to response to the questionnaires at all time points, i.e. 6 weeks, 3, 6 and 12 months (date of first posting to date of questionnaire received by study team) 2. Response rates at 6 weeks, and then at 3 and 12 months (as for primary outcome) 3. Response rates at 6 weeks, 3 months, 6 months and 12 months (return of questionnaire data at any point, including via telephone) 4. Completeness of responses. This will be counted as the number of missing items in the PROMS (OSIS, QuickDASH and EQ5D) and the complications section. 5. Number of reminder notices required. 6. Cost of intervention (phone call or postcard) per participant. |
| Starting date | |
| Contact information | gurmit.dhanjal@warwick.ac.uk |
| Notes |
SWAT #51.
| Study name | Promoting group identity to improve questionnaire return rate |
| Methods | To assess the effect on questionnaire return rate of an intervention to promote group identity in trial participants. |
| Data | |
| Comparisons | Intervention 1: active promotion of a group identity or membership using trial promotional material, such as wristbands, and participant‐friendly newsletters. Intervention 2: no promotional material or newsletters. |
| Outcomes | Primary: Questionnaire return rate Secondary: Measure of group identification |
| Starting date | |
| Contact information | ashley.agus@nictu.hscni.net |
| Notes |
SWAT #54.
| Study name | Giving trial participants a thank you note or card after each study visit |
| Methods | To examine whether giving a thank you note or card to enrolled participants after each study‐related visit improves their retention in the trial. |
| Data | |
| Comparisons | Intervention 1: generic thank you card or note Intervention 2: pPersonalised thank you card or note Intervention 3: no thank you card or note |
| Outcomes | Primary: proportion of participants who remain in the study. Secondary: time that participants remain in the study before they withdraw |
| Starting date | |
| Contact information | ranand01@qub.ac.uk |
| Notes |
SWAT #63.
| Study name | Does local radio and social media advertisement increase recruitment? |
| Methods | To assess the effects on recruitment of local media (radio) or social media (Facebook) advertisement. |
| Data | |
| Comparisons | Intervention 1: Local radio (R) advertisements, lasting two weeks (avoiding school holiday periods) Intervention 2: Facebook (F) advertisements targeted to parents with children aged 6‐12 years in the recruitment city and within a 15‐mile radius (avoiding school holiday periods) Intervention 3: No advertisement (Ø) for 1‐2 months (avoiding school holiday periods) |
| Outcomes | Primary: change in recruitment after each type of advertisement. This change will be assessed as the number of participants recruited during the one month before the start of the advertising intervention and during the one month after it ends. Secondary: changes in recruitment three months before and after the advertisement; retention of participants in the trial; and changes in the number of potentially eligible participants who are assessed or approached for the trial. |
| Starting date | |
| Contact information | a.azuara‐blanco@qub.ac.uk |
| Notes |
SWAT #79.
| Study name | Effect of birthday cards with or without nudge on retention and data completion rates in trials involving children |
| Methods | To determine whether sending a birthday card with or without a nudge improves retention and completion rates in trials involving children |
| Data | |
| Comparisons | Intervention 1: Birthday card. Our PPI (patient and public involvement) group felt that the birthday cards should be as personal as possible but not have anything on the front that could be offensive. The front will therefore have the participant’s age and a gender neutral image linked to the trial. The message on the inside should be from someone they know, such as the treating clinician, or research nurse at their local site and the trial team. Intervention 2: Birthday card (as in intervention 1), but informed by nudge theory to encourage completion of questionnaires Intervention 3: No birthday card |
| Outcomes | Primary: response rate to the participant follow‐up questionnaire at the first time point following receipt of the birthday card. Secondary: 1) Response rate to the participant follow‐up questionnaire at the 12‐month follow‐up: 2) Time to response (number of days from date due to date returned) 3) Completeness of primary outcome measure (defined as providing sufficient data to produce a valid summary score) 4) Need for a postal reminder 5) Cost per participant retained |
| Starting date | |
| Contact information | mike.backhouse@york.ac.uk, adwoa.parker@york.ac.uk |
| Notes |
SWAT #81.
| Study name | A telephone reminder to enhance adherence to interventions in randomised trials |
| Methods | To evaluate the effects of a telephone reminder to enhance the adherence of participants to interventions in randomised trials. |
| Data | |
| Comparisons | Intervention 1: Telephone reminder (maximum three attempts with no messages left on voicemail to protect privacy) the day before their appointment to attend the intervention programme. The telephone reminder will be a scripted text to remind the participant is reminded of their study visit date and time and asking them to confirm their attendance the next day. Intervention 2: No telephone reminder. |
| Outcomes | Primary: Adherence to trial intervention (defined as 100% attendance) Secondary: Number of dropouts, and time to drop out from the host trial. |
| Starting date | |
| Contact information | fionnuala.jordan@nuigalway.ie |
| Notes |
SWAT #82.
| Study name | To evaluate the effect on retention of sending Christmas cards to trial participants. |
| Methods | To evaluate the effect on retention of sending Christmas cards to trial participants. |
| Data | |
| Comparisons | Intervention 1: Christmas card to the trial participant. Intervention 2: no Christmas card. |
| Outcomes | Primary: number of participants retained. Secondary: cost per participant retained. |
| Starting date | |
| Contact information | streweek@mac.com |
| Notes |
SWAT #86.
| Study name | Advance notification of trial participants before outcome data collection to improve retention |
| Methods | To evaluate the effects of a pre‐notification letter or email on completion and return of outcome questionnaires. |
| Data | |
| Comparisons | Intervention 1. Pre‐notification communication in advance of follow‐up questionnaire. Participants who elect to complete follow‐up questionnaires online will be sent a personalised pre‐notification in an email two weeks prior to the mailing of this. Participants who elect to complete follow‐up questionnaires in hard copy form and return by post will be sent a personalised pre‐notification letter. Similar wording and layout will be used in the email and letter. Intervention 2. No pre‐notification communication. |
| Outcomes | Primary: valid response for WORKWELL trial primary outcome (yes/no) (i.e. usable outcome data for the primary outcome measure (WLQ‐25 total score[10]) obtained by any means, no more than 56 days after the scheduled 6‐month follow‐up time‐point. Secondary: 1. Valid response for WORKWELL trial primary outcome (yes/no) without reminder; 2. Number of reminders sent; 3. Time to response [or ceasing follow‐up] (days); 4. Costs per participant retained. |
| Starting date | |
| Contact information | Chris.J.Sutton@manchester.ac.uk |
| Notes |
SWAT #87.
| Study name | Do participants complete the original or the reminder postal follow up questionnaire? |
| Methods | To determine, in people who are sent a reminder postal follow‐up questionnaire, whether they complete the original postal questionnaire or the reminder questionnaire. |
| Data | |
| Comparisons | Intervention 1: ‘Original’ questionnaires will be identified by a green sticker on the front page and a red sticker will be used for ‘reminder’ questionnaires. When the questionnaires are received by the trials office, the date and questionnaire type will be logged using the Trial Central Management system. |
| Outcomes | Primary: proportion of questionnaires returned by people sent a reminder that were the ‘Reminder’ or the ‘Original’ questionnaire. Secondary: time to response, defined as the number of days between the ‘Reminder’ questionnaire being mailed out and a completed questionnaire being received by the trial team. |
| Starting date | |
| Contact information | lucy.cureton@ndorms.ox.ac.uk |
| Notes |
SWAT #89.
| Study name | Including a theoretically informed leaflet in a participant take‐home pack of questionnaires to increase response rate |
| Methods | The joint aims of this study are: (a) To design a leaflet using a theory based behaviour change framework (anticipating the Theoretical Domains Framework) with the aim of maximising participant questionnaire response rates (achieved) (b) To further test this specific approach in a pragmatic setting to provide evidence of its applicability and effectiveness in respect of participant behaviour and adherence |
| Data | |
| Comparisons | Intervention 1. Theoretically informed leaflet in the participant pack Intervention 2: Generic compliments slip in the participant pack |
| Outcomes | Primary: Participant response rates at one, two and twelve weeks post‐intervention |
| Starting date | |
| Contact information | k.starr@abdn.ac.uk |
| Notes |
SWAT #92.
| Study name | Pen incentive to enhance retention in a randomised trial |
| Methods | To evaluate the effects on retention of providing a pen with the 3‐month follow‐up questionnaire. |
| Data | |
| Comparisons | Intervention 1. Pen printed with the trial logo sent along with the 3‐month follow‐up questionnaire. Intervention 2. No pen. |
| Outcomes | Primary: proportion of participants who return the 3‐month questionnaire. Secondary: time to response (length of time taken to return the questionnaire), completeness of response (number of questions completed) and whether a reminder notice is required (number of participants requiring a reminder mailing divided by the number of participants who were sent a questionnaire). |
| Starting date | |
| Contact information | garry.tew@northumbria.ac.uk |
| Notes |
SWAT #97.
| Study name | TRECA (TRials Engagement in Children and Adolescents) |
| Methods | To evaluate multimedia information resources (MMIs) in a series of paediatric trials in the UK, testing their effects on recruitment and retention and decision‐making by comparing the effect of providing standard written participant information with provision of the MMI either in addition to the standard written participant information or the provision of the MMI alone. |
| Data | |
| Comparisons | Intervention 1: MMI only (participants receive information about the trial by viewing a multimedia website) Intervention 2: PIS only (participants receive information about the trial by PIS) Intervention 3: Both MMI and PIS (participants receive information about the trial by both MMI and PIS) |
| Outcomes | Primary: Recruitment rate Secondary: Retention rate; quality of decision making |
| Starting date | |
| Contact information | peter.knapp@york.ac.uk |
| Notes |
PIS: participant information sheet; PPI: patient and public involvement.
Contributions of authors
For this update, PM and AK ran the electronic searches. PM also contributed to screening of titles and abstracts. KG, AK, CK, ST, JH, VB, TC, AH, LM, PC, GR, and MAM contributed to the study design, record screening, full‐text review of retrieved records, and data extraction. KG, MAM, JH, and ST analysed the data. KG and MAM drafted the updated review. All authors approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institue for Health Research Incentive Award, UK
This update was funded by a National Institue for Health Research Incentive Award [NIHR IA 130660].
Declarations of interest
Two of the review authors (ST and GR) are authors on two of the eligible studies (Bailey 2013; Treweek 2020a). There are no other conflicts to declare.
Edited (no change to conclusions)
References
References to studies included in this review
AMBER 2020 {unpublished data only}
- Hagen S. Evaluation of theory informed cover letetr comapred to standard cover letter. Personal Communication.
Arundel 2019 {published data only}
- Arundel C, Coleman E, Fairhurst C, Peckham E, Bailey D, Gilbody S. The effectiveness of a contingent financial incentive to improve trial follow up; a randomised study within a trial (SWAT). F1000Research 2019;8:1937. [DOI: 10.12688/f1000research.21059.2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashby 2011 {published data only}
- Ashby R, Turner G, Cross B, Mitchell N, Torgerson D. A randomized trial of electronic reminders showed a reduction in the time to respond to postal questionnaires. Journal of Clinical Epidemiology 2011;64:208-12. [DOI: 10.1016/j.jclinepi.2010.01.020] [DOI] [PubMed] [Google Scholar]
Avenell 2004 {published data only}
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA, et al. The effects of an open design on trial participant recruitment, compliance and retention - a randomized controlled trial comparison with a blinded, placebo-controlled design. Clinical Trials 2004;1(6):490-8. [DOI: 10.1191/1740774504cn053oa] [DOI] [PubMed] [Google Scholar]
Bailey 2013 {published data only (unpublished sought but not used)}
- Bailey J. Evaluating the effectiveness of incentives in the "Sexunzipped" trial. Personal communication - email.
Bauer 2004 {published data only}
- Bauer JE, Rezaishiraz H, Head K, Cowell J, Bepler G, Aiken M, et al. Obtaining DNA from a geographically dispersed cohort of current and former smokers: use of mail-based mouthwash collection and monetary incentives. Nicotine and Tobacco Research 2004;6(3):439-46. [DOI: 10.1080/14622200410001696583] [DOI] [PubMed] [Google Scholar]
Bean 2019 {published data only}
- Bean MK, Thornton LM, Jeffers AJ, Gow RW, Mazzeo SE. Impact of motivational interviewing on engagement in a parent-exclusive paediatric obesity intervention: randomized controlled trial of NOURISH+MI. Pediatric Obesity 2019;14(4):e12484. [DOI: 10.1111/ijpo.12484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2016 {published data only}
- Bell K, Clark L, Fairhurst C, Mitchell N, Lenaghan E, Blacklock J, et al. Enclosing a pen reduced time to response to questionnaire mailings. Journal of Clinical Epidemiology 2016;74:144-50. [DOI: 10.1016/j.jclinepi.2015.] [DOI] [PubMed] [Google Scholar]
Bradshaw 2020 {published data only}
- Bradshaw LE, Montgomery AA, Williams HC, Chalmers JR, Haines RH. Two-by-two factorial randomised study within a trial (SWAT) to evaluate strategies for follow-up in a randomised prevention trial. Trials. 2020;21(1):529. [DOI: 10.1186/s13063-020-04373-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brubaker 2019 {published data only}
- Brubaker L, Jelovsek JE, Lukacz ES, Balgobin S, Ballard A, Weidner AC, et al. Recruitment and retention: a randomized controlled trial of video-enhanced versus standard consent processes within the E-OPTIMAL study. Clinical Trials 2019;16(5):481-9. [DOI: 10.1177/1740774519865541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2015 {published data only}
- Clark L, Ronaldson S, Dyson L, Hewitt C, Torgerson D, Adamson J. Electronic prompts significantly increase response rates to postal questionnaires: a randomized trial within a randomized trial and meta-analysis. Journal of Clinical Epidemiology 2015;68(12):1446-50. [DOI: 10.1016/j.jclinepi.2015.01.016] [DOI] [PubMed] [Google Scholar]
Cochrane 2020 {published data only}
- Cochrane A, Welch C, Fairhurst C, Cockayne S, Torgerson DJ, OTIS Study Group. An evaluation of a personalised text message reminder compared to a standard text message on postal questionnaire response rates: an embedded randomised controlled trial. F1000Research 2020;9:154. [DOI: 10.12688/f1000research.22361.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cockayne 2005 {published and unpublished data}
- Cockayne S, Torgerson D. A randomised controlled trial to assess the effectiveness of offering study results as an incentive to increase response rates to postal questionnaires. BMC Medical Research Methodology 2005;5(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cockayne 2017 {published data only}
- Cockayne S, Fairhurst C, Adamson J, Hewitt C, Hull R, Hicks K, et al. An optimised patient information sheet did not significantly increase recruitment or retention in a falls prevention study: an embedded randomised recruitment trial. Trials 2017;18(1):144. [DOI: 10.1186/s13063-017-1797-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2020 {unpublished data only}
- Cook J, Cook JA, Bongard E, Heneghan C, Butler CC. SWAT 90 evaluation: Does the time at which a participant incentive is given affect the retention rate? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,902359,en.pdf. [16406421]
Couper 2007 {published data only (unpublished sought but not used)}
- Couper PM, Peytchev A, Strecher JV, Rothert K, Anderson J. Following up non respondents to an online weight management intervention: randomized trial comparing mail versus telephone. Journal of Medical Internet Research 2007;9(2):e16. [DOI: 10.2196/jmir.9.2.e16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 2004 {published and unpublished data}
- Cunningham JA, Wild TC, Cordingley J. Using collaterals to validate self-reports of problem drinkers: any impact on client attrition and quantity of drinking reported? Addictive Behaviors 2004;29(3):615-21. [DOI: 10.1016/j.addbeh.2003.08.023] [DOI] [PubMed] [Google Scholar]
Cunningham‐Burley 2020 {published data only}
- Cunningham-Burley R, Roche J, Fairhurst C, Cockayne S, Hewitt C, Iles-Smith H, et al, SSHeW Study Team. Enclosing a pen to improve response rate to postal questionnaire: an embedded randomised controlled trial. F1000Research 2020;9:577. [DOI: 10.12688/f1000research.23651.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinglas 2015 {published data only}
- Dinglas VD, Huang M, Sepulveda KA, Pinedo M, Hopkins RO, Colantuoni E, et al. Personalized contact strategies and predictors of time to survey completion: analysis of two sequential randomized trials. BMC Medical Research Methodology 2015;15:5. [DOI: 10.1186/1471-2288-15-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorling 2020 {published data only}
- Dorling J, Hewer O, Hurd M, Bari V, Bosiak B, Bowler U, et al. Two speeds of increasing milk feeds for very preterm or very low-birthweight infants: the SIFT RCT. Health Technology Assessment 2020;24(81):1-94. [DOI: 10.3310/hta24180] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorman 1997 {published and unpublished data}
- Dorman P, Slattery J, Farrell B, Dennis MS, Sandercock PA. A randomised comparison of the EuroQol and Short Form-36 after stroke. United Kingdom collaborators in the International Stroke Trial. BMJ 1997;315(7106):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2004 {published data only}
- Edwards P, Roberts I, Sandercock P, Frost C. Follow-up by mail in clinical trials: does questionnaire length matter? Controlled Clinical Trials 2004;25(1):31-52. [DOI: 10.1016/j.cct.2003.08.013] [DOI] [PubMed] [Google Scholar]
Edwards 2016 {published data only}
- Edwards L, Salisbury C, Horspool K, Foster A, Garner K, Montgomery AA. Increasing follow-up questionnaire response rates in a randomized controlled trial of telehealth for depression: three embedded controlled studies. Trials 2016;17(1):107. [DOI: 10.1186/s13063-016-1234-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fouad 2014 {published data only}
- Fouad MN, Johnson RE, Nagy MC, Person SD, Partridge EE. Adherence and retention in clinical trials: a community-based approach. Cancer 2014;120 Suppl 7:1106-12. [DOI: 10.1002/cncr.28572] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2009 {published data only}
- Gates S, Williams M, Withers E, Williamson E, Mt-Isa S, Lamb S. Does a monetary incentive improve the response to a postal questionnaire in a randomised controlled trial? The MINT incentive study. Trials 2009;10(1):44. [DOI: 10.1186/1745-6215-10-44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattellari 2004 {published data only}
- Gattellari M, Ward JE. Does a deadline improve men's participation in self-administered health surveys? A randomized controlled trial in general practice. Journal of Public Health 2004;26(4):384-7. [DOI: 10.1093/pubmed/fdh177] [DOI] [PubMed] [Google Scholar]
Glassman 2020 {published data only}
- Glassman AR, Beaulieu WT, Stockdale CR, Beck RW, Bressler NM, Labriola LT, et al. Effect of telephone calls from a centralized coordinating center on participant retention in a randomized clinical trial. Clinical Trials 2020;17(2):195-201. [DOI: 10.1177/1740774519894229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goulao 2020 {published data only}
- Goulao B, Duncan A, Floate R, Clarkson J, Ramsay C. Three behavior change theory-informed randomized studies within a trial to improve response rates to trial postal questionnaires. Journal of Clinical Epidemiology 2020;122:35-41. [DOI: 10.1016/j.jclinepi.2020.01.018] [DOI] [PubMed] [Google Scholar]
Goulao 2020 (replication of SWAT #2) {published data only}
- Goulao B, Duncan A, Floate R, Clarkson J, Ramsay C. Three behavior change theory-informed randomized studies within a trial to improve response rates to trial postal questionnaires. Journal of Clinical Epidemiology 2020;122:35-41. [DOI: 10.1016/j.jclinepi.2020.01.018] [DOI] [PubMed] [Google Scholar]
Greig 2017 {published data only}
- Greig A, Gardiner MD, Sierakowski A, Zweifel CJ, Pinder RM, Furniss D, et al. Randomized feasibility trial of replacing or discarding the nail plate after nail-bed repair in children. British Journal of Surgery 2017;104(12):1634-9. [DOI: 10.1002/bjs.10673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2019 {published data only}
- Griffin J, Lall R, Bruce J, Withers E, Finnegan S, Lamb SE, PreFIT Study Group. Comparison of alternative falls data collection methods in the Prevention of Falls Injury Trial (PreFIT). Journal of Clinical Epidemiology 2019;106:32-40. [DOI: 10.1016/j.jclinepi.2018.09.006] [DOI] [PubMed] [Google Scholar]
Guarino 2006 {published data only}
- Guarino P, Elbourne D, Carpenter J, Peduzzi P. Consumer involvement in consent document development: a multicenter cluster randomized trial to assess study participants' understanding. Clinical Trials 2006;3(1):19-30. [DOI: 10.1191/1740774506cn133oa] [DOI] [PubMed] [Google Scholar]
Hardy 2016 {published data only}
- Hardy P, Bell JL, Brocklehurst P, Epidural and Position Trial Collaborative Group. Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trial: a randomised study within a trial. BMC Medical Research Methodology 2016;16:82. [DOI: 10.1186/s12874-016-0180-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2010 {published and unpublished data}
- Henderson M, Wight D, Nixon C, Hart G. Retaining young people in a longitudinal sexual health survey: a trial of strategies to maintain participation. BMC Medical Research Methodology 2010;10:9. [DOI: 10.1186/1471-2288-10-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2020 {published data only}
- James S, Parker A, Cockayne S, Rodgers S, Fairhurst C, Torgerson D, et al. Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled Study within a Trial. F1000Research 2020;9:623. [DOI: 10.12688/f1000research.23767.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keding 2016 {published data only}
- Keding A, Brabyn S, MacPherson H, Richmond SJ, Torgerson DJ. Text message reminders to improve questionnaire response rates. Journal of Clinical Epidemiology 2016;79:90-5. [DOI: 10.1016/j.jclinepi.2016.05.011] [DOI] [PubMed] [Google Scholar]
Kenton 2007 {published data only}
- Kenton L, Dennis CL, Weston J, Kiss A. The effect of incentives and high priority mailing on postal questionnaire response rates: a mini-RCT. In: Abstracts from the 28th Meeting of the Society of Clinical Trials; 2007 May 20-23; Montreal. Vol. 4. 2007:371-455.
Kenyon 2005 {published data only}
- Kenyon S, Pike K, Jones D, Taylor D, Salt A, Marlow N, et al. The effect of a monetary incentive on return of a postal health and development questionnaire: a randomised trial. BMC Health Services Research 2005;5(1):55. [DOI: 10.1186/1472-6963-5-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khadjesari 2011 {published and unpublished data}
- Khadjesari Z, Murray E, Kalaitzaki E, White I, Mc Cambridge J, Thompson S. Impact and costs of incentives to reduce attrition in online trials: two randomised controlled trials. Journal of Medical Internet Research 2011;13(1):e26. [DOI: 10.2196/jmir.1523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Land 2007 {published and unpublished data}
- Land SR, Ritter MW, Costantino JP, Julian TB, Cronin WM, Haile SR, et al. Compliance with patient-reported outcomes in multicenter clinical trials: methodologic and practical approaches. Journal of Clinical Oncology 2007;25(32):5113-20. [DOI: 10.1200/jco.2007.12.1749] [DOI] [PubMed] [Google Scholar]
Lewis 2017 {published data only}
- Lewis H, Keding A, Bosanquet K, Gilbody S, Torgerson D. An randomized controlled trial of Post-it® notes did not increase postal response rates in older depressed participants. Journal of Evaluation in Clinical Practice 2017;23(1):102-7. [DOI: 10.1111/jep.12618] [DOI] [PubMed] [Google Scholar]
Lienard 2006 {published data only}
- Liénard JL, Quinaux E, Fabre-Guillevin E, Piedbois P, Jouhaud A, Decoster G, et al. Impact of on-site initiation visits on patient recruitment and data quality in a randomized trial of adjuvant chemotherapy for breast cancer. Clinical Trials 2006;3(5):486-92. [DOI: 10.1177/1740774506070807] [DOI] [PubMed] [Google Scholar]
MacLennan 2014 {published data only}
- MacLennan G, McDonald A, McPherson G, Treweek S, Avenell A, RECORD Trial Group. Advance telephone calls ahead of reminder questionnaires increase response rate in non-responders compared to questionnaire reminders only: The RECORD phone trial. Trials 2014;15:13. [DOI: 10.1186/1745-6215-15-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
MamMOTH 2020 {unpublished data only}
- Beasley M. MamMOTH trial. Personal communication.
Man 2011 {published data only}
- Man MS, Tilbrook HE, Jayakody S, Hewitt CE, Cox H, Cross B, et al. Electronic reminders did not improve postal questionnaire response rates or response times: a randomized controlled trial. Journal of Clinical Epidemiology 2011;64(1001):e1004. [DOI: 10.1016/j.jclinepi.2010.10.013] [DOI] [PubMed] [Google Scholar]
Marques 2013 {published data only}
- Marques E, Johnson EC, Gooberman-Hill R, Blom AW, Noble S. Using resource use logs to reduce the amount of missing data in economic evaluations alongside trials. Value Health 2013;16(1):195-201. [DOI: 10.1016/j.jval.2012.09.008] [DOI] [PubMed] [Google Scholar]
Marsh 1999 {published data only}
- Marsh P, Kendrick D. Using a diary to record near misses and minor injuries?which method of administration is best? Injury Prevention 1999;5:305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marson 2007 {published and unpublished data}
- Marson AG. A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs: the SANAD trial. NIHR HTA Report 2007;11(37):111-3. [DOI: 10.3310/hta11370] [DOI] [PubMed] [Google Scholar]
McCambridge 2011 {published data only}
- McCambridge J, Kalaitzaki E, White RI, Khadjesari Z, Murray E, Linke S. Impact of length or relevance of questionnaires on attrition in online trials: randomized controlled trial. Journal of Medical Internet Research 2011;13(4):e96. [DOI: 10.2196/jmir.1733] [DOI] [PMC free article] [PubMed] [Google Scholar]
McColl 2003 ‐ Trial 1 {published and unpublished data}
- McColl EM, Eccles MP, Rousseau NS, Steen IN, Parkin DW, Grimshaw JM. From the generic to the condition-specific? Instrument order effects in quality of life assessment. Medical Care 2003;41(7):777-90. [DOI: 10.1097/00005650-200307000-00002] [DOI] [PubMed] [Google Scholar]
McColl 2003 ‐ Trial 2 {published and unpublished data}
- McColl EM, Eccles MP, Rousseau NS, Steen IN, Parkin DWD, Grimshaw JM. From the generic to the condition-specific? Instrument order effects in quality of life assessment. Medical Care 2003;41(7):777-90. [DOI: 10.1097/00005650-200307000-00002] [DOI] [PubMed] [Google Scholar]
Mitchell 2011 {published data only}
- Mitchell N, Hewitt CE, Torgerson DJ, SCOOP Trial Group. A controlled trial of envelope colour for increasing response rates in older women. Aging Clinical and Experimental Research 2011;23(3):236-40. [DOI: 10.1007/bf03337749] [DOI] [PubMed] [Google Scholar]
Mitchell 2012 {published data only}
- Mitchell N, Hewitt CE, Lenaghan E, Platt E, Shepstone L, Torgerson DJ, SCOOP study team. Prior notification of trial participants by newsletter increased response rates: a randomized controlled trial. Journal of Clinical Epidemiology 2012;65(12):1348-52. [DOI: 10.1016/j.jclinepi.2012.05.008] [DOI] [PubMed] [Google Scholar]
Mitchell 2020a {published and unpublished data}
- Mitchell AS, Cook L, Dean A, Fairhurst C, Northgraves M, Torgerson D, et al. Using pens as an incentive for questionnaire return in an orthopaedic trial: an embedded randomised controlled retention trial. F1000Research 2020;9:321. [DOI: 10.12688/f1000research.23018.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2020b {published data only}
- Mitchell AS, Cook L, Dean A, Fairhurst C, Northgraves M, Togerson D, et al. An embedded randomised controlled retention trial of personalised text messages compared to non-personalised text messages in an orthopaedic setting. F1000Research 2020;9:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakash 2007 {unpublished data only}
- Nakash RA. A Study of Response and Non-Response to Postal Questionnaire Follow-up in Clinical Trials: Chapter 6: A Randomised Controlled Trial of a Method of Improving Response to Postal Questionnaire Follow-up in a Clinical Trial [PhD thesis]. Warwick, UK: University of Warwick, Warwick Medical School, 2007. [Google Scholar]
OPAL 2020 {unpublished data only}
- Goodman K. Evaluation of theory informed cover letter compared to standard cover letter.. Personal communication.
Renfroe 2002 {published and unpublished data}
- Renfroe EG, Heywood G, Foreman L, Schron E, Powell J, Baessler C, et al. The end-of-study patient survey: methods influencing response rate in the AVID trial. Controlled Clinical Trials 2002;23(5):521-33. [DOI] [PubMed] [Google Scholar]
- Renfroe EG, Powell J, Olarte A, Morris M, Hallstrom A. Method of administering patient surveys affects response rate. Controlled Clinical Trials 1999;20:s62. [DOI: 10.1016/s0197-2456(02)00225-8] [DOI] [Google Scholar]
Rodgers 2019 {published data only}
- Rodgers S, Sbizzera I, Cockayne S, Fairhurst C, Lamb SE, Vernon W, et al. A study update newsletter or Post-it® note did not increase postal questionnaire response rates in a falls prevention trial: an embedded randomised factorial trial. F1000Research 2019;7:1083. [DOI: 10.12688/f1000research.14591.2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvesen 1992 {published and unpublished data}
- Salvesen KA adn Vatten LJ. Effect of a newspaper article on the response to a postal questionnaire. Journal of Epidemiology and Community Health 1992;46(1):86. [DOI: 10.1136/jech.46.1.86] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarathy 2020 {published data only}
- Sarathy PP, Kottam L, Parker A, Brealey S, Coleman E, Keding A, et al. Timing of electronic reminders did not improve trial participant questionnaire response: a randomized trial and meta-analyses. Journal of Clinical Epidemiology 2020;6:eprint. [DOI] [PubMed] [Google Scholar]
Severi 2011 {published data only}
- Severi E, Free C, Knight R, Robertson S, Edwards P, Hoile E. Two controlled trials to increase participant retention in a randomized controlled trial of mobile phone-based smoking cessation support in the United Kingdom. Clinical Trials 2011;8(5):654-60. [DOI: 10.1177/1740774511416524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 2006 {published data only}
- Sharp L, Cochran C, Cotton SC, Gray NM, Gallagher ME, TOMBOLA group. Enclosing a pen with a postal questionnaire can significantly increase the response rate. Journal of Clinical Epidemiology 2006;59(7):747-54. [DOI: 10.1016/j.jclinepi.2005.10.014] [DOI] [PubMed] [Google Scholar]
Starr 2015 {published data only}
- Starr K, McPherson G, Forrest M, Cotton SC. SMS text pre-notification and delivery of reminder e-mails to increase response rates to postal questionnaires in the SUSPEND trial: a factorial design, randomised controlled trial. Trials 2015;16:295. [DOI: 10.1186/s13063-015-0808-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subar 2001 {published data only}
- Subar AF, Ziegler RG, Thompson FE, Johnson CC, Weissfeld JL, Reding D, et al. Is shorter always better? Relative importance of questionnaire length and cognitive ease on response rates and data quality for two dietary questionnaires. American Journal of Epidemiology 2001;153(4):404-9. [DOI: 10.1093/aje/153.4.404] [DOI] [PubMed] [Google Scholar]
Tai 1997 {published data only}
- Tai SS, Nazareth I, Haines A, Jowett C. A randomized trial of the impact of telephone and recorded delivery reminders on the response rate to research questionnaires. Journal of Public Health Medicine 1997;19(2):219-21. [DOI: 10.1093/oxfordjournals.pubmed.a024613] [DOI] [PubMed] [Google Scholar]
Tilbrook 2015 {published data only}
- Tilbrook HE, Becque T, Buckley H, MacPherson H, Bailey M, Torgerson DJ. Randomized trial within a trial of yellow 'post-it notes' did not improve questionnaire response rates among participants in a trial of treatments for neck pain. Journal of Evaluation in Clinical Practice 2015;21(2):202-4. [DOI: 10.1111/jep.12284] [DOI] [PubMed] [Google Scholar]
Tranberg 2018 {published data only}
- Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures - a randomized controlled trial. BMC Cancer 2018;18(1):273. [DOI: 10.1186/s12885-018-4165-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treweek 2020a {unpublished data only}
- Treweek S, Gallant S, Anderson AS. SWAT 76 evaluation: randomised evaluation of sending pre-notification cards to trial participants before a face-to-face primary outcome measurement. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,864300,en.pdf. [DOI] [PMC free article] [PubMed]
Watson 2017 {published data only}
- Watson AJ, Cook J, Hudson J, Kilonzo M, Wood J, Bruhn H, et al. A pragmatic multicentre randomised controlled trial comparing stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease: the eTHoS study. Health Technology Assessment 2017;21(70):1-224. [DOI: 10.3310/hta21700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiteside 2019 {published data only}
- Whiteside K, Flett L, Mitchell A, Fairhurst C, Cockayne S, Rodgers S, et al, OTIS Study Group. Using pens as an incentive for trial recruitment of older adults: An embedded randomised controlled trial. F1000Research 2019;8:315. [DOI: 10.12688/f1000research.18300.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2020 {published data only}
- Young B, Bedford L, das Nair R, Gallant S, Littleford R, Robertson JF, et al. Unconditional and conditional monetary incentives to increase response to mailed questionnaires: A randomized controlled study within a trial (SWAT). Journal of Evaluation in Clinical Practice 2020;26(3):893-902. [DOI: 10.1111/jep.13230] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abboah‐Offei 2020 {published data only}
- Abboah-Offei M, Bristowe K, Vanderpuye-Donton NA, Ansa G, Oppong-Agyei YD, Abas M, et al. Phase II mixed methods' feasibility cluster randomised controlled trial of a novel community-based enhanced care intervention to improve person-centred outcomes for people living with HIV in Ghana. AIDS Care 2020;32(sup 2):107-18. [DOI: 10.1080/09540121.2020.1739217] [DOI] [PubMed] [Google Scholar]
Alexander 2008 {published data only}
- Alexander GL, Divine GW, Couper MP, McClure JB, Stopponi MA, Fortman KK, et al. Effect of incentives and mailing features on online health program enrollment. American Journal of Preventive Medicine 2008;34(5):382-8. [DOI: 10.1016/j.amepre.2008.01.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnevik 2009 {published data only}
- Arnevik E, Wilberg T, Urnes Ø, Johansen M, Monsen J, Karterud S. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy - a randomized controlled study. European Psychiatry 2009;24:71-8. [DOI] [PubMed] [Google Scholar]
Arundel 2017 {published data only}
- Arundel C, Jefferson L, Bailey M, Cockayne S, Hicks K, Loughrey L, et al. A randomized, embedded trial of pre?notification of trial participation did not increase recruitment rates to a falls prevention trial. Journal of Evaluation in Clinical Practice 2017;23(1):73-8. [DOI: 10.1111/jep.12576] [DOI] [PubMed] [Google Scholar]
Atherton 2010 {published data only}
- Atherton H, Oakeshott P, Aghaizu A, Hay P, Kerry S. Use of an online questionnaire for follow-up of young female students recruited to a randomised controlled trial of chlamydia screening. Journal of Epidemiology and Community Health 2010;64(580):e584. [DOI] [PubMed] [Google Scholar]
Aysola 2018 {published data only}
- Aysola J, Tahirovic E, Troxel AB, Asch DA, Gangemi K, Hodlofski AT, et al. A randomized controlled trial of opt-In versus opt-out enrollment into a diabetes behavioral intervention. American Journal of Health Promotion 2018;32(3):745-52. [DOI: 10.1177/0890117116671673] [DOI] [PubMed] [Google Scholar]
Barry 1996 {published and unpublished data}
- Barry MJ, Walker-Corkery E, Chang Y, Tyll LT, Cherkin DC, Fowler FJ. Measurement of overall and disease-specific health status: does the order of questionnaires make a difference. Journal of Health Services Research and Policy 1996;1(1):20-7. [DOI] [PubMed] [Google Scholar]
Bednarek 2008 {published data only}
- Bednarek P, Nichols M, Carlson N, Edelman A, Creinin M, Truitt S, et al. Effect of "observed start" vs. traditional "Sunday start" on hormonal contraceptive continuation rates after medical abortion. Contraception 2008;78:26-30. [DOI] [PubMed] [Google Scholar]
Bisla 2019 {published data only}
- Bisla J, Ambler G, Frank B, Gulati S, Hocken P, James M, et al. Successful and unsuccessful recruitment and retainment strategies in a UK multicentre drug trial for a rare chronic pain condition which performed above target. British Journal of Pain 2020;14(3):171-9. [DOI: 10.1177/2049463719893399] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2000 {published and unpublished data}
- Bowen D, Thornquist M, Anderson K, Barnett M, Valanis B, Goodman G, et al. The use of incentives to increase participation in a chemoprevention trial. Controlled Clinical Trials 1995;16:41s. [Google Scholar]
- Bowen D, Thornquist M, Goodman G, Omenn GS, Anderson K, Barnett M, et al. Effects of incentive items on participation in a randomized chemoprevention trial. Journal of Health and Psychology 2000;5(1):109-15. [DOI] [PubMed] [Google Scholar]
Bromley 2019 {published data only}
- Bromley KJ, Ogollah R, Konstantinou K, Foster N, Lewis M. The use of regular text messaging over one year to collect primary outcome data in a randomised controlled trial. Trials 2019;20(Suppl 1):12. [Google Scholar]
Chaffin 2009 {published and unpublished data}
- Chaffin M, Valle LA, Funderburk B, Gurwitch R, Silovsky J, Bard D. A motivational intervention can improve retention in PCIT for low-motivation child welfare clients. Child Maltreatment 2009;14(4):356-68. [DOI] [PubMed] [Google Scholar]
Chee 2019 {published data only}
- Chee W, Ji X, Kim S, Park S, Zhang J, Chee E, et al. Recruitment and retention of Asian Americans in web-based physical activity promotion programs: a discussion paper. Computers, Informatics, Nursing 2019;37(9):455-62. [DOI: 10.1097/CIN.0000000000000541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2019 {published data only}
- Cheung YT, Weng X, Wang MP, Ho SY, Kwong AC, Lai VM, et al. Effect of prepaid and promised financial incentive on follow-up survey response in cigarette smokers: a randomized controlled trial. BMC Medical Research Methodology 2019;19:138. [DOI: 10.1186/s12874-019-0786-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2003 {published data only}
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40?65 years: the S.W.E.A.T. study (Sedentary Women Exercise Adherence Trial). Preventive Medicine 2003;36:17-29. [DOI] [PubMed] [Google Scholar]
Cox 2006 {published data only}
- Cox KL, Burke V, Beilin LJ, Grove JR, Blanksby BA, Puddey IB. Blood pressure rise with swimming versus walking in older women: the Sedentary Women Exercise Adherence Trial 2 (SWEAT 2). Journal of Hypertension 2006;24(2):307-14. [DOI: 10.1097/01.hjh.0000200514.25571.20] [DOI] [PubMed] [Google Scholar]
Cox 2008 {published and unpublished data}
- Cox KL, Burke V, Beilin LJ, Derbyshire AJ, Grove JR, Blanksby BA, et al. Short and long-term adherence to swimming and walking programs in older women - the Sedentary Women Exercise Adherence Trial (SWEAT 2). Preventive Medicine 2008;46(6):511-7. [DOI] [PubMed] [Google Scholar]
- Cox KL, Burke V, Beilin LJ, Grove JR, Blanksby BA, Puddey IB. Blood pressure rise with swimming versus walking in older women: the Sedentary Women Exercise AdherenceTrial 2 (SWEAT 2). Journal of Hypertension 2006;24(2):307-14. [DOI: 10.1097/01.hjh.0000200514.25571.20] [DOI] [PubMed] [Google Scholar]
Day 1998 {published data only}
- Day R, Cella D, Ganz P, Constantino J. Electronic monitoring of participant adherence in the NSABP breast cancer prevention trial (BCPT). Controlled Clinical Trials 1998;19:69s. [Google Scholar]
Diaz 2001 {published data only}
- Diaz E, Levine HB, Sullivan MC, Sernyak MJ, Hawkins KA, Cramer JA, et al. Use of the Medication Event Monitoring System to estimate medication compliance in patients with schizophrenia. Journal of Psychiatry & Neuroscience 2001;26(4):325-9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Eaker 2004 {published data only}
- Eaker S, Adami H, Granath F, Wilander E, Sparen P. A large population-based randomized controlled trial to increase attendance at screening for cervical cancer. Cancer Epidemiology, Biomarkers & Prevention 2004;13(3):346-54. [PubMed] [Google Scholar]
Edelstein 2005 {published data only}
- Edelstein S, Minutillo A, Pyle L, Anderson B, Wilfley D, Van Buren D, et al. Rewarding youth behaviour: implementation of incentives in the TODAY trial. Clinical Trials 2005;2:s23. [Google Scholar]
Edwards 2013 {published data only}
- Edwards KE, Hagen SM, Hannam J, Kruger C, Yu R, Merry AF. A randomized comparison between records made with an anesthesia information management system and by hand, and evaluation of the Hawthorne effect. Canadian Journal of Anaesthesia 2013;60(10):990-7. [DOI: 10.1007/s12630-013-0003-y] [DOI] [PubMed] [Google Scholar]
Farabee 2016 {published data only}
- Farabee D, Hawken A, Calhoun S, Veliz R, Grossman J, Zhang Y. Tracking and locating itinerant subjects with a rechargeable incentive card: results of a randomized trial. Substance Use & Misuse 2016;51(5):658-63. [DOI: 10.3109/10826084.2015.1126748] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2006 {published and unpublished data}
- Ford ME, Havstad S, Vernon SW, Davis SD, Kroll D, Lamerato L, et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist 2006;46(4):545-50. [DOI] [PubMed] [Google Scholar]
- Ford ME, Randolph V, Hopkins-Johnson L, Eason SL, Havstad S, Jankowski M, et al. Design of a case management approach to enhance cancer screening trial retention among older African American men. Journal of Aging and Health 2004;16(5):39s-57s. [DOI] [PubMed] [Google Scholar]
Galaragga 2017 {published data only}
- Galárraga O, Sosa-Rubí SG, Kuo C, Gozalo P, González A, Saavedra B, et al. Punto Seguro: a randomized controlled pilot using conditional economic incentives to reduce sexually transmitted infection risks in Mexico. AIDS and Behavior 2017;21(12):3440-56. [DOI: 10.1007/s10461-017-1960-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaurino 2006 {published data only}
- Guarino P, Elbourne D, Carpenter J, Peduzzi P. Consumer involvement in consent document development: a multicenter cluster randomized trial to assess study participants' understanding. Clinical Trials 2006;3(1):19-30. [DOI: 10.1191/1740774506cn133oa] [DOI] [PubMed] [Google Scholar]
Grabowski 1995 {published data only}
- Grabowski JP, Rhoades HP, Elk RP, Schmitz JP, Davis CP, Creson DM, et al. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled, double-blind trials. Journal of Clinical Psychopharmacology 1995;15(3):163-74. [DOI] [PubMed] [Google Scholar]
Haines 2019 {published data only}
- Haines RH, Chalmers JR, Swinden R, Bradshaw LE, Montgomery AM, Williams HC. Maximising participant retention in a randomised prevention trial. Trials 2019;20(suppl.1):P-189. [Google Scholar]
Hall 1975 {published data only}
- Hall S, Hall R, Borden B, Hanson R. Follow-up strategies in the behavioural treatment of overweight. Behaviour Research and Therapy 1975;13:167-72. [DOI] [PubMed] [Google Scholar]
Hall 1978 {published data only}
- Hall S, Bass A, Monroe J. Continued contact and monitoring as follow-up strategies: a long-term study of obesity treatment. Addictive Behaviours 1978;3:139-47. [DOI] [PubMed] [Google Scholar]
Henderson 2019 {published data only}
- Henderson M, Waldram M, Thomas A, AlAlmmary F, Di Brito S, Ottman S, et al. Can financial incentives improve living donor follow-up?: a pilot randomized controlled trial. Transplant International 2019;32(suppl.2):199. [Google Scholar]
Hoang 2014 {published data only}
- Hoang U, Sheldenkar A, Leon J, Emmett E, Mckevitt C, Wolfe C. The effect of personalized care on long term loss to follow-up in a stroke register: WSC-0469. International Journal of Stroke 2014;9(Suppl 3):262. [Google Scholar]
Hoffman 1998 {published data only}
- Hoffman S, Burke A, Helzlsouer K, Comstock G. Controlled trial of the effect of length, incentives, and follow-up techniques on response to a mailed questionnaire. American Journal of Epidemiology 1998;148(10):1007-11. [DOI] [PubMed] [Google Scholar]
Hopkins 1983 {published data only}
- Hopkins K, Podolak J. Class-of-mail and the effects of monetary gratuity on the response rates of mailed questionnaires. Journal of Experimental Education 1983;51(4):169-70. [Google Scholar]
Hughes 1989 {published and unpublished data}
- Hughes JFR. Free reprints to increase the return of follow-up questionnaires. In: Abstracts from the Tenth Meeting of the Society of Clinical Trials, 1989 May 15-18; Minneapolis. Vol. 10. 1989:316-54.
Hunter 2018 {published data only}
- Hunter RF, Murray JM, Gough A, Tang J, Patterson C, French DP, et al. Effectiveness and cost-effectiveness of a loyalty scheme for physical activity behaviour change maintenance: results from a cluster randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2018;15(1):127. [DOI: 10.1186/s12966-018-0758-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iglesias 2000 {published data only}
- Iglesias C, Torgerson D. Does length of questionnaire matter? A randomised trial of response rates to a mailed questionnaire. Journal of Health Services Research & Policy 2000;5(4):219-21. [DOI] [PubMed] [Google Scholar]
Iglesias 2001 {published data only}
- Iglesias CP, Birks YF, Torgerson DJ. Improving the measurement of quality of life in older people: the York SF-12. QJM: an International Journal of Medicine 2001;94:695-8. [DOI] [PubMed] [Google Scholar]
Johnson 2004 {published data only}
- Johnson G, Oxman M, Schmader K, Levin M, Concato J, Williams H, et al. Use of an automated telephone response system for follow-up in a long-term multi-centre clinical trial. Clinical Trials 2004;2:211. [Google Scholar]
Juraskova 2014 {published data only}
- Juraskova I, Butow P, Bonner C, Bell ML, Smith AN, Seccombe M, et al. Improving decision making about clinical trial participation - a randomised controlled trial of a decision aid for women considering participation in the IBIS-II breast cancer prevention trial. British Journal of Cancer 2014;111(1):1-7. [DOI: 10.1038/bjc.2014.144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karras‐Jean Gilles 2019 {published data only}
- Karras-Jean Gilles J, Astuto J, Gjicali K, Allen LR. Sample retention in an urban context: exploring influential factors within a longitudinal randomized evaluation. American Journal of Evaluation 2019;40(2):268-90. [DOI: 10.1177/1098214017742719] [DOI] [Google Scholar]
Katz 2001 {published data only}
- Katz K, El-Mohandes A, McNeely Johnson D, Jarrett M, Rose A, et al. Retention of low income mothers in a parenting intervention study. Journal of Community Health 2001;26(3):203-18. [DOI] [PubMed] [Google Scholar]
Kim 2020 {published data only}
- Kim M, Lee H, Kiang P, Aronowitz T, Sheldon LK, Shi L, et al. A Storytelling intervention in a mobile, web-based platform: a pilot randomized controlled trial to evaluate the preliminary effectiveness to promote human papillomavirus vaccination in Korean American college women. Health Education & Behavior 2020;47(2):258-63. [DOI: 10.1177/1090198119894589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiwanuka 2018 {published data only}
- Kiwanuka N, Mpendo J, Asiimwe S, Ssempiira J, Nalutaaya A, Nambuusi B, et al. A randomized trial to assess retention rates using mobile phone reminders versus physical contact tracing in a potential HIV vaccine efficacy population of fishing communities around Lake Victoria, Uganda. BMC Infectious Diseases 2018;18:591. [DOI: 10.1186/s12879-018-3475-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krammer 1986 {published data only}
- Kramer FM, Jeffery RW, Snell MK. Monetary incentives to improve follow-up data collection. Psychological Reports 1986;58(3):739-42. [DOI: 10.2466/pr0.1986.58.3.739] [DOI] [PubMed] [Google Scholar]
Kuhlmann 2017 {published data only}
- Kuhlmann T, Reips UD, Wienert J, Lippke S. Using visual analogue scales in ehealth: non-response effects in a lifestyle intervention. Journal of Medical Internet Research 2016;18(6):e126. [DOI: 10.2196/jmir.5271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lannin 2013 {published data only}
- Lannin NA, Anderson C, Lim J, Paice K, Price C, Faux S, et al. Telephone follow-up was more expensive but more efficient than postal in a national stroke registry. Journal of Clinical Epidemiology 2013;66(8):896-902. [DOI: 10.1016/j.jclinepi.2013.03.005] [DOI] [PubMed] [Google Scholar]
Leidy 2000 {published and unpublished data}
- Leidy NK, Legro M, Schmier J, Zyczynski T, McHorney C, Coyne K. Does order of administration affect subject response on generic and condition-specific measures of health related quality of life? Quality of Life Research 2000;9:337. [Google Scholar]
Leigh Brown 1997 {published and unpublished data}
- Leigh Brown AP, Lawrie HE, Kennedy AD, Webb JA, Torgerson DJ, Grant AM. Cost effectiveness of a prize draw on response to a postal questionnaire: results of a randomised trial among orthopaedic outpatients in Edinburgh. Journal of Epidemiology and Community Health 1997;51(4):463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leighton 2018 {published data only}
- Leighton PA, Brealey SD, Dias JJ. Interventions to improve retention in a surgical, clinical trial: a pragmatic, stakeholder?driven approach. Journal of Evidence-based Medicine 2018;11(1):12-9. [DOI: 10.1111/jebm.12271] [DOI] [PubMed] [Google Scholar]
Litchfield 2005 {published data only}
- Litchfield J, Freeman J, Schou H, Elsley M, Fuller R, Chubb B. Is the future for clinical trials internet-based? A cluster randomized clinical trial. Clinical Trials. 2005;1:72-9. [DOI] [PubMed] [Google Scholar]
Malden 2019 {published data only}
- Malden S, Reilly JJ, Gibson AM, Bardid F, Summerbell C, De Craemer M, et al. A feasibility cluster randomised controlled trial of a preschool obesity prevention intervention: ToyBox-Scotland. Pilot and Feasibility Studies 2019 Dec 1;5(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
McAuley 1994 {published data only}
- McAuley E, Courneya K, Rudolph D, Cox CL. Enhancing exercise in middle aged males and females. Preventive Medicine 1994;23:498-506. [DOI] [PubMed] [Google Scholar]
McBee 2009 {published data only}
- McBee W, Clemons T, Chew E, SanGiovanni JP. A multi-level approach for retention of participants in a long-term clinical trial. Clinical Trials 2009;6:493-524. [Google Scholar]
Munoz 2017 {published data only}
- Muñoz RF, Leykin Y, Barrera AZ, Brown CH, Bunge EL. The impact of phone calls on follow-up rates in an online depression prevention study. Internet interventions. 2017 Jun 1;8:10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2019 {published data only}
- Murray JM, French DP, Patterson CC, Kee F, Gough A, Tang J, et al. Predicting outcomes from engagement with specific components of an internet-based physical activity intervention with financial incentives: process analysis of a cluster randomized controlled trial. Journal of Medical Internet Research 2019;21(4):e11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2020 {published data only}
- Murray JM, French DP, Kee F, Gough A, Tang J, Hunter RF. Mechanisms of physical activity behavior change in an incentive-based intervention: mediation analysis. Health Psychology 2020;39(4):281-97. [DOI: 10.1037/hea0000849] [DOI] [PubMed] [Google Scholar]
Nicholas 2013 {published data only}
- Nicholas A, Bailey JV, Stevenson F, Murray E. The Sexunzipped Trial: young people?s views of participating in an online randomized controlled trial. Journal of Medical Internet Research 2013;15(12):e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielson 2018 {published data only}
- Nielson CM, Rivelli JS, Fuoco MJ, Gawlik VR, Jimenez R, Petrik AF, et al. Effectiveness of automated and live phone reminders after mailed-FIT outreach in a pilot randomized trial. Preventive Medicine Reports 2018;12:210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Novak 2019 {published data only}
- Novak LA, Belsher BE, Freed MC, McCutchan PK, Liu X, Evatt DP, et al. Impact of financial reimbursement on retention rates in military clinical trial research: a natural experiment within a multi-site randomized effectiveness trial with active duty service members. Contemporary Clinical Trials Communications 2019;15:100353. [DOI: 10.1016/j.conctc.2019.100353] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuzzolese 2020 {published data only}
- Nuzzolese I, Montemurro F. Attrition in metastatic breast cancer: a metric to be reported in randomised clinical trials? Lancet Oncology 2020;21(1):21-4. [DOI: 10.1016/S1470-2045(19)30792-2] [DOI] [PubMed] [Google Scholar]
Parker 2019 {published data only}
- Parker A, Arundel C, Beard D, Bower P, Brocklehurst P, Coleman E, et al. PROMoting THE USE OF SWATs (PROMETHEUS): routinely embedding recruitment and retention interventions within randomised trials. Trials 2019;20(Suppl 1):46-7. [Google Scholar]
Paul 2011 {published data only}
- Paul J, Iveson T, Midgley R, Harkin A, Masterton M, Alexander L, et al. Choice of randomisation time-point in non-inferiority studies of reduced treatment duration: experience from the SCOT study. Trials 2011;12:1-2. [DOI: 10.1186/1745-6215-12-S1-A30] [DOI] [Google Scholar]
Phiri 2019 {published data only}
- Phiri SC, Mudhune S, Prust ML, Haimbe P, Shakwelele H, Chisenga T, et al. Impact of the Umoyo mother-infant pair model on HIV-positive mothers? social support, perceived stigma and 12-month retention of their HIV-exposed infants in PMTCT care: evidence from a cluster randomized controlled trial in Zambia. Trials 2019;20(1):505. [DOI: 10.1186/s13063-019-3617-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pieper 2018 {published data only}
- Pieper D, Kotte N, Ober P. The effect of a voucher incentive on a survey response rate in the clinical setting: a quasi-randomized controlled trial. BMC Medical Research Methodology 2018;18(1):1-4. [DOI: 10.1186/s12874-018-0544-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poling 2006 {published data only}
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Archives of General Psychiatry 2006;63:219-28. [DOI] [PubMed] [Google Scholar]
Price 2019 {published data only}
- Price A, Bryson H, Smith A, Mensah F, Goldfeld S. Processes for engaging and retaining women who are experiencing adversity in longitudinal health services research. BMC health services research 2019;19(1):833. [DOI: 10.1186/s12913-019-4698-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puffer 2004 {published data only}
- Puffer S, Porthouse J, Birks Y, Morton V, Torgerson D. Increasing response rates to postal questionnaires: a randomised trial of variations in design. Journal of Health Services Research and Policy 2004;9(4):213?7. [DOI] [PubMed] [Google Scholar]
Rhoades 1998 {published data only}
- Rhoades H, Creson D, Elk R, Schmitz J, Grabowski J. Retention, HIV risk, and illicit drug use during treatment: methadone dose and visit frequency. American Journal of Public Health 1998;88(1):34-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2000 {published data only}
- Roberts P, Roberts C, Sibbald B, Torgerson D. The effect of a direct payment or a lottery on questionnaire response rates: a randomised controlled trial. Journal of Epidemiology and Community Health 2000;54:71-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodgers 2019a {published data only}
- Rodgers S, Sbizzera I, Cockayne S, Fairhurst C, Lamb SE, Vernon W, et al. A study update newsletter or Post-it® note did not increase postal questionnaire response rates in a falls prevention trial: an embedded randomised factorial trial. F1000Research 2019;7:1083. [DOI: 10.12688/f1000research.14591.2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodgers 2019b {published data only}
- Rodgers M, Meisel Z, Wiebe D, Crits-Christoph P, Rhodes KV. Wireless participant incentives using reloadable bank cards to increase clinical trial retention with abused women drinkers: a natural experiment. Journal of Interpersonal Violence 2019;34(13):2774-96. [DOI: 10.1177/0886260516662849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rolfson 2011 {published data only}
- Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery?reliability and response rate. Value in Health 2011;14(2):316-21. [DOI: 10.1016/j.jval.2010.08.004 ] [DOI] [PubMed] [Google Scholar]
Sano 2013 {published data only}
- Sano M, Egelko S, Donohue M, Ferris S, Kaye J, Hayes TL, et al. Developing dementia prevention trials: baseline report of the Home-Based Assessment study. Alzheimer Disease and Associated Disorders 2013;27(4):356-62. [DOI: 10.1097/WAD.0b013e3182769c05] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2005 {published data only}
- Schmitz J, Sayre S, Stotts A, Rothfleisch J, Mooney M. Medication compliance during a smoking cessation clinical trial: a brief intervention using MEMS feedback. Journal of Behavioral Medicine 2005;28(2):139-47. [DOI] [PubMed] [Google Scholar]
Shulman 2019 {published data only}
- Shulman GP, Buck BE, Gahm GA, Reger GM, Norr AM. Effectiveness of the intent to complete and intent to attend intervention to predict and prevent posttraumatic stress disorder treatment drop out among soldiers. Journal of Traumatic Stress 2019;32(5):784-90. [DOI: 10.1002/jts.22427] [DOI] [PubMed] [Google Scholar]
Smeeth 2001ab {published data only}
- Smeeth L, Fletcher AE, Stirling S, Nunes M, Breeze E, Ng E, et al. Randomised comparison of three methods of administering a screening questionnaire to elderly people: findings from the MRC trial of the assessment and management of older people in the community. BMJ 2001;323(7326):1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2015 {published data only}
- Smith V, Clarke M, Begley C, Devane D. SWAT-1: The effectiveness of a?site visit? intervention on recruitment rates in a multi-centre randomised trial. Trials 2015;16(1):211. [DOI: 10.1186/s13063-015-0732-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoner 1998 {published data only}
- Stoner T, Dowd B, Carr W, Maldonado G, Church T, Mandel J. Do vouchers improve breast cancer screening rates? Results from a randomized trial. Health Services Research 1998;33(1):11-28. [PMC free article] [PubMed] [Google Scholar]
Svoboda 2001 {published and unpublished data}
- Svoboda P. A comparison of two questionnaires for assessing outcome after head injury. Personal email communication from P Edwards in April 2010.
Tariq 2019 {published data only}
- Tariq MB, Jones MH, Strnad G, Sosic E, Spindler KP. A last-ditch effort and personalized surgeon letter improves PROMs follow-up rate in sports medicine patients: a crossover randomized controlled trial. Journal of Knee Surgery 2021;34(2):130-136. [DOI: 10.1055/s-0039-1694057] [DOI] [PubMed] [Google Scholar]
Tassopoulos 2007 {published data only}
- Tassopoulos C. The challenges of follow-up in a multi-centre randomised controlled trial. Clinical Trials 2007;4:371-455. [Google Scholar]
Trevena 2006 {published data only}
- Trevena L, Irwig L, Barratt A. Impact of privacy legislation on the number and characteristics of people who are recruited for research: a randomised controlled trial. Journal of Medical Ethics 2006;32(8):473-7. [DOI: 10.1136/jme.2004.011320] [DOI] [PMC free article] [PubMed] [Google Scholar]
von Allmen 2019 {published data only}
- Allmen RS, Tinner C, Schmidli J, Tevaearai HT, Dick F. Randomized controlled comparison of cross-sectional survey approaches to optimize follow-up completeness in clinical studies. PLOS One 2019;14(3):e0213822. [DOI: 10.1371/journal.pone.0213822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagstaff 2019 {published data only}
- Wagstaff A, Doorslaer E, Burger R. SMS nudges as a tool to reduce tuberculosis treatment delay and pretreatment loss to follow-up. A randomized controlled trial. PLOS One 2019 Jun 20;14(6):e0218527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wensing 2005 {published data only}
- Wensing M, Schattenberg G. Initial nonresponders had an increased response rate after repeated questionnaire mailings. Journal of Clinical Epidemiology 2005;58(9):959-61. [DOI: 10.1016/j.jclinepi.2005.03.002] [DOI] [PubMed] [Google Scholar]
Weston 2017 {published data only}
- Weston D, Parsons V, Ntani G, Rushton L, Madan I. Mixed contact methods to improve response to a postal questionnaire. Occupational Medicine 2017;67(4):305-7. [DOI: 10.1093/occmed/kqx032] [DOI] [PubMed] [Google Scholar]
Wood 2015 {published data only}
- Wood J, Bruhn H, Cook JA, McDonald A, Norrie J, Watson AJ. PTU-225 Strategies to improve response rates to patient reported outcome measures in a surgical rct. Gut 2015;64:A161-2. [DOI: 10.1136/gutjnl-2015-309861.340] [DOI] [Google Scholar]
Wood 2017 {published data only}
- Wood J, Cook JA, Hudson J, McDonald A, Bruhn H, Watson AJ. Do higher monetary incentives improve response rates part-way through a randomised control trial? Trials 2017;18(Suppl 1):P65. [DOI: 10.1186/1745-6215-16-S2-P123 ] [Google Scholar]
Wu 1997 {published and unpublished data}
- Wu AW, Jacobson DL, Berzon RA, Revicki DA, Horst C, Fichtenbaum CJ, et al. The effect of mode of administration on medical outcomes study health ratings and EuroQol scores in AIDS. Quality of Life Research 1997;6(1):3-10. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Letley 2000 {published data only}
- Letley L, Garratt A. Questionnaire length and internal reliability: a randomised study. In: Society for Social Medicine 43rd Annual Scientific Meeting; 2000 6-8 Sept; Norwich. 2000.
Sutherland 1996 {published data only}
- Sutherland HJ, Beaton M, Mazer R, Kriukov V, Boyd NF. A randomized trial of the total design method for the postal follow-up of women in a cancer prevention trial. European Journal of Cancer Prevention 1996;5(3):165-8. [DOI: 10.1097/00008469-199606000-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
SWAT #100 {unpublished data only}
- O'Neill L, Knapp P, Doyle S, Guinan E, Hussey J. SWAT 100: Patient and family co-developed participant information to improve recruitment rates, retention, and patient understanding of a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,914713,en.pdf. [DOI] [PMC free article] [PubMed]
SWAT #105 {unpublished data only}
- Hynes S, Dwyer C, Joyce R. SWAT 105: Effects of a patient-designed-and-informed participant information sheet versus a standard, researcher-designed information sheet on recruitment to a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,926069,en.pdf.
SWAT #107 {unpublished data only}
- Shiely F. SWAT 107: Effects of a multi-trial programmable animation platform on the efficiency and success of pre-screening and subsequent recruitment to a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,949779,en.pdf.
SWAT #109 {unpublished data only}
- Parker A, Brealey S, Sharma HK, Welch C, Keding A, Flett L, Hunkins D, Witts J, McDaid C. SWAT 109: The effectiveness of a text message reminder which participants can respond to, compared with a?no reply? text message on questionnaire response rates. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,943284,en.pdf.
SWAT#110 {unpublished data only}
- Ooms A. SWAT 110: Printing the primary outcomE on Pink PapER versus standard paper to increase participant engagement to postal questionnaires (PEPPER). https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,949651,en.pdf.
SWAT#112 {unpublished data only}
- Woodford J & von Essen L. SWAT 112: Effects on recruitment of a personalised compared with a standard study invitation letter. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,939618,en.pdf.
SWAT #119 {unpublished data only}
- Arundel C, Chetter I, Fairhurst C, Joshi K, McCaffery J, Mott A, Wilkinson J. SWAT 119: Effects on retention of giving trial participants a thank you card following each study visit.. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,959362,en.pdf.
SWAT #121 {unpublished data only}
- Dhanjal G. SWAT 121: What are the effects on retention and follow-up of courtesy telephone calls versus postcards to trial participants following enrolment? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,957826,en.pdf.
SWAT #51 {unpublished data only}
- Agus A. SWAT 51: Promoting group identity to improve questionnaire return rate. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,758574,en.pdf.
SWAT #54 {unpublished data only}
- Anand R. SWAT 54: Giving trial participants a thank you note or card after each study visit. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,758633,en.pdf.
SWAT #63 {unpublished data only}
- Azuara-Blanco A & Clarke M. SWAT 63: Does local radio and social media advertisement increase recruitment? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,906043,en.pdf.
SWAT #79 {unpublished data only}
- Backhouse M, Torgerson D, Parker A, Cockayne S. SWAT 79: Effect of birthday cards with or without nudge on retention and data completion rates in trials involving children. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,864304,en.pdf.
SWAT #81 {unpublished data only}
SWAT #82 {published data only}
- Treweek S, Gillies K, Innes K, MacLennan G. SWAT 82: Sending Christmas cards to trial participants to improve retention.. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,846275,en.pdf. [DOI] [PMC free article] [PubMed]
SWAT #86 {unpublished data only}
- Sutton C, Cotterill S, Forshaw, D Rhodes S, Hammond A. SWAT 86: Advance notification of trial participants before outcome data collection to improve retention. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,902355,en.pdf.
SWAT #87 {unpublished data only}
- Hopewell S, Cureton L, Greenall G. SWAT 87: Do participants complete the original or the reminder postal follow up questionnaire? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,902356,en.pdf.
SWAT #89 {unpublished data only}
- Starr K, McRae D, Gillies K, Cooper C, Wolfe A. SWAT 89: Including a theoretically informed leaflet in a participant takehome pack of questionnaires to increase response rate. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,911406,en.pdf.
SWAT #92 {unpublished data only}
- Tew G, Tilbrook H, Paul S, Howe L, Parker A, Bell K. SWAT 92: Pen incentive to enhance retention in a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,903297,en.pdf.
SWAT #97 {unpublished data only}
- kKnapp P. SWAT 97: TRECA (TRials Engagement in Children and Adolescents). https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/FileStore/Filetoupload,903301,en.pdf.
Additional references
Bensaaud 2020
- Bensaaud A, Gibson I, Jones J, Flaherty G, Sultan S, Tawfick W, Jordan F. A telephone reminder to enhance adherence to interventions in cardiovascular randomized trials: a protocol for a study within a trial (SWAT). Journal of Evidence-based Medicine 2020;13(1):81-4. [DOI: 10.1111/jebm.12375] [DOI] [PubMed] [Google Scholar]
Booker 2011
- Booker C, Harding S, Benzeval M. A systematic review of the effect of retention methods on response rates in population-based cohort studies. BMC Public Health. 2011;11:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunsdon 2019
- Brunsdon D, Biesty L, Brocklehurst P, Brueton V, Devane D, Elliott J, et al. What are the most important unanswered research questions in trial retention? A James Lind Alliance Priority Setting Partnership: the PRioRiTy II (Prioritising Retention in Randomised Trials) study. Trials 2019;20(1):593. [DOI: 10.1186/s13063-019-3687-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan A-W, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet 2013;381(9861):91-2. [DOI: 10.1016/S0140-6736(12)62160-6.] [DOI] [PubMed] [Google Scholar]
Edwards 2009
- Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: MR000008. [DOI: 10.1002/14651858.MR000008.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fewtrell 2008
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Archives of Disease in Childhood 2008;93(6):458-61. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck?Ytter Y, Alonso?Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JP, Altman D. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors(s). Cochrane Handbook of Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:188-241. [Google Scholar]
Higgins 2008b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:482-529. [Google Scholar]
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madurasinghe 2016
- Madurasinghe VW, Eldridge S, on behalf of MRC START Group and Gordon Forbes on behalf of the START Expert Consensus Group. Guidelines for reporting embedded recruitment trials. Trials 2016;17:27. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI: 10.1136/bmj.c869] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2009
- Murray E, Khadjesari Z, White IR, Kalaitzaki E, Godfrey C, McCambridge J, et al. Methodological challenges in online trials.. Journal of Medical Internet Research 2009;11(2):e9. [DOI: 10.2196/jmir.1052] [DOI] [PMC free article] [PubMed] [Google Scholar]
PROMETHEUS
- PROMETHEUS. https://www.york.ac.uk/healthsciences/research/trials/research/swats/prometheus/.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robotham 2016
- Robotham D, Satkunanathan S, Reynolds J, Stahl D, Wykes T. Using digital notifications to improve attendance in clinic: systematic review and meta-analysis. BMJ Open 2016;6(10):e012116. [DOI: 10.1136/bmjopen-2016-012116] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019] [DOI] [PubMed] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359(9308):781-5. [DOI] [PubMed] [Google Scholar]
Skea 2019
- Skea ZC, Newlands R, Gillies K. Exploring non-retention in clinical trials: a meta-ethnographic synthesis of studies reporting participant reasons for drop out. BMJ Open 2019;9(6):e021959. [DOI: 10.1136/bmjopen-2018-021959] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2008
- Sterne J, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:297-333. [Google Scholar]
SWAT
- SWAT repository. www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/SWATSWARInformation/Repositories/SWATStore/ Accessed on 1 December 2020.
Treweek 2018
- Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: MR000013. [DOI: 10.1002/14651858.MR000013.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treweek 2020b
- Treweek S, Bevan S, Bower P, Briel M, Campbell M, Christie J, et al. Trial Forge Guidance 2: How to decide if a further Study Within A Trial (SWAT) is needed. Trials 2020;21:33. [DOI: 10.1186/s13063-019-3980-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trial Forge
- Trial Forge - A systematic way to improve trial efficiency. wwww.trialforge.org.
Walsh 2014
- Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. Journal of Clinical Epidemiology 2014;67(6):622-8. [DOI: 10.1016/j.jclinepi.2013.10.019] [DOI] [PubMed] [Google Scholar]
Walsh 2015
- Walsh M, Sackett D, Deveraux PJ. When RCT participants are lost to follow up. Why even a few can matter. Clinical Trials 2015;12:537?9. [DOI: 10.1177/1740774515597702] [DOI] [PubMed] [Google Scholar]
Walters 2016
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7:3. [DOI: 10.1136/bmjopen-2016-015276] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Brueton 2011
- Brueton VC, Rait G, Tierney J, Meredith S, Darbyshire J, Harding S, et al. Strategies to reduce attrition in randomised trials. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: MR000032. [DOI: 10.1002/14651858.MR000032] [DOI] [Google Scholar]
Brueton 2013
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No: MR000032. [DOI: 10.1002/14651858.MR000032.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


