Abstract
Background
Treatment and diagnostic recommendations are often made in clinical guidelines, reports from advisory committee meetings, opinion pieces such as editorials, and narrative reviews. Quite often, the authors or members of advisory committees have industry ties or particular specialty interests which may impact on which interventions are recommended. Similarly, clinical guidelines and narrative reviews may be funded by industry sources resulting in conflicts of interest.
Objectives
To investigate to what degree financial and non‐financial conflicts of interest are associated with favourable recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews.
Search methods
We searched PubMed, Embase, and the Cochrane Methodology Register for studies published up to February 2020. We also searched reference lists of included studies, Web of Science for studies citing the included studies, and grey literature sources.
Selection criteria
We included studies comparing the association between conflicts of interest and favourable recommendations of drugs or devices (e.g. recommending a particular drug) in clinical guidelines, advisory committee reports, opinion pieces, or narrative reviews.
Data collection and analysis
Two review authors independently included studies, extracted data, and assessed risk of bias. When a meta‐analysis was considered meaningful to synthesise our findings, we used random‐effects models to estimate risk ratios (RRs) with 95% confidence intervals (CIs), with RR > 1 indicating that documents (e.g. clinical guidelines) with conflicts of interest more often had favourable recommendations. We analysed associations for financial and non‐financial conflicts of interest separately, and analysed the four types of documents both separately (pre‐planned analyses) and combined (post hoc analysis).
Main results
We included 21 studies analysing 106 clinical guidelines, 1809 advisory committee reports, 340 opinion pieces, and 497 narrative reviews. We received unpublished data from 11 studies; eight full data sets and three summary data sets. Fifteen studies had a risk of confounding, as they compared documents that may differ in other aspects than conflicts of interest (e.g. documents on different drugs used for different populations). The associations between financial conflicts of interest and favourable recommendations were: clinical guidelines, RR: 1.26, 95% CI: 0.93 to 1.69 (four studies of 86 clinical guidelines); advisory committee reports, RR: 1.20, 95% CI: 0.99 to 1.45 (four studies of 629 advisory committee reports); opinion pieces, RR: 2.62, 95% CI: 0.91 to 7.55 (four studies of 284 opinion pieces); and narrative reviews, RR: 1.20, 95% CI: 0.97 to 1.49 (four studies of 457 narrative reviews). An analysis combining all four document types supported these findings (RR: 1.26, 95% CI: 1.09 to 1.44).
One study investigating specialty interests found that the association between including radiologist guideline authors and recommending routine breast cancer screening was RR: 2.10, 95% CI: 0.92 to 4.77 (12 clinical guidelines).
Authors' conclusions
We interpret our findings to indicate that financial conflicts of interest are associated with favourable recommendations of drugs and devices in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews. However, we also stress risk of confounding in the included studies and the statistical imprecision of individual analyses of each document type. It is not certain whether non‐financial conflicts of interest impact on recommendations.
Plain language summary
Conflicts of interest and recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews
Which treatments and diagnostic tests doctors offer to their patients are often based on recommendations expressed in a variety of documents. A common example is clinical guidelines, which are statements providing recommendations on how to diagnose and treat patients on the basis of the best available evidence. The treatments that may be offered to patients are also influenced by which drugs are recommended for approval by drug advisory committees at regulatory drug agencies such as the US Food and Drug Administration (FDA). Finally, doctors may also be influenced by recommendations expressed in opinion pieces, such as editorials, or in narrative review papers in medical journals.
Quite often, publications expressing clinical recommendations are written by authors with conflicts of interest related to a specific product, for example when the author acts as a consultant for the company producing the treatment of interest. Such conflicts of interest may impact on the recommendations made. Similarly, authors may have so‐called non‐financial conflicts of interest such as belonging to a specific profession, for example being an orthopaedic surgeon, which may influence whether a specific intervention is preferred over another. This Cochrane Methodology Review investigated how financial and non‐financial conflicts of interest are associated with the recommendations made in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews.
We included 21 studies and we interpreted our findings to indicate that financial conflicts of interest are associated with favourable recommendations in these documents, although there is some uncertainty around the size of the effect. This means that when such publications are written by authors with financial conflicts of interest, they more often have favourable recommendations than publications written by authors without conflicts of interest. Only a single study investigated the impact of non‐financial conflicts of interest in clinical guidelines and the results were uncertain, but indicated a similar direction of effect.
We suggest that patients, doctors, and healthcare decision makers primarily use clinical guidelines, opinion pieces, and narrative reviews that have been written by authors without financial conflicts of interest. If that is not possible, users should read and interpret the publications with caution. Furthermore, our findings suggest that if committee members are asked to vote on the recommendation of a drug, they may be more likely to vote in favour of the drug when they have financial conflicts of interest.
Background
Recommendations of treatment and diagnostic approaches impact on patient care, especially if they are written by “key opinion leaders” or originate from healthcare authorities. Recommendations may appear in multiple types of documents, for example in clinical guidelines and advisory committee reports (which could include records from meetings in regulatory drug advisory committees or hospital drug and therapeutics committees) as well as in opinion pieces such as editorials, and in narrative reviews.
Quite often, publications with clinical recommendations are written by authors with conflicts of interest related to the drug or device industry. For example, in a sample of 45 clinical guidelines written by 254 authors, Bindslev and colleagues found that 135 (53%) authors had financial conflicts of interest (Bindslev 2013). Similarly, studies report that narrative reviews, editorials and commentaries often (31%) had at least one author with conflicts of interest (Grundy 2018), and around a quarter of committee meetings at the US Food and Drug Administration (FDA) included at least one voting member with financial conflicts of interest (Xu 2017).
Authors may also have non‐financial conflicts of interest. For example, if authors of a guideline were also authors of some of the included studies on which recommendations in a guideline were based, the authors may be more likely to favour the interventions that they previously studied (Akl 2014). Whereas financial conflicts of interest are relatively simple to characterise (i.e. any financial relationship with a party with an interest in the direction of a recommendation), it is more unclear and debated which interests and relationships constitute a non‐financial conflict of interest and whether the term is appropriate (Bero 2016). This lack of consensus regarding non‐financial conflicts of interest is also reflected in journal disclosure policies. Shawwa and colleagues found that only 57% of core clinical journals specifically required disclosure of non‐financial conflicts of interest, and that there was large variation in how journals defined such conflicts (Shawwa 2016).
Numerous studies have investigated the impact of financial conflicts of interest on the interpretation of the results of primary research studies, mainly clinical trials. An updated Cochrane Methodology Review reported an association between industry funding and favourable conclusions in primary research studies (Lundh 2017). This association has been attributed to various factors, including the sponsor's influence on framing the question, study design, and reporting of results (Bero 1996; Bero 2007; Fabbri 2018). Similarly, another Cochrane Methodology Review reported an association between financial conflicts of interest and favourable conclusions in systematic reviews (Hansen 2019a). In contrast, few studies have investigated the association between conflicts of interest and favourable recommendations in clinical guidelines (Norris 2012), advisory committee reports (Pham‐Kanter 2014), opinion pieces (Bariani 2013), and narrative reviews (Dunn 2016). Furthermore, the evidence from such studies has to our knowledge not previously been synthesised in a methodological systematic review. This review fills that gap and is based on the previously published protocol (Hansen 2019b).
How these methods might work
Financial conflicts of interest such as honoraria, consultancies, grants, or advisory board membership can provide a substantial income for physicians and academic researchers. Such relationships may therefore affect how the benefits and harms of the companies' products are perceived by authors and thereby whether they are recommended in publications by the authors. Similarly, non‐financial interests, such as authors’ professional affiliations and personal relationships, may influence the recommendation of a particular intervention.
In contrast to primary research papers and systematic reviews, clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews typically provide specific recommendations concerning treatments and diagnostics. However, the methodological rigour behind such recommendations differs between the types of publications. Clinical guidelines are increasingly based on systematic searches of existing evidence and may follow standardised procedures for grading evidence and recommendations (Guyatt 2011). In contrast, authors of opinion pieces are free to selectively cite studies and interpret the evidence, and editorials often focus on results from a single primary study. Clinical guidelines are also typically written by a broad group of authors who may have differing viewpoints, whereas opinion pieces are often written by single authors. Thus, clinical guidelines may be less susceptible to influence from conflicts of interest compared to opinion pieces. Committee reports and narrative reviews are conducted using more or less systematic procedures, but also involve subjective elements and may therefore be more susceptible to influence from conflicts of interest than clinical guidelines, but less than opinion pieces.
Why it is important to do this review
Recommendations in journal papers or guidelines and decisions about which interventions are approved by regulatory authorities have substantial impact on the interventions offered to patients. It is therefore important that such recommendations are evidence‐based and as little influenced by conflicts of interest as possible. Individual studies have investigated the associations between conflicts of interest and favourable recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews, but these studies differ in methods and conclusions. Despite conflicts of interest being recognised as an important source of influence on clinical recommendations, these studies have, to our knowledge, not previously been summarised in a systematic review. Findings from this review may provide patients, clinicians, and policymakers with guidance on how to interpret recommendations in light of conflicts of interest and may assist journal editors, guideline issuing organisations, and public authorities with managing such conflicts.
Objectives
Our objectives were to investigate to what degree financial and non‐financial conflicts of interests are associated with favourable recommendations in:
clinical guidelines;
advisory committee reports (e.g. records from the Food and Drug Administration (FDA) advisory committee on oncological drugs or hospital drug and therapeutics committees);
opinion pieces (e.g. editorials and commentaries);
narrative reviews.
Terminology
We used the definitions below. All definitions are described in more detail in Appendix 1.
Conflicts of interest: any financial or non‐financial conflicts of interest as specified below.
Financial conflicts of interest: any funding of clinical guidelines, opinion pieces, or narrative reviews by drug or device companies or any authors or committee members with ties to such companies (e.g. advisory board membership).
Non‐financial conflicts of interest: any relationships that differ from what is typically regarded as financial conflicts of interest (i.e. relationships with the drug or device industry). Regardless of the definitions used by the authors of the included studies, we do not focus on studies investigating beliefs (e.g. political or religious), personal experience (e.g. abuse or trauma), or institutional conflicts of interest (Bero 2016).
Drugs: medications that require approval from a regulatory authority.
Devices: instruments used in diagnosis, treatment, or prevention of disease (FDA 2017). This term also includes medical imaging technologies.
Clinical guidelines: “Systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances” (Institute of Medicine 1990).
Advisory committee reports: reports from meetings held in committees, boards, councils, or similar formalised groups that are established to advise an organisation and provide a recommendation concerning an intervention (e.g. the FDA advisory committee on oncological drugs).
Opinion pieces: publications that are not research studies in which an author expresses a personal opinion about a specific intervention (e.g. editorials, commentaries, and letters to the editor).
Narrative reviews: literature reviews without a systematic search of the literature with clear eligibility criteria.
Documents: clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished studies of any design (e.g. cross‐sectional studies) that assessed the association between conflicts of interest and favourable recommendations made in clinical guidelines, advisory committee reports, opinion pieces, or narrative reviews concerning drug or device interventions (which include diagnostic tests for the purposes of this review, see Appendix 1).
Studies in all languages were eligible.
Types of data
We included studies with dichotomous (e.g. favourable or unfavourable recommendations) or continuous data (e.g. percentages) on the association between conflicts of interest and recommendations in favour of the intervention in question.
Types of methods
We included studies that investigated documents with conflicts of interest versus documents without conflicts of interest. For financial conflicts of interest, we included studies regardless of the type of financial conflict. For non‐financial conflicts of interest, we included studies on intellectual, academic, professional, or specialty interests, and personal or professional relationships.
We excluded studies concerning:
financial conflicts of interest not related to the drug or device industry (e.g. tobacco or nutrition industry);
beliefs (e.g. religious) or personal experiences (e.g. suffering from the medical condition), even if the original authors defined these as non‐financial conflicts of interest;
membership of certain groups (e.g. gender or ethnicity), even if the original authors defined this as non‐financial conflicts of interest;
both financial and non‐financial conflicts of interest at the level of an institution;
conflicts of interest related to reports from scientific grant committees.
Types of outcome measures
Primary outcomes
Our primary outcome was the type of recommendation in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews. We defined ‘favourable recommendations’ according to the definitions used by the authors of the included studies.
Search methods for identification of studies
Electronic searches
We searched PubMed, Embase, and the Cochrane Methodology Register (up to February 2020). We searched Web of Science (up to March 2020) for studies that cited any of the included studies.
Search strategy
Our search strategy was based on search terms used in a PubMed search from two previous Cochrane Methodology Reviews on financial conflicts of interest in primary research studies and systematic reviews (Lundh 2017; Hansen 2019a), and tailored it for this review (Appendix 2). The PubMed strategy was adapted for Embase and The Cochrane Methodology Register. All search strategies were developed in collaboration with information specialists.
Searching other resources
Grey literature
Our electronic search in the Cochrane Methodology Register identified relevant grey literature because the database includes conference abstracts. Additionally, we searched for conference abstracts from Peer Review Congresses (American Medical Association 2017), Cochrane Colloquia (Cochrane Community 2017), and Evidence Live (Centre for Evidence‐Based Medicine 2017) (search of all conferences up to February 2020). We searched PROSPERO (up to February 2020) for registered systematic reviews and the ProQuest database (up to February 2020) for dissertations and theses.
Additional searches
We used Google Scholar (up to March 2020) to search for additional eligible studies. We based our search on core search terms from the search strategy defined in Appendix 2 and screened the first 20 records for each search. We searched PubMed for publications by the first and last author of the included studies (up to March 2020). Other sources of data included the files of the authors of this review and checking reference lists of included studies (Horsley 2011).
Data collection and analysis
Selection of studies
One review author (CHN) screened titles and abstracts of all retrieved records for obvious exclusions. Two review authors (CHN and either AWJ or AL) independently assessed potentially eligible studies based on full text. We resolved any disagreements by discussion and used arbitration by a third review author (AL or AH) when needed.
Reasons for exclusion of studies are described in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (CHN and either AWJ, ML, or AL) independently extracted data from included studies. We resolved any differences in data extraction by discussion and used arbitration by a third review author (AH or AL) when needed.
We extracted data on basic characteristics of the included studies and data on the association between conflicts of interest and favourable recommendations. We extracted data for documents with and without conflicts of interest based on the definitions used by the authors of the included studies. When reported, we also extracted effect measures and confidence intervals (CIs) or the raw data to calculate them. We also extracted information on funding sources and conflicts of interest disclosures of authors of the included studies. The full plan for data extraction is reported in Appendix 3.
Assessment of risk of bias in included studies
As there are no published assessment tools for investigating bias in these types of studies, we developed our own criteria based on those used in previous Cochrane Methodology Reviews on financial conflicts of interest in primary research studies and systematic reviews (Lundh 2017; Hansen 2019a). In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020) we use the term ‘risk of bias’ in contrast to ‘methodological quality’. However, we recognise that some of the included items are more related to methodological quality than risk of bias and that inadequate methodological quality (e.g. coding of conflicts of interest information by a single author) is not necessarily biased. In our risk of bias assessment we therefore focused on whether study methodology was appropriate (i.e. appropriate methodology resulted in low risk of bias).
Two review authors (CHN and either AWJ, ML, or AL) independently assessed included studies for risk of bias. We resolved any disagreements by discussion and used arbitration by a third review author (AL or AH) when needed. We used the following criteria.
Whether there was a risk of bias in the inclusion of documents (low risk of bias may, for example, include reporting of clear inclusion criteria with two or more assessors independently selecting documents).
Whether there was a risk of bias in the coding of conflicts of interest (low risk of bias may, for example, include coding done by two or more assessors based on multiple sources of information).
Whether there was a risk of bias in the coding of recommendations (low risk of bias may, for example, include coding done by two or more assessors blinded to the status of conflicts of interest).
Whether there was a risk of confounding (low risk of confounding may, for example, include documents with and without conflicts of interest discussing the same treatment used in similar groups of patients). The documents included in a study may differ on key aspects (e.g. in a sample of clinical guidelines, the guidelines may differ in relation to types of patients and conditions, interventions, the quality of the underlying evidence, and the quality of the guidelines), which could potentially confound the association between conflicts of interest and favourable recommendations.
In assessing risk of bias, our primary aim was to differentiate between studies with higher and lower risk of bias. Thus, we coded, by default, a study as low risk of bias if all criteria were assessed as low risk of bias; otherwise, we coded it as high risk of bias.
Dealing with missing data
We contacted authors of the included studies in an attempt to obtain unpublished data, to clarify issues on our 'Risk of bias' assessments, or to receive copies of unpublished protocols (Appendix 4). When we received unpublished data, we analysed the data according to the methods used in the original studies.
We included one study that investigated a mixture of opinion pieces and narrative reviews, but which did not report results stratified by document type. However, coding of financial conflicts of interest and recommendations were reported separately for each document (Hayes 2019). As the type of document (e.g. opinion piece) was not coded in the original study, two review authors (CHN and AL) independently coded the type of documents to enable inclusion in our meta‐analyses.
Assessment of heterogeneity
Statistical heterogeneity was described using the I2 statistic.
To further address statistical heterogeneity, we calculated prediction intervals for our primary analyses. We only calculated prediction intervals when at least four studies were included in the pooled analysis, because intervals will be imprecise when the effect estimates are based on only a few studies. A prediction interval presents the expected range of true effects in similar studies, is not influenced by sample size, and shows whether the study effects are dispersed over a wide range (IntHout 2016). A prediction interval thereby shows the range of risk ratios (RRs) that can be expected from similar studies, and, thus, a broad prediction interval indicates heterogeneity and uncertainty. To calculate prediction intervals, we used the formula presented by Riley and colleagues (Riley 2011) (Appendix 5).
Data synthesis
Data management of individual studies
In our primary analyses, we used the definitions and coding of recommendations and conflicts of interest used by the authors of the included studies. If an ordinal scale was used to grade recommendations (e.g. highly positive, positive, neutral, negative, and highly negative), we recoded recommendations into two categories (i.e. favourable versus neutral/unfavourable recommendations).
If the sample of documents included in a study contained a mixture of types of documents (e.g. both clinical guidelines and research papers), we only included the study in our pooled analyses if we could get separate data for the types of documents relevant for our review.
In our analyses on clinical guidelines, we included one study that investigated 13 guidelines that each included recommendations on 24 different drugs (Norris 2013). To allow for this type of panel data, we used Poisson Generalised Estimating Equations to calculate effect estimates we could include in our pooled analyses (Lumley 2006).
In our analyses on advisory committee reports, we included studies with two types of analysis units: committee members and their individual votes (individual level) and committee reports and the overall voting outcome (meeting level). In our primary analysis, we analysed data on meeting level as this level of analysis was most comparable to recommendations in the other types of documents (e.g. clinical guidelines).
In some cases the same document was included in two separate studies. When we had access to unpublished data it was possible to remove the duplicate documents and we chose to remove it from the study with the latest publication date. We included two studies that investigated some of the same FDA advisory committee reports (Ackerley 2009; Lurie 2006) and removed duplicates from the study by Ackerley and colleagues (Ackerley 2009). We included two studies that investigated editorials published in some of the same oncology journals in overlapping time periods (Bariani 2013; Lerner 2012) and removed duplicates from the study by Bariani and colleagues (Bariani 2013).
Primary analysis
Due to expected clinical and methodological heterogeneity among the included studies, we used inverse variance random‐effects models to estimate RRs with 95% CIs. We compared recommendations between documents with and without conflicts of interest and ensured uniform directionality so RR > 1 indicated that documents with conflicts of interest more often had favourable recommendations than documents without conflicts of interest. We analysed financial and non‐financial conflicts of interests separately, and analysed clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews separately.
Using the methods for calculating a Number Needed to Treat, we calculated a Number Needed to Read for each document type (Appendix 6) (Schünemann 2020). We defined Number Needed to Read as the expected number of documents with conflicts of interest needed to be read rather than documents without conflicts of interest for one additional document having a favourable recommendation. As describing the 95% CI is difficult for Number Needed to Read when the CI of the RR crosses the boundary of no difference (Altman 1998), we report the 95% CI of the Number Needed to Read in Appendix 6.
Secondary analyses
We analysed advisory committee reports on an individual level.
In a post‐hoc analysis, we combined all four types of documents (i.e. clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews) in an analysis of financial conflicts of interest.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following pre‐planned subgroup analyses for our primary analyses for all document types (Appendix 7):
Documents stratified by different types of financial conflicts of interest (e.g. funding, investigator, author grants, honorarium, consulting, speaker’s bureau, equity/stock, gifts)
Studies assessed as high risk of bias versus studies assessed as low risk of bias
We planned to conduct the following pre‐planned subgroup analysis for our primary analysis on clinical guidelines only:
Clinical guidelines developed using standardised methods (e.g. GRADE (Guyatt 2011) or USPSTF (U.S. Preventive Services Task Force 2015)) versus clinical guidelines not developed using standardised methods. For the stratification of documents, we relied on the coding done by the authors of the included studies
In addition, we conducted the following post‐hoc subgroup analyses for our primary analyses.
Documents stratified by degree of financial conflicts of interest: we compared major financial conflicts of interest (defined as at least half of the authors/committee members having financial conflicts of interest) with minor financial conflicts of interest (defined as less than half of the authors/committee members with financial conflicts of interest). The purpose of this subgroup analysis was to investigate a potential dose‐response relationship between financial conflicts of interest and recommendations.
We only carried out the subgroup analyses when we had sufficient data (i.e. at least five documents in the groups with and without conflicts of interest in the included studies combined).
Sensitivity analysis
We planned to conduct the following pre‐planned sensitivity analyses for our primary analyses (Appendix 8).
Excluding documents with unclear or undisclosed conflicts of interest.
Excluding documents with neutral recommendations.
Excluding all studies which disclosed a relevant conflict of interest. For example, if one of the included studies was funded by a drug company, we excluded the study and re‐analysed our data.
Re‐analysing our primary analyses using a fixed‐effect model.
In addition, we conducted the following post‐hoc sensitivity analyses for our primary analyses.
Re‐categorising documents with financial conflicts of interest into documents with financial conflicts of interest related to the manufacturer of the drug or device of interest or to any for‐profit organisation in two separate analyses.
We only carried out the sensitivity analyses when we had sufficient data (i.e. at least five documents in the groups with and without conflicts of interest in the included studies combined).
We conducted all analyses in Review Manager (RevMan 5.4) and Stata 15.
Assessment of the certainty of the evidence
Based on prior experience, using formal systems such as GRADE for assessing the certainty of evidence from methodological studies is challenging. We therefore focused on interpreting our results in the context of the statistical precision of our estimates (i.e. width of CIs) and risk of confounding. In Appendix 9, we report GRADE assessments employing both an approach similar to observational intervention studies and to prognostic studies (Guyatt 2008; Foroutan 2020).
Results
Description of studies
See: Characteristics of included studies.
Results of the search
See: Figure 1
1.
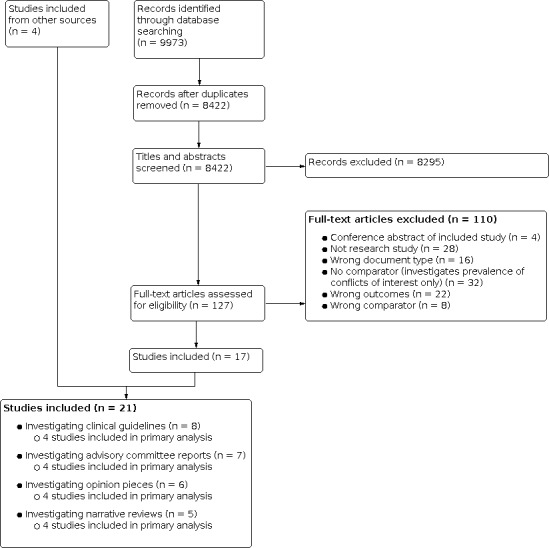
Study flow diagram.
In total, 9973 records were identified through our database searches. After removing duplicates, we screened 8422 records based on titles and abstracts and assessed 127 full‐text papers for inclusion. In total, we included 21 studies. We did not identify any unpublished studies or protocols for planned studies.
Included studies
See: Characteristics of included studies.
The 21 studies were published between 1998 and 2019. Eight studies investigated clinical guidelines (median number of clinical guidelines: nine, range: 2 to 50), seven studies investigated FDA drug and/or device advisory committee reports (median number of advisory committee reports: 376, range: 79 to 416), six studies investigated opinion pieces (editorials, commentaries, and letters; median number of opinion pieces: 44, range: 8 to 131), and five studies investigated narrative reviews (median number of narrative reviews: 84, range: 7 to 213). Sixteen studies investigated documents on drugs, three studies investigated documents on devices, and two studies investigated documents on both drugs and devices. Twenty studies only investigated financial conflicts of interest and one study investigated both financial conflicts of interest and specialty affiliations among guideline authors (i.e. non‐financial conflicts of interest). None of the included studies reported industry funding, but six studies did not report funding information. Seven of the included studies investigating documents with and without financial conflicts of interest were conducted by authors who themselves had financial conflicts of interest.
We received unpublished data from 11 studies. In eight cases, we obtained full unpublished data sets (Ackerley 2009; Bariani 2013; Dunn 2016; Hartog 2012; Lerner 2012; Lurie 2006; Wang 2010; Zhang 2019), and in three cases we obtained additional summary data (Pham‐Kanter 2014; Tibau 2015; Tibau 2016).
Risk of bias in included studies
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
We assessed 20 studies as overall high risk of bias and one study as low risk of bias. Around half of the included studies had low risk of bias in the document inclusion process (n = 10) and the majority had low risk of bias in the coding of conflicts of interest (n = 15) and recommendations (n = 17). We assessed six studies to be low risk of confounding and 15 to be high risk of confounding, because they included documents of different topics (e.g. various cancer drugs for different indications), or included documents on the same drug used for different indications (e.g. antidiabetic drugs used in adults, children, or pregnant women).
We found no published protocols and only received unpublished protocols for two studies (Downing 2014; Lurie 2006). We found no discrepancies between outcomes in these protocols and study publications. Nine of 21 author teams replied that no protocol existed for their study, and two author teams supplied us with reports that we did not consider to be protocols (Appendix 4).
Effect of methods
Financial conflicts of interest: differences in recommendations
Clinical guidelines
Eight studies investigated a total of 106 clinical guidelines and data from four of these studies including 86 clinical guidelines could be used in our pooled primary analysis (Aakre 2012; Norris 2013; Tibau 2015; Wang 2010). The association between financial conflicts of interest and favourable recommendations in clinical guidelines was RR: 1.26, 95% CI: 0.93 to 1.69, I2: 0% (Analysis 1.1). The Number Needed to Read for clinical guidelines was 9.1 (Appendix 6).
1.1. Analysis.

Comparison 1: Primary analyses, Outcome 1: Financial conflicts of interest
The prediction interval for the RR was 0.65 to 2.43 (Appendix 5). Thus, one can expect that clinical guidelines with financial conflicts of interest more often have favourable recommendations compared with clinical guidelines without financial conflicts of interest, but for an individual study of clinical guidelines the association may be reversed.
Four included studies did not report data in a way that enabled us to include them in our pooled analysis. Two studies each investigated one clinical guideline with financial conflicts of interest and one without. In both of these studies the clinical guidelines with financial conflicts of interest had a favourable recommendation, whereas the clinical guidelines without had a unfavourable recommendation (George 2014; Schott 2013). One study investigated 12 clinical guidelines, but only reported the percentage of authors with financial conflicts of interest in each guideline. Three out of eight clinical guidelines with favourable recommendations included authors with financial conflicts of interest (prevalence from 12% to 53%), and two out of four clinical guidelines with unfavourable recommendations included authors with financial conflicts of interest (prevalence 9% and 11%) (Norris 2012). The remaining study investigated a mixture of four clinical guidelines, 23 editorials and commentaries, and 40 reviews (mainly narrative) commenting on a randomised trial on fenofibrate use. The authors found that documents written by authors with conflicts of interest more often recommended fibrate use (RR: 1.69, 95% CI: 1.07 to 2.67) (Downing 2014).
One of the studies included in our pooled analysis adjusted for the specific drug that was evaluated in the guideline (thereby reducing the risk of confounding). The authors found no association between financial conflicts of interest and recommendations of a drug, but did not report any effect estimates in the study publication (Norris 2013).
Advisory committee reports
Seven studies investigated a total of 1809 advisory committee reports and data from five studies could be included in our pooled analyses (Ackerley 2009; Lurie 2006; Pham‐Kanter 2014; Tibau 2016; Zhang 2019). In our primary analysis, including four studies of 629 advisory committee reports, the association between any advisory committee report with members with financial conflicts of interest and voting in favour of approving a drug or device was RR: 1.20, 95% CI: 0.99 to 1.45, I2: 24% (Analysis 1.1). The Number Needed to Read for advisory committee reports was 7.7 (Appendix 6). In our secondary analysis, including three studies of 17,816 votes, the association between financial conflicts of interest of individual advisory committee members and voting in favour of approving a drug or device was RR: 1.14, 95% CI: 1.07 to 1.21, I2: 35% (Analysis 2.1).
2.1. Analysis.

Comparison 2: Secondary analysis: using individual voting level in the analysis on advisory committee reports, Outcome 1: Financial conflicts of interest
The prediction interval for the RR was 0.66 to 2.19 (Appendix 5). Thus, one can expect that advisory committee reports with financial conflicts of interest more often have favourable recommendations compared with advisory committee reports without financial conflicts of interest, but for an individual study of advisory committee reports the association may be reversed.
Two included studies did not report data in a way that enabled us to include them in our pooled analysis. One of the studies investigated the association between conflicts of interest and voting behaviour of 1482 members from 385 advisory committee reports. The authors reported that they found no association between conflicts of interest and voting outcome among members, but did not report any effect estimates on the association (Xu 2017). The remaining study investigated 1483 members from 416 advisory committee reports. The authors found that committee members with financial conflicts of interest had 14.3% greater odds of voting for approval compared with committee members without financial conflicts of interest. However, the estimate was not statistically significant (P value: 0.12) (Cooper 2019).
One of the studies included in the pooled analysis adjusted for medical product and advisory committee meeting characteristics (thereby reducing the risk of confounding) and the association between financial conflicts of interest related to the manufacturing company and favourable recommendations was odds ratio (OR): 4.66, 95% CI: 0.64 to 33.6 (Zhang 2019).
Opinion pieces
Six studies investigated a total of 340 opinion pieces (Bariani 2013; Downing 2014; Hayes 2019; Lerner 2012; Stelfox 1998; Wang 2010) and data from four of these studies including 284 opinion pieces could be included in our pooled primary analysis. The association between financial conflicts of interest and favourable recommendations in opinion pieces was RR: 2.62, 95% CI: 0.91 to 7.55, I2: 78% (Analysis 1.1). The Number Needed to Read for opinion pieces was 2.3 (Appendix 6).
The prediction interval for the RR was 0.03 to 220.56 (Appendix 5). Thus, one can expect that opinion pieces with financial conflicts of interest more often have favourable recommendations compared with opinion pieces without financial conflicts of interest, but for an individual study of opinion pieces the association may be reversed.
Two included studies did not report data in a way that enabled us to include them in our pooled analysis. One study investigated a mixture of 69 authors of original research papers, review articles, and letters. The study found that authors with financial conflicts of interest related to the drug manufacturer more often had favourable recommendations than authors without financial conflicts of interest (RR: 13.91, 95% CI: 1.99 to 96.97) (Stelfox 1998). The remaining study investigated a mixture of four clinical guidelines, 23 editorials and commentaries, and 40 reviews (mainly narrative) and found that documents written by authors with conflicts of interest more often recommended fibrate use (RR: 1.69, 95% CI: 1.07 to 2.67) (Downing 2014).
One of the studies included in the pooled analysis adjusted for characteristics of the trial (e.g. type of intervention and trial conclusion) the editorial commented on (thereby reducing the risk of confounding) and the association between financial conflicts of interest and favourable recommendations was OR: 1.39, 95% CI: 0.52 to 3.70 (Bariani 2013).
Narrative reviews
Five studies investigated a total of 497 narrative reviews and data from four of these studies investigating 457 narrative reviews could be included in our pooled primary analysis (Dunn 2016; Hartog 2012; Hayes 2019; Wang 2010). The association between financial conflicts of interest and favourable recommendations in narrative reviews was RR: 1.20, 95% CI: 0.97 to 1.49, I2: 39% (Analysis 1.1). The Number Needed to Read for narrative reviews was 8.3 (Appendix 6).
The prediction interval for the RR of was 0.56 to 2.59 (Appendix 5). Thus, one can expect that narrative reviews with financial conflicts of interest more often have favourable recommendations compared with narrative reviews without financial conflicts of interest, but for an individual study of narrative reviews the association may be reversed.
One included study did not report data in a way that enabled us to include it in our pooled analysis. The study investigated a mixture of four clinical guidelines, 23 editorials and commentaries, and 40 reviews (mainly narrative). The authors found that documents written by authors with conflicts of interest more often recommended fibrate use (RR: 1.69, 95% CI: 1.07 to 2.67) (Downing 2014).
Post‐hoc analysis combining all document types. Financial conflicts of interest: differences in recommendations
In a post‐hoc analysis, we combined all types of documents and the association between financial conflicts of interest and favourable recommendations was RR: 1.26, 95% CI: 1.09 to 1.44, I2: 38% (Analysis 1.1). The Number Needed to Read was 7.1 (Appendix 6).
The prediction interval for the RR was 0.88 to 1.80 (Appendix 5). Thus, one can expect that documents with financial conflicts of interest more often have favourable recommendations compared with documents without financial conflicts of interest, but for an individual study the association may be reversed.
Non‐financial conflicts of interest: differences in recommendations
One study investigated specialty interests and included 12 clinical guidelines on mammography screening. The focus was whether the guideline author team included a radiologist (Norris 2012). In our analysis based on this study, the association between having radiologists in the guideline panel and recommending routine screening for breast cancer was RR: 2.10, 95% CI: 0.92 to 4.77. The Number Needed to Read was 2.1 (Appendix 6).
Subgroup and sensitivity analyses
We found no differences in effect estimates in relation to the type of financial conflicts of interest or the degree of financial conflicts of interest for any document type. We were not able to conduct the planned subgroup analyses in relation to risk of bias in included studies for all document types and development methods for clinical guidelines (Appendix 7).
Sensitivity analyses were robust in 20 of 23 analyses of financial conflicts of interest and in three analyses the association between financial conflicts of interest and favourable recommendations became stronger (Appendix 8).
Assessment of certainty of the evidence
The evidence on financial conflicts of interest in all four types of documents and non‐financial conflicts of interest in clinical guidelines should be interpreted with some caution as the majority of the studies (15 out of 21) had a risk of confounding and all effect estimates of the primary analyses lacked statistical precision. Using the GRADE approaches for intervention and prognostic studies resulted in low to very low certainty of the evidence depending on type of document and the GRADE system used (Appendix 9).
Discussion
Summary of main results
We included 21 studies investigating 106 clinical guidelines, 1809 advisory committee reports, 340 opinion pieces, and 497 narrative reviews. We found an association between financial conflicts of interest and favourable recommendations of drugs and devices in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews. Our four primary analyses pointed in a consistent direction and provided a fairly similar magnitude of effect, but each with varying degrees of statistical precision. Our post hoc analysis combining all document types confirmed these findings and increased the statistical precision. Our findings on the impact of non‐financial conflicts of interest on recommendations were limited to evidence from a single study of breast cancer screening guidelines with involvement of radiologist authors, with statistically imprecise results. It is therefore uncertain whether specialty interests or other types of non‐financial conflicts of interest impact on recommendations.
Quality of the evidence
All but one of the included studies were assessed as having high risk of bias, mainly due to a high risk of confounding. Documents differed in other aspects than conflicts of interest (e.g. they investigated different drugs used for different patient groups) which could have introduced confounding. For example, if a study included editorials in oncology commenting on numerous drugs. If some drugs are more likely to have editorials written by authors with conflicts of interest (e.g. developed by major drug companies), and if such drugs are more likely to have favourable trial results (i.e. thereby receiving a favourable recommendation in an editorial), this could confound the association between financial conflicts of interest and favourable recommendations.
Strengths and limitations
A major strength of our study is the inclusion of unpublished data from 11 of 21 studies. We retrieved eight full datasets and unpublished summary data for three additional studies which enabled us to ensure high data quality and to conduct comprehensive analyses thereby increasing statistical precision and minimising reporting bias. Furthermore, we did a thorough search for grey literature and attempted to identify published and unpublished protocols. We only obtained two protocols (Downing 2014; Lurie 2006) and a comparison of outcomes in the protocols with outcomes in the study publications gave no indication of selective outcome reporting.
However, six of 21 included studies were reported in a format that did not allow inclusion in meta‐analysis. Four of these studies reported results similar to our meta‐analysis. Two of the four studies combined different types of documents without stratifying results, with estimates RR: 1.69, 95% CI: 1.07 to 2.67 and RR: 13.91, 95% CI: 1.99 to 96.97) in line with our primary analysis (Downing 2014; Stelfox 1998). The other two of the four studies sampled a single pair of clinical guidelines with and without financial conflicts of interest and in both cases guidelines with conflicts were favourable (George 2014; Schott 2013). The last two of the six studies (29% of all documents) (Cooper 2019; Xu 2017) sampled FDA committee reports from the same period as the studies included in our meta‐analysis, implying a considerable risk of overlapping documents between the studies. The two studies reported no results for our primary analysis and if we had access to raw data we would likely have had to exclude a considerable proportion of the documents from our analyses to avoid double‐counting. Thus, we find it unlikely that our result would have been qualitatively different had the six studies reported results in a format suitable for meta‐analysis.
Furthermore, our findings on the influence of financial conflicts of interest were robust in most of our sensitivity analyses. When our analyses were not robust, the sensitivity analyses generally showed a stronger association between financial conflicts of interest and favourable recommendations.
Nevertheless, there are some challenges. First, the different types of documents were described using various terms in the included studies and despite using a comprehensive search strategy we might have missed relevant studies. Furthermore, only four studies were included in each of our four primary analyses. Therefore, our effect estimates have some degree of statistical imprecision and none of our primary analyses were statistically significant at the conventional 5% level. However, the sizes of the effect estimates were similar for clinical guidelines, advisory committee reports, and narrative reviews and slightly higher for opinion pieces, and when we combined all document types in a post hoc analysis including 13 studies, we increased the statistical precision and found a statistically significant association with moderate heterogeneity.
Second, our criteria for assessment of risk of bias in relation to confounding might be viewed as quite strict and others may interpret the risk of bias in studies differently. Nevertheless, the majority of studies had a risk of confounding as they compared documents that may differ in other aspects than conflicts of interest (e.g. documents on different drugs used for different patient groups). While confounding could have influenced our estimates, the association between conflicts of interest and recommendations was fairly consistent across document types despite some studies including quite comparable documents (e.g. clinical guidelines on efalizumab for treatment of psoriasis (Schott 2013)), and others including quite different documents (e.g. advisory committee reports on a wide range of different drugs (Pham‐Kanter 2014)). Moreover, recommendations in guidelines and narrative reviews could have been influenced by conflicts of interest in the underlying evidence. For example, in certain clinical fields such as oncology (Andreatos 2017), conflicts of interest are highly frequent which could have impacted the conclusions of clinical trials and systematic reviews (Lundh 2017; Hansen 2019a) and thereby indirectly affected guideline recommendations and potentially result in effect modification. Furthermore, how conflicts of interest in the primary clinical trials and systematic reviews underpinning a guideline are interpreted could be associated with the conflicts of interest of the guideline authors.
Third, the number of authors with financial conflicts of interest may influence recommendations in a document. Our subgroup analyses comparing documents with the majority of authors with financial conflicts of interest versus a minority of authors found no difference in effect. However, the analyses were somehow simplistic and based on few data with statistically imprecise results. Another important aspect is the role of the author with financial conflicts of interest. For example, the chair of a guideline committee or the lead author of a narrative review likely has greater influence on recommendations than an author with a less prominent role. Unfortunately, none of the included studies reported data that allowed such a comparison.
Fourth, 11 of the 21 included studies relied solely on disclosed information in the included documents for coding conflicts of interest. This could have led to an underestimation of our effect estimates, as conflicts of interest are often underreported in various publication types, including clinical guidelines (Bindslev 2013).
Finally, the interpretation of our results can be debated. There is no published guidance specifically tailored for summarising and interpreting evidence from methodological studies. One approach could be to use the GRADE system (Guyatt 2008), but it is questionable whether using GRADE for observational intervention studies or prognostic studies is best suited for methodological studies, since the methodology of studies or the presence of conflicts of interest cannot be randomised. In Appendix 9, we report assessments using both strategies which resulted in low to very low certainty of evidence depending on type of documents and the system used. Using the GRADE approach for intervention studies resulted in a more conservative interpretation of the certainty of the evidence.
Agreements and disagreements with other studies or reviews
Other systematic reviews have focused on financial conflicts of interest in other types of publications and have reported similar findings. A recent updated Cochrane Methodology Review focused on primary research, mainly trials, and found that industry‐funded studies more often had favourable conclusions compared with non‐industry‐funded studies (RR: 1.34, 95% CI: 1.19 to 1.51) (Lundh 2017). Similarly, another recent Cochrane Methodology Review focused on systematic reviews and found that systematic reviews with industry funding or by authors with financial conflicts of interest more often had favourable conclusions compared with systematic reviews without financial conflicts of interest (RR: 1.98, 95% CI: 1.26 to 3.11) (Hansen 2019a).
Financial conflicts of interest have also been investigated in relation to other industries and in a systematic review, Chartres and colleagues reported that industry‐funded nutrition studies and reviews more often had favourable conclusions than non‐industry‐funded nutrition studies and reviews (RR: 1.31, 95% CI: 0.99 to 1.72) (Chartres 2016).
Meaning of our review
For our analyses, we included studies of four types of documents that both were fairly common and involved authors’ interpretation of external evidence (involving methods less stringent than in a systematic review). Although we had anticipated potential differences between the various types of documents, we found a fairly consistent association between financial conflicts of interest and favourable recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews. One reason could be that authors with conflicts of interest are more prone to confirm prior beliefs by selectively citing and interpreting the literature (DuBroff 2018). This could also explain the somewhat stronger association found in opinion pieces which to some degree allow authors more room for interpretation than narrative reviews, which undergo peer review, and clinical guidelines, which are increasingly done using standardised methods. On an absolute scale, the association between conflicts of interest and recommendations was particularly strong for opinion pieces and specialty interest in clinical guidelines with Numbers Needed to Read of only 2.3 and 2.1, respectively, although the estimates had considerable statistical imprecision.
Authors' conclusions
Implication for systematic reviews and evaluations of healthcare.
We interpreted our findings to indicate that financial conflicts of interest are associated with favourable recommendations of drugs and devices in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews. Although the magnitude of effect is fairly consistent across document types, most studies had a risk of confounding and our individual analyses of each document type had some degree of statistical imprecision. It is more uncertain whether non‐financial conflicts of interest impact on recommendations.
Our findings support conflicts of interest policies from major guideline issuing organisations such as the National Institute for Health and Care Excellence, the US Preventive Services Task Force, and the World Health Organization (NICE 2019; U.S. Preventive Services Task Force 2018; WHO 2014). These policies aim to minimise the number and role of guideline authors with conflicts of interest. Similarly, some high impact journals manage conflicts of interest beyond disclosure, for example New England Journal of Medicine prohibits narrative reviews and editorials with significant financial conflicts of interest (> US$ 10,000), and The Lancet prohibits commentaries, seminars, reviews, and series by authors with relevant stock ownership, employment, or company board membership (Bero 2018; Lundh 2020). Other journals should consider introducing such polices in order to minimise the influence from conflicts of interest on journal content.
In line with this, the FDA introduced more stringent criteria on which types of conflicts of interest where allowed for committee members in 2008 (Ackerley 2009). This could be a possible explanation as to why the study by Zhang and colleagues (Zhang 2019), which exclusively sampled advisory committee reports from 2008 and onwards, found a somewhat weaker association between financial conflicts of interest and recommendations in advisory committee reports than the three other studies included in our pooled analyses (Ackerley 2009; Lurie 2006; Tibau 2016).
To minimise influence from conflicts of interest we suggest that patients, clinicians, and healthcare decision makers primarily use clinical guidelines that are based on rigorous methodology and have clear policies of how to manage conflicts of interest, such as excluding or minimising the role of members with conflicts and ensuring a broad skill set in the panel. If such guidelines are not available, users should interpret such guidelines with caution. Similarly, journal readers should prefer publications written by authors without conflicts of interest.
Implication for methodological research.
Ideally, future studies should try to minimise the risk of confounding, e.g. by using a matched study design (Jørgensen 2006). However, identifying editorials commenting on the same study or guidelines addressing the same question and developed using similar methods might be a challenge. Furthermore, future research could focus on investigating whether specific types of financial conflicts of interest (e.g. advisory board membership) or conflicts of interest related to specific companies (e.g. drug manufacturer) have a greater impact than others. Moreover, the included studies used various definitions of financial conflicts of interest and recommendations, and use of a standardised terminology would be helpful.
Investigating the impact of non‐financial conflicts of interest is challenging because no uniform definition exists. On one hand, a multitude of interests such as specialty interests, intellectual interests, personal beliefs, and personal relationships can be viewed as non‐financial conflicts of interest (The PLoS Medicine Editors 2008; Viswanathan 2014). On the other hand, labelling personal beliefs and theoretical schools of thoughts as conflicts of interest risks muddying the waters since no researcher is completely interest free or free from intellectual pre‐conceptions (Bero 2014; Bero 2016; Bero 2017). Furthermore, the distinction between financial and non‐financial conflicts of interest is not always clear. For example, in relation to the included study on mammography screening guidelines (Norris 2012), it can be debated whether being a radiologist should be considered a purely non‐financial conflict of interest because radiologists may have direct financial income from breast cancer screening. Future studies could focus on investigating the impact of the various types of non‐financial conflicts of interest on favourable recommendations and on the impact of managing such interests using guideline panels with a broad range of skill sets, rather than mainly content area experts.
History
Protocol first published: Issue 6, 2013 Review first published: Issue 12, 2020
| Date | Event | Description |
|---|---|---|
| 3 October 2019 | New citation required but conclusions have not changed | This protocol was re‐published in October 2019 to generate a new citation, reflecting the change in title and authorship from the original version (Lundh A, Jørgensen AW, Bero L. Association between personal conflicts of interest and recommendations on medical interventions. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No.: MR000040). |
| 25 April 2018 | Amended | The text has been updated to align it with other Cochrane Methodology reviews on conflicts of interest. |
Acknowledgements
We thank Gloria Won, from UCSF Medical Center at Mount Zion, for developing our initial search strategy for an earlier version of the protocol. We thank Herdis Foverskov, from University Library of Southern Denmark, for valuable help in developing and adjusting the search strategies. We thank Ulrich Halekoh, from Epidemiology, Biostatistics and Biodemography at University of Southern Denmark, for valuable statistical guidance.
We thank Aakre, Ackerley, Bariani, Downing, Dunn, George, Hartog, Hayes, Lerner, Lurie, Norris, Pham‐Kanter, Schott, Stelfox, Tibau, Wang, and Zhang and their respective colleagues (authors of included studies) for clarifying issues and sharing protocols and data.
An abridged version of this article is published in BMJ. We thank the editors and peer reviewers for both journals for assistance and comments on the review.
Appendices
Appendix 1. Terminology
We use the overall term ‘conflicts of interest’ to refer to both financial and non‐financial conflicts of interest as specified below.
We use the definition by the Institute of Medicine (US) and define ‘conflicts of interest’ as “a set of circumstances that creates a risk that professional judgment or actions regarding a primary interest will be unduly influenced by a secondary interest” (Institute of Medicine 2009). This includes both financial and non‐financial conflicts of interest. By financial conflicts of interest we include authors’ financial relationships, for example employment, research grants, speaker’s bureau membership, stock ownership, and consultancy work and also funding of publication (e.g. a clinical guideline). We focus on financial conflicts of interest related to the drug or device industry. Financial conflicts of interest related to other industries (e.g. tobacco industry) are not included. We define 'drugs' as medications requiring approval from a regulatory authority as a prescription drug. We define 'devices' according to the Food and Drug Administration (FDA) as instruments used in diagnosis, treatment, or prevention of disease (FDA 2017).
As there is no consensus concerning the definition of non‐financial conflicts of interest, we generally use the definition used by the authors of the included studies. If the authors do not use the term non‐financial conflicts of interest, we use the following subcategories: personal and professional relationships (e.g. research collaboration), professional and specialty interests (e.g. belonging to a certain medial subspecialty), or intellectual and academic conflicts of interest (e.g. authorship of studies that are part of the evidence base for reaching a particular recommendation) (Akl 2014). We do not focus on studies investigating beliefs (e.g. political or religious), personal experience (e.g. abuse or trauma), or institutional conflicts of interest (Bero 2016). In some cases an interest can be considered both a financial and non‐financial. For example, a surgeon who uses a particular surgical intervention which he/she then investigates in a clinical guideline. This can be viewed as a financial conflict of interest, because the surgeon might benefit financially if the intervention is recommended. It can also be viewed as a non‐financial conflict of interest, because the surgeon uses the surgical procedure as part of clinical practice (i.e. specialty interest) or may have conducted some of the studies included in the guideline (i.e. academic interest). For this review, we regard such relationships as non‐financial because they differ from what is typically regarded as financial conflicts of interest (i.e. direct financial relationships with the drug or device industry).
We use the term ‘clinical guidelines’ to refer to guidelines. We define ‘clinical guidelines’ as “Systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances” (Institute of Medicine 1990).
We use the term ‘advisory committee reports’ to refer to reports or transcripts from meetings held in committees, boards, councils, or similar that are established to advise an organisation and provide a recommendation concerning an intervention (e.g. the Food and Drug Administration’s advisory committee on oncological drugs).
We define ‘opinion pieces’ as documents that are not research studies in which an author expresses a personal opinion about a specific intervention (e.g. editorials, commentaries, and letters‐to‐the‐editors).
We define ‘narrative reviews’ as literature reviews without a systematic search of the literature with clear eligibility criteria.
We use the term ‘documents’ to refer to clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews included in the studies.
Appendix 2. PubMed search strategy
Block 1A: drug and device industry
1. Drug Industry (MeSH)
2. Manufacturing Industry (MeSH)
3. (Drug [Title/Abstract] OR drugs[Title/Abstract] OR pharmaceutical[Title/Abstract] OR pharmaceutic [Title/Abstract] OR pharmacological[Title/Abstract] OR pharma*[Title/Abstract] OR biotech*[Title/Abstract] OR bio‐tech[Title/Abstract] OR biopharma*[Title/Abstract] OR bio‐pharma*[Title/Abstract] OR biomed*[Title/Abstract] OR bio‐med*[Title/Abstract] OR device[Title/Abstract] OR devices[Title/Abstract] OR imaging[Title/Abstract] OR for‐profit[Title/Abstract] OR private[Title/Abstract]) AND (industry[Title/Abstract] OR industries[Title/Abstract] OR company[Title/Abstract] OR companies[Title/Abstract] OR manufacturer[Title/Abstract] OR manufacturers[Title/Abstract] OR organisation[Title/Abstract] OR organisations[Title/Abstract] OR organization[Title/Abstract] OR organizations[Title/Abstract] OR agency[Title/Abstract] OR agencies[Title/Abstract] OR sector[Title/Abstract] OR sectors[Title/Abstract])
4. Personal[Title] OR self‐reported[Title] OR selfreported[Title] OR author[Title] OR authors[Title] OR authorship[Title] OR ((committee[Title] OR board[Title]) AND (member[Title] OR members[Title])) OR voting[Title] OR votings[Title] OR financial[Title] OR finance[Title]
5. 1 OR 2 OR 3 OR 4
Block 1B: financial conflicts of interest
6. Conflict of interest (MeSH)
7. Financial support (MeSH)
8. Research support as topic (MeSH)
9. (Conflict[Title/Abstract] OR conflicts[Title/Abstract] OR conflicting[Title/Abstract]) AND (interest[Title/Abstract] OR interests[Title/Abstract])
10. (Competing[Title/Abstract] OR vested[Title/Abstract]) AND (interest[Title/Abstract] OR interests[Title/Abstract])
11. (Industry[Title/Abstract] OR industries[Title/Abstract] OR company[Title/Abstract] OR companies[Title/Abstract] OR manufacturer[Title/Abstract] OR manufacturers[Title/Abstract] OR finance[Title/Abstract] OR financial[Title/Abstract]) AND (funded[Title/Abstract] OR funding[Title/Abstract] OR sponsor[Title/Abstract] OR sponsors[Title/Abstract] OR sponsorship[Title/Abstract] OR sponsoring[Title/Abstract] OR support[Title/Abstract] OR supported[Title/Abstract] OR finance[Title/Abstract] OR financial[Title/Abstract] OR involvement[Title/Abstract] OR involving[Title/Abstract] OR payment[Title/Abstract] OR payments[Title/Abstract] OR relationship[Title/Abstract] OR relationships[Title/Abstract] OR relation[Title/Abstract] OR relations[Title/Abstract] OR tie[Title/Abstract] OR ties[Title/Abstract] OR collaboration[Title/Abstract] OR collaborations[Title/Abstract])
12. Industry‐funded[Title/Abstract] OR industry‐funding[Title/Abstract] OR industry‐sponsor*[Title/Abstract] OR company‐funded[Title/Abstract] OR company‐funding[Title/Abstract] OR company‐sponsor*[Title/Abstract] OR industry‐support[Title/Abstract] OR industry‐supported[Title/Abstract] OR company‐support[Title/Abstract] OR company‐supported[Title/Abstract]
13. (Commercial‐academic[Title/Abstract] OR academic‐commercial[Title/Abstract] OR industry‐academic[Title/Abstract] OR academic‐industry[Title/Abstract] OR commercial‐industry[Title/Abstract] OR industry‐commercial[Title/Abstract] OR industry‐physician[Title/Abstract] OR physician‐industry[Title/Abstract]) AND (interaction[Title/Abstract] OR interactions[Title/Abstract] OR relationship[Title/Abstract] OR relationships[Title/Abstract] OR relation[Title/Abstract] OR relations[Title/Abstract] OR collaboration[Title/Abstract] OR collaborations[Title/Abstract])
14. 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13
Block 2A: non‐financial, personal, and academic
15. Non‐financial[Title/Abstract] OR nonfinancial[Title/Abstract]
16. Personal[Title] OR individual[Title] OR self‐reported[Title] OR selfreported[Title] OR author[Title] OR authors[Title] OR authorship[Title]
17. Specialist[Title/Abstract] OR specialists[Title/Abstract] OR specialty[Title/Abstract] OR expert[Title/Abstract] OR experts[Title/Abstract] OR intellectual[Title/Abstract] OR intellectuals[Title/Abstract] OR professional[Title/Abstract] OR professionals[Title/Abstract] OR academic[Title/Abstract] OR academics[Title/Abstract]
18. 15 OR 16 OR 17
Block 2B: non‐financial conflicts of interest
19. Conflict of interest (MeSH)
20. Conflict[Title] OR conflicts[Title] OR conflicting[Title] OR competing[Title] OR vested[Title]
21. Relation[Title] OR relations[Title] OR relationship[Title] OR relationships[Title]
22. Interest[Title] OR interests[Title]
23. 19 OR 20 OR 21 OR 22
Block 3: clinical guidelines, advisory committee reports opinion pieces, and narrative reviews
24. (Opinion[Title/Abstract] OR opinions[Title/Abstract] OR policy[Title/Abstract] OR policies[Title/Abstract] OR statement[Title/Abstract] OR statements[Title/Abstract]) AND (piece[Title/Abstract] OR pieces[Title/Abstract] OR article[Title/Abstract] OR articles[Title/Abstract])
25. (Narrative[Title/Abstract] OR descriptive[Title/Abstract] OR non‐systematic[Title/Abstract] OR non‐systematical[Title/Abstract] OR non‐systematically[Title/Abstract] OR nonsystematic[Title/Abstract] OR nonsystematical[Title/Abstract] OR nonsystematically[Title/Abstract]) AND (review[Title/Abstract] OR reviews[Title/Abstract] OR overview[Title/Abstract] OR overviews[Title/Abstract])
26. Non[Title/Abstract] AND (systematic[Title/Abstract] OR systematical[Title/Abstract] OR systematically[Title/Abstract]) AND (review[Title/Abstract] OR reviews[Title/Abstract] OR overview[Title/Abstract] OR overviews[Title/Abstract])
27. Editorial[Title] OR editorials[Title] OR essay[Title] OR essays[Title] OR commentary[Title] OR commentaries[Title] OR comment[Title] OR comments[Title] OR letter[Title] OR letters[Title]
28. (Treatment[Title/Abstract] OR treatments[Title/Abstract] OR screening[Title/Abstract] OR screen[Title/Abstract] OR testing[Title/Abstract] OR test[Title/Abstract] OR tests[Title/Abstract OR diagnostic[Title/Abstract] OR diagnosis[Title/Abstract] OR therapy[Title/Abstract] OR therapies[Title/Abstract]) AND (recommendation[Title/Abstract] OR recommendations[Title/Abstract])
29. Guidelines as Topic (MeSH)
30. Health Planning Guidelines (MeSH)
31. (Clinical[Title] OR clinic[Title] OR health[Title] OR practice[Title]) AND (guideline[Title] OR guidelines[Title] OR recommendation[Title] OR recommendations[Title])
32. (Advisory[Title/Abstract] OR advising[Title/Abstract] OR formulary[Title/Abstract] OR counselling[Title/Abstract] OR counselling[Title/Abstract] OR drug[Title/Abstract] OR drugs[Title/Abstract]) AND (board[Title/Abstract] OR boards[Title/Abstract] OR committee[Title/Abstract] OR committees[Title/Abstract] OR panel[Title/Abstract] OR panels[Title/Abstract] OR meeting[Title/Abstract] OR meetings[Title/Abstract])
33. 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32
Combined searches
34. 5 AND 14
35. 18 AND 23
36. (34 OR 35) AND 33
Appendix 3. Data extraction
Two review authors independently extracted the following information.
Study characteristics
Title.
Name of lead author.
Name of journal.
Year published.
Primary aim of the study.
Design of study: cohort, cross‐sectional study, systematic review or meta‐analysis, or other.
Study domain ‐ category: clinical guideline, advisory committee report, opinion pieces, narrative review, or mixed.
Sample description: for example, clinical guidelines on treatment of hypertensionStrategy used to collect sample: for example, search of PubMed and time period coveredDefinition of clinical guidelines, advisory committee reports, opinion pieces, or narrative reviews used in the study. Verbatim extraction.
Number of included documents (separate data for clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews).
Types of documents included in the study. Verbatim extraction.
Types of documents included in the study (drug, device or both).
Conflict of interest and outcome data
Definition of financial conflicts of interest used in the study. Verbatim extraction.
Definition of non‐financial conflicts of interest used in the study. Verbatim extraction.
-
Types of financial conflicts of interest investigated, potential categories are:
funding;
author grant;
honorarium;
consulting;
speakers bureau.
Types of non‐financial conflicts of interest investigated.
Definition of favourable recommendations used by the authors of the study. Verbatim extraction.
Definition of primary analysis used in the study. Verbatim extraction.
Total number of documents with and without conflicts of interest. Stratified by type of document (i.e. clinical guideline, advisory committee reports, opinion piece, narrative review) and type of conflicts of interest (i.e. financial, non‐financial).
Number of documents with and without conflicts of interest with favourable recommendations stratified by type of documents (i.e. clinical guideline, advisory committee reports, opinion piece, narrative review) and type of conflicts of interest (i.e. financial, non‐financial).
Any data on estimates of the association between financial conflicts of interest/non‐financial conflicts of interest and recommendations in clinical guidelines, advisory committee reports, opinion pieces, and narrative reviews (for example, adjusted effect estimates and confidence intervals).
Data for informing subgroup analyses or reflection on heterogeneity
Total number of documents with conflicts of interest and number with favourable recommendations. Stratified by document type (i.e. clinical guidelines, advisory committee reports, opinion pieces, narrative reviews) and category of financial conflicts of interest (e.g. investigator, grants, honorarium, consulting, speaker’s bureau, equity/stock, gifts).
Any data on the association between each category of financial conflicts of interest and favourable recommendations.
Total number of clinical guidelines following standardised methods with and without conflicts of interest and number with favourable recommendations. Stratified by type of conflicts of interest (i.e. financial, non‐financial).
Total number of clinical guidelines not following standardised methods with and without conflicts of interest and number with favourable recommendations. Stratified by type of conflicts of interest (i.e. financial, non‐financial).
Any data on the association between conflicts of interest and favourable recommendations for clinical guidelines following standardised methods and clinical guidelines not following standardised methods.
Total number of documents with conflicts of interest and number with favourable recommendations. Stratified by document type (i.e. clinical guidelines, advisory committee reports, opinion pieces, narrative reviews) and degree of financial conflicts of interest (i.e. major and minor).
Any data on the association between major and minor financial conflicts of interest and favourable recommendations.
Data for performing sensitivity analyses
Total number of documents with and without conflicts of interest and number of documents in each group with favourable recommendations, when excluding documents with unclear or undisclosed conflicts of interest. Stratified by document type (i.e. clinical guidelines, advisory committee reports, opinion pieces, narrative reviews) and type of conflicts of interest (i.e. financial, non‐financial).
Any data on the association between conflicts of interest and favourable recommendations, when excluding documents with unclear or undisclosed conflicts of interest.
Total number of documents with and without conflicts of interest and number of documents in each group with favourable recommendations, when excluding documents with neutral recommendations. Stratified by document type (i.e. clinical guidelines, advisory committee reports, opinion pieces, narrative reviews) and type of conflicts of interest (i.e. financial, non‐financial).
Any data on the association between conflicts of interest and favourable recommendations, when excluding documents with neutral recommendations.
Total number of documents with and without financial conflicts of interest and number of documents in each group with favourable recommendations. Stratified by document type (i.e. clinical guidelines, advisory committee reports, opinion pieces, narrative reviews) and type of financial conflict of interest (i.e. related to the manufacturer or related to any for‐profit company).
Any data on the association between financial conflicts of interest and favourable recommendations. Stratified by type of financial conflict of interest (i.e. related to the manufacturer or related to any for‐profit company).
Additional data
Funding and conflicts of interest statement in the study. Verbatim extraction.
Additional relevant information.
Appendix 4. Dealing with missing data
Protocols
We contacted authors in an attempt to obtain published or unpublished protocols for all the studies. All author teams but two responded (Cooper 2019; Xu 2017). Nine author teams replied that no protocol was used (Aakre 2012; Ackerley 2009; Bariani 2013; Dunn 2016; George 2014; Hartog 2012; Hayes 2019; Pham‐Kanter 2014; Zhang 2019), six author teams replied that they had a protocol, but could not locate or access it (Lerner 2012; Norris 2012; Norris 2013; Stelfox 1998; Tibau 2015; Tibau 2016), and two author teams supplied us with their protocol (Downing 2014; Lurie 2006). One author team replied that they had a protocol, but it was incorporated in the study publication (Wang 2010), and one author team supplied us with a master thesis that was used as basis of the study (Schott 2013). However, in both cases these were in our views not protocols (i.e. a document that details the study rationale and proposed methods written prior to study conduct) (Chan 2013).
'Risk of bias' assessment
If the studies did not report their methods in a way that enabled us to conduct our 'Risk of bias' assessment, we contacted the authors to clarify these issues. In total, we contacted authors of all the studies and received clarifications for all but two studies (Cooper 2019; Xu 2017).
Unpublished data
We contacted the authors of the included studies in an attempt to obtain additional individual study data or summary data in the following cases.
If the studies included a mixture of documents, but only reported combined data. For example, if a study included clinical guidelines and randomised trials, we contacted the authors to obtain separate data on clinical guidelines.
If the studies performed unadjusted or adjusted regression analyses, but did not report the raw numbers.
If the studies extracted information on different types of financial conflicts of interest and/or number of authors with and without financial conflicts of interest in each document, but did not report this information.
If the studies included documents with undisclosed conflicts of interest and/or neutral recommendations, but did not report this in a separate category.
In total, we contacted authors of 17 studies (Aakre 2012; Ackerley 2009; Bariani 2013; Cooper 2019; Downing 2014; Dunn 2016; Hartog 2012; Hayes 2019; Lerner 2012; Lurie 2006; Pham‐Kanter 2014; Stelfox 1998; Tibau 2015; Tibau 2016; Wang 2010; Xu 2017; Zhang 2019) and received data for 11 of these studies; eight full data sets (Ackerley 2009; Bariani 2013; Dunn 2016; Hartog 2012; Lerner 2012; Lurie 2006; Wang 2010; Zhang 2019) and in three cases additional summary data (Pham‐Kanter 2014; Tibau 2015; Tibau 2016).
When we received unpublished data, we analysed the data according to the methods used in the original studies. For the study on advisory committee reports by Ackerley and colleagues (Ackerley 2009), we restricted the sample for analysis to standing or temporary committee members that participated in the meeting and the voting in line with the authors’ analysis.
Appendix 5. Calculation of prediction intervals
Formula for prediction interval
We only calculated prediction intervals when at least four studies were included in the pooled analysis, because intervals will be imprecise when the effect estimates are based on only a few studies (IntHout 2016).
To calculate prediction intervals, we used the formula presented in an article by Riley and colleagues (Riley 2011):
û ‐ tk-2· √(Ƭ̂2+SE(û)2), û+tk-2· √(Ƭ̭̂2+SE(û)2)
Where û was the estimate of the average effect measure across studies, SE(û) was the standard error of û, Ƭ̂ was the estimate of between study standard deviation, and tk-2 was the 100(1‐(α/2)) percentile of the t‐distribution with k‐2 degrees of freedom, where k was the number of studies in the meta‐analysis and was 0.05 to give a 95% prediction interval. To meet the assumption on normal distribution, the prediction interval was derived on the natural log scale (Riley 2011). As Ƭ2 is already a measure for the heterogeneity for ln(RR), this was used directly in the calculation (IntHout 2016).
Calculation of prediction interval for clinical guidelines
The prediction interval for the RR of favourable recommendations in clinical guidelines with financial conflicts of interest compared with clinical guidelines without financial conflicts of interest was calculated as: 0.65 to 2.43. Thus, one can expect that clinical guidelines with financial conflicts of interest more often have favourable recommendations compared with clinical guidelines without financial conflicts of interest, but for an individual study of clinical guidelines the association may be reversed.
As our analysis on non‐financial conflicts of interest in clinical guidelines was based on only one study, calculation of a prediction interval was only possible for financial conflicts of interest.
Calculation of prediction interval for advisorycommittee reports
The prediction interval for the RR of favourable recommendations in advisory committee reports with financial conflicts of interest compared with advisory committee reports without financial conflicts of interest was calculated as: 0.66 to 2.19. Thus, one can expect that advisory committee reports with financial conflicts of interest more often have favourable recommendations compared with advisory committee reports without financial conflicts of interest, but for an individual study of advisory committee reports the association may be reversed.
Calculation of prediction interval for opinion pieces
The prediction interval for the RR of favourable recommendations in opinion pieces with financial conflicts of interest compared with opinion pieces without financial conflicts of interest was calculated as: 0.03 to 220.56. Thus, one can expect that opinion pieces with financial conflicts of interest more often have favourable recommendations compared with opinion pieces without financial conflicts of interest, but for an individual study of opinion pieces the association may be reversed.
Calculation of prediction interval for narrative reviews
The prediction interval for the RR of favourable recommendations in narrative reviews with financial conflicts of interest compared with narrative reviews without financial conflicts of interest was calculated as: 0.56 to 2.59. Thus, one can expect that narrative reviews with financial conflicts of interest more often have favourable recommendations compared with narrative reviews without financial conflicts of interest, but for an individual study of narrative reviews the association may be reversed.
Calculation of prediction interval for combined post‐hoc secondary analysis
The prediction interval for the RR of favourable recommendations in documents with financial conflicts of interest compared with documents without financial conflicts of interest was calculated as: 0.88 to 1.80. Thus, one can expect that documents with financial conflicts of interest more often have favourable recommendations compared with documents without financial conflicts of interest, but for an individual study the association may be reversed.
Appendix 6. Number Needed to Read
Number Needed to Read
For each document type, we calculated a Number Needed to Read as 1/Risk Difference. We calculated the Risk Difference based on the estimates presented in the 'Summary of findings' table (Appendix 9). For each estimated Number Needed to Read, we calculated corresponding 95% confidence intervals using the methods described by Altman (Altman 1998) with Number Needed to Read Favourable (NNRF) representing the expected number of documents with conflicts of interest needed to be read rather than documents without conflicts of interest for one additional document having a favourable recommendation, and Number Needed to Read Unfavourable (NNRU) representing the expected number of documents with conflicts of interest needed to be read rather than documents without conflicts of interest for one additional document having an unfavourable recommendation.
The Number Needed to Read for clinical guidelines was 9.1. The corresponding 95% CI was NNRU 33.3 to ∞ to NNRF 3.4.
The Number Needed to Red for advisory committee reports was 7.7. The corresponding 95% CI was NNRU 100.0 to ∞ to NNRF 3.4.
The Number Needed to Read for opinion pieces was 2.3. The corresponding 95% CI was NNRU 50.0 to ∞ to NNRF 1.4.
The Number Needed to Read for narrative reviews was 8.3. The corresponding 95% CI was NNRU 50.0 to ∞ to NNRF 3.4.
The Number Needed to Read for all document types was 7.1. The corresponding 95% CI was NNRF 20 to NNRF 4.2.
The Number Needed to Read for non‐financial conflicts of interest in clinical guidelines was 2.1. The corresponding 95% CI was NNRU 25.0 to ∞ to NNRF 1.8.
Appendix 7. Subgroup analyses
Findings from subgroup analyses on clinical guidelines
Different types of financial conflicts of interest
Of the four studies included in our pooled analysis on financial conflicts of interest, two studies specified subtypes of financial conflicts of interest (Aakre 2012; Wang 2010). We were able to pool data on six different types of financial conflicts of interest: advisory board membership, consultancy, grants, honoraria, industry funding of the clinical guideline, and speaker fees.
We found no difference in recommendations between guidelines with different types of financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.95; Analysis 3.1).
3.1. Analysis.

Comparison 3: Subgroup analyses for clinical guidelines, Outcome 1: Different types of financial conflicts of interest
High risk of bias versus low risk of bias studies
We planned to compare studies assessed as high risk of bias with studies assesses as low risk of bias. However, all four studies included in our pooled analysis on clinical guidelines were assessed as having high risk of bias, and it was not possible to carry out this subgroup analysis.
Clinical guidelines developed using standardised methods versus clinical guidelines not developed using standardised methods
We planned to compare clinical guidelines developed using standardised methods (e.g. through GRADE or US Preventive Services Task Force) with clinical guidelines developed without. Only one of the four studies included in our pooled analysis on financial conflicts of interest in clinical guidelines clearly stated that included clinical guidelines had to provide documentation that a systematic literature search and review was done (Norris 2013). In the remaining three studies, methodological aspects of the included clinical guidelines were not reported and the study samples could potentially be a mixture of clinical guidelines with and without standardised methods. None of the studies had any references to either GRADE or US Preventive Services Task Force. Therefore, our data did not enable us to carry out this subgroup analysis.
Clinical guidelines with major financial conflicts of interest versus clinical guidelines with minor financial conflicts of interest
We were able to assess the number of authors with financial conflicts of interest in each clinical guideline in two studies (Norris 2013; Wang 2010). We found no difference in recommendations between guidelines with major (i.e. at least half of the authors) and minor (i.e. less than half of the authors) financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.20, Analysis 3.2).
3.2. Analysis.

Comparison 3: Subgroup analyses for clinical guidelines, Outcome 2: Clinical guidelines with major financial conflicts of interest versus clinical guidelines with minor financial conflicts of interest
Findings from subgroup analyses on advisory committee reports
Different types of financial conflicts of interest
Of the four studies included in our primary analysis on financial conflicts of interest, one study specified different types of financial conflicts of interest (Ackerley 2009). We were able to pool data on five different types of financial conflicts of interest: consultancy, grants, investments, lecturing and honoraria, and other relationships of committee members (including e.g. patents and expert witness).
We found no difference in recommendations between advisory committee reports with different types of financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.82, Analysis 4.1).
4.1. Analysis.

Comparison 4: Subgroup analyses for advisory committee reports, Outcome 1: Different types of financial conflicts of interest
High risk of bias versus low risk of bias studies
We planned to analyse studies assessed as high risk of bias with studies assesses as low risk of bias. However, all four studies included in our pooled analysis on advisory committee reports were assessed as high risk of bias, and it was not possible to carry out this subgroup analysis.
Advisorycommittee reports with major financial conflicts of interest versusadvisory committee reports with minor financial conflicts of interest
We were able to assess the number of committee members with financial conflicts of interest in each advisory committee report in two studies (Ackerley 2009; Lurie 2006). We found no difference in recommendations between advisory committee reports with major (i.e. at least half of the committee members) and minor (i.e. less than half of the committee members)financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.92, Analysis 4.2).
4.2. Analysis.

Comparison 4: Subgroup analyses for advisory committee reports, Outcome 2: Advisory committee reports with major financial conflicts of interest versus advisory committee reports with minor financial conflicts of interest
Findings from subgroup analyses on opinion pieces
Different types of financial conflicts of interest
Three of the four studies included in our pooled analysis on financial conflicts of interest in opinion pieces investigated different types of financial conflicts of interest. We were able to pool data from the studies on eight types of financial conflicts of interest: advisory board membership, consultancy, employment, grants, honoraria, lecture or speaker fees, other relationships (including royalties, testimony, patents, and travel grants), and stock ownership.
We found no difference in recommendations between opinion pieces with different types of financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.84, Analysis 5.1).
5.1. Analysis.

Comparison 5: Subgroup analyses for opinion pieces, Outcome 1: Different types of financial conflicts of interest
High risk of bias versus low risk of bias studies
We planned to compare studies assessed as high risk of bias with studies assessed as low risk of bias. However, all four studies included in our pooled analysis on opinion pieces were assessed as high risk of bias, and it was not possible to carry out this subgroup analysis.
Opinion pieces with major financial conflicts of interest versus opinion pieces with minor financial conflicts of interest
We were able to assess the number of authors with financial conflicts of interest in each opinion piece in one study (Wang 2010). We found no difference in recommendations between opinion pieces with major (i.e. at least half of the authors)and minor (i.e. less than half of the authors) financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.74, Analysis 5.2).
5.2. Analysis.

Comparison 5: Subgroup analyses for opinion pieces, Outcome 2: Opinion pieces with major financial conflicts of interest versus opinion pieces with minor financial conflicts of interest
Findings from subgroup analyses on narrative reviews
Different types of financial conflicts of interest
Three of the four studies investigating narrative reviews investigated different types of financial conflicts of interest. We were able to pool data on nine types: advisory board membership, assistance provided by industry, consultancy, employment, grants, honoraria, industry funding of the review, lecture or speaker fees, other relationships of review authors, and travel grants.
We found no difference in recommendations between reviews with different types of financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.90, Analysis 6.1).
6.1. Analysis.

Comparison 6: Subgroup analyses for narrative reviews, Outcome 1: Different types of financial conflicts of interest
High risk of bias versus low risk of bias studies
We planned to compare studies assessed as high risk of bias with studies assessed as low risk of bias. However, all four studies included in our pooled analysis on narrative reviews were assessed as high risk of bias, and it was not possible to carry out this subgroup analysis.
Narrative reviews with major financial conflicts of interest versus narrative reviews with minor financial conflicts of interest
We were able to assess the number of authors with financial conflicts of interest in narrative review in two studies (Dunn 2016; Wang 2010). We found no difference in recommendations between reviews with major (i.e. at least half of the authors)and minor (i.e. less than half of the authors) financial conflicts of interest, but estimates were statistically imprecise (P value for interaction test: 0.42, Analysis 6.2).
6.2. Analysis.

Comparison 6: Subgroup analyses for narrative reviews, Outcome 2: Narrative reviews with major financial conflicts of interest versus narrative reviews with minor financial conflicts of interest
Appendix 8. Sensitivity analyses
Findings from sensitivity analyses on clinical guidelines
Excluding clinical guidelines with unclear or undisclosed conflicts of interest
One of the studies included in the pooled analysis on financial conflicts of interest only included clinical guidelines with clear conflicts of interest statements (Norris 2013). In the remaining three studies it was not possible to exclude clinical guidelines with unclear or undisclosed conflicts of interest, because reporting of data did not allow it (Tibau 2015), or the authors did not code this information in their raw datasets (Aakre 2012; Wang 2010). In our analysis excluding clinical guidelines with undisclosed financial conflicts of interest, we found somewhat similar results as the primary analysis (from RR: 1.26, 95% CI: 0.93 to 1.69 in the primary analysis to RR: 1.08, 95% CI: 0.71 to 1.64, Analysis 7.1).
7.1. Analysis.

Comparison 7: Sensitivity analyses for clinical guidelines, Outcome 1: Excluding clinical guidelines with unclear or undisclosed financial conflicts of interest
The one study investigating non‐financial conflicts of interest included no clinical guidelines with undisclosed conflicts of interest (Norris 2012).
Excluding clinical guidelines with neutral recommendations
One of the studies included in our pooled analysis on financial conflicts of interest included no clinical guidelines with neutral recommendations (Norris 2013). In two studies, the sample did not include any clinical guidelines without favourable recommendations (Aakre 2012) or without conflicts of interest (Wang 2010), when we removed clinical guidelines with neutral recommendations. In the remaining study, it was not possible to remove clinical guidelines with neutral recommendations, because reporting of data did not allow it (Tibau 2015). Thus, our sensitivity analysis for financial conflicts of interest was based on one study (Norris 2013). We found somewhat similar results as our primary analysis (from RR: 1.26, 95% CI: 0.93 to 1.69 in the primary analysis to RR: 1.08, 95% CI: 0.71 to 1.64, Analysis 7.2).
7.2. Analysis.

Comparison 7: Sensitivity analyses for clinical guidelines, Outcome 2: Excluding clinical guidelines with neutral recommendations
In the one study investigating specialty interest in clinical guidelines, a neutral category was not used for categorising recommendations. Therefore, it was not possible to undertake a sensitivity analysis excluding clinical guidelines with neutral recommendations (Norris 2012).
Excluding all studies of clinical guidelines which disclosed a relevant conflict of interest of study authors
One of the studies included in our pooled analysis disclosed financial conflicts of interest of study authors (Tibau 2015). Excluding this study from our pooled analysis on financial conflicts of interest did not affect our findings (from RR: 1.26, 95% CI: 0.93 to 1.69 in the primary analysis to RR: 1.23, 95% CI: 0.90 to 1.69, Analysis 7.3).
7.3. Analysis.

Comparison 7: Sensitivity analyses for clinical guidelines, Outcome 3: Excluding all studies of clinical guidelines which disclosed a relevant conflict of interest of study authors
The one study investigating non‐financial conflicts of interest did not disclose any conflicts of interest of the study authors (Norris 2012).
Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Re‐analysing our primary analysis using fixed‐effect models did not affect our findings on financial conflicts of interest (from RR: 1.26, 95% CI: 0.93 to 1.69 in the primary analysis to RR: 1.26, 95% CI: 0.93 to 1.69, Analysis 7.4).
7.4. Analysis.

Comparison 7: Sensitivity analyses for clinical guidelines, Outcome 4: Re‐analysing our primary analyses using fixed‐effect meta‐analyses
As only one study was included in our analysis on non‐financial conflicts of interest, it was not meaningful to carry out this sensitivity analysis.
Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer and financial conflicts of interest related to any for‐profit company
One of the studies included in our pooled analysis measured financial conflicts of interest related to the manufacturer of the investigated drug (Norris 2013), whereas three studies measured financial conflicts of interest related to any for‐profit company (Aakre 2012; Tibau 2015), or included only clinical guidelines with financial conflicts of interest related to any for‐profit company (Wang 2010). Both our sensitivity analyses showed somewhat similar results as our primary analysis (from RR: 1.26, 95% CI: 0.93 to 1.69 in the primary analysis to RR: 1.08, 95% CI: 0.71 to 1.64 for financial conflicts of interest related to the manufacturer, Analysis 7.5; and to RR: 1.46, 95% CI: 0.96 to 2.21 for financial conflicts of interest related to any for‐profit company, Analysis 7.6).
7.5. Analysis.

Comparison 7: Sensitivity analyses for clinical guidelines, Outcome 5: Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer
7.6. Analysis.

Comparison 7: Sensitivity analyses for clinical guidelines, Outcome 6: Re‐categorising financial conflicts of interest into financial conflicts of interest related to any for‐profit company
Findings from sensitivity analyses on advisory committee reports
Excluding advisory committee reports with unclear or undisclosed conflicts of interest
In the three of the four studies included in our pooled analysis on advisory committee reports, it was not possible to remove advisory committee reports with undisclosed conflicts of interest, because the authors did not code this information in their raw dataset (Lurie 2006; Zhang 2019) or reporting of data did not allow it (Tibau 2016). In the remaining study, we excluded all committee members with unclear conflicts of interest declarations. We found similar results as in our primary analysis (from RR: 1.20, 95% CI: 0.99 to 1.45 in the primary analysis to RR: 1.20, 95% CI: 0.77 to 1.87, Analysis 8.1).
8.1. Analysis.

Comparison 8: Sensitivity analyses for advisory committee reports, Outcome 1: Excluding advisory committee reports with unclear or undisclosed conflicts of interest
Excluding advisory committee reports with neutral recommendations
Only one of the studies included in our pooled analysis reported neutral recommendations in a separate category in the primary analysis (Lurie 2006), and additionally one study coded whether the voting outcome of the meetings were unanimous (but did not include any unanimous meetings) (Ackerley 2009). For the remaining studies, the authors did not code neutral recommendations (e.g. unanimous voting outcomes) in their raw dataset (Zhang 2019) or reporting of data did not allow us to exclude advisory committee reports with neutral recommendations (Tibau 2016). We found somewhat similar results as in our primary analysis (from RR: 1.20, 95% CI: 0.99 to 1.45 in the primary analysis to RR: 1.28, 95% CI: 0.96 to 1.70, Analysis 8.2).
8.2. Analysis.

Comparison 8: Sensitivity analyses for advisory committee reports, Outcome 2: Excluding advisory committee reports with neutral recommendations
Excluding all studies of advisory committee reports which disclose a relevant conflict of interest of study authors
One of the studies included in our pooled analysis disclosed financial conflicts of interest of study authors (Zhang 2019). Excluding this study from our pooled analysis on financial conflicts of interest increased the effect estimate and increased statistical precision (from RR: 1.20, 95% CI: 0.99 to 1.45 in the primary analysis to RR: 1.39, 95% CI: 1.08 to 1.80, Analysis 8.3).
8.3. Analysis.

Comparison 8: Sensitivity analyses for advisory committee reports, Outcome 3: Excluding all studies of advisory committee reports which disclose a relevant conflict of interest of study authors
Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Re‐analysing our primary analysis on advisory committee reports using fixed‐effect models did not affect our findings (from RR: 1.20, 95% CI: 0.99 to 1.45 in the primary analysis to RR: 1.15, 95% CI: 1.00 to 1.32, Analysis 8.4).
8.4. Analysis.

Comparison 8: Sensitivity analyses for advisory committee reports, Outcome 4: Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer and financial conflicts of interest related to any for‐profit company
The four studies included in our pooled analysis on advisory committee reports both investigated financial conflicts of interest related to the manufacturer of the investigated drug and any for‐profit company. One of the studies only reported summary odds ratio for financial conflicts of interest related to the manufacturer and competitor and was not included in our pooled analysis (Tibau 2016). Thus, we were able to include data from three studies in our sensitivity analysis restricted to financial conflicts of interest related to the manufacturer (Ackerley 2009; Lurie 2006; Zhang 2019). Our analysis showed similar findings as our primary analysis (from RR: 1.20, 95% CI: 0.99 to 1.45 in the primary analysis to RR: 1.24, 95% CI: 0.99 to 1.54, Analysis 8.5). The remaining study had different effect estimates for financial conflicts of interest related to the manufacturer (OR: 1.79, 95% CI: 0.75 to 4.26) and any for‐profit company (OR: 1.06, 95% CI: 0.78 to 1.44), though with statistical imprecision (Tibau 2016).
8.5. Analysis.

Comparison 8: Sensitivity analyses for advisory committee reports, Outcome 5: Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer
In our primary analysis, all studies included advisory committee reports with financial conflicts of interest related to any for‐profit company (e.g. the manufacturer, competitor, or both) in the financial conflicts of interest group. Thus, we did not perform the sensitivity analysis restricted to any for‐profit company as the results would be identical with the primary analysis.
Findings from sensitivity analyses on opinion pieces
Excluding opinion pieces with unclear or undisclosed conflicts of interest
Two studies coded opinion pieces with unclear or undisclosed financial conflicts of interest (Bariani 2013; Lerner 2012). In the remaining studies, it was not possible to separate opinion pieces with unclear or undisclosed financial conflicts of interest, because the authors did not code this information (Hayes 2019; Wang 2010). Our sensitivity analysis showed somewhat similar results compared with our primary analysis (from RR: 2.62, 95% CI: 0.91 to 7.55 in the primary analysis to RR: 1.47, 95% CI: 0.53 to 4.13, Analysis 9.1).
9.1. Analysis.

Comparison 9: Sensitivity analyses for opinion pieces, Outcome 1: Excluding opinion pieces with unclear or undisclosed conflicts of interest
Excluding opinion pieces with neutral recommendations
We were able to exclude opinion pieces with neutral recommendations for three studies investigating opinion pieces (Bariani 2013; Lerner 2012; Wang 2010). The remaining study did not distinguish between neutral and unfavourable opinion pieces (Hayes 2019). An analysis based on these three studies showed somewhat similar results as our primary analysis (from RR: 2.62, 95% CI: 0.91 to 7.55 in the primary analysis to RR: 2.00, 95% CI: 0.77 to 5.21, Analysis 9.2).
9.2. Analysis.

Comparison 9: Sensitivity analyses for opinion pieces, Outcome 2: Excluding opinion pieces with neutral recommendations
Excluding all studies of opinion pieces which disclose a relevant conflict of interest of study authors
From the four studies included in our primary analysis, one study disclosed financial conflicts of interest of study authors (Bariani 2013). An analysis excluding this study had somewhat different results than our primary analysis (from RR: 2.62, 95% CI: 0.91 to 7.55 in the primary analysis to RR: 3.84, 95% CI: 1.81 to 8.13, Analysis 9.3), though the estimate was statistically imprecise.
9.3. Analysis.

Comparison 9: Sensitivity analyses for opinion pieces, Outcome 3: Excluding all studies of opinion pieces which disclosed a relevant conflict of interest of study authors
Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Our re‐analysis of our primary analysis using a fixed‐effect model showed somewhat similar results as our primary analysis (from RR: 2.62, 95% CI: 0.91 to 7.55 in the primary analysis to RR: 1.27, 95% CI: 0.94 to 1.72, Analysis 9.4).
9.4. Analysis.

Comparison 9: Sensitivity analyses for opinion pieces, Outcome 4: Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer and financial conflicts of interest related to any for‐profit company
Two of the studies included in our pooled analysis investigated financial conflicts of interest related to the manufacturer of the studied drug or device (Hayes 2019; Lerner 2012). Our sensitivity analysis restricted to financial conflicts of interest related to the manufacturer showed a stronger association than our primary analysis (from RR: 2.62, 95% CI: 0.91 to 7.55 in the primary analysis to RR: 14.69, 95% CI: 4.10 to 52.68, Analysis 9.5).
9.5. Analysis.

Comparison 9: Sensitivity analyses for opinion pieces, Outcome 5: Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer
One study solely investigated financial conflicts of interest related to the manufacturer (Hayes 2019). When we excluded this study from the analysis to include only studies on financial conflicts of interest related to any for‐profit companies, we found similar results as our primary analysis (from RR: 2.62, 95% CI: 0.91 to 7.55 in the primary analysis to RR: 2.45, 95% CI: 0.78 to 7.74, Analysis 9.6).
9.6. Analysis.

Comparison 9: Sensitivity analyses for opinion pieces, Outcome 6: Re‐categorising financial conflicts of interest into financial conflicts of interest related to any for‐profit company
Findings from sensitivity analyses on narrative reviews
Excluding narrative reviews with unclear or undisclosed conflicts of interest
We were able to exclude narrative reviews with unclear of undisclosed conflicts of interest from two studies (Dunn 2016; Hartog 2012). An analysis based on these two studies had somewhat similar results as our primary analysis (from RR: 1.20, 95% CI: 0.97 to 1.49 in the primary analysis to RR: 1.37, 95% CI: 1.11 to 1.69, Analysis 10.1).
10.1. Analysis.

Comparison 10: Sensitivity analyses for narrative reviews, Outcome 1: Excluding narrative reviews with unclear or undisclosed conflicts of interest
Excluding narrative reviews with neutral recommendations
We were able to exclude narrative reviews with neutral recommendations from two studies (Dunn 2016; Wang 2010). Additionally, one study investigating narrative reviews did not include any narrative reviews with neutral recommendations (Hartog 2012). The remaining study did not code unfavourable and neutral recommendations separately (Hayes 2019). Our sensitivity analysis had somewhat similar results as our primary analysis (from RR: 1.20, 95% CI: 0.97 to 1.49 in the primary analysis to RR: 1.17, 95% CI: 0.97 to 1.42, Analysis 10.2).
10.2. Analysis.

Comparison 10: Sensitivity analyses for narrative reviews, Outcome 2: Excluding narrative reviews with neutral recommendations
Excluding all studies of narrative reviews which disclose a relevant conflict of interest of study authors
From the studies included in the pooled analysis, one study disclosed conflicts of interest of study authors (Hartog 2012). Our analysis excluding this study showed somewhat similar results as our primary analysis (from RR: 1.20, 95% CI: 0.97 to 1.49 in the primary analysis to RR: 1.39, 95% CI: 0.68 to 2.86, Analysis 10.3).
10.3. Analysis.

Comparison 10: Sensitivity analyses for narrative reviews, Outcome 3: Excluding all studies of narrative reviews which disclosed a relevant conflict of interest of study authors
Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Our re‐analysis of our primary analysis on narrative reviews using a fixed‐effect model had somewhat similar results compared to our primary analysis (from RR: 1.20, 95% CI: 0.97 to 1.49 in the primary analysis to RR: 1.19, 95% CI: 1.05 to 1.36, Analysis 10.4).
10.4. Analysis.

Comparison 10: Sensitivity analyses for narrative reviews, Outcome 4: Re‐analysing our primary analyses using fixed‐effect meta‐analyses
Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer and financial conflicts of interest related to any for‐profit company
Two of the studies on narrative reviews investigated financial conflicts of interest related to the manufacturer of the drug or device of interest (Dunn 2016; Hayes 2019), one study investigated financial conflicts of interest related to both the manufacturer and any for‐profit company (Wang 2010), and the remaining study investigated financial conflicts of interest related to any for‐profit company (Hartog 2012).
Both our sensitivity analyses showed somewhat similar results as our primary analysis (from RR: 1.20, 95% CI: 0.97 to 1.49 in the primary analysis to RR: 1.16, 95% CI: 0.95 to 1.40 for financial conflicts of interest related to the manufacturer, Analysis 10.5; and to: RR: 2.86, 95% CI: 0.35 to 23.30 for financial conflicts of interest related to any for‐profit company, Analysis 10.6).
10.5. Analysis.

Comparison 10: Sensitivity analyses for narrative reviews, Outcome 5: Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer
10.6. Analysis.

Comparison 10: Sensitivity analyses for narrative reviews, Outcome 6: Re‐categorising financial conflicts of interest into financial conflicts of interest related to any for‐profit company
Appendix 9. 'Summary of findings' table
We assessed the certainty of the evidence for our primary outcome using both the GRADE approach for intervention studies (Guyatt 2008) (observational studies preliminary graded as providing low certainty evidence) and prognostic studies (Foroutan 2020) (observational studies preliminary graded as providing high certainty evidence).
Summary of findings table
| Document type | Absolute effect (95% CI)* |
Relative effect RR (95% CI) |
Number of studies | Certainty of the evidence using the GRADE approach for intervention studies** | Certainty of the evidence using the GRADE approach for prognostic studies*** | |
| Event rate in documents with conflicts of interest | Event rate in documents without conflicts of interest | |||||
| Financial conflicts of interest | ||||||
| Clinical guidelines | 54 (40 to 72) clinical guidelines with favourable recommendations per 100 clinical guidelines with financial conflicts of interest**** | 43 clinical guidelines with favourable recommendations per 100 clinical guidelines without financial conflicts of interest |
1.26 (0.93 to 1.69) |
4 studies including 86 clinical guidelines | Very low | Low |
| Downgraded due to study limitations (four studies with high risk of bias) and imprecision (wide CI*****) | ||||||
| Advisory committee reports | 78 (64 to 94) advisory committee reports with favourable recommendations per 100 advisory committee reports with financial conflicts of interest | 65 advisory committee reports with favourable recommendations per 100 advisory committee reports without financial conflicts of interest |
1.20 (0.99 to 1.45) |
4 studies including 629 advisory committee reports | Very low | Low |
| Downgraded due to study limitations (two studies with high risk of bias) and imprecision (wide CI*****) | ||||||
| Opinion pieces | 71 (25 to 100******) opinion pieces with favourable recommendations per 1000 opinion pieces with financial conflicts of interest | 27 opinion pieces with favourable recommendations per 100 opinion pieces without financial conflicts of interest |
2.62 (0.91 to 7.55) |
4 studies including 284 opinion pieces | Very low | Very low |
| Downgraded due to study limitations (three studies with high risk of bias), imprecision (wide CI*****), and inconsistency (substantial statistical heterogeneity) | ||||||
| Narrative reviews | 72 (58‐89) narrative reviews with favourable recommendations per 100 narrative reviews with financial conflicts of interest | 60 narrative reviews with favourable recommendations per 100 narrative reviews without financial conflicts of interest |
1.20 (0.97 to 1.49) |
4 studies including 457 narrative reviews | Very low | Low |
| Downgraded due to study limitations (three studies with high risk of bias) and imprecision (wide CI*****) | ||||||
| Non‐financial conflicts of interest | ||||||
| Clinical guidelines | 90 (39‐100*****) clinical guidelines with favourable recommendations per 100 clinical guidelines with one or more radiology authors | 43 clinical guidelines with favourable recommendations per 100 clinical guidelines without radiology authors |
2.10 (0.92‐4.77) |
1 study including 12 clinical guidelines | Very low | Low |
| Downgraded due to study limitations (one study with high risk of bias) and imprecision (wide CI*****) | ||||||
CI: confidence interval;RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation *The event rate of the control group (i.e. no conflicts of interest group) was calculated as the mean risk (i.e. number of documents with favourable recommendations divided by total number of documents). The event rate (and its 95% CI) in the intervention group (i.e. conflicts of interest group) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). **The procedure for assessing the certainty of the evidence followed the GRADE approach for intervention studies (observational studies preliminary graded as providing low certainty evidence). ***The procedure for assessing the certainty of the evidence followed the GRADE approach for prognostic studies (observational studies preliminary graded as providing high certainty evidence). ****Numbers on clinical guidelines do not account for panel data in the Norris 2013 study (i.e. 13 clinical guidelines with 24 recommendations each). *****We used an effect size of 0.05 on a relative scale (i.e. RR < 0.95 or RR >1.05) as a methodologically important difference (Guyatt 2011). This cut‐off was based on effect sizes of important study design biases in trials (Page 2016). ******Upper event rate truncated at 100.
Data and analyses
Comparison 1. Primary analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Financial conflicts of interest | 13 | Risk Ratio (IV, Random, 95% CI) | 1.26 [1.09, 1.44] | |
| 1.1.1 Clinical guidelines | 4 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.93, 1.69] | |
| 1.1.2 Advisory committee reports | 4 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.99, 1.45] | |
| 1.1.3 Opinion pieces | 4 | Risk Ratio (IV, Random, 95% CI) | 2.62 [0.91, 7.55] | |
| 1.1.4 Narrative reviews | 4 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.97, 1.49] |
Comparison 2. Secondary analysis: using individual voting level in the analysis on advisory committee reports.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Financial conflicts of interest | 3 | 17816 | Risk Ratio (IV, Random, 95% CI) | 1.14 [1.07, 1.21] |
Comparison 3. Subgroup analyses for clinical guidelines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Different types of financial conflicts of interest | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Advisory board membership | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 2.80 [0.24, 33.04] |
| 3.1.2 Consultancy | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 2.80 [0.24, 33.04] |
| 3.1.3 Grants | 1 | 4 | Risk Ratio (IV, Random, 95% CI) | 2.50 [0.20, 31.00] |
| 3.1.4 Honoraria | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 2.80 [0.24, 33.04] |
| 3.1.5 Industry funding of the clinical guideline | 1 | 18 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.88, 2.30] |
| 3.1.6 Speaker fees | 1 | 5 | Risk Ratio (IV, Random, 95% CI) | 2.80 [0.24, 33.04] |
| 3.2 Clinical guidelines with major financial conflicts of interest versus clinical guidelines with minor financial conflicts of interest | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Major financial conflicts of interest | 2 | Risk Ratio (IV, Random, 95% CI) | 1.56 [1.05, 2.33] | |
| 3.2.2 Minor financial conflicts of interest | 1 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.68, 1.65] |
Comparison 4. Subgroup analyses for advisory committee reports.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Different types of financial conflicts of interest | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Consultancy | 1 | 77 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.88, 2.31] |
| 4.1.2 Grants | 1 | 54 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.66, 2.07] |
| 4.1.3 Investments | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 1.49 [0.91, 2.44] |
| 4.1.4 Lecturing and honoraria | 1 | 53 | Risk Ratio (IV, Random, 95% CI) | 1.77 [1.10, 2.87] |
| 4.1.5 Other relationships | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.02, 3.00] |
| 4.2 Advisory committee reports with major financial conflicts of interest versus advisory committee reports with minor financial conflicts of interest | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Major financial conflicts of interest | 2 | 129 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.96, 1.99] |
| 4.2.2 Minor financial conflicts of interest | 2 | 104 | Risk Ratio (IV, Random, 95% CI) | 1.35 [0.97, 1.88] |
Comparison 5. Subgroup analyses for opinion pieces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Different types of financial conflicts of interest | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 Advisory board membership | 2 | 103 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.58, 2.84] |
| 5.1.2 Consultancy | 2 | 156 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.52, 2.27] |
| 5.1.3 Employment | 2 | 132 | Risk Ratio (IV, Random, 95% CI) | 2.71 [0.03, 231.22] |
| 5.1.4 Grants | 3 | 210 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.86, 3.46] |
| 5.1.5 Honoraria | 2 | 132 | Risk Ratio (IV, Random, 95% CI) | 1.35 [0.35, 5.24] |
| 5.1.6 Lecture or speaker fees | 2 | 104 | Risk Ratio (IV, Random, 95% CI) | 1.88 [0.51, 6.94] |
| 5.1.7 Other relationships | 2 | 111 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.51, 1.83] |
| 5.1.8 Stock ownership | 2 | 107 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.22, 1.89] |
| 5.2 Opinion pieces with major financial conflicts of interest versus opinion pieces with minor financial conflicts of interest | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 Major financial conflicts of interest | 1 | 90 | Risk Ratio (IV, Random, 95% CI) | 9.43 [2.24, 39.62] |
| 5.2.2 Minor financial conflicts of interest | 1 | 56 | Risk Ratio (IV, Random, 95% CI) | 5.60 [0.38, 82.41] |
Comparison 6. Subgroup analyses for narrative reviews.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Different types of financial conflicts of interest | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 Advisory board membership | 1 | 130 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.98, 1.69] |
| 6.1.2 Assistance provided by industry | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 3.35 [0.37, 30.16] |
| 6.1.3 Consultancy | 2 | 228 | Risk Ratio (IV, Random, 95% CI) | 2.94 [0.34, 25.62] |
| 6.1.4 Employment | 2 | 275 | Risk Ratio (IV, Random, 95% CI) | 1.36 [1.04, 1.79] |
| 6.1.5 Grants | 2 | 229 | Risk Ratio (IV, Random, 95% CI) | 3.29 [0.25, 42.87] |
| 6.1.6 Honoraria | 1 | 121 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.60, 1.51] |
| 6.1.7 Industry funding of the review | 3 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.34 [0.65, 2.75] |
| 6.1.8 Lecture or speaker fees | 1 | 122 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.06, 1.83] |
| 6.1.9 Other relationships | 1 | 122 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.06, 1.83] |
| 6.1.10 Travel grants | 1 | 125 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.94, 1.71] |
| 6.2 Narrative reviews with major financial conflicts of interest versus narrative reviews with minor financial conflicts of interest | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 Major financial conflicts of interest | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.34, 26.81] |
| 6.2.2 Minor financial conflicts of interest | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.86, 1.71] |
Comparison 7. Sensitivity analyses for clinical guidelines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Excluding clinical guidelines with unclear or undisclosed financial conflicts of interest | 1 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.71, 1.64] | |
| 7.2 Excluding clinical guidelines with neutral recommendations | 1 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.71, 1.64] | |
| 7.3 Excluding all studies of clinical guidelines which disclosed a relevant conflict of interest of study authors | 3 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.90, 1.69] | |
| 7.4 Re‐analysing our primary analyses using fixed‐effect meta‐analyses | 4 | Risk Ratio (IV, Fixed, 95% CI) | 1.26 [0.93, 1.69] | |
| 7.5 Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer | 1 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.71, 1.64] | |
| 7.6 Re‐categorising financial conflicts of interest into financial conflicts of interest related to any for‐profit company | 3 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.96, 2.21] |
Comparison 8. Sensitivity analyses for advisory committee reports.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Excluding advisory committee reports with unclear or undisclosed conflicts of interest | 1 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.77, 1.87] |
| 8.2 Excluding advisory committee reports with neutral recommendations | 2 | 172 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.96, 1.70] |
| 8.3 Excluding all studies of advisory committee reports which disclose a relevant conflict of interest of study authors | 3 | 253 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.08, 1.80] |
| 8.4 Re‐analysing our primary analyses using fixed‐effect meta‐analyses | 4 | 629 | Risk Ratio (IV, Fixed, 95% CI) | 1.15 [1.00, 1.32] |
| 8.5 Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer | 3 | 410 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.99, 1.54] |
Comparison 9. Sensitivity analyses for opinion pieces.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Excluding opinion pieces with unclear or undisclosed conflicts of interest | 2 | 160 | Risk Ratio (IV, Random, 95% CI) | 1.47 [0.53, 4.13] |
| 9.2 Excluding opinion pieces with neutral recommendations | 3 | 187 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.77, 5.21] |
| 9.3 Excluding all studies of opinion pieces which disclosed a relevant conflict of interest of study authors | 3 | 153 | Risk Ratio (IV, Random, 95% CI) | 3.84 [1.81, 8.13] |
| 9.4 Re‐analysing our primary analyses using fixed‐effect meta‐analyses | 4 | 284 | Risk Ratio (IV, Fixed, 95% CI) | 1.27 [0.94, 1.72] |
| 9.5 Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer | 2 | 70 | Risk Ratio (IV, Random, 95% CI) | 14.69 [4.10, 52.68] |
| 9.6 Re‐categorising financial conflicts of interest into financial conflicts of interest related to any for‐profit company | 3 | 276 | Risk Ratio (IV, Random, 95% CI) | 2.45 [0.78, 7.74] |
Comparison 10. Sensitivity analyses for narrative reviews.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Excluding narrative reviews with unclear or undisclosed conflicts of interest | 2 | 201 | Risk Ratio (IV, Random, 95% CI) | 1.37 [1.11, 1.69] |
| 10.2 Excluding narrative reviews with neutral recommendations | 3 | 336 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.97, 1.42] |
| 10.3 Excluding all studies of narrative reviews which disclosed a relevant conflict of interest of study authors | 3 | 304 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.68, 2.86] |
| 10.4 Re‐analysing our primary analyses using fixed‐effect meta‐analyses | 4 | 457 | Risk Ratio (IV, Fixed, 95% CI) | 1.19 [1.05, 1.36] |
| 10.5 Re‐categorising financial conflicts of interest into financial conflicts of interest related to the manufacturer | 3 | 268 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.95, 1.40] |
| 10.6 Re‐categorising financial conflicts of interest into financial conflicts of interest related to any for‐profit company | 2 | 237 | Risk Ratio (IV, Random, 95% CI) | 2.86 [0.35, 23.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aakre 2012.
| Study characteristics | ||
| Methods | To explore to what extent current clinical practice guideline recommendations about use of self‐monitoring blood glucose in patients with diabetes who do not use insulin are based on the principles of evidence‐based medicine. Guidelines published between 1999 and 2011 | |
| Data | 18 guidelines | |
| Comparisons | Clinical guidelines with financial conflicts of interest (defined as funding by industry) and clinical guidelines without financial conflicts of interest | |
| Outcomes | Recommendations (classified by a scale of 1‐4: grade 1, strongly against self‐monitoring; grade 2, weekly against self‐monitoring; grade 3, weakly in favour of self‐monitoring; grade 4, strongly in favour of self‐monitoring) | |
| Funding source | The study was funded by the European Federation of Clinical Chemistry and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two pairs of authors independently assessed clinical guidelines for inclusion |
| Adequate coding of conflicts of interest | Yes | One author extracted data, three authors independently coded each guideline (according to personal correspondence with lead author) |
| Adequate coding of recommendations | Yes | Three authors independently coded the recommendations of each guideline |
| Adequate dealing with confounding | No | Compared clinical guidelines of different types of self‐monitoring with wide range of publication years (1999‐2011) |
Ackerley 2009.
| Study characteristics | ||
| Methods | To analyse whether advisory committee members tend to vote in a manner that is relevant to their financial conflicts‐of‐interest. FDA drug, radiology, device, and biologic advisory committee meetings held between January 2001 and first quarter of 2008. | |
| Data | 98 advisory committee reports and 1191 committee members (611 advisory committee reports included in study (not all had data available in a format for inclusion in analysis) and 221 duplicates also included in Lurie 2006 removed). | |
| Comparisons | Advisory committee reports with financial conflicts of interest (defined as at least one committee member with financial ties to the product manufacturer or competitor) and advisory committee meetings without financial conflicts of interest Advisory committee members with financial conflicts of interest (defined as financial ties to the product manufacturer or competitor) and advisory committee members without financial conflicts of interest |
|
| Outcomes | Recommendations (favourable recommendations defined as votes in favour of the drug) | |
| Funding source | The study was commissioned by Eastern Research Group (ERG) and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | Conflicts of interest not described | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed committee meetings for inclusion (according to personal correspondence with lead author) |
| Adequate coding of conflicts of interest | Yes | The dataset was reviewed by multiple team members (according to personal correspondence with lead author) |
| Adequate coding of recommendations | Yes | The dataset was reviewed by multiple team members (according to personal correspondence with lead author) |
| Adequate dealing with confounding | No | Compared committee meetings of different drugs used for different diseases |
Bariani 2013.
| Study characteristics | ||
| Methods | To identify whether there was any association between conclusions of authors of editorials and self‐reported conflicts of interest or sponsorship. Editorials commenting on phase III oncology clinical trials and published between January 2008 and October 2011 in six clinical oncology journals | |
| Data | 131 editorials (131 opinion pieces included in analysis after removing 19 duplicates also included in the Lerner 2012 study) | |
| Comparisons | Editorials with financial conflicts of interest (defined as at least one author with any self‐reported financial ties with a pharmaceutical company) and editorials without financial conflicts of interest | |
| Outcomes | Recommendations (classified as highly positive, positive, neutral, negative, or highly negative) | |
| Funding source | Funding source not described | |
| Declaration of conflicts of interest | MKK (fourth author) has a consultant or advisory role at Bayer Pharmaceuticals, has received honoraria from Novartis, Sanofi‐Aventis, and AstraZeneca, and has received research funding from AstraZeneca, Novartis, and Exelixis. RPR (last author) has a consultant or advisory role at Novartis, has received honoraria from Novartis, Merck Serono, and Roche, has received research funding from Novartis, and has received other remuneration from Merck Serono, Ipsen, Novartis, Bayer Pharmaceuticals, and Roche | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors independently assessed editorials for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | Yes | Two authors independently coded each editorial (according to personal correspondence with corresponding author) |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each editorial |
| Adequate dealing with confounding | No | Compared editorials of different interventions and outcomes. In regression analyses, the authors adjusted for type of outcome and type of intervention |
Cooper 2019.
| Study characteristics | ||
| Methods | To investigate whether financial ties to drug companies bias FDA drug advisory committee members’ voting on drug approval recommendations. FDA advisory committee meeting held between 1997 and 2012 | |
| Data | 416 advisory committee reports and 1483 advisory committee members | |
| Comparisons | Advisory committee members with financial conflicts of interest (defined as financial ties to any drug company) and advisory committee members without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as votes in favour of the drug) | |
| Funding source | The study received support from the Searle Civil Justice Institute and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | Conflicts of interest not described | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Unclear | Not possible to determine |
| Adequate coding of conflicts of interest | Unclear | Not possible to determine |
| Adequate coding of recommendations | Unclear | Not possible to determine |
| Adequate dealing with confounding | No | Compared committee meetings of different drugs used for different diseases |
Downing 2014.
| Study characteristics | ||
| Methods | To examine whether there was an association between authors’ financial relationships with pharmaceutical companies invested in fenofibrate’s commercial success and their interpretation. Clinical guidelines, opinion pieces, and narrative reviews commenting on a randomised trial of fenofibrate (the ACCORD‐Lipid trial) and published in 2010 and 2011. | |
| Data | 4 clinical guidelines; 23 editorials and commentaries; 40 reviews (mainly narrative) (5 clinical guidelines, 24 editorials and commentaries, and 70 reviews included in the study, but not all had data available in a format for inclusion in analysis). | |
| Comparisons | Documents with financial conflicts of interest (defined as at least one author with financial ties to the manufacturer of fenofibrate or any other drug company with a commercial interest in fenofibrate) and documents without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as recommending use of fibrates) | |
| Funding source | No funding was received for the study | |
| Declaration of conflicts of interest | HMK (third author) and JSR (last author) have received support from Medtronic and Johnson and Johnson to develop methods of clinical trial data sharing | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors independently assessed documents for inclusion |
| Adequate coding of conflicts of interest | Yes | Two authors independently coded conflicts of interest in each document |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each document |
| Adequate dealing with confounding | Yes | Compared documents commenting on the same trial and published within a short period of time |
Dunn 2016.
| Study characteristics | ||
| Methods | To examine the association between author financial competing interests and the conclusions of narrative reviews about neuroaminidase inhibitors. Narrative reviews published between January 2005 and April 2015 | |
| Data | 213 narrative reviews | |
| Comparisons | Narrative reviews with financial conflicts of interest (defined as at least one author with employment, research funding, consulting fees, or speaker fees provided by a pharmaceutical company manufacturing any of the neuraminidase inhibitors of interest) and narrative reviews without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as concluding that one or more of the neuraminidase inhibitors were safe and effective for use in the prophylaxis or treatment of influenza) | |
| Funding source | The study was funded by the National Health and Medical Research Council and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Up to three authors independently assessed narrative reviews for inclusion (according to personal correspondence with lead author) |
| Adequate coding of conflicts of interest | Yes | One author extracted data, and two author independently coded any ambiguous narrative reviews (according to personal correspondence with lead author) |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each narrative review |
| Adequate dealing with confounding | No | Compared narrative reviews of different drugs (all neuraminidase inhibitors), used for different indications (prophylaxis and treatment), and different publication years |
George 2014.
| Study characteristics | ||
| Methods | To compare the methods and outcomes of two guidelines on diagnosis and treatment of primary immune thrombocytopenia published in close proximity | |
| Data | Two clinical guidelines | |
| Comparisons | One clinical guideline with financial conflicts of interest (defined as unrestricted grants from pharmaceutical companies and financial associations among the authors with companies that manufacture products related to primary immune thrombocytopenia) and one clinical guideline without financial conflicts of interest | |
| Outcomes | Recommendations (classified by two different scales: 1) A, strong; B, intermediate; C, weak; or 2) 1, strong; 2, weak) | |
| Funding source | No funding was received for the study | |
| Declaration of conflicts of interest | JNG (lead author) has been a consultant, receiving honoraria, and receiving research funding from pharmaceutical companies. SKV (second author) has served as a biostatistician on an industry funded study | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | No systematic search for guidelines |
| Adequate coding of conflicts of interest | Yes | Three authors agreed that the information provided in the reporting of the two guidelines was concise and clear, and the authors of the study reported this information (according to personal correspondence with lead author) |
| Adequate coding of recommendations | Yes | The two included guidelines graded the recommendations made and the authors of the study reported this grading (according to personal correspondence with lead author) |
| Adequate dealing with confounding | Yes | Compared clinical guidelines of the same disease published within one year of each other in the same scientific journal |
Hartog 2012.
| Study characteristics | ||
| Methods | To examine the relationship between authors’ potential conflicts of interest and the recommendations made in narrative reviews on clinical use of hydroxyethyl starch. Narrative reviews published between 1960 and 21 May 2010 | |
| Data | 153 narrative reviews | |
| Comparisons | Narrative reviews with financial conflicts of interest (defined as at least one author with financial relationships or other kind of support from a manufacturer of any commercially available intravenous fluids) and narrative reviews without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as recommending hydroxyethyl starch use over other fluids) | |
| Funding source | The study was funded by the Intramural Research Program of the U.S. National Institutes of Health and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | KR (last author) has received grants and speaker’s and consultancy fees from B. Braun | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors independently assessed narrative reviews for inclusion |
| Adequate coding of conflicts of interest | Yes | Two authors independently coded each narrative review |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each narrative review |
| Adequate dealing with confounding | No | Compared narrative reviews of hydroxyethyl starch used for many different indications (outcomes may vary) in different populations with different publication years |
Hayes 2019.
| Study characteristics | ||
| Methods | To investigate the association between authors’ financial conflict‐of‐interest and position on the clinical application of a medical device utilising tumour‐treating fields for the treatment of Glioblastoma. Opinion pieces and narrative reviews published up to 2018 | |
| Data | 8 opinion pieces and 7 narrative reviews | |
| Comparisons | Documents with financial conflicts of interest (defined as at least one author with financial ties to the manufacturer of tumour‐treating fields therapy) and documents without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as supporting tumour‐treating fields without caveat) | |
| Funding source | The work of VP (last author) is funded by the Laura and John Arnold Foundation and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed documents for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | Yes | Two authors independently coded conflicts of interest in each document |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each document |
| Adequate dealing with confounding | Yes | Compared documents commenting on the same trial |
Lerner 2012.
| Study characteristics | ||
| Methods | To investigate the possible association between the presence of personal conflicts of interest and favourable opinions. Editorials commenting on phase III clinical trials published between January 2007 and December 2009 in four major oncology journals | |
| Data | 54 editorials | |
| Comparisons | Editorials with financial conflicts of interest (defined as at least one author with financial relationships to a for‐profit organisation) and editorials without financial conflicts of interest | |
| Outcomes | Recommendations (classified as favourable, neutral, and unfavourable) | |
| Funding source | No funding was received for the study | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed editorials for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | Yes | Three authors independently coded each editorial |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each editorial |
| Adequate dealing with confounding | No | Compared editorials of different drugs with different publication years |
Lurie 2006.
| Study characteristics | ||
| Methods | To assess the relationship between conflicts of interest and voting behavior at drug‐related meetings. All FDA Drug Advisory Committee meetings held between January 2001 and December 2004 | |
| Data | 76 advisory committee reports and 886 advisory committee members from (221 advisory committee reports included in the study, but not all had data available in a format for inclusion in analysis) | |
| Comparisons | Advisory committee reports with financial conflicts of interest (defined as at least one committee member with current investments, employment, patents, contracts, grants, cooperative research, development agreements, consulting, speaking/writing arrangements with any for‐profit company within the last 12 months) and advisory committee reports without financial conflicts of interest Advisory committee members with financial conflicts of interest (defined as current investments, employment, patents, contracts, grants, cooperative research, development agreements, consulting, speaking/writing arrangements with any for‐profit company within the last 12 months) and advisory committee members without financial conflicts of interest |
|
| Outcomes | Recommendations (favourable recommendations defined as votes in favour of the drug) | |
| Funding source | Funding source not described | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed advisory committee reports for inclusion based on criteria developed by three authors (according to personal correspondence with lead author) |
| Adequate coding of conflicts of interest | No | Only one author coded each advisory committee report |
| Adequate coding of recommendations | Yes | Two authors developed criteria for which votes to include, then one author extracted the yes/no votes. No interpretation of text |
| Adequate dealing with confounding | No | Compared advisory committee reports of different drugs used for different diseases |
Norris 2012.
| Study characteristics | ||
| Methods | To examine the relationship between guideline recommendations on routine mammography screening and 1) specialty of physician guideline authors and 2) financial disclosures of physician authors. Clinical guidelines published between January 2005 and June 2011 | |
| Data | 12 clinical guidelines | |
| Comparisons | Clinical guidelines with varying percentages of authors with financial conflicts of interest (defined as disclosure of any financial conflicts of interest) Clinical guidelines with at least one radiologists in the guideline author team and clinical guidelines without radiologists in the guideline author team |
|
| Outcomes | Recommendations (favourable recommendations defined as recommending routine screening) | |
| Funding source | The study was funded by the Agency for Healthcare Research and Quality and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors independently assessed clinical guidelines for inclusion |
| Adequate coding of conflicts of interest | Yes | Two authors independently coded each clinical guideline (according to personal lead with corresponding author) |
| Adequate coding of recommendations | Yes | Two author independently coded the recommendations of each clinical guideline (according to personal correspondence with lead author) |
| Adequate dealing with confounding | No | Compared clinical guidelines of the same topic (mammography screening), but with various publication years. Mammography screening is a controversial topic that evolves over time |
Norris 2013.
| Study characteristics | ||
| Methods | To explore whether financial conflicts interests among authors of clinical guidelines on drugs for glycaemic control in type 2 diabetes are associated with recommendation of specific drugs. Clinical guidelines published between February 2012 and June 2012 | |
| Data | 13 clinical guidelines | |
| Comparisons | Clinical guidelines with financial conflicts of interest (defined as having at least one author with financial interests in companies that manufacture the drugs recommended in the clinical guidelines) and clinical guidelines without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as recommending a drug in the guidance portion of the guideline) | |
| Funding source | The study was funded by the Agency for Healthcare Research and Quality and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors independently assessed clinical guidelines for inclusion (according to personal correspondence with lead author) |
| Adequate coding of conflicts of interest | Yes | Two authors independently coded each clinical guideline. A third author checked the coding of a sample of the included clinical guidelines and looked through any outliers or notable information (according to personal correspondence with lead author) |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each clinical guideline |
| Adequate dealing with confounding | No | Compared clinical guidelines of the same drugs, but used for different populations (adults, children, pregnant women) |
Pham‐Kanter 2014.
| Study characteristics | ||
| Methods | To examine the association between financial conflicts of interest among FDA Center for Drug Evaluation and Research advisory committee members and voting behavior. FDA drug advisory committee reports from February 1997 to December 2011 | |
| Data | 379 advisory committee reports and 15,739 advisory committee members | |
| Comparisons | Adviosory committee members with financial conflicts of interest (defined as financial interests in the sponsoring firm, in a firm competing with the sponsor, or in both the sponsoring firm and any of its competitors) and advisory committee members without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as votes favourable to the sponsoring firm) | |
| Funding source | The study was funded by the Edmond J. Safra Philanthropic Foundation and no additional funding related to any for‐profit organisation was disclosed | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Three research assistants assessed advisory committee reports for inclusion (according to personal correspondence with lead author) |
| Adequate coding of conflicts of interest | Yes | One research assistant extracted data and coded each advisory committee report. One author reviewed and audited all data (according to personal correspondence with lead author) |
| Adequate coding of recommendations | Yes | One research assistant coded the recommendations of each advisory committee report. One author reviewed and audited all data (according to personal correspondence with lead author) |
| Adequate dealing with confounding | No | Compared advisory committee reports of different drugs, used for different diseases, and held within a large time span |
Schott 2013.
| Study characteristics | ||
| Methods | To investigate the association between conflicts of interest among guideline authors and the guidelines' recommendations in two clinical guidelines on treatment of psoriasis by gabapentin versus efalizumab | |
| Data | Two clinical guidelines | |
| Comparisons | One clinical guideline with financial conflicts of interest (defined as at least one author with financial ties to drug companies) and one clinical guideline without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as efalizumab being judged more favourable) | |
| Funding source | Funding source not described | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | No systematic search for guidelines |
| Adequate coding of conflicts of interest | No | Only one author coded each guideline |
| Adequate coding of recommendations | No | No systematic procedure for coding the recommendations of each guideline |
| Adequate dealing with confounding | Yes | Compared two guidelines of the same drug used for the same disease and published in the same year |
Stelfox 1998.
| Study characteristics | ||
| Methods | To investigate the association between authors’ positions on the safety of calcium‐channel antagonists and their financial relationships with the pharmaceutical industry. Reports of original research, reviews, and letters to the editor on calcium‐channel antagonists published between March 1995 and September 1996 | |
| Data | 33 letters, 5 research studies, and 32 review articles | |
| Comparisons | Authors of letters to the editors, original research, and review articles with financial conflicts of interest (defined as authors receiving any the following types of funding in the past five years: support to attend a symposium, honoraria, support to organise an educational program, research support, employment, or consultation) and letters to the editor, original research, and review articles without financial conflicts of interest | |
| Outcomes | Recommendations (classified as critical, neutral, and supportive) | |
| Funding source | The authors disclosed that the study was not funded by the pharmaceutical industry | |
| Declaration of conflicts of interest | HTS (lead author) has attended educational rounds sponsored by pharmaceutical manufacturers GC (second author) has received travel fees from manufacturers of calcium‐channel antagonists and manufacturers of competing products. KO (third author) has attended industry‐sponsored functions, when invited by clinicians. ASD (last author) has received honoraria for speeches, consulting fees from manufacturers of calcium‐channel antagonists and manufacturers of competing products, and has received research grants from Rhone‐Poulenc Rorer Pharmaceuticals, Searle, and SmithKline Beecham Pharmaceuticals. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed articles for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | Yes | Conflicts of interest were assessed using a detailed questionnaire |
| Adequate coding of recommendations | Yes | Two authors independently coded the recommendations of each article (according to personal correspondence with corresponding author) |
| Adequate dealing with confounding | Yes | Compared documents commenting of the same controversy and published in a narrow time span |
Tibau 2015.
| Study characteristics | ||
| Methods | To explore whether financial conflicts of interest were associated with greater probability of endorsement of specific anticancer drugs. Clinical guidelines on anticancer drugs for breast, colorectal, lung, and prostate cancers published between January 2003 and October 2013 | |
| Data | 50 clinical guidelines (91 clinical guidelines included in the study, but not all had data available in a format for inclusion in analysis) | |
| Comparisons | Clinical guidelines with financial conflicts of interest (defined as at least one authors with employment, stock ownership, participation in speakers bureaus, consultancy, honoraria, research funding, and expert testimony) and clinical guidelines without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as endorsement of a drug) | |
| Funding source | Funding source not described | |
| Declaration of conflicts of interest | BS (eight author) has received honoraria from Astellas, Janssen Oncology, Novartis, and Sanofi, and has a consulting or advisory role at Astellas, Sanofi, and Janssen Oncology | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed clinical guidelines for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | No | Only one author coded each clinical guideline |
| Adequate coding of recommendations | No | Only one author coded the recommendations of each clinical guideline (according to personal correspondence with corresponding author) |
| Adequate dealing with confounding | No | Compared clinical guidelines of different cancer drugs used for different types of cancers |
Tibau 2016.
| Study characteristics | ||
| Methods | To explore the influence from Drug Advisory Commitee members’ financial conflicts of interest on the meeting recommendations. FDA Oncologic Drugs Advisory Committee meetings between January 2000 and December 2004 | |
| Data | 79 advisory committee reports (82 advisory committee reports included in the study, but not all had data available in a format for inclusion in analysis) | |
| Comparisons | Advisory committee reports with financial conflicts of interest (defined as at least one committee members with investments, employment, consultancy, advisory capacity, research funding, speakers’ bureau activities, or lectures) and advisory committee reports without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as votes in favour of drug approval) | |
| Funding source | Funding source not described | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit organisation | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | No | Only one author assessed advisory committee reports for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | Yes | One author coded each committee member, and another author verified all data |
| Adequate coding of recommendations | Yes | One author coded the recommendations of each advisory committee report, and another author verified all data |
| Adequate dealing with confounding | No | Compared advisory committee reports of different oncology drugs |
Wang 2010.
| Study characteristics | ||
| Methods | To explore the association between authors’ financial conflicts of interest and their position on the association between rosiglitazone in patients with diabetes and cardiovascular events | |
| Data | 5 clinical guidelines, 91 opinion pieces (letters, editorials, commentaries), and 84 narrative reviews | |
| Comparisons | Documents with financial conflicts of interest (defined as at least one author with funding of the document, employment, consultancy, advisory board membership, speaker or lecture feeds, travel grants, stock ownership or honoraria from pharmaceutical companies) and documents without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as recommending the use of rosiglitazone) | |
| Funding source | No funding was received for the study | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related to any for‐profit company | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors independently assessed documents for inclusion (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | No | Only one author coded each document |
| Adequate coding of recommendations | Yes | Two review authors independently coded the recommendations of each document |
| Adequate dealing with confounding | Yes | Compared documents on the same drug used for the same disease |
Xu 2017.
| Study characteristics | ||
| Methods | To examine the association between conflicts of interest and voting behaviour at the FDA advisory committee reports. Committee meetings on drugs and devices held between 2008 and 2014 | |
| Data | 385 advisory committee reports | |
| Comparisons | Advisory committee members with financial conflicts of interest (defined as financial interests or personal and business relationships) and advisory committee members without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined as votes favourable to the product) | |
| Funding source | Funding source not described | |
| Declaration of conflicts of interest | The authors disclosed no conflicts of interest related any for‐profit company | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Unclear | Not possible to determine |
| Adequate coding of conflicts of interest | Unclear | Not possible to determine |
| Adequate coding of recommendations | Unclear | Not possible to determine |
| Adequate dealing with confounding | No | Compared advisory committee reports of different drugs and devices |
Zhang 2019.
| Study characteristics | ||
| Methods | To understand how the FDA interprets the recommendations of its advisory committees and to explore potential contributing factors to cases in which the FDA as an agency disagreed with its advisory committees’ recommendations. FDA advisory committee meetings held between 2008 and 2015 | |
| Data | 376 advisory committee reports | |
| Comparisons | Advisory committee reports with financial conflicts of interest (defined as at least one committee members with financial ties to the drug manufacturer or competitor) and advisory committee reports without financial conflicts of interest | |
| Outcomes | Recommendations (favourable recommendations defined committee votes in favour of the drug) | |
| Funding source | No funding was received for the study | |
| Declaration of conflicts of interest | JSR (last author) has received support from Johnson and Johnson to develop methods of clinical trial data sharing and from Medtronic to develop methods for postmarket surveillance of medical devices | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Support for judgement |
| Adequate document inclusion process | Yes | Two authors developed inclusion criteria, one author primarily assessed committee reports for inclusion, any uncertainties were discussed between two authors (according to personal correspondence with corresponding author) |
| Adequate coding of conflicts of interest | Yes | One author primarily coded conflicts of interest for each committee report, any uncertainties were discussed between two authors (according to personal correspondence with corresponding author) |
| Adequate coding of recommendations | Yes | One author primarily coded recommendations for each committee report, any uncertainties were discussed between two authors (according to personal correspondence with corresponding author) |
| Adequate dealing with confounding | No | Compared committee meetings of different drugs and devices |
FDA: Food and Drug Administration
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abola 2016 | Not research study |
| Abramson 2005 | Not research study |
| Aidara‐Kane 2018 | Wrong document type (includes studies on food‐producing animals) |
| Akl 2014 | No comparator (investigates prevalence of conflicts of interest only) |
| Alhazzani 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Allan 2015 | Wrong outcomes |
| Allan 2015a | Wrong outcomes |
| American Journal of Hospital Pharmacy 1993 | Wrong document type (includes conflicts of interest policies) |
| American Medical Association 1993 | Wrong document type (includes conflicts of interest policies) |
| Bachmann 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Banks 2005 | Not research study |
| Bariani 2012 | Conference abstract of included study |
| Barriocanal 2013 | Wrong outcomes |
| Bastian 2016 | Not research study |
| Bekelman 2003 | Wrong document type (includes systematic reviews and cross‐sectional surveys) |
| Bellomo 2020 | Wrong document type (includes primary research articles) |
| Bennett 2011 | Wrong outcomes |
| Bennett 2019 | Wrong comparator |
| Bero 2014 | Not research study |
| Bhargava 2007 | No comparator (investigates prevalence of conflicts of interest only) |
| Bindslev 2013 | No comparator (investigates prevalence of conflicts of interest only) |
| Biomedical Ethics Committee 1990 | Not research study |
| Bion 2009 | Wrong document type (includes studies investigating conflicts of interest) |
| Burda 2011 | Conference abstract of included study |
| Burki 2016 | Not research study |
| Burklow 1998 | Not research study |
| Campsall 2016 | Wrong outcomes |
| Carlisle 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Checketts 2017 | Wrong outcomes |
| Choudhry 2002 | No comparator (investigates prevalence of conflicts of interest only) |
| Chren 1994 | Not research study |
| Combs 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Combs 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Cosgrove 2006 | Wrong outcomes |
| Cosgrove 2009 | No comparator (investigates prevalence of conflicts of interest only) |
| Cosgrove 2013 | Not research study |
| Cosgrove 2013a | Wrong outcomes |
| Cosgrove 2014 | Wrong document type (includes randomised trials) |
| Cosgrove 2017 | Wrong outcomes |
| Council on Ethical and Judicial Affairs 1991 | Not research study |
| Council on Ethical and Judicial Affairs 1992 | Wrong document type (includes conflicts of interest policies) |
| Coyne 2007 | Not research study |
| DeJong 2018 | Not research study |
| Desai 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Dillon 2016 | Wrong outcomes |
| DuBroff 2018 | Not research study |
| Editors of Annals of Internal Medicine 2004 | Not research study |
| Editors of Canadian Medical Association Journal | Not research study |
| Ferket 2011 | Wrong comparator |
| Finucane 2004 | Wrong document type (includes abstracts, posters, and presentations from a medical conference) |
| Friesen 2019 | Not research study |
| Gasparyan 2013 | Not research study |
| Glazer 2018 | Not research study |
| Graham 2001 | Wrong comparator |
| Greenberg 2012 | Wrong document type (includes conflicts of interest policies) |
| Grindal 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Hart 2019 | Not research study |
| Hayes 2018 | Not research study |
| Holloway 2008 | Wrong outcomes |
| Horn 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Hu 2013 | Wrong comparator |
| Irwig 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Janssen 2015 | Wrong document type (includes medical journal editorial boards) |
| Ji 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Johnson 2020 | Wrong document type (includes public speakers) |
| Jones 2011 | Wrong outcomes |
| Khalil 2012 | Wrong outcomes |
| Khan 2018 | Not research study |
| Klikova 2013 | No comparator (investigates prevalence of conflicts of interest only) |
| Langer 2012 | No comparator (investigates prevalence of conflicts of interest only) |
| Lexchin 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Lexchin 2019a | Wrong document type (includes clinicians making submissions to the pan‐Canadian Oncology Drug Review) |
| Liu 2019 | Wrong outcomes |
| Lopez‐Olivo 2017 | Wrong outcomes |
| Lu 2017 | Wrong outcomes |
| Lurie 2006a | Not research study |
| Lurie 2015 | Wrong comparator |
| MacKenzie 2015 | Wrong comparator |
| Madadi 2012 | No comparator (investigates prevalence of conflicts of interest only) |
| McCoy 2018 | Wrong document type (includes public speakers) |
| Mehlman 2017 | Wrong document type (includes physician editors) |
| Miranda 2011 | Conference abstract of included study |
| Mitchell 2016 | No comparator (investigates prevalence of conflicts of interest only) |
| Mitchell 2019 | Not research study |
| Moynihan 2013 | Wrong outcomes |
| Napierala 2018 | No comparator (investigates prevalence of conflicts of interest only) |
| Neuman 2011 | Wrong outcomes |
| Neuman 2011a | Wrong outcomes |
| Newton 2016 | No comparator (investigates prevalence of conflicts of interest only) |
| Niforatos 2019 | Wrong outcomes |
| Norris 2011 | No comparator (investigates prevalence of conflicts of interest only) |
| Papanikolaou 2001 | Wrong outcomes |
| Pharmaceutical Journal 2005 | Not research study |
| Riechelmann 2007 | Wrong outcomes |
| Roberts 2020 | Wrong document type (includes public speakers) |
| Roland 2009 | Wrong comparator |
| Roundtree 2009 | No comparator (investigates prevalence of conflicts of interest only) |
| Saito 2019 | Not research study |
| Saito 2019a | No comparator (investigates prevalence of conflicts of interest only) |
| Saleh 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Shapiro 2003 | Wrong comparator |
| Shimada 2019 | No comparator (investigates prevalence of conflicts of interest only) |
| Shnier 2016 | No comparator (investigates prevalence of conflicts of interest only) |
| Spithoff 2020 | No comparator (investigates prevalence of conflicts of interest only) |
| Steinbrook 2005 | Not research study |
| Traynor 2002 | Not research study |
| Verma 2017 | No comparator (investigates prevalence of conflicts of interest only) |
| Wang 2010a | Conference abstract of included study |
| Wayant 2019 | No comparator (investigates prevalence of conflicts of interest only) |
Differences between protocol and review
We decided to analyse clinical guidelines and advisory committee reports separately, partly because we had more data than we anticipated for these specific categories, and partly because we wanted to minimise heterogeneity. This decision was taken prior to data analysis.
We included one post hoc secondary analysis analysing advisory committee reports on individual level.
We included one post hoc secondary analysis combining all document types in one analysis.
We decided only to calculate prediction intervals for pooled analyses that included at least five studies, as prediction intervals based on limited data are highly uncertain.
We estimated Number Needed to Read for each document type and the combined analysis of all document types.
We included one new subgroup analysis (referred to as post hoc subgroup analysis). This compared documents by authors with major financial conflicts of interest (defined as at least half of the authors/committee members having financial conflicts of interest) with documents by authors with minor financial conflicts of interest (defined as less than half of the authors/committee members with financial conflicts of interest).
We included one new sensitivity analysis (referred to as post hoc sensitivity analysis). We differentiated between financial conflicts of interest related to the manufacturer and to any for‐profit company in two separate analyses.
We decided only to conduct subgroup and sensitivity analyses when we had sufficient data (i.e. at least five documents in each group).
Contributions of authors
AL conceived the idea for the study. The protocol was developed primarily by CHN, AH, and AL with contribution from LB, KJJ, and AWJ. The protocol was based on a previous protocol developed by AL, AWJ, and LB (Lundh 2013). CHN and either AWJ or AL assessed studies for inclusion; CHN and either ML, AWJ, or AL extracted data and assessed risk of bias. CHN performed the data analysis, and all authors participated in data interpretation. CHN wrote the draft review and all authors contributed in revising the review. CHN is guarantor of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Sources of support
Internal sources
-
Centre for Evidence‐Based Medicine Odense (CEBMO), Odense University Hospital and University of Southern Denmark, Denmark
CHN, AH, and AL were personally salaried by this institution during the period of the review
-
The Nordic Cochrane Centre, Rigshospitalet, Copenhagen, Denmark
CHN and KJJ were personally salaried by this institution during the period of the review
-
Charles Perkins Centre and Faculty of Medicine and Health, The University of Sydney, Sydney, Australia
LB was personally salaried by this institution during periods of the review
-
Center for Bioethics and Humanities, University of Colorado, USA
LB was personally salaried by this institution during periods of the review
-
Otorhinolaryngology and Head & Neck Surgery, Aarhus, Denmark
AWJ was personally salaried by this institution during periods of the review
-
ENT Clinic Hobro, Denmark
AWJ was personally salaried by this institution during periods of the review
-
Department of Infectious Diseases, Hvidovre Hospital, Copenhagen, Denmark
AL was personally salaried by this institution during periods of the review
External sources
No sources of support supplied
Declarations of interest
We declare that we have no conflicts of interest. LB is co‐author of one of the included studies. LB was not involved in the study inclusion, data extraction, and 'Risk of bias' assessment of any studies.
New
References
References to studies included in this review
Aakre 2012 {published data only}
- Aakre KM, Watine J, Bunting PS, Sandberg S, Oosterhuis WP. Self-monitoring of blood glucose in patients with diabetes who do not use insulin - are guidelines evidence-based? Diabetic Medicine 2012;29(10):1226-36. [DOI] [PubMed] [Google Scholar]
Ackerley 2009 {published and unpublished data}
- Ackerley N, Eyraud J, Franz C, Kissel B, Metivier D. Financial conflict-of-interest disclosure and voting patterns at FDA advisory committee meetings. Washington DC: Eastern Research Group (FDA Contract No. HHSF223200810071I), 2009. [Google Scholar]
Bariani 2013 {published and unpublished data}
- Bariani GM, Ferrari AC, Hoff PM, Krzyzanowska MK, Riechelmann RP. Self-reported conflicts of interest of authors, trial sponsorship, and the interpretation of editorials and related phase III trials in oncology. Journal of Clinical Oncology 2013;31(18):2289-95. [DOI] [PubMed] [Google Scholar]
Cooper 2019 {published data only}
- Cooper JC, Golec J. Conflicts of interest on committees of experts: the case of Food and Drug Administration drug advisory committees. Journal of Law & Economics 2019;62(2):321-46. [Google Scholar]
Downing 2014 {published data only}
- Downing NS, Cheng T, Krumholz HM, Shah ND, Ross JS. Descriptions and interpretations of the ACCORD-Lipid trial in the news and biomedical literature: a cross-sectional analysis. JAMA Internal Medicine 2014;174(7):1176-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2016 {published and unpublished data}
- Dunn AG, Zhou X, Hudgins J, Arachi D, Mandl KD, Coiera E, et al. Financial competing interests were associated with favorable conclusions and greater author productivity in nonsystematic reviews of neuraminidase inhibitors. Journal of Clinical Epidemiology 2016;80:43-9. [DOI] [PubMed] [Google Scholar]
George 2014 {published data only}
- George JN, Vesely SK, Woolf SH. Conflicts of interest and clinical recommendations: comparison of two concurrent clinical practice guidelines for primary immune thrombocytopenia developed by different methods. American Journal of Medical Quality 2014;29(1):53-60. [DOI] [PubMed] [Google Scholar]
Hartog 2012 {published and unpublished data}
- Hartog CS, Skupin H, Natanson C, Sun J, Reinhart K. Systematic analysis of hydroxyethyl starch (HES) reviews: proliferation of low-quality reviews overwhelms the results of well-performed meta-analyses. Intensive Care Medicine 2012;38(8):1258-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2019 {published data only}
- Hayes MJ, Prasad V. Association between conflict of interest and published position on tumor-treating fields for the treatment of glioblastoma. Journal of Cancer Policy 2019;21:100189. [Google Scholar]
Lerner 2012 {published and unpublished data}
- Lerner TG, Miranda MC, Lera AT, Ueda A, Briones B, Del Giglio A, et al. The prevalence and influence of self-reported conflicts of interest by editorial authors of phase III cancer trials. Contemporary Clinical Trials 2012;33(5):1019-22. [DOI] [PubMed] [Google Scholar]
Lurie 2006 {published and unpublished data}
- Lurie P, Almeida CM, Stine N, Stine AR, Wolfe SM. Financial conflict of interest disclosure and voting patterns at Food and Drug Administration Drug Advisory Committee meetings. JAMA 2006;295(16):1921-8. [DOI] [PubMed] [Google Scholar]
Norris 2012 {published data only}
- Norris SL, Burda BU, Holmer HK, Ogden LA, Fu R, Bero L, et al. Author's specialty and conflicts of interest contribute to conflicting guidelines for screening mammography. Journal of Clinical Epidemiology 2012;65(7):725-33. [DOI] [PubMed] [Google Scholar]
Norris 2013 {published data only}
- Norris SL, Holmer HK, Ogden LA, Burda BU, Fu R. Conflicts of interest among authors of clinical practice guidelines for glycemic control in type 2 diabetes mellitus. PLOS One 2013;8(10):e75284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pham‐Kanter 2014 {published and unpublished data}
- Pham-Kanter G. Revisiting financial conflicts of interest in FDA advisory committees. Milbank Quarterly 2014;92(3):446-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schott 2013 {published data only}
- Schott G, Dunnweber C, Muhlbauer B, Niebling W, Pachl H, Ludwig WD. Does the pharmaceutical industry influence guidelines?: two examples from Germany. Deutsches Ärzteblatt International 2013;110(35-36):575-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stelfox 1998 {published data only}
- Stelfox HT, Chua G, O'Rourke K, Detsky AS. Conflict of interest in the debate over calcium-channel antagonists. New England Journal of Medicine 1998;338(2):101-6. [DOI] [PubMed] [Google Scholar]
Tibau 2015 {published and unpublished data}
- Tibau A, Bedard PL, Srikanthan A, Ethier JL, Vera-Badillo FE, Templeton AJ, et al. Author financial conflicts of interest, industry funding, and clinical practice guidelines for anticancer drugs. Journal of Clinical Oncology 2015;33(1):100-6. [DOI] [PubMed] [Google Scholar]
Tibau 2016 {published and unpublished data}
- Tibau A, Ocana A, Anguera G, Seruga B, Templeton AJ, Barnadas A, et al. Oncologic Drugs Advisory Committee Recommendations and Approval of Cancer Drugs by the US Food and Drug Administration. JAMA Oncology 2016;2(6):744-50. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published and unpublished data}
- Wang AT, McCoy CP, Murad MH, Montori VM. Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. BMJ 2010;340:c1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu J, Emenanjo O, Ortwerth M, Lurie P. Association of appearance of conflicts of interest with voting behavior at FDA advisory committee meetings-a cross-sectional study. JAMA Internal Medicine 2017;177(7):1038-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019 {published and unpublished data}
- Zhang AD, Schwartz JL, Ross JS. Association between Food and Drug Administration advisory committee recommendations and agency actions, 2008-2015. Milbank Quarterly 2019;97(3):796-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abola 2016 {published data only}
- Abola MV, Prasad V. Characteristics and conflicts of public speakers at meetings of the Oncologic Drugs Advisory Committee to the US Food and Drug Administration. JAMA Internal Medicine 2016;176(3):389-91. [DOI] [PubMed] [Google Scholar]
Abramson 2005 {published data only}
- Abramson J, Starfield B. The effect of conflict of interest on biomedical research and clinical practice guidelines: can we trust the evidence in evidence-based medicine? Journal of the American Board of Family Practice 2005;18(5):414-8. [DOI] [PubMed] [Google Scholar]
Aidara‐Kane 2018 {published data only}
- Aidara-Kane A, Angulo FJ, Conly JM, Minato Y, Silbergeld EK, McEwen SA, et al. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrobial Resistance and Infection Control 2018;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akl 2014 {published data only}
- Akl EA, El-Hachem P, Abou-Haidar H, Neumann I, Schünemann HJ, Guyatt GH. Considering intellectual, in addition to financial, conflicts of interest proved important in a clinical practice guideline: a descriptive study. Journal of Clinical Epidemiology 2014;67(11):1222-8. [DOI] [PubMed] [Google Scholar]
Alhazzani 2018 {published data only}
- Alhazzani W, Lewis K, Jaeschke R, Rochwerg B, Moller MH, Evans L, et al. Conflicts of interest disclosure forms and management in critical care clinical practice guidelines. Intensive Care Medicine 2018;44(10):1691-8. [DOI] [PubMed] [Google Scholar]
Allan 2015 {published data only}
- Allan GM, Kraut R, Crawshay A, Korownyk C, Vandermeer B, Kolber MR. Contributors to primary care guidelines: What are their professions and how many of them have conflicts of interest? Canadian Family Physician Medecin de Famille Canadien 2015;61(1):52-8. [PMC free article] [PubMed] [Google Scholar]
Allan 2015a {published data only}
- Allan GM, Kolber MR, Korownyk C, Kraut R, Crawshay A, Vandermeer B. Contributors to Canadian primary care guidelines profession and conflict of interest. Canadian Family Physician 2015;61 (2 Supplement 1):S28. [PMC free article] [PubMed] [Google Scholar]
American Journal of Hospital Pharmacy 1993 {published data only}
- American Journal of Hospital Pharmacy. FDA finalizing policy statement on industry-supported activities. American Journal of Hospital Pharmacy 1993;50(3):391. [PubMed] [Google Scholar]
American Medical Association 1993 {published data only}
- American Medical Association. Guidelines for interactions with pharmaceutical companies. JAMA 1993;270(10):1250. [PubMed] [Google Scholar]
Bachmann 2019 {published data only}
- Bachmann L, Ulyte A, Dressel H. Clinical practice guidelines of medical societies in Switzerland: analysis of the current state. Swiss Medical Weekly 2019;149:w20134. [DOI] [PubMed] [Google Scholar]
Banks 2005 {published data only}
- Banks D. Pharmacists, pharmaceutical manufacturers, and conflicts of interest. American Journal of Health-System Pharmacy 2005;62(17):1827-32. [DOI] [PubMed] [Google Scholar]
Bariani 2012 {published data only}
- Bariani GM, De Celis Ferrari AC, Hoff PM, Riechelmann R. Self-reported conflicts of interest (sfCOI) of authors and the interpretation of randomized phase III trials (RCT) and related editorials (REd) in cancer research. Journal of Clinical Oncology 2012;30(15):Suppl. 1. [DOI] [PubMed] [Google Scholar]
Barriocanal 2013 {published data only}
- Barriocanal A, Lopez A, Costa J, Montane E. Quality of clinical practice guidelines for peripheral arterial disease:a systematic review. Basic and Clinical Pharmacology and Toxicology 2013;113:11. [Google Scholar]
Bastian 2016 {published data only}
- Bastian H. Nondisclosure of financial interest in clinical practice guideline development: an intractable problem? PLOS Medicine 2016;13(5):e1002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bekelman 2003 {published data only}
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289(4):454-65. [DOI] [PubMed] [Google Scholar]
Bellomo 2020 {published data only}
- Bellomo TR, Hwang C, Spector-Bagdady K, Stanley JC, Corriere MA. Industry compensation and self-reported financial conflicts of interest among authors of highly cited peripheral artery disease studies. Journal of Vascular Surgery 2020;72(2):673-84. [DOI] [PubMed] [Google Scholar]
Bennett 2011 {published data only}
- Bennett WL, Odelola O, Wilson L, Bolen S, Bass EB, Dalal D, et al. Systematic review of clinical practice guidelines on the pharmacologic treatment of type 2 diabetes mellitus: Are guidelines evidence-based? Journal of General Internal Medicine 2011;26:70-1. [Google Scholar]
Bennett 2019 {published data only}
- Bennett AC, Bennett CL, Witherspoon BJ, Knopf KB. An evaluation of reports of ciprofloxacin, levofloxacin, and moxifloxacin-association neuropsychiatric toxicities, long-term disability, and aortic aneurysms/dissections disseminated by the Food and Drug Administration and the European Medicines Agency. Expert Opinion on Drug Safety 2019;18(11):1055-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bero 2014 {published data only}
- Bero L. What is in a name? Nonfinancial influences on the outcomes of systematic reviews and guidelines. Journal of Clinical Epidemiology 2014;67(11):1239-41. [DOI] [PubMed] [Google Scholar]
Bhargava 2007 {published data only}
- Bhargava N, Qureshi J, Vakil N. Funding source and conflict of interest disclosures by authors and editors in gastroenterology specialty journals. American Journal of Gastroenterology 2007;102(6):1146-50. [DOI] [PubMed] [Google Scholar]
Bindslev 2013 {published data only}
- Bindslev JB, Schroll J, Gøtzsche PC, Lundh A. Underreporting of conflicts of interest in clinical practice guidelines: cross sectional study. BMC Medical Ethics 2013;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biomedical Ethics Committee 1990 {published data only}
- Biomedical Ethics Committee. Ethical responsibilities of physicians in their dealings with pharmaceutical companies. Annals (Royal College of Physicians and Surgeons of Canda) 1990;23(1):45-8. [PubMed] [Google Scholar]
Bion 2009 {published data only}
- Bion J. Financial and intellectual conflicts of interest: confusion and clarity. Current Opinion in Critical Care 2009;15(6):583-90. [DOI] [PubMed] [Google Scholar]
Burda 2011 {published data only}
- Burda B, Holmer H, Ogden L, Fu R, Norris S. Conflicting guidelines for screening mammography: influence of author's specialty and conflicts of interest. Poster presentation at the 19th Cochrane Colloquium; 2011 Oct 19-22; Madrid, Spain [abstract]. Cochrane Database of Systematic Reviews 2011;Suppl(Cd000003):136.
Burki 2016 {published data only}
- Burki TK. Oncologic Drugs Advisory Committee and conflicts of interest. Lancet 2016;17(3):e95. [DOI] [PubMed] [Google Scholar]
Burklow 1998 {published data only}
- Burklow J. Journal letters financed by tobacco industry. Journal of the National Cancer Institute 1998;90(17):1259. [DOI] [PubMed] [Google Scholar]
Campsall 2016 {published data only}
- Campsall P, Colizza K, Straus S, Stelfox HT. Financial relationships between organizations that produce clinical practice guidelines and the biomedical industry: a cross-sectional study. PLOS Medicine 2016;13(5):e1002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlisle 2018 {published data only}
- Carlisle A, Bowers A, Wayant C, Meyer C, Vassar M. Financial conflicts of interest among authors of urology clinical practice guidelines. European Urology 2018;74(3):348-54. [DOI] [PubMed] [Google Scholar]
Checketts 2017 {published data only}
- Checketts JX, Sims MT, Vassar M. Evaluating industry payments among dermatology clinical practice guidelines authors. JAMA Dermatology 2017;153(12):1229-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choudhry 2002 {published data only}
- Choudhry NK, Stelfox HT, Detsky AS. Relationships between authors of clinical practice guidelines and the pharmaceutical industry. JAMA 2002;287(5):612-7. [DOI] [PubMed] [Google Scholar]
Chren 1994 {published data only}
- Chren MM. Independent investigators and for-profit companies. Guidelines for biomedical scientists considering funding by industry. Archives of Dermatology 1994;130(4):432-7. [PubMed] [Google Scholar]
Combs 2018 {published data only}
- Combs TR, Scott J, Jorski A, Heavener T, Vassar M. Evaluation of industry relationships among authors of clinical practice guidelines in gastroenterology. JAMA Internal Medicine 2018;178(12):1711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Combs 2019 {published data only}
- Combs T, Tritz D, Ivy H, Borstel D, Horn J, Vassar M. Financial conflicts of interest among authors of clinical practice guidelines for routine screening mammography. Journal of the American College of Radiology 2019;16(11):1598-603. [DOI] [PubMed] [Google Scholar]
Cosgrove 2006 {published data only}
- Cosgrove L, Krimsky S, Vijayaraghavan M, Schneider L. Financial ties between DSM-IV panel members and the pharmaceutical industry. Psychotherapy and Psychosomatics 2006;75(3):154-60. [DOI] [PubMed] [Google Scholar]
Cosgrove 2009 {published data only}
- Cosgrove L, Bursztajn HJ, Krimsky S, Anaya M, Walker J. Conflicts of interest and disclosure in the American Psychiatric Association's Clinical Practice Guidelines. Psychotherapy and Psychosomatics 2009;78(4):228-32. [DOI] [PubMed] [Google Scholar]
Cosgrove 2013 {published data only}
- Cosgrove L, Wheeler EE. Drug firms, the codification of diagnostic categories, and bias in clinical guidelines. Journal of Law, Medicine & Ethics 2013;41(3):644-53. [DOI] [PubMed] [Google Scholar]
Cosgrove 2013a {published data only}
- Cosgrove L, Bursztajn HJ, Erlich DR, Wheeler EE, Shaughnessy AF. Conflicts of interest and the quality of recommendations in clinical guidelines. Journal of Evaluation in Clinical Practice 2013;19(4):674-81. [DOI] [PubMed] [Google Scholar]
Cosgrove 2014 {published data only}
- Cosgrove L, Krimsky S, Wheeler EE, Kaitz J, Greenspan SB, DiPentima NL. Tripartite conflicts of interest and high stakes patent extensions in the DSM-5. Psychotherapy and Psychosomatics 2014;83(2):106-13. [DOI] [PubMed] [Google Scholar]
Cosgrove 2017 {published data only}
- Cosgrove L, Krimsky S, Wheeler EE, Peters SM, Brodt M, Shaughnessy AF. Conflict of Interest Policies and Industry Relationships of Guideline Development Group Members: A Cross-Sectional Study of Clinical Practice Guidelines for Depression. Accountability in research 2017;24(2):99-115. [DOI] [PubMed] [Google Scholar]
Council on Ethical and Judicial Affairs 1991 {published data only}
- Council on Ethical and Judicial Affairs of the American Medical Association. Gifts to physicians from industry. JAMA 1991;265(4):501. [PubMed] [Google Scholar]
Council on Ethical and Judicial Affairs 1992 {published data only}
- Council on Ethical and Judicial Affairs of the American Medical Association. Guidelines on gifts to physicians from industry: an update. Food and Drug Law Journal 1992;47(4):445-58. [PubMed] [Google Scholar]
Coyne 2007 {published data only}
- Coyne DW. Influence of industry on renal guideline development. Clinical journal of the American Society of Nephrology 2007;2(1):3-7. [DOI] [PubMed] [Google Scholar]
DeJong 2018 {published data only}
- DeJong C, Steinbrook R. Continuing problems with financial conflicts of interest and clinical practice guidelines. JAMA Internal Medicine 2018;178(12):1715. [DOI] [PubMed] [Google Scholar]
Desai 2019 {published data only}
- Desai A, Chengappa M, Go RS, Poonacha T. Financial conflicts of interest among the National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline (CPG) Panelists in 2019. Journal of Clinical Oncology 2019;37(Supplement 27):15. [DOI] [PubMed] [Google Scholar]
Dillon 2016 {published data only}
- Dillon A, Rowe IA. Potential conflicts of interest for authors of recent AASLD clinical practice guidelines. Hepatology 2016;64(Supplement 1):857A. [Google Scholar]
DuBroff 2018 {published data only}
- DuBroff R. Confirmation bias, conflicts of interest and cholesterol guidance: can we trust expert opinions? QJM : Monthly Journal of the Association of Physicians 2018;111(10):687-9. [DOI] [PubMed] [Google Scholar]
Editors of Annals of Internal Medicine 2004 {published data only}
- Editors of Annals of Internal Medicine. Publishing commentary by authors with potential conflicts of interest: when, why, and how. Annals of Internal Medicine 2004;141(1):73-4. [DOI] [PubMed] [Google Scholar]
Editors of Canadian Medical Association Journal {published data only}
- Editors of Canadian Medical Association Journal. Clinical practice guidelines and conflict of interest. Canadian Medical Association Journal 2005;173(11):1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferket 2011 {published data only}
- Ferket BS, Genders TS, Colkesen EB, Visser JJ, Spronk S, Steyerberg EW, et al. Systematic review of guidelines on imaging of asymptomatic coronary artery disease. Journal of the American College of Cardiology 2011;57(15):1591-600. [DOI] [PubMed] [Google Scholar]
Finucane 2004 {published data only}
- Finucane TE, Boult CE. Association of funding and findings of pharmaceutical research at a meeting of a medical professional society. American Journal of Medicine 2004;117(11):842-5. [DOI] [PubMed] [Google Scholar]
Friesen 2019 {published data only}
- Friesen P, Caplan AL, Miller JE. Managing conflicts of interest in pharmacy and therapeutics committees: a proposal for multicentre formulary development. Journal of Clinical Pharmacy and Therapeutics 2020;45(2):249-55. [DOI] [PubMed] [Google Scholar]
Gasparyan 2013 {published data only}
- Gasparyan AY, Ayvazyan L, Akazhanov NA, Kitas GD. Conflicts of interest in biomedical publications: considerations for authors, peer reviewers, and editors. Croatian Medical Journal 2013;54(6):600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glazer 2018 {published data only}
- Glazer AM, Siegel DM, Rigel DS. Evaluating industry payments among dermatology clinical practice guideline authors. JAMA Dermatology 2018;154(3):373-4. [DOI] [PubMed] [Google Scholar]
Graham 2001 {published data only}
- Graham ID, Beardall S, Carter AO, Glennie J, Hebert PC, Tetroe JM, et al. What is the quality of drug therapy clinical practice guidelines in Canada? Canadian Medical Association Journal 2001;165(2):157-63. [PMC free article] [PubMed] [Google Scholar]
Greenberg 2012 {published data only}
- Greenberg RD. Conflicts of Interest: can a physician serve two masters? Clinics in Dermatology 2012;30(2):160-73. [DOI] [PubMed] [Google Scholar]
Grindal 2019 {published data only}
- Grindal AW, Khan R, Scaffidi MA, Rumman A, Grover SC. Financial conflicts of interest in inflammatory bowel disease guidelines. Inflammatory Bowel Diseases 2019;25(4):642-5. [DOI] [PubMed] [Google Scholar]
Hart 2019 {published data only}
- Hart KL, Perlis RH, Perlis CS. Conflict of interest and citation impact among dermatology guideline authors. Journal of the American Academy of Dermatology 2019;80(3):813-5. [DOI] [PubMed] [Google Scholar]
Hayes 2018 {published data only}
- Hayes MJ, Prasad V. Financial conflicts of Interest at FDA Drug Advisory Committee Meetings. Hastings Center Report 2018;48(2):10-3. [DOI] [PubMed] [Google Scholar]
Holloway 2008 {published data only}
- Holloway RG, Mooney CJ, Getchius TS, Edlund WS, Miyasaki JO. Invited Article: Conflicts of interest for authors of American Academy of Neurology clinical practice guidelines. Neurology 2008;71(1):57-63. [DOI] [PubMed] [Google Scholar]
Horn 2018 {published data only}
- Horn J, Checketts JX, Jawhar O, Vassar M. Evaluation of industry relationships among authors of otolaryngology clinical practice guidelines. JAMA Otolaryngology-- Head & Neck Surgery 2018;144(3):194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2013 {published data only}
- Hu J, Chen R, Wu S, Tang J, Leng G, Kunnamo I, et al. The quality of clinical practice guidelines in China: a systematic assessment. Journal of Evaluation in Clinical Practice 2013;19(5):961-7. [DOI] [PubMed] [Google Scholar]
Irwig 2018 {published data only}
- Irwig MS, Kyinn M, Shefa MC. Financial conflicts of interest among authors of Endocrine Society Clinical Practice Guidelines. Journal of Clinical Endocrinology and Metabolism 2018;103(12):4333-8. [DOI] [PubMed] [Google Scholar]
Janssen 2015 {published data only}
- Janssen SJ, Bredenoord AL, Dhert W, Kleuver M, Oner FC, Verlaan JJ. Potential conflicts of interest of editorial board members from five leading spine journals. PLOS One 2015;10(6):e0127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2018 {published data only}
- Ji YD, Lahey ET. Conflicts of interest in clinical guidelines: lack of authors and disclosures in the AAOMS White Papers. Journal of Oral and Maxillofacial Surgery 2018;76(9):1946-9. [DOI] [PubMed] [Google Scholar]
Johnson 2020 {published data only}
- Johnson BS, Roberts W, Riddle J, Wayant C, Scott J, Vassar M. Potential financial bias from speakers at US Food and Drug Administration's Bone, Reproductive, and Urologic Drugs Advisory Committee Meetings. Urology 2020;137:1-6. [DOI] [PubMed] [Google Scholar]
Jones 2011 {published data only}
- Jones D, Barkun AN, Lu Y, Enns RA, Sinclair P, Martel M, et al. Is it ethical to silence expertise in the development of clinical practice guidelines? Report & analysis from an international consensus conference. Gastroenterology 2011;140(5 (suppl 1)):S575. [Google Scholar]
Khalil 2012 {published data only}
- Khalil B, Aung K, Mansi IA. Reporting potential conflicts of interest among authors of professional medical societies' guidelines. Southern Medical Journal 2012;105(8):411-5. [DOI] [PubMed] [Google Scholar]
Khan 2018 {published data only}
- Khan R, Scaffidi MA, Rumman A, Grindal AW, Plener IS, Grover SC. Prevalence of financial conflicts of interest among authors of clinical guidelines related to high-revenue medications. JAMA Internal Medicine 2018;178(12):1712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klikova 2013 {published data only}
- Klikova K, Licenik R. Clinical practice guidelines in the Czech and Slovak Republic. BMJ Quality and Safety 2013;22:A62. [Google Scholar]
Langer 2012 {published data only}
- Langer T, Conrad S, Fishman L, Gerken M, Schwarz S, Weikert B, et al. Conflicts of interest among authors of medical guidelines: an analysis of guidelines produced by German specialist societies. Deutsches Arzteblatt International 2012;109(48):836-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lexchin 2019 {published data only}
- Lexchin J. Declarations of interest by members of Health Canada's special advisory committees and panels: a descriptive study. CMAJ Open 2019;7(2):334-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lexchin 2019a {published data only}
- Lexchin J. Financial conflicts of interest of clinicians making submissions to the pan-Canadian Oncology Drug Review: a descriptive study. BMJ Open 2019;9(7):e030750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019 {published data only}
- Liu X, Tang LL, Mao YP, Liu Q, Sun Y, Chen L, et al. Evidence underlying recommendations and payments from industry to authors of the National Comprehensive Cancer Network Guidelines. Oncologist 2019;24(4):498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐Olivo 2017 {published data only}
- Lopez-Olivo MA, Colmegna I, Matusevich A, Qi SR, Zamora N, Sharma R, et al. Recommendations on the management of rheumatoid arthritis in patients with cancer: a systematic review of clinical practice guidelines and consensus statements. Arthritis and Rheumatology 2017;69(Supplement 10):2395. [Google Scholar]
Lu 2017 {published data only}
- Lu Y, Jones DJ, Sharara N, Kaltenbach T, Laine L, McQuaid K, et al. Transparency ethics in practice: revisiting financial conflicts of interest disclosure forms in clinical practice guidelines. PLOS One 2017;12(8):e0182856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lurie 2006a {published data only}
- Lurie P. Financial conflicts of interest are related to voting patterns at FDA Advisory Committee meetings. Medscape General Medicine 2006;8(4):22. [PMC free article] [PubMed] [Google Scholar]
Lurie 2015 {published data only}
- Lurie P, Chahal HS, Sigelman DW, Stacy S, Sclar J, Ddamulira B. Comparison of content of FDA letters not approving applications for new drugs and associated public announcements from sponsors: cross sectional study. BMJ (Clinical Research) 2015;350:h2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 2015 {published data only}
- MacKenzie R, Rogers W. Potential conflict of interest and bias in the RACGP's Smoking Cessation Guidelines: are GPs provided with the best advice on smoking cessation for their patients? Public Health Ethics 2015;8(3):319-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madadi 2012 {published data only}
- Madadi AR, Go RS. Nature and extent of financial conflicts of interest among National Comprehensive Cancer Network (NCCN) clinical practice guidelines panel members. Journal of Clinical Oncology 2012;30(15 Suppl 1):e16555. [Google Scholar]
McCoy 2018 {published data only}
- McCoy MS, Pagan O, Donohoe G, Kanter GP, Litman RS. Conflicts of interest of public speakers at meetings of the Anesthetic and Analgesic Drug Products Advisory Committee. JAMA Internal Medicine 2018;178(7):996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehlman 2017 {published data only}
- Mehlman CT, Okike K, Bhandari M, Kocher MS. Potential financial conflict of interest among Physician Editorial Board Members of Orthopaedic Surgery Journals. Journal of Bone and Joint Surgery 2017;99(5):e19. [DOI] [PubMed] [Google Scholar]
Miranda 2011 {published data only}
- Miranda MC, Lera AT, Ueda A, Briones B, Lerner T, Del Giglio A, et al. The influence of self-reported conflicts of interest on the conclusions of editorial authors of phase III cancer trials. Journal of Clinical Oncology 2011;29(15 Suppl 1):6039. [DOI] [PubMed] [Google Scholar]
Mitchell 2016 {published data only}
- Mitchell AP, Basch EM, Dusetzina SB. Financial relationships with industry among national comprehensive Cancer Network Guideline Authors. JAMA Oncology 2016;2(12):1628-31. [DOI] [PubMed] [Google Scholar]
Mitchell 2019 {published data only}
- Mitchell PB. Financial conflicts of interest andaAuthorship of Clinical Practice Guidelines-trust Is fragile. JAMA Network Open 2019;2(4):e192840. [DOI] [PubMed] [Google Scholar]
Moynihan 2013 {published data only}
- Moynihan RN, Cooke GP, Doust JA, Bero L, Hill S, Glasziou PP. Expanding disease definitions in guidelines and expert panel ties to industry: a cross-sectional study of common conditions in the United States. PLOS Medicine 2013;10(8):e1001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Napierala 2018 {published data only}
- Napierala H, Schafer L, Schott G, Schurig N, Lempert T. Management of financial conflicts of interests in clinical practice guidelines in Germany: results from the public database GuidelineWatch. BMC Medical Ethics 2018;19(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuman 2011 {published data only}
- Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ (Clinical Research) 2011;343:d5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuman 2011a {published data only}
- Neuman J, Korenstein D, Ross JS, Keyhani S. The prevalence of conflicts of interest among guideline panel members. Journal of General Internal Medicine 2011;26:S123. [Google Scholar]
Newton 2016 {published data only}
- Newton A, Lloyd-Williams F, Bromley H, Capewell S. Food for thought? Potential conflicts of interest in academic experts advising government and charities on dietary policies. BMC Public Health 2016;16:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Niforatos 2019 {published data only}
- Niforatos JD, Pescatore RM. Financial relationships with industry among guideline authors for the management of acute ischemic stroke. American Journal of Emergency Medicine 2019;37(5):921-3. [DOI] [PubMed] [Google Scholar]
Norris 2011 {published data only}
- Norris SL, Holmer HK, Ogden LA, Burda BU. Conflict of interest in clinical practice guideline development: a systematic review. PLOS One 2011;6(10):e25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papanikolaou 2001 {published data only}
- Papanikolaou GN, Baltogianni MS, Contopoulos-Ioannidis DG, Haidich AB, Giannakakis IA, Ioannidis JP. Reporting of conflicts of interest in guidelines of preventive and therapeutic interventions. BMC Medical Research Methodology 2001;1(1):3. [DOI] [PMC free article] [PubMed]
Pharmaceutical Journal 2005 {published data only}
- Pharmaceutical Journal. Most US guideline panels have conflicts of interests. Pharmaceutical Journal 2005;275(7372):505. [Google Scholar]
Riechelmann 2007 {published data only}
- Riechelmann RP, Wang L, O'Carroll A, Krzyzanowska MK. Disclosure of conflicts of interest by authors of clinical trials and editorials in oncology. Journal of Clinical Oncology 2007;25(29):4642-7. [DOI] [PubMed] [Google Scholar]
Roberts 2020 {published data only}
- Roberts W, Jellison S, Wayant C, Vassar M. Characteristics and conflicts of interests of public speakers at the Psychopharmacologic Drug and Advisory Committee meetings regarding psychiatric drugs. BMJ Evidence-Based Medicine 2020;25(4):145-6. [DOI] [PubMed] [Google Scholar]
Roland 2009 {published data only}
- Roland KB, Benard VB, Saraiya M, Hawkins NA, Brandt H, Friedman AL. Assessing cervical cancer screening guidelines in patient education materials. Journal of Women's Health 2009;18(1):5-12. [Google Scholar]
Roundtree 2009 {published data only}
- Roundtree AK, Kallen MA, Lopez-Olivo MA, Kimmel B, Skidmore B, Ortiz Z, et al. Poor reporting of search strategy and conflict of interest in over 250 narrative and systematic reviews of two biologic agents in arthritis: a systematic review. Journal of Clinical Epidemiology 2009;62(2):128-37. [DOI] [PubMed] [Google Scholar]
Saito 2019 {published data only}
- Saito H, Tani Y, Ozaki A, Sawano T, Shimada Y, Yamamoto K, et al. Financial ties between authors of the clinical practice guidelines and pharmaceutical companies: an example from Japan. Clinical Microbiology and Infection 2019;25(11):1304-6. [DOI] [PubMed] [Google Scholar]
Saito 2019a {published data only}
- Saito H, Ozaki A, Sawano T, Shimada Y, Tanimoto T. Evaluation of pharmaceutical company payments and conflict of interest disclosures among Oncology Clinical Practice Guideline authors in Japan. JAMA Network Open 2019;2(4):e192834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleh 2019 {published data only}
- Saleh RR, Majeed H, Tibau A, Booth CM, Amir E. Undisclosed financial conflicts of interest among authors of American Society of Clinical Oncology clinical practice guidelines. Cancer 2019;125(22):4069-75. [DOI] [PubMed] [Google Scholar]
Shapiro 2003 {published data only}
- Shapiro J. Software tool reveals financial impact of clinical guideline usage. Performance Improvement Advisor 2003;7(6):83-7. [PubMed] [Google Scholar]
Shimada 2019 {published data only}
- Shimada Y, Ozaki A, Saito H, Sawano T, Tanimoto T. Pharmaceutical company payments to the authors of the Japanese dementia clinical practice guidelines in 2016. Alzheimer's & Dementia 2019;5:228-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shnier 2016 {published data only}
- Shnier A, Lexchin J, Romero M, Brown K. Reporting of financial conflicts of interest in clinical practice guidelines: a case study analysis of guidelines from the Canadian Medical Association Infobase. BMC Health Services Research 2016;16:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spithoff 2020 {published data only}
- Spithoff S, Leece P, Sullivan F, Persaud N, Belesiotis P, Steiner L. Drivers of the opioid crisis: an appraisal of financial conflicts of interest in clinical practice guideline panels at the peak of opioid prescribing. PLOS One 2020;15(1):e0227045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinbrook 2005 {published data only}
- Steinbrook R. Financial conflicts of interest and the Food and Drug Administration's Advisory Committees. New England Journal of Medicine 2005;353(2):116-8. [DOI] [PubMed] [Google Scholar]
Traynor 2002 {published data only}
- Traynor K. Most clinical practice guideline authors receive drug industry support. American Journal of Health-system Pharmacy 2002;59(6):509. [DOI] [PubMed] [Google Scholar]
Verma 2017 {published data only}
- Verma V. Financial relationships with industry of editorial board members of the three journals of the American Society for Radiation Oncology. International Journal of Radiation Oncology 2017;99(2):286-91. [DOI] [PubMed] [Google Scholar]
Wang 2010a {published data only}
- Wang AT, McCoy CP, Murad MH, Montori VM. Association between industry affiliation and position on rosiglitazone and cardiovascular risk: a cross sectional systematic review. Oral presentation at the Joint Cochrane and Campbell Colloquium; 2010 Oct 18-22; Keystone, Colorado, USA [abstract]. Cochrane Database of Systematic Reviews 2010;Suppl(Cd000002):27-8.
Wayant 2019 {published data only}
- Wayant C, Vassar M. A review of the magnitude of financial relationships with industry and disclosure practices among clinical practice guideline authors. BMJ Evidence-Based Medicine 2019;24 (Supplement 1):A2. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1998
- Altman DG. Confidence intervals for the number needed to treat. BMJ 1998;317(7168):1309-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
American Medical Association 2017
- American Medical Association. Eighth International Congress on Peer Review and Scientific Publication. http://www.peerreviewcongress.org/index.html (accessed 19 February 2020).
Andreatos 2017
- Andreatos N, Zacharioudakis IM, Zervou FN, Muhammed M, Mylonakis E. Discrepancy between financial disclosures of authors of clinical practice guidelines and reports by industry. Medicine (Baltimore) 2017;96(2):e5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bero 1996
- Bero LA, Rennie D. Influences on the quality of published drug studies. International Journal of Technology Assessment in Health Care 1996;12(2):209-37. [DOI] [PubMed] [Google Scholar]
Bero 2007
- Bero L, Oostvogel F, Bacchetti P, Lee K. Factors associated with findings of published trials of drug-drug comparisons: why some statins appear more efficacious than others. PLOS Medicine 2007;4(6):e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bero 2016
- Bero LA, Grundy Q. Why Having a (nonfinancial) interest is not a conflict of interest. PLOS Biology 2016;14(12):e2001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bero 2017
- Bero L. Addressing bias and conflict of interest among biomedical researchers. JAMA 2017;317(17):1723-4. [DOI] [PubMed] [Google Scholar]
Bero 2018
- Bero L. More journals should have conflict of interest policies as strict as Cochrane. BMJ Opinion 2018.
Centre for Evidence‐Based Medicine 2017
- Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences. Evidence Live. http://evidencelive.org/ (accessed 19 February 2020).
Chan 2013
- Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chartres 2016
- Chartres N, Fabbri A, Bero LA. Association of industry sponsorship with outcomes of nutrition studies: a systematic review and meta-analysis. JAMA Internal Medicine 2016;176(12):1769-77. [DOI] [PubMed] [Google Scholar]
Cochrane Community 2017
- Cochrane Community. Colloquium. http://community.cochrane.org/news/events/colloquium (accessed 19 February 2020).
Fabbri 2018
- Fabbri A, Lai A, Grundy Q, Bero LA. The influence of industry sponsorship on the research agenda: a scoping review. American Journal of Public Health 2018;108(11):e9-e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2017
- FDA. Medical Device Overview. https://www.fda.gov/ForIndustry/ImportProgram/ImportBasics/RegulatedProducts/ucm510630.htm. Last updated 12 Jan 2017 (accessed 25 Apr 2018).
Foroutan 2020
- Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. Journal of Clinical Epidemiology 2020;121:62-70. [DOI] [PubMed] [Google Scholar]
Grundy 2018
- Grundy Q, Dunn AG, Bourgeois FT, Coiera E, Bero L. Prevalence of disclosed conflicts of interest in biomedical research and associations with journal impact factors and altmetric scores. JAMA 2018;319(4):408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Hansen 2019a
- Hansen C, Lundh A, Rasmussen K, Hróbjartsson A. Financial conflicts of interest in systematic reviews: associations with results, conclusions, and methodological quality. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: MR000047. [DOI: 10.1002/14651858.MR000047.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Horsley 2011
- Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 8. Art. No: MR000026. [DOI: 10.1002/14651858.MR000026.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Institute of Medicine 1990
- Institute of Medicine Committee to Advise the Public Health Service on Clinical Practice Guidelines Field MJ, Lohr KN (editors). Clinical Practice Guidelines: Directions for a New Program. Washington (DC): National Academies Press (US), 1990. [PubMed] [Google Scholar]
Institute of Medicine 2009
- Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice Lo B, Field MJ (editors). Conflict of Interest in Medical Research. Washington (DC): National Academies Press, 2009. [PubMed] [Google Scholar]
IntHout 2016
- IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jørgensen 2006
- Jørgensen AW, Hilden J, Gøtzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: systematic review. BMJ 2006;333(7572):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumley 2006
- Lumley T, Kronmal R, Shuangge M. Relative risk regression in medical research: models, contrasts, estimators, and algorithms. UW Biostatistics Working Paper Series 2006;Working Paper 293:http://biostats.bepress.com/uwbiostat/paper293. [Google Scholar]
Lundh 2013
- Lundh A, Jørgensen AW, Bero L. Association between personal conflicts of interest and recommendations on medical interventions. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: MR000040. [DOI: 10.1002/14651858.MR000040] [DOI] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: MR000033. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2020
- Lundh A, Rasmussen K, Østengaard L, Boutron I, Stewart LA, Hróbjartsson A. Systematic review finds that appraisal tools for medical research studies address conflicts of interest superficially. Journal of Clinical Epidemiology 2020;120:104-15. [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence (NICE). Policy on declaring and managing interests for NICE advisory committees. https://www.nice.org.uk/Media/Default/About/Who-we-are/Policies-and-procedures/declaration-of-interests-policy.pdf (accessed 18 December 2019).
Page 2016
- Page MJ, Higgins JP, Clayton G, Sterne JA, Hróbjartsson A, Savović J. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLOS One 2016;11(7):Ae0159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Shawwa 2016
- Shawwa K, Kallas R, Koujanian S, Agarwal A, Neumann I, Alexander P, et al. Requirements of clinical journals for authors' disclosure of financial and non-financial conflicts of interest: a cross sectional study. PLOS One 2016;11(3):e0152301. [DOI] [PMC free article] [PubMed] [Google Scholar]
The PLoS Medicine Editors 2008
- The PLoS Medicine Editors. Making sense of non-financial competing interests. PLOS Medicine 2008;5(9):e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
U.S. Preventive Services Task Force 2015
- US Preventive Services Task Force. U.S. Preventive Services Task Force Procedure Manual. https://www.uspreventiveservicestaskforce.org/Page/Name/procedure-manual (accessed 31 Jan 2018).
U.S. Preventive Services Task Force 2018
- US Preventive Services Task Force. Conflict of Interest Disclosures. https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures (accessed 31 Jan 2018).
Viswanathan 2014
- Viswanathan M, Carey TS, Belinson SE, Berliner E, Chang SM, Graham E, et al. A proposed approach may help systematic reviews retain needed expertise while minimizing bias from nonfinancial conflicts of interest. Journal of Clinical Epidemiology 2014;67(11):1229-38. [DOI] [PubMed] [Google Scholar]
WHO 2014
- WHO. Handbook for Guideline Development. https://apps.who.int/iris/handle/10665/145714 (accessed 9 November 2020).
References to other published versions of this review
Hansen 2019b
- Hansen C, Bero L, Hróbjartsson A, Jørgensen AW, Jørgensen KJ, Le M, et al. Conflicts of interest and recommendations in clinical guidelines, opinion pieces, and narrative reviews. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: MR000040. [DOI: 10.1002/14651858.MR000040.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


