Abstract
Mitochondrial stress (mitostress) triggered by viral infection or mitochondrial dysfunction causes the release of mitochondrial DNA (mtDNA) into the cytosol and activates the cGAS-mediated innate immune response. The regulation of mtDNA release upon mitostress remains uncharacterized. Here, we identified mitochondria-associated vaccinia virus-related kinase 2 (VRK2) as a key regulator of this process. VRK2 deficiency inhibited the induction of antiviral genes and caused earlier and higher mortality in mice after viral infection. Upon viral infection, VRK2 associated with voltage-dependent anion channel 1 (VDAC1) and promoted VDAC1 oligomerization and mtDNA release, leading to the cGAS-mediated innate immune response. VRK2 was also required for mtDNA release and cGAS-mediated innate immunity triggered by nonviral factors that cause Ca2+ overload but was not required for the cytosolic nucleic acid-triggered innate immune response. Thus, VRK2 plays a crucial role in the mtDNA-triggered innate immune response and may be a potential therapeutic target for infectious and autoimmune diseases associated with mtDNA release.
Keywords: cGAS, Mita/Sting, mitostress, innate immune response, mitochondrial DNA
Subject terms: Innate immunity, Cell signalling
Introduction
It has been well established that the innate immune response is the first line of host defense against viral infection, which is initiated upon the sensing of conserved viral structural components called pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) expressed by host cells.1 The sensing of viral PAMPs by PRRs activates a series of signaling events, leading to the induction of downstream antiviral effector proteins, including type I interferons (IFNs) and proinflammatory cytokines.2,3 The most important viral PAMPs are viral nucleic acids, including viral DNA and/or RNA. After invasion of RNA viruses, cytosolic viral RNA is sensed by retinoic acid-induced gene I or melanoma differentiation-associated gene 5, which then initiates innate antiviral responses via pathways mediated by the mitochondria-associated protein VISA (also called MAVS).4–6 After invasion of DNA viruses, cytosolic viral DNA is mainly sensed by a ubiquitously expressed nucleotidyltransferase called cyclic GMP-AMP (cGAMP) synthase (cGAS).7 Upon binding cytosolic viral or cellular DNA, cGAS catalyzes the synthesis of cGAMP, which then acts as a second messenger to bind to and activate the endoplasmic reticulum (ER)-resident protein MITA (also called STING).8,9 STING/MITA is then translocated from the ER via the ER–Golgi intermediate compartment to the Golgi apparatus, where the kinase TBK1 and the transcription factor IRF3 are recruited to the STING/MITA complex and maximally activated, leading to the induction of type I IFNs, inflammatory cytokines, and other downstream antiviral genes.10,11
Viral infection activates innate immune responses via mechanisms other than viral nucleic acid-dependent mechanisms, such as virus–cell membrane fusion and mitochondrial stress (mitostress).12,13 Recently, it has been shown that viral infection triggers mitostress, which results in the release of mitochondrial DNA (mtDNA) into the cytosol and its recognition by cGAS, leading to STING/MITA-dependent innate immune responses that contribute to host defense against viral infection.14 It has also been shown that mitochondrial dysfunction caused by nonviral factors can cause mtDNA release into the cytosol, leading to cGAS-mediated innate immune responses that are correlated with autoimmune and neurodegenerative diseases.14–18 Recently, it has been demonstrated that upon mitostress, voltage-dependent anion channel (VDAC) proteins oligomerize to form mitochondrial pores, which allow the release of mtDNA into the cytosol to trigger cGAS-mediated innate immune responses.17 The regulation of mtDNA release upon mitostress is not well understood.
Virus-related kinase 2 (VRK2) is a member of the vaccinia VRK family, which is characterized by homology of its catalytic domain to that of vaccinia virus B1R kinase.19 VRK2 is widely expressed in various cell types and tissues and contains an N-terminal kinase domain and a C-terminal transmembrane domain that anchors it to the mitochondrial, ER and nuclear membranes.20 VRK2 is involved in diverse biological processes, such as the response to hypoxic stress,21 interleukin-1β signaling,22 EGF-ERBB2 signaling,23 and tumor cell invasion.24 In this study, we found that VRK2 deficiency inhibited innate antiviral responses and caused earlier and higher mortality in mice after viral infection. VRK2 was not required for the canonical viral nucleic acid-triggered innate immune response but acted as a key regulator of mtDNA release in mitostress-triggered innate immune responses. The results of mechanistic studies suggested that VRK2 regulates the association of mtDNA with VDAC1 as well as the formation of VDAC1 oligomeric pores upon mitostress. Our findings demonstrate a critical role of VRK2 in regulating the mtDNA-triggered innate immune response upon viral infection and mitostress, which facilitates the understanding of the mechanisms underlying cellular stress responses as well as infectious and autoimmune diseases.
Materials and methods
Reagents, viruses, and cells
The following reagents were purchased from the indicated manufacturers: the inhibitors ABT-737 (HY-50907, MCE) and Q-VD-OPh (HY-12305, MCE); mitochondrial membrane potential (MMP) detection kits (JC-10) (CA1310, Solarbio); DCFH-DA (D6470, Solarbio); Fluo 3-AM (F023, DOJINDO); the reagents herring testes (HT)-DNA (D6898, Sigma), ethidium bromide (EB) (1239-45-8, Aladdin), EGS (21565, Thermo), FuGENE (E2311, Promega), poly(I:C) (tlrl-pic-5, Invitrogen), Lipofectamine (LF) (11668019, Invitrogen), and murine IFN-β (8234-MB-010/CF, R&D); ELISA kits for murine IFN-β (42400, PBL) and CXCL10 (ΕΚ0736−ΧΑΠ, BOSTER); a mouse monoclonal antibody against HA (TA180128, OriGene); rabbit monoclonal antibodies against HA (TA591010, OriGene), FLAG (F3165, Sigma), PBR (A4881, Abclonal), TBK1 (ab40676, Abcam), pTBK1S172 (ab109272, Abcam), IRF3 (sc-33641, Santa Cruz), and β-actin (A2228, Sigma); rabbit polyclonal antibodies against VDAC (4661, CST), STING (13647, CST), human p-STINGS366 (43499, CST), and p-IRF3S396 (4947, CST). Sendai virus (SeV), encephalomyocarditis virus (EMCV), herpes simplex virus 1 (HSV-1), and murine cytomegalovirus (MCMV) were previously described.25,26 HEK293 and Raw264.7 cells were obtained from ATCC. Primary wild-type (WT) and Vrk2−/− bone marrow-derived macrophages (BMDMs) and murine lung fibroblasts (MLFs) were prepared as previously described.27
Constructs
Constructs for HA-, Myc-, or Flag-tagged VDAC1, VDAC2, VDAC3, PBR, PPIF, VRK2, and their mutants, as well as CRISPR-Cas9 gRNA plasmids for knockout of Bax, Bak, Vrk2, Sting, Visa, Vdac1, Jip1, Ksr, and Nfat, were constructed by standard molecular biology techniques. CRISPR-Cas9 knockout cell lines were constructed as previously described.28 The following sequences were targeted for the indicated murine genes.
Vrk2-gRNA: 5’-GGCAAGATGATCGGCTCTGG-3’;
Vdac1-gRNA: 5’-TCGTCATTCTCGCCGAACAC-3’;
Bax-gRNA: 5’-AGCGAGTGTCTCCGGCGAAT-3’;
Bak-gRNA: 5’-GGGGCAAGTTGTCCATCTCG-3’;
Sting/Mita-gRNA: 5’-GCACCTAGCCTCGCACGAACT-3’;
Visa/Mavs-gRNA: 5’-GTCGCCAGGTCTCAGGGCCGG-3’;
Jip1-gRNA: 5’-ACTCGTCAGTGATCTCCGAA-3’;
Ksr-gRNA: 5’-GCGTTGCGCGCGGCAGCGAT-3’;
Nfat-gRNA: 5’-CGGGGCCGTCCCCGCCATCG-3’.
Mice
VRK2-deficient mice on a C57BL/6 background were generated by CRISPR-Cas9 genome editing by Cyagen Company. All mice were maintained in SPF rooms. Viral infection experiments were performed in ABSL-2 laboratories. All animal experiments were performed in accordance with the Wuhan University Medical Research Institute Animal Care and Use Committee guidelines.
qPCR
Total RNA was isolated for quantitative PCR (qPCR) analysis to measure the mRNA levels of the indicated genes. The data shown are the relative abundance of each indicated mRNA normalized to that of GAPDH. The qPCR primers used were previously described.29
Isolation of mitochondria and measurement of mtDNA
To isolate the mitochondrial fraction (P5K) and nonmitochondrial fraction (S5K), cells were washed and resuspended in ice-cold hypotonic buffer30 supplemented with protease inhibitors and were then lysed by Dounce homogenization. Cell lysates were centrifuged at 500 x g for 10 min at 4 °C to remove nuclear pellets, and the supernatants were then centrifuged at 5000 × g for 10 min at 4 °C to obtain P5K and S5K. mtDNA was extracted from these fractions and subjected to qPCR analysis with primers corresponding to the D-loop in mtDNA.31 The ratio of S5K to P5K in each sample was used to quantitate the relative release of mtDNA from mitochondria into the cytosol.
mtDNA depletion
MLFs were left untreated or treated with EB (200 ng/ml) for 6 days to deplete mtDNA. The mtDNA depletion efficiency was evaluated by total DNA extraction and qPCR analysis with primers corresponding to the D-loop in mtDNA and to the nuclear genomic gene Tert. The qPCR primers used were previously reported.31
Confocal microscopy
HT1080 cells were transfected with the indicated plasmids. Twenty hours later, cells were stained with MitoTracker, fixed with 4% paraformaldehyde for 20 min and permeabilized for 20 min by incubation with 0.1% Triton X-100. Cells were blocked with 1% BSA and stained with the indicated primary and corresponding secondary antibodies. Cells were imaged using a Zeiss confocal microscope with a 63x oil objective.
Coimmunoprecipitation and immunoblot analysis
Coimmunoprecipitation and immunoblot analysis were performed as previously described.32 Briefly, cells were lysed with lysis buffer33 (20 mM Tris-HCl, pH 7.5; 0.5% Nonidet P-40; 10 mM NaCl; 3 mM EDTA, and 3 mM EGTA) containing protease inhibitors prior to immunoprecipitation with protein G and the indicated antibodies, and the immunoprecipitates were analyzed by immunoblotting with the indicated antibodies.
In vitro pulldown assay
HEK293 cells transfected with the indicated plasmids were lysed, and the lysates were incubated first with biotinylated mtDNA (120 bp)17 at 4 °C for 2 h and then with streptavidin beads at 4 °C for 1 h. The beads were washed five times with lysis buffer and analyzed by immunoblotting with the indicated antibodies. The sequence of the synthetic mtDNA fragment (120 bp) was as follows: 5’-TCCTCCGTGAAACCAACAACCCGCCCACCAATGCCCCTCTTCTCGCTCCGGGCCCATTAAACTTGGGGGTAGCTAAACTGAAACTTTATCAGACATCTGGTTCTTACTTCAGGGCCATCA-3’
mtDNA-binding protein immunoprecipitation
MLFs were infected with HSV-1 or EMCV for an hour, and cell lysates then were immunoprecipitated with anti-VDAC or anti-VRK2 antibodies at 4 °C for 3 h. The bead-bound immunoprecipitates were washed three times with lysis buffer. Protein and DNA complexes were eluted with 200 μl of TE buffer containing 10 mM DTT at 37 °C for 30 min. DNA was extracted using phenol-chloroform-isoamyl alcohol before qPCR analysis of mtDNA.
Viral plaque assay
Viral plaque assays were performed as previously described.32 Eight-week-old mice were infected with HSV-1 or EMCV for 4 days. The brains of infected mice were harvested, weighed, and homogenized. Vero or BHK21 cells were seeded and incubated with serial dilutions of the supernatants at 37 °C for 2 h. The infected cells were overlaid with 1.5% methylcellulose and were then incubated for another 36–48 h prior to fixation with 4% paraformaldehyde and staining with 1% crystal violet before plaque counting.
Statistical analysis
GraphPad Prism and SPSS Statistics were used for statistical analysis. Quantitative data in histograms are shown as the means ± SDs. Data were analyzed using the log-rank (Mantel–Cox) test or Student’s unpaired t-test. The number of asterisks indicates the degree of significance with respect to P values. Statistical significance was assumed at P < 0.05.
Results
VRK2 plays a key role in innate antiviral responses
To identify candidate molecules involved in virus-triggered innate immune responses, we screened ~200 kinases for their ability to regulate cGAS-mediated activation of interferon-stimulated response elements in reporter assays. The preliminary candidate genes in MLFs were knocked out by CRISPR-Cas9 genome editing for further validation. These efforts led to the identification of VRK2. As shown in Supplementary Fig. S1A, qPCR experiments indicated that knockout of Vrk2 dramatically inhibited the transcription of downstream antiviral Ifnb1 and Cxcl10 genes induced by the DNA virus HSV-1 in MLFs. Knockout of Vrk2 also inhibited HSV-1-induced phosphorylation of STING, TBK1, and IRF3 (Supplementary Fig. S1B), which are hallmarks of the activation of downstream signaling components, in MLFs. These results suggest that VRK2 is required for efficient innate immune responses to HSV-1 in MLFs.
To further investigate the roles of VRK2 in vivo, we generated Vrk2−/− mice by CRISPR-Cas9 genome editing (Supplementary Fig. S2A, B). Homozygous Vrk2−/− mice were born at the Mendelian ratio (Supplementary Fig. S2C) and appeared to exhibit normal growth. Hematoxylin and eosin staining indicated that VRK2 deficiency had no effects on brain and lung histomorphology in mice (Supplementary Fig. S2D). The numbers and compositions of cells in the lymph nodes, spleen, and thymus were similar between Vrk2−/− mice and their WT littermates (Supplementary Fig. 2E), suggesting that VRK2 is not required for the development of various types of immune cells.
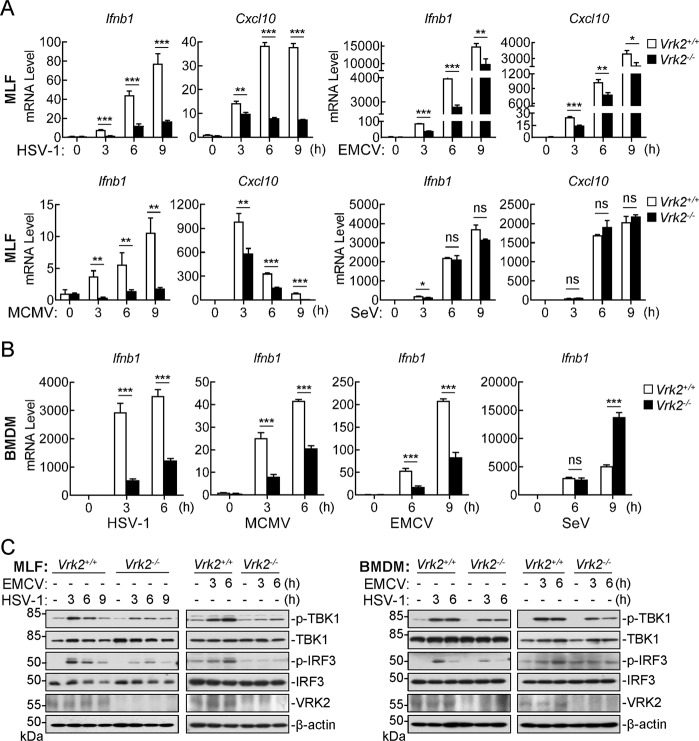
To examine the innate immune responses of primary cells from VRK2-deficient mice to various viruses, we infected WT and Vrk2−/− MLFs and BMDMs with different viruses, including the DNA viruses HSV-1 and MCMV and the RNA viruses EMCV and SeV. qPCR experiments indicated that VRK2 deficiency markedly inhibited the transcription of the Ifnb1 and Cxcl10 genes in MLFs (Fig. 1A) and the Ifnb1 gene in BMDMs (Fig. 1B) induced by HSV-1, MCMV, and EMCV. In these experiments, VRK2 deficiency minimally affected SeV-triggered transcription of the Ifnb1 and Cxcl10 genes in MLFs (Fig. 1A) and increased SeV-triggered transcription of the Ifnb1 gene in BMDMs at 9 h after infection (Fig. 1B). In addition, IFN-β-induced transcription of the Ifit1 and Cxcl10 genes was comparable between Vrk2−/− and WT MLFs (Supplementary Fig. S2F). These results suggest that VRK2 is required for efficient induction of downstream antiviral genes triggered by certain DNA and RNA viruses in different types of cells. Consistent with these findings, phosphorylation of TBK1 and IRF3 induced by HSV-1 or EMCV was markedly inhibited in Vrk2−/− MLFs compared to WT MLFs or BMDMs (Fig. 1C).
Fig. 1.
VRK2 plays a key role in innate antiviral responses. A Effects of VRK2 deficiency on virus-triggered transcription of the Ifnb1 and Cxcl10 genes in MLFs. MLFs (1 × 106) from WT and Vrk2−/− mice were left uninfected or infected with HSV-1, MCMV, EMCV, or SeV (MOI = 1) for the indicated times prior to qPCR analysis of the indicated genes. B Effects of VRK2 deficiency on virus-triggered transcription of the Ifnb1 gene in BMDMs. BMDMs (1 × 106) from WT and Vrk2−/− mice were left uninfected or infected with HSV-1, MCMV, SeV, or EMCV (MOI = 1) for the indicated times prior to qPCR analysis of the Ifnb1 gene. C Effects of VRK2 deficiency on virus-triggered phosphorylation of TBK1 and IRF3. MLFs and BMDMs (1 × 106) from WT and Vrk2−/− mice were left uninfected or infected with HSV-1 or EMCV (MOI = 1) for the indicated times prior to immunoblot analysis with the indicated antibodies. The data shown are the mean ± SD from one representative experiment performed in triplicate (A, B). ns nonsignificant; *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired t-test)
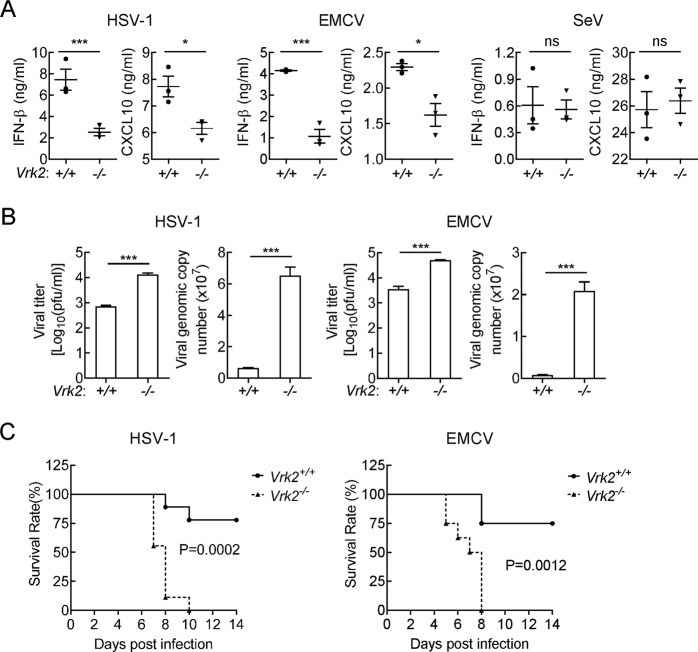
We further evaluated the importance of VRK2 in the host defense against viral infection in vivo. After intraperitoneal infection with HSV-1 or EMCV for 6 h, the serum levels of IFN-β and CXCL10 in Vrk2−/− mice were markedly lower than those in WT mice (Fig. 2A). In similar experiments, the serum IFN-β and CXCL10 levels were compared between WT and Vrk2−/− mice after infection with SeV for 6 h (Fig. 2A). The genomic copies and viral titers of HSV-1 and EMCV in the brain were ~10- and 26-fold higher in Vrk2−/− mice than in WT mice 4 days after infection with the respective viruses (Fig. 2B). Consistent with these findings, Vrk2−/− mice were more susceptible to HSV-1- and EMCV-induced death (Fig. 2C). These results suggest that VRK2 plays important roles in the host defense against various viruses in vivo.
Fig. 2.
VRK2 deficiency impairs host defense against viral infection in vivo. A Effects of VRK2 deficiency on virus-induced production of IFN-β and CXCL10 in serum. WT and Vrk2−/− mice (n = 3) were intraperitoneally infected with HSV-1 (5 × 107 pfu per mouse), EMCV (5 × 107 pfu per mouse), or SeV (5 × 107 pfu per mouse) for 6 h before serum cytokines were measured by ELISA. The results are presented as the means ± SDs. ns nonsignificant; *P < 0.05; ***P < 0.001 (unpaired t-test). B Measurement of viral titers and viral genomic copies in the brains of mice. WT and Vrk2−/− mice (n = 3) were intraperitoneally infected with HSV-1 (5 × 107 pfu per mouse) or EMCV (5 × 106 pfu per mouse) for 4 days, and viral titers and viral genomic copies in the brains of mice were then quantified by plaque assays and qPCR, respectively. The results are presented as the means ± SDs. ***P < 0.001 (unpaired t-test). C Effects of VRK2 deficiency on virus-induced death of mice. WT and Vrk2−/− mice were intraperitoneally infected with HSV-1 (5 × 107 pfu per mouse, n = 9) or EMCV (5 × 105 pfu per mouse, n = 8), and the survival rates of mice were then recorded for 2 weeks. P values were calculated by the log-rank (Mantel–Cox) test. The data are representative of two experiments with similar results
VRK2 regulates mtDNA-triggered innate immune responses
We next explored how VRK2 is involved in innate antiviral responses. Previously, it was shown that viral nucleic acids in the cytosol act as major PAMPs to trigger innate antiviral responses via STING- or VISA-mediated pathways.6 The results of qPCR experiments indicated that STING or VISA deficiency abolished the transcription of the Ifnb1 and Cxcl10 genes induced by transfected HT-DNA and the synthetic double-stranded viral RNA analog poly(I:C), respectively (Fig. 3A). In these experiments, however, VRK2 deficiency had no marked effects on the induction of Ifnb1 and Cxcl10 gene transcription by transfected HT-DNA or poly(I:C) (Fig. 3A). The results of biochemical experiments showed that STING or VISA deficiency but not VRK2 deficiency impaired the phosphorylation of TBK1 and IRF3 induced by transfection of HT-DNA or poly(I:C) (Fig. 3B). These results suggest that VRK2 is not involved in innate antiviral responses triggered by viral nucleic acids. Recently, it has been demonstrated that the processes of viral infection, such as virus-membrane fusion or viral endosomal escape, can induce innate immune responses in a viral nucleic acid-independent manner.12,13,34 This effect is mediated by mitostress and mtDNA release via a viral nucleic acid-independent process.13,14,35 Thus, we next investigated whether VRK2 is involved in innate immune responses triggered by cytosolic mtDNA produced upon mitostress after viral infection. We first examined whether viral infection causes the production of reactive oxygen species (ROS) and a decrease in MMP, which are indicators of mitostress.36 We found that HSV-1 and EMCV caused similar increases in ROS production and decreases in MMP within 2 h of infection in both WT and Vrk2−/− MLFs. In these experiments, SeV infection had no marked effects on ROS production or MMP in either WT or Vrk2−/− MLFs (Fig. 3C, D). In addition, HSV-1 and EMCV but not SeV triggered mtDNA release into the cytosol in WT MLFs, and this effect was abolished in Vrk2−/− MLFs (Fig. 3E). These results suggest that infection with HSV-1 and EMCV but not SeV causes mitostress that is independent of VRK2. However, mtDNA release into the cytosol after viral induction of mitostress requires VRK2.
Fig. 3.
VRK2 regulates mtDNA-triggered innate immune responses. A Effects of STING, VISA, or VRK2 deficiency on the transcription of the Ifnb1 and Cxcl10 genes induced by transfection of HT-DNA or poly(I:C). Control Raw264.7 cells and Raw264.7 cells with knockout of the indicated genes (1 × 106) were transfected with HT-DNA (2 μg) or poly(I:C) (2 μg) via Fugene (4 μg) for 6 h prior to qPCR analysis of the indicated genes. The knockout efficiency of each gRNA is shown in the right panel. The data shown are the mean ± SD from one representative experiment performed in triplicate. B Effects of STING, VISA, or VRK2 deficiency on the phosphorylation of TBK1 and IRF3 induced by transfection of HT-DNA or poly(I:C). The indicated knockout Raw264.7 cells (1 × 106) were transfected with HT-DNA (2 μg) or poly(I:C) (2 μg) via Fugene (4 μg) for the indicated times prior to immunoblot analysis with the indicated antibodies. C Measurement of virus-induced ROS production. WT and Vrk2−/− MLFs (2 × 106) were left uninfected or infected with HSV-1, EMCV, or SeV (MOI = 2) for the indicated times before staining with DCFH-DA (2 μM) for 20 min and subsequent fluorescence detection for ROS measurement. D Measurement of virus-induced changes in MMP. WT and Vrk2−/− MLFs (2 × 106) were left uninfected or infected with HSV-1, EMCV, or SeV (MOI = 2) for the indicated times before staining with JC-10 (4 μM) for 20 min and subsequent fluorescence detection for MMP measurement. E Measurement of virus-induced mtDNA release. WT and Vrk2−/− MLFs (2 × 107) were left uninfected or infected with HSV-1, EMCV, or SeV (MOI = 2) for 1 h prior to subcellular fractionation and measurement of mtDNA release. P5K, mitochondrial fraction; S5K, nonmitochondrial cytosolic fraction. The data shown are the mean ± SD from one representative experiment performed in triplicate. ns nonsignificant; ***P < 0.001 (unpaired t-test). F Measurement of the mtDNA depletion efficiency. WT and Vrk2−/− MLFs were treated with EB (200 ng/ml) for 6 days prior to DNA extraction and qPCR analysis of the indicated genes. The data shown are the mean ± SD from one representative experiment performed in triplicate. ns nonsignificant; ***P < 0.001 (unpaired t-test). G Effects of mtDNA depletion on virus-induced transcription of the Ifnb1 and Cxcl10 genes. Control and EB (200 ng/ml)-treated WT and Vrk2−/− MLFs (1 × 106) were left uninfected or infected with HSV-1 or EMCV (MOI = 1) for 6 h prior to qPCR analysis of the indicated genes. The data shown are the mean ± SD from one representative experiment performed in triplicate. ns nonsignificant; ***P < 0.001 (unpaired t-test)
We next examined whether VRK2-mediated mtDNA release is important for innate antiviral responses. Previously, EB, which intercalates into mtDNA base pairs and prevents DNA replication, has been used to deplete cellular mtDNA without affecting the cellular genomic DNA level.31,37 We found that EB treatment depleted mtDNA but not nuclear DNA in WT and Vrk2−/− MEFs to similar levels (Fig. 3F) and caused a marked decrease in Ifnb1 mRNA expression induced by infection with either HSV-1 or EMCV in WT MLFs (Fig. 3G). In Vrk2−/− MLFs, HSV-1 or EMCV infection-triggered transcription of the Ifnb1 gene was obviously lower than that in WT MLFs. In these experiments, EB treatment increased virus-induced Ifnb1 transcription in Vrk2−/− MLFs (Fig. 3G). One explanation for this observation is that EB treatment damages nuclear DNA and causes the formation of micronuclei, which can weakly trigger cGAS-STING-mediated Ifnb1 transcription. Taken together, these results suggest that VRK2-mediated mtDNA release into the cytosol after viral infection contributes to innate immune responses to HSV-1 and EMCV.
VRK2 regulates VDAC-mediated mtDNA release
Next, we explored how VRK2 regulates mtDNA release and antiviral innate immune responses. Previously, VRK2 was shown to activate NFAT, a transcription factor that induces the transcription of several cytokine genes.24 VRK2 modulates MAPK signaling by interacting with the scaffold proteins KSR and JIP1.21,23 We established MLFs deficient in NFAT, KSR, or JIP1 by CRISPR-Cas9 genome editing (Supplementary Fig. S3A). The results of qPCR experiments showed that deficiency of NFAT or KSR inhibited the transcription of Ifnb1 Cxcl10 genes induced by infection with HSV-1 or EMCV or by transfection of poly(I:C) or HT-DNA (Supplementary Fig. S3B). In these experiments, JIP1 deficiency had no marked effects on the transcription of the Ifnb1 and Cxcl10 genes induced by these stimulators (Supplementary Fig. S3B). These results indicated that NFAT and KSR are involved in innate immune responses triggered by viral nucleic acids, whereas JIP1 is not involved in innate antiviral responses. These results suggest that VRK2, NFAT, and KSR are involved in distinct pathways of innate immune responses.
It has been reported that VRK2 is associated with BAX in mitochondria and that loss of VRK2 increases the transcription of BAX in A549 cells.38 In our experiments, we found that VRK2 was associated with BCL-xL but not BAX in the overexpression system (Supplementary Fig. S4A). In addition, VRK2 deficiency had no marked effects on the transcription of Bcl-xl and Bax in Raw264.7 cells (Supplementary Fig. S4B). Several studies have reported that apoptosis causes mtDNA release via BAX/BAK-forming pores (BBFPs) without immune activation unless apoptosis-activated caspases are inactivated.31,37 Consistent with previous studies, combination treatment with the apoptosis inducer ABT-737 and the pan-caspase inhibitor Q-VD-OPh (A/Q) induced transcription of the Ifnb1 gene, which was inhibited in BAX/BAK double knockout cells (Supplementary Fig. S4C). However, in the same experiments, transcription of the Ifnb1 gene induced by HSV-1 and EMCV was not inhibited in BAX/BAK-deficient cells (Supplementary Fig. S4C). Moreover, BAX/BAK double knockout had no marked effects on HSV-1- and EMCV-triggered mtDNA release (Supplementary Fig. S4D). These results suggest that BBFPs are not required for virus-induced mtDNA release and innate immune responses and that VRK2 regulates mtDNA release and antiviral innate immune responses in a BAX-independent manner.
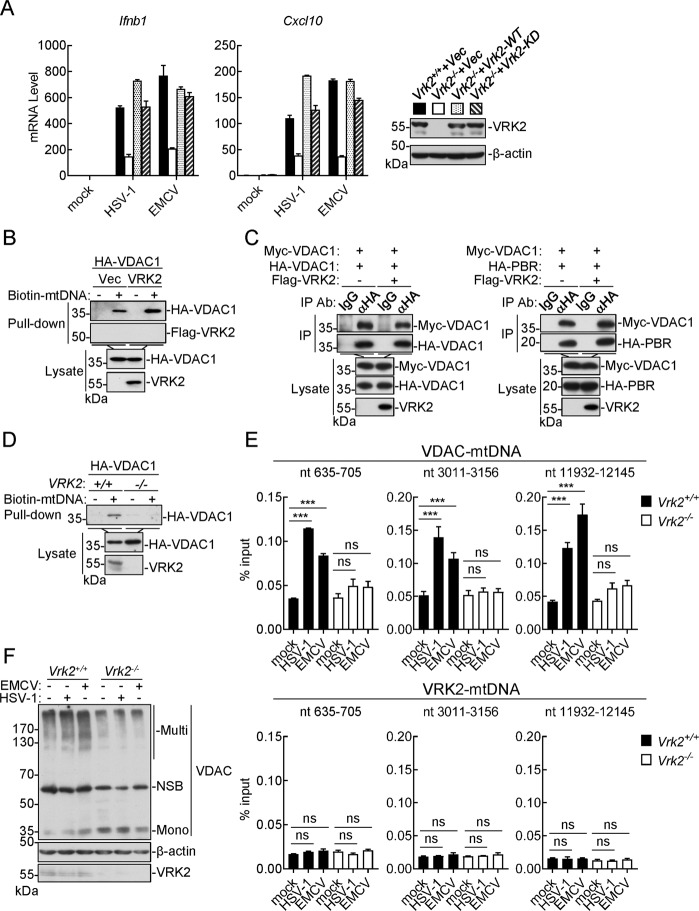
To unambiguously investigate how VRK2 regulates mtDNA release after viral infection, we sought to identify VRK2-associated proteins by biochemical purification and quantitative mass spectrometry. Among the 295 candidate proteins, 41 were mitochondrial proteins, including VDAC1 and VDAC3, which have been shown to form oligomeric pores for mtDNA release upon mitostress (Fig. 4A).17 The results of confocal microscopy experiments showed that VRK2 and VDAC1 were colocalized in mitochondria (Fig. 4B). The results of transient transfection and coimmunoprecipitation experiments confirmed that VRK2 interacted with VDAC1, VDAC2, VDAC3, and the VDAC coupling protein PBR but not with the mitochondrial matrix protein PPIF (Fig. 4C). The results of endogenous coimmunoprecipitation experiments indicated that VRK2 was constitutively associated with VDAC and PBR in uninfected cells and that these association were enhanced after HSV-1 infection (Fig. 4D). In the same experiments, VRK2 was not associated with another mitochondrial membrane-associated protein, VISA, which is involved in cytosolic viral RNA-triggered innate immune responses (Fig. 4D). These results suggest that VRK2 is associated with VDAC complexes.
Fig. 4.
VRK2 regulates VDAC-mediated mtDNA release. A Subcellular location analysis of VRK2-associated proteins identified by quantitative mass spectrometry. B Colocalization of VRK2 with VDAC1. HT1080 cells were transfected with the indicated plasmids for 20 h before incubation with MitoTracker for half an hour, and the cells were then fixed for immunostaining before visualization by confocal microscopy. C Association of VRK2 with VDACs. HEK293 cells were transfected with the indicated plasmids for 20 h and were then lysed for coimmunoprecipitation with IgG or an anti-Flag antibody and subsequent immunoblot analysis with the indicated antibodies. D Association of VRK2 with endogenous VDAC. Flag-VRK2 reconstituted Vrk2−/− MLFs (2 × 107) were left uninfected or infected with HSV-1 (MOI = 2) for the indicated times and were then lysed for coimmunoprecipitation with IgG or an anti-Flag antibody and subsequent immunoblot analysis with the indicated antibodies. The gray value ratio of VDAC1 to VRK2 is shown (lower panel). E Effects of VDAC1 deficiency on virus-induced mtDNA release. Control and VDAC1-deficient MLFs (2 × 107) were left uninfected or infected with HSV-1 or EMCV (MOI = 2) for 1 h prior to subcellular fractionation and mtDNA measurement. P5K, mitochondrial fraction; S5K, nonmitochondrial cytosolic fraction. F Effects of VDAC1 deficiency on the transcription of antiviral genes. Control and VDAC1-deficient MLFs (1 × 106) were stimulated with A/Q (10 μM each), infected with HSV-1 or EMCV (MOI = 1), or transfected with HT-DNA (2 μg) or poly(I:C) (2 μg) via Fugene (4 μg) for 6 h prior to qPCR analysis of the indicated genes. The knockout efficiency of Vdac1-gRNA is shown in the right panels. The data shown are the mean ± SD from one representative experiment performed in triplicate (E, F). ns nonsignificant; *P < 0.05; ***P < 0.001 (unpaired t-test)
We further examined the roles of VDAC complexes in virus-induced mtDNA release and innate immune responses. We found that knockout of Vdac1 markedly inhibited virus-induced mtDNA release (Fig. 4E) and transcription of the Ifnb1 gene (Fig. 4F). In the same experiments, VDAC1 deficiency had no marked effects on transcription of the Ifnb1 gene induced by transfection of HT-DNA or poly(I:C) (Fig. 4F). In addition, transcription of the Ifnb1 gene induced by A/Q was not inhibited by knockout of Vdac1 (Fig. 4F). These results suggest that VDAC complexes are essential for virus-induced mtDNA release and innate immune responses.
VRK2 facilitates mtDNA binding to VDACs
It has been reported that VRK2 functions as a kinase and phosphorylates various substrates involved in different biological processes, including nuclear mobility and autophagy.39,40 To investigate whether the kinase activity of VRK2 is required for virus-triggered innate immune responses, we reconstituted Vrk2−/− MLFs with VRK2 or its kinase-inactive mutant (VRK2-KD). The qPCR results showed that reconstitution with either VRK2 or VRK2-KD rescued HSV-1- and EMCV-induced transcription of the Ifnb1 and Cxcl10 genes in Vrk2−/− MLFs (Fig. 5A). In addition, both VRK2 and VRK2-KD were minimally phosphorylated, and their phosphorylation levels were not affected after viral infection (Supplementary Fig. S5A). VRK2 had no effects on either the expression level or phosphorylation of VDAC1 in either the presence or absence of viral infection (Supplementary Fig. S5B, C). These results suggest that VRK2 regulates mtDNA release and innate immune responses independent of its kinase activity.
Fig. 5.
VRK2 facilitates mtDNA binding to VDACs. A Vrk2−/− MLFs were stably reconstituted with VRK2 or VRK2-KD and were then left uninfected or infected with HSV-1 or EMCV (MOI = 1) for 6 h before qPCR analysis of the indicated genes. The levels of VRK2 and VRK2-KD in the cells were determined by immunoblotting. The data shown are the mean ± SD from one representative experiment performed in triplicate. B Effects of VRK2 on binding of mtDNA to VDAC1. HEK293 cells were transfected with the indicated plasmids for 20 h. The lysates were incubated with biotinylated mtDNA and streptavidin-Sepharose for 2 h. The bead-bound proteins were analyzed by immunoblotting with the indicated antibodies. C Effects of VRK2 on VDAC1 self-association or interaction with PBR. HEK293 cells were transfected with the indicated plasmids for 20 h and were then lysed for coimmunoprecipitation with an anti-HA antibody and subsequent immunoblot analysis with the indicated antibodies. D Effects of VRK2 deficiency on mtDNA binding to VDAC1. Control and VRK2-KO HEK293 cells were transfected with HA-VDAC1 for 20 h, and cells were then lysed for mtDNA pulldown assays as described in (B). E Effects of VRK2 deficiency on virus-induced endogenous mtDNA binding to VDAC1. WT and Vrk2−/− MLFs (2 × 107) were left uninfected or infected with HSV-1 or EMCV (MOI = 2) for an hour, and cells were then lysed for immunoprecipitation with anti-VDAC or anti-VRK2 antibodies and subsequent DNA extraction for qPCR analysis of the indicated genes. The data shown are the mean ± SD from one representative experiment performed in triplicate. ns nonsignificant; ***P < 0.001 (unpaired t-test). F Effects of VRK2 deficiency on virus-induced VDAC oligomerization. Vrk2−/− and WT MLFs (1 × 106) were left uninfected or infected with HSV-1 or EMCV (MOI = 2) for 1 h, and cells were then lysed and crosslinked with EGS (100 μM). The samples were analyzed by immunoblotting with the indicated antibodies. NSB nonspecific band
It has been reported that mitostress triggers the binding of mtDNA to VDACs, leading to VDAC oligomerization and the formation of pores in the mitochondrial outer membrane (MOM) for mtDNA release from the mitochondrial matrix into the cytosol.17 We found that overexpression of VRK2 markedly enhanced mtDNA binding to VDAC1 (Fig. 5B) but had no marked effects on VDAC self-association or the interaction of VDAC with PBR (Fig. 5C). Conversely, VRK2 deficiency impaired mtDNA binding to VDAC1 in pulldown assays (Fig. 5D). Furthermore, VRK2 deficiency inhibited virus-induced binding of endogenous mtDNA to VDAC1 (Fig. 5E) as well as VDAC oligomerization (Fig. 5F). These results suggest that VRK2 facilitates mtDNA binding to VDACs and subsequent VDAC oligomerization.
VRK2 is essential for mtDNA-mediated innate immune responses triggered by nonviral factors
mtDNA release triggered by nonviral factors is associated with autoimmune and neurodegenerative diseases.18,41,42 Under physiological conditions, the cytosolic Ca2+ concentration remains at a relatively low level; however, upon exposure to different stimuli, Ca2+ is transported into the cytosol from intracellular stores or the extracellular environment. It has been shown that an increase in cytosolic Ca2+ also results in an increase in mitochondrial Ca2+, leading to mitostress.17,43 We found that LF, a widely used transfection reagent that causes endolysosomal damage to promote the delivery of nucleic acids into the cytosol,44 caused an increase in cytosolic Ca2+ increase as well as mitostress, as indicated by the increase in ROS production and decrease in MMP, in Raw264.7 cells (Fig. 6A). In these experiments, VRK2 deficiency had no marked effects on the LF-triggered increase in cytosolic Ca2+ or mitostress (Fig. 6A). LF treatment induced mtDNA release from mitochondria into the cytosol in WT cells, which was impaired in Vrk2−/− cells (Fig. 6B). Consistent with these findings, knockout of Vdac1 impaired LF-induced mtDNA release in MLFs (Fig. 6C). In addition, LF potently induced the transcription of the Ifnb1 and Cxcl10 genes, which was impaired in VRK2-, cGAS-, and STING/MITA-deficient but not VISA/MAVS-deficient cells (Fig. 6D) and was also impaired in VDAC1-deficient cells (Fig. 6E). Administration of LF to WT mice increased the CXCL10 level in serum, and this increase was dramatically inhibited in Vrk2−/− mice (Fig. 6F).
Fig. 6.
VRK2 is essential for the mtDNA-mediated innate immune response triggered by Lipofectamine. A. LF-triggered changes in cytosolic Ca2+ levels, ROS production, and MMP. Control and VRK2-deficient Raw264.7 cells (2 × 106) were left untreated or treated with LF (20 μg/ml) for the indicated times before staining with Fluo 3-AM (5 μM), DCFH-DA (2 μM), or JC-10 (4 μM) for 20 min and subsequent fluorescence detection to assess cytosolic Ca2+ levels, ROS production, or MMP. B LF-induced mtDNA release into the cytosol. WT and Vrk2−/− MLFs (2 × 107) were left untreated or treated with LF (20 μg/ml) for an hour prior to subcellular fractionation and mtDNA measurement. P5K, mitochondrial fraction; S5K, nonmitochondrial cytosolic fraction. C Effects of VDAC1 deficiency on LF-induced mtDNA release. Control and VDAC1-deficient MLFs (2 × 107) were left untreated or treated with LF (20 μg/ml) for an hour prior to subcellular fractionation and mtDNA measurement. P5K, mitochondrial fraction; S5K, nonmitochondrial cytosolic fraction. D Effects of STING, VISA, or VRK2 deficiency on LF-induced transcription of antiviral genes. The indicated Raw264.7 cells (1 × 106) were treated with LF (8 μg/ml) for 6 h prior to qPCR analysis of the indicated genes. E Effects of VDAC1 deficiency on LF-induced transcription of antiviral genes. Control and VDAC1-deficient MLFs (1 × 106) were left untreated or treated with LF (8 μg/ml) for 6 h prior to qPCR analysis of the indicated genes. F Effects of VRK2 deficiency on LF-induced production of CXCL10 in the serum of mice. WT and Vrk2−/− mice were left untreated (n = 3 mice per genotype) or intraperitoneally injected with LF (200 μg per mouse) (n = 4 mice per genotype) for 5 h before serum cytokines were measured by ELISA. The data shown are the mean ± SD from one representative experiment performed in triplicate (B–F). ns nonsignificant; *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired t-test)
As shown in Fig. 3A, the transfection reagent Fugene did not induce the transcription of antiviral genes. Consistent with this finding, Fugene did not cause an increase in cytosolic Ca2+ (Supplementary Fig. S6A). In contrast, treatment of cells with the Ca2+ ionophore PMA induced the release of mtDNA and the transcription of antiviral genes (Supplementary Fig. S6B, C). H2O2 also caused a decrease in MMP (Supplementary Fig. S6D), and deficiency of VRK2 or VDAC1 impaired H2O2-induced transcription of the Ifnb1 and Cxcl10 genes (Supplementary Fig. S6E). These results collectively suggest that VRK2 is essential for Ca2+-induced mtDNA release and innate immune responses triggered by nonviral mitostress inducers.
Discussion
For many years, it has been widely believed that innate immune responses to viruses are mostly induced by recognition of viral nucleic acids by cellular PRRs.1,6 Accumulated evidence currently suggests that viral infection also causes mitostress, mtDNA release into the cytosol, and cGAS-mediated innate immune responses.13,45,46 These collectively contribute to the induction of robust innate immune responses for viral clearance. Other nonviral factors that cause mitostress also induce innate immune responses via the mtDNA-cGAS pathway.47 While the mechanisms of viral nucleic acid-triggered innate immune responses have been extensively investigated, the mechanism by which mitostress induces innate immune responses is not well understood. In this study, we identified VRK2 as an essential regulator of mtDNA release and innate immune responses after viral infection and upon mitostress induced by nonviral factors.
We found that VRK2 deficiency dramatically inhibited the phosphorylation of TBK1 and IRF3 as well as antiviral gene transcription induced by different viruses, including HSV-1, MCMV, and EMCV. In vivo experiments showed that VRK2-deficient mice produced lower amounts of serum cytokines and were more susceptible to HSV-1 and EMCV infection. These results suggest that VRK2 plays an important role in robust innate immune responses to different types of DNA and RNA viruses. Although VRK2 plays a role in innate immune responses to various DNA and RNA viruses, VRK2 deficiency had no marked effects on antiviral gene transcription triggered by transfection of viral DNA and RNA analogs, suggesting that VRK2 functions independent of the classical viral nucleic acid-triggered innate immune pathways. Several lines of evidence suggested that VRK2 plays a crucial role in both mitostress and mtDNA-mediated innate immune responses. First, HSV-1 and EMCV infection induced mitostress and mtDNA release into the cytosol, which was impaired in VRK2-deficient cells. Second, both VRK2 deficiency and mtDNA depletion inhibited antiviral gene transcription induced by HSV-1 and EMCV. Third, the transfection reagent LF, which causes endolysosomal damage,44 caused Ca2+ influx, mtDNA release into the cytosol, and cGAS-STING-mediated innate immune responses. Our results suggest that VRK2 is required for LF-triggered mtDNA release and subsequent innate immune responses.
In our study, we examined the roles of VRK2 in innate immune responses to a limited number of viruses, including HSV-1, MCMV, EMCV, and SeV. Our results suggest that VRK2 is important for innate immune responses to HSV-1, MCMV, and EMCV but not SeV. This idea is consistent with our results showing that SeV failed to induce mitostress and mtDNA release into the cytosol in host cells. Previously, other viruses, such as influenza A virus, dengue virus, and severe fever with thrombocytopenia syndrome virus, have been shown to cause mtDNA release into the cytosol after infection.13,45,46,48 It is possible that VRK2 also contributes to innate immune responses to these viruses.
Previously, it has been reported that several types of pores spanning mitochondrial membranes, including pores formed by oligomerized VDACs and BBFPs formed by BAX/BAK, mediate the release of mtDNA of distinct sizes under certain conditions.17,31,37 Our results suggest that VDAC complexes but not BBFPs are specifically required for mitostress-induced innate immune responses. It has been demonstrated that cleavage of cGAS by activated caspases during apoptosis is responsible for the inability of mtDNA released through BBFPs to trigger cGAS-dependent innate immune responses.49
Reconstitution experiments indicated that VRK2 regulates mtDNA release and innate immune responses independent of its kinase activity. Overexpression of VRK2 markedly enhanced mtDNA binding to VDAC1 but had no marked effects on VDAC self-association. Conversely, VRK2 deficiency impaired mtDNA binding to VDAC1 and VDAC oligomerization. The simplest explanation for these observations is that VRK2 facilitates mtDNA binding to VDACs and subsequently promotes VDAC oligomerization/pore formation for mtDNA release into the cytosol upon viral infection or mitostress. Recently, it has been shown that oxidative stress promotes mtDNA binding to VDACs, leading to VDAC pore formation in the MOM and subsequent mtDNA release.17 It will be interesting to investigate whether VRK2 is involved in these processes in the future.
In conclusion, our findings identified a critical role of VRK2 in mediating mtDNA release and cGAS-mediated innate immune responses upon viral infection and mitostress. Since mtDNA-triggered innate immune responses contribute to host defense against various viruses as well as to certain autoimmune and inflammatory diseases, the finding that VRK2 is involved in these processes facilitates the understanding of the complicated mechanisms underlying general innate immune responses and reveals potential targets for intervention in certain immunological and infectious diseases.
Supplementary information
Acknowledgements
This work was supported by grants from the State Key R&D Program of China (2017YFA0505800), the National Natural Science Foundation of China (31830024, 31922021, 31771555, 31630045, and 31801188), and the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-071).
Author contributions
W.R.H., M.M.H., and H.B.S. conceived and designed the study. W.R.H., L.B.C., Y.L.Y., and D.H. performed the experiments. All authors analyzed the data. W.R.H., M.M.H., and H.B.S. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Ming-Ming Hu, Email: mmhu@whu.edu.cn.
Hong-Bing Shu, Email: shuh@whu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00673-0.
References
- 1.Hu MM, Shu HB. Innate immune response to cytoplasmic DNA: mechanisms and diseases. Annu Rev. Immunol. 2020;38:79–98. doi: 10.1146/annurev-immunol-070119-115052. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Hu MM, Shu HB. Cytoplasmic mechanisms of recognition and defense of microbial nucleic acids. Annu Rev. Cell Dev. Biol. 2018;34:357–379. doi: 10.1146/annurev-cellbio-100617-062903. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe. 2015;18:157–168. doi: 10.1016/j.chom.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm CK, et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 2012;13:737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017;17:363–375. doi: 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–1536. doi: 10.1126/science.aav4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sliter DA, et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–262. doi: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Nezu J, Oku A, Jones MH, Shimane M. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics. 1997;45:327–331. doi: 10.1006/geno.1997.4938. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Yue W. VRK2, a candidate gene for psychiatric and neurological disorders. Mol. Neuropsychiatry. 2018;4:119–133. doi: 10.1159/000493941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco S, Santos C, Lazo PA. Vaccinia-related kinase 2 modulates the stress response to hypoxia mediated by TAK1. Mol. Cell Biol. 2007;27:7273–7283. doi: 10.1128/MCB.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanco S, Sanz-Garcia M, Santos CR, Lazo PA. Modulation of interleukin-1 transcriptional response by the interaction between VRK2 and the JIP1 scaffold protein. PLoS One. 2008;3:e1660. doi: 10.1371/journal.pone.0001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez IF, Perez-Rivas LG, Blanco S, Castillo-Dominguez AA, Lozano J, Lazo PA. VRK2 anchors KSR1-MEK1 to endoplasmic reticulum forming a macromolecular complex that compartmentalizes MAPK signaling. Cell Mol. Life Sci. 2012;69:3881–3893. doi: 10.1007/s00018-012-1056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez-Cedeira M, Lazo PA. Human VRK2 (vaccinia-related kinase 2) modulates tumor cell invasion by hyperactivation of NFAT1 and expression of cyclooxygenase-2. J. Biol. Chem. 2012;287:42739–42750. doi: 10.1074/jbc.M112.404285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MM, et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–569. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Hu MM, Liao CY, Yang Q, Xie XQ, Shu HB. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J. Exp. Med. 2017;214:973–989. doi: 10.1084/jem.20161015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo WW, et al. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat. Immunol. 2016;17:1057–1066. doi: 10.1038/ni.3510. [DOI] [PubMed] [Google Scholar]
- 28.Lian H, et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity. 2018;49:438–448.e435. doi: 10.1016/j.immuni.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Shu HB. Dephosphorylation of cGAS by PPP6C impairs its substrate binding activity and innate antiviral response. Protein Cell. 2020;11:584–599. doi: 10.1007/s13238-020-00729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnett KC, et al. Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA. Cell. 2019;176:1432–1446.e1411. doi: 10.1016/j.cell.2019.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rongvaux A, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu MM, et al. Virus-induced accumulation of intracellular bile acids activates the TGR5-beta-arrestin-SRC axis to enable innate antiviral immunity. Cell Res. 2019;29:193–205. doi: 10.1038/s41422-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu ZS, et al. G3BP1 promotes DNA binding and activation of cGAS. Nat. Immunol. 2019;20:18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar CS, Dey D, Ghosh S, Banerjee M. Breach: host membrane penetration and entry by nonenveloped viruses. Trends Microbiol. 2018;26:525–537. doi: 10.1016/j.tim.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Riley JS, Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020;21:e49799. doi: 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monsalve DM, Merced T, Fernandez IF, Blanco S, Vazquez-Cedeira M, Lazo PA. Human VRK2 modulates apoptosis by interaction with Bcl-xL and regulation of BAX gene expression. Cell Death Dis. 2013;4:e513. doi: 10.1038/cddis.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birendra K, et al. VRK2A is an A-type lamin-dependent nuclear envelope kinase that phosphorylates BAF. Mol. Biol. Cell. 2017;28:2241–2250. doi: 10.1091/mbc.E17-03-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirata N, et al. Functional characterization of lysosomal interaction of Akt with VRK2. Oncogene. 2018;37:5367–5386. doi: 10.1038/s41388-018-0330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caielli S, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 2016;213:697–713. doi: 10.1084/jem.20151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lood C, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Degors IMS, Wang C, Rehman ZU, Zuhorn IS. Carriers break barriers in drug delivery: endocytosis and endosomal escape of gene delivery vectors. Acc. Chem. Res. 2019;52:1750–1760. doi: 10.1021/acs.accounts.9b00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun B, et al. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci. Rep. 2017;7:3594. doi: 10.1038/s41598-017-03932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriyama M, Koshiba T, Ichinohe T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 2019;10:4624. doi: 10.1038/s41467-019-12632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benmerzoug S, et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018;9:5226. doi: 10.1038/s41467-018-07425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, et al. SFTSV infection induces BAK/BAX-dependent mitochondrial DNA release to trigger NLRP3 inflammasome activation. Cell Rep. 2020;30:4370–4385.e4377. doi: 10.1016/j.celrep.2020.02.105. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46:393–404. doi: 10.1016/j.immuni.2017.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.