Abstract
Haploidentical stem cell transplantation (haplo-SCT) achieves superior or at least comparable clinical outcomes to HLA-matched sibling donor transplantation (MSDT) in treating hematological malignancies. To define the underlying regulatory dynamics, we analyzed time courses of leukemia burden and immune abundance of haplo-SCT or MSDT from multiple dimension. First, we employed two nonirradiated leukemia mouse models which carried human AML-ETO or MLL-AF9 fusion gene to establish haplo-identical and major histocompatibility (MHC)-matched transplantation models and investigated the immune cell dynamic response during leukemia development in vivo. We found that haplo-matching the MHCs of leukemia cells with recipient mouse T cells prolonged leukemic mice survival and reduced leukemia burden. The stronger graft-versus-leukemia activity in haplo-SCT group mainly induced by decreased apoptosis and increased cytotoxic cytokine secretion including tumor necrosis factor–α, interferon-γ, pore-forming proteins and CD107a secreted by T cells or natural killer cells. Furthermore, we conducted a prospective clinical trial which enrolled 135 patients with t(8;21) acute myeloid leukemia that displayed minimal residual disease before transplantation and underwent either haplo-SCT or MSDT. The results showed that the haplo-SCT slowed the kinetics of the leukemia burden in vivo and reduced the cumulative incidence of relapse compared with MSDT. Ex vivo experiments showed that, 1 year after transplantation, cytotoxic T lymphocytes from the haplo-SCT group had higher cytotoxicity than those from the MSDT group during the same period. Our results unraveled the role of immune cells in superior antileukemia effects of haplo-SCT compared with MSDT.
Keywords: Graft-versus-leukemia, Haplo-SCT, MSDT, AML, MRD
Subject terms: Acute myeloid leukaemia, Allotransplantation
Reinforced immune cell function contributes to superior anti-leukemia effects of haplo-SCT compared with MSDT based on mouse models and clinical study.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is a curative therapy for hematological malignancies.1–3 Evidence from a number of studies have shown that allo-SCT was superior to chemotherapy alone as a post-remission treatment for reducing the cumulative incidence of relapse (CIR).2,4,5 This superiority was ascribed to graft-versus-leukemia (GVL) effects mediated by alloreactive donor T cells and natural killer (NK) cells.6–8 In recent years, haplo-identical SCT (haplo-SCT) has achieved outcomes comparable even superior in certain circumstance to that human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT) in treating patients with acute leukemia. For example, when patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia that exhibited pretransplantation measurable residual disease or patients with AML in first complete remission (CR1) were treated with haplo-SCT, they achieved a lower CIR or cumulative incidences of MRD positivity posttransplantation than those treated with MSDT.9–11 These studies indicate that haplo-SCT might exhibit stronger antileukemia activity compared with MSDT, from the single endpoint of clinical events. However, the regulatory dynamics of immune cells from the allografts during antileukemia process in haplo-SCT or MSDT are still poorly understood.
Since the pioneering demonstration of murine leukemia elimination by homologous bone marrow (BM) transplantation by Barnes et al.,12 traditional GVL mouse models have provided an in vivo platform to explore antileukemia biological processes in transplant because of the well-understood genetic background in different mouse strains and reliable evaluation standards in clinical phenotype. However, the GVL effects in traditional mouse models might be masked by the onset of graft-versus-host disease (GVHD), when leukemia cells and allografts are infused simultaneously. Moreover, the murine leukemia cell lines frequently used to create GVL mouse models (e.g., A20 and P815) originated from mice, and thus, they present limitations for understanding human leukemia, due to the different genetic and biological characteristics.13,14 Therefore, we employed two kinds of mouse models: mouse leukemia was induced by transplanting leukemic cells that carried either the human AML-ETO genetic mutation or the human MLL-AF9 genetic mutation.15,16 These murine AML-ETO or MLL-AF9 leukemic cells were transplanted into either MHC-matched or haplo-identical recipient mice to imitate the antileukemia situation in the clinical context. We hypothesized that these mouse models could provide platform to peruse immune cells reaction when the MHC molecules on T cell were matched or haplo-matched with leukemia cells. It might unravel the mechanisms of superior antileukemia activity from haplo-SCT compared to MSDT without the interference from GVHD.
Besides the mouse models, we also conducted a prospective clinical trial in which the MRD were monitored continuously to reveal the kinetics of the leukemia load of haplo-SCT or MSDT in vivo. The reduction of the leukemia load after allo-SCT compared to pre-transplantation could reflect the GVL effects of immune cells. Therefore, monitoring the dynamic changes of MRD could provide time-resolved evidence of antileukemia activity in haplo-SCT or MSDT. Previous studies showed that patients with t(8;21) AML at high risk could be distinguished based on MRD detection.17,18 Our previous prospective, multicenter study indicated that allo-SCT benefited patients at high risk (defined as a >3-log reduction in RUNX1/RUNX1T1 transcripts, also termed as major molecular remission, MMR or MRD positivity).18 Recommendations from the European LeukemiaNet and from China currently suggest that allo-SCT should be performed in patients with t(8;21) AML that cannot achieve a MMR2,19. Moreover, the MRD of t(8;21) AML could be more accurately determined by leukemia-specific genes RUNX1/RUNX1T1 which is assessed by real-time polymerase chain reactions (RT-PCR).20–22 Thus, we continuously evaluated RUNX1/RUNX1T1 of patients with t(8;21) AML from pre-transplantation to post transplantation to compare the GVL effects between haplo-SCT and MSDT.
In this study, we demonstrated that haplo-matching the MHCs of leukemia cells with recipient mouse T cells prolonged leukemic mouse survival compared to MHC-matching group, mainly via reduced T cell apoptosis and enhanced T cell cytokine secretion, including interferon-γ (IFN-γ) and tumor necrosis factor–α (TNF-α). The NK cells also contributed to stronger GVL effects in the haplo-matched transplantation group evidenced by increased cytokine secretion such as CD107a, IFN-γ, and pore-forming proteins (Perforin). Clinically, we conducted a prospective clinical trial which enrolled 135 patients with t(8;21) AML that displayed MRD before transplantation and underwent either haplo-SCT or MSDT. We monitored the RUNX1/RUNX1T1 transcript levels and found that, after 1 year, patients that received haplo-SCT exhibited a lower leukemia load and higher T-cell cytotoxic activity compared to patients that received MSDT.
Materials and methods
Mice
C57BL/6 (H-2b), BALB/c (H-2d), and CB6F1 (H-2b/d) mice (8–10 weeks old) were purchased from Beijing Vital Laboratory Animal Technology Company, Ltd. (Beijing, China). All mice were maintained in the specific pathogen-free animal facility of Peking University People’s Hospital. All experiments were performed according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
AML-ETO- and MLL-AF9-induced AML mouse models
GFP-expressing AML-ETO primary mouse AML cells were kindly provided by Professor Yue-Ying Wang of Rui-Jin Hospital, Shanghai Jiao Tong University School of Medicine.15 We divided these mice into two groups. The MHC-matched group received a syngenetic transplantation: that is, the leukemia cells (H-2d) were transplanted into BALB/c mice (H-2d). For the haplo-SCT group, the leukemia cells (H-2d) were transplanted into CB6F1 (H-2b/d) mice (Fig. 1a).
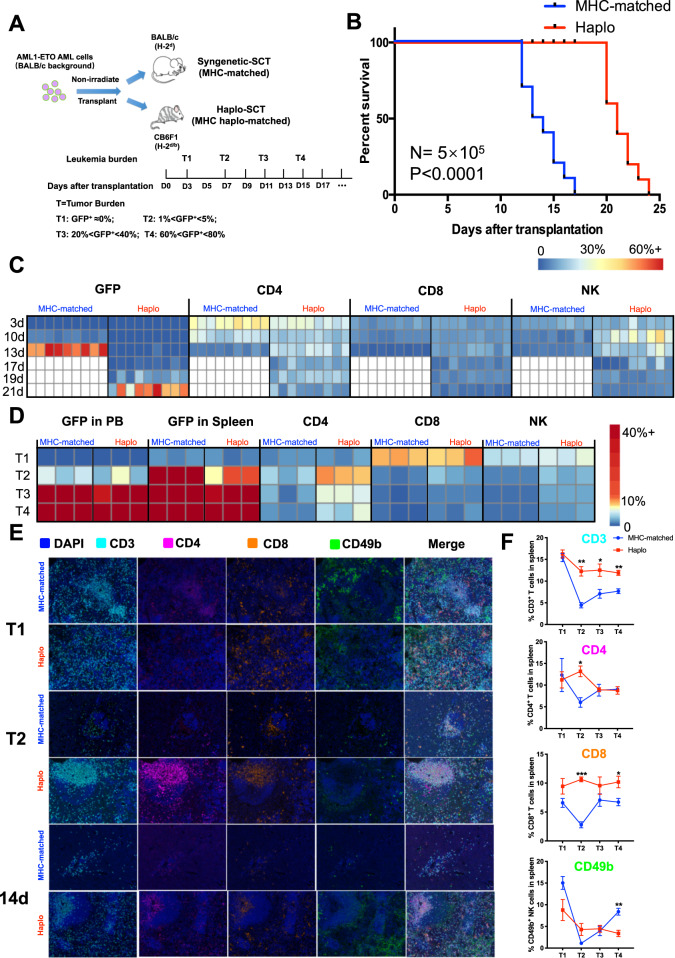
Fig. 1.
Leukemia progression was delayed with haplo-SCT in AML-ETO leukemic mice. a Schematic illustration shows the establishment of nonirradiated leukemia mouse models which carried human AML-ETO fusion gene of haplo-identical and major histocompatibility (MHC)-matched transplantation models. b Kaplan–Meier survival curves compare survival between MHC-matched and haplo-SCT AML-ETO mice. The median survival was 13.5 days in the MHC-matched group and 21 days in the haplo group. Mantel-Cox test; n = 10. Heat map of cluster frequency from peripheral blood (c) or spleen (d) including leukemia cells (GFP+), CD3+CD4+ T cells, CD3+CD8+ T cells and CD3-CD49b+ NK cells. There are 6 animals (for peripheral blood) or 3 animals (for spleen) in each group per timepoint. The white part represents there is no lived mice in the MHC-matched group at that timepoint. e Representative multispectral immunofluorescence images of FFPE spleen sections in different tumor burden (T1 and T2) or timepoint after transplantation. f Quantified immune cells (proportion of all cells) of MHC-matched or haplo group in different tumor burden. n = 3. Error bars represent the mean ± SEM, ***P < 0.001, **P < 0.01, *P < 0.05
GFP-expressing MLL-AF9 primary mouse AML cells were kindly provided by Professor Jianfeng Zhou from Tongji Medical College, Huazhong University of Science and Technology. The generation of MLL-AF9 leukemia mouse models was described in detail previously.16 As described above, we divided mice into two groups. The MHC-matched group comprised syngenetically transplanted C57BL/6 (H-2b) mice, and the haplo-matched group comprised CB6F1 (H-2b/d) mice transplanted with H-2b leukemia cells (Fig. 3a).
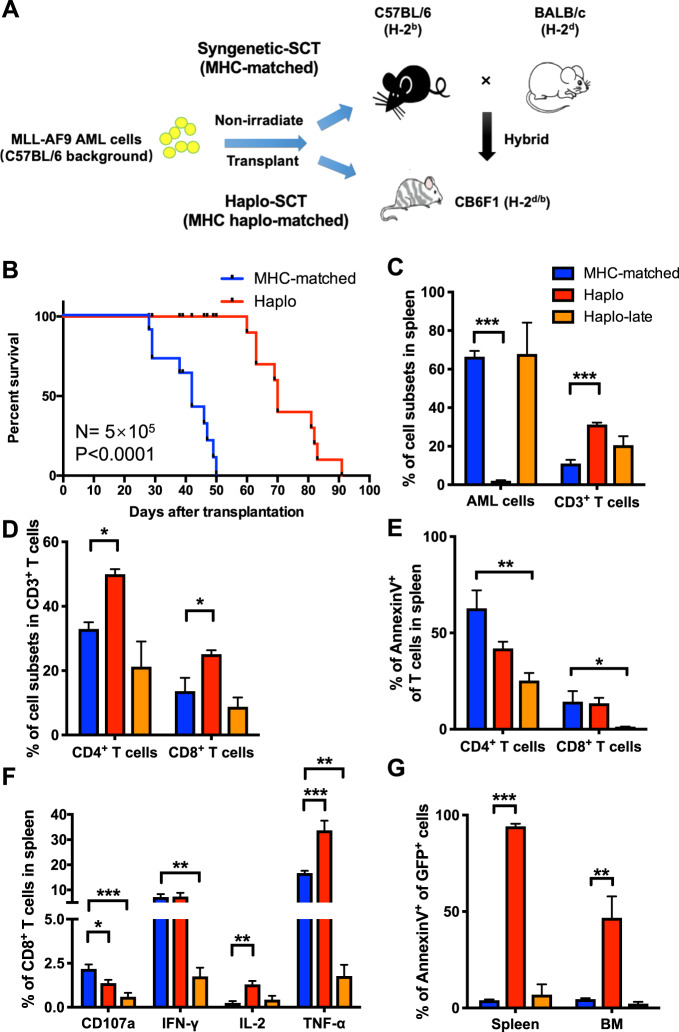
Fig. 3.
Enhanced CTL function in the haplo-SCT group delayed leukemia progression in MLL-AF9 mouse models. a Schematic illustration shows the establishment of nonirradiated leukemia mouse models which carried human MLL-AF9 fusion gene of haplo-identical and MHC-matched transplantation models. b Kaplan–Meier survival curves compare survival between MHC-matched and haplo-SCT MLL-AF9 mice. The median survival was 42 days in the MHC-matched group and 70 days in the haplo group. Mantel-Cox test; n = 10. c Comparison of the percentages of AML cells and T cells in the spleen of MHC-matched, haplo and haplo-late group of MLL-AF9 leukemic mice. d Comparison of the percentages of CD4+ T cells and CD8+ T cells in the splenic CD3+ T cell subsets of MHC-matched, haplo and haplo-late group of MLL-AF9 leukemic mice. e Comparison of the percentages of apoptotic CD4+ T cell and CD8+ T cells in MHC-matched, haplo and haplo-late group. f Cytotoxic cytokine secretion of CD8+ T cells in MHC-matched, haplo and haplo-late group. g Percentages of apoptotic leukemia cells from the spleens or the BM of the MHC-matched, haplo and haplo-late groups. Error bars represent the mean ± SEM, ***P < 0.001, **P < 0.01, *P < 0.05
Transplantation of leukemia cells to create mouse models
Leukemia cells were thawed, and live cells were counted by staining with trypan blue. The ratio of GFP+ cells was detected with flow cytometry. The appropriate amounts of live GFP+ leukemia cells were transplanted into nonirradiated recipient mice, and mouse survival was monitored. A total of 5 × 105 live GFP+ AML-ETO leukemia cells were transplanted into nonirradiated recipient mice in immune functional analyze. Similarly, 1 × 106 live GFP+ MLL-AF9 leukemia cells were transplanted into nonirradiated recipient mice in immune functional analyze.
Multiplex immunofluorescence
Formalin fixed, paraffin embedded (FFPE) spleen sections were processed for 5-plex immunofluorescence. The information of antibodies used to detect T cells and NK cells were supplied in Supplementary Table S8. The original protocol is described detailly in previous studies.23,24
Cell frequency heat map generation
Specified subsets including leukemia cells (GFP+), CD3+CD4+ T cells, CD3+CD8+ T cells, CD3-CD49b+ NK cells were manually gated from peripheral blood, spleen and BM. Cluster frequencies were calculated as a percent of total live nucleated single cells within that subset (excluding erythrocytes). Heat maps of the resulting cluster frequencies were generated in R by pheatmap package.
Flow cytometry analysis
We stained the surfaces of human samples with directly-conjugated monoclonal antibodies. Antibodies were incubated with human samples for 20 min at room temperature. We stained the surfaces of mouse samples with directly-conjugated monoclonal antibodies for 30 min at 4 °C. After incubation, the cells were washed and resuspended in phosphate buffered saline before flow cytometry analysis. After resuspension, intracellular staining was performed with a fixation/permeabilization kit (cat. 562574, BD Bioscience, CA, USA) according to the manufacturer’s instructions. Detailed information on the antibodies used is presented in Supplementary Table S8.
Patients and study design
This prospective study was a substudy of a parent trial (ChiCTR-OCH-12002406). Patients were assigned to groups and transplanted with haplo-identical donor cells (haplo-SCT) or HLA-matched sibling donor (MSD) cells, based on donor availability (biological randomization).25
The study inclusion criteria were as follows: (1) ≤60 years old; (2) patients with t(8;21) AML that had achieved CR before transplantation, but did not achieve a negative pretransplant MRD, with or without mutations in the cytokine receptor, c-KIT; and (3) no contraindications to allo-SCT.
This study was approved by the Institutional Review Board of Peking University. Written informed consent was obtained from all patients and donors, in accordance with the Declaration of Helsinki.
Kinetics of RUNX1/RUNX1T1 monitoring and c-KIT mutation screening
We monitored MRD and screened BM samples for c-KIT mutations, as previously described.18,20
Treatment procedure
All patients were treated similarly during induction and/or consolidation. Each patient underwent an unmanipulated allo-SCT from an MSD or haplo-identical donor, according to protocols described previously.3,26
Definitions and end points
Relapse was defined as previously described.3,11 Patients with any level of residual disease (detectable RUNX1/RUNX1T1 transcripts) after transplantation were considered MRD-positive. We evaluated NRM, relapse, LFS, and OS, as defined previously.3,11,27 Acute and chronic GVHD were diagnosed and graded according to standard criteria27. The primary study end point was the CIR. The secondary end points were the cumulative incidences of NRM and the probabilities of LFS and OS.
Differences in CIR between MSDT and haplo-SCT groups were evaluated with the cumulative incidence approach and a one-sided confidence interval (CI). Briefly, we calculated the difference between groups in the Kaplan–Meier estimate of the 5-year CIR. With a planned sample size of 48 MSDT patients and 87 haplo-SCT patients, 80% power can be achieved against the hypothesis of a 20% absolute increase in LFS after haplo-SCT (73%) from 48% of patients with pre-HSCT MRD positive leukemia free survived after MSDT at a significance level of P = 0.05 in the Student’s one-tailed t test9.
Human cell culture
Human monocytic leukemia cells (THP-1) cells were maintained in our laboratory (purchased from ATCC) and characterized by the Beijing Center for Physical & Chemical Analysis (Beijing, China). THP-1 cells were cultured in RPMI-1640 medium (cat. C11875500BT, Thermo Fisher Scientific, MA, USA) containing 10% FBS (cat. 04-001-1ACS, Biological Industries, Kibbutz Beit-Haemek, Israel), 100 U/ml penicillin, and 100 μg/ml streptomycin. THP-1 cells were infected with GFP-expressing control lentivirus purchased from Sangon Biotech (Shanghai, China).
PBMCs isolated from patients were cultured in IMDM medium (cat. 12440053, Thermo Fisher Scientific, MA, USA) containing 10% BIT Serum Substitute (cat. 9500, STEMCELL Technologies, BC, Canada), and rhIL-2 was added at 100 U/ml.
Functional assays for human T cells
PBMCs were collected from recipients that had received haplo-SCT or MSDT. PBMCs (1 × 106 cells per ml) were stimulated with Dynabeads Human T-Activator CD3/CD28 (cat. 11131D, Thermo Fisher Scientific, MA, USA) and incubated overnight at 37 °C, 5% CO2. Next, PBMCs were cocultured with THP-1 cells at an effector/target ratio of 10:1 for 4–6 h. To investigate cytokine release, we added a Golgi Plug (cat. 555029, BD Bioscience, CA, USA) to the PBMCs together with the THP-1 cells and cultured them for 4–6 h. To detect CD107a expression, we added the CD107a antibody to the PBMCs together with the THP-1 cells and cultured them for 4–6 h.
Statistical analyses
We compared patient characteristics between the two groups with the Mann–Whitney test for continuous variables and with the χ2 statistic for categorical data. The CIR and NRM values were calculated with competing risk analyses. Survival probabilities, including LFS and OS, were estimated with the Kaplan–Meier method. In univariate analyses, potential prognostic factors were evaluated with the log-rank test. In multivariate analyses, Cox proportional hazards regression was performed to evaluate the association between risk factors and outcomes. The univariate analyses were performed to evaluate associations with recipient age, sex, disease stage, c-KIT mutation, pre-transplant MRD status, transplant modality, and GVHD. All factors that showed P values < 0.1 in the univariate analyses were included in the multivariate regression. P values < 0.05 were considered statistically significant. All reported P values were based on two-sided hypothesis tests. Data analyses were performed with SPSS software and R software.
To analyze Kaplan–Meier survival in mouse models, we performed a log-rank (Mantel-Cox) test to determine P values. One-way ANOVA was used for all comparisons among more than two groups in the mouse experiments. Student’s t test was used for two groups analyses. The Mann–Whitney test was performed for all analyses of human data. Data are expressed as the mean ± SEM. P values < 0.05 were considered statistically significant.
Results
Enriched immune cells significantly delayed leukemia progression of AML-ETO leukemic mice in haplo-SCT
Previous clinical studies indicated that GVL effects increased with haplo-SCT compared to MSDT.9–11,28–31 That finding prompted us to investigate whether MHC matching between donor T cells and recipient AML cells might affect the antileukemia effects of T cells. To address this question, we employed a nonirradiated AML-ETO leukemia mouse model created by transferring the humanized fusion gene, AML-ETO, into BALB/c mice (Fig. 1a).15 We prepared an MHC-matched group by transplanting the leukemia cells (H-2d) into the BALB/c mice (H-2d), and a haplo-SCT group by transplanting the leukemia cells (H-2d) into CB6F1 (H-2b/d) mice (Fig. 1a). The AML cells were transplanted in serial doses of 5 × 105, 1 × 105, and 1 × 104. We found that haplo-SCT AML-ETO leukemic mice exhibited significantly prolonged survival compared to MHC-matched AML-ETO leukemic mice (Figs. 1b, and S1A, B). To peruse the population dynamics of leukemia and immune cells, we detected the immune cells abundance and leukemia burden in peripheral blood and spleen by flow cytometry analysis or multiplex immunofluorescence analysis separately. After transplanted 5 × 105 leukemia cells in BALB/c or CB6F1 mice, we detected the CD3+CD4+ T cells, CD3+CD8+ T cells, CD3-CD49b+ NK cells and AML cells (GFP+) in peripheral blood every 2 days by flow cytometry (Fig. 1a). Based on the different leukemia stages reflected by the leukemia burden in peripheral blood, we chose 4 tumor burden to sacrifice leukemic mice to detect the immune abundance in spleen by multiplex immunofluorescence and flow cytometry (Fig. 1a). The results showed that the leukemia progression developed rapidly in MHC-matched group compared with Haplo-SCT group (Fig. 1c). There were higher CD3+CD4+ T cells and lower CD3-CD49b+ NK cells in the peripheral blood of MHC-matched mice compared with haplo-SCT mice at the 3 days after transplanted with leukemia cells (Fig. 1c). However, the immune cells were decreased along with the increased leukemia burden in both mouse models (Figs. 1c, d, and S1C). Surprisingly, in the early stage of leukemia (T2: GFP+ leukemia cells were between 1% and 5% in the peripheral blood), the leukemia burden was much higher in the spleen and BM in the MHC-matched group compared with the haplo-SCT group mice (Figs. 1d and S1C). The multiplex immunofluorescence analysis confirmed this result that there were comparable immune cells in the beginning stage (T1) in two mouse models and sharply decreased immune cells in MHC-matched group in the early stage of leukemia (T2) compared with haplo-SCT group (Figs. 1e, f, and S1D). The same timepoint after transplantation (14d) also showed that decreased immune cells in the spleen of the MHC-matched leukemic mice compared with haplo-SCT mice (Fig. 1e). These results indicate that the higher number of immune cells including CD3+CD4+ T cells, CD3+CD8+ T cells and CD3-CD49b+ NK cells in haplo-SCT group delayed the leukemia progression compared with MHC-matched group mice.
Elevated cytotoxic cytokine secretion by T and NK lymphocytes promoted leukemia cell apoptosis in the haplo-SCT group
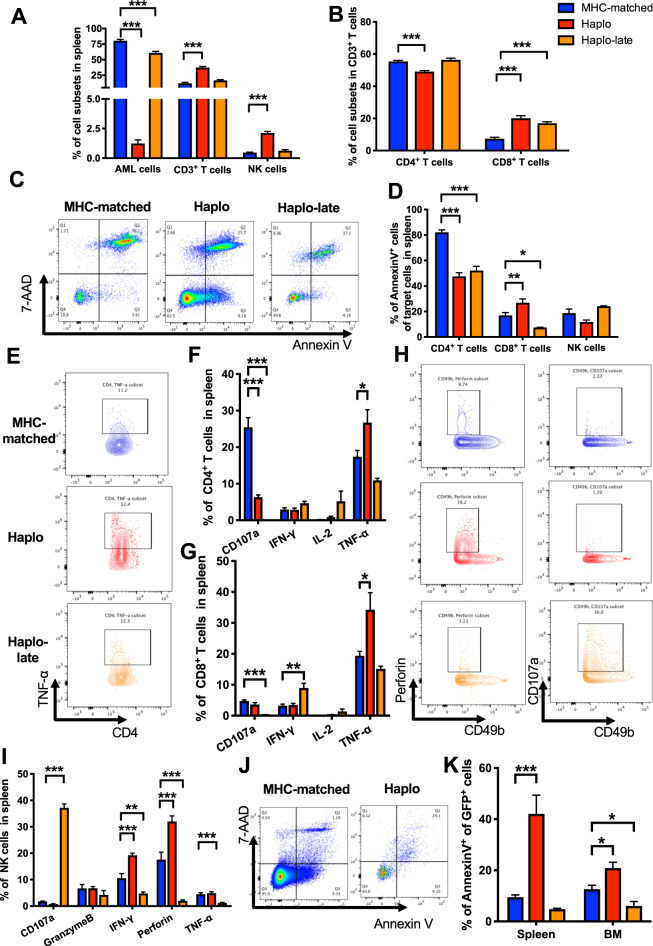
We further compared the immune cell function in haplo-matched leukemic mice with MHC-matched group. Due to the significantly delayed leukemia progression in the haplo-matched group, two stages of immune cells in haplo-matched group were taken into comparison: one is the same timepoint after transplantation compared with MHC-matched group (Haplo); the other is the similar leukemia burden reflected by the ratio of GFP+ cells in the peripheral blood after transplantation compared with MHC-matched group (Haplo-late). The results showed that CD3+ T cells especially the CD3+CD8+ T cells and NK cells from spleen in Haplo group were higher than that in the MHC-matched group (Figs. 2a, b and S2A). Specifically, only the CD3+CD8+ T cells were higher in Haplo-late group compared with the MHC-matched group, which is consistent with the multiplex immunofluorescence results (Figs. 1f and 2b). The CD3+ T cells from BM were lower in haplo group compared with MHC-matched and haplo-late group (Fig. S2B, C). To investigate the reason of higher number of T and NK lymphocytes in haplo-SCT group, we detected the apoptosis of T and NK lymphocytes. The results showed that CD3+CD4+ T cells from spleen in both haplo and haplo-late group were less apoptotic compared with that in MHC-matched group, and the CD3+CD8+ T cells from spleen in haplo-late group were also less apoptotic compared with that in MHC-matched group (Fig. 2c, d). The T cells from BM and NK cells from both spleen and BM exhibited no differences on apoptosis (Figs. 2d and S2D). We further analyzed the cytokine secretion of T and NK lymphocytes to evaluate the function of immune cells in different transplantation models. It is showed that higher level of TNF-α secreted by CD3+CD4+ and CD3+CD8+ T cells from spleen in haplo group compared with that in MHC-matched group, and IFN-γ secreted by CD3+CD8+ T cells was elevated in the haplo-late group compared to the MHC-matched groups (Fig. 2e–g). The NK cells analyzed showed that there were higher levels of IFN-γ and Perforin secretion from spleen in haplo group than that in MHC-matched group, and higher level of CD107a secretion from both spleen and BM in haplo-late group than that in MHC-matched group (Figs. 2h, i and S2E). Furthermore, we detected the apoptosis level of AML cells in different groups and the results showed that AML cells from both spleen and BM in haplo group exhibited higher levels of apoptosis compared with that in MHC-matched group (Fig. 2j, k). These results demonstrated that the elevated immune cell number and reinforced immune cell function promoted leukemia cell apoptosis and prolonged leukemic mice survival in haplo-SCT group compared with MHC-matched group.
Fig. 2.
Elevated CTL levels in haplo group increased leukemia cell apoptosis compared with MHC-matched group. a Comparison of the percentages of AML cells, T cells and NK cells in the spleen of MHC-matched, haplo and haplo-late group of AML-ETO leukemic mice. b Comparison of the percentages of CD4+ T cells and CD8+ T cells in the splenic CD3+ T cell subsets of MHC-matched, haplo and haplo-late group of AML-ETO leukemic mice. c Representative flow cytometry results show apoptotic CD4+ T cells from the spleens of the MHC-matched, haplo and haplo-late groups. d Comparison of the percentages of apoptotic immune cells from spleen including CD4+ T cell, CD8+ T cells and NK cells in MHC-matched, haplo and haplo-late group. e Representative flow cytometry results show the TNF-α secretion by CD4+ T cells from spleen in MHC-matched, haplo and haplo-late group. Cytotoxic cytokine secretion of CD4+ T cells (f) and CD8+ T cells (g) from spleen in MHC-matched, haplo and haplo-late group. h Representative flow cytometry results show the Perforin and CD107a secretion by NK cells from spleen in MHC-matched, haplo and haplo-late group. i Cytotoxic cytokine secretion of NK cells from spleen in MHC-matched, haplo and haplo-late group. Representative flow cytometry results show apoptotic leukemia cells (j) and percentages of apoptotic leukemia cells (k) from the spleens or the BM of the MHC-matched, haplo and haplo-late groups. Error bars represent the mean ± SEM, ***P < 0.001, **P < 0.01, *P < 0.05
Reinforced cytotoxic T lymphocytes function in the haplo-SCT group delayed leukemia progression in MLL-AF9 leukemic mice
Previous results indicated that haplo-SCT showed stronger GVL effects compared with MHC-matched transplantation. To validate this result, we employed another human fusion gene induced mouse leukemia MLL-AF9 which came from the C57BL/6 mouse background, the other parental mouse of CB6F1 (Fig. 3a). We prepared an MHC-matched group by transplanting the leukemia cells (H-2b) into the C57BL/6 mice (H-2b), and a haplo-SCT group by transplanting the leukemia cells (H-2b) into CB6F1 (H-2b/d) mice (Fig. 3a). The AML cells were transplanted in serial doses of 5 × 105, 1 × 105, and 1 × 104. We found that haplo-SCT MLL-AF9 leukemic mice exhibited significantly prolonged survival compared to MHC-matched MLL-AF9 leukemic mice (Figs. 3b and S3A, B). Besides, the splenomegaly was relieved in the haplo group for the remarkably smaller size of spleen than in the MHC-matched group (Fig. S3C). The histological analysis showed reduced leukemic cell infiltrations in the lung, liver, and spleen in the haplo-SCT group compared to the MHC-matched group of MLL-AF9 leukemia mice (Fig. S3D). Then we analyzed the T cell ratio and found that both CD3+CD4+ and CD3+CD8+ spleen T cells were elevated in the haplo group compared with in the MHC-matched group (Figs. 3c, d, and S4A–C). Apoptosis analysis showed that both CD3+CD4+ and CD3+CD8+ T cells from spleen were less apoptotic in haplo-late group compared with in the MHC-matched group, and both CD3+CD4+ and CD3+CD8+ T cells from BM were less apoptotic in the haplo and haplo-late group compared with that in the MHC-matched group (Figs. 3e and S4D). We further analyzed the cytotoxic cytokine secretion and found that IL-2 and TNF-α secreted by CD3+CD8+ T cells from spleen in the haplo group were higher than in the MHC-matched group (Figs. 3f, and S4E). The stronger T cell function in the haplo-SCT group promoted the higher apoptosis level of leukemia cells compared with that in the MHC-matched group (Fig. 3g). These results confirmed that the immune cell in haplo-SCT exhibited higher quantity and stronger cytotoxic activity, leading to stronger GVL activity compared with the MHC-matched transplantation group.
Kinetics of the reduction of leukemia burden after haplo-SCT and MSDT
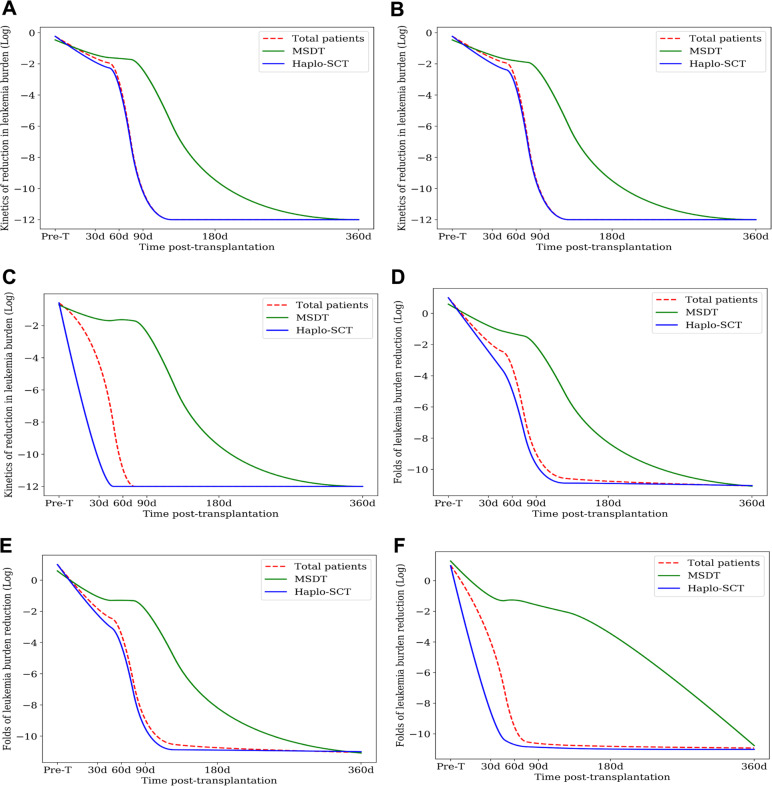
To support the pre-clinical results, we conducted a prospective clinical trial. The patient characteristics are summarized in Table 1. Among the 135 patients included, 48 received MSDT and 87 received haplo-SCT. The median follow-up times were 55 (range, 1–154) months for all patients and 70 (range, 10–154) months for living patients. The prevalence of grade II-IV acute GVHD and chronic GVHD were 33% and 55%, respectively, in the entire cohort. The 5-year CIR and nonrelapse mortality (NRM) values for all 135 patients were 19% and 21%, respectively. The 5-year leukemia-free survival (LFS) and OS values were 61% and 62%, respectively (Tables 2 and S1). The levels of RUNX1/RUNX1T1 after haplo-SCT were significantly lower than those after MSDT, at 60 (P = 0.039) and 90 (P = 0.004) days (Table S2). A repeated measures ANOVA also showed that haplo-SCT significantly reduced the RUNX1/RUNX1T1 levels compared to MSDT (Fig. 4a). Moreover, the RUNX1/RUNX1T1 levels after haplo-SCT remained significantly lower than those observed after MSDT, at 60 (P = 0.021) and 90 (P = 0.004) days after transplantation, when we excluded patients that received a donor lymphocyte infusions (DLI) (Fig. 4b, Table S3) and at 60 (P = 0.010), 90 (P = 0.010), and 180 (P = 0.017) days after transplantation, when we excluded patients with chronic GVHD (Fig. 4c and Table S4).
Table 1.
Patient and donor characteristics
| Characteristics | Total cases | Haplo-SCT | MSDT | P value |
|---|---|---|---|---|
| Number of patients, n (%) | 135 | 87 | 48 | NS |
| Median age (range), years | 30 (4–57) | 23.5 (4–57) | 41.5 (4–55) | <0.0001 |
| Sex, male/female | 70 (51.9%)/65 (48.1%) | 44 (50.6%)/43 (49.4%) ()()(49.4%) | 26 (54.2%)/22 (45.8%) | 0.689 |
| c-KIT mutations, n (%) | 0.122 | |||
| Positive | 36 (26.7%) | 27 (31.0%) | 9 (18.7%) | |
| Negative | 99 (73.3%) | 60 (69.0%) | 39 (81.3%) | |
| Disease status, n (%) | 0.010 | |||
| CR1 | 115 (85.2%) | 69 (79.3%) | 46 (95.8%) | |
| CR≥2 | 20 (14.8%) | 18 (20.7%) | 2 (4.2%) | |
| Disease risk index | NS | |||
| Low | 135 (100%) | 87 (100%) | 48 (100%) | |
| Intermediate | 0 | 0 | 0 | |
| High | 0 | 0 | 0 | |
| Time from diagnosis to transplant, months, median (range) | 6.5 (3-29) | 6.5 (4-29) | 6.5 (3.5-24) | 0.205 |
| Conditioning regimen, n (%) | <0.0001 | |||
| Modified Bu/Cy | 48 (35.6%) | 0 | 48 (100%) | |
| Modified Bu/Cy | 87 (64.4%) | 87 (100%) | 0 | |
| GVHD prophylaxis | NS | |||
| CSA+MTX+MMF | 135 (100%) | 87 (100%) | 48 (100%) | |
| DR sex-matched, n (%) | 0.117 | |||
| Male-male | 46 (34.1%) | 33 (37.9%) | 13 (27.1%) | |
| Male-female | 38 (28.1%) | 26 (29.9%) | 12 (25.0%) | |
| Female-male | 25 (18.5%) | 11 (12.6%) | 14 (29.2%) | |
| Female-female | 26 (19.3%) | 17 (19.5%) | 9 (18.8%) | |
| DR relationship, n (%) | <0.0001 | |||
| Parent-child | 55 (40.7%) | 55 (63.2%) | 0 | |
| Sibling-sibling | 72 (53.3%) | 24 (27.6%) | 48 (100%) | |
| Child-parent | 6 (4.4%) | 6 (6.9%) | 0 | |
| Other | 2 (1.5%) | 2 (2.3%) | 0 | |
| DLI after transplant, n (%) | 0.111 | |||
| Prophylaxis/intervention | 22 (16.3%) | 14 (16.1%) | 8 (16.7%) | |
| Treatment | 5 (3.7%) | 1 (1.1%) | 3 (8.3%) | |
| Cell composition in allografts | ||||
| Infused nuclear cells, (range) 108/kg | 7.76 (3.11–14.93) | 7.82 (3.11–13.56) | 7.58 (4.46–14.93) | 0.065 |
| Infused CD34+ cells, (range) 106/kg | 2.50 (0.32–8.80) | 2.51 (0.32–8.80) | 2.49 (0.9–6.43) | 0.849 |
| Infused CD3+ T cells, (range) 108/kg | 1.78 (0.49–5.90) | 1.83 (0.49–5.90) | 1.55 (0.76–5.26) | 0.337 |
| Infused CD3+CD4+ T cells, (range) 108/kg | 0.93 (0.26–2.37) | 1.02 (0.26–2.37) | 0.84 (0.37–2.26) | 0.373 |
| Infused CD3+CD8+ T cells, (range) 108/kg | 0.58 (0.07–2.38) | 0.58 (0.07–2.38) | 0.57 (0.22–2.25) | 0.547 |
Haplo-SCT haploidentical stem cell transplantation, HLA human leukocyte antigen, MSDT HLA-matched sibling donor transplantation, NS no significance, CR complete remission, DR donor and recipient, DLI donor lymphocyte infusion
Table 2.
Multivariate analysis of factors associated with transplant outcomes in patients undergoing allo-SCT (N = 135)
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Relapse | ||||||
| Disease status (≥CR2 vs. CR1) | 3.454 | 1.430–8.340 | 0.006 | 4.616 | 1.618–13.172 | 0.004 |
| Transplant modality (Haplo-SCT vs. MSDT) | 0.441 | 0.201–0.968 | 0.041 | 0.148 | 0.056–0.386 | <0.0001 |
| c-kit mutation (yes vs. no) | 2.282 | 1.036–5.027 | 0.041 | 3.207 | 1.403–7.331 | 0.006 |
| Platelet engraftment | 0.366 | 0.146–0.916 | 0.032 | |||
| Pre-MRD (≥1.15% vs. <1.15%) | 2.891 | 1.295–6.454 | 0.010 | 3.466 | 1.386–8.667 | 0.008 |
| Non-relapse mortality | ||||||
| Disease status (≥CR2 vs. CR1) | 2.573 | 1.087–6.088 | 0.032 | |||
| Pre-MRD (≥1.15% vs. <1.15%) | 0.370 | 0.128–1.074 | 0.067 | |||
| Grades, II-IV acute GVHD (yes vs. no) | 2.080 | 0.989–4.377 | 0.054 | |||
| HCTCI | ||||||
| 0 | 0.258 | 0.069–0.963 | 0.044 | |||
| 1–2 | 0.394 | 0.134–1.159 | 0.091 | |||
| ≥3 | 1 | |||||
| Platelet engraftment | 0.065 | 0.022–0.189 | <0.0001 | 0.065 | 0.022–0.189 | <0.0001 |
| Leukemia-free survival | ||||||
| Disease status (≥CR2 vs. CR1) | 3.071 | 1.658–5.687 | <0.0001 | 4.813 | 2.052–11.285 | <0.0001 |
| Transplant modality (Haplo-SCT vs. MSDT) | 0.546 | 0.319–0.938 | 0.028 | 0.156 | 0.068–0.361 | <0.0001 |
| Platelet engraftment | 0.131 | 0.049–0.351 | <0.0001 | 0.288 | 0.080–1.032 | 0.056 |
| Pre-MRD ( ≥ 1.15% vs. <1.15%) | 0.293 | 0.139–0.618 | 0.001 | |||
| Grades, II-IV acute GVHD (yes vs. no) | 1.730 | 1.001–2.991 | 0.050 | |||
| Overall survival | ||||||
| Disease status (≥CR2 vs. CR1) | 2.785 | 1.443–5.375 | 0.002 | 4.455 | 1.759–11.284 | 0.002 |
| Transplant modality (Haplo-SCT vs. MSDT) | 0.589 | 0.336–1.034 | 0.065 | 0.129 | 0.051–0.327 | <0.0001 |
| Platelet engraftment | 0.090 | 0.032–0.249 | <0.0001 | 0.115 | 0.028–0.469 | 0.001 |
| Pre-MRD (≥1.15% vs. <1.15%) | 0.333 | 0.154–0.720 | 0.005 | |||
| Grades, II-IV acute GVHD (yes vs. no) | 1.866 | 1.059–3.288 | 0.031 | 2.627 | 1.129–6.112 | 0.025 |
Allo-SCT allogeneic stem cell transplantation, HR hazard ratio, CI confidence interval, CR complete remission, Haplo-SCT haploidentical SCT, MSDT human leukocyte antigen-matched sibling donor transplantation, MRD minimal residual disease, GVHD graft-versus-host disease, HCTCI hematopoietic stem cell transplantation comorbidity index
*All variables were first included in the univariate analysis; only variables with P < 0.1 were included in the Cox proportional hazards model with time-dependent variables
Fig. 4.
Kinetics of leukemia burden evaluated with RT-PCR for RUNX1/RUNX1T1 after transplantation. Log-transformed data show leukemia burden over time in (a) all patients, (b) all patients, except those that received DLI, and (c) all patients, except those with chronic GVHD. Log-transformed data show the fold-reduction in leukemia burden post-transplantation compared with those of pre-transplantation for (d) all patients, (e) all patients, except those that received DLI, and (f) all patients, except those with chronic GVHD
Fold-reduction in the leukemia burden with transplantation
In the total patient group, the fold-reductions in leukemia burden were significantly larger in the haplo-SCT group than in the MSDT group, at 30 (P = 0.068), 60 (P = 0.005), 90 (P < 0.0001), and 180 (P = 0.032) days after transplantation, compared to before transplantation (Table S5). A repeated measures ANOVA showed that the fold-reduction in leukemia burden after haplo-SCT was significantly lower than that after MSDT (Fig. 4d). Moreover, the fold-reductions in leukemia burden remained lower in the haplo-SCT group than in the MSDT group at 30 (P = 0.068), 60 (P = 0.002), 90 (P < 0.0001), and 180 (P = 0.053) days after transplantation, when we excluded patients who received DLI (Fig. 4e and Table S6) and at 30 (P = 0.159), 60 (P = 0.005), 90 (P = 0.003), and 180 (P = 0.003) days after transplantation, when we excluded patients with chronic GVHD (Fig. 4f and Table S7).
Significantly lower incidence of relapse after haplo-SCT compared to MSDT
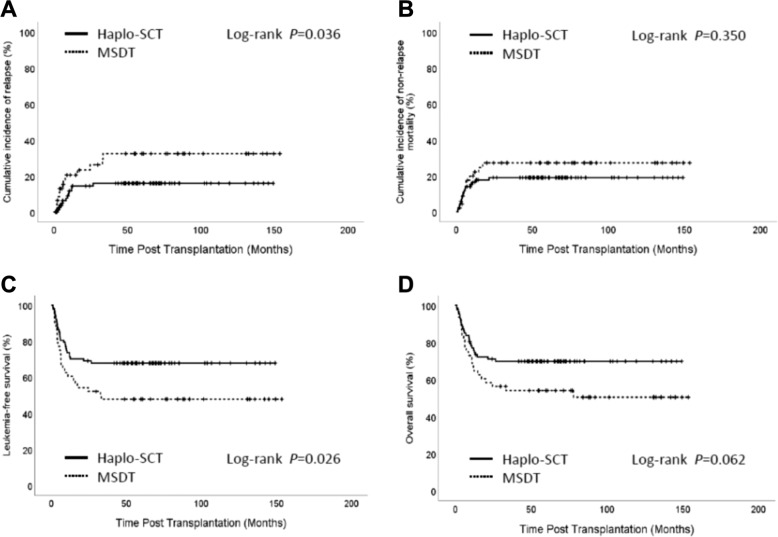
Among the 135 patients, the 5-year CIR rates for the haplo-SCT and MSDT groups were 14% and 25%, respectively (P = 0.036, Fig. 5a). The 5-year incidences of NRM for the haplo-SCT and MSDT groups were 18% and 27%, respectively (P = 0.350, Fig. 5b). The 5-year LFS rates were 48% in the MSDT group and 68% in the haplo-SCT group (P = 0.026, Fig. 5c). The 5-year OS rates were 50% in the MSDT group and 70% in the haplo-SCT group (P = 0.062, Figs. 5d and S5A–D). In the multivariate analysis, haplo-SCT was associated with a lower incidence of relapse (HR: 0.148; 95%CI: 0.056-0.386; P < 0.001), a better LFS (HR: 0.156; 95%CI: 0.068-0.361; P < 0.001), and a better OS (HR: 0.129; 95%CI: 0.051–0.327; P < 0.001) compared to MSDT (Table 2).
Fig. 5.
Impact of transplant modalities on outcomes in humans. Kaplan–Meier curves show the CIR (a), the cumulative incidence of NRM (b), LFS (c), and OS (d) in haplo-SCT and MSDT groups
High cytotoxic T cell activity in the haplo-SCT group after one year
To validate the observation that stronger antileukemia effects were obtained when T cell MHCs haplo-matched those of the leukemia cells in mouse models, we compared primary human T cell function between the MSDT and haplo-SCT groups in vitro. We collected peripheral blood mononuclear cells (PBMCs) from 30 days (30d), 60 days (60d), 90 days (90d), 180 days (180d) and over 1-year post-transplantation (≥365d) patients and cocultured them with GFP-expressing leukemic THP-1 cells, at a ratio of 10:1 for 4-6 h (Fig. S6A). The results showed that T cells from the haplo-SCT group over one-year post-transplantation had higher cytotoxic activity than that from the MSDT group evidenced by the elevated apoptosis level of THP-1 (Fig. 6a, b). However, there were no significant differences between groups in samples collected at other time points after transplantation (Fig. 6a, b). We also observed that ≥365d T cells from the haplo-SCT group secreted higher levels of CD107a than the same period T cells from the MSDT group (Fig. 6c, d). We did not observe any significant differences between groups in the expression of active T-cell markers, like CD25 and CD137, or other cytotoxic function related markers, like IFN-γ, TNF-α, IL-2, or GranzymeB (Fig. S6B–F). These results provided evidence that T cells from recipients of haplo-SCT might exhibit stronger antileukemia effects than T cells from recipients of MSDT over one year after transplantation.
Fig. 6.
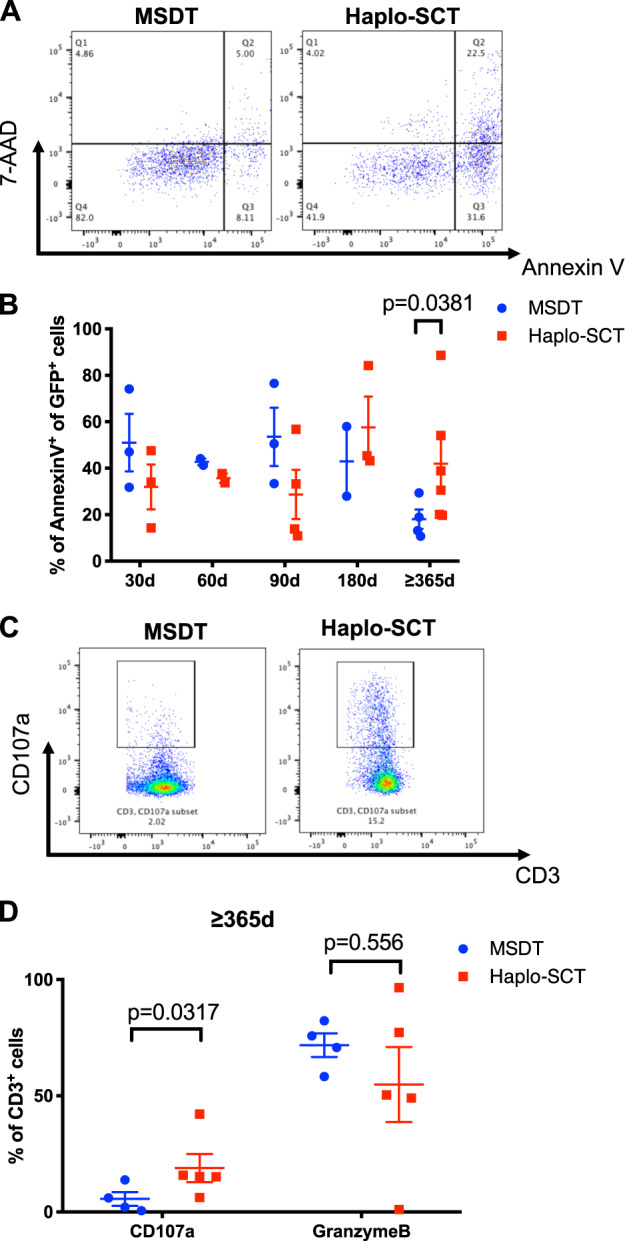
High CTL cytotoxicity in haplo-SCT recipients at over 1 year after transplantation compared with MSDT at the same period. a Representative flow cytometry results compare apoptosis of leukemia cells from MSDT and haplo-SCT groups. b Comparison of apoptotic THP-1 cells after coculturing with PBMCs; PBMCs were isolated from haplo-SCT or MSDT groups at 30 days (30d), 60 days (60d), 90 days (90d), 180 days (180d) and over 1 year (≥365 d) after transplantation. c Representative flow cytometry results compare CD107a-secreting CD3+ T cells from haplo-SCT or MSDT groups at over one year (≥365 d) after transplantation. d Comparison of the percentage of CD3+ T cells from haplo-SCT or MSDT recipients that secrete CD107a and GranzymeB after coculturing with THP-1
Discussion
It has been proved by several clinical studies that haplo-SCT achieved lower relapse and comparable OS in treating patient with AML compared to MSDT,9,11,30,31 indicating there were stronger GVL effects in haplo-SCT compared with MSDT. However, the regulatory dynamics of immune cells in the antileukemia effects of two transplantation models and the mechanism of stronger GVL effects in haplo-SCT have not been discussed. Here, by mouse models, we provided dynamic changes of immune cells and leukemia cells in haplo-SCT and MHC-matched transplantation group during leukemia development. Our results showed that haplo-matching the MHCs of leukemia cells with recipient mouse T cells exhibited higher quantity and cytotoxic activity of immune cells and contributed to the prolonged survival and reduced leukemia burden of leukemic mice. Our prospective clinical trial and ex vivo experiments further provided evidence of stronger antileukemia activity of immune cells from haplo-SCT group than those from the MSDT group.
Due to the genetically homogeneous and very little variation or heterogeneity within a pure inbred mouse strain, it is hard to establish an MSD transplantation models to mimic the MSDT in the clinical situation. Our mouse leukemia cells were established by transplanting retrovirus infected mouse hematopoietic stem/progenitor cells to normal irradiated recipient mouse. The retrovirus carried human fusion gene coding sequence which was either AML-ETO or MLL-AF9.15,16 We transplanted the leukemia cells to the same background strain mice to represent the MHC-matched transplantation group, and transplanted leukemia cells to the hybrid mice to represent the haplo-SCT transplantation group. Although there is limitation of the mouse models, these two nonirradiated mouse models could still provide a platform to peruse immune cells reaction in MHC-matched or haplo-matched group during leukemia development.
Immune infiltration in the tumor microenvironment (TME) have been extensively studied in solid tumors and shed light on the mechanisms of the interaction between immune cells and tumor cells.32 Due to the distinct characteristics of hematological malignances, the interaction between immune cells and leukemia cells in AML was still poorly understood. Previous studies showed BM as a primary lymphoid organ provided support for hematopoietic stem cells and contained most developing and mature immune cell types.33,34 Here in our study, we found the mature T and NK cells which is the main force of antileukemia activity mostly distributed in the periphery and second lymphoid organ like spleen at the early stage of leukemia, and the ratios of mature T and NK cells were quiet low in the BM. This distribution is consistent with previous reference framework which revealed the immune cells constitution in various organs across the body in normal mice.35 Interestingly, with the development of leukemia, the ratios of T and NK cells in periphery and spleen were decreased and increased in BM. It might be aroused by the blockade of T and NK cells in the BM in the late stage of leukemia, and thus, they could not migrate to secondary lymphoid tissues to kill leukemia cells. Another explanation might be the increased leukemia cells in BM formed TME and secreted chemokines to recruit T and NK cells to execute cytotoxic function. It still needs further investigation to explain this phenomenon.
Our studies showed that high quantity and stronger GVL function of CD8+ cytotoxic T lymphocytes (CTL) in haplo-SCT group contributed to prolong leukemic mice survival and reduce leukemia burden. This result is consistent with previous study from Bonnet et al.36 who showed that CD8+ CTL clones specific for minor histocompatibility antigens could inhibit engraftment and proliferation of human AML cells transplanted into nonobese diabetic/SCID mice.36 The results also showed that the predominant cytotoxic cytokine secreted by CD8+ CTL was different in two transplantation models, which is CD107a was higher in MHC-matched group whereas TNF-α and IFN-γ were higher in haplo and haplo-late group separately. It might indicate the MHC matching status induced different cytotoxic function in two transplantation models. Meanwhile, the cytotoxic cytokines including CD107a, Perforin, TNF-α and IFN-γ secreted by NK cells were elevated in haplo or haplo-late group compared with MHC-matched group. These results consist with that when executed cytotoxic function, the cytotoxic T cells depended on MHC-restricted manner whereas NK cells depended on MHC non-restricted manner.37
In our clinical study, we found that haplo-SCT was associated with lower CIRs compared to MSDT, although potential biases in using biologic randomization and the imbalance in donor could not be completely avoided. Moreover, the kinetics of MRD, detected with RT-PCR of RUNX1/RUNX1T1, reflected the dynamic leukemia burden and provided evidence for the stronger GVL effects of haplo-SCT over MSDT. There is one difference between groups that anti-thymocyte globulin (ATG) was used to deplete T cells in patient conditioning for the haplo-SCT group, but not for the MSDT group. Inamoto et al.38 retrospectively investigated the effect of ATG on the outcomes of 23,302 patients with hematological malignancies that underwent allo-SCT as a first-line treatment. They reported that, in adult patients, ATG prophylaxis was associated with a higher CIR compared to no ATG (HR: 1.35, P = 0.05). In patients with acute leukemia. Wakamatsu et al.39 observed that the CIR showed a tendency to increase in patients that underwent MSDT with ATG, compared to patients without ATG (HR: 2.026, P = 0.06). Kröger et al. performed a prospective, multicenter, open-label, phase 3 study that evaluated the effects of ATG prophylaxis on GVHD in patients with hematological malignancies that received MSDT.40 They found that patients in the ATG group experienced a higher relapse rate than patients in the non-ATG group (32.2% vs. 25.5%), although the difference was not statistically significant (P = 0.17). Therefore, the stronger GVL effects of haplo-SCT compared to MSDT could not be explained by the use of ATG, because the use of ATG would be expected to increase the CIR.38,39
Another difference between our groups was that the dose of Ara-C used for chemotherapy in the haplo-SCT group (4 g/m2/d for 2 days) was higher than that used in the MSDT group (2 g/m2/d for two days). Recently, Arai et al.41 conducted a cohort study to compare the prognosis between groups administered HD-Ara-C/CY/TBI (n = 617, group A) or CY/TBI (n = 312, group B) in cord blood transplantations for treating AML/MDS. That study was based on data from a Japanese transplantation registry. They found that, compared to group B, group A had significantly fewer relapses (HR: 0.49; P < 0.01), which resulted in lower tumor-related mortality (HR: 0.50; P < 0.01). Therefore, it could be speculated that a higher dose of Ara-C in the haplo-SCT group (4 g/m2) compared to the MSDT group (2 g/m2) might have contributed to the reduction in RUNX1/RUNX1T1 transcripts. However, studies have shown that a metabolite of Ara-C had an average apparent elimination half-life of 3.75 h after an intravenous infusion at 3 g/m2 Ara-C in patients with leukemia.42 In our study, Ara-C was given on days -10 and -9, and RUNX1/RUNX1T1 transcripts were significantly reduced only at 30, 60, and 90 days after haplo-SCT, compared to MSDT. Therefore, Ara-C might have contributed to the reduction in RUNX1/RUNX1T1 transcripts in the early days after transplantation, but it is unlikely that Ara-C contributed to the enhanced antileukemia activity of haplo-SCT, due to its relatively short half-life.
In our study, haplo-SCT T cells displayed enhanced function compared to MHC-matched T cells, in both our mouse models and the clinical cohort. In the ex vivo experiment, where we collected PBMCs at ≥365 d post transplantation, we found that haplo-SCT T cells had higher cytotoxic activity than MHC-matched T cells. However, we did not observe any differences in T cells between groups at earlier stage after transplantation. Several factors may account for these results. First, the use of immune-suppressive agents for GVHD prophylaxis;3,27 second, the early quantitative immune recovery was delayed after haplo-SCT compared to the recovery observed after MSDT;43 and third, the ex vivo experiments might not have adequately reflected the function of T cells in vivo. Furthermore, our previous study compared the NK cells number and cytotoxic function which was reflected by killing K562 leukemia cells after haplo-SCT or MSDT.44 The results showed that higher CD107a secretion of NK cells from patient received haplo-SCT compared with that from MSDT since 60 days after transplantation.44 These results demonstrated reinforced T and NK lymphocytes cytotoxic function led to stronger GVL effects of haplo-SCT compared with MSDT.
In conclusion, we unraveled the role of immune cells in superior antileukemia effects of haplo-SCT compared with the MHC-matched transplantation group based on mouse models. Moreover, we demonstrated the superiority of haplo-SCT over MSDT for slowing the kinetics of the leukemia burden in vivo and for reducing the CIR, based on a clinical trial. Overall, our study indicated that the enhanced function of T and NK cells might contribute to the stronger antileukemia activity of haplo-SCT compared to MSDT.
Supplementary information
Acknowledgements
This work was partly supported by grants from the National Key Research and Development Program of China (2017YFA0104500), the Beijing Municipal Science and Technology Commission (Z181100009618032), the National Natural Science Foundation of China (Grant No. 81621001, 81530046, 81770189, 81670186, 81870141 and 82070185), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number: 2019-I2M-5-034) and the Peking University Clinical Scientist Program (BMU2019LCKXJ003). We thank all of the faculty members who participated in these studies.
Author contributions
Contributions: X.-J.H. designed the study; X.-J.H., Y.-J.C., and H.-D. G. conceived the project and drafted the manuscript; H.-D. G. and Y. H. performed the in vivo mouse models experiments and ex vivo experiments; Y.-J.C. analyzed the clinical data; all authors contributed to data interpretation and manuscript preparation. All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Hui-Dong Guo, Ying-Jun Chang, Yan Hong
Supplementary information
The online version of this article (10.1038/s41423-020-00597-1) contains supplementary material.
References
- 1.Appelbaum, F. R. Hematopoietic-cell transplantation at 50. N. Engl. J. Med.357, 1472–1475 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Dohner, H. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood129, 424–447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, Y. et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood125, 3956–3962 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Koreth, J. et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA301, 2349–2361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelissen, J. J. et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood109, 3658–3666 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Horowitz, M. M. et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood75, 555–562 (1990). [PubMed] [Google Scholar]
- 7.Lamers, C. H. et al. CD4+ T-cell alloreactivity toward mismatched HLA class II alleles early after double umbilical cord blood transplantation. Blood128, 2165–2174 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Matte-Martone, C. et al. Differential requirements for myeloid leukemia IFN-gamma conditioning determine graft-versus-leukemia resistance and sensitivity. J. Clin. Invest. 127, 2765–2776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Y. J. et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J. Hematol. Oncol.10, 134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, Y. J. et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J. Hematol. Oncol.13, 27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu, S. et al. Haploidentical transplantation might have superior graft-versus-leukemia effect than HLA-matched sibling transplantation for high-risk acute myeloid leukemia in first complete remission: a prospective multicentre cohort study. Leukemia, 10.1038/s41375-019-0686-3 (2019). [DOI] [PubMed]
- 12.Barnes, D. W. & Loutit, J. F. Treatment of murine leukaemia with x-rays and homologous bone marrow. Ii. Br. J. Haematol.3, 241–252 (1957). [DOI] [PubMed] [Google Scholar]
- 13.Schroeder, M. A. & DiPersio, J. F. Mouse models of graft-versus-host disease: advances and limitations. Dis. Model Mech.4, 318–333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy, P., Negrin, R. & Hill, G. R. Mouse models of bone marrow transplantation. Biol. Blood Marrow Transpl.14, 129–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, Y. Y. et al. C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice. Proc. Natl Acad. Sci. USA108, 2450–2455 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, H. et al. PBX3 is essential for leukemia stem cell maintenance in MLL-rearranged leukemia. Int. J. Cancer141, 324–335 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Balsat, M. et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French Association Group. J. Clin. Oncol.35, 185–193 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Zhu, H. H. et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood121, 4056–4062 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Xu, L. et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J. Hematol. Oncol.11, 33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, Y. et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood124, 1880–1886 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Ravandi, F., Walter, R. B. & Freeman, S. D. Evaluating measurable residual disease in acute myeloid leukemia. Blood Adv.2, 1356–1366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thepot, S. et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia24, 1852–1858 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Blom, S. et al. Systems pathology by multiplexed immunohistochemistry and whole-slide digital image analysis. Sci. Rep.7, 15580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruck, O. et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia32, 1643–1656 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y. et al. Haploidentical versus matched-sibling transplant in adults with philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin. Cancer Res. 22, 3467–3476 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Yan, C. H. et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood119, 3256–3262 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Chang, Y. J. et al. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J. Clin. Oncol.34, 1855–1863 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Gauthier, J. et al. Better outcome with haploidentical over HLA-matched related donors in patients with Hodgkin’s lymphoma undergoing allogeneic haematopoietic cell transplantation-a study by the Francophone Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transpl.53, 400–409 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Mariotti, J. et al. T cell-replete haploidentical transplantation with post-transplantation cyclophosphamide for Hodgkin Lymphoma relapsed after autologous transplantation: reduced incidence of relapse and of chronic graft-versus-host disease compared with HLA-identical related donors. Biol. Blood Marrow Transpl.24, 627–632 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Srour, S. A. et al. Haploidentical transplantation for acute myeloid leukemia patients with minimal/measurable residual disease at transplantation. Am. J. Hematol.94, 1382–1387 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Wang, Y. et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol. Blood Marrow Transpl.17, 821–830 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Zhang, Y. & Zhang, Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol.17, 807–821 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercier, F. E., Ragu, C. & Scadden, D. T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol.12, 49–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson-Moncada, J., Viboch, E., Church, S. E., Warren, S. E. & Rutella, S. Dissecting the Immune Landscape of Acute Myeloid Leukemia. Biomedicines6, 10.3390/biomedicines6040110 (2018). [DOI] [PMC free article] [PubMed]
- 35.Spitzer, M. H. et al. Immunology. An interactive reference framework for modeling a dynamic immune system. Science349, 1259425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet, D., Warren, E. H., Greenberg, P. D., Dick, J. E. & Riddell, S. R. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc. Natl Acad. Sci. USA96, 8639–8644 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rees, R. C. MHC restricted and non-restricted killer lymphocytes. Blood Rev.4, 204–210 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Inamoto, Y. et al. Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica101, 1592–1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakamatsu, M. et al. Impacts of thymoglobulin in patients with acute leukemia in remission undergoing allogeneic HSCT from different donors. Blood Adv.3, 105–115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroger, N. et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl. J. Med. 374, 43–53 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Arai, Y. et al. Efficiency of high-dose cytarabine added to CY/TBI in cord blood transplantation for myeloid malignancy. Blood126, 415–422 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breithaupt, H. et al. Clinical results and pharmacokinetics of high-dose cytosine arabinoside (HD ARA-C). Cancer50, 1248–1257 (1982). [DOI] [PubMed] [Google Scholar]
- 43.Chang, Y. J., Zhao, X. Y. & Huang, X. J. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl.20, 440–449 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Hu, L. J. et al. NK cell reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with matched sibling transplantation. Sci. China Life Sci.63, 781–784 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.