Abstract
Purpose of Review
In this review, we summarize current evidence on the association between antibiotics and the subsequent development of obesity through modulation of the gut microbiome. Particular emphasis is given on (i) animal and human studies and their limitations; (ii) the reservoir of antibiotics in animal feed, emerging antibiotic resistance, gut dysbiosis, and obesity; (iii) the role of infections, specifically viral infections, as a cause of obesity; and (iv) the potential therapeutic approaches other than antibiotics to modulate gut microbiome.
Recent Findings
Overall, the majority of animal studies and meta-analyses of human studies on the association between antibiotics and subsequent development of obesity are suggestive of a link between exposure to antibiotics, particularly early exposure in life, and the development of subsequent obesity as a result of alterations in the diversity of gut microbiota. The evidence is strong in animal models whereas evidence in humans is inconclusive requiring well-designed, long-term longitudinal studies to examine this association. Based on recent meta-analyses and epidemiologic studies in healthy children, factors, such as the administration of antibiotics during the first 6 months of life, repeated exposure to antibiotics for ≥ 3 courses, treatment with broad-spectrum antibiotics, and male gender have been associated with increased odds of overweight/obesity.
Early antibiotic exposure in animal models has shown that reductions in the population size of specific microbiota, such as Lactobacillus, Allobaculum, Rikenellaceae, and Candidatus Arthromitus, are related to subsequent adiposity. These data suggest that the loss of diversity of the gut microbiome, especially early in life, may have potential long-term detrimental effects on the adult host gut microbiome and metabolic health. Genetic, environmental, and age-related factors influence the gut microbiome throughout the lifetime. More large-scale, longer-term, longitudinal studies are needed to determine whether changes that occur in the microbiome after exposure to antibiotics, particularly early exposure, are causal of subsequent weight gain or consequent of weight gain in humans.
Summary
Further well-designed, large-scale RCTs in humans are required to evaluate the effects of administration of antibiotics, particularly early administration, and the subsequent development of overweight/obesity. Therapeutic interventions, such as bacteriophage treatment or the use of probiotics, especially genetically engineered ones, need to be evaluated in terms of prevention and management of obesity.
Keywords: Antibiotic, Diet, Gut, Infection, Intestine, Metabolic syndrome, Microbiome, Microbiota, Obesity, Prebiotic, Probiotic, Virus
Introduction
Overweight, defined as BMI ≥ 25 kg/m2, and obesity, defined as BMI ≥ 30 kg/m2, pose a major public health problem worldwide. Specifically, obesity has nearly tripled since 1975. In 2016, more than 1.9 billion adults, aged 18 years and older, were overweight. Of these, individuals with obesity were more than 650 million. In particular, 39% of adults aged 18 years and above were classified in the group of overweight while 13% were classified in the group of obesity [1]. Furthermore, recent data from the National Health and Nutrition Examination Survey have indicated that the age-adjusted prevalence of obesity among US adults was 42.4% in 2017–2018 [2]. Regarding childhood obesity, which is still on the rise, 38 million children under the age of 5 belonged to the group of overweight and obesity in 2019 [3]. Over 340 million children and adolescents aged 5–19 years old belonged to the group of overweight and obesity in 2016 [4]. Notably, most of the world’s population live in countries where overweight and obesity affect more people than underweight [3].
Microbial communities are scattered all over and inside human body, i.e., skin, vagina, intestine, and oral cavity. The abundance, diversity, and features of microorganisms’ genes are collectively known as the human microbiome, a seemingly ‘new actor on stage’, due to its numerous roles in health and disease [5]. However, the majority of microorganisms harbor the gastrointestinal tract. The intestinal microbiota is an ever-changing ecosystem containing more than 105 billion microorganisms that outnumber human cells and includes bacteria, archaea, protozoans, viruses, and fungi [6]. The human adult gut is characterized by six dominant phyla including Firmicutes, Bacteroides, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia, with the first two representing more than 90% [7].
Colonization of the gastrointestinal tract has been demonstrated to begin in utero, and subsequently evolves and reaches maturity within the first years of life [8–10]. Other determinants, such as host genetic factors, oral antibiotic use early in life, diet, smoking, and infections, are fundamental in shaping gut microbiota [11–14].
Even though the composition of the gut microbiota is influenced by factors listed above, as well as gender, geographic location, and race/ethnicity, the delivery mode appears to be the most crucial factor for the acquisition of neonatal microbiota [15, 16]. Newborns are coated by the maternal vaginal and gut microbiota, which are mainly dominated by Lactobacillus, accounting for > 50% of the total microbiota [16, 17]. After birth, newborns acquire their secondary microbiome from their families as well as the surrounding ecosystem. Vaginally delivered newborns acquire a bacterial composition resembling their mother’s vaginal microbiota, dominated by Lactobacillus, Prevotella, or Sneathis [18]. In sharp contrast to vaginal delivery, newborns delivered by cesarean section acquire bacteria, which resemble those present on the skin, such as Staphylococcus, Corynebacterium, and Propionibacterium. These bacteria are not maternally derived, but are acquired from the hospital staff, with whom the newborns have had contact [16]. The period of microbiota acquisition and the development of a child’s immune system are interconnected as they take place at the same time, thus influencing each other strongly [19, 20].
Microbial colonization during neonatal development is characterized by a considerable degree of dynamic variation in its composition, which evolves toward an adult-like configuration within 3 years after birth [19]. Colonization by commensals is related with and required for the maturation of host immunity, leading to an immunometabolic homeostasis of the host [19]. Administration of antibiotics is linked to alterations in the gut microbiota that could lead to alterations in immunometabolic function if they occur in windows of opportunity [19].
Based on recent advances in sequencing technology and bioinformatics analyses summarized in [18], it has been shown that (1) the composition of the gut microbiota may change during life modified by diet, genetics, and the environment; (2) the course of early development of the gut microbiota is highly unstable and idiosyncratic. There is a rapid increase in the diversity of gut microbiota in early childhood with shifts in response to diet and disease. Nevertheless, the reason for this increase in diversity is unknown; (3) there is a difference in the composition of gut microbiota amid children from different countries.
Although very difficult to perform, more age- and region-specific longitudinal studies over a long time period (more than 5–10 years) are necessary to study the composition of gut microbiota throughout the lifespan [18].
Maintaining the variety and balance of gut microbiota are the key points for promoting human health throughout the life cycle. Alterations in the diversity or structure of gut microbiota known as dysbiosis may affect metabolic activities, resulting in metabolic disorders, such as obesity, metabolic syndrome, and diabetes mellitus [21, 22••]. In particular, the gut microbiome can disrupt the gut mucosal barrier, resulting in an increased exposure of the host’s immune system to bacterial products, such as membrane lipopolysaccharides (LPS), a condition known as metabolic endotoxemia. Endotoxemia in conjunction with an increased gut permeability are related to inflammation, which, in turn, may result in weight gain, hyperglycemia, and hyperinsulinemia [23•].
The aim of this review is to present summarize current evidence on the association between antibiotics and the subsequent development of obesity through modulation of the gut microbiome. Particular emphasis is given on (i) animal and human studies and their limitations; (ii) the reservoir of antibiotics in animal feed, emerging antibiotic resistance, gut dysbiosis, and obesity; (iii) the role of infections, specifically viral infections, as a cause of obesity; and (iv) potential therapeutic approaches other than antibiotics to modulate gut microbiome.
Alterations of the Gut Microbiome as a Cause of Obesity
There is mounting evidence highlighting the significance of a well-balanced microbiome in human health [24•, 25]. Disruptions in the diversity and/or structure of the intestinal microbial community could lead from eubiosis to dysbiosis, a condition of imbalance between commensals and pathogenic microbes. Dysbiosis is responsible for numerous alterations in several metabolic pathways leading to metabolic disorders as well as numerous non-communicable diseases [21].
A plethora of animal studies have documented that the decreased diversity and reduced richness in the species and genes of the gut microbiome are correlated with an elevated risk for obesity [11, 12, 23–27]. In 2004, Backhed et al. have shown that germ-free (GF) mice were leaner than the conventional models. In this landmark study, after transplantation of the gut microbiota from conventionally raised mice, GF mice presented an increase in body fat mass despite the reduced food consumption [28]. Notably, in 2006, Turnbaugh et al. have demonstrated that gut microbiota transplantation from obese to GF mice led to fat mass augmentation three times more than the respective transplantation from lean mice [29, 30]. In addition, gavage of mice with B. thetaiotaomicron has been suggested to protect against obesity [31].
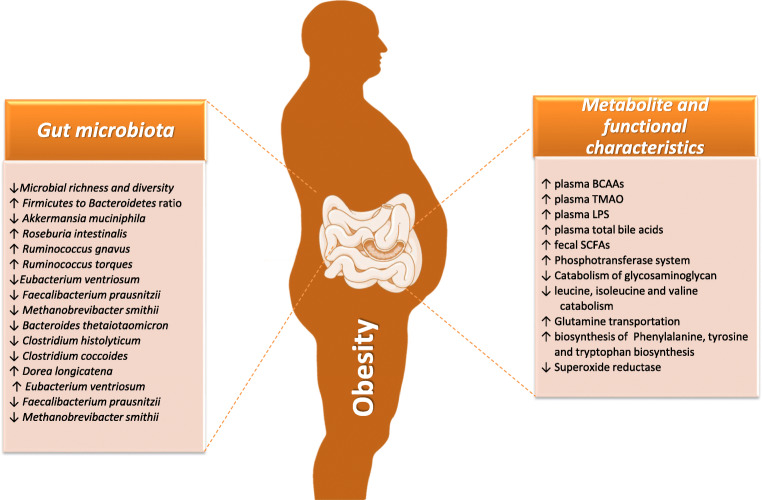
Experimental studies in animal models as well as in humans have shown that obesity is associated with a decrease in the abundance of Bacteroidetes and an increase in the number of Firmicutes [31, 32]. Figure 1 depicts key gut microbiota, metabolite, and functional characteristics associated with obesity. Overall, there is lower microbial richness and diversity as well as lower microbial gene count in obesity compared to individuals of normal weight. A plethora of studies has implicated certain microbial species, metabolic, and functional characteristics in obesity; nevertheless, findings differ between studies. At the species level, several studies have documented the abundance of short chain fatty acids (SCFA) producing-microbes, such as Eubacterium ventriosum and Roseburia intestinalis, which have been linked to obesity [33]. In a metagenome-wide study of lean individuals and individuals with obesity, Bacteroides thetaiotaomicron, a glutamate-fermenting commensal, was reported to be reduced among patients with obesity, being also inversely associated with serum glutamate levels [31]. Noteworthy, diet-induced weight loss is associated with increased gut bacterial gene richness and a reduced subclinical chronic systemic inflammation. When subjects with obesity were either on a carbohydrate-restricted or a fat-restricted low-calorie diet, a decrease in Firmicutes and an increase of Bacteroidetes were documented [29, 34–38].
Fig. 1.
Key gut microbiota, metabolite, and functional characteristics associated with obesity. Overall, there is lower microbial richness and diversity as well as lower microbial gene count in obesity compared to normal weight individuals. A plethora of studies has implicated certain microbial species, metabolic and functional characteristics in obesity; nevertheless, findings differ between studies. This list is not complete regarding the totality of altered taxonomic, metabolite, and functional characteristics but represents frequent patterns observed amid studies. Abbreviation list: BCAA: branched-chain amino acid; LPS: lipopolysaccharide; SCFA: short-chain fatty acid; TMAO: trimethyl-amine-N-oxide; ↓ or ↑: reduced or increased abundance in obesity compared to normal-weight individuals
Microbial metabolites such as bile acids, SCFAs, mainly butyrate, acetate and propionate, branched chain amino acids (BCAAs), aromatic amino acids, and trimethyl-amin-N-oxide (TMAO), play a pivotal role in the metabolic pathways implicated in obesity [22, 39, 40]. Secondary bile acids, mainly derived from the effect of Lactobacilli and Clostridium species on the primary bile acids in the small intestine, seem to modulate glucose homeostasis and energy expenditure [39–42]. SCFAs, as the end products of polysaccharide fermentation in the proximal colon, could serve as an energy warehouse, affecting body weight. Butyrate is a significant player for the integrity of tissue barrier function and for immune regulation [43, 44]. A bulk of evidence, mostly from experimental studies and only one human study examining propionate, has highlighted that SCFAs, through the regulation of appetite, the increase of energy expenditure and the level of anorexic hormones, may influence body weight and prevent the development of obesity [22••, 45–47]. However, experimental in vitro and animal studies may not translate into the human condition. Moreover, human studies are limited by difficulties in determining SCFA production, by differences in the mode of administration and/or the site of production, by the variation in diet composition and metabolic phenotype between subjects [46]. More well-controlled longer-term human SCFA intervention studies are needed to explore SCFA actions in metabolic health [45, 46]. BCAAs and aromatic amino acids are associated with obesity, insulin resistance, and type 2 diabetes mellitus (T2DM). Finally, animal studies have also highlighted the role of TMAO, as a product of the microbial metabolism and food sources, in cardiovascular risk and obesity; however, human studies have yielded conflicting outcomes [22••].
During dysbiosis, the imbalanced gut microflora has the ability to alter the intestinal permeability, leading to amplified exposure of the host’s immune system to microbial metabolites. One of the consequences is metabolic endotoxemia caused by the high levels of LPS, known as endotoxin, of microbial membranes of Gram negative bacteria. There is now compelling evidence that this intestinal leakage contributes to the chronic, low-grade inflammation, which characterizes obesity, by activating the innate immune system with pro-inflammatory molecules and cells [48–51]. Recently, gut microbiome dysbiosis and endotoxemia have been suggested as possible physiological mechanisms for the increased COVID-19 severity in individuals with obesity [52].
There is much interest regarding the potential beneficial effects of functional foods, including probiotics, prebiotics, synbiotics, and postbiotics, in the prevention and treatment of obesity. Gut microbiota modulation through the administration of probiotics or prebiotic dietary fibers seems to be a promising way for obesity prevention and management. In particular, probiotics, such as Bifidobacterium and Lactobacillus, exert beneficial results on weight loss, while reducing inflammation and maintaining glucose control based on animal studies and meta-analyses of human studies [22, 39]. Next-generation probiotics, such as Faecalibacterium prausnitzii, Akkermansia muciniphila, or Clostridia strains, have also demonstrated promising results. However, the use of prebiotics in obesity has yielded inconsistent results, mainly due to the limitations in human studies, such as the heterogeneity of the studied groups in terms of age, sex, race, and the paucity of studies. The use of prebiotics and probiotics is considered safe in immune-competent subjects, while in immune-compromised patients, there is major skepticism, due to many studies confirming infections by probiotics in this category of patients [22••]. Of note, the potential role of pre- and probiotics as well as other nutritional bioactives were suggested as potential immune-modulator factors in patients with COVID-19 and obesity [53].
Further large-scale RCTs in humans are essential for understanding the contribution of all biotics in the prevention and therapeutic management of obesity.
Alterations of the Gut Microbiome by Antibiotics
Antibiotics, as lifesaving medicines for over a century, have been in the front line for combating infections, preventing various medical conditions, and promoting animal growth [54••]. However, they present certain disadvantages including antimicrobial resistance (AR) and adverse drug events (ADEs) [55]. As gut microbiota is characterized by multiple shifts from the endometrial period till the end of life, antibiotics are suggested to represent one of the most pivotal factors for these alterations stimulating or promoting various diseases.
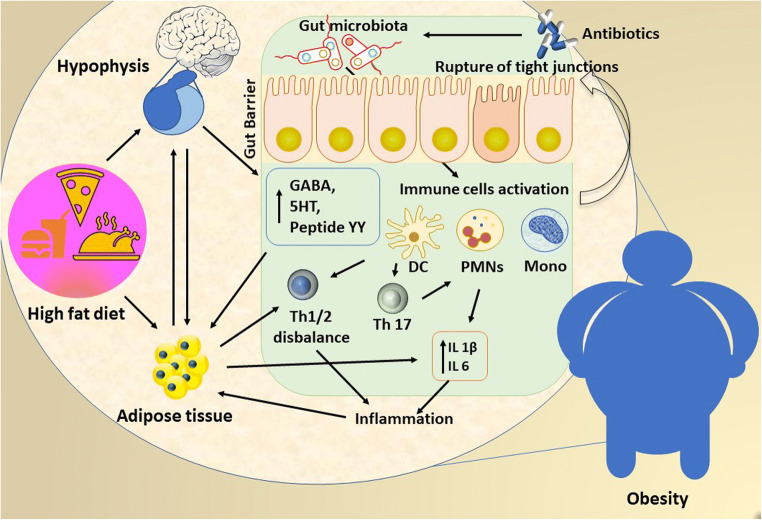
Since 1940, it has been known that antimicrobials may affect the intestinal microbiota. In 1950, terramycin proved to alter the gut microbiota in patients submitted to bowel surgery [56, 57]. Dysbiosis is closely related to the use of medication, being characterized by (i) the flourishing of the pathobionts, i.e., resident microbes with pathogenic potential; (ii) the loss of α-diversity, i.e., the mean species diversity in the intestinal tract; (iii) the recruitment of inflammatory cells; (iv) the ‘leaky gut’ syndrome; and (v) the impaired protection against pathogens [54••]. Figure 2 depicts the main mechanisms interconnecting gut dysbiosis triggered by environmental exposures such as diet and antibiotics and obesity.
Fig. 2.
Gut dysbiosis triggered by environmental exposures such as diet and antibiotics plays an important role in disrupting molecular metabolism and impacting on obesity outcomes. In obesity, the adipose tissue is infiltrated with inflammatory immune cells that produce high amounts of proinflammatory cytokines and chemokines. The gut barrier is disrupted causing gut antigens and PAMPs such as LPS to enter the tissue and stimulate inflammation. DC: dendritic cells, GABA: gamma aminobutyric acid, Mono: monocytes, PYY: peptide YY, PMNs: polymorphonuclear neutrophils, Th: T helper cells; 5HT: 5-hydroxytryptamine
Several studies have demonstrated that the use of antibiotics during pregnancy, infancy, and childhood is strongly correlated to short-term consequences including antibiotic-related diarrhea, infection from Clostridium difficile, and AR emergence, while the long-term effects may comprise allergic, autoimmune, and metabolic disorders [54••, 58–64]. The human intestinal microbiome may also harbor antimicrobial resistance genes (ARGs) as a reservoir undergoing changes in its resistome, i.e., the ARGs from both pathogenic and non-pathogenic bacteria after antibiotic treatment [64, 65]. Moreover, a handful of studies have proposed a transitory dysbiosis, whereas other studies have shown that antibiotics may cause permanent disturbances of the intestinal microbial communities [54••, 65]. Actually, antibiotics lessen the microbial diversity in short-term usage, while they present a variable behavior concerning their long-term effects [66••].
The response of the gut microbiome to antibiotic treatment is a multifactorial process and depends upon the type and spectrum of activity, the route of administration, the duration, the number of doses, the age of subject, the genetic susceptibility and lifestyle, the pharmacological action, and the target bacteria [54••]. Because of the abovementioned factors, there are numerous existing patterns of microbiome shifts due to the use of antibiotics in humans and mouse models as depicted in Table 1 [81, 82••]. In a recent systematic review, it has been shown that changes in the gut microbiome from metronidazole and clarithromycin lasted the longest (4 years), followed by clindamycin (2 years), and ciprofloxacin (1 year). Additionally, antibiotics, particularly macrolides, amoxicillin, amoxicillin/clavulanate, quinolones, clindamycin, lipopolyglycopeptides, ketolides, tigecycline, fosfomycin, and cephalosporins, were associated with elevated numbers of Enterobacteriaceae other than E. coli (mainly Citrobacter spp., Enterobacter spp., and Klebsiella spp.) [82••]. Noteworthy, different classes of antibiotics have variable effects on gut microbiota; for example, β-lactams decrease the abundance of Actinobacteria and Firmicutes and increase Proteobacteria and Bacteroidetes [68, 83]. Elevated abundance of Enterococcus spp. is promoted by amoxicillin, piperacillin and ticarcillin, carbapenems, lipoglycopeptides, and cephalosporins (except fifth generation cephalosporins), while decreased abundance is stimulated by macrolides and doxycycline. Piperacillin and ticarcillin, carbapenems, clindamycin, macrolides, and quinolones reduce significantly the abundance of anaerobic bacteria [65]. There are also studies demonstrating that the intestinal microbiome is resilient after short-term exposure to broad-spectrum antibiotics, but the changes differ among individuals while the restoration of the diversity and composition varies as well [54••, 65, 79, 84].
Table 1.
Existing patterns of microbiome shifts due to antibiotics in humans
| Research/year | Antibiotic category | Main findings |
|---|---|---|
| Dethlefsen L. et al. 2008 [67] | Ciprofloxacin |
Taxonomic richness↓ Diversity ↓ Uniformity↓ |
| Jakobsson HE et al. 2010 [68] | Clarithromycin |
Actinobacteria ↓ Firmicutes ↓ Bacteroides ↑ Proteobacteria ↑ |
| Dethlefsen L. et al. 2011 [65] | Ciprofloxacin |
Microbial diversity↓ Shift in community composition |
| Pérez-Cobas AE et al. 2013 [69] | Moxifloxacin, cefazolin, ampicillin/sulbactam, amoxicillin, penicillin G/clindamycin | Fluctuations in biodiversity parameters for total and growing microbiota |
| Greenwood C et al. 2014 [70] | Ampicillin |
Microbial diversity↓ Enterobacter spp. ↑ |
| Vrieze A et al. 2014 [71] |
Vancomycin Amoxicillin |
Firmicutes (Clostridium, Lactobacillus) ↓ Proteobacteria ↑ |
| Panda S et al. 2014 [72] | Fluoroquinolones and b-lactams |
Microbial diversity↓ Number of taxa↓ Average microbial load↑ |
| Stewardson AJ et al. 2015 [73] | Ciprofloxacin |
Bifidobacterium (Actinobacteria) ↓ Alistipes (Bacteroidetes) ↓ Firmicutes (Faecalibacterium, Oscillospira, Ruminococcus and Dialister) ↓ |
| Mikkelsen K et al. 2015 [74] | Vancomycin, Gentamicin, Meropenem | Gut bacterial population↓ |
| Rashid MU et al. 2015 [75] | Ciprofloxacin | Bifidobacterium (Actinobacteria) ↓Bacteroides ↑ |
| Rashid MU et al. 2015 [75] | Clindamycin | Lactobacilli and Bifidobacteria ↓ |
| Lichtman J et al. 2016 [76] | Streptomycin |
Overall diversity ↓ Ruminococcaceae↑ Bacteroidaceae ↑ |
| Korpela K, et al. 2017 [77] | Macrolide |
Bacteroides ↑ Proteobacteria ↑ Actinobacteria ↓ Firmicutes ↓ Total bacteria diversity ↓ |
| Lankelma JM, et al. 2017 [78] |
Ciprofloxacin Vancomycin Metronidazole |
Gut microbiota diversity↓ |
| Palleja A, et al. 2018 [79] |
Meropenem Gentamicin Vancomycin |
Enterobacteria↑ Enterococcus faecalis↑ Fusobacterium nucleatum↑ Bifidobacterium spp.↓ Other butyrate species↓ |
| Willmann M, et al. 2019 [80] |
Ciprofloxacin Cotrimoxazole |
Diversity↓ Evenness↓ Different effects on gut resistome |
Overall, antibiotics lead to microbiome perturbations and create gut dysbiosis mainly by the increase of the abundance of Proteobacteria (considered as pathobionts) and the decrease of Actinobacteria and Bacteroidetes (considered as synbionts), with great varieties regarding severity and resilience. To overcome the collateral damage of the antibiotic usage, such as dysbiosis and AR, efforts have focused on personalized strategies, including understanding of microbiota-host interactions, rational use of antibiotics, vaccines and non-conventional antimicrobial agents, specifically bacteriophages, antimicrobial peptides, nucleoside-based antibiotics, and monoclonal antibodies [54••].
Administration of Antibiotics as a Cause of Obesity
Antibiotics have been linked to alterations in the gut microbiome, which, in turn, have been suggested to be related to the development of obesity. The mechanisms by which antibiotics may provoke weight gain remain obscure, but several hypotheses have been suggested, such as (i) the increased ability of some gut bacteria to extract energy from indigestible polysaccharides, (ii) the decrease in bacteria that are known to be protective against obesity, (iii) changes in hepatic lipogenesis, and (iv) the decrease in intestinal defense and in beneficial metabolic and immunity pathways [63••, 66••].
Evidence from Animal Studies
There is a growing body of evidence from animal models highlighting the association between the administration of low doses of antibiotics and obesity [66, 84–86]. Table 2 depicts major studies in animal models demonstrating the association between exposure to various classes of antibiotics in mice with the development of obesity. Cho et al. have documented that sub-therapeutic doses of antibiotics resulted in changes in the gut microbiome as well as in weight gain in young rodents [85]. Cox et al. confirmed the notion that around the time of birth, rodents were particularly vulnerable to low-dose antibiotic exposure. In particular, in male mice whose mother was administered penicillin before birth and throughout weaning, there was a significant enhancement in fat mass and total mass that persisted during adulthood, whereas male mice receiving antibiotics after weaning and female mice receiving antibiotics before birth and after weaning had similar body composition, when compared to controls [86]. Interestingly, experiments in animal models have demonstrated that there were reductions in the population size of specific microbiota, such as Lactobacillus, Allobaculum, Rikenellaceae, and Candidatus Arthromitus, suggesting that the abovementioned bacteria could possess protective roles in shaping adult metabolism [86]. Noteworthy, Li et al. have documented a significant decrease in the richness and diversity of the gut microbiota after exposure to antibiotics in mice, a finding which is consistent with similar results among humans [92].
Table 2.
List of main studies in animals associating antibiotics exposure and obesity
| Research/Year | Type of mice Used | Antibiotic Treatment | Main findings | Remarks |
|---|---|---|---|---|
| Backhed et al. 2007 [87] | GF mice | Western diet during 6–10 weeks of life | ✓ ↓ metabolic endotoxemia | |
| Cani et al. 2008 [88] | ob/ob mice | Ampicillin 1 g/L during the first 6 weeks of life | ✓ ↑ weight gain | ✓ Alterations in the gut microbiota control metabolic endotoxemia and inflammation, by means of ↑ intestinal permeability. |
| Cho et al. 2012 [85] | C57BL/6J mice |
I) Sub-therapeutic doses of antibiotics at weaning (age 3 weeks) through life II) Control group (no antibiotics administered) |
Compared to the control group, the group with antibiotics administered had: ✓ ↑ 3% body fat ✓ ↑ GIP ✓ ↑ F/B ratio |
✓ Administration of sub-therapeutic antibiotic therapy resulted in alterations in the gut microbiome and ↑ adiposity in mice. |
| Murphy et al. 2013 [89] | BL6 mice | Vancomycin 2 mg/d, high fed diet during the first 7 weeks of life |
↓ weight gain ↓ fasting plasma glucose. |
|
| Cox et al. 2014 [86] | Experiment 1, 2, 3: C57BL/6J mice Experiment 4: GF Swiss Webster mice |
Experiment. 1: I) Antibiotic treatment with LDP at birth or age 4 weeks and lasting throughout life II) Control group with no antibiotics administered Experiment 2: I) LDP lifelong with a high-fat diet at 17 weeks II)4 groups—all combinations with and without LDP and/ or HFD Experiment 3: I) LDP during first 4 weeks, first 8 weeks or lifelong with a HFD at 6 week II) Control group with no antibiotics administered Experiment 4: I) Transferring antibiotic-treated gut microbiota to GF mice II) Control group with no antibiotics administered |
✓ Experiment 1: ↑ weight if LDP administered at birth rather than at age 4 week, with ↑ effect on male mice. ✓ Experiment 2: ↑ fat mass in both male and female mice Experiment 3: ↑ total, lean and fat mass in all groups, with ↑ effect on female mice Experiment 4: ↑ total and fat mass in recipients of gut microbiome from LDP mice. |
✓ LDP exposure from birth and in early life may result in alterations in metabolism in mice and lead to ↑↑ adiposity. ✓ LDP ↑↑ the effect of HFD on the occurrence of obesity. ✓ The obese phenotype due to LDP-induced microbiome changes is transferrable. |
| Mahana et al. 2016 [90] | BL6 mice | Penicillin G 6.8 mg/L during the first 1–14 days of gestation |
✓ ↑ weight and fat mass ✓ ↑ insulin resistance as well as NAFLD score. |
|
| Rodrigues et al. 2017 [91] | GF Swiss Webster mice | Ampicillin 1 g/l, Metronidazole 1 g/L, Neomycin 1 g/L, Vancomycin 0.5 g/L or all of them | ✓ ↓ FPG | ✓ ↑ Akkermansia muciniphila after Vancomycin administration |
| Li et al. 2017 [92] | C57BL/6 mice |
I) Florfenicol II) Azithromycin III) Control group with no antibiotics administered |
✓ ↑↑ F/B ratio in the two antibiotics groups. ✓ ↓↓ Rikenella in the azithromycin treated group. |
✓ ↓↓ richness and diversity of microbiota in the two antibiotics groups. ✓ ↑↑ adipogenesis in the antibiotics groups. |
| Zarrinpar et al. 2018 [93] |
Ob/ob mice DIO mice |
Norfloxacin and Ampicillin for 2 weeks |
✓ DIO mice exhibited ↓metabolic endotoxemia ✓ ↓ LPS levels |
DIO diet-induced obesity, F/B ratio Firmicutes to Bacteroidetes ratio, FPG fasting plasma glucose, GF germ free, GIP glucose-dependent insulinotropic peptide, HFD high-fat diet, LDP low-dose penicillin, LPS lipopolysaccharide, NAFLD non-alcoholic fatty liver disease
Since the 1950s, antibiotics have been added to food and water in pigs, cows, and chickens as an effective method to improve survival and growth, in particular, weight gain [94]. Notably, early-life exposure to antibiotics in these farm animals has much greater effects on weight gain, than when exposure occurred in later life [95]. Early antibiotic exposure in animal models has shown that reductions in the population size of specific microbiota are related to subsequent adiposity. These data suggest that the loss of diversity of the gut microbiome, especially early in life, may have potential long-term detrimental effects on the adult host gut microbiome and metabolic health. Early exposure of the developing neural circuitry regulating energy homeostasis (input and output) could result in changes in the leptin signaling pathways or other aspects of that circuitry that favor subsequent weight gain. This could occur via effects of SCFAs or other molecules affected by the microbiome [96, 97].
Moreover, in the agricultural setting, efficacy in terms of improving growth and survival with the use of a wide range of antibiotics, such as lincosamides, macrolides, streptogramins, phosphoglycolipids, polyethers, quinoxalines, and sulfonamides, has been documented. This practice has been widely adopted by farmers while a number of different antibiotics have been used for this purpose [95, 98]. The fact that treatment with a wide variety of antibiotic classes leads to increased fat mass, is suggestive of the notion that changes in the gut microbiota may alter host metabolism [63••]. Changes in the intestinal permeability as well as variations in the host immune responses may contribute to the alterations in metabolic outcomes in the host. Of note, administration of high doses of antibiotics early in life in animal models has led to decreased fat mass and body weight as well as improvement in markers of insulin sensitivity. Therefore, it seems likely that variations in metabolic effects are largely dependent on the dose of antibiotics, timing, animal model, and dietary factors. These important parameters are suggested to have differential effects on gut microbiota and, subsequently, on host metabolism [95].
Evidence from Human Studies
To date, not so many studies have been conducted in humans regarding the association of exposure to antibiotics early in life and the subsequent development of childhood obesity. Table 3 depicts the list of meta-analyses and main studies associating antibiotics exposure and obesity in childhood and adulthood.
Table 3.
List of meta-analyses and main studies associating antibiotics exposure and obesity
| Research/year | Population, type of study | Antibiotic treatment | Main findings | Remarks |
|---|---|---|---|---|
| List of meta-analyses | ||||
| Gough et al. 2014 [99] |
4,316 children aged 1 month -12 y.o., from 7 countries (International study) Meta-analyses from 10 RCTs |
I) Antibiotics administration II) Control group (no antibiotics administered) |
↑ Weight by 23.8 g /month among children receiving antibiotics (95% CI: 4.3, 43.3) | ✓ Antibiotics administration was related to increased weight. |
| Shao et al. 2017 [100] |
445,880 children from developed countries, International study Systematic review and meta-analyses of 15 studies |
I) Antibiotics administered prenatal and up to 23 months of age II) Control group (no antibiotics administered) |
✓ ↑ Risk of childhood overweight (RR 1.23; 95% CI: 1.13, 1.35) ✓ ↑ Risk of childhood obesity (RR 1.21; 95% CI: 1.13, 1.30) ✓ For every additional course of antibiotics administered: ↑ 7% Risk of overweight (RR 1.07; 95% CI: 1.01, 1.15) ↑ 6% Risk of obesity (RR 1.06; 95% CI: 1.02, 1.09) |
✓ Administration of antibiotics prenatal to 23 months of age was related to increased risk of obesity during childhood. |
| Wan et al. 2020 [101] |
1,253,035 children, China Meta-analysis from 23 observational studies |
✓ Prenatal exposure to antibiotics was not related to childhood overweight/obesity, whereas a higher risk of overweight/obesity was noted in the subgroup analysis of the second trimester (risk ratio = 1.13; 95% CI: 1.06–1.22; P = 0.001). ✓ Antibiotics administration during infancy may increase the risk of childhood overweight/obesity (risk ratio = 1.14; 95% CI: 1.06–1.23; P = 0.001). |
✓ Antibiotics administration during the second trimester of pregnancy and infancy may increase the risk of childhood overweight/obesity. | |
| List of main studies | ||||
| Thuny et al. 2010 [102] |
96 adults aged 45–77 y.ο with suspected IE, France Case-control study |
I) Gentamicin + vancomycin II) Gentamicin + amoxicillin III) Other antibiotics IV) Control group (no antibiotics administered) |
↑ BMI in patients treated with antibiotics, when compared to controls | ✓ Antibiotic treatment may have an effect on weight gain. |
| Saiman et al. 2010 [103] |
260 children aged 6–18 y.o. with cystic fibrosis, USA Multicenter, double blind placebo-controlled randomized study |
I) Azithromycin II) Placebo |
↑Weight (+ 0.58 kg, 95% Cl 0.14, 1.02) in patients administered with antibiotics | ✓ Slight weight gain with antibiotics administration. |
| Ajslev et al. 2011 [104] |
28,354 children aged 7 y.o. from the Danish National Birth Control, Denmark Prospective longitudinal study |
Antibiotic treatment up to 6 months of age | Conflicting results | ✓ Antibiotics administered early in life have a different effect on children’s weight, which may vary according to maternal BMI. |
| Francois et al. 2011 [105] |
69 adults aged 50–78 y.o. referred for upper gastrointestinal endoscopy, USA Prospective cohort study |
I) Amoxicillin + Clarithromycin + PPI in Helicobacter pylori positive adults II) None in Helicobacter pylori negative adults |
↑ BMI (+ 5%) in patients receiving antibiotics | ✓ ↑ Ghrelin and leptin levels among patients receiving antibiotics. |
| Trasande et al. 2013 [106] |
11,532 children from the Avon Longitudinal Study of Parents and Children Cohort, UK Prospective longitudinal study |
I) Antibiotics administered during the first 2 years of life II) Control group (no antibiotics administered) |
✓ ↑ Odds of overweight at 38 months of age (OR: 1.22) ✓ ↑ BMI at 38 months of age |
✓ Antibiotics administered during the first 6 months of life were related to increased odds of overweight during early childhood. |
| Murphy et al. 2014 [107] |
74,946 children aged 5–8 years old from the International study of asthma and allergies in childhood, (International study) Secondary analysis from a multicenter cross-sectional study |
I) Antibiotics administered during the first 12 months of life II) Control group (no antibiotics administered) |
✓ ↑ BMI (+ 0.11) among boys administered antibiotics | ✓ Antibiotics administration was related to a small increase in BMI in boys, but not girls during childhood. |
| Bailey et al. 2014 [108] |
65,480 children aged 24 months to 59 months, USA Retrospective cohort study |
I) Antibiotics administered during 0-23 months of life II) Control group (no antibiotics administered) |
✓ ↑ Risk of obesity with increased and additive exposure to antibiotics (≥ 4 courses) (RR 1.11; 95% CI: 1.02, 1.21) ✓ ↑ Risk of obesity with antibiotics exposure during 0–5 months of age (RR 1.11; 95% CI: 1.03, 1.19), but lesser with exposure at 6–11 mo of age (RR 1.09; 95% CI: 1.04, 1.14) |
✓ Antibiotics administration during the first 2 years of life was related to obesity in early childhood. |
| Azad et al. 2014 [109] |
723 children aged 9–12 y.o., Canada Case-control study |
I) Antibiotics administered during the 1 year of life II) Control group (no antibiotics administered) |
✓ ↑ Risk of overweight if they received antibiotics during the 1 year of life (32.4% vs 18.2% at age 12) ✓ ↑Odds of overweight more marked in boys than girls (aOR: 5.35; 95% CI: 1.94, 14.72). |
✓ Boys were more likely to be overweight at 12 years old, if they had received antibiotics during the 1 year of life. |
| Angelakis et al. 2014 [110] |
82 adults aged 40–70 y.o. with IE from Q fever, France Case-control study |
I) Doxycycline + hydroxychloroquine administered for at least 18 months II) Control group (no antibiotics administered) |
Conflicting results | ✓ Doxycycline + hydroxychloroquine administration resulted in both weight gain and weight loss. |
| Mikkelsen et al. 2015 [74] |
12 adults aged 18–40 y.o., healthy men, Denmark Interventional study |
I) Administration of gentamicin, meropenem and oral vancomycin | ↑ Weight (+ 1.3 kg) | ✓ A 4 days administration of antibiotics resulted in weight gain as well as increased YY peptide secretion |
| Saari et al. 2015 [111] |
12,062 children aged > 24 months from a Finish birth control, Finland Population-based study |
I) Antibiotics administration during the first 24 months of life II) Control group (no antibiotics administered) |
↑ BMI with antibiotics administration (boys + 0.13 SDS, girls + 0.07 SDS) | ✓ Antibiotics administration during the first 2 years of life was related to increased BMI SDS. The effect was more prominent with macrolides administration during the first 6 months (boys + 0.28 SDS, girls + 0.23 SDS) |
| Edmonson and Ei ckhoff, 2016 [112] |
607 Children aged 2 months to 5.9 y.o. from the Randomized intervention for children with vesicoureteral reflux, USA Secondary analysis of data from a RCT |
I) Trimethoprim-sulfamethoxazole treatment for 2 years II) Control group (no antibiotics administered) |
No effect noted on the prevalence of overweight/obesity at 24 months (Antibiotic 24.8% vs Placebo 25.7%) | ✓ Administration of trimethoprim-sulfamethoxazole for 2 years had no effect on weight among children with recurrent urinary tract infections. |
| Sc Scott et al. 2016 [113] |
21,714 children aged 4 y.o. from the Health improvement network in the UK, UK Retrospective cohort study |
I) Antibiotics administered before 2 years of age II) Control group (no antibiotics administered) |
✓ ↑ Odds of obesity at 4 y (OR: 1.21; 95% CI: 1.07, 1.38) ✓ ↑ Odds of obesity with repeated administration of antibiotics: ✓ 3–5 prescriptions (OR: 1.41; 95% CI: 1.20, 1.65) ✓ ≥ 6 prescriptions (OR: 1.47; 95% CI: 1.19, 1.82) |
✓ Antibiotics administration during the first 2 years of life was related to an increased odds of childhood obesity, especially if repeated exposure to antibiotics occurred. |
| Li et al. 2017 [114••] |
26,0556 children aged 2–18 y.o. from the Kaiser permanente North California population, USA Longitudinal birth control study |
I) Children with infection administered with antibiotics II) Children with infection not administered with antibiotics III) Control group (no infection and not antibiotics administered) |
✓ No relationship between antibiotic use during infancy with odds of childhood obesity (OR: 1.01; 95% CI: 0.98, 1.04) ✓ ↑ Odds of childhood obesity in children with infection compared with controls (OR: 1.25; 95% CI: 1.20, 1.29) |
✓ Infection rather than antibiotics administration during infancy was related to an increased odds of childhood obesity. |
| Stark et al. 2019 [115] |
333,353 children from the US Department of defense TRICARE, USA Cohort study |
I) Antibiotics administered during the first 24 months of age II) Histamine H2 receptors antagonists administration III) PPIs administration |
✓ Antibiotics administration was related to childhood obesity (HR 1.26; 95% CI 1.23 to 1.28). ✓ This association persisted regardless of the antibiotic class, while it strengthened with each additional class of antibiotic administered. |
✓ Antibiotics administered in the first 2 years of life are related to an increased risk of childhood obesity. |
| Kelly et al. 2019 [116] |
8,286 children from the Irish national longitudinal study of children, Ireland Secondary longitudinal study |
I) Antibiotics administration during the second and third years of life II) Control group (no antibiotics administered) |
✓ Any antibiotic administration between 2 and 3 y.o. did not predict risk of overweight or obesity at age 5. ✓ ≥ 4 courses of antibiotics between 2 and 3 years of age were independently related to obesity at age 5 (odds ratio 1.6, 95% confidence interval 1.11–2.31) |
✓ Number of antibiotic courses, rather than antibiotic use per se, is suggested to be an important factor in the link between early antibiotic exposure and childhood obesity. |
| Park et al. 2020 [117] |
31,733 children aged 30–36 months and 4–6 y.o. from Korea, South Korea Retrospective cohort study |
I) Antibiotics administration during the first 24 months of life II) Control group (no antibiotics administered) |
✓ Children who used ≥ 5 antibiotic classes had higher odds of obesity than those who used only 1 class (OR 1.42, 95% CI 1.12–1.8). ✓ Children with > 180 days of antibiotics administration had increased risk of obesity than those with 1-–30 days of antibiotics administration (OR 1.40, 95% CI 1.19–1.64). ✓ Children with earlier antibiotics administration had increased risk of obesity (OR 1.15 per 6 months, 95% CI 1.08–1.22). |
✓ Increased number of antibiotic classes, longer duration of antibiotic administration and earlier antibiotics administration (before 24 months of age) was related to childhood obesity at 30–36 months of age. |
| Chelimo et al. 2020[118] | 6,853 children aged 54 months, New Zealand | I) Antibiotics administration during 48 months of age | ✓ Administration of antibiotics for ≥ 9 times was related to an increased likelihood of obesity, when compared with no exposure (adjusted odds ratio, 2.41; 95% CI, 1.07–5.41). Children whose exposure began in the 1 year of life had a higher adjusted mean (SD) BMI-for-age z score than those not exposed (1.06 [0.05] vs 0.89 [0.09]; P = .03), | ✓ Repeated antibiotics administration in early childhood was related to a higher mean BMI-for-age z score and an increased likelihood of obesity. |
BMI body mass index, CI confidence intervals, HR hazard ratio, IE infective endocarditis, OR odds ratio, PPIs proton pump inhibitors, RCT randomized controlled trials, SDS standard deviation score, y.o. years old
Wan et al. have performed a meta-analysis of 23 observational studies including 1,253,035 children. They have reported that the administration of antibiotics only during the second trimester of pregnancy and during infancy has resulted in childhood overweight/obesity [101]. Noteworthy, increased odds of childhood overweight/obesity were linked to the following parameters: (i) administration of antibiotics during the first 6 months of life, (ii) repeated exposure to antibiotics for ≥ 3 courses, (iii) treatment with broad-spectrum antibiotics, and (iv) male gender [109, 113, 116, 117].
In sharp contrast to previous studies, only two studies have reported no difference between exposure to antibiotics and childhood overweight/obesity [112, 118, 119]. However, in the first study, only a single class of antibiotics was prescribed as prophylaxis; therefore, the results might have been affected by the type and dosage of the administered antibiotic, while in the second study, infection per se rather than the administration of antibiotics accounted for the observed increased body weight.
In adults, only observational studies involving a small number of participants have been conducted. These studies have reported that subjects treated with antibiotics were prone to weight gain, when compared with those not administered any antibiotics. In particular, Mikkelsen et al. have reported weight gain among adult patients receiving gentamicin, meropenem, and oral vancomycin together with an elevation of serum peptide YY levels [74]. In addition, Thuny et al. have demonstrated weight gain among adults receiving antibiotics for suspected endocarditis [102]. Besides, Francois et al. have documented an increase in serum ghrelin and leptin levels as well as an increase in BMI among adults receiving antibiotics for Helicobacter Pylori infection [105].
Limitations of Studies
Unfortunately, almost all of the studies performed in humans have been observational in design; therefore, it is not possible to confirm that antibiotic use is the primary cause of obesity, without excluding other confounding factors, such as dietary parameters, host genetics, maternal BMI, breastfeeding, maternal smoking, and the presence of infection itself [109, 113, 114, 116, 117].
In addition, in some of the abovementioned studies, the data regarding antibiotics usage and compliance have been relatively unreliable, as these have been based upon recalls from the parents of the enrolled children [109, 117, 119]. Furthermore, some of these studies have examined children with infections, and the resolution of infection per se with the administration of antibiotics might account for weight gain.
Overall, although most studies are supposed to link the early-life exposure to antibiotics with childhood overweight/obesity, well controlled and large-scale studies are mandatory to indicate which genetic and environmental factors are fundamental to the development of childhood obesity, that may be targeted to improve the childhood obesity epidemic.
The Reservoir of Antibiotics in Animal Feed, Emerging AR, Gut Dysbiosis, and Obesity
Apart from their usual indications, antibiotics have also been used as ‘animal growth promoters’ for about 70 years [120, 121]. This term implies that the administration concerns low, subtherapeutic dosage and the main target is not the health benefit, but the animal growth. Due to the direct need for animal protein in recent decades with rising human populations, intensive animal husbandry exploited antibiotics as an essential weapon to ward off infection, but also to improve animal health. The intensification of farming led to a greater dependence on antimicrobials in order to convert more efficiently food to animal products and to minimize morbidity/mortality rates. As antimicrobials are added in food or water, they are omnipresent and leave residues in all the livestock and food chain, e.g., meat and milk, causing environmental contamination with potential toxic implications in humans [54••].
Nevertheless, the most perilous side effect is that the inappropriate use of antibiotics in animal feeding represents a leading cause of AR. Cheap antibiotics are easily available in developing countries without prescription and seem to overwhelm farms and environment with multi drug-resistant microbes. AR, characterized as a ‘ticking time bomb’, is closely correlated to antibiotic consumption in food production representing a real scourge given that more than 52% of total antibiotic consumption accounts for animals [122]. The recent colistin resistance globally spread from Chinese pigs highlights the severity of the agricultural AR selection [123]. AR, which is also associated with the increasing use of newer classes of antibiotics, may reduce the diversity and richness of bacteria taxa in the gastrointestinal tract, leading to subsequent alterations in the gut microbiota and dysbiosis with potential detrimental effects on metabolic homeostasis and weight control.
Apart from AR, another growing concern is the contribution of subtherapeutic antibiotic treatment to weight gain in humans. Over the past 20 years, there was a parallel rise of the prevalence of obesity and the industrial intensive farming with increased antibiotic use. Nevertheless, this association has not been thoroughly investigated yet. More well-designed prospective studies with an omics approach are needed to establish a potential causal link between the enormous exposure to food containing low-residue antibiotics and perturbations of gut microbiota associated with dysbiosis and concomitant increase in body weight.
The growing concern for the AR spreading through the food chain forced many countries in Europe and the USA to ban the use of antimicrobial agents for animal growth, especially the medically important for humans [58]. However, legislation is hard to implement, especially if there is no alternative. Promising innovations, such as the use of probiotics and prebiotics as alternative feed additives, activated carbon absorption, membrane filtration, advanced oxidation processes, and silver nanoparticles could supersede antibiotics in animal feed [121]. In this context, various substances present antimicrobial properties; however, more studies are required to investigate their exact mechanism of action and potential toxicity. Additionally, incentives for farmers to produce antibiotic-free food might help tackling the non-human antibiotic use in a more effective way.
Infections or Antibiotics as a Cause of Obesity: the Chicken or the Egg?
As mentioned above, infection per se rather than the administration of antibiotics could be a cofactor to the development of obesity. Specifically, in a large study involving more than 260,000 individuals, Li et al. have reported a relationship between infection and increased risk of obesity during childhood and adolescence, which was found to be independent of antibiotic treatment [114••]. Furthermore, the odds of obesity were demonstrated to be higher in the group with untreated infections, when compared to those who were both uninfected and untreated. However, this study presented a significant limitation; the assessment of obesity was made by comparing BMI among children from 2 to 18 years old, i.e., with a wide variation in their age range, whereas comparisons between children of similar age might have resulted in different outcomes [119].
Apart from bacteria, viruses and other pathogenic microorganisms may be considered as potential causes of obesity. At least ten different viruses have been reported to cause obesity in animals, such as canine distemper virus, Rous-associated virus type 7, Borna disease virus (BDV), scrapie agent, avian adenovirus SMAM1, and human adenoviruses Ad36, Ad5, and Ad37 [124–126]. While some viruses have been documented through polymerase chain reaction (PCR) to affect the central nervous system (CNS) by reducing appetite, affecting energy expenditure and leading to obesity, other viruses seem to directly affect the adipocytes. SMAM-1 and three human adenoviruses, such as adenovirus (Ad) 36, Ad-37, and Ad-5, act in adipocytes and are potentially associated with obesity. In particular, these viruses lead to a faster activation of various enzymes and transcription factors, resulting in the accumulation of triglycerides, in conjunction with a quicker differentiation of pre-adipocytes into mature adipocytes, as shown in in vitro or in vivo findings of lipid accumulation and impaired leptin secretion. Interestingly, SMAM1 and Ad36 have been documented to be related with obesity in humans. Ad-36, the most studied adenovirus, is the only human adenovirus to date that has been related to human obesity. Ad-36 causes obesity in animals, where Ad-36 seropositivity has also been found in 30% of individuals with obesity in comparison to 11% of subjects with normal weight [125, 126].
Nevertheless, the role of viruses as well as bacteria in the development of obesity needs further investigation. These data regarding viral infections suggest the possibility not only that infections may be direct causes of weight gain, but also that the inflammatory processes and their metabolic consequences in general may promote weight gain. The notion of “infectobesity”, although intriguing, should be confirmed or refuted by studies in animal models as well as in humans, with the advent of sophisticated molecular methods.
Therapeutic Approaches Other than Antibiotics to Modulate Gut Microbiome
Probiotics are “live microorganisms, which confer health benefits to the host” and thus, are considered to possess the potential to exert modifications in the composition of the gut microbiota and the host metabolism. The administration of probiotics to pregnant women and in animal models as well has resulted in changes in the growth and weight of their offspring, attributed to the differences in the strains of probiotics together with differences in host species characteristics [125, 127]. Additional studies are anticipated to determine whether probiotics may influence host growth and development after antibiotic treatment. Currently, available probiotics are limited to a relatively small number of phylogenetic species, when compared to the high diversity of the gut microbiota in developing infants and adults. Apart from the potential use of classic probiotics, there is growing interest regarding the use of probiotics, which have been modified by genetic engineering. In particular, engineered Lactococcus lactis has been utilized in order to express and deliver antimicrobial peptides against Enterococcus faecium, thus resulting in reductions of its abundance counts by 10,000-fold in vitro [127–129]. More specifically, there are more sophisticated probiotics, which upon detection of a pathogen, they institute a genetic program that kills their target.
Currently, there is ongoing research regarding the use of bacteriophages, which could be utilized in order to destroy pathogenic bacteria. In addition to its bacterial inhabitants, the gut contains an equally fascinating viral community that exerts a profound effect on the microbiota and, in turn, on the host. As the natural predators of bacteria, phages have been formerly used to treat bacterial infections before the advent of antibiotics [130]. As antibiotics have become less effective due to resistance issues, phages have been the focus of renewed therapeutic interest as they are often highly specific to the targeted bacterium and are self-replicating, reducing the costs of producing phage-based therapeutics. Phages active against Enterococcus faecalis, Bacillus cereus, and Pseudomonas aeruginosa have been identified, among many others. Notably, phages have also been the focus of genetic engineering in order to improve their function in modulating the gut microbiota [130, 131]. Recently, the natural transformation ability of phages has been enhanced by programmable nucleases to enable the development of phages, which specifically kill bacteria with undesirable sequencing, such as ARGs or virulence factors [130, 131]. Based on several reports, probably the ideal diseases for which phage therapy would be appropriate, are those with a specific bacterial cause, refractory to antibiotics, and accessible to phages, such as infections that are caused by Mycobacterium tuberculosis, Vibrio cholerae, Clostridium difficile, enteroaggregative E. coli, and diffusely adherent E. coli [132]. Although there is a long way ahead, natural and engineered phages hold great promise as potential tools in the fight against pathogens and gut dysbiosis.
Conclusions
The gut microbiota expands in parallel with normal human growth and development. It forms a community structure, which is unique for every individual, demonstrating an active potential, including pH, immunity, diet, oxygen supplementation, and microbe-microbe interactions, leading to recovery after environmental intrusions. However, despite these homeostatic mechanisms, the exogenous administration of antibiotics may result in short-term alterations in the gut microbiome, particularly during the development period. The long-term effects of antibiotics on gastro-intestinal microbial composition have been more variable with studies reporting differences in both intra- and inter-individual responses to the same antibiotic.
Overall, the majority of animal studies and meta-analyses of human studies on the association between antibiotics and subsequent development of obesity is suggestive of a significant link between exposure to antibiotics, particularly early exposure, and the development of subsequent obesity as a result of alterations in the variety of the gut microbiota. The evidence is strong in animal models whereas evidence in humans is inconclusive requiring well-designed, long-term longitudinal studies to examine this association.
While early antibiotic exposure in animal models has shown that reductions in the population size of specific microbiota, such as Lactobacillus, Allobaculum, Rikenellaceae, and Candidatus Arthromitus, have been related to adiposity, it seems likely that the loss of diversity of the gut microbiome, especially in early life, may have potential long-term detrimental effects on the adult host gut microbiome and metabolic health. As alterations in the gut microbiome in humans occur during lifetime due to the influence of various environmental and genetic factors as well as the aging process, more large-scale longitudinal studies over a long time period are needed to elucidate whether changes that occur in the microbiome after exposure to antibiotics, particularly early exposure, are causal of subsequent weight gain or consequent of weight gain. In the short-term in humans, changes in caloric intake seem to result in changes in the microbiome that reflect the energy balance (unlike rodents) rather than the other way around [133].
Aiming at reversing some of the metabolic consequences resulting from treatment with antibiotics, there is need to develop strategies restoring the gut microbiota. Several questions still remain unanswered. If the recovery from dysbiosis to homeostasis and equilibrium is timely achieved, is it possible to prevent later metabolic disorders, such as obesity? Do bacteria or viruses cause obesity or is it just the recovery from the infection that leads to obesity? Is there a potential link between the administration of antibiotics, which results in resolution of the infection, and the development of obesity? Is it just a matter of long-term alterations in the gut microbiome due to the use of antibiotics or not? The answer to these questions remains to be elucidated with long-term longitudinal studies which have yet to be done.
There is no doubt that antibiotics are important and life-saving drugs, which have a great impact on human’s morbidity and mortality. Nonetheless, despite their undoubted usefulness, there is now a growing body of evidence that these agents may contribute to the development of obesity via alterations in the gut microbiota [63••]. Additional longitudinal studies in humans that would clarify several parameters of exposure to antibiotics, such as the optimum dose, class, and timing of administration, could further guide clinicians in their clinical setting. More studies are required to enhance our understanding of the extent of usage of antibiotics and their metabolic repercussions which may be long-lasting or amplified. Finally, little is known about the potential to reverse the abovementioned metabolic effects of antibiotics with targeted restoration bacteriotherapy administered at the right chronic period in life.
Abbreviations
- Ad
Adenovirus
- ADEs
Adverse drug events
- AR
Antibiotic resistance
- ARGs
Antimicrobial resistance genes
- BCAA
Branched-chain amino acids
- BDV
Borna disease virus
- BMI
Body mass index
- CRP
C-reactive protein
- DC
Dendritic cells
- DM
Diabetes mellitus
- DNA
Deoxyribonucleic acid
- F/B
Firmicutes to Bacteroidetes ratio
- GF
Germ free
- IBS
Irritable bowel syndrome
- LPS
Lipopolysaccharide
- MS
Metabolic syndrome
- NAFLD
Non-alcoholic fatty liver disease
- PCR
Polymerase chain reaction
- PMNs
Polymorphonuclear neutrophils
- RCT
Randomized controlled trial
- SCFAs
Short chain fatty acids
- T2DM
Type 2 diabetes mellitus
- TMA
Trimethylamine
- TMAO
Trimethyl-amine-N-oxide
- 5HT
5-Hydroxytryptamine
- Th1,2
T helper cells
Declarations
Conflict of Interest
Natalia Vallianou, Maria Dalamaga, Theodora Stratigou Irene Karampela. and Christina Tsigalou declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Metabolism
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Natalia Vallianou, Maria Dalamaga, Theodora Stratigou, Irene Karampela and Christina Tsigalou have contributed equally to the preparation of the manuscript.
Contributor Information
Natalia Vallianou, Email: natalia.vallianou@hotmail.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
Theodora Stratigou, Email: theodorastratigou@yahoo.gr.
Irene Karampela, Email: eikaras1@gmail.com.
Christina Tsigalou, Email: xtsigalou@yahoo.gr.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.NCD Risk Factor Collaboration Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States . NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics; 2017. p. 2020. [PubMed] [Google Scholar]
- 3.https://www.who.int/health-topics/obesity. Accessed on December 20, 2020
- 4.Nishtar S, Gluckman P, Armstrong T. Ending childhood obesity: a time for action. Lancet. 2016;387(10021):825–827. doi: 10.1016/S0140-6736(16)00140-9.4. [DOI] [PubMed] [Google Scholar]
- 5.Tsigalou C, Stavropoulou E, Bezirtzoglou E. Current insights in microbiome shifts in Sjogren's syndrome and possible therapeutic interventions. Front Immunol. 2018;9:1106. doi: 10.3389/fimmu.2018.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11(10):2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivennikov S, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rea D, Coppola G, Palma G, Barbieri A, Luciano A, del Prete P, et al. Microbiota effects on cancer: from risks to therapies. Oncotarget. 2018;9(25):17915–17927. doi: 10.18632/oncotarget.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abreu MT, Peek RM. Gastrointestinal malignancy and the microbiome. Gastroenterology. 2014, 146(6):1534–1546.e3. 10.1053/j.gastro.2014.01.001. [DOI] [PMC free article] [PubMed]
- 12.Vallianou NG, Tzortzatou-Stathopoulou F. Microbiota and cancer: an update. J Chemother. 2019;31(2):59–63. doi: 10.1080/1120009X.2018.1541046. [DOI] [PubMed] [Google Scholar]
- 13.Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: current evidence and perspectives. Curr Obes Rep. 2019;8(3):317–332. doi: 10.1007/s13679-019-00352-2. [DOI] [PubMed] [Google Scholar]
- 14.Rinninela E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasparrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez-Bello MG, Costello EK, Contreras M, Makris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140(6):1713–1719. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15(4):307–310. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- 20.Agace WW, McCoy KD. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity. 2017;46(4):532–548. doi: 10.1016/j.immuni.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Vallianou NG, Geladari E, Kounatidis D. Microbiome and hypertension: where are we now? J Cardiovasc Med. 2020;21(2):83–88. doi: 10.2459/JCM.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 22.Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, Prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. 2020;9(3):179–192. doi: 10.1007/s13679-020-00379-w. [DOI] [PubMed] [Google Scholar]
- 23.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 24.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 25.Charbonneau MR, Blanton LV, DiGiulio DB, Relman DA, Lebrilla CB, Mills DA, et al. A microbial perspective of human developmental biology. Nature. 2016;535(7610):48–55. doi: 10.1038/nature18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: where are we now? Diabetes Res Clin Pract. 2018;146:111–118. doi: 10.1016/j.diabres.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 30.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang X, Xia H, Liu Z, Cui B, Liang P, Xi L, Jin J, Ying X, Wang X, Zhao X, Li W, Jia H, Lan Z, Li F, Wang R, Sun Y, Yang M, Shen Y, Jie Z, Li J, Chen X, Zhong H, Xie H, Zhang Y, Gu W, Deng X, Shen B, Xu X, Yang H, Xu G, Bi Y, Lai S, Wang J, Qi L, Madsen L, Wang J, Ning G, Kristiansen K, Wang W. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 32.Parnell JL, Reimer RA. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes. 2012;3(1):29–34. doi: 10.4161/gmic.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tims S, Derom C, Jonkers DM, Vlietinck R, Saris WH, Kleerebezel M, et al. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707–717. doi: 10.1038/ismej.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 36.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. Febbs Lett. 2014;588(22):4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio. 2016;7(4):e01018–e01016. doi: 10.1128/mBio.01018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Y, Pedersen O. Human gut microbiota in metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 39.Klancic T, Reimer RA. Gut microbiota and obesity: Impact of antibiotics and prebiotics and potential for musculoskeletal health. J Sport Health Sci. 2020;9(2):110–118. doi: 10.1016/j.jshs.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran-Ramos S, Lopez-Contreras BE, Canizales-Quinteros S. Gut microbiota in obesity and metabolic abnormalities: a matter of composition or functionality? Arch Med Res. 2017;48(8):735–753. doi: 10.1016/j.arcmed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Del Chierico F, Abbatini F, Russo A, Quagliariello A, Reddel S, Capoccia D, et al. Gut microbiota markers in obese adolescent and adult patients: age-dependent differential patterns. Front Microbiol. 2018;9:1210. doi: 10.3389/fmicb.2018.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joyce SA, Gahan C. Disease-associated changes in bile acid profiles and links to altered gut microbiota. Dig Dis. 2017;35(3):169–177. doi: 10.1159/000450907. [DOI] [PubMed] [Google Scholar]
- 43.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canfora EE, Meex R, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 46.Blaak E, Canfora E, Theis S, Frost G, Groen A, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benefic Microbes. 2020;11(5):411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 47.Chambers E, Viardot A, Psichas A, Morrison D, Murphy K. Zac-Varghese, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de la Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):440–448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3(4):279–288. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12(1):20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 51.Shi H, Kokoeva MV, Inoue K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belancic A. Gut microbiome dysbiosis and endotoxemia - additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes Med. 2020;20:100302. doi: 10.1016/j.obmed.2020.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinde T, Hansbro PM, Sohal SS, Dingle P, Eri R, Stanley R. Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms. 2020;8(6):921. doi: 10.3390/microorganisms8060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribeiro C, Silveira G, Candido E, Cardoso M, Carvalho C, Franco O. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health. ACS Infect Dis. 2020;6(10):2544–2559. doi: 10.1021/acsinfecdis.0c00036. [DOI] [PubMed] [Google Scholar]
- 55.Hagiya H, Kokado R, Ueda A, Okuno H, Morii D, Hamaguchi S, Yamamoto N, Yoshida H, Tomono K. Association of adverse drug events with broad-spectrum antibiotic use in hospitalized patients: a single-center study. Intern Med. 2019;58(18):2621–2625. doi: 10.2169/internalmedicine.2603-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon MY, Yoon SS. Disruption of the gut ecosystem by antibiotics. Yonsei Med J. 2018;59(1):4–12. doi: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caprio J, Rantz LA. Effects of terramycin on the bacterial flora of the bowel in man. AMA Arch Intern Med. 1950;86(5):649–657. doi: 10.1001/archinte.1950.00230170002001. [DOI] [PubMed] [Google Scholar]
- 58.Konstantinidis T, Tsigalou C, Karvelas A, Stavropoulou E, Voidarou C, Berirtzoglou E. Effects of antibiotics upon the gut microbiome: a review of the literature. Biomedicines. 2020;8(11):502. doi: 10.3390/biomedicines8110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johanesen PA, Mackin KE, Hutton ML, Awad MM, Larcombe S, Amy JM, Lyras D. Disruption of the gut microbiome: clostridium difficile infection and the threat of antibiotic resistance. Genes. 2015;6(4):1347–1360. doi: 10.3390/genes6041347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;373(3):287–288. doi: 10.1056/NEJMc1506004. [DOI] [PubMed] [Google Scholar]
- 61.Iizumi T, Battaglia T, Ruiz V, Perez G. Gut microbiome and antibiotics. Arch Med Res. 2017;48(8):727–734. doi: 10.1016/j.arcmed.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox LM, Blaser MA. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11(3):182–190. doi: 10.1038/nrendo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsigalou C, Konstantinidis T, Stavropoulou E, Bezirtzoglou EE, Tsakris A. Potential elimination of human gut resistome by exploiting the benefits of functional foods. Front Microbiol. 2020;11:50. doi: 10.3389/fmicb.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leong K, Derraik J, Hofman P, Cutfield W. Antibiotics, gut microbiome and obesity. Clin Endocrinol. 2018;88(2):185–200. doi: 10.1111/cen.13495. [DOI] [PubMed] [Google Scholar]
- 67.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez-Cobas AE, Artacho A, Knecht H, Ferrus ML, Friedrichs A, Ott SJ, et al. Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS One. 2013;8(11):e80201. doi: 10.1371/journal.pone.0080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, Newburg DS, Ward DV, Schibler KR. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165(1):23–29. doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, van Nood E, Holleman F, Knaapen M, Romijn JA, Soeters MR, Blaak EE, Dallinga-Thie GM, Reijnders D, Ackermans MT, Serlie MJ, Knop FK, Holst JJ, van der Ley C, Kema IP, Zoetendal EG, de Vos WM, Hoekstra JBL, Stroes ES, Groen AK, Nieuwdorp M. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60(4):824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 72.Panda S, El Khader I, Casellas F, Vivancos JL, Cors MG, Santiago A, et al. Short-term effect of antibiotics on human gut microbiota. PLoS One. 2014;9(4):e95476. doi: 10.1371/journal.pone.0095476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stewardson AJ, Gaia N, Francois P, Malhotra-Kumar S, Delemont C, De Tejada BM, et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect. 2015;21(4):344.e1–344.11. doi: 10.1016/j.cmi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 74.Mikkelsen KH, Frost M, Bahl MI, Licht TR, Jensen US, Rosenberg J, Pedersen O, Hansen T, Rehfeld JF, Holst JJ, Vilsbøll T, Knop FK. Effect of antibiotics on gut microbiota, gut hormones and glucose metabolism. PLoS One. 2015;10(11):e0142352. doi: 10.1371/journal.pone.0142352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rashid MU, Zaura E, Buijs MJ, Keijser BJ, Crielaard W, Nord CE, et al. Determining the long-term effect of antibiotic administration on the human normal intestinal microbiota using culture and pyrosequencing methods. Clin Microbiol Infect. 2015;60(Suppl 2):S77–S84. doi: 10.1093/cid/civ137. [DOI] [PubMed] [Google Scholar]
- 76.Lichtman JS, Ferreyra JA, Ng KM, Smits SA, Sonnenburg JL, Elias JE. Host-microbiota interactions in the pathogenesis of antibiotic-associated diseases. Cell Rep. 2016;4(5):1049–1061. doi: 10.1016/j.celrep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korpela K, Mutanen A, Salonen A, Savilahti E, De Vos W, Pakarinen MP. Intestinal microbiota signatures associated with histological liver steatosis in pediatric-onset intestinal failure. JPEN J Parenter Enteral Nutr. 2017;41(2):238–248. doi: 10.1177/0148607115584388. [DOI] [PubMed] [Google Scholar]
- 78.Lankelma JM, Cranendonk DR, Belzer C, De Vos A, De Vos W, Der Poll T, et al. Antibiotic-induced gut microbiota disruption during human endotoxemia: a randomised controlled study. Gut. 2017;66(9):1623–1630. doi: 10.1136/gutjnl-2016-312132. [DOI] [PubMed] [Google Scholar]
- 79.Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Aline KH, Nielsen T, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255–1265. doi: 10.1038/s41564-018-0257-9. [DOI] [PubMed] [Google Scholar]
- 80.Willmann M, Vehreschild M, Biehl LM, Vogel W, Dorfel D, Hamprecht A, et al. Distinct impact of antibiotics on the gut microbiome and resistome: a longitudinal multicenter cohort study. BMC Biol. 2019;17(1):76. doi: 10.1186/s12915-019-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu L, Surathu A, Raplee I, Chockalingam A, Stewart S, Walker L, Sacks L, Patel V, Li Z, Rouse R. The effect of antibiotics on the gut microbiome: a metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genomics. 2020;21(1):263. doi: 10.1186/s12864-020-6665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Inf Secur. 2019;79(6):471–489. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(Pt 11):3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 84.Isaac S, Scher JU, Djukovic A, Jimenez N, Littman DR, Abramson SB, et al. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72(1):128–136. doi: 10.1093/jac/dkw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cox L, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 89.Murphy EF, Cotter PD, Hogan A, O'Sullivan O, Joyce A, Fouhy F, Clarke SF, Marques TM, O'Toole PW, Stanton C, Quigley EMM, Daly C, Ross PR, O'Doherty RM, Shanahan F. Divergent metabolic outcomes arising from targeted manipulations of the gut microbiota in diet-induced obesity. Gut. 2013;62(2):220–226. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 90.Mahana D, Trend C, Kurtz Z, Boculich N, Battaglia T, Chung J, et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 2016;8(1):48. doi: 10.1186/s13073-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodrigues R, Greer R, Dong X, Dsouza K, Gurung M, Wu J, et al. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017;8:2306. doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li R, Wang H, Shi Q, Wang N, Zhang Z, Xiong C, Liu J, Chen Y, Jiang L, Jiang Q. Effects of oral florfenicol and azithromycin on gut microbiota and adipogenesis in mice. PLoS One. 2017;12(7):e0181690. doi: 10.1371/journal.pone.0181690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zarrinpar A, Chaix A, Xu Z, Chang M, Marotz C, Saghatelian A, et al. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9(1):2872. doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84(4):634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- 95.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: mode of action. Anim Biotechnol. 2002;13(1):29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 96.Mohammadkhah A, Simpson E, Patterson S, Ferguson J. Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children (Basel) 2018;5(12):160. doi: 10.3390/children5120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bliss E, Whiteside E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol. 2018;19:900. doi: 10.3389/fphys.201800900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev. 2003;16(2):175–188. doi: 10.1128/cmr.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gough E, Moodie E, Prendergast A, Johnson S, Humphrey J, Stoltzfus R, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g2267. doi: 10.1136/bmj.g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shao X, Ding X, Wang B, Li L, An X, Yao Q, Song R, Zhang JA. Antibiotic exposure in early life increases risk of childhood obesity: a systematic review and meta-analysis. Front Endocrinol. 2017;8:170. doi: 10.3389/fendo.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wan S, Guo M, Zhang T, Chen Q, Wu M, Teng F, Long Y, Jiang Z, Xu Y. Impact of exposure to antibiotics during pregnancy and infancy on childhood obesity: a systematic review and meta-analysis. Obesity. 2020;28(4):793–802. doi: 10.1002/oby.22747. [DOI] [PubMed] [Google Scholar]
- 102.Thuny F, Richet H, Casalta JP, Angelakis P, Habib G, Raoult D. Vancomycin treatment of infective endocarditis is linked with recently acquired obesity. PLoS One. 2010;5(2):e9074. doi: 10.1371/journal.pone.0009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saiman L, Anstead M, Mayer-Hamblett N, Lands L, Kloster M, Hosevar-Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303(17):1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 104.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35(4):522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 105.Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR, et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011;11:37. doi: 10.1186/1471-230X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes. 2013;37(1):16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murphy R, Stewart AW, Braithwaite I, Beasly R, Hancox RJ, Mitchell EA, et al. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int J Obes. 2014;38(8):1115–1119. doi: 10.1038/ijo.2013.218. [DOI] [PubMed] [Google Scholar]
- 108.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;68(11):1063–1069. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 109.Azad MB, Bridgman SL, Becker AB, Kojurskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes. 2014;38(10):1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 110.Angelakis E, Million M, Kankoe S, Lagier JC, Armougom F, Giorgi R. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob Agents Chemother. 2014;58(6):3342–3347. doi: 10.1128/AAC.02437-14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Saari A, Virta LJ, Sankilampi U, Denkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–626. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 112.Edmonson MB, Eikhoff JC. Weight gain and obesity in infants and young children exposed to prolonged antibiotic prophylaxis. JAMA Pediatr. 2017;171(2):150–156. doi: 10.1001/jamapediatrics.2016.3349. [DOI] [PubMed] [Google Scholar]
- 113.Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology. 2016;151(1):120–129.e5. doi: 10.1053/j.gastro.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li DK, Chen H, Ferber J, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. Lancet Diabetes Endocrinol. 2017;5(1):18–25. doi: 10.1016/S2213-8587(16)30281-9. [DOI] [PubMed] [Google Scholar]
- 115.Stark CM, Susi A, Emerick J, Nylund CD. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68(1):62–69. doi: 10.1136/gutjnl-2017-314971. [DOI] [PubMed] [Google Scholar]
- 116.Kelly D, Kelly A, O’Dowd T, Hays CB. Antibiotic use in early childhood and risk of obesity: longitudinal analysis of a national cohort. World J Pediatr. 2019;15(4):390–397. doi: 10.1007/s12519-018-00223-1. [DOI] [PubMed] [Google Scholar]
- 117.Park YJ, Chang J, Lee G, Son JS, Park SM. Association of class number, cumulative exposure, and earlier initiation of antibiotics during the first two-years of life with subsequent childhood obesity. Metabolism. 2020;112:1543–1548. doi: 10.1016/j.metabol.2020.154348. [DOI] [PubMed] [Google Scholar]
- 118.Chelimo C, Camargo CA, Morton S, Grant CC. Association of repeated antibiotic exposure up to age 4 years with body mass at age 4.5 years. JAMA Netw Open. 2020;3(1):e1917577. doi: 10.1001/jamanetworkopen.2019.17577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Locialo AR, et al. Antibiotic exposure during the first 6 months of life and weight gain during childhood. JAMA. 2016;315(12):1258–1265. doi: 10.1001/jama.2016.2395. [DOI] [PubMed] [Google Scholar]
- 120.Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13(1):7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- 121.Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86(11):2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 122.Taleghani AH, Lim TT, Lin CH, Ericsson AC, Vo PH. Degradation of veterinary antibiotics in swine manure via anaerobic digestion. Bioengineering. 2020;7(4):123. doi: 10.3390/bioengineering7040123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 124.Jess T, Morgen CS, Harpsoe MC, Sorensen T, Ajslev TA, Antvorskov JC, et al. Antibiotic use during pregnancy and childhood overweight: a population-based nationwide cohort study. Sci Rep. 2019;9(1):11528. doi: 10.1038/s41598-019-48065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dhuradhar NV. Infectobesity: obesity of infectious origin. J Nutr. 2001;131(10):2794S–2797S. doi: 10.1093/jn/131.10.2794S. [DOI] [PubMed] [Google Scholar]
- 126.Atkinson RL. Viruses as an etiology of obesity. Mayo Clin Proc. 2007;82(10):1192–1198. doi: 10.4065/82.10.1192. [DOI] [PubMed] [Google Scholar]
- 127.Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12(1):82. doi: 10.1186/s13073-020-00782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Geldart K, Borrero J, Kaznessis Y. Chloride-inducible expression vector for delivery of antimicrobial peptides targeting antibiotic-resistant Enterococcus faecium. Appl Environ Microbiol. 2015;81(11):3889–3897. doi: 10.1128/AEM.00227-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sulakvelitze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45(3):649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Citorik RJ, Mimee M, Lu TK. Bacteriophage-based synthetic biology for the study of infectious diseases. Curr Opin Microbiol. 2014;19:59–69. doi: 10.1016/j.mib.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, Fischetti VA, Marraffini LA. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014;32(11):1146–1150. doi: 10.1038/nbt.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Langdom A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jumpertz R, Son Le D, Turnbauch B, Trinidad C, Bogardus C, Gordon J, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]