Abstract
Background
Bronchiectasis is characterised by excessive sputum production, chronic cough, and acute exacerbations and is associated with symptoms of dyspnoea and fatigue, which reduce exercise tolerance and impair quality of life. Exercise training in isolation or in conjunction with other interventions is beneficial for people with other respiratory diseases, but its effects in bronchiectasis have not been well established.
Objectives
To determine effects of exercise training compared to usual care on exercise tolerance (primary outcome), quality of life (primary outcome), incidence of acute exacerbation and hospitalisation, respiratory and mental health symptoms, physical function, mortality, and adverse events in people with stable or acute exacerbation of bronchiectasis.
Search methods
We identified trials from the Cochrane Airways Specialised Register, ClinicalTrials.gov, and the World Health Organization trials portal, from their inception to October 2020. We reviewed respiratory conference abstracts and reference lists of all primary studies and review articles for additional references.
Selection criteria
We included randomised controlled trials in which exercise training of at least four weeks' duration (or eight sessions) was compared to usual care for people with stable bronchiectasis or experiencing an acute exacerbation. Co‐interventions with exercise training including education, respiratory muscle training, and airway clearance therapy were permitted if also applied as part of usual care.
Data collection and analysis
Two review authors independently screened and selected trials for inclusion, extracted outcome data, and assessed risk of bias. We contacted study authors for missing data. We calculated mean differences (MDs) using a random‐effects model. We used the GRADE approach to assess the certainty of evidence.
Main results
We included six studies, two of which were published as abstracts, with a total of 275 participants. Five studies were undertaken with people with clinically stable bronchiectasis, and one pilot study was undertaken post acute exacerbation. All studies included co‐interventions such as instructions for airway clearance therapy and/or breathing strategies, provision of an educational booklet, and delivery of educational sessions. The duration of training ranged from six to eight weeks, with a mix of supervised and unsupervised sessions conducted in the outpatient or home setting. No studies of children were included in the review; however we identified two studies as currently ongoing. No data were available regarding physical activity levels or adverse events.
For people with stable bronchiectasis, evidence suggests that exercise training compared to usual care improves functional exercise tolerance as measured by the incremental shuttle walk distance, with a mean difference (MD) between groups of 87 metres (95% confidence interval (CI) 43 to 132 metres; 4 studies, 161 participants; low‐certainty evidence). Evidence also suggests that exercise training improves six‐minute walk distance (6MWD) (MD between groups of 42 metres, 95% CI 22 to 62; 1 study, 76 participants; low‐certainty evidence). The magnitude of these observed mean changes appears clinically relevant as they exceed minimal clinically important difference (MCID) thresholds for people with chronic lung disease. Evidence suggests that quality of life improves following exercise training according to St George's Respiratory Questionnaire (SGRQ) total score (MD ‐9.62 points, 95% CI ‐15.67 to ‐3.56 points; 3 studies, 160 participants; low‐certainty evidence), which exceeds the MCID of 4 points for this outcome. A reduction in dyspnoea (MD 1.0 points, 95% CI 0.47 to 1.53; 1 study, 76 participants) and fatigue (MD 1.51 points, 95% CI 0.80 to 2.22 points; 1 study, 76 participants) was observed following exercise training according to these domains of the Chronic Respiratory Disease Questionnaire. However, there was no change in cough‐related quality of life as measured by the Leicester Cough Questionnaire (LCQ) (MD ‐0.09 points, 95% CI ‐0.98 to 0.80 points; 2 studies, 103 participants; moderate‐certainty evidence), nor in anxiety or depression. Two studies reported longer‐term outcomes up to 12 months after intervention completion; however exercise training did not appear to improve exercise capacity or quality of life more than usual care. Exercise training reduced the number of acute exacerbations of bronchiectasis over 12 months in people with stable bronchiectasis (odds ratio 0.26, 95% CI 0.08 to 0.81; 1 study, 55 participants).
After an acute exacerbation of bronchiectasis, data from a single study (N = 27) suggest that exercise training compared to usual care confers little to no effect on exercise capacity (MD 11 metres, 95% CI ‐27 to 49 metres; low‐certainty evidence), SGRQ total score (MD 6.34 points, 95%CI ‐17.08 to 29.76 points), or LCQ score (MD ‐0.08 points, 95% CI ‐0.94 to 0.78 points; low‐certainty evidence) and does not reduce the time to first exacerbation (hazard ratio 0.83, 95% CI 0.31 to 2.22).
Authors' conclusions
This review provides low‐certainty evidence suggesting improvement in functional exercise capacity and quality of life immediately following exercise training in people with stable bronchiectasis; however the effects of exercise training on cough‐related quality of life and psychological symptoms appear to be minimal. Due to inadequate reporting of methods, small study numbers, and variation between study findings, evidence is of very low to moderate certainty. Limited evidence is available to show longer‐term effects of exercise training on these outcomes.
Plain language summary
Exercise training for bronchiectasis
Review question
We wanted to know if exercise training improves exercise tolerance, quality of life, or symptoms, and if it reduces the number of future flare‐ups ('exacerbations'), for people with bronchiectasis compared to people who did not do exercise training. We looked at studies involving people with stable disease and patients in the period following a recent flare‐up. We aimed to include evidence related to both children and adults with bronchiectasis.
Background
People with bronchiectasis suffer from chronic cough and sputum production. They have increased risk of developing acute exacerbations that contribute to poor exercise tolerance and quality of life. When performed by people with other chronic lung conditions, exercise training improves exercise tolerance and reduces symptoms. However, little is understood about the effect of exercise training specifically in bronchiectasis.
Study characteristics
The evidence is current up to October 2020. We included six studies with a total of 275 participants; five studies were related to people with stable disease. No studies involving children were found. Exercise training was delivered in combination with other treatments such as airway clearance therapy, respiratory muscle training, and/or education. Participants were randomly assigned to exercise training or no exercise training. Exercise training was performed for at least six weeks, either in a group setting or at home. None of the included studies were funded by companies with commercial interests in study findings.
Key results
Following completion of exercise training, participants in a stable clinical state walked farther than those who did not do exercise training (an average of 87 metres farther), but our certainty of the evidence is low. Participants also reported improved quality of life (low‐certainty evidence) and less shortness of breath and fatigue. We found evidence of moderate certainty showing that exercise training might not specifically improve cough‐related symptoms, although the incidence of acute exacerbations was lower. Evidence was insufficient to show if the effects of exercise training would last beyond the period of exercise training, and no evidence was available to determine whether exercise training helps people become physically active. No benefits were observed for people who undertook exercise training soon after an acute flare‐up of their bronchiectasis.
Certainty of the evidence
The certainty of evidence was very low to moderate due to uncertainty regarding the true size of observed benefits, poorly conducted studies, and an overall lack of sufficient data. More studies with larger participant numbers are required to determine the long‐term effects of exercise training, irrespective of clinical status.
Summary of findings
Summary of findings 1. Exercise training compared to control in people with stable bronchiectasis.
| Exercise training compared to control in people with stable bronchiectasis | |||||||
| Patient or population: people with stable bronchiectasis Setting: rehabilitation centres, inpatient hospitals, hospital outpatient departments, home‐based exercise settings Intervention: exercise training Comparison: usual care | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with usual care | Risk with exercise training | ||||||
| Change in incremental shuttle walk distance assessed with incremental shuttle walk test | Follow‐up: range 6 weeks to 8 weeks | Mean change in incremental shuttle walk distance ranged from ‐70 to 2.5 metres | MD 87 metres higher (43 higher to 132 higher) | ‐ | 161 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b | |
| Follow‐up: range 3 months to 12 months | Mean change in incremental shuttle walk distance ranged from 5.2 to 343 metres | MD 6.19 metres higher (15.51 lower to 27.9 higher) | ‐ | 82 (2 RCTs) | ⊕⊕⊝⊝ LOWc,d | ||
| Change in 6‐minute walk distance assessed with 6‐minute walk test | Follow‐up: mean 8 weeks | Mean change in 6‐minute walk distance was ‐10.9 metres | MD 42 metres higher (22 higher to 62 higher) | ‐ | 76 (1 RCT) | ⊕⊕⊝⊝ LOWc,e | |
| Follow‐up: mean 12 months | Mean change in 6‐minute walk distance was ‐8.26 metres | MD 6.74 metres lower (29.6 lower to 16.1 higher) | ‐ | 55 (1 RCT) | ⊕⊕⊝⊝ LOWc,e | ||
| Change in endurance shuttle walk time assessed with endurance shuttle walk test | Follow‐up: mean 8 weeks | Mean change in endurance shuttle walk time was 0.2 minutes | MD 5.4 minutes higher (2.7 higher to 8.1 higher) | ‐ | 39 (1 RCT) | ⊕⊕⊝⊝ LOWc,f | |
| Change in endurance shuttle walk distance assessed with endurance shuttle walk test | Follow‐up: mean 8 weeks | Mean change in endurance shuttle walk distance was ‐36.4 metres | MD 311.6 metres higher (42.1 higher to 665.3 higher) | ‐ | 27 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | |
| Follow‐up: mean 3 months | Mean change in endurance shuttle walking distance was 964.3 metres | MD 385.7 metres higher (31.1 higher to 740.3 higher) | ‐ | 27 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | ||
| Change in walking distance during endurance walking test assessed with constant load endurance test | Follow‐up: mean 8 weeks | Mean change in walking distance during endurance walking test was ‐112.6 metres | MD 505.4 metres higher (136.5 higher to 874.3 higher) | ‐ | 19 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | |
| Change in peak oxygen uptake assessed with cardiopulmonary exercise test | Follow‐up: mean 8 weeks | Mean change in peak oxygen uptake was ‐1.91 L/min | MD 3.87 L/min higher (0.12 lower to 7.86 higher) | ‐ | 19 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c,d | |
| Change in quality of life assessed with St George's Respiratory Questionnaire (total score) | Follow‐up: range 6 weeks to 8 weeks | Mean change in quality of life ranged from 4 to 39.2 points | MD 9.62 points lower (15.67 lower to 3.56 lower) | ‐ | 110 (3 RCTs) | ⊕⊕⊝⊝ LOWa,g | Lower scores post intervention are favourable, indicating improvement in quality of life |
| Follow‐up: range 3 months to 12 months | Mean change in quality of life ranged from 33.6 to 45.2 points | MD 6.78 points fewer (14.98 fewer to 1.42 more) | ‐ | 65 (2 RCTs) | ⊕⊕⊝⊝ LOWa,d | Lower scores at follow‐up are favourable, indicating improvement in quality of life | |
| Change in cough‐related quality of life assessed with Leicester Cough Questionnaire | Follow‐up: range 6 weeks to 8 weeks | Mean change in cough‐related quality of life ranged from 14.6 to 15.5 points | MD 0.09 points lower (0.98 lower to 0.8 higher) | ‐ | 103 (2 RCTs) | ⊕⊕⊕⊝ MODERATEd | Higher scores post intervention are favourable, indicating improvement in quality of life |
| Follow‐up: range 3 months to 12 months | Mean change in cough‐related quality of life ranged from 13.6 to 17.9 points | MD 0.97 points lower (8.27 lower to 6.34 higher) | ‐ | 82 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,d,h | Higher scores at follow‐up are favourable, indicating improvement in quality of life | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aWide confidence interval limits exceed (or likely exceed) a clinically relevant threshold for this patient group (imprecision ‐1).
bAllocation bias for 3 studies was unclear, and only 1 study included blinded assessors (bias ‐1).
cLimited data were available for meta‐analysis, reducing its broad representativeness of important factors (e.g. disease severity, settings) (indirectness ‐1).
dAllocation bias in 1 study was unclear (bias ‐1).
eConfidence interval limits exceed minimally important difference for this outcome in people with bronchiectasis (imprecision ‐1).
fBlinding of assessors was not reported and attrition or reporting bias was unclear (bias ‐1).
gAllocation bias and attrition and reporting bias for 1 study were unclear (bias ‐1).
hEffect estimates contrast between benefit and harm in the two included studies (inconsistency ‐1).
Summary of findings 2. Exercise training compared to control for people in the post‐acute period following acute exacerbation of bronchiectasis.
| Exercise training compared to control for people in the post‐acute period following acute exacerbation of bronchiectasis | ||||||
| Patient or population: people in the post‐acute period following acute exacerbation of bronchiectasis Setting: rehabilitation centres, inpatient hospitals, hospital outpatient departments, home‐based exercise settings Intervention: exercise training Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with exercise training | |||||
| Change in 6‐minute walk distance assessed with 6‐minute walk test Follow‐up: mean 8 weeks | Mean change in 6‐minute walk distance was 15 metres | MD 11 metres higher (26.79 lower to 48.79 higher) | ‐ | 27 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | |
| Change in cough‐related quality of life assessed with Leicester Cough Questionnaire Follow‐up: mean 8 weeks | Mean change in cough‐related quality of life was 0.91 units | MD 0.08 units lower (0.94 lower to 0.78 higher) | ‐ | 27 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Higher scores post intervention are favourable, indicating improvement in quality of life |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aRandom sequence generation was unclear and outcome data were incomplete in the sole included study (bias ‐1).
bLimited data were available for meta‐analysis, reducing its broad representativeness of important factors (e.g. disease severity, settings) (indirectness ‐1).
Background
Description of the condition
Bronchiectasis is a chronic and progressive respiratory condition characterised clinically by chronic cough, sputum production, and bronchial infection, and radiologically by abnormal and permanent dilation of the bronchial lumen (Polverino 2017). Peripheral muscle dysfunction is a common feature of the disease associated with muscle weakness, reduced endurance, and high levels of fatigue and dyspnoea (De Carmargo 2018; Inal‐Ince 2014; Ozalp 2012). Difficulty with activities of daily living, as reflected by functional measures of dyspnoea such as the Medical Research Council Dyspnoea Scale, has been reported in people with bronchiectasis (Martinez‐Garcia 2007). Although the causes of dyspnoea in bronchiectasis are multi‐factorial, key factors include altered respiratory mechanics and insufficient gas exchange (Ozalp 2012). Expiratory airflow limitation has been identified in people with moderate to severe bronchiectasis, with commonly recognised functional abnormalities such as air trapping (Radovanovic 2018), along with a corresponding increase in dynamic hyperinflation and heightened levels of dyspnoea (Koulouris 2003; Ozalp 2012). Respiratory muscle weakness has been reported in people with bronchiectasis compared to age‐matched healthy controls (Liaw 2011; Moran 2010; Newall 2005), as has reduced exercise tolerance (Koulouris 2003; Ozalp 2012). Children with bronchiectasis have demonstrated reduced maximal exercise capacity (Swaminathan 2003). Reduced quadriceps strength is common (Ozalp 2012), and fatigue has been reported in 27% to 74% of people with bronchiectasis (Hester 2011; King 2005; King 2006). People with bronchiectasis have been shown to be highly physically inactive, with lower proportions of physical activity undertaken each day compared to healthy controls (Bradley 2015; De Carmargo 2018; Gale 2012).
Although the aetiology of bronchiectasis is heterogeneous and includes severe infection, immune deficiency, autoimmune disorder, and ciliary disorder (Chalmers 2015), a proportion of cases of adults with bronchiectasis are classified as idiopathic (Araugo 2017; Kelly 2003). In children, common aetiologies are immunodeficiency, aspiration, and primary ciliary dyskinesia (Li 2005). Despite the unclear global prevalence of bronchiectasis, various reports provide an estimate according to country. In the USA, between 139 and 1106 cases per 100,000 population have been reported from data collected between 2000 and 2013 (Seitz 2012; Weycker 2017). In the UK, prevalence in 2013 was approximately 566 per 100,000 females and 485 per 100,000 males with a diagnosis of bronchiectasis (Quint 2016), with incidence increasing with age (Quint 2016). Prevalence was slightly lower in Germany in 2013, with an estimated 67 cases per 100,000, but a higher rate has been reported for people over 75 years of age (Ringshausen 2015). Prevalence of bronchiectasis in children has been reported as 1 in 5800 in northeast England (Eastham 2004), and as 1 in 1700 in New Zealand (Twiss 2005). Among some indigenous populations, prevalence is higher. In Australia, an estimated 1470 per 100,000 indigenous children are diagnosed with bronchiectasis (Chang 2002); in New Zealand, between 4.8 and 7.9 per 100,000 in the Maori population; and among the Pacific Islander population, between 17.8 and 18.3 per 100,000 (Edwards 2003). Bronchiectasis is associated with significant mortality, accounting for between 1438 and 1914 deaths per 100,000 people with bronchiectasis in the UK (Quint 2016). In Belgium, over a five‐year follow‐up period, the mortality rate was 20.4% (Goeminne 2014). With bronchiectasis characterised by recurrent acute exacerbations, the rate of hospitalisation is ever increasing, particularly among the older population (Ringshausen 2013; Seitz 2010; Seitz 2012). Acute exacerbations, peripheral and respiratory muscle dysfunction, and respiratory and psychological symptoms of anxiety and depression contribute to reductions in health‐related quality of life (HRQoL) (Giron Moreno 2013; O'Leary 2002; Olveira 2013), as observed in people with bronchiectasis (Chalmers 2018; Martinez‐Garcia 2005; Pifferi 2010).
Description of the intervention
International and national guidelines for managing bronchiectasis have highlighted the importance of minimising inflammation and infection, optimising airway clearance, and addressing structural lung disease (Al‐Jahdali 2017; Chang 2015; Hill 2019; Martinez‐Garcia 2018; Pasteur 2010; Pereira 2019; Polverino 2017). Several interventions are applied to achieve optimal management of bronchiectasis, including antibiotics, anti‐inflammatory agents, mucolytics, airway clearance therapy, and exercise training. Exercise training refers to structured programmes of activities that involve physical exertion and skeletal muscle contractions targeting improvements in physical function or exercise tolerance (or both). Exercise training may be undertaken in isolation or as part of a pulmonary rehabilitation programme. Pulmonary rehabilitation has been defined as "comprehensive intervention based on a thorough patient assessment followed by patient‐tailored therapies that include, but are not limited to, exercise training, education, and behavioural change, designed to improve the physical and psychological conditions of people with chronic respiratory disease and to promote the long‐term adherence to health‐enhancing behaviours" (Spruit 2013). It is well recognised that exercise training is a critical component of pulmonary rehabilitation; this may be complemented by formal educational sessions focusing on self‐management, behavioural modification, and counselling (Spruit 2013). Regardless of the circumstances in which exercise training is provided for people with bronchiectasis, any individually tailored exercise training programme prescribed for people with bronchiectasis may consist of lower and upper limb endurance exercise (of low or high intensity) and strength training (Spruit 2013). Exercise training may be completed in a hospital, in the community, or in a home‐based environment (Jose 2017; Lee 2008; O'Neill 2002; Spruit 2013); may or may not be undertaken in a group setting; and may be prescribed for a person who is in a stable clinical state or is experiencing an acute exacerbation (Greening 2014). Exercise training may also be completed under the supervision of a suitably trained healthcare professional or conducted unsupervised across any of these settings.
The majority of research in exercise training for people with chronic respiratory conditions has been undertaken in those diagnosed with chronic obstructive pulmonary disease (COPD) (Nici 2006; Spruit 2013). For this patient group, clinically significant improvements in respiratory symptoms, functional ability, exercise tolerance, exacerbation frequency, and HRQoL have been reported (Spruit 2013). As many symptoms are commonly seen in people with the two conditions, it has been postulated that exercise training may offer equivalent effects in people with bronchiectasis (Rochester 2015).
How the intervention might work
The theoretical rationale for performing exercise training in people with bronchiectasis relates to the respiratory and peripheral skeletal muscle manifestations of bronchiectasis. Exercise training targets improvements in physical function or exercise tolerance (or both). These are commonly associated with improvements in respiratory symptoms. Endurance and strength exercise training has been associated with improvement in peripheral muscle strength and aerobic capacity; reduced symptoms of dyspnoea and fatigue; and improved HRQoL in other chronic respiratory conditions such as COPD (McCarthy 2015; Spruit 2013). It is hypothesised that a similar effect may occur in bronchiectasis, although the precise mechanisms are unclear. Despite this, clinical guidelines support the inclusion of people with respiratory conditions other than COPD into rehabilitation programmes (Alison 2017; Spruit 2013). Although comparisons of the effects of pulmonary rehabilitation in people with COPD or bronchiectasis have demonstrated similar improvements in exercise tolerance and health status outcomes (Patel 2019), the longevity of these effects is unclear. Exercise training has not been previously associated with modification of the disease process in chronic respiratory conditions (Spruit 2013), so this is not anticipated to be a likely mechanism of action in bronchiectasis (Mandal 2012). Exercise training may additionally benefit respiratory symptoms such as dyspnoea, chronic cough, and sputum expectoration in people with bronchiectasis due to its effects on breathing patterns and sputum clearance. These have been demonstrated in adults with cystic fibrosis during exercise (Dwyer 2017), and they may be evaluated through measures of symptoms or quality of life domains.
Why it is important to do this review
An international policy statement for pulmonary rehabilitation supported the inclusion of people with bronchiectasis within pulmonary rehabilitation programmes (Rochester 2015). Although an earlier version of this review found that inspiratory muscle training improved endurance exercise capacity and quality of life, evidence for the effects of other types of physical training was not available (Bradley 2002). The 2017 Australian and New Zealand pulmonary rehabilitation guidelines also state that people with bronchiectasis can achieve improvements in exercise capacity and HRQoL following pulmonary rehabilitation compared to usual care (Alison 2017). Surveys of clinical practice have indicated that clinicians prescribe exercise training for people with bronchiectasis or refer people to pulmonary rehabilitation programmes (or both) (Lee 2008; O'Neill 2002). Although review authors previously completed a systematic review and meta‐analysis comparing the effects of pulmonary rehabilitation in bronchiectasis to usual care (Lee 2017), this was isolated to pulmonary rehabilitation and did not include broader definitions of exercise training that may be completed in other environments. With lack of ready access to pulmonary rehabilitation for people with chronic respiratory disease (Rochester 2015), including those with diagnosed bronchiectasis, it is important to consider a broad range of options for exercise training and its effects on clinical parameters compared to usual care, to guide future clinical practice.
Objectives
To determine effects of exercise training compared to usual care on exercise tolerance (primary outcome), quality of life (primary outcome), incidence of acute exacerbations and hospitalisation, respiratory and mental health symptoms, physical function, mortality, and adverse events in people with stable or acute exacerbation of bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of a parallel‐group design. We included studies reported in full text, published as an abstract only, and provided as unpublished data. Studies published in languages other than English were eligible for inclusion, with translations sought via the Cochrane Airways network. We recorded studies that are included but are lacking available data as 'awaiting classification'.
Types of participants
We included people of any age with a diagnosis of bronchiectasis according to high‐resolution computed tomography (HRCT) or physician diagnosis (Pasteur 2010). Studies comprising patient groups of mixed respiratory pathology must include at least 75% with a primary diagnosis of bronchiectasis or available data on a bronchiectasis subgroup. No participants were excluded on the basis of coexisting respiratory disease (e.g. COPD). However, people with bronchiectasis due to cystic fibrosis were not eligible for inclusion. Participants were eligible for inclusion irrespective of whether they were experiencing an acute exacerbation of their bronchiectasis or were in a period of disease stability.
Types of interventions
We included studies comparing exercise training with usual care. Exercise training was defined as any structured exercise programme that targets improvements in physical function or exercise tolerance (or both). The intervention must have been applied for a minimum duration of four weeks or eight sessions and may have been undertaken as part of an inpatient, outpatient, community, or home‐based programme, in an individual or group setting. Both supervised and unsupervised exercise training interventions were allowed. Co‐interventions such as respiratory muscle training, airway clearance techniques, and patient education were permitted (Chang 2015; Polverino 2017), as these interventions may be integrated into clinical care for people with bronchiectasis and may be associated with clinical benefit (Martin‐Valero 2020). Such co‐interventions must, however, have been provided to both intervention and usual care groups. The effects of exercise training may endure for differing lengths of time depending upon the duration of the initial intervention. Therefore, we distinguished between studies of 12 weeks' duration or less and longer than 12 weeks' duration. In a previous large Cochrane systematic review of pulmonary rehabilitation for people with COPD (McCarthy 2015), 55 of 64 (86%) included studies involved training programmes of 12 weeks' duration or less, hence the use of this threshold as a marker of 'conventional' versus 'long‐term' interventions appeared justified.
Usual care was defined as treatment that did not include a structured physical exercise training programme. Usual care may have included adjunct therapies, such as medical interventions (i.e. antibiotic prescription), a regimen of airway clearance therapy, respiratory muscle training, or a combination of these.
Types of outcome measures
We evaluated the effects of exercise training on the following outcomes.
Primary outcomes
Exercise tolerance: measured via field walking tests (e.g. incremental shuttle walk test (ISWT), 6‐minute walk test (6MWT), endurance shuttle walk test (ESWT)) or cardiopulmonary exercise testing (e.g. maximal incremental treadmill or cycle ergometer cardiopulmonary exercise test (CPET), constant‐load exercise test (CLET)). The principal units of analysis for these tests were distance (metres) for ISWT and 6MWT; time (minutes) for endurance or CLET; and peak oxygen uptake (VO₂ peak) for maximal incremental CPET. We reported these outcomes separately. Assessment occurred upon completion of the exercise training intervention and at the longest time point available up to 12 months after intervention completion
Health‐related quality of life (HRQoL): measured via disease‐specific questionnaires for bronchiectasis (i.e. Quality of Life‐Bronchiectasis) or respiratory quality of life questionnaires (e.g. St George's Respiratory Questionnaire, Chronic Respiratory Disease Questionnaire (CRDQ), symptom‐specific questionnaires (e.g. Leicester Cough Questionnaire (LCQ)) or generic health questionnaires (e.g. Short Form‐36, EuroQol). Both total scores and symptom‐specific domain scores were used but were reported separately. Data from both disease‐specific and generic instruments were pooled for analysis; however, disease‐specific quality‐of‐life total scores were considered the principal analysis of interest. These were assessed upon completion of the exercise training intervention and at the longest time point available up to 12 months after intervention completion
Secondary outcomes
Exacerbations/hospitalisations: measured as incidence, rate, or time to first acute exacerbation or respiratory‐related hospitalisation, with each defined according to study authors. For this outcome, data were sourced from the longest time point available up to 12 months after intervention completion
Peripheral skeletal muscle force: may have included measures of muscle strength (kilograms), power (Newtons), or torque (Newton.metres). Data from muscle groups of the upper limb were pooled together, and data from muscle groups of the lower limb were pooled together. Upper limb muscle force was analysed separately from lower limb muscle force. This was assessed upon completion of the exercise training intervention and the longest time point available up to 12 months after intervention completion
Physical activity: comprising objectively measured outcomes of movement (e.g. steps, time spent in light/moderate/vigorous activity) but not sedentary behaviour. This was assessed upon completion of the exercise training intervention and the longest time point available up to 12 months after intervention completion
Mental health: comprising measures of anxiety and depression (e.g. Hospital Anxiety and Depression Scale (HADS), Beck Depression Inventory, Hamilton Anxiety/Depression Rating Scale). Anxiety data were analysed distinct from depression data. They were assessed upon completion of the exercise training intervention and the longest time point available up to 12 months after intervention completion
Clinical symptoms: comprising symptoms such as dyspnoea, cough, or fatigue, with all measures of symptoms eligible for inclusion. Symptoms measured at rest were the principal unit of interest; however, data obtained at the end of exercising were accepted when resting data were unavailable provided the outcome was measured in the same manner for each group within individual trials. These were assessed upon completion of the exercise training intervention and the longest time point available up to 12 months after intervention completion
Mortality: measured as the incidence or rate of death, assessed at the longest time point available up to 12 months after intervention completion
Adverse events: comprising events such as falls or injury, measured upon completion of the exercise training intervention
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from searches of the following databases and trial registries.
Cochrane Airways Trials Register (Cochrane Airways 2019), via the Cochrane Register of Studies, all years to 7 October 2020.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9), via the Cochrane Register of Studies, all years to 7 October 2020.
MEDLINE (Ovid SP) ALL, 1946 to 7 October 2020.
Embase (Ovid SP), 1974 to 7 October 2020.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), all years to 7 October 2020.
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch), all years to 9 September 2019.
PEDro (Physiotherapy Evidence Database), all years to 7 October 2020.
The database search strategies are listed in Appendix 1. The Cochrane Airways Information Specialist developed the search strategies, in collaboration with the review authors.
All databases and trial registries were searched from their inception to September 2019, with no restriction on language or type of publication. An additional search was then conducted to update the search yield to 7 October 2020. Handsearched conference abstracts and grey literature were identified through the Cochrane Airways Trials Register and the CENTRAL database.
Searching other resources
We checked the reference lists of all primary studies and review articles as well as respiratory conference abstracts for additional references. We contacted authors of identified trials and experts in the field to identify other published or unpublished studies when possible. We searched for errata or retractions from included studies published in full text on PubMed, on 24 August 2020.
Data collection and analysis
Selection of studies
We used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components.
Known assessments: a service that matches records in the search results to records that have already been screened in Cochrane Crowd (Cochrane's citizen science platform, by which the Crowd help to identify and describe health evidence) and labelled as 'RCT' or 'not an RCT'.
RCT classifier: a machine‐learning model that distinguishes RCTs from non‐RCTs.
Cochrane Crowd, if appropriate (crowd.cochrane.org).
More detailed information about the Screen4Me components can be found in the following publications: McDonald 2017; Thomas 2017; Marshall 2018; and Noel‐Storr 2018.
Following this, two review authors (AL, CG) screened the titles and abstracts of the search results independently and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' We retrieved the full‐text study reports of all potentially eligible studies, and two review authors (AL, CG) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. They used Covidence software (Covidence 2018). We resolved any disagreement through discussion, or, if required, we consulted a third person (CO). We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). Any records identified through the search that involve members of the review team were handled by team members who were not involved with the relevant study to avoid perceived conflicts of interest.
Data extraction and management
We used a data collection form that had been piloted on two studies in the review to record study characteristics and outcome data. Two review authors (CG, AL) independently extracted the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and dates of study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, and inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies, and notable conflicts of interest of trial authors.
Two review authors (CG, AL) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by reaching consensus or by involving a third person (CO). One review author (CG) transferred data into Review Manager Web (RevMan Web 2019). A second review author (CO) spot‐checked study characteristics for accuracy against the study report. We contacted authors of included studies to verify data extracted from their study when required, and to request details of missing data when applicable.
No study data were extracted or analysed by review members directly involved with included studies.
Assessment of risk of bias in included studies
Two review authors (AL, CG) assessed risk of bias independently for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved disagreements by discussion or by consultation with another review author (CO). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as having high, low, or unclear risk and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for outcomes that were self‐reported or were not self‐reported by participants due to the different impact this may have on findings. For example, knowledge of group allocation may influence self‐reported measures of respiratory symptoms but would have plausibly less impact upon an outcome such as all‐cause mortality. For consistency, we considered risk of bias related to blinding for self‐reported outcomes to be uniformly high across all such instances, as is commonly unpreventable in rehabilitation studies. When information on risk of bias related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
We conducted the review according to this published protocol and justified any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We planned to conduct analyses and to report findings from people younger than 18 years of age separately from people 18 years of age or older. However, no studies of people younger than 18 years met the inclusion criteria.
We conducted analyses and reported findings from studies describing interventions commencing during or within two weeks of discharge from an acute exacerbation or diagnosis of an acute exacerbation separately from those applicable to the stable disease state. We accepted study authors' definitions of acute exacerbations or stable disease state.
We conducted analyses and reported findings from interventions of a 'conventional' (12 weeks or less) duration separate from those of a 'long‐term' (greater than 12 weeks) duration.
We reported findings from outcome data collected at more than one time point (e.g. upon completion of the exercise training intervention, at the longest time point available up to 12 months after intervention completion) separately to avoid issues associated with participant double‐counting.
We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs) with 95% confidence intervals (CIs). We used SMDs when outcome data were reported via different metrics but were deemed clinically homogenous (e.g. data from different field walking tests or from different quality‐of‐life instruments). We did not use SMDs when such outcome data comprised a combination of both endpoint and change data. When SMDs were used for outcome data expressed as change from baseline, we used the standard deviation (SD) of baseline values as the unit of measurement to calculate the SMD, and adjusted standard errors to take correlation into account, when appropriate data were available. Results from analyses using SMDs were transformed back to native metrics for ease of interpretation. If data from rating scales were combined in a meta‐analysis, we ensured that they were entered with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only when this was meaningful, that is, when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We described skewed data narratively (e.g. as medians and interquartile ranges for each group).
When multiple trial arms were reported in a single study, we included only the relevant arms. If two comparisons (i.e. exercise training approach one versus usual care and exercise training approach two versus usual care) were combined in the same meta‐analysis, we combined the active arms or halved the control group to avoid double‐counting.
If adjusted analyses were available (ANOVA or ANCOVA), we used these as a preference in our meta‐analyses. If both change from baseline and endpoint scores were available for continuous data, we used change from baseline unless a low correlation between measurements in individuals was reported. If a study reported outcomes at multiple time points, we used the data closest to the primary time point of interest, as defined in the Types of outcome measures section.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses when they were reported (i.e. those in which data had been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For continuous outcomes, we used endpoint, rather than change, data as the principal unit of analysis. Change data were included in pooled meta‐analyses only when endpoint data were not reported, with discussion provided regarding the potential for exaggerated weighting given to such studies.
For dichotomous outcomes, we used the number of people experiencing an event as the unit of analysis (e.g. number of exacerbations). However, if a study reported rate ratios, we analysed them on this basis. We meta‐analysed data from cluster‐RCTs only when available data were adjusted (or could be adjusted), to account for clustering.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study is identified as an abstract only). When this was not possible, and missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis, using a random‐effects or fixed‐effect model, depending on assessment of heterogeneity. When substantial heterogeneity was identified, we reported this but were unable to explore the possible causes by pre‐specified sensitivity analysis due to the absence of subgroups.
Assessment of reporting biases
If we had identified more than 10 studies, we planned to create an example of a funnel plot and to analyse this for small‐study and publication biases (Egger 1997). However, we identified a total of only six studies for this review, which precluded the creation of funnel plots.
Data synthesis
We meta‐analysed data using a random‐effects model, and we performed a sensitivity analysis with a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Exercise training interventions characterised as unicomponent (e.g. exercise training alone) versus those characterised as multi‐component (e.g. exercise training plus at least one adjunct therapy).
We planned to use the following outcomes in subgroup analyses.
Exercise tolerance.
Disease‐specific or generic HRQoL (total scores only).
We identified only multi‐component studies for inclusion in this review; therefore there was no analysis of unicomponent interventions and no possibility of comparisons between types of interventions.
Sensitivity analysis
We planned to carry out the following sensitivity analysis while removing the following from the primary outcome analyses.
Studies identified as being at high risk of bias for domains other than performance bias, considering blinding of participants and personnel to knowledge of group allocation as inherently challenging in studies of exercise interventions.
We planned to compare results from the principal random‐effects model with those from a fixed‐effect model. However, the small number of studies and the abstract reporting of one study precluded sensitivity analysis. If in future updates, a greater number of studies are included, we will perform a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We constructed 'Summary of findings' tables to present the main findings of this review. We reported primary outcomes and presented anticipated absolute effects and 95% CIs. Review author AL performed an assessment of the certainty of evidence for each outcome using the GRADE approach (Schunemann 2019). Review author CO checked GRADE assessments and 'Summary of findings' tables and revised tables to reflect discussions between AL and CO. Analysis findings were interpreted in line with existing minimally important difference threshold for outcomes for which bronchiectasis‐specific guidance was available.
We planned to create separate 'Summary of findings' tables for each of our separate analyses (paediatric and adult populations; acute and stable disease states; intervention duration up to 12 weeks or lasting longer than 12 weeks). However, due to lack of available data for these comparisons, we could present 'Summary of findings' tables only for acute and stable disease. We planned to generate no tables for the level of the two subgroups (as defined in Subgroup analysis and investigation of heterogeneity). We reported on the following primary outcomes for each table.
Exercise tolerance.
Disease‐specific or generic HRQoL (total scores only).
We used the five GRADE considerations (risk of bias, inconsistency, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data for the pre‐specified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), along with GRADEpro software (GRADEpro 2015). We justified any decisions to downgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of studies awaiting classification for complete details.
Results of the search
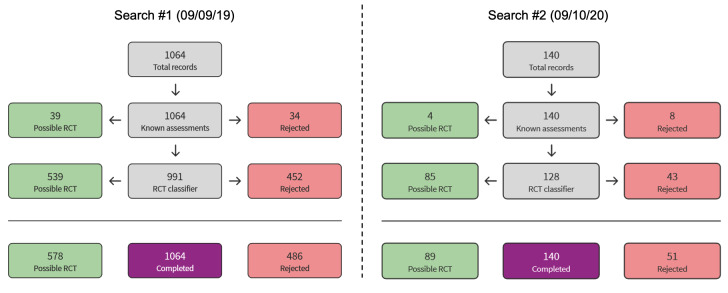
The search of databases and clinical trials registries up to 9 October 2020 yielded a total of 1660 records. After removal of 398 duplicates and 525 records via Cochrane Crowd Known Assessments and Screen4Me (Figure 1), a total of 737 records remained. We excluded 715 records on the basis of title and abstract and evaluated 22 records (15 studies) for eligibility via full text. Six studies were excluded, as they did not meet the review criteria. Four citations pertaining to three studies were identified as suitable for inclusion but are currently ongoing; these were not included in the analysis. A total of six studies were included (Figure 2). Full details of excluded and ongoing studies are outlined in the Excluded studies and Ongoing studies sections.
1.
Overview of Cochrane Crowd Known Assessments and Screen4Me workflows for original and updated searches.
2.
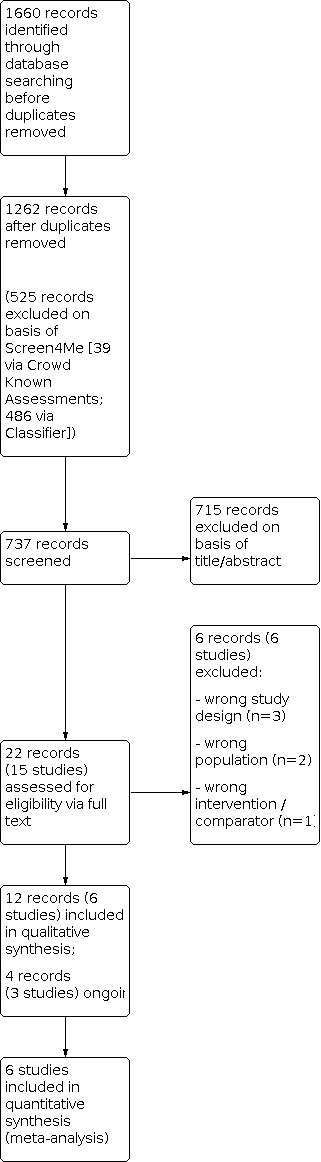
Figure 2. Study flow diagram
Included studies
Design
All studies included in this review were parallel‐group, randomised, controlled trials (Chalmers 2019; Dal Corso 2017; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005). Two of the included studies were described as pilot studies (Chalmers 2019; Mandal 2012).
Participants
The six included studies involved 275 participants, with sample sizes ranging from 20 to 85. Five studies included clinically stable adult participants (Dal Corso 2017; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005), and one study included adult participants who undertook the intervention following an acute exacerbation of bronchiectasis (Chalmers 2019). None of the included studies involved children with bronchiectasis; however two of the three ongoing studies involve children (Joschtel 2014 is a pilot study of Trost 2019, but with different participants, aims, and outcome endpoints (confirmed via email correspondence)). Bronchiectasis was diagnosed on the basis of HRCT in five studies (Chalmers 2019; Dal Corso 2017; Lee 2014; Mandal 2012; Newall 2005), and on the basis of physician diagnosis in two studies (Dal Corso 2017; Kumar 2017). The age range of participants was 63 to 72 years in five studies (Chalmers 2019; Dal Corso 2017; Lee 2014; Mandal 2012; Newall 2005); one study did not report participant age (Kumar 2017). Of the five studies including those who undertook the intervention when clinically stable, disease severity according to spirometry ranged from a forced expiratory volume of one second (FEV₁) of 45% to 77% predicted (Dal Corso 2017; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005). The study in which the intervention was undertaken following an acute exacerbation reported participant FEV₁ between 52% and 96% predicted (Chalmers 2019). Acute exacerbations in this study were community‐managed (Chalmers 2019).
Interventions
All studies compared multi‐component exercise training programmes involving adjunct interventions (ranging from instruction or review of airway clearance therapy to a mix of educational sessions) versus no exercise training. Five studies investigated exercise training in an outpatient setting (Chalmers 2019; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005), and one study evaluated a home‐based exercise training programme (Dal Corso 2017). The duration of exercise training programmes varied from six to eight weeks for outpatient rehabilitation and was eight weeks for home‐based training.
Four studies examined the effects of combined aerobic and resistance training (Chalmers 2019; Dal Corso 2017; Lee 2014; Mandal 2012); one study examined the effects of aerobic training only (Newall 2005), and one study did not specify the modality of training applied (Kumar 2017). Five studies added co‐interventions to exercise training, which were also offered to the control group; these included instruction in airway clearance therapy and/or breathing strategies (Chalmers 2019; Lee 2014; Mandal 2012), provision of an educational booklet (Dal Corso 2017), and delivery of educational sessions (Mandal 2012; Newall 2005). One study did not specify which co‐interventions were offered in conjunction with exercise training (Kumar 2017). A summary of key characteristics of interventions is presented in Table 3.
1. Summary of study interventions.
| Study | Setting | Intervention | Control | Duration (weeks) | Frequency | Follow‐up (weeks) |
| Chalmers 2019 | OP Supervised |
Aerobic: treadmill, bike, walking (80% VO₂ max) Resistance: UL, LL (8 to 10 reps at 1 RM). Education including instruction in daily chest PT |
Guideline concordant ongoing management including instruction in daily chest PT | 6 | 2 OP + 2 HB sessions/week | 12 |
| Dal Corso 2017 | HB Unsupervised |
Aerobic: 20 minutes stepping on platform (60% to 80% maximal stepping rate in IST) Resistance: 20 minutes UL, LL (8 reps, 3 sets at 70% MVIC). Education. Weekly phone call. Home visit every 15 days | Education Recommended to walk at moderate intensity for 30 minutes 3 times/week. Weekly phone call (no exercise or PA discussion) |
8 | 3 times/week Average session duration: 50 minutes |
Nil |
| Kumar 2017 | OP | No details provided | Standard care | 8 | No details provided | Nil |
| Lee 2014 | OP Supervised |
Aerobic: 15 minutes treadmill or walking (75% ISWT speed) and bike (60% maximal work rate) Resistance: 15 minutes UL, LL (10 reps, 1 to 3 sets at 10 RM) Encouraged to maintain HEP over follow‐up period via monthly phone call. Reviewed usual ACT. Taught ACBT If no usual ACT |
Education Twice‐weekly phone call (no exercise or PA discussion) Reviewed usual ACT. Taught ACBT if no usual ACT |
8 | 2 OP + 3 to 5 HB sessions/week | 52 |
| Mandal 2012 | OP Supervised |
Aerobic: 10 minutes treadmill, bike, ski machine (85% VO₂ max) Resistance: UL, LL (10 reps, 3 sets at 60% 1 RM, progressed to 70% at Week 3, 80% at Week 5). Twice‐daily chest PT. Education. Self‐management plan. Encouraged to undertake gym programme for 6 months at end of intervention |
Twice‐daily chest PT. Education. Self‐management plan |
8 | 2 OP + 1 HB session/ week |
12 |
| Newall 2005 | OP Supervised |
OP aerobic: 15 minutes treadmill, cycle ergometry, stair climbing (80% peak HR on maximal test) HB aerobic: 45 minutes walking at target intensity Education. Sham IMT at sub‐therapeutic load (7 cmH₂O) for 15 minutes twice daily at home |
Education | 8 | 2 OP + 1 HB sessions/week Average session duration 45 minutes |
12 |
Abbreviations: ACBT: active cycle of breathing technique; ACT: airway clearance technique; HB: home‐based; HEP: home exercise programme; IMT: inspiratory muscle training; IST: incremental step test; ISWT: incremental shuttle walk test; LL: lower limb; MVIC: maximum voluntary isometric contraction; OP: outpatient; PA: physical activity; PR: pulmonary rehabilitation; PT: physiotherapy; RM: repetition maximum; UL; upper limb; VO₂ max: maximal oxygen uptake.
Outcome measures
All studies reported upon a measure of functional exercise tolerance; most used the six‐minute walk test (Chalmers 2019; Kumar 2017; Lee 2014), or the incremental shuttle walk test (Dal Corso 2017; Lee 2014; Mandal 2012; Newall 2005). Two studies also performed cardiopulmonary exercise testing (Kumar 2017; Newall 2005), two studies applied an endurance shuttle walk test (Dal Corso 2017; Mandal 2012), and one applied a sub‐maximal treadmill‐based exercise test (Newall 2005). Quality of life was assessed by St George's Respiratory Questionnaire (Chalmers 2019; Dal Corso 2017; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005), the Chronic Respiratory Disease Questionnaire (Lee 2014), and the Leicester Cough Questionnaire (Chalmers 2019; Kumar 2017; Lee 2014; Mandal 2012). Health status was measured in one study (Chalmers 2019). Peripheral muscle strength was measured by a dynamometer in one study (Dal Corso 2017), and psychological symptoms of anxiety and depression were measured on the Hospital Anxiety and Depression Scale and the Depression Anxiety Stress Scale in two studies (Kumar 2017; Lee 2014). Time to next exacerbation was measured in two studies (Chalmers 2019; Lee 2014); exacerbation rate over 12 months and mortality were measured in one study only (Lee 2014).
Excluded studies
We excluded six studies after review via full text. Reasons for exclusion related to incorrect study population (investigation of a control group that had a different diagnosis (Choe 1996; Finnerty 1999), incorrect comparator (use of an adjunct co‐intervention that was not applied equally to the usual care arm) (Bradley 2006; Greening 2014), and use of a study design that was not consistent with the inclusion criteria (Kokura 2016; Taylor 2019)).
Risk of bias in included studies
Risk of bias was completed for all six studies. An overview of risk of bias for the domains listed below is outlined in Figure 3 and Figure 4.
3.

Figure 3. Risk of bias summary
4.

Figure 4. Risk of bias graph
Allocation
All studies described participants as randomly allocated to groups; however only four studies described their methods as computer‐generated random sequences (Dal Corso 2017; Lee 2014; Mandal 2012; Newall 2005), resulting in low attributed risk of bias due to sequence generation. Three studies revealed the use of sealed, opaque envelopes as the method of concealment for allocation (Chalmers 2019; Dal Corso 2017; Lee 2014), and three studies did not provide sufficient information to show the method used (Kumar 2017; Mandal 2012; Newall 2005), resulting in unclear ratings of bias due to allocation concealment.
Blinding
The physical nature of exercise training interventions imposes limits to the success of blinding strategies for participants and study personnel. We rated the risk of bias related to performance and detection bias separately for outcomes that were self‐reported or were not self‐reported due to the potentially different impact that participant blinding may have on outcomes such as self‐reported symptoms compared to exacerbations, for example.
All studies included self‐reported outcomes and were consequently rated as having high risk of performance and detection bias for these outcomes due to the risk of participant knowledge of group allocation impacting self‐reported results.
One study stated that participants and personnel were not blinded to group allocation (Dal Corso 2017), and another study inferred that participants and personnel were not blinded to group allocation (Lee 2014). Lee 2014 used an independent, blinded assessor to measure all post‐treatment outcome measures and was therefore rated as having low risk of performance and detection bias for non‐self‐reported outcomes. Dal Corso 2017 stated that due to limited resources, use of a blinded assessor was not possible; this study was therefore rated as having high risk of performance and detection bias for non‐self‐reported outcomes. The remaining four studies did not provide sufficient information regarding blinding of participants and personnel delivering the intervention and undertaking outcome measurement, resulting in unclear risk of bias for these non‐self‐reported outcomes (Chalmers 2019; Kumar 2017; Mandal 2012; Newall 2005). No studies reported whether data analysts were blinded to group allocation.
Incomplete outcome data
Three studies stated withdrawal of participants and reasons for attrition (Lee 2014; Mandal 2012; Newall 2005). One study stated the impact of attrition on the method of statistical analysis applied (Lee 2014), two reported attrition but did not include the reasons or impact on outcomes (Dal Corso 2017; Kumar 2017), and one did not provide sufficient detail on attrition (Chalmers 2019). These latter three studies were rated as possessing uncertain risk of bias related to attrition.
Selective reporting
Four studies were reported prospectively on a clinical trial registry (Chalmers 2019; Dal Corso 2017; Lee 2014; Mandal 2012). Results were reported for all outcomes at each time point for four studies (Chalmers 2019; Lee 2014; Mandal 2012; Newall 2005). Two studies were presented in abstract form only; one had an additional outcome of maximal exercise capacity, for which the findings were not reported (Dal Corso 2017), and one provided insufficient detail to determine selective reporting (Kumar 2017). Both were rated as having uncertain risk for reporting bias.
Other potential sources of bias
Two studies were available in abstract form only (Dal Corso 2017; Kumar 2017), limiting our ability to accurately determine the presence of some sources of bias. It is unclear in one study why the applied method of randomisation resulted in an uneven group allocation (Chalmers 2019). These three studies were consequently rated as having unclear risk of other sources of potential bias. The remaining three studies did not demonstrate any other potential sources of bias (Lee 2014; Mandal 2012; Newall 2005).
Effects of interventions
Refer to the 'Summary of findings' tables for an overview of the main findings related to primary outcome comparisons (Table 1; Table 2). We were able to include data from six studies in a quantitative and narrative synthesis; all studies were conducted in the adult population (Chalmers 2019; Dal Corso 2017; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005).
Exercise training versus usual care in the stable clinical state
Primary outcomes
Exercise tolerance
Five studies involving 234 participants reported findings related to different metrics of exercise tolerance. Pooled data from four studies involving 161 participants with stable bronchiectasis suggest improvements in incremental shuttle walk distance (ISWD) immediately following exercise training (mean difference (MD) in change from baseline 87.12 metres, 95% confidence interval (CI) 42.65 to 131.58 m; I² = 64%; n = 161; low‐certainty evidence; Analysis 1.1; Figure 5). One study did not demonstrate meaningful between‐group differences following training completion (Mandal 2012), but investigators noted large within‐group changes in the intervention group only (which started with a ‐55.8 m mean lower baseline ISWT level). The meta‐analysis for this outcome was considerably influenced by Lee 2014 (weighting 36.1%), which was the only included study to contribute change from baseline rather than endpoint data. Exploratory step‐by‐step removal of studies from the meta‐analysis revealed that Dal Corso 2017 contributed the most to the high degree of statistical heterogeneity; its removal resolved heterogeneity but did not meaningfully alter findings (MD 68.74 metres, 95% CI 44.47 to 93.07 m; I² = 0%; n = 122). For the 6‐minute walk distance (6MWD), evidence suggests that considerable improvement was observed immediately following intervention (MD in change from baseline 42.1 m, 95% CI 21.9 to 62.4 m; n = 76; low‐certainty evidence; Analysis 1.2), with a similar magnitude of change evident for 6MWD (36.9 metres between groups at the conclusion of eight weeks of training) in favour of the intervention (P < 0.001) in another study that could not be included in the meta‐analysis (Kumar 2017). Although improvement in endurance shuttle walk test was noted in minutes in favour of exercise training (MD 5.4 minutes, 95% CI 2.71 to 8.09 minutes; n = 39; low‐certainty evidence; Analysis 1.3), no difference in distance (metres) was evident (very low‐certainty evidence; Analysis 1.4; Mandal 2012). Two studies reported that testing of maximal exercise capacity was performed following exercise training (Kumar 2017; Newall 2005). Exercise training may improve constant‐load exercise test performance, but the evidence is very uncertain (MD 505.4 metres, 95% CI 136.51 to 874.29 m; n = 19; very low‐certainty evidence; Analysis 1.5). No improvement in VO₂ peak was noted between baseline and follow‐up (Analysis 1.6; Newall 2005). In contrast, a mean change in VO₂ peak of 81.7 mL/min between groups post intervention in favour of exercise training (P = 0.006) was reported in one study that could not be included in the meta‐analysis (Kumar 2017).
1.1. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 1: Exercise tolerance ‐ ISWT (metres) at end intervention
5.

Note: Data are expressed as endpoint (end‐intervention) for Mandal 2012, and as change from baseline for all others.
1.2. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 2: Exercise tolerance ‐ 6MWT (metres) at end intervention
1.3. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 3: Exercise tolerance ‐ ESWT (mins) at end intervention
1.4. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 4: Exercise tolerance ‐ ESWT (metres) at end intervention
1.5. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 5: Exercise tolerance ‐ CLET (metres) at end intervention
1.6. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 6: Exercise tolerance ‐ CPET VO 2 peak at end intervention
Two studies whose data were available reported results of the ISWD at three months' follow‐up (Mandal 2012), and at 12 months' follow‐up (Lee 2014). Evidence suggests that exercise training had no effect at either time point (low‐certainty evidence; Analysis 1.7). Similar findings were noted for the 6MWD at 12 months' follow‐up (Analysis 1.8; Lee 2014), and for the constant‐load endurance test, with improvement not maintained at three months' follow‐up in the exercise training group (Newall 2005). In contrast, improvements in endurance shuttle walk distance may have been maintained at three months' follow‐up; however the evidence is very uncertain (very low‐certainty evidence; Analysis 1.9).
1.7. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 7: Exercise tolerance ‐ ISWT (metres) at follow‐up
1.8. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 8: Exercise tolerance ‐ 6MWT (metres) at follow‐up
1.9. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 9: Exercise tolerance ‐ ESWT (metres) at follow‐up
Health‐related quality of life
Five studies involving 234 participants with stable bronchiectasis reported findings related to different metrics for HRQoL. Of the five studies that used the St George's Respiratory Quotient (SGRQ) total score (Dal Corso 2017; Kumar 2017; Lee 2014; Mandal 2012; Newall 2005), data were available for pooling from three studies. Pooled data involving 110 participants suggest improvements in quality of life immediately following exercise training (MD in change from baseline ‐9.62 points, 95% CI ‐15.67 to ‐3.56 points; I² = 16%; n = 101; low‐certainty evidence; Analysis 1.10; Figure 6). Similarly, one study that could not be included in the meta‐analysis demonstrated a mean improvement of 8.43 points between groups at end intervention in favour of exercising training (P = 0.002) (Kumar 2017). In contrast, a mean change in SGRQ total score of 2.3 points (95% CI ‐2.9 to 7.4 points) was demonstrated between groups at end intervention for the other study that could not be included in the meta‐analysis (Newall 2005). Data for longer‐term effects on SGRQ total score were available at three months and at 12 months in two studies (Lee 2014; Mandal 2012). Despite a trend towards maintained improvement in quality of life, evidence suggests this may not occur (low‐certainty evidence; Analysis 1.11). For the CRDQ, improvement in the domains of dyspnoea and fatigue was evident in favour of exercise training, but this was not noted for emotional function and mastery (Analysis 1.12; Figure 7; Lee 2014), nor did any CRDQ domains retain improvement at 12 months (Analysis 1.13).
1.10. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 10: HRQoL ‐ SGRQ total score at end intervention
6.

Note: Data are expressed as change from baseline for Dal Corso 2017, and as endpoint (end intervention) for all others.
1.11. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 11: HRQoL ‐ SGRQ total score at follow‐up
1.12. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 12: HRQoL ‐ CRDQ (all domains) at end treatment
7.

Figure 7: Forest plot Analysis 1.12 ‐ Health‐related quality of life (Chronic Respiratory Disease Questionnaire) at end‐intervention
1.13. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 13: HRQoL ‐ CRDQ (all domains) at follow‐up
Pooled data from two studies involving 103 participants demonstrated that exercise training likely had little to no impact on cough‐related quality of life immediately following treatment as measured by the total LCQ score (MD in change from baseline ‐0.09 points, 95% CI ‐0.98 to 0.80 points; I² = 0%; n = 103; moderate‐certainty evidence) or on LCQ domains of physical, psychological, and social scores (Analysis 1.14; Figure 8). Similar findings were apparent at three months' and at 12 months' follow‐up; however the evidence is very uncertain (very low‐certainty evidence; Analysis 1.15). In contrast, one study that could not be included in the meta‐analysis demonstrated improvement in all LCQ domains with exercise training (all P < 0.001), with only psychological score (P = 0.034) and total score (P = 0.009) improved in the control group (Kumar 2017).
1.14. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 14: HRQoL ‐ LCQ (all domains) at end intervention
8.

Figure 8: Forest plot Analysis 1.14 ‐ Health‐related quality of life (Leicester Cough Questionnaire) at end‐intervention
1.15. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 15: HRQol ‐ LCQ (all domains) at follow‐up
Secondary outcomes
Exacerbation and hospitalisation
The rate of exacerbation in those undertaking exercise training was reduced compared to that in the control group (odds ratio (OR) 0.26, 95% CI 0.08 to 0.81; Analysis 1.16). Time to first exacerbation was longer in those with stable bronchiectasis (log rank 0.49, 95% CI 0.01 to 0.97) (Lee 2014).
1.16. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 16: Exacerbations / hospitalisations ‐ Number of exacerbations
Peripheral muscle strength
Quadriceps muscle strength was measured in one study (Dal Corso 2017). Exercise training improved quadriceps muscle force compared to usual care (MD 7.4 kg, 95% CI 2.81 to 11.99 kg; n = 39) (Analysis 1.17).
1.17. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 17: Peripheral muscle force at end intervention
Physical activity
Physical activity levels were not measured in any studies.
Mental health
Two studies measured anxiety and/or depression, using the Hospital Anxiety and Depression Scale (Lee 2014), or the Depression Anxiety Stress Scale (Kumar 2017). One study demonstrated no effect of exercise training on anxiety or depression immediately post intervention (Analysis 1.18; Analysis 1.19), or at 12 months' follow‐up (Analysis 1.20; Analysis 1.21). In contrast, a second study found that the sub‐scale score for depression and the total score improved, regardless of whether exercise training was undertaken, and anxiety improved with exercise training only (P = 0.01) (Kumar 2017).
1.18. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 18: Anxiety ‐ HADS at end intervention
1.19. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 19: Depression ‐ HADS at end intervention
1.20. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 20: Anxiety ‐ HADS at follow‐up
1.21. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 21: Depression ‐ HADS at follow‐up
Clinical symptoms
Clinical symptoms of dyspnoea, cough, and fatigue were assessed only as part of HRQoL questionnaires.
Mortality
The incidence of mortality was evaluated in Lee 2014, which reported no differences between groups (OR 0.27, 95% CI 0.01 to 6.87; Analysis 1.22).
1.22. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 22: Mortality (all cause)
Adverse events
No studies reported on the occurrence of adverse events.
Exercise training versus usual care post acute exacerbation
Primary outcomes
Exercise tolerance
In one study of 27 participants undertaking exercise training following acute exacerbation of bronchiectasis, evidence suggests that treatment may result in little to no difference in the degree of improvement observed in 6MWD between groups following a 12‐week intervention (MD 11 m, 95% CI ‐26.29 to 48.79 m; n = 27; low‐certainty evidence) (Analysis 2.1; Chalmers 2019).
2.1. Analysis.

Comparison 2: Exercise training vs usual care (post exacerbation), Outcome 1: Exercise tolerance ‐ 6MWT (metres) at end intervention
Health‐related quality of life
Exercise training did not appear to impact the degree of change in quality of life observed between groups immediately post intervention via SGRQ total or sub‐domain scores or by LCQ total score (low‐certainty evidence; Analysis 2.2; Analysis 2.3; Chalmers 2019). Health status was measured via the COPD Assessment Test (CAT), and although exercise training did not appear to impact the difference between groups at any time point, the magnitude of differences between groups at the 12‐week follow‐up time point (3.5 points) was noted as exceeding the minimally important difference threshold for this outcome.
2.2. Analysis.

Comparison 2: Exercise training vs usual care (post exacerbation), Outcome 2: HRQoL ‐ SGRQ total score at end intervention
2.3. Analysis.

Comparison 2: Exercise training vs usual care (post exacerbation), Outcome 3: HRQoL ‐ LCQ total score at end intervention
Secondary outcomes
Exacerbation and hospitalisation
Time to first exacerbation did not change as a result of exercise training (Analysis 2.4).
2.4. Analysis.

Comparison 2: Exercise training vs usual care (post exacerbation), Outcome 4: Exacerbations/Hospitalisations ‐ Time to first exacerbation
Discussion
Summary of main results
This review included six studies comparing exercise training (all multi‐component) to usual care (comprising no exercise training) in adults with bronchiectasis who were in a stable disease state or during a period following an acute exacerbation. Multi‐component interventions comprised physical exercise, educational sessions, and specific instruction regarding airway clearance therapy and/or review of airway clearance therapy techniques. Exercise training was conducted predominantly in an outpatient setting, with programme duration ranging from six to eight weeks. Lack of clinical trial data pertaining to children with bronchiectasis was noted.
Exercise training led to improvement in measures of functional exercise capacity, as measured by the incremental shuttle walk test (ISWT) and the six‐minute walk test (6MWT) in people with stable bronchiectasis. The magnitude of improvement in ISWT following exercise training was 87.12 metres, which is greater than the minimal important difference of 47.5 metres for this outcome for chronic respiratory disease (Singh 2014), and between 35 and 70 metres for people with bronchiectasis (Lee 2014a; Walsh 2020). This reflects a similar magnitude of improvement in functional exercise capacity following pulmonary rehabilitation observed in people with chronic obstructive pulmonary disease (COPD) (Singh 2008). In contrast, the effect on maximal exercise capacity was variable, with contrasting findings between studies. Significant improvements in quality of life were also evident, with the degree of change following exercise training for St George's Respiratory Quotient (SGRQ) total score greater than the minimally important difference threshold of 4 points reported for this outcome in people with chronic respiratory disease (Jones 2005). This was complemented by small reductions in dyspnoea and fatigue immediately following exercise training. Data were insufficient to show the longer‐term effects of exercise training on exercise capacity and quality of life in people with bronchiectasis.
Exercise training appeared to have minimal impact on cough‐related quality of life, anxiety, or depression. Improvement in peripheral muscle strength for the quadriceps was reported in one study of home‐based training. Exercise training was associated with fewer acute exacerbations and longer time to first exacerbation over a 12‐month period in people with stable disease. A single study in people post acute exacerbation of bronchiectasis demonstrated that exercise training was not associated with changes in functional exercise capacity, cough‐related quality of life, or time to first exacerbation (Chalmers 2019). Physical activity was not measured by any studies, and no adverse events related to exercise training were reported.
Overall completeness and applicability of evidence
Five of the included studies involved individuals with stable bronchiectasis, with only one investigating the effects of exercise training in people post acute exacerbation of bronchiectasis. Although rarely specified, it is likely that a diverse range of bronchiectasis aetiologies were included in this review. Specific causes of bronchiectasis may result in more severe physiological impairments, which could impact the benefits of exercise training effectiveness. As the included studies in stable bronchiectasis demonstrated mild to moderate changes in spirometry as a reflection of disease severity, application of these findings to people with more severe disease in a stable clinical state may require further exploration. In the absence of broad application of bronchiectasis severity scores to classify disease severity, measures of airway obstruction may facilitate this interpretation. Guidelines for pulmonary rehabilitation recommend this intervention for people with chronic respiratory disease who demonstrate dyspnoea on exertion (Spruit 2013). In this review, the degree of exertional dyspnoea prior to the intervention was reported by two studies and was stated as ≥ 1 (minimum of 1 to maximum of 3) or ≥ 2 (minimum and maximum not reported) on the modified Medical Research Council Dyspnoea Scale (Kumar 2017; Lee 2014). Therefore caution should be exerted if findings observed in this review are extrapolated to patients with greater levels of exertional dyspnoea.
Pooled outcome data for ISWT reveal that exercise training likely confers clinically relevant benefit compared to usual care. Although this is reassuring and is clinically intuitive, we note the potential for this result to have been influenced by a considerable between‐group difference at baseline in one study (intervention: 287.5 metres; control: 343.3 metres) (Mandal 2012). The review authors noted that this baseline imbalance is not statistically significant; however it does exceed the minimally important difference threshold for people with chronic lung disease (Singh 2014). When considered in light of the lack of between‐group differences at the end of the intervention (mean difference (MD) of 5.5 metres) for this study, it is possible that the true magnitude of effect for this outcome may have been underestimated. The effect estimates for other measures of endurance capacity or maximal exercise testing were more variable. Although improvements were noted in endurance shuttle walk test time, no meaningful change in endurance shuttle walk test distance was noted (Mandal 2012). Similarly, although significant improvement in walking distance was observed during a constant load exercise test, the effect on peak oxygen uptake (VO₂ peak) showed contrast between studies. This may be explained by factors related to individual study methods, with sample size calculations specific for these outcomes not reported in either study, and the approach used for exercise prescription specific to exercise intensity lacking in one study (Kumar 2017). Therefore, it is difficult to determine whether lack of observed effect in such studies reflected true treatment effects or was a consequence of inadequate statistical power to detect appropriate changes. This may also account for one study reporting lack of difference in SGRQ total score at the end of the intervention (Newall 2005), in contrast to pooled improvement in this outcome and in measures of dyspnoea and fatigue immediately post intervention (Lee 2014).
Lack of observed effects of exercise training on psychological measures contrasts with reports of people with other respiratory conditions such as COPD (Gordon 2019). This finding could be attributable to the mean baseline levels of anxiety and depression in the single study that reported this outcome (Lee 2014). In this study, baseline Hospital Anxiety and Depression Scale (HADS) scores did not meet the minimum standard to satisfy diagnostic criteria for the presence of a mood disorder (Snaith 2003), meaning that the opportunity to demonstrate a treatment effect may have been diminished. The improvement in quadriceps muscle force concurs with findings observed in people with other respiratory conditions undergoing exercise training (Spruit 2013). However, given the small volume of data related to this outcome, further research is indicated to confirm this observation in people with bronchiectasis.
All studies in this review incorporated exercise training applied in conjunction with other co‐interventions. These included educational sessions covering a range of topics, regular use of airway clearance therapy, and review of a prescribed technique. It is important to note that all such co‐interventions were applied as part of usual care. Therefore, it is likely that observed effects in this review are attributable to the exercise training stimulus. Studies of self‐management in bronchiectasis, which have included educational sessions on causes of bronchiectasis, disease process, medical investigations, symptom management, exacerbations, health promotion, airway clearance techniques, and support, did not demonstrate beneficial effects on health‐related quality of life (HRQoL) compared to usual care in people with bronchiectasis (Kelly 2018). With some similarities in the educational topics included in the studies in this review, it is possible that their contributions to changes in HRQoL were minimal, but this should be confirmed in future work. In contrast, findings regarding airway clearance therapy, with a previous report of improvement in exercise capacity with this intervention (Murray 2009), are variable, and some studies show no change (Herrero‐Cortina 2019; Munoz 2018). Although the impact of airway clearance interventions on exercise capacity appears controversial and unclear, it cannot be excluded that some observed treatment effects may be partially influenced by the adjunct effect of this co‐intervention. Similarly, effective airway clearance therapy can improve dyspnoea, HRQoL (Lee 2015), and cough‐related quality of life (Murray 2009); the relative contribution of this co‐intervention is therefore somewhat difficult to determine.
Bronchiectasis‐specific quality of life and health status questionnaires, together with measures of bronchiectasis impact, have been recently developed but did not feature in the findings of our review (Crichton 2020; Quittner 2015; Spinou 2017). Application of these tools in evaluating the effects of exercise training in bronchiectasis may further clarify our observed effects on quality of life. The lack of effect on cough‐related quality of life may be related to the instructions provided during exercise training. Optimisation of exercise training to address cough symptoms may be achieved by incorporation of the forced expiratory technique during or immediately following training (Ward 2019). As it is unknown whether this strategy and/or advice was provided to participants in the included studies, it is difficult to determine whether this may account for the lack of change in cough‐related quality of life. Sub‐therapeutic, adjuvant sham respiratory muscle training was another co‐intervention reported in one study (Newall 2005). Although this may have been unlikely to have influenced outcomes related to exercise capacity, it was hypothesised that it may have facilitated improvements in airway clearance. This was not explicitly examined in this study, suggesting that its possible impact on changes in cough‐related quality of life is also unclear.
Although all studies in this review used aerobic exercise training, the inclusion of resistance training was more variable, and its use was not reported in two studies (Kumar 2017; Newall 2005). The use of aerobic exercise training aligns with current recommendations for pulmonary rehabilitation for people with chronic respiratory disease (Spruit 2013), but we are not able to draw consistent inferences regarding the relative contributions of resistance training. Only one study specifically measured peripheral muscle strength. All programmes adhered to current recommendations related to programme length (six to eight weeks), but training sessions varied from unsupervised to supervised, with sessions conducted three to four times per week. Clarity regarding the optimal exercise training prescription for people with bronchiectasis, regardless of clinical state, is currently lacking.
The observed reduction in the incidence of exacerbations following exercise training arose from a single study (Lee 2014; n = 30 included in analysis). This outcome is a common 'downstream' target of exercise training interventions, but data remain scarce for people with bronchiectasis. The ability to affect exacerbations is clinically important due to their negative impact upon subsequent aspects of disease progression such as future hospitalisations, poor quality of life, and mortality (Polverino 2018). It is furthermore interesting to note the magnitude of observed effect (75% reduced odds for experiencing an exacerbation), which is larger than for many other treatments (including pharmacological ones) that may seek to directly impact this outcome. It is challenging, however, to propose a mechanism that accurately explains our observed effect. It is perhaps unlikely attributable to exercise‐induced changes in respiratory‐related symptoms (e.g. dyspnoea, fatigue, cough‐related symptoms) in light of the small magnitude of observed changes noted over time. This may be unsurprising, as exercise training alone in this population is not hypothesised to alter sputum clearance; however further inquiry in this context would appear indicated.
The lack of clinically meaningful benefit of exercise training for people following an acute exacerbation of bronchiectasis seems unlikely to be attributable to intervention timing, as this aligned with international pulmonary rehabilitation commencement recommendations (Alison 2017; Puhan 2016). However, the lack of impact on time to first exacerbation is likely due to an insufficient follow‐up duration following the intervention (three months) (Chalmers 2019). The inclusion of only individuals who had completed a 14‐day course of antibiotics further limits the application of these findings. Although a mix of disease severity was noted for the baseline number of exacerbations or the Bronchiectasis Severity Index (mild to severe), no individuals with severe exacerbations that required hospitalisation were included. Further research is required to explore the effects of exercise training in alternate subgroups with respect to exacerbation severity and the need for hospitalisation.
The lack of data pertaining to children with bronchiectasis is a matter of concern. Exercise training undertaken in the context of pulmonary rehabilitation programmes is most commonly undertaken by adults with chronic lung disease, but this does not imply that children with bronchiectasis may not benefit from exercise training. We ensured that our intervention definition was not constrained to a pulmonary rehabilitation context for this specific reason, but we retrieved only one record of potential relevance (an ongoing clinical trial registered from Australia). It is important that the absence of clinical data for this patient group is not equated to lack of effectiveness of exercise training. Future clinical trials in this space are clearly indicated. Similarly, although no studies of telerehabilitation in people with bronchiectasis were presently identified for inclusion, we anticipate that clinical outcomes from this model of exercise training in this population will emerge in the future. The relatively small number of studies included within this review demonstrates an ongoing indication for additional research regarding the clinical effectiveness of exercise training for people with bronchiectasis across a broad range of clinical outcomes. Although withholding of exercise might pose ethical challenges to conducting such clinical trials in settings where this form of therapy is actively promoted, this is not yet usual care in many places and may be managed through wait‐list control groups (who receive the intervention in a delayed fashion). Additional research data in this area would equip healthcare providers with greater accuracy regarding the likelihood and magnitude of potential benefits from such treatments and, in turn, would assist people affected by bronchiectasis. This is quite different from the research landscape surrounding people affected by COPD, where further trials examining the effectiveness of pulmonary rehabilitation compared to usual care have been discouraged (Lacasse 2015). The modern era of personalised and flexible models of care (including rehabilitation) makes it challenging to identify any single direction of research that may confer the greatest benefit for the greatest number of people affected by bronchiectasis. Considerable opportunity clearly exists to extend inquiry into various aspects of patient care and precision medicine in the field of bronchiectasis rehabilitation.
Quality of the evidence
The evidence provided in this review should be interpreted with regards to some identified sources of bias. Two of the six studies were available only in abstract form (Dal Corso 2017; Kumar 2017); this limits detail on data provided, as well as the ability to adequately assess all parameters of risk of bias for these studies. Due to unclear reporting, selection bias may be present in four studies (Chalmers 2019; Kumar 2017; Mandal 2012; Newall 2005). Lack of blinding of participants to knowledge of treatment allocation was confirmed in two studies (Dal Corso 2017; Lee 2014), but it was rated as unclear for all others. This was not unexpected, as blinding of participants in studies involving physical interventions is inherently difficult. Blinding of outcome assessors was applied in Lee 2014; for the remaining studies, this was not applied (Dal Corso 2017), or it was unclear (Chalmers 2019; Kumar 2017; Mandal 2012; Newall 2005). We attributed high risk of bias to all studies regarding self‐reported outcome measures, as we felt this potential influence was unavoidable. Data related to long‐term treatment effect estimates may have been affected by rates of participant attrition, but this appeared to be of modest magnitude, even for the 12‐month follow‐up data provided in Lee 2014.
We rated the overall quality of evidence according to the GRADE method as very low to low for different measures of exercise capacity, and as very low to moderate for HRQoL. This was predominantly attributed to increased risk of bias due to inadequate reporting of methods and lack of blinding of outcome measures, imprecision, and indirectness due to the small number of studies. Inconsistency was rarely observed across outcome data, with only one outcome (long‐term follow‐up of cough‐related quality of life) demonstrating effect estimates that contrasted between included studies.
Potential biases in the review process
All data were extracted independently by two review authors, and discrepancies were resolved through discussion. Risk of bias ratings were completed by two review authors independently. Data from Lee 2014 were extracted by two review authors who were not involved in this study.
A broad search included handsearching of conference abstracts and trial registries. Studies published in abstract form were included to ensure all available trials were incorporated within the review. When clarification of study characteristics or additional data were required, we attempted to obtain this information from study authors. This was successful on selected occasions, but in some cases, data were not available. This may have influenced the assessment of trial quality and some estimates of treatment effects.
Agreements and disagreements with other studies or reviews
These findings expand upon the conclusions of an earlier Cochrane Review, which found that inspiratory muscle training compared to sham or no training improved endurance exercise capacity and quality of life (Bradley 2002). In addition, results for exercise capacity, quality of life, and cough‐related quality of life are consistent with the overall findings of a previous systematic review of pulmonary rehabilitation for people with bronchiectasis (Lee 2017). The magnitude of change for these primary outcomes is similar to that in people with COPD who are undergoing pulmonary rehabilitation (McCarthy 2015). This supports the international statements and recent pulmonary rehabilitation guidelines for inclusion of people with bronchiectasis in pulmonary rehabilitation (Alison 2017; Spruit 2013). Our findings are also consistent with those of an earlier Cochrane Review of physical training in bronchiectasis, which demonstrated that the combination of exercise training, respiratory muscle training, and education improved endurance exercise capacity and quality of life compared to exercise training and sham respiratory muscle training (Bradley 2002). This previous review, however, incorporated findings from only two reports related to one study published in abstract form only, with limited participant numbers considerably limiting the ability to draw robust implications for clinical practice.
Authors' conclusions
Implications for practice.
This review suggests that exercise training applied with co‐interventions (educational sessions, airway clearance therapy prescription, or review of airway clearance therapy) results in improvements in measures of functional exercise capacity and quality of life immediately following treatment in people with stable bronchiectasis; however the certainty of evidence is low. Evidence to comprehensively support observed long‐term benefits of exercise training is currently lacking, although a beneficial effect on subsequent exacerbations has been observed in one study. Evidence is insufficient to show the effects of exercise training on cough‐related quality of life, psychological symptoms, and peripheral muscle strength, and evidence of low certainty regarding the effect of this intervention in people following acute exacerbations is limited.
Implications for research.
The small number of included studies suggests that additional randomised controlled trials are required to determine the effects of exercise training in isolation or in conjunction with other treatments on exercise capacity and quality of life, irrespective of clinical status. Larger studies with greater participant numbers are required to determine the long‐term effects achieved with exercise training and to reduce the imprecision associated with observed treatment effects.
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2021 | Amended | Updated search dates to reflect the most recent search in the 'Electronic searches' section. |
History
Protocol first published: Issue 8, 2018 Review first published: Issue 4, 2021
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways. The review authors and the Airways Editorial Team are grateful to the following peer reviewers for their time and comments: Rocio Martín‐Valero (Spain); Michal Shteinberg (Israel); Pauline Heus (The Netherlands); Beatriz Herrero‐Cortina (Spain); Neil Greening (UK); and Arietta Spinou (UK).
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service, or the Department of Health.
Appendices
Appendix 1. Database search strategies
CENTRAL and Cochrane Airways Trials Register (via Cochrane Register of Studies)
#1 BRONCH:MISC1 #2 MeSH DESCRIPTOR Bronchiectasis Explode All #3 bronchiect* #4 #1 or #2 or #3 #5 MESH DESCRIPTOR Rehabilitation EXPLODE ALL #6 MESH DESCRIPTOR Respiratory Therapy EXPLODE ALL #7 MESH DESCRIPTOR Exercise EXPLODE ALL #8 MeSH DESCRIPTOR Physical Therapy Modalities Explode All #9 rehabilitat* or fitness* or exercis* or train* or physiotherap* or (physical* NEXT therap*) #10 {OR #5‐#9} #11 #10 AND #4
MEDLINE (Ovid)
1 exp Bronchiectasis/ 2 bronchiect$.ti,ab. 3 1 or 2 4 exp Physical Therapy Modalities/ 5 exp Exercise/ 6 exp Physical Fitness/ 7 Physical Exertion/ 8 exp Exercise Therapy/ 9 (rehabilitat$ or fitness$ or exercis$ or physical$ or train$ or activ$ or physiotherap$ or kinesiotherap$ or exert$).ti,ab. 10 aerobic$.ti,ab. 11 (muscle$ adj3 resist$).ti,ab. 12 or/4‐11 13 3 and 12 14 (controlled clinical trial or randomized controlled trial).pt. 15 (randomized or randomised).ab,ti. 16 placebo.ab,ti. 17 dt.fs. 18 randomly.ab,ti. 19 trial.ab,ti. 20 groups.ab,ti. 21 or/14‐20 22 Animals/ 23 Humans/ 24 22 not (22 and 23) 25 21 not 24 26 13 and 25
Embase (Ovid)
1 exp Bronchiectasis/ 2 bronchiect$.ti,ab. 3 1 or 2 4 exp Physical Therapy Modalities/ 5 exp Exercise/ 6 exp Physical Fitness/ 7 Physical Exertion/ 8 exp Exercise Therapy/ 9 (rehabilitat$ or fitness$ or exercis$ or physical$ or train$ or activ$ or physiotherap$ or kinesiotherap$ or exert$).ti,ab. 10 aerobic$.ti,ab. 11 (muscle$ adj3 resist$).ti,ab. 12 or/4‐11 13 3 and 12 14 Randomized Controlled Trial/ 15 randomization/ 16 controlled clinical trial/ 17 Double Blind Procedure/ 18 Single Blind Procedure/ 19 Crossover Procedure/ 20 (clinica$ adj3 trial$).tw. 21 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 22 exp Placebo/ 23 placebo$.ti,ab. 24 random$.ti,ab. 25 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 26 (crossover$ or cross‐over$).ti,ab. 27 or/14‐26 28 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 29 human/ or normal human/ or human cell/ 30 28 and 29 31 28 not 30 32 27 not 31 33 13 and 32
PEDro
| Abstract and title | Bronchiectasis AND exercise |
| Method | Clinical trial |
ClinicalTrials.gov
| Field | Search term |
| Study type | interventional |
| Condition | Bronchiectasis |
| Intervention | Rehabilitation OR fitness OR exercise OR physical OR training |
WHO Trials Registry
| Field | Search term |
| Condition | Bronchiectasis |
| Intervention | Rehabilitation OR fitness OR exercise OR physical OR training |
Data and analyses
Comparison 1. Exercise training vs usual care (stable disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Exercise tolerance ‐ ISWT (metres) at end intervention | 4 | 161 | Mean Difference (IV, Random, 95% CI) | 87.12 [42.65, 131.58] |
| 1.1.1 Uni‐component | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.1.2 Multi‐component | 4 | 161 | Mean Difference (IV, Random, 95% CI) | 87.12 [42.65, 131.58] |
| 1.2 Exercise tolerance ‐ 6MWT (metres) at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Exercise tolerance ‐ ESWT (mins) at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Exercise tolerance ‐ ESWT (metres) at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Exercise tolerance ‐ CLET (metres) at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Exercise tolerance ‐ CPET VO 2 peak at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 Exercise tolerance ‐ ISWT (metres) at follow‐up | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 6.19 [‐15.51, 27.90] |
| 1.7.1 Uni‐component | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.7.2 Multi‐component | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 6.19 [‐15.51, 27.90] |
| 1.8 Exercise tolerance ‐ 6MWT (metres) at follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9 Exercise tolerance ‐ ESWT (metres) at follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 HRQoL ‐ SGRQ total score at end intervention | 3 | 110 | Mean Difference (IV, Random, 95% CI) | ‐9.62 [‐15.67, ‐3.56] |
| 1.10.1 Uni‐component | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.10.2 Multi‐component | 3 | 110 | Mean Difference (IV, Random, 95% CI) | ‐9.62 [‐15.67, ‐3.56] |
| 1.11 HRQoL ‐ SGRQ total score at follow‐up | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐14.98, 1.42] |
| 1.11.1 Uni‐component | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.11.2 Multi‐component | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐14.98, 1.42] |
| 1.12 HRQoL ‐ CRDQ (all domains) at end treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12.1 CRDQ ‐ Dyspnoea | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12.2 CRDQ ‐ Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12.3 CRDQ ‐ Emotional Function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12.4 CRDQ ‐ Mastery | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13 HRQoL ‐ CRDQ (all domains) at follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.1 CRDQ ‐ Dyspnoea | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.2 CRDQ ‐ Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.3 CRDQ ‐ Emotional function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.4 CRDQ ‐ Mastery | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14 HRQoL ‐ LCQ (all domains) at end intervention | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.14.1 LCQ ‐ Physical | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.28, 0.48] |
| 1.14.2 LCQ ‐ Psychological | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 1.14.3 LCQ ‐ Social | 1 | 76 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.33, 0.53] |
| 1.14.4 LCQ ‐ Total | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.98, 0.80] |
| 1.15 HRQol ‐ LCQ (all domains) at follow‐up | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.15.1 LCQ ‐ Physical | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.45, 0.05] |
| 1.15.2 LCQ ‐ Psychological | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.18, ‐0.02] |
| 1.15.3 LCQ ‐ Social | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.11, ‐2.09] |
| 1.15.4 LCQ ‐ Total | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐8.27, 6.34] |
| 1.16 Exacerbations / hospitalisations ‐ Number of exacerbations | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.16.1 Uni‐component | 0 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.16.2 Multi‐component | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.17 Peripheral muscle force at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.17.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.17.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18 Anxiety ‐ HADS at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.18.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19 Depression ‐ HADS at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.20 Anxiety ‐ HADS at follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.20.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.20.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.21 Depression ‐ HADS at follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.21.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.21.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.22 Mortality (all cause) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.22.1 Uni‐component | 0 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.22.2 Multi‐component | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.23 Sensitivity analysis for ISWT end intervention | 3 | 122 | Mean Difference (IV, Random, 95% CI) | 69.65 [45.32, 93.98] |
| 1.24 Sensitivity analysis for SGRQ total end intervention | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐4.98 [‐13.61, 3.65] |
| 1.25 Heterogeneity for ISWT post intervention | 3 | 122 | Mean Difference (IV, Random, 95% CI) | 68.74 [44.42, 93.07] |
| 1.26 Heterogeneity for SGRQ total score end intervention | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐13.61, 3.65] |
1.23. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 23: Sensitivity analysis for ISWT end intervention
1.24. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 24: Sensitivity analysis for SGRQ total end intervention
1.25. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 25: Heterogeneity for ISWT post intervention
1.26. Analysis.

Comparison 1: Exercise training vs usual care (stable disease), Outcome 26: Heterogeneity for SGRQ total score end intervention
Comparison 2. Exercise training vs usual care (post exacerbation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Exercise tolerance ‐ 6MWT (metres) at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 HRQoL ‐ SGRQ total score at end intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 Uni‐component | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2.2 Multi‐component | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 HRQoL ‐ LCQ total score at end intervention | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 Uni‐component | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.2 Multi‐component | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Exacerbations/Hospitalisations ‐ Time to first exacerbation | 1 | 27 | Hazard Ratio (IV, Random, 95% CI) | 0.83 [0.31, 2.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chalmers 2019.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Total study duration: 8 weeks Study setting: outpatient Withdrawals: not stated |
|
| Participants |
No. participants randomised: 27 (9 intervention, 18 control) No. participants completing study: not stated Baseline characteristics of intervention group: 68 (range 63 to 71) years, FEV₁ 76 (46.5 to 109)% pred, 4 males, 7 never smoked, 1 ex‐smoker, 1 current smoker Baseline characteristics of control group: 68 (range 63 to 73) years, FEV₁ 83 (55.8 to 90.8)% pred, 3 males, 11 never smoked, 7 ex‐smokers, 0 current smokers Inclusion criteria: clinically stable with bronchiectasis; diagnosis of bronchiectasis confirmed by HRCT; clinically significant bronchiectasis confirmed by respiratory physician, with at least 1 documented exacerbation in the year before recruitment Exclusion criteria: inability to give informed consent; age < 18 years; primary diagnosis of chronic obstructive pulmonary disease; significant comorbidity that limits ability to undertake pulmonary rehabilitation; have undergone pulmonary rehabilitation within the previous year |
|
| Interventions |
Intervention: 2 supervised sessions/week and 2 ‘homework’ sessions for 6 weeks. PR contained a mixture of cardiovascular training (e.g. treadmill, bike, walking) at 80% VO₂ max and strength training via free weights at 1 repetition maximum, aiming for 8 to 10 reps, plus educational activities and individual instruction in breathing strategies and chest clearance techniques Control: guideline concordant ongoing management, including instruction in daily chest physiotherapy |
|
| Outcomes | Endpoints were evaluated at baseline, at onset of exacerbation, at end of exacerbation (Day 14), 8 weeks post exacerbation, 12 weeks post exacerbation Primary: change in 6MWD from end of exacerbation (Day 14 post antibiotic) to 8 weeks post exacerbation Secondary: 6MWD at 12 weeks post exacerbation, time to next exacerbation (defined via EXACT‐PRO), change in quality of life (SGRQ), spirometry at 8 and 12 weeks, change in cough symptoms (LCQ) and health status (CAT) at 8 and 12 weeks, sputum microbiology |
|
| Notes | Study was a pilot trial; study authors stated an additional aim of the study was identification of the number of patients required to be recruited to demonstrate clinically meaningful improvement in 6MWD. Study therefore was underpowered for many outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised using sealed opaque envelopes at 1:1 ratio to either pulmonary rehabilitation or standard care" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised using sealed opaque envelopes at 1:1 ratio to either pulmonary rehabilitation or standard care" |
| Blinding of participants and personnel (performance bias) Self reported outcomes | High risk | No information provided but not likely to be preventable |
| Blinding of participants and personnel (performance bias) Non‐self reported outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Self reported outcomes | High risk | No information provided but not likely to be preventable |
| Blinding of outcome assessment (detection bias) Non‐self reported outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided for individual outcome data |
| Selective reporting (reporting bias) | Low risk | Findings related to each outcome reported |
| Other bias | Unclear risk | Unclear why 1:1 randomisation resulted in uneven group allocation (9 in intervention arm, 18 in usual care arm) |
Dal Corso 2017.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Total study duration: 8 weeks Study setting: home‐based Withdrawals: not stated |
|
| Participants |
No. participants randomised: 39 (20 intervention, 19 control) No. participants completing study: not stated Baseline characteristics of intervention group: 44 ± 18 years, FEV₁ 51 ± 22% pred Baseline characteristics of control group: 47 ± 14 years, FEV₁ 45 ± 16% pred Inclusion criteria: NCFB patients >18 years in stable disease state; diagnosis of bronchiectasis confirmed by HRCT or clinical diagnosis Exclusion criteria: smoking; primary diagnosis of other lung disease (asthma, COPD, ILD, CF); severe CVD; inability to perform tests and training protocol due to musculoskeletal limitations; significant levels of desaturation (SpO₂ ≤ 80%) during baseline exercise testing |
|
| Interventions |
Intervention: 3 unsupervised home‐based PR sessions/week for 8 weeks (average session time 50 minutes). Aerobic exercise included stepping on a platform for 20 minutes (intensity: 60% to 80% maximal stepping rate in incremental step test). Resistance training for quadriceps, hamstrings, deltoids, and biceps for 20 minutes (load: 70% maximum voluntary isometric contraction; dose: 3 sets/8 reps). Education booklet. Weekly phone call. Home visit every 15 days Control: education booklet; recommended to walk at moderate intensity for 30 minutes 3 times/week. Weekly phone call (no exercise or physical activity discussion) |
|
| Outcomes |
Primary: ISWT at baseline and on completion of intervention (8 weeks) Secondary: ESWT, SGRQ, and peripheral muscle strength at baseline (using a dynamometer) and on completion of intervention (8 weeks) |
|
| Notes | Abstract only. Study details available from published protocol. Results taken from abstract of interim analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study protocol reports, “The randomisation sequence will be generated using the website www.randomization.com” |
| Allocation concealment (selection bias) | Low risk | Study protocol reports randomisation concealed by using “consecutively numbered, sealed and opaque envelopes” |
| Blinding of participants and personnel (performance bias) Self reported outcomes | High risk | Study protocol reports, “due to the nature of the interventions, it is not possible to blind patients and therapists” |
| Blinding of participants and personnel (performance bias) Non‐self reported outcomes | High risk | Study protocol reports, “due to the nature of the interventions, it is not possible to blind patients and therapists” |
| Blinding of outcome assessment (detection bias) Self reported outcomes | High risk | Study protocol reports, “due to lack of human resources, the outcomes of this trial will be collected by an unblinded outcomes assessor” |
| Blinding of outcome assessment (detection bias) Non‐self reported outcomes | High risk | Study protocol reports, “due to lack of human resources, the outcomes of this trial will be collected by an unblinded outcomes assessor” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not stated for all outcomes (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol refers to outcome measures including maximal exercise capacity (incremental step test) not reported in abstract results. All primary and secondary outcomes reported in abstract reflect protocol |
| Other bias | Unclear risk | Insufficient detail provided to accurately determine other bias risk (abstract only) |
Kumar 2017.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Total study duration: 8 weeks Study setting: outpatient Withdrawals: 18 (11 intervention, 7 control); no reason provided |
|
| Participants |
No. participants randomised: 60 (31 intervention, 29 control) No. participants completed study: 42 (20 intervention, 22 control) Baseline characteristics of intervention and control groups: not reported Inclusion criteria: NCFB patients with at least 2 exacerbations in 1 year and mMRC score ≥ 2. Diagnostic criteria for bronchiectasis not stated Exclusion criteria: not reported |
|
| Interventions |
Intervention: 8 weeks outpatient PR. No further details reported Control: standard care |
|
| Outcomes | 6MWT, CPET, SGRQ, LCQ, DASS at baseline and on completion of intervention (8 weeks) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reports, "patients were randomised"; however insufficient details provided to accurately assess risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) Self reported outcomes | High risk | No information provided but not likely to be preventable |
| Blinding of participants and personnel (performance bias) Non‐self reported outcomes | Unclear risk | No details provided. Would assume participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) Self reported outcomes | High risk | No information provided but not likely to be preventable |
| Blinding of outcome assessment (detection bias) Non‐self reported outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18 participants "dropped out" (11 from intervention, 7 from control) without reasons or effects on outcomes examined |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available; insufficient detail provided in abstract to accurately determine risk |
| Other bias | Unclear risk | Study protocol not available; insufficient detail provided in abstract to accurately determine risk |
Lee 2014.
| Study characteristics | ||
| Methods |
Study design: multi‐centre RCT Total study duration: 14 months Study setting: outpatient Withdrawals: following intervention phase: 4 in control, 5 in intervention; at 12 months' follow‐up: additional 14 in control, 4 in intervention |
|
| Participants |
No. participants randomised at start: 85 (42 intervention, 43 control) No. participants randomised to long‐term follow‐up: 64 (32 intervention, 32 control) for all outcomes, with the exception of SGRQ (21 intervention, 23 control) Baseline characteristics of intervention group: mean age 63 ± 13 years, FEV₁ 70 ± 23% pred, 12 males Baseline characteristics of control group: mean age 65 ± 12 years, FEV₁ 77 ± 18% pred, 12 males Inclusion criteria: adults > 18 years of age with stable NCFB; self‐reported at least 2 exacerbations requiring antibiotics per year within previous 2 years; ambulant; reporting dyspnoea on exertion (mMRC ≥ 1); diagnosis of bronchiectasis confirmed on HRCT Exclusion criteria: concurrent respiratory disease including COPD (smoking history > 10 pack‐years and evidence of emphysema on HRCT); ILD; asthma (diagnosis and reversibility > 12%); comorbidities that preclude safe exercise; participation in PR in previous 12 months |
|
| Interventions |
Intervention: 2 supervised outpatient exercise training sessions/week for 8 weeks. Aerobic exercise included 15 minutes each of treadmill or land‐based walking and bike (intensity: 75% of ISWT speed for walking and 60% maximal work rate for bike). Resistance training of UL and LL for 15 minutes (load: 10 RM; dose: 1 to 3 sets/10 reps). Home programme prescribed in Week 1, encouraged to undertake 3 to 5 sessions per week (unsupervised). Encouraged to maintain HEP over follow‐up period via monthly phone calls. Reviewed usual ACT. If no usual ACT, participants taught ACBT Control: informed that undertaking 30 minutes exercise most days of the week was associated with health benefits. Phone call twice weekly (no discussion of exercise or physical activity). Reviewed usual ACT. If no usual ACT, participants taught ACBT |
|
| Outcomes |
Primary: ISWT and CRDQ at baseline, on completion of intervention (8 weeks), and at 12 months post intervention follow‐up Secondary: 6MWT, LCQ, HADS at baseline, on completion of intervention (8 weeks), and at 12 months post intervention follow‐up; mortality and exacerbation incidence at 12 months post intervention follow‐up |
|
| Notes | Data pertaining to ISWT, 6MWT, and CRDQ represent change from baseline and were extracted from figures. No characteristics or data pertaining to this study were extracted by review author ALL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...participants were randomised to the intervention or control group at a central location using a computer‐generated randomisation sequence, which was concealed using sealed, opaque envelopes and prepared by an independent individual" |
| Allocation concealment (selection bias) | Low risk | "...participants were randomised to the intervention or control group at a central location using a computer‐generated randomisation sequence, which was concealed using sealed, opaque envelopes and prepared by an independent individual" |
| Blinding of participants and personnel (performance bias) Self reported outcomes | High risk | Participants and treating therapists were not blinded to knowledge of intervention, as this was "a multi‐site, randomised, single blinded, controlled trial..." Whilst this is an inherently challenging issue to control for in rehabilitation studies, lack of participant blinding may have impacted self‐reported outcome measures such as quality of life, symptoms, and mental health |
| Blinding of participants and personnel (performance bias) Non‐self reported outcomes | Low risk | Participants and treating therapists were not blinded to knowledge of intervention, as this was "a multi‐site, randomised, single blinded, controlled trial..." This is, however, unlikely to have affected outcomes that were not self‐reported |
| Blinding of outcome assessment (detection bias) Self reported outcomes | High risk | "...primary and secondary outcomes were measured ... by an assessor blinded to group allocation" However, this is unlikely to prevent self‐reported outcome measures |
| Blinding of outcome assessment (detection bias) Non‐self reported outcomes | Low risk | "...primary and secondary outcomes were measured ... by an assessor blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for participant dropout provided (unrelated to intervention) "Data analysis was completed based on intention‐to‐treat principles ... Missing data were replaced by the last observation carried forward method ... with the robustness of this analysis checked with a sensitivity analysis in which the observed means from the exercise and control groups were substituted for missing data in the opposite arm" |
| Selective reporting (reporting bias) | Low risk | Data for all pre‐specified outcomes were reported |
| Other bias | Low risk | No other sources of bias were identified |
Mandal 2012.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Total study duration: 20 weeks Study setting: outpatient Withdrawals: 3 participants in intervention group (2 family bereavement, 1 new diagnosis of terminal disease) |
|
| Participants |
No. participants randomised: 30 (15 intervention, 15 control) No. participants completed study: 27 (12 intervention, 15 control) Baseline characteristics of intervention group: mean age 65 ± 4 years, FEV₁ 76% pred, 6 males, 33% never smoked Baseline characteristics of control group: mean age 65 ± 3 years, FEV₁ 72% pred, 8 males, 58% never smoked Inclusion criteria: diagnosis of bronchiectasis confirmed by radiology; clinically significant bronchiectasis expectorating sputum when clinically stable; clinically stable disease with no requirement of antibiotics in previous 4 weeks; exercise capacity reduced due to disease process; practising chest clearance regularly Exclusion criteria: current smoker or ex‐smoker for < 1 year; > 15 pack‐year smoking history; CF; active allergic bronchopulmonary aspergillosis; active tuberculosis; poorly controlled asthma |
|
| Interventions |
Intervention: 2 supervised outpatient PR sessions and 1 unsupervised home‐based session/week for 8 weeks. Aerobic exercise included 10 minutes on each of treadmill, bike, and ski machine (intensity: 85% of VO₂ max). Resistance training of UL and LL (load: 60% 1 RM, progressed to 70% at Week 3, then to 80% at Week 5; dose: 3 sets/10 reps). Twice‐daily chest physiotherapy for 8 weeks. Education provided on coping with breathlessness and importance of regular chest physiotherapy. Provided with self‐management plan, dietician advice, taught ACT, and inhaler technique. Encouraged to undertake gym programme at end of intervention period Control: twice‐daily chest physiotherapy with Acapella (20 to 30 minutes per session) for 8 weeks. Technique checked at each visit. Education provided on coping with breathlessness and importance of regular chest physiotherapy. Provided with self‐management plan, dietician advice, taught ACT, and inhaler technique |
|
| Outcomes |
Primary: ISWT at baseline, at completion of intervention (8 weeks), and at 12 weeks' follow‐up Secondary: ESWT, SGRQ, and LCQ at baseline, at completion of intervention (8 weeks), and at 12 weeks' follow‐up |
|
| Notes | Data reported as endpoint rather than as change from baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were "allocated randomly by a computer generated random number sequence" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) Self reported outcomes | High risk | No details provided. Participants and personnel unlikely to have been blinded, which may have impacted self‐reported outcomes |
| Blinding of participants and personnel (performance bias) Non‐self reported outcomes | Unclear risk | No details provided. Participants and personnel unlikely to have been blinded but unlikely to have impacted outcomes that were not self‐reported |
| Blinding of outcome assessment (detection bias) Self reported outcomes | High risk | No information provided but not likely to be preventable |
| Blinding of outcome assessment (detection bias) Non‐self reported outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. Number of participants who withdrew from intervention group provided |
| Selective reporting (reporting bias) | Low risk | Study protocol not available; however all primary and secondary outcomes reported as per methods |
| Other bias | Low risk | No other sources of bias could be identified |
Newall 2005.
| Study characteristics | ||
| Methods |
Study design: single‐centre RCT Total study duration: 20 weeks Study setting: outpatient Withdrawals: 1 participant in intervention group (disease exacerbation) |
|
| Participants |
No. participants randomised: 20 (11 intervention, 9 control) No. participants completed study: 19 (10 intervention, 9 control) Baseline characteristics of intervention group: mean age 63 ± 4 years, FEV₁ 64% pred, 4 males, 4 ex‐smokers, 7 never smoked Baseline characteristics of control group: mean age 63 ± 4 years, FEV₁ 69% pred, 9 females, 2 ex‐smokers, 7 never smoked Inclusion criteria: diagnosis of bronchiectasis confirmed by HRCT; stable clinical and functional state Exclusion criteria: evidence of emphysema; endocrine, orthopaedic, primary cardiac disorders; coronary artery disease; hypertension; cor pulmonale. Experienced an acute exacerbation within previous 6 weeks or receiving long‐term oral corticosteroids |
|
| Interventions |
Intervention: 2 supervised outpatient PR sessions and 1 unsupervised home‐based session/week for 8 weeks (average session 45 minutes). Outpatient exercise included 15 minutes on treadmill, cycle ergometry, and stair climbing (intensity: 80% peak HR on maximal test). Home exercise included 45 minutes walking at targeted intensity. Education provided on disease pathology, rationale for training, medication, coping strategies, relaxation, nutrition, physiotherapy, and smoking cessation. Sham inspiratory muscle training performed at sub‐therapeutic load (7 cmH₂O) for 15 minutes twice daily at home Control: education provided on disease pathology, rationale for training, medication, coping strategies, relaxation, nutrition, physiotherapy, and smoking cessation |
|
| Outcomes | ISWT, CPET, submaximal exercise test (endurance exercise capacity test on treadmill at 85% peak VO₂ achieved on baseline CPET), SGRQ at baseline and on completion of intervention (8 weeks). Intervention group (not control group) followed up at 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were allocated randomly by a “computer generated random number sequence” |
| Allocation concealment (selection bias) | Unclear risk | No specific details provided |
| Blinding of participants and personnel (performance bias) Self reported outcomes | High risk | No specific details provided. Participants and personnel unlikely to have been blinded, which may have impacted self‐reported outcomes |
| Blinding of participants and personnel (performance bias) Non‐self reported outcomes | Unclear risk | No specific details provided. Participants and personnel unlikely to have been blinded, but unlikely to have impacted outcomes that were not self‐reported |
| Blinding of outcome assessment (detection bias) Self reported outcomes | High risk | No specific details provided. Study authors stated that when participants completed the SGRQ, they “were blinded to their responses on previous visits to minimise bias”; however, participants still likely had knowledge of their group allocation |
| Blinding of outcome assessment (detection bias) Non‐self reported outcomes | Unclear risk | No specific details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. Number of participants who withdrew from intervention group during intervention period (and reasons why) reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available; however all outcomes reported, per methods |
| Other bias | Low risk | No other sources of bias could be identified |
Abbreviations: 6MWD: six‐minute walk test distance; 6MWT: six‐minute walk test; ACBT: active cycle of breathing technique; ACT: airway clearance technique; CAT: COPD Assessment Test; CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; CPET: cardiopulmonary exercise test; CRDQ: Chronic Respiratory Disease Questionnaire; CVD: cardiovascular disease; DASS: Depression Anxiety Stress Scale; ESWT: endurance shuttle walk test; EXACT‐PRO: The EXAcerbations of Chronic Pulmonary Disease Tool patient‐reported outcome; FEV₁: forced expiratory volume in one second; HADS: Hospital Anxiety and Depression Scale; HRCT: high‐resolution computerised tomography; ILD: interstitial lung disease; ISWT: incremental shuttle walk test; LCQ: Leicester Cough Questionnaire; LL: lower limb; mMRC: Modified Medical Research Council; NCFB: non‐cystic fibrosis bronchiectasis PR: pulmonary rehabilitation; RCT: randomised controlled trial; RM: repetition maximum; SGRQ: St George's Respiratory Questionnaire; SpO₂: oxyhaemoglobin saturation; UL: upper limb; VO₂ max: maximum rate of oxygen consumption.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bradley 2006 | Wrong intervention (adjunctive inspiratory muscle training not performed in usual care group) |
| Choe 1996 | Wrong participants |
| Finnerty 1999 | Wrong patient group (COPD) |
| Greening 2014 | Wrong intervention ('SPACE' self‐management and education programme not administered to usual care group) |
| Kokura 2016 | Wrong study design (letter) |
| Taylor 2019 | Wrong study design |
Abbreviation: COPD: chronic obstructive pulmonary disease.
Characteristics of ongoing studies [ordered by study ID]
Araújo 2019.
| Study name | The impact of pulmonary rehabilitation on inflammatory markers and functional capacity in patients with bronchiectasis |
| Methods | Treatment and efficient clinical trial; randomised, open, parallel, with 2 arms |
| Participants | Inclusion criteria: patients of both genders with clinical diagnosis of bronchiectasis, with moderate or severe disease according to FACED score; age between 18 and 80 years; clinically stable (without hospitalisations or infection in the last 3 months since start of the study) Exclusion criteria: patients who presented other associated respiratory chronic disease and needed treatment or urgent intervention; those with other disabling disease, severe, or with difficult control; patients who did not finish the study protocol and/or those who presented exacerbations during their period of participation in the research |
| Interventions | Experimental group: 70 individuals with bronchiectasis who will do physical exercises during 12 weeks, 3 times a week, 36 attendances in total with appropriate medication. The protocol includes muscle‐stretching, exercises of interval warming, arm and leg strengthening on treadmill or bicycle, and orientation about domiciliary use of the technique of oral oscillation of high frequency and autogenic drainage at home Control group: 70 individuals with bronchiectasis followed up in the outpatient clinic of the locale of the study during 12 weeks with appropriate medication and orientation about the benefits of practising physical exercise regularly and domiciliary use of the technique of oral oscillation of high frequency and autogenic drainage. All participants will be evaluated before and immediately after the protocol about their pulmonary function, respiratory muscle strength, physical capacity, quality of life, fatigue, chronic cough, anxiety and depression, and dyspnoea. Blood analysis of inflammatory markers will also be investigated |
| Outcomes |
Primary outcomes: Significant improvement in inflammatory markers (fibrinogen, albumin, and PCR) after 12 weeks of the intervention protocol Significant increase in functional capacity from the increase in walked distance in the 6‐minute walking test after 12 weeks of the intervention protocol Secondary outcomes: Significant decrease in anxiety and depression score punctuation using the Beck Questionnaire of Anxiety and Depression after 12 weeks of the intervention protocol Significant improvement in quality of life seen in quality of life questionnaires (SGRQ and LCQ) after 12 weeks of the intervention protocol Significant improvement in fatigue and dyspnoea on fatigue severity scale and index of modified dyspnoea (MMRC) after 12 weeks of the intervention protocol Significant improvement in respiratory muscle strength through manovacuometry and maximum strength of upper and lower limbs using 1 maximum repetition after 12 weeks of the intervention protocol |
| Starting date | 01/02/2017 |
| Contact information | Name: Amanda Souza Araújo Address: Av. Frei Cirilo, 3480 ‐ Messejana 60846‐190, Fortaleza, Brazil Telephone: +55(85)997364603 Email: amandasafisio@hotmail.com Affiliation: Hospital de Messejana, Dr. Carlos Alberto Studart Gomes |
| Notes | Information accessed 11/08/2020 from clinical trials registry: https://apps.who.int/trialsearch/Trial2.aspx?TrialID=RBR-85hvqq |
Joschtel 2014.
| Study name | A randomised clinical trial of a group exercise program for improving cardiovascular fitness, fundamental movement skills, global self esteem and quality of life in school aged children with chronic pulmonary disease. |
| Methods | Purpose: education/counselling/training; allocation: randomised controlled trial |
| Participants | Inclusion criteria: children with diagnosis of chronic pulmonary disease: non‐CF bronchiectasis, cystic fibrosis, asthma; between 8 and 12 years of age; able to follow directions and to complete the exercise programme Exclusion criteria: unstable medical condition; unstable emotional or behavioural status; inability to complete the exercise programme; recent musculoskeletal injury (e.g. sprain, fracture, muscle strain) |
| Interventions | Aim: to determine the effects of a specialised exercise programme on physical and psychosocial health in children and adolescents with chronic respiratory disease Hypothesis: exercise programme will enhance cardiovascular fitness, fundamental movement skills (FMS), self‐esteem, and quality of life Participants will complete the Motor Active exercise programme offered by the University of Queensland, School of Human Movement Studies. Motor Active is an exercise programme designed for children aged 4 to 12 years who have difficulty moving and playing in a controlled and coordinated manner. The programme offers weekly small group sessions with an assigned instructor with expert knowledge of exercise physiology, and health and physical education. Each session includes specialised activities individually tailored for each child and group interaction activities (warm‐up, movement to music, cool‐down, and relaxation). Individual activities incorporate fundamental motor skills important for child development, including balance, strength, flexibility, agility, kicking, catching, batting, passing, coordination, locomotion, body awareness, and fine motor activities The exercise programme will run for 7 weeks. Children will complete 1 session per week. Each session is 60 minutes in duration and is divided into separate parts targeting cardiovascular fitness, fundamental motor skill development, and social interaction * 3 group games (warm‐up/middle game/warm down) * Participation at different developmentally appropriate stations targeting cardiovascular fitness and motor skill development Children will work in small groups of 2 or 3 at each station with 1 instructor who has expert knowledge of Exercise Physiology, and Health and Physical Education. |
| Outcomes |
Primary outcomes: Cardiovascular fitness will be assessed by the PWC 170‐bicycle test. This is an exercise test measuring aerobic fitness by estimating VO₂. Participants will be asked to ride an exercise bike in the lab for a total 15 minutes. Intensity will be increased gradually. Pre and post results will be compared to each other [baseline/immediately after intervention (8 weeks)] Fundamental movement Skills will be assessed by the Test of Gross Motor Development 2 (TGMD 2). This is a skill battery assessing motor skills of children 3 to 10 years of age. Pre and post scores will be compared to each other [baseline/immediately after intervention (8 weeks)] Secondary outcomes: Global self‐esteem and self‐perception will be assessed by Harter’s Self‐Perception Profile for CChildren. Pre and post scores will be compared to each other [baseline/immediately after intervention (8 weeks)] Quality of life will be assessed by the PedsQL. Pre and post scores will be compared to each other [baseline/immediately after intervention (8 weeks)] |
| Starting date | 1/09/2014 |
| Contact information | Name: Barbara Joschtel Address: School of Human Movement Studies University of Queensland St Lucia QLD 4072, Australia Telephone: +61 (7) 3365 6117 Email: barbara.joschtel@uqconnect.edu.au |
| Notes | Information accessed 11/08/2020 from clinical trial registry: https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12614000920695 |
Trost 2019.
| Study name | Short and long‐term effects of therapeutic exercise in children with bronchiectasis: a multi‐centre randomised controlled trial. |
| Methods | Purpose: treatment; allocation: randomised controlled trial |
| Participants | Inclusion criteria: children aged 6 to 12 years with diagnosis of bronchiectasis (bronchiectasis confirmed on high‐resolution computed tomography (HRCT)), or if more than 5 years since HRCT, under the regular care of a respiratory paediatrician; ≥ 1 acute pulmonary exacerbation in the past 12‐months; medically able to complete an exercise programme as determined by treating physician Exclusion criteria: medically unstable as determined by treating physician; unstable emotional and/or behavioural problems; recent musculoskeletal injury (e.g. muscle strain, sprains, fractures); underlying chronic illness other than BE (e.g. asthma, cystic fibrosis (CF), neurological or cardiac disorder); knowingly unable to attend any exercise sessions or follow‐up clinical visits over 12 months (e.g. planned movement from the area); active participation in a clinical trial of another investigational drug/device or interventional therapy; other medical/psychosocial condition that investigators/treating physicians consider should be excluded from the trial to prevent potential harm/risk to the subject or that may adversely affect study outcomes |
| Interventions | A tailored therapeutic exercise programme delivered by trained exercise therapists. The programme focuses on developing and enhancing children’s confidence, motivation, functional motor skills (FMS) proficiency, and aerobic fitness through the use of developmentally appropriate, play‐based activity stations designed specifically to suit individual needs. It comprises a combination of supervised and unsupervised exercise therapy sessions. Therapists and parents will be required to follow a detailed programme manual and standard operating procedures. Sessions will be conducted at local community centres or at the Centre for Children's Health Research South Brisbane The supervised component consists of 60‐minute group sessions, completed on a weekly basis for 8 weeks, led by a clinical exercise physiologist or physiotherapist. During supervised group sessions, children rotate through at least 6 activity stations, where they complete developmentally appropriate games targeting specific FMSs. Activity stations are sequenced so that a high‐intensity game is always followed by a low‐intensity game, thus ensuring that aerobic fitness is promoted in a safe and effective manner. Activities are tailored to the child’s fitness and skill level by modifying intensity and duration of each session and/or by modifying the equipment. Up to 6 children will be included in each session Children will participate in a warm‐up followed by a circuit of different activities and games targeting functional movement skills and cardiovascular fitness. A group activity will occur at the halfway point, and the session will end with a cool‐down. Children will spend 6 minutes at each activity; 30 seconds to switch stations, 30 seconds to orientate to the game, 5 minutes to complete the activity |
| Outcomes |
Primary outcomes: Acute respiratory exacerbations of bronchiectasis assessed by parent report and medical review. An exacerbation is defined as treatment by clinic or hospital staff with antibiotics for any of the following (as recorded in the medical chart or parent report): increased cough (must be wet and of at least 3 days' duration), dyspnoea, increased sputum volume or colour intensity, new chest examination or radiographic findings, deterioration in FEV₁ percentage by more than 10%, or haemoptysis [12 months following enrolment] Secondary outcomes: Aerobic fitness: will be measured using the Modified Shuttle Test (MST). This test requires participants to move back and forth on a 10 metre course in time with a sound signal from a pre‐recorded tape. Speed for the first minute is set to 1.8 km/hr and is increased by 0.61 km/hr every minute thereafter. When participants can no longer follow the pace, the last stage completed is used to predict maximal oxygen uptake (peak VO₂) measured as per SOPs [9 weeks, 6 and 12 months post enrolment] Cost‐effectiveness of the intervention. This will focus on health resource use and costs as reported in medical records and parent report at each follow‐up time point. Staff administering the intervention will also record associated costs such as time, travel, and equipment [12 months following enrolment] Fundamental motor skills (FMSs) proficiency. Test of Gross Motor Development 3rd Edition (TGMD‐3) will be used to measure movement competency relative to 13 FMSs subdivided into 2 subscales: locomotor and object control (ball) skills as per SOPs [9 weeks, 6 and 12 months post enrolment] Health‐related quality of life measured on the Peds‐QoL questionnaire [9 weeks, 6 and 12 months following enrolment] Lung function (measured by forced expiratory volume at 1 second (FEV₁)) assessed by routine spirometry at each time point [9 weeks, 6 and 12 months following enrolment] Moderate to vigorous physical activity (MVPA) proficiency: measured using the ActiGraph GT3X+ accelerometer. Monitors will be attached to adjustable elastic belts and worn on the right hip for 7 days. Accelerometer data will be uploaded to a customized Excel macro for determination of time spent in sedentary, light, moderate, and vigorous intensity PA. Recorded activity counts will be classified into the aforementioned intensity categories using the cut‐points developed by Evenson [9 weeks, 6 and 12 months post enrolment] Safety of the intervention: staff and parents will document the number, type, and severity of adverse events that occur during and following the intervention. These will focus on exercise‐induced wheeze and injury [Weeks 1 to 9 while the intervention is taking place] |
| Starting date | 01/02/2021 |
| Contact information | Name: Prof Stewart Trost Address: Queensland University of Technology L6, Centre for Children's Health Research 62 Graham Street South Brisbane QLD 4101 Australia Phone: +61 7 3069 7301 Email: s.trost@qut.edu.au |
| Notes | Email contact with study author confirmed a parallel‐group study design and the following details of the control group: "the therapeutic exercise group will be compared to a wait‐list control group receiving usual care, which in the target patient group, is currently limited to little or no exercise therapy. Children allocated to the wait‐list control group will receive the exercise program at the conclusion of their 12‐month follow‐up period" Also confirmed the ongoing study of Joschtel 2014 is a pilot study informing this one, but distinct participants, aims, outcomes, and study endpoints noted |
Differences between protocol and review
We made minor amendments to clarify expression in our 'Risk of bias' method section, to better articulate the different approach we took to appraise studies that involved self‐reported and non‐self‐reported outcomes. Minor amendments to phrasing were made to the Background section following peer review comments, to strengthen the rationale for undertaking this review (including addition of references not originally included at protocol stage).
Contributions of authors
AL: protocol drafting, critical review, all aspects of review production.
CG: protocol drafting, critical review, all aspects of review production.
CO: protocol conception and drafting, critical review, all aspects of review production.
Contributions of editorial team
Chris Cates (Coordinating Editor) checked data entry prior to the full write‐up of the review; edited the review; advised on methods; approved the review prior to publication.
Anne Holland (Contact Editor): edited the review; advised on methods, interpretation, and content.
Emma Dennett (Managing Editor): coordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; edited references and other sections of the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
-
Monash University, Department of Physiotherapy, Australia
Salary support for AL and CO.
-
Monash Health, Department of Physiotherapy, Australia
Salary support for CG.
External sources
-
Lung Foundation Australia/Boehringer‐Ingelheim COPD Research Fellowship, Australia
Salary support for CO.
Declarations of interest
AL: none known. AL was a trialist for one of the included studies in the review ‐ Lee 2014 ‐ but was not involved in determining its eligibility for inclusion within the review, extraction of its data, or interpretation of its findings.
CG: none known.
CO: reports receiving project grants from Lung Foundation Australia/Boehringer‐Ingelheim, Pat Cosh Trust Fund, Rebecca L Cooper Medical Research Foundation, and Royal Australian College of General Practitioners. Also reports a position as Editor with Cochrane Airways. All declarations are unrelated to the present research and involve no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Chalmers 2019 {published and unpublished data}
- Chalmers JD, Crichton ML, Brady G, Finch S, Lonergan M, Fardon TC. Pulmonary rehabilitation after exacerbation of bronchiectasis: a pilot randomized controlled trial. BMC Pulmonary Medicine 2019;19(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02179983. Tayside rehabilitation in bronchiectasis exacerbations (TRIBE): a randomized controlled trial. clinicaltrials.gov/show/nct02179983 (first received 2 July 2014).
Dal Corso 2017 {published and unpublished data}
- Dal Corso S, Jose A, Holland AE, Selman JPR, Castro RAS, Camargo AA, et al. Home-based pulmonary rehabilitation in patients with bronchiectasis: a randomized controlled trial. European Respiratory Journal 2017;50(suppl 61):OA4668. [Google Scholar]
Kumar 2017 {published data only}
- Kumar R, Guleria R, Khilnani GC, Mohan A, Madan K, Hadda V, et al. The effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: a randomised controlled trial. European Respiratory Journal 2017;50(Suppl 61):OA307. [Google Scholar]
Lee 2014 {published and unpublished data}
- Lee A, Hill C, Cecins N, Jenkins S, McDonald C, Burge A, et al. Exercise training is beneficial in patients with non-cystic fibrosis bronchiectasis. A multi-centre, randomised controlled trial. European Respiratory Journal 2012;40(Suppl 56):2834. [Google Scholar]
- Lee AL, Hill CJ, Cecins N, Jenkins S, Mcdonald CF, Burge AT, et al. Exercise training is beneficial in non-CF bronchiectasis: a multi-centre, randomized controlled trial. Respirology 2012;17(Suppl 1):35. [Google Scholar]
- Lee AL, Hill CJ, Cecins N, Jenkins S, McDonald CF, Burge AT, et al. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis - a randomised controlled trial. Respiratory Research 2014;15:44. [DOI: doi: 10.1186/1465-9921-15-44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandal 2012 {published data only}
- Mandal M, Sidhu MK, Kope L, Pollock W, Stevenson LM, Pentland JL, et al. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Respiratory Medicine 2012;106:1647-54. [DOI] [PubMed] [Google Scholar]
- Mandal P, Sidhu MK, Kope L, Pollock W, Stevenson LM, Pentland JL, et al. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Respiratory Medicine 2012;106(12):1647-54. [DOI] [PubMed] [Google Scholar]
Newall 2005 {published data only}
- Newall C, Henson M, McConnell AK, Stockley RA, Hill SL. The effects of pulmonary rehabilitation (PR) in patients with bronchiectasis (BE). In: World Congress on Lung Health and 10th ERS Annual Congress; 2000 30 Aug-3 Sep, Florence. Vol. 16. 2000:330s.
- Newall C, Stockley RA, Hill SL. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax 2005;60(11):943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newall C, Stockley RA, Hill SL. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax 2005;60(11):943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bradley 2006 {published data only}
- Bradley J, Moran F. Pulmonary rehabilitation improves exercise tolerance in patients with bronchiectasis. Australian Journal of Physiotherapy 2006;52(1):65. [DOI] [PubMed] [Google Scholar]
Choe 1996 {published data only}
- Choe KH, Park YJ, Cho WK, Lim CM, Lee SD, Koh YS, et al. The effect of pulmonary rehabilitation in patients with chronic lung disease. Tuberculosis and Respiratory Diseases 1996;43(5):736-45. [Google Scholar]
Finnerty 1999 {published data only}
- Finnerty JP, Keeping IM, Bullough I, Jones J. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease: a randomised controlled trial. Chest 2001;119(6):1705-10. [DOI] [PubMed] [Google Scholar]
Greening 2014 {published data only}
- Greening NJ, Williams JEA, Hussain SF, Harvey-Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014;349:g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kokura 2016 {published data only}
- Kokura Y, Maeda K, Wakabayashi H. Pulmonary rehabilitation and oral nutritional supplement enriched with beta-hydroxy-beta-methylbutyrate for bronchiectasis participants: a prospective, randomised study. Clinical Nutrition 2016;35(3):767-8. [DOI] [PubMed] [Google Scholar]
Taylor 2019 {published data only}
- NCT03920124. Exploring the feasibility and effectiveness of minimal-equipment high-intensity interval exercise (HIIE) interventions in bronchiectasis patients. clinicaltrials.gov/show/nct03920124 (first received 18 April 2019).
References to ongoing studies
Araújo 2019 {published data only}
- RBR-85hvqq. The impact of physical exercise on inflammatory markers and functional capacity in people with chronic respiratory disease [The impact of pulmonary rehabilitation on inflammatory markers and functional capacity in patients with bronchiectasis]. www.ensaiosclinicos.gov.br/rg/RBR-85hvqq/ (first received 23 October 2018).
- WHO RBR-85hvqq. The impact of physical exercise on inflammatory markers and functional capacity in people with chronic respiratory disease [The impact of pulmonary rehabilitation on inflammatory markers and functional capacity in patients with bronchiectasis]. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-85hvqq (first received 27 December 2018).
Joschtel 2014 {published data only}
- ACTRN12614000920695. Effects of an exercise program for children with chronic pulmonary disease: a pilot study [A randomised clinical trial of a group exercise program for improving cardiovascular fitness, fundamental movement skills, global self esteem and quality of life in school aged children with chronic pulmonary disease.]. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12614000920695 (first received 28 August 2014).
Trost 2019 {published data only}
- ACTRN12619001008112. Short and long-term effects of therapeutic exercise in children with bronchiectasis: a multi-centre randomised controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619001008112 (first received 15 July 2019).
Additional references
Alison 2017
- Alison JA, McKeough Z, Johnston K, McNamara R, Spencer L, Jenkins S, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology 2017;22:800-19. [DOI] [PubMed] [Google Scholar]
Al‐Jahdali 2017
- Al Jahdali H, Alshimemeri A, Mobeireek A, Albanna AS, Al Shirawi NN, Wali S, et al. The Saudi Thoracic Society guidelines for diagnosis and management of noncystic fibrosis bronchiectasis. Annals of Thoracic Medicine 2017;12(3):135-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Araugo 2017
- Araugo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon T, et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. European Respiratory Journal 2017;50:1701289. [DOI] [PubMed] [Google Scholar]
Bradley 2002
- Bradley J, Moran F, Greenstone M. Physical training for bronchiectasis. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD002166. [DOI: 10.1002/14651858.CD002166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2015
- Bradley J, Wilson J, Hayes K, Kent L, McDonough S, Tully M, et al. Sedentary behaviour and physical activity in bronchiectasis: a cross-sectional study. BMC Pulmonary Medicine 2015;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalmers 2015
- Chalmers J, Elborn JS. Reclaiming the name 'bronchiectasis'. Thorax 2015;70(5):399-400. [DOI] [PubMed] [Google Scholar]
Chalmers 2018
- Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterisation of the 'frequent exacerbator phenotype' in bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2018;197(11):1410-20. [DOI: 10.1164/rccm.201711-2202OC] [DOI] [PubMed] [Google Scholar]
Chang 2002
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ, for the Working Group on Indigenous Paediatric Respiratory Health. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200-4. [DOI] [PubMed] [Google Scholar]
Chang 2015
- Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Medical Journal of Australia 2015;202(1):21-3. [DOI] [PubMed] [Google Scholar]
Covidence 2018 [Computer program]
- Veritas Health Innovation Covidence. Version accessed prior to 10 September 2019. Melbourne, Australia: Veritas Health Innovation, 2018. Available at www.covidence.org.
Crichton 2020
- Crichton ML, Dudgeon EK, Shoemark A, Chalmers JD. Validation of the bronchiectasis impact measure (BIM) - a novel patient report outcome measure. European Respiratory Journal 2020;2003156. [DOI: 10.1183/13993003.03156-2020] [DOI] [PubMed] [Google Scholar]
De Carmargo 2018
- De Carmargo A, Boldorini J, Holland A, Castro R, Lanza F, Athanazio R, et al. Determinants of peripheral muscle strength and activity in daily life in people with bronchiectasis. Physical Therapy 2018;98(3):153-61. [DOI: 10.1093/ptj/pzx123.] [DOI] [PubMed] [Google Scholar]
Dwyer 2017
- Dwyer T, Zainuldin R, Daviskas E, Bye PT, Alison JA. Effects of treadmill exercise versus Flutter on respiratory flow and sputum properties in adults with cystic fibrosis: a randomised, controlled, cross-over trial. BMC Pulmonary Medicine 2017;17(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eastham 2004
- Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax 2004;59(4):324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2003
- Edwards EA, Metcalf R, Milne DG, Thompson J, Byrnes CA. Retrospective review of children presenting with non cystic fibrosis bronchiectasis: HRCT features and clinical relationships. Pediatric Pulmonology 2003;36:87-93. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gale 2012
- Gale N, Bolton C, Duckers J, Enright S, Cockcroft J, Shale D. Systemic comorbidities in bronchiectasis. Chronic Respiratory Disease 2012;9(4):231-8. [DOI] [PubMed] [Google Scholar]
Giron Moreno 2013
- Giron Moreno RM, Fernandes Vasconcelos G, Cisneros C, Gomez-Punter RM, Segrelles Calvo G, Ancochea J. Presence of anxiety and depression in patients with bronchiectasis unrelated to cystic fibrosis. Archivos de Bronconeumologia 2013;49(10):415-20. [DOI] [PubMed] [Google Scholar]
Goeminne 2014
- Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respiratory Medicine 2014;108(2):287-96. [DOI] [PubMed] [Google Scholar]
Gordon 2019
- Gordon CS, Waller JW, Cook RM, Cavalera SL, Lim WT, Osadnik CR. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD: a systematic review and meta-analysis. Chest 2019;156(1):80-91. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. McMaster University (developed by Evidence Prime), Version accessed 10 August 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Herrero‐Cortina 2019
- Herrero-Cortina B, San Miguel-Pagola M, Francin-Gallego M, Saez-Perez JA, Polverino E. Long-term efficacy of a home-based airway clearance programme to improve cough severity in patients with bronchiectasis: a randomised controlled trial. European Respiratory Journal 2019;54:OA4948. [Google Scholar]
Hester 2011
- Hester K, Macfarlane J, Tedd H, Jary H, McAlinden P, Rostron L, et al. Fatigue in bronchiectasis. QJM: Monthly Journal of the Association of Physicians 2011;105(3):235-40. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hill 2019
- Hill AT, Sullivan AL, Chalmers JD, De Souza A, Elborn JS, Floto RA, et al. British Thoracic Society Guidelines for bronchiectasis in adults. Thorax 2019;74(Suppl 1):1-69. [DOI] [PubMed] [Google Scholar]
Inal‐Ince 2014
- Inal-Ince D, Sabasi S, Saglam M, Calik Kutukcu E, Vardar Yagli N, Arikan H, et al. Effects of endurance on strength, fatigue and quality of life in bronchiectasis. European Respiratory Journal 2014;44:P4278. [Google Scholar]
Jones 2005
- Jones PW. St George's Respiratory Questionnaire - MCID. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2:75-9. [DOI] [PubMed] [Google Scholar]
Jose 2017
- Jose A, Holland A, Oliveira C, Selman J, Castro R, Athanazio R, et al. Does home-based pulmonary rehabilitation improve functional capacity, peripheral muscle strength and quality of life in patients with bronchiectasis compared to standard care? Brazilian Journal of Physical Therapy 2017;21(6):473-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2003
- Kelly MG, Murphy S, Elborn JS. Bronchiectasis in secondary care: a comprehensive profile of a neglected disease. European Journal of Internal Medicine 2003;14:488-92. [DOI] [PubMed] [Google Scholar]
Kelly 2018
- Kelly C, Grundy S, Lynes D, Evans DJW, Gudur S, Milan SJ, et al. Self-management for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012528. [DOI: 10.1002/14651858.CD012528.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2005
- King P, Holdsworth S, Freezer N, Villanueva E, Gallagher M, Holmes P. Outcome in adult bronchiectasis. Chronic Obstructive Pulmonary Disease 2005;2(1):27-34. [DOI] [PubMed] [Google Scholar]
King 2006
- King P, Holdsworth S, Freezer N, Holmes P. Bronchiectasis. Internal Medicine Journal 2006;36(11):729-37. [DOI] [PubMed] [Google Scholar]
Koulouris 2003
- Koulouris N, Retsou S, Kosmas E, Dimakou K, Malagari K, Mantzikopoulos G, et al. Tidal expiratory flow limitation, dyspnoea and exercise capacity in patients with bilateral bronchiectasis. European Respiratory Journal 2003;21(5):743-8. [DOI] [PubMed] [Google Scholar]
Lacasse 2015
- Lacasse Y, Cates CJ, McCarthy B, Welsh EJ. This Cochrane Review is closed: deciding what constitutes enough research and where next for pulmonary rehabilitation in COPD. Cochrane Database of Systematic Reviews 2015;11. [DOI: 10.1002/14651858.ED000107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2008
- Lee AL, Button BM, Denehy L. Current Australian and New Zealand physiotherapy practice in the management of patients with bronchiectasis and COPD. New Zealand Journal of Physiotherapy 2008;36(2):49-58. [Google Scholar]
Lee 2014a
- Lee AL, Hill CJ, Cecins N, Jenkins SJ, McDonald CF, Burge AT, et al. Minimal important difference for field walking tests in non-cystic fibrosis bronchiectasis following exercise training. Respiratory Medicine 2014;108:1303-9. [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD008351. [DOI: 10.1002/14651858.CD008351.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017
- Lee AL, Hill CJ, McDonald CF, Holland AE. Pulmonary rehabilitation in non-cystic fibrosis bronchiectasis: a systematic review. Archives of Physical Medicine and Rehabilitation 2017;98(4):774-82. [DOI] [PubMed] [Google Scholar]
Li 2005
- Li AM, Sonnappa S, Lex C, Wong E, Zacharasiewicz A, Bush A, et al. Non-CF bronchiectasis: does knowing the aetiology lead to changes in management? European Respiratory Journal 2005;26(1):8. [DOI] [PubMed] [Google Scholar]
Liaw 2011
- Liaw MY, Tsai YC, Huang KT, Chang PW, Chen YC, Lin MC. Inspiratory muscle training in bronchiectasis patients: a prospective randomised controlled study. Clinical Rehabilitation 2011;25(6):524-36. [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Garcia 2005
- Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, Soler-Cataluña JJ. Quality-of-life determinants in patients with clinically stable bronchiectasis. Chest 2005;128:739-45. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2007
- Martinez-Garcia MA, Perpina-Tordera M, Soler-Cataluna JJ, Roman-Sanchez P, Lloris-Bayo A, Gonzalez-Molina A. Dissociation of lung function, dyspnoea ratings and pulmonary extension in bronchiectasis. Respiratory Medicine 2007;101(11):2248-53. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2018
- Martinez-Garcia MA, Maiz L, Olveira C, Giron RM, la Rosa D, Blanco M, et al. Spanish guidelines on treatment of bronchiectasis in adults. Archivos de Bronconeumologia 2018;54(2):88-98. [DOI] [PubMed] [Google Scholar]
Martin‐Valero 2020
- Martín-Valero R, Jimenez-Cebrian AM, Moral-Munoz JA, de-la-Casa-Almeida M, RodriguezHuguet M, Casuso-Holgado MJ. The efficacy of therapeutic respiratory muscle training interventions in people with bronchiectasis: a systematic review and meta-analysis. Journal of Clinical Medicine 2020;9(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [CD003793] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the Efficiencies of Machine Learning and Cochrane Crowd to Identify Randomised Trials for Individual Cochrane Reviews. Cape Town, South Africa: Global Evidence Summit, 2017.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 2010
- Moran F, Piper A, Elborn JS, Bradley J. Respiratory muscle pressures in non-CF bronchiectasis: repeatability and reliability. Chronic Respiratory Disease 2010;7:165-71. [DOI] [PubMed] [Google Scholar]
Munoz 2018
- Munoz G, Gracia J, Buxo M, Alvarez A, Vendrell M. Long-term benefits of airway clearance in bronchiectasis: a randomised placebo-controlled trial. European Respiratory Journal 2018;51(1):1701926. [DOI] [PubMed] [Google Scholar]
Murray 2009
- Murray MP, Pentland JL, Hill AT. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. European Respiratory Journal 2009;34:1086-92. [DOI] [PubMed] [Google Scholar]
Nici 2006
- Nici L, Donner C, Wouters E, ZuWallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2006;173(12):1390-413. [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2018
- Noel-Storr AH, Project Transform Team. Cochrane Crowd: New Ways of Working Together to Produce Health Evidence. Oxford, England: Evidence Live, 2018.
O'Leary 2002
- O'Leary CJ, Wilson CB, Hansell DM, Cole PJ, Wilson R, Jones PW. Relationship between psychological well-being and lung health status in patients with bronchiectasis. Respiratory Medicine 2002;96(9):686-92. [DOI] [PubMed] [Google Scholar]
O'Neill 2002
- O'Neill B, McArdle N, MacMahon J. The current physiotherapy management of patients with bronchiectasis: a UK survey. International Journal of Clinical Practice 2002;56(1):34-5. [PubMed] [Google Scholar]
Olveira 2013
- Olveira C, Gaspar I, Dorado A, Cruz I, Sorignuer F, Quittner AL, et al. Depression and anxiety symptoms in bronchiectasis: association with health-related quality of life. Quality of Life Research 2013;22:597-605. [DOI] [PubMed] [Google Scholar]
Ozalp 2012
- Ozalp O, Inal-Ince D, Calik E, Vardar-Yagli N, Saglam M, Savci S, et al. Extrapulmonary features of bronchiectasis: muscle function, exercise capacity, fatigue, and health status. Multidisciplinary Respiratory Medicine 2012;7:3. [DOI: 10.1186/2049-6958-7-3.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65(Suppl 1):1-58. [DOI] [PubMed] [Google Scholar]
Patel 2019
- Patel S, Cole AD, Nolan CM, Barker RE, Jones SE, Kon S, et al. Pulmonary rehabilitation in bronchiectasis: a propensity-matched study. European Respiratory Journal 2019;53:1801264. [DOI] [PubMed] [Google Scholar]
Pereira 2019
- Pereira MC, Athanazio RA, Dalcin P, Figueriedo MRF, Gomes M, Guimaraes de Freitas C, et al. Brazilian consensus on non-cystic fibrosis bronchiectasis. Jornal Brasileiro de Pneumologia 2019;45(4):e20190122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pifferi 2010
- Pifferi M, Bush A, Di Cicco M, Pradal U, Ragazzo V, Macchia P, et al. Health-related quality of life and unmet needs in patients with primary ciliary dyskinesia. European Respiratory Journal 2010;35(4):787-94. [DOI] [PubMed] [Google Scholar]
Polverino 2017
- Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Leobinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. European Respiratory Journal 2017;50(3):1-23. [DOI] [PubMed] [Google Scholar]
Polverino 2018
- Polverino E, Blasi F, Ringshausen F, De Soyza A, Vendrell M, Goeminne P, et al. Determinants of quality of life in bronchiectasis using the Quality Of Life Bronchiectasis (QOL-B) questionnaire: data from the EMBARC registry. European Respiratory Journal 2018;52(Suppl 62):OA4951. [DOI: 10.1183/13993003.congress-2018.OA4951] [DOI] [Google Scholar]
Puhan 2016
- Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005305. [DOI: 10.1002/14651858.CD005305.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quint 2016
- Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. European Respiratory Journal 2016;47(1):186-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quittner 2015
- Quittner AL, O'Donnell A, Salathe MA, Lewis SA, Li X, Montgomery AB, et al. Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax 2015;70:12-20. [DOI] [PubMed] [Google Scholar]
Radovanovic 2018
- Radovanovic D, Santus P, Blasi F, Sotgiu G, D'Arcangelo F, Simonetta E, et al. A comprehensive approach to lung function in bronchiectasis. Respiratory Medicine 2018;145:120-9. [DOI] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevManWeb). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Ringshausen 2013
- Ringshausen FC, Roux A, Pletz MW, Hamalainen N, Welte T, Rademacher J. Bronchiectasis-associated hospitalisations in Germany, 2005-2011: a population-based study of disease burden and trends. PloS One 2013;8(8):e71109. [DOI: 10.1371/journal.pone.0071109] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringshausen 2015
- Ringshausen FC, Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population-based estimation of disease prevalence. European Respiratory Journal 2015;46:1805-7. [DOI] [PubMed] [Google Scholar]
Rochester 2015
- Rochester C, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. An official American Thoracic Society/European Respiratory Society Policy statement: enhancing implementation, use and delivery of pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2015;192(11):1373-86. [DOI] [PubMed] [Google Scholar]
Schunemann 2019
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14, Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In:Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Seitz 2010
- Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burdens of bronchiectasis-associated hospitalisations in the United States, 1993-2006. Chest 2010;138(4):944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seitz 2012
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest 2012;142(2):432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2008
- Singh SJ, Jones PW, Evans R, Morgan MDL. Minimum clinically important improvement for the incremental shuttle walk test. Thorax 2008;63:75-7. [DOI] [PubMed] [Google Scholar]
Singh 2014
- Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. European Respiratory Journal 2014;44(6):1447-78. [DOI] [PubMed] [Google Scholar]
Snaith 2003
- Snaith R. The Hospital Anxiety and Depression Scale. Health and Quality of Life Outcomes 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinou 2017
- Spinou A, Siegert R, Guan W, Patel AS, Gosker HR, Lee KK, et al. The development and validation of the Bronchiectasis Health Questionnaire. European Respiratory Journal 2017;49:1601532. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188:13-64. [DOI] [PubMed] [Google Scholar]
Swaminathan 2003
- Swaminathan S, Kuppurao KV, Somu N, Vijayan VK. Reduced exercise capacity in non-cystic fibrosis bronchiectasis. Indian Journal of Pediatrics 2003;70(7):553-6. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr A, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Twiss 2005
- Twiss J, Metcalfe R, Edwards E, Byrnes C. New Zealand national incidence of bronchiectasis "too high" for a developed country. Archives of Disease in Childhood 2005;90(7):737-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2020
- Walsh JA, Patel S, Barker RE, Jones SE, Wynne SE, Kon SCC, et al. The minimum clinically important difference of the incremental shuttle walk test in bronchiectasis: a prospective cohort study. Annals of American Thoracic Society 2020;17(3):375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2019
- Ward N, Stiller K, Holland AE. Exercise is commonly used as a substitute for traditional airway clearance techniques by adults with cystic fibrosis in Australia: a survey. Journal of Physiotherapy 2019;65(1):43-50. [DOI] [PubMed] [Google Scholar]
Weycker 2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of non-cystic fibrosis bronchiectasis among US adults in 2013. Chronic Respiratory Disease 2017;14(4):377-84. [DOI] [PMC free article] [PubMed] [Google Scholar]