Abstract
Background
Urgent‐start peritoneal dialysis (PD), defined as initiation of PD within two weeks of catheter insertion, has been emerging as an alternative mode of dialysis initiation for patients with chronic kidney disease (CKD) requiring urgent dialysis without established permanent dialysis access. Recently, several small studies have reported comparable patient outcomes between urgent‐start and conventional‐start PD.
Objectives
To examine the benefits and harms of urgent‐start PD compared with conventional‐start PD in adults and children with CKD requiring long‐term kidney replacement therapy.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 May 2020 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov.
For non‐randomised controlled trials, MEDLINE (OVID) (1946 to 27 June 2019), EMBASE (OVID) (1980 to 27 June 2019), Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov (up to 27 June 2019) were searched.
Selection criteria
All randomised controlled trials (RCTs) and non‐RCTs comparing the outcomes of urgent‐start PD (within 2 weeks of catheter insertion) and conventional‐start PD ( ≥ 2 weeks of catheter insertion) treatment in children and adults CKD patients requiring long‐term dialysis were included. Studies without a control group were excluded.
Data collection and analysis
Data were extracted and quality of studies were examined by two independent authors. The authors contacted investigators for additional information. Summary estimates of effect were examined using random‐effects model and results were presented as risk ratios (RR) with 95% confidence intervals (CI) as appropriate for the data. The certainty of evidence for individual outcome was assessed using the GRADE approach.
Main results
A total of 16 studies (2953 participants) were included in this review, which included one multicentre RCT (122 participants) and 15 non‐RCTs (2831 participants): 13 cohort studies (2671 participants) and 2 case‐control studies (160 participants). The review included unadjusted data for analyses due to paucity of studies reporting adjusted data.
In low certainty evidence, urgent‐start PD may increase dialysate leak (1 RCT, 122 participants: RR 3.90, 95% CI 1.56 to 9.78) compared with conventional‐start PD which translated into an absolute number of 210 more leaks per 1000 (95% CI 40 to 635).
In very low certainty evidence, it is uncertain whether urgent‐start PD increases catheter blockage (4 cohort studies, 1214 participants: RR 1.33, 95% CI 0.40 to 4.43; 2 case‐control studies, 160 participants: RR 1.89, 95% CI 0.58 to 6.13), catheter malposition (6 cohort studies, 1353 participants: RR 1.63, 95% CI 0.80 to 3.32; 1 case‐control study, 104 participants: RR 3.00, 95% CI 0.64 to 13.96), and PD dialysate flow problems (3 cohort studies, 937 participants: RR 1.44, 95% CI 0.34 to 6.14) compared to conventional‐start PD.
In very low certainty evidence, it is uncertain whether urgent‐start PD increases exit‐site infection (2 cohort studies, 337 participants: RR 1.43, 95% CI 0.24 to 8.61; 1 case‐control study, 104 participants RR 1.20, 95% CI 0.41 to 3.50), exit‐site bleeding (1 RCT, 122 participants: RR 0.70, 95% CI 0.03 to 16.81; 1 cohort study, 27 participants: RR 1.58, 95% CI 0.07 to 35.32), peritonitis (7 cohort studies, 1497 participants: RR 1.00, 95% CI 0.68 to 1.46; 2 case‐control studies, 160 participants: RR 1.09, 95% CI 0.12 to 9.51), catheter readjustment (2 cohort studies, 739 participants: RR 1.27, 95% CI 0.40 to 4.02), or reduces technique survival (1 RCT, 122 participants: RR 1.09, 95% CI 1.00 to 1.20; 8 cohort studies, 1668 participants: RR 0.90, 95% CI 0.76 to 1.07; 2 case‐control studies, 160 participants: RR 0.92, 95% CI 0.79 to 1.06).
In very low certainty evidence, it is uncertain whether urgent‐start PD compared with conventional‐start PD increased death (any cause) (1 RCT, 122 participants: RR 1.49, 95% CI 0.87 to 2.53; 7 cohort studies, 1509 participants: RR 1.89, 95% CI 1.07 to 3.3; 1 case‐control study, 104 participants: RR 0.90, 95% CI 0.27 to 3.02; very low certainty evidence). None of the included studies reported on tunnel tract infection.
Authors' conclusions
In patients with CKD who require dialysis urgently without ready‐to‐use dialysis access in place, urgent‐start PD may increase the risk of dialysate leak and has uncertain effects on catheter blockage, malposition or readjustment, PD dialysate flow problems, infectious complications, exit‐site bleeding, technique survival, and patient survival compared with conventional‐start PD.
Plain language summary
Is urgent‐start peritoneal dialysis safe for patients with chronic kidney disease?
What is the issue?
Peritoneal dialysis is a form of kidney replacement therapy in which the lining of the abdomen is used as a filter for dialysis. The dialysis fluid is introduced via a tube which is placed into the abdomen, called a peritoneal dialysis catheter. Traditionally, dialysis is delayed for two weeks after catheter placement, in order to allow proper wound healing. However, some studies reported that patients with chronic kidney disease who urgently need to start dialysis within two weeks of catheter insertion (urgent‐ start peritoneal dialysis) experienced comparable outcomes to others who commenced dialysis more than two weeks after catheter insertion (conventional‐start peritoneal dialysis).
What did we do?
We conducted a systematic review to examine the complications and outcomes of patients with chronic kidney disease who started peritoneal dialysis urgently within two weeks of insertion of peritoneal dialysis catheter.
What did we find?
We identified 16 studies (2953 participants) examining the outcomes of urgent versus conventional start peritoneal dialysis. When we compared results from patients who initiated dialysis two weeks after catheter insertion, patients who initiated dialysis urgently were more likely to have leakage of dialysis fluid outside the abdominal cavity into the skin near the exit site of peritoneal dialysis catheter. The differences in infection of the lining of the abdomen (peritonitis), infection at the exit point of the peritoneal dialysis catheter (exit‐site infection), mechanical complications of peritoneal dialysis (including catheter blockage, catheter malposition and catheter readjustment), patients remaining on peritoneal dialysis (technique survival), and death between patients who started dialysis urgently and those who waited for two weeks after catheter insertion remain unclear.
Conclusions
In patients with chronic kidney disease who require dialysis urgently without ready‐to‐use dialysis access in place, peritoneal dialysis may increase dialysate leak. However, the overall risks of infectious and other non‐infectious complications between urgent‐start peritoneal dialysis and conventional‐start peritoneal dialysis remains unclear.
Summary of findings
Background
Description of the condition
People with chronic kidney disease (CKD) requiring long‐term kidney replacement therapy (KRT) is a common and growing problem affecting over two million people worldwide (AIHW 2016; Couser 2011; Gilg 2016). In the USA, CKD consumes 6.7% of total Medicare budget to care for less than 1% of the covered population (ISN 2015). Increasing utilization of home‐based dialysis, such as peritoneal dialysis (PD), can lead to an annual cost saving of up to 40% compared to facility haemodialysis (HD) (KHA 2012; KHA 2016).
PD is a type of dialysis that uses the peritoneum in a person’s abdomen as the membrane through which fluid and dissolved substances are exchanged with the blood. Even though PD is a relatively simple technique to master and has been shown to improve many patient‐level clinical outcomes (e.g. an initial survival advantage compared to HD, better preservation of residual kidney function, superior patient‐level satisfaction, and preservation of vascular access for future use (Mehrotra 2016; Tokgoz 2009), only approximately 11% of the global dialysis population are currently receiving PD as their dialysis modality (Jain 2012; Li 2017). One of the main impediments to growth in PD uptake has been a clinician reluctance to utilize PD as the preferred dialysis modality of choice when there is no established functional dialysis access in place (e.g. PD catheter or mature arteriovenous fistula/graft). This has been driven by traditional practice to delay commencement of PD by at least two weeks from the time of PD catheter insertion to prevent complications, as recommended by the International Society for Peritoneal Dialysis (ISPD) and European Renal Best Practice (ERBP) guidelines (Dombros 2005; Figueiredo 2010). These recommendations are based on a weak level of evidence (Dombros 2005; Figueiredo 2010), which has variably shown that early use of a PD catheter shortly after its insertion is associated with increased risks of early complications, such as dialysate leaks, infection and catheter dysfunction (See 2017; Yang 2011). However, there are several advantages of urgent‐start PD, for example, avoiding temporary vascular catheters and their attendant risks (including bloodstream infections), avoiding initiation of a dialysis modality that is contrary to patient choice, and possibly avoiding the risk of patients remaining on in‐centre HD rather than home dialysis. These would have to be balanced against the apparent increased risk of dialysate leaks versus elective PD start. The effect of urgent‐start PD, defined as initiation of PD to treat patients who require dialysis imminently without established dialysis access, on long‐term patient outcomes remains uncertain.
Unfortunately, the need to commence dialysis without mature permanent dialysis access in situ is a relatively common phenomena, affecting approximately 20% of patients who present ‘late’ to nephrology service whereby patients need to commence dialysis within three months of being first reviewed by nephrologist (Foote 2014). The practice of urgent‐start PD has been increasingly adopted across both developed (Arramreddy 2014; See 2017) and developing countries (Bitencourt Dias 2017). However, at present, there is no universally agreed definition regarding the duration between PD catheter insertion and commencement that qualifies as urgent‐start PD. Moreover, whether there exists a 'necessary' wait period to minimise the risk of complications within this clinical context is unknown. The ISPD recommends to use PD catheters at least two weeks after their insertion (Figueiredo 2010). Moreover, recently published randomised controlled trial (RCT) demonstrated an increased risk of leaks in patients who started PD at one week compared to those starting at two weeks or later (Timely PD 2010). The variation in duration between PD catheter insertion and commencement, fill volume, methods of catheter insertion across the studies involving urgent‐start PD might potentially influence the observed outcomes. Therefore, it is important to conduct a detailed examination of the effect of urgent‐start PD on both short‐ and long‐term patient‐level outcomes compared to conventional‐start PD treatment regimens including subgroup analyses (e.g. duration between PD catheter placement and commencement, fill volume and insertion technique) to examine their relationship.
Description of the intervention
Unlike conventional‐start PD where initiation of PD occurs > two weeks after PD catheter placement, urgent‐start PD takes an approach to initiate PD within two weeks of PD catheter insertion.
How the intervention might work
Recommendation to delay the initiation of PD till two weeks after PD catheter insertion in conventional‐start PD is to minimize the early complication of PD including leak (Yang 2011). Urgent‐start PD is initiated with low fill volumes in the supine position using a cycler to reduce the intra‐abdominal pressure in order to minimize the risk of pericatheter leak. Treatment can be delivered in both inpatient and outpatient settings.
Why it is important to do this review
Although urgent‐start PD has been received as a conceptually positive initiative with reassuring early outcomes, the vast majority of evidence has been generated from single‐centre observational studies with relatively small patient numbers (Casaretto 2012; Ghaffari 2012; Jo 2007; Koch 2012; Lobbedez 2008), which has resulted in ad hoc implementation rather than a ‘standard’ care across the world. The objective of this review is to conduct a comprehensive examination of the literature to examine all possible outcomes from urgent‐start PD compared to those of conventional‐start PD treatments.
Objectives
This review aims to look at the benefits and harms of urgent‐start PD compared with conventional‐start PD in adults and children with CKD requiring long‐term KRT.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and non‐RCTs comparing urgent‐start PD to conventional‐start PD treatments.
Types of participants
Inclusion criteria
Participants included in this review were both adults and children with CKD who required dialysis treatment. Participants had a PD catheter inserted, which could be their first PD catheter or any subsequent catheter.
Exclusion criteria
The review did not include data obtained from patients with acute kidney injury as recovery of kidney function may potentially introduce the risk of detection bias (i.e. those with rapid recovery may not remain on PD for 30 or 90 days to capture clinical events of interest).
Types of interventions
Studies comparing two different PD therapy commencement types were included in this review. They could be broadly divided into urgent‐start PD and conventional‐start PD groups.
Intervention: patients commenced on urgent‐start PD, defined as initiation of PD therapy within two weeks of catheter placement.
Comparator: patients commenced on conventional‐start PD, defined as initiation of PD therapy at or after two weeks of catheter placement.
Types of outcome measures
Primary outcomes
-
Mechanical complications occurring within 30 days (early complication) and 90 days (late complication) of commencement of PD (proportion of patients for each relevant outcome listed below).
Dialysate leak
Catheter blockage
Catheter malposition
PD dialysate flow problem.
-
Exit‐site complications occurring within 30 days (early complication) and 90 days (late complication) of commencement of PD.
Exit‐site infection (proportion of patients with exit‐site infection and episodes of exit‐site infections per patient‐year)
Tunnel tract infection (proportion of patients with tunnel tract infection and episodes of tunnel tract infections per patient‐year)
Exit‐site bleeding (proportion of patients developing exit‐site bleeding).
Technique survival (number of patients remaining on PD at study completion).
Secondary outcomes
Peritonitis occurring within 30 days (early complication) and 90 days (late complication) of commencement of PD (proportion of patients developing peritonitis and episodes of peritonitis per patient‐year)
Catheter re‐adjustment within 30 days (early complication) and 90 days (late complication) of commencement of PD (proportion of patients requiring intervention for catheter malfunction
Catheter survival
Interim HD (number of patients requiring temporary HD after PD commencement)
Hospitalisation (average days spent in hospital or number of hospitalisation episodes)
PD training duration (number of days from PD commencement to PD at home)
Death (all causes)
Adverse effects (including pain/discomfort)
Quality of life (QoL)
Cost of dialysis
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 May 2020 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of hand searched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
For non‐randomised controlled trials, MEDLINE (OVID) (1946 ‐ 27 June 2019), EMBASE (OVID) (1980 ‐ 27 June 2019), International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov were searched.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts will be screened independently by two authors, who will discard studies that are not applicable; however, studies and reviews that might include relevant data or information on studies will be retained initially. Two authors will independently assess retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study exists, reports were grouped together and the publication with the most complete data were used in the analyses. Where relevant outcomes are only published in earlier versions, these data were used.
Assessment of risk of bias in included studies
Randomised controlled trials
The following items were independently assessed by two authors using the risk of bias assessment tool for RCTs (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Non‐randomised controlled trials
The Newcastle‐Ottawa Scale (www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf) for assessing the quality of non‐randomised studies was used.
-
For case control studies the following items were evaluated.
Selection (adequacy of definition, representativeness of the cases, selection of controls, definition of controls)
Comparability (comparability of cases and controls on the basis of the design or analysis)
Exposure (ascertainment of exposure, same method of ascertainment for cases and controls, non‐response rate).
-
For cohort studies the following items were evaluated.
Selection (representativeness of the exposed cohort, selection of the non‐exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at start of study)
Comparability (comparability of cohorts on the basis of the design or analysis)
Outcome (assessment of outcome, adequacy of follow‐up and duration of follow‐up).
Measures of treatment effect
For dichotomous outcomes (e.g. death, mechanical complications within one month of commencement of PD) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. duration of hospitalisation, duration of PD training), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used. SMD was re‐expressed using a familiar instrument, by applying the calculated SMD back into one of the original studies and depicted on the scale used in that study. Studies may report different risk measures such as hazards ratios, odds ratio (OR) or relative risk. We analysed the studies by the type of measure reported whenever possible. However, in the present review, the event rate was very small for case‐control studies which led to similar values obtained for odds ratio and risk ratio were similar. Hence, we used risk ratio instead of the OR for case‐control studies. The results of studies with the same risk measure were combined using the generic inverse‐variance method and a random effect model.
Unit of analysis issues
The present review included only one RCT, which adopted parallel design. There was no issue with unit of analysis.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author/s) and any relevant information obtained in this manner were included in the review. Evaluation of important numerical data, such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population, was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals, were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
The heterogeneity was first assessed by visual inspection of the forest plot. We quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was based on the following:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Studies with different designs, RCT and non‐RCT or non‐RCT with dissimilar study design were analysed separately. For dichotomous outcomes, a random effects model was performed to measure treatment effects. In sensitivity analyses, adjusted effect estimates (whenever possible) and their standard errors were used for combining studies in meta‐analyses and the generic inverse‐variance method was used .
Subgroup analysis and investigation of heterogeneity
Subgroup analysis were planned to explore possible sources of heterogeneity (e.g. participants, interventions and study quality including method of PD catheter insertion). Heterogeneity among participants could be related to age and renal pathology (e.g. children versus adults). Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose, and duration of therapy (e.g. initial fill volume). Therefore, subgroup analyses were conducted to evaluate the source of heterogeneity.
-
Participants
Adults versus children
Incident versus prevalent patients
-
Setting
Single‐centre versus multi‐centre
-
Type of treatment utilised
According to initial fill volume
Days to PD commencement (e.g. within 24 hours versus 7 days).
Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. Where possible, the risk difference with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
'Summary of findings' tables
The main results of the review were presented in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We plan to present the following outcomes in the 'Summary of findings' tables.
Mechanical complications: dialysate leak, catheter blockage, catheter malposition, PD dialysate flow problems
Exit‐site complications: exit‐site infection, exit‐site bleeding, tunnel tract infection
Peritonitis
Catheter re‐adjustment within a month of commencement of PD
Technique survival
Interim HD
Duration of hospitalisation.
Results
Description of studies
Results of the search
An electronic search (last search date for RCTs: 25 May 2020; non‐RCTs: 26 June 2019) identified total 219 potentially relevant reports. After removing duplicates and screening through 210 titles and abstracts, 160 reports were excluded. Full text review was conducted of the remaining 56 records (53 studies); 16 studies (19 records) were included, 36 studies (36 records) were excluded, and one study is awaiting classification and will be assessed in a future update of this review (Figure 1).
1.
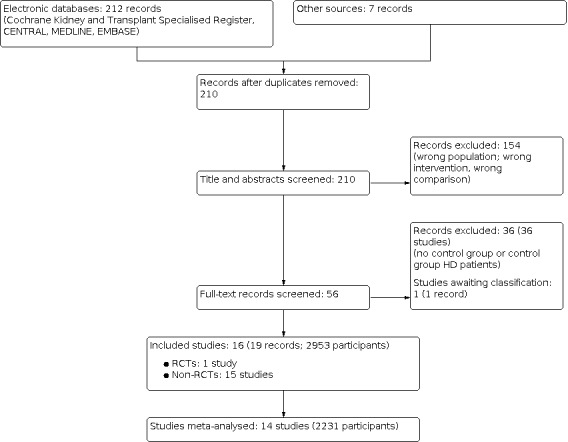
Study flow diagram.
Included studies
Sixteen studies (2953 participants) (Ghaffari 2012; Jaivid 2017; Kim 2018; Liu 2014; Nayak 2018; Pai 2016; Povlsen 2016; Salari 2018; See 2017; Serrano 2019; Silva 2018; Timely PD 2010; Vlasak 2017; Wojtaszek 2018; Yang 2011; Zhang 2017) were included in this review (see Table 4).
1. Description of studies included in the review.
| Study | Country | Study design | Time frame | No. participants (DM) | Follow‐up duration | Break‐in periods for USPD | Break‐in periods for CSPD | Insertion methods | Initial PD regimen |
| Ghaffari 2012 | USA | Prospective cohort (SC) | 2010‐2011 | 27 (52%) | 90 days | Not reported | Not reported | Percutaneous (laparoscopic for control) |
Based on BSA & GFR* |
| Jaivid 2017 | Singapore | Retrospective cohort (SC) | 2015 | 50 (58%) | 180 days | 1 to 10 days | 2 to 4 weeks | Percutaneous | Not available |
| Kim 2018 | Korea | Retrospective cohort (SC) | 2007‐2014 | 87 (40%) | 6 months | 0.4 to 5.9 days | 20 days | Laparotomy | Day 2: 0.5 L Day 5: 0.75 to 1 L |
| Liu 2014 | China | Retrospective cohort (SC) | 2001‐2010 | 657 (26%) | 6 months | ≤ 7 days | > 14 days | Laparotomy | 0.75 to 1.2 L |
| Nayak 20181 | India | Case control (SC) | 2016‐2017 | 56 (not reported) | 90 days | Within 48 hours of presentation | > 14 days | Not reported | Not reported |
| Pai 2016 | Taiwan, China | Retrospective cohort (SC) | 2006‐2012 | 149 (43%) | 30.5 ± 24.9 months | 11 (6 to 13) days | 20.7 (14 to 76) days | Laparotomy | Not reported |
| Povlsen 2016 | Denmark | Prospective cohort (multicentre) | 2005‐2009 | 643 (not reported) | Follow‐up till 2012 | Not reported | Not reported | Not reported | Not reported |
| Salari 2018 | USA | Retrospective cohort (SC) | 2010‐2017 | 159 (57%) | USPD: 986 ± 634 days CSPD: 1010 ± 732 days | Not reported | Not reported | Not reported | Not reported |
| See 2017 | Australia | Case control (SC) | 2010‐2015 | 104 (35%) | 4 weeks | 4 (1 to 7) days | Not reported | Laparoscopic | 1 to 1.2 L |
| Serrano 2019 | USA | Retrospective cohort (SC) | Not reported | Not reported | 6 months | 7.3 days | Not reported | Not reported | Not reported |
| Silva 2018 | Brazil | Prospective cohort (SC) | 2010‐2018 | Not reported | 381 days | 3 to 14 days | Not reported | Percutaneously inserted | Week 1: 1 L Titrate week 4: 2 L |
| Timely PD 2010 | Australia | RCT (multicentre) | 2008‐2013 | 122 (35%) | 180 days | 7 days | ≥ 14 days | Laparotomy | Day 1: 1 L Day 2: 1.5 L Day 3: 2 L |
| Vlasak 2017 | Czech Republic | Retrospective cohort (SC) | 2011 | 89 (not reported) | 4 weeks | Not reported | Not reported | Laparoscopic | Not reported |
| Wojtaszek 2018 | Poland | Retrospective cohort (SC) | 2005‐2015 | Not reported | USPD: 19 months CSPD: 19.5 months |
3.5 ± 2.3 days | 16.2 ± 1.7 days | Not reported | Not reported |
| Yang 2011 | Taiwan, China | Retrospective cohort (SC) | 2003‐2007 | Not reported | USPD: 823 ± 591 days CSPD: 522 ± 319 days |
2.0 ± 2.7 days | 40.6 ± 42.8 days | Laparotomy | Day 1: 0.5 L Day 6: 0.75 L Day 8: 1 L |
| Zhang 2017 | China | Retrospective cohort (SC) | 2014‐2016 | Not reported | 90 days | 1 to 3 days | ≥ 14 days | Not reported | Not reported |
BSA ‐ body surface area; CSPD ‐ conventional‐start peritoneal dialysis; DM ‐ diabetes mellitus; GFR ‐ glomerular filtration rate; RCT ‐ randomised controlled trial; SC ‐ single centre study; USPD ‐ urgent‐start peritoneal dialysis;
* For GFR > 7 (BSA < 1.65 m2: 500 mL, 4 cycles, BAS 1.65 to 1.8 m2: 750 mL, 5 cycles, BSA > 1.8 m2: 1000 mL, 6 cycles) , for GFR < 7 (BSA < 1.65 m2: 500 mL, 6 cycles, BSA: 1.65 to 1.8 m2: 750 mL, 6 cycles, BSA: > 1.8 m2: 1250 mL, 6 cycles)
1 treatment group is emergent‐start PD
One multicentre RCT (Timely PD 2010) (122 participants) and fifteen non‐RCTs (13 cohort studies: Ghaffari 2012; Jaivid 2017; Kim 2018; Liu 2014; Pai 2016; Povlsen 2016; Salari 2018; Serrano 2019; Silva 2018; Vlasak 2017; Wojtaszek 2018; Yang 2011; Zhang 2017 (2671 participants); 2 case‐control studies: Nayak 2018; See 2017 (160 participants)) were included. Of these, four studies were conducted in China (Liu 2014; Pai 2016; Yang 2011; Zhang 2017), two in Australia (Timely PD 2010; See 2017), one in Brazil (Silva 2018), three in the USA (Ghaffari 2012; Salari 2018; Serrano 2019), one in the Czech Republic (Vlasak 2017), one in Denmark (Povlsen 2016), one in India (Nayak 2018), one in Korea (Kim 2018), one in Poland (Wojtaszek 2018), and one in Singapore (Jaivid 2017). Study were conducted between 2001 to 2018. Catheter insertion techniques also varied across studies: three studies used the percutaneous approach (Ghaffari 2012; Jaivid 2017; Silva 2018), five studies used laparotomy (Liu 2014; Kim 2018; Pai 2016; Timely PD 2010; Yang 2011), two studies used laparoscopic insertion methods (See 2017; Vlasak 2017), and two studies (Serrano 2019; Wojtaszek 2018) did not report the insertion technique (see Table 4).
Ten studies (1604 participants) examined dialysate leak (Ghaffari 2012; Jaivid 2017; Kim 2018; Liu 2014; Nayak 2018; See 2017; Serrano 2019; Timely PD 2010; Vlasak 2017; Yang 2011)
Seven studies (1457 participants) examined catheter malposition (Jaivid 2017; Kim 2018; Liu 2014; See 2017; Vlasak 2017; Yang 2011; Zhang 2017)
Six studies (1374 participants) examined catheter blockage (Kim 2018; Liu 2014; Nayak 2018; See 2017; Yang 2011; Zhang 2017)
Three studies (937 participants) examined PD dialysate flow problem (Liu 2014; Serrano 2019; Yang 2011)
Two studies (739 participants) examined catheter readjustment (Kim 2018; Liu 2014)
Two studies (149 participants) examined exit‐site bleeding (Ghaffari 2012; Timely PD 2010)
Three studies (441 participants) examined the incidence of exit‐site infection (Ghaffari 2012; See 2017; Yang 2011)
Eight studies (1492 participants) examined the incidence of peritonitis (Ghaffari 2012; Kim 2018; Liu 2014; Nayak 2018; Pai 2016; See 2017; Serrano 2019; Yang 2011)
Eleven studies (1950 participants) examined technique survival and death‐censored technique survival (Jaivid 2017; Kim 2018; Liu 2014; Nayak 2018; Pai 2016; Salari 2018; See 2017; Serrano 2019; Silva 2018; Timely PD 2010; Yang 2011)
Nine studies (1735 participants) examined death (any cause) (Jaivid 2017; Kim 2018; Liu 2014; Pai 2016; See 2017; Serrano 2019; Silva 2018; Timely PD 2010; Yang 2011).
Excluded studies
A total of 36 studies were excluded from this review. The reasons for exclusion included; lack of a control group (conventional‐start PD group), wrong comparison (comparison with another intervention e.g. HD), different definition of conventional‐start PD, and being a review paper.
Risk of bias in included studies
Risk of bias for randomised controlled trials
The risk of bias for the RCT is presented in Table 5.
2. Assessment of quality of studies (randomised controlled studies).
| Study | Selection bias | Blinding (performance bias and detection bias) | Attrition bias | Reporting bias | Other | ||
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome of data | Selective reporting | ||
| Timely PD 2010 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
Allocation
One RCT (Timely PD 2010) was included in the review, which demonstrated a low risk of selection bias risk based on utilisation of randomly varying block (permuted block) method where sequence of randomisation was generated using STATA software by an independent research nurse.
Allocation concealment was achieved using sealed envelopes.
Blinding
Blinding of participants and investigators was not possible due to the nature of the study.
Incomplete outcome data
There was a low risk of attrition bias because all patients were followed till the end of the study. Data were analysed using intention‐to‐treat method.
Selective reporting
Risk of reporting bias was low based on the published protocol, and the study reported most of the pre‐specified outcomes.
Risk of bias for observational studies
Risk of bias for all cohort studies is presented in Table 6 and the risk of bias of case‐control studies is presented in Table 7.
3. Assessment of quality of studies (cohort studies).
| Study | Selection | Comparability | Outcome | Evidence of quality | |||||
| Representativeness of exposed cohort | Selection of non‐exposed cohort | Ascertainment of exposure | Outcomes not present at start | Assessment of outcome | Length of follow‐up | Adequacy of follow‐up | |||
| Ghaffari 2012 | * | * | * | * | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 4 |
| Jaivid 2017 | * | * | * | * | ‐‐ | * | * | 6 | |
| Kim 2018 | * | * | * | * | ‐‐ | * | * | ‐‐ | 6 |
| Liu 2014 | * | * | * | * | * | * | * | ‐‐ | 7 |
| Povlsen 2016 | ‐‐ | ‐‐ | ‐‐ | * | ‐‐ | ‐‐ | * | ‐‐ | 2 |
| Pai 2016 | * | * | * | * | * | * | * | * | 8 |
| Salari 2018 | * | * | ‐‐ | * | ‐‐ | ‐‐ | * | ‐‐ | 4 |
| Serrano 2019 | * | * | ‐‐ | * | ‐‐ | ‐‐ | * | * | 5 |
| Silva 2018 | * | * | ‐‐ | * | ‐‐ | ‐‐ | * | ‐‐ | 4 |
| Vlasak 2017 | * | * | ‐‐ | * | ‐‐ | ‐‐ | * | ‐‐ | 4 |
| Wojtaszek 2018 | * | * | ‐‐ | * | ‐‐ | ‐‐ | * | ‐‐ | 4 |
| Yang 2011 | * | * | * | * | ‐‐ | * | * | * | 7 |
| Zhang 2017 | * | * | ‐‐ | * | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 3 |
4. Assessment of quality of studies (case‐control study).
| Study | Selection | Comparability | Exposure | Quality score | |||||
| Case definition | Representativeness of cases | Control selection | Control definition | Ascertainment of exposure | Methods of ascertainment | Non‐exposure rate | |||
| Nayak 2018 | ‐‐ | ‐‐ | * | * | ‐‐ | ‐‐ | ‐‐ | ‐‐ | 2 |
| See 2017 | * | * | * | * | * | * | * | ‐‐ | 7 |
Selection
There were four criteria in the selection domain including: a) representativeness of the exposed cohort, b) selection of the non‐exposed cohort, c) ascertainment of exposure, and d) demonstration that the outcome of interest was not present at start of study. Six included cohort studies (Ghaffari 2012; Jaivid 2017; Kim 2018; Liu 2014; Pai 2016; Yang 2011) met three Newcastle‐Ottawa Scale criteria for the domains of selection which included representativeness of the exposed cohort (truly representative), selection of the non‐exposed cohort (dawn from the same community as the exposed cohort) and ascertainment of exposure (secure record). There was insufficient information to assess the selection domain for one study (Povlsen 2016). Six studies (Salari 2018; Serrano 2019; Silva 2018; Vlasak 2017; Wojtaszek 2018; Zhang 2017 ) met two (representativeness of exposed cohort and selection of non‐exposed cohort) out of three criteria for selection domain. There was insufficient information to assess the ascertainment of exposure for these studies. Outcome of interest was unlikely to be present at the start of study in 13 cohort studies (Ghaffari 2012; Jaivid 2017; Kim 2018; Liu 2014; Pai 2016; Povlsen 2016; Salari 2018; Serrano 2019; Silva 2018; Vlasak 2017; Wojtaszek 2018; Yang 2011; Zhang 2017).
There were two case control studies (Nayak 2018; See 2017). See 2017 met four Newcastle‐Ottawa Scale criteria for selection which included case definition, representativeness of the cases, control selection and control definition. There was insufficient information to assess the two selection criteria (case definition and representativeness) for Nayak 2018.
Comparability of groups of study
This domain assessed the comparability of cohorts/case and controls on the basis of the design or analysis. Two cohort studies (Liu 2014) (adjusted for potential confounders) and (Pai 2016) (cohorts were comparable and adjusted for confounders) met the criteria, however, five cohort studies (Ghaffari 2012; Jaivid 2017; Kim 2018; Silva 2018; Yang 2011) did not met these criteria and six studies did not report the comparability between the two groups (Povlsen 2016; Salari 2018; Serrano 2019; Vlasak 2017; Wojtaszek 2018; Zhang 2017).
One case control study (See 2017) met the criteria given the study matched between case and control by age and co‐morbidities (diabetes mellitus), however, one case control study (Nayak 2018) did not match between case and control groups.
Outcome
There were three criteria included in this domain, method of assessment of outcome, duration, and adequacy of follow‐up of cohorts. Four cohort studies (Kim 2018; Liu 2014; Pai 2016; Yang 2011) met the criterion for assessment of outcome. Outcome assessment method was not reported in nine cohort studies (Ghaffari 2012; Jaivid 2017; Povlsen 2016; Salari 2018; Serrano 2019; Silva 2018; Vlasak 2017; Wojtaszek 2018; Zhang 2017).
Eleven of 13 cohort studies (85%) studies (Jaivid 2017; Kim 2018; Liu 2014; Pai 2016; Povlsen 2016; Salari 2018; Serrano 2019; Silva 2018; Wojtaszek 2018; Yang 2011) had follow‐up duration of at least six months. Nine cohort studies (Ghaffari 2012; Kim 2018; Liu 2014; Povlsen 2016; Salari 2018; Silva 2018; Vlasak 2017; Wojtaszek 2018; Zhang 2017) did not report number lost to follow‐up and remaining four cohort studies (Jaivid 2017; Pai 2016; Serrano 2019; Yang 2011) reported low percentage of lost to follow‐up.
Exposure
One case control study (See 2017) met the two Newcastle‐Ottawa Scale criteria for exposure; the ascertainment of exposure was based on secure record and same method of ascertainment was applied for case and controls. One case control study (Nayak 2018) did not report ascertainment of exposure, method of ascertainment, or non‐response rate.
Other potential sources of bias
There was potential other bias as authors of one of the included studies (See 2017) were involved in the present review. The majority of included studies did not adjust the potential confounders including age, presence of diabetes mellitus, cardiovascular disease, malignancy, aetiology of kidney failure, nutritional status of patients, and residual kidney function. In general, patients who required urgent dialysis were sicker and more likely to have worse outcomes compared to patients who had planned dialysis initiation, regardless of modality of dialysis (confounding by indication).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Summary of findings of randomised controlled trials.
| Urgent‐start peritoneal dialysis versus conventional‐start peritoneal dialysis for people with chronic kidney disease | |||||
|
Patient or population: people with CKD Settings: community Intervention: USPD Comparison: CSPD | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with CSPD | Risk with USPD | ||||
| Dialysate leak | 72 per 1,000 |
282 per 1,000 (113 to 707) |
RR 3.90 (1.56 to 9.78) |
122 (1) | ⊕⊕⊝⊝ LOW1 |
| Catheter blockage | ‐ | ‐ | ‐ | No studies | Absent |
| Catheter malposition | ‐ | ‐ | ‐ | No studies | Absent |
| PD dialysate flow problem | ‐ | ‐ | ‐ | No studies | Absent |
| Exit‐site infection | ‐ | ‐ | ‐ | No studies | Absent |
| Exit‐site bleeding | 12 per 1,000 | 8 per 1,000 (0 to 203) |
RR 0.70 (0.03 to 16.81) |
122 (1) | ⊕⊝⊝⊝ VERY LOW 2 |
| Tunnel tract infection | ‐ | ‐ | ‐ | No studies | Absent |
| Peritonitis | ‐ | ‐ | ‐ | No studies | Absent |
| Catheter readjustment | ‐ | ‐ | ‐ | No studies | Absent |
| Technique survival | 892 per 1,000 |
972 per 1,000 (892 to 1,000) |
RR 1.09 (1.00 to 1.20) |
122 (1) | ⊕⊝⊝⊝ VERY LOW 2 |
| *The risk in the USPD group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; PD: peritoneal dialysis;USPD: urgent‐start PD; CSPD: conventional‐start PD | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 downgrade two levels for imprecision (small number of study and participants) and indirectness (all patients in USPD initiated PD only at day‐7 of catheter insertion and the majority of patients (69%) required bridging hemodialysis prior to USPD)
2 downgrade three levels for imprecision: small number of study and participants and suboptimal follow‐up duration to assess technique survival
Summary of findings 2. Summary of findings of non‐randomised study interventions: cohort studies.
| Urgent‐start peritoneal dialysis versus conventional‐start peritoneal dialysis for people with chronic kidney disease | |||||
|
Patient or population: people with CKD Settings: community Intervention: USPD Comparison: CSPD | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (cohort studies) | Quality of the evidence (GRADE) | |
| Risk with CSPD | Risk with USPD | ||||
| Dialysate leak | 9 per 1,000 |
18 per 1,000 (7 to 47) |
RR 2.06 (0.80 to 5.28) |
1322 ( 7) | ⊕⊝⊝⊝ VERY LOW1 |
| Catheter blockage | 8 per 1,000 |
11 per 1,000 (3 to 37) |
RR 1.33 (0.40 to 4.43) |
1214 (4) | ⊕⊝⊝⊝ VERY LOW1 |
| Catheter malposition | 28 per 1,000 |
45 per 1,000 (22 to 93) |
RR 1.63 (0.80 to 3.32) |
1353 (6) | ⊕⊝⊝⊝ VERY LOW1 |
| PD dialysate flow problem | 23 per 1,000 |
33 per 1,000 (8 to 140) |
RR 1.44 (0.34 to 6.14) |
937 (3) | ⊕⊝⊝⊝ VERY LOW1 |
| Exit‐site infection | 11 per 1,000 |
15 per 1,000 (3 to 93) |
RR 1.43 (0.24 to 8.61) |
337 (2) | ⊕⊝⊝⊝ VERY LOW1 |
| Exit‐site bleeding | 0 per 1,000 | 0 per 1,000 (0 to 0) |
RR 1.58 (0.07 to 35.32) |
27 (1) | ⊕⊝⊝⊝ VERY LOW1 |
| Tunnel tract infection | ‐ | ‐ | ‐ | No studies | Absent |
| Peritonitis | 107 per 1,000 |
107 per 1,000 (73 to 157) |
RR 1.00 (0.68 to 1.46) |
1497 (7) | ⊕⊝⊝⊝ VERY LOW1 |
| Catheter readjustment | 20 per 1,000 |
25 per 1,000 (8 to 78) |
RR 1.27 (0.40 to 4.02) |
739 (2) | ⊕⊝⊝⊝ VERY LOW 1 |
| Technique survival | 757 per 1,000 |
681 per 1,000 (575 to 810) |
RR 0.90 (0.76 to 1.07) |
1668 (8 studies) | ⊕⊝⊝⊝ VERY LOW 1,2 |
| *The risk in the USPD group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; PD: peritoneal dialysis;USPD: urgent‐start PD; CSPD: conventional‐start PD | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 All studies were observational studies, downgrade one level for imprecision (small number of study and participants)
2 Downgrade one level for inconsistency
Summary of findings 3. Summary of findings of non‐randomised study interventions: case‐control studies.
| Urgent‐start peritoneal dialysis versus conventional‐start peritoneal dialysis for people with chronic kidney disease | |||||
|
Patient or population: people with CKD Settings: community Intervention: USPD Comparison: CSPD | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (case‐control studies) | Quality of the evidence (GRADE) | |
| Risk with CSPD | Risk with USPD | ||||
| Dialysate leak | 10 per 1,000 |
73 per 1,000 (12 to 425) |
RR 7.41 (1.27 to 43.36) |
160 (2) | ⊕⊝⊝⊝ VERY LOW1 |
| Catheter blockage | 39 per 1,000 |
74 per 1,000 (23 to 240) |
RR 1.89 (0.58 to 6.13) |
160 (2) | ⊕⊝⊝⊝ VERY LOW1 |
| Catheter malposition | 38 per 1,000 |
115 per 1,000 (25 to 537) |
RR 3.00 (0.64 to 13.96) |
104 (1) | ⊕⊝⊝⊝ VERY LOW1 |
| PD dialysate flow problem | ‐ | ‐ | ‐ | No studies | Absent |
| Exit‐site infection | 128 per 1,000 |
154 per 1,000 (53 to 449) |
RR 1.20 (0.41 to 3.50) |
104 (1) | ⊕⊝⊝⊝ VERY LOW1 |
| Exit‐site bleeding | ‐ | ‐ | ‐ | No studies | Absent |
| Tunnel tract infection | ‐ | ‐ | ‐ | No studies | Absent |
| Peritonitis | 284 per 1,000 |
310 per 1,000 (34 to 1,000) |
RR 1.09 (0.12 to 9.51) |
160 (2) | ⊕⊝⊝⊝ VERY LOW1 |
| Catheter readjustment | ‐ | ‐ | ‐ | No studies | Absent |
| Technique survival | 627 per 1,000 |
577 per 1,000 (496 to 665) |
RR 0.92 (0.79 to 1.06) |
160 (2) | ⊕⊝⊝⊝ VERY LOW 1 |
| *The risk in the USPD group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; PD: peritoneal dialysis;USPD: urgent‐start PD; CSPD: conventional‐start PD | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 All studies were observational studies, downgrade one level for imprecision (small number of study and participants)
2 Downgrade one level for inconsistency
Mechanical complications
Dialysate leak
In low certainty evidence, urgent‐start PD may increase dialysate leak (Analysis 1.1) (1 RCT, 122 participants: RR 3.90, 95% CI 1.56 to 9.78) compared with conventional‐start PD (Table 1) which translated into an absolute number of 210 more leaks per 1000 (95% CI 40 to 635). It is uncertain whether urgent‐start PD increased dialysate leak compared to conventional‐start PD in analysis of non‐RCTs (Analysis 1.2) (7 cohort studies, 1322 participants): RR 2.06, 95% CI 0.80 to 5.28; I² = 0%, very low certainty evidence; 2 case‐control studies, 160 participants: RR 7.41, 95% CI 1.27 to 43.36; I² = 0%, very low certainty evidence).
1.1. Analysis.

Comparison 1: Mechanical complications, Outcome 1: Dialysate leak (RCT)
1.2. Analysis.

Comparison 1: Mechanical complications, Outcome 2: Dialysate leak (non‐RCT)
We graded the evidence as low certainty based on one RCT, although the evidence for non‐RCTs was uncertain.
Catheter blockage
It is uncertain whether urgent‐start PD increases catheter blockage compared to conventional‐start PD (Analysis 1.3) (4 cohort studies, 1214 participants: RR 1.33, 95% CI 0.40 to 4.43, I² = 0%; 2 case‐control studies, 160 participants; RR 1.89, 95% CI 0.58 to 6.13,I² = 7%; very low certainty evidence) (Table 2). All included studies were observational studies with a small number of events which resulted in imprecision.
1.3. Analysis.

Comparison 1: Mechanical complications, Outcome 3: Catheter blockage (non‐RCT)
Catheter malposition
It is uncertain whether urgent‐start PD increase catheter malposition compared with conventional‐start PD (Analysis 1.4) (6 cohort studies, 1353 participants: RR 1.63, 95% CI 0.80 to 3.32, I² = 0%; 1 case‐control study, 104 participants: RR 3.00, 95% CI 0.64 to 13.96; very low certainty evidence). We graded the evidence as very low certainty because all included studies were observational studies and a small number of events resulted in imprecision.
1.4. Analysis.

Comparison 1: Mechanical complications, Outcome 4: Catheter malposition (non‐RCT)
Peritoneal dialysis dialysate flow problem
It is uncertain whether urgent‐start PD increases dialysate flow problem compared to conventional‐start PD (Analysis 1.5) (3 cohort studies, 937 participants: RR 1.44, 95% CI 0.34 to 6.14; I² = 55%). We graded very low certainty evidence because all included studies were observational studies and moderate heterogeneity was observed. Further sub‐group and sensitivity analyses were not able to be meaningfully performed due to insufficient data.
1.5. Analysis.

Comparison 1: Mechanical complications, Outcome 5: PD dialysate flow problem (non‐RCT)
Exit‐site complications
Exit‐site infection
It is uncertain whether urgent‐start PD increased exit‐site infection (Analysis 2.1) (2 cohort studies, 337 participants: RR 1.43, 95% CI 0.24 to 8.6, I² = 0%; 1 case‐control study, 104 participants: RR 1.20, 95% CI 0.41 to 3.50; very low certainty evidence) or exit‐site infection rate (Analysis 2.2) (2 cohort studies, 8048 patient‐months: RR 1.06, 95% CI 0.17 to 6.75, I² = 0%; very low certainty evidence) compared to conventional‐start PD. See 2017 reported comparable risk of early exit‐site infection (day 30) between urgent‐start and conventional‐start groups (15% versus 13% respectively). Similarly, Ghaffari 2012 reported comparable risk of late exit‐site infection (day 90) between the urgent‐start and conventional‐start PD groups (1 over 55 versus 1 over 42 patient‐months, respectively). Tunnel tract infection was not reported separately in any of the included studies.
2.1. Analysis.

Comparison 2: Exit‐site complications, Outcome 1: Exit‐site infection (non‐RCT)
2.2. Analysis.

Comparison 2: Exit‐site complications, Outcome 2: Exit‐site infection rate (non‐RCT)
Exit‐site bleeding
It is uncertain whether urgent‐start PD increased exit‐site bleeding compared with conventional‐start PD (Analysis 2.3) (1 RCT, 122 participants: RR 0.70, 95% CI 0.03 to 16.81; 1 cohort study, 27 participants: RR 1.58, 95% CI 0.07 to 35.32; very low certainty evidence). We graded the evidence as very low certainty because of imprecision due to small number of participants and studies.
2.3. Analysis.

Comparison 2: Exit‐site complications, Outcome 3: Exit‐site bleeding (RCT)
Peritonitis
It is uncertain whether urgent‐start PD increased the risk of peritonitis compared with conventional‐start PD (Analysis 3.1) (7 cohort studies, 1497 participants; RR 1.00, 95% CI 0.68 to 1.46, I² = 18%; 2 case‐control studies, 160 participants: RR 1.09, 95% CI 0.12 to 9.51, I² = 57%; very low certainty evidence). We graded very low certainty evidence because all included studies were non‐RCTs. In addition, imprecision and/or inconsistency were observed. A similar result was observed in secondary analyses of day‐30 peritonitis (Analysis 3.3) (2 cohort studies, 627 participants: RR 1.02, 95% CI 0.59 to 1.74; I² = 0%; 1 case‐control study, 104 participants: RR 0.52, 95% CI 0.22 to 1.20; I² = 0%) and day‐90 peritonitis (Analysis 3.4) (2 cohort studies, 192 participants: RR 0.67, 95% CI 0.17 to 2.57; I² = 0%; 1 case‐control study, 56 participants: RR 5.30, 95% CI 0.29 to 98.06; I² = 0%). Similarly, it is uncertain whether urgent‐start PD increases peritonitis rate compared to conventional‐start PD (Analysis 3.2) (2 cohort studies, 8048 patient‐months: RR 0.88, 95% CI 0.23 to 3.34, I² = 0%; very low certainty evidence). Two studies (Timely PD 2010; Vlasak 2017) reported similar in the PD‐related infection between urgent‐start and conventional‐start PD groups.
3.1. Analysis.

Comparison 3: Peritonitis, Outcome 1: Peritonitis (non‐RCT)
3.3. Analysis.

Comparison 3: Peritonitis, Outcome 3: Peritonitis (secondary analysis: day 30)
3.4. Analysis.

Comparison 3: Peritonitis, Outcome 4: Peritonitis (secondary analysis: day 90)
3.2. Analysis.

Comparison 3: Peritonitis, Outcome 2: Peritonitis rate (non‐RCT)
Tunnel tract infection
No studies reported tunnel tract infection.
Catheter readjustment
It is uncertain whether urgent‐start PD compared with conventional‐start PD increases catheter readjustment (Analysis 4.1) (2 cohort studies, 739 participants: RR 1.27, 95% CI 0.40 to 4.02, I² = 0%; very low certainty evidence). There was no study specifically reported catheter readjustment within one month of commencement of PD.
4.1. Analysis.

Comparison 4: Catheter re‐adjustment (non‐RCT), Outcome 1: Catheter readjustment
Technique and patient survival
Technique survival
It is uncertain whether urgent‐start PD compared with conventional‐start PD reduces technique survival. This analysis included one RCT (Analysis 5.1) (1 RCT, 122 participants: RR 1.09, 95% CI 1.00 to 1.20, very low certainty evidence), and 10 non‐RCTs (Analysis 5.2) (8 cohort studies, 1668 participants: RR 0.90, 95% CI 0.76 to 1.07, I² = 88%; 2 case‐control studies, 160 participants: RR 0.92, 95% CI 0.79 to 1.06, I² = 0%; very low certainty evidence ). We graded the evidence as very low certainty because this analysis only included one RCT with inadequate follow‐up duration for the outcome and substantial heterogeneity was observed in analysis of cohort studies. The heterogeneity was unable to be resolved when analyses were repeated according to the method of catheter insertion (laparotomy) (Analysis 5.3) (4 cohort studies, 1198 participants: RR 0.74, 95% CI 0.58 to 0.94, I² = 84%) or follow‐up duration of up to 6 months (Analysis 5.4) (4 cohort studies, 896 participants: RR 0.94, 95% CI 0.78 to 1.12; I² = 85%) or more than 6 months (Analysis 5.5) (4 cohort studies, 772 participants: RR 0.87, 95% CI 0.58 to 1.30, I² = 92%). Sensitivity analysis was performed after excluding studies with a high risk of bias and a large study: urgent‐start PD had little or no effect on technique survival compared to conventional‐start PD (Analysis 5.6) (3 cohort studies, 418 participants: RR 0.87, 95% CI 0.76 to 1.00; I² = 0%; 1 case‐control study, 104 participants: RR 0.95, 95% CI 0.61 to 1.47).
5.1. Analysis.

Comparison 5: Technique survival, Outcome 1: Technique survival (RCT)
5.2. Analysis.

Comparison 5: Technique survival, Outcome 2: Technique survival (non‐RCT)
5.3. Analysis.

Comparison 5: Technique survival, Outcome 3: Technique survival: secondary analysis (cohort studies ‐ laparotomy)
5.4. Analysis.

Comparison 5: Technique survival, Outcome 4: Technique survival: sensitivity analysis (cohort studies ‐ up to 6 months follow‐up)
5.5. Analysis.

Comparison 5: Technique survival, Outcome 5: Technique survival: sensitivity analysis (cohort studies ‐ more than 6 months follow‐up)
5.6. Analysis.

Comparison 5: Technique survival, Outcome 6: Technique survival: sensitivity analysis (cohort studies ‐ low risk of bias)
Death‐censored technique survival
It is uncertain whether urgent‐start PD compared with conventional‐start PD reduces death‐censored technique survival (Analysis 5.7) (1 RCT, 122 participants: RR 1.08, 95% CI 0.99 to 1.18, very low certainty evidence) and (Analysis 5.8) (7 cohort studies,1509 participants: RR 0.99, 95% CI 0.88 to 1.10; I² = 82%; very low certainty evidence; 1 case‐control study, 104 participants: RR 0.94, 95% CI 0.67 to 1.33; I² = 0%, very low certainty evidence). We graded the evidence as very low certainty because substantial heterogeneity (inconsistency) was observed during analysis of cohort studies. Heterogeneity was not resolved when analyses were repeated according to the method of catheter insertion (laparotomy) (Analysis 5.9) (4 cohort studies, 1198 participants: RR 0.90, 95% CI 0.81 to 1.00; I² = 61%) or according to follow‐up duration of up to 6 months (Analysis 5.10) (4 cohort studies, 896 participants: RR 0.98, 95% CI 0.91 to 1.07; I² = 59%), or more than 6 months (Analysis 5.11) (3 cohort studies, 613 participants: RR 1.00, 95% CI 0.69 to 1.46; I² = 92%).
5.7. Analysis.

Comparison 5: Technique survival, Outcome 7: Death‐censored technique survival (RCT)
5.8. Analysis.

Comparison 5: Technique survival, Outcome 8: Death‐censored technique survival (non‐RCT)
5.9. Analysis.

Comparison 5: Technique survival, Outcome 9: Death‐censored technique survival: secondary analysis (cohort studies ‐ laparotomy)
5.10. Analysis.

Comparison 5: Technique survival, Outcome 10: Death‐censored technique survival: sensitivity analysis (cohort studies ‐ up to 6 months follow‐up)
5.11. Analysis.

Comparison 5: Technique survival, Outcome 11: Death‐censored technique survival: sensitivity analysis (cohort studies ‐ more than 6 months follow‐up)
Death (any cause)
It is uncertain whether urgent‐start PD compared with conventional‐start PD increased death (any cause) (Analysis 6.1) (1 RCT, 122 participants: RR 1.49, 95% CI 0.87 to 2.53; very low certainty evidence); (Analysis 6.2) (7 cohort studies, 1509 participants: RR 1.89, 95% CI 1.07 to 3.32; I² = 42%; very low certainty evidence; 1 case‐control study, 104 participants: RR 0.90, 95% CI 0.27 to 3.02; I² = 0%;very low certainty evidence). We graded the evidence as very low certainty because inconsistency was observed.
6.1. Analysis.

Comparison 6: Death (any cause), Outcome 1: Death (any cause) (RCT)
6.2. Analysis.

Comparison 6: Death (any cause), Outcome 2: Death (any cause) (non‐RCT)
Interim haemodialysis
Only Timely PD 2010 reported that eight patients from the urgent‐start PD group and four patients from the conventional‐start PD group required interim HD after commencement of PD (Analysis 8.1).
8.1. Analysis.

Comparison 8: Interim haemodialysis, Outcome 1: Interim HD
Hospitalisation
Hospitalisation was reported in 2 studies (Jaivid 2017; Yang 2011). Due to inconsistently reported outcome measures, results from these studies could not be directly compared. Jaivid 2017 reported no difference in hospitalisation between the urgent‐start PD and conventional‐start PD group (7.3 versus 7.29 patient‐months), whereas Yang 2011 reported longer mean length of hospitalisation in the urgent‐start PD group compared to the conventional‐start PD group (11.8 ± 10.2 versus 7.5 ± 6.2 days, P < 0.001). Continuous scale of measurement of effect was unable to be used given the insufficient studies on duration of hospitalisation.
Adverse events
Yang 2011 reported pericatheter hernia (Analysis 7.1) and hemoperitoneum (Analysis 7.2) and Timely PD 2010 reported delayed wound healing (Analysis 7.3)
7.1. Analysis.

Comparison 7: Adverse events, Outcome 1: Pericatheter hernia
7.2. Analysis.

Comparison 7: Adverse events, Outcome 2: Haemoperitoneum
7.3. Analysis.

Comparison 7: Adverse events, Outcome 3: Delayed wound healing
See Table 8.
5. Adverse events.
| Adverse events | USPD | CSPD | Study | ||
| Events | Total | Events | Total | ||
| Pericatheter hernia | 1 | 226 | 0 | 84 | Yang 2011 |
| Haemoperitoneum | 1 | 226 | 0 | 84 | Yang 2011 |
| Delayed wound healing | 1 | 39 | 1 | 83 | Timely PD 2010 |
USPD ‐ urgent‐start peritoneal dialysis; CSPD ‐ conventional‐start peritoneal dialysis
Cost of dialysis
No studies compared the cost of urgent‐start PD with conventional PD.
Peritoneal dialysis training duration
No studies compared PD training durations between urgent‐start PD and conventional‐start PD.
Quality of life
No studies compared patient QoL between urgent‐start PD and conventional‐start PD.
Overall, there were insufficient data to allow other subgroup or sensitivity analyses for most of the outcomes.
Discussion
Summary of main results
Urgent‐start PD may increase the risk of dialysate leak compared with conventional‐start PD (RR 3.90, 95% CI 1.56 to 9.78; low certainty evidence). It is uncertain whether urgent‐start PD compared with conventional‐start PD increased other mechanical complications including catheter blockage, catheter malposition, poor flow of catheter, and catheter readjustment because of the suboptimal quality of included studies and a small number of events resulting in imprecision. It is uncertain whether urgent‐start PD compared with conventional‐start PD increased exit‐site complications including exit‐site infection, exit‐site bleeding, and peritonitis, or reduced technique survival compared with conventional‐start PD. Substantial heterogeneity was observed when analysed for technique survival and heterogeneity was unable to resolve despite after secondary analyses accordingly to methods of insertion (laparotomy), duration of follow‐up (≤ or > 6 months). Similarly, it is uncertain whether urgent‐start PD compared with conventional‐start PD increases death (any cause) because the certainty of this evidence was very low.
Overall completeness and applicability of evidence
The present review included a total of 16 studies (2953 participants) comparing the outcomes between urgent‐start and conventional‐start PD. Only one RCT was identified (Timely PD 2010), the remaining studies were observational studies. Most studies did not adjust for potential confounders including age, gender, body mass index, comorbidities of patients (such as diabetes mellitus and cardiovascular disease), methods of catheter insertion, skills of interventionists, initial dialysate fill volume and initial PD regimen, and PD modality. The review performed analyses using unadjusted estimates instead of adjusted estimates because most studies did not adjust for potential confounders. Therefore, the pooled analysis of adjusted estimates was not available for the present review.
In this review, initiation of PD within 2 weeks of catheter insertion may increase the risk of pericatheter or subcutaneous dialysate leak compared with initiation of PD at least 2 weeks after catheter insertion. This is based on one RCT with a low risk of bias, whereas the results of observational studies on this outcome are uncertain. These results are supported by previous literature where an increase in the risk of dialysate leak was observed in conditions associated with poor wound healing and impaired tensile strength of tissues (Tzallaloukas 1990). Therefore, initiation of urgent PD, as early as within 24 hours of catheter implantation, would not have allowed for sufficient time for the operative wound to heal, resulting in an increase in risk of dialysate leak (Povlsen 2006). Other recognised risk factors for dialysate leak include catheter insertion technique (median versus lateral catheter insertion) (Stegmayr 1990), larger initial fill volume, history of previous abdominal surgery, chronic steroid use, and multiple pregnancies (Tzallaloukas 1990). Early leak, which is defined as leak within 30 days of catheter insertion, is usually manifested as dialysate loss externally. Two included studies (See 2017; Vlasak 2017) reported early leak. See 2017 reported that early leak was more likely to occur in urgent‐start PD than conventional‐start PD (12% versus 1%). See 2017 reported that patients with early leak were successfully managed with conservative approaches including using lower dwell volume or temporary interruption of PD. See 2017 also reported that long‐term (up to 3 years) technique survival rates were comparable between urgent‐start PD and conventional‐start PD. Other studies included in the review did not distinguish between early and late leaks.
The effects of urgent‐start PD compared with conventional‐start PD on catheter blockage, catheter malposition, and PD dialysate flow problems remain uncertain. Previous small individual studies (See 2017; Bitencourt Dias 2017) reported a trend of higher odds of catheter migration in the urgent‐start PD group. All included studies reporting catheter migration/malposition were retrospective, single centre, observational studies. We graded low certainty evidence in view of suboptimal quality of included studies and a small number of events resulting in imprecision.
There were only two studies (Ghaffari 2012; Timely PD 2010) that reported bleeding/haematoma at the catheter exit site. In Ghaffari 2012, the method of catheter placement was different between the urgent‐start and conventional‐start PD groups (percutaneous versus laparoscopic insertion), with one case of exit‐site bleeding/haematoma observed in a patient who received urgent‐start PD. Based on the available evidence, it is uncertain whether urgent‐start PD increases exit‐site bleeding/haematoma compared with conventional‐start PD.
It is uncertain whether urgent‐start PD increases risks of exit‐site infection compared with conventional‐start PD groups. The risk of early exit‐site infection (day‐30 of PD initiation) was reported in one study (See 2017), which found no difference between the two groups. Similarly, the risk of late exit‐site infection (day‐90 of PD initiation) was reported to be comparable between the two groups in one study (Ghaffari 2012). It is uncertain whether urgent‐start PD increased risk of peritonitis or peritonitis rate. Similarly, it is uncertain whether urgent‐start PD increases early peritonitis (day 30) and late peritonitis (day 90) compared with conventional‐start PD.
Urgent‐start PD has uncertain effect on technique survival compared with conventional‐start PD groups. There was a considerable heterogeneity in the analysis of technique survival between urgent‐start and conventional‐start PD groups. Heterogeneity was not resolved despite subgroup analyses according to method of catheter insertion (laparotomy) and follow‐up duration (up to 6 months versus more than 6 months). Based on the available evidence, it is uncertain whether urgent‐start PD increases technique survival compared with conventional‐start PD.
This review observed that it is uncertain whether urgent‐start PD increases death (any cause) compared with conventional‐start PD, because all but one of the included studies were observational studies and there was inadequate follow‐up duration, and most studies did not adjust for potential confounders. Generally, patients with CKD who required urgent dialysis were more unwell and had a higher risk of experiencing adverse outcomes than patients who required planned dialysis, regardless of type of dialysis initiation (confounding by indication).
In general, the majority of included studies were retrospective, observational studies, with only one RCT included in the review. In addition, most studies had small numbers of participants and the follow‐up durations varied among the included studies. Moreover, there was considerable variation in the duration of time between catheter placement and initiation of dialysis (break‐in period) among patients in the urgent‐start PD group, ranging from within 24 hours to 14 days of catheter insertion. Furthermore, there were various catheter placement methods, PD modalities, and initial PD fill volumes, which would have likely contributed to the observed heterogeneity in outcomes. Lastly, some of the included studies did not clearly distinguish whether urgent‐start PD was initiated in patients who were initially planned for conventional‐start PD, but used their catheters early or in those who had an unplanned, urgent‐ PD start. At present, it is unknown whether the outcomes of these patients are different. In summary, due to the suboptimal methodological quality of the included reviews, and the imprecision and inconsistency of results, the majority of outcomes were graded as low to very low level of evidence. There is no strong recommendation that can be made for or against urgent‐start PD based on the available evidence.
Quality of the evidence
There was only one RCT identified, which is perhaps not surprising as the need to initiate dialysis urgently is driven by clinical indication. However, prior to any broader implementation of urgent‐start PD, it is important to ensure that this approach confers at least comparable outcomes to those of the more traditional, conventional‐start approach. In this review the majority of studies were retrospective cohort studies. All included observational studies met the criteria for the selection domain of risk of bias assessment. There were only 2 out of 12 cohort studies (17%) and one case‐control (50%) that met the criteria for the comparability domain. The majority of cohort studies did not report the method of outcome assessment and only one case‐control study met most of the criteria for the exposure domain. The one RCT had a low risk of bias. In general, studies were limited by non‐RCTs, small sample sizes, retrospective study designs, and lack of comparability of cohorts.
There were potential systemic differences between participants in urgent‐start and conventional‐start PD groups (selection bias). An attempt to counter bias introduced from confounding by analysing with adjusted estimates of intervention effects was not possible because only a few studies adjusted for potential confounders and different studies adjusted different confounders means that pooling analysis is inappropriate.
Potential biases in the review process
The review included comprehensive systematic review of publications through MEDLINE, EMBASE, and CENTRAL search. The review processes, including data extraction, data analysis and assessment of study quality, were performed by two independent investigators and any differences were resolved by checking with additional two authors. The authors of previous publications were approached for additional data for the review. However, there was a potential risk of bias as authors from one of the included studies were also authors for this review.
Agreements and disagreements with other studies or reviews
A previous narrative literature review of urgent‐start PD by Alkatheeri 2016 reported that there was no significant difference in the risk of leak between urgent‐start and conventional‐start PD groups. However, a previous meta‐analysis by Zang 2019, which included only six observational studies, reported that urgent‐start PD was associated with a higher risk of dialysate leak. Similarly, the present meta‐analysis, which included one RCT and 15 observational studies, concluded that urgent‐start PD may slightly increase the risk of dialysate leak compared with conventional‐start PD.
The risk of catheter readjustment was reported to be higher in urgent‐start PD patients, who initiated PD within 24 hours of catheter insertion, compared with conventional‐start PD patients, who initiated PD > 12 days after catheter insertion, in a small single centre study (Povlsen 2006). Zang 2019, including only 2 observational studies (Liu 2014; Povlsen 2006), reported the comparable risk of catheter readjustment between the two groups. The present review including two studies (Kim 2018; Liu 2014) for the outcomes of catheter readjustment, it was observed that urgent‐start PD had an uncertain effect on the risk of catheter readjustment compared with conventional‐start PD because the certainty of this evidence is very low given that a few included studies with small events resulting in imprecision. This review did not include Povlsen 2006 because the control arm initiated PD > 12 days instead of ≥ 14 days.
Zang 2019 reported no difference in death between urgent‐start PD and conventional‐start PD. This review included a larger number of studies and observed that urgent‐start PD had an uncertain effect on death (any cause) compared with conventional‐start PD. Most of the included observational studies had different, and relatively short follow‐up durations, and the studies were unadjusted for potential confounders. In summary, the certainty of evidence is very low for the mortality outcome.
Authors' conclusions
Implications for practice.
In patients with CKD requiring urgent commencement of dialysis, either due to late referral to nephrologists or unexpected rapid progression of kidney disease, and who are suitable for PD, clinicians and patients should be aware that based on one small RCT, urgent‐start PD may slightly increase dialysate leak (results from the observational studies are uncertain – imprecise and based on unadjusted data) and has uncertain effects on catheter blockage, catheter malposition, catheter readjustment, infectious complications, technique survival, and patient survival compared with conventional‐start PD.
Implications for research.
Future studies need to specify and report the early versus late mechanical or infection‐related complications to allow for a better understanding of the timing and types of complications. In addition, future studies should clearly indicate the technique of catheter insertion, urgency of PD initiation (early versus urgent use of PD catheter) to allow for adjustment of potential confounders and better understanding of the outcomes of urgent‐start PD
Future studies should examine cost effectiveness, QoL and other patient‐reported outcomes in addition to clinical outcomes in patients with kidney failure on urgent‐start PD.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2020 | Amended | Addition of number of participants for case‐control studies for the outcome peritonitis |
History
Protocol first published: Issue 1, 2018 Review first published: Issue 12, 2020
Acknowledgements
The authors wish to thank Cochrane Kidney and Transplant for their support and advice in during the development of this protocol. The authors gratefully acknowledge the contribution of Dr Johan V. Povlsen, Wei Fang, Ya‐Fei Yang and Huang Chiu‐Ching. The authors gratefully acknowledge Gail Higgins from Cochrane Kidney and Transplant for her contribution.
The authors are grateful to the following peer reviewers for their time and comments: Davide Bolignano (CNR‐Institute of Clinical Physiology, Reggio Calabria, Italy), Dr Mark Lambie (Keele University, UK), Peter G Blake (Western University, London, Ontario, Canada), Dr Stanley Fan (Renal Unit, Barts Health NHS Trust, UK).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Mechanical complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Dialysate leak (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 Dialysate leak (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Dialysate leak (non‐RCT) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Dialysate leak (cohort studies) | 7 | 1322 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.80, 5.28] |
| 1.2.2 Dialysate leak (case‐control studies) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 7.41 [1.27, 43.36] |
| 1.3 Catheter blockage (non‐RCT) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Catheter blockage (cohort studies) | 4 | 1214 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.40, 4.43] |
| 1.3.2 Catheter blockage (case‐control studies) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.58, 6.13] |
| 1.4 Catheter malposition (non‐RCT) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Catheter malposition (cohort studies) | 6 | 1353 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.80, 3.32] |
| 1.4.2 Catheter malposition (case‐control studies) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.64, 13.96] |
| 1.5 PD dialysate flow problem (non‐RCT) | 3 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.34, 6.14] |
Comparison 2. Exit‐site complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Exit‐site infection (non‐RCT) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Exit‐site or tunnel infection (cohort studies) | 2 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.24, 8.61] |
| 2.1.2 Exit‐site or tunnel infection (case‐control studies) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.41, 3.50] |
| 2.2 Exit‐site infection rate (non‐RCT) | 2 | 8048 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.17, 6.75] |
| 2.3 Exit‐site bleeding (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3.1 Exit‐site bleeding (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Exit‐site bleeding (non‐RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4.1 Exit‐site bleeding (cohort studies) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.4. Analysis.

Comparison 2: Exit‐site complications, Outcome 4: Exit‐site bleeding (non‐RCT)
Comparison 3. Peritonitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Peritonitis (non‐RCT) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 Peritonitis (cohort studies) | 7 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.68, 1.46] |
| 3.1.2 Peritonitis (case‐control studies) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.12, 9.51] |
| 3.2 Peritonitis rate (non‐RCT) | 2 | 8048 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.23, 3.34] |
| 3.3 Peritonitis (secondary analysis: day 30) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.3.1 Peritonitis (cohort studies) | 2 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.74] |
| 3.3.2 Peritonitis (case‐control studies) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.22, 1.20] |
| 3.4 Peritonitis (secondary analysis: day 90) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.4.1 Peritonitis (cohort studies) | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.17, 2.57] |
| 3.4.2 Peritonitis (case‐control studies) | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 5.30 [0.29, 98.06] |
Comparison 4. Catheter re‐adjustment (non‐RCT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Catheter readjustment | 2 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.40, 4.02] |
Comparison 5. Technique survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Technique survival (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1.1 Technique survival (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Technique survival (non‐RCT) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Technique survival (cohort studies) | 8 | 1668 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.07] |
| 5.2.2 Technique survival (case‐control studies) | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.06] |
| 5.3 Technique survival: secondary analysis (cohort studies ‐ laparotomy) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 Technique survival (cohort studies) | 4 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.58, 0.94] |
| 5.4 Technique survival: sensitivity analysis (cohort studies ‐ up to 6 months follow‐up) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.4.1 Technique survival (cohort studies) | 4 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.12] |
| 5.5 Technique survival: sensitivity analysis (cohort studies ‐ more than 6 months follow‐up) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.5.1 Technique survival (cohort studies) | 4 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| 5.6 Technique survival: sensitivity analysis (cohort studies ‐ low risk of bias) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.6.1 Technique survival (cohort studies) | 3 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 1.00] |
| 5.6.2 Technique survival (case‐control studies) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.47] |
| 5.7 Death‐censored technique survival (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.7.1 Death‐censored technique survival (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.8 Death‐censored technique survival (non‐RCT) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.8.1 Death‐censored technique survival (cohort studies) | 7 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.10] |
| 5.8.2 Death‐censored technique survival (case‐control studies) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.33] |
| 5.9 Death‐censored technique survival: secondary analysis (cohort studies ‐ laparotomy) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.9.1 Death‐censored technique survival (cohort studies) | 4 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 1.00] |
| 5.10 Death‐censored technique survival: sensitivity analysis (cohort studies ‐ up to 6 months follow‐up) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.10.1 Death‐censored technique survival (cohort studies) | 4 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.07] |
| 5.11 Death‐censored technique survival: sensitivity analysis (cohort studies ‐ more than 6 months follow‐up) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.11.1 Death‐censored technique survival (cohort studies) | 3 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.46] |
Comparison 6. Death (any cause).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Death (any cause) (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1.1 Death (any cause) (RCT) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Death (any cause) (non‐RCT) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.2.1 Death (any cause) (cohort studies) | 7 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.07, 3.32] |
| 6.2.2 Death (any cause) (case‐control studies) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.27, 3.02] |
| 6.3 Death (any cause): secondary analysis (cohort studies ‐ laparotomy) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.3.1 Death (any cause) (cohort studies) | 4 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [1.14, 4.61] |
| 6.4 Death (any cause): sensitivity analysis (cohort studies‐up to 6 months follow‐up) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.4.1 Death (any cause) (cohort studies) | 4 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.47, 6.48] |
| 6.5 Death (any cause): sensitivity analysis (cohort studies ‐ more than 6 months follow‐up) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.5.1 Death (any cause) (cohort studies) | 3 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.01, 3.26] |
| 6.6 Death (any cause): sensitivity analysis (cohort studies ‐ low risk of bias) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.6.1 Death (any cause) (cohort studies) | 3 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.81, 2.30] |
6.3. Analysis.

Comparison 6: Death (any cause), Outcome 3: Death (any cause): secondary analysis (cohort studies ‐ laparotomy)
6.4. Analysis.

Comparison 6: Death (any cause), Outcome 4: Death (any cause): sensitivity analysis (cohort studies‐up to 6 months follow‐up)
6.5. Analysis.

Comparison 6: Death (any cause), Outcome 5: Death (any cause): sensitivity analysis (cohort studies ‐ more than 6 months follow‐up)
6.6. Analysis.

Comparison 6: Death (any cause), Outcome 6: Death (any cause): sensitivity analysis (cohort studies ‐ low risk of bias)
Comparison 7. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Pericatheter hernia | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2 Haemoperitoneum | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.3 Delayed wound healing | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Interim haemodialysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Interim HD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ghaffari 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Cohorts are true representative of CKD 5 patients in the community |
| Selection: non exposed cohort | Low risk | Non‐exposed drawn from the same community as exposed cohorts |
| Selection: ascertainment of exposure | Low risk | Ascertainment of exposure by secure record |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome of interest was unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | High risk | Data were not adjusted for potential confounders, used percutaneous method for urgent‐start group and laparoscopic method for non‐urgent start group. In addition, study did not adjust for potential confounders. |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Unclear risk | Follow‐up: 90 days |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
Jaivid 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Representative of the average CKD stage 5 patients without having planned dialysis in the community |
| Selection: non exposed cohort | Low risk | Same cohort |
| Selection: ascertainment of exposure | Low risk | Secure record |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome of interest was unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | High risk | Significantly higher number of laparoscopic assisted catheter insertion in control group than urgent‐start group, and data were not adjusted for all potential confounders |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Low risk | 180 days, long enough to examine the main outcomes of interest |
| Outcome: adequacy of follow‐up | Low risk | 8% dropout |
Kim 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Representative of CKD stage 5 patients who initiated PD urgently in a hospital |
| Selection: non exposed cohort | Low risk | These patients were selected from the same hospital as exposed cohort |
| Selection: ascertainment of exposure | Low risk | Secure record |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome of interest was unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | High risk | Baseline characteristics were significantly different between the two groups, the outcomes were not adjusted for potential confounders |
| Outcome: assessment | Low risk | Medical record |
| Outcome: follow‐up length | Low risk | Adequate for study of short term outcomes |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
Liu 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
The choice of when to start dialysis after catheter implantation was made by the nephrologists based on the clinical condition of individual patients |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Representative of the average CKD stage 5 patients in the community |
| Selection: non exposed cohort | Low risk | Same cohort |
| Selection: ascertainment of exposure | Low risk | Secure record |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | All patients were not on dialysis before the study |
| Comparability of cohorts on basis of design or analysis | Low risk | Age and baseline albumin were different between groups, however, data were adjusted for potential confounders including age, gender, comorbidities |
| Outcome: assessment | Low risk | Medical record |
| Outcome: follow‐up length | Low risk | 6 months, long enough to examine the primary outcomes of interest |
| Outcome: adequacy of follow‐up | Unclear risk | No description of lost to follow‐up |
Nayak 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition | Unclear risk | Insufficient information to permit judgement |
| Representativeness of the case | Unclear risk | Insufficient information to permit judgement |
| Selection: control | Low risk | Control from same community |
| Definition of control | Low risk | Initiation of PD 14 days after catheter insertion |
| Comparability of cases and controls on basis of design or analysis | High risk | Data were not matched, PVD was significantly higher in emergent start group, data were not adjusted for potential confounders |
| Exposure: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Exposure: same methods for case and control | Unclear risk | Insufficient information to permit judgement |
| Exposure: non‐response rate | Unclear risk | insufficient information to permit judgement |
Pai 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Representative of average CKD stage 5 patients who choose to do PD in the community |
| Selection: non exposed cohort | Low risk | Same cohort |
| Selection: ascertainment of exposure | Low risk | Secure record |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | All are CKD stage 5 patients not on dialysis before the study |
| Comparability of cohorts on basis of design or analysis | Low risk | Study adjusted for diabetes, age, albumin |
| Outcome: assessment | Low risk | Medical record review |
| Outcome: follow‐up length | Low risk | The average period of follow‐up was 30.5 ± 24.9 months |
| Outcome: adequacy of follow‐up | Low risk | Description provided for lost to follow up, similar dropout in both groups |
Povlsen 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Unclear risk | Insufficient information to permit judgement |
| Selection: non exposed cohort | Unclear risk | Insufficient information to permit judgement |
| Selection: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome of interest was unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | Unclear risk | Insufficient information to permit judgement |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Low risk | Adequate for outcomes of interest |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
Salari 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Included all patients underwent PD in a single centre, during the study period |
| Selection: non exposed cohort | Low risk | Same cohort, underwent PD in the same hospital |
| Selection: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome of interest was unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | Unclear risk | Insufficient information to permit judgement |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Low risk | Duration of follow‐up was adequate |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
See 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition | Low risk | Independent validation – from unit database, further confirmed using hospital medical records |
| Representativeness of the case | Low risk | All eligible cases with outcome of interest over a defined period of time were included |
| Selection: control | Low risk | Control from same community |
| Definition of control | Low risk | Initiation of PD > 2 weeks after catheter placement |
| Comparability of cases and controls on basis of design or analysis | Low risk | Matched for confounders: age, diabetic status |
| Exposure: ascertainment of exposure | Low risk | Hospital records |
| Exposure: same methods for case and control | Low risk | Yes |
| Exposure: non‐response rate | Unclear risk | Unclear |
Serrano 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | All patients from single centre who required urgent initiation of PD within 2 weeks of catheter insertion were included |
| Selection: non exposed cohort | Low risk | Same cohort, underwent traditional PD in the same hospital |
| Selection: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcomes are unlikely to be present at the start of the study |
| Comparability of cohorts on basis of design or analysis | Unclear risk | Insufficient information to permit judgement |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Low risk | long enough to determine the early complications |
| Outcome: adequacy of follow‐up | Low risk | low dropout < 5% (5 out of 107 patients dropout from the programme) |
Silva 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | CKD stage 5 patients admitted to hospital requiring urgent‐dialysis |
| Selection: non exposed cohort | Low risk | Recruited from the same hospital as exposed cohort |
| Selection: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | High risk | Unequal baseline characteristics between groups with higher number of heart failure among urgent‐start group. The analysis only adjusted for DM, BMI and mode of PD imitation (USPD versus CSPD) |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Low risk | Median follow‐up duration 381 days |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Reporting bias |
Timely PD 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised (permuted block) |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not blinded, unlikely to influence the outcomes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, unlikely to influence the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, unlikely to influence the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed till at the end of study period |
| Selective reporting (reporting bias) | Low risk | Published protocol before the study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Vlasak 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Represent kidney failure patients who chose PD from the single centre |
| Selection: non exposed cohort | Low risk | Same cohort |
| Selection: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcomes of interest were unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | Unclear risk | Insufficient information to permit judgement |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | High risk | Follow‐up 4 weeks only |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
Wojtaszek 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | The study included all incident PD patients from the same hospital during the study period |
| Selection: non exposed cohort | Low risk | Non‐exposed cohorts were from the same hospital as exposed cohorts |
| Selection: ascertainment of exposure | Unclear risk | Insufficient information to permit judgement |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcomes unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | Unclear risk | Insufficient information to permit judgement |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | Low risk | Long enough for short‐term outcomes |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
Yang 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Representative of new CKD stage 5 patients in the community |
| Selection: non exposed cohort | Low risk | Same community |
| Selection: ascertainment of exposure | Low risk | Secure record |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcome of interest was unlikely to be present before the study |
| Comparability of cohorts on basis of design or analysis | High risk | No adjustment was done for potential confounders. There were significant different in baseline data, serum albumin, gender, HD before PD etc. Different follow up period between the two groups |
| Outcome: assessment | Low risk | Medical record review |
| Outcome: follow‐up length | Low risk | 6 months, enough to examine the outcome of interest |
| Outcome: adequacy of follow‐up | Low risk | 5% lost to follow‐up |
Zhang 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection: representativeness of exposed cohort | Low risk | Kidney failure patients treated with emergency dialysis |
| Selection: non exposed cohort | Low risk | Selected from the same hospital |
| Selection: demonstration that outcome of interest was not present at the start of the study | Low risk | Outcomes unlikely to be present at the start of the study |
| Comparability of cohorts on basis of design or analysis | High risk | Results were not adjusted for potential confounders |
| Outcome: assessment | Unclear risk | Insufficient information to permit judgement |
| Outcome: follow‐up length | High risk | Followed up to 90 days only |
| Outcome: adequacy of follow‐up | Unclear risk | Insufficient information to permit judgement |
BMI ‐ body mass index; BUN ‐ blood urea nitrogen; CKD ‐ chronic kidney disease; Cr ‐ creatinine; CSPD ‐ conventional‐start PD; DM ‐ diabetes mellitus; KRT ‐ kidney replacement therapy; PD ‐ peritoneal dialysis; PVD ‐ peripheral vascular disease; SD ‐ standard deviation; USPD ‐ urgent‐start PD
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel 2018 | Wrong comparison: compared different methods of catheter insertion |
| Abid 2014 | Control group was not CSPD |
| Alkatheeri 2016 | Control group was not CSPD |
| Banli 2005 | No control group for the study |
| Bhalla 2017 | Wrong comparison: control group was HD patients |
| Bitencourt Dias 2017 | No control group for the study |
| Brabo 2018 | Wrong comparison: control group was HD patients |
| Casaretto 2012 | No control group for the study |
| Davis 2018 | No control group for the study |
| Dias 2016 | No control group for the study |
| Ghaffari 2015 | Wrong comparison: control group was HD patients |
| Jin 2016 | Wrong comparison: control group was HD patients |
| Jin 2018 | Wrong comparison: control group was HD patients |
| Jin 2019 | Wrong comparison: control group was HD patients |
| Jo 2007 | No control group for the study |
| Kim 2016 | No control group for the study |
| Koch 2012 | Wrong comparison: control group was HD patients |
| Li 2017a | Wrong comparison: treatment group was emergent start group which required emergency HD using central venous catheter, followed by insertion of PD catheter but authors did not indicate the urgent PD initiation in this group |
| Liu 2014a | Wrong comparison: control group was HD patients |
| Liu 2018a | Wrong comparison: compared different modality of PD |
| Lobbedez 2008 | Wrong comparison: control group is urgent‐start HD |
| Machowska 2017 | Wrong intervention: study the impact of educational programme on the choice of KRT modality in unplanned kidney failure patients |
| Masseur 2014 | Control group was not CSPD |
| Naljayan 2018 | No control group for the study |
| NCT02946528 | Wrong comparison: compared USPD and HD |
| NCT03474367 | Wrong comparison: compared USPD and HD |
| Povlsen 2006 | Wrong comparison: conventional‐start group initiated PD > 12 days |
| Povlsen 2009 | Review article |
| Serrano 2014 | Wrong definition of USPD: average break‐in period was 15 days for USPD |
| Song 2000 | Wrong intervention: compared the effect of different fill volume on outcomes in USPD patients |
| Soto‐Vargas 2017 | No control group for the study |
| Tannus 2017 | Control arm was HD patients |
| TCTR20140814001 | Comparison of early versus late initiation of dialysis, which are not the subject of the review |
| Vlasak 2017b | No control arm which is CSPD group |
| Wang 2017 | Wrong comparison: control group was HD patients |
| Wong 2016 | No control group for the study |
| Xu 2017 | No control group for the study |
CSPD ‐ conventional‐start peritoneal dialysis; HD ‐ haemodialysis; KRT ‐ kidney replacement therapy; PD ‐ peritoneal dialysis; USPD ‐ urgent‐start peritoneal dialysis
Characteristics of studies awaiting classification [ordered by study ID]
Abdel 2018a.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
|
| Outcomes |
|
| Notes |
|
Differences between protocol and review
The review was conducted as specified in the protocol.
Contributions of authors
Draft the protocol: YC, HH, CH, JC, AT, DJ
Study selection: YC, HH
Extract data from studies: YC, HH
Enter data into RevMan: YC, HH
Carry out the analysis: YC, HH, JC, AT
Interpret the analysis: YC, HH, CH, JC, AT, DJ
Draft the final review: YC, HH, CH, JC, AT, DJ
Disagreement resolution: JC, DJ
Update the review: YC
Sources of support
Internal sources
-
National Health and Medical Research Council, Australia
DJ is supported by Practitioner Fellowship; YC is supported by Early Career Fellowship
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ghaffari 2012 {published data only}
- Ghaffari A. Urgent-start peritoneal dialysis: a quality improvement report. American Journal of Kidney Diseases 2012;59(3):400-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jaivid 2017 {published data only}
- Javaid MM, Lee E, Khan BA, Subramanian S. Description of an urgent-start peritoneal dialysis program in Singapore. Peritoneal Dialysis International 201;37(5):500-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2018 {published data only}
- Kim K, Son KY, Lee SM, Kim SE, An WS. Early technical complications and long-term survival of urgent peritoneal dialysis according to break-in periods. PLoS ONE [Electronic Resource] 2018;13(10):e0206426. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu Y, Zhang L, Lin A, Ni Z, Qian J, Fang W. Impact of break-in period on the short-term outcomes of patients started on peritoneal dialysis. Peritoneal Dialysis International 2014;34(1):49-56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nayak 2018 {published data only}
- Nayak KS, Subhramanyam SV, Pavankumar N, Antony S, Sarfaraz Khan MA. Emergent start peritoneal dialysis for end-stage renal disease: outcomes and advantages. Blood Purification 2018;45(4):313-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pai 2016 {published data only}
- Pai MF, Yang JY, Chen HY, Hsu SP, Chiu YL, Wu HY, et al. Comparing long-term outcomes between early and delayed initiation of peritoneal dialysis following catheter implantation. Renal Failure 2016;38(6):875-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Povlsen 2016 {published data only}
- Holm MO, Ivarsen P, Bech J, Gabor G, Jensen JE, Otte KE, et al. Unplanned start on PD right after PD catheter implantation is associated with inferior PD catheter survival [abstract]. Peritoneal Dialysis International 2010;30(Suppl 2):S106. [EMBASE: 71928087] [Google Scholar]
- Povlsen JV, Otte KE, Larsen PV, Bech JN, Graehn G and Ivarsen P. Unplanned start on PD with less than two weeks of peritoneal rest between PD catheter implantation and initiation of PD is not associated with inferior PD catheter survival [abstract no: MP499]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i506. [EMBASE: 72327350] [Google Scholar]
Salari 2018 {published data only}
- Salari A, Ghaffari A. Catheter-related infections in urgent-start peritoneal dialysis compared to conventional start peritoneal dialysis [abstract]. Peritoneal Dialysis International 2018;38(Suppl 1):S8. [EMBASE: 621290484] [Google Scholar]
- Salari A, Ghaffari A. Long-term technique failure urgent-start peritoneal dialysis compared to conventional start peritoneal dialysis [abstract no: 261]. American Journal of Kidney Diseases 2018;71(4):580-1. [EMBASE: 621595904] [Google Scholar]
See 2017 {published data only}
- See EJ, Cho Y, Hawley CM, Jaffrey LR, Johnson DW. Early and late patient outcomes in urgent-start peritoneal dialysis. Peritoneal Dialysis International 2017;37(4):414-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Serrano 2019 {published data only}
- Serrano A. Urgent-start PD: excellent outcomes at 6 months of dialysis initiation [abstract no: 340]. American Journal of Kidney Diseases 2019;73(5):732. [EMBASE: 2001804437] [Google Scholar]
Silva 2018 {published data only}
- Silva BC, Adelina E, Pereira BJ, Cordeiro L, Rodrigues CE, Duarte RJ, et al. Early start peritoneal dialysis: technique survival in long-term follow-up. Kidney Blood Press Research 2018;43(6):1699-705. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Timely PD 2010 {published data only}
- Ranganathan D, Baer R, Fassett RG, Williams N, Han T, Watson M, et al. Randomised controlled trial to determine the appropriate time to initiate peritoneal dialysis after insertion of catheter to minimise complications (Timely PD study). BMC Nephrology 2010;11:11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan D, John GT, Yeoh E, Williams N, O'Loughlin B, Han T, et al. A randomized controlled trial to determine the appropriate time to initiate peritoneal dialysis after insertion of catheter (Timely PD Study). Peritoneal Dialysis International 2017;37(4):420-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vlasak 2017 {published data only}
- Vlasak J. Are we worried about early complications in urgent-start peritoneal dialysis? [abstract no: SA-PO734]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):868. [Google Scholar]
Wojtaszek 2018 {published data only}
- Wojtaszek E, Grzejszczak A, Grygiel K, Malyszko J, Matuszkiewicz-Rowinska J. Urgent- start peritoneal dialysis as a bridge to definitive chronic renal replacement therapy: short- and long-term outcomes. Frontiers in Physiology 2018;9:1830. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang YF, Wang HJ, Yeh CC, Lin HH, Huang CC. Early initiation of continuous ambulatory peritoneal dialysis in patients undergoing surgical implantation of Tenckhoff catheters. Peritoneal Dialysis International 2011;31(6):551-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- Zhang C, Zhengrong Z, Yuhan G, Jun D, Jinjin R. Application of automated peritoneal dialysis in patients with end-stage renal disease. Chinese Community Doctors 2017;33:13-4. [Google Scholar]
References to studies excluded from this review
Abdel 2018 {published data only}
- Abdel Aal A, Mahmoud K, Moawad S, Ertel N, Hamed B, Ali D, et al. Urgent-start peritoneal dialysis catheter placement: Outcomes of radiologic versus laparoscopic techniques [abstract]. Journal of Vascular and Interventional Radiology 2018;29(4 Suppl 1):S243. [EMBASE: 621352804] [Google Scholar]
Abid 2014 {published data only}
- Abid F, Tevar AD, Bender FH, Piraino B. Successful urgent start peritoneal dialysis [abstract no: 5]. American Journal of Kidney Diseases 2014;63(5):A20. [EMBASE: 71448272] [Google Scholar]
Alkatheeri 2016 {published data only}
- Alkatheeri AM, Blake PG, Gray D, Jain AK. Success of urgent-start peritoneal dialysis in a large Canadian renal program. Peritoneal Dialysis International 2016;36(2):171–6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banli 2005 {published data only}
- Banli O, Altun H, Oztemel A. Early start of CAPD with the Seldinger technique. Peritoneal Dialysis International 2005;25(6):556-9. [MEDLINE: ] [PubMed] [Google Scholar]
Bhalla 2017 {published data only}
- Bhalla NM, Arora N, Darbinian JA, Zheng S. Urgent start dialysis: peritoneal dialysis versus hemodialysis via a central venous catheter [abstract no: TH-PO827]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):314. [Google Scholar]
Bitencourt Dias 2017 {published data only}
- Bitencourt Dias D, Mendes ML, Burgugi Banin V, Barretti P, Ponce D. Urgent-start peritoneal dialysis: the first year of Brazilian experience. Blood Purification 2017;44(4):283–7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brabo 2018 {published data only}
- Brabo AM, Menezes FG, Morgado F, Ponce D. A pilot study on cost evaluation of urgent start automated peritoneal dialysis and hemodialysis in the treatment of end-stage renal disease in Sao Paulo, Brazil [abstract]. Value in Health 2018;21(Suppl 1):S266. [Google Scholar]
Casaretto 2012 {published data only}
- Casaretto A, Rosario R, Kotzker WR, Pagan-Rosario Y, Groenhoff C, Guest S. Urgent-start peritoneal dialysis: report from a U.S. private nephrology practice. Advances in Peritoneal Dialysis 2012;28:102-5. [MEDLINE: ] [PubMed] [Google Scholar]
Davis 2018 {published data only}
- Davis T. Is an urgent start program a sustainable model for initiating peritoneal dialysis? [abstract]. Peritoneal Dialysis International 2018;38(Suppl 1):S3. [EMBASE: 621290453] [Google Scholar]
Dias 2016 {published data only}
- Dias DB, Banin V, Mendes ML, Barretti P, Ponce D. Peritoneal dialysis can be an option for unplanned chronic dialysis: initial results from a developing country. International Urology & Nephrology 2016;48(6):901–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ghaffari 2015 {published data only}
- Ghaffari A, Hashemi N, Ghofrani H, Adenuga G. Urgent-start peritoneal dialysis versus other modalities of dialysis: long-term outcomes [abstract no: 89]. American Journal of Kidney Diseases 2015;65(4):A37. [EMBASE: 71874973] [Google Scholar]
Jin 2016 {published data only}
- Jin H, Fang W, Zhu M, Yu Z, Fang Y, Yan H, et al. Urgent-start peritoneal dialysis and hemodialysis in ESRD patients: complications and outcomes. PLoS ONE [Electronic Resource] 2016;11(11):e0166181. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jin 2018 {published data only}
- Jin H, Ni Z, Mou S, Lu R, Fang W, Huang J, et al. Feasibility of urgent-start peritoneal dialysis in older patients with end-stage renal disease: a single-center experience. Peritoneal Dialysis International 2018;38(2):125-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jin 2019 {published data only}
- Jin H, Ni Z, Che X, Gu L, Zhu M, Yuan J, et al. Peritoneal dialysis as an option for unplanned dialysis initiation in patients with end-stage renal disease and diabetes mellitus. Blood Purification 2019;47(1-3):52-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jo 2007 {published data only}
- Jo YI, Shin SK, Lee JH, Song JO, Park JH. Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Peritoneal Dialysis International 2007;27(2):179-83. [MEDLINE: ] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim SY, Jeong HJ, Lee JY, Lee SM, Oh YJ, Nam HK, et al. Early technical complications and long term survival of urgent peritoneal dialysis according to break-in period [abstract no: SP452]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i243. [EMBASE: 72326554] [Google Scholar]
Koch 2012 {published data only}
- Koch M, Kohnle M, Trapp R, Haastert B, Rump LC, Aker S. Comparable outcome of acute unplanned peritoneal dialysis and haemodialysis. Nephrology Dialysis Transplantation 2012;27(1):375-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2017a {published data only}
- Li WY, Wang YC, Hwan SJ, Lin SH, Wu KD, Chen YM. Comparison of outcomes between emergent-start and planned-start peritoneal dialysis in incident ESRD patients: a prospective observational study. BMC Nephrology 2017;18(1):359. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014a {published data only}
- Liu FX, Ghaffari A, Dhatt H, Kumar V, Balsera C, Wallace E, et al. Economic evaluation of urgent-start peritoneal dialysis versus urgent-start hemodialysis in the United States. Medicine 2014;93(8):e293. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2018a {published data only}
- Liu S, Zhuang X, Zhang M, Wu Y, Liu M, Guan S, et al. Application of automated peritoneal dialysis in urgent-start peritoneal dialysis patients during the break-in period. International Urology & Nephrology 2018;50(3):541-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobbedez 2008 {published data only}
- Lobbedez T, Lecouf A, Ficheux M, Henri P, Hurault de Ligny B, Ryckelynck JP. Is rapid initiation of peritoneal dialysis feasible in unplanned dialysis patients? a single-centre experience. Nephrology Dialysis Transplantation 2008;23(10):3290–4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Machowska 2017 {published data only}
- Machowska A, Alscher MD, Vanga SR, Koch M, Aarup M, Qureshi AR, et al. Offering Patients Therapy Options in Unplanned Start (OPTiONS): Implementation of an educational program is feasible and effective. BMC Nephrology 2017;18(1):18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masseur 2014 {published data only}
- Masseur A, Guest S, Kumar V. Early technique success after initiation of treatment with urgent-start peritoneal dialysis. Advances in Peritoneal Dialysis 2014;30:36-9. [MEDLINE: ] [PubMed] [Google Scholar]
Naljayan 2018 {published data only}
- Naljayan MV, Yazdi F, Reisin E. Using manual exchanges for an urgent-start peritoneal dialysis program. Clinical Kidney Journal 2018;11(5):720-3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02946528 {published data only}
- Ni Z. Urgent-start peritoneal dialysis in ESRD patients: a multi-center study. www.clinicaltrials.gov/ct2/show/NCT02946528 (first received 27 October 2016).
NCT03474367 {published data only}
- Brabo AM, Menezes FG, Morgado F, Ponce D. A pilot study on cost evaluation of urgent start automated peritoneal dialysis and hemodialysis in the treatment of end-stage renal disease in Sao Paulo, Brazil [abstract]. Value in Health 2018;21(Suppl 1):S266. [EMBASE: 623584854] [Google Scholar]
Povlsen 2006 {published data only}
- Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrology Dialysis Transplantation 2006;21 Suppl 2:ii56–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Povlsen 2009 {published data only}
- Povlsen JV. Unplanned start on assisted peritoneal dialysis. Contributions to Nephrology 2009;163:261-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Serrano 2014 {published data only}
- Serrano A. Urgent initiation of peritoneal dialysis: a safe and viable alternative [abstract no: 321]. American Journal of Kidney Diseases 2014;63(5):A99. [EMBASE: 71448588] [Google Scholar]
Song 2000 {published data only}
- Song JH, Kim GA, Lee SW, Kim MJ. Clinical outcomes of immediate full-volume exchange one year after peritoneal catheter implantation for CAPD. Peritoneal Dialysis International 2000;20(2):194-9. [MEDLINE: ] [PubMed] [Google Scholar]
Soto‐Vargas 2017 {published data only}
- Soto-Vargas J, Lopez HR, Vargas Ezquivel MD, Jimenez Mejía CD, Garca-Vera AL, Cortina RA, et al. Urgent-start peritoneal dialysis by the nephrologist [abstract no: TH-PO828]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):314. [Google Scholar]
Tannus 2017 {published data only}
- Tannus G, Sansone D, Farah D, Ramirez MG, Fonseca M. A budget impact analysis of increasing peritoneal dialysis in adults experiencing unplanned start dialysis (urgent start) in Brazil [abstract]. Value in Health 2017;20(9):A577. [EMBASE: 619025783] [Google Scholar]
TCTR20140814001 {unpublished data only}
- Witoon R. A randomized, controlled trial of early versus late initiation of peritoneal dialysis. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1151 (first received 13 August 2014).
Vlasak 2017b {published data only}
- Serrano A, Philip M. Urgent-start peritoneal dialysis in the outpatient setting in an underserved urban area [abstract no: SA-PO740]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):869-70. [Google Scholar]
Wang 2017 {published data only}
- Wang D, Kerns ES, Licht J, Hu SL. The choice of urgent-start peritoneal dialysis versus hemodialysis through a tunneled central venous catheter: a single center experience in the United States [abstract no: SA-PO706]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):861. [Google Scholar]
Wong 2016 {published data only}
- Wong LP, Li NC, Kansal S, Lacson E Jr, Maddux F, Kessler J, et al. Urgent peritoneal dialysis starts for ESRD: initial multicenter experiences in the United States. American Journal of Kidney Diseases 2016;68(3):500-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu D, Liu T, Dong J. Urgent-start peritoneal dialysis complications: prevalence and risk factors. American Journal of Kidney Diseases 2017;70(1):102-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdel 2018a {published data only}
- Abdel Aal A, Mahmoud K, Moawad S, Oser R, Ertel N, Rageeb P, et al. Outcomes of elective versus urgent-start peritoneal dialysis catheter placement [abstract]. Journal of Vascular and Interventional Radiology 2018;29(4 Suppl 1):S103-4. [EMBASE: 621352697] [Google Scholar]
Additional references
AIHW 2016
- Australian Institute of Health and Welfare. Incidence of end-stage kidney disease in Australia 1997–2013. Cat. no. PHE 211. Canberra: AIHW. 2016. www.aihw.gov.au/getmedia/491a8af1-559e-43a0-9862-28b052720777/20044.pdf.aspx?inline=true (accessed 09 November 2020).
Arramreddy 2014
- Arramreddy R, Zheng S, Saxena AB, Liebman SE, Wong L. Urgent-start peritoneal dialysis: a chance for a new beginning. American Journal of Kidney Diseases 2014;63(3):390-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Couser 2011
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney International 2011;80(12):1258-70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dombros 2005
- Dombros N, Dratwa M, Feriani M, Gokal R, Heimburger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 3 Peritoneal access. Nephrology Dialysis Transplantation 2005;20 Suppl 9:ix8-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Figueiredo 2010
- Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Peritoneal Dialysis International 2010;30(4):424-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foote 2014
- Foote C, Clayton PA, Johnson DW, Jardine M, Snelling P, Cass A. Impact of estimated GFR reporting on late referral rates and practice patterns for end-stage kidney disease patients: a multilevel logistic regression analysis using the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). American Journal of Kidney Diseases 2014;64(3):359-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gilg 2016
- Gilg J, Caskey F, Fogarty D. UK Renal Registry 18th Annual Report: Chapter 1 UK Renal Replacement Therapy Incidence in 2014: National and Centre-specific Analyses. Nephron 2016;132 Suppl 1:9-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
ISN 2015
- ISN. World Kidney Day: Chronic Kidney Disease. 2015. www.worldkidneyday.org/faqs/chronic-kidney-disease/ (accessed 9 November 2020).
Jain 2012
- Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. Journal of the American Society of Nephrology 2012;23(3):533-44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
KHA 2012
- KHA. Kidney Health Australia. A model for Home Dialysis. 2012. www.kidney.org.au/cms_uploads/docs/a-model-for-home-dialysis-2012-kha-web.pdf (last accessed 7 December 2017).
KHA 2016
- KHA. Kidney Health Australia. State of the Nation: 2016 Kidney Health Week. Chronic Kidney Disease Hot Spots. www.kidney.org.au/uploads/resources/state-of-the-nation-kidney-health-week-2016-chronic-kidney-disease-hot-spots.pdf (accessed 9 November 2020).
Li 2017
- Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nature Reviews Nephrology 2017;13(2):90-103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehrotra 2016
- Mehrotra R, Devuyst O, Davies SJ, Johnson DW. The current state of peritoneal dialysis. Journal of the American Society of Nephrology 2016;27(11):3238-52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Stegmayr 1990
- Stegmayr B, Hedberg B, Sandzen B, Wikdahl AM. Absence of leakage by insertion of peritoneal dialysis catheter through the rectus muscle. Peritoneal Dialysis International 1990;10(1):53-5. [MEDLINE: ] [PubMed] [Google Scholar]
Tokgoz 2009
- Tokgoz B. Clinical advantages of peritoneal dialysis. Peritoneal Dialysis International 2009;29 Suppl 2:S59-61. [MEDLINE: ] [PubMed] [Google Scholar]
Tzallaloukas 1990
- Tzallaloukas AH, Gibel L J, Eisenberg B, Goldllian RS, Kanig SP, Zager PG et al. Early and Late Peritoneal Dialysate Leaks in Patients on CAPD. Advances in Peritoneal Dialysis 1990;6:64-71. [MEDLINE: ] [PubMed] [Google Scholar]
Zang 2019
- Zang XJ, Yang B, Du X, Mei CL. Urgent-start peritoneal dialysis and patient outcomes: a systematic review and meta-analysis. European Review for Medical & Pharmacological Sciences 2019;23(5):2158-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Htay 2018
- Htay H, Johnson DW, Craig JC, Teixeira-Pinto A, Hawley C, Cho Y. Urgent-start peritoneal dialysis versus conventional-start peritoneal dialysis for people with chronic kidney disease. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012913. [DOI: 10.1002/14651858.CD012913] [DOI] [PMC free article] [PubMed] [Google Scholar]


