Abstract
Background
People with chronic kidney disease (CKD) requiring dialysis are at a particularly high risk of cardiovascular death and morbidity. Several clinical studies suggested that aldosterone antagonists would be a promising treatment option for people undergoing dialysis. However, the clinical efficacy and potential harm of aldosterone antagonists for people with CKD on dialysis has yet to be determined.
Objectives
This review aimed to evaluate the benefits and harms of aldosterone antagonists, both non‐selective (spironolactone) and selective (eplerenone), in comparison to control (placebo or standard care) in people with CKD requiring haemodialysis (HD) or peritoneal dialysis (PD).
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 5 August 2020 using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We included parallel randomised controlled trials (RCTs), cross‐over RCTs, and quasi‐RCTs (where group allocation is by a method that is not truly random, such as alternation, assignment based on alternate medical records, date of birth, case record number, or other predictable methods) that compared aldosterone antagonists with placebo or standard care in people with CKD requiring dialysis.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias for included studies. We used a random‐effects model meta‐analysis to perform a quantitative synthesis of the data. We used the I² statistic to measure heterogeneity among the studies in each analysis. We indicated summary estimates as a risk ratio (RR) for dichotomous outcomes, mean difference (MD) for continuous outcomes, or standardised mean differences (SMD) if different scales were used, with their 95% confidence interval (CI). We assessed the certainty of the evidence for each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) approach.
Main results
We included 16 studies (14 parallel RCTs and two cross‐over RCTs) involving a total of 1446 participants. Thirteen studies compared spironolactone to placebo or standard care and one study compared eplerenone to a placebo. Most included studies had an unclear or high risk of bias. Compared to control, aldosterone antagonists probably reduced the risk of death (any cause) for people with CKD requiring dialysis (9 studies, 1119 participants: RR 0.45, 95% CI 0.30 to 0.67; I² = 0%; moderate certainty of evidence). Aldosterone antagonist probably decreased the risk of death due to cardiovascular disease (6 studies, 908 participants: RR 0.37, 95% CI 0.22 to 0.64; I² = 0%; moderate certainty of evidence) and cardiovascular and cerebrovascular morbidity (3 studies, 328 participants: RR 0.38, 95% CI 0.18 to 0.76; I² = 0%; moderate certainty of evidence). While aldosterone antagonists probably increased risk of gynaecomastia compared with control (4 studies, 768 participants: RR 5.95, 95% CI 1.93 to 18.3; I² = 0%; moderate certainty of evidence), aldosterone antagonists may make little or no difference to the risk of hyperkalaemia (9 studies, 981 participants: RR 1.41, 95% CI 0.72 to 2.78; I² = 47%; low certainty of evidence). Aldosterone antagonists had a marginal effect on left ventricular mass among participants undergoing dialysis (8 studies, 633 participants: SMD ‐0.42, 95% CI ‐0.78 to 0.05; I² = 77%).
In people with CKD requiring dialysis received aldosterone antagonists compared to control, there were 72 fewer deaths from all causes per 1000 participants (95% CI 47 to 98) with a number needed to treat for an additional beneficial outcome (NNTB) of 14 (95% CI 10 to 21) and for gynaecomastia were 26 events per 1000 participants (95% CI 15 to 39) with a number need to treat for an additional harmful outcome (NNTH) of 38 (95% CI 26 to 68).
Authors' conclusions
Based on moderate certainty of the evidence, aldosterone antagonists probably reduces the risk of all‐cause and cardiovascular death and probably reduces morbidity due to cardiovascular and cerebrovascular disease in people with CKD requiring dialysis. For the adverse effect of gynaecomastia, the risk was increased compared to control. For this outcome, the absolute risk was lower than the absolute risk of death. It is hoped the three large ongoing studies will provide better certainty of evidence.
Plain language summary
Aldosterone antagonists for people with chronic kidney disease requiring dialysis
What is the issue?
People with chronic kidney disease (CKD) requiring dialysis are at an increased risk of death and other illnesses. One particular cause of this is an increased risk of heart disease. Aldosterone antagonists (e.g. spironolactone or eplerenone) are one kind of drug for treating high blood pressure that may be a promising treatment option for these people, but which can be associated with adverse effects such as high potassium concentration in the blood (also called hyperkalaemia). This high potassium concentration in the blood may be potentially harmful in people undergoing dialysis.
What did we do?
We searched the literature up until 5 August 2020 and identified 16 studies enrolling 1446 patients with kidney failure undergoing dialysis. These studies compared aldosterone antagonists to placebo (inactive treatment) or usual care.
What did we find?
Based on moderate certainty of the evidence, this review showed that people with CKD taking aldosterone antagonists probably had a reduced risk of death from any cause and a reduced risk of death specifically arising from heart disease. Aldosterone antagonists did not appear to increase potassium concentrations in the blood. However, there is moderate evidence that some men with CKD undergoing dialysis may have an increase development of breast tissue (also called gynaecomastia) as a result of taking aldosterone antagonists.
Conclusions
We concluded that aldosterone antagonists probably reduce the risk of death and heart disease but could increase the risk of gynaecomastia for people on dialysis. More studies are needed to clarify how effective and safe aldosterone antagonists are in people with CKD requiring dialysis.
Summary of findings
Summary of findings 1. Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or standard care) for people with chronic kidney disease requiring dialysis.
| Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or standard care) for people with chronic kidney disease requiring dialysis | |||||
| Patient or population: people with chronic kidney disease requiring dialysis Setting: haemodialysis and peritoneal dialysis Intervention: aldosterone antagonists (spironolactone or eplerenone) Comparison: control (placebo or standard care) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Certainty of the evidence (GRADE) | |
| Risk with control (placebo or standard care) | Risk with aldosterone antagonists (spironolactone or eplerenone) | ||||
| Death (any cause) | 131 per 1,000 | 59 per 1,000 (39 to 88) | RR 0.45 (0.30 to 0.67) | 1119 (9) | ⊕⊕⊕⊝ MODERATE 1 |
| Death (cardiovascular) | 101 per 1,000 | 37 per 1,000 (22 to 65) | RR 0.37 (0.22 to 0.64) | 908 (6) | ⊕⊕⊕⊝ MODERATE 1 |
| Cardiovascular and cerebrovascular morbidity | 133 per 1,000 | 51 per 1,000 (24 to 101) | RR 0.38 (0.18 to 0.76) | 328 (3) | ⊕⊕⊕⊝ MODERATE 1 |
| Hyperkalaemia | 91 per 1,000 | 128 per 1,000 (66 to 253) | RR 1.41 (0.72 to 2.78) | 981 (9) | ⊕⊕⊝⊝ LOW 1 2 |
| Gynaecomastia | 5 per 1,000 | 31 per 1,000 (10 to 95) | RR 5.95 (1.93 to 18.28) | 768 (4) | ⊕⊕⊕⊝ MODERATE 1 |
| Left ventricular mass Measured with different units in the different studies. Lower number mean less hypertrophy |
Left ventricular mass in the aldosterone antagonist group was 0.42 standard deviations lower (0.05 to 0.78 lower) compared to placebo or standard care* | ‐‐ | 562 (7) | ⊕⊝⊝⊝ LOW 1 3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial; RR: Risk ratio; SMD: standardised mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 The total size of the included in the analysis for this outcome were less than optimal information size.
2 There is inconsistency in the definition of hyperkalaemia between the included studies.
3 There is inconsistency in the measurement and calculation methods of left ventricular mass between the included studies.
* Rule of thumb according to Cohen's interpretation of effect size
- < 0.41 represents a small effect
- 0.40 to 0.70 represents and moderate effect
- > 0.70 represents a large effect
Background
Description of the condition
Over 2.6 million people were estimated to have end‐stage kidney disease (ESKD) requiring dialysis worldwide in 2010, with this number expected to more than double by 2030 (Liyanage 2015). Accordingly, the global health burden arising from people with ESKD needing kidney replacement therapy (KRT) has been rapidly increasing in recent years.
People with ESKD requiring dialysis have especially high risk of cardiovascular death and morbidity (Foley 1998; USRDS 2017a). Hypertension, heart failure, and left ventricular hypertrophy are particularly common among people with chronic kidney disease (CKD) undergoing dialysis. The recent epidemiological data based on the US Renal Data System (USRDS) (USRDS 2017a) revealed that the prevalence of any cardiovascular disease was more common in people receiving haemodialysis (HD) (69.8%) than those receiving peritoneal dialysis (PD) (56.6%). The USRDS also showed that 48% of deaths in this population were due to cardiovascular events including arrhythmia/cardiac arrest, acute myocardial infarction and atherosclerotic heart disease, and congestive heart failure (USRDS 2017b). These features greatly contribute to their death and morbidity (Foley 1995; Harnett 1995; Salem 1995; USRDS 2017a; USRDS 2017b).
In healthy people, aldosterone is a mineral corticosteroid hormone produced by the adrenal gland, which regulates water and salt balance in the body by stimulating absorption of sodium and excretion of potassium by the kidneys. However, excess aldosterone induces cardiac fibrosis and vascular damage, and consequently can result in heart failure and kidney injury. In people requiring dialysis, serum aldosterone concentration can be elevated and independently associated with left ventricular hypertrophy (Sato 1999; Steigerwalt 2007). Therefore, it has been postulated that aldosterone antagonists would be a promising therapeutic option for people on dialysis with cardiovascular disease. Moreover, the decreased residual kidney function could result in possible harm because potassium excretion by the kidneys needs tubular flow of urine.
Description of the intervention
Aldosterone antagonists, both non‐selective (spironolactone) and selective (eplerenone), are oral antihypertensive pharmaceutical agents that block the effect of aldosterone at the mineralocorticoid receptors in myocardium, endothelium, and vascular smooth muscles. Administration of aldosterone antagonists may raise serum potassium especially in people without residual kidney function, and the major concern of its use in people requiring dialysis is life‐threatening hyperkalaemia. However, several investigations have suggested that aldosterone antagonists can be safely given to people with ESKD on dialysis (Gross 2005; PHASE 2015; Saudan 2003).
How the intervention might work
Previous studies have shown protective effects of aldosterone antagonists on death and morbidity in people with cardiovascular disease (Pitt 1999; Pitt 2003). Addition of aldosterone antagonists to renin‐angiotensin system (RAS) inhibitors may address problems with the ‘aldosterone escape phenomenon' (Staessen 1981) ‐ a phenomenon which describes the incomplete suppression of serum aldosterone levels with RAS inhibitors alone ‐ and could further improve clinical outcomes of people with heart failure (Yancy 2013).
Recent randomised controlled trials (RCTs) involving people with ESKD who are on dialysis have evaluated the role of both spironolactone and eplerenone in death, morbidity, blood pressure, serum potassium, and on echocardiographic findings including left ventricular mass and ejection fraction (Feniman‐De‐Stefano 2015; Gross 2005; Saudan 2003; Taheri CAPD 2012; Taheri HD 2009). It has been reported that spironolactone may lower blood pressure without also resulting in hyperkalaemia in people receiving HD (Gross 2005; Ni 2014). On the other, one RCT found that eplerenone increased serum potassium levels slightly with no effect on blood pressure (PHASE 2015). Other RCTs have found that spironolactone prevented progression of left ventricular hypertrophy irrespective of blood pressure control in people on PD (Ito 2014) and HD (Feniman‐De‐Stefano 2015). Furthermore, Matsumoto 2014 indicated the effects of spironolactone on cardiovascular and cerebrovascular morbidity and death in people receiving HD in an RCT with a three‐year follow‐up. A more recent RCT has also shown that spironolactone reduced the risk of cardiovascular death and morbidity and improved cardiovascular‐related indexes in people requiring dialysis without heart failure (Lin 2016a).
Why it is important to do this review
Previous systematic reviews have shown that aldosterone antagonists reduces proteinuria and blood pressure but increases hyperkalaemia in people with CKD who are not receiving dialysis, and that it improves survival of patients with chronic heart failure in combination with CKD (Bolignano 2014). Recently, Quach 2016 published a non‐Cochrane systematic review that involved a meta‐analysis of nine studies (829 patients) testing the safety and efficacy of aldosterone antagonists in people receiving dialysis. This investigation reported that patients undergoing dialysis who were treated with aldosterone antagonists had a reduced relative risk (RR) for cardiovascular and death (any cause) compared with control patients (RR 0.34, 95% CI 0.15 to 0.75 and RR 0.40, 95% CI 0.23 to 0.69, respectively). The authors also indicated that aldosterone antagonists tended to cause hyperkalaemia (RR 3.05, 95% CI 1.21 to 7.70). However, there is still some uncertainty about both the benefits and harms of aldosterone antagonists for people with CKD requiring dialysis. Indeed, since completion of the review by Quach 2016, a new, large RCT (n = 258) has been published, which assesses the long‐term (two years) effects and adverse events in this population (Lin 2016a). Furthermore, the meta‐analysis by Quach 2016 only evaluated cardiovascular and death (any cause), hyperkalaemia, blood pressure including hypotension events, and gynaecomastia. The other important surrogate outcomes such as cardiovascular‐related indexes, including left ventricular mass based on echocardiographic findings (which would consequently affect the prognosis of the people requiring dialysis) have never been systematically evaluated. There are also several ongoing trials including ACHIEVE 2017 and ALCHEMIST 2014 which will provide addition data on this important issue. Finally, the financial burden of cardiovascular death and morbidity among dialysis population is huge. Aldosterone antagonists may provide an important low‐cost treatment option if they are found to be clinically effective.
Objectives
This review aimed to assess the benefits and harms of aldosterone antagonists, both non‐selective (spironolactone) and selective (eplerenone), in comparison to placebo or no intervention or standard care in people with ESKD requiring HD or PD.
Methods
Criteria for considering studies for this review
Types of studies
All parallel‐group RCTs, cross‐over trials, and quasi‐RCTs (where group allocation is by a method that is not truly random, such as alternation, assignment based on alternate medical records, date of birth, case record number, or other predictable methods) looking at aldosterone antagonists use, both non‐selective (spironolactone) and selective (eplerenone), in comparison to placebo or no intervention or standard care in people with ESKD requiring HD or PD.
Types of participants
Inclusion criteria
People with ESKD (CKD stage 5D) defined by Kidney Disease: Improving Global Outcomes Clinical Practice Guidelines (Levey 2011), who are being treated with KRT including HD or PD.
Exclusion criteria
We excluded studies involving people with ESKD who have received a kidney transplant.
Types of interventions
We included studies comparing aldosterone antagonists, both non‐selective (spironolactone) and selective (eplerenone), to placebo, no intervention, standard care, or head‐to head studies.
Types of outcome measures
The outcomes selected included the relevant SONG core outcome sets as specified by the Standardised Outcomes in Nephrology initiative (SONG 2017). The outcome measures in studies did not form part of the inclusion/exclusion criteria.
Primary outcomes
Death (any cause)*
Death (cardiovascular)*
Cardiovascular* and cerebrovascular morbidity including but not limited to myocardial infarction, stroke, congestive heart failure
Hyperkalaemia
Secondary outcomes
Left ventricular mass based on echocardiographic findings
Ejection fraction based on echocardiographic findings
Residual kidney function
Serum potassium
Vascular access failure*
Fatigue score*
Gynaecomastia
Peritoneal function (only in people undergoing PD)
*SONG core outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 5 August 2020 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
We used the search strategy described above to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. Studies and reviews that might have included relevant data or information on trials were retained. Two authors independently assessed retrieved abstracts and, if necessary, the full text, of these studies to determine which studies satisfy the inclusion criteria. Any disagreements were resolved through consensus with two authors or by a third author if consensus was not reached.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Depending on available resources, we translated studies reported in non‐English language journals before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions was highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
We expressed dichotomous outcomes (e.g. death, cardiovascular and cerebrovascular morbidity, hyperkalaemia) as RR. We reported continuous outcome data (e.g. left ventricular mass and ejection fraction based on echocardiographic findings, residual kidney function, serum potassium) as mean differences (MD) if the same measurement scales were used, or as standardised mean differences (SMD) if different scales were used. We reported 95% confidence intervals (CI) for all outcomes.
Unit of analysis issues
We did not consider unit of analysis issues because we used data from parallel RCTs only.
Dealing with missing data
We requested further information from the original authors of included studies if this was required (e.g. emailing corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). We interpreted the I² values as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or a confidence interval for I²) (Higgins 2011).
Assessment of reporting biases
Where possible, we used funnel plots to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
We pooled data using the random‐effects model but examined the influence of the fixed‐effect model to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis to explore possible sources of heterogeneity (e.g. participants, interventions, and study quality). We tested the following subgroups.
Dialysis modality: HD or PD.
Adverse effects were tabulated and assessed with descriptive techniques as they were likely to be different for the various agents used. Where possible, the risk difference with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size: repeating the analysis excluding unpublished studies and excluding the studies at high risk of bias for allocation concealment and incomplete outcome data.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables for comparing aldosterone antagonists, both non‐selective (spironolactone) and selective (eplerenone), to placebo, no intervention, or standard care. These tables presented key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also included an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Death (any cause)*
Death(cardiovascular)*
Cardiovascular and cerebrovascular morbidity including but not limited to myocardial infarction, stroke, and congestive heart failure.
Hyperkalaemia
Gynaecomastia
Left ventricular mass based on echocardiographic finding
Results
Description of studies
Results of the search
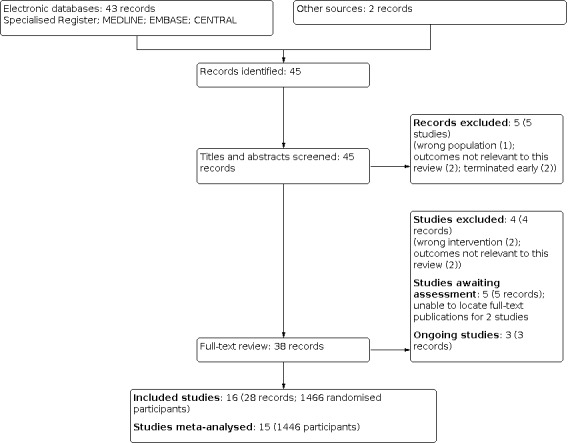
We identified 43 publications (31 studies) from the Cochrane Kidney and Transplant Register of Studies. After screening, nine studies were excluded (Eklund 2016; EPURE 2018; Essaian 2007; Host 2000; Koh 2010; LAST‐D 2013; Nakao 2007; NCT00328809; Witoon 2019), two studies are awaiting classification (Gueiros 2019; NCT02190318), and three studies are ongoing (ACHIEVE 2017; ALCHEMIST 2014: NCT00277693). We identified two additional studies (Song 2017; Wang 2018) from a recent non‐Cochrane systematic review (Li 2019). These studies are awaiting assessment while we attempt to access the full text publications.
A total of 16 studies (28 records, 1466 participants) were included, 9 excluded, 5 studies are awaiting assessment, and there are 3 ongoing studies (Figure 1).
1.
Study flow diagram.
Included studies
See Characteristics of included studies.
Fourteen studies were parallel RCTs (Feniman De Stefano 2015; MiREnDa 2014; Ito 2014; Lin 2016a; Matsumoto 2014; Ni 2014; PHASE 2015; SPIN‐D 2019; Taheri CAPD 2012; Taheri HD 2009; Vazquez‐Rangel 2014; Vukusich 2010; Zaripova 2012; Ziaee 2019), and two were cross‐over studies (Gross 2005; Yongsiri 2015). The results of Vazquez‐Rangel 2014 could not be meta‐analysed because outcomes of interest for this review were not available.
Eleven studies only included HD patients (Feniman De Stefano 2015; Gross 2005; Matsumoto 2014; MiREnDa 2014; Ni 2014; PHASE 2015; SPIN‐D 2019; Taheri HD 2009; Vukusich 2010; Zaripova 2012; Ziaee 2019), four studies only included PD patients (Ito 2014; Taheri CAPD 2012; Vazquez‐Rangel 2014; Yongsiri 2015), and one study included both HD and PD patients (Lin 2016a).
Sample size ranged from 8 to 309 participants. Seven studies were multicentre (Ito 2014; Lin 2016a; Matsumoto 2014; MiREnDa 2014; Ni 2014; PHASE 2015; SPIN‐D 2019) and eight were single‐centre studies (Feniman De Stefano 2015; Gross 2005; Taheri CAPD 2012; Taheri HD 2009; Vukusich 2010; Yongsiri 2015; Zaripova 2012; Ziaee 2019). There were 1147 participants undergoing HD and 299 undergoing PD.
Fourteen studies compared non‐selective aldosterone antagonist (spironolactone) to either placebo (Feniman De Stefano 2015; Gross 2005; MiREnDa 2014; Ni 2014; SPIN‐D 2019; Taheri CAPD 2012; Taheri HD 2009; Vukusich 2010; Yongsiri 2015) or standard care (Ito 2014; Lin 2016a; Matsumoto 2014; Zaripova 2012; Ziaee 2019). One study (PHASE 2015) compared selective aldosterone antagonist (eplerenone) to placebo. The doses were as follows:
-
Spironolactone
25 mg/day: 10 studies (Feniman De Stefano 2015; Ito 2014; Lin 2016a; Matsumoto 2014; Ni 2014; Taheri CAPD 2012; Taheri HD 2009; Vazquez‐Rangel 2014; Yongsiri 2015; Zaripova 2012)
12.5 mg, 25 mg or 50 mg/day: 1 study (SPIN‐D 2019)
50 mg/day: 1 study (MiREnDa 2014)
100 mg/day: 1 study (Gross 2005)
50 mg/HD: 1 study (Vukusich 2010)
25 mg/HD: 1 study (Ziaee 2019).
-
Eplerenone
50 mg/day: 1 study (PHASE 2015).
The following reported outcomes were included in quantitative syntheses for this review:
Death (any cause): nine studies (Ito 2014; Lin 2016a; Matsumoto 2014; MiREnDa 2014; PHASE 2015; Taheri CAPD 2012Taheri HD 2009; Vukusich 2010; Ziaee 2019) (1119 participants)
Death (cardiovascular): six studies (Ito 2014; Lin 2016a; Matsumoto 2014; PHASE 2015; Taheri CAPD 2012Taheri HD 2009) (908 participants)
-
Cardiovascular and cerebrovascular morbidity: three studies ( Ito 2014; PHASE 2015; Taheri HD 2009) (328 participants)
Ito 2014 only reported acute myocardial infarction events
Taheri HD 2009 reported hospitalisation due to cardiovascular events
PHASE 2015 reported fatal or nonfatal cardiovascular events.
-
Hyperkalaemia: nine studies (SPIN‐D 2019; MiREnDa 2014; Ito 2014; Matsumoto 2014; Ni 2014; Taheri HD 2009; Taheri CAPD 2012; PHASE 2015; Yongsiri 2015) (981 participants)
Hyperkalaemia was defined as serum potassium level > 5.0 mEq/L (Ni 2014); > 5.5 mEq/L (Taheri CAPD 2012; Taheri HD 2009; Yongsiri 2015); > 6.0 mEq/L (MiREnDa 2014; Ito 2014); > 6.5 mEq/L (Matsumoto 2014; SPIN‐D 2019); and > 6.8 mEq/L (Matsumoto 2014).
Gynaecomastia: four studies (Ito 2014; Lin 2016a; Matsumoto 2014; Ziaee 2019) (768 participants)
Left ventricular mass: eight studies (Feniman De Stefano 2015; Ito 2014; Lin 2016a; MiREnDa 2014; SPIN‐D 2019; Taheri HD 2009; Zaripova 2012; Ziaee 2019) (633 participants)
Ejection fraction: seven studies (Feniman De Stefano 2015; MiREnDa 2014; Ito 2014; Lin 2016a; Taheri HD 2009; Taheri CAPD 2012; Ziaee 2019) (458 participants)
Serum potassium: seven studies (SPIN‐D 2019; Feniman De Stefano 2015; Gross 2005; Lin 2016a; Ni 2014; ; Yongsiri 2015; Ziaee 2019) (519 participants)
Residual kidney function (urine volume/day): three studies (Gross 2005; Ni 2014; Yongsiri 2015) (132 participants)
Vascular access failure, fatigue score, and peritoneal function: the data for these outcomes were either not reported in an extractable format, or not reported in any of the included studies.
Excluded studies
See Characteristics of excluded studies.
We excluded nine studies for the following reasons.
Wrong population (EPURE 2018)
Wrong interventions (Nakao 2007; Witoon 2019)
Not designed to measure the outcomes of interest (Eklund 2016; Essaian 2007; Host 2000; Koh 2010)
Withdrawn without being completed (LAST‐D 2013; NCT00328809).
Risk of bias in included studies
The assessment of risk of bias in the included studies are shown in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Random sequence generation was judged to be at low risk of bias in five studies (Lin 2016a; MiREnDa 2014; PHASE 2015; SPIN‐D 2019; Yongsiri 2015) and at high risk of bias in one quasi‐RCT (odd or even days) (Ziaee 2019). The risk of bias was unclear in 10 studies (Feniman De Stefano 2015; Gross 2005; Ito 2014; Matsumoto 2014; Ni 2014; Taheri CAPD 2012; Taheri HD 2009; Vazquez‐Rangel 2014; Vukusich 2010; Zaripova 2012).
Allocation concealment
Allocation concealment was judged to be at low risk of bias in five studies (Lin 2016a; MiREnDa 2014; PHASE 2015; SPIN‐D 2019; Yongsiri 2015) and at high risk of bias in one quasi‐randomised study (odd or even days) (Ziaee 2019). The risk of bias was unclear in 10 studies (Feniman De Stefano 2015; Gross 2005; Ito 2014; Matsumoto 2014; Ni 2014; Taheri CAPD 2012; Taheri HD 2009; Vazquez‐Rangel 2014; Vukusich 2010; Zaripova 2012).
Blinding
Performance bias
Performance bias (blinding of participants and research personnel) was judged to be at low risk of bias in 12 studies (Feniman De Stefano 2015; Gross 2005; Lin 2016a; MiREnDa 2014; Ni 2014; PHASE 2015; SPIN‐D 2019; Taheri CAPD 2012; Taheri HD 2009; Vazquez‐Rangel 2014; Vukusich 2010; Yongsiri 2015). Three studies were open‐label and judged to be high risk of bias (Ito 2014; Matsumoto 2014; Zaripova 2012), and in Ziaee 2019 the intervention group were given spironolactone (aldactone) acquired on the market after each HD session and was also judged to be at high risk of bias.
Detection bias
Detection bias (blinding of outcome assessors) was judged to be at low risk of bias in 13 studies (Feniman De Stefano 2015; Gross 2005; Lin 2016a; MiREnDa 2014; Ni 2014; PHASE 2015; SPIN‐D 2019; Taheri CAPD 2012; Taheri HD 2009; Vazquez‐Rangel 2014; Vukusich 2010; Yongsiri 2015; Ziaee 2019). Three studies were open‐label and judged to be high risk of bias (Ito 2014; Matsumoto 2014; Zaripova 2012).
Incomplete outcome data
Eight studies were judged to be at as low risk for attrition bias because almost all of the participants were followed up and missing outcome data balanced across the intervention and control groups (Feniman De Stefano 2015; Gross 2005; MiREnDa 2014; Ni 2014; PHASE 2015; SPIN‐D 2019; Taheri CAPD 2012; Taheri HD 2009). Six studies were judged to be at high risk for attrition bias as more than 10% of participants were not followed up and there was imbalance in numbers for missing data across the intervention and control groups (Ito 2014; Lin 2016a; Matsumoto 2014; Vukusich 2010; Yongsiri 2015; Ziaee 2019). One study with incomplete outcome data was judged unclear (Zaripova 2012).
Selective reporting
All the prespecified outcomes were reported in seven studies (SPIN‐D 2019; Feniman De Stefano 2015; MiREnDa 2014; Ito 2014; Vazquez‐Rangel 2014; Vukusich 2010; Yongsiri 2015). The remaining nine studies were classified as unclear for reporting bias because their study protocol was not available (Gross 2005; Lin 2016a; Matsumoto 2014; Ni 2014; Taheri CAPD 2012Taheri HD 2009; PHASE 2015; Ziaee 2019; Zaripova 2012).
Other potential sources of bias
We judged eight studies to be at low risk of bias due to funding (Feniman De Stefano 2015; MiREnDa 2014; Ito 2014; SPIN‐D 2019; Taheri HD 2009; Vazquez‐Rangel 2014; Vukusich 2010; Yongsiri 2015). One study was judged to be at high risk of bias because it was partly funded by a pharmaceutical company (PHASE 2015). The risk of bias were judged to be unclear in the remaining seven studies as there was no reported information on funding (Gross 2005; Lin 2016a; Matsumoto 2014; Ni 2014; Taheri HD 2009; Ziaee 2019; Zaripova 2012).
Effects of interventions
See: Table 1
See Table 1 (Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or standard care) for people with chronic kidney disease requiring dialysis)
Death (any cause)
Aldosterone antagonists probably reduces the risk of death (any cause) compared to control interventions (Analysis 1.1 (9 studies, 1119 participants): RR 0.45, 95% CI 0.30 to 0.67; I² = 0%; moderate certainty of evidence).
1.1. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 1: Death (any cause)
In people with CKD requiring dialysis received aldosterone antagonists compared to control, there were 72 fewer deaths from all causes per 1000 participants (95% CI 47 to 98) with a number needed to treat for an additional beneficial outcome (NNTB) of 14 (95% CI 10 to 21).
Death (cardiovascular)
Aldosterone antagonists probably reduces the risk of death due to cardiovascular events compared to control interventions (Analysis 1.2 (6 studies, 908 participants): RR 0.37, 95% CI 0.22 to 0.64; I² = 0%; moderate certainty of evidence).
1.2. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 2: Death: cardiovascular
In people receiving aldosterone antagonists compared to control, there were 64 fewer deaths from cardiovascular events per 1000 participants (95% CI 42 to 87) with a NNTB of 16 (95% CI 12 to 24).
Cardiovascular and cerebrovascular morbidity
Aldosterone antagonists probably reduces the risk of cardiovascular and cerebrovascular morbidity compared to control interventions (Analysis 1.3 (3 studies, 328 participants): RR 0.38, 95% CI 0.18 to 0.76; I² = 0%; moderate certainty of evidence).
1.3. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 3: Cardiovascular and cerebrovascular morbidity
In people receiving aldosterone antagonists compared to control, there were 82 fewer cardiovascular and cerebrovascular events per 1000 participants (95% CI 57 to 108) with a NNTB of 12 (95% CI 9 to 18).
Hyperkalaemia
Aldosterone antagonists may make little or no difference to the risk of hyperkalaemia compared to control interventions (Analysis 1.4 (9 studies, 981 participants): RR 1.41, 95% CI 0.72 to 2.78; I² = 47%; low certainty of evidence).
1.4. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 4: Hyperkalaemia
In people receiving aldosterone antagonists compared to control, there were 37 more hyperkalaemia per 1000 participants (95% CI 10 to 65) with a number needed to treat for an additional harmful outcome (NNTH) of 27 (95% CI 16 to 104).
Gynaecomastia
Aldosterone antagonists probably increases the risk of gynaecomastia compared to control interventions (Analysis 1.5 (4 studies, 768 participants): RR 5.95, 95% CI 1.93 to 18.28; I² = 0%; moderate certainty of evidence).
1.5. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 5: Gynaecomastia
In people receiving aldosterone antagonists compared to control, there were 26 more gynaecomastia per 1000 participants (95% CI 15 to 39) with a NNTH of 38 (95% CI 26 to 68).
Left ventricular mass
Aldosterone antagonists may prevent left ventricular mass hypertrophy compared to control interventions (Analysis 1.6 (8 studies, 633 participants): SMD ‐0.42, 95% CI ‐0.78 to ‐0.05; I² = 77%; low certainty evidence).
1.6. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 6: Left ventricular mass
Because of the significant heterogeneity among the included studies for left ventricular mass, we carried out the subgroup analyses by dialysis modality (HD or PD). In this analysis Lin 2016a was excluded as it included both HD and PD patients. There were no differences in left ventricular mass found between the aldosterone antagonists and control interventions for either HD participants (Analysis 1.7.1 (6 studies, 342 participants): SMD ‐0.40, 95% CI ‐0.92 to 0.12; I² = 79%) or in Ito 2014 which only included PD participants (Analysis 1.7.2 (1 study, 93 participants): SMD ‐0.37, 95% CI ‐0.78 to 0.04). Heterogeneity was still high.
1.7. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 7: Left ventricular mass: haemodialysis or peritoneal dialysis
Ejection fraction
Aldosterone antagonists may make little or no difference to preserving ejection fraction compared to control interventions (Analysis 1.8 (7 studies, 458 participants): MD 3.15, 95% CI ‐0.74 to 7.04; I² = 84%).
1.8. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 8: Ejection fraction
Subgroup analyses (HD or PD) were also performed because of the significant heterogeneity among the included studies for left ejection fraction. There was no evidence that aldosterone antagonists altered ejection fraction in comparison to control interventions for either HD participants (Analysis 1.9.1 (4 studies, 156 participants): MD 3.13, 95% CI ‐4.25 to 10.51; I² = 84%) or PD participants (Analysis 1.9.2 (2 studies, 104 participants): MD 2.31, 95% CI ‐1.99 to 6.61; I² = 0%).
1.9. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 9: Ejection fraction: haemodialysis or peritoneal dialysis
Serum potassium
Aldosterone antagonists may make little or no difference to serum potassium concentration compared to control interventions (Analysis 1.10 (7 studies, 519 participants): MD 0.21, 95% CI ‐0.06 to 0.47; I² = 84%).
1.10. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 10: Serum potassium
Subgroup analyses (HD or PD) were also performed because there was significant heterogeneity among the included studies for serum potassium due to Lin 2016a which included both HD and PD patients. There was no evidence of differences in serum potassium concentration between the intervention and control groups for HD participants (Analysis 1.11.1 (5 studies, 281 participants): MD 0.07 mmol/L, 95% CI ‐0.05 to 0.20; I² = 0%) or in Ito 2014 which only included PD participants (Analysis 1.11.2 (1 study, 40 participants): MD ‐0.01 mmol/L, 95% CI ‐0.35 to 0.33).
1.11. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 11: Serum potassium: haemodialysis or peritoneal dialysis
Residual kidney function
Aldosterone antagonists may make little or no difference to residual kidney function compared to control interventions (Analysis 1.12 (3 studies, 132 participants): MD ‐36.23 urine volume/day, 95% CI ‐114.61 to 42.15; I² = 0%).
1.12. Analysis.

Comparison 1: Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care), Outcome 12: Residual kidney function [urine volume/day]
Discussion
Summary of main results
Based on the results from our systematic reviews and meta‐analysis, we found that aldosterone antagonists (spironolactone or eplerenone) probably reduced all‐cause and cardiovascular death and probably reduced cardiovascular and cerebrovascular morbidity compared to control interventions (placebo or standard care) for people with CKD requiring dialysis.
Aldosterone antagonists made little or no difference to the risk of hyperkalaemia and increased serum potassium. However, aldosterone antagonists probably increased the risk of gynaecomastia compared with control interventions. Although the relative risk of gynaecomastia with aldosterone antagonists was increased compared with controls, in people receiving aldosterone antagonists there were 64 fewer deaths from cardiovascular events per 1000 participants (95% CI 42 to 87) with a NNTB of 16 (95% CI 12 to 24) and for gynaecomastia there were 26 more reports of gynaecomastia per 1000 participants (95% CI 15 to 39) with a NNTH of 38 (95% CI 26 to 68).
Aldosterone antagonists may prevent left ventricular mass hypertrophy. Aldosterone antagonists may make little or no difference to ejection fraction or residual kidney function among participants undergoing dialysis.
Overall completeness and applicability of evidence
In terms of completeness, we performed a comprehensive systematic search strategy of the Cochrane Kidney and Transplantation's Register. As shown in Characteristics of included studies, the analyses included studies with participants receiving HD (Feniman De Stefano 2015; Gross 2005; Matsumoto 2014; MiREnDa 2014; Ni 2014; PHASE 2015; SPIN‐D 2019; Taheri HD 2009; Vukusich 2010; Zaripova 2012; Ziaee 2019), PD (Ito 2014; Taheri CAPD 2012; Yongsiri 2015) or both (Lin 2016a), and most studies reported the inclusion and exclusion criteria. These included studies were conducted from the late 2000s through to the late 2010s. During this period, only spironolactone was available for people with CKD requiring dialysis. Therefore, we judged that our review had high external validity. However, there was little evidence for selective aldosterone antagonists (eplerenone) (PHASE 2015).
Quality of the evidence
We assessed the certainty of the evidence using the grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (GRADE 2008; GRADE 2011). As shown in the Table 1, we graded the overall certainty of the body of the evidence in this review. We rated the certainty of the evidence for the patient‐focused outcomes including death (any cause), death (cardiovascular), cardiovascular and cerebrovascular morbidity, and adverse events (gynaecomastia) as moderate because the total size of the included in the analysis for these outcomes were less than the optimal information size (OIS) of 400 (Guyatt 2011) and because three of the 14 included studies did not used a placebo intervention for the control group. The certainty of the evidence for the adverse events (hyperkalaemia) was also rated as low because the number of the included participants did not meet the OIS and because there was inconsistency in the definition of hyperkalaemia between the included studies. The certainty of the evidence for surrogate endpoint (left ventricular mass) was assessed as very low because of the risk of bias issue regarding the lack of blinding of outcomes, inconsistency in the definition of left ventricular mass between the included studies, and the number of the included participants did not meet the OIS. As shown in Figure 2 and Figure 3, some of the included articles did not adequately reported on key aspects of the study methods which could result in an increased risk of selection, reporting, and other (especially due to funding) bias. Moreover, attrition bias due to incomplete outcome data should be considered in this review.
Potential biases in the review process
We conducted this review on the basis of the peer‐reviewed protocol and a comprehensive systematic search strategy using the Cochrane Kidney and Transplant's Specialised Register. Two independent authors evaluated the quality of the studies, extracted, and analysed the data. However, this review has several limitations. First, long‐term data on the effects of aldosterone antagonists was limited for patient‐focused outcomes including mortality (all‐cause or cardiovascular), and cardiovascular and cerebrovascular morbidity. Further, some of the analysed data for primary outcomes of death (all‐cause) from the included studies were considered at high risk of selection bias due to obscure or inadequate random sequence allocation and allocation concealment (Ito 2014; Matsumoto 2014; Taheri CAPD 2012; Taheri HD 2009; Vukusich 2010; Ziaee 2019). Some studies were also at risk of performance and detection bias due to lack of blinding ((Ito 2014; Matsumoto 2014; Ziaee 2019), and attrition bias due to incomplete outcome data (Ito 2014; Lin 2016a; Matsumoto 2014; Vukusich 2010; PHASE 2015; Ziaee 2019). Therefore, although the point estimates of risk reduction were substantial, we recognised that the data of this review are insufficient to reliably determine whether aldosterone antagonists are truly beneficial for the participants with CKD requiring dialysis. We also consider the integration of the results of hyperkalaemia incidence, defined at different thresholds in each study, as one of the limitations in the interpretation of this systematic review. Finally, despite a comprehensive systematic search strategy of Cochrane Kidney and Transplant's Specialised Register, publication bias may exist in this review (Figure 4).
4.

Funnel plot of comparison: 1 Aldosterone antagonists (Spironolactone or eplerenone) versus control (placebo or standard care), outcome: 1.1 Death (any cause).
Agreements and disagreements with other studies or reviews
Findings from our review were similar to those reported in a previous systematic review (Quach 2016). Compared to control, aldosterone antagonists reduced the risk of all‐cause and cardiovascular death among participants with CKD undergoing dialysis with a similar effect size reported in both this and the prior review. Although these findings suggested that aldosterone antagonists could provide clinical benefit to people with CKD requiring dialysis, the potential adverse events such as hyperkalaemia should also be considered. The previous meta‐analysis by Quach 2016 showed that aldosterone antagonists could increase the risk of severe hyperkalaemia among participants with CKD undergoing dialysis. However, the association between increased risk of hyperkalaemia with aldosterone antagonists was uncertain in this review. Quach 2016 only reported the greater overall incidence of gynaecomastia in the aldosterone antagonist arms compared to controls.
Gynaecomastia, a known side effect of aldosterone antagonists, was increased compared to controls in one study reported in Quach 2016. Our analysis included three additional studies for this outcome with a similar finding. Of note, all of the studies included in the gynaecomastia analysis used spironolactone and this outcome was not reported in the one study using eplerenone (PHASE 2015).
A non‐Cochrane systematic review was recently published that examined the clinical effects of aldosterone antagonists on the following outcomes: death (any cause), cardiovascular death, adverse effects, and cardiac function (left ventricular mass, ejection fraction) in patients on dialysis (Li 2019). There were some differences between the results of our systematic review and that of Li 2019. One key reason for these differences arises from the variation in the studies included in the review. In particular, we included three studies published in 2019 (SPIN‐D 2019; MiREnDa 2014; Ziaee 2019) that were not included in Li 2019. Our search strategy (based on the Cochrane Kidney and Transplant Register) differed from Li 2019, and did not identify two studies written in Chinese (Song 2017; Wang 2018). Li 2019 identified these studies through the Chinese Biomedical Literature Database and the China National Knowledge Infrastructure, which are not searched as part of the methods used for the Cochrane Kidney and Transplant Register. We were not able to access the full text reports on these additional two studies identified by Li 2019 at the time of our Cochrane review, however, so have categorised these studies as awaiting classification and will be assessed in a future update of this review. In summary, our systematic review includes all the studies included in Li 2019 (with the exception of Song 2017 and Wang 2018), plus an additional six studies (Table 2).
1. Comparison of included studies between previous non‐Cochrane systematic reviews and current Cochrane systematic review.
| Systematic reviews | Quach 2016 | Li 2019 | Hasegawa 2021 a |
| Number of included studies | 9 | 10 | 16 |
| Number of analysed participants | 829 | 1172 | 1446 |
| Included studies | ‐ | Feniman De Stefano 2015 | Feniman De Stefano 2015 |
| Gross 2005 | ‐ | Gross 2005 | |
| Ito 2014 | Ito 2014 | Ito 2014 | |
| ‐ | Lin 2016a | Lin 2016a | |
| Matsumoto 2014 | Matsumoto 2014 | Matsumoto 2014 | |
| ‐ | ‐ | MiREnDa 2014 | |
| Ni 2014 | ‐ | Ni 2014 | |
| PHASE 2015 | PHASE 2015 | PHASE 2015 | |
| ‐ | Song 2017b | ‐ | |
| ‐ | ‐ | SPIN‐D 2019 | |
| Taheri CAPD 2012 | Taheri CAPD 2012 | Taheri CAPD 2012 | |
| Taheri HD 2009 | Taheri HD 2009 | Taheri HD 2009 | |
| ‐ | ‐ | Vazquez‐Rangel 2014c | |
| Vukusich 2010 | ‐ | Vukusich 2010 | |
| ‐ | Wang 2018b | ‐ | |
| Yongsiri 2015 | ‐ | Yongsiri 2015 | |
| ‐ | Zaripova 2012 | Zaripova 2012 | |
| ‐ | ‐ | Ziaee 2019 |
a Current Cochrane review
b No data available for this review
c Not meta‐analysed
In this review we dichotomized the onset of hyperkalaemia according to the definition used in each study and meta‐analysed the results whereas Li 2019 use a threshold of K > 6 mEq/L to define hyperkalaemia. In PHASE 2015, significant hyperkalaemia was defined as K > 6.5 mEq/L, above the threshold of adopted by Li 2019 for their sensitivity analysis. We therefore used the threshold of hyperkalaemia of K > 6.5 mEq/L in the PHASE study (PHASE 2015), which is consistent with the prior non‐Cochrane systematic review (Quach 2016). In addition, Li 2019 used per‐protocol analysis to estimate the RR of each study in a meta‐analysis of gynaecomastia. This method differs from those adopted by us and Quach 2016 which used the principles of intention‐to‐treat analysis.
We found a small effect of aldosterone antagonists on preventing left ventricular mass hypertrophy based on echocardiographic findings, which is similar to that of the systematic review by Li 2019. We observed a more conservative effect of aldosterone antagonists on ejection fraction based on echocardiographic findings in our systematic review compared to Li 2019. We consider this difference attributable to Li 2019 not including the two most recent studies (MiREnDa 2014; Ziaee 2019). We consider that the differences in analysis methods described above led to small differences in point estimates and 95% CI in Li 2019 and our systematic review.
Authors' conclusions
Implications for practice.
In people with CKD requiring dialysis, aldosterone antagonists probably reduces the risk of death (any cause and cardiovascular) and morbidity due to cardiovascular and cerebrovascular disease. Although non‐selective aldosterone antagonists (spironolactone) may increase the risk of gynaecomastia in people with CKD requiring dialysis, its incidence and clinical significance are not comparable to those of aldosterone antagonists in reducing the risk of death or cardiovascular disease. However, there was no clear association between hyperkalaemia and/or serum potassium elevation with aldosterone antagonists in this review. There is currently insufficient data to draw conclusions about the effect of aldosterone antagonists on echocardiographic findings such as left ventricular mass and ejection fraction. We should recognise that these findings were based on very low to moderate certainty of the evidence. There are three ongoing studies investigating this issue and these will strengthen the evidence.
Implications for research.
Some of the included studies in this review were affected by certain selection bias due to inappropriate random sequence generation or/and allocation concealment. Lack of blinding of participants and outcome assessors also biased the findings from some studies. There was more concern that the potential outcomes of participants in the included studies could largely affect the results of the meta‐analysis in this review. Moreover, the low sample size in studies included in this review might mean some clinically significant findings may have been missed. Therefore, it is important to plan the methods carefully in future studies to avoid these selection and information biases.
Following spironolactone and eplerenone, the third‐generation of aldosterone antagonists has recently become available for clinical use. It is expected that the third‐generation aldosterone antagonists would be better tolerated in individuals with kidney dysfunction compared to spironolactone and eplerenone. Future systematic reviews should incorporate the findings from clinical studies of the third‐generation aldosterone antagonists not yet published.
Due to the small number of included studies eligible for subgroup analysis and the lack of information on each study, we were not able to perform the following subgroup analyses (residual kidney function, diabetic kidney disease, and concomitant use of RAS inhibitors) as planned in the protocol. It would also be important to investigate the difference in the clinical efficacy of aldosterone antagonists in subgroups of patients with or without pre‐existing comorbidities such as cardiovascular disease and heart failure.
We believe that patient‐oriented outcome measures as listed in the SONG core outcomes (SONG 2017) such as fatigue score are also required to be examined in the future.
Additionally, there has been no cost‐effective analyses of aldosterone antagonists among people undergoing dialysis. These gaps in the evidence base indicate that larger and well‐designed clinical RCTs are required to clarify the efficacy of aldosterone antagonists in people with CKD requiring dialysis.
History
Protocol first published: Issue 8, 2018 Review first published: Issue 2, 2021
Acknowledgements
We would like to thank Gail Higgins, the Information Specialist for the Cochrane Kidney and Transplant Group for assistance in the development of our search strategy. The authors are grateful to Fiona Russell and Narelle Willis of the Cochrane Kidney and Transplant Group, for their help in coordinating this review and the peer reviewers for their editorial advice and feedback during the preparation of this review. We also thank Dr Yasuhiko Ito for providing additional detailed information regarding his publication included in this review. The authors are grateful to the following peer reviewers for their time and comments: Bertram Pitt MD (University of Michigan School of Medicine); Anna Mathew, MD, MPH, FRCP(C) (Associate Professor of Medicine, McMaster University). This work was supported by JSPS KAKENHI Grant Number 19K03092 and undertaken as part of a program (Showa University Next Leaders in Study and Education: SUNLiSE) organized by Showa University Research Administration Center (SURAC). We also thank Tomomi Kodama, Tomoki Ishikawa, Toru Takei, Kenji Suzuki, and Kenji Ishizaki of SURAC for their assistance as University Research Administrator.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Aldosterone antagonists (spironolactone or eplerenone) versus control (placebo or usual care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death (any cause) | 9 | 1119 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.30, 0.67] |
| 1.2 Death: cardiovascular | 6 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.22, 0.64] |
| 1.3 Cardiovascular and cerebrovascular morbidity | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.18, 0.76] |
| 1.4 Hyperkalaemia | 9 | 981 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.72, 2.78] |
| 1.5 Gynaecomastia | 4 | 768 | Risk Ratio (M‐H, Random, 95% CI) | 5.95 [1.93, 18.28] |
| 1.6 Left ventricular mass | 8 | 633 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.78, ‐0.05] |
| 1.7 Left ventricular mass: haemodialysis or peritoneal dialysis | 7 | 435 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.79, 0.04] |
| 1.7.1 Haemodialysis only | 6 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.92, 0.12] |
| 1.7.2 Peritoneal dialysis only | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.78, 0.04] |
| 1.8 Ejection fraction | 7 | 458 | Mean Difference (IV, Random, 95% CI) | 3.15 [‐0.74, 7.04] |
| 1.9 Ejection fraction: haemodialysis or peritoneal dialysis | 6 | 260 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐2.34, 7.48] |
| 1.9.1 Haemodialysis only | 4 | 156 | Mean Difference (IV, Random, 95% CI) | 3.13 [‐4.25, 10.51] |
| 1.9.2 Peritoneal dialysis only | 2 | 104 | Mean Difference (IV, Random, 95% CI) | 2.31 [‐1.99, 6.61] |
| 1.10 Serum potassium | 7 | 519 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.06, 0.47] |
| 1.11 Serum potassium: haemodialysis or peritoneal dialysis | 6 | 321 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.18] |
| 1.11.1 Haemodialysis only | 5 | 281 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.05, 0.20] |
| 1.11.2 Peritoneal dialysis only | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.35, 0.33] |
| 1.12 Residual kidney function [urine volume/day] | 3 | 132 | Mean Difference (IV, Random, 95% CI) | ‐36.23 [‐114.61, 42.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Feniman De Stefano 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind", "placebo‐controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind", "placebo‐controlled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported in ClinicalTrials.gov identifier NCT01128101 |
| Other bias | Low risk | Funded by FUNDUNESP (Foundation for the Development of UNESP, Process 0090910) and FAPESP (Foundation for Research Support of São Paulo, Process 2010/10439‐1) |
Gross 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind", "placebo‐controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind", "placebo‐controlled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Funding: not reported |
Ito 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported in The University Hospital Medical Information Network Clinical Trials Registry as UMIN000492 |
| Other bias | Low risk | This study was supported in part by a Grant‐in‐Aid for Progressive Renal Disease Research, Research on Rare and Intractable Diseases, from the Ministry of Health, Labor and Welfare of Japan |
Lin 2016a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation sequence was generated independently by a nurse and concealed in opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "Both investigators and participants were not aware of the allocations." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "placebo‐controlled" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol: not reported |
| Other bias | Unclear risk | Funding: not reported |
Matsumoto 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label" study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open‐label" study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol: not reported |
| Other bias | Unclear risk | Funding: not reported |
MiREnDa 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated 1:1 to either 50 mg spironolactone once daily or matching placebo using an algorithm for balanced randomised assignment for stratified randomizations." |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly allocated 1:1 to either 50 mg spironolactone once daily or matching placebo using an algorithm for balanced randomised assignment for stratified randomizations." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "MiREnDa was an investigator‐initiated, randomised, double‐blind, placebo‐ controlled trial to study the efficacy and safety of spironolactone in HD patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "MiREnDa was an investigator‐initiated, randomised, double‐blind, placebo‐ controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported in ClinicalTrials.gov identifier NCT01691053 |
| Other bias | Low risk | The MiREnDa trial was funded by the German Federal Ministry of Education and Research (01KG1202) and conducted under the auspices of the German Society of Nephrology. Additional funding was obtained from E.N.D.I.—the European Nephrology and Dialysis Institute |
Ni 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind" study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unclear |
| Other bias | Unclear risk | Funding: not reported |
PHASE 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible participants were randomly allocated to eplerenone or placebo using a computerized web‐based randomisation system maintained by the coordinating canter (the Population Health Research Institute)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible participants were randomly allocated to eplerenone or placebo using a computerized web‐based randomisation system maintained by the coordinating centre (the Population Health Research Institute)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, health care providers, study personnel, and outcome assessors were all blind to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants, health care providers, study personnel, and outcome assessors were all blind to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified protocol unclear |
| Other bias | High risk | Funding: this trial was partly funded by pharmaceutical company |
SPIN‐D 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random number generator, the data coordinating centre prepared permuted blocks of random sizes with stratification by centre" |
| Allocation concealment (selection bias) | Low risk | Quote: "Web‐based randomisation was performed with participants and all research personnel masked to the treatment assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Web‐based randomisation was performed with participants and all research personnel masked to the treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Web‐based randomisation was performed with participants and all research personnel masked to the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Quote: "The complete list of primary and secondary outcomes with definitions is provided in Supplementary Table S1." |
| Other bias | Low risk | Quote: "This trial was funded by the following cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases: U01DK096189, U01DK099923, U01DK099914, and U01DK099919." |
Taheri CAPD 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind placebo‐controlled" clinical study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind placebo‐controlled" clinical study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol unclear |
| Other bias | Low risk | Funding: internally funded |
Taheri HD 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind placebo‐controlled" study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind placebo‐controlled" study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol unclear |
| Other bias | Unclear risk | Funding: not reported |
Vazquez‐Rangel 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind placebo‐controlled" study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind placebo‐controlled" study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported in ClinicalTrials.gov identifier NCT00865449 |
| Other bias | Low risk | This research was supported by the Mexican Kidney Foundation and the Miguel Aleman Foundation |
Vukusich 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo‐controlled randomised clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "placebo‐controlled randomised clinical trial" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes have been reported in study protocol in materials and methods section |
| Other bias | Low risk | This research was supported by grants FONDECYT 1040338 and Fondo Ayuda Investigacion Universidad Los Andes MED 002/06 |
Yongsiri 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation was done by a computerized program." |
| Allocation concealment (selection bias) | Low risk | Quote: "Block randomisation was done by a computerized program." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomised, double‐blind, placebo‐controlled cross‐over study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "randomised, double‐blind, placebo‐controlled cross‐over study" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% of participants were not followed up |
| Selective reporting (reporting bias) | Low risk | Quote: "The study protocol was approved by the institutional review board of Burapha University and registered at http://www.clinicaltrials.in.th, identification number TCTR20130314001." |
| Other bias | Low risk | Quote: "This study was supported by the Office of the Higher Education Commission of Thailand and Faculty of Medicine" |
Zaripova 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how sequence generation was done |
| Allocation concealment (selection bias) | Unclear risk | Only states the study was randomised, does not ensure how allocation concealment was done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label" study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open‐label" study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up rate was not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Funding: not reported |
Ziaee 2019.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Identification |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "In the allocation of the patients to the 2 groups, those who referred on even days were assigned to the control group, and those who referred on odd days were assigned to the case group (receiving spironolactone)." |
| Allocation concealment (selection bias) | High risk | Quote: "In the allocation of the patients to the 2 groups, those who referred on even days were assigned to the control group, and those who referred on odd days were assigned to the case group (receiving spironolactone)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients in the case group, after each HD session, were given 25 mg of spironolactone by a nurse, who was unaware of the grouping of the patients. Spironolactone used in this protocol was acquired on the market as Aldactone 25 mg. The patients in the second group were given no medication." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In order to follow up the patients and measure the study variables at baseline and after 9 months, echocardiography was conducted by a single examiner, who was unaware of the study procedure and the allocation of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers for missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Funding: not reported |
ACEi ‐ angiotensin converting enzyme inhibitors; ARB ‐ angiotensin receptor blockers; CKD ‐ chronic kidney disease; DBP ‐ diastolic blood pressure; EF ‐ ejection fraction; ESKD ‐ end‐stage kidney disease; (m)GFR ‐ (measured) glomerular filtration rate; Hb ‐ haemoglobin; HD ‐ haemodialysis; KtV ‐ dialysis adequacy; LVM ‐ left ventricular mass; M/F ‐ male/female; MI ‐ myocardial infarction; NYHA ‐ New York Heart Association; PD ‐ peritoneal dialysis; RCT ‐ randomised control trial; SBP ‐ systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Eklund 2016 | Wrong outcomes: vascular stiffness |
| EPURE 2018 | Wrong participants: patients undergoing kidney transplantation |
| Essaian 2007 | Wrong outcomes: plasma level of plasminogen‐activator inhibitor type 1 |
| Host 2000 | Wrong outcomes: left ventricular cavity |
| Koh 2010 | Wrong outcomes: estimated sodium intake |
| LAST‐D 2013 | This study was withdrawn due to funding issue |
| Nakao 2007 | Wrong interventions: combination of carvedilol with spironolactone |
| NCT00328809 | This study has been withdrawn due to personnel shortage |
| Witoon 2019 | Wrong interventions: combination of furosemide, hydrochlorothiazide, and spironolactone |
Characteristics of studies awaiting classification [ordered by study ID]
Gueiros 2019.
| Methods | Single‐centre parallel RCT (open label) |
| Participants | PD patients |
| Interventions | Spironolactone |
| Outcomes | Relative and absolute progression of the coronary calcium score; hyperkalaemia; adverse effects (hypotension, hyperpotassaemia, gynaecomastia); mineral metabolism at 12 months; change in inflammation markers at 12 months; causes of study discontinuation |
| Notes | Protocol registration only; recruitment completed, current status is unknown |
NCT02190318.
| Methods | Single‐centre parallel RCT (open label) |
| Participants | PD patients |
| Interventions | Losartan plus spironolactone |
| Outcomes | Residual kidney function, peritoneal membrane function |
| Notes | Protocol registration only; current status is unknown |
NCT03953950.
| Methods | Parallel RCT (open label) |
| Participants | PD patients |
| Interventions | Losartan plus spironolactone versus losartan alone |
| Outcomes | Peritoneal dialysate effluent CA‐125; PET indices; dialysate adequacy; creatinine clearance; serum albumin; serum potassium; waist circumference; BMI; nutritional status; Malnutrition‐Inflammation Score; quality of life score; health utility; disease‐specific Thai quality of life score; participant well‐being score; disability; blood pressure; PD technique failure; PD‐related infections; peritonitis or exit‐site and tunnel infection; medicinal products‐related adverse events |
| Notes | Registered May 2019; not yet recruiting (December 2020) |
Song 2017.
| Methods | Not available at this time |
| Participants | Not available at this time |
| Interventions | Not available at this time |
| Outcomes | Not available at this time |
| Notes | Not available at this time |
Wang 2018.
| Methods | Not available at this time |
| Participants | Not available at this time |
| Interventions | Not available at this time |
| Outcomes | Not available at this time |
| Notes | Not available at this time |
BMI ‐ body mass index; PD ‐ peritoneal dialysis; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACHIEVE 2017.
| Study name | Aldosterone bloCkade for Health Improvement EValuation in End‐stage Renal Disease (ACHIEVE) |
| Methods | Multi‐centre parallel RCT |
| Participants | Estimated enrolment: 2750 Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention: spironolactone 25 mg/day Control: placebo |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | 7 July 2017 |
| Contact information | Michael Walsh, MD, PhD Population Health Research Institute, McMaster University, Canada |
| Notes |
ALCHEMIST 2014.
| Study name | ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (ALCHEMIST) |
| Methods | Multi‐centre parallel RCT |
| Participants | Estimated enrolment: 825 Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention
Control
|
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | June 2013 |
| Contact information | Prof Patrick ROSSIGNOL University Hospital, Brest, France |
| Notes | Status: active, not recruiting (28 October 2020) |
NCT00277693.
| Study name | Cardiovascular protective effect of spironolactone in hemodialysis |
| Methods | Parallel RCT |
| Participants | Estimated enrolment: not reported Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention
Control
|
| Outcomes | Not reported |
| Starting date | Not reported |
| Contact information | Antonio Vukusich, MD Faculty of Medicine, University Los Andes, Chile |
| Notes | Status: unknown (last update posted 16 January 2006) |
ACEi ‐ angiotensin‐converting enzyme inhibitor; AF ‐ atrial fibrillation; ARB ‐ angiotensin receptor blocker; BP ‐ blood pressure; CRP ‐ C‐reactive protein, CV ‐ cardiovascular; DBP ‐ diastolic blood pressure; ECG ‐ echocardiography; EF ‐ ejection fraction; HD ‐ haemodialysis; LVH ‐ left ventricular hypertrophy; LVM ‐ left ventricular mass; PD ‐ peritoneal dialysis; MI ‐ myocardial infarction; NSAID ‐ non‐steroidal anti‐inflammatory drugs; RCT ‐ randomised controlled trial; SBP ‐ systolic blood pressure
Differences between protocol and review
Non‐selective (spironolactone) and selective (eplerenone) interventions were combined and not analysed separately as planned in the protocol because only one study compared eplerenone to a control intervention. Death was set as a composite outcome for all‐cause and cardiovascular In the protocol. Because the cause of death was not distinguished in each case in the included studies, we set the outcomes for death (any cause) and death (cardiovascular) separately in the review. We kept the various definition of hyperkalaemia together due to the small number of the eligible included studies. Information on blood pressure as an outcome was insufficient and lacked a standardised method of reporting (e.g. pre‐dialysis systolic or diastolic blood pressure or mean 24 hours blood pressure or mean morning systolic or diastolic blood pressure), we therefore were not able to carry out a meta‐analysis on these data as planned. Additionally, we could not conduct prespecified subgroup analyses including residual kidney function, diabetic kidney disease, and concomitant use of RAS inhibitors except for dialysis modality due to the small number of the eligible included studies and the lack of information reported.
Contributions of authors
Draft the protocol: TH, EO, WL
Study selection: TH, H Nishiwaki
Extract data from studies: TH, H Nishiwaki
Enter data into RevMan: TH, H Nishiwaki, EO, WL, H Noma
Carry out the analysis: TH, EO, WL, H Noma
Interpret the analysis: TH, H Nishiwaki
Draft the final review: TH, H Nishiwaki, EO, WL, H Noma
Disagreement resolution: TH, H Nishiwaki, EO
Update the review: TH, H Nishiwaki, EO, WL, H Noma
Sources of support
Internal sources
No sources of support supplied
External sources
-
JSPS KAKENHI, Japan
This work was supported by JSPS KAKENHI Grant Number 19K03092.
Declarations of interest
TH: has a grant by JSPS KAKENHI Grant Number 19K03092 and consultancy agreement with Kyowa Kirin and has received speaker honoraria from Kyowa Kirin, Astellas, Baxter, Terumo, and Torii Pharmaceutical for activities unrelated to this review
H Nishiwaki: has declared that they have no conflict of interest
EO: has declared that they have no conflict of interest
WL: has declared that they have no conflict of interest
H Noma: has received consultant fees from Kyowa Kirinand lecture fees from Boehringer Ingelheim and Kyowa Kirin
New
References
References to studies included in this review
Feniman De Stefano 2015 {published data only}
- Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, Xavier PS, Castro AD, Caramori JC, et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Therapeutic Advances in Cardiovascular Disease 2015;9(4):158-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gross 2005 {published data only}
- Gross E, Rothstein M, Dombek S, Juknis HI. Effect of spironolactone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric hemodialysis patients. American Journal of Kidney Diseases 2005;46(1):94-101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ito 2014 {published data only}
- Ito Y, Mizuno M, Suzuki Y, Kasuga H, Maruyama S, Yuzawa Y, et al. Spironolactone prevents cardiac hypertrophy in peritoneal dialysis patients without significant adverse effects [abstract no: SA-OR127]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):100A. [Google Scholar]
- Ito Y, Mizuno M, Suzuki Y, Tamai H, Hiramatsu T, Ohashi H, et al. Long-term effects of spironolactone in peritoneal dialysis patients. Journal of the American Society of Nephrology 2014;25(5):1094-102. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2016a {published data only}
- Lin C, Zhang Q, Zhang H, Lin A. Long-term effects of low-dose spironolactone on chronic dialysis patients: a randomized placebo-controlled study. Journal of Clinical Hypertension 2016;18(2):121-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsumoto 2014 {published data only}
- Matsumoto Y, Kageyama S, Mori Y, Yakushigawa T, Arihara K. Spironolactone reduces cardio-and cerebrovascular morbidity and mortality in hemodialysis patients [abstract no: FR-OR025]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):36A. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. Journal of the American College of Cardiology 2014;63(6):528-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MiREnDa 2014 {published data only}
- Hammer F, Krane V, Stork S, Roser C, Hofmann K, Pollak N, et al. Rationale and design of the Mineralocorticoid Receptor Antagonists in End-Stage Renal Disease Study (MiREnDa). Nephrology Dialysis Transplantation 2014;29(2):400-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hammer F, Malzahn U, Donhauser J, Betz C, Schneider MP, Grupp C, et al. A randomized controlled trial of the effect of spironolactone on left ventricular mass in hemodialysis patients. Kidney International 2019;95(4):983-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hauser T, Malzahn U, Betz C, Grupp C, Doeltz T, Grebe S, et al. The prognostic value of nephro-cardiac biomarkers in hemodialysis patients-the randomized-controlled MiREnDa trial [abstract no: FP532]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i218. [EMBASE: 622606301] [Google Scholar]
- Wanner C, Malzahn U, Donhauuser J, Betz C, Grupp C, Doltz T, et al. Effect of spironolactone on the left ventricular mass in hemodialysis patients: the MiREnDa study- a randomized controlled trial. [abstract no: SA-PO849]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):899. [Google Scholar]
Ni 2014 {published data only}
- Ni X, Zhang J, Zhang P, Wu F, Xia M, Ying G, et al. Effects of spironolactone on dialysis patients with refractory hypertension: a randomized controlled study. Journal of Clinical Hypertension 2014;16(9):658-63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PHASE 2015 {published data only}
- Walsh M, Manns B, Garg AX, Bueti J, Rabbat C, Smyth A, et al. The safety of eplerenone in hemodialysis patients: a noninferiority randomized controlled trial. Clinical Journal of the American Society of Nephrology: CJASN 2015;10(9):1602-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M, Manns BJ, Garg AX, Bueti JA, Rabbat CG, Smyth A, et al. Safety of eplerenone in hemodialysis patients: a randomized controlled trial [abstract no: FR-PO1016]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):605A. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPIN‐D 2019 {published data only}
- Charytan D, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, et al. Safety and cardiovascular efficacy of spironolactone (SPL) in dialysis-dependent ESRD (SPin-D): a pilot trial of the NIDDK Hemodialysis Novel Therapies Consortium [abstract no: FR-PO1078]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):B9. [Google Scholar]
- Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, et al. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney International 2019;95(4):973-82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taheri CAPD 2012 {published data only}
- Najafabadi MM, Taheri S, Seyrafian S, Alipoor Z, Karimi S, Poormodhadas A, et al. Spironolactone in continuous ambulatory peritoneal dialysis patients, improves cardiac function [abstract no: SU475]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Taheri S, Mortazavi M, Pourmoghadas A, Seyrafian S, Alipour Z, Karimi S. A prospective double-blind randomized placebo-controlled clinical trial to evaluate the safety and efficacy of spironolactone in patients with advanced congestive heart failure on continuous ambulatory peritoneal dialysis. Saudi Journal of Kidney Diseases & Transplantation 2012;23(3):507-12. [MEDLINE: ] [PubMed] [Google Scholar]
Taheri HD 2009 {published data only}
- Mortazavi M, Taheri S, Shahidi S, Pourmoghaddas A, Yaraghi MG, Seyrafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function [abstract no: FP379]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi145. [CENTRAL: CN-00716090] [PubMed] [Google Scholar]
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi Journal of Kidney Diseases & Transplantation 2009;20(3):392-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function [abstract no: S-PO-0370]. In: 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:148.
Vazquez‐Rangel 2014 {published data only}
- Vazquez-Rangel A, Soto V, Escalona M, Toledo RG, Castillo EA, Polanco Flores NA, et al. Spironolactone to prevent peritoneal fibrosis in peritoneal dialysis patients: a randomized controlled trial. American Journal of Kidney Diseases 2014;63(6):1072-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vazquez-Rangel A, Soto V, Toledo RG, Castillo EA, Polanco Flores NA, Falcon-Chavez I, et al. Spironolactone to prevent peritoneal fibrosis in peritoneal dialysis patients: a randomized controlled trial [abstract no: FR-PO789]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):549A. [DOI] [PubMed] [Google Scholar]
Vukusich 2010 {published data only}
- Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, et al. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(8):1380-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yongsiri 2015 {published data only}
- Yongsiri S, Thammakumpee J, Prongnamchai S, Tengpraettanakorn P, Chueansuwan R, Tangjaturonrasme S, et al. Randomized, double-blind, placebo-controlled trial of spironolactone for hypokalemia in continuous ambulatory peritoneal dialysis patients. Therapeutic Apheresis & Dialysis 2015;19(1):81-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yongsiri S, Thammakumpee J, Prongnamchai S, Tengpraettanakorn P, Tangjaturonrasme S, Dinchuthai P, et al. Spironolactone for hypokalia in CAPD patients, a randomized, double-blind, placebo controlled trial [abstract no: 390]. American Journal of Kidney Diseases 2014;63(5):A116. [EMBASE: 71448657] [Google Scholar]
Zaripova 2012 {published data only}
- Zaripova I, Kayukov I, Essaian A, Nimgirova A. Renin angiotensin aldosterone system blockade and left ventricular hypertrophy in maintenance hemodialysis patients [abstract no: FP497]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii238. [EMBASE: 70765993] [Google Scholar]
Ziaee 2019 {published data only}
- Ziaee SA, Karvandi M, Ziaee NS, Ghozloujeh ZG, Shahrbaf MA, Roshan A. Effects of spironolactone on cardiovascular complications in hemodialysis patients of Taleghani Hospital during the period of 2016-2017: a randomized double-blind controlled clinical trial. Iranian Heart Journal 2019;20(1):45-52. [EMBASE: 2001576782] [Google Scholar]
References to studies excluded from this review
Eklund 2016 {published data only}
- Eklund M, Furuland H, Hellberg O, Nilsson E. Effect of spironolactone on vascular stiffness in hemodialysis - a randomized crossover study [abstract no: TH-PO913]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):305-6A. [Google Scholar]
EPURE 2018 {published data only}
- Girerd S, Frimat L, Ducloux D, Le Meur Y, Mariat C, Moulin B, et al. EPURE Transplant (Eplerenone in Patients Undergoing Renal Transplant) study: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2018;19(1):595. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Essaian 2007 {published data only}
- Essaian A, Kaukov I, Karabayeva A, Kadinskaya M, Katysheva N. The influence of spironolactone on plasma level of plasminogen-activator inhibitor type 1 (PAI-1) in anuric hemodialysis patients [abstract no: SaP266]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi322. [CENTRAL: CN-00716089] [Google Scholar]
Host 2000 {published data only}
- Host U, Egfjord M, Holm E, Jespersen B, Lokkegaard H, Skagen K, et al. Long term spironolactone and cardiac function in chronic uremia [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A215. [CENTRAL: CN-00460961] [Google Scholar]
Koh 2010 {published data only}
- Koh KH, Lee J, Chai NS, Tan C. Randomized controlled trial on the antihypertensive effect of spironolactone in addition to salt restriction in peritoneal dialysis patients [abstract]. Peritoneal Dialysis International 2010;30(Suppl 2):S137. [Google Scholar]
LAST‐D 2013 {published data only}
- Charytan D. L-arginine and spironolactone trial in dialysis-dependent ESRD (LAST-D). www.clinicaltrials.gov/ct2/show/NCT01855334 (first received 16 May 2013).
Nakao 2007 {published data only}
- Nakao N, Hasegawa H, Fujimori A, Seno H, Toriyama T, Kawahara H. Effects of combined b-blocker and anti-aldosterone antagonist treatment for cardiovascular prevention in patients receiving maintenance hemodialysis [abstract no: SU-PO573]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):709A. [Google Scholar]
NCT00328809 {published data only}
- Narsipur SS. Spironolactone safety in dialysis patients. www.clinicaltrials.gov/ct2/show/NCT00328809 (first received 22 May 2006).
Witoon 2019 {published data only}
- Witoon R, Yongsiri S, Buranaburidej P, Nanna P. Efficacy of triple diuretic treatment in continuous ambulatory peritoneal dialysis patients: a randomized controlled trial. Kidney Research & Clinical Practice 2019;38(1):108-15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gueiros 2019 {published data only}
- Gueiros AP, Gueiros JE, Nobrega KT, Calado EB, Matta MC, Torres LC, et al. Effect of spironolactone on the progression of coronary calcification in peritoneal dialysis patients: a pilot study. Jornal Brasileiro de Nefrologia 2019;41(3):345-55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02190318 {published data only}
- Liu H. Residual renal function preservation in peritoneal dialysis patients. www.clinicaltrials.gov/ct2/show/NCT02190318 (first received 15 July 2014).
NCT03953950 {published data only}
- Ruengorn C. Effect of add-on spironolactone to losartan versus losartan alone on peritoneal membrane among peritoneal dialysis patients (ESCAPE-PD). www.clinicaltrials.gov/show/nct03953950 (received 17 May 2019).
Song 2017 {published data only}
- Song, et al. Effect of spironolactone on cardiac function, dialysis adequacy and complications in hemodialysis. Journal of Modern Medicine & Health 2017;33(19):2947-9. [Google Scholar]
Wang 2018 {published data only}
- Wang CC, et al. Effects of spironolacone on cardiac and residual renal function in patients with peritoneal dialysis. Chinese Journal of General Practice 2018;16(8):1303-7. [Google Scholar]
References to ongoing studies
ACHIEVE 2017 {published data only}
- Walsh M, Devereaux PJ. Aldosterone bloCkade for Health Improvement EValuation in End-stage Renal Disease (ACHIEVE). www.clinicaltrials.gov/ct2/show/NCT03020303 (first received 13 January 2017).
ALCHEMIST 2014 {published data only}
- Rossignol P. ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial (ALCHEMIST). www.clinicaltrials.gov/ct2/show/NCT01848639 (first received 7 May 2013).
NCT00277693 {published data only}
- Michea LF. Cardiovascular protective effect of spironolactone in hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00277693 (first received 16 January 2006).
Additional references
Bolignano 2014
- Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD007004. [DOI: 10.1002/14651858.CD007004.pub3] [DOI] [PubMed] [Google Scholar]
Feniman‐De‐Stefano 2015
- Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, Xavier PS, Castro AD, Caramori JC, et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Therapeutic Advances in Cardiovascular Disease 2015;9(4):158-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 1995
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney International 1995;47(1):186-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 1998
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. American Journal of Kidney Diseases 1998;32(5 Suppl 3):S112-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence-imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harnett 1995
- Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney International 1995;47(3):884-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Levey 2011
- Levey AS, Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report.[Erratum appears in Kidney Int. 2011 Nov;80(9):1000], [Erratum appears in Kidney Int. 2011 Nov 1;80(9):1000; PMID: 30036909]. Kidney International 2011;80(1):17-28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2019
- Li Y, Xie N, Liang M. Aldosterone antagonists reduce the risk of cardiovascular mortality in dialysis patients: a meta-analysis. Evidence-Based Complementary & Alternative Medicine: eCAM 2019:1925243. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liyanage 2015
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385(9981):1975-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pitt 1999
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 1999;341(10):709-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pitt 2003
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.[Erratum in: N Engl J Med. 2003 May 29;348(22):2271]. New England Journal of Medicine 2003;348(14):1309-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quach 2016
- Quach K, Lvtvyn L, Baigent C, Bueti J, Garg AX, Hawley C, et al. The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: a systematic review and meta-analysis. American Journal of Kidney Diseases 2016;68(4):591-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salem 1995
- Salem MM. Hypertension in the hemodialysis population: a survey of 649 patients. American Journal of Kidney Diseases 1995;26(3):461-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sato 1999
- Sato A, Funder JW, Saruta T. Involvement of aldosterone in left ventricular hypertrophy of patients with end-stage renal failure treated with hemodialysis. American Journal of Hypertension 1999;12(9 Pt 1):867-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saudan 2003
- Saudan P, Mach F, Perneger T, Schnetzler B, Stoermann C, Fumeaux Z, et al. Safety of low-dose spironolactone administration in chronic haemodialysis patients. Nephrology Dialysis Transplantation 2003;18(11):2359-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
SONG 2017
- SONG Initiative. The SONG Handbook Version 1.0. www.songinitiative.org/reports-and-publications/ (accessed 13 August 2018).
Staessen 1981
- Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise in plasma concentration of aldosterone during long-term angiotensin II suppression. Journal of Endocrinology 1981;91(3):457-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Steigerwalt 2007
- Steigerwalt S, Zafar A, Mesiha N, Gardin J, Provenzano R. Role of aldosterone in left ventricular hypertrophy among African-American patients with end-stage renal disease on hemodialysis. American Journal of Nephrology 2007;27(2):159-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2017a
- US Renal Data System. 2017 Annual Data Report. Chapter 8: Cardiovascular Disease in Patients with ESRD. www.usrds.org/media/1687/v2_c08_cvd_17.pdf (accessed 14 December 2020).
USRDS 2017b
- US Renal Data System. 2017 Annual Data Report. Chapter 5: Mortality. www.usrds.org/media/1681/v2_c05_mortality_17.pdf (accessed 14 December 2020).
Yancy 2013
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62(16):e147-239. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hasegawa 2018
- Hasegawa T, Nishiwaki H, Ota E, Levack WMM, Noma H. Aldosterone antagonists for people with chronic kidney disease requiring dialysis. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD013109. [DOI: 10.1002/14651858.CD013109] [DOI] [PMC free article] [PubMed] [Google Scholar]


