Abstract
Background
This is the third update of this review, first published in July 2009. All major guidelines on treatment of hypertension recommend weight loss; anti‐obesity drugs may be able to help in this respect.
Objectives
Primary objectives:
To assess the long‐term effects of pharmacologically‐induced reduction in body weight in adults with essential hypertension on all‐cause mortality, cardiovascular morbidity, and adverse events (including total serious adverse events, withdrawal due to adverse events, and total non‐serious adverse events)..
Secondary objectives:
To assess the long‐term effects of pharmacologically‐induced reduction in body weight in adults with essential hypertension on change from baseline in systolic and diastolic blood pressure, and on body weight reduction.
Search methods
For this updated review, the Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to March 2020: the Cochrane Hypertension Specialised Register, CENTRAL, MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. The searches had no language restrictions. We contacted authors of relevant papers about further published and unpublished work.
Selection criteria
Randomised controlled trials of at least 24 weeks' duration in adults with hypertension that compared approved long‐term weight‐loss medications to placebo.
Data collection and analysis
Two review authors independently selected studies, assessed risks of bias, and extracted data. Where appropriate and in the absence of significant heterogeneity between studies (P > 0.1), we pooled studies using a fixed‐effect meta‐analysis. When heterogeneity was present, we used the random‐effects method and investigated the cause of the heterogeneity.
Main results
This third update of the review added one new trial, investigating the combination of naltrexone/bupropion versus placebo. Two medications, which were included in the previous versions of this review (rimonabant and sibutramine) are no longer considered relevant for this update, since their marketing approval was withdrawn in 2010 and 2009, respectively. The number of included studies in this review update is therefore six (12,724 participants in total): four RCTs comparing orlistat to placebo, involving a total of 3132 participants with high blood pressure and a mean age of 46 to 55 years; one trial comparing phentermine/topiramate to placebo, involving 1305 participants with high blood pressure and a mean age of 53 years; and one trial comparing naltrexone/bupropion to placebo, involving 8283 participants with hypertension and a mean age of 62 years. We judged the risks of bias to be unclear for the trials investigating orlistat or naltrexone/bupropion. and low for the trial investigating phentermine/topiramate. Only the study of naltrexone/bupropion included cardiovascular mortality and morbidity as predefined outcomes.
There were no differences in the rates of all‐cause or cardiovascular mortality, major cardiovascular events, or serious adverse events between naltrexone/bupropion and placebo. The incidence of overall adverse events was significantly higher in participants treated with naltrexone/bupropion. For orlistat, the incidence of gastrointestinal side effects was consistently higher compared to placebo. The most frequent side effects with phentermine/topiramate were dry mouth and paraesthesia. After six to 12 months, orlistat reduced systolic blood pressure compared to placebo by mean difference (MD) −2.6 mm Hg (95% confidence interval (CI) −3.8 to −1.4 mm Hg; 4 trials, 2058 participants) and diastolic blood pressure by MD −2.0 mm Hg (95% CI −2.7 to −1.2 mm Hg; 4 trials, 2058 participants). After 13 months of follow‐up, phentermine/topiramate decreased systolic blood pressure compared to placebo by −2.0 to −4.2 mm Hg (1 trial, 1030 participants) (depending on drug dosage), and diastolic blood pressure by −1.3 to −1.9 mm Hg (1 trial, 1030 participants) (depending on drug dosage). There was no difference in the change in systolic or diastolic blood pressure between naltrexone/bupropion and placebo (1 trial, 8283 participants). We identified no relevant studies investigating liraglutide or lorcaserin in people with hypertension.
Authors' conclusions
In people with elevated blood pressure, orlistat, phentermine/topiramate and naltrexone/bupropion reduced body weight; the magnitude of the effect was greatest with phentermine/topiramate. In the same trials, orlistat and phentermine/topiramate, but not naltrexone/bupropion, reduced blood pressure. One RCT of naltrexone/bupropion versus placebo showed no differences in all‐cause mortality or cardiovascular mortality or morbidity after two years. The European Medicines Agency refused marketing authorisation for phentermine/topiramate due to safety concerns, while for lorcaserin the application for European marketing authorisation was withdrawn due to a negative overall benefit/risk balance. In 2020 lorcaserin was also withdrawn from the US market. Two other medications (rimonabant and sibutramine) had already been withdrawn from the market in 2009 and 2010, respectively.
Plain language summary
Do medicines for weight loss affect blood pressure, and reduce the effects of high blood pressure (hypertension)?
What is high blood pressure (hypertension)?
Blood pressure is a measure of the force that your heart uses to pump blood around your body. It is usually given as two figures: the pressure when your heart pushes blood out (systolic pressure), and the pressure when your heart rests between beats (diastolic pressure). Blood pressure is considered to be high when systolic pressure is over 140 and/or diastolic pressure is over 90, often written as '140 over 90' and measured in millimetres of mercury (mm Hg). The risk of developing high blood pressure increases as you get older.
Hypertension can increase people's risk of developing serious long‐term health problems, such as heart attack or stroke. Lowering blood pressure in people with hypertension reduces the number of people who develop diseases of the heart and blood vessels (cardiovascular disease), which leads to fewer deaths and cardiovascular problems.
Weight and hypertension
Hypertension treatment guidelines recommend keeping to a healthy weight and losing weight when needed. Some people may take medicines to help reduce their weight.
Why we did this Cochrane Review
Medicines licensed for use in weight loss in the USA and Europe include orlistat and naltrexone combined with bupropion. Another combination, phentermine with topiramate, is licensed in the USA only. We wanted to find out if weight‐loss medicines have long‐lasting effects on blood pressure, and whether they could reduce the unwanted effects of high blood pressure on people's health.
What did we do?
We searched for studies about the effects of taking weight‐loss medicines in people with high blood pressure. We were interested in how these medicines affected blood pressure and body weight. We also wanted to know how many people experienced any unwanted effects, how many people developed cardiovascular disease, and if any people died.
We looked for randomised controlled studies, in which the treatments people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a treatment.
We assessed the reliability of the evidence we found. We considered factors such as: how the studies were conducted, how many people they involved, and whether their findings were consistent across studies.
Search date: we included evidence published up to March 2020.
What we found
We found six studies in 12,724 people with high blood pressure (average age 46 to 62 years). The studies were conducted in the USA (3 studies) and Europe (3 studies), and lasted from 6 months to 28 months.
All studies compared the effects of taking a weight‐loss medicine with effects of taking a dummy medicine (placebo).
What are the results of our review?
Orlistat may reduce weight and probably reduces blood pressure (4 studies; 2058 people).
Phentermine plus topiramate may reduce weight and may reduce blood pressure (1 study; 1305 people).
Naltrexone plus bupropion probably reduces weight but probably does not reduce blood pressure (1 study; 8283 people).
One study looked at the risk of death and major unwanted cardiovascular effects; it showed no differences between naltrexone plus bupropion treatment and a placebo after two years.
People taking weight‐loss medicines reported more unwanted effects than those taking a placebo. The most common unwanted effects were digestive problems (for orlistat, and phentermine plus topiramate), dry mouth and skin tingling or numbness (for naltrexone plus bupropion).
How reliable are these results?
Results are from a small number of studies. In some studies, there were few events for some measures we were interested in.
We are moderately confident about how orlistat and naltrexone plus bupropion affected weight loss and blood pressure. However, results might change if more evidence becomes available.
We are less confident about the effects of phentermine plus topiramate; the unwanted effects of orlistat, and the risk of unwanted cardiovascular events associated with naltrexone plus bupropion. Results are likely to change if more evidence becomes available.
Conclusions
Some weight‐loss medicines reduce weight and blood pressure in people with high blood pressure, but may cause unwanted effects. We did not find enough evidence about whether taking weight‐loss medicines to lose weight could reduce death and cardiovascular disease.
Summary of findings
Background
Description of the condition
Hypertension is a chronic condition associated with an increased risk of cardiovascular mortality and morbidity. It is estimated that raised blood pressure affects 1.13 billion people worldwide and leads to over 9 million deaths each year (WHO 2013). Lowering blood pressure levels in hypertensive people has been shown to be an effective means of reducing cardiovascular morbidity and mortality.
Epidemiological investigations have consistently found an association between high blood pressure and different lifestyles, one of them being excess body weight. Major guidelines recommend weight reduction as a first‐step intervention in the therapy of hypertensive people (Hypertension Canada 2018; ACC/AHA 2017; ESC/ESH 2018; NICE 2019). Body weight may be reduced by lifestyle modifications as well as pharmacological and invasive interventions.
Description of the intervention
For a select group of overweight or obese people for whom lifestyle interventions are unsuccessful, anti‐obesity drugs may be an option to help reduce body weight. Orlistat, sibutramine, and rimonabant were formerly the most commonly used anti‐obesity drugs, but only orlistat, which was approved in 1998, still has market approval for the long‐term treatment of obesity. Since 2012, four additional drugs (lorcaserin, liraglutide, phentermine/topiramate, and naltrexone/bupropion) have been approved by the US Food and Drug Administration (FDA) for obese (body mass index (BMI) ≥ 30 kg/m2) and overweight (BMI ≥ 27 kg/m2) people with at least one obesity‐related comorbidity (FDA 2012a; FDA 2012b; FDA 2014a; FDA 2014b). These medications have been quoted in guidelines for the long‐term pharmacological treatment of obesity (Apovian 2015). In Europe, liraglutide, which has also been approved for the treatment of diabetes mellitus type 2, and naltrexone/bupropion were approved for weight management in March 2015 by the European Medicines Agency (EMA) (EMA 2015a; EMA 2015b). In 2013, the manufacturer of lorcaserin withdrew its application to the EMA after the Committee for Medicinal Products for Human Use raised safety concerns (EMA 2013a), while the EMA refused marketing authorisation for phentermine/topiramate due to safety concerns (EMA 2013c).
Sibutramine and rimonabant both lost their marketing approval about 10 years ago.Sibutramine was approved by the FDA in 1997 and by the EMA in 1999. Preliminary results of the Sibutramine Cardiovascular Outcomes Trial (SCOUT 2010), presented in October 2009, showed an increased risk of serious cardiovascular events (such as heart attack or stroke) among people with known cardiovascular disease who were taking sibutramine. This led the FDA and EMA to recommend suspension of the marketing authorisation (EMA 2010a; EMA 2010b; FDA 2010a). In January 2010, Abbot Laboratories agreed to voluntarily withdraw sibutramine from the European market (Abbott 2010), and in October 2010 from the US market (FDA 2010b). Rimonabant received regulatory approval in several European countries in 2006, but failed to receive FDA approval after preclinical and clinical data raised concerns about an association between rimonabant intake and the increased incidence of psychiatric adverse events, including suicidality, an ill‐defined constellation of neurological signs and symptoms, and seizures (FDA 2007). In October 2008, the EMA recommended the suspension of rimonabant from the market because of newly‐available post‐marketing analyses demonstrating detrimental effects compared with placebo (EMA 2008a; EMA 2008b). In January 2009, the European Commission decided to withdraw market authorisation for rimonabant in all countries of the European Union (EMA 2009a). In February 2020, lorcaserin was withdrawn from the US market by the manufacturer after a request by the FDA (Eisai Inc. 2020). This action was taken after an FDA analysis of data from a safety clinical trial showed an increased occurrence of cancer in participants treated with lorcaserin (FDA 2020).
How the intervention might work
Anti‐obesity drugs aim to reduce body weight and to maintain the weight reduction over a longer period. Orlistat is a gastric and pancreatic lipase inhibitor that blocks the absorption of about 30% of dietary fat (Padwal 2007). Liraglutide, a glucagon‐like peptide 1 (GLP‐1) receptor agonist, appears to regulate appetite by increasing feelings of satiety (Russell‐Jones 2009). The combination of phentermine, a neurostabiliser, and topiramate, an antiseizure medication, appears to have an additive effect on weight reduction (Aronne 2013). In combination with naltrexone, bupropion, a dopamine and norepinephrine reuptake inhibitor, reduces appetite and increases energy expenditure (Caixas 2014). The mechanisms by which these three medications cause weight loss are not yet fully understood. Lorcaserin is a selective serotonin receptor agonist and increases the sense of fullness (Taylor 2013). Dietary‐intervention studies in hypertensive people have shown a positive association between weight loss and blood pressure reduction (Horvath 2008). It therefore seems reasonable to suppose that medical weight‐reducing treatment may also lead to a fall in blood pressure.
Why it is important to do this review
For overweight or obese people with established hypertension, blood pressure should first be managed with non‐pharmacological interventions such as weight reduction (Hypertension Canada 2018; ACC/AHA 2017; ESC/ESH 2018; NICE 2019). Since anti‐obesity drugs may support the efforts of people to reduce body weight, it is important that the physician be informed about the efficacy and potential harms of these drugs before prescribing them.
Systematic reviews and meta‐analyses have shown that pharmacological weight‐reducing interventions with orlistat, lorcaserin, phentermine/topiramate,and liraglutide reduce both blood pressure and body weight (Chan 2013; Khera 2018; LeBlanc 2018; Zhang 2015). Treatment with naltrexone/bupropion reduced body weight but did not lower blood pressure (Caixas 2014; Khera 2018, LeBlanc 2018). None of these reviews investigated the efficacy and safety of weight‐reducing drugs in the subgroup of overweight or obese people with hypertension. Furthermore, only limited data were available on the question of whether pharmacological weight reduction lowers the risk of mortality and other patient‐relevant endpoints. Results from a few RCTs showed no beneficial effect on cardiovascular events for liraglutide or phentermine/topiramate compared to placebo (LeBlanc 2018). Two studies examining clinical endpoints for rimonabant (CRESCENDO 2010) and sibutramine (SCOUT 2010) have resulted in the drugs being withdrawn from the market. Neither sibutramine nor rimonabant are therefore considered relevant for this review update.This currently leaves orlistat, liraglutide and naltrexone/bupropion as approved for the long‐term treatment of obesity in Europe and the USA, and lorcaserin and phentermine/topiramate as approved for the long‐term treatment of obesity in the USA only.
This is an update of a previously‐published Cochrane Review (Siebenhofer 2016).
Objectives
Primary objectives:
To assess the long‐term effects of pharmacologically‐induced reduction in body weight in adults with essential hypertension on all‐cause mortality, cardiovascular morbidity, and adverse events (including total serious adverse events, withdrawal due to adverse events, and total non‐serious adverse events).
Secondary objectives:
To assess the long‐term effects of pharmacologically‐induced reduction in body weight in adults with essential hypertension on change from baseline in systolic blood pressure, change from baseline in diastolic blood pressure, and body weight reduction.
Methods
Criteria for considering studies for this review
Types of studies
The study design must meet the following criteria: all randomised controlled trials (RCTs) comparing pharmacologic interventions approved for long‐term weight management versus placebo, with a follow‐up of at least 24 weeks. The reason for including only studies with a follow‐up of at least 24 weeks is that studies of shorter duration cannot show long‐term effects. Any additional active care (for example, antihypertensive medication) must have been applied to the active treatment group and to the control group.
Types of participants
Men and non‐pregnant women aged 18 years or older with essential hypertension (i.e. a baseline blood pressure of at least 140 mm Hg systolic or a diastolic blood pressure of at least 90 mm Hg, or both, or people on antihypertensive treatment), for whom at least one of the following outcomes was reported: mortality, cardiovascular outcomes, adverse events, or blood pressure.
Types of interventions
Monotherapy with drugs for long‐term weight management (orlistat, phentermine/topiramate, lorcaserin, naltrexone/bupropion, or liraglutide).
Types of outcome measures
We included the following outcomes:
Primary outcomes
Total mortality
Cardiovascular morbidity
Adverse events (withdrawals due to adverse events, adverse events related to a particular anti‐obesity drug)
Secondary outcomes
Change in systolic blood pressure
Change in diastolic blood pressure
Change in body weight
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist searched the following databases, without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 20 March 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web; searched 17 March 2020);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 17 March 2020);
Embase Ovid (from 1974 onwards) (searched 17 March 2020);
ClinicalTrials.gov (www.clinicaltrials.gov); (searched 17 March 2020);
World Health Organization International Clinical Trials Registry Platform (www.who.it.trialsearch); (searched 17 March 2020).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 6 (Handbook 2019; Lefebvre 2019)). We present search strategies for major databases in Appendix 4.
The search strategy used in the previous review versions is documented in Appendix 5, Appendix 6 and Appendix 7.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches for controlled trials in the Allied and Complementary Medicine Database (AMED), CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses and Web of Science.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials
Data collection and analysis
Selection of studies
Two review authors (from SW, TS, KH, KJ) independently screened the title and abstract of each reference identified by the search and applied the inclusion criteria. We retrieved potentially relevant studies in full, and again two review authors (SW, TS) independently decided whether they met the inclusion criteria. In case of disagreement, we also obtained the full article and the two review authors inspected it independently, resolving differences in opinion by recourse to a third party. If a resolution of the disagreement was not possible, we classified the article as 'awaiting assessment' and contacted the authors of the study for clarification.
Data extraction and management
Two review authors (from SW, TS, KH, KJ, CK) independently extracted data using a data extraction form. We resolved differences in data extraction by consensus, referring back to the original article. We sought information from the authors of the primary studies if necessary. We extracted, checked, and recorded the following data.
1. General information
This included all publications of a single trial, the sponsor of the trial (specified, known or unknown), and the country in which the trial was conducted.
2. Methods section
The information on the methods summarised the characteristics of the trial, participants, and interventions, and the outcome measures used and reported in the publication.
2.1. Characteristics of the trial
The reported items included the design and duration of the trial, randomisation (and method), allocation concealment (and method), blinding (participants, people administering treatment, outcome assessors), and checking of blinding.
2.2. Characteristics of participants
This included the number of participants in each group, how the participants were selected (random, convenience), the exclusion criteria used, and their demographic characteristics (for example, age, gender, nationality, ethnicity). We extracted disease‐related information about duration of hypertension. We checked the similarity of groups at baseline as well as reports about withdrawals and losses to follow‐up (reasons/description), and described them in the 'Risk of bias' tables in Characteristics of included studies. If subgroups were analysed, we noted and reported the reasons and methods.
2.3. Characteristics of interventions
The relevant information to be extracted was the duration of the intervention, length of follow‐up (in months), the type of anti‐obesity drug (orlistat, lorcaserin, phentermine/topiramate, naltrexone/bupropion, or liraglutide), the dose, and the administration route.
2.4. Characteristics of outcome measures
We reported the measures mentioned in the outcome section and any other outcomes measured in the study.
3. Data from clinical trial registers
If data from included trials were available as study results in clinical trial registers such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted the data. If there was also a full publication of the trial, we collated and critically appraised all available data. If an included trial was marked as a completed study in a clinical trial register but no additional information (study results, publication or both) was available, we added this trial to the table Characteristics of excluded studies.
Assessment of risk of bias in included studies
Two review authors (from SW, TS, KH, KJ, CK) independently assessed trials fulfilling the inclusion criteria in order to evaluate methodological quality, resolving any differences in opinion by discussion with a third review author (from TS, KH, KJ). We assessed all trials using the 'Risk of bias' assessment tool (Higgins 2019), and we judged the 'Risk of bias' criteria as having low, high or unclear risk. We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions according to the categories: adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other potential biases. We evaluated the risk of bias for the categories of blinding and incomplete outcome data separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures.
Measures of treatment effect
When at least two included trials were available for a comparison and a given outcome, we tried to express dichotomous data as a risk ratio (RR) with a 95% confidence interval (CI). For continuous outcomes (changes in blood pressure and body weight), we estimated the intervention effect using the mean difference with a 95% CI.
Unit of analysis issues
We intended to consider the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single pair‐wise comparison or appropriately reduced the sample size so that the same participants did not contribute more than once (splitting the 'shared' group into two or more groups). While the latter approach offers some solution to adjusting the precision of the comparison, it does not account for correlation arising because the same set of participants was included in multiple comparisons (Deeks 2019).
Dealing with missing data
We obtained relevant missing data from authors and from the Institute for Quality and Efficiency in Health Care report (IQWiG 2006). We evaluated important numerical data such as screened, eligible, and randomised participants as well as intention‐to‐treat (ITT) and per‐protocol (PP) populations. We investigated attrition rates, for example dropouts, losses to follow‐up, and withdrawals. We critically appraised issues of missing data, ITT, and PP and, if available, compared them to the specification of primary outcome parameters and power calculations.
Assessment of heterogeneity
We assessed heterogeneity using Higgins I2.
Assessment of reporting biases
If we included 10 or more trials that investigated a particular outcome, we assessed publication bias and small‐study effects using the funnel plot. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore interpreted the results cautiously (Sterne 2011).
Data synthesis
We summarised data statistically when they were available, sufficiently similar, and of sufficient quality. We performed analyses separately for each drug. We performed statistical analysis according to the statistical guidelines referenced in the current version of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). We considered fixed‐effect and random‐effects models for the meta‐analyses. In case of between‐study variability, we present the results of the random‐effects model. If standard deviations were unavailable, we approximated them on the basis of P values and sample sizes.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses where appropriate. Heterogeneity among participants could be related to, for example, sex, age, body mass index, concomitant diseases, ethnicity, blood pressure at baseline, blood pressure goals, concomitant antihypertensive therapy, and socioeconomic status.
Sensitivity analysis
We tested the robustness of our results where appropriate, using several sensitivity analyses (for example, study quality or PP versus ITT analyses, studies with large dropout rates and losses to follow‐up). In case of substantial heterogeneity (I2 greater than 50%), we planned to perform sensitivity analyses for the following items: study quality, PP versus ITT analyses, sex, age, body mass index, concomitant diseases, ethnicity, blood pressure at baseline, blood pressure goals, concomitant antihypertensive therapy, and socioeconomic status.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence (Guyatt 2011). We present the main results of the review, including a summary of the data, the magnitude of the effect and the overall certainty of the evidence, for each type of weight‐reducing drug separately in Table 1, Table 2, and Table 3.
Summary of findings 1. Orlistat versus placebo for weight reduction.
| Orlistat compared with placebo for weight reduction | ||||||
|
Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Orlistat Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with orlistat | |||||
|
Total mortality Follow‐up: 24 ‐ 208 weeks |
2 deaths (OD subgroup) and 1 death (OS subgroup) with orlistat, and no deaths with placebo in 1 trial; no deaths with orlistat or placebo in 2 other trials | ‐ | 1488 (3 studies) | ⊕⊕⊝⊝ lowa | Very low event rate. | |
|
Cardiovascular morbidity Follow‐up: 24 weeks |
see comment | ‐ | 1811 (3 studies) | ⊕⊝⊝⊝ very lowb |
Reporting of results too diverse to allow a meta‐analysis and small number of events. The effects of orlistat compared with placebo for this outcome are uncertain. | |
|
Serious adverse events Follow‐up: 24 ‐ 208 weeks |
101 per 1000 | 146 per 1000 (111 to 192) | RR 1.45 (1.10 to 1.91) | 1476 (3 studies) | ⊕⊕⊕⊝ moderatec | ‐ |
|
All adverse events Follow‐up: 24‐208 weeks |
865 per 1000 | 977 per 1000 (727 to 1000) | RR 1.13 (0.84 to 1.54) | 1386 (2 studies) | ⊕⊝⊝⊝ very lowd | 98% heterogeneity |
|
Change in systolic blood pressure compared to placebo (mm Hg) from baseline to end of study Follow‐up: 24 ‐ 52 weeks |
Reduction of systolic blood pressure ranged across control groups from 0.9 to 11.0 mm Hg | MD 2.58 mm Hg lower (3.78 lower to 1.37 lower) | ‐ | 2058 (4 studies) | ⊕⊕⊕⊝ moderatec | ‐ |
|
Change in diastolic blood pressure compared to placebo (mm Hg) from baseline to end of study Follow‐up: 24 ‐ 52 weeks |
Reduction of diastolic blood pressure ranged across control groups from 0.8 to 9.2 mm Hg | MD 1.97 mm Hg lower (2.72 lower to 1.22 lower) | ‐ | 2058 (4 studies) | ⊕⊕⊕⊝ moderatec | ‐ |
|
Change in body weight compared to placebo (kg) from baseline to end of study Follow‐up: 24 ‐ 52 weeks |
Reduction of body weight ranged across control groups from 1.8 to 6.93 kg | MD 3.74 kg lower (4.70 lower to 2.78 lower) | ‐ | 2080 (4 studies) | ⊕⊕⊝⊝ lowd | ‐ |
| CI: confidence interval; MD: mean difference; OD: orlistat and diastolic blood pressure ≥ 90 mm Hg; OS: orlistat and systolic blood pressure ≥ 140 mm Hg; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels because of serious imprecision (small number of trials, very low event rates) ‐ see Appendix 1. bDowngraded by two levels because of serious imprecision (small number of trials, very low event rates) and by one level because of high risk of bias (attrition bias) ‐ see Appendix 1. cDowngraded by one level because of imprecision (small number of trials) ‐ see Appendix 1. dDowngraded by one level because of imprecision (small number of trials) and by two levels because of severe inconsistency (98% heterogeneity) ‐ see Appendix 1.
Summary of findings 2. Phentermine/topiramate versus placebo for weight reduction.
| Phentermine/topiramate compared with placebo for weight reduction | ||||||
|
Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Phentermine/topiramate Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with phentermine/topiramate | |||||
|
Total mortality Follow‐up: 56 weeks |
see comment | ‐ | 1305 (1 study) | ⊕⊕⊝⊝ lowa | No death occurred in the hypertensive subgroup of the only included RCT. | |
|
Cardiovascular morbidity Follow‐up: 56 weeks |
see comment | ‐ | 1305 (1 study) | ⊕⊝⊝⊝ very lowb |
2.3% of the hypertensive participants in the low‐dose phen/top group, 3.7% in the high‐dose phen/top group, and 1.7% in the placebo group experienced treatment‐emergent cardiovascular adverse events. | |
|
Serious adverse events Follow‐up: 56 weeks |
42 per 1000 | 36 per 1000 (21 to 62) | RR 0.85 (0.49 to 1.48) | 1305 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
|
All adverse events Follow‐up: 56 weeks |
773 per 1000 | 873 per 1000 (835 to 927) | RR 1.13 (1.08 to 1.20) | 1305 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
|
Change in systolic blood pressure compared to placebo (mm Hg) from baseline to end of study Follow‐up: 56 weeks |
Reduction of systolic blood pressure was −4.9 mm Hg | Low dose: MD 2.0 mm Hg lower (3.97 lower to 0.03 lower) |
‐ | 772 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| High dose: MD 4.2 mm Hg lower (5.85 lower to 2.55 lower) |
‐ | 1030 (1 study) | ⊕⊕⊝⊝ lowa | ‐ | ||
|
Change in diastolic blood pressure compared to placebo (mm Hg) from baseline to end of study Follow‐up: 56 weeks |
Reduction of diastolic blood pressure was −4.9 mm Hg | Low dose: MD 1.3 mm Hg lower (2.6 lower to 0.0 lower) | ‐ | 772 (1 study) | ⊕⊕⊝⊝ lowa | ‐ |
| High dose: MD 1.9 mm Hg lower (2.88 lower to 0.92 lower) | ‐ | 1030 (1 study) | ⊕⊕⊝⊝ lowa | ‐ | ||
|
Change in body weight as compared to placebo (%) from baseline to end of study Follow‐up: 56 weeks |
Reduction of body weight was −1.9 % | Low dose: MD 6.3 % lower (7.37 lower to 5.23 lower) | ‐ | 772 (1 study) | ⊕⊕⊝⊝ lowa | Percentage change from initial body weight. |
| High dose: MD 8.2 % lower (9.09 lower to 7.31 lower) | ‐ | 1030 (1 study) | ⊕⊕⊝⊝ lowa | Percentage change from initial body weight. | ||
| CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels because of serious imprecision (single study) ‐ see Appendix 2. bDowngraded by two levels because of serious imprecision (single study) and by one level because of high risk of bias (attrition bias) ‐ see Appendix 2.
Summary of findings 3. Naltrexone/bupropion versus placebo for weight reduction.
| Naltrexone/bupropion compared with placebo for weight reduction | ||||||
|
Patient or population: Men and non‐pregnant women ≥ 18 years old with essential hypertension Intervention: Naltrexone/bupropion Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with naltrexone/bupropion | |||||
|
Total mortality Follow‐up: 121 weeks |
15 per 1000 | 15 per 1000 (11 to 21) | RR 0.99 (0.70 to 1.40) | 8283 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
|
Cardiovascular morbidity Follow‐up: 121 weeks |
29 per 1000 | 32 per 1000 (25 to 40) | RR 1.11 (0.87 to 1.41) | 8283 (1 study) | ⊕⊕⊝⊝ lowb | MI, stroke + hospitalisation for unstable angina. |
|
Serious adverse events Follow‐up: 121 weeks |
205 per 1000 | 215 per 1000 (196 to 233) | RR 1.05 (0.96 to 1.14) | 8283 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
|
All adverse events Follow‐up: 121 weeks |
256 per 1000 | 432 per 1000 (404 to 460) | RR 1.69 (1.58 to 1.80) | 8283 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
|
Change in systolic blood pressure as compared to placebo (mm Hg) from baseline to end of study Follow‐up: 56 weeks |
Increase of systolic blood pressure was 2.2 mm Hg | MD 0.0 mm Hg higher (0.6 lower to 0.6 higher) | ‐ | 8283 (1 study) | ⊕⊕⊕⊕⊝ moderatea | ‐ |
|
Change in diastolic blood pressure as compared to placebo (mm Hg) from baseline to end of study Follow‐up: 121 weeks |
Increase of diastolic blood pressure was 1.1 mm Hg | MD 0.3 mm Hg higher (0.08 lower to 0.68 higher) | ‐ | 8283 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
|
Change in body weight as compared to placebo (kg) from baseline to end of study Follow‐up: 121 weeks |
Reduction of body weight was 0.0 kg | MD 1.9 kg lower (2.07 lower to 1.73 lower) | ‐ | 8283 (1 study) | ⊕⊕⊕⊝ moderatea | ‐ |
| CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by one level because of imprecision (single study). bDowngraded by two levels because of imprecision (single study) and increased risk of bias (other bias: only 50% of the planned number of cardiovascular events were reported) ‐ see Appendix 3
We include all primary and secondary outcomes in the 'Summary of findings' tables, listed according to priority:
Total mortality
Cardiovascular morbidity
Adverse events
Change in systolic blood pressure
Change in diastolic blood pressure
Change in body weight
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our search of the electronic databases for this third update in 2020 yielded 739 records after correcting for duplication. The consensus was that on the basis of their abstracts, 649 of these were not relevant to the question under study and should be excluded. We identified no further studies from the reference lists of the included trials nor from relevant systematic reviews and meta‐analyses. We therefore identified 90 publications for further examination.
In the previous version of the review (Siebenhofer 2016) we retrieved 146 articles for full‐text evaluation. Among them was a report from the German Institute for Quality and Efficiency in Health Care (IQWiG), which provided data for an unpublished subgroup analysis of hypertensive people in the Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) study (IQWiG 2006; XENDOS 2001‐2006).
In summary, we identified 236 publications for further examination. After screening the full text of these selected publications and after contacting authors of potentially relevant studies, we excluded 198 articles. We also excluded five publications (four studies) from the previous version of this review that investigated the effect of sibutramine, since this drug is no longer considered relevant for the current update. Finally, three additional publications referring to the CONQUER 2013 trial (which had been included in the previous version of the review), and five articles referring to one new trial could be included from the 2020 update search. Together with the remaining 25 publications (five studies) from the previous version of the review (Siebenhofer 2016), we include in this third update 33 publications describing six completed studies (see Figure 1 for details of the PRISMA statement; PRISMA 2009).
1.
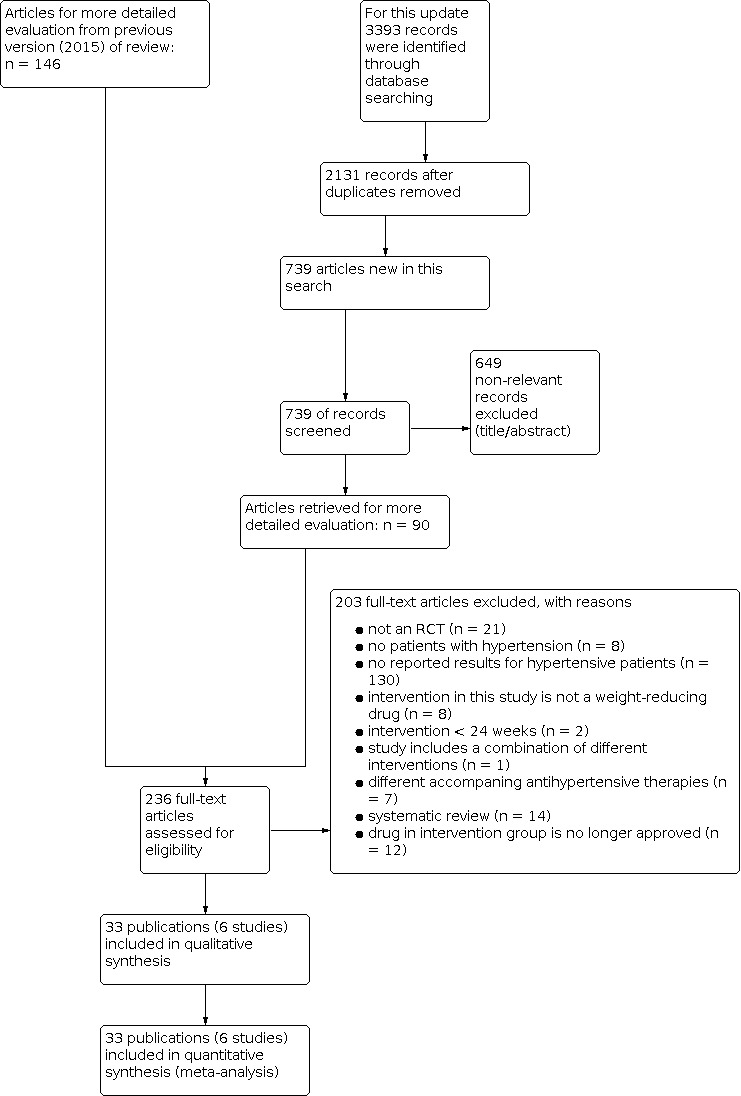
Study flow diagram.
All relevant studies were published after the year 2000 and were written in English, except for the report from the Institute for Quality and Efficiency in Health Care (IQWiG 2006), which was published in German.
Included studies
We have provided details of the included studies in the Characteristics of included studies table and in Table 4; Table 5; Table 6. The following gives a brief overview of the comparisons between orlistat and placebo (four RCTs), naltrexone/bupropion and placebo (one RCT), and phentermine/topiramate and placebo (one RCT). We found no relevant studies investigating liraglutide or lorcaserin, since no results for participants with hypertension were available.
1. Overview of study populations.
| Study | Intervention(s) and comparator(s) | Description of power and sample size calculation | Randomised (N) | Safety (N) | ITT (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up (extended follow‐up) | |
| Orlistat vs placebo | |||||||||
| Bakris 2002 | Orlistat | The sample size determination for the present trial was based on a 2‐sample t‐test (2‐tailed). Since there were 2 primary efficacy parameters in this trial, body weight reduction and sitting diastolic BP reduction, Holm’s sequential rejection procedure was used to project the overall type I error rate 0.05, and α = 0.025 was chosen for the calculation of sample size for each parameter. A mean body weight change of 2.1 kg and a within‐group standard deviation of 6.1 kg would require 161 participants per group to provide a power of 0.8 at α = 0.025. A mean change of 3.0 mm Hg in sitting diastolic BP with a within‐group standard deviation of 8.5 mm Hg would require 153 participants per group to provide power of 0.8 with α = 0.025. Based on these calculations, and assuming a dropout rate of 35%, 496 participants (248 participants per group) had to be enrolled to ensure an adequate statistical power of at least 80% in either of the 2 primary efficacy parameters. | 278 | nr | 267 | 162 | 58 | 24 weeks | |
| Placebo | 276 | nr | 265 | 108 | 36 | ||||
| Cocco 2005 | Orlistat | nr | 45 | 45 | 45 | 45 | 100 | 24 weeks | |
| Placebo | 45 | 45 | 45 | 45 | 100 | ||||
| Guy‐Grand 2004 | Orlistat | Power calculations indicated that, with a power of 80% at the 0.05 significance level, 408 participants were needed to detect a 2.5 mm Hg difference in diastolic BP, 152 participants were needed to detect a 0.05% difference in HbA1c. 140 participants were needed to detect a 0.35 mmol/l difference in LDL‐cholesterol. | 499 (HT: 304) | nr | 499 (HT: 304) | 458 (HT: nr) | 91.6% (HT: nr) | 24 weeks | |
| Placebo | 505 (HT: 310) | nr | 505 (HT: 310) | 458 (HT: nr) | 90.7% (HT: nr) | ||||
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD] |
A 2‐sided log‐rank test would require a minimum of ~ 95 primary cases of type 2 diabetes in both study groups combined to have 90% power of detecting a significant outcome at α = 0.05. With this event‐based design, 3305 participants were randomised and followed until sufficient events occurred. As a consequence of the design, study power would be unaffected by dropout rate. | 1650 | (HT: 408) | nr | 1640 (HT: nr) | (HT: 386) | 94.6 | 208 weeks |
| Orlistat (SBP ≥ 140mm Hg) [OS] |
(HT: 516) | nr | (HT: 491) | 95.2 | |||||
| Placebo (DBP ≥ 90 mm Hg) [PD] |
1655 | (HT: 441) | nr | 1637 (HT: nr) | (HT: 421) | 95.5 | |||
| Placebo (SBP ≥ 140 mm Hg) [PS] |
(HT: 509) | nr | (HT: 487) | 95.7 | |||||
| Phentermine/topiramate vs placebo | |||||||||
| CONQUER 2013 | Phen/Top [LD] | Power analysis based on data from a previous study suggested that 250 participants in each group would provide > 95% power to detect a difference of 4.4% in weight loss between placebo and active treatments at a significance level of 0.05. To enhance the power for detecting differences in safety outcomes, we planned to enrol about 2500 participants. | 498 (HT: 261) | 398 (HT: 261) | 488 (HT: 261) | 374 (HT: 256) | 75.1 (HT: 98.1) | 56 weeks | |
| Phen/Top [HD] | 995 (HT: 520) | 994 (HT: 520) | 981 (HT: 520) | 733 (HT: 514) | 73.7 (HT: 98.8) | ||||
| Placebo | 994 (HT: 524) | 993 (HT: 524) | 976 (HT: 524) | 616 (HT: 516) | 61.9 (HT: 98.5) | ||||
| Naltrexone/bupropion vs placebo | |||||||||
| Nissen 2016 | Nal/Bup | The trial was designed to provide 90% power to rule out the 1.4 margin (i.e. the upper limit of the confidence interval would not exceed 1.4) when the true HR is 1.0, which required 378 primary events. The early pre‐approval analysis to rule out the 2.0 margin required 87 primary events to provide 90% power when the true HR is 1.0. In both settings, a 1‐sided type I error (α) of 2.5% was used. To obtain sample sizes, an annualised rate of primary events of 1.5% in the placebo group was assumed. The recruitment was assumed to take 1.5 years, with maximum participant follow‐up of 4 years. It was assumed that 7% of the study population would discontinue during the lead‐in period, with a loss–to–follow‐up rate of 1.2% annually. | 4456 (HT: 4164) | 4455 (HT: 4164) | 4455 (HT: 4164) | 705 (HT: nr) | 15.8% (HT: nr) | 121 weeks | |
| Placebo | 4454 (HT: 4123) | 4450 (HT: 4119) | 4450 (HT: 4119) | 275 (HT: nr) | 6.2% (HT: nr) | ||||
DBD: diastolic blood pressure; HR: hazard ratio; HT: hypertensive subgroup; ITT: intention‐to‐treat; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure.
2. Baseline characteristics (I).
| Study | Intervention(s) and comparator(s) | Description of participants | Nationality | Setting | Ethnic groups (%) | Duration of disease (mean years (SD)) | Antihypertensive treatment (%) |
| Orlistat vs placebo | |||||||
| Bakris 2002 | Orlistat | Obese individuals with insufficiently controlled hypertension | USA | Outpatient clinic | White (85) African American (11) Hispanic (4) Other (0) |
nr | Antihypertensive‐treatment at trial onset: 95% ACE‐inhibitor (27) Ca‐antagonists (29) β‐blocker (27) Diuretic (19) AT‐II‐receptor antagonists (6) α‐blocker (10) Other (11), |
| Placebo | White (86) African American (12) Hispanic (1) Other (1) |
nr | antihypertensive‐treatment at trial onset: 94% ACE‐inhibitor (35) Ca‐antagonist (30) β‐blocker (26) Diuretic (14) AT‐II‐receptor antagonists (10) α‐blocker (8) Other (9) |
||||
| Cocco 2005 | Orlistat | Obese individuals with metabolic syndrome, diabetes type 2, hypertension, mostly with coronary heart disease (77%) and concomitant cardiac dysfunction | Switzerland | Outpatient clinic | White (100) | nr | Antihypertensive‐treatment at trial onset: 100% ACE‐inhibitor (36) Ca‐antagonist (18) β‐blocker (49) Diuretic (low‐dose hydrochlorothiazide) (60) AT‐II‐receptor antagonists (49) |
| Placebo | |||||||
| Guy‐Grand 2004 | Orlistat | Obese individuals with diabetes type 2, hypertension or hypercholesterolaemia | France | Private practices (endocrinologists) | nr | nr | Antihypertensive‐treatment at trial onset: 70% |
| Placebo | |||||||
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD] | Obese individuals with normal or impaired glucose tolerancea | Sweden | Medical centres | nr | nr | ACE‐inhibitors (7) Ca‐antagonists (6) β‐blockers (17) Diuretics (7) AT‐II‐receptor antagonists (2) |
| Orlistat (SBP ≥ 140 mm Hg) [OS] | ACE‐inhibitors (6) Ca‐antagonists (6) β‐blockers (14) Diuretics (8) AT‐II‐receptor antagonists (2) |
||||||
| Placebo (DBP ≥ 90 mm Hg) [PD] | ACE‐inhibitors (7) Ca‐antagonists (7) β‐blockers (13) Diuretics (11) AT‐II‐receptor antagonists (1) |
||||||
| Placebo (SBP ≥ 140 mm Hg) [PS] | ACE‐inhibitors (8) Ca‐antagonists (8) β‐blockers (13) Diuretics (10) AT‐II‐receptor antagonists (2) |
||||||
| Phentermine/topiramate vs placebo | |||||||
| CONQUER 2013 | Phen/Top [LD] | Obese or overweight individuals with 2 or more comorbidities (hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity)a | USA | Outpatient clinic | White (83) African American (15) Hispanic or Latino (10) |
nr | ACE inhibitors alone (26.9) β‐blockers alone (24.1) AT‐II‐receptor antagonists alone (15.5) ACE inhibitors + diuretics (5.8) ACE inhibitors + Ca‐antagonists (3.5), AT‐II‐receptor antagonists + diuretics (12.4) AT‐II‐receptor antagonists + Ca‐antagonists (0.9) |
| Phen/Top [HD] | |||||||
| Placebo | |||||||
| Naltrexone/bupropion vs placebo | |||||||
| Nissen 2016 | Nal/Bup | Overweight or obese people at increased risk of adverse cardiovascular outcomes | USA | Medical sites | White (83.3) Black (15.3) Other (1.4) |
12.2 (9.40) | 97.1 |
| Placebo | White (82.3) Black (15.3) Other (2.4) |
11.6 (9.03) | 97.2 | ||||
a only data for the predefined subgroup of hypertensive participants are reported here.
ACE inhibitors: angiotensin‐converting enzyme inhibitors; AT‐II‐receptor antagonists: angiotensin II‐receptor antagonists; DBP: diastolic blood pressure; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top; [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure; SD: standard deviation.
3. Baseline characteristics (II).
| Study | Intervention(s) and comparator(s) | Age (mean years (SD)) | Sex (female %) | BMI (mean kg/m² (SD)) | Body weight (mean kg (SD)) | Sitting systolic blood pressure (mean mm Hg (SD)) | Sitting diastolic blood pressure (mean mm Hg (SD)) | Comorbid conditions (%) |
| Orlistat vs placebo | ||||||||
| Bakris 2002 | Orlistat | 53.2 (0.5) | 63 | 35.8 (3.9) | 101.2 (1.0) | 154.2 (13.4) | 98.4 (3.7) | diabetes (8) |
| Placebo | 52.5 (0.5) | 59 | 35.4 (4.0) | 101.5 (1.0) | 150.8 (12.7) | 98.3 (35) | diabetes (8) | |
| Cocco 2005 | Orlistat | 54.9 (5.1) | 51 | 36.5 (1.9) | 107.0 (5.7) | 145.8 (9.8) | 87.8 (7.3) | metabolic syndrome (100) coronary heart disease (77) myocardial infarction (47) |
| Placebo | 54.5 (4.5) | 51 | 36.1 (1.8) | 106.0 (5.9) | 142.1 (6.2) | 85.3 (5.6) | ||
| Guy‐Grand 2004 | Orlistat | 49.1 (0.6) | 69 | 34.3 (0.2) | 93.9 (0.8) | 150.0 (0.8) | 96.9 (0.3) | nr |
| Placebo | 49.5 (0.5) | 65 | 33.9 (0.2) | 93.5 (0.8) | 152.2 (0.9) | 97.0 (0.3) | nr | |
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD] | 46 (7) | 3862 | nr | 116 (17) | 146 (13) | 95 (5) | nr |
| Orlistat (SBP ≥ 140mm Hg) [OS] | 47(7) | 42 | nr | 116 (17) | 149 (10) | 91 (9) | nr | |
| Placebo (DBP ≥ 90 mm Hg) [PD] | 46 (7) | 4456 | nr | 114 (18) | 142 (126) | 95 (5) | nr | |
| Placebo (SBP ≥ 140 mm Hg) [PS] | 47(7) | 42 | nr | 115 (18) | 149 (8) | 91 (8) | nr | |
| Phentermine/topiramate vs placebo | ||||||||
| CONQUER 2013 | Phen/Top [LD] | 53.0 (9.8) | 65.9% | 36.7 (4.6) | 104.4 (18.4) | 134.2 (13.0) | 83.7 (9.1) | nr |
| Phen/Top [HD] | ||||||||
| Placebo | ||||||||
| Naltrexone/bupropion vs placebo | ||||||||
| Nissen 2016 | Nal/Bup | 61.8 (7.27) | 54.5 | 37.1 (5.27) | 106 (19.09) | 126.1 (12.55) | 74.5 (9.01) | History of cardiovascular disease (30.3) History of Type 2 diabetes (86.4) History of dyslipidaemia (92.4) History of low LDL (27.8) Current smoker (8.4) |
| Placebo | 61.6 (7.38) | 54.4 | 37.3 (5.42) | 106.6 (19.17) | 125.7 (12.62) | 74.4 (9.14) | History of cardiovascular disease (31.3) History of type 2 diabetes (86.9) History of dyslipidemia (92.1) History of low LDL (28.2) Current smoker (8.5) |
|
BMI: body mass index; LDL: low‐density lipoprotein; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure; SD: standard deviation.
Orlistat versus placebo
All four included studies had a parallel, double‐blind design (Bakris 2002; Cocco 2005; Guy‐Grand 2004; XENDOS 2001‐2006). Only Cocco 2005 did not mention any industry sponsorship, and it was the only study that was performed as a single‐centre trial.
Participants and duration
The four included studies involved a total of 3132 hypertensive participants with a mean age of 46 to 55 years, a baseline systolic blood pressure (BP) of 142 to 154 mm Hg and a baseline diastolic BP of 85 to 98 mm Hg. Mean treatment duration was 6 to 48 months. All participants in Bakris 2002 and Cocco 2005 were overweight and hypertensive. In Cocco 2005 all participants also had diabetes mellitus type 2. One trial (Guy‐Grand 2004) included overweight people with diabetes mellitus type 2, hypercholesterolaemia or hypertension, and reported results for the predefined subgroup of participants with hypertension. XENDOS 2001‐2006 included both normotensive and hypertensive obese participants. Data are available for two predefined subgroups of hypertensive participants (first subgroup: diastolic BP at baseline ≥ 90 mm Hg; second subgroup: systolic BP at baseline ≥ 140 mm Hg). Participants in one subgroup may also be included in the other subgroup. We present the data for the two subgroups separately in this review. For meta‐analyses we used the results of the subgroup with participants having a diastolic BP of ≥ 90 mm Hg at baseline.
Interventions
Participants received either 120 mg orlistat three times daily or placebo in all studies. In Bakris 2002, Cocco 2005 and XENDOS 2001‐2006, participants in all study groups were also encouraged to modify their lifestyle and increase physical activity.
Outcomes
Primary outcomes
No study included mortality or cardiovascular morbidity as predefined outcomes. All studies reported adverse events.
Secondary outcomes
All studies described the mean change in systolic and diastolic BP and the mean change in body weight.
Phentermine/topiramate versus placebo
The only included study was multicentre, with 93 study sites in the USA (CONQUER 2013). The study had a parallel, double‐blind design and the industry sponsor was mentioned.
Participants and duration
The included study involved 2487 obese or overweight people with two or more comorbidities (hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity). The hypertensive subgroup included 1305 participants with a mean age of 53 years, a mean baseline systolic BP of 134 mm Hg and a mean baseline diastolic BP of 84 mm Hg. About 90% of the included participants were taking antihypertensive medication. Among the hypertensive subgroup, 216 participants had uncontrolled hypertension at baseline. The treatment duration was 56 weeks.
Interventions
The study compared two different dose regimens of phentermine/topiramate once daily versus placebo. All participants in the active groups received an initial dose of 7.5 mg phentermine and 23 mg topiramate. During an initial four‐week titration period, doses were increased weekly (3.75 mg phentermine and 23 mg topiramate) until the assigned dosages of 7.5 mg phentermine/46.0 mg topiramate (group low dose) or 15 mg phentermine/92.0 mg topiramate (group high dose) were achieved. The assigned dosages were maintained for 52 weeks. In addition, all participants received a standardised counselling for diet (to reduce caloric intake by 500 kcal/day) and lifestyle modification.
Outcomes
Primary outcomes
The study did not include mortality or cardiovascular morbidity as predefined outcomes. Adverse events were reported.
Secondary outcomes
The study described the mean change in systolic and diastolic BP and the mean percentage change in body weight.
Naltrexone/bupropion versus placebo
The included trial was a parallel, double‐blind placebo‐controlled multicentre trial with 266 study sites in the USA (Nissen 2016). The trial was funded by Orexigen Therapeutics Inc. and Takeda Pharmaceuticals International.
Participants and duration
The trial involved 8910 obese or overweight people aged at least 45 years (men) or 50 years (women), with an increased risk of adverse cardiovascular events. Results for the subgroup of participants with hypertension were provided by the authors upon request. The hypertensive subgroup included 8287 participants (93% of the total study population), with a mean age of 62 years, a mean baseline systolic BP of 126 mm Hg and a mean baseline diastolic BP of 75 mm Hg. About 97% of the included participants were taking antihypertensive medication. Among the hypertensive subgroup, about 87% of the participants had diabetes mellitus type 2 and about 30% had cardiovascular disease. The median duration of follow‐up was 121 weeks (interquartile range,114 to 128 weeks).
Interventions
The participants received either a fixed‐dose combination of naltrexone (8 mg) and bupropion (90 mg) or placebo once or twice a day. During an initial four‐week titration period, the number of tablets were increased weekly (one tablet a day during the first week, two tablets a day during week two, three tablets a day during week three, and four tablets a day during week four and thereafter). All participants also received an internet‐based weight management programme focused on healthy eating and physical activity.
Outcomes
Primary outcomes
All‐cause mortality was reported as an additional outcome. Cardiovascular death, fatal or nonfatal stroke, fatal or nonfatal myocardial infarction were reported as primary and secondary outcomes.
Secondary outcomes
The study described the mean change in systolic and diastolic BP and the mean change in body weight.
Excluded studies
Full‐text evaluation in the study selection process of this review resulted in the exclusion of 124 studies (203 publications/records). The main reason for exclusion was a lack of data for the hypertensive subgroup in studies including normotensive as well as hypertensive participants. Other reasons for exclusion were not describing a randomised controlled trial, not including participants with essential hypertension, having a duration of intervention of less than 24 weeks, or including different accompanying antihypertensive therapies in the study groups. We provide reasons for excluding each trial in the Characteristics of excluded studies table.
Risk of bias in included studies
The judgements of the risk of bias for all included studies are shown in the 'Risk of bias' summary figures (Figure 2; Figure 3). For details see the 'Risk of bias' tables in Characteristics of included studies. The following provides a brief overview.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Orlistat versus placebo
All included studies were randomised controlled trials involving randomised individuals. For method of randomisation, we judged three trials (Cocco 2005; Guy‐Grand 2004; XENDOS 2001‐2006) to have a low risk of bias based on the information from journal publications or the IQWIG report (IQWiG 2006). In one study the method of randomisation was not reported and therefore unclear (Bakris 2002). The method of allocation concealment was adequate in two studies (Guy‐Grand 2004; XENDOS 2001‐2006), while it was unclear in the other two studies (Bakris 2002; Cocco 2005).
Phentermine/topiramate versus placebo
The included study adequately described the method of randomisation and concealment (CONQUER 2013).
Naltrexone/bupropion versus placebo
The study adequately described the method of randomisation and concealment (Nissen 2016).
Blinding
Orlistat versus placebo
While all included trials were described as double‐blind, three trials provided too little information, and the blinding of participants and key study personnel was uncertain (Bakris 2002; Cocco 2005; Guy‐Grand 2004). Based on the authors' information, we can only assume that blinding took place throughout the duration of one study (Cocco 2005).
Phentermine/topiramate versus placebo
The included study was described as double‐blind. The investigators, participants, and study sponsors were masked to treatment assignment, and all study drugs were administered as capsules that were identical in size and appearance (CONQUER 2013).
Naltrexone/bupropion versus placebo
The included study was described as double‐blind. The investigators and participants were masked to treatment assignment. A blinded independent clinical events committee adjudicated clinical outcomes, including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, hospitalisation for unstable angina, and all‐cause mortality (Nissen 2016).
Incomplete outcome data
Orlistat versus placebo
In two studies, the outcome data description was complete for total mortality, adverse events, change in blood pressure and change in body weight: Cocco 2005 had no losses to follow‐up, and Bakris 2002 described all reasons for withdrawals and losses to follow‐up. Cardiovascular morbidity was reported only in the context of adverse events in these two RCTs, so the completeness of the information is unclear. For this endpoint we judged the risk of bias to be high.
In Guy‐Grand 2004, withdrawals were only reported for the whole study population and not for the hypertensive subgroup, and in XENDOS 2001‐2006 the reasons for withdrawals were incompletely reported. We rated the risk of bias for these two studies as unclear for all reported outcomes. Cardiovascular morbidity was reported only in the context of adverse events in the XENDOS 2001‐2006, so the completeness of the information is unclear. For this endpoint we judged the risk of bias to be high.
Phentermine/topiramate versus placebo
The total number of withdrawals was only reported for the whole study population and not for the hypertensive subgroup of the study (CONQUER 2013). For the hypertensive subgroup, only the number of withdrawals due to adverse events was reported, so the reasons for withdrawals were incompletely reported. We therefore judged the risk of bias to be unclear for all reported outcomes, except for cardiovascular morbidity. Cardiovascular morbidity was reported only in the context of treatment‐emergent adverse events, so the completeness of the information is unclear. For this endpoint we judged the risk of bias to be high.
Naltrexone/bupropion versus placebo
All primary analyses were performed for the intention‐to‐treat (ITT) population, defined as participants who underwent randomisation into the treatment period and were dispensed study medication. No imputation was performed for missing data. The number of withdrawals and reasons were only reported for the whole study population, but not for the subset of participants with hypertension (Nissen 2016).
Selective reporting
Orlistat versus placebo
As either no study protocol was provided (Bakris 2002; Cocco 2005; Guy‐Grand 2004), or no full publication was obtainable (XENDOS 2001‐2006), or more outcomes were reported than were prespecified (Bakris 2002), we classified the risk of bias for selective reporting as uncertain for all studies.
Phentermine/topiramate versus placebo
No study protocol was available for CONQUER 2013. We therefore classified the risk of bias for selective reporting as uncertain.
Naltrexone/bupropion versus placebo
A study protocol was provided and all prespecified outcomes were reported. The study was terminated prematurely after the unplanned release of the 25% interim data, which was judged as compromising the scientific integrity of the ongoing study. Outcome measures data based on the 50% interim analysis were designated as the primary analysis. Safety data were based on all available data at the time of database lock, which occurred after the 50% interim analysis (Nissen 2016).
Other potential sources of bias
Orlistat versus placebo
No trial included in the review reported any significant differences between groups in the main characteristics of participants at baseline. However, in Bakris 2002 and XENDOS 2001‐2006, the combination of a high withdrawal rate and the unknown length of involvement of participants in the trial increases the risk of bias, even when last observation carried forward (LOCF) analysis is used. In addition, within Bakris 2002 there were inconsistencies between the text and flowchart for numbers of participants who finished the study.
Phentermine/topiramate versus placebo
We could identify no other potential source of bias in the included study (CONQUER 2013).
Naltrexone/bupropion versus placebo
There were no significance differences between groups in the main characteristics of participants at baseline. There was an increased risk of bias because of very high withdrawal rates (84% and 94%, respectively), without imputation for missing data (Nissen 2016).
Effects of interventions
See: Table 1; Table 2; Table 3
1. Orlistat versus placebo
See: Table 1.
Primary outcomes
For details on primary outcome data see Table 7 and Table 8.
4. Adverse events (I).
| Study | Intervention(s) and comparator(s) | Randomised/Safety (N) | Death (n (%)) | All adverse events (n (%)) | Leading to withdrawal (n (%)) | Serious adverse events (n (%)) |
| Orlistat vs placebo | ||||||
| Bakris 2002 | Orlistat | 278/268a | 0 (0) | 239 (89) | 18 (6.7) | 31 (11.7) |
| Placebo | 276/274a | 0 (0) | 195 (71) | 20 (7.3) | 24 (8.6) | |
| Cocco 2005 | Orlistat | 45/45 | 0 (0) | nr | nr | 0 (0) |
| Placebo | 45/45 | 0 (0) | nr | nr | 0 (0) | |
| Guy‐Grand 2004 | Orlistat | 304/304 | nr | nr | nr | nr |
| Placebo | 310/310 | nr | nr | nr | nr | |
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD] | 408/407 | 2 (0.5) | 403 (99) | 37 (9) | 73 (18) |
| Orlistat (SBP ≥ 140mm Hg) [OS] | 516/513 | 1 (0.2) | 508 (99) | 46 (9) | 92 (18) | |
| Placebo (DBP ≥ 90 mm Hg) [PD] | 441/437 | 0 (0) | 420 (96) | 17 (4) | 52 (12) | |
| Placebo (SBP ≥ 140 mm Hg) [PS] | 509/508 | 0 (0) | 493 (97) | 20 (4) | 60 (12) | |
| Phentermine/topiramate vs placebo | ||||||
| CONQUER 2013 | Phen/Top [LD] | 261/261 | 0 (0) | 223 (85.4) | 31 (11.9) | 9 (3.4) |
| Phen/Top [HD] | 520/520 | 0 (0) | 462 (88.8) | 103 (19.8) | 19 (3.7) | |
| Placebo | 524/524 | 0 (0) | 405 (77.3) | 51 (9.7) | 22 (4.2) | |
| Naltrexone/bupropion vs placebo | ||||||
| Nissen 2016 | Nal/Bup | 4164/4164 | 63 (1.5) / P = 0.916 | 1796 (43.1) / P < 0.001 | 1273 (30.6) / P < 0.001 | 891 (21.4) / P = 0.297 |
| Placebo | 4119/4119 | 63 (1,5) | 1053 (25.6) | 379 (9.2) | 843 (20.5) | |
aCalculated from percentage rates
DBP: diastolic blood pressure; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure.
5. Adverse events (II).
| Study | Intervention(s) and comparator(s) | Randomised/Safety /N) | Gastrointestinal AE (n (%)) | Musculoskeletal AE (n (%)) | Dermatological AE (n (%)) | Vascular AE (n (%)) | Cardiac AE (n (%)) | Nervous system AE (n (%)) | Respiratory AE (n (%)) |
| Orlistat vs placebo | |||||||||
| Bakris 2002 | Orlistat | 278/nr | 200 (72.5) | nr (22.8) | nr | nr | 5 (nr)a | nr | nr |
| Placebo | 276/nr | 120 (43.6) | nr (15.5) | nr | nr | 5 (nr)a | nr | nr | |
| Cocco 2005 | Orlistat | 45/45 | 16 (35.6)b | nr | nr | nr | nr | nr | nr |
| Placebo | 45/45 | 11 (24.4)b | nr | nr | nr | nr | nr | nr | |
| Guy‐Grand 2004 | Orlistat | 304/304 | nr | nr | nr | nr | nr | nr | nr |
| Placebo | 310/310 | nr | nr | nr | nr | nr | nr | nr | |
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD] | 408/407 | 379 (93) | 265 (65) | 81 (20) | 69 (17) | nr | 159 (39) | nr |
| Orlistat (SBP ≥ 140mm Hg) [OS] | 516/513 | 477 (93) | 333 (65) | 113 (22) | 87 (17) | nr | 205 (40) | nr | |
| Placebo (DBP ≥ 90 mm Hg) [PD] | 441/437 | 306 (70) | 271 (62) | 74 (17) | 83 (19) | nr | 170 (39) | nr | |
| Placebo (SBP ≥ 140 mm Hg) [PS] | 509/508 | 361 (71) | 320 (63) | 86 (17) | 97 (19) | nr | 188 (37) | nr | |
| Phentermine/topiramate vs placebo | |||||||||
| CONQUER 2013 | Phen/Top [LD] | 261/261 | Constipation: 41 (15.7) | nr | nr | nr | 6 (2.3) | Dry mouth: 37 (14.2) Paresthesia: 37 (14.2) Dysgeusia: 20 (7.7) Insomnia: 15 (5.7) Headache: 13 (5.0) Dizziness: 17 (6.5) |
Upper respiratory tract infection: 33 (12.6) Nasopharyngitis: 27 (10.3) Sinusitis: 14 (5.4) |
| Phen/Top [HD] | 520/520 | Constipation: 94 (18.1) | nr | nr | nr | 19 (3.7) | Dry mouth: 118 (22.7) Paresthesia: 116 (22.3) Dysgeusia: 57 (11.0) Insomnia: 57 (11.0) Headache: 56 (10.8) Dizziness: 63 (12.1) |
Upper respiratory tract infection: 63 (12.1) Nasopharyngitis: 53 (10.2) Sinusitis: 43 (8.3) |
|
| Placebo | 524/524 | Constipation: 29 (5.5) | nr | nr | nr | 9 (1.7) | Dry mouth: 12 (2.3) Paresthesia: 12 (2.3) Dysgeusia: 4 (0.8) Insomnia: 25 (4.8) Headache: 44 (8.4) Dizziness: 16 (3.1) |
Upper respiratory tract infection: 62 (11.8) Nasopharyngitis: 46 (8.8) Sinusitis: 34 (6.5) |
|
| Naltrexone/bupropion vs placebo | |||||||||
| Nissen 2016 | Nal/Bup | 4164/4164 | 718 (17.2) / P < 0.001 | 181 (4.3) / P = 0.749 | 45 (1.1) / P = 0.002 | 158 (3.8)c / P = 0.978 | 313 (7.5) / P < 0.001 | 74 (1.8) / P = 0.947 | |
| Placebo | 4119/4119 | 142 (3.4) | 185 (4.5) | 20 (0.5) | 155 (3.8)c | 111 (2.7) | 74 (1.8) | ||
aOnly serious cardiac events. bNo data on adverse events were reported for the whole study duration. The data above refer to 4 and 3 weeks of treatment in the orlistat and placebo group, respectively. After 3 months, the number of participants with events decreased to 5 (11%) [O] with flatulence and mild abdominal cramps versus 6 (13%) [P] with nausea and hunger feeling. c Major adverse cardiovascular events (cardiovascular death, non‐fatal stroke, non‐fatal myocardial infarction) + hospitalisation for unstable angina.
AE: adverse events; DBP: diastolic blood pressure; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure.
Total mortality
Three of four studies reported on mortality. No deaths were reported in either Bakris 2002 or Cocco 2005. In XENDOS 2001‐2006, there were two deaths in the orlistat‐treated group in the first subgroup analysis (diastolic BP ≥ 90 mm Hg) and one death in the orlistat group in the second subgroup analysis (systolic BP ≥ 140 mm Hg); no deaths occurred in the placebo group.
Cardiovascular morbidity
Three studies presented data on cardiovascular morbidity. In Bakris 2002, two participants in the orlistat group suffered a myocardial infarction, two had chest pain, and one had an episode of atrial fibrillation. In the placebo group, one participant had a myocardial infarction, one had worsening atherosclerotic coronary artery disease, and two had an episode of chest pain. Cocco 2005 reported that in participants with resting left ventricular ejection fraction (LVEF) below 50% at baseline, LVEF did not change with placebo (0.6%), but was increased by 4.3% in the orlistat group (P < 0.001). In XENDOS 2001‐2006, vascular complications were reported in the context of adverse events. In both subgroups (diastolic BP ≥ 90 mm Hg and systolic BP ≥ 140 mm Hg), 17% of the participants treated with orlistat and 19% treated with placebo reported a vascular adverse event.
Adverse events
Data on serious adverse events (SAEs) were available from three trials (Bakris 2002; Cocco 2005; XENDOS 2001‐2006), with significantly more SAEs in participants treated with orlistat compared to those who received placebo: RR 1.45 (95% CI 1.10 to 1.91; 3 trials, 1476 participants; Analysis 1.1). Overall adverse events were reported in two RCTs (Bakris 2002; XENDOS 2001‐2006), with no significant difference between the orlistat and the placebo group: RR 1.13 (95% CI 0.84 to 1.54; 2 trials, 1386 participants; Analysis 1.2).
1.1. Analysis.

Comparison 1: Orlistat versus placebo, Outcome 1: Serious adverse events
1.2. Analysis.

Comparison 1: Orlistat versus placebo, Outcome 2: All adverse events
Bakris 2002: At least one adverse event was reported by significantly more participants in the orlistat‐treated group (89%) than in the placebo‐treated group (71%), with a P value < 0.001. Of those, 7% of participants in the orlistat group versus 7% of participants in the placebo group withdrew. Twelve per cent of all adverse events in the orlistat‐treated group versus 9% in the placebo‐treated group were classified as serious adverse events, and none was classified as being related to study medication. Gastrointestinal side effects were significantly higher in the orlistat‐treated group than in the placebo group (73% versus 44%; P < 0.001); 8% of those participants in the orlistat group and 5% in the placebo group stopped taking the medication for this reason. Musculoskeletal side effects were also reported significantly more often in the orlistat versus the placebo group (23% versus 16%; P < 0.05).
Cocco 2005: As reported by the authors, side effects were mild. No overall adverse events were reported. Gastrointestinal side effects were the most common adverse events and were described for 24% of the placebo group within the first three weeks and for 36% of the orlistat group within the first four weeks.
Guy‐Grand 2004: Data were only presented for the whole study group, with no information provided on the hypertensive subgroup.
XENDOS 2001‐2006: First subgroup (diastolic BP ≥ 90 mm Hg): Side effects were reported in 99% of participants in the orlistat and 96% of participants in the placebo group. Eighteen per cent of participants in the orlistat group and 12% of participants in the placebo group experienced severe adverse events. Gastrointestinal side effects were more common in the orlistat (93%) versus the placebo group (70%). Musculoskeletal, nervous, dermatological, and vascular events were comparable in both treatment groups. Nine per cent withdrew due to side effects in the orlistat versus 4% in the placebo group, but it is not clear whether the reported side effects were study drug‐related. Second subgroup (systolic BP ≥ 140 mm Hg): Side effects were reported in 99% of participants in the orlistat and 97% of participants in the placebo group. Eighteen per cent of participants in the orlistat group and 12% of participants in the placebo group experienced severe adverse events. Gastrointestinal side effects were more common in the orlistat (93%) versus the placebo group (71%). Musculoskeletal, nervous, dermatological, and vascular events were comparable in both treatment groups. Nine per cent withdrew due to side effects in the orlistat versus 4% in the placebo group. It is not clear whether the reported side effects were study drug‐related.
Secondary outcomes
For details on secondary outcome data see Table 9 (body weight), Table 10 (systolic blood pressure), and Table 11 (diastolic blood pressure). For XENDOS 2001‐2006, we used the results after 12 months study duration for the subgroup of participants with diastolic BP ≥ 90 mm Hg at baseline for the meta‐analyses. Due to between‐study variability, we have presented results from random‐effects models in the analysis of body weight.
6. Body weight.
| Study | Intervention(s) and comparator(s) | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | ||||||
| Bakris 2002b | Orlistat | 101.2 (1.0)c | nr | nr | na | −5.4 (6.4) / P < 0.001 (after 12 months) |
| Placebo | 101.5 (1.0)c | nr | nr | na | −2.7 (6.4) (after 12 months) | |
| Cocco 2005 | Orlistat | 106.9 (5.7) | 101.6(4.5) | na | na | −5.4c /P < 0.001 (after 6 months) |
| Placebo | 105.9 (5.9) | 103.5 (5.3) | na | na | −2.5c (after 6 months) | |
| Guy‐Grand 2004 | Orlistat | 93.9 (0.8)d | nr | na | na | −5.8 (0.3) / P < 0.0001 (after 6 months) |
| Placebo | 93.5 (0.8)d | nr | na | na | −1.8 (0.2) (after 6 months) | |
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD]e | 117 (18) | 106 (17) | 105 (18) | 110 (19) | −11.9 (8.2) / P = nr (after 12 months)f −6.6 (8.6) / P < 0.001 (after 48 months) |
| Orlistat (SBP ≥ 140mm Hg ) [OS]e | 117 (17) | 106 (17) | 105 (17) | 110 (18) | −6.8 (8.7) / P < 0.001 (after 48 months) | |
| Placebo (DBP ≥ 90 mm Hg) [PD]e | 115 (18 | 108 (18) | 108 (19) | 111 (20) | −6.9 (7.6) (after 12 months)f −3.8 (7.8) (after 48 months) |
|
| Placebo (SBP ≥ 140 mm Hg) [PS]e | 116 (18) | 109 (18) | 110 (19) | 113 (19) | −3.2 (7.4) (after 48 months) | |
| Phentermine/topiramate vs placebo | ||||||
| CONQUER 2013 | Phen/Top [LD] | 104 (18)g | nr | nr | na | −8.2% (95% CI 7.3; 9.0) / P < 0.0001 (after 13 months) |
| Phen/Top [HD] | nr | nr | na | −10.1% (95% CI 9.5; 10.7) / P < 0.0001 (after 13 months) | ||
| Placebo | nr | nr | na | −1.9% (95% CI 1.3; 2.6) (after 13 months) | ||
| Naltrexone/bupropion vs placebo | ||||||
| Nissen 2016 | Nal/Bup | 106 (19.09) | nr | nr | nr | −1.9 (4.26) / P < 0.001 (after 28 months) |
| Placebo | 106.6 (19.17) | nr | nr | nr | −0.0 (3.50) (after 13 months) | |
aMean kg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cPublished values are different, but data were corrected after personal communication with the author. dReported as being the standard deviation but probably the standard error due to its small number. eBased on data of 407 [OD], 437 [PD], 513 [OS], and 508 [PS] participants. f12 months results used for meta‐analysis. gReported only combined for all three study groups.
CI: confidence interval; DBP: diastolic blood pressure; mo: months; na: not applicable; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure; SD: standard deviation.
7. Systolic blood pressure.
| Study | Intervention(s) and comparator(s) | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | ||||||
| Bakris 2002b | Orlistat | 154 (13) | nr | nr | na | −13.3 (15.2) / ns (after 12 months) |
| Placebo | 151 (13) | nr | nr | na | −11.0 (15.0) (after 12 months) | |
| Cocco 2005 | Orlistat | 145.8 (9.8) | 141.5 (12.5) | na | na | −4.3 / P = 0.025 (after 6 months) |
| Placebo | 142.1 (6.2) | 141.2 (8.8) | na | na | −0.9 (after 6 months) | |
| Guy‐Grand 2004 | Orlistat | 150.0 (0.8)c | nr | na | na | −9.8 (1) / ns (after 6 months) |
| Placebo | 152.2 (0.9)c | nr | na | na | −9.8 (1) (after 6 months) | |
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD]d | 146 (13) | 135 (14) | 135 (14) | 137 (15) | −11.2 (13.5) / P = nr (after 12 months)e −8.8 (14.8) / P = 0.024 (after 48 months) |
| Orlistat (SBP ≥ 140mm Hg) [OS]d | 149 (10) | 125 (14) | 135 (14) | 138 (15) | −11.5 (14.9) / P < 0.002 (after 48 months) | |
| Placebo (DBP ≥ 90 mm Hg) [PD]d | 146 (12) | 136 (15) | 138 (16) | 139 (16) | −7.7 (13.8) (after 12 months)e −6.4 (15.1) (after 48 months) |
|
| Placebo (SBP ≥ 140 mm Hg) [PS]d | 149 (8) | 138 (14) | 140 (14) | 140 (15) | −8.6 (14.3) (after 48 months) | |
| Phentermine/topiramate vs placebo | ||||||
| CONQUER 2013 | Phen/Top [LD] | 134.3 (nr) | nr | nr | na | −6.9 (95% CI 5.3; 8.5) / P = 0.0475 (after 13 months) |
| Phen/Top [HD] | 133.1 (nr) | nr | nr | na | −9.1 (95% CI 7.9; 10.3) / P < 0.001 (after 13 months) | |
| Placebo | 135.2 (nr) | nr | nr | na | −4.9 (95% CI 3.7; 6.1) (after 13 months) | |
| Naltrexone/bupropion vs placebo | ||||||
| Nissen 2016 | Nal/Bup | 126.1 (12.55) | nr | nr | nr | −2.2 (14.45) / P = 0.706 (after 28 months) |
| Placebo | 125.7 (12.62) | nr | nr | nr | −2.2 (13.51) (after 28 months) | |
aMean mm Hg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cReported as being the standard deviation but probably the standard error due to its small number. dBased on last observation carried forward data on 399 [OD], 423 [PD], 493 [OS], and 504 [PS] participants. e12 months results used for meta‐analysis.
DBP: diastolic blood pressure; mo: months; na: not applicable; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure; SD: standard deviation.
8. Diastolic blood pressure.
| Study | Intervention(s) and comparator(s) | Baselinea | 6 moa | 12 moa | 48 moa | Change from baseline to endpointa |
| Orlistat vs placebo | ||||||
| Bakris 2002b | Orlistat | 98.4 (3.7) | nr | nr | na | −11.4 (8.3) / P = 0.002 (after 12 months) |
| Placebo | 98.3 (3.5)c | nr | nr | na | −9.2 (8.4) (after 12 months) | |
| Cocco 2005 | Orlistat | 87.8 (7.3) | 84.2 (8.6) | na | na | −3.6 / P = 0.012 (after 6 months) |
| Placebo | 85.3 (5.6) | 84.5 (7.3) | na | na | −0.8 (after 6 months) | |
| Guy‐Grand 2004 | Orlistat | 96.9 (0.3)d | nr | na | na | −7.5 (0.6) / ns (after 6 months) |
| Placebo | 97.0 (0.3)d | nr | na | na | −7.3 (0.6) (after 6 months) | |
| XENDOS 2001‐2006 | Orlistat (DBP ≥ 90 mm Hg) [OD]e | 95 (6) | 86 (8) | 86 (8) | 87 (9) | −9.1 (7.9) / P = nr (after 12 months)f −8.1 (9.3) / P < 0.006 (after 48 months) |
| Orlistat (SBP ≥ 140mm Hg) [OS]e | 91 (9) | 84 (9) | 85 (9) | 86 (9) | −5.0 (9.9) / P < 0.001 (after 48 months) | |
| Placebo (DBP ≥ 90 mm Hg) [PD]e | 95 (5) | 88 (9) | 88 (10) | 89 (10) | −6.7 (9.6) (after 12 months)f −6.2 (9.9) (after 48 months) |
|
| Placebo (SBP ≥ 140 mm Hg) [PS]e | 91 (8) | 87 (9) | 88 (10) | 88 (10) | −3.0 (10.4) (after 48 months) | |
| Phentermine/topiramate vs placebo | ||||||
| CONQUER 2013 | Phen/Top [LD] | 83.4 (nr) | nr | nr | na | −5.2 (95% CI 4.1; 6.3) / P = 0.04 (after 13 months) |
| Phen/Top [HD] | 83.2 (nr) | nr | nr | na | −5.8 (95% CI 5.1; 6.5) / P = 0.0003 (after 13 months) | |
| Placebo | 84.5 (nr) | nr | nr | na | −3.9 (95% CI 3.2; 4.6) (after 13 months) | |
| Naltrexone/bupropion vs placebo | ||||||
| Nissen 2016 | Nal/Bup | 74.5 (9.01) | nr | nr | na | 1.4 (9.05) / P = 0.326 (after 28 months) |
| Placebo | 74.4 (9.14) | nr | nr | na | 1.1 (8.50) (after 28 months) | |
aMean mm Hg (SD), unless otherwise indicated. bData are reported for 267 of 278 [O] and 265 of 276 [P] participants only. cThe standard deviation was published as being 35 but should probably be 3.5. dReported as being the standard deviation but probably the standard error due to its small number. eBased on last observation carried forward data on 399 [OD], 423 [PD], 493 [OS], and 504 [PS] participants. f12 months results used for meta‐analysis.
DBP: diastolic blood pressure; mo: months; na: not applicable; nr: not reported; [OD]: orlistat and diastolic blood pressure ≥ 90 mm Hg; [OS]: orlistat and systolic blood pressure ≥ 140 mm Hg; Phen/Top [HD]: phentermine/topiramate high dose (15 mg/92 mg); Phen/Top [LD]: phentermine/topiramate low dose (7.5 mg/46 mg); [PD]: placebo and diastolic blood pressure ≥ 90 mm Hg; [PS]: placebo and systolic blood pressure ≥ 140 mm Hg; SBD: systolic blood pressure; SD: standard deviation.
Body weight
Orlistat was found to lower body weight significantly more effectively than placebo in all studies. The meta‐analysis of orlistat studies yielded a MD of −3.7 kg (95% CI −4.7 to −2.8; 4 trials, 2080 participants; Analysis 1.3) after a study duration of six to 12 months. The test for heterogeneity gave a P value of 0.02 (I2 = 68%) (see Figure 4). Differences in study quality could not explain the heterogeneity. We could deduce no plausible explanation for heterogeneity from differences in study design, study duration, sample sizes, interventions, or characteristics of included participants. In XENDOS 2001‐2006, results after 48 months of follow‐up were reported for the subgroups of participants with diastolic BP ≥ 90 mm Hg at baseline and systolic BP ≥ 140 mm Hg, with a mean reduction in body weight with orlistat compared to placebo of −2.8 kg (95% CI −3.9 to −1.7) and −3.6 kg (95% CI −4.6 to −2.6), respectively.
1.3. Analysis.

Comparison 1: Orlistat versus placebo, Outcome 3: Change in body weight from baseline to endpoint (6 to 12 months follow‐up)
4.

Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.3 Change in body weight from baseline to endpoint (6 to 12 months follow‐up) [kg].
Changes in systolic blood pressure
We could include all four studies in the meta‐analysis investigating the effects of orlistat on systolic BP. After six to 12 months, there was a significant reduction in systolic BP with a mean difference (MD) of −2.6 mm Hg (95% CI −3.8 to −1.4; 4 trials, 2058 participants; Analysis 1.4) in favour of orlistat. The test for heterogeneity gave a P value of 0.19 (I2 = 36%) (see Figure 5). Differences in study quality could not explain the heterogeneity. We could deduce no plausible explanation for heterogeneity from differences in study design, study duration, sample sizes, interventions, or characteristics of included participants. In XENDOS 2001‐2006, results after 48 months of follow‐up were reported for the subgroups of participants with diastolic BP ≥ 90 mm Hg at baseline and systolic BP ≥ 140 mm Hg, with a mean reduction in systolic BP with orlistat compared to placebo of −2.4 mm Hg (95% CI −4.4 to −0.4) and −2.9 mm Hg (95% CI −4.7 to −1.1), respectively.
1.4. Analysis.

Comparison 1: Orlistat versus placebo, Outcome 4: Change in systolic blood pressure from baseline to endpoint (6 to 12 months follow‐up)
5.

Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.4 Change in systolic blood pressure from baseline to endpoint (6 to 12 months follow‐up) [mm Hg].
Changes in diastolic blood pressure
We could include all four studies in the meta‐analysis investigating the effects of orlistat on diastolic BP. After six to 12 months, diastolic BP was also significantly reduced in participants treated with orlistat, with a MD of −2.0 mm Hg (95% CI −2.7 to −1.2; 4 trials, 2058 participants; Analysis 1.5). The test for heterogeneity gave a P value of 0.13 (I2 = 47%) (see Figure 6). Differences in study quality could not explain the heterogeneity. We could deduce no plausible explanation for heterogeneity from differences in study design, study duration, sample sizes, interventions, or characteristics of included participants. In XENDOS 2001‐2006, results after 48 months of follow‐up were reported for the subgroups of participants with diastolic BP ≥ 90 mm Hg at baseline and systolic BP ≥ 140 mm Hg, with a mean reduction in diastolic BP with orlistat compared to placebo of −1.9 mm Hg (95% CI −3.2 to −0.6) and −2.0 mm Hg (95% CI −3.3 to −0.7), respectively.
1.5. Analysis.

Comparison 1: Orlistat versus placebo, Outcome 5: Change in diastolic blood pressure from baseline to endpoint (6 to 12 months follow‐up)
6.

Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.5 Change in diastolic blood pressure from baseline to endpoint (6 to 12 months follow‐up) [mm Hg].
Subgroup analyses
Not performed due to lack of data.
Sensitivity analyses
Not performed due to lack of data.
Publication and small‐study bias
We did not draw funnel plots due to the limited number of trials (n = 4).
2. Phentermine/topiramate versus placebo
See Table 2.
Primary outcomes
Total mortality
Only one participant in the placebo group in the dyslipidaemia subgroup died during the study. There were no deaths in the hypertensive subgroup (CONQUER 2013).
Cardiovascular morbidity
In CONQUER 2013, 2.3% of the hypertensive participants in the 7.5 mg phentermine/46 mg topiramate group, 3.7% in the 15 mg phentermine/92 mg topiramate group, and 1.7% of the placebo group experienced treatment‐emergent cardiovascular adverse events. Palpitations occurred in 0.8%, 1.2%, and 0.6% of the hypertensive participants, respectively. Serious adverse cardiac events occurred in six hypertensive participants and serious adverse vascular events in two hypertensive participants, but it was not reported to which treatment group these participants belonged.
Adverse events
In the hypertensive subgroup of CONQUER 2013, treatment‐emergent adverse events were reported in 85.4% of participants in the 7.5 mg/46 mg phentermine/topiramate group, 88.8% of participants in the 15 mg/92 mg phentermine/topiramate group and 77.3% of participants in the placebo group (1305 participants; Analysis 2.1). Serious adverse events occurred in 3.4% (7.5 mg/46 mg group) and 3.7% (15 mg/92 mg group) of the hypertensive participants treated with phentermine/topiramate, and in 4.2% of participants in the placebo group (1305 participants; Analysis 2.2). The most common treatment‐emergent adverse events in the phentermine/topiramate groups were dry mouth (14.2% (7.5 mg/46 mg group) and 22.7% (15 mg/92 mg group)) and paraesthesia (14.2% (7.5 mg/46 mg group) and 22.3%(15 mg/92 mg group)) when compared to placebo (2.3% for each outcome).
2.1. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 1: All adverse events
2.2. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 2: Serious adverse events
Secondary outcomes
For details on secondary outcome data see Table 9 (body weight), Table 10 (systolic blood pressure), and Table 11 (diastolic blood pressure).
Body weight
Compared to placebo, phentermine/topiramate produced a significantly greater percentage weight loss in participants with hypertension at baseline (−8.1%, −10.1%, and −1.9% for 7.5 mg/46 mg, 15 mg/92 mg, and placebo respectively, P < 0.001; 256, 514 and 516 participants respectively; Analysis 2.3; Analysis 2.4). In addition, significantly more participants in the hypertensive subgroup achieved weight loss of ≥ 5%, ≥ 10%, and ≥ 15% with phentermine/topiramate compared with placebo (CONQUER 2013).
2.3. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 3: Change in body weight from baseline to endpoint: Phen/Top low dose (13 months follow‐up)
2.4. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 4: Change in body weight from baseline to endpoint: Phen/Top high dose (13 months follow‐up)
Changes in systolic blood pressure
In hypertensive participants, greater reductions in systolic BP were noted in the phentermine/topiramate groups than in the placebo group (−6.9 mm Hg (7.5 mg/46 mg group) and −9.1 mm Hg (15 mg/92 mg group) versus −4.9 mm Hg; P = 0.047 and P < 0.0001 for comparisons; 256, 514 and 516 participants respectively; Analysis 2.5; Analysis 2.6). Compared with placebo, a greater percentage of phentermine/topiramate‐treated participants with uncontrolled hypertension at baseline (≥ 140/90 mm Hg; placebo n = 104; 7.5 mg/46 mg n = 40; 15 mg /92 mg n = 72) achieved the BP goal of 140/90 mm Hg by week 56: 52.9% in the placebo group, 62.5% in the 7.5 mg/46 mg group, and 75.0% in the 15 mg/92 mg group (P = 0.2996 for 7.5/46 versus placebo; P = 0.0034 for 15/92 versus placebo) (CONQUER 2013).
2.5. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 5: Change in systolic blood pressure from baseline to endpoint: Phen/Top low dose (13 months follow‐up)
2.6. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 6: Change in systolic blood pressure from baseline to endpoint: Phen/Top high dose (13 months follow‐up)
Changes in diastolic blood pressure
Both doses of phentermine/topiramate were associated with a significantly greater reduction in diastolic BP compared to placebo in hypertensive participants (−5.2 mm Hg (7.5 mg/46 mg group) and −5.8 mm Hg (15 mg/92 mg group) versus −3.9 mm Hg; P = 0.04 and < 0.001 for comparisons; 256, 514 and 516 participants respectively; Analysis 2.7; Analysis 2.8) (CONQUER 2013).
2.7. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 7: Change in diastolic blood pressure from baseline to endpoint: Phen/Top low dose (13 months follow‐up)
2.8. Analysis.

Comparison 2: Phentermine/topiramate versus placebo, Outcome 8: Change in diastolic blood pressure from baseline to endpoint: Phen/Top high dose (13 months follow‐up)
Heterogeneity
Only one study; no meta‐analysis performed.
Subgroup analyses
Only one study; no meta‐analysis performed.
Sensitivity analyses
Only one study; no meta‐analysis performed.
Publication and small‐study bias
Only one study; not applicable.
3. Naltrexone/bupropion versus placebo
See Table 3.
Primary outcomes
Total mortality
During the study period, there were 63 deaths in the naltrexone/bupropion group (1.51%) and 63 deaths in the placebo group (1.53%) reported for the subgroup of participants with hypertension (8283 participants; Analysis 3.1; Nissen 2016). The number of cardiovascular death were 26 (0.62%) in the naltrexone/bupropion group and 37 (0.90%) in the placebo group.
3.1. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 1: Total mortality
Cardiovascular morbidity
There were 64 participants with hypertension who had a non‐fatal myocardial infarction (1.54%), 28 had a non‐fatal stroke (0.67%) and 49 participants were hospitalised for unstable angina (1.18%) in the naltrexone/bupropion group. In the placebo group the respective numbers were 61 non‐fatal myocardial infarctions (1.48%), 20 non‐fatal strokes (0.49%), and 45 hospitalisations for unstable angina (1.09%) (8283 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 2: Cardiovascular morbidity
Adverse events
In the hypertensive subgroup, adverse events were reported in 43.1% of participants in the naltrexone/bupropion group and in 25.6% of participants in the placebo group (P < 0.001; 8283 participants; Analysis 3.3). Serious adverse events occurred in 21.4% of the hypertensive participants treated with naltrexone/bupropion and in 20.5% of participants in the placebo group (P = 0.3; 8283 participants; Analysis 3.4). Gastrointestinal adverse events were significantly higher in the naltrexone/bupropion group than in the placebo group (17.2% versus 3.4%; P < 0.001). Nervous system adverse events (7.5% versus 2.7%, P < 0.001) and dermatological adverse events (1.1% versus 0.5%, P = 0.002) were also reported significantly more often in the naltrexone/bupropion versus the placebo group. In addition, adverse events leading to withdrawal of study medication occurred significantly more often in the naltrexone/bupropion group than in the placebo group (30.6% versus 9.2%; P < 0.001).
3.3. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 3: All adverse events
3.4. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 4: Serious adverse events
Secondary outcomes
For details on secondary outcome data see Table 9 (body weight), Table 10 (systolic blood pressure), and Table 11 (diastolic blood pressure).
Body weight
Compared to placebo, naltrexone/bupropion led to a significantly greater reduction in body weight in participants with hypertension (−1.9 kg versus −0.0 kg, P < 0.001; 8283 participants; Analysis 3.5).
3.5. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 5: Change in body weight from baseline to endpoint (28 months follow‐up)
Changes in systolic blood pressure
In hypertensive participants, there was an increase in systolic BP in both study groups during the study period, with no difference between the naltrexone/bupropion group and the placebo group (+2.2 mm Hg versus +2.2 mm Hg; P = 0.1; 8283 participants; Analysis 3.6).
3.6. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 6: Change in systolic blood pressure from baseline to endpoint (28 months follow‐up)
Changes in diastolic blood pressure
In hypertensive participants, there was an increase in diastolic BP in both study groups during the study period, with no difference between the naltrexone/bupropion group and the placebo group (+1.4 mm Hg versus +1.1 mm Hg, P = 0.12; 8283 participants; Analysis 3.7).
3.7. Analysis.

Comparison 3: Naltrexone/bupropion versus placebo, Outcome 7: Change in diastolic blood pressure from baseline to endpoint (28 months follow‐up)
Heterogeneity
Only one study; no meta‐analysis performed.
Subgroup analyses
Only one study; no meta‐analysis performed.
Sensitivity analyses
Only one study; no meta‐analysis performed.
Publication and small‐study bias
Only one study; not applicable.
Discussion
Summary of main results
This updated systematic review attempted to determine the long‐term effects of weight loss through approved pharmacological intervention on patient‐relevant endpoints, namely death and cardiovascular complications, in the antihypertensive therapy of people with essential hypertension. We found no randomised controlled trials designed to answer this question. Our search revealed only studies focusing mainly on the evaluation of effects on body weight, blood pressure (BP), and adverse events. Four relevant trials investigating orlistat and one investigating the combination of phentermine/topiramate were already included in the previous version of our review. For this update we could include one additional RCT investigating the combination of naltrexone/bupropion versus placebo (Nissen 2016) and we identified three additional publications belonging to the included phentermine/topiramate study (CONQUER 2013), with none of them reporting any relevant new results for the subgroup of people with hypertension. We still could not include any study examining liraglutide or lorcaserin in this review, as all potentially relevant trials failed to present analyses of hypertensive participants. In addition, for this review update we exclude studies investigation sibutramine or rimonabant, since these two products lost their market approval nearly 10 years ago and are therefore no longer relevant for long‐term weight loss.
We found that of the four studies on the effects of orlistat that we include in our analyses, only one was considered to have major deficiencies in study quality. The meta‐analyses showed that participants on therapy with orlistat could reduce their weight and blood pressure levels to a statistically significantly greater degree than participants in the placebo group. While these results show that orlistat may be a helpful option in the antihypertensive therapy of obese hypertensive people, some questions still remain. First of all, participants undergoing orlistat therapy experienced substantial side effects, mainly gastrointestinal. This may limit the effectiveness of the medication in settings outside of scientific studies. Furthermore it remains unclear whether blood pressure levels will remain low over a longer period of time or once the medication is discontinued, since some investigations have found that body weight increased after one year, whether or not orlistat was continued (Davidson 1999; Sjöström 1998).
For the combination therapy of phentermine/topiramate we could include only one trial involving a hypertensive subgroup in our analysis (CONQUER 2013). An additional 56‐week trial compared two different doses of phentermine/topiramate to placebo in obese participants with blood pressure below 140/90 mm Hg (EQUIP 2012). In these trials, the mean weight loss with phentermine/topiramate was about 6 to 8 kg, which is higher than in the case of orlistat, while the antihypertensive effect of phentermine/topiramate is comparable with orlistat. After completion of the CONQUER trial, participants could take part in a further 52‐week extension trial (SEQUEL 2014). Since this extension trial did not report any results for the hypertensive subgroup, and enrolment in the study occurred at participants' request, we did not include the results in our analysis. Although there was significant sustained weight loss in the phentermine/topiramate groups compared to placebo, the reduction in blood pressure did not differ significantly between the groups after two years (SEQUEL 2014). Contrary to the USA, where the Food and Drug Administration (FDA) approved phentermine/topiramate for the long‐term pharmacological treatment of obesity in 2012 (FDA 2012a), the European Medicines Agency (EMA) refused approval in Europe on 21 February 2013, because of major concerns about the long‐term effects of the drug on the heart and blood vessels, and particularly because phentermine is known to increase heart rate. There were also concerns about long‐term psychiatric and cognitive effects related to the topiramate component (EMA 2013d).
Only one trial could be included for the combination of naltrexone/bupropion in our analysis, since the author provided results for the subgroup of participants with hypertension (Nissen 2016). The results in this study showed a modest weight reduction with naltrexone/bupropion compared to placebo, but blood pressure was slightly raised in both study groups. While there were no differences in the numbers of deaths or cardiovascular events between the naltrexone/bupropion and placebo groups, participants in the naltrexone/bupropion group experienced significantly more gastrointestinal, nervous system or dermatological adverse events. As well as this RCT, naltrexone/bupropion was also assessed in four 56‐week trials of the Contrave Obesity Research (COR) study programme, including 4536 overweight or obese people with or without hypertension, dyslipidaemia, or diabetes mellitus type 2. Results for the subgroup of participants with hypertension were not available. In two of the trials, the percentage of participants with hypertension at baseline was about 20% (COR‐I 2010; COR‐II 2013); in the other two trials it was not reported (COR‐BMOD 2011; COR‐Diabetes 2013). A statistically significant weight reduction of about 4 to 5 kg was reported for naltrexone/bupropion compared to placebo in addition to diet and exercise counselling (COR‐Diabetes 2013; COR‐I 2010; COR‐II 2013), or to an intensive behaviour modification programme (COR‐BMOD 2011). Blood pressure remained unchanged or was slightly reduced in the naltrexone/bupropion groups, but there was a larger reduction in the placebo groups. The group differences were significant for systolic BP in the COR‐I 2010, COR‐II 2013, and COR‐BMOD 2011 trials and for diastolic BP in the COR‐I 2010 and COR‐BMOD 2011 trials. None of these studies investigated the effects on patient‐related outcomes. The dropout rates in the four trials ranged from 42% to 50%. Nausea, dizziness, constipation, and tinnitus were the most common adverse events in the naltrexone/bupropion groups.
As for the two remaining drugs (lorcaserin and liraglutide) that are currently approved for long‐term weight reduction, most studies enrolled only overweight or obese people without hypertension or a mixed population of normo‐ and hypertensive people, without providing separate results for the hypertensive subgroup. We therefore excluded the results of these trials from our analysis and only briefly discuss them here.
Lorcaserin
Three large phase III trials assessed the efficacy and safety of lorcaserin in obese people with or without weight‐related comorbidities (BLOOM 2010; BLOOM‐DM 2012; BLOSSOM 2011). The Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trial enrolled 4008 participants who were randomly assigned to two different daily doses of lorcaserin or placebo (BLOSSOM 2011). About 24% of the participants were hypertensive. In the Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) study, 3182 overweight or obese normotensive participants received either 10 mg lorcaserin twice daily or placebo (BLOOM 2010), while the Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus (BLOOM‐DM) trial investigated the effect of two different daily doses of lorcaserin versus placebo in 604 overweight or obese people with diabetes mellitus type 2 (BLOOM‐DM 2012). In all three trials, the study medication was combined with a reduced‐calorie diet and increased physical activity. None of the studies were designed to assess the effects on patient‐relevant endpoints. In all the studies, the lorcaserin groups showed a significant mean weight loss of about 3 to 4 kg compared with the placebo groups after 52 weeks. In contrast, blood pressure did not change to a statistically significant degree in the BLOSSOM and the BLOOM‐DM trials. In the BLOOM trial, which included only normotensive participants, there was a significant reduction in systolic BP and diastolic BP in the lorcaserin groups compared to placebo (systolic BP: −0.6 mm Hg; diastolic BP: −0.5 mm Hg). The most common adverse events in the lorcaserin groups were headache, nasopharyngitis, and nausea. In addition, large discontinuation rates of up to 50% were reported in the trials (Taylor 2013). Although there was no difference in the incidence of valvulopathy between lorcaserin and placebo, the FDA has requested that a post‐approval trial be conducted to assess the long‐term cardiovascular effects of lorcaserin (Yanovski 2014). In January 2013, a Committee for Medicinal Products for Human Use review of the EMA described some concerns about the potential risk of psychiatric disorders and valvulopathy, and the potential risk of tumours, particularly when used over the long term, based on the results of laboratory tests (EMA 2013b). As a result of this negative overall benefit/risk balance for lorcaserin, the manufacturers withdrew their application for marketing approval in Europe (EMA 2013a). In 2018 results have been published on the cardiovascular safety of lorcaserin from another trial including 12,000 overweight or obese participants with cardiovascular risk factors (CAMELLIA‐TIMI 2018). About 90% of the participants were hypertensive. After one year, the lorcaserin group showed a significant mean weight loss of 1.9 kg and slight reductions in systolic and diastolic blood pressure compared with the placebo group. There were no differences in major cardiovascular events, cardiovascular death, or death from any cause between the two study groups. An FDA review of the study data found a numeric imbalance in the number of participants with malignancies, with one additional cancer observed per 470 persons treated with lorcaserin for one year. The FDA therefore concluded that the potential risks of lorcaserin outweigh its benefits and recommended its withdrawal from the market (FDA 2020). In February 2020, the manufacturers announced their voluntary withdrawal of lorcaserin from the US market (Eisai Inc. 2020).
Liraglutide
Liraglutide was first approved by the EMA in 2009 and by the FDA in 2010 to improve glycaemic control in adults with type 2 diabetes mellitus (EMA 2009b; FDA 2010c). A Cochrane Review of glucagon‐like peptide 1 (GLP‐1) receptor agonists for type 2 diabetes mellitus showed a significant weight reduction of 1.33 kg (P < 0.0001) and a marginally significant reduction in systolic BP of −2.42 mm Hg (P = 0.05) for 1.8 mg liraglutide compared to placebo after 24 weeks. No difference in diastolic BP was reported. The most common adverse events of liraglutide were gastrointestinal, such as nausea or vomiting (Shyangdan 2011). There were also more cases of pancreatitis among liraglutide users compared with comparators, and in 2011 the FDA issued a warning that liraglutide may cause pancreatitis and thyroid carcinoma (NEJM Journal Watch 2011). In 2016, the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial, a double‐blind, placebo‐controlled randomised controlled trial including 9340 participants with type 2 diabetes mellitus at high risk of cardiovascular disease, investigated the long‐term effects of 1.8 mg liraglutide in comparison to placebo on cardiovascular outcomes as well as on neoplasms and other adverse events (LEADER 2013). After a mean follow‐up of 3.8 years, the primary outcome, a composite endpoint including the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, occurred in significantly fewer participants in the liraglutide group compared to the placebo group. On the other hand, significantly more participants in the liraglutide group discontinued the study due to adverse events, and the rate of pancreatic carcinoma was twice as high in the liraglutide group as in the placebo group. In addition to glycaemic control, liraglutide was approved by the FDA and EMA at a dose of 3.0 mg for the treatment for obesity in 2014/2015 (EMA 2015b; FDA 2014b). It was investigated for this indication in the SCALE study programme, in which 5358 obese or overweight people with or without weight‐related comorbidities participated (SCALE diabetes 2014; SCALE maintenance 2013; SCALE obesity and prediabetes 2014; SCALE sleep apnoea 2014). Although the percentage of hypertensive participants was as high as 70% in these trials, no results for a hypertensive subgroup were reported. At the end of the studies (32 to 56 weeks), mean body weight was significantly reduced among liraglutide users compared to placebo (−3 to −6 kg). Systolic BP was also significantly reduced in all four trials (−3 mm Hg), while for diastolic BP only one trial showed a significant difference between liraglutide and placebo (−1 mm Hg) (SCALE obesity and prediabetes 2014). The dropout rates in the studies were about 23% to 34% and therefore lower than in other weight‐management trials. As in the glycaemic control trials, the most common adverse events were nausea, diarrhoea, and vomiting. Pancreatitis also occurred more frequently in the liraglutide group compared to placebo.
For this review update, we also excluded trials investigating sibutramine or rimonabant, since their marketing approval was withdrawn in 2010 and 2009, respectively. Results of trials investigating these two products are briefly discussed in the following paragraphs.
Sibutramine
Results for participants with hypertension were reported in four trials investigating sibutramine (Fanghänel 2003; Faria 2002; McMahon 2000; McMahon 2002). Results of clinical outcomes were sparse, with only one study reporting that no death occurred during the study period (McMahon 2002). In the four included studies, body weight was reduced by about 4 kg, which corresponds to the weight reduction with orlistat. However, sibutramine did not show the same beneficial effects on blood pressure. In two studies using a dosage of 20 mg a day, which is higher than the currently approved dosage (in Germany) of 10 to 15 mg a day, blood pressure actually rose in participants treated with sibutramine. This finding is further underlined by the result of a head–to‐head comparison of orlistat versus sibutramine (Derosa 2005). It was found that while in participants in the orlistat group (120 mg three times a day) a reduction of −8.4 kg body weight resulted in a reduction of systolic BP and diastolic BP of −4.0 and −3.0 mm Hg respectively, the same 8.3 kg decline in body weight in the sibutramine group did not lead to a change in BP in participants treated with 10 mg sibutramine a day (0.0 and 0.0 mm Hg respectively). In a meta‐analysis by Kim 2003. comparing sibutramine with placebo in participants with or without hypertension at baseline, a significant increase in systolic BP (+1.6 mm Hg) and diastolic BP (+1.8 mm Hg) were also found in the sibutramine‐treatment group despite a large effect on weight loss in this group. As there are safety concerns in hypertensive people, the EMA demanded a long‐term trial of people at high cardiovascular risk, and the Sibutramine Cardiovascular Outcome Trial (SCOUT) was initiated. This double‐blind, randomised, placebo‐controlled outcome trial in approximately 10,000 overweight/obese high‐risk cardiovascular participants started recruitment in December 2002 and was designed to determine the impact of weight loss in 10,742 participants (SCOUT 2010). After a mean duration of 3.5 years, 11.4% of participants in the sibutramine and 10.0% in the placebo group had a primary outcome event. These were either a non‐fatal myocardial infarction, a non‐fatal stroke, resuscitation after cardiac arrest, or cardiovascular death (hazard ratio 1.16, 95% CI 1.03 to 1.31; P = 0.02; number needed to treat for an additional harmful outcome: 71). As any observed loss in body weight was only modest, the FDA and EMA concluded that the risk of an adverse cardiovascular event outweighed any benefit resulting from taking sibutramine. Even though about one‐third of the study population was hypertensive, the SCOUT study is not included in our review, as no hypertensive subgroup analyses were presented. In addition, as marketing approval for sibutramine has been withdrawn, we did not attempt to contact the authors to obtain the necessary data for this subgroup.
Rimonabant
Four studies (the Rimonabant in Obesity (RIO) studies) investigated the effects of a daily therapy of 5 and 20 mg rimonabant in comparison to placebo in study populations including normo‐ and hypertensive (30% to 60%) participants (Despres 2005; Pi‐Sunyer 2006; Scheen 2006; Van Gaal 2005). None of the RIO studies were designed to assess the effects on patient‐relevant endpoints. Participants receiving treatment with rimonabant reduced their body weight to a statistically significantly greater degree than placebo participants, regardless of the dosage. Contrary to these findings, the studies yielded heterogeneous results for blood pressure changes. In particular, therapy with 5 mg rimonabant showed inconsistent results, with a higher reduction in blood pressure compared to placebo in some studies, but less of a reduction in others. Treatment with 20 mg rimonabant daily showed more uniform findings, with a higher reduction in systolic BP in all four studies (the difference was statistically significant in two). Furthermore, in three studies the reduction in diastolic BP was more pronounced in participants taking 20 mg rimonabant than in the placebo group (the difference was statistically significant in one), but slightly less pronounced in one study. Only the publication of the RIO Lipid study reported information on a hypertensive subgroup (Despres 2005). In hypertensive participants treated with 20 mg rimonabant, blood pressure was reduced to a statistically significantly greater degree than in participants in the placebo group (systolic BP: −5.9 mm Hg; diastolic BP: −3.9 mm Hg). Since no other information on the hypertensive subgroup is provided (the paper does not even report the percentage of participants with hypertension at baseline), the relevance of these findings remains unclear. In addition, it must be noted that rimonabant was never approved by the FDA because of serious safety concerns (FDA 2007). Original data analysis performed by the FDA showed that the incidence of suicidality was doubled in the rimonabant group versus placebo. Similarly, incidences of psychiatric adverse events, neurological adverse events, and seizures were consistently higher for 20 mg rimonabant compared to placebo. Rimonabant received marketing approval from the EMA on 19 June 2006. However, new data derived from post‐marketing experience and ongoing clinical trials led the EMA on 23 October 2008 to recommend suspension of the marketing authorisation for rimonabant in Europe as well (EMA 2008a; EMA 2008b). In January 2009, the European Commission issued a decision to withdraw the market authorisation for rimonabant in all EU countries (EMA 2009a). Many more results from long‐term data will have to be published before a decision on the usefulness of rimonabant can be made. The results of the Comprehensive Rimonabant Evaluation Study of Cardiovascular Endpoints and Outcomes (CRESCENDO) trial, published in 2010, did not change the recommendation made by EMA in 2009 (CRESCENDO 2010). This study was closed early in November 2008 due to high suicide rates and other psychiatric side effects related to rimonabant. It was a multicentre, double‐blind, placebo‐controlled trial with a mean follow‐up of 13.8 months, aimed at determining whether long‐term treatment with 20 mg rimonabant could potentially reduce the risk of cardiovascular events in 9314 participants with previously manifest or increased risk of vascular disease. Even though about 88% of participants were hypertensive, no hypertensive subgroup analyses were presented. The primary endpoints were cardiovascular death, myocardial infarction, or stroke, which occurred in 3.9% of participants assigned to rimonabant, compared with 4.0% of participants assigned to placebo (hazard ratio 0.97, 95% CI 0.84 to 1.12; P = 0.68). Among participants taking rimonabant, the incidence of gastrointestinal (33% versus 22%), neuropsychiatric (32% versus 21%), and serious psychiatric side effects (2.5% versus 1.3%) was significantly increased compared with placebo. Four participants in the rimonabant group and one in the placebo group committed suicide.
Overall completeness and applicability of evidence
We searched four electronic databases and the clinical trials registries ClinicalTrials.gov and WHO ICTRP up to March 2020 and examined the reference lists of included trials and relevant systematic reviews and meta‐analyses. We contacted authors of studies with mixed study populations (normo‐ and hypertensive participants) for further information about the subset of people with hypertension. We assessed each study's quality and summarised the results. The results of this review can therefore be taken to be complete and applicable. For full information please see details in the relevant sections.
Quality of the evidence
Of the four orlistat studies included in our analyses, we judged only one to have major deficiencies in study quality (Bakris 2002). We regarded the two included trials investigating phentermine/topiramate (CONQUER 2013) or naltrexone/bupropion (Nissen 2016) as having no major deficiencies in study quality. Full details are provided in the 'Risk of bias' tables in Characteristics of included studies.
Potential biases in the review process
A major limitation of this review is that due to insufficient information in the included studies, we can draw no conclusions on the effects of the different pharmaceutical weight‐loss interventions on patient‐relevant long‐term outcomes.
Agreements and disagreements with other studies or reviews
Systematic reviews on the long‐term effects of weight‐reducing drugs in people with hypertension are rare. One systematic review (Aucott 2005) reached the same conclusion as us, that only short‐term trials were available. The authors also warned "that extrapolation of short‐term blood pressure changes with weight loss to the longer term is potentially misleading. The weight/hypertension relationship is complex and needs well‐conducted studies with long‐term follow‐up to examine the effects of weight loss on hypertension outcomes". Systematic reviews on long‐term effects of weight‐reducing drugs in a mixed population of overweight or obese people with or without hypertension reported blood pressure‐reducing effects for orlistat, liraglutide, lorcaserin, and phentermine/topiramate, but not for naltrexone/bupropion (Khera 2018; LeBlanc 2018). Although LeBlanc 2018 could include more than 30 trials that addressed medications for weight loss, no reliable conclusions could be drawn about clinical outcomes. A recently‐published systematic review (Kane 2019) investigated the effect of pharmacological weight reduction versus placebo on all‐cause mortality and cardiovascular risk factors. The meta‐analyses, including up to seven RCTs with normo‐ and hypertensive participants, showed a significant benefit for the pharmacological weight‐loss interventions in cardiovascular mortality, but no effect on all‐cause mortality.
We were also involved in the preparation of the scientific report on the evaluation of the benefits and harms of non‐drug treatment strategies in people with essential hypertension (IQWiG 2006), and published a paper on this topic (Horvath 2008).We are aware of all major and relevant systematic reviews and studies, and can say with confidence that our findings are in good agreement with recently‐published reviews and studies.
Authors' conclusions
Implications for practice.
Of the three anti‐obesity drugs initially included in the first update of this review, only orlistat is still on the market. Four additional drugs have been approved by the FDA since 2012, but only two of them (liraglutide and naltrexone/bupropion) have also been approved for use in the EU. Trials of orlistat in people with raised blood pressure have demonstrated statistically significant decreases in weight and a decline in blood pressure. The only trial reporting results on phentermine/topiramate in a subgroup of hypertensive people also showed significant reductions in body weight and blood pressure in comparison to placebo, while results from one trial of naltrexone/bupropion showed a reduction in body weight, but no effect on blood pressure for the subgroup of participants with hypertension. In Europe, the EMA refused marketing authorisation for phentermine/topiramate because of safety concerns. Approval for sibutramine and rimonabant was withdrawn due to potential cardiovascular risks (sibutramine) and psychiatric side effects (rimonabant), while the application for European marketing approval for lorcaserin was withdrawn by the manufacturers after the overall benefit/risk balance was judged to be negative by the Committee for Medicinal Products for Human Use. In 2020 lorcaserin was also withdrawn from the US market, after an increased number of malignancies with lorcaserin compared with placebo in a large safety trial.
Implications for research.
Long‐term trials assessing the effects of the currently‐approved drugs used for weight reduction on mortality and morbidity in people with raised blood pressure are very much needed. Long‐term trials assessing the effects of sibutramine and rimonabant on mortality and morbidity have confirmed concerns about the potentially severe side effects that led to marketing withdrawal of these two drugs throughout the world.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2021 | New citation required but conclusions have not changed | Update published with changed authors, additional Background information and one additional study investigating weight‐reducing drugs in people with hypertension. |
| 4 January 2021 | New search has been performed | We updated the electronic search to March 2020. We found one additional study investigating weight‐reducing drugs in people with hypertension. Studies investigating sibutramine or rimonabant are no longer included in this review update, since these products lost their market approval nearly 10 years ago and are therefore no longer relevant for long‐term weight loss. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 26 February 2016 | New citation required and conclusions have changed | Update published with changed authors, additional background information, and one additional study included. |
| 26 February 2016 | New search has been performed | The electronic search for new studies was extended to include four new weight‐reducing drugs and updated to April 2015. We identified one additional study investigating phentermine/topiramate versus placebo that met the inclusion criteria of this review. |
| 15 January 2013 | New search has been performed | The electronic search for new studies was updated to August 2012. No new studies were identified that met the inclusion criteria of this review. |
Acknowledgements
We would like to thank Douglas Salzwedel for helping to update the literature search, Phillip Elliott for the final editing of the manuscript, Ulrich Siering for assisting in the development of the original review, and Nicole Posch and Jutta Meschik for assisting in the development of the previous update of the review. We also thank Prof. Le Roux, Dr. Kolotkin, Dr. Finkelstein, Dr. Fidler, Dr. Wadden, Dr. Greenway, Mr. Quesenberry and Mr. Gould from Currax Pharmaceuticals, and Mrs. Olsen from Novo Nordisk Pharmaceuticals for replying to our information requests, and Dr. Cocco, Dr. Nissen and Mrs. Wolski for providing additional data with relevance to our review.
Appendices
Appendix 1. Checklist to aid consistency and reproducibility of GRADE assessments (Orlistat vs placebo)
| Orlistat vs. placebo | Total mortality | Cardiovascular morbidity | Serious adverse events | All adverse events | Systolic blood pressure | Diastolic blood pressure | Body weight | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was an objective outcome used? | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?b | Yes | No (↓) | Yes | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Inconsistencyc | Point estimates did not vary widely? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | N/A | Substantial | Substantial | Substantial | Substantial | Substantial | |
| Was the direction of effect consistent? | Yes | N/A | Yes | Yes | Yes | Yes | Yes | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | N/A | N/A | Low | High (↓↓) | Low | Moderate | High (↓) | |
| Was the test for heterogeneity statistically significant (P < 0.1)? | N/A | N/A | Not statistically significant | Statistically significant | Not statistically significant | Not statistically significant | Statistically significant | |
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Was the included outcome not a surrogate outcome? | No | No | No | No | Yes | Yes | No | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | N/A | No | Yes | No | No | No |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?b | Intermediate | Intermediate | Intermediate | Intermediate | Intermediate | Intermediate | Intermediate | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?b | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | No (↓↓) | No (↓↓) | Yes | Yes | N/A | N/A | N/A | |
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | No | No | No | No | No | No | No | |
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bDepends on the context of the systematic review area. cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². dWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s). HbA1c: glycosylated haemoglobin A1c; N/A: not applicable; | ||||||||
Appendix 2. Checklist to aid consistency and reproducibility of GRADE assessments (Phentermine/topiramate vs placebo)
| Phentermine/topiramate vs placebo | Total mortality | Cardiovascular morbidity | Serious adverse events | All adverse events | Systolic blood pressure | Diastolic blood pressure | Body weight | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?b | Yes | No (↓) | Yes | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Inconsistencyc | Point estimates did not vary widely? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Was the direction of effect consistent? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Was the test for heterogeneity statistically significant (P < 0.1)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Was the included outcome not a surrogate outcome? | No | No | No | No | Yes | Yes | No | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?b | High | High | High | High | High | High | High | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 ‐ 10 studies, small: < 5 studies)?b | Small (↓↓) | Small (↓↓) | Small (↓↓) | Small (↓↓) | Small (↓↓) | Small (↓↓) | Small (↓↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | No (↓↓) | Yes | Yes | Yes | N/A | N/A | N/A | |
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | No | No | No | No | No | No | No | |
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bDepends on the context of the systematic review area. cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². dWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s). HbA1c: glycosylated haemoglobin A1c; N/A: not applicable; | ||||||||
Appendix 3. Checklist to aid consistency and reproducibility of GRADE assessments (Naltrexone/bupropion vs placebo)
| Phentermine/topiramate vs. placebo | Total mortality | Cardiovascular morbidity | Serious adverse events | All adverse events | Systolic blood pressure | Diastolic blood pressure | Body weight | |
| Trial limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?b | Yes | No (↓) | Yes | Yes | Yes | Yes | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | No | No | No | No | No | No | No | |
| Inconsistencyc | Point estimates did not vary widely? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Was the direction of effect consistent? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40%‐60%), high I² > 60%)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Was the test for heterogeneity statistically significant (P < 0.1)? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | |
| Was the included outcome not a surrogate outcome? | No | No | No | No | Yes | Yes | No | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?b | High | High | High | High | High | High | High | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 ‐ 10 studies, small: < 5 studies)?b | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | Yes | Yes | N/A | N/A | N/A | |
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| There was no industry influence on studies included in the review? | No | No | No | No | No | No | No | |
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials.
bDepends on the context of the systematic review area. cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². dWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s) HbA1c: glycosylated haemoglobin A1c; N/A: not applicable; | ||||||||
Appendix 4. Search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to March 16, 2020> Search Date: 17 March 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (orlistat$ or tetrahydrolipstatin or thlp or "96829‐58‐2").mp. 2 (ro180647 or "ro 180647" or "ro 18‐0647").mp. 3 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical).mp. 4 or/1‐3 5 (sibutramin$ or "106650‐56‐0").mp. 6 (bts54524 or "bts 54524" or "bts‐54524" or "bts‐54‐524" or "bts54‐524").mp. 7 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex).mp. 8 or/5‐7 9 (rimonabant$ or "168273‐06‐1" or "158681‐13‐1").mp. 10 (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).mp. 11 (acomplia or accomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti).mp. 12 or/9‐11 13 (lorcaserin or "616202‐92‐7").mp. 14 ("apd 356" or apd356).mp. 15 (beliviq or lorqess).mp. 16 or/13‐15 17 (liraglutide or "204656‐20‐2").mp. 18 ("nn 2211" or nn2211 or "nnc 90‐1170" or "nnc90 1170").mp. 19 (saxenda or victoza).mp. 20 or/17‐19 21 (phentermine or "1197‐21‐3" or "122‐09‐8").mp. 22 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or lomaira or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl).mp. 23 or/21‐22 24 (topiramate or "97240‐79‐4").mp. 25 (mcn4853 or "mcn 4853" or rwj17021 or "rwj17021‐000" or "rwj 17021" or "rwj 17021‐000").mp. 26 (epitomax or qudexy or topamax or topimax or trokendi).mp. 27 or/24‐26 28 (qnexa or qsiva or qsymia).mp. 29 (23 and 27) or 28 30 (bupropion or "31677‐93‐7" or "34911‐55‐2").mp. 31 (bw323 or "bw 323" or "bw323u66 bw 323u66").mp. 32 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban).mp. 33 or/30‐32 34 (naltrexone or "16590‐41‐3" or "16676‐29‐2").mp. 35 (en1639a or "en 1639a").mp. 36 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol).mp. 37 or/34‐36 38 (contrave or mysimba).mp. 39 (33 and 37) or 38 40 4 or 8 or 12 or 16 or 20 or 29 or 39 41 hypertension/ 42 essential hypertension/ 43 (antihypertens$ or hypertens$).tw,kf,ot. 44 exp blood pressure/ 45 (blood pressur$ or bloodpressur$).tw,kf,ot. 46 or/41‐45 47 randomized controlled trial.pt. 48 controlled clinical trial.pt. 49 randomized.ab. 50 placebo.ab. 51 clinical trials as topic/ 52 randomly.ab. 53 trial.ti. 54 or/47‐53 55 animals/ not (humans/ and animals/) 56 54 not 55 57 40 and 46 and 56 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) Search Date: 20 March 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (orlistat* OR tetrahydrolipstatin OR thlp) AND INREGISTER #2 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical) AND INREGISTER #3 (#1 OR #2) AND INREGISTER #4 sibutramin* AND INREGISTER #5 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex) AND INREGISTER #6 (#4 OR #5) AND INREGISTER #7 rimonabant* AND INREGISTER #8 (acomplia or accomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti) AND INREGISTER #9 (#7 OR #8) AND INREGISTER #10 lorcaserin AND INREGISTER #11 (belviq OR lorqess) AND INREGISTER #12 (#10 OR #11) AND INREGISTER #13 liraglutide AND INREGISTER #14 (saxenda OR victoza) AND INREGISTER #15 (#13 OR #14) AND INREGISTER #16 phentermine AND INREGISTER #17 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or lomaira or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl) AND INREGISTER #18 (#16 OR #17) AND INREGISTER #19 topiramate AND INREGISTER #20 (epitomax OR qudexy OR topamax OR topimax OR trokendi) AND INREGISTER #21 (#19 OR #20) AND INREGISTER #22 (qnexa OR qsiva OR qsymia) AND INREGISTER #23 ((#18 AND #21) OR #22) AND INREGISTER #24 bupropion AND INREGISTER #25 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban) AND INREGISTER #26 (#24 OR #25) AND INREGISTER #27 naltrexone AND INREGISTER #28 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol) AND INREGISTER #29 (#27 OR #28) AND INREGISTER #30 (contrave OR mysimba) AND INREGISTER #31 ((#26 AND #29) OR #30) AND INREGISTER #32 (#3 OR #6 OR #9 OR #12 OR #15 OR #23 OR #31) AND INREGISTER #33 RCT:DE AND INREGISTER #34 Review:ODE AND INREGISTER #35 (#33 OR #34) AND INREGISTER #36 #32 AND #35 AND INREGISTER #37 * AND INREGISTER AND 01/04/2018_TO_20/03/2020:CRSCREATED #38 #36 AND #37 AND INREGISTER ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials (Issue 3, 2020) via the Cochrane Register of Studies (CRS‐Web) Search Date: 17 March 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ((orlistat* OR tetrahydrolipstatin or thlp)) AND CENTRAL:TARGET #2 ((alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical)) AND CENTRAL:TARGET #3 (#1 OR #2) AND CENTRAL:TARGET #4 sibutramin* AND CENTRAL:TARGET #5 ((adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex)) AND CENTRAL:TARGET #6 (#4 OR #5) AND CENTRAL:TARGET #7 rimonabant* AND CENTRAL:TARGET #8 ((acomplia or accomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti)) AND CENTRAL:TARGET #9 (#7 OR #8) AND CENTRAL:TARGET #10 lorcaserin AND CENTRAL:TARGET #11 (belviq OR lorqess) AND CENTRAL:TARGET #12 (#10 OR #11) AND CENTRAL:TARGET #13 MESH DESCRIPTOR Liraglutide AND CENTRAL:TARGET #14 liraglutide AND CENTRAL:TARGET #15 (saxenda OR victoza) AND CENTRAL:TARGET #16 (#13 OR #14 OR #15) AND CENTRAL:TARGET #17 MESH DESCRIPTOR Phentermine AND CENTRAL:TARGET #18 phentermine AND CENTRAL:TARGET #19 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or lomaira or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl) AND CENTRAL:TARGET #20 (#17 OR #18 OR #19) AND CENTRAL:TARGET #21 topiramate AND CENTRAL:TARGET #22 (epitomax OR qudexy OR topamax OR topimax OR trokendi) AND CENTRAL:TARGET #23 (#21 OR #22) AND CENTRAL:TARGET #24 (qnexa OR qsiva OR qsymia) AND CENTRAL:TARGET #25 ((#20 AND #23) OR #24) AND CENTRAL:TARGET #26 MESH DESCRIPTOR Bupropion AND CENTRAL:TARGET #27 bupropion AND CENTRAL:TARGET #28 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban) AND CENTRAL:TARGET #29 (#26 OR #27 OR #28) AND CENTRAL:TARGET #30 MESH DESCRIPTOR Naltrexone AND CENTRAL:TARGET #31 naltrexone AND CENTRAL:TARGET #32 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol) AND CENTRAL:TARGET #33 #30 OR #31 OR #32 AND CENTRAL:TARGET #34 (contrave or mysimba) AND CENTRAL:TARGET #35 ((#29 AND #33) OR #34) AND CENTRAL:TARGET #36 (#3 OR #6 OR #9 OR #12 OR #16 OR #25 OR #35) AND CENTRAL:TARGET #37 (antihypertens* OR hypertens*) AND CENTRAL:TARGET #38 (blood pressur* OR bloodpressur*) AND CENTRAL:TARGET #39 (#37 OR #38) AND CENTRAL:TARGET #40 #36 AND #39 AND CENTRAL:TARGET #41 * AND 01/04/2018_TO_17/03/2020:CRSCREATED AND CENTRAL:TARGET ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2020 March 16> Search Date: 17 March 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (orlistat$ or tetrahydrolipstatin or thlp or "96829‐58‐2").mp. 2 (ro180647 or "ro 180647" or "ro 18‐0647").mp. 3 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical).mp. 4 or/1‐3 5 (sibutramin$ or "106650‐56‐0").mp. 6 (bts54524 or "bts 54524" or "bts‐54524" or "bts‐54‐524" or "bts54‐524").mp. 7 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex).mp. 8 or/5‐7 9 (rimonabant$ or "168273‐06‐1" or "158681‐13‐1").mp. 10 (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).mp. 11 (acomplia or accomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti).mp. 12 or/9‐11 13 (lorcaserin or "616202‐92‐7").mp. 14 ("apd 356" or apd356).mp. 15 (beliviq or lorqess).mp. 16 or/13‐15 17 (liraglutide or "204656‐20‐2").mp. 18 ("nn 2211" or nn2211 or "nnc 90‐1170" or "nnc90 1170").mp. 19 (saxenda or victoza).mp. 20 or/17‐19 21 (phentermine or "1197‐21‐3" or "122‐09‐8").mp. 22 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or lomaira or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl).mp. 23 or/21‐22 24 (topiramate or "97240‐79‐4").mp. 25 (mcn4853 or "mcn 4853" or rwj17021 or "rwj17021‐000" or "rwj 17021" or "rwj 17021‐000").mp. 26 (epitomax or qudexy or topamax or topimax or trokendi).mp. 27 or/24‐26 28 (qnexa or qsiva or qsymia).mp. 29 (23 and 27) or 28 30 (bupropion or "31677‐93‐7" or "34911‐55‐2").mp. 31 (bw323 or "bw 323" or "bw323u66 bw 323u66").mp. 32 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban).mp. 33 or/30‐32 34 (naltrexone or "16590‐41‐3" or "16676‐29‐2").mp. 35 (en1639a or "en 1639a").mp. 36 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol).mp. 37 or/34‐36 38 (contrave or mysimba).mp. 39 (33 and 37) or 38 40 4 or 8 or 12 or 16 or 20 or 29 or 39 41 exp hypertension/ 42 (antihypertens$ or hypertens$).tw,ot. 43 exp blood pressure/ 44 (blood pressur$ or bloodpressur$).tw,ot. 45 or/41‐44 46 randomized controlled trial/ 47 crossover procedure/ 48 double‐blind procedure/ 49 (randomi$ or randomly).tw. 50 (crossover$ or cross‐over$).tw. 51 placebo.ab. 52 (doubl$ adj blind$).tw. 53 assign$.ab. 54 allocat$.ab. 55 or/46‐54 56 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 57 55 not 56 58 40 and 45 and 57 59 58 and (2018$ or 2019$ or 2020$).dc,dd. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov Search Date: 17 March 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition or disease: Hypertension Other terms: randomized Study type: Interventional Studies (Clinical Trials) Intervention/treatment: liraglutide OR lorcaserin OR orlistat OR rimonabant OR sibutramine OR phentermine OR topiramate OR qnexa OR qsiva or qsymia ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 17 March 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition: Hypertension: Intervention: liraglutide OR lorcaserin OR orlistat OR rimonabant OR sibutramine OR phentermine OR topiramate OR qnexa OR qsiva or qsymia
Appendix 5. Search strategies used in the 2015 update of the review
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 13 April 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (orlistat$ or tetrahydrolipstatin or thlp or "96829‐58‐2").mp. 2 (ro180647 or "ro 180647" or "ro 18‐0647").mp. 3 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical).mp. 4 or/1‐3 5 (sibutramin$ or "106650‐56‐0").mp. 6 (bts54524 or "bts 54524" or "bts‐54524" or "bts‐54‐524" or "bts54‐524").mp. 7 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex).mp. 8 or/5‐7 9 (rimonabant$ or "168273‐06‐1" or "158681‐13‐1").mp. 10 (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).mp. 11 (acomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti).mp. 12 or/9‐11 13 (lorcaserin or "616202‐92‐7").mp. 14 ("apd 356" or apd356).mp. 15 (beliviq or lorqess).mp. 16 or/13‐15 17 (liraglutide or "204656‐20‐2").mp. 18 ("nn 2211" or nn2211 or "nnc 90‐1170" or "nnc90 1170").mp. 19 (saxenda or victoza).mp. 20 or/17‐19 21 (phentermine or "1197‐21‐3" or "122‐09‐8").mp. 22 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl).mp. 23 or/21‐22 24 (topiramate or "97240‐79‐4").mp. 25 (mcn4853 or "mcn 4853" or rwj17021 or "rwj17021‐000" or "rwj 17021" or "rwj 17021‐000").mp. 26 (epitomax or qudexy or topamax or topimax or trokendi).mp. 27 or/24‐26 28 (qnexa or qsiva or qsymia).mp. 29 (23 and 27) or 28 30 (bupropion or "31677‐93‐7" or "34911‐55‐2").mp. 31 (bw323 or "bw 323" or "bw323u66 bw 323u66").mp. 32 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban).mp. 33 or/30‐32 34 (naltrexone or "16590‐41‐3" or "16676‐29‐2").mp. 35 (en1639a or "en 1639a").mp. 36 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol).mp. 37 or/34‐36 38 (contrave or mysimba).mp. 39 (33 and 37) or 38 40 4 or 8 or 12 or 16 or 20 or 29 or 39 41 hypertension/ 42 (antihypertens$ or hypertens$).ti,ab,ot. 43 exp blood pressure/ 44 (blood pressure$ or bloodpressure$).ti,ab,ot. 45 or/41‐44 46 randomized controlled trial.pt. 47 controlled clinical trial.pt. 48 randomi?ed.ab. 49 placebo.ab. 50 clinical trials as topic/ 51 randomly.ab. 52 trial.ti. 53 or/46‐52 54 animals/ not (humans/ and animals/) 55 53 not 54 56 40 and 45 and 55 57 remove duplicates from 56 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 4 via the Cochrane Register of Studies Online Search Date: 13 April 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (orlistat* or tetrahydrolipstatin or thlp) #2 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical) #3 #1 OR #2 #4 sibutramin* #5 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex) #6 #4 OR #5 #7 rimonabant* #8 (acomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti) #9 #7 OR #8 #10 lorcaserin #11 (belviq or lorqess) #12 #10 OR #11 #13 liraglutide #14 (saxenda or victoza) #15 #13 OR #14 #16 phentermine #17 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl) #18 #16 OR #17 #19 topiramate #20 (epitomax or qudexy or topamax or topimax or trokendi) #21 #19 OR #20 #22 (qnexa or qsiva or qsymia) #23 #18 AND #21 OR #22 #24 bupropion #25 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban) #26 #24 OR #25 #27 naltrexone #28 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol) #29 #27 OR #28 #30 (contrave or mysimba) #31 #26 AND #29 OR #30 #32 #3 OR #6 OR #9 OR #12 OR #15 OR #23 OR #31 #33 antihypertens* or hypertens*36 #34 blood pressure* or bloodpressure* #35 #33 OR #34 #36 #32 AND #35 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1980 to 2015 April 10> Search Date: 13 April 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (orlistat$ or tetrahydrolipstatin or thlp or "96829‐58‐2").mp. 2 (ro180647 or "ro 180647" or "ro 18‐0647").mp. 3 (alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical).mp. 4 or/1‐3 5 (sibutramin$ or "106650‐56‐0").mp. 6 (bts54524 or "bts 54524" or "bts‐54524" or "bts‐54‐524" or "bts54‐524").mp. 7 (adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex).mp. 8 or/5‐7 9 (rimonabant$ or "168273‐06‐1" or "158681‐13‐1").mp. 10 (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).mp. 11 (acomplia or accomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti).mp. 12 or/9‐11 13 (lorcaserin or "616202‐92‐7").mp. 14 ("apd 356" or apd356).mp. 15 (beliviq or lorqess).mp. 16 or/13‐15 17 (liraglutide or "204656‐20‐2").mp. 18 ("nn 2211" or nn2211 or "nnc 90‐1170" or "nnc90 1170").mp. 19 (saxenda or victoza).mp. 20 or/17‐19 21 (phentermine or "1197‐21‐3" or "122‐09‐8").mp. 22 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl).mp. 23 or/21‐22 24 (topiramate or "97240‐79‐4").mp. 25 (mcn4853 or "mcn 4853" or rwj17021 or "rwj17021‐000" or "rwj 17021" or "rwj 17021‐000").mp. 26 (epitomax or qudexy or topamax or topimax or trokendi).mp. 27 or/24‐26 28 (qnexa or qsiva or qsymia).mp. 29 (23 and 27) or 28 30 (bupropion or "31677‐93‐7" or "34911‐55‐2").mp. 31 (bw323 or "bw 323" or "bw323u66 bw 323u66").mp. 32 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban).mp. 33 or/30‐32 34 (naltrexone or "16590‐41‐3" or "16676‐29‐2").mp. 35 (en1639a or "en 1639a").mp. 36 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol).mp. 37 or/34‐36 38 (contrave or mysimba).mp. 39 (33 and 37) or 38 40 4 or 8 or 12 or 16 or 20 or 29 or 39 41 exp hypertension/ 42 (antihypertens$ or hypertens$).ti,ab,ot. 43 exp blood pressure/ 44 (blood pressure$ or bloodpressure$).ti,ab,ot. 45 or/41‐44 46 randomized controlled trial/ 47 crossover procedure/ 48 double‐blind procedure/ 49 (randomi?ed or randomly).tw. 50 (crossover$ or cross‐over$).tw. 51 placebo.ab. 52 (doubl$ adj blind$).tw. 53 assign$.ab 54 allocat$.ab. 55 or/46‐54 56 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 57 55 not 56 58 40 and 45 and 57 59 remove duplicates from 58
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Hypertension Group Specialised Register Search Date: 13 April 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ((orlistat* or tetrahydrolipstatin or thlp)) #2 ((alli or crisplus or lipiblock or liposol or lipstatin or oleofin or ordiet or orlipastat or tetrahydro or xenical)) 34 #3 #1 OR #2 #4 sibutramin* #5 ((adisar or apo‐sibutramin or arcalion or atenix or ectiva or medaria or meridia or raductil or reductil or reduten or sacietyl or sibutral or sibutrex)) #6 #4 OR #5 #7 rimonabant* #8 ((acomplia or bethin or monoslim or remonabent or resibant or riobant or rimoslim or riomont or slimona or zimulti)) #9 #7 OR #8 #10 lorcaserin #11 (belviq or lorqess) #12 #10 OR #11 #13 liraglutide #14 (saxenda or victoza) #15 #13 OR #14 #16 phentermine #17 (adipex or duromine or "ex adipos" or exadipos or fastin or ionamine or "miobese‐forte" or obermine or "obestin‐30" or "oby‐cap" or "oby‐trim" or "ona‐mast" or panbesy or panbesyl or phentercot or phentermide or phentermin or phentermine or phentrol or "pro‐fast" or reducyl or redusa or suprenza or "t‐diet" or terbutylamine or umine or wilpo or zantryl) #18 #16 OR #17 #19 topiramate #20 (epitomax or qudexy or topamax or topimax or trokendi) #21 #19 OR #20 #22 (qnexa or qsiva or qsymia) #23 (#18 AND #21) OR #22 #24 bupropion #25 (amfebutamone or aplenzin or budeprion or buprion or bupropin or bupropion or buxon or forfivo or odranal or quomen or wellbatrin or wellbutrin or zyban) #26 #24 OR #25 #27 naltrexone #28 (antaxone or celupan or nemexin or nalerona or nalorex or naltrel or nemexin or nodict or nutrexon or phaltrexia or regental or revez or revia or trexan or vivitrex or vivitrol) #29 #27 OR #28 #30 (contrave or mysimba) #31 (#26 AND #29) OR #30 #32 #3 OR #6 OR #9 OR #12 OR #15 OR #23 OR #31 #33 #32 AND (RCT OR Review OR Meta‐Analysis):DE ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search Date: 13 April 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: randomized Study type: Interventional Studies Conditions: hypertension Intervention: liraglutide OR lorcaserin OR orlistat OR rimonabant OR sibutramine OR phentermine‐topiramate OR (phentermine AND topiramate) OR qnexa OR qsiva or qsymia ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Appendix 6. Search strategies used in the 2009 update of the review
Ovid MEDLINE(R) <1946 to August Week 2 2012>
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations <August 16, 2012>
Embase <1988 to 2012 Week 32>
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <August 2012>
Search Date: 17 August 2012
1. exp hypertension/ or exp blood pressure/ 2. (hypertens$ or antihypertens$ or anti hypertens$).ti,ab,ot. 3. ((systolic or diastolic or arterial) adj pressur$).ti,ab,ot. 4. (blood pressur$ or bloodpressur$).ti,ab,ot. 5. or/1‐4 6. (orlistat$ or tetrahydrolipstatin or thlp).ti,ab,ot,tn,sh. 7. (orlistat or 96829‐58‐2).rn. 8. (ro180647 or "ro 180647" or "ro 18‐0647").ti,ab,ot,tn. 9. (xenical or alli).ti,ab,ot,tn. 10. or/6‐9 11. sibutramin$.ti,ab,ot,tn,sh. 12. (sibutramine or 106650‐56‐0).rn. 13. (BTS‐54524 or BTS‐54‐524 or BTS54‐524).ti,ab,ot,tn. 14. (reductil or medaria or meridia or arcalion).ti,ab,ot,tn. 15. or/11‐14 16. rimonabant$.ti,ab,ot,tn,sh. 17. (rimonabant or "168273‐06‐1" or "158681‐13‐1").rn. 18. (sr141716 or sr141716a or (sr adj ("141716" or 141716a))).ti,ab,ot,tn. 19. (acomplia or accomplia or zimulti).ti,ab,ot,tn. 20. or/16‐19 21. or/10,15,20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. clinical trials as topic.sh. 27. randomly.ab. 28. trial.ti. 29. or/22‐28 30. exp animals/ not humans.sh. 31. 29 not 30 32. crossover procedure/ 33. Double Blind Procedure/ 34. Randomized Controlled Trial/ 35. Single Blind Procedure/ 36. random$.ti,ab. 37. factorial$.ti,ab. 38. (crossover$ or cross‐over$).ti,ab. 39. placebo$.ti,ab. 40. (doubl$ adj blind$).ti,ab. 41. (singl$ adj blind$).ti,ab. 42. assign$.ti,ab. 43. allocat$.ti,ab. 44. volunteer$.ti,ab. 45. or/32‐44 46. 5 and 21 and 31 use prem 47. 5 and 21 and 31 use mesz 48. 5 and 21 and 45 use emed 49. 5 and 21 use cctr 50. or/46‐49
Appendix 7. Search strategy used in original review
1. exp hypertension/ or exp blood pressure/ 2. (hypertens$ or antihypertens$ or anti hypertens$).ti,ab,ot. 3. ((systolic or diastolic or arterial) adj pressur$).ti,ab,ot. 4. (blood pressur$ or bloodpressur$).ti,ab,ot. 5. or/1‐4 6. (orlistat$ or tetrahydrolipstatin or thlp).ti,ab,ot,tn,sh. 7. (xenical or alli).ti,ab,ot,tn. 8. or/6,7 9. sibutramin$.ti,ab,ot,tn,sh. 10. (reductil or medaria or meridia or arcalion).ti,ab,ot,tn. 11. or/9,10 12. rimonabant$.ti,ab,ot,tn,sh. 13. (acomplia or zimulti).ti,ab,ot,tn. 14. or/12,13 15. or/8,11,14 16. controlled clinical trial.pt. 17. controlled clinical trials/ 18. randomized controlled trial.pt. 19. randomized controlled trials/ 20. random allocation/ 21. cross‐over studies/ 22. double‐blind method/ 23. single‐blind method/ 24. or/16‐23 25. ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ti,ab,ot. 26. ((random$ or cross‐over or crossover) adj25 (trial$ or study or studies or intervention$ or investigat$ or experiment$ or design$ or method$ or group$ or evaluation$ or evidenc$ or data or test$ or condition$)).ti,ab,ot. 27. (random$ adj25 (cross over or crossover)).ti,ab,ot. 28. (RCT or placebo$).ti,ab,ot. 29. or/25‐28 30. 24 or 29 31. 5 and 15 32. 31 use prem 33. 31 use mesz 34. 31 use emed 35. or/32‐34 36. 35 and 30 37. 31 use cctr 38. 36 or 37
Appendix 8. Survey of trial investigators providing information on included trials
| Study | Study author contacted | Study author replied | Study author asked for additional information | Study author provided data |
| Bakris 2002 | 24.11.2005a | ‐ | ‐ | No |
| Cocco 2005 | 24.11.2005a | ‐ | 24.11.2005a | Yes |
| Guy‐Grand 2004 | 24.11.2005a | ‐ | ‐ | No |
| XENDOS 2001‐2006 | 24.11.2005a | ‐ | ‐ | No |
| CONQUER 2013 | 05.07.2018 | 06.07.2018 | 11.07.2018 | No |
| SCALE Diabetes | 30.03.2020 | 31.03.2020 | 01.04.2020 | No |
| SCALE Obesity | 30.03.2020 | 31.03.2020 | 01.04.2020 | No |
| SCALE Sleep Apnoe | 30.03.2020 | 31.03.2020 | 01.04.2020 | No |
| SCALE Maintenance | 30.03.2020 | 31.03.2020 | 01.04.2020 | No |
| BLOOM 2010 | 30.03.2020 | 30.03.2020 | ‐ | No |
| BLOOM‐DM 2012 | 30.03.2020 | 30.03.2020 | ‐ | No |
| BLOSSOM 2011 | 30.03.2020 | 30.03.2020 | ‐ | No |
| COR‐I 2010 | 30.03.2020 | 30.03.2020 | 01.04.2020 | No |
| COR‐II 2013 | 30.03.2020 | 30.03.2020 | 01.04.2020 | No |
| COR‐BMOD 2011 | 30.03.2020 | 30.03.2020 | 01.04.2020 | No |
| COR‐Diabetes 2013 | 30.03.2020 | 30.03.2020 | 01.04.2020 | No |
| Nissen 2016 | 30.03.2020 | 30.03.2020 | 01.04.2020 | Yes |
| Lu 2018 | 03.04.2020 | ‐ | ‐ | No |
| CAMELLIA‐TIMI 2018 | 03.04.2020 | ‐ | ‐ | No |
Notes
aTrial investigators were contacted during the preparation of the IQWiG report (IQWiG 2006).
Appendix 9. Selection bias decisions
| Selection bias decisions for trials reporting unadjusted analyses ‐ comparison of results obtained using method details alone with results using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | Risk of bias judgement using methods reporting | Information gained from study characteristics data | Risk of bias using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly random, or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
|
Footnotes aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias, compared with using methods reporting alone. bImbalance identified which appears likely to be due to chance. cDetails for the remaining important prognostic variables not reported. | |||
Data and analyses
Comparison 1. Orlistat versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Serious adverse events | 3 | 1476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.10, 1.91] |
| 1.2 All adverse events | 2 | 1386 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.84, 1.54] |
| 1.3 Change in body weight from baseline to endpoint (6 to 12 months follow‐up) | 4 | 2080 | Mean Difference (IV, Random, 95% CI) | ‐3.74 [‐4.70, ‐2.78] |
| 1.4 Change in systolic blood pressure from baseline to endpoint (6 to 12 months follow‐up) | 4 | 2058 | Mean Difference (IV, Fixed, 95% CI) | ‐2.58 [‐3.78, ‐1.37] |
| 1.5 Change in diastolic blood pressure from baseline to endpoint (6 to 12 months follow‐up) | 4 | 2058 | Mean Difference (IV, Fixed, 95% CI) | ‐1.97 [‐2.72, ‐1.22] |
Comparison 2. Phentermine/topiramate versus placebo.
Comparison 3. Naltrexone/bupropion versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total mortality | 1 | 8283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.40] |
| 3.2 Cardiovascular morbidity | 1 | 8283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.87, 1.41] |
| 3.3 All adverse events | 1 | 8283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.58, 1.80] |
| 3.4 Serious adverse events | 1 | 8283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.14] |
| 3.5 Change in body weight from baseline to endpoint (28 months follow‐up) | 1 | 8283 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐2.07, ‐1.73] |
| 3.6 Change in systolic blood pressure from baseline to endpoint (28 months follow‐up) | 1 | 8283 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.60, 0.60] |
| 3.7 Change in diastolic blood pressure from baseline to endpoint (28 months follow‐up) | 1 | 8283 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.08, 0.68] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Bakris 2002.
| Study characteristics | ||
| Methods |
Design: parallel, randomised, double‐blind Date: not stated Duration: 12 months Number of study centres:41 Country of publication: USA Setting: outpatient clinic |
|
| Participants | 544 obese people with insufficiently‐controlled hypertension Main inclusion criteria:age ≥ 40 years, BMI 28 to 43 kg/m2, at least 1 antihypertensive medication, sitting DBP 96 to 109 mm Hg Main exclusion criteria:recent initiation or change in diuretic therapy, previous gastrointestinal surgery for weight reduction, active gastrointestinal disorders, use of nicotine replacement therapy, appetite suppressants, fish oil supplements, chronic systemic steroids, acute antidepressant or anxiolytic therapy Subgroup: diabetic participants (no information on reasons and method are noted) |
|
| Interventions |
Intervention (route, total dose/day, frequency):Orlistat 120 mg 3 times a day with meals (N = 278) Control (route, total dose/day, frequency): Placebo 3 times a day with meals (N = 276) Additional treatment: Hypocaloric diet, lifestyle intervention, moderate physical activity |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Additional outcomes measured in the study:
|
|
| Study details |
Run‐in period: No Length of follow‐up: 12 months Study terminated before regular end (for benefit / because of adverse events): No |
|
| Publication details |
Language of publication: English Funding: Commercial (Roche Laboratories, New Jersey, USA) Publication status: Full‐text journal publication |
|
| Study aim | Quote from publication: “to investigate the hypothesis that weight reduction with orlistat plus mild caloric restriction leads to better blood pressure control than diet alone in obese individuals with inadequately controlled hypertension” | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details on sequence generation are provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment is not described |
| Blinding of participants and personnel (performance bias) Total mortality | Low risk | Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” |
| Blinding of participants and personnel (performance bias) Cardiovascular morbidity | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Body weight | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Total mortality | Low risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: Investigator assessed |
| Blinding of outcome assessment (detection bias) Cardiovascular morbidity | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: Investigator assessed; no indication that blinding of key study personnel was not broken |
| Blinding of outcome assessment (detection bias) Body weight | Unclear risk |
Quote: “We conducted a randomised, double‐blind, placebo‐controlled study…” Comment: Investigator assessed; no indication that blinding of key study personnel was not broken |
| Incomplete outcome data (attrition bias) Total mortality | Low risk |
Quote: "... statistical analyses were performed on an ITT basis ..." Comment:Last observation was carried forward and reasons and description for withdrawals are provided Withdrawals: and reasons/descriptions (orlistat vs placebo): 116 vs 168 (orlistat vs placebo)
|
| Incomplete outcome data (attrition bias) Cardiovascular morbidity | High risk |
Quote: "... statistical analyses were performed on an ITT basis ..." Comment: Last observation was carried forward and reasons and description for withdrawals are provided Withdrawals: and reasons/descriptions (orlistat vs placebo): 116 vs 168 (orlistat vs placebo)
Cardiovascular morbidity was reported only in the context of adverse events. The completeness of the information is unclear. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk |
Quote: "... statistical analyses were performed on an ITT basis ..." Comment:Last observation was carried forward and reasons and description for withdrawals are provided Withdrawals: and reasons/descriptions (orlistat vs placebo): 116 vs 168 (orlistat vs placebo) · adverse events: 18 vs 20 · refused treatment: 40 vs 91 · lost to follow‐up: 48 vs 47 · other: 8 vs 9 · excluded: 2 vs 1 |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote: "... statistical analyses were performed on an ITT basis ..." Last observation was carried forward and reasons and description for withdrawals are provided Comment: Withdrawals: and reasons/descriptions (orlistat vs placebo): 116 vs 168 (orlistat vs placebo)
|
| Incomplete outcome data (attrition bias) Body weight | Low risk |
Quote: "... statistical analyses were performed on an ITT basis ..." Comment:Last observation was carried forward and reasons and description for withdrawals are provided Withdrawals: and reasons/descriptions (orlistat vs placebo): 116 vs 168 (orlistat vs placebo)
|
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol, outcomes reported that were not prespecified (e.g. systolic blood pressure). |
| Other bias | High risk | Comment: The combination of a high withdrawal rate and the unknown duration of participation in the trial increases risk of bias even using the LOCF analysis. There are inconsistencies between text and flowchart for numbers of participants who finished the study. |
Cocco 2005.
| Study characteristics | ||
| Methods |
Design: parallel, randomised, double‐blind Date: January 2000 ‐ June 2002 Duration of intervention: 6 months Number of study centres: 1 Country of publication: Switzerland Setting: outpatient |
|
| Participants | 90 obese people with metabolic syndrome, diabetes type 2, hypertension, mostly with coronary heart disease and concomitant cardiac dysfunction Main inclusion criteria: age ≥ 35 years, BMI 31 to 40 kg/m2, left ventricular function 42% to 50% Main exclusion criteria: inability of participants to monitor their own glucose and blood pressure, active participation in a dietary programme, use of weight‐losing medication Subgroup: ‐ |
|
| Interventions |
Intervention (route, total dose/day, frequency):Orlistat 120 mg 3 times a day (N = 45) Control (route, total dose/day, frequency): Placebo 3 times a day (N = 45) Additional treatment: Hypocaloric diet, teaching sessions for lifestyle intervention, moderate physical activity |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Additional outcomes measured in the study:
|
|
| Study details |
Run‐in period: No Length of follow‐up: 6 months Study terminated before regular end (for benefit / because of adverse events): No |
|
| Publication details |
Language of publication: English Funding: Not reported Publication status: Full‐text journal publication |
|
| Study aim | Quote from publication: “…show that…orlistat could also be an adjunct to lifestyle changes in adipose, diabetic patients with the metabolic syndrome an established heart pathology and signs of incipient cardiac dysfunctions. Furthermore, we hypothesize that following a sufficient weight reduction improvements in metabolic pathology and, more important to us, in cardiac dysfunction should be observed.” | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure that each group would contain approximately equal numbers of risk factors, stratified randomisation was used with an algorithm generated from a random set of numbers." |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment is not described |
| Blinding of participants and personnel (performance bias) Total mortality | Low risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. |
| Blinding of participants and personnel (performance bias) Cardiovascular morbidity | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Body weight | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. No indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Total mortality | Low risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. Investigator assessed |
| Blinding of outcome assessment (detection bias) Cardiovascular morbidity | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. Investigator assessed; no indication that blinding of key study personnel was not broken |
| Blinding of outcome assessment (detection bias) Body weight | Unclear risk | Comment: Authors’ reply gave further detailed information that participants, study personnel, and outcome assessors were blinded. Investigator assessed; no indication that blinding of key study personnel was not broken |
| Incomplete outcome data (attrition bias) Total mortality | Low risk |
Quote:“All 90 patients completed the trial” Comment: No missing outcome data |
| Incomplete outcome data (attrition bias) Cardiovascular morbidity | High risk |
Quote: “All 90 patients completed the trial” Comment: No missing outcome data Cardiovascular morbidity was reported only in the context of adverse events. The completeness of the information is unclear. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk |
Quote:“All 90 patients completed the trial” Comment: No missing outcome data |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote:“All 90 patients completed the trial” Comment: No missing outcome data |
| Incomplete outcome data (attrition bias) Body weight | Low risk |
Quote:“All 90 patients completed the trial” Comment: No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol or reporting on prespecified endpoint, no primary endpoint reported |
| Other bias | Low risk | Comment: Small inconsistencies between changes in body weight reported in the table and text in comparison with changes in body weight calculated from "baseline" and "after therapy" measures reported in the table |
Guy‐Grand 2004.
| Study characteristics | ||
| Methods |
Design: parallel, randomised, double‐blind Date: not stated Duration of intervention: 6 months Number of study centres: 253 Country of publication: France Setting: private endocrinologists |
|
| Participants | 1004 obese people with diabetes type 2, hypertension, or hypercholesterolaemia Main inclusion criteria: age 18 to 65 years, BMI 28 to 40 kg/m2, DBP 90 to 110 mm Hg or untreated hypertensives or treatment stopped for more than 3 months or insufficiently controlled by the last 6 months of treatment Main exclusion criteria: secondary hypertension; history or presence of drug and alcohol abuse; significant cardiac, renal, hepatic gastrointestinal, endocrine. and psychiatric disorders Subgroups: participants with diabetes, with hypertension, or participants with hypercholesterolaemia (only the predefined subgroup of hypertensive participants is reported in this review) |
|
| Interventions |
Intervention (route, total dose/day, frequency): Orlistat 120 mg 3 times a day with meals (N = 499; hypertensive subgroup: N = 304) Control (route, total dose/day, frequency):Placebo 3 times a day with meals (N = 504; hypertensive subgroup: N = 310) Additional treatment: Hypocaloric diet |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Additional outcomes measured in the study:
|
|
| Study details |
Run‐in period: No Length of follow‐up: 6 months Study terminated before regular end (for benefit / because of adverse events):No |
|
| Publication details |
Language of publication: English Funding: Roche Pharma, Paris, France Publication status: Full‐text journal publication |
|
| Study aim | Quote from publication: “to assess the effect of orlistat on body weight and concomitant diseases in patients with body mass index (BMI) of > 28 kg/m² and poorly controlled type 2 diabetes, hypertension or hypercholesterolaemia. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a centralized procedure in which patients were stratified by both centre and comorbidity. According to minimization procedure, patients were randomised to ensure that treatment groups were well balanced by both centre and by concomitant disease. Patients who met the inclusion criteria for more than one group were assigned to the group that the investigator considered to be the most important treatment objective for weight loss." |
| Allocation concealment (selection bias) | Low risk | Comment: Central allocation |
| Blinding of participants and personnel (performance bias) Total mortality | Unclear risk | Comment: Outcome not reported |
| Blinding of participants and personnel (performance bias) Cardiovascular morbidity | Unclear risk | Comment: Outcome not reported |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: Outcome not reported |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote: “... randomised, double‐blind, placebo controlled, parallel‐group study...” Comment: no indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Body weight | Unclear risk |
Quote: “... randomised, double‐blind, placebo controlled, parallel‐group study...” Comment: no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Total mortality | Unclear risk | Comment: Outcome not reported |
| Blinding of outcome assessment (detection bias) Cardiovascular morbidity | Unclear risk | Comment: Outcome not reported |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: Outcome not reported |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote: “... randomised, double‐blind, placebo controlled, parallel‐group study...” Comment: Investigator assessed; no indication that blinding of key study personnel was not broken |
| Blinding of outcome assessment (detection bias) Body weight | Unclear risk |
Quote: “... randomised, double‐blind, placebo controlled, parallel‐group study...” Comment: Investigator assessed; no indication that blinding of key study personnel was not broken |
| Incomplete outcome data (attrition bias) Total mortality | Unclear risk | Comment: Outcome not reported |
| Incomplete outcome data (attrition bias) Cardiovascular morbidity | Unclear risk | Comment: Outcome not reported |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: Outcome not reported |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote: "Efficacy was assessed on an intent‐to‐treat (ITT) basis. The ITT population included all randomised patients and the safety population consisted of all randomised patients who had received at least one dose of study treatment. Principal criteria missing at six months were replaced by the last‐measured value (last observation carried forward, LOCF)." Comment: Withdrawals or lost to follow‐up / reasons/descriptions: Only reported for the whole study population, which had diabetes type 2, hypertension, or hypercholesterolaemia. Orlistat vs placebo: 8.4% vs 9.3% adverse events: 19 vs 13
|
| Incomplete outcome data (attrition bias) Body weight | Unclear risk |
Quote: "Efficacy was assessed on an intent‐to‐treat (ITT) basis. The ITT population included all randomised patients and the safety population consisted of all randomised patients who had received at least one dose of study treatment. Principal criteria missing at six months were replaced by the last‐measured value (last observation carried forward, LOCF)." Comment: Withdrawals or lost to follow‐up / reasons/descriptions: Only reported for the whole study population, which had diabetes type 2, hypertension, or hypercholesterolaemia. Orlistat vs placebo: 8.4% vs 9.3%
|
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol |
| Other bias | Low risk | Comment: None identified |
XENDOS 2001‐2006.
| Study characteristics | ||
| Methods |
Design: parallel, randomised, double‐blind Date: 1997 ‐ 2002 Duration of intervention: 48 months Number of study centres: 22 Country of publication: Sweden Setting: medical centres |
|
| Participants | 3305 obese people with normal or impaired glucose tolerance. Only the predefined subgroup of hypertensive participants is reported here. Data were obtained from the publicly‐available scientific report of the Institute for Quality and Efficiency in Health Care (IQWiG 2006) Main inclusion criteria: age 30 to 60 years, BMI ≥ 30 kg/m2, normal or impaired glucose tolerance, either DBP ≥ 90 mm Hg or SBP ≥ 140 mm Hg Main exclusion criteria: SBP > 165 mm Hg or DBP > 105 mm Hg, diabetes mellitus, ongoing and active cardiovascular and gastrointestinal disease Subgroups: DBP ≥ 90 mm Hg (first predefined subgroup) or SBP ≥ 140 mm Hg (second predefined subgroup) |
|
| Interventions |
Intervention (route, total dose/day, frequency):Orlistat 120 mg 3 times a day with meals (N = 1650; hypertensive subgroup: N = 924) Control (route, total dose/day, frequency): Placebo 3 times a day with meals (N = 1655; hypertensive subgroup: N = 950) Additional treatment: Hypocaloric diet, teaching sessions for lifestyle intervention, moderate physical activity |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Additional outcomes measured in the study:
|
|
| Study details |
Run‐in period: No Length of follow‐up: 48 months Study terminated before regular end (for benefit / because of adverse events): No |
|
| Publication details |
Language of publication: English Funding: Hoffmann‐La Roche, Nutley, (NJ) USA Publication status: Full‐text journal publication |
|
| Study aim | Quote from publication: “It is well established that the risk of developing type 2 diabetes is closely linked to the presence and duration of overweight and obesity. A reduction in the incidence of type 2 diabetes with lifestyle changes has previously been demonstrated. We hypothesized that adding a weight‐reducing agent to lifestyle changes may lead to an even greater decrease in body weight, and thus the incidence of type 2 diabetes, in obese patients.” | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: no information from publication, but according to IQWIG report adequate (IQWiG 2006) |
| Allocation concealment (selection bias) | Low risk | Comment: no information from publication, but according to IQWIG report adequate (IQWiG 2006) |
| Blinding of participants and personnel (performance bias) Total mortality | Low risk | Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” |
| Blinding of participants and personnel (performance bias) Cardiovascular morbidity | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: no indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: no indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: no indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Body weight | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Total mortality | Low risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: Investigator assessed |
| Blinding of outcome assessment (detection bias) Cardiovascular morbidity | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: Investigator assessed; no indication that blinding of key study personnel was not broken |
| Blinding of outcome assessment (detection bias) Body weight | Unclear risk |
Quote: “…4‐year, double‐blind, randomised, placebo controlled prospective study…” Comment: Investigator assessed; no indication that blinding of key study personnel was not broken |
| Incomplete outcome data (attrition bias) Total mortality | Unclear risk |
Quote: "Efficacy was assessed on an intention‐to‐treat (ITT) basis with a last observation carried forward principle (LOCF). The ITT population included all randomised patients who had received at least one dose of study treatment and one follow up examination." Comment: Withdrawals and reasons/descriptions: First subgroup: 183 (45%) vs 268 (61%) (orlistat vs placebo)
Second subgroup: 215 (42%) vs 307 (60%) (orlistat vs placebo)
|
| Incomplete outcome data (attrition bias) Cardiovascular morbidity | Unclear risk |
Quote: "Efficacy was assessed on an intention‐to‐treat (ITT) basis with a last observation carried forward principle (LOCF). The ITT population included all randomised patients who had received at least one dose of study treatment and one follow up examination." Comment: Withdrawals and reasons/descriptions: First subgroup: 183 (45%) vs 268 (61%) (orlistat vs placebo)
Second subgroup: 215 (42%) vs 307 (60%) (orlistat vs placebo)
|
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote: "Efficacy was assessed on an intention‐to‐treat (ITT) basis with a last observation carried forward principle (LOCF). The ITT population included all randomised patients who had received at least one dose of study treatment and one follow up examination." Comment: Withdrawals and reasons/descriptions: First subgroup: 183 (45%) vs 268 (61%) (orlistat vs placebo)
Second subgroup: 215 (42%) vs 307 (60%) (orlistat vs placebo)
|
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote: "Efficacy was assessed on an intention‐to‐treat (ITT) basis with a last observation carried forward principle (LOCF). The ITT population included all randomised patients who had received at least one dose of study treatment and one follow up examination." Comment: Withdrawals and reasons/descriptions: First subgroup: 183 (45%) vs 268 (61%) (orlistat vs placebo)
Second subgroup: 215 (42%) vs 307 (60%) (orlistat vs placebo)
|
| Incomplete outcome data (attrition bias) Body weight | Unclear risk |
Quote: "Efficacy was assessed on an intention‐to‐treat (ITT) basis with a last observation carried forward principle (LOCF). The ITT population included all randomised patients who had received at least one dose of study treatment and one follow up examination." Comment: Withdrawals and reasons/descriptions: First subgroup: 183 (45%) vs 268 (61%) (orlistat vs placebo)
Second subgroup: 215 (42%) vs 307 (60%) (orlistat vs placebo)
|
| Selective reporting (reporting bias) | Unclear risk | Comment: Information was obtained only from the scientific report (IQWiG 2006); no full publication was available to allow judgement of either yes or no |
| Other bias | Unclear risk | Comment: Power calculation is only provided for the whole group. The combination of a high withdrawal rate and the unknown duration of participation in the trial increases risk of bias even using the LOCF analysis. |
CONQUER 2013.
| Study characteristics | ||
| Methods |
Design: parallel, randomised, double‐blind Date: November 2007 ‐ June 2009 Duration of intervention: 56 weeks Number of study centres: 93 Country of publication: USA Setting: outpatient clinic |
|
| Participants | 2487 obese or overweight people with 2 or more comorbidities Main inclusion criteria: age 18 to 70 years, BMI 27 to 45 kg/m2, 2 or more of the following comorbidities at baseline: SBP 140 to 160 mm Hg (130 to 160 mm Hg in people with diabetes), DBP 90 to 100 mm Hg (85 to 100 mm Hg in people with diabetes), or taking at least 2 antihypertensive drugs; triglycerides 2.26 to 4.52 mmol/l or using at least 2 lipid‐lowering drugs; fasting blood glucose > 5.55 mmol/l, blood glucose > 7.77 mmol/l at 2 h after oral glucose load during oral glucose tolerance test, or diagnosed type 2 diabetes managed with lifestyle changes or metformin monotherapy; and waist circumference of at least 102 cm for men or at least 88 cm for women Main exclusion criteria: blood pressure > 160/100 mm Hg, fasting glucose > 13.32 mmol/l or triglycerides > 4.52 mmol/l at randomisation, type 1 diabetes, use of antidiabetic drugs other than metformin, history of nephrolithiasis, recurrent major depression, presence or history of suicidal behaviour or ideation with intent to act, and current substantial depressive symptoms (Patient Health Questionnaire (PHQ‐9) total score ≥ 10) Subgroups: Participants with hypertension, participants with dyslipidaemia (only the predefined subgroup of hypertensive participants is reported in this review) |
|
| Interventions |
Intervention (route, total dose/day, frequency):Phentermine/topiramate: dose titration during the first 4 weeks; initial dosage of 3.75 mg phentermine and 23 mg topiramate, increasing weekly (3.75 mg phentermine and 23 mg topiramate) until the assigned dosage of phentermine 7.5 mg/topiramate 46.0 mg (group 1 – low dose) (N = 498; hypertensive subgroup: N = 261) or phentermine 15 mg/topiramate 92.0 mg (group 2 –high dose) was achieved, maintained at assigned dosages once daily for 52 weeks (N = 995; hypertensive subgroup: N = 520) Control (route, total dose/day, frequency): Placebo once daily (N = 994; hypertensive subgroup: N = 524) Additional treatment: Standardised counselling for diet (to reduce caloric intake by 500 kcal/day) and lifestyle modification |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Additional outcomes measured in the study:
· Mean change in BMI (kg/m2) · Change in waist circumference (cm) · Change in heart rate · Changes in lipids, glycaemic measures, biomarkers · Change concomitant drugs for comorbidities · Depressive symptoms (Patient Health Questionnaire (PHQ‐9) · Response to Columbia Suicide Severity Rating Scale (C‐SSRS) |
|
| Study details |
Run‐in period: 4 week Length of follow‐up: 56 weeks Study terminated before regular end (for benefit / because of adverse events): No |
|
| Publication details |
Language of publication: English Funding: VIVUS Inc., Mountain View (CA) USA Publication status: Full‐text journal publication |
|
| Study aim | Quote from publication: “Obesity is associated with a reduction in life expectancy and an increase in mortality from cardiovascular diseases, cancer, and other causes. We therefore assessed the efficacy and safety of two doses of phentermine plus topiramate controlled‐release combination as an adjunct to diet and lifestyle modification for weight loss and metabolic risk reduction in individuals who were overweight and obese, with two or more risk factors.” | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... used a computer‐generated algorithm that was implemented through an interactive voice response system to assign patients according to the random allocation sequence, with a block size of eight." |
| Allocation concealment (selection bias) | Low risk | Comment: Central allocation |
| Blinding of participants and personnel (performance bias) Total mortality | Low risk | Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” |
| Blinding of participants and personnel (performance bias) Cardiovascular morbidity | Low risk | Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk | Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” |
| Blinding of participants and personnel (performance bias) Body weight | Low risk | Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” |
| Blinding of outcome assessment (detection bias) Total mortality | Low risk |
Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” Comment: Investigator assessed |
| Blinding of outcome assessment (detection bias) Cardiovascular morbidity | Low risk |
Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” Comment: Investigator assessed or self‐reported |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” Comment: Investigator assessed or self‐reported |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” Comment: Investigator assessed |
| Blinding of outcome assessment (detection bias) Body weight | Low risk |
Quote: “…a randomised, double‐blind, placebo controlled study…”. “Study drugs were administered as capsules that were identical in size and appearance. Investigators, patients, and study sponsors were masked to treatment assignment.” Comment: Investigator assessed |
| Incomplete outcome data (attrition bias) Total mortality | Unclear risk |
Quote: "The primary analyses were done on the intention‐to‐treat sample, consisting of all patients who were randomly assigned, took at least one dose of the study drug or placebo, and had one post baseline bodyweight measurement." Comment: Withdrawals and reasons/descriptions: Total number of withdrawals and reasons only reported for the whole study population having hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity Phen/Top – low dose vs Phen/Top – high dose vs placebo: 30.9% vs 36.2% vs 43.2%
Hypertensive subgroup:
|
| Incomplete outcome data (attrition bias) Cardiovascular morbidity | High risk |
Quote: "The primary analyses were done on the intention‐to‐treat sample, consisting of all patients who were randomly assigned, took at least one dose of the study drug or placebo, and had one post baseline bodyweight measurement." Comment: Withdrawals and reasons/descriptions: Total number of withdrawals and reasons only reported for the whole study population having hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity Phen/Top – low dose vs Phen/Top – high dose vs placebo: 30.9% vs 36.2% vs 43.2%
Hypertensive subgroup:
Cardiovascular morbidity was reported only in the context of adverse events. The completeness of the information is unclear. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote: "The primary analyses were done on the intention‐to‐treat sample, consisting of all patients who were randomly assigned, took at least one dose of the study drug or placebo, and had one post baseline bodyweight measurement." Comment: Withdrawals and reasons/descriptions: Total number of withdrawals and reasons only reported for the whole study population having hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity Phen/Top – low dose vs Phen/Top – high dose vs placebo: 30.9% vs 36.2% vs 43.2%
Hypertensive subgroup:
|
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote: "The primary analyses were done on the intention‐to‐treat sample, consisting of all patients who were randomly assigned, took at least one dose of the study drug or placebo, and had one post baseline bodyweight measurement." Comment: Withdrawals and reasons/descriptions: Total number of withdrawals and reasons only reported for the whole study population having hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity Phen/Top – low dose vs Phen/Top – high dose vs placebo: 30.9% vs 36.2% vs 43.2%
Hypertensive subgroup:
|
| Incomplete outcome data (attrition bias) Body weight | Unclear risk |
Quote: "The primary analyses were done on the intention‐to‐treat sample, consisting of all patients who were randomly assigned, took at least one dose of the study drug or placebo, and had one post baseline bodyweight measurement." Comment: Withdrawals and reasons/descriptions: Total number of withdrawals and reasons only reported for the whole study population having hypertension, dyslipidaemia, diabetes or prediabetes, or abdominal obesity Phen/Top – low dose vs Phen/Top – high dose vs placebo: 30.9% vs 36.2% vs 43.2%
Hypertensive subgroup:
|
| Selective reporting (reporting bias) | Unclear risk | Comment: No study protocol provided |
| Other bias | Low risk | Comment: None detected |
Nissen 2016.
| Study characteristics | ||
| Methods |
Design: parallel, randomised, double‐blind Date: June 2012 ‐ January 2013 Duration of intervention: 121 weeks Number of study centres: 266 Country of publication: USA Setting:medical sites |
|
| Participants | 8910 overweight or obese patients at increased risk of adverse cardiovascular outcomes. Main inclusion criteria: age ≥ 50 years (women) or ≥ 45 years (men), BMI 27 to 50 kg/m2, waist circumference ≥ 88 cm (women) or ≥ 102 cm (men), increased risk of adverse cardiovascular outcomes, diagnosed type 2 diabetes with 2 or more comorbidities as hypertension, dyslipidaemia requiring pharmacotherapy, low high‐density lipoprotein cholesterol (< 50 mg/dL (1.30 mmol/L) in women or < 40 mg/dL (1.04 mmol/L) in men) or current tobacco smoking Main exclusion criteria: myocardial infarction within 3 months prior to screening, severe angina pectoris, New York Heart Association class 3 or 4 heart failure, history of stroke or blood pressure of ≥ 145/95 mm Hg, unstable weight within 3 months prior to screening (weight gain or loss of > 3%), planned bariatric or cardiac surgery or percutaneous coronary intervention, severe renal impairment (estimated glomerular filtration rate < 30 mL/min), elevated liver enzymes more than 3 times the upper limit of normal, long‐term opioid use, history of seizures or cranial trauma, mania, bulimia, anorexia nervosa, acute depressive illness or suicidality, patients taking monoamine oxidase inhibitors within 14 days prior to screening Subgroup: hypertensive participants with antihypertensive medication at baseline |
|
| Interventions |
Intervention (route, total dose/day, frequency): Naltrexone/bupropion: extended‐release, fixed‐dose formulation containing 8 mg naltrexone and 90 mg bupropion (1 tablet): dose titration during the first 4 weeks; 1 tablet/day during the first week, 2 tablets/day during week 2, 3 tablets/day during week 3 and 4 tablets/day during week 4 and thereafter. Dosage levels requiring more than 1 tablet daily were administered twice a day (morning and evening). (N = 4456; hypertensive subgroup: N = 4164) Control (route, total dose/day, frequency): Placebo once or twice a day (N = 4454; hypertensive subgroup: N = 4123) Additional treatment: Internet‐based weight management programme that included educational resources on healthy eating, exercise and behavioral modifications. Programme information was presented in the form of weekly lessons that the study participants could access online, and they also received weekly emails with healthy lifestyle tips and reminders. Participants also had access to a personal weight‐loss coach; programmes to track weight, meals, and physical activity; and a low‐fat, low‐calorie meal plan |
|
| Outcomes |
Primary outcomes: (ST1)
Secondary outcomes:
Additional outcomes measured in the study:
|
|
| Study details |
Run‐in period: 2 weeks Length of follow‐up: 121 weeks Study terminated before regular end (for benefit / because of adverse events): Yes The study was terminated prematurely after the unplanned release of the 25% interim data |
|
| Publication details |
Language of publication: English Funding: Orexigen Therapeutics Inc (La Jolla, California) and Takeda Pharmaceuticals International (Deerfield, Illinois) Publication status:Full‐text journal publication |
|
| Study aim |
Quote from publication: “to determine whether the combination of naltrexone and bupropion increases major adverse cardiovascular events (MACE, defined as cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction) compared with placebo in overweight and obese patients” |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Randomization occurred via an interactive voice recognition system using a permuted block size of 4 with no stratification factors.” |
| Allocation concealment (selection bias) | Low risk | Comment:Central allocation |
| Blinding of participants and personnel (performance bias) Total mortality | Low risk | Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” |
| Blinding of participants and personnel (performance bias) Cardiovascular morbidity | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of participants and personnel (performance bias) Body weight | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: No indication that blinding of participants and key study personnel were not broken |
| Blinding of outcome assessment (detection bias) Total mortality | Low risk | Quote: “A blinded independent clinical events committee at the Cleveland Clinic Center for Clinical Research adjudicated clinical outcomes, including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, and all‐cause mortality.” |
| Blinding of outcome assessment (detection bias) Cardiovascular morbidity | Low risk | Quote: “A blinded independent clinical events committee at the Cleveland Clinic Center for Clinical Research adjudicated clinical outcomes, including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, and all‐cause mortality.” |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken. |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken. |
| Blinding of outcome assessment (detection bias) Body weight | Unclear risk |
Quote: “The study was a phase 3b, multicenter, randomized, double‐blind, placebo‐controlled trial …” Comment: Investigator assessed or self‐reported; no indication that blinding of participants and key study personnel were not broken. |
| Incomplete outcome data (attrition bias) Total mortality | Unclear risk |
Quote: “The primary analyses were performed for the intention‐to‐treat (ITT) population, defined as patients who underwent randomization into the treatment period and were dispensed study medication. No imputation was performed for missing data. Analyses were also performed for an “on‐treatment” population, defined as ITT patients who took at least 1 dose of study medication, with censoring of events occurring more than 30 days after a patient’s last confirmed dose of study medication.” Comment: Withdrawals: and reasons/descriptions (naltrexone/bupropion vs placebo): 4455 vs 4450 (naltrexone/bupropion vs placebo):
Number of withdrawals and reasons only reported for the whole study population which had hypertension, diabetes type 2 and/or dyslipidemia. |
| Incomplete outcome data (attrition bias) Cardiovascular morbidity | High risk |
Quote: “The primary analyses were performed for the intention‐to‐treat (ITT) population, defined as patients who underwent randomization into the treatment period and were dispensed study medication. No imputation was performed for missing data. Analyses were also performed for an “on‐treatment” population, defined as ITT patients who took at least 1 dose of study medication, with censoring of events occurring more than 30 days after a patient’s last confirmed dose of study medication.” Comment: The cardiovascular safety of naltrexone‐bupropion remains inconclusive following publication of the final results for the prematurely terminated outcomes study; only 50% of the planned number of cardiovascular events were reported. Withdrawals: and reasons/descriptions (naltrexone/bupropion vs placebo): 4455 vs 4450 (naltrexone/bupropion vs placebo):
Number of withdrawals and reasons only reported for the whole study population which had hypertension, diabetes type 2 and/or dyslipidemia. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote: “The primary analyses were performed for the intention‐to‐treat (ITT) population, defined as patients who underwent randomization into the treatment period and were dispensed study medication. No imputation was performed for missing data. Analyses were also performed for an “on‐treatment” population, defined as ITT patients who took at least 1 dose of study medication, with censoring of events occurring more than 30 days after a patient’s last confirmed dose of study medication.” Comment: Withdrawals: and reasons/descriptions (naltrexone/bupropion vs placebo): 4455 vs 4450 (naltrexone/bupropion vs placebo):
Number of withdrawals and reasons only reported for the whole study population which had hypertension, diabetes type 2 and/or dyslipidemia. |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote: “The primary analyses were performed for the intention‐to‐treat (ITT) population, defined as patients who underwent randomization into the treatment period and were dispensed study medication. No imputation was performed for missing data. Analyses were also performed for an “on‐treatment” population, defined as ITT patients who took at least 1 dose of study medication, with censoring of events occurring more than 30 days after a patient’s last confirmed dose of study medication.” Comment: Withdrawals: and reasons/descriptions (naltrexone/bupropion vs placebo): 4455 vs 4450 (naltrexone/bupropion vs placebo):
Number of withdrawals and reasons only reported for the whole study population which had hypertension, diabetes type 2 and/or dyslipidemia. |
| Incomplete outcome data (attrition bias) Body weight | Unclear risk |
Quote: “The primary analyses were performed for the intention‐to‐treat (ITT) population, defined as patients who underwent randomization into the treatment period and were dispensed study medication. No imputation was performed for missing data. Analyses were also performed for an “on‐treatment” population, defined as ITT patients who took at least 1 dose of study medication, with censoring of events occurring more than 30 days after a patient’s last confirmed dose of study medication.” Comment: Withdrawals: and reasons/descriptions (naltrexone/bupropion vs placebo): 4455 vs 4450 (naltrexone/bupropion vs placebo):
revoked contact with primary care physician or designated contact: 193 vs 192 Number of withdrawals and reasons only reported for the whole study population which had hypertension, diabetes type 2 and/or dyslipidemia. |
| Selective reporting (reporting bias) | Unclear risk | Comment: A study protocol is available. All prespecified outcomes are reported. The study was terminated prematurely after the unplanned release of the 25% interim data. Outcome measures data are based on the 50% interim analysis, designated as the primary analysis. Safety data are based on all available data at the time of database lock, which occurred after the 50% interim analysis |
| Other bias | High risk | Comment: Very high withdrawal rates (84% and 94%, respectively), without imputation for missing data |
BMI: body mass index; DBP: diastolic blood pressure; ITT: intention to treat; LOCF: last observation carried forward analysis; SBP: systolic blood pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Apfelbaum 1999 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Bach 1999 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Bain 2015 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| BLOOM 2010 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| BLOOM‐DM 2012 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| BLOSSOM 2011 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Bray 1999 | The study does not include participants with essential hypertension |
| Broom 2002 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| CAMELLIA‐TIMI 2018 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Christou 2015 | The study is not a randomised controlled trial |
| Cohen 2019 | The study is not a randomised controlled trial |
| COR‐BMOD 2011 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| COR‐Diabetes 2013 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| COR‐I 2010 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| COR‐II 2013 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| CTRI/2017/05/008655 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Davidson 1999 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| De Castro 2009 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Derosa 2003 | The study does not include participants with essential hypertension |
| Derosa 2008 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Derosa 2010 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Despres 2005 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Despres 2009 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Dujovne 2001 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| EQUIP 2012 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Erdmann 2004 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| EUCTR2013‐002348‐99 | The intention of the intervention is the reduction of body weight |
| Fanghänel 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Fanghänel 2003 | Weight‐reducing drug in intervention group no longer approved |
| Faria 2002 | Weight‐reducing drug in intervention group no longer approved |
| Finer 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Fujioka 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Fujioka 2016 | The study is not a randomised controlled trial |
| Gadde 2017 | The study is not a randomised controlled trial |
| Garvey 2009 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Gentile 2005 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Ginsberg 2004 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Greenway 2009 | The study does not include participants with essential hypertension |
| Greenway 2016 | The study is not a randomised controlled trial |
| Halpern 2002 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Halpern 2003 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Halseth 2018 | The study includes different accompanying antihypertensive therapies in the study groups |
| Hauner 2004 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Hauptman 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Heinonen 2009 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Hollander 2007 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Hung 2005 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Jain 2011 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| James 1997 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| James 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Jia 2010 | The study is not a randomised controlled trial |
| Jordan 2012 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Kaukua 2004 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| KCT0002097 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Kelley 2002 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Kern 2017 | The study is not a randomised controlled trial |
| Kim 2016 | The study is not a randomised controlled trial |
| Klassen 2016 | The study does not include participants with essential hypertension |
| LEADER 2013 | The intention of the intervention is the reduction of body weight |
| Le Roux 2018 | The study is not a randomised controlled trial |
| Lewis 2014 | The study is not a randomised controlled trial |
| Lindgarde 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Lindgarde 2001a | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Lindgarde 2001b | The study is not a randomised controlled trial |
| Lu 2018 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Madsen 2009 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| McMahon 2000 | Weight‐reducing drug in intervention group no longer approved |
| McMahon 2002 | Weight‐reducing drug in intervention group no longer approved |
| McNulty 2003 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Miles 2002 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Moore 2016 | The study is not a randomised controlled trial |
| NCT01764386 | The study includes a combination of different interventions |
| Niskanen 2010 | The duration of the intervention is less than 24 weeks |
| Nissen 2008 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| O'Leary 2011 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Pathan 2004 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Patschan 2007 | The duration of the intervention is less than 24 weeks |
| Pi‐Sunyer 2006 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Reaven 2001 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Rossner 2000 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Rossner 2001 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Samuelsson 2003 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| SCALE diabetes 2014 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| SCALE maintenance 2013 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| SCALE obesity and prediabetes 2014 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| SCALE sleep apnoea 2014 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Scheen 2002 | The study is not a randomised controlled trial |
| Scott 2015 | The study is not a randomised controlled trial |
| SCOUT 2010 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| SEQUEL 2014 | The study is not a randomised controlled trial |
| Sharif 2016 | The study is not a randomised controlled trial |
| Shaw Tronieri 2018 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Sjöström 1998 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Starling 2001 | The study does not include participants with essential hypertension |
| Strebkova 2015 | The study does not include participants with essential hypertension |
| Suyog 2011 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Svendsen 2008 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Swinburn 2005 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Tambascia 2003 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Tiikkainen 2004 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Triay 2012 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Tunyan 2007 | The study includes different accompanying antihypertensive therapies in the study groups |
| Van Gaal 2005 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Van Gaal 2008 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Von Scholten 2015 | The study is not a randomised controlled trial |
| Wang 2003 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Winslow 2012 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Wirth 2001 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Ye 2017 | The study does not include participants with essential hypertension |
| Zannad 2002 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
| Zavoral 1998 | The study includes normotensive and hypertensive participants but reports no or insufficient results for the hypertensive subgroup |
Differences between protocol and review
Two review authors (Anne Stich and Eva Matyas) did not contribute to the 2013 update of this review and were removed from the list of authors.
Thomas Semlitsch joined the team of review authors for the 2013 version of this review and provided substantive intellectual contributions that justify his inclusion as author.
Ulrich Siering did not contribute to the 2015 update of this review and was removed from the list of authors.
Jutta Meschik joined the team of review authors for the 2015 version of this review and provided substantive intellectual contributions that justify her inclusion as author.
Nicole Posch and Jutta Meschik did not contribute to the 2020 update of this review and were removed from the list of authors.
Sebastian Winterholer and Cornelia Krenn joined the team of review authors for the 2020 version of this review and provided substantive intellectual contributions that justify their inclusion as authors.
Since current guidelines for the pharmacological management of obesity quote four additional medications (liraglutide, lorcaserin, phentermine/topiramate, or naltrexone/bupropion) for long‐term weight reduction, we extended the search to include these drugs in the 2015 version of this review.
Since the market approvals of rimonabant and sibutramine have been withdrawn since 2009 and 2010, respectively, these two drugs were no longer considered as relevant for long‐term weight management and were therefore excluded from the 2020 version of this review.
Contributions of authors
Andrea Siebenhofer: protocol development, quality assessment of trials, data extraction, development of final review and review update, corresponding author.
Klaus Jeitler: protocol development, searching for trials, quality assessment of trials, data extraction, development of review update.
Karl Horvath: protocol development, quality assessment of trials, data extraction, development of final review and review update.
Andrea Berghold: statistical analysis, development of final review and review update.
Sebastian Winterholer: selection of studies, quality assessment of trials, data extraction, development of review update.
Cornelia Krenn: selection of studies, quality assessment of trials, data extraction, development of review update.
Thomas Semlitsch: searching for trials, selection of studies, quality assessment of trials, data extraction, development of review update.
Sources of support
Internal sources
-
Medical University of Graz, Austria
Salary, office space, computer support, library resources
External sources
No sources of support supplied
Declarations of interest
Andrea Siebenhofer, Klaus Jeitler, and Karl Horvath were involved in the preparation of a report on the evaluation of the benefits and harms of non‐drug treatment strategies in people with essential hypertension: weight reduction for the Institute for Quality and Efficiency in Health Care (iqwig.de/).
Andrea Berghold: none known.
Sebastian Winterholer: none known.
Cornelia Krenn: none known.
Thomas Semlitsch: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bakris 2002 {published data only}
- Bakris G, Calhoun D, Egan B, Hellmann C, Dolker M, Kingma I, et al. Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. Journal of Hypertension 2002;20(11):2257-67. [DOI] [PubMed] [Google Scholar]
Cocco 2005 {published data only}
- Cocco G, Pandolfi S, Rousson V. Sufficient weight reduction decreases cardiovascular complications in diabetic patients with the metabolic syndrome: a randomized study of orlistat as an adjunct to lifestyle changes (diet and exercise). Heart Drug 2005;5(2):68-74. [Google Scholar]
CONQUER 2013 {published data only}
- Astrup AV, Oppert JM, Peterson CA. Weight loss and improvements in cardiometabolic outcomes with extended-release phentermine/topiramate treatment in overweight/obese subjects with type 2 diabetes. Diabetologia 2012;55(Suppl1):S284-S285. [EMBASE: 70888716] [Google Scholar]
- Cheskin J, Bowden CH. Reduction of cardiovascular risk factors by magnitude of weight loss in obese and overweight subjects with >2 comorbidities. Journal of General Internal Medicine 2013;28(Suppl1):S168. [EMBASE: 71292986] [Google Scholar]
- Cheskin LJ, Peterson CA. Improved cardiometabolic risk factors in overweight/obese subjects receiving controlled-release phentermine/topiramate (PHEN/TPM CR). Diabetes 2012;61(Suppl1):A710. [EMBASE: 70799295] [Google Scholar]
- Davidson M, Bowden CH, Day WW. Weight loss and cardiovascular risk reduction over 2 years with controlled-release phentermine-topiramate. Journal of the American College of Cardiology 2011;57(14, Supplement):E545. [EMBASE: 70400222] [Google Scholar]
- Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index >27 kg/m(2). American Journal of Cardiology 2013;111(8):1131-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Finer N, Jordan J, Dvorak R. Weight loss and improvements in blood pressure (BP) status associated with phentermine and topiramate extended-release (PHEN/TPM ER) treatment in obese and overweight adults. Obesity Facts 2013;6(Suppl1):45. [EMBASE: 71300090] [Google Scholar]
- Finkelstein EA, Kruger E, Karnawat S. Cost-effectiveness analysis of Qsymia for weight loss. PharmacoEconomics 2015;33(7):699-706. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde K, Najarian T, Day WW. Magnitude of weight loss experienced with a low-dose, controlled-release formulation of phentermine/topiramate (PHEN/TPM) may drive degree of cardiometabolic benefit. Diabetes 2010;Conference Publication:(var. pagings). [EMBASE: 71602039] [Google Scholar]
- Gadde K, Peterson C, Troupin B, Day WW. 12-month weight loss and antihypertensive benefits with PHEN/TPM in overweight and obese subjects with hypertension. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17(2 Suppl):S2. [EMBASE: 70349685] [Google Scholar]
- Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial.[Erratum appears in Lancet. 2011 Apr 30;377(9776):1494]. Lancet 2011;377(9774):1341-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gadde KM, Day WW. Low-dose, controlled-release phentermine/topiramate for reduction of weight, related risks in overweight/obese adults with >2 comorbidities. Obesity Reviews 2010;11(Suppl1):42-3. [EMBASE: 70226591] [Google Scholar]
- Garvey WT, Gadde K, Tam P, Peterson C. Changes in insulin sensitivity in overweight/obese patients treated with low-dose, controlled-release phentermine/topiramate (PHEN/TPM). Diabetes 2010;Conference Publication:(var. pagings). [EMBASE: 71602034] [Google Scholar]
- Garvey WT, Gadde K, Wilson LF, Peterson C. Improvements in dyslipidemia and other cardiometabolic disease risk factors with low-dose, controlled-release phentermine/topiramate (PHEN/TPM). Diabetes 2010;Conference Publication:(var. pagings). [EMBASE: 71601293] [Google Scholar]
- Garvey WT, Henry RR, Peterson CA, Bowden CH. Weight loss with controlled-release phentermine/topiramate (PHEN/TPM CR) reverses metabolic syndrome (MetS) and improves associated traits. Diabetes 2011;60(Suppl1):A506. [EMBASE: 70629626] [Google Scholar]
- Garvey WT, Troupin B, Day WW. Once-daily, low-dose, controlled-release phentermine/topiramate results in significant clinical improvements in overweight/obese patients with type 2 diabetes. Diabetologia 2010;53(1 Supplement):S308. [EMBASE: 70262814] [Google Scholar]
- Gupta AK, Church T, Day WW. Obesity treatment-induced weight loss improves resting blood pressure and reduces progression from prehypertension to hypertension in overweight/obese adults. Circulation 2013;127:(12 Meeting Abstracts). [EMBASE: 71026610] [Google Scholar]
- Kahan S, Karnawat S. Effect of weight loss (Wl) on concomitant medication costs in obese/overweight individuals with metabolic syndrome (METS) receiving phentermine/topiramate extended-release (PHEN/TPM ER). Value in Health 2015;18(3):A297. [EMBASE: 1819490] [Google Scholar]
- Kushner RF, Varghese ST. Weight loss with phentermine and topiramate extended-release in obese and overweight subjects over 56 weeks. Journal of General Internal Medicine 2013;28(Suppl1):S241. [EMBASE: 71293155] [Google Scholar]
- Rossner SK, Sharma AM, Troupin B, Padwal RS. Effects of extended-release phentermine/topiramate on weight loss and blood pressure in obese subjects with type 2 diabetes mellitus. Diabetologia 2012;55(Suppl1):S285. [EMBASE: 70888717] [Google Scholar]
- Ryan DH, Garvey WT, Day WW. Achievement of composite of recommended goals in obese subjects with type 2 diabetes mellitus using extended-release phentermine/topiramate in a 56-week weight loss intervention. Endocrine Reviews 2012;33:(3 Meeting Abstracts). [EMBASE: 70832584] [Google Scholar]
- Troupin B, Hankin CS. Low-dose controlled-release phentermine/topiramate (PHEN/TPM CR) results in significant weight loss and improves atherosclerotic biomarkers in overweight/obese patients with obesity-related comorbidities. Clinical Pharmacology and Therapeutics 2011;89(Suppl 1):S39. [EMBASE: 70343842] [Google Scholar]
- Troupin B, Hankin CS. Magnitude of weight loss conferred by controlled-release low-dose phentermine/topiramate (PHEN/TPM CR) determines extent of cardiometabolic benefit. Clinical Pharmacology and Therapeutics 2011;89(S1):S38-S39. [EMBASE: 4272365] [Google Scholar]
Guy‐Grand 2004 {published data only}
- Guy-Grand B, Drouin P, Eschwege E, Gin H, Joubert JM, Valensi P. Effects of orlistat on obesity-related diseases - a six-month randomized trial. Diabetes, Obesity & Metabolism 2004;6(5):375-83. [DOI] [PubMed] [Google Scholar]
Nissen 2016 {published data only}
- Buse JB, Smith SR, Gilder K, Shan K, Halseth A. Effect of naltrexone/bupropion on cardiovascular events in overweight and obese participants with type 2 diabetes and cardiovascular risk factors in a large randomized, double-blind study. Diabetes 2017;66(Supplement 1):A557. [EMBASE: 8024951] [Google Scholar]
- Halseth A, Gilder K, Shan K, Acevedo L, Buse J. Effect on body weight of naltrexone/bupropion in overweight and obese participants with cardiovascular risk factors in a large randomized double-blind study. Obesity Facts 2017;10(Supplement 1):46. [EMBASE: 8024954] [Google Scholar]
- Halseth A, Gilder K, Shan K, Acevedo L, Smith S. Naltrexone/bupropion is well tolerated and had no effect on serious adverse events in participants receiving antidepressant medication, including SSRIs, in a large randomized double-blind study. Obesity Facts 2017;10(Supplement 1):46. [EMBASE: 6521721] [Google Scholar]
- Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA 2016;315(10):990-1004. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Smith SR, Buse JB, Gilder K, Shan K, Halseth A. Effect on body weight of naltrexone/bupropion in overweight and obese participants with type 2 diabetes and cardiovascular risk factors in a large, randomized, double-blind study. Diabetes 2017;66(Supplement 1):A556. [EMBASE: 8024952] [Google Scholar]
XENDOS 2001‐2006 {published data only}
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Evaluation of the benefits and harms of non-drug weight-reducing treatment strategies in patients with essential hypertension [Nutzenbewertung nichtmedikamentöser Behandlungsstrategien bei Patienten mit Bluthochdruck: A-Gewichtsreduktion]. iqwig.de/download/A05-21A_Abschlussbericht_Gewichtsreduktion_bei_Bluthochdruck_neu.pdf 2006:19-119.
- Torgerson JS, Arlinger K, Kappi M, Sjöström L. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clinical Trials 2001;22(5):515-25. [DOI] [PubMed] [Google Scholar]
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27(1):155-61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Apfelbaum 1999 {published data only}
- Apfelbaum M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long-term maintenance of weight loss after a very-low-calorie diet: a randomized blinded trial of the efficacy and tolerability of sibutramine. American Journal of Medicine 1999;106(2):179-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bach 1999 {published data only}
- Bach DS, Rissanen AM, Mendel CM, Shepherd G, Weinstein SP, Kelly F, et al. Absence of cardiac valve dysfunction in obese patients treated with sibutramine. Obesity Research 1999;7(4):363-9. [DOI] [PubMed] [Google Scholar]
Bain 2015 {published data only}
- Bain SC, Ahmann A, Rodbard H, Rosenstock J, Lahtela J, De Loredo L, et al. A randomised, placebo-controlled trial of liraglutide as adjunct to basal insulin analogues in subjects with Type 2 diabetes (LIRA-ADD2BASAL). Diabetic Medicine 2015;32(Suppl 1):71. [EMBASE: 71821007] [Google Scholar]
BLOOM 2010 {published data only}
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. New England Journal of Medicine 2010;363(3):245-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Stubbe S, Anderson CM, Shanahan WR. Lorcaserin reduces body weight in obese and overweight subjects: behavioral modification and lorcaserin for overweight and obesity management, the BLOOM trial. Diabetes 2009;58:LB24. [EMBASE: 70134408] [Google Scholar]
BLOOM‐DM 2012 {published data only}
- Fidler MC, Shazer R, Sanchez, M, Stubbe S, Anderson CM, Shanahan WR. Lorcaserin, a selective 5-HT2C agonist, evaluated as a weight loss agent in obese and overweight patients with type 2 diabetes treated with sulfonylureas or metformin. Diabetes 2011;60:A515. [EMBASE: 70629658]
- O'Neil PM, Fidler MC, Sanchez, M, Weissman NJ, Smith SR, Anderson CM, et al. Randomized, placebo-controlled trial of lorcaserin for weight loss in patients with type 2 diabetes. Diabetes 2011;60:A507. [EMBASE: 70629629]
- O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity 2012;20(7):1426-36. [PMID: ] [DOI] [PubMed]
BLOSSOM 2011 {published data only}
- Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al, BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. Journal of Clinical Endocrinology and Metabolism 2011;96(10):3067-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bray 1999 {published data only}
- Bray GA, Blackburn GL, Ferguson JM, Greenway FL, Jain AK, Mendel CM, et al. Sibutramine produces dose-related weight loss. Obesity Research 1999;7(2):189-98. [DOI] [PubMed] [Google Scholar]
Broom 2002 {published data only}
- Broom I, Wilding J, Stott P, Myers N, UK Multimorbidity Study Group. Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. International Journal of Clinical Practice 2002;56(7):494-9. [PubMed] [Google Scholar]
CAMELLIA‐TIMI 2018 {published data only}
- Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. New England Journal of Medicine 2018;379(12):1107-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
- EUCTR2013-000324-34-PL. A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the effect of long-term treatment with BELVIQ (lorcaserin HCl) on the incidence of major adverse cardiovascular events and conversion to type 2 diabetes mellitus in obese and overweight Subjects with cardiovascular disease or multiple cardiovascular risk factors - CAMELLIA. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-000324-34-PL (first received 07 July 2014).
- NCT02019264. A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the effect of long-term treatment with BELVIQ (Lorcaserin HCl) on the incidence of major adverse cardiovascular events and conversion to type 2 diabetes mellitus in obese and overweight subjects with cardiovascular disease or multiple cardiovascular risk fFactors. clinicaltrials.gov/ct2/show/NCT02019264 (first received 24 December 2013).
Christou 2015 {published data only}
- Christou GA, Kiortsis DN. The efficacy and safety of the naltrexone/bupropion combination for the treatment of obesity: an update. Hormones 2015;14(3):370-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 2019 {published data only}
- Cohen JB, Gadde KM. Weight loss medications in the treatment of obesity and hypertension. Current Hypertension Reports 2019;21(2):16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
COR‐BMOD 2011 {published data only}
- O'Neil PM, Wadden TA, Foreyt JP, Clapper B, Burns C, Klassen P, et al. Naltrexone SR/bupropion SR and intensive behavioral modification combination increases the likelihood of achieving early and sustained weight loss and associated improvement in markers of cardiometabolic risk. Obesity 2011;19(Suppl1):S179-80. [EMBASE: 70680332] [Google Scholar]
- Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity 2011;19(1):110-20. [EMBASE: 2011434989] [DOI] [PMC free article] [PubMed] [Google Scholar]
COR‐Diabetes 2013 {published data only}
- Hollander P, Gupta AK, Plodkowski R, Greenway F, Bay H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013;36:4022-9. [EMBASE: 2014021906] [DOI] [PMC free article] [PubMed] [Google Scholar]
COR‐I 2010 {published data only}
- Fujioka K, Billes SK, Burns C, Harris-Collazo R, Kim DD, Dunayevich E, et al. Weight loss and improvements in markers of metabolic risk in overweight and obese subjects completing 56 weeks of treatment with naltrexone SR/bupropion SR. Diabetes 2011;60:A520-1. [EMBASE: 70629675] [Google Scholar]
- Fujioka K, Greenway FL, Rubino D, Clapper B, Burns C, Klassen P, et al. Completion of 56 weeks of naltrexone SR/bupropion SR combination therapy increases likelihood of achieving improvements in markers of cardiometabolic risk associated with clinically meaningful weight loss. Obesity 2011;19(Suppl1):S179. [EMBASE: 70680330] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376(9741):595-605. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00532779. A multicenter, randomized, double blind, placebo controlled study comparing the safety and efficacy of two doses of naltrexone sustained release (SR)/bupropion sustained release (SR) and placebo in obese subjects. clinicaltrials.gov/ct2/show/NCT00532779 (first received 20 September 2007).
- Plutzky J, Buse J, Chilton R, Mignon L, Burns C, Harris-Collazo R, et al. Response to NB at week 16 is a good predictor of significant week 56 weight loss and improvements in weight-related risk factors. Diabetes 2011;60:A700. [EMBASE: 70630321] [Google Scholar]
COR‐II 2013 {published data only}
- Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity 2013;21(5):935-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Wyatt H, Billes SK, Burns C, Harris-Collazo R, Dunayevich E, et al. Naltrexone SR/bupropion SR combination therapy improves control of eating and reduces food cravings in overweight and obese subjects. Diabetes 2011;60:A506. [EMBASE: 70629625] [Google Scholar]
- Kolotkin RL, Still C, Maier H, Burns C, Harris-Collazo R, Dunayevich E, et al. Naltrexone SR/bupropion SR combination therapy reduced weight and improved weight-related quality of life and physical health in overweight and obese subjects. Diabetes 2011;60:A506-7. [EMBASE: 70629628] [Google Scholar]
- Plutzky J, Chilton R, Still C, Burns C, Kim D, Dunayevich E. Weight loss, blood pressure, pulse and circadian patterns with naltrexone sustained-release/bupropion sustained-release combination therapy for obesity. Journal of the American College of Cardiology 2011;57(14, Supplement):E517. [EMBASE: 70400194] [Google Scholar]
- Rubino D, Apovian C, Mignon L, Burns C, Harris-Collazo R, Kim D. Effects of naltrexone SR/bupropion SR on weight and obesity-related health risks in overweight/obese women from the COR-II trial. Diabetes 2011;60:A504. [EMBASE: 70629619] [Google Scholar]
CTRI/2017/05/008655 {published data only}
- CTRI/2017/05/008655. A clinical study to study the effect of lorcaserin tablets in the treatment of obesity. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/05/008655 (first received 25 May 2017).
Davidson 1999 {published data only}
- Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial [see comment] [erratum appears in JAMA 1999 Apr 7;281(13):1174]. JAMA 1999;281(3):235-42. [DOI] [PubMed] [Google Scholar]
De Castro 2009 {published data only}
- De Castro JJ, Dias T, Chambel P, Carvalheiro M, Correia LG, Guerreiro L, et al. A randomized double-blind study comparing the efficacy and safety of orlistat versus placebo in obese patients with mild to moderate hypercholesterolemia. Revista Portuguesa de Cardiologia 2009;28(12):1361-74. [PubMed] [Google Scholar]
Derosa 2003 {published data only}
- Derosa G, Mugellini A, Ciccarelli L, Fogari R. Randomized, double-blind, placebo-controlled comparison of the action of orlistat, fluvastatin, or both ao anthropometric measurements, blood pressure, and lipid profile in obese patients with hypercholesterolemia prescribed a standardized diet. Clinical Therapeutics 2003;25(4):1107-22. [DOI] [PubMed] [Google Scholar]
Derosa 2008 {published data only}
- Derosa G, D'Angelo A, Salvadeo SA, Ferrari I, Gravina A, Fogari E, et al. Sibutramine effect on metabolic control of obese patients with type 2 diabetes mellitus treated with pioglitazone. Metabolism: Clinical & Experimental 2008;57(11):1552-7. [DOI] [PubMed] [Google Scholar]
Derosa 2010 {published data only}
- Derosa G, Maffioli P, Ferrari I, Palumbo I, Randazzo S, D'Angelo A, et al. Effects of one year treatment of sibutramine on insulin resistance parameters in type 2 diabetic patients. Journal of Pharmacy and Pharmaceutical Sciences 2010;13(3):378-90. [DOI] [PubMed] [Google Scholar]
- Derosa G, Maffioli P, Ferrari I, Palumbo I, Randazzo S, D'Angelo A, et al. Variation of inflammatory parameters after sibutramine treatment compared to placebo in type 2 diabetic patients. Journal of Clinical Pharmacy and Therapeutics 2011;36(5):592-601. [DOI] [PubMed] [Google Scholar]
Despres 2005 {published data only}
- Despres JP, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. New England Journal of Medicine 2005;353(20):2121-34. [DOI] [PubMed] [Google Scholar]
Despres 2009 {published data only}
- Despres JP, Ross R, Boka G, Almeras N, Lemieux I. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arteriosclerosis, Thrombosis, and Vascular Biology 2009;29(3):416-23. [DOI] [PubMed] [Google Scholar]
Dujovne 2001 {published data only}
- Dujovne CA, Zavoral JH, Rowe E, Mendel CM. Effects of sibutramine on body weight and serum lipids: a double-blind, randomized, placebo-controlled study in 322 overweight and obese patients with dyslipidemia. American Heart Journal 2001;142(3):489-97. [DOI] [PubMed] [Google Scholar]
EQUIP 2012 {published data only}
- Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity 2012;20(2):330-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erdmann 2004 {published data only}
- Erdmann J, Lippl F, Klose G, Schusdziarra V. Cholesterol lowering effect of dietary weight loss and orlistat treatment - Efficacy and limitations. Alimentary Pharmacology and Therapeutics 2004;19(11):1173-9. [DOI] [PubMed] [Google Scholar]
EUCTR2013‐002348‐99 {published data only}
- EUCTR2013-002348-99. A randomised, double blind, placebo-controlled study of the effect of liraglutide on arterial blood pressure in hypertensive patients with type 2 diabetes mellitus. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2013-002348-99 (first received 22 September 2014).
Fanghänel 2000 {published data only}
- Fanghänel G, Cortinas L, Sanchez-Reyes L, Berber A. A clinical trial of the use of sibutramine for the treatment of patients suffering essential obesity. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2000;24(2):144-50. [DOI] [PubMed] [Google Scholar]
- Fanghänel G, Cortinas L, Sanchez-Reyes L, Berber A. Second phase of a double-blind study clinical trial on Sibutramine for the treatment of patients suffering essential obesity: 6 months after treatment cross-over. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2001;25(5):741-7. [DOI] [PubMed] [Google Scholar]
Fanghänel 2003 {published data only}
- Fanghänel G, Cortinas L, Sanchez-Reyes L, Gomez-Santos R, Campos-Franco E, Berber A. Safety and efficacy of sibutramine in overweight Hispanic patients with hypertension. Advances in Therapy 2003;20(2):101-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Faria 2002 {published data only}
- Faria AN, Ribeiro Filho FF, Kohlmann NE, Gouvea F Sr, Zanella MT. Effects of sibutramine on abdominal fat mass, insulin resistance and blood pressure in obese hypertensive patients. Diabetes, Obesity & Metabolism 2005;7(3):246-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Faria AN, Ribeiro Filho FF, Lerario DD, Kohlmann N, Gouvea Ferreira SR, Zanella MT. Effects of sibutramine on the treatment of obesity in patients with arterial hypertension. Arquivos Brasileiros de Cardiologia 2002;78(2):172-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Finer 2000 {published data only}
- Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2000;24(3):306-13. [DOI] [PubMed] [Google Scholar]
Fujioka 2000 {published data only}
- Fujioka K, Seaton TB, Rowe E, Jelinek CA, Raskin P, Lebovitz HE, et al. Weight loss with sibutramine improves glycaemic control and other metabolic parameters in obese patients with type 2 diabetes mellitus. Diabetes, Obesity & Metabolism 2000;2(3):175-87. [DOI] [PubMed] [Google Scholar]
Fujioka 2016 {published data only}
- Fujioka K, Plodkowski R, O'Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone er/bupropion er combination therapy. International Journal of oOesity 2016;40(9):1369-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gadde 2017 {published data only}
- Gadde KM, Pritham Raj Y. Pharmacotherapy of obesity: clinical trials to clinical practice. Current Diabetes Reports 2017;17(5):34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garvey 2009 {published data only}
- Garvey WT, Troupin B, Peterson C, Najarian T, Tam P, Day W. Treatment with VI-0521 (phentermine and topiramate) leads to one year durable glycaemic benefit and weight loss in subjects with type 2 diabetes. Diabetologia 2009;52(S1):S77. [EMBASE: 70067664] [Google Scholar]
- Garvey WT, Troupin B, Tam P, Najarian T, Peterson C, Day WW. One-year treatment with VI-0521 in type 2 diabetes demonstrates continued glycemic improvement and weight loss. Diabetes 2009;58(Suppl. 1):A95. [EMBASE: 70136156] [Google Scholar]
Gentile 2005 {published data only}
- Gentile S, Guarina G, Padovano B, Buonocunto F, Gruppo Campano Obesita. Efficacy and safety of a short-time orlistat treatment in obese subjects [Efficacia e sicurezza di impiego di un trattamento a breve termine con orlistat in soggetti obesi]. Annali Italiani di Medicina Interna 2005;20(2):90-6. [PubMed] [Google Scholar]
Ginsberg 2004 {published data only}
- Ginsberg DL. Add-on sibutramine for olanzapine-induced weight gain. Primary Psychiatry 2004;11(7):24. [Google Scholar]
Greenway 2009 {published data only}
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. Journal of Clinical Endocrinology and Metabolism 2009;94:4898-906. [EMBASE: 2010011828] [DOI] [PubMed] [Google Scholar]
Greenway 2016 {published data only}
- Greenway FL, Shanahan Wm Fain R, Ma T, Rubino D. Safety and tolerability review of lorcaserin in clinical trials. Clinical Obesity 2016;6(5):285-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Halpern 2002 {published data only}
- Halpern A, Leite CC, Herszkowicz N, Barbato A, Costa AP. Evaluation of efficacy, reliability, and tolerability of sibutramine in obese patients, with an echocardiographic study. Revista do Hospital das Clinicas; Faculdade de Medicina Da Universidade de Sao Paulo 2002;57(3):98-102. [DOI] [PubMed] [Google Scholar]
Halpern 2003 {published data only}
- Halpern A, Mancini MC, Suplicy H, Zanella MT, Repetto G, Gross J, et al. Latin-American trial of orlistat for weight loss and improvement in glycaemic profile in obese diabetic patients. Diabetes, Obesity & Metabolism 2003;5(3):180-8. [DOI] [PubMed] [Google Scholar]
Halseth 2018 {published data only}
- Acevedo LM, Shan K, Fujioka K. Improvement in patient-assessed quality of life, eating behavior, and sexual function after 26 weeks of naltrexone/ bupropion compared with usual care. Obesity Facts 2016;9(Suppl 1):177. [EMBASE: 72315993] [Google Scholar]
- Fujioka, K, Walsh B, Halseth AE, Shan K, Wadden TA. Improvement in patient-assessed quality of life, eating behavior, and sexual function after 26 weeks of naltrexone/bupropion compared with usual care. Diabetes 2015;64(Suppl 1):A308. [EMBASE: 71940993] [Google Scholar]
- Halseth A, Shan K, Chen S. Long-term efficacy of naltrexone/bupropion, administered as recommended in clinical practice. Obesity Facts 2016;9(Suppl 1):178. [EMBASE: 72315729] [Google Scholar]
- Halseth A, Shan K, Fujioka K. Efficacy of naltrexone/bupropion, administered asS recommended in clinical practice, compared with usual care for weight management. Obesity Facts 2016;9(Suppl 1):80-1. [EMBASE: 72315729] [Google Scholar]
- Halseth A, Shan K, Gilder K, Malone M, Acevedo L, Fujioka K. Quality of life, binge eating and sexual function in participants treated for obesity with sustained release naltrexone/bupropion. Obesity Science and Practice 2018;4(2):141-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Walsh B, Halseth AE, Shan K, Fujioka K. Efficacy of naltrexone/bupropion, administered as recommended in clinical practice, compared with usual care. Diabetes 2015;64(Suppl 1):A334. [EMBASE: 71941083] [Google Scholar]
Hauner 2004 {published data only}
- Hauner H, Meier M, Wendland G, Kurscheid T, Lauterbach K, The SAT Study Group. Weight reduction by sibutramine in obese subjects in primary care medicine: the SAT Study. Experimental and Clinical Endocrinology & Diabetes 2004;112(4):201-7. [DOI] [PubMed] [Google Scholar]
Hauptman 2000 {published data only}
- Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Archives of Family Medicine 2000;9(2):160-7. [DOI] [PubMed] [Google Scholar]
Heinonen 2009 {published data only}
- Heinonen MV, Laaksonen DE, Karhu T, Karhunen L, Laitinen T, Kainulainen S, et al. Effect of diet-induced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutrition, Metabolism and Cardiovascular Diseases 2009;19(9):626-33. [DOI] [PubMed] [Google Scholar]
Hollander 2007 {published data only}
- Hollander P. Endocannabinoid blockade for improving glycemic control and lipids in patients with type 2 diabetes mellitus. American Journal of Medicine 2007;120(2 Suppl 1):S18-28. [DOI] [PubMed] [Google Scholar]
Hung 2005 {published data only}
- Hung YJ, Chen YC, Pei D, Kuo SW, Hsieh CH, Wu LY, et al. Sibutramine improves insulin sensitivity without alteration of serum adiponectin in obese subjects with Type 2 diabetes. Diabetic Medicine 2005;22(8):1024-30. [DOI] [PubMed] [Google Scholar]
Jain 2011 {published data only}
- Jain SS, Ramanand SJ, Ramanand JB, Akat PB, Patwardhan MH, Joshi SR. Evaluation of efficacy and safety of orlistat in obese patients. Indian Journal of Endocrinology and Metabolism 2011;15(2):99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 1997 {published data only}
- James WP, Avenell A, Broom J, Whitehead J. A one-year trial to assess the value of orlistat in the management of obesity. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 1997;21 Suppl 3:S24-30. [PubMed] [Google Scholar]
James 2000 {published data only}
- James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rossner S, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance [see comment]. Lancet 2000;356(9248):2119-25. [DOI] [PubMed] [Google Scholar]
Jia 2010 {published data only}
- Jia J, Xiong L, Chen S. Trial of lorcaserin for weight management. New England Journal of Medicine 2010;363(25):2468-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jordan 2012 {published data only}
- Jordan J, Astrup AV, Day WW. Effects of phentermine and extended-release topiramate alone and in combination on cardiovascular risk factors. Diabetologia 2012;55:S285. [EMBASE: 70888718] [Google Scholar]
Kaukua 2004 {published data only}
- Kaukua JK, Pekkarinen TA, Rissanen AM. Health-related quality of life in a randomised placebo-controlled trial of sibutramine in obese patients with type II diabetes. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2004;28(4):600-5. [DOI] [PubMed] [Google Scholar]
KCT0002097 {published data only}
- KCT0002097. A 48 weeks, double-blind, randomized, placebo-controlled clinical trial for the evaluation of the efficacy and safety of obesity medication on morbid obese and obese patients. www.who.int/trialsearch/Trial2.aspx?TrialID=KCT0002097 (first received 07 October 2016).
Kelley 2002 {published data only}
- Kelley DE, Bray GA, Pi-Sunyer FX, Klein S, Hill J, Miles J, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial [erratum appears in Diabetes Care 2003 Mar;26(3):971]. Diabetes Care 2002;25(6):1033-41. [DOI] [PubMed] [Google Scholar]
Kern 2017 {published data only}
- Kern W. Obesity and weight reduction: current findings on liraglutid 3.0 mg [Gewichtsreduktion bei Adipositas: Aktueller Erkenntnisstand zu Liraglutid 3,0 mg]. Diabetes, Stoffwechsel und Herz 2017;26(2):75-83. [Google Scholar]
Kim 2016 {published data only}
- Kim S. Drugs to treat obesity: do they work? Postgraduate Medical Journal 2016;92(1089):401-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Klassen 2016 {published data only}
- Klassen P, Halseth A, Chilton R. Weight loss, blood pressure, pulse, and circadian patterns with prolonged-release naltrexone /bupropion combination therapy for obesity. Obesity Facts 2016;9:245. [EMBASE: 4300320] [Google Scholar]
- Klassen P, Halseth AE, Chilton R. Weight loss, blood pressure, pulse, and circadian patterns with combination prolonged-release naltrexone/bupropion therapy for obesity. Obesity Reviews 2016;17(Supplement 2):69. [EMBASE: 7455004] [Google Scholar]
LEADER 2013 {published data only}
- Bain SC, Petrie J. Liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER™) trial: rationale and study design. Diabetic Medicine 2012;29:68. [EMBASE: 70695949] [Google Scholar]
- Bergenstal R, Daniels G, Mann J, Nissen S, Pocock S, Zinman B, et al. Liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (LEADER™) trial: Rationale and study design. Diabetes 2011;60:A612-3. [EMBASE: 70630008] [Google Scholar]
- Consoli A, Bergenstal R, Daniels G, Mann J, Nissen S, Pocock S, et al. Liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (leader) trial: Rationale and study design. High Blood Pressure and Cardiovascular Prevention 2012;19(2):104. [EMBASE: 713987964] [Google Scholar]
- Mannucci E, Bergenstal R, Daniels G, Mann J, Nissen S, Zinman B, et al. Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) Trial: Rationale and study design. Italian Journal of Medicine 2012;6(1 Suppl 1):86. [EMBASE: 70799751] [Google Scholar]
- Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, et al. Design of the liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (LEADER) trial. American Heart Journal 2013;166:823-30. [EMBASE: 2013691152] [DOI] [PubMed] [Google Scholar]
- Petrie JR, Marso SP, Bain SC, Franek E, Jacob S, Masmiquel L, et al. LEADER-4: Blood pressure control in patients with type 2 diabetes and high cardiovascular risk: Baseline data from the LEADER randomized trial. Journal of Hypertension 2016;34(6):1140-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie JR. LEADER-4: blood pressure control in patients with Type 2 diabetes and high cardiovascular risk: baseline data from the LEADER trial. Diabetic Medicine 2016;33(Suppl 1):67. [EMBASE: 72230725] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Roux 2018 {published data only}
- Le Roux C, Aroda V, Hemmingsson J, Cancino AP, Christensen R, Pi-Sunyer X. Comparison of efficacy and safety of liraglutide 3.0 mg in Individuals with BMI above and below 35 kg/m2: a post-hoc analysis. Obesity Facts 2018;10(6):531-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2014 {published data only}
- Lewis K. Weight loss achieved with medication can delay onset of type 2 diabetes in at-risk individuals. Journal of Clinical Outcomes Management 2014;21:393-5. [EMBASE: 2014910274] [Google Scholar]
Lindgarde 2000 {published data only}
- Lindgarde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish Multimorbidity Study [see comment]. Journal of Internal Medicine 2000;248(3):245-54. [DOI] [PubMed] [Google Scholar]
Lindgarde 2001a {published data only}
- Lindgarde F. Orlistat with diet was effective and safe for weight loss and coronary risk reduction in obesity. Evidence-based Medicine 2001;6(2):54. [Google Scholar]
Lindgarde 2001b {published data only}
- Lindgarde F. Effect of orlistat on coronary heart disease risk in obese patients with hypertension and/or hyperlipidemia. American Heart Journal 2001;141(1):171. [Google Scholar]
Lu 2018 {published data only}
- Lu CW, Chang CJ, Yang YC, Lin WY, Huang KC. Multicentre, placebo-controlled trial of lorcaserin for weight management in Chinese population. Obesity Research & Clinical Practice 2018;12(5):465-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Madsen 2009 {published data only}
- Madsen EL, Bruun JM, Skogstrand K, Hougaard DM, Christiansen T, Richelsen B. Long-term weight loss decreases the nontraditional cardiovascular risk factors interleukin-18 and matrix metalloproteinase-9 in obese subjects. Metabolism 2009;58(7):946-53. [DOI] [PubMed] [Google Scholar]
McMahon 2000 {published data only}
- McMahon FG, Fujioka K, Singh BN, Mendel CM, Rowe E, Rolston K, et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension: a 1-year, double-blind, placebo-controlled, multicenter trial. Archives of Internal Medicine 2000;160(14):2185-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
McMahon 2002 {published data only}
- McMahon FG, Weinstein SP, Rowe E, Ernst KR, Johnson F, Fujioka K, et al. Sibutramine is safe and effective for weight loss in obese patients whose hypertension is well controlled with angiotensin-converting enzyme inhibitors. Journal of Human Hypertension 2002;16(1):5-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
McNulty 2003 {published data only}
- McNulty SJ, Ur E, Williams G, Multicenter Sibutramine Study Group. A randomized trial of sibutramine in the management of obese type 2 diabetic patients treated with metformin. Diabetes Care 2003;26(1):125-31. [DOI] [PubMed] [Google Scholar]
Miles 2002 {published data only}
- Miles JM, Leiter L, Hollander P, Wadden T, Anderson JW, Doyle M, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin [erratum appears in Diabetes Care 2002 Sep;25(9):1671]. Diabetes Care 2002;25(7):1123-8. [DOI] [PubMed] [Google Scholar]
Moore 2016 {published data only}
- Moore KG, Shealy K, Clements JN. Liraglutide, GLP-1 receptor agonist, for chronic weight loss. Expert Review of Endocrinology & Metabolism 2016;11(5):373-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT01764386 {published data only}
- NCT01764386. A multicenter, randomized, open-label, controlled, method-of-use study assessing the effect of naltrexone sustained release (SR)/bupropion SR on body weight and cardiovascular risk factors in overweight and obese subjects (The Ignite Study). clinicaltrials.gov/ct2/show/NCT01764386 (first received 09 January 2013).
Niskanen 2010 {published data only}
- Niskanen L, Astrup A, Hakim MA, Carraro R, Finer N, Hartvig H, et al. Liraglutide once daily reduces the prevalence of prediabetes and metabolic syndrome in obese non-diabetic adults: a 2-year randomized trial. Obesity 2010;18:S56. [EMBASE: 71774529] [Google Scholar]
Nissen 2008 {published data only}
- Nissen SE, Nicholls SJ, Wolski K, Rodes-Cabau J, Cannon CP, Deanfield JE, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 2008;299(13):1547-60. [DOI] [PubMed] [Google Scholar]
O'Leary 2011 {published data only}
- O'Leary DH, Reuwer AQ, Nissen SE, Despres JP, Deanfield JE, Brown MW, et al. Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: the AUDITOR Trial. Heart 2011;97(14):1143-50. [DOI] [PubMed] [Google Scholar]
Pathan 2004 {published data only}
- Pathan MF, Latif ZA, Nazneen NE, Mili SU. Orlistat as an adjunct therapy in type 2 obese diabetic patients treated with sulphonylurea: a Bangladesh experience. Bangladesh Medical Research Council Bulletin 2004;30(1):1-8. [PubMed] [Google Scholar]
Patschan 2007 {published data only}
- Patschan S, Scholze J. Obesity-related hypertension. Cardiology Review 2007;24(12):32-5. [Google Scholar]
Pi‐Sunyer 2006 {published data only}
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial [see comment] [erratum appears in JAMA 2006 Mar 15;295(11):1252]. JAMA 2006;295(7):761-75. [DOI] [PubMed] [Google Scholar]
Reaven 2001 {published data only}
- Reaven G, Segal K, Hauptman J, Boldrin M, Lucas C. Effect of orlistat-assisted weight loss in decreasing coronary heart disease risk in patients with syndrome X. American Journal of Cardiology 2001;87(7):827-31. [DOI] [PubMed] [Google Scholar]
Rossner 2000 {published data only}
- Rossner S, Sjöström L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obesity Research 2000;8(1):49-61. [DOI] [PubMed] [Google Scholar]
Rossner 2001 {published data only}
- Rossner S. Sibutramine - antidepressive agent tested against obesity [Sibutramin – antidepressivt läkemedel prövat mot fetma]. Lakartidningen 2001;98(15):1802-3. [PubMed] [Google Scholar]
Samuelsson 2003 {published data only}
- Samuelsson L, Gottsater A, Lindgarde F. Decreasing levels of tumour necrosis factor alpha and interleukin 6 during lowering of body mass index with orlistat or placebo in obese subjects with cardiovascular risk factors. Diabetes, Obesity and Metabolism 2003;5(3):195-201. [DOI] [PubMed] [Google Scholar]
SCALE diabetes 2014 {published data only}
- Bode B, DeFronzo R, Bergenstal R, Kushner R, Lewin A, Skjoth TV, et al. Effect of liraglutide 3.0/1.8 mg on body weight and cardiometabolic risk factors in overweight/obese adults with type 2 diabetes: SCALE diabetes randomised, double-blind, 56-week trial. Diabetologia 2014;57(Suppl 1):S83. [EMBASE: 71594831] [Google Scholar]
- Davies MJ, DeFronzo RA, Bergenstal RM, Bode BW, Kushner R, Noctor M, et al. Liraglutide 3.0mg results in significant weight loss and improvements in cardiometabolic risk factors compared with liraglutide 1.8mg or placebo: a randomised, double-blind, 56 week trial in overweight/obese adults with Type 2 diabetes (SCALE diabetes). Diabetic Medicine 2015;32:72. [EMBASE: 71821008] [Google Scholar]
- DeFronzo RA, Bergenstal RM, Bode B, Kushner R, Lewin AJ, Skjoth TV, et al. Effects of liraglutide 3.0 mg and 1.8 mg on body weight and cardiometabolic risk factors in overweight and obese adults with type 2 diabetes mellitus (T2DM): the SCALE diabetes randomized, double-blind, placebo-controlled, 56-week trial. Endocrine Reviews 2014;35:var. [EMBASE: 4344566] [Google Scholar]
- Pedersen S, Defronzo RA, Bergenstal R, Bode B, Kushner R, Lewin A, et al. Effects of liraglutide 3.0 mg and 1.8 mg on body weight and cardiometabolic risk factors in adults with overweight or obesity and type 2 diabetes (T2D): The SCALE diabetes randomized, double-blind, placebo-controlled, 56-week trial. Canadian Journal of Diabetes 2015;39:S36. [EMBASE: 1860363] [Google Scholar]
SCALE maintenance 2013 {published data only}
- Hollander P, Aronne L, Klein S, Niswender K, Jensen CB, Woo V, et al. Diet-induced weight loss and subsequent addition of liraglutide 3.0 mg reduces impaired fasting glucose in overweight/obese adults in the SCALE™ maintenance 56-week randomised trial. Journal of Diabetes 2013;5:124. [EMBASE: 71034943] [Google Scholar]
- Klein S, Aronne LJ, Hollander P, Niswender K, Woo V, Hale PM, et al. Effect of liraglutide on cardiovascular (CV) risk factors in adults without diabetes after diet-induced weight loss: the SCALE 56-week randomized study. Obesity 2011;19:S177. [Google Scholar]
SCALE obesity and prediabetes 2014 {published data only}
- Astrup A, Fujioka K, Violante Ortiz R, Jensen CB, Lillerre SK, O'Neil P. Efficacy and safety of liraglutide 3.0 mg in adult overweight and obese weight loss responders without diabetes: results of the 56-week randomised, controlled, SCALE Obesity and Prediabetes trial. Obesity Facts 2015;8(Suppl 1):42. [EMBASE: 71907401] [Google Scholar]
- Astrup A, Greenway F, Krempf M, Le Roux CW, Vettor R, Shapiro Manning L, et al. Weight loss and associated improvements in cardiometabolic risk factors with liraglutide 3.0 mg in the SCALE Obesity and Prediabetes randomised, double-blind, placebo-controlled 3-year trial. Obesity Facts 2016;9(Suppl 1):182. [EMBASE: 72316004] [Google Scholar]
- Bjorner JB, Brett JH, Meincke HH, Kolotkin RL. The effect of liraglutide 3.0 mg for weight management on hrqol, as measured by SF-36: 3-year data. Diabetologia 2016;59(1 Suppl 1):S330. [EMBASE: 612314178] [Google Scholar]
- Fujioka K, Greenway FL, Krempf M, Le Roux CW, Vettor R, Manning LS, et al. Liraglutide 3.0 mg reduces body weight and improves cardiometabolic risk factors in adults with obesity or overweight and prediabetes: the SCALE Obesity and Prediabetes randomized, double-blind, placebo-controlled 3-year trial. Endocrine Reviews 2016;37(2 Suppl 1):no pagination. [EMBASE: 613520086] [Google Scholar]
- Fujioka K, Wilding JP, Astrup A, Greenway F, Halpern A, Krempf M, et al. Efficacy and safety of liraglutide 3.0 mg for weight management in overweight/obese adults: The SCALE Obesity and Prediabetes trial. Diabetologia 2014;57(Suppl1):S368-9. [EMBASE: 71595554] [Google Scholar]
- Kolotkin R, Manning LS, Meincke HH, Bjorner JB. Improvements in impact of weight on quality of life-lite (Iwqollite) with liraglutide 3.0 mg (LIRA) vs. placebo (PBO) for weight management: 3-year data. Diabetes 2016;65(Suppl 1):A69-A70. [EMBASE: 620236438] [Google Scholar]
- Kolotkin R, Shapiro Manning L, Meincke HH, Bjorner JB. Improvements in 'impact of weight on quality of life-lite' score with liraglutide 3.0 mg vs placebo for weight management: 3-year data. Diabetologia 2016;59(1 Suppl 1):S331. [EMBASE: 612314201] [Google Scholar]
- Kolotkin RL, Fujioka K, Wolden ML, Brett JH, Bjorner JB. Improvements in health-related quality of life with liraglutide 3.0 mg compared with placebo in weight management. Clinical Obesity 2016;6(4):233-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotkin RL, Smolarz G, Meincke HH, Bjorner JB. Improvement in iwqol-lite-derived health utility score with liraglutide 3.0 mg versus placebo over 3 years in prediabetes. Value in Health 2016;19(3):A251-A252. [EMBASE: 72312063] [Google Scholar]
- Kolotkin RL, Smolarz G, Meincke HH, Bjorner JB. Improvement in SF-36-derived health utility score with liraglutide 3.0 mg versus placebo over 3 years in prediabetes. Value in Health 2016;19(3):A251. [EMBASE: 72312059] [Google Scholar]
- Krempf M, Astrup A, Le Roux C, Fujioka K, Greenway F, Halpern A, et al. Liraglutide 3.0 mg reduces body weight and improves cardiometabolic risk factors in overweight/obese adults: The SCALE Obesity and Prediabetes randomised trial. Diabetologia 2014;57(Suppl 1):S368. [EMBASE: 71595553] [Google Scholar]
- Lau DC, Astrup A, Fujioka K, Greenway FL, Halpern A, Krempf M, et al. Safety and tolerability of liraglutide 3.0 mg in overweight and obese adults: the SCALE Obesity and Prediabetes randomized, double-blind, placebo-controlled 56-week trial. Endocrine Reviews 2014;35(Suppl 3):no pagination. [EMBASE: 72338382] [Google Scholar]
- Lau DC, Blueher M, Van Gaal L, Rubino DM, Guerrero G, Manning LS, et al. Characteristics of individuals developing type 2 diabetes in the SCALE Obesity and Prediabetes randomized, double-blind, liraglutide vs. placebo trial. Diabetes 2016;65(Suppl 1):A510. [Google Scholar]
- Lau DC, Fujioka K, Astrup A, Greenway F, Halpern A, Krempf M, et al. Liraglutide 3.0 mg reduces body weight and improves HRQoL in overweight or obese adults without diabetes: SCALE Obesity and Prediabetes randomized, double-blind, placebo controlled, 56-week trial. Obesity Facts 2015;8(Suppl 1):185. [EMBASE: 71907826] [Google Scholar]
- Lau DC, Krempf M, Astrup A, Le Roux CW, Fujioka K, Greenway F, et al. Liraglutide 3.0 mg reduces body weight and improves cardiometabolic risk factors in adults with overweight/obesity: The SCALE Obesity and Prediabetes randomised trial. Canadian Journal of Diabetes 2015;39(Suppl 1):S48-S49. [EMBASE: 72016947] [Google Scholar]
- Le Roux C, Astrup A, Fujioka K, Greenway FL, Halpern A, Krempf M, et al. Liraglutide 3.0 mg improves body weight and cardiometabolic risk factors in overweight or obese adults without diabetes: the SCALE Obesity and Prediabetes randomized, double-blind, placebo-controlled 56-week trial. Endocrine Reviews 2014;35(Suppl 3):no pagination. [EMBASE: 72338390] [Google Scholar]
- Le Roux C, Lau DC, Astrup A, Fujioka K, Greenway F, Halpern A, et al. Safety and tolerability of liraglutide 3.0 mg in overweight and obese adults: The SCALE Obesity and Prediabetes randomised trial. Diabetologia 2014;57(1 Suppl 1):S338. [EMBASE: 71595483] [Google Scholar]
- Le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389(10077):1399-409. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Le Roux CW, Astrup A, Greenway F, Krempf M, Vettor R, Shapiro Manning L, et al. Liraglutide 3.0 mg reduces body weight and improves cardiometabolic risk factors: SCALE Obesity and Prediabetes randomised, double-blind, placebo-controlled 3-year trial. Diabetologia 2016;59(1 Suppl 1):S329. [EMBASE: 612314122] [Google Scholar]
- Madsbad S, Lieberman G, Skjoth TV, Lilleore SK, De Fronzo RA. Comparable efficacy and safety of liraglutide 3.0 mg for weight management across baseline BMI subgroups: results from the SCALE Obesity and Prediabetes trial. Diabetologia 2016;59(1 Suppl 1):S40-S41. [EMBASE: 612313893] [Google Scholar]
- NCT01272219. Effect of liraglutide on body weight in non-diabetic obese subjects or overweight subjects with co-morbidities: a randomised, double-blind, placebo controlled, parallel group, multi-centre, multinational trial with stratification of subject to either 56 or 160 weeks of treatment based on pre-diabetes status at randomisation. clinicaltrials.gov/ct2/show/NCT01272219 (first received 07 January 2011).
- O'Neil P, Fujioka K, Ortiz RV, Claudius B, Jensen CB, Astrup A. Efficacy and safety of liraglutide 3.0 mg in adult overweight and obese weight loss responders without diabetes: results of the randomised, double-blind, placebo-controlled 56-week SCALE Obesity and Prediabetes trial. Endocrine Reviews 2015;36(Suppl 2):no pagination. [EMBASE: 613818371] [Google Scholar]
- Pi-Sunyer FX, Astrup A, Fujioka K, Greenway FL, Halpern A, Krempf M, et al. Liraglutide 3.0 mg reduces the prevalence of prediabetes and delays onset of type 2 diabetes in overweight and obese adults: results from SCALE Obesity and Prediabetes, a randomized, double-blind and placebo-controlled 56-week trial. Endocrine Reviews 2014;35(Suppl 3):no pagination. [EMBASE: 72338378] [Google Scholar]
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine 2015;373(1):11-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Proietto J, Le Roux CW, Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, et al. Efficacy and safety of liraglutide 3.0mg for weight management in overweight and obese adults: The SCALE Obesity and Prediabetes, a randomised, double-blind and placebo-controlled trial. Obesity Research and Clinical Practice 2014;8:117. [EMBASE: 71741424] [Google Scholar]
- Van Gaal L, Astrup A, Fujioka K, Greenway F, Lau DC, Ortiz RV, et al. Liraglutide 3.0 mg reduced the risk of developing type 2 diabetes and decreased body weight in the SCALE Obesity and Prediabetes randomised, double-blind, placebo controlled trial. Obesity Facts 2016;9(Suppl 1):182. [EMBASE: 72316005] [Google Scholar]
- Vettor R, Le Roux C, Astrup A, Fujioka K, Greenway F, Lau D, et al. Reduction in the risk of developing type 2 diabetes with liraglutide 3.0 mg in people with prediabetes from the SCALE Obesity and Prediabetes randomized, double-blind, placebo-controlled trial. Italian Journal of Medicine 2016;10(Suppl 2):121. [EMBASE: 614931673] [Google Scholar]
- Wilding J, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. Efficacy and safety of liraglutide 3.0 mg for weight management in overweight and obese adults: the SCALE Obesity and Prediabetes, a randomised, double-blind and placebo-controlled trial. Obesity Facts 2014;7:15. [EMBASE: 71497901] [Google Scholar]
- Wilding J, Astrup A, Fujioka K, Greenway FL, Halpern A, Krempf M, et al. Liraglutide 3.0 mg improves insulin secretion and action in overweight and obese adults without diabetes: results from SCALE Obesity and Prediabetes, a randomized, double-blind and placebo-controlled 56-week trial. Endocrine Reviews 2014;35(Suppl 3):no pagination. [EMBASE: 72338384] [Google Scholar]
- Wilding J, Lau D, Astrup A, Fujioka K, Greenway F, Krempf M, et al. Safety and tolerability of liraglutide 3.0mg following 56 weeks of randomised treatment in overweight and obese adults: SCALE Obesity and Pre-diabetes trial. Diabetic Medicine 2015;32:70. [EMBASE: 71821004] [Google Scholar]
- Wittert G, Le Roux CW, Astrup A, Fujioka K, Greenway F, Halpern A, et al. Liraglutide 3.0mg improves body weight and cardiometabolic risk factors in overweight or obese adults without diabetes: The SCALE Obesity and Prediabetes randomised, double-blind, placebo-controlled 56-week trial. Obesity Research and Clinical Practice 2014;8:118. [EMBASE: 71742425] [Google Scholar]
SCALE sleep apnoea 2014 {published data only}
- Blackman A, Foster G, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Liraglutide 3.0 mg reduces severity of obstructive sleep apnea and body weight in obese individuals with moderate or severe disease: SCALE sleep apnoea tria. Sleep Medicine 2015;16(Suppl 1):S24. [EMBASE: 4282911] [Google Scholar]
- Blackman A, Foster G, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Liraglutide 3.0 mg reduces severity of obstructive sleep apnoea and body weight in obese individuals with moderate or severe disease: SCALE sleep apnoea trial. Diabetologia 2014;57(1 Suppl 1):S85. [EMBASE: 1745360] [Google Scholar]
- Blackman A, Foster G, Zammit G, Rosenberg R, Wadden T, Aronne L, et al. Liraglutide 3.0 mg reduces severity of obstructive sleep apnea and body weight in individuals with obesity and moderate or severe disease: SCALE sleep apnoea trial. Canadian Journal of Diabetes 2015;39(Suppl 1):S35. [EMBASE: 1826652] [Google Scholar]
- Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. International Journal of Obesity 2016;40(8):1310-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A, Blackman A, Foster G, Zammit G, Rosenberg R, Wadden T, et al. Liraglutide 3.0 mg reduces severity of obstructive sleep apnoea and body weight in obese individuals with moderate or severe disease: SCALE sleep apnoea trial. Thorax 2014;69(Suppl 2):A16-7. [EMBASE: 71688686] [Google Scholar]
Scheen 2002 {published data only}
- Scheen AJ, Ernest P. New antiobesity agents in type 2 diabetes: Overview of clinical trials with sibutramine and orlistat. Diabetes and Metabolism 2002;28(6 I):437-45. [PubMed] [Google Scholar]
Scott 2015 {published data only}
- Scott LJ. Liraglutide: a review of its use in the management of obesity. Drugs 2015;75(8):899-910. [PMID: ] [DOI] [PubMed] [Google Scholar]
SCOUT 2010 {published data only}
- James P, Caterson I, Coutinho W, Finer N, Van Gaal L, Maggioni A, et al. Effects on mortality and morbidity in overweight/obese subjects: the sibutramine cardiovascular outcomes (SCOUT) trial. Journal of the American College of Cardiology 2010;Conference: American College of Cardiology's 59th Annual Scientific Session and i2 Summit: Innovation in Intervention Atlanta, GA United States. Conference Start: 20100314 Conference End: 20100316. Conference Publication:(var.pagings). 55 (10 SUPPL 1):A141.E1326. [Google Scholar]
- James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. New England Journal of Medicine 2010;363(10):905-17. [DOI] [PubMed] [Google Scholar]
- Kamil S, Finer N, James WP, Caterson ID, Andersson C, Torp-Pedersen C. Influence of sibutramine in addition to diet and exercise on the relationship between weight loss and blood glucose changes. European Heart Journal - Cardiovascular Pharmacotherapy 2017;3(3):134-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Maggioni AP, Caterson I, Coutinho W, Finer N, Gaal LV, Sharma AM, et al. Tolerability of sibutramine during a 6-week treatment period in high-risk patients with cardiovascular disease and/or diabetes: a preliminary analysis of the Sibutramine Cardiovascular Outcomes (SCOUT) Trial. Journal of Cardiovascular Pharmacology 2008;52(5):393-402. [DOI] [PubMed] [Google Scholar]
- Seimon R, Espinoza D, Ivers L, Gebski V, Finer N, Legler U, et al. Changes in body weight and blood pressure: Paradoxical outcome events in overweight and obese subjects with cardiovascular disease. Obesity Reviews 2014;15:173. [EMBASE: 71516191] [DOI] [PubMed] [Google Scholar]
- Seimon RV, Espinoza D, Ivers L, Gebski V, Finer N, Legler UF, et al. Changes in body weight and blood pressure: paradoxical outcome events in overweight and obese subjects with cardiovascular disease. International Journal of Obesity 2014;38:1165-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
SEQUEL 2014 {published data only}
- Garvey WT, Najarian T, Peterson CA. Significant weight loss with controlled-release phentermine/topiramate (PHEN/TPM CR) is associated with significant reductions in use of concomitant medications for cardiometabolic diseases over 108 weeks. Obesity 2011;19:S176. [EMBASE: 70680319] [Google Scholar]
- Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care 2014;37:912-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. American Journal of Clinical Nutrition 2012;95:297-308. [EMBASE: 2012064424] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Najarian T, Troupin B, Day WW. Weight loss (WL) with low-dose, controlled-release phentermine/topiramate (PHEN/TPM CR) correlates with improvements in liver function in overweight/obese adults with elevated alanine aminotransferase (ALT). Obesity 2011;19:S177. [EMBASE: 70680321] [Google Scholar]
- Toplak H, Rossner S, Garvey WT, Troupin B, Bowden CH. Long-term weight loss with controlled-release phentermine/topiramate reverses metabolic syndrome and improves associated traits. Diabetologia 2011;54:S370. [EMBASE: 70563050] [Google Scholar]
Sharif 2016 {published data only}
- Sharif Z, Peikanpour M, Rangchian M, Foroughi Moghadam MJ. Cost-utility analysis of lorcaserin in the treatment of obesity. Value in Health 2017;7:A590-A591. [EMBASE: 613234809] [Google Scholar]
Shaw Tronieri 2018 {published data only}
- Shaw Tronieri J, Wadden TA, Berkowitz RI, Chao AM, Pearl RL, Alamuddin N, et al. A randomized trial of lorcaserin and lifestyle counseling for maintaining weight loss achieved with a low-calorie diet. Obesity 2018;26(2):299-309. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sjöström 1998 {published data only}
- Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group [see comment]. Lancet 1998;352(9123):167-72. [DOI] [PubMed] [Google Scholar]
Starling 2001 {published data only}
- Starling RD, Liu X, Sullivan DH. Influence of sibutramine on energy expenditure in African American women. Obesity Research 2001;9(4):251-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strebkova 2015 {published data only}
- Strebkova E, Solovyova I, Sharapova E, Mkrtumyan A, Alekseeva L, Nasonov E. Impact of body weight loss on the clinical manifestations of knee osteoarthritis and metabolic syndrome parameters in female patients with obesity taking orlistat. Annals of the Rheumatic Diseases 2015;74:1183-4. [EMBASE: 4338732] [Google Scholar]
Suyog 2011 {published data only}
- Suyog J, Milind P, Karuna R. Comparison of efficacy and safety of rimonabant with orlistat in obese and overweight patients. International Journal of Pharma and Bio Sciences 2011;2(1):179-87. [Google Scholar]
Svendsen 2008 {published data only}
- Svendsen M, Rissanen A, Richelsen B, Rossner S, Hansson F, Tonstad S. Effect of orlistat on eating behavior among participants in a 3-year weight maintenance trial. Obesity (Silver Spring) 2008;16(2):327-33. [DOI] [PubMed] [Google Scholar]
Swinburn 2005 {published data only}
- Swinburn BA, Carey D, Hills AP, Hooper M, Marks S, Proietto J, et al. Effect of orlistat on cardiovascular disease risk in obese adults. Diabetes, Obesity & Metabolism 2005;7(3):254-62. [DOI] [PubMed] [Google Scholar]
Tambascia 2003 {published data only}
- Tambascia MA, Geloneze B, Repetto EM, Geloneze SR, Picolo M, Magro DO. Sibutramine enhances insulin sensitivity ameliorating metabolic parameters in a double-blind, randomized, placebo-controlled trial. Diabetes, Obesity & Metabolism 2003;5(5):338-44. [DOI] [PubMed] [Google Scholar]
Tiikkainen 2004 {published data only}
- Tiikkainen M, Bergholm R, Rissanen A, Aro A, Salminen I, Tamminen M, et al. Effects of equal weight loss with orlistat and placebo on body fat and serum fatty acid composition and insulin resistance in obese women. American Journal of Clinical Nutrition 2004;79(1):22-30. [DOI] [PubMed] [Google Scholar]
Triay 2012 {published data only}
- Triay J, Mundi M, Klein S, Toledo FG, Smith SR, Abu-Lebdeh H, et al. Does rimonabant independently affect free fatty acid and glucose metabolism? Journal of Clinical Endocrinology and Metabolism 2012;97(3):819-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunyan 2007 {published data only}
- Tunyan AM. Cardioprotective effects of antihypertensive therapy with orlistat treatment and hypocaloric diet in combination in obese hypertensive patients. Circulation 2007;115(8):E248. [CENTRAL: CN-00868400] [Google Scholar]
Van Gaal 2005 {published data only}
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study [see comment] [erratum appears in Lancet 2005 Jul 30-Aug 5;366(9483):370]. Lancet 2005;365(9468):1389-97. [DOI] [PubMed] [Google Scholar]
Van Gaal 2008 {published data only}
- Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O, et al. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. European Heart Journal 2008;29(14):1761-71. [DOI] [PubMed] [Google Scholar]
Von Scholten 2015 {published data only}
- Von Scholten BJ, Hansen TW, Goetze JP, Persson F, Rossing P. Glucagon-like peptide 1 receptor agonist (GLP-1 RA): long-term effect on kidney function in patients with type 2 diabetes. Journal of Diabetes and its Complications 2015;29(5):670-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang YJ, Liu C, Liu YM. Orlistat for adjuvant treatment of fatty type 2 diabetes mellitus in 32 patients. Zhongguo Xin Yao Yu Lin Chuang Za Zhi [Chinese journal of new drugs and clinical remedies] 2003;22(11):651-3. [Google Scholar]
Winslow 2012 {published data only}
- Winslow DH, Bowden CH, DiDonato K, McCullough PA. The effects of once-daily 15mg phentermine plus 92mg topiramate extended-release (PHEN/TPM ER) on cardiometabolic endpoints in obese patients with obstructive sleep apnea. Sleep 2013;36:A297. [EMBASE: 71513682] [Google Scholar]
- Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep 2012;35:1529-39. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wirth 2001 {published data only}
- Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286(11):1331-9. [DOI] [PubMed] [Google Scholar]
Ye 2017 {published data only}
- Ye J, Yanqin W, Xuan H, Bihui Z. Effect of orlistat on total liver fat quantification by novel magnetic resonance imaging in obese patients with non-alcoholic steatohepatitis: interim analysis of a prospective, randomized, single-center, open-label trial. United European Gastroenterology Journal 2017;5(Supplement 1):A625. [EMBASE: 7471868] [Google Scholar]
Zannad 2002 {published data only}
- Zannad F, Gille B, Grentzinger A, Bruntz JF, Hammadi M, Boivin JM, et al. Effects of sibutramine on ventricular dimensions and heart valves in obese patients during weight reduction. American Heart Journal 2002;144(3):508-15. [DOI] [PubMed] [Google Scholar]
Zavoral 1998 {published data only}
- Zavoral JH. Treatment with orlistat reduces cardiovascular risk in obese patients. Journal of Hypertension 1998;16(12 Pt 2):2013-7. [DOI] [PubMed] [Google Scholar]
Additional references
Abbott 2010
- Abbott Germany Theobald F. Press release: [Abbott setzt Vertrieb seiner Medikamente mit dem Wirkstoff Sibutramin zur Behandlung von Adipositas in den Ländern der Europäischen Union aus.] 2010. Available from: www.abbott.de/press/show/e7340/e19695/e18095/Sibutramin_210110_Theobald_de.pdf (cited 14 January 2013).
ACC/AHA 2017
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.. Hypertension 2018;71(6):1269-1324. [PMID: ] [DOI] [PubMed] [Google Scholar]
Apovian 2015
- Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2015;100(2):342-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aronne 2013
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring, Md.) 2013;21(11):2163-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aucott 2005
- Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension 2005;45(6):1035-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Caixas 2014
- Caixas A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Design, Development and Therapy 2014;8:1419-27. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obesity Reviews: an official journal of the International Association for the Study of Obesity 2013;14(5):383-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
CRESCENDO 2010
- Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 2010;376(9740):517-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Derosa 2005
- Derosa G, Cicero AF, Murdolo G, Piccinni MN, Fogari E, Bertone G, et al. Efficacy and safety comparative evaluation of orlistat and sibutramine treatment in hypertensive obese patients. Diabetes, Obesity & Metabolism 2005;7(1):47-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eisai Inc. 2020
- Eisai Incorporation. Eisai to Voluntarily Withdraw BELVIQ®/BELVIQ XR® in the U.S.. Available from: eisai.mediaroom.com/2020-02-13-Eisai-to-Voluntarily-Withdraw-BELVIQ-R-BELVIQ-XR-R-in-the-U-S (13 February 2020; cited 30 March 2020).
EMA 2008a
- European Medicines Agency (EMA). Questions and answers on the recommendation to suspend the marketing authorisation of Acomplia (rimonabant). Available from: www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/11/WC500014779.pdf (updated 23 October 2008; cited 17 December 2012).
EMA 2008b
- European Medicines Agency (EMA). Press Release - The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia. Available from: www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500014774.pdf (updated 23 October 2008; cited 17 December 2012).
EMA 2009a
- European Medicines Agency (EMA). Public Statement on Acomplia (rimonabant) - Withdrawal of the marketing authorisation in the European Union. Available from: www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/11/WC500012189.pdf (updated 30 January 2009; cited 17 December 2012).
EMA 2009b
- European Medicines Agency (EMA). Victoza (liraglutide) - EPAR - Summary for the public. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/001026/WC500050013.pdf (updated 8 July 2009; cited 17 December 2012).
EMA 2010a
- European Medicines Agency (EMA). Press Release - European Medicines Agency recommends suspension of marketing authorisations for sibutramine. Available from: www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/01/WC500069995.pdf (updated 21 January 2010; cited 17 December 2012).
EMA 2010b
- European Medicines Agency (EMA). Questions and answers on the suspension of medicines containing sibutramine. Available from: www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Sibutramine_107/WC500094238.pdf (updated 6 August 2010; cited 17 December 2012).
EMA 2013a
- European Medicines Agency (EMA). Questions and answers on the withdrawal of the marketing authorisation application for Belviq (lorcaserin). Available from: www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2013/05/WC500143811.pdf (updated 31 May 2013; cited 20 July 2015).
EMA 2013b
- European Medicines Agency (EMA). Withdrawal assessment report for Belviq. Available from: www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/human/002597/WC500148196.pdf (updated 20 August 2013; cited 20 July 2015).
EMA 2013c
- European Medicines Agency (EMA). Questions and answers on the refusal of the marketing authorisation for Qsiva (phentermine/topiramate). Available from: www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002350/WC500139215.pdf (updated 22 February 2013; cited 20 July 2015).
EMA 2013d
- European Medicines Agency (EMA). Scientific conclusions and grounds for refusal of the marketing authorisation for Qsiva (13 June 2013). Available from: www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002350/human_med_001607.jsp&mid=WC0b01ac058001d124# (updated 13 June 2013; cited 20 July 2015).
EMA 2015a
- European Medicines Agency (EMA). Mysimba (naltrexone/bupropion) - EPAR summary for the public. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003687/WC500185583.pdf (updated 15 April 2015; cited 20 July 2015).
EMA 2015b
- European Medicines Agency (EMA). Saxenda (liraglutide) - EPAR summary for the public. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003780/WC500185789.pdf (updated 16 April 2015; cited 20 July 2015).
ESC/ESH 2018
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018;39(33):3021-3104. [PMID: ] [DOI] [PubMed] [Google Scholar]
FDA 2007
- US Food and Drug Administration Egan AG, Colman EG. FDA Rimonabant Briefing Document. Endocrine and Metabolic Drugs Advisory Committee. 2007. Available from: https://wayback.archive-it.org/7993/20170405052424/https://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounder.pdf (updated 13 June 2007; cited 18 December 2012).
FDA 2010a
- US Food and Drug Administration (FDA). Recommendation on a regulatory decision for Meridia (sibutramine). http://wayback.archive-it.org/7993/20170113112219/http:/www.fda.gov/downloads/Drugs/DrugSafety/UCM228795.pdf (updated 7 October 2010; cited 18 December 2012).
FDA 2010b
- US Food and Drug Administration (FDA). FDA News Release: Abbott Laboratories agrees to withdraw its obesity drug Meridia. http://wayback.archive-it.org/7993/20170112215610/http:/www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm228812.htm (updated 8 October 2010; cited 18 December 2012).
FDA 2010c
- US Food and Drug Administration (FDA). Victoza (Liraglutide (rDNA)) - Approval letter. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022341s000TOC.cfm (updated 25 January 2010; cited 20 July 2015).
FDA 2012a
- US Food and Drug Administration (FDA). Qsymia (phentermine and topiramate extended-release) - Approval letter. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000Approv.pdf (updated 17 July 2012; cited 20 July 2015).
FDA 2012b
- US Food and Drug Administration (FDA). Belviq (lorcaserin hydrochloride) - Approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/022529Orig1s000ltr.pdf (updated 27 June 2012; cited 20 July 2015).
FDA 2014a
- US Food and Drug Administration (FDA). Contrave (naltrexone hydrochloride/bupropion hydrochloride) - Approval letter. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2014/200063Orig1s000Approv.pdf (updated 10 September 2014; cited 20 July 2015).
FDA 2014b
- US Food and Drug Administration (FDA). Saxenda (liraglutide) - Approval letter. Available from: www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/206321Orig1s000ltr.pdf (updated 23 December 2014; cited 20 July 2015).
FDA 2020
- US Food and Drug Administration (FDA). Drug Safety Communication - FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. Available from: www.fda.gov/media/135189/download (updated 13 February 2020; cited 30 March 2020).
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Handbook 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Horvath 2008
- Horvath K, Jeitler K, Siering U, Stich AK, Skipka G, Gratzer TW, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Archives of Internal Medicine 2008;168(6):571-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hypertension Canada 2018
- Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, et al. Hypertension Canada's 2018 Guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Canadian Journal of Cardiology 2018;34(5):506-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
IQWiG 2006
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Evaluation of the benefits and harms of non-drug weight-reducing treatment strategies in patients with essential hypertension [Nutzenbewertung nichtmedikamentöser Behandlungsstrategien bei Patienten mit Bluthochdruck: A-Gewichtsreduktion]. Available from: iqwig.de/download/A05-21A_Abschlussbericht_Gewichtsreduktion_bei_Bluthochdruck_neu.pdf 2006:19-119.
Kim 2003
- Kim SH, Lee YM, Jee SH, Nam CM. Effect of sibutramine on weight loss and blood pressure: a meta-analysis of controlled trials. Obesity Research 2003;11(9):1116-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
NEJM Journal Watch 2011
- NEJM Journal Watch. FDA reminds clinicians of liraglutide’s risks for pancreatitis and cancer. Available from: www.jwatch.org/fw201106140000002/2011/06/14/fda-reminds-clinicians-liraglutides-risks (cited 20 July 2015).
NICE 2019
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management (NG136). Available from: //www.nice.org.uk/guidance/ng136 (last updated 28 August 2019; cited 3 April 2020).
Padwal 2007
- Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 2007;369(9555):71-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
PRISMA 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9, W64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Russell‐Jones 2009
- Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Molecular and Cellular Endocrinology 2009;297(1-2):137-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scheen 2006
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368(9548):1660-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shyangdan 2011
- Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD006423. [DOI: 10.1002/14651858.CD006423.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2013
- Taylor JR, Dietrich E, Powell J. Lorcaserin for weight management. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2013;6:209-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. World Health Day 2013 – A global brief on hypertension. 2013. Available from: www.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf (cited 20 July 2015).
Yanovski 2014
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311(1):74-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Siebenhofer 2009
- Siebenhofer A, Horvath K, Jeitler K, Berghold A, Stich A K, Matyas E, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007654. [DOI: 10.1002/14651858.CD007654.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Siebenhofer 2009a
- Siebenhofer A, Horvath K, Jeitler K, Berghold A, Stich AK, Matyas E, et al. Long‐term effects of weight‐reducing drugs in hypertensive patients. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007654. [DOI: 10.1002/14651858.CD007654] [DOI] [PubMed] [Google Scholar]
Siebenhofer 2013
- Siebenhofer A, Jeitler K, Horvath K, Berghold A, Siering U, Semlitsch T. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD007654. [DOI: 10.1002/14651858.CD007654.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Siebenhofer 2016
- Siebenhofer A, Jeitler K, Horvath K, Berghold A, Posch N, Meschik J, Semlitsch T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD007654. [DOI: 10.1002/14651858.CD007654.pub4] [PMID: ] [DOI] [PubMed] [Google Scholar]


