Abstract
Background
Treatment with angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) is used to reduce proteinuria and retard the progression of chronic kidney disease (CKD). However, resolution of proteinuria may be incomplete with these therapies and the addition of an aldosterone antagonist may be added to further prevent progression of CKD. This is an update of a Cochrane review first published in 2009 and updated in 2014.
Objectives
To evaluate the effects of aldosterone antagonists (selective (eplerenone), non‐selective (spironolactone or canrenone), or non‐steroidal mineralocorticoid antagonists (finerenone)) in adults who have CKD with proteinuria (nephrotic and non‐nephrotic range) on: patient‐centred endpoints including kidney failure (previously know as end‐stage kidney disease (ESKD)), major cardiovascular events, and death (any cause); kidney function (proteinuria, estimated glomerular filtration rate (eGFR), and doubling of serum creatinine); blood pressure; and adverse events (including hyperkalaemia, acute kidney injury, and gynaecomastia).
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 13 January 2020 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that compared aldosterone antagonists in combination with ACEi or ARB (or both) to other anti‐hypertensive strategies or placebo in participants with proteinuric CKD.
Data collection and analysis
Two authors independently assessed study quality and extracted data. Data were summarised using random effects meta‐analysis. We expressed summary treatment estimates as a risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes, or standardised mean difference (SMD) when different scales were used together with their 95% confidence interval (CI). Risk of bias were assessed using the Cochrane tool. Evidence certainty was evaluated using GRADE.
Main results
Forty‐four studies (5745 participants) were included. Risk of bias in the evaluated methodological domains were unclear or high risk in most studies. Adequate random sequence generation was present in 12 studies, allocation concealment in five studies, blinding of participant and investigators in 18 studies, blinding of outcome assessment in 15 studies, and complete outcome reporting in 24 studies.
All studies comparing aldosterone antagonists to placebo or standard care were used in addition to an ACEi or ARB (or both). None of the studies were powered to detect differences in patient‐level outcomes including kidney failure, major cardiovascular events or death.
Aldosterone antagonists had uncertain effects on kidney failure (2 studies, 84 participants: RR 3.00, 95% CI 0.33 to 27.65, I² = 0%; very low certainty evidence), death (3 studies, 421 participants: RR 0.58, 95% CI 0.10 to 3.50, I² = 0%; low certainty evidence), and cardiovascular events (3 studies, 1067 participants: RR 0.95, 95% CI 0.26 to 3.56; I² = 42%; low certainty evidence) compared to placebo or standard care. Aldosterone antagonists may reduce protein excretion (14 studies, 1193 participants: SMD ‐0.51, 95% CI ‐0.82 to ‐0.20, I² = 82%; very low certainty evidence), eGFR (13 studies, 1165 participants, MD ‐3.00 mL/min/1.73 m², 95% CI ‐5.51 to ‐0.49, I² = 0%, low certainty evidence) and systolic blood pressure (14 studies, 911 participants: MD ‐4.98 mmHg, 95% CI ‐8.22 to ‐1.75, I² = 87%; very low certainty evidence) compared to placebo or standard care.
Aldosterone antagonists probably increase the risk of hyperkalaemia (17 studies, 3001 participants: RR 2.17, 95% CI 1.47 to 3.22, I² = 0%; moderate certainty evidence), acute kidney injury (5 studies, 1446 participants: RR 2.04, 95% CI 1.05 to 3.97, I² = 0%; moderate certainty evidence), and gynaecomastia (4 studies, 281 participants: RR 5.14, 95% CI 1.14 to 23.23, I² = 0%; moderate certainty evidence) compared to placebo or standard care.
Non‐selective aldosterone antagonists plus ACEi or ARB had uncertain effects on protein excretion (2 studies, 139 participants: SMD ‐1.59, 95% CI ‐3.80 to 0.62, I² = 93%; very low certainty evidence) but may increase serum potassium (2 studies, 121 participants: MD 0.31 mEq/L, 95% CI 0.17 to 0.45, I² = 0%; low certainty evidence) compared to diuretics plus ACEi or ARB. Selective aldosterone antagonists may increase the risk of hyperkalaemia (2 studies, 500 participants: RR 1.62, 95% CI 0.66 to 3.95, I² = 0%; low certainty evidence) compared ACEi or ARB (or both). There were insufficient studies to perform meta‐analyses for the comparison between non‐selective aldosterone antagonists and calcium channel blockers, selective aldosterone antagonists plus ACEi or ARB (or both) and nitrate plus ACEi or ARB (or both), and non‐steroidal mineralocorticoid antagonists and selective aldosterone antagonists.
Authors' conclusions
The effects of aldosterone antagonists when added to ACEi or ARB (or both) on the risks of death, major cardiovascular events, and kidney failure in people with proteinuric CKD are uncertain. Aldosterone antagonists may reduce proteinuria, eGFR, and systolic blood pressure in adults who have mild to moderate CKD but may increase the risk of hyperkalaemia, acute kidney injury and gynaecomastia when added to ACEi and/or ARB.
Plain language summary
Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease
What is the issue?
People who have chronic kidney disease (CKD) have a higher risk of heart disease and declining kidney function. Increased amounts of protein in the urine is a sign of kidney stress and is linked to declining kidney function. Medications used to lower blood pressure and reduce protein levels in the urine ‐ in particular, angiotensin‐converting enzyme Inhibitors (ACEi) and angiotensin receptor blockers (ARBs) ‐remain the core treatment to prevent the declining of kidney function in CKD.
Protecting kidney function with these medications may however be incomplete and adding an aldosterone antagonist (blocker) (for example, spironolactone, canrenone, eplerenone, or finerenone) may better protect kidney function in the long‐term. By blocking the production of aldosterone, the kidneys excrete more water which can lead to a lowering of blood pressure. However, they can cause side effects including enlargement of male breast tissue, and when used with ACEi or ARBs may cause high levels of potassium in the blood or a decline in kidney function.
What did we do? We reviewed the available studies looking at the addition of aldosterone blockers to standard treatment in people with CKD to see if they slowed the decline of kidney function and the subsequent need for dialysis or a kidney transplant. We looked at whether they reduced heart disease, the amount of protein in urine, or improved blood pressure. We also looked at whether aldosterone blockers were safe in terms of risks of male breast enlargement, potassium levels in the blood, and short‐term effects on kidney function.
What did we find? We found that adding aldosterone blockers to a patient's current medications (ACEi or ARBs), lowered both protein in the urine and systolic blood pressure. Kidney function declined, however the effects on survival were uncertain. The addition of aldosterone blockers increased the amount of potassium in the blood. This may require medication changes, extra blood tests, and may be potentially harmful. Treatment with aldosterone blockers also increased the chance of short‐term decline in kidney function and enlargement of male breast tissue.
Conclusions It is unclear as to whether aldosterone blockers protect kidney function or prevent heart disease in people who have CKD.
Summary of findings
Background
Description of the condition
Chronic kidney disease (CKD) has a global prevalence of 10% to 12% and progression to kidney failure (previously known as end‐stage kidney disease (ESKD)) is rising due to the global diabetes and hypertension pandemics (Mills 2015; Nugent 2011). There is a significant associated economic burden to patients, caregivers, and society, which increases throughout disease progression (Wang 2016). Angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) are the standard of care to slow progression of CKD and reduce incidence of kidney failure in patients with proteinuria irrespective of primary kidney disease (Jafar 2001; Strippoli 2006) because they lower proteinuria and blood pressure, which are both independent predictors of death in adults with CKD (Brenner 2001; GISEN 1997; Mathiesen 1999). However, ACEi or ARB slow, but may not completely retard, the progression of CKD (Schieppati 2003).
Description of the intervention
Animal studies have shown that aldosterone has an independent role in the development of hypertensive kidney disease and vascular injury resulting in myocardial and renal fibrosis (Figure 1), and exacerbates glomerulosclerosis resulting in severe proteinuria (Bomback 2007), which is reduced with aldosterone blockade (Aldigier 2005; Green 1996; Rocha 1998; Silvestre 1998). Renin‐angiotensin‐aldosterone system blockade with ACEi or ARB result in incomplete suppression of serum aldosterone levels and is known as the 'aldosterone escape phenomenon' (Staessen 1981). Further experimental studies have established this theory and in humans, the treatment of adults with CKD exhibiting aldosterone escape phenomenon with aldosterone antagonists reduces proteinuria (Fritsch Neves 2003). However, aldosterone antagonism may increase risks of hyperkalaemia and gynaecomastia (Nappi 2011). Novel non‐steroidal mineralocorticoid receptor antagonists such as finerenone are more selective for the mineralocorticoid receptor than other steroid receptors including the glucocorticoid receptor, androgen receptor and progesterone receptor (Bramlage 2016) and may provide similar efficacy as non‐selective aldosterone antagonist but improved safety profile.
1.
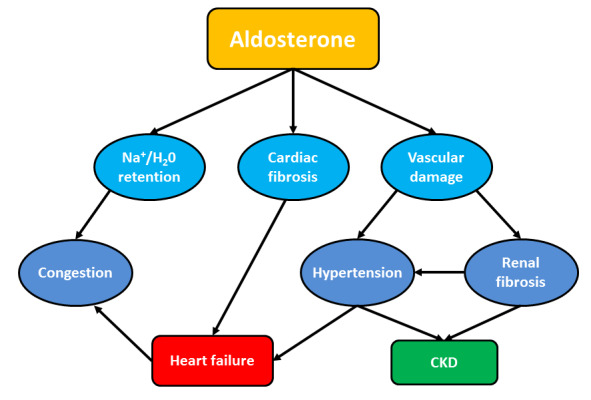
Mechanisms of cardiac and kidney damage induced by aldosterone excess
How the intervention might work
Multiple aldosterone‐mediated mechanisms have been shown to contribute to renal vascular injury and fibrosis in animal studies. These include aldosterone‐mediated increases in plasminogen activator inhibitor‐1 (PAI‐1) which inhibits the fibrinolytic system and activates latent growth factors; up‐regulation of transforming growth factor‐b and associated fibroblast differentiation, up‐regulation of collagen synthesis and down regulation of matrix metalloproteinase collagenase; generation of oxygen‐free radicals and hydrogen peroxide; and up regulation of endothelin‐1 with resultant vasoconstriction (Hollenberg 2004). However, a common pathway is yet to be clearly defined. In a rat model, renal radiation injury resulted in an eight‐fold increase in the expression of PAI‐1 messenger RNA (mRNA) and non‐selective aldosterone blockade (spironolactone) significantly decreased PAI‐1 mRNA expression, development of glomerulosclerosis and proteinuria (Brown 2000). In human studies, beneficial effects of aldosterone blockade (non‐selective and selective) have been established in congestive cardiac failure (Hostetter 2003; Pitt 1999; Pitt 2003) and proteinuric CKD (Bianchi 2006; Chrysostomou 2006; Epstein 2006; Rossing 2005; Schjoedt 2005). In animal studies, finerenone had a more potent natriuretic response than eplerenone but no impact on urinary potassium levels (Kolkhof 2014). Therefore, it is hypothesised that non‐steroidal mineralocorticoid receptor antagonists will exhibit the benefits of aldosterone blockade without the risk of hyperkalaemia.
Why it is important to do this review
Aldosterone blockade in combination with ACEi or ARB may reduce proteinuria but their effects on patient‐level outcomes such as kidney failure requiring dialysis or kidney transplantation or major cardiovascular events and their safety in regards to risk of hyperkalaemia and acute kidney injury, particularly in adults who have coexisting CKD, remain uncertain. Thus, we analysed the benefits and harms of aldosterone antagonists in adults who had CKD and who were or who were not already treated with ACEi or ARB (or in combination). We specifically focused on treatment effects for patient‐level outcomes including kidney failure and major cardiovascular events, proteinuria, and kidney function. New relevant studies on CKD patients receiving aldosterone antagonists, including non‐steroidal mineralocorticoid antagonists, have recently been completed and their inclusion to update the previous published versions of this review (Bolignano 2014; Navaneethan 2009) would be valuable.
Objectives
To evaluate the effect of aldosterone antagonists (selective (eplerenone), non‐selective (spironolactone), and non‐steroidal (finerenone)) in combination with ACEi or ARB in adults who have CKD with proteinuria (nephrotic and non‐nephrotic range) on:
Patient‐centred endpoints including kidney failure, major cardiovascular events, and death (any cause)
Kidney function (proteinuria, estimated glomerular filtration rate (eGFR), and doubling of serum creatinine (SCr)
Adverse events (including hyperkalaemia, acute kidney injury, and gynaecomastia).
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs of aldosterone antagonists used in combination with ACEi or ARB (or both) were included. Data from the first period of randomised cross‐over studies was also included.
Types of participants
Inclusion criteria
Studies enrolling participants with CKD stages 1 to 4, as defined by the by Kidney Disease Outcomes Quality Initiative (K‐DOQI) guidelines (Levey 2003) and who had albuminuria or proteinuria were considered for inclusion. We included studies in adults who had CKD regardless of aetiology. The K/DOQI categories for kidney disease are as follows.
CKD stage 1: eGFR > 90 mL/min/1.73 m² and evidence of clinically relevant structural or urinary abnormalities including haematuria or proteinuria (or both)
CKD stage 2: eGFR 60 to 89 mL/min/1.73 m²
CKD stage 3: eGFR 30 to 59 mL/1.73 m²
CKD stage 4: eGFR 15 to 29 mL/min/1.73 m².
Exclusion criteria
We excluded studies in adults on dialysis, recipients of a kidney transplant, participants without evidence of CKD or proteinuria, studies less than 4 weeks of duration, and studies not evaluating any outcome of interest.
Types of interventions
We included studies evaluating aldosterone antagonist treatment given in combination with an ACEi or ARB (or both). We considered studies in which treatment duration was 4 weeks or longer. If any studies compared aldosterone antagonists alone (i.e. no additional RAS antagonists), these studies were also included,
We considered the following treatment comparisons.
Aldosterone antagonists with RAS antagonists versus placebo or standard care
Aldosterone antagonist with RAS antagonists versus diuretic plus ACEi or ARB
Non‐selective aldosterone antagonist with RAS antagonists versus calcium channel blocker
Selective aldosterone antagonist with RAS antagonists versus ACEi or ARB (or both)
Selective aldosterone antagonist with RAS antagonists versus ACEi or ARB (or both) versus ACEi or ARB (or both) plus nitrate
Selective aldosterone antagonist with RAS antagonists versus non‐steroidal mineralocorticoid antagonist.
If disaggregated outcome data were not available for the three groups (ACEi alone, ARB alone or the combination separately), we used combined data when available.
Types of outcome measures
Primary outcomes
Kidney failure (defined as permanent worsening in eGFR requiring kidney replacement therapy)
Hyperkalaemia (defined as serum potassium > 5.0 mEq/L or mmol/L)
Secondary outcomes
Death (any cause)
Major cardiovascular events as defined by the investigators (including but not limited to myocardial infarction, stroke, congestive heart failure)
Urinary protein excretion rate (24‐hour proteinuria, 24‐hour albuminuria in mg/dL, urine protein:creatinine ratio, or urine albumin:creatinine ratio)
Kidney function: estimated GFR (mL/min or mL/min/1.73 m²); doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria
Blood pressure: systolic and diastolic blood pressure (mmHg)
Serum potassium
Acute kidney injury
Gynaecomastia
Fatigue
Falls
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 13 January 2020 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review update.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Disagreements were resolved in consultation with two authors who also provided methodological assistance throughout the review process.
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. For this update, titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that may have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary, the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data were used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancies between published versions were highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Discrepancies were resolved by discussion with a third author.
Measures of treatment effect
For dichotomous outcomes (kidney failure, death (any cause), cardiovascular events, doubling of SCr, hyperkalaemia, acute kidney injury, and gynaecomastia) results were expressed as a risk ratio (RR) with 95% confidence intervals (CI). When continuous scales of measurement were used to assess the effects of treatment (end of treatment protein excretion rate or albumin excretion rate, eGFR or creatinine clearance, blood pressure, and serum potassium), we used the mean difference (MD) or the standardised mean difference (SMD) when different measurement scales were used.
Dealing with missing data
We contacted study authors to seek additional information. We were successful in obtaining additional data from Drs KJ Schjoedt, K Rossing, A Chrysostomou, S Bianchi, S Nielsen, and K Takebayashi. These data were included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) population were performed. Attrition rates, such as drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi² test, or a confidence interval for I²) (Higgins 2011).
Assessment of reporting biases
We planned to assess for the potential existence of small study bias (Higgins 2011) for outcomes in which sufficient data observations were available (10 or more studies) and in which there was low or no statistical heterogeneity between studies.
Data synthesis
Data were pooled using random effects meta‐analysis, but the fixed effects model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity. Heterogeneity among participants could be related to age, stage of kidney disease, aetiology of kidney disease and amount of proteinuria. Heterogeneity in treatments could be related to prior agent(s) used and the agent (selective or non‐selective aldosterone antagonist), dose, duration of aldosterone antagonists and the concomitant use of ACEi or ARB (or both).
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables. The absolute treatment effects for dichotomous outcomes was estimated using the risk estimate and 95% CI obtained from the corresponding meta‐analysis.
Primary efficacy outcome
Kidney failure
Primary safety outcome
Hyperkalaemia
Secondary outcomes
Death (any cause)
Cardiovascular events
Doubling SCr
Acute kidney injury
Proteinuria
eGFR
Results
Description of studies
Results of the search
For this 2020 update a search of The Cochrane Kidney and Transplant Register of Studies identified 83 reports. We identified 18 new included studies (32 reports), and four reports of two existing included studies; three new ongoing studies (four reports); 13 new excluded studies (40 reports), and two reports of two existing excluded studies. One study (one report) has been recently completed but no results have been published and is awaiting assessment.
In addition to the new reports, one previously included study has been move to excluded (Schjoedt 2006) as a proportion of the patients have been reported in two other included studies (Schjoedt 2005; Rossing 2005). Two previous ongoing studies have now been included (Abolghasmi 2011; EVALUATE 2010) and one study, while complete, is yet to publish any results and is awaiting assessment (NCT00315016). Four non‐RCTs have been removed from this update.
See Figure 2.
2.

Study flow diagram.
Included studies
For this 2020 update we included 18 new studies (4248 participants) (ARTS 2012; ARTS‐DN 2015; ARTS‐HF 2015; Bianchi 2010; Boesby 2013; Chen 2018b; Esteghamati 2013; EVALUATE 2010; Fogari 2014; Hamid 2017a; Hase 2013; Horestani 2012; Ito 2019a; Kato 2015; Morales 2015; Tylicki 2012; Wang 2013g; Ziaee 2013). This brings the total number of included studies to 44 (85 reports, 5745 participants).
Ten studies were cross‐over studies (Boesby 2011; Morales 2009; Morales 2015; Nielsen 2012; Saklayen 2008; Smolen 2006; Rossing 2005; Schjoedt 2005; Tylicki 2008; Tylicki 2012).
Twenty‐three studies included participants who had kidney disease secondary to diabetes mellitus (ARTS‐DN 2015; Chen 2018b; Chrysostomou 2006; Epstein 2002; Epstein 2006; Esteghamati 2013; Fogari 2014; Hamid 2017a; Hase 2013; Horestani 2012; Ito 2019a; Kato 2015; Koroshi 2010; Mehdi 2009; Nielsen 2012; Ogawa 2006a; Rossing 2005; Saklayen 2008; Schjoedt 2005; Takebayashi 2006; van den Meiracker 2006; Zheng 2011; Ziaee 2013). Two studies included participants with heart failure with associated proteinuric CKD (ARTS 2012; ARTS‐HF 2015). The remaining studies included participants with non‐diabetic kidney disease encompassing IgA nephropathy, benign nephrosclerosis, membranous nephropathy, or idiopathic chronic glomerulonephritis (Abolghasmi 2011; Bianchi 2006; Bianchi 2010; Boesby 2011; Boesby 2013; Cohen 2010; CRIBS II 2009; Furumatsu 2008; Guney 2009; Haykal 2007; Lv 2009a; Morales 2009; Morales 2015; Smolen 2006; Tokunaga 2008a; Tylicki 2008; Tylicki 2012; Wang 2013g). All studies excluded participants with an eGFR below 15 mL/min/1.73 m². For studies measuring 24‐hour urine protein or albumin, the baseline albuminuria/proteinuria excretion rates ranged from 0.15 to 3.6 g/day. Study duration varied from one to 36 months with a median duration of 3 months. Sample size of all studies was variable (range 16 to 1055) and none were powered to detect hard primary outcomes including kidney failure, death, or major cardiovascular events.
Among studies using non‐selective aldosterone antagonists, 22 studies (1441 participants) compared spironolactone plus ACEi or ARB (or both) to ACEi or ARB (or both) (Abolghasmi 2011; Bianchi 2006; Chen 2018b; Chrysostomou 2006; CRIBS II 2009; Furumatsu 2008; Guney 2009; Kato 2015; Koroshi 2010; Lv 2009a; Mehdi 2009; Nielsen 2012; Ogawa 2006a; Rossing 2005; Saklayen 2008; Schjoedt 2005; Tokunaga 2008a; Tylicki 2008; van den Meiracker 2006; Wang 2013g; Zheng 2011; Ziaee 2013). Five studies (220 participants) compared spironolactone plus ACEi or ARB to diuretics plus ACEi or ARB (Hamid 2017a; Hase 2013, Horestani 2012; Morales 2015; Smolen 2006); one study (37 participants) compared spironolactone to calcium channel blockers (Takebayashi 2006); one study (136 participants) compared spironolactone plus ARB to ACEi plus ARB (Esteghamati 2013); one study (120 participants) compared canrenone plus ARB and calcium channel blockers to hydrochlorothiazide plus ARB and calcium channel blockers (Fogari 2014); and one study (128 participants) compared spironolactone plus ACEi and ARB to ACEi (Bianchi 2010). In the studies that analysed the efficacy of non‐selective aldosterone antagonists, 25 mg/day of spironolactone was used throughout the study period except for Abolghasmi 2011, Saklayen 2008 and van den Meiracker 2006 who used 25 to 50 mg/day. Chen 2018b, Lv 2009a, Wang 2013g and Zheng 2011 used 20 mg/day; Horestani 2012, Koroshi 2010and Takebayashi 2006 used 50 mg/day; Mehdi 2009 used 12.5 to 25 mg/day of spironolactone; and Bianchi 2010 used 25 mg three times/week to 50 mg/day of spironolactone. Fogari 2014 used 25 mg/day of canrenone. In Hamid 2017a, the dose of spironolactone was not defined.
Six studies (925 participants) compared the selective aldosterone antagonist eplerenone plus ACEi or ARB (or both) to ACEi or ARB (or both) (Boesby 2011; Haykal 2007; Epstein 2002; Epstein 2006; EVALUATE 2010; Tylicki 2012). One study (34 participants) compared eplerenone plus ACEi or ARB (or both) to ACEi or ARB (or both) and to ACEi or ARB (or both) plus nitrate (Cohen 2010), and one study (54 participants) compared the selective aldosterone antagonist eplerenone to placebo (Boesby 2013). Studies that analysed the efficacy of selective aldosterone antagonists used eplerenone at the dose of 200 mg/day (Epstein 2002), 50 to 100 mg/day (Epstein 2006), 25 to 50 mg/day (Boesby 2011; Boesby 2013; Haykal 2007), and 50 mg/day (EVALUATE 2010; Tylicki 2012). In Cohen 2010 the dose of eplerenone administered was not defined.
One cross‐over study (12 participants) compared eplerenone alone (25 mg/day) to ACEi alone (20 mg/day) or ACEi (10 mg/day) plus ARB (16 mg/day) (Morales 2009).
Among studies using non‐steroidal mineralocorticoid antagonists, one study (821 participants) compared finerenone to placebo (ARTS‐DN 2015), one study (392 participants) compared finerenone to placebo or spironolactone (ARTS 2012), one study (1055 participants) compared finerenone to eplerenone (ARTS‐HF 2015), and one study (358 participants) compared esaxerenone to placebo (Ito 2019a). Studies that analysed the efficacy of non‐steroidal mineralocorticoid antagonists used finerenone at the dose of 2.5 to 10 mg/day (ARTS 2012), 1.25 to 25 mg/day (ARTS‐DN 2015), 5 to 20 mg/day (ARTS‐HF 2015), and esaxerenone 0.625 to 5 mg/day (Ito 2019a). Other characteristics of the participants and the interventions of the included studies are detailed in the Characteristics of included studies.
Excluded studies
Twenty‐seven studies (61 reports) were excluded because; they did not include adults with CKD (17 studies); were not studies comparing aldosterone antagonists with or without ACEi or ARB (3); were of short duration (1); included participants already reported in other included studies (1); were terminated early with no reported outcomes (1); or they did not examine outcomes of interest (e.g. pharmacokinetic studies) (1). One study was retracted (1).
For this 2020 update, non‐RCTs have been deleted.
Risk of bias in included studies
Risks of bias in the available studies are shown in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was judged to be at low risk of bias in 12 studies (ARTS‐DN 2015; ARTS‐HF 2015; Bianchi 2006; Chen 2018b; EVALUATE 2010; Mehdi 2009; Morales 2015; Nielsen 2012; Schjoedt 2005; Tylicki 2008; Tylicki 2012; van den Meiracker 2006) and unclear in the remaining 32 studies.
Allocation concealment was judged to be at low risk in five studies (ARTS‐DN 2015; ARTS‐HF 2015; Boesby 2011; Chrysostomou 2006; EVALUATE 2010), one study was judged to be a high risk of bias (Bianchi 2010), and unclear in the remaining 38 studies.
Blinding
Participants and investigators were blinded in 18 studies (Abolghasmi 2011; ARTS 2012; ARTS‐DN 2015; ARTS‐HF 2015; Chrysostomou 2006; CRIBS II 2009; Epstein 2002; Epstein 2006; EVALUATE 2010; Kato 2015; Lv 2009a; Mehdi 2009; Nielsen 2012; Rossing 2005; Saklayen 2008; Schjoedt 2005; Tylicki 2012; van den Meiracker 2006) and not blinded in 13 studies (Bianchi 2006; Bianchi 2010; Boesby 2011; Boesby 2013; Chen 2018b; Esteghamati 2013; Fogari 2014; Furumatsu 2008; Hase 2013; Morales 2009; Tokunaga 2008a; Tylicki 2008; Wang 2013g); blinding was unclear in the remaining 13 studies.
Outcome assessors were not aware of treatment allocation or outcomes were unlikely influenced by treatment allocation in 15 studies (ARTS‐DN 2015; Boesby 2013; CRIBS II 2009; Epstein 2006; Esteghamati 2013; EVALUATE 2010; Fogari 2014; Furumatsu 2008; Guney 2009; Morales 2009; Morales 2015; Nielsen 2012; Saklayen 2008; Smolen 2006; Zheng 2011). Blinding of outcome assessors was unclear in the remaining 29 studies.
Incomplete outcome data
Twenty‐four studies were judged to be at low risk of bias (Abolghasmi 2011; ARTS‐DN 2015; ARTS‐HF 2015; Bianchi 2006; Boesby 2011; Chrysostomou 2006; Cohen 2010; CRIBS II 2009; Epstein 2006; EVALUATE 2010; Fogari 2014; Furumatsu 2008; Hase 2013; Haykal 2007; Kato 2015; Morales 2009; Nielsen 2012; Rossing 2005; Saklayen 2008; Schjoedt 2005; Takebayashi 2006; Tylicki 2008; Tylicki 2012; Zheng 2011). Seven studies where there was some loss to follow‐up (ARTS‐DN 2015; Bianchi 2006; Boesby 2011; Chen 2018b; Epstein 2006; Cohen 2010; CRIBS II 2009) were analysed on an intention‐to‐treat basis. Ten studies were judged to be at high risk of bias (ARTS 2012; Bianchi 2010; Boesby 2013; Chen 2018b; Esteghamati 2013; Guney 2009; Ito 2019a; Mehdi 2009; Morales 2015; van den Meiracker 2006). The dropout rate from study follow‐up ranged from 0% to 37% and did not differ between the treatment and control groups.
Selective reporting
All the pre‐specified outcomes and all relevant outcomes were reported in 18 studies (ARTS‐DN 2015; ARTS‐HF 2015; Bianchi 2006; Chen 2018b; Chrysostomou 2006; Epstein 2006; Esteghamati 2013; EVALUATE 2010; Fogari 2014; Furumatsu 2008; Guney 2009; Hase 2013; Kato 2015; Lv 2009a; Mehdi 2009; Tokunaga 2008a; Tylicki 2008; Tylicki 2012). Selective reporting was judged to be at high risk of bias in 23 studies (Abolghasmi 2011; Bianchi 2006; Bianchi 2010; Boesby 2011; CRIBS II 2009; Epstein 2002; Hamid 2017a; Haykal 2007; Ito 2019a; Koroshi 2010; Morales 2009; Morales 2015; Nielsen 2012; Ogawa 2006a; Rossing 2005; Saklayen 2008; Schjoedt 2005; Smolen 2006; Takebayashi 2006; van den Meiracker 2006; Wang 2013g; Zheng 2011; Ziaee 2013), and unclear in the remaining three studies.
Other potential sources of bias
Eighteen studies were judged to be at low risk of bias due to funding (Bianchi 2006; Bianchi 2010; Boesby 2011; Boesby 2013; Chrysostomou 2006; CRIBS II 2009; Fogari 2014; Furumatsu 2008; Guney 2009; Hase 2013; Mehdi 2009; Morales 2015; Nielsen 2012; Rossing 2005; Schjoedt 2005; Tylicki 2008; van den Meiracker 2006; Ziaee 2013); six studies were funded by a pharmaceutical company (ARTS 2012; ARTS‐DN 2015; ARTS‐HF 2015; Epstein 2006; EVALUATE 2010; Ito 2019a;); one study excluded participants after randomisation due change in treatment (Horestani 2012) and the risk of bias was unclear in the remaining 19 studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Aldosterone antagonists versus placebo or standard care for proteinuric chronic kidney disease.
| Aldosterone antagonist versus placebo or standard care for proteinuric CKD | |||||
| Patient or population: proteinuric CKD Intervention: aldosterone antagonist Comparison: placebo or standard care | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo or standard care | Risk with aldosterone antagonist | ||||
| Kidney failure | 0 per 1,000 | 0 per 1,000 (0 to 0) | RR 3.00 (0.33 to 27.65) | 84 (2) | ⊕⊝⊝⊝ VERY LOW 1, 2 |
| Hyperkalaemia | 25 per 1,000 | 55 per 1,000 (37 to 81) | RR 2.17 (1.47 to 3.22) | 3001 (17) | ⊕⊕⊕⊝ MODERATE 3 |
| Death | 14 per 1,000 | 8 per 1,000 (1 to 50) | RR 0.58 (0.10 to 3.50) | 421 (3) | ⊕⊕⊝⊝ LOW 2, 4 |
| Cardiovascular events | 32 per 1,000 | 31 per 1,000 (8 to 115) | RR 0.95 (0.26 to 3.56) | 1067 (3) | ⊕⊕⊝⊝ LOW 2, 5 |
| Doubling serum creatinine | 83 per 1,000 | 107 per 1,000 (57 to 202) | RR 1.30 (0.69 to 2.44) | 875 (2) | ⊕⊕⊝⊝ LOW 2, 5 |
| AKI | 30 per 1,000 | 61 per 1,000 (31 to 119) | RR 2.04 (1.05 to 3.97) | 1446 (5) | ⊕⊕⊕⊝ MODERATE 6 |
| Proteinuria | The SMD was 0.51 lower with aldosterone antagonists (0.82 lower to 0.20 lower) than placebo or standard care | ‐ | 1193 (14) | ⊕⊝⊝⊝ VERY LOW 7, 8, 9, 10 | |
| eGFR (mL/min/1.73 m²) | The mean eGFR was 3.00 mL/min/1.73 m² lower with aldosterone antagonists (5.51 lower to 0.49 lower) than placebo or standard care | ‐ | 1144 (12) | ⊕⊕⊝⊝ LOW 2, 11 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKD: chronic kidney disease; CI: confidence interval; RR: risk ratio; AKI: acute kidney injury; eGFR: estimated glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Evidence quality was downgraded because of study risks of bias. Allocation concealment in no studies, blinding of outcome assessment in one study and complete outcome data in no studies
2 Treatment estimate had a wide CI
3 Evidence quality was downgraded because of study risks of bias. Allocation concealment in three studies, blinding of outcome assessment in seven studies, complete outcome data in 11 studies
4 Evidence quality was downgraded because of study risks of bias. Allocation concealment in one study, blinding of outcome assessment in one study, and complete outcome data in one study
5 Evidence quality was downgraded because of study risks of bias. Allocation concealment in one study, blinding of outcome assessment in one study, complete outcome data in one study
6 Evidence quality was downgraded because of study risks of bias. Allocation concealment in one study, blinding of outcome assessment in three studies, complete outcome data in one study
7 Evidence quality was downgraded because of suspected small study effects from asymmetry on inverted funnel plot
8Evidence quality was downgraded because of study risks of bias. Allocation concealment in one study, blinding of outcome assessment in seven studies, complete outcome data in nine studies
9 There was significant heterogeneity between studies
10 Evidence quality was downgraded because proteinuria is a surrogate outcome for CKD progression
11 Evidence quality was downgraded because of study risks of bias. Allocation concealment in one study, blinding of outcome assessment in four studies, complete outcome data in six studies
Summary of findings 2. Aldosterone antagonists versus diuretics for proteinuric chronic kidney disease.
| Aldosterone antagonist versus diuretics for proteinuric CKD | |||||
| Patient or population: proteinuric CKD Intervention: aldosterone antagonist Comparison: diuretics | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with diuretics | Risk with aldosterone antagonist | ||||
| Kidney failure | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Hyperkalaemia | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Death | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Cardiovascular events | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Doubling serum creatinine | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| AKI | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Proteinuria | The SMD was 1.59 lower (3.8 lower to 0.62 higher) with aldosterone antagonists than diuretics | ‐ | 139 (2) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3, 4 | |
| eGFR (mL/min/1.73 m²) |
The mean eGFR was 2 mL/min/1.73 m² higher with aldosterone antagonists (26.31 lower to 30.31 higher) than diuretics | ‐ | 12 (1) | ⊕⊕⊝⊝ LOW 4, 5 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKD: chronic kidney disease; CI: confidence interval; RR: risk ratio; AKI: acute kidney injury; eGFR: estimated glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Evidence quality was downgraded due to study risks of bias. Allocation concealment in no studies, blinding of outcome assessment in one study, complete data in one study
2 There was significant heterogeneity between the studies
3 Evidence quality was downgraded because proteinuria is a surrogate outcome for CKD progression
4 Treatment estimate had a wide confidence interval
5 Single study with unclear allocation concealment
Summary of findings 3. Aldosterone antagonists versus calcium channel blockers for proteinuric chronic kidney disease.
| Aldosterone antagonists versus calcium channel blocker for proteinuric CKD | |||||
| Patient or population: proteinuric CKD Intervention: aldosterone antagonist Comparison: calcium channel blocker | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with calcium channel blocker | Risk with aldosterone antagonist | ||||
| Kidney failure | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Hyperkalaemia | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Death | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Cardiovascular events | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Doubling serum creatinine | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| AKI | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Proteinuria | Data could not to be meta‐analysed | ‐ | 37 (1) | ⊕⊕⊝⊝ LOW 1, 2 | |
| eGFR (mL/min/1.73 m²) |
not reported | ‐‐ | ‐‐ | ‐‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CKD: chronic kidney disease; CI: Confidence interval; RR: Risk ratio; AKI: acute kidney injury; eGFR: estimated glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Single study with unclear allocation concealment
2 Evidence quality was downgraded because proteinuria is a surrogate outcome for CKD progression
Summary of findings 4. Aldosterone antagonists versus ACEi or ACEi plus ARB for proteinuric chronic kidney disease.
| Aldosterone antagonists versus ACEi or ACEi plus ARB for proteinuric chronic kidney disease | |||||
| Patient or population: proteinuric chronic kidney disease Intervention: aldosterone antagonist Comparison: ACEi or ACEi plus ARB | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with ACEi or ACEi plus ARB | Risk with aldosterone antagonist | ||||
| Kidney failure | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Hyperkalaemia | 37 per 1,000 | 60 per 1,000 (24 to 146) | RR 1.62 (0.66 to 3.95) | 500 (2) | ⊕⊕⊝⊝ LOW 1, 2 |
| Death | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Cardiovascular events | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Doubling serum creatinine | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| AKI | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Proteinuria | Data could not be meta‐analysed | ‐ | 465 (4) | ⊕⊝⊝⊝ VERY LOW 3, 4, 5 | |
| GFR (mL/min/1.73 m²) |
Data could not be meta‐analysed | ‐ | 18 (1) | ⊕⊕⊝⊝ LOW 6, 7 | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEi: angiotensin‐converting enzyme inhibitors; ARB: angiotensin receptor blocker; CI: confidence interval; RR: risk ratio; AKI: acute kidney injury; eGFR: estimated glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Evidence quality was downgraded due to risks of bias. Allocation concealment in no studies, blinding of outcome assessment in one study, complete outcome data in one study
2 Treatment estimates had wide confidence intervals
3 Evidence quality was downgraded due to risks of bias. Allocation concealment in no studies, blinding of outcome assessment in one study, complete outcome data in two studies
4 Evidence quality was downgraded as proteinuria is a surrogate outcome for CKD progression
5 Raw data was not available in studies to allow pooling of treatment estimates
6 Single study with unclear allocation concealment
7 Insufficient studies to inform precision
Summary of findings 5. Aldosterone antagonist versus nitrate for proteinuric chronic kidney disease.
| Aldosterone antagonist versus nitrate for proteinuric chronic kidney disease | |||||
| Patient or population: proteinuric chronic kidney disease Intervention: aldosterone antagonist Comparison: nitrate | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with nitrate | Risk with aldosterone antagonist | ||||
| Kidney failure | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Hyperkalaemia | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Death | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Cardiovascular events | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Doubling serum creatinine | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| AKI | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Proteinuria | Data could not be meta‐analysed | ‐ | 29 (1) | ⊕⊕⊝⊝ LOW 1, 2 | |
| eGFR | not reported | ‐‐ | ‐‐ | ‐‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; AKI: acute kidney injury; eGFR: estimated glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Single study with unclear allocation concealment and unclear blinding of outcome assessment
2 Evidence quality was downgraded as proteinuria is a surrogate outcome for CKD progression
Summary of findings 6. Non‐steroidal mineralocorticoid receptor antagonist versus selective aldosterone antagonist for proteinuric chronic kidney disease.
| Non‐steroidal mineralocorticoid receptor antagonist versus selective aldosterone antagonist for proteinuric chronic kidney disease | |||||
| Patient or population: proteinuric chronic kidney disease Intervention: non‐steroidal mineralocorticoid receptor antagonist Comparison: selective aldosterone antagonist | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with selective aldosterone antagonist | Risk with non‐steroidal mineralocorticoid receptor antagonist | ||||
| Kidney failure | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Hyperkalaemia | 47 per 1,000 | 42 per 1,000 (21 to 83) | RR 0.89 (0.45 to 1.77) | 1023 (1) | ⊕⊕⊝⊝ LOW 1, 2 |
| Death | 36 per 1,000 | 25 per 1,000 (11 to 56) | RR 0.70 (0.31 to 1.55) | 1055 (1) | ⊕⊕⊝⊝ LOW 1, 2 |
| Cardiovascular events | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Doubling serum creatinine | 1/834 | 0/221** | RR 0.80 (0.03 to 19.51) | 1055 (1) | ⊕⊕⊝⊝ LOW 1, 2 |
| AKI | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Proteinuria | not reported | ‐‐ | ‐‐ | ‐‐ | |
| eGFR | not reported | ‐‐ | ‐‐ | ‐‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the non‐steroidal mineralocorticoid receptor antagonist group CI: confidence interval; RR: risk ratio; AKI: acute kidney injury; eGFR: estimated glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Single study
2 Treatment estimate had a wide confidence interval
Aldosterone antagonists (selective or non‐selective) versus placebo or standard care
Kidney failure
In very low certainty evidence, aldosterone antagonists have uncertain effects on kidney failure (Analysis 1.1 (2 studies, 84 participants): RR 3.00, 95% CI 0.33 to 27.65; I² = 0%) (Figure 5) compared to placebo or standard care.
1.1. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 1: Kidney failure
5.

Effect of aldosterone antagonists versus placebo or standard care on kidney failure
Hyperkalaemia
In moderate certainty evidence, aldosterone antagonists probably increases risk of hyperkalaemia (Analysis 1.2 (17 studies, 3001 participants): RR 2.17, 95% CI 1.47 to 3.22; I² = 0%) (numbers needed to treat for an additional harmful outcome (NNTH) 41) (Figure 6) compared to placebo or standard care, regardless of whether aldosterone antagonists were combined with one ACEi or ARB (Analysis 1.3.1 (11 studies, 1828 participants): RR 2.05, 95% CI 1.28 to 3.28; I² = 0%), or combined with ACEi plus ARB (Analysis 1.3.2 (4 studies, 149 participants): RR 4.30, 95% CI 1.12 to 16.51; I² = 0%).
1.2. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 2: Hyperkalaemia
6.

Effect of aldosterone antagonists versus placebo or standard care on hyperkalaemia
1.3. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 3: Subgroup analysis: hyperkalaemia ‐ number of RAS inhibitors used
Death
In low certainty evidence, aldosterone antagonists have uncertain effects on death (any cause) (Analysis 1.5 (3 studies, 421 participants): RR 0.58, 95% CI 0.10 to 3.50; I² = 0%) compared to placebo or standard care.
1.5. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 5: Death
Cardiovascular events
In low certainty evidence, aldosterone antagonists have uncertain effects on cardiovascular events (Analysis 1.6 (3 studies, 1067 participants): RR 0.95, 95% CI 0.26 to 3.56; I² = 42%) compared to placebo or standard care.
1.6. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 6: Cardiovascular events
Mehdi 2009 reported one myocardial infarction in the aldosterone antagonist group. Meta‐analysis was not performed.
Aldosterone antagonists had uncertain effects on stroke compared to placebo or standard care (Analysis 1.8 (3 studies, 1233 participants): RR 0.65, 95% CI 0.12 to 3.44; I² = 11%).
1.8. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 8: Stroke
Proteinuria
In very low certainty evidence, aldosterone antagonists may reduce proteinuria (Analysis 1.9 (14 studies, 1193 participants): SMD ‐0.51, 95% CI ‐0.82 to ‐0.20; I² = 82%) (Figure 7) compared to placebo or standard care. There was significant heterogeneity.
1.9. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 9: Proteinuria
7.

Effect of aldosterone antagonists versus placebo or standard care on proteinuria.
Kidney function
Glomerular filtration rate
In low certainty evidence, aldosterone antagonists may reduce eGFR (Analysis 1.12 (13 studies, 1165 participants): MD ‐3.00 mL/min/1.73 m², 95% CI ‐5.51 to ‐0.49; I² = 0%) (Figure 8) compared to placebo or standard care.
1.12. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 12: eGFR [mL/min/1.73 m²]
8.

Effect of aldosterone antagonists versus placebo or standard care on GFR [mL/min/1.73 m²].
Doubling of serum creatinine
Two studies (ARTS‐DN 2015; Mehdi 2009) reported doubling of SCr (or equivalent eGFR decline ≥ 57%) with events only occurring in Mehdi 2009. Meta‐analysis was not performed.
Blood pressure
In very low certainty evidence, aldosterone antagonists may reduce systolic blood pressure (Analysis 1.16 (14 studies, 911 participants): MD ‐4.98 mmHg, 95% CI ‐8.22 to ‐1.75; I² = 87%) but had uncertain effects on diastolic blood pressure (Analysis 1.17 (13 studies, 875 participants): MD ‐1.04 mmHg, 95% CI ‐2.82 to 0.73; I² = 79%) compared to placebo or standard care. There was significant heterogeneity in both analyses.
1.16. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 16: Systolic BP
1.17. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 17: Diastolic BP
Serum potassium
In very low certainty evidence, aldosterone antagonists may increase serum potassium (Analysis 1.20 (17 studies, 1326 participants): MD 0.19 mEq/L, 95% CI 0.10 to 0.29; I² = 81%) compared to placebo or standard care. There was significant heterogeneity.
1.20. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 20: Serum potassium
Acute kidney injury
In moderate certainty evidence, aldosterone antagonists probably increases the risk of acute kidney injury (Analysis 1.22 (5 studies, 1446 participants): RR 1.94, 95% CI 0.99 to 3.79; I² = 0%) compared to placebo or standard care.
1.22. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 22: Acute kidney injury
Gynaecomastia
In moderate certainty evidence, aldosterone antagonists probably increases the risk of gynaecomastia (Analysis 1.23 (4 studies, 281 participants): RR 5.14, 95% CI 1.14 to 23.23; I² = 0%) compared to placebo or standard care.
1.23. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 23: Gynaecomastia
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria; regression from micro‐ to normoalbuminuria; falls; and fatigue.
Analysis of heterogeneity
Heterogeneity in the effects of spironolactone on proteinuria was explored through sub‐group analyses.
Baseline kidney disease
Aldosterone antagonists reduced proteinuria in diabetic kidney disease (Analysis 1.9.1 (7 studies, 572 participants): SMD ‐0.46, 95% CI ‐0.64 to ‐0.27; I² = 0%) but had unclear effects on non‐diabetic kidney disease (Analysis 1.9.2 (5 studies, 367 participants): SMD ‐0.68, 95% CI ‐1.57 to 0.21; I² = 93%) compared to placebo or standard care.
Aldosterone antagonists reduced systolic blood pressure to a greater extent in non‐diabetic kidney disease (Analysis 1.16.2 (5 studies, 367 participants): MD ‐3.35, 95% CI ‐5.06 to ‐1.65; I² = 0%) than diabetic kidney disease (Analysis 1.16.1 (5 studies, 228 participants): MD ‐1.07, 95% CI ‐1.82 to ‐0.32; I² = 0%) compared to placebo or standard care.
Aldosterone antagonists reduced diastolic blood pressure in non‐diabetic kidney disease (Analysis 1.17.2 (4 studies, 331 participants): MD ‐1.62, 95% CI ‐2.86 to ‐0.38; I² = 0%) but had unclear effects on diabetic kidney disease (Analysis 1.17.1 (5 studies, 249 participants): MD ‐1.06, 95% CI ‐1.80 to ‐0.31; I² = 25%) compared to placebo or standard care.
Aldosterone antagonists increased serum potassium in both diabetic kidney disease (Analysis 1.20.1 (9 studies, 664 participants): MD 0.21 mEq/L, 95% CI 0.14 to 0.28; I² = 0%) and non‐diabetic kidney disease (Analysis 1.20.2 (6 studies, 367 participants): MD 0.30 mEq/L, 95% CI 0.10 to 0.50; I² = 92%) compared to placebo or standard care.
Study duration
Aldosterone antagonists reduced proteinuria in studies reporting follow‐up of less than six months (Analysis 1.24.1 (9 studies, 822 participants): SMD ‐0.39, 95% CI ‐0.54 to ‐0.24; I² = 0%) but had unclear effects in studies reporting follow‐up of longer than six months (Analysis 1.24.2 (4 studies, 331 participants): SMD ‐0.59, 95% CI ‐1.68 to 0.50; I² = 95%) compared to placebo or standard care.
1.24. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 24: Subgroup analysis: proteinuria ‐ duration of follow‐up
Aldosterone antagonists reduced systolic blood pressure in both studies reporting follow‐up of less than six months (Analysis 1.25.1 (10 studies, 580 participants): MD ‐5.65, 95% CI ‐10.96 to ‐0.33; I² = 90%) and studies reporting follow‐up of longer than six months (Analysis 1.25.2 (4 studies, 331 participants): MD ‐3.62, 95% CI ‐6.09 to ‐1.15; I² = 15%).
1.25. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 25: Subgroup analysis: systolic BP ‐ duration of follow‐up
Aldosterone antagonists reduced diastolic blood pressure in studies reporting follow‐up longer than six months (Analysis 1.26.2 (4 studies, 331 participants): MD ‐1.62, 95% CI ‐2.86 to ‐0.38; I² = 0%) but had unclear effect in studies reporting follow‐up less than six months (Analysis 1.26.1 (9 studies, 553 participants): MD ‐0.98, 95% CI ‐3.71 to 1.75; I² = 83%).
1.26. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 26: Subgroup analysis: diastolic BP ‐ duration of follow‐up
Aldosterone antagonists increased serum potassium in both studies reporting follow up less than six months (Analysis 1.27.1 (12 studies, 954 participants): MD 0.16 mEq/L, 95% CI 0.10 to 0.22; I² = 23%) and studies reporting follow‐up longer than six months (Analysis 1.27.2 (4 studies, 331 participants): MD 0.35 mEq/L, 95% CI 0.04 to 0.65; I² = 93%).
1.27. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 27: Subgroup analysis: serum potassium ‐ duration of follow‐up
Aldosterone antagonist selectivity
A single study (Boesby 2013) reported the effect of the selective aldosterone antagonist eplerenone on proteinuria, systolic blood pressure, diastolic blood pressure and serum potassium. All other studies reported the effect of the non‐selective aldosterone antagonist spironolactone on these outcomes. Subgroup analysis was not performed.
Baseline proteinuria or albuminuria
Single studies specified baseline albuminuria > 100mg/g (Kato 2015), > 300mg/g (Rossing 2005), > 300mg/day (Schjoedt 2005) and 45 to 300 mg/day (Ito 2019a), and which reported on proteinuria and serum potassium. Subgroup analysis was not performed.
Single studies specified baseline proteinuria > 150mg/day (Horestani 2012), > 0.3g/day (Tylicki 2008), >1g/g (Bianchi 2006), and > 1.5g/day (Chrysostomou 2006), and which reported on proteinuria, systolic blood pressure, diastolic blood pressure, and serum potassium. All other studies specified baseline proteinuria > 0.5g/day or did not specific baseline proteinuria. Subgroup analysis was not performed.
Baseline kidney function
Single studies specified baseline eGFR 15 to 60 mL/min/1.73 m² (Boesby 2013), eGFR 25 to 50 mL/min/1.73 m² (Abolghasmi 2011), eGFR > 45 mL/min/1.73 m² (Tylicki 2008), and which reported on proteinuria, systolic blood pressure, diastolic blood pressure, and serum potassium. All other studies specified baseline eGFR > 30 mL/min/1.73 m² or did not specific baseline kidney function. Subgroup analysis was not performed.
Non‐selective aldosterone antagonists (spironolactone or canrenone) plus ACEi or ARB versus diuretics plus ACEi or ARB
Proteinuria
In very low certainty evidence, non‐selective aldosterone antagonists plus ACEi or ARB had an uncertain effect on proteinuria (Analysis 2.1 (2 studies, 139 participants): SMD ‐1.59, 95% CI ‐3.80 to 0.62; I² = 93%) compared to diuretics plus ACEi or ARB. There was significant heterogeneity.
2.1. Analysis.

Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 1: Proteinuria
Glomerular filtration rate
One study reported eGFR (Morales 2015) and one cross‐over study (Smolen 2006) did not report individual study periods. Meta‐analysis was not performed due to inability to combine study data.
Blood pressure
In very low certainty evidence, non‐selective aldosterone antagonists plus ACEi or ARB had an uncertain effect on systolic blood pressure (Analysis 2.8 (3 studies, 151 participants): MD ‐3.79, 95% CI ‐14.36 to 6.79; I² = 90%) and diastolic blood pressure (Analysis 2.7 (3 studies, 151 participants): MD ‐1.56, 95% CI ‐3.52 to 0.41; I² = 3%) compared to diuretics plus ACEi or ARB. There was significant heterogeneity in the analysis for systolic blood pressure.
2.8. Analysis.

Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 8: Systolic BP
2.7. Analysis.

Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 7: Diastolic BP
Serum potassium
In low certainty evidence, non‐selective aldosterone antagonists plus ACEi or ARB may increase serum potassium (Analysis 2.11 (2 studies, 121 participants): MD 0.31, 95% CI 0.17 to 0.45; I² = 0%) compared to diuretics plus ACEi or ARB.
2.11. Analysis.

Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 11: Serum potassium
Fatigue
Fogari 2014 reported no difference in fatigue between the two groups. Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; hyperkalaemia; death (any cause); cardiovascular events; doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria; acute kidney injury; gynaecomastia; and falls.
Non‐selective aldosterone antagonists (spironolactone) versus calcium channel blockers
Proteinuria
Takebayashi 2006 reported spironolactone reduced urinary albumin excretion but did not change in amlodipine group. Meta‐analysis was not performed.
Blood pressure
Takebayashi 2006reported no change in systolic or diastolic blood pressure between the two groups. Meta‐analysis was not performed.
Serum potassium
Takebayashi 2006 reported serum potassium was lower in the calcium channel blocker group compared to the spironolactone group. Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; hyperkalaemia; death (any cause); cardiovascular events; eGFR; doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria; blood pressure; acute kidney injury; gynaecomastia; fatigue; and falls.
Selective aldosterone antagonists (eplerenone) alone versus ACEi alone
Hyperkalaemia
One cross‐over study reported hyperkalaemia (Morales 2009). Meta‐analysis was not performed.
Proteinuria
One cross‐over study reported proteinuria (Morales 2009). Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; death (any cause); cardiovascular events; eGFR; doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria; blood pressure: serum potassium; acute kidney injury; gynaecomastia; fatigue; and falls.
Selective aldosterone antagonists (eplerenone) alone versus ACEi plus ARB
Hyperkalaemia
One cross‐over study reported hyperkalaemia (Morales 2009). Meta‐analysis was not performed.
Proteinuria
One cross‐over study reported proteinuria (Morales 2009). Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; death (any cause); cardiovascular events; eGFR; doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria; blood pressure: serum potassium; acute kidney injury; gynaecomastia; fatigue; and falls.
Selective aldosterone antagonists (eplerenone) plus ACEi or ARB (or both) versus ACEi or ARB (or both)
Hyperkalaemia
Selective aldosterone antagonists plus ACEi or ARB (or both) may increase the risk of hyperkalaemia (Analysis 6.1 (2 studies, 500 participants): RR 1.62, 95% CI 0.66 to 3.95; I2 = 0%; low certainty evidence).
6.1. Analysis.

Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 1: Hyperkalaemia
Proteinuria
Six studies reported proteinuria, however data could not be meta‐analysed (Boesby 2011; Cohen 2010; Epstein 2002; Epstein 2006; Haykal 2007; Tylicki 2012).
Blood pressure
Four studies reported blood pressure, however data could not be meta‐analysed (Cohen 2010; Epstein 2002; Epstein 2006; Haykal 2007).
Glomerular filtration rate
One cross‐over study (Tylicki 2012) reported no difference in eGFR between the two groups. Meta‐analysis was not performed.
Serum potassium
One cross‐over study (Tylicki 2012) reported no difference in serum potassium between the two groups. Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; death (any cause); cardiovascular events; doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria; acute kidney injury; gynaecomastia; fatigue; and falls.
Selective aldosterone antagonists (eplerenone) plus ACEi or ARB (or both) versus ACEi or ARB (or both) plus nitrate
Proteinuria
Cohen 2010 reported urine protein excretion was significantly reduced after four weeks of eplerenone while it increased in the comparator group. We could not conduct a meta‐analysis as additional data could not be obtained from the investigators.
Blood pressure
Cohen 2010 reported systolic blood pressure was reduced by 9.7 ± 6.4 mmHg in the eplerenone group and by 1.0 ± 5.4 mmHg in the ACEi/ARB plus isosorbide group at 4 weeks. No data were available about diastolic blood pressure. We could not conduct a meta‐analysis as additional data could not be obtained from the investigators.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; hyperkalaemia; death (any cause); cardiovascular events; eGFR; doubling of SCr; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria: serum potassium; acute kidney injury; gynaecomastia; fatigue; and falls.
Non‐steroidal mineralocorticoid antagonists (finerenone) versus selective aldosterone antagonist (eplerenone)
Hyperkalaemia
ARTS‐HF 2015 reported no difference in the risk of hyperkalaemia between the two groups. Meta‐analysis was not performed.
Death
ARTS‐HF 2015 reported no difference in the risk of death between the two groups. Meta‐analysis was not performed.
Glomerular filtration rate
ARTS‐HF 2015 reported no significant change in GFR from baseline in either group. Meta‐analysis was not performed.
Doubling of serum creatinine
ARTS‐HF 2015 reported no difference in the risk of doubling of SCr between the two groups. Meta‐analysis was not performed.
Blood pressure
ARTS‐HF 2015 reported no significant change in systolic blood pressure from baseline in either group. Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; cardiovascular events; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria: serum potassium; acute kidney injury; gynaecomastia; fatigue; and falls.
Non‐selective aldosterone antagonists (spironolactone) plus ACEi and ARB versus ACEi
Hyperkalaemia
Bianchi 2010 reported hyperkalaemia in 9/64 patients in the spironolactone plus ACEi and ARB group and 3/64 patients in the ACEi group. Meta‐analysis was not performed.
Proteinuria
Bianchi 2010 reported proteinuria was significantly lower in the spironolactone plus ACEi and ARB group compared to ACEi group. Meta‐analysis was not performed.
Glomerular filtration rate
Bianchi 2010 reported eGFR was lower in ACEi group compared to the spironolactone plus ACEi and ARB group. Meta‐analysis was not performed.
Blood pressure
Bianchi 2010 systolic and diastolic blood pressure was lower in the spironolactone plus ACEi and ARB group compared to the ACEi group. Meta‐analysis was not performed.
Serum potassium
Bianchi 2010 reported serum potassium was lower in the ACEi group compared to the spironolactone plus ACEi and ARB group. Meta‐analysis was not performed.
Gynaecomastia
Bianchi 2010 reported gynaecomastia in 9/64 patients in the spironolactone plus ACEi and ARB group and 0/64 patients in the ACEi group. Meta‐analysis was not performed.
Other outcomes
Data for the following outcomes were not extractable in a format required for inclusion in analyses or not reported in the available studies: kidney failure; death; cardiovascular events; progression from micro‐ to macroalbuminuria; regression from macro‐ to microalbuminuria and regression from micro‐ to normoalbuminuria: acute kidney injury; fatigue; and falls.
Publication bias
Overall, there were sufficient data and lack of statistical heterogeneity for the outcomes eGFR and hyperkalaemia for the comparison of aldosterone antagonists and placebo or standard care. There was no evidence of small study effects in the analysis of eGFR (Figure 9) or hyperkalaemia (Figure 10).
9.

Funnel plot of comparison studies comparing aldosterone antagonist versus control for the study endpoint of GFR [mL/min/1.73 m²].
10.

Funnel plot of comparison studies comparing aldosterone antagonist versus control for the study endpoint of hyperkalaemia.
Discussion
Summary of main results
In this review of the evidence for aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of CKD, 44 studies involving 5637 participants were available. Studies included follow‐up for generally three to 12 months. Compared to ACEI or ARB (or both), the addition of an aldosterone antagonist has uncertain effects on progression to kidney failure, major cardiovascular events, and death (any cause) while probably doubling the risk of hyperkalaemia and probably increasing risk of acute kidney injury and gynaecomastia in adults who have proteinuric CKD stages 1 to 4. Aldosterone blockade may reduce proteinuria and kidney function in addition to co‐intervention with ACEi or ARB over a median treatment duration of 3.5 months and may lower systolic blood pressure but had little or no effect on diastolic blood pressure. Aldosterone antagonists appeared to lower systolic pressure to a greater extent in non‐diabetic kidney disease compared to diabetic kidney disease. While aldosterone antagonists appeared to lower proteinuria in diabetic kidney disease, its anti‐proteinuric effect in non‐diabetic kidney disease is less certain. Compared to diuretics, non‐selective aldosterone antagonists (spironolactone or canrenone) had uncertain effects on proteinuria and systolic blood pressure but may increase serum potassium. Furthermore, data comparing aldosterone antagonists to calcium channel blockers or nitrates, and data for treatment effects of selective aldosterone antagonists (eplerenone) and non‐steroidal mineralocorticoid antagonists (finerenone) were sparse leading to serious imprecision in treatment estimates or a lack of sufficient data for meta‐analysis.
Overall completeness and applicability of evidence
This review examined the evidence, updated to 2020, for the benefits of aldosterone antagonists in proteinuric CKD focusing on patient‐centred outcomes (including kidney failure, death (any cause), and major cardiovascular events) and potential harms (including hyperkalaemia, acute kidney injury, and gynaecomastia). Similar to the previous versions of this review in 2009 and 2014, evidence for aldosterone antagonists in preventing the progression of CKD has allowed primarily the evaluation of surrogate outcomes such as proteinuria and blood pressure rather than patient‐centred outcomes. The Standardised Outcomes in Nephrology (SONG) initiative aims to establish a set of core outcomes based on the shared priorities of patients, caregivers, clinicians, researchers, and policy makers. The SONG‐Glomerular Disease core outcomes are being established and will be essential in standardising outcome reporting in future studies (SONG‐GD 2019). The clinical relevance of proteinuria to predict progression of CKD has been shown in multiple studies where reduction in proteinuria was associated with reduced decline in eGFR and kidney failure for both non‐diabetic kidney disease (AASK 2002; REIN 1998) and diabetic kidney disease (IDNT 2004; RENAAL 2001). However, it is ultimately a surrogate outcome and it remains uncertain whether reduction in proteinuria with aldosterone antagonists in addition to ACEi or ARB reduces risk of kidney failure, death (any cause), and major cardiovascular events. Furthermore, reported measures of proteinuria in the included studies were heterogeneous necessitating the use of SMD, which is difficult to apply clinically since it summarises the intervention effect in each study relative to the standard deviation observed in each study. The use of SMD also assumes differences in standard deviations amongst the studies reflect differences in the measurement scales of proteinuria rather than differences in the study populations, which may not be true due to differences in severity of CKD, cause of CKD and co‐interventions between studies. Caution should also be advised since aldosterone antagonists may increase risk of hyperkalaemia, and probably increased risks of acute kidney injury and gynaecomastia. The risk of hyperkalaemia reported in the included studies may be influenced by the dose of spironolactone used, mostly 25 mg/day or higher with no studies evaluating lower dose spironolactone (e.g. 12.5 mg/day), which could reduce proteinuria and minimise risk of hyperkalaemia in the CKD population.
Study duration was mostly between three to 12 months, which limits the ability of the current evidence to inform clinical practice on hard endpoints such as kidney failure, death (any cause) and major cardiovascular events, and on long‐term safety. Few studies specified baseline kidney function and proteinuria; therefore, it is unclear whether aldosterone antagonists had different efficacy and safety based on the severity of underlying kidney disease or proteinuria. A recent phase II RCT showed spironolactone did not increase risk of hyperkalaemia in maintenance haemodialysis patients compared to placebo until dosages of 50 mg/day or higher (SPin‐D 2019). However, no effect on diastolic function assessed by Doppler echocardiography was detected in this small study (SPin‐D 2019) and further phase III studies are needed to evaluate the safety and efficacy of aldosterone antagonists in kidney failure.
Quality of the evidence
The evidence identified in this review for the primary efficacy and safety outcomes (kidney failure and hyperkalaemia) was of very low to low certainty. This evidence for the effect of aldosterone antagonists on preventing kidney failure was downgraded primarily due to methodological limitations in the included studies and serious imprecision in estimated treatment effects. The evidence for the increased risk of hyperkalaemia due to aldosterone antagonists in CKD was downgraded primarily due to study limitations.
Most studies enrolled few patients and were powered to observe differences in surrogate end points rather than patient‐focused outcomes. Ten studies had a cross‐over design (Boesby 2011; Morales 2009; Morales 2015; Nielsen 2012; Rossing 2005; Saklayen 2008; Schjoedt 2005; Smolen 2006; Tylicki 2008, Tylicki 2012), none of which reported on endpoints at the completion of each study phase to enable inclusion into the meta‐analysis. The majority of studies did not adequately report study methods, such as the methods of allocation concealment and blinding of outcome assessment, sufficient to assess study quality. For most studies, the study protocol was not available to assess selective reporting of outcomes. Of the 15 studies reporting systolic blood pressure in the comparison between aldosterone antagonists and placebo or standard care, only six studies defined the method of measuring blood pressure (Boesby 2013; CRIBS II 2009; Furumatsu 2008; Guney 2009; Saklayen 2008; Tylicki 2008). Of the five studies reporting acute kidney injury, only one study specified a definition (ARTS 2012). Of the four studies reporting gynaecomastia, none provided a definition.
Potential biases in the review process
Our review has a number of strengths and weaknesses. The review examined aldosterone antagonists for their benefits on patient‐centred outcomes and potential harms, and was conducted with a peer‐reviewed protocol, a systematic search of electronic databases including the Cochrane Kidney and Transplant Specialised Register of studies, data extraction and analysis and study quality assessment by two independent authors, and adjudication of evidence certainty using the GRADE process. The key limitation of the review is the data provided by available studies. First, long‐term data for the effects of aldosterone antagonists on major patient‐centred outcomes including death, kidney failure, and major cardiovascular events were absent or sparse, with most studies primarily focused on surrogate outcomes such as proteinuria. In the cross‐over studies and many other RCTs, outcomes were not reported in ways that could be extracted, which lowered confidence in the results. Whilst authors of all studies were contacted, most no longer had access to the raw data. Second, the duration of follow‐up in most studies was likely sub‐optimal for detecting these hard endpoints. The 10‐year risk of kidney failure in 50 year old persons with an eGFR of 30 to 44 mL/min/1.73 m² is 11% to 21% depending on gender and only 1% to 2% with eGFR 45 to 59 mL/min/1.73 m² (Turin 2012), whilst the 25‐year risk of kidney failure in persons with an eGFR less than 60 mL/min/1.73 m² and 2+ proteinuria or higher on urinalysis is 41% compared to 6% for 1+ proteinuria and 4% in the absence of proteinuria (Ishani 2006). Overall, in our systematic review of persons with CKD and variable levels of proteinuria, the median follow‐up of three months in the included studies is likely sub‐optimal for detecting the development of kidney failure. Third, data on stage 5 CKD were not available in the included studies. Finally, there was a significant heterogeneity between studies in treatment effects on proteinuria. Although we explored causes of heterogeneity by type of kidney disease and study duration, insufficient data were available for testing the effect of baseline kidney function and proteinuria. Treatment duration and other factors may therefore modify the treatment effects we observed.
Agreements and disagreements with other studies or reviews
In this systematic review, aldosterone antagonist therapy in addition to ACEi or ARB therapy was found to reduce proteinuria in participants with stage 1‐4 proteinuric CKD, increase risk of hyperkalaemia and decline in eGFR, but had no detectable effect on kidney failure, cardiovascular events, and death, which is consistent with existing literature. One previous systematic review of aldosterone antagonists in both diabetic and non‐diabetic CKD found a reduction in proteinuria, eGFR, systolic and diastolic blood pressure, and an increased risk of hyperkalaemia (Currie 2016). Similar to our review, Currie 2016l could not conclude the effect of aldosterone antagonists on kidney failure, cardiovascular events and death, due to underreporting of these outcomes in included studies. However, apart from hyperkalaemia, other potential harms of aldosterone antagonists such as acute kidney injury and gynaecomastia were not explored. Furthermore, our review found the effect of aldosterone antagonists on diastolic blood pressure were uncertain. The discordance between the current Cochrane review and Currie 2016 is possibly explained by the inclusion of more recent trials in our review. Four other previous systematic reviews of aldosterone antagonists in diabetic CKD reported a reduction in proteinuria, and systolic and diastolic blood pressure, an increased risk of hyperkalaemia, and a reduction or unclear effect on eGFR (Hou 2015; Mavrakanas 2014; Sun 2017; Takahashi 2016). One review (Takahashi 2016) performed meta‐analyses on blood pressure and serum potassium but not on the kidney outcomes such as proteinuria and eGFR, despite an adequate number of included studies, decreasing the strengths of its conclusions due to potential selective reporting bias. One review (Mavrakanas 2014) did not perform a meta‐analysis due to the heterogeneous reporting of outcomes in the limited number of included studies, and did not specify risk of bias assessment by two independent authors, limiting the strength of its conclusions. None of the four reviews on diabetic CKD examined patient‐centred efficacy outcomes such as kidney failure, major cardiovascular events, or death.
The results of our review also agree with existing RCTs and review articles showing an increased risk of hyperkalaemia when spironolactone is combined with ACEi or ARB (or both) in individuals with heart failure (Phillips 2007; RALES 1995). There is good evidence that ACEi or ARBs reduce progression of CKD to kidney failure requiring dialysis or transplantation by approximately 20% and have cardioprotective effects for adults with diabetic or non‐diabetic CKD (RENAAL 2004; Strippoli 2005; Strippoli 2006). Some patients treated with ACEi with or without ARB exhibit aldosterone escape (Staessen 1981) and the addition of aldosterone antagonists has been shown to have anti‐fibrotic and antihypertensive effects in animal and human studies (Nakhoul 2008; Tylicki 2008). However, our review was unable to conclude whether aldosterone antagonists in addition to ACEi or ARB (or both) had any effect on risk of kidney failure, major cardiovascular events, or death.
Authors' conclusions
Implications for practice.
In adults with CKD who have an eGFR between 15 and 90 mL/min/1.73 m² and who have persistent proteinuria despite being on maximal doses of ACEi or ARB, aldosterone antagonists may reduce proteinuria, eGFR and systolic blood pressure. However, treatment effects on patient‐relevant outcomes including progression to kidney failure, major cardiovascular events, and death are uncertain. Treatment using aldosterone antagonists in combination with ACEi or ARB (or both) may increase risk of hyperkalaemia and probably increases risk of acute kidney injury. Treatment using spironolactone probably increases risk of gynaecomastia. Evidence for the efficacy and safety of aldosterone antagonists compared to other interventions (diuretics, calcium channel blockers, nitrates, or ACEI or ARB (or both)) and evidence for the relative efficacy between different aldosterone antagonists are sparse. Patients and clinicians may reasonably choose not to use an aldosterone antagonist due to the uncertain benefits of treatment and identified risks of harm and adverse events.
Implications for research.
Existing evidence on the effect of aldosterone antagonists on progression of CKD, cardiovascular events and death are uncertain. Whilst there is strong evidence supporting the association between reduction of proteinuria with ACEi or ARB and reduced risk of kidney failure, data supporting the benefit of further reduction in proteinuria using aldosterone antagonists in addition to ACEi or ARB on risk of kidney failure are lacking. Clinical evidence for the cardioprotective benefits of aldosterone antagonists in addition to ACEi or ARB is also sparse. Therefore, future high‐quality studies with adequate follow‐up that are powered to detect differences kidney failure and major cardiovascular events are necessary. The concurrent use of potassium‐binding agents such as patiromer may reduce the risk of hyperkalaemia associated with aldosterone antagonists (AMBER 2019) though whether this will lead to improvements in long‐term patient‐level outcomes requires further study. Two ongoing studies of finerenone (FIDELIO‐DKD 2019; FIGARO‐DKD 2019) will provide important insights into whether non‐steroidal mineralocorticoid antagonists in addition to ACEi or ARB may reduce the risk of patient‐level cardiovascular and kidney endpoints without increasing the risk of hyperkalaemia.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2020 | New citation required but conclusions have not changed | New studies added; no change to conclusions |
| 13 January 2020 | New search has been performed | New search update |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 28 April 2014 | New citation required and conclusions have changed | 10 new studies added, new comparisons added |
| 30 January 2013 | New search has been performed | New update search completed, new studies identified |
| 22 February 2012 | Amended | Update search completed |
| 20 February 2012 | Amended | Search methods & search strategies updated |
| 2 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Sagar Nigwekar and Ashwini Sehgal who contributed to the design, quality assessment, data collection, entry, analysis and interpretation, and writing of the first version (Navaneethan 2009) of this review.
The authors are grateful to the following peer reviewers for their time and comments: Ionut Nistor (Nephrology Department, "Grigore T. Popa" University of Medicine and Pharmacy, Iasi, Romania), Dr Pauline A Swift (Nephrologist, Epsom & St. Helier University Hospitals NHS Trust, UK). We thank Narelle Willis of Cochrane kidney and Transplant, for her help in co‐ordinating and editing this review and Ruth Mitchell and Gail Higgins of Cochrane Kidney and Transplant for assistance in the development of search strategies. We also thank Drs KJ Schjoedt, K Rossing, A Chrysostomou, S Bianchi, S Nielsen, and K Takebayashi for providing additional details about their studies which were included in this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Aldosterone antagonist versus placebo/standard care (all studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Kidney failure | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.33, 27.65] |
| 1.1.1 Diabetes | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.53] |
| 1.1.2 No diabetes | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 1.2 Hyperkalaemia | 17 | 3001 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.47, 3.22] |
| 1.2.1 Diabetes | 10 | 2122 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [1.20, 2.91] |
| 1.2.2 No diabetes | 6 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 3.43 [1.35, 8.72] |
| 1.2.3 Diabetes not reported | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 6.14 [0.82, 46.20] |
| 1.3 Subgroup analysis: hyperkalaemia ‐ number of RAS inhibitors used | 13 | 1977 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.42, 3.46] |
| 1.3.1 Aldosterone antagonist plus 1 RAS inhibitor | 11 | 1828 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.28, 3.28] |
| 1.3.2 Aldosterone antagonist plus 2 RAS inhibitors | 4 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [1.12, 16.51] |
| 1.4 Hyperkalaemia data from cross‐over studies | 1 | Other data | No numeric data | |
| 1.5 Death | 3 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.50] |
| 1.5.1 Diabetes | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.46] |
| 1.5.2 No diabetes | 1 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.12, 68.60] |
| 1.6 Cardiovascular events | 3 | 1067 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.33, 3.99] |
| 1.6.1 Diabetes | 2 | 875 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [0.57, 8.55] |
| 1.6.2 No diabetes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.3 Diabetes not reported | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.11, 2.47] |
| 1.7 Myocardial infarction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Diabetes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 No diabetes | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Stroke | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 Diabetes | 3 | 1233 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.12, 3.44] |
| 1.8.2 No diabetes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Proteinuria | 14 | 1193 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.82, ‐0.20] |
| 1.9.1 Diabetes | 7 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.64, ‐0.27] |
| 1.9.2 No diabetes | 5 | 367 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.57, 0.21] |
| 1.9.3 Diabetes not reported | 2 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.49, 0.01] |
| 1.10 Proteinuria: descriptive outcome data | 13 | Other data | No numeric data | |
| 1.11 Proteinuria data from cross‐over studies | 2 | Other data | No numeric data | |
| 1.12 eGFR [mL/min/1.73 m²] | 13 | 1165 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐5.51, ‐0.49] |
| 1.12.1 Diabetes | 8 | 610 | Mean Difference (IV, Random, 95% CI) | ‐4.43 [‐8.35, ‐0.51] |
| 1.12.2 No diabetes | 3 | 301 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐8.69, 4.18] |
| 1.12.3 Diabetes not reported | 2 | 254 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐5.85, 4.95] |
| 1.13 eGFR: descriptive outcome data | 6 | Other data | No numeric data | |
| 1.14 eGFR data from cross‐over studies | 1 | Other data | No numeric data | |
| 1.15 Doubling serum creatinine | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.1 Diabetes | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.2 No diabetes | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.16 Systolic BP | 14 | 911 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐8.22, ‐1.75] |
| 1.16.1 Diabetes | 6 | 249 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.80, ‐0.31] |
| 1.16.2 No diabetes | 5 | 367 | Mean Difference (IV, Random, 95% CI) | ‐3.35 [‐5.06, ‐1.65] |
| 1.16.3 Diabetes not reported | 3 | 295 | Mean Difference (IV, Random, 95% CI) | ‐12.07 [‐29.27, 5.12] |
| 1.17 Diastolic BP | 13 | 875 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.82, 0.73] |
| 1.17.1 Diabetes | 6 | 249 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.95, 1.44] |
| 1.17.2 No diabetes | 4 | 331 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.86, ‐0.38] |
| 1.17.3 Diabetes not reported | 3 | 295 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐10.75, 7.71] |
| 1.18 Blood pressure: descriptive outcome data | 9 | Other data | No numeric data | |
| 1.19 Blood pressure data from cross‐over studies | 2 | Other data | No numeric data | |
| 1.20 Serum potassium | 17 | 1326 | Mean Difference (IV, Random, 95% CI) | 0.19 [0.10, 0.29] |
| 1.20.1 Diabetes | 9 | 664 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.14, 0.28] |
| 1.20.2 No diabetes | 5 | 367 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.10, 0.50] |
| 1.20.3 Diabetes not reported | 3 | 295 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.21, 0.15] |
| 1.21 Potassium: descriptive outcome data | 6 | Other data | No numeric data | |
| 1.22 Acute kidney injury | 5 | 1446 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.99, 3.79] |
| 1.22.1 Diabetes | 2 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.26, 3.69] |
| 1.22.2 No diabetes | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 67.06] |
| 1.22.3 Diabetes not reported | 2 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.08, 5.39] |
| 1.23 Gynaecomastia | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 5.14 [1.14, 23.23] |
| 1.23.1 Diabetes | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 70.53] |
| 1.23.2 No diabetes | 3 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 6.02 [1.08, 33.57] |
| 1.24 Subgroup analysis: proteinuria ‐ duration of follow‐up | 13 | 1153 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.83, ‐0.17] |
| 1.24.1 Less than 6 months | 9 | 822 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.54, ‐0.24] |
| 1.24.2 At least 6 months | 4 | 331 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.68, 0.50] |
| 1.25 Subgroup analysis: systolic BP ‐ duration of follow‐up | 14 | 911 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐8.22, ‐1.75] |
| 1.25.1 Less than 6 months | 10 | 580 | Mean Difference (IV, Random, 95% CI) | ‐5.65 [‐10.96, ‐0.33] |
| 1.25.2 At least 6 months | 4 | 331 | Mean Difference (IV, Random, 95% CI) | ‐3.62 [‐6.09, ‐1.15] |
| 1.26 Subgroup analysis: diastolic BP ‐ duration of follow‐up | 13 | 884 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.81, 0.73] |
| 1.26.1 Less than 6 months | 9 | 553 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐3.71, 1.75] |
| 1.26.2 At least 6 months | 4 | 331 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.86, ‐0.38] |
| 1.27 Subgroup analysis: serum potassium ‐ duration of follow‐up | 16 | 1285 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.13, 0.31] |
| 1.27.1 Less than 6 months | 12 | 954 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.10, 0.22] |
| 1.27.2 At least 6 months | 4 | 331 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.04, 0.65] |
1.4. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 4: Hyperkalaemia data from cross‐over studies
| Hyperkalaemia data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Nielsen 2012 | Spironolactone versus placebo | Two patients had severe hyperkalaemia (plasma potassium = 5.7 mmol/L). Four patients experienced light to moderate hyperkalaemia (plasma potassium = 5.0 to 5.4 mmol/L). Hyperkalaemia was mainly observed 2 weeks after the start of spironolactone treatment |
1.7. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 7: Myocardial infarction
1.10. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 10: Proteinuria: descriptive outcome data
| Proteinuria: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| ARTS 2012 | Finerenone or spironolactone versus placebo | Mean UACR decreased in all BAY 94‐8862 dose groups (geometric mean ratio versus baseline UACR 0.77 for 2.5 mg/day, 0.69 for 5 mg/day, 0.72 for 10 mg/day and 0.86 for 5 mg twice/day) and in the spironolactone group (geometric mean ratio versus baseline UACR 0.61), compared with a small increase in the placebo group (geometric mean ratio versus baseline UACR 1.04). No P value or CI reported |
| ARTS‐DN 2015 | Finerenone plus ACEi or ARB versus ACEi or ARB alone | The mean placebo‐corrected ratios of UACR at day 90 versus baseline in the finerenone 7.5, 10, 15, and 20 mg/day groups were 0.79 (90% CI, 0.68 to 0.91; P = .004), 0.76 (90% CI, 0.65 to 0.88; P = 0.001), 0.67 (90% CI, 0.58 to 0.77; P < 0.001), and 0.62 (90% CI, 0.54 to 0.72; P < 0.001), respectively |
| Chen 2018b | Spironolactone plus irbesartan (low or high dose) versus irbesartan (low or high dose) | At 72 weeks, UAER significant decreased in the spironolactone + high dose irbesartan group (‐30 μg/min) and spironolactone + low dose irbesartan group (‐23 μg/min) compared to low dose irbesartan group (‐15 μg/min) (P < 0.05). However, UAER was significantly reduced in high dose irbesartan (‐30 μg/min) compared to spironolactone + low dose irbesartan (‐23 μg/min) (P < 0.05) |
| Cohen 2010 | Eplerenone plus ACEi or ARB versus ACEi plus ARB | Urine protein excretion was reduced by 1.04 ± 0.4 g/24 h in the eplerenone and by 0.32 ± 0.2 g/24 h in the ACEi plus ARB group |
| Epstein 2002 | Eplerenone plus ACEi versus ACEi | UAE was reduced by 74% in the eplerenone and by 45% in the control group |
| Epstein 2006 | Eplerenone plus ACEi versus ACEi | Eplerenone treatment significantly reduced albuminuria from baseline as early as week 4 and continued throughout weeks 8 and 12. ACEi treatment did not result in any significant decrease from baseline in albuminuria |
| EVALUATE 2010 | Eplerenone plus ACEi and/or ARB versus ACEi and/or ARB | UACR reduced significantly more with eplerenone (‐17.3 mg/g) than placebo (+10.3 mg/g) (P = 0.0222) |
| Haykal 2007 | Eplerenone plus ACEi versus ACEi | Eplerenone treatment reduced proteinuria after 4 weeks. The effect continued throughout weeks 8 and 12 (P < 0.001) |
| Kato 2015 | Spironolactone plus ACEi or ARB versus ACEi or ARB | At week 8, spironolactone reduced proteinuria compared to control (UACR ‐519.7 ± 129.4 mg/g) (P = 0.001) |
| Koroshi 2010 | Spironolactone + ACEi versus ACEi | In comparison with the placebo group, proteinuria decreased by 42.3% (95% CI, P = 0.004) in the group assigned to spironolactone |
| Lv 2009a | Spironolactone + ACEi or ARB versus ACEi or ARB alone | After 9 months therapy, proteinuria decreased significantly (1.25 ± 0.61 g/day at baseline, 0.85 ± 0.56 g/day at the 3rd month, 0.81 ± 0.61 g/day at the 6th month, and 0.64 ± 0.42 g/day, at the 9th month, P < 0.05) in patients treated with spironolactone, while it didn't change in control group |
| Mehdi 2009 | Spironolactone + ACEi versus ARB | During the 48 weeks of treatment, albuminuria (UACR) decreased significantly from baseline in the ARB (P = 0.001) and spironolactone (P < 0.0001) groups but not in the placebo group (P = 0.08). At 48 weeks, the percentage change from the baseline was 24.6% (95% CI 54.8% to 25.9%) in those assigned to placebo, 38.2% (95% CI 59.3% to 5.9%) in those assigned to ARB, and 51.6% (95% CI 70.2% to 21.4%) in those assigned to spironolactone |
| Tokunaga 2008a | Spironolactone + ARB versus ARB alone | Spironolactone reduced proteinuria from 1.70 ± 1.12 g/g to 1.11 ± 1.13 g/g Cr (P < 0.05) |
1.11. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 11: Proteinuria data from cross‐over studies
| Proteinuria data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Boesby 2011 | Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) | Albuminuria was significantly lower during the add‐on eplerenone period as compared with the control period with a 22% (95% CI 14 to 28, P < 0.001), lower excretion. The mean 24 hour excretion was 1481 mg (95% CI 1192 to 1840) during the control period and 1163 mg (95% CI 921 to 1468) during add‐on eplerenone. No significant carry‐over, P = 0.3 or time effect, P = 0.3, was detected for the UAE |
| Nielsen 2012 | Spironolactone versus placebo | During spironolactone treatment, urinary albumin excretion was reduced by 60% (21% to 80%) from 90 mg/24 h to 35 mg/24 h when compared with placebo (P = 0.01) |
1.13. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 13: eGFR: descriptive outcome data
| eGFR: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| ARTS 2012 | Finerenone or spironolactone versus placebo | There was a decrease in eGFR in all finerenone groups (mean change from baseline eGFR ranging ‐0.85 to ‐2.69 mL/min/1.73 m2) and the spironolactone group (‐6.70 mL/min/1.73 m2), compared with a small increase in the placebo group (0.87 mL/min/1.73 m2). However, the decrease in the spironolactone group was significantly greater than in all finerenone groups (P = 0.0002 to 0.0133) |
| ARTS‐DN 2015 | Finerenone plus ACEi or ARB versus ACEi or ARB alone | There was absolute decrease in eGFR in all finerenone groups (mean change from baseline eGFR ranging ‐1.9 to ‐3.9 mL/min/1.73 m2) as well as placebo (‐1.5 mL/min/1.73 m2). No P value was reported |
| Chen 2018b | Spironolactone plus irbesartan (low or high dose) versus irbesartan (low or high dose) | At 72 weeks, eGFR was lower in the spironolactone + high dose irbesartan group (‐3.8 mL/min/1.73 m2) compared to low dose irbesartan (‐0.3 mL/min/1.73 m2) and high dose irbesartan group (‐1.5 mL/min/1.73 m2) (P < 0.05), but not significantly different in the spironolactone + low dose irbesartan group (‐0.6 mL/min/1.73 m2) |
| EVALUATE 2010 | Eplerenone plus ACEi and/or ARB versus ACEi and/or ARB | eGFR was significantly lower with eplerenone (‐4.6 mL/min/1.73 m2) than placebo (+0.47 mL/min/1.73 m2) (P = 0.0041) |
| Kato 2015 | Spironolactone plus ACEi or ARB versus ACEi or ARB | At week 8, there was no difference in eGFR between spironolactone and control (‐3.2 ± 9.7 mL/min/1.73 m2) (P = 0.052) |
| Lv 2009a | Spironolactone + ACEi or ARB versus ACEi or ARB alone | By the end of the 9th month, the monthly rate of decrease of eGFR was similar in the two groups (‐0.66 mL/min/1.73 m2 in spironolactone group versus ‐0.94 mL/min/1.73 m2 in control group, P = 0.28) |
1.14. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 14: eGFR data from cross‐over studies
| eGFR data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Nielsen 2012 | Spironolactone versus placebo | Significant decline in GFR from 78 mL/min/1.73 m2 to 72 mL/min/1.73 m2 (P = 0.003) during spironolactone treatment |
1.15. Analysis.

Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 15: Doubling serum creatinine
1.18. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 18: Blood pressure: descriptive outcome data
| Blood pressure: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| ARTS 2012 | Finerenone or spironolactone versus placebo | Spironolactone significantly decreased systolic BP (‐10.1 mmHg) at day 29 ± 2 compared with either placebo (‐3.1 mmHg, P = 0.0104) or all doses of finerenone (range ‐1.9 to ‐4.2 mmHg, P = 0.0023 to 0.0255) |
| ARTS‐DN 2015 | Finerenone plus ACEi or ARB versus ACEi or ARB alone | Systolic BP from baseline to day 90 decreased in all finerenone groups (placebo corrected least squares mean differences range from −2.8 to −5.1 mmHg). Decreases only significant in 15 mg daily and 20 mg daily groups |
| Chen 2018b | Spironolactone plus irbesartan (low or high dose) versus irbesartan (low or high dose) | At 72 weeks, there was no difference between systolic of diastolic BP between the spironolactone + high dose irbesartan group (change in systolic BP ‐24 mmHg, diastolic BP ‐14 mmHg) or spironolactone + low dose irbesartan (change in systolic BP ‐24 mmHg, diastolic BP ‐13 mmHg), compared to low dose irbesartan (change in systolic BP ‐23 mmHg, diastolic BP ‐13 mmHg) and high dose irbesartan group (change in systolic BP ‐24 mmHg, diastolic BP ‐14 mmHg) |
| Cohen 2010 | Eplerenone plus ACEi or ARB versus ACEi plus ARB | Systolic BP was reduced by 9.7 ± 6.4 mmHg in the eplerenone group and by 13.4 ± 14.9 mmHg in the ACEi plus ARB group |
| Epstein 2002 | Eplerenone plus ACEi versus ACEi | Systolic BP was reduced by 21.8% and 20.4% in the eplerenone and control group respectively. Diastolic BP was reduced by 16.2% and 15% in the eplerenone and control group respectively |
| Epstein 2006 | Eplerenone plus ACEi versus ACEi | Both systolic and diastolic BP decreased at weeks 4, 8, and 12 in eplerenone and control groups. There were no significant differences in BP reduction between groups |
| EVALUATE 2010 | Eplerenone plus ACEi and/or ARB versus ACEi and/or ARB | Systolic BP was lower with eplerenone (128.8 mmHg) than placebo (132.2 mmHg) (P = 0.0035). Diastolic BP was also lower with eplerenone (76.7 mmHg) than placebo (78.4 mmHg) (P < 0.0370) |
| Haykal 2007 | Eplerenone plus ACEi versus ACEi | Both systolic and diastolic BP decreased in the two groups at weeks 4, 8 and 12 (P < 0.001). BP reduction was slightly higher in eplerenone group |
| Kato 2015 | Spironolactone plus ACEi or ARB versus ACEi or ARB | At week 8, there was no difference in blood pressure between spironolactone (systolic BP ‐2.68 ± 25.3 mmHg, diastolic BP ‐3.44 ± 14.3 mmHg) and control (no change but exact values not reported) (P value not reported) |
1.19. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 19: Blood pressure data from cross‐over studies
| Blood pressure data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Boesby 2011 | Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) alone | Systolic and diastolic BP was significantly lower during add‐on eplerenone treatment when compared to the control period. There was a significant reduction of systolic BP after 2 weeks of eplerenone treatment (P = 0.003). The diastolic BP was significantly reduced after 4 weeks of eplerenone treatment (P = 0.002), and there was a significant difference in diastolic BP between the treatment period and control period at the same time point (P = 0.004).There were no significant differences between diastolic BP at the end of the 2 periods. There were no significant carry‐over, P = 0.4 and P = 0.9, or time effects, P = 0.5 and P = 0.2 for systolic BP or diastolic BP |
| Nielsen 2012 | Spironolactone versus placebo | No significant changes in diastolic and systolic BP after the placebo or spironolactone period |
1.21. Analysis.
Comparison 1: Aldosterone antagonist versus placebo/standard care (all studies), Outcome 21: Potassium: descriptive outcome data
| Potassium: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| ARTS 2012 | Finerenone or spironolactone versus placebo | By day 29 ± 2, finerenone 10 mg/day and 5 mg twice/day showed significantly greater mean increases in serum potassium concentration from baseline than placebo
(P = 0.02 and P = 0.0003, respectively) Finerenone: 5mg/day and 2.5 mg/day groups were not significantly different from placebo |
| ARTS‐DN 2015 | Finerenone plus ACEi or ARB versus ACEi or ARB alone | Potassium non‐significantly increased in all finerenone groups (absolute mean change from baseline to day 90 range 0.07 to 0.23) and non‐significantly decreased with placebo (‐0.004) |
| Chen 2018b | Spironolactone plus irbesartan (low or high dose) versus irbesartan (low or high dose) | At 72 weeks, potassium was higher in the spironolactone + high dose irbesartan group (+ 0.5 mmol/L) compared to low dose irbesartan (+ 0.11 mmol/L) and high dose irbesartan group (+ 0.29 mmol/L) (P < 0.05). Potassium was higher in the spironolactone + low dose irbesartan group (+ 0.27 mmol/L) compared to low dose irbesartan group (+ 0.11 mmol/L) (P < 0.05) |
| EVALUATE 2010 | Eplerenone plus ACEi and/or ARB versus ACEi and/or ARB | Potassium increased from baseline more with eplerenone (+0.17 mmol/L) than placebo (+0.02 mmol/L) (P = 0.0043) |
| Lv 2009a | Spironolactone + ACEi or ARB versus ACEi or ARB alone | Spironolactone caused an increase in serum potassium after 9 months of treatment (from 3.8 ± 0.4 mEq/L to 4.1 ± 0.3 mEq/L, P = 0.029) |
| Tokunaga 2008a | Spironolactone + ARB versus ARB alone | Spironolactone produced a significant increase in serum potassium levels (from 4.31 ± 0.53 mmol/L to 4.67 ± 0.68 mmol/L, P < 0.05) |
Comparison 2. Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proteinuria | 2 | 139 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐3.80, 0.62] |
| 2.2 Proteinuria: descriptive outcome data | 2 | Other data | No numeric data | |
| 2.3 Proteinuria data from cross‐over studies | 1 | Other data | No numeric data | |
| 2.4 eGFR [mL/min/1.73 m²] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 GFR: descriptive outcome data | 1 | Other data | No numeric data | |
| 2.6 eGFR data from cross‐over studies | 1 | Other data | No numeric data | |
| 2.7 Diastolic BP | 3 | 151 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐3.52, 0.41] |
| 2.8 Systolic BP | 3 | 151 | Mean Difference (IV, Random, 95% CI) | ‐3.79 [‐14.36, 6.79] |
| 2.9 Blood pressure: descriptive outcome data | 1 | Other data | No numeric data | |
| 2.10 Blood pressure data from cross‐over studies | 1 | Other data | No numeric data | |
| 2.11 Serum potassium | 2 | 121 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.17, 0.45] |
| 2.12 Potassium: descriptive outcome data | 1 | Other data | No numeric data | |
| 2.13 Potassium data from cross‐over studies | 1 | Other data | No numeric data | |
| 2.14 Fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
2.2. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 2: Proteinuria: descriptive outcome data
| Proteinuria: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Hase 2013 | Spironolactone plus ACEi or ARB versus trichlormethiazide plus ACEi or ARB | At week 24, UAER decreased in both spironolactone group (‐57.6% from baseline) and trichlormethiazide group (‐48.4% from baseline) but there was no between group difference |
| Morales 2015 | Spironolactone plus ACEi versus hydrochlorothiazide plus ACEi | At week 4, 24 h urine protein decreased in both spironolactone group (median proteinuria 1.7 g to 1.5 g) and hydrochlorothiazide group (median proteinuria 1.7 g to 1.3 g) but there was no between group difference |
2.3. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 3: Proteinuria data from cross‐over studies
| Proteinuria data from cross‐over studies | ||
| Study | Comparison | Descriptive outcome data |
| Smolen 2006 | Spironolactone verus hydrochlorothiazide | At the start of treatment with spironolactone or hydrochlorothiazide, PCR was 1.65 ± 1.39 and 1.46 ± 1.28 g/g, respectively. After 8 weeks of treatment with spironolactone proteinuria was significantly reduced to 0.99 ± 1.03 g/g (P = 0.03; a decrease of 0.66 ± 0.64 g/g) but not after hydrochlorothiazide (1.28 ± 1.18 g/g; P = 0.35, a decrease of 0.18 ± 0.83 g/g) |
2.4. Analysis.

Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 4: eGFR [mL/min/1.73 m²]
2.5. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 5: GFR: descriptive outcome data
| GFR: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Hase 2013 | Spironolactone plus ACEi or ARB versus trichlormethiazide plus ACEi or ARB | At week 24, eGFR decreased in both spironolactone group (‐9.3 mL/min/1.73 m2 from baseline) and trichlormethiazide group (‐9.4 mL/min/1.73 m2 from baseline) but there was no between group difference |
2.6. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 6: eGFR data from cross‐over studies
| eGFR data from cross‐over studies | ||
| Study | Comparison | Descriptive outcome data |
| Smolen 2006 | Spironolactone versus hydrochlorothiazide | Mean baseline GFR was 94 ± 25 mL/min in the spironolactone and 95 ± 28 mL/min in the hydrochlorothiazide group. After treatment the GFR remained similar in the two groups (91 ± 28 mL/min versus 93 ± 29 mL/min respectively) |
2.9. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 9: Blood pressure: descriptive outcome data
| Blood pressure: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Hase 2013 | Spironolactone plus ACEi or ARB versus trichlormethiazide plus ACEi or ARB | At week 24, systolic BP decreased in both spironolactone group (‐12 mmHg from baseline) and trichlormethiazide group (‐10 mmHg from baseline) but there was no between group difference. Diastolic BP also decreased in both spironolactone group (‐7 mmHg from baseline) and trichlormethiazide group (‐3 mmHg from baseline) and there was no between group difference |
2.10. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 10: Blood pressure data from cross‐over studies
| Blood pressure data from cross‐over studies | ||
| Study | Comparison | Descriptive outcome data |
| Smolen 2006 | Spironolactone verus hydrochlorothiazide | Mean 24 h BP was similar at the start of treatment with spironolactone or hydrochlorothiazide (95.7 ± 10.2 and 95.6 ± 9.1 mmHg, respectively). Both drugs did not significantly influence the 24 h BP (post‐treatment values were 95.6 ± 10.4 and 96.4 ± 12.1 mmHg, respectively) |
2.12. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 12: Potassium: descriptive outcome data
| Potassium: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Hase 2013 | Spironolactone plus ACEi or ARB versus trichlormethiazide plus ACEi or ARB | At week 24, serum potassium increased in the spironolactone group (+ 0.3 mmol/L from baseline) but remained unchanged in the trichlormethiazide group (P = 0.035) |
2.13. Analysis.
Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 13: Potassium data from cross‐over studies
| Potassium data from cross‐over studies | ||
| Study | Comparison | Descriptive outcome data |
| Smolen 2006 | Spironolactone verus hydrochlorothiazide | Serum potassium concentration was 4.23 ± 0.39 and 4.29 ± 0.37 mmol/L, in the spironolactone and hydrochlorothiazide group, respectively. It tended to increase in the spironolactone group after treatment (0.29 ± 0.39 mmol/L, P = 0.01) while it remained stable in the hydrochlorothiazide group (‐0.13 ± 0.4 mmol/L, P = 0.18) |
2.14. Analysis.

Comparison 2: Aldosterone antagonist plus RAS inhibitor versus other diuretic plus RAS inhibitor, Outcome 14: Fatigue
Comparison 3. Spironolactone versus calcium channel blockers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proteinuria: descriptive outcome data | 1 | Other data | No numeric data | |
| 3.2 Systolic BP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Diastolic BP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3: Spironolactone versus calcium channel blockers, Outcome 1: Proteinuria: descriptive outcome data
| Proteinuria: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Takebayashi 2006 | Spironolactone versus amlodipine | Spironolactone reduced UAE from 543.7 mg/g Cr (170 to 1146) to 376.7 mg/g Cr (135 to 794) (P = 0.003). UAE did not change in amlodipine group (P = 0.38) |
3.2. Analysis.

Comparison 3: Spironolactone versus calcium channel blockers, Outcome 2: Systolic BP
3.3. Analysis.

Comparison 3: Spironolactone versus calcium channel blockers, Outcome 3: Diastolic BP
3.4. Analysis.

Comparison 3: Spironolactone versus calcium channel blockers, Outcome 4: Serum potassium
Comparison 4. Eplerenone versus ACEi.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Hyperkalaemia data from cross‐over studies | 1 | Other data | No numeric data | |
| 4.2 Proteinuria data from cross‐over studies | 1 | Other data | No numeric data |
4.1. Analysis.
Comparison 4: Eplerenone versus ACEi, Outcome 1: Hyperkalaemia data from cross‐over studies
| Hyperkalaemia data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Morales 2009 | Eplerenone versus ACEi | The number of patients in which serum potassium was above 5.5 mEq/L after treatment was 2/12 (16%) with ACEi (lisinopril) while none of the patients treated with eplerenone reached this level of potassium |
4.2. Analysis.
Comparison 4: Eplerenone versus ACEi, Outcome 2: Proteinuria data from cross‐over studies
| Proteinuria data from cross‐over studies | ||
| Study | Comparison | Descriptive outcome data |
| Morales 2009 | Eplerenone versus ACEi | ACEi (lisinopril) induced a reduction in proteinuria (11.3 ± 34.8%) which was not statistically significant with respect to baseline values (P = 0.158), while that induced by eplerenone (28.4 ± 31.6%) was significant with respect to baseline values (comparison P = 0.034) and to the lisinopril group (P = 0.034) |
Comparison 5. Eplerenone versus ACEi plus ARB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Hyperkalaemia data from cross‐over studies | 1 | Other data | No numeric data | |
| 5.2 Proteinuria data from cross‐over studies | 1 | Other data | No numeric data |
5.1. Analysis.
Comparison 5: Eplerenone versus ACEi plus ARB, Outcome 1: Hyperkalaemia data from cross‐over studies
| Hyperkalaemia data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Morales 2009 | Eplerenone versus ACEi plus ARB | The number of patients in which serum potassium was above 5.5 mEq/L after treatment was 2/12 (16%) with ACEi plus ARBs (lisinopril plus candesartan) while none of the patients treated with eplerenone reached this level of potassium |
5.2. Analysis.
Comparison 5: Eplerenone versus ACEi plus ARB, Outcome 2: Proteinuria data from cross‐over studies
| Proteinuria data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Morales 2009 | Eplerenone versus ACEi plus ARB | Both eplerenone and the combination of ACEi plus ARB (lisinopril and candesartan) obtained a significant reduction of proteinuria from baseline (26.9 ± 30.6% and 28.4 ± 31.6%, P = 0.045 and P = 0.034 respectively) |
Comparison 6. Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Hyperkalaemia | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.66, 3.95] |
| 6.2 Proteinuria: descriptive outcome data | 4 | Other data | No numeric data | |
| 6.3 Proteinuria data from cross‐over studies | 2 | Other data | No numeric data | |
| 6.4 eGFR: data from cross‐over studies | 1 | Other data | No numeric data | |
| 6.5 Blood pressure: descriptive outcome data | 4 | Other data | No numeric data | |
| 6.6 Blood pressure data from cross‐over studies | 2 | Other data | No numeric data | |
| 6.7 Serum potassium: data from cross‐over studies | 1 | Other data | No numeric data |
6.2. Analysis.
Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 2: Proteinuria: descriptive outcome data
| Proteinuria: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Cohen 2010 | Eplerenone plus ACEi or ARB versus ACEi plus ARB | Urine protein excretion was reduced by 1.04 ± 0.4 g/24 h in the eplerenone and by 0.32 ± 0.2 g/24 h in the ACEi plus ARB group |
| Epstein 2002 | Eplerenone plus ACEi versus ACEi | UAE was reduced by 74% in the eplerenone and by 45% in the control group |
| Epstein 2006 | Eplerenone plus ACEi versus ACEi | Eplerenone treatment significantly reduced albuminuria from baseline as early as week 4 and continued throughout weeks 8 and 12. ACEi treatment did not result in any significant decrease from baseline in albuminuria |
| Haykal 2007 | Eplerenone plus ACEi versus ACEi | Eplerenone treatment reduced proteinuria after 4 weeks. The effect continued throughout weeks 8 and 12 (P < 0.001) |
6.3. Analysis.
Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 3: Proteinuria data from cross‐over studies
| Proteinuria data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Boesby 2011 | Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) | Albuminuria was significantly lower during the add‐on eplerenone period as compared with the control period with a 22% (95% CI 14 to 28, P < 0.001), lower excretion. The mean 24 h excretion was 1481 mg (95% CI 1192 to 1840) during the control period and 1163 mg (95% CI 921 to 1468) during add‐on eplerenone. No significant carry‐over, P = 0.3 or time effect, P = 0.3, was detected for the UAE |
| Tylicki 2012 | Eplerenone plus telmisartan 80 mg daily versus telmisartan 160 mg daily | During the 24 week study period, albuminuria was higher in the eplerenone plus telmisartan 80 mg/day group versus telmisartan 160 mg/day group (mean UACR 707 mg/g (95% CI 502‐1204)) versus 525 mg/g (95% CI 318 to 763)) though statistical significance was not reported |
6.4. Analysis.
Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 4: eGFR: data from cross‐over studies
| eGFR: data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Tylicki 2012 | Eplerenone plus telmisartan 80 mg daily versus telmisartan 160 mg daily | During the 24 week study period, there was no difference between eplerenone plus telmisartan 80 mg daily group versus telmisartan 160 mg daily in creatinine clearance (97.3 ± 8.1 mL/min versus 97.9 ± 8.3 mL/min) |
6.5. Analysis.
Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 5: Blood pressure: descriptive outcome data
| Blood pressure: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Cohen 2010 | Eplerenone plus ACEi or ARB versus ACEi plus ARB | Systolic BP was reduced by 9.7 ± 6.4 mmHg in the eplerenone group and by 13.4 ± 14.9 mmHg in the ACEi plus ARB group |
| Epstein 2002 | Eplerenone plus ACEi versus ACEi | Systolic BP was reduced by 21.8% and 20.4% in the eplerenone and control group respectively. Diastolic BP was reduced by 16.2% and 15% in the eplerenone and control group respectively |
| Epstein 2006 | Eplerenone plus ACEi versus ACEi | Both systolic and diastolic BP decreased in at weeks 4, 8, and 12 in eplerenone and control groups. There were no significant differences in BP reduction between groups |
| Haykal 2007 | Eplerenone plus ACEi versus ACEi | Both systolic and diastolic BP decreased in the two groups at weeks 4, 8 and 12 (P < 0.001). BP reduction was slightly higher in eplerenone group |
6.6. Analysis.
Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 6: Blood pressure data from cross‐over studies
| Blood pressure data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Boesby 2011 | Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) alone | Systolic and diastolic BP was significantly lower during add‐on eplerenone treatment when compared to the control period. There was a significant reduction of systolic BP after 2 weeks of eplerenone treatment (P = 0.003). The diastolic BP was significantly reduced after 4 weeks of eplerenone treatment (P = 0.002), and there was a significant difference in diastolic BP between the treatment period and control period at the same time point (P = 0.004).There were no significant differences between diastolic BP at the end of the 2 periods. There were no significant carry‐over, P = 0.4 and P = 0.9, or time effects, P = 0.5 and P = 0.2 for systolic BP or diastolic BP |
| Tylicki 2012 | Eplerenone plus telmisartan 80 mg daily versus telmisartan 160 mg daily | During the 24 week study period, there was no difference between eplerenone plus telmisartan 80 mg daily group versus telmisartan 160 mg daily for both systolic BP (121.5 ± 2.6 mmHg versus 120.6 ± 2.4 mmHg) and diastolic BP (76.6 ± 1.9 mmHg versus 75.8 ± 2.0 mmHg) |
6.7. Analysis.
Comparison 6: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both), Outcome 7: Serum potassium: data from cross‐over studies
| Serum potassium: data from cross‐over studies | ||
| Study | Comparison | Description of outcome |
| Tylicki 2012 | Eplerenone plus telmisartan 80 mg daily versus telmisartan 160 mg daily | During the 24 week study period, there was no difference between eplerenone plus telmisartan 80 mg/day group versus telmisartan 160 mg/day in serum potassium (4.28 ± 0.08 mmol/L versus 4.45 ± 0.01 mmol/L) |
Comparison 7. Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) plus nitrate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Proteinuria: descriptive outcome data | 1 | Other data | No numeric data | |
| 7.2 Systolic BP: descriptive outcome data | 1 | Other data | No numeric data |
7.1. Analysis.
Comparison 7: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) plus nitrate, Outcome 1: Proteinuria: descriptive outcome data
| Proteinuria: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Cohen 2010 | Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) plus isosorbide | Urine protein excretion was reduced by 1.04 ± 0.4 g/24 h in the eplerenone group but increased in the ACEi/ARB plus isosorbide group by 0.2 ± 0.3 g/24 h |
7.2. Analysis.
Comparison 7: Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) plus nitrate, Outcome 2: Systolic BP: descriptive outcome data
| Systolic BP: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| Cohen 2010 | Eplerenone plus ACEi or ARB (or both) versus ACEi or ARB (or both) plus isosorbide | Systolic blood pressure was reduced by 9.7 ± 6.4 mm Hg in the eplerenone group and by 1.0 ± 5.4 mm Hg in the ACEi/ARB plus isosorbide group |
Comparison 8. Finerenone versus eplerenone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Hyperkalaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.3 eGFR: descriptive outcome data | 1 | Other data | No numeric data | |
| 8.4 Doubling serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.5 Blood pressure: descriptive outcome data | 1 | Other data | No numeric data |
8.1. Analysis.

Comparison 8: Finerenone versus eplerenone, Outcome 1: Hyperkalaemia
8.2. Analysis.

Comparison 8: Finerenone versus eplerenone, Outcome 2: Death
8.3. Analysis.
Comparison 8: Finerenone versus eplerenone, Outcome 3: eGFR: descriptive outcome data
| eGFR: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| ARTS‐HF 2015 | Finerenone versus eplerenone | At day 90, there was no significant change in eGFR from baseline in finerenone groups (mean change in eGFR range ‐2.4 to 1.3 mL/min/1.73 m2) versus eplerenone (mean change in eGFR ‐1.1 mL/min/1.73 m2) |
8.4. Analysis.

Comparison 8: Finerenone versus eplerenone, Outcome 4: Doubling serum creatinine
8.5. Analysis.
Comparison 8: Finerenone versus eplerenone, Outcome 5: Blood pressure: descriptive outcome data
| Blood pressure: descriptive outcome data | ||
| Study | Comparison | Description of outcome |
| ARTS‐HF 2015 | Finerenone versus eplerenone | In patients with worsening heart failure with reduced ejection fraction, there was no difference between the systolic BP in the finerenone groups (LS mean change in systolic BP from baseline range ‐0.8 to ‐2.7 mmHg) compared to eplerenone group (LS mean change in systolic BP from baseline ‐2.4 mmHg) |
Comparison 9. Spironolactone plus ACEi and ARB versus ACEi.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Hyperkalaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.2 Proteinuria | 1 | Other data | No numeric data | |
| 9.3 eGFR [mL/min/1.73 m²] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.4 Systolic BP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.5 Diastolic BP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.6 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.7 Gynaecomastia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
9.1. Analysis.

Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 1: Hyperkalaemia
9.2. Analysis.
Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 2: Proteinuria
| Proteinuria | ||
| Study | Comparison | Description of outcome |
| Bianchi 2010 | Spironolactone, ramipril, irbesartan (intensive group) versus spironolactone, ramipril (conventional group) | At month 36, proteinuria was significantly lower in the intensive group (end of study median proteinuria 0.45 g/g Cr, IQR 0.14 to 1.51) compared to conventional group (end of study median proteinuria 1.23 g/g Cr, IQR 0.36 to 3.42) |
9.3. Analysis.

Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 3: eGFR [mL/min/1.73 m²]
9.4. Analysis.

Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 4: Systolic BP
9.5. Analysis.

Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 5: Diastolic BP
9.6. Analysis.

Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 6: Serum potassium
9.7. Analysis.

Comparison 9: Spironolactone plus ACEi and ARB versus ACEi, Outcome 7: Gynaecomastia
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abolghasmi 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly divided. No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Randomly divided into two groups in a double‐blind fashion". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, however outcome measurement unlikely to be affected by awareness of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | The study fails to include results for key outcomes that would be expected to have been reported for such a study |
| Other bias | Unclear risk | Funding and other sources of bias were unclear |
ARTS 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Part A treatment groups (1, 2, 3)
Part A: control group
Part B: treatment groups (1, 2, 3, 4)
Part B: control group
Co‐interventions
|
|
| Outcomes | Part A
Part B
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in sufficient detail to perform adjudication. Some outcomes might have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 34/265 participants assigned to BAY 94‐8862 did not complete study follow‐up; 20/128 participants assigned to spironolactone and placebo did not complete |
| Selective reporting (reporting bias) | Unclear risk | The study fails to include results for key outcomes that would be expected to have been reported for such a study. Outcomes were reported according to the published protocol |
| Other bias | High risk | Funded by pharmaceutical company. |
ARTS‐DN 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (7 groups)
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, and the sponsor's clinical team were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No reported, although key outcomes were laboratory measures conducted centrally, and investigators were unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants except 9 were included in the analysis set. Those that were excluded related to no valid post‐baseline urine albumin to creatinine ratio. Missing participants were evenly split across treatment groups |
| Selective reporting (reporting bias) | Low risk | Key outcomes expected for this type of study including kidney outcomes and adverse events were systematically reported |
| Other bias | High risk | Funded by pharmaceutical company |
ARTS‐HF 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (5 groups)
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice/web response system |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, and the sponsor’s clinical team was blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinical endpoints have been adjudicated by the event committee. Blinding of outcome assessment was not reported in sufficient detail to perform adjudication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45/842 allocated to finerenone were not included in full analysis; 17/224 allocated to eplerenone were not included in full analysis |
| Selective reporting (reporting bias) | Low risk | This study reported outcomes according to published protocol and as expected for a study of this type |
| Other bias | High risk | Funded by pharmaceutical company |
Bianchi 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed using a computer‐generated system |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/83 patients in the spironolactone group and 4/82 in the control group withdrew. They were included in the analysis as intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | No other sources of bias were identified |
Bianchi 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | High risk | Randomisation was performed by investigators who were aware of group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators aware of group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% drop out with no description of differences in drop out between groups. No mention of analysis by ITT |
| Selective reporting (reporting bias) | High risk | No protocol available. The study did not report all outcomes that might be expected for this type of study |
| Other bias | Low risk | No other sources of bias identified |
Boesby 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Randomisation was done through drawing sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/42 patients were not included in analysis |
| Selective reporting (reporting bias) | High risk | The study did not report all outcomes that might be expected for this type of study. Data were not extractable due to cross‐over study design |
| Other bias | Low risk | No other sources of bias were identified |
Boesby 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done by the GCP‐unit, University of Copenhagen |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 54 patients were included and 46 completed the study. Three patients were withdrawn prior to the first visit due to unexpected serious non‐kidney disease. Five patients did not complete the study, four in the eplerenone group and one in the control group. Three patients in the eplerenone group were withdrawn due to serious side‐effects. In the control group, one was withdrawal due to relapse of GN. There was an imbalance in study attrition based on treatment allocation and for reasons that were potentially due to the study intervention |
| Selective reporting (reporting bias) | High risk | The study did not report all outcomes that might be expected for this type of study |
| Other bias | Low risk | Public grant funding |
Chen 2018b.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generated using statistical software |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in exclusion from analysis based on adverse events in the high‐dose ARB and spironolactone group. Imbalance in follow up may have been related to treatment |
| Selective reporting (reporting bias) | Low risk | Outcomes aligned with those expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Chrysostomou 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2 (control group)
Treatment group 3
Treatment group 4 (treatment group)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation process was undertaken by the Royal Melbourne Hospital Pharmacy Department. Method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Clinical trial pharmacists, who were otherwise not involved in the clinical trial, performed treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | No other sources of bias were identified |
Cohen 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/7 patients in one comparator group withdrew |
| Selective reporting (reporting bias) | Unclear risk | The study did not report all outcomes that might be expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
CRIBS II 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/112 patients withdrew from the study |
| Selective reporting (reporting bias) | High risk | The study did not report all outcomes that might be expected for this type of study |
| Other bias | Low risk | No other sources of bias were identified |
Epstein 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | The study did not report all outcomes that might be expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Epstein 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/91 patients in the control group, 8/91 and 9/86 in the EPL 50 and 100 mg groups respectively, withdrew. The overall percentage of withdrawal was 13%. These patients were analysed on an ITT basis |
| Selective reporting (reporting bias) | Low risk | Outcomes aligned with those expected for this type of study |
| Other bias | High risk | Study funded by Pfizer Inc |
Esteghamati 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation software, not further defined |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/74 discontinued in treatment arm directly due to treatment side‐effects. No participants lost excluded in control arm due to side effects. Marked loss to follow up at 18 months (22/74 in treatment arm and 17/62 in control arm) |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Unclear risk | Other sources of bias and funding source unclear |
EVALUATE 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer‐generated allocation procedure |
| Allocation concealment (selection bias) | Low risk | Web‐based allocation system used by UMIN. Block size was concealed to investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | DBcaps capsules were used to mask the test drugs (eplerenone and placebo). Encapsulated study drugs were prepared and packed centrally by the pharmacy of the University of Tokyo and distributed to participating hospitals. The study investigators, patients, data collection and management personnel and statisticians were all masked to treatment assignment throughout the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study investigators, patients, data collection and management personnel and statisticians were all masked to treatment assignment throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/170 lost to follow up in the treatment group for safety analysis and 14/166 lost to follow up in the control group |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all the study prespecified primary and secondary outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | High risk | Funded by pharmaceutical company. |
Fogari 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded endpoint evaluation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/60 patients in the treatment group discontinued therapy while 5/60 patients in the control group discontinued therapy |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | No payment received for manuscript preparation |
Furumatsu 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient in the spironolactone and one in the control group were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | No other sources of bias identified |
Guney 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/30 participants did not complete the study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | No other sources of bias were identified |
Hamid 2017a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | High risk | Protocol not available and study did not report all outcomes expected for a study of this type. |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Hase 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group:
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33/35 participants completed treatment |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | No other sources of bias and funding source unclear |
Haykal 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/15 patients in both treatment and control groups withdrew |
| Selective reporting (reporting bias) | High risk | Study did not report all expected outcomes for a study of this type. No pre‐published protocol |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Horestani 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment not reported, although outcomes unlikely to be affected by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | High risk | Participants were excluded after randomisation if their antihypertensive treatment was changed |
Ito 2019a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dynamic allocation (minimisation method) but randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10% drop out rate without ITT analysis |
| Selective reporting (reporting bias) | High risk | No cardiovascular outcomes |
| Other bias | High risk | Multiple authors received personal fees from Daiichi‐Sankyo and 2 authors were employees of Daiichi‐Sankyo |
Kato 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/52 did not complete the study |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes |
| Other bias | Unclear risk | No other sources of bias were identified. Funding by University which also receives payment from pharmaceutical company |
Koroshi 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | The study did not report all expected outcomes for this type of study. |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lv 2009a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | No protocol was available. The study reported many of the outcomes that would be expected for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mehdi 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation stratified by diabetes type was programmed to determine treatment assignment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Adjudication of some outcomes may have been influenced by knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/27 patients assigned to spironolactone, 6/27 assigned to placebo and 9/27 assigned to losartan withdrew |
| Selective reporting (reporting bias) | Low risk | All defined outcomes expected for this type of study have been reported |
| Other bias | Low risk | No other apparent sources of bias were identified |
Morales 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was carried out by means of envelopes containing the order of treatment which the patients was to receive. Unclear whether sealed, opaque, or sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients completed the study |
| Selective reporting (reporting bias) | High risk | All defined outcomes have been reported. Data were not extractable due to crossover study design |
| Other bias | Unclear risk | No other sources of potential bias were identified |
Morales 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised assignment list was generated by a computer |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21 of 29 selected patients were randomised |
| Selective reporting (reporting bias) | High risk | No protocol was pre‐published. The study appeared to report all outcomes expected for this type of study. Data were not extractable due to crossover study design |
| Other bias | Low risk | No other sources of bias were identified and public grant funding |
Nielsen 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "randomisation was ensured with computer‐generated envelopes with an unknown block size and frequency" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | High risk | All defined outcomes expected for this type of study have been reported. Data were not extractable due to cross‐over study design |
| Other bias | Low risk | No other potential sources of risk were identified |
Ogawa 2006a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐published protocol. The study did not report all expected outcomes |
| Other bias | Unclear risk | No other potential sources of bias |
Rossing 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | QUOTE "Randomisation was concealed with computer‐generated envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient withdrew |
| Selective reporting (reporting bias) | High risk | All defined outcomes have been reported. Data were not extractable due to cross‐over study design |
| Other bias | Low risk | No other sources of potential bias were identified |
Saklayen 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators and the study nurse remained blind regarding the assignment until the code was broken at the end of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/30 randomised patients withdrew and were not included in the final analysis (no ITT) |
| Selective reporting (reporting bias) | High risk | There was no pre‐published protocol. The study did not report all expected outcomes. Data were not extractable due to cross‐over study design |
| Other bias | Unclear risk | No other sources of bias |
Schjoedt 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "Randomization was concealed with computer generated envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/22 patients completing the study were included in the final analysis |
| Selective reporting (reporting bias) | High risk | There was no pre‐published protocol. The study reported all expected outcomes for this type of study. Data were not extractable due to cross‐over study design |
| Other bias | Low risk | No other sources of bias were identified |
Smolen 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported but outcomes unlikely influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol was identified. The study did not report all expected outcomes for this type of study. Data were not extractable due to cross‐over study design |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Takebayashi 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/25 patients in the spironolactone group were excluded from the analysis due to symptoms of common cold. 1/15 patient in the control group was excluded due to poor compliance |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol was identified. The study did not report all expected outcomes for this type of study |
| Other bias | Unclear risk | No other sources of bias were identified |
Tokunaga 2008a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported. Outcomes adjudication was possibly influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol was identified. The study reported expected outcomes for this type of study |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tylicki 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "Allocation was performed independent of the research team person according to a computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | not reported. Outcomes adjudication was possibly influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients completed the study |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol was identified. The study reported expected outcomes for this type of study |
| Other bias | Low risk | No other sources of bias were identified |
Tylicki 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Outcomes adjudication was possibly influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study |
| Selective reporting (reporting bias) | Low risk | No pre‐published protocol was identified. The study reported expected outcomes for this type of study |
| Other bias | Unclear risk | No other sources of bias were identified |
van den Meiracker 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "Eligible participants were randomised for spironolactone or placebo using a computerized randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Outcomes adjudication was possibly influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/29 patients in the spironolactone group and 2/30 in the control group withdrew. Apparently, they were not included in the final analysis |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol was identified. The study did not report all expected outcomes for this type of study |
| Other bias | Low risk | No other sources of bias were identified |
Wang 2013g.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12/221 were excluded after randomisation due to adverse effects, failure to attend follow‐up. not reported which treatment group |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol was identified. The study did not report all expected outcomes for this type of study |
| Other bias | Unclear risk | No other sources of bias were identified |
Zheng 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Outcomes adjudication was unlikely to be influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients apparently completed the study |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol was identified. The study did not report all expected outcomes for this type of study |
| Other bias | Unclear risk | No other sources of bias were identified and funding source unclear |
Ziaee 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group:
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | No pre‐published protocol was identified. The study did not report all expected outcomes for this type of study |
| Other bias | Low risk | No other sources of bias were identified and public grant funding. |
ACR ‐ albumin creatinine ratio; ACEi ‐ angiotensin converting enzyme inhibitors; ARB ‐ angiotensin receptor blockers; BMI ‐ body mass index; BP ‐ blood pressure; CCB ‐ calcium channel blocker; CKD ‐ chronic kidney disease; CHF ‐ chronic heart failure; CrCl ‐ creatinine clearance; DKD ‐ diabetic kidney disease; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; EPL ‐ eplerenone; Hb ‐ haemoglobin; HbA1c ‐ haemoglobin A1c (glycated); GN ‐ glomerulonephritis; HFrEF ‐ heart failure and reduced ejection fraction; HRQoL‐ health‐related quality of life; IV ‐ intravenous; KRT ‐ kidney replacement therapy; L‐FABP ‐ urinary L‐type fatty acid‐binding protein; LVEF ‐ left ventricular ejection fraction; LVM ‐ left ventricular mass; M/F ‐ male/female; MAP ‐ mean arterial pressure; MI ‐ myocardial infarction; NSAIDs ‐ non‐steroidal anti‐inflammatory drugs; NT‐proBNP ‐ N terminal pro‐brain natriuretic peptide; NYHA ‐ New York Heart Association; RAS ‐ renin‐angiotensin system; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SEM ‐ standard error of the mean; SPL ‐ spironolactone; UACR ‐ urinary albumin creatinine ratio; UAE ‐ urinary albumin excretion; UAER ‐ urinary albumin excretion ratio; UPCR ‐ urinary protein creatinine ratio; UPE ‐ urinary protein excretion; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ATHENA‐HF 2019 | Treatment duration only 96 hours |
| Barr 1995 | Wrong population: heart failure patients without proteinuria or CKD |
| Berry 2007 | Wrong population: heart failure patients without proteinuria or CKD |
| Blanchard 2015 | Wrong population: only patients with Gitelman syndrome |
| EMPHASIS‐HF 2010 | Wrong population: heart failure patients; not reported whether CKD and proteinuria was present |
| Epstein 1998 | Wrong population: hypertensive patients; not reported whether CKD was present |
| Essaian 2007 | Wrong population: HD patients |
| Hollenberg 2003a | Wrong population: hypertensive patients; not reported whether CKD was present |
| Karalliedde 2006 | Wrong population: diabetic patients; not reported whether CKD and proteinuria was present |
| Makhlough 2014 | Wrong intervention: both groups used aldosterone antagonists. Only difference between groups was the use of ARB |
| Medeiros 2017 | Wrong population: children with chronic allograft nephropathy |
| Oxlund 2015 | Wrong study design: experimental study; no outcomes of interest |
| Preston 2009 | Wrong intervention: potassium was administered to patients |
| PRIORITY 2014 | Wrong population: diabetic patients with no proteinuria |
| Rachmani 2004 | Study retracted QUOTE: "Diabetic Medicine has been advised by the Chief Executive Officer of the Mair Medical Center that the above study, done in collaboration with the Sackler University of Tel Aviv, has raised concerns over ethical conduct and security of findings. Specifically, a local investigatory committee found that ethical permission was only given approximately 3 months after Diabetic Medicine accepted the paper for publication. In addition, whilst the investigatory committee believed that the research had been undertaken, they were unable to access research records and thus confirm the results, even though the study was conducted as recently as 2001. In these circumstances, Diabetic Medicine regrets publication of the article, and suggests that its readers treat its findings with caution. The senior author has been advised of our wish to have the article retracted and has made a statement of confidence in the findings." |
| RALES 1995 | Wrong population: heart failure patients; not reported whether CKD was present |
| Schjoedt 2006 | 12 of 20 participants were also reported in Schjoedt 2005 and Rossing 2005 |
| Schjoedt 2009 | Wrong study design: experimental study; no outcomes of interest |
| Schmidt 2005 | Wrong population: healthy subjects and hypertensive patients without a clear diagnosis of CKD |
| Schmidt 2005a | No outcomes of interest |
| Schmidt 2008 | Wrong population: CKD not present |
| STOP‐CKD 2014 | Study terminated early due to futility with no outcomes reported |
| Swift 2006 | Wrong population: only patients with Liddle syndrome |
| Taheri HD 2009 | Wrong population: HD patients |
| TOPCAT 2014 | Wrong population: heart failure patients; not reported whether CKD and proteinuria was present |
| Toto 2005 | Wrong intervention: no aldosterone antagonists has been used |
| Viswanathan 2013 | Wrong population: diabetic patients; not reported whether CKD and proteinuria was present; no outcome of interest |
CKD ‐ chronic kidney disease; HD ‐ haemodialysis
Characteristics of studies awaiting classification [ordered by study ID]
NCT00315016.
| Methods | Interventional randomised phase II study |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
SPIRO‐CKD 2017.
| Methods | Interventional randomised phase IV study |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
ACE‐ angiotensin‐converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker; BP ‐ blood pressure; CrCl ‐ creatinine clearance; DKD ‐ diabetic kidney disease; DM ‐ diabetes mellitus; Hb ‐ haemoglobin; MI ‐ myocardial infarction; NSAIDs ‐ nonsteroidal anti‐inflammatory drug/s; SCr ‐ serum creatinine
Characteristics of ongoing studies [ordered by study ID]
BARACK D 2014.
| Study name | Benefits of Aldosterone Receptor Antagonism in Chronic Kidney Disease (BARACK D) |
| Methods | Interventional, randomised, open label (but with blinding of outcome assessment) phase IV trial |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | Not reported |
| Contact information | Richard Hobbs, richard.hobbs@phc.ox.ac.uk, University of Oxford |
| Notes | Currently recruiting |
FIDELIO‐DKD 2019.
| Study name | Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial |
| Methods | Double‐blind placebo‐controlled RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2015 |
| Contact information | Bayer |
| Notes | Estimated primary completion date: July 2021 |
FIGARO‐DKD 2019.
| Study name | A randomised, double‐blind, placebo‐controlled, parallel‐group, multicenter, event‐driven phase 3 study to investigate efficacy and safety of finerenone on the reduction of cardiovascular morbidity and mortality in subjects with type 2 diabetes mellitus and the clinical diagnosis of diabetic kidney disease in addition to standard of care (FIGARO‐DKD) |
| Methods | Double‐blind placebo‐controlled RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2015 |
| Contact information | Bayer Study Director |
| Notes | Estimated primary completion date: July 2021 |
NCT00870402.
| Study name | Aldosterone in diabetic nephropathy (ALDODN) |
| Methods | Interventional, double blind phase IV RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | March 2009 |
| Contact information | Francisco G Espinoza, fespinoz@mi.cl, Universidad Los Andes |
| Notes | Estimated primary completion date: December 2009. No recent updates on the trial status |
ACR ‐ albumin creatinine ratio; BNP ‐ brain natriuretic peptide; BP ‐ blood pressure; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; DKD ‐ diabetic kidney disease; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; HbA1c ‐ haemoglobin A1c (glycolated); MDRD ‐ modification of diet in renal disease; MI ‐ myocardial infarction; NYHA ‐ New York Heart Association; RAS ‐ renin‐angiotensin system; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; UACR ‐ urinary albumin creatinine ratio
Contributions of authors
Writing of protocol and review: EC, SP, GS
Screening of titles and abstracts: EC, MR, PN, SP
Assessment for inclusion: EC, MR, PN, SP
Quality assessment: EC, MR, PN, SP
Data extraction: EC, MR, PN, SP
Data entry into RevMan: EC, MR, PN, SP
Data analysis and interpretation: All authors
Disagreement resolution: GS
Declarations of interest
Edmund YM Chung: none known
Marinella Ruospo: none known
Patrizia Natale: none known
Davide Bolignano: none known
Sankar D Navaneethan: none known
Suetonia C Palmer: none known
Giovanni FM Strippoli: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abolghasmi 2011 {published data only}
- Abolghasmi R, Taziki O. Efficacy of low dose spironolactone in chronic kidney disease with resistant hypertension. Saudi Journal of Kidney Diseases & Transplantation 2011;22(1):75-8. [MEDLINE: ] [PubMed] [Google Scholar]
ARTS 2012 {published data only}
- Pitt B, Filippatos G, Gheorghiade M, Kober L, Krum H, Ponikowski P, et al. Rationale and design of ARTS: a randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. European Journal of Heart Failure 2012;14(6):668-75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. European Heart Journal 2013;34(31):2453-63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ARTS‐DN 2015 {published data only}
- Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015;314(9):884-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Katayama S, Yamada D, Nakayama M, Yamada T, Myoishi M, Kato M, et al. A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. Journal of Diabetes & its Complications 2017;31(4):758-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effects of finerenone in patients with diabetic nephropathy [abstract]. In: ISN World Congress of Nephrology; 2015 March 13-17; Cape Town, South Africa. 2015.
- Ruilope LM, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Rationale, design, and baseline characteristics of ARTS-DN: a randomized study to assess the safety and efficacy of finerenone in patients with type 2 diabetes mellitus and a clinical diagnosis of diabetic nephropathy. American Journal of Nephrology 2015;40(6):572-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ARTS‐HF 2015 {published data only}
- Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. European Heart Journal 2016;37(27):2105-14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. European Journal of Heart Failure 2015;17(2):224-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sato N, Ajioka M, Yamada T, Kato M, Myoishi M, Yamada T, et al. A randomized controlled study of finerenone vs. eplerenone in Japanese patients with worsening chronic heart failure and diabetes and/or chronic kidney disease. Circulation Journal 2016;80(5):1113-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bianchi 2006 {published data only}
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney International 2006;70(12):2116-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bianchi S, Bigazzi R, Campese VM. Spironolactone reduces proteinuria and retards the progression of kidney disease: a long-term study [abstract no: TH-FC152]. Journal of the American Society of Nephrology 2006;17(Abstracts):33A. [CENTRAL: CN-00615828] [Google Scholar]
Bianchi 2010 {published data only}
- Bianchi S, Bigazzi R, Campese VM. Intensive versus conventional therapy to slow the progression of idiopathic glomerular diseases. American Journal of Kidney Diseases 2010;55(4):671-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boesby 2011 {published data only}
- Boesby L, Elung-Jensen T, Klausen TW, Strandgaard S, Kamper AL. Moderate antiproteinuric effect of add-on aldosterone blockade with eplerenone in non-diabetic chronic kidney disease. A randomized cross-over study. PLoS ONE [Electronic Resource] 2011;6(11):e26904. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesby L, Elung-Jensen T, Strandgaard S, Kamper A. Aldosterone blockade in patients with non-diabetic chronic kidney disease [abstract no: SA153]. NDT Plus 2009;2(Suppl 2):ii882. [CENTRAL: CN-01912397] [Google Scholar]
Boesby 2013 {published data only}
- Boesby L, Elung-Jensen T, Strandgaard S, Kamper AL. Eplerenone attenuates pulse wave reflection in chronic kidney disease stage 3-4--a randomized controlled study. PLoS ONE [Electronic Resource] 2013;8(5):e64549. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2018b {published data only}
- Chen Y, Liu P, Chen X, Li Y, Zhang F, Wang Y. Effects of different doses of irbesartan combined with spironolactone on urinary albumin excretion rate in elderly patients with early type 2 diabetic nephropathy. American Journal of the Medical Sciences 2018;355(5):418-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chrysostomou 2006 {published data only}
- Chrysostomou A, Pedagogos E, Briganti E, Becker GJ. A double blind, placebo controlled study on the effect of the aldosterone receptor blocker: spironolactone, in patients with persistent proteinuria on longterm ACE inhibitor therapy, with or without an ATRB [abstract no: T-PO20016]. Nephrology 2005;10(Suppl 1):A137. [CENTRAL: CN-00602127] [DOI] [PubMed] [Google Scholar]
- Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(2):256-62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 2010 {published data only}
- Cohen DL, Sterling K, Townsend RR. Discordant changes in vascular compliance and proteinuria with drug therapy in CKD patients [abstract no: PUB556]. Journal of the American Society of Nephrology 2010;21(Abstracts):936A. [CENTRAL: CN-01657694] [Google Scholar]
- Cohen DL, Sterling KA, Townsend RR. Addition of eplerenone to RAAS blockade in patients with CKD and proteinuria correlates with baseline vascular compliance [abstract no: PO247]. Journal of Clinical Hypertension 2011;13(4 Suppl 1):A116. [CENTRAL: CN-01657695] [Google Scholar]
CRIBS II 2009 {published data only}
- Cabrera SE, Edwards NC, Steeds RP, Townend JN, Ferro CJ. Spironolactone increases serum uric acid levels in patients with chronic kidney disease. Journal of Human Hypertension 2014;28(3):210-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Edwards NC, Ferro CJ, Kirkwood H, Chue CD, Young AA, Stewart PM, et al. Effect of spironolactone on left ventricular systolic and diastolic function in patients with early stage chronic kidney disease. American Journal of Cardiology 2010;106(10):1505-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Edwards NC, Steeds RP, Chue CD, Stewart PM, Ferro CJ, Townend JN. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. British Journal of Clinical Pharmacology 2012;73(3):447-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. Journal of the American College of Cardiology 2009;54(6):505-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hammer F, Edwards NC, Hughes BA, Steeds RP, Ferro CJ, Townend JN, et al. The effect of spironolactone upon corticosteroid hormone metabolism in patients with early stage chronic kidney disease. Clinical Endocrinology 2010;73(5):566-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Epstein 2002 {published data only}
- Epstein M, Buckalew V, Martinez F. Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination therapy in diabetic hypertensives with microalbuminuria [abstract no: OR54]. American Journal of Hypertension 2002;15(4 Suppl 1):24A. [CENTRAL: CN-00689079] [Google Scholar]
Epstein 2006 {published data only}
- Epstein M, Weinberger M, Lewin A, Martinez F, He W, Krause S. The selective aldosterone blocker eplerenone reduces proteinuria without concomitant hyperkalemia [abstract no: F-FC026]. Journal of the American Society of Nephrology 2003;14(Nov):6A. [CENTRAL: CN-00550622] [Google Scholar]
- Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(5):940-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Esteghamati 2013 {published data only}
- Esteghamati A, Noshad S, Jarrah S, Mousavizadeh M, Khoee SH, Nakhjavani M. Long-term effects of addition of mineralocorticoid receptor antagonist to angiotensin II receptor blocker in patients with diabetic nephropathy: a randomized clinical trial. Nephrology Dialysis Transplantation 2013;28(11):2823-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EVALUATE 2010 {published data only}
- Ando K, Ohtsu H, Arakawa Y, Kubota K, Yamaguchi T, Nagase M, et al. Rationale and design of the Eplerenone combination Versus conventional Agents to Lower blood pressure on Urinary Antialbuminuric Treatment Effect (EVALUATE) trial: a double-blinded randomized placebo-controlled trial to evaluate the antialbuminuric effects of an aldosterone blocker in hypertensive patients with albuminuria. Hypertension Research - Clinical & Experimental 2010;33(6):616-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T, et al. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes & Endocrinology 2014;2(12):944-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fujita T, Uchida S, Seki G, Kaname S. The antialbuminuric effects of an aldosterone blocker in hypertensive patients with albuminuria: a double-blinded, randomized, placebo-controlled (EVALUATE) trial [abstract no: SA-PO1081]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):3B. [Google Scholar]
Fogari 2014 {published data only}
- Fogari R, Derosa G, Zoppi A, Lazzari P, D'Angelo A, Mugellini A. Comparative effect of canrenone or hydrochlorothiazide addition to valsartan/amlodipine combination on urinary albumin excretion in well-controlled type 2 diabetic hypertensive patients with microalbuminuria. Expert Opinion on Pharmacotherapy 2014;15(4):453-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furumatsu 2008 {published data only}
- Furumatsu Y, Nagasawa Y, Tomida K, Mikami S, Kaneko T, Okada N, et al. Effect of renin-angiotensin-aldosterone system triple blockade on non-diabetic renal disease: addition of an aldosterone blocker, spironolactone, to combination treatment with an angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker. Hypertension Research - Clinical & Experimental 2008;31(1):59-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furumatsu Y, Shoji T, Hayashi D, Tomida K, Kaneko T, Tsubakihara Y, et al. Antiproteiuric effect of triple blockade with angiotensin-converting-enzyme inhibitor, angiotensin-II receptor blocker and spironolactone: one year follow up [abstract no: SU-FC069]. Journal of the American Society of Nephrology 2004;15(Oct):58A. [CENTRAL: CN-00653797] [Google Scholar]
- Furumatsu Y, Shoji T, Tomida K, Mikami S, Kaneko T, Togawa M, et al. Antiproteinuric effect of triple blockage with angiotensin-converting-enzyme inhibitor, angiotensin-II receptor blocker and spironolactone [abstract no: SU-PO1031]. Journal of the American Society of Nephrology 2003;14(Suppl S):762A. [CENTRAL: CN-00653796] [Google Scholar]
Guney 2009 {published data only}
- Guney I, Selcuk NY, Altintepe L, Atalay H, Basarali MK, Buyukbas S. Antifibrotic effects of aldosterone receptor blocker (spironolactone) in patients with chronic kidney disease. Renal Failure 2009;31(9):779-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hamid 2017a {published data only}
- Hamid N, Taher M. Effect of hydrochlorothiazide and spironolactone on reducing proteinuria among patients with diabetic nephropathy treated by angiotensin converting enzyme inhibitors [abstract no: P113]. Iranian Journal of Kidney Diseases 2017;11(Suppl 1):11-2. [EMBASE: 616611416] [Google Scholar]
- Noshad H, Manzari T. Hydrochlorothiazide or spironolacton in diabetic nephropathy [abstract no: SP446]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii273. [EMBASE: 617291179] [Google Scholar]
Hase 2013 {published data only}
- Hase M, Babazono T, Ujihara N, Uchigata Y. Comparison of spironolactone and trichlormethiazide as add-on therapy to renin-angiotensin blockade for reduction of albuminuria in diabetic patients. Journal of Diabetes Investigation 2013;4(3):316-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haykal 2007 {published data only}
- Haykal WJ, Kuryata OV, Frolova EO, Shvedenko OO. The effects of aldosterone blockade on proteinuria and glomerular filtration rate in patients with chronic kidney disease [abstract no: SAP075]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi257. [CENTRAL: CN-01657693] [Google Scholar]
Horestani 2012 {published data only}
- Horestani MK, Behradmanesh S, Momeni A, Baradaran A. Effects of spironolactone on proteinuria of patients with type 2 diabetes. Journal of Isfahan Medical School 2012;30(200). [EMBASE: 2012617320] [Google Scholar]
- Momeni A, Behradmanesh MS, Kheiri S, Karami Horestani M. Evaluation of spironolactone plus hydrochlorothiazide in reducing proteinuria in type 2 diabetic nephropathy. Journal of the Renin-Angiotensin-Aldosterone System 2015;16(1):113-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ito 2019a {published data only}
- Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clinical Journal of the American Society of Nephrology: CJASN 2019;14(8):1161-72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2015 {published data only}
- Kato S, Maruyama S, Makino H, Wada J, Ogawa D, Uzu T, et al. Anti-albuminuric effects of spironolactone in patients with type 2 diabetic nephropathy: a multicenter, randomized clinical trial. Clinical & Experimental Nephrology 2015;19(6):1098-106. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Maruyama S, Makino H, Wada J, Uzu T, Araki H, et al. Renoprotective effects of combining RAS blockade drugs with spironolactone in patients with diabetic mephropathy and overt albuminuria [abstract no: MP302]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii428. [EMBASE: 71492704] [Google Scholar]
- Kato S, Maruyama S, Makino H, Wada J, Uzu T, Araki H, et al. Spironolactone improving intrarenal renin-angiotensin system activation in type II diabetic nephropathy patients with overt albuminuria [abstract no: PS3-039]. Nephrology 2014;19(Suppl 2):172. [Google Scholar]
Koroshi 2010 {published data only}
- Koroshi A, Idrizi A, Gjergji Z, Hoxha B, Emrullaj E, Spahia E, et al. Combination of angiotensin converting enzyme inhibitors with mineralocorticoid antagonists in diabetic nephropathy [abstract no: SU342]. NDT Plus 2010;3(Suppl 3):iii424. [EMBASE: 70484562] [Google Scholar]
Lv 2009a {published data only}
- Lv J, Qin S, Li Y, Zhang H, Liu L, Shi S, et al. Change of proteinuria after adding spironolactone ACE inhibitor or angiotensin receptor blockers in IGA nephropathy, a pilot study [abstract no: SA309]. NDT Plus 2009;2(Suppl 2):ii1029. [CENTRAL: CN-01657692] [Google Scholar]
Mehdi 2009 {published data only}
- Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. Journal of the American Society of Nephrology 2009;20(12):2641-50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Effect of an angiotensin receptor blocker (ARB) or a mineralocorticoid antagonist (MRA) added-on to maximally-dosed angiotensin converting enzyme inhibitor (ACEi) regimen in diabetic nephropathy (DN) [abstract no: TH-FC128]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):28A. [CENTRAL: CN-00756304] [Google Scholar]
- Srivastava A, Adams-Huet B, Vega GL, Toto RD. Effect of losartan and spironolactone on lipoprotein metabolism in diabetic nephropathy [abstract no: 266]. American Journal of Kidney Diseases 2012;59(4):A81. [EMBASE: 71074879] [Google Scholar]
- Srivastava A, Adams-Huet B, Vega GL, Toto RD. Effect of losartan and spironolactone on triglyceride-rich lipoproteins in diabetic nephropathy. Journal of Investigative Medicine 2016;64(6):1102-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren PN, Adams-Huet B, Nguyen M, Molina C, Toto RD. Potassium handling with dual renin-angiotensin system inhibition in diabetic nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(2):295-301. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren PN, Adams-Huet B, Toto RD. Effective antihypertensive strategies for high-risk patients with diabetic nephropathy. Journal of Investigative Medicine 2010;58(8):950-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morales 2009 {published data only}
- Morales E, Huerta A, Gutierrez E, Gutierrez SE, Segura J, Praga M. The antiproteinuric effect of the blockage of the renin-angiotensin-aldosterone system (RAAS) in obese patients. Which treatment option is the most effective? [Efecto antiproteinurico del bloqueo del sistema renina-angiotensina-aldosterona (SRAA) en los pacientes obesos. Cual es la opcion terapeutica mas eficaz]. Nefrologia 2009;29(5):421-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales E, Huerta A, Gutierrez-Millet V, Gutierrez E, Gutierrez-Solis E, Hernandez E, et al. Antiproteinuric effect of renin-angiotensin-aldosterone system (RAAS) in obese patients. Which is the most effective option? [abstract no: SA131]. NDT Plus 2009;2(Suppl 2):ii882. [CENTRAL: CN-01657690] [Google Scholar]
- Morales E, Huerta A, Gutierrez-Solis E, Gutierrez E, Polanco N, Gutierrez-Millet V, et al. Antiproteinuric effect of renin-angiotensin-aldosterone system (RAAS) in obese patients. Which is the most effective option? [abstract no: F-PO1941]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):549A. [CENTRAL: CN-00790676] [Google Scholar]
Morales 2015 {published data only}
- Morales E, Caro J, Gutierrez E, Sevillano A, Aunon P, Fernandez C, et al. Diverse diuretics regimens differentially enhance the antialbuminuric effect of renin-angiotensin blockers in patients with chronic kidney disease. Kidney International 2015;88(6):1434-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales E, Caro J, Gutierrez E, Sevillano A, Aunon P, Praga M. Crossover randomized clinical trial to evaluate the albuminuric effect of three different types of diuretics (spironolactone, hydroclorothiazide and hydroclorothiazide+amiloride) on top of RAAS blockade in proteinuric nephropathies [abstract no: SP309]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii482. [EMBASE: 72207635] [Google Scholar]
- Morales E, Espada PJ, Gutierrez-Martinez E, Praga M. Crossover randomised clinical trial to evaluate the antialbuminuric effect of three different types of diuretics (spironolactone, hydrochlorothiazide and hydrochlorothiazide + amiloride) on top of RAAS blockade in proteinuric nephropathies [abstract no: TH-PO655]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):257A. [Google Scholar]
Nielsen 2012 {published data only}
- Nielsen S, Persson F, Frandsen E, Sugaya T, Hess G, Zdunek D, et al. Spironolactone diminishes urinary albumin excretion in type 1 diabetic patients with microalbuminuria: a randomised placebo-controlled crossover study [abstract no: 1099]. Diabetologia 2011;54(Suppl 1):S445. [EMBASE: 70563244] [DOI] [PubMed] [Google Scholar]
- Nielsen S, Persson F, Frandsen E, Sugaya T, Zdunek DW, Pedersen K, et al. Spironolactone diminishes urinary albumin excretion in type 1 diabetic patients with microalbuminuria: a randomized placebo-controlled crossover study [abstract no: TH-PO548]. Journal of the American Society of Nephrology 2011;22(Abstracts):238A. [CENTRAL: CN-01912458] [Google Scholar]
- Nielsen SE, Persson F, Frandsen E, Sugaya T, Hess G, Zdunek D, et al. Spironolactone diminishes urinary albumin excretion in patients with type 1 diabetes and microalbuminuria: a randomized placebo-controlled crossover study. Diabetic Medicine 2012;29(8):e184-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ogawa 2006a {published data only}
- Ogawa S, Takeuchi K, Mori T, Nako K, Ito S. Spironolactone further reduces urinary albumin excretion and plasma B-type natriuretic peptide levels in hypertensive type II diabetes treated with angiotensin-converting enzyme inhibitor. Clinical & Experimental Pharmacology & Physiology 2006;33(5-6):477-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossing 2005 {published data only}
- Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care 2005;28(9):2106-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saklayen 2008 {published data only}
- Saklayen M, Gyebi L, Tasosa J, Yap J. Spironolactone reduces proteinuria further in diabetic patients who are already on ACE inhibitor or ARB therapy: results of a randomized, placebo-controlled, double-blind cross-over trial [abstract no: TH-PO240]. Journal of the American Society of Nephrology 2006;17(Abstracts):157A. [CENTRAL: CN-00615896] [Google Scholar]
- Saklayen MG, Gyebi LK, Tasosa J, Yap J. Effects of additive therapy with spironolactone on proteinuria in diabetic patients already on ACE inhibitor or ARB therapy: results of a randomized, placebo-controlled, double-blind, crossover trial. Journal of Investigative Medicine 2008;56(4):714-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schjoedt 2005 {published data only}
- Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Parving HH. Beneficial impact of spironolactone in diabetic nephropathy. Kidney International 2005;68(6):2829-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schjoedt KJ, Rossing K, Tarnow L, Parving H. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy [abstract no: F-FC090]. Journal of the American Society of Nephrology 2005;16(Abstracts):57A. [CENTRAL: CN-00689347] [DOI] [PubMed] [Google Scholar]
Smolen 2006 {published data only}
- Smolen E, Nowicki M. Contrasting effects of spironolactone and hydrochlorothiazide in patients with persistent glomerular proteinuria [abstract no: MP260]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv386. [CENTRAL: CN-00716094] [Google Scholar]
Takebayashi 2006 {published data only}
- Matsumoto S, Takebayashi K, Aso Y. The effect of spironolactone on circulating adipocytokines in patients with type 2 diabetes mellitus complicated by diabetic nephropathy. Metabolism: Clinical & Experimental 2006;55(12):1645-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Matsumoto S, Aso Y, Inukai T. Aldosterone blockade attenuates urinary monocyte chemoattractant protein-1 and oxidative stress in patients with type 2 diabetes complicated by diabetic nephropathy. Journal of Clinical Endocrinology & Metabolism 2006;91(6):2214-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tokunaga 2008a {published data only}
- Tokunaga M, Tamura M, Kabashima N, Serino R, Shibata T, Matsumoto M, et al. Add-on spironolactone in patients with advanced chronic kidney disease treated with angiotensin II receptor blockers [abstract no: F-PO1840]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):525A. [CENTRAL: CN-01657689] [Google Scholar]
Tylicki 2008 {published data only}
- Larczynski W, Tylicki L, Rutkowski P, Renke M, Rutkowski B. The triple renin-angiotensin-aldosterone system blockade - new promising nephroprotective strategy in patients with proteinuric nondiabetic renal disease [abstract no: SAP081]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi259. [CENTRAL: CN-00653742] [Google Scholar]
- Tylicki L, Rutkowski P, Renke M, Larczynski W, Aleksandrowicz E, Lysiak-Szydlowska W, et al. Triple pharmacological blockade of the renin-angiotensin-aldosterone system in nondiabetic CKD: an open-label crossover randomized controlled trial. American Journal of Kidney Diseases 2008;52(3):486-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tylicki L, Rutkowski P, Renke M, Rutkowski B. Addition of aldosterone receptor blocker to dual renin-angiotensin-aldosterone blockade leads to limitation of tubulointerstitial injury of kidney. Kidney International 2007;72(9):1164-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tylicki 2012 {published data only}
- Lizakowski S, Rutkowski P, Tylicki L, Renke M, Sulikowska B, Donderski R, et al. The effect of various types of the renin-angiotensin-aldosteron system blockade on proteinuria in chronic non-diabetic kidney disease: a double-blind cross-over randomised controlled trial [abstract no: FP112]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii109. [EMBASE: 70765606] [Google Scholar]
- Lizakowski S, Tylicki L, Rutkowski P, Renke M, Sulikowska B, Heleniak Z, et al. Safety of enhanced renin-angiotensin-aldosterone system inhibition with aliskiren in nondiabetic patients with chronic kidney disease. Polskie Archiwum Medycyny Wewnetrznej 2013;123(5):221-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tylicki L, Lizakowski S, Rutkowski P, Renke M, Sulikowska B, Heleniak Z, et al. The enhanced renin-angiotensin-aldosteron system pharmacological blockade--which is the best? Kidney & Blood Pressure Research 2012;36(1):335-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van den Meiracker 2006 {published data only}
- den Meiracker AH, Baggen RG, Pauli S, Lindemans A, Vulto AG, Poldermans D. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. Journal of Hypertension 2006;24(11):2285-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2013g {published data only}
- Wang W, Li L, Zhou Z, Gao J, Sun Y. Effect of spironolactone combined with angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers on chronic glomerular disease. Experimental & Therapeutic Medicine 2013;6(6):1527-31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2011 {published data only}
- Zheng H, Qiu H, Liu X, Jiang B. Clinical investigation of combined therapy with benazepril and spironolactone in diabetic nephropathy. Pharmaceutical Care & Research 2011;11(4):266-8. [EMBASE: 2011509942] [Google Scholar]
Ziaee 2013 {published data only}
- Ziaee A, Abbas Vaezi A, Oveisi S, Javadi A, Hashemipour S, Kazemifar AM. Effects of additive therapy with spironolactone on albuminuria in diabetes mellitus: a pilot randomized clinical trial. Caspian Journal of Internal Medicine 2013;4(2):648-53. [EMBASE: 369112192] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ATHENA‐HF 2019 {published data only}
- Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiology 2017;2(9):950-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Konstam MA, et al. Rationale and design of the ATHENA-HF Trial: aldosterone targeted neurohormonal combined with natriuresis therapy in heart failure. JACC Heart Failure 2016;4(9):726-35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SJ, Felker GM, Giczewska A, Kalogeropoulos AP, Ambrosy AP, Chakraborty H, et al. Spironolactone in acute heart failure patients with renal dysfunction and risk factors for diuretic resistance: from the ATHENA-HF trial. Canadian Journal of Cardiology 2019;35(9):1097-105. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SJ, Felker GM, Giczewska A, Kalogeropoulos AP, Ambrosy AP, Chakraborty H, et al. Spironolactone in acute heart failure patients with renal dysfunction and risk factors for diuretic resistance: from the ATHENA-HF trial [abstract no: 10456]. Circulation 2018;138(Suppl 1):A10456. [EMBASE: 627102576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr 1995 {published data only}
- Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. American Journal of Cardiology 1995;76(17):1259-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berry 2007 {published data only}
- Berry C, Murphy NF, De Vito G, Galloway S, Seed A, Fisher C, et al. Effects of aldosterone receptor blockade in patients with mild-moderate heart failure taking a beta-blocker.[Erratum in: Eur J Heart Fail. 2007 Oct;9(10):1074 Note: Murphy, Niamh [corrected to Murphy, Niamh F]]. European Journal of Heart Failure 2007;9(4):429-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blanchard 2015 {published data only}
- Blanchard A, Vargas-Poussou R, Vallet M, Caumont-Prim A, Allard J, Desport E, et al. Indomethacin, amiloride, or eplerenone for treating hypokalemia in Gitelman syndrome. Journal of the American Society of Nephrology 2015;26(2):468-75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EMPHASIS‐HF 2010 {published data only}
- Eschalier R, McMurray JJ, Swedberg K, Veldhuisen DJ, Krum H, Pocock SJ, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). Journal of the American College of Cardiology 2013;62(17):1585-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferreira JP, Abreu P, McMurray JJ, Veldhuisen DJ, Swedberg K, Pocock SJ, et al. Renal function stratified dose comparisons of eplerenone versus placebo in the EMPHASIS-HF trial. European Journal of Heart Failure 2019;21(3):345-51. [EMBASE: 626398757] [DOI] [PubMed] [Google Scholar]
- Krum H, Shi H, Pitt B, McMurray J, Swedberg K, Veldhuisen DJ, et al. Clinical benefit of eplerenone in patients with mild symptoms of systolic heart failure already receiving optimal best practice background drug therapy: analysis of the EMPHASIS-HF study. Circulation: Heart Failure 2013;6(4):711-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rogers JK, McMurray JJ, Pocock SJ, Zannad F, Krum H, Veldhuisen DJ, et al. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation 2012;126(19):2317-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circulation: Heart Failure 2014;7(1):51-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zannad F, McMurray JJ, Drexler H, Krum H, Veldhuisen DJ, Swedberg K, et al. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF). European Journal of Heart Failure 2010;12(6):617-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zannad F, McMurray JJ, Krum H, Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. New England Journal of Medicine 2011;364(1):11-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Epstein 1998 {published data only}
- Epstein M, Alexander JC, Roniker B. Eplerenone, a new selective aldosterone receptor antagonist (SARA): efficacy in patients with mild to moderate hypertension [abstract no: A1640]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):322-3A. [CENTRAL: CN-00445239] [Google Scholar]
Essaian 2007 {published data only}
- Essaian A, Kaukov I, Karabayeva A, Kadinskaya M, Katysheva N. The influence of spironolactone on plasma level of plasminogen-activator inhibitor type 1 (PAI-1) in anuric hemodialysis patients [abstract no: SaP266]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi322. [CENTRAL: CN-00716089] [Google Scholar]
Hollenberg 2003a {published data only}
- Hollenberg NK, Williams GH, Anderson R, Akhras KS, Bittman RM, Krause SL. Symptoms and the distress they cause: comparison of an aldosterone antagonist and a calcium channel blocking agent in patients with systolic hypertension. Archives of Internal Medicine 2003;163(13):1543-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karalliedde 2006 {published data only}
- Karalliedde J, Buckingham R, Starkie M, Lorand D, Stewart M, Viberti G, et al. Effect of various diuretic treatments on rosiglitazone-induced fluid retention. Journal of the American Society of Nephrology 2006;17(12):3482-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Makhlough 2014 {published data only}
- Makhlough A, Kashi Z, Akha O, Zaboli E, Yazdanicharati J. Effect of spironolactone on diabetic nephropathy compared to the combination of spironolactone and losartan. Nephrourology Monthly 2014;6(1):e12148. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlough A, Kashi Z, Akha O. Effect of spironolactone-placebo and spironolacton-losartan on microalbuminuria in type II diabetes patients [abstract]. Iranian Journal of Kidney Diseases 2011;5(Suppl 2):46. [EMBASE: 70673884] [Google Scholar]
Medeiros 2017 {published data only}
- Medeiros M, Ramirez S, Velasquez L, Hernandez AM, Valverde S, Vargas A, et al. Aldosterone blockade in children with chronic allograft nephropathy [abstract no: O-10]. Pediatric Nephrology 2010;25(9):1808. [EMBASE: 70438169] [Google Scholar]
- Medeiros M, Sosa J, Velasquez L, Hernandez AM, Ramirez S, Valverde S, et al. Aldosterone blockade in children with chronic allograft nephropathy [abstract no: 1296]. American Journal of Transplantation 2010;10(Suppl 4):411. [EMBASE: 70464672] [Google Scholar]
- Medeiros M, Velasquez-Jones L, Hernandaz AM, Ramirez S, Fuentes Y, Valverdes S, et al. Aldosterone blockade in children with chronic allograft nephropathy [abstract no: 234]. Pediatric Transplantation 2011;15(Suppl 1):98-9. [EMBASE: 70508755] [Google Scholar]
- Medeiros M, Velasquez-Jones L, Hernandez AM, Ramon-Garcia G, Valverde S, Fuentes Y, et al. Randomized controlled trial of mineralocorticoid receptor blockade in children with chronic kidney allograft nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2017;12(8):1291-300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros M, Velasquez-Jones L, Hernandez AM, Valverde S, Fuentes Y, Barrera-Chimal J, et al. Mineralocorticoid receptor blockade in children with chronic allograft injury [abstract no: 116]. Pediatric Transplantation 2013;17(Suppl 1):71. [EMBASE: 71163526] [Google Scholar]
Oxlund 2015 {published data only}
- Lindhardt M, Oxlund CS, Mischak H, Jacobsen IA, Rossing P, Lambers Heerspink HJ. A urinary peptide classifier predicts albuminuria response to spironolactone in patients with type 2 diabetes and hypertension [abstract no: SA-PO255]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):694A. [Google Scholar]
- Lindhardt M, Persson F, Oxlund C, Jacobsen IA, Zurbig P, Mischak H, et al. Predicting albuminuria response to spironolactone treatment with urinary proteomics in patients with type 2 diabetes and hypertension. Nephrology Dialysis Transplantation 2018;33(2):296-303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oxlund C, Kurt B, Schwarzensteiner I, Hansen MR, Staehr M, Svenningsen P, et al. Albuminuria is associated with an increased prostasin in urine while aldosterone has no direct effect on urine and kidney tissue abundance of prostasin. Pflugers Archiv - European Journal of Physiology 2017;469(5-6):655-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Oxlund CS, Cangemi C, Henriksen JE, Jacobsen IA, Gram J, Schousboe K, et al. Low-dose spironolactone reduces plasma fibulin-1 levels in patients with type 2 diabetes and resistant hypertension. Journal of Human Hypertension 2015;29(1):28-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Preston 2009 {published data only}
- Preston RA, Afshartous D, Garg D, Medrano S, Alonso AB, Rodriguez R. Mechanisms of impaired potassium handling with dual renin-angiotensin-aldosterone blockade in chronic kidney disease. Hypertension 2009;53(5):754-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PRIORITY 2014 {published data only}
- Lindhardt M, Persson F, Currie G, Pontillo C, Beige J, Delles C, et al. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. Trials 2016;6(3):e010310. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwy J, Schanstra JP, Argiles A, Bakker SJ, Beige J, Boucek P, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrology Dialysis Transplantation 2014;29(8):1563-70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofte N, Lindhardt M, Adamova K, Beige J, Beulens JW, Birkenfeld AL, et al. Characteristics of high- and low-risk individuals in the PRIORITY study: urinary proteomics and mineralocorticoid receptor antagonism for prevention of diabetic nephropathy in Type 2 diabetes. Diabetic Medicine 2018;35(10):1375-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tofte N, PRIORITY Study Group. Baseline characteristics in PRIORITY study: proteomics and mineralocorticoid receptor antagonism for prevention of diabetic nephropathy in type 2 diabetes [abstract no: TH-PO718]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):284. [Google Scholar]
Rachmani 2004 {published data only}
- Anonymous. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled study.[Retraction of: Rachmani R, Slavachevsky I, Amit M, Levi Z, Kedar Y, Berla M, Ravid M. Diabet Med. 2004 May;21(5):471-5; PMID: 15089793]. Diabetic Medicine 2006;23(7):818. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rachmani R, Slavachevsky I, Amit M, Levi Z, Kedar Y, Berla M, et al. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: A randomized controlled study. Diabetic Medicine 2004;21(5):471-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RALES 1995 {published data only}
- Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). American Journal of Cardiology 1996;78(8):902-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Glick HA, Orzol SM, Tooley JF, Remme WJ, Sasayama S, Pitt B. Economic evaluation of the randomized aldactone evaluation study (RALES): treatment of patients with severe heart failure. Cardiovascular Drugs & Therapy 2002;16(1):53-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hauben M, Reich L, Gerrits CM, Madigan D. Detection of spironolactone-associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES). Drug Safety 2007;30(12):1143-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 1999;341(10):709-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pitt D. ACE inhibitor co-therapy in patients with heart failure: rationale for the Randomized Aldactone Evaluation Study (RALES). European Heart Journal 1995;16 Suppl N:107-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S, et al. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy.[Erratum in: J Am Coll Cardiol. 2003 Nov 19;42(10):1865]. Journal of the American College of Cardiology 2002;40(9):1596-601. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schwinger RH. The aldosterone antagonist spironolactone prolongs the survival of chronic heart failure patients. The results of the RALES study. The Randomized Aldactone Evaluation Study [Der Aldosteronantagonist Spironolacton verlangert das Uberleben chronisch herzinsuffizienter Patienten. Ergebnisse der RALES-Studie]. Deutsche Medizinische Wochenschrift 1999;124(34-35):987-8. [MEDLINE: ] [PubMed] [Google Scholar]
- Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, et al. Influence of baseline and worsening renal function on efficacy of spironolactone in patients With severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). Journal of the American College of Cardiology 2012;60(20):2082-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wittes J, Palensky J, Asner D, Julian D, Boissel JP, Furberg CD, et al. Experience collecting interim data on mortality: an example from the RALES study. Current Controlled Trials in Cardiovascular Medicine 2001;2(1):59-62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators.[Erratum in: Circulation 2001 Jan 23;103(3):476]. Circulation 2000;102(22):2700-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schjoedt 2006 {published data only}
- Schjoedt KJ, Rossing K, Juhl TR, Boomsa F, Tarnow L, Rossing P, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney International 2006;70(3):536-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schjoedt 2009 {published data only}
- Schjoedt KJ, Christensen PK, Jorsal A, Boomsma F, Rossing P, Parving HH. Autoregulation of glomerular filtration rate during spironolactone treatment in hypertensive patients with type 1 diabetes: a randomized crossover trial. Nephrology Dialysis Transplantation 2009;24(11):3343-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schjoedt KJ, Rossing K, Christensen PK, Parving HH, Rossing P. Autoregulation of the glomerular filtration rate during spironolactone treatment [abstract no: PUB561]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):953A. [CENTRAL: CN-00689324] [Google Scholar]
Schmidt 2005 {published data only}
- Schmidt BM, Schneider A, Kalizki T, Rastatter A, Schwarz TK, Schmieder RE. Low dose spironolactone rapidly reduces left ventricular mass [abstract no: F-FC099]. Journal of the American Society of Nephrology 2005;16:60A. [CENTRAL: CN-00716088] [Google Scholar]
Schmidt 2005a {published data only}
- Schmidt B, Sammer U, Schaufele T, Schmieder R. Effects of aldosterone on kidney blood flow. Medizinische Klinik 2005;100(Abstract-Band):68. [CENTRAL: CN-00777290] [Google Scholar]
Schmidt 2008 {published data only}
- Schmidt BM, Raff U, Schwab J, Bar I, Schmieder RE. Eplerenone at low dose induces regression of left ventricular hypertrophy in resistant hypertension [abstract no: SA-PO2251]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):617A. [CENTRAL: CN-01657688] [Google Scholar]
STOP‐CKD 2014 {published data only}80658312
- Ng KP, Jain P, Gill PS, Heer G, Townend JN, Freemantle N, et al. Results and lessons from the Spironolactone To Prevent Cardiovascular Events in Early Stage Chronic Kidney Disease (STOP-CKD) randomised controlled trial. BMJ Open 2016;6(2):e010519. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Jain P, Heer G, Redman V, Chagoury OL, Dowswell G, et al. Spironolactone to prevent cardiovascular events in early-stage chronic kidney disease (STOP-CKD): study protocol for a randomized controlled pilot trial. Trials [Electronic Resource] 2014;15(1):158. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swift 2006 {published data only}
- Swift PA, Markandu ND, Wood M, Sagnella GA, MacGregor GA. A double blind randomized crossover trial of amiloride and spironolactone in black hypertensives with and without the T594M b-subunit epithelial sodium channel polymorphism [abstract no: TH-FC153]. Journal of the American Society of Nephrology 2006;17(Abstracts):33-4A. [CENTRAL: CN-00716092] [Google Scholar]
Taheri HD 2009 {published data only}
- Mortazavi M, Taheri S, Shahidi S, Pourmoghaddas A, Yaraghi MG, Seyrafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function [abstract no: FP379]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi145. [CENTRAL: CN-00716090] [PubMed] [Google Scholar]
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi Journal of Kidney Diseases & Transplantation 2009;20(3):392-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function [abstract no: S-PO-0370]. In: 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21-25; Rio de Janeiro, Brazil. 2007:148.
TOPCAT 2014 {published data only}
- Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT Trial. JACC Heart Failure 2017;5(4):241-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beldhuis IE, Myhre PL, Claggett B, Damman K, Fang JC, Lewis EF, et al. Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. JACC Heart Failure 2019;7(1):25-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bristow MR, Sharma K, Assmann SF, Linas S, Gersh BJ, Grady C, et al. Data and Safety Monitoring Board evaluation and management of a renal adverse event signal in TOPCAT. European Journal of Heart Failure 2017;19(4):457-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dalzell JR. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;371(2):179. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Efthimiadis GK, Zegkos T, Karvounis H. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;371(2):179-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kristensen S, Mogensen UM, Jhund PS, Rorth R, Anand IS, Carson PE, et al. N-terminal pro-B-type natriuretic peptide levels for risk prediction in patients with heart failure and preserved ejection fraction according to atrial fibrillation status. Circulation: Heart Failure 2019;12(3):e005766. [EMBASE: 627472409] [DOI] [PubMed] [Google Scholar]
- Kumar N, Garg N. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;371(2):180. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morawietz H, Bornstein SR. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;371(2):181. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131(1):34-42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Pitt B, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;371(2):181-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine 2014;370(15):1383-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, O'Meara E, et al. Prognostic value of albuminuria and influence of spironolactone in heart failure with preserved ejection fraction. Circulation: Heart Failure 2018;11(11):e005288. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation: Heart Failure 2014;7(5):740-51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circulation: Heart Failure 2013;6(2):184-92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardeny O, Claggett B, Vaduganathan M, Beldhuis I, Rouleau J, O'Meara E, et al. Influence of age on efficacy and safety of spironolactone in heart failure. JACC: Heart Failure 2019;7(12):1022-28. [EMBASE: 2003801409] [DOI] [PubMed] [Google Scholar]
Toto 2005 {published data only}
- Toto R, Mortazavi K, Jones K, Roman-Latorre J, Dominguez M, Blackstone S, et al. Combined blockade with the renin-angiotensin-aldosterone system to improve outcomes in diabetics with nephropathy (DN) [abstract no: PUB582]. Journal of the American Society of Nephrology 2005;16:908A. [Google Scholar]
Viswanathan 2013 {published data only}
- Viswanathan V, Mohan V, Subramani P, Parthasarathy N, Subramaniyam G, Manoharan D, et al. Effect of spironolactone and amiloride on thiazolidinedione-induced fluid retention in South Indian patients with type 2 diabetes. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(2):225-32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00315016 {published data only}
- Deinum J. Eplerenone, ACE inhibition and albuminuria. www.clinicaltrials.gov/ct2/show/NCT00315016 (first received 17 April 2006).
SPIRO‐CKD 2017 {published data only}94696478
- Hayer MK, Edwards NC, Slinn G, Moody WE, Steeds RP, Ferro CJ, et al. A randomized, multicenter, open-label, blinded end point trial comparing the effects of spironolactone to chlorthalidone on left ventricular mass in patients with early-stage chronic kidney disease: Rationale and design of the SPIRO-CKD trial. American Heart Journal 2017;191:37-46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
BARACK D 2014 {published data only}44522369
- Hill NR, Lasserson D, Thompson B, Perera-Salazar R, Wolstenholme J, Bower P, et al. Benefits of Aldosterone Receptor Antagonism in Chronic Kidney Disease (BARACK D) trial-a multi-centre, prospective, randomised, open, blinded end-point, 36-month study of 2,616 patients within primary care with stage 3b chronic kidney disease to compare the efficacy of spironolactone 25 mg once daily in addition to routine care on mortality and cardiovascular outcomes versus routine care alone: study protocol for a randomized controlled trial. Trials 2014;15:160. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Ogburn E, Yu L, Begum N, Long A, Hobbs FDR. On-site monitoring of primary outcomes is important in primary care clinical trials: Benefits of Aldosterone Receptor Antagonism in Chronic Kidney Disease (BARACK-D) Trial a case study [abstract]. Trials 2019;20(Suppl 1). [EMBASE: 629760839] [Google Scholar]
FIDELIO‐DKD 2019 {published data only}
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Nowack C, et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. American Journal of Nephrology 2019;50(5):333-44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FIGARO‐DKD 2019 {published data only}
- Ruilope LM, Agarwal R, Anker SD, Bakris GL, Filippatos G, Nowack C, et al. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. American Journal of Nephrology 2019;50(5):345-56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00870402 {published data only}
- Espinoza FG. Effect of aldosterone antagonism in the reduction of albuminuria and diastolic disfunction of patients with diabetic nephropathy. www.clinicaltrials.gov/ct2/show/NCT00870402 (first received 27 March 2009).
Additional references
AASK 2002
- Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial.[Erratum in: JAMA. 2006 Jun 21;295(23):2726]. Journal of the American Medical Association 2002;288(19):2421-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aldigier 2005
- Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. Journal of the American Society of Nephrology 2005;16(11):3306-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AMBER 2019
- Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019;394(10208):1540–50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bomback 2007
- Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nature Clinical Practice Nephrology 2007;3(9):486-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bramlage 2016
- Bramlage P, Swift SL, Thoenes M, Minguet J, Ferrero C, Schmieder RE. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. European Journal of Heart Failure 2016;18(1):28-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brenner 2001
- Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 2001;345(12):861-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 2000
- Brown NJ, Nakamura S, Ma L, Nakamura I, Donnert E, Freeman M, et al. Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney International 2000;58(3):1219-27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Currie 2016
- Currie G, Taylor AH, Fujita T, Ohtsu H, Lindhardt M, Rossing P, et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrology 2016;17(1):127. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fritsch Neves 2003
- Fritsch Neves M, Schiffrin EL. Aldosterone: a risk factor for vascular disease. Current Hypertension Reports 2003;5(1):59-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GISEN 1997
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997;349(9069):1857-63. [MEDLINE: ] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Green 1996
- Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. Journal of Clinical Investigation 1996;98(4):1063-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hollenberg 2004
- Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney International 2004;66(1):1-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hostetter 2003
- Hostetter TH, Ibrahim HN. Aldosterone in chronic kidney and cardiac disease. Journal of the American Society of Nephrology 2003;14(9):2395-401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hou 2015
- Hou J, Xiong W, Cao L, Wen X, Li A. Spironolactone add-on for preventing or slowing the progression of diabetic nephropathy: a meta-analysis. Clinical Therapeutics 2015;37(9):2086-103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IDNT 2004
- Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Crespigny PJ, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. American Journal of Kidney Diseases 2004;45(2):281-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ishani 2006
- Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. Journal of the American Society of Nephrology 2006;17(5):1444-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jafar 2001
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Annals of Internal Medicine 2001;135(2):73-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kolkhof 2014
- Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. Journal of Cardiovascular Pharmacology 2014;64(1):69-78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levey 2003
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification.[Erratum in: Ann Intern Med. 2003 Oct 7;139(7):605]. Annals of Internal Medicine 2003;139(2):137-47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathiesen 1999
- Mathiesen ER, Hommel E, Hansen HP, Smidt UM, Parving HH. Randomised controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ 1999;319(7201):24-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavrakanas 2014
- Mavrakanas TA, Gariani K, Martin PY. Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: an emerging paradigm in diabetic nephropathy. A systematic review. European Journal of Internal Medicine 2014;25(2):173–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mills 2015
- Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney International 2015;88(5):950-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakhoul 2008
- Nakhoul F, Khankin E, Yaccob A, Kawachi H, Karram T, Awaad H, et al. Eplerenone potentiates the antiproteinuric effects of enalapril in experimental nephrotic syndrome. American Journal of Physiology - Renal Physiology 2008;294(3):F628-37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nappi 2011
- Nappi JM, Sieg A. Aldosterone and aldosterone receptor antagonists in patients with chronic heart failure. Vascular Health & Risk Management 2011;7:353-63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nugent 2011
- Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: s 21st century challenge in global health. Nephron Clinical Practice 2011;118(3):c269–277. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phillips 2007
- Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Archives of Internal Medicine 2007;167(18):1930-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pitt 1999
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 1999;341(10):709-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pitt 2003
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction.[Erratum in: N Engl J Med. 2003 May 29;348(22):2271]. New England Journal of Medicine 2003;348(14):1309-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
REIN 1998
- Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. "Gruppo Italiano di Studi Epidemiologici in Nefrologia" (GISEN). Kidney International 1998;53(5):1209–16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RENAAL 2001
- Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 2001;345(12):861-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RENAAL 2004
- Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney International 2004;65(6):2309-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rocha 1998
- Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT Jr. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 1998;31(1 Pt 2):451-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schieppati 2003
- Schieppati A, Remuzzi G. The June 2003 Barry M. Brenner Comgan lecture. The future of renoprotection: frustration and promises. Kidney International 2003;64(6):1947-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Silvestre 1998
- Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, et al. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. Journal of Biological Chemistry 1998;273(9):4883-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SONG‐GD 2019
- Carter SA, Lightstone L, Cattran D, Bagga A, Barbour SJ, Barratt J, et al. Standardized Outcomes in Nephrology-Glomerular Disease (SONG-GD): establishing a core outcome set for trials in patients with glomerular disease. Kidney International 2019;95(6):1280–3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SPin‐D 2019
- Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR, et al. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney International 2019;95(4):973-82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Staessen 1981
- Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise in plasma concentration of aldosterone during long-term angiotensin II suppression. Journal of Endocrinology 1981;91(3):457-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strippoli 2005
- Strippoli GF, Craig M, Schena FP, Craig JC. Antihypertensive agents for primary prevention of diabetic nephropathy. Journal of the American Society of Nephrology 2005;16(10):3081-91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strippoli 2006
- Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006257. [DOI: 10.1002/14651858.CD006257] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2017
- Sun LJ, Sun YN, Shan JP, Jiang GR. Effects of mineralocorticoid receptor antagonists on the progression of diabetic nephropathy. Journal of Diabetes Investigation 2017;8(4):609-18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2016
- Takahashi S, Katada J, Daida H, Kitamura F, Yokoyama K. Effects of mineralocorticoid receptor antagonists in patients with hypertension and diabetes mellitus: a systematic review and meta-analysis. Journal of Human Hypertension 2016;30(9):534–42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turin 2012
- Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, et al. Lifetime risk of ESRD. Journal of the American Society of Nephrology 2012;23(9):1569-78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016
- Wang V, Vilme H, Maciejewski ML, Boulware LE. The economic burden of chronic kidney disease and end-stage renal disease. Seminars in Nephrology 2016;36(4):319-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bolignano 2014
- Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD007004. [DOI: 10.1002/14651858.CD007004.pub3] [DOI] [PubMed] [Google Scholar]
Navaneethan 2008
- Navaneethan SD, Nigwekar SU, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD007004. [DOI: 10.1002/14651858.CD007004] [DOI] [PubMed] [Google Scholar]
Navaneethan 2009
- Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(3):542-51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


