Abstract
Background
Medications used to treat inflammatory bowel disease (IBD) have significantly improved patient outcomes and delayed time to surgery. However, some of these therapies are recognized to increase the general risk of infection and have an unclear impact on postoperative infection risk.
Objectives
To assess the impact of IBD medications on postoperative infection risk within 30 days of surgery.
Search methods
We searched the Cochrane IBD Groups Specialized Register (29 October 2019), MEDLINE (January 1966 to October 2019), EMBASE (January 1985 to October 2019), the Cochrane Library, Clinicaltrials.gov and the WHO International Clinical Trials Registry Platform from inception up to October 2019 and reference lists of articles.
Selection criteria
Randomized controlled trials, quasi‐randomized controlled trials, non‐randomized controlled trials, prospective cohort studies, retrospective cohort studies, case‐control studies and cross‐sectional studies comparing patients treated with an IBD medication preoperatively or within 30 days postoperatively to patients who were not taking that medication. Manuscripts and abstracts were included.
Data collection and analysis
Two authors independently screened titles and abstracts and extracted data. The primary outcome was postoperative infection within 30 days of surgery. Secondary outcomes included incisional infections and wound dehiscence, intra‐abdominal infectious complications and extra‐abdominal infections. Three authors assessed risk of bias using the Newcastle‐Ottawa scale. We contacted authors for additional information when data were missing. For the primary and secondary outcomes, we calculated odds ratios (ORs) and corresponding 95% confidence intervals (95% CI) using the generic inverse variance method. When applicable, we analyzed adjusted and unadjusted data separately. The certainty of the evidence was evaluated using GRADE.
Main results
Sixty‐eight non‐randomized studies were included. Twenty‐four studies had low risk of bias while the remaining had very high risk. Based on pooling of adjusted data, overall infectious complications were increased in patients who received anti‐TNF agents (OR 1.60; 95% CI 1.20 to 2.13; very low certainty evidence) and corticosteroids (OR 1.70; 95% CI 1.38 to 2.09; low certainty evidence). Use of 5‐ASA (OR 0.76; 95% CI 0.51 to 1.14; very low certainty evidence), immunomodulators (OR 1.29; 95% CI 0.95 to 1.76; low certainty evidence) and anti‐integrin agents (OR 1.04; 95% CI 0.79 to 1.36; low certainty evidence) had no impact on overall infectious complications. No difference in the odds of wound‐related complications was seen in patients using corticosteroids, 5‐ASA, immunomodulators, anti‐TNF or anti‐integrin agents when compared to controls. Both corticosteroids and anti‐TNF agents increased odds of intra‐abdominal infection (OR 1.53; 95% CI 1.28 to 1.84; very low certainty evidence and OR 1.38; 95% CI 1.04 to 1.82; very low certainty evidence, respectively) whereas no impact was observed with 5‐ASA, immunomodulators or anti‐integrin agents. The rate of extra‐abdominal infections was not affected by corticosteroids, immunomodulators, anti‐TNF or anti‐integrin agents.
Authors' conclusions
The evidence regarding corticosteroids, 5ASA, immunomodulators, anti‐TNF mediations and anti‐integrin medications was low or very low in certainty. Thus, the impact of these medications on postoperative infectious complications is uncertain and no firm conclusions can be drawn regarding their safety in the perioperative period. Decisions regarding preoperative IBD medications should be tailored to each patient’s unique circumstances. Future studies should focus on controlling for potential confounding factors to generate higher quality evidence.
Keywords: Adult; Female; Humans; Male; Adrenal Cortex Hormones; Adrenal Cortex Hormones/adverse effects; Aminosalicylic Acids; Aminosalicylic Acids/adverse effects; Bias; Colitis, Ulcerative; Colitis, Ulcerative/drug therapy; Confidence Intervals; Crohn Disease; Crohn Disease/drug therapy; Immunologic Factors; Immunologic Factors/adverse effects; Infections; Infections/chemically induced; Infections/epidemiology; Inflammatory Bowel Diseases; Inflammatory Bowel Diseases/drug therapy; Integrins; Integrins/antagonists & inhibitors; Observational Studies as Topic; Observational Studies as Topic/statistics & numerical data; Odds Ratio; Postoperative Complications; Postoperative Complications/chemically induced; Postoperative Complications/epidemiology; Surgical Wound Dehiscence; Surgical Wound Dehiscence/chemically induced; Surgical Wound Dehiscence/epidemiology; Surgical Wound Infection; Surgical Wound Infection/chemically induced; Surgical Wound Infection/epidemiology; Time Factors; Tumor Necrosis Factor-alpha; Tumor Necrosis Factor-alpha/antagonists & inhibitors
Plain language summary
Infection risk after surgery in patients using medications for inflammatory bowel disease
Background
More than 1.2 million individuals in North America are affected by inflammatory bowel disease (IBD). It is a condition that involves inflammation in the large and/or small intestine(s), resulting in symptoms such as diarrhea and abdominal pain. Many of the medications used to treat IBD suppress the immune system. As a result, use of these medications increases the risk of infection. This increased risk of infection is particularly concerning in patients undergoing surgery.
Review Question
This systematic review examined the combined data from 68 previously published studies to determine whether patients using IBD medications around the time of surgery had more infections compared to those not using the same medications.
Study Characteristics
This systematic review is current up to 29 October 2019. It included 68 studies in patients with IBD who underwent surgery. Most participants were 18 years or older and both men and women were included. Five IBD medication groups were examined within our study. Infections were tracked up to 30 days after surgery.
Key Results
Analyses of this large set of data revealed that infection risk around the time of surgery varied depending on which type of IBD medication the patients were on. Patients being treated with corticosteroids or anti‐TNF agents seemed to have more infections after surgery, while those on 5‐ASA, immunomodulators or anti‐integrin agents did not seem to have more infections after surgery. These findings should be taken with caution as our review included studies which were of limited quality, and therefore we were not able to draw any firm conclusions. These findings could help doctors choose which medications to treat IBD patients with before surgery. Decisions should be tailored to each patient's unique health needs. In addition, this study suggests the need to carefully monitor for infections after surgery in patients who are on certain types of IBD medications.
Limitations
One limitation of this systematic review was its dependence on data from a wide range of previously published studies, with various approaches and quality control standards. Most studies examined had very low certainty regarding its conclusions. This review illustrates the need for future high‐quality research examining the impact of medications used to treat IBD on infection risk after surgery.
Summary of findings
Summary of findings 1. Risk of postoperative infectious complications: corticosteroids compared to control.
| Risk of postoperative infectious complications: corticosteroids compared to control | |||||
| Patient or population: inflammatory bowel disease Setting: Intervention: Corticosteroids Comparison: control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with Corticosteroids | ||||
| Overall infectious complications within 30 days of surgery | Study population | OR 1.40 (1.23 to 1.60) | 41 observational studies | ⊕⊝⊝⊝ VERY LOW 1 | |
| 141 per 1,000 | 187 per 1,000 (168 to 209) | ||||
| Overall infectious complications within 30 days of surgery (Adjusted Analysis) | Study population | OR 1.70 (1.38 to 2.09) | 17 observational studies | ⊕⊕⊝⊝ LOW | |
| 141 per 1,000 | 219 per 1,000 (185 to 256) | ||||
| Overall infectious complications within 30 days of surgery (Unadjusted Analysis) | Study population | OR 1.22 (1.03 to 1.45) | 24 observational studies | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| 141 per 1,000 | 167 per 1,000 (145 to 193) | ||||
| Incisional infections and wound dehiscence within 30 days of surgery | Study population | OR 1.41 (0.72 to 2.74) | 7 observational studies | ⊕⊝⊝⊝ VERY LOW 1 3 4 | |
| 20 per 1,000 | 28 per 1,000 (15 to 53) | ||||
| Intra‐abdominal infectious complications within 30 days of surgery | Study population | OR 1.53 (1.28 to 1.84) | 28 observational studies | ⊕⊝⊝⊝ VERY LOW 1 | |
| 60 per 1,000 | 89 per 1,000 (75 to 105) | ||||
| Extra‐abdominal infections within 30 days of surgery | Study population | OR 1.23 (0.97 to 1.55) | 4 observational studies | ⊕⊝⊝⊝ VERY LOW 1 3 | |
| 51 per 1,000 | 62 per 1,000 (50 to 77) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Many studies did not adjust results for important variables such as other medications.
2 Studies did not adjust results for important variables such as other medications.
3 Wide confidence interval
4 High heterogeneity
Summary of findings 2. Risk of postoperative infectious complications: 5‐ASA compared to control.
| Risk of postoperative infectious complications: 5‐ASA compared to control | |||||
| Patient or population: inflammatory bowel disease Setting: Intervention: 5‐ASA Comparison: control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with 5‐ASA | ||||
| Overall infectious complications within 30 days of surgery | Study population | OR 0.76 (0.51 to 1.14) | 6 observational studies | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| 148 per 1,000 1 | 116 per 1,000 (81 to 165) | ||||
| Overall infectious complications within 30 days of surgery (Adjusted Analysis) 1 | Study population | ‐ | 0 studies | ‐ | |
| ‐ | ‐ | ||||
| Overall infectious complications within 30 days of surgery (Unadjusted Analysis) | Study population | OR 0.76 (0.51 to 1.14) | 6 observational studies | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| 148 per 1,000 | 116 per 1,000 (81 to 165) | ||||
| Incisional infections and wound dehiscence within 30 days of surgery | Study population | OR 0.53 (0.30 to 0.95) | 1 study | ‐ | |
| 265 per 1,000 | 160 per 1,000 (98 to 255) | ||||
| Intra‐abdominal infectious complications within 30 days of surgery | Study population | OR 0.77 (0.45 to 1.33) | 3 observational studies | ⊕⊝⊝⊝ VERY LOW 2 3 | |
| 108 per 1,000 | 86 per 1,000 (52 to 139) | ||||
| Extra‐abdominal infections within 30 days of surgery 1 | Study population | ‐ | 0 studies | ‐ | |
| ‐ | ‐ | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Analysis was not performed as no appropriate studies were identified
2 All studies were considered very high risk of bias according to the Newcastle Ottawa Scale
3 Wide confidence interval
4 Unable to assess GRADE as only 1 study was identified
Summary of findings 3. Risk of postoperative infectious complications: immunomodulators compared to control.
| Risk of postoperative infectious complications: immunomodulators compared to control | |||||
| Patient or population: inflammatory bowel disease Setting: Intervention: Immunosuppressive agents Comparison: control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with Immunosuppressive agents | ||||
| Overall infectious complications within 30 days of surgery | Study population | OR 1.11 (0.97 to 1.26) | 31 observational studies | ⊕⊝⊝⊝ VERY LOW 1 | |
| 151 per 1,000 | 165 per 1,000 (147 to 183) | ||||
| Overall infectious complications within 30 days of surgery (Adjusted Analysis) | Study population | OR 1.29 (0.95 to 1.76) | 9 observational studies | ⊕⊕⊝⊝ LOW | |
| 151 per 1,000 | 187 per 1,000 (145 to 239) | ||||
| Overall infectious complications within 30 days of surgery (Unadjusted Analysis) | Study population | OR 1.07 (0.93 to 1.24) | 22 observational studies | ⊕⊝⊝⊝ VERY LOW 1 | |
| 151 per 1,000 | 160 per 1,000 (142 to 181) | ||||
| Incisional infections and wound dehiscence within 30 days of surgery | Study population | OR 1.35 (0.96 to 1.89) | 11 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 62 per 1,000 | 82 per 1,000 (60 to 111) | ||||
| Intra‐abdominal infectious complications within 30 days of surgery | Study population | OR 0.86 (0.66 to 1.12) | 20 observational studies | ⊕⊝⊝⊝ VERY LOW 1 | |
| 82 per 1,000 | 71 per 1,000 (55 to 91) | ||||
| Extra‐abdominal infections within 30 days of surgery | Study population | OR 1.17 (0.80 to 1.71) | 4 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 0 per 1,000 | 0 per 1,000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Many studies did not perform adjusted analyses and were considered to have a high risk of bias according to the Newcastle Ottawa Scale
2 Wide confidence interval
Summary of findings 4. Risk of postoperative infectious complications: anti‐TNF agents compared to control.
| Anti‐TNF‐α agents compared to control in inflammatory bowel disease | |||||
| Patient or population: inflammatory bowel disease Setting: Intervention: Anti‐TNF‐α agents Comparison: control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with Anti‐TNF‐α agents | ||||
| Overall infectious complications within 30 days of surgery | Study population | OR 1.27 (1.09 to 1.47) | 54 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| 112 per 1,000 | 138 per 1,000 (121 to 156) | ||||
| Overall infectious complications within 30 days of surgery (Adjusted Analysis) | Study population | OR 1.60 (1.20 to 2.13) | 17 observational studies | ⊕⊝⊝⊝ VERY LOW 3 | |
| 112 per 1,000 | 167 per 1,000 (131 to 211) | ||||
| Overall infectious complications within 30 days of surgery (Unadjusted Analysis) | Study population | OR 1.14 (0.96 to 1.36) | 37 observational studies | ⊕⊝⊝⊝ VERY LOW 2 3 4 5 | |
| 112 per 1,000 | 125 per 1,000 (108 to 146) | ||||
| Incisional infections and wound dehiscence within 30 days of surgery | Study population | OR 1.18 (0.83 to 1.68) | 24 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| 45 per 1,000 | 53 per 1,000 (38 to 74) | ||||
| Intra‐abdominal infectious complications within 30 days of surgery | Study population | OR 1.38 (1.04 to 1.82) | 39 observational studies | ⊕⊝⊝⊝ VERY LOW 1 3 6 | |
| 66 per 1,000 | 89 per 1,000 (69 to 115) | ||||
| Extra‐abdominal infections within 30 days of surgery | Study population | OR 1.34 (0.96 to 1.87) | 13 observational studies | ⊕⊝⊝⊝ VERY LOW 1 3 | |
| 13 per 1,000 | 18 per 1,000 (13 to 25) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Many studies did not perform adjusted analyses and were considered to have a high risk of bias according to the Newcastle Ottawa Scale
2 Many confidence intervals do not overlap
3 Wide confidence interval
4 Many studies did not performed adjusted analyses and were considered to have a high risk of bias according to the Newcastle Ottawa Scale
5 Studies did not perform adjusted analyses and were considered to have a high risk of bias according to the Newcastle Ottawa Scale
6 High degree of heterogeneity
Summary of findings 5. Risk of postoperative infectious complications: anti‐integrin agents compared to control.
| Risk of postoperative infectious complications: anti‐integrin agents compared to control | |||||
| Patient or population: inflammatory bowel disease Setting: Intervention: Anti‐integrin agents Comparison: control | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with control | Risk with Anti‐integrin agents | ||||
| Overall infectious complications within 30 days of surgery | Study population | OR 1.11 (0.76 to 1.62) | 9 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 136 per 1,000 | 149 per 1,000 (107 to 203) | ||||
| Overall infectious complications within 30 days of surgery (Adjusted Analysis) | Study population | OR 1.04 (0.79 to 1.36) | 2 observational studies | ⊕⊕⊝⊝ LOW | |
| 136 per 1,000 | 141 per 1,000 (111 to 176) | ||||
| Overall infectious complications within 30 days of surgery (Unadjusted Analysis) | Study population | OR 1.06 (0.54 to 2.10) | 7 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 136 per 1,000 | 143 per 1,000 (78 to 248) | ||||
| Incisional infections and wound dehiscence within 30 days of surgery | Study population | OR 1.64 (0.77 to 3.50) | 6 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| 20 per 1,000 | 32 per 1,000 (15 to 67) | ||||
| Intra‐abdominal infectious complications within 30 days of surgery | Study population | OR 0.40 (0.14 to 1.20) | 5 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 | |
| 88 per 1,000 | 37 per 1,000 (13 to 104) | ||||
| Extra‐abdominal infections within 30 days of surgery | Study population | OR 1.15 (0.43 to 3.08) | 5 observational studies | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |
| 28 per 1,000 | 32 per 1,000 (12 to 81) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Many studies did not performed adjusted analyses and were considered to have a high risk of bias according to the Newcastle Ottawa Scale
2 Wide confidence interval
3 High degree of heterogeneity
Background
Description of the condition
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic and incurable disorder characterized by inflammation of the gastrointestinal tract. Inflammation in UC is generally limited to the mucosa of the colon and rectum while Crohn’s disease is associated with transmural inflammation in any portion of the gastrointestinal tract. In addition, both conditions can be associated with extraintestinal manifestations in areas such as the skin, joints, and eyes. Over 1.2 million individuals have a diagnosis of IBD in North America and the worldwide prevalence of this disease is projected to increase exponentially over the next decade (Kaplan 2015).
The goal of IBD treatment is to achieve remission of clinical symptoms and resolution of gut inflammation. Several pharmacological and, if necessary, surgical options are available for the treatment of IBD. Traditionally, depending on the severity of inflammation and symptoms, 5‐aminosalicylates, corticosteroids, immunomodulators and biologic medications have been used. More recently, biosimilars and small molecules have also been incorporated into the treatment algorithm for IBD.
Description of the intervention
A diverse array of medications are available for the treatment of IBD. These medications can be categorized into several broad categories: aminosalicylates, corticosteroids, immunomodulators, biologics, and small molecules.
Some of the oldest drugs used for the treatment of IBD are aminosalicylates. Aminosalicylates refers to a group of drugs that contain the active ingredient 5‐aminosalicylic acid (Sales‐Campos 2015). Commonly used aminosalicylates include mesalamine, balsalazide, olsalazine and sulfasalazine and these drugs can be administered orally in pill form or topically as suppositories and enemas. Aminosalicylates are mainly used for the induction and maintenance of remission in mild to moderate UC. Evidence for the use of aminosalicylates in CD is limited.
Another category of medication used to treat IBD is corticosteroids. Commonly prescribed corticosteroids include prednisone, prednisolone, methylprednisolone and budesonide. Corticosteroids can be administered orally, intravenously or rectally. Corticosteroids are effective at inducing remission of CD and UC but are less suitable as long‐term therapy due to numerous adverse effects such as increased risk of infection, hyperglycemia, osteoporosis, and hypertension (Sales‐Campos 2015).
Immunomodulators include thiopurines, methotrexate, cyclosporine and tacrolimus. Thiopurines include 6‐mercaptopurine and its prodrug, azathioprine. Thiopurines are commonly used maintenance therapies for UC and CD but are not suitable for induction of remission given the slow onset of action of these drugs (Zenlea 2014). Patients treated with thiopurines require regular monitoring due to the potential for serious adverse effects such as hepatotoxicity and bone marrow suppression. Patients are also at increased risk of infections and malignancies such as non‐melanoma skin cancers with long‐term use (Zenlea 2014).
Methotrexate is a folic acid antagonist that can be used for the induction and maintenance of remission of CD. Its role in UC is limited (Herfarth 2018; Sales‐Campos 2015). Uncommon but important adverse effects include opportunistic infections, hypersensitivity pneumonitis, leukopenia and hepatotoxicity (Zenlea 2014). Methotrexate should also be used cautiously in women of childbearing age, as it is teratogenic.
There is limited literature on the use of calcineurin inhibitors such as cyclosporine and tacrolimus for the treatment of IBD. Tacrolimus has been used for the treatment of fistulizing CD and refractory UC but data are limited to small studies (Triantafillidis 2011). Cyclosporine is associated with potentially serious adverse effects such as seizure and permanent nephrotoxicity, and has a narrow therapeutic range. Thus, it is reserved as a rescue therapy for steroid resistant, acute severe UC and as a bridge to other immunosuppressive medications (Zenlea 2014).
Biologics are medications derived partly or completely from living cells (Rawla 2018). The introduction of biologic medications in the late 1990s revolutionized the treatment of IBD. While biologics are effective, these drugs can cause undesired adverse effects such as infections, antibody formation and malignancies. Biologics used for the treatment of IBD include anti‐tumor necrosis factor‐alpha (TNF‐α) antibodies, anti‐integrin antibodies (natalizumab and vedolizumab), and anti‐interleukin antibodies (ustekinumab). Anti‐TNF‐α medications approved for use in CD include infliximab, adalimumab and certolizumab pegol. Infliximab, adalimumab and golimumab are approved medications for UC.
Natalizumab and vedolizumab are anti‐integrins. Natalizumab’s use is limited due to its association with progressive multifocal leukoencephalopathy (PML) (Reinglas 2018; Zenlea 2014). Vedolizumab is approved for treatment of moderate to severe CD and UC. Natalizumab inhibits both α4β1 integrin and α4β7 integrin as opposed to vedolizumab, which acts only on the α4β7 integrin. As it is more selective, vedolizumab does not carry the same level of risk for PML (Zenlea 2014). However, theoretical concerns have been raised that vedolizumab could impair postoperative wound healing because it targets leukocyte migration, a necessary component of wound healing (Law 2018).
Biosimilars have also entered treatment algorithms. There are four biosimilars approved by the FDA for infliximab and four for adalimumab as of February 2020. Indications for these biosimilars are the same as the licensed indications for the originator product. Studies evaluating switching from originator drugs to biosimilars have generally not shown inferiority (Reinglas 2018).
Lastly, small molecules are an emerging class of IBD therapy. Tofacitinib is a new oral medication approved for the treatment of UC in the United States in 2018. Studies of tofacitinib in UC patients reported an elevated risk of herpes zoster, particularly in patients treated with higher dosing (i.e. 10mg BID) (Reinglas 2018).
How the intervention might work
The aim of medical therapy in IBD is to decrease inflammation and hence alleviate symptoms and allow mucosal healing (Rawla 2018). Current medications target different stages of the inflammatory cascade that is believed to underpin IBD pathogenesis. Aminosalicylates topically decrease inflammation in the colon through three main ways: inhibition of macrophage chemotaxis, increase in intestinal epithelial cell proliferation, and activation of peroxisome proliferator activated receptor γ (Sales‐Campos 2015). Corticosteroids systemically suppress inflammation by down regulating the transcription of proinflammatory genes involved in cytokine production and inhibiting the recruitment of immune cells (Sales‐Campos 2015). Thiopurines inhibit lymphocyte proliferation and induce apoptosis of activated T‐lymphocytes (Sales‐Campos 2015; Zenlea 2014). Methotrexate is a folic acid antagonist, which increases adenosine, inhibits interleukin‐1 and suppresses T cell function (Zenlea 2014). Cyclosporine and tacrolimus are calcineurin inhibitors. These drugs act by suppressing cytokine production and T‐cell activation (Triantafillidis 2011; Zenlea 2014).
Biologics work by targeting various pro‐inflammatory molecules. Anti‐TNF drugs inhibit tumor necrosis factor‐α, a key cytokine in the pathogenesis of IBD (Sales‐Campos 2015). Infliximab is a chimeric human‐mouse monoclonal antibody. It has increased specificity and affinity to the TNF receptor and hence blocks TNF‐α from binding (Rawla 2018). Adalimumab is a fully human monoclonal antibody that inhibits TNF‐α and its ability to interact with p55 and p75 cell surface receptors (Rawla 2018). Other anti‐TNF medications used to treat IBD include certolizumab, a recombinant antigen‐binding fragment antibody against TNF‐α conjugated to polyethylene glycol, and golimumab, a fully human monoclonal antibody that binds to and inhibits soluble and transmembrane forms of anti‐TNF (Rawla 2018). Ustekinumab functions by blocking the activity of interleukin 12 and interleukin 23, which play a role in the activation of natural killer cells and CD4 T lymphocytes (Rawla 2018; Reinglas 2018). Natalizumab is a humanized monoclonal antibody that is an antagonist to both α4β1 integrins and α4β7 integrins. It works by inhibiting the translocation of leukocytes across blood vessel membranes (Rawla 2018). In comparison, vedolizumab is a monoclonal antibody to only the α4β7 integrin. As a result, vedolizumab is gut‐selective. It prevents T cell activation and adhesion through blocking the binding of mucosal addressin cell adhesion molecule‐1 to the integrin receptor (Rawla 2018).
Biosimilars are biological medications that are highly similar to the reference product and work in the same ways. There are minor differences in clinically inactive components with no clinically meaningful differences in safety and efficacy (Reinglas 2018). Tofacitinib is an inhibitor of janus kinase enzymes, and functions by suppressing cytokine signaling in mucosal cells (Reinglas 2018).
Why it is important to do this review
The growth of medical treatment options has improved physicians’ ability to manage IBD medically and in many cases, delay or avoid surgery (Frolkis 2013; Lichtenstein 2005; Rungoe 2014). However, despite these advances, a meta‐analysis found that nearly half of CD patients and 16% of UC patients required surgery within 10 years of diagnosis (Frolkis 2013). Many medications commonly used to treat IBD such as corticosteroids, immunomodulators, and biologics are recognized to increase the general risk of infection (Rawla 2018). However, the impact of these medications on surgical outcomes is controversial. Concerns have been raised that preoperative treatment with these medications could theoretically impair wound healing and in turn, increase postoperative infections and other complications (Appau 2008; Lightner 2017b; Magro 2017). Of particular concern are biologic medications, as long‐term information on safety, especially with regards to the perioperative setting, is scarce and limited mostly to observational studies. Given the important role TNF‐α plays in stimulating dermal fibroblast proliferation and activity, investigators have examined its impact on wound healing in rat models. Lee et al demonstrated that continuous suppression of TNF‐α decreased wound breaking strength in rats, raising the possibility of a similar outcome in humans treated with anti‐TNF medications (Lee 2000). Additionally, anti‐integrins such as vedolizumab function by blocking leukocyte migration to the gut. However, leukocytes are also critical to wound healing, and thus theoretically could impair anastomotic and stoma healing (Argollo 2018; Lightner 2017b). Current studies evaluating this topic have yielded conflicting results (Argollo 2018; Kopylov 2012; Law 2018; Narula 2013; Yang 2012; Yang 2014; Xu 2019). Therefore, a systematic review of the literature would be valuable to study the impact of perioperative IBD medications on the risk of postoperative infectious complications.
Objectives
The primary objective of this review was to assess the impact of perioperative IBD medications on the risk of postoperative infections within 30 days of surgery.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials, quasi‐randomized controlled trials, non‐randomized controlled trials, prospective cohort studies, retrospective cohort studies, case‐control studies and cross‐sectional studies were considered for inclusion. Meta‐analyses, systematic reviews, case series, and case reports were excluded. Studies lacking a comparison or control group were also excluded. Studies reporting complications that occurred greater than 30 days after surgery were excluded as infections outside this time period may be less likely to be related to the surgery. Manuscripts as well as abstracts were considered for inclusion.
Types of participants
The majority of patients in each study were required to be adults (at least 18 years in age). Patients needed to have a diagnosis of Crohn’s disease, ulcerative colitis, or indeterminate colitis and have undergone surgery, including both abdominal and non‐abdominal surgeries.
Types of interventions
We included studies comparing patients treated with an IBD medication (preoperatively or within 30 days postoperatively, as treatment during this time period could potentially influence rates of early infectious complications) to patients who were not taking that medication. What constituted preoperative treatment was not fixed and was based on the definitions used by the authors of the primary studies. Comparison groups could include another active medication, placebo, or a no treatment control. Studies that compared post‐operative outcomes between two biologics (e.g. vedolizumab versus infliximab) were excluded as these studies generally all suffered from confounding by indication. We examined the following classes of medications: 1. Aminosalicylates (5‐ASA): balsalazide, mesalamine, olsalazine, sulfasalazine; 2. Corticosteroids: budesonide, methylprednisolone, prednisolone, prednisone; 3. Immunomodulators: azathioprine, 6‐mercaptopurine, methotrexate, cyclosporine, tacrolimus; 4. Anti‐TNF medications: adalimumab, certolizumab, golimumab, infliximab; 5. Anti‐interleukin medications: ustekinumab; 6. Anti‐integrin medications: vedolizumab, natalizumab; and 7. Small Molecules: tofacitinib.
Types of outcome measures
We investigated the following postoperative infectious complications.
Primary outcomes
The primary outcome was postoperative infection within 30 days of surgery.
Secondary outcomes
The secondary outcomes were:
1. Incisional infections and wound dehiscence;
2. Intra‐abdominal infectious complications including anastomotic leak, intra‐abdominal abscess and enterocutaneous fistula; and
3. Extra‐abdominal infections including pneumonia, urinary tract infection, bacteremia, catheter associated infections and other infections.
Search methods for identification of studies
Electronic searches
We searched the following databases from inception up to October 29, 2019: MEDLINE, Embase, the Cochrane Library, the Cochrane IBD Group Specialized Register, Clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform. The search strategies for each database are reported in Appendix 1.
Searching other resources
To identify additional studies, we screened the bibliographies of applicable systematic reviews.
Data collection and analysis
Selection of studies
Two investigators (CL and YB) independently screened the titles and abstracts identified by the literature search. Potentially relevant articles were reviewed in full to determine eligibility for inclusion. When necessary, we attempted to contact study authors for clarification. Any disagreements were resolved through consensus and evaluation by a third investigator (NN).
Data extraction and management
Three investigators (CL, CB and NN) performed data extraction independently. In cases where data were missing, we attempted to contact authors for additional information. The following information was extracted from the studies: 1. Study Characteristics: Author, year of publication, time period of study, country of origin, format (paper/abstract), study design, inclusion and exclusion criteria; 2. Patient and IBD Disease Characteristics: Mean age, gender, number of patients by IBD subtype, type of surgery performed, perioperative IBD medication(s), last dose of medication prior to surgery, emergency versus elective surgery; and 3. Outcome Assessment: Length of follow‐up period, rate of overall postoperative infectious complications, rate of incisional infections/wound dehiscence, rate of intra‐abdominal infectious complications, rate of extra‐abdominal infections.
Assessment of risk of bias in included studies
Four investigators (CL, CB, DK and YB) independently assessed the methodological quality of included studies using the Newcastle‐Ottawa Scale (Wells 2019). Studies were evaluated based on the selection of the study groups (four questions), the comparability of the groups (two questions), and the ascertainment of either the exposure or outcome of interest (three questions) for case‐control or cohort studies respectively. A maximum of 1 point was awarded for each question. Studies with 3 points in the selection domain, and 1 point in the comparability domain, and 2 points in the outcome domain were considered to have a low risk of bias. Studies with 2 points in the selection domain, and 1 point in the comparability domain, and 2 points in the outcome domain were deemed to have a high risk of bias. Finally, studies with 1 point in the selection domain, or 0 points in the comparability domain, or 1 point in the outcome domain were considered to have a very high risk of bias. We planned to have four authors (CL, CB, DK and YB) independently assess the risk of bias of RCTs using the Cochrane risk of bias tool. Each study was to be assessed based on sequence generation, allocation sequence concealment, incomplete outcome data, selective outcome reporting and other potential sources of bias. However, no RCTs were identified for inclusion.
Measures of treatment effect
Data was analyzed using Review Manager 5.3. Odds ratio (OR) with corresponding 95% confidence intervals (95% CI) were calculated. Since adjusted odds ratios reported by studies were used where available, the generic inverse variance method was used for obtaining overall pooled OR estimates. For continuous data, we planned to calculate the mean difference (MD) or standardized mean difference (SMD) with corresponding 95% CI as appropriate. If only the MD was reported by a study, the generic inverse variance method was used. However, no continuous data were reported by included studies.
Unit of analysis issues
Whenever possible, we analyzed count data as dichotomous data by extracting the proportion of participants who experienced at least one infection. We attempted to contact authors for clarification whenever necessary. For studies with multiple treatment groups, depending on the situation, one of three strategies was used. If only one of the treatment arms was relevant to the study, the other treatment arms were ignored and the remaining treatment arm was compared to the control group. If two or more treatment arms were relevant and similar (e.g. two types of immunomodulators), these treatment arms were combined into one group. If it was not appropriate to combine the treatment arms (e.g. immunomodulator and anti‐TNF medication), data were analyzed separately. We did not divide the control group between the treatment groups, as the data for each treatment group were used in entirely separate analyses. We planned to use paired analysis with the generic inverse variance method for cross‐over studies, however, these were not encountered. We also did not encounter any cluster‐randomized trials.
Dealing with missing data
For missing dichotomous outcomes, an intention‐to‐treat analysis was used. Patients who were lost to follow‐up or have missing outcome data were considered to have experienced an infection. We attempted to contact authors to provide missing data. We planned to estimate the value of missing continuous outcomes from other values provided in the applicable study. If this was not possible, we planned to impute the value from the mean of the standard deviations of the other studies in the meta analysis. If possible, we also planned to perform a sensitivity analysis of per protocol data. As no studies reported continuous outcomes, these methods were not required in our analysis.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of forest plots and by calculating the Chi² and I² statistics. For the Chi² test, we considered a P value less than 0.10 to be statistically significant. I² values of greater than 50% were considered to indicate substantial heterogeneity. A priori subgroup analyses were performed to explore potential sources of heterogeneity.
Assessment of reporting biases
Publication bias was assessed using funnel plots, provided at least 10 studies were included.
Data synthesis
Data were pooled by like interventions. We planned to conduct separate analyses for corticosteroids, immunosuppressive agents, anti‐TNF agents, biosimilars of anti‐TNF agents, anti‐integrin agents, anti‐interleukin agents, and small molecules, provided at least 2 studies were available for each type of medication. Additionally, data from individual studies were pooled for meta‐analysis only if the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). We planned to analyze randomized and observational data separately but this was not necessary as no randomized studies were identified. For evaluation of the primary outcome, we analyzed adjusted and unadjusted data separately. For dichotomous outcomes, we calculated a pooled OR and 95% CI. For continuous outcomes, we planned to calculate the pooled MD or SMD with corresponding 95% CI. A random‐effects model was used as we anticipated significant heterogeneity in the studies.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses: 1. Crohn’s disease patients versus ulcerative colitis patients; 2. Studies conducted prior to 1998 (year of introduction of the first biologic for IBD) versus studies conducted after 1998; 3. Last dose of biologic within eight weeks prior to surgery versus last dose of biologic greater than eight weeks prior to surgery.
Sensitivity analysis
Sensitivity analysis excluding studies with very high risk of bias according to the Newcastle‐Ottawa Scale, abstracts, and non‐randomized studies was performed. For this analysis, unadjusted and adjusted odds ratios were c ombined. We performed two ad‐hoc sensitivity analyses. The first analysis excluded studies with patients who underwent surgery to alleviate a fistula/abscess or who were found to have an intra‐abdominal abscess intraoperatively. The second analysis excluded studies with potential unit of analysis issues (i.e. studies for which we estimated the overall rate of infection by summation of different types of infections).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence in the review (Guyatt 2008). Evidence for the primary (overall postoperative infectious complications) and secondary outcomes (incisional infections and wound dehiscence, intra‐abdominal infectious complications and extra‐abdominal infections) were evaluated and reported in the 'Summary of findings' tables. Data from RCTs begin as high‐certainty and observational randomized studies begin as low‐certainty evidence. The certainty of the evidence can be downgraded due to risk of bias, inconsistency, indirectness, imprecision or publication bias. The certainty of the evidence can be upgraded due to a large magnitude of effect, dose response gradient, and a result that opposes any plausible residual confounding (Guyatt 2008). Ultimately, the certainty of the evidence for each outcome was determined to be high (further research is unlikely to change our confidence in the estimate of effect), moderate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), low (further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate), or very low (any estimate of effect is very uncertain). We resolved disagreements by discussion and consensus.
Results
Description of studies
We included 68 studies in total in this review; forty one evaluated perioperative corticosteroid therapy, six evaluated perioperative 5ASA therapy, thirty one evaluated perioperative immunomodulator therapy, fifty four evaluated perioperative anti‐TNF therapy, nine evaluated perioperative anti‐integrin therapy and one evaluated perioperative anti‐interleukin therapy.
Results of the search
We conducted a literature search on August 30, 2018, which identified 12,248 citations. Two additional studies were identified through other sources. Duplicate studies were counted as secondary publications of those studies that were included. 9709 studies remained for screening. 9594 studies were excluded after review of the titles and abstracts. We retrieved the full text of the remaining 115 studies. Of these, 52 studies were excluded and 63 studies were included in the review (Figure 1). An updated literature search was performed on October 29, 2019. The time period of the updated search was August 1, 2018 to October 29, 2019 and it identified 1726 citations. Duplicate studies were counted as secondary publications of studies that were included. 1690 studies were excluded after review of titles and abstracts. 21 studies remained for full text review and of these, 5 studies were included (Figure 2).
1.
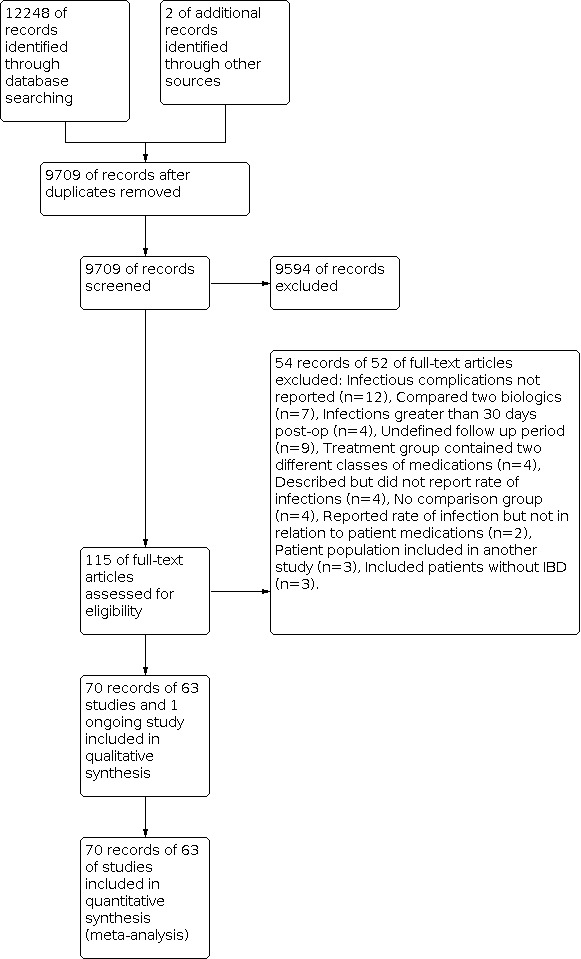
Flow diagram of original search (inception to August 30, 2018).
2.
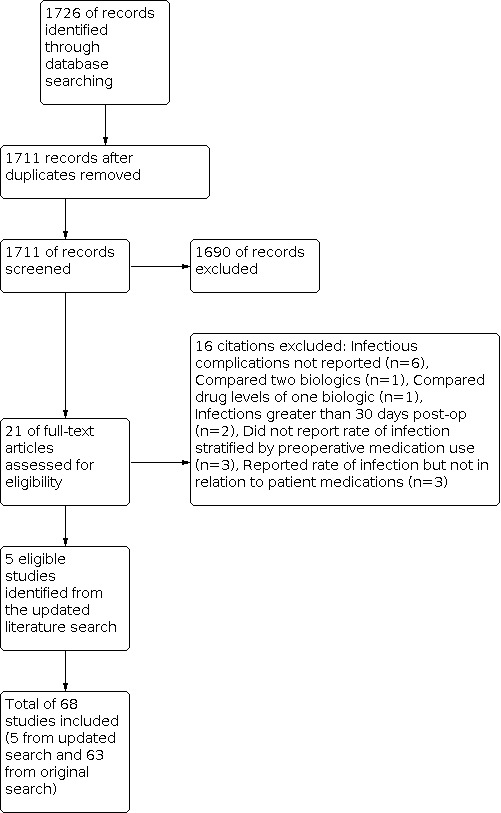
Flow diagram of updated search from August 1, 2018 to October 29, 2019.
Included studies
A total of 68 studies were included in the review. All were observational studies. There were 5 prospective studies (Araki 2014; Brouquet 2018; Fumery 2017; Myrelid 2009; Cohen 2019) and 63 retrospective studies. From these, 60 were manuscripts and 8 were abstracts. All the prospective studies except for one (Cohen 2019) are currently published as manuscripts. The included studies were heterogenous in their patient selection criteria. The specific criteria for each study are outlined in the Characteristics of included studies section. Some studies focused on only CD or UC patients, while others included a combination of the two. A small percentage of studies also included patients with indeterminate colitis. Both elective and emergent surgeries were included in our study. All selected studies examined patients who underwent abdominal surgery.
With regards to the type of preoperative medication studied, 41 studies examined corticosteroids (Aberra 2003; Alves 2007; Appau 2008; Bregnbak 2012; Colombel 2004; De Buck Van Overstraeten 2017; El‐Hussuna 2012; Ferrante 2009; Ferrante 2017; Fumery 2017; Gainsbury 2011; Guo 2017; Jouvin 2018; Krane 2013; Kunitake 2008; Liang 2017; Lightner 2018 B; McKenna 2018; Mor 2008; Morar 2015; Myrelid 2009; Myrelid 2014; Nasir 2010; Nguyen 2014; Regadas 2011; Rizzo 2011; Schils 2017; Selvasekar 2007; Serradori 2013;Shaib 2017; Tzivanakis 2012; Uchino 2015; Uchino 2019; Wilson 2014; Yamada 2017; Yamamoto 2000; Yamamoto 2016; Yu 2019; Zittan 2016; Ziv 1996; Zuo 2014), 6 examined 5ASAs (Ferrante 2017; Guo 2017; Liang 2017; Morar 2015; Myrelid 2009; Uchino 2013a), 31 examined immunomodulators (Aberra 2003; Afzali 2016; Araki 2014; Appau 2008; Colombel 2004; El‐Hussuna 2012; Ferrante 2009; Ferrante 2017; Gainsbury 2011; Guo 2017; Jouvin 2018; Krane 2013; Liang 2017; Lightner 2018b; Mahadevan 2002; McKenna 2018; Mor 2008; Morar 2015; Myrelid 2009; Myrelid 2014; Nasir 2010; Regadas 2011; Rizzo 2011; Selvasekar 2007; Uchino 2010; Uchino 2013a; Uchino 2013b; Uchino 2015; Uchino 2019; Yamamoto 2016, Yu 2019), 54 examined anti‐TNF agents (Appau 2008; Ayoub 2018; Bregnbak 2012; Brouquet 2018; Cohen 2019; Canedo 2011; Colombel 2004; Coquet‐Reinier 2010; De Buck Van Overstraeten 2017; El‐Hussuna 2012; Eshuis 2013; Ferrante 2009; Ferrante 2017; Fumery 2017; Gainsbury 2011; Gu 2013; Guasch 2016; Gudsoorkar 2018; Guo 2017; Jouvin 2018; Kim 2018; Kotze 2017; Krane 2013; Kunitake 2008; Liang 2017; Lightner 2018 A; Lightner 2018 B; Marchal 2004; McKenna 2018; Mor 2008; Morar 2015; Myrelid 2014; Nasir 2010; Norgard 2012; Norgard 2013; Novello 2020; Regadas 2011; Rizzo 2011; Schils 2017; Schluender 2007; Selvasekar 2007; Serradori 2013; Shwaartz 2016; Syed 2013; Uchino 2013a; Uchino 2013b; Uchino 2015; Uchino 2019; Ward 2018; Waterman 2013; Yamada 2017; Yamamoto 2016; Yu 2019; Zittan 2016), 9 examined anti‐integrin agents (Ayoub 2018; Ferrante 2017; Gudsoorkar 2018; Kim 2018; Liang 2017; Lightner 2018 A; Novello 2020; Schils 2017; Yamada 2017) and only 1 study examined ustekinumab (Liang 2017). No studies regarding small molecules or biosimilars were found.
Among the studies examining preoperative corticosteroids, a variety of doses were used. Among the studies examining anti‐TNF medications, the timing of the last dose prior to surgery also varied from study to study. For instance, 17 studies considered a patient to have been treated preoperatively with an anti‐TNF agent only if they received a dose within 8 weeks of surgery. Thirty four studies used a longer cut off time and the remaining 3 did not specify the time of last dose of anti‐TNF medication. All postoperative infectious outcomes occurred within 30 days of surgery. A diverse array of infections was reported by each study. Commonly reported outcomes included overall infectious complications, wound infections, anastomotic leaks, intra‐abdominal abscesses, pneumonia and urinary tract infections. While some studies grouped infections into categories such as intra‐abdominal infections and extra‐abdominal infections, others studies reported each type of infection individually. To allow for comparability between trials, we categorized infections as incisional, intra‐abdominal and extra‐abdominal. Some studies also provided an overall rate of infection. When this was not available, we estimated the overall rate of infections by combining the rates of individual infections reported in a study. As some patients could have had more than one postoperative infection, this could potentially create a unit of analysis error. The corresponding author of Schils 2017 kindly provided additional data, which allowed us to identify patients that experienced more than one postoperative infection.
Excluded studies
Following full‐text review, 68 studies were excluded for the following reasons:
Overall postoperative complications but not specifically infectious complications were reported in 18 studies (Achkasov 2015; Bafford 2013; Braun 2018, Chaparro 2018; Coscia 2012; Fronda 1999; Gamaleldin 2018; Gonzalez 2013; Grant 2019, Justiniano 2019; Kamel 2019; Li 2016; Melo‐Pinto 2018; Monsinjon 2017; Quade 2013; Scarpa 2015; Watson 2018; Weber 2017). We attempted to contact the authors for additional information but were unsuccessful.
Seven studies did not provide numerical data regarding incidence of infections stratified by patients’ preoperative medication use (Adegbola 2018; Benichou 2018; Desai 2012; Kimura 2019; Lau 2013; Oh 2014; Yamamoto 2016a). We attempted to contact authors for additional information but were unsuccessful. Eight studies were excluded because they compared postoperative outcomes between two biologic medications (Aelvoet 2016; Lightner 2017a; Lightner 2018a; Lightner 2018b; Novello 2019; Park 2018; Poylin 2018; Shim 2018). One study was excluded because it compared preoperative drug levels in patient treated with ustekinumab and their subsequent rates of postoperative infection (Parrish 2019). Six studies were excluded for including postoperative infections that occurred more than 30 days after surgery (Andrew 2017; Balachandran 2015; Bewtra 2013; Gregory 2019; Kulaylat 2017; Rizvi 2019). Nine studies did not define their follow up period (Bruewer 2003;De Silva 2011; Eisner 2014; Hyde 2001; Kasparek 2012; Krupa 2012; Nagao 2016; Sahami 2016; Shimada 2016). Four studies were excluded because the treatment group included multiple medications (e.g. anti‐TNF medications and tacrolimus) (Abou‐Khalil 2016; Stewart 2009; Valizadeh 2017; Yamamoto 2018). Four studies were excluded because they lacked a comparison group (Chiplunker 2015; Domenech 2016; Labidi 2018; Stringfield 2016). Five studies did not explore the relation between infectious complications and preoperative medications (Abelson 2018; Fu 2014; Heimann 1985; Kline 2020; Lim 2018). Three studies were excluded due to overlap of patients with another, larger study that is included in this review (Kotze 2017a; Kotze 2011; Lightner 2017). Finally, 3 studies were excluded because they included non‐IBD patients (George 2017; Kotze 2018; Strassle 2017).
Risk of bias in included studies
Risk of bias was assessed using the Newcastle‐Ottawa Scale and the results are summarized in Figure 3.
3.

Risk of bias summary for cohort studies (Newcastle Ottawa Scale): review authors' judgements about each risk of bias item for each included study.
Selection
All 63 studies received 1 point for representativeness of the exposed cohort. All the studies included unselected adult patients with CD or UC who underwent surgery, which is representative of the average IBD patient requiring surgery.
All studies received 1 point for selection of the non‐exposed cohort. The non‐exposed cohorts were all drawn from either the same hospitals or databases as the exposed cohort.
Five studies (Ayoub 2018; El‐Hussuna 2012; Gudsoorkar 2018; Jouvin 2018; Uchino 2010) received 0 points for ascertainment of exposure due to not explicitly stating the source of their data.
Another point criterion was for demonstrating that infection was not present at the start of the study, however, no study qualified. In fact, in 25 studies, surgery was performed to alleviate a fistula/abscess, or an intra‐abdominal abscess was discovered intraoperatively (Alves 2007; Appau 2008; Brouquet 2018; Canedo 2011; Cohen 2019; El‐Hussuna 2012; Fumery 2017; Guo 2017; Krane 2013; Kunitake 2008; Lightner 2018 B; Marchal 2004; McKenna 2018; Morar 2015; Myrelid 2009; Myrelid 2014; Rizzo 2011; Serradori 2013; Tzivanakis 2012; Uchino 2013a; Wilson 2014; Yamamoto 2000; Yamamoto 2016; Yu 2019; Zuo 2014).
Comparability
One point was awarded if the study controlled for use of a concomitant medication(s) as we considered it to be the most significant potential confounding factor. Another point was awarded if the study controlled for any other potential confounding factor(s) such as age or length of surgery.
The majority of studies reported the event rate of infectious complications and therefore did not control for other variables. Adjusted analyses were performed in 26 studies (Aberra 2003; Afzali 2016; Alves 2007; Appau 2008; Ayoub 2018; Brouquet 2018; Cohen 2019; Coquet‐Reinier 2010; De Buck Van Overstraeten 2017; Gainsbury 2011; Kim 2018; Krane 2013; Marchal 2004; McKenna 2018; Mor 2008; Novello 2020; Selvasekar 2007; Serradori 2013; Shaib 2017; Syed 2013; Tzivanakis 2012; Uchino 2019; Waterman 2013; Wilson 2014; Yamamoto 2016; Zuo 2014). Of these, 9 studies controlled for concomitant immunomodulator use (Aberra 2003, Appau 2008, Cohen 2019, Gainsbury 2011, Krane 2013, McKenna 2018, Mor 2008, Yamamoto 2016, Selvasekar 2007), 15 studies controlled for concomitant corticosteroid use (Aberra 2003,Afzali 2016,Appau 2008, Ayoub 2018, Brouquet 2018, Cohen 2019, Gainsbury 2011, Krane 2013, McKenna 2018, Mor 2008, Waterman 2013, Yamamoto 2016, Zuo 2014, Selvasekar 2007, Serradori 2013) and 5 studies controlled for concomitant anti‐TNF use (Appau 2008, McKenna 2018, Yamamoto 2016, Selvasekar 2007, Serradori 2013). In addition, studies controlled for a variety of other potential confounding factors including age (Aberra 2003, Appau 2008, Cohen 2019, Coquet‐Reinier 2010, Kim 2018, Marchal 2004, Novello 2020, Selvasekar 2007, Serradori 2013, Tzivanakis 2012, Waterman 2013, Yamamoto 2016, Uchino 2019), gender (Appau 2008, Cohen 2019, Coquet‐Reinier 2010, Kim 2018, Marchal 2004, Novello 2020, Shaib 2017, Tzivanakis 2012, Yamamoto 2016), BMI (Afzali 2016, Gainsbury 2011, Syed 2013, Wilson 2014, Zuo 2014), smoking (Afzali 2016, Shaib 2017, Wilson 2014, Yamamoto 2016, Zuo 2014), combordities (Appau 2008, Cohen 2019, Krane 2013, Wilson 2014) and duration of surgery (Aberra 2003, Brouquet 2018, Shaib 2017, Wilson 2014, Uchino 2019). More information about the factors each study controlled for can be found in the Characteristics of included studies tables.
Outcome
Five studies (Ayoub 2018; El‐Hussuna 2012; Gudsoorkar 2018; Jouvin 2018; Uchino 2010) received 0 points for assessment of outcomes as they did not specify their data collection methodology.
One point for length of follow up was awarded to all studies except Marchal 2004 as patients in this study were followed for only 10 days post‐surgery.
Only thirteen studies were awarded a point for adequacy of follow up as most studies did not comment on this area. Three studies reported a negligible percentage of patients lost to follow up (Afzali 2016; Mahadevan 2002; Morar 2015), while the presence of complete patient data was mandatory in 10 studies (Canedo 2011; Guasch 2016; Krane 2013; Lightner 2018 A; Norgard 2012; Norgard 2013; Waterman 2013; Yamada 2017; Yamamoto 2016; Zittan 2016).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Analysis 1 Corticosteroids vs Control
Pooling of data from 41 studies comparing preoperative corticosteroids to a no treatment control demonstrated an increase in postoperative infectious complications (OR 1.40; 95% CI 1.23 to 1.60, very low certainty evidence; Analysis 1.1). Low heterogeneity was observed in the overall analysis (I2= 40%). Based on 17 of the studies, the adjusted pooled OR was 1.70 (95% CI 1.38 to 2.09; I2 35%; low certainty evidence; Analysis 1.1.1). Unadjusted OR, based on 24 studies was 1.22 (95% CI 1.03 to 1.45; I2 34%; very low certainty evidence; Analysis 1.1.2) Eleven studies included only patients with ulcerative colitis (OR 1.49; 95% CI 1.10 to 2.02, very low certainty evidence) and 23 studies included only patients with Crohn’s disease (OR 1.32; 95% CI 1.11 to 1.57, very low certainty evidence) (Analysis 1.2). A statistically significant subgroup difference was not detected (p=0.50). Increased postoperative infectious complications were seen in studies performed prior to 1998 (OR 1.79; 95% CI 1.20 to 2.66, very low certainty evidence) as well as after 1998 (OR 1.35; 95% CI 1.17 to 1.56, very low certainty evidence) (Analysis 1.3). A statistically significant subgroup difference was not detected (p=0.20). In terms of secondary outcomes, there was no difference in incidence of incisional infection and wound dehiscence with preoperative corticosteroid use in the seven trials which reported this (OR 1.41; 95% CI 0.72 to 2.74, very low certainty evidence) (Analysis 1.4). The incidence of intra‐abdominal infection (28 studies; OR 1.53; 95% CI 1.28 to 1.84, very low certainty evidence) (Analysis 1.5) was significantly higher in the corticosteroid group, while there was no difference observed in extra‐abdominal infections (4 studies; OR 1.23 (95% CI 0.97 to 1.55, very low certainty evidence) (Analysis 1.6). Primary outcome findings were not affected by excluding very high risk of bias studies (1 5 studies; OR 1.4 3 (95% CI 1.1 3 to 1.8 1 ) (Analysis 1.7) and studies published as a full manuscript (36 studies; OR 1.48 (95% CI 1.28 to 1.72) (Analysis 1.8). A sensitivity analysis was also performed excluding studies that did not adjust for patients who underwent surgery to repair an intrabdominal abscess/fistula or were found to have an abscess intraoperatively and our findings remained significant (21 studies; OR 1.37 (95% CI 1.14 to 1.65) (Analysis 1.9).
1.1. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 1: Postoperative infection within 30 days of surgery
1.2. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 2: Postoperative infection within 30 days of surgery: subgroup UC vs CD
1.3. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 3: Postoperative infection within 30 days of surgery: subgroup pre 1998 versus 1998 or after
1.4. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 4: Incisional infections and wound dehiscence
1.5. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 5: Intra‐abdominal infectious complications
1.6. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 6: Extra‐abdominal infections
1.7. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 7: Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias
1.8. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 8: Postoperative infection within 30 days of surgery: sensitivity exclude abstract
1.9. Analysis.

Comparison 1: Corticosteroids versus control, Outcome 9: Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess
Analysis 2 5ASA vs Control
Pooling of data from the 6 studies (all unadjusted outcomes) comparing preoperative 5ASA versus no 5ASA demonstrated no increase in overall postoperative infectious complications (OR 0.76; 95% CI 0.51 to 1.14, very low certainty evidence) (Analysis 2.1). High heterogeneity was observed in the overall analysis (I2= 60%). One study included only patients with ulcerative colitis and 4 studies included only patients with Crohn’s disease (OR 0.70; 95% CI 0.45 to 1.07, very low certainty evidence) (Analysis 2.2). A statistically significant subgroup difference was not detected (p=0.41). Studies performed prior to 1998 (OR 1.08; 95% CI 0.47 to 2.51, very low certainty evidence) and studies performed after 1998 (OR 0.71; 95% CI 0.45 to 1.14, very low certainty evidence) both demonstrated no difference in postoperative infectious complications (Analysis 2.3). A statistically significant subgroup difference was not detected (p=0.39). In terms of secondary outcomes, only 1 study reported rates of incisional infections and wound dehiscence (Analysis 2.4). Data regarding intra‐abdominal infections was reported in 3 studies. The OR was 0.77 (95% CI 0.45 to 1.33, very low certainty evidence) (Analysis 2.5). No studies reported rates of extra‐abdominal infections. Primary outcome findings were not significantly changed within a sensitivity analysis excluding studies that did not adjust for patients who underwent surgery to repair an intrabdominal abscess/fistula or were found to have an abscess intraoperatively (2 studies; OR 0.79; 95% CI 0.36 to 1.73) (Analysis 2.7). Other pre‐planned sensitivity analyses were not performed as no studies were classified as low risk of bias and all 6 studies were manuscripts.
2.1. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 1: Postoperative infection within 30 days of surgery
2.2. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 2: Postoperative infection within 30 days of surgery: subgroup UC vs CD
2.3. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 3: Postoperative infection within 30 days of surgery: subgroup pre 1998 versus 1998 or after
2.4. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 4: Incisional infections and wound dehiscence
2.5. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 5: Intra‐abdominal infectious complications
2.7. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 7: Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess
Analysis 3 Immunomodulators vs Control
Pooling of data from the 31 studies comparing preoperative immunomodulators versus no immunomodulator treatment demonstrated no difference in the incidence postoperative infectious complications (OR 1.11; 95% CI 0.97 to 1.26, very low certainty evidence) (Analysis 3.1). Low heterogeneity was observed in the overall analysis (I2= 0%). Based on 9 of the studies, the adjusted pooled OR was 1.29 (95% CI 0.95 to 1.76; I2 0%; low certainty evidence; Analysis 3.1.1). Unadjusted OR, based on 22 studies, was 1.07 (95% CI 0.93 to 1.24; I2 0%; very low certainty evidence; Analysis 1.1.2) Eleven studies included only patients with ulcerative colitis (OR 1.10; 95% CI 0.86 to 1.39, very low certainty evidence) and 14 studies included patients with Crohn’s disease only (OR 1.11; 95% CI 0.90 to 1.36, very low certainty evidence) (Analysis 3.2). A statistically significant subgroup difference was not detected (p=0.95) Studies performed prior to 1998 showed an increased postoperative infectious complications incidence (OR 1.85; 95% CI 1.14 to 3.01, very low certainty evidence) while studies performed after 1998 demonstrated no difference (OR 1.06; 95% CI 0.93 to 1.22, very low certainty evidence) (Analysis 3.3). The test of subgroup differences suggests that there is a statistically significant subgroup effect (p=0.03) In terms of secondary outcomes, eleven studies reported rates of incisional infection and wound dehiscence and demonstrated no increase with preoperative immunomodulator use (OR 1.35; 95% CI 0.96 to 1.89, very low certainty evidence) (Analysis 3.4). Similar findings were seen in regards to rates of intra‐abdominal infections (20 studies; OR 0.86; 95% CI 0.66 to 1.12, very low certainty evidence) (Analysis 3.5) and extra‐abdominal infections (4 studies; OR1.17 (95% CI 0.80 to 1.71, very low certainty evidence;) (Analysis 3.6) in the immunomodulator group. Primary outcome findings were not affected by excluding very high risk of bias studies (9 studies; OR 1.29 (95% CI 0.95 to 1.76) (Analysis 3.7) and studies published as a full manuscript (30 studies; OR 1.11 (95% CI 0.97 to 1.27) (Analysis 3.8). A sensitivity analysis was also performed excluding studies that included patients who underwent surgery to repair an intrabdominal abscess/fistula or were found to have an abscess intraoperatively and our findings remained stable (18 studies; OR 1.09; 95% CI 0.93 to 1.29) (Analysis 3.9). The findings were also not affected by excluding studies with potential unit of analysis error (overall rate of infection estimated by summation of different types of infection) (30 studies; OR 1.11; 95% CI 0.97 to 1.26) (Analysis 3.10).
3.1. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 1: Postoperative infection within 30 days of surgery
3.2. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 2: Postoperative infection within 30 days of surgery: subgroup UC vs CD
3.3. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 3: Postoperative infection within 30 days of surgery: subgroup pre 1998 vs 1998 or after
3.4. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 4: Incisional infections and wound dehiscence
3.5. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 5: Intra‐abdominal infectious complications
3.6. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 6: Extra‐abdominal infections
3.7. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 7: Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias
3.8. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 8: Postoperative infection within 30 days of surgery: sensitivity exclude abstract
3.9. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 9: Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess
3.10. Analysis.

Comparison 3: Immunosuppressive agents versus control, Outcome 10: Postoperative infection within 30 days of surgery: sensitivity excluding sum of infection studies
Analysis 4 Anti‐TNF agents vs Control
Pooling of data from the 54 studies comparing preoperative anti‐TNF therapy versus no anti‐TNF treatment demonstrated a modestly increased incidence of postoperative infectious complications (OR 1.27; 95% CI 1.09 to 1.47, very low certainty evidence) (Analysis 4.1). Substantial heterogeneity was not observed in the overall analysis (I2= 46%). Based on 17 of the studies, the adjusted pooled OR was 1.60 (95% CI 1.20 to 2.13; I2 48%; low certainty evidence; Analysis 4.1.1). Unadjusted OR, based on 37 studies was 1.14 (95% CI 0.96 to 1.36; I2 42%; very low certainty evidence; Analysis 4.1.2) Seventeen studies included only patients with ulcerative colitis (OR 1.04; 95% CI 0.79 to 1.36, very low certainty evidence) and 27 studies included patients with Crohn’s disease only (OR 1.43; 95% CI 1.09 to 1.87, very low certainty evidence) (Analysis 4.2). A statistically significant subgroup difference was not detected (p=0.10) In the 17 studies that included patients treated with anti‐TNF therapy within 8 weeks of surgery, increased incidence of postoperative infectious complications was found (OR 1.44; 95% CI 1.08 to 1.94, very low certainty evidence) (Analysis 4.3). This was not the case in the 34 studies with patients whose last dose of TNF‐therapy was more than 8 weeks before surgery (OR 1.18 95% CI 0.99 to 1.40, very low certainty evidence). A statistically significant subgroup difference was not detected (p=0.25) In terms of secondary outcomes, twenty four studies reported rates of incisional infection and wound dehiscence and demonstrated no increase with preoperative anti‐TNF therapy (OR 1.18; 95% CI 0.83 to 1.68, very low certainty evidence) (Analysis 4.4). While the incidence of intra‐abdominal infections was higher in the anti‐TNF group (39 studies; OR 1.38; 95% CI 1.04 to 1.82, very low certainty evidence) (Analysis 4.5), there was no difference in extra‐abdominal infections (13 studies; OR 1.34 (95% CI 0.96 to 1.87, very low certainty evidence) (Analysis 4.6). Primary outcome findings were not affected by excluding very high risk of bias studies (16 studies; OR 1.67 (95% CI 1.31 to 2.13) (Analysis 4.7) and studies published as a full manuscript (47 studies; OR 1.26 (95% CI 1.07 to 1.48) (Analysis 4.8). A sensitivity analysis was also performed restricting the analysis to studies that did not include patients who underwent surgery to repair an intrabdominal abscess/fistula or were found to have an abscess intraoperatively and the findings remained significant (37 studies; OR 1.31; 95% CI 1.10 to 1.56) (Analysis 4.9). The findings were also not affected by excluding studies with potential unit of analysis error (overall rate of infection estimated by summation of different types of infection) (46 studies; OR 1.22; 95% CI 1.02 to 1.45) (Analysis 4.10).
4.1. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 1: Postoperative infection within 30 days of surgery
4.2. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 2: Postoperative infection within 30 days of surgery: subgroup UC vs CD
4.3. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 3: Postoperative infection within 30 days of surgery: subgroup biologics < 8 weeks before surgery vs > 8 weeks before surgery
4.4. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 4: Incisional infections and wound dehiscence
4.5. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 5: Intra‐abdominal infectious complications
4.6. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 6: Extra‐abdominal infections
4.7. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 7: Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias
4.8. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 8: Postoperative infection within 30 days of surgery: sensitivity exclude abstract
4.9. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 9: Postoperative infection within 30 days of surgery: sensitivity exclude surgery for abscess
4.10. Analysis.

Comparison 4: Anti‐TNF‐α agents versus control, Outcome 10: Postoperative infection within 30 days of surgery: sensitivity excluding sum of infection studies
Analysis 5 Anti‐integrin agents vs Control
Pooling of data from the 9 studies comparing preoperative anti‐integrin therapy versus no anti‐integrin treatment demonstrated no difference in postoperative infectious complications (OR 1.11; 95% CI 0.76 to 1.62, very low certainty evidence) (Analysis 5.1). Substantial heterogeneity was observed in the overall analysis (I2= 55%). Based on 2 of the studies, the adjusted pooled OR was 1.04 (95% CI 0.79 to 1.36; I2 22%; low certainty evidence; Analysis 5.1.1). Unadjusted OR, based on 7 studies was 1.06 (95% CI 0.54 to 2.10; I2 62%; very low certainty evidence; Analysis 5.1.2) Two studies included only patients with ulcerative colitis (OR 0.61; 95% CI 0.28 to 1.36, very low certainty evidence) and 4 studies included only patients with Crohn’s disease (OR 1.32; 95% CI 0.51 to 3.42, very low certainty evidence) (Analysis 5.2). A statistically significant subgroup difference was not detected (p=0.22). In terms of secondary outcomes, six studies reported rates of incisional infection and wound dehiscence and demonstrated no increased incidence with preoperative anti‐integrin therapy (OR 1.64; 95% CI 0.77 to 3.50, very low certainty evidence) (Analysis 5.3). Similarly, there was no increase in intra‐abdominal infections (5 studies; OR 0.40; 95% CI 0.14 to 1.20, very low certainty evidence) (Analysis 5.4) or extra‐abdominal infections (5 studies; OR 1.15 (95% CI 0.43 to 3.08, very low certainty evidence) (Analysis 5.5) in the anti‐integrin group. Primary outcome findings were not affected by excluding very risk of bias studies (3 studies, OR 1.10 (95% CI 0.79 to 1.52) (Analysis 5.6) and studies published as a full manuscript (5 studies, OR 1.06 (95% CI 0.58 to 1.96) (Analysis 5.7). A sensitivity analysis was also performed restricting the analysis to studies that did not include patients who underwent surgery to repair an intrabdominal abscess/fistula or were found to have an abscess intraoperatively and the findings remained non‐significant (9 studies; OR 1.11; 95% CI 0.76 to 1.62) (Analysis 5.8).The findings were also not affected by excluding studies with potential unit of analysis error (overall rate of infection estimated by summation of different types of infection) (7 studies; OR 0.97; 95% CI 0.73 to 1.29) (Analysis 5.9).
5.1. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 1: Postoperative infection within 30 days of surgery
5.2. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 2: Postoperative infection within 30 days of surgery: subgroup UC vs CD
5.3. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 3: Incisional infections and wound dehiscence
5.4. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 4: Intra‐abdominal infectious complications
5.5. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 5: Extra‐abdominal infections
5.6. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 6: Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias
5.7. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 7: Postoperative infection within 30 days of surgery: sensitivity exclude abstract
5.8. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 8: Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess
5.9. Analysis.

Comparison 5: Anti‐integrin agents versus control, Outcome 9: Postoperative infection within 30 days of surgery: sensitivity excluding sum of infection studies
Analysis 6 Anti‐interleukin agents vs Control
As only 1 study regarding anti‐interleukin agents met the inclusion criteria, a meta‐analysis was not performed. Liang et al. reported no difference in risk of overall postoperative infection (OR 0.80; 95% CI 0.10 to 6.51, very low certainty evidence) (Liang 2017).
Analysis 7 Small molecules vs Control
No studies regarding small molecules meeting our inclusion criteria were identified.
Analysis 8 Biosimilars versus Control
No studies regarding biosimilars meeting our inclusion criteria were identified.
Discussion
Summary of main results
This systematic review included 68 observational studies that evaluated the risk of postoperative infectious complications from IBD medications. Separate analyses were performed for corticosteroids, 5ASAs, immunomodulators, anti‐TNF medications, and anti‐integrin medications. Meta‐analysis was not performed for anti‐interleukin medications, small molecules and biosimilars due to lack of appropriate studies. Patients taking corticosteroids were found to have an increase in postoperative infectious complications within our meta‐analysis. These findings were associated with low heterogeneity and low imprecision. Furthermore, the results remained similar in subgroup analyses of UC and CD patients, as well as subgroup analyses of studies performed before and after 1998. With regards to the secondary outcomes, we observed increased intra‐abdominal infections within the group treated with preoperative corticosteroids but no difference in the incidence of wound infections/dehiscence and of extra‐abdominal infections. The lack of difference discovered for wound infections/dehiscence and for extra‐abdominal infections may have been related to inadequate power to detect a difference, as from 41 studies, only 7 and 4 reported these outcomes, respectively. Of note, we included patients treated with any dose of corticosteroids. The strength of these findings could potentially be enhanced by evaluating whether there is a dose dependent impact on postoperative infectious complications. We found no difference in postoperative infectious complications in patients treated with 5ASA. This result was associated with high heterogeneity and high imprecision. No difference was found in incidence of postoperative intra‐abdominal infections. Analysis of incisional infections/wound dehiscence and extra‐abdominal infections was not performed as an insufficient number of studies reported this information. This meta‐analysis did not find increased postoperative infectious complications in patients using immunomodulators. This result was associated with low heterogeneity and low imprecision. The findings remained stable in subgroup analyses of UC and CD patients. Interestingly, subgroup analysis of studies performed prior to 1998 revealed an increase in postoperative infectious complications that was not seen after 1998. Prior to the biologic era, immunomodulators were used more often for those with severe disease, so it is possible this finding is due to confounding by disease severity rather than a true biological effect. There was no difference observed in wound infection/dehiscence, intra‐abdominal infections, or extra‐abdominal infections within the group treated with immunomodulators. For patients prescribed anti‐TNF therapy, the incidence of postoperative infectious complications was higher within studies that reported adjusted data, but no different in the studies that reported unadjusted data. The risk of confounding within the unadjusted data must be considered, therefore it is more likely that anti‐TNF therapy is associated with increased postoperative infectious complications based on our results. In subgroup analyses, the increased incidence of postoperative infectious complications remained significant only in CD patients and in patients who were treated with anti‐TNF medications within 8 weeks of surgery. The increase in infectious complications seen only in patients receiving anti‐TNF therapy within eight weeks before surgery could be explained by the pharmacokinetics of the drug class. Patients receiving their last dose of anti‐TNF therapy greater than eight weeks before surgery may not have had significant drug levels at the time of surgery. Further studies could look at testing serum drug levels in postoperative IBD patients to compare infection rates in patients with and without therapeutic anti‐TNF levels. It is not entirely clear why postoperative infectious complications on anti‐TNF therapy was only increased in patients with CD, especially as no differences in results were found for CD compared to UC patients for other outcomes within this review. Secondary outcomes revealed mixed results, which may be explained by lower sample sizes, inclusion of both UC and CD patients, as well as variation in the timing of the last dose of medication. We found no difference in postoperative infectious complications in patients treated with anti‐integrin medications compared to those not using these therapies. This finding was associated with low heterogeneity but was also imprecise, likely due to a small sample size. Results were consistent in all subgroup analyses and analyses of secondary outcomes. Given the assumed gut selective mechanism of vedolizumab, intra‐abdominal infections were of particular interest. We did not find an increase in intra‐abdominal infections in patients prescribed anti‐integrin agents. Preplanned analyses for anti‐interleukin medications, small molecule and biosimilars were not performed due to insufficient number of studies.
Overall completeness and applicability of evidence
The literature search was designed with the assistance of an experienced research librarian and was designed to include studies from around the world regardless of publication form, language, or date of publication. Hence, we believe this review is comprehensive and reflects the available evidence. There were 41 studies on corticosteroids, 31 on immunomodulators and 54 reporting on anti‐TNF medications. Data regarding 5ASA (6 studies), anti‐integrin medications (9 studies), and anti‐interleukin medications (1 study) was sparse and completely lacking in the case of biosimilars and small molecules. As anti‐integrins, anti‐interleukins, biosimilars and small molecules were relatively recently approved for the treatment of IBD, there will likely be more data regarding these medications in coming years. We believe the results of this study to be generalizable to UC and CD patients but with some caveats. As most of the studies were performed at tertiary centers, the results may be less applicable to hospitals with less expertise in IBD‐related surgeries. Also, the results of this study may not be applicable to patients undergoing non‐abdominal surgery. We originally planned to perform subgroup analyses of studies containing abdominal and non‐abdominal surgeries. However, this analysis could not be performed as patients only underwent abdominal procedures within the included studies. It is also important to be mindful that some studies excluded emergency surgeries and surgeries performed for the management of dysplasia/malignancy, and thus applicability of the results of this study to these specific patient populations may be limited. Furthermore, as many studies did not control for concomitant medication use, it may be difficult to apply these findings to clinical contexts where patients may be on multiple IBD medications. This is especially true regarding decisions around anti‐TNF agents and corticosteroids since they were associated with increased postoperative infectious complications.
Quality of the evidence
Risk of bias according to the Newcastle‐Ottawa Scale was deemed low in 24 studies and very high in 44 studies. Studies commonly lost points in the comparability section because many studies did not control for important factors such as disease severity and concomitant medications. Fourteen studies (Aberra 2003; Afzali 2016; Brouquet 2018; Cohen 2019; Gainsbury 2011; Krane 2013; McKenna 2018; Selvasekar 2007; Serradori 2013; Yamamoto 2016; Ayoub 2018; Appau 2008; Mor 2008; Waterman 2013) controlled for concomitant steroid us and 5 studies controlled for anti‐TNF agents (McKenna 2018; Selvasekar 2007; Serradori 2013; Yamamoto 2016; Appau 2008). Also, many studies did not account for pre‐existing infections. We acknowledge it may be impossible to guarantee that infections were not present prior to surgery as infections require some time to incubate before symptoms manifest. However, in cases where there are known preoperative infections such as when surgery is performed for management of an abdominal abscess, it may be prudent to adjust for this factor. Overall, our assessment based on GRADE suggests that the certainty of evidence supporting the outcomes of this review is very low due to the observational nature of the data and very serious risk of bias. As well, many outcomes also demonstrated substantial levels of imprecision.
Potential biases in the review process
We explored publication bias by creating funnel plots for the primary outcome of each class of medication. Funnel plots were not created for the 5ASA and anti‐integrin analyses due to insufficient (<10) number of studies. Publication bias was not detected based on visual inspection of these funnel plots (Figure 4; Figure 5; Figure 6). Limitations of this review include the paucity of prospective studies, small number of studies regarding 5ASA and anti‐integrin medications, as well as sparse reporting of secondary outcomes. Several studies did not provide an overall rate of infection, but rather reported different types of infections separately. In these situations, we contacted the authors for additional information, but if they did not provide the requested details, we estimated the overall rate of infection by summation of the different types of infection reported. This created the possibility of unit of analysis error. Reassuringly, sensitivity analyses excluding studies with potential unit of analysis issues did not result in significant changes to our findings. In addition, 18 studies reported overall postoperative complications but not specifically infectious complications and 7 studies did not provide numerical data regarding incidence of infections stratified by patients’ preoperative medication use. Efforts to contact authors for additional information were made. We did not hear back from the authors and thus, the above studies were excluded from our analysis. The data may be unavailable because the above studies had different areas of focus and did not specifically examine postoperative infections. It is also possible that data on postoperative infections was recorded but not reported, which would result in a publication bias. Lastly, there were variations between studies as to what constituted an infectious complication. For example, some studies focused on only intra‐abdominal infections, while others reported both intra‐abdominal and extra‐abdominal outcomes. Standardization in the method of reporting postoperative infections would allow for more accurate comparisons between studies.
4.

Funnel plot of comparison: 1 Corticosteroids versus control, outcome: 1.1 Postoperative infection within 30 days of surgery.
5.

Funnel plot of comparison: 3 Immunosuppressive agents versus control, outcome: 3.1 Postoperative infection within 30 days of surgery.
6.

Funnel plot of comparison: 4 Anti‐TNF‐α agents versus control, outcome: 4.1 Postoperative infection within 30 days of surgery.
Agreements and disagreements with other studies or reviews
Our findings were similar to those reported in previous meta‐analyses on this topic. Subramanian et al. performed a meta‐analysis on the risk of postoperative complications in IBD patients treated with corticosteroids. It examined infectious outcomes in addition to total postoperative outcomes (Subramanian 2008). In their analysis of postoperative infections, 5 studies were included and an increase in odds of infections within 30 days of surgery was reported (OR 1.68; 95% CI 1.24 to 2.28). Ali et al also studied the association between corticosteroid and postoperative infections (Ali 2014). They included 10 studies and reported a RR of 1.55 (95% CI 1.23 to 1.95). Our findings were in agreement with both studies and also had a narrower confidence interval, likely due to the larger sample size of our analysis. To our knowledge, there are no previous meta‐analyses examining the association between 5ASA therapy and postoperative infections in IBD patients. We found two previous systematic reviews studying preoperative immunomodulator use and postoperative infections (Ali 2014; Subramanian 2006). Ali et al pooled data from 7 studies and found no difference in infectious complications (RR 1.23; 95% CI 0.66 to 2.29). Subramanian et al did not perform a meta‐analysis but identified two studies (Aberra 2003; Colombel 2004) that both reported no significant increase in the risk of postoperative infection. Numerous meta‐analyses have been performed examining the association between anti‐TNF therapy and postoperative infections (Billioud 2013; Ehteshami‐Afshar 2011; El‐Hussuna 2013; Kopylov 2012; Narula 2013; Waterland 2016; Xu 2019; Yang 2014; Yang 2012). For unclear reasons, conclusions of these studies have been inconsistent. Three studies examined odds of infection in IBD patients (UC and CD combined): Billoud et al (OR 1.27; 95% CI 0.87 to 1.85) and Ehtshami et al (OR 1.56; 95% CI 0.71 to 3.44) reported no difference in infection, while Narula et al (OR 1.56; 95% CI 1.09 to 2.24) reported a modest increase. However, both Billoud et al (OR 1.45; 95% CI 1.03 to 2.05) and Narula et al (OR 1.94; 95% CI 1.28 to 2.89) found a significant increase in CD patients with no difference in UC patients. One potential reason for the difference in findings amongst these reviews could be related to the variation in the accepted definition of preoperative anti‐TNF administration. For example, Billioud et al included studies that reported the last dose of anti‐TNF infusion anywhere from 2‐24 weeks preoperatively. In contrast, Ehtshami et al did not provide information on the time of last infusion but did provide the total length of time a patient had been on anti‐TNF therapy. Among studies that examined CD patients exclusively, El‐Hussana et al (RR 0.91; 95% CI 0.56 to 1.47) did not find an increase in risk, but Kopylov et al (OR 1.50; 95% CI 1.14 to 2.03), Waterland et al (OR 1.52; 95% CI 1.14 to 2.03), Xu et al (OR 1.23; 95% CI 0.87 to 1.74) and Yang et al (OR 1.47; 95% CI 1.08 to 1.99) all reported an increased odds. In a separate publication, Yang et al also studied UC patients and reported no difference (OR 1.10; 95% CI 0.51 to 2.38) (Yang 2012). Our study found that there appears to be a mildly increased risk of postoperative infection in IBD patients overall and particularly in CD patients, which is consistent with the majority of these previous meta‐analyses. Two systematic reviews studying anti‐integrin medications and postoperative infection were identified (Law 2018; Yung 2018). Neither identified any increase in risk of infectious complications (Law: RR 0.92; 95% CI 0.44 to 1.92; Yung: OR 0.72; 95% CI 0.19 to 2.74), which is consistent with the results of our study.
Authors' conclusions
Implications for practice.
This is the largest and most comprehensive meta‐analysis to date on this topic. The evidence regarding corticosteroids, 5ASA, immunomodulators, anti‐TNF mediations and anti‐integrin medications was very low in certainty. Thus, the impact of these medications on postoperative infectious complications is uncertain and no firm conclusions can be drawn regarding their safety in the perioperative period. The decision to stop IBD medications prior to surgery involves potential risks and benefits. Holding therapies prior to surgery could result in a disease flare and in the case of biologic medications, could lead to sensitization and the formation of antibodies. Thus, the decision should involve careful consideration of each patient’s circumstances, treatment history, preferences and values.
Implications for research.
This review has highlighted areas for further research. Prospective studies and studies controlling for potential confounding factors such as disease severity and concomitant medications are required to generate higher quality evidence. Furthermore, data regarding anti‐interleukin and anti‐integrin medications was sparse and no studies are currently available on small molecules and biosimilars. Undoubtedly, more studies are required before firm conclusions can be drawn regarding these medications. Incorporation of preoperative drug levels in future studies would also be helpful as it could delineate a potential dose‐dependent risk and help stratify patients' risks for potential infection. Lastly, standardization of which and how postoperative infections are reported would greatly aid in comparison of studies.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2021 | Amended | This amendment addresses post‐publication feedback. Changes include updates to the analyses and risk of bias assessments. These changes did not impact the conclusions or certainty of the evidence. |
History
Protocol first published: Issue 2, 2019 Review first published: Issue 10, 2020
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
We would like to acknowledge John K MacDonald for providing editorial, statistical and logistic support as well as Tran Nguyen for assistance with the literature search and logistic support. We would like to thank Dr. Marylise Boutros, Dr. David Dietz, and Dr. Marc Ferrante for kindly providing additional information regarding their studies.
Appendices
Appendix 1. Search strategies
MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11 assign$.tw.
12. allocat$.tw.
13. randomized controlled trial/
14. or/1‐13
15. exp cohort studies/
16. exp case‐control studies/
17. exp retrospective studies/
18. exp Epidemiologic Studies/
19. case‐control studies/ or longitudinal studies/ or follow‐up studies/ or prospective studies/ or cross‐sectional studies/
20.(cohort$ or prospective$ or retrospective$).mp.
21. or/15‐20
22. 14 or 21
23. Exp Inflammatory bowel disease/
24. (inflammatory bowel disease* or IBD).mp.
25. Exp Crohn disease/ or crohn*.mp.
26. Exp ulcerative colitis/ or (colitis and ulcerat*).mp.
27. or/23‐26
28. (Anti‐TNF* OR anti TNF* or Biologic*).mp.
29. Integrin receptor antagonist.mp.
30. (Corticosteroid* or steroid*).mp.
31. (immunosuppress* or immunomodulator*).mp.
32. Antibiotic*.mp.
33. Aminosalicylate*.mp.
34. (Adalimumab or Certolizumab* or Golimumab or Infliximab or Natalizumab or Ustekinumab or Vedolizumab).mp.
35. (Tofacitinib or Ozanimod).mp.
36. (Budesonide or Methylprednisolone or Prednisolone or Prednisone).mp.
37. (Azathioprine or 6‐MP or 6‐mercaptopurine or Cyclosporine or Mercaptopurine or Methotrexate or Tacrolimus).mp.
38. (Ciprofloxain or Metronidazole).mp.
39. (5‐ASA or Balsalazide or Mesalamine or Olsalazine or Sulfasalazine).mp.
40. or/28‐39
41. (Post‐operation or Post‐operative or Post‐op* or postoperative* or postsurgical* or post‐surg*).mp.
42. (operation* or surg* or strictureplasty or resection or colectomy or proctocolectomy).mp.
43. 41 or 42
44. (Infect* or complication* or heal* or re‐operation or reoperation or outcome* or adverse* or adverse event* or side effect*).mp.
45. 22 and 27 and 40 and 43 and 44
Embase
1. random$.mp.
2. factorial$.mp.
3. (crossover$ or cross over$ or cross‐over$).mp.
4. placebo$.mp.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).mp.
9. (double$ adj blind$).mp.
10. (tripl$ adj blind$).mp.
11. assign$.mp.
12. allocat$.mp.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. exp cohort studies/
20. exp case‐control studies/
21. exp retrospective studies/
22. exp Epidemiologic Studies/
23. case‐control studies/ or longitudinal studies/ or follow‐up studies/ or prospective studies/ or cross‐sectional studies/
24. (cohort$ or prospective$ or retrospective$).mp.
25. or/19‐24
26. 18 or 25
27. Exp Inflammatory bowel disease/
28. (inflammatory bowel disease* or IBD).mp.
29. Exp Crohn disease/ or crohn*.mp.
30. Exp ulcerative colitis/ or (colitis and ulcerat*).mp.
31. or/27‐30
32. (Anti‐TNF* OR anti TNF* or Biologic*).mp.
33. Integrin receptor antagonist.mp.
34. (Corticosteroid* or steroid*).mp.
35. (immunosuppress* or immunomodulator*).mp.
36. Antibiotic*.mp.
37. (Aminosalicylate* or Aminosalicylic*).mp.
38. (Adalimumab or Certolizumab* or Golimumab or Infliximab or Natalizumab or Ustekinumab or Vedolizumab).mp.
39. (Tofacitinib or Ozanimod).mp.
40. (Budesonide or Methylprednisolone or Prednisolone or Prednisone).mp.
41. (Azathioprine or 6‐MP or 6‐mercaptopurine or Cyclosporine or Mercaptopurine or Methotrexate or Tacrolimus).mp.
42. (Ciprofloxain or Metronidazole).mp.
43. (5‐ASA or Balsalazide or Mesalamine or Olsalazine or Sulfasalazine).mp.
44. or/32‐43
45. (Post‐operation or Post‐operative or Post‐op* or postoperative* or postsurgical* or post‐surg*).mp.
46. (operation* or surg* or strictureplasty or resection or colectomy or proctocolectomy).mp.
47. 45 or 46
48. (Infect* or complication* or heal* or re‐operation or reoperation or outcome* or adverse* or adverse event* or side effect*).mp.
49. 26 and 31 and 44 and 47 and 48
CENTRAL
#1 MeSH: [Inflammatory bowel disease] explode all trees
#2 IBD
#3 Crohn
#4 ulcerative colitis
#5 #1 or #2 or #3 or #4
#6 MeSH: [Biological factors] explode all trees
#7 MeSH: [Receptors, Steroid] explode all trees
#8 MeSH: [Immunosuppressive Agents] explode all trees
#9 MeSH: [Anti‐bacterial agents] explode all trees
#10 MeSH: [Aminosalicylic Acids] explode all trees
#11 Adalimumab or Certolizumab* or Golimumab or Infliximab or Natalizumab or Ustekinumab or Vedolizumab
#12 Tofacitinib or Ozanimod
#13 Budesonide or Methylprednisolone or Prednisolone or Prednisone
#14 Azathioprine or 6MP or mercaptopurine or Cyclosporine or Mercaptopurine or Methotrexate or Tacrolimus
#15 Ciprofloxain or Metronidazole
#16 5ASA or Balsalazide or Mesalamine or Olsalazine or Sulfasalazine
#17 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 Post‐operation or Post‐operative or Post‐op* or postoperative* or postsurgical* or post‐surg*
#19 operation* or surg* or strictureplasty or resection or colectomy or proctocolectomy
#20 #18 or #19
#21 Infect* or complication* or heal* or re‐operation or reoperation or outcome* or adverse* or adverse event* or side effect*
#22 #5 and #17 and #20 and #21
Cochrane IBD Group Specialized Register
1. Operation and infection
2. Post‐opera and outcome
3. Operation and complication
4. Surgery and Crohn’s disease
5. Surgery and ulcerative colitis
Clinicaltrials.gov
1. Inflammatory bowel disease and operation/surgery
2. Inflammatory bowel disease and surgical complication
WHO trials registry (ICTRP)
1. Inflammatory bowel disease and operation/surgery
2. Inflammatory bowel disease and surgical complication
Data and analyses
Comparison 1. Corticosteroids versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Postoperative infection within 30 days of surgery | 41 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Adjusted Analysis | 17 | Odds Ratio (IV, Random, 95% CI) | 1.70 [1.38, 2.09] | |
| 1.1.2 Unadjusted Analysis | 24 | Odds Ratio (IV, Random, 95% CI) | 1.22 [1.03, 1.45] | |
| 1.2 Postoperative infection within 30 days of surgery: subgroup UC vs CD | 33 | Odds Ratio (IV, Random, 95% CI) | 1.36 [1.18, 1.57] | |
| 1.2.1 Ulcerative colitis | 11 | Odds Ratio (IV, Random, 95% CI) | 1.49 [1.10, 2.02] | |
| 1.2.2 Crohn's disease | 23 | Odds Ratio (IV, Random, 95% CI) | 1.32 [1.11, 1.57] | |
| 1.3 Postoperative infection within 30 days of surgery: subgroup pre 1998 versus 1998 or after | 15 | Odds Ratio (IV, Random, 95% CI) | 1.72 [1.37, 2.16] | |
| 1.3.1 Pre 1998 | 2 | Odds Ratio (IV, Random, 95% CI) | 4.22 [1.67, 10.64] | |
| 1.3.2 1998 or after | 13 | Odds Ratio (IV, Random, 95% CI) | 1.62 [1.30, 2.01] | |
| 1.4 Incisional infections and wound dehiscence | 7 | Odds Ratio (IV, Random, 95% CI) | 1.41 [0.72, 2.74] | |
| 1.5 Intra‐abdominal infectious complications | 28 | Odds Ratio (IV, Random, 95% CI) | 1.53 [1.28, 1.84] | |
| 1.6 Extra‐abdominal infections | 4 | Odds Ratio (IV, Random, 95% CI) | 1.23 [0.97, 1.55] | |
| 1.7 Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias | 15 | Odds Ratio (IV, Random, 95% CI) | 1.43 [1.13, 1.81] | |
| 1.8 Postoperative infection within 30 days of surgery: sensitivity exclude abstract | 36 | Odds Ratio (IV, Random, 95% CI) | 1.48 [1.28, 1.72] | |
| 1.9 Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess | 21 | Odds Ratio (IV, Random, 95% CI) | 1.37 [1.14, 1.65] | |
| 1.9.1 Adjusted Analysis | 9 | Odds Ratio (IV, Random, 95% CI) | 1.79 [1.35, 2.38] | |
| 1.9.2 Unadjusted Analysis | 12 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.81, 1.40] |
Comparison 2. 5‐ASA versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Postoperative infection within 30 days of surgery | 6 | 5030 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.51, 1.14] |
| 2.1.1 Unadjusted Analysis | 6 | 5030 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.51, 1.14] |
| 2.2 Postoperative infection within 30 days of surgery: subgroup UC vs CD | 5 | Odds Ratio (IV, Random, 95% CI) | 0.63 [0.45, 0.89] | |
| 2.2.1 Ulcerative colitis | 1 | Odds Ratio (IV, Random, 95% CI) | 0.50 [0.26, 0.96] | |
| 2.2.2 Crohn's disease | 4 | Odds Ratio (IV, Random, 95% CI) | 0.70 [0.45, 1.07] | |
| 2.3 Postoperative infection within 30 days of surgery: subgroup pre 1998 versus 1998 or after | 6 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.51, 1.14] | |
| 2.3.1 Pre 1998 | 1 | Odds Ratio (IV, Random, 95% CI) | 1.08 [0.47, 2.51] | |
| 2.3.2 1998 or after | 5 | Odds Ratio (IV, Random, 95% CI) | 0.71 [0.45, 1.14] | |
| 2.4 Incisional infections and wound dehiscence | 1 | Odds Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.95] | |
| 2.5 Intra‐abdominal infectious complications | 3 | Odds Ratio (IV, Random, 95% CI) | 0.77 [0.45, 1.33] | |
| 2.6 Postoperative infection within 30 days of surgery: sensitivity exclude abstract | 6 | Odds Ratio (IV, Random, 95% CI) | 0.76 [0.51, 1.14] | |
| 2.7 Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess | 2 | Odds Ratio (IV, Random, 95% CI) | 0.79 [0.36, 1.73] | |
| 2.7.1 Unadjusted Analysis | 2 | Odds Ratio (IV, Random, 95% CI) | 0.79 [0.36, 1.73] |
2.6. Analysis.

Comparison 2: 5‐ASA versus control, Outcome 6: Postoperative infection within 30 days of surgery: sensitivity exclude abstract
Comparison 3. Immunosuppressive agents versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Postoperative infection within 30 days of surgery | 31 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Adjusted Analysis | 9 | Odds Ratio (IV, Random, 95% CI) | 1.29 [0.95, 1.76] | |
| 3.1.2 Unadjusted Analysis | 22 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.93, 1.24] | |
| 3.2 Postoperative infection within 30 days of surgery: subgroup UC vs CD | 25 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.95, 1.29] | |
| 3.2.1 Ulcerative colitis | 11 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.86, 1.39] | |
| 3.2.2 Crohn's disease | 14 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.90, 1.36] | |
| 3.3 Postoperative infection within 30 days of surgery: subgroup pre 1998 vs 1998 or after | 31 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.97, 1.26] | |
| 3.3.1 Pre 1998 | 4 | Odds Ratio (IV, Random, 95% CI) | 1.85 [1.14, 3.01] | |
| 3.3.2 1998 or after | 27 | Odds Ratio (IV, Random, 95% CI) | 1.06 [0.93, 1.22] | |
| 3.4 Incisional infections and wound dehiscence | 11 | Odds Ratio (IV, Random, 95% CI) | 1.35 [0.96, 1.89] | |
| 3.5 Intra‐abdominal infectious complications | 20 | Odds Ratio (IV, Random, 95% CI) | 0.86 [0.66, 1.12] | |
| 3.6 Extra‐abdominal infections | 4 | Odds Ratio (IV, Random, 95% CI) | 1.17 [0.80, 1.71] | |
| 3.7 Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias | 9 | Odds Ratio (IV, Random, 95% CI) | 1.29 [0.95, 1.76] | |
| 3.8 Postoperative infection within 30 days of surgery: sensitivity exclude abstract | 30 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.97, 1.27] | |
| 3.9 Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess | 18 | Odds Ratio (IV, Random, 95% CI) | 1.09 [0.93, 1.29] | |
| 3.9.1 Adjusted Analysis | 5 | Odds Ratio (IV, Random, 95% CI) | 1.34 [0.83, 2.16] | |
| 3.9.2 Unadjusted Analysis | 13 | Odds Ratio (IV, Random, 95% CI) | 1.06 [0.89, 1.27] | |
| 3.10 Postoperative infection within 30 days of surgery: sensitivity excluding sum of infection studies | 30 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.97, 1.26] | |
| 3.10.1 Adjusted Analysis | 9 | Odds Ratio (IV, Random, 95% CI) | 1.29 [0.95, 1.76] | |
| 3.10.2 Unadjusted Analysis | 21 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.92, 1.24] |
Comparison 4. Anti‐TNF‐α agents versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Postoperative infection within 30 days of surgery | 54 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Adjusted Analysis | 17 | Odds Ratio (IV, Random, 95% CI) | 1.60 [1.20, 2.13] | |
| 4.1.2 Unadjusted Analysis | 37 | Odds Ratio (IV, Random, 95% CI) | 1.14 [0.96, 1.36] | |
| 4.2 Postoperative infection within 30 days of surgery: subgroup UC vs CD | 43 | Odds Ratio (IV, Random, 95% CI) | 1.26 [1.03, 1.53] | |
| 4.2.1 Ulcerative colitis | 17 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.79, 1.36] | |
| 4.2.2 Crohn's disease | 27 | Odds Ratio (IV, Random, 95% CI) | 1.43 [1.09, 1.87] | |
| 4.3 Postoperative infection within 30 days of surgery: subgroup biologics < 8 weeks before surgery vs > 8 weeks before surgery | 51 | Odds Ratio (IV, Random, 95% CI) | 1.25 [1.08, 1.46] | |
| 4.3.1 < 8 weeks before surgery | 17 | Odds Ratio (IV, Random, 95% CI) | 1.44 [1.08, 1.94] | |
| 4.3.2 > 8 weeks before surgery | 34 | Odds Ratio (IV, Random, 95% CI) | 1.18 [0.99, 1.40] | |
| 4.4 Incisional infections and wound dehiscence | 24 | Odds Ratio (IV, Random, 95% CI) | 1.18 [0.83, 1.68] | |
| 4.5 Intra‐abdominal infectious complications | 39 | Odds Ratio (IV, Random, 95% CI) | 1.38 [1.04, 1.82] | |
| 4.6 Extra‐abdominal infections | 13 | Odds Ratio (IV, Random, 95% CI) | 1.34 [0.96, 1.87] | |
| 4.7 Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias | 16 | Odds Ratio (IV, Random, 95% CI) | 1.67 [1.31, 2.13] | |
| 4.8 Postoperative infection within 30 days of surgery: sensitivity exclude abstract | 47 | Odds Ratio (IV, Random, 95% CI) | 1.26 [1.07, 1.48] | |
| 4.9 Postoperative infection within 30 days of surgery: sensitivity exclude surgery for abscess | 37 | Odds Ratio (IV, Random, 95% CI) | 1.31 [1.10, 1.56] | |
| 4.9.1 Adjusted Analysis | 8 | Odds Ratio (IV, Random, 95% CI) | 1.61 [0.98, 2.65] | |
| 4.9.2 Unadjusted Analysis | 29 | Odds Ratio (IV, Random, 95% CI) | 1.24 [1.03, 1.50] | |
| 4.10 Postoperative infection within 30 days of surgery: sensitivity excluding sum of infection studies | 46 | Odds Ratio (IV, Random, 95% CI) | 1.22 [1.02, 1.45] | |
| 4.10.1 Adjusted Analysis | 17 | Odds Ratio (IV, Random, 95% CI) | 1.60 [1.20, 2.13] | |
| 4.10.2 Unadjusted Analysis | 29 | Odds Ratio (IV, Random, 95% CI) | 1.01 [0.82, 1.24] |
Comparison 5. Anti‐integrin agents versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Postoperative infection within 30 days of surgery | 9 | 5157 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.76, 1.62] |
| 5.1.1 Adjusted Analysis | 2 | 1022 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.79, 1.36] |
| 5.1.2 Unadjusted Analysis | 7 | 4135 | Odds Ratio (IV, Random, 95% CI) | 1.06 [0.54, 2.10] |
| 5.2 Postoperative infection within 30 days of surgery: subgroup UC vs CD | 5 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.52, 2.07] | |
| 5.2.1 Ulcerative colitis | 2 | Odds Ratio (IV, Random, 95% CI) | 0.61 [0.28, 1.36] | |
| 5.2.2 Crohn's disease | 4 | Odds Ratio (IV, Random, 95% CI) | 1.32 [0.51, 3.42] | |
| 5.3 Incisional infections and wound dehiscence | 6 | Odds Ratio (IV, Random, 95% CI) | 1.64 [0.77, 3.50] | |
| 5.4 Intra‐abdominal infectious complications | 5 | Odds Ratio (IV, Random, 95% CI) | 0.40 [0.14, 1.20] | |
| 5.5 Extra‐abdominal infections | 5 | Odds Ratio (IV, Random, 95% CI) | 1.15 [0.43, 3.08] | |
| 5.6 Postoperative infection within 30 days of surgery: sensitivity excluding very high risk of bias | 3 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.79, 1.52] | |
| 5.7 Postoperative infection within 30 days of surgery: sensitivity exclude abstract | 5 | Odds Ratio (IV, Random, 95% CI) | 1.06 [0.58, 1.96] | |
| 5.8 Postoperative infection within 30 days of surgery: sensitivity excluding surgery for abscess | 9 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.76, 1.62] | |
| 5.8.1 Adjusted Analysis | 2 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.79, 1.36] | |
| 5.8.2 Unadjusted Analysis | 7 | Odds Ratio (IV, Random, 95% CI) | 1.06 [0.54, 2.10] | |
| 5.9 Postoperative infection within 30 days of surgery: sensitivity excluding sum of infection studies | 7 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.73, 1.29] | |
| 5.9.1 Adjusted Analysis | 2 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.79, 1.36] | |
| 5.9.2 Unadjusted Analysis | 5 | Odds Ratio (IV, Random, 95% CI) | 0.78 [0.42, 1.47] |
Comparison 6. Anti‐interleukin agents versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Postoperative infection within 30 days of surgery | 1 | 3360 | Odds Ratio (IV, Random, 95% CI) | 0.80 [0.10, 6.51] |
6.1. Analysis.

Comparison 6: Anti‐interleukin agents versus control, Outcome 1: Postoperative infection within 30 days of surgery
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aberra 2003.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1992 to 2000 | |
| Participants | Country: USA. Patients with UC or CD who underwent elective bowel surgery. Patients treated with infliximab, mycophenolate, or tacrolimus were excluded | |
| Interventions | 1. Azathioprine/6‐mercaptopurine within 2 weeks of surgery (n=52) 2. Preoperative corticosteroids (n=90) 3. No preoperative immunosuppressants or corticosteroids (n=51) |
|
| Outcomes | Wound infection, sepsis, p n e umonia, peritonitis, abdominal abscess and wound dehiscence within 30 days of surgery | |
| Notes | NOS low risk of bias overall. Adjusted O Rs for corticosteroids and azathioprine/6‐mercaptopurine were obtained from multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All UC and CD patients undergoing elective bowel surgery at University of Pennsylvania Health system from 1992 to 2000 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | All charts were manually searched to determine medication exposure |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information was provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Azathioprine/6‐mercaptopurine analysis controlled for corticosteroids and vice versa |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also controlled for CD, disease refractory to medication, age >38 years, and surgery duration >241 minutes |
| Assessment of outcome | Low risk | Inpatient medical records were examined |
| Was follow‐up long enough for outcomes to occur | Low risk | Patients were followed until discharge from hospital (median 8 days, range 1‐37 days) |
| Adequacy of follow up of cohorts | Unclear risk | No information was provided |
Afzali 2016.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1992 to 2012 | |
| Participants | Country: USA. UC and CD patients who underwent either urgent or elective abdominal surgery. | |
| Interventions | 1.Preoperative methotrexate (n=15) 2. Preoperative azathioprine or 6‐mercaptopurine (n= 52) 3. No preoperative methotrexate (n=165) 4. No preoperative azathioprine or 6‐mercaptopurine (n=128) |
|
| Outcomes | Wound infection, anastomotic leak, abscess, fistula and extraabdominal infection within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for methotrexate and azathioprine were obtained from multivariate logistic regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All CD and UC who underwent abdominal surgery at the Univerity of Washington Medical Center from 1993 to 2012 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records were examined |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information was provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Adjusted for steroid use |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also adjusted for albumin, hematocrit, smoking status, and BMI |
| Assessment of outcome | Low risk | Medical records were examined |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | 61 patients excluded from analysis due to missing BMI measurement |
Alves 2007.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1984 to 2004. | |
| Participants | Country: France. CD patients who underwent their first ileocecal resection with primary anastomosis. Patients with temporary stomas were excluded. | |
| Interventions | 1. Preoperative steroids (n= 59) 2. No preoperative steroids (n=102) |
|
| Outcomes | Anastomotic leak and intra‐abdominal abscess within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted OR for st eroids was obtained from stepwise multivariate analysis model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD database of patients who underwent surgery from 1984 to 2004 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | CD database |
| Demonstration that outcome of interest was not present at start of study | High risk | Some patients were found to have Intra‐abdominal abscesses during surgery |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not report controlling for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for poor nutritional status |
| Assessment of outcome | Low risk | CD database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | NOS low risk of bias overall |
Appau 2008.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1998 to 2007. | |
| Participants | Country: USA. CD patients who underwent ileocolonic resection with anastomosis. | |
| Interventions | 1. Preoperative infliximab (n= 60) within 3 months of surgery 2. No preoperative infliximab (n=329) |
|
| Outcomes | Wound infection, wound complications, anastomotic leak, sepsis and intraabdominal abscess within 30 days of surgery | |
| Notes | NOS low risk of bias overall A djusted OR s for infliximab, 6MP/azathioprine/ methotrexate, and steroids were obtained from multivariate logistic regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD database of all patients who underwent ileocolonic resection at the Cleveland Clinic |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medication use verified with pharmacy department and patients were called to confirm last dose of infliximab infusion |
| Demonstration that outcome of interest was not present at start of study | High risk | Some patient were noted to have intraabdominal abscesses preoperatively |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Adjusted for methotrexate, 6‐mercaptopurine, azathioprine, infliximab and steroid use |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also adjusted for age, gender, comorbidities, penetrating abscess before surgery, diverting stoma, and disease phenotype |
| Assessment of outcome | Low risk | Medical charts were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information was provided |
Araki 2014.
| Study characteristics | ||
| Methods | Prospective, multicenter (13 institutions), observational cohort study. Study period: 2009 to 2010 | |
| Participants | Country: Japan. UC patients who underwent colorectal surgery | |
| Interventions | 1. Preoperative immunomodulator therapy (n= 92) 2. No preoperative immunomodulator (n= 103) |
|
| Outcomes | Superficial, deep and organ space surgical site infections within 30 days | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All UC patients who underwent colorectal surgery from 2009 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Data collection prospectively on a standardized form by physicians |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information was provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data collection prospectively on a standardized form by physicians |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information was provided |
Ayoub 2018.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2014 to 2016. | |
| Participants | Country: USA. CD and UC patients who underwent major abdominal surgery. Patients actively treated with steroids were excluded | |
| Interventions | 1. Vedolizumab (n= 16) within 8 weeks of surgery 2. Biologics other than vedolizumab (n= 37) within 8 weeks of surgery 3. No preoperative biologic therapy (n=18) |
|
| Outcomes | Surgical site infection within 30 days of surgery | |
| Notes | This study is an abstract. NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All patients who underwent major abdominal procedures due to IBD were screened |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Unclear risk | No information provided |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Excluded patients actively treated with steroids |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for any other variables |
| Assessment of outcome | Unclear risk | No information provided |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Bregnbak 2012.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2005 to 2010 | |
| Participants | Country: Denmark. UC patients who underwent colectomy. Patients with colonic malignancy or dysplasia were excluded. | |
| Interventions | 1. Preoperative infliximab (n=20) within 90 days of surgery. 2. Preoperative corticosteroids (n=48) 3. No preoperative infliximab (n=51) 4. No preoperative corticosteroids (n=23) |
|
| Outcomes | Infectious complications within 30 days postoperative | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All patients with UC based on clinical, endoscopic and histological criteria who underwent colectomy from 2005 to 2010. |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Danish National Health Reigster and examination of patient records |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other factors |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for other factors |
| Assessment of outcome | Low risk | Medical records were examined |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Brouquet 2018.
| Study characteristics | ||
| Methods | Prospective, multicenter (19 institutions), cohort study. Study period: 2013 to 2015 | |
| Participants | Country: France. Patients with ileocolonic CD who underwent abdominal surgery. Patients with perianal CD or colonic CD were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 143) within 3 months of surgery 2. No preoperative anti‐TNF therapy (n= 449) |
|
| Outcomes | Peritonitis, anastomotic leak, and intraabdominal abscess within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted OR for anti‐TNF medications was obtained from multivariate m odel with propensity s core analysis . |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All patients who underwent surgery for ileocolonic CD from 2013 to 2015 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Data prospectively collected on an electronic dedicated clinical research form |
| Demonstration that outcome of interest was not present at start of study | High risk | 18% patients had intraoperative finding of abscess |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Controlled for systemic steroids and budesonide |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also controlled for recurrent CD, hemoglobin, TPN, laparoscopic approach, and operative time |
| Assessment of outcome | Low risk | Prospectively collected on an electronic dedicated clinical research form |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Canedo 2011.
| Study characteristics | ||
| Methods | Retrospective cohort study. Study period: 2000 to 2008 | |
| Participants | Country: USA. CD patients who underwent intestinal or colorectal resection. Patients who had stoma creation without resection, stoma reversal and lysis of adhesions were excluded | |
| Interventions | 1. Preoperative infliximab (n= 65) within 3 months of surgery 2. No preoperative biologics (n= 75) |
|
| Outcomes | Wound infection, pulmonary infection, abscess and anastomotic leak within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Consecutive patients with CD who underwent surgical intestinal and colorectal resection from 2000 to 2008 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Retrospective analysis of a prospective surgical database |
| Demonstration that outcome of interest was not present at start of study | High risk | Firstula/abscess was indication for surgery for 72 patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other factors |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for other factors |
| Assessment of outcome | Low risk | Retrospective analysis of a prospective surgical database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Patients with incomplete data were excluded |
Cohen 2019.
| Study characteristics | ||
| Methods | Multicentre prospective cohort study. Study period 2014 to 2017. | |
| Participants | Country: USA. Crohn's and ulcerative colitis patients undergoing intra‐abdominal surgery. | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 382) within 12 weeks of surgery. 2. No preoperative anti‐TNF exposure (n= 573) |
|
| Outcomes | 30 post operative infectious complications (any infection and surgical site infection) | |
| Notes | NOS low risk of bias overall Adjusted OR for preoperative anti‐TNF therapy was obtained from m ultivariable logistic regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Prospective cohort of IBD patients who underwent intra‐abdominal surgery from 2014 to 2017 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from same centres and time period |
| Ascertainment of exposure | Low risk | Prospective database. Data obtained by patient interview and chart abstraction |
| Demonstration that outcome of interest was not present at start of study | Low risk | Controlled for pre‐operative non‐abdominal infection |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Performed multivariable analysis controlling for preoperative methotrexate and steroids |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for multiple other factors including age, BMI, gender and comorbid disease |
| Assessment of outcome | Low risk | Prospective database. Data obtained by patient interview and chart abstraction |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Authors did not comment whether any patients were lost to follow up |
Colombel 2004.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1998 to 2001 | |
| Participants | Country: USA. CD patients who underwent surgical resection, stricturoplasty, or intestinal bypass. Patients who received cyclosporine, tacrolimus, or investigational therapy within 8 weeks of surgery were excluded. Patients who underwent perianal surgery were also excluded. | |
| Interventions | 1. Preoperative infliximab (n= 52) 8 weeks prior to surgery and within 30 days after surgery 2. Preoperative azathioprine/6‐mercatopurine/methotrexate (n= 105) 3. Preoperative moderate/high dose steroids (n= 77) 4. No preoperative infliximab (n= 218) 5. No preoperative azathioprine/6‐mercatopurine/methotrexate (n= 165) 6. Low dose/no preoperative steroids (n=193) |
|
| Outcomes | Wound sepsis, intraabdominal infections and extraabdominal infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent surgical resection, stricturoplastly or intestinal bypass from 1998 to 2001 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records were examined |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis only |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis only |
| Assessment of outcome | Low risk | Medical records were examined |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Coquet‐Reinier 2010.
| Study characteristics | ||
| Methods | Matched retrospective cohort study. Study period: 1998 to 2008 | |
| Participants | Country: France. UC patients who underwent laproscopic restorative proctocolectomy with ileal pouch anal anastomosis | |
| Interventions | 1. Preoperative infliximab (n=13) 2. No preoperative infliximab (n=13) |
|
| Outcomes | Pelvic abscess and anastomotic leak within 30 days of surgery | |
| Notes | NOS low risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent laparoscopic IPAA since 1999 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records were examined |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Matched for gender, age and procedure type (2 or 3 stage) |
| Assessment of outcome | Low risk | Medical records were examined |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
De Buck Van Overstraeten 2017.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1998 to 2013 | |
| Participants | Country: Belgium. CD patients who underwent primary ileocecal resection | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 111) within 12 weeks of surgery 2. Preoperative steroids (n=278) 3. No preoperative anti‐TNF therapy (n=427) 4. No preopera tive steroids (n= 260) |
|
| Outcomes | Anastomotic leak within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for preoperative steroids and anti‐TNF therapy were obtained from multivariate logistic regression model . |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Patients with CD operated on for terminal ileocecal disease |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical charts reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Corrected for institution and time since first surgical procedure |
| Assessment of outcome | Low risk | Medical charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
El‐Hussuna 2012.
| Study characteristics | ||
| Methods | Retrospective, multicenter (4 institutions) cohort. Study period: 2000 to 2007. | |
| Participants | Country: Denmark. CD patients who underwent resection and anastomosis or with stricturoplasty | |
| Interventions | 1. Preoperative infliximab/certolizumab (n= 32) within 3 months of surgery 2. Azathioprine/6‐mercaptopurine/methotrexate (n= 166) within 1 month o surgery 3. Preoperative steroids (n= 66) 4. No preoperative anti‐TNF medication (n= 385) 5. No preoperative immunosuppressants (n= 251) 6. No preoperative steroids (n= 351) |
|
| Outcomes | Intraabdominal septic complications (anastomotic dehiscence, fistula, abscess), septic complications, other infective complications within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent resection form 2000 to 2007 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Unclear risk | No information provided |
| Demonstration that outcome of interest was not present at start of study | High risk | 20% patients had preoperative intraabdominal infection |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Unclear risk | No information provided |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Eshuis 2013.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2006 to 2010 | |
| Participants | Country: Netherlands. UC patients who underwent restorative proctocolectomy. Patients who had surgery for dysplasia or malignancy were excluded. | |
| Interventions | 1. Preoperative infliximab (n= 38) 2. No preoperative infliximab (n =34) |
|
| Outcomes | Pelvic sepsis, intraabdominal asbcess, surgical site infection, extraabdominal infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All patients requiring restorative proctocolectomy for refactory UC from 2006 to 2010. |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical charts reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for other factors |
| Assessment of outcome | Low risk | Medical charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Ferrante 2009.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1998 to 2008 | |
| Participants | Country: Belgium. UC and unclassified IBD patients who underwent restorative proctocolectomy | |
| Interventions | 1. Preoperative infliximab (n= 22) within 12 weeks of surgery 2. Preoperative immunomodulators (n= 78) 3. Preoperative steroids(n= 64) 4. No preoperative infliximab (n= 119) 5. No preoperative immunomodulators (n= 63) 6. no preoperative steroids (n= 77) |
|
| Outcomes | Pouch sepcific complications, surgical site infections, and non‐surgical site infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR for preoperative steroids was obtained from multiv ariate model. Unadjusted ORs for preoperative infliximab and immunomodulators were obtained from univariate analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Consecutive UC/IBDU patients who underwent restorative proctocolectomy from 1998 to 2008 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Clinical charts reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Clinical charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Ferrante 2017.
| Study characteristics | ||
| Methods | Retrospective cohort. Enrolment period: 2006 to 2016 | |
| Participants | Country: Belgium. UC patients who underwent colectomy. Patients who were treated with investigational products and who were anticipated to have a permant ileostomy were excluded | |
| Interventions | 1. Preoperative mesalamine (n= 97) 2. Preoperative thiopurine/methotrexate (n= 38) 3. Preoperative steroids (n= 32) 4. Preoperative anti‐TNF medications within 8 weeks of surgery (n= 60) 5. Preoperative vedolizumab within 16 weeks of surgery (n= 34) 6. No preoperative mesalamine (n= 73) 7. No preoperative thiopurine/methotrexate (n= 132) 8. No preoperative steroids (n= 138) 9. No preoperative anti‐TNF medications (n= 110) 10. No preoperative vedolizumab (n= 136) |
|
| Outcomes | Pouch specific infectious complications, surgical site infections, non‐surgical site infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Unadjusted ORs for preoperative mesalamine, thiopurine/methotrexate, steroids, anti‐TNF therapy, and vedolizumab were obtained from univariate regre ssion models. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All UC patients who underwent colectomy from 2006 to 2016 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Patient charts reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not adjust for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not adjust for additional factors |
| Assessment of outcome | Low risk | Patient charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Fumery 2017.
| Study characteristics | ||
| Methods | Prospective, multicenter (9 institutions) cohort. Study period: 2010 to 2014 | |
| Participants | Country: France. CD patients who underwent ileocecal resection. Pregnant patients and those who had surgery for dysplasia were excluded | |
| Interventions | 1. Preoperative steroids (n= 45) 2. Preoperative anti‐TNF therapy (n= 93) within 4 weeks of surgery 3. No preoperative steroid (n= 164) 4. No preoperative anti‐TNF therapy (n= 165) |
|
| Outcomes | Abdominal infections and extaabdominal infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All adult CD patients who underwent ileocecal resection from 2010 to 2014 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Data collected prospectively in a standardized format by gastroenterologist |
| Demonstration that outcome of interest was not present at start of study | High risk | 42% surgeries performed due to fistula/abscess |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control of other factors |
| Assessment of outcome | Low risk | Data collected prospectively in a standardized format and reviewed in detail |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Gainsbury 2011.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2005 to 2009 | |
| Participants | Country: USA. UC patients who underwent ileal pounch‐anal anastomosis | |
| Interventions | 1. Preoperative infliximab (n= 29) within 12 weeks of surgery 2. No preoperative infliximab (n= 52) |
|
| Outcomes | Pelvic/intraabdominal abscess or wound infection | |
| Notes | NOS low risk of bias overall Adjusted ORs f or preoperative infliximab, 6MP and corticosteroids were obtained from multivariate logistic regression models. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent ileal pounch‐anal anastomosis from 2005 to 2009 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Medical records were reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Adjusted for steroids, 6‐mercaptopurine, and methotrexate |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also adjusted for BMI, laproscopic colectomy, and failed medical therapy |
| Assessment of outcome | Low risk | Medical records were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Gu 2013.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period 2006 to 2010 | |
| Participants | Country: USA. UC or indeterminate colitis ptints who underwent total proctocolectomy or subtotal colectomy with end ileostomy. Patients who underwent surgery for toxic megacolon, massive hemorrhage, colonic perforation, dyplasia or malignancy were exlcuded | |
| Interventions | 1. Anti‐TNF medication (n= 167) within 12 weeks of surgery for infliximab and within 4 weeks of surgery for adalimumab/certolizumab 2. No preoperative anti‐TNF medication (n= 421) |
|
| Outcomes | Pelvic sepsis, wound infection, anastomotic leak within 30 days | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC or indeterminate colitis ptints who underwent total proctocolectomy or subtotal colectomy with end ileostomy |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Information obtained from prospective database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Did not provide information |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control of other factors |
| Assessment of outcome | Low risk | Information obtained from prospective database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Guasch 2016.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2009 to 2014 | |
| Participants | Country: Spain. CD patients who underwent intestinal resection | |
| Interventions | 1. Preoperative anti‐TNF treatment (n= 44) within 3 months of surgery 2. No preoperative anti‐TNF treatment (n= 56) |
|
| Outcomes | Septic complications within 30 days of surgery | |
| Notes | This study is an abstract. NOS risk of bias very high overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | All CD patients who underwent intestinal resection from 2009 to 2014 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Electronic medical record reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for additional factor) | Unclear risk | No information provided |
| Assessment of outcome | Low risk | Electronic medical record reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | All patients had at least 1 year of follow up |
Gudsoorkar 2018.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2015 to 2017 | |
| Participants | CD and UC patients who underwent IBD‐related surgery | |
| Interventions | 1. Preoperative anti‐TNF medication (n= 20) 2. Preoperative vedolizumab (n= 16) 3. No preoperative biologic therapy (n= 12) |
|
| Outcomes | Infectious complications within 30 days | |
| Notes | This study is an abstract. NOS risk of bias very high overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD and UC patients who underwent IBD‐related surgery |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Unclear risk | Information not provided |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not adjust for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not adjust for additional factors |
| Assessment of outcome | Unclear risk | Information not provided |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Guo 2017.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2013 to 2015 | |
| Participants | Country: China. Patients with fistulizing CD who underwent abodminal surgery. Excluded stricturoplasty, resection of perianal disease, ileostomy/colostomy closure, reoperations for postoperative complications | |
| Interventions | 1. Preoperative 5ASA (n= 45) 2. Preoperative immunomodulator (n= 7) 3. Preoperative steroids (n= 22) 4. Preoperative anti‐TNF medication (n= 11) 5. No preoperative 5ASA (N= 73) 6. No preoperative immunomodulator (n= 111) 7. No preoperative steroids (n= 96) 8. No preoperative anti‐TNF medication (n= 107) |
|
| Outcomes | Surgical site infection within 30 days | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Patients with fistulizing CD who underwent abodminal surgery. |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Medical records reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | 13 patients had preoperative intraabdominal abscess |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factors |
| Assessment of outcome | Low risk | Medical records reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | Surgical site infection within 30 days of surgery |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Jouvin 2018.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2002 to 2013 | |
| Participants | Country: France. CD patients who underwent ileocolic resection. Patients requiring ileorectal or ileoanal anastomosis were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 55) within 8 weeks of surgery 2. Preoperative immunomodulator (n= 147) 3. Preoperative steroids (n= 154) 4. No preoperative anti‐TNF therapy (n= 305) 5. No preoperative immunomodulator (n= 213) 6. No preoperative steroids (n= 206) |
|
| Outcomes | Intraabdominal septic complications, septic complications within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR for preoperative anti‐TNF therapy was obtained from multivaria ble regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent ileocolic resection from 2002 to 2013 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Unclear risk | Information not provided |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for other factors |
| Assessment of outcome | Unclear risk | Information not provided |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Kim 2018.
| Study characteristics | ||
| Methods | Matched cohort study. Enrolment period: unknown | |
| Participants | CD patients who underwent colorectal surgery. Patients matched for age and gender | |
| Interventions | 1. Preoperative vedolizumab (n= 13) 2. Preoperative anti‐TNF (n= 39) 3. No preoperative biologic (n= 29) |
|
| Outcomes | Surgical site infection within 30 days of surgery | |
| Notes | This study is an abstract. NOS low risk of bias Adjusted OR for preoperative vedolizumab was obtained from a multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent colorectal surgery |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period. Patients matched for age and gender |
| Ascertainment of exposure | Low risk | Information obtained from prospective database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for age and gender |
| Assessment of outcome | Low risk | Information obtained from prospective database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Kotze 2017.
| Study characteristics | ||
| Methods | Retrospective, multicenter (2 institutions) cohort. Study period: 2007 to 2014 | |
| Participants | Country: Brazil. CD patients who underwent elective intestinal resection. Stricuroplasty, diverting stomas and emergency surgeries were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 81) within 8 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 52) |
|
| Outcomes | Intraabdominal abscess, anastomotic dehiscence, surgical site infection, other infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent elective intestinal resection from 2007 to 2014 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Medical records examined |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factors |
| Assessment of outcome | Low risk | Medical records examined |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Krane 2013.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2004 to 2011 | |
| Participants | Country: USA. CD, UC and indeterminate colitis patients who underwent laparascopic resection. Stricturoplasty, multiorgan resection, stoma formation without colorectal resection, emergency surgery, and robot‐assisted surgery were excluded. | |
| Interventions | 1. Preoperative infliximab (n= 142) within 12 weeks of surgery 2. No preoperative infliximab (n= 376) |
|
| Outcomes | Surgical site infections and non‐surgical site infections within 30 days of surgery | |
| Notes | Only patients with minimum of 6 months of follow were included. NOS low risk of bias overall Adjusted ORs for preoperative infliximab, steroi ds and immunomodulators were obtained from a multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD, UC and indeterminate colitis patients who underwent laparascopic resection |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospitals and time period |
| Ascertainment of exposure | Low risk | Prospectively collected database |
| Demonstration that outcome of interest was not present at start of study | High risk | 24 patients had preoperative C. difficile infection |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Adjusted for steroids and immunosuppressants |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Adjusted for type of IBD, disease activity, comorbidities |
| Assessment of outcome | Low risk | Prospectively collected database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Only patients with minimum of 6 months of follow were included |
Kunitake 2008.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1993 to 2007 | |
| Participants | Country: USA. CD, UC and indeterminate colitis patients who underwent abdominal surgery | |
| Interventions | 1. Preoperative infliximab (n= 101) within 12 weeks of surgery 2. No preoperative infliximab (n= 312) |
|
| Outcomes | Anastomotic leak, sepsis, intraabdominal abscess, wound infection, wound dehiscence, pneumonia | |
| Notes | NOS very high risk of bias overall Adjusted ORs for preoperative infliximab and c orticosteroids were obtained fr om multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD, UC and indeterminate colitis patients who underwent abdominal surgery |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Electronic medical records were reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | Indication for surgery was intraabdominal abscess in 38 patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Reported event rates |
| Comparability of cohorts (Controlled for additional factor) | High risk | Reported event rates |
| Assessment of outcome | Low risk | Electronic medical records were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Liang 2017.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2014 to 2016 | |
| Participants | Country: USA. UC and CD patients who underwent lower GI surgery | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 686) within 12 weeks of surgery 2. Preoperative vedolizumab (n= 114) 3. Preoperative ustekinumab (n= 8) 4. Preoperative 5ASA (n= 733) 5. Preoperative corticosteroids (n= 674) 6. Preoperative immunosuppressants (n= 382) 7. No preoperative anti‐TNF therapy (n= 2674) 8. No preoperativ vedolizumab (n= 3246) 9. No preoperative ustekinumab (n= 3352) 10. No preoperative 5ASA (n= 2627) 11. No preoperative corticosteroids (n= 2686) 12. No preoperative immunosuppressants (n= 2978) |
|
| Outcomes | Wound infection, peritonitis, retroperitoneal infection, sepsis within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR for preoperative corticosteroids was obtained from multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC and CD patients who underwent lower GI surgery |
| Selection of the non exposed cohort | Low risk | Both groups obtained from Clinformatics DataMart database and time period |
| Ascertainment of exposure | Low risk | Identified exposures using medical and pharmacy claims |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Did not provide information |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Identified outcomes using ICD codes, facility claims and provider claims |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Did not provide information |
Lightner 2018 A.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2014 to 2016 | |
| Participants | Country: USA. CD patients who underwent elective major abdominal surgery. | |
| Interventions | 1. Preoperative vedolizumab (n= 100) within 12 weeks of surgery 2. Preoperative anti‐TNF therapy (n= 107) within 12 weeks of surgery 3. No preoperative biologic therapy (n= 105) |
|
| Outcomes | Surgical site infection, mucocutaneous separation, anastomotic leak, extraabdominal infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent elective major abdominal surgery from 2014 to 2016 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Electronic medical records reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factors |
| Assessment of outcome | Low risk | Electronic medical records reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Excluded patients with less than 30 days of follow up |
Lightner 2018 B.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period 2010 to 2017. | |
| Participants | Country: USA. Adult patients undergoing ileocolic resection with primary anastomosis for Crohn's disease | |
| Interventions | 1. Preoperative steroid use 2. Preoperative immunomodulator use 3. Preoperative biologics 4. Dual therapy 5. Triple therapy 6. No preoperative medication |
|
| Outcomes | Intra‐abdominal sepsis, defined as an intraperitoneal abscess or anastomotic leak | |
| Notes | NOS very high risk of bias overall Unadjusted ORs for preoperative steroids, immunomodulators and biologics were obtained from univari ate analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Retrospective cohort of patients undergoing ileocolic resection for Crohn's disease |
| Selection of the non exposed cohort | Low risk | Both groups obtained from same centres and time period |
| Ascertainment of exposure | Low risk | Chart review was performed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | No information provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Unadjusted odds ratios were reported |
| Comparability of cohorts (Controlled for additional factor) | High risk | Unadjusted odd ratios were reported |
| Assessment of outcome | Low risk | Chart review was performed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Mahadevan 2002.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1997 to 1999 | |
| Participants | Country: USA. UC patients who underwent proctocolectomy with IPAA | |
| Interventions | 1. Preoperative immunosuppressants (n= 58) 2. No preoperative immunosuppressants (n= 151) |
|
| Outcomes | Anastomotic leak, wound dehiscence, pelvic sepsis, perianal fistula/abscess, other infectious complications within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent proctocolectomy with IPAA from 1997 to 1999 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factors |
| Assessment of outcome | Low risk | Medical records reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | All 209 patients were included in the analysis of early complications |
Marchal 2004.
| Study characteristics | ||
| Methods | Matched retrospective cohort study. Study period: 1998 to 2002 | |
| Participants | Country: Belgium. CD patients who underwent intestinal resection | |
| Interventions | 1. Preoperative infliximab (n= 40) 2. No preoperative infliximab (n= 39) |
|
| Outcomes | Sepsis, anastomotic leak, peritonitis, fistula, abscess, wound infection within 10 days of surgery | |
| Notes | NOS low risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent intestinal resection |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | Indication for surgery was fistula/abscess in 31 patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Control group adjusted for age, gender and surgical procedure |
| Assessment of outcome | Low risk | Medical records reviewed |
| Was follow‐up long enough for outcomes to occur | Unclear risk | 10 days only |
| Adequacy of follow up of cohorts | Unclear risk | Information was not provided |
McKenna 2018.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2007 to 2017 | |
| Participants | Country: USA. CD patients who underwent ileocolic resection with primary anastomosis. Patients who underwent ileocolic resection with primary anastomosis and diverting loop ileostomy were excluded | |
| Interventions | 1. Preoperative steroids (n= 37) 2. Preoperative immunomodulators (n =57) 3. Preoperative anti‐TNF therapy (n= 322) within 12 weeks of surgery 4. No preoperative steroids (n= 584) 5. No preoperative immunomodulators (n= 564) 6. No preoperative anti‐TNF therapy (n= 299) |
|
| Outcomes | Intraabdominal sepsis within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for preoperative steroids, immunomodulators, and anti‐TNF therapy were obtained from multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent ileocolic resection with primary anastomosis from 2007 to 2017 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical charts were reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | 61 patients had an abscess at the time of surgery |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Controlled for other medications (corticosteroids, immunomodulators, anti‐TNF) |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for previous intestinal resection, tobacco use |
| Assessment of outcome | Low risk | Medical charts were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Mor 2008.
| Study characteristics | ||
| Methods | Matched retrospective cohort study. Study period: 2000 to 2006 | |
| Participants | Country: USA. UC and indeterminate colitis patients who underwent two stage restorative proctocolectomy | |
| Interventions | 1. Preoperative infliximab (n= 46) 2. No preoperative infliximab (n= 46) |
|
| Outcomes | Pelvis sepsis within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for preoperative steroids, immunomodulators, and anti‐TNF were obtained from multiv ariate regression analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC and indeterminate colitis patients who underwent two stage restorative proctocolectomy from 2000 to 2006 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records were reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Adjusted for dose of steroids and other immunomodulators |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Patients matched for age, gender, date of operation and indication for surgery |
| Assessment of outcome | Low risk | Medical records were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Morar 2015.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2005 to 2010 | |
| Participants | Country: United Kingdom. CD patients who underwent ileocolonic resection. Patients with previous segmental or subtotal colectomy were excluded | |
| Interventions | 1. Preoperative steroids (n= 34) 2. Preoperative immunomodulators (n= 64) 3. Preoperative 5ASA (n= 43) 4. Preoperative anti‐TNF therapy (n= 4) within 4 weeks of surgery 5. No preoperative steroids (n= 104) 6. No preoperative immunomodulators (n= 193) 7. No preoperative 5ASA (n= 82) 8. No preoper a tive anti‐TNF therapy (n= 126) |
|
| Outcomes | Intrabdominal septic complication including anastomotic leak, intraabdominal collection, enter o cutaneous fistula formation within 30 days of surgery | |
| Notes | NOS very high risk of bias ov erall Adjusted OR for preoperative anti‐TNF therapy was obtained from multivariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent ileocolonic resection from 2005 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same databases and time period |
| Ascertainment of exposure | Low risk | Clinical case records were examined |
| Demonstration that outcome of interest was not present at start of study | High risk | Fistula/abscess was indication for surgery for some patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Clinical case records were examined |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Low percentage of patients had missing data (<15%) |
Myrelid 2009.
| Study characteristics | ||
| Methods | Prospective cohort. Enrolment period: 1989 to 2002 | |
| Participants | Country: Sweden. CD patients who underwent a surgery for CD involving an anastomosis/strictureplasty. Proceudres including diverting or permantent stomas and reconstructions after stomas were excluded | |
| Interventions | 1. Preoperative steroids (n=87) 2. Preoperative 5ASA (n= 113) 3. Preoperative azathioprine (n= 51) 4. No preoperative steroids (n= 256) 5. No preoperative 5ASA (n= 230) 6. No preoeprative azathioprine (n= 292) |
|
| Outcomes | Intraabdominal septic complications (including fistula, abscess, and dehiscence) and extraabdominal infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent a surgery for CD involving an anastomosis/strictureplasty |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database |
| Ascertainment of exposure | Low risk | Data obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | High risk | 12% of patients had preoperative intraabdominal infection |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Myrelid 2014.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period not provided | |
| Participants | Country: Sweden. CD patients who underwent resection with primary anastomoses and/or stirctureplasties. Patients with stomas were excluded | |
| Interventions | 1. Preoperative infliximab (n= 111) within 2 months of surgery 2. No preoperative infliximab (n= 187) |
|
| Outcomes | Infectious complications within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Unadjusted ORs for preoperative steroids, immunomodulators and anti‐TNF were o btained from regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent resection with primary anastomoses and/or stirctureplasties. |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital database and time period |
| Ascertainment of exposure | Low risk | Data obtained from database |
| Demonstration that outcome of interest was not present at start of study | High risk | Indication for surgery was abscess/fistula in 61 patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data obtained from database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | No information provided |
Nasir 2010.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2005 to 2009 | |
| Participants | Country: USA. CD patients who underwent operations resulting in an anastomosis. Emergency surgeries and proximal diversions were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 119) within 8 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 251) |
|
| Outcomes | Intraabdominal abscess and anastomotic leak within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent operations resulting in an anastomosis from 2005 to 2009 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital database and time period |
| Ascertainment of exposure | Low risk | Data obtained from prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factors |
| Assessment of outcome | Low risk | Data obtained from prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Nguyen 2014.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2005 to 2012. | |
| Participants | Country: Canada. CD and UC patients who underwent abdominal surgery in the ACS‐NSQIP database. | |
| Interventions | 1. Preoperative steroids (n= 6281) 2. No preoperative steroids (n= 9214) |
|
| Outcomes | Superficial and deep wound infection, intraabdominal infection, extraabdominal infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD and UC patients who underwent abdominal surgery in the ACS‐NSQIP database from 2005 to 2012 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Patients identified by ICD‐9 codes |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factor |
| Assessment of outcome | Low risk | Obtained fron ACS‐NSQIP database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Norgard 2012.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2003 to 2010 | |
| Participants | Country: Denmark. UC patients who underwent colectomy in the Danish National Patient Registry | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 199) within 12 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 1027) |
|
| Outcomes | Anastomotic leak, intraabdominal asbcess within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent colectomy from 2003 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Exposure identified by codes from the National Patient Registry and prescription database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | OR adjusted for age, gender, comorbidity, steroids, duration of UC, year and length of inpatient stay was available for anastomotic leak and intraabodminal abscess outcomes separately. Combined event rate of anastomotic leak and intraabdominal abscess was used for our analysis. |
| Comparability of cohorts (Controlled for additional factor) | High risk | See above |
| Assessment of outcome | Low risk | Outcome identified by codes from the National Patient Registry |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | All patients had 30 and 60 day follow up data after surgery |
Norgard 2013.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2003 to 2010 | |
| Participants | Country: Denmark. CD patients who underwent abdominal surgery in the Danish National Patient Registry | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 214) within 12 weeks 2. No preoperative anti‐TNF therapy (n= 2079) |
|
| Outcomes | Anastomotic leak, intraabdominal abscess, bacteremia within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent abdominal surgery from 2003 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Exposure identified by codes from the National Patient Registry and prescription database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | OR adjusted for age, gender, comorbidity, steroids, duration of CD, year and length of inpatient stay was available for anastomotic leak and intraabodminal abscess outcomes separately. Combined event rate of anastomotic leak and intraabdominal abscess was used for our analysis. |
| Comparability of cohorts (Controlled for additional factor) | High risk | See above |
| Assessment of outcome | Low risk | Outcome identified by codes from the National Patient Registry |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | All patients had 30 and 60 day follow up data after surgery |
Novello 2020.
| Study characteristics | ||
| Methods | Retrospective cohort study. Study period 2012 to 2017 | |
| Participants | IBD patiests who underwent elective abdominal surgery. Excluded patients undergoing anorectal surgery alone. | |
| Interventions | 1. Preoperative vedolizumab use within 12 weeks of surgery 2. Preoperative infliximab use within 12 weeks of surgery 3. No preoperative biologic use |
|
| Outcomes | Infectious complications within 30 days of surgery, including organ space SSI, superficial SSI, perineal wound infection, pneumonia, UTI, anastomotic leak, rectal stump leak, other enteric leak, stoma separation and Clostridium difficile infection | |
| Notes | Case‐matched analysis was performed but we used the data from the analysis conducted on the full, unmatched sample. NOS low risk of bias overall Adjusted ORs were obtained for preoperative vedolizumab and infliximab treatment from multivariable logistic regression models. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Consecutive patients with IBD who underwent abdominal surgery |
| Selection of the non exposed cohort | Low risk | Both groups obtained from same centres and time period |
| Ascertainment of exposure | Low risk | Chart review performed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Multivariate analysis did not control for other preoperative medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for other factors including hemoglobin, BMI, surgery time and diagnosis (CD vs UC) |
| Assessment of outcome | Low risk | Chart review performed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Regadas 2011.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2001 to 2008 | |
| Participants | Country: USA. CD, UC and indeterminate colitis patients who underwent ileostomy reversal | |
| Interventions | 1. Preoperative infliximab (n= 28) within 2 months of surgery 2. Preoperative steroids (n= 72) 3. Preoperative immunosuppressive therapy (n= 35) 4. No preoperative immunosuppressive therapy (n= 114) |
|
| Outcomes | Wound infection, anastomotic leak, intraabdominal abscess and enterocutaneous fistula within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD, UC and indeterminate colitis patients who underwent ileostomy reversal from 2001 to 2008 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Data obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control for additional factors |
| Assessment of outcome | Low risk | Data obtained from a prospectively maintained databas |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Rizzo 2011.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2004 to 2010 | |
| Participants | Country: Italy. CD or UC patients who underwent abdominal surgery | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 54) within 12 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 60) |
|
| Outcomes | Wound infection, intraabdominal abscess, anastomotic leak, pelvic abscess, extraabdominal infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD or UC patients who underwent abdominal surgery from 2004 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Medical records were reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | Indication for surgery was perforation in 6 patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Medical records were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Schils 2017.
| Study characteristics | ||
| Methods | Matched retrospective cohort study. Study period 2006 to 2016 | |
| Participants | Country: Belgium. CD patients who underwent right hemicolectomy with ileocolonic anastomosis | |
| Interventions | 1. Preoperative vedolizumab (n= 12) within 14 weeks of surgery 2. Preoperative anti‐TNF therapy (n= 12) within 8 weeks of surgery 3. Preoperative steroids (n= 12) 4. No preoperative therapy (n= 12) |
|
| Outcomes | Infectious complications and anastomotic leak within 30 days of surgery | |
| Notes | This study is an abstract. Additional information was provided by the authors of Schils et al. NOS low risk of bias overall. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent right hemicolectomy with ileocolonic anastomosis from 2006 to 2016 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period. Matched for age and gender |
| Ascertainment of exposure | Low risk | Medical charts were reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for age and gender |
| Assessment of outcome | Low risk | Medical charts were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Schluender 2007.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2000 to 2005 | |
| Participants | Country: USA. UC patients who underwent colectomy. Patients with indeterminate colitis, surgery for dysplasia or carcinoma were excluded | |
| Interventions | 1. Preoperative infliximab (n= 17) 2. No preoperative infliximab (n= 134) |
|
| Outcomes | Infectious complications within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent colectomy from 2000 to 2005 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Information obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | High risk | Did not control of additional factors |
| Assessment of outcome | Low risk | Information obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Selvasekar 2007.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2002 to 2005 | |
| Participants | Country: USA. UC patients who underwent ileal pouch anal anastomosis | |
| Interventions | 1. Preoperative infliximab (n= 47) 2. No preoperative infliximab (n= 254) |
|
| Outcomes | Anastomotic leak, intraabdominal abscess, superficial and deep wound infections within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for steroids, azathioprine and anti‐TNF were obtained from multivariate regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent ileal pouch anal anastomosis from 2002 to 2005 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Medical charts reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Adjusted for steroids, infliximab and azathioprine use |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also adjusted for age and severity of colitis |
| Assessment of outcome | Low risk | Medical charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Serradori 2013.
| Study characteristics | ||
| Methods | Retrospective, multicenter (3 institutions) cohort. Study period: 2000 to 2010 | |
| Participants | Country: France. CD patients who underwent ileocecal or ileocolonic resection. Patients with temporary stomas were excluded. | |
| Interventions | 1. Preoperative anti‐TNF within 8 weeks of surgery 2. Preoperative steroids |
|
| Outcomes | Intraabdominal abscess, anastomotic leak, fistula within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for preoperative anti‐TNF and corticosteroids were obtained from multivariate regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent ileocecal or ileocolonic resection from 2000 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Medical charts reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | 30 patients had an abscess at the time of surgery |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Controlled for steroids and anti‐TNF use |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also controlled for age, Montreal classification |
| Assessment of outcome | Low risk | Medical charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Shaib 2017.
| Study characteristics | ||
| Methods | Retrospective cohort (NSQIP database). Study period: not provided | |
| Participants | Country: USA. UC and CD patients who underwent colectomy | |
| Interventions | 1. Preoperative steroid 2. No preoperative steroid |
|
| Outcomes | Anastomotic leak within 30 days of surgery | |
| Notes | This study is an abstract. NOS very high risk of bias overall Adjusted OR for preoperative corticosteroids was obtained from multivariate regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC and CD patients who underwent colectomy |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database |
| Ascertainment of exposure | Low risk | Information obtained from NSQIP database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for gender, operation time, smoking, emergency surgeries, subtype of IBD |
| Assessment of outcome | Low risk | Information obtained from NSQIP database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Shwaartz 2016.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2013 to 2015 | |
| Participants | Country: USA. UC and CD patients who underwent intestinal surgery with primary anastomosis. Emergency surgeries were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 73). Last dose of infliximab within 8 weeks and last dose of adalimumab and certolizumab within 4 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 209) |
|
| Outcomes | Anastomotic leak, intraabdominal abscess, extraabdominal infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC and CD patients who underwent intestinal surgery with primary anastomosis from 2013 to 2015 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Analysis for overall infectious complications did not control for other factors. |
| Comparability of cohorts (Controlled for additional factor) | High risk | See above |
| Assessment of outcome | Low risk | Medical records reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Syed 2013.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period 2004 to 2011 | |
| Participants | Country: USA. CD patients who underwent abdominal surgery. Isolated perianal surgieres were excluded. | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 150) within 8 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 175) |
|
| Outcomes | Intraabdominal infectious complication, surgical site complication, any infectious complication within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted OR for preoperative anti‐TNF was obtained from multivariate regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent abdominal surgery from 2004 to 2011 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Electronic medical record and clinic charts were reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for BMI, stricture, open surgery, perforation, ASA >2 |
| Assessment of outcome | Low risk | Electronic medical record and clinic charts were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Tzivanakis 2012.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2000 to 2010 | |
| Participants | Country: UK. CD patients who underwent ileocecal and ileocolic resection. Strictureplasties, mulitple resections, subtotal or isolated colonic resections were excluded | |
| Interventions | 1. Preoperative steroids (n= 56) 2. No preoperative steroids (n= 117) |
|
| Outcomes | Anastomotic leak, intraabdominal abscess, fistula within 30 days of surgery | |
| Notes | NOS low risk of bias overall Unadjusted OR for preoperative corticosteroids was ob tained from regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent ileocecal and ileocolic resection from 2000 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Data from propsective database that was audited weekly to ensure accurate entry of data |
| Demonstration that outcome of interest was not present at start of study | High risk | Preoperative abscess was found to an independent predictor of anastomotic‐associated complication |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for age, sex, malnutrition, preoperative serum albumin, smoking, urgency of surgery, mode of surgery, presence of abscess or fistula at the time of surgery and previous resection for CD |
| Assessment of outcome | Low risk | Data from propsective database that was audited weekly to ensure accurate entry of data |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Uchino 2010.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2006 to 2008 | |
| Participants | Country: Japan. UC patients who underwent laparotomy. Patients who had subtotal colectomy with mucous fistula before IPAA or ileostomy closure were excluded. | |
| Interventions | 1. Preoperative immunosuppressants (n= 20) 2. No preoperative immunosuppressants (n= 172) |
|
| Outcomes | Superficial incisional, deep incisional, and organ space surgical site infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent laparotomy from 2006 to 2008 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Unclear risk | Information not provided |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univerariate analysis |
| Assessment of outcome | Unclear risk | Information not provided |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Uchino 2013a.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2008 to 2011 | |
| Participants | Country: Japan. CD patients who underwent laparotomy. Perianal procedures were excluded | |
| Interventions | 1. Preoperative infliximab (n= 79) within 12 weeks of surgery 2. Preoperative immunosuppressants (n= 6) 3. Preoperative 5ASA (n= 322) 4. No preoperative infliximab (n= 326) 5. No preoperative immunosuppressants (n= 399) 6. No preoperative 5ASA (n= 83) |
|
| Outcomes | Incisional surgical site infection, organ space surgical site infeciton within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent laparotomy from 2008 to 2011 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Data was obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | High risk | 27 patients classified as wound class IV (dirty/infected) |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data was obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Uchino 2013b.
| Study characteristics | ||
| Methods | Retrospective cohort. 2010 to 2012 | |
| Participants | Country: Japan. UC patients who underwent laparotomy. Patients who had subtotal colectomy with mucous fistula before IPAA or ileostomy closure were excluded. | |
| Interventions | 1. Preoperative infliximab (n= 22) within 12 weeks of surgery 2. Properative immunomodulators (n= 94) 3. No preoperative infliximab (n= 174) 4. No preoperative immunomodulators (n= 102) |
|
| Outcomes | Superficial incisional, deep incisional, organ space surgical site infection, and extraabdominal infections within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent laparotomy from 2010 to 2012 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Data was obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Included patients with contaminated wounds |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univiariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data was obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Uchino 2015.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2012 to 2014 | |
| Participants | Country: Japan. UC patients who underwent IPAA. Patients who underwent with total colectomy without IPAA were ex c luded | |
| Interventions | 1. Preoperative biologic (n=44) within 12 weeks of surgery 2. Preoperative immunomodulator (n= 105) 3. Preoperative steroids (n= 113) |
|
| Outcomes | Surgical site infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR preoperative steroids, immunomodulators, and biologics were obtained from multivariate regression analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent IPAA from 2012 to 2014 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Data was obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data was obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Uchino 2019.
| Study characteristics | ||
| Methods | Retrospective cohort study. Study period 2015 to 2018 | |
| Participants | Country: Japan. Patients with ulcerative colitis who underwent total colectomy with ileostomy, total proctocolectomy with end ileostomy or ileal pouch anal anastomosis | |
| Interventions | 1. Preoperative steroids (n=117) 2. Preoperative azathioprine/6MP (n=121) 3. Preoperative anti‐TNF agents within 12 weeks of surgery (n=146) |
|
| Outcomes | Surgical site infection (incisional infection and organ/space infection) within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR for preoperative anti‐TNF therapy was obtained from multivariable regression model. Unadjusted OR for preoperative steroid therapy was obtained from univariate regression model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | 301 consecutive patients with UC who underwent abdominal surgery |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Retrospective evaluation of a prospective database at Hyogo College of Medicine |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not perform |
| Comparability of cohorts (Controlled for additional factor) | High risk | Multivariate analysis only for anti‐TNF administration (age, pre‐op albumin, transfusion, ASA score, wound class, urgent/emergent surgery, intra‐operative blood loss and duration of surgery). Univariate analysis for steroids and azathioprine/6M |
| Assessment of outcome | Low risk | Retrospective evaluation of a prospective database at Hyogo College of Medicine |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Ward 2018.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2006 to 2015 | |
| Participants | Country: UK. UC patients who underwent subtotal colectomy | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 418) within 4 weeks of surgery 2. No preoperative anti‐TNF therapy (n= 5807) |
|
| Outcomes | Infectious complications within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent subtotal colectomy from 2006 to 2015 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period |
| Ascertainment of exposure | Low risk | Information obtained using Office of Population Censuses and Surveys codes |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Information obtained using ICD codes |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Waterman 2013.
| Study characteristics | ||
| Methods | Matched retrospective cohort study. Study period: 2000 to 2010 | |
| Participants | Country: Canada. CD and UC patients who underwent abdominal surgery. | |
| Interventions | 1. Preoperative infliximab (n= 195) within 180 days of surgery 2. No preoperative infliximab (n= 278) |
|
| Outcomes | Wound infection, anastomotic leak, intraabdominal abscess, extraabdominal infections within 30 days of surgery | |
| Notes | NOS low risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD and UC patients who underwent abdominal surgery from 2000 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period. |
| Ascertainment of exposure | Low risk | Patient charts reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Groups matched for steroids |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Groups also matched for surgical procedure, IBD subtype and patient age |
| Assessment of outcome | Low risk | Patient charts reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Patients without adequate clinical records documenting 30 day outcomes were excluded |
Wilson 2014.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2005 to 2010 | |
| Participants | Country: USA. CD patients in National Surgical Quality Improvement Project database who underwent ileocolectomy | |
| Interventions | 1. Preoperative steroid therapy (n= 954) 2. No preoperative steroid therapy (n= 1515) |
|
| Outcomes | Organ space surgical site infection within 30 days of surgery | |
| Notes | NOS low risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients in National Surgical Quality Improvement Project database who underwent ileocolectomy from 2005 to 2010 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period. |
| Ascertainment of exposure | Low risk | NSQIP database |
| Demonstration that outcome of interest was not present at start of study | High risk | Wound class classification IV in 12% of patients |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for BMI, diabetes, smoking, ASA classification, extended operative time, open procedure, preoperative SIRS, emergent procedure, and wound classification |
| Assessment of outcome | Low risk | NSQIP database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Yamada 2017.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2014 to 2016 | |
| Participants | Country: USA. UC and CD patients who underwent major and minor abdominal surgeries. Patients with less than 30 days of follow up data or missing data were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 129) within 4 weeks of surgery 2. Preoperative vedolizumab (n= 64) within 4 weeks 3. No preoperative biologic therapy (n= 250) |
|
| Outcomes | Infectious complications (wound infection or dehiscence, anastomotic leak, abscess, sepsis, fistula, pulmonary infection, UTI) within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR for preoperative steroids was obtained from multivariate model. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC and CD patients who underwent major and minor abdominal surgeries from 2014 to 2016 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same database and time period. |
| Ascertainment of exposure | Low risk | Data was obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Data was obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Patients with less than 30 days of follow up data or missing data were excluded |
Yamamoto 2000.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1980 to 1997 | |
| Participants | Country: UK. CD patients who underwent at least one intestinal anastomosis. Closure of loop ileostomy/colostomy and reoperations for early postoperative complications were excluded | |
| Interventions | 1. Preoperative steroids (n= 220) 2. No preoperative steroids (n= 346) |
|
| Outcomes | Anastomotic leak, intraabdominal abscess, fistula within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent at least one intestinal anastomosis from 1980 to 1997 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period. |
| Ascertainment of exposure | Low risk | Medical records were reviewed |
| Demonstration that outcome of interest was not present at start of study | High risk | 121 patients were found to have an abscess at the time of laparotomy |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Medical records were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Yamamoto 2016.
| Study characteristics | ||
| Methods | Retrospective multicenter (7 institutions) cohort. Study period: 2008 to 2013 | |
| Participants | Countries: Japan, Brazil and Italy. CD patients who underwent ileocolonic resection with primary anastomosis. Patients who had covering ileostomy or with insufficient data for analysis were excluded | |
| Interventions | 1. Preoperative steroids (n= 68) 2. Preoperative immunomodulators (n= 65) 3. Preoperative biologics (n= 79) within 8 weeks of surgery 4. No preoperative steroids (n= 163) 5. No preoperative immunomodulators (n= 166) 6. No preoperative biologics (n= 152) |
|
| Outcomes | Anastomotic leak, intraabdominal abscess, fistula within 30 days of surgery | |
| Notes | NOS low risk of bias overall Adjusted ORs for preoperative steroids, immunomodulators and biologics were obtained from multivariate analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD patients who underwent ileocolonic resection with primary anastomosis from 2008 to 2013 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Data was obtained from a prospectively maintained database |
| Demonstration that outcome of interest was not present at start of study | High risk | 43% of patients had perforating disease at baseline |
| Comparability of cohorts (Controlled for critical factor/other medications) | Low risk | Controlled for steroids, immunosuppressants, biologics |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Also controlled for age, gender, behavior of CD, smoking, previous resections, blood transfusions, surgical approach and type of anastomosis |
| Assessment of outcome | Low risk | Data was obtained from a prospectively maintained database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Patients with insuffi ci ent data for analysis were excluded |
Yu 2019.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period 2006 to 2015 | |
| Participants | Country: Korea. Patients with Crohn's disease who underwent bowel resection with or without anastomosis or strictureplasty. Patients excluded if they had open biopsy or stoma formation/reversal only | |
| Interventions | 1. Preoperative steroids (n= 39) 2. Preoperative immunomodulators (azathioprine, 6MP, methotrexate) (n= 211) 3. Preoperative anti‐TNF medications within 8 weeks of surgery (n= 32) |
|
| Outcomes | 30 day infectious and non‐infectious postoperative complications. Infectious complications include intra‐abdominal sepsis, enterocutaneous fistula, wound infection and extra‐abdominal infection | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | Patients with Crohn's disease who underwent bowel resection with or without anastomosis or strictureplasty. |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Medical records reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Zittan 2016.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 2002 to 2013 | |
| Participants | Country: Canada. UC patients who underwent IPAA. Patients with less than 30 days of follow up data were excluded. Patients without adequate clinical documentation of the 30 day postoperative period were excluded | |
| Interventions | 1. Preoperative anti‐TNF therapy (n= 196) within 180 days of surgery 2. No preoperative anti‐TNF therapy (n= 562) |
|
| Outcomes | Pelvic abcess, anastomotic leak, and wound infection within 30 days of surgery | |
| Notes | NOS very high risk of bias overall Adjusted OR for preoperative corticosteroid s was obtained from multivariable analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent IPAA from 2002 to 2013 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical charts were reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Medical charts were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Low risk | Patients without adequate clinical documentation of the 30 day postoperative period were excluded |
Ziv 1996.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1983 to 1992 | |
| Participants | Country: USA. UC patients who underwent IPAA | |
| Interventions | 1. Preoperative steroids (n= 361) 2. No preoperative steroids (n= 310) |
|
| Outcomes | Sepsis, anastomotic leak, fistula, parapouch abscess within 30 days of surgery | |
| Notes | NOS very high risk of bias overall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | UC patients who underwent IPAA from 1983 to 1992 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Medical records were reviewed |
| Demonstration that outcome of interest was not present at start of study | Unclear risk | Information not provided |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Univariate analysis |
| Comparability of cohorts (Controlled for additional factor) | High risk | Univariate analysis |
| Assessment of outcome | Low risk | Medical records were reviewed |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Zuo 2014.
| Study characteristics | ||
| Methods | Retrospective cohort. Study period: 1999 to 2004 | |
| Participants | Country: China. CD who underwent abdominal surgery. Patients with intestinal TB, gastrointestinal malignancy, Behcet's or who underwent perianal surgery were excluded | |
| Interventions | 1. Preoperative steroids (n= 32) 2. No preoperative steroids (n= 684) |
|
| Outcomes | Intraabdominal abscess, fistula, anastomotic leak within 30 days | |
| Notes | NOS low risk of bias overall Adjusted OR for preoperative corticosteroids was obtained from multivariate analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representativeness of the exposed cohort | Low risk | CD who underwent abdominal surgery from 1999 to 2004 |
| Selection of the non exposed cohort | Low risk | Both groups obtained from the same hospital and time period |
| Ascertainment of exposure | Low risk | Data extracted from hospital database |
| Demonstration that outcome of interest was not present at start of study | High risk | Proportion of patients were found to have abscesses at the time of surgery |
| Comparability of cohorts (Controlled for critical factor/other medications) | High risk | Did not control for other medications |
| Comparability of cohorts (Controlled for additional factor) | Low risk | Controlled for preoperative albumin, CRP, enteral nutrition, preoperative infection, ostomy, resection and anastomosis |
| Assessment of outcome | Low risk | Data extracted from hospital database |
| Was follow‐up long enough for outcomes to occur | Low risk | 30 days |
| Adequacy of follow up of cohorts | Unclear risk | Information not provided |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelson 2018 | Compared rates of surgical complications in IBD patients who underwent surgery between 1995 and 2005 vs 2005 and 2013. Did not compare preoperative medications. |
| Abou‐Khalil 2016 | Studied patients with CD who were treated with immunosuppressants compared with those who were not. Authors clarified that the immunosuppressant group included patients treated with any of the following: mycophenolate mofetil, adalimumab, etanercept, azathioprine, cyclosporine, tacrolimus, sirolimus, infliximab, natalizumab, methotrexate and certolizumab pegol. |
| Achkasov 2015 | Reported combined outcome of surgical site infection and parastomal complications. Unable to contact authors to obtain rate of only the infectious complications. |
| Adegbola 2018 | Reported rates of intra‐abdominal septic complications in IBD patients who underwent ileocolonic resection. Did not report pre‐operative medications in patients who developed septic complications and those who did not develop septic complications. Unable to contact author for more information. |
| Aelvoet 2016 | Compared patients treated with vedolizumab to those treated with infliximab. |
| Andrew 2017 | Infectious outcomes were collected within the first 60 postoperative days. |
| Bafford 2013 | Reported overall postoperative complications and not specifically infectious complications. Unable to contact authors for additional information. |
| Balachandran 2015 | Recorded complications occurring within 3 months of surgery. |
| Benichou 2018 | Did not specify number of patients with postoperative infections who were treated with and without anti‐TNF agents. |
| Bewtra 2013 | Recorded complications occurring within 90 days of surgery. |
| Braun 2018 | Did not report postoperative infectious complications. |
| Bruewer 2003 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Chaparro 2018 | Described characteristics and indications for surgical interventions. Did not report postoperative complications. |
| Chiplunker 2015 | No comparison group |
| Coscia 2012 | Reported overall postoperative complications. Unable to contact authors to obtain rate of infectious complications. |
| Desai 2012 | Postoperative infection was a secondary outcome but results were not reported in the abstract. Unable to obtain information from authors. |
| De Silva 2011 | Complications were recorded from time of surgery to time of discharge from hospital. Range or mean length of stay in hospital was not reported. While infectious complications were reported, patients' medications were not specified. Unable to contact authors. |
| Domenech 2016 | No comparison group. |
| Eisner 2014 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Fronda 1999 | Did not specifically report infectious complications. |
| Fu 2014 | Study did not compare rates of postoperative infection in IBD patients treated with different medications |
| Gamaleldin 2018 | Did not report postoperative infections |
| George 2017 | Included patients who did not have a diagnosis of IBD |
| Gonzalez 2013 | Did not report postoperative infections |
| Grant 2019 | Reported rates of wound healing following proctectomy but not postoperative infectious complications. |
| Gregory 2019 | Reported 90 day postoperative infectious complications. |
| Heimann 1985 | Reported postoperative infections but did not report how many of these patients were treated with steroids and how many were not treated with steroids. Unable to contact authors for confirmation. |
| Hyde 2001 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Justiniano 2019 | Only reported postoperative mortality. |
| Kamel 2019 | Reported effect of biologic agents on operative outcomes (length of small bowel resection, intraoperative blood loss and total operative time) but not postoperative infectious complications. |
| Kasparek 2012 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Kimura 2019 | Did not provide rates of postoperative infectious complications for each category of preoperative medication. |
| Kline 2020 | Did not examine relationship between preoperative medications and postoperative infectious complications. |
| Kotze 2011 | Patient population included in Kotze 2017. |
| Kotze 2017a | Patient population included in Kotze 2017. |
| Kotze 2018 | Comparison group included patients without IBD |
| Krupa 2012 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Kulaylat 2017 | Recorded complications occurring within 90 days of surgery. |
| Labidi 2018 | There was no comparison group. |
| Lau 2013 | Reported rates of postoperative infection in a graph, which was illegible. Unable to contact authors for additional information. |
| Li 2016 | Reported overall postoperative complications but not specifically infectious complications. |
| Lightner 2017 | Significant overlap with patients in Lightner 2018. |
| Lightner 2017a | Compared vedolizumab treated patients to anti‐TNF treated patients. |
| Lightner 2018a | Compared patients treated with ustekinumab to patients treated with anti‐TNF medications. |
| Lightner 2018b | Compared patients treated with vedolizumab to patients treated with anti‐TNF medications. |
| Lim 2018 | Reported surgical trends over time. Did not examine relationship between preoperative medications and postoperative infectious complications. |
| Melo‐Pinto 2018 | Reported overall postoperative complications but not specifically infectious complications. |
| Monsinjon 2017 | Reported overall postoperative complications but not specifically infectious complications. |
| Nagao 2016 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Novello 2019 | Compared patients treated with ustekinumab to patients treated with vedolizumab. |
| Oh 2014 | Mentioned that the rate of infectious complications was similar between treatment and comparison groups but did not actually report the rate. Unable to contact authors for additional information. |
| Park 2018 | Compared patients treated with ustekinumab to patients treated with vedolizumab. |
| Parrish 2019 | Did not compare different preoperative medications. Compared patients treated with ustekinumab with detectable levels before surgery to patients treated with ustekinumab with undetectable levels. |
| Poylin 2018 | Compared patients treated with vedolizumab to those treated with an anti‐TNF agent. |
| Quade 2013 | Reported overall postoperative complications but not specifically infectious complications. |
| Rizvi 2019 | Reported postoperative infectious complications up to 6 months after surgery. |
| Sahami 2016 | Did not specify time frame for postoperative complications. Unable to contact authors for confirmation. |
| Scarpa 2015 | Reported overall postoperative complications but not specifically infectious complications. |
| Shim 2018 | Compared patients treated with ustekinumab to patients treated with anti‐TNF medications. |
| Shimada 2016 | Did not specify time frame for postoperative complications or rate of complications in the comparison group. Unable to contact authors for confirmation. |
| Stewart 2009 | Reported infectious complications in a group of patients on some form of immunosuppression such as infliximab, cyclosporine, prednisone, or azathioprine. Contacted authors for separate rates of infectious complications in patients treated with each of these medications. However, the authors were unable to obtain this data. |
| Strassle 2017 | Included patients with ulcerative colitis, Crohn's disease, or familial adenomatous polyposis. |
| Stringfield 2016 | Lacked a comparison group. |
| Valizadeh 2017 | Reported infectious complications in patients treated with either immunosuppressant medications or steroids. Did not analyse each type of medications separately. |
| Watson 2018 | Reports rates of any postoperative complication but not specifically infectious complications. |
| Weber 2017 | Did not report postoperative infectious complications. |
| Yamamoto 2016a | Stated that neither immunosuppressive nor biologic therapy prior to surgery was significantly associated with the incidence of septic complications. Also stated that high dose steroid therapy significantly increased the risk of septic complications. Did not report any rates, odd ratio or risk ratio. Unable to contact author for additional information. |
| Yamamoto 2018 | Included patients treated with either anti‐TNF medication or tacrolimus into one group in their analysis. |
Differences between protocol and review
Several modifications were made to the protocol.
Firstly, we clarified that patients with a diagnosis of indeterminate colitis could be included. With regards to the classes of medications included in the study, we separated the biologics category into three separate categories entitled anti‐TNF medications, anti‐interleukin medications, and anti‐integrin medications. Also, we decided that studies comparing two biologics (i.e. vedolizumab versus infliximab) would be excluded due to issues with confounding by indication. Furthermore, we clarified that we would exclude studies that reported complications outside the 30‐day postoperative period.
In the Data extraction and management section, we updated that the investigators CL, CB and NN performed data extraction instead of CL, DK, and YB. We clarified that we attempted to contact authors in cases where data was missing. In the Assessment of risk of bias in included studies section, we updated that four investigators assessed the methodological quality of the studies instead of three. Definitions of low, high, and very high risk of bias for the Newcastle‐Ottawa Scale were added to provide clarification to readers.
In instances where a study included two treatment arms (e.g. immunomodulator and anti‐TNF medication), we had originally stated in the protocol that we would divide the control group between the treatment groups. For our analysis, we elected not to divide the control group between the treatment groups because the data for each treatment group was used in separate analyses and thus, would not create a unit of analysis error.
Information on the GRADE analysis was moved from this section to the 'Summary of findings and assessment of the certainty of the evidence' section. Two sensitivity analyses were added. We excluded studies that included patients who underwent surgery to alleviate a fistula/abscess or who were found to have an intra‐abdominal abscess intraoperatively. We performed this sensitivity analysis as the presence of a pre‐operative abscess is likely a confounding factor. Another ad‐hoc sensitivity analysis was performed excluding studies with potential unit of analysis issues (studies for which we estimated the overall rate of infection by summation of different types of infections). In the protocol, we stated that data from randomized and non‐randomized studies would be pooled. We amended the Methods section to reflect that, if applicable, we would analyze randomized and non‐randomized studies separately. In addition, we had planned to select a random effects or fixed effects model based on the amount of heterogeneity in the results. However, we later decided that a random effects model would be used for all analyses given that significant heterogeneity was anticipated in the studies and this would be best accounted for using a random effects model. Lastly, some studies reported adjusted data while others reported unadjusted results. We modified our Methods so that adjusted and unadjusted data would be analyzed separately for the primary outcome of interest.
Contributions of authors
Cindy CY Law: Data acquisition, data analysis and drafting of the manuscript.
Conor Bell: Data acquisition and drafting of the manuscript.
Deborah Koh: Data acquisition and drafting of the manuscript.
Yueyang Bao: Data acquisition and critical revision of the manuscript.
Vipul Jairath: Study concept and critical revision of the manuscript.
Neeraj Narula: Study concept, data analysis and critical revision of the manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Cindy CY Law: None known
Conor Bell: None known
Deborah Koh: None known
Yueyang Bao: None known
Vipul Jairath has received has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speakers fees from Takeda, Janssen, Shire, Ferring, Abbvie, and Pfizer.
Neeraj Narula has received fees for consultancy from Abbvie, Takeda. Janssen, and Lupin; payment for lectures from Abbvie, Takeda, Janssen, and Pfizier; and payment for educational presentations from Abbvie and Janssen.
Edited (no change to conclusions)
References
References to studies included in this review
Aberra 2003 {published data only}
- Aberra FN, Lewis JD, Hass D, Rombeau JL, Osborne B, Lichtenstein GR. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology 2003;125(2):320-7. [DOI] [PubMed] [Google Scholar]
Afzali 2016 {published data only}
- Afzali A, Park CJ, Hu J, Lee S. Preoperative methotrexate and risk for early postoperative complications in inflammatory bowel disease: A single-center academic experience. The American Journal of Gastroenterology 2013;108(S1):561. [Google Scholar]
- Afzali A, Park CJ, Zhu K, Hu JK, Sharma P, Sinanan MN. Preoperative use of methotrexate and the risk of early postoperative complications in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2016;22(8):1887-95. [DOI] [PubMed] [Google Scholar]
Alves 2007 {published data only}
- Alves A, Panis Y, Bouhnik Y, Pocard M, Vicaut E, Valleur P. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn's disease: a multivariate analysis in 161 consecutive patients. Diseases of the Colon & Rectum 2007;50(3):331-6. [DOI] [PubMed] [Google Scholar]
Appau 2008 {published data only}
- Appau KA, Fazio VW, Shen B, Church JM, Lashner B, Remzi F, et al. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn's patients. Journal of Gastrointestinal Surgery 2008;12(10):1738-44. [DOI] [PubMed] [Google Scholar]
Araki 2014 {published data only}
- Araki T, Okita Y, Uchino M, Ikeuchi H, Sasaki I, Funayama Y, et al. Risk factors for surgical site infection in Japanese patients with ulcerative colitis: A multicenter prospective study. Surgery Today 2014;44(6):1072-8. [DOI] [PubMed] [Google Scholar]
Ayoub 2018 {published data only}
- Ayoub F, Ewelukwa O, Brar T, Forde J, Mramba L, Glover S, et al. Evaluating the impact of vedolizumab on postoperative complications in inflammatory bowel disease patients. Diseases of the Colon and Rectum 2018;61(5):e38-333. [Google Scholar]
Bregnbak 2012 {published data only}
- Bregnbak D, Mortensen C, Bendtsen F. Infliximab and complications after colectomy in patients with ulcerative colitis. Journal of Crohn's & colitis 2012;6(3):281-6. [DOI] [PubMed] [Google Scholar]
Brouquet 2018 {published data only}
- Brouquet A, Maggiori L, Zerbib P, Lefevre JH, Denost Q, Germain A, et al. Anti-TNF therapy is associated with an increased risk of postoperative morbidity after surgery for ileocolonic Crohn disease: Results of a prospective nationwide cohort. Annals of Surgery 2018;267(2):221-8. [DOI] [PubMed] [Google Scholar]
Canedo 2011 {published data only}
- Canedo J, Lee SH, Pinto R, Murad-Regadas S, Rosen L, Wexner SD. Surgical resection in Crohn's disease: is immunosuppressive medication associated with higher postoperative infection rates? Colorectal Disease 2011;13(11):1294-8. [DOI] [PubMed] [Google Scholar]
Cohen 2019 {published data only}
- Cohen BL, Fleshner P, Kane SV, Herfarth HH, Palekar N, Farraye FA, Leighton JA, Katz J, Cohen RD, Gerich ME, Cross RK, Higgins PD, Tinsley A, Glover SC, Siegel CA, Bohl JL, Iskandar H, Raymond S, Huang R, Suarez-Farinnas M, Sands BE. Anti-tumor necrosis factor therapy is not associated with post-operative infection: results from prospective cohort of ulcerative colitis and Crohn's disease patients undergoing surgery to identify risk factors for post-operative infection I (PUCCINI). Gastroenterology 2019;156(6 Supplement 1):S80. [Google Scholar]
Colombel 2004 {published data only}
- Colombel JF, Loftus EV Jr, Tremaine WJ, Pemberton JH, Wolff BG, Young-Fadok T, et al. Early postoperative complications are not increased in patients with Crohn's disease treated perioperatively with infliximab or immunosuppressive therapy. American Journal of Gastroenterology 2004;99(5):878-83. [DOI] [PubMed] [Google Scholar]
Coquet‐Reinier 2010 {published data only}
- Coquet-Reinier B, Berdah SV, Grimaud JC, Birnbaum D, Cougard PA, Barthet M, et al. Preoperative infliximab treatment and postoperative complications after laparoscopic restorative proctocolectomy with ileal pouch-anal anastomosis: A case-matched study. Surgical Endoscopy and Other Interventional Techniques 2010;24(8):1866-71. [DOI] [PubMed] [Google Scholar]
De Buck Van Overstraeten 2017 {published data only}
- De Buck Van Overstraeten A, Eshuis EJ, Vermeire S, Van Assche GA, Ferrante M, D'Haens GR. Short and medium-term outcomes following primary ileocaecal resection for Crohn's disease in two specialist centres. British Journal of Surgery 2017;104(12):1713-22. [DOI] [PubMed] [Google Scholar]
El‐Hussuna 2012 {published data only}
- El-Hussuna A, Andersen J, Bisgaard T, Jess P, Henriksen M, Oehlenschlager J, et al. Biologic treatment or immunomodulation is not associated with postoperative anastomotic complications in abdominal surgery for Crohn's disease. Scandinavian Journal of Gastroenterology 2012;47(6):662-8. [DOI] [PubMed] [Google Scholar]
Eshuis 2013 {published data only}
- Eshuis EJ, Al Saady RL, Stokkers PC, Ponsioen CY, Tanis PJ, Bemelman WA. Previous infliximab therapy and postoperative complications after proctocolectomy with ileum pouch anal anastomosis. Journal of Crohn's & colitis 2013;7(2):142-9. [DOI] [PubMed] [Google Scholar]
Ferrante 2009 {published data only}
- Ferrante M, D'Hoore A, Vermeire S, Declerck S, Noman M, Van Assche G, et al. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflammatory Bowel Diseases 2009;15(7):1062-70. [DOI] [PubMed] [Google Scholar]
Ferrante 2017 {published data only}
- Ferrante M, Buck van Overstraeten A, Schils N, Moens A, Van Assche G, Wolthuis A, et al. Perioperative use of Vedolizumab is not associated with postoperative infectious complications in patients with Ulcerative Colitis undergoing colectomy. Journal of Crohn's & colitis 2017;11(11):1353-61. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Schils N, Buck van Overstraeten A, Vermeire S, Assche G, Wolthuis A, et al. Perioperative use of vedolizumab is not associated with postoperative infectious complications in patients with ulcerative colitis undergoing (procto) colectomy with ileal pouch-anal anastomosis. Gastroenterology 2017;152(5):S581-2. [Google Scholar]
Fumery 2017 {published data only}
- Fumery M, Seksik P, Auzolle C, Munoz-Bongrand N, Gornet JM, Boschetti G, et al. Postoperative complications after ileocecal resection in Crohn's disease: A prospective study from the REMIND group. American Journal of Gastroenterology 2017;112(2):337-45. [DOI] [PubMed] [Google Scholar]
- Fumery M, Seksik P, Chirica M, Gornet JM, Boschetti G, Cotte E et al. Postoperative complications after ileo-caecal resection in Crohn’s Disease: a prospective multicentre study. Journal of Crohn's and Colitis 2015;9(S1):S239. [Google Scholar]
Gainsbury 2011 {published data only}
- Gainsbury ML, Chu DI, Howard LA, Coukos JA, Farraye, FA, Stucchi AF, et al. Preoperative infliximab is not associated with an increased risk of short-term postoperative complications after restorative proctocolectomy and ileal pouch-anal anastomosis. Journal of Gastrointestinal Surgery 2011;15(3):397-403. [DOI] [PubMed] [Google Scholar]
Gu 2013 {published data only}
- Gu J, Remzi FH, Shen B, Vogel JD, Kiran RP. Operative strategy modifies risk of pouch-related outcomes in patients with ulcerative colitis on preoperative anti-tumor necrosis factor-alpha therapy. Diseases of the Colon & Rectum 2013;56(11):1243-52. [DOI] [PubMed] [Google Scholar]
Guasch 2016 {published data only}
- Guasch M, Clos A, Manyosa M, Lobaton T, Gomez JR, Pinol M, et al. Prevalence and risk factors for postoperative septic complications in Crohn's disease. Journal of Crohn's and Colitis 2016;10(S1):S350. [Google Scholar]
Gudsoorkar 2018 {published data only}
- Gudsoorkar V, Ibarra S, Gonzalez-Almada A, Oglat A, Koduru P, Abraham B, et al. An analysis of surgical outcomes in IBD patients treated with and without biologic therapy. Gastroenterology 2018;154(S1):S68-9. [Google Scholar]
Guo 2017 {published data only}
- Guo K, Ren J, Li G, Hu Q, Wu X, Wang Z, et al. Risk factors of surgical site infections in patients with Crohn's disease complicated with gastrointestinal fistula. International Journal of Colorectal Disease 2017;32(5):635-43. [DOI] [PubMed] [Google Scholar]
Jouvin 2018 {published data only}
- Jouvin I, Lefevre JH, Creavin B, Pitel S, Chafai N, Tiret E, et al. Postoperative morbidity risks following ileocolic resection for Crohn's disease treated with anti-TNF alpha therapy: A retrospective study of 360 patients. Inflammatory Bowel Diseases 2018;24(2):422-32. [DOI] [PubMed] [Google Scholar]
Kim 2018 {published data only}
- Kim JY, Zaghiyan K, Fleshner P. Risk of post-operative complications among Crohn's disease patients treated pre-operatively with vedolizumab. A matched case-control study. Gastroenterology 2018;154(6):S1294. [Google Scholar]
Kotze 2017 {published data only}
- Kotze PG, Saab MP, Saab B, da Silva Kotze LM, Olandoski M, Pinheiro LV, et al. Tumor necrosis factor alpha inhibitors did not influence postoperative morbidity after elective surgical resections in Crohn's disease. Digestive Diseases & Sciences 2017;62(2):456-64. [DOI] [PubMed] [Google Scholar]
Krane 2013 {published data only}
- Krane MK, Allaix ME, Zoccali M, Umanskiy K, Rubin MA, Villa A, et al. Preoperative infliximab therapy does not increase morbidity and mortality after laparoscopic resection for inflammatory bowel disease. Diseases of the Colon & Rectum 2013;56(4):449-57. [DOI] [PubMed] [Google Scholar]
Kunitake 2008 {published data only}
- Kunitake H, Hodin R, Shellito PC, Sands BE, Korzenik J, Bordeianou L. Perioperative treatment with infliximab in patients with Crohn's disease and ulcerative colitis is not associated with an increased rate of postoperative complications. Journal of Gastrointestinal Surgery 2008;12(10):1736-7. [DOI] [PubMed] [Google Scholar]
Liang 2017 {published data only}
- Liang H, Jiang B, Manne S, Lissoos T, Bennett D, Dolin P. Risk factors for post-operative infection after gastrointestinal surgery among adult patients with inflammatory bowel disease: Findings from a large observational US cohort study. JGH Open 2018;2:182-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lightner 2018 A {published data only}
- Lightner AL, McKenna NP, Tse CS, Raffals LE, Loftus EV Jr, Mathis KL. Postoperative outcomes in vedolizumab-treated Crohn's disease patients undergoing major abdominal operations. Alimentary Pharmacology & Therapeutics 2018;47(5):573-80. [DOI] [PubMed] [Google Scholar]
Lightner 2018 B {published data only}
- Lightner AL, McKenna NP, Warusavitarne J, Spinelli A. Intra-abdominal sepsis following ileocolic resection for Crohn's disease: what are the risk factors and are they consistent internationally? Gastroenterology 2018;154(6 Supplement 1):S1294. [Google Scholar]
Mahadevan 2002 {published data only}
- Mahadevan U, Loftus EV Jr, Tremaine WJ, Pemberton JH, Harmsen WS, Schleck CD, et al. Azathioprine or 6-mercaptopurine before colectomy for ulcerative colitis is not associated with increased postoperative complications. Inflammatory Bowel Diseases 2002;8(5):311-6. [DOI] [PubMed] [Google Scholar]
Marchal 2004 {published data only}
- Marchal L, D'Haens G, Van Assche G, Vermeire S, Noman M, Ferrante M, et al. The risk of post-operative complications associated with infliximab therapy for Crohn's disease: a controlled cohort study. Alimentary pharmacology & therapeutics 2004;19(7):749-54. [DOI] [PubMed] [Google Scholar]
McKenna 2018 {published data only}
- McKenna NP, Habermann EB, Glasgow AE, Dozois EJ, Lightner AL. Intra-Abdominal sepsis after ileocolic resection in Crohn's Disease: The role of combination immunosuppression. Diseases of the Colon and Rectum 2018;61(12):1393-402. [DOI] [PubMed] [Google Scholar]
Mor 2008 {published data only}
- Mor IJ, Vogel JD, da Luz Moreira A, Shen B, Hammel J, Remzi FH. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy. Diseases of the Colon & Rectum 2008;51(8):1202-7. [DOI] [PubMed] [Google Scholar]
Morar 2015 {published data only}
- Morar PS, Hodgkinson JD, Thalayasingam S, Koysombat K, Purcell M, Hart AL. Determining predictors for intra-abdominal septic complications following ileocolonic resection for Crohn's disease-considerations in pre-operative and peri-operative optimisation techniques to improve outcome. Journal of Crohn's & colitis 2015;9(6):483-91. [DOI] [PubMed] [Google Scholar]
Myrelid 2009 {published data only}
- Myrelid P, Olaison G, Sjodahl R, Nystrom PO, Almer S, Andersson P. Thiopurine therapy is associated with postoperative intra-abdominal septic complications in abdominal surgery for crohn's disease. Diseases of the Colon and Rectum 2009;52(8):1387-94. [DOI] [PubMed] [Google Scholar]
Myrelid 2014 {published data only}
- Myrelid P, Marti-Gallostra M, Ashraf S, Sunde ML, Tholin M, Oresland TL, et al. Complications in surgery for Crohn's disease after preoperative antitumour necrosis factor therapy. British Journal of Surgery 2014;101(5):539-45. [DOI] [PubMed] [Google Scholar]
Nasir 2010 {published data only}
- Nasir BS, Dozois EJ, Cima RR, Pemberton JH, Wolff BG, Sandborn WJ, et al. Perioperative anti-tumor necrosis factor therapy does not increase the rate of early postoperative complications in Crohn's disease. Journal of Gastrointestinal Surgery 2010;14(12):1859-65. [DOI] [PubMed] [Google Scholar]
Nguyen 2014 {published data only}
- Nguyen GC, Elnahas A, Jackson TD. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. Journal of Crohn's & colitis 2014;8(12):1661-7. [DOI] [PubMed] [Google Scholar]
Norgard 2012 {published data only}
- Norgard, B M, Nielsen, J, Qvist, N, Gradel, K O, de Muckadell, O B, Kjeldsen, J. Pre-operative use of anti-TNF-alpha agents and the risk of post-operative complications in patients with ulcerative colitis - a nationwide cohort study. Alimentary Pharmacology & Therapeutics 2012;35:1301-1309. [DOI] [PubMed] [Google Scholar]
Norgard 2013 {published data only}
- Norgard, B M, Nielsen, J, Qvist, N, Gradel, K O, de Muckadell, O B, Kjeldsen, J. Pre-operative use of anti-TNF-alpha agents and the risk of post-operative complications in patients with Crohn's disease--a nationwide cohort study. Alimentary Pharmacology & Therapeutics 2013;37:214-224. [DOI] [PubMed] [Google Scholar]
Novello 2020 {published data only}
- Novello M, Stocchi L, Steele SR, Holubar SD, Duraes LC, Kessler H, Shawki S, Hull LT. Case-matched comparison of postoperative outcomes following surgery for inflammatory bowel disease after exposure to vedolizumab vs other biologics. Journal of Crohn's and Colitis 2020;14(2):185-191. [DOI] [PubMed] [Google Scholar]
Regadas 2011 {published data only}
- Regadas FS, Pinto RA, Murad-Regadas SM, Canedo JA, Leal M, Nogueras JJ, et al. Short-term outcome of infliximab and other medications on patients with inflammatory bowel disease undergoing ileostomy reversal. Colorectal Disease 2011;13(5):555-60. [DOI] [PubMed] [Google Scholar]
Rizzo 2011 {published data only}
- Rizzo G, Armuzzi A, Pugliese D, Verbo A, Papa A, Mattana C, et al. Anti-TNF-alpha therapies do not increase early postoperative complications in patients with inflammatory bowel disease. An Italian single-center experience. International Journal of Colorectal Disease 2011;26(11):1435-44. [DOI] [PubMed] [Google Scholar]
Schils 2017 {published data only}
- Schils N, De Buck Van Overstraeten A, Vermeire S, Van Assche G, Wolthuis A, D'Hoore A, et al. Perioperative use of vedolizumab seems not associated with short-term postoperative infectious complications in patients with Crohn's disease undergoing right hemicolectomy with ileocolonic anastomosis. Journal of Crohn's and Colitis 2017;11(S1):S304. [DOI] [PubMed] [Google Scholar]
Schluender 2007 {published data only}
- Schluender SJ, Ippoliti A, Dubinsky M, Vasiliauskas EA, Papadakis KA, Mei L, et al. Does infliximab influence surgical morbidity of ileal pouch-anal anastomosis in patients with ulcerative colitis? Diseases of the Colon and Rectum 2007;50(11):1747-53. [DOI] [PubMed] [Google Scholar]
Selvasekar 2007 {published data only}
- Selvasekar CR, Cima RR, Larson DW, Dozois EJ, Harrington JR, Harmsen WS, et al. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. Journal of the American College of Surgeons 2007;204(5):956-62. [DOI] [PubMed] [Google Scholar]
Serradori 2013 {published data only}
- Serradori T, Germain A, Scherrer ML, Ayav C, Perez M, Romain B, et al. The effect of immune therapy on surgical site infection following Crohn's Disease resection. British Journal of Surgery 2013;100(8):1089-93. [DOI] [PubMed] [Google Scholar]
Shaib 2017 {published data only}
- Shaib YH, Karaoui W, Rahal M, Tamim H, Mailhac A, et al. Surgical outcomes among inflammatory bowel disease patients undergoing colectomy: Results from a national database. Gastroenterology 2017;152(5):S363-4. [PubMed] [Google Scholar]
Shwaartz 2016 {published data only}
- Divino CM, Shwaartz C, Fields AC, Sobrero M, Cohen B. Do anti-TNF agents affect postoperative outcomes in patients undergoing surgery for inflammatory bowel disease? Gastroenterology 2016;150(4):S1261. [DOI] [PubMed] [Google Scholar]
- Shwaartz C, Fields AC, Sobrero M, Cohen BD, Divino CM. Effect of anti-TNF agents on postoperative outcomes in inflammatory bowel disease patients: a single institution experience. Journal of Gastrointestinal Surgery 2016;20(9):1636-42. [DOI] [PubMed] [Google Scholar]
Syed 2013 {published data only}
- Syed A, Cross RK, Flasar MH. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn's disease patients. American Journal of Gastroenterology 2013;108(4):583-93. [DOI] [PubMed] [Google Scholar]
Tzivanakis 2012 {published data only}
- Tzivanakis A, Singh JC, Guy RJ, Travis SP, Mortensen NJ, George BD. Influence of risk factors on the safety of ileocolic anastomosis in crohn's disease surgery. Diseases of the Colon and Rectum 2012;55(5):558-62. [DOI] [PubMed] [Google Scholar]
Uchino 2010 {published data only}
- Uchino M, Ikeuchi H, Matsuoka H, Tsuchida T, Tomita N, Takesue Y. Risk factors associated with surgical site infection after ileal pouch-anal anastomosis in ulcerative colitis. Diseases of the Colon & Rectum 2010;53(2):143-9. [DOI] [PubMed] [Google Scholar]
Uchino 2013a {published data only}
- Uchino M, Ikeuchi H, Matsuoka H, Bando T, Ichiki K, Nakajima K, et al. Risk factors for surgical site infection and association with infliximab administration during surgery for Crohn's disease. Diseases of the Colon & Rectum 2013;56(10):1156-65. [DOI] [PubMed] [Google Scholar]
Uchino 2013b {published data only}
- Uchino M, Ikeuchi H, Matsuoka H, Bando T, Ichiki K, Nakajima K, et al. Infliximab administration prior to surgery does not increase surgical site infections in patients with ulcerative colitis. International Journal of Colorectal Disease 2013;28(9):1295-306. [DOI] [PubMed] [Google Scholar]
Uchino 2015 {published data only}
- Uchino M, Ikeuchi H, Bando T, Hirose K, Hirata A, Chohno T, et al. Does pre-operative multiple immunosuppressive therapy associate with surgical site infection in surgery for ulcerative colitis. Digestion 2015;92(3):121-9. [DOI] [PubMed] [Google Scholar]
Uchino 2019 {published data only}
- Uchino M, Ikeuchi H, Bnado T, Sasaki H, Horio Y, Minagawa T, Kuwahara R, Goto Y, Chohno T, Takesue Y. Associations between multiple immunosuppressive treatments before surgery and surgical morbidity in patients with ulcerative colitis during the era of biologics. International Journal of Colorectal Disease 2019;34:699-710. [DOI] [PubMed] [Google Scholar]
Ward 2018 {published data only}
- Ward ST, Mytton J, Henderson L, Amin V, Tanner JR, Evison F, et al. Anti-TNF therapy is not associated with an increased risk of post-colectomy complications, a population-based study. Colorectal Disease 2018;20(5):416-23. [DOI] [PubMed] [Google Scholar]
Waterman 2013 {published data only}
- Waterman M, Xu W, Dinani A, Steinhart AH, Croitoru K, Nguyen GC, et al. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut 2013;62(3):387-94. [DOI] [PubMed] [Google Scholar]
Wilson 2014 {published data only}
- Wilson MZ, Connelly TM, Hollenbeak CS, Messaris E. Organ space infection following ileocolectomy for Crohn's disease: a national surgical quality improvement project study. American Journal of Surgery 2014;208(5):749-55. [DOI] [PubMed] [Google Scholar]
Yamada 2017 {published data only}
- Yamada A, Komaki Y, Patel N, Komaki F, Aelvoet AS, Tran AL, et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with anti-integrin and anti-tumor necrosis factor agents. Gastroenterology 2017;152(5):S574. [DOI] [PubMed] [Google Scholar]
- Yamada A, Komaki Y, Patel N, Komaki F, Aelvoet AS, Tran AL, et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. American Journal of Gastroenterology 2017;112(9):1423-9. [DOI] [PubMed] [Google Scholar]
Yamamoto 2000 {published data only}
- Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn's disease. Diseases of the Colon & Rectum 2000;43(8):1141-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Keighley MR. Factors affecting the incidence of postoperative septic complications and recurrence after strictureplasty for jejunoileal Crohn's disease. American Journal of Surgery 1999;178(3):240-5. [DOI] [PubMed] [Google Scholar]
Yamamoto 2016 {published data only}
- Yamamoto T, Kotze P, Suzuki Y, Spinelli A, Danese S, Saad-Hossne R, et al. The risk of postoperative complications following preoperative immunosuppressive therapy in patients undergoing ileocolonic resection for Crohn's disease. Journal of Crohn's and Colitis 2014;8:S62. [Google Scholar]
- Yamamoto T, Spinelli A, Suzuki Y, Saad-Hossne R, Teixeira FV, Albuquerque IC. Risk factors for complications after ileocolonic resection for Crohn's disease with a major focus on the impact of preoperative immunosuppressive and biologic therapy: A retrospective international multicentre study. United European Gastroenterology Journal 2016;4(6):784-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2019 {published data only}
- Yu CS, Jung SW, Lee JL, Lim SB, Park IJ, Yoon YS, Kim CW, Yang SK, Ye BD, Park SH, Han M, Kim JC. The influence of preoperative medications on postoperative complications in patients after intestinal surgery for Crohn's disease. Inflammatory Bowel Diseases 2019;25(9):1559-1568. [DOI] [PubMed] [Google Scholar]
Zittan 2016 {published data only}
- Zittan E, Milgrom R, Ma GW, Wong-Chong N O'Connor, B, McLeod RS, et al. Preoperative anti-tumor necrosis factor therapy in patients with ulcerative colitis is not associated with an increased risk of infectious and noninfectious complications after ileal pouch-anal anastomosis. Inflammatory Bowel Diseases 2016;22(10):2442-7. [DOI] [PubMed] [Google Scholar]
Ziv 1996 {published data only}
- Ziv Y, Church JM, Fazio VW, King TM, Lavery IC. Effect of systemic steroids on ileal pouch-anal anastomosis in patients with ulcerative colitis. Diseases of the Colon & Rectum 1996;39(5):504-8. [DOI] [PubMed] [Google Scholar]
Zuo 2014 {published data only}
- Zuo L, Zhu W, Li Y, Gong J, Cao L, Gu L, et al. Risk factors for postoperative intra-abdominal septic complications in Crohn's disease. Chinese Journal of Gastroenterology 2014;19(8):454-457. [Google Scholar]
References to studies excluded from this review
Abelson 2018 {published data only}
- Abelson JS, Michelassi F, Mao J, Sedrakyan A, Yeo H. Higher surgical morbidity for ulcerative colitis patients in the era of biologics. Annals of Surgery 2018;268(2):311-317. [DOI] [PubMed] [Google Scholar]
Abou‐Khalil 2016 {published data only}
- Abou Khalil M, Abou Khalil J, Motter J, Vasilevsky C, Morin N, Gordon P, et al. Impact of immunosuppressants on postoperative complications following colectomies for Crohn's disease: results from the ACS-NSQIP database. Canadian Journal of Surgery 2016;59(4):S133. [Google Scholar]
Achkasov 2015 {published data only}
- Achkasov S, Kashnikov V, Vardanyan A, Kalashnikova I. The influence of steroid therapy (ST) on the postoperative frequency of development and peculiarities of the wound infection behavior in patients with ulcerative colitis (UC). Colorectal Disease 2015;17(S2):48. [Google Scholar]
Adegbola 2018 {published data only}
- Adegbola S, Sahnan K, Fareleira A, Hiles S, Faiz O, Hart et al. Are there patient or operative factors associated with septic complications following ileocolonic resection for Crohn’s disease? - single centre contemporaneous cohort. Colorectal Disease 2018;20(S4):46. [Google Scholar]
- Adegbola SO, Sahnan K, Morar P, Fareleira A, Basset P, Tozer PJ et al. Ileocolonic resection for Crohn's disease - perioperative outcomes in a contemporary cohort from a single tertiary centre. Colorectal Disease 2018;20(S7):47. [Google Scholar]
Aelvoet 2016 {published data only}
- Aelvoet AS, Tran AL, Rubin DT. The influence of vedolizumab on postoperative outcomes in IBD patients undergoing abdominal surgery. The American Journal of Gastroenterology 2016;111(S1):S297. [Google Scholar]
Andrew 2017 {published data only}
- Andrew RE, Lauria A, Puleo FJ, Berg A, Steward DB. Inpatient infliximab is ineffective at preventing colectomy for steroid refractory extensive colitis. Journal of Surgical Research 2017;219:18-24. [DOI] [PubMed] [Google Scholar]
Bafford 2013 {published data only}
- Bafford AC, Powers S, Ha C, Kruse D, Gorfine SR, Chessin DB, et al. Immunosuppressive therapy does not increase operative morbidity in patients with Crohn's disease. Journal of Clinical Gastroenterology 2013;47(6):491-5. [DOI] [PubMed] [Google Scholar]
Balachandran 2015 {published data only}
- Balachandran R, Tøttrup A. Safety of proctocolectomy for ulcerative colitis under elective and non-elective circumstances: preoperative corticosteroid treatment worsens outcome. Digestive Surgery 2015;32(4):251-7. [DOI] [PubMed] [Google Scholar]
Benichou 2018 {published data only}
- Benichou B, Rahili MA, Bernard J, Hebuterne X, Benizri E. Effect of anti TNFα on postoperative outcomes after ileocolic resection for Crohn disease. Colorectal Disease 2018;20(S4):47. [Google Scholar]
Bewtra 2013 {published data only}
- Bewtra M, Newcomb C, Wu Q, Lewis JD. Immunosuppressant therapy prior to elective surgery is not associated with increased post-operative morbidity in ulcerative colitis. Gastroenterology 2013;144(5):S188. [Google Scholar]
Braun 2018 {published data only}
- Braun I, Heitland W, Bader F, Huber F, Janelidze S, Schnitzler F, Ochsenkuhn T. The preoperative use of anti-TNF α-antibodies in IBD patients undergoing resective abdominal surgery has no influence on peri- and postoperative complications. United European Gastroenterology Journal 2018;6(8S):A652. [Google Scholar]
Bruewer 2003 {published data only}
- Bruewer M, Utech M, Rijcken EJM, Anthoni C, Laukoetter MG, Kersting S, et al. Preoperative steroid administration: effect on morbidity among patients undergoing intestinal bowel resection for Crohn's disease. World Journal of Surgery 2003;27(12):1306-10. [DOI] [PubMed] [Google Scholar]
Chaparro 2018 {published data only}
- Chaparro M, Barreiro de-Acosta M, Benitez JM, Cabriada JL, Casanova MJ, Ceballos D, Esteve M, Fernandez Rosaenz H, Ginard D, Gomollon F, Lorente Poyatos R, Nos P, Riestra Menendez S, Rivero M, Robledo P, Rodriguez C, Sicilia B, Torrella Cortes E, Garcia-Esquinas EG, Rodriguez-Artalejo F, Gisbert JP. Surgical and hospital admission in adults newly diagnosed with inflammatory bowel disease in the biological era in Spain: results of the nationwide EpidemIBD study of Geteccu. United European Gastroenterology Journal 2018;6(8S):A254. [Google Scholar]
Chiplunker 2015 {published data only}
- Chiplunker AJ, Dharmarajan S, Gutierrez A. Vedolizumab therapy in Crohn's disease and ulcerative colitis: a retrospective review of post-procedural complications. American Journal of Gastroenterology 2015;110(S1):S773. [Google Scholar]
Coscia 2012 {published data only}
- Coscia M, Vitali G, Laureti S, Gentilini L, Ugolini F, Calafiore A, et al. Risk of short-term postoperative complications in patients treated with azathioprine for Crohn's disease. A single center experience. Journal of Crohn's and Colitis 2012;6:S165. [Google Scholar]
- Vitali G, Gentilini L, Coscia M, Vittori L, Ugolini F, Laureti S, et al. Short-term postoperative complications in patients treated with azathioprine for Crohn's disease. Colorectal Disease 2012;14(S2):6. [Google Scholar]
Desai 2012 {published data only}
- Desai PN, Sharma A, Naik AS, Otterson MF, Zadvornova Y, Perera LP, et al. Timing of pre-operative anti-tumor necrosis factor therapy does not affect early post-operative complication rates in inflammatory bowel disease patients undergoing intestinal resection. Gastroenterology 2012;142(5):S1063. [Google Scholar]
De Silva 2011 {published data only}
- De Silva S, Ma C, Proulx MC, Crespin M, Kaplan BS, Hubbard J, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clinical Gastroenterology and Hepatology 2011;9(11):972-80. [DOI] [PubMed] [Google Scholar]
Domenech 2016 {published data only}
- Domenech E, Barreiro-de Acosta M, Gutierrez A, Garcia V, Martin Arranz MD, Cea-Calvo L, et al. Postoperative complications after intestinal resection in Crohn's disease: analysis from the Practicrohn study. Journal of Crohn's and Colitis 2016;10(S1):S431. [Google Scholar]
Eisner 2014 {published data only}
- Eisner F, Küper M A, Ziegler F, Zieker D, Königsrainer A, Glatzle J. Impact of perioperative immunosuppressive medication on surgical outcome in Crohn's disease (CD). Zeitschrift fur Gastroenterologie 2014;52(5):436-40. [DOI] [PubMed] [Google Scholar]
Fronda 1999 {published data only}
- Fronda GR, Resegotti A, Astegiano M, Sostegni R, Giustetto A, Soldati T, et al. Postoperative complications in Crohn's disease. Are they related to recurrence promoting factors? A preliminary study. Chirurgia 1999;12(2):101-6. [Google Scholar]
Fu 2014 {published data only}
- Fu YTN, Hong T, Round A, Bressler B. Impact of medical therapy on patients with Crohn's disease requiring surgical resection. World Journal of Gastroenterology 2014;20(33):11808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamaleldin 2018 {published data only}
- Gamaleldin M, Holubar S, Stocchi L, Hull T. Safety of stricturoplasty for Crohn’s disease in the era of biologics. Journal of Crohn's and Colitis 2018;12(S1):S211. [Google Scholar]
George 2017 {published data only}
- George MD, Baker JF, Hsu JY, Wu Q, Xie F, Chen L, et al. Perioperative timing of infliximab and the risk of serious infection after elective hip and knee arthroplasty. Arthritis Care & Research 2017;69(12):1845-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2013 {published data only}
- Gonzalez R, Pereyra L, Omodeo M, Gomez EJ, Panigadi GN, Mella JM, et al. Clinical risk factors for complications after laparoscopic ileal-pouch-anal anastomosis in patients with ulcerative colitis. Gastroenterology 2013;146(5):S585. [Google Scholar]
Grant 2019 {published data only}
- Grant R, Bouri S, Gonzalez AE, Dilke S, Sahnan K, Adegbola S, Warusavitarne J, Tozer P, Hart A. Rates of wound healing in patients with Crohn's disease undergoing proctectomy. Gut 2019;68:A77. [Google Scholar]
- Grant R, Bouri S, Gonzalez AE, Dilke S, Sahnan K, Adegbola S, Warusavitarne J, Tozer P, Hart A. Rates of wound healing in patients with Crohn's disease undergoing proctectomy. Journal of Crohn's and Colitis 2019;13 Supplement 1:S198. [Google Scholar]
Gregory 2019 {published data only}
- Gregory MH, McKinnon A, Stwalley D, Hippensteel KJ, Loftus EV, Ciorba MA, Olsen MA, Deepak P. Anti-tumor necrosis factor therapy for inflammatory bowel disease do not impact serious infections after arthroplasty. Journal of Crohn's and Colitis 2019;13(2):182-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MH, McKinnon A, Stwalley D, Loftus EV, Hippensteel KJ, Ciorba MA, Olsen MA, Deepak P. Outcomes after joint replacement surgery in patients with inflammatory bowel disease. Gastroenterology 2018;154(6 Supplement 1):S401-402. [Google Scholar]
Heimann 1985 {published data only}
- Heimann TM, Greenstein AJ, Mechanic L, Aufses AH. Early complications following surgical treatment for Crohn's disease. Annals of Surgery 1985;201(4):494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyde 2001 {published data only}
- Hyde GM, Jewell DP, Kettlewell MGW, Mortensen NJ. Cyclosporin for severe ulcerative colitis does not increase the rate of perioperative complications. Diseases of the Colon and Rectum 2001;44(10):1436-40. [DOI] [PubMed] [Google Scholar]
Justiniano 2019 {published data only}
- Justiniano CF, Aquina CT, Becerra AZ, Xu Z, Boodry CI, Swanger AA, Monson JRT, Fleming FJ. Postoperative mortality after nonelective surgery for inflammatory bowel disease patients in the era of biologics. Annals of Surgery 2019;269(4):686-691. [DOI] [PubMed] [Google Scholar]
Kamel 2019 {published data only}
- Kamel AY, Ayoub F, Banerjee D, Chaudhry N, Ader Y, Tan S, Zimmermann EM, Glover SC, Iqbal A. Effects of preoperative use of biologic agents on operative outcomes in Crohn's disease patients. The American Surgeon 2018;84:1526-1530. [PubMed] [Google Scholar]
Kasparek 2012 {published data only}
- Kasparek MS, Bruckmeier A, Beigel F, Müller MH, Brand S, Mansmann U, et al. Infliximab does not affect postoperative complication rates in Crohn's patients undergoing abdominal surgery. Inflammatory Bowel Diseases 2012;18(7):1207-13. [DOI] [PubMed] [Google Scholar]
Kimura 2019 {published data only}
- Kimura C, Sobrado Junior C, Borba M, Queiroz N, Scanavini Neto A, Nahas S et al. Risk factors for complications after abdominal surgery in Crohn's disease. Diseases of the Colon & Rectum 2019;62(6):469. [Google Scholar]
Kline 2020 {published data only}
- Kline B, Weaver T, Berg A, Brinton D, Deiling S, Koltun W. Clinical and genetic factors associated with complications after Crohn's ileocolectomy. Diseases of the Colon & Rectum 2020;63(3):375-364. [DOI] [PubMed] [Google Scholar]
Kotze 2011 {published data only}
- Kotze P, Albuquerque I, Sobrado C. Biological therapy does not increase post operative complications after major abdominal surgery in Crohn's disease Brazilian patients. Inflammatory Bowel Diseases 2011;17:S43. [Google Scholar]
Kotze 2017a {published data only}
- Kotze PG, Magro DO, Martinez CAR, Saab B, Saab MP, Pinheiro LV, et al. Adalimumab and postoperative complications of elective intestinal resections in Crohn's disease: a propensity score case-matched study. Colorectal Disease 2017;20:211-8. [DOI] [PubMed] [Google Scholar]
Kotze 2018 {published data only}
- Almutairdi AA, Kotze PG, Ma C, McKenna NP, Raffals LH, Loftus EV, Panaccione R, Lightner AL. Vedolizumab and early postoperative complications in non-intestinal surgery: a case-matched analysis. Gastroenterology 2018;154(6):S358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze PG, Ma C, McKenna N, Almutairdi A, Kaplan GG, Raffals LE, et al. Vedolizumab and early postoperative complications in nonintestinal surgery: a case-matched analysis. Therapeutic Advances in Gastroenterology 2018;11:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krupa 2012 {published data only}
- Krupa L, Dave P, Hart A, Igali L, Tighe R, Tremelling M. Preoperative ciclosporin therapy and appropriate steroid use does not increase risk of infective complications following colectomy for ulcerative colitis. Gut 2012;61(S2):A227. [Google Scholar]
Kulaylat 2017 {published data only}
- Kulaylat AS, Kulaylat AN, Schaefer EW, Tinsley A, Williams E, Koltun W, et al. Association of preoperative anti-tumor necrosis factor therapy with adverse postoperative outcomes in patients undergoing abdominal surgery for ulcerative colitis. JAMA Surgery 2017;152(8):e171538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labidi 2018 {published data only}
- Labidi A, Ben Abbes M, Hamdi S, Maghrebi H, Ben Mustapha N, Fekih M, et al. Risk factors for poor postoperative outcome in patients with Crohn’s disease undergoing ileocaecal resection. Journal of Crohn's and Colitis 2018;12:S296. [Google Scholar]
Lau 2013 {published data only}
- Lau CC, Dubinsky M, Melmed GY, Vasiliauskas EA, McGovern DP, Berel D, et al. Influence of biologic agents on short-term postoperative complications in patients with Crohn's disease: a prospective, single-surgeon cohort study. Gastroenterology 2013;144(5):S407. [Google Scholar]
Li 2016 {published data only}
- Li J, Lyu H, Yang H, Li Y, Tan B, Wei MM, et al. Preoperative corticosteroid usage and hypoalbuminemia increase occurrence of short‑term postoperative complications in Chinese patients with ulcerative colitis. Chinese Medical Journal 2016;129:435-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lightner 2017 {published data only}
- Lightner AL, Raffals LE, Mathis KL, Cima RR, Tse CS, Pemberton JH, et al. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. Journal of Crohn's and Colitis 2017;11(2):185-90. [DOI] [PubMed] [Google Scholar]
Lightner 2017a {published data only}
- Lightner AL, McKenna NP, Moncrief S, Pemberton JH, Raffals LE, Mathis KL. Surgical outcomes in vedolizumab-treated patients with ulcerative colitis. Inflammatory Bowel Diseases 2017;23(12):2197-201. [DOI] [PubMed] [Google Scholar]
Lightner 2018a {published data only}
- Lightner AL, McKenna NP, Tse CS, Hyman N, Smith R, Ovsepyan G, et al. Postoperatve outcomes in ustekinumab-treated patients undergoing abdominal operations for Crohn's disease. Journal of Crohn's and Colitis 2018;12(4):402-7. [DOI] [PubMed] [Google Scholar]
Lightner 2018b {published data only}
- Lightner A, Mathis K, Sang Tse C, Pemberton J, Shen B, Kocklar G, et al. A multi-institutional report of postoperative outcomes in vedolizumab-treated patients undergoing major abdominal operations for inflammatory bowel disease. Journal of Crohn's and Colitis 2017;11(S1):S258-9. [DOI] [PubMed] [Google Scholar]
- Lightner AL, Mathis KL, Tse CS, Pemberton JH, Shen B, Kochlar G, et al. Postoperative outcomes in vedolizumab-treated patients undergoing major abdominal operations for inflammatory bowel disease: retrospective multicenter cohort study. Inflammatory Bowel Diseases 2018;24(4):871-6. [DOI] [PubMed] [Google Scholar]
Lim 2018 {published data only}
- Lim MH, Lord A, Simms L, Hanigan K, Edmundson A, Rickard M, Stitz R, Clark D, Radford-Smith GL. Have changes in medical therapy and surgical techniques for the treatment of ulcerative colitis altered post-colectomy outcomes over the past 26 years? Journal of Gastroenterology and Hepatology 2018;33(S2):101. [Google Scholar]
Melo‐Pinto 2018 {published data only}
- Melo-Pinto D, Santos JV, Barbosa E. Risk factors for postoperative complications in Crohn disease: analysis of 173 patients. Journal of Coloproctology 2018;38(3):214-20. [Google Scholar]
Monsinjon 2017 {published data only}
- Monsinjon M, Mege D, Maggiori L. Postoperative course of laparoscopic subtotal colectomy is affected by prolonged preoperative anti-TNF therapy in patients with acute colitis complicating inflammatory bowel disease. International Journal of Colorectal Disease 2017;32:1499-502. [DOI] [PubMed] [Google Scholar]
Nagao 2016 {published data only}
- Nagao M, Watanabe K, Karasawa H, Abe T, Ohnuma S, Musha H, et al. Does tacrolimus affect the outcome after surgery for ulcerative colitis? Gastroenterology 2016;150(4):S1218-9. [Google Scholar]
Novello 2019 {published data only}
- Novello M, Stocchi L, Holubar S, Shawki S, Lipman J, Gorgun E, et al. Surgical outcomes of patients treated with ustekinumab vs. vedolizumab in inflammatory bowel disease: a matched case analysis. International Journal of Colorectal Disease 2019;34(3):451-7. [DOI] [PubMed] [Google Scholar]
Oh 2014 {published data only}
- Oh, SN Hong, MJ Kim, ER Kim, DK Chang. The risk of preoperative anti-TNF-alpha treatment on early postoperative complications in patients with Crohn's disease. Journal of Crohn's and Colitis 2014;8(S1):S196. [Google Scholar]
Park 2018 {published data only}
- Park KT, Sceats L, Dehghan M, Trickey AW, Wren A, Wong JJ, et al. Risk of post-operative surgical site infections after vedolizumab vs anti-tumour necrosis factor therapy: a propensity score matching analysis in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2018;48(3):340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KT, Sceats L, Dehghan MS, Trickey AW, Wren A, Bensen R, Limketkai B, Kin C. Risk of postoperative surgical site infections after vedolizumab vs anti-TNF therapy: a propensity-matched cohort analysis. Gastroenterology 2018;154(6 Supplement 1):S364-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parrish 2019 {published data only}
- Parrish AB, Zaghiyan K, Melmed G, McGovern D, Jain A, Landers C et al. A pilot study comparing postoperative outcomes after ustekinumab in patients with inflammatory bowel disease. Gastroenterology 2019;156(S1):636-637. [Google Scholar]
Poylin 2018 {published data only}
- Poylin VY, Cataneo J, Pastrana J, Feuerstein JD. Vedolizumab does not increase perioperative surgical complications in patients with inflammatory bowel disease. Gastroenterology 2018;154(6):S402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quade 2013 {published data only}
- Quade SJ, Mourot J, Afzali A, Sinanan MN, Lee SD, Hu JK, et al. Assessment of postoperative complications in patients with IBD. A single academic medical center experience. Gastroenterology 2013;144(5):S1109. [Google Scholar]
Rizvi 2019 {published data only}
- Rizvi A, Kayal M, Plietz M, Zylberberg H, Radcliffe M, Yzet C et al. Pre-operative biologics significantly reduce post-operative leaks after staged restorative proctocolectomy. Gastroenterology 2019;156(6):S154. [Google Scholar]
Sahami 2016 {published data only}
- Sahami S, Bartels SAL, D'Hoore A, Fadok TY, Tanis PJ, Lindeboom R, et al. A multicentre evaluation of risk factors for anastomotic leakage after restorative proctocolectomy with ileal pouch-anal anastomosis for inflammatory bowel disease. Journal of Crohn's and Colitis 2016;10(7):773-8. [DOI] [PubMed] [Google Scholar]
Scarpa 2015 {published data only}
- Scarpa M, Martinato M, Bertin E, Da Roit A, Pozza A, Ruffolo C, et al. Intestinal surgery for Crohn's disease: role of preoperative therapy in postoperative outcome. Digestive Surgery 2015;32:243-50. [DOI] [PubMed] [Google Scholar]
Shim 2018 {published data only}
- Shim HH, Ma C, Kotze PG, Seow CH, Al-Farhan H, Al-Darmaki AK, et al. Preoperative ustekinumab treatment is not associated with increased postoperative complications in Crohn’s disease: a Canadian multicentre observational cohort study. Journal of the Canadian Association of Gastroenterology 2018;1(3):115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimada 2016 {published data only}
- Shimada N, Ohge H, Watadani Y, Uegami S, Shigemoto N, Murao N, et al. Impact of preoperative tacrolimus on the postoperative course in patients with ulcerative colitis. Diseases of the Colon and Rectum 2016;59(5):94. [Google Scholar]
Stewart 2009 {published data only}
- Stewart D, Chao A, Kodner I, Birnbaum E, Fleshman J, Dietz D. Subtotal colectomy for toxic and fulminant colitis in the era of immunosuppressive therapy. Colorectal Disease 2009;11(2):184-90. [DOI] [PubMed] [Google Scholar]
Strassle 2017 {published data only}
- Strassle PD, Samples J, Sickbert-Bennett EE, Weber DJ, Sadiq TS, Chaumont N. Incidence and risk factors of Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Pharmacoepidemiology and Drug Safety 2017;26(S2):541. [Google Scholar]
Stringfield 2016 {published data only}
- Stringfield S, Parry L, Sandborn W, Ramamoorthy S, Eisenstein S. Patients on vedolizumab have a high rate of postoperative complications. Diseases of the Colon and Rectum 2016;59(5):33. [Google Scholar]
Valizadeh 2017 {published data only}
- Valizadeh N, Murray ACA, Suradkar K, Al-Mazrou A, Kiran RP. Impact of preoperative steroid or immunosuppressant use on short-term outcomes following colectomy in Crohn’s disease patients. Techniques in Coloproctology 2017;21(3):217-23. [DOI] [PubMed] [Google Scholar]
Watson 2018 {published data only}
- Watson A, Labott A, Yarur AJ. Rate and risk factors for post-operative complications in Crohn's and colitis patients undergoing non-inflammatory bowel disease surgery. Gastroenterology 2018;154(6):S633-634. [Google Scholar]
Weber 2017 {published data only}
- Weber AT, Sack J, Ha CY. Anti-TNF exposure is not associated with increased post-operative morbidity or reoperation among Crohn's disease patients. American Journal of Gastroenterology 2017;112:S319-S428. [Google Scholar]
Yamamoto 2016a {published data only}
- Yamamoto T, Shimoyama T, Himan T, Umegae S. The impact of preoperative immunosuppressive and biologic therapy on complications after surgery for inflammatory bowel disease. Colorectal Disease 2016;18(S1):55. [Google Scholar]
Yamamoto 2018 {published data only}
- Yamamoto T, Shimoyama T, Umegae S. Surgery for severely active ulcerative colitis in the era of new medical treatment: the impacts of medications on surgery avoidance, and short-term and long-term surgical outcomes. Journal of Crohn's and Colitis 2018;12(S1):S423-24. [Google Scholar]
Additional references
Ali 2014
- Ali UA, Martin ST, Rao AD, Kiran RP. Impact of preoperative immunosuppressive agents on postoperative outcomes in Crohn's disease. Diseases of the Colon and Rectum 2014;57(5):663-74. [DOI] [PubMed] [Google Scholar]
Argollo 2018
- Argollo MC, Kotze PG, Spinelli A, Gomes TNF, Danese S. The impact of biologics in surgical outcomes in ulcerative colitis. Best Practice & Research Clinical Gastroenterology 2018;32-33:79-87. [DOI] [PubMed] [Google Scholar]
Billioud 2013
- Billioud V, Ford AC, Tedesco ED, Colombel JF, Roblin X, Peyrin-Biroulet L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: A meta-analysis. Journal of Crohn's and Colitis 2013;7(11):853-67. [DOI] [PubMed] [Google Scholar]
Ehteshami‐Afshar 2011
- Ehteshami-Afshar S, Nikfar S, Rezaie A, Abdollahi M. A systematic review and meta-analysis of the effects of infliximab on the rate of colectomy and post-operative complications in patients with inflammatory bowel disease. Archives of Medical Science 2011;7(6):1000-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Hussuna 2013
- El-Hussuna A, Krag A, Olaison G, Bendtsen F, Gluud LL. The effect of anti-tumor necrosis factor alpha agents on postoperative anastomotic complications in Crohn's disease: A systematic review. Diseases of the Colon and Rectum 2013;56(12):1423-33. [DOI] [PubMed] [Google Scholar]
Frolkis 2013
- Frolkis AD, Dykeman J, Negron ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996-1006. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Flack-Ytter Y, Aonso-Coello A, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herfarth 2018
- Herfarth H, Branes EL, Valentine JF, Hanson J, Higgins PDR, Isaacs KL, Jackson S, Osterman MT, Anton K, Ivanova A, Long MD, Martin C, Sandler RS, Abraham B, Cross RK, Dryden G, Fischer M, Harlan W, Levy C, McCabe R, Polyak S, Saha S, Williams E, Yajnik V, Serrano J, Sands BE, Lewis JD, Clinical Research Alliance of the Crohn's and Colitis Foundation. Methotrexate in not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology 2018;155(4):1098-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2015
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature Reviews Gastroenterology & Hepatology 2015;12:720-7. [DOI] [PubMed] [Google Scholar]
Kopylov 2012
- Kopylov U, Ben-Horin S, Zmora O, Eliakim R, Katz LH. Anti-tumor necrosis factor and postoperative complications in Crohn's disease: systematic review and meta-analysis. Inflammatory Bowel Diseases 2012;18:2404-13. [DOI] [PubMed] [Google Scholar]
Law 2018
- Law CCY, Narula A, Lightner AL, McKenna NP, Colombel JF, Narula N. Systematic review and meta-analysis: preoperative vedolizumab treatment and postoperative complications in patients with inflammatory bowel disease. Journal of Crohn's and Colitis 2018;12(5):538-45. [DOI] [PubMed] [Google Scholar]
Lee 2000
- Lee RH, Efron DT, Tantry U, Stuelten C, Moldawer LL, Abouhamze A, et al. Inhibition of tumor necrosis factor-ɑ attenuates wound breaking strength in rats. Wound Repair and Regeneration 2000;8(6):547-53. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2005
- Lichtenstein G, Yan S, Bala M, Blank M, Sands B. Infliximab maintenance treatment reduces hospitalizations, surgeries and procedures in fistulizing Crohn's disease. Gastroenterology 2005;128:862-9. [DOI] [PubMed] [Google Scholar]
Lightner 2017b
- Lightner AL, Raffals LE, Mathis KL, Cima RR, Tse CS, Pemberton JH, et al. Postoperative outcomes in vedolizumab-treated patients undergoing abdominal operations for inflammatory bowel disease. Journal of Crohn's and Colitis 2017;11(2):185-90. [DOI] [PubMed] [Google Scholar]
Magro 2017
- Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. Journal of Crohn's and Colitis 2017;11(6):649-70. [DOI] [PubMed] [Google Scholar]
Narula 2013
- Narula N, Charleton D, Marshall JK. Meta-analysis: peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2013;37(11):1057-64. [DOI] [PubMed] [Google Scholar]
Rawla 2018
- Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. Journal of Inflammation Research 2018;11:215-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinglas 2018
- Reinglas J, Gonczi L, Kurt Z, Bessissow T, Lakatos PL. Positioning of old and new biologicals and small molecules in the treatment of inflammatory bowel diseases. World Journal of Gastroenterology 2018;24(32):3567-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rungoe 2014
- Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut 2014;63:1607-16. [DOI] [PubMed] [Google Scholar]
Sales‐Campos 2015
- Sales-Campos H, Basso PJ, Alves VBF, Fonseca MTC, Bonfa G, Nardini V, et al. Classical and recent advances in the treatment of inflammatory bowel diseases. Brazilian Journal of Medical and Biological Research 2015;48(2):96-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramanian 2006
- Subramanian V, Pollok RCG, Kang J, Kumar D. Systematic review of postoperative complications in patients with inflammatory bowel disease treated with immunomodulators. British Journal of Surgery 2006;93:793-9. [DOI] [PubMed] [Google Scholar]
Subramanian 2008
- Subramanian V, Saxena S, Kang JY, Pollok RCG. Preoperative steroid use and risk of postoperative complications in patients with inflammatory bowel disease undergoing abdominal surgery. American Journal of Gastroenterology 2008;103:2373-81. [DOI] [PubMed] [Google Scholar]
Triantafillidis 2011
- Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Design, Development and Therapy 2011;5:185-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterland 2016
- Waterland P, Athanasiou T, Patel H. Post-operative abdominal complications in Crohn’s disease in the biological era: Systematic review and meta-analysis. World Journal of Gastrointestinal Surgery 2016;8(3):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2019
- Wells GA Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 28 January 2019).
Xu 2019
- Xu YY, Yang LS, An P, Zhou B, Liu G. Meta-analysis: The influence of preoperative infliximab use on postoperative complications of Crohn's disease. Inflammatory Bowel Diseases 2019;25(2):261-9. [DOI] [PubMed] [Google Scholar]
Yang 2012
- Yang Z, Wu Q, Wang F, Wu K, Fan D. Meta-analysis: effect of preoperative infliximab use on early postoperative complications in patients with ulcerative colitis undergoing abdominal surgery. Alimentary Pharmacology and Therapeutics 2012;36(10):922-8. [DOI] [PubMed] [Google Scholar]
Yang 2014
- Yang ZP, Hong L, Wu Qiong, Wu KC, Fan DM. Preoperative infliximab use and postoperative complications in Crohn's disease: a systematic review and meta-analysis. International Journal of Surgery 2014;12:224-30. [DOI] [PubMed] [Google Scholar]
Yung 2018
- Yung DE, Horesh N, Lightner AL, Ben-Horin S, Eliakim R, Koulaouzidis A et al. Systematic review and meta-analysis: Vedolizumab and postoperative complications in inflammatory bowel disease. Inflammatory Bowel Disease 2018;24(11):2327-38. [DOI] [PubMed] [Google Scholar]
Zenlea 2014
- Zenlea T, Peppercorn MA. Immunosuppressive therapies for inflammatory bowel disease. World Journal of Gastroenterology 2014;20(12):3146-52. [DOI] [PMC free article] [PubMed] [Google Scholar]


