Abstract
Background
Several comparative randomised controlled trials (RCTs) have been performed including combinations of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors since the publication of a Cochrane Review on targeted therapy for metastatic renal cell carcinoma (mRCC) in 2008. This review represents an update of that original review.
Objectives
To assess the effects of targeted therapies for clear cell mRCC in patients naïve to systemic therapy.
Search methods
We performed a comprehensive search with no restrictions on language or publication status. The date of the latest search was 18 June 2020.
Selection criteria
We included randomised controlled trials, recruiting patients with clear cell mRCC naïve to previous systemic treatment. The index intervention was any TKI‐based targeted therapy.
Data collection and analysis
Two review authors independently assessed the included studies and extracted data for the primary outcomes: progression‐free survival (PFS), overall survival (OS) and serious adverse events (SAEs); and the secondary outcomes: health‐related quality of life (QoL), response rate and minor adverse events (AEs). We performed statistical analyses using a random‐effects model and rated the certainty of evidence according to the GRADE approach.
Main results
We included 18 RCTs reporting on 11,590 participants randomised across 18 comparisons. This abstract focuses on the primary outcomes of select comparisons.
1. Pazopanib versus sunitinib
Pazopanib may result in little to no difference in PFS as compared to sunitinib (hazard ratio (HR) 1.05, 95% confidence interval (CI) 0.90 to 1.23; 1 study, 1110 participants; low‐certainty evidence). Based on the control event risk of 420 per 1000 in this trial at 12 months, this corresponds to 18 fewer participants experiencing PFS (95% CI 76 fewer to 38 more) per 1000 participants. Pazopanib may result in little to no difference in OS compared to sunitinib (HR 0.92, 95% CI 0.80 to 1.06; 1 study, 1110 participants; low‐certainty evidence). Based on the control event risk of 550 per 1000 in this trial at 12 months, this corresponds to 27 more OSs (95% CI 19 fewer to 70 more) per 1000 participants. Pazopanib may result in little to no difference in SAEs as compared to sunitinib (risk ratio (RR) 1.01, 95% CI 0.94 to 1.09; 1 study, 1102 participants; low‐certainty evidence). Based on the control event risk of 734 per 1000 in this trial, this corresponds to 7 more participants experiencing SAEs (95% CI 44 fewer to 66 more) per 1000 participants.
2. Sunitinib versus avelumab and axitinib
Sunitinib probably reduces PFS as compared to avelumab plus axitinib (HR 1.45, 95% CI 1.17 to 1.80; 1 study, 886 participants; moderate‐certainty evidence). Based on the control event risk of 550 per 1000 in this trial at 12 months, this corresponds to 130 fewer participants experiencing PFS (95% CI 209 fewer to 53 fewer) per 1000 participants. Sunitinib may result in little to no difference in OS (HR 1.28, 95% CI 0.92 to 1.79; 1 study, 886 participants; low‐certainty evidence). Based on the control event risk of 890 per 1000 in this trial at 12 months, this would result in 29 fewer OSs (95% CI 78 fewer to 8 more) per 1000 participants. Sunitinib may result in little to no difference in SAEs (RR 1.01, 95% CI 0.93 to 1.10; 1 study, 873 participants; low‐certainty evidence). Based on the control event risk of 705 per 1000 in this trial, this corresponds to 7 more SAEs (95% CI 49 fewer to 71 more) per 1000 participants.
3. Sunitinib versus pembrolizumab and axitinib
Sunitinib probably reduces PFS as compared to pembrolizumab plus axitinib (HR 1.45, 95% CI 1.19 to 1.76; 1 study, 861 participants; moderate‐certainty evidence). Based on the control event risk of 590 per 1000 in this trial at 12 months, this corresponds to 125 fewer participants experiencing PFS (95% CI 195 fewer to 56 fewer) per 1000 participants. Sunitinib probably reduces OS (HR 1.90, 95% CI 1.36 to 2.65; 1 study, 861 participants; moderate‐certainty evidence). Based on the control event risk of 880 per 1000 in this trial at 12 months, this would result in 96 fewer OSs (95% CI 167 fewer to 40 fewer) per 1000 participants. Sunitinib may reduce SAEs as compared to pembrolizumab plus axitinib (RR 0.90, 95% CI 0.81 to 1.02; 1 study, 854 participants; low‐certainty evidence) although the CI includes the possibility of no effect. Based on the control event risk of 604 per 1000 in this trial, this corresponds to 60 fewer SAEs (95% CI 115 fewer to 12 more) per 1000 participants.
4. Sunitinib versus nivolumab and ipilimumab
Sunitinib may reduce PFS as compared to nivolumab plus ipilimumab (HR 1.30, 95% CI 1.11 to 1.52; 1 study, 847 participants; low‐certainty evidence). Based on the control event risk of 280 per 1000 in this trial at 30 months' follow‐up, this corresponds to 89 fewer PFSs (95% CI 136 fewer to 37 fewer) per 1000 participants. Sunitinib reduces OS (HR 1.52, 95% CI 1.23 to 1.89; 1 study, 847 participants; high‐certainty evidence). Based on the control event risk 600 per 1000 in this trial at 30 months, this would result in 140 fewer OSs (95% CI 219 fewer to 67 fewer) per 1000 participants. Sunitinib probably increases SAEs (RR 1.37, 95% CI 1.22 to 1.53; 1 study, 1082 participants; moderate‐certainty evidence). Based on the control event risk of 457 per 1000 in this trial, this corresponds to 169 more SAEs (95% CI 101 more to 242 more) per 1000 participants.
Authors' conclusions
Based on the low to high certainty of evidence, several combinations of immune checkpoint inhibitors appear to be superior to single‐agent targeted therapy in terms of PFS and OS, and with a favourable AE profile. Some single‐agent targeted therapies demonstrated a similar or improved oncological outcome compared to others; minor differences were observed for AE within this group. The certainty of evidence was variable ranging from high to very low and all comparisons were based on single trials.
Keywords: Adult; Humans; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/adverse effects; Antibodies, Monoclonal, Humanized/therapeutic use; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Antineoplastic Agents/therapeutic use; Antineoplastic Agents, Immunological; Antineoplastic Agents, Immunological/therapeutic use; Axitinib; Axitinib/adverse effects; Axitinib/therapeutic use; Bevacizumab; Bevacizumab/adverse effects; Bevacizumab/therapeutic use; Bias; Carcinoma, Renal Cell; Carcinoma, Renal Cell/drug therapy; Carcinoma, Renal Cell/mortality; Everolimus; Everolimus/adverse effects; Everolimus/therapeutic use; Indazoles; Ipilimumab; Ipilimumab/adverse effects; Ipilimumab/therapeutic use; Kidney Neoplasms; Kidney Neoplasms/drug therapy; Kidney Neoplasms/mortality; Kidney Neoplasms/pathology; Phenylurea Compounds; Phenylurea Compounds/adverse effects; Phenylurea Compounds/therapeutic use; Progression-Free Survival; Protein Kinase Inhibitors; Protein Kinase Inhibitors/adverse effects; Protein Kinase Inhibitors/therapeutic use; Pyrimidines; Pyrimidines/adverse effects; Pyrimidines/therapeutic use; Quality of Life; Quinolines; Quinolines/adverse effects; Quinolines/therapeutic use; Randomized Controlled Trials as Topic; Receptors, Vascular Endothelial Growth Factor; Receptors, Vascular Endothelial Growth Factor/antagonists & inhibitors; Sirolimus; Sirolimus/adverse effects; Sirolimus/analogs & derivatives; Sirolimus/therapeutic use; Sorafenib; Sorafenib/adverse effects; Sorafenib/therapeutic use; Sulfonamides; Sulfonamides/adverse effects; Sulfonamides/therapeutic use; Sunitinib; Sunitinib/adverse effects; Sunitinib/therapeutic use
Plain language summary
Targeted drug treatment for kidney cancer which has spread
Review question
How effective is targeted drug treatment for patients with kidney cancer which has spread compared with other targeted drug treatments?
Background
Kidney cancer which has spread was treated over the last decade with a group of drugs called targeted therapy which act specifically on molecular pathways. However, the last few years have seen the emergence of a promising, newer group of drugs called immune checkpoint inhibitors which exploit the immune system (hence called immunotherapy). Some of these drugs are currently used in combinations. This review assesses how effective targeted therapies are in comparison with different targeted therapies, immune checkpoint inhibitors or different combinations of these drugs.
Study characteristics
We included only studies in which chance determined whether people got a targeted drug or other targeted drug, and which were reported in medical literature up to 18 June 2020. Most of the studies examined the effects on kidney cancer growth (called progression), survival (life expectancy) and serious unwanted effects.
Key results
We found 18 studies that answered our review question. Participants included in these trials had metastatic (cancer that has spread to other parts of the body) or advanced cancer that could not be removed by surgery. We reported up‐to‐date comparisons that are most important to doctors and participants.
1. Pazopanib versus sunitinib (targeted therapy versus targeted therapy)
Pazopanib may make little to no difference in progression, survival, and serious unwanted effects compared to sunitinib.
2. Sunitinib versus avelumab and axitinib (targeted agent versus immunotherapy + targeted agent)
Sunitinib probably results in more progression but may make little to no difference on death and serious unwanted effects compared to avelumab and axitinib.
3. Sunitinib versus pembrolizumab and axitinib (targeted agent versus immunotherapy + targeted agent)
Sunitinib probably results in more progression and death but may slightly reduce serious unwanted effects compared to pembrolizumab and axitinib.
4. Sunitinib versus nivolumab and ipilimumab (targeted therapy versus combinations of immunotherapy)
Sunitinib may result in more progression and serious unwanted effects compared to nivolumab and ipilimumab. Sunitinib results in more deaths compared to combinations.
Certainty of the evidence
The certainty of the evidence for most outcomes was low to high, meaning that there is some uncertainty regarding the findings. Nevertheless, there is sufficient data for us to make definitive conclusions regarding how these drugs should be used in the management of patients with kidney cancer which has spread.
Summary of findings
Summary of findings 1. Sorafenib compared to sunitinib (targeted agent versus targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type) Setting: Germany and the Netherlands/muticentre/likely outpatient Intervention: Sorafenib Comparison: Sunitinib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Sunitinib | Risk difference with Sorafenib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 10 months) follow‐up: mean 10.3 months |
365 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 1.19 (0.92 to 1.53) | Study population | |
| 340 per 1000 | 63 fewer per 1000 (148 fewer to 31 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: mean 10.3 months |
365 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 | HR 0.99 (0.74 to 1.33) | Study population | |
| 550 per 1000 | 3 more per 1000 (98 fewer to 92 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 353 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 0.99 (0.85 to 1.14) | Study population | |
| 670 per 1000 | 7 fewer per 1000 (101 fewer to 94 more) | ||||
| Health‐related quality of life5 | not reported | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; high risk of performance and detection bias and unclear risk of other bias
2 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included harm and no harm)
3 Downgraded by 1 level for study limitations; unclear risk of other bias
4 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
5 Health‐related quality of life: no available data
Summary of findings 2. Pazopanib compared to sunitinib (targeted agent versus targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Pazopanib Comparison: Sunitinib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Sunitinib | Risk difference with Pazopanib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: not reported |
1110 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 1.05 (0.90 to 1.23) | Study population | |
| 420 per 1000 | 18 fewer per 1000 (76 fewer to 38 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
1110 (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | HR 0.92 (0.80 to 1.06) | Study population | |
| 550 per 1000 | 27 more per 1000 (19 fewer to 70 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 1102 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 1.01 (0.94 to 1.09) | Study population | |
| 734 per 1000 | 7 more per 1000 (44 fewer to 66 more) | ||||
|
Health‐related quality of life (mean change value)
assessed with: FACIT‐F (higher scores indicating less fatigue)
Scale from: 0 to 52 follow‐up: after 4 cycle |
467 (1 RCT) | ⊕⊕⊝⊝ LOW 5 6 | ‐ | The mean health‐related quality of life (mean change value) was ‐6.5 | MD 3.6 higher (1.76 higher to 5.44 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;FACIT‐F: Functional Assessment of Chronic Illness Therapy–Fatigue scale; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; high risk of performance and detection bias and unclear risk of other bias
2 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included harm and no harm)
3 Downgraded by 1 level for study limitations; unclear risk of other bias
4 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included no benefit and benefit)
5 Downgraded by 1 level for study limitations; high risk of performance, detection and attrition bias and unclear risk of other bias
6 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (3 points, included benefit and little benefit)
Summary of findings 3. Tivozanib compared to sorafenib (targeted agent versus targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Tivozanib Comparison: Sorafenib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Sorafenib | Risk difference with Tivozanib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: not reported |
517 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 0.79 (0.64 to 0.99) | Study population | |
| 360 per 1000 | 86 more per 1000 (4 more to 160 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
517 (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | HR 1.25 (0.95 to 1.64) | Study population | |
| 620 per 1000 | 70 fewer per 1000 (163 fewer to 15 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 516 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 0.85 (0.74 to 0.97) | Study population | |
| 689 per 1000 | 103 fewer per 1000 (179 fewer to 21 fewer) | ||||
| Health‐related quality of life assessed with: EQ‐5D Health State Index Scale from: ‐0.59 (worst health state) to 1 (best health state) follow‐up: 12 months | 506 (1 RCT) | ⊕⊕⊝⊝ LOW 2 5 | ‐ | The mean health‐related quality of life was ‐0.06 | MD 0.01 higher (0.05 lower to 0.07 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;EQ‐5D: EuroQol‐5D; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included benefit and little benefit)
2 Downgraded by 1 level for study limitations; high risk of performance, detection and other bias
3 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included harm and no harm)
4 Downgraded by 1 level for study limitations; high risk of other bias
5 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (0.06 points, included benefit and no benefit)
Summary of findings 4. Sorafenib compared to pazopanib (targeted agent versus targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sorafenib Comparison: Pazopanib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Pazopanib | Risk difference with Sorafenib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: not reported |
377 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 1.92 (1.74 to 2.11) | Study population | |
| 380 per 1000 | 224 fewer per 1000 (250 fewer to 194 fewer) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
377 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | HR 1.22 (0.91 to 1.64) | Study population | |
| 520 per 1000 | 70 fewer per 1000 (178 fewer to 32 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.03 | 366 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 0.92 (0.78 to 1.09) | Study population | |
| 639 per 1000 | 51 fewer per 1000 (141 fewer to 58 more) | ||||
|
Health‐related quality of life
(mean change value)
assessed with: FACIT‐F (higher scores indicating less fatigue)
Scale from: 0 to 52 follow‐up: not reported |
267 (1 RCT) | ⊕⊕⊝⊝ LOW 5 6 | ‐ | The mean health‐related quality of life was ‐9.9 | MD 3.1 higher (1.82 lower to 8.02 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;FACIT‐F: Functional Assessment of Chronic Illness Therapy–Fatigue scale; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; unclear risk of selection, detection, and reporting bias and high risk of performance bias
2 Downgraded by 1 level for study limitations; unclear risk of selection, and reporting bias
3 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included harm and no harm)
4 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
5 Downgraded by 1 level for study limitations; unclear risk of selection, detection, and reporting bias and high risk of performance and attrition bias
6 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (3 points, included benefit and no benefit)
Summary of findings 5. Sunitinib compared to everolimus (targeted agent versus targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Everolimus | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Everolimus | Risk difference with Sunitinib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: not reported |
471 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 0.71 (0.59 to 0.87) | Study population | |
| 300 per 1000 | 125 more per 1000 (51 more to 191 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
471 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | HR 0.90 (0.72 to 1.11) | Study population | |
| 470 per 1000 | 37 more per 1000 (37 fewer to 111 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 469 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | RR 1.34 (1.14 to 1.59) | Study population | |
| 471 per 1000 | 160 more per 1000 (66 more to 278 more) | ||||
| Health‐related quality of life assessed with: EORTC QLQ‐C30 (Global health status scale: high score represent better functioning) Scale from: 0 to 100 follow‐up: 16 weeks | 288 (1 RCT) | ⊕⊕⊝⊝ LOW 1 4 | ‐ | The mean health‐related quality of life was 65.5 | MD 5 lower (10.4 lower to 0.4 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; high risk of performance, detection and other bias
2 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included benefit and no benefit)
3 Downgraded by 1 level for study limitations; high risk of other bias
4 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (10 points, included harm and no harm)
Summary of findings 6. Sunitinib compared to avelumab + axitinib (targeted agent versus immunotherapy + targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Avelumab + Axitinib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Avelumab + Axitinib | Risk difference with Sunitinib | ||||
|
Progression‐free survival
(absolute effect size estimates based on survival rate at 12 months) follow‐up: median 10.8 months |
886 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 1.45 (1.17 to 1.80) | Study population | |
| 550 per 1000 | 130 fewer per 1000 (209 fewer to 53 fewer) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: median 12.0 months |
886 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | HR 1.28 (0.92 to 1.79) | Study population | |
| 890 per 1000 | 29 fewer per 1000 (78 fewer to 8 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.03 | 873 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 1.01 (0.93 to 1.10) | Study population | |
| 705 per 1000 | 7 more per 1000 (49 fewer to 71 more) | ||||
| Health‐related quality of life4 | Not reported | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations: high risk of performance bias and unclear risk of reporting bias
2 Downgraded by 1 level for imprecision: confidence interval crossed the assumed threshold of a clinically important difference (included no benefit and harm)
3 Downgraded by 1 level for study limitations: unclear risk of reporting bias
4 Health‐related quality of life: no available data
Summary of findings 7. Sunitinib compared to pembrolizumab + axitinib (targeted agent versus immunotherapy + targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Pembrolizumab + Axitinib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Pembrolizumab + Axitinib | Risk difference with Sunitinib | ||||
|
Progression‐free survival
(absolute effect size estimates based on survival rate at 12 months) follow‐up: median 12.8 months |
861 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 1.45 (1.19 to 1.76) | Study population | |
| 590 per 1000 | 125 fewer per 1000 (195 fewer to 56 fewer) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: median 12.8 months |
861 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | HR 1.90 (1.36 to 2.65) | Study population | |
| 880 per 1000 | 96 fewer per 1000 (167 fewer to 40 fewer) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.0 | 854 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | RR 0.90 (0.81 to 1.02) | Study population | |
| 604 per 1000 | 60 fewer per 1000 (115 fewer to 12 more) | ||||
| Health‐related quality of life4 | Not reported | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; high risk of performance bias
2 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included harm and little harm)
3 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included benefit and no benefit)
4 Health‐related quality of life: no available data
Summary of findings 8. Sunitinib compared to atezolizumab + bevacizumab (targeted agent versus immunotherapy + targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Atezolizumab + Bevacizumab | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Atezolizumab + Bevacizumab | Risk difference with Sunitinib | ||||
|
Progression‐free survival
(absolute effect size estimates based on survival rate at 12 months) follow‐up: range 15 months to 20.7 months |
1117 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | HR 1.18 (1.02 to 1.36) | Study population | |
| 480 per 1000 | 59 fewer per 1000 (111 fewer to 7 fewer) | ||||
|
Overall survival
(absolute effect size estimates based on survival rate at 24 months) follow‐up: range 20.7 months to 24 months |
1117 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 | HR 0.99 (0.73 to 1.33) | Study population | |
| 630 per 1000 | 3 more per 1000 (89 fewer to 84 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.0 | 1098 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 | RR 1.22 (1.00 to 1.49) | Study population | |
| 446 per 1000 | 98 more per 1000 (0 fewer to 218 more) | ||||
| Health‐related quality of life assessed with: MDASI (high score indicates worse QoL) Scale from: 0 to 10 follow‐up: 12 weeks | 691 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ‐ | The mean health‐related quality of life ranged from 0.56 to 1.57 | MD 1 higher (0.68 higher to 1.32 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; MDASI: MD Anderson Symptom Inventory; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included harm and little harm)
2 Downgraded by 1 level for study limitations; high and unclear risk of 1 or more domains.
3 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
4 Downgraded by 1 level for inconsistency; moderate to substantial heterogeneity: unexplained differences between study results
5 Downgraded by 1 level for imprecision; confidence interval reached the line of no difference and crossed the assumed threshold of a clinically important difference (included harm and no harm)
Summary of findings 9. Sunitinib compared to IMA901 + sunitinib (targeted agent versus tumour vaccine + targeted agent).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: IMA901 + Sunitinib | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with IMA901 + Sunitinib | Risk difference with Sunitinib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: median 33.27 months |
339 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | HR 0.95 (0.70 to 1.30) | Study population | |
| 590 per 1000 | 16 more per 1000 (86 fewer to 101 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: median 33.27 months |
339 (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | HR 0.75 (0.54 to 1.04) | Study population | |
| 800 per 1000 | 46 more per 1000 (7 fewer to 86 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.0 | 334 (1 RCT) | ⊕⊕⊝⊝ LOW 2 5 | RR 0.74 (0.59 to 0.95) | Study population | |
| 550 per 1000 | 143 fewer per 1000 (225 fewer to 27 fewer) | ||||
| Health‐related quality of life6 | Not reported | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
2 Downgraded by 1 level for study limitations; high risk of performance and other bias
3 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included benefit and no benefit)
4 Downgraded by 1 level for study limitations: high risk of other bias
5 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included benefit and little benefit)
6 Health‐related quality of life: no available data
Summary of findings 10. Sunitinib compared to interferon‐α (IFN‐α) (targeted agent versus classic immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Interferon‐α (IFN‐α) | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Interferon‐α (IFN‐α) | Risk difference with Sunitinib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 6 months) follow‐up: median 31 months |
750 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 0.54 (0.45 to 0.64) | Study population | |
| 400 per 1000 | 210 more per 1000 (156 more to 262 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: median 31 months |
750 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 | HR 0.82 (0.67 to 1.00) | Study population | |
| 480 per 1000 | 68 more per 1000 (0 fewer to 132 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed as: CTCAE v3.0 | 735 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | RR 1.75 (1.43 to 2.16) | Study population | |
| 258 per 1000 | 194 more per 1000 (111 more to 300 more) | ||||
|
Health‐related quality of life
assessed with: EQ‐5D Health State Index
Scale from: ‐0.59 (worst health state) to 1 (best health state) follow‐up: after 2 cycle |
544 (1 RCT) | ⊕⊕⊕⊝ MODERATE 4 | ‐ | The mean health‐related quality of life was 0.74 | MD 0.01 lower (0.05 lower to 0.03 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;EQ‐5D: EuroQol‐5D; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; unclear risk of selection bias and high risk of performance and other bias
2 Downgraded by 1 level for study limitations; unclear risk of selection bias and high risk of other bias
3 Downgraded by 1 level for imprecision; confidence interval reached the line of no difference and crossed the assumed threshold of a clinically important difference (included benefit and no benefit)
4 Downgraded by 1 level for study limitations; unclear risk of selection and attrition bias and high risk of performance and other bias
Summary of findings 11. Temsirolimus compared to IFN‐α (targeted agent versus classic immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type [80% clear cell]) Setting: Multinational muticentre/likely outpatient Intervention: Temsirolimus Comparison: IFN‐α | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with IFN‐α | Risk difference with Temsirolimus | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: up to 80 months |
416 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 0.74 (0.60 to 0.91) | Study population | |
| 100 per 1000 | 82 more per 1000 (23 more to 151 more) | ||||
|
Overall survival survival (absolute effect size estimates based on survival rate at 12 months ) follow‐up: up to 80 months |
416 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 0.78 (0.63 to 0.97) | Study population | |
| 300 per 1000 | 91 more per 1000 (11 more to 168 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE version: not reported | 408 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 0.86 (0.76 to 0.97) | Study population | |
| 780 per 1000 | 109 fewer per 1000 (187 fewer to 23 fewer) | ||||
|
Health‐related quality of life
assessed with: EQ‐5D Health State Index
Scale from: ‐0.59 (worst health state) to 1 (best health state) follow‐up: not reported |
401 (1 RCT) | ⊕⊕⊝⊝ LOW 3 4 | ‐ | The mean health‐related quality of life was 0.66 | MD 0.03 higher (0.01 lower to 0.07 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;EQ‐5D: EuroQol‐5D; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included benefit and no benefit)
2 Downgraded by 1 level for study limitations; high risk of performance and detection bias
3 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included benefit and no benefit)
4 Downgraded by 1 level for study limitations; high risk of performance, detection and attrition bias
Summary of findings 12. Sunitinib compared to atezolizumab (targeted therapy versus immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Atezolizumab | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Atezolizumab | Risk difference with Sunitinib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: median 20.7 months |
204 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | HR 0.84 (0.58 to 1.22) | Study population | |
| 420 per 1000 | 63 more per 1000 (73 fewer to 185 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: median 20.7 months |
204 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | HR 0.94 (0.58 to 1.54) | Moderate | |
| 630 per 1000 6 | 18 more per 1000 (139 fewer to 135 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.0 | 203 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | RR 1.73 (1.32 to 2.27) | Study population | |
| 398 per 1000 | 291 more per 1000 (127 more to 506 more) | ||||
| Health‐related quality of life assessed with: MDASI (high score indicates worse QoL) Scale from: 0 to 10 follow‐up: 12 weeks | 157 (1 RCT) | ⊕⊕⊝⊝ LOW 4 5 | ‐ | The mean health‐related quality of life was 1.04 | MD 1.46 higher (0.8 higher to 2.12 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; MDASI: MD Anderson Symptom Inventory; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
2 Downgraded by 1 level for study limitations; high risk of selection, performance and detection bias and unclear risk of other bias
3 Downgraded by 1 level for study limitations; high risk of selection and unclear risk of other bias
4 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (1 point, included harm and little harm)
5 Downgraded by 1 level for study limitations; high risk of selection, performance and detection bias and unclear risk of other bias
6 Baseline risk for overall survival in the atezolizumab group was assumed to be 63% (moderate risk) at 24 months as reported in Rini 2019b
Summary of findings 13. Bevacizumab + IFN compared to IFN (+ placebo) (targeted agent + classic immunotherapy versus classic immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Bevacizumab + IFN Comparison: IFN (+ placebo) | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with IFN (+ placebo) | Risk difference with Bevacizumab + IFN | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: intervention: 13.3 months comparator: 12.8 months |
1381 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | HR 0.68 (0.60 to 0.77) | Study population | |
| 200 per 1000 | 135 more per 1000 (90 more to 181 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: intervention: 23 months comparator: 21 months |
1381 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | HR 0.88 (0.79 to 0.99) | Study population | |
| 500 per 1000 | 43 more per 1000 (3 more to 78 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 1356 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | RR 1.31 (1.20 to 1.42) | Study population | |
| 536 per 1000 | 166 more per 1000 (107 more to 225 more) | ||||
| Health‐related quality of life3 | Not reported | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; high and unclear risk of 1 or more domains
2 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included benefit and little benefit)
3 Health‐related quality of life: no available data
Summary of findings 14. Temsirolimus + IFN‐α compared to IFN‐α (targeted agent + classic immunotherapy versus classic immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type [80% clear cell]) Setting: Multinational muticentre/likely outpatient Intervention: Temsirolimus + IFN‐α Comparison: IFN‐α | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with IFN‐α | Risk difference with Temsirolimus + IFN‐α | ||||
| Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: up to 80 months | 417 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 0.76 (0.62 to 0.93) | Study population | |
| 100 per 1000 | 74 more per 1000 (17 more to 140 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: up to 80 months |
417 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | HR 0.93 (0.75 to 1.15) | Study population | |
| 300 per 1000 | 26 more per 1000 (50 fewer to 105 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE version: not reported | 408 (1 RCT) | ⊕⊕⊝⊝ LOW 2 4 | RR 1.12 (1.02 to 1.22) | Study population | |
| 780 per 1000 | 94 more per 1000 (16 more to 172 more) | ||||
|
Health‐related quality of life
assessed with: EQ‐5D Health State Index
Scale from: ‐0.59 (worst health state) to 1 (best health state) follow‐up: not reported |
394 (1 RCT) | ⊕⊕⊝⊝ LOW 5 6 | ‐ | The mean health‐related quality of life was 0.66 | MD 0.03 higher (0.01 lower to 0.07 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;EQ‐5D: EuroQol‐5D; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included benefit and no benefit)
2 Downgraded by 1 level for study limitations; high risk of performance and detection bias
3 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
4 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included harm and no harm)
5 Downgraded by 1 level for study limitations; high risk of performance, detection and attrition bias
6 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included benefit and no benefit)
Summary of findings 15. Temsirolimus + bevacizumab compared to bevacizumab + IFN‐α (targeted agent + targeted agent versus targeted agent + classic immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type [80% clear cell]) Setting: Multinational muticentre/likely outpatient Intervention: Temsirolimus + Bevacizumab Comparison: Bevacizumab + IFN‐α | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Bevacizumab + IFN‐α | Risk difference with Temsirolimus + Bevacizumab | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: not reported |
791 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 1.10 (0.90 to 1.34) | Study population | |
| 420 per 1000 | 35 fewer per 1000 (107 fewer to 38 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
791 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 1.08 (0.90 to 1.30) | Study population | |
| 550 per 1000 | 26 fewer per 1000 (90 fewer to 34 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 784 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 1.05 (0.98 to 1.13) | Study population | |
| 760 per 1000 | 38 more per 1000 (15 fewer to 99 more) | ||||
|
Health‐related quality of life3 assessed with: FKSI–15 Scale from: 0 to 60 |
no available data | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;FKSI: Functional Assessment of Cancer Therapy–Kidney Symptom Index; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included harm and no harm)
2 Downgraded by 1 level for study limitations; high risk of performance bias (we are not concerned with unclear risk of other bias)
3 Health‐related quality of life: no available data
Summary of findings 16. Everolimus + bevacizumab compared to IFN α‐2a + bevacizumab (targeted agent + targeted agent versus targeted agent + classic immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (any cell type) Setting: Multinational muticentre/likely outpatient Intervention: Everolimus + Bevacizumab Comparison: IFN α‐2a + Bevacizumab | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with IFN α‐2a + Bevacizumab | Risk difference with Everolimus + Bevacizumab | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 18 months) follow‐up: not reported |
365 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | HR 0.91 (0.69 to 1.20) | Study population | |
| 250 per 1000 | 33 more per 1000 (61 fewer to 134 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
365 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 | HR 1.01 (0.75 to 1.36) | Study population | |
| 533 per 1000 | 3 fewer per 1000 (108 fewer to 91 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 361 (1 RCT) | ⊕⊕⊝⊝ LOW 1 4 | RR 1.06 (0.95 to 1.18) | Study population | |
| 762 per 1000 | 46 more per 1000 (38 fewer to 137 more) | ||||
| Health‐related quality of life5 assessed with: EORTC QLQ‐C30 (Global health status scale: high score represent better functioning) Scale from: 0 to 100 | no available data | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for study limitations; unclear risk of selection, performance and other bias and high risk of detection bias
2 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference: wide confidence interval (included both benefit and harm)
3 Downgraded by 1 level for study limitations; unclear risk of selection and other bias
4 Downgraded by 1 level for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included harm and no harm)
5 Health‐related quality of life: no available data
Summary of findings 17. Sunitinib compared to nivolumab + ipilimumab (targeted agent versus combinations of immunotherapy).
| Patient or population: Treatment‐naïve metastatic renal cell carcinoma (clear cell type); IMDC intermediate, poor risk patients only. Setting: Multinational muticentre/likely outpatient Intervention: Sunitinib Comparison: Nivolumab + Ipilimumab | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Nivolumab + Ipilimumab | Risk difference with Sunitinib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 30 months) follow‐up: median 32.4 months |
847 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | HR 1.30 (1.11 to 1.52) | Study population | |
| 280 per 1000 | 89 fewer per 1000 (136 fewer to 37 fewer) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 30 months) follow‐up: median 32.4 months |
847 (1 RCT) | ⊕⊕⊕⊕ HIGH | HR 1.52 (1.23 to 1.89) | Study population | |
| 600 per 1000 | 140 fewer per 1000 (219 fewer to 67 fewer) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v4.0 | 1082 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | RR 1.37 (1.22 to 1.53) | Study population | |
| 457 per 1000 | 169 more per 1000 (101 more to 242 more) | ||||
|
Health‐related quality of life
assessed with: FKSI‐19 (higher scores indicating fewer symptoms) Scale from: 0 to 76 follow‐up: 24 weeks |
460 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | ‐ | The mean health‐related quality of life was 2.6 | MD 4.1 lower (5.75 lower to 2.45 lower) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;FKSI: Functional Assessment of Cancer Therapy–Kidney Symptom IndexHR: Hazard ratio; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 level for imprecision; confidence interval crossed the assumed threshold of a clinically important difference (included harm and no harm)
2 Downgraded by 1 level for study limitations; high risk of performance and detection bias
3 Downgraded by 1 level for study limitations; high risk of performance and detection bias and unclear risk of attrition bias
Summary of findings 18. Pazopanib compared to placebo (targeted agent versus placebo).
| Patient or population: Previous treated and treatment‐naïve (54%) metastatic renal cell carcinoma (clear cell type) Setting: Multinational muticentre/likely outpatient Intervention: Pazopanib Comparison: Placebo | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with Placebo | Risk difference with Pazopanib | ||||
|
Progression‐free survival (absolute effect size estimates based on survival rate at 12 months) follow‐up: not reported |
435 (1 RCT) | ⊕⊕⊕⊕ HIGH | HR 0.46 (0.34 to 0.62) | Study population | |
| 180 per 1000 | 274 more per 1000 (165 more to 378 more) | ||||
|
Overall survival (absolute effect size estimates based on survival rate at 24 months) follow‐up: not reported |
435 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | HR 0.91 (0.72 to 1.16) | Study population | |
| 480 per 1000 | 33 more per 1000 (53 fewer to 110 more) | ||||
| Serious adverse events (Grade 3 or 4) assessed with: CTCAE v3.0 | 435 (1 RCT) | ⊕⊕⊕⊕ HIGH | RR 2.00 (1.40 to 2.85) | Study population | |
| 200 per 1000 | 200 more per 1000 (80 more to 370 more) | ||||
| Health‐related quality of life assessed with: EORTC QLQ‐C30 (Global health status scale: high score represent better functioning but negative change from baseline represents a worsening condition) Scale from: 0 to 100 follow‐up: 12 weeks | 300 (1 RCT) | ⊕⊕⊕⊕ HIGH | ‐ | The mean health‐related quality of life was ‐0.5 | MD 3.1 lower (7.76 lower to 1.56 higher) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTCAE: Common Terminology Criteria for Adverse Events;EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; HR: Hazard ratio; RCT: Randomized controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 2 levels for imprecision; confidence interval crossed the line of no difference and the assumed threshold of a clinically important difference (included both benefit and harm)
Background
Description of the condition
Renal cell carcinoma (RCC) incidence represents about 2.2% of all invasive cancers and has a projected 2018 population age‐standardised mortality rate of 1.8 per 100,000 worldwide (GLOBOCAN 2018; Howlader 2017). Two‐thirds of cases occur in men. These figures include both renal cell carcinoma and the less common urothelial carcinoma of the renal pelvis: the latter is biologically related to bladder cancer and we do not consider it here. Renal cell carcinoma is divided into different pathologic subtypes, of which the clear cell subtype represents about 75% (Srigley 2013). The more uncommon subtypes are collectively referred to by clinicians as non‐clear renal cell carcinomas: they respond differently to treatment as compared to clear cell renal cell carcinoma (Fernández‐Pello 2017). Death from renal cell carcinoma is usually from metastases, either detected during staging of newly‐diagnosed patients (Stage IV) or detected during follow‐up after nephrectomy. A minority of patients are diagnosed with locally advanced disease which is too advanced for surgical resection but without metastatic findings. The term 'advanced renal cell carcinoma' has been used by authors to include both metastatic and locally advanced disease that have aspects that require separate consideration.
There has been great interest in finding more effective treatments for metastatic renal cell carcinoma. The search for specific targets for therapy goes back at least to Paul Ehrlich's 'magic bullet' over a century ago (Strebhardt 2008). This concept has gained renewed interest owing to the identification of multiple molecular targets and the potential for associated therapies that are target‐specific and therefore might have greater efficacy with less toxicity (Sawyers 2004). Clinical proof of concept came with the remarkable success of single‐agent imatinib for chronic myeloid leukaemia (Deininger 2005). Here we review the subsequent development of targeted therapy for metastatic renal cell carcinoma.
Description of the intervention
Prior to the development of targeted agents, renal cell carcinoma was one of the most drug‐resistant malignancies. Hormonal and cytotoxic chemotherapy agents have not been demonstrated to improve overall survival (OS) for this condition, and remissions with those agents occur at a frequency similar to that seen with no therapy or with placebo (Gleave 1998; Oliver 1989). Until the past decades, immunotherapy was the main focus of the search for an effective drug therapy for renal cell carcinoma and was the main initial comparator for targeted therapy; it was the subject of a companion Cochrane Review (Coppin 2004). In summary, classic immunotherapy, for example interferon‐alpha or interleukin‐2, has been associated with very modest survival benefit at best. When targeted agents were first being evaluated, the immunotherapy agent interferon‐alpha was considered the standard comparator for first‐line therapy of metastatic renal cell carcinoma (Mickisch 2003; Motzer 2002); placebo‐controlled trials have been appropriate in the second‐line setting. One should be aware that the distribution of prognostic risk strata in clinical trials is changing to a more favourable profile, such that direct comparisons of interventions through head‐to‐head clinical trials remain essential (Patil 2010).
Molecular pathways with multiple targets that are of particular interest in renal cell carcinoma currently fall into two major groups: angiogenesis (Rini 2005), and intracellular signal transduction pathways (Adjei 2005). The presence of a target may or may not translate into benefit from a targeted agent (Bergsland 2006). Some agents have activity against multiple targets. Classic immunotherapies such as interferon‐alpha may have anti‐angiogenic activity but are considered a separate class of agent (Coppin 2004). Suitably large randomised controlled trials have a high financial and resource cost, so that selection of agents for phase III testing requires strategic decision‐making (Roberts 2003).
A new class of drugs has been introduced into the treatment paradigm of clear cell RCC (Motzer 2015a). Immune checkpoint inhibitors are a new type of targeted immunotherapy and have been very successfully tested in other immunogenic tumours such as melanoma.
Since neither multi‐kinase inhibitors nor immune checkpoint inhibitors are necessarily cytotoxic, it is possible that tumour shrinkage may not be a reliable indicator of drug activity (Stadler 2006); for example, objective stabilisation of previously progressive disease might result in extension of OS. This is especially the case for immune checkpoint inhibition which in second‐line RCC treatment leads to prolonged OS without benefit in progression‐free survival (PFS).
Drug therapy for metastatic renal cell carcinoma has yet to demonstrate curative potential. Improvement in OS is the preferred and definitive outcome of interest to patients, and is a realistic outcome if there is only one effective intervention for an incurable cancer, as was the situation for metastatic renal cell carcinoma at the beginning of the targeted era (i.e. from 2000 onwards). However, when participants with progressive cancer in one arm of a randomised trial are permitted cross‐over to the other arm, as is commonly done for ethical reasons or to enhance recruitment, then any survival benefit (or detriment) of the investigational agent might be obscured; the same problem might happen if sequential active therapies are applied. For these reasons and as in other cancer sites, the duration of freedom from cancer progression may be accepted by regulatory bodies as adequate evidence of benefit for drug approval purposes (Johnson 2011). Surrogate endpoints such as PFS should preferably be accompanied by patient‐reported outcomes.
How the intervention might work
Molecular analysis of renal cell carcinoma has shown that this cancer is not a homogeneous condition (Hacker 2010; Linehan 2005). A high proportion of sporadic clear cell renal cell carcinomas have biallelic abnormalities of the Von Hippel‒Lindau (VHL) tumour‐suppressor gene (Young 2009), whereas other subtypes do not. Absence of the active VHL gene produces results in unregulated activation of the hypoxia‐inducible system and accumulation of growth factors such as vascular endothelial growth factor (VEGF). In subtypes such as papillary and chromophobe RCC, other pathways such as MET proto‐oncogene (MET) and tuberous sclerosis (TSC) alterations have been identified through investigation of hereditary and sporadic forms. Therefore the mainstays of first‐line therapy until now are multi‐kinase inhibitors targeting predominantly the VEGF‐receptor kinases but other targets are included to various degrees, such as MET, AXL receptor tyrosine kinase (AXL), platelet‐derived growth factor receptor (PDGFR) and epidermal growth factor receptor (EGFR). Immune checkpoint inhibitors targeting the programmed death‐ligand (PD‐L1) or its receptor (PD‐1) have been tested successfully in second‐ and third‐line treatments after failure of one or two lines of VEGFR‐targeting therapies (Motzer 2015a). These drugs counteract the tumour‐driven inhibition of T‐cell receptor‐mediated activation of IL‐2 production and T‐cell proliferation which leads to a successful anti‐tumour T‐cell‐mediated immune activity. Currently, these drugs are tested in first‐line trials in combination with either multi‐kinase inhibitors or other monoclonal antibodies targeting circulating VEGF or anti‐CTLA4 against the current first‐line monotherapy with VEGFR‐targeted therapies. With more treatment options being approved and investigated, it will be necessary to distinguish the impact of therapy on different molecularly‐defined tumour types as well as on tumours which have been treated with previous lines of therapy to better select patients for a given drug based on their predicted outcome. Although available, the necessary technology is not yet used in clinical routine. The molecular complexities of both the disease (renal cell carcinoma) and the treatment (targeted therapy) are resulting in a rapidly‐evolving and exciting phase in the history of the treatment of metastatic disease. According to Uzzo 2003, "an understanding of the basic biology of renal cell carcinoma is more advanced than that of any other solid malignancy." Further molecular subclassification within clear cell renal cell carcinoma may well become feasible (Kaelin 2008).
Why it is important to do this review
The topic of this review is systemic therapy of treatment‐naïve metastatic renal cell carcinoma, an important type of malignancy for which the therapy has changed greatly over the past decade and continues to be a strong focus of development of new agents and comparative studies. This review is needed to provide an objective and up‐to‐date resource for researchers, clinicians and consumers.
This is an update of a Cochrane Review first published in 2008 and previously updated in 2011 (Coppin 2008; Coppin 2011). Since the last date of full literature search, a number of additional studies have been published and there is an evolving shift to using previously validated targeted agents as the comparator rather than placebo, quasi‐placebo such as hormone therapy, or immunotherapy such as interferon‐alpha. There is also increasing emphasis on second‐line therapy now that targeted agents are established for first‐line therapy of metastatic renal cell carcinoma. In addition, new agents such as immune checkpoint inhibitors are increasingly being compared against first‐line standard therapies (Kuusk 2017).
This updated review reflects a restriction of scope in order to focus on metastatic renal cell carcinoma within the broader category of 'advanced disease' that additionally included locally‐advanced cancers without metastases. The main reason for this change of scope is because the management of locally‐advanced disease may include both systemic and surgical interventions, and therefore the complex interaction between the two modalities as well as additional outcomes such as resectability and local control rates. Other reasons include lack of criteria for inoperability that include both cancer and patient factors, and the possibility that drug response to the primary tumour might be different from the response of its metastases.
This review originates from a collaboration between the previous Cochrane Review authorship and the Renal Cell Carcinoma Guideline Panel of the European Association of Urology (EAU panel). Preliminary discussions with the EAU panel demonstrated a high level of overlap between the protocols of the two groups. This review is designed to minimise residual differences.
Objectives
To assess the effects of targeted therapies for clear cell mRCC in patients naïve to systemic therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, including randomised discontinuation trials in which treatment was stopped early because of obvious benefits or harms (Stadler 2006). Quasi‐randomised trials such as alternate allocation were eligible for consideration. We excluded randomised phase I trials as well as cluster‐randomised trials or trials of factorial design. Additionally, we imposed a stricter inclusion criterion of more than 100 patients per arm for study inclusion. This was a decision driven by pragmatic and methodological considerations, to avoid including small, methodologically‐flawed and underpowered studies with low internal and external validity and high clinical and methodological heterogeneity, whose findings are highly unlikely to inform, guide or influence clinical practice.
Types of participants
Participants were eligible if: older than 18 years of age; they had mRCC histologically or pathologically verified at presentation or relapse; they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2 or equivalent. No prior systemic treatment was allowed.
Exclusion criteria were: the presence of symptomatic brain metastases; a life expectancy of less than 12 weeks; a serious acute or chronic illness or recent history of cardiac event.
Studies which allow solid tumours other than renal cell carcinoma were eligible only if participants with renal cell carcinoma were stratified and reported separately from other tumour types.
Diagnosis must be reported using the standard criteria (e.g. TNM Classification of Malignant Tumors) valid at the time that the trial began.
A predominant clear cell renal cell carcinoma histology was required.
We excluded studies for analysis of oncological outcomes that are designed for or include more than 20% of participants without metastases (i.e. locally‐advanced disease or unfit for nephrectomy). We included evaluation of adverse events if reported, however.
Types of interventions
Agents with known or presumed molecular targets were part of the therapeutic regimen of at least one study arm. Non‐specific agents considered previously were no longer eligible, as they are of historic interest only; these include ABT‐510, AE‐941, and carboxyaminoimidazole. We excluded classic immunotherapy agents, including recombinant cytokines and their predecessors, from the definition of targeted therapy, but they were allowed as part of combined regimens in any study arm (i.e. either as index interventions or as comparators).
Our approach to targeting immunotherapies (including PD1 or PD‐L1 checkpoint inhibitors) deserves special mention. As the main focus of the review was on VEGF‐targeted therapies on the basis of the earlier version of our review, and the fact that VEGF‐targeted therapies have been established as the mainstay of treatment for mRCC at the inception of this review, we considered targeting immunotherapies as a comparator intervention. In addition, targeted immunotherapies for mRCC are being considered as an index intervention in a separate Cochrane Review (Unverzagt 2017).
See Table 19 for a list of targeted agents to be sought, although additional targeted agents were identified during the search process. Studies in which maintenance therapy by a targeted agent was the randomised variable were eligible. Studies of dose or schedule of a targeted agent were eligible. There were no restrictions on drug route, dose, or schedule.
1. Individual targeted agents to be searched.
| Axitinib |
| Bevacizumab |
| Dovitinib |
| Erlotinib |
| Everolimus |
| Lapatinib |
| Pazopanib |
| Sorafenib |
| Sunitinib |
| Temsirolimus |
| Tivozanib |
| Other agents identified during search |
We investigated the following comparisons of target agents listed in Table 19 versus control/comparator.
Intervention
Targeted agent
Comparator
Targeted agent other than the ones used in intervention
Targeted agent in combination with immunotherapy
Immunotherapy
Combinations of immunotherapy
Placebo
We considered whether the control arm has been validated by a prior randomised study.
Minimum duration of intervention
Minimum duration of intervention was four weeks.
Minimum duration of follow‐up
Minimum duration of follow‐up was 12 weeks. We evaluated extended follow‐up periods after the trial termination only for adverse events.
Specific exclusion criteria
Studies observing neoadjuvant or adjuvant treatment or both with targeted agents were not eligible for analysis.
Types of outcome measures
Studies had to assess at least one efficacy outcome by allocation arm. We examined 'quality of life' outcomes where available, with reference to minimally important clinical differences where known for the assessment tools used. We evaluated adverse events in all studies. Our selection of outcomes for GRADE assessment was based on discussions amongst an expert panel (EAU panel) and authors of the previous review, and reflects outcomes of importance to stakeholders including patients, clinicians and healthcare providers.
Primary outcomes
Progression‐free survival (PFS)
Overall survival (OS)
Serious adverse events (SAEs; Grade 3 or 4)
Secondary outcomes
Health‐related quality of life (QoL)
Response rate
Minor adverse events (minor AEs; Grade 1 or 2)
Method and timing of outcome measurement
PFS: time from date of randomisation to date of clinical or radiological progression
OS: length of time from date of randomisation that participants are still alive
SAEs: all adverse events measured at any time that needed surgical, endoscopic, radiological or anesthesiological intervention, as well as any life‐threatening complications after participants received at least one treatment in intervention or comparator groups, classified by Common Terminology Criteria for Adverse Events (CTCAE)
QoL: evaluated by a validated instrument such as Supplementary Quality of Life Questionnaire (SQLQ), Functional Assessment of Cancer Therapy (FACT), Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI) or European Quality of Life‐5 Dimensions (EQ‐5D). If available, we focused on data of pre‐ to post‐treatment evaluation
Response rate: measured by Response Evaluation Criteria in Solid Tumors (RECIST) or modified RECIST criteria (Eisenhauer 2009)
Minor AEs: all adverse events measured at any time that could be managed by observation or pharmacological treatment after participants received at least one treatment in intervention or comparator groups, classified by Common Terminology Criteria for Adverse Events (CTCAE)
We considered a 5% absolute risk difference as clinically important for primary outcomes (PFS, OS and SAEs); we considered a 10% absolute risk difference as clinically important for the secondary outcomes of response rate and minor AEs. We used published threshold for QoL instruments.
If time‐to‐event data were not available, we tried to assess the number of events per total for dichotomised outcomes at certain time points (e.g. at one, two, three, four, five years, or at the longest reported follow‐up).
Main outcomes for 'Summary of findings' table
Progression‐free survival
Overall survival
Serious adverse events
Quality of life
Search methods for identification of studies
Overall time frame: we conducted a search from 1 January 2000 (we found no earlier studies in the previous version of this review) to an agreed cut‐off date that was at least one month before the date of search, to allow for indexing. We initially compared duplicate searches from separate time segments for consistency. For example the current authors have completed a search to 18 June 2020 using the algorithm in Appendix 1, and the Canadian authors have searched to 30 June 2010 as described previously (Coppin 2008, electronically updated to 30 June 2011 for Coppin 2011).
There were no restrictions by language or publication status.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and LILACS databases, as well as trial registers ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Searching other resources
We handsearched abstracts in the proceedings of the annual meetings of the American Urological Association, the European Cancer Conference (ECCO), the European Society of Medical Oncology (ESMO), and the American Society of Clinical Oncology (ASCO), all from 2000 to current year; and the annual ASCO genitourinary meeting (2008 to current year)
We handsearched the bibliographies of included primary studies and of recent systematic reviews of targeted therapies for metastatic or advanced renal cell carcinoma
We consulted clinical experts (EAU panel) to identify additional potentially important or seminal studies which may have been missed by the electronic searches
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, systematic reviews, meta‐analyses and health technology assessment reports. We also contacted authors of included trials to identify any additional information on the retrieved trials, and to determine if further trials exist that we may have missed. We also searched databases from regulatory agencies (European Medicines Agency (EMA) and US Food and Drugs Administration (FDA)) (Hart 2012; Schroll 2015).
Data collection and analysis
Selection of studies
Inclusion and exclusion of studies
Two review authors (FH; and LM or TL or AB or BL) independently conducted searches, assessed full‐text records, and independently mapped records to potentially eligible studies for inclusion/exclusion. We resolved disagreements by discussion.
We referred to trials by their eight‐digit NCT number where known. We classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a). We documented the search process in a study flow diagram.
We document reasons for exclusion of identified studies not suitable for this review in the Excluded studies table. We included studies that did not report on our primary or secondary outcomes, and considered them for qualitative analysis.
Data extraction and management
Two review authors (FH; and LM or TL or AB) independently extracted data using an agreed template which we had piloted, and resolved any disagreements by consensus, with recourse to a third review author (TL or AB) if needed. We constructed a master database of consensus‐agreed data, which was available to all review authors.
Data extraction fields for each study included:
basic study design features (e.g. parallel‐group randomised trial);
dates when the study was conducted;
study setting;
participant eligibility criteria and actual accrual by arm for age, race, gender, performance status, prior nephrectomy, prior systemic therapy, histologic subtype, and prognostic risk method and distribution;
stratification parameters, if any;
detailed interventions, including criteria for discontinuing therapy and cross‐over to the investigational arm;
the sample size for each included study and for each intervention/comparator group;
details (such as dose, route, frequency, duration, as applicable) of each intervention/comparator relevant to this review;
treatment delivery evaluation such as time point of administration and masking of treatment in interventional/comparator groups;
frequency and protocol status (e.g. planned versus later protocol modification) of cross‐over to the investigational arm;
details of the outcome definition for outcomes relevant to this review that were assessed in each study, method of outcome measurement for each outcome, timing of outcome measurement for each outcome, subgroups relevant to this review that were assessed for each outcome;
reported statistics for each time‐dependent outcome, i.e. hazard ratio and two‐sided log rank P value;
all adverse events reported by allocation;
study funding sources;
details of declarations of interest among the trialists.
We attempted to contact study investigators to obtain missing data for primary outcomes for eligible studies.
We report identified studies in the Characteristics of included studies table. If an eligible trial was ongoing and did not report any results, we collected information in the Characteristics of ongoing studies table.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximised the information yield by collating all available data and used the most complete data set aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary trial and trial documents of included trials (such as trial registry information) as secondary references under the study ID of the included trial. We also listed duplicate publications, companion documents, multiple reports of a trial and trial documents of excluded trials (such as trial registry information) as secondary references under the study ID of the excluded trial.
Data from clinical trial registers
In cases where data of included trials were available as study results in clinical trial registers such as ClinicalTrials.gov or similar resources, we made full use of this information and extracted data. If there was also a full publication of the trial, we collated and critically appraised all available data. If an included trial was marked as a completed study in a clinical trial register but no additional information (study results, publication or both) was available, we added this trial to the table Characteristics of studies awaiting classification.
Assessment of risk of bias in included studies
Two review authors (FH; and TL or ECH) independently used the latest version of the Cochrane tool for assessing risk of bias to construct a 'Risk of bias' table for each study, resolving disagreements by discussion (Higgins 2017b). If needed, a third review author (either AB or BL) was involved to enable us to reach consensus. We rated the following domains at low, high, or unclear risk of bias.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective reporting
Other potential sources of bias
We assessed the 'Risk of bias' domains 'Blinding of participants and personnel', 'Blinding of outcome assessment', and 'Incomplete outcome data' on an outcome‐specific basis, grouping subjective outcomes and objective outcomes for the blinding domains, and grouping outcomes according to similar completeness of data for the outcome‐specific assessment of 'Incomplete outcome data'. We regarded all outcomes except for 'Overall survival of the total population' as susceptible to performance bias and detection bias. We summarised the risk of bias across domains for each outcome in each included study. We assessed the risk of attrition bias in three combined outcome groups that we defined by oncological, adverse event and quality‐of‐life outcomes. We present our judgements in a 'Risk of bias' summary and 'Risk of bias' graph.
Measures of treatment effect
When at least two included trials were available for a comparison and a given outcome, we tried to express dichotomous data as a risk ratio (RR) or odds ratio (OR) with 95% confidence intervals (CIs). For continuous outcomes measured on the same scale we estimated the intervention effect using the mean difference (MD) with 95% CIs. For continuous outcomes measuring the same underlying concept but using different measurement scales, we calculated the standardised mean difference (SMD). We expressed time‐to‐event data as a hazard ratio (HR) with 95% CIs.
Unit of analysis issues
If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single pairwise comparison or appropriately reduced the sample size so that the same participants do not contribute to multiple comparisons (splitting the 'shared' group into two or more groups). While the latter approach offered some solution to adjusting the precision of the comparison, it did not account for correlation arising from the same set of participants being in multiple comparisons (Higgins 2017a).
Dealing with missing data
We planned to perform intention‐to‐treat analyses where data were available; however, we did not impute missing data. We included studies that combine outcomes from metastatic and locally‐advanced disease in tabulations if the locally‐advanced subgroup is documented as less than 20% of the total participants randomised; we considered other studies separately.
Whenever possible, we obtained missing data from the authors of the included trials. We carefully evaluated important numerical data such as screened, randomly‐assigned participants as well as intention‐to‐treat, and 'as treated' and 'per protocol' populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up, withdrawals), and we critically appraised issues concerning missing data and use of imputation methods (e.g. last observation carried forward).
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis. We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also considered the I² statistic, which quantifies inconsistency across trials, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpret the I² statistic as follows.
0% to 40%: may not be important
30% to 60%: represents moderate heterogeneity
50% to 90%: represents substantial heterogeneity
75% to 100%: represents considerable heterogeneity
We attempted to determine possible reasons for heterogeneity by examining individual study and subgroup characteristics.
Assessment of reporting biases
We did not find 10 or more trials that investigate a particular outcome, and did not use funnel plots to assess small‐trial effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias, so we were cautious in our interpretation of results (Sterne 2011).
Data synthesis
We conducted (or displayed) a meta‐analysis only if we judged participants, interventions, comparisons and outcomes to be sufficiently similar to ensure an answer that is clinically meaningful. Unless good evidence showed homogeneous effects across trials, we primarily summarised low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration for the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). This specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). For rare events such as event rates below 1% we planned to use the Peto odds ratio, provided that there was no substantial imbalance between intervention and comparator group sizes, and that intervention effects were not exceptionally large. We also performed statistical analyses using Review Manager 5 software provided by Cochrane (Review Manager 2020), according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a).
Statistical analysis
We anticipated analysis of four types of outcomes: categorical outcomes, such as tumour remission; single time‐dependent outcomes, such as OS; quality‐of‐life surveys; and toxicity tables. Of these, methods for analysis of dichotomous outcomes were fully covered by standard Cochrane procedures (Deeks 2017). We considered multidimensional quality‐of‐life and toxicity outcomes individually. Time‐dependent outcomes were potentially problematic. Where only a single study was available for a comparison, we accepted any standard statistical analysis, such as the log‐rank test used by the author, but we preferred the hazard ratio and log‐rank testing. For meta‐analysis of multiple studies of the same type, we used extraction of a dichotomous endpoint such as survival at one year from randomisation (see also Measures of treatment effect above).
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis for the following.
Nephrectomy done or not done prior to treatment
ECOG performance status (0, 1 or 2)
Sensitivity analysis
We planned a sensitivity analysis for studies that were at a high risk of bias for sequence generation, allocation concealment and blinding versus studies at low risk of bias. We planned to conduct a separate meta‐analysis for validation of results of studies at low risk of bias only.
Summary of findings and assessment of the certainty of the evidence
We present results for the outcomes as described in the Types of outcome measures section. We present the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account five criteria not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (ECH, FH) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low', using GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT 2015). We resolved any disagreements by discussion or, if needed, by recourse to a third review author (TL, AB). For each comparison, we present a summary of the evidence for the main outcomes in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2017). We justified all decisions to downgrade the quality of trials using footnotes, and we made comments to aid the reader's understanding of the Cochrane Review where necessary.
Results
Description of studies
Our literature search identified 7738 records eligible for screening. We excluded 7668 records that did not meet our predefined inclusion criteria from further evaluation. Of these, we marked four as ongoing and included them in the Characteristics of ongoing studies section.
Results of the search
We found 70 titles and abstracts to be eligible for full‐text evaluation. Of these, we selected 18 trials for qualitative synthesis and included four of them in a quantitative analysis. We excluded 52 articles; our reasons are presented in Figure 1 and under Characteristics of excluded studies.
1.
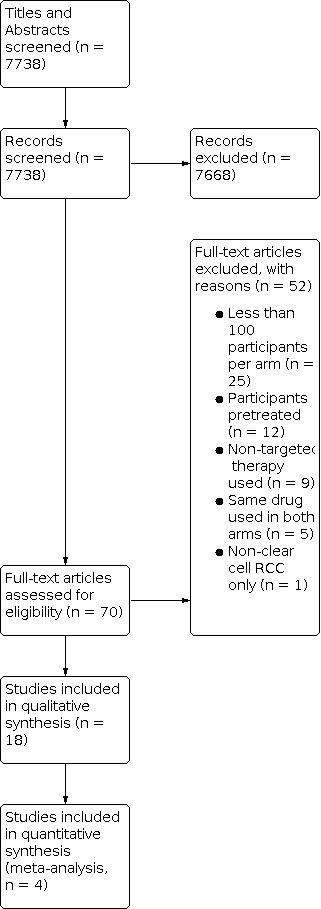
Study flow diagram.
Included studies
Additional information on included studies is available in the following tables: Characteristics of included studies; Participants disposition (Table 20); and Baseline characteristics (Table 21).
2. Participants disposition.
| Studies | Intervention(s)/ Comparator(s) | Randomised (N) | Received treatment (N) | Discontinued treatment (N) | Efficacy analysis (N) | Safety analysis (N) |
| Eichelberg 2015 | Soreafenib/ Sunitinib | 182 | 177 | 161 | 182 | 177 |
| Sunitinib/ Sorafenib | 183 | 176 | 156 | 183 | 176 | |
| Escudier 2010 | Bevacizumab + IFN‐a2a | 327 | 325 | 206 | 327 | 337 |
| IFN‐a2a + Placebo | 322 | 316 | 274 | 322 | 304 | |
| Escudier 2017 1 | Sunitinib | 546 | 535 | 438 | 546 | 535 |
| Nivolumab + Ipilimumab | 550 | 547 | 419 | 550 | 547 | |
| Hudes 2007 | Temsirolimus | 209 | 208 | 199 | 209 | 208 |
| Temsirolimus + Interferon | 210 | 208 | 193 | 210 | 208 | |
| Interferon | 207 | 200 | 194 | 207 | 200 | |
| McDermott 2017 | Sunitinib | 101 | 100 | 83 | 101 | 100 |
| Atezolizumab + Bevacizumab | 101 | 101 | 69 | 101 | 101 | |
| Atezolizumab | 103 | 103 | 80 | 103 | 103 | |
| Motzer 2010 | Sunitinib | 375 | 375 | 127 | 375 | 375 |
| IFN‐a2a | 375 | 360 | 234 | 375 | 360 | |
| Motzer 2013a | Pazopanib | 557 | 554 | 486 | 557 | 554 |
| Sunitinib | 553 | 548 | 483 | 553 | 548 | |
| Motzer 2013b | Tivozanib | 260 | 259 | 154 | 260 | 259 |
| Sorafenib | 257 | 257 | 192 | 257 | 257 | |
| Motzer 2014 | Sunitinib/ Everolimus | 233 | 231 | 192 | 233 | 231 |
| Everolimus/ Sunitinib | 238 | 238 | 201 | 238 | 238 | |
| Motzer 2019 | Sunitinib | 444 | 439 | 227 | 444 | 439 |
| Avelumab + Axitinib | 442 | 434 | 187 | 442 | 434 | |
| Ravaud 2015 | Everolimus + Bevacizumab | 182 | 180 | 175 | 182 | 180 |
| Interferon + Bevacizumab | 183 | 181 | 175 | 183 | 181 | |
| Retz 2019 | Sorafenib/ Pazopanib | 189 | 183 | 115 | 189 | 183 |
| Pazopanib/ Sorafenib | 188 | 183 | 110 | 188 | 183 | |
| Rini 2008 | Bevacizumab + IFN‐a2b | 369 | 366 | 355 | 369 | 366 |
| IFN‐a2b | 363 | 350 | 355 | 363 | 349 | |
| Rini 2014 | Temsirolimus + Bevacizumab | 400 | 393 | 372 | 400 | 393 |
| Temsirolimus + Interferon | 391 | 391 | 354 | 391 | 391 | |
| Rini 2016 | Sunitinib | 135 | 130 | 23 | 135 | 132 |
| IMA901 + Sunitinib | 204 | 185 | 28 | 204 | 202 | |
| Rini 2019a | Sunitinib | 429 | 425 | 242 | 429 | 425 |
| Pembrolizumab + Axitinib | 432 | 429 | 176 | 432 | 429 | |
| Rini 2019b | Sunitinib | 461 | 446 | 308 | 461 | 446 |
| Atezolizumab + Bevacizumab | 454 | 451 | 265 | 454 | 451 | |
| Sternberg 2010 | Pazopanib | 290 | 290 | 227 | 290 | 290 |
| Placebo | 145 | 145 | 131 | 145 | 145 | |
| Total | 11590 | 11419 | 8366 | 11590 | 11437 | |
1 Included overall population; but in the data and analyses section and summary of findings table, we used IMDC intermediate and poor risk patients for efficacy analysis.
3. Baseline characteristics.
| Studies | Phase of study | Accrual | Blinding | RCC subtype | Prior therapy | Intervention |
| Comparator | ||||||
| Eichelberg 2015 | 3 | Feb 2009 to Dec 2011 | open label study | any, 87% clear cell | naïve | Soreafenib/Sunitinib |
| Sunitinib/Sorafenib | ||||||
| Escudier 2010 | 3 | Jun 2004 to Oct 2005 | double‐blind study | clear cell | naïve | Bevacizumab + IFN‐a2a |
| IFN‐a2a + Placebo | ||||||
| Escudier 2017 | 3 | Oct 2014 to Feb 2016 | open label study | clear cell | naïve | Sunitinib |
| Nivolumab + Ipilimumab | ||||||
| Hudes 2007 | 3 | Jul 2003 to Apr 2005 | open label study | any, 80% clear cell | naïve | Temsirolimus |
| Temsirolimus + Interferon | ||||||
| Inferferon | ||||||
| McDermott 2017 | 2 | Jan 2014 to Mar 2015 | open label study | clear cell | naïve | Sunitinib |
| Atezolizumab + Bevacizumab | ||||||
| Atezolizumab | ||||||
| Motzer 2010 | 3 | Aug 2004 to Oct 2005 | radiologic assessment | clear cell | naïve | Sunitinib |
| IFN‐a2a | ||||||
| Motzer 2013a | 3 | Aug 2008 to Sep 2011 | open label study | clear cell | naïve | Pazopanib |
| Sunitinib | ||||||
| Motzer 2013b | 2 | Feb 2010 to Aug 2010 | open label study | clear cell | naïve | Tivozanib |
| Sorafenib | ||||||
| Motzer 2014 | 2 | Sep 2009 to Jun 2012 | open label study | any, 85% clear cell | naïve | Sunitinib/ Everolimus |
| Everolimus/ Sunitinib | ||||||
| Motzer 2019 | 3 | Mar 2016 to Dec 2017 | open label study | clear cell | naïve | Sunitinib |
| Avelumab + Axitinib | ||||||
| Ravaud 2015 | 2 | ‐ | open label study | any 96% clear cell | naïve | Everolimus + Bevacizumab |
| Interferon + Bevacizumab | ||||||
| Retz 2019 | 3 | Jun 2012 to Nov 2016 | open label study | any, 87% clear cell | naïve | Sorafenib/ Pazopanib |
| Pazopanib/ Sorafenib | ||||||
| Rini 2008 | 3 | Oct 2003 to Jul 2005 | open label study | clear cell | naïve | Bevacizumab + IFN‐a2b |
| IFN‐a2b | ||||||
| Rini 2014 | 3 | Apr 2008 to Oct 2010 | open label study | any, 80% clear cell | naïve | Temsirolimus + Bevacizumab |
| Bevacizumab + Inferferon | ||||||
| Rini 2016 | 3 | Dec 2010 to Dec 2012 | open label study | clear cell | naïve | Sunitinib |
| IMA901 + Sunitinib | ||||||
| Rini 2019a | 3 | Oct 2016 to Jan 2018 | open label study | clear cell | naïve | Sunitinib |
| Pembrolizumab + Axitinib | ||||||
| Rini 2019b | 3 | May 2015 to Oct 2016 | open label study | clear cell | naïve | Sunitinib |
| Atezolizumab + Bevacizumab | ||||||
| Sternberg 2010 | 3 | Apr 2006 to Apr 2007 | double‐blind study | clear cell | 54% naive | Pazopanib |
| Placebo | ||||||
| ‐ denotes not reported | ||||||
IFN: interferon; RCC: renal cell carcinoma
Source of data
In total we included 18 trials in this review, all of which we identified by electronic database search. All trials were available as peer‐reviewed publications and published in English. We contacted corresponding authors of nine trials to obtain additional information on results (Escudier 2017; Hudes 2007; Motzer 2010; Motzer 2014; Ravaud 2015; Retz 2019; Rini 2008; Rini 2014; Sternberg 2010). We received three replies (Retz 2019; Rini 2014; Sternberg 2010); and could include additional data for one trial (Retz 2019).
Study design and settings
Two of the 18 included randomised controlled trials were conducted with a double blind design in which participants and personnel were unaware of allocated intervention (Escudier 2010; Sternberg 2010). All other studies were open label without masking treatments. Three trials used a phase 2 trial design (McDermott 2018; Motzer 2014; Ravaud 2015); all others were performed in a phase 3 setting. Eight trials allowed a cross‐over between treatment arms or to active treatment if disease progression occurred (Eichelberg 2015; Escudier 2010; McDermott 2018; Motzer 2010; Motzer 2013b; Motzer 2014; Retz 2019; Sternberg 2010). All trials were multicentre studies with an accrual period between 2003 and 2019.
Participants
Combining the numbers of all studies, there were 11,590 participants included of which 11,419 received the allocated treatment. All randomised participants were included in the efficacy analysis, 98% were assessed for safety. Most trials allowed only participants with a clear cell histology or a clear cell component. Six trials allowed any histology but 85% of the participants were diagnosed with clear cell renal cell carcinoma (Eichelberg 2015; Hudes 2007; Motzer 2014; Ravaud 2015; Retz 2019; Rini 2014). Median age amongst the total population was 61 years; 72% were males; and 9048 were treated with a nephrectomy prior to systemic therapy.
Interventions
Most trials had two arms comparing either two active treatments or an active treatment against placebo. Two trials had a design with multiple comparisons (Hudes 2007; McDermott 2018).
Five trials compared two different targeted therapies against each other; sorafenib versus sunitinib (Eichelberg 2015), pazopanib versus sunitinib (Motzer 2013a), tivozanib versus sorafenib (Motzer 2013b), sorafenib versus pazopanib (Retz 2019), sunitinib versus everolimus (Motzer 2014).
One trial compared pazopanib against a placebo (Sternberg 2010).
Four trials assessed the effectiveness of sunitinib against targeted immunotherapy in combination with targeted therapy. Avelumab and axitinib (Motzer 2019), pembrolizumab and axitinib (Rini 2019a), and atezolizumab and bevacizumab (Rini 2019b; McDermott 2018) were used as comparators. McDermott 2018 had a three‐arm design which even included a direct comparison of sunitinib against atezolizumab. Another trial compared sunitinib against the combination of nivolumab and ipilimumab (Escudier 2017).
One trial compared sunitinib against a combination of tumour vaccine, chemotherapy and sunitinib (Rini 2016).
Interferon alpha was compared to targeted therapy or combinations of targeted therapy in four studies: sunitinib (Motzer 2010); temsirolimus (Hudes 2007); temsirolimus and interferon alpha (Hudes 2007); and bevacizumab and interferon alpha (Escudier 2010; Rini 2008).
Combinations of targeted therapies were used in two trials. Rini 2014 compared temsirolimus and bevacizumab against bevacizumab and interferon alpha; Ravaud 2015 compared everolimus and bevacizumab against interferon alpha and bevacizumab.
Outcomes
All included studies reported our primary outcomes PFS, OS and SAEs as well as response rates and minor AEs which were predefined secondary outcomes. An assessment of QoL was performed in 11 trials (Escudier 2017; Hudes 2007; McDermott 2018; Motzer 2010; Motzer 2013a; Motzer 2013b; Motzer 2014; Retz 2019; Rini 2014; Rini 2019b; Sternberg 2010).
Excluded studies
We excluded 52 trials at the stage of full‐text screening. The main reasons for exclusion were: less than 100 participants in one treatment arm in 25 trials (Broom 2015; Bukowski 2007; Choueiri 2017; Cirkel 2016; Eisen 2015; Escudier 2009; Flaherty 2015; Hainsworth 2015; Jonasch 2010; Mulders 2012; Négrier 2011; Nosov 2012; Pal 2015; Pili 2015; Powles 2014; Powles 2016a; Powles 2016b; Procopio 2011; Ratain 2006; Rini 2013; Tannir 2016; Tannir 2018; Tomita 2014; Twardowski 2015; Yang 2003); participants were not treatment naïve in 12 studies (Choueiri 2015; Dorff 2015; Escudier 2010a; Hutson 2013; Hutson 2014; Jonasch 2017; Motzer 2008; Motzer 2014b; Motzer 2015a; Motzer 2015b; Motzer 2015c; Rini 2011); the intervention or comparison did not include a targeted therapy in nine trials (Escudier 2007; Gordon 2004; Hawkins 2016; Lee 2006; Madhusudan 2004; Ravaud 2008, Rini 2012; Srinivas 2005; Stadler 2005); both intervention and comparison arms used the same drug with different dosages in five trials (Atkins 2004; Bracarda 2010; Ebbinghaus 2007; Lee 2015; Motzer 2012); and one trial included only non‐clear cell renal cell carcinoma participants (Armstrong 2016).
Risk of bias in included studies
For details, please refer to 'Characteristics of included studies' section, the 'Risk of bias' table, and each 'Summary of findings table', as well as Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Categories: green point (+) = low risk of bias; yellow point (?) = unclear risk of bias; red point (‐) = high risk of bias.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We judged 15 trials to have a low risk of bias (Eichelberg 2015; Escudier 2010; Escudier 2017; Hudes 2007; McDermott 2018; Motzer 2013a; Motzer 2013b; Motzer 2014; Motzer 2019; Rini 2008; Rini 2014; Rini 2016; Rini 2019a; Rini 2019b; Sternberg 2010). The remaining three trials had an unclear risk of bias.
Allocation concealment
We judged 14 trials to have a low risk of bias (Eichelberg 2015; Escudier 2010; Escudier 2017; Hudes 2007; Motzer 2013a; Motzer 2013b; Motzer 2014; Motzer 2019; Rini 2008; Rini 2014; Rini 2016; Rini 2019a; Rini 2019b; Sternberg 2010). Three trials had an unclear risk of bias (Motzer 2010; Ravaud 2015; Retz 2019); and we judged one trial to have a high risk of bias (McDermott 2018).
Blinding
Blinding of participants and personnel
For objective outcomes we judged all studies to have a low risk of bias. For subjective outcomes we rated only two trials as low risk of bias (Escudier 2010; Sternberg 2010); and one we rated unclear risk of bias (Ravaud 2015). The remaining trials had a high risk of bias.
Blinding of outcome assessment
We rated all trials at low risk of bias for assessment of objective outcomes. Ten trials had a high risk of bias (Eichelberg 2015; Escudier 2017; Hudes 2007; McDermott 2018; Motzer 2013a; Motzer 2013b; Motzer 2014; Ravaud 2015; Rini 2008; Rini 2019b); one we rated unclear risk of bias (Retz 2019); and seven had a low risk of bias for subjective outcomes.
Incomplete outcome data
All studies had a low risk of bias for OS and PFS. One trial we judged to have a high risk of bias for tumour response data (Hudes 2007); the remaining trials we judged as low risk of bias. For QoL, we rated four trials as unclear risk of bias (Escudier 2017; Motzer 2010; Ravaud 2015; Rini 2019b); three trials at high risk of bias (Hudes 2007; Motzer 2013a; Retz 2019); five trials we judged at low risk of bias (.McDermott 2018; Motzer 2013b; Motzer 2014; Rini 2014; Sternberg 2010); and the remaining trials did not assess the QoL.
Selective reporting
Two studies had an unclear risk of bias (Motzer 2019; Retz 2019); the remaining 16 we judged at low risk because both the outcomes reported and the analytic approach in the published report matched those of the predefined protocol.
Other potential sources of bias
We found eight trials to have a low risk of bias (Escudier 2017; Hudes 2007; Motzer 2019; Retz 2019; Rini 2008; Rini 2019a; Rini 2019b; Sternberg 2010); six trials we rated unclear risk of bias (Eichelberg 2015; Escudier 2010; McDermott 2018; Motzer 2013a; Ravaud 2015; Rini 2014); and four studies had a high risk of bias (Motzer 2010; Motzer 2013b; Motzer 2014; Rini 2016)
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18
We performed two meta‐analyses using studies which had the same population and the same comparison (Escudier 2010; McDermott 2018; Rini 2008; Rini 2019b). The remaining trials had considerable clinical heterogeneity and we judged them inappropriate for pooling of data and meta‐analysis.
1. Sorafenib versus sunitinib
Please refer to Table 1.
Primary outcomes
Progression‐free survival (PFS)
Sorafenib may reduce PFS when compared to sunitinib (HR 1.19, 95% CI 0.92 to 1.53; 1 study, 365 participants; low‐certainty evidence; Analysis 1.1) although the CI also includes the possibility of no effect. Based on a control event risk of 340 per 1000 in this trial at 10 months, this corresponds to 63 fewer participants experiencing PFS (95% CI 148 fewer to 31 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with little to no increase in PFS.
1.1. Analysis.

Comparison 1: Sorafenib versus Sunitinib, Outcome 1: Progression‐free survival
Overall survival (OS)
We are very uncertain how sorafenib compares to sunitinib for OS (HR 0.99, 95% CI 0.74 to 1.33; 1 study, 365 participants; very low‐certainty evidence; Analysis 1.2). We rated the certainty of evidence as very low due to study limitations (other bias, downgraded one level) and imprecision (downgraded two levels), given that the CI was compatible with both an appreciable reduction in OS as well as an appreciable increase in OS.
1.2. Analysis.

Comparison 1: Sorafenib versus Sunitinib, Outcome 2: Overall survival
Serious adverse events (SAEs, assessed with: CTCAE v3.0)
We are very uncertain how sorafenib compares to sunitinib with regard to SAEs (RR 0.99, 95% CI 0.85 to 1.14; 1 study, 353 participants; very low certainty evidence; Analysis 1.3). We rated the certainty of evidence as very low due to study limitations (performance, detection and other bias, downgraded one level) and imprecision (downgraded two levels), given that the CI was compatible with both an appreciable reduction in SAEs as well as an appreciable increase in SAEs.
1.3. Analysis.

Comparison 1: Sorafenib versus Sunitinib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
Quality of life (QoL)
We found no studies that reported this outcome.
Response rate (assessed with: RECIST version not reported)
Sorafenib may result in little to no difference in response rate as compared to sunitinib (RR 1.07, 95% CI 0.78 to 1.47; 1 study, 353 participants; low‐certainty evidence; Analysis 1.4). Based on the control event risk of 290 per 1000 in this trial, this corresponds to 20 more response (95% CI 64 fewer to 136 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in the response rate.
1.4. Analysis.

Comparison 1: Sorafenib versus Sunitinib, Outcome 4: Response rate
Minor adverse events (AEs, assessed with: CTCAE v3.0)
Sorafenib may result in little to no difference in minor AEs as compared to sunitinib (RR 1.13, 95% CI 0.77 to 1.65; 1 study, 353 participants; low‐certainty evidence; Analysis 1.5). Based on the control event risk of 216 per 1000 in this trial, this corresponds to 28 more minor AEs (95% CI 50 fewer to 140 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in minor AEs.
1.5. Analysis.

Comparison 1: Sorafenib versus Sunitinib, Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
2. Pazopanib versus sunitinib
Please refer to Table 2.
Primary outcomes
PFS
Pazopanib may result in little to no difference in PFS as compared to sunitinib (HR 1.05, 95% CI 0.90 to 1.23; 1 study, 1110 participants; low‐certainty evidence; Analysis 2.1). Based on the control event risk of 420 per 1000 in this trial at 12 months, this corresponds to 18 fewer participants experiencing PFS (95% CI 76 fewer to 38 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable reduction in PFS.
2.1. Analysis.

Comparison 2: Pazopanib versus Sunitinib, Outcome 1: Progression‐free survival
OS
Pazopanib may result in little to no difference in OS compared to sunitinib (HR 0.92, 95% CI 0.80 to 1.06; 1 study, 1110 participants; low‐certainty evidence; Analysis 2.2). Based on the control event risk of 550 per 1000 in this trial at 12 months, this corresponds to 27 more participants experiencing OS (95% CI 19 fewer to 70 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (other bias) and imprecision, given that the CI was also compatible with an appreciable increase in OS.
2.2. Analysis.

Comparison 2: Pazopanib versus Sunitinib, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v3.0)
Pazopanib may result in little to no difference in SAEs as compared to sunitinib (RR 1.01, 95% CI 0.94 to 1.09; 1 study, 1102 participants; low‐certainty evidence; Analysis 2.3). Based on the control event risk of 734 per 1000 in this trial, this corresponds to seven more participants experiencing SAEs (95% CI 44 fewer to 66 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in SAEs.
2.3. Analysis.

Comparison 2: Pazopanib versus Sunitinib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Pazopanib may increase QoL as compared to sunitinib (MD 3.60, 95% CI 1.76 to 5.44; 1 study, 467 participants; low‐certainty evidence; Analysis 2.4) assessed with FACIT‐F (scale 0 to 52; higher scores indicating less fatigue; MCID: 3 points). We rated the certainty of evidence as low due to study limitations (performance, detection, attrition and other bias), and imprecision, given that the CI was also compatible with no increase in QoL.
2.4. Analysis.

Comparison 2: Pazopanib versus Sunitinib, Outcome 4: Health‐related quality of life
Response rate (assessed by RECIST v1.0)
Pazopanib may result in little to no difference in response rate as compared to sunitinib (RR 1.24, 95% CI 1.02 to 1.50; 1 study, 1110 participants; low‐certainty evidence; Analysis 2.5). Based on the control event risk of 248 per 1000 in this trial, this corresponds to 59 more response (95% CI 5 more to 124 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in the response rate.
2.5. Analysis.

Comparison 2: Pazopanib versus Sunitinib, Outcome 5: Response rate
Minor AEs (assessed with: CTCAE v3.0)
Pazopanib probably results in little to no difference in minor AEs as compared to sunitinib (RR 1.00, 95% CI 0.81 to 1.23; 1 study, 1102 participants; moderate‐certainty evidence; Analysis 2.6). Based on the control event risk of 237 per 1000 in this trial, this corresponds to no fewer minor AEs (95% CI 45 fewer to 55 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance, detection and other bias).
2.6. Analysis.

Comparison 2: Pazopanib versus Sunitinib, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analyses
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analyses
We were unable to perform a sensitivity analysis because there was only one study.
3. Tivozanib versus sorafenib
Please refer to Table 3.
Primary outcomes
PFS
Tivozanib may extend PFS as compared to sorafenib (HR 0.79, 95% CI 0.64 to 0.99; 1 study, 517 participants; low‐certainty evidence; Analysis 3.1). Based on the control event risk of 360 per 1000 in this trial at 12 months, this corresponds to 86 more participants experiencing PFS (95% CI 4 more to 160 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with a little to no increase in PFS.
3.1. Analysis.

Comparison 3: Tivozanib versus Sorafenib, Outcome 1: Progression‐free survival
OS
Tivozanib may reduce OS as compared to sorafenib (HR 1.25, 95% CI 0.95 to 1.64; 1 study, 517 participants; low‐certainty evidence; Analysis 3.2) although the CI also includes the possibility of no effect. Based on the control event risk of 620 per 1000 in this trial at 24 months, this would result in 70 fewer OSs (95% CI 163 fewer to 15 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (other bias) and imprecision, given that the CI was also compatible with little to no increase in OS.
3.2. Analysis.

Comparison 3: Tivozanib versus Sorafenib, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v3.0)
Tivozanib may reduce SAEs as compared to sorafenib (RR 0.85, 95% CI 0.74 to 0.97; 1 study, 516 participants; low‐certainty evidence; Analysis 3.3). Based on the control event risk of 689 per 1000 in this trial, this corresponds to 103 fewer SAEs (95% CI 179 fewer to 21 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with little to no reduction in SAEs.
3.3. Analysis.

Comparison 3: Tivozanib versus Sorafenib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Tivozanib may result in little to no difference in QoL (assessed with EQ‐5D; scale: −0.59 to 1; higher values reflect better QoL; MCID 0.06) as compared to sorafenib (MD 0.01, 95% CI −0.05 to 0.07; 1 study, 506 participants; low‐certainty evidence; Analysis 3.4). We rated the certainty of evidence as low due to study limitations (performance, detection and other bias), and imprecision, given that the CI was also compatible with an increase in QoL.
3.4. Analysis.

Comparison 3: Tivozanib versus Sorafenib, Outcome 4: Health‐related quality of life
Response rate (assessed by RECIST v1.0)
Tivozanib may increase the response rate as compared to sorafenib (RR 1.42, 95% CI 1.07 to 1.88; 1 study, 517 participants; low‐certainty evidence; Analysis 3.5). Based on the control event risk of 233 per 1000 in this trial, this corresponds to 98 more responses (16 more to 205 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with little to no increase in the response rate.
3.5. Analysis.

Comparison 3: Tivozanib versus Sorafenib, Outcome 5: Response rate
Minor AEs (assessed with: CTCAE v3.0)
Tivozanib may result in little to no difference in minor AEs as compared to sorafenib (RR 1.16, 95% CI 0.89 to 1.51; 1 study, 516 participants; low‐certainty evidence; Analysis 3.6). Based on the control event risk of 280 per 1000 in this trial, this corresponds to 45 more minor AEs (95% CI 31 fewer to 143 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in minor AEs.
3.6. Analysis.

Comparison 3: Tivozanib versus Sorafenib, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analyses
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analyses
We were unable to perform a sensitivity analyses because there was only one study.
4. Sorafenib versus pazopanib
Please refer to Table 4.
Primary outcomes
PFS
Sorafenib probably reduces PFS as compared to pazopanib (HR 1.92, 95% CI 1.74 to 2.11; 1 study, 377 participants; moderate‐certainty evidence; Analysis 4.1). Based on the control event risk of 380 per 1000 in this trial at 12 months, this corresponds to 224 fewer participants experiencing PFS (95% CI 250 fewer to 194 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance, detection, and reporting bias).
4.1. Analysis.

Comparison 4: Sorafenib versus Pazopanib, Outcome 1: Progression‐free survival
OS
Sorafenib may reduce OS as compared to pazopanib (HR 1.22, 95% CI 0.91 to 1.64; 1 study, 377 participants; low‐certainty evidence; Analysis 4.2) although the CI also includes the possibility of no effect. Based on the control event risk of 520 per 1000 in this trial at 24 months, this would result in 70 fewer OSs (95% CI 178 fewer to 32 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (selection and reporting bias) and imprecision, given that the CI was also compatible with little to no reduction in OS.
4.2. Analysis.

Comparison 4: Sorafenib versus Pazopanib, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v4.03)
We are very uncertain how sorafenib compares to pazopanib (RR 0.92, 95% CI 0.78 to 1.09; 1 study, 366 participants; very low certainty evidence; Analysis 4.3) with regard to SAEs. We rated the certainty of evidence as very low due to study limitations (selection, performance, detection, and reporting bias, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with both an appreciable reduction in SAEs as well as an appreciable increase in SAEs.
4.3. Analysis.

Comparison 4: Sorafenib versus Pazopanib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Sorafenib may increase QoL slightly (assessed with FACIT‐F; scale 0 to 52; higher scores indicating less fatigue; MCID: 3 points) as compared to pazopanib (MD 3.10, 95% CI −1.82 to 8.02; 1 study, 267 participants; low‐certainty evidence; Analysis 4.4). We rated the certainty of evidence as low due to study limitations (selection, performance, detection, attrition and reporting bias) and imprecision, given that the CI was also compatible with no increase in QoL.
4.4. Analysis.

Comparison 4: Sorafenib versus Pazopanib, Outcome 4: Health‐related quality of life
Response rate (assessed with RECIST v1.1)
Sorafenib may reduce the response rate as compared to pazopanib (RR 0.62, 95% CI 0.47 to 0.81; 1 study, 377 participants; low‐certainty evidence; Analysis 4.5). Based on the control event risk of 463 per 1000 in this trial, this corresponds to 176 fewer response (95% CI 245 fewer to 88 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (selection, performance, detection, and reporting bias) and imprecision, given that the CI was also compatible with no reduction in the response rate.
4.5. Analysis.

Comparison 4: Sorafenib versus Pazopanib, Outcome 5: Response rate
Minor AEs (assessed with: CTCAE v4.03)
Sorafenib may result in little to no difference in minor AEs (assessed with: CTCAE v4.03) as compared to pazopanib (RR 1.15, 95% CI 0.87 to 1.52; 1 study, 366 participants; low‐certainty evidence; Analysis 4.6). Based on the control event risk of 328 per 1000 in this trial, this corresponds to 49 more minor AEs (95% CI 43 fewer to 170 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (selection, performance, detection, and reporting bias) and imprecision, given that the CI was also compatible with an appreciable increase in minor AEs.
4.6. Analysis.

Comparison 4: Sorafenib versus Pazopanib, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analyses
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analyses
We were unable to perform a sensitivity analysis because there was only one study.
5. Sunitinib versus everolimus
Please refer to Table 5.
Primary outcomes
PFS
Sunitinib probably increases PFS as compared to everolimus (HR 0.71, 95% CI 0.59 to 0.87; 1 study, 471 participants; moderate‐certainty evidence; Analysis 5.1). Based on the control event risk of 300 per 1000 in this trial at 12 months, this corresponds to 125 participants experiencing PFS (95% CI 51 more to 191 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance, detection and other bias).
5.1. Analysis.

Comparison 5: Sunitinib versus Everolimus, Outcome 1: Progression‐free survival
OS
Sunitinib may result in little to no difference in OS as compared to everolimus (HR 0.90, 95% CI 0.72 to 1.11; 1 study, 471 participants; low‐certainty evidence; Analysis 5.2). Based on the control event risk of 470 per 1000 in this trial at 24 months, this would result in 37 more OSs (95% CI 37 fewer to 111 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (other bias) and imprecision, given that the CI was also compatible with an appreciable increase in OS.
5.2. Analysis.

Comparison 5: Sunitinib versus Everolimus, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v3.0)
Sunitinib probably increases SAEs as compared to everolimus (RR 1.34, 95% CI 1.14 to 1.59; 1 study, 469 participants; moderate‐certainty evidence; Analysis 5.3). Based on the control event risk of 471 per 1000 in this trial, this corresponds to 160 more SAEs (95% CI 66 more to 278 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance, detection and other bias).
5.3. Analysis.

Comparison 5: Sunitinib versus Everolimus, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Sunitinib may result in little to no difference in QoL (assessed with EORTC QLQ‐C30; scale: 0 to 100; high score represent better functioning, MCID: 10 points) as compared to everolimus (MD −5.00, 95% CI −10.40 to 0.40; 1 study, 288 participants; low‐certainty evidence; Analysis 5.4). We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with a decrease in QoL.
5.4. Analysis.

Comparison 5: Sunitinib versus Everolimus, Outcome 4: Health‐related quality of life
Response rate (assessed with RECIST v1.0)
Sunitinib may increase response rate as compared to everolimus (RR 3.33, 95% CI 2.06 to 5.39; 1 study, 471 participants; low‐certainty evidence; Analysis 5.5). Based on the control event risk of 80 per 1000 in this trial, this corresponds to 186 more responses (95% CI 85 more to 350 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and other bias) and imprecision, given that the CI was also compatible with little to no increase in response rate.
5.5. Analysis.

Comparison 5: Sunitinib versus Everolimus, Outcome 5: Response rate
Minor AEs (assessed with: CTCAE v3.0)
Sunitinib probably results in little to no difference in minor AEs as compared to everolimus (RR 1.02, 95% CI 0.99 to 1.04; 1 study, 469 participants; moderate‐certainty evidence; Analysis 5.6). Based on the control event risk of 971 per 1000 in this trial, this corresponds to 19 more minor AEs (95% CI 10 fewer to 39 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance, detection and other bias).
5.6. Analysis.

Comparison 5: Sunitinib versus Everolimus, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analyses
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analyses
We were unable to perform a sensitivity analysis because there was only one study.
6. Sunitinib versus avelumab + axitinib
Please refer to Table 6.
Primary outcomes
PFS
Sunitinib probably reduces PFS as compared to avelumab plus axitinib (HR 1.45, 95% CI 1.17 to 1.80; 1 study, 886 participants; moderate‐certainty evidence; Analysis 6.1). Based on the control event risk of 550 per 1000 in this trial at 12 months, this corresponds to 130 fewer participants experiencing PFS (95% CI 209 fewer to 53 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias and reporting bias).
6.1. Analysis.

Comparison 6: Sunitinib versus Avelumab + Axitinib, Outcome 1: Progression‐free survival
OS
Sunitinib may result in little to no difference in OS as compared to avelumab plus axitinib (HR 1.28, 95% CI 0.92 to 1.79; 1 study, 886 participants; low‐certainty evidence; Analysis 6.2). Based on the control event risk of 890 per 1000 in this trial at 12 months, this would result in 29 fewer OSs (95% CI 78 fewer to 8 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (reporting bias) and imprecision given that the CI was also compatible with an appreciable reduction in OS.
6.2. Analysis.

Comparison 6: Sunitinib versus Avelumab + Axitinib, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v4.03)
Sunitinib may result in little to no difference in SAEs as compared to avelumab plus axitinib (RR 1.01, 95% CI 0.93 to 1.10; 1 study, 873 participants; low‐certainty evidence; Analysis 6.3). Based on the control event risk of 705 per 1000 in this trial, this corresponds to 7 more SAEs (95% CI 49 fewer to 71 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance bias and reporting bias) and imprecision given that the CI was also compatible with an increase in SAEs.
6.3. Analysis.

Comparison 6: Sunitinib versus Avelumab + Axitinib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
We found no studies that reported this outcome.
Response rate (assessed with: RECIST v1.1)
Sunitinib probably reduces the response rate as compared to avelumab plus axitinib (RR 0.50, 95% CI 0.42 to 0.60; 1 study, 886 participants; moderate‐certainty evidence; Analysis 6.4). Based on the control event risk of 514 per 1000 in this trial, this corresponds to 257 fewer responses (95% CI 298 fewer to 205 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias and reporting bias).
6.4. Analysis.

Comparison 6: Sunitinib versus Avelumab + Axitinib, Outcome 4: Response rate
Minor AEs (assessed with CTCAE v4.03)
Sunitinib probably results in little to no difference in minor AEs as compared to avelumab plus axitinib (RR 0.97, 95% CI 0.78 to 1.19; 1 study, 873 participants; moderate‐certainty evidence; Analysis 6.5). Based on the control event risk of 290 per 1000 in this trial, this corresponds to nine fewer minor AEs (95% CI 64 fewer to 55 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias and reporting bias)
6.5. Analysis.

Comparison 6: Sunitinib versus Avelumab + Axitinib, Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analyses
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analyses
We were unable to perform a sensitivity analysis because there was only one study.
7. Sunitinib versus pembrolizumab + axitinib
Please refer to Table 7.
Primary outcomes
PFS
Sunitinib probably reduces PFS as compared to pembrolizumab plus axitinib (HR 1.45, 95% CI 1.19 to 1.76; 1 study, 861 participants; moderate‐certainty evidence; Analysis 7.1). Based on the control event risk of 590 per 1000 in this trial at 12 months, this corresponds to 125 fewer participants experiencing PFS (95% CI 195 fewer to 56 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias).
7.1. Analysis.

Comparison 7: Sunitinib versus Pembrolizumab + Axitinib, Outcome 1: Progression‐free survival
OS
Sunitinib probably reduces OS as compared to pembrolizumab plus axitinib (HR 1.90, 95% CI 1.36 to 2.65; 1 study, 861 participants; moderate‐certainty evidence; Analysis 7.2). Based on the control event risk of 880 per 1000 in this trial at 12 months, this would result in 96 fewer OSs (95% CI 167 fewer to 40 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to imprecision, given that the CI was also compatible with little to no reduction in OS.
7.2. Analysis.

Comparison 7: Sunitinib versus Pembrolizumab + Axitinib, Outcome 2: Overall survival
SAEs (assessed with CTCAE v4.0)
Sunitinib may reduce SAEs as compared to pembrolizumab plus axitinib (RR 0.90, 95% CI 0.81 to 1.02; 1 study, 854 participants; low‐certainty evidence; Analysis 7.3) although the CI also includes the possibility of no effect. Based on the control event risk of 604 per 1000 in this trial, this corresponds to 60 fewer SAEs (95% CI 115 fewer to 12 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance bias) and imprecision, given that the CI was also compatible with little to no increase in SAEs.
7.3. Analysis.

Comparison 7: Sunitinib versus Pembrolizumab + Axitinib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
We found no studies that reported this outcome.
Response rate (assessed with: RECIST v1.1)
Sunitinib probably reduces the response rate as compared to pembrolizumab plus axitinib (RR 0.60, 95% CI 0.52 to 0.70; 1 study, 861 participants; moderate‐certainty evidence; Analysis 7.4). Based on the control event risk of 593 per 1000 in this trial, this corresponds to 237 fewer response (95% CI 284 fewer to 178 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias)
7.4. Analysis.

Comparison 7: Sunitinib versus Pembrolizumab + Axitinib, Outcome 4: Response rate
Minor AEs (assessed with CTCAE v4.0)
Sunitinib may result in little to no difference in minor AEs as compared to pembrolizumab plus axitinib (RR 1.19, 95% CI 0.99 to 1.42; 1 study, 854 participants; low‐certainty evidence; Analysis 7.5). Based on the control event risk of 333 per 1000 in this trial, this corresponds to 63 more minor AEs (95% CI 3 fewer to 140 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance bias) and imprecision, given that the CI was also compatible with an increase in minor AEs.
7.5. Analysis.

Comparison 7: Sunitinib versus Pembrolizumab + Axitinib, Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analyses
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analyses
We were unable to perform a sensitivity analysis because there was only one study.
8. Sunitinib versus atezolizumab + bevacizumab
Please refer to Table 8.
Primary outcomes
PFS
Sunitinib may reduce PFS as compared to atezolizumab plus bevacizumab (HR 1.18, 95% CI 1.02 to 1.36; 2 studies, 1117 participants; I² = 0%; low‐certainty evidence; Analysis 8.1). Based on the control event risk of 480 per 1000 in this trial at 12 months, this corresponds to 59 fewer PFSs (95% CI 111 fewer to 7 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (high and unclear risk of one or more domains) and imprecision, given that the CI was also compatible with no reduction in PFS.
8.1. Analysis.

Comparison 8: Sunitinib versus Atezolizumab + Bevacizumab, Outcome 1: Progression‐free survival
OS
We are very uncertain how sunitinib compares to atezolizumab plus bevacizumab for OS (HR 0.99, 95% CI 0.73 to 1.33; 2 studies, 1117 participants; I² = 37%; very low certainty evidence; Analysis 8.2). We rated the certainty of evidence as very low due to study limitations (high and unclear risk of one or more domains, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with an appreciable reduction in the OS as well as an appreciable increase in OS.
8.2. Analysis.

Comparison 8: Sunitinib versus Atezolizumab + Bevacizumab, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v4.0)
We are very uncertain how sunitinib compares to atezolizumab plus bevacizumab for SAEs as compared to atezolizumab + bevacizumab (RR 1.22, 95% CI 1.00 to 1.49; 2 studies, 1098 participants; I² = 64%; very low certainty evidence; Analysis 8.3). We rated the certainty of evidence as very low due to study limitations (high and unclear risk of one or more domains), inconsistency (unexplained differences between study results) and imprecision, given that the CI was compatible with no increase in SAEs as well as an appreciable increase in SAEs.
8.3. Analysis.

Comparison 8: Sunitinib versus Atezolizumab + Bevacizumab, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Sunitinib may decrease QoL (assessed with MD Anderson Symptom Inventory Interference Score (MDASI); scale 0 to 10; higher scores indicate worse QoL; MCID 1.0) as compared to atezolizumab plus bevacizumab (MD 1.00, 95% CI 0.68 to 1.32; 2 studies, 691 participants; I² = 0%; low‐certainty evidence; Analysis 8.4). We rated the certainty of evidence as low due to study limitations (high and unclear risk of one or more domains) and imprecision, given that the CI was compatible with possibly no decrease in QoL.
8.4. Analysis.

Comparison 8: Sunitinib versus Atezolizumab + Bevacizumab, Outcome 4: Health‐related quality of life
Response rate (assessed with: RECIST v1.1)
Sunitinib probably results in little to no difference in response rate as compared to atezolizumab plus bevacizumab (RR 0.91, 95% CI 0.77 to 1.07; 2 studies, 1117 participants; I² = 0%; moderate‐certainty evidence; Analysis 8.5). Based on the control event risk of 357 per 1000 in this trial, this corresponds to 32 fewer responses (95% CI 82 fewer to 25 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (high and unclear risk of one or more domains).
8.5. Analysis.

Comparison 8: Sunitinib versus Atezolizumab + Bevacizumab, Outcome 5: Response rate
Minor AEs (assessed with CTCAE v4.0)
Sunitinib may result in little to no difference in minor AEs as compared to atezolizumab plus bevacizumab (RR 0.85, 95% CI 0.74 to 0.97; 2 studies, 1098 participants; I² = 0%; low‐certainty evidence; Analysis 8.6). Based on the control event risk of 467 per 1000 in this trial, this corresponds to 70 fewer minor AEs (95% CI 112 fewer to 14 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (high and unclear risk of one or more domains) and imprecision, given that the CI was also compatible with an appreciable reduction in minor AEs.
8.6. Analysis.

Comparison 8: Sunitinib versus Atezolizumab + Bevacizumab, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We rated all of the included studies as high or unclear risk of bias and were unable to perform a sensitivity analysis.
9. Sunitinib versus IMA901 + sunitinib
Please refer to Table 9.
Primary outcomes
PFS
We are very uncertain about the effect of sunitinib on PFS as compared to IMA901 plus sunitinib (HR 0.95, 95% CI 0.70 to 1.30; 1 study, 339 participants; very low certainty evidence; Analysis 9.1). We rated the certainty of evidence as very low due to study limitations (performance and other bias, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with an appreciable reduction in PFS as well as an appreciable increase in PFS.
9.1. Analysis.

Comparison 9: Sunitinib versus IMA901 + Sunitinib, Outcome 1: Progression‐free survival
OS
Sunitinib may result in little to no difference in OS as compared to IMA901 plus sunitinib (HR 0.75, 95% CI 0.54 to 1.04; 1 study, 339 participants; low‐certainty evidence; Analysis 9.2). Based on the control event risk of 800 per 1000 in this trial at 12 months, this would result in 46 more OSs (95% CI 7 fewer to 86 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (other bias) and imprecision, given that the CI was also compatible with an increase in OS.
9.2. Analysis.

Comparison 9: Sunitinib versus IMA901 + Sunitinib, Outcome 2: Overall survival
SAEs (assessed with CTCAE v4.0)
Sunitinib may reduce SAEs as compared to IMA901 plus sunitinib (RR 0.74, 95% CI 0.59 to 0.95; 1 study, 334 participants; low‐certainty evidence; Analysis 9.3). Based on the control event risk of 550 per 1000 in this trial, this corresponds to 143 fewer SAEs (95% CI 225 fewer to 27 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and other bias) and imprecision, given that the CI was also compatible with a small to no reduction in SAEs.
9.3. Analysis.

Comparison 9: Sunitinib versus IMA901 + Sunitinib, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
We found no studies that reported this outcome.
Response rate (assessed with: RECIST v1.1)
Sunitinib may result in little to no difference in response rate as compared to IMA901 plus sunitinib (RR 0.87, 95% CI 0.64 to 1.19; 1 study, 339 participants; low‐certainty evidence; Analysis 9.4). Based on the control event risk of 358 per 1000 in this trial, this corresponds to 47 fewer response (95% CI 129 fewer to 68 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and other bias) and imprecision, given that the CI was also compatible with an appreciable reduction in response rate.
9.4. Analysis.

Comparison 9: Sunitinib versus IMA901 + Sunitinib, Outcome 4: Response rate
Minor AEs (assessed with CTCAE v4.0)
Sunitinib may result in little to no difference in minor AEs as compared to IMA901 plus sunitinib (RR 1.29, 95% CI 0.96 to 1.72; 1 study, 334 participants; low‐certainty evidence; Analysis 9.5). Based on the control event risk of 312 per 1000 in this trial, this corresponds to 90 more minor AEs (95% CI 12 fewer to 225 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in minor AEs.
9.5. Analysis.

Comparison 9: Sunitinib versus IMA901 + Sunitinib, Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
10. Sunitinib versus interferon‐α (IFN‐α) (targeted agent versus classic immunotherapy)
Please refer to Table 10.
Primary outcomes
PFS
Sunitinib probably increases PFS as compared to IFN‐α (HR 0.54, 95% CI 0.45 to 0.64; 1 study, 750 participants; moderate‐certainty evidence; Analysis 10.1). Based on the control event risk of 400 per 1000 in this trial at six months, this corresponds to 210 more PFSs (95% CI 156 more to 262 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance and other bias).
10.1. Analysis.

Comparison 10: Sunitinib versus Interferon‐α (IFN‐α), Outcome 1: Progression‐free survival
OS
Sunitinib may increase OS as compared to IFN‐α (HR 0.82, 95% CI 0.67 to 1.00; 1 study, 750 participants; low‐certainty evidence; Analysis 10.2) although the CI also includes the possibility of no effect. Based on the control event risk of 480 per 1000 in this trial at 24 months, this would result in 68 more OSs (95% CI 0 fewer to 132 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (selection and other bias) and imprecision, given that the CI was also compatible with no increase in OS.
10.2. Analysis.

Comparison 10: Sunitinib versus Interferon‐α (IFN‐α), Outcome 2: Overall survival
SAEs (assessed with CTCAE v3.0)
Sunitinib probably increases SAEs as compared to IFN‐α (RR 1.75, 95% CI 1.43 to 2.16; 1 study, 735 participants; moderate‐certainty evidence; Analysis 10.3). Based on the control event risk of 258 per 1000 in this trial, this corresponds to 194 more SAEs (95% CI 111 more to 300 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance and other bias).
10.3. Analysis.

Comparison 10: Sunitinib versus Interferon‐α (IFN‐α), Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Sunitinib probably results in little to no difference in QoL (assessed with EQ‐5D; scale: −0.59 to 1.00 with higher scores indicating better QoL; MCID 0.06) as compared to IFN‐α (MD −0.01, 95% CI −0.05 to 0.03; 1 study, 544 participants; moderate‐certainty evidence; Analysis 10.4). We rated the certainty of evidence as moderate due to study limitations (selection, performance and other bias).
10.4. Analysis.

Comparison 10: Sunitinib versus Interferon‐α (IFN‐α), Outcome 4: Health‐related quality of life
Response rate (assessed with RECIST v1.0)
Sunitinib probably increases response rate as compared to IFN‐α (RR 3.83, 95% CI 2.86 to 5.12; 1 study, 750 participants; moderate‐certainty evidence; Analysis 10.5). Based on the control event risk of 123 per 1000 in this trial, this corresponds to 347 more response (95% CI 228 more to 505 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance and other bias).
10.5. Analysis.

Comparison 10: Sunitinib versus Interferon‐α (IFN‐α), Outcome 5: Response rate
Minor AEs (assessed with CTCAE v3.0)
Sunitinib probably results in little to no difference in minor AEs as compared to IFN‐α (RR 1.03, 95% CI 1.00 to 1.05; 1 study, 735 participants; moderate‐certainty evidence; Analysis 10.6). Based on the control event risk of 956 per 1000 in this trial, this corresponds to 29 more minor AEs (95% CI 0 fewer to 48 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance and other bias).
10.6. Analysis.

Comparison 10: Sunitinib versus Interferon‐α (IFN‐α), Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
11. Temsirolimus versus IFN‐α (targeted agent versus classic immunotherapy)
Please refer to Table 11.
Primary outcomes
PFS
Temsirolimus may increase PFS as compared to IFN‐α (HR 0.74, 95% CI 0.60 to 0.91; 1 study, 416 participants; low‐certainty evidence; Analysis 11.1). Based on the control event risk of 100 per 1000 in this trial at 12 months, this corresponds to 82 more PFSs (95% CI 23 more to 151 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was also compatible with a small or no increase in PFS.
11.1. Analysis.

Comparison 11: Temsirolimus versus IFN‐α, Outcome 1: Progression‐free survival
OS
Temsirolimus probably increases OS as compared to IFN‐α (HR 0.78, 95% CI 0.63 to 0.97; 1 study, 416 participants; moderate‐certainty evidence; Analysis 11.2). Based on the control event risk of 300 per 1000 in this trial at 12 months, this would result in 91 more OSs (95% CI 11 more to 168 more) per 1000 participants. We rated the certainty of evidence as moderate due to imprecision, given that the CI was also compatible with a small or no increase in OS.
11.2. Analysis.

Comparison 11: Temsirolimus versus IFN‐α, Outcome 2: Overall survival
SAEs (assessed with CTCAE version not reported)
Temsirolimus may reduce SAEs as compared to IFN‐α (RR 0.86, 95% CI 0.76 to 0.97; 1 study, 408 participants; low‐certainty evidence; Analysis 11.3). Based on the control event risk of 780 per 1000 in this trial, this corresponds to 109 fewer SAEs (95% CI 187 fewer to 23 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was also compatible with a small or no reduction in SAEs.
11.3. Analysis.

Comparison 11: Temsirolimus versus IFN‐α, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Temsirolimus may result in little to no difference in QoL (assessed with EQ‐5D; scale −0.59 to 1.0 with higher values indicating better QoL; MCID: 0.06) as compared to IFN‐α (MD 0.03, 95% CI −0.01 to 0.07; 1 study, 401 participants; low‐certainty evidence; Analysis 11.4). We rated the certainty of evidence as low due to study limitations (performance, detection and attrition bias), and imprecision, given that the CI was also compatible with an increase in QoL.
11.4. Analysis.

Comparison 11: Temsirolimus versus IFN‐α, Outcome 4: Health‐related quality of life
Response rate (assessed with RECIST version not reported)
Temsirolimus may result in little to no difference in response rate as compared to IFN‐α (RR 1.78, 95% CI 0.84 to 3.77; 1 study, 416 participants; low‐certainty evidence; Analysis 11.5). Based on the control event risk of 48 per 1000 in this trial, this corresponds to 38 more response (95% CI 8 fewer to 134 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and attrition bias), and imprecision, given that the CI is also compatible with an appreciable increase in response rate.
11.5. Analysis.

Comparison 11: Temsirolimus versus IFN‐α, Outcome 5: Response rate
Minor AEs (assessed with CTCAE version not reported)
Temsirolimus probably results in little to no difference in minor AEs as compared to IFN‐α (RR 1.02, 95% CI 1.00 to 1.04; 1 study, 408 participants; moderate‐certainty evidence; Analysis 11.6). Based on the control event risk of 985 per 1000 in this trial, this corresponds to 20 more minor AEs (95% CI 0 fewer to 39 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance and detection bias).
11.6. Analysis.

Comparison 11: Temsirolimus versus IFN‐α, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
12. Sunitinib versus atezolizumab (targeted therapy versus immunotherapy)
Please refer to Table 12.
Primary outcomes
PFS
We are very uncertain how sunitinib affects PFS as compared to atezolizumab (HR 0.84, 95% CI 0.58 to 1.22; 1 study, 204 participants; very low certainty evidence; Analysis 12.1). We rated the certainty of evidence as very low due to study limitations (selection, performance, detection and other bias, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with both an appreciable reduction and increase in PFS.
12.1. Analysis.

Comparison 12: Sunitinib versus Atezolizumab, Outcome 1: Progression‐free survival
OS
We are very uncertain how sunitinib affects OS as compared to atezolizumab (HR 0.94, 95% CI 0.58 to 1.54; 1 study, 204 participants; very low certainty evidence; Analysis 12.2). We rated the certainty of evidence as very low due to study limitations (selection and other bias, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with both an appreciable reduction and increase in OS.
12.2. Analysis.

Comparison 12: Sunitinib versus Atezolizumab, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v4.0)
Sunitinib probably increases SAEs as compared to atezolizumab (RR 1.73, 95% CI 1.32 to 2.27; 1 study, 203 participants; moderate‐certainty evidence; Analysis 12.3). Based on the control event risk of 398 per 1000 in this trial, this corresponds to 291 more SAEs (95% CI 127 more to 506 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance, detection and other bias).
12.3. Analysis.

Comparison 12: Sunitinib versus Atezolizumab, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Sunitinib may decrease QoL slightly (assessed with MD Anderson Symptom Inventory Interference Score (MDASI); scale 0 to 10; higher scores indicate worse QoL; MCID 1.0) as compared to atezolizumab (MD 1.46, 95% CI 0.80 to 2.12; 1 study, 157 participants; low‐certainty evidence; Analysis 12.4). We rated the certainty of evidence as low due to study limitations (selection, performance, detection, attrition and other bias) and imprecision, given that the CI was also compatible with no decrease in QoL.
12.4. Analysis.

Comparison 12: Sunitinib versus Atezolizumab, Outcome 4: Health‐related quality of life
Response rate (assessed with: RECIST v1.1)
Sunitinib may result in little to no difference in response rate as compared to atezolizumab (RR 1.14, 95% CI 0.72 to 1.79; 1 study, 204 participants; low‐certainty evidence; Analysis 12.5). Based on the control event risk of 252 per 1000 in this trial, this corresponds to 35 more response (95% CI 71 fewer to 199 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (selection, performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in response rate.
12.5. Analysis.

Comparison 12: Sunitinib versus Atezolizumab, Outcome 5: Response rate
Minor AEs (assessed with: CTCAE v4.0)
Sunitinib probably reduces minor AEs as compared to atezolizumab (RR 0.50, 95% CI 0.35 to 0.71; 1 study, 203 participants; moderate‐certainty evidence; Analysis 12.6). Based on the control event risk of 563 per 1000 in this trial, this corresponds to 282 fewer minor AEs (95% CI 366 fewer to 163 fewer) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance, detection and other bias).
12.6. Analysis.

Comparison 12: Sunitinib versus Atezolizumab, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
13. Bevacizumab + IFN versus IFN (+ placebo)
Please refer to Table 13.
Primary outcomes
PFS
Bevacizumab plus IFN probably increases PFS as compared to IFN (+ placebo) (HR 0.68, 95% CI 0.60 to 0.77; 2 studies, 1381 participants; I² = 14%; moderate‐certainty evidence; Analysis 13.1). Based on the control event risk of 200 per 1000 in this trial at 12 months, this corresponds to 135 more PFSs (95% CI 90 more to 181 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (high and unclear risk of one or more domains).
13.1. Analysis.

Comparison 13: Bevacizumab + IFN versus IFN (+ placebo), Outcome 1: Progression‐free survival
OS
Bevacizumab + IFN may result in little to no difference in OS as compared to IFN (+ placebo) (HR 0.88, 95% CI 0.79 to 0.99; 2 studies, 1381 participants; I² = 0%; low‐certainty evidence; Analysis 13.2). Based on the control event risk of 500 per 1000 in this trial at 24 months, this would result in 43 more OSs (95% CI 3 more to 78 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (high and unclear risk of one or more domains) and imprecision, given that the CI was also compatible with an increase in OS.
13.2. Analysis.

Comparison 13: Bevacizumab + IFN versus IFN (+ placebo), Outcome 2: Overall survival
SAEs (assessed with: CTCAE v3.0)
Bevacizumab plus IFN probably increases SAEs as compared to IFN (+ placebo) (RR 1.31, 95% CI 1.20 to 1.42; 2 studies, 1356 participants; I² = 0%; moderate‐certainty evidence; Analysis 13.3). Based on the control event risk of 536 per 1000 in this trial, this corresponds to 166 more SAEs (95% CI 107 more to 225 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (high and unclear risk of one or more domains).
13.3. Analysis.

Comparison 13: Bevacizumab + IFN versus IFN (+ placebo), Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
We found no studies that reported this outcome.
Response rate (assessed with: RECIST v1.0)
Bevacizumab plus IFN may increase response rate as compared to IFN (+ placebo) (RR 2.45, 95% CI 1.74 to 3.45; 1 study, 595 participants; low‐certainty evidence; Analysis 13.4). Based on the control event risk of 128 per 1000 in this trial, this corresponds to 186 more response (95% CI 95 more to 314 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (unclear risk of other bias) and imprecision, given that the CI was also compatible with a small or no increase response rate.
13.4. Analysis.

Comparison 13: Bevacizumab + IFN versus IFN (+ placebo), Outcome 4: Response rate
Minor AEs (assessed with: CTCAE v3.0)
Bevacizumab plus IFN may reduce minor AEs (grade 1 or 2) as compared to IFN (+ placebo) (RR 0.75, 95% CI 0.63 to 0.90; 1 study, 641 participants; low‐certainty evidence; Analysis 13.5). Based on the control event risk of 493 per 1000 in this trial, this corresponds to 123 fewer minor AEs (95% CI 183 fewer to 49 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (unclear risk of other bias) and imprecision, given that the CI was also compatible with a small or no reduction in minor AEs.
13.5. Analysis.

Comparison 13: Bevacizumab + IFN versus IFN (+ placebo), Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We rated all of the included studies as high or unclear risk of bias and were unable to perform a sensitivity analysis.
14. Temsirolimus + IFN‐α versus IFN‐α
Please refer to Table 14.
Primary outcomes
PFS
Temsirolimus plus IFN‐α may increase PFS as compared to IFN‐α (HR 0.76, 95% CI 0.62 to 0.93; 1 study, 417 participants; low‐certainty evidence; Analysis 14.1). Based on the control event risk of 100 per 1000 in this trial at 12 months, this corresponds to 74 more PFSs (95% CI 17 more to 140 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was also compatible with little to no increase in PFS.
14.1. Analysis.

Comparison 14: Temsirolimus + IFN‐α versus IFN‐α, Outcome 1: Progression‐free survival
OS
Temsirolimus plus IFN‐α may result in little to no difference in OS as compared to IFN‐α (HR 0.93, 95% CI 0.75 to 1.15; 1 study, 417 participants; low‐certainty evidence; Analysis 14.2). Based on the control event risk of 300 per 1000 in this trial at 12 months, this would result in 26 more OSs (95% CI 50 fewer to 105 more) per 1000 participants. We rated the certainty of evidence as low due to imprecision (downgrade two levels), given that the CI was also compatible with both an appreciable reduction or increase in OS.
14.2. Analysis.

Comparison 14: Temsirolimus + IFN‐α versus IFN‐α, Outcome 2: Overall survival
SAEs (assessed with: CTCAE version not reported)
Temsirolimus plus IFN‐α may increase SAEs as compared to IFN‐α (RR 1.12, 95% CI 1.02 to 1.22; 1 study, 408 participants; low‐certainty evidence; Analysis 14.3). Based on the control event risk of 780 per 1000 in this trial, this corresponds to 94 more SAEs (95% CI 16 more to 172 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was also compatible with little to no increase in SAEs.
14.3. Analysis.

Comparison 14: Temsirolimus + IFN‐α versus IFN‐α, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Temsirolimus plus IFN‐α may result in little to no difference in QoL (assessed with EQ‐5D; scale −0.59 to 1.0 with higher scores indicating better QoL; MCID 0.06) as compared to IFN‐α (MD 0.03, 95% CI −0.01 to 0.07; 1 study, 394 participants; low‐certainty evidence; Analysis 14.4). We rated the certainty of evidence as low due to study limitations (performance, detection and attrition bias), and imprecision, given that the CI was also compatible with an increase in QoL.
14.4. Analysis.

Comparison 14: Temsirolimus + IFN‐α versus IFN‐α, Outcome 4: Health‐related quality of life
Response rate (assessed with: RECIST version not reported)
Temsirolimus plus IFN‐α may result in little to no difference in response rate as compared to IFN‐α (RR 1.68, 95% CI 0.79 to 3.57; 1 study, 417 participants; low‐certainty evidence; Analysis 14.5). Based on the control event risk of 48 per 1000 in this trial, this corresponds to 33 more response (95% CI 10 fewer to 124 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance, detection and attrition bias), and imprecision, given that the CI was also compatible with an appreciable increase in response rate.
14.5. Analysis.

Comparison 14: Temsirolimus + IFN‐α versus IFN‐α, Outcome 5: Response rate
Minor AEs (assessed with: CTCAE version not reported)
Temsirolimus plus IFN‐α probably results in little to no difference in minor AEs (grade 1 or 2) as compared to IFN‐α (RR 1.00, 95% CI 0.98 to 1.02; 1 study, 408 participants; moderate‐certainty evidence; Analysis 14.6). Based on the control event risk of 985 per 1000 in this trial, this corresponds to zero fewer minor AEs (95% CI 20 fewer to 20 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance and detection bias).
14.6. Analysis.

Comparison 14: Temsirolimus + IFN‐α versus IFN‐α, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
15. Temsirolimus + bevacizumab versus bevacizumab + IFN‐α
Please refer to Table 15.
Primary outcomes
PFS
Temsirolimus plus bevacizumab may result in little to no difference in PFS as compared to bevacizumab plus IFN‐α (HR 1.10, 95% CI 0.90 to 1.34; 1 study, 791 participants; low‐certainty evidence; Analysis 15.1). Based on the control event risk of 420 per 1000 in this trial at 12 months, this corresponds to 35 fewer PFSs (95% CI 107 fewer to 38 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance bias) and imprecision, given that the CI was also compatible with an appreciable reduction in PFS.
15.1. Analysis.

Comparison 15: Temsirolimus + Bevacizumab versus Bevacizumab + IFN‐α, Outcome 1: Progression‐free survival
OS
Temsirolimus plus bevacizumab probably results in little to no difference in OS as compared to bevacizumab plus IFN‐α (HR 1.08, 95% CI 0.90 to 1.30; 1 study, 791 participants; moderate‐certainty evidence; Analysis 15.2). Based on the control event risk of 550 per 1000 in this trial at 24 months, this would result in 26 fewer OSs (95% CI 90 fewer to 34 more) per 1000 participants. We rated the certainty of evidence as moderate due to imprecision, given that the CI was also compatible with a reduction in OS.
15.2. Analysis.

Comparison 15: Temsirolimus + Bevacizumab versus Bevacizumab + IFN‐α, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v3.0)
Temsirolimus plus bevacizumab may result in little to no difference in SAEs (grade 3 or 4) as compared to bevacizumab plus IFN‐α (RR 1.05, 95% CI 0.98 to 1.13; 1 study, 784 participants; low‐certainty evidence; Analysis 15.3). Based on the control event risk of 760 per 1000 in this trial, this corresponds to 38 more SAEs (95% CI 15 fewer to 99 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance bias) and imprecision, given that the CI was also compatible with an appreciable increase in SAEs.
15.3. Analysis.

Comparison 15: Temsirolimus + Bevacizumab versus Bevacizumab + IFN‐α, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Rini 2014 reported QoL measured by Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI)–15, which contains 15 questions representing concerns specific to patients with advanced kidney cancer and FKSI‐Disease Related Symptoms (FKSI‐DRS) subscale. We could not obtain a mean and standard deviation in each arm, however, and therefore we were unable to estimate this outcome.
Response rate (assessed with: RECIST version not reported)
Temsirolimus plus bevacizumab probably results in little to no difference in response rate as compared to bevacizumab plus IFN‐α (RR 0.99, 95% CI 0.79 to 1.24; 1 study, 791 participants; moderate‐certainty evidence; Analysis 15.4). Based on the control event risk of 274 per 1000 in this trial, this corresponds to 3 fewer response (95% CI 57 fewer to 66 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias).
15.4. Analysis.

Comparison 15: Temsirolimus + Bevacizumab versus Bevacizumab + IFN‐α, Outcome 4: Response rate
Minor AEs (assessed with: CTCAE v3.0)
Temsirolimus plus bevacizumab probably results in little to no difference in minor AEs as compared to bevacizumab plus IFN‐α (RR 1.01, 95% CI 0.98 to 1.03; 1 study, 784 participants; moderate‐certainty evidence; Analysis 15.5). Based on the control event risk of 967 per 1000 in this trial, this corresponds to 10 more minor AEs (95% CI 19 fewer to 29 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance bias).
15.5. Analysis.

Comparison 15: Temsirolimus + Bevacizumab versus Bevacizumab + IFN‐α, Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
16. Everolimus + bevacizumab versus IFN α‐2a + bevacizumab
Please refer to Table 16.
Primary outcomes
PFS
We are very uncertain how everolimus plus bevacizumab affects PFS as compared to IFN α‐2a plus bevacizumab (HR 0.91, 95% CI 0.69 to 1.20; 1 study, 365 participants; very low certainty evidence; Analysis 16.1). We rated the certainty of evidence as very low due to study limitations (selection, performance, detection and other bias, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with both an appreciable reduction and increase in PFS.
16.1. Analysis.

Comparison 16: Everolimus + Bevacizumab versus IFN α‐2a + Bevacizumab, Outcome 1: Progression‐free survival
OS
We are very uncertain how everolimus plus bevacizumab affects OS as compared to IFN α‐2a plus bevacizumab (HR 1.01, 95% CI 0.75 to 1.36; 1 study, 365 participants; very low certainty evidence; Analysis 16.2). We rated the certainty of evidence as very low due to study limitations (selection and other bias, downgrade one level) and imprecision (downgrade two levels), given that the CI was compatible with both an appreciable reduction and increase in OS.
16.2. Analysis.

Comparison 16: Everolimus + Bevacizumab versus IFN α‐2a + Bevacizumab, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v3.0)
Everolimus plus bevacizumab may result in little to no difference in SAEs as compared to IFN α‐2a plus bevacizumab (RR 1.06, 95% CI 0.95 to 1.18; 1 study, 361 participants; low‐certainty evidence; Analysis 16.3). Based on the control event risk of 762 per 1000 in this trial, this corresponds to 46 more SAEs (95% CI 38 fewer to 137 more) per 1000 participants. We rated the certainty of evidence as low due to study limitations (selection, performance, detection and other bias) and imprecision, given that the CI was also compatible with an appreciable increase in SAEs.
16.3. Analysis.

Comparison 16: Everolimus + Bevacizumab versus IFN α‐2a + Bevacizumab, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Ravaud 2015 reported time to deterioration of global health status measured by the European Organisation for the Research and Treatment of Cancer (EORTC)‐Core Quality of Life Questionnaire (QLQ‐C30) (NCT00719264). We could not obtain a mean and standard deviation in each arm, however, and therefore we were unable to estimate this outcome.
Response rate (assessed with: RECIST v1.0)
Everolimus plus bevacizumab probably results in little to no difference in response rate as compared to IFN α‐2a plus bevacizumab (RR 0.97, 95% CI 0.69 to 1.35; 1 study, 365 participants; moderate‐certainty evidence; Analysis 16.4). Based on the control event risk of 279 per 1000 in this trial, this corresponds to 8 fewer response (95% CI 86 fewer to 98 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance, detection and other bias).
16.4. Analysis.

Comparison 16: Everolimus + Bevacizumab versus IFN α‐2a + Bevacizumab, Outcome 4: Response rate
Minor AEs (assessed with CTCAE v3.0)
Everolimus plus bevacizumab probably results in little to no difference in minor AEs as compared to IFN α‐2a plus bevacizumab (RR 0.63, 95% CI 0.34 to 1.16; 1 study, 361 participants; moderate‐certainty evidence; Analysis 16.5). Based on the control event risk of 133 per 1000 in this trial, this corresponds to 49 fewer minor AEs (95% CI 88 fewer to 21 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (selection, performance, detection and other bias).
16.5. Analysis.

Comparison 16: Everolimus + Bevacizumab versus IFN α‐2a + Bevacizumab, Outcome 5: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
17. Sunitinib versus nivolumab + ipilimumab (Targeted agent versus combinations of immunotherapy)
Please refer to Table 17.
Primary outcomes
PFS
Sunitinib may reduce PFS as compared to nivolumab plus ipilimumab (HR 1.30, 95% CI 1.11 to 1.52; 1 study, 847 participants; low‐certainty evidence; Analysis 17.1). Based on the control event risk of 280 per 1000 in this trial at 30 months, this corresponds to 89 fewer PFSs (95% CI 136 fewer to 37 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was also compatible with no reduction in PFS.
17.1. Analysis.

Comparison 17: Sunitinib versus Nivolumab + Ipilimumab, Outcome 1: Progression‐free survival
OS
Sunitinib reduces OS as compared to nivolumab plus ipilimumab (HR 1.52, 95% CI 1.23 to 1.89; 1 study, 847 participants; high‐certainty evidence; Analysis 17.2). Based on the control event risk 600 per 1000 in this trial at 30 months, this would result in 140 fewer OSs (95% CI 219 fewer to 67 fewer) per 1000 participants. We rated the certainty of evidence as high.
17.2. Analysis.

Comparison 17: Sunitinib versus Nivolumab + Ipilimumab, Outcome 2: Overall survival
SAEs (assessed with: CTCAE v4.0)
Sunitinib probably increases SAEs (grade 3 or 4) as compared to nivolumab plus ipilimumab (RR 1.37, 95% CI 1.22 to 1.53; 1 study, 1082 participants; moderate‐certainty evidence; Analysis 17.3). Based on the control event risk of 457 per 1000 in this trial, this corresponds to 169 more SAEs (95% CI 101 more to 242 more) per 1000 participants. We rated the certainty of evidence as moderate due to study limitations (performance and detection bias).
17.3. Analysis.

Comparison 17: Sunitinib versus Nivolumab + Ipilimumab, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Sunitinib probably reduces QoL (assessed with FKSI‐19; scale 0 to 76 with higher scores indicating better QoL; MCID: 2) as compared to nivolumab plus ipilimumab (MD −4.10, 95% CI −5.75 to −2.45; 1 study, 460 participants; moderate‐certainty evidence; Analysis 17.4). We rated the certainty of evidence as moderate due to study limitations (performance, detection and attrition bias).
17.4. Analysis.

Comparison 17: Sunitinib versus Nivolumab + Ipilimumab, Outcome 4: Health‐related quality of life
Response rate (assessed with: RECIST v1.1)
Sunitinib may reduce response rate as compared to nivolumab plus ipilimumab (RR 0.70, 95% CI 0.58 to 0.84; 1 study, 847 participants; low‐certainty evidence; Analysis 17.5). Based on the control event risk of 419 per 1000 in this trial, this corresponds to 126 fewer response (95% CI 176 fewer to 67 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was also compatible with little to no reduction in response rate.
17.5. Analysis.

Comparison 17: Sunitinib versus Nivolumab + Ipilimumab, Outcome 5: Response rate
Minor AEs (assessed with CTCAE v4.0)
Sunitinib may reduce minor AEs as compared to nivolumab plus ipilimumab (RR 0.74, 95% CI 0.64 to 0.86; 1 study, 1082 participants; low‐certainty evidence; Analysis 17.6). Based on the control event risk of 459 per 1000 in this trial, this corresponds to 119 fewer minor AEs (95% CI 165 fewer to 64 fewer) per 1000 participants. We rated the certainty of evidence as low due to study limitations (performance and detection bias) and imprecision, given that the CI was compatible with little to no reduction in minor AEs.
17.6. Analysis.

Comparison 17: Sunitinib versus Nivolumab + Ipilimumab, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
18. Pazopanib versus placebo (targeted agent versus placebo)
Please refer to Table 18.
Primary outcomes
PFS
Pazopanib increases PFS as compared to placebo (HR 0.46, 95% CI 0.34 to 0.62; 1 study, 435 participants; high‐certainty evidence; Analysis 18.1). Based on the control event risk of 180 per 1000 in this trial at 12 months, this corresponds to 274 more PFSs (95% CI 165 more to 378 more) per 1000 participants.
18.1. Analysis.

Comparison 18: Pazopanib versus Placebo, Outcome 1: Progression‐free survival
OS
Pazopanib may result in little to no difference in OS as compared to placebo (HR 0.91, 95% CI 0.72 to 1.16; 1 study, 435 participants; low‐certainty evidence; Analysis 18.2). Based on the control event risk of 480 per 1000 in this trial at 24 months, this would result in 33 more OSs (95% CI 53 fewer to 110 more) per 1000 participants. We rated the certainty of evidence as low due to imprecision (downgrade two levels), given that the CI was both compatible with an appreciable reduction and increase in OS.
18.2. Analysis.

Comparison 18: Pazopanib versus Placebo, Outcome 2: Overall survival
SAEs (assessed with CTCAE v3.0)
Pazopanib increases SAEs as compared to placebo (RR 2.00, 95% CI 1.40 to 2.85; 1 study, 435 participants; high‐certainty evidence; Analysis 18.3). Based on the control event risk in this trial of 200 per 1000, this corresponds to 200 more SAEs (95% CI 80 more to 370 more) per 1000 participants.
18.3. Analysis.

Comparison 18: Pazopanib versus Placebo, Outcome 3: Serious adverse events (Grade 3 or 4)
Secondary outcomes
QoL
Pazopanib results in little to no difference in QoL (assessed with EORTC QLQ‐C30; scale 0 to 100 with higher values reflecting better QoL; MCID 10) as compared to placebo (MD −3.10, 95% CI −7.76 to 1.56; 1 study, 300 participants; high‐certainty evidence; Analysis 18.4).
18.4. Analysis.

Comparison 18: Pazopanib versus Placebo, Outcome 4: Health‐related quality of life
Response rate (assessed with: RECIST v1.0)
Pazopanib probably increases response rate as compared to placebo (RR 8.80, 95% CI 3.65 to 21.19; 1 study, 435 participants; moderate‐certainty evidence; Analysis 18.5). Based on the control event risk of 34 per 1000 in this trial, this corresponds to 269 more response (95% CI 91 more to 696 more) per 1000 participants. We rated the certainty of evidence as moderate due to imprecision, given that the CI was also compatible with little to no increase in response rate.
18.5. Analysis.

Comparison 18: Pazopanib versus Placebo, Outcome 5: Response rate
Minor AEs (assessed with CTCAE v3.0)
Pazopanib probably increases minor AEs as compared to placebo (RR 1.31, 95% CI 1.13 to 1.52; 1 study, 435 participants; moderate‐certainty evidence; Analysis 18.6). Based on the control event risk of 600 per 1000 in this trial, this corresponds to 186 more minor AEs (95% CI 78 more to 312 more) per 1000 participants. We rated the certainty of evidence as moderate due to imprecision, given that the CI was also compatible with little to no increase in minor AEs.
18.6. Analysis.

Comparison 18: Pazopanib versus Placebo, Outcome 6: Minor adverse events (Grade 1 or 2)
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses.
Sensitivity analysis
We were unable to perform a sensitivity analysis because there was only one study.
Discussion
For this review, we considered targeted therapies as the index intervention. This group of drugs included VEGFR‐TKIs (e.g. sunitinib, sorafenib, pazopanib, tivozanib and axitinib), VEGF‐inhibitors (e.g. bevacizumab) and mTOR inhibitors (e.g. everolimus or temsirolimus). Comparators included placebo, alternative targeted therapy (i.e. targeted therapy vs targeted therapy), cytokines (i.e. classic non‐targeted immunotherapy, e.g. interferon‐alpha), immune checkpoint inhibitors, or combinations of different classes of drugs. Immune checkpoint inhibitors included programmed death 1 inhibitors (PD‐1), programmed death ligand 1 inhibitors (PD‐L1) and cytotoxic T‐lymphocyte associated antigen 4 (CTLA‐4) inhibitors (e.g. avelumab, pembrolizumab, atezolizumab, nivolumab and ipilimumab).
Summary of main results
One trial compared targeted agent (pazopanib) against placebo (Table 18). There was high quality evidence showing pazopanib was significantly superior to placebo in terms of PFS, but inferior in terms of incidence of SAEs. There was low certainty evidence showing no difference between the groups in terms of OS.
For the intra‐group comparison of single‐agent targeted therapy against each other, we found some differences between them (Table 1; Table 2; Table 3; Table 4; Table 5). Pazopanib and tivozanib were both superior to sorafenib in terms of PFS but there were some differences in terms of OS; the certainty of evidence was moderate for pazopanib for PFS and low for OS but low for tivozanib for both PFS and OS. Sunitinib was superior to everolimus for PFS although there was no difference for OS, with moderate to low certainty of evidence for both outcomes. Sorafenib was inferior to sunitinib for PFS but there was no difference for OS (low and very low certainty evidence, respectively), nor between pazopanib versus sunitinib for PFS and OS (low certainty evidence for both). For AE, there was very low to low‐certainty evidence that there were no statistically significant differences between the drugs, except for sunitinib versus everolimus; sunitinib appeared to have a higher incidence of SAEs compared with everolimus (moderate‐certainty evidence).
Next, for the comparison of targeted therapy versus cytokines (i.e. classic non‐targeted immunotherapy, Table 10; Table 11; Table 13; Table 14; Table 15; Table 16), there was low‐ to moderate‐certainty evidence showing that single‐agent targeted therapy was superior to single‐agent interferon‐alpha for PFS and OS (for sunitinib and temsirolimus). Regarding the incidence of SAEs, temsirolimus was better than interferon‐alpha (low‐certainty evidence), but sunitinib was worse than interferon‐alpha (moderate‐certainty evidence). The results for the comparison between combination of targeted therapies with each other or with interferon‐alpha versus single‐agent interferon‐alpha suggest combinations of targeted therapy involving either bevacizumab or temsirolimus with interferon‐alpha are superior to single‐agent interferon‐alpha for PFS (low‐ to moderate‐certainty evidence) but there was no difference in OS (low‐certainty evidence); however the targeted therapy combinations had a significantly higher incidence of SAEs (low‐ to moderate‐certainty evidence). The comparison of combinations of targeted therapy (temsirolimus and bevacizumab, and everolimus and bevacizumab) versus combination of bevacizumab and interferon‐alpha did not show any differences in PFS, OS nor SAEs (very low to moderate‐certainty evidence).
For the comparison of targeted therapy versus combination of tumour vaccine (IMA901) with sunitinib, (Table 9), there was low‐certainty evidence that single‐agent sunitinib had significantly better OS and better SAEs profile compared with combination of IMA901 plus sunitinib.
For the comparison of single‐agent targeted therapy (all based on sunitinib) versus immune checkpoint inhibitor, the results can be summarised into three sub‐sections: (1) sunitinib versus single‐agent immune checkpoint inhibitor (atezolizumab, Table 12); (2) sunitinib versus combination of targeted drug with immune checkpoint inhibitor (axitinib + avelumab; axitinib + pembrolizumab; and bevacizumab + atezolizumab; Table 6; Table 7; Table 8); and (3) sunitinib versus combination of immune checkpoint inhibitors (nivolumab + ipilimumab, Table 17). For the comparison of sunitinib versus atezolizumab, there were no significant differences between them for PFS and OS (very low certainty evidence); however, sunitinib had worse incidence of SAEs (moderate certainty evidence). For the comparison of sunitinib versus combination of targeted therapy with immune checkpoint inhibitor, sunitinib appeared to have worse PFS than two of the combinations (moderate‐certainty evidence) and worse OS (moderate‐certainty evidence) than one combination of targeted drug and immune checkpoint inhibitor; Sunitinib may have no difference or less SAEs compared to combinations (low‐certainty evidence). Finally, for the comparison of sunitinib versus combination of immune checkpoint inhibitors (nivolumab + ipilimumab), sunitinib had worse PFS (low‐certainty evidence), worse OS (high‐certainty evidence) and worse incidence of SAEs (moderate‐certainty evidence) compared with the combination of nivolumab and ipilimumab. Sunitinib was also associated with worse QoL (moderate‐certainty evidence) and worse response rate (low‐certainty evidence).
In summary, when comparing targeted therapy (i.e. sunitinib) against immune checkpoint inhibitor either singly or in combination, there was high‐ to moderate‐quality evidence (from two trials) demonstrating sunitinib was inferior to a combination of targeted therapy with immune checkpoint inhibitor (i.e. axitinib + pembrolizumab), and a combination of immune checkpoint inhibitors (i.e. nivolumab + ipilimumab), in terms of OS. The result for PFS was also worse for sunitinib compared with the other combinations involving immune checkpoint inhibitors, with low‐ to moderate‐certainty evidence across all studies. There was also moderate‐quality evidence from two studies that sunitinib had worse incidence of SAEs compared with immune checkpoint inhibitors (involving atezolizumab as single agent, and nivolumab + ipilimumab combination). Although sunitinib was the comparator in all of those trials, a meta‐analysis was only possible for the two studies which used the combination of atezolizumab and bevacizumab. This was due to considerable clinical heterogeneity across the trials namely in the use of eligibility criteria, primary endpoints (e.g. results reported only for the intention‐to‐treat the population or those with PD‐L1 expression on tumour cells or tumour‐infiltrating lymphocytes), inconsistent use validated risk scores such as the Memorial Sloan Kettering Cancer Center (MSKCC) or International Metastatic RCC Database Consortium (IMDC) risks models, and experimental drugs used.
Overall completeness and applicability of evidence
We identified studies to include in this review by independent searches. Results were comparable and we resolved disagreements by discussion within the author group. We also identified trials through hand searching of conference proceedings and tried to acquire additional data whenever they were needed by contacting authors.
Participants were comparable amongst the identified studies, which all had a multicentre design. All except two trials (Motzer 2013b and Sternberg 2010) had a homogeneous population which had not received previous treatment. A phase 3 study was the favoured trial design which was used in 15 trials. Another three used a phase 2 design. All included studies investigated our primary and secondary outcomes except for QoL data, which was not available in seven trials.
We imposed strict criteria for study inclusion for methodological reasons which excluded studies with less than 100 participants per arm. This meant excluding the CABOSUN trial comparing cabozantinib versus sunitinib, an initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk (n = 157 in total) (Choueiri 2017). Guidelines panels of ESMO and EAU consider cabozantinib an alternative for IMDC intermediate‐ and poor‐risk patients only who cannot receive immune checkpoint inhibitor combination therapies but on the same par as sunitinib and pazopanib, which have been tested in randomised controlled phase 3 trials in this setting (EAU Guidelines 2020; ESMO Clinical Practice Guidelines 2019). This decision was taken because CABOSUN was a randomised phase 2 trial in which cabozantinib had a PFS but no OS benefit compared to sunitinib. This was considered not enough evidence to argue that cabozantinib is qualitatively superior to sunitinib.
Quality of the evidence
We used GRADE to rate the quality of evidence and created 18 'Summary of findings' tables for the different comparisons. We rated most of the comparisons at a low to moderate level of evidence. The main reasons for downgrading were study limitations, especially those due to lack of blinding with the risk of performance and detection bias. Imprecision was another important factor for lowering the certainty of evidence in efficacy outcomes.
Potential biases in the review process
Two reviewers screened all search results independently. There were no language restrictions but all identified studies were published in the English language. We did not receive additional data after contacting authors except for one study, which could be a source of bias.
After publication of our protocol we amended our inclusion criteria. We chose to limit the number of participants to a minimum of 100 per study arm to ensure our evidence synthesis was based on more robust data by reducing the risk of small‐study bias. We also chose to only include participants who were naïve to systemic therapy. This decision was mainly driven by the large number of studies assessing patients with metastatic renal cell carcinoma in the second‐line treatment setting or beyond. Including these trials would have distracted us from the main focus of our review which was on the first‐line treatment setting, and the burden of work would have become unfeasibly high.
For the interpretation of clinically important effect sizes, we used absolute effect estimates that were informed by the input of expert clinicians on our team; unless there were published thresholds (as was the case for quality of life instruments) we used 5% for the most patient‐important primary outcomes of PFS and OS and 10% for the secondary outcomes of response rates and minor AEs. We recognise that different thresholds might lead to different interpretations and have therefore made all our judgments as transparent as possible.
Agreements and disagreements with other studies or reviews
A systematic review with a broader approach on systemic treatment for metastatic renal cell carcinoma was published in 2018 (Lalani 2018). In total 26 trials were identified for first and later line treatments. All studies of that review that met our inclusion criteria were also identified and included in our review. The authors expected combinations therapies to become the new promising standard of care in treatment‐naïve renal cell carcinoma.
Another systematic review, also published in 2018, was focusing on first line systemic therapy for metastatic renal cell carcinoma (Wallis 2018). Of the 37 identified trials for a qualitative synthesis 13 were eligible for a quantitative synthesis. All trials that meet our inclusion criteria were part of our review. Cabozantinib was judged as being highly likely to provide the greatest PFS benefit, while the combination of nivolumab plus ipilimumab was most likely to provide the greatest OS benefit. The later combination was rated likely to have the most beneficial tolerance profile.
Authors' conclusions
Implications for practice.
Single‐agent vascular endothelial growth factor receptor‐tyrosine kinase inhibitors (VEGFR TKI), having being the first‐line treatment option in the management of metastatic RCC for years, appear to have been superseded by combinations of immune checkpoint inhibitors. However targeted therapy drugs have proved to be an effective treatment for those who cannot receive or tolerate immune checkpoint inhibition.
At present two immune checkpoint inhibitor‐based combinations with proven OS benefit are available as new standard of care for first‐line treatment of clear‐cell mRCC (EAU Guidelines 2020; ESMO Clinical Practice Guidelines 2019). In terms of comparative effectiveness within this new group of drugs, interpretation of data is limited by the short follow‐up of studies. ORR and PFS appear higher for the pembrolizumab plus axitinib combination than for ipilimumab plus nivolumab. With regard to complete response rates, it is possible they may improve for pembrolizumab plus axitinib, but decisive conclusions cannot be drawn at this stage.
Implications for research.
Results of this review underpin the trend towards a wider field of application for targeted immunotherapy agents alone or in combination with classic targeted therapy in the first‐line setting. However, some of these drugs are already successfully used in further lines for treating metastatic renal cell carcinoma patients. Further research is needed to answer the question of how to sequence therapies. This is of special importance now that combinations of immune checkpoint inhibitors (pembrolizumab) and VEGFR‐TKI (axitinib) are being used in treatment‐naive patients. While it would be intuitive to use a VEGFR‐TKI upon progression with dual immune checkpoint inhibitor combination such as ipilimumab and nivolumab this is less clear for combinations with VEGFR‐TKI in the first‐line treatment setting. In addition, some trials are investigating triple combinations such as ipilimumab plus nivolumab plus cabozantinib (Choueiri 2019), based on the emerging evidence that immune checkpoint inhibitor combination therapies are more effective than the same drugs in sequence.
What's new
| Date | Event | Description |
|---|---|---|
| 27 August 2020 | New citation required and conclusions have changed | In this update, we added new studies such as targeted therapy versus combinations of immunotherapy. We applied current MECIR standards as well as GRADE to assess the certainty of evidence. The conclusions of this review have changed. |
History
Protocol first published: Issue 9, 2017 Review first published: Issue 10, 2020
| Date | Event | Description |
|---|---|---|
| 1 July 2010 | New search has been performed | Complete update with additional studies, revised analysis, risk of bias assessment, and revised conclusions. Specifically, the search has been updated from the end of 2007 to June 2010, with 5 new eligible studies identified; analyses are now based on the nature of the control arm. Targeted agents have now been validated as first and second‐line therapy choices for patients with advanced renal cancers of the clear cell subtype. |
| 8 April 2010 | New search has been performed | Converted to new review format. |
| 14 January 2008 | New citation required and conclusions have changed | Substantive amendment |
Notes
This is review is developed from the existing Cochrane Review entitled, "Targeted therapy for advanced renal cell carcinoma" (Coppin 2008).
We have based parts of the Methods section of this Cochrane protocol on a standard template established by the CMED Group.
Acknowledgements
We thank Cochrane Urology Review Group representatives Philipp Dahm and Robert Lane for facilitating this collaboration and for editorial suggestions, and Molly M Neuberger, for administrative assistance. We wish to acknowledge additional authors contributing to previous versions of this review: Chris Coppin (Canada), Christian Kollmannsberger (Canada), Lyly Le (Canada), Franz Porzsolt (Germany), and Timothy J Wilt (USA). We also thank Fiona Stewart for the initial search methods, as well as all members of the EAU Renal Cell Cancer Guideline Panel for support and individual contributions.
We are most grateful to Federico Cayol, Arjun K Nambiar, Mark Klein and Petri Bono for their thorough and accurate feedback on this review.
Appendices
Appendix 1. EAU panel search strategy
Courtesy of the EAU panel, reproduced with permission.
MEDLINE 1946 to 2020 18 June MEDLINE‐In‐Process and other Non‐Indexed Citations 18 June 2020 1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomi?ed.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. Carcinoma, Renal Cell/ 11. (metastas* adj5 ((kidney or renal) adj2 (cancer* or carcinoma* or neoplasm* or tum?or* or mass*))).tw. 12. or/10‐11 13. Chemotherapy, Cancer, Regional Perfusion/ 14. thalidomide/ 15. exp Antineoplastic Protocols/ 16. exp Antineoplastic agents/ 17. (axitinib or bevacizumab or dovitinib or erlotinib or everolimus or lapatinib or pazopanib or sorafenib or sunitinib or temsirolimus or thalidomide or tivozanib).tw. 18. antineoplastic$.tw. 19. or/13‐18 20. 9 and 12 and 19 21. (conference or letter or editorial or comment*).pt. 22. exp animals/ not humans/ 23. 20 not (21 or 22) 24. Limit 23 to yr="2001 ‐Current" Embase 1974 to 2020 June 18 1. kidney carcinoma/ 2. (metastas* adj5 ((kidney or renal) adj2 (cancer* or carcinoma* or neoplasm* or tum?or* or mass*))).tw. 3. 1 or 2 4. exp cancer chemotherapy/ 5. exp Antineoplastic agent/ 6. sorafenib/ 7. sunitinib/ 8. bevacizumab/ 9. axitinib/ 10. pazopanib/ 11. everolimus/ 12. temsirolimus/ 13. interferon/ 14. interleukin 2/ 15. dovitinib/ 16. tivozanib/ 17. erlotinib/ 18. (axitinib or bevacizumab or dovitinib or erlotinib or everolimus or lapatinib or pazopanib or sorafenib or sunitinib or temsirolimus or thalidomide or tivozanib).tw. 19. antineoplastic$.tw. 20. or/4‐19 21. random.tw. 22. placebo.mp. 23. double‐blind.tw. 24. or/21‐23 25. 3 and 20 and 24 26. exp animals/ not humans/ 27. (conference or letter or editorial or comment*).pt. 28. 25 not (26 or 27) 29. Limit 28 to yr="2001 ‐Current" Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (The Cochrane Library, 18 June 2020) www.thecochranelibrary.com 1. MeSH descriptor Carcinoma, Renal Cell, this term only 2. (metastas* near/5 ((kidney or renal) near/2 (cancer* or carcinoma* or neoplasm* or tum?or* or mass*))) 3. (#1 OR #2) 4. (#3), from 2001 to current LILACS 18 June 2020 http://lilacs.bvsalud.org/en/ (tw:(renal cell carcinoma or renal cancer or renal tumour$ or renal tumor$ or renal carcinoma$ or renal neoplasm$ or renal mass$ or kidney cancer or kidney tumour$ or kidney tumor$ or kidney neoplasm$ or kidney mass$)) OR (mh:(kidney neoplasms)) Type of study: Controlled Clinical Trial
Clinicaltrials.gov: http://clinicaltrials.gov Basic search: metastatic renal cell carcinoma WHO International Clinical Trials Registry Platform http://apps.who.int/ Basic search: metastatic renal cell carcinoma
Appendix 2. Survey of study investigators providing information on included studies
| Study | Date trial author contacted (first) | Date trial author provided data (latest) | Data trial author provided (short summary) |
| Retz 2019 | 19 May 2019 | 27 May 2019 | Health‐related quality of life standard deviation |
Data and analyses
Comparison 1. Sorafenib versus Sunitinib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Pazopanib versus Sunitinib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Tivozanib versus Sorafenib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Sorafenib versus Pazopanib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Sunitinib versus Everolimus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Sunitinib versus Avelumab + Axitinib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Sunitinib versus Pembrolizumab + Axitinib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Sunitinib versus Atezolizumab + Bevacizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Progression‐free survival | 2 | 1117 | Hazard Ratio (IV, Random, 95% CI) | 1.18 [1.02, 1.36] |
| 8.2 Overall survival | 2 | 1117 | Hazard Ratio (IV, Random, 95% CI) | 0.99 [0.73, 1.33] |
| 8.3 Serious adverse events (Grade 3 or 4) | 2 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.00, 1.49] |
| 8.4 Health‐related quality of life | 2 | 691 | Mean Difference (IV, Random, 95% CI) | 1.00 [0.68, 1.32] |
| 8.5 Response rate | 2 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.77, 1.07] |
| 8.6 Minor adverse events (Grade 1 or 2) | 2 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.97] |
Comparison 9. Sunitinib versus IMA901 + Sunitinib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 9.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 9.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Sunitinib versus Interferon‐α (IFN‐α).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Temsirolimus versus IFN‐α.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 11.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 11.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. Sunitinib versus Atezolizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 12.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 12.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 13. Bevacizumab + IFN versus IFN (+ placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Progression‐free survival | 2 | 1381 | Hazard Ratio (IV, Random, 95% CI) | 0.68 [0.60, 0.77] |
| 13.2 Overall survival | 2 | 1381 | Hazard Ratio (IV, Random, 95% CI) | 0.88 [0.79, 0.99] |
| 13.3 Serious adverse events (Grade 3 or 4) | 2 | 1356 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.20, 1.42] |
| 13.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 14. Temsirolimus + IFN‐α versus IFN‐α.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 14.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 14.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 15. Temsirolimus + Bevacizumab versus Bevacizumab + IFN‐α.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 15.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 15.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 16. Everolimus + Bevacizumab versus IFN α‐2a + Bevacizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 16.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 16.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16.4 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 16.5 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 17. Sunitinib versus Nivolumab + Ipilimumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 17.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 17.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 17.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 17.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 18. Pazopanib versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Progression‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 18.2 Overall survival | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 18.3 Serious adverse events (Grade 3 or 4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18.4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.5 Response rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18.6 Minor adverse events (Grade 1 or 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eichelberg 2015.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: February 2009 to December 2011 Blinding: open label study Strata: MSKCC risk category IMC: data not found Crossover: from first to second line after disease progression |
|
| Participants |
Histology: all histologies Prior systemic therapy: treatment‐naïve Measurable disease: required Non‐metastatic %: combined data not found M/F: 274/91 Eligible PS: ECOG PS 1 or better Age median (range): 65 (39 to 84) Prior nephrectomy: 335 Prognostic strata: system, good/intermediate/poor risk: MSKCC; 153/202/2 |
|
| Interventions | sorafenib 400 mg po twice daily followed, on progression or toxicity, by sunitinib 50 mg po daily 4 wks on, 2 wks off or vice versa | |
| Outcomes |
PFS: primary outcome (time from randomisation to confirmed progression or death during second‐line therapy); secondary outcome (time from randomisation to confirmed progression or death during first‐line therapy) OS: secondary endpoint AE: reported in toxicity table QoL: not analysed RR: secondary outcome Other: disease control rate, total time to progression, time to first‐line treatment failure, cardiotoxicity |
|
| Funding Sources | German Cancer Society (DKG), Austrian Social Security Institutions, grants from industry study sponsor | |
| Declarations of interest | Reported | |
| Notes | Planned as a non‐inferiority study, amended to a superiority design; power reduced from 90% to 85% due to slower rate of events than expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central randomisation via fax" |
| Allocation concealment (selection bias) | Low risk | "the person who generated the randomisation list was not involved in the study project management, monitoring, or data management." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Open label study design, participants and personnel not blinded to treatment; no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Open label study design, participants and personnel not blinded to treatment; both arms treated with same drugs in different sequence |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessed by investigator; no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | PFS assessed by investigator |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | All patients reported, 5/7 did not receive treatment in first line |
| Incomplete outcome data (attrition bias) Response rate | Low risk | All patients reported, 5/7 did not receive treatment in first line |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated patients in first and second line reported |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Unclear risk | Study design changed from non‐inferiority design to superiority design after randomisation of 138 participants |
Escudier 2010.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: June 2006 to October 2005 Blinding: double‐blind study Strata: country, risk group IMC: data safety monitoring board used Crossover: not planned but at preplanned interim OS analysis, difference in PFS clinically and statistically significant and DSMB recommended that patients in the control group who had not experienced progression should cross over to receive bevacizumab |
|
| Participants |
Histology: clear cell Prior systemic therapy: treatment‐naïve Measurable disease: required Non metastatic %: data not found M/F: 457/192 Eligible PS: Karnofsky > 60 Age median (range): 61 (18 to 82) Prior nephrectomy: required Prognostic strata: system, good/intermediate/poor risk %: MSKCC 30/61/9 |
|
| Interventions | Interferon‐a2a 9 MU sc tiw (subcutaneous thrice weekly) plus either (1) BEVACIZUMAB 10 mg/kg IV q2w, or (2) placebo [crossed over at final PFS analysis if not progressed] | |
| Outcomes |
PFS: reported [protocol modified to permit final PFS analysis before mature OS data] OS: primary endpoint AE: reported in toxicity table QoL: not assessed RR: secondary outcome Other: ‐ |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomization was done centrally” |
| Allocation concealment (selection bias) | Low risk | Clearly described; interactive voice recognition system |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "Double blind placebo controlled trial". Participants and personnel were blinded to treatment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | "Double blind placebo controlled trial". Participants and personnel were blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Investigator assessed, no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | PFS assessed by investigator who were blinded to treatment |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | 4% in each arm lost or withdrew consent; intention to treat population analysed |
| Incomplete outcome data (attrition bias) Response rate | Low risk | 4% in each arm lost or withdrew consent; intention to treat population analysed |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants analysed |
| Selective reporting (reporting bias) | Low risk | All protocol endpoints reported |
| Other bias | Unclear risk | Early unblinding and addition of bevacizumab; recommended for unprogressed placebo‐assigned patients by independent monitoring committee based on unplanned final PFS and preplanned interim survival analysis |
Escudier 2017.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: October 2014 to February 2016 Blinding: open label study design Strata: IMDC score, region IMC (independent monitoring committee): a data and safety monitoring committee reviewed efficacy and safety Crossover: not planned |
|
| Participants |
Histology: clear cell Prior systemic therapy: treatment‐naïve Measurable disease: required Non metastatic %: data not found; at least 78% of participants had 2 or more target or non‐target lesions M/F: 808/288 Eligible PS: Karnofsky PS 70% or better Age median: 62 (21 to 85) Prior nephrectomy: 890 Prognostic strata: system, % good/intermediate/poor/NA risk: IMDC risk score (0 vs. 1 or 2 vs. 3 to 6) and geographic region |
|
| Interventions | nivolumab 3 mg/kg + ipilimumab 1 mg/kg every 3 wk for 4 doses followed by nivolumab 3 mg/kg every 2 wk vs sunitinib 50 mg daily orally for 4 wk (6‐wk cycles) | |
| Outcomes |
PFS: primary endpoint OS: primary endpoint AE: toxicity table reported QoL: reported RR: primary endpoint Other: ‐ |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Published online | |
| Notes | Stopped early | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Begins with the randomization call to the Interactive Voice Response System (IVRS)" (from protocol) |
| Allocation concealment (selection bias) | Low risk | "Begins with the randomization call to the Interactive Voice Response System (IVRS)" (from protocol) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "Open‐label, phase 3 trial" participants and personnel were not blinded to treatment; no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "Open‐label, phase 3 trial" participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | (from protocol) Progression free survival; Tumor assessments for ongoing study treatment decisions will be completed by the investigator using RECIST (Response Evaluation Criteria in Solid Tumors) 1.1 criteria Health related quality of life, Minor adverse events; Open label trial |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | All IMDC intermediate and poor risk patients analysed for efficacy as predefined outcome |
| Incomplete outcome data (attrition bias) Response rate | Low risk | All IMDC intermediate and poor risk patients analysed for efficacy as predefined outcome |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants analysed |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | More than 80% completion rate in both groups but final analysed participants were 44/425 (10.3%) in Nivolumab + Ipilimumab arm and 26/422 (6.1%) in sunitinib arm. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | Completed planned accrual |
Hudes 2007.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: June 2003 to April 2005 Blinding: imaging Strata: according to the geographic location of the centre and nephrectomy status IMC: an independent data and safety monitoring committee reviewed the study at 6‐month intervals Crossover: not allowed |
|
| Participants |
Histology: all histologies Prior therapy: naïve Measurable disease: required (RECIST)Non metastatic %: <20M/F: 432/194 Eligible PS: Karnofsky > 50; actual KPS( > 70) = 17% Age median (range): 59 (23 to 86) Prior nephrectomy: 419 Prognostic strata: system, % good/intermediate/poor risk: MSKCC, ‐/26/74% |
|
| Interventions | TEMSIROLIMUS 25mg IV weekly, Interferon‐a2a 3‐18MU sc tiw, or both | |
| Outcomes |
PFS: secondary endpoint OS: primary endpoint AE: reported in toxicity table QoL: reported RR: (RECIST) Other: ‐ |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported in main publication | |
| Notes | Only poor risk (76%) and intermediate risk (26%) patients included in trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned in equal proportions, with the use of permuted blocks of three, to one of three treatment groups." Central randomisation presumed |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned in equal proportions, with the use of permuted blocks of three, to one of three treatment groups." Central randomisation presumed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants and personnel were not blinded to treatment, no effects on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel were not blinded to treatment, active treatment in all 3 groups |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Unblinded clinical investigator assessment, no effects on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Shorter investigator assessed PFS in comparison to independent radiologic assessment; which is explained by different inclusion criteria |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | Intention‐to‐treat population reported |
| Incomplete outcome data (attrition bias) Response rate | High risk | 82% of ITT population reported; only selected participants included in analysis which underwent tumour assessment after the baseline |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants reported |
| Incomplete outcome data (attrition bias) Quality of life | High risk | 65% of participants were evaluable for QoL analysis |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | Completed planned accrual; stopped early at the second interim analysis |
McDermott 2018.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 2 Accrual period: January 2014 to March 2015 Blinding: open label design Strata: MSKCC risk category, prior nephrectomy status, and PD‐L1 status IMC: "Independent review facility‐assessed efficacy endpoints" Crossover: allowed |
|
| Participants |
Histology: clear cell Prior systemic therapy: treatment‐naïve Eligible PS: Karnowsky PS 70% or better Measurable disease: required Non metastatic %: not specified M/F: 230/75 Age median (range): 61 Prior nephrectomy: 184 Prognostic strata: system, good/intermediate/poor risk: MSKCC, 77/201/27 |
|
| Interventions | atezolizumab 1200 mg IV q3w + bevacizumab 15 mg/kg IV q3w, atezolizumab alone or sunitinib 50 mg PO QD 4 wk on/2 wk off | |
| Outcomes |
PFS: co‐primary endpoint OS: secondary endpoint AE: reported QoL: exploratory outcome RR: secondary endpoint Other: primary endpoint: percentage of participants with disease progression per response evaluation |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Published online | |
| Notes | Crossover to atezolizumab + bevacizumab arm allowed on progression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "interactive voice/web response system (IxRS)" used; "Stratified permuted block randomization was used to assign patients in a 1:1:1 ratio to one of three treatment arms" |
| Allocation concealment (selection bias) | High risk | "allocation was unmasked" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "The study was open‐label"; participants and personnel were not blinded to treatment; no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "The study was open‐label"; participants and personnel were not blinded to treatment, all participants received an active treatment with different administration forms |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Independent review facility (IRF)‐assessed efficacy |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Independent review facility (IRF)‐assessed efficacy, no blinding used |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population reported, 1 patient did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT population reported, 1 patient did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants included in safety analysis |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | "96 (95%) of 101 patients in the atezolizumab plus bevacizumab group and 93 (92%) of 101 patients in the sunitinib group completed the MDASI at baseline" |
| Selective reporting (reporting bias) | Low risk | All planned outcomes except for quality of life reported |
| Other bias | Unclear risk | Industry sponsored |
Motzer 2010.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: August 2004 to October 2005 Blinding: imaging Strata: LDH, PS, nephrectomy status IMC: used for data and safety Crossover: study amended when sunitinib approved in January 2006 to allow cross‐over of patients on IFN on documented disease progression as primary endpoint of PFS had been met ‒ agreed with IMC |
|
| Participants |
Histology: clear cell Prior systemic therapy: treatment‐naïve Measurable disease: required Non metastatic %: metastatic disease required M/F %: 536/214 Eligible PS: ECOG 0 to 1 Age median (range): 61 (27 to 87) Prior nephrectomy: 675 Prognostic strata: system, good/intermediate/poor risk %: MSKCC, 37/56/7% |
|
| Interventions | (1) SUNITINIB 50 mg oral daily for 4 weeks of 6‐week cycle; (2) Interferon‐alfa2a 9 MU sc tiw (with cross‐over to SUNITINIB at disease progression, after second interim analysis) | |
| Outcomes |
PFS: primary endpoint OS: secondary endpoint AE: toxicity table, secondary endpoint QoL: secondary endpoint (FACT‐G, FKSI) RR: secondary endpoint Other: cross‐over post study |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “patients were randomly assigned”, presumed central randomisation |
| Allocation concealment (selection bias) | Unclear risk | Data not found |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Open label study design, participants and personnel were not blinded to treatment, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Open label study design, participants and personnel were not blinded to treatment, 2 active treatments used with different administration forms |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessed by investigator, no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "A blinded central review of radiologic images was used to assess the primary end point and the objective response rate" |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | 8% of patients withdrew consent on the control arm (vs 1%, P < 0.001) but primary end‐ point was analysed by allocation |
| Incomplete outcome data (attrition bias) Response rate | Low risk | All randomised participants analysed |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants analysed |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | No information on how many participants completed questionnaire found |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | High risk | Cross‐over permitted after second interim analysis but planned accrual had been completed, OS analysis secondary endpoint |
Motzer 2013a.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: Aug 2008 to Sep 2011 Blinding: open label design Strata: Karnofsky PS, LDH, nephrectomy status IMC: safety reviewed Crossover: not allowed |
|
| Participants |
Histology: clear cell component Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky 70% or more Measurable disease: required Non metastatic %: < 20 M/F: 813/297 Age median (range): 61 (18 to 88) Prior nephrectomy: 924 Prognostic strata: system, good/intermediate/poor risk: MSKCC, 303/650/119 |
|
| Interventions | pazopanib 800 mg po vs sunitinib 50 mg po 4 wks on, 2 wks off | |
| Outcomes |
PFS: primary outcome as non‐inferiority measure OS: secondary outcome AE: reported in toxicity table, secondary outcome QoL: secondary outcome RR: secondary outcome Other: medical resource utilization |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Published online | |
| Notes | Non‐inferiority design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “GSK interactive voice response system called RAMOS (Registration And Medication Ordering System), by the investigator or authorized site staff for stratification and central randomization.” from protocol |
| Allocation concealment (selection bias) | Low risk | “GSK interactive voice response system called RAMOS (Registration And Medication Ordering System), by the investigator or authorized site staff for stratification and central randomization.” from protocol |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Open label study design, participants and personnel were not blinded to treatment, OS reported as secondary outcome |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Open label study design, participants and personnel were not blinded to treatment, oral administered, active drugs used in both arms |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Not specifically reported but no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "The primary end point was progression‐free survival as assessed by independent review"; tumour response and PFS assessed independently, lack of blinding |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population assessed, 8 patients not treated |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT population assessed, 8 patients not treated |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants assessed |
| Incomplete outcome data (attrition bias) Quality of life | High risk | High losses in questionnaire completion rates |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Unclear risk | Protocol amended to increase sample size for planned events, non‐inferiority design |
Motzer 2013b.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: February 2010 to August 2010 Blinding: open label design Strata: region, number of prior treatments, number of metastatic sites and organs involved IMC: data monitored not specified Crossover: at progression |
|
| Participants |
Histology: clear cell component Prior systemic therapy: treatment‐naïve; except for immunotherapy, chemotherapy, or hormonal therapy Eligible PS: ECOG PS 1 or better Measurable disease: required Non metastatic %: ≤ 21 M/F: 374/143 Age median (range): 59 (23 to 85) Prior nephrectomy: required Prognostic strata: system, good/intermediate/poor risk: MSKCC 157/333/27 |
|
| Interventions | tivozanib 1.5 mg, 3 wks on/1 wks off vs sorafenib 400 mg bid | |
| Outcomes |
PFS: primary outcome OS: secondary outcome AE: secondary outcome QoL: secondary outcome RR: secondary outcome Other: tolerability, kidney specific symptoms |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An Interactive Voice Response / Interactive Web Response (IVR/IWR) system will be used for enrolment, randomization and drug management” from protocol |
| Allocation concealment (selection bias) | Low risk | “An Interactive Voice Response / Interactive Web Response (IVR/IWR) system will be used for enrolment, randomization and drug management” from protocol; central randomization |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "This was an open‐label, randomized phase III trial"; participants and personnel were not blinded to treatment, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "This was an open‐label, randomized phase III trial"; participants and personnel were not blinded to treatment; oral administered, active drugs used in both arms |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessment not specified; no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "The primary end point was progression‐free survival (PFS) by independent review", open label trial design |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population analysed, 1 patient not treated in experimental arm |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT population analysed, 1 patient not treated in experimental arm |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants assessed for safety |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | "HRQoL questionnaires were completed by more than 99% of patients in both arms at baseline. Completion rates decreased over time, in line with study dropout, falling below 50% after cycle 13" |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | High risk | Possible confounding of OS data by cross‐over design |
Motzer 2014.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 2 Accrual period: October 2009 to June 2011 Blinding: open label design Strata: MSKCC risk category IMC: data not found Crossover: within treatment arms from first to second line |
|
| Participants |
Histology: any Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky PS 70% or better Measurable disease: required Non metastatic %: not specified M/F: 342/129 Age median (range): 62 (20 to 89) Prior nephrectomy: 315 Prognostic strata: system, good/intermediate/poor risk: MSKCC, 139/263/67 |
|
| Interventions | first line: everolimus 10 mg po; second line: sunitinib 50 mg po 4 wks on, 2 wks off vs first line: sunitinib 50 mg po 4 wks on, 2 wks off; second line everolimus 10 mg po | |
| Outcomes |
PFS: primary outcome for first line treatment OS: secondary outcome AE: reported in toxicity table QoL: reported RR: assessed as secondary outcome Other: tolerability, PFS after second line treatment |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | Non‐inferiority design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned in a 1:1 manner; Interactive Voice Response System (IVRS) to randomize the patient" |
| Allocation concealment (selection bias) | Low risk | Patient randomisation list used; described in detail |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "RECORD‐3 [...] was an open‐label, randomized, multicenter, phase II study...", no effects on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "RECORD‐3 [...] was an open‐label, randomized, multicenter, phase II study..."; same drugs with different sequence used in both arms but participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessment not specified, no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "The primary end point was to assess progression‐free survival (PFS) non‐inferiority of first‐line everolimus compared with sunitinib by investigator assessment" |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population reported, 2 patients in control arm did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT population reported, 2 patients in control arm did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants reported |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | High initial questionnaire completion rate, decreased in both arms over time |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | High risk | Difference in baseline performance status, non‐inferiority design |
Motzer 2019.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: March 2016 to December 2017 Blinding: open label design Strata: ECOG PS (0 or 1), geographic region IMC: external data monitoring committee used for efficacy and safety Crossover: not planned |
|
| Participants |
Histology: clear cell renal cell carcinoma Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky PS 70% or better Measurable disease: required Non metastatic %: not specified M/F: 660/226 Age median (range): 61 (27 to 88) Prior nephrectomy: 707 Prognostic strata: system, good/intermediate/poor risk: MSKCC, 196/576/96 |
|
| Interventions | Avelumab administered at 10 mg/kg IV every 2 weeks in combination with Axitinib, 5 mg PO BID versus Sunitinib given at 50 mg PO QD on schedule 4/2 | |
| Outcomes |
PFS: primary outcome among patients with PD‐L1–positive tumours, secondary endpoint for overall population OS: primary outcome among patients with PD‐L1–positive tumours, secondary endpoint for overall population AE: reported in toxicity table QoL: not assessed RR: assessed as secondary outcome Other: pharmacokinetic measures, tumour‐tissue biomarker |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported online | |
| Notes | Primary endpoints changed after protocol amendment in June 2017 to evaluate PFS or OS for PD‐L1 positive participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the study treatment assignment using the Interactive Response Technology (IRT) system (interactive web‐based response [IWR]/interactive voice response [IVR] system)" from protocol |
| Allocation concealment (selection bias) | Low risk | "the study treatment assignment using the Interactive Response Technology (IRT) system (interactive web‐based response [IWR]/interactive voice response [IVR] system)" from protocol |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "This was a multicenter, randomized, open‐label, phase‐3 trial..."; open label trial design, no effects on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "This was a multicenter, randomized, open‐label, phase‐3 trial..."; different drugs and administration form, participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | OS assessment of PD‐L1 positive population. The evaluation of PD‐L1 status a priori to assessment of OS is not considered as objective. Trial design is open‐label. However, we extracted OS result from overall population. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Primary and main secondary endpoints determined by blinded independent central review |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT and PD‐L1 population reported separately for PFS, 8 and 5 participants did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT and PD‐L1 population reported separately, 8 and 5 participants did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants reported in toxicity table |
| Selective reporting (reporting bias) | Unclear risk | OS data not mature at time of publication, protocol amended after study start but initially planned outcomes reported |
| Other bias | Low risk | Planned accrual completed, protocol changes after study start. |
Ravaud 2015.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 2 Accrual period: data not found Blinding: open label design Strata: MSKCC risk category IMC: used to analyse tumour responses Crossover: not allowed |
|
| Participants |
Histology: predominantly clear‐cell mRCC Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky PS 70% or better Measurable disease: required Non metastatic %: 0, metastatic disease mandatory M/F: 269/96 Age median (range): 60 (20 to 84) Prior nephrectomy: partial or radical nephrectomy mandatory Prognostic strata: system, good/intermediate/poor risk: MSKCC, 131/208/26 |
|
| Interventions | Everolimus 10 mg po and bevacizumab 10mg/kg iv every 2 wks vs IFN 9 MIU 3 times per wk plus bevacizumab 10 mg/kg every 2 weeks | |
| Outcomes |
PFS: primary endpoint OS: secondary outcome AE: secondary outcome, reported in toxicity table QoL: not assessed RR: secondary outcome Other: duration of response |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized 1:1" |
| Allocation concealment (selection bias) | Unclear risk | Data not found |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "open‐label, phase II RECORD‐2 trial"; personnel and participants were not blinded to treatment, different drug administration forms used, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | "open‐label, phase II RECORD‐2 trial"; personnel and participants were not blinded to treatment, different drug administration forms used |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Tumor response and progression were evaluated by the local radiologist and independent central review |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Tumor response and progression were evaluated by the local radiologist and independent central review; trial design is open‐label |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population reported, 3 patients did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT population reported, 3 patients did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All but 1 participant who received treatment were included in safety analysis |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | No available data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Study not powered to show significant treatment effect |
Retz 2019.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: June 2012 to November 2016 Blinding: open label design Strata: low versus intermediate MSKCC risk score, clear cell versus non‐clear cell histology IMC: data not found Crossover: from first to second‐line after disease progression |
|
| Participants |
Histology: any Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky PS 70% or better Measurable disease: required Non metastatic %: data not found M/F: 189/188 Age median (range): 68 (26 to 86) Prior nephrectomy: 328 Prognostic strata: system, good/intermediate/poor risk: MSKCC, 186/179/9 |
|
| Interventions | Sorafenib 400 mg bid orally until progression or intolerable toxicity, followed by pazopanib 800 mg once daily orally until progression or intolerable toxicity or vice versa | |
| Outcomes |
PFS: primary outcome OS: reported AE: reported QoL: planned RR: planned Other: time to first‐line treatment failure, biomarker |
|
| Funding Sources | Technische Universität München | |
| Declarations of interest | Reported online | |
| Notes | MSKCC PS low or intermediate patients only, non‐inferiority study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "377 patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Data not found |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "Phase III randomized, sequential, open‐label study"; participants and personnel were not masked to treatment; no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "Phase III randomized, sequential, open‐label study"; participants and personnel were not masked to treatment; same drugs used in both arms |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Open label study design but no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Data not found |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | Intention to treat population analysed |
| Incomplete outcome data (attrition bias) Response rate | Low risk | Intention to treat population analysed |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | 83/189 (96.8%) and 183/188 (97.3%) population analysed in each arm |
| Incomplete outcome data (attrition bias) Quality of life | High risk | 136/189 (71.9%) and 131/188 (69.6%) population analysed in each arm |
| Selective reporting (reporting bias) | Unclear risk | Quality of life outcome not published in conference abstract |
| Other bias | Low risk | Not detected |
Rini 2008.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: October 2003 to July 2005 Blinding: open label design Strata: nephrectomy status; prognostic risk IMC: Data Safety Monitoring Board Crossover: data not found |
|
| Participants |
Histology: clear cell Prior systemic therapy: naïve Measurable disease: measurable or non‐measurable disease Non metastatic %: not specified M/F %: 508/224 Eligible PS: Karnofsky; 100 to 70% Age median: 62 Prior nephrectomy: 620 Prognostic strata: system, good/intermediate/poor risk %: MSKCC, 26/64/10 |
|
| Interventions | INTERFERON alfa‐2b (Intron, Schering‐Plough) 9 MU SC TIW (1) with, or (2) without, BEVACIZUMAB 10 mg/kg IV Q2weeks | |
| Outcomes |
PFS: secondary endpoint OS: primary endpoint AE: secondary endpoint, reported in toxicity table QoL: not assessed RR: secondary endpoint Other: ‐ |
|
| Funding Sources | Independent sponsoring | |
| Declarations of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A stratified random block design was used"; Central randomisation by cooperative groups (CALGB and NCI‐Canada) |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "There was no placebo infusion in this non blinded trial", participants and personnel were not blinded to treatment, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "There was no placebo infusion in this non blinded trial", participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "no independent review of radiographs"; Investigator assessment of total population, overall survival primary outcome assumed reliable |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "no independent review of radiographs", assessed by investigator |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | All patients accounted for with small symmetric losses |
| Incomplete outcome data (attrition bias) Response rate | Low risk | All patients accounted for with small symmetric losses |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | On treated participant not reported |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes reported |
| Other bias | Low risk | Planned accrual completed; independently sponsored and conducted |
Rini 2014.
| Study characteristics | ||
| Methods | Study design: multicentre RCT Phase: 3 Accrual period: April 2008 to October 2010 Blinding: open label design Strata: nephrectomy status, MSKCC risk category IMC: external data monitoring committee used Crossover: not allowed | |
| Participants | Histology: clear cell component Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky PS 70% or better Measurable disease: yes Non metastatic %: data not found M/F: 556/235 Age median (range): 58 (22 to 87) Prior nephrectomy: 673 Prognostic strata: system, good/intermediate/poor risk: MSKCC, 237/467/87 | |
| Interventions | Temsirolimus 25 mg iv weekly plus Bevacizumab 10 mg/kg iv every 2 weeks or IFN 9 million U[MIU] subcutaneously thrice weekly plus Bevacizumab 10 mg/kg IV every 2 weeks | |
| Outcomes | PFS: independent assessment as primary endpoint OS: secondary endpoint AE: secondary endpoint QoL: assessed as exploratory objective RR: secondary endpoint Other: "Disease‐related symptoms and quality of life were assessed as exploratory objectives." | |
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported in main publication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerized centrally located randomization system was used" |
| Allocation concealment (selection bias) | Low risk | "A computerized centrally located randomization system was used" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "randomized, open‐label, multicenter, phase III study"; participants and personnel were not blinded to treatment, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "randomized, open‐label, multicenter, phase III study"; participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "Secondary end points were investigator‐assessed PFS, independently assessed ORR, OS, and safety" no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "Radiographic evaluations were conducted at screening and every 8 weeks, and tumor progression was assessed both by investigators and by an independent blinded assessment" |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population reported, 7 patients in 1 arm did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | ITT population reported, 7 patients in 1 arm did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants reported in toxicity table |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | "Completion rate for each questionnaire was uniformly high in both treatment arms, with rates above 90% among patients on treatment up to the end of treatment visit" assessed as exploratory outcome only |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Unclear risk | Planned accrual almost completed (791 versus 800) |
Rini 2016.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: December 2010 to December 2012 Blinding: open label design Strata: IMDC risk group, region, nephrectomy status IMC: data and safety monitored Crossover: not allowed |
|
| Participants |
Histology: clear cell component Prior systemic therapy: treatment‐naïve Eligible PS: Karnowsky PS 80% or better Measurable disease: required Non metastatic %: ≤ 28 M/F: 230/109 Age median (range): 61 (54 to 69) Prior nephrectomy: 306 Prognostic strata: system, good/intermediate/poor risk %: IMDC, 91/241/7 |
|
| Interventions | Sunitinib 50 mg po 4 wks on, 2 wks off vs sunitinib 50 mg po 4 wks on, 2 wks off and Cyclophosphamide (1 dose of 300 mg/m² iv) at visit D (3 days before the first vaccination at visit 1) and the vaccination schedule (GM‐CSF 75 μg and IMA901 4.13 mg intradermally at each vaccination) included 6 vaccinations within the first 3 weeks (visits 1 to 6 on days 1, 2, 3, 8, 15, and 22, respectively) and a further 4 vaccinations at 3‐week intervals (visits 7 to 10 on days 43, 64, 85, and 106, respectively) | |
| Outcomes |
PFS: secondary outcome OS: primary endpoint AE: reported in toxicity table, secondary outcome QoL: not assessed RR: secondary outcome Other: biomarker related OS |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | Only favourable or intermediate risk according to the International Metastatic Database Consortium (IMDC) risk criteria patients included, all patients treated with sunitinib and evaluated for efficacy before randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomised centrally with an interactive web response system..." |
| Allocation concealment (selection bias) | Low risk | "...randomised centrally with an interactive web response system..." |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "patients and investigators were not masked to treatment allocation", open label study design, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "patients and investigators were not masked to treatment allocation", open label study design |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "The primary outcome was overall survival from randomisation until death of any cause as determined by local investigators"; no effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "progression‐free survival from randomisation according to blinded, independent central review", "Best tumour response was assessed according to RECIST 1.1 and was based on centrally reviewed tumour images" |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | ITT population reported, 8 patients did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | All randomised participants analysed |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants analysed |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | High risk | All participants treated with 1 of the study drugs in run in phase, only responders randomised |
Rini 2019a.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: October 2016 to January 2018 Blinding: open label design Strata: IMDC risk group (favourable, intermediate, or poor risk) and geographic region IMC: an independent data and safety monitoring committee oversaw the trial Crossover: not planned |
|
| Participants |
Histology: clear‐cell renal‐cell carcinoma Prior systemic therapy: treatment‐naïve Eligible PS: Karnowsky PS 70% or better Measurable disease: required Non metastatic %: < 99 M/F: 628/233 Age median (range): 62 (26 to 90) Prior nephrectomy: 715 Prognostic strata: system, good/intermediate/poor risk %: IMDC, 269/484/108 |
|
| Interventions | Pembrolizumab 200 mg intravenously every 3 weeks plus Axitinib 5 mg orally twice daily versus Sunitinib 50 mg orally once daily for 4 weeks and then are off treatment for 2 weeks | |
| Outcomes |
PFS: co‐primary outcome assessed by blinded, independent central review OS: co‐primary outcome assessed by blinded, independent central review AE: reported in toxicity table, secondary outcome QoL: not assessed RR: secondary outcome assessed by blinded, independent central review Other: duration of response |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported online | |
| Notes | Planned accrual completed, primary endpoint for OS not met because of short follow up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment randomization will occur centrally using an interactive voice response system / integrated web response system (IVRS/IWRS)" from protocol |
| Allocation concealment (selection bias) | Low risk | "Treatment randomization will occur centrally using an interactive voice response system / integrated web response system (IVRS/IWRS)" from protocol; central randomisation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "In this open‐label, phase 3 trial..." participants and personnel were not blinded to treatment, no effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "In this open‐label, phase 3 trial..." different drugs and administration form, participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | OS assessed by blinded, independent central review |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Subjective primary and secondary efficacy endpoints assessed by blinded, independent central review |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | Intention to treat population analysed; < 1% in both groups did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | Intention to treat population analysed; < 1% in both groups did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants reported in toxicity table |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported, no mature OS data available |
| Other bias | Low risk | Not detected |
Rini 2019b.
| Study characteristics | ||
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: May 2015 to October 2016 Blinding: open label design Strata: PD‐L1 expression, presence of liver metastasis, MSKCC risk category IMC: "An independent data monitoring committee reviewed safety data during the study on a periodic basis" Crossover: "No prespecified crossover was planned per protocol" |
|
| Participants |
Histology: "clear‐cell histology and/or a component of sarcomatoid carcinoma" Prior systemic therapy: treatment‐naïve Eligible PS: Karnowsky PS 70% or better Measurable disease: required Non metastatic %: not specified M/F: 669/246 Age median (range): 61 (54 to 69) Prior nephrectomy: 664 Prognostic strata: system, good/intermediate/poor risk %: MSKCC, 179/629/107 |
|
| Interventions | Atezolizumab administered at a fixed dose of 1200 milligrams (mg) via intravenous (IV) infusion on Days 1 and 22 of each 42‐day cycle in combination with Bevacizumab administered at a dose of 15 milligrams per kilogram (mg/kg) via IV infusion on Days 1 and 22 of each 42‐day cycle versus Sunitinib administered at a dose of 50 mg once daily, orally via capsule, on Day 1 through Day 28 of each 42‐day cycle. | |
| Outcomes |
PFS: co‐primary outcome by investigator assessment in patients with PD‐L1 positive disease, secondary outcome in the ITT population OS: co‐primary outcome in the intention‐to‐treat population, secondary outcome in the PD‐L1 positive population AE: secondary outcome QoL: reported RR: secondary outcome Other: |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported in main publication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned (1:1) via an interactive voice and web response system to receive Atezolizumab plus Bevacizumab or Sunitinib." central randomisation presumed |
| Allocation concealment (selection bias) | Low risk | Central randomisation presumed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "The study was open label, and investigators and participants were not masked to treatment allocation" No effect on OS expected |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "The study was open label, and investigators and participants were not masked to treatment allocation", different drugs and forms of administration used in experimental and control arm |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessment not specified but not effect on OS expected |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | "Co‐primary endpoints were progression‐free survival (RECIST 1.1) by investigator assessment in patients with PD‐L1 positive disease" PFS results in PD‐L1 population differ from independent assessment |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | Intention to treat population reported, 3 participants in experimental and 15 participants in control arm did not receive treatment |
| Incomplete outcome data (attrition bias) Response rate | Low risk | Intention to treat population reported, 3 participants in experimental and 15 participants in control arm did not receive treatment |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants reported |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | "386 (86%) of 451 patients in the atezolizumab plus bevacizumab group and 369 (83%) of 446 patients in the sunitinib group completed the MDASI at baseline" |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | Planned accrual completed |
Sternberg 2010.
| Study characteristics | ||
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: April 2006 to April 2007 Blinding: double‐blind, placebo‐controlled Strata: ECOG PS 0vs1; nephrectomy status; prior cytokine IMC: responsible for safety monitoring and to review interim overall survival data Crossover: allowed from placebo to active treatment |
|
| Participants |
Histology: clear cell Prior systemic therapy: 1 line of cytokines permitted. Measurable disease: required Non metastatic %: < 18 M/F: 307/128 Eligible PS: ECOG 0 to 1 Age median(range): 59 (25 to 85) Prior nephrectomy: 385 Prognostic strata: system, good/intermediate/poor risk %: MSKCC; 39/54/3 |
|
| Interventions | (1) PAZOPANIB 800 mg PO daily, vs (2) matched PLACEBO (2:1 randomization) Cross‐over 48% | |
| Outcomes |
PFS: primary endpoint OS: principal secondary end point AE: toxicity table available, additional secondary end point QoL: reported RR: additional secondary end point Other: ‐ |
|
| Funding Sources | Industry sponsored | |
| Declarations of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomly assigned in a 2:1 ratio..."; central randomisation |
| Allocation concealment (selection bias) | Low risk | "Patients were centrally randomly assigned in a 2:1 ratio..."; central randomisation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "was a placebo‐controlled, randomized, double‐blind, global, multicenter, phase III study"; participants and personnel were masked to treatment |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | "was a placebo‐controlled, randomized, double‐blind, global, multicenter, phase III study"; participants and personnel were masked to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "All imaging scans were evaluated by an independent imaging‐review committee (IRC) blinded to study treatment" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "All imaging scans were evaluated by an independent imaging‐review committee (IRC) blinded to study treatment" |
| Incomplete outcome data (attrition bias) PFS, OS | Low risk | All patients accounted for, losses 6% (investigational) and 3% (control) unlikely significant, “completion rates > 90% across most of the assessment time points” |
| Incomplete outcome data (attrition bias) Response rate | Low risk | All patients accounted for, losses 6% (investigational) and 3% (control) unlikely significant, “completion rates > 90% across most of the assessment time points” |
| Incomplete outcome data (attrition bias) Serious and minor adverse events | Low risk | All treated participants analysed |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | "Completion rates for QoL questionnaires were high across most of the assessment time points for each instrument" |
| Selective reporting (reporting bias) | Low risk | All protocol specified endpoints reported |
| Other bias | Low risk | Planned accrual completed |
AE: adverse event; ECOG: Eastern Cooperative Oncology Group; F: female; IMC: independent monitoring committee; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; ITT: intention to treat; M: male; MSKCC: Memorial Sloan Kettering Cancer Center; OS: overall survival; PFS: progression‐free survival; PS: performance status; QoL: quality of life; RCT: randomised controlled trial; RR: response rate; wks: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armstrong 2016 | Non‐clear cell renal cell carcinoma participants only, less than 100 participants per treatment arm included |
| Atkins 2004 | Same targeted drug used in all treatment arms, less than 100 participants per treatment arm included, cytokine pretreatment required |
| Bracarda 2010 | Same targeted treatment used in both arms, less than 100 participants per treatment arm included |
| Broom 2015 | Less than 100 participants per treatment arm included |
| Bukowski 2007 | Less than 100 participants per treatment arm included |
| Choueiri 2015 | Participants were not treatment‐naïve |
| Choueiri 2017 | Less than 100 participants per treatment arm included |
| Cirkel 2016 | Less than 100 participants per treatment arm included |
| Dorff 2015 | Participants not treatment‐naïve, less than 100 participants per treatment arm included |
| Ebbinghaus 2007 | Same drug used in both treatment arms, less than 100 participants per treatment arm included |
| Eisen 2015 | Less than 100 participants per treatment arm included |
| Escudier 2007 | Treatment is not considered as targeted therapy |
| Escudier 2009 | Less than 100 participants per treatment arm included |
| Escudier 2010a | Participants were not treatment naïve |
| Flaherty 2015 | Less than 100 participants per treatment arm included |
| Gordon 2004 | No treatment that is considered as targeted therapy was used in trial |
| Hainsworth 2015 | Less than 100 participants per treatment arm included |
| Hawkins 2016 | Treatment not considered as targeted therapy, any renal cell carcinoma histologies included |
| Hutson 2013 | Participants were not treatment naïve |
| Hutson 2014 | Participants were not treatment naïve |
| Jonasch 2010 | Less than 100 participants per treatment arm included |
| Jonasch 2017 | Participants were not treatment naïve |
| Lee 2006 | Treatment not considered as targeted therapy |
| Lee 2015 | Same study drugs used in both arms, less than 100 participants per treatment arm included |
| Madhusudan 2004 | Treatment not considered as targeted therapy |
| Motzer 2008 | Participants were not treatment‐naïve |
| Motzer 2012 | Same study drug used in both arms |
| Motzer 2014b | Participants were not treatment‐naïve |
| Motzer 2015a | Participants were not treatment‐naïve |
| Motzer 2015b | Participants were not treatment‐naïve |
| Motzer 2015c | Participants were not treatment‐naïve |
| Mulders 2012 | Less than 100 participants per treatment arm included |
| Nosov 2012 | Less than 100 participants per treatment arm included |
| Négrier 2011 | Less than 100 participants per treatment arm included |
| Pal 2015 | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
| Pili 2015 | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
| Powles 2014 | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
| Powles 2016a | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
| Powles 2016b | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
| Procopio 2011 | Less than 100 participants per treatment arm included |
| Ratain 2006 | Less than 100 participants per treatment arm included, 84% of participants pretreated |
| Ravaud 2008 | Treatment in control arm not eligible for comparisons |
| Rini 2011 | Participants were not treatment‐naïve |
| Rini 2012 | Treatment in observational and control arm not eligible for comparison |
| Rini 2013 | Less than 100 participants per treatment arm included |
| Srinivas 2005 | Treatment not considered as targeted therapy |
| Stadler 2005 | Treatment is not considered as targeted therapy |
| Tannir 2016 | Less than 100 participants per treatment arm included, non‐clear cell renal cell carcinoma only |
| Tannir 2018 | Less than 100 participants per treatment arm included |
| Tomita 2014 | Less than 100 participants per treatment arm included |
| Twardowski 2015 | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
| Yang 2003 | Less than 100 participants per treatment arm included, participants were not treatment‐naïve |
Characteristics of ongoing studies [ordered by study ID]
Choueiri 2018.
| Study name | NCT03141177, CheckMate 9ER |
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: data not found Blinding: open label study design Strata: IMDC risk score, PD‐1 ligand 1 (PD‐L1) tumour expression and geographic region IMC: data not found Crossover: data not found |
| Participants |
Histology: clear cell component Prior systemic therapy: no prior systemic therapy for RCC Measurable disease: required Eligible PS: data not found Non metastatic %: data not found M/F %: data not found Age median(range): data not found Prior nephrectomy: data not found Prognostic strata: system, good/intermediate/poor risk %: data not found |
| Interventions | Nivolumab and cabozantinib versus sunitinib |
| Outcomes |
PFS: primary endpoint OS: secondary end point AE: secondary end point QoL: not planned RR: secondary end point Other: ‐ |
| Starting date | July 2017 |
| Contact information | |
| Notes |
Choueiri 2019.
| Study name | NCT03937219, Cosmic‐313 |
| Methods |
Study type: multicentre RCT Phase: 3 Accrual period: 25 June 2019 ‐ Blinding: double‐blind study design Strata: IMDC risk score and geographic region IMC: data not found Crossover: data not found |
| Participants |
Histology: clear cell component Prior systemic therapy: no prior systemic therapy for RCC Measurable disease: required Eligible PS: Karnofsky PS 70% or better Non metastatic %: data not found M/F %: data not found Age median (range): data not found Prior nephrectomy: data not found Prognostic strata: system, good/intermediate/poor risk %: data not found |
| Interventions | Cabozantinib + nivolumab + ipilimumab (4 doses) followed by cabozantinib + nivolumab vs Cabozantinib‐matched placebo + nivolumab + ipilimumab (4 doses) followed by cabozantinib‐matched placebo + nivolumab |
| Outcomes |
PFS: primary endpoint OS: secondary end point AE: ‐ QoL:‐ RR:‐ Other: ‐ |
| Starting date | June 2019 |
| Contact information | |
| Notes |
Grünwald 2018.
| Study name | NCT02959554, NIVOSWITCH |
| Methods |
Study design: multicentre RCT Phase: 2 Accrual period: data not found Blinding: open label study design Strata: data not found IMC: data not found Crossover: data not found |
| Participants |
Histology: clear cell component Prior systemic therapy: First‐line treatment with a TKI for 10‐12 weeks (limited to sunitinib or pazopanib) Eligible PS: ECOG‐PS 0‐2 Measurable disease: required Non metastatic %: data not found M/F %: data not found Age median, years: data not found Prior nephrectomy: data not found Prognostic strata: system, good/intermediate/poor risk %: data not found |
| Interventions | Nivolumab: 240 mg iv on D1 of every cycle (Q2W) for 16 weeks. After 16 weeks 480 mg iv on D1 of every cycle (Q4W) until disease progress, intolerable toxicity, withdrawal of consent or end of study versus Sunitinib: According to Standard of Care (SOC). Recommended dose is 50 mg PO once daily for 4 consecutive weeks followed by a 2‐week rest period (schedule 4/2) to comprise a complete cycle of 6 weeks (until disease progress, intolerable toxicity, withdrawal of consent or end of study) or Pazopanib: According to Standard of Care (SOC). Recommended dose is 800 mg PO daily continuously (until disease progress, intolerable toxicity, withdrawal of consent or end of study) |
| Outcomes |
PFS: secondary endpoint OS: primary end point AE: secondary end point QoL: secondary endpoint RR: secondary end point Other: ‐ |
| Starting date | December 2016 |
| Contact information | Principal Investigator: Prof. Dr. Viktor Grünwald |
| Notes |
Motzer 2018.
| Study name | NCT02811861 |
| Methods |
Study design: multicentre RCT Phase: 3 Accrual period: data not found Blinding: open label design Strata: data not found IMC: data not found Crossover: data not found |
| Participants |
Histology: clear cell carcinoma Prior systemic therapy: treatment‐naïve Eligible PS: Karnofsky PS 70% or better Measurable disease: required Non metastatic %: data not found M/F %: data not found Age median, years: data not found Prior nephrectomy: data not found Prognostic strata: system, good/intermediate/poor risk %: data not found |
| Interventions | lenvatinib 18 milligrams (mg) administered orally, once daily, plus everolimus 5 mg administered orally, once daily; Lenvatinib 20 mg administered orally, once daily, plus pembrolizumab 200 mg administered intravenously (IV), every 3 weeks; Sunitinib 50 mg administered orally, once daily, on a schedule of 4 weeks on treatment followed by 2 weeks off treatment |
| Outcomes |
PFS: primary endpoint OS: planned AE: planned QoL: planned RR: primary endpoint Other: ‐ |
| Starting date |
Trial start date: 13 October 2016 Trial completion date: Estimated 15 January 2020 |
| Contact information | Responsible party/ principal investigator: Eisai Inc. |
| Notes | Study based on results of phase 2 study data |
AE: adverse event; ECOG: Eastern Cooperative Oncology Group; F: female; IMC: independent monitoring committee; IMDC: International Metastatic Renal Cell Carcinoma Database Consortium; IV; intravenous; M: male; OS: overall survival; PFS: progression‐free survival; PO; per oral; PS: performance status; QoL: quality of life; RCC: renal cell carcinoma; RR: response rate; TKI: tyrosine kinase inhibitor
Differences between protocol and review
This review is based on a published protocol (Hofmann 2017) and underwent some changes during the process of completion.
We expanded the scope to include newer immunotherapy agents. Several of these drugs have been compared to targeted agents mainly in first line treatment and the comparisons are included in this review.
We initially planned to include second and further line treatments into this review. Since the first publication of the protocol there has been a dramatic increase in available treatment options mainly in first, but also in second and further lines. We therefore chose to focus on treatment‐naïve patients to retain a manageable review scope.
We restricted the number of participants per study arm to at least 100 which we considered as sufficient to make clinically substantial conclusions and at the same time limit small study bias.
Contributions of authors
Contributions to the protocol
This protocol version concept and design: Fabian Hofmann, Thomas BL Lam, Axel Bex
Submitted and revised protocol: final approval by all authors.
Contributions to the review
Fabian Hofmann (FH): conception and study design, drafting the protocol, searching for trials, study selection, extracting data, assessing risk of bias, performing data analysis, interpretation of data, and drafting the review.
Eu Chang Hwang (ECH): extracting data, assessing risk of bias, performing data analysis, interpretation of data.
Thomas BL Lam (TB): conception and study design, drafting the protocol, searching for trials, study selection, extracting data, assessing risk of bias, drafting the review and providing methodological advices on the review.
Axel Bex (AB): conception and study design, drafting the protocol, searching for trials, study selection, drafting the review and providing clinical advices on the review.
Yuhong Yuan (YY): creating search strategies, searching for trials and drafting the review.
Lorenzo SO Marconi (LM): searching for trials, study selection, extracting data, assessing risk of bias
Börje Ljungberg (BL): searching for trials, study selection, drafting the review and providing clinical advices on the review.
Sources of support
Internal sources
-
Fabian Hofmann, Sweden
No sources of support supplied for Fabian Hofmann
-
Eu Chang Hwang, Korea, South
Chonnam National University Hwasun Hospital, Hwasun, Korea, South Salary support for Eu Chang Hwang
-
Thomas BL Lam, UK
Aberdeen Royal Infirmary, NHS Grampian, Aberdeen, Scotland, United Kingdom provided salary support for Dr. Thomas B. L. Lam
-
Axel Bex, UK
No sources of support supplied for Axel Bex
-
Lorenzo Marconi, Portugal
No sources of support supplied for Lorenzo Marconi
-
Yuhong Yuan, Canada
No sources of support supplied for Yuhong Yuan
-
Börje Ljungberg, Sweden
Department of surgical and perioperative sciences, Umeå University, Umeå, Sweden provided salary support for Börje Ljungberg
External sources
-
Fabian Hofmann, Sweden
No sources of support supplied for Fabian Hofmann
-
Eu Chang Hwang, Korea, South
No sources of support supplied for Eu Chang Hwang
-
Thomas BL Lam, UK
No sources of support supplied for Dr. Lam
-
Axel Bex, UK
No sources of support supplied for Axel Bex
-
Yuhong Yuan, Canada
No sources of support supplied for Yuhong Yuan
-
Lorenzo Marconi, Portugal
No sources of support supplied for Lorenzo Marconi
-
Börje Ljungberg, Sweden
No sources of support supplied for Börje Ljungberg
Declarations of interest
F Hofmann: declares the following relevant activities outside the submitted work: employed as a urologist, serves as guideline associate of European Association of Urology Renal Cell Carcinoma Guideline Panel and reports receiving no compensation for panel membership. Received payment from Ipsen for presenting at Ipsen‐sponsored symposia and conferences.
EC Hwang: none known
LSO Marconi: none known
Yuhong Y: none known.
TBL Lam: declares the following relevant activity outside the submitted work: serves as member of European Association of Urology Renal Cell Carcinoma Guideline Panel and reports receiving no compensation for panel membership.
A Bex: declares the following relevant activities outside the submitted work: received consultancy support paid to his institution by Pfizer and Novartis for taking part in advisory boards; received payment from Pfizer and GlaxoSmithKline for presenting at Pfizer and GlaxoSmithKline sponsored symposia and conferences. These companies produce interventions (mTOR inhibitors and VEGF‐targeting therapy) that are researched in the review. Dr. Bex also reports that he is principal investigator of the European Organisation for Research and Treatment of Cancer (EORTC) SURTIME trial, a randomised phase III trial comparing immediate versus deferred nephrectomy in patients with synchronous metastatic renal cell carcinoma, which is in part supported by a grant from Pfizer to the sponsor (EORTC).
B Ljungberg: declares the following relevant activities outside the submitted work: received support from Pfizer, GlaxoSmithKline and Novartis for advisory board attendance, most recently in early 2013, on the topic of renal cell carcinoma. Most interventions assessed in the review are produced by these companies.
New
References
References to studies included in this review
Eichelberg 2015 {published data only}
- Eichelberg C, Goebell PJ, Vervenne WL, De Santis M, Fischer von Weikersthal L, Lerchenmüller C, et al. Updated overall survival, multivariate, and Q-TWiST analyses of a randomized, sequential, open-label study (SWITCH) to evaluate the efficacy and safety of sorafenib (So)-sunitinib (Su) versus Su-So in the treatment of metastatic renal cell cancer. Annals of Oncology 2014;25(suppl 4):iv290. [DOI: 10.1093/annonc/mdu337.28] [DOI] [Google Scholar]
- *.Eichelberg C, Vervenne WL, De Santis M, Fischer von Weikersthal L, Goebell PJ, Lerchenmüller C, et al. SWITCH: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the treatment of metastatic renal cell cancer. European Urology 2015;68(5):837-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Goebell PJ, Vervenne W, De Santis M, Fischer Von Weikersthal L, Lerchenmuller CA, Zimmermann U, et al. Subgroup analyses of a randomized sequential open-label study (SWITCH) to evaluate efficacy and safety of sorafenib (SO)/sunitinib (SU) versus SU/SO in the treatment of metastatic renal cell cancer (mRCC). Journal of Clinical Oncology 2014;32(15 suppl):4567. [DOI: 10.1200/jco.2014.32.15_suppl.4567] [DOI] [Google Scholar]
- Michel MS, Vervenne W, Santis M, Fischer von Weikersthal L, Goebell PJ, Lerchenmueller J, et al. SWITCH: A randomized sequential open-label study to evaluate efficacy and safety of sorafenib (SO)/sunitinib (SU) versus SU/SO in the treatment of metastatic renal cell cancer (mRCC). Journal of Clinical Oncology 2014;32(4 suppl):393. [DOI: 10.1200/jco.2014.32.4_suppl.393] [DOI] [Google Scholar]
- NCT00732914. Sequential study to treat renal cell carcinoma [A phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by sunitinib versus sunitinib followed by sorafenib in the treatment of first-line advanced / metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT00732914 (first received 12 August 2008). [NCT00732914]
Escudier 2010 {published data only}
- Bajetta E, Ravaud A, Bracarda S, Négrier S, Szczylik C, Bellmunt J, et al. Efficacy and safety of first-line bevacizumab (BEV) plus interferon-α2a (IFN) in patients (pts) ≥65 years with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2008;26(15 suppl):5095. [DOI: 10.1200/jco.2008.26.15_suppl.5095] [DOI] [Google Scholar]
- Bellmunt J, Melichar B, Bracarda S, Negrier SN, Ravaud A, Laeufle R, et al. Bevacizumab (BEV) and interferon (IFN) therapy does not increase risk of cardiac events in metastatic renal cell carcinoma(mRCC). European Journal of Cancer Supplements 2009;7(2):429. [DOI: 10.1016/S1359-6349(09)71454-5] [DOI] [Google Scholar]
- Bracarda S, Bellmunt J, Negrier SN, Melichar B, Ravaud A, Jethwa S, et al. What is the impact of subsequent antineoplastic therapy on overall survival (OS) following first-line bevacizumab (BEV)/interferonalpha2a (IFN) in metastatic renal cell carcinoma (mRCC)? – Experience from AVOREN. European Journal of Cancer Supplements 2009;7(2):431. [DOI: 10.1016/S1359-6349(09)71459-4] [DOI] [Google Scholar]
- Bracarda S, Koralewski P, Pluzanska A, Ravaud A, Szczylik C, Chevreau C, et al. Bevacizumab/interferon-alpha2a provides a progression-free survival benefit in all prespecified patient subgroups as first-line treatment of metastatic renal cell carcinoma (AVOREN). European Journal of Cancer Supplements 2007;5(4):281-2. [DOI: 10.1016/S1359-6349(07)71076-5] [DOI] [Google Scholar]
- Escudier B, Koralewski P, Pluzanska A, Ravaud A, Bracarda S, Szczylik C, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-a2a vs placebo/interferon-α2a as first-line therapy in metastatic renal cell carcinoma. Journal of Clinical Oncology 2007;25(18 suppl):3. [DOI: 10.1200/jco.2007.25.18_suppl.3] [DOI] [Google Scholar]
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomized double-blind phase III trial. Lancet 2007;370(9605):2103-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- *.Escudier BB, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. Journal of Clinical Oncology 2010;28(13):2144-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Escudier BJ, Bellmunt J, Negrier S, Melichar B, Bracarda S, Ravaud A, et al. Final results of the phase III, randomized, double-blind AVOREN trial of first-line bevacizumab (BEV) + interferon-α2a (IFN) in metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2009;27(15 suppl):5020. [DOI: 10.1200/jco.2009.27.15_suppl.5020] [DOI] [Google Scholar]
- Escudier BJ, Negrier S, Chevreau C, et al. Analysis of prognostic risk categories in the phase III AVOREN trial of first-line bevacizumab plus interferon-α2a in patients with metastatic renal cell carcinoma: French prognostic scoring system. In: Genitourinary Cancers Symposium, ASCO, abst 417. 2010.
- Escudier BJ, Ravaud A, Negrier S, Szczylik C, Bellmunt J, Bracarda S, et al. Update on AVOREN trial in metastatic renal cell carcinoma (mRCC): Efficacy and safety in subgroups of patients (pts) and pharmacokinetic (PK) analysis. Journal of Clinical Oncology 2008;26(15 suppl):5025. [DOI: 10.1200/jco.2008.26.15_suppl.5025] [DOI] [Google Scholar]
- Karakiewicz P, Sun M, Sneller V, Escudier B. Use of a nomogram to quantify overall survival (OS) benefit in patients with metastatic renal cell carcinoma receiving bevacizumab (BEV) with interferon (IFN) versus IFN alone. Journal of Clinical Oncology 2010;28(15 suppl):4592. [DOI: 10.1200/jco.2010.28.15_suppl.4592 ] [Google Scholar]
- Karakiewicz PI, Sun M, Sneller V, et al. Use of a nomogram to quantify progression-free survival benefit in metastatic renal cell carcinoma patients receiving bevacizumab plus interferon or interferon alone. In: Genitourinary Cancers Symposium, ASCO, abstr 392. 2010.
- Melichar B, Koralewski P, Pluzanska A, Ravaud A, Bracarda S, Szczylik C, et al. First-line bevacizumab improves progression-free survival with lower doses of interferon-α2a in the treatment of patients with metastatic renal cell carcinoma (AVOREN). European Journal of Cancer Supplements 2007;5(4):304. [DOI: 10.1016/S1359-6349(07)71149-7] [DOI] [Google Scholar]
- Melichar B, Koralewski P, Ravaud A, Pluzanska A, Bracarda S, Szczylik C, et al. First-line bevacizumab combined with reduced dose interferon-α2a is active in patients with metastatic renal cell carcinoma. Annals of Oncology 2008;19(8):1470-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00738530. A study of avastin (bevacizumab) added to interferon alfa-2a (roferon) therapy in patients with metastatic renal cell cancer with nephrectomy [A randomised, double-blind study to evaluate the efficacy and safety of avastin plus roferon compared with placebo plus roferon on overall survival and tumor assessment in nephrectomised patients with metastatic clear cell renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT00738530 (first received 20 August 2008). [NCT00738530]
Escudier 2017 {published data only}
- Cella D, Grunwald V, Escudier B, Hammers HJ, George S, Nathan P, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncology 2019;20(2):297-310. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Tannir N, McDermott DF, Frontera OA, Melichar B, Plimack ER, et al. CheckMate 214: Efficacy and safety of nivolumab + ipilimumab (N+I) v sunitinib (S) for treatment-naive advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups. Annals of Oncology 2017;28(suppl 5):621-2. [DOI: 10.1093/annonc/mdx440.029] [DOI] [Google Scholar]
- Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncology 2019;S1470-2045(19):30413-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Motzer RJ, Tannir N, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New England Journal of Medicine 2018;378(14):1277-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02231749. Nivolumab combined with ipilimumab versus sunitinib in previously untreated advanced or metastatic renal cell carcinoma (CheckMate 214) [A phase 3, randomized, open-label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in subjects with previously untreated, advanced or metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT02231749 (first received 4 September 2014). [NCT02231749]
- Tannir NM, Hammers HJ, Amin A, Grimm M-O, Rini BI, Mekan S, et al. Characterization of the benefit-risk profile of nivolumab + ipilimumab (N+I) v sunitinib (S) for treatment-naïve advanced renal cell carcinoma (aRCC; CheckMate 214). Journal of Clinical Oncology 2018;28(6 suppl):686. [DOI: 10.1200/JCO.2018.36.6_suppl.686] [DOI] [Google Scholar]
- Vyas C, Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, et al. Nivolumab + ipilimumab (N+I) vs sunitinib (S) for treatment-naive advanced or metastatic renal cell carcinoma (aRCC): results from checkmate 214, including overall survival by subgroups. Journal of Oncology Pharmacy Practice 2018;24(5_suppl):17-8. [Google Scholar]
Hudes 2007 {published data only}
- Alemao E, Rajagopalan S, Yang S, et al. Quality adjusted survival in randomized, controlled trials: application of inverse probability weighting in renal cell cancer. In: Genitourinary Cancers Symposium, ASCO, abstr 399. 2010.
- Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase as a biomarker for survival with mTOR inhibition in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2010;28(15 suppl):4361. [DOI: 10.1200/jco.2010.28.15_suppl.4631] [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Szczylik C, Tannir N, Benedetto P, Ruff P, Hsu A, et al. Correlation of survival with tumor histology, age, and prognostic risk group for previously untreated patients with advanced renal cell carcinoma receiving temsirolimus or interferon-alpha. Journal of Clinical Oncology 2007;25(18 suppl):5033. [DOI: 10.1200/jco.2007.25.18_suppl.5033] [DOI] [Google Scholar]
- Dutcher JP, Souza P, Figlin R, et al. Effect of temsirolimus versus interferon- on survival of patients with advanced renal cell carcinoma of different histologies. In: Genitourinary Cancers Symposium, ASCO, abstr 384. 2008.
- Dutcher JP, Souza P, McDermott D, Figlin RA, Berkenblit A, Thiele A, et al. Effect of temsirolimus versus interferon- on outcome of patients with advanced renal cell carcinoma of different histologies. Medical Oncology 2009;26(2):202–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. A phase 3, randomized, 3-arm study of temsirolimus or interferon alpha or the combination in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma. Journal of Clinical Oncology 2006;24(18 suppl):LBA4. [DOI: 10.1200/jco.2006.24.18_suppl.lba4] [DOI] [Google Scholar]
- *.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New England Journal of Medicine 2007;356(22):2271–81. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Logan T, McDermott D, Dutcher J, et al. Exploratory analysis of the influence of nephrectomy status on temsirolimus efficacy in patients with advanced renal cell carcinoma and poor-risk features. In: Genitourinary Cancers Symposium, ASCO, abstr 281. 2009.
- NCT00065468. Study Evaluating Interferon And CCI-779 In Advanced Renal Cell Carcinoma (ARCC). clinicaltrials.gov/ct2/show/results/NCT00065468 (first received July 25 2003). [NCT00065468]
- Pablo M, Hudes G, Dutcher J, White C, Krygowski M, Cincotta M, et al. Radiographic findings of drug-induced pneumonitis and clinical correlation inpatients with advanced renal cell carcinoma treated with temsirolimus. European Journal of Cancer Supplements 2009;7(2):426-7. [DOI: 10.1016/S1359-6349(09)71446-6] [DOI] [Google Scholar]
- Parasuraman S, Hudes G, Levy D, Strahs A, Moore L, De Marinise R, et al. Comparison of quality-adjusted survival in patients with advanced renal cell carcinoma receiving first-line treatment with temsirolimus (TEMSR) or interferon- (IFN) or the combination of IFN+TEMSR. Journal of Clinical Oncology 2007;25(18 suppl):5049. [DOI: 10.1200/jco.2007.25.18_suppl.5049] [DOI] [Google Scholar]
- Yang S, Hudes G, Souza P, Alemao E, Strahs A, Purvis J. Evaluation of quality of life in patients with advanced renal cell carcinoma treated with temsirolimus vs interferon-alfa: results from a phase III randomized trial. European Journal of Cancer Supplements 2009;7(2):433. [DOI: 10.1016/S1359-6349(09)71467-3] [DOI] [Google Scholar]
- Zbrozek AS, Hudes G, Levy D, Strahs A, Berkenblit A, DeMarinis R, et al. Q-TWiST analysis of patients receiving temsirolimus or interferon alpha for treatment of advanced renal cell carcinoma. Pharmacoeconomics 2010;28(7):577–84. [PMID: ] [DOI] [PubMed] [Google Scholar]
McDermott 2018 {published data only}
- Atkins MB, McDermott DF, Powles T, Motzer RJ, Rini BI, Fong L, et al. IMmotion150: A phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun). Journal of Clinical Oncology 2017;35(15 suppl):4505. [DOI: 10.1200/JCO.2017.35.15_suppl.4505] [DOI] [Google Scholar]
- McDermott DF, Atkins MB, Motzer RJ, Rini BI, Escudier BJ, Fong L, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Journal of Clinical Oncology 2017;35(6 suppl):431. [DOI: 10.1200/JCO.2017.35.6_suppl.431] [DOI] [Google Scholar]
- *.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nature Medicine 2018;24(6):749-57. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01984242. A study of atezolizumab (an engineered anti-programmed death-ligand 1 [PD-L1] antibody) as monotherapy or in combination with bevacizumab (avastin®) compared to sunitinib (Sutent®) in participants with untreated advanced renal cell carcinoma (IMmotion150) [A phase II, randomized study of atezolizumab (anti-PD-L1 antibody) administered as monotherapy or in combination with bevacizumab versus sunitinib in patients with untreated advanced renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT01984242 (first received 14 November 2013). [NCT01984242]
- Pal SK, McDermott DF, Atkins MB, Escudier B, Rini BI, Motzer RJ, et al. Patient-reported outcomes in a phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma. BJU Int 2020;126:73-82. [DOI: 10.1111/bju.15058] [DOI] [PMC free article] [PubMed] [Google Scholar]
- PowlesT, McDermott DF, Rini B, Motzer RJ, Atkins MB, Fong L, et al. IMmotion150: Novel radiological endpoints and updated data from a randomized phase II trial investigating atezolizumab (atezo) with or without bevacizumab (bev) vs sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC). Annals of Oncology 2017;28(suppl 5):624. [DOI: 10.1093/annonc/mdx440.033] [DOI] [Google Scholar]
Motzer 2010 {published data only}
- Castellano D, Muro XG, Pérez-Gracia JL, González-Larriba JL, Abrio MV, Ruiz MA, et al. Patient-reported outcomes in a phase III, randomized study of sunitinib versus interferon-{alpha} as first-line systemic therapy for patients with metastatic renal cell carcinoma in a European population. Annals of Oncology 2009;20(11):1803-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Cappelleri JC, Bushmakin A, Charbonneau C, Li JZ, Kim ST, et al. Quality of life predicts progression-free survival in patients with renal cell carcinoma treated with sunitinib versus interferon alfa. Journal of Oncology Practice 2009;5(2):66-70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Li JZ, Cappelari JC, Bushmakin A, Charbonneau C, Kim ST, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. Journal of Clinical Oncology 2008;26(22):3763-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cella D, Li JZ, Cappelleri JC, Bushmakin A, Charbonneau C, Kim ST, Chen I, et al. Quality of life (QOL) predicts for progression-free survival (PFS) in patients with metastatic renal cell carcinoma (mRCC) treated with sunitinib compared to interferon-alpha (IFN-α). Journal of Clinical Oncology 2007;25(18 suppl):6594. [DOI: 10.1200/jco.2007.25.18_suppl.6594] [DOI] [Google Scholar]
- Cella D, Michaelson MD, Bushmakin AG, Cappelleri JC, Charbonneau C, Kim ST, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-a in a phase III trial: final results and geographical analysis. British Journal of Cancer 2010;102(4):658-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figin RA, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Negrier S, et al. Overall survival with sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2008;26(15 suppl):5024. [DOI: 10.1200/jco.2008.26.15_suppl.5024] [DOI] [Google Scholar]
- Motzer RJ, Bukowski RM, Figlin RA, Hutson TE, Michaelson MD, Kim ST, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer 2008;113(7):1552-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Figlin RA, Hutson TE, Tomczak P, Bukowski RM, Rixe O, et al. Sunitinib versus interferon-alfa (IFN-α) as first-line treatment of metastatic renal cell carcinoma (mRCC): Updated results and analysis of prognostic factors. Journal of Clinical Oncology 2007;25(18 suppl):5024. [DOI: 10.1200/jco.2007.25.18_suppl.5024] [DOI] [Google Scholar]
- Motzer RJ, Figlin RA, Hutson TE, Tomczak P, Bukowski RM, Rixe O, et al. Sunitinib versus interferon-alfa as first-line treatment of metastatic renal cell carcinoma: updated results and analysis of prognostic factors. In: www.asco.org/virtual meeting. 2007.
- *.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(22):3584-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Phase III randomized trial of sunitinib malate (SU11248) versus interferon-alfa (IFN-α) as first-line systemic therapy for patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2006;24(18 suppl):LBA3. [DOI: 10.1200/jco.2006.24.18_suppl.lba3] [DOI] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New England Journal of Medicine 2007;356(2):115-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Michaelson MD, Hutson TE, Tomczak P, Bukowski RM, Rixe O, et al. Sunitinib versus interferon (IFN)-alfa as first-line treatment of metastatic renal cell carcinoma (mRCC): updated efficacy and safety results and further analysis of prognostic factors. European Journal of Cancer Supplements 2007;5(4):301. [DOI: 10.1016/S1359-6349(07)71140-0] [DOI] [Google Scholar]
- NCT00083889. SU011248 versus interferon-alfa as first-line systemic therapy for patients with metastatic renal cell carcinoma [A phase 3, randomized study of SU011248 versus interferon-alfa as first-line systemic therapy for patients with metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT00083889 (first received 4 June 2004). [NCT00083889]
- Patil S, Figlin RA, Hutson TE, Michaelson D, Negrier S, Kim ST, et al. TWiST analysis to estimate overall benefit for metastatic renal cell carcinoma (mRCC) patients (pts) treated in a phase III trial of sunitinib versus interferon-alfa (IFN-α). Journal of Clinical Oncology 2010;28(15 suppl):4594. [DOI: 10.1200/jco.2010.28.15_suppl.4594] [DOI] [Google Scholar]
- Wilkerson J, Stein WD, Kim ST, Huang X, Motzer RJ, Fojo AT, et al. Validation of kinetic analysis of renal cancer regression and growth following treatment with sunitinib and interferon-alfa: analysis of the pivotal randomized trial. Journal of Clinical Oncology 2010;28(15 suppl):4597. [DOI: 10.1200/jco.2010.28.15_suppl.4597] [DOI] [Google Scholar]
Motzer 2013a {published data only}
- *.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. New England Journal of Medicine 2013;369(8):722-1. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. New England Journal of Medicine 2014;370(18):1769-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00720941. Pazopanib versus sunitinib in the treatment of locally advanced and/or metastatic renal cell carcinoma (COMPARZ) [Study VEG108844, A study of pazopanib versus sunitinib in the treatment of subjects with locally advanced and/or metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT00720941 (first received 23 July 2008). [NCT00720941]
Motzer 2013b {published data only}
- Hutson T, Nosov D, Tomczak P, Bondarenko I, Lipatov ON, Sternberg CN, et al. Tivozanib vs sorafenib targeted therapy for advanced renal cell carcinoma: Final results of a phase III trial (901) and efficacy results of a 2nd line tivozanib extension study (902). Journal of Clinical Oncology 2015;33(15 suppl):4557. [DOI: 10.1200/jco.2015.33.15_suppl.4557] [DOI] [Google Scholar]
- *.Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. Journal of Clinical Oncology 2013;31(30):3791-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01030783. A study to compare tivozanib (AV-951) to sorafenib in subjects with advanced renal cell carcinoma (TIVO-1) [A phase 3, randomized, controlled, multi-center, open-label study to compare tivozanib (AV-951) to sorafenib in subjects with advanced renal cell carcinoma (TIVO-1)]. clinicaltrials.gov/ct2/show/NCT01030783 (first received 11 December 2009). [NCT01030783]
Motzer 2014 {published data only}
- Knox JJ, Barrios CH, Kim TM, Cosgriff T, Srimuninnimit V, Pittman K, et al. Final overall survival analysis for the phase II RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC. Annals of Oncology 2017;28(6):1339-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JJ, Barrios CH, Kim TM, Cosgriff T, Srimuninnimit V, Pittman KB, et al. Final overall survival analysis for the RECORD-3 study of first-line everolimus followed by sunitinib versus first-line sunitinib followed by everolimus in metastatic RCC (mRCC). Journal of Clinical Oncology 2015;33(15 suppl):4554. [DOI: 10.1200/jco.2015.33.15_suppl.4554] [DOI] [Google Scholar]
- *.Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2014;32(25):2765-72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00903175. Efficacy and safety comparison of RAD001 versus sunitinib in the first-line and second-line treatment of patients with metastatic renal cell carcinoma (RECORD-3) [An open-label, multicenter phase II study to compare the efficacy and safety of RAD001 as first-line followed by second-line sunitinib versus sunitinib as First-line followed by second-line RAD001 in the treatment of patients with metastatic renal cell carcinoma.]. clinicaltrials.gov/ct2/show/NCT00903175 (first received 18 May 2009). [NCT00903175]
Motzer 2019 {published data only}
- *.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal cell carcinoma. New England Journal of Medicine 2019;380(12):1103-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02684006. A study of avelumab with axitinib versus sunitinib in advanced renal cell cancer (JAVELIN Renal 101) [A phase 3, multinational, randomized, open-label, parallel-arm study of avelumab (MSB0010718C) in combination with axitinib (Inlyta(registered)) versus sunitinib (Sutent(registered)) monotherapy in the first-line treatment of patients with advanced renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT02684006 (first received 17 February 2016). [NCT02684006]
Ravaud 2015 {published data only}
- NCT00719264. Safety and efficacy of bevacizumab plus RAD001 versus interferon alfa-2a and bevacizumab for the first-line treatment in adult patients with kidney cancer [A randomized, open-label, multi-center phase II study to compare bevacizumab plus RAD001 versus interferon alfa-2a plus bevacizumab for the first-line treatment of patients with metastatic clear cell carcinoma of the kidney]. clinicaltrials.gov/ct2/show/study/NCT00719264 (first received 21 July 2008). [NCT00719264]
- *.Ravaud A, Barrios CH, Alekseev B, Tay MH, Agarwala SS, Yalcin S, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon α-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Annals of Oncology 2015;26(7):1378-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Retz 2019 {published data only}
- NCT01613846. Phase III sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the treatment of advanced / metastatic renal cell carcinoma (SWITCH-II) [Phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the treatment of advanced / metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/study/NCT01613846 (first received 7 June 2012). [NCT01613846]
- *.Retz M, Bedke J, Bögemann M, Grimm MO, Zimmermann U, Müller L, et al. SWITCH II: Phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib-sorafenib in the treatment of advanced or metastatic renal cell carcinoma (AUO AN 33/11). European Journal of Cancer 2019;107:37-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Retz M, BedkeJ, Herrmann E, Grimm M, Zimmermann U, Müller L, et al. Phase III randomized, sequential, open-label study to evaluate the efficacy and safety of sorafenib-pazopanib versus pazopanib sorafenib in the treatment of metastatic renal cell carcinoma(SWITCH-II). Annals of Oncology 2017;28(suppl 5):295. [DOI: 10.1093/annonc/mdx371] [DOI] [PubMed] [Google Scholar]
Rini 2008 {published data only}
- Halabi S, Rini BI, Stadler WM, Small EJ. Use of progression-free survival (PFS) to predict overall survival (OS) in patients with metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2010;28(15 suppl):4525. [DOI: 10.1200/jco.2010.28.15_suppl.4525] [DOI] [Google Scholar]
- Harzstark AL, Halabi S, Stadler WM, et al. Hypertensionis associated with clinical outcome for patients with metastatic renal cell carcinoma treated with interferon and bevacizumab on CALGB 90206. In: Genitourinary Cancers Symposium, ASCO, abstr 351. 2010.
- NCT00072046. Interferon alfa-2b with or without bevacizumab in treating patients with advanced renal cell carcinoma (Kidney Cancer) [A randomized phase III trial of interferon alfa-2B or interferon alfa-2B plus bevacizumab in patients with advanced renal carcinoma]. clinicaltrials.gov/ct2/show/study/NCT00072046 (first received 6 November 2003). [NCT00072046]
- Rini BI, Halabi S, Rosenberg J, Stadler WM, Vaena DA, Atkins JN, et al. Bevacizumab plus interferon-alfa versus interferon-alfa monotherapy in patients with metastatic renal cell carcinoma: results of overall survival for CALGB 90206. Journal of Clinical Oncology 2009;27(18 suppl):LBA5019. [DOI: 10.1200/jco.2009.27.18_suppl.lba5019 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB90206. Journal of Clinical Oncology 2008;26(33):5422-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, et al. CALGB 90206:a phase III trial of bevacizumab plus interferon-alfa versus interferon-alfa monotherapy in metastatic renal cell carcinoma. In: Genitourinary Cancers Symposium, ASCO, abstr 350. 2008.
- Rini BI, Halabi S, Taylor J, Small EJ, Schilsky RL, Cancer and Leukemia Group B . Cancerand Leukemia Group B 90206: a randomized phase III trial of interferon-a or interferon-a plus anti-vascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma. Clinical Cancer Research 2004;10(8):2584-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2014 {published data only}
- NCT00631371. Study Comparing Bevacizumab + Temsirolimus vs. Bevacizumab + Interferon-Alfa In Advanced Renal Cell Carcinoma Subjects (INTORACT). linicaltrials.gov/ct2/show/NCT00631371 (first received 20 May 2019). [NCT00631371]
- *.Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. Journal of Clinical Oncology 2014;32(8):752-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2016 {published data only}
- NCT01265901. IMA901 in patients receiving sunitinib for advanced/metastatic renal cell carcinoma [A randomized, controlled phase III study investigating IMA901 multipeptide cancer vaccine in patients receiving sunitinib as first-line therapy for advanced/metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/study/NCT01265901 (first received 23 December 2010). [NCT01265901]
- *.Rini BI, Stenzl A, Zdrojowy R, Kogan M, Shkolnik M, Oudard S, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncology 2016;17(11):1599-611. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2019a {published data only}
- NCT02853331. Study to evaluate the efficacy and safety of pembrolizumab (MK-3475) in combination with axitinib versus sunitinib monotherapy in participants with renal cell carcinoma (MK-3475-426/KEYNOTE-426) [A phase III randomized, open-label study to evaluate efficacy and safety of pembrolizumab (MK-3475) in combination with axitinib versus sunitinib monotherapy as a first-line treatment for locally advanced or metastatic renal cell carcinoma (mRCC) (KEYNOTE-426)]. clinicaltrials.gov/ct2/show/NCT02853331 (first received 2 August 2016). [NCT02853331]
- Powles T, Plimack ER, Stus V, Gafanov RA, Hawkins RE, Nosov D. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): phase III KEYNOTE-426 study. Journal of Clinical Oncology 2019;37(7 suppl):543-543. [Google Scholar]
- *.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New England Journal of Medicine 2019;380(12):1116-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2019b {published data only}
- Atkins MB, Rini BI, Motzer RJ, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes from the phase 3 randomized IMmotion151 trial: atezolizumab + bevacizumab vs sunitinib in treatment-naive metastatic renal cell carcinoma. Clinical Cancer Research 2020;26(11):2506-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Motzer RJ, Rini BI, Powles T, McDermott DF, Suarez C, et al. Patient-reported outcomes (PROs) in IMmotion151: atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun) in treatment (tx) naive metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2018;36(15_suppl):4511. [DOI: 10.1200/JCO.2018.36.15_suppl.4511] [DOI] [Google Scholar]
- Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology 2018;36(6 suppl):578. [DOI: 10.1200/JCO.2018.36.6_suppl.578] [DOI] [Google Scholar]
- NCT02420821. A study of atezolizumab in combination with bevacizumab versus sunitinib in participants with untreated advanced renal cell carcinoma (RCC) (IMmotion151) [A phase III, open-label, randomized study of atezolizumab (Anti-PD-L1 Antibody) in combination with bevacizumab versus sunitinib in patients with untreated advanced renal cell carcinoma]. clinicaltrials.gov/ct2/show/NCT02420821 (first received 20 April 2015). [NCT02420821]
- *.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393(10189):2404-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sternberg 2010 {published data only}
- Cella D, Pickard AS, Duh MS, Guerin A, Mishagina N, Antràs L, et al. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomised phase III trial. European Journal of Cancer 2012;48(3):311-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hutson TE, Bukowski RM. A phase II study of GW786034 using a randomized discontinuation design in patients with locally recurrent or metastatic clear-cell renal cell carcinoma. Clinical Genitourinary Cancer 2006;4(4):296-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hutson TE, Davis ID, Machiels JP, Souza PL, Hong BF, Rottey S, et al. Pazopanib (GW786034) is active in metastatic renal cell carcinoma (RCC): Interim results of a phase II randomized discontinuation trial (RDT). Journal of Clinical Oncology 2007;25(18 suppl):5031. [DOI: 10.1200/jco.2007.25.18_suppl.5031] [DOI] [Google Scholar]
- NCT00334282. Safety and efficacy of GW786034 (pazopanib) In metastatic renal cell carcinoma [A randomised, double-blind, placebo controlled, multi-center phase III study to evaluate the efficacy and safety of pazopanib (GW786034) compared to placebo in patients with locally advanced and/or metastatic renal cell carcinoma]. clinicaltrials.gov/ct2/show/study/NCT00334282 (first received 7 June 2006). [NCT00334282]
- *.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of Clinical Oncology 2010;28(6):1061-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. European Journal of Cancer 2013;49(6):1287-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Szczylik C, Lee E, Salman PV, Mardiak J, Davis ID, et al. A randomized, double-blind phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). Journal of Clinical Oncology 2009;27(15S):5021. [DOI: 10.1200/jco.2009.27.15s.5021] [DOI] [Google Scholar]
References to studies excluded from this review
Armstrong 2016 {published data only}
- Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncology 2016;17(3):378-88. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004 {published data only}
- Atkins MB, Hidalgo M, Stadler W, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin inhibitor, in patients with advanced refractory renal cell carcinoma. Journal of Clinical Oncology 2004;22:909-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bracarda 2010 {published data only}
- Bracarda S, Porta C, Boni C, Santoro A, Mucciarini C, Pazzola A, et al. Could interferon still play a role in metastatic renal cell carcinoma? A randomized study of two schedules of sorafenib plus interferon-alpha 2a (RAPSODY). European Urology 2013;63(2):254-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Broom 2015 {published data only}
- Broom RJ, Hinder V, Sharples K, Proctor J, Duffey S, Pollard S, et al. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: A randomized first-line phase II trial. Clinical Genitourinary Cancer 2015;13(1):50-8. [PMID: 25163397] [DOI] [PubMed] [Google Scholar]
Bukowski 2007 {published data only}
- Bukowski RM, Kabbinavar F, Figlin RA, Flaherty K, Srinivas S, Vaishampayan UN, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. Journal of Clinical Oncology 2007;25(29):4536–41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Choueiri 2015 {published data only}
- Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine 2015;373(19):1814–23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choueiri 2017 {published data only}
- Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. Journal of Clinical Oncology 2017;35(6):591-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cirkel 2016 {published data only}
- Cirkel GA, Hamberg P, Sleijfer S, Loosveld OJL, Dercksen MW, Los M, et al. Alternating treatment with pazopanib and everolimus vs continuous pazopanib to delay disease progressionin patients with metastatic clear cell renal cell cancer: The ROPETAR randomized clinical trial. JAMA Oncology 2017;3(4):501-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dorff 2015 {published data only}
- Dorff TB, Longmate JA, Pal SK, Stadler WM, Fishman MN, Vaishampayan UN, et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 2017;123(23):4566-73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbinghaus 2007 {published data only}
- Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, et al. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clinical Cancer Research 2007;13(22 Pt 1):6689–95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eisen 2015 {published data only}
- Eisen T, Loembé A-B, Shparyk Y, MacLeod N, Jones RJ, Mazurkiewicz M, Temple G, et al. A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-year results. British Journal of Cancer 2015;113(8):1140-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Escudier 2007 {published data only}
- Escudier B, Choueiri TK, Oudard S, Szczylik C, Négrier S, Ravaud A, et al. Prognostic factors of metastatic renal cell carcinoma after failure of immunotherapy: new paradigm from a large phase III trial with shark cartilage extract AE 941. Journal of Urology 2007;178:1901-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Escudier 2009 {published data only}
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27:1280–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Escudier 2010a {published data only}
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New England Journal of Medicine 2007;356(2):125-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flaherty 2015 {published data only}
- Flaherty KT, Manola JB, Pins M, McDermott DF, Atkins MB, Dutcher JJ, et al. BEST: A randomized phase II study of vascular endothelial growth factor, RAF Kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma--A trial of the ECOG-ACRIN cancer research group (E2804). Journal of Clinical Oncology 2015;33(21):2384-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2004 {published data only}
- Gordon MS, Manola J, Fairclough D, Cella D, Richardson R, Sosman J, et al. Low dose interferon-α2b + thalidomide in patients with previously untreated renal cell cancer. Improvement in progression-free survival but not quality of life or overall survival. A phase III study of the Eastern Cooperative Oncology Group(E2898). Journal of Clinical Oncology 2004;22(14 suppl):4516. [DOI: 10.1200/jco.2004.22.90140.4516] [DOI] [Google Scholar]
Hainsworth 2015 {published data only}
- Hainsworth JD, Reeves JA, Mace JR, Crane EJ, Hamid O, Stille JR, et al. A randomized, open-Label phase 2 study of the CXCR4 Inhibitor LY2510924 in combination with sunitinib versus sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Targeted Oncology 2016;11(5):643-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hawkins 2016 {published data only}
- Hawkins R, Gore M, Shparyk Y, Bondar V, Gladkov O, Ganev T, et al. A randomized phase II/III study of naptumomab estafenatox þ IFNa versus IFNa in renal cell carcinoma: final analysis with baseline biomarker subgroup and trend analysis. Clinical Cancer Research 2016;22(13):3172-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hutson 2013 {published data only}
- Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncology 2013;14(13):1287-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hutson 2014 {published data only}
- Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2014;32(8):760-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonasch 2010 {published data only}
- Jonasch E, Corn P, Pagliaro LC, Warneke CL, Johnson MM, Tamboli P, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma. Cancer 2010;116(1):57-65. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonasch 2017 {published data only}
- Jonasch E, Hasanov E, Corn PG, Moss T, Shaw KR, Stovall S, et al. A randomized phase 2 study of MK-2206 versus everolimus in refractory renal cell carcinoma. Annals of Oncology 2017;28(4):804-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee CP, Patel PM, Selby PJ, Hancock BW, Mak I, Pyle L, et al. Randomized phase II study comparing thalidomide with medroxyprogesterone acetate in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2006;24(6):898-903. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee JL, Kim MK, Park I, Ahn JH, Lee DH, Ryoo HM, et al. Randomized phase II trial of sunitinib four weeks on and two weeks off versus Two weeks on and One week off in metastatic clear-cell type Renal cell carcinoma: RESTORE trial. Annals of Oncology 2015;26(11):2300-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Madhusudan 2004 {published data only}
- Madhusudan S, Protheroe A, Vasey P, Patel P, Selby P, Altman D, et al. A randomized phase II study of interferon alpha alone or in combination with thalidomide in metastatic renal cancer. Journal of Clinical Oncology 2004;22(14_suppl):4742. [DOI: 10.1200/jco.2004.22.90140.4742] [DOI] [Google Scholar]
Motzer 2008 {published data only}
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomized, placebo-controlled phase III trial. Lancet 2008;372(9637):449-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Motzer 2012 {published data only}
- Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. Journal of Clinical Oncology 2012;30(12):1371-7. [PMID: 22430274 ] [DOI] [PubMed] [Google Scholar]
Motzer 2014b {published data only}
- Motzer RJ, Porta C, Vogelzang NJ, Sternberg CN, Szczylik C, Zolnierek J, et al. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma:an open-label, randomised phase 3 trial. Lancet Oncology 2014;15(3):286-96. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Motzer 2015a {published data only}
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2015;373(19):1803-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Motzer 2015b {published data only}
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel T, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): Results of a randomized, dose-ranging phase II trial. Journal of Clinical Oncology 2014;32(15_suppl):5009. [DOI: 10.1200/jco.2014.32.15_suppl.5009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Motzer 2015c {published data only}
- Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncology 2015;16(15):1473-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mulders 2012 {published data only}
- Mulders P, Hawkins R, Nathan P, Jong I, Osanto S, Porfiri E, et al. Cediranib monotherapy in patients with advanced renal cell carcinoma: results of a randomised phase II study. European Journal of Cancer 2012;48(4):527-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Négrier 2011 {published data only}
- Négrier S, Gravis G, Pérol D, Chevreau C, Delva R, Bay JO, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncology 2011;12(7):673-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nosov 2012 {published data only}
- Nosov DA, Esteves B, Lipatov ON, Lyulko AA, Anischenko AA, Chacko RT, et al. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. Journal of Clinical Oncology 2012;30(14):1678-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pal 2015 {published data only}
- Pal S, Azad A, Bhatia S, Drabkin H, Costello B, Sarantopoulos J, et al. A Phase I/II trial of BNC105P with everolimus in metastatic renal cell carcinoma. Clinical Cancer Research 2015;21(15):3420-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pili 2015 {published data only}
- Pili R, Manola J, Carducci MA, Dutcher JP, Appleman LJ, Groteluschen DL, et al. Randomized phase II study of two different doses of AVE0005 (VEGF Trap, aflibercept) in patients (pts) with metastatic renal cell carcinoma (RCC): An ECOG-ACRIN study [E4805]. Journal of Clinical Oncology 2015;33(15_suppl):4549. [DOI: 10.1200/jco.2015.33.15_suppl.4549] [DOI] [Google Scholar]
Powles 2014 {published data only}
- Powles T, Oudard S, Escudier BJ, Brown JE, Hawkins RE, Castellano DE, et al. A randomized phase II study of GDC-0980 versus everolimus in metastatic renal cell carcinoma (mRCC) patients (pts) after VEGF-targeted therapy (VEGF-TT). Journal of Clinical Oncology 2014;32(15_suppl):4525. [DOI: 10.1200/jco.2014.32.15_suppl.4525] [DOI] [Google Scholar]
Powles 2016a {published data only}
- Powles T, Wheater M, Din O, Geldart T, Boleti E, Stockdale A, et al. A randomised phase 2 study of AZD2014 versus everolimus in patients with VEGF-refractory metastatic clear cell renal cancer. European Urology 2016;69(3):450-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Powles 2016b {published data only}
- Powles T, Brown J, Larkin J, Jones R, Ralph C, Hawkins R, et al. A randomized, double-blind phase II study evaluating cediranib versus cediranib and saracatinib in patients with relapsed metastatic clear-cell renal cancer(COSAK). Annals of Oncology 2016;27(5):880–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Procopio 2011 {published data only}
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. British Journal of Cancer 2011;104(8):1256-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratain 2006 {published data only}
- Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2006;24(16):2505-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ravaud 2008 {published data only}
- Ravaud A, Hawkins R, Gardner JP, Maase H, Zantl N, Harper P, et al. Lapatinib versus hormone therapy in patients with advanced renal cell carcinoma: a randomized phase III clinical trial. Journal of Clinical Oncology 2008;26(14):2285-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2011 {published data only}
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378(9807):1931-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2012 {published data only}
- Rini B, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: a randomized, double-blind, placebo-controlled, phase 2 study. Cancer 2012;118(24):6152-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rini 2013 {published data only}
- Rini BI, Melichar B, Ueda T, Grünwald V, Fishman MN, Arranz JA, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncology 2013;14(12):1233-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivas 2005 {published data only}
- Srinivas S, Guarding AE. A lower dose of thalidomide is better than a high dose in metastatic renal cell carcinoma. BJU International 2005;96(4):536-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stadler 2005 {published data only}
- Stadler WM, Rosner G, Small E, Hollis D, Rini B, Zaentz DS, et al. Successful implementation of the randomized discontinuation trial design: an application to the study of the putative antiangiogenic agent carboxyaminoimidazole in renal cell carcinoma -CALGB 69901. Journal of Clinical Oncology 2005;23(16):3726-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tannir 2016 {published data only}
- Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. European Urology 2016;69(5):866-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tannir 2018 {published data only}
- Tannir NM, Ross JA, Devine CE, Chandramohan A, Wang X, Lim ZD, et al. A randomized phase II trial of pazopanib (PAZ) versus temsirolimus (TEM) in patients (pts) with advanced clear-cell renal cell carcinoma (aCCRCC) of intermediate and poor-risk (the TemPa trial). Journal of Clinical Oncology 2018;36(6_suppl):583. [DOI: 10.1200/JCO.2018.36.6_suppl.583] [DOI] [Google Scholar]
Tomita 2014 {published data only}
- Tomita Y, Naito S, Sassa N, Takahashi A, Kondo T, Koie T, et al. Sunitinib versus sorafenib as first-line therapy for patients with metastatic renal cell carcinoma with favourable or intermediate MSKCC risk factors: A multicenter randomized trial, CROSS-J-RCC. Journal of Clinical Oncology 2014;32(4_suppl):502. [DOI: 10.1200/jco.2014.32.4_suppl.502] [DOI] [Google Scholar]
Twardowski 2015 {published data only}
- Twardowski P, Plets M, Plimack ER, Agarwal N, Tangen CM, Vogelzang NJ, et al. SWOG 1107: Parallel (randomized) phase II evaluation of tivantinib (ARQ-197) and tivantinib in combination with erlotinib in patients (Pts) with papillary renal cell carcinoma (pRCC). Journal of Clinical Oncology 2015;33(15_suppl):4523. [DOI: 10.1200/jco.2015.33.15_suppl.4523] [DOI] [Google Scholar]
Yang 2003 {published data only}
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New England Journal of Medicine 2003;349(5):427-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Choueiri 2018 {published and unpublished data}
- *.Choueiri TK, Apolo AB, Powles T, Escudier B, Aren OR, Shah A, et al. A phase 3, randomized, open-label study of nivolumab combined with cabozantinib vs sunitinib in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC; CheckMate 9ER). Journal of Clinical Oncology 2018;36(15_suppl):TPS4598. [DOI: 10.1200/JCO.2018.36.15_suppl.TPS4598] [DOI] [Google Scholar]
- NCT03141177. A study of nivolumab combined with cabozantinib compared to sunitinib in previously untreated advanced or metastatic renal cell carcinoma (CheckMate 9ER). clinicaltrials.gov/ct2/show/NCT03141177 (first received May 2019).
Choueiri 2019 {published and unpublished data}
- *.Choueiri TK, Albiges L, Powles T, Scheffold C, Wang F, Motzer RJ, et al. A phase III study (COSMIC-313) of cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal cell carcinoma of intermediate or poor-risk. Journal of Clinical Oncology 2020;38(6_suppl):TPS767. [DOI: 10.1200/JCO.2020.38.6_suppl.TPS767] [DOI] [Google Scholar]
- NCT03937219. Study of Cabozantinib in Combination With Nivolumab and Ipilimumab in Patients With Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (COSMIC-313). https://clinicaltrials.gov/ct2/show/NCT03937219 (first received June 2020).
Grünwald 2018 {published and unpublished data}
- Grunwald V, Bergmann L, Steiner T, Grullich C, Muller L, Staib P, et al. A randomized phase II study with NIVOlumab or continuation of therapy as an early SWITCH approach in patients with advanced or metastatic renal cell carcinoma (RCC) and disease control after 3 months of treatment with a tyrosine kinase inhibitor (NIVOSWITCH). Oncology Research and Treatment 2018;41:75. [Google Scholar]
- *.NCT02959554. Study in Which Therapy is Either Switched to Nivolumab After 3 Months of Treatment or Therapy is Continued With a Tyrosine Kinase Inhibitor in Patients With Metastatic Renal Cell Carcinoma (RCC) and Disease Control (NIVOSWITCH) [A randomized phase II study with NIVOlumab or continuation of therapy as an early SWITCH approach in patients with advanced or metastatic renal cell carcinoma (RCC) and disease control after 3 months of treatment with a tyrosine kinase inhibitor (NIVOSWITCH)]. https://clinicaltrials.gov/ct2/show/NCT02959554.
Motzer 2018 {published and unpublished data}
- *.Motzer RJ, Grünwald V, Hutson TE, Porta C, Powles T, Eto M, et al. A phase 3 trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab versus sunitinib alone in first-line treatment of patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2018;36(6 suppl):TPS706. [DOI: 10.1200/JCO.2018.36.6_suppl.TPS706] [DOI] [Google Scholar]
- NCT02811861. Lenvatinib/everolimus or Lenvatinib/pembrolizumab versus sunitinib alone as treatment of advanced renal cell carcinoma (CLEAR) [A multicenter, open-label, randomized, phase 3 trial to compare the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab versus sunitinib alone in first-line treatment of subjects with advanced renal cell carcinoma]. clinicaltrials.gov/ct2/show/study/NCT02811861 (first received 23 June 2016).
Additional references
Adjei 2005
- Adjei AA, Hidalgo M. Intracellular signal transduction pathway proteins as targets for cancer therapy. Journal of Clinical Oncology 2005;23(23):5386-403. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bergsland 2006
- Bergsland EK. When does the presence of the target predict response to the targeted agent? Journal of Clinical Oncology 2006;24(2):213-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coppin 2004
- Coppin C, Porzsolt F, Autenrieth M, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD001425. [DOI: 10.1002/14651858.CD001425.pub2] [DOI] [PubMed] [Google Scholar]
CTCAE
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed 18 May 2019).
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Deininger 2005
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukeamia. Blood 2005;105(7):2640-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
EAU Guidelines 2020
- Ljungberg B, Albiges L, Bensalah K, Bex A, Giles RH, Hora M, et al. EAU Guidelines on Renal Cell Carcinoma. Edn. presented at the EAU Annual Congress Amsterdam. EAU Guidelines Office, 2020. 978-94-92671-07-3. [Google Scholar]
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
ESMO Clinical Practice Guidelines 2019
- Escudier B, Porta C, Schmidinger M, Rioux-Leclerq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019;30(5):706-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fernández‐Pello 2017
- Fernández-Pello S, Hofmann F, Tahbaz R, Marconi L, Lam TB, Albiges L, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. European Urology 2017;71(3):426-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gleave 1998
- Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. New England Journal of Medicine 1998;338(18):1265-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394-424. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Version accessed 10 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hacker 2010
- Hacker KE, Rathmell WK. Emerging molecular classification in renal cell carcinoma: implications for drug development. Targeted Oncology 2010;5(2):75-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2012
- Hart B, Lundh A, Bero L. Effect of reporting bias on meta-analyses of drug trials: reanalysis of meta-analyses. BMJ 2012;344:d7202. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137-59. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017a
- Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2017b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Howlader 2017
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD. seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
Johnson 2011
- Johnson JR, Ning Y-M, Farrell A, Justice R, Keegan P, Pazdur R. Accelerated approval of oncology products: the Food and Drug Adminstration experience. Journal of the National Cancer Institute 2011;103(8):636-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaelin 2008
- Kaelin WG Jr. Kidney cancer: now available in a new flavor. Cancer Cell 2008;14(6):423-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuusk 2017
- Kuusk T, Albiges L, Escudier B, Grivas N, Haanen J, Powles T, et al. Antiangiogenic therapy combined with immune checkpoint blockade in renal cancer. Angiogenesis 2017;20(2):205-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lalani 2018
- Lalani AA, McGregor BA, Albiges L, Choueiri TK, Motzer R, Powles T, et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: Current paradigms, use of immunotherapy, and future directions. European Urology 2018;75(1):100-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Linehan 2005
- Linehan WM, Grubb RL, Coleman JA, Zbar B, Walther MM. The genetic basis of cancer of kidney cancer: implications for gene-specific clinical management. BJU International 2005;95 Suppl 2:2-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mickisch 2003
- Mickisch GH. Rational selection of a control arm for randomised trials in metastatic renal cell carcinoma. European Urology 2003;43(6):670-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Motzer 2002
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. Journal of Clinical Oncology 2002;20(1):289-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Oliver 1989
- Oliver RTD, Nethersell ABW, Bottomly JM. Unexplained spontaneous regression and alpha-interferon as treatment for metastatic renal carcinoma. British Journal of Urology 1989;63(2):128-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patil 2010
- Patil S, Ishill N, Deluca J, Motzer RJ. Stage migration and increasing proportion of favorable-prognosis metastatic renal cell carcinoma patients. Cancer 2010;116(2):347-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rini 2005
- Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. Journal of Clinical Oncology 2005;23(5):1028-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roberts 2003
- Roberts TG Jr, Lynch TJ Jr, Chabner BA. The phase III trial in the era of targeted therapy: unravelling the "go or no go" decision. Journal of Clinical Oncology 2003;21(19):3683-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sawyers 2004
- Sawyers C. Targeted cancer therapy. Nature 2004;432(7015):294-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schroll 2015
- Schroll J, Bero L. Regulatory agencies hold the key to improving Cochrane Reviews of drugs. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.ED000098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Srigley 2013
- Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. American Journal of Surgical Pathology 2013;37(10):1469-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stadler 2006
- Stadler WM. New targets, therapies, and toxicities: lessons to be learned. Journal of Clinical Oncology 2006;24(1):4-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strebhardt 2008
- Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nature Reviews Cancer 2008;8(6):473-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Unverzagt 2017
- Unverzagt S, Moldenhauer I, Nothacker M, Roßmeißl D, Hadjinicolaou AV, Peinemann F, et al. Immunotherapy for metastatic renal cell carcinoma. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011673. [DOI: 10.1002/14651858.CD011673.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uzzo 2003
- Uzzo RG, Cairns P, Al-Saleem T, Hudes G, Haas N, Greenberg RE, et al. The basic biology and immunobiology of renal cell carcinoma: considerations for the clinician. Urologic Clinics of North America 2003;30(3):423-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wallis 2018
- Wallis CJD, Klaassen Z, Bhindi B, Ye XY, Chandrasekar T, Farrell AM, et al. First-line systemic therapy for metastatic renal cell carcinoma: A systematic review and network meta-analysis. European Urology 2018;74(3):309-321. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2009
- Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P, et al. Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clinical Cancer Research 2009;15(24):7582-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Coppin 2006
- Coppin C, Porzolt F, Le L, Autenrieth M, Wilt T. Targeted therapy for advanced renal cell carcinoma. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No: CD006017. [DOI: 10.1002/14651858.CD006017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coppin 2008
- Coppin C, Le L, Wilt TJ, Kollmannsberger C. Targeted therapy for advanced renal cell carcinoma. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD006017. [DOI: 10.1002/14651858.CD006017.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coppin 2011
- Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU International 2011;108(10):1556-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hofmann 2017
- Hofmann F, Marconi L, Stewart F, Lam T, Bex A, Canfield SE, et al. Targeted therapy for metastatic renal cell carcinoma. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012796. [DOI: 10.1002/14651858.CD012796] [DOI] [PMC free article] [PubMed] [Google Scholar]


