Abstract
Background
Leg cramps are a common problem in pregnancy. Various interventions have been used to treat them, including drug, electrolyte and vitamin therapies, and non‐drug therapies. This Cochrane Review is an update of a review first published in 2015.
Objectives
To assess the effectiveness and safety of different interventions for treating leg cramps in pregnancy.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (25 September 2019), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) of any intervention for the treatment of leg cramps in pregnancy compared with placebo, no treatment or other treatments. Quinine was excluded for its known adverse effects. Cluster‐RCTS were eligible for inclusion. Quasi‐RCTs and cross‐over studies were excluded.
Data collection and analysis
Three review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The certainty of the evidence was assessed using the GRADE approach.
Main results
We included eight small studies (576 women). Frequency of leg cramps was our primary outcome and secondary outcomes included intensity and duration of leg cramps, adverse outcomes for mother and baby and health‐related quality of life. Overall, the studies were at low or unclear risk of bias. Outcomes were reported in different ways, precluding the use of meta‐analysis and thus data were limited to single trials. Certainty of evidence was assessed as either low or very‐low due to serious limitations in study design and imprecision.
Oral magnesium versus placebo/no treatment
The results for frequency of leg cramps were inconsistent. In one study, results indicated that women may be more likely to report never having any leg cramps after treatment (risk ratio (RR) 5.66, 95% confidence interval (CI) 1.35 to 23.68, 1 trial, 69 women, low‐certainty evidence); whilst fewer women may report having twice‐weekly leg cramps (RR 0.29, 95% CI 0.11 to 0.80, 1 trial, 69 women); and more women may report a 50% reduction in number of leg cramps after treatment (RR 1.42, 95% CI 1.09 to 1.86, 1 trial, 86 women, low‐certainty evidence). However, other findings indicated that magnesium may make little to no difference in the frequency of leg cramps during differing periods of treatment.
For pain intensity, again results were inconsistent. Findings indicated that magnesium may make little or no difference: mean total pain score (MD 1.80, 95% CI ‐3.10 to 6.70, 1 trial, 38 women, low‐certainty evidence). In another study the evidence was very uncertain about the effects of magnesium on pain intensity as measured in terms of a 50% reduction in pain. Findings from another study indicated that magnesium may reduce pain intensity according to a visual analogue scale (MD ‐17.50, 95% CI ‐34.68 to ‐0.32,1 trial, 69 women, low‐certainty evidence). For all other outcomes examined there may be little or no difference: duration of leg cramps (low to very‐low certainty); composite outcome ‐ symptoms of leg cramps (very‐low certainty); and for any side effects, including nausea and diarrhoea (low certainty).
Oral calcium versus placebo/no treatment
The evidence is unclear about the effect of calcium supplements on frequency of leg cramps because the certainty was found to be very low: no leg cramps after treatment (RR 8.59, 95% CI 1.19 to 62.07, 1 study, 43 women, very low‐certainty evidence). In another small study, the findings indicated that the mean frequency of leg cramps may be slightly lower with oral calcium (MD ‐0.53, 95% CI ‐0.72 to ‐0.34; 1 study, 60 women; low certainty).
Oral vitamin B versus no treatment
One small trial, did not report on frequency of leg cramps individually, but showed that oral vitamin B supplements may reduce the frequency and intensity (composite outcome) of leg cramps (RR 0.29, 95% CI 0.11 to 0.73; 1 study, 42 women). There were no data on side effects.
Oral calcium versus oral vitamin C
The evidence is very uncertain about the effect of calcium on frequency of leg cramps after treatment compared with vitamin C (RR 1.33, 95% CI 0.53 to 3.38, 1 study, 60 women, very low‐certainty evidence).
Oral vitamin D versus placebo
One trial (84 women) found vitamin D may make little or no difference to frequency of leg cramps compared with placebo at three weeks (MD 2.06, 95% CI 0.58 to 3.54); or six weeks after treatment (MD 1.53, 95% CI 0.12 to 2.94).
Oral calcium‐vitamin D versus placebo
One trial (84 women) found oral calcium‐vitamin D may make little or no difference to frequency of leg cramps compared with placebo after treatment at three weeks (MD ‐0.30, 95% CI ‐1.55 to 0.95); and six weeks (MD 0.03, 95% CI ‐1.3 to 1.36).
Oral calcium‐vitamin D versus vitamin D
One trial (84 women) found oral calcium‐vitamin D may make little or no difference to frequency of leg cramps compared with vitamin D after treatment at three weeks (MD ‐1.35, 95% CI ‐2.84 to 0.14); and six weeks after treatment (MD ‐1.10, 95% CI ‐2.69 to 0.49).
Authors' conclusions
It is unclear from the evidence reviewed whether any of the interventions provide an effective treatment for leg cramps. This is primarily due to outcomes being measured and reported in different, incomparable ways so that data could not be pooled. The certainty of evidence was found to be low or very‐low due to design limitations and trials being too small to address the question satisfactorily.
Adverse outcomes were not reported, other than side effects for magnesium versus placebo/no treatment. It is therefore not possible to assess the safety of these interventions.
The inconsistency in the measurement and reporting of outcomes meant that meta‐analyses could not be carried out. The development of a core outcome set for measuring the frequency, intensity and duration of leg cramps would address these inconsistencies and mean these outcomes could be investigated effectively in the future.
Plain language summary
Interventions for leg cramps during pregnancy
What is the issue?
Leg cramps are experienced as sudden, intense involuntary contractions of the leg muscles. They are a common problem in pregnancy, especially in the third trimester. They are painful and can interfere with daily activities, disrupt sleep, and reduce quality of life. Various interventions have been used during pregnancy to treat leg cramps, including drug, electrolyte (magnesium, calcium, sodium) and vitamin therapies, and non‐drug therapies such as muscle stretching.
Why is this important?
The goal of this review was to find out what is effective and safe for treating leg cramps during pregnancy.
What evidence did we find?
We searched for evidence in September 2019 and identified eight randomised controlled studies, with a total of 576 women who were 14 to 36 weeks pregnant, comparing either magnesium, calcium, calcium‐vitamin D or vitamin B with placebo or no treatment, and comparing vitamin C with calcium. All treatments were given as tablets to be chewed or swallowed.
Magnesium supplements may reduce how often women experienced leg cramps when compared with placebo or no treatment, although findings were not consistent. Studies measured this in different ways, sometimes showing that magnesium helped reduce the number of leg cramps but sometimes showing that it made little or no difference. Likewise, evidence about whether magnesium reduced the intensity of pain was inconclusive with one study showing a reduction while others showed no difference. There was little or no difference in the experience of side effects, such as nausea and diarrhoea.
Calcium did not consistently reduce how often women experienced leg cramps after treatment compared to women who did not receive any treatment. The evidence was also found to be of very low quality and so we cannot be sure of the results.
More women who received vitamin B supplements fully recovered compared with those women receiving no treatment; however these results were from a small sample and the study had design limitations.
The frequency of leg cramps was no different between women treated with calcium and those treated with vitamin C.
The calcium‐vitamin D and the vitamin D supplements had no effect on the frequency, length, and pain intensity of leg cramps after treatment compared to women who received placebo.
What does this mean?
The level of evidence was found to be of low or very low quality. This was mainly due to the small sample size of studies and poor study design. Four studies were well‐conducted and reported. The other four had design limitations: women were not allocated to different treatment groups in the best way in several studies, and in two studies women knew whether they were receiving treatment or not. Adverse effects such as any effect of the treatment on pregnancy complications, labour and the baby were not reported. Several of the studies focused mainly on serum calcium and magnesium levels. The frequency and intensity of cramps and the duration of pain were not reported in a consistent way and often information was lacking on how they were measured, either during treatment, at the end of treatment or after treatment had stopped.
It is not clear from the evidence reviewed whether any of the oral interventions (magnesium, calcium, calcium‐vitamin D, vitamin B vitamin D or vitamin C) provide an effective and safe treatment for leg cramps in pregnancy. Supplements may have different effects depending on women's usual intake of these substances. No trials considered therapies such as muscle stretching, massage, relaxation or heat therapy.
Summary of findings
Background
This Cochrane Review is a further update of a review first published in 2015.
Description of the condition
Leg cramps in pregnancy are a common problem characterised by sudden, intense, painful, and involuntary contractions of the leg muscles. Leg cramps may occur in pregnancy secondary to other diseases (e.g. amyotrophic lateral sclerosis, hypothyroidism, restless legs syndrome) or due to medication (e.g. diuretics) or procedures (e.g. undergoing haemodialysis (Allen 2012; Miller 2005; Young 2009). They are different from restless legs syndrome, an involuntary movement in legs without muscle contractions or pain (Allen 2003; Allen 2012), although both conditions can occur in pregnant women (Hensley 2009). Up to 30% to 50% of pregnant women suffer from leg cramps, especially in the third trimester. Almost two‐thirds of these women experience leg cramps twice per week and they can occur at any time, particularly at night (Sohrabvand 2009). Unfortunately, the aetiology and the precise mechanism of leg cramps in pregnancy is still unclear. It is possible that they are associated with metabolic disorders in pregnancy, inactivity or excessive exercise, electrolyte imbalances (e.g. magnesium, calcium, and sodium) and vitamin (E and D) deficiency (Miller 2005; Page 1953; Parisi 2003; Young 2009). One possible pathophysiological explanation is that leg cramps are caused by lower motor neurons with hyperactive, high‐frequency, involuntary nerve spontaneous discharge (Allen 2012; Miller 2005; Minetto 2013). To date, there is no guideline to clarify the diagnostic criteria of leg cramps in pregnancy, but clinical history, physical examination and laboratory tests are useful (McGee 1990; Miller 2005; Shaker 2005). In most cases, leg cramps only last for seconds, but in severe cases, leg cramps in pregnancy will last for minutes with severe pain, which can affect daily activities, limit exercise and performance, cause sleep disturbance and reduce the quality of life (Allen 2012; Hertz 1992; Soares 2006). Leg cramps are considered to be part of sleep‐related movement disorders (Merlino 2012). For pregnant women, leg cramps overnight can cause sleep disorders such as sleep loss and insomnia, which may affect the outcome of labour including the length of labour and mode of delivery (Hensley 2009; Hertz 1992; Lee 2004; Mindell 2000). One prospective, observational study including 131 pregnant women, found that pregnant women sleeping less than six hours per night and those with a severe sleep problem were, respectively, 4.5 times and 5.2 times more likely to undergo a caesarean delivery (Lee 2004). Leg cramps in pregnancy may also be related to depression which can increase placental corticotropin‐releasing factor and initiate uterine contractions and cervical ripening, and might eventually cause labour difficulty, fetal hypoxia and increased risks of neonatal asphyxia and postpartum haemorrhage (Dayan 2002; Hickey 1995; Marcus 2003; Rondo 2003).
Description of the intervention
A number of interventions are available for leg cramps in pregnancy. The most commonly used can be divided into two categories: drug/electrolyte/vitamin therapies and non‐drug therapies. Historically, quinine and its derivatives were the effective mainstay therapy for idiopathic muscle cramps, including leg cramps in pregnancy (Katzberg 2010). Quinine is effective in reducing the number and intensity of cramps (El‐Tawil 2015; Man‐Son‐Hing 1998). Unfortunately, quinine is associated with many severe side effects, such as visual toxicity, auditory toxicity (e.g. hearing loss), cardiotoxicity, fetal teratogenicity (e.g. central nervous system, limb, facial and cardiac defects, optic nerve hypoplasia and deafness), gastrointestinal symptoms, and renal impairment (Langford 2003; Nishimura 1976; Pedersen 1985). Because of these serious adverse effects, multiple drug regulatory agencies have banned the use of quinine for muscle cramps (ADRAC 2002; FDA 2006; Medsafe 2007). Other commonly used drug/electrolyte/vitamin therapies include magnesium, calcium, sodium, vitamins (vitamin E, vitamin D) supplement and pycnogenol (Garrison 2020; Hammar 1987; Kohama 2006; Miller 2005; Nygaard 2008; Page 1953). In addition, one study also found anticonvulsants such as gabapentin were helpful for leg cramps (Serrao 2000). However, one observational controlled trial found oral magnesium supplementation during pregnancy did not reduce the occurrence and frequency of leg cramps (Leal de Araújo 2020). A lot of research has been done with these drug therapies, however, there are still no consistent conclusions for treating leg cramps in pregnancy. Non‐drug therapies commonly used in treating acute cramps and preventing cramps include muscle stretching, massage, relaxation, heat therapy and dorsiflexion of the foot (Blyton 2012; Kanaan 2001; Miller 2005). Muscle stretching is a simple intervention and is suggested as the first line treatment in some studies (McGee 1990; Miller 2005). However, the effectiveness and safety of all therapies are not known.
How the intervention might work
Different interventions work in different ways. Quinine increases the refractory period of muscle and reduces the excitability of the motor end plate, thereby reducing its response to repetitive stimulation, nerve stimulation and acetylcholine, resulting in suppression of muscle cramps (El‐Tawil 2015; Goodman 2001; Harvey 1939). Magnesium deficiency increases neuronal excitability and enhances neuromuscular transmission with muscle cramps as it has a curariform action on the neuromuscular junction and is associated with the release of acetylcholine from motor nerve terminals (Wacker 1968). Hence, magnesium supplementation may suppress excitable tissue and suppress muscle cramps (Frusso 1999; Garrison 2020). However, the mechanism of many other interventions for leg cramps in pregnancy is unclear.
Why it is important to do this review
Leg cramps in pregnancy are a common problem, with the potential for adverse effects on the mother and baby. Other than quinine, which is not recommended in pregnancy, the effectiveness and safety of interventions for this problem have not been addressed (Allen 2012; El‐Tawil 2015; Hensley 2009; Lee 2004).
Five Cochrane Reviews have investigated muscle cramps (including one previous review by Young 2002, which looked at interventions for leg cramps in pregnancy ‐ the topic of our review). One review of non‐drug therapies for lower limb muscle cramps did not focus on pregnant women (Blyton 2012). The Garrison 2020 review looked at magnesium for muscle cramps and carried out subgroup analysis on pregnant women but only compared placebo with no treatment. Another review assessed all interventions for muscle cramps in amyotrophic lateral sclerosis, but pregnancy‐associated leg cramps were excluded (Baldinger 2012). In contrast, the Cochrane Review by Young 2002 looked at interventions for leg cramps in pregnancy but there have since been new studies published in this area. Consequently, we prepared a protocol (Zhou 2013) for a new review team to prepare an updated Cochrane Review on this topic.
A Cochrane Review by El‐Tawil 2015 focused on quinine and found it could significantly reduce the number and intensity of cramps in the general population, but was associated with significant gastrointestinal symptoms, haematological and cardiac toxicity events and fatal adverse effects. Quinine is excluded from our review because of its known adverse effects. Magnesium and non‐drug therapies are included in our review as we want to find the most effective intervention. In addition, other common therapies (e.g. calcium, sodium, various vitamins) still need to be evaluated.
Objectives
To assess the effectiveness and safety of different interventions for treating leg cramps in pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any intervention (except for quinine seeBackground) for treatment of leg cramps in pregnancy. Studies for prevention of leg cramps in pregnancy were excluded. Cluster‐randomised studies were considered as mentioned in the Unit of analysis issues. Quasi‐RCTs were excluded due to obvious selection bias. Cross‐over studies were also excluded. RCTs published as abstracts were eligible for inclusion.
Types of participants
Pregnant women who were experiencing leg cramps in pregnancy. However, pregnant women with leg cramps secondary to another disease (e.g. amyotrophic lateral sclerosis, hypothyroidism), receiving medication (e.g. diuretics), undergoing haemodialysis and pregnant women with restless legs syndrome were excluded.
Types of interventions
We included all therapeutic interventions for leg cramps in pregnancy, including:
drug/electrolyte/vitamin therapies, for example, calcium salts, magnesium salts, sodium salts, vitamins (vitamin D, vitamin E), calcium‐vitamin D and mineral supplements compared with placebo, no treatment or other treatment. We planned to exclude any trials of quinine, for its known adverse effects (teratogenicity);
non‐drug therapies, for example, muscle stretching, massage, relaxation, heat therapy, dorsiflexion of the foot compared with placebo, no treatment or other treatment.
Types of outcome measures
Primary outcomes
Frequency of leg cramps. For example, measured as the number of leg cramps per week.
Secondary outcomes
Intensity of leg cramps. For example, level of pain intensity measured by validated instruments.
Duration of leg cramps. For example, measured by seconds per leg cramp.
Composite outcome: symptoms of leg cramps, including two or more of: frequency, pain intensity or duration of leg cramps (not prespecified).
-
Adverse outcomes:
maternal side effects (e.g. nausea, vomiting, diarrhoea, constipation);
labour outcome (e.g. mode of birth);
pregnancy complications (e.g. hypertension, pre‐eclampsia, antepartum haemorrhage);
pregnant outcomes: fetal death, including spontaneous abortion (before 20 weeks' gestation), preterm labour and stillbirth;
neonatal outcomes: neonatal asphyxia, neonatal death: a baby death within 28 days of live birth;
congenital abnormalities (e.g. biochemical defects, genetic and chromosomal abnormalities).
Health‐related quality of life, as measured by validated instruments.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (25 September 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (25 September 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeZhou 2015.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
If information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were identified for this review. Had we found any cluster‐randomised trials, we would have included them along with the individually‐randomised trials.Their sample sizes or standard errors would have been adjusted using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Multiple pregnancies studies
No trials focused on multiple pregnancies were identified for this version of the review. Had we included studies involving women with multiple pregnancies, we would have treated the infants as independent and noted effects of estimates of confidence intervals in the review.
Multi‐arm studies
We included two multi‐arm studies (Mansouri 2017; Sohrabvand 2006). We sought statistical advice on how to present the results of these two studies. The participants assigned to no treatment have been used as a comparison with magnesium, calcium and vitamin B in Sohrabvand 2006 and presented in separate comparisons. The other participants in Mansouri 2017 assigned to placebo have been used in separate comparisons with oral vitamin D and calcium‐vitamin D. These strategies have been used in order to avoid unit of analysis issues caused by either double counting of participant data or by omitting any relevant data.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014), but we did not combine data in meta‐analysis due to insufficient data. In future updates of this review, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We did not combine data in meta‐analysis due to insufficient data. However, in future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
In future updates we plan to undertake the following subgroup analyses by types of interventions:
gestational age at the end of the treatment: (1) 28 weeks or less; or (2) more than 28 weeks.
Subgroup analysis will be restricted to the primary outcome.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
There were insufficient data in any one comparison to undertake sensitivity analysis to assess the effect of missing data or trial quality. In future updates of this review, if appropriate, we will carry out sensitivity analysis to explore the effects of trial quality assessed by allocation concealment and other 'Risk of bias' components, by omitting studies rated as inadequate for these components. We will also use sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity and the effects of any assumptions made. Sensitivity analysis will be restricted to the primary outcome.
Assessing the certainty of the evidence using the GRADE approach
For this update the certainty of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison (oral magnesium versus placebo/no treatment), and the primary outcome for other comparisons (oral calcium versus no treatment, oral vitamin B versus no treatment, and oral calcium versus oral vitamin C).
Frequency of leg cramps, (e.g. measured as the number of leg cramps per week).
Intensity of leg cramps, (e.g. pain intensity measured by validated instruments).
Duration of leg cramps, (e.g. measured by seconds per leg cramp).
Composite outcome: symptoms of leg cramps, including two or more of: frequency, pain intensity or duration of leg cramps (not prespecified).
Maternal side effects (e.g. nausea, vomiting, diarrhoea, constipation).
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for important outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See: Figure 1.
1.
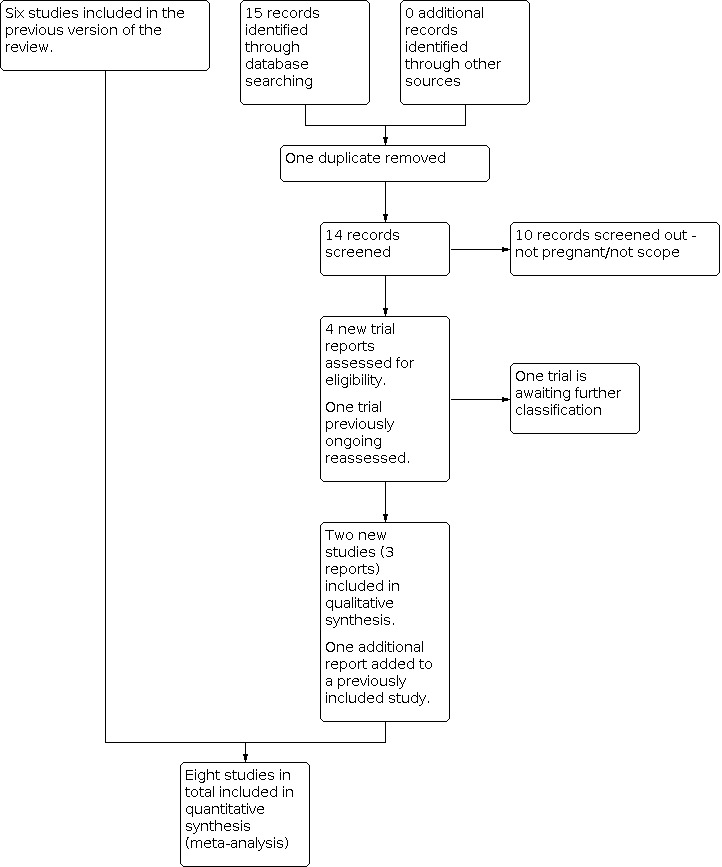
Study flow diagram.
For this 2020 update, we retrieved five new trial reports to assess and we reassessed the trial that was ongoing in the previous version of the review. One report was a duplicate. We included two new trials (three reports) and added an additional report to a trial already included. One trial is awaiting further classification.
Included studies
Eight studies (involving 576 women) were included in this review update. Six studies were included in the first previous version of the review. In this update, another two studies were included after assessment. See Characteristics of included studies.
Design
Six studies were two‐arm randomised controlled trials. Three of these trials compared magnesium with placebo (Dahle 1995; Nygaard 2008; Supakatisant 2015), one compared calcium with no treatment (Hammar 1981), one compared calcium with placebo (Khoranrodi 2011), and one compared calcium with vitamin C (Hammar 1987). One study was a four‐arm randomised controlled trial, in which women were allocated to receive calcium, magnesium, vitamin B or no treatment (Sohrabvand 2006). One study was a three‐arm randomised controlled trial, in which women were allocated to receive vitamin D, calcium‐vitamin D or placebo (Mansouri 2017). Two trials included an additional control group of pregnant women without leg cramps, who were not included in this review (Hammar 1981; Hammar 1987).
Sample sizes
The total number of women recruited to the trials was 576. Studies had a sample size ranging from 42 (Hammar 1981) to 126 (Mansouri 2017).
Setting
Studies were carried out in Sweden (Dahle 1995; Hammar 1981; Hammar 1987), Norway (Nygaard 2008), Iran (Sohrabvand 2006; Mansouri 2017), Taleb (Khoranrodi 2011) and Thailand (Supakatisant 2015). Recruitment and treatment took place in outpatient clinics (Dahle 1995; Khoranrodi 2011; Mansouri 2017; Nygaard 2008; Supakatisant 2015), or was not described (Hammar 1981; Hammar 1987; Sohrabvand 2006).
Participants
Pregnant women who were experiencing leg cramps were included in all studies. The inclusion criteria specified that women had experienced leg cramps at least twice a week (Hammar 1981; Nygaard 2008; Supakatisant 2015), for at least two weeks (Hammar 1981; Hammar 1987), and that they were painful (Nygaard 2008; Supakatisant 2015). Dahle 1995, Khoranrodi 2011, Mansouri 2017 and Sohrabvand 2006 did not specify the frequency, duration or intensity of leg cramps previously experienced by women eligible for the study. Women were eligible to participate if their gestation was 22 to 36 weeks (Dahle 1995), 18 to 36 weeks (Nygaard 2008), 25 to 30 weeks(Mansouri 2017), third trimester of pregnancy (Khoranrodi 2011), and 14 to 34 weeks (Supakatisant 2015).
Women were excluded from participation if they had already received treatment for leg cramps (Dahle 1995; Supakatisant 2015), and if they had concurrent medical conditions (Dahle 1995; Nygaard 2008; Supakatisant 2015). The inclusion and exclusion criteria were not described by Hammar 1981; Hammar 1987 and Sohrabvand 2006.
Interventions
All therapies were given orally.
The dose of magnesium was three chewable tablets of magnesium 120 mg (5 mmol) per day, one tablet in the morning and two each evening (primarily magnesium lactate and magnesium citrate) (Dahle 1995; Nygaard 2008), two tablets of magnesium 183.2 mg (7.5 mmol) per day (magnesium aspartate) (Sohrabvand 2006), and three tablets of 100 mg magnesium bisglycinate chelate per day (Supakatisant 2015). Treatment was for two weeks (Nygaard 2008; Sohrabvand 2006), three weeks (Dahle 1995), or four weeks (Supakatisant 2015).
Oral calcium preparations used were calcium gluconate, calcium lactate and calcium carbonate corresponding to a calcium dose of 1 g twice daily (Hammar 1981; Hammar 1987), and 500 mg calcium carbonate tablets once daily (Khoranrodi 2011; Sohrabvand 2006). Treatment was for two weeks (Hammar 1981; Sohrabvand 2006), or three weeks (Hammar 1987) and four weeks (Khoranrodi 2011).
The dose of vitamin C was 1 g twice daily for three weeks (Hammar 1987), and vitamin B was 100 mg of thiamine (vitamin B1) plus 40 mg of pyridoxine (vitamin B6) once daily for two weeks (Sohrabvand 2006).
The dose of vitamin D was 1000 units once daily for three weeks and the dose of calcium‐vitamin D was 300 mg of calcium carbonate and 1000 units of vitamin D once daily for three weeks (Mansouri 2017).
Five studies used placebo tablets (Dahle 1995; Khoranrodi 2011; Mansouri 2017; Nygaard 2008; Supakatisant 2015), and two studies used no treatment as a comparison (Hammar 1981; Sohrabvand 2006). Comparisons between different treatments were made in two studies (Hammar 1987; Sohrabvand 2006).
Outcomes
Most studies measured biochemical outcomes, such as serum calcium and serum magnesium levels (Dahle 1995; Hammar 1981; Hammar 1987; Nygaard 2008), which are not of relevance to this review. Clinical outcomes were not reported in a consistent way and often there was a lack of information on how they had been measured. For example "frequency of leg cramps" (our primary outcome) was given as mean episodes during the treatment period (Khoranrodi 2011; Nygaard 2008); one study showed frequency of leg cramps by comparison of the leg cramps length before and after treatment (Mansouri 2017); number of cramps per week after treatment (Dahle 1995); number of cramps during the three weeks and six weeks after treatment (Mansouri 2017); 50% reduction in the number of leg cramps (Supakatisant 2015); and whether leg cramps had ceased after treatment. The outcome "intensity of leg cramps" (our secondary outcome) was given as a mean intensity of pain score during the treatment period (Khoranrodi 2011; Nygaard 2008), 50% reduction in pain score of leg cramps (Supakatisant 2015), and the specific intensity pain score points (Dahle 1995). Only one study reported the outcome relating to "duration of the leg cramps", however, it was given as persisting leg cramps after night‐time (Dahle 1995). The "composite outcome" was reported as whether the frequency and intensity of leg cramps showed partial improvement or complete recovery (Sohrabvand 2006). Maternal side effects of nausea and diarrhoea were reported in two studies (Nygaard 2008; Supakatisant 2015), however, other adverse events were not reported.
Studies measured the frequency and intensity of leg cramps at different time points. Outcomes were assessed during treatment (Mansouri 2017; Nygaard 2008; Supakatisant 2015), at the end of the treatment period (Dahle 1995; Hammar 1981; Hammar 1987; Khoranrodi 2011), or in a time period after treatment had ceased (for example, Sohrabvand 2006).
The authors of Sohrabvand 2006 and Supakatisant 2015 were contacted for additional information on the studies. A response was received from Sohrabvand 2006, with details of trial methodology and results not provided in the published report. At the time of writing, no response has been received from Supakatisant 2015.
Dates of study
Four studies did not report study dates (Dahle 1995; Hammar 1981; Hammar 1987; Sohrabvand 2006). Other studies took place between 2000 and 2014: August 2000 to January 2003 (Nygaard 2008); June 2010 to August 2011 (Supakatisant 2015); July 2013 to April 2014 (Mansouri 2017). One study was unclear about describing study dates, but it is likely to have taken place between March 2006 and November 2007 (Khoranrodi 2011).
Funding sources
Two studies did not report funding sources (Hammar 1981; Sohrabvand 2006). Two studies reported having no funding sources (Mansouri 2017; Nygaard 2008). Other studies were funded by Linkoping University Faculty Grant and by ACO & Pharmacia Lakemedel (Dahle 1995); ACO research Fund (Hammar 1987); Research Managment Affairs, Bushehr University Of Medical Sciences (Khoranrodi 2011); and Grant for Development of New Faculty Staff, Chulalongkorn University (Supakatisant 2015).
Declarations of interest
Four studies did not report conflicts of interest (Dahle 1995; Hammar 1981; Hammar 1987; Sohrabvand 2006). Three studies reported to have no conflicts (Khoranrodi 2011; Mansouri 2017; Supakatisant 2015). One study reported that an author (Thomas Bøhmer) received compensation 'for effort' from the company that provided the intervention and placebo tablets, Nycomed Pharma. The rest of the authors had no conflicts of interest.
Excluded studies
We excluded nine studies (see Characteristics of excluded studies). They were excluded because group allocation was not randomised (Mukherjee 1997; Robinson 1947; Shahraki 2006); a cross‐over design was used (Mauss 1970); some women received more than one course of treatment, not necessarily the same treatment (Odendaal 1974); the pregnant women did not have leg cramps (Griffith 1998; Thauvin 1992); participants with leg cramps were combined with pregnant women experiencing other types of pain such as lower back pain and pelvic pain (Kohama 2006); or participants were not pregnant women (Rougin 2012).
Ongoing studies
There are no ongoing studies in this updated paper.
Risk of bias in included studies
See Characteristics of included studies, 'Risk of bias' graph (Figure 2) and Risk of bias' summary (Figure 3).
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In four studies, women were randomised using a random number table or randomisation programme to generate the sequence (low risk of bias, Mansouri 2017; Nygaard 2008; Sohrabvand 2006; Supakatisant 2015). Three studies state that pregnant women were randomly allocated, but give no description of the method (unclear risk of bias, Dahle 1995; Hammar 1981; Hammar 1987). Only one study states that it was “randomised”, but there is no description of the method (Khoranrodi 2011).
Allocation concealment was achieved by using sequentially numbered drug containers of identical appearance in three studies (low risk of bias, Dahle 1995; Nygaard 2008; Supakatisant 2015), and numbered envelopes in two studies (low risk of bias, Mansouri 2017; Sohrabvand 2006). The code was not broken until women had completed the investigation, which suggests that allocation was concealed in Hammar 1987. The remaining two studies did not provide information on allocation concealment (unclear risk of bias, Hammar 1981; Khoranrodi 2011).
Blinding
Five studies are described as double‐blind, with the code not being broken until all women had completed the investigation (low risk of bias, Dahle 1995; Hammar 1987; Khoranrodi 2011; Nygaard 2008; Supakatisant 2015). In one study, the control group received no treatment, so participants, personnel and outcome assessors were aware of whether or not they were receiving the intervention (high risk of bias, Hammar 1981). In another study, healthcare providers and the statistician were blinded, but women may have been aware of the group allocation as the timing and size of treatments was different (unclear risk of bias, Sohrabvand 2006). One study was conducted as double‐blinded, participants did not know which group they were in, but no information on blinding of personnel (unclear risk of bias, Mansouri 2017).
Incomplete outcome data
Five women who were recruited by Dahle 1995; Mansouri 2017 were subsequently excluded; the report does not state which group they had been allocated to, and the analysis is not intention‐to‐treat. It is unclear whether this would bias the results (unclear risk of bias). All women were accounted for in the other included studies (low risk of bias), however some studies had missing data. Results are presented for 84% of women recruited in Nygaard 2008, for 93% of women in Supakatisant 2015 and for 94% of women in Khoranrodi 2011. These three studies used intention‐to‐treat analyses.
Selective reporting
All outcomes pre‐specified in the study protocols were reported in Khoranrodi 2011, Mansouri 2017, Nygaard 2008;Supakatisant 2015 (low risk of bias). The other studies were assessed from published reports without access to the study protocol, so the level of reporting bias is unclear (Dahle 1995; Hammar 1981; Hammar 1987; Sohrabvand 2006).
Other potential sources of bias
One author declared a conflict of interest in Nygaard 2008, having contributed to developing the magnesium tablet used and received payment from the pharmaceutical company. Groups appear similar at baseline, where this information was given (Dahle 1995; Supakatisant 2015), however in most studies there was insufficient information to assess whether any other potential sources of bias existed (unclear risk of bias for all studies).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Oral magnesium compared with placebo/no treatment for treating leg cramps in pregnancy.
| Oral magnesium compared with placebo/no treatment for treating leg cramps in pregnancy | ||||||
| Patient or population: treating leg cramps in pregnancy Settings: outpatient clinics in Norway, Sweden and Thailand Intervention: oral magnesium Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with placebo/ no treatment | Corresponding risk with oral magnesium | |||||
| Frequency of leg cramps during treatment | The mean frequency of leg cramps during treatment in the control group was 7.7 | The mean frequency of leg cramps during treatment in the intervention group was 1.8 higher (1.32 lower to 4.92 higher) | ‐ | 38 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| Frequency of leg cramps after treatment: never | Study population | RR 5.66 (1.35 to 23.68) | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 2,3 | ||
| 57 per 1000 | 323 per 1000 (77 to 1000) | |||||
| Frequency: 50% reduction in number of leg cramps | Study population | RR 1.42 (1.09 to 1.86) | 86 (1 RCT) | ⊕⊕⊝⊝ LOW 4,5 | ||
| 605 per 1000 | 859 per 1000 (659 to 1000) | |||||
| Intensity of pain during treatment: mean total scale points The degree of pain was noted in the form, on a scale from 0 to 4 measured over 4 days and nights (0 = no pain, 1 = light pain, 2 = medium pain, 3=strong pain, 4=extreme pain) |
The mean intensity of pain during treatment: mean total scale points in the control group was 11.4 | The mean intensity of pain during treatment: mean total scale points in the intervention group was 1.8 higher (3.1 lower to 6.7 higher) | ‐ | 38 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| Intensity of pain: 50% reduction in pain score 100‐mm visual analogue scale (0 = no pain, 100 = worst pain) |
Study population | RR 1.43 (0.99 to 2.06) | 86 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,4 | ||
| 488 per 1000 | 698 per 1000 (483 to 1000) | |||||
| Intensity of pain: visual analogue scale 100 mm Visual analogue scale where 0=insignificant and 100 = extremely painful. |
The mean intensity of pain: visual analogue scale in the control group was 47.8 | The mean intensity of pain: visual analogue scale in the intervention group was 17.5 lower (34.68 lower to 0.32 lower) | ‐ | 69 (1 RCT) | ⊕⊕⊝⊝ LOW 2,5 | |
| Duration of leg cramps: persisting symptoms after night‐time cramps Always |
Study population | RR 0.23 (0.05 to 0.98) | 69 (1 RCT) |
⊕⊕⊝⊝ LOW 2,3 | ||
| 257 per 1000 | 59 per 1000 (13 to 252) |
|||||
| Duration of leg cramps: persisting symptoms after night‐time cramps Sometimes |
Study population | RR 0.59 (0.19 to 1.83) | 69 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 200 per 1000 | 118 per 1000 (38 to 366) |
|||||
| Composite outcome: symptoms of leg cramps (intensity and frequency) Partial improvement: decrease in intensity and frequency |
Study population | RR 1.07 (0.71 to 1.61) |
42 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 667 per 1000 | 713 per 1000 (473 to 1000) |
|||||
| Composite outcome: symptoms of leg cramps (intensity and frequency) Complete recovery: no leg cramps after treatment |
Study population | RR 3.00 (0.68 to 13.20) |
42 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 95 per 1000 | 286 per 1000 (65 to 1000) |
|||||
| Side effects ‐ nausea | Study population | RR 1.83 (0.75 to 4.51) |
86 (1 RCT) |
⊕⊕⊝⊝ LOW 1 | ||
| 140 per 1000 | 255 per 1000 (105 to 629) |
|||||
| Side effects ‐ diarrhoea | Study population | RR 6.00 (0.75 to 47.76) |
86 (1 RCT) |
⊕⊕⊝⊝ LOW 1 | ||
| 23 per 1000 | 140 per 1000 (17 to 1000) |
|||||
| Side effects ‐ any | Study population | RR 0.96 (0.36 to 2.52) |
45 (1 RCT) |
⊕⊕⊝⊝ LOW 1 | ||
| 273 per 1000 | 262 per 1000 (98 to 687) |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1Wide CI crossing the line of no effect and small sample size (‐2).
2Design limitations (‐1).
3Few events and small sample size (‐1).
4Outcome is assessed using an arbitrary cut‐off (‐1).
5Small sample size (‐1).
Summary of findings 2. Oral calcium compared with no treatment for treating leg cramps in pregnancy.
| Oral calcium compared with no treatment for treating leg cramps in pregnancy | ||||||
| Patient or population: treating leg cramps in pregnancy Settings: outpatient clinic in Sweden Intervention: oral calcium Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with no treatment | Corresponding risk with oral calcium | |||||
| Frequency of leg cramps after treatment: never | Study population | RR 8.59 (1.19 to 62.07) | 43 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 48 per 1000 | 409 per 1000 (57 to 1000) | |||||
| Frequency of leg cramps after treatment | Study population | ‐ | 60 (1 RCT) |
⊕⊕⊝⊝ LOW | ||
| The mean frequency of leg cramps in the control group was 0.95 | The mean frequency of leg cramps in the intervention group was 0.53 lower (0.72 lower to 0.34 lower) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1Serious design limitations (‐2).
2Few events and small sample size (‐1).
3Some concerns with design limitations (unclear selection bias) (‐1)
4Small sample size (‐1)
Summary of findings 3. Oral vitamin B compared with no treatment for treating leg cramps in pregnancy.
| Oral calcium compared with no treatment for treating leg cramps in pregnancy | ||||||
| Patient or population: treating leg cramps in pregnancy Settings: Iran Intervention: oral vitamin B Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with no treatment | Corresponding risk with oral calcium | |||||
| Frequency of leg cramps after treatment | Study population | ‐ | 0 (0 RCTs) |
‐ | No data | |
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
Summary of findings 4. Oral calcium compared with oral vitamin C for leg cramps in pregnancy.
| Oral calcium compared with oral vitamin C for leg cramps in pregnancy | ||||||
| Patient or population: leg cramps in pregnancy Settings: outpatient clinic in Sweden Intervention: oral calcium Comparison: oral vitamin C | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with oral vitamin C | Corresponding risk with oral calcium | |||||
| Frequency of leg cramps after treatment: never | Study population | RR 1.33 (0.53 to 3.38) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 200 per 1000 | 266 per 1000 (106 to 676) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1Design limitations (‐1).
2Wide CI crossing the line of no effect, few events and small sample size (‐2).
Oral magnesium versus placebo/no treatment
Primary outcomes
See Table 1.
Three studies (193 women) comparing oral magnesium with placebo or no treatment reported the frequency of leg cramps, however they did so in different ways, which meant data could not be pooled in meta‐analysis. The results for frequency of leg cramps were inconsistent. In one study, results indicated that women may be more likely to report never having any leg cramps after treatment (risk ratio (RR) 5.66, 95% confidence interval (CI) 1.35 to 23.68, 1 trial, 69 women, low‐certainty evidence,Analysis 1.3); whilst fewer women may report having twice‐weekly leg cramps (RR 0.29, 95% CI 0.11 to 0.80, 1 trial, 69 women, Analysis 1.2); and more women may report a 50% reduction in number of leg cramps after treatment (RR 1.42, 95% CI 1.09 to 1.86, 1 trial, 86 women, low‐certainty evidence, Analysis 1.4). However, other findings indicated that magnesium may make little to no difference in the frequency of leg cramps during differing periods of treatment: during two weeks of treatment (mean. difference (MD) 1.80, 95% CI ‐1.32 to 4.92, 1 trial, 38 women, evidence graded low,Analysis 1.1); after daily treatment (RR 1.20, 95% CI 0.45 to 3.21, 1 trial, 69 women, Analysis 1.2); after treatment every other day (RR 0.44, 95% CI 0.12 to 1.57, 1 trial, 69 women, Analysis 1.2); and after once weekly treatment (RR 1.54, 95% CI 0.62 to 3.87, 1 trial, 69 women, Analysis 1.2).
1.3. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 3: Frequency of leg cramps after treatment: never
1.2. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 2: Frequency of leg cramps after treatment
1.4. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 4: Frequency: 50% reduction in number of leg cramps
1.1. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 1: Frequency of leg cramps during treatment
Secondary outcomes
For pain intensity, again results were inconsistent. Findings indicated that magnesium may make little or no difference: mean total pain score (MD 1.80, 95% CI ‐3.10 to 6.70, 1 trial, 38 women, low‐certainty evidence, Analysis 1.5). In another study the evidence was very uncertain about the effects of magnesium on pain intensity as measured in terms of a 50% reduction in pain (50% reduction in pain score RR 1.43, 95% CI 0.99 to 2.06, 1 trial, 86 women, Analysis 1.6). Findings from another study indicated that magnesium may reduce pain intensity according to a visual analogue scale (MD ‐17.50, 95% CI ‐34.68 to ‐0.32,1 trial, 69 women, low‐certainty evidence, Analysis 1.7).
1.5. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 5: Intensity of pain during treatment: mean total score
1.6. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 6: Intensity of pain: 50% reduction in pain score
1.7. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 7: Intensity of pain: visual analogue scale
For duration of leg cramps, one study of 69 women suggested that women in the oral magnesium group may be less likely to have symptoms that persist after night‐time cramps (duration: persisting symptoms after night‐time cramps: always RR 0.23, 95% CI 0.05 to 0.98; sometimes RR 0.59, 95% CI 0.19 to 1.83, Analysis 1.8).
1.8. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 8: Duration: persisting symptoms after night‐time cramps
One trial reported a composite outcome of intensity and frequency of leg cramps (42 women, two arms of a four‐arm trial, Sohrabvand 2006). There may be little to no differences in the levels of partial improvement (decrease in intensity and frequency of leg cramps) or complete recovery between groups receiving oral magnesium and no treatment (partial improvement: RR 1.07, 95% CI 0.71 to 1.61; complete recovery: RR 3.00, 95% CI 0.68 to 13.20; Analysis 1.9).
1.9. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 9: Composite outcome: symptoms of leg cramps (intensity and frequency)
There may be little to no differences in the occurrence of side effects, (including nausea, diarrhoea, flatulence and intestinal air) in the results from two trials (131 women), although these results could not be pooled due to the method of reporting (nausea: RR 1.83, 95% CI 0.75 to 4.51, 1 trial, 86 women; diarrhoea: RR 6.00, 95% CI 0.75 to 47.76, 1 trial, 86 women; any side effect (including nausea and diarrhoea): RR 0.96, 95% CI 0.36 to 2.52, 1 trial, 45 women, Analysis 1.10).
1.10. Analysis.

Comparison 1: Oral magnesium versus placebo/no treatment, Outcome 10: Side effects
Other secondary outcomes (adverse outcomes including pregnancy complications, and health‐related quality of life) were not reported in the included studies.
Oral calcium versus placebo/no treatment
Primary outcomes
See Table 2.
The evidence is unclear about the effect of calcium supplements on frequency of leg cramps because the certainty was found to be very low: no leg cramps after treatment (RR 8.59, 95% CI 1.19 to 62.07, 1 study, 43 women, very low‐certainty evidence, Analysis 2.1). In another small study, the findings indicated that the mean frequency of leg cramps may be slightly lower with oral calcium (MD ‐0.53, 95% CI ‐0.72 to ‐0.34; 1 study, 60 women; low certainty, Analysis 2.2). There were may
2.1. Analysis.

Comparison 2: Oral calcium versus placebo/no treatment, Outcome 1: Frequency of leg cramps after treatment: never
2.2. Analysis.

Comparison 2: Oral calcium versus placebo/no treatment, Outcome 2: Frequency of leg cramps after treatment
Secondary outcomes
There may be little to no difference in the levels of partial improvement (decrease in the composite outcome of intensity and frequency of leg cramps) between groups receiving oral calcium versus no treatment (RR 0.64, 95% CI 0.36 to 1.15, 1 trial, 42 women, Analysis 2.3), however in the same trial, it was found that there a greater proportion of women may experience no leg cramps after treatment with calcium compared with no treatment (RR 5.50, 95% CI 1.38 to 21.86, Analysis 2.3). These results are from a four‐arm trial (Sohrabvand 2006).
2.3. Analysis.

Comparison 2: Oral calcium versus placebo/no treatment, Outcome 3: Composite outcome: symptoms of leg cramps (intensity and frequency)
Other secondary outcomes (intensity of leg cramps, duration of leg cramps, adverse outcomes including side effects and pregnancy complications, and health‐related quality of life) were not reported in the included studies.
Oral vitamin B versus no treatment
Primary outcomes
The frequency of leg cramps was not reported in any included studies for this comparison.
Secondary outcomes
One four‐arm trial reported on the composite outcome (intensity and frequency of leg cramps) for the comparison of oral vitamin B with no treatment (42 women. More women receiving oral vitamin B may fully recover compared with those allocated to no treatment (RR 7.50, 95% CI 1.95 to 28.81, Analysis 3.1). Those women receiving no treatment were may be more likely to experience a partial improvement in the intensity and frequency of leg cramps than those taking vitamin B supplements (RR 0.29, 95% CI 0.11 to 0.73, 1 trial, 42 women, Analysis 3.1), or to see no change in their condition.
3.1. Analysis.

Comparison 3: Oral vitamin B versus no treatment, Outcome 1: Composite outcome: symptoms of leg cramps (intensity and frequency)
Other secondary outcomes (intensity of leg cramps, duration of leg cramps, adverse outcomes including side effects and pregnancy complications, and health‐related quality of life) were not reported in the included study.
Oral calcium versus oral vitamin C
Primary outcomes
See Table 4.
One trial of 60 women compared these interventions. The evidence was very uncertain about the effects of oral calcium in comparison to oral vitamin C on frequency of leg cramps after treatment: never (RR 1.33, 95% CI 0.53 to 3.38, very‐low certainty, Analysis 4.1).
4.1. Analysis.

Comparison 4: Oral calcium versus oral vitamin C, Outcome 1: Frequency of leg cramps after treatment: never
Secondary outcomes
No secondary outcomes were reported in the included study (intensity of leg cramps, duration of leg cramps, composite outcome for symptoms of leg cramps, adverse outcomes including side effects and pregnancy complications, and health‐related quality of life).
Oral vitamin D versus placebo
Primary outcomes
Vitamin D did not appear to reduce leg cramps number after treatment compared with placebo. There may be little to no differences for the following: number of leg cramps after three and six weeks of treatment: three weeks (MD 2.06, 95% CI 0.58 to 3.54, 1 trial, 84 women) and six weeks (MD 1.53, 95% CI 0.12 to 2.94, 1 trial, 84 women (Analysis 5.1).
5.1. Analysis.

Comparison 5: Oral vitamin D versus placebo, Outcome 1: Frequency of leg cramps
Secondary outcomes
There may be little to no difference between groups for severity of leg cramps after three weeks (MD ‐0.4, 95% CI ‐0.59 to 0.21, 1 trial, 84 women) and six weeks of treatment (MD ‐0.1, 95% CI ‐0.29 to 0.09, one trial, 84 women) (Analysis 5.2). There may be little to no difference in the duration of leg cramps (min) after three weeks (MD 0.55, 95% CI ‐0.95 to 2.05, 1 trial, 84 women) and six weeks of treatment between these two groups (MD 2.73, 95% CI 0.27 to 5.19, 1 trial, 84 women) (Analysis 5.3).
5.2. Analysis.

Comparison 5: Oral vitamin D versus placebo, Outcome 2: Severity of leg cramps pain
5.3. Analysis.

Comparison 5: Oral vitamin D versus placebo, Outcome 3: Duration of leg cramps
Other secondary outcomes, including adverse outcomes including side effects and pregnancy complications, and health‐related quality of life were not reported.
Oral calcium‐vitamin D versus placebo
Primary outcomes
Oral calcium‐vitamin D did not appear to reduce leg cramps number after treatment compared with oral vitamin D. There may be little to no differences for: number of leg cramps after three and six weeks of treatment: three weeks (MD ‐0.30, 95% CI ‐1.55 to 0.95, 1 trial, 84 women) and six weeks (MD 0.03, 95% CI ‐1.30 to 1.36, 1 trial, 84 women) (Analysis 6.1).
6.1. Analysis.

Comparison 6: Oral Calcium‐vitamin D versus placebo, Outcome 1: Frequency of leg cramps
Secondary outcomes
There may be a slight reduction in the severity of leg cramps after three weeks (MD ‐0.20, 95% CI ‐0.39 to ‐0.01, 1 trial, 84 women), but little to no difference after six weeks of treatment (MD 0.00, 95% CI ‐0.17 to 0.17, 1 trial, 84 women) (Analysis 6.2). There may be little to no difference in the duration leg cramps length (minutes) after three weeks (MD ‐0.30, 95% CI ‐1.55 to 0.95, 1 trial, 84 women) and six weeks of treatment (MD 0.03, 95% CI ‐1.30 to 1.36, 1 trial, 84 women) (Analysis 6.3) between these two groups.
6.2. Analysis.

Comparison 6: Oral Calcium‐vitamin D versus placebo, Outcome 2: Severity of leg cramps pain
6.3. Analysis.

Comparison 6: Oral Calcium‐vitamin D versus placebo, Outcome 3: Duration of leg cramps
Other secondary outcomes, including adverse outcomes including side effects and pregnancy complications, and health‐related quality of life were not reported.
Oral calcium‐vitamin D versus vitamin D
Primary outcomes
Oral calcium‐vitamin D may make little to no difference in the frequency of leg cramps compared with oral vitamin D. Outcomes that showed little or no differences were: number of leg cramps after three and six weeks of treatment: three weeks (MD ‐1.35, 95% CI ‐2.84 to 0.14, 1 trial, 84 women) and six weeks (MD ‐1.10, 95% CI ‐2.69 to 0.49, 1 trial, 84 women) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Oral Calcium‐vitamin D versus vitamin D, Outcome 1: Frequency of leg cramps
Secondary outcomes
There may be little to no difference between groups for severity of leg cramps after three weeks (MD 0.20, 95% CI ‐0.01 to 0.41, 1 trial, 84 women) and six weeks of treatment (MD 0.10, 95% CI ‐0.09 to 0.29, 1 trial, 84 women) (Analysis 7.2). There may be little to no difference in the duration of leg cramps (minutes) after three weeks of treatment (MD ‐0.85, 95% CI ‐2.30 to 0.60, 1 trial, 84 women), although after six weeks of treatment there may be a reduction in favour of the oral calcium‐vitamin D group (MD ‐2.70, 95% CI ‐5.21 to ‐0.19, 1 trial, 84 women) (Analysis 7.3).
7.2. Analysis.

Comparison 7: Oral Calcium‐vitamin D versus vitamin D, Outcome 2: Severity of leg cramps pain
7.3. Analysis.

Comparison 7: Oral Calcium‐vitamin D versus vitamin D, Outcome 3: Duration of leg cramps
Other secondary outcomes, including adverse outcomes including side effects and pregnancy complications, and health‐related quality of life were not reported.
Discussion
Summary of main results
We included eight randomised controlled trials with a total of 576 pregnant women (14 to 36 weeks). These trials contributed results to the comparison of oral magnesium, oral calcium or oral vitamin B or oral vitamin D and oral calcium‐vitamin D with placebo or no treatment, oral vitamin D with calcium‐vitamin D and oral calcium with oral vitamin C. The level of evidence was graded low or very low, see Table 1; Table 2; Table 3; and Table 4. This was mainly due to the small sample size of studies and limitations in study design. Outcomes were reported in different ways, precluding the pooling of results and the use of meta‐analysis, and limiting the strength of our conclusions.
The results for frequency of leg cramps were inconsistent for oral magnesium compared with placebo or no treatment. For some outcome measures there appeared to be a reduction in the frequency of leg cramps in women randomised to receive magnesium, while in other studies, there were little to no differences between groups. There were little to no differences in the occurrence of side effects (including nausea, diarrhoea, flatulence and intestinal air) between pregnant women receiving oral magnesium compared with placebo or no treatment.
The evidence is unclear about the effect of calcium supplements on frequency of leg cramps because the certainty was found to be very low. In another small study, the findings indicated that the mean frequency of leg cramps may be slightly lower with oral calcium. Side effects were not reported in studies of this intervention.
Only one small study showed that oral vitamin B supplements may reduce the frequency and intensity (composite outcome) of leg cramps. However, frequency was not reported individually, and there were no data on side effects.
The evidence is very unclear about the effect of calcium on frequency of leg cramps after treatment compared with vitamin C, again because the certainty was found to be very low.
For oral calcium‐vitamin D in comparison to placebo or vitamin D alone, there may be little to no differences on the frequency, duration, and pain intensity of leg cramps after treatment.
Overall completeness and applicability of evidence
The review only considers trials of interventions to treat leg cramps in pregnancy, not interventions to prevent leg cramps. This evidence is therefore not applicable to the population of pregnant women interested in avoiding this condition.
Supplements may have different effects depending on the baseline intake of the compounds, and pre‐existing deficiencies. In different cultures, pregnant women consume different amounts of the dietary vitamins and minerals considered as interventions in this review, therefore treatment of leg cramps may vary depending on individual and cultural variables.
Several of the trials included in this review focused primarily on biochemical markers in the blood as indirect evidence of leg‐cramp symptoms (Dahle 1995; Hammar 1981; Hammar 1987; Nygaard 2008). This objective may explain some of the inadequacies in the reporting of clinical data. The lack of reporting of adverse outcomes, such as maternal side effects, labour outcome, pregnancy complications, and neonatal outcomes, means that the safety of the interventions cannot be assessed.
Trials were not consistent in when they assessed the effects of treatment. Studies measured the frequency and intensity of leg cramps during treatment (Mansouri 2017; Nygaard 2008; Supakatisant 2015), at the end of the treatment period (Dahle 1995; Hammar 1981; Hammar 1987; Khoranrodi 2011), or in a time period after treatment has ceased (for example Sohrabvand 2006). Depending on how the treatment acts, these may show different effects.
The small number of included studies (eight), and small sample sizes of those studies (42 to 126 women, 576 in total), mean that the evidence is incomplete and not generalisable.
No trials considered non‐drug therapies, for example, muscle stretching, massage, relaxation, heat therapy, dorsiflexion of the foot compared with placebo, no treatment or other treatment.
Quality of the evidence
This review includes three well‐conducted and reported trials, with few known design limitations (Mansouri 2017; Nygaard 2008; Supakatisant 2015). The other included studies had design limitations. The descriptions of randomisation and allocation procedures were not optimal in four studies (Dahle 1995; Hammar 1981; Hammar 1987; Khoranrodi 2011). It is unclear whether this is due to omissions in the reporting of the studies, or limitations of study design. Correspondance with the author of Sohrabvand 2006 revealed that, although the published report did not give details, randomisation and allocation were well‐conducted. Two studies had the design limitations with blinding (Hammar 1981; Sohrabvand 2006). So women would have been aware of the intervention and this may have affected their perception or reporting of pain and side effects.
The level of evidence for oral magnesium versus placebo/no treatment was graded low (frequency of leg cramps during treatment, frequency of leg cramps after treatment: never, frequency: 50% reduction in number of leg cramps, intensity of pain during treatment: mean total scale points, intensity of pain: visual analogue scale) or very low (intensity of pain: 50% reduction in pain score) (Table 1). For oral calcium versus no treatment it was graded very low (frequency of leg cramps after treatment: never) (Table 2). No primary outcomes were reported for oral vitamin B versus no treatment, (Table 3). For oral vitamin C versus oral calcium, a grading of very low was made (frequency of leg cramps after treatment: never) (Table 4). All graded outcomes were downgraded for imprecision due to small sample size, and some also for wide confidence intervals. Several outcomes were downgraded due to design limitations in the studies. Outcomes reporting 50% reduction were downgraded for indirectness, as they used an arbitrary cut‐off for frequency and intensity of leg cramps.
The inconsistency in the measurement and reporting of frequency, intensity, and duration of pain, and the experience of side effects, meant that data could not be pooled in meta‐analyses, so comparisons between studies was not possible.
Potential biases in the review process
The assessment of risk of bias involves subjective judgements. This potential limitation is minimised by following the procedures in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with review authors independently assessing studies and resolving any disagreement through discussion, and if required involving a third assessor in the decision.
Several trial authors were contacted with requests for additional data, in the hope that unpublished results might yield comparable outcomes. Additional information on methodology and results was received from Mansouri 2017 and Sohrabvand 2006. No response has yet been received from Supakatisant 2015.
Agreements and disagreements with other studies or reviews
This review does not agree with a previous version of this review (Young 2002), which concluded that magnesium may have benefits for leg cramps in pregnancy, and calcium did not appear to have benefits, according to the limited evidence. The conclusions of this review may alter in the future with evidence from more studies.
Authors' conclusions
Implications for practice.
The evidence is currently unclear and inconsistent. Evidence from these studies was too limited in quantity and quality to provide clinical direction for the use of oral magnesium, oral calcium, oral vitaminD, oral calcium‐vitamin D, oral vitamin B or oral vitamin C for treating leg cramps in pregnancy. Adverse effects were also unclear in these studies, with side‐effect data only available for the comparison of oral magnesium with placebo or no treatment. There was no evidence available to include in this review on other interventions, including non‐drug therapies such as muscle stretching, massage, relaxation, heat therapy, or dorsiflexion.
It is difficult to provide accurate advice for pregnant women based on the evidence presented in this review. Current guidelines and other reviews often offer incomplete evidence, without comment on the quality of the evidence. It is not possible at present to identify, with confidence, safe and effective interventions for leg cramps in pregnancy.
Implications for research.
The development of a standardised set of core outcomes for measuring the frequency, intensity and duration of leg cramps are needed for this area to be investigated. Well‐conducted randomised controlled trials would then be able to evaluate interventions for treating leg cramps in pregnancy.
The safety of interventions should be assessed, by including analysis of adverse outcomes in the mother and baby in trial outcomes.
High‐quality randomised controlled trials of non‐drug therapies would also be a valuable addition to the field.
What's new
| Date | Event | Description |
|---|---|---|
| 25 September 2019 | New search has been performed | Two new randomised controlled trials have been added (Khoranrodi 2011; Mansouri 2017). |
| 25 September 2019 | New citation required but conclusions have not changed | Although two new trials were included, conclusions remain unchanged. |
History
Protocol first published: Issue 7, 2013 Review first published: Issue 8, 2015
Acknowledgements
We would like to thank Helen West for her contribution as an author on the previous version of this review (2015). We would like to thank Anna Cuthbert, Research Associate, Cochrane Pregnancy and Childbirth for her help with the 'Summary of findings' tables for the 2020 update.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
Each line was run separately
leg AND cramp AND pregnancy
leg AND cramps AND pregnancy
leg AND cramp AND pregnant
leg AND cramps AND pregnancy
ClinicalTrials.gov
Advanced search
pregnancy | Interventional Studies | Cramp
pregnant | Interventional Studies | Cramp
pregnancy | Interventional Studies | Leg Cramp, Nocturnal
pregnant | Interventional Studies | Leg Cramp, Nocturnal
Data and analyses
Comparison 1. Oral magnesium versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Frequency of leg cramps during treatment | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐1.32, 4.92] |
| 1.2 Frequency of leg cramps after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Daily | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.45, 3.21] |
| 1.2.2 Every other day | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.57] |
| 1.2.3 Twice a week | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.80] |
| 1.2.4 Once a week | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.62, 3.87] |
| 1.3 Frequency of leg cramps after treatment: never | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.66 [1.35, 23.68] |
| 1.4 Frequency: 50% reduction in number of leg cramps | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.09, 1.86] |
| 1.5 Intensity of pain during treatment: mean total score | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐3.10, 6.70] |
| 1.6 Intensity of pain: 50% reduction in pain score | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.99, 2.06] |
| 1.7 Intensity of pain: visual analogue scale | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐17.50 [‐34.68, ‐0.32] |
| 1.8 Duration: persisting symptoms after night‐time cramps | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Always | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 0.98] |
| 1.8.2 Sometimes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.19, 1.83] |
| 1.9 Composite outcome: symptoms of leg cramps (intensity and frequency) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 Partial improvement: decrease in intensity and frequency | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.61] |
| 1.9.2 Complete recovery: no leg cramps after treatment | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.68, 13.20] |
| 1.10 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Nausea | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.75, 4.51] |
| 1.10.2 Diarrhoea | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.00 [0.75, 47.76] |
| 1.10.3 Any side effect (including nausea, flatulence, diarrhoea and intestinal air) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.36, 2.52] |
Comparison 2. Oral calcium versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Frequency of leg cramps after treatment: never | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.59 [1.19, 62.07] |
| 2.2 Frequency of leg cramps after treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.72, ‐0.34] |
| 2.3 Composite outcome: symptoms of leg cramps (intensity and frequency) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Partial improvement: decrease in intensity and frequency | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.15] |
| 2.3.2 Complete recovery: no leg cramps after treatment | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.50 [1.38, 21.86] |
| 2.4 Severity of leg cramps after treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐6.06 [‐18.93, 6.81] |
2.4. Analysis.

Comparison 2: Oral calcium versus placebo/no treatment, Outcome 4: Severity of leg cramps after treatment
Comparison 3. Oral vitamin B versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Composite outcome: symptoms of leg cramps (intensity and frequency) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Partial improvement: decrease in intensity and frequency | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.73] |
| 3.1.2 Complete recovery: no leg cramps after treatment | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.50 [1.95, 28.81] |
Comparison 4. Oral calcium versus oral vitamin C.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Frequency of leg cramps after treatment: never | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.53, 3.38] |
Comparison 5. Oral vitamin D versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Frequency of leg cramps | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 2.06 [0.58, 3.54] |
| 5.1.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 1.53 [0.12, 2.94] |
| 5.2 Severity of leg cramps pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.59, ‐0.21] |
| 5.2.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.29, 0.09] |
| 5.3 Duration of leg cramps | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.95, 2.05] |
| 5.3.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 2.73 [0.27, 5.19] |
Comparison 6. Oral Calcium‐vitamin D versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Frequency of leg cramps | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.55, 0.95] |
| 6.1.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐1.30, 1.36] |
| 6.2 Severity of leg cramps pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.2.1 Three weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.39, ‐0.01] |
| 6.2.2 Six weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.17, 0.17] |
| 6.3 Duration of leg cramps | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.55, 0.95] |
| 6.3.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐1.30, 1.36] |
Comparison 7. Oral Calcium‐vitamin D versus vitamin D.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Frequency of leg cramps | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐2.84, 0.14] |
| 7.1.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.69, 0.49] |
| 7.2 Severity of leg cramps pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 At 3 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.01, 0.41] |
| 7.2.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.09, 0.29] |
| 7.3 Duration of leg cramps | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 At 3 weeks after treament | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐2.30, 0.60] |
| 7.3.2 At 6 weeks after treatment | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐5.21, ‐0.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dahle 1995.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial. | |
| Participants | Setting: 2 prenatal care units in Sweden.
Inclusion criteria: pregnant women complaining of leg cramps during pregnancy, at 22‐36 weeks' gestation. Exclusion criteria: with other pregnancy complications or intercurrent medical problems. Previous treatment had been given for leg cramps in the current pregnancy. |
|
| Interventions | Experimental intervention: oral magnesium 5 mmol (primarily magnesium lactate, magnesium citrate) chewable tablet. 1 tablet each morning, and 2 each evening, for 3 weeks (34 women). Comparison intervention: placebo (primarily sorbitol, fructose‐dextrose) chewable tablet, same treatment regimen as intervention (35 women). Four women were randomised but did not complete the study and were excluded from analyses. It is not clear to which group they belonged. |
|
| Outcomes | Leg cramps duration, frequency, diurnal variation, distress, and whether nocturnal cramps persisted the following day. Whether the condition improved, deteriorated, or remained unchanged and side effects. Serum calcium, serum magnesium, 24‐hour urine calcium, magnesium and creatinine. | |
| Notes | Dates of study: this information is not reported by the trial authors Funding sources: Linkoping University Faculty Grant and by ACO & Pharmacia Lakemedel Declarations of interest: this information is not reported by the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were randomly allocated to either magnesium or placebo.” However no description of the method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Quote: "A magnesium‐placebo tablet batch of 90 numbered bottles was prepared by AC0 Lakemedel (Stockholm)...permitting blinded statistical analysis at the end of the study." Sequentially‐numbered drug containers of identical appearance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study Design:.....in a prospective, double‐blind, randomized trial." Blinding of participants and key study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...permitting blinded statistical analysis at the end of the study." No blinding of outcome assessment, but the outcome measurement is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 women were excluded. Reasons are given, e.g. premature labour, but it is not clear which group they were from. The analysis was not by intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | Assessed only from published report, with insufficient information to permit judgement. |
| Other bias | Unclear risk | Groups appear to be similar at baseline. Insufficient information to assess whether another important risk of bias exists. |
Hammar 1981.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial (with an additional control group of pregnant women without cramps, not included in meta‐analysis). | |
| Participants | Setting: Sweden. no further information.
Inclusion criteria: pregnant women who had leg cramps occurring at least twice a week during the last fortnight. Exclusion criteria: not described. |
|
| Interventions | Experimental intervention: oral calcium preparation with calcium gluconate, calcium lactate and calcium carbonate corresponding to a calcium dose of 1 g twice daily for 2 weeks (21 women). Comparison intervention: no treatment (21 women). |
|
| Outcomes | Serum calcium concentrations. Frequency of cramps. | |
| Notes | Dates of study: this information is not reported by the trial authors Funding sources: this information is not reported by the trial authors Declarations of interest: this information is not reported by the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions “randomization”, but no information on method. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. The effect of this is likely to vary by outcome. A standardised questionnaire was used to assess persistence of leg cramps, which may have been influenced by lack of blinding. Serum calcium levels are unlikely to have been affected by lack of blinding, however these are not included in the review. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women included in analyses (although symptoms are described for 22 women in treatment group, when only 21 were randomised). |
| Selective reporting (reporting bias) | Unclear risk | The focus of the study is on serum calcium concentrations. Assessed from published report without access to protocol, so reporting bias difficult to assess. |
| Other bias | Unclear risk | Insufficient information to assess whether another important risk of bias exists. |
Hammar 1987.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, with 13 additional controls without leg cramps (not included in analysis). | |
| Participants | Setting: Sweden.
Inclusion criteria: pregnant women who had experienced leg cramps for more than 2 weeks. Exclusion criteria: not described |
|
| Interventions | Experimental intervention: oral calcium preparation containing calcium gluconate, calcium lactate and calcium carbonate corresponding to a calcium dose of 1 g twice daily for 3 weeks (30 women). Comparison intervention: vitamin C 1 g twice daily for 3 weeks (30 women). |
|
| Outcomes | Frequency of cramps, serum calcium, magnesium and albumin concentrations. | |
| Notes | Dates of study: this information is not reported by the trial authors Funding sources: ACO Research Fund Declarations of interest: this information is not reported by the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that it was “randomised”, but there is no description of the method. |
| Allocation concealment (selection bias) | Low risk | Not specifically described, but the code was not broken until all women had completed the investigation, which suggests that allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blinded, and the code was not broken until all women had completed the investigation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code was not broken until all women had completed the investigation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessed from published report, without protocol. Focus is on biochemical outcomes, but all prespecified outcomes appear to be reported. |
| Other bias | Unclear risk | Insufficient information to assess whether another important risk of bias exists. |
Khoranrodi 2011.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial. | |
| Participants | Setting: health centre ‐ Ali Ebn Abi Taleb Inciusion criteria: third trimester of pregnancy, single pregnancy, no consumption of any drug excepts for iron and multivitamins Exclusion criteria: irregular use of medication, PLP, unwilling to complete forms |
|
| Interventions | Intervention group: 500 mg calcium, daily for 4 weeks Control group: 1 placebo capsule (lactose Merk) daily for 4 weeks |
|
| Outcomes | Frequency and severity of leg cramp | |
| Notes | Clinical Trials.gov ID IRCT201009274824N1 Dates of study: 2011‐02‐05 Funding sources: Research Managment Affairs, Bushehr University Of Medical Sciences Declarations of interest: there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that it was “randomised”, but there is no description of the method. |
| Allocation concealment (selection bias) | Unclear risk | Not specifically described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both healthcare providers and women were masked to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women self‐reported outcomes. The treatment assignment was not revealed until data collection was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women were excluded. Reasons are given |
| Selective reporting (reporting bias) | Low risk | Assessed from study protocol and published report, all prespecified outcomes reported. |
| Other bias | Unclear risk | Insufficient information available to assess whether another important risk of bias exists. |
Mansouri 2017.
| Study characteristics | ||
| Methods | 3‐arm randomised controlled trial. | |
| Participants | Setting: Tabriz‐Iran Inciusion criteria: pregnant women,14–34 weeks of gestation, between 18 and 35 years of age who suffered from leg cramps at least twice a week. Exclusion criteria: history of chronic diseases, kidney problems, osteomalacia, active thyroid or parathyroid diseases, endocrine disorders, hypertension, use of diuretics, calcium‐blockers intake, chronic hypertension, and history of allergy to the study supplements. |
|
| Interventions | Experimental intervention: vitamin D (1000 units) (42 women) or vitamin D plus calcium complements (42 women) (300 mg of calcium carbonate and 1000 units of vitamin D) Comparison intervention: placebo (42 women) |
|
| Outcomes | The frequency, length, and pain intensity of leg cramps | |
| Notes | Dates of study: July 2013 to April 2014 Funding sources: nil Declarations of interest: there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study used a randomised block design with block sizes of 3 and 6 with the allocation ratio 1:1:1. |
| Allocation concealment (selection bias) | Low risk | Each participant received 2 small envelopes, each with enough medicine for 3 weeks, inside a large matte‐coloured envelope of the same shape that were serially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was conducted as a double‐blind. Participants did not know which group they were in, but no information on blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women self‐reported outcomes. The treatment assignment was not revealed until data collection was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 women were excluded. Reasons are given and it is clear which group they were from. The analysis was not by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Assessed from study protocol and published report, all prespecified outcomes reported. |
| Other bias | Unclear risk | Insufficient information available to assess whether another important risk of bias exists. |
Nygaard 2008.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial. | |
| Participants | Setting: outpatients, Norway. Inclusion criteria: healthy pregnant women between 18 and 36 weeks of pregnancy suffering painful leg cramps, at least twice a week. Norwegian as first language. Exclusion criteria: women with restless legs symptoms. Women with pregnancy complications or other medical diseases. Twin pregnancy, oedema, pre‐eclampsia, magnesium supplements beyond the trial treatment. |
|
| Interventions | Experimental intervention: 120 mg (5 mmol) oral magnesium citrate, magnesium lactate chewable tables. 1 tablet in the morning and 2 tablets in the evening, for 2 weeks. 23 women were randomised, 2 subsequently dropped out. Control: chewable placebo tablets. 1 tablet in the morning and 2 tablets in the evening, for 2 weeks. 22 women were randomised, 5 dropped out. |
|
| Outcomes | Serum magnesium and calcium, urine magnesium and magnesium‐creatinine, leg cramp frequency and intensity. Side effects (nausea, flatulence, diarrhoea, intestinal air). | |
| Notes | Clinical Trials.gov ID NCT00525317. Dates of study: from August 2000 to January 2003 Funding sources: nil Declarations of interest: Thomas Bøhmer received a compensation from Nycomed for effort. The rest of the authors had no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation program was provided by Medstat Research AS.” No further information on method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered drug containers of identical appearance. Code was only broken after completion. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Code was only broken after completion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Code was broken for statistical analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 women dropped out of the trial (2 treatment group, 5 placebo), and an intention‐to‐treat analysis was carried out. The risk of bias is low for clinical outcomes. Laboratory specimens were lost, leaving results for 64% of women for some biochemical outcomes, however these data are not included in the review. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available (Clinical Trials.gov ID NCT00525317) and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Conflict of interest declared: 1 author contributed to developing the magnesium tablet used and received payment from the pharmaceutical company. |
Sohrabvand 2006.
| Study characteristics | ||
| Methods | 4‐arm randomised controlled trial. | |
| Participants | Setting: Iran. Inclusion criteria: pregnant women. From 401 pregnant women, 217 (54.5%) had leg cramps with different intensity and frequency and amongst them 84 who had an acceptable nutrition and no associated medical problem and agreed to enter the trial were recruited and randomly assigned to the 4 groups. Exclusion criteria: not described. |
|
| Interventions | Group 1: oral 500 mg calcium carbonate tablets (Tehran Chimie, Iran) once daily for 2 weeks. 21 women. Group 2: oral 7.5 mmol magnesium aspartate (Magnesiocard; Verla, Germany) twice daily for 2 weeks. 21 women. Group 3: oral 100 mg of thiamine (vitamin B1) plus 40 mg of pyridoxine (vitamin B6) (Tehran Chimie, Iran) once daily for 2 weeks. 21 women. Group 4: no treatment. 21 women. |
|
| Outcomes | Assessed after 4 weeks. A decrease in the intensity and frequency of muscle cramps was considered a relative improvement and a complete absence of muscle cramps was considered an absolute improvement. | |
| Notes | Helen West contacted the authors to check that participants had leg cramps at the point of randomisation. They confirmed that they did, and provided additional information on the methodology and results. Dates of study: this information is not reported by the trial authors Funding sources: this information is not reported by the trial authors Declarations of interest: this information is not reported by the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used. |
| Allocation concealment (selection bias) | Low risk | A series of envelopes numbered from 1 to 84 had been prepared. Each patient was invited to pull out an envelope and was placed by the clinic secretary in 1 of the 4 groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The drugs were given in similar boxes to the participants, but since the timing and size of the tablets was different complete blinding was not possible. The healthcare providers and statistician were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The healthcare providers and statistician were blinded. Women self‐reported on their symptoms, and may have been aware of their group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data appear to be presented for all women recruited. |
| Selective reporting (reporting bias) | Unclear risk | No information provided on whether outcomes were prespecified. |
| Other bias | Unclear risk | Insufficient information available to assess whether another important risk of bias exists. |
Supakatisant 2015.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial. | |
| Participants | Setting: antenatal care clinic at the Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Inclusion criteria: pregnant women with leg cramps (defined as: sudden tonic or clonic involuntary contraction of the gastrocnemius muscle associated with severe pain). 14–34 weeks of gestation, having pregnancy‐induced leg cramps at least twice a week. Exclusion criteria: other medical disease, concurrent obstetrics complication, other prescriptions for leg cramps, history of magnesium allergy, pregnant women with multifetal gestation, subsequently developed pregnancy‐induced hypertension and preterm labour treated with tocolytic agent. |
|
| Interventions | Experimental intervention: oral magnesium bisglycinate chelate (100 mg magnesium), 1 tablet, 3 times a day with meals, for 4 weeks. 43 women randomised (data for 41). Control: placebo, 1 tablet, 3 times a day with meals, for 4 weeks. 43 women randomised (data for 39). |
|
| Outcomes | 50% reduction of number of leg cramps, 50% reduction of pain score of leg cramps. Side effects. | |
| Notes | ISRCTN0389660. HW contacted the authors on 23/3/15 to request additional data on frequency and intensity of leg cramps after treatment, and side effects ‐ no response received. Dates of study: between June 2010 and August 2011 Funding sources: Grant for Development of New Faculty Staff, Chulalongkorn University. Declarations of interest: the authors declare that they have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table using a block‐of‐4 technique, generated by co‐investigator who did not have patient contact. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque plastic containers of identical size, shape and colour tablets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both healthcare providers and women were masked to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women self‐reported outcomes. The treatment assignment was not revealed until data collection was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three women left the study because of personal reasons and other 3 women were lost to follow‐up. 86 women were included in the intention‐to‐treat analysis by a ‘worst‐case’ scenario. |
| Selective reporting (reporting bias) | Low risk | Assessed from study protocol and published report, all prespecified outcomes reported. |
| Other bias | Unclear risk | Baseline characteristics appear similar between groups, although possibly placebo group had less frequent but more severe leg cramps. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Griffith 1998 | The method of randomisation was unclear. Not an intervention for leg cramps, the participants included pregnant women without leg cramps. |
| Kohama 2006 | This is not a randomised controlled trial, and included women with other types of pain (including lower back pain, hip joint pain and pelvic pain) in addition to leg cramps. |
| Mauss 1970 | Cross‐over study, not a randomised controlled trial. |
| Mukherjee 1997 | Quasi‐randomised controlled trial (alternate allocation). |
| Odendaal 1974 | Some women received more than 1 course of treatment, not necessarily the same treatment. |
| Robinson 1947 | Quasi‐randomised controlled trial (alternate allocation). |
| Rougin 2012 | The participants were not pregnant women. |
| Shahraki 2006 | Quasi‐randomised controlled trial (alternate allocation Quote: "The total number of samples was 120 persons, whose divided into 3 groups and each group was included 40 persons which were divided randomly and turn of coming" p980). |
| Thauvin 1992 | Not an intervention for leg cramps. The participants included pregnant women without leg cramps. |
Characteristics of studies awaiting classification [ordered by study ID]
Mirghafourvand 2015.
| Methods | 3‐arm randomised controlled trial. |
| Participants | Describe setting: Tabriz‐Iran Inciusion criteria: pregnant women,14–34 weeks of gestation, between 18 and 35 years of age who suffered from leg cramps at least twice a week. Exclusion criteria: history of chronic diseases, kidney problems, osteomalacia, active thyroid or parathyroid diseases, endocrine disorders, hypertension, use of diuretics, calcium‐blockers intake, chronic hypertension, and history of allergy to the study supplements. |
| Interventions | Experimental intervention: vitamin D (1000 units) (42 women) or vitamin D plus calcium complements (42 women) (300 mg of calcium carbonate and 1000 units of vitamin D) Comparison intervention: placebo (42 women) |
| Outcomes | Sleep quality in pregnant women with leg cramps |
| Notes | Dates of study: July 2013 to April 2014 Funding sources: nil Declarations of interest: there are no conflicts of interest. |
Differences between protocol and review
We added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
The secondary outcome "Composite outcome: symptoms of leg cramps, including two or more of: frequency, pain intensity or duration of leg cramps" was not prespecified, and has been added.
The outcomes were changed from specifying the measure to be used, to giving that measure as an example, so that other measures could be accommodated in the review,
"Frequency of leg cramps. Measured as the number of leg cramps per week" was changed to "Frequency of leg cramps. For example, measured as the number of leg cramps per week".
"Intensity of leg cramps. Level of pain intensity measured by validated instruments" was changed to "Intensity of leg cramps. For example, level of pain intensity measured by validated instruments".
"Duration of leg cramps. For example, measured by seconds per leg cramp" was changed to "Duration of leg cramps. For example, measured by seconds per leg cramp".
Comparison of treatments with "other treatment" in addition to no treatment and placebo has been added.
Clarification that studies of prevention of leg cramps in pregnancy have been excluded has been added to "Types of studies", and the word "treatment" has been added to the "Objectives".
Methods for use of GRADE and producing 'Summary of findings' tables have been added to the review.
The 'Summary of findings' table is restricted to seven lines for each comparison. The outcomes in this review were measured in a variety of ways. A selection from the prespecified outcomes therefore had to be made to fit this requirement. Frequency of leg cramps and intensity of leg cramps are presented. Duration, composite symptoms, and side effects are not included.
Planned subgroups were not listed correctly in the protocol. They have therefore been removed, and a subgroup for future versions of this review has been added.
Helen West has been added as an author since the protocol was published.
Contributions of authors
Li Luo and Kunyan Zhou assessed studies for inclusion, assessed risk of bias and extracted data. Li Luo conducted the analysis and drafted the update. Kunyan Zhou and Liangzhi Xu gave proposals for the update. Jing Zhang and Weiyao Yin amended the review.
Sources of support
Internal sources
(HW) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK
External sources
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland
2015 update
Declarations of interest
Kunyan Zhou: none known.
Li Luo: none known.
Jing Zhang: none known.
Liangzhi Xu: none known.
Wenjuan Li: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dahle 1995 {published data only}
- Dahle LO, Berg G, Hammar M, Hurtig M, Larsson L. The effect of oral magnesium substitution on pregnancy-induced leg cramps. American Journal of Obstetrics and Gynecology 1995;173:175-80. [DOI] [PubMed] [Google Scholar]
Hammar 1981 {published data only}
- Hammar M, Larsson L, Tegler L. Calcium treatment of leg cramps in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1981;60:345-7. [DOI] [PubMed] [Google Scholar]
Hammar 1987 {published data only}
- Hammar M, Berg G, Solheim F, Larsson L. Calcium and magnesium status in pregnant women. A comparison between treatment with calcium and vitamin C in pregnant women with leg cramps. International Journal of Vitamin and Nutrition Research 1987;57:179-83. [PubMed] [Google Scholar]
Khoranrodi 2011 {published data only}
- Khoranrodi R, IRCT201009274824N1. Comparative study of calcium efficacy and placebo on leg muscle cramps during pregnancy in patients referred to clinics supervised by Bushehr University of Medical Sciences. https://en.irct.ir/trial/5172 (first received 5 February 2011).
Mansouri 2017 {published data only}
- Mansouri A, Mirghafourvand M, Charandabi SM, Najafi M. The effect of vitamin d and calcium plus vitamin d on leg cramps in pregnant women: a randomized controlled trial. Journal of Research in Medical Sciences 2017;22(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghafourvand M. The effect of vitamin D and calcium plus vitamin D for leg cramps in pregnant women: a randomised controlled trial. https://en.irct.ir/trial/10789 (first received 4 May 2013). [DOI] [PMC free article] [PubMed]
Nygaard 2008 {published data only}
- Bohmer T, NCT00525317. Does oral magnesium substitution relieve magnesium induced leg cramps ? https://clinicaltrials.gov/ct2/show/NCT00525317 (first received 5 September 2007).
- Nygaard IH, Valbo A, Pethick SV, Bohmer T. Does oral magnesium substitution relieve pregnancy-induced leg cramps? European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;141(1):23-6. [DOI] [PubMed] [Google Scholar]
Sohrabvand 2006 {published data only}
- Sohrabvand F, Shariat M, Haghollahi F. Vitamin B supplementation for leg cramps during pregnancy. International Journal of Gynecology & Obstetrics 2006;95(1):48-9. [DOI] [PubMed] [Google Scholar]
Supakatisant 2015 {published data only}ISRCTN03989660
- Phupong V. A randomized, double-blinded, placebo-controlled trial of oral magnesium for relief in pregnancy-induced leg cramps. Current Controlled Trials (http://www.controlled-trials.com/ISRCTN03989660) 2011 [accessed 31 January 2013].
- Supakatisant C, Phupong V. Oral magnesium for relief in pregnancy-induced leg cramps: a randomised controlled trial. Maternal & Child Nutrition 2015;11(2):139-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Griffith 1998 {published data only}
- Griffith EC, Crowther CA, Hiller JE, Wilson KJ, ACT Study Group. Leg cramps in pregnancy: ineffectiveness of calcium supplementation. In: 2nd Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30-April 4; Alice Springs, Australia. 1998:99.
Kohama 2006 {published data only}
- Kohama T, Inoue M. Pycnogenol alleviates pain associated with pregnancy. Phytotherapy Research 2006;20(3):232-4. [DOI] [PubMed] [Google Scholar]
Mauss 1970 {published data only}
- Mauss HJ. Muscular cramp in the calf caused by pregnancy. Therapy in a blind study [Schwangerschaftsbedingte Wadenkrampfe. Therapie im Blindversuch.]. Medizinische Welt 1970;36:1570-1. [PubMed] [Google Scholar]
Mukherjee 1997 {published data only}
- Mukherjee J, Jong A, Wu MY, Tsim YL. Leg cramps in pregnancy and calcium supplementation. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):89. [Google Scholar]
Odendaal 1974 {published data only}
- Odendaal HJ. Calcium for the treatment of leg cramps during pregnancy [Kalsium vir die Behandeling van Beenkrampe tydens Swangerskap]. South African Medical Journal 1974;48:780-1. [PubMed] [Google Scholar]
Robinson 1947 {published data only}
- Robinson M. Cramps in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1947;54:826-9. [DOI] [PubMed] [Google Scholar]
Rougin 2012 {published data only}
- Rougin M. Magnesium oxide monohydrate for nocturnal leg cramps (MgNLC); a prospective, randomized, double blind, placebo controlled clinical trial. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 31 January 2014] 2012.
Shahraki 2006 {published data only}
- Shahraki AD. Effects of vitamin E, calcium carbonate and milk of magnesium on muscular cramps in pregnant women. Journal of Medical Sciences 2006;6(6):979-83. [Google Scholar]
Thauvin 1992 {published data only}
- Thauvin E, Fusselier M, Arnaud J, Faure H, Favier M, Coudray C, et al. Effects of multivitamin mineral supplement on zinc and copper status during pregnancy. Biological Trace Element Research 1992;32:405-14. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mirghafourvand 2015 {published data only}
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Mansouri A, Najafi M, Khodabande F. The effect of vitamin D and calcium plus vitamin D on sleep quality in pregnant women with leg cramps: a controlled randomized clinical trial. Journal of Isfahan Medical School 2015;32(320):2444-53. [Google Scholar]
Additional references
ADRAC 2002
- Adverse Drug Reactions Advisory Committee. Quinine and profound thrombocytopenia. Australian Adverse Drug Reactions Bulletin 2002;21(3):10. [Google Scholar]
Allen 2003
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine 2003;4(2):101-19. [DOI] [PubMed] [Google Scholar]
Allen 2012
- Allen RE, Kirby KA. Nocturnal leg cramps. American Family Physician 2012;86(4):350-5. [PubMed] [Google Scholar]
Baldinger 2012
- Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD004157. [DOI: 10.1002/14651858.CD004157.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blyton 2012
- Blyton F, Chuter V, Walter KE, Burns J. Non-drug therapies for lower limb muscle cramps. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD008496. [DOI: 10.1002/14651858.CD008496.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dayan 2002
- Dayan J, Creveuil C, Herlicoviez M, Herbel C, Baranger E, Savoye C, et al. Role of anxiety and depression in the onset of spontaneous preterm labor. American Journal of Epidemiology 2002;155:293-301. [DOI] [PubMed] [Google Scholar]
El‐Tawil 2015
- El‐Tawil S, Al Musa T, Valli H, Lunn MPT, Brassington R, El‐Tawil T, Weber M. Quinine for muscle cramps. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD005044. [DOI: 10.1002/14651858.CD005044.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2006
- Food, Drug Administration. Quinine: important warning. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108799.htm [accessed 20 July 2008] 2006 11 Dec.
Frusso 1999
- Frusso R, Zárate M, Augustovski F, Rubinstein A. Magnesium for the treatment of nocturnal leg cramps: a crossover randomized trial. Journal of Family Practice 1999;48(11):868-71. [PubMed] [Google Scholar]
Garrison 2020
- Garrison SR, Korownyk CS, Kolber MR, Allan GM, Musini VM, Sekhon RK, Dugré N. Magnesium for skeletal muscle cramps. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD009402. [DOI: 10.1002/14651858.CD009402.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 2001
- Goodman L, Gilman A. The Pharmacological Basis of Therapeutics. 10th edition. New York: McGraw-Hill, 2001. [Google Scholar]
Harvey 1939
- Harvey A. The mechanism of action of quinine in myotonia and myasthenia. JAMA 1939;112:1562-3. [Google Scholar]
Hensley 2009
- Hensley JG. Leg cramps and restless legs syndrome during pregnancy. Journal of Midwifery and Women's Health 2009;54(3):211-8. [DOI] [PubMed] [Google Scholar]
Hertz 1992
Hickey 1995
- Hickey CA, Cliver SP, Goldenberg RL, McNeal SF, Hoffman HJ. Relationship of psychosocial status to low prenatal weight gain among nonobese black and white women delivering at term. Obstetrics and Gynecology 1995;86:177-83. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Kanaan 2001
- Kanaan N, Sawaya R. Nocturnal leg cramps. Clinically mysterious and painful--but manageable. Geriatrics 2001;56(6):39-42. [PubMed] [Google Scholar]
Katzberg 2010
- Katzberg HD, Khan AH, So YT. Assessment: symptomatic treatment for muscle cramps (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American Academy of neurology. Neurology 2010;74(8):691-6. [DOI] [PubMed] [Google Scholar]
Langford 2003
- Langford NJ, Good AM, Laing WJ, Bateman DN. Quinine intoxications reported to the Scottish Poisons Information Bureau 1997-2002: a continuing problem. British Journal of Clinical Pharmacology 2003;56(5):576-8. [DOI] [PMC free article] [PubMed]
Leal de Araújo 2020
- Leal de Araújo CA, Lorena SB, Sousa Cavalcanti GC, Souza Leão GL, Tenόrio GP, B Alves JG. Oral magnesium supplementation for leg cramps in pregnancy-An observational controlled trial. POLoS One 2020 Jan 10;15(1):e0227497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2004
- Lee KA, Gay CL. Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics and Gynecology 2004;191(6):2041-6. [DOI] [PubMed] [Google Scholar]
Man‐Son‐Hing 1998
- Man-Son-Hing M, Wells G, Lau A. Quinine for nocturnal leg cramps a meta-analysis including unpublished data. Journal of General Internal Medicine 1998;13(9):600-6. [DOI] [PMC free article] [PubMed]
Marcus 2003
- Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women's Health 2003;12:373-80. [DOI] [PubMed] [Google Scholar]
McGee 1990
- McGee SR. Muscle cramps. Archives of Internal Medicine 1990;150:511-8. [PubMed] [Google Scholar]
Medsafe 2007
- Medsafe Pharmacovigilance Team, New Zealand Medicines and Medical Devices Safety Authority. Quinine - not for leg cramps anymore. http://www.medsafe.govt.nz/profs/PUArticles/watchingbriefsNov07.htm Prescriber Update 2007;28(1):2-6.
Merlino 2012
- Merlino G, Gigli GL. Sleep-related movement disorders. Neurological Sciences 2012;33(3):491-513. [DOI] [PubMed] [Google Scholar]
Miller 2005
- Milller TM, Layzer RB. Muscle cramps. Muscle and Nerve 2005;32(4):431-42. [DOI] [PubMed] [Google Scholar]
Mindell 2000
- Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2000;29(6):590-7. [DOI] [PubMed] [Google Scholar]
Minetto 2013
- Minetto MA, Holobar A, Botter A, Farina D. Origin and development of muscle cramps. Exercise and Sport Sciences Reviews 2013;41(1):3-10. [DOI] [PubMed] [Google Scholar]
Nishimura 1976
- Nishimura H, Tanimura T. Clinical Aspects of the Teratogenicity of Drugs. Excerpta Medica Amsterdam, 1976. [Google Scholar]
Page 1953
- Page EW, Page EP. Leg cramps in pregnancy; etiology and treatment. Obstetrics and Gynecology 1953;1:94-100. [PubMed] [Google Scholar]
Parisi 2003
- Parisi L, Pierelli F, Amabile G, Valente G, Calandriello E, Fattapposta F, et al. Muscular cramps: proposals for a new classification. Acta Neurologica Scandinavica 2003;107:176-86. [DOI] [PubMed] [Google Scholar]
Pedersen 1985
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rondo 2003
- Rondo PH, Ferreira RF, Nogueira F, Ribeiro MC, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. European Journal of Clinical Nutrition 2003;57:266-72. [DOI] [PubMed] [Google Scholar]
Serrao 2000
- Serrao M, Rossi P, Cardinali P, Valente G, Parisi L, Pierelli F. Gabapentin treatment for muscle cramps: an open-label trial. Clinical Neuropharmacology 2000;23(1):45-9. [DOI] [PubMed] [Google Scholar]
Shaker 2005
- Shaker HK, Mackler L, Huber TE. What is the diagnostic approach to a patient with leg cramps? Journal of Family Practice 2005;54:817-8. [PubMed] [Google Scholar]
Soares 2006
- Soares CN, Murray BJ. Sleep disorders in women: clinical evidence and treatment strategies. Psychiatric Clinics of North America 2006;29(4):1095-113. [DOI] [PubMed] [Google Scholar]
Sohrabvand 2009
- Sohrabvand F, Karimi M. Frequency and predisposing factors of leg cramps in pregnancy: a prospective clinical trial. Tehran University Medical Journal 2009;67(9):661-4. [Google Scholar]
Wacker 1968
- Wacker WE, Parisi AF. Magnesium metabolism. New England Journal of Medicine 1968;278(13):712-7. [DOI] [PubMed] [Google Scholar]
Young 2009
- Young G. Leg cramps. Clinical Evidence 2009;03:1113. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Young 2002
- Young G, Jewell D. Interventions for leg cramps in pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD000121. [DOI: 10.1002/14651858.CD000121] [DOI] [PubMed] [Google Scholar]
Zhou 2013
- Zhou K, Xu L, Li W, Zhang J. Interventions for leg cramps in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD010655. [DOI: 10.1002/14651858.CD010655] [DOI] [PubMed] [Google Scholar]
Zhou 2015
- Zhou K, West HM, Zhang J, Xu L, Li W. Interventions for leg cramps in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD010655. [DOI: 10.1002/14651858.CD010655.pub2] [DOI] [PubMed] [Google Scholar]


