Abstract
Background
This is a second update of a Cochrane Review originally published in Issue 2, 2009. Transcutaneous Electrical Nerve Stimulation (TENS) is a non‐pharmacological agent, based on delivering low voltage electrical currents to the skin. TENS is used by people to treat a variety of pain conditions.
Objectives
To assess the analgesic effectiveness of TENS, as a sole treatment, for acute pain in adults.
Search methods
We searched the following databases up to 3 December 2014: the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE; EMBASE; CINAHL; and AMED. We also checked the reference lists of included trials.
Selection criteria
We included randomised controlled trials (RCTs) of adults with acute pain (< 12 weeks) if they examined TENS given as a sole treatment and assessed pain with subjective pain scales. Trials were eligible if they compared TENS to placebo TENS, no treatment controls, pharmacological interventions or non‐pharmacological interventions. We excluded trials on experimental pain, case reports, clinical observations, letters, abstracts or reviews. Also we excluded trials investigating the effect of TENS on pain during childbirth (labour), primary dysmenorrhoea or dental procedures. Studies where TENS was given with another treatment as part of the formal trial design were excluded. We did not restrict any articles based on language of publication.
Data collection and analysis
Two review authors independently assessed study eligibility and carried out study selection, data extraction, 'Risk of bias' assessment and analyses of data. We extracted data on the following: types of participants and pain condition, trial design and methods, treatment parameters, adverse effects, and outcome measures. We contacted trial authors for additional information if necessary.
Main results
We included 12 trials in the original review (2009) and included no further trials in the first update (2011). An additional seven new trials met the inclusion criteria in this second update. In total, we included 19 RCTs involving 1346 participants at entry, with 11 trials awaiting classification either because the full text was unavailable or information in the full text failed to clarify eligibility. We excluded most trials because TENS was given in combination with another treatment as part of the formal study design or TENS was not delivered using appropriate TENS technique. The types of acute pain included in this Cochrane Review were procedural pain, e.g. cervical laser treatment, venepuncture, screening flexible sigmoidoscopy and non‐procedural pain, e.g. postpartum uterine contractions and rib fractures. We pooled data for pain intensity for six trials (seven comparisons) comparing TENS with placebo but the I2 statistic suggested substantial heterogeneity. Mean difference (MD) with 95% confidence intervals (CIs) on a visual analogue scale (VAS, 100 mm) was ‐24.62 mm (95% CI ‐31.79 to ‐17.46) in favour of TENS. Data for the proportion of participants achieving ≥ 50% reduction in pain was pooled for four trials (seven comparisons) and relative risk was 3.91 (95% CI 2.42 to 6.32) in favour of TENS over placebo. We pooled data for pain intensity from five trials (seven comparisons) but the I2 statistic suggested considerable heterogeneity. MD was ‐19.05 mm (95% CI ‐27.30 to ‐10.79) in favour of TENS using a random‐effects model. It was not possible to pool other data. There was a high risk of bias associated with inadequate sample sizes in treatment arms and unsuccessful blinding of treatment interventions. Seven trials reported minor adverse effects, such as mild erythema and itching underneath the electrodes and participants disliking TENS sensation.
Authors' conclusions
This Cochrane Review update includes seven new trials, in addition to the 12 trials reviewed in the first update in 2011. The analysis provides tentative evidence that TENS reduces pain intensity over and above that seen with placebo (no current) TENS when administered as a stand‐alone treatment for acute pain in adults. The high risk of bias associated with inadequate sample sizes in treatment arms and unsuccessful blinding of treatment interventions makes definitive conclusions impossible. There was incomplete reporting of treatment in many reports making replication of trials impossible.
Plain language summary
Transcutaneous Electrical Nerve Stimulation (TENS) to treat acute pain in adults
Background
Acute pain is pain of recent onset and limited duration. Acute pain is associated with surgery, physical trauma (e.g. broken bones, burns and cuts) and medical procedures (e.g. venepuncture and sigmoidoscopy). Transcutaneous Electrical Nerve Stimulation (TENS) is a treatment to relieve pain by administering mild electrical currents to the body using electrode pads attached to the surface of the skin.
Review question
Does TENS relieve acute pain in adults?
Study characteristics
We included 19 clinical trials published up to 3 December 2014, which examined 1346 people. The trials administered TENS to produce a strong non painful 'tingling' sensation at the site of acute pain. The trials assessed TENS for cervical laser treatment, venepuncture, sigmoidoscopy, rib fractures and uterine contractions after childbirth. We did not include trials that assessed TENS for pain associated with childbirth, dental procedures and menstruation because they have been the subject of other Cochrane Reviews. Eleven trials are awaiting classification.
Key results
TENS was better than placebo TENS (delivering no electrical current) at reducing the intensity of acute pain but the reduction in pain was not consistent across all trials. This finding was based on an analysis of only six of the 19 trials. There was an insufficient number patients to make a firm conclusion.
A small number of patients experienced itching and redness beneath the TENS pads or disliked the sensation produced by TENS.
Overall we concluded that TENS may reduce the intensity of acute pain in some patients but the quality of evidence was weak. TENS is inexpensive, safe and can be self‐administered. We recommended that TENS should be considered as a treatment option given on its own or in combination with other treatments.
Quality of the evidence
The quality of the evidence was moderate to low because sample sizes were small and some patients were aware that they were receiving TENS or placebo.
Background
This Cochrane Review is a second update of Walsh 2009, and replaces the 2011 update.
Description of the condition
The International Association for the Study of Pain defines pain as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage" (Merskey 1994). Acute pain is defined as pain "of recent onset and probable limited duration which usually has an identifiable temporal and causal relationship to the injury or disease". In clinical practice acute pain is categorised as pain of less than three months duration (Strong 2002). Current approaches to acute pain management include pharmacological agents (drugs) and a number of non‐pharmacological agents, one of which is Transcutaneous Electrical Nerve Stimulation (TENS) (Schug 2014).
Description of the intervention
TENS is the delivery of pulsed electrical currents across the intact surface of the skin to stimulate peripheral nerves principally for pain relief (Johnson 2014). In clinical practice TENS is administered using a portable, battery‐powered device that generates electrical currents that are delivered to the body via electrodes attached to the intact surface of the skin. TENS is inexpensive and can be self‐administered. The safety profile of TENS compares positively compared with medication. Safety guidelines published by professional bodies guide judgements about whether it is appropriate to use TENS (Houghton 2010). Contradictions include TENS for patients who also have electronic implants, such as cardiac pacemakers and implantable cardioverter defibrillators. Precautions include pregnancy, epilepsy, active malignancy, deep‐vein thrombosis, and frail or damaged skin.
How the intervention might work
Natural forms of electricity (e.g. electrogenic fish) have been used as a method of pain relief since the Egyptian era (Johnson 2014). A theoretical foundation for electroanalgesia (pain relief by electrical methods) was established in 1965 through the publication of Melzack and Wall's gate control theory of pain (Melzack 1965). This theory proposed that a metaphorical gate consisting of excitatory and inhibitory synapses existed in the dorsal horn of the spinal cord. The gate could regulate the amount of nociceptive traffic (painful stimuli) being transmitted onwards to the brain. This gate could be opened by noxious stimuli that excited high threshold small diameter peripheral afferents and could be closed by non‐noxious stimuli (e.g. touch, pressure and electrical currents) that excited low threshold large diameter peripheral afferents.
Technological advances have produced a variety of TENS devices with a wide range of stimulation parameters for clinicians and patients to choose from (e.g. pulse frequency, pulse amplitude, pulse duration and electrode placement site). TENS interventions tend to be described according to technical characteristics as either high frequency, low intensity (conventional TENS) or low frequency, high intensity (acupuncture‐like TENS, AL‐TENS). This technical approach fails to specify the physiological intention of delivering TENS. In this regard, the physiological intention when administering conventional TENS is to activate selectively non‐noxious low threshold afferent nerve fibres in the skin (Aβ‐fibres) which are claimed to inhibit transmission of nociceptive information at the level of the spinal cord (i.e. segmental modulation) (DeSantana 2008). In practice, Aβ nerve fibre activity is recognised by the user reporting strong electrical paraesthesia (pins and needles) beneath the electrodes. The physiological intention of AL‐TENS is to generate a muscle twitch which is believed to increase activity in small diameter afferent nerve fibres in muscles (Aδ) leading to activation of descending pain inhibitory pathways. In practice, AL‐TENS is achieved by administering low frequency and high intensity, but non‐painful, currents over muscles (Francis 2011). Interestingly, experimental evidence to establish the roles of different afferent fibres in TENS outcome is inconclusive (Garrison 1994; Levin 1993; Radhakrishnan 2005). Research suggests that different frequencies of TENS may act through different neurotransmitter systems. Sluka and colleagues conducted a series of animal studies that have shown that low frequency TENS‐induced antihyperalgesia (decreased sensitivity to pain) is mediated by activation of serotonin and mu opioid receptors, while high frequency TENS activates delta opioid receptors (Kalra 2001; Radhakrishnan 2003; Sluka 1999). In 2008, a systematic review evaluating frequency dependent effects on experimentally induced pain in humans was inconclusive due to an insufficient number of high quality trials (Chen 2008). In recent years frequency‐dependent effects have been confirmed in human subjects by high quality research studies (Chen 2010a; Chen 2010b; Chen 2011; Claydon 2011; Leonard 2010; Léonard 2011; Liebano 2011).
Why it is important to do this review
TENS is used extensively by people with acute and chronic pain (DeSantana 2008; Johnson 2011). Meta‐analyses on the effectiveness of TENS for chronic musculoskeletal pain (Johnson 2007) and for osteoarthritis of the knee (Bjordal 2007) demonstrated a significant effect on pain over placebo. Cochrane Reviews on TENS for specific chronic pain conditions have been hindered by methodological weaknesses in randomised controlled trials (RCTs) (Bennett 2011; Johnson 2010; Johnson 2014; Sluka 2013). An all‐encompassing Cochrane Review on TENS for a variety of chronic pain conditions (i.e. pain > three months' duration) reported inconclusive results (Nnoaham 2008). However, this review has now been withdrawn and is being replaced by new reviews on TENS for neuropathic pain in adults, led by Gibson (protocol in press) and TENS for fibromyalgia, led by Claydon et al (protocol in press). There is also a title registered for an overview of Cochrane Reviews of TENS for chronic pain (protocol in press).
Cochrane Reviews on TENS for specific types of acute pain have been inconclusive for labour pain (Dowswell 2009) and dysmenorrhoea (Proctor 2002). An early systematic review of TENS for post‐operative pain found TENS to be no better than controls for postoperative pain (Carroll 1996) although pain measures were taken when patients were allowed free access to analgesic medication. This compromises pain scores because patients in placebo and TENS groups titrate analgesic medication to achieve effective pain relief, and therefore exhibit similar pain scores. Review authors also included trials that underdosed TENS or used an inappropriate TENS technique, or both. A meta‐analysis with subgroup analysis demonstrated a significantly better outcome for TENS when applied using adequate (optimal) stimulation techniques when compared to non‐adequate stimulation techniques (Bjordal 2003); optimal TENS techniques were defined as an intensity that was strong enough to generate a strong paraesthesia and electrodes applied at the site of the operative scar. Recent evidence from systematic reviews suggests that TENS is superior to placebo TENS when used in combination with analgesic medication for thoracotomy and post‐sternotomy pain (Freynet 2010; Sbruzzi 2012). To date, there has been no all‐encompassing systematic review on TENS for acute pain. A systematic review, which takes account of adequate TENS techniques, is necessary to assist clinicians and researchers to make informed decisions on the effectiveness of this modality for acute pain. TENS can be given either as a sole treatment, i.e. stand alone treatment, or combined with other interventions. This Cochrane Review will focus on TENS given as a sole treatment only to see if it has sufficient efficacy in its own right.
Objectives
Primary objective
To assess the analgesic effectiveness of TENS, as a sole treatment, for acute pain in adults.
Secondary objectives
To assess whether:
TENS effectiveness is influenced by the type of TENS (i.e. conventional TENS versus AL‐TENS);
TENS effectiveness is influenced by the time of recording the outcome measure, i.e. if outcome is influenced by measurements taken when TENS is switched on (during TENS measurement) compared to when TENS has been turned off after the treatment (post‐TENS measurement);
TENS effectiveness is influenced by duration of TENS treatment;
TENS effectiveness differs for different acute pain conditions; and,
TENS is safe for the treatment of acute pain.
Methods
Criteria for considering studies for this review
Types of studies
We included all prospective RCTs. Both cross‐over and parallel trial designs were acceptable. We excluded data from the following: trials that were non‐randomised; studies of experimental pain; case reports; clinical observations; and letters, abstracts and reviews (unless they provided additional information from published RCTs that met the criteria).
Types of participants
Study participants were required to be adults (i.e. 16 years and over) with a diagnosis of acute pain (less than 12 weeks) by any cause including injury or surgical intervention. Acute pain conditions included, but were not limited to, the following: angina; back pain; fractures; headache; musculoskeletal pain and procedural pain. We included postpartum pain trials if the pain investigated was due to episiotomy or Caesarean section irrespective of the presence of uterine cramps. We excluded trials including patients with pain due to uterine contractions (i.e. labour) alone and trials including patients with acute pain due to primary dysmenorrhoea as these conditions have been covered by previous Cochrane Reviews (Dowswell 2009; Proctor 2002). In addition, we excluded trials on electrical stimulation for dental procedures as this is a subject for a separate review.
Types of interventions
We only included trials which evaluated surface electrical nerve stimulation for the treatment of acute pain (i.e. transcutaneous as opposed to percutaneous electrical stimulation). We defined appropriate delivery of TENS as follows:
A 'standard TENS device' was used which delivered biphasic or monophasic (type of waveform) pulsed electrical currents in the mA range. TENS had to be delivered using at least two surface electrodes. We excluded TENS delivered using single probes (i.e. TENS pens). Neuromuscular electrical stimulation (NMES) devices and Interferential Current devices were excluded;
TENS was administered to produce a strong electrical paraesthesia that was felt by the patient. We included AL‐TENS delivered at strong intensities to generate muscle twitches. We excluded trials if the active TENS intervention was delivered at intensities reported to be 'barely perceptible', 'faint' or 'mild';
TENS was administered on an area of the body which was sensate (where pain is being felt) at either (a) the site of pain or (b) over nerve bundles proximal (or near) to the site of pain. We only included TENS delivered at acupuncture stimulation points if the point was lying over nerve bundles proximal (or near) to the site of pain. We considered any parameters of treatment meeting these criteria as were any duration or frequency of treatment and either self‐applied or therapist‐applied treatments.
The interventions to be compared included the following:
TENS versus placebo TENS (i.e. use of a sham TENS device). We defined a sham TENS device as a device similar to the one used in the active group but the output was modified in some way so that either no electrical current or a barely perceptible electrical current is delivered through the electrodes;
TENS versus no treatment controls;
TENS versus a pharmacological intervention;
TENS versus a non‐pharmacological intervention.
We excluded trials if TENS was given in combination with any other treatment as part of the formal trial design, e.g. analgesic medication, exercise.
Types of outcome measures
Primary outcomes
Standard subjective scales for pain intensity, pain relief or both (e.g. visual analogue scales (VAS), numerical rating scales (NRS); verbal rating scales (VRS) McGill Pain Questionnaire (MPQ)).
Secondary outcomes
Other measures of pain.
We recorded adverse events associated with the intervention. Also, we sought information on the level of compliance with the intervention, the magnitude and duration of effect.
Search methods for identification of studies
Electronic searches
We developed detailed search strategies for each electronic database searched. We based these on the search strategy developed for MEDLINE but revised each strategy appropriately for each database. The search strategy combined the subject specific search with phase one and two of the Cochrane Sensitive Search Strategy for RCTs (as published in chapter sections 6.4.11.1, 6.3.2.1 and 6.3.3.2 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The subject specific search used a combination of MeSH (upper case) and free text (lower case) terms based on the MEDLINE search strategy via OVID which can be seen in Appendix 1. We attempted to identify all relevant trials irrespective of language. We assessed non‐English papers and translated articles when necessary.
We performed the literature search for Walsh 2009 up to 8 August 2008 and subsequent searches up to 7 January 2011 for the 2011 review update. For this second search update we performed searches up to 3 December 2014. We searched the following databases:
Cochrane Pain, Palliative and Supportive Care Group (PaPaS) Specialized Register (4 August 2008; as data are captured in CENTRAL, we did not include this database in the 2011 or 2013 update search) Appendix 2;
Cochrane Central Register of Controlled Trials, CENTRAL (the Cochrane Library, Issue 11 of 12, 2014) Appendix 3;
MEDLINE (1950 to Nov week 3 2014) Appendix 1;
EMBASE (1980 to 2 Dec 2014) Appendix 4;
CINAHL (1982 to 6 Dec 2014) Appendix 5;
AMED (1985 to 6 November 2014) Appendix 6;
PEDro (www.pedro.org.au) accessed 7 January 2011. We excluded this database from the 2013 update search Appendix 7;
OTseeker (www.otseeker.com) accessed 7 January 2011. We excluded this database from the 2013 update search Appendix 8; and,
OpenSIGLE (http://opensigle.inist.fr) accessed 7 January 2011. We excluded this database from the 2013 update search Appendix 9.
Searching other resources
We searched the reference lists of all included trials, key textbooks and previous systematic reviews for additional trials.
Data collection and analysis
Selection of studies
From the title, abstract, and descriptors, pairs of review authors independently reviewed the results of the literature searches to identify potentially relevant trials for full review. We resolved any disagreements by consensus. We did not blind the review authors from authors' names, institutions, and journal name or trial results at this stage or any stage of the review. After screening full text articles, we included trials that met the inclusion criteria. We sought additional information or clarification from the primary trial author if incompletely reported.
Data extraction and management
Pairs of review authors independently extracted data using a customised data extraction tool tested prior to use. We resolved any disagreements by consensus or by consulting a third review author. We contacted trial authors where there was incomplete reporting of data. We extracted data on the following trial characteristics for entry into RevMan 2014:
Study participants: age, gender, condition, inclusion/exclusion criteria, number of participants randomised, number of, and reasons for, withdrawals or dropouts;
Study: design and location, methods of sequence generation and allocation concealment, blinding, intention‐to‐treat (ITT) or per protocol analysis, outcome measures for pain, and results of statistical analysis;
Interventions used: where TENS was applied and by whom, stimulation parameters (frequency, waveform, pulse amplitude/intensity, pulse duration), electrode details, treatment time and frequency, and adverse effects.
Assessment of risk of bias in included studies
We originally intended to assess the methodological quality of trials using the scale devised by Jadad 1996 as detailed in the protocol. However, with the launch of Review Manager (RevMan) in 2008, we decided to use the Cochrane Collaboration's 'Risk of bias' assessment tool as described in Chapter 8 of Higgins 2011. Two review authors independently assessed the following: sequence generation, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, and other sources of bias (funding and size of trial). We resolved any disagreement by consensus or by consulting a third review author.
Measures of treatment effect
Where available and appropriate, we presented quantitative data for the outcomes listed in the inclusion criteria. For each trial, we calculated relative risk and 95% confidence intervals (CI) for dichotomous outcomes. For continuous outcomes reported using the same scale, we determined mean differences (MD) and 95% CIs. Where results for continuous outcomes were presented on different scales, we calculated standardised mean differences (SMD) and 95% CIs. We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) for treatment effect.
Dealing with missing data
In cases of missing data due to withdrawals or dropouts, we only used the data analysed in the trial for analysis in this Cochrane Review.
Assessment of heterogeneity
We had intended that, where appropriate, we would pool results of comparable groups of trials using the fixed‐effect model and calculate 95% CIs. We planned to test heterogeneity between comparable trials using a standard Chi² test considered statistically significant at a P value < 0.1, after due consideration of the I2 statistic value. We interpreted the I2 statistic value according to the following thresholds (Higgins 2011): 0% to 40%, might not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; and 75% to 100%, considerable heterogeneity. We planned to investigate any evidence of heterogeneity to determine if there were obvious differences in the trials that were likely causes of the heterogeneity. If the heterogeneity was regarded as likely to have serious effects on the validity of the results, then we did not combine the data. Where there was significant heterogeneity, we intended to view the results of the random‐effects model and present these when appropriate.
Subgroup analysis and investigation of heterogeneity
Where the data allowed, we planned separate outcome analyses to test the following null hypotheses that there is no difference in analgesia:
Between AL‐TENS (visible phasic muscle contractions) and conventional TENS (no visible muscle contraction);
If the outcome measure is recorded during TENS application;
Between different TENS treatment durations; and,
Between different acute pain conditions
Results
Description of studies
Results of the search
For the 2011 update we identified 1775 reports in the literature searches. For this update, 1421 records were identified through database searching between 2011 and 2014. After removal of duplicates we screened the abstracts of 1065 reports (Figure 1). Of these 1065 reports, 1038 were removed because they were not relevant, did not meet the inclusion criteria, had administered TENS in combination with another treatment as part of the formal trial design (n=120) or had not administered TENS using appropriate technique as defined in the Types of interventions section (n=32). Hence this update included seven new trials (Amer‐Cuenca 2011; de Sousa 2014; Ekblom 1987; Gregorini 2010; Keskin 2012; Kim 2012; Pitangui 2012), including two of the trials that were awaiting classification in the 2011 update (Ekblom 1987; Gregorini 2010). In total there were 19 trials included for review (Characteristics of included studies) and all were published in English. Eleven trials were awaiting classification (Cambiaghi 2013; de Paiva Tosato 2007; França 2012; Hsueh 1997; Liebano 2013; Park 2014; Rajpurohit 2010; Salvador 2005; Salvino 2013; Silva 2012; Treacy 2011).
1.
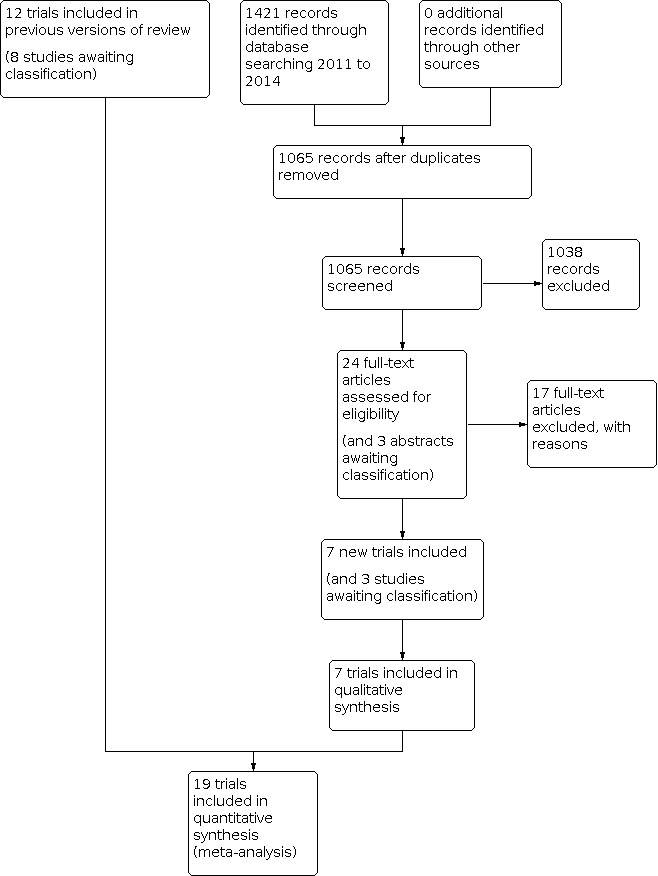
Study flow diagram.
Included studies
Participants
The 19 included trials had 1346 participants at entry (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Crompton 1992; De Angelis 2003; de Sousa 2014; Ekblom 1987; Gregorini 2010; Hansson 1983; Hruby 2006; Keskin 2012; Kim 2012; Limoges 2004; Liu 1985; Olsén 2007; Oncel 2002; Ordog 1987; Pitangui 2012; Roche 1985). Two trials did not indicate the gender of participants (Ordog 1987; Roche 1985), six trials included only women (Crompton 1992; De Angelis 2003; de Sousa 2014; Keskin 2012; Olsén 2007; Pitangui 2012) and the remaining 11 trials included women and men. There were 429 males and 759 females with an age range of 11 to 81 years in reports that provided information about age. One report did not provide details about the age of participants (Ordog 1987). Two trials included at least one participant under 16 years of age in the sample population (age range: Cheing 2005: 15 to 58 years; Oncel 2002: 11 to 81 years) but we included these trials because the mean age for both sample populations was > 30 years. Seven trials investigated the effect of TENS on procedural pain. Procedures included cervical laser treatment (Crompton 1992), office hysteroscopy (De Angelis 2003), screening flexible sigmoidoscopy (Limoges 2004), flexible cystoscopy (Hruby 2006), unsedated colonoscopy (Amer‐Cuenca 2011) and venepuncture (Coyne 1995; Kim 2012). The remaining trials investigated the effect of TENS on haemophilia pain (Roche 1985), acute trauma such as sprains or fractures (Ordog 1987), postpartum uterine contractions (de Sousa 2014; Olsén 2007), acute low back pain (LBP) during pregnancy (Keskin 2012) acute orofacial pain (Ekblom 1987; Hansson 1983), post thoracotomy (Liu 1985), post‐cardiac surgery (Gregorini 2010), post‐episiotomy (Pitangui 2012), rib fractures (Oncel 2002) and neuropathic pain (Cheing 2005).
Setting
Studies were conducted in Europe (UK, Sweden, Turkey, Italy, Spain), North America, Brazil and China, Hong Kong and South Korea. Eighteen trials were conducted in a hospital or specialised clinic with participants in one of these trials continuing to use TENS at home after discharge (Oncel 2002). In one trial, participants received TENS instruction in hospital but only used it at home (Ordog 1987).
Design
All included RCTs used a parallel group design.
Sample sizes
The number of participants randomised to each treatment group ranged from eight (Olsén 2007; Roche 1985) to 71 (De Angelis 2003). Ten trials had at least 20 participants in each of the treatment groups (Amer‐Cuenca 2011; Crompton 1992; De Angelis 2003; Hansson 1983; Hruby 2006; Kim 2012; Limoges 2004; Oncel 2002; Ordog 1987; Pitangui 2012). Four trials performed a prospective sample size calculation to determine the appropriate number of participants required (Amer‐Cuenca 2011; Crompton 1992; de Sousa 2014; Keskin 2012). Olsén 2007 reported that they based their sample size on results from previous trials in the area but did not provide a priori power analysis details; they performed a post hoc power analysis on the data they collected and claimed that the numbers they recruited (N = 13 and 8 in the two groups) were adequate.
TENS device and application
Electrodes were placed at the painful site in all trials except Amer‐Cuenca 2011, where electrodes were placed over the sensory nerves supplying the colon for unsedated colonoscopy, and Roche 1985, where electrodes were placed over the painful area or close to the area of bleeding for pain associated with haemophilia. Five trials did not provide full details of the type, size, number of electrodes used (Crompton 1992; De Angelis 2003; Hruby 2006; Liu 1985; Ordog 1987). TENS was administered using two self‐adhesive electrodes or two rubber/silicone electrodes smeared with gel in most trials. Crompton 1992 used four electrodes over the anterior abdominal wall (painful area) and two over the sacrum for pain experienced during cervical laser treatment. Limoges 2004 placed two electrodes over the abdomen (painful area) and two electrodes parallel to the spinal cord at L1‐S3 level for screening flexible sigmoidoscopy pain. Ordog 1987 used metal electrodes. Details of the model or manufacturer of the TENS device used, or both, was provided in all reports. Two trials used a device from the same Swedish manufacturer (Hansson 1983; Olsén 2007) and two trials used a Chattanooga Intelect Advanced combination Therapy System (Amer‐Cuenca 2011; Keskin 2012).
Only three reports described both the intensity (i.e. subjective description) and current amplitude (mA) of TENS (Hruby 2006; Liu 1985; Olsén 2007). Twelve reports described the intensity but not current amplitude (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Crompton 1992; De Angelis 2003; de Sousa 2014; Hansson 1983; Keskin 2012; Kim 2012; Oncel 2002; Ordog 1987; Roche 1985) and one report described pulse amplitude but not intensity (Limoges 2004). Two trials delivered TENS using a fixed pulse amplitude: Limoges 2004 used 30 mA; Olsén 2007 used 50 mA in the high pulse amplitude TENS group and 10 to 15 mA in the low pulse amplitude TENS group. Seven reports indicated that the pulse amplitude was adjusted during treatment (Amer‐Cuenca 2011; Coyne 1995; De Angelis 2003; Gregorini 2010; Hansson 1983; Hruby 2006; Pitangui 2012). This information was unclear or not provided in the remaining trials. A variety of subjective descriptors were used to describe the intensity of TENS including: tingling, non‐painful sensation from stimulated area (high frequency TENS group) or non‐painful muscular contractions in stimulated area (low frequency TENS group (Hansson 1983); strong but tolerable tingling (Amer‐Cuenca 2011; Cheing 2005); subjective level of comfort (Liu 1985); highest level that did not make participants uncomfortable (Oncel 2002); definite but comfortable perception with no muscle activation (Roche 1985); and below pain threshold (Coyne 1995). De Angelis 2003 used the term 'tickle' to describe the level of intensity. This is an unusual term and may be a result of translation from non‐English language. Most trials used high pulse frequencies, ranging from 51 Hz (Liu 1985) to 160 Hz (Coyne 1995). Two trials used trains of pulses delivered at a low frequency (Hansson 1983; Roche 1985). One trial, Ekblom 1987, had two TENS groups, one with a pulse frequency of 2 Hz and one with a frequency of 100 Hz. Pulse duration ranged between 50 μs (Oncel 2002) and 400 μs (Amer‐Cuenca 2011). One trial used a pulse duration of 310 to 400 μs (Coyne 1995). de Sousa 2014 reported using a pulse duration of 75 msec, which seems excessively large. We suspect that this is a typographical error in the trial report as technical specifications for the device used was listed as 45 to 300 μs by the manufacturer. Ordog 1987 did not specify frequency or pulse duration settings.
There was a wide variation in the number of treatments and individual treatment times across the included trials. TENS was administered in a single treatment session in 14 trials (Amer‐Cuenca 2011; Coyne 1995; Crompton 1992; De Angelis 2003; de Sousa 2014; Ekblom 1987; Gregorini 2010; Hansson 1983; Hruby 2006; Kim 2012; Limoges 2004; Pitangui 2012; Roche 1985; Olsén 2007) and in multiple treatment sessions in five trials (Cheing 2005; Keskin 2012; Liu 1985; Oncel 2002; Ordog 1987). Often it was difficult to ascertain exactly how often and for how long TENS was administered in trials using multiple TENS treatment sessions. Three of the seven reports of trials on procedural pain did not specify treatment duration (Crompton 1992; De Angelis 2003; Hruby 2006); in those that did, treatment duration varied from five minutes to four hours (Coyne 1995; Kim 2012; Limoges 2004) or was described as being for the duration of the procedure (Amer‐Cuenca 2011). In the non‐procedural pain trials, treatment duration varied from one minute (Olsén 2007) to applying TENS as often as required (Ordog 1987). Only two trials involved TENS being self‐administered at home where compliance could be assessed (Oncel 2002; Ordog 1987). In these trials participants continued to use TENS at home for two days (Oncel 2002) or used TENS at home for as long as needed with mean duration of use being three days and no participants using TENS at one month follow‐up (Ordog 1987).
Comparison groups
Eleven trials included a placebo TENS intervention (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Ekblom 1987; Gregorini 2010; Hansson 1983; Hruby 2006; Kim 2012; Limoges 2004; Ordog 1987; Roche 1985) and one trial included a placebo pill (Oncel 2002). In most trials placebo TENS was operationalised as a sham TENS device with no current output that was similar in appearance to the active TENS device but had no batteries, or the internal circuit disconnected, or the device was not switched on. Gregorini 2010 administered placebo TENS using an active device with an inter‐pulse interval of 33 seconds and claimed that this would avoid an analgesic effect. Liu 1985 applied a low pulse amplitude stimulus (fixed at 2.5 mA) as they felt this was a more valid control than a no stimulus placebo; for the purposes of this review, this was treated as low pulse amplitude TENS rather than placebo TENS. Only four of the trials that included placebo TENS also included TENS naive participants. Coyne 1995 specified "no previous TENS exposure" as an inclusion criterion and Cheing 2005 and Amer‐Cuenca 2011 had previous experience of TENS as an exclusion criterion. Ordog 1987 indicated that none of their participants had used TENS previously. Olsén 2007 did not include a placebo group but did use TENS naive participants. Eight trials included a no treatment comparison group (Amer‐Cuenca 2011; Coyne 1995; De Angelis 2003; de Sousa 2014; Hruby 2006; Keskin 2012; Limoges 2004; Pitangui 2012). Four trials included a pharmacological intervention as a comparison group: acetaminophen (paracetamol; Keskin 2012); local anaesthetic (Lignocaine with Octopressin, Crompton 1992); non‐steroidal anti‐inflammatory drug (NSAID) (Naproxen Sodium; Oncel 2002) and Tylenol (Ordog 1987). Two trials included a non‐pharmacological comparison group: exercise (Keskin 2012) and vibration (Ekblom 1987). Two active TENS groups were compared by Ekblom 1987 (2 Hz versus 100 Hz); Hansson 1983 (conventional TENS (100 Hz) to AL‐TENS (2 Hz trains with 71 Hz internal frequency); Olsén 2007 (high (50 mA) versus low (10 to 15 mA) pulse amplitude); and Liu 1985 (high (5.86 + 0.96 mA) versus low (2.5 mA) pulse amplitude.
Adverse effects
Ten reports included information about the occurrence of adverse effects with three indicating that there were no adverse effects (Oncel 2002; Ordog 1987; Roche 1985) and seven indicating adverse effects. De Angelis 2003 compared TENS with no treatment in participants undergoing office hysteroscopy and reported nausea (8.5% of TENS group; 11.3% of control group, sample size of 71 per group); shoulder pain (3% of TENS group; 0% of control group); bradycardia (0% of TENS group; 2.8% of control group) and dizziness (8.5% of TENS group; 10% of control group). They did not specifically link these effects to TENS. Limoges 2004 reported that 29 out of 30 participants in the TENS group and six out of 30 participants in the placebo TENS group reported pain, burning or tingling at the electrode site. Hruby 2006 reported that two out of 48 participants could not tolerate TENS and Keskin 2012 reported discomfort with the TENS treatment as an adverse effect in one participant. Kim 2012 reported erythema and itching as adverse effects in seven out of 50 participants in the placebo TENS group and eight out of 50 in the TENS group. Olsén 2007 reported that TENS was discontinued due to discomfort during stimulation in one out of 13 participants receiving high pulse amplitude TENS. Hansson 1983 reported that most of the 20 participants receiving low frequency TENS found muscle twitch uncomfortable.
Outcomes
All trials used standard pain scales/questionnaires to record pain (VAS; NRS; McGill Pain Questionnaire, MPQ; VRS) but many trials did not provide sufficient information about the exact instruction given to participants about how to rate pain scores. Thus, it was difficult to determine whether pain scores were taken at a specific moment in time (e.g. present pain intensity) or retrospectively for over a specified period of time (e.g. pain intensity for the previous 24 hours) and if taken retrospectively whether scores were for 'average' pain or worst pain episode. Other outcomes included time in minutes until first report of pain reduction and maximum pain reduction (Hansson 1983), overall impression of using TENS (de Sousa 2014; Liu 1985), discomfort during TENS (Amer‐Cuenca 2011; Crompton 1992; de Sousa 2014; Olsén 2007). One trial used a Roland Morris Disability Questionnaire (Keskin 2012). It was only possible to ascertain that three trials measured pain intensity whilst TENS was switched on and generating an electrical paraesthesia (Amer‐Cuenca 2011; Ekblom 1987; Hruby 2006). Amer‐Cuenca 2011 measured pain intensity during non‐sedated colonoscopy; Ekblom 1987 measured pain intensity in participants experiencing acute dental pain due to pulpal inflammation, apical periodontitis, pericoronitis or postoperative pain following operative removal of an impacted tooth; and Hruby 2006 measured pain intensity during TENS for discomfort during office‐based flexible cystoscopy. Many trials recorded pain after TENS had finished.
Excluded studies
For this update we retrieved 1065 reports from the literature searches after we removed duplicates, of which we considered 1038 irrelevant or excluded against eligibility criteria based on screening of abstracts (Figure 1). We obtained 24 full‐text trial reports, of which we excluded 17. Overall we excluded 120 trials on the basis that TENS was given in combination with another treatment as part of the formal trial design, of which 73 were postoperative pain trials (Table 1). In most trials, TENS was given with analgesic medication as part of the formal trial design but some provided TENS in conjunction with non‐pharmacological interventions, e.g. TENS given as part of a physiotherapy package of treatment. The reasons for excluding the remaining trials included not using a standard TENS device or TENS intensity in the active intervention was too low (Characteristics of excluded studies).
1. Studies excluded as TENS given in combination with other treatments.
| Akyuz G, Kayhan O, Babacan A, Gener FA. Transcutaneous electrical nerve stimulation (TENS) in the treatment of postoperative pain and prevention of paralytic ileus. Clinical Rehabilitation 1993; 7(3): 218‐21. |
| Ali J, Yaffe CS, Serrette C. The effect of transcutaneous electric nerve stimulation on postoperative pain and pulmonary function. Surgery 1981; 89(4): 507‐12. |
| Alm WA, Gold ML, Weil LS. Evaluation of transcutaneous electrical nerve stimulation (TENS) in podiatric surgery. Journal of the American Podiatry Association 1979; 69(9): 537‐42. |
| Anderson AF, Lipscomb AB. Analysis of rehabilitation techniques after anterior cruciate reconstruction. American Journal of Sports Medicine 1989; 17(2): 154‐60. |
| Angulo DL, Colwell CWJr. Use of postoperative TENS and continuous passive motion following total knee replacement. The Journal of Orthopaedic and Sports Physical Therapy 1990; 11(12): 599‐604. |
| Ardic F, Sarhus M, Topuz O. Comparison of two different techniques of electrotherapy on myofascial pain. Journal of Back and Musculoskeletal Rehabilitation 2002; 16(1): 11‐6. |
| Arena M, Savoca G, Lednyiczky G. Percutaneous paravertebral infiltration of O2‐O3, bioresonance magnetotherapy, transcutaneous electrical nerve stimulation and psychosomatic postural rehabilitation in the treatment of degenerative joint disease of the lumbar spine with functional insufficiency of the vertebral motor unit. International Journal of Ozone Therapy 2008; 7(1): 29‐36. |
| Avraham F, Aviv S, Ya'akobi P, Faran H, Fisher Z, Goldman Y, Neeman G, et al. The efficacy of treatment of different intervention programs for patellofemoral pain syndrome‐a single blinded randomized clinical trial. Pilot study. The Scientific World Journal 2007; 7:1256‐62. |
| Baker SB, Wong CC, Wong PC, Jenkins LC. Transcutaneous electrostimulation in the management of postoperative pain: initial report. Canadian Anaesthetists' Society Journal 1980; 27(2): 150‐5. |
| Bayindir O, Paker T, Akpinar B, Erenturk S, Askin D, Aytac A. Use of transcutaneous electrical nerve stimulation in the control of postoperative chest pain after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 1991; 5(6): 589‐91. |
| Bennett MI, Johnson MI, Brown SR, Radford H, Brown JM, Searle RD. Feasibility study of transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. Journal of Pain 2010; 11(4): 351‐9. |
| Benedetti F, Amanzio M, Casadio C, Cavallo A, Cianci R, Giobbe R, et al. Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Annals of Thoracic Surgery 1997; 63(3): 773‐6. |
| Bicer A, Ozisik S, Aksik SC, Erdogan C. Comparison of local corticosteroid injection and conventional physical therapy in management of the painful shoulder. Turkiye Klinikleri Tip Bilimleri Dergisi 2005; 25(4): 506‐12. |
| Bjersa K, Andersson T. High frequency TENS as a complement for pain relief in postoperative transition from epidural to general analgesia after pancreatic resection. Complementary Therapies in Clinical Practice 2014; 20(1): 5‐10. |
| Borjesson M, Eriksson P, Dellborg M, Eliasson T, Mannheimer C. Transcutaneous electrical nerve stimulation in unstable angina pectoris. Coronary Artery Disease 1997; 8(8‐9): 543‐50. |
| Breit R, Van Der Wall H. Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty. Journal of Arthroplasty 2004; 19(1): 45‐8. |
| Cekmen N, Salman B, Keles Z, Aslan M, Akcabay M. Transcutaneous electrical nerve stimulation in the prevention of postoperative nausea and vomiting after elective laparoscopic cholecystectomy. Journal of Clinical Anesthesia 2007; 19(1): 49‐52. |
| Celik D, Akyuz G, Yeldan I. Comparison of the effects of two different exercise programs on pain in subacromial impingement syndrome. Acta Orthopaedica Et Traumatologica Turcica 2009; 43(6): 504‐9. |
| Chen L, Tang J, White PF, Sloninsky A, Wender RH, Naruse R, et al. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesthesia & Analgesia 1998; 87(5): 1129‐34. |
| Chitsaz A, Janghorbani M, Shaygannejad V, Ashtari F, Heshmatipour M, Freeman J. Sensory complaints of the upper extremities in multiple sclerosis: relative efficacy of nortriptyline and transcutaneous electrical nerve stimulation. Clinical Journal of Pain 2009; 25(4): 281‐5. |
| Chiu JH, Chen WS, Chen CH, Jiang JK, Tang GJ, Lui WY, et al. Effect of transcutaneous electrical nerve stimulation for pain relief on patients undergoing hemorrhoidectomy: prospective, randomized, controlled trial. Diseases of the Colon & Rectum 1999; 42(2): 180‐5. |
| Cipriano G, Jr, de Camargo Carvalho AC, Bernardelli GF, Tayar Peres PA. Short‐term transcutaneous electrical nerve stimulation after cardiac surgery: effect on pain, pulmonary function and electrical muscle activity. Interactive Cardiovascular & Thoracic Surgery 2008; 7(4): 539‐43. |
| Conn IG, Marshall AH, Yadav SN. Transcutaneous electrical nerve stimulation following appendicectomy: the placebo effect. Annals of the Royal College of Surgeons of England 1986; 68(4): 191‐2. |
| Cooperman AM, Hall B, Mikalacki K, Hardy R, Sadar E. Use of transcutaneous electrical stimulation in the control of postoperative pain. Result of a prospective, randomized, controlled study. American Journal of Surgery 1977; 133: 185‐7. |
| Cornell PE, Lopez AL, Malofsky H. Pain reduction with transcutaneous electrical nerve stimulation after foot surgery. Journal of Foot Surgery 1984; 23(4): 326‐33. |
| Cuschieri RJ, Morran CG, McArdle CS. Transcutaneous electrical stimulation for postoperative pain. Annals of the Royal College of Surgeons of England 1985; 67(2): 127‐9. |
| de la Rocha AG, Chambers K. Pain amelioration after thoracotomy: a prospective, randomized study. Annals of Thoracic Surgery 1984; 37(3): 239‐42. |
| DeSantana JM, Santana‐Filho VJ, Guerra DR, Sluka KA, Gurgel RQ, da Silva Jr, WM. Hypoalgesic effect of the transcutaneous electrical nerve stimulation following inguinal herniorrhaphy: a randomized, controlled trial. Journal of Pain 2008; 9(7): 623‐9. |
| DeSantana JM, Sluka KA, Lauretti GR. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: a randomized controlled trial. Clinical Journal of Pain 2009; 25(1): 12‐9. |
| Domaille M, Reeves B. TENS and pain control after coronary artery bypass surgery. Physiotherapy (London) 1997; 83(10): 510‐6. |
| Dusunceli Y, Ozturk C, Atamaz F, Hepguler S, Durmaz B. Efficacy of neck stabilization exercises for neck pain: a randomized controlled study. Journal of Rehabilitation Medicine 2009; 41(8): 626‐31. |
| Emmiler M, Solak O, Kocogullari C, Dundar U, Ayva E, Ela Y, Cekirdekci A, Kavuncu V. Control of acute postoperative pain by transcutaneous electrical nerve stimulation after open cardiac operations: a randomized placebo‐controlled prospective study. Heart Surgery Forum 2008; 11(5): E300‐3. |
| Erdogan M, Erdogan A, Erbil N, Karakaya H, Demircan A. Prospective, randomized, placebo‐controlled study of the effect of TENS on postthoracotomy pain and pulmonary function. World Journal of Surgery 2005; 29(12): 1563‐70. |
| Fagade OO, Oginni FO, Obilade TO. Comparative study of the therapeutic effect of a systemic analgesic and transcutaneous electrical nerve stimulation (TENS) on post‐IMF trismus and pain in Nigerian patients. Nigerian Postgraduate Medical Journal 2005; 12(2): 97‐101. |
| Ferraz FS, Moreira CMC. Electroanalgesia with TENS in postoperative of cardiac surgery [Portuguese]. Fisioterapia em Movimento 2009; 22(1): 133‐9. |
| Finsen V, Persen L, Lovlien M, Veslegaard EK, Simensen M, Gasvann AK, et al. Transcutaneous electrical nerve stimulation after major amputation. Journal of Bone & Joint Surgery ‐ British Volume 1988; 70(1): 109‐12. |
| Fiorelli A, Aurilio C, Morgillo F, Fasano M, Milione R, et al. Control of post‐thoracotomy pain by transcutaneous electrical nerve stimulation (tens): Effect on serum cytokine levels, visual analogue scale, pulmonary function, and medication. Interactive Cardiovascular and Thoracic Surgery 2011; 13: S2 |
| Fodor‐Sertl B, Miller K, Hohenfellner B. Transcutaneous electric nerve block in postoperative pain‐therapy. Zeitschrift Fur Physikalische Medizin Balneologie Med. Klimatologie 1990; 19(3): 132‐7. |
| Forster EL, Kramer JF, Lucy SD, Scudds RA, Novick RJ. Effects of TENS on pain, medications, and pulmonary function following coronary artery bypass graft surgery. Chest 1994; 106(5): 1343‐8. |
| Galloway DJ, Boyle P, Burns HJ, Davidson PM, George WD. A clinical assessment of electroanalgesia following abdominal operations. Surgery, Gynecology & Obstetrics, 1984; 159(5): 453‐6. |
| Geler Kulcu D, Gulsen G. Effect of physical therapy program on insomnia severity in a patient population with fibromyalgia syndrome. Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2009; 55(2): 64‐7. |
| Ghoname EA, Craig WF, White PF, Ahmed HE, Hamza MA, Henderson BN, et al. Percutaneous electrical nerve stimulation for low back pain: a randomized crossover study. Journal of the American Medical Association 1999; 281(9): 818‐23. |
| Ghoname EA, White PF, Ahmed HE, Hamza MA, Craig WF, Noe CE. Percutaneous electrical nerve stimulation: an alternative to TENS in the management of sciatica. Pain, 1999; 83(2): 193‐9. |
| Gilbert JM, Geldhill T, Law N, George C. Controlled trial of transcutaneous electrical nerve stimulation (TENS) for postoperative pain relief following inguinal herniorrhaphy. British Journal of Surgery 1986; 73(9): 749‐51. |
| Gonzalez‐Iglesias J, Fernandez‐de‐las‐Penas C, Cleland JA, Alburquerque‐Sendin F, Palomeque‐del‐Cerro L, Mendez‐Sanchez R. Inclusion of thoracic spine thrust manipulation into an electro‐therapy/thermal program for the management of patients with acute mechanical neck pain: a randomized clinical trial. Manual Therapy 2009; 14(3): 306‐13. |
| Gupta AK, Gupta S, Meena DS, Sharma U. Post ‐ tonsillectomy pain: different modes of pain relief. Indian Journal of Otolaryngology 2002; 54(2): 136‐9. |
| Guler H, Turhanoglu AD, Inanoglu K, Inanoglu D, Ozer C. Comparison of ketoprofen phonophoresis with ketoprofen and lidocaine‐prilocaine phonophoresis in patients with subacromial impingement syndrome. Turkish Journal of Rhuematology 2009; 24(2): 88‐93. |
| Hamza MA, White PF, Ahmed HE, Ghoname EA. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 1999; 91(5): 1232‐8. |
| Hargreaves A, Lander J. Use of transcutaneous electrical nerve stimulation for postoperative pain. Nursing Research 1989; 38(3): 159‐61. |
| Hazneci B, Tan AK, Ozdem T, Dincer K, Kalyon TA. The effects of transcutaneous electroneurostimulation and ultrasound in the treatment of reflex sympathetic dystrophy syndrome. Ftr ‐ Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2005; 51(3): 83‐9. |
| Hershman MJ, Cheadle WG, Swift RI, Reilly DT, Gompertz H, Wood CB. Transcutaneous electrical nerve stimulation (TENS) as adjunctive analgesia in patients undergoing elective abdominal procedures. Surgical Research Communications 1989; 7(1): 65‐9. |
| Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger‐point sensitivity. Archives of Physical Medicine & Rehabilitation 2002; 83(10): 1406‐14. |
| Hsieh RL, Lee WC. One‐shot percutaneous electrical nerve stimulation vs. transcutaneous electrical nerve stimulation for low back pain: comparison of therapeutic effects. American Journal of Physical Medicine and Rehabilitation 2002; 81(11): 838‐43. |
| Jaafarpour M, Khani A, Javadifar N, Taghinejad H, Mahmoudi R, Saadipour KH. The analgesic effect of transcutaneous electrical nerve stimulation (TENS) on caesarean under spinal anaesthesia. Journal of Clinical and Diagnostic Research 2008; 2(3): 815‐9. |
| Jensen JE, Conn RR, Hazelrigg G, Hewett JE. The use of transcutaneous neural stimulation and isokinetic testing in arthroscopic knee surgery. American Journal of Sports Medicine 1985; 13(1): 27‐33. |
| Jones AYM, Hutchinson RC. A comparison of the analgesic effect of transcutaneous electrical nerve stimulation and Entonox. Physiotherapy 1991; 77(8): 526‐30. |
| Kararmaz A, Kaya S, Karaman H, Turhanoglu S. Effect of the frequency of transcutaneous electrical nerve stimulation on analgesia during extracorporeal shock wave lithotripsy. Urological Research 2004: 32(6): 411‐5. |
| Kavuncu V, Danisger S, Kozakcioglu M, Omer SR, Aksoy C, Yucel K. Comparison of the efficacy of TENS and ultrasound in temporomandibular joint dysfunction syndrome. Journal of Rheumatology & Medical Rehabilitation 1994; 5(1): 38‐42. |
| Khan AA, Mowla A, Shakoor MA, Rahman MR. Arthrographic distension of the shoulder joint in the management of frozen shoulder. Mymensingh Medical Journal 2005; 14(1): 67‐70. |
| Kho HG, Eijk RJ, Kapteijns WM, van Egmond J. Acupuncture and transcutaneous stimulation analgesia in comparison with moderate‐dose fentanyl anaesthesia in major surgery. Clinical efficacy and influence on recovery and morbidity. Anaesthesia 1991; 46(2): 129‐35. |
| Kimball KL, Drews JE, Walker S, Dimick AR. Use of TENS for pain reduction in burn patients receiving travase. Journal of Burn Care & Rehabilitation 1987; 8: 28‐31. |
| Klin B, Uretzky G, Magora F. Transcutaneous electrical nerve stimulation (TENS) after open heart surgery. Journal of Cardiovascular Surgery (Torino) 1984; 25(5): 445‐8. |
| Kruger LR, Van der Linden WJ, Cleaton‐Jones PE. Transcutaneous electrical nerve stimulation in the treatment of myofascial pain dysfunction. South African Journal of Surgery 1998; 36(1): 35‐8. |
| Laitinen J, Nuutinen L. Failure of transcutaneous electrical nerve stimulation and indomethacin to reduce opiate requirement following cholecystectomy. Acta Anaesthesiologica Scandinavica 1991; 35(8): 700‐5. |
| Leblebici B, Adam M, Yapgu S, Bags S, Akman MN. Comparing the effects of open versus closed kinetic chain scapulohumeral stability exercises in rotator cuff problems. Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2007; 53(4): 134‐7. |
| Leung WW, Ng SS, Jones A, Chan SK, Wong CY, et al. The application of transcutaneous electric nerve stimulation on acupoints (ACU‐TENS) for pain relief during colonoscopy: A prospective, randomized, placebo‐controlled study. Gastrointestinal Endoscopy 2014; 75(5): AB222. |
| Likar R, Molnar M, Pipam W, Koppert W, Quantschnigg B, Disselhoff B, Sittl R. Postoperative transcutaneous electrical nerve stimulation (TENS) in shoulder surgery (randomized, double blind, placebo controlled pilot trial). Schmerz 2001; 15(3): 158‐63. |
| Lim AT, Edis G, Kranz H. Postoperative pain control: contribution of psychological factors and transcutaneous electrical stimulation. Pain 1983; 17(2): 179‐88. |
| Luchesa CA, Greca FH, Guarita‐Souza LC, dos Santos JL, Aquim EE. The role of electroanalgesia in patients undergoing coronary artery bypass surgery. Revista Brasileira De Cirurgia Cardiovascular: Orgao Oficial Da Sociedade Brasileira De Cirurgia Cardiovascular 2009; 24(3): 391‐6. |
| Martelete M, Fiori AM. Comparative study of the analgesic effect of transcutaneous nerve stimulation (TNS); electroacupuncture (EA) and meperidine in the treatment of postoperative pain. Acupuncture & Electro‐Therapeutics Research 1985; 10(3): 183‐93. |
| McCallum MI, Glynn CJ, Moore RA, Lammer P, Phillips AM. Transcutaneous electrical nerve stimulation in the management of acute postoperative pain. British Journal of Anaesthesia 1988; 61(3): 308‐12. |
| Mehlhorn G, Beckmann MW, Schild RL, Binder H. Analgesia of afterpains with transcutaneous nerve stimulation (TENS) vs. metamizole. A prospective, randomized placebo controlled double‐blind study. Geburtshilfe Und Frauenheilkunde 2005; 65(3): 266‐71. |
| Moffet H, Richards CL, Malouin F, Bravo G, Paradis G. Early and intensive physiotherapy accelerates recovery postarthroscopic meniscectomy: results of a randomized controlled study. Archives of Physical Medicine & Rehabilitation 1994; 75(4): 415‐26. |
| Morgan B, Jones AR, Mulcahy KA, Finlay DB, Collett B. Transcutaneous electric nerve stimulation (TENS) during distension shoulder arthrography: a controlled trial. Pain 1996; 64(2): 265‐7. |
| Naumann C, Lange A. The application of the transcutaneous electric nerve stimulation for analgesia in the postoperative phase. Zeitschrift für Physiotherapie 1989; 41(1): 9‐13. |
| Navarathnam RG, Wang IY, Thomas D, Klineberg PL. Evaluation of the transcutaneous electrical nerve stimulator for postoperative analgesia following cardiac surgery. Anaesthesia & Intensive Care 1984; 12(4): 345‐50. |
| Navarro Nunez C, Pacheco Carrasco M. Transcutaneous electric stimulation (TENS) to reduce pain after cesarean section. Ginecologia y Obstetricia De Mexico 2000; 68: 60‐3. |
| Ng S, Leung WW, Mak T, Jones A, Chan S, et al. The application of transcutaneous electrical nerve stimulation on acupoints (Acu‐TENS) for pain relief during colonoscopy: A prospective, randomised, placebo‐controlled study. Colorectal Disease 2014; 6: 115. |
| Nordemar R, Thorner C. Treatment of acute cervical pain ‐ a comparative group study. Pain 1981; 10(1): 93‐101. |
| Norrbrink C. Transcutaneous electrical nerve stimulation for treatment of spinal cord injury neuropathic pain. Journal of Rehabilitation Research and Development 2009; 46(1): 85‐93. |
| Pike PM. Transcutaneous electrical stimulation. Its use in the management of postoperative pain. Anaesthesia 1978; 33(2): 165‐71. |
| Platon B, Andrell P, Raner C, Rudolph M, Dvoretsky A, Mannheimer C. High‐frequency, high‐intensity transcutaneous electrical nerve stimulation as treatment of pain after surgical abortion. Pain 2010; 148(1): 114‐9. |
| Poulain P, Leandri EP, Laplanche A, Montagne F, Bouzy J, TruffaBachi J. Electroacupuncture analgesia in major abdominal and pelvic surgery: a randomised study. Acupuncture in Medicine 1997; 15(1): 10‐3. |
| Presser M, Birkhan J, Adler R, Hanani A, Eisenberg E. Transcutaneous electrical nerve stimulation (TENS) during epidural steroids injection: a randomized controlled trial. Pain Clinic 2000; 12(2): 77‐80. |
| Quinton DN, Sloan JP, Theakstone J. Transcutaneous electrical nerve stimulation in acute hand infections. Journal of Hand Surgery ‐ British Volume 1987; 12B(2): 267‐8. |
| Rainov NG, Heidecke V, Albertz C, Burkert W. Transcutaneous electrical nerve stimulation (TENS) for acute postoperative pain after spinal surgery. European Journal of Pain 1994; 15(2‐3): 44‐9. |
| Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. Journal of Pain 2003; 4(8): 455‐64. |
| Reuss R, Cronen P, Abplanalp L. Transcutaneous electrical nerve stimulation for pain control after cholecystectomy: lack of expected benefits. Southern Medical Journal 1988; 81(11): 1361‐3. |
| Robinson R, Darlow S, Wright SJ, Watters C, Carr I, Gadsby G, et al. Is transcutaneous electrical nerve stimulation an effective analgesia during colonoscopy? Postgraduate Medical Journal 2001; 77(909): 445‐6. |
| Rooney SM, Jain S, Goldiner PL. Effect of transcutaneous nervous stimulation on postoperative pain after thoracotomy. Anesthesia & Analgesia 1983; 62(11): 1010‐2. |
| Rosenberg M, Curtis L, Bourke DL. Transcutaneous electrical nerve stimulation for the relief of postoperative pain. Pain 1978; 5(2): 129‐33. |
| Schomburg FL, Carter‐Baker SA. Transcutaneous electrical nerve stimulation for post laparotomy pain. Physical Therapy 1983; 63(2): 188‐93. |
| Schuster GD, Infante MC. Pain relief after low back surgery: the efficacy of transcutaneous electrical nerve stimulation. Pain 1980; 8(3): 299‐302. |
| Seraia EV, Smirnov SV, Lapshin VP. Exercise therapy in combination with transcutaneous electroneurostimulation in patients with inhalation burns during intensive care. Voprosy Kurortologii, Fizioterapii i Lechebnoi Fizicheskoi Kultury 2004; 1: 38. |
| Shehab D, Adham N. Comparative effectiveness of ultrasound and transcutaneous electrical stimulation in treatment of periarticular shoulder pain. Physiotherapy Canada 2000; 52(3): 208‐10, 214. |
| Sim DT. Effectiveness of transcutaneous electrical nerve stimulation following cholecystectomy. Physiotherapy 1991; 77(10): 715‐22. |
| Sloan JP, Muwanga CL, Waters EA, Dove AF, Dave SH. Multiple rib fractures: transcutaneous nerve stimulation versus conventional analgesia. Journal of Trauma‐Injury Infection & Critical Care 1986; 26(12): 1120‐2. |
| Smedley F, Taube M, Wastell C. Transcutaneous electrical nerve stimulation for pain relief following inguinal hernia repair: a controlled trial. European Surgical Research 1988; 20(4): 233‐7. |
| Smith CM, Guralnick MS, Gelfand MM, Jeans ME. The effects of transcutaneous electrical nerve stimulation on post‐cesarean pain. Pain 1986; 27(2): 181‐93. |
| Sodipo JO, Adedeji SA, Olumide O. Postoperative pain relief by transcutaneous electrical nerve stimulation (TENS). American Journal of Chinese Medicine 1980; 8(1‐2): 190‐4. |
| Solak O, Turna A, Pekcolaklar A, Metin M, Sayar A, Solak O, Gurses A. Transcutaneous electric nerve stimulation for the treatment of postthoracotomy pain: a randomized prospective study. Thoracic & Cardiovascular Surgeon 2007; 55(3): 182‐5. |
| Solak O, Emmiler M, Ela Y, Dundar U, Kocoiullari CU, Eren N, Gokce IY, Cekirdekci A, Kavuncu V. Comparison of continuous and intermittent transcutaneous electrical nerve stimulation in postoperative pain management after coronary artery bypass grafting: a randomized, placebo‐controlled prospective study. Heart Surgery Forum 2009; 12(5): E266‐71. |
| Solomon RA, Viernstein MC, Long DM. Transcutaneous electrostimulation in the management of postoperative pain: initial report. Surgery 1980; 87(2): 142‐6. |
| Stubbing JF, Jellicoe JA. Transcutaneous electrical nerve stimulation after thoracotomy. Pain relief and peak expiratory flow rate ‐ a trial of transcutaneous electrical nerve stimulation. Anaesthesia 1988; 43(4): 296‐8. |
| Tabak Y, Entok E, Tascioglu F, Zor E, Armagan O, Oner C, et al. The efficacy of intranasal salmon calcitonin in the treatment of reflex sympathetic dystrophia. Journal of Rheumatology & Medical Rehabilitation 2004; 15(4): 234‐40. |
| Tascioglu F, Dalkiran I, Oner C. The efficacy of low‐level laser in the treatment of subacromial impingement syndrome due to partial rupture of the supraspinatus tendon. Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2003; 49(6): 18‐22. |
| Taspinar S, Sahin F, Ercalik C, Kuran B, Barkut K, Celik M, et al. Comparison of the efficacy of corticosteroid injection, night splint and physiotherapy in diabetic carpal tunnel syndrome. Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2007; 53(2): 54‐60. |
| Taylor AG, West BA, Simon B, Skelton J, Rowlingson JC. How effective is TENS for acute pain? American Journal of Nursing 1983; 83(8): 1171‐4. |
| Tokuda M, Tabira K, Masuda T, Nishiwada T, Shomoto K. Effect of modulated‐frequency and modulated‐intensity transcutaneous electrical nerve stimulation after abdominal surgery: A randomized controlled trial. Clinical Journal of Pain 2014; 30: 565‐70. |
| Tonella RM, Araujo S, and Da Silva AMO [Transcutaneous electrical nerve stimulation in the relief of pain related to physical therapy after abdominal surgery]. Estimulacao eletrica nervosa transcutanea no alivio da dor pos‐operatoria relacionada com procedimentos fisioterapeuticos em pacientes submetidos a intervencoes cirurgicas abdominais. Revista Brasileira de Anestesiologia 2006; 56(6): 630‐42. |
| Tunc M, Gunal H, Bilgili T, Ulus F, Tunc H, Savkilioglu E. The effect of TENS on epidural patient controlled analgesia with tramadol for postthoracotomy pain relief. Turk Anesteziyoloji Ve Reanimasyon 2002; 30(7): 315‐21. |
| Unterrainer AF, Friedrich C, Krenn MH, Piotrowski WP, Golaszewski SM, Hitzl W. Postoperative and preincisional electrical nerve stimulation TENS reduce postoperative opioid requirement after major spinal surgery. Journal of Neurosurgical Anesthesiology 2010; 22(1): 1‐5. |
| VanderArk GD, McGrath KA. Transcutaneous electrical stimulation in treatment of postoperative pain. American Journal of Surgery 1975; 130(3): 338‐40. |
| Walker RH, Morris BA, Angulo DL, Schneider J, Colwell Jr CW. Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation, and continuous cooling pad following total knee arthroplasty. Journal of Arthroplasty 1991; 6(2): 151‐6. |
| Wang B, Tang J, White PF, Naruse R, Sloninsky A, Kariger R, et al. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesthesia & Analgesia 1997; 85(2): 406‐13. |
| Wang WC, George SL, Wilimas JA. Transcutaneous electrical nerve stimulation treatment of sickle cell pain crises. Acta Haematologica 1988: 80(2): 99‐102. |
| Warfield CA, Stein JM, Frank HA. The effect of transcutaneous electrical nerve stimulation on pain after thoracotomy. Annals of Thoracic Surgery 1985; 39(5): 462‐5. |
| Xu G, Feng Y, Tang WZ, Lv ZW. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: a single‐center randomized controlled trial. American Journal of Physical Medicine & Rehabilitation 2014; 93(4): 287‐98. |
| Yip YB, Tse HM, Wu KK. An experimental study comparing the effects of combined transcutaneous acupoint electrical stimulation and electromagnetic millimetre waves for spinal pain in Hong Kong. Complementary Therapies in Clinical Practice 2007; 13(1): 4‐14. |
| Zhang Q, Zhang JH, Tong PJ. Application of transcutaneous electrical nerve stimulation to multimodal analgesia after total knee arthroplasty. China Journal of Orthopaedics and Traumatology 2014; 27(4); 283‐6. |
Please note we may have had other reasons for exclusion of above studies in addition to the fact that TENS was used in combination with other treatments.
Studies awaiting classification
Eleven trials are awaiting classification (Characteristics of studies awaiting classification). Nine were written in English (Cambiaghi 2013; França 2012; Hsueh 1997; Liebano 2013; Park 2014; Rajpurohit 2010; Treacy 2011; Salvino 2013; Silva 2012) and two in Portuguese that required translation (de Paiva Tosato 2007; Salvador 2005). We contacted the trial authors by e‐mail to clarify their eligibility based on three of our inclusion criteria (i.e. if the trial involved acute pain, if it was a randomised trial, or if other treatment was given in addition to TENS). The full trial report of the abstract by Liebano 2013 has been submitted for publication. We have not obtained the information required to classify the other studies yet.
Risk of bias in included studies
The 'Risk of bias' table provides details of judgements on the following items: allocation; blinding; incomplete outcome data; and, sources of funding bias. We have provided the overall 'Risk of bias' assessment of the 19 trials in Figure 2. We have listed details of the judgments about each methodological quality item for each included trial in Figure 3.
2.

Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgments about each methodological quality item for each included trial.
Allocation
We considered sequence generation to be adequate in 11 trials (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; De Angelis 2003; de Sousa 2014; Keskin 2012; Limoges 2004; Olsén 2007; Oncel 2002; Ordog 1987; Pitangui 2012), and unclear or inadequate in the other eight trials. Six trials used a computer generated list for sequence generation (Amer‐Cuenca 2011; De Angelis 2003; de Sousa 2014; Olsén 2007; Oncel 2002; Pitangui 2012). Ordog 1987 mixed active and sham TENS devices during allocation and unblinded group allocation when all devices were returned to the researcher after the trial was completed. Gregorini 2010 used a 'sealed' box for randomisation but did not give specific operational details. Coyne 1995 and Keskin 2012 used a randomisation table. We rated the remaining trials as either inadequate (dividing participants alternatively into groups; Liu 1985) or unclear in their methods of sequence generation (Ekblom 1987; Gregorini 2010; Kim 2012). Only three trials had adequate allocation concealment (Keskin 2012; Olsén 2007; Ordog 1987). Olsén 2007 and Keskin 2012 were the only trials to use pre‐sealed opaque envelopes. Ordog 1987 revealed which of the TENS units were active or sham only after they had been returned to the researcher when the trial was completed. Most trials were unclear regarding how allocation was concealed (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Crompton 1992; De Angelis 2003; de Sousa 2014; Gregorini 2010; Hansson 1983; Hruby 2006; Kim 2012; Oncel 2002; Pitangui 2012; Roche 1985) and deemed inadequate in two trials (Limoges 2004; Liu 1985).
Blinding
Participant blinding
It is impossible to fully blind participants to an electrical current that generates a sensory experience, although participants can be made to be uncertain whether the sensations that they experience are likely to be effective. Four trials that included a placebo control specified that participants were TENS naive (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Ordog 1987). Studies that used a sham TENS device ensured that it was similar in appearance to the active TENS device but delivered no current (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Hansson 1983; Hruby 2006; Kim 2012; Limoges 2004; Oncel 2002; Ordog 1987; Roche 1985) or a very low pulse amplitude current (Liu 1985; Gregorini 2010). In addition, participants were told they may or may not feel a sensation during the treatment (Cheing 2005; Kim 2012; Limoges 2004; Oncel 2002; Roche 1985) or that some people may not experience the stimulation (Hansson 1983). Olsén 2007 did not use a placebo TENS intervention and participants experienced TENS sensation in both of the active TENS interventions.
Assessor blinding
In six trials, the person who recorded the outcomes was blind to group allocation (Amer‐Cuenca 2011; Cheing 2005; Coyne 1995; Kim 2012; Liu 1985; Ordog 1987). Five trials did not have blinded assessors (de Sousa 2014; Hansson 1983; Limoges 2004; Olsén 2007; Oncel 2002). Oncel 2002 recorded pain scores using an investigator not blinded to group allocation and also by nurses who were blinded to group allocation. The remaining trials did not provide sufficient details to judge assessor blinding (Crompton 1992; De Angelis 2003; Ekblom 1987; Gregorini 2010; Hruby 2006; Keskin 2012; Pitangui 2012).
Follow‐up and exclusions
Amer‐Cuenca 2011, Kim 2012, Limoges 2004, Pitangui 2012 and Roche 1985 did not report any participant withdrawals. de Sousa 2014 evaluated 44 participants for eligibility, of which five did not meet the inclusion criteria, six were excluded and one refused to participate in the trial. All 32 participants randomised completed the trial. Coyne 1995 withdrew ten participants post‐randomisation as they did not meet blood donor criteria, although such screening for eligibility should have been conducted before randomisation. Crompton 1992 provided details of two withdrawals (one participant failed to record a pain score and another found the cervical laser treatment uncomfortable) but there were no details of which treatment group they belonged to. Oncel 2002 reported that eight participants were withdrawn due to complications from respiratory distress associated with their minor rib fractures but they did not state which treatment group they belonged to. These withdrawals were replaced. Liu 1985 reported the number of participants that data were recorded from on each postoperative day but did not give specific reasons for the incomplete data set. Olsén 2007 reported that one participant dropped out due to discomfort of TENS (high pulse amplitude TENS group). Keskin 2012 reported dropouts due to non‐compliance, loss to follow‐up or pregnancy‐related complications but gave no information on how this data was dealt with. Six trials did not provide details on whether there were any incomplete data (Cheing 2005; De Angelis 2003; Gregorini 2010; Hansson 1983; Hruby 2006; Ordog 1987).
Other potential sources of bias
There was a high risk of bias associated with inadequate sample sizes in treatment arms. Four trials acknowledged sources of funding: loan of TENS units from a TENS manufacturer (Crompton 1992); TENS units provided by a TENS manufacturer and university project grant (Limoges 2004); research foundation (Hansson 1983); and a research council grant (Roche 1985). None of these sources were thought to introduce bias.
Effects of interventions
Primary objective
The primary objective of this Cochrane Review was to assess the analgesic effectiveness of TENS, as a sole treatment, for acute pain in adults. We were unable to extract data from included trials for the following reasons: data presented as median and interquartile (IQ) range (Crompton 1992; Keskin 2012); insufficient data provided (Coyne 1995). We felt that there was sufficient information in reports to assume that De Angelis 2003 and Hruby 2006 presented means with standard deviations (SDs). We also decided to extract data from the two trials that included at least one participant under 16 years (age range: Cheing 2005 = 15 to 58 years; Oncel 2002 = 11 to 81 years) because the mean ages for the sample populations were above 30 years. We contacted the following authors in an attempt to obtain the data: Crompton 1992 (responded but unable to provide data as mean and SD); Coyne 1995 (responded but unable to provide data); Hruby 2006 and De Angelis 2003 (no response). There were insufficient extractable data to allow us to pool data for meta‐analysis for most planned comparisons. We decided to pool data for pain intensity (100 mm VAS) and proportion of participants achieving ≥ 50% reduction in pain, although there was variability in procedures used to measure pain scores including whether scores were for present or retrospective pain and whether TENS was switched on during pain ratings.
TENS versus placebo TENS
Eleven trials included a comparison between active and placebo TENS. Eight trials reported a statistically significant improvement in favour of TENS of at least one outcome measure at one or more time points (Amer‐Cuenca 2011; Cheing 2005; Ekblom 1987; Gregorini 2010; Hansson 1983; Kim 2012; Ordog 1987; Roche 1985). Cheing 2005 reported lower pain scores (VAS) for neuropathic pain in the hand during TENS. Hansson 1983 claimed that more patients experienced > 50% relief of orofacial pain post treatment using a VAS but only reported details of a descriptive analysis. Ordog 1987 reported a significant decrease in pain intensity during TENS after two days of treatment (VAS, WMD ‐2.44 cm, 95% CI ‐3.85 to ‐1.03, P = 0.0007). Roche 1985 reported that more patients achieved 50% relief of pain associated with haemophilia haemorrhage using TENS (P < 0.02). Ekblom 1987 reported that more patients experienced reduction of acute orofacial pain using 100 Hz TENS following statistical analysis using the Chi² test but there was insufficient information to evaluate the analysis. Gregorini 2010 reported a significant reduction in post‐operative pain intensity (VAS) following cardiac surgery during TENS group (P < 0.001). Amer‐Cuenca 2011 reported that more patients achieved > 50% relief of pain associated with colonoscopy during TENS (P < 0.001). Kim 2012 reported significantly lower pain intensity (VAS) during venous cannulation during TENS. Studies that reported no differences in pain outcomes between TENS and placebo TENS found no significant differences between active and placebo TENS for procedural pain associated with venipuncture (Coyne 1995), flexible cytoscopy (Hruby 2006) and flexible sigmoidoscopy (Limoges 2004). One trial included a comparison between active TENS and placebo pill and reported a statistically significant improvement in favour of TENS (Oncel 2002).
We pooled data for pain intensity for six trials (seven comparisons) but the I2 statistic (67%) suggested substantial heterogeneity (Figure 4). The MD was ‐24.62 mm (95% CI ‐31.79 to ‐17.46; six trials, 436 participants; Analysis 1.1) in favour of TENS using a random‐effects model. We pooled data for the proportion of participants achieving ≥ 50% reduction in pain from four trials (seven comparisons). The relative risk was 3.91 (95% CI 2.42 to 6.32; four trials, 280 participants; Analysis 1.2) in favour of TENS with a NNTB of 2.49 (Figure 5). We were unable to pool other data.
4.

Forest plot of comparison: 1 TENS versus placebo TENS, outcome: 1.1 Pain intensity (100 mm VAS).
1.1. Analysis.

Comparison 1: TENS versus placebo TENS, Outcome 1: Pain intensity (100 mm VAS)
1.2. Analysis.

Comparison 1: TENS versus placebo TENS, Outcome 2: > 50% reduction in pain
5.

Forest plot of comparison: 1 TENS versus placebo TENS, outcome: 1.2 > 50% reduction in pain.
TENS versus no treatment control
Seven trials included a comparison between TENS and a no treatment control. Five trials reported an improvement in favour of TENS of at least one outcome measure at one or more time points (Amer‐Cuenca 2011; De Angelis 2003; de Sousa 2014; Keskin 2012; Pitangui 2012). de Sousa 2014 found that TENS reduced post‐partum uterine contraction pain during breast‐feeding compared with the no treatment control. De Angelis 2003 found that TENS reduced the intensity of pain during hysteroscopy compared with a no treatment control. Amer‐Cuenca 2011 reported that more patients achieved > 50% relief of pain associated with colonoscopy during TENS compared with a no treatment control. Keskin 2012 reported that the pain intensity associated with LBP during pregnancy was lower during TENS compared with a no treatment control. Pitangui 2012 found a significant reduction in resting, sitting and ambulating pain (NRS) following episiotomy immediately after TENS and 60 minutes later when compared with the control group (P < 0.001). Hruby 2006 found no significant differences between TENS and no treatment control for the intensity of pain during flexible cytoscopy. Limoges 2004 found no significant difference between TENS and no treatment control groups during screening flexible sigmoidoscopy (NRS, WMD ‐0.23 points, 95% CI ‐0.72 to 0.26, P = 0.36). We were unable to ascertain whether Coyne 1995 used a no treatment control or an unspecified 'placebo' for procedural pain associated with venipuncture. Coyne 1995 found no significant differences between TENS and the control/placebo.
We pooled data for pain intensity were pooled from five trials (seven comparisons) but the I2 statistic value (71%) suggested considerable heterogeneity (Figure 6). MD was ‐19.05 mm (95% CI ‐27.30 to ‐10.79; five trials, 473 participants; Analysis 2.1) in favour of TENS using a random‐effects model. We were unable to pool other data.
6.

Forest plot of comparison: 2 TENS versus no treatment control, outcome: 2.1 Pain intensity (100 mm VAS).
2.1. Analysis.

Comparison 2: TENS versus no treatment control, Outcome 1: Pain intensity (100 mm VAS)
TENS versus a pharmacological intervention
Four trials included a comparison between TENS and a pharmacological treatment. Three trials reported an improvement in favour of TENS of at least one outcome measure at one or more time points. Crompton 1992 reported that TENS was superior to local anaesthetic for procedural cervical laser treatment. Oncel 2002 reported that TENS was superior to NSAID for rib fractures. Keskin 2012 reported that TENS was superior to acetaminophen (2 x 500 mg/day) for LBP during pregnancy. Ordog 1987 reported that there was no difference between TENS and acetaminophen (300 to 600 mg) plus codeine (30 to 60 mg) for pain associated with acute traumatic injuries including sprains, lacerations, fractures, haematomas and contusions but did not make a direct comparison of TENS alone versus medication. We were unable to pool other data.
TENS versus a non‐pharmacological intervention
Two trials included a comparison between TENS and a non‐pharmacological treatment. Keskin 2012 reported that TENS produced greater pain relief than exercise for LBP during pregnancy. Ekblom 1987 reported that there were no differences in pain relief between TENS and vibration for acute orofacial pain. We were unable to pool data.
Conventional TENS versus AL‐TENS
Two trials included a comparison between conventional and AL‐TENS. Hansson 1983 and Ekblom 1987 reported that there were no significant differences in the proportion of participants achieving > 50% reduction in orofacial pain between high frequency, low intensity (conventional) TENS (100 Hz, intensity of two to three times perception threshold) and low frequency, high intensity (acupuncture‐like) TENS (2 Hz pulse train, intensity of three to five times perception threshold). We pooled data for the proportion of participants achieving ≥ 50% reduction in pain. The relative risk was 0.72 (95% CI 0.37 to 1.39; two trials, 64 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: Conventional TENS versus AL‐TENS, Outcome 1: > 50% reduction in pain
High pulse amplitude TENS versus low pulse amplitude TENS
Two trials included a comparison between high and low pulse amplitude TENS. Olsén 2007 reported that high intensity (50 mA) high frequency (70 to 100 Hz) TENS produced a larger decrease in the intensity of pain associated with postpartum uterine contractions than low intensity (15 mA) high frequency (70 to 100 Hz) TENS just above sensory detection threshold. The trial authors reported a significantly higher number of participants reported discomfort with the higher pulse amplitudes (P < 0.01). Liu 1985 delivered active TENS at a "subjective level of comfort" with a mean ± SD amplitude of 5.86 ± 0.96 mA across the sample. For this analysis we interpret this 'high pulse amplitude'. They also administered 'control' TENS fixed at 2.5 mA as they believed that this was a more valid control than a no stimulus placebo. For the purposes of this Cochrane Review, we treated this as low pulse amplitude TENS rather than placebo TENS. Liu 1985 found no significant differences between these two pulse amplitudes (VAS, WMD ‐1.53 cm, 95% CI ‐3.37 to 0.31; P = 0.1). In addition, De Angelis 2003 measured pain intensity (VAS) during hysteroscopy in one group of participants and reported that pain was reduced to a greater extent when participants increased pulse amplitude and associated intensity by pressing a 'plus' switch on the device. MD for these latter two trials was ‐23.47mm (95% CI ‐29.60 to ‐17.34) in favour of high TENS using a random‐effects model (Analysis 4.1).
4.1. Analysis.

Comparison 4: High pulse amplitude TENS versus low pulse amplitude TENS, Outcome 1: Pain intensity (100 mm VAS)
Secondary objectives
Insufficient extractable data meant that it was not possible to perform any planned subgroup analysis for any secondary objectives. We were unable to determine whether TENS effectiveness was influenced by the time of recording the outcome measure, i.e. during TENS measurement compared to post TENS measurement, or to compare the duration of TENS treatment or comparisons for different acute pain conditions.
Discussion
Summary of main results
This updated Cochrane Review examined the effectiveness of TENS as a sole intervention for the treatment of acute pain in adults. We retrieved 1065 reports from literature searches for this update, in addition to the 1775 reports identified for the 2011 update. We included seven new trials and 11 studies are awaiting classification. Thus, 19 RCTs involving 1346 participants at entry met the inclusion criteria. We were able to extract data from 13 of the 19 included trials (Amer‐Cuenca 2011; Cheing 2005; De Angelis 2003; de Sousa 2014; Ekblom 1987; Hansson 1983; Kim 2012; Limoges 2004; Liu 1985; Olsén 2007; Oncel 2002; Ordog 1987; Roche 1985). Eight of 11 trial reports with a placebo TENS comparison identified a statistically significant improvement in favour of TENS of at least one outcome measures at one or more measurement time point. Pooled data from six trials found a MD of ‐24.62 mm (95% CI ‐31.79 to ‐17.46) on 100mm VAS in favour of TENS. Pooled data from four trials (seven comparisons) found a relative risk of 3.91 (95% CI 2.42 to 6.32), in favour of TENS with a NNTB of 2.49 for the proportion of participants achieving ≥ 50% reduction in pain. The NNTB is remarkably low and most likely to have been exaggerated by the high risk of bias associated with small sample sizes and various other biases as highlighted in the risk of bias analysis. We do not attribute statistical credibility to the effect sizes because of statistical heterogeneity but it is noteworthy that the direction of effect is consistent.
Five of the seven trial reports with a no treatment control identified an improvement in favour of TENS in at least one outcome measure at one or more time point. Pooled data from five studies produced a MD of ‐19.05mm (95% CI ‐27.30 to ‐10.79) in favour of TENS. Three out of four trials that compared TENS with an analgesic drug and one out of two studies that compared TENS with a non‐pharmacological treatment found an improvement in favour of TENS of at least one outcome measure at one or more measurement time point. Three trials included a comparison between high and low pulse amplitude and all found that higher pulse amplitudes were superior. This finding is consistent with recent experimental pain studies that indicated high pulse amplitude (irrespective of the applied frequency) is the key parameter for effective TENS applications (Aarskog 2007; Chen 2008; Claydon 2008). Furthermore, a meta‐analysis of TENS for postoperative pain by Bjordal 2003 highlighted the relevance of optimal (strong or maximal non‐painful) intensity levels for pain relief in this clinical population. There were no differences in the proportion of participants achieving ≥ 50% reduction in pain between conventional and AL‐TENS in the two included trials. Three trial reports indicated that there were no adverse effects and seven reports indicated a range of adverse effects that were primarily related to sensations experienced at the electrode site or the muscle contractions associated with low frequency TENS. We judged these as minor. The methodological quality of the trials varied considerably: we judged sequence generation to be adequate in ten trials, allocation concealment was adequate in three trials and only five had adequate assessor blinding. There was a high risk of bias associated with inadequate sample sizes with only two trials having sample sizes ≥ 50 per treatment arm.
Overall completeness and applicability of evidence
The range of acute pain conditions included in this review was limited by eligibility criteria that excluded trials of acute pain during childbirth and primary dysmenorrhoea because these conditions have been covered by previous Cochrane Reviews (Dowswell 2009; Proctor 2002). In addition, we excluded trials that evaluated TENS in combination with any other treatment as part of the formal trial design (e.g. analgesic medication, exercise) on the basis that addition of another treatment would compromise pain relief measures making it impossible to ascertain the contribution of TENS. The highest number of excluded trials were on postoperative pain as they gave analgesic medication in addition to TENS for pain management. The effect of TENS in combination with other treatments for acute pain is the subject for another systematic review. We categorised the 19 included trials into procedural and non‐procedural pain but were unable to pool data for subgroup analyses. All trials were in the English language with most based in Europe. Only one trial described the use of TENS by the participants solely at home (Ordog 1987). As TENS can easily be self‐applied for most conditions, this limits the evidence for comparison of self‐applied versus therapist‐applied TENS. The range of outcome measures used provided limited data that could be extracted from the included trials.
The reporting of TENS treatments showed wide variations across the included trials. Several trials failed to report full details of the TENS parameters used or technique of application, thus making replication impossible. Attempts to combine outcomes in a meta‐analysis were undermined by substantial heterogeneity, a lack of available data, and a lack of specific information on procedures used to measure pain scores, especially whether scores were taken for present pain or retrospective pain, during or after TENS. This seriously limits the interpretation of the results. Both experimental pain and clinical studies suggest that maximum pain relief is obtained while TENS is switched on (Johnson 1991; Johnson 1999; Tong 2007). Thus the timing of pain measurement is crucial, particularly for procedural pain; some included trials measured pain post procedure but asked participants to record 'during procedure' pain thus relying on recall (De Angelis 2003; Limoges 2004). Often it was impossible to ascertain the exact instruction given to participants about the nature of the pain score required. As TENS has been shown to have maximum pain relieving effects during application, it is important to record pain outcome whilst it is being applied. Few trials continued to record the effect of TENS on pain outcome for more than a few days thus limiting any conclusions regarding the duration of effect of TENS on acute pain.
Quality of the evidence
The 19 included trials involved 1346 participants at entry. In general, the quality of the evidence was weak due to inadequate methods or lack of information on: allocation concealment; blinding of the outcome assessors; incomplete outcome data; and method of analysis (per protocol or ITT). There was a high risk of bias from inadequate sample sizes. Sample sizes ranged from eight to 71 per group and nine trials had fewer than 20 participants in each treatment arm. Only three trials had a prospective sample size calculation. Blinding participants to active TENS is challenging because treatment necessitates a perceptual experience (i.e. TENS sensation) yet investigators should make every attempt to introduce uncertainty about which treatment arm is active through carefully worded pre‐trial instructions. TENS naïvety is an important inclusion criteria in trials attempting to blind participants. Only four of the trials that compared TENS to placebo used participants that were TENS naïve. Typically placebo TENS was administered using a sham TENS device with no electrical output and no perceptual experience and this can be a credible approach to achieve at least partial blinding (Deyo 1990). However, there was no attempt in included trials to monitor the success or otherwise of blinding using an assessment tool, such as that developed by Deyo 1990. Rakel 2010 developed and tested a new sham TENS device that delivered a current for 30 seconds, which then declined in amplitude to 0 mA over 15 seconds. This output allowed the clinician to set the pulse amplitude without knowing if the unit was an active or sham device. Thus, the method of delivery of treatment by the clinician was identical for each participant and this type of sham TENS device may be useful for future trials. Hrobjartsson 2007 highlighted this issue of monitoring blinding in RCTs and analysed a random sample of 1599 blinded RCTs indexed in CENTRAL and found that only 2% of trials included tests for the success of blinding.
Potential biases in the review process
Review authors were not blinded from authors' names, institutions and journal name or trial results at any stage of the review process. However, pairs of review authors undertook each stage of the review process independently and we compared the outcomes.
Agreements and disagreements with other studies or reviews
Cochrane Reviews on TENS for specific types of acute pain have been inconclusive for labour pain (Dowswell 2009) and dysmenorrhoea (Proctor 2002).
Authors' conclusions
Implications for practice.
In this update we identified seven additional trials to the 12 trials reviewed in 2011. The analysis of 19 RCTs with 1346 participants provides tentative evidence that TENS reduces pain intensity over and above that seen with placebo (no current) TENS when administered as a stand‐alone treatment for acute pain in adults. However, the high risk of bias associated with inadequate sample sizes in treatment arms and unsuccessful blinding of treatment interventions makes definitive conclusions impossible. The additional analyses conducted in this second update strengthen evidence presented in Walsh 2009. Whether TENS should be considered as a potential treatment option for patients and clinicians managing acute pain remains a matter for debate, although TENS compares favourably to many alternatives because it can be self‐administered, safe, inexpensive and readily available to patients over the counter.
Implications for research.
There was incomplete reporting of treatment in many reports, making replication of trials impossible. Further adequately powered research trials are required to provide a comprehensive assessment of the role of TENS as a sole treatment in acute pain management. Bennett 2011 has provided criteria and operational guidelines for the design of a robust RCT on TENS. PaPaS guidance suggests that a sample size of ≥ 200 participants per treatment arm is necessary for a low risk of bias in RCTs. The Consolidated Standards of Reporting Trials (CONSORT) statement has been revised for non‐pharmacological treatments (Boutron 2008); this should be adopted to ensure better reporting of all aspects of trial design and subsequent reporting. In particular, appropriate sequence generation and allocation concealment methods should be used and reported. Sample size calculations should be performed to determine appropriate participant numbers. Complete details of the TENS application should be provided to allow subgroup analysis between trials. Appropriate TENS technique should be used including a strong non‐painful TENS sensation at the site of pain. A clear description of missing data and how they are analysed is required. Outcome assessor blinding should be adopted as a key element of future trial design. Blinding of participants is accepted as a challenge in TENS trials but should be addressed nevertheless. Finally, future trials should adopt a common policy of reporting means and SDs for continuous data to enable data extraction for subsequent meta‐analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 3 February 2021 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 12 June 2015 | Review declared as stable | At 2015, the authors and editors agreed to reassess this review for further updating in 2020. |
| 20 November 2014 | New search has been performed | We updated the review using a search conducted up to 3 December 2014. |
| 17 January 2014 | New citation required but conclusions have not changed | We included seven new trials in this update. In total, there were 19 included RCTs with 1346 participants at entry, and 11 trials awaiting classification. The analysis provides tentative evidence that TENS reduces pain intensity over and above that seen with placebo (no current) TENS when administered as a stand‐alone treatment for acute pain in adults. However, there is high risk of bias associated with inadequate sample sizes in treatment arms and unsuccessful blinding of treatment interventions. This makes definitive conclusions impossible. |
| 7 January 2011 | New search has been performed | Updated search done in January 2011. No new included studies but two new studies are awaiting classification (Gregorini 2010; Rajpurohit 2010) and an additional 12 studies were assessed and excluded from this review (Akhmadeeva 2010; Andersen 2009a; Andersen 2009b; Barbarisi 2010; Dogu 2009; Durmus 2009; Gul 2009; Korkmaz 2010; Murina 2008; Stratton 2009; Tsai 2010; Wang 2009). A further 17 studies were excluded as TENS was given with another treatment (see Table 1). |
| 1 May 2008 | Amended | Protocol converted to new review format |
Notes
This review has been stabilised permanently following discussion with the authors and editors. The review is correct at the date of publication, but the question is no longer considered current and is of historical interest only. We are scoping titles for potential future reviews to update and replace this review.
Acknowledgements
We acknowledge the following individuals: Professor Deirdre Walsh (DW) was responsible for co‐ordinating the development of Walsh 2009 and the 2011 update; Dr Fidelma Moran for the 2011 update; Ms Anna Hobson, Managing Editor, Cochrane Pain Palliative and Supportive Care Review Group for her invaluable support throughout the entire process of this update; Sylvia Bickley, former Trials Search Co‐ordinator for the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group, for her assistance in writing the search strategy; Ms. Caroline Struthers and Ms Jane Hayes, Trials Search Co‐ordinators for the Cochrane PaPaS Review Group, for their assistance in the final database searches; Miss Jessica Thomas, Managing Editor, Cochrane PaPaS Review Group for her support through Walsh 2009 and the 2011 update; Mr. Phil Wiffen, Cochrane PaPaS Review Group for his invaluable advice and feedback; Mrs. Jenny Allen, Senior Library Assistant, University of Ulster for her tremendous support in retrieving inter‐library loans; Prof. Suzanne McDonough, University of Ulster for acting as local supervisor for the Cochrane Fellowship awarded to DW. We thank the following people for their roles in translating foreign language papers: Ms. Guio Garcia, University of Ulster; Dr. Jing Yuan, formerly University of Ulster; Dr. Sebastian Straube, Churchill Hospital, Oxford; Ms. Simona Vecchi, ASL RME, Italy; Ms. Anabela Silva, Leeds Beckett University; Dr. Tiina Saarto, Helsinki University Central Hospital, Finland; Dr. Richard Liebano, University of the City of São Paulo, Brazil; and Dr. Suzie Xu Wang, Leeds Beckett University.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Ovid MEDLINE search strategy
1. exp Pain/
2. Pain Measurement/
3. Pain Threshold/
4. Pain Clinics/
5. Myofascial Pain Syndromes/
6. Hyperalgesia/
7. exp Headache Disorders/
8. (toothache$ or tooth‐ache$ or ear‐ache$ or earache$ or sciatic$ or neuralgi$ or migraine$ or headache$ or neuralgi$ or cephalalgi$ or metatarsalgia$ or bursitis or hyperalg$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
9. pain$.ti.
10. pain$.ab.
11. exp Angina Pectoris/
12. angina.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
13. Metatarsalgia/
14. or/1‐13
15. exp Transcutaneous Electric Nerve Stimulation/
16. "TENS".ti.
17. "TENS".ab.
18. "TNS".ti.
19. "TNS".ab.
20. "ENS".ti.
21. "ENS".ab.
22. ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
23. ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
24. ("electric$ nerve therap$" or electroanalgesi$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
25. transcutaneous electric$ stimulation.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
26. TES.ti,ab.
27. or/15‐26
28. 14 and 27
29. RANDOMIZED CONTROLLED TRIAL.pt.
30. CONTROLLED CLINICAL TRIAL.pt.
31. RANDOMIZED CONTROLLED TRIALS.sh.
32. RANDOM ALLOCATION.sh.
33. DOUBLE BLIND METHOD.sh.
34. SINGLE BLIND METHOD.sh.
35. or/29‐34
36. (ANIMALS not HUMAN).sh.
37. 35 not 36
38. CLINICAL TRIAL.pt.
39. exp CLINICAL TRIALS/
40. (clin$ adj25 trial$).ti,ab.
41. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
42. PLACEBOS.sh.
43. placebo$.ti,ab.
44. random$.ti,ab.
45. RESEARCH DESIGN.sh.
46. or/38‐45
47. 46 not 36
48. 47 not 37
49. 37 or 48
50. 28 and 49
Appendix 2. PaPaS Specialized Register search strategy
((pain* or hyperalgesi* or headache* or migrain* or toothache or "tooth ache*" or earache or "ear ache*" or sciatic* or neuralgi* or cephalgi* or metatarsalg* or bursitis or angina) AND ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation" or "electric* nerve stimulation" or "electrostimulation therap*" or electroanalgesi* or TENS))
Appendix 3. CENTRAL (the Cochrane Library) search strategy
MeSH descriptor Pain explode all trees in MeSH products
MeSH descriptor Pain Measurement, this term only in MeSH products
MeSH descriptor Pain Threshold, this term only in MeSH products
MeSH descriptor Pain Clinics, this term only in MeSH products
MeSH descriptor Myofascial Pain Syndromes, this term only in MeSH products
MeSH descriptor Hyperalgesia, this term only in MeSH products
MeSH descriptor Headache Disorders explode all trees in MeSH products
(Toothache* or tooth‐ache* or ear‐ache* or earache* or sciatic* or neuralgi* or migrain* or headache* or neuralgi* or cephalalgia or metatarsalgia* or bursitis or hyperalg*) in All Fields in all products
pain* in Record Title in all products
pain* in Abstract in all products
MeSH descriptor Angina Pectoris explode all trees in MeSH products
angina in All Fields in all products
MeSH descriptor Metatarsalgia, this term only in MeSH products
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13)
MeSH descriptor Transcutaneous Electric Nerve Stimulation explode all trees in MeSH products
"TENS" in Record Title in all products
"TENS" in Abstract in all products
"TNS" in Record Title in all products
"TNS" in Abstract in all products
"ENS" in Record Title in all products
"ENS" in Abstract in all products
(transcutaneous next electric* next nerve next stimulation or "transcutaneous nerve stimulation" ) in All Fields in all products
("electric* nerve stimulation" or "electrostimulation therap*" ) in All Fields in all products
("electric* nerve therap*" or electroanalgesi*) in All Fields in all products
"TES" in Record Title in all products
"TES" in Abstract in all products
(transcutaneous next electric* next stimulation) in All Fields in all products
(#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27)
(#14 AND #28)
Appendix 4. Ovid EMBASE search strategy
1. exp PAIN/
2. Pain Assessment/
3. Pain Threshold/
4. Pain Clinic/
5. Myofascial Pain/
6. HYPERALGESIA/
7. exp "Headache and Facial Pain"/
8. (toothache$ or tooth‐ache$ or ear‐ache$ or earache$ or sciatic$ or neuralgi$ or migraine$ or headache$ or neuralgi$ or cephalalgi$ or metatarsalgia$ or bursitis or hyperalg$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
9. pain$.ti.
10. pain$.ab.
11. exp Angina Pectoris/
12. angina.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
13. METATARSALGIA/
14. or/1‐13
15. exp Transcutaneous Nerve Stimulation/
16. "TENS".ti.
17. "TENS".ab.
18. "TNS".ti.
19. "TNS".ab.
20. "ENS".ti.
21. "ENS".ab.
22. ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
23. ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
24. ("electric$ nerve therap$" or electroanalgesi$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
25. transcutaneous electric$ stimulation.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
26. TES.ti,ab.
27. or/15‐26
28. 14 and 27
29. random$.ti,ab.
30. factorial$.ti,ab.
31. (crossover$ or cross over$ or cross‐over$).ti,ab.
32. placebo$.ti,ab.
33. (doubl$ adj blind$).ti,ab.
34. (singl$ adj blind$).ti,ab.
35. assign$.ti,ab.
36. allocat$.ti,ab.
37. volunteer$.ti,ab.
38. CROSSOVER PROCEDURE.sh.
39. DOUBLE‐BLIND PROCEDURE.sh.
40. RANDOMIZED CONTROLLED TRIAL.sh.
41. SINGLE BLIND PROCEDURE.sh.
42. or/29‐41
43. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/
44. HUMAN/
45. 44 and 43
46. 43 not 45
47. 42 not 46
48. 28 and 47
Appendix 5. EBSCO CINAHL search strategy
1 exp PAIN/
2 PAIN MEASUREMENT/
3 PAIN CLINICS/
4 MYOFASCIAL PAIN SYNDROMES/
5 HYPERALGESIA/
6 exp HEADACHE/
7 (toothache* OR tooth‐ache* OR ear‐ache* OR earache* OR sciatic* OR neuralgi* OR migraine* OR headache* OR neuralgi* OR cephalalgi* OR metatarsalgia* OR bursitis OR hyperalg*).ti,ab
8 pain*.ti,ab
9 exp ANGINA PECTORIS/
10 angina.ti,ab
11 PAIN THRESHOLD/
12 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11
13 exp TRANSCUTANEOUS ELECTRIC NERVE STIMULATION/
14 (TENS OR TNS OR ENS).ti,ab
15 (transcutaneous AND stimulation).ti,ab
16 TES.ti,ab
17 ((electric* AND stimulation) OR electrostimulation OR electro‐stimulation).ti,ab
18 ((electric* nerve therap*) OR electroanalgesi*).ti,ab
19 13 OR 14 OR 15 OR 16 OR 17 OR 18
20 12 AND 19
21 RANDOM ASSIGNMENT/
22 SINGLE‐BLIND STUDIES/
23 DOUBLE‐BLIND STUDIES/
24 TRIPLE‐BLIND STUDIES/
25 CROSSOVER DESIGN/
26 FACTORIAL DESIGN/
27 ((multicentre OR multicenter OR multi‐centre OR multi‐center) AND stud*).ti,ab
28 random*.ti,ab
29 (latin AND square).ti,ab
30 (cross‐over OR crossover).ti,ab
31 PLACEBOS/
32 placebo*.ti,ab
33 ((singl* OR doubl* OR trebl* OR tripl*) AND (blind* OR mask*)).ti,ab
34 exp CLINICAL TRIALS/
35 (clin* AND trial*).ti,ab
36 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35
37 20 AND 36
Appendix 6. Ovid AMED search strategy
1. exp Pain/
2. Pain measurement/
3. Pain threshold/
4. PAIN CLINICS.mp.
5. Myofascial pain syndromes/
6. Hyperalgesia/
7. exp Headache/
8. (toothache$ or tooth‐ache$ or ear‐ache$ or earache$ or sciatic$ or neuralgi$ or migraine$ or headache$ or neuralgi$ or cephalalgi$ or metatarsalgia$ or bursitis or hyperalg$).mp. [mp=title, subject heading word, abstract, instrumentation]
9. pain$.ti.
10. pain$.ab.
11. exp angina pectoris/
12. angina.mp. [mp=title, subject heading word, abstract, instrumentation]
13. Metatarsalgia/
14. or/1‐13
15. exp Transcutaneous electric nerve stimulation/
16. "TENS".ti.
17. "TENS".ab.
18. "TNS".ti.
19. "TNS".ab.
20. "ENS".ti.
21. "ENS".ab.
22. ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp. [mp=title, subject heading word, abstract, instrumentation]
23. ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp. [mp=title, subject heading word, abstract, instrumentation]
24. ("electric$ nerve therap$" or electroanalgesi$).mp. [mp=title, subject heading word, abstract, instrumentation]
25. transcutaneous electric$ stimulation.mp. [mp=title, subject heading word, abstract, instrumentation]
26. TES.ti,ab.
27. or/15‐26
28. 14 and 27
29. RANDOMIZED CONTROLLED TRIAL.pt.
30. CONTROLLED CLINICAL TRIAL.pt.
31. RANDOMIZED CONTROLLED TRIALS.sh.
32. RANDOM ALLOCATION.sh.
33. DOUBLE BLIND METHOD.sh.
34. "single blind method".mp. [mp=title, subject heading word, abstract, instrumentation]
35. or/29‐34
36. (ANIMALS not HUMANS).sh.
37. 35 not 36
38. CLINICAL TRIAL.pt.
39. exp CLINICAL TRIALS/
40. (clin$ adj25 trial$).ti,ab.
41. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
42. PLACEBOS.sh.
43. placebo$.ti,ab.
44. random$.ti,ab.
45. RESEARCH DESIGN.sh.
46. or/38‐45
47. 46 not 36
48. 47 not 37
49. 37 or 48
50. 28 and 49
Appendix 7. PEDro search strategy
Abstract & Title:"electrical stimulation" pain
Therapy: electrotherapies, heat and cold
Problem: pain
Method: Clinical Trial
Note: check “match all search terms”
Appendix 8. OTseeker search strategy
Keywords: electrical stimulation
Methods: clinical trial
Appendix 9. OpenSIGLE search strategy
((pain OR toothache* OR tooth‐ache* OR ear‐ache* OR earache* OR sciatic* OR neuralgi* OR migraine* OR headache* OR neuralgi* OR cephalalgi* OR metatarsalgia* OR bursitis OR hyperalg* OR myofascial OR angina*) AND (transcutaneous electric nerve stimulation OR tens OR tns OR ens OR transcutaneous electric* OR transcutaneous nerve stimulation OR electric* nerve stimulation OR electrostimulation therap* OR electro‐stimulation therap* OR electro‐stimulation OR electrostimulation OR electric* nerve therap* OR electroanalgesi*))
Appendix 10. Search strategies for 2014 update
CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Pain] explode all trees
#2 MeSH descriptor: [Pain Measurement] this term only
#3 MeSH descriptor: [Pain Threshold] this term only
#4 MeSH descriptor: [Pain Clinics] this term only
#5 MeSH descriptor: [Myofascial Pain Syndromes] this term only
#6 MeSH descriptor: [Hyperalgesia] this term only
#7 MeSH descriptor: [Headache Disorders] explode all trees
#8 (toothache* or tooth‐ache* or ear‐ache* or earache* or sciatic* or neuralgi* or migraine* or headache* or neuralgi* or cephalalgi* or metatarsalgia* or bursitis or hyperalg*):ti,ab,kw (Word variations have been searched)
#9 pain*:ab or pain*:ti (Word variations have been searched)
#10 MeSH descriptor: [Angina Pectoris] explode all trees
#11 angina:ti,ab,kw (Word variations have been searched)
#12 MeSH descriptor: [Metatarsalgia] this term only
#13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 MeSH descriptor: [Transcutaneous Electric Nerve Stimulation] explode all trees
#15 ("TENS" or "TNS" or "ENS" or "TES"):ti,ab,kw (Word variations have been searched)
#16 ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation"):ti,ab,kw (Word variations have been searched)
#17 ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*"):ti,ab,kw (Word variations have been searched)
#18 ("electric* nerve therap*" or electroanalgesi*):ti,ab,kw (Word variations have been searched)
#19 "transcutaneous electric* stimulation":ti,ab,kw (Word variations have been searched)
#20 #14 or #15 or #16 or #17 or #18 or #19
#21 #13 and #20 from 2011 to 2014
MEDLINE (OVID) & Medline In‐Process (OVID)
1. exp Pain/
2. Pain Measurement/
3. Pain Threshold/
4. Pain Clinics/
5. Myofascial Pain Syndromes/
6. Hyperalgesia/
7. exp Headache Disorders/
8. (toothache$ or tooth‐ache$ or ear‐ache$ or earache$ or sciatic$ or neuralgi$ or migraine$ or headache$ or neuralgi$ or cephalalgi$ or metatarsalgia$ or bursitis or hyperalg$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
9. pain$.ti.
10. pain$.ab.
11. exp Angina Pectoris/
12. angina.mp.
13. Metatarsalgia/
14. or/1‐13
15. exp Transcutaneous Electric Nerve Stimulation/
16. ("TENS" or "TNS" or "ENS").ti.
17. ("TENS" or "TNS" or "ENS").ab.
18. ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp.
19. ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp.
20. ("electric$ nerve therap$" or electroanalgesi$).mp.
21. transcutaneous electric$ stimulation.mp.
22. TES.ti,ab.
23. or/15‐22
24. 14 and 23
25. randomized controlled trial.pt.
26. controlled clinical trial.pt.
27. randomized.ab.
28. placebo.ab.
29. drug therapy.fs.
30. randomly.ab.
31. trial.ab.
32. or/25‐31
33. exp animals/ not humans.sh.
34. 32 not 33
35. 24 and 34
36. (2011* or 2012* or 2013* or 2014*).ed.
37. 35 and 36
EMBASE (OVID)
1. exp Pain/
2. Pain Measurement/
3. Pain Threshold/
4. Pain Clinics/
5. Myofascial Pain Syndromes/
6. Hyperalgesia/
7. exp Headache Disorders/
8. (toothache$ or tooth‐ache$ or ear‐ache$ or earache$ or sciatic$ or neuralgi$ or migraine$ or headache$ or neuralgi$ or cephalalgi$ or metatarsalgia$ or bursitis or hyperalg$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
9. pain$.ti.
10. pain$.ab.
11. exp Angina Pectoris/
12. angina.mp.
13. Metatarsalgia/
14. or/1‐13
15. exp Transcutaneous Electric Nerve Stimulation/
16. ("TENS" or "TNS" or "ENS").ti.
17. ("TENS" or "TNS" or "ENS").ab.
18. ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp.
19. ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp.
20. ("electric$ nerve therap$" or electroanalgesi$).mp.
21. transcutaneous electric$ stimulation.mp.
22. TES.ti,ab.
23. or/15‐22
24. 14 and 23
25. random$.tw.
26. factorial$.tw.
27. crossover$.tw.
28. cross over$.tw.
29. cross‐over$.tw.
30. placebo$.tw.
31. (doubl$ adj blind$).tw.
32. (singl$ adj blind$).tw.
33. assign$.tw.
34. allocat$.tw.
35. volunteer$.tw.
36. Crossover Procedure/
37. double‐blind procedure.tw.
38. Randomized Controlled Trial/
39. Single Blind Procedure/
40. or/25‐39
41. (animal/ or nonhuman/) not human/
42. 40 not 41
43. 24 and 42
AMED (OVID)
1. exp Pain/
2. Pain Measurement/
3. Pain Threshold/
4. Pain Clinics/
5. Myofascial Pain Syndromes/
6. Hyperalgesia/
7. (toothache$ or tooth‐ache$ or ear‐ache$ or earache$ or sciatic$ or neuralgi$ or migraine$ or headache$ or neuralgi$ or cephalalgi$ or metatarsalgia$ or bursitis or hyperalg$).mp. [mp=abstract, heading words, title]
8. pain$.ti.
9. pain$.ab.
10. exp Angina Pectoris/
11. angina.mp.
12. Metatarsalgia/
13. (or/1‐6) or 7 or 8 or 9 or 10 or 11 or 12
14. exp Transcutaneous Electric Nerve Stimulation/
15. ("TENS" or "TNS" or "ENS").ti.
16. ("TENS" or "TNS" or "ENS").ab.
17. ("transcutaneous electric$ nerve stimulation" or "transcutaneous nerve stimulation").mp.
18. ("electric$ nerve stimulation" or "electrostimulation therap$" or "electro‐stimulation therap$").mp.
19. ("electric$ nerve therap$" or electroanalgesi$).mp.
20. transcutaneous electric$ stimulation.mp.
21. TES.ti,ab.
22. or/14‐21
23. 13 and 22
CINAHL (EBSCO)
S32 S30 AND S31
S31 EM 20110101‐20141231
S30 S20 AND S29
S29 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
S28 (allocat* random*)
S27 (MH "Quantitative Studies")
S26 (MH "Placebos")
S25 placebo*
S24 (random* allocat*)
S23 (MH "Random Assignment")
S22 (Randomi?ed control* trial*) Limiters ‐ Published Date: 20090101‐20130231
S21 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S20 S12 AND S19
S19 S13 OR S14 OR S15 OR S16 OR S17 OR S18
S18 "transcutaneous electric* stimulation"
S17 ("electric* nerve therap*" or electroanalgesi*)
S16 ("electric* nerve stimulation" or "electrostimulation therap*" or "electro‐stimulation therap*")
S15 ("transcutaneous electric* nerve stimulation" or "transcutaneous nerve stimulation")
S14 ("TENS" or "TNS" or "ENS" or "TES")
S13 (MH "Transcutaneous Electric Nerve Stimulation")
S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
S11 (MH "Metatarsalgia")
S10 angina
S9 (MH "Angina Pectoris+")
S8 TI pain* OR AB pain*
S7 (toothache* or tooth‐ache* or ear‐ache* or earache* or sciatic* or neuralgi* or migraine* or headache* or neuralgi* or cephalalgi* or metatarsalgia* or bursitis or hyperalg*)
S6 (MH "Hyperalgesia")
S5 (MH "Myofascial Pain Syndromes")
S4 (MH "Pain Clinics")
S3 (MH "Pain Threshold")
S2 (MH "Pain Measurement")
S1 (MH "Pain+")
Data and analyses
Comparison 1. TENS versus placebo TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain intensity (100 mm VAS) | 6 | 436 | Mean Difference (IV, Random, 95% CI) | ‐24.62 [‐31.79, ‐17.46] |
| 1.2 > 50% reduction in pain | 4 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [2.42, 6.32] |
Comparison 2. TENS versus no treatment control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain intensity (100 mm VAS) | 5 | 473 | Mean Difference (IV, Random, 95% CI) | ‐19.05 [‐27.30, ‐10.79] |
Comparison 3. Conventional TENS versus AL‐TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 > 50% reduction in pain | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.39] |
Comparison 4. High pulse amplitude TENS versus low pulse amplitude TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain intensity (100 mm VAS) | 2 | 172 | Mean Difference (IV, Random, 95% CI) | ‐23.47 [‐29.60, ‐17.34] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amer‐Cuenca 2011.
| Study characteristics | ||
| Methods |
Type of study: double‐blind, placebo controlled RCT. Condition and number of participants randomised: 90 participants attending for unsedated colonoscopy were randomised. Groups: TENS group 30; placebo TENS group 30; no‐treatment control group 30. |
|
| Participants |
Demographics: N = 90, mean age 50.2 years, TENS group mean age 49.5 years ± 2.4, 14F/16M; placebo group mean age 51.3 years ± 2.5, 19 female/11 male; control group mean age 49.9 years ± 2.4, 17 female/13 male. Setting: outpatients. Inclusion: attending unsedated screening colonoscopy, ASA I or II status, age > 18, no visual or hearing impairments, no neuropsychiatric disorders. Exclusion: refusal to consent, non‐Spanish speakers, colonic resection or stenosis of the colon, previous experience of TENS, cutaneous damage on application sites, pacemaker or cardiac defibrillator. Withdrawals/dropouts: no withdrawals. |
|
| Interventions |
Where applied: in hospital. Applied by: not stated. Waveform: not stated. Frequency: 80 to 100 Hz. Pulse duration: 400 μs. Pulse amplitude/Intensity: adjusted to the maximum sensory level without muscle contraction. Placebo Group: procedures identical to those for TENS group, except that a sham unit was used. Internal circuit of the sham TENS unit disconnected but the indicator lamp lit when unit switched on. All participants told that they might or might not feel a tingling sensation during treatment (Rx). Electrodes: 2 rectangular autoadhesive electrodes, 7 cm x 13 cm, applied parallel to the lumbo‐sacral spine. Duration and frequency of Rx: for the duration of the procedure. Device/manufacturer: Intellect Advance (Chattanooga) Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: VAS, Likert Scale. ITT/per protocol analysis: statistical analysis done according to ITT. Statistical analysis: Intergroup and intragroup differences calculated using one‐way ANOVA for continuous variables, followed by Tukey's post‐hoc test and Chi² test for proportional variables. Mean pain intensity VAS scores were no different from placebo and control groups at 5 minutes. The active TENS group was significantly different at 5 minutes when compared against placebo or control groups (P < 0.001). At the end of the procedure the TENS group VAS scores were significantly lower than the other two groups (P < 0.001) The differences between the placebo and control groups were not significant at 5 minutes and at the end. Spearman's correlation coefficient between the VAS and Likert scales was performed. There were significant differences when the TENS group was compared with either the placebo or the control groups. The scores were significantly lower in the TENS group compared with the other two groups (P = 0.009). There was a strong correlation between VAS and Likert scales in measuring pain at both 5 minutes and at the end of the procedure (P < 0.001). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation with stratification for gender. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not applicable ‐ no withdrawals or dropouts. |
| Source of funding bias | Low risk | No funding bias apparent. |
| Blinding (Participant) | Low risk | TENS and Placebo participants blinded, medics blinded. |
| Blinding (Outcome Assessor) | Low risk | Blinded assessor. |
| Sample Size | High risk | N = 30 per treatment arm. |
Cheing 2005.
| Study characteristics | ||
| Methods |
Type of study: double blind, placebo‐controlled, parallel RCT. Condition and number of participants randomised: clinical diagnosis of hypersensitive hands due to peripheral nerve injuries (N = 19). Groups: TENS group (N = 10); placebo group (N = 9). |
|
| Participants |
Demographics: N = 19, mean 35 yrs, range 15 to 58 yrs, 16 male/3 female. TENS group, 32 ± 11 yrs; placebo group, 38 ± 13 yrs (mean ± SD). Setting: outpatients. Inclusion: people who complained of hypersensitive hands within or adjacent to the site of the injury, and who were able to complete the VAS independently. Exclusion: people who had general manifestations of pain as seen in causalgia or shoulder‐hand syndrome; people who had received any TENS or undergone a desensitization programme 1 month prior to the trial; cardiac pacemaker or who had experienced sensory loss in their hands prior to the trial. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in hospital. Applied by: presume by clinician. Waveform: square pulses. Frequency: 100 Hz. Pulse duration: 200 μs. Pulse amplitude/Intensity: adjusted to produce a tingling sensation that was strong but tolerable. Placebo Group: procedures identical to those for TENS group, except that a sham unit was used. Internal circuit of the sham TENS unit disconnected but the indicator lamp lit when unit switched on. All participants told that they might or might not feel a tingling sensation during Rx. Electrodes: 2 rectangular carbon rubber electrodes with gel, 2 cm x 3 cm, anode applied directly over the hypersensitive area and cathode placed proximally along the distribution of the same peripheral nerve. Duration and frequency of Rx: 20 mins, 10 Rxs. Device/manufacturer: 120Z TENS unit (ITO, Tokyo). Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: pain intensity using VAS for a brush‐evoked stimulus with a toothbrush. Recorded before Rx on days 1, 4, 7 and 11. ITT/per protocol analysis: not detailed. Statistical analysis: no evaluable data for this review as mixed age population (adults and children). Significantly lower pain scores were found in the TENS group than in the placebo group by Day 7 and Day 11. Both groups demonstrated significant decreases in VAS scores across treatment sessions. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were matched by age, history of developing hypersensitivity and baseline VAS scores, and then randomly assigned into either the TENS (n = 10) or placebo group (n = 9) by drawing lots". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. "All subjects were blind to group allocation. The placebo group had received no active treatment (just placebo TENS) throughout the trial. The treatment procedures for the placebo group were identical to those for the real TENS group, except that a sham unit was used. The appearance of the sham unit was identical to that of a real TENS unit, but the internal circuit of the sham TENS unit was disconnected. When the machine was switched on, there was no output of current, but the indicator lamp lit up. All subjects were told that they might or might not feel a tingling sensation during the treatment". "People who had received any TENS" was an exclusion criteria. |
| Blinding (Outcome Assessor) | Low risk | "The blinded assessor repeatedly practiced applying the same brushing force on a digital balance prior to the study". |
| Sample Size | High risk | TENS (N =10); placebo (N = 9). |
Coyne 1995.
| Study characteristics | ||
| Methods |
Type of study: double blind, placebo‐controlled, parallel RCT. Condition and number of participants randomised: procedural IV needlestick pain in blood donors, 71. Groups: TENS group (N = 19); placebo TENS group (N = 21); control group (N = 21), these are numbers after 10 participants were dropped due to not meeting Virginia Blood Service criteria for blood donation. |
|
| Participants |
Demographics: N = 71 randomised, 26 male/35 female post dropout. TENS group, 36 yrs; placebo TENS group, 37 yrs; control group, 35 yrs (mean). Setting: blood donor clinic. Inclusion: blood donors meeting Virginia Blood Service criteria for donation; previous IV insertion; no previous TENS exposure; upper extremity exposure for electrode placement; appropriate consent obtained; having venipuncture to the right or left antecubital site. Exclusion: not detailed. Withdrawals/dropouts: 10 participants were dropped as they did not meet the Virginia Blood Service criteria for blood donation. |
|
| Interventions |
Where applied: in clinic. Applied by: clinician. Waveform: balanced and biphasic. Frequency: 160 pulses/s. Pulse duration: 310 to 400 μs on the strength‐duration mode. Pulse amplitude/Intensity: below the participant’s pain threshold, adjusted during stimulation to maintain this level. Placebo TENS Group: TENS unit without batteries. Control Group: no treatment. Electrodes: 4 carbon electrodes, 4 cm, applied at site of venipuncture in a square fashion. Duration and frequency of Rx: min 12 mins and max 32 mins, 1 Rx. Device/manufacturer: Maxima III TENS unit. Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: pain assessed by a subjective and an affective VAS. Recorded before intravenous (IV) insertion, after Rx, and at end of needle insertion phase. ITT/per protocol analysis: per protocol. Statistical analysis: no evaluable data for this review as unable to extract data from paper. No significant difference among groups for sensory or affective VAS scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "It was a convenient sample of 71 volunteer donors from the Virginia Blood Service who were randomized into one of the following three groups". Author response "a randomization table was how the participants were selected as participants arrived and consented to the trial". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "However, ten subjects were dropped because they did not meet the Virginia Blood Service criteria for blood donation (i.e. low haemoglobin)". |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. "No previous TENS exposure" was an inclusion criteria. Author responded "both were blinded" to the question "who was blinded, was it the patient and person recording VAS?" Author response: "TENS unit without batteries were the sham". Control group received no treatment so these participants could not be blinded. |
| Blinding (Outcome Assessor) | Low risk | Author responded "both were blinded" to the question "who was blinded, was it the patient and person recording VAS?". |
| Sample Size | High risk | TENS (N = 19); placebo (N = 21); control (N = 21). |
Crompton 1992.
| Study characteristics | ||
| Methods |
Type of study: parallel RCT. Condition and number of participants randomised: women undergoing cervical laser treatment (N = 100). Two participants were excluded from analysis because they failed to record pain score or found treatment too uncomfortable. Groups: TENS group (N = 34); local anaesthetic group (N = 35); TENS and local anaesthetic group (N = 29). NB 10 more participants recruited than initially intended as researchers lost count of number recruited and failed to stop the trial. |
|
| Participants |
Demographics: N = 100, all female. TENS group, 31.8 ± 9 yrs; local anaesthetic group, 32.6 ± 9 yrs; TENS and local anaesthetic group, 30.1 ± 8 yrs (mean ± SD). Setting: colposcopy unit. Inclusion: colposcopic diagnosis of cervical intra‐epithelial neoplasia (CIN). Exclusion: past history of treatment for CIN; other cervical surgery or pelvic inflammatory disease; postmenopausal women; cardiac pacemakers. Withdrawals/dropouts: 1 woman excluded as she failed to record pain score. Another found treatment too uncomfortable so direct local infiltration was added. |
|
| Interventions |
Where applied: in hospital. Applied by: clinician. Waveform: not detailed. Frequency: 80 Hz. Pulse duration: 210 μs. Pulse amplitude/Intensity: activated by participants under instruction, told to increase it until it became uncomfortable. Electrodes: 4, conductive silicone polymer electrodes and gel, size not detailed. 2 applied anteriorly to abdominal wall just above symphysis pubis, and 1 on each side of sacrum. Duration and frequency of Rx: participants given approximately 20 min to experiment with TENS until they were called into another room for laser treatment. Duration of TENS during laser treatment not detailed, 1 Rx. Device/manufacturer: Microtens (Neen Pain Management, UK). Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: pain assessed by a VAS after the procedure. After procedure, participants asked to complete questionnaire on TENS, one question was "did they find TENS pain relieving?". ITT/per protocol analysis: ITT. Statistical analysis: no evaluable data for this review as data presented as medians and IQ ranges. Median pain score for TENS group was significantly higher than that for local anaesthetic. Combining TENS with local anaesthesia did not further reduce the median pain score. 51 women who used TENS completed questionnaire: of the coherent responses 75% thought it was pain relieving. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Suitable subjects were then allocated to one of the following three groups according to a block randomised code". It is unclear how this code was generated. |
| Allocation concealment (selection bias) | Unclear risk | "The block randomisation code was held by one investigator who then allocated treatment. The nurses, clerical officers responsible for the computerized appointments, and the laser surgeon did not have access to this code". It is unclear how this code was kept concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "One woman was excluded because she failed to record pain score. Another found the treatment too uncomfortable and therefore direct local infiltration was added". No indication what group these individuals were randomised to. "Fifty‐one women who used TENS completed the questionnaire. Six responses were incoherent and nine women claimed the treatment was not painful and they did not need to turn the TENS on". No indication what group these individuals were randomised to. |
| Source of funding bias | Low risk | "We are indebted to Roy Sherlock of Neen Pain Management Systems (Old Pharmacy Yard, Church Street, Dereham, Norfolk NR16 1DJ) for lending us the TENS units". |
| Blinding (Participant) | High risk | "As it is impossible to conceal the use of TENS from the attendants a sham instrument was not used in group 3". Groups were: TENS group; local anaesthetic group; TENS and local anaesthetic group. There was no placebo group. |
| Blinding (Outcome Assessor) | Unclear risk | No details provided. |
| Sample Size | High risk | TENS (N = 24); local anaesthetic (N = 35); TENS and local anaesthetic (N = 29). |
De Angelis 2003.
| Study characteristics | ||
| Methods |
Type of study: parallel RCT. Condition and number of participants randomised: participants undergoing office hysteroscopy, N = 142. Groups: TENS group (N = 71); control group (N = 71). |
|
| Participants |
Demographics: N = 142, all female. TENS group, 47.9 ± 10 yrs; control group, 50 ± 10 yrs (mean ± SD). Setting: gynaecological endoscopy centre. Inclusion: outpatient hysteroscopy. Exclusion: not detailed. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in hospital. Applied by: clinician. Waveform: symmetric rectangular biphasic waveform. Frequency: 100 pulses/s. Pulse duration: 100 μs. Pulse amplitude/Intensity: device set at basal level of stimulation, participant felt mild tickle in area between electrodes. Participant instructed when she felt pain to gently press plus switch once or several times. If feeling was unpleasant she could reduce amplitude by pressing minus switch until discomfort disappeared. Control Group: no TENS applied. Electrodes: 2, type and size not detailed, on abdomen in middle of line joining iliac spine and pubic tubercle. Duration and frequency of Rx: during procedure, 1 Rx. Device/manufacturer: Freelady TENS, Life Care, Tiberias, Israel. Adverse effects: nausea, shoulder pain and dizziness reported in both groups, not specifically linked to TENS. |
|
| Outcomes |
Pain outcome: pain experienced during procedure assessed by VAS, after procedure. For TENS group, pain at basal level of stimulation was compared with pain felt after participant increased amplitude at least once. ITT/per protocol analysis: not detailed. Statistical analysis: no evaluable data for this review as unclear if SD data are presented. Significantly lower pain experienced during procedure by TENS group vs control group. Within TENS group, pain at basal level of stimulation vs after participants had increased amplitude at least once was significantly higher. Pelvic pain evaluated 5 mins after examination ‐ significant reduction in TENS group vs control group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomised, computer‐generated list was used to divide the subjects into two equal groups (A and B) of 71 patients". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. No details provided. Groups were TENS group and no treatment control group. There was no placebo group. As the control group received no treatment, these participants could not be blinded. |
| Blinding (Outcome Assessor) | Unclear risk | No details provided. |
| Sample Size | Unclear risk | TENS (N =71); control (N =71). |
de Sousa 2014.
| Study characteristics | ||
| Methods |
Type of study: RCT. Condition and number of participants randomised: 32 post‐partum multiparous women were randomised. Groups: TENS group (N = 16); no‐treatment control group (N = 16). |
|
| Participants |
Demographics: N = 32, mean age 26.84 ± 5.14 years. Setting: hospital. Inclusion: aged over 18 years, without post‐partum complications, exclusively breastfeeding, who experienced uterine contraction pain while breast‐feeding. The women were also literate and able to understand the pain rating scales used. Exclusion: intolerance to the stimulus generated by TENS or complications requiring medical intervention, such as haemorrhage and infection. Withdrawals/dropouts: no withdrawals were reported. |
|
| Interventions |
Where applied: in hospital. Applied by: not stated. Waveform: asymmetrical. Frequency: 100 Hz. Pulse duration: 75 μs. Pulse amplitude/Intensity: adjusted strong and tolerable sensation without muscular contraction. Control Group: no TENS administered. Electrodes: four 5 x 3 cm silicone and carbon rubber electrodes. Two electrodes were placed in parallel in the T10–L1 region; the other two were placed in the S2–S4 region. Duration and frequency of Rx: 40 mins. Device/manufacturer: KW Indústria Nacional de Tecnologia e Eletrônica, São Paulo, Brazil. Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: numerical rating scale (NRS). ITT/per protocol analysis: statistical analysis done according to ITT. Statistical analysis: the Mann–Whitney U‐test was used for comparison of pain between the groups before and after application of TENS, and the Wilcoxon rank sum test for intra group analysis. The results showed that the pain intensity of the uterine contraction during breastfeeding in the TENS group showed a reduction of 2.00 compared with 0.69 in the control group. In both groups, the reduction of the intra group pain was significant, as well as the inter group reduction. However, the assessment of the reduction of pain in the TENS group showed clinically relevant pain relief, which was not obtained in the control group. In addition, although the CG showed a significant reduction of pain, it was not clinically significant. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation spreadsheet used ‐ no further detail available. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or withdrawals reported. |
| Source of funding bias | Unclear risk | No details. |
| Blinding (Participant) | High risk | Patients would be aware that a no treatment control was being used as comparison. |
| Blinding (Outcome Assessor) | High risk | No details provided of any attempts to blind assessor to group. |
| Sample Size | High risk | TENS (N = 16); control (N = 16). |
Ekblom 1987.
| Study characteristics | ||
| Methods |
Type of study: Randomised, placebo‐controlled, parallel design. Condition and number of participants randomised: acute pain from teeth or surrounding tissues, N = 40. Groups: 100 Hz Vibration Group (N = 8); placebo vibration group (N = 5); 2 Hz TENS group (N = 11); 100 Hz TENS group (N = 11); placebo TENS group (N = 5). |
|
| Participants |
Demographics: N = 40, 20 to 58 yrs, 23 male/17 female. Setting: emergency clinic for dental and oral surgery. Inclusion: acute pain from teeth or surrounding tissues, or both. Exclusion: not detailed. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in clinic. Applied by: presume by clinician. Waveform: monopolar square wave pulses. Frequency: high frequency (HF) group, 100 Hz; low frequency (LF) group, 71 Hz pulse train (duration 84 ms) delivered at 2 Hz. Pulse duration: 0.2 ms. Pulse amplitude/Intensity: HF set to produce a tingling sensation. LF set to produce prominent muscular contractions. Placebo TENS Group: electrodes applied to skin but no stimulation transmitted. Participants informed that some people might not experience the stimulation. Electrodes: two 3 cm x 3 cm conducting rubber, skin overlying painful area, anode distal. Duration and frequency of Rx: 30 min, 1 Rx. Device/manufacturer: not detailed. Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: VAS and 5 level verbal scale for pain intensity, before and after Rx. Heat pain threshold recorded before, during and after Rx. ITT/per protocol analysis: not detailed. Statistical analysis: no active stimulation was superior to the others re number of participants reporting pain reduction; placebo significantly less effective than active stimulation. No significant effects of Rx on heat pain threshold. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | Unclear risk | Participants informed that they may or may not experience a sensation associated with treatment. |
| Blinding (Outcome Assessor) | Unclear risk | No details provided. |
| Sample Size | High risk | 100 Hz Vibration (N = 8); placebo vibration (N = 5); 2 Hz TENS (N = 11); 100Hz TENS (N = 11); placebo TENS (N = 5). |
Gregorini 2010.
| Study characteristics | ||
| Methods |
Type of study: placebo‐controlled, parallel RCT. Condition and number of participants randomised: postoperative period of cardiac surgery (N = 25). Groups: placebo group (N = 12); TENS group (N = 13). |
|
| Participants |
Demographics: N = 25, 59.9 ± 10.3 yrs (mean ±?SD), 18 male/7 female. Setting: inpatient. Inclusion: patients aged between 35 to 80 years who had undergone elective cardiac surgery via longitudinal median sternotomy. Exclusion: patients with pacemaker; pregnant women; cognitive or intellectual impairment; absence of pain in the postoperative period; sensitivity disorders; and patients undergoing any type of analgesia in the eight‐hour period preceding the beginning of the protocol. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in hospital. Applied by: participant. Waveform: not detailed. Frequency: 80 Hz. Pulse duration: 150 μs. Pulse amplitude/intensity: participants adjusted the intensity of stimulation at the point at which they felt a strong, although yet comfortable, prickling sensation, and were told to reduce the intensity if they felt uncomfortable. Electrodes: 2 pairs of adhesive electrodes, 10 x 3.5 cm. Placed one on each side of the surgical wound in the subclavian region. Duration and frequency of Rx: 4 hrs, 1 Rx. Device/manufacturer: TENS Device, KLD, Amparo, São Paulo, Brazil. Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: numerical VAS for pain intensity at rest and with cough, before and after Rx. ITT/per protocol analysis: not detailed. Statistical analysis: data were analysed using means and SDs and non‐parametric data was analysed as medians and quartiles. Categorical data was expressed as absolute numbers and relative (%) frequency). TENS significantly reduced pain in the postoperative period with an improvement of 40% at rest and 42.9% with cough compared with the placebo group. No statistical differences were found in the placebo group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of "sealed box" for randomisation but specific details not given. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Low risk | No apparent funding bias. |
| Blinding (Participant) | Unclear risk | No details provided. |
| Blinding (Outcome Assessor) | Unclear risk | No details provided. |
| Sample Size | High risk | TENS (N =13); placebo (N = 12). |
Hansson 1983.
| Study characteristics | ||
| Methods |
Type of study: randomised, placebo‐controlled, parallel design. Condition and number of participants randomised: acute oro‐facial pain (N = 62). Groups: HF TENS group (N = 22); LF TENS group (N = 20); placebo TENS group (N = 20). |
|
| Participants |
Demographics: N = 62, 19 to 54 yrs, 26 male/36 female. Setting: emergency clinic for dental surgery. Inclusion: acute oro‐facial pain. Exclusion: not detailed. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in clinic. Applied by: presume by clinician. Waveform: monopolar square wave pulses. Frequency: HF Group, 100 Hz; LF Group, 2 Hz, 71 Hz pulse train with total duration of 84 ms delivered at 2 /sec. Pulse duration: 0.2 ms. Pulse amplitude/Intensity: HF, adjusted to 2 to 3 times perception threshold to produce a tingling non‐painful sensation from the stimulated area. Output adjusted during TENS in order to maintain a constant tingling sensation. LF, adjusted to 3 to 5 times perception threshold which produced non‐painful muscular contractions in the stimulated area. Placebo TENS Group: same as for other TENS groups except no batteries in units and participants told some people may not experience the stimulation. Electrodes: two, 2 cm x 3 cm conducting rubber, skin overlying painful area. Duration and frequency of Rx: 30 min, 1 Rx. Device/manufacturer: CEFAR SIII, Lund, Sweden. Adverse effects: most participants found the muscle twitches produced by LF TENS uncomfortable. |
|
| Outcomes |
Pain outcome: 5‐graded verbal scale for pain intensity before Rx. VAS for pain intensity before and after Rx. During Rx pain rated continuously using a graphic rating scale‐ consistent results obtained with both methods. Time until first report of subjective pain reduction and maximal pain reduction recorded. ITT/per protocol analysis: not detailed. Statistical analysis: HF TENS: 7/22 reported pain reduction > 50%, includes 2 who had total pain reduction. LF TENS: 9/20 reported pain reduction > 50%, includes 2 who had total pain reduction. Placebo TENS: 8/20 reported some degree of pain relief, includes 2 who had pain reduction > 50%. In the two active TENS groups, approx 80% reported a reduction of pain within less than 5 mins after onset of stimulation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were assigned randomly to one of the three groups". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Low risk | "This work has been supported by grants from Magnus Bergwalls Stiftelse". This is a research foundation. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. "For practical reasons a double‐blind technique could not be used." For the placebo TENS group: "Twenty patients received in all ways, except two, the same treatment as the two groups receiving TENS. One difference was that the TENS stimulators used were not equipped with batteries; and the other difference was that these patients were told that some people may not experience the stimulation". The exclusion criteria were not provided so we do not know if participants had to be TENS naïve. |
| Blinding (Outcome Assessor) | High risk | Study appears to be designed as single blind (i.e. participants blind). |
| Sample Size | High risk | HF TENS (N = 22); LF TENS (N = 20); placebo TENS (N =20). |
Hruby 2006.
| Study characteristics | ||
| Methods |
Type of study: double blind, placebo‐controlled, parallel RCT. Condition and number of participants randomised: participants undergoing flexible cystoscopy (N = 148). Groups: active TENS group (N = 48); placebo TENS group (N = 49); control group (N = 51). |
|
| Participants |
Demographics: N = 148, 108 male/40 female. Active TENS Group, 62.23 yrs; placebo TENS Group, 61.53 yrs; control group, 60.98 yrs (? mean). Setting: office‐based. Inclusion: flexible cystoscopy for surveillance of transitional cell carcinoma; voiding symptoms; hematuria, or stent removal. Exclusion: participants with a neobladder; cystoscopy with biopsy or with dilation of strictures; participants taking chronic analgesics or with pain syndromes; and participants who required post procedure catheterization. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in hospital. Applied by: clinician. Waveform: symmetric rectangular biphasic. Frequency: 100 pulses/s. Pulse duration: 180 μs. Pulse amplitude/Intensity: at the initial settings, the participant typically felt a slight tickle at the site of the electrodes. The tickling sensation is greater than the sensory threshold but less than the pain threshold. The starting point for pulse amplitude was 20 mA. During flexible cystoscopy, participants were able to change the amplitude on the TENS device at will. Placebo TENS Group: unit identical to active unit but without any nerve stimulation. Control Group: no analgesia. Electrodes: 2, type and size not detailed, each electrode was placed halfway along an imaginary line drawn from the ASIS to pubis. Duration and frequency of Rx: duration not detailed, 1 Rx. Device/manufacturer: Prometheus Group, Dover, NH. Adverse effects: 2 participants in the Active TENS group could not tolerate the TENS unit as the amplitude was gradually increased to the starting point of 20 mA; 1 participant in the Placebo TENS group reported severe abdominal pain several hours after the procedure. |
|
| Outcomes |
Pain outcome: VAS, 30 seconds and 1 min into the procedure, 5 mins after procedure finished. ITT/per protocol analysis: not detailed. Statistical analysis: no evaluable data for this review as unclear if SD data are presented. No significant changes in VAS between groups at each of the 3 time points. |
|
| Notes | Abbreviation: ASIS‐anterior superior iliac spine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A total of 148 patients were prospectively randomised into one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. Text says it was a double‐blind study but no details provided ‐ assume they intended to blind the participants. The placebo TENS group was described as "a control group with a placebo TENS unit (unit identical to active unit but without any nerve stimulation)". The inclusion/exclusion criteria did not state that participants had to be TENS naïve. Control group received no treatment so these participants could not be blinded. |
| Blinding (Outcome Assessor) | Unclear risk | Text states that it was a double‐blind study but no details provided if the outcome assessor was blinded. |
| Sample Size | High risk | TENS (N = 48); placebo TENS (N = 49); control (N = 51). |
Keskin 2012.
| Study characteristics | ||
| Methods |
Type of study: prospective RCT. Condition and number of participants randomised: 88 pregnant women suffering from LBP with no previous history of LBP or lumbar pathology. Groups: active TENS (N = 22); exercise (N = 22); acetaminophen (N = 22); no‐Rx control (N = 22). |
|
| Participants |
Demographics: N = 88, all female. Age: TENS group 29.1yrs ± 5.0; exercise group 30.7 ±4.3; acetaminophen 29.7 ± 4.2, control 29.2 ± 4.0. Setting: outpatient antenatal care unit, Turkey. Inclusion: uncomplicated pregnancy with LBP. Exclusion: history of Lumbar pathology pre‐pregnancy or pathology detected during physical examination; pain due to non‐musculoskeletal factors; declined to take part. Withdrawals/dropouts: TENS (N = 2); exercise (N = 3); acetaminophen (N = 3); control (N = 1). |
|
| Interventions |
Where applied: on the painful lumbar region. Applied by: not stated. Waveform: not stated. Frequency: 120 Hz Pulse duration: 100 μs Pulse amplitude/Intensity: adjusted to produce a tingling sensation approx 2 to 3 times above the sensory threshold. Placebo TENS Group: N/A. Exercise group: completed a home exercise programme set by a physical therapist and including pelvic tilting, stretching for the lower extremity and mild isometric abdominal contractions x 10 of each per session, twice daily for 3 weeks. Acetaminophen group: one 500 mg paracetamol tablet 2 x daily for 3 weeks. Control Group: no Rx administered Electrodes: 4 surface electrodes 5 cm² Duration and frequency of Rx: duration not stated. 2 sessions weekly for 3 weeks. Device/manufacturer: Intelect TENS, Chattanooga Medical Supplies Inc., Taiwan). Adverse effects: discomfort using TENS and gastric effect with medication. |
|
| Outcomes |
Pain outcome: VAS scores and Roland‐Morris Disability Questionnaire (RMDQ). ITT/per protocol analysis: not stated. Statistical analysis: median pre‐treatment VAS scores differed significantly between groups (P = 0.004; Kruskal‐Wallis test). These scores were significantly higher in the TENS group (P = 0.002; post‐hoc Mann‐Whitney) and acetaminophen groups (P = 0.009). Median pre‐treatment RMDQ scores were similar across all groups. At the end of the trial pain intensity had increased in control group (57%), and decreased in exercise group(95%). In acetaminophen and TENS groups 100% had a decrease in pain. All treatment groups showed a significant improvement in both VAS and RMDQ scores (P < 0.0001) using the Wilcoxon test. Differences in pre and post‐Rx VAS and RMDQ scores were significant in all treatment groups using Kruskal Wallis (VAS; P < 0.001; RMDQ, P < 0.001). This difference was caused by markedly higher scores in the TENS group (P < 0.001 for both comparisons; Mann‐Whitney test). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals and dropouts were reported but no information was included as to how the data was dealt with. |
| Source of funding bias | Low risk | No apparent funding bias. |
| Blinding (Participant) | High risk | No TENS placebo group so not possible to blind participants as to which group they were allocated to. |
| Blinding (Outcome Assessor) | Unclear risk | No details provided. |
| Sample Size | High risk | TENS (N = 22); exercise (N = 22); acetaminophen (N = 22); control (N = 22). |
Kim 2012.
| Study characteristics | ||
| Methods |
Type of study: single‐blind, placebo‐controlled RCT. Condition and number of participants randomised: 100 patients undergoing plastic surgery. Groups: 2 groups: active TENS (N = 50); placebo TENS (N = 50). |
|
| Participants |
Demographics: N = 100; TENS group 21 male/29 female; age 48.2 yrs ± 13.0; placebo group 19 male/31 female; age 51.2 yrs ± 11.7. Setting: Hospital outpatient, Korea. Inclusion: patients undergoing plastic surgery. Exclusion: concomitant sedative or analgesic medication and neurological disease, or potentially serious internal diseases (ASA physical status > 3). Withdrawals/dropouts: none. |
|
| Interventions |
Where applied: radial side of the dominant forearm ‐ cathode over cephalic vein 1cm proximal to radial styloid process; anode 3 cm away proximal to cathode. Applied by: anaesthesiologist. Waveform: not stated. Frequency: 80Hz. Pulse duration: 200 μs. Pulse amplitude/Intensity: maximum tolerable level below pain threshold without noticeable muscle contraction. Placebo TENS Group: TENS device without current output but with power indicator light illuminated. Control Group: none. Electrodes: 2 TensCare electrodes, 5 cm² Duration and frequency of Rx: 20 minutes immediately prior to venous cannulation. 1 single Rx. Device/manufacturer: select TENS unit (Empi, St Paul, Minnesota). Adverse effects: itching and erythema reported. |
|
| Outcomes |
Pain outcome: pain incidence; VAS scores. ITT/per protocol analysis: not stated. Statistical analysis: pain incidence was similar between the 2 groups (P > 0.05); 45 (90%) in the TENS group experienced pain against 50 (100%) in the placebo group using the X² test or Fisher exact test. Pain intensity (VAS) in TENS group was significantly lower than placebo, with TENS VAS scores 1.9 ± 1.2 (P < 0.01) against placebo VAS scores 4.8 ± 1.5 using Wlcoxon rank sum test with continuity correction. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated but no withdrawals/dropouts reported. |
| Source of funding bias | Low risk | No apparent funding bias. |
| Blinding (Participant) | Low risk | Placebo tens applied to blind participants. |
| Blinding (Outcome Assessor) | Low risk | "study‐blinded anaesthesiologist". |
| Sample Size | Unclear risk | TENS (N = 50); placebo TENS (N = 50). |
Limoges 2004.
| Study characteristics | ||
| Methods |
Type of study: double blind, placebo‐controlled, parallel RCT. Condition and number of participants randomised: participants undergoing screening flexible sigmoidoscopy (N = 90). Groups: TENS group (N = 30); placebo TENS group (N = 30); control group (N = 30). |
|
| Participants |
Demographics: N = 90, 51 male/39 female. TENS group, 57.18 ± 7.787 yrs; placebo TENS group, 55.97 ± 5.411 yrs; control group, 58.6 ± 9.073 yrs (mean ± SD). Setting: screening flexible sigmoidoscopy (SFS) speciality clinic. Inclusion: over 50 yrs; presenting for screening flexible sigmoidoscopy. Exclusion: cardiac pacemakers; automated implanted cardiac defibrillators; pre procedural skin irritation at electrode placement site; pre procedural sedation or analgesia. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in clinic. Applied by: clinician. Waveform: biphasic waveform and asymmetric pulse pattern. Frequency: 100 Hz. Pulse duration: 190 μs. Pulse amplitude/Intensity: 30 mA, setting chosen after progressively increasing amplitude and testing tolerability of each level on volunteers. Same intensity used for all participants. Placebo TENS Group: unit same as active group, attached to participant but not turned on. All participants told they may or may not feel tingling sensation at electrode site. Control Group: received only verbal encouragement. Electrodes: 4 self‐adhesive, 2 x 5 inch rectangular, 2 on left upper and lower quadrants of abdomen and 2 parallel to spinal cord at L1‐S3 level. Duration and frequency of Rx: varied 5 to 15 mins, 1 Rx. Device/manufacturer: Empi EPIX VT TENS. Adverse effects: 29 participants in TENS group and 6 participants in placebo TENS group reported pain/burning/tingling at electrode site. |
|
| Outcomes |
Pain outcome: pain experienced during procedure assessed by a NRS of 1 to 5 for pain intensity after procedure finished. ITT/per protocol analysis: not detailed. Statistical analysis: no significant difference between groups for pain experienced during the procedure. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author response: "Randomization was done by drawing numbers out of a hat. We picked a number out of the hat after the patient arrived and consented to participate". |
| Allocation concealment (selection bias) | High risk | Author response: "Randomization was done by drawing numbers out of a hat. We picked a number out of the hat after the patient arrived and consented to participate". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Ninety subjects were enrolled and completed the study". |
| Source of funding bias | Low risk | "Funding for this study was provided by the Innovative Pilot Project Grant Program at the University of California Davis Medical Center. The TENS unit was provided by EMPI, Inc." |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. "Subjects in the sham TENS group were connected to the TENS unit exactly the same as subjects in the TENS group. The research assistant manipulated the programming buttons on the TENS unit exactly as with the TENS group, but without actually turning the TENS units on beforehand. This step was performed in an effort to maintain blinding of both the endoscopist and subject. Subjects in the control group received only verbal encouragement." The inclusion/exclusion criteria did not state that participants had to be TENS naïve. Control group received no active treatment so these participants could not be blinded. |
| Blinding (Outcome Assessor) | High risk | Author response regarding the placebo TENS group: "the TENS unit was attached to the subject but never turned on by the RA (I and the subject were blinded to this)". "My RA administered the questionnaires". |
| Sample Size | High risk | TENS (N = 30); placebo TENS (N = 30); control (N = 30). |
Liu 1985.
| Study characteristics | ||
| Methods |
Type of study: randomised, double blind, controlled, parallel design. Condition and number of participants randomised: post thoracotomy, 30. Groups: TENS group, 15; control group, 15. |
|
| Participants |
Demographics: N = 30, 18 to 72 yrs, 22 male/8 female. TENS group, 51.73 yrs; control group, 52.73 yrs (mean). Setting: hospital. Inclusion: post thoracotomy. Exclusion: participants who had cardiac surgery. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: in hospital. Applied by: clinician. Waveform: not detailed. Frequency: mean was 75.75 Hz for TENS Group, 51 Hz for Control Group. Pulse duration: 0.1 ms. Pulse amplitude/Intensity: set at a subjective level of comfort, not adjusted during treatment, mean pulse amplitude was 7.33 mA for TENS Group. Control Group: TENS applied at fixed pulse amplitude of 2.5 mA. All participants told how TENS worked to control pain and what to expect from TENS after surgery. Electrodes: 2 carbon rubber and gel, size not detailed, placed on most painful area along incision wound. Duration and frequency of Rx: 20 min, daily treatment from 1st post‐op day until pain disappeared or participant discharged or Rx rejected by participant. Device/manufacturer: HRS Neuro‐Pulse Model HME‐12. Adverse effects: not detailed. |
|
| Outcomes |
Pain outcome: overall impression with TENS rated using 4 categories, after TENS discontinued. Pain rated using a 0 to 10 scale before and after each TENS Rx. Recorded daily (for 10 days) until pain disappeared, or patient discharged or treatment rejected by the patient. ITT/per protocol analysis: not detailed. Statistical analysis: significant alleviation of pain after TENS every day in the TENS group. No significant change in the Control group except on days 4 and 6. Significant difference between groups for post TENS pain scores on days 2/5/6/7/8. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Author response: "The patients were enrolled to the study consecutively before the surgery, divided into experimental and control groups alternatively". "Males and females were counted separately". |
| Allocation concealment (selection bias) | High risk | See under randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Table 2 gives pain scores on days 1 to 10. The table details the number of participants from whom data were recorded on each day ‐ shows a decline as the days progress. The text says that stimulation was given everyday from first postop day until pain disappeared, or the participant was discharged or the treatment was rejected by the participant. Table shows data collected for all participants (N = 15/group) for days 1 and 2 only. Figure 1 shows number of participants in each group that continued with TENS for each postop day. Specific reasons for each participant not recording pain scores was not given. |
| Source of funding bias | Unclear risk | No funding source detailed. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. Author response: "The study design was double blinded. The patients and I (the evaluator) were blinded. All patients were explained how TENS worked to control pain and what the patient should expect from TENS after operation". The control group received low intensity TENS. The exclusion criteria were not provided so we do not know if participants had to be TENS naïve. |
| Blinding (Outcome Assessor) | Low risk | Author response: "The study design was double blinded. The patients and I (the evaluator) were blinded". |
| Sample Size | High risk | TENS (N = 15); control (N = 15) |
Olsén 2007.
| Study characteristics | ||
| Methods |
Type of study: parallel RCT. Condition and number of participants randomised: newly delivered women with pain from postpartum uterine contractions (N = 21). Groups: HI TENS group (N = 13); LI TENS group (N = 8). |
|
| Participants |
Demographics: N = 21, all female, 31 yrs (mean). HI TENS Group, 31 ± 4.2 yrs; LI TENS Group, 31 ± 4.8 yrs (mean ± SD). Setting: Department of Obstetrics and Gynecology. Inclusion: newly delivered healthy women; well integrated in the Swedish language with uncomplicated vaginal delivery; painful postpartum uterine contractions that required pain relief. Exclusion: systemic disorders; abnormal pregnancy; operative delivery; other treatments for the pain should not have been initiated. Withdrawals/dropouts: 1 in HI TENS group dropped out due to discomfort of stimulation. |
|
| Interventions |
Where applied: in hospital. Applied by: clinician. Waveform: not detailed. Frequency: 80 Hz. Pulse duration: 0.2 ms. Pulse amplitude/Intensity: HI, set at 50 mA. LI, set at just above the sensory threshold (10 to 15 mA). Electrodes: 2 carbon rubber and gel, 53 x 34 mm, placed on the lower part of the abdomen, bilaterally over the uterus. Duration and frequency of Rx: 1 minute, 1 Rx repeated twice if no effect occurred. Device/manufacturer: Cefar AB, Lund, Sweden. Adverse effects: no adverse effects except for discomfort during stimulation were recorded. |
|
| Outcomes |
Pain outcome: measurement of discomfort on a 5‐point verbal scale, before and after Rx. VAS for present pain intensity, before and after Rx. Discomfort of Rx recorded on a 5‐point verbal scale. ITT/per protocol analysis: not detailed. Statistical analysis: median decrease in VAS pain ratings before and after treatment was larger in the HI TENS group than in the LI TENS group. Post Rx, women in the HI TENS group had less pain from the uterine contractions than the women in the LI TENS group. HI TENS group experienced significantly less discomfort from uterine contractions after treatment compared with the LI TENS group. Discomfort from TENS itself was significantly greater in HI group than in LI group. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After informed written consent, the women were randomised to either high‐intensity (HI) or low intensity (LI) high‐frequency (80 Hz) TENS. The allocation sequence was determined before the study by a research assistant using a computer generated random table." |
| Allocation concealment (selection bias) | Low risk | "Groups were coded and the allocation transferred to a series of pre‐sealed opaque envelopes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient in the HI TENS group dropped out from the study immediately after commencing TENS treatment because of discomfort of the stimulation." |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | Low risk | Study described as single‐blind. Groups were high‐intensity (HI) or low intensity (LI) high‐frequency (80 Hz) TENS. There was no placebo TENS group. "Before treatment the women were informed that they might experience pain or discomfort from the electrical stimulation." Author response: "it was the participants who were blinded to the treatment". Author response: "The patients had no previous experience of TENS". |
| Blinding (Outcome Assessor) | High risk | Study was designed as single blind. |
| Sample Size | High risk | HI TENS (N = 13); LI TENS (N = 8). |
Oncel 2002.
| Study characteristics | ||
| Methods |
Type of study: randomised, placebo‐controlled, parallel design. Condition and number of participants randomised: minor rib fractures, 100. Groups: NSAID group, 25; TENS group, 25; NSAID and placebo TENS group, 25; placebo tablets group, 25. |
|
| Participants |
Demographics: N = 100, 11 to 81 yrs, 41 female/59 male, 40 ± 16 yrs (mean ± SD). NSAID group, 35 ± 19 yrs; TENS group, 44 ± 15 yrs; NSAID and placebo TENS group, 41 ± 14 yrs; Placebo tablets group, 40 ± 16 yrs. Setting: hospital emergency service. Inclusion: minor rib fractures. Exclusion: 1st or 2nd rib fracture; more than 3 rib fractures or flail chest; requiring hospitalisation for cranial or abdominal trauma; patient refusal; undergoing any kind of surgery (including tube thoracostomy); cardiac or psychiatric illness; < 10 yrs; history of gastrointestinal bleeding or ulcer or other contraindications for NSAIDS; being pregnant. Withdrawals/dropouts: 8 participants were excluded because of complications and they were replaced. 7 had respiratory distress during the hospitalisation period; 3 had haemothorax and 4 had pneumothorax. All were treated with tube thoracostomy. Right haemothorax was diagnosed on the eighth patient the day after he had been discharged. He was re‐hospitalised and underwent a tube thoracostomy procedure. |
|
| Interventions |
Where applied: in hospital and at home. Applied by: clinician in hospital and by participant at home. Waveform: not detailed. Frequency: 80 Hz. Pulse duration: 50 μs. Pulse amplitude/Intensity: participants asked to turn up to the highest level that did not make them uncomfortable. Placebo TENS Group: TENS unit without batteries and no sign on unit that showed it was on. Participants in the TENS and NSAID and Inactive TENS group told they might or might not feel a sensation of tingling. Electrodes: 2 or 4 carbon rubber electrodes with adhesive gel, 3.4 x 4.2 cm, placed on both sides of fractures along lines of intercostal nerves. Duration and frequency of Rx: 30 mins, 6 Rxs. 2 treatments in hospital: within 2 hrs after admission and 12 hrs later. On discharge, home TENS twice a day for 2 days. Device/manufacturer: dual channel TENS, Biotens Inc Istanbul, Turkey. Adverse effects: no complications seen during trial. |
|
| Outcomes |
Pain outcome: pain assessed by 0 to 10 scoring system. Recorded when hospitalised ‐pre Rx, next day before they were discharged (after 2 phases of Rx) and third day after therapy had ended. ITT/per protocol analysis: no. Statistical analysis: no evaluable data for this review as mixed age population (adults and children). Day 0: no significant difference between groups. Day 1: pain in placebo group significantly higher than other groups. Pain in TENS group significantly less than NSAID and NSAID and inactive TENS groups. Day 3: pain in TENS group significantly less than all other groups and no significant difference between these 3 groups. All participants except the placebo group had significantly less pain on days 1 and 3 than day 0. In the placebo group, pain was significantly less on day 3 than 0 but no difference between pain levels on day 0 and 1. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One hundred consecutive patients admitted to Kartal Education and Research Hospital Emergency Service, were randomized into four groups". Author response: "A computerized randomization protocol had been received prior to the beginning of the trial, and the randomization of the patients was done accordingly". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Eight patients were excluded because of complications and they were replaced. Seven had respiratory distress during the hospitalisation period; three had haemothorax and four had pneumothorax. All were treated with tube thoracostomy. Right haemothorax was diagnosed on the eighth patient the day after he had been discharged. He was re‐hospitalized and underwent a tube thoracostomy procedure". No indication which group these individuals were randomised to. |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. "These patients were told that they might or might not feel a sensation of tingling, and this instruction was carefully standardized. The same blinded nurses performed two phases of TENS therapy during the hospitalisation period and instructed the patients how to use the machine at home. These nurses were told that every patient would be treated with active TENS units and that they were not to know about the content of the trial. Inactive TENS units were out of battery and there were no signs on the machines that showed they were 'on'.' Author response ‘As mentioned in the paper, the patients were completely unaware that the cases in the control group would not feel a sensation, and both the patients and the nurses assumed that all cases would have a TENS treatment." The inclusion/exclusion criteria did not state that participants had to be TENS naïve. |
| Blinding (Outcome Assessor) | High risk | Author response: "The pain scores were recorded by one of the authors (HY) or by educated nurses. The nurses were blinded to the randomisation but the author was not". Not all of the outcome assessors were blind to group allocation. |
| Sample Size | High risk | NSAID (N = 25); TENS (N = 25); NSAID and placebo TENS (N = 25); placebo tablets (N = 25). |
Ordog 1987.
| Study characteristics | ||
| Methods |
Type of study: randomised, double blind, placebo‐controlled, parallel design. Condition and number of participants randomised: acute trauma outpatients, 100. Groups: functioning TENS group (N = 25); placebo TENS group (N = 25); functioning TENS plus Tylenol (N = 25); placebo TENS plus Tylenol (N = 25). |
|
| Participants |
Demographics: N = 100, age/gender not detailed. Setting: outpatients. Inclusion: acute trauma outpatients. Exclusion: < 21 yrs; hx cardiac disease or pacemaker; insufficient aptitude or personality for operation of apparatus; allergies to acetaminophen or codeine; pregnancy. Withdrawals/dropouts: not detailed. |
|
| Interventions |
Where applied: at home by participant. Applied by: participant. Waveform: not detailed. Frequency: not detailed. Pulse duration: not detailed. Pulse amplitude/Intensity: instructed to adjust energy knob to level at which pain disappeared or until they felt a mild electric shock from the unit. Placebo TENS group: unit appeared like active but no electrical current transmitted to the skin. It produced the slight hum and vibration that active unit produced. Participants were not told that the functioning units could produce a mild electrical shock by turning up the unit. Electrodes: 2 metal electrodes and a disposable sterile skin pad, size not detailed. Applied over area of injury or as close to it as practical. Duration and frequency of Rx: could be worn at all times or as often as required for pain control. Device/manufacturer: disposable TENS‐PAC unit measures ½ x 3 x 4 inches. Dow Corning, Arlington, Tennessee. Adverse effects: no complications and no side effects except a mild tingling sensation at higher output levels, 20% of participants reported this effect. |
|
| Outcomes |
Pain outcome: 11 point VAS for pain intensity, administered pre Rx, after two days of Rx, and a month after initial injury. ITT/per protocol analysis: not detailed. Statistical analysis: statistically significant reduction in pain severity in functioning TENS vs placebo group at day 2, not at 1 month. No significant difference between functioning TENS unit and Tylenol group when either the subjective levels of pain versus time or pre‐Rx and post‐Rx pain levels at 2 days and 1 month were compared. Mean length of use of TENS in all groups was 3 days versus a mean of 5 days for the oral analgesics in the 2 Tylenol groups. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One hundred consecutive consenting acute trauma outpatients seen by the researcher were randomly assigned to four pain treatment groups. Randomization of the TENS‐PAC units was achieved by mixing the two boxes of 50 functioning and 50 placebo units together. A decoding process was released when all of the TENS‐PAC units were returned after the trial was completed. All of the units were returned to the researcher following the trial to determine which units the patient had and also to assure their function". |
| Allocation concealment (selection bias) | Low risk | "A decoding process was released when all of the TENS‐PAC units were returned after the trial was completed. All of the units were returned to the researcher following the trial to determine which units the patient had and also to assure their function". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Source of funding bias | Unclear risk | No details provided. |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. "In the study, 50% of the patients received a functioning TENS‐PAC, and the other 50% received a ‘placebo’ unit, which appeared and operated in all ways similar to the functioning unit except that no electrical current was transmitted to the skin. This ‘placebo’ unit was originally a functioning TENS‐PAC, but in this unit, an internal wire that supplied the electrical current to the skin was cut. The TENS‐PAC produces a slight hum and vibration that the ‘placebo’ unit also produced. The ‘placebo’ units were prepared by an independent source, and neither the researcher nor the patient was able to identify which unit was given until the trial was completed. The possibility that the patients might have figured out whether they had the placebo units seems remote, as patients were not told that the functioning units can produce a mild electrical shock by turning up the unit. As none of the patients had used TENS previously, it is unlikely that they would have known that an electrical shock could be produced only by the functioning units". |
| Blinding (Outcome Assessor) | Low risk | "The 'placebo' units were prepared by an independent source, and neither the researcher nor the patient was able to identify which unit was given until the study was completed". |
| Sample Size | High risk | TENS (N = 25); placebo TENS (N = 25); TENS plus Tylenol (N = 25); placebo TENS plus Tylenol (N = 25). |
Pitangui 2012.
| Study characteristics | ||
| Methods |
Type of study: RCT. Condition and number of participants randomised: 40 primiparous women who had experienced spontaneous vaginal delivery were randomised. Groups: N = 40, all female. HF TENS (N = 20), no‐Rx control (N = 20). |
|
| Participants |
Demographics: all female (N = 40). Age 18 to 31 years (median 20.5 years) with no statistical differences in age, education or colour between groups. Setting: hospital maternity ward, Brazil. Inclusion: low‐risk, primiparous pregnancy, older than 18 years of age, literate and understanding of Portuguese language,aware of time and space, post‐vaginal spontaneous delivery, experienced an episiotomy with stitches, presenting with pain in the episiotomy area, absence of any genitourinary pathology. Exclusion: contraindications to TENS, puerperal complications, previous exposure to TENS, morbid obesity, instrumental delivery (e.g. use of forceps). Withdrawals/dropouts: none reported |
|
| Interventions |
Where applied: parallel to the episiotomy site. Applied by: not stated. Waveform: biphasic, asymmetrical. Frequency: 100 Hz. Pulse duration: 75 μs. Pulse amplitude/Intensity: strong numbing sensation but no muscle contractions. Placebo TENS Group: N/A. Control Group: no intervention received. Electrodes: 4 silicone‐carbon electrodes 5.5 cm x 3 cm. Duration and frequency of Rx: 60 mins, single‐session. Device/manufacturer: Tens KW Compact, KW Industria Nacional Tecnologia e Electronica, San Paulo, Brazil. Adverse effects: none reported. |
|
| Outcomes |
Pain outcome: numerical rating scale (NRS) 11 point (0 to 10) carried out at the beginning of the trial (1st evaluation), at 60 mins (2nd evaluation) and 120 mins (3rd evaluation). Pain was measured during resting, sitting and ambulation at each evaluation. McGill pain questionnaire used to obtain pain descriptors. ITT/per protocol analysis: not stated. Statistical analysis: data for the groups were compared using the unpaired t‐test and intragroup differences analysed using a repeated‐measures ANOVA with a post‐hoc Tukey test. Mann‐Whitney test was used for analysing continuous variables such as neonatal or obstetric data and Pearson's Chi² test or Fisher's exact test was used for categorical variables. Groups presented similar pain scores at baseline. The application of TENS significantly reduced pain intensity in resting, sitting and ambulating (P > 0.001) immediately after TENS and 60 mins later compared with the control group. Comparing the 1st evaluation with the 3rd there was only a significant difference in the TENS group. On the McGill pain questionnaire at baseline there were no significant differences. After TENS there was a decrease in NWC (P > 0.001) in the TENS group and PRI for the sensory, affective, evaluative, miscellaneous and total categories (P > 0.001). The TENS group also showed a reduction in the NWC. The control group did not show a similar alteration in the PRI or NWC. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. |
| Source of funding bias | Low risk | No apparent funding bias. |
| Blinding (Participant) | High risk | No blinding. |
| Blinding (Outcome Assessor) | Unclear risk | No details provided. |
| Sample Size | High risk | HF TENS (N = 20); control (N = 20). |
Roche 1985.
| Study characteristics | ||
| Methods |
Type of study: placebo‐controlled, parallel RCT. Condition and number of participants randomised: haemophiliac participants (N = 36). Groups: active TENS group (N = 28); placebo TENS group (N = 8). |
|
| Participants |
Demographics: N = 36, 35 ± 12 yrs (mean ±?SD), gender not detailed. Setting: specialised outpatient clinic at hospital. Inclusion: haemophiliac participants suffering from unilateral haemorrhage into a joint. Exclusion: participants attending for dental care or for treatment to haemorrhage in the region of the face, abdomen or cranium. Withdrawals/dropouts: none. |
|
| Interventions |
Where applied: in hospital. Applied by: clinician. Waveform: square wave pulses. Frequency: internal pulse frequency of trains was 100 Hz and repetition rate of trains was 5 Hz. In initial stage of trial, trains of pulses rather than continuous TENS reported by participants as being more tolerable, consequently this form of TENS was adopted throughout the trial. Pulse duration: 1 ms pulses, 100 ms train duration. Pulse amplitude/Intensity: raised to a level of definite but comfortable perception with no presence of muscle activation. Placebo TENS Group: as for active group but no stimulation applied. Participants informed that a very high frequency of stimulation was being used which they might or might not feel. Electrodes: 2 or 4, flexible carbon electrodes layered with electrode gel, 2x2 cm, over the major sensory nerves supplying affected area or as close as possible to area of bleed. Duration and frequency of Rx: 25 min, 1 Rx. Device/manufacturer: Digitimer Ltd, Model DS2. Adverse effects: none. |
|
| Outcomes |
Pain outcome: MPQ (PRI, PPI, group scores for each category) before and after Rx for current pain. ITT/per protocol analysis: no. Statistical analysis: over 71% of participants receiving TENS reported changes in MPQ scores which represented pain relief > 50%. Only 2 placebo participants (25%) reported this amount of pain relief. The difference between participants reporting at least 50% relief was significantly different between groups using PRI and PPI. 9 TENS participants reported > 80% pain relief, 4 of these reported 100% pain relief. 2 placebo participants reported > 50% pain relief, neither reported 100%. Pre Rx PRI data divided into mild‐medium (PRI score of 0 to 25) and medium‐severe (PRI score of 26 to 50) based on highest recorded PRI score of 50. For TENS participants, difference between these 2 groups of scores was not significant. |
|
| Notes | Abbreviation: MPQ‐ McGill pain questionnaire; PPI‐ present pain index; PRI‐ pain rating index. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were randomly assigned to one of two groups". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Trial author responded "no" to question "Were there any dropouts/withdrawals?". |
| Source of funding bias | Low risk | "The research was supported by a grant from The British Medical Research Council (Grant No. 0979/723/N) awarded to K. Gijsbers". |
| Blinding (Participant) | High risk | It is impossible to adequately blind participants who receive electrical stimulation. Author response "The study was single blind. The same researcher took measures and applied TENS. Specific TENS settings were screened from participants". "The same apparatus and electrodes were used for the placebo group, but no stimulation was applied. These subjects were informed that a very high frequency of stimulation was being used which they might or might not feel". The exclusion criteria were not provided so we do not know if participants had to be TENS naïve. |
| Blinding (Outcome Assessor) | High risk | Author response: "The study was single blind. The same researcher took measures and applied TENS. Specific TENS settings were screened from participants". |
| Sample Size | High risk | N = 28 TENS; N = 8 placebo TENS |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhmadeeva 2010 | RCT but chronic pain. |
| Andersen 2009a | RCT but not a standard TENS device. |
| Andersen 2009b | RCT but not a standard TENS device. |
| Barbarisi 2010 | RCT but chronic pain. |
| Barker 2006 | RCT but intensity too low. |
| Baskurt 2006 | RCT but chronic pain. |
| Bertalanffy 2005 | RCT but intensity too low. |
| Celik 2013 | RCT but chronic pain. |
| Chee 1986 | RCT but microcurrent used. |
| Coletta 1988 | RCT but intensity too low. |
| Doğu 2009 | RCT but chronic pain. |
| Durmus 2009 | RCT but chronic pain. |
| Ekblom 1985 | RCT but TENS delivered at distal acupuncture point. |
| Eyigor 2012 | RCT but chronic pain. |
| Fengler 2007 | RCT but microcurrent used/chronic condition. |
| Gemmell 2011 | RCT but 'latent' myofascial trigger points used on otherwise asymptomatic adults. |
| Gül 2009 | RCT but chronic pain. |
| Gupta 2002 | RCT but concurrent 'rescue' medication given. |
| Herman 1994 | RCT but not a standard TENS device. |
| Izadpanah 2005 | RCT but needle electrode used/not standard TENS device. |
| Korkmaz 2010 | RCT but chronic pain. |
| Kumar 2014 | RCT but chronic pain. |
| Lang 2007 | RCT but intensity too low. |
| Lee 1997 | RCT but not a standard TENS device. |
| Lee 2012 | RCT but concurrent pain medication. |
| Leo 1986 | RCT but mixed acute and chronic pain. |
| Mora 2006 | RCT but intensity too low. |
| Murina 2008 | RCT but chronic pain. |
| Myśliwiec 2011 | RCT but chronic pain. |
| Peng‐fei 2011 | This is a letter a letter in response to study by Korkmaz et al which was excluded in first screening because chronic pain. |
| Pope 1994 | RCT but not acute pain. |
| Reichstein 2005 | RCT but H‐wave device used. |
| Rodarti 2012 | Duplicate of another study. Pitangui 2012 |
| Rodríguez‐Fernández 2011 | Use of 'latent' myofascial trigger points on otherwise asymptomatic individuals. |
| Sahin 2011 | RCT but chronic pain. |
| Solomon 1985 | RCT but not a standard TENS device. |
| Stratton 2009 | RCT but chronic pain. |
| Sunshine 1996 | RCT but APS therapy used/chronic condition. |
| Taskaynatan 2007 | RCT but IFT used. |
| Tsai 2010 | RCT but chronic pain. |
| Tulgar 1991a | RCT but chronic conditions included. |
| Tulgar 1991b | RCT but chronic conditions included. |
| Wang 2009 | RCT but chronic pain. |
Characteristics of studies awaiting classification [ordered by study ID]
Cambiaghi 2013.
| Methods |
Type of study: RCT. Condition and number of participants randomised: 40 females submitted for office diagnostic hysteroscopy and endometrial biopsy. Groups: active TENS with Tanyx and no‐treatment control. |
| Participants |
Demographics: N = 40, female participants. Age not available. Setting: Brazil. Inclusion: information not available. Exclusion: information not available. Withdrawals/dropouts: information not available. |
| Interventions |
Where applied: infra‐umbilical area. Applied by: information not available. Waveform: information not available. Frequency: information not available. Pulse duration: information not available. Pulse amplitude/Intensity: information not available. Electrodes: information not available. Duration and frequency of Rx: information not available. Device/manufacturer: information not available. Adverse effects: information not available. |
| Outcomes |
Pain outcome: VAS for pain intensity, during Rx. ITT/per protocol analysis: not detailed. Statistical analysis: statistically significant reduction in VAS scores during both procedures in the TENS group. |
| Notes |
de Paiva Tosato 2007.
| Methods |
Type of study: parallel RCT. Condition and number of participants randomised: temporomandibular pain (? acute pain), 20. Groups: massage group, 10; TENS group, 10. |
| Participants |
Demographics: N = 20, 22 to 46 yrs, 31.75 ± 8.71 (mean ± SD), all female. Setting: not detailed. Inclusion: signs and symptoms of temporomandibular disorders; females. Exclusion: no temporomandibular pain; males; dental problems; systemic disease; patients having other treatment (dental treatment, physiotherapy, medication). Withdrawals/dropouts: not detailed. |
| Interventions |
Where applied: not detailed. Applied by: not detailed. Waveform: not detailed. Frequency: not detailed. Pulse duration: not detailed. Pulse amplitude/Intensity: participants told the sensation should be pleasant and were told to report whenever the intensity of the current decreased. Electrodes: not detailed. Placed over masseter muscle, anterior portion of temporal muscle. Duration and frequency of Rx: 30 min, 1 Rx. Device/manufacturer: Quark. Adverse effects: not detailed. |
| Outcomes |
Pain outcome: VAS for pain intensity, before and after Rx. ITT/per protocol analysis: not detailed. Statistical analysis: statistically significant reduction in VAS scores post Rx in both groups. |
| Notes |
França 2012.
| Methods |
Type of study: RCT. Condition and number of participants randomised: 23 patients randomized into two groups. Groups: TENS group, stabilization group (received exercises of lumbar segmental stabilization ‐ transversus abdominis and lumbar multifidus muscles exercises). |
| Participants |
Demographics: N = 23. Stabilization group (SG N = 12; age 43.58 + 7.17; BMI 26.47 + 3.39). TENS group (TG N = 11; age 46.45 + 5.14; BMI 26.92 + 3.02). Setting: information not available. Inclusion: information not available. Exclusion: information not available. Withdrawals/dropouts: information not available. |
| Interventions | Both groups received 16 sessions, lasting 60 minutes, twice a week and evaluated before and after 8 weeks. TENS Group Where applied: Information not available. Applied by: information not available. Waveform: information not available. Frequency: information not available. Pulse duration: information not available. Pulse amplitude/intensity: information not available. Electrodes: information not available. Duration and frequency of Rx: 16 sessions, lasting 60 minutes, twice a week. Device/manufacturer: information not available. Adverse effects: information not available. |
| Outcomes |
Pain outcome: Visual Analog Pain Scale, Oswestry disability questionnaire for functional disability and pressure biofeedback unit for the ability to contract the TrA muscle. ITT/per protocol analysis: information not available. Statistical analysis: intragroup statistical analysis using t‐test and Wilcoxon Signed Rank tests. "After eight weeks, Stabilization Group showed statistically significant improvement in pain (6.16+1.26; 1.58+1.24; p<0.001), functional disability (15.50+3.77; 4.83+2.94; p<0,001) and the ability to contract the TrA muscle (‐0.83+1.49;‐3.16+0.77; p<0,001). There was no statistically significant difference in TENS Group for functional disability (18.09+4.27;17.09+7.96; p=0.569) and ability to contract the TrA muscle (‐1.40+0.83; ‐1.54+0.93; p=0.557), however it demonstrated improvement in pain (6.90+2.30;4.81+2.52; p=0.004)". |
| Notes | . |
Hsueh 1997.
| Methods |
Type of study: randomised, double blind, placebo‐controlled, parallel design. Condition and number of participants randomised: myofascial trigger points of upper trapezius muscle (? acute pain), N = 60. Groups: placebo group (N = 18); ENS group (N = 20); EMS therapy (N = 22). |
| Participants |
Demographics: N = 60, 44.4 ± 13.9 yrs (mean ± ?SD), 25 male/35 female. Placebo group, 41.4 ± 13.0 yrs; ENS group, 42.7 ± 13.8 yrs; EMS therapy, 44.4 ± 14.5 yrs (mean ±?SD). Setting: outpatient clinic at hospital. Inclusion: myofascial trigger points in one side of upper trapezius muscles. Exclusion: < 18 yrs or > 80 yrs; acute or serious illness; mental retardation; neurologic deficits involving the investigated upper limb; advanced osteopathic or arthropathic disorder of the cervical spine or the shoulder of the investigated side; participants should have had no therapy, such as physical therapy or injection therapy, within the last 2 months on MTrPs selected for this trial. Withdrawals/dropouts: not detailed. |
| Interventions |
Where applied: in clinic. Applied by: presume by clinician. Waveform: not detailed. Frequency: 60 Hz. Pulse duration: not detailed. Pulse amplitude/Intensity: at a level that the participant could feel but was not strong enough to induce muscle contraction. Placebo Group: participant told that a certain type of therapy would be given to treat MTrPs, but was not told what treatment was to be given. Electrodes were applied on the upper trapezius muscle as in other groups, 0 mA current intensity. Electrodes: 2, type and number not detailed, negative electrode placed on MTrP of upper trapezius muscle and positive one on its acromial tendon insertional site. Duration and frequency of Rx: 20 min, 1 Rx. Device/manufacturer: not detailed. Adverse effects: not detailed. |
| Outcomes |
Pain outcome: VAS for pain intensity, before and after Rx. PT of MTrP of the upper trapezius muscle before and after Rx. ITT/per protocol analysis: not detailed. Statistical analysis: improvement in PI and PT was significantly greater in the ENS Group than the other 2 groups. |
| Notes | ENS‐ electrical nerve stimulation; EMS‐ electrical muscle stimulation; MTrPs‐ myofascial trigger points; PI‐ pain intensity; PT‐ pain threshold. |
Liebano 2013.
| Methods |
Type of study: information not available. Condition and number of participants randomised: information not available. Groups: information not available. |
| Participants |
Demographics: N =74; gender and age not known. Setting: information not available. Inclusion: information not available. Exclusion: information not available. Withdrawals/dropouts: information not available. |
| Interventions |
Where applied: information not available. Applied by: information not available. Waveform: information not available. Frequency: information not available. Pulse duration: information not available. Pulse amplitude/Intensity: information not available. Electrodes: information not available. Duration and frequency of Rx: information not available. Device/manufacturer: information not available. Adverse effects: information not available. |
| Outcomes |
Pain outcome: VAS for pain intensity, before and after Rx. ITT/per protocol analysis: information not available. Statistical analysis:information not available. |
| Notes |
Park 2014.
| Methods |
Type of study: RCT. Condition and number of participants randomised: 20 to 60 year‐old women undergoing thyroidectomy. Groups: control or TENS. |
| Participants |
Demographics: 20 to 60 year‐old women undergoing thyroidectomy without history of headache or neck pain within six months. Setting: information not available. Inclusion: information not available. Exclusion: information not available. Withdrawals/dropouts: information not available. |
| Interventions |
TENS group Intraoperative TENS. Where applied: in the upper trapezius during thyroidectomy. Applied by: information not available. Waveform: information not available. Frequency: information not available. Pulse duration: information not available. Pulse amplitude/Intensity: information not available. Electrodes: information not available. Duration and frequency of Rx: information not available. Device/manufacturer: information not available. Adverse effects: information not available. |
| Outcomes |
Pain outcome: numerical rating scale of posterior neck pain and wound pain at 30 minutes, 6, 24 and 48 hours after surgery. ITT/per protocol analysis: information not available. Statistical analysis: information not available. |
| Notes |
Rajpurohit 2010.
| Methods |
Type of study: randomised, controlled, parallel design. Condition and number of participants randomised: bruxism with masticatory muscle pain (? acute pain), 60. Groups: MENS group (N = 30); TENS group (N = 30). |
| Participants |
Demographics: N = 60, age not detailed, 36 male/24 female. Setting: physiotherapy department in a hospital. Inclusion: clinical diagnosis of bruxism; muscle tenderness over masseter muscle; early morning temporomandibular joint stiffness and pain; duration of pain more than three weeks; and, age ranged from 19 to 60 years. Exclusion: wearing any removable restoration; treated with analgesic and antiinflammatory drugs; having muscle pain without bruxism; presence of any tumour or cancer around jaws or infection. Withdrawals/dropouts: not detailed. |
| Interventions |
Where applied: in hospital. Applied by: not detailed. Waveform: not detailed. Frequency: 50 Hz. Pulse duration: 0.5 ms. Pulse amplitude/Intensity: intensity was as per the participant's tolerance. Electrodes: carbon electrodes, number not detailed, 40 x 54 mm2. Placed over the affected side of masseter muscle. Duration and frequency of Rx: 20 minutes, 1 Rx daily for 7 days. Device/manufacturer: not detailed. Adverse effects: not detailed. |
| Outcomes |
Pain outcome: VAS for pain intensity, pre‐TENS and post‐TENS at the end of the 7th day of treatment. Tenderness by using digital pressometer of 2 KgF, pre‐TENS and post‐TENS at the end of the seventh day of treatment. ITT/per protocol analysis: not detailed. Statistical analysis: statistically significant pain relief and decrease in tenderness in MENS group compared to TENS group. |
| Notes |
Salvador 2005.
| Methods |
Type of study: Randomised, blinded, controlled, parallel design. Condition and number of participants randomised: acute LBP, 28. Groups: muscle energy technique group (N = 14); TENS group (N = 14). |
| Participants |
Demographics: N = 28, age not detailed, all male. Setting: clinic. Inclusion: acute LBP (constant pain present for no more than 3 weeks); shortening of at least one of the muscle groups assessed; no treatment (physiotherapy or tablets) in the last 2 weeks for the LBP. Exclusion: chronic LBP; rheumatological problems (arthritis, osteoporosis); no muscle shortening; positive Valsalva. Withdrawals/dropouts: not detailed. |
| Interventions |
Where applied: in clinic. Applied by: clinician. Waveform: not detailed. Frequency: not detailed. Pulse duration: not detailed. Pulse amplitude/Intensity: not detailed. Electrodes: not detailed. Duration and frequency of Rx: 5 min, 1 Rx. Device/manufacturer: Quark. Adverse effects: not detailed. |
| Outcomes |
Pain outcome: VAS for pain intensity, before and after Rx. ITT/per protocol analysis: not detailed. Statistical analysis: significant reduction in pain intensity after treatment in TENS group when compared to muscle energy technique group. |
| Notes |
Salvino 2013.
| Methods |
Type of study: randomised, placebo controlled. Condition and number of participants randomised: 145 consecutive headache sufferers grouped in 2 groups according to cutaneous allodynia total score. Groups: real or sham TENS. |
| Participants |
Demographics: information not available. Setting: information not available. Inclusion: information not available. Exclusion: information not available. Withdrawals/dropouts: information not available. |
| Interventions |
TENS group Where applied: at the back of the head bilaterally. Applied by: information not available. Waveform: information not available. Frequency: information not available. Pulse duration: information not available. Pulse amplitude/Intensity: information not available. Electrodes: information not available. Duration and frequency of Rx: 30 minutes, three times a day for two consecutive weeks. Device/manufacturer: information not available. Adverse effects: information not available. Sham TENS group: information not available. |
| Outcomes |
Pain outcome: number of headache free‐days (> 50%) at 15, 30 and 60 days. ITT/per protocol analysis: information not available. Statistical analysis: information not available. "A significant change in number of headache free‐days above 50% was observed in 53 (49%) out of l08 patients treated with real TENS. Of these patients thirty‐seven respondents (82%) were non allodynic. While 47 (75%) out of the 63 non respondents were allodynic patients. Only 2 (5%) out of the 37 patients were responsive to sham TENS therapy." |
| Notes | Objectives: to test if cutaneous allodynia influences the response to treatment with TENS in headache sufferers. |
Silva 2012.
| Methods |
Type of study: single‐blind, randomised design. Condition and number of participants randomised: patients post‐laparoscopic cholecystectomy (N = ?). Groups: active TENS and placebo TENS. |
| Participants |
Demographics: N = ? Age and gender not available. Setting: not available. Inclusion: not available. Exclusion: not available. Withdrawals/dropouts: not available. |
| Interventions |
Where applied: information not available. Applied by: information not available. Waveform: biphasic square pulse TENS current. Frequency:150 Hz. Pulse duration: 75 μs. Pulse amplitude/Intensity: information not available. Electrodes: information not available. Duration and frequency of Rx: information not available. Device/manufacturer: information not available. Adverse effects: information not available. |
| Outcomes |
Pain outcome: VAS for pain intensity, post Rx. ITT/per protocol analysis: information not available. Statistical analysis: statistically significant reduction in VAS scores post Rx in active TENS group. |
| Notes |
Treacy 2011.
| Methods |
Type of study: randomised, placebo controlled design (pilot study). Condition and number of participants randomised: 12 adults admitted for IV antibiotics with acute lung pain (VAS score > 4/10) Groups: active TENS and placebo TENS |
| Participants |
Demographics: N = 12; age and gender information not available. Setting: Northern Ireland; hospital inpatient setting. Inclusion: TENS naive. Exclusion: information not available. Withdrawals/dropouts: information not available. |
| Interventions |
Where applied: information not available. Applied by: information not available. Waveform: information not available. Frequency: 150 Hz. Pulse duration: 200 ms. Pulse amplitude/Intensity: information not available. Electrodes:Information not available. Duration and frequency of Rx: the duration of the lung pain. Device/manufacturer: information not available. Adverse effects: information not available. |
| Outcomes |
Pain outcome: VAS for pain intensity, before and after Rx. ITT/per protocol analysis: not detailed. Statistical analysis: statistically significant reduction in VAS scores post Rx in both groups. |
| Notes |
Differences between protocol and review
In the 2011 update we decided to use the Cochrane Collaboration's 'Risk of bias' assessment tool to ascertain the methodological quality of trials (instead of Jadad's scale) as this is now the Cochrane Collaboration's recommended tool for all Cochrane Reviews. We excluded trials if TENS was given in combination with any other treatment, either pharmacological or non‐pharmacological. We have listed the trials we excluded for this reason in Table 1.
Contributions of authors
DW was responsible for co‐ordinating the development of Walsh 2009 and the 2011 update. Professor Mark I. Johnson was responsible for co‐ordinating the development of this 2014 update and is its guarantor. DW conducted the original database searches. Dr Fidelma Moran joined the review team for the 2011 update. Dr Carole Paley joined the review team for the 2014 update. All review authors participated in the screening of studies against eligibility criteria, data extraction, interpretation of the data, formulation of the results and their clinical interpretation. All review authors developed and commented on the review drafts.
Sources of support
Internal sources
-
University of Ulster, UK
For Walsh 2009 and the 2011 update
-
Glasgow Caledonian University, UK
For Walsh 2009 and the 2011 and 2014 updates
-
Leeds Beckett University, UK
For Walsh 2009 and the 2011 and 2014 updates
-
University of Iowa, USA
For Walsh 2009 and the 2011 and 2014 updates
-
Airedale NGS Foundation Trust, UK
For the 2014 update
External sources
-
Cochrane Fellowship, Research and Development Office, Northern Ireland, UK
For Professor Deirdre Walsh in Walsh 2009 and the 2011 update
Strategic Priority Grant, Department for Employment and Learning, Northern Ireland, UK
Scottish Funding Council, UK
Chief Scientist Office Scotland, UK
NHS Education for Scotland, UK
Scottish Executive Health Department, UK
Declarations of interest
Mark I Johnson has no conflicts of interest to declare. Carole A Paley has no conflicts of interest to declare. Tracey E Howe has no conflicts of interest to declare. Kathleen A Sluka acts as a consultant for DJO, Inc. (declaration approved by the Cochrane Funding Arbiter).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Amer‐Cuenca 2011 {published data only}
- Amer-Cuenca JJ, Goicoechea C, Girona-López A, Andreu-Plaza JL, Palao-Román R, Martínez-Santa G, et al. Pain relief by applying transcutaneous electrical nerve stimulation (TENS) during unsedated colonoscopy: a randomized double-blind placebo-controlled trial. European Journal of Pain 2011;15(1):29-35. [DOI] [PubMed] [Google Scholar]
Cheing 2005 {published data only}
- Cheing GL, Luk ML. Transcutaneous electrical nerve stimulation for neuropathic pain. Journal of Hand Surgery - British Volume 2005;30(1):50-5. [DOI] [PubMed] [Google Scholar]
Coyne 1995 {published data only}
- Coyne PJ, MacMurren M, Izzo T, Kramer T. Transcutaneous electrical nerve stimulator for procedural pain associated with intravenous needlesticks. Journal of Intravenous Nursing 1995;18(5):263-7. [PubMed] [Google Scholar]
Crompton 1992 {published data only}
- Crompton AC, Johnson N, Dudek U, Batra N, Tucker A. Is transcutaneous electrical nerve stimulation of any value during cervical laser treatment. British Journal of Obstetrics & Gynaecology 1992;99(6):492-4. [DOI] [PubMed] [Google Scholar]
De Angelis 2003 {published data only}
- De Angelis C, Perrone G, Santoro G, Nofroni I, Zichella L. Suppression of pelvic pain during hysteroscopy with a transcutaneous electrical nerve stimulation device. Fertility & Sterility 2003;79(6):1422-7. [DOI] [PubMed] [Google Scholar]
de Sousa 2014 {published data only}
- Sousa L, Gomes-Sponholz FA, Nakano AMS. Transcutaneous electrical nerve stimulation for the relief of post-partum uterine contraction pain during breast-feeding: a randomized clinical trial. Journal of Obstetrics and Gynaecology Research 2014;40(5):1317-23. [DOI] [PubMed] [Google Scholar]
Ekblom 1987 {published data only}
- Ekblom A, Hansson P. Thermal sensitivity is not changed by acute pain or afferent stimulation. Journal of Neurology, Neurosurgery & Psychiatry 1987;50(9):1216-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregorini 2010 {published data only}
- Gregorini C, Cipriano JG, De Aquino LM, Rodrigues Branco JN, Bernardelli GF. Short-duration transcutaneous electrical nerve stimulation in the postoperative period of cardiac surgery. Arquivos Brasileiros De Cardiologia 2010;94(3):325-31. [DOI] [PubMed] [Google Scholar]
Hansson 1983 {published data only}
- Hansson P, Ekblom A. Transcutaneous electrical nerve stimulation (TENS) as compared to placebo TENS for the relief of acute oro-facial pain. Pain 1983;15(2):157-65. [DOI] [PubMed] [Google Scholar]
Hruby 2006 {published data only}
- Hruby G, Ames C, Chen C, Yan Y, Sagar J, Baron P, et al. Assessment of efficacy of transcutaneous electrical nerve stimulation for pain management during office-based flexible cystoscopy. Urology 2006;67(5):914-7. [DOI] [PubMed] [Google Scholar]
Keskin 2012 {published data only}
- Keskin EA, Onur O, Keskin HL, Gumus II, Kafali H, Turhan N. Transcutaneous electrical nerve stimulation improves low back pain during pregnancy. Gynecologic & Obstetric Investigation 2012;74(1):76-83. [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim S, Park K, Son B, Jeon Y. The effect of transcutaneous electrical nerve stimulation on pain during venous cannulation. Current Therapeutic Research, Clinical and Experimental 2012;73(4-5):134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Limoges 2004 {published data only}
- Limoges MF, Rickabaugh B. Evaluation of TENS during screening flexible sigmoidoscopy. Gastroenterology Nursing 2004;27(2):61-8. [DOI] [PubMed] [Google Scholar]
Liu 1985 {published data only}
- Liu YC, Liao WS, Lien IN. Effect of transcutaneous electrical nerve stimulation for post-thoracotomic pain. Journal of the Formosan Medical Association 1985;84(7):801-9. [PubMed] [Google Scholar]
Olsén 2007 {published data only}
- Olsén MF, Elden H, Janson ED, Lilja H, Stener-Victorin E. A comparison of high- versus low-intensity, high-frequency transcutaneous electric nerve stimulation for painful postpartum uterine contractions. Acta Obstetricia et Gynecologica Scandinavica 2007;86(3):310-4. [DOI] [PubMed] [Google Scholar]
Oncel 2002 {published data only}
- Oncel M, Sencan S, Yildiz H, Kurt N. Transcutaneous electrical nerve stimulation for pain management in patients with uncomplicated minor rib fractures. European Journal of Cardio-Thoracic Surgery 2002;22(1):13-7. [DOI] [PubMed] [Google Scholar]
Ordog 1987 {published data only}
- Ordog GJ. Transcutaneous electrical nerve stimulation versus oral analgesic: a randomized double-blind controlled study in acute traumatic pain. American Journal of Emergency Medicine 1987;5(1):6-10. [DOI] [PubMed] [Google Scholar]
Pitangui 2012 {published data only}
- Pitangui ACR, De Sousa L, Gomes FA, Ferreira CHJ, Nakano AMS. High-frequency TENS in post-episiotomy pain relief in primiparous puerpere: a randomized, controlled trial. Journal of Obstetrics and Gynaecology Research 2012;38(7):980-7. [DOI] [PubMed] [Google Scholar]
Roche 1985 {published data only}
- Roche PA, Gijsbers K, Belch JJ, Forbes CD. Modification of haemophiliac haemorrhage pain by transcutaneous electrical nerve stimulation. Pain 1985;21(1):43-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhmadeeva 2010 {published data only}
- Akhmadeeva LR, Setchenkova NM, Magzhanov RV, Abdrashitova EV, Bulgakova AZ. Randomized blind placebo-controlled study of the effectiveness of transcutaneous adaptive electrostimulation in the treatment of nonspecific low back pain [Russian]. Zhurnal nevrologii i psikhiatrii i S.S. Korsakova/Ministerstvo zdravookhraneniia i meditsinskoĭ promyshlennosti Rossiĭskoĭ Federatsii, Vserossiĭskoe obshchestvo nevrologov [i] Vserossiĭskoe obshchestvo psikhiatrov 2010;110(4):57-62. [PubMed] [Google Scholar]
Andersen 2009a {published data only}
- Andersen T, Christensen FB, Ernst C, Fruensgaard S, Østergaard J, Andersen JL et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: a randomized, controlled, multi-center trial: part 1: functional outcome. Spine 2009;34(21):2241-7. [DOI] [PubMed] [Google Scholar]
Andersen 2009b {published data only}
- Andersen T, Christensen FB, Egund N, Ernst C, Fruensgaard S, Østergaard J, et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: a randomized, controlled, multi-center trial: part 2: fusion rates. Spine 2009;34(21):2248-53. [DOI] [PubMed] [Google Scholar]
Barbarisi 2010 {published data only}
- Barbarisi M, Pace MC, Passavanti MB, Maisto M, Mazzariello L, Pota V et al. Pregabalin and transcutaneous electrical nerve stimulation for postherpetic neuralgia treatment. Clinical Journal of Pain 2010;26(7):567-72. [DOI] [PubMed] [Google Scholar]
Barker 2006 {published data only}
- Barker R, Lang T, Steinlechner B, Mora B, Heigel P, Gauss N, et al. Transcutaneous electrical nerve stimulation as prehospital emergency interventional care: treating acute pelvic pain in young women. Neuromodulation 2006;9(2):136-42. [DOI] [PubMed] [Google Scholar]
Baskurt 2006 {published data only}
- Baskurt Z, Baskurt F, Ozcan A, Yilmaz O. The immediate effects of heat and TENS on pressure pain threshold and pain intensity in patients with stage I shoulder impingement syndrome. Pain Clinic 2006;18(1):81-5. [Google Scholar]
Bertalanffy 2005 {published data only}
- Bertalanffy A, Kober A, Bertalanffy P, Gustorff B, Gore O, Adel S, et al. Transcutaneous electrical nerve stimulation reduces acute low back pain during emergency transport. Academic Emergency Medicine 2005;12(7):607-11. [DOI] [PubMed] [Google Scholar]
Celik 2013 {published data only}
- Celik EC, Erhan B, Gunduz B, Lakse E. The effect of low-frequency TENS in the treatment of neuropathic pain in patients with spinal cord injury. Spinal Cord 2013;51(4):334-7. [DOI] [PubMed] [Google Scholar]
Chee 1986 {published data only}
- Chee EK, Walton H. Treatment of trigger points with microamperage transcutaneous electrical nerve stimulation (TENS)--(the Electro-Acuscope 80). Journal of Manipulative & Physiological Therapeutics 1986;9(2):131-4. [PubMed] [Google Scholar]
Coletta 1988 {published data only}
- Coletta R, Maggiolo F, Di Tizio S. Etofenamate and transcutaneous electrical nerve stimulation treatment of painful spinal syndromes. International Journal of Clinical Pharmacology Research 1988;8(4):295-8. [PubMed] [Google Scholar]
Doğu 2009 {published data only}
- Doğu B, Yilmaz F, Karan A, Ergöz E, Kuran B. Comparative the effectiveness of occlusal splint and TENS treatments on clinical findings and pain threshold of temporomandibular disorders secondary to bruxism. Türkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2009;55(1):1-7. [Google Scholar]
Durmus 2009 {published data only}
- Durmus D, Akyol Y, Alayli G, Tander B, Zahiroglu Y, Canturk F. Effects of electrical stimulation program on trunk muscle strength, functional capacity, quality of life, and depression in the patients with low back pain: a randomized controlled trial. Rheumatology International 2009;29(8):947-54. [DOI] [PubMed] [Google Scholar]
Ekblom 1985 {published data only}
- Ekblom A, Hansson P. Extrasegmental transcutaneous electrical nerve stimulation and mechanical vibratory stimulation as compared to placebo for the relief of acute oro-facial pain. Pain 1985;23(3):223-9. [DOI] [PubMed] [Google Scholar]
Eyigor 2012 {published data only}
- Eyigor C, Eyigor S, Kivilcim Korkmaz O. Are intra-articular corticosteroid injections better than conventional TENS in treatment of rotator cuff tendinitis in the short run? A randomized study. European Journal of Physical and Rehabilitation Medicine 2012;46(3):315-24. [PubMed] [Google Scholar]
Fengler 2007 {published data only}
- Fengler RKB, Jacobs JWG, Bac M, Wijck AJM, Meeteren NLU. Action potential simulation (APS) in patients with fibromyalgia syndrome (FMS): a controlled single subject experimental design. Clinical Rheumatology 2007;26(3):322-9. [DOI] [PubMed] [Google Scholar]
Gemmell 2011 {published data only}
- Gemmell H, Hilland A. Immediate effect of electric point stimulation (TENS) in treating latent upper trapezius trigger points: a double blind randomised placebo-controlled trial. Journal of Bodywork and Movement Therapies 2011;15(3):348-54. [DOI] [PubMed] [Google Scholar]
Gül 2009 {published data only}
- Gül K, Onal SA. Comparison of non-invasive and invasive techniques in the treatment of patients with myofascial pain syndrome. Ağri Derneği'nin Yayin organidir 2009;21(3):104-12. [PubMed] [Google Scholar]
Gupta 2002 {published data only}
- Gupta, AK, Gupta S, Meena, DS, Sharma U. Post-tonsillectomy pain: Different modes of pain relief. Indian Journal of Otolaryngology and Head and Neck Surgery 2002;54(2):136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 1994 {published data only}
- Herman E, Williams R, Stratford P, Fargas-Babjak A, Trott M. A randomized controlled trial of transcutaneous electrical nerve stimulation (CODETRON) to determine its benefits in a rehabilitation program for acute occupational low back pain. Spine 1994;19(5):561-8. [DOI] [PubMed] [Google Scholar]
Izadpanah 2005 {published data only}
- Izadpanah A, Hosseini SV. Comparison of electrotherapy of hemorrhoids and Ferguson hemorrhoidectomy in a randomized prospective study. International Journal of Surgery 2005;3(4):258-62. [DOI] [PubMed] [Google Scholar]
Korkmaz 2010 {published data only}
- Korkmaz OK, Capaci K, Eyigor C, Eyigor S. Pulsed radiofrequency versus conventional transcutaneous electrical nerve stimulation in painful shoulder: a prospective, randomized study. Clinical Rehabilitation 2010;24(11):1000-8. [DOI] [PubMed] [Google Scholar]
Kumar 2014 {published data only}
- Kumar S, Raje A. Effect of progressive muscular relaxation exercises versus transcutaneous electrical nerve stimulation on tension headache: A comparative study. Hong Kong Physiotherapy Journal 2014;32(2):86-91. [Google Scholar]
Lang 2007 {published data only}
- Lang T, Barker R, Steinlechner B, Gustorff B, Puskas T, Gore O, et al. TENS relieves acute posttraumatic hip pain during emergency transport. Journal of Trauma 2007;62(1):184-8. [DOI] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee JC, Lin DT, Hong C-Z. The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger points. Journal of Musculoskeletal Pain 1997;5(1):81-90. [Google Scholar]
Lee 2012 {published data only}
- Lee J, Rakel B, Dailey D, Vance C, Broderick A, Sluka K, et al. The effectiveness of TENS for head and neck cancer pain and function: a randomized and placebo-controlled double blind pilot study. Journal of Pain 2012;13(4):S63. [Google Scholar]
Leo 1986 {published data only}
- Leo KC, Dostal WF, Bossen DG, Eldridge VL, Fairchild ML, Evans RE. Effect of transcutaneous electrical nerve stimulation characteristics on clinical pain. Physical Therapy 1986;66(2):200-5. [DOI] [PubMed] [Google Scholar]
Mora 2006 {published data only}
- Mora B, Giorni E, Dobrovits M, Barker R, Lang T, Gore C, et al. Transcutaneous electrical nerve stimulation: an effective treatment for pain caused by renal colic in emergency care. Journal of Urology 2006;175(5):1737-41. [DOI] [PubMed] [Google Scholar]
Murina 2008 {published data only}
- Murina F, Bianco V, Radici G, Felice R, Di Martino M, Nicolini U. Transcutaneous electrical nerve stimulation to treat vestibulodynia: a randomised controlled trial. British Journal of Obstetrics & Gynaecology 2008;115(9):1165-70. [DOI] [PubMed] [Google Scholar]
Myśliwiec 2011 {published data only}
- Myśliwiec A, Saulicz E, Kuszewski M, Kokosz M, Wolny T. Assessment of the influence of Saunders traction and transcutaneous electrical nerve stimulation on hand grip force in patients with neck pain. Ortopedia, Traumatologia, Rehabilitacja 2011;13(1):37-44. [DOI] [PubMed] [Google Scholar]
Peng‐fei 2011 {published data only}
- Peng-fei S, Yu-hua J. Pulsed radiofrequency versus conventional transcutaneous electrical nerve stimulation in painful shoulder: a prospective, randomized study - can you really have the conclusion? Clinical Rehabilitation 2011;25(9):856-7. [DOI] [PubMed] [Google Scholar]
Pope 1994 {published data only}
- Pope MH, Phillips RB, Haugh LD, Hsieh CY, MacDonald L, Haldeman S. A prospective randomized three-week trial of spinal manipulation, transcutaneous muscle stimulation, massage and corset in the treatment of subacute low back pain. Spine 1994;19(22):2571-7. [DOI] [PubMed] [Google Scholar]
Reichstein 2005 {published data only}
- Reichstein L, Labrenz S, Ziegler D, Martin S. Effective treatment of symptomatic diabetic polyneuropathy by high-frequency external muscle stimulation. Diabetologia 2005;48(5):824-8. [DOI] [PubMed] [Google Scholar]
Rodarti 2012 {published data only}
- Pitangui AC, Sousa L, Gomes FA, Ferreira CH, Nakano AM. High-frequency TENS in post-episiotomy pain relief in primiparous puerpere: a randomized, controlled trial. Journal of Obstetrics and Gynaecology Research 2012;38(7):980-7. [DOI] [PubMed] [Google Scholar]
Rodríguez‐Fernández 2011 {published data only}
- Rodríguez-Fernández ÁL, Garrido-Santofimia V, Güeita-Rodríguez J, Fernández-de-las-Peñas C. Effects of burst-type transcutaneous electrical nerve stimulation on cervical range of motion and latent myofascial trigger point pain sensitivity. Archives of Physical Medicine and Rehabilitation 2011;92(9):1353-8. [DOI] [PubMed] [Google Scholar]
Sahin 2011 {published data only}
- Sahin N, Albayrak I, Ugurlu H. Effect of different transcutaneous electrical stimulation modalities on cervical myofascial pain syndrome. Journal of Musculoskeletal Pain 2011;19(1):18-23. [Google Scholar]
Solomon 1985 {published data only}
- Solomon S, Guglielmo KM. Treatment of headache by transcutaneous electrical stimulation. Headache 1985;25(1):12-5. [DOI] [PubMed] [Google Scholar]
Stratton 2009 {published data only}
- Stratton M, McPoil TG, Cornwall MW, Patrick K. Use of low-frequency electrical stimulation for the treatment of plantar fasciitis. Journal of the American Podiatric Medical Association 2009;99(6):481-8. [DOI] [PubMed] [Google Scholar]
Sunshine 1996 {published data only}
- Sunshine W, Field TM, Quintino O, Fierro K, Kuhn C, Burman I, et al. Fibromyalgia benefits from massage therapy and transcutaneous electrical stimulation. Journal of Clinical Rheumatology 1996;2(1):18-22. [DOI] [PubMed] [Google Scholar]
Taskaynatan 2007 {published data only}
- Taskaynatan MA, Ozgul A, Ozdemir A, Tan AK, Kalyon TA. Effects of steroid iontophoresis and electrotherapy on bicipital tendonitis. Journal of Musculoskeletal Pain 2007;15(4):47-54. [Google Scholar]
Tsai 2010 {published data only}
- Tsai C, Chang W, Lee J. Effects of short-term treatment with kinesiotaping for plantar fasciitis. Journal of Musculoskeletal Pain 2010;18(1):71-80. [Google Scholar]
Tulgar 1991a {published data only}
- Tulgar M, McGlone F, Bowsher D, Miles JB. Comparative effectiveness of different stimulation modes in relieving pain. Part I. A pilot study. Pain 1991;47(2):151-5. [DOI] [PubMed] [Google Scholar]
Tulgar 1991b {published data only}
- Tulgar M, McGlone F, Bowsher D, Miles JB. Comparative effectiveness of different stimulation modes in relieving pain. Part II. A double-blind controlled long-term clinical trial. Pain 1991;47(2):157-62. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang ZX. Clinical observation on electroacupuncture at acupoints for treatment of senile radical sciatica [Chinese]. Zhongguo zhen jiu (Chinese Acupuncture & Moxibustion) 2009;29(2):126-8. [PubMed] [Google Scholar]
References to studies awaiting assessment
Cambiaghi 2013 {published data only}
- Cambiaghi AS, Leao RBF, Alvarez AV, Nascimento PF. Transcutaneous electrical nerve stimulation can reduce pain during diagnostic hysteroscopy and endometrial biopsy: a randomized, controlled trial. Fertility and Sterility 2013;100(3):S377. [Google Scholar]
de Paiva Tosato 2007 {published data only}
- Paiva Tosato J, Biasotto-Gonzalez DA, Caria PHF. Effect of massage therapy and of transcutaneous electrical nerve stimulation on pain and electromyographic activity in patients with temporomandibular dysfunction [Portugese]. Fisioterapia e Pesquisa 2007;14(2):21-6. [Google Scholar]
França 2012 {published data only}
- França FR, Ramos LV, Burke TN, Caffaro RR, Marques AP. Lumbar stabilization and transcutaneous electrical nerve stimulation in lumbar disc herniation: Preliminary study. Annals of the Rheumatic Disease. Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2012;71(Suppl 3):757. [Google Scholar]
Hsueh 1997 {published data only}
- Hsueh TC, Cheng PT, Kuan TS, Hong CZ. The immediate effectiveness of electrical nerve stimulation and electrical muscle stimulation on myofascial trigger points. American Journal of Physical Medicine & Rehabilitation 1997;76(6):471-6. [DOI] [PubMed] [Google Scholar]
Liebano 2013 {published data only}
- Liebano R, Galli T, Chiavegato, L. Effects of transcutaneous electrical nerve stimulation on pain, mobility and pulmonary function in kidney donors: a randomized, double-blind and controlled trial. Journal of Pain 2013;14(4):S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park C, Han D. Intraoperative transcutaneous electric nerve stimulation for postoperative posterior neck pain after thyroidectomy: A randomized trial. Pain Practice 2014;14:94. [Google Scholar]
Rajpurohit 2010 {published data only}
- Rajpurohit B, Khatri SM, Metgud D, Bagewadi A. Effectiveness of transcutaneous electrical nerve stimulation and microcurrent electrical nerve stimulation in bruxism associated with masticatory muscle pain - a comparative study. Indian Journal of Dental Research 2010;21(1):104-6. [DOI] [PubMed] [Google Scholar]
Salvador 2005 {published data only}
- Salvador D, El Daher Neto PD, Ferrari FP. Application of muscle energy technique in garbage collectors with acute mechanical lumbar pain Portuguese [Portuguese]. Fisioterapia e Pesquisa 2005;12(2):20-7. [Google Scholar]
Salvino 2013 {published data only}
- Salvino D, Curcio M, Trimboli M, Paletta R, Mazza MR, Quattrone A, et al. Cutaneous allodynia influences the analgesic effect of transcutaneous electrical nerve stimulation therapy in headache sufferers. Cephalalgia 2013;33(8):197. [Google Scholar]
Silva 2012 {published data only}
- Silva MB, Melo PR, Oliveira NML, Crema E, Fernandes LFRM. Analgesic effect of transcutaneous electrical nerve stimulation after laparoscopic cholecystectomy. American Journal of Physical Medicine & Rehabilitation 2012;91(8):652-7. [DOI] [PubMed] [Google Scholar]
Treacy 2011 {published data only}
- Treacy K, Walsh DM, Kent L, McCrory L, Rendall J, Elborn JS, et al. Transcutaneous electrical nerve stimulation (TENS) for acute lung pain in cystic fibrosis. Journal of Cystic Fibrosis 2011;10:S66. [Google Scholar]
Additional references
Aarskog 2007
- Aarskog R, Johnson MI, Demmink JH, Lofthus A, Iversen V, Lopes-Martins R, et al. Is mechanical pain threshold after transcutaneous electrical nerve stimulation (TENS) increased locally and unilaterally? A randomized placebo-controlled trial in healthy subjects. Physiotherapy Research International 2007;12(4):251-63. [DOI] [PubMed] [Google Scholar]
Bennett 2011
- Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain 2011;152(6):1226-32. [DOI] [PubMed] [Google Scholar]
Bjordal 2003
- Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. European Journal of Pain 2003;7(2):181-8. [DOI] [PubMed] [Google Scholar]
Bjordal 2007
- Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskeletal Disorders 2007;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, CONSORT group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295-309. [DOI] [PubMed] [Google Scholar]
Carroll 1996
- Carroll D, Tramèr M, McQuay H, Nye B, Moore A. Randomization is important in studies with pain outcomes: systematic review of transcutaneous electrical nerve stimulation in acute postoperative pain. British Journal of Anaesthesia 1996;77(6):798-803. [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen C, Tabasam G, Johnson MI. Does the pulse frequency of transcutaneous electrical nerve stimulation (TENS) influence hypoalgesia? A systematic review of studies using experimental pain and healthy human participants. Physiotherapy 2008;94(1):11-20. [Google Scholar]
Chen 2010a
- Chen CC, Johnson MI. An investigation into the hypoalgesic effects of high and low frequency transcutaneous electrical nerve stimulation (TENS) on experimentally-induced blunt pressure pain in healthy human participants. Journal of Pain 2010;11(1):53-61. [DOI] [PubMed] [Google Scholar]
Chen 2010b
- Chen CC, Johnson MI. A comparison of transcutaneous electrical nerve stimulation (TENS) at 3 and 80 pulses per second on cold-pressor pain in healthy human participants. Clinical Physiology and Functional Imaging 2010;30(4):260-8. [DOI] [PubMed] [Google Scholar]
Chen 2011
- Chen CC, Johnson MI. Differential frequency effects of strong non-painful transcutaneous electrical nerve stimulation (TENS) on experimentally-induced ischaemic pain in healthy human participants. Clinical Journal of Pain 2011;27(5):434-41. [DOI] [PubMed] [Google Scholar]
Claydon 2008
- Claydon LS, Chesterton LS, Barlas P, Sim J. Effects of simultaneous dual-site TENS stimulation on experimental pain. European Journal of Pain 2008;12(6):696-704. [DOI] [PubMed] [Google Scholar]
Claydon 2011
- Claydon LS, Chesterton LS, Barlas P, Sim J. Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: a systematic review. Clinical Journal of Pain 2011;27(7):635–47. [DOI] [PubMed] [Google Scholar]
DeSantana 2008
- DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports 2008;10(6):492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deyo 1990
- Deyo RA, Walsh NE, Schoenfeld LS, Ramamurthy S. Can trials of physical treatments be blinded? The example of transcutaneous electrical nerve stimulation for chronic pain. American Journal of Physical Medicine and Rehabilitation 1990;69(1):6-10. [DOI] [PubMed] [Google Scholar]
Dowswell 2009
- Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain management in labour. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007214. [DOI: 10.1002/14651858.CD007214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 2011
- Francis RP, Johnson MI. The characteristics of acupuncture-like transcutaneous electrical nerve stimulation (acupuncture-like TENS): a literature review. Acupuncture and Electrotherapeutics Research 2011;36(3-4):231-58. [DOI] [PubMed] [Google Scholar]
Freynet 2010
- Freynet A, Falcoz PE. Is transcutaneous electrical nerve stimulation effective in relieving postoperative pain after thoracotomy? Interactive Cardiovascular Thoracic Surgery 2010;10(2):283-8. [DOI] [PubMed] [Google Scholar]
Garrison 1994
- Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS). Pain 1994;58(3):309-15. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Houghton 2010
- Houghton P, Nussbaum E, Hoens A. Electrophysical agents - Contraindications and precautions: an evidence-based approach to clinical decision making in physical therapy. Physiotherapy Canada 2010;62(5):1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrobjartsson 2007
- Hrobjartsson A, Forfang E, Haahr MT, Als-Nielsen B, Brorson S. Blinded trials taken to the test: an analysis of randomized clinical trials that report tests for the success of blinding. International Journal of Epidemiology 2007;36(3):654-63. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Johnson 1991
- Johnson MI, Ashton CH, Thompson JW. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain 1991;44(3):221-9. [DOI] [PubMed] [Google Scholar]
Johnson 1999
- Johnson MI, Tabasam G. A double blind placebo controlled investigation into the analgesic effects of interferential currents (IFC) and transcutaneous electrical nerve stimulation (TENS) on cold induced pain in healthy subjects. Physiotherapy Theory and Practice 1999;15(4):217-33. [Google Scholar]
Johnson 2007
- Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain 2007;130(1-2):157-65. [DOI] [PubMed] [Google Scholar]
Johnson 2010
- Johnson MI, Walsh DM. Pain: continued uncertainty of TENS' effectiveness for pain relief. Nature Reviews Rheumatology 2010;6(6):314-6. [DOI] [PubMed] [Google Scholar]
Johnson 2011
- Johnson MI, Bjordal JM. Transcutaneous Electrical Nerve Stimulation for the management of painful conditions: focus on neuropathic pain. Expert Review of Neurotherapeutics 2011;11(5):735–53. [DOI] [PubMed] [Google Scholar]
Johnson 2014
- Johnson MI. Transcutaneous Electrical Nerve Stimulation (TENS). Research to Support Clinical Practice. Oxford: Oxford University Press, 2014. [Google Scholar]
Kalra 2001
- Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). Journal of Pharmacology and Experimental Therapeutics 2001;298(1):257-63. [PubMed] [Google Scholar]
Leonard 2010
- Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain 2010;151(1):215-9. [DOI] [PubMed] [Google Scholar]
Léonard 2011
- Léonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. Journal of Pain 2011;12(2):213-21. [DOI] [PubMed] [Google Scholar]
Levin 1993
- Levin MF, Hui-Chan CW. Conventional and acupuncture-like transcutaneous electrical nerve stimulation excite similar afferent fibers. Archives of Physical Medicine and Rehabilitation 1993;74(1):54-60. [PubMed] [Google Scholar]
Liebano 2011
- Liebano RE, Rakel B, Vance CG, Walsh DM, Sluka KA. An investigation of the development of analgesic tolerance to TENS in humans. Pain 2011;152(2):335-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150(3699):971-9. [DOI] [PubMed] [Google Scholar]
Merskey 1994
- Merskey H, Bogduk N (editors). Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd edition. Seattle: IASP Press, 1994. [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD003222. [DOI: 10.1002/14651858.CD003222.pub2] [DOI] [PubMed] [Google Scholar]
Proctor 2002
- Proctor ML, Smith CA, Farquhar CM, Stones RW. Transcutaneous electrical nerve stimulation and acupuncture for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD002123. [DOI: 10.1002/14651858.CD002123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radhakrishnan 2003
- Radhakrishnan R, King EW, Dickman JK, Herold CA, Johnston NF, Spurgin ML, et al. Spinal 5-HT(2) and 5-HT(3) receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain 2003;105(1-2):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radhakrishnan 2005
- Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-induced antihyperalgesia. Journal of Pain 2005;6(10):673-80. [DOI] [PubMed] [Google Scholar]
Rakel 2010
- Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. Journal of Pain 2010;11(3):230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre; The Cochrane Collaboration Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre; The Cochrane Collaboration, 2014.
Sbruzzi 2012
- Sbruzzi G, Silveira SA, Silva DV, Coronel CC, Plentz RD. Transcutaneous electrical nerve stimulation after thoracic surgery: systematic review and meta-analysis of 11 randomized trials. Revista Brasileira de Cirurgia Cardiovascular 2012;27(1):75-87. [DOI] [PubMed] [Google Scholar]
Schug 2014
- Schug SA, Watson D, Pogatzki-Zahn EM. Acute pain. In: Griensven H, Strong J, Unrih AM, editors(s). Pain. A Textbook for Health Professionals. 2 edition. Edinburgh: Churchill Livingstone Elsevier, 2014:395-405. [Google Scholar]
Sluka 1999
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. Journal of Pharmacology and Experimental Therapeutics 1999;289(2):840-6. [PubMed] [Google Scholar]
Sluka 2013
- Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Physical Therapy 2013;93(10):1397-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strong 2002
- Strong J. Chronic pain problems. In: Strong J, Unruh A, Wright A, Baxter GD, editors(s). Pain: a Textbook for Therapists. Edinburgh: Churchill Livingstone, 2002:397-410. [Google Scholar]
Tong 2007
- Tong KC, Lo SK, Cheing GL. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Archives of Physical Medicine and Rehabilitation 2007;88(10):1344-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walsh 2009
- Walsh DM, Howe TE, Johnson MI, Moran F, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD006142. [DOI: 10.1002/14651858.CD006142.pub2] [DOI] [PubMed] [Google Scholar]


