Abstract
Background
Diarrhoea accounts for 1.8 million deaths in children in low‐ and middle‐income countries (LMICs). One of the identified strategies to prevent diarrhoea is hand washing.
Objectives
To assess the effects of hand‐washing promotion interventions on diarrhoeal episodes in children and adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, nine other databases, the World Health Organization (WHO) International Clinical Trial Registry Platform (ICTRP), and metaRegister of Controlled Trials (mRCT) on 8 January 2020, together with reference checking, citation searching and contact with study authors to identify additional studies.
Selection criteria
Individually‐randomized controlled trials (RCTs) and cluster‐RCTs that compared the effects of hand‐washing interventions on diarrhoea episodes in children and adults with no intervention.
Data collection and analysis
Three review authors independently assessed trial eligibility, extracted data, and assessed risks of bias. We stratified the analyses for child day‐care centres or schools, community, and hospital‐based settings. Where appropriate, we pooled incidence rate ratios (IRRs) using the generic inverse variance method and a random‐effects model with a 95% confidence interval (CI). We used the GRADE approach to assess the certainty of the evidence.
Main results
We included 29 RCTs: 13 trials from child day‐care centres or schools in mainly high‐income countries (54,471 participants), 15 community‐based trials in LMICs (29,347 participants), and one hospital‐based trial among people with AIDS in a high‐income country (148 participants). All the trials and follow‐up assessments were of short‐term duration.
Hand‐washing promotion (education activities, sometimes with provision of soap) at child day‐care facilities or schools prevent around one‐third of diarrhoea episodes in high‐income countries (incidence rate ratio (IRR) 0.70, 95% CI 0.58 to 0.85; 9 trials, 4664 participants, high‐certainty evidence) and may prevent a similar proportion in LMICs, but only two trials from urban Egypt and Kenya have evaluated this (IRR 0.66, 95% CI 0.43 to 0.99; 2 trials, 45,380 participants; low‐certainty evidence). Only four trials reported measures of behaviour change, and the methods of data collection were susceptible to bias. In one trial from the USA hand‐washing behaviour was reported to improve; and in the trial from Kenya that provided free soap, hand washing did not increase, but soap use did (data not pooled; 3 trials, 1845 participants; low‐certainty evidence).
Hand‐washing promotion among communities in LMICs probably prevents around one‐quarter of diarrhoea episodes (IRR 0.71, 95% CI 0.62 to 0.81; 9 trials, 15,950 participants; moderate‐certainty evidence). However, six of these nine trials were from Asian settings, with only one trial from South America and two trials from sub‐Saharan Africa. In seven trials, soap was provided free alongside hand‐washing education, and the overall average effect size was larger than in the two trials which did not provide soap (soap provided: RR 0.66, 95% CI 0.58 to 0.75; 7 trials, 12,646 participants; education only: RR 0.84, 95% CI 0.67 to 1.05; 2 trials, 3304 participants). There was increased hand washing at major prompts (before eating or cooking, after visiting the toilet, or cleaning the baby's bottom) and increased compliance with hand‐hygiene procedure (behavioural outcome) in the intervention groups compared with the control in community trials (data not pooled: 4 trials, 3591 participants; high‐certainty evidence).
Hand‐washing promotion for the one trial conducted in a hospital among a high‐risk population showed significant reduction in mean episodes of diarrhoea (1.68 fewer) in the intervention group (mean difference −1.68, 95% CI −1.93 to −1.43; 1 trial, 148 participants; moderate‐certainty evidence). Hand‐washing frequency increased to seven times a day in the intervention group versus three times a day in the control arm in this hospital trial (1 trial, 148 participants; moderate‐certainty evidence).
We found no trials evaluating the effects of hand‐washing promotions on diarrhoea‐related deaths or cost effectiveness.
Authors' conclusions
Hand‐washing promotion probably reduces diarrhoea episodes in both child day‐care centres in high‐income countries and among communities living in LMICs by about 30%. The included trials do not provide evidence about the long‐term impact of the interventions.
Plain language summary
Does encouraging people to wash their hands stop them having diarrhoea?
Key messages
Encouraging hand washing probably reduces the number of times children have diarrhoea, by around 30%, in communities in low‐ to middle‐income countries and in child‐care centres in high‐income countries.
We did not find evidence about the long‐term effects of hand‐washing programmes.
What causes diarrhoea?
‘Diarrhoea’ is the name for frequent bowel movements or the passing of unusually soft or watery faeces. Infections of the gut by bacteria, viruses, or parasites commonly cause diarrhoea, and are mostly spread through water contaminated with faeces.
The symptoms of diarrhoea usually improve in a couple of days. However, in severe or long‐lasting diarrhoea, too much water, salts, and nutrients may be lost from the body. This loss can cause dehydration and even death. Diarrhoea is a leading cause of death and sickness among children under five years of age.
Preventing diarrhoea
Most deaths associated with diarrhoea are caused by pathogens acquired as a result of unsafe drinking water, poor sanitary conditions, and lack of hygiene. Washing hands with soap and water removes the bacteria, viruses, and parasites that cause disease. Programmes and activities encouraging people to wash their hands have been developed for use in communities and schools, including hygiene training, posters, leaflets, comic books, songs, and drama.
Why we did this Cochrane Review
We know that hand washing at appropriate times can prevent diarrhoea, but we do not know how best to encourage the practice. We wanted to find out if programmes and activities that had been studied for this purpose were effective at increasing hand washing and reducing diarrhoea.
What did we do?
We searched for studies that investigated the use of programmes to encourage hand washing in communities, day‐care centres, schools, hospitals, and households. We were interested in whether taking part in the programmes affected the number of times people in the study reported having diarrhoea.
We looked for studies in which the treatments people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a treatment.
Search date
We included evidence published up to 8 January 2020.
What we found
We found 29 studies:
13 studies (in 54,471 people) took place in child day‐care centres or schools in mainly high‐income countries; 15 studies (in 29,347 people) were community‐based in low‐ to middle‐income countries; and 1 study (in 148 people) was hospital‐based.
The studies looked at the effects of hand‐washing programmes on the number of times people in the study reported having diarrhoea. The effects of the programmes were followed for four months to one year.
No studies reported the effects of hand‐washing programmes on how many people died from diarrhoea, how many children under five years of age died (of any cause), or whether the benefits associated with the programme outweighed any extra costs.
What are the results of our review?
All studies compared the effects of programmes to encourage hand washing with not having any programmes about hand washing.
In child‐care centres and schools: in high‐income countries, encouraging hand washing reduced the number of times children had diarrhoea (9 studies, 4664 children); and in low‐ to middle‐income countries may have reduced the number of times children had diarrhoea (2 studies, 45,380 children).
In communities in low‐ to middle‐income countries, encouraging hand washing probably reduced the number of times children (up to 15 years of age) had diarrhoea (9 studies,; 15,950 children).
In hospitalized adults with AIDS, encouraging hand washing probably reduced the number of times they had diarrhoea and probably improved hand‐washing behaviour (washing hands more often) over one year of follow‐up (1 study, 148 people).
How reliable are these results?
We are confident that, in high‐income countries, hand‐washing programmes in schools and child‐care centres reduced the number of times children had diarrhoea. This result is unlikely to change with more evidence. We are less confident about our result for low‐ to middle‐income countries, which is based on a small number of studies and might or might not change with more evidence.
We are moderately confident about our results for children in communities and in hospitalized adults with AIDS. These results might change if more evidence becomes available.
Summary of findings
Background
Description of the condition
Diarrhoeal disease ranks among the top 1% of causes of death, particularly at the two extremes of life (Mbakaya 2017). Diarrhoea remains a serious global public health problem, accounting for 1.8 million deaths annually, especially among children under five years of age (Walker 2013). The yearly global diarrhoeal disease burden is estimated at 72.8 million disability‐adjusted life years lost through incapacitation and premature deaths, mainly in low‐ and middle‐income countries (LMICs) (Murray 2012).
A synergistic relationship exists between diarrhoea and malnutrition, often demonstrated through a combination of forced low‐nutrient intake, reduced absorption, and increased nutrient excretion (WHO 2003; Luby 2018 BGD). The malnutrition‐infection complex is clearly reinforced during diarrhoea episodes, as poor nutritional status predisposes children to more severe and persistent diarrhoea, impaired growth and development, and higher case fatality rates (UNICEF/WHO 2009; Lee 2012; Luby 2018 BGD).
Diarrhoeal disease pathogens are usually transmitted through the faecal‐oral route (Curtis 2000). The pathways include ingestion of food and water contaminated by faecal matter, person‐to‐person contact, and direct contact with infected faeces (Eisenberg 2012). Some trials estimate that over 75% of all diarrhoea cases can be attributed to contaminated food and water (Curtis 2000; Maxwell 2012). Poor hygiene behaviours and improper handling practices of caregivers are associated with high levels of bacterial contamination of food and water (Iroegbu 2000; Mannan 2010; Pickering 2011).
Behaviours that encourage human contact with faecal matter include the following: improper disposal of faeces; children defaecating on the floor; rags being used to cleanse the child after defaecation; and lack of hand washing after defaecation, handling faeces (including children's faeces), or cleansing the child's perineum before handling food by caregivers and children (Pickering 2011). In particular, hand contact with ready‐to‐eat food (i.e. food consumed without further washing, cooking, or processing or preparation by the consumer) represents a potentially important mechanism by which diarrhoea‐causing pathogens contaminate food and water (UNICEF/WHO 2009). In addition, flies serve as vectors of diarrhoea‐causing pathogens to humans. Thus, consumption of food exposed to flies is associated with a high risk of diarrhoea (Marino 2007).
Household economic status is significantly associated with diarrhoea prevalence (Woldemicael 2001), especially in low‐income countries. Households may lack basic infrastructure for proper hygiene practices, such as facilities for proper disposal of excreta. In addition, even where available, these may not be adapted for children's use (Tumwine 2002; UNICEF/WHO 2009). This often leads to indiscriminate defaecation in and around the premises and to increased risk of excreta handling by mothers, caregivers, and children (Nielsen 2001). A trial in Eritrea found that the availability of a toilet facility in households was associated with a 27% reduction in the risk of diarrhoea (Woldemicael 2001). The same trial also found associations between the number of children living in the house and diarrhoea morbidity. In some cultures children's faeces are regarded as innocuous. For this reason adults may not wash their hands after handling children's faeces and may cleanse a child with their bare hands (Traoré 1994; Curtis 2000). However, evidence suggests that children's faeces are as hazardous as adult faeces and may contain even higher concentrations of pathogens than those of adults, due to the children's increased interactions with contaminated materials in their surroundings (Oketcho 2012).
Description of the intervention
Hygiene‐promotion interventions constitute one of a number of strategies identified by the World Health Organization (WHO) for control of diarrhoea (UNICEF/WHO 2009). These constitute a range of activities aimed at encouraging individuals and communities to adopt safer practices within domestic and community settings to prevent hygiene‐related diseases that lead to diarrhoea (WELL 1999; Ehiri 2001); hand washing is one such intervention. The practice of hand washing and the factors that influence hand‐washing behaviour among individuals in communities are complex and include psychosocial, contextual, and infrastructural reasons (Whitby 2007; Mbakaya 2017); for example, washing hands with water only or with soap may be influenced both by knowledge of best practice and by the availability of water and soap (Curtis 2011). Also, hand washing may require infrastructural, cultural, and behavioural changes, which take time to develop, as well as substantial resources (e.g. trained personnel, community organization, provision of water supply and soap) (Luby 2001a; UNICEF/WHO 2009). Consideration of the wide applicability and sustainability of hygiene interventions continues to come under critical review (Luby 2006 PAK; Ejemot‐Nwadiaro 2008; Gould 2017; Curtis 2011; Huis 2012; Madhu 2012; Ejemot‐Nwadiaro 2015; Luby 2018 BGD; Null 2018 KEN). For example, maintenance of the new hand‐washing behaviours that result from promotional interventions is vital to maximizing the associated potential health benefits. Apart from the challenges of sustaining new behaviour (hand washing) among the target communities, cost has been identified as a major factor that limits the sustainability of hand‐hygiene behaviour (Langford 2007 NPL; Hartinger 2011 PER). For example, to sustain the health benefits of newly‐acquired hand‐washing behaviours, it is also important that individuals and communities have access to resources that support hand washing, including water and soap. Lack of access to hand‐washing resources may therefore limit the potential impact of hand washing on health, particularly for low‐income households and communities.
How the intervention might work
Hand washing helps decontaminate the hands and prevent cross‐transmission of diarrhoeal‐causing pathogens (Ehiri 2001; Gurjeet 2013). Hand‐washing promotion uses direct approaches, such as training and educating individuals or groups of individuals about hygiene, diarrhoea transmission, the relationship between germs and health and demonstrating this relationship through leaflets, posters, drama, and songs (Whitby 2007; Curtis 2011). Washing hands with soap and water removes pathogens mechanically and may also chemically kill contaminating and colonizing flora, making hand washing more effective (Hugonnet 2000). Washing hands with soap under running water or large quantities of water with vigorous rubbing was found to be more effective than several members of a household dipping their hands into the same bowl of water (often without soap) (Luby 2005), which is a common practice in many low‐income countries, especially before household meals (Ehiri 2001). This may contribute to, rather than prevent, food contamination, as pathogens present on the contaminated hands of household members can be transferred to those who subsequently dip their hands in the same bowl of water (Prüss 2002).
Why it is important to do this review
Hand washing is an effective intervention in the control of diarrhoeal diseases. It is listed in the UNICEF/WHO 2009 seven‐point plan for comprehensive control of diarrhoea. Hand washing requires infrastructural, cultural, and behavioural changes that take time and substantial resources to develop (Cave 1999; Yeager 1999; Luby 2001b). Given that resources spent on interventions to promote hand washing could be invested in other public health programmes, it is important to ascertain whether hand‐washing promotion is an efficient use of scarce health resources. In 2008, we published a review that assessed in RCTs the broader question of the effectiveness of hand washing with soap in preventing diarrhoea compared with other interventions, such as provision of water and improvement of water quality (treatment of water) (Ejemot‐Nwadiaro 2008). A review by Curtis 2003, which examined the effectiveness of hand washing with soap in community‐based trials, estimated that hand washing could reduce diarrhoea risk by up to 47%. Similarly, Fewtrell 2005 examined a range of water, sanitation, and hygiene interventions in LMICs, and estimated that hygiene interventions reduced diarrhoea incidence by 44%. However, both reviews included non‐randomized trials. Curtis 2003 included cross‐sectional trials, which have inherent limitations on the establishment of causal relationships. Fewtrell 2005 presented evidence of publication bias in included trials. In this Cochrane Review, we assess whether the estimate of effect observed only in RCTs is of similar magnitude to those seen in previous reviews, and the applicability of hand‐washing promotion in reducing diarrhoeal diseases across wide population groups. We also include both institution‐based and community‐based trials in countries of any income level.
In 2015, we published a review update that provided evidence that interventions to promote hand hygiene observed only in RCTs can decrease diarrhoea rates by approximately 30% (Ejemot‐Nwadiaro 2015). However, there were few studies of high methodological quality to make a strong statement on the effect of the intervention in each of the identified settings. In addition, it is important to assess the sustainability of hand‐washing practices or behaviours and effects on diarrhoeal illness in the long term. Single or multiple hand‐washing intervention pathways to reducing diarrhoea still remain a key issue, especially for scaling up (large‐scale and long‐duration studies) and for cost benefit or cost effectiveness analysis. Evidence on these aspects remains scant (Ejemot‐Nwadiaro 2015), with these intricately related to issues of intervention sustainability. Given that diarrhoea remains a significant public health problem in LMICs, there is a need for robust evidence to improve precision in the magnitude of effect obtained and the certainty of the evidence presented in the last update.
Objectives
To assess the effects of hand‐washing promotion interventions on diarrhoeal episodes in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), including cluster‐RCTs.
Types of participants
Individuals (adults and children) in day‐care centres (DCCs) or schools, communities, or households, and patients in hospitals.
Types of interventions
Intervention
Activities that promoted hand washing after defaecation or after disposal of children's faeces and before eating, preparing, or handling foods; for example, small‐group discussions and larger meetings on hygiene education, germs‐health awareness interventions, multimedia communication campaigns with posters, radio and TV campaigns, leaflets, comic books, songs, slide shows, use of T‐shirts and badges, pictorial stories, dramas, and games. We included trials that focused exclusively on hand washing and those that had hand washing as part of a broader package of hygiene interventions if they undertook analyses of the effects of hand washing on diarrhoea.
Control
No hand‐washing promotion.
Types of outcome measures
Primary outcomes
Episodes of diarrhoea (self‐reports collected through home visits; hospital or health‐centre or clinic records, including admissions for diarrhoea‐related dehydration).
We defined diarrhoea as:
acute or primary diarrhoea: passage of three or more loose or watery stools in a 24‐hour period, a loose stool being one that would take the shape of a container, or definitions used by trial authors consistent with this standard definition;
persistent diarrhoea: diarrhoea lasting 14 days or more;
dysentery: stool with blood.
Secondary outcomes
diarrhoea‐related death among children or adults;
behavioural changes, such as changes in the proportion of people who reported or are observed washing their hands after defaecation, disposal of children's faeces, or before preparing or handling foods;
changes in knowledge, attitudes, and beliefs about hand washing;
all‐cause under‐five mortality;
cost effectiveness.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress) (Lefebvre 2020).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (8 January 2020); Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (2020, Issue 1); PubMed (MEDLINE), 1966 to 8 January 2020); Embase (OVID; 1974 to 8 January 2020); PsycINFO (EBSCOHost, 1967 to 8 January 2020); Science Citation Index, Social Sciences Citation Index, SSCI, CPCI‐S, CPCI‐SSH (1981 to 8 January 2020); ERIC (Educational Resources Information Center; 1966 to 8 January 2020), and LILACS (Bireme; 1982 to 8 January 2020).
We also searched the following databases using a simplified strategy (diarrhea, diarrhoea, handwashing): SPECTR (The Campbell Collaboration's Social, Psychological, Educational, and Criminological Trials Register; 2000 to 8 January 2020); Bibliomap and TRoPHI (The Trials Register of Promoting Health Interventions) maintained by the Evidence for Policy and Practice Information and Co‐ordinating Centre www.eppi.ioe.ac.uk) (1990 to 8 January 2020); and The Grey Literature (www.nyam.org/library/grey.shtml; 2002 to 8 January 2020). We also searched the World Health Organization (WHO) International Clinical Trial Registry Platform (ICTRP) and the metaRegister of Controlled Trials (mRCT) for ongoing trials on 8 January 2020 using diarrhoea, diarrhea, and hand washing as search terms. The PRISMA flow diagram is shown in Figure 1 below.
Searching other resources
Researchers and organizations
To obtain further information, we contacted researchers in the field for unpublished and ongoing trials (October 2019).
Reference lists
We also examined the reference lists of articles for relevant trials.
Data collection and analysis
Three review authors (RIE, JAC, and DA) independently screened titles and abstracts of relevant articles to assess their eligibility for inclusion in the review.
Selection of studies
We retrieved full texts of articles that were deemed potentially relevant to the review for further assessment. We decided on inclusion by consensus among all review authors. We scrutinized each trial report to ensure that we included multiple publications from the same trial only once. We listed the excluded trials and the reasons for their exclusion.
Data extraction and management
Three review authors (RIE, DA, and JAC) independently extracted data on methods, types of participants, interventions, and outcomes from the selected trials using a standardized data extraction form. We resolved any disagreements by discussion and consensus among review authors. We requested unpublished data and additional information from published trials from relevant individuals, groups, and organizations.
We extracted the year of completion of the trial rather than the year of publication for identification of included trials. When such data were not reported, we used the year of publication. This was to give a clear time frame for this Cochrane Review (1977 to 2019). In addition, we used the three‐letter international code of the country where the trial was conducted in the study ID. We extracted data on each trial site, including any measures of availability of water and soap, and literacy level of the communities. Where data were available, we extracted the socioeconomic status of trial participants, since resources for effective hand washing (e.g. running water and soap) may be more accessible to higher‐income households. We carefully summarized details of the intervention including type of promotional activity, whether soap and water provision was part of the intervention, method of hand washing promoted (washing in a bowl or under running water), and procedure for hand washing.
We had intended to analyze episodes of diarrhoea as a dichotomous outcome, but the data reported by the trials did not permit this type of analysis. We analyzed the outcome as count data, when either the incidence rate ratio (IRR) and its 95% confidence interval (CI) or the number of episodes of diarrhoea and the person‐time at risk was reported, or as continuous data when the mean number of diarrhoea episodes and standard deviation (SD) were presented.
For RCTs which randomized individuals, when continuous outcomes data were summarized as arithmetic means we extracted the arithmetic means, SDs, and numbers of participants for the treatment and control groups. For count (rate) outcome data, we extracted the number of episodes, the number of person‐years at risk, and the number of participants for each intervention group, or we extracted a rate ratio and measure of variation (e.g. CI) directly from the publication.
Cluster‐RCTs required the use of different data extraction methods and analysis methods, because trials with a cluster design require more complex analysis than trials that randomized individuals. Observations on participants in the same cluster tend to be correlated, so the intracluster variation must be accounted for during the analysis. If this correlation is ignored in the analysis and the same techniques are employed as for RCTs that randomized individuals, the resulting measure of effect remains a valid estimate, but the associated variance of the estimate will be underestimated, leading to unduly narrow CIs. For meta‐analysis this means that trials analyzed without allowing for this design effect will receive too much weight.
For the cluster‐RCTs, we extracted information on the number of clusters, average size of clusters, unit of randomization, whether the trials adjusted for clustering, and the statistical method used to analyze cluster trials. When a trial's analysis had adjusted for clustering, we extracted the point estimate and 95% CI. For count data we extracted the IRR. If a trial had not adjusted for clustering, we extracted the same data as for RCTs that randomized individuals.
Assessment of risk of bias in included studies
Two review authors (RIE and DA) independently assessed the risks of bias in included trials using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed the risks of bias across the following domains: randomization sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other potential biases. We classified our judgements as 'high', 'unclear', or 'low' risk of bias using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
In the blinding domain, we acknowledged that double blinding is not possible in trials of hand‐washing interventions since there is no obvious placebo. However, outcome assessors could be blinded, and we assessed whether or not this had occurred. It is also difficult to assess losses to follow‐up (incomplete outcome data) in open cluster‐RCTs. Some adults and children may leave the trial, but others are born or enter the trial during the follow‐up period, hence participant numbers are in constant flux. Inclusion of all randomized participants in the analysis is thus most clearly represented as the person‐time at risk accrued as a percentage of maximum possible person‐time at risk in each trial arm. We therefore reported on this measure and also on any loss to follow‐up of both clusters and participants. We assessed this as low risk if it was at least 90%. We also assessed whether baseline characteristics were comparable across the intervention groups and whether data were collected at similar time points for the intervention and control sites with a view to identifying selective reporting and other possible biases. The details are shown in Figure 2 and Figure 3.
Measures of treatment effect
We qualitatively compared included trials to ascertain the feasibility of pooling them in a meta‐analysis. Thus we identified three distinct settings, covering child DCCs, community‐based interventions, and hospital‐based trials, since the factors that affect hand‐washing practice may vary in these settings. We stratified the trials based on these settings for the meta‐analysis and calculated incidence rate ratios (IRRs) for dichotomous outcomes, mean differences (MDs) for continuous outcome measures on the same scale, and standardized mean differences (SMDs) for continuous outcomes measured using different scales.
Unit of analysis issues
For all trials that did not adjust for clustering, we made approximate adjustments using estimates of the intra‐cluster correlation coefficient (ICC) from other trials that did adjust for clustering and reported this statistic. We did this by multiplying the standard error (SE) for each trial by the square root of the design effect. We estimated the design effect as 1 + (m ‐ 1) * ICC, where 'm' is the average cluster size and 'ICC' is the intra‐cluster correlation coefficient (Higgins 2020).
Dealing with missing data
We contacted authors of eligible trials for missing data or for additional information when the trials were less than 15 years old.
Assessment of heterogeneity
We checked for heterogeneity by visually inspecting the forest plots, applying the Chi2 test, with a P value of 0.10 indicating statistical significance, and also implementing the I2 statistic with a value of 50% used to denote moderate levels of heterogeneity. We used the random‐effects model to pool data if we detected heterogeneity and it was still considered clinically meaningful to combine the trials. Due to the limited number of trials in each setting, we were unable to explore potential sources of heterogeneity in depth. We explored and attempted to explain heterogeneity where possible using a predefined trial characteristic (provision of hand‐washing material (soap) as part of intervention and type of promotional activity employed) and quality characteristics (whether or not outcome assessors were blinded and whether or not trials had adjusted for clustering) (Deeks 2020).
Assessment of reporting biases
We planned to assess the possibility of publication bias by producing a funnel plot if at least 10 trials contributed to the treatment comparison. However, we did not undertake this, due to an insufficient number of included trials.
Data synthesis
We analyzed the data using RevMan 5 (Review Manager 2019) and present all results with 95% CIs. We stratified the analysis into three categories of settings: child DCCs and school‐based interventions (DCCs or primary schools), community‐based interventions, and hospital‐based intervention (in persons at high risk of diarrhoea ‐ people with AIDS). Also we stratified the analyses by the income status of the countries where the trials were conducted. Since the outcomes and methods of measuring behaviour changes were too variable to make meta‐analysis meaningful, we tabulated the results.
RCTs that randomize individuals
We summarized continuous outcome data from RCTs randomizing individuals using the MD value. We did not undertake meta‐analysis of RCTs randomizing individuals, due to their limited number.
Cluster‐RCTs that adjusted for clustering
For count outcomes, we pooled the IRR in RevMan 5, using the generic inverse variance method with the random‐effects model. We used standard techniques for calculating SEs from 95% CIs (Deeks 2020). When the outcomes and methods of measuring outcomes were too variable to make meta‐analysis meaningful (for changes in hand‐washing behaviour), we tabulated the results. One trial performed child‐ and site‐level analyses (Haggerty 1988 COD), without providing the 95% CI for the site‐level analysis. We therefore estimated the denominator from the number of children by trial arm by assuming that all those who had remained in the trial for at least nine weeks had a total of 12 weeks of follow‐up. The numerator (average number of episodes per child) was provided at the cluster level. We classified this trial as cluster‐adjusted. Several community studies in LMICs reported changes in diarrhoea as the difference in the 'mean longitudinal prevalence' of diarrhoea episodes over a certain period of time (Luby 2006 PAK; Galiani 2016 PER; Kapoor 2016 IND; Briceno 2017 TZA; Luby 2018 BGD; Null 2018 KEN). Where feasible (i.e. where the period of time was consistently one week and sufficient details were reported to estimate a SE), we combined these in a meta‐analysis using the generic inverse variance method.
Cluster‐RCTs that did not adjust for clustering
For trials that did not report on or were unclear on the method used to adjust for clustering, we either extracted information on the rate ratio and unadjusted 95% CI or, wherever possible, estimated the unadjusted rate ratios and 95% CIs from the total number of diarrhoea episodes and person‐time at risk in each trial arm. Where data on person‐time at risk were not directly provided by the trial authors, we estimated this as accurately as possible from the follow‐up duration multiplied by the total number of children as the denominator for both intervention and control groups, respectively. The measures of effect and CIs are presented in tables. One trial adjusted for clustering by comparing the mean incidence rate of intervention and non‐intervention classrooms (Kotch 1989 USA), but presented only cluster‐adjusted 95% CIs for a different outcome (excess mean episodes) and not a rate ratio. We took the cluster‐adjusted estimate of the numerator (the mean incidence rate across the clusters) from the published data and estimated the person‐time at risk crudely by multiplying the number of contacts every two weeks by the number of children, assuming this was equally distributed between the intervention and control groups. We classified this trial as not having adjusted for clustering.
For all trials that did not adjust for clustering, we attempted to make an approximate adjustment using estimates of the ICC from one of the trials that did adjust for clustering and reported this statistic. Only two trials reported this statistic: one community‐based trial (Luby 2003b PAK), and one trial in a child DCC (Roberts 1996 AUS). We assumed that these ICC estimates could be generalized to other community‐based and child DCCs or to school‐based trials, respectively. We extracted the number of children and number of clusters from each unadjusted trial to estimate the average cluster size. We then followed standard methods to estimate the design effect for each trial and multiplied the SE for each trial by the square root of this design effect (Higgins 2020). This approximate adjustment increases the SE (and hence the width of the CIs for the unadjusted trials) and appropriately reduces the weight given to such trials in the meta‐analysis. We performed meta‐analyses by pooling the estimates of the cluster‐adjusted and approximately‐adjusted trials together.
Certainty of evidence
We assessed the certainty of the evidence using the GRADE approach (Guyatt 2008; Schünemann 2020). We imported data from RevMan 5 to GRADEpro 2014 to create a 'Summary of findings' table containing relevant information on the outcomes of interest. We then appraised the certainty of the evidence for each outcome across the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias for each trial that contributed to the outcome. Where we identified deficiencies that were sufficient to decrease our confidence in the estimates of effect, we downgraded the certainty of evidence for RCTs from ‘high' to either ‘moderate', ‘low', or ‘very low', and explained our reasons for doing so in footnotes. We have included the prespecified outcomes for the three independent settings in Table 1; Table 2; and Table 3.
Summary of findings 1. Summary of findings table 1.
| Hand‐washing promotion at child care centres and schools compared to no intervention for preventing diarrhoea | ||||||
| Patient or population: children Setting: child day‐care centres or schools Intervention: hand‐washing promotion Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with hand‐washing promotion at child care centres and schools | |||||
| Episodes of diarrhoea | High‐income countries | IRR 0.70 (0.58 to 0.85) | 4664 (9 RCTs) | ⊕⊕⊕⊕ HIGHb,c,d,e | Hand‐washing promotion reduces the risk of diarrhoea in high‐income countries compared to no hand‐washing promotion | |
| 4 episodes per 100 children per yeara | 2 episodes per 100 children per year (2 to 3) | |||||
| Low‐ or middle‐income countries | IRR 0.66 (0.43 to 0.99) | 45,380 (2 RCTs) | ⊕⊕⊝⊝ LOWf,g,h | Hand‐washing promotion may reduce the risk of diarrhoea in low‐ or middle‐income countries compared to no hand‐washing promotion | ||
| 22 episodes per 100 children per year | 15 per 1000 (9 to 22) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IRR: Incidence rate ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aThe median incidence of diarrhoea in the control groups was 4 episodes per 100 children per year. bNo serious risk of bias: most trials are at high or unclear risk of detection or reporting bias due to no description of blinding of outcome assessors. Restriction of the analysis to just the blinded trials finds a slightly smaller effect size, but the result remains statistically significant. Not downgraded. cNo serious inconsistency: although statistical heterogeneity was high, this heterogeneity was related to the size of the effect, not the direction of effect. The individual effect sizes in trials ranged from a 10% relative reduction in diarrhoea to a 50% reduction. dNo serious indirectness: these nine trials were conducted in day‐care centres/schools in high‐income countries (USA, Denmark, Australia, Netherlands and Canada). eNo serious imprecision: the result is statistically significant and the meta‐analysis adequately powered to detect this result. fNo serious inconsistency: while both trials found reductions in diarrhoea incidence, the reduction was only statistically significant in the trials from Egypt. However, we did not downgrade. gThe incidence of diarrhoea in the control group in the trial from Egypt was 22 per 100 children per year. The incidence in the control group in the Kenya trial was not stated. hDowngraded by two levels for serious indirectness: only one trial was conducted in a low‐income country (Pickering 2013 KEN). This trial from an urban slum in Nairobi did not find a statistically significant benefit on diarrhoea incidence.
Summary of findings 2. Summary of findings table 2.
| Hand‐washing intervention in the community compared to no intervention for preventing diarrhoea | ||||||
| Patient or population: children up to 15 years of age Setting: community Intervention: hand‐washing promotion Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with Hand washing intervention in the community | |||||
| Episodes of diarrhoea: rate ratios | Low‐ or middle‐income countries | Incidence rate ratio 0.71 (0.62 to 0.81) | 15,950 (9 RCTs) | ⊕⊕⊕⊝ MODERATEb,c,d,e | Hand‐washing promotion probably reduces the risk of diarrhoea in low‐ or middle‐income countries compared to no hand‐washing promotion | |
| 3 episodes per 100 children per yeara | 2 episodes per 100 children per yeara (2 to 2) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IRR: Incidence rate ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aThe median incidence of diarrhoea in the control groups was three episodes per 100 children per year. bNo serious risk of bias: most trials are at high or unclear risk of detection or reporting bias, due to no description of blinding of outcome assessors. Restriction of the analysis to just the blinded trials finds a slightly smaller effect size, but the result remains statistically significant. Not downgraded. cNo serious inconsistency: although statistical heterogeneity was high, this heterogeneity was related to the size of the effect, not the direction of effect. The individual effect sizes in trials ranged from a 6% relative reduction in diarrhoea to a 29% reduction. dNo serious imprecision: the result is statistically significant and the meta‐analysis adequately powered to detect this result. eDowngraded by one level for serious indirectness: eight trials were conducted in low‐ and middle‐income countries (the Democratic Republic of Congo, Pakistan, Bangladesh, Myanmar, Peru, India, and Nepal), and one trial was conducted in a low‐income country (Ethiopia).
Summary of findings 3. Summary of findings table 3.
| Hand‐washing intervention in a hospital setting compared to no intervention for preventing diarrhoea | ||||||
| Patient or population: adults with AIDS Setting: hospital Intervention: Hand‐washing promotion Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with hand‐ washing intervention in hospital setting | |||||
| Episodes of diarrhoea assessed with: self‐reports collected through home visits; hospital/health centre/clinic records, including admission for diarrhoea‐related dehydration follow up: mean 1 year | The mean episodes of diarrhoea was 2.92 | The mean episodes of diarrhoea was 1.24 |
Mean difference 1.68 lower (1.93 lower to 1.43 lower) | 148 (1 RCT) | ⊕⊕⊕⊝ MODERATEa,b,c,d | Hand‐washing promotion probably reduces the risk of diarrhoea in adults with AIDS compared to no hand‐washing promotion |
| Hand‐washing behavioural changes/changes in knowledge, attitude and practice assessed with: frequency of hand washing per day follow‐up: mean 1 year | 4 times daily | 7 times daily | ‐ | (1 RCT) | ⊕⊕⊕⊝ MODERATEc,d,e | Hand‐washing promotion probably improves hand‐washing behaviour, knowledge, attitude, and practice in adults with AIDS compared to no hand‐ washing promotion |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aOutcomes assessed in adults in high‐risk group (people with AIDS). bThe mean episodes in the control groups was 2.92, while that of the intervention group was 1.24 episodes over the 1‐year trial period. cDowngraded by one level for serious risk of bias: the trial is at an unclear risk of selection bias due to failure to describe a process of allocation concealment. This trial is also at high risk of detection or reporting bias due to no description of blinding of outcome assessors. Blinding of participants would not have been possible. dEvidence from this setting was very limited, since it is from only one trial (Huang 2007 USA). eHand‐washing rates: intervention: seven times daily from three times at baseline; control: four times daily from three times.
Subgroup analysis and investigation of heterogeneity
If we detected any heterogeneity, we planned to explore its possible causes using subgroup analysis. We conducted subgroup analyses for trial setting, provision of hand‐washing material (soap) as part of intervention, type of promotional activity employed (focused or multiple hygiene interventions), and quality characteristics (whether outcome assessors were blinded).
Sensitivity analysis
We undertook a sensitivity analysis to explore the robustness of our findings, including the trial size, duration of follow‐up, differences in method of assessing the primary outcome, and differences in methodological quality (blinding of outcome assessors) of the included trials.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Guyatt 2008; Schünemann 2020). We imported data from RevMan 5 to GRADEpro 2014 to create a 'Summary of findings' table containing relevant information on the outcomes of interest. We then appraised the certainty of the evidence for each outcome across the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias for each trial that contributed to the outcome. Where we identified deficiencies that were sufficient to decrease our confidence in the estimates of effect, we downgraded the certainty of evidence for RCTs from ‘high' to either ‘moderate', ‘low', or ‘very low', and explained our reasons for doing so in footnotes. We have included the prespecified outcomes for the three independent settings in Table 1; Table 2; and Table 3.
Results
Description of studies
Results of the search
Our search yielded 206 potentially relevant trials, making a total of 290 when combined with the 47 search results of the first review update (Ejemot‐Nwadiaro 2015), and the 37 search results of the original review (Ejemot‐Nwadiaro 2008). Twenty‐nine trials met the inclusion criteria: 14 trials were included in the original version of the review (Ejemot‐Nwadiaro 2008), eight new trials were added to the first review update, and we included seven new trials based on our updated search. See Figure 1. We describe them in the Characteristics of included studies tables. One trial was in Danish (Ladegaard 1999 DEN), and the rest were written in English. Thirteen trials were DCC‐ or school‐based, 15 were community‐based (one of the trials had both community‐based and school‐based components, but the community‐based component predominated), and one trial (Huang 2007 USA) was in a high‐risk group. We have listed reasons for excluding 84 trials in the Characteristics of excluded studies table.
1.
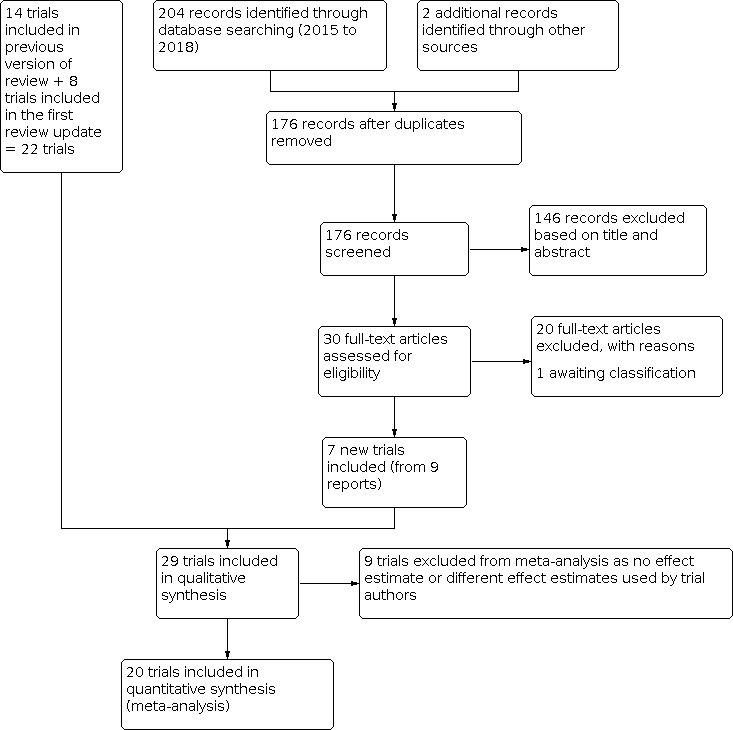
PRISMA flow diagram.
Included studies
Child DCCs or schools
All 13 trials in this group were randomized by cluster using primary schools (Bowen 2004 CHN; Talaat 2008 EGY; Pickering 2013 KEN), DCCs (Black 1977 USA; Bartlett 1984 USA; Butz 1990 USA; Roberts 1996 AUS; Carabin 1997 CAN; Ladegaard 1999 DEN; Kotch 2003 USA; Zomer 2015 NED), or classrooms in DCCs as the unit of randomization (Kotch 1989 USA; Ban 2015 CHN). These trials were all conducted in high‐income countries except for two trials conducted in an upper‐middle income country (UMIC): Bowen 2004 CHN and Ban 2015 CHN (conducted in Hubei province and Fujian province in China, respectively), and another two conducted in LMICs: Talaat 2008 EGY (conducted in Cairo, Egypt) and Pickering 2013 KEN (conducted in Nairobi, Kenya). The other trials were performed in Australia (Roberts 1996 AUS), Europe (Ladegaard 1999 DEN; Zomer 2015 NED), and North America (Black 1977 USA; Bartlett 1984 USA; Kotch 1989 USA; Butz 1990 USA; Carabin 1997 CAN; Kotch 2003 USA), where resources and materials for hand washing were relatively available and accessible.
Interventions
All trials used multiple hygiene interventions, except for Black 1977 USA, Bowen 2004 CHN, and Pickering 2013 KEN, which used only a hand‐washing intervention. Although Pickering 2013 KEN was a three‐arm trial that investigated hand sanitizer and hand washing with soap, we considered only the hand‐washing arm with soap in this Cochrane Review; it is therefore categorized as a hand washing‐only intervention. Kotch 2003 USA assessed the impact of the provision of hand‐washing and diapering equipment on the incidence and duration of infectious illness (including diarrhoea) in both children and staff. We describe the interventions in more detail in Table 4. In addition to instruction on proper hand‐hygiene techniques in Ban 2015 CHN, parents or guardians and teachers were given antimicrobial supplies with which to regularly clean hard surfaces and disinfect the classrooms and homes of the participants.
1. Description of hand‐washing interventions at child day‐care centres and schools.
| Trial | Promotional activity | Classificationa | Message content | Hand‐ washing method | Hand‐ washing styleb | Material provision | Water availability |
| Ban 2015 CHN | Parents or guardians and teachers in the intervention group were instructed, in person, on:
1. proper hand‐hygiene techniques 2. how to use all of the antibacterial products which were distributed 3. Hard surfaces in kindergartens were to be cleaned or disinfected every day using antiseptic‐germicide or bleach 4. hard surfaces in homes were to be cleaned or disinfected at least twice every week. Items such as children’s toys, house furnishings, frequently‐touched objects (doorknobs, tables or desks), kitchen surfaces (utensils, cutlery, countertops, chopping boards, sinks, floors, etc.), bathroom surfaces (toilet, sink, floor, etc.) were also included in the weekly cleaning or disinfecting practices |
|
Children, their family members and teachers were advised to wash their hands daily using liquid antimicrobial soap, especially 1. before eating 2. after using the bathroom 3. after blowing their nose 4. after outdoor activities. Instant hand sanitizer was to be carried daily and used without running water |
1. water with liquid antimicrobial soap 2. hand sanitizers used without running water |
Not specified | Families and kindergartens in the intervention group were provided with antibacterial products for hand hygiene and surface cleaning or disinfection produced by the Whealthfields Lohmann (Guangzhou) Company Ltd. Items distributed included liquid antimicrobial soap for hand washing, instant hand sanitizer for hand‐disinfecting antiseptic‐germicide and bleach for surface disinfecting. | Unknown |
| Bartlett 1984 USA |
|
|
Staff and child hand washing, diapering, food handling, and environmental cleaning | Unclear | Not specified | Not specified | Adequate |
| Black 1977 USA | Large‐group education | Hygiene education | Staff and child hand‐washing before handling food and after defaecation | Water with bar soap and paper towels | Unclear | By the day‐care centres' management | Adequate |
| Bowen 2004 CHN |
|
|
Hand washing before eating and after toileting | Water with soap | Under running water | Supplies of soap to schools in 'expanded Intervention'; 1 bar of soap to homes in both expanded and standard intervention | Adequate (criteria for taking part in trial) |
| Butz 1990 USA | Large‐group training (in‐home instruction to day‐care providers) |
|
|
Water with soap | Not specified | All supplies provided by researchers | Adequate |
| Carabin 1997 CAN |
|
Hygiene education |
|
Unclear | Not specified | Unclear | Adequate |
| Kotch 1989 USA |
|
Hygiene education |
|
Water with soap plus disposable towel | Under running water | Unclear | Adequate |
| Kotch 2003 USA |
|
|
Training to improve and standardize the hand‐washing, sanitation, diapering, and food preparation procedures in both intervention and control centres by addressing knowledge, attitudes, and behaviours of child‐care providers and promoting use of the equipment | Not described | Not described | Diapering, hand‐washing, and food preparation equipment was provided by the researchers | Adequate |
| Ladegaard 1999 DEN | Small‐group practical demonstration |
|
|
Water with soap | Under running water | Unclear | Adequate |
| Pickering 2013 KEN |
|
|
|
Water with soap | Not described | Researchers provided liquid soap and water tank | Adequate |
| Roberts 1996 AUS |
|
|
|
Water with soap | Under running water | Unclear | Adequate |
| Talaat 2008 EGY |
|
Hygiene education |
|
Water with soap | Under running water |
|
Adequate (Cairo governate was chosen because of the continuous availability of water in school settings) |
| Zomer 2015 NED |
|
|
|
Water with soap | Not described | Trial investigators provided hand‐hygiene products free of charge (dispenser for paper towels, soap, alcohol‐based hand sanitizer and hand cream, with refills for 6 months) | Adequate |
aMessage classification. bWhether done under running water; in a bowl, by an individual, or by several people. cParticipatory learning involves a process that helps engage learners in an active role of inquiry in which they share experiences and reflect critically on practice in a context that many group members find stimulating and relatively safe (Martin 1997).
All but one of the included trials based in child DCCs or schools had intervention and control arms (monitoring only). Bowen 2004 CHN had three arms for the standard intervention, expanded intervention (which included the standard intervention and peer monitoring of hand washing), and control. It is important to note that the control group in most cases received quite frequent monitoring (estimating diarrhoea illness episodes typically on a two‐week basis). This monitoring itself may have influenced hand‐washing behaviour. Carabin 1997 CAN attempted to tease out the effects of the intervention alone from 'monitoring'. The monitoring effect in this trial was estimated as the difference in diarrhoea incidence rates within each arm over one year of the trial (September 1996 to November 1997). The crude effectiveness of intervention was estimated as the difference between the monitoring effect in the intervention group and control group.
Participants
Thirteen trials including 54,471 children met the inclusion criteria. Seven trials included children under three years of age, another trial included children less than five years of age (Ban 2015 CHN), one trial was in children under six years of age (Ladegaard 1999 DEN), and one trial was with children under seven years of age (Butz 1990 USA). Bowen 2004 CHN involved children in the first grade at school in China; Talaat 2008 EGY included children in government elementary schools in Cairo, Egypt; and Pickering 2013 KEN involved children aged five to 10 in primary schools in Nairobi, Kenya. Hand‐washing behavioural changes and changes in knowledge, attitude, and beliefs on hygiene were assessed in the day‐care providers (number not precisely reported) and children, while the primary outcome measures were assessed in the children.
The number of clusters ranged from 4 to 87 (Black 1977 USA; Bowen 2004 CHN). Primary outcome measures were assessed across 278 DCCs, two kindergartens, and 151 schools. Participants were exposed to mainly small‐ and large‐group training sessions on hygiene education and germs‐health theory that used multiple promotional techniques (e.g. audio and video tapes, pamphlets, practical demonstrations, drama, posters, songs, games, or peer monitoring). Kotch 2003 USA used the 'Keep‐it‐clean' module in training caregivers to standardize the interventions across the trial arms. The aim was to provide education about personal hygiene, diarrhoea transmission, treatment, and prevention, and the importance of techniques for hand washing. Intervention and control groups were generally comparable in important characteristics at baseline (Table 4).
Outcome measures
All included trials measured our primary outcome of episodes of diarrhoea. Three trials reported the proportion of people washing their hands or changes in knowledge, attitude, and beliefs about hand washing, or both (Kotch 1989 USA; Roberts 1996 AUS; Pickering 2013 KEN). No trials reported diarrhoea‐related deaths, all‐cause under‐five mortality, or cost‐effectiveness data. However, Kotch 2003 USA reported that the cost of purchasing and installing one unit of the hand‐washing and diapering equipment was USD 10,385 (USD 7500 for the equipment and the rest for installation) by classroom. Follow‐up periods ranged from two to 12 months.
Adjustment for clustering
Six trials did not appear to have accounted for clustering in the analysis for any outcome measure (Black 1977 USA; Bartlett 1984 USA; Butz 1990 USA; Ladegaard 1999 DEN; Talaat 2008 EGY; Ban 2015 CHN). Kotch 1989 USA adjusted for clustering by comparing the mean incidence rate of intervention and non‐intervention classrooms, but only a cluster‐adjusted 95% CI for a difference outcome (excess mean episodes) was presented, and not a rate ratio. Kotch 2003 USA reported controlling for clustering by estimating a random effect for the centres, but this does not seem to have been reflected in the results. In the other five cluster‐adjusted trials. Bowen 2004 CHN presented only the school‐level analysis (mean illness and absence rates by school); Carabin 1997 CAN adjusted for clustering using a Bayesian hierarchical model, while Roberts 1996 AUS, Pickering 2013 KEN, and Zomer 2015 NED estimated robust SEs in a Poisson regression model.
Community‐based trials
We included 15 community‐based trials. Fourteen were cluster‐RCTs that used entire communities (generally villages, squatter settlements, or neighbourhoods, except for Han 1985 MMR and Kapoor 2016 IND, which used households) as units of randomization. These trials were conducted in low‐ and middle‐income countries (LMICs) in Africa (Haggerty 1988 COD; Briceno 2017 TZA; Hashi 2017 ETH; Null 2018 KEN), Asia (Han 1985 MMR; Stanton 1985 BGD; Luby 2003a PAK; Luby 2003b PAK; Luby 2006 PAK; Langford 2007 NPL; Nicholson 2008 IND; Galiani 2016 PER; Kapoor 2016 IND; Luby 2018 BGD), and South America (Hartinger 2011 PER). Galiani 2016 PER was a community‐based trial that also had a school component.
Interventions
Five trials evaluated hand‐washing‐only interventions (Han 1985 MMR; Luby 2003a PAK; Luby 2003b PAK; Langford 2007 NPL; Nicholson 2008 IND). Luby 2003a PAK had two hand‐washing arms, one with plain soap and one with antibacterial soap. These two arms had similar results and are combined in this review. Han 1985 MMR used plain soap. Luby 2003b PAK was a five‐arm trial that investigated water‐quality interventions, hand washing, and a combination of the two; only the arm with antibacterial soap and hand‐washing education is considered in this review. Luby 2006 PAK conducted a follow‐up trial to the Luby 2003b PAK trial, maintaining the initial randomization process to assess if learned hygiene behaviours could be sustained over time without additional hygiene‐promotion intervention. Three other trials used multiple hygiene interventions that included hand washing with soap (the type of soap used is not described) (Stanton 1985 BGD; Haggerty 1988 COD; Hartinger 2011 PER). We have provided more detailed descriptions of the interventions in Table 5.
2. Description of hand‐washing intervention in communities.
| Trial | Promotional activity | Classificationa | Message content | Hand‐ washing method | Hand‐washing styleb | Material provision | Water availability |
| Briceno 2017 TZA | Intensive social marketing, including: 1. hand‐washing promotion events with women on market days, during prenatal clinic visits, and village meetings 2. Distribution of promotional materials 3. face‐to‐face interactions 4. Helping households build 'tippy' taps 5. Travelling road shows 6. Mass media radio campaigns |
1. Hygiene education 2. Behaviour modification |
1. importance of hand washing 2. hand washing after faecal contact: after defaecating, after toileting, after cleaning child post‐toileting 3. hand washing before handling food: before cutting or preparing food, before eating, before serving food, before breastfeeding |
Water with soap | Under running water ('tippy' taps) | Not specified | Unknown |
| Galiani 2016 PER | hand‐washing promotion through: 1. radio 2. printed materials 3. promotional events 4. educational sessions |
1. Hygiene education 2. Behaviour modification |
1. hand washing with soap | Water with soap | Unclear | Adequate | Unknown |
| Haggerty 1988 COD | Large‐group training | Hygiene education | 1. Hand washing before meal preparation and eating 2. Hand washing after defaecation (wash both hand and buttocks for children) 3. Proper disposal of children's faeces 4. Disposal of animal faeces from yard |
Unclear | Not specified | Unclear | Unknown |
| Han 1985 MMR | Small‐group education (households) | 1. Hygiene education 2. Provision of hand‐washing material |
Hand washing: 1. after defaecation; 2. before preparing or eating food. |
Water with bar soap | Not specified | Plain bar soap provided by researcher | Unknown |
| Hartinger 2011 PER | 1. Hygiene education 2. Provision of an Integrated home‐based intervention package (IHIP) |
1. Hygiene education 2. Home‐hygiene intervention package including OPTIMA‐improved stove, kitchen sink, hand washing, and solar drinking water disinfection (SODIS) household water treatment |
Hand washing: 1. After stool contact/ defaecation 2. Before food preparation/ handling 3. Before eating and feeding infants and small children 4. After changing diapers 5. Correct use of improved stoves, including clearing and removing ashes and wood residues that could obstruct ventilation 6. Correct application of the solar drinking water disinfection (SODIS) method 7. Elimination of animal excreta and isolation of animals from the kitchen environment |
Water with soap | Not specified | IHIP provided by researchers | Unknown |
| Hashi 2017 ETH | 1. Sessions on health education 2. Provision of soap 3. community meetings 4. Distribution of pamphlets 5. Hygiene education 6. Demonstration |
1. Hygiene education 2. Behaviour modification |
1. hand washing messages · wash your hands before meal preparation · wash your hands before eating food · wash your children's hands with the soap (provided) after defaecation, before meal preparation, and before eating 2. Water storage behaviour messages: · cover your water storage container properly · use narrow‐mouthed storage container · Clean your water storage container regularly · have a latrine 3. Latrine availability messages ‐ If you don't have a latrine, share with the nearest neighbourhood and build a latrine · use your latrine properly · make a hand‐washing site with soap, and use properly regularly by washing your hands every time you use 4. Safe waste disposal messages · dispose of liquid waste properly · dispose of solid waste properly · dispose of your children's waste properly |
Water with soap | Not specified | Saop provided by the researchers | Unknown |
| Kapoor 2016 IND | 1. Hygiene education 2. Flip books 3. Distribution of pamphlets |
1. Hygiene education 2. Behaviour modification |
1. hand washing 2. personal hygiene of mother and child 3. clean storage of drinking water 4. food hygiene 5. sanitation |
Not specified | Key observations reported: 1. wet hands under running water 2. apply soap/sanitizer; 3. rub hands together for 15 ‐ 30 seconds |
Not reported | Unknown |
| Langford 2007 NPL | 1. Larger meetings 2. Small group meetings: focus group discussion of 6 to 8 women 3. Posters 4. Dramas |
1. Behaviour modification 2. Hygiene education |
Hand washing: 1. After stool contact/defaecation including wiping bottoms of babies 2. After refuse disposal 3. Before food preparation/ handling 4. Before eating |
Water with soap | Not specified | Soap provided by researcher (community motivators distributed a new bar of soap to each mother at these meetings) | Adequate (water for hygienic purposes, but was always available from these tubes and deep wells) |
| Luby 2003a PAK | 1. Large group training using slide shows, pamphlets, and video tapes 2. Education at weekly field visits |
Hygiene education | Hand washing: 1. Before preparing food 2. Before eating food |
Water with plain or antibacterial soap | Water from a pitcher (though not clearly stated) | Soap provided by researchers | Unknown |
| Luby 2003b PAK | 1. Large group training using slide shows, pamphlets, and video tapes 2. Education at twice‐weekly visits |
1. Hygiene education 2. Provision of hand washing material |
Hand washing: 1. After stool contact/ defaecation 2. Before food preparation/handling/eating 3. Before feeding infants |
Water with antibacterial soap | Not specified | Soap provided by researchers | Unknown |
| Luby 2006 PAK | Follow‐up trial of Luby 2003b PAK | No intervention was conducted | Follow‐up trial of Luby 2003b PAK above | No intervention | No intervention | No intervention | Follow‐up trial |
| Luby 2018 BGD | 1. Discussions 2. video dramas 3. storytelling 4. games 5. songs 6. training on hardware maintenance |
1. Hygiene education 2. Behaviour modification |
Wash hands with soapy water 1. before preparing food 2. before eating or feeding a child 3. after defaecating 4. after cleaning a child who has defaecated. |
Water with soap | Not specified | Promoters also provided a regular supply of detergent sachets for making soapy water. Intervention households received 2 hand‐washing stations, 1 with a 40 L water reservoir placed near the latrine and a 16 L reservoir for the kitchen. Each hand‐washing station included a basin to collect rinse water and a soapy water bottle.16 Promoters also provided a regular supply of detergent sachets for making soapy water | Unknown |
| Nicholson 2008 IND | 1. Large group training 2. Establishment of a 'Good Mums' Club 3. Environmental cues (wall hangers, danglers, etc) 4. Reward system from mothers to children (stickers, toy animals, coins, etc) |
1. Hygiene education 2. Behaviour modification (intervention designed according to behaviour change principles of (Claessen 2008) |
1. Hand washing after stool contact/defaecation 2. Hand washing before eating 3. Hand washing during bathing |
Water with soap | Not specified | Soap provided by researchers | Unknown |
| Null 2018 KEN | 1. Guided discussions 2. using visual aids 3. song 4. storytelling 5. resupply of consumable intervention materials; |
1. Hygiene education 2. Behaviour modification |
Hand washing with soap 1. before handling food 2. after defaecation (including assisting a child) |
Water with soap |
Not specified | Soap provided by researchers. Study compounds were given 2 permanent, water‐frugal hand‐washing stations intended to be installed near the food preparation area and the latrine. Hand‐washing stations were constructed of painted metal, with 2 foot‐pedal‐operated jerry‐cans that dispensed a light flow of rinse water and soapy water. Promoters added chunks of bar soap to the soapy water container quarterly |
Unknown |
| Stanton 1985 BGD | 1. Small group discussion (only women or children) 2. Larger demonstrations (mixed audience) 3. Posters, games, pictorial stories, and 'flexiflans' for illustrations |
Hygiene education | 1. Hand washing before food preparation 2. Defaecation away from the house and in a proper site 3. Suitable disposal of waste and faeces |
Unclear | Not specified | Unclear | Inadequate |
aMessage classification. bWhether done under running water; in a bowl by an individual, or by several people.
Participants
We included 15 trials with about 29,347 children. In the community‐based trials, seven trials were with very young children (under three years) (Haggerty 1988 COD; Langford 2007 NPL; Hartinger 2011 PER; Galiani 2016 PER; Kapoor 2016 IND; Luby 2018 BGD; Null 2018 KEN); four trials were with children less than five years of age (Han 1985 MMR; Briceno 2017 TZA; Hashi 2017 ETH), or less than six years of age (Stanton 1985 BGD); and three involved older children, up to 15 years of age (Luby 2003a PAK; Luby 2003b PAK; Luby 2006 PAK). Nicholson 2008 IND had four categories of participants: targeted children five years old, children less than five years old, children 6 to 15 years old, and adults in the families. The primary outcome measure (incidence of diarrhoea) was assessed in each of these categories with their corresponding control groups, except for the adults reported as the 'whole family'. In this review, we considered results only from the target group, as the first three categories had similar effect sizes. Hand‐washing behavioural changes and changes in knowledge, attitude, and beliefs about hygiene were assessed in the mothers (number not precisely reported), while the primary outcome measures were assessed in the children.
The number of clusters varied from 18 to 1923 (Haggerty 1988 COD; Stanton 1985 BGD). The participants were provided with hand‐washing materials and were involved in large‐group hygiene education training, except for Luby 2006 PAK, which was a follow‐up trial. The intervention and control groups were socioeconomically comparable at baseline.
Outcome measures
All included trials measured diarrhoea episodes except for Luby 2006 PAK; Galiani 2016 PER; Kapoor 2016 IND; Briceno 2017 TZA; Luby 2018 BGD; Null 2018 KEN, which measured mean longitudinal prevalence of diarrhoea. Some trials also assessed different types of diarrhoea: Han 1985 MMR measured dysentery rates, and Luby 2003a PAK and Luby 2003b PAK also assessed the rate of persistent diarrhoea. Two of the included trials reported all‐cause under‐five mortality (Luby 2018 BGD; Null 2018 KEN). None of the included trials reported diarrhoea‐related deaths or cost‐effectiveness data. However, Briceno 2017 TZA also estimated the associated cost‐per‐household gaining access to improved sanitation to be USD 194. Langford 2007 NPL reported changes in hand washing from baseline to end‐line at hand‐washing junctures, Stanton 1985 BGD reported on changes in hand‐washing behaviour, while Nicholson 2008 IND reported hand washing using soap wrappers collected as an indirect measure of soap consumption. Length of follow‐up ranged from 4 to 12 months.
Adjustment for clustering
All trials adjusted for clustering in some way, except for Han 1985 MMR; Langford 2007 NPL; Nicholson 2008 IND; Hartinger 2011 PER; and Kapoor 2016 IND. Stanton 1985 BGD and Luby 2003a PAK adjusted for clustering by estimating rates at the group level; Luby 2003b PAK adjusted for clustering by calculating an ICC based on an analysis of variance level and design effect. Luby 2006 PAK, measuring mean longitudinal prevalence of diarrhoea, accounted for clustering using generalized estimating equations. Luby 2018 BGD and Null 2018 KEN adjusting for covariates using targeted maximum likelihood estimation. Haggerty 1988 COD performed child‐ and site‐level analyses, without providing the 95% CI for the site‐level analysis. The numerator (average number of episodes per child) was provided at the cluster level. Galiani 2016 PER and Briceno 2017 TZA used clustered SEs.
Hospital‐based trial (high‐risk group)
We identified only one trial in a high‐risk group (Huang 2007 USA). It individually randomized 148 adults with AIDS from one HIV clinic in the USA to receive intensive hand‐washing promotion delivered by specialist nurses (Huang 2007 USA). The intervention included hygiene education, hand‐washing demonstrations by nurses and participants, and weekly telephone calls to reinforce hand‐washing messages (Table 6). The major outcomes reported were mean episodes of diarrhoea in each group and the number of hand‐washing episodes per day. They reported the mean hand‐washing frequency per day at baseline and at the end of the intervention (Table 7).
3. Description of hand‐washing intervention among high‐risk group (AIDS patients).
| Trial | Promotional activity | Classificationa | Message content | Hand washing method | Hand washing styleb | Material provision | Water availability |
| Huang 2007 USA | Demonstration by nurses and patients | Hygiene education |
|
Water with soap | Under running water | Unclear (probably not relevant in this population) | Adequate |
aMessage classification. bWhether done under running water; in a bowl by an individual or by several people.
4. Hand washing in high‐risk group (AIDS patients): behavioural change outcomes and KAB.
| Trial | Cluster adjusted? | KAPa changes | Outcome | Intervention | Control | Effect size/P value |
| Huang 2007 USA | Individual randomization | Frequency of hand washing per day | Mean hand‐washing frequency per day at baseline | 3.3 | 3.4 | P value not significant |
| Mean hand washing frequency per day at end‐line | 7 | 4 | P value not provideda |
Abbreviations; KAB = knowledge, attitude, and beliefs. aPercentage change in the mean frequency of hand washing in the intervention arm is 109% versus 18% in the control arm.
Excluded studies
We have listed the excluded trials and the reasons for exclusion in the Characteristics of excluded studies section.
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the 'Risk of bias' assessments for all included trials.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.

Allocation
Child DCCs or school‐based trials
Five of the 13 trials used an adequate method to generate the allocation sequence (Roberts 1996 AUS; Carabin 1997 CAN; Bowen 2004 CHN; Talaat 2008 EGY; Zomer 2015 NED); the method was unclear in the others. The method used to conceal allocation was unclear in all trials. In cluster‐RCTs, lack of concealment of allocation is not considered a major risk of bias, since all clusters are usually randomized at the same time (Higgins 2020).
Community‐based trials
Eleven of the 15 included community‐based trials reported adequate methods for generating allocation sequence (Stanton 1985 BGD; Luby 2003a PAK; Luby 2003b PAK; Luby 2006 PAK; Langford 2007 NPL; Nicholson 2008 IND; Hartinger 2011 PER; Briceno 2017 TZA; Hashi 2017 ETH; Luby 2018 BGD; Null 2018 KEN). Only Luby 2003a PAK reported adequate allocation concealment; it was unclear in the other trials.
Hospital‐based trial (high‐risk group)
Huang 2007 USA did not clearly report the method of randomization or allocation concealment, and we adjudged this trial as having an unclear risk of selection bias.
Blinding
Child DCCs or school‐based trials
Three trials reported blinding of the outcome assessors (Bartlett 1984 USA; Kotch 1989 USA; Roberts 1996 AUS); the rest were open trials.
Community‐based trials
Eight trials reported blinding of outcome assessors, and the rest were open trials (Han 1985 MMR; Haggerty 1988 COD; Langford 2007 NPL; Hartinger 2011 PER; Briceno 2017 TZA; Hashi 2017 ETH; Luby 2018 BGD; Null 2018 KEN).
Hospital‐based trial (high‐risk group)
Huang 2007 USA was at an unclear risk of performance and detection bias, because the trial authors did not provide enough information to make a judgement about the blinding of participants and personnel or of outcome assessors.
Incomplete outcome data
Child DCCs or school‐based trials
It was difficult to assess the number of randomized participants included in the analysis, as this was reported at different levels (cluster, child, person‐time at risk). However, all trials were able to account for the number of randomized clusters included in the analysis. Six trials were at low risk of attrition bias because they reported outcome data for at least 90% of their participants (Butz 1990 USA; Carabin 1997 CAN; Kotch 2003 USA; Bowen 2004 CHN; Talaat 2008 EGY; Zomer 2015 NED). Roberts 1996 AUS; Ban 2015 CHN and Nicholson 2008 IND were at high risk of attrition bias, as they had attrition rates greater than 10%. The rest of the trials were at unclear risk of attrition bias (Black 1977 USA; Bartlett 1984 USA; Kotch 1989 USA; Ladegaard 1999 DEN; Pickering 2013 KEN). It was also unclear in Kotch 1989 USA why one of the DCCs withdrew from the trial.
Community‐based trials
Inclusion of all randomized participants in the analysis was unclear, as it was reported at different levels of analysis (cluster, household, child), except for Nicholson 2008 IND, which reported 18% average attrition bias for all the subgroups in both arms. Luby 2003b PAK and Null 2018 KEN were at unclear risk of attrition bias because the trial authors did not provide sufficient information to make a judgement. Attrition bias was unclear in Galiani 2016 PER and Briceno 2017 TZA because data were collected from a sample, and different participants were surveyed at baseline and follow‐up.
Hospital‐based trial (high‐risk group)
Attrition bias was unclear in Huang 2007 USA.
Selective reporting
Child DCCs or school‐based trials
We note that in Butz 1990 USA the intervention arm received combined interventions, including a hand‐washing educational programme, use of vinyl gloves, use of disposable diaper‐changing pads, and use of alcohol‐based hand rinse by the day‐care provider. The trials did not measure the relative contribution of each component of intervention, so we adjudged reporting bias in this trial to be unclear. The trial authors, however, reported that to reduce reporting bias, all day‐care providers were aware that the intervention programme was being tested in certain homes.
Community‐based trials
We did not detect any evidence of selective outcome reporting in any of the included studies.
Other potential sources of bias
Child DCCs or school‐based trials
Eight trials reported adequate comparability between the intervention and control groups for diarrhoea incidence and sociodemographic characteristics at baseline (including mean total enrolment; percentage of dropouts; sex, age, and race composition of children enrolled; diapering; and toilet facilities) (Black 1977 USA; Bartlett 1984 USA; Butz 1990 USA; Ladegaard 1999 DEN; Bowen 2004 CHN; Talaat 2008 EGY; Pickering 2013 KEN; Ban 2015 CHN). Investigators in Bowen 2004 CHN were forced to over‐ or undersample certain regions to obtain more 'control' schools after the original control schools were sent intervention packs by mistake and thus were excluded. This trial reported small differences in household sanitation and piped water at baseline but no differences between schools in the number of students, class size, or hygiene infrastructure. Comparability at baseline was unclear in the two other trials (Kotch 1989 USA; Roberts 1996 AUS), while it was considered inadequate in two trials. Kotch 2003 USA reported baseline differences in total number of children and boys in favour of the intervention, which they believed may have influenced the outcome measure. Zomer 2015 NED acknowledged baseline imbalance in crude incidence of diarrhoeal episodes per child‐year of 3.0 for intervention versus 5.1 for the control, but they applied statistical adjustments for this baseline characteristic. All trials reported collecting data at the same time for both the intervention and control groups.
Community‐based trials
Eleven trials reported baseline similarity of diarrhoea morbidity and socioeconomic characteristics (including population and household size, socioeconomic status, hand washing and sanitary facilities, and sources of water supply) between the intervention and control groups (Han 1985 MMR; Stanton 1985 BGD; Luby 2003a PAK; Luby 2003b PAK; Luby 2006 PAK; Langford 2007 NPL; Nicholson 2008 IND; Hartinger 2011 PER; Hashi 2017 ETH; Luby 2018 BGD; Null 2018 KEN). There were some differences at baseline in Haggerty 1988 COD (controls had diarrhoea episodes of longer duration than the intervention group), therefore the study was assessed to be at a high risk of other bias. Briceno 2017 TZA and Langford 2007 NPL were also assessed to be at high risk of other bias because of possible cross contamination between the study sites. All the trials reported collecting data at the same period for intervention and control groups.
Hospital‐based trial (High‐risk group)
All 148 randomized participants (Huang 2007 USA) were followed for the trial's one‐year duration. Participants were similar at the start of the trial in terms of age, sex, ethnicity, hand‐washing episodes per day, CD4 count, HIV load, and prophylaxis for opportunistic infections. The results were presented as a continuous outcome only (mean and SD of number of diarrhoea episodes in each arm over the year). This should be viewed with caution, as it is likely that the distribution of diarrhoea episodes may be highly skewed (the mean of 1.24 and SD of 0.9 episodes in the intervention arm imply a non‐normal distribution of diarrhoea episodes). If so, the mean may not be the most appropriate measure of the 'average number' of episodes per participant. The trial reported collecting data at the same period for intervention and control groups.
Effects of interventions
See: Table 1; Table 2; Table 3
We have presented the results as reported by each trial in Table 7 (behavioural change), Table 8, Table 9, Table 10 (incidence of diarrhoea), Table 11, and Table 12. For trials with cluster‐adjusted results and trials that were individually randomized, we summarized the data in forest plots. For trials where this was not possible, we summarized the data in tables in the Data and analyses section.
5. Incidence of diarrhoea in child day‐care centres and schools.
| Trial | Cluster‐ adjusted? | Outcome and result | Method of assessment | Sample size |
| Ban 2015 CHN | No | Number of person‐months for diarrhoea OR = 0.37 (95% CI 0.22 to 0.60). Protective effect of the intervention on diarrhoea Intervention −0.11, control −0.11, protection rate 60.83 (95% CI 42.73 to 78.94) |
|
221 children from 1 kindergarten as the intervention group, and 245 children from the other kindergarten as the control group |
| Bartlett 1984 USA | No | Diarrhoea rate per child‐year of observation Intervention: 0.71 (95% CI 0.65 to 0.77) Control: 0.81 (95% CI 0.75 to 0.87) |
|
26 day‐care centres with 374 children (196 intervention, 178 control) aged 0 to 3 years |
| Black 1977 USA | No | Diarrhoea incidence/100/child‐weeks of observation Intervention: 4.2/100/child‐week Control: 8.1/100/child‐week |
Daily record of attendance plus diarrhoea occurrence for each child by day‐care personnel | 4 day‐care centres (2 intervention, 2 control) with 116 children < 3 years |
| Bowen 2004 CHN | Yes | Median episodes of diarrhoea per 100 student weeks Expanded intervention: 0 per 100 student‐weeks Standard intervention: 0 per 100 student‐weeks Control: 0 per 100 student‐weeks |
Teachers trained using standardized case definitions to identify 10 symptoms or signs of illness and record these among students in class, 1 day per week; if parent's reported infection as cause of absence, teachers recorded name of syndrome and asked parent if child suffered any of 10 individual symptoms; verified verbally that reports of diarrhoea met case definition | 3962 children within 87 primary schools |
| Butz 1990 USA | No | Proportion of diarrhoea days per month Diarrhoea episodes/child‐days Intervention: 93/10,159 Control: 133/10,424 |
Daily symptom record for each child by care providers | 24 family day‐care homes with 108 children (58 intervention, 50 control) aged 1 month to 7 years |
| Carabin 1997 CAN | Yes | Diarrhoea incidence: episodes/100 child‐days at risk Incidence rate ratio (95% Bayesian credible interval) 1.10 (0.81 to 1.50), adjusted for age and gender Intervention alone: 0.77 (0.51 to 1.18) Monitoring alone: 0.73 (0.54 to 0.97) |
Daily record of diarrhoea episodes on calendar by educators | 52 day‐care centres with 1729 children aged 18 months to 3 years |
| Kotch 1989 USA | Yes | Diarrhoea rates: incidence density (episodes/child‐year) Intervention (< 2 years): 4.54 Intervention (> 2 years): 2.85 Control (< 2 years): 5.12 Control (> 2 years): 2.79 All: RR 1.19, 95% CI −0.48 to 1.96 |
|
24 day‐care centres with 389 children < 3 years |
| Kotch 2003 USA | No | Intervention group experienced significantly lower episodes of diarrhoea Incidence density score: Intervention: 0.90 diarrhoea illness per 100 child‐days. P < 0.001 Control: 1.58 diarrhoea illness per 100 child‐days. P < 0.001 Children in the intervention group sick with diarrhoea a lower proportion of days than children in the control group: Days ill with diarrhoea: Intervention: 4.0% Control: 5.0% P < 0.001 |
|
46 child‐care centres (23 child‐care centres in the intervention arm and 23 child‐care centres in the control arm) with 388 children (infants and toddlers < 36 months) |
| Ladegaard 1999 DEN | No | Diarrhoea episodes/child‐month Intervention: 33/848 Control: 61/1052 (34% reduction from 3.25 days per child in favour of children 3 years or more) |
Information on absenteeism recorded on a form by child‐care provider | 8 day‐care centres with 475 children (212 intervention, 263 control) aged 6 years and below |
| Pickering 2013 KEN | Yes | Hierarchical (Poisson) model result soap versus control; Diarrhoea (defined as 3 or more loose/watery stool in 24 hours): RR 0.84, 95% CI 0.58 to 1.22; P = 0.36 Any loose/watery stool in 24 hours: RR 1.09 (95% CI 0.92 to 1.30). P = 0.33 Loose/watery stool identified on Bristol stool Chart: RR 1.04 (95% CI (0.85 to 1.29); P = 0.69 |
|
6 primary schools (2 each for Hand washing with soap (HWWS), Hand sanitizer and control) with a total of 1364 children participants. However, the intervention of interest (HWWS = 460; control = 469) therefore total = 929). aged between 5 and 10 years. |
| Roberts 1996 AUS | Yes | Diarrhoeal rates: episodes/child‐year Intervention: 1.9 episodes/child‐year Control: 2.7 episodes/child‐year All: RR 0.50, 95% CI 0.36 to 0.68 < 2 years: RR 0.90, 95% CI, 0.67 to 1.19 > 2 years: RR 0.48, 95% CI 0.29 to 0.78 (Adjusted for clustering by centre, confounding variables (age, sex, weight at birth, breastfeeding status, child care history, and home factors), and interactions between age and intervention status, and between having a sibling who attends child care and intervention status) |
|
23 day‐care centres (11 intervention, 12 control) with 558 children under 3 years |
| Talaat 2008 EGY | No | Diarrhoea episodes Intervention: 639 episodes Median IQR: 0.2 (0.0 to 0.5) Control: 1316 episodes Median IQR: 0.3 (0.1 to 0.6) 33% reduction P < 0.0001 Incidence of absenteeism caused by diarrhoea was 33% lower in school children in the intervention schools |
|
60 elementary schools (30 intervention, 30 control) with 44,451 children (20,882 intervention; 23,569 control) Median age: 8 years |
| Zomer 2015 NED | Yes | Diarrhoeal rates: episodes/child‐year (7 symptom‐free days between episodes) Intervention: 3.0 episodes/child‐year Control: 3.4 episodes/child‐year IRR 0.90, 95% CI 0.73 to 1.11 P value: 0.32 |
|
71 day‐care centres (36 intervention; 35 control 35) with 545 children (278 from 34 intervention DCC and 267 from 35 control DCC) |
Abbreviations: CI: confidence interval; IQR: interquartile range.
6. Incidence of diarrhoea in communities.
| Trial | Cluster‐adjusted? | Outcome and result | Method of assessment | Sample size |
| Briceno 2017 TZA | Yes | Health indices Diarrhoea in the past 7 days = −0.004 (SE 0.012), control mean = 0.086 Diarrhoea in the past 14 days (listing data) = −0.013 (SE 0.011), control mean = 0.168 |
|
10 districts with 181 wards (44 wards with 88 villages and 1433 children < 5 assigned to sanitation‐only treatment, 45 wards with 90 villages and 1452 children < 5 assigned to hand washing‐only treatment, 46 wards with 92 villages and 1431 children < 5 assigned to combined treatment, 46 wards with 92 villages and 1481 children < 5 assigned to control) |
| Galiani 2016 PER | Yes | Diarrhoea prevalence in 48 hours A. Community treatment sample Effect of treatment −0.002 (SE 0.011), control mean = 0.040 B. School component sample Effect of treatment 0.001 (SE 0.009), control mean = 0.033 Diarrhoea prevalence in 7 days A. Community treatment sample Effect of treatment 0.001 (SE 0.015), control mean = 0.060 B. School component sample Effect of treatment −0.005 (SE 0.014), control mean = 0.069 |
|
85 districts (44 intervention, 41 control) |
| Haggerty 1988 COD | Yes | Diarrhoea rates (mean episodes of diarrhoea) Intervention site: 0.071 Control site: 0.075 (RR 0.94, 95% CI 0.85 to 1.05; P = 0.3) |
|
18 sites (9 intervention, 9 control) with 1954 children aged 3 months to 35 months |
| Han 1985 MMR | No | Incidence rate per 1000 child days of observation Intervention: 3.5 Control: 4.9 Incidence density ratio 1. Diarrhoea < 2 years: 0.69 (95% CI 0.48 to 1.10) > 2 years: 0.67 (95% CI 0.45 to 0.98) All: 0.70 (95% CI 0.54 to 0.92) 2. Dysentery < 2 years: 0.59 (95% CI 0.22 to 1.55) > 2 years: 1.21 (95% CI 0.52 to 2.80) All: 0.93 (95% CI 0.39 to 2.23) |
Daily surveillance (24‐hour recall) for diarrhoea and dysentery | 350 households (162 intervention, 188 control) with 494 children (236 intervention; 258 control) under 5 years |
| Hashi 2017 ETH | Yes | Longitudinal adjusted incidence rate ratio (IRR) 0.65 (95% CI 0.57, 0.73) Episodes of diarrhoea per 100 person‐weeks of observation Intervention: 594 episodes (4.1 episodes per 100 person‐weeks observation) Control: 905 episodes (6.3 episodes per 100 person‐weeks observation) |
2‐weekly data collection. Data collectors recorded episodes of diarrhoea over the previous 2 weeks based on primary care takers report | 24 sub‐Kebelles districts with 1224 children (12 sub‐Kebelles with 612 children < 5 assigned to the intervention group, 12 sub‐Kebelles with 612 children < 5 assigned to the control group) Follow‐up: intervention: 603 children, control: 596 children |
| Hartinger 2011 PER | Unclear | Diarrhoea episodes: Intervention: 287 diarrhoea episodes or a mean of 1.7 episodes per child‐year at risk Control: 365 diarrhoea episodes or a mean of 2.3 episodes per child‐year at risk |
Records and observations through monthly home visits | 534 children (267 intervention, 267 control) |
| Kapoor 2016 IND | No | Episodes of diarrhoea Intervention: reduced from 90% to 52% Control: reduced from 88.7% to 83.2% Post‐intervention prevalence of diarrhoea was 4.3 times higher in control group compared to intervention group (non‐adjusted odds ratio). After controlling the confounding variables the prevalence of diarrhoea was 3.9 times higher in the control group compared to intervention group (adjusted odds ratio) |
Self‐reporting/ records collected by health workers during home visits | 101 mothers with children below 2 years (50 intervention, 51 control) |
| Langford 2007 NPL | No | Diarrhoea episodes: children from intervention areas experienced on average 31% fewer episodes of diarrhoea than control counterparts Intervention: 3.0 episodes Control: 4.33 episodes P = 0.049 Intervention children also experienced 41% fewer days of diarrhoea than children in control areas Diarrhoea incidence: Intervention: 9.67 person‐days Control: 16.33 person‐days P = 0.023 |
|
88 children (45 intervention, 43 control) aged 3 to 12 months old had complete data sets |
| Luby 2003a PAK | Yes | Incidence density of diarrhoea (number of new episodes of diarrhoea divided by the at‐risk person‐weeks of observation) Mean incidence 1. Primary diarrhoea Intervention: Antibacterial soap: 2.02 Plain soap: 1.91 Control: 4.06 2. Persistent diarrhoea Intervention: Antibacterial soap: 0.14 Plain soap: 0.12 Control: 0.17 |
Weekly observational visits to households | 36 neighbourhoods (25 intervention, 11 control) with 4691 children (3163 intervention, 1528 control) aged < 15 years |
| Luby 2003b PAK | Yes | Diarrhoea episodes/100 child‐weeks: for diarrhoea and persistent diarrhoea Intervention: 3.71 Control: 6.56 RR 0.57, 95% CI 0.35 to 0.86 Diarrhoea, mean incidence: 3.71 Persistent diarrhoea, mean incidence: 0.09 −52% (−100% to 100%) |
Weekly observational visits to households | 18 clusters (544 households; 262 intervention; 282 control) with children < 15 years |
| Luby 2006 PAK | Yes | Crude diarrhoea longitudinal prevalence (%) 1.58 Modeled risk difference (%) vs. control −0.16 (95% CI −0.92 to 0.60) |
Weekly observational visits to household/self‐reports | 577 households including the hand‐washing promotion (195 households), hand‐washing promotion plus water treatment (186 households) and control arm (195 households) |
| Luby 2018 BGD | Yes | Diarrhoea prevalence Mean prevalence ‐ intervention 3.5%, control 5.7% Unadjusted prevalence difference intervention = −2.3 (−3.4 to −1.1) Adjusted prevalence difference intervention = −2.5 (−3.6 to −1.3) |
1. Caregiver‐reported diarrhoea during caregiver interview | 5551 pregnant women in 720 clusters (Control: 1382, water: 698, sanitation: 696, hand washing: 688; water, sanitation, and hand washing: 702, nutrition: 699; and water, sanitation, hand washing, and nutrition: 686) |
| Nicholson 2008 IND | No | Per protocol analyses for diarrhoea incidence; episodes per 100 person‐weeks
|
|
35 matched pairs communities (70 in total for intervention and control); 30 households from each of the communities. Target children (5 year olds) = 2052 (intervention: 1026; control: 1026); < 5 years of age = 2469 (intervention: 1190; Control: 1279); 6 to 15 years = 3519 (intervention: 1784; control: 1735); adults = 3685 (intervention: 1892; control: 1793) All participants = 11,725 (intervention: 5892; control: 5833) Age: 5 year old children (Target); under‐5 years of age, children 6 to15 years and adults (non‐targets) |
| Null 2018 KEN | Yes | Diarrhoea prevalence Mean prevalence: intervention 26.1%, control 27.1% Unadjusted prevalence difference intervention = −0.6 (−3.5 to −2.3) Adjusted prevalence difference intervention = −1.1 (−4.0 to −1.8) |
1. Caregiver‐reported diarrhoea during end‐line survey | 702 village clusters (active control arm: 158 clusters; passive control arm: 80 clusters; water arm: 77 clusters; sanitation arm: 77 clusters; hand‐washing arm: 77 clusters; combined water, sanitation, and hand‐washing arm: 76 clusters; nutrition arm: 78 clusters; and the combined water, sanitation, hand‐washing, and nutrition arm: 79 clusters |
| Stanton 1985 BGD | Yes | Rate of diarrhoea per 100 person‐weeks of observation Intervention: 4.29 Control: 5.78 Incidence density ratio 0.75 (95% CI 0.66 to 0.84; P < 0.0001) < 2 years: 0.54 (95% CI 0.43 to 0.66) > 2 years: 0.68 (95% CI 0.54 to 0.85) |
|
1923 families (937 intervention, 986 control) with children aged < 6 years |
Abbreviations: CI: confidence interval.
7. Incidence of diarrhoea in high‐risk group (AIDS patients).
| Trial | Cluster adjusted? | Outcome and result | Method of assessment | Sample size |
| Huang 2007 USA | Not applicable | Mean episodes of diarrhoea over trial period (1 year) Intervention group: 1.24 (± 0.9) Control group: 2.92 (± 0.6) |
Daily hand‐washing diary to record number of hand‐washing episodes per day and diarrhoea diary to record stool frequency and characteristics; weekly telephone calls from trial nurse to ascertain episodes of these outcomes | 75 in hand‐washing group, 73 controls |
Abbreviations: CI: confidence interval.
8. Hand washing in child day‐care centres and schools: behavioural change outcomes and KAB.
| Trial | Cluster‐adjusted? | Measured by | Outcome | Intervention | Control | Effect size or P value |
| Galiani 2016 PER | Yes | Self‐reported hand‐washing behaviour for hand washing with soap and water before eating, food preparation, feeding child/baby, and after faecal contact | Behaviour index | 0.0694 | 0.000 | ‐ |
| Structured observations for hand washing with soap and water before eating, food preparation, feeding child/baby, and after faecal contact | Structured observations index | 0.0643 | 0.000 | ‐ | ||
| Kotch 1989 USA | Yes | Recorded observations at 5‐week intervals | Mean hand‐washing behaviour score after changing a diaper (0 = none, 0.5 = partial, 1 = correct) | 0.75 | 0.37 | P < 0.01 |
| Mean hand‐washing behaviour score after contact with child's mucus, saliva, vomit, etc. (0 = none, 0.5 = partial, 1 = correct) | 0.66 | 0.21 | P < 0.01 | |||
| Pickering 2013 KEN | Yes | Hand‐washing events observed 2 to 4 days per week per school | Proportion of people washing hands after toilet use |
38% | 37% | P > 0.05 |
| Proportion of people washing hands with soap after toilet use | 37% | 2% | P < 0.05 | |||
| Proportion of people washing hands before lunch |
82% | 69% | P > 0.05 | |||
| Proportion of students washing hands with soap before lunch | 70% | 1% | P < 0.05 | |||
| Roberts 1996 AUS | Yes | Observation for compliance of recommended practices every 6 weeks | Compliance of children washing their hands | 53% to > 80% | Not reported | Not reported |
| Zomer 2015 NED | Yes | Observation for hand hygiene compliance at 6 months follow‐up | Compliance of caregivers with hand‐hygiene guidelines | 59% | 44% | OR 4.13, 95% CI 2.33 to 7.32 |
Abbreviations: KAB = knowledge, attitude, and beliefs; OR = odds ratio
9. Hand washing in communities: behavioural change outcomes and KAB.
| Trial | Cluster‐adjusted? | Measured by | Outcome | Intervention | Control | Effect size/P value |
| Briceno 2017 TZA | Yes | Structure observations of hand‐washing behaviour at critical junctures | Hygiene index | 0.096 | 0.010 | P < 0.01 |
| Observation and caregiver reported information | Sanitation index | 0.029 | −0.001 | ‐ | ||
| Galiani 2016 PER | Yes | Self‐reported hand‐washing behaviour for hand washing with soap and water before eating, food preparation, feeding child/baby, and after faecal contact | Behaviour index | 0.0454 | 0.000 | ‐ |
| Kapoor 2016 IND | Unclear | Observations recorded using an observation checklist | Hand‐washing practices pre‐intervention assessment Median score (IQR) | 47.0 (44.0 to 51.0) | 48.0 (43.0 to 51.0) | P 0.086 |
| Hand‐washing practices post‐intervention assessment Median score (IQR) | 51.0 (46.0 to 52.0) | 48.0 (43.0 to 50.0) | P < 0.001 | |||
| Langford 2007 NPL | Approximately adjusted | Trial staff completed questionnaires with mothers' self‐reporting their hand‐washing behaviour | Proportion washing hands after visiting the toilet |
100% | 90.7% | 0.500 |
| Proportion washing hands after cleaning baby's bottom | 100% | 83.7% | 0.031 | |||
| Proportion washing hands before cooking | 71.1% | 2.3% | < 0.001 | |||
| Proportion washing hands before feeding the baby | 62.2% | 18.6% | 0.004 | |||
| Proportion washing hands before eating | 60% | 0% | 0.003 | |||
| Nicholson 2008 IND | Approximately adjusted | Hand‐washing behaviour indirectly assessed using soap consumption (soap wrapper collection) | Median soap consumption per household per week |
235 g | 45 g | ‐ |
| Stanton 1985 BGD | Yes | Comparison of hygienic practices after intervention | Proportion of mothers who wash their hands before preparing food |
39/79 (49%) | 25/75 (33%) | RR 1.48, 95% CI 1.01 to 2.21; P = 0.056 |
Abbreviations; KAB = knowledge, attitude, and beliefs
1. Child DCCs or schools
Primary outcomes
1.1. Incidence of diarrhoea
Overall, hand‐washing promotion reduced diarrhoea episodes by about one‐third (incident rate ratio (IRR) 0.69, 95% CI 0.59 to 0.81; 11 trials, 50,044 children (Bowen 2004 CHN and Ban 2015 CHN not included in analysis); Analysis 1.1). Most data were from high‐income countries (IRR 0.70, 95% CI 0.58 to 0.85; 9 trials, 4664 participants; high‐certainty evidence; Analysis 1.1), with only two trials from LMICs (IRR 0.66, 95% CI 0.43 to 0.99; 2 trials, 45,380 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Hand washing intervention at child care centres and schools versus no intervention, Outcome 1: Incidence of diarrhoea; subgrouped by country income strata
All trials showed a benefit for the intervention, except for Bowen 2004 CHN, which showed no difference between each arm and for which it was not possible to calculate a rate ratio (the median episodes of diarrhoea were 0 per 100 student‐weeks in the control group, standard intervention group, and expanded intervention) (Table 8). Roberts 1996 AUS showed greater risk reduction than other trials (IRR 0.50, 95% CI 0.36 to 0.69; 1 trial, 558 participants), possibly due to a more specific technique of hand washing used (an approximate 'count to 10' to wash and 'count to 10' to rinse). Ban 2015 CHN (not included in the analysis) reported an odds ratio of 0.37 (95% CI 0.22 to 0.60) person‐months of illness due to diarrhoea.
All participants were monitored at least every two weeks to collect data on diarrhoea episodes, while Ban 2015 CHN reported quarterly home visits. This monitoring itself may have helped to improve compliance with hand washing. Only Carabin 1997 CAN attempted to investigate this effect by assessing rates in both groups compared to the pre‐intervention period. They found that monitoring alone appeared to reduce the incidence of diarrhoea (IRR 0.73, 95% CI 0.54 to 0.97; Table 8) and that the intervention effect did not appear to have any benefits beyond this monitoring effect when adjusted for age and gender (IRR 0.77, 95% CI 0.51 to 1.18; Table 8) or when adjusted for age, gender, season, and baseline incidence rate in each cluster (IRR 1.10, 95% CI 0.81 to 1.50; Table 8). However, monitoring was particularly frequent (daily) in this trial. In Bowen 2004 CHN among first‐grade students in schools in China, monitoring may have been less intensive, as in‐class monitoring was carried out one day a week by teachers; reasons for absenteeism were noted when recorded. As the trial was school‐based, no illness information was collected during weekends or school holidays. This design reduced the teachers' burden of data collection, but it may also have reduced the ability of the trial to detect differences in the incidence of diarrhoea between each trial arm.
Black 1977 USA and Pickering 2013 KEN focused only on a hand‐washing intervention, and found no significant difference in the effect estimate (IRR 0.69, 95% CI 0.43 to 1.09; 2 trials, 1045 participants). Nine trials involved multiple hygiene interventions (Bartlett 1984 USA; Kotch 1989 USA; Butz 1990 USA; Roberts 1996 AUS; Carabin 1997 CAN; Ladegaard 1999 DEN; Kotch 2003 USA; Talaat 2008 EGY; Zomer 2015 NED) (IRR 0.69, 95% CI 0.57 to 0.84; 9 trials, 48,999 participants; Analysis 1.2). The implication of this aspect of hand‐hygiene interventions should be further investigated, as we had too few trials in each category to make a statement.
1.2. Analysis.

Comparison 1: Hand washing intervention at child care centres and schools versus no intervention, Outcome 2: Incidence of diarrhoea; subgrouped by co‐interventions
Three trials attempted blinding of outcome assessors (Bartlett 1984 USA; Kotch 1989 USA; Roberts 1996 AUS), with the benefit of hand washing seeming to be less in a 26% reduction (rate ratio 0.74, 95% CI 0.56 to 0.98; 3 trials, 1303 participants; Analysis 1.3), than in the trials that did not blind outcome assessors (Black 1977 USA; Butz 1990 USA; Carabin 1997 CAN; Ladegaard 1999 DEN; Kotch 2003 USA; Talaat 2008 EGY; Pickering 2013 KEN; Zomer 2015 NED), a 33% reduction (IRR 0.67, 95% CI 0.56 to 0.80; 8 trials, 48,741 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: Hand washing intervention at child care centres and schools versus no intervention, Outcome 3: Incidence of diarrhoea; subgrouped by blinding
Secondary outcomes
1.2. Behavioural changes
Four trials reported measures of behavioural change (Kotch 1989 USA; Roberts 1996 AUS; Pickering 2013 KEN; Zomer 2015 NED). As described in Table 11, Kotch 1989 USA reported that hand‐washing behaviour based on 'event sampling scores' improved in the intervention classrooms compared with control classrooms. Roberts 1996 AUS reported that the intervention improved compliance with infection‐control procedures from 53% at baseline to more than 80% at end‐line. Pickering 2013 KEN reported a statistically significant rate of hand washing with soap at intervention schools: 37% against 2% for the control for all toilet events (prevalence ratio 17.2, 95% CI 4.4 to 67.5), while the mean proportion (intervention 0.70, control 0.01) of students hand washing with soap before lunch events was equally significantly different between schools (prevalence ratio 143.0, 95% CI 38.9 to 525.6) (data not pooled; 3 trials, 1845 participants; Table 11).Zomer 2015 NED reported a significant increase in hand‐hygiene compliance for caregivers in intervention DCCs than in control groups, but this did not seem to have any effect on the incidence of diarrhoea episodes. Kotch 1989 USA; Roberts 1996 AUS; Pickering 2013 KEN also reported changes in knowledge, attitude, and beliefs about hand washing (Table 11).
1.3. Diarrhoea‐related deaths, all‐cause under‐five mortality
None of the included trials reported diarrhoea‐related deaths or all‐cause under‐five mortality.
1.4 Cost effectiveness
None of the included trials reported cost‐effectiveness data. However, Kotch 2003 USA reported that the cost of purchasing and installing one unit of the hand‐washing and diapering equipment was high, at USD 10,385 (USD 7500 for the equipment and the rest for installation) per classroom.
2. Community‐based trials
Primary outcomes
2.1. Incidence of diarrhoea
Overall, community‐based hand‐washing promotion reduced the incidence of diarrhoea by around one‐quarter (IRR 0.71, 95% CI 0.62 to 0.81; 9 trials, 15,950 participants; moderate‐certainty evidence; Analysis 2.1). All the trials were conducted in eight LMICs (six from Asia, one from South America, and one from Africa) and one low‐income country (LIC) (Ethiopia).
2.1. Analysis.

Comparison 2: Hand washing intervention in the community versus no intervention, Outcome 1: Incidence of diarrhoea: rate ratios
Three trials assessed the effect of intervention on the incidence rate of different categories of diarrhoea (Han 1985 MMR; Luby 2003a PAK; Luby 2003b PAK). Han 1985 MMR reported on dysentery, and Luby 2003a PAK and Luby 2003b PAK reported on persistent diarrhoea. None of the results were statistically significant (Table 9). Some trials reported the results by participant age (Han 1985 MMR; Stanton 1985 BGD; Luby 2003a PAK; Luby 2003b PAK; Nicholson 2008 IND), with no discernible trend of which age group intervention had greater diarrhoeal reductions (Table 9). Han 1985 MMR and Stanton 1985 BGD reported greater diarrhoeal reduction in children under two years of age, while Luby 2003a PAK and Luby 2003b PAK reported greater reductions in older children. For Nicholson 2008 IND, the effect for the different age groups (five years old, less than five years old, and 6 to 15 years old) were similar.
Five trials promoted hand washing only (Han 1985 MMR; Luby 2003a PAK; Luby 2003b PAK; Langford 2007 NPL; Nicholson 2008 IND), while four trials promoted multiple hygiene interventions (Stanton 1985 BGD; Haggerty 1988 COD; Hartinger 2011 PER; Hashi 2017 ETH). The reduction in the risk of diarrhoea was greater in the trials that promoted hand washing only (IRR 0.63, 95% CI 0.52 to 0.78; 5 trials, 10,888 participants) than in the trials that promoted multiple hygiene interventions (IRR 0.77, 95% CI 0.65 to 0.90; 4 trials, 5062 participants; Analysis 2.3). This aspect of hand‐hygiene interventions should be interpreted with caution, as we had too few trials in each category to make a strong statement. Luby 2006 PAK; Galiani 2016 PER; Kapoor 2016 IND; Briceno 2017 TZA; Luby 2018 BGD; and Null 2018 KEN also promoted multiple hygiene interventions, but we did not include them in the analyses because of the way the data were presented. For instance, the outcome measure for Luby 2003b PAK was mean incidence, while Luby 2006 PAK (which was a follow‐up to Luby 2003b PAK) reported a different outcome measure of longitudinal prevalence. It is therefore difficult to make meaningful direct comparisons. However, Luby 2006 PAK reported that each arm of their intervention significantly reduced diarrhoea, but that simultaneously combining hand‐washing promotion and water treatment had no apparent benefits.
2.3. Analysis.

Comparison 2: Hand washing intervention in the community versus no intervention, Outcome 3: Incidence of diarrhoea; subgrouped by co‐interventions
Five trials attempted blinding of outcome assessors, with the benefit of hand washing appearing to be lower than in trials which did not blind outcome assessors (IRR 0.76, 95% CI 0.64 to 0.90; 5 trials, 4294 participants) versus (IRR 0.63, 95% CI 0.48 to 0.83; 4 trials, 11,656 participants; Analysis 2.4).
2.4. Analysis.

Comparison 2: Hand washing intervention in the community versus no intervention, Outcome 4: Incidence of diarrhoea; subgrouped by blinding
Seven trials provided soap alongside hand‐hygiene promotional activities, and the effect seemed to be larger in these trials than in those which did not provide soap (IRR 0.66, 95% CI 0.58 to 0.75; 7 trials, 12,646 participants) versus (IRR 0.84, 95% CI 0.67 to 1.05; 2 trials, 3304 participants; Analysis 2.5). With only a small number of trials, these differences may be due to chance or, even if real, it is difficult to discern which components (providing soap or focusing on hand washing only) are most effective.
2.5. Analysis.

Comparison 2: Hand washing intervention in the community versus no intervention, Outcome 5: Incidence of diarrhoea; subgrouped by provision of soap
2.1.1 Mean longitudinal prevalence of diarrhoea
Six studies reported the mean longitudinal prevalence of diarrhoea. All of these showed a reduction in the prevalence of diarrhoea favouring the intervention arm, but not all were statistically significant (Analysis 2.6). One study did not report sufficient information about the method of measuring diarrhoea, time period, or study sample size to extract data for a meta‐analysis (Kapoor 2016 IND). The study results showed a reduction in the episodes of diarrhoea from 90% to 52% in the intervention group, and from 88.7% to 83.2% in the control group, as well as a post‐intervention prevalence of diarrhoea 3.9 times higher in the control group after adjusting for confounding variables. Similarly, Luby 2006 PAK reported a mean prevalence of 4.73 person‐weeks with diarrhoea in the soap and hand‐washing promotion arm compared to 8.62 person‐weeks with diarrhoea in the control arm following the intervention. The other four studies all reported the mean longitudinal prevalence over a one‐week period (Galiani 2016 PER; Briceno 2017 TZA; Luby 2018 BGD; Null 2018 KEN). Overall, the meta‐analysis showed a reduction in the prevalence of diarrhoea of over four percentage points (4.60% reduction, 95% CI 1.19 to 8.02; 4 studies, 14,577 participants; Analysis 2.2).
2.6. Analysis.
Comparison 2: Hand washing intervention in the community versus no intervention, Outcome 6: Mean longitudinal prevalence
| Mean longitudinal prevalence | ||||||
| Study | Mean longitudinal prevalence of diarrhoea for all children under observation (Hand washing promotion) | Control | Standard error | Adjusted prevalence difference (study Author reported) | Time period of measurement | How the measurement was made |
| Briceno 2017 TZA | ‐0.004 (‐0.4%) | 0.086 (8.6%) | 0.012 | ‐ | Diarrhoeal symptoms in the past 7 days | Caregiver‐reported diarrhoea |
| Galiani 2016 PER | 0.001 (0.1%) | 0.060 (6%) | 0.015 | ‐ | Diarrhoeal symptoms in the past 7 days | Caregiver‐reported diarrhoea |
| Kapoor 2016 IND | 38% | 5.5% | ‐ | "After controlling the confounding variables the prevalence of diarrhoea was 3.9 times higher in control group as compared to intervention group (adjusted odds ratio)" | "End line survey in both groups was carried after 3
months to record mothers’ behaviour and number of
episodes of diarrhoea among children." There were also five visits where the episodes of diarrhoea where recorded |
Caregiver‐reported |
| Luby 2006 PAK | 4.73% | 8.62% | 1.232 | ‐ | 7 days (weekly longitudinal prevalence of diarrhoea) | Person‐weeks with diarrhoea/Person‐weeks of observation |
| Luby 2018 BGD | 3.5% | 5.7% | 0.561 | Adjusted prevalence difference (95% CI) ‐2.5 (‐3.6 to ‐1.3) Prevalence ratio (95%CI) 0.60(0.45 to 0.80) |
Diarrhoeal symptoms in the past 7 days | Caregiver‐reported diarrhoea |
| Null 2018 KEN | 26.1% | 27.1% | 1.479 | Adjusted prevalence difference (95% CI) ‐1.1 (‐4.0 to ‐1.8) Prevalence ratio (95%CI) 0.98 (0.87 to 1.09) |
Diarrhoeal symptoms in the past 7 days | Caregiver‐reported diarrhoea |
2.2. Analysis.

Comparison 2: Hand washing intervention in the community versus no intervention, Outcome 2: Mean longitudinal prevalence (pooled)
Secondary outcomes
2.2. Behavioural changes
Stanton 1985 BGD adjusted for clustering and reported that the intervention group exhibited a greater increase in hygiene practices (IRR 1.48, 95% CI 1.01 to 2.21), although this increase is of borderline statistical significance (P = 0.056; Table 12). Langford 2007 NPL reports that at the end of the intervention, hand washing after cleaning the baby's bottom or before cooking, eating, or feeding the baby had increased in mothers from the intervention areas (McNemar's test, P < 0.01 for all four junctures), while hand‐washing practices remained unchanged in the control areas. Nicholson 2008 IND measured hand‐washing behaviour between trial groups indirectly by assessing soap consumption (soap wrapper collection) and reported median soap consumption per household per week of 235 g for intervention households compared with 45 g for the controls. Kapoor 2016 IND reported improvements in the hand‐washing practices of mothers in the intervention group. (Data not pooled; 4 trials, 3591 participants; trials reporting mean longitudinal prevalence Galiani 2016 PER and Briceno 2017 TZA are not included; Table 12).
2.3. Diarrhoea‐related deaths and all‐cause under‐five mortality
Two trials assessed all‐cause mortality as tertiary outcomes in their trials (Luby 2018 BGD; Null 2018 KEN). All‐cause mortality was higher in the intervention arm – hand‐washing only (5.3%) than in the active control arm (3.9%). All‐cause mortality was lower in the combined water sanitation, hand‐washing, and nutrition group (2.8%) than in the control arm.
Luby 2018 BGD showed no difference in all‐cause mortality between the hand‐washing arm and the control arm, respectively (4.5% vs 4.7%). All‐cause mortality was lower in the combined nutrition, water, sanitation, and hygiene arm than the control arm (2.9% versus 4.7%).
None of the included trials reported diarrhoea‐related deaths.
2.4 Cost effectiveness
None of the included trials reported cost‐effectiveness data.
3. Hospital‐based trial (high‐risk group)
3.1. Episodes of diarrhoea
In Huang 2007 USA, the intensive hand‐washing intervention reduced the mean number of episodes of diarrhoea over the one‐year trial period (2.92 in control group, 1.24 in intervention group; a reduction of 1.68 episodes, 95% CI −1.93 to −1.43; 148 participants; moderate‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Hand washing intervention in hospital setting versus no intervention, Outcome 1: Episodes of diarrhoea
3.2. Behavioural changes
At the beginning of the trial, there was no difference in daily hand‐washing frequency between intervention and control groups (3.4 ± 1.1 in control group; 3.3 ± 0.98 in intervention group; Table 7), but at the end of the trial the intervention group reported hand washing seven times a day compared with four times daily in the control group (P < 0.05; moderate‐certainty evidence).
Discussion
In the original review (Ejemot‐Nwadiaro 2008), 14 trials met the inclusion criteria. Eight other trials were included in the first review update (Ejemot‐Nwadiaro 2015). We have included seven additional trials in this review update, making a total of 29 included trials. Luby 2006 PAK was a follow‐up trial to Luby 2003b PAK. This trial involved no primary interventions, but assessed the sustainability of the Luby 2003b PAK hand‐hygiene interventions in preventing diarrhoea. The other trials had primary interventions.
Summary of main results
Hand‐washing promotion at child day‐care facilities or schools prevents around one‐third of diarrhoea episodes in high‐income countries (high‐certainty evidence; Table 1). It may prevent a similar proportion in LMICs, but only two trials (from urban Egypt and Kenya) have evaluated this (low‐certainty evidence).
Hand‐washing promotion among communities in LMICs probably prevents around one‐quarter of diarrhoea episodes (moderate‐certainty evidence; Table 2). However, six of these eight trials were from Asian settings, with one trial from South America and two trials (one of them (Ethiopia) a LIC) from sub‐Saharan Africa. In seven trials, soap was provided free alongside education and behavioural‐change interventions. The overall effect size was larger in the trials that provided soap (34%) than in the two trials that did not provide soap (16%). The influence of this on the intervention effect estimate is not well understood. This underscores the need for data on the long‐term sustainability of hand‐washing promotion to inform research design and policy decisions, especially for LMICs, which tend to have a higher burden of diarrhoea but lack the resources to address it (Ejemot‐Nwadiaro 2015; Luby 2018 BGD).
The effect of hand‐washing promotion in a hospital‐based setting among a high‐risk population had a significant reduction in mean episodes of diarrhoea that favoured the intervention group (moderate‐certainty evidence; Table 3). This is from only one trial.
The effect of the intervention on the hand hygiene‐related behavioural outcome in all settings showed an increase in the proportion of hand‐washing or hand‐hygiene compliance at essential junctures (before eating and cooking and after visiting the toilet or cleaning the baby's bottom), favouring the intervention groups (unpooled data, reflecting a range of low‐ to high‐certainty evidence; Table 1; Table 2; Table 3). The effectiveness of each type of hand‐washing promotion or a combination of different types was not assessed and is therefore unknown. We found no trials evaluating or reporting the effects of hand‐washing interventions on diarrhoea‐related deaths or cost effectiveness. The effect of hand‐washing interventions on all‐cause under‐five mortality in community‐based trials in LMICs is unclear. One trial reported no difference between the intervention and control arm, while the other reported a higher all‐cause mortality in the intervention arm. In both trials, the cumulative incidence was lower in the combined nutrition, water, sanitation, and hygiene arm than control arm. The results suggest that there may be other modifiers of all‐cause mortality than hand‐washing interventions alone.
Overall completeness and applicability of evidence
We believe we identified all RCTs that met our inclusion criteria. We further categorized the included trials into three distinct settings: child DCCs or schools, community, and hospital. Although there were only a few trials included in each category, evidence favours hand‐washing interventions in preventing diarrhoea in all the settings. This suggests that the intervention exhibits population‐wide health gains. However, most included trials in the institution category were from childcare settings in high‐income countries. We are therefore not confident that this finding can be applied to schools in LMIC settings or alternative institutions. Also, only one hospital‐based trial met the inclusion criteria, so evidence from this setting was limited.
Ninety‐five per cent of the participants for whom the primary outcome was measured were below five years of age. Talaat 2008 EGY measured the primary outcome in participants with a mean age of eight years but did not stratify the results by age. Nicholson 2008 IND measured the primary outcome in participants of various ages (target children five years of age, children below five years of age, children between 6 and 15 years of age, and adults) and stratified results by these independent subgroups and reported effect sizes, with no significant trend observed. Although children under five years of age are most at risk of diarrhoeal infection, understanding the effect of this intervention in participants above five years of age and in adults would provide better comparative evidence.
All included trials were relatively small and had short follow‐up durations, including intensive monitoring, and they demonstrated significant reductions in the risk of diarrhoea after hand‐hygiene intervention. However, in one relatively large trial (Bowen 2004 CHN), and in three with longer follow‐up (Luby 2006 PAK; Luby 2018 BGD; Null 2018 KEN), there were no apparent benefits, as no significant differences between the incidence or longitudinal prevalence of diarrhoea were found. We are therefore unclear if the reductions in incidence of diarrhoea would be maintained if these trials had been larger and conducted over a longer period.
The effect size was lower in child DCCs and school‐based trials that attempted blinding outcome assessors than in trials that did not (26% versus 33% reduction in the incidence of diarrhoea, respectively). The same trend was observed for community‐based trials, with a 24% reduction for five trials that attempted blinding of outcome assessors and a 37% reduction for four trials that did not attempt blinding. This suggests a possible introduction of bias in trials that did not attempt blinding. However, there were too few trials in each category to draw strong conclusions.
Community‐based trials that focused only on hand‐washing interventions showed a greater effect size than those that involved multiple hygiene interventions (37% versus 23%), whereas in child DCC and school‐based trials there was no detectable difference in effect size. Although there were few trials in both settings to suggest direction in intervention designs, Luby 2018 BGD opined that a single intervention may provide greater health benefits than multiple interventions that are likely to reach fewer people. However, a lack of evidence on which to make design decisions or support this assertion remains a challenge, particularly in light of scarce and competing health resources.
Quality of the evidence
We assessed the certainty of the evidence using the GRADE approach (GRADEpro 2014). In general, the evidence that hand washing promotion reduces the incidence of diarrhoea in both child DCCs in high‐income countries and community settings in LMICs is considered high‐certainty (Table 1; Table 2). Most trials were at high or unclear risk of detection or reporting bias because there was no description of outcome assessors blinding. However, this made a negligible difference to our findings, as restriction of the analysis to just the blinded trials found a slightly smaller but statistically significant effect size. In addition, the trials' results showed a lot of statistical heterogeneity. However, these inconsistencies did not affect the certainty of evidence in these settings, since all trials favoured the intervention, albeit with varying effect sizes. We are therefore confident in the estimate of effect, and further research is very unlikely to change our confidence in the estimate.
For the trials conducted in LMIC schools, we considered the certainty of evidence to be low due to indirectness, as this limits our confidence in the effect estimate. Talaat 2008 EGY and Pickering 2013 KEN were conducted under experimentally‐controlled conditions. Although they showed benefits in favour of the intervention groups, we are unsure if these benefits would be maintained if trials were longer, with minimal provision of hand‐washing materials and less intense follow‐up.
Certainty of evidence from unpooled data for the behavioural outcomes ranged from low to high in all settings. These should be interpreted with caution, as there were too few trials in each setting and the methods of assessment were too varied to make strong statements. The benefit of adopting an explicit behavioural change model is still unclear; this may influence the maintenance and sustainability of hand‐hygiene behaviour, as Whitby 2007 has opined that the strongest determinant of hand‐washing behaviour may be its habituation.The certainty of evidence about the other outcomes (diarrhoea‐related deaths, all‐cause under‐five mortality, and cost effectiveness) were not determined due to a paucity of included trials providing data on which to make such judgements. Further research is therefore necessary to provide a basis for assessment of evidence for these factors critical to hand‐washing interventions in preventing diarrhoea.
Potential biases in the review process
We did not identify any potential biases in the review process. We only included peer‐reviewed and published randomized controlled trials in this review. We also included a trial published in Danish (Ladegaard 1999 DEN). We also searched grey literature and clinical trials registers to identify eligible ongoing trials. One of the settings (hospital‐based) had only one trial (Huang 2007 USA) and small number of participants ‐ 148. This limits our interpretation of the effect of the intervention in this setting.
Agreements and disagreements with other studies or reviews
The magnitude of the intervention effect (≃ 30%) in both child DCCs or schools and community settings that we observed in this review did not differ significantly from that of the original review (Ejemot‐Nwadiaro 2008), or the first update (Ejemot‐Nwadiaro 2015). The effect size, however, remains lower in magnitude than previous reviews of hand‐washing interventions, which was 47% (Curtis 2003), and about 44% in the reviews of Fewtrell 2004 and Fewtrell 2005. These differences may be attributable to the choice of effect measure, mixed trial designs, and a single setting. Curtis 2003 used odds ratios, which are known to inflate effects sizes for conditions such as diarrhoea with common event rates in the analyses. In our review, we reported only rate ratios, which Guevara 2004 opines improves clinical interpretation of pooled effect estimates. Fewtrell 2005 presented evidence of publication bias, while Curtis 2003 included case‐control and cross‐sectional trials as well as prospective interventions. Both reviews considered only hand‐hygiene interventions conducted in LMICs. In our review, we included only RCTs and mixed settings (child DCC‐ or school‐, community‐, and hospital‐based trials conducted in both developing and developed countries). However, they are all in agreement that hand‐hygiene interventions are effective for reducing diarrhoeal diseases.
Authors' conclusions
Implications for practice.
Hand‐washing promotion leads to a reduction in diarrhoea episodes by about 30%, both in child DCCs in high‐income countries and among communities in LMICs. We have little evidence about the sustainability of changes in hand‐washing behaviour or how best to promote this over a longer period.
Implications for research.
The findings of this Cochrane Review show that further research to determine the efficacy of hand‐washing intervention in preventing diarrhoea will be unnecessary in child DCCs in high‐income countries and in communities in LMICs, although only one of these trials was conducted in Africa. Most of the included studies were of short duration and follow‐up. They could therefore be described as high intensity, since trial participants were contacted at least twice weekly during the intervention. There is a need to assess the effect of the intervention in trials of longer duration and follow‐up, and to ascertain the sustainability of hand‐washing behaviours. This presents an evidence gap and rationale for further research to guide practice and policy directions. This becomes all the more critical in areas of limited competing resources, particularly in LMICs.
More trials conducted in child DCCs or schools in LMICs are needed to enhance our ability to generalize the intervention effects. The need to conduct research with longer follow‐ups that uses a structured method of assessing the primary outcome is pertinent, since it has been observed that an arbitrary use of methods may have a significant effect on the precision of estimates (Morgado 2017). Outcome assessors should be blinded to reduce the bias in estimates of effect size. Evidence of the effects of hand washing interventions on diarrhoea incidence in hospital‐based settings is still limited, as we found only one trial that met the inclusion criteria. Further research in this area is therefore warranted.
Feedback
Search strategy, December 2011
Summary
I have read the interesting Cochrane Review 'Hand washing for preventing diarrhoea' conducted by you and your colleagues, published in The Cochrane Library 2009, issue 3. I would like to take the liberty to comment on the search strategies shown in Table 1:
Search set 8 and 9 are identical for MEDLINE and EMBASE – I assume one of them should be upper case to indicate MeSH/EMTREE, or? (The correct MeSH/EMTREE is DIARRHEA, not DIARRHOEA – but either maps to the correct term, and thus gives the same result)
I suggest you include hand washing$, diarrhoea$ and diarrhoea$ as free text terms.
From the attached search sets it appears that you may have missed 98 and 61 potentially relevant records in MEDLINE and EMBASE respectively. Of course, this does not mean that you have not identified all relevant and available trials but it still poses a risk which I suggest you address in your next update of the review. How I searched MEDLINE and EMBASE, via Ovid (other databases were not searched):
Set 1‐11: Identical to the search shown in Table 1 (I assumed set 9 should be in upper case)
Set 12‐16: I added hand washing$ as free text term and show how many records are missed (set 16: records published before 2008)
Set 17‐22: Same as above, but added diarrhoea$ and diarrhoea$ to the search (set 22: records published before 2008)
Also, it would be helpful to know how many records your retrieved in your initial searches, how many were excluded due to lack of relevance, methodological flaws etc., i.e. presented in a flowchart.
Best regards,
Ole Nørgaard
Reply
We agree with the contributor that there was an error in Table 1. We have corrected this. We do not believe that we have missed any relevant records, but as this review is due to be updated, we will investigate this further during the updating process. With regard to presenting the results in a flowchart, PRISMA diagrams were not expected in Cochrane Reviews at the time this review was initially produced. This will again be dealt with during the updating process.
Contributors
Ole Frandsen Nørgaard of the Department of Computer Science, Faculty of Health Sciences, University of Copenhagen, Denmark identified slight anomalies in the search strategy used in preparing the original review (Ejemot‐Nwadiaro 2008). We have incorporated his suggestions appropriately into this review update.
What's new
| Date | Event | Description |
|---|---|---|
| 5 January 2021 | New search has been performed | Review updated and 7 new trials included. |
| 5 January 2021 | New citation required but conclusions have not changed | No change to conclusions. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 26 August 2015 | New citation required but conclusions have not changed | Review updated and eight new trials included. |
| 26 August 2015 | New search has been performed | We updated the literature search and eight new trials met the inclusion criteria. We used GRADEpro 2014 to assess the certainty of the evidence and have included 'Summary of findings' tables in this review update. Also, we have introduced the term 'promotion' into the review title. |
| 17 January 2012 | Feedback has been incorporated | We are grateful to an observant reader who identified an error in the search strategy. We have now corrected this. |
| 8 August 2008 | Amended | We converted to new review format with minor editing. |
| 2 July 2008 | Amended | We removed trials that did not adjust for clustering from the meta‐analysis and presented the data in tables. Trials that did not adjust for clustering are clearly labelled in the Results, tables, and 'Characteristics of included studies' sections. We amended the Methods and Results to reflect these changes. |
Acknowledgements
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We thank all trial authors that assisted us with information and clarifications about their trials. We are particularly grateful to Dr S Luby of the Centers for Disease Control and Prevention and Jonathan Kotch of the University of North Carolina at Chapel Hill, USA.
We thank Karin Schiöler and Jeppe Schroll for assisting with translation of the Danish trial.
The first version of this review was technically completed during the Cochrane Review Finishing School attended by Regina Ejemot‐Nwadiaro at Liverpool School of Tropical Medicine and organized by the Cochrane Infectious Diseases Group in June 2005 (Ejemot‐Nwadiaro 2008).
Appendices
Appendix 1. Detailed search strategies
MEDLINE (PubMed)
| Search | Query |
| #1 | Search hand AND (wash* or disifect* or clean* or hygiene) Field: Title/Abstract |
| #2 | Search "Hand Disinfection"[Mesh] |
| #3 | Search handwashing Field: Title/Abstract |
| #4 | Search ((#3) OR #2) OR #1 |
| #5 | Search diarrh* Field: Title/Abstract |
| #6 | Search "Diarrhea, Infantile"[Mesh] |
| #7 | Search "Diarrhea"[Mesh] |
| #8 | Search gastroenteritis Field: Title/Abstract |
| #9 | Search dysenter* Field: Title/Abstract |
| #10 | Search (((#9) OR #8) OR #7) OR #6 OR #5 |
| #11 | Search "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] |
| #12 | Search random* Filters: Field: Title/Abstract |
| #13 | Search randomized controlled trial Field: Title/Abstract |
| #14 | Search (((#13) OR #12) OR #11) OR (placebo [Title/Abstract] OR double‐blind*[Title/Abstract] OR single‐blind*[Title/Abstract]) |
| #15 | Search (#10) AND #4 |
| #16 | Search (#15) AND #14 |
Search Name: Cochrane Central Register of Controlled Trials
#1 handwash*
#2 hand wash*
#3 hand‐wash*
#4 hand and (sanitation or cleaning or cleansing or hygiene or disinfect*)
#5 #1 or #2 or #3 or #4
#6 diarrh*
#7 gastroenteritis
#8 dysenter*
#9 #6 or #7 or #8
#10 #9 and #5, in Trials
Database: Embase
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 handwashing.mp. or hand washing/
2 ((Hygiene or handwash* or " hand wash*") adj2 (educat* or promot* or communicat* or behavior)).mp.
3 (hand adj2 (wash* or hygiene or disinfect* or clean* or sanit*)).mp.
4 1 or 2 or 3
5 diarrhea/ or acute diarrhea/ or diarrhea.mp.
6 acute gastroenteritis/ or gastroenteritis.mp. or gastroenteritis/
7 5 or 6
8 4 and 7
9 (randomized or randomised or placebo or double‐blind* or single‐blind*).ti. or (randomized or randomised or placebo or double‐blind* or single‐blind*).ab.
10 randomized controlled trial/ or controlled clinical trial/
11 9 or 10
12 8 and 11
PsycINFO, ERIC (EBSCOhost)
| S1 | TX ( handwashing or hand washing or hand hygiene ) AND TX diarrhea* |
SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH (Web of Science Core Collection)
TOPIC: (handwashing or hand hygiene or hand sanitation) AND TOPIC: (diarrhea or gastroenteritis) AND TOPIC: (randomized or trial or double‐blind*)
Appendix 2. Prespecified changes for review update
| Protocol section | Refreshed protocol |
| Background and research question |
|
| Inclusion criteria |
|
| Methods |
|
| Detailed search strategies | The search strategies have been moved from a table to Appendix 1, to accurately report the format search terms are used in different databases |
Data and analyses
Comparison 1. Hand washing intervention at child care centres and schools versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of diarrhoea; subgrouped by country income strata | 11 | 50044 | Rate Ratio (IV, Random, 95% CI) | 0.69 [0.59, 0.81] |
| 1.1.1 High‐income countries | 9 | 4664 | Rate Ratio (IV, Random, 95% CI) | 0.70 [0.58, 0.85] |
| 1.1.2 Low‐ or middle‐income countries | 2 | 45380 | Rate Ratio (IV, Random, 95% CI) | 0.66 [0.43, 0.99] |
| 1.2 Incidence of diarrhoea; subgrouped by co‐interventions | 11 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Focused: hand washing only | 2 | 1045 | Rate Ratio (IV, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 1.2.2 Multiple hygiene interventions | 9 | 48999 | Rate Ratio (IV, Random, 95% CI) | 0.69 [0.57, 0.84] |
| 1.3 Incidence of diarrhoea; subgrouped by blinding | 11 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Blinding of outcome assessors | 3 | 1303 | Rate Ratio (IV, Random, 95% CI) | 0.74 [0.56, 0.98] |
| 1.3.2 No blinding of outcome assessors | 8 | 48741 | Rate Ratio (IV, Random, 95% CI) | 0.67 [0.56, 0.80] |
Comparison 2. Hand washing intervention in the community versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of diarrhoea: rate ratios | 9 | 15950 | Incidence rate ratio (IV, Random, 95% CI) | 0.71 [0.62, 0.81] |
| 2.2 Mean longitudinal prevalence (pooled) | 4 | 14577 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐8.02, ‐1.19] |
| 2.3 Incidence of diarrhoea; subgrouped by co‐interventions | 9 | 15950 | Rate Ratio (IV, Random, 95% CI) | 0.71 [0.62, 0.81] |
| 2.3.1 Focused: hand washing only | 5 | 10888 | Rate Ratio (IV, Random, 95% CI) | 0.63 [0.52, 0.78] |
| 2.3.2 Multiple hand hygiene interventions | 4 | 5062 | Rate Ratio (IV, Random, 95% CI) | 0.77 [0.65, 0.90] |
| 2.4 Incidence of diarrhoea; subgrouped by blinding | 9 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Blinding of outcome assessors | 5 | 4294 | Rate Ratio (IV, Random, 95% CI) | 0.76 [0.64, 0.90] |
| 2.4.2 No blinding of outcome assessors | 4 | 11656 | Rate Ratio (IV, Random, 95% CI) | 0.63 [0.48, 0.83] |
| 2.5 Incidence of diarrhoea; subgrouped by provision of soap | 9 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Soap provided | 7 | 12646 | Rate Ratio (IV, Random, 95% CI) | 0.66 [0.58, 0.75] |
| 2.5.2 No soap provided | 2 | 3304 | Rate Ratio (IV, Random, 95% CI) | 0.84 [0.67, 1.05] |
| 2.6 Mean longitudinal prevalence | 6 | Other data | No numeric data |
Comparison 3. Hand washing intervention in hospital setting versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Episodes of diarrhoea | 1 | 148 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐1.93, ‐1.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ban 2015 CHN.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: 84% included Length of follow‐up: 12 months Cluster‐adjustment method: not adjusted |
|
| Participants | Number: 2 kindergartens, with 465 children (intervention: 221 children from 5 classes; control: 244 children from 6 classes) Inclusion criteria: not stated Exclusion criteria: not stated Age: < 5 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Illness symptoms for
Not used in this review:
|
|
| Notes | Location: Xianto city, Hubei province, China Duration: October 2010 to September 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We did not adopt the use of individual randomized design. Randomization was based on the kindergarten, 221 children from one kindergarten as the intervention group, and 245 children from the other kindergarten as the control group." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the participants nor the investigators were blinded. However, keeping these limitations in mind, we attempted to reduce ascertainment bias through the use of Daily Record Calendars for both the homes and kindergartens while maintaining close contact with the parents, or guardians, and teachers from both groups." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Neither the participants nor the investigators were blinded. However, keeping these limitations in mind we attempted to reduce ascertainment bias through the use of Daily Record Calendars for both the homes and kindergartens while maintaining close contact with the parents, or guardians, and teachers from both groups." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition more than 10%. 466 enrolled, 72 not analysed (16% attrition) |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Bartlett 1984 USA.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 12 months Cluster‐adjustment method: not adjusted |
|
| Participants | Number: 26 day‐care centres, with 374 children (196 intervention and 178 control) Inclusion criteria: not stated Exclusion criteria: not stated Age: < 3 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Diarrhoea rates | |
| Notes | Location: Maricopa County, Arizona, USA Duration: October 1981 to September 1984 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "22 day care centres were randomly selected from the 108 day care centres in Maricopa county licensed to care for infants and toddlers. The 22 trial day care centres were divided into three strata, based on surveillance rates of infant‐toddler diarrhoea in the preceding 12 months. Half of the centres in each stratum were then randomly assigned to intervention groups." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Student nurses were blinded in regard to intervention or control status of the day‐care centres |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Black 1977 USA.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 6 months Cluster‐adjustment method: not adjusted |
|
| Participants | Number: 4 day‐care centres, with 116 children Inclusion criteria: not stated Exclusion criteria: not stated Age: < 3 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: suburban Atlanta, Georgia, USA Duration: June 1976 to April 1977 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Bowen 2004 CHN.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of all participants in the analysis: 93% (3962/4256) agreed to participate Length of follow‐up: 2003/2004 school year Cluster‐adjustment method: adjusted |
|
| Participants | Number: 87 schools (57 intervention; 30 control); with 3962 children (2670 intervention; 1292 control) Inclusion criteria: public primary schools; at least 20 students in first‐grade year in 2003 to 2004; no overnight boarders; at least 1 running water tap for every 30 first grade students Exclusion criteria: no compulsory hand washing or provision of hand‐cleaning products before school lunch; no commercial hand‐washing promotion programmes at school during previous 5 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: 3 counties in Fujian province, Southeast China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3962 (93%) first grade students from the 4256 first graders attending the enrolled schools agreed to participate and were included in the analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Briceno 2017 TZA.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 1 year and 3 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 10 districts with 181 wards (44 wards with 88 villages and 1433 children < 5 years assigned to sanitation only treatment, 45 wards with 90 villages and 1452 children < 5 years assigned to hand washing only treatment, 46 wards with 92 villages and 1431 children < 5 years assigned to combined treatment, 46 wards with 92 villages and 1481 children < 5 years assigned to control) Inclusion criteria: largest rural wards Exclusion criteria: not described Age: children < 5 |
|
| Interventions | Intervention (see Table 5 for detailed description): Intensive social marketing including
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: rural Tanzania Duration: February 2009 to May 2012 (3 years and 3 months) Data were collected via surveys, with 10 households randomly selected from each village |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The ward‐level randomization was stratified by district and population size using Stata." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "It was not possible to blind participants, although they were never told explicitly about the link between the survey and interventions, and any questions on program exposure were included only at the end of the survey." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To mitigate enumerator bias, survey firms were never provided information on treatment status of participating wards." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were collected by surveys from 10 randomly‐selected households in each village |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | High risk | Quote: "These districts were purposively targeted because of operational feasibility for program implementation, taking into account the existence of ongoing MoW and MoHSW projects." Comment: Purposively selecting districts with ongoing related projects may make it difficult to isolate the effects of the intervention. Possible ‘contamination’ |
Butz 1990 USA.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 12 months Cluster‐adjustment method: not adjusted |
|
| Participants | Number: 24 family day‐care centres, with 108 children (58 intervention, 50 control) Inclusion criteria: not stated Exclusion criteria: unclear Age: 1 month to 7 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Incidence of infectious disease symptoms (diarrhoea) | |
| Notes | Location: Baltimore, Maryland, USA Duration: 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28 children (114 children were enrolled from the FDCHs but actual number of children used in the analysis is 86). |
| Selective reporting (reporting bias) | Unclear risk | Did not measure the relative contribution of each component of intervention, but "to reduce reporting bias, all day care providers were aware that the intervention program was being tested in certain homes" |
| Other bias | Low risk | None observed |
Carabin 1997 CAN.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 6 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 52 day‐care centres, with 1729 children Inclusion criteria: presence of at least 1 sandbox and 1 play area; at least 12 available toddler places Exclusion criteria: not stated Age: 18 months to 3 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Diarrhoea rates | |
| Notes | Location: Quebec, Canada Duration: September 1996 to November 1997 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomized |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 children lost to follow‐up (5 day‐care centres excluded from the analysis) |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Galiani 2016 PER.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 3 years Cluster‐adjustment method: adjusted |
|
| Participants | Number: 85 districts (intervention: 44; control: 41), 3756 households included in the baseline and end‐line survey Inclusion criteria: families that had at least 1 child under 2 years of age Exclusion criteria: not stated Age: children under 5 years |
|
| Interventions | Intervention (see Table 5 for detailed description):
Control:
|
|
| Outcomes |
|
|
| Notes | Location: Peru Duration: 2008 ‐ 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | From these provinces, 85 districts (with between 1500 and 100,000 inhabitants) were randomly selected, with 44 randomly assigned to receive the district‐level community treatment and the other 41 randomly assigned to serve as the control group |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Our surveys record an overall attrition rate of 20% after 3 years." "To make up for the loss in sample size due to attrition, we included 688 new households in the follow‐up survey to replace households that had dropped out." |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Haggerty 1988 COD.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 6 months Cluster‐adjustment method: adjusted and unadjusted results given |
|
| Participants | Number: 18 sites (intervention: 9; control: 9), with 1954 children (intervention: 977; control: 977) Inclusion criteria: not stated Exclusion criteria: not stated Age: 3 months to 35 months |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Diarrhoeal rates | |
| Notes | Location: Kikwit, Bandundu Province, Zaire (Democratic Republic of Congo) Duration: October 1987 to December 1988 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers blind to the diarrhoea histories of families |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 190 children enrolled in the follow‐up were excluded from the analysis due to incomplete data; 1954 children were enrolled in the follow‐up trial but 1764 were retained for analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | High risk | Reported some baseline differences (control group had diarrhoea episodes of longer duration than the intervention group) |
Han 1985 MMR.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 4 months Cluster‐adjustment method: not adjusted |
|
| Participants | Number: 350 households (intervention: 162 intervention; control: 188) with 494 children (intervention: 236; control: 258) Inclusion criteria: households with 1 or more children between 6 and 59 months; those in which regular follow‐up was possible; not allergic to soap; gave informed consent Exclusion criteria: not stated Age: < 5 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
|
|
| Notes | Location: Nga‐Kha ward of Thin‐Gun‐Kyun township, Rangoon, Burma (now Myanmar) Duration: June to November 1985 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "to avoid bias staff were blind to which households were intervention or otherwise" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 children (7 from intervention, 5 from control households) of the 494 enrolled |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Hartinger 2011 PER.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 12 months Cluster‐adjustment method: unclear |
|
| Participants | Number: 51 communities, 534 households (intervention: 267; control: 267) with 534 children (intervention: 267; control: 267) Inclusion criteria:
Exclusion criteria:
Age: 6 to 35 months |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: San Marcos province, Cajamarca region, Peru Duration of trial: March 2008 to January 2010 (23 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized "...using covariate‐based constrained randomisation as proposed by Moulton (2004)". Researchers went to extra lengths to ensure integrity of the randomizations. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "As a strategy to reduce non‐blinding bias, a child psychomotor development intervention was implemented in the control arm as an equivalent to the IHIP in the intervention arm" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...and data collection was done by an independent team of field workers, which was not part of the initial education and re‐enforcement of the interventions during the follow‐up period". Comment: We consider this an attempt to blinding outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Researchers presented a detailed account of the randomization and follow‐up in a PRISMA flow diagram |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Hashi 2017 ETH.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: adequate Length of follow‐up: 6 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 24 sub‐Kebelles districts with 1224 children (12 sub‐Kebelles with 612 children < 5 years assigned to the intervention group; 12 sub‐Kebelles with 612 children < 5 years assigned to the control group) Inclusion criteria: at least 1 child aged 1 – 59 months living in the home, and not a model health extension household Exclusion criteria: see inclusion criteria above Age: children < 5 years |
|
| Interventions | Intervention (see Table 5 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: rural areas of Jigjiga district of Ethiopian‐Somali Regional State (ESRS), Eastern Ethiopia Duration: February 1, 2009 to July 30, 2015 (4 years and 5 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sub‐Kebelles were then randomly selected from the 56 total sub‐Kebelles by using simple randomisation (computer generated numbers)" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the community (both control and intervention group) nor the field workers knew the intervention purpose." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the community (both control and intervention group) nor the field workers knew the intervention purpose." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study accounted for all the participants included in the study and attrition was < 10% |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Huang 2007 USA.
| Study characteristics | ||
| Methods | RCT with individual randomization Inclusion of participants in the analysis: 100% Length of follow‐up: 1 year |
|
| Participants | Number: intervention: 73; control: 75 Inclusion criteria: people with AIDS at local HIV clinic; HIV‐1 infection verified by both ELISA and Western blot; AIDS by CD4 counts and plasma HIV RNA; been on highly active anti‐retroviral therapy (HAART) for at least 6 weeks and without diarrhoea for at least 3 months |
|
| Interventions | Both groups: 3 dedicated trial nurses educated participants on health problem associated with contaminated hands and provided specific hand‐washing instructions at enrolment; hand‐washing technique demonstrated by nurses, including wetting hands, lathering completely with soap, rubbing together for at least 15 seconds, and drying hands with towels; all 148 participants then demonstrated adequate hand‐washing technique intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: USA (location unclear) Duration: 1 year (exact dates unclear) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Kapoor 2016 IND.
| Study characteristics | ||
| Methods | RCT Inclusion of participants in the analysis: 100% Length of follow‐up: 6 months |
|
| Participants | Number: 101 mothers with children below 2 years (50 mothers assigned to the intervention group, 51 mothers assigned to the control group) Inclusion criteria: not explicitly stated Exclusion criteria: not explicitly stated Age: children < 2 years |
|
| Interventions | Intervention (see Table 5 for detailed description):
Control:
|
|
| Outcomes |
|
|
| Notes | Location: a resettlement colony, northwest of Chandiagrh, India Duration: July to November 2014 (6 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "3 strata were randomly allocated to intervention and control group" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for and included in the analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Kotch 1989 USA.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 7 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 24 day‐care centres, with 389 children Inclusion criteria: children < 3 years; present in the day care at least 20 hours per week; absence of chronic illness or medication that would predispose to infection; youngest of potentially eligible children in the same family; consenting English‐speaking parents with access to a telephone; intending to remain in day‐care centre throughout trial Exclusion criteria: not stated Age: < 3 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Diarrhoeal rates | |
| Notes | Location: Cumberland County, North Carolina, USA Duration: October 1988 to May 1989 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: " specifically, parental illness reports were blind to the intervention status of their children's DCCs, potential confounders were controlled for and effect modifiers were examined" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18 children out of the 389 enrolled were lost to follow‐up. 1 day‐care centre withdrew from the trial |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Unclear risk | Comparability at baseline is unclear |
Kotch 2003 USA.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 7 months (November 2002 to May 2003) Cluster‐adjustment method: unclear |
|
| Participants | Number: 46 child‐care centres (intervention: 23; control: 23) with 388 infants and toddlers Inclusion criteria: Child expected to remain in the child‐care centre for the duration of trial and < 36 months of age at the end of data collection; at least 1 family member contact could participate in a telephone survey in English Exclusion criteria: not stated Age: Infants and toddlers < 36 months |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: North Carolina, USA Duration: September 2002 to May 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Applied different statistical tests for different nature of variables: Quote: "No control variables are included in these descriptive comparisons". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Attrition form the intervention and control groups during the course of the trial was comparable" |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | High risk | Quote: "Two significant differences between the 2 trial groups were noted. The total number of children and the number of boys were larger in the intervention classrooms. These differences may have reduced the overall effect of the intervention, because number of children per classroom is a risk factor, and boys tend to stay in diapers longer. In addition, control centres were working hard to get their perceived reward (the free equipment that they were promised at the end of the trial). These 3 factors should have reduced the difference in outcomes between the intervention and control groups, suggesting that the significant differences in illnesses and absences that were found favouring the intervention group are all the more impressive" |
Ladegaard 1999 DEN.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 4 months Cluster‐adjustment method: not adjusted |
|
| Participants | Number: 8 day‐care centres, with 475 children (intervention: 212; control: 263) Inclusion criteria: not stated Exclusion criteria: not stated Age: < 6 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Diarrhoeal rates | |
| Notes | Location: Odense, Denmark Duration: 6 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear whether they were divided in 2 groups manually and then randomized or randomized stratified. Quote: "The 8 institutions were allocated based on likeliness and randomized to intervention or control with 4 institutions in each" |
| Allocation concealment (selection bias) | Unclear risk | Randomization not described in detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One institution had not written down attendance for the children 0 ‐ 2 years. There were 212 children in the intervention group and 263 in the control group but no account of what happened to the children over time |
| Selective reporting (reporting bias) | Low risk | No evidence of outcomes not presented |
| Other bias | Low risk | None observed |
Langford 2007 NPL.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: adequate (11 of 99 lost) Length of follow‐up: 6 months Cluster‐adjustment method: unclear |
|
| Participants | Number: 88 children (intervention: 45; control: 43) Inclusion criteria: not stated Exclusion criteria: not stated Age: 3 to 12 months old |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: Kathmandu, Nepal Duration: May to November 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups were randomly allocated by flipping a coin to intervention or control groups |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To prevent bias in data collection, these field workers were never involved in any aspect of the program to promote hand washing". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 children from 99 originally recruited were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | High risk | Quote: "It was not possible to randomly allocate each separate settlement to control/intervention conditions as many sites were situated very close to one another (e.g. separated just by road or stream) such that the intervention message could easily have crossed over into control settlements." Comment: cross‐contamination possible |
Luby 2003a PAK.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 12 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 36 neighbourhoods (intervention: 25; control: 11), with 4691 children (intervention: 3163; control: 1528) Inclusion criteria: household located in the trial area; have at least 2 children < 5 years; intention to reside in the house for the duration of trial Exclusion criteria: not stated Age: < 15 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes | Diarrhoeal rates | |
| Notes | Location: low‐income squatter settlements, Karachi, Pakistan Duration: April 2002 to April 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 139 children from the intervention arm and 85 from the control arm of the 4691 children originally enrolled were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Luby 2003b PAK.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 9 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 18 clusters, with 544 households (intervention: 262; control: 282) Inclusion criteria: households with at least 1 child < 5 years; provided informed consent Exclusion criteria: not stated Age range: < 15 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
|
|
| Notes | Location: Multi‐ethnic squatter settlements in Central Karachi, Pakistan Duration: April 2003 to December 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 5 trial groups were assigned a random number generated by a computer spreadsheet. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described (open trial) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described (open trial) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly stated |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Luby 2006 PAK.
| Study characteristics | ||
| Methods | Cluster‐RCT Length of follow‐up: 14 months (63 weeks) Cluster‐adjustment method: adjusted |
|
| Participants | Number: 577 households: hand‐washing promotion (195 households), hand‐washing promotion plus water treatment (187 households) and control arm (195 households) Inclusion criteria: same used in Luby 2003b PAK Exclusion criteria: same used in Luby 2003b PAK Age: children under 5 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
|
|
| Outcomes |
|
|
| Notes | Location: Karachi, Pakistan Duration: 63 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | None. Trial is a follow‐up to Luby 2003a PAK |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 household was not accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Luby 2018 BGD.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: 100% Length of follow‐up: 2 years Cluster‐adjustment method: adjusted |
|
| Participants | Number: 5551 pregnant women in 720 clusters were randomly allocated to 1 of 7 groups. 1382 women were assigned to the control group; 698 to water; 696 to sanitation; 688 to hand washing; 702 to water, sanitation, and hand washing; 699 to nutrition; and 686 to water, sanitation, hand washing, and nutrition Inclusion criteria: in utero children of enrolled pregnant women (index children) were eligible for inclusion if their mother was planning to live in the study village for the next 2 years, regardless of where she gave birth. Only 1 pregnant woman was enrolled per compound, but if she gave birth to twins, both children were enrolled. Children who were younger than 3 years at enrolment and lived in the compound were included in diarrhoea measurements Exclusion criteria: not described Age: unclear |
|
| Interventions | Hand‐washing interventions, sanitation intervention, drinking water interventions, nutrition intervention hand‐washing intervention (see Table 5 for detailed description):
Each hand‐washing station included:
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: Bangladesh Duration: February 2009 to May 2012 (3 years and 3 months) Data were collected via surveys, with 10 households randomly selected from each village |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Clusters were randomly allocated to treatment using a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Interventions included distinct visible components so neither participants nor data collectors were masked to intervention assignment, although the data collection and intervention teams were different individuals. Two investigators (BFA and JBC) did independent, masked statistical analyses from raw data sets to generate final estimates, with the true group assignment variable replaced with a re‐randomised uninformative assignment variable. The results were unmasked after all analyses were replicated" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Interventions included distinct visible components so neither participants nor data collectors were masked to intervention assignment, although the data collection and intervention teams were different individuals. Two investigators (BFA and JBC) did independent, masked statistical analyses from raw data sets to generate final estimates, with the true group assignment variable replaced with a re‐randomised uninformative assignment variable. The results were unmasked after all analyses were replicated". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "331 (6%) women were lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Nicholson 2008 IND.
| Study characteristics | ||
| Methods | Cluster‐RCT Length of follow‐up: 41 weeks Cluster‐adjustment method: not adjusted |
|
| Participants | 35 matched pairs communities (70 in total for intervention and control); 30 households from each of the communities. Target children: 2052 (intervention: 1026; control: 1026); < 5 years of age: 2469 (intervention: 1190; control: 1279); 6 ‐ 15 years: 3519 (Intervention: 1784; control: 1735); adults: 3685 (intervention: 1892; control: 1793) All participants: 11,725 (intervention: 5892; control: 5833) Inclusion criteria: informed consent Exclusion criteria: not stated Age: 5 years (target); < 5 years, children 6 ‐ 15 years, and adults (non‐targets) |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: West and South Mumbai, India Duration: 22 October, 2007 to 2 August 2008 (41 weeks) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Repeated coin‐tossing |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was impossible to 'blind' either the participants or those responsible for data collection." Comment: None (open trial) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "It was impossible to 'blind' either the participants or those responsible for data collection." Comment: None (open trial) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up in both arms and for all the subgroups were more than 10% (average attrition in all groups 18%) |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Null 2018 KEN.
| Study characteristics | ||
| Methods | Cluster‐RCT Length of follow‐up: 2 years Cluster‐adjustment method: adjusted |
|
| Participants | Number: 702 village clusters with 158 clusters assigned to the active control arm; 80 clusters assigned to the passive control arm; 77 clusters assigned to the water arm; 77 assigned to the sanitation arm; 77 clusters assigned to the hand‐washing arm; 76 clusters assigned to the combined water, sanitation, and hand‐washing arm; 78 clusters assigned to the nutrition arm; and 79 clusters assigned to the combined water, sanitation, hand‐washing, and nutrition arm Inclusion criteria: villages were eligible for selection into the study if they were rural, most of the population relied on communal water sources and had unimproved sanitation facilities, and there were no other ongoing water, sanitation, hand‐washing, or nutrition programmes. Within selected villages, women were eligible to participate if they reported that they were in their second or third trimester of pregnancy, planned to continue to live at their current residence for the next 2 years, and could speak Kiswahili, Luhya, or English well enough to respond to an interviewer‐administered survey Exclusion criteria: see inclusion criteria above Age: 0‐2 years |
|
| Interventions | Hand‐washing interventions, sanitation intervention, drinking water interventions, nutrition intervention Hand‐washing intervention (see Table 5 for detailed description):
Control:
|
|
| Outcomes | Outcomes were assessed in the children of the enrolled pregnant women (index children), including twins. Primary outcomes:
Not used in this review:
Secondary and tertiary outcomes:
|
|
| Notes | Location: rural villages in Bungoma, Kakamega, and Vihiga counties in Kenya’s western region Duration: November 2012 to May 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Clusters were randomly allocated to treatment using a random number generator with reproducible seed" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "investigators remained blinded to treatment assignments. Blinding of participants was not possible. Participants were informed of their treatment assignment after baseline data collection and might have known the treatment assignment of nearby villages. The health promoters and staff who delivered the interventions were not involved in data collection, but the data collection team could have inferred treatment status if they saw intervention materials in study communities." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "investigators remained blinded to treatment assignments. Blinding of participants was not possible. Participants were informed of their treatment assignment after baseline data collection and might have known the treatment assignment of nearby villages. The health promoters and staff who delivered the interventions were not involved in data collection, but the data collection team could have inferred treatment status if they saw intervention materials in study communities." Comment: 2 biostatisticians, blinded to treatment assignment, independently replicated the analyses following the prespecified analysis plan with minor updates |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. Not explicitly reported |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Pickering 2013 KEN.
| Study characteristics | ||
| Methods | Cluster‐RCT Length of follow‐up: 2 months (8 weeks) Cluster‐adjustment method: adjusted |
|
| Participants | Number: 6 schools (2 hand sanitizer; 2 hand washing with soap; 2 control). Student numbers: hand washing with soap: 460; hand sanitizer: 435; control: 469 Inclusion criteria: schools with > 100 student population; written consent from parents/teachers Exclusion criteria: schools that shared latrines with community members Age: 5‐ to 10‐year‐old‐schoolchildren |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: Kibera, Nairobi, Kenya Duration: 2 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "schools randomly assigned to receive" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial. Quote: "Treatment assignment was not blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open trial. Quote: "Treatment assignment was not blinded". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly stated, as the trial authors only reported total observations |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Roberts 1996 AUS.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 9 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 23 day‐care centres, with 558 children Inclusion criteria: Day‐care centres licensed in the Australian Capital Territory; children < 3 years as at January 1996; attendance for at least 3 days per week; have no underlying chronic illness that predisposes to infection Exclusion criteria: not stated Age: < 3 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
|
|
| Notes | Location: Australian Capital Territory, Australia Duration: March to November 1996 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random‐number table generated using EpiInfo |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | None described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The observer was not informed of the content of the training sessions or the intervention status of the centres". "The staff members in the centres were aware the observer was watching hygiene practices but not which specific practices were being recorded". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% (123 children) from 558 children enrolled were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Unclear risk | Baseline comparable data not given |
Stanton 1985 BGD.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: unclear Length of follow‐up: 6 months Cluster‐adjustment method: adjusted |
|
| Participants | Number: 1923 families (intervention: 937; control: 986) with 1350 children (intervention: 675; control: 675) Inclusion criteria: not stated Exclusion criteria: not stated Age: < 6 years |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
|
|
| Notes | Location: Urban Dhaka, Bangladesh Duration: October 1984 to May 1985 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomized allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal number of emigrant and immigrant included in effectiveness analyses but not in behavioral assessment |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | None observed |
Talaat 2008 EGY.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: adequate Length of follow‐up: 12 weeks (February to May 2008) Cluster‐adjustment method: adjusted |
|
| Participants | Number: 60 government elementary schools (intervention: 30; control: 30), with 44,451 children (intervention: 20,882; control: 23,569) Inclusion criteria: not stated Exclusion criteria: not stated Age: children in elementary schools (median age 8 years) |
|
| Interventions | Intervention (see Table 4 for detailed description):
School's contribution:
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: Cairo, Eygpt Duration: February to May 2008 (12 weeks) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 60 elementary schools were randomly selected by using a computer‐generated random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for number enrolled for the trial in the analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | Low risk | Quote: "No significant differences were found for the 2 groups in median (8 years), sex distribution (51% male) or the median number of students per school (635 [interquartile range 394‐978])" |
Zomer 2015 NED.
| Study characteristics | ||
| Methods | Cluster‐RCT Inclusion of participants in the analysis: adequate Length of follow‐up: November 2011 to March 2012 Cluster‐adjustment method: adjusted |
|
| Participants | Number: 71 day‐care centres (DCC) (intervention: 36; control: 35) with 545 children (278 from 34 intervention DCCs and 267 from 35 control DCCs) Inclusion criteria: children attended the DCC at least 2 days a week; aged between 6 months and 3 ‐ 5 years; intended to attend the DCC throughout the trial period; parents consented; Dutch‐speaking and had access to e‐mail or regular post Exclusion criteria: child had chronic illness; child was on medication that predisposed him/her to infection and sibling is taking part in the trial (1 per child per family participant) Age: children between 6 months and 60 months |
|
| Interventions | Intervention (see Table 4 for detailed description):
Control:
|
|
| Outcomes |
Not used in this review:
|
|
| Notes | Location: Rotterdam‐Rijnmond, Gouda, and Leiden regions of Netherlands Duration: September 2011 to April 2012 (7 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomized allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 553 children included in the trial; 545 included in the analysis |
| Selective reporting (reporting bias) | Low risk | None observed |
| Other bias | High risk | Quote: "...the crude incidence of diarrhoeal episodes differed between intervention and control DCCs at baseline..." Comment: There were some differences in baseline characteristics between intervention and control groups. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 1993 | Observational trial examining risk factors for diarrhoeal infections |
| Aiello 2008 | Combined both randomized and quasi‐experimental trials in the analysis. Outcome measure was on general GIT illnesses including diarrhoea |
| Alam 1989 | Main intervention was provision of water supply through hand pumps |
| Andrade 2017 | Quasi‐RCT and Incidence of diarrhoea was not assessed |
| Arnold 2009 | Cross‐sectional cohort intervention trial (non‐randomized study) |
| Arnold 2013 | Description of planned intervention trial design and rationale |
| Azor‐Martinez 2014 | Acute gastroenteritis (AGE) outcome assessed, not specific to diarrhoea |
| Barros 1999 | Observational trial examining risk factors for diarrhoeal infections |
| Benjamin‐Chung 2017 | Hand‐washing promotion was part of a group of interventions administered. No handwashing‐only arm |
| Bieri 2013 | Hand washing not an intervention and diarrhoea not an outcome |
| Biran 2009 | Hand washing an outcome not an intervention |
| Biran 2014 | Diarrhoea not an outcome, assessed emotional drivers of behaviour for improving hand‐washing behaviours |
| Boubacar Maïnassara 2014 | Mixed hygiene interventions not specific to hand washing |
| Bowen 2012 | Diarrhoea not an outcome, assessed child growth and development |
| Bowen 2013 | Did not assess diarrhoeal outcomes but assessed hand‐washing behaviours – 1 of our secondary outcome measures |
| Burns 2018 | Diarrhoea not an outcome |
| Burton 2011 | Measures effect on hand contamination not diarrhoeal rates |
| Caruso 2014 | Diarrhoea not an outcome; assessed the effect of latrine cleaning and hand washing with soap intervention on school absenteeism |
| Chard 2018 | Diarrhoea not an outcome |
| Chard 2019 | Hand‐washing promotion was part of a group of interventions administered including drinking water filters. No handwashing‐only arm |
| Clasen 2014a | Hand‐washing promotion not an intervention |
| Clasen 2014b | Hand‐washing promotion not specific intervention but latrine use/coverage |
| Clemens 1987 | Observational trial examining risk factors for diarrhoeal infections |
| Contzen 2015 | Non‐randomized trial. Diarrhoea incidence not assessed |
| Correa 2012 | Trial did not promote handwashing but alcohol‐based hand rubs as complement to handwashing; control continued existing handwashing practices |
| Curtis 2001 | No concurrent control |
| Doebbeling 1992 | Outcome measure (incidence of nosocomial infection) not specific to diarrhoea episodes but to incidence of gastrointestinal infections in general |
| Dreibelbis 2014 | Mixed hygiene intervention, not specific to hand washing |
| Dreibelbis 2016 | Not an RCT and Incidence of diarrhoea was not assessed |
| Duijster 2017 | Not an RCT and Incidence of diarrhoea was not assessed |
| Dyer 2000 | Intervention was instant hand sanitizer |
| Ecrumen 2018 | Incidence of diarrhoea not assessed |
| Ecrumen 2019 | Incidence of diarrhoea not assessed |
| Enebeli 2017 | Not an RCT |
| Erismann 2017 | Incidence of diarrhoea not assessed |
| Fan 2011 | Non‐randomized study |
| Freeman 2014 | Mixed water, sanitation and hygiene intervention, not specific to hand washing |
| Greene 2012 | Measured exposure to faecal pathogen (risk of Escherichia coli). Hand contamination of E. coli |
| Greenland 2016 | Incidence of diarrhoea was not assessed. The trial assessed use of zinc and ORS to treat diarrhoea, and reported incidence of diarrhoea in children that were treated with zinc |
| Guinan 2002 | Observational study |
| Hammond 2000 | Intervention did not involve hand washing |
| Hovi 2017 | Incidence of diarrhoea not assessed. Trial reports the effect of the intervention on weekly prevalence of reported symptoms of gastrointestinal infection in general |
| Hübner 2010 | Hand washing not an intervention (but measured the effectiveness of hand disinfection with alcoholic rubs) |
| Huda 2012 | Assessed observed hand‐washing hygiene behaviours |
| Jinadu 2007 | Diarrhoea episodes not assessed, but rather hygiene behavioural change. |
| Johansen 2015 | Outcome measure not directly on diarrhoea but on infectious illness and school absenteeism. Paper describes the design of the RCT |
| Kamm 2016 | Incidence of diarrhoea was not assessed |
| Kang 2017 | Incidence of diarrhoea not assessed |
| Khan 1982 | Case‐control study |
| Larson 2003 | No relevant outcome measures. Assessed colony‐forming units of bacteria |
| Larson 2004 | Outcome measure not specific to incidence of diarrhoea |
| Lee 1991 | Controlled before‐and‐after study |
| Luby 2001b | Observational trial |
| Luby 2004 | Non‐randomized trial |
| Luby 2007 | Diarrhoea episodes not assessed. Measured hand contamination |
| Luby 2008 | Hand washing not an intervention but use of flocculant‐disinfectant for treating drinking water |
| Luby 2010 | Diarrhoea episodes not assessed. Measured hand contamination |
| Manjang 2018 | Incidence of diarhoea not assessed. Study reports baseline characteristics only |
| Master 1997 | Outcome measure not specific on diarrhoeal episodes |
| Morton 2004 | Outcome measure not specific on diarrhoeal episodes |
| Najnin 2017 | None of the arms received hand‐washing promotion only |
| Naluonde 2018 | Incidence of diarrhoea not assessed |
| Oncu 2018 | Incidence of diarrhoea not assessed |
| Oughton 2009 | Diarrhoea episodes not assessed but removal of Clostridium difficile |
| Overgaard 2016 | Incidence of diarrhoea not assessed. Study reports incidence of episodes of school absence ascribed to diarrhoea per school year |
| Patel 2012 | Non‐randomized trial |
| Peterson 1998 | Observational trial examining risk factors for diarrhoeal infections |
| Pinfold 1996 | No comparable baseline information on diarrhoeal episodes provided |
| Priest 2014 | Diarrhoea episodes not the outcome, but illness absence including general GIT infection |
| Rosen 2009 | Diarrhoea episodes not assessed. Tested effect of hand‐washing intervention on psychosocial measures |
| Saboori 2013 | Diarrhoea episodes not assessed. Assessed hand‐washing episodes and E. coli hand contamination |
| Savolainen‐Kopra 2012 | Outcome measure not specific to diarrhoeal morbidity but to incidence of GIT infection |
| Shafique 2013 | Hand sanitizer not hand washing the intervention. Mean duration of diarrhoea and not diarrhoea episodes the main outcome measure |
| Shahid 1996 | No comparable baseline information provided |
| Sinharoy 2017 | Hand washing was part of a group of interventions administered to the 2 intervention arms in the trial |
| Sircar 1987 | No comparable baseline information on diarrhoea episodes provided |
| Slayton 2013 | Hand towels the main intervention, not hand washing |
| Vally 2019 | Mixed water, sanitation and hygiene intervention, not specific to hand washing |
| Vindigni 2011 | Combined both randomized and quasi‐experimental trials in the analysis. Measured hand washing adherence |
| White 2003 | Outcome measure not specific to diarrhoeal morbidity |
| Wilson 1991 | Controlled before‐and‐after study |
| Zhang 2013 | Diarrhoea not the direct outcome; Proxy data of 'stomach pain' was reported |
GIT: gastro‐intestinal tract
Characteristics of studies awaiting classification [ordered by study ID]
Denbaek 2018.
| Methods | 3‐armed cluster‐randomized controlled trial using school‐based multicomponent interventions to improve hand washing among schoolchildren |
| Participants | Schoolchildren in Danish schools; 2 intervention arms involving 14 schools each and 15 control schools |
| Interventions | A curriculum component addressing knowledge and skills, daily hand washing before lunch, extra cleaning of school toilets during the school day |
| Outcomes | Infectious illness days, infectious illness episodes, and illness‐related absenteeism |
| Notes |
Differences between protocol and review
We introduced the term 'promotion' into the title of the first review update, and we retain it in this current review update.
We added methods for assessing blinding and changed our primary outcome measure in the protocol from the relative risk of at least one diarrhoea episode to the incidence rate ratio for diarrhoea episodes. We pooled rate ratios in our analyses rather than relative risks, since all trials presented diarrhoea as episodes, and we removed 'or standard hygiene promotion' as a control because it is included in the 'no hand‐washing promotion' control group.
We added all‐cause under‐five mortality and cost effectiveness as secondary outcome measures for this review update. We used GRADEpro 2014 to assess the certainty of the evidence. We have also included Table 1; Table 2; and Table 3 in this update.
Henry Ejere, a co‐author on the protocol, did not participate in preparing the original review or this review update. Dachi Arikpo joined as a co‐author in the first review update published in 2015.
Differences between review update (2015) and review update (2021)
We have updated the literature search methods, changed some of the terms used, and reported the search strategies in Appendix 1. The prespecified changes to the protocol for this review update (2021) are given in Appendix 2.
Contributions of authors
Regina Ejemot‐Nwadiaro and Dachi Arikpo extracted and analysed data and drafted the review. John Ehiri developed the protocol and drafted and commented on the review. Julia Critchley extracted and analyzed data and edited the review. Martin Meremikwu helped finalize the data extraction form and drafted and commented on the review.
Sources of support
Internal sources
University of Calabar, Nigeria
Institute of Tropical Diseases Research and Prevention (ITDR&P), Calabar, Nigeria
Liverpool School of Tropical Medicine (LSTM), UK
Division of Health Promotion Sciences, University of Arizona, Mel & Enid Zuckerman College of Public Health, Tucson, Arizona, USA
Higher Education Funding Council for England, UK
Cochrane Nigeria, Nigeria
External sources
-
Foreign, Commonwealth and Development Office (FCDO), UK
Project number 300342‐104
Declarations of interest
Regina Ejemot‐Nwadiaro: none known. John Ehiri: none known. Dachi Arikpo: none known. Martin Meremikwu: none known. Julia Critchley: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ban 2015 CHN {published data only}
- Ban HQ, Li T, Shen J, Li J, Peng PZ, Ye HP, et al. Effects of multiple cleaning and disinfection interventions on infectious diseases in children: a group randomized trial in China. Biomedical and Environmental Sciences 2015;28(11):779-87. [DOI] [PubMed] [Google Scholar]
Bartlett 1984 USA {published data only}
- Bartlett AV, Jarvis BA, Ross V, Katz TM, Dalia MA, Englender SJ, et al. Diarrheal illness among infants and toddlers in day care centers: effects of active surveillance and staff training without subsequent monitoring. American Journal of Epidemiology 1988;127(4):808-17. [DOI] [PubMed] [Google Scholar]
Black 1977 USA {published data only}
- Black RE, Dykes AC, Anderson KE, Wells JG, Sinclair SP, Gary GW Jr, et al. Handwashing to prevent diarrhea in day-care centers. American Journal of Epidemiology 1981;113(4):445-51. [DOI] [PubMed] [Google Scholar]
Bowen 2004 CHN {published data only}
- Bowen A, Ma H, Ou J, Billhimer W, Long T, Mintz E, et al. A cluster-randomized controlled trial evaluating the effect of a handwashing-promotion program in Chinese primary schools. American Journal of Tropical Medicine and Hygiene 2007;76(6):1166-73. [PubMed] [Google Scholar]
Briceno 2017 TZA {published data only}
- Briceno B, Coville A, Gertler P, Martinez S. Are there synergies from combining hygiene and sanitation promotion campaigns: evidence from a large-scale cluster randomized trial in rural Tanzania. PLOS One 2017;12(11):e0186228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butz 1990 USA {published data only}
- Butz AM, Larson E, Fosarelli P, Yolken R. Occurrence of infectious symptoms in children in day care homes. American Journal of Infection Control 1990;18(6):347-53. [DOI] [PubMed] [Google Scholar]
Carabin 1997 CAN {published data only}
- Carabin H, Gyorkos TW, Soto JC, Joseph L, Payment P, Collect JP. Effectiveness of a training program in reducing infections in toddlers attending day care centers. Epidemiology 1999;10(3):219-27. [PubMed] [Google Scholar]
Galiani 2016 PER {published data only}
- Galiani S, Gertlerb P, Ajzenmanc N, Orsola-Vidal A. Promoting handwashing behavior: the effects of large scale community and school-level interventions. Health Economics 2016;25(12):1545-59. [DOI] [PubMed] [Google Scholar]
Haggerty 1988 COD {published data only}
- Haggerty PA, Muladi K, Kirkwood BR, Ashworth A, Manunebo M. Community-based hygiene education to reduce diarrhoeal disease in rural Zaire: impact of the intervention on diarrhoeal morbidity. International Journal of Epidemiology 1994;23(5):1050-9. [DOI] [PubMed] [Google Scholar]
Han 1985 MMR {published data only}
- Han AM, Hlaing T. Prevention of diarrhoea and dysentery by hand washing. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(1):128-31. [DOI] [PubMed] [Google Scholar]
Hartinger 2011 PER {published data only}
- Hartinger S, Hattendorf J, Lanata C, Gil A, Verastegui H, Mausezahl D. Integrating home-based environmental interventions (IHIP): a community-randomised trial to improve indoor air pollution, drinking water quality and child nutrition in rural Peru. Tropical Medicine & International Health 2011;16(Suppl 1):97-384. [Google Scholar]
- Hartinger SM, Lanata CF, Hattendorf J, Gil AI, Verastegui H, Mäusezahl D. A community randomized controlled trial of an integrated home-based intervention improving household-air pollution, drinking water quality and hygiene in rural Peru. 61st Annual Meeting of the American Society of Tropical Medicine and Hygiene (ASTMH); 2012 Nov 11-15; Atlanta. American Journal of Tropical Medicine and Hygiene 2012;87((5 Suppl 1)):285. [Google Scholar]
- Hartinger SM, Lanata CF, Hattendorf J, Gil AI, Verastegui H, Ochoa T, et al. A community randomized controlled trial evaluating a home-based environmental intervention package of improved stoves, solar water disinfection and kitchen sinks in rural Peru: rationale, trial design and baseline findings. Contemporary Clinical Trials 2011;32(6):864-73. [DOI] [PubMed] [Google Scholar]
Hashi 2017 ETH {published data only}
- Hashi A, Kumie A, Gasana J. Hand washing with soap and WASH educational intervention reduces under-five childhood diarrhoea incidence in Jigjiga District, Eastern Ethiopia: a community-based cluster randomized controlled trial. Preventive Medicine Reports 2017;6:361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2007 USA {published data only}
- Huang DB, Zhou J. Effect of intensive handwashing in the prevention of diarrhoeal illness among patients with AIDS: a randomized controlled study. Journal of Medical Microbiology 2007;56(Pt 5):659-63. [DOI] [PubMed] [Google Scholar]
Kapoor 2016 IND {published data only}
- Kapoor N, Saini SK, Kaur M. Effectiveness of package for behaviour change of mothers in prevention of diarrhoea in children less than 2 years of age in a resettlement colony. Indian Journal of Public Health Research & Development 2016;7(2):90-6. [Google Scholar]
Kotch 1989 USA {published data only}
- Kotch JB, Weigle KA, Weber DJ, Clifford RM, Harms TO, Loda FA, et al. Evaluation of an hygienic intervention in child day-care centers. Pediatrics 1994;94(6 Pt 2):991-4. [PubMed] [Google Scholar]
Kotch 2003 USA {published data only}
- Kotch JB, Isbell P, Weber DJ, Nguyen V, Savage E, Gunn E, et al. Hand-washing and diapering equipment reduces disease among children in out-of-home child care centers. Pediatrics 2007;120(1):e29-36. [DOI] [PubMed] [Google Scholar]
Ladegaard 1999 DEN {published data only}
- Ladegaard MB, Stage V. Hand-hygiene and sickness among small children attending day care centres. An intervention study [Handhygiejne og smabornssygelighed i daginstitutioner: En interventionsundersogelse]. Ugeskrift for Laeger 1999;161(31):4396-400. [PubMed] [Google Scholar]
Langford 2007 NPL {published data only}
- Langford R, Lunn P, Panter-Brick C. Hand-washing, subclinical infections, and growth: a longitudinal evaluation of an intervention in Nepali slums. America Journal of Human Biology 2011;23(5):621-9. [DOI] [PubMed] [Google Scholar]
Luby 2003a PAK {published data only}
- Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, et al. Effect of handwashing in child health: a randomised controlled trial. Lancet 2005;366(9481):225-33. [DOI] [PubMed] [Google Scholar]
- Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer WL, Hoekstra RM. Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA 2004;291(21):2547-54. [DOI] [PubMed] [Google Scholar]
Luby 2003b PAK {published and unpublished data}
- Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer W, Keswick B, et al. Combining drinking water treatment and hand washing for diarrhea prevention, a cluster randomised control trial. Tropical Medicine & International Health 2006;11(4):479-89. [DOI] [PubMed] [Google Scholar]
Luby 2006 PAK {published data only}
- Luby SP, Agboatwalla M, Bowen A, Kenah E, Sharker Y, Hoekstra RM. Difficulties in maintaining improved handwashing behavior, Karachi, Pakistan. American Journal of Tropical Medicine and Hygiene 2009;81(1):140-5. [PubMed] [Google Scholar]
Luby 2018 BGD {published data only}
- Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Global Health 2018;6(3):e302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicholson 2008 IND {published data only}
- Nicholson JA, Naeeni M, Hoptroff M, Matheson JR, Roberts AJ, Taylor D, et al. An investigation of the effects of a hand washing intervention on health outcomes and school absence using a randomised trial in Indian urban communities. Tropical Medicine & International Health 2014;19(3):284-92. [DOI] [PubMed] [Google Scholar]
Null 2018 KEN {published data only}
- Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Global Health 2018;6:e316-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Kariger P, Fernald L, Pickering AJ, Arnold CD, Arnold BF, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on child development in rural Kenya (WASH Benefits Kenya): a cluster-randomised controlled trial. Lancet Child Adolescent Health 2018;2(4):269-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pickering 2013 KEN {published data only}
- Pickering AJ, Davis J, Blum AG, Scalmanini J, Oyier B, Okoth G, et al. Access to waterless hand sanitizer improves student hand hygiene behavior in primary schools in Nairobi, Kenya. American Journal of Tropical Medicine and Hygiene 2013;89(3):411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1996 AUS {published data only}
- Roberts L, Jorm L, Patel M, Smith W, Douglas RM, McGilchrist C. Effect of infection control measures on the frequency of diarrheal episodes in child care: a randomized, controlled trial. Pediatrics 2000;105(4 Pt 1):743-6. [DOI] [PubMed] [Google Scholar]
Stanton 1985 BGD {published data only}
- Stanton BF, Clemens JD. An educational intervention for altering water-sanitation behaviors to reduce childhood diarrhea in urban Bangladesh. II. A randomized trial to assess the impact of the intervention on hygienic behaviors and rates of diarrhea. American Journal of Epidemiology 1987;125(2):292-301. [DOI] [PubMed] [Google Scholar]
Talaat 2008 EGY {published data only}
- Talaat M, Afifi S, Dueger E, El-Ashry N, Marfin A, Kandeel A, et al. Effects of hand hygiene campaigns on incidence of laboratory-confirmed influenza and absenteeism in school children, Cairo, Egypt. Emerging Infectious Diseases 2011;17(4):619-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zomer 2015 NED {published data only}
- Zomer TP, Erasmus V, Looman CW, Tjon-a-Tsien A, Van Beeck EF, De Graaf JM, et al. A hand hygiene intervention to reduce infections in child daycare: a randomized controlled trial. Epidemiology and Infection 2015;143(12):2494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer TP, Erasmus V, Vlaar N, Van Beeck EF, Tjon-A-Tsien A, Richardus JH, et al. A hand hygiene intervention to decrease infections among children attending day care centers: design of a cluster randomized controlled trial. BMC Infectious Diseases 2013;13:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 1993 {published data only}
- Ahmed NU, Zeitlin MF, Beiser AS, Super CM, Gershoff SN. A longitudinal study of the impact of behavioural change intervention on cleanliness, diarrhoeal morbidity and growth of children in rural Bangladesh. Social Science & Medicine 1993;37(2):159-71. [DOI] [PubMed] [Google Scholar]
Aiello 2008 {published data only}
- Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. American Journal of Public Health 2008;98(8):1372-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alam 1989 {published data only}
- Alam N, Wojtyniak B, Henry FJ, Rahaman MM. Mothers' personal and domestic hygiene and diarrhoea incidence in young children in rural Bangladesh. International Journal of Epidemiology 1989;18(1):242-7. [DOI] [PubMed] [Google Scholar]
Andrade 2017 {published data only}
- Andrade EL, Bingenheimer JB, Edberg MC, Zoerhoff KL, Putzer EM. Evaluating the effectiveness of a community-based hygiene promotion program in a rural Salvadoran setting. Global Health Promotion 2017;26(1):69-80. [DOI] [PubMed] [Google Scholar]
Arnold 2009 {published data only}
- Arnold B, Arana B, Mäusezahl D, Hubbard A, Colford JM Jr. Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. International Journal of Epidemiology 2009;38(6):1651-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 2013 {published data only}
- Arnold BF, Null C, Luby SP, Unicomb L, Stewart CP, Dewey KG, et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open 2013;3(8):e003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azor‐Martinez 2014 {published data only}
- Azor-Martinez E, Cobos-Carrascosa E, Gimenez-Sanchez F, Martinez-Lopez JM, Garrido-Fernandez P, Santisteban-Martinez J, et al. Effectiveness of a multifactorial handwashing program to reduce school absenteeism due to acute gastroenteritis. Pediatric Infectious Disease Journal 2014;33(2):e34-9. [DOI] [PubMed] [Google Scholar]
Barros 1999 {published data only}
- Barros AJ, Ross DA, Fonseca WV, Williams LA, Moreira-Filho DC. Preventing acute respiratory infections and diarrhoea in child care centres. Acta Paediatrica 1999;88(10):1113-8. [DOI] [PubMed] [Google Scholar]
Benjamin‐Chung 2017 {published data only}
- Benjamin-Chung J, Amin N, Ercumen A, Arnold BF, Hubbard A, Unicomb L, et al. A randomized controlled trial to measure spillover effects of a combined water, sanitation, and handwashing intervention in rural Bangladesh. American Journal of Epidemiology 2018;187(8):1733-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin-Chung J, Amin N, Ercumen A, Arnold BF, Hubbard A, Unicomb L, et al. Spillover effects of a combined water, sanitation and handwashing intervention in rural Bangladesh: a randomized controlled trial. American Journal of Tropical Medicine and Hygiene. Conference: 66th annual meeting of the American Society of Tropical Medicine and Hygiene, ASTMH 2017. United States 2017;97(5 Supplement 1):213. [Google Scholar]
Bieri 2013 {published data only}
- Bieri FA, Gray DJ, Williams GM, Raso G, Li YS, Yuan L, et al. Health-education package to prevent worm infections in Chinese schoolchildren. New England Journal of Medicine 2013;368(17):1603-12. [DOI] [PubMed] [Google Scholar]
Biran 2009 {published data only}
- Biran A, Schmidt WP, Wright R, Jones T, Seshadri M, Isaac P, et al. The effect of a soap promotion and hygiene education campaign on handwashing behaviour in rural India: a cluster randomised trial. Tropical Medicine & International Health 2009;14(10):1303-14. [DOI] [PubMed] [Google Scholar]
Biran 2014 {published data only}
- Biran A, Schmidt WP, Varadharajan KS, Rajaraman D, Kumar R, Greenland K, et al. Effect of a behaviour-change intervention on handwashing with soap in India (SuperAmma): a cluster-randomised trial. Lancet Global Health 2014;2(3):e145-54. [DOI] [PubMed] [Google Scholar]
Boubacar Maïnassara 2014 {published data only}
- Boubacar Maïnassara H, Tohon Z. Assessing the health impact of the following measures in schools in Maradi (Niger): Construction of latrines, clean water supply, establishment of hand washing stations, and health education. Journal of Parasitology Research 2014;2014:190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2012 {published data only}
- Bowen A, Agboatwalla M, Luby S, Tobery T, Ayers T, Hoekstra R. Association between intensive handwashing promotion and child development in Karachi, Pakistan: a cluster randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2012;166(11):1037-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2013 {published data only}
- Bowen A, Agboatwalla M, Ayers T, Tobery T, Tariq M, Luby S. Sustained improvements in handwashing indicators more than 5 years after a cluster-randomised, community-based trial of handwashing promotion in Karachi, Pakistan. Tropical Medicine & International Health 2013;18(3):259-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2018 {published data only}
- Burns J, Maughan-Brown B, Mouzinho A. Washing with hope: evidence of improved handwashing among children in South Africa from a pilot study of a novel soap technology. BMC Public Health 2018;18(1):709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2011 {published data only}
- Burton M, Cobb E, Donachie P, Judah G, Curtis V, Schmidt WP. The effect of handwashing with water or soap on bacterial contamination of hands. International Journal of Environmental Research and Public Health 2011;8(1):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caruso 2014 {published data only}
- Caruso BA, Freeman MC, Garn JV, Dreibelbis R, Saboori S, Muga R, et al. Assessing the impact of a school-based latrine cleaning and handwashing program on pupil absence in Nyanza Province, Kenya: a cluster-randomized trial. Tropical Medicine & International Health 2014;19(10):1185-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chard 2018 {published data only}
- Chard AN, Freeman MC. Design, intervention fidelity, and behavioral outcomes of a school-based water, sanitation, and hygiene cluster-randomized trial in Laos. International Journal of Environmental Research and Public Health 2018;15(4):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chard 2019 {published data only}
- Chard AN, Garn JV, Chang HH, Clasen T, Freeman MC. Impact of a school-based water, sanitation, and hygiene intervention on school absence, diarrhoea, respiratory infection, and soil-transmitted helminths: results from the WASH HELPS cluster-randomized trial. Journal of Global Health 2019;9(2):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clasen 2014a {published data only}
- Clasen T, Boisson S, Routray P, Torondel B, Bell M, Cumming O, et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Global Health 2014;2(11):e645-53. [DOI] [PubMed] [Google Scholar]
Clasen 2014b {published data only}
- Clasen T, Boisson S, Routray P, Torondel B, Jenkins M, Freeman M. The effectiveness of a rural sanitation intervention on health and ORISSA, India: a cluster randomized, controlled trial. 63rd Annual Meeting of the American Society of Tropical Medicine and Hygiene (ASTMH); 20141102-06; New Orleans, In: American Journal of Tropical Medicine and Hygiene 2014;91(5 SUPPL):215. [Google Scholar]
Clemens 1987 {published data only}
- Clemens JD, Stanton BF. An educational intervention for altering water-sanitation behaviors to reduce childhood diarrhea in urban Bangladesh. 1. Application of the case-control method for development of an intervention. American Journal of Epidemiology 1987;125(2):284-91. [DOI] [PubMed] [Google Scholar]
Contzen 2015 {published data only}
- Contzen N, Meili IH, Mosler HJ. Changing handwashing behaviour in southern Ethiopia: a longitudinal study on infrastructural and commitment interventions. Social Science & Medicine 2015;124:103-14. [DOI] [PubMed] [Google Scholar]
Correa 2012 {published data only}
- Correa JC, Pinto D, Salas LA, Camacho JC, Rondón M, Quintero J. A cluster-randomized controlled trial of hand rubs for prevention of infectious diseases among children in Colombia. Revista Panamericana de Salud Pública 2012;31(6):476-84. [DOI] [PubMed] [Google Scholar]
Curtis 2001 {published data only}
- Curtis V, Kanki B, Cousen S, Diallo I, Kpozehouen A, Sangaré M, et al. Evidence of behaviour change following a hygiene promotion programme in Burkina Faso. Bulletin of the World Health Organization 2001;79(6):518-27. [PMC free article] [PubMed] [Google Scholar]
Doebbeling 1992 {published data only}
- Doebbeling BN, Stanley GL, Sheetz CT, Pfaller MA, Houston AK, Annis L, et al. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. New England Journal of Medicine 1992;327(2):88-93. [DOI] [PubMed] [Google Scholar]
Dreibelbis 2014 {published data only}
- Dreibelbis R, Freeman MC, Greene LE, Saboori S, Rheingans R. The impact of school water, sanitation, and hygiene interventions on the health of younger siblings of pupils: a cluster-randomized trial in Kenya. American Journal of Public Health 2014;104(1):e91-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreibelbis 2016 {published data only}
- Dreibelbis R, Kroeger A, Hossain K, Venkatesh M, Ram PK. Behavior change without behavior change communication: nudging handwashing among primary school students in Bangladesh. International Journal of Environmental Research and Public Health 2016;13(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duijster 2017 {published data only}
- Duijster D, Monse B, Dimaisip-Nabuab J, Djuharnoko P, Heinrich-Weltzien R, Hobdell M. 'Fit for school' - a school-based water, sanitation and hygiene programme to improve child health: results from a longitudinal study in Cambodia, Indonesia and Lao PDR. BMC Public Health 2017;17(302):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dyer 2000 {published data only}
- Dyer DL, Shinder A, Shinder F. Alcohol-free instant hand sanitizer reduces elementary school illness absenteeism. Family Medicine 2000;32(9):633-8. [PubMed] [Google Scholar]
Ecrumen 2018 {published data only}
- Ercumen A, Mertens A, Arnold BF, Benjamin-Chung J, Hubbard AE, Ahmed MA, et al. Effects of single and combined water, sanitation and handwashing interventions on fecal contamination in the domestic environment: a cluster-randomized controlled trial in rural Bangladesh. Environmental Science Technology 2018;52(21):12078-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercumen A, Pickering A, Kwong LH, Mertens A, Arnold BF, Benjamin-Chung J, et al. Do sanitation improvements reduce fecal contamination of water, hands, food, soil and flies? Evidence from a cluster-randomized controlled trial in rural Bangladesh. Environmental Science Technology 2018;52(21):2089-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ecrumen 2019 {published data only}
- Ercumen A, Benjamin-Chung J, Arnold BF, Lin A, Hubbard AE, Stewart C, et al. Effects of water, sanitation, handwashing and nutritional interventions on soil-transmitted helminth infections in young children: a cluster-randomized controlled trial in rural Bangladesh. PLOS Neglected Tropical Diseases 2019;3(5):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enebeli 2017 {published data only}
- Enebeli UU. The influence of sanitation on childhood diarrhoea in 2016 and its implications on integrated community case management of endemic childhood diseases in Abia State, Nigeria. American Journal of Tropical Medicine and Hygiene. Conference: 66th annual meeting of the American Society of Tropical Medicine and Hygiene, ASTMH 2017. United States 2017;97(5 Supplement 1):586-7. [Google Scholar]
Erismann 2017 {published data only}
- Erismann S, Diagbouga S, Schindler C, Odermatt P, Knoblauch AM, Gerold J, et al. School children's intestinal parasite and nutritional status one year after complementary school garden, nutrition, water, sanitation, and hygiene interventions in Burkina Faso. American Journal of Tropical Medicine and Hygiene 2017;97(3):904-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2011 {published data only}
- Fan VY, Mahal A. What prevents child diarrhoea? The impacts of water supply, toilets, and hand-washing in rural India. Journal of Development Effectiveness 2011;3(3):340-70. [Google Scholar]
Freeman 2014 {published data only}
- Freeman MC, Clasen T, Dreibelbis R, Saboori S, Greene LE, Brumback B, et al. The impact of a school-based water supply and treatment, hygiene, and sanitation programme on pupil diarrhoea: a cluster-randomized trial. Epidemiology and Infection 2014;142(2):340-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greene 2012 {published data only}
- Greene LE, Freeman MC, Akoko D, Saboori S, Moe C, Rheingans R. Impact of a school-based hygiene promotion and sanitation intervention on pupil hand contamination in Western Kenya: a cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2012;87(3):385-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenland 2016 {published data only}
- Greenland K, Chipungu J, Curtis V, Schmidt WP, Siwale Z, Mudenda M, et al. Multiple behaviour change intervention for diarrhoea control in Lusaka, Zambia: a cluster randomised trial. Lancet Global Health 2016;4(12):E966-77. [DOI] [PubMed] [Google Scholar]
Guinan 2002 {published data only}
- Guinan M, McGuckin M, Ali Y. The effect of a comprehensive handwashing program on absenteeism in elementary schools. American Journal of Infection Control 2002;30(4):217-20. [DOI] [PubMed] [Google Scholar]
Hammond 2000 {published data only}
- Hammond B, Ali Y, Fendler E, Dolan M, Donovan S. Effect of hand sanitizer use on elementary school absenteeism. American Journal of Infection Control 2000;28(5):340-6. [DOI] [PubMed] [Google Scholar]
Hovi 2017 {published data only}
- Hovi T, Ollgren J, Savolainen-Kopra C. Intensified hand-hygiene campaign including soap-and-water wash may prevent acute infections in office workers, as shown by a recognized-exposure-adjusted analysis of a randomized trial. BioMed Central Infectious Diseases 2017;17(47):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hübner 2010 {published data only}
- Hübner NO, Hübner C, Wodny M, Kampf G, Kramer A. Effectiveness of alcohol-based hand disinfectants in a public administration: impact on health and work performance related to acute respiratory symptoms and diarrhoea. BMC Infectious Diseases 2010;10:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huda 2012 {published data only}
- Huda TM, Unicomb L, Johnston RB, Halder AK, Yushuf Sharker MA, Luby SP. Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Social Science & Medicine 2012;75(4):604-11. [DOI] [PubMed] [Google Scholar]
Jinadu 2007 {published data only}
- Jinadu MK, Adegbenro CA, Esmai AO, Ojo AA, Oyeleye BA. Health promotion intervention for hygienic disposal of children's faeces in a rural area of Nigeria. Health Education Journal 2007;66(3):222-8. [Google Scholar]
Johansen 2015 {published data only}
- Johansen A, Denbaek AM, Bonnesen CT, Due P. The Hi Five study: design of a school-based randomized trial to reduce infections and improve hygiene and well-being among 6-15 year olds in Denmark. BMC Public Health 2015;15:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamm 2016 {published data only}
- Kamm KB, Vujcic J, Nasreen S, Luby SP, Zaman K, El Arifeen S, et al. Is pregnancy a teachable moment to promote handwashing with soap among primiparous women in rural Bangladesh? Follow-up of a randomised controlled trial. Tropical Medicine & International Health 2016;21(12):1562-71. [DOI] [PubMed] [Google Scholar]
Kang 2017 {published data only}
- Kang Y, Suh YK, Debele L, Juon HS, Christian P. Effects of a community-based nutrition promotion programme on child feeding and hygiene practices among caregivers in rural Eastern Ethiopia. Public Health Nutrition 2017;20(8):1461-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1982 {published data only}
- Khan MU. Interruption of shigellosis by hand washing. Transactions of the Royal Society of Tropical Medicine and Hygiene 1982;76(2):164-8. [DOI] [PubMed] [Google Scholar]
Larson 2003 {published data only}
- Larson E, Aiello A, Lee LV, Della-Latta P, Gomez-Duarte C, Lin S. Short- and long-term effects of handwashing with antimicrobial or plain soap in the community. Journal of Community Health 2003;28(2):139-50. [DOI] [PubMed] [Google Scholar]
Larson 2004 {published data only}
- Larson EL, Lin SX, Gomez-Pichardo C, Della-Latta P. Effect of antibacterial home cleaning and handwashing products on infectious disease symptoms: a randomized, double-blind trial. Annals of Internal Medicine 2004;140(5):321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 1991 {published data only}
- Lee W, Stoeckel J, Jintaganont P, Romanarak T, Kullavanijaya S. The impact of a community based health education program on the incidence of diarrheal disease in Southern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1991;22(4):548-56. [PubMed] [Google Scholar]
Luby 2001b {published data only}
- Luby SP, Agboatwalla M, Raza A, Sobel J, Mintz ED, Baier K, et al. Microbiologic effectiveness of hand washing with soap in an urban squatter settlement, Karachi, Pakistan. Epidemiology and Infection 2001;127(2):237-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luby 2004 {published data only}
- Luby SP, Agboatwalla M, Hoekstra RM, Rahbar MH, Billhimer W, Keswick BH. Delayed effectiveness of home-based intervention in reducing childhood diarrhea, Karachi, Pakistan. American Journal of Medicine and Hygiene 2004;71(4):420-7. [PubMed] [Google Scholar]
Luby 2007 {published data only}
- Luby SP, Agboatwalla M, Billhimer W, Hoekstra RM. Field trial of a low cost method to evaluate hand cleanliness. Tropical Medicine & International Health 2007;12(6):765-71. [DOI] [PubMed] [Google Scholar]
Luby 2008 {published data only}
- Luby SP, Mendoza C, Keswick BH, Chiller TM, Hoekstra RM. Difficulties in bringing point-of-use water treatment to scale in rural Guatemala. American Journal of Tropical Medicine and Hygiene 2008;78(3):382-7. [PubMed] [Google Scholar]
Luby 2010 {published data only}
- Luby SP, Kadir MA, Yushuf Sharker MA, Yeasmin F, Unicomb L, Sirajul Islam M. A community-randomised controlled trial promoting waterless hand sanitizer and handwashing with soap, Dhaka, Bangladesh. Tropical Medicine & International Health 2010;15(12):1508-16. [DOI] [PubMed] [Google Scholar]
Manjang 2018 {published data only}
- Manjang B, Hemming K, Bradley C, Ensink J, Martin J, Sowe J, et al. Promoting hygienic weaning food handling practices through a community based programme: intervention implementation and baseline characteristics for a cluster randomised controlled trial in rural Gambia. British Medical Journal Open 2018;8:e017573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Master 1997 {published data only}
- Master D, Hess Longe SH, Dickson H. Scheduled hand washing in an elementary school population. Family Medicine 1997;29(5):336-9. [PubMed] [Google Scholar]
Morton 2004 {published data only}
- Morton JL, Schultz AA. Healthy Hands: Use of alcohol gel as an adjunct to handwashing in elementary school children. Journal of School Nursing 2004;20(3):161-7. [DOI] [PubMed] [Google Scholar]
Najnin 2017 {published data only}
- Najnin N, Leder K, Qadri F, Forbes A, Unicomb L, Winch PJ, et al. Impact of adding hand-washing and water disinfection promotion to oral cholera vaccination on diarrhoea-associated hospitalization in Dhaka, Bangladesh: evidence from a cluster randomized control trial. International Journal of Epidemiology 2017;46(6):2056-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naluonde 2018 {published data only}
- Naluonde T, Wakefield C, Markle L, Martin A, Tresphor C, Abdullah R, et al. A disruptive cue improves handwashing in school children in Zambia. Health Promotion International 2018;34(6):e119-28. [DOI] [PubMed] [Google Scholar]
Oncu 2018 {published data only}
- Oncu E, Vayisoglu SK, Lafci D, Yurtsever D, Bulut ER, Peker E. Comparison of interactive education versus fluorescent concretization on hand hygiene compliance among primary school students: a randomized controlled trial. Journal of School Nursing 2018;35(5):337-47. [DOI] [PubMed] [Google Scholar]
Oughton 2009 {published data only}
- Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD. Hand hygiene with soap and water Is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infection Control and Hospital Epidemiology 2009;30(10):939-44. [DOI] [PubMed] [Google Scholar]
Overgaard 2016 {published data only}
- Overgaard H, Alexander N, Matiz MI, Jaramillo JF, Olano VA, Vargas S, et al. A cluster-randomized controlled trial to reduce diarrheal disease and dengue entomological risk factors in rural primary schools in Colombia. PLOS Neglected Tropical Diseases 2016;10(11):e005106. 1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2012 {published data only}
- Patel MK, Harris JR, Juliao P, Nygren B, Were V, Kola S, et al. Impact of a hygiene curriculum and the installation of simple handwashing and drinking water stations in rural Kenyan primary schools on student health and hygiene practices. American Journal of Tropical Medicine and Hygiene 2012;87(4):594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 1998 {published data only}
- Peterson EA, Roberts L, Toole MJ, Peterson DE. Effect of soap distribution on diarrhoea: Nyamithuthu Refugee Camp. International Journal of Epidemiology 1998;27(3):520-4. [DOI] [PubMed] [Google Scholar]
Pinfold 1996 {published data only}
- Pinfold JV, Horan NJ. Measuring the effect of a hygiene behaviour intervention by indicators of behaviour and diarrhoeal disease. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(4):366-71. [DOI] [PubMed] [Google Scholar]
Priest 2014 {published data only}
- Priest P, McKenzie JE, Audas R, Poore M, Brunton C, Reeves L. Hand sanitizer provision for reducing illness absences in primary school children: a cluster randomised trial. PLOS Medicine 2014;11(8):e1001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen 2009 {published data only}
- Rosen L, Zucker D, Brody D, Engelhard D, Manor O. The effect of a handwashing intervention on preschool educator beliefs, attitudes, knowledge and self-efficacy. Health Education Resources 2009;24(4):686-98. [DOI] [PubMed] [Google Scholar]
Saboori 2013 {published data only}
- Saboori S, Greene LE, Moe CL, Freeman MC, Caruso BA, Akoko D, et al. Impact of regular soap provision to primary schools on hand washing and E. coli hand contamination among pupils in Nyanza Province, Kenya: a cluster-randomized trial. American Journal of Tropical Medicine and Hygiene 2013;89(4):698-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savolainen‐Kopra 2012 {published data only}
- Savolainen-Kopra C, Haapakoski J, Peltola PA, Ziegler T, Korpela T, Anttila P, et al. Hand washing with soap and water together with behavioural recommendations prevents infections in common work environment: an open cluster-randomized trial. Trials 2012;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shafique 2013 {published data only}
- Shafique S, Jalal CS, Jolly SP, Shikder H, Sellen DW, Zlotkin S. Effects of water-based hand sanitizers and micronutrient powders along with nutrition and hygiene education to prevent infections and linear growth faltering among low birth weight infants in Bangladesh. FASEB Journal Conference 2013;27(Meeting Abstract Supplement):243.1. [Google Scholar]
Shahid 1996 {published data only}
- Shahid NS, Greenough WB 3rd, Samadi AR, Huq MI, Rahaman N. Handwashing with soap reduces diarrhoea and spread of bacterial pathogens in a Bangladesh village. Journal of Diarrhoeal Diseases Research 1996;14(2):85-9. [PubMed] [Google Scholar]
Sinharoy 2017 {published data only}
- Sinharoy SS, Schmidt WP, Wendt R, Mfura L, Crossett E, Grepin KA, et al. Effect of community health clubs on child diarrhoea in western Rwanda: cluster-randomised controlled trial. Lancet Global Health 2017;5(7):e699-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinharoy SS, Schmidt WP, Wendt R, Mfura L, Crossett E, Grepin KA, et al. Impact of community health clubs on diarrhea and anthropometry in western Rwanda: cluster-randomized controlled trial. FASEB Journal. Conference: Experimental Biology 2017;31:(1 Supplement 1). [Google Scholar]
Sircar 1987 {published data only}
- Sircar BK, Sengupta PG, Mondal SK, Gupta DN, Saha NC, Ghosh S, et al. Effect of handwashing on the incidence of diarrhoea in a Calcutta slum. Journal of Diarrhoeal Diseases Research 1987;5(2):112-4. [PubMed] [Google Scholar]
Slayton 2013 {published data only}
- Slayton RB, Murphy JL, Faith SH, Oremo J, Odhiambo A, Ayers T, et al. A cluster randomized controlled evaluation of the health impact of a novel antimicrobial hand towel on the health of children under two years old in rural communities in Nyanza province, Kenya. American Journal of Tropical Medicine and Hygiene (Conference Publication) 2013;85(Suppl 1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vally 2019 {published data only}
- Vally H, McMichael C, Doherty C, Li X, Guevarra G, Tobias P. The impact of a school-based water, sanitation and hygiene intervention on knowledge, practices, and diarrhoea rates in the Philippines. International Journal of Environmental Research and Public Health 2019;16(21):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vindigni 2011 {published data only}
- Vindigni SM, Riley PL, Jhung M. Systematic review: handwashing behaviour in low- to middle-income countries: outcome measures and behaviour maintenance. Tropical Medicine & International Health 2011;16(4):466-77. [DOI] [PubMed] [Google Scholar]
White 2003 {published data only}
- White C, Kolble R, Carlson R, Lipson N, Dolan M, Ali Y, et al. The effect of hand hygiene on illness rate among students in university residence halls. American Journal of Infection Control 2003;31(6):364-70. [DOI] [PubMed] [Google Scholar]
Wilson 1991 {published data only}
- Wilson JM, Chandler GN, Muslihatun, Jamiluddin. Hand-washing reduces diarrhoea episodes: a study in Lombok, Indonesia. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991;85(6):819-21. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Chandler GN. Sustained improvements in hygiene behaviour amongst village women in Lombok, Indonesia. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(6):615-6. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang C, Mosa AJ, Hayward AS, Matthews SA. Promoting clean hands among children in Uganda: a school-based intervention using 'tippy-taps'. Public Health 2013;127(6):586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Denbaek 2018 {published data only}
- Denbaek AM, Andersen A, Bonnesen CT, Laursen B, Ersboll AK, Due P, et al. Effect evaluation of a randomized trial to reduce infectious illness and illness-related absenteeism among schoolchildren: the Hi Five Study. Pediatric Infectious Disease Journal 2018;37(1):16-21. [DOI] [PubMed] [Google Scholar]
Additional references
Cave 1999
- Cave B, Curtis V. Effectiveness of promotional techniques in environmental health. WELL Study. Task No.165. March 1999. https://www.ircwash.org/sites/default/files/Cave-1999-Effectiveness.pdf (accessed 1 September 2007).
Claessen 2008
- Claessen J-P, Bates S, Sherlock K, Seeparsand F, Wright R. Designing interventions to improve tooth brushing. International Dental Journal 2008;58:307-20. [Google Scholar]
Curtis 2000
- Curtis V, Cairncross S, Yonli R. Domestic hygiene and diarrhoea - pinpointing the problem. Tropical Medicine & International Health 2000;5(1):22-32. [DOI] [PubMed] [Google Scholar]
Curtis 2003
- Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infectious Diseases 2003;3(5):275-81. [DOI] [PubMed] [Google Scholar]
Curtis 2011
- Curtis V, Schmidt W, Luby S, Florez R, Touré O, Biran A. Hygiene: new hopes, new horizons. Lancet Infectious Diseases 2011;11(4):312-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Ehiri 2001
- Ehiri JE, Azubuike MC, Ubbaonu CN, Anyanwu EC, Ibe KM, Ogbonna MO. Critical control points of complementary food preparation and handling in eastern Nigeria. Bulletin of World Health Organization 2001;79(5):423-33. [PMC free article] [PubMed] [Google Scholar]
Eisenberg 2012
- Eisenberg JN, Trostle J, Sorensen RJ, Shields KF. Toward a systems approach to enteric pathogen transmission: from individual independence to community interdependence. Annual Review of Public Health 2012;33:239-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fewtrell 2004
- Fewtrell L, Colford JM. Water, Sanitation and Hygiene: Interventions and Diarrhoea: A Systematic Review and Meta-analysis [Report No 34960]. Washington DC: The International Bank for Reconstruction and Development/The World Bank, 2004. [Google Scholar]
Fewtrell 2005
- Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infectious Diseases 2005;5(1):42-52. [DOI] [PubMed] [Google Scholar]
Gould 2017
- Gould DJ, Moralejo D, Drey N, Chudleigh JH, Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD005186. [DOI: 10.1002/14651858.CD005186.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro. Hamilton (ON): McMaster University (developed by Evidence Prime), Accessed 01 November 2020. Available at gradepro.org.
Guevara 2004
- Guevara JP, Berlin JA, Wolf FM. Meta-analytic methods for pooling rates when follow-up duration varies: a case study. BMC Medical Research Methodology 2004;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurjeet 2013
- Gurjeet S, Urhekar AD. Effects of hand wash agents: prevent the laboratory associated infections. International Journal of Medical Research & Health Sciences 2013;2(3):564-8. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, AlonsoCoello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2020
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Hugonnet 2000
- Hugonnet S, Pittet D. Hand hygiene - beliefs or science. Clinical Microbiology and Infection 2000;6(7):350-6. [DOI] [PubMed] [Google Scholar]
Huis 2012
- Huis A, Van Achterberg T, De Bruin M, Grol R, Schoonhoven L, Hulscher M. A systematic review of hand hygiene improvement strategies: a behavioural approach. Implementation Science 2012;7:92. [DOI: 10.1186/1748-5908-7-92] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iroegbu 2000
- Iroegbu CU, Ene-Obong HN, Uwaegute AC, Amazigo UV. Bacteriological quality of weaning food and drinking water given to children of market women in Nigeria: implications for control of diarrhea. Journal of Health, Population, and Nutrition 2000;18(3):157-62. [PubMed] [Google Scholar]
Lee 2012
- Lee G, Yori P, Olortegui MP, Pan W, Caulfield L, Gilman RH, et al. Comparative effects of vivax malaria, fever and diarrhoea on child growth. International Journal of Epidemiology 2012;41(2):531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Luby 2001a
- Luby S. The role of hand washing in improving hygiene and health in low-income countries. American Journal of Infection Control 2001;29(4):239-40. [DOI] [PubMed] [Google Scholar]
Luby 2005
- Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet 2005;366(9481):225-33. [DOI] [PubMed] [Google Scholar]
Madhu 2012
- Madhu R, Van Beinum A. A systematic review of community hand washing interventions leading to changes in hygiene behavior in the developing world. Global Journal on Medicine and Public Health 2012;1(4):49-55. [Google Scholar]
Mannan 2010
- Mannan SR, Rahman MA. Exploring the link between food-hygiene practices and diarrhoea among the children of garments worker mothers in Dhaka. Anwer Khan Modern Medical College Journal 2010;1(2):4-11. [Google Scholar]
Marino 2007
- Marino DD. Water and food safety in the developing world: global implications for health and nutrition of infants and young children. Journal of the American Dietetic Association 2007;107(11):1930-4. [DOI] [PubMed] [Google Scholar]
Martin 1997
- Martin M. Critical education for participatory research. www.socresonline.org.uk/2/2/8.html (accessed 28 September 2007).
Maxwell 2012
- Maxwell O, Oklo A, Bernard A. Profile of water related diseases in Benue State, Nigeria. American Journal of Human Ecology 2012;1(3):87-94. [Google Scholar]
Mbakaya 2017
- Mbakaya BC, Lee PH, Lee RL. Hand hygiene intervention strategies to reduce diarrhoea and respiratory infections among schoolchildren in developing countries: a systematic review. International Journal of Environmental Research and Public Health 2017;14(4):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgado 2017
- Morgado FF, Meireles JF, Neves CM, Amaral AC, Ferreira ME. Scale development: ten main limitations and recommendations to improve future research practices. Psicologia: Reflexão e Crítica volume 2017;30(3 - 2018):1-20. [DOI: 10.1186/s41155-016-0057-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2012
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman A, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197–223. [DOI] [PubMed] [Google Scholar]
Nielsen 2001
- Nielsen M, Hoogvorst A, Konradsen F, Mudasser M, Van der Hoek W. Childhood diarrhea and hygiene: mothers' perceptions and practices in the Punjab, Pakistan. Working Paper 25. Colombo, Sri Lanka: International Water Management Institute. www.iwmi.cgiar.org/Publications/Working_Papers/working/WOR25.pdf (accessed 01 September 2014):1-21.
Oketcho 2012
- Oketcho R, Nyaruhucha CN, Taybali S, Karimuribo ED. Influence of enteric bacteria and parasite infection and nutritional status on diarrhoea occurrence in six to 60 month old children admitted at Morogoro Regional Hospital of Tanzania. Tanzania Journal of Health Research 2012;14(2):1-14. [DOI] [PubMed] [Google Scholar]
Pickering 2011
- Pickering AJ, Julian TR, Mamuya S, Boehm AB, Davis J. Bacterial hand contamination among Tanzanian mothers varies temporally and following household activities. Tropical Medicine & International Health 2011;16(2):233-9. [DOI] [PubMed] [Google Scholar]
Prüss 2002
- Prüss A, Kay D, Fewtrell L, Bartram J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environmental Health Perspectives 2002;110(5):537-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2019 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2019.
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane Collaboration, 2020. Available from www.training.cochrane.org/handbook.
Traoré 1994
- Traoré E, Cousens S, Curtis V, Mertens T, Tall F, Traoré A, et al. Childhood defecation behaviour, stool disposal practices, and childhood diarrhoea in Burkina Faso: results from a case-control study. Journal of Epidemiology and Community Health 1994;48(3):270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tumwine 2002
- Tumwine JK, Thompson J, Katua-Katua M, Mujwajuzi M, Johnstone N, Porras I. Diarrhoea and effects of different water sources, sanitation and hygiene behaviour in East Africa. Tropical Medicine & International Health 2002;7(9):750-6. [DOI] [PubMed] [Google Scholar]
UNICEF/WHO 2009
- United Nations Children's Fund (UNICEF), World Health Organization (WHO). Diarrhoea: why children are still dying and what can be done. Maternal, newborn, child and adolescent health. 2009. whqlibdoc.who.int/publications/2009/9789241598415_eng.pdf?ua=1 (accessed 18 July 2013):1-68.
Walker 2013
- Walker C, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
WELL 1999
- Water and Environmental Health at London and Loughborough (WELL). DFID guidance manual on water supply and sanitation programmes. 1998. www.eenet.org.uk/resources/docs/prelims.pdf (accessed 14 June 2014).
Whitby 2007
- Whitby M, Pessoa-Silva CL, McLaws ML, Allegranzi B, Sax H, Larson E, et al. Behavioural considerations for hand hygiene practices: the basic building blocks. Journal of Hospital Infection 2007;65(1):1-8. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. The Treatment of Diarrhoea: a Manual for Physicians and Other Senior Health Workers [WHO/FCH/CAH/03.7]. Geneva: World Health Organization, 2003. [Google Scholar]
Woldemicael 2001
- Woldemicael G. Diarrhoeal morbidity among young children in Eritrea: environmental and socioeconomic determinants. Journal of Health, Population, and Nutrition 2001;19(2):83-90. [PubMed] [Google Scholar]
Yeager 1999
- Yeager BA, Huttly SR, Bartolini R, Rojas M, Lanata CF. Defecation practices of young children in a Peruvian shanty town. Social Science and Medicine 1999;49(4):531-41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ehiri JE
- Ehiri JE, Ejere HOD. Hand washing for preventing diarrhoea. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD004265. [DOI: 10.1002/14651858.CD004265] [DOI] [PubMed] [Google Scholar]
Ejemot‐Nwadiaro 2008
- Ejemot-Nwadiaro RI, Ehiri JE, Meremikwu MM, Critchley JA. Hand washing for preventing diarrhoea. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004265. [DOI: 10.1002/14651858.CD004265.pub2] [DOI] [PubMed] [Google Scholar]
Ejemot‐Nwadiaro 2015
- Ejemot‐Nwadiaro RI, Ehiri JE, Arikpo D, Meremikwu MM, Critchley JA. Hand washing promotion for preventing diarrhoea. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004265. [DOI: 10.1002/14651858.CD004265.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


