Abstract
Inhibitor of growth 4 (ING‐4) is a tumor suppressor gene that interacts with nuclear factor‐kappaB (NF‐κB) and represses its transcriptional activity. Several lines of evidence suggest that the tumor suppressor gene ING‐4, the transcription factor NF‐κB and its target genes matrix metalloproteases MMP‐2, MMP‐9 and urokinase plasminogen activator (u‐PA) are critically involved in tumor invasion. The aim of the present study was to investigate immunohistochemically the expression pattern of ING‐4, NF‐κB and the NF‐κB downstream targets MMP‐2, MMP‐9 and u‐PA in human astrocytomas from 101 patients. We found that ING‐4 expression was significantly decreased in astrocytomas, and ING‐4 loss was associated with tumor grade progression. Expression of p65, a NF‐κB subunit, was significantly higher in grade IV than in grade III and grade I/II tumors, and a statistical significant negative correlation between expression of ING‐4 and expression of nuclear p65 was noticed. MMP‐9, MMP‐2 and u‐PA were overexpressed in human astrocytomas. Of note, astrocytomas of advanced histologic grades (grade III, IV) displayed significantly higher expression levels of these proteins compared to tumors of lower grades (grade I, II). Collectively, our data suggest an essential role for ING‐4 in human astrocytoma development and progression possibly through regulation of the NF‐κB‐dependent expression of genes involved in tumor invasion.
Keywords: human astrocytomas, ING‐4, NF‐κB
INTRODUCTION
Astrocytomas are the most common primary central nervous system tumors. The most prominent characteristic of these tumors is their ability to invade adjacent normal brain parenchyma by degrading components of extracellular matrix. The highly invasive nature of malignant astrocytomas often prevents curative surgical resections and complicates effective delivery of therapy, leading to serious clinical deficits and poor prognosis. Tumor invasion is a complex biologic process involving specific well‐orchestrated events, namely adhesion, cell motility and degradation of extracellular matrix (25). Recent molecular studies suggest the implication of inhibitor of growth ING‐4 (inhibitor of growth family, member 4) in astrocytomas invasion process (13). ING‐4 is a member of the ING tumor suppressor gene family. The ING family involves five evolutionarily conserved proteins (ING‐1–ING5) characterized by a highly conserved plant homeodomain finger motif in their carboxy‐terminal end (7). Previous studies have shown that this motif is found in transcription factors (ie, AF10, AF17, Trx) that modulate chromatin structure (1) and that ING proteins interact with multiprotein complexes containing histone acetyltransferase and deacetylase (12). These data indicate that ING proteins participate in transcriptional regulation through chromatin remodeling (12). ING‐4 is located in chromosomal locus 12p13 and encodes for a 249‐amino acid protein containing a C‐terminal plant homeodomain finger motif and two nuclear localization signals. ING‐4 has been shown to induce cell cycle arrest and p53‐dependent apoptosis, to suppress cell migration and angiogenesis and to enhance chemosensitivity (16, 21). Additionally, in vitro studies in cell lines have suggested that ectopic expression of ING‐4 decreases cell spreading and migration (42). In vitro data link the tumor suppressor function of ING‐4 to nuclear factor‐kappaB (NF‐κB) signaling pathway (13). NF‐κB is a collective name for dimeric transcriptional factors comprising the Rel family of proteins that involve RelA (p65), c‐Rel, RelB, NF‐κB1 (p50) and NF‐κB2 (p52). The most common heterodimer in activated cells is the NF‐κB1‐RelA that contains the p65 and p50 proteins (24). In quiescent cells, NF‐κB remains in the cytoplasm bound to its inhibitory component IκB. Activation of NF‐κB occurs after IκB phosphorylation, ubiquitination and subsequently degradation. This results in NF‐κB translocation to the nucleus, where it binds to specific binding sites of the promoters of target genes involved in a variety of processes including proliferation and survival (2, 20). In addition, previous studies have shown that ING‐4 overexpression suppresses tumor invasion by decreasing the expression of both matrix metalloproteases (MMP)‐2 and MMP‐9 (26).
Many of the genes transcriptionally regulated by NF‐κB [ie, MMP‐2, MMP‐9 and urokinase‐type plasminogen activators (u‐PA)] are known to be involved in the process of invasion (5, 35). Accordingly, inhibition of NF‐κB activity suppresses invasion and metastasis through u‐PA, MMP‐2 and MMP‐9 down‐regulation (18, 32, 36). However, extensive in vivo evidence supporting a role of ING‐4–NF‐κB–MMPs and u‐PA signaling axis in human astrocytoma invasion process is lacking.
In the present study we examined the expression pattern of ING‐4, p65, MMP‐2, MMP‐9 and u‐PA in a well‐characterized set of human astrocytomas of all grades and in normal brain. In addition, we investigated their implication in the pathogenesis and progression of astrocytic tumors and correlated their expression levels with clinicopathologic parameters such as age, sex and tumor grade. Finally, we assessed the potential of role of ING‐4 as a biomarker for the prediction of the grade of astrocytic neoplasms.
MATERIALS AND METHODS
Tissue specimens
Formalin‐fixed paraffin‐embedded tissue samples from 101 patients with astrocytomas of all grades were obtained from the Department of Pathology of “Agios Andeas” and “Nikaia” General Hospitals, of Patras and Athens, Greece. The study was approved by the Committee on Research and Ethics and the Scientific Committee of the University Hospital of Patras, Greece. The mean age of the patients was 56 years ranging from 12 to 80 years, whereas the male‐to‐female ratio was 0.8. For statistical purposes we categorized patients' age into three groups: group A (10–39 years), group B (40–69 years) and group C (70–100 years). All cases were reviewed by two experienced pathologists (H.P. and J.V.), who assigned a histologic grade according to the latest World Health Organization Classification (28). Our cohort included four (3.9%) pilocytic (grade I), seven (6.9%) grade II, 18 (17.8%) grade III and 72 (71.3%) grade IV astrocytomas. Because of the relatively small number of grade I and grade II astrocytomas of our series, theses tumors were grouped together for the statistical analyses. We used normal brain cortex obtained from 15 autopsy cases as controls.
Immunohistochemical staining
Paraffin‐embedded sections of tumor tissue fixed in 10% buffered formalin from 101 patients were used for immunohistochemistry. Four‐micrometer serial sections were obtained from each specimen on SuperFrost® (Menzel‐Gläser, Braunschweig, Germany) plus glass slides. In brief, after deparaffinization and rehydration in graded alcohols, immunohistochemistry was performed using the two‐step peroxidase technique with a peroxidase‐conjugated polymer (DAKO Envision kit, DAKO, Carpinteria, CA). The following primary antibodies were used: anti‐p65 mouse monoclonal (Santa Cruz Biotechnology, Santa Cruz, CA), anti‐MMP‐2 (Chemicon, Hampshire, UK), anti‐MMP‐9 mouse monoclonal (Novocastra, Newcastle, UK), anti‐u‐PA mouse monoclonal (Calbiochem, Darmstadt, Germany), anti‐ING‐4 polyclonal (Proteintech, 10617‐1‐AP‐Proteintech Group, Inc., Chicago, IL, USA).
Sections were counterstained with Harris' hematoxylin, dehydrated and mounted permanently. For each antiserum, all sections from different studied cases were immunostained concurrently. Negative controls were performed in all cases by omitting the primary antibodies. We have attained optimal reproducible results for each antibody, employing dilutions/incubation time and pretreatment conditions.
Immunohistologic evaluation
Each slide was independently evaluated by two experienced pathologists (E.P. and J.V.) and one investigator (G.K.). The intensity of the immunostaining for all the antibodies was scored as follows: 0, negative staining; 1, weak staining; 2, moderate staining and 3, strong staining. The distribution of immunostaining was scored according to the following assumption: 0, less than 10% of neoplastic cells display positive immunoreactivity; 1, 10–30% of neoplastic cells display positive immunoreactivity; 2, 30–80% of neoplastic cells display positive immunoreactivity; and 3, >80% of neoplastic cells display positive immunoreactivity for the examined protein. The total score combined the intensity and the distribution of immunostaining and was calculated according to the following assumption: 0: intensity and distribution of staining 0; 1: intensity 1+, distribution 2+/3+ or intensity 2+, distribution 1+; 2: intensity 2+, distribution 2+/3+ or intensity 3+, distribution 1+; and 3: intensity 3+, distribution 2+/3+. The immunohistochemical profile of all the tested antibodies was similar in all the areas of the examined tissue sections. No differences were observed between the invasive front and the central core of the examined tumors.
Statistical analysis
Statistical analysis was performed using spss for Windows, release 12.0 (SPSS Inc., Chicago, IL). To test the significance of differences among groups of clinicopathologic parameters, ordinal data were analyzed with non‐parametric Kruskal–Wallis or Mann–Whitney tests, whereas correlations between expression of proteins (immunohistologic scores) were evaluated by Spearman rank order correlation coefficient. Tumor grade was modeled using binary logistic regression analysis. All ranking tests were performed with correction for ties. The significance level was defined as P < 0.05.
RESULTS
ING‐4 protein expression
The results of immunohistochemical investigation are summarized in Table 1. Glial cells of normal brain tissue and tissue adjacent to neoplastic brain parenchyma showed strong nuclear expression of ING‐4. In contrast, only 28 of 101 (27.7%) cases of astrocytomas cells showed nuclear ING‐4 immunopositivity (Figure 1). In 21 of 101 (20.8%) of tumor cases, ING‐4 displayed weak and in seven of 101 cases (6.9%) strong immunoreactivity. Loss of ING‐4 expression correlated significantly with tumor grade. More specifically, the expression of ING‐4 was significantly lower in grade IV than in grade III tumors, and in grade III than in grade I/II tumors (P < 0.001, for both correlations). No significant difference was observed in ING‐4 expression levels between grade I and grade II neoplasms. The expression levels of ING‐4 did not reveal any significant difference between different age groups, as well as between male and female patients. By using binary logistic regression analysis we found that loss of ING‐4 immunoreactivity could predict a high‐grade astrocytoma of our series with an overall accuracy of 89%.
Table 1.
Expression of ING‐4 and NF‐κB (p65) in astrocytomas. Correlation with the clinicopathologic parameters age, sex and histologic grade.
| Gliomas | Total | ING‐4 expression | Nuclear p65 expression | Cytoplasmic p65 expression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| 101 | 6 (8.7) | 19 (27.5) | 24 (34.8) | 20 (29) | 7 (6,9) | 38 (37.6) | 45 (44.5) | 11 (10.8) | 9 (8.9) | 25 (24.7) | 36 (35.6) | 31 (30.6) | ||
| Age | <20 | 5 | 4 (80) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 3 (60) | 2 (40) | 0 (0) | 0 (0) | 2 (40) | 2 (40) | 1 (20) |
| 20–50 | 32 | 22 (68.8) | 8 (25) | 2 (6.3) | 0 (0) | 2 (6.3) | 13 (40.6) | 15 (46.9) | 2 (6.3) | 2 (6.3) | 7 (21.9) | 14 (43.8) | 9 (28.1) | |
| >50 | 64 | 47 (73) | 12 (18.8) | 5 (7.8) | 0 (0) | 5 (7.8) | 22 (34.4) | 28 (43.8) | 9 (14.1) | 7 (6.9) | 16 (25) | 20 (31.3) | 21 (32.8) | |
| Sex | M | 45 | 33 (73.3) | 8 (17.8) | 4 (8.9) | 0 (0) | 3 (6.7) | 15 (33.3) | 22 (48.9) | 5 (11.i) | 5 (11.1) | 13 (28.9) | 14 (31.1) | 13 (28.9) |
| F | 56 | 40 (71.4) | 13 (23.2) | 3 (5.4) | 0 (0) | 4 (7.1) | 23 (41.1) | 23 (41.1) | 6 (10.7) | 4 (7.1) | 12 (21.4) | 22 (39.3) | 18 (32.1) | |
| Grade | Grade 1/II | 11 | 2 (18.2) | 7 (63.6) | 2 (18.2) | 0 (0) | 1 (9.1) | 8 (72.7) | 2 (18.2) | 0 (0) | 1 (9.1) | 7 (63.6) | 3 (27.3) | 0 (0) |
| Grade III | 18 | 13 (72.2) | 2 (11.1) | 3 (16.7) | 0 (0) | 4 (4.22) | 8 (44.7) | 5 (27.5) | 1 (5.6) | 4 (22.2) | 6 (33.3) | 6 (33.3) | 2 (11.1) | |
| Grade IV | 72 | 58 (80.6) | 12 (16.7) | 2 (2.8) | 0 (0) | 2 (2.8) | 22 (30.6) | 38 (52.8) | 10 (13.9) | 4 (5.6) | 12 (16.7) | 27 (37.5) | 29 (40.3) | |
Figure 1.
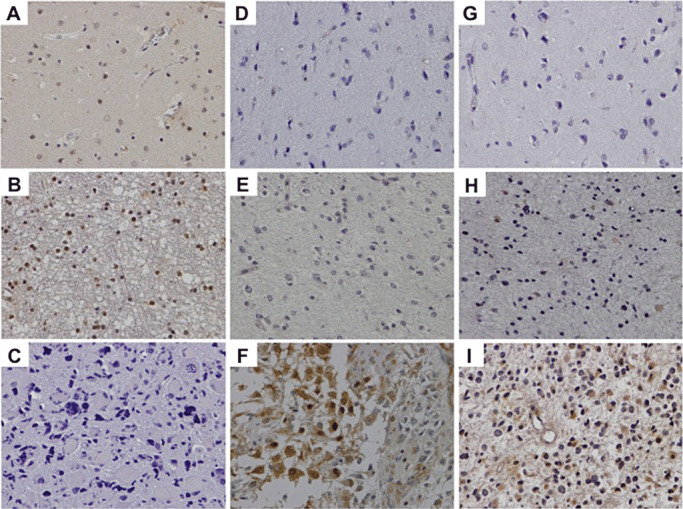
Loss of ING‐4 expression in a glioblastoma (C). Note the increased nuclear expression of ING‐4 in normal brain parenchyma (A) and low‐grade astrocytoma (B). Elevated NF‐κB (p65) expression is evident in a high‐ and in a low‐grade astrocytoma (F and E, respectively). Note that normal brain parenchyma is negative for p65 (D). A case of glioblastoma revealing augmented cytoplasmic u‐PA expression (I). A low‐grade astrocytoma (H) shows weak u‐PA immunostaining, whereas normal brain does not show any immunoreactivity for this protein (F).
NF‐κB (p65) protein expression
NF‐κB (p65) was detected in both the cytoplasm and the nucleus of 94 of 101 (93.1%) of the examined astrocytomas (Table 1). On the contrary, this protein was not detected in normal brain cells, under the conditions employed. Cytoplasmic p65 immunostaining was found in 92 of 101 (91.1%) tumors while nuclear p65 immunolocalization was observed in 94 of 101 (93.1%). Both nuclear and cytoplasmic immunoreactivity was detected in 92 of 101 (91.1%) astrocytomas. p65 showed both nuclear and cytoplasmic immunolocalization (Figure 1D–F). Importantly, its cellular levels (both nuclear and cytoplasmic) were significantly higher in grade IV compared to grade III and in grade III compared to grade I and II tumors (P < 0.001, for all correlations). We did not observe any significant difference among the p65 immunoexpression levels of grade I and grade II tumors. There was no significant correlation between p65 expression levels and patient gender and age.
MMP‐9 protein expression
Cytoplasmic expression of MMP‐9 was observed in 93 of 101 (92.1%) of the examined tumors (Table 2), while in normal brain tissue MMP‐9 immunostaining was absent. MMP‐9 expression levels correlated significantly with tumor grade. Indeed, grade IV astrocytomas demonstrated significantly higher expression of MMP‐9 compared to grade III and grade III compared to grade I/II astrocytomas (P < 0.001) (Figure 2D–F). We did not detect significant differences in the MMP‐9 expression levels between grade I and grade II neoplasms. No significant correlation was observed between expression levels of MMP‐9 and patient age or gender.
Table 2.
Expression of MMP‐9, MMP‐2 and u‐PA in astrocytomas in relation to age, sex and grade.
| Gliomas | Total | MMP‐9 expression | MMP‐2 expression | u‐PA expression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| 101 | 8 (7.9) | 23 (22.7) | 32 (31.6) | 38 (37.6) | 2 (1.9) | 17 (16.8) | 46 (45.5) | 36 (35.6) | 12 (11.8) | 22 (21.7) | 55 (54.4) | 12 (11.8) | ||
| Age | <20 | 5 | 1 (20) | 1 (20) | 0 (0) | 3 (7.9) | 0 (0) | 2 (40) | 2 (40) | 1 (20) | 2 (40) | 1 (20) | 2 (40) | 0 (0) |
| 20–50 | 32 | 4 (12.5) | 12 (37.5) | 4 (12.5) | 12 (37.5) | 1 (3.1) | 7 (21.9) | 13 (40.6) | 11 (34.4) | 5 (15.6) | 8 (25) | 15 (46.9) | 4 (12.5) | |
| >50 | 64 | 3 (4.7) | 10 (15.6) | 28 (43.8) | 23 (35.9) | 1 (1.6) | 8 (12.5) | 31 (48.4) | 24 (37.5) | 5 (7.8) | 13 (20.3) | 38 (59.4) | 8 (12.5) | |
| Sex | M | 45 | 3 (6.7) | 10 (22.2) | 14 (31.1) | 18 (40) | 0 (0) | 9 (20) | 22 (48.9) | 14 (31.1) | 3 (6.7) | 11 (24.4) | 26 (57.8) | 5 (11.1) |
| F | 56 | 5 (8.9) | 13 (23.2) | 18 (32.1) | 20 (35.7) | 2 (3.6) | 8 (14.3) | 24 (42.9) | 22 (39.3) | 9 (16.1) | 1 (19.6) | 29 (51.8) | 7 (12.5) | |
| Grade | Grade 1/II | 11 | 5 (45.5) | 5 (45.5) | 1 (9.1) | 0 (0) | 1 (9.1) | 7 (63.6) | 2 (18.2) | 1 (9.1) | 4 (36.4) | 6 (54.5) | 1 (9.1) | 0 (0) |
| Grade III | 18 | 2 (11.1) | 6 (33.3) | 5 (27.8) | 5 (27.8) | 1 (5.6) | 5 (27.8) | 7 (38.9) | 5 (27.8) | 2 (11.1) | 4 (22.2) | 8 (44.4) | 4 (22.2) | |
| Grade IV | 72 | 1 (1.4) | 12 (16.7) | 26 (36.1) | 33 (45.8) | 0 (0) | 5 (6.9) | 37 (51.4) | 30 (41.7) | 6 (8.3) | 12 (16.7) | 46 (63.9) | 8 (11.1) | |
Figure 2.
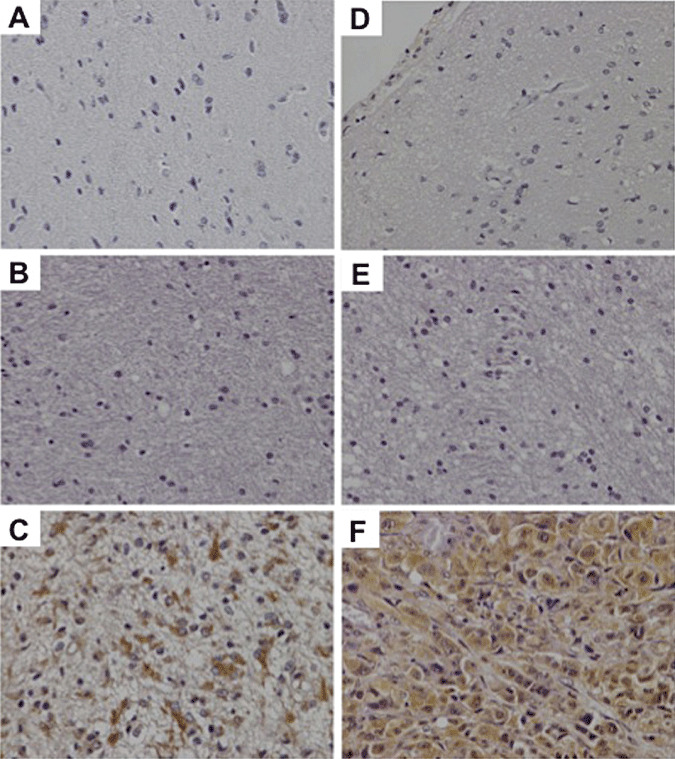
Normal brain tissue shows negative immunoreactivity for MMP‐2 (A) and MMP‐9 (D). Weak cytoplasmic expression of MMP‐2 (B) and MMP‐9 (E) in a low‐grade astrocytoma. Strong cytoplasmic expression of MMP‐2 (C) and MMP‐9 (F) in a glioblastoma.
MMP‐2 protein expression
In normal brain tissue, immunostaining for MMP‐2 was negative. With regard to astrocytomas 99 of 101 (98%) of the cases had cytoplasmic MMP‐2 expression (Figure 2A–C). There was a statistical significant correlation between MMP‐2 expression and tumor grade. MMP‐2 expression was significantly elevated in grade IV compared to grade III and in grade III in comparison to grade I/II gliomas (P < 0.001) (Table 2). No significant difference was observed in MMP‐2 expression levels among grade I and grade II astrocytomas. MMP‐2 immunoexpression levels did not differ among different patient age groups or gender.
u‐PA protein expression
The results of immunohistochemical evaluation are summarized in Table 2. Immunohistochemical analysis revealed cytoplasmic immunoreactivity for u‐PA in 89 of 101 (88.1%) of gliomas, while normal brain did not show expression of u‐PA, under the conditions employed. The expression levels of u‐PA were significantly elevated in tumors of higher grades (grades III and IV) in comparison to lower grade astrocytomas (P < 0.001). In fact, low‐grade astrocytomas display positive immunoreactivity in seven of 11 (63%) of the cases, whereas anaplastic astrocytomas and glioblastoma display positive cytoplasmic immunoreactivity in 16 of 18 (88%) and 66 of 72 (91%) of the examined tumors, respectively (Figure 1G–I). We did not observe any significant difference in u‐PA expression levels between grade I and grade II brain tumors. u‐PA immunostaining levels were not correlated with patient gender and age.
Correlations between the expression levels of the examined proteins
In our study there was a statistical significant negative correlation between expression of ING‐4 and of p65 (r = −326, P = 0.001 for total p65, r = −290, P = 0.003 for nuclear p65 and r = −0.323, P = 0.001 for cytoplasmic p65). MMP‐9 protein expression levels were strongly associated with p65 expression (r = 0.415, P < 0.001 for total p65, r = 0.399, P < 0.001 for nuclear p65 and r = 0.393, P < 0.001 for cytoplasmic p65). Interestingly statistically significant negative correlation was found between MMP‐9 and ING‐4 expression levels in glioma cells (r = −0.264, P = 0.008). There was no significant correlation between MMP‐2 and ING‐4 expression; nevertheless, a trend toward a negative correlation was observed (r = −0.186, P = 0.062). However, the expression levels of MMP‐2 were strongly associated with the cellular levels of p65 (r = 0.300, P = 0.002 for total p65, r = 0.270, P = 0.006 for nuclear p65 and r = 0.261, P = 0.008 for cytoplasmic p65). In addition, MMP‐2 staining was robustly and positively correlated with MMP‐9 (r = 0.393, <0.001). There was no significant correlation among u‐PA and ING‐4, although a trend toward a negative correlation was noted (r = −0.192, P = 0.054). The expression levels of u‐PA were strongly correlated with the cellular levels of p65 (r = 0.277, P = 0.005 for total p65, r = 0.248, P = 0.12 for nuclear p65 and r = 0.265, P = 0.007 for cytoplasmic p65), MMP‐9 (r = 0.427, P < 0.001) and MMP‐2 (r = 0.358, P < 0.001).
DISCUSSION
In the present study we demonstrated significantly reduced levels of ING‐4, a candidate tumor suppressor gene, in human astrocytomas compared to normal brain tissue. This finding indicates that down‐regulation of this protein might be involved in the pathogenesis of human astrocytic tumors, a notion that is in concert with recent molecular studies on human breast cancer cell lines and head and neck squamous cell carcinomas having shown loss of ING‐4 heterozygosity (15). This hypothesis is further supported by previous reports suggesting that genomic alterations of the chromosomal locus 12p13, which involves ING‐4 gene, occur frequently in human neoplasias (9, 15, 26).
Our results have demonstrated that decreased ING‐4 expression in human astrocytomas correlated significantly with tumor grade progression, with lower expression levels of ING‐4 observed in cases of high‐grade neoplasms.
These in vivo finding parallels data from previous in vitro studies on glioblastoma and multiple myeloma cell lines showing a negative correlation between ING‐4 expression and tumor grade (10, 13). Taken together, these results indicate that loss of ING‐4 might be implicated not only in the development but also in the progression of astrocytic tumors to more aggressive phenotypes.
A remarkable volume of studies has shown that NF‐κB regulates the expression of genes that govern major cancer‐related processes, namely apoptosis, angiogenesis, metastasis, proliferation, tumor growth and survival (33).
In this study we have shown that NF‐κB is highly expressed in astrocytomas as compared with normal human brain tissue. These results provide further evidence that NF‐κB is probably involved in the development of astrocytic tumors in humans.
In addition, we revealed a positive and significant correlation between NF‐κB expression levels and tumor grade. Indeed, grade III and IV astrocytomas showed remarkably elevated NF‐κB immunoreactivity compared to grade I and II neoplasms, suggesting that NF‐κB might also contribute to histologic aggressiveness of astrocytomas. Similar immunohistochemical analyses have shown that NF‐κB/p50 is also highly expressed in human astrocytic neoplasms and that its expression is robustly correlated with tumor grade (23). This points toward a critical role of the NF‐κB family members in astrocytoma pathobiology.
ING‐4 has been proposed to regulate tumorigenesis by modulating NF‐κB signaling (13). In the present study we demonstrated an inverse relationship between ING‐4 and NF‐κB protein expression in human astrocytomas, indicating that the ING‐4–NF‐κB axis might be involved the pathobiology of these neoplasms. In accordance with our data, it has been recently shown that one of the upstream regulators of the NF‐κB is ING‐4 in experiments performed on the glioblastoma cell line U87MG. That study showed that ING‐4 expression was significantly suppressed at both the RNA and the protein levels and that ING‐4 binding to RelA subunit of NF‐κB acts as a negative regulator of NF‐κB activity (13). In addition, functional biochemical studies have revealed that ING‐4 suppresses brain tumor angiogenesis, and thus progression, through physical interaction and transcriptional repression of NF‐κB (13). We further assessed the cellular levels of ING‐4 as biologic marker that could distinguish between high‐ and low‐grade astrocytomas. By applying binary logistic regression analysis we revealed that loss of ING‐4 expression could reliably predict a high‐grade tumor of our series with an overall accuracy of 89%. This distinction might contribute to the individualization of prognosis and eventually treatment of astrocytoma patients.
Tumor invasion is a complex biologic process involving adhesion, motility and degradation of extracellular matrix (25). Many of the genes regulated by NF‐κB, such as MMPs and u‐PA, are known to be involved in invasion. MMP‐2, ‐9 and u‐PA have the ability to promote ECM remodeling, since they are key components of the tumor‐associated proteolysis. Hence, many studies have proposed that these molecules could probably use therapeutic targets in oncology (14). We demonstrated increased expression levels of u‐PA in human astrocytomas. More importantly, u‐PA cellular levels exhibited significant correlation with progression of the astrocytomas to higher histologic grade. This is not a surprising finding, since there are reports indicating that u‐PA activity is augmented in brain tumors in vivo (39). Additionally, studies on other human malignancies, such as lung, breast and colon carcinomas have clearly demonstrated a significant correlation between the production of u‐PA and tumor invasiveness and metastatic propensity (19, 44). A considerable number of proteins, including NF‐κB, serve as essential regulators of u‐PA (11, 40). In fact, molecular studies have shown that u‐PA gene harbors NF‐κB binding site within its promoter region (37). Consistently with previous molecular studies that have linked NF‐κB with the regulation of u‐PA (22, 27, 45), we observed that NF‐κB and u‐PA immunoexpression levels were strongly and positively associated to each other, suggesting that in human astrocytomas u‐PA may be influenced by NF‐κB.
In line with evidence suggesting that u‐PA functions as an important physiologic activator of MMPs (29) we found a strong correlation between u‐PA and MMP‐2/MMP‐9 expression in human gliomas, indicating that u‐PA is probably involved in activation of MMPs in brain astrocytic tumors (38).
Similar to the plasminogen activator system, members of the MMP family of proteins are involved in extracellular matrix degradation and linked to various steps of tumor invasion and metastasis (3, 6, 30, 41). Among them MMP‐2 and MMP‐9 seem to have a central role in astrocytoma invasive potential (8, 31). In the present study we demonstrated increased expression of MMP‐2 and MMP‐9 in the majority of the astrocytomas of our sample, while this immunoreactivity was absent in normal brain tissue, suggesting a role of these enzymes in astrocytoma initiation. In addition, we observed augmented MMP‐2 and MMP‐9 expression levels in grade IV in comparison to grade III and grade I/II gliomas. Increased immunoexpression of MMP‐2 and MMP‐9 in human gliomas has also been reported by other study groups (8, 34), further strengthening the hypothesis that these proteins participate in the progression of astrocytic neoplasms. Since MMPs are activated by u‐PA, a synergetic role of these enzymes in tumor progression and invasion has been considered (29).
NF‐κB also plays an important role in regulating tumor cell infiltration as it is essential for MMPs expression (4). In the current study we observed positive correlation between the expression levels of the evaluated MMPs and NF‐κB. This is consistent with earlier studies showing that the expression of several MMPs is regulated by a NF‐κB (17, 43).
Collectively, our findings provide evidence that the down‐regulation of ING‐4 in human gliomas may induce MMP‐2 and MMP‐9 expression possibly via enhancement of NF‐κB. Clearly, more studies are required to clarify the role of these proteins in the pathobiology of human astrocytomas. If ING‐4 proves to be essential in the pathogenesis of astrocytomas, drugs that could selectively modulate the activity of this protein might constitute a novel alternative therapeutic approach for the treatment of central nervous system tumors.
REFERENCES
- 1. Aasland R, Gibson TJ, Stewart AF (1995) The PHD finger: implications for chromatin‐mediated transcriptional regulation. Trends Biochem Sci 20:56–59. [DOI] [PubMed] [Google Scholar]
- 2. Baldwin AS Jr (2001) Series introduction: the transcription factor NF‐kappaB and human disease. J Clin Invest 107:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birkedal‐Hansen H (1995) Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 7:728–735. [DOI] [PubMed] [Google Scholar]
- 4. Bjorklund M, Koivunen E (2005) Gelatinase‐mediated migration and invasion of cancer cells. Biochim Biophys Acta 1755:37–69. [DOI] [PubMed] [Google Scholar]
- 5. Brat DJ, Bellail AC, Van Meir EG (2005) The role of interleukin‐8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol 7:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown PD, Bloxidge RE, Stuart NS, Gatter KC, Carmichael J (1993) Association between expression of activated 72‐kilodalton gelatinase and tumor spread in non‐small‐cell lung carcinoma. J Natl Cancer Inst 85:574–578. [DOI] [PubMed] [Google Scholar]
- 7. Campos EI, Chin MY, Kuo WH, Li G (2004) Biological functions of the ING family tumor suppressors. Cell Mol Life Sci 61:2597–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choe G, Park JK, Jouben‐Steele L, Kremen TJ, Liau LM, Vinters HV et al (2002) Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res 8:2894–2901. [PubMed] [Google Scholar]
- 9. Coles AH, Jones SN (2009) The ING gene family in the regulation of cell growth and tumorigenesis. J Cell Physiol 218:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colla S, Tagliaferri S, Morandi F, Lunghi P, Donofrio G, Martorana D et al (2007) The new tumor‐suppressor gene inhibitor of growth family member 4 (ING4) regulates the production of proangiogenic molecules by myeloma cells and suppresses hypoxia‐inducible factor‐1 alpha (HIF‐1alpha) activity: involvement in myeloma‐induced angiogenesis. Blood 110:4464–4475. [DOI] [PubMed] [Google Scholar]
- 11. Das R, Philip S, Mahabeleshwar GH, Bulbule A, Kundu GC (2005) Osteopontin: its role in regulation of cell motility and nuclear factor kappa B‐mediated urokinase type plasminogen activator expression. IUBMB Life 57:441–447. [DOI] [PubMed] [Google Scholar]
- 12. Feng X, Hara Y, Riabowol K (2002) Different HATS of the ING1 gene family. Trends Cell Biol 12:532–538. [DOI] [PubMed] [Google Scholar]
- 13. Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E et al (2004) The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature 428:328–332. [DOI] [PubMed] [Google Scholar]
- 14. Gondi CS, Rao JS (2009) Therapeutic potential of siRNA‐mediated targeting of urokinase plasminogen activator, its receptor, and matrix metalloproteinases. Methods Mol Biol 487:267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunduz M, Magatsuka H, Demiran K, Gunduz E, Cenqiz B, Ouchida M et al (2005) Frequent deletion and down‐regulation of ING4, a candidate tumor suppressor gene at 12p13, in head and neck squamous cell carcinomas. Gene 356:109–117. [DOI] [PubMed] [Google Scholar]
- 16. Gunduz M, Gunduz E, Rivera RS, Nagatsuka H (2008) The inhibitor of growth (ING) gene family: potential role in cancer therapy. Curr Cancer Drug Targets 8:275–284. [DOI] [PubMed] [Google Scholar]
- 17. Han YP, Tuan TL, Wu H, Hughes M, Garner WLTN (2001) F‐alpha stimulates activation of pro‐MMP2 in human skin through NF‐(kappa)B mediated induction of MT1‐MMP. J Cell Sci 114:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ (2001) Blockade of NF‐kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20:4188–4197. [DOI] [PubMed] [Google Scholar]
- 19. Janicke F, Schmitt M, Ulm K, Gossner W, Graeff H (1989) Urokinase‐type plasminogen activator antigen and early relapse in breast cancer. Lancet 2:1049. [DOI] [PubMed] [Google Scholar]
- 20. Kim HJ, Hawke N, Baldwin AS (2006) NF‐kappaB and IKK as therapeutic targets in cancer. Cell Death Differ 13:738–747. [DOI] [PubMed] [Google Scholar]
- 21. Kim S, Chin K, Gray JW, Bishop JM (2004) A screen for genes that suppress loss of contact inhibition: identification of ING4 as a candidate tumor suppressor gene in human cancer. Proc Natl Acad Sci USA 101:16251–16256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH (2007) Inhibition of angiogenesis and invasion by 3,3′‐diindolylmethane is mediated by the nuclear factor‐kappaB downstream target genes MMP‐9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res 67:3310–3319. [DOI] [PubMed] [Google Scholar]
- 23. Korkolopoulou P, Levidou G, Saetta AA, Habr EI, Eftichiadis C, Demenagas P et al (2008) Expression of nuclear factor‐kappaB in human astrocytomas: relation to pI kappa Ba, vascular endothelial growth factor, Cox‐2, microvascular characteristics, and survival. Hum Pathol 39:1143–1152. [DOI] [PubMed] [Google Scholar]
- 24. Lee CH, Jeon YT, Kim SH, Song YS (2007) NF‐kappaB as a potential molecular target for cancer therapy. Biofactors 29:19–35. [DOI] [PubMed] [Google Scholar]
- 25. Lefranc F, Brotchi J, Kiss R (2005) Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol 23:2411–2422. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Martinka M, Li G (2008) Role of ING4 in human melanoma cell migration, invasion and patient survival. Carcinogenesis 29:1373–1379. [DOI] [PubMed] [Google Scholar]
- 27. Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM et al (2003) Mitogenic and antiapoptotic role of constitutive NF‐kappaB/Rel activity in pancreatic cancer. Int J Cancer 105:735–746. [DOI] [PubMed] [Google Scholar]
- 28. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mignatti P, Rifkin DB (1993) Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 73:161–195. [DOI] [PubMed] [Google Scholar]
- 30. Mott JD, Werb Z (2004) Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H et al (1999) Expression and tissue localization of membrane‐type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol 154:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Novak U, Cocks BG, Hamilton JA (1991) A labile repressor acts through the NFkB‐like binding sites of the human urokinase gene. Nucleic Acids Res 19:3389–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins ND (2004) NF‐kappaB tumor promoter or suppressor? Trends Cell Biol 14:64–69. [DOI] [PubMed] [Google Scholar]
- 34. Raithatha SA, Muzik H, Muzik H, Rewcastle NB, Johnston RN, Edwards DR et al (2000) Localization of gelatinase‐A and gelatinase‐B mRNA and protein in human gliomas. Neuro-oncol 2:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3:489–501. [DOI] [PubMed] [Google Scholar]
- 36. Raychaudhuri B, Han Y, Lu T, Vogelbaum MA (2007) Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drives invasive phenotype. J Neurooncol 85:39–47. [DOI] [PubMed] [Google Scholar]
- 37. Reuning U, Wilhelm O, Nishiguchi T, Guerrini L, Blasi F, Graeff H et al (1995) Inhibition of NF‐kappa B‐Rel A expression by antisense oligodeoxynucleotides suppresses synthesis of urokinase‐type plasminogen activator (uPA) but not its inhibitor PAI‐1. Nucleic Acids Res 23:3887–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sappino AP, Busso N, Belin D, Vassalli JD (1987) Increase of urokinase‐type plasminogen activator gene expression in human lung and breast carcinomas. Cancer Res 47: 4043–4046. [PubMed] [Google Scholar]
- 39. Sawaya R, Ramo OJ, Shi ML, Mandybur G (1991) Biological significance of tissue plasminogen activator content in brain tumors. J Neurosurg 74:480–486. [DOI] [PubMed] [Google Scholar]
- 40. Sliva D (2004) Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets 4:327–336. [DOI] [PubMed] [Google Scholar]
- 41. Talvensaari‐Mattila A, Paakko P, Turpeenniemi‐Hujanen T (2003) Matrix metalloproteinase‐2 (MMP‐2) is associated with survival in breast carcinoma. Br J Cancer 89:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Unoki M, Shen JC, Zheng ZM, Harris CC (2006) Novel splice variants of ING4 and their possible roles in the regulation of cell growth and motility. J Biol Chem 281:34677–34686. [DOI] [PubMed] [Google Scholar]
- 43. Vincenti MP, Coon CI, Brinckerhoff CE (1998) Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin‐1beta‐stimulated synovial fibroblasts. Arthritis Rheum 41:1987–1994. [DOI] [PubMed] [Google Scholar]
- 44. Wang W, Abbruzzese JL, Evans DB, Chiao PJ (1999) Overexpression of urokinase‐type plasminogen activator in pancreatic adenocarcinoma is regulated by constitutively activated RelA. Oncogene 18:4554–4563. [DOI] [PubMed] [Google Scholar]
- 45. Wu JM, Sheng H, Saxena R, Skill NJ, Bhat‐Nakshatri P, Yu M et al (2009) NF‐kappaB inhibition in human hepatocellular carcinoma and its potential as adjunct to sorafenib based therapy. Cancer Lett 278:145–155. [DOI] [PubMed] [Google Scholar]


