Abstract
Background
Symptoms of autism spectrum disorder (ASD) have been associated, in part, with the dysfunction of N‐methyl‐D‐aspartate (NMDA) glutamate receptors at excitatory synapses and glutamate abnormalities. Medications related to glutamatergic neurotransmission, such as D‐cycloserine ‐ which is a partial agonist of the NMDA glutamate receptor ‐ are potential treatment options for the core features of ASD. However, the potential effect of D‐cycloserine on the social and communication skills deficits of individuals with ASD has not been thoroughly explored and no systematic reviews of the evidence have been conducted.
Objectives
To assess the efficacy and adverse effects of D‐cycloserine compared with placebo for social and communication skills in individuals with ASD.
Search methods
In November 2020, we searched CENTRAL, MEDLINE, Embase, six other databases and two trials registers. We also searched the reference lists of relevant publications and contacted the authors of the included study, Minshawi 2016, to identify any additional studies. In addition, we contacted pharmaceutical companies, searched manufacturers' websites and sources of reports of adverse events.
Selection criteria
All randomised controlled trials (RCTs) of any duration and dose of D‐cycloserine, with or without adjunct treatment, compared to placebo in individuals with ASD.
Data collection and analysis
Two review authors independently selected studies for inclusion, extracted relevant data, assessed the risk of bias, graded the certainty of the evidence using the GRADE approach, and analysed and evaluated the data. We provide a narrative report of the findings as only one study is included in this review.
Main results
We included a single RCT (Minshawi 2016) funded by the United States Department of Defense. It was conducted at two sites in the USA: Indiana University School of Medicine and Cincinnati Children's Hospital Medical Centre. In the included study, 67 children with ASD aged between 5 and 11 years were randomised to receive either 10 weeks (10 doses) of (50 mg) D‐cycloserine plus social skills training, or placebo plus social skills training. Randomisation was carried out 1:1 between D‐cycloserine and placebo arms, and outcome measures were recorded at one‐week post‐treatment. The 'risk of bias' assessment for the included study was low for five domains and unclear for two domains.
The study (67 participants) reported low certainty evidence of little to no difference between the two groups for all outcomes measured at one week post‐treatment: social interaction impairment (mean difference (MD) 3.61 (assessed with the Social Responsiveness Scale), 95% confidence interval (CI) ‐5.60 to 12.82); social communication impairment (MD ‐1.08 (measured using the inappropriate speech subscale of the Aberrant Behavior Checklist (ABC)), 95% CI ‐2.34 to 0.18); restricted, repetitive, stereotyped patterns of behaviour (MD 0.12 (measured by the ABC stereotypy subscale), 95% CI ‐1.71 to 1.95); serious adverse events (risk ratio (RR) 1.11, 95% CI 0.94 to 1.31); non‐core symptoms of ASD (RR 0.97 (measured by the Clinical Global Impression‐Improvement scale), 95% CI 0.49 to 1.93); and tolerability of D‐cycloserine (RR 0.32 (assessed by the number of dropouts), 95% CI 0.01 to 7.68).
Authors' conclusions
We are unable to conclude with certainty whether D‐cycloserine is effective for individuals with ASD. This review included low certainty data from only one study with methodological issues and imprecision. The added value of this review compared to the included study is we assessed the risk of bias and evaluated the certainty of evidence using the GRADE approach. Moreover, if we find new trials in future updates of this review, we could potentially pool the data, which may either strengthen or decrease the evidence for our findings.
Keywords: Child; Child, Preschool; Female; Humans; Male; Autism Spectrum Disorder; Autism Spectrum Disorder/drug therapy; Communication; Cycloserine; Cycloserine/adverse effects; Cycloserine/therapeutic use; Indiana; Multicenter Studies as Topic; Ohio; Patient Dropouts; Patient Dropouts/statistics & numerical data; Placebos; Placebos/therapeutic use; Randomized Controlled Trials as Topic; Social Skills; Stereotyped Behavior; Stereotyped Behavior/drug effects
Plain language summary
Is D‐cycloserine effective and safe for social and communication skills deficits in people with ASD?
What is the aim of this review?
Autism spectrum disorder (ASD) is a relatively common disorder involving the abnormal development of the brain. It often leads to repetitive behaviours, restrictive activities, limited interests, reduced social functioning and language skills. There are no effective treatments for these features of ASD but recent research suggests that D‐cycloserine might improve social and communication skills in people with ASD. D‐cycloserine is a type of medicine used to treat tuberculosis (a contagious infection that usually affects the lungs) and schizophrenia (a serious mental disorder of thought, emotion and behaviour). We wanted to know whether D‐cycloserine, alone or in combination with other treatment, was better or worse than placebo (dummy pill) at improving social and communication skills in individuals with ASD. We also wanted to know if there were any harmful side effects from using this medication.
Key messages
There appears to be no clear difference between D‐cycloserine plus social skills training and social skills training alone on social and communication skills in individuals with ASD. However, we are uncertain about these results.
What was studied in the review?
We searched databases of scientific studies and found one relevant study to include in this review. The study took place in the USA and did not have industry funding. A total of 67 children aged 5 to 11 years were included in the study. One group took a medicine called D‐cycloserine once a week plus social skills training, and the other group took a placebo pill (dummy pill which does not include medicine) plus social skills training. The treatment lasted for 10 weeks.
What are the main results of the review?
One week post‐treatment, there was no difference in social interaction, repetitive behaviours and language skills between the D‐cycloserine and placebo groups. Compared to placebo treatment, D‐cycloserine may not increase the number of harmful side effects, the number of people dropping out of the study and treatment responsiveness.
D‐cycloserine may make little or no difference to social and communication skills deficits in individuals with ASD. These findings may change if more studies are included. We do not know the long‐term effects of D‐cycloserine due to the short duration of the study.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to November 2020.
Summary of findings
Summary of findings 1. D‐cycloserine versus placebo for the treatment of social skills deficits in people with autism spectrum disorder.
| D‐cycloserine versus placebo for the treatment of social skills deficits in people with autism spectrum disorder | ||||||
|
Patient or population: children aged 5 to 11 years with ASD Setting: academic autism treatment centres, local schools and community organisations from Indiana University School of Medicine and Cincinnati Children's Hospital Medical Centre Intervention: D‐cycloserine 50 mg weekly for 10 weeks plus social skills training Comparison: placebo weekly for 10 weeks plus social skills training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with D‐cycloserine | |||||
|
Social interaction impairment
Assessed with: Social Responsiveness Scale (SRS)
Scale range: 0 to 195 Follow‐up: 1 week after the end of treatment |
The mean change scores between baseline and 1 week post‐treatment was 17 points lower (better) in the placebo group. | The mean change in SRS score in the D‐cycloserine group was 3.61 points higher (worsening) than in placebo group (5.6 lower to 12.82 higher). | ‐ | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Positive scores reflect a greater improvement in social communication for participants in the placebo group compared to the intervention group. A negative score would reflect a greater improvement in the intervention group compared with the placebo group. |
|
Social communication impairment
Assessed with: ABC parent‐rated scores on the inappropriate speech subscale
Scale from: 0 to 12 Follow‐up: 1 week after the end of treatment |
The mean change from baseline to 1 week post‐treatment was an increase (worsening) of 0.35 points in the control group. | The mean change in ABC scores on inappropriate speech subscale in the D‐cycloserine group was 1.08 points lower (better) than in the placebo group (2.34 lower to 0.18 higher). | ‐ | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Positive scores reflect a greater improvement in inappropriate speech for participants in the placebo group compared to the intervention group. A negative score would reflect a greater improvement in the intervention group compared with the placebo group. |
|
Stereotyped patterns of behaviour and interests
Assessed with: ABC, parent‐rated scores on the stereotypy subscale
Scale from: 0 to 21 Follow‐up: 1 week after the end of treatment |
The mean change from baseline to 1 week post‐treatment was a decline (better) of 0.45 points in the control group. | The mean change in ABC scores on stereotypy subscale in the D‐cycloserine group was 0.12 points higher (worsening) than in the placebo group (1.71 lower to 1.95 higher). | ‐ | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Positive scores reflect a greater improvement in stereotyped patterns of behaviour and interests for participants in the placebo group compared to the intervention group. A negative score would reflect a greater improvement in the intervention group compared with the placebo group. |
| Total adverse events Assessed with: reports of adverse events | Study population | RR 1.11 (0.94 to 1.31) | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR > 1 means increased adverse outcomes in D‐cycloserine group | |
| 879 in 1000 | 975 in 1000 (826 to 1000) | |||||
|
Non‐core symptoms of ASD
Assessed with: Clinical Global Impression‐Improvement (CGI‐I) scale Scale from: 1 to 7 (Responders to treatment = CGI‐I score of 1 or 2, or non‐responders to treatment = CGI‐I score of 3 or higher) Follow‐up: 1 week after the end of treatment |
Study population | RR 0.97 (0.49, 1.93) | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR < 1 means reduced non‐core symptoms in D‐cycloserine group. | |
| 333 in 1000 | 324 in 1000 (145 to 570) | |||||
| Tolerability Assessed with: number of dropouts | Study population | RR 0.32 (0.01, 7.68) | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR < 1 means reduced number of dropouts in D‐cycloserine group. | |
| 30 in 1000 | 10 in 1000 (0 to 200) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: Randomised controlled trial; RR: Rate ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded by one level due to risk of bias: we rated the potential of selection bias as unclear due to the method of randomisation and allocation being unclear in the report. bWe downgraded the evidence by one level due to imprecision: small sample size.
Background
Description of the condition
'Autism' is derived from the Greek word autos, which means 'self'. The term was first used by the German psychiatrist Eugen Bleuler in 1911 to describe social withdrawal in people with schizophrenia (Moskowitz 2011). The child psychiatrist Dr Leo Kanner described infantile autism, a lifelong neurodevelopmental condition, in 1943 (Kanner 1943). However, the first two editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM) labelled it as a type of schizophrenia occurring in childhood (DSM; DSM‐II). It was not until 1980 that infantile autism was officially recognised as a separate, stand‐alone category in the DSM‐III. When the DSM‐IV was published in 1994, people could be diagnosed with four pervasive developmental disorders (PDDs): autistic disorders; Asperger’s disorder; childhood disintegrative disorder; and PDD–not otherwise specified. Recently, the DSM‐5 has subsumed all four PDDs under the umbrella term 'autism spectrum disorder' (ASD). This classification is in line with the 10th revision of the International Classification of Diseases (ICD‐10).
The definition of ASD comprises five criteria in the DSM‐5, and the ICD‐11:
social communication and social interaction impairment;
restricted, repetitive, stereotyped patterns of behaviour and interests;
symptoms must be present in the early developmental period;
symptoms cause clinically significant impairment in current functioning; and
these disturbances are not better explained by intellectual disability or global developmental delay.
Prevalence
It is estimated that one in 160 children worldwide are currently diagnosed with ASD (WHO 2018). In 2014, the prevalence of ASD in eight‐year olds in the USA was 16.8 per 1000, or 1 in 59 (Baio 2018). Brugha 2011 reported a prevalence among adults in the UK as one in 100. In a middle‐income country such as Iran, the overall prevalence of ASD was 6.26 per 10,000 for five‐year olds (Samadi 2011). By contrast, in Nepal, a low‐income country, the prevalence of ASD in children aged nine to 13 years was three in 1000 (Heys 2018). The prevalence of ASD increases to 28% in people with learning disabilities (Bryson 2008). In general, the prevalence of ASD has been increasing in recent decades, due to increased recognition, awareness and changes in diagnostic criteria for ASD (Elsabbagh 2012).
ASD is more common in males, with a male‐to‐female ratio of 3:1 reported in a recent meta‐analysis (Loomes 2017). Many girls with ASD may be undiagnosed or diagnosed late (Gould 2017). According to Gould 2017, girls tend to be more passive, and the interests of girls with ASD are similar to those of typically developing girls (for example, a special interest in animals, music, art and literature). Therefore, it can be more difficult for parents, teachers or paediatricians to identify girls who are struggling in social situations (Cola 2020).
Aetiology
ASD is a neurodevelopmental disorder, with symptoms becoming noticeable in early childhood. Genetic problems, such as mutations, syndromes, and de novo copy number variations (a genetic alteration in which specific sections of genome are deleted or duplicated and can lead to phenotypic diversity among individuals), are thought to be the underlying cause in 10% to 20% of people with the condition (Abrahams 2008). The chance of people with ASD also having significant medical conditions ranges from 0% to 16.7%; tuberous sclerosis and fragile X syndrome are the conditions most commonly associated with ASD (Lyall 2017). Neuroimaging findings of abnormalities in the brain structure (dysfunctional activation and abnormal connectivity) of people with ASD reflect the clinical diversity of the condition (Ha 2015). Advanced paternal age, birth complications associated with trauma or ischaemia and hypoxia, vitamin D deficiency, and heavy metals (mercury and lead) poisoning have shown a strong association with ASD (Modabbernia 2017). Immune abnormalities, such as maternal infection during pregnancy, maternal autoimmune diseases, immune dysfunction and gastrointestinal dysfunction, are consistently reported in ASD (Matelski 2016). A recent review found that immune activation may be a cause of ASD or may just be an epiphenomenon (a secondary effect that occurs simultaneously with a disease but is not directly related to it) (Estes 2015).
ASD is comorbid with a number of other conditions. For example, 20% to 30% of children with ASD have seizure disorders (Tuchman 2010), 14% to 78% have attention deficit hyperactivity disorder (ADHD) (Gargaro 2011), and 11% to 84% have anxiety disorders (White 2009). A systematic review that focused on outcomes for people with ASD found that children with ASD whose early language acquisition was not considerably behind those of their peers at the age of five or six have better adult outcomes than those who showed a delay in language acquisition (Magiati 2014). That review reported positive associations between these predictors and better adaptive outcomes particularly in daily living skills and possibly communication skills in adulthood (Magiati 2014).
A glutamate dysfunction has been implicated in comorbidities of ASD. For example, there is an increase in the downstream effects of glutamate signalling in people with tuberous sclerosis, of whom 20% to 40% have ASD (Rojas 2014). Blaylock 2009 theorised that the male predominance in ASD is attributable to foetal testosterone, which enhances glutamate receptor hyperactivity. Although glutamate is implicated in the pathophysiology of ASD, it is still not clear how glutamate dysfunction leads to the core symptoms of ASD (Rojas 2014). As such, glutamate, a major excitatory neurotransmitter, and its receptor, N‐methyl‐D‐aspartate (NMDA), have become targets in the treatment of ASD (Posey 2008).
Impact
Most children with ASD have reduced social functioning, cognitive ability and language skills, and increased mental health problems such as schizophrenia, obsessive compulsive disorder, depression and anxiety (Magiati 2014). Because of these problems, adolescents and young adults with ASD can face many challenges with independent living, friendship, education and job opportunities (Magiati 2014). Parents of children and adolescents with ASD have reported lower quality of life and higher levels of distress, anxiety and depression than parents of typically‐developing children (Padden 2017). In addition, parents may experience high levels of stress related to finding diagnostic and treatment services, the financial burden, and adapting family routines and daily activities (Schiltz 2018). Mothers of children with ASD have been found to exhibit higher levels of parenting stress than fathers (Pisula 2017). Parenting stress is often associated with poor mental health and emotional well‐being, which reduce involvement in social activities and worsen the negative social, emotional, academic and behavioral outcomes for both children with ASD and their parents (Schiltz 2018). For example, heightened parental stress and depression can worsen challenging behaviours in children with ASD, such as internalising (anxiety, sadness, reticence, fearfulness and oversensitivity) and externalising behaviours (aggression towards others, hyperactivity, self‐harm and conduct problems), because parents who are under stress may have difficulty in coping with the demands of parenting a child with ASD, and may not know how to respond best to challenging behaviours (Schiltz 2018).
The large social and financial burden of ASD affects the families of children with ASD, through lower levels of savings and reduced income, and is a potential burden for society as a whole (Ganz 2007). In the USA, the total annual societal costs of autism were estimated to be 35 billion US dollars (USD) for the entire cohort of individuals with autism in 2007 (Ganz 2007). In the UK, the annual cost of supporting people with ASD was estimated to be 2.7 billion pounds sterling (GBP) for children and GBP 25 billion for adults; cost calculation adjusted to 2005/2006 price levels (Knapp 2009). In the USA, the annual cost of caring for people with ASD is expected to exceed USD 461 billion by 2025 (Leigh 2015).
Description of the intervention
Pharmacological interventions have been shown to be ineffective in treating the core features of ASD (NICE 2013), and drug treatment is currently considered a short‐ to medium‐term intervention for co‐existing psychiatric or neurodevelopmental conditions in individuals with ASD (SIGN 2016). D‐cycloserine has been widely researched in neuropsychiatric studies and findings suggest it may improve the negative symptoms of schizophrenia, such as affective flattening (lack of emotional reactions), loss of drive, anhedonia (loss of interest), and alogia (loss of fluency of thought) (Heresco‐Levy 2002). D‐cycloserine acts as a dose‐dependent agonist or antagonist to the NMDA receptor, and thus its dose needs to be optimised wisely (Goff 2012). Only low‐dose D‐cycloserine (50 mg/day) is associated with an improvement in the negative symptoms of people with schizophrenia. A higher dosage (more than 250 mg/day) does not show any greater improvement than 50 mg dosage and has more side effects (Goff 2012). Similarly, low‐dose D‐cycloserine given intermittently (once a week) up to 11 times appears to be effective in the extinction of fear in anxiety disorders (Rodrigues 2014).
Research in neuropsychiatric diseases suggests that D‐cycloserine may be of use in ASD, given the similarity between the negative symptoms of people with schizophrenia and the social impairment of people with ASD (Goff 1999), as well as the glutamate dysfunction found in both disorders (McDougle 2005). Specifically, an intermittent, once‐weekly dosage of D‐cycloserine persistently improves memory consolidation and the negative symptoms of schizophrenia, whereas the efficacy of D‐cycloserine is lowered in schizophrenia with regular and repeated use, due to tachyphylaxis (receptor down‐regulation) (Goff 2008).
For over 50 years, D‐cycloserine has been approved by the US Food and Drug Administration for use as a second‐line drug for the treatment of advanced tuberculosis, at a dose of up to 1 g (Schade 2016). The most common adverse effects of D‐cycloserine occur at doses higher than 500 mg (Schade 2016). With high‐dose D‐cycloserine, the reported rate of neurological adverse effects (commonly, seizure and neuropathy) is 1.1%, whereas the proportion of psychiatric adverse effects (notably psychosis, depression, risk of suicide) is higher, at 5.7% (Hwang 2013). Pyridoxine deficiency may also occur during treatment with D‐cycloserine, since D‐cycloserine is a pyridoxine antagonist and causes increased renal excretion of pyridoxine. D‐cycloserine is safe at low doses because, at low doses, the side effects are minimal or almost negligible (Schade 2016).
How the intervention might work
The specific mechanism by which D‐cycloserine may improve social and communication problems in ASD is not clear. D‐cycloserine is a partial agonist of the NMDA glutamate receptor with subunits known as NMDA Receptor 1 (NR1) and NMDA Receptor 2 (NR2) (A, B, C, D), which are activated by glycine and glutamate, respectively (Sheinin 2001). In addition, D‐cycloserine has partial agonistic action at NR1/NR2C receptors at low doses, and antagonistic action on NR1/NR2A and NR1/NR2B receptors at high doses (Danysz 1998).
NMDA receptors are mainly found in the frontal cortex and hippocampus, and are responsible for sociability. Diminished NMDA receptor–mediated signalling activity is associated with impaired sociability (Crino 2011). Blood glutamate levels are higher in people with ASD than in neurotypical people (Zheng 2016), and dysfunction of NMDA receptors at excitatory synapses has been found in people with ASD (Zhou 2013). As such, low‐dose D‐cycloserine, as an NMDA receptor agonist, has been used in the treatment of ASD for the improvement of social and communication skills.
Why it is important to do this review
ASD is one of the most common neurodevelopmental disorders, which can significantly reduce quality of life for both the person and his or her family. At present, there are no first‐line, evidence‐based pharmacological treatments recommended for the core features of ASD. However, some medications are used for associated characteristics; for example, behaviours of concern, hyperactivity (Sturman 2017), and anxiety or depression (Hurwitz 2012; Williams 2013).
There is some suggestion that D‐cycloserine may be an effective treatment for people with ASD. However, the effectiveness of the drug has not been thoroughly explored and there are currently no systematic reviews of the evidence. At present, any decisions about dosage and schedule of treatment rest on assumptions that what works in schizophrenia might work in ASD, because of some symptom similarities (Goff 2012). This review will, for the first time, collate all randomised controlled trial (RCT) evidence of the effect of D‐cycloserine compared to placebo for people with ASD. We will also explore the effectiveness of different dosages and drug administration frequencies.
Objectives
To assess the efficacy and adverse effects of D‐cycloserine compared with placebo for social and communication skills in individuals with ASD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials, including both parallel‐group and cross‐over designs, of any duration and dose of D‐cycloserine, with or without adjunct treatment, compared to placebo in individuals with ASD.
Types of participants
Individuals of any age diagnosed with ASD according to standard diagnostic classification systems, including: DSM‐III; DSM‐IV; DSM‐IV‐TR; DSM‐5; ICD‐10; ICD‐11; Autism Diagnostic Interview‐Revised (Lord 1994), and the Autism Diagnostic Observation Schedule, Second Edition (ADOS‐2) (Maddox 2017).
We intended to include participants with any intelligence quotient (IQ), comorbid disorders (e.g. anxiety, depression), and pharmacological co‐interventions (e.g. antipsychotics, psychostimulants, antidepressants or mood stabilisers) with a stable dosage for a minimum of two weeks before randomisation. We also included non‐pharmacological co‐interventions such as behavioural therapy, speech therapy, occupational therapy and dietary modifications. However, we excluded participants with more than two psychotropic medications and glutamatergic modulators (e.g. riluzole, memantine, etc.). We also excluded participants with social and communication problems due to other established causes such as acquired brain injury or neurodegenerative diseases. We planned to exclude studies that included initial treatment with a different medication because of potential carry‐over effects (Evans 2010).
Types of interventions
D‐cycloserine for the treatment of ASD, irrespective of dose or duration and with or without adjunct treatment, versus placebo.
Types of outcome measures
Primary outcomes
Social communication and social interaction impairment, measured by validated instruments, such as the Social Responsiveness Scale (SRS) (Constantino 2005), Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II) (Sparrow 2005), and other valid scales
Restricted, repetitive, stereotyped patterns of behaviour and interests, assessed by valid scales, such as the Aberrant Behaviour Checklist (ABC) for stereotypies (Aman 1985), and other validated instruments
Adverse events associated with D‐cycloserine, assessed by the number of non‐serious adverse events (headache, vomiting, cold, cough, restlessness, sadness, sedation, etc.) and serious adverse events (suicidal ideation etc.)
Secondary outcomes
Non‐core symptoms of ASD (e.g. anxiety, depression, parental stress level, sleep disorders, etc.), assessed using validated instruments, such as the Clinical Global Impressions ‐ Improvement (CGI‐I) scale (Busner 2007), and other validated scales
Tolerability of D‐cycloserine (adherence to treatment and follow‐up), assessed by the number of participants dropping out from studies, as well as the timing and reason for exclusion from studies, before study endpoints
Search methods for identification of studies
Electronic searches
We searched the following electronic databases and trials registers in November 2019, and ran top‐up searches in November 2020. We did not limit the searches by date, language or publication type. Search details, including search strategies for each source, are reported in Appendix 1.
Cochrane Central Register of Controlled trials (CENTRAL; 2020, Issue 11) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 17 November 2020).
MEDLINE Ovid (1946 to November Week 1 2020).
MEDLINE In‐Process and Other Non‐indexed Citations Ovid (1946 to 16 November 2020).
MEDLINE EPub Ahead of Print Ovid (16 November 2020; searched 17 November 2020).
Embase Ovid (1974 to 16 November 2020; searched 17 November 2020).
APA PsycINFO Ovid (1967 to November Week 2 2020).
Conference Proceedings Citation Index – Science Web of Science, Clarivate (1990 to 17 November 2020).
Conference Proceedings Citation Index – Social Sciences and Humanities Web of Science, Clarivate (1990 to 17 November 2020).
Cochrane Database of Systematic Reviews (CDSR; 2020, Issue 11), part of the Cochrane Library (searched 17 November 2020).
Database of Abstracts of Reviews of Effects (DARE final issue; www.crd.york.ac.uk/CRDWeb; searched 12 November 2019).
Epistemonikos (www.epistemonikos.org; searched 18 November 2020).
ClinicalTrials.gov (www.clinicaltrials.gov; searched 18 November 2020).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 12 November 2019. Database did not respond in November 2020);
WorldCat (limited to dissertations and theses) (www.worldcat.org; searched 12 November 2019; replaced in 2020 by ProQuest Dissertations & Theses Global).
ProQuest Dissertations & Theses Global (all available years searched 18 November 2020).
Searching other resources
We checked the bibliographies of all included studies for additional references to potentially relevant studies. We contacted experts working in the field, as well as the drug companies (Lilly Corporate Center, Indianapolis, Indiana, USA) that make D‐cycloserine, to determine whether there were any ongoing or unpublished trials in this area. We searched manufacturers’ websites for trial information, and also the following specialised sources of adverse events reports: Australian Adverse Drug Reactions Bulletin (tga.gov.au/publication/australian-adverse-drug-reactions-bulletin); Current Problems in Pharmacovigilance (www.mhra.gov.uk); European Medicines Evaluation Agency (www.emea.eu); and the US Food and Drug Administration (FDA) Medwatch (www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program). On 17 November 2020, we searched MEDLINE, Embase and Retraction Watch (retractionwatch.com) to identify retractions, errors or corrections of the included study. See Potential biases in the review process section.
Data collection and analysis
We were unable to use all of our preplanned methods (Aye 2019), due to only one RCT being included in the review. These methods have been archived for use in future updates of this review in Table 2, pending the inclusion of more studies.
1. Unused methods.
| Method | Approach |
| Types of outcome measures | In keeping with time frames of other ASD intervention reviews (e.g. Williams 2013), we would have assessed outcomes at the following time points: short‐term (up to three months); medium‐term (three to 12 months); and long term (over 12 months). |
| Measures of treatment effects |
Dichotomous data We planned to calculate the number needed to treat for an additional beneficial or harmful outcome (NNTB or NNTH); that is, the number of participants needed to obtain a beneficial or harmful outcome with the intervention, using the pooled RR. |
|
Continuous data We planned to report continuous data as either mean differences (MD), when studies assessed the same outcome using the same scale, or standardised mean differences (SMD) when studies used different scales to measure the same outcome, along with their 95% confidence intervals. For the SMD, we planned to interpret the effect sizes using Cohen's 'rule of thumb', i.e. 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988). | |
| Unit of analysis issues |
Cross‐over trials For cross‐over trials, we believe that there might have be a carry‐over effect of D‐cycloserine that outlasts any washout period, thereby making it impossible to gain 'clean' results unaffected by the first period (Attari 2014). Hence, we had planned to include data from the first period only. |
|
Studies with different doses of D‐cycloserine Had we identified studies assessing different doses of D‐cycloserine in one trial, we would have combined all dose groups and compared them with the results of the placebo group, making single pair‐wise comparisons; for dichotomous outcomes, we would have summed the sample sizes and the numbers of people with events across groups, whereas for continuous outcomes, we would have combined the means and standard deviations using the formula described in Section 6.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). If primary studies had reported the results as a single value for the placebo arm (e.g. in a trial of D‐cycloserine with low‐dose, high‐dose and placebo arms, where the outcomes for the placebo group were reported as one whole value), we would have split the data proportionately according to the number of dosage groups, to avoid double counting the participants. For dichotomous outcomes, we would have divided both the number of participants and the number of events by the number of dosage groups in the study. For continuous outcomes, we would have divided the total number of participants only, leaving the means and standard deviations unchanged. | |
| Assessment of heterogeneity | If we had included at least two studies, we would have assessed clinical, methodological and statistical heterogeneity. For clinical heterogeneity, we would have compared the distribution of participant characteristics between studies (e.g. age, gender), whereas for methodological heterogeneity, we would have compared trial characteristics such as randomisation, allocation concealment, blinding, and loss to follow‐up. For statistical heterogeneity, we would have visually identified the horizontal lines representing each trial on the forest plot for overlapping CIs. We would have used the Chi2 test for homogeneity (interpreting a P value less than 0.10 as heterogeneity) and the I2 statistic to quantify the degree of heterogeneity. We would have interpreted the I2 values using the following thresholds:
As these thresholds can be misleading, when considering the importance of I2, we would have taken into account the magnitude and direction of effects, and strength of evidence for heterogeneity based on the P value from the Chi2 test (Deeks 2019). Had the I2 value exceeded 50%, we would have used a random‐effects model for meta‐analysis. We would have reported Tau2, a measure of between‐study variance, when reporting results from the random‐effects model (Data synthesis). Had we considered the studies to be too clinically heterogeneous, we would not have conducted a primary meta‐analysis but provided a narrative synthesis of the results instead (Data synthesis). We would have explored the possible sources of heterogeneity by conducting prespecified subgroup analyses (Subgroup analysis and investigation of heterogeneity). |
| Assessment of reporting biases | Had we been able to pool more than 10 studies in a meta‐analysis, we would have drawn a funnel plot and examined it for any asymmetry, which might have been due to the following reasons stated in Section 13.3.5.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Page 2019).
While funnel plots might be useful in investigating reporting biases, there is some concern that tests for funnel plot asymmetry (e.g. Egger's regression test; Egger 1997) have limited power to detect small‐study effects, particularly when there are fewer than 10 studies or where all studies are of similar sample size. We would have consulted with a statistician before implementing such tests (Page 2019). |
| Data synthesis | Had we been able to identify two or more studies with sufficient clinical and methodological homogeneity, we would have pooled the data in a meta‐analysis using a fixed‐effect model in Review Manager 5 (RevMan Web 2020). |
| Subgroup analysis and investigation of heterogeneity | We would have carried out subgroup analyses if a sufficient number of trials had reported the following factors.
|
| Sensitivity analysis | We would have performed sensitivity analyses by repeating the meta‐analyses after excluding trials with:
We would have conducted additional sensitivity analyses if we had identified other issues that might have impeded our confidence in the estimated pooled effect sizes such as source of recruitment (population or clinical), non‐compliance or protocol violation, different definitions for the outcomes, outliers or baseline imbalance across the intervention arms in primary studies. Had we found substantial differences in effect estimates in any of the sensitivity analyses, we would have presented the results separately; that is, we would not have presented the pooled data. |
ADHD: attention deficit hyperactivity disorder ASD: autism spectrum disorder CI: confidence interval
Selection of studies
Two review authors (SZA; HN) independently screened titles and abstracts using Covidence. Next, we retrieved the full‐text reports of all potentially relevant records and assessed them against the inclusion criteria (Criteria for considering studies for this review). Then, we categorised them as included studies, excluded studies, studies awaiting classification, or ongoing studies (Higgins 2017). We resolved any disagreements through discussion or, if required, by consulting a third review author (HHS).
We recorded the reasons for excluding ineligible studies that appeared relevant in the second stage of screening (Characteristics of excluded studies tables).
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
Data extraction and management
Using a pre‐designed, piloted, standardised data collection form, two review authors (SZA, HN) independently extracted the following information from the one included study.
Methods: study design; total duration of study; number of study centres and locations; study settings; withdrawals; date of study.
Participants: number; mean age; age range; gender; severity of condition; diagnostic criteria; inclusion and exclusion criteria.
Interventions: intervention; comparator; concomitant medications; excluded medications.
Outcomes: primary and secondary outcomes, with reported time points.
Two review authors (SZA; HN) compared the extracted data for accuracy, resolving any discrepancies through discussion, or by involving a third review author (HHS). One review author (QZ) transferred data into Review Manager 5.4 (Review Manager 2020) and a second review author (HHS) checked the data entry for accuracy (RevMan Web 2020).
Assessment of risk of bias in included studies
Following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), two review authors (SZA, HHS) independently assessed the risk of bias in the included study across each of the following seven domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other bias. We considered blinding of outcome assessment separately for different outcomes, where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality might be very different to that for participant‐reported stress levels). We assigned a rating of low, high or unclear (uncertain) risk of bias for each domain using the criteria set out in Appendix 2, and provided a quote from the study report together with an explanation for the judgment in the 'Risk of bias' table. We resolved any disagreements through discussion or by involving a third review author (STM). When we considered the risk of bias to be unclear due to insufficient information, we contacted the study authors for additional detail and recorded this in the 'Risk of bias' table.
Measures of treatment effect
Dichotomous data
We report dichotomous outcome data as risk ratios (RR) and present these with 95% confidence intervals (CIs).
Continuous data
As we have included only one study, we report mean difference (MD) with 95% CIs.
Unit of analysis issues
We did not encounter any unit of analysis issues.
Dealing with missing data
We contacted the study authors and requested them to supply any missing numerical outcome data, or to provide an explanation about why they were missing and not available in the study report. If data were made available to us, we included them in the analyses. If not, we reported our attempts to obtain the missing outcome data and its potential impact on the findings of the review in the Discussion section. We did not impute the missing data, and we only analysed the available data, to avoid any potential artificial inflation of the precision of the effect estimate (Higgins 2019).
Assessment of heterogeneity
We could not test for heterogeneity in this review due to only one RCT being included in the analysis.
Assessment of reporting biases
We obtained the protocol of the included study, to compare the outcomes reported in the protocol with those reported in the published paper. We made explicit judgements about whether the included study was at high risk of reporting bias, according to the criteria for selective outcome reporting set out in Appendix 2.
Data synthesis
We were unable to perform a meta‐analysis in this review due to only one RCT being included. Consequently, we provide a narrative summary of the results, as presented in the original study report, in the Effects of interventions section. To illustrate the findings, we entered the data into RevMan 5.4 (RevMan Web 2020) and generated forest plots. We present the data using a fixed‐effect model with inverse‐variance weighting because we included continuous data from only one study that had assessed different outcomes from the same participants.
Subgroup analysis and investigation of heterogeneity
We could not carry out subgroup analyses in this review due to only one RCT being included in the analysis.
Sensitivity analysis
We could not perform sensitivity analysis in this review due to only one RCT being included in the analysis.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using GRADEpro GDT software for the comparison: D‐cycloserine plus social skills training group versus placebo plus social skills training group. We included in this table all primary and secondary outcomes, assessed at one week post‐treatment follow‐up:
social communication and social interaction impairment;
restricted, repetitive, stereotyped patterns of behaviours and interests;
adverse events;
non‐core symptoms of ASD; and
tolerability of D‐cycloserine.
Following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017; Schünemann 2019), two review authors (SZA; HN) independently evaluated the certainty of the evidence for each outcome as high, moderate, low or very low, according to the five GRADE criteria: study limitations, consistency of effect, imprecision, indirectness, and publication bias. High certainty of evidence reflected that we are very confident that the true effect is close to the estimated effect, and low certainty of evidence indicated that we have very little confidence in the effect we found. We resolved any conflicts in certainty of evidence by consulting a third review author (QZ). We report these ratings in the table, along with our decisions for downgrading the certainty of the evidence, if relevant. We also include comments to facilitate better understanding of our review by readers.
Results
Description of studies
See Characteristics of included studies table, Characteristics of excluded studies table, and Characteristics of ongoing studies table for details.
Results of the search
We ran searches for this review up to November 2020. We found a total of 688 records and discarded 177 duplicates. We screened the remaining 511 titles and abstracts in Covidence and excluded 501 irrelevant records (Figure 1). We obtained the full‐text reports of the remaining 10 records for eligibility assessment. Of these, we excluded three studies (six reports) and reported the reasons for their exclusion in the Characteristics of excluded studies tables. We identified one ongoing study in which recruitment of participants was completed but results were not yet available (Characteristics of ongoing studies table). Finally, we identified one randomised, placebo‐controlled trial (three reports) that was eligible for inclusion according to our selection criteria (Included studies).
1.
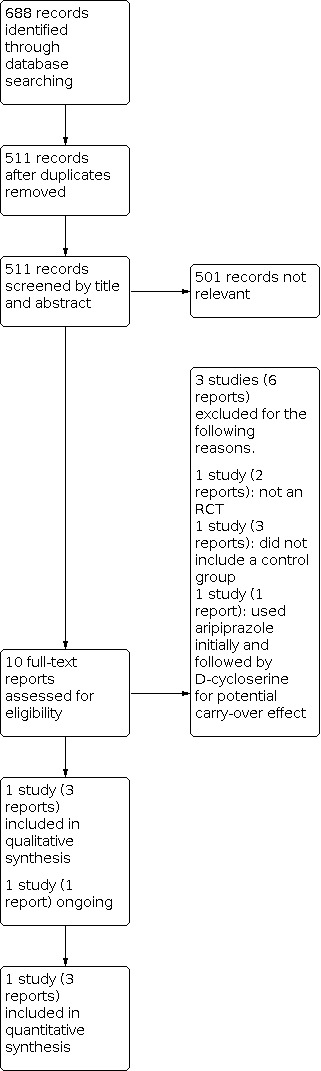
Study flow diagram.
Included studies
We identified two publications and one trial registry record of one randomised controlled trial in this review (Minshawi 2016). This clinical trial reported ASD core symptoms at one week post‐treatment and 12 weeks post‐treatment (see Characteristics of included studies table).
Study design
The included trial, Minshawi 2016, was of parallel design and compared D‐cycloserine plus social skills training with placebo plus social skills training; randomisation was carried out 1:1 between D‐cycloserine and placebo arms.
Location and funding
The included study, Minshawi 2016, was carried out in the USA and was funded by the United States Department of Defense. The participants were recruited from academic autism treatment centres, local schools and community organisations of the two sites (University of Cincinnati and Indiana University).
Participants
The trial included 67 children with ASD (Minshawi 2016). The age of the children ranged from 5 to 11 years, with 55 males and 12 females. All 67 children completed the one‐week post‐treatment assessment and 60 completed assessments at 12 weeks post‐treatment.
All the children were diagnosed with ASD by clinical assessment, based on diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR), and corroborated by administration of the Autism Diagnostic Observation Schedule (ADOS) (Lord 2000) and the Autism Diagnostic Interview‐Revised (ADI‐R) (Lord 1994).
In order to be eligible for inclusion, the children also had to exhibit an intellectual quotient of more than 70 on the Stanford‐Binet Intelligence Scales, Fifth Edition, and a standard score of more than 70 on the Communication domain of the Vineland Adaptive Behavioural Scale, Second Edition (VABS‐II). These criteria were used to ensure that the children had the minimum cognitive or language skills for group social skills training.
Interventions
Treatment took place over 10 weeks, with children receiving either one dose of D‐cycloserine (50 mg) or one dose of placebo per week, 30 minutes prior to a weekly, group social skills training using a curriculum that included Applied Behaviour Analysis (ABA) methodologies (Baer 1968). Social skills sessions focused on shaping, incidental teaching, positive reinforcement, visual schedules, social stories, and weekly, parent‐mediated homework assignments.
Concurrent psychotropic medication use was permitted if the children were required to be treated for symptoms associated with ASD (e.g. insomnia, inattention, hyperactivity, anxiety, irritability). However, the children had to have been on stable doses for a minimum of two weeks (four weeks for fluoxetine) before randomisation. In addition, concomitant psychotropic treatment had to remain stable throughout the treatment intervention and the follow‐up period (Minshawi 2016), for the durability of treatment effect analysis.
Outcomes
The included study reported four different standardised primary outcomes for the core features of ASD and adverse effects at one week post‐treatment and 12 weeks post‐treatment. The first primary outcome for Minshawi 2016 was social interaction impairment measured by: Social Responsiveness Scale (SRS); Aberrant Behavior Checklist (ABC); Triad Social Skills Assessment (TSSA); and Vineland Adaptive Behaviour Scales, Second Edition (VABS‐II). Minshawi 2016 used the Aberrant Behavior Checklist (ABC) and Vineland Adaptive Behaviour Scales, Second Edition (VABS‐II) to assess the outcome of social communication impairment in children with ASD. The primary outcome for restricted, repetitive, stereotyped patterns of behaviour and interests was measured by Aberrant Behaviour Checklist (ABC) stereotypic behaviour subscale. According to the stated primary outcomes of our review, we selected the SRS and ABC as our main primary outcomes measures assessed at one week post‐treatment (Characteristics of included studies).
In Minshawi 2016, the site physician recognised and recorded adverse events in a log, along with the date of onset and resolution, severity and relationship to study intervention (e.g. definite, probable, possible, remote or none).
In order to measure the secondary outcome of treatment, non‐core symptoms of ASD, Minshawi 2016 used the Clinical Global Impression‐Improvement (CGI‐I) at one week post‐treatment follow‐up. Individuals with a CGI‐I score of 1 or 2 were considered as having a significant improvement in non‐core symptoms of ASD while those with a CGI‐I score of 3 or higher were not considered to have had a significant improvement. The last secondary outcome of tolerability to D‐cycloserine was measured by the number of dropouts in both treatment and placebo groups.
Excluded studies
We excluded three studies (six reports) after full‐text review. We excluded one study (two reports) because it was a pilot study and not an RCT (Posey 2004). We excluded another study (three reports) because it did not include the control group (Urbano 2014). We also excluded NCT00198107 because the study participants received 24 weeks of initial treatment with aripiprazole, immediately followed by eight weeks subsequent treatment with D‐cycloserine. There could be a potential carry‐over effect on D‐cycloserine. See the Characteristics of excluded studies table.
Ongoing studies
One study, NCT00198120, is an ongoing RCT entitled 'Safety and effectiveness of D‐cycloserine in children with autism'. It is being conducted at the Indiana University School of Medicine, USA, with 80 participants with ASD aged 3 to 12 years. This study compared eight weeks of treatment with D‐cycloserine with placebo in children with ASD. The outcome measures used were the lethargy subscale of the Aberrant Behavior Checklist (ABC) and the Clinical Global Impression‐Improvement (CGI‐I) scale. The results of the study are not yet available and the trial author did not respond to our requests for further information. For further details, please see the Characteristics of ongoing studies table.
Risk of bias in included studies
We judged the risk of bias for the included trial across the seven domains of the Cochrane 'risk of bias' assessment tools (Higgins 2017). We stated the detailed assessment methods for the risk of bias in our protocol (Aye 2019) and Appendix 2. An overview of the risk of bias is summarised below and in a graph (Figure 2). Further details can be found in the 'Risk of bias' table beneath the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study, Minshawi 2016, did not clearly disclose the method of randomisation. It was reported as "randomly assigned the participants to two groups" but no further details were provided about how the randomisation process was executed. In addition, no details were available on method of allocation concealment after contacting the trial investigators for additional details. Thus, we rated the study at unclear risk of bias for random sequence generation and allocation concealment.
Blinding
Participants and caregivers remained blinded to study group assignment and treatment for the included study. We judged performance bias to be at low risk because it was clearly stated as triple blind in the trial register, www.ClinicalTrials.gov, before full publication.
Outcome assessors were unaware of the treatment allocation until after 12 weeks post‐treatment follow‐up visits and the data set was locked. We judged the potential for detection bias to be low.
Incomplete outcome data
We considered Minshawi 2016 to be at low risk of attrition bias. Minshawi 2016 reported outcome data for at least 98% of randomised children at the one week post‐treatment time point, with only one child dropping out before treatment. At the 12 week post‐intervention assessment, 60 children completed the assessments. Two children were lost from the treatment group and six from the placebo group. The reasons for these dropouts were stated as follows: five children were lost during follow‐up, one was excluded due to schedule conflict, one child discontinued due to increased irritability, and one moved to another area. We judged Minshawi 2016 to be at low risk of attrition bias because the investigators provided sufficient information for loss of participants.
Selective reporting
The study protocol was available online at www.ClinicalTrials.gov (NCT01086475). Across all of the publications for this study, data were provided for all outcomes listed on the trial register. We rated the included study at low risk of selective reporting bias as the outcome reports were comprehensive.
Other potential sources of bias
We identified no additional sources of bias and thus rated the study at low risk of bias on this domain.
Effects of interventions
See: Table 1
See Table 1 for the comparison: D‐cycloserine plus social skills training versus placebo plus social skills training for individuals with ASD. We extracted change from baseline to one week post‐treatment data from the included study.
For this review, we were not able to perform a meta‐analysis as only one study was included. In Minshawi 2016, 67 children completed the study and were assessed for the outcomes at one week post‐intervention. Of these, 60 children also completed assessments at 12 weeks post‐intervention, which was reported in a secondary publication of Minshawi 2016.
D‐cycloserine plus social skills training versus placebo plus social skills training
Primary outcomes
Social communication and interaction impairment: social interaction
Social Responsiveness Scale (SRS), parent‐rated total scores
One week post‐treatment
Minshawi 2016 reported change from baseline to one week post‐treatment scores on the parent‐rated Social Responsiveness Scale (SRS) (range = 0 to 195) using the items that measure the core features of ASD, including social awareness, social cognition, social communication, social motivation and autistic mannerisms. In Minshawi 2016, SRS scores suggested that D‐cycloserine plus social skills training (SST) resulted in little or no difference in the SRS scores compared to placebo plus SST (MD 3.61, 95% CI ‐5.60 to 12.82; 67 participants; low certainty evidence; Analysis 1.1). A positive score would reflect a greater improvement in social communication for participants in the placebo group compared to the intervention group, whereas a negative score would reflect a greater improvement in the intervention group compared to the placebo group.
1.1. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 1: Social interaction impairment measured by Social Responsiveness Scale (SRS) parent total score at one week post‐treatment
12 weeks post‐treatment
Compared to the placebo plus SST group, the D‐cycloserine plus SST group reported a large increase in SRS total scores. This suggested that children who have received active treatment with D‐cycloserine were more likely to show sustained benefits of SST at 12 weeks post‐treatment (MD ‐6.40, 95% CI ‐10.18 to ‐2.62; 60 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 2: Social interaction impairment measured by Social Responsiveness Scale (SRS) parent total score at 12 weeks post‐treatment
Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II), parent‐rated scores for the socialisation subscale
One week post‐treatment
VABS‐II parent‐rated scores for the socialisation subscale (range = 0 to 198 points) were reported at one week post‐treatment and not reported at 12 weeks post‐treatment. The study reported little or no difference in parent‐rated scores on the socialisation subscale of the VABS‐II between the D‐cycloserine plus SST group and the placebo plus SST group at one week post‐treatment (MD 0.24, 95% CI ‐9.91 to 10.39; 67 participants; Analysis 1.3). A positive VABS‐II score would reflect greater improvement in socialisation skills for participants in the intervention group compared to the placebo group, whereas a negative score would reflect a greater improvement in the placebo group compared to the intervention group.
1.3. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 3: Social interaction impairment measured by Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II) parent scores for socialization subscale
Triad Social Skills Assessment (TSSA), parent‐rated scores for social interaction
One week post‐treatment
TSSA parent‐rated scores (range = 4 to 140) for social interaction were reported at one week post‐treatment and not reported at 12 weeks post‐treatment. The study authors of Minshawi 2016 reported little or no difference in parent‐rated TSSA scores for social interaction between the D‐cycloserine plus SST group and the placebo plus SST group at one week post‐treatment (MD ‐1.95, 95% CI ‐5.46 to 1.56; 67 participants; Analysis 1.4). A positive TSSA score would reflect greater improvement in social interaction for participants in the intervention group compared to the placebo group, whereas a negative score would reflect a greater improvement in the placebo group compared to the intervention group.
1.4. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 4: Social interaction impairment measured by Triad Social Skills Assessment (TSSA) parent score
Social communication and interaction impairment: social communication
Aberrant Behavior Checklist (ABC), parent‐rated scores for the inappropriate speech subscale
One week post‐treatment
ABC parent‐rated scores (range = 0 to 12) for inappropriate speech were reported at one week post‐treatment and not reported at 12 weeks post‐treatment. In Minshawi 2016, ABC parent‐rated scores for inappropriate speech at one week post‐treatment suggested little or no difference between the D‐cycloserine plus SST group and the placebo plus SST group (MD ‐1.08, 95% CI ‐2.34 to 0.18; 67 participants; low certainty evidence; Analysis 1.5). A positive ABC parent‐rated score for the inappropriate speech subscale would reflect a greater improvement in inappropriate speech for participants in the placebo group compared to the intervention group, whereas a negative score would reflect a greater improvement in the intervention group compared to the placebo group.
1.5. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 5: Social communication impairment measured by Aberrant Behaviour Checklist (ABC) parent score ‐ inappropriate speech subscale
Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II), parent‐rated scores for the communication subscale
One week post‐treatment
Minshawi 2016 used parent‐rated scores (range = 0 to 194) on the Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II) communication subscale to rate overall change in communication skills of children with ASD at one week post‐treatment assessment. There was little or no difference in the efficacy index of this scale between the D‐cycloserine plus SST group and the placebo plus SST group (MD ‐3.14, 95% CI ‐7.85 to 1.57; 67 participants; Analysis 1.6). A positive VABS‐II score would reflect a greater improvement in communication skills for participants in the intervention group compared to the placebo group, whereas a negative score would reflect a greater improvement in the placebo group compared to the intervention group.
1.6. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 6: Social communication impairment measured by Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II) parent scores for communication subscale
Restricted, repetitive, stereotyped patterns of behaviour and interests
Aberrant Behavior Checklist (ABC), parent‐rated scores for the stereotypy subscale
One week post‐treatment
Minshawi 2016 used the parent‐rated scores (range = 0 to 21) on the Aberrant Behavior Checklist (ABC) stereotypy subscale. The analysis showed little or no difference in stereotypy symptoms for the D‐cycloserine plus SST group as opposed to the placebo plus SST group (MD 0.12, 95% CI ‐1.71 to 1.95; 67 participants; low certainty evidence; Analysis 1.7). Higher ABC scores reflected severe problem in stereotyped patterns of behaviours whereas negative scores reflected improvement of the stereotyped behaviour. A positive ABC parent‐rated score for the stereotypy subscale would reflect a greater improvement in stereotyped behaviour for participants in the placebo group compared to the intervention group, whereas a negative score would reflect a greater improvement in the intervention group compared to the placebo group.
1.7. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 7: Stereotype patterns of behaviour and interests measured by Aberrant Behaviour Checklist (ABC) parent score ‐ stereotypy subscale
Adverse events
One week post‐treatment
The site physician monitored adverse events at all visits and recorded them in a log that included the date of onset, date of resolution, severity and relationship to study intervention (e.g. definite, probable, possible, remote, or none). Only one serious adverse event (a suicidal comment at school when angry) was reported in the placebo plus SST training group. No serious adverse events were reported in the D‐cycloserine plus SST group. There was little or no difference in non‐serious adverse events (headache, vomiting, cold, cough, increased motor activity, sleep problems, aggression, restlessness, irritability, sadness, sedation, etc.) between the D‐cycloserine plus SST group and the placebo plus SST group (RR 1.11, 95% CI 0.94 to 1.31; 67 participants; low certainty evidence; Analysis 1.8). The number needed to treat for an additional harmful outcome was 11 (95% CI ‐20 to 4).
1.8. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 8: Total adverse events measured by reports of adverse events
Secondary outcomes
Non‐core symptoms of ASD
Clinical Global Impression‐Severity (CGI‐S) and Clinical Global Impression‐Improvement (CGI‐I) clinician‐rated scores for treatment response
One week post‐treatment
In Minshawi 2016, the trained clinician blinded to treatment used the CGI‐S at baseline to rate symptom severity and the CGI‐I at each follow‐up visit to assess treatment response and efficacy of D‐cycloserine in children with ASD. Treatment responders were those with a CGI‐I score of 1 or 2, while non‐responders to treatment were those with a CGI‐I score of 3 or higher. At one week post‐treatment, 33.3% of the D‐cycloserine plus SST group were responders to treatment compared to 32.3% in the placebo plus SST group. There was little or no difference in responder analysis of CGI‐I, which assessed the therapeutic effect of D‐cycloserine (RR 0.97, 95% CI 0.49 to 1.93; 67 participants; low certainty evidence; Analysis 1.9). The number needed to treat for an additional beneficial outcome (NNTB) was 100 (95% CI ‐5 to 4).
1.9. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 9: Responders to treatment assessed with Clinical Global Impression‐Improvement (CGI‐I) scale
Tolerability of D‐cycloserine
One week post‐treatment
In Minshawi 2016, 67 children completed the course of treatment and the assessments at one week post‐treatment. Only one child dropped out before treatment began in the placebo plus SST arm while none of the children were lost in the D‐cycloserine plus SST group. There was little or no difference in tolerability of D‐cycloserine between the two groups at one week post‐treatment assessment (RR 0.32, 95% CI 0.01 to 7.68; 67 participants; low certainty evidence; Analysis 1.10). The NNTB was 33 (95% CI ‐20 to 9).
1.10. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 10: Tolerability of D‐cycloserine assessed with numbers of dropouts at one week post‐treatment
12 weeks post‐treatment
Sixty out of 67 children completed the 12 weeks post‐treatment assessment in the second phase. Two children were lost to follow‐up in the D‐cycloserine plus SST group. Of the six children lost in the placebo plus SST group, one child was lost due to increased irritability, three were lost during follow‐up, one due to schedule conflict, and one moved to another area. There was little or no difference in tolerability of D‐cycloserine between the two groups at 12 weeks post‐treatment assessment (Minshawi 2016) (RR 0.29, 95% CI 0.06 to 1.33; 60 participants; Analysis 1.11). The NNTB was 3 (95% CI 1 to 17).
1.11. Analysis.

Comparison 1: D‐cycloserine versus placebo, Outcome 11: Tolerability of D‐cycloserine assessed with numbers of dropouts at 12 weeks post‐treatment
Discussion
Summary of main results
We found only one completed trial, which precluded performing a meta‐analysis. The included study reported the benefits and harms of D‐cycloserine in the treatment of ASD (Minshawi 2016). It was conducted in the USA with a total of 67 children from 5 to 11 years old. Minshawi 2016 compared D‐cycloserine plus social skills training to placebo plus social skills training in children with ASD. D‐cycloserine was given to the children before social skills training ‐ with the hypothesis being that it may boost the effects of this training ‐ rather than being used as a standalone intervention in individuals with ASD. The duration of treatment was 10 weeks. At short‐term follow‐up (one week post‐intervention), we found no clear evidence that D‐cycloserine plus social skills training might improve the primary outcomes of social interaction, communication and stereotyped patterns of behaviour, although we rated the certainty of the evidence as low and so we are uncertain as to whether these findings reflect the true effect. Although no serious adverse event was reported in the D‐cycloserine plus social skills training group, one serious adverse event (a suicidal comment at school when angry) was reported in the placebo plus social skills training group. However, there was little or no difference in non‐serious adverse events such as headache, vomiting, cold, cough, increased motor activity, sleep problems, aggression, restlessness, irritability, sadness or sedation between the two groups at short‐term follow‐up of one week post‐treatment.
We also found little or no difference between the D‐cycloserine plus social skills training and the placebo plus social skills training groups for the secondary outcomes defined in this review, such as non‐core symptoms of ASD and tolerability at one week post‐treatment. See Table 1.
We are unable to draw a confident conclusion about the effectiveness of D‐cycloserine due to the low certainty of the evidence, meaning that the actual effect may differ from the predicted effect at one week post‐treatment and short‐term follow‐up.
We identified one ongoing trial in which recruitment of participants is completed (NCT00198120). However, the results of this trial are not yet available.
Overall completeness and applicability of evidence
When interpreting the results of this review for completeness and applicability, we need to consider the following issues. Only one randomised controlled trial (RCT), with a relatively small sample size of 67 children (Minshawi 2016), is not adequate to provide clear evidence on the effectiveness of D‐cycloserine for people with ASD. The study included children ranging in age from 5 to 11 years and was conducted in the USA. Although our inclusion criteria did not place any limit on age, we still do not have any evidence regarding effectiveness in adults. These issues limit the applicability of the results to the wider population because different subgroups of population may have different thresholds of effect. Moreover, this study used D‐cycloserine in conjunction with social skills training in order to assess the impact of the former on the latter. Hence, it is unclear what impact, if any, D‐cycloserine alone has on the social skills deficits in individuals with ASD. The inclusion criteria for participants in the included study was limited to people with ASD who are high functioning. There was no available evidence regarding the effectiveness of D‐cycloserine for people with ASD who are low functioning. Reporting of the methods of randomisation and allocation was incomplete, meaning we were unclear as to whether there was the potential for selection bias. Outcomes assessments in Minshawi 2016 were at one week and 12 weeks post‐treatment follow‐up. Hence, the completeness and applicability of evidence was limited, as long‐term efficacy data was not reported.
Quality of the evidence
We downgraded the quality of the evidence to low for all outcomes on the basis of our GRADE analysis because of imprecision (there was only one study with a small sample size of 67 participants) and unclear risk of selection bias (insufficient reporting of random sequence generation and allocation concealment). More randomised controlled trials (RCTs) with large sample sizes, from multiple locations, are required to confirm the effectiveness of D‐cycloserine in people with ASD.
Potential biases in the review process
We used a comprehensive search strategy to find all relevant RCTs, without limiting by language or publication status. We searched multiple sources for ongoing and unpublished studies, but found only one study with three reports. We also contacted the authors of the included trial to obtain additional information for that study and any ongoing study. Therefore, it is unlikely that we missed relevant trials. We attempted to minimise the bias in review process by using pairs of independent review authors to screen studies for eligibility, and to extract data, assess the risk of bias and rate the certainty of the evidence from the included study. Any discrepancies arising from this process were resolved by a third review author.
Agreements and disagreements with other studies or reviews
We were unable to compare our results with other systematic reviews because there were no systematic reviews addressing a similar question. We found two published randomised trials of D‐cycloserine in individuals with ASD (Posey 2004; Urbano 2014). We excluded these studies because they had no placebo group. Posey 2004 was a prospective and single‐blind trial with 10 participants aged 5 to 27 years old. D‐cycloserine was effective in improving ABC social withdrawal score from baseline (P = 0.02). However, there was no improvement in SRS and ABC (irritability, stereotypic behaviour, hyperactivity, inappropriate speech) (Posey 2004). No serious adverse events were reported in Posey 2004. Analysis of data from a double‐blind, randomised, 10‐week trial revealed that D‐cycloserine was effective in improving stereotypic symptoms in 21 participants with ASD aged 14 to 25 years old (P = 0.003) (Urbano 2014). It also reported D‐cycloserine may improve social skills in terms of both the SRS and ABC lethargy/social withdrawal scores (P < 0.001). Additionally, Urbano 2014 found that D‐cycloserine was well tolerated in both daily and weekly dosage groups. The results of our included study, Minshawi 2016, with respect to social skills improvement and adverse events of D‐cycloserine were similar to those provided by Posey 2004 and Urbano 2014.
Authors' conclusions
Implications for practice.
This review provided low certainty evidence that D‐cycloserine plus social skills training might result in little or no difference in social skills deficits for individuals with ASD. The evidence was judged as being of low certainty due to methodological issues (e.g. only one included study, low participant numbers, and uncertain risk of bias). We are unable to make any firm conclusions regarding the benefits and harms of D‐cycloserine in terms of social interaction, communication and stereotypical behaviour in individuals with ASD.
Implications for research.
The present review highlighted the critical need for more randomised controlled trials (RCTs) ‐ with bigger sample sizes and better reporting of methodology to aid judgements of bias ‐ for D‐cycloserine in people with ASD. One main area of improvement for future trials is to separate targeted participants by age (children versus adolescents versus adults) due to the potential psychopharmacological effects of D‐cycloserine on the developing brain. Future research for ASD should be conducted by considering the effect of D‐cycloserine in combination with other medications (e.g. fluoxetine, methylphenidate (Ritalin), sertraline) and other behavioural interventions because many young people with ASD have co‐morbid medicated conditions. Moreover, future trials should compare various doses (e.g. low dose < 50 mg versus high dose > 50 mg) and schedules of D‐cycloserine (e.g. once‐weekly dose versus daily dose), and head‐to‐head comparison of D‐cycloserine with other medications. Furthermore, future studies should utilise social skills as outcome measures of interest for D‐cycloserine because it is the main area of improvement through this medication. To ensure maximum generalisability of the outcomes, long‐term follow‐up (up to 12 months) should be planned in future trials.
History
Protocol first published: Issue 10, 2019 Review first published: Issue 2, 2021
Acknowledgements
The review was developed under the support and guidance of the Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) Editorial Team. We would like to acknowledge Margaret Anderson, Information Specialist of CDPLP, for her input in writing the search strategy, and Joanne Duffield for advice and guidance. In addition, we would like to thank the following reviewers for their helpful comments on this review: Dr Clare S Allely, Reader in Forensic Psychology, School of Health Sciences, University of Salford, UK and affiliate member of the Gillberg Neuropsychiatry Centre, Sahlgrenska Academy, University of Gothenburg, Sweden; Professor Michael Fitzgerald, Trinity College Dublin, Ireland; Dimitris Mavridis, Department of Primary Education, University of Ioannina, Greece; and Danial Sayyad, Iran. We would also like to thank the following reviewer for his helpful comments on the protocol for this review: Edward S Brodkin MD, Associate Professor of Psychiatry, Perelman School of Medicine at the University of Pennsylvania, USA.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
Searched 11 November 2019 (25 records) Searched 17 November 2020 (1 new record added since previous search)
#1[mh "Child Development Disorders, Pervasive"] #2(pervasive NEXT development* NEXT disorder*) #3(pervasive NEAR/3 child*) #4(PDD or PDDs or PDD‐NOS or ASD or ASDs) #5autis* #6asperger* #7childhood NEXT schizophrenia #8Rett* #9{or #1‐#8} #10MeSH descriptor: [Cycloserine] explode all trees #11D NEXT cycloserin* #12cycloserin* #13MeSH descriptor: [N‐Methylaspartate] explode all trees #14methyl NEXT d NEXT aspart* #15Seromycin #16{or #10‐#15} #17#9 AND #16 #18#17 in Trials
MEDLINE Ovid
Searched 11 November 2019 (142 records) Searched 17 November 2020 (156 records)
1 exp child development disorders, pervasive/ 2 pervasive development$ disorder$.tw,kf. 3 (pervasive adj3 child$).tw,kf. 4 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 5 autis$.tw,kf. 6 asperger$.tw,kf. 7 childhood schizophrenia.tw,kf. 8 Rett$.tw,kf. 9 or/1‐8 10 Cycloserine/ 11 D‐cycloserin$.mp. 12 cycloserin$.mp. 13 N‐Methylaspartate/ 14 N‐methyl‐d‐aspart$.mp. 15 Seromycin.mp. 16 or/10‐15 17 9 and 16 18 exp animals/ not humans/ 19 17 not 18
MEDLINE IN‐Process and Other Non‐Indexed Citations Ovid
Searched 11 November 2019 (43 records) Searched 17 November 2020 (44 records)
1 autis$.tw,kf. 2 asperger$.tw,kf. 3 (pervasive adj3 child$).tw,kf. 4 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 5 childhood schizophrenia.tw,kf. 6 Rett$.tw,kf. 7 or/1‐6 8 cycloserin$.mp. 9 D‐cycloserin$.mp. 10 Seromycin.mp. 11 N‐methyl‐d‐aspart$.mp. 12 or/8‐11 13 7 and 12
MEDLINE EPub ahead of Print Ovid
Searched 11 November 2019 (8 records) Searched 17 November 2020 (7 records)
1 autis$.tw,kf. 2 asperger$.tw,kf. 3 (pervasive adj3 child$).tw,kf. 4 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kf. 5 childhood schizophrenia.tw,kf. 6 Rett$.tw,kf. 7 or/1‐6 8 cycloserin$.mp. 9 D‐cycloserin$.mp. 10 Seromycin.mp. 11 N‐methyl‐d‐aspart$.mp. 12 or/8‐11 13 7 and 12
Embase Ovid
Searched 11 November 2019 (257 records) Searched 17 November 2020 (287 records)
1 exp autism/ 2 pervasive development$ disorder$.tw,kw. 3 (pervasive adj3 child$).tw,kw. 4 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw,kw. 5 autis$.tw,kw. 6 asperger$.tw,kw. 7 childhood schizophrenia.tw,kw. 8 Rett$.tw,kw. 9 or/1‐8 10 cycloserine/ 11 D‐cycloserin$.mp. 12 cycloserin$.mp. 13 n methyl dextro aspartic acid receptor stimulating agent/ 14 N‐methyl‐d‐aspart$.mp. 15 Seromycin.mp. 16 or/10‐15 17 9 and 16 18 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 19 human/ or normal human/ or human cell/ 20 18 and 19 21 18 not 20 22 17 not 21
PsycINFO Ovid
Searched 11 November 2019 (167 records) Searched 17 November 2020 (175 records)
1 exp autism spectrum disorders/ 2 pervasive development$ disorder$.tw. 3 (pervasive adj3 child$).tw. 4 autis$.tw. 5 asperger$.tw. 6 Rett$.tw. 7 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 8 childhood schizophreni$.tw. 9 or/1‐8 10 D‐cycloserin$.mp. 11 Cycloserin$.mp. 12 N‐Methyl‐D‐Aspartate/ 13 N‐methyl‐d‐aspart$.mp. 14 Seromycin.mp. 15 or/10‐14 16 9 and 15
Conference Proceedings Citation Index – Science and Conference Proceedings Citation Index – Social Sciences and Humanities (Web of Science; Clarivate)
Searched 12 November 2019 (9 records) Searched 17 November 2020 (9 records)
# 14 #13 AND #8 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 13 #12 OR #11 OR #10 OR #9 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 12 TS=(Cycloserin*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 11 TS=(N‐methyl‐d‐aspart*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 10 TS=(Cycloserin*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 9 TS=(D‐cycloserin*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 7 TS=(childhood schizophreni*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 6 TS=(PDD or PDDs or PDD‐NOS or ASD or ASDs) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 5 TS=(Rett*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 4 TS= (pervasive NEAR/3 child*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 3TS=[pervasive development* disorder*] Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 2 TS=[asperger*] Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 1 TS=[autis*]
Cochrane Database of Systematic Reviews
Searched 11 November 2019 (1 record) Searched 17 November 2020 (1 record)
#1[mh "Child Development Disorders, Pervasive"] #2(pervasive NEXT development* NEXT disorder*):ti,ab,kw #3(pervasive NEAR/3 child*):ti,ab,kw #4(PDD or PDDs or PDD‐NOS or ASD or ASDs):ti,ab,kw #5autis*:ti,ab,kw #6asperger*:ti,ab,kw #7(childhood NEXT schizophrenia):ti,ab,kw #8Rett*:ti,ab,kw #9{or #1‐#8} #10MeSH descriptor: [Cycloserine] explode all trees #11(D NEXT cycloserin*):ti,ab,kw #12cycloserin*:ti,ab,kw #13MeSH descriptor: [N‐Methylaspartate] explode all trees #14(methyl NEXT d NEXT aspart*):ti,ab,kw #15Seromycin:ti,ab,kw #16{or #10‐#15} #17#9 AND #16 #18#17 in Cochrane Reviews, Cochrane Protocols
Database of Abstracts of Reviews of Effects (DARE; www.crd.york.ac.uk/CRDWeb)
Searched 12 November 2019 (0 records)
(Cycloserine OR Seromycin OR D‐Cycloserine) AND (AUTIS* OR ASPERGER* OR PERVASIVE)
Epistemonikos (www.epistemonikos.org)
Searched 12 November 2019 (2 records) Searched 18 November 2020 (4 records)
Full query:(title:((title:(cycloserin* OR D‐cycloserin* OR Seromycin) OR abstract:(cycloserin* OR D‐cycloserin* OR Seromycin)) AND (title:(AUTIS* OR ASPERGER* OR PERVASIVE) OR abstract:(AUTIS* OR ASPERGER* OR PERVASIVE))) OR abstract:((title:(cycloserin* OR D‐cycloserin* OR Seromycin) OR abstract:(cycloserin* OR D‐cycloserin* OR Seromycin)) AND (title:(AUTIS* OR ASPERGER* OR PERVASIVE) OR abstract:(AUTIS* OR ASPERGER* OR PERVASIVE))))
ClinicalTrials.gov (www.ClinicalTrials.gov)
Searched 12 November 2019 (3 records) Searched 18 November 2020 (3 records)
Autism OR asperger OR PDD OR pervasive | Cycloserine OR D‐cycloserine OR Seromycin
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en)
Searched 12 November 2019 (3 records after duplicates were removed) 18 November 2020 Not available for searching .
Cycloserine AND Asperger* OR D‐cycloserine AND Asperger* OR Seromycin AND Asperger* (1) Cycloserine AND Autis* OR D‐cycloserine AND Autis* OR Seromycin AND Autis* (3) Cycloserine AND Pervasive OR D‐cycloserine AND Pervasive OR Seromycin AND Pervasive (1) Cycloserine AND PDD* OR D‐cycloserine AND PDD* OR Seromycin AND PDD* (0)
WorldCat (www.worldcat.org)
Searched 12 November 2019 (limited to dissertations and theses; 0 records)
'kw:((Cycloserine OR D‐cycloserine ) AND (autis* OR asperger* OR pervasive or pdd*))' > 'Thesis/dissertation'
ProQuest Dissertations & Theses Global
Searched 18 November 2020 (1 record) noft(Cycloserine OR D‐cycloserine or Seromycin) AND noft((autis* OR asperger* OR pervasive or pdd*))
Appendix 2. 'Risk of bias' assessment
We judged the risk of bias in the included studies according to the criteria described below (see Higgins 2017).
Random sequence generation (checking for possible selection bias)
We judged the risk of selection bias related to random sequence generation as follows.
Low: investigators described a random component in the sequence generation process such as referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, or minimisation.
High: investigators described a non‐random component in the sequence generation process, for example, sequence generated by odd date or even date of birth, sequence generated by some rule based on date (or day) of admission, or sequence generated by some rule based on hospital or clinic record number. Other non‐random approaches that could have happened much less frequently would usually have involved judgement or some method of non‐random categorisation of participants, such as allocation by judgement of the clinician, allocation by preference of the participant, allocation based on the results of a laboratory test or a series of tests, and allocation by availability of the intervention.
Unclear: information about the sequence generation process was not clearly stated or was insufficient to permit a judgement of low or high risk of bias.
Allocation concealment (checking for possible selection bias)
We judged the risk of selection bias related to allocation concealment as follows.
Low: participants and investigators enrolling participants could not have foreseen assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, or sealed envelopes.
High: participants or investigators enrolling participants could possibly have foreseen assignments and thus introduced selection bias; for example, allocation based on: using an open random allocation schedule (e.g. a list of random numbers); using assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear: information about allocation concealment was not clearly stated or was insufficient to permit a judgement of low or high risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We judged the risk of performance bias as follows.
Low: blinding of participants and key study personnel was ensured and it was unlikely that the blinding could have been broken; no blinding or incomplete blinding, but the outcome was not likely to have been influenced by the lack of blinding.
High: no blinding or incomplete blinding and the outcome was likely to have been influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to have been influenced by the lack of blinding.
Unclear: study did not address this outcome; or there was insufficient information to permit a judgement of low or high risk of bias.
Blinding of outcome assessors (checking for possible detection bias)
We judged the risk of detection bias as follows.
Low: no blinding of outcome assessment, but the outcome measurement was not likely to have been influenced by the lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
High: no blinding of outcome assessment and the outcome measurement was likely to have been influenced by the lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to have been influenced by the lack of blinding.
Unclear: study did not address this outcome; or there was insufficient information to permit a judgement of low or high risk of bias.
Incomplete outcome data (checking for possible attrition bias)
We judged the risk of attrition bias as follows.
Low: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, proportion of missing outcomes compared with observed event risk was not enough to have had a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have had a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods.
High: reasons for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, proportion of missing outcomes compared with observed event risk was enough to have induced clinically relevant bias in the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to have induced clinically relevant bias in observed effect size; 'as‐treated' analysis was done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Unclear: study did not address this outcome; or there was insufficient reporting of attrition or exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
Selective outcome reporting (checking for possible reporting bias)
We judged the risk of reporting bias as follows.
Low: study protocol was available and all of the study’s pre‐specified (primary and secondary) outcomes that were of interest to the review were reported in the pre‐specified way; or the study protocol was not available, but it was clear that the published reports included all expected outcomes, including those that were pre‐specified.
High: not all of the study’s pre‐specified primary outcomes were reported; one or more primary outcomes were reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review was reported incompletely so that it could not be entered in a meta‐analysis; or the study report failed to include results for a key outcome that was expected to be reported for such a study.
Unclear: information was insufficient to permit a judgement of low or high risk of bias.
Other sources of bias (checking for other potential sources of bias)
We judged the risk of other bias as follows.
Low: study appeared to be free of other sources of bias.
High: at least one important risk of bias existed (for example, the study had a potential source of bias related to the specific study design used, or had been claimed to have been fraudulent, or had some other problem).
Unclear: potential risk of bias, but there was either insufficient information to assess whether an important risk of bias existed or insufficient rationale or evidence that an identified problem would have introduced bias.
Data and analyses
Comparison 1. D‐cycloserine versus placebo.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Minshawi 2016.
| Study characteristics | ||
| Methods |
Study design: randomised, parallel‐group, placebo‐controlled trial Masking: double‐blind (participant, care provider and investigator) Study start date: March 2010 Study end date: January 2014 Setting: recruitment from academic autism treatment centres, local schools and community organizations Country: USA Study location: Indiana University School of Medicine (n = 52) and Cincinnati Children's Hospital Medical Centre (n = 15) |
|
| Participants |
Number recruited: 68 children (154 participants assessed for eligibility and 86 participants excluded: 35 = not meeting inclusion criteria; 40 = refused to participate; 11 = other reason) Number randomised: 68 children Number completed: 67 children Number dropouts/withdrawals: 1 child with ASD was excluded from the analyses due to early dropout prior to taking study drug Inclusion criteria
Exclusion criteria
Pretreatment: differences in age, sex and number of participants between the two groups are small Baseline characteristics
|
|
| Interventions |
Experimental intervention: D‐cycloserine, 50 mg weekly
Control intervention: placebo
|
|
| Outcomes |
Primary outcomes Outcome 1: social interaction impairment, measured by:
Outcome 2: social communication impairment, measured by:
Outcome 3: restricted, repetitive, stereotyped patterns of behaviour and interests, measured by:
Outcome 4: adverse events
Secondary outcomes Outcome 1: non‐core symptoms of ASD, measured by Clinical Global Impression‐Improvement (CGI‐I) scale
Outcome 2: tolerability of D‐cycloserine
|
|
| Identification |
Author's name: Noha F Minshawi (contact author) Institution: Indiana University School of Medicine, Department of Psychiatry Email: craig.erickson@cchmc.org Address: Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, 3333 Burnet Avenue MLC 4002, Cincinnati, OH 45229, USA |
|
| Notes | Sponsorship source: United States Department of Defense, Award Number W81XWH‐09‐1‐0091 Comments: This RCT was approved by the Institutional review board at each site. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Children with ASD were randomised to receive 10 weeks (10 doses) of DCS or placebo in a 1:1 ratio." Judgement comment: it was a randomised, double‐blind, placebo‐controlled trial but did not state the method of randomisation clearly. We asked the authors for clarification, but did not receive a response. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: the method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "10‐week, double‐blind, placebo‐controlled trial of low dose (50 mg) DCS given 30 min prior to weekly group social skills training was conducted at two sites, Indiana University School of Medicine and Cincinnati Children’s Hospital Medical Center." Judgement comment: it is clearly stated that there was blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Outcome assessors were unaware of the treatment allocation until after 12 weeks post‐treatment follow‐up visits and the data set was locked." Judgement comment: it was stated as triple blind in the trial register. (clinicaltrials.gov/ct2/show/NCT01086475) before full publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "One subject with ASD was excluded from the analyses due to early dropout prior to taking the study drug." Judgement comment: only one participant was excluded. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: the trial reported all outcomes listed on the trial registration. |
| Other bias | Low risk |
Quote: "The authors declare that they have no competing interests." Judgement comment: the principal investigators are not employed by the organisation sponsoring the study. The study was supported by the United States Department of Defense. The authors declared no conflict of interest. |
ASD: autism spectrum disorder DCS: D‐cycloserine DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders‐Fourth Edition‐Text Revision. IQ: intelligence quotient min: minute(s) SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT00198107 | The study was a randomised controlled trial (RCT) of 81 participants younger than 18 years. The study participants were randomised to receive 8 weeks of initial treatment with aripiprazole or placebo. If responsive to treatment, participants might have been assigned to an additional 16 weeks of aripiprazole and then an additional 8 weeks of aripiprazole with D‐cycloserine. This study was excluded because the initial treatment of aripiprazole could have a potential carry‐over effect on D‐cycloserine treatment. |
| Posey 2004 | The study was a prospective single‐blind pilot study which lasted for 8 weeks. Twelve participants aged older than 5 years and with ASD were enrolled. Following a 2‐week placebo lead‐in phase, the participants were administered three different ascending doses of D‐cycloserine, 0.7, 1.4 and 2.8 mg/kg/day, each for 2 weeks. D‐cycloserine treatment resulted in significant improvement in social withdrawal. This study was excluded because it was not a randomised controlled trial. |
| Urbano 2014 | The study was a double‐blind randomised 10‐week trial with no control group. Twenty‐one participants, aged 14‐25 year, were enrolled and randomised to either 50 mg weekly or 50 mg daily dosage of D‐cycloserine for 8 weeks. At 2‐week post‐treatment follow‐up, D‐cycloserine resulted in an improvement in social skills deficit in individuals with ASD in both daily and weekly groups. This study was excluded because there was no control group. |
Characteristics of ongoing studies [ordered by study ID]
NCT00198120.
| Study name |
Public title: Safety and effectiveness of D‐Cycloserine in children with autism Scientific title: A randomized controlled trial of D‐cycloserine in autism |
| Methods |
Study design: randomised, double‐blind controlled trial Country: USA Study location: Riley Hospital for Children, Christian Sarkine Autism Treatment Center, Indianapolis, Indiana |
| Participants | 80 participants (age range = 3 to 12 years) |
| Interventions |
Experimental intervention: D‐cycloserine
Control intervention: placebo
|
| Outcomes |
Primary outcome: core symptoms of ASD, social and communication impairment, measured by the Lethargy subscale of the Aberrant Behavior Checklist (ABC) Primary outcome: non‐core symptoms of ASD, measured by the Clinical Global Impression‐Improvement (CGI‐I) scale |
| Starting date | February 2004 |
| Contact information |
Principal investigator: Christopher J McDougle, MD Affiliation: Indiana University School of Medicine Telephone: 781‐860‐1783 Email: cmcdougle@partners.org |
| Notes |
Sponsorship source: the study was funded by Indiana University. Comment: recruitment completed. No results posted on the trial register (clinicaltrials.gov/ct2/show/NCT00198120). The investigator did not respond to any of the three emails that we sent requesting further details on the trial. |
ASD: autism spectrum disorder.
Differences between protocol and review
Title
We included ‘social and communication skills’ in the title of the review, as these are the key ASD symptoms that D‐cycloserine is hypothesised to target.
Objectives
We modified the objective of this review, by referring to the social skills deficits in people with ASD, as these are postulated to be targeted by D‐cycloserine.
Background > Description of the condition
Based on a suggestion from an Editor with Cochrane Developmental, Psychosocial and Learning Problems (CDPLP), we added a paragraph on ASD in girls, the fact that it is often diagnosed later, and the different ways in which this presents (e.g. with masking of social skills deficits).
Methods > Criteria for considering studies for inclusion in this review > Type of participants
Based on a suggestion from an Editor with CDPLP, we added more details about the types of participants eligible and not eligible for inclusion. Specifically, we added the text below.
"We intended to include participants with any intelligence quotient (IQ), comorbid disorders (e.g. anxiety, depression), and pharmacological co‐interventions (e.g. antipsychotics, psychostimulants, antidepressants or mood stabilisers) with a stable dosage for a minimum of two weeks before randomisation. We also included non‐pharmacological co‐interventions such as behavioural therapy, speech therapy, occupational therapy and dietary modifications. However, we excluded participants with more than two psychotropic medications and glutamatergic modulators (e.g. riluzole, memantine, etc.). We also excluded participants with social and communication problems due to other established causes such as acquired brain injury or neurodegenerative diseases. We planned to exclude studies that included initial treatment with a different medication because of potential carry‐over effects (Evans 2010)."
Methods > Criteria for considering studies for inclusion in this review > Types of interventions
In our protocol (Aye 2019), we wrote that we intended to compare D‐cycloserine for the treatment of ASD, irrespective of dose or duration, versus placebo. For clarity and to specify that adjunct treatments were eligible for inclusion, we added that this could have been with or without adjunct treatment.
Methods > Criteria for considering studies for inclusion in this review > Types of outcome measures
We included the Vineland Adaptive Behaviour Scales 2nd Edition (VABS‐II) scale to assess the social communication and social interaction status of ASD because Vineland is often used to assess functional status of ASD.
Methods > Search methods for identification of studies > Electronic searches
WHO ICTRP was not available to search in November 2020 because the website was experiencing heavy traffic due to COVID‐related activity.
We replaced WorldCat with ProQuest Dissertations & Theses Global which recently became available to us and is a larger database.
Methods > Data collection and analysis
We were not able to use all of our preplanned methods due to including only one eligible study. See Table 2 of unused methods.
Methods > Data collection and analysis > Data extraction and management
We had originally planned to spot check the data entry for accuracy. However, we actually checked all data extraction thoroughly, as only one study was included in the review. We have revised this sentence in this section of the review accordingly.
Methods > Data collection and analysis > Summary of findings and assessment of the certainty of the evidence
We planned to assess outcomes at three‐ to six‐months follow‐up. However, we changed this to one week post‐treatment follow‐up, as the included study used this time frame for follow‐up assessment.
We changed 'quality of evidence' to 'certainty of evidence' throughout the review, in keeping with current recommendations.
Contributions of authors
Swe Zin Aye (SZA) is the guarantor of the review and wrote the draft protocol and full text of the review. SZA and Han Ni (HN) independently selected trials for inclusion and extracted data using Covidence. SZA and Htwe Htwe Sein (HHS) assessed risk of bias using the Cochrane ‘risk of bias’ tool. HHS resolved differences in opinion regarding study selection and data extraction, while San Thidar Mon (STM) resolved differences in opinion regarding the ‘risk of bias’ assessment. Qishi Zheng (QZ) entered the data into RevMan, which HHS checked for accuracy. SZA and QZ prepared the 'Summary of findings' table. QZ and Yoko Kin Yoke Wong (YKYW) contributed methodological advice on the review and in interpreting the data. SZA and HN conducted the GRADE assessment, which was subsequently approved by QZ. All review authors contributed to writing the protocol and review.
Sources of support
Internal sources
-
QUEST International University Perak, Malaysia
Permitted Swe Zin Aye and Htwe Htwe Sein to work on this review during office hours
External sources
-
None, Other
No external sources of support.
Declarations of interest
Swe Zin Aye: none known.
Han Ni: none known.
Htwe Htwe Sein: none known.
San Thidar Mon: none known.
Qishi Zheng: none known.
Yoko Kin Yoke Wong: none known.
New
References
References to studies included in this review
Minshawi 2016 {published data only}
- *.Minshawi NF, Wink LK, Shaffer R, Plawecki MH, Posey DJ, Liu H, et al. A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in autism spectrum disorders. Molecular Autism 2016;7:2. [DOI: 10.1186/s13229-015-0062-8] [PMC4712595] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01086475. D-cycloserine and social skills training in autism spectrum disorders [A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in pervasive developmental disorders]. clinicaltrials.gov/ct2/show/NCT01086475 (first received 10 March 2010).
- Wink LK, Minshawi NF, Shaffer RC, Plawecki MH, Posey DJ, Horn PS, et al. D-cycloserine enhances durability of social skills training in autism spectrum disorder. Molecular Autism 2017;8:2. [DOI: 10.1186/s13229-017-0116-1] [PMC5264460] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
NCT00198107 {published data only}
- NCT00198107. Evaluating the effectiveness of Aripiprazole and D-cycloserine to treat symptoms associated with autism [Novel pharmacological strategies in autism]. clinicaltrials.gov/ct2/show/results/NCT00198107 (first received 12 September 2005).
Posey 2004 {published data only}
- *.Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of D-cycloserine in subjects with autistic disorder. American Journal of Psychiatry 2004;161(11):2115-7. [DOI: 10.1176/appi.ajp.161.11.2115] [DOI] [PubMed] [Google Scholar]
- Posey DJ, Swiezy NB, Kern DL, Sweeten TL, McDougle C. A pilot study of D-cycloserine in the treatment of autistic disorder. Biological Psychiatry 2003;53(8):S170-1. [DOI: 10.1016/S0006-3223(03)00164-1] [Abstract #484] [DOI] [Google Scholar]
Urbano 2014 {published data only}
- Urbano M, Okwara L, Manser P, Hartmann K, Deutsch SI. A trial of D-cycloserine to treat the social deficit in older adolescents and young adults with autism spectrum disorders. Journal of Neuropsychiatry and Clinical Neurosciences 2015;27(2):133-8. [DOI: 10.1176/appi.neuropsych.13070155.] [PMID: ] [DOI] [PubMed] [Google Scholar]
- *.Urbano M, Okwara L, Manser P, Hartmann K, Herndon A, Deutsch SI. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clinical Neuropharmacology 2014;37(3):69-72. [DOI: 10.1097/WNF.0000000000000033] [PMC4354861] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano MR, Okwara L, Manser P, Hartmann, K, Deutsch S. A trial of D-cycloserine to treat the social deficit in older adolescents and young adults with autism spectrum disorders. Neuropsychopharmacology 2013;38:S505-6. [DOI: 10.1038/npp.2013.281] [Abstract #W106] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00198120 {published data only (unpublished sought but not used)}
- NCT00198120. Safety and effectiveness of D-Cycloserine in children with autism [A randomized controlled trial of D-Cycloserine in autism]. clinicaltrials.gov/ct2/show/study/NCT00198120 (first received 12 September 2005).
Additional references
Abrahams 2008
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews. Genetics 2008;9(5):341-55. [DOI: 10.1038/nrg2346] [PMC2756414] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aman 1985
- Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency 1985;89(5):485-91. [PMID: ] [PubMed] [Google Scholar]
Attari 2014
- Attari A, Rajabi F, Maracy MR. D-cycloserine for treatment of numbing and avoidance in chronic post traumatic stress disorder: a randomized, double blind, clinical trial. Journal of Research in Medical Sciences 2014;19(7):592-8. [PMC4214015] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Baer 1968
- Baer DM, Wolf MM, Risley TR. Some current dimensions of applied behavior analysis. Journal of Applied Behavior Analysis 1968;1(1):91-7. [DOI: 10.1901/jaba.1968.1-91] [PMC1310980] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baio 2018
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report - Surveillance Summaries 2018;67(6):1-23. [DOI: 10.15585/mmwr.ss6706a1] [PMC5919599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blaylock 2009
- Blaylock RL, Strunecka A. Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Current Medicinal Chemistry 2009;16(2):157-70. [DOI: 10.2174/092986709787002745] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brugha 2011
- Brugha TS, McManus S, Bankart J, Scott F, Purdon S, Smith J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Archives of General Psychiatry 2011;68(5):459-65. [DOI: 10.1001/archgenpsychiatry.2011.38] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bryson 2008
- Bryson SE, Bradley EA, Thompson A, Wainwright A. Prevalence of autism among adolescents with intellectual disabilities. Canadian Journal of Psychiatry [Revue Canadienne de Psychiatrie] 2008;53(7):449-59. [DOI: 10.1177/070674370805300710] [PMID: ] [DOI] [PubMed] [Google Scholar]
Busner 2007
- Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry 2007;4(7):28-37. [PMC2880930] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Cola 2020
- Cola ML, Plate S, Yankowitz L, Petrulla V, Bateman L, Zampella CJ, et al. Sex differences in the first impressions made by girls and boys with autism. Molecular Autism 2020;11(1):49. [DOI: 10.1186/s13229-020-00336-3] [PMC7298946] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Constantino 2005
- Constantino JN, Gruber CP. The Social Responsiveness Scale. Torrance (CA): Western Psychological Services, 2005. [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 20 November 2020. Available at www.covidence.org.
Crino 2011
- Crino PB. mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends in Molecular Medicine 2011;17(12):734-42. [DOI: 10.1016/j.molmed.2011.07.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Danysz 1998
- Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacological Reviews 1998;50(4):597-664. [PMID: ] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DSM
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 1st edition. Washington (DC): American Psychiatric Association, 1952. [Google Scholar]
DSM‐5
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
DSM‐II
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-II). 2nd edition. Washington (DC): American Psychiatric Association, 1968. [Google Scholar]
DSM‐III
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): American Psychiatric Association, 1980. [Google Scholar]
DSM‐IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
DSM‐IV‐TR
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Scheider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsabbagh 2012
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Research 2012;5(3):160-79. [DOI: 10.1002/aur.239] [PMC3763210] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Estes 2015
- Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nature reviews. Neuroscience 2015;16(8):469-86. [DOI: 10.1038/nrn3978] [PMC5650494] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2010
- Evans SR. Clinical trial structures. Journal of Experimental Stroke & Translational Medicine 2010;3(1):8-18. [DOI: 10.6030/1939-067x-3.1.8] [PMC3059315] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2007
- Ganz ML. The lifetime distribution of the incremental societal costs of autism. Archives of Pediatrics & Adolescent Medicine 2007;161(4):343-9. [DOI: 10.1001/archpedi.161.4.343] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gargaro 2011
- Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, Sheppard DM. Autism and ADHD: how far have we come in the comorbidity debate? Neuroscience and Biobehavioral Reviews 2011;35(5):1081-8. [DOI: 10.1016/j.neubiorev.2010.11.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goff 1999
- Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, et al. A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Archives of General Psychiatry 1999;56(1):21-7. [DOI: 10.1001/archpsyc.56.1.21] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goff 2008
- Goff DC, Cather C, Gottlieb JD, Evins AE, Walsh J, Raeke L, et al. Once-weekly d-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophrenia Research 2008;106(2-3):320-7. [DOI: 10.1016/j.schres.2008.08.012] [PMC2628436] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goff 2012
- Goff DC. D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophrenia Bulletin 2012;38(5):936-41. [DOI: 10.1093/schbul/sbs012] [PMC3446239] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gould 2017
- Gould J. Towards understanding the under-recognition of girls and women on the autism spectrum. Autism 2017;21(6):703-5. [DOI: 10.1177/1362361317706174] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 12 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Ha 2015
- Ha S, Sohn IJ, Kim N, Sim HJ, Cheon KA. Characteristics of brains in autism spectrum disorder: structure, function and connectivity across the lifespan. Experimental Neurobiology 2015;24(4):273-84. [DOI: 10.5607/en.2015.24.4.273] [PMC4688328] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heresco‐Levy 2002
- Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC. Placebo-controlled trial of d-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. American Journal of Psychiatry 2002;159(3):480-2. [DOI: 10.1176/appi.ajp.159.3.480] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heys 2018
- Heys M, Gibbons F, Haworth E, Medeiros E, Tumbahangphe KM, Wickenden M, et al. The estimated prevalence of autism in school-aged children living in rural Nepal using a population-based screening tool. Journal of Autism and Developmental Disorders 2018;48(10):3483-98. [DOI: 10.1007/s10803-018-3610-1] [PMC6153945] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook.
Hurwitz 2012
- Hurwitz R, Blackmore R, Hazell P, Williams K, Woolfenden S. Tricyclic antidepressants for autism spectrum disorders (ASD) in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD008372. [DOI: 10.1002/14651858.CD008372.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hwang 2013
- Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. International Journal of Tuberculosis and Lung Disease 2013;17(10):1257-66. [DOI: 10.5588/ijtld.12.0863] [PMID: ] [DOI] [PubMed] [Google Scholar]
ICD‐10
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD-10). 10th edition. Geneva (CH): World Health Organization, 2007. [Google Scholar]
ICD‐11
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics (ICD-11). 11th edition. Geneva (CH): World Health Organization, 2018. [Google Scholar]
Kanner 1943
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943;2:217-50. [psycnet.apa.org/record/1943-03624-001] [Google Scholar]
Knapp 2009
- Knapp M, Romeo R, Beecham J. Economic cost of autism in the UK. Autism 2009;13(3):317-36. [DOI: 10.1177/1362361309104246] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leigh 2015
- Leigh JP, Du J. Brief report: forecasting the economic burden of autism in 2015 and 2025 in the United States. Journal of Autism and Developmental Disorders 2015;45(12):4135-9. [DOI: 10.1007/s10803-015-2521-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Loomes 2017
- Loomes R, Hull L, Mandy WP. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(6):466-74. [DOI: 10.1016/j.jaac.2017.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lord 1994
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lord 2000
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule - Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders 2000;30(3):205-23. [PMID: ] [PubMed] [Google Scholar]
Lyall 2017
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annual Review of Public Health 2017;38:81-102. [DOI: 10.1146/annurev-publhealth-031816-044318] [PMC6566093 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddox 2017
- Maddox BB, Brodkin ES, Calkins ME, Shea K, Mullan K, Hostager J, et al. The accuracy of the ADOS-2 in identifying autism among adults with complex psychiatric conditions. Journal of Autism and Developmental Disorders 2017;47(9):2703-9. [DOI: 10.1007/s10803-017-3188-z] [PMC5813679] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magiati 2014
- Magiati I, Tay XW, Howlin P. Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: a systematic review of longitudinal follow-up studies in adulthood. Clinical Psychology Review 2014;34(1):73-86. [DOI: 10.1016/j.cpr.2013.11.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Matelski 2016
- Matelski L, Van de Water J. Risk factors in autism: thinking outside the brain. Journal of Autoimmunity 2016;67:1-7. [DOI: 10.1016/j.jaut.2015.11.003] [PMC5467975] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDougle 2005
- McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. Journal of Clinical Psychiatry 2005;66 Suppl 10:9-18. [PMID: ] [PubMed] [Google Scholar]
Modabbernia 2017
- Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Molecular Autism 2017;8:13. [DOI: 10.1186/s13229-017-0121-4] [PMC5356236] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [PMC2707599] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moskowitz 2011
- Moskowitz A, Heim G. Eugen Bleuler's dementia praecox or the group of schizophrenias (1911): a centenary appreciation and reconsideration. Schizophrenia Bulletin 2011;37(3):471-9. [DOI: 10.1093/schbul/sbr016] [PMC3080676] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01086475
- NCT01086475. D-cycloserine and social skills training in autism spectrum disorders [A randomized, placebo-controlled trial of D-cycloserine for the enhancement of social skills training in pervasive developmental disorders]. clinicaltrials.gov/ct2/show/NCT01086475 (first received 10 March 2010).
NICE 2013
- National Institute for Health and Care Excellence. Autism spectrum disorder in under 19s: support and management. nice.org.uk/guidance/cg170 (accessed 23 September 2019). [PubMed]
Padden 2017
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. Journal of Developmental and Physical Disabilities 2017;29(4):567-86. [DOI: 10.1007/s10882-017-9547-z] [PMC5502228] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Pisula 2017
- Pisula E, Porębowicz-Dörsmann A. Family functioning, parenting stress and quality of life in mothers and fathers of Polish children with high functioning autism or Asperger syndrome. PLoS One 2017;12(10):e0186536. [DOI: 10.1371/journal.pone.0186536] [PMC5643111] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Posey 2008
- Posey DJ, Erickson CA, McDougle CJ. Developing drugs for core social and communication impairment in autism. Child and Adolescent Psychiatric Clinics of North America 2008;17(4):787-801. [DOI: 10.1016/j.chc.2008.06.010] [PMC2566849] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rodrigues 2014
- Rodrigues H, Figueira I, Lopes A, Goncalves R, Mendlowicz MV, Coutinho ES, et al. Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PloS One 2014;9(7):e93519. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rojas 2014
- Rojas DC. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. Journal of Neural Transmission 2014;121(8):891-905. [DOI: 10.1007/s00702-014-1216-0] [PMC4134390] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samadi 2011
- Samadi SA, McConkey R. Autism in developing countries: lessons from Iran. Autism Research and Treatment 2011;2011:1-11. [DOI: 10.1155/2011/145359] [Article ID 145359] [PMC3420542] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schade 2016
- Schade S, Paulus W. D-cycloserine in neuropsychiatric diseases: a systematic review. International Journal of Neuropsychopharmacology 2016;19(4):pii: pyv102. [DOI: 10.1093/ijnp/pyv102] [PMC4851259] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiltz 2018
- Schiltz HK, McVey AJ, Magnus B, Dolan BK, Willar KS, Pleiss S, et al. Examining the links between challenging behaviors in youth with ASD and parental stress, mental health, and involvement: applying an adaptation of the Family Stress Model to families of youth with ASD. Journal of Autism and Developmental Disorders 2018;48(4):1169-80. [DOI: 10.1007/s10803-017-3446-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019.. Available from www.training.cochrane.org/handbook.
Sheinin 2001
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist d-cycloserine. Neuropharmacology 2001;41(2):151-8. [DOI: 10.1016/s0028-3908(01)00073-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
SIGN 2016
- Scottish Intercollegiate Guidelines Network. SIGN 145. Assessment, diagnosis and interventions for autism spectrum disorders. A national clinical guideline. www.sign.ac.uk/assets/sign145.pdf (accessed 23 September 2019).
Sparrow 2005
- Sparrow SS, Balla DA, Cicchetti DV, Edgar AD. Vineland Adaptive Behavior Scales. 2nd edition. Circle Pines (MN): American Guidance Service, 2005. [Google Scholar]
Sturman 2017
- Sturman N, Deckx L, Van Driel ML. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD011144. [DOI: 10.1002/14651858.CD011144.pub2] [PMC6486133 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuchman 2010
- Tuchman R, Alessandri M, Cuccaro M. Autism spectrum disorders and epilepsy: moving towards a comprehensive approach to treatment. Brain and Development 2010;32(9):719-30. [DOI: 10.1016/j.braindev.2010.05.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
White 2009
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review 2009;29(3):216-29. [DOI: 10.1016/j.cpr.2009.01.003] [PMC2692135] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. Autistic spectrum disorder. www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (assessed 24 August 2018).
Williams 2012
- Williams K, Thomson D, Seto I, Contopoulos-Ioannidis DG, Ioannidis JP, Curtis S, et al. Standard 6: age groups for pediatric trials. Pediatrics 2012;129 Suppl 3:S153-60. [DOI: 10.1542/peds.2012-0055I] [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2013
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004677. [DOI: 10.1002/14651858.CD004677.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2016
- Zheng Z, Zhu T, Qu Y, Mu D. Blood glutamate levels in autism spectrum disorder: a systematic review and meta-analysis. PLoS One 2016;11(7):e0158688. [DOI: 10.1371/journal.pone.0158688] [PMC4938426] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2013
- Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology 2013;74:69-75. [DOI: 10.1016/j.neuropharm.2013.03.030] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Aye 2019
- Aye SZ, Ni H, Sein HH, Mon ST, Zheng Q, Wong YK. D-cycloserine for autism spectrum disorder. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013457. [DOI: 10.1002/14651858.CD013457] [DOI] [PMC free article] [PubMed] [Google Scholar]


