Abstract
Background
Itch in patients with chronic kidney disease (CKD) is common, often very distressing and associated with depression, reduced quality of life, and increased death. The most common first‐line treatment has been the use of antihistamines despite the lack of substantial evidence for its use for uraemic itch. Few recommendations and guidelines exist for treatment.
Objectives
We aimed to determine: 1) the benefits and harms (both absolute and relative) of all topical and systemic interventions for the treatment of uraemic itch, either alone or in combination, when compared with placebo or standard care; and, 2) the dose strength or frequency, stage of kidney disease or method of dialysis used (where applicable) in cases where the effects of these interventions vary depending on co‐interventions.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 17 December 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials (RCTs) in adults with CKD stages 4 or 5 comparing treatments (pharmacological, topical, exposure, dialysis modality) for CKD associated itch to either placebo or other established treatments.
Data collection and analysis
Two authors independently abstracted study data and assessed study quality. Data were analysed using a random effects meta‐analysis design estimating the relative effects of treatment versus placebo. Estimates of the relative effects between treatments are included where possible. For continuous measures of severity of itch up to three months, mean difference (MD) or standardised mean difference (SMD) were used. When reported, adverse effects were tabulated. The certainty of the evidence was estimated using GRADE.
Main results
Ninety‐two RCTs, randomising 4466 participants were included. Fifty‐eight studies (3285 participants) provided sufficient data to be meta‐analysed. Of these, 30 compared an intervention to a placebo or control. The 10 cm Visual Analogue Scale (VAS) was the dominant instrument utilized for itch reporting and the Duo score was used in a minority of studies.
GABA analogues including, gabapentin and pregabalin, reduce itch in patients with CKD (5 studies, 297 participants: 4.95 cm reduction, 95% CI 5.46 to 4.44 lower in VAS compared to placebo; high certainty evidence). Kappa opioid agonists, including nalfurafine also reduced itch in this population (6 studies, 661 participants: 1.05 cm reduction, 95% CI 1.40 to 0.71 lower in VAS compared to placebo; high certainty evidence). Ondansetron had little or no effect on itch scores (3 studies, 183 participants: 0.38 cm reduction, 95% CI 1.04 lower to 0.29 higher in VAS compared to placebo; high certainty evidence). Reduction in the severity of itch was reported with oral montelukast, turmeric, zinc sulfate and topical capsaicin. For all other interventions, the certainty of the evidence was low to moderate, and the interventions had uncertain effects on uraemic pruritus.
Six studies have disclosed significant financial support from their respective manufacturers, six were affected by lack of blinding, and 11 studies have 15 participants or less. Older, smaller RCTs often failed to follow intention‐to‐treat protocols with unexplained dropouts after randomisation.
Adverse effects were generally poorly and inconsistently reported across all RCTs. No severe adverse events were reported for any intervention.
Authors' conclusions
The RCTs of this meta‐analysis contain a large array of interventions with a diverse set of comparators. For many interventions, trials are sparse. This served to make informative meta‐analysis challenging.
Of all treatments for uraemic pruritus, gabapentinoids (gabapentin and pregabalin) were the most studied and show the greatest reduction in itch scores. Further RCTs, even of the scale of the largest trials included in this review, are unlikely to significantly change this finding. Kappa‐opioid agonists (mainly nalfurafine) also may reduce itch, but indirect comparison suggests a much more modest effect in comparison to GABA analogues.
Evidence for oral montelukast, turmeric, zinc sulfate, and topical capsaicin also showed an itch score reduction. However, these reductions were reported in small studies, and warrant further investigation. Ondansetron did not reduce itch. It is somewhat unlikely that a further study of ondansetron will change this result.
Plain language summary
What is the best treatment for itch in people with chronic kidney disease?
What is the issue? Itch (medical term pruritus) is a common problem for people with chronic kidney disease (CKD). Itch can greatly affect quality of life and may lead to depression or increased risk of death. There are no widely used or agreed upon treatment guidelines for itch associated with CKD.
What did we do? We found 92 studies involving 4466 people investigating 30 treatments for CKD‐associated itch. The control treatment was either placebo or (less commonly) another treatment for CKD‐associated itch.
What did we find? One type of drug (gabapentin and pregabalin), an analogue to a common neurotransmitter appear to reduce itch in patients with CKD. Ondansetron, an anti‐nausea drug, was another well studied treatment and appears have no significant association with itch reduction. Kappa‐opioid drugs (nalfurafine) appear to slightly reduce itch. There is too little information on the remaining treatments for any thorough assessment of their efficacy in relieving itch or whether there is any anti‐itch effect at all.
The three drugs mentioned above are well studied with higher quality evidence. The other treatments studied are of lower to moderate quality.
The studies seldom document a comprehensive list of adverse or side effects incurred during treatment. However, none of the adverse effects documented were severe. Further meaningful assessment on harm cannot be made.
Conclusions Drugs that work like neurotransmitters (gabapentin and pregabalin) reduce itch in patients with CKD. Other intervention either do not work, do not work as well, or need further study to make a conclusion.
Summary of findings
Background
Description of the condition
Itch (uraemic pruritus) is a common symptom in people with end‐stage kidney disease (ESKD) and affects 42% to 57% of people on dialysis (Mistik 2006; Patel 2007; Pisoni 2006; Zucker 2003). Itch has significant adverse effects on quality of life (QoL) due to discomfort, disordered sleep, anxiety and depression (Narita 2006; Pisoni 2006). Despite its high prevalence, mechanisms driving uraemic itch remain poorly understood; two common theories implicate hyperactive and disordered immune (Mettang 2002) or opioid systems (Peer 1996). However, roles have also been proposed for hyperparathyroidism (Hampers 1968; Massry 1968), abnormal serum chemistry (Carmichael 1988), mast cell hyperactivity (Kaku 1990), and dialysis technique (Kato 2001; Tan 1991).
Description of the intervention
Itch has generally been used to refer to a symptom that is an intense sensation of the skin, either local or generalized, which triggers repeated scratching in an attempt to relieve the discomfort. Due to the commonality of itch in general, a formal definition in the context of chronic kidney disease (CKD) has been proposed (Zucker 2003). This defines uraemic itch as a) itch appearing shortly before the onset of dialysis, or at any time, without evidence of any other active disease that could explain the itch, b) three or more episodes of itch during a period of less than two weeks, with the symptom appearing a few times a day, lasting at least few minutes, and troubling the patient, and c) appearance of an itch in a regular pattern during a period of six months, but less frequently than listed above.
How the intervention might work
Given the variety of potential mediators in the pathophysiology of uraemic itch, a diverse range of interventions addressing the varied hypotheses has been investigated. These range from topical, symptomatic treatments to systemic treatments aimed at alleged underlying mechanisms. They largely target neurons (thought to be C‐fibres transmitting to the posterior spinothalamic tract and onto the thalamus and somatosensory cortex), their receptors, or their various local inflammatory triggers in the skin. They are presented here by mechanism of action.
Opioid receptor mediation
Recent studies have recognised spinal Mu‐receptor agonism as the mechanism of opioid‐associated itch (Liu 2011), supporting the theory that uraemic itch could represent ‘hyperactivity’ of mu‐receptors. A case report of successful treatment of uraemic itch with naloxone (Andersen 1984), a mu‐receptor antagonist, appeared to supported this concept leading to the conduct of several trials to further define this effect (Pauli‐Magnus 2000; Peer 1996). Mu agonism is typically associated with analgesia. Kappa agonism is typically associated with dysphoria and mu‐antagonism. It has also been suggested that excessive mu‐receptor or inadequate kappa‐receptor activity, with systemic imbalance rather than isolated mu‐receptor hyperactivity, may stimulate itch (Kumagai 2010). Thus, kappa‐receptor agonism such a nalfurafine may also be a therapeutic target (Kumagai 2010; Wikstrom 2005a).
Anti‐inflammatory immunomodulator mediation
A deregulated pro‐inflammatory immune system has also been implicated in the development of uraemic itch. Histamine is the best‐known immune trigger of pruritus. Preformed histamine is present in large amounts in mast cell granules. For this reason, after mast cell activation, it can be immediately released into the surrounding area where it can induce pruritus via H1 receptors on nerve fibres. Antihistamines act via prevention of the histamine fixation on the surface of the histamine receptors. Doxepin, a tricyclic antidepressant with anti‐H1 receptor effect has been investigated with this presumed mechanism (Pour‐Reza‐Gholi 2007).
Increased mast cell numbers have been observed in the skin of patients with CKD (Dimkovic 1992; Matsumoto 1985) leading to speculation that this excess was associated with increased mast cell and histamine activity (Stockenhuber 1987). Antagonising histamine or inhibiting mast cell degranulation would block this pathway. Cromolyn sodium is a drug that blocks mast cell degranulation in response to antigens, leading to decreased release of histamine, leukotrienes, and other inflammatory mast cell products. Another purported mechanism of excessive mast cell degranulation is by relative zinc deficiency. By supplementing zinc, degranulation and histamine release may be prevented (Marone 1986). Leukotriene antagonists prevent the role of leukotrienes in sustaining the inflammatory response after degranulation.
The observation that sun exposure could relieve undifferentiated itch led to trials of ultraviolet radiation in uraemic itch (Gilchrest 1977; Ko 2011). Early positive results were eventually attributed to the effect of ultraviolet B radiation in altering T helper subsets (Garssen 1999). These conclusions led to several controlled and non‐controlled trials of immunomodulators that could suppress T cell responses, such as tacrolimus, pimecrolimus, and thalidomide.
Thalidomide is a drug with anti‐inflammatory properties by modification the immune systems The exact mechanism of action of thalidomide is unknown, but it inhibits TNF‐α, IL‐6, IL‐10 and IL‐12 and other pro‐inflammatory cytokines. It modulates natural killer cell cytotoxicity and also inhibits NF‐κB and COX‐2 activity.
Nicotinamide (vitamin B3/niacin), and it is a member of the vitamin B family. It has no side‐effects like its relative, nicotinic acid such as vasodilation or flushing, and it is considered generally safe as a food additive or as a component in cosmetics and medications (Narita 2006). Nicotinamide has been used for a diverse range of conditions, including acne, rosacea, autoimmune bullous dermatoses, photo‐aging and photo immunosuppression by playing a significant role in DNA repair, maintenance of genomic stability and cellular response to injury, including inflammation and apoptosis (Cho 1997). It has been shown to be capable of inhibition of the expression of MHC‐II and the production of IL‐12, TNF‐α and IL‐1 and to be a potent stabilizer of mast cells and leukocytes (Namazi 2003).
Erythropoietin (EPO), a hormone produced by the kidneys that stimulates the production of red blood cells. The kidney synthetic function of EPO is impaired in CKD. EPO may have some anti‐itch properties as it is has been shown to reduce plasma histamine concentrations (Bohlius 2009).
Turmeric, a powder of the rhizomes of Curcuma longa L. (Zingiberaceae), commonly used as a dietary spice, is also used in Asian and Iranian medicine ordinarily for treatment of inflammation and skin wounds (Baliga 2006). Curcumin (diferuloylmethane), the most active and non‐toxic component of turmeric, is a polyphenol that has been extensively studied for its therapeutic benefits including anti‐ inflammatory activities (Aggarwal 2007).
Neuronal pathways
Gabapentin and pregabalin are structural analogues of the neurotransmitter gamma‐aminobutyric acid (GABA). The exact mechanisms of their antipruritic effects are not clear but may be related to the hindrance of C‐fibre mediated nociceptive sensations to the brain and thus pruritus (Patel 2007). Gabapentin may be particularly useful in forms of peripheral neuropathic pruritus, itch related to cholestasis, and post‐burn itch in addition to uraemic itch (Rayner 2013).
Ondansetron is a 5‐HT3 serotonin receptor antagonist to both the central and peripheral nervous system. 5‐HT3 is known to be an activator of neuronal receptors along the C‐fibre/spinothalamic pathway. The medication’s possible efficacy in uraemic itch has been attributed to this mechanism (Yue 2015).
Capsaicin has been demonstrated to deplete substance P, a principal neurotransmitter regulating passage of noxious stimuli (Burks 1985), and may therefore block transmission of pruritic sensation.
Chilled baby oil can also interrupt the transmission of C nerve fibres and can minimize inflammation and chemical stimulation (Kennet 2007; Wang 2006). This is thought to be mediated by temperature induced vasoconstriction, reduced cell metabolism and nerve transmission speed, and paralysis of neural receptors (Chiu 2008).
Other interventions
Ergocalciferol is a precursor in the local production of active vitamin D in the skin of HD patients after exposure to sunlight. One hypothesis, supported by trials, claims anti‐itch benefit from the positive effect of UVB exposure on uraemic pruritus (Shirazian 2013).
Activated charcoal is an agent that can bind many poisons in the stomach preventing them from being absorbed. Charcoal has been studied for possible effectiveness in uraemic pruritus (Giovannetti 1995).
Several agents have also been trialled on an empiric basis with identifiable mechanism. Cholestyramine and lidocaine have been trialled after published RCTs showed benefit with cholestatic itch (Villamil 2005). L‐carnitine has been suspected as the causative agent in other symptoms of uraemia (Bohmer 1978). Pramoxine is a commercially available topical local anaesthetic that has been shown to have antipruritic properties when used both alone and in combination with lactic acid (Grove 2004). L‐arginine ointment, a semi‐essential amino acid, has been shown to improve skin dryness and, in particular, improve pruritus in haemodialysis (HD) patients (Durant‐Finn 2008). Essential fatty acids and their derivatives have a protective function and influence skin structure and physiological characteristics (Andreassi 1997).
Why it is important to do this review
Itch affects the majority of CKD patients. The majority of patients on HD report itch symptoms. One fifth of all those on HD reported significant sleep disturbances (Narita 2006). Typically, trials investigating itch treatments are single centre studies with small numbers and often have conflicting results. The conclusions from past meta‐analyses were that there was insufficient data to recommend one treatment compared with another, and further rigorous trials were needed. Therefore, it is important that a modern systematic assessment of the existing evidence be conducted to summarise the effect of current studies. The aim of this systematic review is to summarise randomised controlled trials (RCTs) in patients with ESKD comparing any topical or systemic intervention with placebo or usual care in the management of uraemic itch.
Objectives
Our objectives are to determine:
the benefits and harms (both absolute and relative) of all topical and systemic interventions for the treatment of uraemic itch, either alone or in combination, when compared with placebo or standard care; and
the dose strength or frequency, stage of kidney disease or method of dialysis used (where applicable) in cases where the effects of these interventions vary depending on co‐interventions.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at evaluating interventions involving uraemic itch. Some studies allocated treatment based only on dialysis schedule (e.g. Monday, Wednesday, Friday) which also represent a systemic change in treatment and environment. These studies have not been included.
Types of participants
Inclusion criteria
Patients with advanced CKD defined as CKD stages 4, 5, or 5D were included.
Exclusion criteria
Patients with CKD stages 1, 2 and 3 were excluded. In studies before 2002, patients with CKD not on dialysis were excluded.
Types of interventions
All interventions, administered by any method (oral, intravenous (IV), topical, or otherwise), in any frequency and at any dose strength are included. Among people undergoing dialysis, the intervention may be administered on dialysis or non‐dialysis days. Complementary interventions (such as acupuncture or massage) were excluded because they are not easily comparable or categorised with other interventions.
Participants in included study control arms received no intervention, placebo, a different dose strength or frequency from the experimental intervention, or any other intervention not administered to experimental arm participants.
We included studies of the type:
Intervention versus placebo
Intervention A versus intervention B
Co‐intervention A versus co‐intervention B.
To simplify interpretation, each intervention was assigned a GRADE evidence profile in a summary of findings table (Guyatt 2011).
Types of outcome measures
We assessed outcome measures at the end of the treatment period or up to two weeks post‐treatment, or as reported by investigators.
Primary outcomes
-
Post treatment itch
Measured by visual analogue scale (VAS), Duo score or any other validated score for itch
Other recognised numerical or categorical itch measurement scores.
Secondary outcomes
QoL as measured by any validated QoL scale
Death
Length of treatment in hospital or outpatient clinic
Length of time to itch relief
-
Adverse events
Sleep disturbances
Dermatological reactions
Other adverse effects (e.g. neurological, gastrointestinal).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 17 December 2019 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Additional data sources included clinical study reports and direct correspondence with study authors.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were potentially relevant to the review. Two authors independently screened titles and abstracts, and discarded studies that were not applicable; however, studies and reviews that potentially included relevant data or information on studies were initially retained. The two authors independently assessed retrieved abstracts and appropriate full texts of these studies to determine which studies satisfied our inclusion criteria.
Data extraction and management
Two authors carried out data extraction independently using standardised data extraction forms. Studies reported in non‐English language journals were translated before assessment. The translators are noted in the acknowledgements. When more than one publication of one study exists, reports were grouped together and the publication with the most complete data was used in the analyses. When relevant outcomes are only published in earlier versions then these data were used. Any discrepancy between published versions were to be noted and there were no significant instances in this meta‐analysis.
Assessment of risk of bias in included studies
The following items are independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. any itch versus no itch) results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. Duo score or VAS), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales needed to be resolved.
Any validated tool for the quantification of itch was used. These included, but were not limited, to VAS and the Duo scoring system, which were the most commonly reported measurement tools for itch. VAS was scored on a 10‐point scale and the Duo scoring system is based on severity, distribution, and sleep disturbance up to a maximum score (usually 45). RCTs with clearly documented, but non‐validated scoring systems were considered as non‐ideal evidence.
Unit of analysis issues
The unit of focus was the quantities and qualities affecting a single person. For example, itch episodes/person was preferable to total number of itch episodes affecting an unspecified number of people or time frame.
Dealing with missing data
Further information required from the original author was requested by written correspondence (e.g. emailing corresponding author/s) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were to be performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated.
For missing data of the second stage of a cross‐over RCT, assuming appropriate data can be acquired from the first (pre‐cross‐over) stage, the second stage was dropped from the analysis. The "first" stage was treated at as a parallel RCT. When all the means and SD for both groups and both periods were available with an incomplete paired data analysis, all measurements from both periods were treated as parallel group studies. If this analysis in consistent with the data provided within the study, we accepted this with the acknowledgement of risk of bias in both the inflation of confidence intervals and study heterogeneity. Finally, if paired data were available (or able to be fully reconstructed) then the generic inverse variance method was used to incorporate the studies into the meta‐analysis.
Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) was critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Given the size and organisation of participants in this review, funnel plots (used to assess for the potential existence of small study bias) were not included. Reporting bias was discussed on an individual study basis (Characteristics of included studies).
Data synthesis
Data was pooled using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. participants, interventions, and study quality). Heterogeneity among participants could be related to age, geography, and stage of CKD. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose, and duration of therapy (such as increased tolerance after prolonged use of anti‐itch agents). Additionally, cross‐over studies may represent an independent source of bias due to their paired design. Adverse effects have been tabulated and assessed using descriptive techniques, because they are likely to differ among agents used. We planned to calculate the 95% risk difference for each adverse effect. However, due to the variety of interventions used and the inadequate reporting of adverse events, this was not done.
Sensitivity analysis
We planned to undertake sensitivity analyses however due the wide variety of interventions this was not performed.
Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; Guyatt 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Itch severity: a patient's subjective rating of their sensation of itch. Severity is measured on a continuous scale or as a binary response. The most common itch scales included were the VAS and Duo score. Few studies included their own holistic scale based on varying degrees of validated evidence.
VAS: a 0 to 10 cm rating using the horizontal or vertical numeric rating scale for subjective characteristics or attitudes that cannot be directly measured. It was developed originally to assess the intensity of pain, but subsequently it was also adopted for pruritus evaluation. A number of studies dealing with itch have demonstrated that VAS is a reliable method of pruritus severity measurement (Reich 2012)
Duo score: a numerical measure of itching scoring according to severity, frequency, and distribution with roughly equal contributions from each category. Originally proposed by Duo 1987, modified by Mettang 1990, and again by (Hiroshige 1995), the structure has remained consistent, while the range of score has varied from 0 to 10 to 3 to 81.
Adverse events: adverse effects were poorly and inconsistently reported across all studies. These have been documented in the results section and 'additional tables' (Table 4; Table 5; Table 6; Table 7; Table 8). Further meaningful assessments on harm could not be made and were not included in the 'Summary of findings' tables.
1. Adverse events: pharmacological interventions.
| Intervention | Participants (studies) | Route/dose | Intervention adverse effects (dropouts/participants)* | Control adverse effects (dropouts/participants)* |
| GABA analogue (pregabalin or gabapentin) versus placebo |
271 (6) | Pregabalin (a) Oral: 75 mg, twice/week Gabapentin (b) Oral: 400 mg, twice/week (c) Oral: 300 mg, 3 times/week (d) Oral: 300 mg/day (e) Oral: dose not reported |
Gunal 2004 (c): somnolence, dizziness, fatigue Naghibi 2007 (e): somnolence Naini 2007 (b): somnolence, dizziness, nausea Nofal 2016 (d): somnolence (9/27), dizziness (5/27) Tol 2010 (c): not reported Yue 2015 (a): somnolence (3/67), loss of balance (2/67) |
Gunal 2004: not reported Naghibi 2007: not reported Naini 2007: not reported Nofal 2016: not reported Tol 2010: not reported Yue 2015: not reported |
| Ondansetron versus placebo | 161 (3) | (a) Oral: 8 mg, 3 times/day (b) Oral: 8 mg, once/day (c) Oral: 8 mg, twice/day |
Ashmore 2000 (a): not reported Murphy 2003 (b): constipation (1/14), ischaemic stroke (1/18), line sepsis (1/17) Yue 2015 (c): nausea and vomiting (2/64) |
Ashmore 2000: not reported Murphy 2003: not reported Yue 2015: none |
| Kappa opioid agonists versus placebo | 626 (4) | Nalfurafine (a) Oral: 2.5 µg once/day (b) Oral: 5 µg once/day (c) IV: 5 µg, 3 times/week (d) IV: 2.5, 5 µg with dialysis CR845 (e) IV: 0.5 to 1.5 µg/kg with dialysis |
Kumagai 2010 (a, b) 2.5 µg (oral): somnolence (4.5%); insomnia (7.1%), diarrhoea (4.5%), nasopharyngitis (8.0%) 5 µg (oral): constipation (7.9%), somnolence (3.5%), insomnia (14.9%), nasopharyngitis (12.3%) Spencer 2015 (e): not reported Spencer 2017 (e) (0.5 to 1.5 µg/kg): somnolence (9/129), dizziness (12/129), headache (5/129), diarrhoea (16/129), nausea (11/129) Bhaduri 2006 (d): not reported Wikstrom 2005 (c): headache (3/26), nausea (3/26), vomiting (2/26), insomnia (2/26), vertigo (2/26) |
Kumagai 2010: nasopharyngitis (17.1%), headache (3.6%), vomiting (3.6%) Spencer 2015: not reported Spencer 2017: somnolence (1/45), dizziness (2/45), headache (1/45), diarrhoea (0/45) Wikstrom 2005: 13/25 (type not reported) |
| Mu opioid antagonists versus placebo | 31 (2) | Oral: 50 mg once/day |
Pauli‐Magnus 2000: loss of appetite and nausea (9) Peer 1996: heartburn (2), abdominal discomfort (3) |
Pauli‐Magnus 2000: nausea (1) Peer 1996: not reported |
| Nalbuphine versus placebo | 373 (1) | Oral: 60 or 120 mg, twice/day |
TREVITR02 2017 60 mg: serious adverse events (12.7%), adverse events leading to discontinuation (33/128) 120 mg: serious adverse events (6.7%), adverse events leading to discontinuation (27/120) |
TREVITR02 2017: serious adverse events (15.4%), adverse events leading to discontinuation (7/123) |
| EPO versus placebo | 39 (2) | (a) IV: 36 U/kg/dialysis (b) SC: 2000 IU twice/day |
De Marchi 1992 (a): not reported Sja'bani 1997 (b): not reported |
De Marchi 1992: not reported Sja'bani 1997: not reported |
| Nicotinamide versus placebo | 50 (1) | Oral: 500 mg twice/day | Omidian 2013: not reported | Omidian 2013: not reported |
| Lidocaine versus placebo | 20 (1) | IV: 200 mg | Tapia 1977: not reported | Tapia 1977: not reported |
| Cholestyramine | 20 (2) | Oral: 5 mg, twice/day |
Silverberg 1977: constipation (1/5), nausea (1/5) van Leusen 1978: not reported |
Silverberg 1977: not reported van Leusen 1978: not reported |
| Montelukast versus placebo | 89 (2) | Oral: 10 mg/day |
Mahmudpour 2017: not reported Nasrollahi 2007: myelodysplastic syndrome (1/8) |
Mahmudpour 2017: Not reported Nasrollahi 2007: myocardial infarction (1/8) |
| Sertraline versus placebo | 50 (1) | Oral: 50 mg twice/day | Pakfetrat 2018: not reported | Pakfetrat 2018: not reported |
| Sodium thiosulfate versus placebo | 45 (1) | IV: 12.5 mg/dialysis session | Mohamed 2012: not reported | Mohamed 2012: not reported |
| Doxepin versus placebo | 24 (1) | Oral: 10 mg, twice/day | Pour‐Reza‐Gholi 2007: drowsiness (12/24) | Pour‐Reza‐Gholi 2007: not reported |
| Thalidomide versus placebo | 29 (1) | Oral: 100 mg/day | Silva 1994: not reported | Silva 1994: not reported |
| Cimetidine versus placebo | 13 (1) | Oral: 600 mg/day | Aubia 1980: not reported | Aubia 1980: not reported |
| Cromolyn versus placebo |
62 (1) | Oral: 135 mg, 3 times/day | Vessal 2010: flatulence (1/32) | Vessal 2010: nausea (5/30), diarrhoea (4/30) |
| Gabapentin versus pregabalin | 50 (1) | Oral gabapentin (300 mg, once/day) versus oral pregabalin (75 mg, once/day) |
Solak 2012 Gabapentin: not reported Pregabalin: not reported |
‐‐ |
| GADA versus ondansetron | 131 (1) | Oral pregabalin (75 mg twice/week) versus oral ondansetron (8 mg/day) |
Yue 2015 Pregabalin: somnolence (3/67), loss of balance (2/67) Ondansetron: not reported |
‐‐ |
| GABA analogue versus doxepin | 90 (1) | Oral pregabalin (50 mg every other night) versus oral doxepin (10 mg/night) |
Foroutan 2017 Pregabalin: intolerable adverse events (3/46), somnolence (6/37), oedema (3/37), drowsiness (3/27), imbalance (1/37), numbness (1/37) Doxepin: intolerable adverse events (1/44), nervousness (1/35) |
‐‐ |
| GABA analogue versus antihistamine | 212 (4) | (a) Oral gabapentin (100 mg/day) versus oral ketotifen (1 mg, twice/day) (b) Oral gabapentin (300 mg, 3 times/week) versus oral dexchlorpheniramine (6 mg, 3 times/week) (c) Oral gabapentin (300 mg/day) versus oral loratadine (10 mg/day) (d) Oral gabapentin (100 to 200 mg/day) versus oral hydroxyzine (10 mg/day) (e) Oral gabapentin (100 mg/day) versus oral hydroxyzine (10 mg/day) |
Amirkhanlou 2016 (a) Gabapentin: drowsiness (4/26), dizziness (1/26) Ketotifen: drowsiness (4/26), dizziness (1/26) Gobo‐Oliveira 2018 (b) Gabapentin: total (11/30), drowsiness (17%) Dexchlorpheniramine: total (8/30), drowsiness (1/30) Marin 2013 (c) Gabapentin: somnolence (8/30) Loratadine: none reported Noshad 2011 (d) Gabapentin: complications (7/20) Hydroxyzine: complications (10/20) Suwanpidokkul 2007 (e) Gabapentin: (9/18) Loratadine: (4/16) |
‐‐ |
| Mu opioid antagonists versus antihistamine | 52 (1) | Oral naltrexone (50 mg/day) versus oral loratadine (10 mg/day) |
Legroux‐Crespel 2004 Naltrexone (26): vomiting (2), nausea (9), anorexia (1), abdominal distention (1), malaise (1), cramps (2), sleep disturbances (5), vertigo (5), headache (2), somnolence (1), paraesthesia (1), withdrawn (10) Loratadine (26): vomiting (2), malaise (1), withdrawn from study (2) |
‐‐ |
| Ondansetron versus antihistamine | 20 (1) | (a) Ondansetron tablet (8 mg/day) versus cyproheptadine syrup (8 mg/day) (b) "3 doses ondansetron 8mg" versus "diphenhydramine 25mg" (c) Oral ondansetron (8 mg, 3 times/day) versus oral loratadine (10 mg twice/day) |
Ozaykan 2001 (a): not reported Subach 2001 (b): not reported Mirnezami 2013 (c): not reported |
‐‐ |
*when reported
GABA ‐ gamma‐aminobutyric acid
2. Adverse events: topical interventions.
| Intervention | Participants (studies) | Route/dose | Intervention adverse effects (dropouts/participants)* | Control adverse effects (dropouts/participants)* |
| Cromolyn cream versus placebo | 60 (1) | 4% cream | Feily 2012: burning sensation (6/30) | Feily 2012: none |
| Capsaicin cream versus placebo | 91 (4) | (a) 0.025%, 4 times/day (b) 0.03%, 4 times/day |
Breneman 1992 (a): burning and stinging sensation (5), decrease in xerosis (3), dryness (2) Cho 1997 (a): not reported Makhlough 2010 (b): skin burning Tarng 1996 (a): local burning and/or stinging sensations |
Breneman 1992: not reported Cho 1997: not reported Makhlough 2010: none Tarng 1996: local burning and/or stinging sensations |
| Pramoxine lotion versus placebo | 28 (1) | 1.0% twice/day | Young 2009: none | Young 2009: none |
| Baby oil versus placebo | 92 (1) | Chilled and unchilled 15 min application at least once/day | Lin 2012: not reported | Lin 2012: not reported |
| Dead Sea lotion versus placebo | 50 (1) | Entire body, twice/day | Boaz 2009: total adverse events (2/25) | Boaz 2009: total adverse events (3/25) |
| Sericin cream versus placebo | 50 (1) | 1 g, twice/day | Aramwit 2012a: not reported | Aramwit 2012a: not reported |
| L‐arginine salve versus placebo | 24 (1) | 25 µg/2.5 cm2 twice/day | Durant‐Finn 2008: not reported | Durant‐Finn 2008: not reported |
| Calcineurin inhibitors versus placebo | 80 (2) | TAC: 0.1% twice/day Pimecrolimus: 1.0% twice/day |
Duque 2005: warmth sensation (6/12) Ghorbani 2012a: burning sensation which disappeared by the end of 8 weeks |
Duque 2005: warmth sensation (3/8) Ghorbani 2012a: none |
| Sweet almond oil versus no intervention | 44 (1) | 100 mg/day | Afrasiabifar 2017: not reported | Afrasiabifar 2017: not reported |
| Gamma‐linoleic acid versus placebo | 17 (1) | 2.2%, 30 mL/day | Chen 2006e: allergic reaction (1/8) | Chen 2006e: none |
| Calcineurin inhibitors versus cromolyn | 60 (1) | Pimecrolimus: 2% twice/day Cromolyn: 4%, twice/day |
Ghorbani Birgani 2011: unknown | Ghorbani Birgani 2011: unknown |
| Avena sativa versus diluted vinegar versus hydroxyzine | 23 (1) | Avena sativa: variable dose, twice/day Dilute vinegar: 30 mL twice/day Oral hydroxyzine: 10 mg/day |
Nakhaee 2015: not reported | ‐‐ |
| Sarna versus eurax | 30 (1) | Sarna: 0.5% each of camphor, menthol, and phenol "as required" for 7 days Eurax: 10% crotamiton "as required" for 7 days |
Tan 1990 Sarna: none Eurax: rash (1) |
‐‐ |
*when reported
3. Adverse events: oral and IV supplements.
| Intervention | Participants (studies) | Dose/route | Intervention adverse effects (dropouts/participants)* | Placebo adverse effects (dropouts/participants)* |
| Polyunsaturated fatty acids versus placebo | 89 (4) | Fish oil (a) Oral: 6 g/day (b) Oral: 3 g/day Omega‐3 fatty acids (c) Oral: 3 g/day |
Begum 2004 (a): not reported Ghanei 2012 (c): not reported Mojgan 2017 (b): not reported Peck 1996 (a): not reported |
Begum 2004: not reported Ghanei 2012: not reported Mojgan 2017: not reported Peck 1996: not reported |
| L‐carnitine versus placebo | 17 (1) | IV: 10 mg/kg, once/day | Mettang 1997: not reported | Mettang 1997: not reported |
| Zinc sulfate versus placebo | 80 (2) | (a) Oral: 220 mg/day (b) Oral: 200 mg twice/day |
Mapar 2015 (a): none Najafabadi 2012 (b): none “attributable to zinc sulfate” |
Mapar 2015: vomiting (1/20) Najafabadi 2012: not reported |
| Ergocalciferol versus placebo | 50 (1) | Oral: 50,000 IU/week | Shirazian 2013: none | Shirazian 2013: not reported |
| Turmeric (curcumin) versus placebo | 100 (1) | Oral: 500 mg (22.1 mg), 3 times/day | Pakfetrat 2014: none | Pakfetrat 2014: not reported |
| Fumaria parviflora versus placebo | 79 (1) | Oral: 1000 mg, 3 times/day | Akrami 2017: Gastric pain (4/39), rash (1/39) | Akrami 2017: abdominal pain (1/40), constipation (1/40) |
| Senna versus placebo | 60 (1) | Oral: dose and frequency not reported | Fallahzadeh 2015: not reported | Fallahzadeh 2015: not reported |
| Evening primrose oil | 16 (1) | Oral: 2 capsules/day (containing 360 mg of linoleic acid, 50 mg oleic acid and 45 mg of gamma‐linoleic acid) | Yoshimoto‐Furuie 1999: none | Yoshimoto‐Furuie 1999: none |
| Activated charcoal versus placebo | 20 (1) | Oral: 6 g/day | Pederson 1980: not reported | Pederson 1980: not reported |
| Charcoal versus aluminium hydroxide | 30 (1) | Charcoal: 6 g, 3 times/day Aluminium hydroxide: 30 mL, 3 times/day |
Shariati 2010: not reported | ‐‐ |
*when reported
4. Adverse events: dialysis modality.
| Intervention | Participants (studies) | Dose/route | Intervention adverse effects (dropouts/participants)* | Control adverse effects (dropouts/participants)* |
| High flux/ high permeability/high flow HD |
252 (4) | (a) High‐flow HD (b) High‐permeability HD (c) High‐flux HD |
Aliasgharpour 2018 (a): not reported Chen 2009 (b): not reported Hui 2011 (c): not reported Jiang 2016 (c): not reported |
Aliasgharpour 2018: not reported Chen 2009: not reported Hui 2011: not reported Jiang 2016: not reported |
| HD with haemoperfusion | 90 (1) | Haemoperfusion HA130‐RHA HA330‐RHA |
Li 2017a: not reported | Li 2017a: not reported |
| Haemoperfusion plus HD versus haemoperfusion plus HDF | 40 (1) | Haemoperfusion plus HD Haemoperfusion plus HDF |
Zhang 2016a Haemoperfusion plus HD: not reported Haemoperfusion plus HDF: not reported |
‐‐ |
| Magnesium‐free HD versus standard HD | 17 (1) | Standard HD: 0.85 mmol/L magnesium solution for 2 weeks | Carmichael 1988: not reported | Carmichael 1988: not reported |
| Calcium dialysate HD | 4 (1) | Calcium concentration 1.0 mmol/L 1.25 mmol/L 1.75 mmol/L |
Kyriazis 2000: not reported | Kyriazis 2000: not reported |
| Cool versus normal dialysate | 60 (1) | Cool dialysate: 35.5oC, 3 times/week Normal dialysate: 37oC, 3 time/week |
Rad 2017: not reported | Rad 2017: not reported |
*when reported
5. Adverse events: other interventions.
| Intervention | Participants (studies) | Dose/route | Intervention adverse effects (dropouts/participants)* | Control adverse effects (dropouts/participants)* |
| UV‐B exposure | 75 (4) | (a) 0.19 nJ/cm2/sec, 3 times/week (b) Minimal erythema dose, twice/week (c) 4.4 watts/m2, twice/week (d) 200 mJ/cm2, 3 times/week |
Blachley 1985 (a): not reported Chan 1995 (b): not reported Gilchrest 1977 (c): sunburn (3/10), tanning (5/10) Gilchrest 1979 (c): mild sunburn and tanning Ko 2011 (d): erythema (2/11) |
Blachley 1985: not reported Chan 1995: not reported Gilchrest 1977: not reported Gilchrest 1979: not reported Ko 2011: not reported |
| UV‐A exposure | 11 (1) | UV‐A (exposure): 40 min exposure (10, 180 cm 85W UV‐A lamps) 3 times/week | Taylor 1983: not reported | Taylor 1983: not reported |
| Thermal therapy | 41 (1) | 40oC thermal therapy, twice/week | Hsu 2009: not reported | Hsu 2009: not reported |
| UV‐B exposure versus cetirizine | 30 (1) | UV‐B Whole body: 200 to 1038 mJ/cm2 every 3rd day for 15 sessions Cetirizine Oral: 10 mg/day for the same duration |
Sherjeena 2017: not reported | ‐‐ |
*when reported
Results
Description of studies
Results of the search
The process of selecting records and studies for inclusion in this systematic review is outlined in Figure 1. The titles, abstracts, and summaries of 689 records were evaluated from three separate databases and the Specialised Register. Overlap within the database searches resulted in 196 records removed as duplicate records. An additional 312 records were excluded due to failing to meet study design, intervention, participant, or outcome criteria prior to full‐text review.
1.
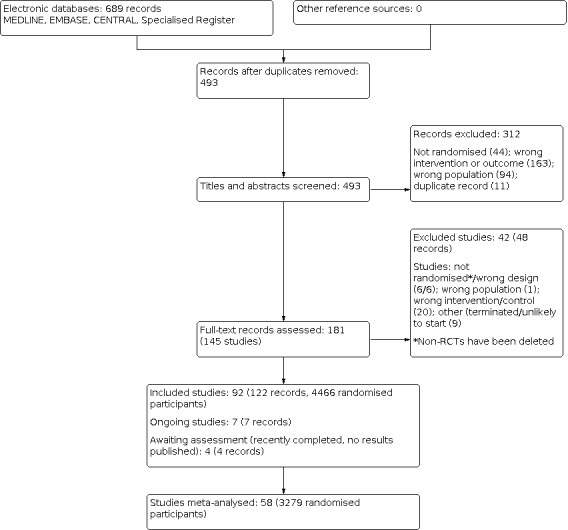
Study flow diagram.
We contacted 27 authors of papers with conflicting or missing data. Of all authors contacted for further clarification seven responded including Dr. Tol, Dr. Ashmore, Dr. Tarng, Dr. Pornanong Aramwit. Dr. Fleicher, Dr. Peer, and Dr. Haghverdi. Five authors provided supplementary data incorporated into the review.
In total we identified 144 studies (181 records). Ninety‐one studies met our inclusion criteria and 42 studies (48 records) were excluded; six non‐RCTs were subsequently deleted. There are seven ongoing studies (ACTRN12614000677606; DON'T ITCH 2015; IRCT201311152417N14; IRCT2015051411940N3; NCT03422653; NCT03636269; SNUG 2019 and three studies (NCT01513161; NCT02696499; NCT02747979) have recently been completed but have yet to report results. These 10 studies will be assessed in a future update of this review.
Included studies
Ninety‐two studies (122 records) randomising 4466 participants met our inclusion criteria. All were RCTs that evaluated changes in itch (the primary outcome) associated with CKD before and after an intervention. Almost 90% of all RCTs originated from the USA, UK, Israel, Taiwan, Iran, Germany, or Japan. Translated non‐English study languages included Farsi, French, Mandarin, Turkish, German, and Spanish.
The identified RCTs yielded a broad spectrum of different interventions for the treatment of itch associated with different underlying diseases. A total of 78 studies were placebo‐controlled, five studies compared gabapentinoids versus antihistamines or gabapentin versus pregabalin, and nine studies compared different dialysis modalities or dialysis solutions.
The most common reason for studies not to be included in this review's quantitative analysis was inadequate reporting that precluded a meaningful comparison (e.g. SD or placebo results not explicitly reported). Thirty additional studies were included in the qualitative analysis.
All but 23 studies described adverse effects in at least the intervention group. Just over half of the studies failed to specify adverse effects (or lack thereof) in the control population. A handful of studies also measured QoL, sleep quality, depression, dialysis quality, or patient satisfaction. Two studies with pharmacological interventions measured the interaction of dialysis modality with their intervention. No meaningful qualitative or quantitative analysis could be made from secondary outcomes other than adverse events.
For additional information on all included studies see Characteristics of included studies.
Excluded studies
Forty‐two studies were excluded from this review after comprehensive full text analysis. The most common reasons for exclusion were not meeting proper criteria for a true RCT, followed by inappropriate intervention. Four studies did not meet our protocol's criteria for the target population. Finally, eight excluded studies appeared to have never been initiated or stopped prematurely without publishing results.
Across all searched studies the most common reasons for exclusion were:
Outcome not truly itch‐related (e.g. serum PTH level used as a surrogate monitor)
Lack of a true control, self‐control, or comparison group
Wrong intervention
Selected studies were not truly randomised or pseudo‐randomised.
Gross omission of data based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selected studies include patients without CKD
Study was never initiated or stopped prematurely without publishing results.
For additional information on all excluded studies see Characteristics of excluded studies
Risk of bias in included studies
All studies included in the meta‐analyses were RCTs, either parallel or cross‐over. Each explicitly reported patients as randomised to an intervention or placebo group. Sensitivity analyses were conducted for each intervention that included both only parallel RCTs versus cross‐over with parallel RCT data. No significant differences in effect size of heterogeneity were observed. See Figure 2; Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Forty‐two studies reported a specific method of randomisation, either computer‐generated or the use of a random number table. Three studies were judged to be at high risk of bias (Ghanei 2012; Lin 2012; Sherjeena 2017), and the remaining 47 studies were considered to be uncertain risk.
Allocation concealment
Nineteen studies were judged to be al low risk of bias for allocation concealment, two were at high risk of bias ((Marin 2013; Sherjeena 2017), and the remaining 71 studies were considered to be of uncertain risk.
Blinding
Two studies (Ko 2011; Nasrollahi 2007) only blinded the participants (single blind), and three studies (Lin 2012; Marin 2013; Ozaykan 2001) were open‐label studies. Complicated equipment for emitting UVB radiation (Ko 2011; Sherjeena 2017), administering new dialysis modalities (Zhang 2016a), or the absolute temperature of the intervention (Lin 2012; Rad 2017) may have precluded any blinding efforts. The majority of blinded studies utilized unlabelled pills/infusions for oral/IV interventions or a comparable unlabelled vehicle of similar consistency for blinding of a topical agent.
For performance bias, 10 studies were judged to be at high risk of bias (Afrasiabifar 2017; Hui 2011; Legroux‐Crespel 2004; Li 2017a; Marin 2013; Nakhaee 2015; Ozaykan 2001; Sherjeena 2017; Solak 2012; Zhang 2016a), 59 studies were at low risk of bias, and the remaining 23 studies were considered to be of uncertain risk.
For detection bias, 14 studies were judged to be at high risk of bias (Gilchrest 1977; Gilchrest 1979; Legroux‐Crespel 2004; Li 2017a; Lin 2012; Marin 2013; Mohamed 2012; Nakhaee 2015; Nasrollahi 2007; Ozaykan 2001; Sherjeena 2017; Solak 2012; Taylor 1983; Zhang 2016a), 47 were at low risk of bias, and the remaining 30 studies were considered to be of uncertain risk.
Incomplete outcome data
Eight studies (Carmichael 1988; Gilchrest 1979; Murphy 2003; Peck 1996; Pederson 1980; Silva 1994; Tapia 1977; Vessal 2010) had a greater than 10% dropout rate, mainly reflective of low sample sizes. The average post randomisation size of these eight studies was 28 participants. Only one of these studies analysed on an intention‐to‐treat basis. Twenty‐two of the remaining studies were completed with one or more dropouts after randomisation; half were analysed on an intention‐to‐treat basis.
For attrition bias, 12 studies were judged to be at high risk of bias (Breneman 1992; Carmichael 1988; Gilchrest 1977; Gilchrest 1979; Murphy 2003; Peck 1996; Pederson 1980; Silva 1994; Tamimi 1999; Tapia 1977; Vessal 2010), 52 studies were at low risk of bias, and 28 studies were considered to be of uncertain risk.
Selective reporting
Two studies appeared to only report and collect categorical or binary endpoints such as “significant itch reduction” versus “no significant itch reduction (Gilchrest 1979; Tapia 1977). Silva 1994 clearly collected continuous itch outcomes, but only reported and analysed binomial outcomes. Tol 2010, Pederson 1980, and Tarng 1996 did not report placebo results and could not be included in the quantitative analysis.
For reporting bias, 25 studies were judged to be at high risk of reporting bias (Amirkhanlou 2016; Aubia 1980; Baumelou 1993; Breneman 1992; Carmichael 1988; Chan 1995; De Marchi 1992; Duque 2005; Gilchrest 1977; Kyriazis 2000; Legroux‐Crespel 2004; Mohamed 2012; Mojgan 2017; Nakhaee 2015; Pederson 1980; Rad 2017; Rivory 1984; Silva 1994; Spencer 2017; Tamimi 1999; Tapia 1977; Tarng 1996; Taylor 1983; Tol 2010; Young 2009), 46 studies were at low risk of bias, and 21 studies were considered to be of uncertain risk.
Other potential sources of bias
In all the included studies, post‐randomisation dropout rates were balanced (no statistically significant difference in dropout rates) between intervention and control with the exception of Pauli‐Magnus 2000 which had five dropouts (2.5%) in the intervention group for the indication of opioid pain relief. However, this was anticipated in pretrial planning and the patients were included in the analysis on an intention‐to‐treat basis.
The authors of six studies (Boaz 2009; Duque 2005; Spencer 2015; Spencer 2017; TREVITR02 2017; Young 2009) had financial backing from the respective pharmaceutical manufacturers. One study (Legroux‐Crespel 2004) reported conflicting results and used arbitrary definitions of improvement. These seven studies were judged to be at high risk of bias. Sixty studies were judged to be at low risk of bias and 25 studies were considered to of uncertain risk.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Pharmacological interventions versus placebo for the relief of itch in people with advanced chronic kidney disease.
| Pharmacological interventions versus placebo for the relief of itch in people with advanced chronic kidney disease | |||||
|
Patient or population: uraemic pruritus Settings: outpatient and multi‐centre Intervention: pharmacological treatments Comparison: placebo | |||||
| Outcomes |
Anticipated absolute effects* (95% CI) |
Relative Effect (95% CI) | No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Reduction of risk of placebo | Reduction of risk with pharmacological interventions | ||||
| GABA analogue VAS(0 to 10 cm) |
The mean VAS score of the placebo group ranged from 0.8 to 2 cm lower than pretreatment scores | The mean reduction in VAS score of the GABA analogue group was 4.95 cm lower (5.46 to 4.44 lower) than placebo | ‐ | 297 (5) | ⊕⊕⊕⊕ HIGH |
| Ondansetron VAS(0 to 10 cm) |
The mean VAS score of the placebo group ranged from 0.1 to 2 cm lower than pretreatment scores | The mean reduction in VAS score of the ondansetron agonist group was 0.38 cm lower (1.04 lower to 0.27 higher) than placebo | ‐ | 183 (3) | ⊕⊕⊕⊕ HIGH |
| Kappa‐opioid agonist VAS(0 to 10 cm) |
The mean VAS score of the placebo group ranged from 1.3 to 1.9 cm lower than pretreatment scores | The mean reduction in VAS score of the kappa‐opioid agonist group was 1.05 cm lower (1.40 to 0.70 lower) than placebo | ‐ | 661 (5) | ⊕⊕⊕⊕ HIGH |
| Mu‐opioid antagonist VAS(0 to 10 cm) |
The mean VAS score of the placebo group ranged from 0.5 to 1 cm lower than pretreatment scores | The mean reduction in VAS score of the mu‐opioid antagonist group was 4.29 cm lower (10.24 lower to 1.66 higher) than placebo | ‐ | 62 (2) | ⊕⊕⊝⊝ LOW1,2 |
| Nalbuphine VAS(0 to 10 cm) |
The mean VAS score of the placebo group was 3.2 cm lower than pretreatment scores | The mean reduction in VAS score of the nalbuphine group was 0.75 cm lower (1.70 lower to 0.20 higher) than placebo | ‐ | 179 (1) | ⊕⊕⊝⊝ LOW2,3 |
| Cromolyn VAS(0 to 10 cm) |
The mean VAS score of the placebo group was 3 cm lower than pretreatment scores | The mean reduction in VAS score of the cromolyn group was 4.8 cm lower (7.03 to 2.57 lower) than placebo | ‐ | 40 (1) | ⊕⊕⊝⊝ LOW1,2 |
| Nicotinamide VAS(0 to 5 cm) |
The mean VAS score of the placebo group was 1.7 cm lower than pretreatment scores | The mean reduction in VAS score of the nicotinamide group was 0.47 cm higher (0.32 lower to 1.26 higher) than placebo | ‐ | 50 (1) | ⊕⊕⊝⊝ LOW1,2 |
| EPO Duo score(0 to 40) |
The mean Duo score of the placebo group was 1.5 lower than pretreatment scores | The mean reduction in Duo score of the EPO group was 14.5 lower (38.78 lower to 9.78 higher) than placebo | ‐ | 20 (1) | ⊕⊝⊝⊝ VERY LOW1,2,3 |
| Cholestyramine 0 to 3 severity scale |
The mean itch score of the placebo group ranged from 1.3 to 0.7 lower than pretreatment scores | The mean reduction in VAS score of the cholestyramine group was 0.24 higher (0.38 lower to 0.86 higher) than placebo | ‐ | 15 (2) | ⊕⊕⊝⊝ LOW1,4 |
| Montelukast Duo score (0 to 81) and VAS (0 to 10 cm) |
The mean Duo score and VAS of the placebo group was 7 points and 0.5 cm lower (respectively) than pretreatment scores. | TheSMD reduction of the montelukast group was 1.4 lower (1.87 to 0.92 lower) than placebo | ‐ | 87 (2) | ⊕⊕⊕⊝ MODERATE5 |
| Sertraline VAS(0 to 10 cm) |
The mean VAS score of the placebo group was 3.7 lower than pretreatment scores | The mean reduction in VAS score of the sertraline group was 1.8 cm lower (3.65 lower to 0.05 higher) than placebo | ‐ | 46 (1) | ⊕⊕⊝⊝ LOW1,2 |
| Lidocaine Itch relief |
167 per 1000 | 800 per 1000 (221 to 1000) | 4.80 (0.78 to 29.50) | 16 (1) | ⊕⊝⊝⊝ VERY LOW1,2,3 |
| Sodium thalidomide Itch relief |
133 per 1000 | 556 per 1000 (177 to 1000) | 4.17 (1.08 to 16.15) | 33 (1) | ⊕⊝⊝⊝ VERY LOW1,2,3 |
| Doxepin Itch relief |
208 per 1000 |
875 per 1000 (396 to 1000) |
4.20 (1.90 to 9.30) |
48 (1) | ⊕⊕⊝⊝ LOW1,2 |
| The reduction of risk of pharmacological versus placebo (column 3) is the additional risk reduction in addition to the benefit provided by the placebo. "Lower" indicates a reduction or negative numerical change versus baseline. CI: Confidence interval; SMD: standardised mean difference; RR: Risk Ratio; VAS: visual analogues scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Evidence of certainty was downgraded one level because of the reliance of the estimated effect on a small number of participants
2Evidence of certainty was downgraded one level because of the imprecise treatment estimate
3Evidence of certainty was downgraded one level because of study risks of bias
4Evidence of certainty was downgraded one level because heterogeneous results utilizing nonvalidated itch scoring methods
5Evidence of certainty was downgraded one level as homogeneity was difficult to assess (due to well validated but different itch scoring methods) and that the analysis would benefit from a greater number of participants
Summary of findings 2. Topical treatments versus placebo for the relief of itch in people with advanced chronic kidney disease.
| Topical treatments versus placebo for the relief of itch in people with advanced chronic kidney disease | ||||
|
Patient or population: uraemic pruritus Settings: outpatient and multi‐centre Intervention: topical treatments Comparison: placebo | ||||
| Outcomes |
Anticipated absolute effects* (95% CI) |
No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Reduction of risk of placebo | Reduction of risk with topical treatments | |||
| Capsaicin cream VAS and Duo’s score |
The mean VAS and Duo score of this vehicle group was 1.7 cm and 13.4 lower (respectively) than pretreatment scores. | The SMD of the capsaicin group was 0.84 lower (1.22 to 0.45 lower) than vehicle | 112 (2) | ⊕⊕⊕⊝ MODERATE1 |
| Pramoxine lotion VAS(0 to 10 cm) |
The mean VAS score of this vehicle group was 1.4 cm lower than pretreatment scores. | The mean reduction in VAS score of the pramoxine lotion group was 1.97 lower (6.06 lower to 2.12 higher) than vehicle | 27 (1) | ⊕⊝⊝⊝ VERY LOW2,3,4 |
| Calcineurin inhibitor VAS(0 to 10 cm) |
The mean VAS score of this vehicle group was 7.1 cm lower than pretreatment scores. | The mean reduction in VAS score of the calcineurin inhibitor group was 1.2 higher (0.36 lower to 2.76 higher) than vehicle | 80 (2) | ⊕⊝⊝⊝ VERY LOW2,3,4 |
| Dead Sea lotion 1 to 5 severity score |
The mean severity score of this vehicle group was 3 lower than pretreatment scores. | The mean reduction in severity score of the Dead Sea Lotion group was 2 lower (4.31 lower to 0.31 higher) than vehicle | 41 (1) | ⊕⊝⊝⊝ VERY LOW2,3,4 |
| Cromolyn cream VAS (0 to 5 cm) |
The mean VAS score of this vehicle group was 1.4 cm lower than pretreatment scores. | The mean reduction in VAS score of the cromolyn cream group was 0.8 cm lower (1.98 lower to 0.38 higher) than vehicle | 60 (1) | ⊕⊕⊝⊝ LOW2,3 |
| Baby oil Itch Severity Scale(0 to 21) |
The mean Itch Severity Scale of this vehicle group was 1 lower than pretreatment scores. | The mean reduction in Itch Severity Scale of the baby oil group was 2.36 lower (3.29 to 1.44 lower) than vehicle | 125 (2) | ⊕⊕⊝⊝ LOW5 |
| L‐arginine salve 0 to 3 severity score |
The mean severity score of this vehicle group was 3.4 lower than pretreatment scores. | The mean reduction in severity score of the L‐arginine salve group was 0.58 lower (1.86 lower to 0.7 higher) than vehicle | 48 (1) | ⊕⊕⊝⊝ LOW2,3 |
| Polyunsaturated fatty acids VAS (0 to 10 cm) Duo score |
The mean VAS and Duo score of this vehicle group was 1 cm lower and 5 points higher (respectively) than pretreatment scores. | The SMD of the polyunsaturated fatty acids group was 0.91 lower (1.99 lower to 0.17 higher) than vehicle | 78 (2) | ⊕⊕⊝⊝ LOW2,6 |
| The reduction of risk of pharmacological versus placebo (column 3) is the additional risk reduction in addition to the benefit provided by the placebo. "Lower" indicates a reduction or negative numerical change versus baseline. CI: Confidence interval; SMD: standardised mean difference; VAS: visual analogue scale | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Evidence of certainty was downgraded one level as homogeneity was difficult to assess (due to well validated but different itch scoring methods) and that the analysis would benefit from a greater number of participants
2Evidence of certainty was downgraded one level because of the reliance of the estimated effect on a small number of participants
3Evidence of certainty was downgraded one level because of the imprecise treatment estimate
4Evidence of certainty was downgraded one level because of study risks of bias
5Evidence of certainty was downgraded two levels because of study risks of bias and use of a non‐validated itch scoring method.
6Evidence of certainty was downgraded one level because of the imprecise and small treatment estimate
Summary of findings 3. Supplements, haemodialysis modalities, and other treatments for the relief of itch in people with advanced chronic kidney disease.
| Supplements, HD modalities, and other treatments for the relief of itch in people with advanced chronic kidney disease | ||||
|
Patient or population: uraemic pruritus Settings: outpatient and multi‐centre Intervention: supplements, HD modalities, and other treatments Comparison: placebo; other HD comparators | ||||
| Outcomes | Anticipated absolute effects* (95% CI) |
No. of participants (RCTs) | Quality of the evidence (GRADE) | |
| Reduction of risk of comparator | Reduction of risk with supplements, HD modalities, and other treatments | |||
| Polyunsaturated fatty acids 0 to 5severity score |
The mean severity score of this placebo group was 1.6% lower than pretreatment scores. | The mean reduction in 0 to 5 severity score of the polyunsaturated fatty acids group was 11.3% lower (9.0 to 3.6 lower) than placebo | 22 (1) | ⊕⊕⊝⊝ LOW1,2 |
| L‐carnitine VAS (0 to 6 cm) |
The mean VAS score of this placebo group was 0.2 higher than pretreatment scores. | The mean reduction in VAS score of the L‐carnitine group was 0.26 lower (2.85 lower to 2.43 higher) than placebo | 12 (1) | ⊕⊕⊝⊝ LOW1,2 |
| Zinc sulfate VAS (0 to 10 cm) |
The mean VAS and Duo score of this vehicle group was 4.3cm and 6.1 lower (respectively) than pretreatment scores. | The mean reduction of the zinc sulfate group was 1.77 lower (2.88 to 0.66 lower) than placebo | 76 (2) | ⊕⊕⊕⊝ MODERATE1 |
| Ergocalciferol 21 point scale |
The mean score of this vehicle group was 6.1 lower than pretreatment scores. | The mean reduction in VAS score of the ergocalciferol group was 0.4 higher (2.52 lower to 3.32 higher) than placebo | 50 (1) | ⊕⊕⊝⊝ LOW1,2 |
| Turmeric Duo score (5 to 40) |
The mean Duo’s score of this vehicle group was 2 lower than pretreatment scores. | The mean reduction in VAS score of the turmeric group was 6.4 lower* (7.42 to 5.38 lower) than placebo | 100 (1) | ⊕⊕⊕⊝ MODERATE1 |
| Fumaria parviflora VAS (0 to 10 cm) |
The mean VAS score of this vehicle group was 2.2lower than pretreatment scores. | The mean reduction in VAS score of the Fumaria parviflora group was 3.90lower (5.04 to 2.76 lower) than placebo | 63 (1) | ⊕⊕⊝⊝ LOW1,3 |
| High flux/permeability dialysis VAS (0 to 10 cm) |
The mean VAS score of this control group ranged from 0.6 cm to 5.6 cm lower than pretreatment scores. | The mean reduction in VAS score of the high flow/permeability group was 2.60 cm lower (3.22 to 1.97 lower) than placebo | 202 (3) | ⊕⊕⊝⊝ LOW3,4 |
| HD with haemoperfusion VAS (0 to 10 cm) |
The mean VAS score of this control group was 0.6 cm lower than pretreatment scores. | The mean reduction in VAS score of the HD with haemoperfusion group was 2.37 cm lower (2.89 to 1.85 lower) than placebo | 90 (1) | ⊕⊕⊝⊝ LOW1,3 |
| UV‐B Duo score, VAS, and %improvement |
The mean Duo score and VAS of this control group was 2.2 points and 0.3 cm lower (respectively) than pretreatment scores. | The SMD of the UV‐B group was 2.49 lower (4.62 to 0.36 lower) than placebo | 86 (4) | ⊕⊕⊝⊝ LOW1,3 |
| Thermal therapy VAS (0 to 10 cm) |
The mean VAS score of this control group was 5.8 lower than pretreatment scores. | The mean reduction in VAS score of the thermal therapy group was 2.06 lower (6.98 lower to 2.84 higher) than placebo | 41 (1) | ⊕⊕⊝⊝ LOW1,2 |
| The reduction of risk of pharmacological versus placebo (column 3) is the additional risk reduction in addition to the benefit provided by the placebo. "Lower" indicates a reduction or negative numerical change versus baseline. CI: Confidence interval; RR: Risk Ratio; SMD: standardised mean difference; VAS: visual analogue scale | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Evidence of certainty was downgraded one level because of the reliance of the estimated effect on a small number of participants
2Evidence of certainty was downgraded one level because of the imprecise treatment estimate
3Evidence of certainty was downgraded one level because of study risks of bias
4Evidence of certainty was downgraded one level because heterogeneity between studies
We organised the studies into the following groups:
Pharmacological interventions
Topical interventions
Oral or IV supplements
Dialysis modality
All other interventions.
1. Pharmacological interventions
See Table 1; adverse effects Table 4.
GABA analogues
Twelve studies (Amirkhanlou 2016; Foroutan 2017; Gobo‐Oliveira 2018; Gunal 2004; Marin 2013; Naghibi 2007; Naini 2007; Nofal 2016; Noshad 2011; Solak 2012; Tol 2010; Yue 2015) involving 618 patients and 13 comparisons, investigating the effects of either oral gabapentin or pregabalin. Dosing included 300 mg twice weekly oral gabapentin (Gunal 2004; Nofal 2016), 400 mg of twice weekly oral gabapentin (Naini 2007) or 75 mg twice weekly oral pregabalin (Yue 2015) compared to placebo. Naghibi 2007 did not explicitly state the dose of gabapentin. These five studies all reported itch on a 10 cm VAS.
GABA analogues versus placebo
GABA analogues reduced symptoms of uraemic itch compared to placebo (Analysis 1.1.1 (5 studies, 297 participants): MD ‐4.95 cm, 95% CI ‐5.46 to ‐4.44 on VAS; I² = 0%; high certainty evidence). The overall certainty of the evidence was high as these results were taken from multiple RCTs with large, homogeneous magnitudes of effect and narrow (%% CI demonstrating precision and efficacy. Risk of bias was uncommon and low overall.
1.1. Analysis.

Comparison 1: Pharmacological interventions (oral or IV), Outcome 1: Itch
Tol 2010, a placebo controlled cross‐over RCT involving 14 patients taking gabapentin 300 mg/HD session, did not report placebo results and could not be included in the quantitative analysis. Tol 2010 reported a significant absolute reduction in itch during gabapentin treatment: 6.3 cm (95% CI 3.8 to 8.8) versus baseline in VAS similar to the other gabapentin studies.
GABA analogues versus antihistamines
Five studies examined the efficacy of gabapentin versus various antihistamines. Marin 2013 compared 300 mg gabapentin every two days versus 10 mg oral loratidine every two days; Noshad 2011 studied 100 to 200 gabapentin mg/day versus oral hydroxyzine; Amirkhanlou 2016 measured a binary response of itch improvement from gabapentin versus oral ketotifen; Gobo‐Oliveira 2018 compared 300 mg gabapentin 3 times/week versus 6 mg oral dexchlorpheniramine twice/day; and Suwanpidokkul 2007 studied 100 mg gabapentin/day versus 10 mg loratidine/day. Overall, GABA analogues (gabapentin) may reduce symptoms of uraemic itch (Analysis 1.1.2 (5 studies, 220 participants): SMD 0.44 reduction, 95% CI 0.75 to 0.14 lower; I² = 22%; low certainty evidence) compared to antihistamines.
While these are four separate independent RCTs, there was low to moderate heterogeneity (the efficacy of oral antihistamines was highly variable), and two studies are at high risk of bias. Amirkhanlou 2016 does not report baseline scores and Marin 2013 was an open‐label study.
Yue 2015 (in addition to the placebo comparison above) reported a relative reduction of 4.1 cm (95% CI 1.98 to 6.22) with pregabalin over ondansetron. Solak 2012 compared 300 mg gabapentin/day to 75 mg pregabalin/day and reported no significant difference in itch reduction between the two treatments.
Adverse effects
Across the studies, few mild adverse effects occurred. Somnolence, dizziness, and fatigue are reported in less than 5% of patients in the intervention groups. No moderate or severe adverse effects are reported.
Ondansetron
Ondansetron versus placebo
Three studies (Ashmore 2000; Murphy 2003; Yue 2015) investigated the effects of 8 mg oral ondansetron 3 times/day. All three studies reported itch on a 10 cm VAS, however Ashmore 2000 employed a cross‐over design and reported VAS with only medians and interquartile ranges. This analysis extrapolates means and SDs according to the standard practice recommendations of Cochrane. Based on the inherent variability of these changes, a sensitivity analysis of ondansetron interventions without Ashmore 2000 was performed.
Ondansetron did not reduce symptoms of uraemic itch (Analysis 1.1.3 (3 studies, 183 participants): MD ‐0.38 cm, 95% CI ‐1.04 to 0.27 on VAS; high certainty evidence) compared to placebo. These finding remain valid with the exclusion of Ashmore 2000. The placebo group experienced a non‐significant mean decrease in VAS ranging from 0.1 to 2 cm.
Ondansetron versus antihistamine
Ozaykan 2001 compared ondansetron to the antihistamine cyproheptadine. The authors report a slight improvement in itch reduction with ondansetron compared to cyproheptadine.
Subach 2001 and Mirnezami 2013 compared ondansetron to diphenhydramine and loratidine, respectively. Neither study found any difference in measured itch.
Adverse effects
Nausea and vomiting were reported as uncommon and mild in severity.
Kappa‐opioid agonists versus placebo
Six studies investigated the effects of either 5 µg/day or 2.5 µg/day nalfurafine (oral or IV) (Bhaduri 2006; Kumagai 2010; Wikstrom 2005 (1); Wikstrom 2005 (2)) and a newly synthesized agent "CR845" (Spencer 2015; Spencer 2017) at 0.5, 1.0, and 1.5 µg/kg IV with dialysis. All studies reported itch on a 10 cm VAS.
Kappa opioid agonists reduced symptoms of uraemic itch (Analysis 1.1.4 (5 studies, 661 participants): MD ‐1.05 cm, 95% CI ‐1.40 to ‐0.71 on VAS; I² = 0%; high certainty evidence). Bhaduri 2006 reported no decrease in itch on VAS.
Both studies examining CR845 were funded by Cara Therapeutics and were judged to be at high risk of bias. A sensitivity analysis without the CR845 studies yields the similar result. The additional power from these two high risk studies are not required to maintain the high certainty of the evidence.
Adverse effects
Adverse effects were common and were mild to moderate in severity. Somnolence, headache, insomnia, diarrhoea, and nausea/vomiting were reported in 2% to 10% of the intervention group.
Mu opioid antagonists versus placebo
Two cross‐over studies (Pauli‐Magnus 2000; Peer 1996) compared 50 mg naltrexone once/day with placebo. Both studies evaluated itch on a 10 cm VAS.
Mu opioid antagonists may not improve symptoms of uraemic itch (Analysis 1.1.5 (2 studies, 62 participants): MD ‐4.29 cm, 95% CI ‐10.24 to 1.66 on VAS; low certainty evidence).
Pauli‐Magnus 2000 reported interquartile ranges and Peer 1996 reported only percentage changes of VAS. Results are merged according to the standard practice recommendations of Cochrane. Additionally, Peer 1996 evaluated the effect of naltrexone on uraemic itch using Duo scale as well as VAS. There was no significant difference reported between naltrexone and placebo in these studies.
Adverse effects
These studies found that the adverse effects of Mu opioid antagonists are both somewhat common and mild to moderate in severity. Symptoms reported included loss of appetite, nausea, heartburn, and other gastrointestinal symptoms in approximately one third of the intervention groups. In addition, patients ceased any opioid medication for the duration of the trial period. Acute pain management became a common reason for cessation of natrexone during the studies resulting in many dropouts post randomisation.
Nalbuphine versus placebo
TREVITR02 2017 compared nalbuphine, a combined kappa‐opioid agonist and mu‐opioid antagonist, to placebo. Nalbuphine may make little or no difference to uraemic itch (Analysis 1.1.6 (1 study, 179 participants): MD ‐0.75 cm, 95% CI ‐1.70 to 0.20 on VAS; low certainty evidence).
This study did not report on adverse effects.
Cromolyn versus placebo
Vessal 2010 reported oral cromolyn may reduce symptoms of uraemic itch compared to placebo (Analysis 1.1.7 (1 study, 40 participants): MD ‐4.8 cm, 95% CI ‐7.03 to‐ 2.57; low certainty evidence).
Adverse effects
The adverse effects reported were flatulence in one patient in the cromolyn group and three gastrointestinal complaints in the placebo group.
Nicotinamide versus placebo
A four‐week study by Omidian 2013 evaluated nicotinamide versus placebo. Nicotinamide may make little or no difference to the symptoms of uraemic itch (Analysis 1.1.8 (1 study, 50 participants): 0.47 cm, 95% CI ‐0.32 to 1.26; low certainty evidence).
No adverse effects were reported in either the nicotinamide to placebo groups.
Erythropoietin versus placebo
A four‐week study by De Marchi 1992 evaluated erythropoietin versus placebo. Erythropoietin had uncertain effects on the symptoms of uraemic itch (Analysis 1.1.9 (1 study, 29 participants): MD ‐14.50, 95% CI ‐38.78 to 9.78 on 40 point Duo score; very low certainty evidence).
Sja'bani 1997 reported that the erythropoietin group experienced a significantly greater mean reduction in itch than the placebo group. However, baseline itch scores are not fully reported to allow for inclusion in the quantitative review.
These studies did not report on adverse effects.
Cholestyramine versus placebo
Two cross‐over studies (Silverberg 1977; van Leusen 1978) compared cholestyramine and placebo. Cholestyramine may make little or no difference to the symptoms of uraemic itch (Analysis 1.1.10 (2 studies, 15 participants): MD 0.00, 95% CI ‐0.49 to 0.49 on a 0 to 3 severity scale; low certainty evidence).
These studies did not report on adverse effects.
Montelukast versus placebo
One cross‐over study (Nasrollahi 2007) and one parallel study (Mahmudpour 2017) compared montelukast to placebo. Duo score and VAS were measured, respectively.
Montelukast may slightly reduce symptoms of uraemic itch (Analysis 1.1.11 (2 studies, 87 participants): SMD ‐1.40, 95% CI ‐1.87 to ‐0.92; moderate certainty evidence).
Homogeneity was difficult to assess as the RCTs used well validated but slightly different itch severity scores.
Adverse effects
One patient in the intervention group of Nasrollahi 2007 developed myelodysplastic syndrome, but this was not considered an adverse effect of Montelukast. In comparison, one patient in the placebo first group developed a myocardial infarction prior to being allocated to Montelukast. No other adverse effects were reported.
Sertraline versus placebo
Pakfetrat 2018 compared sertraline and placebo. Sertraline may make little or no difference to the symptoms of uraemic itch (Analysis 1.1.12 (1 study, 46 participants): MD ‐1.80 cm, 95% CI ‐3.65 to 0.05 on VAS; low certainty evidence).
This study did not report on adverse effects.
Lidocaine versus placebo
Tapia 1977 compared 600 mg IV lidocaine once/day and placebo. Only acute (15 to 30 minutes) relief of pruritus was included in the analysis. It is unclear whether lidocaine relieved itch (within 30 minutes) compared to placebo due to very low certainty evidence (Analysis 1.1.13 (1 study, 16 participants): MD ‐0.63 cm, 95% CI ‐1.46 to 0.19). Longer term assessment was not reported. Improvement in itch was reported in 8/10 participants receiving lidocaine and 1/6 participants receiving placebo (Analysis 1.2.1); the definition of improvement was not reported.
1.2. Analysis.

Comparison 1: Pharmacological interventions (oral or IV), Outcome 2: Itch (dichotomous)
This study did not report on adverse effects.
Thalidomide versus placebo
Silva 1994 compared 100 mg thalidomide/day for one week with placebo. Thalidomide may relieve itch following administration (Analysis 1.2.2 (1 study, 18 participants): RR 4.17, 95% CI 1.08 to 16.15) compared to placebo, however, the certainty of the evidence was very low as these results were taken from a single study with a small number of participants, and a high number of dropouts (11).
No adverse effects were reported in either the placebo or thalidomide groups.
Sodium thiosulfate versus placebo
Mohamed 2012compared 12.5 mg sodium thiosulfate/dialysis session and placebo. Overall, there was no reported significant difference comparing sodium thiosulfate and placebo. The study was not included in the quantitative analysis due to incomplete reporting of results.
The study did not report on adverse effects.
Doxepin versus placebo
Pour‐Reza‐Gholi 2007 compared 10 mg doxepin twice/day and placebo in a cross‐over study. Complete improvement was achieved in 58% of participants on doxepin which was significantly higher than placebo (P > 0.001).
Adverse effects
Mild drowsiness was a commonly reported complaint and resulted in one dropout. Placebo adverse effects were not reported.
Antihistamines
Aubia 1980compared 400 mg oral cimetidine once/day (over and up to 1 hour), a different unspecified "classical antihistamine", and placebo. The study found no significant differences between the three groups. No measures of variability (e.g. standard error) were reported.
Antihistamines were compared with four other interventions: ondansetron, topically applied dilute vinegar, GABA agonists (gabapentin; see above section on GABA analogues), and Mu opioid antagonists (naltrexone).
Ozaykan 2001 reported the ondansetron group experienced a significantly greater mean reduction: 9 point (95% CI 16.34 to 1.64) compared to cyproheptadine (first generation antihistamine) using Duo pruritus score.
Nakhaee 2015 reported no significant difference between hydroxyzine and topically applied dilute vinegar.
Legroux‐Crespel 2004 reported no significant difference between loratidine and the Mu opioid antagonist naltrexone.
Baumelou 1993 reported no significant difference between the two antihistamines cetirizine and dexchlorpheniramine. However, both significantly improved itch compared to placebo.
Other therapies
Several isolated interventions could not be included in the quantitative analysis due to insufficient reporting of results.
Fallahzadeh 2015 reported a significant improvement with oral senna compared to placebo in patients with uraemic pruritus.
Pederson 1980reported a significant reduction with oral charcoal compared to placebo.
Rivory 1984 reported a significant improvement in itch with nicergoline compared to placebo.
Shariati 2010 reported oral charcoal was significantly more effective in reducing VAS in patients with uraemic pruritus than oral aluminium hydroxide.
2. Topical interventions
See Table 2; adverse effects Table 5.
Capsaicin cream versus vehicle cream
Three studies tested the efficacy of the topical agent capsaicin in treating CKD‐related uraemic pruritus: 0.025% (Cho 1997) or 0.03% (Makhlough 2010; Tarng 1996) capsaicin cream applied 4 times/day versus vehicle cream (placebo). Evaluation of itch was reported on a 10 cm VAS and a customized 4‐point itch scale (Makhlough 2010). Capsaicin cream application probably reduced the symptoms of uraemic itch (Analysis 2.1.1 (2 studies, 112 participants): SMD ‐0.84, 95% CI ‐1.22 to ‐0.45; I2 = 0%; moderate certainty evidence) than during the vehicle application period.
2.1. Analysis.

Comparison 2: Topical interventions, Outcome 1: Itch
Tarng 1996 did not provide any results for the placebo cross‐over periods and could not be included in the quantitative analysis. Within the intervention group of that study, approximately 80% of patients initially reported moderate to severe pruritus and then none or mild symptoms post‐intervention.
Adverse effects
All studies reported mild local burning sensations and cutaneous erythema as adverse effects.
Pramoxine cream versus vehicle cream
Young 2009) compared 1% pramoxine cream twice/day with vehicle cream. It is uncertain whether pramoxine cream decreased uraemic itch (Analysis 2.1.2 (1 study, 28 participants): MD ‐1.97 cm, 95% CI ‐6.06 to 2.12; very low certainty evidence) compared to vehicle.
This study did not report on adverse effects.
Calcineurin inhibitor cream versus vehicle cream
Two studies compared 0.1% tacrolimus (Duque 2005) and 1% pimecrolimus (Ghorbani 2012a) with vehicle cream. Duque 2005 did not report SD (or any measurement of variability/error) and was not included in the quantitative analysis. Duque 2005 reported pimecrolimus cream application resulted in a non‐significant, but greater reduction in VAS compared to the vehicle cream.
It is uncertain whether 1% pimecrolimus reduced uraemic itch (Analysis 2.1.3 (1 study, 60 participants): MD 1.2 cm, 95% CI ‐0.36 to 2.76; very low certainty evidence) compared to vehicle.
Adverse effects
Adverse effects of the tacrolimus cream included a burning sensation over the area of skin applied with cream.
Dead Sea lotion versus vehicle lotion
Boaz 2009 compared Dead Sea lotion, containing Dead Sea water and sea silt (Dead Sea mud), and two related vehicle lotion groups. Itch was quantified using a 5‐point itch severity scale. It is uncertain whether Dead Sea lotion reduced uraemic itch (Analysis 2.1.4 (1 study, 41 participants): MD ‐2.00, 95% CI ‐4.31 to 0.31 on 5‐point severity scale; very low certainty evidence) compared to vehicle.
This study did not report on adverse effects.
Cromolyn cream
Cromolyn cream versus vehicle cream
Feily 2012 compared 4% cromolyn cream twice/day and vehicle cream. Cromolyn cream may not reduce uraemic itch (Analysis 2.1.5 (1 study, 60 participants): MD 0.8 cm, 95% CI ‐1.98 to 0.38; low certainty evidence) compared to vehicle.
This study did not report on adverse effects.
Cromolyn cream versus calcineurin inhibitor cream
Ghorbani Birgani 2011 compared 4% cromolyn cream with 1% pimecrolimus cream. This study reported both interventions significantly reduced pruritus on a VAS with a non‐significant difference between the two.
Sericin cream versus vehicle cream
Aramwit 2012a compared sericin cream and vehicle cream. Itch was reported on a 10 cm VAS. This study reported the sericin cream group experienced a significant absolute mean decrease in itch: 2.8 cm reduction (95% CI 0.5 lower to 5.1 lower) in VAS. Placebo results were not reported and the study could not be included in the quantitative analysis.
This study did not report on adverse effects.
Baby oil versus placebo
Lin 2012 compared chilled and unchilled baby oil with a common vehicle. Itch was evaluated on with a customized 21‐point itch severity scale that incorporated itching, dryness, peeling, tightness, and sleep disturbances. The itch severity scale does not appear to be well validated unlike VAS or Duo score. The placebo group experienced a 1‐point non‐significant decrease in itch severity scale.
Overall, baby oil application may reduce uraemic itch (Analysis 2.1.6 (1 study, 93 participants): MD ‐2.38, 95% CI ‐3.49 to ‐1.27; low certainty evidence) compared to vehicle.
The report documented that no adverse effects occurred using either intervention or vehicle.
L‐arginine versus vehicle salve
Durant‐Finn 2008compared L‐arginine salve and vehicle salve groups. Itch was quantified using a 3‐point itch severity scale. L‐arginine may make little or no difference to uraemic itch (Analysis 2.1.7 (1 study, 48 participants): MD ‐0.58, 95% CI ‐1.86 to 0.7 on 3‐point severity scale; low certainty evidence) compared to vehicle.
This study did not report on adverse effects.
Polyunsaturated fatty acids versus vehicle cream
Chen 2006e and Afrasiabifar 2017 compared topically applied polyunsaturated fatty acids of varying concentrations and quantity with vehicle cream. Itch was reported with a 10 cm VAS in both studies.
Topically applied polyunsaturated fatty acids may make little or no difference to uraemic itch (Analysis 2.1.8 (2 studies, 78 participants): SMD ‐0.91, 95% CI ‐1.99 to 0.17; I2 = 88% low certainty evidence) compared to vehicle.
These studies did not report on adverse effects.
Eurax cream versus Sarna lotion
Tan 1990 compared Eurax cream with Sarna lotion and reported a statistically significant reduction of uraemic itch for both the Eurax cream and Sarna lotion periods.
This study did not report on adverse effects.
3. Oral or IV supplements
See Table 3; adverse effects Table 6.
Oral polyunsaturated fatty acids versus placebo
Three studies (Ghanei 2012; Peck 1996; Yoshimoto‐Furuie 1999) tested the efficacy of 1 g polyunsaturated fatty acids 3 time/day versus placebo. Itch was evaluated with a customized 5‐point itch scale with continuous or binary results reported. Only Ghanei 2012 also reported complete placebo results.
Ghanei 2012 reported oral polyunsaturated fatty acids may decrease uraemic itch (Analysis 3.1.1 (1 study, 22 participants): MD ‐11.30%, 95% CI ‐19.01% to ‐3.59%; low certainty evidence) compared to placebo. Two additional studies (Peck 1996; Yoshimoto‐Furuie 1999) also reported reductions in itch scores versus baseline, but did not include sufficient reporting of placebo results.
3.1. Analysis.

Comparison 3: Oral or IV supplements, Outcome 1: Itch
Mojgan 2017 examined fish oil supplements versus placebo and reported a small but significant benefit versus placebo; neither CIs nor SDs were reported.
Begum 2004 compared fish oil and safflower oil (both polyunsaturated fatty acids) and found neither significantly reduced itch on a VAS.
These studies did not report on adverse effects.
IV L‐carnitine versus placebo
Mettang 1997 compared 10 mg IV L‐carnitine/kg and IV placebo once/dialysis session. Evaluation of itch used a 10 cm VAS. IV L‐carnitine may make little or no difference to uraemic itch (Analysis 3.1.2 (1 study, 12 participants): MD ‐0.26 cm, 95% CI ‐2.85 to 2.43 on VAS; low certainty evidence) compared to IV placebo.
This study did not report on adverse effects.
Oral zinc sulfate versus placebo
Two studies (Mapar 2015; Najafabadi 2012) compared 220 mg oral zinc sulfate twice/day and placebo. Evaluation of itch was reported on a 10 cm VAS. Zinc sulfate probably reduces uraemic itch (Analysis 3.1.3 (2 studies, 76 participants): MD ‐1.77 cm, 95% CI ‐2.88 to ‐0.66 on VAS; moderate certainty evidence) compared to placebo.
Adverse effects
Mapar 2015 reported vomiting in one participant in the placebo group and Najafabadi 2012 did not specify exact adverse effects, only that none were "attributable to zinc sulfate".
Oral ergocalciferol versus placebo
Shirazian 2013 compared 50,000 IU oral ergocalciferol/week and placebo. Itch was reported with the results of a 21‐point customised itch questionnaire. Ergocalciferol may make little or no difference to uraemic itch (Analysis 3.1.4 (1 study, 50 participants): MD 0.40, 95% CI ‐2.48 to 3.28; low certainty evidence) compared to placebo.
No adverse effects were reported in the ergocalciferol group.
Oral turmeric versus placebo
Pakfetrat 2014compared 22 mg oral turmeric 3 times/day and placebo. Itch was evaluated with a 40‐point modified Duo scale. Turmeric probably reduces uraemic itch (Analysis 3.1.5 (1 study, 100 participants): MD ‐6.40, 95% CI ‐7.42 to ‐5.38 on modified Duo scale; moderate certainty evidence) compared to placebo.
No adverse effects are reported in the intervention group.
Oral Fumaria parviflora versus placebo
Akrami 2017 compared 1 g oral Fumaria parviflora 3 times/day of with placebo. Itch was evaluated on a 10 cm VAS. Fumaria parviflora may reduce uraemic itch (Analysis 3.1.6 (1 study, 63 participants): MD ‐3.90 cm, 95% CI ‐5.04 to ‐2.76 on modified Duo scale; low certainty evidence) compared to placebo.
A few mild abdominal symptoms were observed in both the Fumaria parviflora and placebo groups.
4. Dialysis modality
See Table 3; adverse effects Table 7.
High flux/permeability haemodialysis versus conventional haemodialysis
Three studies (Chen 2009; Hui 2011; Jiang 2016) compared high flux/permeability HD to conventional HD. Evaluation of itch used a 10 cm VAS. High flux/permeability HD may decrease uraemic itch (Analysis 4.1.1 (3 studies, 202 participants): MD ‐2.62 cm, 95% CI ‐3.72 to ‐1.52; I2 = 67%; low certainty evidence) compared to conventional HD.
4.1. Analysis.

Comparison 4: Haemodialysis modality, Outcome 1: Itch
These studies did not report on adverse effects.
Haemodialysis with haemoperfusion versus conventional haemodialysis
Li 2017a compared conventional HD with HD using neutral macroporous resin haemoperfusion with one of two different resin perfusers. Evaluation of itch used a 10 cm VAS. HD with haemoperfusion therapy may decrease uraemic itch (Analysis 4.1.2 (1 study, 202 participants): MD ‐2.37 cm, 95% CI ‐2.89 to ‐1.85; low certainty evidence) compared to conventional HD
This study did not report on adverse effects.
Haemodiafiltration with haemoperfusion against high‐flux haemodialysis
Zhang 2016a compared haemodiafiltration with haemoperfusion to high‐flux HD. They reported that haemodiafiltration with haemoperfusion was significantly more effective in relieving itch than high‐flux HD measured on a VAS.
This study did not report on adverse effects.
High‐flow versus conventional flow haemodialysis
Aliasgharpour 2018 compared high‐flow HD with conventional flow HD. They reported a significant reduction in severity in itch with high‐flow HD measured on a 4‐point VAS.
This study did not report on adverse effects.
Haemodialysis solutions
Carmichael 1988 compared magnesium‐free dialysate with conventional dialysate containing magnesium. They reported no significant itch reduction on a VAS.
Rad 2017 compared cool dialysate with conventional dialysate at a normal temperature. They reported cool dialysate was significantly more effective in relieving uraemic pruritus.
Kyriazis 2000 crossed over four patients with variable concentrations of calcium in their dialysate and reported a non‐significant trend towards lower calcium concentrations reducing uraemic itch.
5. Other interventions
See Table 3; adverse effects Table 8.
UV‐B radiation
Four studies (Blachley 1985; Gilchrest 1979; Ko 2011; Sherjeena 2017) compared UV‐B radiation versus placebo (typically UV‐A) exposure 3 times/week for the reduction of CKD‐related uraemic pruritus. Due to the mechanism of the intervention there was often inherent difficulties in blinding the administrators and patients. Outcomes included both Duo score and percent of patients experiencing absolute relief.
UV‐B radiation may make little or no difference to uraemic itch (Analysis 5.1.1 (4 studies, 86 participants): SMD ‐2.49, 95% CI ‐4.62 to ‐0.41; I2 = 93%; low certainty evidence) compared to UV‐A/placebo
5.1. Analysis.

Comparison 5: Other interventions, Outcome 1: Itch
UV‐A radiation was not originally included in this systemic review as an intervention category. During the 1980s some RCTs (including Sanchez 1986 and Taylor 1983) investigated UV‐A and found that it likely does not decrease uraemic itch. UV‐A has been commonly used as a placebo in RCTs of analogous interventions.
Chan 1995 reported a significant reduction in itch in the UV‐B group but did not report the results of the placebo intervention.
Common adverse effects across all studies included sunburn and tanning; these were also seen in the control UV exposures.
Thermal therapy
Hsu 2009 compared thermal (warming) therapy with a placebo patch. Evaluation of itch used a 10 cm VAS. Thermal therapy may make little or no difference to uraemic itch (Analysis 5.1.2 (1 study, 42 participants): MD ‐2.06 cm, 95% CI ‐6.54 to 2.42; low certainty evidence) compared to the placebo patch.
This study did not report on adverse effects.
Discussion
Summary of main results
This systematic review assesses 92 RCTs evaluating 43 different interventions. Evidence for most interventions include only a single placebo controlled trial, often underpowered. However, the number of studies, participants, statistical power, and evidence quality significantly improves for several interventions. Less often, one intervention was compared to another allowing for some informal indirect comparisons between treatments. Fortunately, the majority of interventions include studies reporting itch with a well‐validated VAS or Duo’s scores aiding in the interpretation of the results. These results allowed reporting as MD or SMD with most interventions.
The results are reported in Table 1; Table 2; Table 3. The grouping of the GABA analogues, kappa opioid agonists, Mu opioid antagonists, polyunsaturated fatty acids, and UV‐B radiation assumed their class effect corroborated by previous studies on their effectiveness in uraemic pruritus, non‐specified pruritus, and related pathophysiology such as pain. For instance pregabalin and gabapentin, known to have similar and highly correlative downstream effects, are studied together for their classed effect on uraemic pruritus (Matsuda 2016). They have also been shown through a head‐to‐head RCT to have similar efficacy in treating uraemic pruritus (Solak 2012). Finally, the results of this review's placebo‐controlled gabapentin and pregabalin RCTs are homogeneous, again supporting this classification.
Five interventions included multiple and/or larger studies with a combined sample size of over 100 participants: GABA analogues, kappa opioid agonists, ondansetron, capsaicin cream, and turmeric. Each of these has no identified major sources of bias limiting their interpretation. Of the five, only ondansetron was not found to be associated with a reduction in uraemic itch versus placebo. GABA analogues achieved the largest effect size of all interventions. The effect size of kappa opioid agonists and capsaicin cream are both modest in comparison. One direct comparison (GABA analogues versus. Ondansetron) was consistent with above with similar effect size to those of the GABA analogue versus placebo RCTs. Supplementing this data on gabapentin and pregabalin are five mixed quality RCTs favouring gabapentin in direct comparison to various antihistamines.
The small sample sizes and often significant sources of bias limit the conclusions drawn from the majority of this review's other interventions. No meaningful quantitative analysis can be drawn from the adverse effects of the interventions due to insufficient and disorganised reporting. As a global assessment, adverse effects of nearly all antipruritic interventions are somewhat uncommon and non severe. One exception may be kappa opioid agonists where adverse effects were slightly more common.
While most studies provided adequate data to contribute to an analysis of itch reduction, few reported on any of the secondary outcomes (e.g. sleep, QoL) described by our protocol. Of the secondary data reported, the conclusions are limited by heterogeneous outcomes and low individual study quality.
Overall completeness and applicability of evidence
Recruited patients included only those already on HD or those with an expectation to begin shortly. All studies outlined prolonged and ongoing significant itch coinciding with CKD as inclusion criteria. Nearly every RCT also outlined exclusion criteria to exclude patients with pathology that potentially otherwise explains their itch symptoms (e.g. dermatological or liver disease). The applicability of the evidence derived from this meta‐analysis may be weaker in populations who have potential non‐renal causes to their itch pathology. This was notable as many patients living with CKD do not have the disease in isolation.
Given the diversity of the interventions and relatively modest number of studies per intervention, it was not possible to make comparisons on the effectiveness of all interventions. For instance, Solak 2012 found both gabapentin and pregabalin to be equally and highly efficacious in reducing uraemic itch. Missing data and inconsistent reporting did not allow us to include data from all studies in the quantitative meta‐analyses. Approximately 70% of all participants (in studies that met protocol inclusion criteria) contributed to our meta‐analyses and the remainder were qualitatively analysed. Patient characteristics in the quantitative and qualitative analyses are very similar.
Multiple studies noted that recruited patients had already failed one anti‐itch treatment prior to being randomised. The most common previous treatment was an antihistamine despite the lack of substantial evidence for its use for uraemic itch. It is unclear if prior antihistamine treatment could be a confounding factor.
Some interventions that are yet to be studied via an RCT are currently recommended by guidelines and authorities for uraemic itch. Often, they are also routinely used in clinical practice. Without at least one placebo‐controlled RCT it is beyond the scope of this systematic review to assess this evidence in a quantitative manner.
There have not been sufficient RCTs using different dosing regimens to give definitive recommendations about the doses of specific interventions. The populations included in the RCT’s tend to be younger than the typical population with CKD. The elderly may be more susceptible to side effects from these drugs. In the case of GABA analogues, evidence from Noshad 2011 and Rayner 2012 suggest that a low dose of gabapentin (100 mg/day) or pregabalin (25 mg/day) should be used initially and then titrated up.
This systematic review's recommendation on individual interventions as monotherapy are generalisable to patients with CKD and chronic itching with no other obvious cause. Thus, there is strong external validity extending to this review's outlined population (patients with stage 4 and 5 CKD and established CKD‐related itch).
Quality of the evidence
Certainty of the evidence varied widely. High quality evidence exists for GABA analogues, kappa opioid agonists, and ondansetron. These interventions draw conclusions from multiple independent well‐powered RCTs with no significant biases identified. There was moderate quality evidence for several other inventions Table 1; Table 2; Table 3. The most common factor limiting the certainty of the evidence was the reliance on a single underpowered RCT. Many of these studies are clearly underpowered with limited participants and large standard error. Other common reasons include the use of a non‐validated itch severity outcome measures, insignificant magnitude of effect, and other significant sources of bias.
Most studies had low or unclear risk of bias across the majority of domains (Figure 2 and Figure 3), however results of this review should be interpreted with caution. Increased risk of bias appears correlated with earlier dates of publication. In this review, underpowered interventions often had increased risk across most of the bias categories. This aside, there are many interventions (both of small and large sample sizes) with low overall risk of bias profiles Figure 2.
Potential biases in the review process
Several intervention are grouped (most notably GABA analogues, Kappa agonists, Mu antagonists, and antihistamines) within the quantitative and qualitative analysis. Opioids, GABA analogues, and antihistamines all have a body of literature externally supporting this "class effect". Additionally, within this systematic review, consistent effects sizes, standard error, and adverse effects provide strong internal validity to this categorisation. However, this inevitably poses a potential for bias and warrants highlighting.
Several cross‐over studies within this review reported results consistent with parallel RCTs. This approach gives rise to a unit‐of‐analysis error with CIs that are likely to be too wide, and the study would receive too little weight, with the possible consequence of disguising clinically important heterogeneity. This was somewhat mitigated by verifying that our calculated results match those that are partially reported and also by an overall sensitivity analysis targeting these "approximated" studies.
This review only examined RCTs. All included studies, save one, were blinded. Chilled baby, some UV‐B, and some HD modality interventions are unlikely to have been able to blind their participants due to the inherent nature of the intervention. Six studies included significant statements of declaration; all declared significant financial conflicts of interest relating to the pharmaceutical manufacturers of those interventions. However, these were unlikely to bias the major findings of this review.
This systematic review addressed a clear research questions and used predefined inclusion criteria to select and appraise studies. We conducted extensive and sensitive searches but the possibility of publication bias remains. This was especially true for interventions with only one RCT identified. Our protocol did not include exhaustive exclusion criteria for patients potentially with pathology associated with non‐uraemic itch. It should be noted that the majority of RCTs in this review excluded such patients.
The review did not impose language restrictions. Seven studies were translated prior to data extraction.
A comprehensive search of the literature was performed by searching multiple databases and well as handsearching for potential RCTs in the grey literature. All possible relevant data was extracted and whenever studies’ reporting proved insufficient the relevant author(s) were contacted or studies were cross checked in the relevant clinical trials registry. Approximately half of all such cases recovered additional original data. Registers of ongoing trials and available conference proceedings were also searched.
Of the studies qualifying for this review, many did not, or only superficially, reported on adverse effects. Overall, the adverse effects reported were somewhat uncommon and generally mild in nature. Often no adverse effects occurred. It was possible that in some studies the authors did not bother to report the lack of adverse effects occurring, however this was not helpful for drawing accurate conclusions. Other secondary outcomes investigated were rarely reported. Significant results of either adverse effects or important secondary outcomes that go unreported may bias the results of this review.
Agreements and disagreements with other studies or reviews
This systematic review found similar results relative to other reviews on the treatment uraemic pruritus. Our search revealed three recent reviews on pathological itch in general. Two are specifically CKD‐related.
Siemens 2016 examined 947 CKD participants in 36 trials. The review included all patients in the palliative care setting, did not focus on non‐pharmacological interventions, and excluded trials comparing interventions. The review did not exclusively focus on patients with CKD. Additionally, substantial new evidence on GABA analogues, ondansetron, and new pharmacological interventions have been published since their search. This new evidence and our review is consistent with the overall findings of Siemens 2016, but notably provided increase power to the positive findings on GABA analogues, kappa opioid agonists, and the non‐efficacy of ondansetron.
Pongcharoen 2015 examined participants in 26 trials in a quasi‐systematic review of all systemic anti‐itch treatments. Again, this review did not exclusively focus on patients with CKD. Less than half of all trials involved patients with CKD.
Simonsen 2017 examined participants in 44 trials examining pharmacological, alternative, and adjunctive interventions. These included interventions such as acupuncture which was not included in our review. The limited number and degree of heterogeneity of the studies did not permit formal meta‐analysis. While the authors did not comment on kappa agonists and ondansetron their results on gabapentin are consistent with the findings of our review.
Other more focused reviews examined the effect of the GABA analogue gabapentin (Lau 2016), opioid receptor antagonist (Phan 2010), and topical capsaicin (Gooding 2010) on uraemic pruritus. Again, the results of this systematic review are consistent with these reviews. Of note, this is the first quantitative meta‐analysis of uraemic pruritus on this scale.
Authors' conclusions
Implications for practice.
A large number of interventions were examined in this review. Some treatment modalities appear to be effective in the reduction of uraemic itch, others may be of some possible effectiveness, and several appear to have minimal or no effectiveness.
Of all treatments for uraemic pruritus GABA analogues have been studies by the greatest number of RCTs and each have been shown to have the greatest effect size versus all other inventions studied. GABA analogues reduce itch in patients with CKD. Within GABA analogues most of the evidence was for gabapentin with the rest for pregabalin. Even with the removal of pregabalin trials, these results remain consistent. A further RCT, even of on the scale of the largest GABA analogue trials included in this review, is unlikely to substantially change this result.
There have not been sufficient RCTs using different dosing regimens to give definitive recommendations about dosage. Both scheduled dosing and titrating dosages frequency occur.
Evidence in this review show that Kappa opioid agonists slightly reduce itch in patients with CKD. Additionally, indirect comparisons to other interventions suggest a much more modest effect in comparison to GABA analogues. Nalfurafine is the kappa opioid agonist with the largest and highest quality body of evidence.
Ondansetron was also well studied in multiple RCTs, bur does not appear to reduce uraemic itch. This was again with high certainty of evidence.
Oral montelukast, turmeric, zinc sulfate, and topical capsaicin all probably reduce uraemic pruritus, but additional high quality evidence is required before a decisive conclusion can be made.
Guidelines do not often recommend gabapentin as first line treatment in uraemic itch. Many of the included RCTs note that it is often standard practice to prescribe antihistamines initially. Research has shown most medical directors continue to prescribe antihistamines as first line in the majority of cases (Rayner 2017). Conclusions from this systematic review may influence this policy.
This review may also be a guide for a changing role for treatment modalities where evidence was lacking. Erythropoietin, thalidomide, cromolyn, doxepin, nicergoline, cholestyramine, nicotinamide, sodium thiosulfate, and lidocaine are of questionable utility in the treatment of uraemic pruritus. Ondansetron was not efficacious. It is somewhat unlikely the further study on ondansetron will change this result. Currently, there is insufficient data for the other interventions to infer in either direction.
Implications for research.
The effectiveness of GABA analogues may guide future study into the underlying mechanisms of uraemic pruritus. GABA analogues may also serve as a target for research in non‐uraemic pruritus which has mostly focused on interventions with unrelated mechanisms of action. While shown to be efficacious, the optimal dosing of gabapentin and pregabalin would benefit from targeted study. Finally, several interventions investigated by this systemic review would benefit from additional appropriately powered RCTs. In particular the interventions turmeric, topical capsaicin, montelukast, high flux or permeability HD, and oral cromolyn have limited, but potentially promising preliminary trials.
History
Protocol first published: Issue 11, 2014 Review first published: Issue 12, 2020
Acknowledgements
We would like to acknowledge the referees for their feedback and comments.
We would like to acknowledge the contributions of Tanvir Kapoor for his contributions as co‐author of this review's protocol as well translators Israel Berger and Nazan Kocak.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Pharmacological interventions (oral or IV).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Itch | 30 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 GABA analogues | 5 | 297 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐2.43, ‐1.85] |
| 1.1.2 GABA analogues versus antihistamine | 5 | 220 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.75, ‐0.14] |
| 1.1.3 Ondansetron | 3 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.49, 0.15] |
| 1.1.4 Kappa‐opioid agonist | 4 | 661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.60, ‐0.27] |
| 1.1.5 Mu‐opioid antagonist | 2 | 62 | Std. Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐11.05, 2.85] |
| 1.1.6 Nalbuphine | 1 | 179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.54, 0.10] |
| 1.1.7 Cromolyn | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.00, ‐0.62] |
| 1.1.8 Nicotinamide | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.23, 0.88] |
| 1.1.9 EPO | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.39, 0.39] |
| 1.1.10 Cholestyramine | 2 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.89, 0.89] |
| 1.1.11 Montelukast | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.87, ‐0.92] |
| 1.1.12 Sertraline | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.15, 0.03] |
| 1.1.13 Lidocaine | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.87, 0.25] |
| 1.1.14 Gabapentin versus pregabalin | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.61, 0.63] |
| 1.1.15 GABA analogues versus doxepin | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.33, ‐0.36] |
| 1.2 Itch (dichotomous) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2.1 Lidocaine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2.2 Thalidomide | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2.3 Doxepin | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Topical interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Itch | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Capsaicin cream | 2 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.22, ‐0.45] |
| 2.1.2 Pramoxine cream | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.11, 0.41] |
| 2.1.3 Calcineurin Inhibitor cream | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.13, 0.90] |
| 2.1.4 Dead Sea lotion | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.14, 0.10] |
| 2.1.5 Cromolyn cream | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 2.1.6 Baby oil | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.32, ‐0.43] |
| 2.1.7 L‐arginine salve | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.82, 0.32] |
| 2.1.8 Polyunsaturated fatty acids | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.99, 0.17] |
Comparison 3. Oral or IV supplements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Itch | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Polyunsaturated fatty acids | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐11.30 [‐19.01, ‐3.59] |
| 3.1.2 L‐carnitine (IV) | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐2.85, 2.33] |
| 3.1.3 Zinc sulfate | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐2.88, ‐0.66] |
| 3.1.4 Ergocalciferol | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.48, 3.28] |
| 3.1.5 Turmeric | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐6.40 [‐7.42, ‐5.38] |
| 3.1.6 Fumaria parviflora | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐3.90 [‐5.04, ‐2.76] |
Comparison 4. Haemodialysis modality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Itch | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 High flux or permeability HD | 3 | 202 | Mean Difference (IV, Random, 95% CI) | ‐2.62 [‐3.72, ‐1.52] |
| 4.1.2 NMR haemoperfusion | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐2.37 [‐2.89, ‐1.85] |
Comparison 5. Other interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Itch | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 UV‐B | 4 | 86 | Mean Difference (IV, Random, 95% CI) | ‐4.06 [‐8.40, 0.28] |
| 5.1.2 Thermal therapy | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐6.54, 2.42] |
Comparison 6. Cross‐over studies with paired data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Cholestyramine | 2 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.86, 0.38] |
6.1. Analysis.

Comparison 6: Cross‐over studies with paired data, Outcome 1: Cholestyramine
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afrasiabifar 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "...allocated to two groups test and control using block randomization." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No placebo group, not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than %10 attrition per study protocol |
| Selective reporting (reporting bias) | Low risk | Specified results clearly reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Akrami 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Balanced blocked randomization with a block size of four was used." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Each set of eight bottles were packed into one container, each of which was numbered for each patient." "Code‐breaking was carried out after data analysis." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "All the participants and the investigator were blinded to group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "All the participants and the investigator were blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: treatment group (9); control group (7) |
| Selective reporting (reporting bias) | Low risk | Results clearly and fully reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Aliasgharpour 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "They were divided into two groups of experimental and control as random allocation block" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Single blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "The interviewer did not know the patients grouping into intervention and control" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 dropouts post‐randomisation |
| Selective reporting (reporting bias) | Unclear risk | Not specified what metrics of severity are being tested |
| Other bias | Unclear risk | During the study 72% and 52% of patients in the experimental and control group consumed medications such as antihistamines, Renagel, hydroxyzine, erythropoietin, and gabapentin No evidence of publication or funding bias |
Amirkhanlou 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "double‐blind randomised, Patients were randomly assigned to two groups " |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "double‐blind randomised" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double‐blind randomised, patients and drug distributors were not aware of the prescribed medications " |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "double‐blind randomised, patients and drug distributors were not aware of the prescribed medications" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised completed the study |
| Selective reporting (reporting bias) | High risk | Baseline scores not reported, raw scores not reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Aramwit 2012a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "The physician investigator enrolled the subjects into this study, and using a computer‐generated block of four, another investigator generated the random allocation sequence that divided the patients into two groups. The identities of the patients in each group were concealed from both the investigators and the patients." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "The physician investigator enrolled the subjects into this study, and using a computer‐generated block of four, another investigator generated the random allocation sequence that divided the patients into two groups. The identities of the patients in each group were concealed from both the investigators and the patients." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "The identities of the patients in each group were concealed from both the investigators and the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "The identities of the patients in each group were concealed from both the investigators and the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts (6%) "due to relocation". Unlikely to influence patients' body part/sides served as controls |
| Selective reporting (reporting bias) | Unclear risk | Split body trial with only aggregate intervention level data without patient level comparisons provided |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Ashmore 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Participants were randomised to receive active drug and placebo in a double‐blind crossover study." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Participants were randomised to receive active drug and placebo in a double‐blind crossover study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Patients recorded the intensity of pruritus each day on a 0‐to‐10 visual analogue scale" Patient assessed VAS |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/19 dropouts. Dropouts were balanced. Not ITT |
| Selective reporting (reporting bias) | Low risk | Cross‐over study, protocol in advance, both periods combined reported |
| Other bias | Unclear risk | Supported by grant from Glaxo Group Research and Yorkshire Kidney Research Fund |
Aubia 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "...included in a double blind randomised study that evaluated the effects of classic antihistaminic (group AH) before the effects of a placebo (P) during 4 weeks." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "...included in a double blind randomised study that evaluated the effects of classic antihistaminic (group AH) before the effects of a placebo (P) during 4 weeks." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts post randomisation |
| Selective reporting (reporting bias) | High risk | Only P‐values and t‐scores reported; unable to meta‐analyse |
| Other bias | Low risk | No evidence of publication or funding bias |
Baumelou 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "determined by randomization" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 dropouts |
| Selective reporting (reporting bias) | High risk | Only percentage change and P‐values reported |
| Other bias | Unclear risk | Abstract‐only publication |
Begum 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "randomised" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "packaged in similar soft gel capsules containing 1 g ethyl ester each" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "packaged in similar soft gel capsules containing 1 g ethyl ester each" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts and complete reporting |
| Selective reporting (reporting bias) | Low risk | No dropouts and complete reporting |
| Other bias | Low risk | No evidence of publication or funding bias |
Bhaduri 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Baseline, percent change and CI reported |
| Other bias | Unclear risk | Abstract‐only publication |
Blachley 1985.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "17 pruritic hemodialysis patients were randomised to one of two treatment groups: UVA (placebo) or UVB phototherapy." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "In a single blinded fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient reported VAS scores |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No post randomisation dropouts |
| Selective reporting (reporting bias) | Unclear risk | No placebo results explicitly reported. Reported in bar graph |
| Other bias | Low risk | QUOTE: "Supported by the United States Veterans Administration." No evidence of publication, funding, or other confounding bias |
Boaz 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group (DS)
Control group 1 (P1)
Control group 2 (P2)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization was conducted using an online randomiser (http://www.randomization.com) following stratification for gender and age (in 5‐year categories)" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "All were packaged in containers void of labelling except for the treatment code number and were identical in terms of shape, size and colour so that identification of treatment assignment was unknowable to the participant, study investigators and medical personnel. The code for treatment identification was held by a company representative and revealed only after data were analysed." ‐Treatments were unlabeled. coded, and held by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "DS, P1 and P2 were identical in colour, texture and scent. All were packaged in containers void of labelling except for the treatment code number and were identical in terms of shape, size and colour so that identification of treatment assignment was unknowable to the participant, study investigators and medical personnel. The code for treatment identification was held by a company representative and revealed only after data were analysed." ‐Treatments were virtually identical unlabeled. coded, and held by a third party |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "DS, P1 and P2 were identical in colour, texture and scent. All were packaged in containers void of labelling except for the treatment code number and were identical in terms of shape, size and colour so that identification of treatment assignment was unknowable to the participant, study investigators and medical personnel. The code for treatment identification was held by a company representative and revealed only after data were analysed." ‐Treatments were virtually identical unlabeled. coded, and held by a third party |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4,5,4 dropouts from DS, P1, P2 |
| Selective reporting (reporting bias) | Low risk | Baseline and post interventions results fully reported |
| Other bias | High risk | Ahava Dead Sea Laboratories, Ein Bokek, Israel, provided a research grant to the research fund of the Institute of Nephrology and the Epidemiology and Research Unit at E. Wolfson Medical Center, Holon, Israel. Two of the co‐authors, Miriam Oron and Zeevi Maor, are employees at Ahava Dead Sea Laboratories |
Breneman 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Multiple patient dropouts |
| Selective reporting (reporting bias) | High risk | No statistics reported |
| Other bias | Low risk | No evidence for publication or funding bias |
Carmichael 1988.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: '"randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "double blinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15% dropout rate, unclear allocation |
| Selective reporting (reporting bias) | High risk | Only "p > 0.1" reported |
| Other bias | Unclear risk | No washout period. No evidence of publication or funding bias |
Chan 1995.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "...were randomised for a six‐week UVB(N=10) double‐blind non‐crossover study against placebo (UVA, N=9)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double‐blind non‐crossover..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Assessment was made by a single investigator who was blind‐folded for the type of UV therapy to avoid observer variation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One post‐randomisation patient in the UVB group died of a stroke |
| Selective reporting (reporting bias) | High risk | Distribution of VAS reported as bimodal and nonlinear. No means were reported. Only P‐values (Fisher exact test) and graphs |
| Other bias | Unclear risk | Abstract‐only publication; insufficient information to permit judgement |
Chen 2006e.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "At the end of the baseline day, patients were randomly assigned to group A or group B." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "patients applied topical GLA‐rich cream or placebo cream in a double‐blind fashion to their entire body" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient recorded pruritus score, double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout (allergic reaction to GLA cream) |
| Selective reporting (reporting bias) | Unclear risk | Median and IQR clearly reported for each treatment phase. Group level data without individual patient level comparisons provided |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Chen 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "An independent technician allocated the patients into one of two groups, either HPHD or CHD, according to a random‐number table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts after randomisation |
| Selective reporting (reporting bias) | Low risk | All results clearly and fully reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Cho 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Treatment order was arranged from computer generated numbers" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "by a coauthor who did not participate in observations" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blinded" and "were unknown by the observers and patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, patients made self‐evaluations, base creams ""were unknown by the observers and patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients completed the trial and were analysed. |
| Selective reporting (reporting bias) | Low risk | Baseline and post interventions results fully reported Intervention level data report with patient level graphical comparison comparisons provided. Correlation may inflate standard error. Carry‐over effects unlikely due to washout periods. |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
De Marchi 1992.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "patients were randomly assigned" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "All placebo and intervention labelling hidden by treatment code." "Code broken only after completion" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "All treatment was hidden by treatment code. Both placebo and intervention delivered in the same way." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment performed by a "single investigator who was unaware of treatment assignments" "treatment code was only broken after the trial had ended" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One withdrawal in second crossover period (recorded as non‐responding for at least the first week). Unclear if ITT, but unlikely to significantly influence results |
| Selective reporting (reporting bias) | High risk | All entered patients completed the trial and were analysed VAS documented directly from patient diaries Intervention level data without patient level comparisons provided No washout period specified |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Duque 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "randomised, double‐blind, vehicle controlled study" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "randomised, double‐blind, vehicle controlled study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient recorded VAS |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropout in vehicle (kidney transplantation and lack of improvement) Unclear if ITT |
| Selective reporting (reporting bias) | High risk | No SDs reported |
| Other bias | High risk | Supported by Fujisawa Health Care Inc, Deerfield, Ill. |
Durant‐Finn 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unclear of specific method in translation, but a randomisation technique is likely used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 24 patients reported on for each 2‐week period |
| Selective reporting (reporting bias) | Low risk | Main outcomes fully reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Fallahzadeh 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as "randomised double‐blind placebo‐controlled"; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "double‐blind"; insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "double‐blind"; insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Abstract‐only publication; insufficient information to permit judgement |
Feily 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization was performed by using a simple random table," |
| Allocation concealment (selection bias) | Low risk | QUOTE: "The placebo was formulated by a pharmacist to have a similar base with the drug but not containing the active ingredient and stored in a tube without any labelling. A similar tube was used to store CS 4% to make both creams to look physically identical." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "The placebo was formulated by a pharmacist to have a similar base with the drug but not containing the active ingredient and stored in a tube without any labelling. A similar tube was used to store CS 4% to make both creams to look physically identical." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The medications used were not revealed to their physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All entered patients completed the trial and were analysed |
| Selective reporting (reporting bias) | Low risk | Baseline and results clearly reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Foroutan 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "randomly assigned to pregabalin or doxepin based on block randomization" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Patients were not blind to their treatment, but who evaluated the participants and who statistically analyzed the results did not know the allocated medication of each patient" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "Patients were not blind to their treatment, but who evaluated the participants and who statistically analyzed the results did not know the allocated medication of each patient" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Patients were not blind to their treatment, but who evaluated the participants and who statistically analyzed the results did not know the allocated medication of each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not ITT, 9 dropouts in each arm all with justifications |
| Selective reporting (reporting bias) | Low risk | Clear reporting of scores at all time points |
| Other bias | Low risk | No evidence of publication or funding bias |
Ghanei 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | QUOTE: "Patients were divided into two groups randomly by alternation method" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blinded", "Fish oil and placebo capsules with the same shape and volume" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes came from "observation and interview", "double blinded" No other specific information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All entered patients completed the trial and were analysed |
| Selective reporting (reporting bias) | Unclear risk | Results reported as percent reduction of a customised itch score Correlation may inflate standard error. Carry‐over effects unlikely due to washout periods. |
| Other bias | Low risk | No evidence for publication, funding, or other confounding bias |
Ghorbani 2012a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization was performed by using a simple random table." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double Blind", Patients given unlabelled medication as start of trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient recorded VAS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients completed the trial and were analysed |
| Selective reporting (reporting bias) | Low risk | Baseline and results clearly reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Ghorbani Birgani 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "Blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "Blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if any patient dropped out |
| Selective reporting (reporting bias) | Low risk | Full results clearly reported |
| Other bias | Low risk | No evidence of publication or funding bias |
Gilchrest 1977.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "were randomly assigned to one of two treatment schedules" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported, similar control treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, likely known to assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 dropouts due to unspecified concurrent illness. Unknown which arm they were randomised to. Not Intention to treat |
| Selective reporting (reporting bias) | High risk | Unclear grading of pruritis and not classified by patient; unable to meta‐analyse |
| Other bias | Unclear risk | Poor/minimal exclusion criteria; no evidence of publication or funding bias |
Gilchrest 1979.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "were randomly assigned to one of two treatment schedules" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported, similar control treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, likely known to assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 dropouts due to unspecified concurrent illness. Unknown which arm they were randomised to. Not Intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Unclear grading of pruritis and not classified by patient |
| Other bias | Unclear risk | Poor/minimal exclusion criteria; no evidence of publication or funding bias |
Gobo‐Oliveira 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomisation was performed by an individual unrelated to the clinical follow‐up using specific software" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Randomisation was performed by an individual unrelated to the clinical follow‐up using specific software, and the information was held in a sealed opaque envelope containing the name of the therapeutic agent proposed for each group. The randomisation list was under the care of the researchers and patients were labelled as “Group 1” or “Group 2” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Both groups were instructed to take one tablet every 12 hours and received two bottles identified as “Home” and “Dialysis”. The “Home” bottle was taken at home by the patient who was directed to take medication twice a day on non‐HD days and once daily on HD days. To maintain blinding of the study, for the GABA group, the “Home” bottle contained a placebo identical to the gabapentin capsule, and the medication was stored in the “Dialysis” bottle. The “Dialysis” bottle remained in the Dialysis Unit, and the medication was administered to patients at the end of the session by the responsible technician. Participants and assessors were blinded to the treatment groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Participants and assessors were blinded to the treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Statistical analysis was conducted by intention to treat (ITT). The missing data (dropouts) were replaced by the last recorded values (LOCF) 1 dropout in each arm post randomisation |
| Selective reporting (reporting bias) | Low risk | Results clearly reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Gunal 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "On a random and blinded basis, patients were assigned to" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "On a random and blinded basis, patients were assigned", "We conducted a double‐blind," |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double‐blinded", "The daily pruritus scores of patients were collected VAS from patient diaries.", "On a random and blinded basis, patients were assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients completed the trial and were analysed. Multiple 1 week washout periods preceding intervention and control periods. |
| Selective reporting (reporting bias) | Unclear risk | All entered patients completed the trial and were analysed Both periods combined reported with mean change and standard deviations reported in full Intervention level data without patient level comparisons provided. Correlation may inflate standard error. Carry‐over effects unlikely due to washout periods |
| Other bias | Low risk | No intervention first group (however 1 week washout). No evidence of publication, funding, or other confounding bias |
Hsu 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "A staff team not involved in the trial organized and held the randomisation list and serially numbered envelopes." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "A staff team not involved in the trial organized and held the randomisation list and serially numbered envelopes. They passed envelopes to the principal investigator after demonstrating that the patient has consented to the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "The non‐thermal therapy group received a plain adhesive patch placed on the same acupoint and routine care. The principal investigator stayed with these patients for the same duration as the thermal therapy group." QUOTE: "The staff team was did not know to which treatment group a patient would be allocated. The principal investigator opened envelopes to reveal the study treatment allocation and then administered the intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "The staff team was did not know to which treatment group a patient would be allocated. The principal investigator opened envelopes to reveal the study treatment allocation and then administered the intervention." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Eight participants (thermal group = 3, non‐thermal group = 5) declined or were unable to participate in the study for various reasons (e.g. dermatological disorders and other medical conditions). Not ITT. |
| Selective reporting (reporting bias) | Low risk | Baseline and results reported for both arms |
| Other bias | Low risk | No evidence of publication or funding bias |
Hui 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by random serial number generated from a random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No change to dialysis for control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Baseline and final scores fully reported |
| Other bias | Low risk | No evidence of publication or funding bias |
Jiang 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "randomly allocated to two groups with the aid of ClinStat software" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% dropout in both groups and balanced |
| Selective reporting (reporting bias) | Unclear risk | All results clearly reported |
| Other bias | Low risk | No evidence for publication or funding bias |
Ko 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "The enrolled patients were randomly assigned to the treatment and control groups, with an allocation ratio of 1: 1, according to a sequence of computer‐generated randomised codes" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "The control group received time‐matched exposures to long‐ wave UVA. The doses of UVA were approximately 1– 6 J cm‐2, which was an appropriate control in this study." "Single blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "Single blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An allocation ratio of 1:1 of is reported |
| Selective reporting (reporting bias) | Low risk | Baseline and results reported for both arms |
| Other bias | Low risk | No evidence of publication or funding bias |
Kumagai 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "receive 5 µg, 2.5 µg nalfurafine or a placebo using a variable size permuted block design stratified by centre" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "variable size permuted block design" this implies the assignments are coded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "The patients took the soft capsules containing the drug or placebo once daily" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double blinded". Patient's directly recorded their VAS scores. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | QUOTE: "Each arm had 2‐3 patients discontinue due to adverse effects. 1 patient in each arm who did not received any treatment were not analysed." QUOTE: "The full analysis set (FAS), defined as all patients who were randomised and received at least one dose of study drug and were as close as possible to the intention‐to‐treat ideal, was chosen for examining the primary end point." ‐ Few dropouts and followed ITT |
| Selective reporting (reporting bias) | Low risk | Baseline and post interventions results fully reported |
| Other bias | Low risk | No evidence for publication, funding, or other confounding bias |
Kyriazis 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the trial and are reported on |
| Selective reporting (reporting bias) | High risk | Pre and post intervention scores not reported |
| Other bias | Unclear risk | No female participants; no evidence of publication or funding bias |
Legroux‐Crespel 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "This was a randomised study (drawing of lots)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported. Likely not blinded. No discussion for treatment concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear number of dropouts, at least 10 |
| Selective reporting (reporting bias) | High risk | Missing raw data (No standard deviations for either group or baseline scores score for the natrexone group reported) |
| Other bias | High risk | Conflicting results and arbitrary definitions of improvement; no evidence of publication or funding bias |
Li 2017a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patient randomly selected letters |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Sealed letters" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of their intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants aware of their intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Specified pre and post intervention scores not reported, but some surrogate statistics are |
| Selective reporting (reporting bias) | Low risk | < 10% dropouts post randomisation |
| Other bias | Unclear risk | Patients recruited mid study to replace all dropout as specified in their protocol |
Lin 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | QUOTE: "All qualified participants were recruited. Those currently receiving haemodialysis treatment every Monday, Wednesday and Friday were enrolled in experimental group 1; those currently receiving haemodialysis treatment on Tuesday, Thursday and Saturday, were enrolled in experimental group 2. The control group consisted of patients randomly selected from the above two groups." Quasi‐RCT |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Doctor administered questionnaire with no blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 skin rash, privacy concerns and hospitalisation. Unclear which treatment arms they were in |
| Selective reporting (reporting bias) | Low risk | Change in pruritus and baseline pruritus reported |
| Other bias | Unclear risk | Poor exclusion criteria. Blinding likely not possible as intended for intervention type. No evidence of publication or funding bias |
Mahmudpour 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "...enrolled in the study and based on block randomization method, were randomised into 2 groups of 40 participants" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "All medication and placebo tablets were similar in size, shape, weight, color, and package. Clinical investigators, laboratory personnel, and patients were all masked to the treatment assignment and code breaking was done at the end of study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "All medication and placebo tablets were similar in size, shape, weight, color, and package. Clinical investigators, laboratory personnel, and patients were all masked to the treatment assignment and code breaking was done at the end of study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "All medication and placebo tablets were similar in size, shape, weight, color, and package. Clinical investigators, laboratory personnel, and patients were all masked to the treatment assignment and code breaking was done at the end of study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% dropout in each arm, roughly equal, with explanation |
| Selective reporting (reporting bias) | Low risk | Outcomes clearly reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Makhlough 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomly assigned by lottery into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blind" QUOTE: "The placebo was prepared in a same size and colour packages as Capsian 0.03% ointment tubes." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "Double blind" QUOTE: "The placebo was prepared in a same size and colour packages as Capsian 0.03% ointment tubes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All entered patients completed the trial and were analysed |
| Selective reporting (reporting bias) | Low risk | Baseline and results reported for both arms |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Mapar 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised, triple‐blind study" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "randomised, triple‐blind study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "randomised, triple‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "randomised, triple‐blind study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% dropouts per arm with explanation |
| Selective reporting (reporting bias) | Low risk | Clear results |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Marin 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A simple randomization will be carried out by computer using the medcalc software" |
| Allocation concealment (selection bias) | High risk | "open, comparative clinical trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open, comparative clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "open, comparative clinical trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% attrition rate (2 drop out in the gabapentin group and none in the Loratidine group) |
| Selective reporting (reporting bias) | Low risk | All results fully and clearly reported |
| Other bias | Low risk | "The study is financed by the Hospital de Concentración ISSEMyM Satélite" No evidence of publication or funding bias |
Mettang 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "A Double‐Blind randomised Trial" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "Double‐Blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "Double‐Blind" and patient recorded diary |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, but with implication of no dropouts |
| Selective reporting (reporting bias) | Low risk | Baseline and postintervention results reported |
| Other bias | Unclear risk | QUOTE: "Supported in part by research grants from Fresenius AG, Oberursel; the Khalil Foundation; the Robert‐Bosch Foundation, Stuttgart; and Fa Medice, Iserlohn, Germany" |
Mirnezami 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE "Double Blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE "Double Blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Change in pruritus and baseline pruritus reported |
| Other bias | Unclear risk | Abstract only |
Mohamed 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | No numeric results |
| Other bias | Unclear risk | Abstract‐only publication; poorly explained inclusion/exclusion criteria |
Mojgan 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Double blind" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "Double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear dropout rate |
| Selective reporting (reporting bias) | High risk | Only group means and a nonspecific P value reported |
| Other bias | Unclear risk | Abstract‐only publication; insufficient information to permit judgement |
Murphy 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "On a random basis, 24 patients were blindly allocated..." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "On a random basis, 24 patients were blindly allocated..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "... were blindly allocated to the ondansetron‐placebo sequence and 10 to the placebo‐ondansetron sequence" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUUOTE: "Double blind", VAS directly recorded by patients. Investigator independent from implementation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT. ~25% attrition. Non‐compliance and complications partially addressed. Cross‐over design likely limits the severity of the bias |
| Selective reporting (reporting bias) | Low risk | VAS from patient diaries. All baselines and results reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Naghibi 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "On a random and blinded basis" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "On a random and blinded basis" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "On a random and blinded basis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | QUOTE: "All of the patients completed the study" |
| Selective reporting (reporting bias) | Low risk | The mean difference of pruritus score (VAS) before and after treatment was fully reported. One week washout in between all interventions and controls. Carry‐over effects unlikely |
| Other bias | Unclear risk | Abstract‐only publication; group level data without patient level comparisons provided; correlation may inflate SE |
Naini 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "The patients were randomly allocated to receive either gabapentin 400 mg or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "to prepare the placebo, we emptied gabapentin capsules and refilled them with flour, thus making them indistinguishable" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double blind" VAS from patient diaries. Investigator independent |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All entered patients completed the trial and were analysed |
| Selective reporting (reporting bias) | Low risk | Baseline and mean decreases reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Najafabadi 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "The patients were then randomly assigned into treatment and placebo groups." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "At the end of the study the drug and placebo groups were determined by decoding." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blind", "while the other group received a similar shaped and coloured capsule which was a placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Neither the patients nor the physicians had any knowledge of the group to which patients were assigned. The patients were assigned codes, and at the end of the study the drug and placebo groups were determined by decoding." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patient completed the trial and were analysed |
| Selective reporting (reporting bias) | Low risk | Baseline and postintervention results clearly recorded |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Nakhaee 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: Assigned by random numbers to 3 groups (two with 8 patients and one with 9). The CONSORT flowchart that describes the progress of the patients through the trial" |
| Allocation concealment (selection bias) | Unclear risk | QUOTE: "Assigned by random numbers to 3 groups (two with 8 patients and one with 9). The CONSORT flowchart that describes the progress of the patients through the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Topical and scented intervention versus oral |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Topical and scented intervention versus oral |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts post randomisation due to kidney transplantation |
| Selective reporting (reporting bias) | High risk | Only 3‐day washout. Intervention level data without patient level comparisons provided |
| Other bias | Low risk | No evidence of publication or funding bias |
Nasrollahi 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "The patients were randomly divided into groups 1 and 2" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "single‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | QUOTE: "single‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Anaemia from myelodysplasic syndrome in montelukast arm (1); death but to myocardial infarction in placebo (1) Not ITT, but followed the Good Clinical Practices guidelines in RCTs which recommended including the MI patient and excluding the myelodysplasic patient |
| Selective reporting (reporting bias) | Unclear risk | Only percent changes recorded with no baseline Intervention level data without patient level comparisons provided Carry‐over effects unlikely due to washout periods |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Nofal 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization was done by random number list" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "single‐blinded trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "single‐blinded trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patient analysed |
| Selective reporting (reporting bias) | Low risk | All results clearly reported |
| Other bias | Low risk | No evidence for publication, funding, or other confounding bias |
Noshad 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "...randomised in two groups..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double‐blind", "Patients and investigators were not aware of the medications prescribed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Double‐blind", "Patients and investigators were not aware of the medications prescribed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients randomised and analysed at trial completion. No dropouts |
| Selective reporting (reporting bias) | Low risk | Mean and SE of VAS at baseline and after the intervention reported in full for both placebo and Gabapentin groups |
| Other bias | Unclear risk | Abstract‐only publication; insufficient information to permit judgement |
Omidian 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization was performed by using a simple random table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "The used medications were not revealed to the treating physicians." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "The patients were oriented as to how to interpret their pruritus based on Visual Analogue Scale (VAS)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout from Nicotinamide group |
| Selective reporting (reporting bias) | Low risk | All baseline and weekly results reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Ozaykan 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "open, randomised and comparative study" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts in either group |
| Selective reporting (reporting bias) | Unclear risk | Baseline and weekly results all reported Group level data without individual patient level comparisons provided |
| Other bias | Low risk | No evidence of publication or funding bias |
Pakfetrat 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Factorial block randomisation was used for allocation sequence" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "The allocation sequence was concealed from the researcher enrolling and assessing participants in sequentially numbered, opaque, sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Clinical investigators, laboratory personnel, and patients were all masked to the treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Clinical investigators, laboratory personnel, and patients were all masked to the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout (1% attrition rate), unlikely to change study results |
| Selective reporting (reporting bias) | Low risk | All baseline and final results reported |
| Other bias | Low risk | No evidence for publication, funding, or other confounding bias |
Pakfetrat 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "...randomly we divided patients into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "This double blinded clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "This double blinded clinical trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | During the course of study one patient from control group died due to an accident and three patients of this group quit the study as a result of feeling no relief in their symptom. Twenty‐one patients remained in control group |
| Selective reporting (reporting bias) | Low risk | Clearly reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Pauli‐Magnus 2000.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised, double‐blind, placebo‐controlled crossover study" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Double blind". Patient recorded their own scores "on a daily basis by marking a visual analogue scale (VAS)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 dropouts. Mostly from developing an indication for opiates. ITT protocol followed |
| Selective reporting (reporting bias) | Unclear risk | Means and CIs from each week reported for each of naltrexone and placebo Group level data without patient level comparisons provided. Correlation may inflate standard error. Carry‐over effects unlikely due to washout periods |
| Other bias | Low risk | No evidence of publication or funding bias |
Peck 1996.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Patients were randomly assigned into three groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded, patient reported Duo score |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 dropouts out of 41 enrolled |
| Selective reporting (reporting bias) | Low risk | Detail table of results (mean, standard error) at baseline, postintervention, and net change for all groups |
| Other bias | Low risk | No evidence of publication or funding bias |
Pederson 1980.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Patients were randomly assigned..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blinded, "treatments "administered orally in identical opaque capsules", "iron pills masked the charcoal stained stools" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind", unclear is assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Likely 9 dropouts/20, patients dropped for low compliance |
| Selective reporting (reporting bias) | High risk | Incomplete results with arbitrary markers for improvement |
| Other bias | Unclear risk | No washout indicated, unlike other naltrexone studies; no evidence of publication or funding bias |
Peer 1996.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "entered a randomised double‐blind placebo controlled crossover study (figure 1)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind. Patient recorded their own scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients completed the trial and were analysed |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting of placebo itch score SDs Group level data without patient level comparisons provided. Correlation may inflate standard error. Carry‐over effects unlikely due to washout periods. |
| Other bias | Low risk | No evidence of publication or funding bias |
Pour‐Reza‐Gholi 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "They were randomly assigned..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "...placed in another capsule in order to provide placebo capsules similar in shape, size, and colour." "The patients and the physicians involving in their management were blind to the randomization." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "The patients and the physicians involving in their management were blind to the randomization. Assessments based on clinician subjective reports." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient dropout from doxepin group; did not complete placebo portion |
| Selective reporting (reporting bias) | Unclear risk | Aggregate results reported, arbitrary and subjective reporting of outcomes Group level data without patient level comparisons provided. Correlation may inflate standard error. Carry‐over effects unlikely due to washout periods. |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Rad 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "The random permuted block method was used" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "the [researcher] was unaware of whether they were assigned to the intervention or control", "triple blinded" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | QUOTE: "triple blinded"; unclear how one can blind patients to temperature |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "triple blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post randomisation dropouts |
| Selective reporting (reporting bias) | High risk | Only baseline VAS reported. Quantitative results of the regression not reported |
| Other bias | Unclear risk | No evidence of publication or funding bias |
Rivory 1984.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE " in a random order" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE "in double blind manner" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE "in double blind manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | High risk | Only nonspecific, interpreted results reported |
| Other bias | Unclear risk | Abstract only publication |
Shariati 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Blinded" while discussing participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "Blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% dropouts |
| Selective reporting (reporting bias) | Low risk | Full results reported with paired testing |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Sherjeena 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | QUOTE: "By alternation" |
| Allocation concealment (selection bias) | High risk | QUOTE: "By alternation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | QUOTE: "Unblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | QUOTE: "Unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Results reported in full |
| Other bias | Low risk | No evidence of publication or funding bias |
Shirazian 2013.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "A research pharmacist prepackaged ergocalciferol and placebo tablets into opaque bottles. A research nurse, who did not participate in con sent, pruritus surveys, or study analysis assigned patients to the appropriate pill bottle. The research nurse also dispensed the medication to the patient (within 1 week of the prerandomization visit and randomisation assignment)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Patients and investigators were blinded to the allocation of the study drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Patients and investigators were blinded to the allocation of the study drug." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT protocol, 6 dropout (4 in Ergocalciferol group) |
| Selective reporting (reporting bias) | Low risk | Baseline and result fully reported at www.clinicaltrial.gov |
| Other bias | Low risk | No evidence of publication or funding bias |
Silva 1994.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18/29 completed the study after randomisation, no ITT |
| Selective reporting (reporting bias) | High risk | Only subjective responder rates recorded with arbitrary cut offs. Group level data without patient level comparisons provided. Carry‐over effects unlikely due to washout periods. |
| Other bias | Low risk | No evidence for publication or funding bias |
Silverberg 1977.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "patients were randomly assigned to two treatments" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patient completed the trial and were analysed |
| Selective reporting (reporting bias) | Low risk | All patient outcomes reported |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Sja'bani 1997.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "randomised double blind study design" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 dropout (2 placebo, 1 rHuEPO) reasons not reported |
| Selective reporting (reporting bias) | Unclear risk | Baseline VAS scores not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Solak 2012.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Patients were randomised into either gabapentin (25 patients) or pregabalin (25 patients) treatment arms using computer generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 dropouts from each group. ITT unclear |
| Selective reporting (reporting bias) | Unclear risk | Change (mean and SD) in VAS clearly reported for each treatment type and period Group level data without patient level comparisons provided. Carry‐over effects unlikely due to washout periods. |
| Other bias | Low risk | No evidence of publication or funding bias |
Spencer 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Multi‐center (21 US sites), randomised (1:1), double‐blind, placebo‐controlled, parallel‐group Phase 2 study" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double‐blind, placebo‐controlled, parallel‐group Phase 2 study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "double‐blind, placebo‐controlled, parallel‐group Phase 2 study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One dropout in the placebo group, unlikely to affect outcomes |
| Selective reporting (reporting bias) | Low risk | Mean and SD of changes and baseline VAS score reported for both CR845 and placebo |
| Other bias | High risk | The present study was fully supported by Cara Therapeutics, Inc. The authors received medical writing assistance from Edward Weselcouch, PhD, of PharmaWrite (Princeton, NJ), which was funded by Cara Therapeutics, Inc. RHS, JWS, and FM are employees of Cara Therapeutics, Inc |
Spencer 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported |
| Selective reporting (reporting bias) | High risk | 1.0 µg/kg and placebo results not fully reported |
| Other bias | High risk | Abstract‐only publications; funded by Cara Therapeutics |
Subach 2001.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "in a randomised..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear reporting. Assumed to be results from 120 min, but not clear. No results of the ANOVA reported |
| Other bias | Unclear risk | Abstract only; no declaration relating to conflicts of interest |
Suwanpidokkul 2007.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Route not specified but implied oral |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Patients were randomised assigned" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double‐blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Partial reporting on 5 dropout |
| Selective reporting (reporting bias) | Low risk | Within and between group changes clearly reported |
| Other bias | Low risk | Abstract only; no declaration relating conflicts of interest |
Tamimi 1999.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/33 patients failed to complete study |
| Selective reporting (reporting bias) | High risk | No data available to meta‐analyse |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tan 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "The order of study medicaments used was randomly assigned for consecutive patients according to a computer‐generated randomization code." |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Both observer and patient were blinded to the identity of the medications, which were contained in identical opaque plastic bottles." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Both observer and patient were blinded to the identity of the medications, which were contained in identical opaque plastic bottles." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Both observer and patient were blinded to the identity of the medications, which were contained in identical opaque plastic bottles." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout, unlikely to change results |
| Selective reporting (reporting bias) | Unclear risk | Baseline and final scores recorded in full Group level data without patient level comparisons provided. Carry‐over effects unlikely due to washout periods |
| Other bias | Low risk | Interventions used "as required". No evidence of publication or funding bias |
Tapia 1977.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Table of random numbers" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Vial arranged in order and patient enters study area with unlabelled vials" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double Blind", "Identical vials" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "investigator unaware of vial order" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four placebo patients unaccounted for in analysis |
| Selective reporting (reporting bias) | High risk | Simple binary response fully reported, only 6 placebo patients reported on with no explanation |
| Other bias | Low risk | No evidence of publication or funding bias |
Tarng 1996.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Treatment order is block‐randomized with the use of computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double blind, doctor evaluated, complex assignments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropouts, not ITT |
| Selective reporting (reporting bias) | High risk | Placebo results not reported Group level data without patient level comparisons provided |
| Other bias | Low risk | No evidence of publication or funding bias |
Taylor 1983.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised into control and treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded (used a radiation barrier) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded (used a radiation barrier) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | High risk | Qualitative results only |
| Other bias | Low risk | No evidence of publication or funding bias |
Tol 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "On a random basis..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient recorded VAS independent of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient enrolled completed the trial |
| Selective reporting (reporting bias) | High risk | Placebo results not reported Intervention level data without patient level comparisons provided. Carry‐over effects unlikely due to washout periods |
| Other bias | Low risk | No evidence of publication or funding bias |
TREVITR02 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 3
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Randomization was performed by site personnel, using a centralized interactive web‐based randomization system, which assigned unique blister card numbers reflecting the blinded treatment assignment" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Randomization was performed by site personnel, using a centralized interactive web‐based randomization system, which assigned unique blister card numbers reflecting the blinded treatment assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "The sponsor, study site personnel, and all contract research organization personnel involved in the conduct of the trial were blinded to treatment assignment. Matching placebo was used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "The sponsor, study site personnel, and all contract research organization personnel involved in the conduct of the trial were blinded to treatment assignment. Matching placebo was used" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT, high number of post‐randomisation dropout with explanation |
| Selective reporting (reporting bias) | Low risk | Numerical rate scale clearly reported |
| Other bias | High risk | For‐profit pharmaceutical development |
van Leusen 1978.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Results clearly reported |
| Other bias | Unclear risk | Washout period unclear; no evidence of publication or funding bias |
Vessal 2010.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | QUOTE: "Stratified randomization method where the prognostic factor was the gender variable" |
| Allocation concealment (selection bias) | Low risk | QUOTE: "Drug packages were prepared by the principal investigator (G.V.). Both the participants and the investigator that administered the interventions and assessed the outcomes were blinded to group assignment. Code breaking was performed at the end of data analysis." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Drug packages were prepared by the principal investigator (G.V.). Both the participants and the investigator that administered the interventions and assessed the outcomes were blinded to group assignment. Code breaking was performed at the end of data analysis." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QUOTE: "Drug packages were prepared by the principal investigator (G.V.). Both the participants and the investigator that administered the interventions and assessed the outcomes were blinded to group assignment. Code breaking was performed at the end of data analysis." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 dropouts from each arm. Not analysed on ITT
|
| Selective reporting (reporting bias) | Low risk | Clearly reported full results |
| Other bias | Low risk | No evidence of publication or funding bias. |
Wikstrom 2005.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Study 1 treatment group
Study 1 control group
Study 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "patients were randomly assigned in this study" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded, Patient recorded VAS independent of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% attrition and balanced, analysed with ITT |
| Selective reporting (reporting bias) | Low risk | Cross‐over period 2 ignored, but mentioned in protocol |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Yoshimoto‐Furuie 1999.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "The patients were randomly assigned into two study groups:" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "in a double‐blind manner." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient enrolled completed the trial |
| Selective reporting (reporting bias) | Unclear risk | No actual itch scores reported. Only bar graph and P‐values |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Young 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "a randomised, double‐blind, controlled comparative trial" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "a randomised, double‐blind, controlled comparative trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinical evaluation, double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout (~3%), unlikely to changes study results |
| Selective reporting (reporting bias) | High risk | Only a regression slope result reported |
| Other bias | High risk | Financial conflicts of interest ‐ Funding obtained from Stiefel Laboratories (GSK), a manufacturer of skin care products |
Yue 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "Patients were randomly assigned to 12 weeks of treatment" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | QUOTE: "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | QUOTE: "prescription of pregabalin for UP was not mentioned in the dispensatory." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ~5% dropout rate. Unclear in following ITT |
| Selective reporting (reporting bias) | Low risk | Baseline and final itch results reported in full for all interventions and placebo (mean and standard error) |
| Other bias | Low risk | No evidence of publication, funding, or other confounding bias |
Zhang 2016a.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | QUOTE: "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts post randomisation |
| Selective reporting (reporting bias) | Low risk | VAS clearly reported for both groups |
| Other bias | Low risk | No evidence of publication or funding bias |
APD ‐ automated peritoneal dialysis; BP ‐ blood pressure; CKD ‐ chronic kidney disease; DM ‐ diabetes mellitus; (rHu)EPO ‐ (recombinant human) erythropoietin; ESKD ‐ end‐stage kidney disease; Hb ‐ haemoglobin; HCT ‐ haematocrit; HD ‐ haemodialysis; HDF ‐ haemodiafiltration; ITT ‐ intention‐to‐treat; IV ‐ intravenous; Kt/V ‐ dialysis adequacy; M/F ‐ male/female; PD ‐ peritoneal dialysis; (i)PTH ‐ (intact) parathyroid hormone; RCT ‐ randomised controlled trial; SBP ‐ systolic blood pressure; SC ‐ subcutaneous; SD ‐ standard deviation; SE ‐ standard error; SLE ‐ systemic lupus erythematosus; UV ‐ ultraviolet; VAS ‐ visual analogue scale; WBC ‐ white blood cell/s
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bousquet 1989 | QUOTE: "All patients with pruritus entered in a crossover, double‐blind trial with nicergoline. In a first period of six dialyses, they received either nicergoline (daily oral dose, 30 mg, and intravenous dose during dialyses, 5 mg) or placebo. In the second period of six dialyses, patients received the crossover treatment" COMMENT: Randomisation unclear; unable to confirm |
| Burrai 2014 | Wrong intervention: not applicable pruritus intervention for this review (music) |
| Cavalcanti 2003 | Wrong intervention: not applicable pruritus intervention for this review (homeopathy) |
| Che‐Yi 2005 | Wrong intervention: not applicable pruritus intervention for this review (acupuncture) |
| CTRI/2016/04/006870 | Wrong intervention: not applicable pruritus intervention for this review (self care) |
| CYCLE‐HD 2016 | Wrong intervention: not applicable pruritus intervention for this review (exercise for cardiovascular health) |
| Gao 2002 | Wrong intervention: not applicable pruritus intervention for this review (acupuncture) |
| Ghura 1998 | Wrong study design: no control |
| IRCT201303093560N2 | Wrong intervention: not applicable pruritus intervention for this review (massage) |
| IRCT2015091010076N6 | Wrong intervention: not applicable pruritus intervention for this review (massage) |
| Jedras 2003 | Wrong intervention: not applicable pruritus intervention for this review (acupuncture) |
| Joffe 1985 | Other: study terminated due to lack of enrolment |
| Kilic Akca 2016 | Wrong intervention: not pruritus intervention (acupuncture) |
| Legat 2017 | Wrong population: includes all pruritus, not just uraemic pruritus |
| Little 1995 | QUOTE: " At entry patients were selected to receive loratidine or placebo for two weeks after which crossover occurred" COMMENT: Randomisation unclear and no mention of dose |
| Lücker 1986 | Protocol only. No update in > 30 years |
| Marquez 2012 | We did not consider allocation based on dialysis schedule as quasi‐randomisation. More than alternation or other forms of quasi‐RCT this introduces additional bias |
| NCT00577967 | Recruitment status unknown (not yet recruiting as of 7 July 2007) |
| NCT00793156 | Recruitment status unknown (not yet recruiting as of 4 February 2010) |
| NCT01073501 | Recruitment status unknown (not yet recruiting as of 23 February 2010) |
| NCT01620580 | Recruitment status unknown (not yet recruiting as of 4 February 2010) |
| NCT01660243 | Recruitment status: terminated due to insufficient patient recruitment (17 March 2016) |
| NCT01852318 | Recruitment status unknown (not yet recruiting as of 15 April 2014) |
| NCT02032537 | Recruitment status unknown (not yet recruiting as of 10 January 2014) |
| NCT02432508 | Wrong intervention: not applicable pruritus intervention for this review (acupuncture) |
| Och 2000 | Wrong intervention: not applicable pruritus intervention for this review (acupressure) |
| Rehman 2018 | Wrong intervention: not applicable pruritus intervention for this review (acupressure) |
| Ro 2002 | Wrong intervention: not applicable pruritus intervention for this review (aromatherapy) |
| Rui 2002 | Wrong intervention: not applicable pruritus intervention for this review (acupuncture) |
| Sanchez 1986 | Wrong control: UVA versus PUVA are indistinguishable interventions |
| Wang 2014e | We did not consider allocation based on dialysis schedule as quasi‐randomisation. More than alternation or other forms of quasi‐RCT this introduces additional bias |
| Weisshaar 2003 | Areas on each patient are randomised to treatment rather than patients |
| Yan 2015 | Wrong intervention: not applicable pruritus intervention for this review (acupressure) |
| Yoshida 2017 | We did not consider allocation based on dialysis schedule as quasi‐randomisation. More than alternation or other forms of quasi‐RCT this introduces additional bias |
| Zadeh 2015 | Wrong intervention: not applicable pruritus intervention for this review (massage) |
| Zhang 2011d | Wrong intervention: not applicable pruritus intervention for this review (acupuncture) |
Characteristics of studies awaiting classification [ordered by study ID]
Bai 2002.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
NCT01513161.
| Methods |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
| Outcomes |
|
| Notes |
|
NCT02696499.
| Methods |
|
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Notes |
|
NCT02747979.
| Methods | Parallel, open‐label, RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
ADAMA ‐ asymmetric dimethylarginine; BMI ‐ body mass index; BMP2 ‐ bone morphogenetic protein 2; CABG ‐ coronary artery bypass grafting; CKD ‐ chronic kidney disease; CRP ‐ C‐reactive protein; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; MOS ‐ medical outcomes study; NRS ‐ numerical rating scale; NYHA ‐ New York Heart Association; PCI ‐ percutaneous coronary intervention; PGIC ‐ Patient Global Impression of Change; (i)PTH ‐ (intact) parathyroid hormone; QoL ‐ quality of life; RCT ‐ randomised control trial; URR ‐ urea reduction ratio; VAS ‐ visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000677606.
| Study name | In patients with end stage renal failure on dialysis, does evening primrose oil, compared to omega‐3 fish oil and placebo improve pruritis? |
| Methods | Double blinded, placebo controlled RCT |
| Participants | Patients with ESKD undergoing dialysis (in hospital or at home) |
| Interventions |
|
| Outcomes | VAS, rule of nines and questions involving QoL |
| Starting date | Not yet recruiting |
| Contact information | Dr Jane Holt Department of Renal Medicine Wollongong Hospital Dudley Street Wollongong NSW 2500 + 61 02 4222 5443 |
| Notes |
DON'T ITCH 2015.
| Study name | A phase IV, randomised, double‐blind, controlled, parallel group trial to evaluate the effectiveness and safety of balneum plus vs emollient in the treatment of uraemic pruritus in haemodialysis patients |
| Methods | Double‐blind, controlled, parallel RCT |
| Participants | Receiving HD for the treatment of ESKD for at least 3 months; aged > 18 years |
| Interventions |
|
| Outcomes | The primary outcome measure will be reduction in itch intensity as measured by VAS |
| Starting date | 13 November 2015 |
| Contact information | Jacqueline Nevols Queen Alexandra Hospital Portsmouth PO6 3LY UK 02392286000 jacqueline.nevols@porthosp.nhs.uk |
| Notes |
IRCT201311152417N14.
| Study name | Effect of omega‐3 on pruritus scale in hemodialysis patients |
| Methods | Double‐blinded, parallel RCT |
| Participants | HD for at least 3 months; pruritus duration > 8 weeks; without any dermatologic problems; no hypersensitivity to omega‐3; no malabsorption or other gastrointestinal problems (chronic diarrhoea > 2 weeks); not using anticoagulant and antiplatelet drugs Exclusion criteria: non‐compliance; kidney transplantation; antihistamine or gabapentin using; anaemia (Hb < 7 g/dL); PTH > 300 µg/L; phosphorus >7 mg/dL; INR rising; aged > 16 years |
| Interventions | Omega‐3 fatty acid supplementation |
| Outcomes | Questionnaire (VAS) |
| Starting date | 22 November 2013 |
| Contact information | Firouzeh Moeinzadeh University of Medical Sciences Iran, Islamic Republic of +98 31 1625 5555 addressmoinzade@resident.mui.ac.ir |
| Notes |
IRCT2015051411940N3.
| Study name | The effect of aloe vera gel on pruritus severity of hemodialysis patients |
| Methods | Double‐blind, controlled, parallel RCT |
| Participants | Receiving HD for the treatment of ESKD for at least 3 months; aged > 18 years |
| Interventions | Aloe vera gel will be used 2 times in a day for 1 month |
| Outcomes | 5‐D pruritus scale |
| Starting date | 23 July 2015 |
| Contact information | Azam Malek Hoseini Arak University of Medical Sciences, Alamolhoda St, Arak Arak 3817834467 Iran +98 86 3226 7892 malekhoseni.aram@gmail.com |
| Notes |
NCT03422653.
| Study name | A multicenter, double‐blind, randomised, placebo‐controlled study to evaluate the safety and efficacy of intravenous CR845 in hemodialysis patients with moderate‐to‐severe pruritus, with a 52‐week open label extension |
| Methods | Double‐blind, parallel RCT |
| Participants | Receiving HD for the treatment of ESKD for at least 3 months; aged > 18 years |
| Interventions | IV CR845 0.5 µg/kg administered after each dialysis session (3 times/week) versus IV placebo |
| Outcomes | Reduction in itch intensity Improvement in itch‐related QoL |
| Starting date | 20 February 2018 |
| Contact information | Frédérique Menzaghi, PhD, Cara Therapeutics |
| Notes |
NCT03636269.
| Study name | A multicenter, double‐blind, randomised, placebo‐controlled study to evaluate the safety and efficacy of intravenous CR845 in hemodialysis patients with moderate‐to‐severe pruritus, with a 52‐week open label extension |
| Methods | Parallel, double blind, RCT |
| Participants | 350 |
| Interventions | CR845 0.5 µg/kg versus placebo |
| Outcomes | 24‐hour worst itching intensity (NRS) |
| Starting date | 17 July 17 2018 |
| Contact information | Georgine Ragsdale, PharmD 203‐406‐3700 clinicaltrials.gov@caratherapeutics.com |
| Notes |
SNUG 2019.
| Study name | Safety and efficacy of PG102P for the coNtrol of prUritus in patients underGoing hemodialysis (SNUG Trial): study protocol for a randomised control trial |
| Methods | Parallel, double blind, RCT |
| Participants | 80 |
| Interventions | PG102P 1.5 g/day |
| Outcomes | VAS |
| Starting date | May 1, 2018 |
| Contact information | Yong Chul Kim, MD +82‐2‐2072‐1724 imyongkim@gmail.com Seoul National University Boramae Medical Center |
| Notes |
ESKD ‐ end‐stage kidney disease; Hb ‐ haemoglobin; HD ‐ haemodialysis; INR ‐ international normalised ratio; NRS ‐ numerical rating scale; PTH ‐ parathyroid hormone; QoL ‐ quality of life; RCT ‐ randomised controlled trial; VAS ‐ visual analogue scale
Contributions of authors
Draft the protocol: DH, SJ
Study selection: DH, SJ
Extract data from studies: DH
Enter data into RevMan: DH
Carry out the analysis: DH, AW
Interpret the analysis: DH
Draft the final review: DH, AW
Disagreement resolution: AW
Update the review: DH
Declarations of interest
Daniel Hercz: none known
Simon H Jiang: none known
Angela C Webster: none known
New
References
References to studies included in this review
Afrasiabifar 2017 {published data only}
- Afrasiabifar A, Mehri Z, Hosseini N. Efficacy of topical application of sweet almond oil on reducing uremic pruritus in hemodialysis patients: A randomized clinical trial study. Iranian Red Crescent Medical Journal 2017;19(2):e34695. [EMBASE: 614585273] [Google Scholar]
Akrami 2017 {published data only}
- Akrami R, Hashempur MH, Tavakoli A, Nimrouzi M, Sayadi M, Roodaki M, et al. Effects of Fumaria parviflora L on uremic pruritus in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Jundishapur Journal of Natural Pharmaceutical Products 2017;12(3 Suppl):e39744. [EMBASE: 621978284] [Google Scholar]
Aliasgharpour 2018 {published data only}
- Aliasgharpour M, Zabolypour S, Asadinoghabi A, Haghani H, Lesanpezeshki M. The effect of increasing blood flow rate on severity of uremic pruritus in hemodialysis patients: a single clinical trial. Journal of the National Medical Association 2018;110(3):270-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amirkhanlou 2016 {published data only}
- Amirkhanlou S, Rashedi A, Taherian J, Hafezi AA, Parsaei S. Comparison of gabapentin and ketotifen in treatment of uremic pruritus in hemodialysis patients. Pakistan Journal of Medical Sciences 2016;32(1):22-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aramwit 2012a {published data only (unpublished sought but not used)}16019033
- Aramwit P, Keongamaroon O, Siritientong T, Bang N, Supasyndh O. Sericin cream reduces pruritus in hemodialysis patients: a randomized, double-blind, placebo-controlled experimental study. BMC Nephrology 2012;13:119. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashmore 2000 {published and unpublished data}
- Ashmore SD, Jones CH, Newstead CG, Daly MJ, Chrystyn H. Ondansetron therapy for uraemic pruritus in maintenance haemodialysis patients [abstract]. In: 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6-9; Rimini, Italy. 1998:223. [CENTRAL: CN-00483053]
- Ashmore SD, Jones CH, Newstead CG, Daly MJ, Chrystyn H. Ondansetron therapy for uremic pruritus in hemodialysis patients. American Journal of Kidney Diseases 2000;35(5):827-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aubia 1980 {published data only}
- Aubia J, Aguilera J, Llorach I, Garcia C, Rius E, LLoveras J, et al. Dialysis pruritus: effect of cimetidine. Journal of Dialysis 1980;4(4):141-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baumelou 1993 {published data only}
- Baumelou A, Melac M, French Cetirizine in Uraemic Pruritus Multicenter Study Group. Double blind placebo controlled study of cetirizine in the treatment of pruritus in patients receiving maintenance hemodialysis [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:381.
Begum 2004 {published data only}
- Begum R, Belury MA, Burgess JR, Peck LW. Supplementation with n-3 and n-6 polyunsaturated fatty acids: effects on lipoxygenase activity and clinical symptoms of pruritus in hemodialysis patients. Journal of Renal Nutrition 2004;14(4):233-41. [MEDLINE: ] [PubMed] [Google Scholar]
Bhaduri 2006 {published data only}
- Bhaduri S, Mathur V, Fellmann J, Rosen D, Ueno K, Ueno Y. Nalfurafine (TRK-820) as a treatment for uremic pruritus (UP): patients are responsive independent of baseline itch - data from a multicenter, randomized, placebo controlled trial [abstract no: SA-PO1014]. Journal of the American Society of Nephrology 2006;17(Abstracts):787A. [Google Scholar]
- Bhaduri S, Mathur V, Fellmann J, Rosen D, Ueno K, Ueno Y. Nalfurafine (TRK-820) as a treatment for uremic pruritus (UP): persistence of effect on dialytic and non-dialytic days - data from a multicenter, randomized, placebo controlled trial [abstract no: SA-PO1015]. Journal of the American Society of Nephrology 2006;17(Abstracts):787A. [Google Scholar]
Blachley 1985 {published data only}
- Blachley JD, Blankenship DM, Menter A, Parker TF 3rd, Knochel JP. Uremic pruritus: skin divalent ion content and response to ultraviolet phototherapy. American Journal of Kidney Diseases 1985;5(5):237-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boaz 2009 {published data only}
- Boaz M, Shtendik L, Oron M, Portugal-Cohen M, Kohen R, Biro A, et al. A randomized controlled clinical trial comparing the efficacy of dead sea mineral-enriched body lotion versus two types of placebo in the treatment of cutaneous dryness, itching, peeling and tightness in hemodialysis patients (EDIT). Nephron 2009;113(3):c169-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Breneman 1992 {published data only}
- Breneman DL, Cardone JS, Blumsack RF, Lather RM, Searle EA, Pollack VE. Topical capsaicin for treatment of hemodialysis-related pruritus. Journal of the American Academy of Dermatology 1992;26(1):91-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carmichael 1988 {published data only}
- Carmichael AJ, Dickinson F, McHugh MI, Martin AM, Farrow M. Magnesium free dialysis for uraemic pruritus. BMJ 1988;297(6663):1584-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael AJ. Investigation of a low magnesium dialysate in uraemic pruritus [abstract no: 16]. British Journal of Dermatology 1988;119(Suppl 33):50. [CENTRAL: CN-00509120] [Google Scholar]
Chan 1995 {published data only}
- Chan CM, Leung CY, Lam TY, Lo KK, Tong KL. Use of phototherapy (UVB) in the treatment uraemic pruritus, a randomized controlled trial [abstract no: OP2-3]. In: 6th Asian Pacific Congress of Nephrology; 1995 Dec 5-9; Hong Kong. 1995:28. [CENTRAL: CN-00460521]
Chen 2006e {published data only (unpublished sought but not used)}
- Chen YC, Chiu WT, Wu MS. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. American Journal of Kidney Diseases 2006;48(1):69-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2009 {published data only}
- Chen ZJ, Cao G, Tang WX, Lv XY, Huang SM, Qin W, et al. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clinical & Experimental Dermatology 2009;34(6):679-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cho 1997 {published data only}
- Cho YL, Liu HN, Huang TP, Tarng DC. Uremic pruritus: roles of parathyroid hormone and substance P. Journal of the American Academy of Dermatology 1997;36(4):538-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Marchi 1992 {published data only}
- De Marchi S, Cecchin E, Villalta D, Sepiacci G, Santini G, Bartoli E. Effect of erythropoietin therapy on uraemic pruritus [abstract]. Nephrology Dialysis Transplantation 1992;7(7):767. [CENTRAL: CN-00260739] [Google Scholar]
- De Marchi S, Cecchin E, Villalta D, Sepiacci G, Santini G, Bartoli E. Relief of pruritus and decreases in plasma histamine concentrations during erythropoietin therapy in patients with uremia. New England Journal of Medicine 1992;326(15):969-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duque 2005 {published data only (unpublished sought but not used)}
- Duque MI, Yosipovitch G, Fleischer AB Jr, Willard J, Freedman BI. Lack of efficacy of tacrolimus ointment 0.1% for treatment of hemodialysis-related pruritus: a randomized, double-blind, vehicle-controlled study. Journal of the American Academy of Dermatology 2005;52(3 Pt 1):519-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Durant‐Finn 2008 {published data only}
- Durrant-Finn U, Osten B, Nenoff P. Topical L-arginine hydrochloride ointment improves skin dryness and pruritus in patients under chronic haemodialysis - a vehicle-controlled prospective randomised study [Topisch appliziertes L-argininhydrochlorid verbessert hauttrockenheit und pruritus bei patienten unter chronischer hamodialyse - Eine vehikelkontrollierte prospektive randomisierte studie im halbseitenvergleich]. Aktuelle Dermatologie 2008;34(5):175-87. [EMBASE: 2008337532] [Google Scholar]
Fallahzadeh 2015 {published data only}
- Fallahzadeh MK, Faridi P, Sarvestani AK, Sagheb MM, Blondin J, Mohagheghzadeh A, et al. Effect of senna on reduction of uremic pruritus in hemodialysis patients: a randomized double-blind placebo-controlled trial [abstract]. American Journal of Kidney Diseases 2015;65(4):A33. [EMBASE: 71875063] [Google Scholar]
Feily 2012 {published data only}
- Feily A, Dormanesh B, Ghorbani AR, Moosavi Z, Kouchak M, Cheraghian B, et al. Efficacy of topical cromolyn sodium 4% on pruritus in uremic nephrogenic patients: a randomized double-blind study in 60 patients.[Erratum in: Int J Clin Pharmacol Ther. 2013 Nov;51(11):910]. International Journal of Clinical Pharmacology & Therapeutics 2012;50(7):510-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foroutan 2017 {published data only}
- Foroutan N, Etminan A, Nikvarz N, Shojai Shahrokh Abadi M. Comparison of pregabalin with doxepin in the management of uremic pruritus: a randomized single blind clinical trial. Hemodialysis International 2017;21(1):63-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ghanei 2012 {published data only}
- Ghanei E, Zeinali J, Borghei M, Homayouni M. Efficacy of omega-3 fatty acids supplementation in treatment of uremic pruritus in hemodialysis patients: a double-blind randomized controlled trial. Iranian Red Crescent Medical Journal 2012;14(9):515-22. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Ghorbani 2012a {published data only}
- Ghorbani AR, Feily A, Khalili A, Dormanesh B. Lack of efficacy of topical calcineurin inhibitor pimecrolimus 1% on pruritus of severely uremic patients: a randomized double-blind study in 60 patients. Dermatitis 2012;22(3):167-8. [MEDLINE: ] [PubMed] [Google Scholar]
Ghorbani Birgani 2011 {published data only}
- Ghorbani Birgani AR, Khalili A, Zamani L. A comparison between the effect of cromolyn sodium gel 4% and pimecrolimus cream 1% in treatment of pruritus of patients with end stage renal disease. Avicenna Journal of Nursing & Midwifery Care 2011;19(2):11-22. [Google Scholar]
Gilchrest 1977 {published data only}
- Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Relief of uremic pruritus with ultraviolet phototherapy. New England Journal of Medicine 1977;297(3):136-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gilchrest BA. Ultraviolet phototherapy of uremic pruritus. International Journal of Dermatology 1979;18(9):741-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gilchrest 1979 {published data only (unpublished sought but not used)}
- Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Ultraviolet phototherapy of uremic pruritus. Long-term results and possible mechanism of action. Annals of Internal Medicine 1979;91(1):17-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gilchrest BA. Ultraviolet phototherapy of uremic pruritus. International Journal of Dermatology 1979;18(9):741-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gobo‐Oliveira 2018 {published data only}
- Gobo-Oliveira M, Pigari VG, Ogata MS, Miot HA, Ponce D, Abbade LP. Gabapentin versus dexchlorpheniramine as treatment for uremic pruritus: a randomised controlled trial. European Journal of Dermatology 2018;28(4):488-95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gunal 2004 {published data only}
- Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrology Dialysis Transplantation 2004;19(12):3137-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hsu 2009 {published data only}
- Hsu MC, Chen HW, Hwu YJ, Chanc CM, Liu CF. Effects of thermal therapy on uremic pruritus and biochemical parameters in patients having haemodialysis. Journal of Advanced Nursing 2009;65(11):2397-408. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hui 2011 {published data only}
- Hui B, Min Z, Cai-lan Y, Gang L. Effect of high-flux dialysis membrane on uremic pruritus and solute clearance of maintenance hemodialysis patients. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15(29):5493-6. [DOI: 10.3969/j.issn.1673-8225.2011.29.043] [DOI] [Google Scholar]
Jiang 2016 {published data only}
- Jiang X, Ji F, Chen Z, Huang Q. Comparison of high-flux hemodialysis with hemodialysis filtration in treatment of uraemic pruritus: a randomized controlled trial. International Urology & Nephrology 2016;48(9):1533-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ko 2011 {published data only}
- Ko MJ, Yang JY, Wu HY, Hu FC, Chen SI, Tsai PJ, et al. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: a randomized controlled trial. British Journal of Dermatology 2011;165(3):633-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumagai 2010 {published data only}
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, et al. Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. American Journal of Nephrology 2012;36(2):175-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrology Dialysis Transplantation 2010;25(4):1251-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kyriazis 2000 {published data only}
- Kyriazis J, Glotsos J. Dialysate calcium concentration of</=1.25 mmol/l: is it effective in suppressing uremic pruritus? Nephron 2000;84(1):85-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Legroux‐Crespel 2004 {published data only}
- Legroux-Crespel E, Cledes J, Misery L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology 2004;208(4):326-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2017a {published data only}
- Li WH, Yin YM, Chen H, Wang XD, Yun H, Li H, et al. Curative effect of neutral macroporous resin hemoperfusion on treating hemodialysis patients with refractory uremic pruritus. Medicine 2017;96(12):e6160. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin TC, Lai YH, Guo SE, Liu CF, Tsai JC, Guo HR, et al. Baby oil therapy for uremic pruritus in haemodialysis patients. Journal of Clinical Nursing 2012;21(1-2):139-48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mahmudpour 2017 {published data only}
- Mahmudpour M, Rouzbeh J, Jalali QA, Pakfetrat M, Zadegan SE, Sagheb MM. Therapeutic effect of montelukast for treatment of uremic pruritus in hemodialysis patients. Iranian Journal of Kidney Diseases 2017;11(1):50-5. [MEDLINE: ] [PubMed] [Google Scholar]
Makhlough 2010 {published data only}
- Makhlough A, Ala S, Haj-Heydari Z, Kashi Z, Bari A. Topical capsaicin therapy for uremic pruritus in patients on hemodialysis.[Erratum in: Iran J Kidney Dis. 2010 Jul;4(3):273 Note: Ala, Shahram [added]; Haj-Heydari, Zohreh [added]; Kashi, Zahra [added]; Bari, Alireza [added]]. Iranian Journal of Kidney Diseases 2010;4(2):137-40. [MEDLINE: ] [PubMed] [Google Scholar]
Mapar 2015 {published data only}
- Mapar MA, Pazyar N, Siahpoosh A, Latifi SM, Beladi Mousavi SS, Khazanee A. Comparison of the efficacy and safety of zinc sulfate vs. placebo in the treatment of pruritus of hemodialytic patients: a pilot randomized, triple-blind study. Giornale Italiano di Dermatologia e Venereologia 2015;150(4):351-5. [MEDLINE: ] [PubMed] [Google Scholar]
Marin 2013 {published data only}
- Marin AR. Gabapentin therapy for pruritus in automated peritoneal dialysis patients: a randomized controlled trial [abstract no: SA-PO936]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):841A. [Google Scholar]
Mettang 1997 {published data only}
- Mettang T, Thomas S, Kuhlmann U. L-carnitine does not alleviate uremic pruritus in hemodialysis patients. Nephron 1997;75(3):372. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thomas S, Fischer FP, Mettang T, Pauli-Magnus C, Weber J, Kuhlmann U. Effects of L-carnitine on leukocyte function and viability in hemodialysis patients: a double-blind randomized trial. American Journal of Kidney Diseases 1999;34(4):678-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mirnezami 2013 {published data only}
- Mirnezami M. Effect of ondasetron on pruritus in hemodialysis patients. Arak Medical University Journal (AMUJ) 2013;16(3):80-4. [Google Scholar]
Mohamed 2012 {published data only (unpublished sought but not used)}
- Mohamed WA, Zaki FM, Bekhit WH, Sherif IS. Sodium thiosulfate (STS): a new option for hemodialysis patients with uremic pruritus [abstract no: SAP598]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii511-2. [EMBASE: 70766834] [Google Scholar]
Mojgan 2017 {published data only}
- Mojgan M, Masoud M, Shahrzad S, Zahra PH, Firouzeh M, Jinoos Z, et al. Pruritus-reducing effects of omega-3 fatty acids in hemodialysis patients [abstract no: P106]. Iranian Journal of Kidney Diseases 2017;11(Suppl 1):7-8. [EMBASE: 616611409] [Google Scholar]
Murphy 2003 {published data only}75728112
- Murphy M, Reaich D, Pai P, Finn P, Carmichael AJ. A randomized, placebo-controlled, double-blind trial of ondansetron in renal itch. British Journal of Dermatology 2003;148(2):314-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murphy MD, Reaich D, Pai P, Finn P, Carmichael AJ. A randomised, placebo-controlled, double-blind trial of ondansetron in renal itch [abstract]. British Journal of Dermatology 2001;145(Suppl 59):20-1. [CENTRAL: CN-00509373] [DOI] [PubMed] [Google Scholar]
Naghibi 2007 {published data only}
- Naghibi M, Nazemian F, Mohammad-Poor A, Morovat-Dar Z, Javidi-Dasht-Bayaz A, Azmoodeh H. The effect of gabapentin on uremic pruritus in hemodialysis patients [abstract no: FP479]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi181. [Google Scholar]
Naini 2007 {published data only}
- Naini AE, Harandi AA, Khanbabapour S, Shahidi S, Seirafiyan S, Mohseni M. Gabapentin: a promising drug for the treatment of uremic pruritus. Saudi Journal of Kidney Diseases & Transplantation 2007;18(3):378-81. [MEDLINE: ] [PubMed] [Google Scholar]
- Naini AE, Shahidi S, Seirafian S, Atapoor A, Khanbabapoor S, Harandi AA, et al. Gabapentin: a promising treatment for uremic pruritus [abstract no: SP471]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv174. [Google Scholar]
Najafabadi 2012 {published data only}
- Mortazavi M, Faghihi G, Naeini AE, Monghad M, Hosseini SM. Zinc sulfate for the relief of pruritus in patients on maintenance hemodialysis [abstract no: SA590]. NDT Plus 2010;3(Suppl 3):iii241. [EMBASE: 70484056] [Google Scholar]
- Najafabadi MM, Faghihi G, Emami A, Monghad M, Moeenzadeh F, Sharif N, et al. Zinc sulfate for relief of pruritus in patients on maintenance hemodialysis. Therapeutic Apheresis & Dialysis 2012;16(2):142-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakhaee 2015 {published data only}
- Nakhaee S, Nasiri A, Waghei Y, Morshedi J. Comparison of Avena sativa, vinegar, and hydroxyzine for uremic pruritus of hemodialysis patients: a crossover randomized clinical trial. Iranian Journal of Kidney Diseases 2015;9(4):316-22. [MEDLINE: ] [PubMed] [Google Scholar]
Nasrollahi 2007 {published and unpublished data}
- Nasrollahi AR, Miladipour A, Ghanei E, Yavari P, Haghverdi F. Montelukast for treatment of refractory pruritus in patients on hemodialysis. Iranian Journal of Kidney Diseases 2007;1(2):73-7. [MEDLINE: ] [PubMed] [Google Scholar]
Nofal 2016 {published data only}
- Nofal E, Farag F, Nofal A, Eldesouky F, Alkot R, Abdelkhalik Z. Gabapentin: a promising therapy for uremic pruritus in hemodialysis patients: a randomized-controlled trial and review of literature. Journal of Dermatological Treatment 2016;27(6):515-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solak B, Solak Y. Reply to: Gabapentin: a promising therapy for uremic pruritus in hemodialysis patients: a randomized-controlled trial and review of literature. Journal of Dermatological Treatment 2017;28(3):280. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noshad 2011 {published data only}
- Noshad H. Comparison of gabapentin and antihistamins in treatment of uremic pruritus and its psychological problems [abstract no: P209]. Iranian Journal of Kidney Diseases 2011;5(Suppl 2):27-8. [EMBASE: 70673855] [Google Scholar]
Omidian 2013 {published data only}
- Omidian M, Khazanee A, Yaghoobi R, Ghorbani AR, Pazyar N, Beladimousavi SS, et al. Therapeutic effect of oral nicotinamide on refractory uremic pruritus: a randomized, double-blind study. Saudi Journal of Kidney Diseases & Transplantation 2013;24(5):995-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozaykan 2001 {published data only}
- Ozaykan S, Mansur T, Gunduz S, Guney O. Comparison of ondansetron and cyproheptadine in treatment of uremic pruritus [Uremi kasintisi olan hastalarda ondansetron ve siproheptadinin etkinliginin karsilastirilmasi]. Turkderm Deri Hastaliklari Ve Frengi Arsivi 2001;35(2):130-4. [EMBASE: 2001415704] [Google Scholar]
Pakfetrat 2014 {published data only}
- Pakfetrat M, Basiri F, Malekmakan L, Roozbeh J. Effects of turmeric on uremic pruritus in end stage renal disease patients: a double-blind randomized clinical trial. Journal of Nephrology 2014;27(2):203-7. [EMBASE: 2014347658] [DOI] [PubMed] [Google Scholar]
Pakfetrat 2018 {published data only}
- Pakfetrat M, Malekmakan L, Hashemi N, Tadayon T. Sertraline can reduce the uremic pruritus in hemodialysis patient: A double blind randomized clinical trial from Southern Iran. Hemodialysis International 2018;22(1):103-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pauli‐Magnus 2000 {published data only}
- Pauli-Magnus C, Mikus G, Alscher DM, Kirschner T, Nagel W, Gugeler N, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. Journal of the American Society of Nephrology 2000;11(3):514-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peck 1996 {published data only}
- Peck LW, Monsen ER, Ahmad S. Effect of three sources of long-chain fatty acids on the plasma fatty acid profile, plasma prostaglandin E2 concentrations, and pruritus symptoms in hemodialysis patients. American Journal of Clinical Nutrition 1996;64(2):210-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pederson 1980 {published data only}
- Pederson JA, Matter BJ, Czerwinski AW, Llach F. Relief of idiopathic generalized pruritus in dialysis patients treated with activated oral charcoal. Annals of Internal Medicine 1980;93(3):446-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peer 1996 {published and unpublished data}
- Peer G, Kivity S, Agami O, Fireman E, Silverberg D, Blum M, et al. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet 1996;348(9041):1552-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Peer G, Silverberg DS, Blum M, Kaplan E, Iaina A. Naltrexone (NX) an opiate antagonist relieves pruritus in dialysis patients [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:560. [CENTRAL: CN-00509406]
Pour‐Reza‐Gholi 2007 {published data only}
- Pour-Reza-Gholi F, Nasrollahi A, Firouzan A, Nasli EE, Farrokhi F. Low-dose doxepin for treatment of pruritus in patients on hemodialysis. Iranian Journal of Kidney Diseases 2007;1(1):34-7. [MEDLINE: ] [PubMed] [Google Scholar]
- Pour Reza Gholi F, Reza Nasrollahi A, Nafar M, Firoozan A, Esfahani EN, Farrokhi F. A randomized crossover trial: low-dose doxepin reduces pruritus in dialysis patients [abstract no: MP428]. In: 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15-18; Lisbon, Portugal. 2004:374. [CENTRAL: CN-00509425]
Rad 2017 {published data only}
- Rad M, Jaghouri E, Sharifipour F, Rakhshani MH. The effects of cool dialysate on pruritus status during hemodialysis of patients with chronic renal failure: a controlled randomized clinical trial. Iranian Red Crescent Medical Journal 2017;19(1):e34759. [EMBASE: 614362274] [Google Scholar]
Rivory 1984 {published data only}
- Rivory JP, Maheut H. Favorable effect of nicergoline on pruritus in chronic hemodialysis patients. Role of a hyper-alpha-adrenergic system? [Effet favorable de la nicergoline sur le prurit des hemodialyses chroniques. Role de l'hyperalpha-adrenergie?]. Presse Medicale 1984;13(44):2703. [MEDLINE: ] [PubMed] [Google Scholar]
Shariati 2010 {published data only}
- Shariati A, Abbasi A, Mojer Lou M, Ghorbani M. Comparison of the effects of oral charcoal capsule with aluminum hydroxide syrup on pruritus in hemodialysis patients. Journal of the Guilan University of Medical Sciences 2010;18(72):22-9. [Google Scholar]
Sherjeena 2017 {published data only}
- Sherjeena PB, Binitha MP, Rajan U, Sreelatha M, Sarita S, Nirmal C, et al. A controlled trial of narrowband ultraviolet B phototherapy for the treatment of uremic pruritus. Indian Journal of Dermatology, Venereology & Leprology 2017;83(2):247-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shirazian 2013 {published data only}
- Shirazian S, Kline M, Sakhiya V, Schanler M, Moledina D, Patel C, et al. Longitudinal predictors of uremic pruritus. Journal of Renal Nutrition 2013;23(6):428-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shirazian S, Schanler M, Drakakis J, Miyawaki NB, Fishbane S. The effect of vitamin D insufficiency on uremic pruritis [abstract no: PUB388]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):982A. [Google Scholar]
- Shirazian S, Schanler M, Shastry S, Dwivedi S, Kumar M, Rice K, et al. The effect of ergocalciferol on uremic pruritus severity: a randomized controlled trial. Journal of Renal Nutrition 2013;23(4):308-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silva 1994 {published data only}
- Lugon JR, Silva SR, Viana PC, Lugon NV, Hoette M, Ruzany F. Thalidomide (TH) as a new perspective for the treatment of uremic pruritus (UP): a crossed randomized double-blind trial [abstract no: 14P]. Journal of the American Society of Nephrology 1992;3(3):377. [CENTRAL: CN-00461215] [Google Scholar]
- Silva SR, Viana PC, Lugon NV, Hoette M, Ruzany F, Lugon JR. Thalidomide for the treatment of uremic pruritus: a crossover randomized double-blind trial. Nephron 1994;67(3):270-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silverberg 1977 {published data only}
- Silverberg DS, Iaina A, Reisin E, Rotzak R, Eliahou HE. Cholestyramine in uraemic pruritus. BMJ 1977;1(6063):752-3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sja'bani 1997 {published data only}
- Sja'bani M, Asdie AH. Effect of erythropoietin on pruritus, anemia and quality of life, in chronic hemodialyzed end stage renal disease patients [abstract]. In: ISN XIII International Congress of Nephrology; 1995 Jul 2-6; Madrid, Spain. 1995:501. [CENTRAL: CN-00509480]
- Sja'bani M, Asdie AH. Effect of erythropoietin on pruritus and quality of life in chronic hemodialyzed end stage renal disease patients [abstract]. Journal of Clinical Epidemiology 1997;50(Suppl 1):10S. [CENTRAL: CN-00550491] [Google Scholar]
Solak 2012 {published data only}
- Atalay H, Solak Y, Biyik Z, Gaipov A, Guney F, Turk S. Cross-over, open-label trial of the effects of gabapentin versus pregabalin on painful peripheral neuropathy and health-related quality of life in haemodialysis patients. Clinical Drug Investigation 2013;33(6):401-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Biyik Z, Solak Y, Atalay H, Gaipov A, Guney F, Turk S. Gabapentin versus pregabalin in improving sleep quality and depression in hemodialysis patients with peripheral neuropathy: a randomized prospective crossover trial. International Urology & Nephrology 2013;45(3):831-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Solak Y, Biyik Z, Atalay H, Gaipov A, Guney F, Turk S, et al. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: a prospective, crossover study. Nephrology 2012;17(8):710-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spencer 2015 {unpublished data only}
- Mathur VS, Spencer RH, Illidge J, Stauffer JW, Munera C, Menzaghi F. Improvement of quality of life in hemodialysis patients with uremic pruritus as measured by the skindex-10 questionnaire: effect of a novel kappa opiod receptor agonist, CR845 [abstract no: TH-PO1040]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):338A. [Google Scholar]
- Spencer R, Mathur VS, Tumlin JA, Stauffer JW, Menzaghi F. CR845, a novel kappa opiod receptor agonist reduces moderate-to-severe pruritus and improves quality of life in chronic kidney disease patients undergoing hemodialysis [abstract no: SA-PO1117]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):B9. [Google Scholar]
Spencer 2017 {published data only}
- Menzaghi F, Munera C, Oberdick MS, Stauffer JW, Spencer RH. Randomized, placebo-controlled study on the efficacy of CR845 in improving the quality of life of hemodialysis patients with CKD- associated pruritus [abstract no: SA-OR032]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):80. [Google Scholar]
- Munera C, Vernon MK, Stauffer JW, Spencer RH, Menzaghi F. Psychometric validation and meaningful change threshold of the worst itching intensity numerical rating scale for use in hemodialysis patients with pruritus [abstract]. Journal of Investigative Dermatology 2018;138(5 Suppl 1):S99. [EMBASE: 622252595] [Google Scholar]
- Spencer RH, Munera C, Oberdick MS, Stauffer JW, Menzaghi F. Randomized, placebo-controlled study on the efficacy of CR845 in reducing CKD- associated pruritus in hemodialysis patients [abstract no: FR-PO875]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):629. [Google Scholar]
- Spencer RH, Munera C, Vernon MK, Stauffer JW, Menzaghi F. Clinically meaningful itch reduction by CR845 an 8-week randomized, placebo-controlled study in hemodialysis patients [abstract no: 280]. American Journal of Kidney Diseases 2018;71(4):585. [EMBASE: 621596159] [Google Scholar]
Subach 2001 {published data only}
- Subach RA, Radabaugh RS, Williams DK, Marx MA. Ondansetron versus diphenhydramine versus placebo for hemodialysis-associated itching [abstract no: A1790]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):348A. [CENTRAL: CN-00447890] [Google Scholar]
Suwanpidokkul 2007 {published data only}
- Suwanpidokkul P, Chaiprasert A, Supasyndh O, Choovichian P, Luesuthiviboon L. Effects of gabanpentin and loratadine on uremic pruritus in hemodialysis patients: a randomized controlled trial [abstract no: F-PO896]. Journal of the American Society of Nephrology 2007;18(Abstracts):300A. [Google Scholar]
Tamimi 1999 {published data only}
- Tamimi NA, Mikhail AI, Stevens PE. Role of gamma-linolenic acid in uraemic pruritus. Nephron 1999;83(2):170-1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 1990 {published data only}
- Tan CC, Wong KS, Thirumoorthy T, Lee E, Woo K. A randomized, crossover trial of sarna and eurax lotions in the treatment of haemodialysis patients with uraemic pruritus. Journal of Dermatological Treatment 1990;1(5):235-8. [EMBASE: 1991052418] [Google Scholar]
Tapia 1977 {published data only}
- Tapia L, Cheigh JS, David DS, Sullivan JF, Saal S, Reidenberg MM, et al. Pruritus in dialysis patients treated with parenteral lidocaine. New England Journal of Medicine 1977;296(5):261-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tapia L, Cheigh JS, David DS, Sullivan JF, Saal S, Reindenberg MM, et al. Treatment of pruritus in dialysis patients with parenteral lidocaine [abstract]. Kidney International 1976;10(6):527. [CENTRAL: CN-00583216] [Google Scholar]
Tarng 1996 {published data only}
- Tarng DC, Cho YL, Liu HN, Huang TP. Hemodialysis-related pruritus: a double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron 1996;72(4):617-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taylor 1983 {published data only}
- Taylor R, Taylor AE, Diffey BL, Hindson TC. A placebo-controlled trial of UV-A phototherapy for the treatment of uraemic pruritus. Nephron 1983;33(1):14-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tol 2010 {published data only (unpublished sought but not used)}
- Tol H, Atalay H, Guney I, Gokbel H, Altintepe L, Buyukbas S, et al. The effects of gabapentin therapy on pruritus, quality of life, depression and sleep quality in pruritic hemodialysis patients. Trakya Universitesi Tip Fakultesi Dergisi 2010;27(1):1-5. [EMBASE: 2010207038] [Google Scholar]
TREVITR02 2017 {published data only}
- Kumar J, Crawford P, Mathur V, Sciascia T. Nalbuphine ER tablets in hemodialysis patients with severe uremic pruritus: multicenter, randomized, double-blind, placebo-controlled trial [abstract no: 185]. American Journal of Kidney Diseases 2016;67(5):A65. [EMBASE: 72313488]
- Mathur VS, Germain MJ, Duncan R, Sciascia T. The rationale for and design of TREVITR02: a multicenter randomized, double-blind, placebo-controlled trial of nalbuphine ER for the treatment of uremic pruritus in hemodialysis patients [abstract no: PUB113]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):912A. [Google Scholar]
- Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T, TR02 Study Investigators. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. American Journal of Nephrology 2017;46(6):450-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T. A multicenter, phase2/3 randomized, double-blind, placebo-controlled tiral of nalbuphine ER tablets for the treatment of uremic pruritus: baseline population characteristics [abstract no: TH-PO956]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):316a. [Google Scholar]
- Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T. Randomized, double-blind, placebo-controlled, parallel, 3-arm study of safety and anti-pruritic efficacy of nalbuphine HCI ER tablets in hemodialysis patients with uremic pruritus [abstract no: HI-OR07]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):B2. [Google Scholar]
van Leusen 1978 {published data only}
- Leusen R, Kutsch Lojenga JC, Ruben AT. Is cholestyramine helpful in uraemic pruritus? British Medical Journal 1978;1(6117):918-9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vessal 2010 {published data only}
- Vessal G, Sagheb MM, Shilian S, Jafari P, Samani SM. Effect of oral cromolyn sodium on CKD-associated pruritus and serum tryptase level: a double-blind placebo-controlled study. Nephrology Dialysis Transplantation 2010;25(5):1541-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wikstrom 2005 {published data only}
- Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. Journal of the American Society of Nephrology 2005;16(12):3742-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoshimoto‐Furuie 1999 {published data only}
- Yoshimoto-Furuie K, Yoshimoto K, Tanaka T, Saima S, Kikuchi Y, Shay J, et al. Effects of oral supplementation with evening primrose oil for six weeks on plasma essential fatty acids and uremic skin symptoms in hemodialysis patients. Nephron 1999;81(2):151-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Young 2009 {published data only}
- Fleischer AB, Kaur M, Clark A, Yosipovitch G. A controlled comparative study of the efficacy of 1% pramoxine hydrochloride lotion for the treatment of uremic pruritus in adult hemodialysis patients [abstract no: P591]. Journal of the American Academy of Dermatology 2007;56(2):AB63. [CENTRAL: CN-00615966] [Google Scholar]
- Young TA, Patel TS, Camacho F, Clark A, Freedman BI, Kaur M, et al. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. Journal of Dermatological Treatment 2009;20(2):76-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yue 2015 {published data only}
- Yue J, Jiao S, Xiao Y, Ren W, Zhao T, Meng J. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: a prospective, randomized, double-blind study. International Journal of Urology & Nephrology 2015;47(1):161-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2016a {published data only}
- Zhang J, Yuan Y, An X, Ouyang C, Ren H, Yang G, et al. Comparison of combined blood purification techniques in treatment of dialysis patients with uraemic pruritus. International Journal of Clinical & Experimental Medicine 2016;9(5):8563-8. [EMBASE: 610545436] [Google Scholar]
References to studies excluded from this review
Bousquet 1989 {published data only}
- Bousquet J, Rivory JP, Maheut M, Michel FB, Mion C. Double-blind, placebo-controlled study of nicergoline in the treatment of pruritus in patients receiving maintenance hemodialysis. Journal of Allergy & Clinical Immunology 1989;83(4):825-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burrai 2014 {published data only}
- Burrai F, Micheluzzi V, Zito MP, Pietro G, Sisti D. Effects of live saxophone music on physiological parameters, pain, mood and itching levels in patients undergoing haemodialysis. Journal of Renal Care 2014;40(4):249-56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cavalcanti 2003 {published data only}
- Cavalcanti AM, Rocha LM, Carillo R, Lima LU, Lugon JR. Effects of homeopathic treatment on pruritus of haemodialysis patients: a randomised placebo-controlled double-blind trial. Homeopathy: the Journal of the Faculty of Homeopathy 2003;92(4):177-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Che‐Yi 2005 {published data only}
- Che-Yi C, Wen CY, Min-Tsung K, Chiu-Ching H. Acupuncture in haemodialysis patients at the Quchi (LI11) acupoint for refractory uraemic pruritus. Nephrology Dialysis Transplantation 2005;20(9):1912-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CTRI/2016/04/006870 {published data only}
- Ruby A. Effectiveness of self care management support intervention on medication adherence, pruritus severity, sleep quality and quality of life in patients with chronic kidney disease associated pruritus. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=13708&EncHid=&modid=&compid=%27,%2713708det%27 (first received 22 April 2016).
CYCLE‐HD 2016 {published data only}11299707
- Careless A, March D, Churchward D, Grantham C, Highton P, Tomlinson C, et al. Intradialytic exercise: a non-pharmacological solution to a uraemic problem? [abstract no: MP465]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii599-600. [EMBASE: 617290883] [Google Scholar]
- Graham-Brown MP, March DS, Churchward DR, Young HM, Dungey M, Lloyd S, et al. Design and methods of CYCLE-HD: improving cardiovascular health in patients with end stage renal disease using a structured programme of exercise: a randomised control trial. BMC Nephrology 2016;17(1):69. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- March DS, Grantham CE, Graham-Brown MP, Young HM, Cooper N, Burton J. A six month program of intradialytic exercise is effective in reducing length of hospital stay in hemodialysis patients [abstract no: SA-PO787]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):882. [Google Scholar]
- Tomlinson C, Churchward D, Grantham C, Young H, Highton P, Graham-Brown M, et al. A six month programme of intradialytic exercise improves resting heart rate in haemodialysis patients [abstract no: MP612]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii658-9. [EMBASE: 617291352] [Google Scholar]
Gao 2002 {published data only}
- Gao H, Zhang W, Wang Y. Acupuncture treatment for 34 cases of uremic cutaneous pruritus. Journal of Traditional Chinese Medicine 2002;22(1):29-30. [MEDLINE: ] [PubMed] [Google Scholar]
Ghura 1998 {published data only}
- Ghura HS. Naltrexone in the treatment of renal itch [abstract]. British Journal of Dermatology 1998;139(Suppl 51):64. [CENTRAL: CN-00550970] [Google Scholar]
IRCT201303093560N2 {published data only}
- Shahgholian N. The effect of massage with and without aromatic oils on pruritus relief In hemodialysis patient. en.search.irct ir/view/3653 (first received 8 May 2013).
IRCT2015091010076N6 {published data only}
- Saeedi M. The effect of progressive muscle relaxation on pruritus severity of hemodialysis patients. en.search.irct.ir/view/10559 (first received 13 August 2016).
Jedras 2003 {published data only}
- Jedras M, Bataa O, Gellert R, Ostrowski G, Wojtaszek E, Lange J, et al. Acupressure in the treatment of uremic pruritus. Dialysis & Transplantation 2003;32(1):8-10. [EMBASE: 2003030067] [Google Scholar]
Joffe 1985 {published data only}
- Joffe P, Andersen LW, Molwig J, Kyst A, Johannessen A. Intravenous lidocaine in the treatment of pruritus in hemodialysis patients. Clinical Nephrology 1985;24(4):214. [MEDLINE: ] [PubMed] [Google Scholar]
Kilic Akca 2016 {published data only}
- Kilic Akca N, Tasci S. Acupressure and transcutaneous electrical acupoint stimulation for improving uremic pruritus: a randomized, controlled trial. Alternative Therapies in Health & Medicine 2016;22(3):18-24. [MEDLINE: ] [PubMed] [Google Scholar]
Legat 2017 {published data only}
- Legat FJ, Hofer A, Gruber-Wackernagel A, Quehenberger F, Waltner K, Wolf P. Both narrowband-UVB and broadband UVB are equally effective in reducing itch in chronic pruritus patients [abstract]. Acta Dermato-Venereologica 2017;97(8):1056-7. [EMBASE: 620192493] [Google Scholar]
Little 1995 {published data only}
- Little PJ, Assban S, Addous A, Sidahmed A, Iman M. Loratidine to relieve pruritus in dialysis patients [abstract no: PP2-35]. In: 6th Asian Pacific Congress of Nephrology; 1995 Dec 5-9; Hong Kong. 1995:71. [CENTRAL: CN-00461191]
Lücker 1986 {published data only}
- Lücker PW, Kiehn R. Treatment of pruritus in renal insufficiency. Die Medizinische Welt 1986;37:1590-2. [CENTRAL: CN-00237953] [Google Scholar]
Marquez 2012 {published data only}
- Marquez D, Orias M, Peixoto A, Novoa P, Ramonda C, Vukelic V, et al. Hemodialysis pruritus: efficacy of treatment with desloratadine vs gabapentin [abstract no: SU583]. In: World Congress of Nephrology; 2009 May 22-26; Milan, Italy. 2009.
- Marquez D, Ramonda C, Lauxmann JE, Romero CA, Vukelic VL, Martinatto C, et al. Uremic pruritus in hemodialysis patients: treatment with desloratidine versus gabapentin. Jornal Brasileiro de Nefrologia 2012;34(2):148-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00577967 {published data only}
- Siu YP. Gabapentin - a solution to uremic pruritus? www.clinicaltrials.gov/show/NCT00577967 (first received 19 December 2007).
NCT00793156 {published data only}
- McGuire D. A randomized-withdrawal phase 3 study evaluating the safety and efficacy of oral nalfurafine HCl (AC-820) in subjects on hemodialysis with uremic pruritus (renal itch) (AC120-8231). www.clinicaltrials.gov/show/NCT00793156 (first received 19 November 2008).
NCT01073501 {published data only}
- Shavit L. Efficacy of pregabalin in the management of chronic uremic pruritus. www.clinicaltrials.gov/show/NCT01073501 (first received 23 February 2010).
NCT01620580 {published data only}
- Danquah FV. Symptom management program for hemodialysis patients. www.clinicaltrials.gov/ct2/show/NCT01620580 (first received 24 October 2011).
NCT01660243 {published data only}
- NCT01660243. Efficacy and safety of MT-9938 for treatment of uremic pruritus in subjects with end-stage renal disease receiving hemodialysis. www.clinicaltrials.gov/ct2/show/NCT01660243 (first received 8 August 2012).
NCT01852318 {published data only}
- Chiu HY. Pregabalin for the treatment of uremic pruritus. www.clinicaltrials.gov/ct2/show/NCT01852318 (first received 13 May 2013).
NCT02032537 {published data only}
- Shavit L. Efficacy of calmmax cream in the management of chronic uremic pruritus. www.clinicaltrials.gov/show/NCT02032537 (first received 6 January 2014).
NCT02432508 {published data only}
- NCT02432508, Chang CT. Efficacy of laser acupuncture on pruritus in patients with chronic kidney disease undergoing hemodialysis. www.clinicaltrials.gov/ct2/show/NCT02432508 (first received 4 May 2015).
Och 2000 {published data only}
- Och B, Jedras M, Gellert R. Is acupressure effective in the treatment of uremic pruritus? [abstract]. In: 37th Congress. European Renal Association. European Dialysis and Transplantation Association; 2000 Sep 17-20; Nice, France. 2000:157. [CENTRAL: CN-00461428]
Rehman 2018 {published data only}
- Rehman IU, Bin-Chia Wu D, Ahmed R, Khan NA, Rahman AU, Munib S, et al. A randomized controlled trial for effectiveness of zolpidem versus acupressure on sleep in hemodialysis patients having chronic kidney disease-associated pruritus. [Erratum in: Medicine (Baltimore). 2018 Sep;97(37):e12527; PMID: 30213024]. Medicine 2018;97(31):e10764. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ro 2002 {published data only}
- Ro YJ, Ha HC, Kim CG, Yeom HA. The effects of aromatherapy on pruritus in patients undergoing hemodialysis. Dermatology Nursing 2002;14(4):231-56. [MEDLINE: ] [PubMed] [Google Scholar]
Rui 2002 {published data only}
- Rui H, Lin W, Sha J. Observation on therapeutic effect of 80 cases of uremic cutaneous pruritus treated with acupuncture. Zhongguo Zhenjiu [Chinese Acupuncture & Moxibustion] 2002;22(4):235-6. [CENTRAL: CN-01912287] [Google Scholar]
Sanchez 1986 {published data only}
- Sanchez HG. Comparison of UVA and PUVA in pruritis due to renal failure. Actas Dermo Sifiliograficas 1986;77(10):621-4. [CENTRAL: CN-00550484] [Google Scholar]
Wang 2014e {published data only}
- Wang TJ, Lan LC, Lu CS, Lin KC, Tung HH, Wu SF, et al. Efficacy of narrowband ultraviolet phototherapy on renal pruritus. Journal of Clinical Nursing 2014;23(11-12):1593-602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weisshaar 2003 {published data only}
- Weisshaar E, Dunker N, Domrose U, Neumann KH, Gollnick H. Plasma serotonin and histamine levels in hemodialysis-related pruritus are not significantly influenced by 5-HT3 receptor blocker and antihistaminic therapy. Clinical Nephrology 2003;59(2):124-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Dunker N, Gollnick H. Topical capsaicin therapy in humans with hemodialysis-related pruritus. Neuroscience Letters 2003;345(3):192-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Dunker N, Rohl FW, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Experimental Dermatology 2004;13(5):298-304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yan 2015 {published data only}
- Yan CN, Yao WG, Bao YJ, Shi XJ, Yu H, Yin PH, et al. Effect of auricular acupressure on uremic pruritus in patients receiving hemodialysis treatment: a randomized controlled trial. Evidence-based Complementary & Alternative Medicine 2015;2015:593196. [EMBASE: 2015445985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshida 2017 {published data only}
- Yoshida Y, Hashimoto K, Saeki H, Fujimoto S, Tsuruoka S. The moisturizer improves pruritus of dialysis patients by increasing water content in stratum corneum [abstract no: FR-PO876]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):630. [Google Scholar]
Zadeh 2015 {published data only}
- Zadeh MH, Moradi H. Assessment of the impact of massaging with aromatic oil on relieving itchy skin in the patients undergoing dialysis [abstract]. Avicenna Journal of Phytomedicine 2015;5(Suppl 1):101-2. [EMBASE: 72156813] [Google Scholar]
Zhang 2011d {published data only}
- Zhang F, Qiu ZL, Huang HX, Fang XX, Shwm Y. The efficacy of acupuncture plus hemodiafiltration treatment of uremic with cutaneous pruritus. Journal of Practical Medicine 2011;27(9):1687-9. [Google Scholar]
References to studies awaiting assessment
Bai 2002 {published data only}
- Bai YP, Jia HZ, Zhang LX. Analysis of clinical effect of lifu paste in treating patients of long-term dialysis complicated with cutaneous pruritis. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional & Western Medicine] 2002;22(4):301-2. [MEDLINE: ] [PubMed] [Google Scholar]
NCT01513161 {published data only}
- Kim SG. Efficacy and safety study of TRK-820 to treat conventional-treatment-resistant pruritus in patients receiving hemodialysis (TRK-820). www.clinicaltrials.gov/show/NCT01513161 (first received 20 January 2012).
NCT02696499 {published data only}
- NCT02696499. Treatment of uremic pruritus with PA101B. www.clinicaltrials.gov/show/NCT02696499 (first received 2 March 2016).
NCT02747979 {published data only}
- Yu XQ. The effect and safety of hemodialysis and hemoperfusion on severe renal osteopathy and itching in uremia patients. www.clinicaltrials.gov/show/NCT02747979 (first received 22 April 2016).
References to ongoing studies
ACTRN12614000677606 {published data only}
- Holt J, Meyer B. Does evening primrose oil Improve pruritis (itching) in a dialysis population? www.anzctr org au/Trial/Registration/TrialReview.aspx?id=366382 (first received 26 June 2014).
DON'T ITCH 2015 {published data only}13971661
- Nevols J. Balneum Plus cream for the treatment of itchy skin in renal patients. www.isrctn.com/ISRCTN13971661 (first received 15 January 2015).
IRCT201311152417N14 {published data only}
- Mortazavi M. Effect of omega-3 on pruritus scale in hemodialysis patients. www.en.irct.ir/trial/2125 (first received 3 October 2016).
IRCT2015051411940N3 {published data only}
- Hoseini AM. The effect of aloe vera gel on pruritus severity of Hemodialysis patients. www.en.irct.ir/trial/12117 (first received 4 May 2016).
NCT03422653 {published data only}
- Menzaghi F. A study to evaluate the safety and efficacy of CR845 in hemodialysis patients with moderate-to-severe pruritus (KALM-1). www.clinicaltrials gov/show/nct03422653 (first received 16 February 2018).
NCT03636269 {published data only}
- NCT03636269. CR845-CLIN3103: a global study to evaluate the safety and efficacy of CR845 in hemodialysis patients with moderate-to-severe pruritus (KALM-2). www.clinicaltrials.gov/show/nct03636269 (first received 17 August 2018).
SNUG 2019 {published data only}
- Kim YC, Park JY, Oh S, Cho JH, Chang JH, Choi DE, et al. Safety and efficacy of PG102P for the control of pruritus in patients undergoing hemodialysis (SNUG trial): study protocol for a randomized controlled trial. Trials [Electronic Resource] 2019;20(1):651. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aggarwal 2007
- Aggarwal BB Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Advances in Experimental Medicine & Biology 2007;595:1-75. [MEDLINE: 17569205] [DOI] [PubMed] [Google Scholar]
Andersen 1984
- Andersen LW, Friedberg M, Lokkegaard N. Naloxone in the treatment of uremic pruritus: a case history. Clinical Nephrology 1984;21(6):355-6. [MEDLINE: ] [PubMed]
Andreassi 1997
- Andreassi M, Forleo P, Di Lorio A, Masci S, Abate G, Amerio P. Efficacy of gamma-linolenic acid in the treatment of patients with atopic dermatitis. Journal of International Medical Research 1997;25(5):266-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baliga 2006
- Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochemical & Photobiological Sciences 2006;5(2):243-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bohlius 2009
- Bohlius J Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer - meta-analysis based on individual patient data. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007303. [DOI: 10.1002/14651858.CD007303.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohmer 1978
- Bohmer T, Bergrem H, Eiklid K. Carnitine deficiency induced during intermittent haemodialysis for renal failure. Lancet 1978;311(8056):126-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burks 1985
- Burks TF, Buck SH, Miller MS. Mechanisms of depletion of substance P by capsaicin. Federation Proceedings 1985;44(9):2531-4. [MEDLINE: ] [PubMed] [Google Scholar]
Chiu 2008
- Chiu YL CH, Chuang YF, Hsu SP, Lai CF, Pai MF, Yang SY, et al. Association of uraemic pruritus with inflammation and hepatitis infection in haemodialysis patients. Nephrology Dialysis Transplantation 2008;23(11):3685-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dimkovic 1992
- Dimković N, Djukanović L, Radmilović A, Bojić P, Juloski T. Uremic pruritus and skin mast cells. Nephron 1992;61(1):5-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duo 1987
- Duo LJ. Electrical needle therapy of uremic pruritus. Nephron 1987;47(3):179-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garssen 1999
- Garssen J, Vandebriel RJ, De Gruijl FR, Wolvers DA, Van Dijk M, Fluitman A, et al. UVB exposure-induced systemic modulation of Th1- and Th2-mediated immune responses. Immunology 1999;97(3):506-14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giovannetti 1995
- Giovannetti S, Barsotti G, Cupisti A, Dani L, Bandini S, Angelini D, et al. Oral activated charcoal in patients with uremic pruritus. Nephron 1995;70(2):193-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gooding 2010
- Gooding SM, Canter PH, Coelho HF, Boddy K, Ernst E. Systematic review of topical capsaicin in the treatment of pruritus. International Journal of Dermatology 2010;49(8):858-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grove 2004
- Grove G, Zerweck C. An evaluation of the moisturizing and anti-itch effects of a lactic acid and pramoxine hydrochloride cream. Cutis 2004;73(2):135-9. [MEDLINE: ] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hampers 1968
- Hampers CL, Katz AI, Wilson RE, Merrill JP. Disappearance of "uremic" itching after subtotal parathyroidectomy. New England Journal of Medicine 1968;279(13):695-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hiroshige 1995
- Hiroshige K, Kabashima N, Takasugi M, Kuroiwa A. Optimal dialysis improves uremic pruritus. American Journal of Kidney Diseases 1995;25(3):413-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaku 1990
- Kaku H, Fujita Y, Yago H, Naka F, Kawakubo H, Nakano K, et al. Study on pruritus in hemodialysis patients and the antipruritic effect of neurotropin: plasma levels of substance P, somatostatin, IgE, PTH and histamine. Nihon Jinzo Gakkai Shi [Japanese Journal of Nephrology] 1990;32(3):319-26. [MEDLINE: ] [PubMed] [Google Scholar]
Kato 2001
- Kato A, Takita T, Furuhashi M, Takahashi T, Watanabe T, Maruyama Y, et al. Polymethylmethacrylate efficacy in reduction of renal itching in hemodialysis patients: crossover study and role of tumor necrosis factor-alpha. Artificial Organs 2001;25(6):441-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kennet 2007
- Kennet J, Hardaker N, Hobbs S, Selfe J. Cooling efficiency of 4 common cryotherapeutic agents. Journal of Athletic Training 2007;42(3):343-8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Lau 2016
- Lau T, Leung S, Lau W. Gabapentin for uremic pruritus in hemodialysis patients: a qualitative systematic review. Canadian Journal of Kidney Health & Disease 2016;3:14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 2011;147(2):447-58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marone 1986
- Marone G, Columbo M, Paulis A, Cirillo R, Giugliano R, Condorelli M. Physiological concentrations of zinc inhibit the release of histamine from human basophils and lung mast cells. Agents & Actions 1986;18(1-2):130-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Massry 1968
- Massry SG, Popovtzer MM, Coburn JW, Makoff DL, Maxwell MH, Kleeman CR. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. New England Journal of Medicine 1968;279(13):697-700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsuda 2016
- Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. American Academy of Dermatology 2016;75(3):619-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsumoto 1985
- Matsumoto M, Ichimaru K, Horie A. Pruritus and mast cell proliferation of the skin in end stage renal failure. Clinical Nephrology 1985;23(6):285-8. [MEDLINE: ] [PubMed] [Google Scholar]
Mettang 1990
- Mettang T, Fritz P, Weber J, Machleidt C, Hubel E, Kuhlmann U. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The role of plasma histamine and skin mast cells. Clinical Nephrology 1990;34(3):136-41. [MEDLINE: ] [PubMed] [Google Scholar]
Mettang 2002
- Mettang T, Pauli-Magnus C, Alscher DM. Uraemic pruritus--new perspectives and insights from recent trials. Nephrology Dialysis Transplantation 2002;17(9):1558-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mistik 2006
- Mistik S, Utas S, Ferahbas A, Tokgoz B, Unsal G, Sahan H, et al. An epidemiology study of patients with uremic pruritus. Journal of the European Academy of Dermatology & Venereology 2006;20(6):672-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Namazi 2003
- Namazi MR. Nicotinamide: a potential addition to the anti-psoriatic weaponry. FASEB Journal 2003;17(11):1377-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Narita 2006
- Narita I, Alchi B, Omori K, Sato F, Ajiro J, Saga D, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney International 2006;69(9):1626-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patel 2007
- Patel TS, Freedman BI, Yosipovitch G. An update on pruritus associated with CKD. American Journal of Kidney Diseases 2007;50(1):11-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phan 2010
- Phan NQ, Bernhard JD, Luger TA, Ständer S. Antipruritic treatment with systemic μ-opioid receptor antagonists: a review. Journal of the American Academy of Dermatology 2010;63(4):680-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pisoni 2006
- Pisoni RL, Wikström B, Elder SJ, Akizawa T, Asano Y, Keen ML, et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 2006;21(12):3495-505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pongcharoen 2015
- Pongcharoen P, Fleischer AB Jr. An evidence-based review of systemic treatments for itch. European Journal of Pain 2016;20(1):24-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rayner 2012
- Rayner H, Baharani J, Smith S, Suresh V, Dasgupta I. Uraemic pruritus: Relief of itching by gabapentin and pregabalin. Nephron Clinical Practice 2012;122:75-79. [DOI: 10.1159/000349943] [DOI] [PubMed] [Google Scholar]
Rayner 2013
- Rayner HC. Itching in renal failure: a curse with a cure. Journal of Renal Nursing 2013;5(4):75-9. [DOI: 10.12968/jorn.2013.5.4.178] [DOI] [Google Scholar]
Rayner 2017
- Rayner HC, Larkina M, Wang M, Graham-Brown M, Veer SN, Ecder T, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(12):2000-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reich 2012
- Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Dermato-Venereologica 2012;92(5):497-501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Siemens 2016
- Siemens W, Xander C, Meerpohl JJ, Buroh S, Antes G, Schwarzer G, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD008320. [DOI: 10.1002/14651858.CD008320.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simonsen 2017
- Simonsen E, Komenda P, Lerner B, Askin N, Bohm C, Shaw J, et al. Treatment of uremic pruritus: a systematic review. American Journal of Kidney Diseases 2017;70(5):638-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stockenhuber 1987
- Stockenhuber F, Sunder-Plassmann G, Balcke P. Increased plasma histamine levels in chronic renal failure. New England Journal of Medicine 1987;317(6):386. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 1991
- Tan JK, Haberman HF, Coldman AJ. Identifying effective treatments for uremic pruritus. Journal of the American Academy of Dermatology 1991;25(5 Pt 1):811-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Villamil 2005
- Villamil AG, Bandi JC, Galdame OA, Gerona S, Gadano, AC. Efficacy of lidocaine in the treatment of pruritus in patients with chronic cholestatic liver diseases. American Journal of Medicine 2005;118(10):1160-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang WY, Tang YY, Chu PL, Chao CM, Wang KY. Physiology, pathology and clinical practice in uremic pruritus. Kidney & Dialysis 2006;18:99-104. [Google Scholar]
Wikstrom 2005a
- Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. Journal of the American Society of Nephrology 2005;16(12):3742-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zucker 2003
- Zucker I, Yosipovitch G, David M, Gafter U, Boner G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. Journal of the American Academy of Dermatology 2003;49(5):842-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hercz 2014
- Hercz D, Jiang SH, Kapoor T, Webster AC. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD011393. [DOI: 10.1002/14651858.CD011393] [DOI] [PMC free article] [PubMed] [Google Scholar]


