Abstract
Background
Dementia is a progressive syndrome characterised by deterioration in memory, thinking and behaviour, and by impaired ability to perform daily activities. Two classes of drug ‐ cholinesterase inhibitors (donepezil, galantamine and rivastigmine) and memantine ‐ are widely licensed for dementia due to Alzheimer's disease, and rivastigmine is also licensed for Parkinson's disease dementia. These drugs are prescribed to alleviate symptoms and delay disease progression in these and sometimes in other forms of dementia. There are uncertainties about the benefits and adverse effects of these drugs in the long term and in severe dementia, about effects of withdrawal, and about the most appropriate time to discontinue treatment.
Objectives
To evaluate the effects of withdrawal or continuation of cholinesterase inhibitors or memantine, or both, in people with dementia on: cognitive, neuropsychiatric and functional outcomes, rates of institutionalisation, adverse events, dropout from trials, mortality, quality of life and carer‐related outcomes.
Search methods
We searched the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register up to 17 October 2020 using terms appropriate for the retrieval of studies of cholinesterase inhibitors or memantine. The Specialised Register contains records of clinical trials identified from monthly searches of a number of major healthcare databases, numerous trial registries and grey literature sources.
Selection criteria
We included all randomised, controlled clinical trials (RCTs) which compared withdrawal of cholinesterase inhibitors or memantine, or both, with continuation of the same drug or drugs.
Data collection and analysis
Two review authors independently assessed citations and full‐text articles for inclusion, extracted data from included trials and assessed risk of bias using the Cochrane risk of bias tool. Where trials were sufficiently similar, we pooled data for outcomes in the short term (up to 2 months after randomisation), medium term (3‐11 months) and long term (12 months or more). We assessed the overall certainty of the evidence for each outcome using GRADE methods.
Main results
We included six trials investigating cholinesterase inhibitor withdrawal, and one trial investigating withdrawal of either donepezil or memantine. No trials assessed withdrawal of memantine only. Drugs were withdrawn abruptly in five trials and stepwise in two trials. All participants had dementia due to Alzheimer's disease, with severities ranging from mild to very severe, and were taking cholinesterase inhibitors without known adverse effects at baseline. The included trials randomised 759 participants to treatment groups relevant to this review. Study duration ranged from 6 weeks to 12 months. There were too few included studies to allow planned subgroup analyses. We considered some studies to be at unclear or high risk of selection, performance, detection, attrition or reporting bias.
Compared to continuing cholinesterase inhibitors, discontinuing treatment may be associated with worse cognitive function in the short term (standardised mean difference (SMD) ‐0.42, 95% confidence interval (CI) ‐0.64 to ‐0.21; 4 studies; low certainty), but the effect in the medium term is very uncertain (SMD ‐0.40, 95% CI ‐0.87 to 0.07; 3 studies; very low certainty). In a sensitivity analysis omitting data from a study which only included participants who had shown a relatively poor prior response to donepezil, inconsistency was reduced and we found that cognitive function may be worse in the discontinuation group in the medium term (SMD ‐0.62; 95% CI ‐0.94 to ‐0.31). Data from one longer‐term study suggest that discontinuing a cholinesterase inhibitor is probably associated with worse cognitive function at 12 months (mean difference (MD) ‐2.09 Standardised Mini‐Mental State Examination (SMMSE) points, 95% CI ‐3.43 to ‐0.75; moderate certainty).
Discontinuation may make little or no difference to functional status in the short term (SMD ‐0.25, 95% CI ‐0.54 to 0.04; 2 studies; low certainty), and its effect in the medium term is uncertain (SMD ‐0.38, 95% CI ‐0.74 to ‐0.01; 2 studies; very low certainty). After 12 months, discontinuing a cholinesterase inhibitor probably results in greater functional impairment than continuing treatment (MD ‐3.38 Bristol Activities of Daily Living Scale (BADLS) points, 95% CI ‐6.67 to ‐0.10; one study; moderate certainty). Discontinuation may be associated with a worsening of neuropsychiatric symptoms over the short term and medium term, although we cannot exclude a minimal effect (SMD ‐ 0.48, 95% CI ‐0.82 to ‐0.13; 2 studies; low certainty; and SMD ‐0.27, 95% CI ‐0.47 to ‐0.08; 3 studies; low certainty, respectively). Data from one study suggest that discontinuing a cholinesterase inhibitor may result in little to no change in neuropsychiatric status at 12 months (MD ‐0.87 Neuropsychiatric Inventory (NPI) points; 95% CI ‐8.42 to 6.68; moderate certainty).
We found no clear evidence of an effect of discontinuation on dropout due to lack of medication efficacy or deterioration in overall medical condition (odds ratio (OR) 1.53, 95% CI 0.84 to 2.76; 4 studies; low certainty), on number of adverse events (OR 0.85, 95% CI 0.57 to 1.27; 4 studies; low certainty) or serious adverse events (OR 0.80, 95% CI 0.46 to 1.39; 4 studies; low certainty), and on mortality (OR 0.75, 95% CI 0.36 to 1.55; 5 studies; low certainty). Institutionalisation was reported in one trial, but it was not possible to extract data for the groups relevant to this review.
Authors' conclusions
This review suggests that discontinuing cholinesterase inhibitors may result in worse cognitive, neuropsychiatric and functional status than continuing treatment, although this is supported by limited evidence, almost all of low or very low certainty. As all participants had dementia due to Alzheimer's disease, our findings are not transferable to other dementia types. We were unable to determine whether the effects of discontinuing cholinesterase inhibitors differed with baseline dementia severity. There is currently no evidence to guide decisions about discontinuing memantine. There is a need for further well‐designed RCTs, across a range of dementia severities and settings. We are aware of two ongoing registered trials. In making decisions about discontinuing these drugs, clinicians should exercise caution, considering the evidence from existing trials along with other factors important to patients and their carers.
Plain language summary
Stopping or continuing anti‐dementia drugs in patients with dementia
Background
Dementia is the term used to describe a group of illnesses, usually developing in late life, in which there is a deterioration in a person’s ability to think, remember, communicate and manage daily activities independently. It can be caused by several different brain diseases, but the most common form is dementia due to Alzheimer’s disease. At the moment, there are no medical treatments which can prevent dementia or stop it from progressing, but there are two classes of drugs – the cholinesterase inhibitors (donepezil, rivastigmine and galantamine) and memantine ‐ which are approved and widely prescribed to treat some of the symptoms. They are used mainly for dementia due to Alzheimer's disease but also sometimes for other types of dementia. Most of the trials studying the effects of these drugs have been quite short (typically six months) even though dementia usually lasts for years. The drugs can have unwanted side effects in some people. There is uncertainty about their long‐term effects and about how useful they are for severe dementia, with different countries making different recommendations. Therefore it can be difficult for doctors and patients to decide if and when these drugs should be stopped once they have been started.
What was the aim of this review?
In this review, we aimed to summarise the best evidence about whether stopping cholinesterase inhibitors or memantine was beneficial or harmful to people with dementia who had been taking them for at least two months.
What we did
We searched up to October 2020 for trials which had: recruited people with dementia who were taking a cholinesterase inhibitor or memantine, or both; divided them randomly into a group of patients who continued treatment and a group of patients who stopped treatment; and compared what happened in the two groups.
What we found
We found seven trials (759 participants) to include in the review. All of the participants had dementia due to Alzheimer’s disease, but in some trials, the disease was mild to moderate and in others, it was moderate to severe or very severe. Six trials investigated the effects of stopping a cholinesterase inhibitor and one trial investigated stopping either a cholinesterase inhibitor (specifically, donepezil) or memantine. We decided not to pool its results with the other six trials. Effects were measured over different periods of time in different trials. We looked separately at effects in the first 2 months (short term), between 3 and 11 months (medium term), and after a year or more (long term).
When we looked at the effect on thinking skills and memory, we found that, compared to stopping treatment, continuing treatment with a cholinesterase inhibitor may be beneficial in the short term and medium term and is probably beneficial in the long term. For ability to carry out daily activities, there may be little or no effect in the short term, and the effect in the medium term was very uncertain, but there is probably a benefit to continuing treatment over the longer term. For mood and behavioural problems, continuing treatment may have benefits in the short term and medium term, but not in the long term. We found no clear evidence about the effects of stopping these drugs on patients’ physical health or risk of dying. There was very little evidence about effects on quality of life or on the likelihood of moving to a care home to live. There was not enough evidence for us to see whether results differed with the severity of dementia.
Our certainty in the results varied from moderate to very low, mainly because of small numbers of trials and participants, some problems with the way the trials were conducted, and imprecise statistical results.
Our conclusions
Although there was uncertainty about the results, most of the evidence pointed to benefits of continuing treatment with cholinesterase inhibitors. There was no evidence about types of dementia other than Alzheimer’s disease, and we were unable to draw specific conclusions about continuing or stopping treatment at different stages of the illness. We found no trials that just investigated stopping memantine.
These results may help patients and their doctors to make decisions about whether or not to continue treatment, although other factors, such as side effects in an individual patient and the patient’s preferences, are also important.
Summary of findings
Summary of findings 1. Discontinued cholinesterase inhibitor compared to continued cholinesterase inhibitor in patients with dementia (short term, up to 2 months).
| Discontinued cholinesterase inhibitor compared to continued cholinesterase inhibitor for patients with dementia (short term, up to 2 months) | ||||||
|
Patient or population: patients with dementia Settings: all healthcare settings Intervention: withdrawal of Cholinesterase Inhibitor Comparison: continuation of Cholinesterase Inhibitor | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Continued cholinesterase inhibitor | Discontinued cholinesterase inhibitor | |||||
|
Cognitive function (change from baseline, short term) Standardised mean difference (ADAS‐Cog/11, SMMSE, MMSE) |
‐ | SMD 0.42 lower (0.64 lower to 0.21 lower; P < 0.001). Lower SMD means a greater decline in cognitive function from baseline |
‐ | 344 (4) | ⊕⊕⊝⊝ lowa,b | Discontinuing a ChEI may reduce cognitive function compared to continuing treatment |
|
Functional status (change from baseline, short term) Standardised mean difference (BADLS, ADCS‐ADL‐sev) |
‐ | SMD 0.25 lower (0.54 to 0.04 higher; P = 0.09). Lower SMD means a greater decline in function from baseline |
‐ | 183 (2) |
⊕⊕⊝⊝ lowc,d |
Discontinuing a ChEI may result in increased functional impairment compared to continuing ChEI treatment. However, the 95% confidence interval indicates that discontinuation might make little or no difference to functional status. |
|
Neuropsychiatric status (change from baseline, short term) Standardised mean difference (NPI, NPI‐NH) |
‐ | SMD 0.48 lower (0.82 lower to 0.13 lower; P = 0.007). Lower SMD means a greater deterioration in neuropsychiatric status from baseline |
‐ | 136 (2) | ⊕⊕⊝⊝ lowe,f | Discontinuation may result in increased neuropsychiatric symptoms compared to continuing ChEI treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog/11: Alzheimer's Disease Assessment Scale‐Cognitive subscale/11; ADCS‐ADL‐sev: Alzheimer's Disease Co‐operative Study ‐ Activities of Daily Living Inventory, modified for severe dementia; BADLS: Bristol Activities of Daily Living scale; ChEI: Cholinesterase inhibitor; CI: Confidence interval; MMSE: Mini‐Mental State Examination; NPI: Neuropsychiatric Inventory; NPI‐NH: Neuropsychiatric Inventory ‐ Nursing Home version; OR: Odds Ratio; SMD: Standardised Mean Difference; SMMSE: Standardised Mini‐Mental State Examination | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aSerious risk of bias: one study had unclear risk of selection bias associated with random sequence generation, detection bias in blinding of outcome assessment, reporting bias in selective reporting, and other bias. One study had high risk of attrition bias (incomplete outcome data) and unclear risks of selection bias (allocation concealment) and reporting bias, and one study had unclear risks of selection bias (allocation concealment), detection bias (blinding of participants and personnel, and blinding of outcome assessment), attrition bias (incomplete outcome data) and other bias. bSerious imprecision: there were 344 participants in the three studies. cSerious risk of bias: one study had high risk of attrition bias (incomplete outcome data) and unclear risks of selection bias (allocation concealment) and reporting bias (selective reporting). dSerious imprecision: there were 183 participants in the two studies, and a wide confidence interval including both no effect and a large effect. eSerious risk of bias: one study had high risk of attrition bias (incomplete outcome data), and unclear risks of selection bias (allocation concealment) and reporting bias (selective reporting). One study had unclear risks of selection bias (allocation concealment), performance bias (blinding of participants and personnel, and blinding of outcome assessment), attrition bias (incomplete outcome data) and other bias. fSerious imprecision: there were 136 participants in the two studies.
Summary of findings 2. Discontinued cholinesterase inhibitor compared to continued cholinesterase inhibitor in patients with dementia (medium‐term, 3‐11 months).
| Discontinued cholinesterase inhibitor compared with continued cholinesterase inhibitor for patients with dementia (medium term, 3 to 11 months) | ||||||
|
Patient or population: patients withdementia Settings: all healthcare settings Intervention: withdrawal of cholinesterase Inhibitor Comparison: continuation of cholinesterase Inhibitor | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Continued cholinesterase inhibitor | Discontinued cholinesterase inhibitor | |||||
|
Cognitive function (change from baseline, medium term) Standardised mean difference (SMMSE, MMSE) |
‐ | SMD 0.40 lower (0.87 lower to 0.09 higher; P = 0.10). Lower SMD means a greater decline in cognitive function from baseline |
‐ | 411 (3) | ⊕⊝⊝⊝ verylowa,b,c | It is uncertain whether discontinuing a ChEI reduces cognitive function compared to continuing treatment. The 95% confidence interval indicates that discontinuation might make little or no difference to cognitive function, and the certainty of the evidence is very low. On removing data from one study which only included participants who had shown a poor response to donepezil, inconsistency was reduced and the SMD was ‐0.62 (95% CI ‐0.94 to ‐0.31); P < 0.001. |
|
Functional status (change from baseline, medium term) Standardised mean difference (BADLS, DAD) |
‐ | SMD 0.38 lower (0.74 lower to 0.01 lower; P = 0.04). Lower SMD means a greater decline in function from baseline |
‐ | 314 (2) | ⊕⊝⊝⊝ verylowd,e,f | It is uncertain whether discontinuing a ChEI may result in increased functional impairment compared to continuing ChEI treatment, because the certainty of the evidence is very low. |
|
Neuropsychiatric status (change from baseline, medium term) Standardised mean difference (10 and 12‐item NPI) |
‐ | SMD 0.27 lower (0.47 lower to 0.08 lower; P = 0.007). Lower SMD means a greater deterioration in neuropsychiatric status from baseline |
‐ | 410 (3) | ⊕⊕⊝⊝ lowc,g | Discontinuation may result in increased neuropsychiatric symptoms compared to continuing ChEI treatment. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BADLS: Bristol Activities of Daily Living Scale; ChEI: Cholinesterase inhibitor; CI: Confidence interval; DAD: Disability Assessment for Dementia Scale; MD: Mean Difference; MMSE: Mini‐Mental State Examination; NPI: Neuropsychiatric Inventory; OR: Odds Ratio; SMD: Standardised Mean Difference; SMMSE: Standardised Mini‐Mental State Examination | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aSerious inconsistency: the confidence intervals did not all overlap, P = 0.005 and I2 = 81%. bSerious imprecision: wide confidence interval including both no effect and a large effect cSerious risk of bias: one study had unclear risks of selection bias (allocation concealment), performance bias, detection bias, attrition bias and other bias, and one study had unclear risks of selection bias (allocation concealment), performance bias, detection bias, attrition bias and other bias, and high risk of reporting bias. dSerious imprecision: there were 314 participants in the two studies, and the upper confidence interval was close to the null effect value. eSerious inconsistency: I2 = 60% fSerious risk of bias: one study had unclear risks of selection bias (allocation concealment), performance bias, detection bias, attrition bias and other bias, and high risk of reporting bias. gSerious imprecision: the CI includes essentially no effect, and an effect of moderate size, which may be clinically important.
Summary of findings 3. Discontinuing cholinesterase inhibitor compared with continuing cholinesterase inhibitor for patients with dementia (long‐term, 12 months or longer).
| Discontinuing cholinesterase inhibitor compared with continuing cholinesterase inhibitor for patients with dementia (long term, 12 months or longer) | ||||||
|
Patient or population: patients with dementia Settings: all healthcare settings Intervention: withdrawal of cholinesterase inhibitor Comparison: continuation of cholinesterase inhibitor | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Continued cholinesterase inhibitor | Discontinued cholinesterase inhibitor | |||||
|
Cognitive function (change from baseline, long term) SMMSE |
‐ | MD 2.09 lower (3.43 lower to 0.75 lower; P = 0.002) Lower MD means a greater decline in cognitive function from baseline |
‐ | 108 (1) | ⊕⊕⊕⊝ moderatea | Discontinuing a ChEI probably reduces cognitive function compared to continuing treatment |
|
Functional status (change from baseline, long term) BADLS |
‐ | MD 3.38 lower (6.67 lower to 0.10 lower; P = 0.04) Lower MD means a greater decline in function from baseline |
‐ | 109 (1) | ⊕⊕⊕⊝ moderateb | Discontinuing a ChEI probably results in increased functional impairment compared to continuing ChEI treatment |
|
Neuropsychiatric status (change in NPI from baseline, long term) NPI |
‐ | MD 0.87 lower (8.42 lower to 6.68 higher; P = 0.82) Lower MD means a greater deterioration in neuropsychiatric status from baseline |
‐ | 108 (1) |
⊕⊕⊕⊝ moderatea | Discontinuing a ChEI may result in little to no change in neuropsychiatric status compared to continuing treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BADLS: Bristol Activities of Daily Living scale; ChEI: Cholinesterase inhibitor; CI: Confidence interval; MD: Mean difference; NPI: Neuropsychiatric Inventory; OR: Odds Ratio; SMMSE: Standardised Mini‐Mental State Examination | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aSerious imprecision: there were 108 participants in one study, and a wide confidence interval. bSerious imprecision: there were 109 participants in one study, and a wide confidence interval.
Summary of findings 4. Discontinuing cholinesterase inhibitor compared to continued cholinesterase inhibitor for patients with dementia (all trial durations) Summary of findings.
| Discontinuing cholinesterase inhibitor compared with continuing cholinesterase inhibitor for patients with dementia (all trial durations) | ||||||
|
Patient or population: patients with dementia Settings: all healthcare settings Intervention: withdrawal of cholinesterase Inhibitor Comparison: continuation of cholinesterase Inhibitor | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Continued cholinesterase inhibitor | Continued cholinesterase inhibitor | |||||
| Dropout due to lack of efficacy of trial medication or deterioration in overall medical condition | 9.7% | 14.1% (95% CI = 8.3% to 22.8%) 6.0% more (95% CI = 1.7% less to 23.3% more) |
OR 1.53 (0.84 to 2.76); P = 0.16 | 583 (4) | ⊕⊕⊝⊝ lowa,b | Discontinuing a ChEI may make little or no difference to the number of dropouts due to lack of efficacy of trial medication or deterioration in overall medical condition in those who discontinued ChEIs vs. those who continued ChEIs |
| Adverse events (any) | 38.7% | 34.9% (95% CI = 26.4% to 44.5%) 8.6% less (95% CI = 21.3% less to 20.5% more) |
OR 0.85 (0.57 to 1.27); P = 0.43 | 446 (4) | ⊕⊕⊝⊝ lowb,c | Discontinuing a ChEI may make little or no difference to the number of adverse events between those who discontinued ChEIs vs. those who continued ChEIs |
| Serious adverse events (SAEs) | 29.6% | 25.2% (95% CI = 16.2% to 36.9%) 7.8% less (95% CI = 18.5% less to 19.7% more) |
OR 0.80 (0.46 to 1.39); P = 0.43 | 390 (4) | ⊕⊕⊝⊝ lowd,e | Discontinuing a ChEI may make little or no difference to the number of adverse events between those who discontinued ChEIs vs. those who continued ChEIs |
| Deaths | 6.3% | 4.8% (95% CI = 2.4% to 9.5%) 1.7% less (95% CI = 4.1% less to 3.8% more) |
OR 0.75 (0.36 to 1.55);P = 0.43 | 598 (5) |
⊕⊕⊝⊝ lowb,f | Discontinuing a ChEI may make little or no difference to the number of deaths between those who discontinued ChEIs vs. those who continued ChEIs |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ChEI: Cholinesterase inhibitor; CI: Confidence interval; OR: Odds Ratio; SAE: Serious Adverse Event | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aSerious risk of bias: one study had unclear risks of performance bias, detection bias, reporting bias and other bias, and high risk of attrition bias. One study had unclear risk of bias in the following domains: selection bias (allocation concealment), performance bias, detection bias, attrition bias, and other bias, and one study had high risk of reporting bias and unclear risks of selection bias (allocation concealment), performance bias, detection bias, attrition bias, and other bias. bSerious imprecision: the CI includes no effect, and an effect which may be clinically important. cSerious risk of bias: one study had unclear risks of performance bias, detection bias, reporting bias and other bias, and high risk of attrition bias. One study had unclear risk of selection bias (random sequence generation), detection bias, reporting bias and other bias, one had unclear risk of selection and reporting bias and high risk of attrition bias, and one study had unclear risk of selection bias (allocation concealment), performance bias, detection bias, attrition bias and other bias and high risk of reporting bias. dSerious risk of bias: one study had unclear risks of performance bias, detection bias, reporting bias and other bias, and high risk of attrition bias. One study had unclear risks of selection bias (random sequence generation), detection bias, reporting bias and other bias, and one had unclear risk of selection and reporting bias and high risk of attrition bias. eSerious imprecision: there were 390 participants in the four studies, and the CI includes no effect, and an effect which may be clinically important. fSerious risk of bias: one study had unclear risk of performance bias, detection bias, reporting bias and other bias, and high risk of attrition bias. One study had unclear risks of selection bias (random sequence generation), detection bias, reporting bias and other bias, and one had unclear risks of selection bias (allocation concealment) and reporting bias, and high risk of attrition bias. One study had unclear risks of selection bias (allocation concealment), performance bias, detection bias, attrition bias and other bias and high risk of reporting bias.
Background
Description of the condition
Dementia is a global public health problem which will continue to grow as the proportion of older people in the population increases. It has been estimated that 46.8 million people worldwide were living with dementia in 2015 and that this number will rise to 74.7 million in 2030, and to 131.5 million in 2050 (Alzheimer's Disease International 2016). Whilst it has been estimated that between 2% and 10% of all cases start before the age of 65 years, dementia predominantly affects older people (Alzheimer's Disease International 2009; Winblad 2016).
Dementia is defined as “a progressive and largely irreversible clinical syndrome that is characterised by a widespread impairment of mental function” (NICE 2006). It is characterised by a cluster of symptoms and signs including difficulties in memory, disturbances in language, psychosocial and psychiatric changes, and impairments in activities of daily living (Burns 2009; Wu 2016). The intellectual decline is usually progressive and spares the level of consciousness until the very late stages of the illness.
There are different subtypes of dementia associated with a large number of underlying brain pathologies (Alzheimer's Disease International 2009; Burns 2009). The most common subtypes in older patients are dementia in Alzheimer’s disease, vascular dementia (VaD), dementia with Lewy Bodies (DLB) and Parkinson's Disease Dementia (PDD). Alzheimer's disease is the most common subtype, accounting for between 50% and 75% of dementia cases. It is characterised by cortical amyloid “plaques” and neurofibrillary “tangles” which develop in the structure of the brain (Tomlinson 1982; Hardy 2002; Querferth 2010; Puri 2011; Scheltens 2016). Vascular dementia is the next most common subtype, accounting for 20% to 30% of all dementia cases. It is caused by a variety of cerebrovascular pathologies, either single infarcts in critical regions of the brain or more diffuse small vessel or multi‐infarct disease (Alzheimer's Disease International 2009; World Health Organization 2010; O'Brien 2015). Post‐mortem studies suggest that many people with dementia have mixed Alzheimer’s disease and vascular dementia pathology and that this ‘mixed dementia’ is under‐diagnosed (Alzheimer's Disease International 2009; Winblad 2016 ). Prevalence figures for DLB vary widely; it is thought to be responsible for anything up to 30% of dementia cases (Zaccai 2005; Vann Jones 2014) and is caused by cortical Lewy Bodies (alpha‐synuclein) in the brain (Kalra 1996; Jacques 2000; Alzheimer's Disease International 2009; Walker 2015). It has been suggested that DLB is also under‐diagnosed in clinical practice (Mok 2004; Toledo 2013). PDD is related pathologically to DLB and many investigators consider them to lie on a spectrum of Lewy Body disorders.
Description of the intervention
Currently, there are no drugs available which can modify the course of Alzheimer's disease or the Lewy Body dementias. However, an important advance has been the introduction of drugs to delay symptomatic progression and ‐ to some extent ‐ treat the symptoms (Qaseem 2008; Raina 2008; Lopez 2009). Five drugs have United States Food and Drug Administration (FDA) approval for managing Alzheimer's disease: four cholinesterase inhibitors (ChEIs; donepezil, galantamine, rivastigmine, and tacrine) and memantine. Donepezil, galantamine, rivastigmine and memantine are licensed in the United Kingdom (UK) and throughout Europe for the management of Alzheimer's disease (Rodda 2012), though they are no longer remunerated in France, due to concern that the small benefit they offer shifts clinicians' attention from other interventions (Livingston 2020). Rivastigmine is currently the only ChEI licensed in the UK and the USA for the treatment of mild to moderate PDD (Rolinski 2012). This represents a limited armoury of therapeutic agents available for pharmacological management of dementia.
In this review, we identified and appraised trials which included patients who were on stable treatment with a ChEI or memantine and were then randomised to withdrawal or continuation of the drug. Although the only regulatory approvals are of ChEIs and memantine for Alzheimer's disease, and rivastigmine for PDD, in clinical practice they are also prescribed for other dementias (Raina 2008). Therefore, we examined studies relating to withdrawal of ChEIs and memantine in people with Alzheimer's disease, vascular dementia, mixed dementias, PDD and DLB.
ChEIs work by inhibiting acetylcholinesterase at cholinergic synapses, thereby raising synaptic levels of acetylcholine (a neurotransmitter critical to the neurons involved in cognition) (Hsiung 2008; Raina 2008). Donepezil, galantamine and rivastigmine are the most widely used and have received regulatory approval for the treatment of people with mild to moderate Alzheimer's disease in all jurisdictions (Voisin 2009). There is also evidence to suggest that donepezil can improve the cognitive, functional and neuropsychiatric status of patients with more advanced Alzheimer's disease (Feldman 2001; Schmitt 2006; Winblad 2006a; Winblad 2006c; Black 2007; Herrmann 2007a; Winblad 2009) and it is approved by the FDA in the USA for this indication. Rivastigmine, administered transdermally in a patch formulation, may also benefit cognition, activities of daily living and global functioning in people with severe Alzheimer's disease and it has received FDA approval for use in such patients (Farlow 2013). However, numerous drug agencies have not approved use of ChEIs in patients at this stage of the disease(Voisin 2009). There is therefore controversy surrounding their use in people with severe disease (Parsons 2010), due in part to the lack of RCT data in people with more severe illness (Herrmann 2007b; Hong 2018).
Rivastigmine is licensed for the treatment of mild and moderate PDD in the UK and the USA and available evidence suggests that it has a positive impact on global assessment, cognitive function, behavioural disturbance and activities of daily living (Rolinski 2012). Although use of ChEIs in DLB is common practice among clinicians, the effect of these agents on patients with DLB has not been widely investigated and evidence for their use in this patient group is not clear (Rolinski 2012).
There is considerable debate over the benefits and risks of extended use of these agents. Trial durations of 3, 6 or 12 months are the most common for assessing efficacy of dementia medications (Winblad 2006a; Rodda 2009; Schneider 2012; Deardorff 2015; Winblad 2016), with a consequent lack of evidence for longer‐term treatment (Seltzer 2007; Schneider 2012; Winblad 2016). There has been a handful of placebo‐controlled trials of ChEIs for people with Alzheimer's disease which have followed participants for a year or more (Mohs 2001; Winblad 2001; Courtney 2004; Suh 2008; DOMINO AD Howard). Their interpretation is complicated by restricted inclusion criteria, high discontinuation rates, and questionable statistical analyses (Hogan 2014). A number of open‐label extension studies have also been conducted in order to evaluate the long‐term efficacy of ChEIs (Rogers 2000; Doody 2001; Geldmacher 2003a; Winblad 2003; Pirttila 2004; Raskind 2004; Farlow 2005; Small 2005; Winblad 2006b; Burns 2007; Feldman 2009; Rountree 2009; Kavanagh 2011; Wattmo 2011; Atri 2012; Rountree 2013; Farlow 2015). Analysis of the data from such studies has shown that cognitive measures of ChEI‐treated patients remain higher (often significantly) than those predicted for a hypothetical placebo group for periods of up to four or five years (Winblad 2004; Bullock 2005; Seltzer 2007). An observational study examining long‐term use of ChEIs, in which patients were monitored for six years from the early stages of Alzheimer's disease, has also demonstrated longer time to reaching functional end points and death (Zhu 2013). Another long‐term study over the course of 20 years reported that increased duration and persistence of treatment was associated with better performance on global, functional and cognitive outcome measures (Rountree 2009). Such studies provide useful data on long‐term effects of these agents in a more authentic setting than RCTs, but they are unable to provide evidence with the same level of certainty (Deardorff 2015). The impression of sustained benefit of these drugs must be interpreted in light of the various limitations and sources of bias inherent in the design of such studies (Schneider 2012). Further, there is evidence that the efficacy of donepezil, and to a lesser extent galantamine, decreases over time due to its ability to induce up‐regulation of acetylcholinesterase (Amici 2001; Davidsson 2001; Parnetti 2002; Darreh‐Shori 2006; Nordberg 2009), and that treatment with the rapidly‐reversible ChEIs (donepezil, galantamine and tacrine) is associated with a marked and significant up‐regulation of acetylcholinesterase in patients with Alzheimer's disease (Darreh‐Shori 2010). Hence, there is continuing uncertainty regarding the long‐term efficacy of ChEIs (Schneider 2012).
As well as questions about long‐term efficacy, concerns have been raised in the literature about adverse events associated with use of ChEIs. Population‐based studies have demonstrated increased rates of syncope, bradycardia, pacemaker insertion and hip fracture in older adults with dementia who are taking ChEIs (Gill 2009; Hernandez 2009; Park‐Wyllie 2009). A meta‐analysis of RCTs has reported an association between use of ChEIs and greater risk of syncope, but not of falls, fracture or accidental injury (Kim 2011). These studies in combination highlight the importance of careful monitoring due to the potential for these serious adverse events in this vulnerable patient population (Deardorff 2015). Discontinuing ChEIs in patients with moderate to severe Alzheimer's disease has been common practice in some places. However, if these drugs retain efficacy over the long term, then this may lead to worsening cognitive function and greater functional impairment. This risk must be balanced against the risk of side effects and the costs involved in continuing these agents (Herrmann 2013). Deciding when to discontinue a ChEI remains an area of uncertainty for clinicians (Parsons 2010; Herrmann 2013; Parsons 2014; Deardorff 2015; Hong 2018; Renn 2018).
Memantine is an agonist‐antagonist (partial agonist and uncompetitive antagonist) of the N‐methyl‐D‐aspartic acid (NMDA) receptor. It partially blocks the NMDA receptor and prevents excessive stimulation of the glutamate system, which influences memory and learning (Hsiung 2008; Raina 2008). It is licensed for the treatment of moderate and severe Alzheimer's disease in North America, Europe and Australia (Reisberg 2006), but is not licensed for treatment of mild Alzheimer's disease. Along with cholinesterase inhibitors, memantine was removed from state funding in France in 2018. There is uncertainty about the efficacy of memantine in end‐stage dementia (Herrmann 2008), and about the most appropriate time to discontinue treatment (Puangthong 2009). The long‐term efficacy of memantine also remains to be confirmed (Puangthong 2009), as the duration of most trials evaluating memantine efficacy has been only three to six months (Wilcock 2008; Förstl 2011; Herrmann 2011; Rainer 2011; Schulz 2011; Fox 2012; Grossberg 2013; Nakamura 2014; McShane 2019), although several follow‐up and open‐label extension studies have reported clinically relevant benefits for patients at one year and two years, respectively (Reisberg 2006; Sinforiani 2012). However, prolonged treatment with memantine may be associated with serious adverse effects in some patients: there have been case reports of loss of consciousness or seizure‐like episodes, or both (Savic 2013). A recent Cochrane Review of memantine for dementia concluded that there is a substantial volume of high certainty evidence for a small, beneficial and clinically detectable effect in moderate to severe Alzheimer's disease at six months, and moderate certainty evidence of no benefit in mild Alzheimer's disease over six months, with increased possibility of discontinuation due to adverse events (McShane 2019). The review authors identified a need for a large trial of at least two to three years' duration in mild Alzheimer's disease to definitely rule out benefit of long‐term treatment in earlier dementia, and highlighted that a three‐year study in moderate to severe Alzheimer's disease is required to determine whether there are any continuing effects beyond six months' treatment with memantine (McShane 2019).
These clinical questions surrounding long‐term treatment with ChEIs and memantine are further complicated by the challenge of detecting ongoing benefit of treatment which is not disease‐modifying for patients in whom dementia progresses. In addition, socioeconomic considerations must be taken into account. A study examining the cost‐effectiveness of continuing donepezil in patients with moderate to severe Alzheimer's disease already treated with the drug reported that continuation of donepezil treatment for a further 52 weeks was more cost‐effective than discontinuation, regardless of whether outcomes were measured in terms of improvements in cognitive impairment, functional impairment or health‐related quality of life, and whether costs were measured for the health and social care system or for society as a whole (Knapp 2017). The majority of economic analyses of ChEIs make projections for fairly long periods of time (four to five years), and support persistent use (Seltzer 2007). Studies examining longer durations of treatment with memantine are lacking. Such studies, together with transparent economic analyses, are required to determine the long‐term cost‐effectiveness of memantine (Puangthong 2009).
Finally, the increasing interest in deprescribing medications, where deprescribing is defined as a systematic process of identifying and discontinuing drugs where the potential harms outweigh the potential benefits of continued treatment (Scott 2015), focuses on older adults taking multiple medicines and particularly on individuals with dementia (Herrmann 2018). In many countries, initiatives are underway to deprescribe medications considered to be of questionable benefit and to guide deprescribing priorities where multiple medications may be considered for deprescribing. It is within this context that this review aims to address the effects of withdrawing ChEIs or memantine, or both, on clinical and humanistic outcomes for people with dementia and their carers.
How the intervention might work
Interventions to withdraw ChEIs or memantine, or both, in people with dementia may reduce adverse effects and improve quality of life for the patient and carer. However, they may also cause withdrawal symptoms or worsening of cognitive, neuropsychiatric and functional outcomes, and may accelerate institutionalisation. Conversely, continuation of these drugs may prevent deterioration in the clinical outcomes just mentioned, but may increase mortality and adverse events and have a negative impact on the patient's quality of life.
Why it is important to do this review
Little direction is provided within treatment guidelines on how to determine the benefit of ChEIs or memantine in people with dementia, how long treatment should be continued and under what conditions to discontinue treatment. There has been ongoing debate regarding the benefit of continuing therapy, and it remains unclear whether patients who decline despite continuing treatment or those in more severe stages of the disease should have treatment withdrawn. Questions therefore remain unanswered regarding withdrawal or continuation of ChEIs or memantine, or both, for patients with dementia. A systematic review will help to identify the benefits and risks of withdrawal or continuation of these medications in this vulnerable population and may also identify important gaps in the evidence base.
Objectives
To evaluate the effects of withdrawal or continuation of cholinesterase inhibitors or memantine, or both, in people with dementia on: cognitive, neuropsychiatric and functional outcomes, rates of institutionalisation, adverse events, dropout from trials, mortality, quality of life and carer‐related outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, controlled clinical trials.
Types of participants
Participants had dementia of any severity, diagnosed using a recognised and validated tool or by clinical assessment, and were taking a cholinesterase inhibitor or memantine, or both, at baseline. Eligible dementia subtypes were Alzheimer's disease, vascular dementia, mixed dementia, DLB and PDD.
Alzheimer's disease: diagnosis of probable or possible Alzheimer's disease according to National Institute of Neurological and Communicative Disorders and Stroke / the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria or acceptable equivalent
Vascular dementia: diagnosis of probable or possible vascular dementia according to National Institute of Neurological Disorders and Stroke / Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NIND‐AIREN) criteria or acceptable equivalent
PDD: diagnosis of probable or possible PDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria (American Psychiatric Association 1994), or acceptable equivalent
DLB: diagnosis of probable or possible DLB according to international consensus criteria for DLB (McKeith 2006)
Patients could be resident in any setting (including acute hospitals, nursing and residential homes and the community).
Types of interventions
Control Intervention
Continuation of cholinesterase inhibitor or memantine, or both, beyond the time of randomisation.
Experimental interventions
Discontinuation of cholinesterase inhibitor or memantine, or both, after randomisation, with or without placebo substitution. Treatment may have been discontinued abruptly or by gradual tapering of the dose.
Types of outcome measures
We selected the following primary and secondary outcomes of interest.
Primary outcomes
Cognitive, neuropsychiatric and functional outcomes, measured with validated scales
Rates of institutionalisation
Adverse effects
Dropouts from the trial, including total number of dropouts and number of dropouts due to deterioration or lack of efficacy
Secondary outcomes
Quality of life of patients (measured with a validated scale)
Quality of life of caregivers (measured with a validated scale)
Mortality
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register ‐ up to 17 October 2020. We used search terms appropriate for the identification of reports of trials using the cholinesterase inhibitors (donepezil, rivastigmine, galantamine and tacrine) and memantine.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment and cognitive improvement. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly searches of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL); and
six‐monthly searches of a number of grey literature sources from ISI Web of Science Core Collection.
To view a list of all sources searched by ALOIS see 'About ALOIS' on the ALOIS website (alois.medsci.ox.ac.uk/about-alois). Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Cochrane Dementia and Cognitive Improvement Group. We performed additional searches in many of the sources listed above, to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used are described in Appendix 1. The most recent search was carried out in October 2020. No language restrictions were applied.
Searching other resources
We inspected the references of all identified studies for other studies.
Data collection and analysis
Selection of studies
Three review authors (CP, CH, WYL) independently screened titles and abstracts of citations identified by the searches, discarding obviously irrelevant articles. At this stage, we were overly inclusive: any article that suggested a relevant trial was retrieved in full‐text form for further assessment. Two review authors then independently assessed the full‐text articles against the predefined inclusion criteria. We resolved discrepancies by consensus.
Data extraction and management
Three review authors (CP, CH, WYL) independently extracted data from the included trials and resolved discrepancies by consensus.
For continuous data, we extracted the mean change from baseline, the standard error or standard deviation of the mean change, and the number of patients in each treatment group at each time point. Where changes from baseline were not reported, we extracted the mean, standard deviation and the number of people in each treatment group at each time point. For ordinal variables, such as cognitive, neuropsychiatric, functional and quality of life scales, where there are large numbers of possible scores, we treated the measures as continuous. Where there were differences in the direction of the scales used to measure an outcome (e.g. the Alzheimer's Disease Assessment Scale ‐ Cognitive Subscale (ADAS‐Cog/11) and the Mini‐Mental State Examination (MMSE) as measures of cognitive function, where a decrease in MMSE and an increase in ADAS‐Cog/11 indicate poorer function), we multiplied the mean values by ‐1 as appropriate to ensure that all the scales pointed in the same direction.
For dichotomous data, we extracted the number in each treatment group and the numbers experiencing the outcome of interest.
For each outcome measure, we sought to extract data on every patient randomised, irrespective of compliance, whether or not the person was subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. If these 'intention‐to‐treat' data were not available in the publications, then we extracted 'on‐treatment' data of those who completed the trial.
Assessment of risk of bias in included studies
We assessed the risk of bias in each of the included trials using the following criteria of internal validity: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, adequate reporting and handling of missing outcome data, selective outcome reporting and other risks of bias. We followed the guidelines in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011, hereafter referred to as the Cochrane Handbook). Three reviewers (CP, CH, WYL) independently assessed the risk of bias of the included studies. We followed GRADE recommendations to determine the certainty of the evidence (Guyatt 2008). This involved considering risk of bias together with inconsistency, indirectness, imprecision and publication bias. Two reviewers (CP, WYL) completed this assessment.
Measures of treatment effect
For continuous outcomes, the measure of treatment effect was the standardised mean difference (SMD, the absolute mean difference divided by the standard deviation) due to a range of outcome measures being employed by the included studies. For dichotomous outcomes, the measure of treatment effect was the Mantel‐Haenszel odds ratio. A 95% confidence interval was calculated for all effect estimates.
Unit of analysis issues
Individual patients were randomised in all included trials. No unit of analysis issues were encountered.
Dealing with missing data
We described any data which were missing from the published report of a trial.
Where participant‐level data were missing, then we sought intention‐to‐treat data and, if these were not reported, analysed available case data. We reported any statistical method used by the study authors (e.g. multiple imputation analysis, last observation carried forward) to deal with non‐missing‐at‐random data. We excluded studies from meta‐analysis if there was a differential loss to follow‐up between groups greater than 20%.
We also encountered missing data required for our analyses. Where change‐from‐baseline scores were missing for a time point in a study, we extracted numerical post‐intervention data from the appropriate graphs, calculated mean change scores and imputed standard deviations using a correlation coefficient determined using change scores at another time point in the study. Where data were presented as mean change scores with standard errors, we transformed the standard errors into standard deviations.
Assessment of heterogeneity
We assessed potential differences between the included studies in the types of participants, interventions or controls used before pooling data.
We assessed heterogeneity between studies using the Chi2 test (with a significance level set at P < 0.10) and the I2 statistic, which calculates the percentage of variability due to heterogeneity rather than to chance, with I2 values over 50% suggesting substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We included studies published in any language to avoid any risk of language bias. In order to minimise the risk of publication bias, we performed a comprehensive search in multiple databases, including searching for unpublished studies in trial registries. We compared outcomes reported in a trial with the protocol, wherever possible, to examine whether all of the study's prespecified outcomes that were of interest to the review had been reported.
Data synthesis
The duration of trials varied from 6 weeks up to 24 months from the time of randomisation. We conducted separate meta‐analyses where possible for short‐term (up to 2 months) and medium‐term (3 to 11 months) outcomes. It was not possible to conduct meta‐analyses for long‐term (12 months or longer) outcomes due to the limited number of studies reporting these outcomes. Durations for short‐ and medium‐term outcomes are reflective of observation in the literature that the first six weeks following ChEI discontinuation are particularly important when monitoring patients for changes in cognition and neuropsychiatric symptoms (O'Regan 2015; Herrmann 2018). Some trials contributed data to more than one meta‐analysis if multiple assessments were performed. We were not able to conduct separate meta‐analyses for short‐, medium‐ and long‐term outcomes relating to dropouts, adverse events, serious adverse events or deaths as the data available on these outcomes did not allow these distinctions to be determined.
We intended to conduct separate analyses for different dementia subtypes, but in fact all the included studies focused on Alzheimer's disease.
A weighted estimate of the typical treatment effect across trials was calculated using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We had prespecified subgroup analyses for severity of dementia (mild to moderate and moderate to severe), but there were too few included studies to allow meaningful subgroup analysis. We were also unable to undertake subgroup analysis by duration of treatment prior to enrolment in the discontinuation trial or by method of discontinuation (abrupt versus tapered) due to the low numbers of included studies.
Sensitivity analysis
We performed sensitivity analyses to determine the impact of including Johannsen 2006, in which participants who showed the best response to donepezil were excluded, and of using data from differing scales measuring the same outcome, to assess the robustness of our results.
Summary of findings and assessment of the certainty of the evidence
We used 'Summary of findings' tables to summarise the data comparing withdrawal and continuation of cholinesterase inhibitors at short‐, medium‐ and long‐term time points on cognitive, functional and neuropsychiatric outcome measures. We also included data on dropout due to lack of efficacy of trial medication or deterioration in overall medical condition, adverse events, serious adverse events and mortality across the duration of the trials; it was not possible to separate these by time point. We used GRADE methods to assess the overall certainty of the evidence for each outcome (Guyatt 2008).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The electronic searches identified 10,806 potentially relevant citations (Figure 1). Following removal of duplicates and review of titles and abstracts by Cochrane Dementia and Cognitive Improvement Group information specialists, we screened 1810 records. Of these, 42 publications were identified as potentially eligible and were examined in full‐text form. We identified seven completed studies which were eligible for inclusion. We also identified two ongoing studies from clinical trial registers (See Characteristics of ongoing studies).
1.
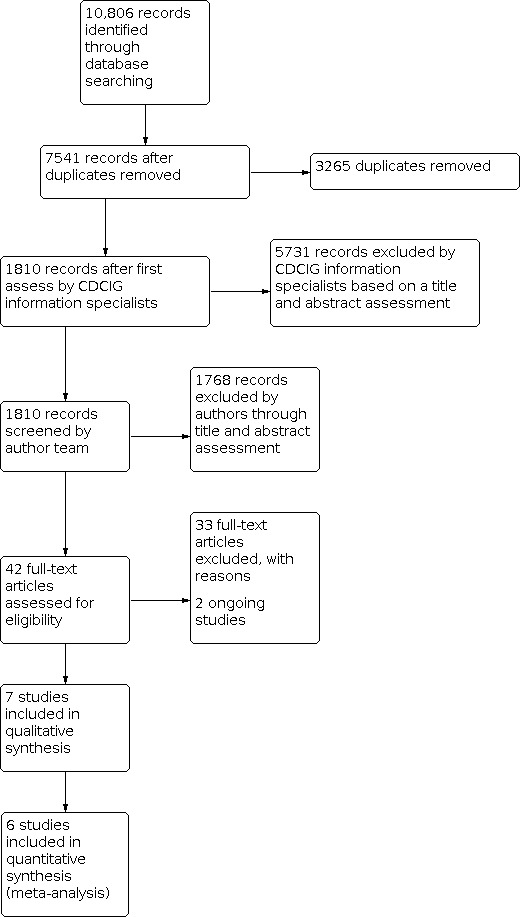
Study flow diagram.
Included studies
Seven studies met the inclusion criteria (the DOMINO‐AD trial (DOMINO AD Howard); GAL‐USA‐5 Gaudig; GAL‐ITA‐2 Scarpini; Herrmann 2016; Holmes 2004; Hong 2018; Johannsen 2006). The characteristics of these studies are described in detail in the Characteristics of included studies table.
Number of participants and setting
There were a total of 955 participants randomised in the included studies, of whom 759 were assigned to groups relevant to this review. The included studies were conducted in the UK (DOMINO AD Howard; Holmes 2004), USA (GAL‐USA‐5 Gaudig), Canada (Herrmann 2016), Italy (GAL‐ITA‐2 Scarpini), South Korea (Hong 2018) and across multiple countries (Belgium, Denmark, Greece, Hungary, Iceland, the Netherlands, Poland and the USA; Johannsen 2006). Participants in the studies were resident at home or in an assisted home care or long‐term care setting. Participants in GAL‐USA‐5 Gaudig had previously completed a three‐month, randomised, multicentre, international clinical trial (GAL‐INT‐2; Tariot 2000). Government or charitable foundations, or both, funded two studies (DOMINO AD Howard; Herrmann 2016), and the pharmaceutical industry funded four studies (GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Holmes 2004; Johannsen 2006). Hong 2018 received a combination of government and pharmaceutical industry funding.
Dementia diagnoses and severity
Participants in four trials had a diagnosis of probable Alzheimer's disease, according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Assocation (NINCDS‐ADRDA) criteria (GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Hong 2018; Johannsen 2006), and in three trials, a diagnosis of probable or possible Alzheimer's disease according to the same criteria (DOMINO AD Howard; Herrmann 2016; Holmes 2004). In addition, patients in Herrmann 2016, Hong 2018 and Johannsen 2006 were required to meet Diagnostic and Statistical Manual of Mental Disorders‐Fourth Edition (DSM‐IV) criteria for dementia. Severity of dementia among participants, and the measures used to determine severity, varied across studies: Hong 2018 included patients with extremely severe Alzheimer's disease; DOMINO AD Howard and Herrmann 2016 included patients with moderate to severe dementia; GAL‐ITA‐2 Scarpini, GAL‐USA‐5 Gaudig, Johannsen 2006 and Holmes 2004 included patients with mild to moderate dementia.
Additional criteria for inclusion and exclusion
Other inclusion criteria were also stipulated in each of the included studies. These covered prescribing of ChEIs and other medications, comorbid medical conditions, and regular contact with a caregiver. In general, patients with severe, unstable or poorly controlled medical conditions and with neurodegenerative disorders other than Alzheimer's disease were excluded. In addition, patients were excluded from Johannsen 2006 if they resided in a nursing home. Importantly, participants included in Johannsen 2006 were those who had had a poorer response (uncertain clinical benefit) to prior open‐label treatment with donepezil over 12 to 24 weeks; those thought to have derived clear benefit from the treatment were excluded. Conversely, patients were included in the double blind placebo‐controlled withdrawal phase of GAL‐ITA‐2 Scarpini only if they had had a good response to galantamine ‐ defined as cognitive deterioration from baseline of less than 4 points on the ADAS‐Cog/11 ‐ in the prior 12‐month open‐label treatment phase.
Description of interventions
Trial duration varied across included studies, ranging from 6 weeks up to 24 months from the time of randomisation.
All studies compared continuing ChEI or memantine to discontinuing treatment. Six of the studies used placebo substitution for the discontinued ChEI; Hong 2018 was the only study which was not placebo‐controlled.
Participants in Hong 2018 had been taking anti‐dementia drugs for at least two months prior to randomisation. The minimum duration of ChEI treatment prior to randomisation in the other trials was: 3 months, with at least 6 weeks on a stable dose (DOMINO AD Howard; GAL‐USA‐5 Gaudig; Holmes 2004); 6 months (Johannsen 2006); 12 months (GAL‐ITA‐2 Scarpini); or 24 months (Herrmann 2016). All treatments consisted of doses regarded as being within the therapeutic range.
In DOMINO AD Howard, participants were randomly assigned to one of four treatment groups, two of which were relevant to this review. In the first group, participants continued to take donepezil 10 mg/day, with placebo memantine, starting in week one, and in the second group, participants took donepezil at a dose of 5 mg/day during weeks one to four and then placebo donepezil starting in week five, plus placebo memantine starting in week one.
Participants entering GAL‐USA‐5 Gaudig were assigned treatment according to the group into which they had been randomised in GAL‐INT‐2. Participants who had received placebo were continued on it; this group was not relevant to the review. Participants who had received galantamine were randomised into two groups: a withdrawal group, in which galantamine was discontinued abruptly and participants received a placebo, or a continuation group, in which galantamine treatment was continued at the same dosage as in the previous trial (24 mg/day or 32 mg/day, in two divided doses). These withdrawal and continuation groups were included in this review.
In Hong 2018, no differentiation was made between donepezil and memantine, both of which were classed as anti‐dementia drugs. Participants were randomly assigned to continuation or abrupt discontinuation of anti‐dementia drug treatment.
In Holmes 2004, Johannsen 2006 and GAL‐ITA‐2 Scarpini, ChEI treatment was also discontinued abruptly, from 10 mg/day donepezil (Holmes 2004 and Johannsen 2006) or 16 mg/day galantamine in two divided doses (GAL‐ITA‐2 Scarpini).
In Herrmann 2016, participants in the discontinuation group were tapered off their baseline dose of ChEI (donepezil, galantamine or rivastigmine) for two weeks and then took placebo for the remaining six weeks of the study period.
Outcomes
The included studies examined cognitive, functional and global outcomes, neuropsychiatric symptoms, quality of life and safety, tolerability and adverse effects, and mortality. Measures used to assess many of these outcomes varied across studies; Appendix 2 provides a list of the assessment scales used. Institutionalisation was also considered by DOMINO AD Howard, in which nursing home placement was a secondary outcome measure. Time to dropout was considered by two studies (GAL‐ITA‐2 Scarpini and Hong 2018). All included studies considered adverse events, safety and tolerability. Quality of life was considered for the patient by DOMINO AD Howard and Herrmann 2016, and the caregiver by DOMINO AD Howard.
Excluded studies
Excluded publications that were read in full are summarised along with the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
Two review authors independently assessed the risk of bias in all included studies, across the following domains: random sequence generation and allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other potential sources of bias.
See 'Risk of bias' tables in Risk of bias in included studies, overall 'Risk of bias' graph (Figure 2), and risk of bias summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged six studies to be at low risk of selection bias due to random sequence generation (DOMINO AD Howard; GAL‐ITA‐2 Scarpini; Herrmann 2016; Holmes 2004; Hong 2018; Johannsen 2006). GAL‐USA‐5 Gaudig was considered to be at unclear risk of bias for this domain as the Clinical Research Report indicated that 28 patients were randomised out of sequence.
We judged four studies to be at low risk of selection bias due to allocation concealment (DOMINO AD Howard; GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Hong 2018), with the remaining three studies considered to be at unclear risk (Herrmann 2016; Holmes 2004; Johannsen 2006), as no details were reported regarding allocation concealment.
Blinding
We judged three studies to be at low risk of performance bias (DOMINO AD Howard; GAL‐USA‐5 Gaudig; Herrmann 2016), three at unclear risk due to lack of adequate descriptive detail (GAL‐ITA‐2 Scarpini; Holmes 2004; Johannsen 2006), and one study (Hong 2018) to be at high risk of bias in this domain, as participants and personnel were not blinded to treatment allocation, and it was possible that participants' perceptions may have been affected by their knowledge of the treatment group to which they were allocated. This may have indirectly made them more aware of side effects of drug withdrawal or may have resulted in more severe rating of symptoms. Similarly, investigators may have been influenced by knowledge of treatment allocation when advising patients to look out for certain side effects of drug withdrawal.
We judged three studies to be at low risk of detection bias (DOMINO AD Howard; Herrmann 2016; Hong 2018). We considered the remaining four studies to be at unclear risk of bias in this domain (GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Holmes 2004; Johannsen 2006), as no details were provided of how blinding of outcome assessment was undertaken.
Incomplete outcome data
We deemed three studies (DOMINO AD Howard; GAL‐USA‐5 Gaudig; Hong 2018) to be at low risk of attrition bias. We considered two studies to be at unclear risk of attrition bias (Holmes 2004; Johannsen 2006). In these studies, the results of the Intent‐to‐Treat with Last Observation Carried Forward (ITT‐LOCF) analyses differed from those of the observed case (OC) analyses, and discontinuations could be linked to participants' health status. We judged two studies to be at high risk of bias (GAL‐ITA‐2 Scarpini; Herrmann 2016) due to the proportion of missing data differing in the continuation and discontinuation groups and because discontinuations could be linked to participants' health status. Furthermore, in Herrmann 2016, there was no evidence in the analysis methods or sensitivity analyses that correction for bias was undertaken, and no information was provided on the imputation of missing data, despite Figure 1 clearly demonstrating that there were dropouts after randomisation.
Selective reporting
We judged risk of reporting bias to be low for three studies (DOMINO AD Howard; Holmes 2004; Hong 2018), and deemed three studies to have an unclear risk of bias. In GAL‐ITA‐2 Scarpini, Clinical Interview Based Impression of Change‐Plus Caregiver Input (CIBIC‐plus) scores (one of the specified secondary outcome measures) were not reported, and the study was not sufficiently powered for ADAS‐Cog/11 survival analysis. In GAL‐USA‐5 Gaudig, the clinical study report stated that both Traditional Division of Neuropharmacological Drug Product with Last Observation Carried Forward (Traditional DNDP‐LOCF) and OC analyses were performed, but the published paper reported only the OC analyses. In Herrmann 2016, the number of 'as needed' medications used to treat behavioural and psychological symptoms of dementia (BPSD) was not reported, for the Clinician's Global Impression (CGI) outcome measure, it was not made explicit whether this was the Clinician's Global Impression‐Severity (CGI‐S) measure which considers severity, and the baseline Cornell Depression Scale for Dementia scores were not reported. Johannsen 2006 was considered to have a high risk of bias because ADAS‐Cog/11, MMSE, Neuropsychiatric Inventory (NPI) and Disability Assessment in Dementia (DAD) were measured at weeks 6 and 12, but only results at week 12 were reported.
Other potential sources of bias
We considered DOMINO AD Howard, Herrmann 2016 and Hong 2018 to be at low risk of other sources of bias. We considered GAL‐ITA‐2 Scarpini, GAL‐USA‐5 Gaudig, Holmes 2004, and Johannsen 2006 to be at unclear risk of bias since pharmaceutical companies which manufactured galantamine (GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig) and donepezil (Holmes 2004; Johannsen 2006) funded the analysis and writing of the manuscripts.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Six of the seven included trials investigated the effect of withdrawal of ChEIs. Three trials investigated withdrawal of donepezil and two trials examined withdrawal of galantamine. One trial was a pilot study in 40 patients, which investigated withdrawal of any ChEI (Herrmann 2016). In this trial, there were very small numbers of patients taking donepezil (N = 17; with 7 in the placebo group and 10 in the continuation group), galantamine (N = 16; with 8 in the placebo group and 8 in the continuation group) and rivastigmine (N = 7; with 4 in the placebo group and 3 in the continuation group) at baseline. We were able to include between two and six trials in each meta‐analysis, depending on the outcome and time point being considered. A seventh trial (Hong 2018) investigated the effect of withdrawing either a cholinesterase inhibitor (donepezil) or memantine, and did not report results for the two drug classes separately. We did not include this trial in the quantitative syntheses. The meta‐analysis results and evidence quality for each outcome for the main comparison (discontinuation compared to continuation of ChEI) are described in the Table 1, Table 2, Table 3 and Table 4.
Cognitive function
The seven included trials considered a range of time points and cognitive outcomes.
In the meta‐analyses, we considered the effect of discontinuation versus continuation of ChEIs at two different time points, short term (up to 2 months) and medium term (3 to 11 months), using pooled data from four studies and three studies respectively. As different cognitive outcome measures were employed by the included studies, we used standardised mean differences as the measure of effect size.
For the short‐term effect, four studies (344 participants) reported data relevant to this outcome (DOMINO AD Howard; GAL‐USA‐5 Gaudig; Herrmann 2016; Holmes 2004). Discontinuation may reduce cognitive function compared to continuing ChEI treatment (SMD ‐0.42, 95% CI ‐0.64 to ‐0.21; 344 participants, 4 studies; Analysis 1.1). We assessed the overall certainty of the evidence for this outcome as low, downgraded one level for risk of bias and one level for imprecision. Johannsen 2006 also measured cognitive function at six weeks, but did not report the results.
1.1. Analysis.

Comparison 1: Cognitive function, Outcome 1: Cognitive function (change from baseline, short term)
For the medium‐term effect, three studies (411 participants) reported data relevant to this outcome (DOMINO AD Howard; Holmes 2004; Johannsen 2006). For DOMINO AD Howard, we included data from two treatment groups: continuation of donepezil with placebo memantine, and discontinuation of donepezil with placebo memantine. We considered the evidence behind our main analysis to be very low certainty, downgraded for inconsistency, imprecision and risk of bias. Therefore we are very uncertain of the effect of discontinuation of ChEI on cognitive function (SMD ‐0.40, 95% CI ‐0.87 to 0.07; 411 participants, 3 studies; Analysis 1.2). Heterogeneity in this analysis was high (I2 = 81%). We conducted a sensitivity analysis, excluding data from Johannsen 2006 which had included only participants with a poor response to donepezil, who might be expected to show less effect of discontinuation. When these data were omitted, the heterogeneity was reduced (I2 = 24%) and the result favoured continuation (SMD ‐0.62, 95% CI ‐0.94 to ‐0.31; 219 participants, 2 studies; P < 0.001). We also conducted a sensitivity analysis to determine the impact of using MMSE data rather than ADAS‐Cog/11 data from Johannsen 2006 on the conclusions regarding cognition. We found that using MMSE data made little difference to the effect estimate (SMD ‐0.31, 95% CI ‐0.96 to 0.34; P = 0.35) and heterogeneity remained very high (I2 = 90%).
1.2. Analysis.

Comparison 1: Cognitive function, Outcome 2: Cognitive function (change from baseline, medium term)
For the long‐term effect, data relevant to this outcome were only available for one study (DOMINO AD Howard). Discontinuation probably reduces cognitive function compared to continuing donepezil treatment (MD ‐2.09 SMMSE points, 95% CI ‐3.43 to ‐0.75; 108 participants, 1 study; Analysis 1.3). The wide confidence interval and data from a single study affect our certainty about the result (moderate certainty evidence, downgraded one level due to imprecision).
1.3. Analysis.

Comparison 1: Cognitive function, Outcome 3: Cognitive function (change in SMMSE from baseline, long term)
We did not include data from GAL‐ITA‐2 Scarpini and Hong 2018 in the quantitative syntheses due to the nature of the study design or the outcome measures selected, or both. GAL‐ITA‐2 Scarpini withdrew participants when they experienced a deterioration in ADAS‐Cog/11 score of 4 points or more, and reported time to deterioration as the study endpoint. Hong 2018 did not differentiate between participants who were discontinuing donepezil and those discontinuing memantine.
In GAL‐ITA‐2 Scarpini, there was no statistically significant difference in the likelihood of premature study discontinuation due to a confirmed deterioration in ADAS‐Cog/11 score of 4 points or more between participants switched to placebo and those continuing galantamine (HR 1.66, 95% CI 0.78 to 3.54; P = 0.19). The authors examined the number of participants in each group who experienced lack of efficacy (defined as the subjective impression of their caregiver or general practitioner, and deterioration of 4 points or more in the ADAS‐Cog/11 score). Participants taking placebo were more likely to discontinue the study prematurely than those continuing galantamine (HR 1.80, 95% CI 1.02 to 3.18; P = 0.04). The authors reported that 47% of participants who continued galantamine completed the 24‐month follow‐up without showing a change of ADAS‐Cog/11 score of 4 points or more, compared to 30% of those in the discontinuation group, and concluded that galantamine was effective in delaying time to cognitive deterioration in people with mild to moderate Alzheimer's disease.
Hong 2018 reported the change in Baylor Profound Mental State Examination (BPMSE) scores from baseline to 12 weeks in the drug‐continuation group (0.4 point improvement) and the drug‐discontinuation group (0.5 point decline). Our analysis suggests that there was no evidence of a difference between groups (MD ‐0.90 BPMSE points; 95% CI ‐2.18 to 0.38; 57 participants, 1 study; Analysis 1.4). The authors also reported no significant difference between the groups in changes from baseline on the MMSE. There was a 0.3 point decline in the continuation group and a 0.3 point improvement in the discontinuation group, and our analysis concurs with their conclusion (MD 0.60 MMSE points, 95% CI ‐0.09 to 1.29; 57 participants, 1 study; Analysis 1.5).
1.4. Analysis.

Comparison 1: Cognitive function, Outcome 4: Cognitive function (change in BPMSE from baseline, medium term) for Hong 2018
1.5. Analysis.

Comparison 1: Cognitive function, Outcome 5: Cognitive function (change in MMSE from baseline, medium term) for Hong 2018
Functional outcomes (performance of activities of daily living)
GAL‐ITA‐2 Scarpini, GAL‐USA‐5 Gaudig and Holmes 2004 did not measure functional outcomes. The included trials which did consider functional status used a range of outcome measures at varying time points. We used standardised mean differences because different functional outcome measures were employed by the included studies.
Two studies (183 participants) reported functional outcomes in the short term (Herrmann 2016 and DOMINO AD Howard; SMD ‐0.25, 95% CI ‐0.54 to 0.04; 183 participants, 2 studies; Analysis 2.1). The meta‐analysis suggests that discontinuation may result in slightly more functional impairment than continuing ChEI treatment, but we cannot exclude no effect. We assessed the overall certainty of evidence to be low, downgraded one level for risk of bias and one for imprecision. For the medium‐term effect, two studies (314 participants) reported data relevant to this outcome (DOMINO AD Howard; Johannsen 2006). As before, data from DOMINO AD Howard only included two groups: continuation of donepezil with placebo memantine, and discontinuation of donepezil with placebo memantine. For the medium‐term effect, it is uncertain whether discontinuation results in increased functional impairment compared to continuing ChEI treatment (SMD ‐0.38, 95% CI ‐0.74 to ‐0.01; 314 participants, 2 studies; Analysis 2.2). We assessed the overall certainty of evidence to be very low, downgraded one level for imprecision, one for inconsistency and one for risk of bias.
2.1. Analysis.

Comparison 2: Functional status, Outcome 1: Functional status (change from baseline, short term)
2.2. Analysis.

Comparison 2: Functional status, Outcome 2: Functional status (change from baseline, medium term)
The data from Hong 2018 were not included in the meta‐analysis as no differentiation was made between ChEI or memantine; patients were randomly assigned to an antidementia drug continuation or discontinuation group. Hong 2018 reported no significant differences in change from baseline to week 12 in Alzheimer's Disease Cooperative Study‐Activities of Daily Living Inventory, modified for severe dementia (ADCS‐ADL‐sev), Functional Assessment Staging (FAST) or Barthel Index of Activities of Daily Living scores between antidementia drug continuation and antidementia drug discontinuation groups (P = 0.89, P = 0.14 and P = 0.54 respectively).
For the long‐term effect, data relevant to this outcome were only available for one study (DOMINO AD Howard). Discontinuing donepezil probably results in increased functional impairment compared to continuing treatment (MD ‐3.38 Bristol Activities of Daily Living Scale (BADLS) points, 95% CI ‐6.67 to ‐0.10; 109 participants, 1 study; Analysis 2.3). However, the wide confidence interval and single study affect our certainty in the evidence (moderate certainty evidence, downgraded one level due to imprecision).
2.3. Analysis.

Comparison 2: Functional status, Outcome 3: Functional status (change from baseline, long term)
Neuropsychiatric outcomes
A number of studies considered neuropsychiatric outcomes, using a range of outcome scales. In the meta‐analysis, the effect of discontinuation versus continuation of ChEIs was considered at two different time points, short term (up to 2 months) and medium term (3 to 11 months). As different neuropsychiatric outcome measures were employed by the included studies, we used standardised mean differences.
For the short‐term effect, two studies (136 participants) reported data relevant to this outcome (Herrmann 2016; Holmes 2004). We selected the Neuropsychiatric Inventory‐Nursing Home version (NPI‐NH) scores in Herrmann 2016 and NPI total scores in Holmes 2004 for the meta‐analysis, and found that discontinuation may result in increased neuropsychiatric symptoms compared to continuing ChEI treatment, although the effect may be very small (SMD ‐0.48, 95% CI ‐0.82 to ‐0.13; 136 participants, 2 studies; Analysis 3.1). We assessed the overall certainty of evidence for this outcome at this time point as low, downgraded one level for risk of bias and one level for imprecision.
3.1. Analysis.

Comparison 3: Neuropsychiatric status, Outcome 1: Neuropsychiatric status (change from baseline, short term)
For the medium‐term effect, three studies (410 participants) reported data relevant to this outcome (DOMINO AD Howard; Holmes 2004; Johannsen 2006). We selected NPI scores for the meta‐analysis but due to the variation in the use of the 10‐item (Holmes 2004; Johannsen 2006) or 12‐item versions (DOMINO AD Howard), standardised mean differences were determined. Discontinuation may increase neuropsychiatric symptoms compared to continuing ChEI treatment, although the effect may be minimal (SMD ‐0.27, 95% CI ‐0.47 to ‐0.08; 410 participants, 3 studies; Analysis 3.2). We assessed the overall certainty of evidence for this outcome at this time point as low, downgraded one level for imprecision and one level for risk of bias.
3.2. Analysis.

Comparison 3: Neuropsychiatric status, Outcome 2: Neuropsychiatric status (change from baseline, medium term)
As with cognitive and functional outcomes, we did not include data from Hong 2018 in the meta‐analysis as no differentiation was made between ChEI or memantine: patients were randomly assigned to an antidementia drug continuation or discontinuation group. Hong 2018 reported no significant differences between antidementia drug continuation and antidementia drug discontinuation groups in changes from baseline to week 12 in NPI scores (P = 0.22).
For the long‐term effect, data relevant to this outcome were only available for one study (DOMINO AD Howard). Discontinuing donepezil may result in little to no change in neuropsychiatric status in the long term, compared to continuing treatment (MD ‐0.87 NPI points, 95% CI ‐8.42 to 6.68; 108 participants, 1 study; Analysis 3.3 ). The wide confidence interval and data from a single study affect the certainty of the evidence (moderate certainty, downgraded one level due to imprecision).
3.3. Analysis.

Comparison 3: Neuropsychiatric status, Outcome 3: Neuropsychiatric status (change in NPI from baseline, long term)
Quality of life
DOMINO AD Howard reported participant quality of life as Dementia Quality of Life Proxy Measure (DEMQOL‐Proxy) scores at weeks 18 and 52. However, the data reported for continuation and discontinuation groups were average values across patients who received active and placebo memantine, and therefore included all four treatment groups rather than the two of specific interest to this review. The difference in scores between donepezil continuation and discontinuation groups was ‐1.1 (99% CI ‐4.6 to 2.4) at 18 weeks, and ‐2.4 (99% CI ‐6.4 to 1.6) at 52 weeks, indicating that discontinuation may result in little to no change in patient health‐related quality of life. Herrmann 2016 reported quality of life as change in Quality of Life in late stage Dementia (QUALID) scores from baseline to 8 weeks, and there was no evidence of a difference in scores between ChEI discontinuation and continuation groups (MD 0.40 QUALID points, 95% CI ‐2.08 to 2.88; 40 participants, 1 study; Analysis 4.1). This was a small pilot study with 40 participants, which was not powered to detect statistically significant differences in quality of life or in any of the outcomes measured.
4.1. Analysis.

Comparison 4: Quality of life, Outcome 1: Quality of life (change in QUALID from baseline, short term)
DOMINO AD Howard reported caregiver quality of life in terms of health status, using the General Health Questionnaire 12‐ item (GHQ‐12), at weeks 6, 30 and 52. Again, these data represented average values across patients who received active and placebo memantine, and therefore included all four treatment groups rather than the two of specific interest to this review. The differences in scores between donepezil continuation and discontinuation groups were ‐0.5 (99% CI ‐1.2 to 0.1) at 6 weeks, ‐0.5 (99% CI ‐1.3 to 0.4) at 30 weeks, and ‐0.7 (99% CI ‐1.7 to 0.3) at 52 weeks. These differences indicate that discontinuation may result in little to no change in caregiver health status.
Dropout and adverse events
The included studies considered the effect of ChEI discontinuation on dropout for a range of different reasons (total dropout during trial and follow‐up, death, adverse events, lack of efficacy of trial medication or deterioration in overall medical condition). We were unable to extract data for these outcomes at our predetermined time points, so we present data across the trial durations.
More participants in the discontinuation group dropped out during the trial or follow‐up than in the continuation group (OR 1.48, 95% CI 1.01 to 2.17; 694 participants, 6 studies; Analysis 5.1). We assessed the overall quality of the evidence for this outcome as low, downgraded one level for imprecision and one level for risk of bias. We found no evidence that discontinuing a ChEI affects numbers of participants who dropped out due to adverse events (OR 0.82, 95% CI 0.42 to 1.61; 694 participants, 6 studies; Analysis 5.2) or due to lack of efficacy of trial medication or deterioration in overall medical condition (OR 1.53, 95% CI 0.84 to 2.76; 583 participants, 4 studies; Analysis 5.3), compared to continuing treatment. We assessed the overall quality of the evidence for these outcomes as low, downgraded one level for imprecision and one level for risk of bias.
5.1. Analysis.

Comparison 5: Safety, tolerability and dropout, Outcome 1: Total dropout during trial and follow‐up
5.2. Analysis.

Comparison 5: Safety, tolerability and dropout, Outcome 2: Dropout due to adverse event
5.3. Analysis.

Comparison 5: Safety, tolerability and dropout, Outcome 3: Dropout due to lack of efficacy of trial medication or deterioration in overall medical condition
Four of the included studies (GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Herrmann 2016; Johannsen 2006) considered the occurrence of any adverse events and serious adverse events, and did not find any evidence of difference between discontinuation and continuation groups (OR 0.85, 95% CI 0.57 to 1.27; 446 participants, 4 studies; Analysis 5.4; and OR 0.80, 95% CI 0.46 to 1.39; 390 participants, 4 studies; Analysis 5.5, respectively). We considered the overall quality of evidence for these outcomes to be low, again downgraded one level for imprecision and one for risk of bias.
5.4. Analysis.

Comparison 5: Safety, tolerability and dropout, Outcome 4: Adverse events (any)
5.5. Analysis.

Comparison 5: Safety, tolerability and dropout, Outcome 5: Serious adverse events (SAEs)
Institutionalisation
DOMINO AD Howard reported institutionalisation as a secondary outcome of the trial. The specific outcomes were number of nursing home placement events, nursing home placement rate per 10 person‐years, centiles of time to nursing home placement in months, probability of nursing home placement by time after randomisation (6, 12, 24, 36 and 48 months) and cumulative probability of nursing home placement over time for the two groups relevant to this review. Cumulative probability of nursing home placement over time was not significantly different between discontinuation and continuation groups (HR 1.46, 95% CI 0.94 to 2.29; P = 0.06). Further analyses were undertaken for combined donepezil discontinuation and continuation groups, each group including participants who started memantine and those who started placebo memantine at the time of randomisation; it was not possible to extract data only for the groups receiving placebo memantine. Overall, there was significant (P = 0.010) heterogeneity of treatment effect over time, with significantly more nursing home placements in the combined donepezil discontinuation groups during the first year (HR 2.09, 95% CI 1.29 to 3.39) than in the combined donepezil continuation groups, and no difference during the next three years (HR 0.89, 95% CI 0.58 to 1.35). The trial was not powered to show differences for time to nursing home placement.
Mortality
Five studies (598 participants) reported the number of deaths in the discontinuation and continuation groups over study duration (DOMINO AD Howard; GAL‐ITA‐2 Scarpini; Herrmann 2016; Hong 2018; Johannsen 2006) or within 30 days of the last intake of trial medication (GAL‐USA‐5 Gaudig). No evidence of difference was found (OR 0.75, 95% CI 0.36 to 1.55; 598 participants, 5 studies; Analysis 5.6). We assessed the overall quality of the evidence for this outcome as low, downgraded one level for imprecision and one for risk of bias.
5.6. Analysis.

Comparison 5: Safety, tolerability and dropout, Outcome 6: Deaths
Discussion
Summary of main results
Of the seven included trials, we excluded one from the quantitative syntheses (Hong 2018) as data reported did not differentiate between participants discontinuing a cholinesterase inhibitor (donepezil) and those discontinuing memantine. We were not able to include all six remaining studies in meta‐analyses for each of the outcomes, and due to the variation in outcome measures used for cognitive function, neuropsychiatric and functional status, standardised mean differences rather than mean differences were determined. The results and quality of evidence assessment for each outcome in the main comparison (discontinuation of ChEI versus continuation) are described in the Table 1; Table 2; Table 3 and Table 4.
Primary outcomes
We found low certainty evidence in the four pooled studies for the short‐term cognitive function outcome suggesting that discontinuing ChEIs may reduce cognitive function compared to continuing ChEI treatment for two months. We found very low certainty evidence in the three pooled studies for the medium‐term cognitive function outcome; we are therefore uncertain whether discontinuation reduces cognitive function compared to continuing ChEI treatment for 3 to 11 months. We conducted a sensitivity analysis omitting data from a study which excluded participants who showed the best response to donepezil: heterogeneity significantly reduced and the evidence suggested a detrimental effect of discontinuation on cognitive function in the medium term. We also found moderate certainty evidence from one study that discontinuing a ChEI probably reduces cognitive function compared to continuing treatment over the long term (12 months). Low certainty evidence from two pooled studies suggests that ChEI discontinuation may result in increased functional impairment over the short term, compared to continuing ChEI treatment, but we could not exclude no effect. In the medium term, due to very low certainty in the evidence, it remains uncertain whether discontinuation results in increased functional impairment. Moderate certainty evidence from one study demonstrated worse functional status after discontinuing treatment after 12 months. Low certainty evidence from two pooled studies and three pooled studies in the short and medium term respectively, suggested that discontinuation may result in increased neuropsychiatric symptoms compared to continuing ChEI treatment, although effects may be minimal. However, moderate certainty evidence from one study suggests that after 12 months, there may be little to no difference in neuropsychiatric status between groups who continue and discontinue ChEIs. There was low certainty evidence from four studies to suggest that discontinuation may have little or no effect, compared to continuing treatment, on dropouts due to lack of efficacy of medication or to deterioration in overall medical condition, or on numbers of adverse events or serious adverse events.
Secondary outcomes
We found low certainty evidence from five pooled studies that there was little or no difference in the number of deaths during the study period (four studies) or within 30 days of last intake of trial medication (one study) in ChEI discontinuation and continuation groups.
Findings in the study of antidementia drug (donepezil or memantine) discontinuation (Hong 2018) ‐ which were unsuitable for inclusion in the quantitative synthesis ‐ are similar to the findings from the meta‐analysis. Continuation of antidementia drugs over 12 weeks was reported not to be equivalent, and possibly to be superior to, discontinuation in terms of effects on general cognition as measured by the Baylor Profound Mental State Examination (BPMSE). However, there were no significant differences between antidementia drug continuation and antidementia drug discontinuation groups in changes from baseline in other cognitive, global, functional or neuropsychiatric outcomes (Mini‐Mental State Examination (MMSE), Clinician's Global Impression of Change (CGIC), Clinical Dementia Rating Sum of Boxes (CDR‐SB), Neuropsychiatric Inventory (NPI), Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living Inventory modified for severe dementia (ADCS‐ADL‐sev), the Barthel Index of Activities of Daily Living, or Funtional Assessment Staging (FAST). Study withdrawals due to adverse events and related to study procedures were more frequent in the antidementia drug discontinuation group. Agitation was the most frequent adverse event that led to study withdrawals in the discontinuation group. However, this must be interpreted with caution, particularly in light of the risk of performance bias in this study.
Overall completeness and applicability of evidence
There is a paucity of well‐designed randomised controlled trials examining withdrawal of cholinesterase inhibitors and memantine, in which continuation of the medication is the control intervention. This is demonstrated by the inclusion of only seven trials in this review, all of which examine withdrawal of cholinesterase inhibitors in patients with dementia due to Alzheimer's disease. Only one of these trials (Hong 2018) also considered withdrawal of memantine. However, the authors did not report results for each medication separately but rather considered both donepezil and memantine under the umbrella of 'antidementia drugs'. Participants in all the trials had dementia due to Alzheimer's disease with a range of severities from mild to very severe. None of the trials included have examined withdrawal of cholinesterase inhibitors in patients with dementia subtypes other than Alzheimer's disease (vascular dementia, mixed dementia, PDD or DLB).
We conducted separate meta‐analyses where possible for short‐term (up to 2 months) and medium‐term (3 to 11 months) cognitive, functional and neuropsychiatric outcomes. It was not possible to conduct meta‐analyses for long‐term (12 months or longer) outcomes due to the limited number of studies reporting these outcomes. We were not able to conduct separate meta‐analyses for short‐, medium‐ and long‐term outcomes relating to dropouts, adverse events, serious adverse events or deaths as the data available on these outcomes did not allow these distinctions to be determined. We therefore analysed these data across all trial durations, including follow‐up.
We prespecified subgroup analyses for severity of dementia (mild to moderate, and moderate to severe), but there were too few included studies to allow meaningful subgroup analysis. We were also unable to undertake subgroup analysis by duration of treatment prior to enrolment in the discontinuation trial or by method of discontinuation (abrupt versus tapered) due to the low numbers of included studies.
We found two further trials which are currently ongoing or have completed and results are yet to be published (NCT02248636; ISRCTN12134230). NCT02248636 recruited participants with advanced Alzheimer's disease and randomised participants into a discontinuation arm, in which they continued receiving half their cholinesterase inhibitor dose for two weeks and then received placebo, and a continuation arm, in which participants continued their previous dose of cholinesterase inhibitor (total study duration: 6 weeks). This trial has completed but the results have not been published to date. A further trial is comparing the effect of maintaining treatment with a ChEI (with or without memantine) to discontinuing treatment, at 1, 3, 6 and 12 months (ISRCTN12134230).
Quality of the evidence
The findings of this review must be interpreted in light of the methodological limitations and sources of bias inherent in the studies reporting these findings, which have been outlined in the text of the review and in Figure 2 and Figure 3, and form part of our assessment of the certainty of the evidence (Table 1; Table 2; Table 3; Table 4). All but two of the included studies were industry‐sponsored or industry‐funded.
Data on the effect of discontinuation of ChEIs and memantine remain very sparse. The certainty of evidence ranges from very low to moderate for the outcomes considered. The reasons for downgrading were risk of bias, imprecision, and inconsistency. We summarised the certainty of evidence for each outcome in each comparison in the Table 1; Table 2; Table 3; Table 4.
Potential biases in the review process
We performed sensitivity analyses to determine the impact of including Johannsen 2006, in which participants who showed the best response to donepezil were excluded. This decision was made after examination of the detail of the included studies which could introduce bias. However, we considered that the different population in this study could bias the pooled results (against continuation of treatment).
Agreements and disagreements with other studies or reviews
We are aware of a number of other systematic reviews on this topic, some of which considered RCTs and non‐randomised studies, while others focused on clinical practice guidelines and recommendations (O'Regan 2015; Reeve 2018; Renn 2018). The meta‐analysis by O'Regan and colleagues focused on randomised, double‐blind, placebo‐controlled studies of ChEI discontinuation in patients with Alzheimer's disease, and included five studies, all of which were included in our review (DOMINO AD Howard; GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Holmes 2004; Johannsen 2006). The authors converted ADAS‐Cog/11 scores for cognitive function employed in some studies into MMSE scores, and extracted NPI scores for three of the included studies. They calculated standardised mean differences using a fixed‐effects model and determined the clinical impact of the meta‐analyses by converting SMDs for each outcome into the respective test score units. Selected studies were analysed for reporting quality by two independent raters using the Newcastle‐Ottawa Scale and the Cochrane Collaboration's risk of bias tool. The authors judged each of the five included studies to have likelihood of high overall quality, though they did present in a supplementary table judgements on individual risk of bias items and reporting indicators. In so doing, they highlighted risk of biased participant selection in GAL‐ITA‐2 Scarpini, Holmes 2004 and Johannsen 2006, uncertain risk of bias in blinding of participants and personnel and of outcome assessment in GAL‐ITA‐2 Scarpini, GAL‐USA‐5 Gaudig, and Johannsen 2006, uncertainty risk of bias in gender proportion and other characteristics in GAL‐ITA‐2 Scarpini, and issues with reporting in all studies. O'Regan 2015 concluded that ChEI discontinuation may be associated with a statistically significant deterioration in cognition and neuropsychiatric symptoms in patients with Alzheimer's disease, and that these changes may be clinically relevant. Further, they reported that for the three studies that followed participants for longer than six weeks, the greatest rate of cognitive decline was observed in the six weeks following ChEI discontinuation regardless of the length of previous ChEI use. They concluded that the majority of the statistical difference in outcomes may be accounted for during the first six weeks of discontinuation, and suggested that when discontinuation is attempted, patients should be closely monitored for six weeks for significant declines in cognition or worsening of neuropsychiatric symptoms. Our review similarly suggests that discontinuing ChEIs may reduce cognitive function compared to continuing ChEI treatment in the short term (low certainty evidence). Although we were uncertain whether discontinuation reduces cognitive function in the medium term (3 to 11 months) (very low quality evidence), we did see a detrimental effect of ChEI withdrawal when we omitted data from a study which excluded those participants considered to have shown a prior good response to donepezil. Our review was consistent with O'Regan 2015, who found that discontinuation of ChEIs may result in increased neuropsychiatric symptoms compared to continuing ChEI treatment in the short term and the medium term. However, our assessment of certainty of evidence differed to O'Regan 2015, in which all included studies were considered likely to be of high overall quality; we considered the certainty of evidence for this outcome to be low.
A systematic review undertaken by Reeve 2018 considered randomised controlled trials, non‐randomised controlled studies, pilot or feasibility interventional studies, before‐after interventional studies, and observational, prospective or retrospective before and after studies. The search was undertaken in July 2016, and similar to O'Regan 2015, ADAS‐Cog/11 scores were converted to MMSE scores. The Cochrane risk of bias tool was used to assess risk of bias for the randomised controlled trials identified for inclusion, and GRADE recommendations were followed to determine the quality of the evidence. The authors included seven RCTs of continuation versus discontinuation of ChEIs and, similar to our review, did not identify any RCTs of continuation versus discontinuation of memantine. They included one study (Kertesz 2008) which was excluded from our review as it did not fall within our defined eligible dementia subtypes of Alzheimer's disease, vascular dementia, mixed dementia, DLB and PDD. The remaining six studies (DOMINO AD Howard; GAL‐ITA‐2 Scarpini; GAL‐USA‐5 Gaudig; Herrmann 2016; Holmes 2004; Johannsen 2006) were all included in our review. The authors' judgements of risk of bias were broadly similar to ours in risk of selection bias in random sequence generation and performance bias in blinding of participants and personnel and in blinding of outcome assessment. There were some differences in risk of bias judgements for allocation concealment, incomplete outcome data addressed, selective reporting and other bias. Similar to our findings, the GRADE summary of findings in Reeve 2018 reported low quality evidence of a significant greater decrease in cognitive function among those who discontinued versus those who continued, and low quality evidence of a non‐significant greater change in NPI scores in discontinuation versus continuation groups, expressed as standardised mean differences.
Renn and colleagues conducted a systematic review of professional, practice and clinical guidelines and textbook recommendations regarding ChEI discontinuation in Alzheimer's disease, published in the English language from 2005 onwards (Renn 2018). They included 16 practice guidelines (United States, Western Europe, Canada, Singapore, Australia and multinational), and 36 textbooks across a range of disciplines (dementia, neurology, psychiatry, geriatric psychiatry and neuropsychiatry, family medicine, geriatrics, and pharmacology). The authors concluded that there was a lack of informative clinical trial data to provide an evidence base for practice, and considerable variability and inconsistency across practice guidelines and recommendations regarding clinical findings or situations which warrant ChEI discontinuation. They therefore argued against the use of a formulaic approach, and advocated individualised decisions about stopping ChEIs, based on patients' and families' preferences and values, and a balanced discussion of potential risks and benefits to discontinuation. They called for rigorous studies of the effects of discontinuation to inform clearer practice guidelines, which would in turn assist clinicians in making informed deprescribing decisions, in agreement with the conclusions of our review.
Authors' conclusions
Implications for practice.
There is still clinical uncertainty about the effects of withdrawing cholinesterase inhibitors due to the limited evidence available in the literature. We found low certainty evidence suggesting that discontinuing ChEIs may result in worse cognitive function and more functional impairment than continuing ChEI treatment in the short term (up to 2 months). Evidence for a medium‐term effect on cognition over 3 to 11 months was even less certain, but there may also be a detrimental effect on this timescale of withdrawing ChEI treatment among participants who had previously responded to a ChEI. We also found low certainty evidence suggesting that, compared to continuing treatment, discontinuation may result in more neuropsychiatric symptoms in the short term and medium term. There was low certainty evidence to suggest that discontinuation may have little or no effect on dropouts due to lack of efficacy of medication or to deterioration in overall medical condition, on numbers of adverse events (any) and serious adverse events, or on numbers of deaths. Therefore, although certainty is low, the small body of evidence is consistent in suggesting that discontinuing ChEIs may be associated with worse outcomes than continuing treatment at least over the short term (up to 2 months), indicating that clinicians should approach discontinuation of ChEIs with caution. If withdrawal is to be attempted, careful re‐evaluation of the cognitive, functional and neuropsychiatric status of the patient is advisable. There is currently no available evidence to influence clinical practice regarding withdrawal or continuation of memantine. In making decisions regarding discontinuing these drugs, clinicians should consider the evidence from existing trials, or the lack thereof, in combination with other important individualised patient‐centred considerations, including patient and carer preferences and values, changing goals of care as individuals approach the end of life, and potential adverse events.
Implications for research.
The findings of this Cochrane review highlight the lack of high certainty evidence available in the literature regarding withdrawal or continuation of cholinesterase inhibitors or memantine, or both, in people with dementia. The available studies have methodological limitations and there is imprecision and some inconsistency in the evidence as a whole. There is therefore a pressing need for more well‐designed, randomised, placebo‐controlled trials examining withdrawal of cholinesterase inhibitors and memantine, in which continuation of the medication is the control intervention. Evidence is needed in people with a range of severities of dementia and in community and institutional care settings. Studies should examine cognitive function, functional ability, neuropsychiatric status, quality of life and effects on caregivers. They should include both short‐term and longer‐term adverse effects. Economic analysis of withdrawal versus continuation, including a measure of impact on rates of institutionalisation of community‐dwelling patients, would be valuable.
History
Protocol first published: Issue 4, 2011 Review first published: Issue 2, 2021
Acknowledgements
We would like to acknowledge the valuable input of Anna Noel‐Storr (Dementia and Cognitive Improvement Group) and Alexandra McIlroy (Queen's University Belfast) into the development of the search strategy. We would also like to thank Professor Martin Dempster (Queen's University Belfast) for his assistance with the statistical aspects of the review. Thanks also to peer reviewers Michal Rolinski and Dallas Seitz, and consumer reviewers Gordon Kennedy and Catherine Hofstetter for their feedback and comments. We would also like to thank Dr Jenny McCleery (Dementia and Cognitive Improvement Group) for her input at the editorial review stage, and Sue Marcus for her assistance as Managing Editor of the Dementia and Cognitive Improvement Group.
Appendices
Appendix 1. Sources searched and search strategies used
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 17 October 2020] |
Keyword search: donepezil OR galantamine OR rivastigmine OR tacrine OR memantine | Dec 2012: 531 Jan 2014: 23 May 2015: 0 Oct 2015: 9 Jul 2016: 11 May 2017: 0 Dec 2017: 0 Oct 2018: 1 Oct 2020: 128 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 17 October 2020] |
1. exp Dementia/ 2. Wernicke Encephalopathy/ 3. Delirium, Dementia, Amnestic, Cognitive Disorders/ 4. dement*.mp. 5. (alzheimer* or AD).mp. 6. (chronic adj2 cerebrovascular).mp. 7. ("organic brain disease" or "organic brain syndrome").mp. 8. ("normal pressure hydrocephalus" and "shunt*").mp. 9. "benign senescent forgetfulness".mp. 10. (cerebr* adj2 deteriorat*).mp. 11. (cerebr* adj2 insufficient*).mp. 12. (VaD or VCI or "vascular cognitive impair*").mp. 13. or/1‐12 14. exp *Cholinesterase Inhibitors/ 15. "cholinesterase inhibitor*".mp. 16. "acetylcholinesterase inhibitor*".mp. 17. donepezil*.mp. 18. aricept*.mp. 19. E2020.mp. 20. donezepil.mp. 21. exp *Galantamine/ 22. galantamin*.mp. 23. galanthamin*.mp. 24. nivalin*.mp. 25. razadyne*.mp. 26. reminyl*.mp. 27. rivastigmin*.mp. 28. exelon*.mp. 29. "SDZ ENA 713".mp. 30. exp *Tacrine/ 31. tacrin*.mp. 32. cognex*.mp. 33. exp *Memantine/ 34. memantin*.mp. 35. axura*.mp. 36. akatinol*.mp. 37. namenda*.mp. 38. ebixa*.mp. 39. abixa*.mp. 40. memox*.mp. 41. memary*.mp. 42. memani*.mp. 43. ("D‐145" or DMAA or "DRG‐0267" or DRG0267).mp. 44. or/14‐43 45. withdraw*.mp. 46. cessat*.mp. 47. (reduce* or reducing* or reduct*).mp. 48. taper*.mp. 49. stop*.mp. 50. "carr* on".mp. 51. continu*.mp. 52. (maintain* or maintenance).mp. 53. "come off".mp. 54. remain*.mp. 55. or/45‐54 56. 13 and 44 and 55 57. randomised controlled trial.pt. 58. controlled clinical trial.pt. 59. random*.ab. 60. placebo.ab. 61. drug therapy.fs. 62. trial.ab. 63. groups.ab. 64. or/57‐63 65. (animals not (humans and animals)).sh. 66. 64 not 65 67. 56 and 66 |
Dec 2012: 1350 Jan 2014: 95 May 2015: 68 Oct 2015: 80 Jul 2016: 77 May 2017: 155 Dec 2017: 99 Oct 2018: 154 Nov 2019: 163 Oct 2020: 90 |
| 3. EMBASE 1980‐2011 week 21 (Ovid SP) [Date of most recent search: 17 October 2020] |
1. exp dementia/ 2. dement*.mp. 3. (alzheimer* or AD).mp. 4. (chronic adj2 cerebrovascular).mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. "benign senescent forgetfulness".mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebr* adj2 insufficient*).mp. 9. (VaD or VCI or "vascular cognitive impair*").mp. 10. or/1‐9 11. exp *cholinesterase inhibitor/ 12. "cholinesterase inhibitor*".mp. 13. "acetylcholinesterase inhibitor*".mp. 14. donepezil/ 15. donepezil*.mp. 16. aricept*.mp. 17. E2020.mp. 18. donezepil.mp. 19. galantamine/ 20. galantamin*.mp. 21. galanthamin*.mp. 22. nivalin*.mp. 23. razadyne*.mp. 24. reminyl*.mp. 25. rivastigmine/ 26. rivastigmin*.mp. 27. exelon*.mp. 28. "SDZ ENA 713".mp. 29. tacrine/ 30. tacrin*.mp. 31. cognex*.mp. 32. memantine/ 33. memantin*.mp. 34. axura*.mp. 35. akatinol*.mp. 36. namenda*.mp. 37. ebixa*.mp. 38. abixa*.mp. 39. memox*.mp. 40. memary*.mp. 41. memani*.mp. 42. ("D‐145" or DMAA or "DRG‐0267" or DRG0267).mp. 43. or/11‐42 44. withdraw*.mp. 45. cessat*.mp. 46. (reduce* or reducing* or reduct*).mp. 47. taper*.mp. 48. stop*.mp. 49. "carr* on".mp. 50. continu*.mp. 51. (maintain* or maintenance).mp. 52. "come off".mp. 53. remain*.mp. 54. or/44‐53 55. 10 and 43 and 54 56. randomised controlled trial/ 57. controlled clinical trial/ 58. random*.ab. 59. placebo.ab. 60. trial.ab. 61. groups.ab. 62. or/56‐61 63. 55 and 62 |
Dec 2012: 1607 Jan 2014: 171 May 2015: 233 Oct 2015: 133 Jul 2016: 93 May 2017: 170 Dec 2017: 118 Oct 2018: 417 Nov 2019: 311 Oct 2020: 123 |
| 4. PSYCINFO 1806‐May week 5 2011 (Ovid SP) [Date of most recent search: 17 October 2020] |
1. exp Dementia/ 2. dement*.mp. 3. (alzheimer* or AD).mp. 4. (chronic adj2 cerebrovascular).mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. (cerebr* adj2 deteriorat*).mp. 7. (cerebr* adj2 insufficient*).mp. 8. (VaD or VCI or "vascular cognitive impair*").mp. 9. or/1‐8 10. exp *Cholinesterase Inhibitors/ 11. "cholinesterase inhibitor*".mp. 12. "acetylcholinesterase inhibitor*".mp. 13. donepezil*.mp. 14. aricept*.mp. 15. E2020.mp. 16. donezepil.mp. 17. exp Galanthamine/ 18. galantamin*.mp. 19. galanthamin*.mp. 20. nivalin*.mp. 21. razadyne*.mp. 22. reminyl*.mp. 23. rivastigmin*.mp. 24. exelon*.mp. 25. "SDZ ENA 713".mp. 26. tacrin*.mp. 27. cognex*.mp. 28. memantin*.mp. 29. axura*.mp. 30. akatinol*.mp. 31. namenda*.mp. 32. ebixa*.mp. 33. abixa*.mp. 34. memox*.mp. 35. memary*.mp. 36. memani*.mp. 37. ("D‐145" or DMAA or "DRG‐0267" or DRG0267).mp. 38. or/10‐37 39. withdraw*.mp. 40. cessat*.mp. 41. (reduce* or reducing* or reduct*).mp. 42. taper*.mp. 43. stop*.mp. 44. "carr* on".mp. 45. continu*.mp. 46. (maintain* or maintenance).mp. 47. "come off".mp. 48. remain*.mp. 49. or/39‐48 50. 9 and 38 and 49 51. exp Clinical Trials/ 52. random*.ab. 53. placebo.ab. 54. trial.ab. 55. groups.ab. 56. or/51‐55 57. 50 and 56 |
Dec 2012: 399 Jan 2014: 29 May 2015: 44 Oct 2015: 18 Jul 2016: 14 May 2017: 18 Dec 2017: 7 Oct 2018: 18 Nov 2019: 28 Oct 2020: 21 |
| 5. CINAHL (EBSCOhost) [Date of most recent search: 17 October 2020] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 |
Dec 2012: 256 Jan 2014: 8 May 2015: 11 Oct 2015: 0 Jul 2016: 2 May 2017: 7 Dec 2017: 4 Oct 2018: 13 Nov 2019: 37 Oct 2020: 17 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] [Date of most recent search: 17 October 2020] |
Topic=(dement* OR alzheimer* OR AD OR VCI OR VaD OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL) AND Topic=(donepezil OR galantamine OR glanthamin* OR rivastigmine OR tacrine OR memantine) AND Topic=(withdraw* OR reduce OR reduction OR reducing OR taper* OR cessat* OR contin* OR "carr* on") AND Topic=(random* OR trial OR placebo OR "double‐blind*" OR "single‐blind*" OR RCT OR "control group*") Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. |
Dec 2012: 861 Jan 2014: 49 May 2015: 66 Oct 2015: 47 Jul 2016: 103 May 2017: 76 Dec 2017: 46 Oct 2018: 86 Nov 2019: 87 Oct 2020: 31 |
| 7. LILACS (BIREME) [Date of most recent search: 17 October 2020] |
donepezil OR rivastigmin$ OR galantamin$ OR galanthamin$ OR tacrine OR memantin$ [Words] and random$ OR placeb$ OR trial OR study [Words] | Dec 2012: 14 Jan 2014: 0 May 2015: 0 Oct 2015: 0 Jul 2016: 1 May 2017: 3 Dec 2017: 0 Oct 2018: 1 Nov 2019: 5 Oct 2020: 0 |
| 8. CENTRAL (The Cochrane Library) (Issue 1 of 4, 2011) [Date of most recent search: 17 October 2020] |
#1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 MeSH descriptor Cholinesterase Inhibitors explode all trees #22 donepezil OR galanthamin* OR rivastigmine OR tacrine OR memantine OR galantamine #23 aricept* OR E2020 OR nivalin* OR razadyne* OR reminyl* OR exelon* OR "SDZ ENA 713" OR cognex* OR axura* OR akatinol* OR namenda* OR ebixa* OR abixa* OR memox* OR memary* OR memani* OR "D‐145" OR DMAA OR "DRG‐0267" OR DRG0267 #24 (#21 OR #22 OR #23) #25 withdraw* OR cessat* OR reduce* OR reducing* OR reduct* OR taper* OR stop* OR "com* off" #26 "carr* on" OR continu* OR maintain* OR maintenance OR remain* #27 (#25 OR #26) #28 (#20 AND #24 AND #27) |
Dec 2012: 330 Jan 2014: 1 May 2015: 37 Oct 2015: 13 Jul 2016: 38 May 2017: 70 Dec 2017: 84 Oct 2018: 127 Nov 2019: 151 Oct 2020: 57 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 17 October 2020] |
#1 Interventional Studies | dementia OR alzheimer OR alzheimer's OR AD | donepezil OR E2020 OR aricept OR rivastigmine OR exelon OR tacrine OR nivalin OR galantamine OR galanthamine OR cognex OR razadyne OR reminyl #2 Interventional Studies | dementia OR alzheimer OR alzheimer's OR AD | memantine OR axura OR akatinol OR namenda OR ebixa OR memox OR memary OR memani OR D‐145 OR DMAA OR DRG‐0267 |
Dec 2012: 259 Jan 2014: 17 May 2015: 63 Oct 2015: 0 Jul 2016: 0 May 2017: 29 Dec 2017: 14 Oct 2018: 20 Nov 2019: 24 Oct 2020: 6 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 17 October 2020] |
#1 Interventional Studies | dementia OR alzheimer OR alzheimer's OR AD | donepezil OR E2020 OR aricept OR rivastigmine OR exelon OR tacrine OR nivalin OR galantamine OR galanthamine OR cognex OR razadyne OR reminyl #2 Interventional Studies | dementia OR alzheimer OR alzheimer's OR AD | memantine OR axura OR akatinol OR namenda OR ebixa OR memox OR memary OR memani OR D‐145 OR DMAA OR DRG‐0267 |
Jan 2014: 35 May 2015: 8 Oct 2015: 9 Jul 2016: 14 May 2017: 49 Dec 2017: 73 Oct 2018: 151 Nov 2019: 202 Oct 2020: 0 |
| TOTAL before de‐duplication | 9237 Nov 2019: 1096 Oct 2020: 473 TOTAL: 10,806 |
|
| TOTAL after de‐duplication and first‐assessment by the CDCIG Information Specialists | 890 Nov 2019: 880 Oct 2020: 40 TOTAL: 1810 |
|
| TOTAL full‐text screening | 42 | |
Appendix 2. Assessment scales used in included studies
| Outcomes measured | Assessment scales |
| Cognitive | The Alzheimer's DIsease Assessment Scale‐Cognitive Subscale (ADAS‐Cog/11) Rosen 1984 The Alzheimer's Disease Assessment Scale–Cognitive Subscale plus Concentration and Distractability and Delayed Word Recall items (ADAS‐Cog/13) Mohs 1997 The non‐memory Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS‐Cog/10) The memory Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS‐Cog/mem) Baylor Profound Mental State Examination (BPSME) Doody 1999 Mini‐Mental State Examination (MMSE) Folstein 1975 Standardised Mini‐Mental State Examination (SMMSE) Molloy 1991 Severe Impairment Battery (SIB) Saxton 1990 |
| Functional | Alzheimer's Disease Cooperative Study‐Activities of Daily Living Inventory, modified for severe dementia (ADCS‐ADL‐sev) Galasko 2005 Barthel Index of Activities of Daily Living Wade 1988 Bristol Activities of Daily Living Scale (BADLS) Bucks 1996 Disability Assessment for Dementia (DAD) Gelinas 1999 Functional Assessment Staging scale (FAST) Reisberg 1988 |
| Neuropsychiatric | Apathy Evaluation Scale (AES) Marin 1991
Cohen‐Mansfield Agitation Inventory (CMAI) Cohen‐Mansfield 1988 Cornell Depression Scale for Dementia (CDSD) Alexopoulos 1988 Neuropsychiatric Inventory (NPI) Cummings 1994 Neuropsychiatric Inventory‐Nursing Home version (NPI‐NH) Iverson 2002 Neuropsychiatry Inventory caregiver Distress (NPI‐D) Kaufer 1998 |
| Global | Clinical Interview Based Impression of Changes‐Plus Caregiver Input (CIBIC‐Plus) Schneider 1997 Clinician's Global Impression (CGI) Guy 1976 Clinician's Global Impression of Change (CGI‐C) Guy 1976 Clinical Dementia Rating Sum of Boxes score (CDR‐SB) Morris 1993 |
| Quality of Life | General Health Questionnaire (GHQ‐12) Goldberg 1997 Dementia Quality of Life Proxy Measure (DEMQOL‐Proxy) Smith 2005 EuroQol‐5 Dimension (EuroQoL EQ‐5D) Quality of Life in late stage Dementia (QUALID) Weiner 2000 |
| Adverse events, safety and tolerability | Udvaig for Kliniske Undersogelser Seide Effect Rating Scale (UKU SERS Clin) Lingjaerde 1987 |
| Institutionalisation | Client Service Receipt Inventory (CSRI) Beecham 2001 |
Data and analyses
Comparison 1. Cognitive function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cognitive function (change from baseline, short term) | 4 | 344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.64, ‐0.21] |
| 1.2 Cognitive function (change from baseline, medium term) | 3 | 411 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.87, 0.07] |
| 1.3 Cognitive function (change in SMMSE from baseline, long term) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐3.43, ‐0.75] |
| 1.4 Cognitive function (change in BPMSE from baseline, medium term) for Hong 2018 | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.18, 0.38] |
| 1.5 Cognitive function (change in MMSE from baseline, medium term) for Hong 2018 | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.09, 1.29] |
Comparison 2. Functional status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Functional status (change from baseline, short term) | 2 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.54, 0.04] |
| 2.2 Functional status (change from baseline, medium term) | 2 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.74, ‐0.01] |
| 2.3 Functional status (change from baseline, long term) | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐3.38 [‐6.67, ‐0.10] |
Comparison 3. Neuropsychiatric status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Neuropsychiatric status (change from baseline, short term) | 2 | 136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.82, ‐0.13] |
| 3.2 Neuropsychiatric status (change from baseline, medium term) | 3 | 410 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.47, ‐0.08] |
| 3.3 Neuropsychiatric status (change in NPI from baseline, long term) | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐8.42, 6.68] |
Comparison 4. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Quality of life (change in QUALID from baseline, short term) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.08, 2.88] |
Comparison 5. Safety, tolerability and dropout.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Total dropout during trial and follow‐up | 6 | 694 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.17] |
| 5.2 Dropout due to adverse event | 6 | 694 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.42, 1.61] |
| 5.3 Dropout due to lack of efficacy of trial medication or deterioration in overall medical condition | 4 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.84, 2.76] |
| 5.4 Adverse events (any) | 4 | 446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.27] |
| 5.5 Serious adverse events (SAEs) | 4 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.46, 1.39] |
| 5.6 Deaths | 5 | 598 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DOMINO AD Howard.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, placebo‐controlled, clinical trial with a two‐by‐two factorial design | |
| Participants |
Setting: UK, 15 centres between February 2008 and March 2010, with the last participant completing follow‐up in April 2014. Sample size: 295 patients in four treatment arms, of which two are relevant to this review (146 patients; 48 male and 98 female) Age: mean 77.1 years for all participants, 77.5 years for patients in two arms relevant to review Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Patients were randomly assigned to one of four treatments, two of which are relevant to this review:
|
|
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Service use, informal care and other aspects of accommodation and care
4. Health‐related quality of life
5. Neuropsychiatric symptoms
6. Institutionalisation (defined as permanent transition to a care home, continuing care unit or hospital)
7. Safety and tolerability
Outcomes were measured at randomisation, 6, 18, 30 and 52 weeks, with the exception of GHQ‐12 which was not assessed at 18 weeks, and DEMQOL‐Proxy, which was not assessed at 6 weeks or 30 weeks. |
|
| Source of funding | UK Medical Research Council (MRC) and the Alzheimer's Society. Pfizer‐Eisai and Lundbeck donated supplies of the drugs. | |
| Declaration of interest | Pfizer‐Eisai and Lundbeck had no involvement in the design or conduct of the study or the analysis or the reporting of the data | |
| Notes | BADLS: Bristol Activities of Daily Living Scale DEMQOL‐Proxy: Dementia Quality of Life Proxy measure EuroQol EQ‐5D: EuroQol‐5 Dimension GHQ‐12: twelve‐item General Health Questionnaire NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association NPI: Neuropsychiatric Inventory SMMSE: Standardised Mini‐Mental State Examination |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment assignments were made (by telephone) by the UK Medical Research Council Clinical Trials Unit with the use of randomised minimisation". Full details were given in the Supplementary Appendix. |
| Allocation concealment (selection bias) | Low risk | "[To] maintain concealment of treatment assignments, the first 80 participants were assigned with the use of a prepared list of simple randomised assignments". Further details were provided in the Supplementary Appendix. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain blinding, identical placebos for both memantine and donepezil hydrochloride were co‐administered as indicated by randomisation. Donepezil and donepezil placebo, however, appeared different from memantine and memantine placebo. All trial staff, patients and carers were blinded, but statisticians and randomising staff were unblinded (details given in the Supplementary Appendix) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Figure 1 shows the number of patients who were enrolled, were assigned to a study group, and completed follow‐up". Figure 1 also included the number of patients in each of the study groups who were included in the primary intention‐to‐treat analysis. "Unless otherwise specified, we performed the analyses on data from all patients who underwent randomisation and who received at least one dose of study drug, applying the principle of intention to treat as much as was practically possible, given any missing data". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for the co‐primary outcomes (SMMSE scores and BADLS scores) were available for 99% of participants randomised. A modified intention‐to‐treat analysis (mITT) was performed on participants who were randomised and received at least one dose of the study drug (i.e. excluded eligible participants post‐randomisation who did not start assigned treatment). Total exclusion post‐randomisation was 1.4%, and is unlikely to affect the outcome. |
| Selective reporting (reporting bias) | Low risk | Three secondary outcome measures specified in the protocol ‐ the Client Service Receipt Inventory (CSRI, which describes service use, informal care and other aspects of accommodation and care pertinent to the costing of interventions and their implications), the EuroQol EQ‐D measure of health‐related quality of life and institutionalisation ‐ were not reported. |
| Other bias | Low risk | Appears to be free of other sources of bias |
GAL‐ITA‐2 Scarpini.
| Study characteristics | ||
| Methods | Multicentre randomised double‐blind placebo‐controlled withdrawal trial | |
| Participants |
Setting: Italy, 29 study sites, between July 2001 and November 2005 Sample size: 139 patients, 56 male and 83 female Age: 74.6 years Inclusion criteria for double blind phase
Exclusion criteria
|
|
| Interventions |
Patients were titrated from 4 mg twice daily (8 mg/day) of immediate‐release galantamine to 8 mg twice daily (16 mg/day) during the first 4 weeks of the 12‐month open‐label phase. |
|
| Outcomes | 1. Cognitive function
2. Time to dropout for lack of efficacy, defined as subjective impression of caregiver or general practitioner 3. Time to dropout for any reason 4. Global measure of change in cognition, function and neuropsychiatric status
5. Measure of disability
6. Safety and tolerability
All outcomes were assessed at the start of the double‐blind continuation or withdrawal phase and at 6‐month intervals thereafter for 24 months |
|
| Source of funding | "Trial medication was provided by Janssen Cilag SpA". "Janssen‐Cilag EMEA provided funding for this manuscript and was involved in the design and review of the manuscript". | |
| Declaration of interest | "Trial medication was provided by Janssen Cilag SpA". "Janssen‐Cilag EMEA provided funding for this manuscript and was involved in the design and review of the manuscript, and approved it with regard to consistency with the scientific and safety information of Reminyl® galantamine. Ute Richarz, Maren Gaudig, Marina Adami and Barbara Schäuble are employees and stockholders of Johnson and Johnson". (Janssen Cilag is part of the Johnson and Johnson family of companies) | |
| Notes | AD: Alzheimer's disease ADAS‐Cog/11: The Alzheimer's Disease Assessment Scale–Cognitive Subscale CIBIC‐Plus:Clinician's Interview‐Based Impression of Change‐Plus DAD: Disability Assessment for Dementia scale NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At the beginning of the open label phase, subjects were given a subject number corresponding to a computer generated randomization code. Subjects were randomly allocated to treatment 1 : 1 (galantamine: placebo)". The allocation sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | "The randomization code was generated by Janssen Pharmaceutica, Beerse, Belgium. Randomization was balanced between the centers. Each study center received trial medication in blocks of 4 and assigned subject numbers consecutively starting with the lowest available number". Randomisation was undertaken at the beginning of the open‐label phase of the study rather than at the start of the double‐blind phase; the authors acknowledged that it "was an unusual design feature that randomization took place at the beginning of the open label phase rather than the double blind phase; however, this does not appear to have negatively affected the study, and the subjects were still well balanced". The allocation sequence was generated by an independent party. Allocation was conducted at the study site according to the randomisation schedule in sequence with the subject number. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Trial medication was provided by Janssen Cilag SpA....It was not possible to distinguish boxes containing active drug from those containing placebo. Galantamine and placebo tablets were identical in appearance, taste and smell". It was not explicitly stated that participants, carers and trial personnel were blind to assigned intervention. There is no information on whether the randomisation code generated by the pharmaceutical company was available to on‐site personnel. The use of central randomisation and matching placebo might suggest the blinding of participants and personnel. Frequency of side effects was high but similar between groups and would not have caused unblinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no information reported on whether outcome assessors were blind to the treatment. There is no information on whether th randomisation code generated by the pharmaceutical company was available to on‐site personnel. The use of identical placebo might suggest the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 126 of the 139 participants (69 patients of the 76 allocated to galantamine and 57 of the 63 allocated to placebo) who entered the double‐blind phase were included in the final efficacy analysis. This was defined as the ITT population. A modified intention‐to‐treat (mITT) analysis was performed on patients who were randomised and received at least one dose of the study drug and who completed at least one ADAS‐Cog/11 score post‐randomisation. Data from the primary outcome (time to dropout due to deterioration or increase in ADAS‐Cog/11 score of ≥ 4 points) were available for 40% (55/139) of randomised participants (completers). Missing data differed between groups: 53% (40/76) galantamine vs. 70% (44/63) placebo. Discontinuations and dropouts from the study could be related to participants' health status, as the main reason for dropouts was poor efficacy. Although not explicitly stated, data for the 71 participants who discontinued or did not complete the double‐blind phase but had at least one post‐randomisation ADAS‐Cog/11 assessment, had been imputed and therefore were included in the mITT analysis. Total exclusion post‐randomisation was 60% (84/139) ‐ i.e. completers, but 9% (13/139) were excluded from the ITT analysis because they did not have at least one ADAS‐Cog/11 assessment post‐randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The clinical study report detailed change over time in DAD scores as a secondary efficacy measure. However, this was not defined as a secondary outcome by the study authors, and scores were not reported. CIBIC‐Plus scores were not reported; however, the authors did provide a summary statement that there was no difference in mean values between treatment groups. Neither the primary endpoint defined by the authors, time to deterioration (defined as deterioration in the ADAS‐Cog/11 score of ≥ 4 points relative to the start of the double‐blind phase, and confirmed after one month) nor deterioration in the ADAS‐Cog/11 scale were reported. The authors acknowledged that the study was not sufficiently powered for the ADAS‐Cog/11 survival analysis and stated that "Many subjects dropped out before they reached a difference in ADAS‐cog/11 ≥ 4; only 27 subjects dropped out when looking at measured cognitive decline (difference in ADAS‐cog ≥ 4)". |
| Other bias | Unclear risk | "Trial medication was provided by Janssen Cilag SpA". "Janssen‐Cilag EMEA provided funding for this manuscript and was involved in the design and review of the manuscript, and approved it with regard to consistency with the scientific and safety information of Reminyl® galantamine. Ute Richarz, Maren Gaudig, Marina Adami and Barbara Schäuble are employees and stockholders of Johnson and Johnson" (Janssen Cilag is part of the Johnson and Johnson family of companies). |
GAL‐USA‐5 Gaudig.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, 6‐week, parallel arm withdrawal study involving patients who had completed a preceding randomised multicentre clinical trial GAL‐INT‐2 | |
| Participants |
Setting: USA; 15 sites, conducted between December 1997 and May 1998 Sample size: 118 participants (49 male, 69 female) Age: 75.1 ± 1.01 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | 1. Cognitive function
2. Safety and tolerability
Efficacy outcomes (cognitive function) were measured at the initial visit and at week 6. |
|
| Source of funding | Janssen‐Cilag EMEA, a division of Janssen Pharmaceutica NV | |
| Declaration of interest | Withdrawal study was sponsored by Janssen‐Cilag EMEA, a division of Janssen Pharmaceutica NV. Post hoc analyses were funded by Janssen Pharmaceutica NV. Assistance with writing the manuscript (Gaudig 2011) was provided by Bioscript Stirling Ltd, UK, and funded by Janssen EMEA Medical Affairs, Beerse, Belgium. | |
| Notes | ADAS‐Cog/11: The Alzheimer's Disease Assessment Scale–Cognitive Subscale ADAS‐Cog/13: The Alzheimer's Disease Assessment Scale–Cognitive Subscale plus Concentration and Distractability and Delayed Word Recall items ADAS‐Cog/10: The non‐memory Alzheimer's Disease Assessment Scale–Cognitive Subscale ADAS‐Cog/mem: The memory Alzheimer's Disease Assessment Scale–Cognitive Subscale ECG: Electrocardiogram MMSE: Mini‐Mental State Examination NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients entering GAL‐INT‐2 were randomised to receive galantamine or placebo in a 2:1 ratio using a computer generated code. Of the 111 patients who went on to complete GAL‐USA‐5, 70 patients were included in the withdrawal study: 31 were assigned to continue galantamine 24 mg/day or 32 mg/day, and 39 patients were switched from galantamine to placebo, representing a 1:1 ratio for galantamine:placebo. The Clinical Research Report indicated that 28 patients were randomised out of sequence. |
| Allocation concealment (selection bias) | Low risk | Assignments were kept in sealed, opaque envelopes until the point of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The medication was formulated in tablets that were identical in appearance, taste, and smell, and which contained either no active ingredient, or 12 mg or 16 mg of galantamine. For each patient, the investigator was provided with a blinded code containing details of the treatment in the withdrawal phase. This code could only be broken in case of an emergency where further treatment of the patient depended on knowledge of the trial medication he or she had been receiving. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear who the assessors were, or whether they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Patients with missing data for Week 6 were not included in the analysis...3 in the GAL/PLA group, and 1 in the GAL/GAL group, did not have any ADAS data collected at the initial visit...1 in the GAL/PLA group, and 2 in the GAL/GAL group did not have ADAS data collected at week 6." Of the 71 patients randomised (GAL/PLA N = 39, GAL/GAL N = 32), 4 did not have outcome data at the initial visit, and another 3 did not have outcome data at week 6. Therefore, data for the primary outcome (ADAS‐Cog/11) were available for 90% (64/71) of randomised participants (completers). |
| Selective reporting (reporting bias) | Unclear risk | Efficacy results were presented for comparisons with the baseline of the parent trial GAL‐INT‐2 rather than the start of the withdrawal study. In the post hoc analyses undertaken, changes in ADAS‐Cog/11 score were evaluated over time, from the baseline of the 3‐month parent trial to the end of the 6‐week withdrawal study. Analyses were in accordance with prespecified plans in the Statistical Analysis section of the publication. However, information in the published paper differs from that in the clinical study report. In the clinical study report, it is stated that both Traditional Division of Neuropharmacological Drug Product with Last Observation Carried Forward (Traditional DNDP‐LOCF) and Observed Case (OC) analyses were performed, but in the published paper, it is stated that only OC analyses were performed, and DNDP‐LOCF analyses were not reported. |
| Other bias | Unclear risk | The post hoc analyses were funded by Janssen Pharmaceutica NV, the drug company which manufactures Reminyl® galantamine. Assistance with the writing of the manuscript was provided by a medical‐writing company and funded by Janssen EMEA Medical Affairs, Beerse, Belgium. |
Herrmann 2016.
| Study characteristics | ||
| Methods | 8‐week randomised, double‐blind, placebo‐controlled pilot trial | |
| Participants |
Setting: 2 long‐term care facilities in Canada between July 2010 and August 2015 Sample size: 40 patients were randomised to ChEI continuation (N = 21) or placebo (N = 19); 32 male and 8 female Age: mean 89.2 years Inclusion criteria
Exclusion criteria
|
|
| Interventions | Patients were randomised with a 1:1 ratio balanced by ChEI to continue receiving their ChEI (continuation) at their current dose, or to receive an identical‐looking placebo substitution. Patients randomised to placebo were tapered off their ChEI for the first 2 weeks and continued on placebo for the remaining 6 weeks. | |
| Outcomes | 1. Clinician's global impression
2. Cognition
3. Side effects
4. Neuropsychiatric status
5. Apathy
6. Function
7. Quality of life
8. Safety
9. Adverse events, measured at 2, 4 and 8 weeks |
|
| Source of funding | Alzheimer's Society of Canada and internal funding, Sunnybrook Health Sciences Centre, Toronto, Canada | |
| Declaration of interest | None declared | |
| Notes | AD: Alzheimer's Disease ADCS‐ADL‐sev: Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory modified for severe dementia AES: Apathy Evaluation Scale CGI: Clinical Global Impressions scale CGI‐C: Clinical Global Impression of Change scale ChEI: Cholinesterase Inhibitor CMAI: Cohen Mansfield Agitation Inventory NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association NPI‐NH: Neuropsychiatric Inventory‐Nursing Home version QUALID:Quality of Life in Late‐Stage Dementia scale SIB: Severe Impairment Battery SMMSE: Standardised Mini‐Mental State Examination UKU‐SERS: Kliniske Undersøgelser (UKU) Side Effects Rating Scale |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was completed independently by the pharmacy at Sunnybrook Health Sciences Center in permuted blocks using a computer generated code". |
| Allocation concealment (selection bias) | Unclear risk | Methods used to conceal the allocation sequence were not described; therefore it was not possible to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, family members, nurses, clinicians, outcome assessors, and investigators were unaware of treatment group assignments or block size". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, family members, nurses, clinicians, outcome assessors, and investigators were unaware of treatment group assignments or block size". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 patients were randomised to ChEI continuation (N = 21) or placebo (N = 19), and all were included in the analysis. The authors reported that all baseline characteristics were comparable, with the exception that patients randomised to ChEI continuation had lower SMMSE scores (P = 0.03). Of the 40 randomised patients, 33 patients (82.5%) completed the study (85.7% continuation, N = 18; 78.9% placebo, N = 15); 1 died prior to study completion (unrelated to study; placebo); 1 was terminated early because of a serious adverse effect (continuation); 1 was lost to follow‐up (continuation); 1 had clinically significant cognitive decline (placebo); and 3 had clinically significant neuropsychiatric deterioration (2 placebo, 1 continuation). Data for the primary outcome (CGI‐C; worsening, improvement/no change) were available for 83% of participants (86% continuation, 79% placebo). Overall, the number of participants with the event of interest (worsening on the CGI‐C) was 13 (13/40 = 33%), while the number of participants with missing data was 7 (7/40 = 18%). There may be possible bias because: 1. the proportion of missing data in the 2 groups is different (14% continuation, 21% placebo), and 2. the number of participants with the event of interest is not relatively greater in comparison to the number with missing data (N = 13 vs. N = 7). There is no evidence in analysis methods that correct for bias, or sensitivity analyses. The authors stated that the primary assessment of efficacy was based on an ITT comparison of CGI‐C ratings at week 8. However, there was no information on imputation of missing data, despite Figure 1 clearly showing that there were dropouts after randomisation, and the results stating that "forty institutionalised patients with moderate to severe AD were randomised..... and all were included in the analyses". Although not stated anywhere in the paper, there may have been imputation of missing data using LOCF. Reasons for missing data could be related to participants' health status: death, adverse events, loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The study's prespecified primary, secondary and other outcomes (as detailed in the clinical trials registration documentation) were reported, with the exception of the number of 'prn' (as needed) medications used to treat behavioural and psychological symptoms of dementia (BPSD) at 0, 2, 4 and 8 weeks. For the CGI outcome measure, it was not made explicit whether this was the CGI‐S measure which considers severity. The authors reported that the UKU‐SERS scale was completed by primary nurses, but the scores were not reported in the manuscript. The documentation on the clinical trials registration website indicated that the Cornell Depression Scale for Dementia (CDSD) was to be administered at 0, 4 and 8 weeks. While the authors did report that the CDSD was administered by primary nurses, it was not clear whether this was performed at baseline only or at all time points, and no data were reported in the manuscript. |
| Other bias | Low risk | The authors acknowledged in the results section that patients randomised to the continuation group had lower SMMSE scores, and adjusted for this baseline SMMSE in the between‐group comparison. |
Holmes 2004.
| Study characteristics | ||
| Methods | 24‐week, double‐blind randomised placebo‐controlled withdrawal study | |
| Participants |
Setting: 16 sites in the United Kingdom Sample size: 134 patients entered the study, 96 were randomised at 12 weeks to receive placebo (N = 55) or donepezil 10mg/day (N = 41) Age: mean age entering randomisation phase = 78.8 ± 1.5 years (placebo) and 78.6 ± 1.4 years (donepezil) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Patients were treated in an open‐label phase with 5 mg/day donepezil for 6 weeks followed by 10mg/day donepezil for a further 6 weeks. Patients were then randomised to placebo or 10 mg/day donepezil on a 3:2 ratio for a further 6 weeks. Provided there was no further deterioration in cognitive function (defined as a loss of greater than 2 points on the MMSE compared with baseline), then the randomised treatment with placebo or 10 mg/day donepezil was continued for a further 6 weeks. | |
| Outcomes | 1. NPI 2. NPI‐D 3. MMSE 4. Safety and tolerability Psychometric evaluations, medication compliance checks and adverse event monitoring was undertaken at screening, at baseline, and at weeks 6, 12, 18 and 24. |
|
| Source of funding | Unrestricted project grant in excess of $10,000 from Pfizer/Eisai to Drs C Holmes and D Wilkinson | |
| Declaration of interest | Both Dr C Holmes and Dr D Wilkinson have received sponsorship from Pfizer/Eisai to attend educational meetings and as speakers. | |
| Notes | AD: Alzheimer's disease NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association MMSE: Mini‐Mental State Examination NPI: Neuropsychiatry Inventory NPI‐D: Neuropsychiatry Inventory caregiver Distress |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by an independent pharmacist using a computer‐generated randomisation protocol. |
| Allocation concealment (selection bias) | Unclear risk | Methods used to conceal the allocation sequence were not described; therefore it was not possible to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All participants were blind to the treatment being offered in the randomisation phase of the study. An independent pharmacist provided numbered containers of identical tablets for each patient. Blinding of personnel was not described; the authors only mentioned that patients were blind to the treatment. It seems likely that carers, clinicians and trial personnel were blinded to the assigned treatment due to the use of central randomisation and matching placebo tablets, but this was not explicitly stated. There may be a small risk of unblinding due to the relatively high proportion of participants with marked cognitive deterioration (6/10 = 60% placebo vs. 2/6 = 33% donepezil). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were provided of how blinding of outcome assessment was undertaken, although the use of central randomisation and identical placebo make blinding seem likely. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 96 patients were included in the randomisation phase of the study, 16 patients withdrew during the randomisation phase (10 subjects on placebo and 6 subjects on donepezil). Completion rates were 82% and 85% for patients taking placebo and donepezil, respectively. The authors reported that placebo and donepezil treatment groups were similar with respect to demographic characteristics and psychometric test scores. Data for the primary outcome (NPI) were available for 83% of randomised participants (82% placebo, 85% donepezil). The remaining 17% missing data were imputed using the LOCF approach (ITT was defined as randomised, dosed with at least one outcome post‐randomisation). The proportion of missing data are similar between groups: 18% placebo, 15% donepezil. Results of the ITT‐LOCF analysis (statistically significant; P = 0.02) differed from that of the OC analysis (not statistically significant; P = 0.14) at week 24. Discontinuation from the study could be linked to participants' health status: of the 10 participants who discontinued from the placebo group, 6 (60%) had marked cognitive deterioration (loss of ≥ 2 points on MMSE) with marked increase in neuropsychiatric symptoms (increase of > 15 points on NPI). Of the 6 participants who discontinued from the donepezil group, 2 (33%) had marked cognitive deterioration (loss of ≥ 2 points on MMSE), 3 (50%) had adverse events. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes have been reported. All reported results for the primary outcome correspond to all intended outcome measurements (time points ‐ weeks 18 and 24) and all intended analyses (ITT‐LOCF and OC analyses). |
| Other bias | Unclear risk | The study was supported by an unrestricted project grant in excess of $10,000 from Pfizer/Eisai to Drs C Holmes and D Wilkinson. Drs Holmes and Wilkinson have both received sponsorship from Pfizer/Eisai to attend educational meetings and as speakers. |
Hong 2018.
| Study characteristics | ||
| Methods | 12‐week, multicentre, randomised, single‐blind, parallel group study | |
| Participants |
Setting: Neurology clinics of 3 university hospitals and 2 geriatric hospitals in South Korea Sample size: 67 patients were screened for eligibility, 65 were randomised to antidementia drug continuation group (N = 30) or antidementia drug discontinuation group (N = 35) Age: mean age = 81.3 ± 8.7 years (discontinuation) and 80.8 ± 7.4 years (continuation) Inclusion criteria
Exclusion criteria
|
|
| Interventions | The current use of donepezil or memantine was maintained at a stable dose during the 12‐week study period in the antidementia drug continuation group.The use of donepezil or memantine was discontinued during the study period after baseline in the antidementia drug discontinuation group. Patients were required to maintain medication with the potential to affect cognition (including anxiolytics, sedatives, hypnotics, antipsychotics and antidepressants) at a stable dose regimen for at least 30 days prior to screening and for the duration of the study. | |
| Outcomes | 1. Change from baseline on the Baylor Profound Mental State Examination (BPMSE) 2. MMSE 3. CGI‐C 4. CDR‐SB 5. NPI 6. CMAI 7. Barthel Index 8. ADCS‐ADL‐sev 9. FAST Efficacy assessments were performed at baseline (week 0) and the end of the study (week 12), and safety was monitored at all visits: weeks 0, 4, 8, and 12. All adverse events (AEs) and serious AEs were recorded at each study visit. |
|
| Source of funding | 2012 Research Awards of Korean Society of Geriatric Neurology, Korea Healthcare Technology R&D Project, the Ministry of Health and Welfare South Korea, the Original Technology Research Program for Brain Science, National Research Foundation of Korea, Korean Government, Ildong Pharmaceutical Company Ltd. | |
| Declaration of interest | All authors declared nothing to disclose | |
| Notes | AD: Alzheimer's disease ADCS‐ADL‐sev: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living Inventory, modified for severe dementia BPMSE: Baylor Profound Mental State Examination CGI‐C: Clinical Global Impression of Change scale CDR‐SB: Clinical Dementia Rating Scale Sum of Boxes CMAI: Cohen Mansfield Agitation Inventory DSM‐IV: The Diagnostic and Statistical Manual of Mental Disorders Fourth Edition FAST: Functional Assessment Staging scale MMSE: Mini Mental State Examination NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association NPI: Neuropsychiatric Inventory |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned in a 1:1 ratio to an ADD [antidementia drug]‐continuation group or an ADD‐discontinuation group by the block randomisation method using SAS [Statistical Analysis Software] and stratified according to current ADD (donepezil versus memantine)". |
| Allocation concealment (selection bias) | Low risk | "The randomisation sequence was known only to the clinical trial coordination center, which was contacted by the local principal investigator or co‐investigator at the participating center after enrollment of a patient". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to treatment allocation. It is possible that the perception of the participants may be affected by their knowledge of the group to which they were assigned, therefore possibly indirectly affecting self‐reported measures such as, for example, side effects of drug withdrawal or rating of symptoms. Similarly, investigators involved in the care of the patient might have reminded them to be more aware of certain side effects of drug withdrawal. A higher rate of adverse events was reported in the ADD‐discontinuation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Outcome measures were assessed by raters who were unaware of group assignment. The same rater assessed outcome measures at baseline and the end of the study in each patient". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 30 patients randomised to the ADD‐continuation group, 26 (86.7%) completed the study, while 30 of 35 patients (85.7%) allocated to the ADD‐discontinuation group completed. The difference in study withdrawal rates was not significant (P = 0.91). Study discontinuations due to adverse events (4/35, 11.4% versus 2/30, 6.7%, P = 0.51) and study discontinuations related to ADD usage or discontinuation (3/35, 8.6% versus 0/30, 0.0%, P = 0.10) were more frequent in the ADD‐discontinuation group than in the ADD‐continuation group, but the differences were not statistically significant. All deaths and serious adverse events were assessed by the investigators as not related to study medication or the study process. Primary and secondary efficacy analyses were based on the intention‐to‐treat (ITT) population using last‐observation‐carried‐forward (LOCF) imputation, where ITT was defined as randomised, dosed, with at least one outcome post‐randomisation. Per‐protocol (PP) analyses were also performed. Results of the ITT‐LOCF and PP analyses were similar. |
| Selective reporting (reporting bias) | Low risk | The study's specified outcomes were reported. Both ITT‐LOCF and PP analyses were reported as planned. |
| Other bias | Low risk | Appears to be free of other sources of bias |
Johannsen 2006.
| Study characteristics | ||
| Methods | 3‐phase study comprising a 12‐week pre‐randomisation, open‐label donepezil treatment phase, a 12‐week randomised double‐blind placebo‐controlled phase and a 12‐week single‐blind donepezil treatment phase (continuation or rechallenge). | |
| Participants |
Setting: 57 investigational sites in Belgium, Denmark, Greece, Hungary, Iceland, the Netherlands, Poland, and the USA. All sites were outpatient dementia and/or memory clinics and patients were living at home or in an assisted home care facility prior to study entry. Sample size: 619 patients completed the open‐label phase, 202 were randomised to continued donepezil treatment (N = 99) or placebo (N = 103), and 171 entered the single‐blind phase (N = 88 continued treatment and N = 83 were rechallenged with donepezil). Age: mean 72.7 ± 8.6 years for patients randomised into double‐blind phase Inclusion criteria
Exclusion criteria
|
|
| Interventions | During the open‐label phase, all patients received donepezil 5 mg/day for 4 weeks, increased to 10 mg/day thereafter. Patients who showed a clear clinical benefit after 24 weeks of open‐label donepezil treatment were considered to have completed the study and were not followed further. Patients who did not show a clear clinical benefit were randomised into the 12‐week double‐blind phase to continue with donepezil 10 mg/day or to receive placebo. After 12 weeks of double‐blind treatment, patients receiving placebo were rechallenged with donepezil in a single‐blind manner, beginning with 5 mg/day and increasing to 10mg/day after 4 weeks. The patients treated with donepezil during the double‐blind phase continued to receive donepezil at 10 mg/day for the remaining 12 weeks of the study. Only the double‐blind phase of this study is relevant to this review. |
|
| Outcomes | 1. Cognitive function
2. Neuropsychiatric status
3. Activities of daily living
4. Safety
|
|
| Source of funding | Pfizer Inc., New York, NY, USA and Eisai Inc., Teaneck, NJ, USA | |
| Declaration of interest | Dr Johannsen has received honoraria from the study sponsors. Dr Hampel has received an investigator‐initiated research grant and honoraria from the study sponsors. Dr Salmon has received honoraria for participating in conferences organised by the study sponsors. Drs Xu and Schlndler are employees of Pfizer Inc., and also hold equity in the company. Dr Qvitzau was an employee of Pfizer Denmark when the study was being conducted. Dr Richardson is an employee of Eisai Inc. PPS International Communications (Worthing, UK) assisted in the development of the manuscript. | |
| Notes | AD: Alzheimer's disease ADAS‐Cog/11: Alzheimer's Disease Assessment Scale–Cognitive Subscale DAD: Disability Assessment for Dementia DSM‐IV: The Diagnostic and Statistical Manual of Mental Disorders Fourth Edition MMSE: Mini‐Mental State Examination NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association NPI: Neuropsychiatric Inventory |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated randomisation list provided by Pfizer Inc |
| Allocation concealment (selection bias) | Unclear risk | Methods used to conceal the allocation sequence were not described; therefore it was not possible to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was implemented with identical film‐coated tablets within a blister‐packaged card. The use of identical placebo would ensure that participants were blind to the assigned intervention. However, it is not known whether trial personnel were blinded as the randomisation list provided by the independent party was available to them. No further information on blinding was provided in the publication. The 12‐week double‐blind phase was followed by a single‐blind phase, and there was no information on how unblinding was done. Treatment‐related adverse events were similar between groups and so the risk of unblinding due to adverse events among participants is small. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear who the assessors were, or whether they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 202 patients randomised, 171 patients completed the trial, corresponding to a completion rate of 85%. Withdrawal rates were 11% for patients randomised to receive donepezil and 19% for patients randomised to receive placebo. Data for the primary outcome (ADAS‐Cog/11) were therefore available for 85% of randomised participants (89% donepezil, 81% placebo; Figure 2). The proportions of missing data were similar between groups: 11% donepezil, 19% placebo. In the OC analysis, outcome data were available for 83% of randomised participants (87% donepezil, 80% placebo; Table 4). ITT was defined as randomised, dosed, with at least one outcome post‐randomisation. Results of the ITT‐LOCF analyses differed qualitatively (change scores from baseline to week 12) from that of OC analysis, although both were not statistically significant (Table 4). Discontinuation from the study could be related to participants' health status: of the 11 participants who discontinued from the donepezil group, 2 (18%) had insufficient clinical response (decline/no change in MMSE) and 3 (27%) withdrew consent for undocumented reasons. Of the 20 participants who discontinued from the placebo group, 3 (15%) had adverse events related to the study drug and 9 (45%) withdrew consent for undocumented reasons. |
| Selective reporting (reporting bias) | High risk | The study's specified outcomes were reported. Both ITT‐LOCF and OC analyses were reported as planned. However, although ADAS‐Cog/11, MMSE, NPI and DAD were measured at weeks 6 and 12 (end of double‐blind phase), only results at week 12 were reported. |
| Other bias | Unclear risk | This study was funded by Pfizer Inc., New York, NY, USA and Eisai Inc., Teaneck, NJ, USA. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adami 2011 | Conference proceeding of GAL‐ITA‐2 Scarpini |
| Antonanzas 2006 | Not a randomised controlled discontinuation trial |
| Bogardus 2001 | Hypothetical case study in Psychopharmacology for the Clinician series |
| Daiello 2009 | Retrospective cohort study; not a randomised controlled clinical trial |
| Doody 2004 | Conference proceeding for an open‐label donepezil extension study |
| Farlow 2003 | Not a randomised controlled discontinuation trial |
| Feldman 2003 | Conference proceedings; not a discontinuation trial |
| Ferris 2001 | Not a controlled clinical discontinuation trial |
| Frankfort 2006 | Not a randomised controlled discontinuation trial |
| GAL‐USA‐11 Gaudig | Not truly randomised: although patients were randomised upon entering the parent study GAL‐USA‐10, they were assigned treatment on entry into this study based on the group into which they had previously been randomised |
| Gaudig 2011 | Conference proceedings; not a discontinuation trial |
| Grossberg 2003 | Not a randomised controlled discontinuation trial |
| Howard 2011 | Not a randomised controlled discontinuation trial |
| Howard 2012 | Author reply to letter to Editor on clinically important differences in DOMINO AD Howard |
| Kwak 2009 | Case report of discontinuation syndrome following cessation of memantine |
| Le Couteur 2012 | Not randomised controlled discontinuation trial |
| Maclure 2009 | This was a conference proceeding of the Memory Medication Study; the authors were contacted as this study is unpublished. Randomised allocation was suspended during the study and was not re‐instated prior to study completion. |
| Mansour 2011 | Not a randomised controlled discontinuation trial |
| Moo 2019 | Abstract for a poster presentation on a subset of 6 patients from NCT02248636 |
| Morris 2001a | Not a randomised controlled discontinuation trial |
| Morris 2001b | Not a randomised controlled discontinuation trial |
| Pariente 2008 | Not a randomised controlled discontinuation trial |
| Perhach 2011 | Conference proceeding; not a randomised controlled discontinuation trial |
| Peyro Saint‐Paul 2015 | Not a randomised controlled discontinuation trial |
| Raskind 2004 | Not a randomised controlled discontinuation trial |
| Richarz 2011 | Conference proceedings |
| Rockwood 2001 | Not a randomised controlled discontinuation trial |
| Rockwood 2008 | Not a randomised controlled discontinuation trial |
| Schaeuble 2011a | Conference proceeding; not a randomised controlled discontinuation trial |
| Schaeuble 2011b | Not a randomised controlled discontinuation trial |
| Schneider 2012 | Not a randomised controlled discontinuation trial |
| Schwalen 2004 | Not a controlled clinical trial; predicted rate of decline was utilised as a control |
| Singh 2003 | Case reports of discontinuation syndrome following cessation of donepezil treatment |
| Umegaki 2008 | Not a randomised controlled discontinuation trial |
| Waldemar 2008 | Not a randomised controlled discontinuation trial |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN12134230.
| Study name | Continuation versus discontinuation of treatment for severe dementia: randomised, pragmatic, open‐label, clinical trial to evaluate the efficacy of continuing drug treatment in patients with severe dementia (STOP‐DEM) |
| Methods | Randomised, pragmatic, open‐label, clinical trial |
| Participants | 302 community‐dwelling patients with advanced dementia due to Alzheimer's disease (AD) who have been taking a stable dose of a ChEI for three months or more, randomised to intervention or control and assessed after 1, 3, 6 and 12 months. Inclusion criteria
Exclusion criteria
|
| Interventions | Continuation versus cessation of pharmacological treatment. |
| Outcomes | Time to institutionalisation and/or progression of disability (defined as a loss of 2 of 4 basic functions, or 6 of 11 instrumental functions using the Bristol Activities of Daily Living Scale [BADLS]), functional assessment using the FAST scale, cognitive assessment using the SMMSE, quality of life (QUALID), behavioural and psychological symptoms of dementia (NPI‐Q), clinical global impression of change, cost‐effectiveness, caregiver burden, mortality, adverse events and complications associated with dementia. |
| Starting date | January 2017 |
| Contact information | Aina Soler, Primary Care Research Unit of Mallorca, Palma, Spain and Instituto de Investigacion Sanitaria de Palma, Palma, Spain ainasoler@ibsalut.caib.es |
| Notes |
NCT02248636.
| Study name | Cholinesterase inhibitor discontinuation (CID) |
| Methods | Randomised, double‐blind efficacy study, using single group assignment |
| Participants | 72 patients in 2 arms Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: real discontinuation. This group receives a half‐dose of their previous cholinesterase inhibitor medication (in overencapsulated form) for 2 weeks, then receives placebo. Sham comparator: sham discontinuation. This groups receives their previous dose of cholinesterase inhibitor medication, but in an overencapsulated form. |
| Outcomes | Successful completion, medical events, caregiver burden (Zarit caregiver burden scale), cognition (Severe Cognitive Impairment Profile), functioning (Alzheimer's Disease Cooperative Study ADL Scale (ADCS‐ADL)), neuropsychiatric symptoms (Neuropsychiatric Inventory (NPI), brief version), post‐study treatment choice (patient and caregiver decision about what treatment to use (pre‐study medication, no treatment)) |
| Starting date | January 2015 |
| Contact information | Stephen M Thielke MD, stephen.thielke@va.gov; Erica Martinez BS, erica.martinez@va.gov, USA |
| Notes |
Differences between protocol and review
The background has been updated and modified. The inclusion criterion that participants must have been taking a cholinesterase inhibitor or memantine, or both, at a stable dose for three months prior to study participation was removed, in order to make best use of the available evidence. In response to reviewer comments, withdrawals were added as an outcome at the review stage, and eligible dementia subtypes were increased to include DLB and PDD. The protocol stated that trials which were not placebo‐controlled would only be included if outcome assessors were blinded. At full review stage, we decided to include all controlled withdrawal trials, with or without placebo substitution, and to treat blinding of outcome assessors as a potential contributor to risk of bias in included trials. In practice, this change did not affect inclusion or exclusion of any trials.
Three further authors have joined the author team since the publication of the protocol: WYL, CL and SAW.
We were not able to conduct separate meta‐analyses for short‐, medium‐ and long‐term outcomes relating to dropouts, adverse events, serious adverse events or deaths as the data available on these outcomes did not allow these distinctions to be determined. We therefore analysed these data across all trial durations, including follow‐up.
Contributions of authors
C Parsons (CP): conceiving and designing the review, coordinating the review, study selection, data extraction, data entry into Review Manager 5, risk of bias assessment for the included studies, data interpretation and assessment of the certainty in the body of evidence, writing of the review
WY Lim (WYL): study selection, data extraction, verification of data entry into Review Manager 5, risk of bias assessment for the included studies, data interpretation and assessment of the certainty in the body of evidence, writing of the review
C Hughes (CH): conceiving and designing the review, study selection, data extraction, risk of bias assessment for the included studies, data interpretation, writing of the review
C Loy (CL): risk of bias assessment for the included studies, data interpretation, writing of the review
B McGuinness (BMcG), P Passmore (PP): conceiving and designing the review, data interpretation, writing of the review
SA Ward (SAW): data interpretation, writing of the review
Sources of support
Internal sources
Queen's University Belfast, School of Pharmacy, UK
External sources
-
HSC R&D, Public Health Agency, Northern Ireland, UK
Fellowship awarded to Dr Carole Parsons to undertake the review for 2 years, 2 days per week.
-
NIHR, UK
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
CP, CH, BMcG, WYL, SAW have no known conflicts of interest.
PP was an investigator in a donepezil‐licensing European study. He has received honoraria and educational and clinical trial grants from manufacturers of acetylcholinesterase inhibitors and memantine.
CL has received a Wellcome Trust Travelling Award in relation to his work with Cochrane.
New
References
References to studies included in this review
DOMINO AD Howard {published and unpublished data}
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. The New England Journal of Medicine 2012;366:893-903. [DOI] [PubMed] [Google Scholar]
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer's disease (DOMINO-AD) trial: secondary and post-hoc analyses. The Lancet Neurology 2015;14(12):1171-81. [DOI] [PubMed] [Google Scholar]
- Jones R, Sheehan B, Phillips P, Juszczak E, Adams J, Baldwin A, et al. DOMINO-AD protocol: donepezil and memantine in moderate to severe Alzheimer's disease - a multicentre RCT. Trials 2009;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M, King D, Romeo R, Adams J, Baldwin A, Ballard C, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer's Disease (the DOMINO-AD trial). International Journal of Geriatric Psychiatry 2017;32:1205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane R [pers comm]. Email to: C Parsons 2 December 2015.
GAL‐ITA‐2 Scarpini {published data only}
- Janssen Research and Development. Clinical Study Report Synopsis GAL ITA 2; Phase III JNJ-17335630-AAD (Galantamine). The Yale University Open Data Access (YODA) Project. Accessed November 2015.
- Scarpini E, Bruno G, Zappalà G, Adami M, Richarz U, Gaudig M, et al. Cessation versus continuation of galantamine treatment after 12 months of therapy in patients with Alzheimer's Disease: a randomized, double blind, placebo controlled withdrawal trial. Journal of Alzheimer's Disease 2011;26:211-20. [DOI] [PubMed] [Google Scholar]
GAL‐USA‐5 Gaudig {published and unpublished data}
- Gaudig M, Richarz U, Van Baelen B, Schauble B. Effects of galantamine in Alzheimer's disease: double-blind withdrawal studies evaluating sustained versus interrupted treatment. Current Alzheimer Research 2011;8:771-80. [DOI] [PubMed] [Google Scholar]
- Janssen Research Foundation. Safety and efficacy of galantamine during its withdrawal in the treatment of Alzheimer's Disease. Clinical Research Report February 12, 1999.
Herrmann 2016 {published data only}
- Hermann N, O'Regan J, Ruthirakuhan M, Kiss A, Eryavec G, Williams E, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. Journal of the American Medical Directors Association 2016;17(2):142-7. [DOI] [PubMed] [Google Scholar]
Holmes 2004 {published data only}
- Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, Langley A, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology 2004;63:214-19. [DOI] [PubMed] [Google Scholar]
Hong 2018 {published data only (unpublished sought but not used)}
- Hong YJ, Choi SH, Jeong JH, Park KW, Na HR. Effectiveness of anti-dementia drugs in extremely severe Alzheimer's disease: a 12-week, multicenter, randomized, single-blind study. Journal of Alsheimer's Disease 2018;63:1035-44. [DOI] [PubMed] [Google Scholar]
Johannsen 2006 {published data only}
- Johannsen P, Salmon E, Hampel H, Xu Y, Richardson S, Qvitzau S, et al. Assessing therapeutic efficacy in a progressive disease: a study of donepezil in Alzheimer's Disease. CNS Drugs 2006;20(4):311-25. [DOI] [PubMed] [Google Scholar]
- Kozubski W, Hasselbach C, Jakab G, Kalisvaart CJ, Kurz A, McCarthy J, et al. Donepezil-treated Alzheimer's disease patients with apparent initial cognitive decline demonstrate significant benefits when therapy is continued: results from a randomised, placebo-controlled trial. European Neuropsychopharmacology 2003;13(4):S405. [Google Scholar]
References to studies excluded from this review
Adami 2011 {published data only}
- Adami M, Scarpini E, Bruno G, Zappala G, Richarz U, Gaudig M, et al. Cessation versus continuation of 12 months galantamine therapy in patients with Alzheimer's disease: a randomised, double blind, placebo controlled withdrawal trial. Alzheimer's and Dementia 2011;7(4S Part 23):S794. [DOI] [PubMed] [Google Scholar]
Antonanzas 2006 {published data only}
- Antonanzas F, Rive B, Badenas JM, Gomez-Lus S, Guilhaume C. Cost-effectiveness of memantine in community-based Alzheimer's disease patients: an adaptation in Spain. European Journal of Health Economics 2006;7:137-44. [DOI] [PubMed] [Google Scholar]
Bogardus 2001 {published data only}
- Bogardus ST. When should one stop cholinesterase inhibitors in patients with Alzheimer's disease? Journal of Psychiatry and Neuroscience 2001;26(5):425. [PMC free article] [PubMed] [Google Scholar]
Daiello 2009 {published data only}
- Daiello LA, Ott BR, Lapane KL, Reinert SE, Machan JT, Dore DD. Effect of discontinuing cholinesterase inhibitor therapy on behavioral and mood symptoms in nursing home patients with dementia. The American Journal of Geriatric Pharmacotherapy 2009;7:74-83. [DOI] [PubMed] [Google Scholar]
Doody 2004 {published data only}
- Doody R, Pratt RD, Posner H, Kumar D. Vascular dementia patients who receive donepezil treatment for 12 to 18 months maintain cognitive benefits. Neurobiology of Aging 2004;25(2):S469-70. [Google Scholar]
Farlow 2003 {published data only}
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26-week, Alzheimer disease trial. Archives of Neurology 2003;60:843-8. [DOI] [PubMed] [Google Scholar]
Feldman 2003 {published data only}
- Feldman H, Gauthier S, Vellas B, Hecker J, Xu Y, Jeni J, et al. Donepezil has significant benefits on behavior in patients with severe Alzheimer's Disease. American Psychiatric Association 2003 Annual Meeting Research Abstracts 2003;1:330. [Google Scholar]
Ferris 2001 {published data only}
- Ferris SH. Switching previous therapies for Alzheimer's Disease to galantamine. Clinical Therapeutics 2001;23(Suppl A):A3-7. [DOI] [PubMed] [Google Scholar]
Frankfort 2006 {published data only}
- Frankfort SV, Appels BA, De Boer A, Tulner LR, Van Campen JP, Kwoks CH, et al. Treatment effects of rivastigmine on cognition, performance of daily living activities and behaviour in Alzheimer's disease in an outpatient geriatric setting. International Journal of Clinical Practice 2006;60(6):646-54. [DOI] [PubMed] [Google Scholar]
GAL‐USA‐11 Gaudig {published data only}
- Gaudig M, Richarz U, Han J, Van Baelen B, Schauble B. Effects of galantamine in Alzheimer's Disease: double-blind withdrawal studies evaluating sustained versus interrupted treatment. Current Alzheimer Reserach 2011;8:771-80. [DOI] [PubMed] [Google Scholar]
Gaudig 2011 {published data only}
- Gaudig M, Richarz U, Rettig K, Djelani M, Schauble B. Effectiveness of galantamine in patients with mild-to-moderate dementia: 12 months' outcome data. Alzheimer's & Dementia 2012;8(4):S592. [Google Scholar]
Grossberg 2003 {published data only}
- Grossberg GT. Cholinesterase inhibitors for the treatment of Alzheimer's disease: getting on and staying on. Current Therapeutic Research 2003;64(4):216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2011 {published data only}
- Howard R, Phillips P, Johnson T, O'Brien J, Lindesay J, Bentham P, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. International Journal of Geriatric Psychiatry 2011;26:812-7. [DOI] [PubMed] [Google Scholar]
Howard 2012 {published data only}
- Howard R, Phillips P, Gray R. Discontinuing donepezil or starting memantine for Alzheimer's disease. The New England Journal of Medicine 2012;366(23):2227-8. [DOI] [PubMed] [Google Scholar]
Kwak 2009 {published data only}
- Kwak YT, Han IW, Suk SH, Koo MS. Two cases of discontinuation syndrome following cessation of memantine. Geriatrics & Gerontology International 2009;9:203-5. [DOI] [PubMed] [Google Scholar]
Le Couteur 2012 {published data only}
- Le Couteur DG, Robinson M, Leverton A, Creasey H, Waite L, Atkins A, et al. Adherence, persistence and continuation with cholinesterase inhibitors in Alzheimer's disease. Australasian Journal on Ageing 2012;31(3):164-9. [DOI] [PubMed] [Google Scholar]
Maclure 2009 {published data only}
- Maclure M, Green CJ, Jacova C, Hsiung RG. Pragmatic randomized trial of continuing cholinesterase inhibitors among indeterminate responders. Pharmacoepidemiology and Drug Safety 2009;18:S45-6. [Google Scholar]
Mansour 2011 {published data only}
- Mansour D, Wong R, Kuskowski M, Dysken M. Discontinuation of acetylcholinesterase inhibitor treatment in the nursing home. The American Journal of Geriatric Pharmacotherapy 2011;9(5):345-50. [DOI] [PubMed] [Google Scholar]
Moo 2019 {published data only (unpublished sought but not used)}
- Moo L, Martinez E, Dunay M, Thielke S. Cholinesterase inhibitor taper worsens symptoms in patients with Parkinson's disease. Neurology 2019;92(15 Supplement):P2.8-046. [Google Scholar]
Morris 2001a {published data only}
- Morris JC. Overview. Clinical Therapeutics 2001;23(Suppl A):A1-2. [DOI] [PubMed] [Google Scholar]
Morris 2001b {published data only}
- Morris JC, Farlow MR, Ferris SH, Kurz AF, Maelicke A, Rasmusen L, et al. Panel discussion: recommendations for prescribers. Clinical Therapeutics 2001;23(Suppl A):A31-A39. [DOI] [PubMed] [Google Scholar]
Pariente 2008 {published data only}
- Pariente A, Helmer C, Merliere Y, Moore N, Fourrier-Reglat A, Dartigues JF. Prevalence of cholinesterase inhibitors in subjects with dementia in Europe. Pharmacoepidemiology and Drug Safety 2008;17:655-60. [DOI] [PubMed] [Google Scholar]
Perhach 2011 {published data only}
- Perhach J, Graham S. A long-term, open-label extension study evaluating the safety of extended-release memantine (28 mg) in patients with moderate to severe Alzheimer's disease. Alzheimer's & Dementia 2011;7(4):Se70. [Google Scholar]
Peyro Saint‐Paul 2015 {published data only}
- Peyro Saint-Paul L, Martin J, Gaillard C, Garnier A, Mosquet B, Guillamo JS, et al. Sudden discontinuation of anti-dementia drugs in moderate and severe Alzheimer's disease in a residency for dependent elderly people: a longitudinal descriptive pilot study. Therapie 2015;70(4):313-9. [DOI] [PubMed] [Google Scholar]
Raskind 2004 {published data only}
- Raskind MA, Peskind ER, Trueyn L, Kershaw P, Damaraju CR. The cognitive benefits of galantamine are sustained for at least 36 months. Archives of Neurology 2004;61:252-6. [DOI] [PubMed] [Google Scholar]
Richarz 2011 {published data only}
- Richarz U, Gaudig M, Adami M, Jacobs A, Schreiner A. Predictors of outcome in patients with mild to moderate Alzheimer's disease treated with galantamine. European Journal of Neurology 2011;18(S2):354. [Google Scholar]
Rockwood 2001 {published data only}
- Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer's disease: a randomised, controlled trial. Journal of Neurology, Neurosurgery and Psychiatry 2001;71:589-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rockwood 2008 {published data only}
- Rockwood K, Dai D, Mitnitski A. Patterns of decline and evidence of subgroups in patients with Alzheimer's disease taking galantamine for up to 48 months. International Journal of Geriatic Psychiatry 2008;23:207-14. [DOI] [PubMed] [Google Scholar]
Schaeuble 2011a {published data only}
- Schauble B, Kavanagh S, Van Baelan B. Long-term effects of galantamine on cognitive function in Alzheimer's disease: a large-scale international retrospective study. Alzheimer's & Dementia 2011;7(4):S788. [DOI] [PubMed] [Google Scholar]
Schaeuble 2011b {published data only}
- Schauble B, Gaudig M, Richarz U, Kavanagh S, Van Baelan B. Predictors to long-term treatment response to galantamine. Alzheimer's & Dementia 2011;7(4):S665. [Google Scholar]
Schneider 2012 {published data only}
- Schneider LS. Could cholinesterase inhibitors be harmful over the long term? International Psychogeriatrics 2012;24(2):171-4. [DOI] [PubMed] [Google Scholar]
Schwalen 2004 {published data only}
- Schwalen S, Blesa R, Van Baelen B, Kavanagh S, Janssen-Cilag GmBH. Effects of continuous, interrupted and delayed galantamine treatment on long-term cognitive function in patients with advanced-moderate Alzheimer's disease. The Journal of Nutrition, Health and Aging 2004;8(4):262. [Google Scholar]
Singh 2003 {published data only}
- Singh S, Dudley C. Discontinuation syndrome following donepezil cessation. International Journal of Geriatric Psychiatry 2003;18:282-4. [DOI] [PubMed] [Google Scholar]
Umegaki 2008 {published data only}
- Umegaki H, Itoh A, Suzuki Y, Nabeshima T. Discontinuation of donepezil for the treatment of Alzheimer's disease in geriatric practice. International Psychogeriatrics 2008;20(4):800-6. [DOI] [PubMed] [Google Scholar]
Waldemar 2008 {published data only}
- Waldemar G, Hyvarinen M, Josiassen MK, Korner A, Lehto H, Wetterberg P. Tolerability of switching from donepezil to memantine treatment in patients with moderate to severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2008;23:979-81. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN12134230 {published data only}
- Soler A, Amer G, Leiva A, Ripoli J, Llorente MA, Leiva A, et al. Continuation versus discontinuation of treatment for severe dementia: randomized, pragmatic, open-label, clinical trial to evaluate efficacy of continuing drug treatment in patients with severe dementia (STOP-DEM). BMC Geriatrics 2019;19(101):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02248636 {published data only}
- Cholinesterase inhibitor discontinuation (CID). Ongoing study. January 2015. Contact author for more information.
Additional references
Alexopoulos 1988
- Alexopoulos G, Abrams R, Young R, Shamoian C. Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23:271-84. [DOI] [PubMed] [Google Scholar]
Alzheimer's Disease International 2009
- Alzheimer's Disease International. World Alzheimer Report. London, UK: Alzheimer's Disease International; 2009.
Alzheimer's Disease International 2016
- Alzheimer's Disease International. World Alzheimer Report. London (UK): Alzheimer's Disease International; 2016.
American Psychiatric Association 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington DC, USA: American Psychiatric Association, 1994. [Google Scholar]
Amici 2001
- Amici S, Lanari A, Romani R, Antognelli C, Gallai V, Parnetti L. Cerebrospinal fluid acetylcholinesterase activity after long-term treatment with donepezil and rivastigmine. Mechanisms of Ageing and Development 2001;122:2057-62. [DOI] [PubMed] [Google Scholar]
Atri 2012
- Atri A, Rountree SD, Lopez OL, Doody RS. Validity, significance, strengths, limitations, and evidentiary value of real-world clinical data for combination therapy in Alzheimer's disease. Neurodegenerative Diseases 2012;10(1-4):170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beecham 2001
- Beecham J, Knapp M. Costing psychiatric interventions. In: Thornicroft G, editors(s). Measuring mental health needs. London: Gaskell, 2001:200-24. [Google Scholar]
Black 2007
- Black SE, Doody RS, Li H. Donepezil preserves cognition and global function in severe Alzheimer's disease patients. Neurology 2007;69:459-469. [DOI] [PubMed] [Google Scholar]
Bucks 1996
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age and Ageing 1996;25:113-20. [DOI] [PubMed] [Google Scholar]
Bullock 2005
- Bullock R, Dengiz A. Cognitive performance in patients with Alzheimer's disease receiving cholinesterase inhibitors for up to 5 years. International Journal of Clinical Practice 2005;59(7):817-22. [DOI] [PubMed] [Google Scholar]
Burns 2007
- Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 2007;22:806-12. [DOI] [PubMed] [Google Scholar]
Burns 2009
- Burns A, Iliffe S. Dementia. British Medical Journal 2009;338:405-9. [Google Scholar]
Cohen‐Mansfield 1988
- Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. Journal of Gerontology 1988;44(3):M77-84. [DOI] [PubMed] [Google Scholar]
Courtney 2004
- Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomized double blind trial. Lancet 2004;363:2105-15. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308-14. [DOI] [PubMed] [Google Scholar]
Darreh‐Shori 2006
- Darreh-Shori T, Meurling L, Petersson T, Hugosson K, Hellstrom-Lindahl E, Anreasen N, et al. Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer's disease following chronic donepezil treatment. Journal of Neural Transmission 2006;113:1791-801. [DOI] [PubMed] [Google Scholar]
Darreh‐Shori 2010
- Darreh-Shori T, Sioninen H. Effects of cholinesterase inhibitors on the activities and protein levels of cholinesterases in the cerebrospinal fluid of patients with Alzheimer's disease: a review of recent clinical studies. Current Alzheimer Research 2010;7:67-73. [DOI] [PubMed] [Google Scholar]
Davidsson 2001
- Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, Hesse C. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer's disease. Neuroscience Letters 2001;300:157-60. [DOI] [PubMed] [Google Scholar]
Deardorff 2015
- Deardorff WJ, Feen E, Grossberg GT. The use of cholinesterase inhibitors across all stages of Alzheimer's disease. Drugs and Aging 2015;32:537-47. [DOI] [PubMed] [Google Scholar]
Doody 1999
- Doody RS, Strehlow SL, Massman PJ, Feher EP, Clark C, Roy JR. Baylor profound mental status examination: a brief staging measure for profoundly demented Alzheimer disease patients. Alzheimer Disease and Associated Disorders 1999;13(1):53-9. [DOI] [PubMed] [Google Scholar]
Doody 2001
- Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD, for the Donepezil Study Group. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer Disease. Archives of Neurology 2001;58:427-33. [DOI] [PubMed] [Google Scholar]
Farlow 2005
- Farlow MR, Lilly ML, ENA713 B352 Study Group. Rivastigmine: an open-label, observational study of safety and effectiveness in treating patients with Alzheimer's disease for up to 5 years. BMC Geriatrics 2005;5(3):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farlow 2013
- Farlow MR, Grossberg GT, Sadowsky CH, Meng X, Somogyi M. A 24-week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6 mg/24 h in severe Alzheimer's Dementia. CNS Neuroscience & Therapeutics 2013;19:745-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farlow 2015
- Farlow MR, Grossberg GT, Sadowsky CH, Meng X, Velting DM. A 24-week open-label extension study to investigate the long-term safety, tolerability and efficacy of 13.3mg/24h rivastigmine patch in patients with severe Alzheimer disease. Alzheimer Disease and Associated Disorders 2015;29(2):110-16. [DOI] [PubMed] [Google Scholar]
Feldman 2001
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer's disease. Neurology 2001;57(4):613-20. [DOI] [PubMed] [Google Scholar]
Feldman 2009
- Feldman HH, Pirttila T, Dartigues JF, Everitt B, Van Baelen B, Schwalen S, et al. Treatment with galantamine and time to nursing home placement in Alzheimer's disease patients with and without cerebrovascular disease. International Journal of Geriatric Psychiatry 2009;24:479-88. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein M, Folstein S, McHugh P. Mini-mental state: a practical method for grading the cognitive status of patients for the clinician. Journal of Psychiatric Research 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
Förstl 2011
- Förstl H, Stamouli SS, Janetzky W, Galanopoulos A, Karageorgiou C, Tzanakaki M. Memantine in everyday clinical practice: a comparison of studies in Germany and Greece. Dementia and Geriatric Cognitive Disorders 2011;32(4):267-72. [DOI] [PubMed] [Google Scholar]
Fox 2012
- Fox C, Crugel M, Maidment I, Auestad BH, Coulton S, Treloar A, et al. Efficacy of memantine for agitation in Alzheimer's dementia: a randomised double-blind placebo controlled trial. PLoS ONE 2012;7(5):Article ID e35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galasko 2005
- Galasko DR, Schmitt FA, Thomas R, Jin S, Bennett D, Ferris S. Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease. Journal of the International Neuropsychological Society 2005;11:446-53. [DOI] [PubMed] [Google Scholar]
Geldmacher 2003a
- Geldmacher DS, Provenanzo G, McRae T, Mastey V, Ieni JR. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. Journal of the American Geriatrics Society 2003;51:937-44. [DOI] [PubMed] [Google Scholar]
Gelinas 1999
- Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the Disability Assessment for Dementia. The American Journal of Occupational Therapy 1999;53:471-81. [DOI] [PubMed] [Google Scholar]
Gill 2009
- Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand ST, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Archives of Internal Medicine 2009;169:867-73. [DOI] [PubMed] [Google Scholar]
Goldberg 1997
- Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychological Medicine 1997;27:191-7. [DOI] [PubMed] [Google Scholar]
Grossberg 2013
- Grossberg GT, Manes F, Allegri RF, Gutierrez-Robledo LM, Gloger S, Xie L, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer's disease taking cholinesterase inhibitors. CNS Drugs 2013;27:469-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W. CGI: Clinical Global Impressions. In: ECDEU Assessment manual for psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare, ADAMHA, MIMH Psyhcopharmacology Research Branch, 1976:218-22. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 2002
- Hardy J, Selkoe SJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297(5580):353-6. [DOI] [PubMed] [Google Scholar]
Hernandez 2009
- Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the veterans affairs new England healthcare system. Journal of the American Geriatrics Society 2009;57(11):1997-2003. [DOI] [PubMed] [Google Scholar]
Herrmann 2007a
- Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer's disease. Alzheimer's and Dementia 2007;3(4):385-97. [DOI] [PubMed] [Google Scholar]
Herrmann 2007b
- Herrmann N, Gill SS, Bell CM, Anderson GM, Bronskill SE, Shulman K I, et al. A population-based study of cholinesterase inhibitor use for dementia. Journal of the American Geriatrics Society 2007;55(10):1517-23. [DOI] [PubMed] [Google Scholar]
Herrmann 2008
- Herrmann N, Gauthier S. Management of severe Alzheimer disease. Canadian Medical Association Journal 2008;179(12):1279-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrmann 2011
- Herrmann N, Cappell J, Eryavec GM, Lanctôt K. Changes in nursing burden following memantine for agitation and aggression in long-term care residents with moderate to severe Alzheimer's disease: an open label pilot study. CNS Drugs 2011;25(5):425-433. [DOI] [PubMed] [Google Scholar]
Herrmann 2013
- Herrmann N, Lanctôt KL, Hogan DB. Pharmacological recommendations for the symptomatic treatment of dementia: the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 2012. Alzheimer's Research &Therapy 2013;5(S1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrmann 2018
- Herrmann N. Cholinesterase inhibitor discontinuation: the buck stops here. American Journal of Geriatric Psychiatry 2018;26(2):148-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hogan 2014
- Hogan DB. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer Disease. Canadian Journal of Psychiatry 2014;59(12):618-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsiung 2008
- Hsiung GY, Feldman HH. Pharmacological treatment in moderate-to-severe Alzheimer's disease. Expert Opinion on Pharmacotherapy 2008;9(15):2575-82. [DOI] [PubMed] [Google Scholar]
Iverson 2002
- Iverson GL, Hopp GA, DeWolfe K, Solomons K. Measuring change in psychiatric symptoms using the Neuropsychiatric Inventory: Nursing Home version. International Journal of Geriatric Psychiatry 2002;17:438-43. [DOI] [PubMed] [Google Scholar]
Jacques 2000
- Jacques A, Jackson G. Understanding dementia. 3rd edition. Edinburgh: Churchill Livingstone, 2000. [Google Scholar]
Kalra 1996
- Kalra S, Bergeron C, Lang A. Lewy body disease and dementia. Archives of Internal Medicine 1996;156:487-93. [PubMed] [Google Scholar]
Kaufer 1998
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon C, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: the Neuropsychiatric Inventory caregiver Distress scale. Journal of the American Geriatrics Society 1998;46(2):210-5. [DOI] [PubMed] [Google Scholar]
Kavanagh 2011
- Kavanagh S, Van Baelen B, Schauble B. Long-term effects of galantamine on cognitive function in Alzheimer's disease: a large-scale international retrospective study. Journal of Alzheimer's disease 2011;27(3):521-30. [DOI] [PubMed] [Google Scholar]
Kertesz 2008
- Kertesz A, Morlg D, Light M, Blair M, Davidson W, Jesso S, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dementia and Geriatric Cognitive Disorders 2008;25(2):178-85. [DOI] [PubMed] [Google Scholar]
Kim 2011
- Kim DH, Brown RT, Ding EL, Kiel DP, Berry SD. Dementia medications and risk of falls, syncope and related adverse events: meta-analysis of randomized controlled trials. Journal of the American Geriatrics Society 2011;59(6):1019-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knapp 2017
- Knapp M, King D, Romeo R, Adams J, Baldwin A, Ballard C, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer's Disease (the DOMINO-AD trial). International Journal of Geriatric Psychiatry 2017;32:1205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lingjaerde 1987
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs, and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatrica Scandinavica 1987;s334:1-100. [DOI] [PubMed] [Google Scholar]
Livingston 2020
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2009
- Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. Journal of Neurology, Neurosurgery & Psychiatry 2009;80:600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin 1991
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research 1991;38(2):143-62. [DOI] [PubMed] [Google Scholar]
McKeith 2006
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy Bodies (DLB): report of the Consortium on DLB International Workshop. Journal of Alzheimer's Disease 2006;9(Suppl 3):417-23. [DOI] [PubMed] [Google Scholar]
McShane 2019
- McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, et al. Memantine for dementia. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD003154. [DOI: 10.1002/14651858.CD003154.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohs 1997
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope.The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11(S2):S13–21. [PubMed] [Google Scholar]
Mohs 2001
- Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 2001;57:489-95. [DOI] [PubMed] [Google Scholar]
Mok 2004
- Mok W, Chow TW, Zheng L, Mack WJ, Miller C. Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. American Journal of Alzheimer's Disease and Other Dementias 2004;19:161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molloy 1991
- Molloy DW, Alemayehu E, Roberts R. Reliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. American Journal of Psychiatry 1991;148(1):102-5. [DOI] [PubMed] [Google Scholar]
Morris 1993
- Morris J. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-2414. [DOI] [PubMed] [Google Scholar]
Nakamura 2014
- Nakamura Y, Kitamura S, Homma A, Shiosakai K, Matsui D. Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer's disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan. Expert Opinion in Pharmacotherapy 2014;15(7):913-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2006
- National Institute for Health and Clinical Excellence. Dementia: Supporting people with dementia and their carers in health and social care. London (UK): National Institute for Health and Clinical Excellence; 2006. Clinical guideline [CG42].
Nordberg 2009
- Nordberg A, Darreh-Shori T, Peskind E, Soininen H, Mousavi M, Lane R. Different cholinesterase inhibitor effects on CSF cholinesterases in Alzheimer patients. Current Alzheimer Research 2009;6:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2015
- O'Brien JT, Thomas A. Vascular dementia. Lancet Neurology 2015;386:1698-706. [DOI] [PubMed] [Google Scholar]
O'Regan 2015
- O'Regan J, Lanctôt KL, Mazareeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer's disease: a meta-analysis of randomized controlled trials. Journal of Clinical Psychiatry 2015;76(11):e1424-31. [DOI] [PubMed] [Google Scholar]
Park‐Wyllie 2009
- Park-Wyllie LY, Mamdani MM, Li P, Gill SS, Laupacis A, Juurlink DN. Cholinesterase inhibitors and hospitalization for bradycardia: a population-based study. PLoS Medicine 2009;6(9):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parnetti 2002
- Parnetti L, Amici S, Lanari A, Romani C, Antognelli C, Adreasen N, et al. Cerebrospinal fluid levels of biomarkers and activity of acetylcholinesterase (AChE) and butyrylcholinesterase in AD patients before and after treatment with different AChE inhibitors. Neurological Sciences 2002;23:S95-6. [DOI] [PubMed] [Google Scholar]
Parsons 2010
- Parsons C, Hughes CM, Passmore AP, Lapane KL. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs and Aging 2010;27(6):435-49. [DOI] [PubMed] [Google Scholar]
Parsons 2014
- Parsons C, McCorry N, Murphy K, Byrne S, O'Sullivan D, O'Mahony, et al. Assessment of factors that influence physician decision making regarding medication use in patients with dementia at the end of life. International Journal of Geriatric Psychiatry 2014;29(3):281-90. [DOI] [PubMed] [Google Scholar]
Pirttila 2004
- Pirttila T, Wilcock G, Truyen L, Damaraju CV. Long-term efficacy and safety of galantamine in patients with mild-to-moderate Alzheimer's disease: multicenter trial. European Journal of Neurology 2004;11:734-41. [DOI] [PubMed] [Google Scholar]
Puangthong 2009
- Puangthong U, Hsiung GR. Critical appraisal of the long-term impact of memantine in treatment of moderate to severe Alzheimer's disease. Neuropsychiatric Disease and Treatment 2009;5:553-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puri 2011
- Puri B, Treasaden I. Textbook of Psychiatry. 3rd edition. London: Churchill Livingstone, 2011. [Google Scholar]
Qaseem 2008
- Qaseem A, Snow V, Cross JT, Forciea MA, Hopkins R, Shekelle P, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Annals of Internal Medicine 2008;148:370-8. [DOI] [PubMed] [Google Scholar]
Querferth 2010
- Querferth H, LaFerla F. Alzheimer's disease: mechanisms of disease. The New England Journal of Medicine 2010;362:329-44. [DOI] [PubMed] [Google Scholar]
Raina 2008
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Annals of Internal Medicine 2008;148(5):379-97. [DOI] [PubMed] [Google Scholar]
Rainer 2011
- Rainer M, Wuschitz A, Jagsch C, Erb C, Chirikdjian JJ, Mucke HA. Memantine in moderate to severe Alzheimer's disease: an observational post-marketing study. Journal of Neural Transmission 2011;118(8):1255-9. [DOI] [PubMed] [Google Scholar]
Reeve 2018
- Reeve E, Farrell B, Thompson W, Herrmann N, Sketris I, Magin P, et al. Evidence-based clinical practice guideline for deprescribing cholinesterase inhibitors and memantine. Sydney (Australia). The University of Sydney, NHMRC Partnership Centre: Dealing with Cognitive and Related Functional Decline in Older People (Cognitive Decline Partnership Centre), Bruyère Research Institute, Deprescribing Guidelines in the Elderly Project; 2018 February.
Reisberg 1988
- Reisberg B. Functional assessment staging (FAST). Psychopharmacology Bulletin 1988;24:653-9. [PubMed] [Google Scholar]
Reisberg 2006
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Archives of Neurology 2006;63(1):49-54. [DOI] [PubMed] [Google Scholar]
Renn 2018
- Renn BN, Asgbar-Ali AA, Thielke S, Catic A, Martini SR, Mitchell BG, Kunik ME. A systematic review of practice guidelines and recommendations for discontinuation of cholinesterase inhibitors in dementia. American Journal of Geriatric Psychiatry 2018;26(2):134-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodda 2009
- Rodda J, Walker Z. Ten years of cholinesterase inhibitors. International Journal of Geriatric Psychiatry 2009;24(5):437-42. [DOI] [PubMed] [Google Scholar]
Rodda 2012
- Rodda J, Carter J. Cholinesterase inhibitors and memantine for symptomatic treatment of dementia. BMJ 2012;344(e2986):1-6. [DOI] [PubMed] [Google Scholar]
Rogers 2000
- Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: final analysis of a US multicentre open-label study. European Neuropsychopharmacology 2000;10:195-203. [DOI] [PubMed] [Google Scholar]
Rolinski 2012
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD006504. [DOI: 10.1002/14651858.CD006504.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356-64. [DOI] [PubMed] [Google Scholar]
Rountree 2009
- Rountree SD, Cahn W, Pavlik VN, Darby EJ, Siddiqui S, Doody RS. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimer's Research & Therapy 2009;1(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rountree 2013
- Rountree SD, Atri A, Lopez OL, Doody RS. Effectiveness of antidementia drugs in delaying Alzheimer's disease progression. Alzheimer's and Dementia 2013;9(3):338-45. [DOI] [PubMed] [Google Scholar]
Savic 2013
- Savic A, Mimica N. Two cases of loss of consciousness after long-term memantine treatment. Journal of the American Medical Directors Association 2013;14:375-376. [DOI] [PubMed] [Google Scholar]
Saxton 1990
- Saxton J, McGonigle-Gibson K, Swihart A, Miller M, Boller F. Assessment of the severely impaired patient: description and validation of a new neuropsychological Test Battery. Psychological Assessment: A Journal of Consulting and Clinical Psychology 1990;2:298-303. [Google Scholar]
Scheltens 2016
- Scheltens P, Blennow K, Breteler MMB, Strooper B, Frisoni BG, Salloway S, et al. Alzheimer's Disease. Lancet 2016;388:505-17. [DOI] [PubMed] [Google Scholar]
Schmitt 2006
- Schmitt FA, Wichems CH. A systematic review of assessment and treatment of moderate to severe Alzheimer's disease. Primary Care Companion to the Journal of Clinical Psychiatry 2006;8(3):158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 1997
- Schneider L, Olin J, Doody R, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study - Clinical Global Impression of Change. Alzheimer Diease and Associated Disorders 1997;11(Suppl 2):22-32. [DOI] [PubMed] [Google Scholar]
Schulz 2011
- Schulz JB, Rainer M, Klünemann HH, Kurz A, Wolf S, Sternberg K, et al. Sustained effects of once-daily memantine treatment on cognition and functional communication skills in patients with moderate to severe Alzheimer's disease: results of a 16-week open-label trial. Journal of Alzheimer's Disease 2011;25(3):463-75. [DOI] [PubMed] [Google Scholar]
Scott 2015
- Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Internal Medicine 2015;175(8):827-34. [DOI] [PubMed] [Google Scholar]
Seltzer 2007
- Seltzer B. Is long-term treatment of Alzheimer's disease with cholinesterase inhibitor therapy justified? Drugs & Aging 2007;24(11):881-90. [DOI] [PubMed] [Google Scholar]
Sinforiani 2012
- Sinforiani E, Pasotti C, Chiapella L, Malinverni P, Zucchella C. Memantine in Alzhiemer's disease: experience in an Alzheimer's disease assessment unit. Aging Clinical and Experimental Research 2012;24(2):193-6. [DOI] [PubMed] [Google Scholar]
Small 2005
- Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R. Cognitive performance in Alzheimer's disease patients receiving rivastigmine for up to 5 years. International Journal of Clinical Practice 2005;59:473-7. [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, Cook JC, Murray J, Prince M, Levin E, Mann A, Knapp M. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technology Assessment 2005;9(10):1-93. [DOI] [PubMed] [Google Scholar]
Suh 2008
- Suh G-H, Jung HY, Lee CU, Choi S, the Korean Galantamine Study Group. Economic and clinical benefits of galantamine in the treatment of mild to moderate Alzheimer's disease in a Korean population: a 52-week prospective study. Journal of Korean Medical Science 2008;23:10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tariot 2000
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfield S, Ding C, the Galantamine USA-10 study group. A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology 2000;54:2269-76. [DOI] [PubMed] [Google Scholar]
Toledo 2013
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathologica Communications 2013;1:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomlinson 1982
- Tomlinson B. Plaques, tangles and Alzheimer's disease. Psychological Medicine 1982;12:449-49. [DOI] [PubMed] [Google Scholar]
Vann Jones 2014
- Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychological Medicine 2014;44:673-83. [DOI] [PubMed] [Google Scholar]
Voisin 2009
- Voisin T, Vellas B. Diagnosis and Treatment of Patients with Severe Alzheimer's Disease. Drugs & Aging 2009;26(2):135-44. [DOI] [PubMed] [Google Scholar]
Wade 1988
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? International Disability Studies 1988;10(2):64-7. [DOI] [PubMed] [Google Scholar]
Walker 2015
- Walker Z, Possin KL, Boeve BF, Aarsland D. Lewy body dementias. Lancet Neurology 2015;386:1683-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wattmo 2011
- Wattmo C, Wallin A, Londos E, Minthon L. Long-term outcome and prediction models of activities of daily living in Alzheimer disease with cholinesterase inhibitor treatment. Alzheimer Disease and Associated Disorders 2011;25(1):63-72. [DOI] [PubMed] [Google Scholar]
Weiner 2000
- Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. Journal of the American Medical Directors Association 2000;1(3):114-6. [PubMed] [Google Scholar]
Wilcock 2008
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer's disease: a pooled analysis of 3 studies. Journal of Clinical Psychiatry 2008;69(3):341-8. [DOI] [PubMed] [Google Scholar]
Winblad 2001
- Winblad B, Engedal K, Soinenen H, Verhey F, Waldemar G, Wimo A, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001;57:489-95. [DOI] [PubMed] [Google Scholar]
Winblad 2003
- Winblad B, Engedal K, Sioninen H, Waldemar G, Verhey F, Wimo A, et al. Long-term efficacy of donepezil in patients with mild to moderate Alzheimer's Disease: results from a one-year placebo-controlled study and two-year follow-up study. International Psychogeriatrics 2003;15(S2):293-4. [Google Scholar]
Winblad 2004
- Winblad B, Jelic V. Long-term treatment of Alzheimer Disease: efficacy and safety of acetylcholinesterase inhibitors. Alzheimer Disease and Associated Disorders 2004;18(S1):S2-8. [DOI] [PubMed] [Google Scholar]
Winblad 2006a
- Winblad B, Kilander L, Eriksson S, Minthon L, Båtsman S, Wetterholm AL, et al. Donepezil in patients with severe Alzheimer's disease: double-blind, parallel-group, placebo-controlled study. Lancet 2006;367(9516):1057-65. [DOI] [PubMed] [Google Scholar]
Winblad 2006b
- Winblad B, Wimo A, Engedal K, Soininen H, Verhey F, Waldemar G, et al. 3-Year Study of Donepezil Therapy in Alzheimers Disease: Effects of Early and Continuous Therapy. Dementia and Geriatric Cognitive Disorders 2006;21:353-63. [DOI] [PubMed] [Google Scholar]
Winblad 2006c
- Winblad B. Severe Alzheimer's disease: benefits of donepezil therapy. International Psychogeriatrics 2006;18(S1):25-31. [Google Scholar]
Winblad 2009
- Winblad B. Review: Donepezil in Severe Alzheimer's Disease. American Journal of Alzheimer's Disease and Other Dementias 2009;24(3):185-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winblad 2016
- Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurology 2016;15:455-532. [DOI] [PubMed] [Google Scholar]
World Health Organization 2010
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010. Geneva: World Health Organization, Division of Mental Health, 2010. [Google Scholar]
Wu 2016
- Wu YT, Fratiglioni L, Matthews FE, Lobo A, Breteler MM, Skoag I, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurology 2016;15(1):116-24. [DOI] [PubMed] [Google Scholar]
Zaccai 2005
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age and Ageing 2005;34:561-6. [DOI] [PubMed] [Google Scholar]
Zhu 2013
- Zhu CW, Livote EE, Scarmeas N, Albert M, Brandt J, Blacker D, et al. Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer's disease. Alzheimer's and Dementia 2013;9:733-40. [DOI] [PMC free article] [PubMed] [Google Scholar]


