Abstract
Background
Cancer cachexia is a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass, with or without a loss of fat mass, leading to progressive functional impairment. Physical exercise may attenuate cancer cachexia and its impact on patient function. This is the first update of an original Cochrane Review published in Issue 11, 2014, which found no studies to include.
Objectives
To determine the effectiveness, acceptability and safety of exercise, compared with usual care, no treatment or active control, for cancer cachexia in adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, and eight other databases to March 2020. We searched for ongoing studies in trial registries, checked reference lists and contacted experts to seek relevant studies.
Selection criteria
We sought randomised controlled trials in adults with cancer cachexia, that compared a programme of exercise alone or in combination with another intervention, with usual care, no treatment or an active control group.
Data collection and analysis
Two review authors independently assessed titles and abstracts for relevance and extracted data on study design, participants, interventions and outcomes from potentially relevant articles. We used standard methodological procedures expected by Cochrane. Our primary outcome was lean body mass and secondary outcomes were adherence to exercise programme, adverse events, muscle strength and endurance, exercise capacity, fatigue and health‐related quality of life. We assessed the certainty of evidence using GRADE and included two Summary of findings tables.
Main results
We included four new studies in this update which overall randomised 178 adults with a mean age of 58 (standard deviation (SD) 8.2) years. Study sample size ranged from 20 to 60 participants and in three studies the proportion of men ranged from 52% to 82% (the fourth study was only available in abstract form). Three studies were from Europe: one in the UK and Norway; one in Belgium and one in Germany. The remaining study was in Canada. The types of primary cancer were head and neck (two studies), lung and pancreas (one study), and mixed (one study).
We found two comparisons: exercise alone (strength‐based exercise) compared to usual care (one study; 20 participants); and exercise (strength‐based exercise/endurance exercise) as a component of a multimodal intervention (pharmacological, nutritional or educational (or a combination) interventions) compared with usual care (three studies, 158 participants). Studies had unclear and high risk of bias for most domains.
Exercise plus usual care compared with usual care
We found one study (20 participants). There was no clear evidence of a difference for lean body mass (8 weeks: MD 6.40 kg, 95% CI –2.30 to 15.10; very low‐certainty evidence).
For our secondary outcomes, all participants adhered to the exercise programme and no participant reported any adverse event during the study. There were no data for muscle strength and endurance, or maximal and submaximal exercise capacity. There was no clear evidence of a difference for either fatigue (4 to 20 scale, lower score was better) (8 weeks: MD –0.10, 95% CI –4.00 to 3.80; very low‐certainty evidence) or health‐related quality of life (0 to 104 scale, higher score was better) (8 weeks: MD 4.90, 95% CI –15.10 to 24.90; very low‐certainty evidence).
Multimodal intervention (exercise plus other interventions) plus usual care compared with usual care
We found three studies but outcome data were only available for two studies. There was no clear evidence of a difference for lean body mass (6 weeks: MD 7.89 kg, 95% CI –9.57 to 25.35; 1 study, 44 participants; very low‐certainty evidence; 12 weeks: MD –2.00, 95% CI –8.00 to 4.00; one study, 60 participants; very low‐certainty evidence).
For our secondary outcomes, there were no data reported on adherence to the exercise programme, endurance, or maximal exercise capacity. In one study (44 participants) there was no clear evidence of a difference for adverse events (patient episode report) (6 weeks: risk ratio (RR) 1.18, 95% CI 0.67 to 2.07; very low‐certainty evidence). Another study assessed adverse events but reported no data and the third study did not assess this outcome. There was no clear evidence of a difference in muscle strength (6 weeks: MD 3.80 kg, 95% CI –2.87 to 10.47; 1 study, 44 participants; very low‐certainty evidence; 12 weeks MD –5.00 kg, 95% CI –14.00 to 4.00; 1 study, 60 participants; very low‐certainty evidence), submaximal exercise capacity (6 weeks: MD –16.10 m walked, 95% CI –76.53 to 44.33; 1 study, 44 participants; very low‐certainty evidence; 12 weeks: MD –62.60 m walked, 95% CI –145.87 to 20.67; 1 study, 60 participants; very low‐certainty evidence), fatigue (0 to 10 scale, lower score better) (6 weeks: MD 0.12, 95% CI –1.00 to 1.24; 1 study, 44 participants; very low‐certainty evidence) or health‐related quality of life (0 to 104 scale, higher score better) (12 weeks: MD –2.20, 95% CI –13.99 to 9.59; 1 study, 60 participants; very low‐certainty evidence).
Authors' conclusions
The previous review identified no studies. For this update, our conclusions have changed with the inclusion of four studies. However, we are uncertain of the effectiveness, acceptability and safety of exercise for adults with cancer cachexia. Further high‐quality randomised controlled trials are still required to test exercise alone or as part of a multimodal intervention to improve people's well‐being throughout all phases of cancer care. We assessed the certainty of the body of evidence as very low, downgraded due to serious study limitations, imprecision and indirectness. We have very little confidence in the results and the true effect is likely to be substantially different from these. The findings of at least three more studies (one awaiting classification and two ongoing) are expected in the next review update.
Plain language summary
What are the benefits and risks of exercise for adults with cancer who experience loss of appetite and weight loss?
Key message
We do not know if exercise is helpful or safe for people with cancer who experience loss of appetite and weight loss. This is because too few robust studies have tested exercise with this group of patients. We need researchers to conduct rigorous and better designed studies in this area in future to help patients and clinicians decide if exercise could be beneficial.
Why did we set out to review the literature?
Many people with cancer experience loss of appetite and weight loss (cancer cachexia), because of the cancer itself or its treatment. Cachexia is more common in some types of cancer, such as lung and pancreatic, and in advanced stages of cancer. It can compromise the ability to live independently and increase the need for care due to fatigue, muscle weakness and impaired quality of life.
There is currently no standard treatment for cachexia. One treatment option would be for patients to exercise and see if that helps to strengthen their muscles and stop or slow down their weight loss and muscle wasting.
We reviewed the evidence from clinical trials to find out if exercise, alone or in combination with other treatments (such as medicines, health education or information, and practical advice about nutrition) is beneficial for people with cancer cachexia. We wanted to know if exercise improved:
· lean body mass (total body weight minus body fat);
· muscle strength and muscle endurance (ability of the muscle to repeat an exercise over an extended time);
· exercise capacity (maximum amount of physical effort that someone can sustain);
· fatigue; and
· health‐related quality of life (ability to participate in family and social life as well as some degree of self‐care and the perception of self‐efficacy)
We also looked at whether:
· people did the amount of exercise they were prescribed and
· exercise was associated with any risks (unwanted effects).
How did we identify and evaluate the evidence?
We searched the medical literature for studies that evaluated the effects of exercise, alone or with other treatments, in people with cancer cachexia. We then compared and summarised the results. We rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found four studies that included 178 people (average age: 58 years; 52% to 82% of people in each study were men). The studies lasted for six weeks to three months. Two studies included people with head and neck cancer, one study included people with lung and pancreas cancer, and the fourth study included various cancer types.
The studies compared:
· exercise plus usual care against usual care alone (one study, 20 people);
· exercise combined with other treatments (medicines, health education or nutrition) plus usual care against usual care alone (three studies, 158 people).
The studies did not provide enough robust evidence to determine if exercise is associated with benefits or risks in people with cancer cachexia.
How‐up‐to date is this review?
The evidence in this Cochrane Review is current to March 2020.
Summary of findings
Background
This systematic review is an update of Grande 2014. We conducted this systematic review following the protocol previously published in the Cochrane Database of Systematic Reviews (Grande 2013). For explanations of methodological terms, see the main glossary on the Cochrane website (community.cochrane.org/glossary).
Description of the condition
Cancer cachexia is defined as "a multi‐factorial syndrome characterized by an ongoing loss of skeletal muscle mass, with or without a loss of fat mass, that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment" (Fearon 2011). Prevalence varies with cancer type, but is highest in people with cancer arising from the upper gastrointestinal tract or lung, where over half of those people are affected by cachexia around the time of their cancer diagnosis (Fearon 2011; Laviano 2005). The pathophysiology of cancer cachexia is complex but is characterised by a negative energy balance and abnormal metabolism (Baracos 2018; Fearon 2012).
The combination of a persistent inflammatory response, tumour‐derived catabolic factors and a stress response leads to reduced food intake, increased resting energy expenditure and an overall loss of skeletal muscle mass, which is the result of reduced protein synthesis, increased protein breakdown and reduced insulin sensitivity (Evans 2008; Fearon 2011; Tisdale 2009). The loss of lean body mass contributes to a progressive decline in muscle performance (Stephens 2012; Weber 2009), functional exercise capacity (England 2012; Jones 2012), and physical activity level (Dodson 2011; Wilcock 2008), and is associated with increased risk of dose‐limiting chemotherapy toxicities (Prado 2007; Prado 2008; Prado 2009), accelerated functional decline (LeBlanc 2015), disability in activities of daily living (Naito 2017), and poor survival (Antoun 2013; Martin 2013).
Description of the intervention
There is no current usual intervention for cancer cachexia and some consider it refractory once established, for example, in people with progressive disease and a limited prognosis (Fearon 2011). As such, it is recommended that greater emphasis be placed on applying a proactive approach, early in the course of the disease, with the aim being to maintain or slow down the loss of function (Fearon 2011; Muscaritoli 2010). Due to the complex nature of cancer cachexia, multimodal intervention is also considered necessary as it is unlikely that any single intervention will increase food intake, attenuate the metabolic disturbances, and address the imbalance between muscle protein synthesis and breakdown (Fearon 2008). Three main component interventions are being developed alone or in combination (Solheim 2012): nutritional therapies to increase energy and protein intake (Dewey 2007); drug therapies to stimulate appetite and reduce inflammation (Lee 2011; Reid 2012; Ruiz Garcia 2013); and physical exercise.
Exercise is defined by the American College of Sports Medicine as a "planned, structured and repetitive bodily movement done to maintain or improve one or more components of physical fitness" (Thompson 2010). Different types of exercise may use everyday activities, such as walking, or specialist equipment, such as free‐weights, for the purposes of training. Exercise programmes vary widely according to the frequency, intensity, type and timing of training used, as well as contextual factors such as the programme setting and level of supervision (Thompson 2010).
How the intervention might work
Exercise may attenuate the effects of cancer cachexia via several mechanisms, including the modulation of muscle metabolism, insulin sensitivity and levels of inflammation (Maddocks 2012). Resistance exercise is a potent stimulator of muscle protein synthesis, particularly when performed in conjunction with the supplentation of amino acids (Glover 2010; Marimuthu 2011). Improved insulin action in peripheral tissues following exercise may inhibit muscle protein breakdown (Wang 2006). Exercise also triggers the formation of a cohort of cytokines from muscle fibres, including interleukin‐6, which increases insulin sensitivity and reduces the production of proinflammatory cytokines (Starkie 2003). Repeated exercise has an overall anti‐inflammatory effect, which has been observed in healthy populations (Gleeson 2011), and people with early‐stage cancer (Betof 2013). This effect would be beneficial in cancer cachexia as levels of systemic inflammation are associated with reduced weight, exercise capacity and survival (McMillan 2013; Moses 2009; Proctor 2011). Thus, by preventing or slowing down the loss of lean body mass, exercise may ultimately help people with or at risk of cancer cachexia maintain their independence for longer.
Why it is important to do this review
Despite a growing evidence base for nutritional and drug interventions for cancer cachexia, including Cochrane Reviews (Dewey 2007; Payne 2017; Reid 2012; Ruiz Garcia 2013), studies of exercise interventions in the field are few in number. Reviews examining the use of exercise in cancer cachexia are generally narrative (Gould 2013), opinion based (Argilés 2012; Maddocks 2011; Maddocks 2012), or based on animal models (Argilés 2012). Nonetheless, there are increasingly reports of published, ongoing and planned studies. Thus, there is a need to synthesise the evidence for the use of exercise for cancer cachexia and, if data permit, explore the optimal programme characteristics for this group.
Objectives
To determine the effectiveness, acceptability and safety of exercise, compared with usual care, no treatment or active control, for cancer cachexia in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a parallel, single‐stage or cross‐over design, including studies with a quasi‐randomised allocation in cases where allocation concealment was described.
Types of participants
Study participants were adults (aged 18 years or older) with a histological or clinical diagnosis of cancer, meeting international criteria for cancer cachexia of any stage (Fearon 2011), which included:
precachexia, defined as weight loss of 5% or less with anorexia and metabolic changes;
cachexia, defined as weight loss greater than 5% in the past six months or body mass index (BMI) less than 20 kg/m2 and ongoing weight loss greater than 2% or sarcopenia, anorexia or systemic inflammation; and
refractory cachexia, defined as active catabolism, ongoing weight loss, unresponsive to treatment and life expectancy of less than three months.
We contacted study authors to seek additional data where baseline demographic data were insufficient to assess participants against these criteria.
We considered studies that were relevant to the review objectives but which were not performed specifically to address cancer cachexia (e.g. studies in people with advanced cancer). As such, we included studies in which at least half of the study population fell within the cachexia definitions above. Participants could be studied in any hospital or community setting. We excluded studies relating to participants during or following treatment with curative intent, or with no evidence of current disease.
Types of interventions
Studies examining any programme of exercise offered as a sole intervention or in combination with another intervention were eligible. We considered programmes using aerobic/endurance training, resistance training or a combination of both. We expected programmes to vary in terms of session length (minutes) and frequency (sessions/week), intensity of training (low, moderate, high) and overall duration (weeks). There were no restrictions on these or other programme characteristics including the setting in which the programme was offered (hospital/centre/home) and level of supervision (none, minimal, close). Interventions could be compared to either usual care, no treatment or an active control group (e.g. a nutritional or drug intervention). We used the definition of exercise by the American College of Sports Medicine as a "planned, structured and repetitive bodily movement done to maintain or improve one or more components of physical fitness" (Thompson 2010).
Types of outcome measures
We considered only outcome measures from the primary studies that were validated and used count/rate of events.
Primary outcomes
Lean body mass, assessed at the first study time point following the end of an exercise programme.
Secondary outcomes
Adherence to prescribed exercise programme.
Occurrence of adverse events.
Muscle strength and endurance.
Maximal and submaximal exercise capacity.
Fatigue.
Health‐related quality of life.
Outcome measures
Lean body mass: any validated scale such as computed tomography, dual‐energy x‐ray absorptiometry scan or bioelectrical impedance.
Adherence to prescribed exercise programmes outcome measure: number of participants in each arm at different moments of studies measurements and observed dropout rates.
Occurrence of adverse events: participant self‐reported non‐occurrence.
Muscle strength and endurance: any validated scale such as dynamometer and sit and stand test.
Maximal and submaximal exercise capacity: any validated scale such as the six‐minute walk test (6MWT).
Fatigue: any validated scale such as the Fatigue Severity Scale and Multidimensional Fatigue Inventory (MFI).
Health‐related quality of life as measured by any validated scale such as Functional Assessment of Cancer Therapy (FACT).
Search methods for identification of studies
Electronic searches
We developed an electronic search strategy using a combination of terms based on the target population, intervention, comparator and outcomes. See Appendix 1 for the search strategies. We adapted these where necessary for the other databases listed below. We searched the following electronic databases from their start date until March 2020:
CENTRAL (the Cochrane Library) 2020, Issue 3;
MEDLINE (Ovid) 1946 to March 2020;
Embase (Ovid) 1974 to March 2020;
DARE (the Cochrane Library) 2015, Issue 2. It was not searched after 2015, since it is no longer updated/available;
HTA – Health Technology Assessments (the Cochrane Library) 2016, Issue 4;
ISI Web of Science (SCI‐Expanded and CPCI) 1900 to March 2020;
LILACS (Latin American and Caribbean Health Sciences) (BIREME) 1985 to March 2020;
PEDro (the Physiotherapy Evidence Database) 24 March 2020;
SciVerse SCOPUS 24 March 2020;
Biosis Previews PreMEDLINE 1969 to March 2020;
Open Grey (System for Information on Grey Literature) 24 March 2020.
Searching other resources
We identified ongoing studies using:
ClinicalTrials.gov (clinicaltrials.gov/);
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/);
MetaRegister of Controlled Trials (mRCT) (www.controlled-trials.com/) updated to ISRCTN registry (www.isrctn.com/);
Pan African Clinical Trials (www.pactr.org); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
We handsearched the following sources: the Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD); the American Cancer Society; the British Association for Cancer Research (BACR) and the European Clinical Guidelines. We checked reference lists of relevant studies and reports citing all retrieved studies. In addition, we contacted corresponding authors of retrieved studies, experts and organisations in the field to seek potentially relevant research material, including unpublished and ongoing studies.
Data collection and analysis
Selection of studies
We used reference management software to merge results from different electronic databases and remove duplicate studies. Two review authors (AJG, VS) independently assessed titles and abstracts of articles for relevance (Higgins 2011). We obtained full‐text reports of potentially relevant studies for assessment against the inclusion criteria. We contacted the study authors by email to clarify the necessary information if missing information impaired the study selection. The two review authors discussed any disagreement in the selection of studies and resolved it by consensus. In cases of persistent disagreement, they consulted a third review author (MSP or MM). We applied no language restrictions in the selection of studies.
Data extraction and management
Two review authors (AJG and VS) independently extracted data from the included studies. We developed an online extraction form to store data relating to the study source and eligibility, methods and bias (study design, sequence generation, allocation sequence concealment, blinding), participants (number, age, sex, ethnicity, diagnosis, disease severity, setting) and intervention (exercise type and intensity, session length and frequency, and overall programme duration), adherence to the exercise programme (either self‐reported or objective) and the occurrence of any adverse events. We discussed and resolved any disagreements by consensus.
Outcome data collected at baseline immediately following a programme of exercise and at first follow‐up were:
lean body mass, generally assessed by anthropometry (e.g. skin fold thickness, or imaging, e.g. dual x‐ray absorptiometry and expressed as a weight (e.g. kilograms, kg), cross‐sectional area (square centimetres, cm2) or volume (cubic centimetres, cm3) normalised to height);
muscle strength, either isometric or isotonic, generally assessed using myometry and expressed as a measure of force (e.g. kilograms, kg, or Newton metres, Nm); muscle endurance, generally assessed as time or number of repetitions to a specified decline in muscle performance;
maximal and submaximal exercise capacity, generally assessed by a walking or cycling test and expressed as a measure of oxygen uptake (VO2) or performance (e.g. distance walked in metres, m);
fatigue, generally assessed on a numerical or categorical scale with a higher score representing more severe fatigue;
health‐related quality of life, generally assessed on a numerical or categorical scale with a higher score representing a better quality of life.
Assessment of risk of bias in included studies
Two review authors (AJG and VS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), with any disagreements resolved by discussion. We completed a 'Risk of bias' table and summary using the 'Risk of bias' tool in Review Manager 5 (Review Manager 2014).
We assessed the following for each study.
Random sequence generation (checking for possible selection bias): we assessed the method used in the studies to generate the randomised sequence process. We assessed the methods as: low risk of bias (e.g. random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots); unclear risk of bias (method not clearly described); high risk of bias (e.g. sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number; or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests or availability of the intervention).
Allocation concealment (checking for possible selection bias): we assessed the method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); unclear risk of bias (method not clearly stated); high risk of bias (studies that did not conceal allocation (e.g. open list)).
Blinding of participants and personnel (checking for possible performance bias): we assessed the methods used to blind trial participants and personnel from knowledge of which intervention they received. We assessed the methods as: low risk of bias (trial stated that personnel were blinded to participant's condition and intervention); unclear risk of bias (trial did not mention it or provide an adequate description of how it was achieved); high risk of bias (trial stated that participants or personnel were not blinded).
Blinding of outcome assessment (checking for possible detection bias): we assessed the methods used to blind outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, e.g. outcome assessor was someone not involved in the study); unclear risk of bias (trial stated that it was blinded but did not provide an adequate description of how it was achieved); high risk of bias (e.g. the study mentioned that it was not blinded).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data): we assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or missing data imputed using appropriate methods, or both); unclear risk of bias (used 'last observation carried forward for analysis without detailed time point' or lacking information to judge it); high risk of bias (used 'completer' analysis).
Selective reporting (checking for reporting bias): we assessed the methods used to report the outcomes of the study as: low risk of bias (if all planned outcomes in the study protocol or methods were reported in the results of the published study); unclear risk of bias (if there was no clear distinction between planned outcomes and reported outcomes); or high risk of bias (if some planned outcomes from the study protocol or methods were clearly not reported in the results).
Other bias (checking for possible bias not explored in the categories above): we assessed the methods as low risk of bias (e.g. if the study appeared free of other sources of bias (e.g. study funding described, balanced groups for characteristics, and carryover effect or blocking described in the study); unclear risk of bias (e.g. lack of information regarding participants, study funding not described, and carryover effect or blocking not clearly reported); high risk of bias (unbalanced groups and carryover effect or blocking not being conducted).
Measures of treatment effect
We considered the review objectives and extracted relevant counts, dichotomous, categorical and continuous data from included studies. We extracted dichotomous data and calculated risk ratio (RR) with 95% confidence intervals (CI) for adherence to prescribed programmes and occurrence of adverse events. We extracted continuous data and calculated mean difference (MD) with 95% CI for the outcomes of lean body mass, fatigue and health‐related quality of life, muscle strength and muscle endurance, maximal and submaximal exercise capacity (Deeks 2011). If studies had used different scales, we would have expressed the treatment effect as standardised mean difference (SMD) and 95% CI.
Unit of analysis issues
In the parallel‐group RCTs, we considered the individual participant as the unit of analysis. If we had included cross‐over RCTs, we would have analysed both periods, separated by periods and together. If we had included cluster‐RCTs, we would have considered each cluster group as the unit of analysis.
Dealing with missing data
We contacted authors of included studies via email to request further information when the published study report did not provide sufficient information (e.g. did not describe randomisation or intention‐to‐treat analysis, or had missing data). If no answer had been obtained from study authors, we planned to present the findings in the main discussion.
Assessment of heterogeneity
We explored clinical, methodological and statistical heterogeneity. We conducted an initial assessment of clinical heterogeneity (participants, intervention and outcome), as well as methodological heterogeneity (study design). Based on this analysis and the decision to include exercise as part of multicomponent interventions, we chose a random‐effects model to better estimate effects (Deeks 2011; Higgins 2002; Higgins 2003). We planned visual inspection of any forest plots and testing for statistical heterogeneity for any meta‐analysis to inform the interpretation of the findings.
Assessment of reporting biases
We planned to use a funnel plot and examine for asymmetry to assess for evidence of reporting bias if there were 10 or more included studies.
Data synthesis
We planned to meta‐analyse data from similar comparisons using a random‐effect model in Review Manager 5 (Review Manager 2014). Due to the limited available data, we used Review Manager 5 to report individual studies with different time points, random effects and without group totals.
Subgroup analysis and investigation of heterogeneity
We planned to use descriptive comparisons to consider differences in effect between subgroups according to the following participant characteristics: type of cancer (lung, pancreatic, etc.); stage of cachexia (precachexia, cachexia, refractory cachexia (Fearon 2011)); and intervention characteristics: type of exercise (aerobic, resistance, combined), intensity of exercise (low, moderate, high), duration of exercise programme (six weeks, 12 weeks, etc.), where there were sufficient data.
Sensitivity analysis
We planned to perform a sensitivity analysis to consider the difference in pooled effects when studies were at high or unclear risk of bias, or those with substantial (greater than 20%) missing data, were omitted from analyses, where there were sufficient data. However, we did not perform such analysis due to the number of included studies, which did not allow us to explore the results any further.
Summary of findings and assessment of the certainty of the evidence
Two review authors (AJG, VS) independently rated the certainty of the evidence for each outcome using the GRADE system and GRADEprofiler Guideline Development Tool software (GRADEpro GDT), and the guidelines provided in Chapter 14 of the CochraneHandbook for Systematic Reviews of Interventions (Schünemann 2019).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome, using the following criteria (Guyatt 2008):
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system uses the following criteria for assigning a certainty level to a body of evidence (Chapter 14 of the CochraneHandbook for Systematic Reviews of Interventions; Schünemann 2019).
High: RCTs or double‐upgraded observational studies.
Moderate: downgraded RCTs or upgraded observational studies.
Low: double‐downgraded RCTs or observational studies.
Very low: triple‐downgraded RCTs or downgraded observational studies; or case series/case reports.
Factors may decrease the certainty level of a body of evidence such as downgrading the evidence by one (–1) or two (–2) levels for the five considerations:
serious (–1) or very serious (–2) limitations in the design and implementation of available studies suggesting high likelihood of bias;
serious (–1) or very serious (–2) indirectness of evidence (indirect population, intervention, control, outcomes);
serious (–1) or very serious (–2) unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
serious (–1) or very serious (–2) imprecision of results (wide CIs);
serious (–1) or very serious (–2) high probability of publication bias.
We included two 'Summary of findings' tables to present the main findings in a transparent and simple tabular format, for the comparisons:
exercise plus usual care compared with usual care;
multimodal intervention with exercise plus usual care compared with usual care.
In particular, we included key information concerning the certainty of the evidence, the magnitude of effect of the interventions examined and the sum of available data on the outcomes lean body mass (eight weeks for comparison 1 and up to 12 weeks for comparison 2); adherence to prescribed programmes (eight weeks for comparison 1 and no data for comparison 2), occurrence of adverse events (eight weeks for comparison 1 and six weeks for comparison 2); muscle strength and endurance (no data for comparison 1 and six ‐ 12 weeks for comparison 2); maximal and submaximal exercise capacity (no data for comparison 1 and six ‐ 12 weeks for comparison 2); fatigue (eight weeks for comparison 1 and six weeks for comparison 2); and health‐related quality of life (eight weeks for comparison 1 and 12 weeks for comparison 2).
In circumstances where there were no data for an outcome, the certainty of the evidence was unknown and we reported this as 'no evidence to support or refute'.
Results
Description of studies
Results of the search
We updated the search for studies up to March 2020. We identified 216 new references since the initial search in Grande 2014, plus 19 references from specialists in the area (see Figure 1). There were 149 references after removal of duplicates, and we removed 132 studies after title screening. We viewed the remaining 17 references as potentially relevant and retrieved full‐text reports. We included four studies (14 reports) in this review, we classified two as ongoing studies, and one study is awaiting classification. We excluded three references: two studies did not investigate cancer cachexia and one study was not a RCT (see Excluded studies), resulting in 21 excluded studies overall.
1.
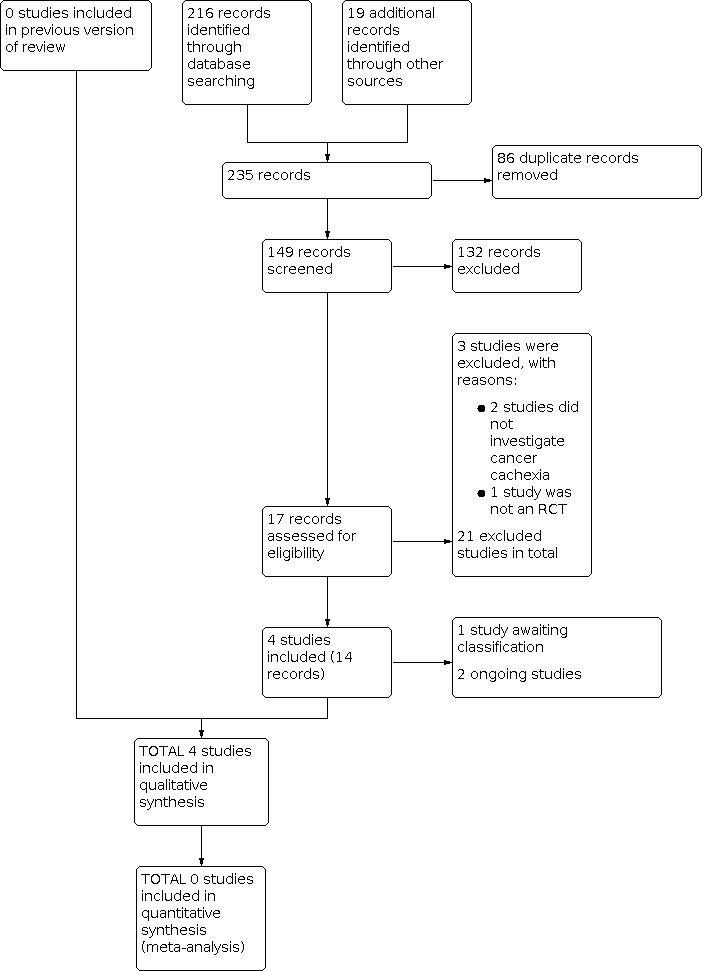
Study flow diagram.
Included studies
We included four studies that enrolled 178 adults, published between 2014 and 2018 in English (Capozzi 2016, 60 participants; Forget 2014, 54 participants; Grote 2018, 20 participants; Solheim 2017, 44 participants) (see Characteristics of included studies table). All four included studies were parallel RCTs. The study sample size ranged from 20 (Grote 2018) to 60 participants (Capozzi 2016).
Settings
Three studies enrolled participants from a single centre (Capozzi 2016; Forget 2014; Grote 2018), and one study was multicentre (Solheim 2017). Three studies were from Europe: one in the UK and Norway (Solheim 2017), one in Belgium (Forget 2014), and one in Germany (Grote 2018). The remaining study was in Canada (Capozzi 2016).
Participants
Participants had a mean age of 58.1 years (standard deviation (SD) 8.2). The proportion of men in three of the studies ranged from 52.4% to 81.7%. One study presented in abstract form only and did not report participants' sex or gender (Forget 2014). The types of primary cancer were: head and neck cancer (Capozzi 2016; Grote 2018), lung and pancreas (Solheim 2017), and mixed cancer types (Forget 2014).
Experimental and comparator interventions
Exercise alone
The Grote 2018 intervention comprised eight weeks of three‐times weekly progressive resistance training. Each session consisted of a 5‐minute warm‐up, followed by leg press, latissimus pull‐down and chest press exercises using fixed equipment performed with eight to 12 repetitions and three sets, with 60 seconds of rest between sets. Progression using fixed‐weight increments (2.5 kg to 5 kg) occurred if people reported a rating of perceived exertion of less than seven out of 10, and supervision was provided by a physiotherapist.
Multimodal interventions (exercise in combination with pharmacological, nutritional and educational interventions)
Three studies used multimodal interventions (Capozzi 2016; Forget 2014; Solheim 2017).
The Capozzi 2016 intervention comprised 12 weeks of twice‐weekly progressive resistance training. Each session included a 2‐ to 7‐minute warm‐up, followed by two or three sets of exercises of eight to 10 repetitions at a self‐rated intensity of three to five out of 10 using the Borg rating of perceived exertion, with 60 seconds of rest between sets. Progression was applied at four, six, and nine weeks, as appropriate and supervision was provided by a physiologist/personal trainer. Exercise was combined with group‐based health education and behaviour change support.
The Forget 2014 intervention comprised 12 weeks of daily exercise, with weekly supervision by a physiotherapist, combined with mirtazapine 30 mg/day, weekly advice provided by a dietician and psychological support as required.
The Solheim 2017 intervention comprised six weeks of twice‐weekly aerobic training and three‐times weekly resistance training. Aerobic sessions included 30‐minutes of exercise of the participant's choice, usually walking, and resistance sessions included six exercises targeting major muscle groups in the upper body and legs (wall push‐ups, overhead presses, biceps curls, squats, lunges, calf raises using weights), performed over approximately 20 minutes. Exercise was combined with celecoxib 300 mg once daily, oral nutritional supplements (ONS), two 220 mL cartons of ProSure (Abbott, each containing eicosapentaenoic acid (EPA) 1 g, giving a net protein intake of 2 g/day), plus nutritional counselling with advice on meal frequency and energy‐dense food provided by a dietician or trial nursing staff or both.
Comparator interventions
The comparator interventions were usual care (Capozzi 2016; Solheim 2017) or best supportive care (Forget 2014), with additional nutritional support by a professional dietician (Solheim 2017) or inpatient physiotherapy (Grote 2018) only when medically indicated, as judged by the treating physician. Capozzi 2016 used a wait list design where control participants received a delayed intervention after the primary study endpoint.
Outcome measures
Lean body mass
Three studies reported lean body mass. Solheim 2017 used computed tomography measuring muscle surface area. Capozzi 2016 used dual‐energy x‐ray absorptiometry scan measuring T score or a Z score. Grote 2018 used bioelectrical impedance analysis measuring frequency of 50 kHz and 400 μA constant current in electrical resistance of the body.
Adherence to prescribed exercise programmes
Two studies reported adherence to prescribed exercise programmes. Solheim 2017 measured the frequency of participants still completing exercise at the end of study. Capozzi 2016 measured using weekly logs.
Occurrence of adverse events
One study reported data on occurrence of adverse events. Solheim 2017 measured any adverse events self‐reported by the participants.
Muscle strength and endurance
Four studies reported muscle strength and endurance. Solheim 2017 measured muscle strength using a hand‐held dynamometer as kilogram force. Forget 2014 measured handgrip strength and provided no additional information. Capozzi 2016 measured muscle strength using a dynamometer. Grote 2018 used strength of the functional muscle group for elbow flexion in supine position as well as of knee extension in sitting position tested via hand‐held dynamometry at baseline only.
One study measured muscle endurance. Capozzi 2016 used the sit and stand test.
Maximal and submaximal exercise capacity
Three studies reported maximal and submaximal exercise capacity.Capozzi 2016, Grote 2018, and Solheim 2017 used the 6MWT.
Fatigue
Two studies reported fatigue. Solheim 2017 used the Fatigue Severity Scale. Grote 2018 used two measurements (rating of perceived exertion (RPE) from 0 to 10) and dyspnoea (RPE 0–10) and the MFI.
Health‐related quality of life
Three studies reported health‐related quality of life. Forget 2014 mentioned but did not provide information regarding outcome measurements. Capozzi 2016 used Functional Assessment of Cancer Therapy‐Anemia (FACT‐An) scale and the Functional Assessment of Cancer Therapy Head/Neck Symptom Index‐22 (FHNSI‐22). Grote 2018 used the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire.
Funding and potential conflicts
Three studies specified funding sources (Capozzi 2016; Grote 2018; Solheim 2017); the remaining study did not report this information (Forget 2014). We identified unrelated but potential conflicts of interest in two studies (Capozzi 2016; Solheim 2017). In Solheim 2017, one researcher received fees from pharmaceutical companies. In Capozzi 2016 and Solheim 2017, there were donations of ONSs or drugs (or both) from pharmaceutical companies. For additional information, see the Characteristics of included studies table.
Excluded studies
For this 2021 update, we excluded three studies, two because they did not investigate cancer cachexia (Arrieta 2019; Jain 2019), and one because it was not an RCT (Solheim 2019). There was a total of 21 excluded studies.
From the previous review, 16 studies examined an exercise intervention in adults with cancer and may have collected data on cachexia domains (Battaglini 2010; Carnaby‐Mann 2012; Cheville 2010; Courneya 2009; Elter 2009; Irwin 2009; Kuehr 2014; Litterini 2013; Mantovani 2010; Oldervoll 2006; Oldervoll 2011; Saarto 2012; Schwartz 2007; Uster 2018; Vanderbyl 2017; Zatarain 2013). We attempted to contact corresponding authors via email to determine the proportion of the sample meeting precachexia or cachexia criteria. Most authors did not explore this concept (Battaglini 2010; Courneya 2009; Elter 2009; Irwin 2009; Kuehr 2014; Mantovani 2010; Oldervoll 2006; Oldervoll 2011; Schwartz 2007; Zatarain 2013); others did not respond and are unlikely to respond, and so we made the assumption that they did not investigate cancer cachexia (Carnaby‐Mann 2012; Cheville 2010; Litterini 2013; Saarto 2012).
Two studies did not investigate exercise (Fouladiun 2007; Op den Kamp 2012).
See Characteristics of excluded studies table.
Studies awaiting classification
There was one additional completed study, but the full article or results were not yet published (Rogers 2011). A feasibility study was planned to assess the effect of a multimodal intervention (resistance training and oral ingestion of essential amino acids (EAA) plus EPA and cyclo‐oxygenase‐2 (COX‐2) inhibitor) versus usual care (EPA and COX‐2 inhibitor) for people with lung cancer with cancer cachexia. We will consider this study in future updates of this review. For more information, see the Characteristics of studies awaiting classification table.
Ongoing studies
We identified two ongoing studies (ACTRN12619000426189; Solheim 2018). For more information, see the Characteristics of ongoing studies table.
Risk of bias in included studies
The risk of bias graphs for all four studies are presented in Figure 2. Summary details for each trial are given in Figure 3. All included studies were at high or unclear risk of bias for at least three domains.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We classified two studies at low risk of bias as they appropriately described randomisation procedures (Capozzi 2016; Solheim 2017). One used computer generated numbers (Capozzi 2016), and the other used blocked randomisation (Solheim 2017). We classified two studies at unclear risk of bias as they did not provide sufficient information to enable assessment (Forget 2014; Grote 2018).
Allocation concealment
We classified three studies at unclear risk of bias since they did not provide sufficient information to enable assessment (Capozzi 2016; Forget 2014; Grote 2018). We assessed one study as low risk of bias, because personnel independent from the administrative centre allocated participants (Solheim 2017).
Blinding
Performance bias
We considered two studies at high risk of bias for blinding participants and personnel, since they reported that participants were not blinded (Grote 2018; Solheim 2017). We considered the other two studies at unclear risk, since they did not provide any information to be judged (Capozzi 2016; Forget 2014).
Detection bias
We considered Capozzi 2016 at low risk of bias because the exercise physiologist was blinded to the outcome assessment. Two studies reported that outcome assessors were not blinded (Grote 2018; Solheim 2017), thus we considered these at high risk of bias. One study did not provide sufficient information to enable assessment (Forget 2014).
Incomplete outcome data
We classified two studies at low risk of bias (Grote 2018; Solheim 2017). In Grote 2018, there were no losses to follow‐up. In Solheim 2017, there were losses to follow‐up, but adjustments were made to the analysis and the reasons were fully explained in the reporting. One study was at unclear risk of bias, since the report did not provide the analysis for 48 weeks (Capozzi 2016). We classified Forget 2014 at high risk of bias due to high loss of follow‐up (over 50%).
Selective reporting
We judged three studies at high risk of bias (Capozzi 2016; Grote 2018; Solheim 2017), and one study at unclear risk of bias (Forget 2014). Capozzi 2016 wrote in the protocol that they would report data for 12, 24, 36 and 48 weeks but did not report this in the study. Solheim 2017 planned to report six and 12 weeks but did not report 12‐week data. Grote 2018 did not fully report data for the outcomes 6MWT and muscle strength. Forget 2014 did not report enough information and so we judged it at unclear risk of bias.
Other potential sources of bias
We considered Capozzi 2016 at high risk of bias since imbalance might have affected adherence to treatment and outcomes, the experimental intervention group had higher surgery rates (20/31 participants) compared to control (6/29 participants). Forget 2014 did not provide enough information since it is a conference abstract and so we judged it at unclear risk of bias. We judged Grote 2018 and Solheim 2017 at low risk of bias as they were free from other sources of bias.
Effects of interventions
Summary of findings 1. Exercise plus usual care compared to usual care for cancer cachexia in adults.
| Exercise plus usual care compared to usual care for cancer cachexia in adults | ||||||
|
Patient or population: cancer cachexia in adults Setting: cancer centres Intervention: exercise plus usual care Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | № of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Risk with usual care | Risk with exercise plus usual care | |||||
|
Lean body mass Assessed with: bioimpedance Follow‐up: 8 weeks |
The mean lean body mass was 52.7 kg (95% CI 44.04 to 61.36) |
MD 6.4 kg higher (2.3 lower to 15.1 higher) |
— | 20 (1 study) |
⊕⊝⊝⊝ Very lowa,b,c |
— |
|
Adherence to prescribed exercise programmes Assessed with: counting of participants finishing the study Follow‐up: 8 weeks |
Study population | RR 1.00 (0.83 to 1.20 | 20 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
The outcome was not planned in the protocol or presented in methods, but it was reported descriptively in the results and conclusion. | |
| 1000 per 1000 | 1000 per 1000 (830 to 1000) | |||||
|
Occurrence of adverse events Assessed with: patient's self‐report Follow‐up: 8 weeks |
Study population | Not estimable | 20 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
No adverse events were reported during or after the training. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Muscle strength and endurance | No data | No data | — | — | — | Only baseline data for muscle strength. No evidence to support or refute. |
|
Maximal and submaximal exercise capacity Functional capacity assessed with: 6MWT |
No data | No data | — | — | — | Only baseline data. No evidence to support or refute. |
|
Fatigue Assessed with: MFI questionnaire (scale 4–20; lower score better) Follow‐up: 8 weeks |
The mean fatigue score was 11.90 (95% CI 8.61 to 15.19) | MD 0.1 lower (4 lower to 3.8 higher) | — | 20 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
|
Health‐related quality of life Assessed with: FAACT (scale 0–104; higher score better) Follow‐up: 8 weeks |
The mean health‐related quality of life score was 59.50 (95% CI 8.61 to 15.19) | MD 4.9 higher (15.1 lower to 24.9 higher) | — | 20 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWT: six‐minute walk test; CI: confidence interval; FAACT: Functional Assessment of Anorexia/Cachexia Therapy; MD: mean difference; MFI: Multidimensional Fatigue Inventory; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious study limitations: high risk of bias: blinding of participants and personnel; blinding of outcome assessment; other bias. bDowngraded one level for indirectness: outcome timeframe insufficient to produce benefits attributed to exercise. cDowngraded one level for imprecision: wide confidence intervals; few events and studies.
Summary of findings 2. Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care.
| Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care | ||||||
| Patient or population: cancer cachexia in adults Setting: cancer centres Intervention: multimodal intervention (exercise plus other interventions) plus usual care Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with multimodal intervention plus usual care | |||||
|
Lean body mass Assessed with: muscle surface area (cm2) and DEXA (kg) Follow‐up: 6–12 weeks |
The mean of lean body mass was 123.07 cm2 (95% CI 108.60 to 137.56) at 6 weeks | MD 7.89 cm2 higher (9.57 lower to 25.35 higher) at 6 weeks | — | 44 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
| The mean lean body mass was 63.80 kg (95% CI 59.39 to 68.20) at 12 weeks | MD 2.00 kg lower (8 lower to 4 higher) at 12 weeks | — | 60 (1 study) | |||
| Adherence to prescribed exercise programmes | No data | No data | — | — | — | No evidence to support or refute. |
|
Occurrence of adverse events Assessed with: self‐reported Follow‐up: 6 weeks |
Study population | RR 1.18 (0.67 to 2.07) | 44 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— | |
| 476 per 1000 | 562 per 1000 (319 to 986) | |||||
|
Muscle strength and endurance** Strength assessed with: hand‐held dynamometry (kg) Follow‐up: 6 to 12 weeks |
The mean muscle strength was 31.5 kg (95% CI 25.85 to 37.14) at 6 weeks | MD 3.8 kg higher (2.87 lower to 10.47 higher) at 6 weeks | — | 44 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
| The mean muscle strength was 90.0 kg (95% CI 83.48 to 96.51) at 12 weeks | MD 5 kg lower (14 lower to 4 higher) at 12 weeks | — | 60 (1 study) | — | — | |
|
Maximal and submaximal exercise capacity** Submaximal capacity assessed with: 6MWT distance walked in meters Follow‐up: 6 to 12 weeks |
The mean muscle endurance submaximal exercise capacity was 490.50 m (95% CI 444.47 to 536.52) at 6 weeks | MD 16.1 m lower (76.53 lower to 44.33 higher) at 6 weeks | — | 44 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
| The mean muscle endurance submaximal exercise capacity was 690.80 m (95% CI 641.31 to 740.28) at 12 weeks | MD 62.6 m lower (145.87 lower to 20.67 higher) at 12 weeks | — | 60 (1 study) | |||
|
Fatigue Assessed with: Fatigue Severity (scale: 0–10; lower score better) Follow‐up: 6 weeks |
The mean fatigue score was 3.73 (95% CI 2.84 to 4.61) | MD 0.12 higher (1 lower to 1.24 higher) | — | 44 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
|
Health‐related quality of life Assessed with: FAACT (scale: 0–136; higher score better) Follow‐up: 12 weeks |
The mean health‐related quality of life score was –19.1 (95% CI –27.49 to –10.70) | MD 2.20 lower (13.99 lower to 9.59 higher) | — | 60 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Endurance, and maximal exercise capacity, not measured/reported. 6MWT: six‐minute walk test; CI: confidence interval; DEXA: dual‐energy x‐ray absorptiometry; FAACT: Functional Assessment of Anorexia/Cachexia Therapy; kg: kilograms; m: meters; MD: mean difference; MFI: Multidimensional Fatigue Inventory; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious study limitations: high risk of bias: blinding of participants and personnel (Solheim 2017); blinding of outcome assessment (Solheim 2017); incomplete outcome data (Capozzi 2016; Solheim 2017); selective reporting (Capozzi 2016; Solheim 2017); other bias (Capozzi 2016; Solheim 2017). bDowngraded one level for indirectness: the interventions in the included studies may not be applicable to the decision context (i.e. effects may be attributed to other components of multimodal intervention rather than to exercise; outcome timeframe insufficient to produce benefits attributed to exercise). cDowngraded one level for imprecision: wide confidence intervals; few events and studies.
Exercise plus usual care compared with usual care
One study compared exercise plus usual care versus usual care (Grote 2018).
Primary outcome
Lean body mass
Data from the seven‐week and eight‐week time periods indicated there was no clear evidence of a difference for lean body mass in kilograms, but the evidence was very uncertain (7 weeks: MD 4.30 kg, 95% CI –4.91 to 13.51; 20 participants; 8 weeks: MD 6.40 kg, 95% CI –2.30 to 15.10; 20 participants). We assessed the certainty of evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness due to insufficient time for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (Table 1).
Secondary outcomes
Adherence to prescribed exercise programmes
Grote 2018 did not present this measure in the methods section; however, it was reported descriptively in the results and conclusion. After eight weeks of the intervention, there was no clear evidence of a difference, but the evidence was very uncertain (RR 1.00, 95% CI 0.83 to 1.20; 20 participants). In Solheim 2017, adherence to components of the intervention was 76% for celecoxib, 60% for exercise and 48% for nutritional supplements. We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness due to insufficient time for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (Table 1).
Occurrence of adverse events
None of the participants reported occurrence of adverse events in the exercise group or the control group. We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness due to insufficient time for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (Table 1).
Muscle strength and endurance
Grote 2018 reported muscle strength only at baseline and there were no data to support or refute the effect of exercise on this outcome.
Maximal and submaximal exercise capacity
Grote 2018 reported only the baseline information for functional capacity with the 6MWT and there were no data to support or refute the effect of exercise on this outcome.
Fatigue
Data from seven weeks and eight weeks indicated no clear evidence of a difference for fatigue (4 to 20 scale, the lower the better), but the evidence was very uncertain (7 weeks: MD –1.80, 95% CI –5.74 to 2.14; 20 participants; 8 weeks: MD –0.10, 95% CI –4.00 to 3.80; 20 participants). We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness due to insufficient time for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (Table 1)
Health‐related quality of life
Data from seven weeks and eight weeks indicated no clear evidence of a difference (0 to 104 scale, the higher the better), but the evidence was very uncertain (7 weeks: MD 3.10, 95% CI –13.65 to 19.85; 20 participants; 8 weeks: MD 4.90, 95% CI –15.10 to 24.90; 20 participants). We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness due to insufficient time for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (Table 1).
Multimodal intervention (exercise combined with other interventions) plus usual care compared with usual care
Three studies assessed multimodal intervention plus usual care versus usual care (Capozzi 2016; Forget 2014; Solheim 2017). Forget 2014 did not provide enough data for analysis.
Primary outcome
Lean body mass
Two studies reported lean body mass (Capozzi 2016; Solheim 2017). There was no clear evidence of a difference for lean body mass, but the evidence was very uncertain (6 weeks: MD 7.89 kg, 95% CI –9.57 to 25.35; 1 study, 44 participants; Analysis 2.1; 12 weeks: MD –2.00 kg, 95% CI –8.00 to 4.00; 1 study, 60 participants; Analysis 2.1). We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness due to insufficient time for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (see Table 2).
2.1. Analysis.

Comparison 2: Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care, Outcome 1: Lean body mass
Secondary outcomes
Adherence to prescribed exercise programmes
One study reported adherence to prescribed exercise programmes (Solheim 2017). Compliance to the individual components of the intervention was 76% for celecoxib, 60% for exercise and 48% for nutritional supplements
Occurrence of adverse events
One study reported occurrence of adverse events (Solheim 2017). The other two studies reported no data for this outcome (Capozzi 2016; Forget 2014). There was no clear evidence of a difference for occurrence of adverse events (patient episode report), but the evidence was very uncertain (RR 1.18, 95% CI 0.67 to 2.07; 44 participants; Analysis 2.2).We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness since the interventions may not have been applicable to the decision context, and the insufficient outcome timeframe for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (see Table 2).
2.2. Analysis.

Comparison 2: Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care, Outcome 2: Occurrence of adverse events
Muscle strength and endurance
Two studies reported muscle strength (assessed using hand‐held dynamometry) but not endurance (Capozzi 2016; Solheim 2017). There was no clear evidence of a difference for muscle strength, but the evidence was very uncertain (6 weeks: MD 3.80, 95% CI –2.87 to 10.47; 1 study, 44 participants; 12 weeks: MD –5.00, 95% CI –14.00 to 4.00; 1 study, 60 participants; Analysis 2.3). We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness since the interventions may not have been applicable to the decision context, and the insufficient outcome timeframe for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (see Table 2).
2.3. Analysis.

Comparison 2: Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care, Outcome 3: Muscle strength and endurance
Maximal and submaximal exercise capacity
Two studies reported submaximal exercise capacity (6MWT) only (Capozzi 2016; Solheim 2017). Data from six‐week and 12‐week endpoints indicated no clear evidence of a difference for distance walked, but the evidence was very uncertain (6 weeks: MD –16.10 m, 95% CI –76.53 to 44.33; 1 study, 44 participants; 12 weeks: MD –62.60 m, 95% CI –145.87 to 20.67; 1 study, 60 participants; Analysis 2.4). We assessed the certainty of evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness since the interventions may not have been applicable to the decision context, and the insufficient outcome timeframe for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (see Table 2).
2.4. Analysis.

Comparison 2: Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care, Outcome 4: Maximal and submaximal exercise capacity
Fatigue
One study reported fatigue (Solheim 2017). There was no clear evidence of a difference for fatigue (0 to 10 scale, lower score better), but the evidence was very uncertain at six weeks (MD 0.12, 95% CI –1.00 to 1.24; 44 participants; Analysis 2.5). We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness since the interventions may not have been applicable to the decision context, and the insufficient outcome timeframe for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (see Table 2).
2.5. Analysis.

Comparison 2: Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care, Outcome 5: Fatigue
Health‐related quality of life
One study reported quality of life at 12 weeks (Capozzi 2016). There was no clear evidence of a difference for health‐related quality of life (0 to 104 scale, the higher the better), but the evidence was very uncertain (MD –2.20, 95% CI –13.99 to 9.59; 60 participants; Analysis 2.6). We assessed the certainty of the evidence as very low, downgrading once for serious study limitations due to high risk of bias; once for indirectness since the interventions may not been applicable to the decision context, and the insufficient outcome timeframe for the intervention to have physiological changes (adaptations) from exercise; and once for imprecision due to wide CIs and few events and studies (see Table 2).
2.6. Analysis.

Comparison 2: Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care, Outcome 6: Health‐related quality of life
Exercise compared with no treatment
We found no studies.
Exercise compared with active control
We found no studies.
Discussion
Summary of main results
We aimed to determine the effectiveness, acceptability and safety of exercise, compared to usual care, no treatment or active control, on biomarkers and clinical outcomes of cachexia in adults living with cancer. We analysed findings from four RCTs and summarised the direction of effects for each outcome. There was no clear evidence of a difference for lean body mass in exercise plus usual care compared with usual care and multimodal intervention (exercise plus other interventions) plus usual care compared with usual care, but the evidence was very uncertain.
Exercise plus usual care compared with usual care
There was no clear evidence of a difference for lean body mass, adherence, fatigue and health‐related quality of life, but the evidence was very uncertain. There were no data for muscle strength and endurance and maximal and submaximal exercise capacity. There were no adverse events reported during or after the training by the participants.
Multimodal intervention (exercise combined with other interventions) plus usual care compared with usual care
There was no clear evidence of a difference for lean body mass, occurrence of adverse events, muscle strength and endurance, and maximal and submaximal exercise capacity, fatigue and health‐related quality of life, but the evidence was very uncertain. There were no data for prescribed exercise programmes.
Exercise compared with no treatment
We found no studies.
Exercise compared with active control
We found no studies.
Overall completeness and applicability of evidence
This review identified only four studies. The primary outcomes of the included studies were not always specific to cancer cachexia (e.g. health‐related quality of life), and, therefore, the interventions being examined were not always designed to address cachexia symptoms alone. Our main objective was to assess the effectiveness, acceptability and safety of exercise intervention on cancer cachexia and, according to our original protocol, we included studies where exercise was used as a standalone intervention or used in combination with other component interventions. Studies in this field are shifting towards using multicomponent approaches as a standard, therefore, studies of exercise as a sole intervention are unlikely to emerge in the future. This does limit the extent to which the specific effects of exercise can be delineated outside of laboratory/acute studies. In future updates of this review, we will consider amending the protocol towards multimodal interventions.
Another feature of the included studies was the inconsistency of the interventions in terms of specific component, and exercise intensity, volume and frequency. While we could describe 'what' was offered in sufficient detail, studies lacked detail on 'who' delivered what and 'how' components were delivered, for example which materials were used or if there were prompts or scripted interactions. Detail on dosing and adherence in particular was brief and only reported at the group level limiting the extent to which this could be assessed. We recommend authors in this field adhere to reporting standards for interventions such as the TIDieR checklist to improve transparency and utility of published studies (Hoffmann 2014). The short duration of some interventions may have been a factor in the lack of evidence for an effect on some outcomes including lean body mass (Roeland 2020).
Quality of the evidence
The overall certainty of the evidence was very low. We based this assessment on serious study limitations, indirectness and imprecision. We downgraded for serious study limitations due to a lack of blinding, risk of selection bias (allocation concealment not reported in three studies) and suspicion of reporting bias. We identified high risk of performance bias due to the non‐blinding of participants and personnel. Blinding of outcome assessor (detection bias) in half of the studies was high risk and selective outcome reporting was high risk of bias. When considering other possible biases, we noted imbalances between study groups in terms of cancer stage and the frequency of intervention contacts, both of which might have affected adherence to treatment and study outcomes.
The comparison of exercise plus usual care compared with usual care included only one study (Grote 2018), and the certainty of the evidence was very low for all outcomes analysed. The comparison of multimodal intervention (exercise plus other interventions) plus usual care compared with usual care included three studies (Capozzi 2016; Forget 2014; Solheim 2017), but outcome data were only available for two studies. The certainty of the evidence was very low for all outcomes; therefore, we have very little confidence in the results and the true effect may be substantially different.
Potential biases in the review process
We used a comprehensive and highly sensitive search strategy in the major databases and clinical trial registries. We consider it unlikely that we missed relevant studies from the search, though the poor availability of data on parameters to diagnose cachexia (e.g. weight loss reporting) was limiting. We followed usual Cochrane methods and there were no contestable decisions relating to the inclusion or exclusion of studies, data analyses or assessing risk of bias. The overall objective of this review was to determine the effectiveness, acceptability and safety of exercise, compared to usual care, no treatment or active control, for cancer cachexia in adults. Including studies of exercise combined with other interventions in this review did not permit the identification of the effectiveness of exercise alone. There are different time points published for different outcomes in the included studies. Since there is no clear agreement on the most appropriate follow‐up or adequate duration of an intervention, this could be considered a bias.
Agreements and disagreements with other studies or reviews
There are few data available for exercise or multimodal interventions for people with cancer cachexia. The lack of evidence and number of unanswered research questions are acknowledged by an international guideline (Radbruch 2010) and 2019 systematic review (Hall 2019), yet it is widely agreed malnutrition and a loss of bodyweight (muscle mass and fat) has a negative impact on function and quality of life in people with cancer (Fearon 2011; Fearon 2012; Hall 2019; Maddocks 2012). While the mechanisms of cancer cachexia are complex, existing theory indicates the potential for benefit from exercise, nutrition and anti‐inflammatory medication (Fearon 2012; Hall 2019; Maddocks 2012).
Due to the complexity of cancer cachexia, screening, assessing and monitoring may currently be conducted to increase quality of care and raise awareness in clinical practice (Arends 2017). Wilms 2016 explored the evidence on exercise and nutrition for the prevention and treatment of cachexia in a narrative review. The authors' main findings reflected that exercise interventions currently lack a strong evidence base, and nutritional interventions alone show minimal effect on the natural course and outcomes of cancer cachexia. Hall 2019 explored the optimal components for rehabilitation in people with incurable cancer using the principles of exercise and nutrition‐based interventions. The authors similarly found limited data (only two RCTs) for multimodal rehabilitation programmes combining exercise and nutritional interventions in people with incurable cancer and called for further high‐quality studies.
The American Society of Clinical Oncology published evidence‐based guidance on the clinical management of cancer cachexia in adults with advanced cancer (Roeland 2020). The guidance considered 20 systematic reviews and 13 additional RCTs, spanning three broad intervention groups: 1. nutritional interventions where evidence remained limited despite increased bodyweight in some trials; 2. pharmacological interventions with improvements in appetite or bodyweight (or both) found with progesterone analogues and corticosteroids; and 3. other interventions including exercise, all of which had insufficient evidence of benefit to draw conclusions on efficacy (Roeland 2020).
Authors' conclusions
Implications for practice.
For clinicians and people with cancer cachexia
There is insufficient evidence to support or refute the use of exercise alone, or as part of a complex multimodal intervention, from this review. The studies identified provided insufficient evidence to support or refute an effect on clinical outcomes of lean body mass, muscle strength and endurance, maximal and submaximal exercise capacity, fatigue, and health‐related quality of life when compared to usual care. The immediate practice implications for the management of cachexia in people with head and neck, lung or pancreatic cancer are therefore limited.
For policy makers
We found no clear evidence of a difference for lean body mass, and no clear evidence of a difference for occurrence of adverse events, muscle strength and endurance, maximal and submaximal exercise capacity, fatigue and health‐related quality of life of exercise for multimodal interventions including exercise, compared with usual care, for adults with cancer cachexia. However, the available evidence is very low certainty.
For funders of the intervention
Exercise alone, or as part of a multimodal intervention, with usual care, showed no clear evidence of a difference for lean body mass; and no clear evidence of a difference for occurrence of adverse events, muscle strength and endurance, maximal and submaximal exercise capacity, fatigue or health‐related quality of life when compared to usual care. However, the evidence available is very low certainty.
Implications for research.
General implications
Agreement on the criteria to diagnose and classify cancer cachexia provides a strong basis for studies to develop interventions to improve the management of this condition including examination of safety, feasibility and effectiveness of exercise (Fearon 2011). Interventions must have clear theoretical reasoning linking them to cancer cachexia mechanisms. Health behaviour changes strategies may also be required to optimise adherence and should be clearly articulated within the intervention description. Randomised controlled trials are required to test exercise alone or as part of multimodal intervention to improve people's well‐being throughout all phases of cancer care. Randomised controlled trials are required to test exercise alone or as part of multimodal intervention to improve management of cancer cachexia in a broad range of cancer types.
Design
Carefully designed, adequately powered, high‐quality randomised controlled trials are needed, paying special attention to the definition of cancer cachexia, cancer stage, randomisation, blinding and intention‐to‐treat analysis. There also needs to be accurate reporting of adverse events in all study arms to ensure that any risks do not outweigh the benefits. We encourage researchers to include follow‐up measurements beyond the immediate end of the intervention period, to assess the longevity of any effect(s). Consensus regarding appropriate time points for long‐term follow‐up of outcomes needs to be reached. There is one ongoing study, the findings of which may have a substantial impact on the overall interpretation of the results presented in this review (Solheim 2018).
Intervention
Future studies need to report detailed intervention protocols, considering aspects related to: frequency of the intervention, intensity, duration, description of the exercise and details of the personnel delivering the intervention. Issues of intervention fidelity should also be considered and reported. Future studies should make use of the TIDieR checklist (Hoffmann 2014).
Measurement (endpoints)
Exercise interventions may be offered alone or as part of complex interventions, with the aim being to impact on key domains of cancer cachexia, including nutritional status (anthropometry), muscle mass (dual energy x‐ray absorptiometry), physical function (laboratory tests), mental health and functioning, and overall quality of life (self‐assessment). The use of assessment of these domains in populations where cancer cachexia may be present would improve the interpretation and implementation of findings into the cachexia setting.
What's new
| Date | Event | Description |
|---|---|---|
| 24 March 2020 | New citation required and conclusions have changed | In this update we included four new studies (178 participants) published between 2014 and March 2020. The original review identified no studies meeting our eligibility criteria. We have added GRADE and created 'Summary of findings' tables. |
| 24 March 2020 | New search has been performed | This review has been updated to include the results of a new search on 24 March 2020. This is the first update of this Cochrane Review published in Issue 11, 2014. |
History
Protocol first published: Issue 10, 2013 Review first published: Issue 11, 2014
Acknowledgements
The authors acknowledge Professor Fiona Cramp and Yvonne Roy for their comments on the protocol and Jane Hayes for developing the search strategy. The authors acknowledge Rachel Riera, Alessandra Medeiros and Simone GP Vitoriano for the collaboration in the 2014 version of the review (Grande 2014). For the 2021 update, the authors acknowledge the input of Professor Fiona Cramp, Kerry Harding, Anna Erskine, Neil O’Connell and Joanne Abbott.
We would like to acknowledge the following peer reviewers: Roberta W Scherer, Adrian Tookman, Stella Maria O’Brien for the 2021 update.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategies
The Cochrane Library (CENTRAL, DARE and HTA)
#1 MeSH descriptor: [Neoplasms] explode all trees
#2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*):ti,ab,kw (Word variations have been searched)
#3 #1 and #2
#4 MeSH descriptor: [Weight Loss] explode all trees
#5 (cachexia or cachexic):ti,ab,kw (Word variations have been searched)
#6 MeSH descriptor: [Malnutrition] explode all trees
#7 (weight or underweight or malnutrition or wasting):ti,ab,kw (Word variations have been searched)
#8 #4 or #5 or #6 or #7
#9 MeSH descriptor: [Exercise] explode all trees
#10 MeSH descriptor: [Exercise Movement Techniques] explode all trees
#11 MeSH descriptor: [Exercise Therapy] explode all trees
#12 MeSH descriptor: [Physical Fitness] this term only
#13 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*):ti,ab,kw (Word variations have been searched)
#14 (physical* near/5 (fit* or activ* or movement*)):ti,ab,kw (Word variations have been searched)
#15 #9 or #10 or #11 or #12 or #13 or #14
#16 #3 and #8 and #15
MEDLINE (Ovid)
1 exp Neoplasms/
2 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp.
3 1 or 2
4 exp Weight Loss/
5 (cachexia or cachexic).mp.
6 exp Malnutrition/
7 (weight or underweight or malnutrition or wasting).mp.
8 4 or 5 or 6 or 7
9 exp Exercise/
10 exp Exercise Movement Techniques/
11 exp Exercise Therapy/
12 Physical Fitness/
13 (exercis* or aerobic* or resistance* or strength* or walk* or endurance*).mp.
14 (physical* adj5 (fit* or activ* or movement*)).mp.
15 9 or 10 or 11 or 12 or 13 or 14
16 3 and 8 and 15
17 randomized controlled trial.pt.
18 controlled clinical trial.pt.
19 randomized.ab.
20 placebo.ab.
21 clinical trials as topic.sh.
22 randomly.ab.
23 trial.ti.
24 17 or 18 or 19 or 20 or 21 or 22 or 23
25 16 and 24
key:
mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
pt=publication type
ab=abstract
sh=subject heading
ti=title
Embase (Ovid)
1. exp Neoplasms/
2. (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
3. 1 or 2
4. exp Weight Loss/
5. (cachexia or cachexic).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
6. exp Malnutrition/
7. (weight or underweight or malnutrition or wasting).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
8. 4 or 5 or 6 or 7
9. exp Exercise/
10. exp Exercise Movement Techniques/
11. exp Exercise Therapy/
12. Physical Fitness/
13. (exercis* or aerobic* or resistance* or strength* or walk* or endurance*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
14. (physical* adj5 (fit* or activ* or movement*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
15. or/9‐14
16. 3 and 8 and 15
17. random$.tw.
18. factorial$.tw.
19. crossover$.tw.
20. cross over$.tw.
21. cross‐over$.tw.
22. placebo$.tw.
23. (doubl$ adj blind$).tw.
24. (singl$ adj blind$).tw.
25. assign$.tw.
26. allocat$.tw.
27. volunteer$.tw.
28. Crossover Procedure/
29. double‐blind procedure.tw.
30. Randomized Controlled Trial/
31. Single Blind Procedure/
32. or/17‐31
33. (animal/ or nonhuman/) not human/
34. 32 not 33
35. 16 and 34
ISI Web of Science and BIOSIS:
#15 #14 AND #8
#14 #13 AND #12
#13 Topic=(human*)
#12 #11 OR #10 OR #9
#11 Topic=(((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)))
#10 Topic=((controlled clinical trial OR controlled trial OR clinical trial OR placebo))
#9 Topic=((randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial))
#8 #7 AND #4 AND #1
#7 #6 OR #5
#6 Topic=((physical* near/5 (fit* or activ* or movement*)))
#5 Topic=((exercis* or aerobic* or resistance* or strength* or walk* or endurance*))
#4 #3 OR #2
#3 Topic=((weight or underweight or malnutrition or wasting))
#2 Topic=((cachexia or cachexic))
Databases=SCI‐EXPANDED, CPCI‐S Timespan=All years
#1 Topic=((cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*))
LILACS (BIREME)
(cancer$ or tumor$ or tumour$ or neoplas$ or malignan$ or carcinoma$ or adenocarcinoma$ or choricarcinoma$ or leukemia$ or leukaemia$ or metastat$ or sarcoma$ or teratoma$) [Words] and (cachexia or cachexic) or (weight or underweight or malnutrition or wasting) and (fit* or activ* or movement* or exercis* or aerobic* or resistance* or strength* or walk* or endurance*) [Words] and ((PT randomized controlled trial OR PT controlled clinical trial OR PT multicenter study OR MH randomized controlled trials as topic OR MH controlled clinical trials as topic OR MH multicenter study as topic OR MH random allocation OR MH double‐blind method OR MH single‐blind method ) OR (( ensaio $ OR ensayo $ OR trial $) AND ( azar OR acaso OR placebo OR control $ OR aleat $ OR random $ OR enmascarado $ OR simpleciego OR (( simple $ OR single OR duplo $ OR doble $ OR double $) AND ( cego OR ciego OR blind OR mask ))) AND clinic $)) AND NOT (MH animals))
PEDro (the Physiotherapy Evidence Database)
cachexia AND exercise
SciVerse SCOPUS
KEY("exercise") OR KEY("physical activity") AND KEY("cachexia")
Open Grey (System for Information on Grey Literature)
cachexia AND exercise
Data and analyses
Comparison 1. Exercise plus usual care compared with usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Lean body mass | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1.1 At 7 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1.2 At 8 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Adherence to prescribed exercise programmes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Occurrence of adverse events | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4.1 At 7 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4.2 At 8 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 At 7 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.2 At 8 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: Exercise plus usual care compared with usual care, Outcome 1: Lean body mass
1.2. Analysis.

Comparison 1: Exercise plus usual care compared with usual care, Outcome 2: Adherence to prescribed exercise programmes
1.3. Analysis.

Comparison 1: Exercise plus usual care compared with usual care, Outcome 3: Occurrence of adverse events
1.4. Analysis.

Comparison 1: Exercise plus usual care compared with usual care, Outcome 4: Fatigue
1.5. Analysis.

Comparison 1: Exercise plus usual care compared with usual care, Outcome 5: Health‐related quality of life
Comparison 2. Multimodal intervention (exercise plus other interventions) plus usual care compared to usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Lean body mass | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.1 At 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1.2 At 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Occurrence of adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Muscle strength and endurance | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.1 At 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.3.2 At 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Maximal and submaximal exercise capacity | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 At 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.2 At 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5.1 At 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.6.1 At 12 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Capozzi 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, single centre Study dates: June 2012 to January 2014 Country: Canada |
|
| Participants |
Total participants: 60 Inclusion criteria: aged > 18 years; newly diagnosed with nasopharyngeal, oropharyngeal or hypopharyngeal cancer; scheduled to receive radiation or concurrent chemoradiation treatment; able to walk without assistance; received clearance for exercise from their treating oncologist; lived in the Calgary area; could speak and write English Exclusion criteria: not reported Participants characteristics: Gender (men/women) · Experimental intervention group: 26/5 · Comparator intervention group: 23/6 Age (years) (Mean ± SD) · Experimental intervention group: 55.9 ± 9.4 · Comparator intervention group: 56.4 ± 9.2 Race/ethnicity · Experimental intervention group: not reported · Comparator intervention group: not reported Weight (kg) (Mean ± SD) · Experimental intervention group: 81.6 ± 2.9 · Comparator intervention group: 84.8 ± 3.0 BMI (kg/m2) (Mean ± SD) · Experimental intervention group: 26.6 ± 0.8 · Comparator intervention group: 28.0 ± 0.8 Type of cancer (oral/larynx or hypopharynx/nasopharynx/oropharynx/major salivary glands/nasal cavity or paranasal sinuses/unknown primary) · Experimental intervention group: 4/2/1/16/3/2/3 · Comparator intervention group: 4/4/3/13/0/1/4 Cancer staging (Eastern Cooperative Oncology Group/Zubrod score: I/II/III/IV) · Experimental intervention group: 1/2/2/26 · Comparator intervention group: 0/5/1/22 Stage of cachexia (PG‐SGA total) (Mean ± SD) · Experimental intervention group: 6.6 ± 5.8 · Comparator intervention group: 5.9 ± 4.9 Comorbidities · Experimental intervention group: not reported · Comparator intervention group: not reported |
|
| Interventions |
Experimental group
All participants prescribed a progressive resistance‐training programme with a short, moderate‐intensity warm‐up (5–7 minutes). Basic exercise prescription included: 8–10 repetitions at 8–10 RM, for 2–3 sets, rest: 60 seconds between sets. Participants used Borg Rated Perceived Exertion Scale (0–10) to monitor intensity. All participants worked within moderate‐to‐hard range. Progression applied at 4 weeks, 6 weeks and 9 weeks, as appropriate, with participants advancing to 3 sets of 8 repetitions at 8 RM
Comparator group: 12‐week delayed lifestyle intervention group acted as the control during the first 12‐week phase Concomitant interventions: none Follow‐up: 12 weeks |
|
| Outcomes |
Primary outcomes Body composition BMI. Height and total body mass How measured: Health Carter Balance Beam scale with height rod. Mean of 2 consecutive measurements recorded for each outcome. Time points measured: baseline, 12 weeks and 24 weeks Lean body mass How measured: dual‐energy x‐ray absorptiometry scan (QDR 4500; Hologic Inc, Bedford, MA) Time points measured: baseline, 12 weeks and 24 weeks Secondary outcomes Muscular fitness‐grip strength How measured: handgrip dynamometer (Smedley Dynamometer; TTM, Tokyo, Japan). Sum of best 2 trials recorded for each hand was combined according to the Canadian Physical Activity Fitness and Lifestyle Approach protocol Time points measured: baseline, 12 weeks and 24 weeks Functional lower body strength How measured: 30‐second sit to stand test. Number of times a participant could stand from a seated position in 30 seconds Time points measured: baseline, 12 weeks and 24 weeks Functional aerobic capacity How measured: sit to stand and 6MWT. Participants walked as far as they could around a 400‐m track for 6 minutes, and the distance covered was recorded to the nearest 0.5 m Time points measured: baseline, 12 weeks and 24 weeks Flexibility How measured: standardised trunk‐forward‐flexion sit‐and‐reach test using the Wells‐Dillon flexometer, following the standard protocol and recording the best of 2 trials to the nearest 0.5 cm Time points measured: baseline, 12 weeks and 24 weeks Quality of life How measured: FACT‐An scale and FHNSI‐22 Time points measured: baseline, 12 weeks and 24 weeks Depression How measured: CES‐D. Score > 16 indicated clinically meaningful depression Time points measured: baseline, 12 weeks and 24 weeks Nutrition How measured: PG‐SGA, which has been validated in the cancer population Time points measured: baseline, 12 weeks and 24 weeks Weekly physical activity logs How measured: information about the type, duration, intensity and frequency of activities in which they participated, including adverse events Time points measured: completed weekly physical activity logs during the 12‐week intervention Lifestyle satisfaction and staff's competence and support How measured: participants completed survey assessing satisfaction with the lifestyle intervention, rated the staff's competence and support, and indicated whether they would recommend the programme to other cancer survivors. Participants reported responses on a 5‐point scale from 1 (not at all satisfied/no) to 5 (very satisfied/definitely yes). Time points measured: after end of programme |
|
| Notes | Study supported by the Alberta Cancer Foundation's Joe's Team Donor‐Directed Funds. Lauren C Capozzi was supported by a Vanier Canada Graduate Scholarship from the Canadian Institute of Health Research, an Izaak Walton Killam Predoctoral Scholarship, an Alberta Innovates Health Solutions MD/PhD Studentship, a Psychosocial Oncology Research Training Program Scholarship and University of Calgary Scholarships. Margaret L McNeely was supported by the Canadian Institute of Health Research, the Canadian Breast Cancer Foundation–Prairies Northwest Territory chapter, Alberta Cancer Foundation Investigator Initiated Trials, the University of Alberta Hospital Foundation and the M.S.I. Foundation. Raylene A Reimer was supported by the Canadian Institutes of Health Research. Janine Giese‐Davis received salary funding provided by The Enbridge Chair for Psychosocial Oncology Research held by Linda E Carlson. Nicole Culos‐Reed was supported by the Canadian Imperial Bank of Commerce (operating funds) and by the Alberta Cancer Foundation's Joe's Team Donor‐Directed Funds for head and neck cancer research. The units of measurements were missing in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐based random number program. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Certified exercise physiologists conducting the assessments were blind to each participant's randomisation status." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were included in final analyses reported at 24 weeks; however, final analysis was not presented for 48 weeks. |
| Selective reporting (reporting bias) | High risk | Study was designed to assess the participants at baseline, 12 weeks, 24 weeks, 36 weeks and 48 weeks; only baseline, 12 weeks and 24 weeks were reported. |
| Other bias | High risk | The experimental intervention group had higher surgery rates (20/31 participants) compared to control (6/29 participants) and this imbalance might have affected adherence to treatment. The reported results were based in interim analysis. |
Forget 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel, single centre Study dates: not reported Country: not reported |
|
| Participants |
Total participants: 54 Inclusion criteria: people with performance status 0, 1 or 2 on chemotherapy for various cancer; have cachexia (weight loss ≥ 5% bodyweight within prior 6 months) or at high risk for cachexia (radiochemotherapy planned on upper part of digestive tract) Exclusion criteria: not reported Participants characteristics: not reported |
|
| Interventions |
Experimental group: participants received mirtazapine 30 mg/day, weekly advice from a dietician and physical exercise. Psychological support available if needed
Comparator group: best supportive care (no other information) Concomitant interventions: unclear Follow‐up: 3 months |
|
| Outcomes |
Primary outcomes Body mass index How measured: not reported Time points measured: baseline, 3 months Secondary outcomes Hand grip strength How measured: not reported Time points measured: baseline, 3 months Mid‐upper muscle circumference How measured: not reported Time points measured: baseline, 3 months Quality of life How measured: QLQc‐30 Time points measured: baseline, 3 months PG‐SGA How measured: not reported Time points measured: baseline, 3 months |
|
| Notes | Conference abstract It was not possible to extract any data since authors presented only mean values with no measure of variability. No funding or conflict of interest were stated in the conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23/54 participants were included in final analysis; 9 participants died. |
| Selective reporting (reporting bias) | Unclear risk | The data provided in the table regarded only mean values, authors did not provide confidence interval, standard deviation or standard errors. |
| Other bias | Unclear risk | Lack of information to make a judgement. |
Grote 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel, single centre Study dates: June 2013 and January 2015 Country: Germany |
|
| Participants |
Total participants: 20 Inclusion criteria: planned inpatient or outpatient resistance training; aged ≥ 18 years; diagnosed state of cachexia (weight loss > 5% over past 6 months) or precachexia (unintentional weight loss of ≤ 5% of usual bodyweight during last 6 months) Exclusion criteria: metastatic disease; severe neurological problems or other contraindications for progressive resistance training. 80 people were excluded for following reasons: metastases (20), second cancer diagnosis or relapse, or both (18), no exercise therapy possible due to other comorbidities (8), no interest (16), no time (4), resistance training at non‐study site (3) and other reasons (11) Participants characteristics: Gender (men/women) · Experimental intervention group: 9/1 · Comparator intervention group: 6/4 Age (years) (mean, standard deviation) · Experimental intervention group: 60.2, 4.7 · Comparator intervention group: 61.5, 15.7 Race/Ethnicity (caucasian/other) · Experimental intervention group: not reported · Comparator intervention group: not reported Weight (kg) (mean ± SD) · Experimental intervention group: not reported · Comparator intervention group: not reported BMI (kg/m2) (median, interquartile range) · Experimental intervention group: not reported · Comparator intervention group: not reported Type of cancer · Experimental intervention group: 10 (9 upper aerodigestive tract neoplasms and 1 tumor of the esophagus) · Comparator intervention group: 10 (8 head and neck squamous cell carcinoma and 2 tumors of the salivary gland) Cancer staging (Eastern Cooperative Oncology Group/Zubrod score: I/II/III/IV) · Experimental intervention group: 3 Stadium I/II and 7 Stadium III/IV · Comparator intervention group: 3 Stadium I/II and 7 Stadium III/IV Stage of cachexia (% of weight loss in the previous 6 months) (median, interquartile range) · Experimental intervention group: not reported · Comparator intervention group: not reported Comorbidities · Experimental intervention group: not reported · Comparator intervention group: not reported |
|
| Interventions |
Experimental group: all exercises were carried out within the participant's given limb range of motion and in a dynamic way without defined execution time for the concentric and eccentric phase. Goal of the very first training was to determine the training load set for hypertrophic adaption. Because of the vulnerable patient group, a submaximal step‐by‐step approach within 3 sets and 8–12 repetitions was chosen. The first 2 sets acted as both muscular and movement adaption. The last set served as submaximal 1‐RM estimation. The evaluation of the training prescription was done in real‐time by a supervising physiotherapist. The training protocol consisted of a warm‐up period for 5 minutes on a bicycle ergometer or an upper body cycle with individual selectable wattage. A leg press, a latissimus pull‐down and a chest press (Kaphingst) formed the 3 equipment‐supported core exercises. All exercises were performed with 8–12 repetitions and 3 sets. After each set and during the changes to another exercise machine, a break of maximum 60 seconds was assured. After each machine, the participants rated their perceived exertion from 0 to 10. The weight loading was increased at the next workout if RPE < 7. For the 2 upper limbs exercise, a progression of 2.5 kg weight loading and for the lower limbs exercise a progression of 5.0 kg weight loading was implemented. The exercises were supervised for performance and safety reasons by physiotherapists experienced in oncology rehabilitation and certified in medical training therapy. Comparator group: usual care. This could have included inpatient physiotherapy if prescribed by the ward physician. Muscle strengthening techniques were not part of usual care. Concomitant interventions: unclear Follow‐up: 8 weeks |
|
| Outcomes |
Primary outcomes Lean mass How measured: bioelectrical impedance analysis (AKERN SRL, BIA 101 New Edition) was executed to assess the adaption in body composition. Participants were lying supine on a therapy table. The measurements took place at the right side of the body with the tetrapolar‐technique of 4 standard electrodes on the surface of the hand and the foot. Constant frequency of 50 kHz and 400 μA constant current in electrical resistance of the body were used to measure resistance and reactance Time points measured: baseline (t1), after 7 weeks of radiotherapy (t2) and 8 weeks after radiotherapy (t3) Secondary outcomes Maximal isometric strength How measured: elbow flexion in supine position as well as of knee extension in sitting position (in each case right and left) was tested via hand‐held dynamometry (Mecmesin Ltd., Broadbridge Heath, West Sussex, UK) for isometric maximal muscle strength Time points measured: baseline (t1), after 7 weeks of radiotherapy (t2) and 8 weeks after radiotherapy (t3) Functional capacity How measured: 6MWT, heart rate, pulse oximetry (Contec Medical Systems CO., Ltd., Model: CMS50E), fatigue (RPE 0–10) and dyspnoea (RPE 0–10) before‐and‐after test Time points measured: baseline (t1), after 7 weeks of radiotherapy (t2) and 8 weeks after radiotherapy (t3) Fatigue How measured: MFI Time points measured: baseline (t1), after 7 weeks of radiotherapy (t2) and 8 weeks after radiotherapy (t3) Quality of lifein anorexia/cachexia How measured: FAACT questionnaire Time points measured: baseline (t1), after 7 weeks of radiotherapy (t2) and 8 weeks after radiotherapy (t3) |
|
| Notes | The study stated that they received no funding for the research. No conflicts of interest for any authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants allocated at random to control or exercise group via blocked randomisation in sealed envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Participants allocated at random to control or exercise group via blocked randomisation in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "non‐blinding of assessors and therapists are major limitations which reflect lack of time and budget in pilot studies." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "non‐blinding of assessors and therapists are major limitations which reflect lack of time and budget in pilot studies." For 'Adherence to prescribed programmes', although the RCT authors did not plan the evaluation in the protocol, it was reported descriptively in the results and conclusion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up. |
| Selective reporting (reporting bias) | High risk | 6MWT and muscle strength were not fully report for baseline, postintervention and follow‐up for both groups. 'Adherence to prescribed programmes' was not planned in the protocol, but was reported descriptively in the results and conclusion. |
| Other bias | Low risk | No other bias found. |
Solheim 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial, parallel, multicentre, open‐label Study dates: July 2011 to April 2014 Countries: Norway, the UK |
|
| Participants |
Total participants: 44 Inclusion criteria: aged 18–80 years; stage III/IV non‐small cell lung cancer or inoperable pancreatic cancer; due to commence chemotherapy; Karnofsky Performance Status > 70; no contraindication to the study interventions (primarily the anti‐inflammatory medication); body mass index < 30 kg/m2; and < 20% weight loss in the previous 6 months Exclusion criteria: received systemic anticancer therapy in the preceding 4 weeks; taking regular oral steroid medication; participating in other interventional clinical trials or who within 30 days prior to inclusion were taking other agents for the prevention or treatment of cachexia (e.g. megestrol acetate, progestational agents, growth hormone, dronabinol, marijuana or other anabolic agent); with renal impairment defined as creatinine clearance < 30 mL/minute; with potential contraindications to celecoxib (New York Heart Association Functional class III or IV heart failure, uncontrolled hypertension (diastolic blood pressure > 95 mmHg at screening), history of previous myocardial infarction, unstable angina, coronary revascularisation, uncontrolled arrhythmia, cerebrovascular accident, previous gastrointestinal inflammatory disease and history of gastrointestinal ulceration, history of bronchospasm, asthma, rhinitis, nasal polyps, angioneurotic oedema or urticaria with intake of NSAID or aspirin therapy, history of hypersensibility related to intake of acetylsalicylic acid or NSAIDs) Participants characteristics: Gender (men/women) · Experimental intervention group: 15/10 · Comparator intervention group: 11/10 Age (years) (median, interquartile range) · Experimental intervention group: 63.0, 54.5–68.0 · Comparator intervention group: 59.0, 52.5–67.0 Race/Ethnicity (caucasian/other) · Experimental intervention group: 24/1 · Comparator intervention group: 21/0 Weight (kg) (mean ± SD) · Experimental intervention group: 70.18 ± 13.03 · Comparator intervention group: 66.63 ± 10.46 BMI (kg/m2) (median, interquartile range) · Experimental intervention group: 24.2, 21.4–27.0 · Comparator intervention group: 24.0, 21.9–25.3 Type of cancer (non‐small cell lung cancer/pancreatic) · Experimental intervention group: 15/10 · Comparator intervention group: 11/10 Cancer staging (Karnofsky performance status) (median, interquartile range) · Experimental intervention group: 90.0, 80.0–100.0 · Comparator intervention group: 90.0, 80.0–90.0 Stage of cachexia (% of weight loss in the previous 6 months) (median, interquartile range) · Experimental intervention group: 5.7, 0.6–13.3 · Comparator intervention group: 5.4, 1.6–11.7 Comorbidities · Experimental intervention group: not reported · Comparator intervention group: not reported |
|
| Interventions |
Experimental group
Comparator group: usual care without exercise intervention. They were asked to keep their everyday habits without changing daily physical activity level. Nutritional support by a professional dietician was only provided when so‐called medically indicated (as judged by the treating physician) Concomitant interventions: regular oncology review: outpatient appointments prior to chemotherapy (prechemotherapy assessments) and hospital visits (single day) for chemotherapy delivery (most commonly every 3 weeks). The most common chemotherapy regimens were Folfirinox (folinic acid, 5‐fluorouracil, irinotecan and oxaliplatin), vinorelbine‐carboplatin/cisplatin, gemcitabine mono, and pemetrexed‐carboplatin/cisplatin. All participants had their symptoms managed according to guidelines at each centre. Follow‐up: 6 weeks |
|
| Outcomes |
Primary outcomes Feasibility How measured: assessed by proportion of participants screened vs those consented and attrition rates. In cancer trials, the percentage of participants recruited vs those screened varies: we accepted 10% recruitment and an attrition rate of < 26% as feasible Time points measured: 6 weeks Secondary outcomes Bodyweight How measured: not reported Time points measured: baseline, 6 weeks Body mass index How measured: not reported Time points measured: baseline, 6 weeks Muscle mass (muscle surface area, cm2) How measured: computed tomography Time points measured: baseline, 6 weeks Adherence to prescribed exercise programmes How measured: self‐reported compliance Time points measured: baseline, 6 weeks Muscle strength How measured: hand‐held dynamometer assessing grip strength Time points measured: baseline, 6 weeks Physical function How measured: number of steps using ActivPAL (physical activity meter worn for 7 days and 6MWT) Time points measured: baseline, 6 weeks Nutritional status How measured: aPG‐SGA Time points measured: baseline, 6 weeks Nutritional intake How measured: 10‐point verbal scale assessment of nutritional intake (AveS) Time points measured: baseline, 6 weeks Fatigue How measured: Fatigue Severity Scale Time points measured: baseline, 6 weeks Safety and survival How measured: Fatigue Severity Scale Time points measured: baseline, 6 weeks |
|
| Notes | 1 author received research funding from pharmaceutical industry (Abbott Laboratories). The oral nutritional supplements (ONS; ProSure) was received free of charge from Abbott Nutrition, a subsidiary company of Abbott Laboratories. Participants were recruited from 3 centres of 2 countries: Norway
UK
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A web‐based randomisation system developed and administered by a research centre were undertaken in a 1:1 ratio with stratification by centre and tumour type." |
| Allocation concealment (selection bias) | Low risk | Quote: "administered by a research centre." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This unblinded design." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This unblinded design." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were losses to follow‐up described in the study. 2 participants died (1 in each group). For 'endurance', > 50% losses occurred. Intention to treat used do adjust losses of follow‐up. |
| Selective reporting (reporting bias) | High risk | Exclusion criteria were not reported in protocol. There were differences between the 2 publications for this study. Lack of reporting at all follow‐ups time points. |
| Other bias | Low risk | No other bias found. |
6MWT: six‐minute walk test; aPG‐SGA: abridged Patient Generated Subjective Global Assessment; AveS: Analogue verbal scale; CES‐D: Center for Epidemiological Studies Depression Scale; EPA: eicosapentaenoic acid; FAACT: Functional Assessment of Anorexia/Cachexia Therapy; FACT‐An: The Functional Assessment of Cancer Therapy‐Anemia; FHNSI‐22: Functional Assessment of Cancer Therapy Head/Neck Symptom Index‐22; MFI: Multidimensional Fatigue Inventory; NSAID: non‐steroidal anti‐inflammatory drug; PG‐SGA: Patient Generated‐Subjective Global Assessment; RM: repetition maximum; RPE: rating of perceived exertion.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arrieta 2019 | Authors did not investigate cancer cachexia. |
| Battaglini 2010 | Authors did not investigate cancer cachexia. |
| Carnaby‐Mann 2012 | Authors did not investigate cancer cachexia. |
| Cheville 2010 | Authors did not investigate cancer cachexia. |
| Courneya 2009 | Authors did not investigate cancer cachexia. |
| Elter 2009 | Authors did not investigate cancer cachexia. |
| Fouladiun 2007 | Not a randomised controlled trial with exercise. |
| Irwin 2009 | Authors did not investigate cancer cachexia. |
| Jain 2019 | Authors did not investigate cancer cachexia. |
| Kuehr 2014 | Authors did not investigate cancer cachexia. |
| Litterini 2013 | Authors did not investigate cancer cachexia. |
| Mantovani 2010 | Authors did not investigate cancer cachexia. |
| Oldervoll 2006 | Authors did not investigate cancer cachexia. |
| Oldervoll 2011 | Authors did not investigate cancer cachexia. |
| Op den Kamp 2012 | Not a randomised controlled trial with exercise. |
| Saarto 2012 | Authors did not investigate cancer cachexia. |
| Schwartz 2007 | Authors did not investigate cancer cachexia. |
| Solheim 2019 | Not a randomised controlled trial. |
| Uster 2018 | Authors did not investigate cancer cachexia. |
| Vanderbyl 2017 | Authors did not investigate cancer cachexia. |
| Zatarain 2013 | Authors did not investigate cancer cachexia. |
Characteristics of studies awaiting classification [ordered by study ID]
Rogers 2011.
| Methods | After patients complete the screening procedures at the screening visit and the principal investigator has confirmed that all inclusion/exclusion criteria have been met, all eligible patients will be randomly assigned to a treatment arm. Enclosed treatment assignments will be serially numbered in opaque, sealed envelopes and opened sequentially after the participant's name and other details have been written on the appropriate envelope. Simple randomisation by using a randomisation table created by computer software (i.e. computerised sequence generation) |
| Participants |
Inclusion criteria: people who have diagnosed NSCLC and have received ≥ 1 first‐line anticancer treatment, e.g. surgery, chemotherapy, radiotherapy or a targeted therapy (i.e. gefitinib, erlotinib and crizotinib) and fulfil the following cachexia definition:
Exclusion criteria: patients who are, in the opinion of a doctor or nurse in the department, unlikely to be suitable to participate by virtue of mental incapacity, severe current psychological or psychiatric disorder; estimated prognosis < 1 month; concurrent use of other investigational agents and patients who have received investigational agents in the last 4 weeks prior to randomisation; concurrent use of other appetite stimulants e.g. MPA or MA and dexamethasone 4 mg once daily or prednisolone 30 mg once daily; systolic BP > 160 mmHg or diastolic BP > 90 mmHg (or both); pleural effusion that causes a CTC grade 2 dyspnoea; radiotherapy in last 2 weeks prior to randomisation, patients must have recovered from all radiotherapy‐related toxicities; history of another primary malignancy within last 5 years with exception of non‐melanoma skin cancer or cervical cancer in situ; CNS metastases (patients having any clinical signs of CNS metastases must have a CT or MRI of the brain performed to rule out CNS metastases to be eligible for study participation. Patients who have had brain metastases surgically removed or irradiated with no residual disease confirmed by imaging are allowed); recent haemoptysis associated with NSCLC (> 1 teaspoon in a single episode within 4 weeks); abnormal baseline 12‐lead ECG; concurrent severe or uncontrolled (or both) medical disease (i.e. uncontrolled diabetes, chronic renal failure, chronic liver disease); patient unwilling or unable to comply with the study protocol Age minimum: 18 years Age maximum: no limit Gender: men and women |
| Interventions |
Experimental group: EPA 2 g/day orally, celecoxib 300 mg/day orally and PRT (2 episodes per week, involving 5‐ to 10‐minute warm‐up, 10‐ to 15‐minute resistance training followed by 5‐minute cool‐down, approximately 30 minutes increasing in time to 1 hour per session, commencing on a 1‐to‐1 basis and moving onto group sessions if required by participant, under supervision of a trained exercise therapist) plus essential amino acids 20 g high in leucine over 3 days commencing 1 hour after exercise, orally, for 20 weeks Comparator group: international best supportive care: EPA 2 g/day orally and celecoxib 300 mg/day (COX‐2 inhibitor) orally for 20 weeks |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Trial ID: ACTRN12611000870954 Stephen P Bird is a consultant to Musashi & PowerBar, Nestle Performance Nutrition. EPA was donated by Health World Ltd. Celecoxib was donated by Pfizer Australia and New Zealand. Essential amino acids were donated by Musashi (Notting Hill, Australia). Study funded by University of Auckland and the Louisa & Patrick Emmet Murphy Foundation, Managed by Public Trust (New Zealand). Study classified as awaiting classification due date of last data collection (13 October 2015), but the results are not still available. |
BMI: body mass index; BP: blood pressure; CNS: cental nervous system; CRP: C‐reactive protein; CT: computed tomography; CTC: Common Terminology Criteria; ECG: electrocardiogram; ECOG: Eastern Cooperative Oncology Group; FAACT: Functional Assessment of Anorexia‐Cachexia Therapy; IL: interleukin; LLN: lower limit of normal; MA: megesterol acetate; MFSI‐SF: The Multidimensional Fatigue Symptom Inventory‐Short Form; MPA: medroxyprogesterone acetate; MRI: magnetic resonance imaging; NSCLC: non‐small‐cell lung carcinoma; PRT: progressive resistance training; RECIST: Response Evaluation Criteria In Solid Tumors; TNFalpha: tumour necrosis factor alpha; ULN: upper limit of normal; VO2: rate of oxygen consumption; WHOQOL‐BREF: World Health Organization Quality of Life.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619000426189.
| Study name | ACE Trial: the Advanced cancer & Cachexia Exercise trial |
| Methods |
Study design: parallel randomised controlled trial, multicentre Study dates: April 2019 to July 2021 Country: Australia |
| Participants |
Inclusion criteria: aged > 18 years; men and women; confirmed diagnosis of advanced cancer (i.e. locally advanced or metastatic disease); BMI < 30 mg/m2; Australian‐modified Karnofsky Performance Status > 70; weight loss > 5% within the past 6 months, or BMI < 20 kg/m2 and weight loss > 2% within the past 6 months, or presence of sarcopenia and weight loss > 2% within the past 6 months Exclusion criteria: refractory cachexia; expected survival < 3 months; receiving parenteral nutrition or enteral nutrition via feeding tube; scheduled to undergo surgery within study period; neuroendocrine carcinoma (e.g. neuroendocrine pancreatic cancer); use of mobility aids including a brace, walking stick/cane or wheelchair; contraindication to exercise as determined by treating medical physician (e.g. uncontrolled medical condition); inability to read and communicate in English. Sample size: intervention group: 28; comparator group: 28 |
| Interventions |
Experimental group: supervised, small group‐based (1–4 participants) aerobic and resistance exercise training 3 times per week over 12 weeks. Exercise sessions led by exercise physiologists with experience working with people with cancer and delivered in community fitness centres. Continuous aerobic exercise performed on a stationary exercise bike or treadmill for 10–20 minutes at 55–65% estimated HRmax, or a Borg RPE of 12–13 out of 20, for the first 2 weeks and will progress to moderate‐intensity interval training at 70–80% HRmax, or an RPE of 15–16. High‐intensity interval training sessions at 85–95% HRmax or RPE of 18–19 will be introduced in week 4. Resistance exercise will include up to 6 exercises to target major upper and lower body muscle groups (e.g. leg press, chest press, seated row) using machines, free weights or bodyweight. Resistance exercise will start at 2 sets at 10–12 RM, progressing to 3–4 sets of 6–10 RM by week 9. Exercise will be modified and progressed according to individual tolerability. Compliance and attendance (including the reasons for missed sessions or inability to meet exercise targets) will be tracked throughout the intervention by the supervising exercise physiologist. A 30 g serving of whey protein isolate powder (26 g of protein) mixed with 250–300 mL of water will be provided to all participants following each exercise session. Exercise physiologists will administer the protein supplement and track adherence.
Comparator group: usual care group will not be told to refrain from physical activity; however, they will have no exposure to a formalised exercise intervention during the main research study period. Following the study period, the usual care arm will be offered an identical 12‐week supervised intervention (delayed exercise intervention). Both groups: subgroup analyses will be performed on a select number of intervention and usual care participants who choose to opt‐in to additional assessments upon enrolment in the trial. Concomitant interventions: both groups will continue to receive usual medical care. Follow‐up: 24 weeks |
| Outcomes |
Primary outcomes Lean body mass How measured: dual‐energy x‐ray absorptiometry scan Time points measured: baseline, 12 weeks and 24 weeks Secondary outcomes Muscle strength How measured: maximal leg press 1 RM, maximal hand grip strength and the 30‐second chair rise Time points measured: baseline, 12 weeks and 24 weeks Muscle endurance maximal and submaximal exercise capacity How measured: submaximal incremental aerobic exercise test on a cycle ergometer and 6MWT Time points measured: baseline, 12 weeks and 24 weeks Fatigue Not reported Health‐related quality of life How measured: FAACT questionnaire and SF‐36 questionnaire Time points measured: baseline, 12 weeks and 24 weeks |
| Starting date | 1 April 2019 |
| Contact information |
Principal investigator: Prof Prue Cormie; Email: prue.cormie@acu.edu.au Contact person for scientific queries: Ms Kelcey Bland; Email: kelcey.bland@acu.edu.au |
| Notes | Funding: Mary MacKillop Institute for Health Research, Australian Catholic University |
Solheim 2018.
| Study name | Cancer cachexia: rationale for the MENAC (Multimodal‐Exercise, Nutrition and Anti‐inflammatory medication for Cachexia) trial |
| Methods |
Study design: parallel randomised controlled trial, multicentre Study dates: 5 January 2015 to December 2020 Country: Canada, Germany, Norway, Switzerland, the UK |
| Participants |
Inclusion criteria: diagnosis of lung cancer, pancreatic cancer or cholangiocarcinoma where the diagnosis is based on histological, radiological or multidisciplinary team evaluation; non‐small cell lung cancer (stage III or IV), pancreatic adenocarcinoma (stage III or IV), due to commence first‐ or second‐line anticancer treatment (defined as chemotherapy, chemoradiotherapy, targeted therapy or immunotherapy); staging CT within 4 weeks of commencement of anticancer therapy (in patients where staging CT is without this period, further CT scanning will be undertaken. PET‐CT is also appropriate); completed all other baseline assessments within 1 week prior to first course of anticancer treatment; written informed consent; able to comply with trial interventions (in the opinion of referring clinician), e.g. willing and able to do light exercise and take oral nutritional supplements as well as no major contraindications against ibuprofen; Karnofsky Performance Status > 70 Exclusion criteria: neuro‐endocrine pancreatic cancer; creatinine clearance < 30 mL/minute; receiving parenteral nutrition or enteral nutrition via feeding tube; receiving neo‐adjuvant anticancer therapy; BMI > 30 kg/m2; use of appetite stimulants or anabolic/anticatabolic agents (such as megestrol acetate, progestational agents, marijuana growth hormone, dronabinol or other anabolic agent) within 30 days prior to study baseline; concomitant steroid (prednisolone > 10 mg/day or equivalent) treatment for < 3 months prior to inclusion (inhaled, optical or pulsed oral steroids (up to 10 days use) are permitted); concomitant long‐term (> 1 week) non‐steroidal anti‐inflammatory drugs or aspirin treatment; pregnancy, breast‐feeding or of child‐bearing potential (that is not postmenopausal or permanently sterilised) age and not using adequate contraception (oral, injected, implanted or hormonal methods of contraception, intrauterine device and barrier method); concomitant anticoagulant treatment (e.g. warfarin or heparin) Sample size: 240 participants |
| Interventions |
Experimental group: usual care plus multimodal intervention consisting of nutritional supplements and advice, home‐based self‐assisted exercise programme, and anti‐inflammatory medication (ibuprofen) Comparator group: usual palliative care |
| Outcomes |
Weight loss How measured: CT scan Time point measured: week 0, week 3, week 6 and week 12 Weight gain How measured: CT scan Time point measured: week 0, week 3, week 6 and week 12 Muscle mass How measured: CT scan Time point measured: week 0, week 3, week 6 and week 12 Physical activity level How measured: ActivPAL and 6MWT Time point measured: week 0, week 3, week 6 and week 12 Patient‐reported outcome measure How measured: appetite, physical activity and fatigue using the European Organisation for the Research and treatment of Cancer Quality of Life Questionnaire C30 and Health Status (EQ‐5D‐3L). Time point measured: week 0, week 3, week 6 and week 12 |
| Starting date | 5 January 5 2015 |
| Contact information | Trude R Balstad, PhD; trude.r.balstad@ntnu.no Tora S Solheim, MD PhD; tora.l.skeidsvoll@ntnu.no |
| Notes | Funding: Norwegian University of Science and Technology; St. Olavs Hospital; Oslo University Hospital; Cantonal Hospital of St. Gallen; Ottawa Regional Cancer Centre; Jewish General Hospital; Cross Cancer Institute; The Beatson West of Scotland Cancer Centre; Queen Margaret Hospital, Dunfermline; Cancer Research UK Edinburgh Centre; Malteser Krankenhaus Seliger Gerhardt; Tumor Biology Center Freiburg; Tumor Zentrum Aarau Trial register ID: NCT02330926 |
6MWT: six‐minute walk test; BMI: body mass index; CT: computed tomography; FAACT: Functional Assessment Anorexia/Cachexia Treatment; HRmax: maximum heart rate; PET‐CT: positron emission tomography‐computed tomography; RM: repetition maximum; RPE: rating of perceived exertion; SF‐36: 36‐item Short Form.
Differences between protocol and review
In the protocol, we did not plan to use GRADE and 'Summary of findings' tables, though we included both in this review, as important tools to assess and present the certainty of evidence produced.
In the protocol, we did not include the term multimodal intervention, though we described our intention to include exercise in combination with other interventions. As we observed the term being used in the literature and understand its concept, we added the term multimodal intervention when exercise was part of a multicomponent intervention.
In the protocol, we did not name the comparisons we conducted in the review. To make fair comparisons in the synthesis of findings from studies, we separated the comparisons to: exercise plus usual care compared with usual care and multimodal intervention (exercise plus other interventions) plus usual care compared with usual care.
In the protocol, we did not include the outcome measures section in the methods, thus we included it in the full review, it is very important to describe how each outcome was assessed.
In Assessment of heterogeneity, we added to the limited protocol text. In the review, we included details of an initial assessment of clinical and methodological heterogeneity and indicated the use of random‐effects models given the inclusion of multicomponent interventions.
Changes to the review team: L Sawaris Neto and JP Teixeira Basmage joined and R Riera, A Medeiros and SG Vitoriano left the team.
Contributions of authors
AJG co‐ordinated the protocol and review; retrieved papers; and wrote the background, methods, results, discussion and conclusions.
VS participated in the protocol and review; retrieved papers; and co‐wrote the background, methods, results, discussion and conclusions.
LSN co‐wrote the background, methods, results, discussion and conclusions.
JPTB co‐wrote the background, methods, results, discussion and conclusions.
MSP co‐wrote the protocol and review, worked on the background, methods, results, discussion and conclusions.
MM participated in the protocol and review, retrieved papers and co‐wrote the background, methods, results, discussion and conclusions.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK
MM is supported by a National Institute for Health Research (NIHR) Career Development Fellowship (CDF‐2017‐009) and the NIHR Applied Research Collaboration South London at King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care.
Declarations of interest
AJG: none.
VS: none.
LSN: none.
JPTB: none.
MSP: none.
MM: received personal fees for lectures and consultancy with Chugai UK (2015), Helsinn (2015 to 2017) and Fresenius Kabi (2016 to 2019) relating to cancer cachexia.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Capozzi 2016 {published data only}
- Capozzi LC, Lau H, Reimer RA, McNeely M, Giese-Davis J, Culos-Reed SN. Exercise and nutrition for head and neck cancer patients: a patient oriented, clinic-supported randomized controlled trial. BMC Cancer 2012;12:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi LC, McNeely ML, Lau HY, Reimer RA, Giese-Davis J, Fung TS, et al. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: results from an exploratory randomized controlled exercise trial. Cancer 2016;122(8):1185-200. [DOI] [PubMed] [Google Scholar]
- NCT01681654. Exercise and Nutrition for Head And Neck Cancer Patients (ENHANCE). clinicaltrials.gov/show/NCT01681654 (first received 10 September 2012).
Forget 2014 {published data only}
- Forget F, Frusch N, Trokay L, Archen C, Courtois a-C. A randomized trial comparing best supportive care (BSC) versus multimodality approach (MA) to fight against cachexia in patients with cancer treated with chemotherapy. Journal of Clinical Oncology 2014;32(15 Suppl 1):e20655. [DOI: 10.1200/jco.2014.32.15_suppl.e20655 ] [Google Scholar]
Grote 2018 {published data only}
- Grote M, Maihöfer C, Weigl M, Davies-Knorr P, Belka C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial. Radiation Oncology 2018;13(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03524755. Physical exercise for patients who suffer from weight loss due to head and neck cancer undergoing medical treatment. clinicaltrials.gov/show/NCT03524755 (first received 15 May 2018).
Solheim 2017 {published data only}
- EudraCT 2010-022897-14. PreMENAC: multimodal exercise/nutrition/anti-inflammatory treatment for cachexia: a feasibility study (phase II). www.clinicaltrialsregister.eu/ctr-search/trial/2010-022897-14/results (first received 18 September 2016).
- Kaasa S, Solheim T, Laird BJ, Balstad T, Stene GB, Bye A, et al. A randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to prevent/attenuate cachexia in advanced cancer patients undergoing chemotherapy. Journal of Clinical Oncology 2015;33(15 Suppl):9628. [Google Scholar]
- NCT01419145. A feasibility study of multimodal exercise/nutrition/anti-inflammatory treatment for cachexia – the pre-MENAC Study. clinicaltrials.gov/show/NCT01419145 (first received 17 August 2011).
- Rakel Balstad T, Solheim TS, Laird B, Fearon K, Kaasa S, Bye A. PP090-SUN: Feasibility of dietary counseling to attenuate cachexia in patients with advanced cancer. Clinical Nutrition 2015;33:S53. [Google Scholar]
- Solheim TS, Laird BJ, Balstad TR, Stene GB, Bye A, Johns N, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. Journal of Cachexia, Sarcopenia and Muscle 2017;8(5):778-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Arrieta 2019 {published data only}
- Arrieta H, Astrugue C, Regueme S, Durrieu J, Maillard A, Rieger A, et al. Effects of a physical activity programme to prevent physical performance decline in onco-geriatric patients: a randomized multicentre trial. Journal of Cachexia, Sarcopenia and Muscle 2019;10(2):287-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Battaglini 2010 {published data only}
- Battaglini C, Groff D, Shields E, Evans E, Naumann F, Peppercorn J, et al. Exercise and psychosocial interventions in breast cancer survivors: preliminary results of a randomized controlled trial. Medicine and Science in Sports and Exercise 2010;42(5):344-5. [Google Scholar]
Carnaby‐Mann 2012 {published data only}
- Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. "Pharyngocise": randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. International Journal of Radiation Oncology, Biology, Physics 2012;83(1):210-9. [DOI] [PubMed] [Google Scholar]
Cheville 2010 {published data only}
- Cheville AL, Girardi J, Clark MM, Rummans TA, Pittelkow T, Brown P, et al. Therapeutic exercise during out patient radiation therapy for advanced cancer: feasibility and impact on physical well-being. American Journal of Physical Medicine and Rehabilitation 2010;89(8):611-9. [DOI] [PubMed] [Google Scholar]
Courneya 2009 {published data only}
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. Journal of Clinical Oncology 2009;27(27):4605-12. [DOI] [PubMed] [Google Scholar]
Elter 2009 {published data only}
- Elter T, Stipanov M, Heuser E, Bergwelt-Baildon M, Bloch W, Hallek M, et al. Is physical exercise possible in patients with critical cytopenia undergoing intensive chemotherapy for acute leukaemia or aggressive lymphoma? International Journal of Hematology 2009;90(2):199-204. [DOI] [PubMed] [Google Scholar]
Fouladiun 2007 {published data only}
- Fouladiun M, Körner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clinical Cancer Research 2007;13(21):6379-85. [DOI] [PubMed] [Google Scholar]
Irwin 2009 {published data only}
- Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009;17(8):1534-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2019 {published data only}
- Jain R, Handorf E, Khare V, Blau M, Chertock Y, Hall MJ. Impact of baseline nutrition and exercise status on toxicity and outcomes in phase I and II oncology clinical trial participants. Oncologist 2019;289:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuehr 2014 {published data only}
- Kuehr L, Wiskemann J, Abel U, Ulrich CM, Hummler S, Thomas M. Exercise inpatients with non-small cell lung cancer. Medicine and Science in Sports and Exercise 2014;46(4):656-63. [DOI] [PubMed] [Google Scholar]
Litterini 2013 {published data only}
- Litterini AJ, Fieler VK, Cavanaugh JT, Lee JQ. Differential effects of cardiovascular and resistance exercise on functional mobility in individuals with advanced cancer: a randomized trial. Archives of Physical Medicine and Rehabilitation 2013;94(12):2329-35. [DOI] [PubMed] [Google Scholar]
Mantovani 2010 {published data only}
- Mantovani G, Macciò A, Madeddu C, Dessì M, Serpe R, Antoni G, et al. Focus on the assessment of physical activity level of patients with cancer cachexia enrolled in a randomized phase III clinical trial. Journal of Clinical Oncology 2010;28(15):9164. [Google Scholar]
- Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. European Review for Medical and Pharmacological Sciences 2010;14(4):292-301. [PubMed] [Google Scholar]
Oldervoll 2006 {published data only}
- Oldervoll LM, Loge JH, Paltiel H, Asp MB, Vidvei U, Wiken AN, et al. The effect of a physical exercise program in palliative care: a phase II study. Journal of Pain and Symptom Management 2006;31(5):421-30. [DOI] [PubMed] [Google Scholar]
Oldervoll 2011 {published data only}
- Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist 2011;16(11):1649-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Op den Kamp 2012 {published data only}
- Op den Kamp CM, Langen RC, Minnaard R, Kelders MC, Snepvangers FJ, Hesselink MK, et al. Pre-cachexia in patients with stages I-III non-small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer 2012;76(1):112-7. [DOI: 10.1016/j.lungcan.2011.09.012] [DOI] [PubMed] [Google Scholar]
Saarto 2012 {published data only}
- Saarto T. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporosis International 2012;23(5):1601-12. [DOI] [PubMed] [Google Scholar]
Schwartz 2007 {published data only}
- Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 2007;34(3):627-33. [DOI] [PubMed] [Google Scholar]
Solheim 2019 {published data only}
- Solheim TS, Vagnildhaug OM, Laird BJ, Balstad TR. Combining optimal nutrition and exercise in a multimodal approach for patients with active cancer and risk for losing weight: rationale and practical approach. Nutrition 2019;Nov-Dec:67-8. [DOI] [PubMed] [Google Scholar]
Uster 2018 {published data only}
- Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clinical Nutrition 2018;37(4):1202-9. [DOI: 10.1016/j.clnu.2017.05.027] [DOI] [PubMed] [Google Scholar]
Vanderbyl 2017 {published data only}
- Vanderbyl BL, Mayer MJ, Nash C, Tran AT, Windholz T, Swanson T, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Supportive Care in Cancer 2017;25(6):1749-58. [DOI: 10.1007/s00520-017-3579-x] [DOI] [PubMed] [Google Scholar]
Zatarain 2013 {published data only}
- Zatarain LA. Body composition in head and neck cancer patients undergoing concurrent chemoradiation. Journal of Clinical Oncology 2013;31 Suppl 1:15. [Google Scholar]
References to studies awaiting assessment
Rogers 2011 {published data only}
- ACTRN12611000870954. Anti-inflammatory and nutritional support, with simple exercises in lung cancer patients with weight loss. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343304 (first received 16 August 2011).
- Rogers ES, MacLeod RD, Stewart J, Bird SP, Keogh JW. A randomised feasibility study of EPA and COX-2 inhibitor (Celebrex) versus EPA, COX-2 inhibitor (Celebrex), resistance training followed by ingestion of essential amino acids high in leucine in NSCLC cachectic patients – ACCeRT study. BMC Cancer 2011;11:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12619000426189 {published data only}
- ACTRN12619000426189. ACE trial: the Advanced cancer & Cachexia Exercise trial. mmihr.acu.edu.au/projects/ace-trial/ (accessed 31 July 2019).
- ACTRN12619000426189. Feasibility and efficacy of exercise in advanced cancer patients with cachexia. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12619000426189 (first received 19 February 2019).
Solheim 2018 {published data only}
- EudraCT 2013-002282-19. A randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to prevent / attenuate cachexia in advanced cancer patients undergoing chemotherapy. www.clinicaltrialsregister.eu/ctr-search/trial/2013-002282-19/GB (first received 7 April 2016).
- MENAC-2013-05. A randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to prevent/attenuate cachexia in advanced cancer patients undergoing chemotherapy. bonn.clinicalsite.org/de/cat/457/trial/3212 (accessed 7 August 2019).
- NCT02330926. Multimodal intervention for cachexia in advanced cancer patients undergoing chemotherapy (MENAC). clinicaltrials.gov/ct2/show/NCT02330926 (first received 5 January 2015).
- Solheim TS, Laird BJ, Balstad TR, Bye A, Stene G, Baracos V, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Supportive Palliative Care 2018;8(3):258-65. [DOI] [PubMed] [Google Scholar]
Additional references
Antoun 2013
- Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013;25:2821-8. [DOI] [PubMed] [Google Scholar]
Arends 2017
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clinical Nutrition 2017;1:11-48. [DOI] [PubMed] [Google Scholar]
Argilés 2012
- Argilés JM, Busquets S, López-Soriano FJ, Costelli P, Penna F. Are there any benefits of exercise training in cancer cachexia? Journal of Cachexia, Sarcopenia and Muscle 2012;3:73-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baracos 2018
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KC. Cancer-associated cachexia. Nature Reviews Disease Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
Betof 2013
- Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, Behavior, and Immunity 2013;30:S75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from training.cochrane.org/handbook/archive/v5.1/.
Dewey 2007
- Dewey A, Baughan C, Dean TP, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD004597. [DOI: 10.1002/14651858.CD004597.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodson 2011
- Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annual Review of Medicine 2011;62:265-79. [DOI] [PubMed] [Google Scholar]
England 2012
- England R, Maddocks M, Manderson C, Wilcock A. Factors influencing exercise performance in thoracic cancer. Respiratory Medicine 2012;106:294-9. [DOI] [PubMed] [Google Scholar]
Evans 2008
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clinical Nutrition 2008;27:793-9. [DOI: 10.1016/j.clnu.2008.06.013] [DOI] [PubMed] [Google Scholar]
Fearon 2008
- Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. European Journal of Cancer 2008;44(8):1124-32. [DOI] [PubMed] [Google Scholar]
Fearon 2011
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncology 2011;12(5):489-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fearon 2012
- Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signalling and metabolic pathways. Cell Metabolism 2012;16:153-66. [DOI] [PubMed] [Google Scholar]
Gleeson 2011
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews. Immunology 2011;9:607-15. [DOI] [PubMed] [Google Scholar]
Glover 2010
- Glover EI, Phillips SM. Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Current Opinion in Clinical Nutrition and Metabolic Care 2010;13:630-4. [DOI] [PubMed] [Google Scholar]
Gould 2013
- Gould DW, Lahart I, Carmichael AR, Koutedakis Y, Metsios GS. Cancer cachexia prevention via physical exercise: molecular mechanisms. Journal of Cachexia, Sarcopenia and Muscle 2013;4(2):111-24. [DOI: 10.1007/s13539-012-0096-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 5 February 2021. Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendation. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2019
- Hall CC, Cook J, Maddocks M, Skipworth RJ, Fallon M, Laird BJ. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: a systematic review. Support Care Cancer 2019;27(7):2371-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-59. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2 (updated June 2017). Cochrane, 2017. training.cochrane.org/handbook/archive/v5.2.
Hoffmann 2014
Jones 2012
- Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012;76:248-52. [DOI: 10.1016/j.lungcan.2011.10.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laviano 2005
- Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: cancer anorexia-cachexia syndrome – when all you can eat is yourself. Nature Clinical Practice Oncology 2005;2:159-65. [DOI] [PubMed] [Google Scholar]
LeBlanc 2015
- LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, Kamal AH, et al. Correlation between the international consensus definition of the Cancer Anorexia-Cachexia Syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. Journal of Pain and Symptom Management 2015;49(4):680-9. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee SJ, Glass DJ. Treating cancer cachexia to treat cancer. Skeletal Muscle 2011;1(2):1-5. [DOI: 10.1186/2044-5040-1-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddocks 2011
- Maddocks M, Murton AJ, Wilcock A. Improving muscle mass and function in cachexia: non-drug approaches. Current Opinion in Supportive and Palliative Care 2011;5(4):361-4. [DOI: 10.1097/SPC.0b013e32834bdde3] [DOI] [PubMed] [Google Scholar]
Maddocks 2012
- Maddocks M, Murton AJ, Wilcock A. Therapeutic exercise in cancer cachexia. Critical Reviews in Oncogenesis 2012;17:285-92. [DOI] [PubMed] [Google Scholar]
Marimuthu 2011
- Marimuthu K, Murton AJ, Greenhaff PL. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. Journal of Applied Physiology 2011;110:555-60. [DOI] [PubMed] [Google Scholar]
Martin 2013
- Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of Clinical Oncology 2013;31(12):1539-47. [DOI] [PubMed] [Google Scholar]
McMillan 2013
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treatment Reviews 2013;39:534-40. [DOI] [PubMed] [Google Scholar]
Moses 2009
- Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncology Reports 2009;21:1091-5. [DOI] [PubMed] [Google Scholar]
Muscaritoli 2010
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Specialist Interest Groups (SIG) 'cachexia – anorexia in chronic wasting diseases' and 'nutrition in genetics'. Clinical Nutrition 2010;29:154-9. [DOI] [PubMed] [Google Scholar]
Naito 2017
- Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer 2017;17(1):800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Payne 2017
- Payne C, Wiffen PJ, Martin S. Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD008427. [DOI: 10.1002/14651858.CD008427.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prado 2007
- Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clinical Cancer Research 2007;13:3264-8. [DOI] [PubMed] [Google Scholar]
Prado 2008
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncology 2008;9:629-35. [DOI] [PubMed] [Google Scholar]
Prado 2009
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clinical Cancer Research 2009;15:2920-6. [DOI] [PubMed] [Google Scholar]
Proctor 2011
- Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. British Journal of Cancer 2011;104:726-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radbruch 2010
- Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical Practise Guidelines on Cancer Cachexia in Advanced Cancer Patients. Aachen: Department of Palliative Medicine/European Palliative Care Research Collaborative, 2010. [Google Scholar]
Reid 2012
- Reid J, Mills M, Cantwell M, Cardwell CR, Murray LJ, Donnelly M. Thalidomide for managing cancer cachexia. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD008664. [DOI: 10.1002/14651858.CD008664.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roeland 2020
- Roeland EJ, Bohlke K, Baracos VE, Bruera E, Fabbro Ed, Dixon S, et al. Management of cancer cachexia: ASCO guideline. Journal of Clinical Oncology 2020;38(21):2438-53. [DOI] [PubMed] [Google Scholar]
Ruiz Garcia 2013
- Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Martí S. Megestrol acetate for treatment of anorexia‐cachexia syndrome. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD004310. [DOI: 10.1002/14651858.CD004310.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Solheim 2012
- Solheim TS, Laird BJ. Evidence base for multimodal therapy in cachexia. Current Opinion in Supportive and Palliative Care 2012;6(4):424-31. [DOI] [PubMed] [Google Scholar]
Starkie 2003
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNFα production in humans. FASEB Journal 2003;17:884-6. [DOI] [PubMed] [Google Scholar]
Stephens 2012
- Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, Skipworth RJ, et al. Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clinical Nutrition 2012;31:499-505. [DOI] [PubMed] [Google Scholar]
Thompson 2010
- Thompson WR, Gordon NF, Pescatello LS. ACSM's Guidelines for Exercise Testing and Prescription. 8th edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2010. [Google Scholar]
Tisdale 2009
- Tisdale MJ. Mechanisms of cancer cachexia. Physiology Review 2009;89:381-410. [DOI: 10.1152/physrev.00016.2008] [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang X, Hu Z, J Hu, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006;147(9):4160-8. [DOI] [PubMed] [Google Scholar]
Weber 2009
- Weber MA, Krakowski-Roosen H, Schroder L, Kinscherf R, Krix M, Kopp-Schneider A, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncologica 2009;48:116-24. [DOI] [PubMed] [Google Scholar]
Wilcock 2008
- Wilcock A, Maddocks M, Lewis M, England R, Manderson C. Symptoms limiting activity in cancer patients with breathlessness on exertion: ask about muscle fatigue. Thorax 2008;63:91-2. [DOI] [PubMed] [Google Scholar]
Wilms 2016
- Wilms B, Schmid SM, Luley K, Wiskemann J, Lehnert H. Prevention and treatment of cachexia: exercise and nutritional therapy. Der Internist 2016;57(10):971-7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Grande 2013
- Grande AJ, Silva V, Maddocks M, Riera R, Medeiros A, Vitoriano SG, et al. Exercise for cancer cachexia in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD010804. [DOI: 10.1002/14651858.CD010804] [DOI] [PubMed] [Google Scholar]
Grande 2014
- Grande AJ, Silva V, Riera R, Medeiros A, Vitoriano SG, Peccin MS, et al. Exercise for cancer cachexia in adults. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD010804. [DOI: 10.1002/14651858.CD010804.pub2] [DOI] [PubMed] [Google Scholar]
Grande 2015
- Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: executive summary of a Cochrane Collaboration systematic review. Journal of Cachexia, Sarcopenia and Muscle 2015;6(3):208-11. [DOI: 10.1002/jcsm.12055] [Only an executive summary of Grande 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]


