Abstract
Our understanding of the neural basis of somatosensation is based largely on studies of the whisker system of mice and rats and the hands of macaque monkeys. Results across these animal models are often interpreted as providing direct insight into human somatosensation. Work on these systems has proceeded in parallel, capitalizing on the strengths of each model, but has rarely been considered as a whole. This lack of integration promotes a piecemeal understanding of somatosensation. Here, we examine the functions and morphologies of whiskers of mice and rats, the hands of macaque monkeys, and the somatosensory neuraxes of these three species. We then discuss how somatosensory information is encoded in their respective nervous systems, highlighting similarities and differences. We reflect on the limitations of these models of human somatosensation and consider key gaps in our understanding of the neural basis of somatosensation.
Introduction
How do humans process a restricted collection of sensory inputs to generate an extraordinarily complex and diverse repertoire of behaviors? While our desire is to understand our own species, much of what we know about the human brain comes from work on other animals, and most of what we know about sensory processing in mammals comes from work on a few animal models, namely mice, rats, and macaque monkeys. Since extrapolation across species is essential to understanding the human brain – and nervous system function more broadly – cross-species comparisons should be made in a well-informed manner (Figure 1). Somatosensation offers a useful case study of this comparative process, given the extensive work on the organization and function of this sensory system in mice, rats, and macaques.
Figure 1.
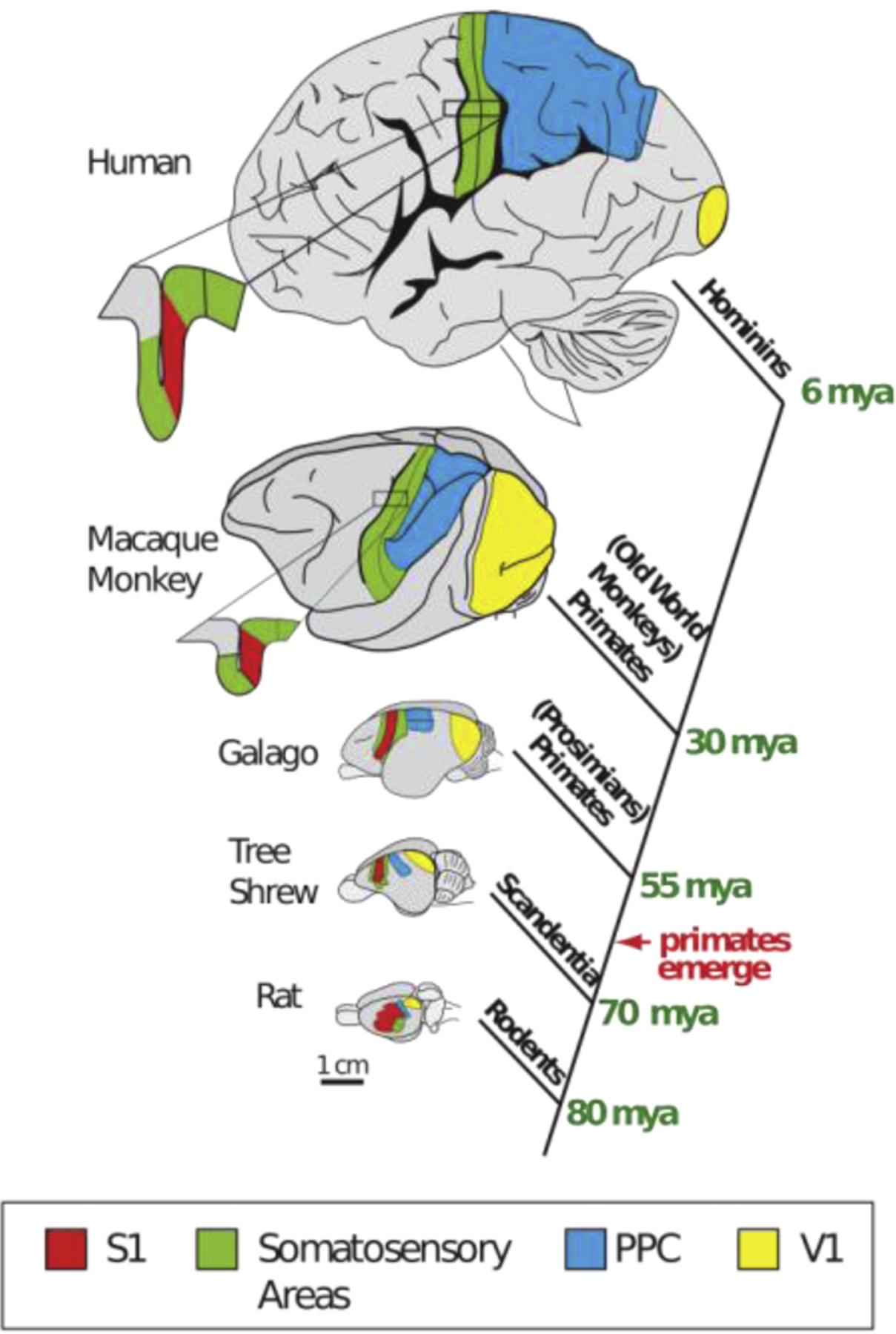
A cladogram showing the phylogenetic relationship of five different mammals with each branching point indicating the time of the last common ancestor. Brains are drawn to (relative) scale except the human brain, which is substantially scaled down. The location of the primary somatosensory area, S1 (green), the primary visual area, V1 (yellow), and the posterior parietal cortex (PPC; blue) are depicted. Note that the relative size of the PPC is greatly expanded in primates, particularly in humans. From Goldring and Krubitzer, 2017.
With this in mind, we explore the neural basis of touch and proprioception in these three species, focusing on the hand in macaques and the whisker system of mice and rats, because these sensory systems have received the bulk of the experimental attention. The adoption of the whisker system of mice and rats as a model for understanding the somatosensory system of humans seems counterintuitive, given the vast differences between whiskers and hands. While rats have been used in experimental studies for over 150 years, and genetic studies in mice have been done since the early part of the last century, the use of these murine rodents as models for somatosensory processing is likely due to historic happenstance. Traditionally, the barrel system was critical for understanding how sensory input contributes to the development of the neocortex and aspects of cortical organization1,2. Indeed, the one-to-one correspondence between barrels and individual whiskers allowed for a variety of straightforward experimental manipulations to the whiskers (e.g. trimming individual whiskers or rows of whiskers at different developmental time points) coupled with direct examination of the cortical manifestations of these manipulations. The resulting surge in studies on the barrel system was followed by the development of genetic tools and other sophisticated techniques to examine cells and circuits within the mouse brain. Rodents have thus become the dominant animal model in neuroscience, including somatosensory neuroscience.
Our objective is to assess the degree to which mice, rat, and macaque models are synergistic in a quest to understand somatosensation, and to place similarities and differences in a phylogenetic and ecological context. To this end, we examine the biomechanics of the hands and whiskers, and their sensory innervation, and the neuroanatomy of the associated neuraxes. We then discuss how information is encoded in neuronal populations at different stages of processing to assess whether these three animal models convey a consistent picture of somatosensation.
This review is written from three very distinct perspectives – a mouse neurophysiologist, a monkey neurophysiologist, and an evolutionary neurobiologist – in an attempt to synthesize findings from three widely used animal models and to generate a common framework from which we can understand the somatosensory system of mammals in general. One motivation for this endeavor is the misconception that the somatosensory system is well understood, especially in comparison to the much more advanced understanding of the visual system. Indeed, most information on the somatosensory system comes from textbooks and older reviews, which focus on well-established anatomical pathways or specific features of processing in a few animals. This limited focus impedes a broader understanding of the fundamental, organizational principles of the somatosensory system in mammals in general.
Functions of whiskers and hands
The innervation of an effector is dependent on the function of that effector. To understand and compare somatosensory processing in mice and monkeys thus requires that we first place the respective sensory systems in the context of their function (Figure 2). Although frequently studied in mice and rats, whiskers are nearly ubiquitous among mammals. The principal function of whiskers is to explore peripersonal space for the purposes of navigation, localization, and identification of nearby objects and surfaces. Whiskers are also used for tracking the contours of a wall during locomotion or rapid prey motions during close-range hunting and pursuit. Some evidence suggests rats also use whiskers to sense airflow3, akin to the function of whiskers as hydrodynamic sensors in seals4. While hands can be used for navigation and for object identification (stereognosis) when vision is not available, their principal function is to grasp and manipulate objects.
Figure 2. Functions of hands and whiskers.
Both whiskers and hands can sense object properties such as texture and shape, but only hands can grasp and manipulate objects.
Distinct but overlapping forms of sensory information are required to support the various functions of whiskers and hands. Navigation requires information about the spatial location and extent of a landmark. Tracking requires information about object motion to guide rapid compensatory changes in posture. Stereognosis, an ability achieved via both whiskers and hands, requires information about the three-dimensional structure of an object. Manipulation requires information about the location and dynamics of contact events. Of course, these functional domains share informational requirements to a degree – for example some information about the three-dimensional structure of an object is required for manipulation – but their relative importance varies. For comparison, the most conspicuous example of a somatosensory specialization is the snout of the star nosed mole, whose exploratory and grasping function has endowed it with a hand-like sensory innervation5. These functional requirements have shaped the somatosensory systems of mammals and underscore the importance of the interplay between brain, body, and behavior for understanding how sensory systems evolve.
Biomechanics of whiskers and hands
Interactions with objects cause deformations of the whiskers or skin, which then transmit stresses to mechanoreceptors, which in turn convert these stresses into electrical signals. The mechanical properties of the whiskers and skin thus play a role in shaping the sensory signals transmitted to the central nervous system. Whiskers are tapered beams that transmit to the whisker follicle stresses produced during whisker-object interactions6,7. A variety of different types of receptors in the whisker follicle, each sensitive to different aspects of the deflection, transduce local stresses into action potentials. Whisker follicles have a complex structure into which receptors take up formation at specific loci that likely expose them to distinct features of the mechanical input8. The transmission of stresses applied to different locations along the whisker, and the dependence of this process on the geometry of the whiskers, which varies systematically across the face, has been successfully modeled using quantitative approaches6,7,9.
The glabrous skin of the hand provides an obviously different coupling between the outside world and mechanoreceptors than does the whisker. Simple biomechanical models of the skin based on continuum mechanics capture some essential features of how the skin deforms when indented, including the enhancement of external features of an object and the obscuration of internal ones due to the way forces propagate through the tissue10,11. Finite element models12–14 involve more detailed descriptions of the skin and, unlike their continuum mechanical counterparts, take into account the influence of the bone on the skin’s response, particularly near the joints. Regardless, the response properties of mechanoreceptors are critically dependent on skin mechanics. For example, nerve fibers that innervate receptors located deeper in the skin tend to have larger receptive fields, a phenomenon that can be attributed to skin mechanics, as can the enhanced afferent responses to edges and corners10,11.
The respective morphologies and mechanical properties of the whiskers and skin differ substantially, and these differences can lead to important differences in the resulting neuronal representations. For example, whiskers sample an object sparsely and provide a time-varying signal at each contact point that is determined by the forces applied on the whisker. In contrast, hands provide a spatio-temporal signal at each point of contact – reflecting the spatio-temporal pattern of skin deformation – a signal that is shaped by skin biomechanics as forces applied to the skin’s surface propagate through the tissue. The disparate morphologies and mechanical properties lead to differences in the resulting sensory representations, as discussed below.
Peripheral innervation
The peripheral innervation of whisker follicles and the fingertip are highly distinct, although both share Merkel-type afferents (Figure 3). In mice, each follicle is innervated by 100 to 300 sensory fibers, each of which terminates in one of roughly 8 distinct types of mechanoreceptors8,15. The cell bodies of these mechanoreceptors are located in the trigeminal ganglion, and each includes a single whisker in its receptive field16. Nerve fibers can be categorized electrophysiologically into slowly adapting and rapidly adapting classes based on whether or not they produce a sustained response to a sustained whisker deflection. However, this dichotomy only captures one of many ways in which afferent responses differ across classes17. So far, only Merkel afferents of the follicle have been studied during behavior,18 but advances in genetic labeling will likely lead to rapid progress in characterizing other afferent types.
Figure 3. Innervation of the whisker follicle and fingertip.
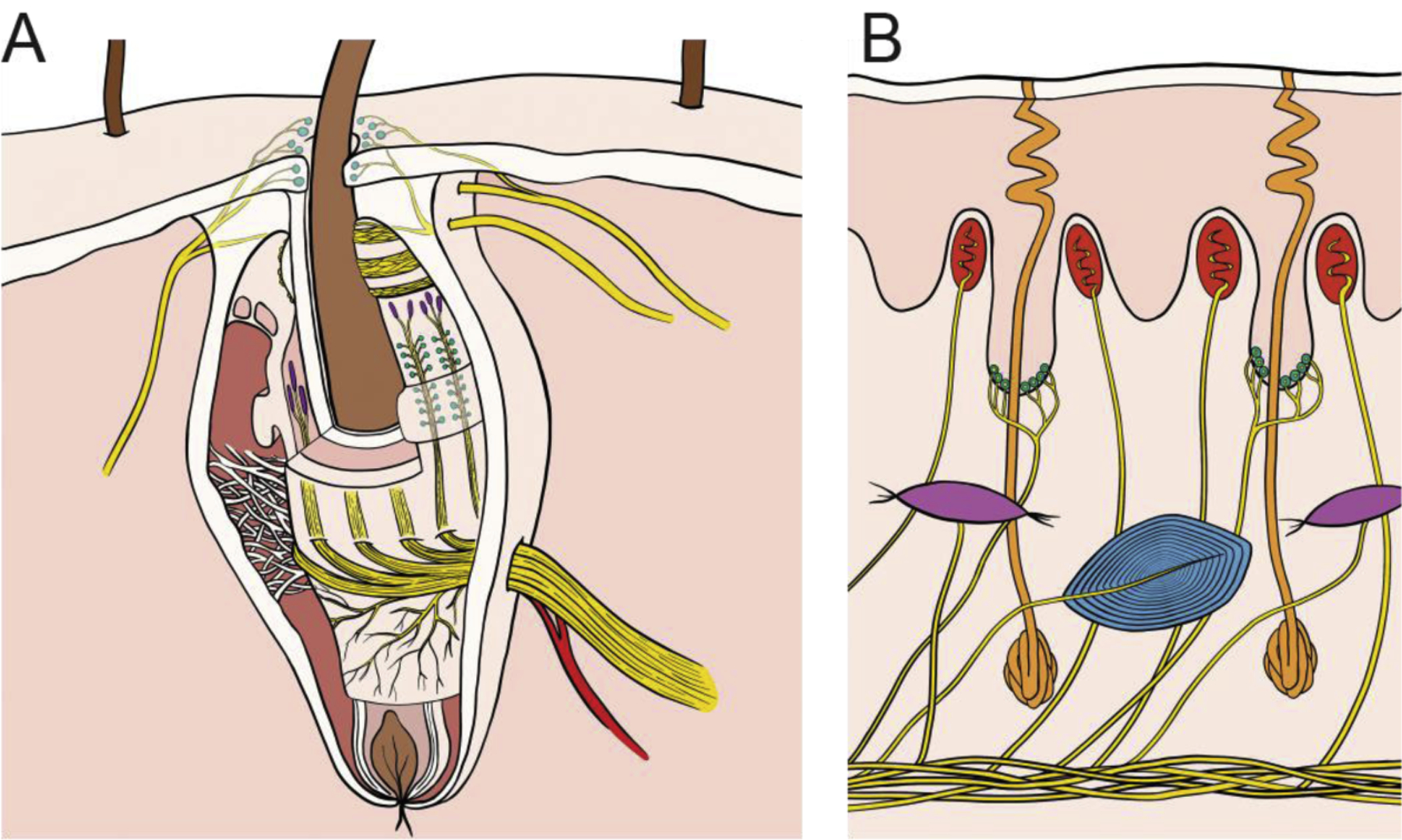
A| Whisker follicles in mice and rats are complex encapsulated structures containing ~8 different mechanoreceptor types6,13, including Merkel cell (cyan) and lanceolate-type (purple) endings. The responses of many types to sensory input remain unknown. The entire follicle rotates together with the whisker during whisking, due to the action of intrinsic and extrinsic muscles. B| The primate fingertip contains three main types of mechanoreceptors including Merkel cell (cyan), innervated by slowly adapting type 1 nerve fibers, Meissner corpuscles (red), innervated by rapidly adapting fibers, Pacinian corpuscles (blue), innervated by Pacinian corpuscle associated fibers. A fourth type, Ruffini endings (purple), innervated by slowly adapting type 2 fibers, is absent in macaques and sparse in human glabrous skin.
The glabrous skin of the macaque hand is innervated by three types of tactile nerve fibers, each of which innervates a different type of mechanoreceptor and responds best to different aspects of skin deformations. The cutaneous innervation of the palm is similar in macaques and humans except that macaques lack the Ruffini-type nerve fiber, which sparsely innervates human glabrous skin19. Analogously to whisker follicle afferents, cutaneous nerve fibers can be split based on their adaptation properties: rapidly adapting fibers respond only to the onset and offset of an indentation whereas slowly adapting fibers respond throughout the stimulus presentation. Nerve fibers are also classically divided according to the size of their receptive fields (RF): type 1 fibers have small RFs, type 2 have large ones. During most manual interactions with objects, all three types of nerve fiber are activated and convey distinct but overlapping information about object features20,21.
While the substrates are very different – whisker vs. skin – the peripheral innervation is qualitatively similar across rodents and macaques, consisting of an overlapping set of afferent classes. The hand and whiskers are innervated by approximately 18,000 and 5,000 tactile nerve fibers, respectively, making them the most densely innervated regions of their respective organisms. As a point of comparison, however, the star of the star-nosed mole is innervated by 100,000 nerve fibers, making it the most densely innervated organ in the animal kingdom22.
Organization of the somatosensory neuraxis
Cortical organization
Perhaps the most profound differences in the somatosensory systems of rats, mice, and macaque monkeys are at the level of the neocortex (Figure 4). While these three species all have a primary somatosensory area (S1 or area 3b), a secondary somatosensory area (S2), and a parietal ventral area (PV) that process cutaneous inputs, there the similarities end. The extent of cortex that processes somatic input, the number of cortical fields involved in this network, and the interconnections of this network are radically different in rats, mice, and macaque monkeys. In addition to the areas noted above, macaque monkeys have three additional anterior parietal fields including area 1, which processes cutaneous input, area 3a, which processes proprioceptive input, and area 2, which integrates cutaneous and proprioceptive inputs23–25. While many use the term “primary somatosensory cortex” (“S1”) to refer collectively to areas 3a, 3b, 1, and 2 in macaque monkeys, this is an outmoded concept since it has been well established for decades that only area 3b is homologous to S1 in a range of other mammals26. Although the dysgranular zone in mice and rats has been proposed to be the homologue of area 3a in primates27, this is still speculative, and there are no clear homologues of macaque areas 1 and 2 in rats and mice. In addition to the expansion of anterior parietal cortex, the number of somatosensory areas in the lateral sulcus of macaque monkeys has increased to include the ventral somatosensory area (VS), the parietal rostral region (PR), and the retroinsular area (Ri)28. Finally, similar changes have emerged in the posterior parietal cortex (PPC) of monkeys (Figure 1 and 4), which spans several centimeters of cortex and includes multiple cortical areas associated with hand use29,30 (see31,32 for a review). In rats and mice, a small strip of cortex – located between S1 and primary visual cortex and occupying about 1 × 2 mm of cortical territory33 – may be the homologue of some parts of PPC in macaque monkeys (Figure 4). Because of these extreme differences, it is difficult to make comparisons of somatosensory cortical areas between commonly used rodent models and macaque monkeys outside of S1, S2 and PV.
Figure 4. The organization of somatosensory cortex in mice, rats and, macaque monkeys.
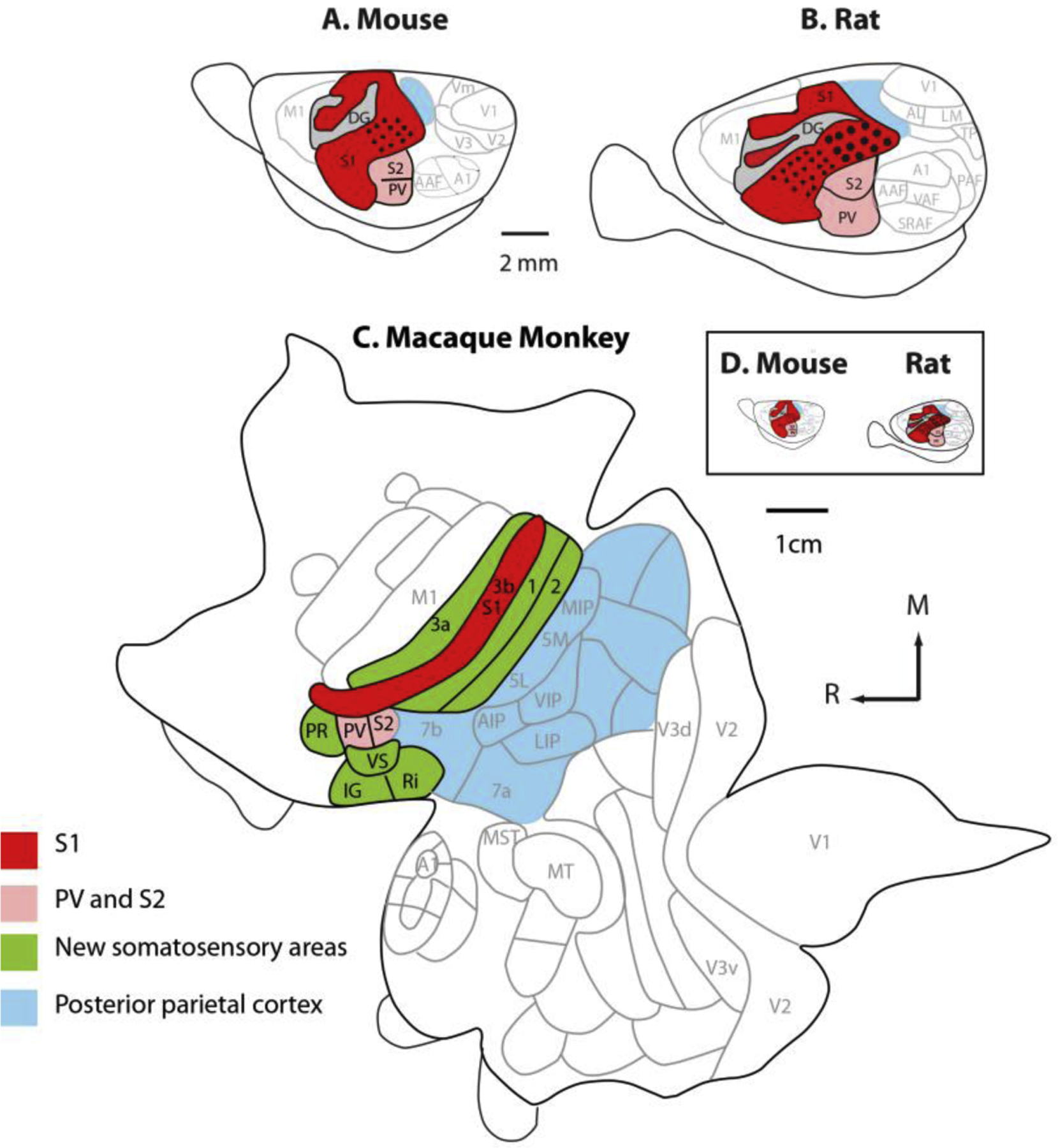
All three species have a primary somatosensory area (red), a second somatosensory area, and a parietal ventral area (pink). In macaque monkeys, additional areas that process somatosensory inputs have emerged over the course of evolution (green). Posterior parietal cortex has greatly expanded and includes multiple cortical fields in macaque monkeys. When mice and rat cortices are drawn to scale (D) the enormous difference in the size of the cortical sheet in these rodent models and macaque monkeys is striking. Adapted from Dooley and Krubitzer, 2013.
In addition to the differences in the number of cortical areas that process tactile and proprioceptive inputs, the somatotopic organization is also distinct in these animal models, even in homologous cortical areas. In macaque monkeys, the primary somatosensory cortex (area 3b) is dominated by the representation of the glabrous hand and comprises a detailed representation of the individual digits and the palmar surface of the hand23. In macaque monkeys, S1 neurons that respond to stimulation of the glabrous hand have very small receptive fields, particularly on the digit tips. In contrast, approximately 50% of S1 in mice and rats is devoted to representing the vibrissae and perioral structures and the RFs of these neurons are small34, like their hand counterparts in macaques. While there is a wealth of data on the whisker representation (barrel cortex) in mice, to our knowledge there are no detailed maps of the entire body representation in S1 of mice; however early studies by Woolsey indicate that the overall organization of S1 in mice is much like that in rats27,35. Differences in somatotopic organization and cortical magnification are maintained to some degree in higher order fields such as S2 and PV in macaque monkeys, rats, and mice28,36,37. In areas 3a, 1, and 2 in macaque monkeys, the dominance of the hand and digit representation is maintained such that a huge swath of anterior parietal cortex is devoted to representing the glabrous skin, reflecting the importance of the palmar surface of the hand in touch-mediated behaviors23–25. Similarly, higher order somatosensory cortices in mice and rats comprise an oversized representation of the whiskers27. Finally, PPC in monkeys occupies a huge expanse of cortex and is composed of multiple, well defined cortical fields31,38–40. Most of the rostral portions of posterior parietal cortex in macaques are associated with the somatosensory system. These cortical fields are devoted almost exclusively to representing the forelimb and are involved in the planning of reaching and grasping31,32,41. The proposed homologue of PPC in rats and mice features a coarse topography of the whiskers42 and is proposed to be involved in a number of functions including encoding behaviorally relevant tactile information, postural control, and sensory memory to name a few42–44.
These differences in cortical field number and magnification between mice and rats compared to macaque monkeys are not necessarily a “rodent” phenomenon. In squirrels, for example, a cortical field rostral to and partially embedded in S1 comprises neurons that respond to joint and muscle manipulation, and this region may be homologous to area 3a in macaque monkeys and other primates45. In a field caudal to S1, neurons are responsive to stimulation of both cutaneous receptors and joint and muscle manipulation. This field, termed the parietal medial area (PM)27,45, may be homologous to area 2 or to some portion of PPC in macaque monkeys. Furthermore, S1 in squirrels has a large representation of the lower and upper lips, a small representation of the vibrissae, and a large representation of the digits, and forelimb46. The pattern of cortical magnification in squirrels is thus more similar to that in primates than to that in mice and rats.
These types of differences exist in the somatosensory cortex of other rodents as well27. Thus, mice and rats are not necessarily good “models” of the rodent order when considering somatosensory cortex.
Somatosensory networks
Differences in the number and organizational features of cortical fields in mice, rats, and macaque monkeys are accompanied by differences in the cortical and subcortical networks involved in somatosensory processing. While detailing the specific patterns of connectivity in these animal models falls outside the scope of this review, several fundamental differences in connectivity patterns between these species are noteworthy. The first is in their thalamocortical connections. In macaque monkeys, a large portion of the dorsal thalamus is devoted to somatosensory processing and multiple thalamic nuclei beyond the ventral posterior nucleus (VP) are associated with the somatosensory system47. In contrast, the somatosensory thalamus of mice and rats is limited to VP and the posterior medial nucleus (POm)48,49. Thalamocortical connections between VP and S1 in macaques, mice, and rats are similar, but there the similarities end. Specifically, in macaque monkeys, thalamic projections to S1 are highly convergent and divergent from a number of nuclei including VP, the superior and inferior ventral posterior nuclei, the posterior division of the ventrolateral nucleus, and the anterior pulvinar47,50. This divergence and convergence in thalamocortical connectivity is also a defining feature of other anterior parietal areas, and of cortical areas in the lateral sulcus of macaque monkeys47,50. Given that mice and rats have many fewer cortical fields and thalamic nuclei associated with somatosensory processing, their thalamocortical networks are relatively simple compared to their macaque counterparts48,49. Specifically, the patterns of interconnections between higher order somatosensory nuclei of the thalamus in mice and rats (POm) and higher order cortical fields (S2) are limited compared to macaque monkeys, given the huge expanse of the higher order fields associated with somatosensory processing in monkeys (e.g. S2, PV, area 3a, area 2, area 5, VS, PRR) compared to mice and rats (S2 and PV)24,25,28,31.
Second, although S1 has strong cortico-cortical connections with S2 and PV in mice, rats, and macaque monkeys49,51,52, comparisons of cortical connections across these species are defined more by differences than similarities. For example, because there are no homologs of areas 3a, 1,2, VS, PR, and Ri in mice and rats, the network of cortical connections of these fields is a feature in macaque monkeys (and other primates) that does not exist in mice and rats. In addition, there are profound differences in corticocortical connections in mice, rats, and macaque monkeys between homologous fields. In mice and rats, S1 has strong connections with primary motor cortex (M1)53,54, while S1 (area 3b) in macaque monkeys has little to no connections with M1, and areas 3a and 1 have sparse connections with M155–57. Rather, area 2 and posterior parietal area 5 are interconnected with motor cortex forming what is commonly called the frontoparietal network39,56,58,59.
Finally, in mice and rats, corticospinal neurons originate primarily in M1 and to a lesser extent in S260, while in macaque monkeys corticospinal inputs arise from M1, as well as from areas 3a, 3b, 1, 2, and 5, and from areas in the lateral sulcus39,61. These differences indicate that somatosensory areas play a relatively large role in the motor control of the body in macaque monkeys, particularly the hand. Consistent with this view, movements of the hand and other body parts can be evoked not only from motor and premotor cortex, but also from all anterior parietal areas (3a, 3b, 1 and 2) as well as from posterior parietal cortex (areas 5 and 7) in macaque monkeys30,62. Many of the evoked movement of the hand in motor cortex and parietal areas are complex digit movements including multiple types of precision grips as well as power grips. In mice and rats, movements can only be evoked from M1 and S163,64 and S1 has been implicated in the motor control of whisker retraction movements65,66. While stimulation in M1 and S1 can evoke grasp like movements in rats, unlike macaque monkeys there are no evoked movements of individual digits.
Neural coding
One of the main insights of modern sensory neuroscience is that sensory information can be encoded by neurons in different ways. Some sensory information is encoded in the strength of the neural response – a rate code – some in the spatial layout of the neural response – a spatial code – some in the precise timing of the response – a temporal code. These different coding schemes allow information to be multiplexed in neural activity: information about one stimulus feature might be encoded in the rate of firing of the activated neurons, while information about another feature might be encoded in its temporal patterning, for example.
Through the whiskers, mice and rats acquire information about the environment that overlaps, at least in part, with the information that monkeys acquire through the glabrous skin of the hand. One might then ask whether sensory information is encoded in the same way across these organisms given the differences in the sensory organs and in their respective functions. To address this question, we examine in turn how different stimulus features are encoded in the different somatosensory systems.
Vibration
Vibratory sensitivity provides a window into somatosensory innervation and into the ability of the nervous system to resolve the temporal structure of whisker or skin deformations. In mice, rats, and monkeys, nerve fibers exhibit different frequency preferences, with some nerve fibers more sensitive at low frequencies and others at high frequencies. A prominent feature of afferent responses to vibrations is their phase locking: Nerve fibers will produce one spike or a burst of spikes within a restricted phase of each stimulus cycle such that the frequency composition of the vibration is reflected in the temporal sequence of the evoked spikes67–71. Even complex multi-harmonic skin vibrations give rise to very repeatable temporal spiking patterns in the nerves (Figure 5A). The temporal precision of vibratory responses is such that they can encode some types of stimuli with a precision measured in the milliseconds or even microseconds70,72. Similarly, neurons in somatosensory cortex produce phase locked responses to vibrations delivered to the whiskers73 or skin74,75, though with reduced precision.
Figure 5. Temporal precision and repeatability are hallmarks of both whisker and fingertip mechanoreceptors.
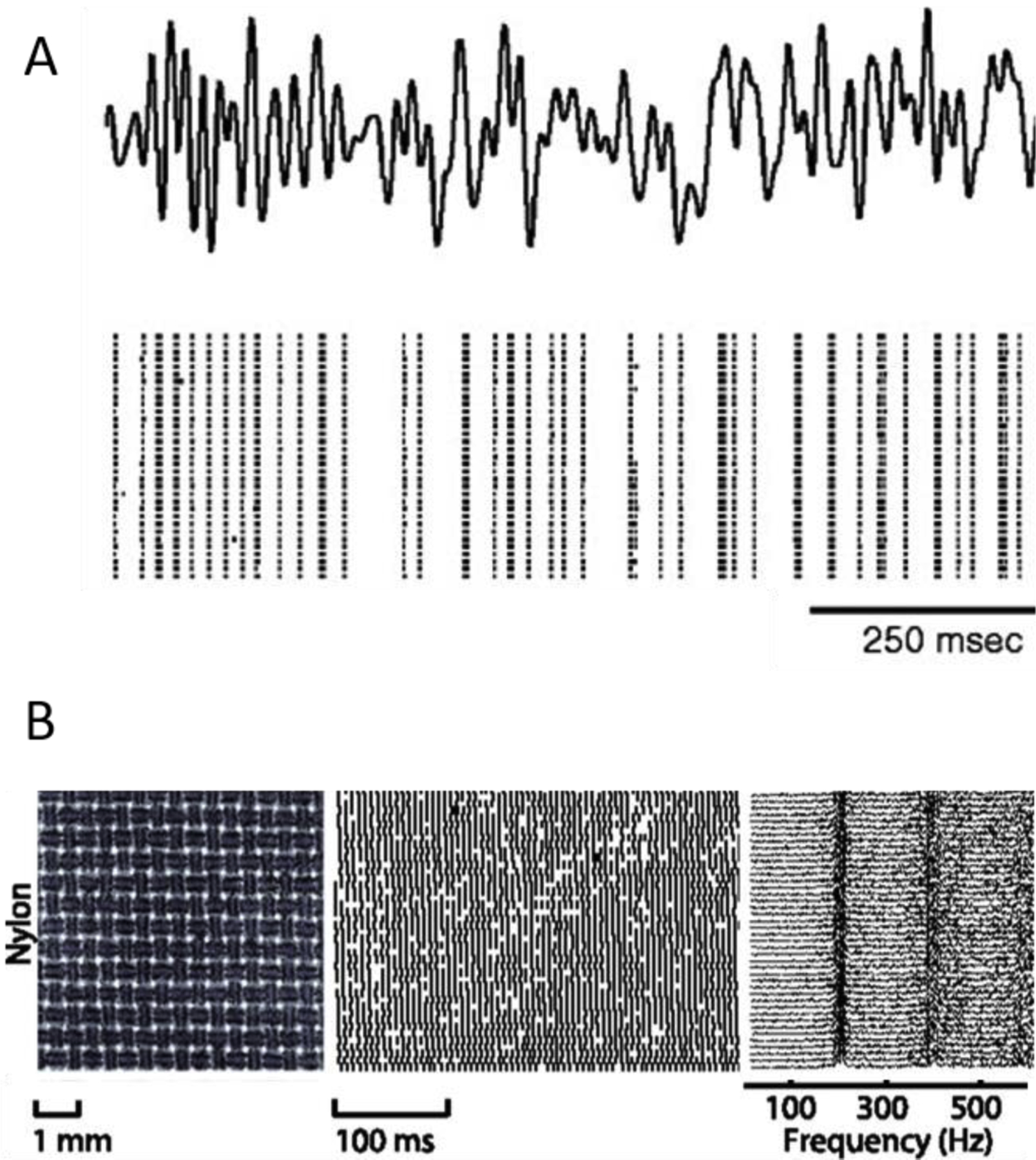
A| Responses evoked in primary afferents by repeated presentations of the same mechanical noise stimulus delivered to a whisker. The response is temporally patterned and highly repeatable. The same phenomenon is observed when analogous stimuli are delivered to the skin of macaque monkeys. Reproduced from Jones et al., 2004. B| Responses of a PC fiber to repeated presentations of a finely textured fabric. Left: Laser microscope image of the texture. Middle: Spiking response over 40 repeated presentations Right: Power spectra of the neuronal responses. When textures are scanned across the skin, PC fibers produce texture-specific temporal spiking patterns. Modified from Weber et al. (2013).
The specific behavioral relevance of different features of the response to vibrations has been extensively studied in macaques and to a lesser degree in rats and mice. In macaques, vibratory amplitude has been shown to be encoded in the strength of the response evoked in the nerve76 and in somatosensory cortex74. In both the nerve70 and cortex74, the frequency composition of a skin vibration is encoded in the temporal patterning of the response: In the absence of changes in firing rate, the perceived frequency can be extracted from the phase-locked spiking of nerve fibers or of cortical neurons (Figure 5A). However, while monkeys (and humans) can discriminate vibrations on the basis of frequency independent of amplitude75,77, rats cannot78, bringing into question the behavioral significance of precise timing periodicity of the whisker vibratory response in rodents.
Texture
In the primate fingertip, texture processing relies on two neural mechanisms distributed over three populations of tactile nerve fibers: Coarse textural features – measured in millimeters – are encoded in the spatial pattern of activation across type 1 fibers79. At this scale, individual textural features produce localized and relatively large scale deformations in the skin, which are in turn reflected in the localized activation of tactile fibers with RFs under the deformation80. The nerve fibers thus carry a neural “image” of the stimulus. The spatial resolution of this coding mechanism – limited by the innervation density and the filtering properties of the skin – is too low to discern fine surface features, with sizes and spatial periods measured in the hundreds of nanometers to hundreds of micrometers. At these spatial scales, texture perception requires movement between skin and surface81, which drives vibrations in the skin whose characteristics depend on both the texture and the speed at which it is scanned82–84. In turn, these skin vibrations elicit temporal spiking patterns in vibration sensitive nerve fibers, which are also dependent on surface and scanning speed79,85 (Figure 5B). In rats and mice, the sparseness of the whiskers precludes a spatial mechanism of texture processing. Rather, texture information is carried in the time-varying profile of whisker deformations associated with stick-slip events of individual whiskers as they brush against the surface. These texture-dependent whisker deflections activate nerve fibers that innervate the follicle, and texture information is encoded in the pattern of such activations over time, drawing a strong analogy to the temporal mechanism of texture processing in primates.
In somatosensory cortex of rats and monkeys, texture information is encoded in firing rates evoked during contact with the surface86–90 and in the temporal features of the response87,91. In areas 3b, 1, and 2 of primates, the texture signal carried by the firing rate response of cortical neurons reflects a spatial and temporal differentiation of the afferent input87,92. These differentiation operations confer to individual neurons a selectivity for specific spatial or temporal features in the afferent input. Because the computations differ across neurons, individual neurons are selective for different textural features, and the neuronal population carries a high-dimensional representation of texture87. A similar mechanism of differentiation – limited to the time domain – may also underlie the texture signal in barrel cortex given the similarities of the peripheral representation of texture in rats to its primate counterpart.
Shape and spatial localization
When we grasp and manipulate an object, we can recognize its three-dimensional structure even with vision obscured. This perceptual ability, known as stereognosis93, involves the integration of cutaneous and proprioceptive signals. Signals carried by cutaneous nerve fibers convey information about the local geometry of the object at each point of contact94,95 (Figure 6). These signals are analogous to those that carry information about coarse textural features, described above. In somatosensory cortex (areas 3b, 1, and 2), neurons respond selectively to specific local features of the object, for example edges, via the aforementioned spatial differentiation mechanism96. Signals about the configuration of the hand are carried by different sets of nerve fibers that primarily innervate muscles and ultimately impinge on area 3a97,98. This representation thus provides a substrate for the configural component of stereognosis, which carries information about the global shape of the object. However, little is known about how tactile signals about local shape and proprioceptive signals about global shape are combined95.
Figure 6. Spatial coding by fingertip mechanoreceptors.

Spatial pattern of activation evoked in populations of SA1 (top row), RA (middle row), and PC fibers when embossed letters are scanned across the fingertip of macaques. The spatial layout of the stimulus is reflected in the spatial layout of the response it evokes in SA1 and RA fibers. Modified from Phillips et al., 1988.
The neural processing of shape or form in the whisker system has largely been studied indirectly, via the processing of object localization. Indeed, localization can be construed as an elemental computation that underlies shape processing. A shape can be viewed as a collection of points; if all the points are localized, the shape is determined. Because whiskers form an array on the face, the x-y location (azimuth and elevation) of an object within reach of the whiskers can be coarsely determined by the identity of the contacting whiskers99. For whisking animals such as rats and mice100, additional strategies for localization beyond a whisker identity code are available. When whiskers sweep across space, the location of the whiskers can be tracked, and spikes arising from whisker-object contacts can be referenced against whisker position to determine the location of the touched object. In this way, information about whisker position is combined with contact information to compute location and shape. However, unlike in the primate hand, contact information is carried together with proprioceptive signals in individual nerve fibers. How whisker position is extracted using afferent responses is an active area of research18,101–106.
While many questions remain about the neural basis of stereognosis with hands and its analogue with whiskers, the underlying mechanisms are likely very different because local features and global shape of objects are processed by two sensory modalities – touch and proprioception – that are largely served by separate fibers in the macaque hand, while whisker position and contact are carried largely by the same nerve fibers. There are two important caveats, however. First, cutaneous afferents of the hand – which carry touch signals – also respond to stretching of the hand’s skin and may therefore carry proprioceptive information107. Second, the fact that hand sensing relies on dedicated proprioceptive fibers that are absent in the whisker system may not reflect a difference in species so much as a difference between the trigeminal and spinal somatosensory systems. Further work on macaque facial sensing and mouse paw sensing should resolve this uncertainty.
Motion
Somatosensory signals associated with object interactions typically arise during actively generated movements. Not surprisingly, then, the movement of objects across the skin or whiskers is explicitly encoded in the responses of neurons along the somatosensory neuraxis. In macaque somatosensory cortex, many neurons respond preferentially when a stimulus moves in their so-called preferred direction and less so when it moves in other directions (Figure 7)108–110. Similarly, neurons in barrel cortex exhibit dramatic selectivity for the direction in which individual whiskers are deflected. This direction tuning is to a large extent inherited from the periphery, as follicle afferents often exhibit such tuning. In contrast, direction tuning is weak or absent in the primate nerve and emerges downstream108. In macaque cortex, local motion signals – which are often ambiguous – are integrated to achieve a global motion signal that is largely independent of the spatial characteristics of the moving object108,109. Some neurons in barrel cortex exhibit selectivity for patterns of motion across multiple whiskers111–114 and these multi-whisker representations may play a role in the computation of global motion moving across the whisker array. The nature of these computations remains to be elucidated, however.
Figure 7. Direction tuning in area 1.
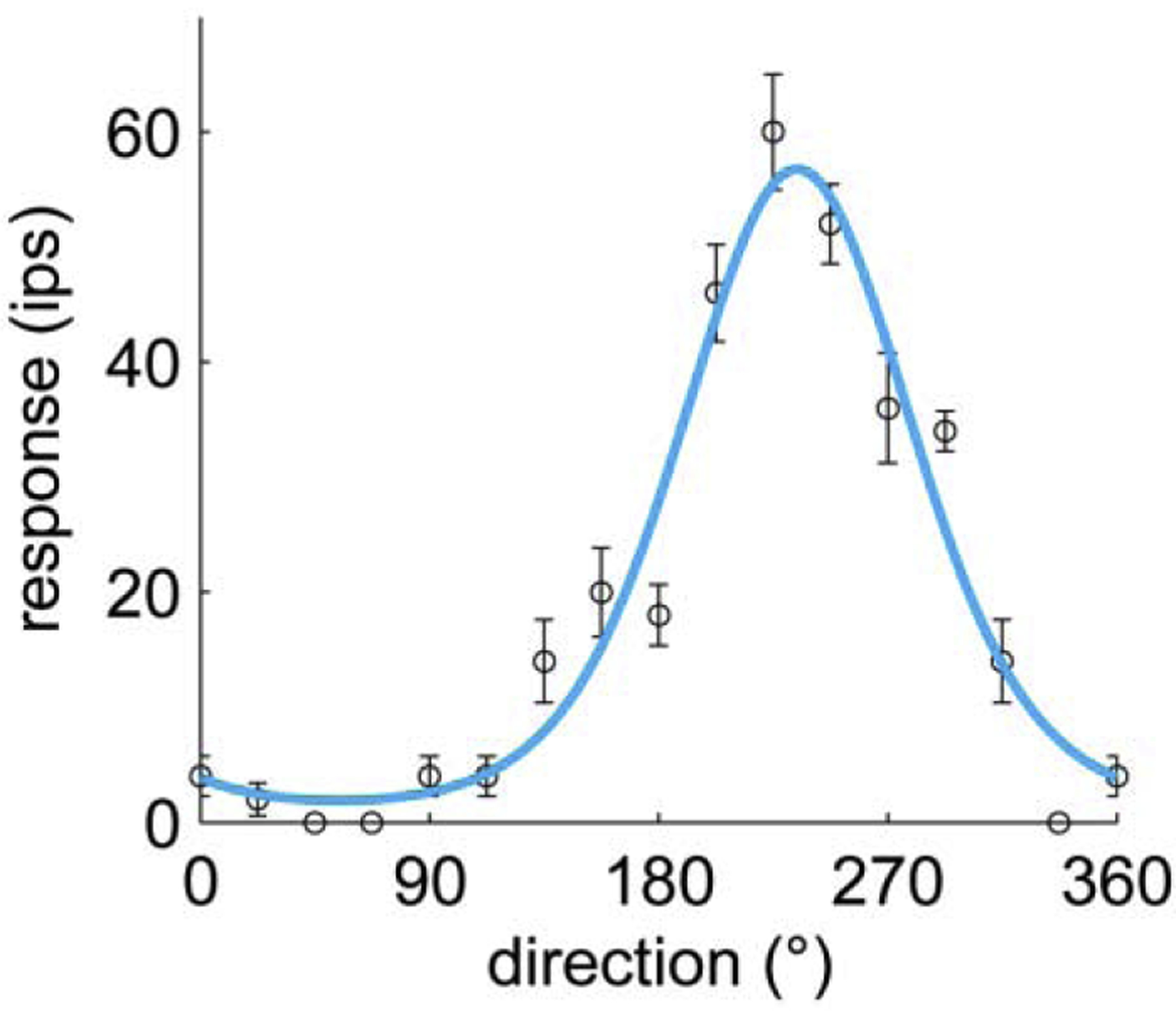
This neuron responds most strongly to an edge scanned across the skin in the distal to proximal direction and does not respond at all to the same edge scanned in the opposite direction. From Pei et al., 2010
Active touch
While touch is sometimes imposed, such as when we are touched by someone or when a breeze blows against our face, most tactile input occurs as a consequence of self-generated movements. Such “active touch” is the dominant mode of sensing for both whiskers and hands. Despite this, the vast majority of neurophysiological studies investigating primate touch have focused on passive sensing, in which stimuli are applied to the immobilized fingertip. Indeed, nearly all of our textbook knowledge about neural coding of touch has arisen from such studies. The challenge is that touch is very sensitive – detectable touches on the primate hand are measured in microns or even tenths of a micron76,115,116. Contact events during active manual interactions with objects, which involve skin deflections orders of magnitude larger, have not been characterized with sufficient precision to understand how they are encoded. Moreover, self-motion creates patterns of skin strain that evoke activity in tactile nerve fibers107,117,118 upon which contact signals are superimposed. The few studies of active manual touch have thus yielded only very qualitative conclusions about which areas preferentially respond during object contact, and some general observations about the neural dynamics during a contact event119–121.
In the whisker system, in contrast, methods for estimating forces and moments from whisker bending in high-speed video permit relating minuscule stresses to spiking of single-units18,122,123, despite the fact that thresholds are measured in the tens of μN105. Indeed, the detailed understanding of whisker biomechanics has made it possible to reconstruct, from measured patterns of whisker deformation, the forces acting at the whisker base during active touch6,124 (Figure 8). Direct interrogation of neural activity during active touch has revealed that active whisker sensing differs from its passive counterpart even at the level of mechanoreceptors. Indeed, whisker motion in rats and mice is actuated by muscles that pull on the whisker follicle125,126, and the actuation affects the coupling between whisker and mechanoreceptor. As a result, the same deflection will elicit a different neuronal response if it is actively generated vs. passively imposed122.
Figure 8. Forces and moments can be estimated during active touch in the whisker system.
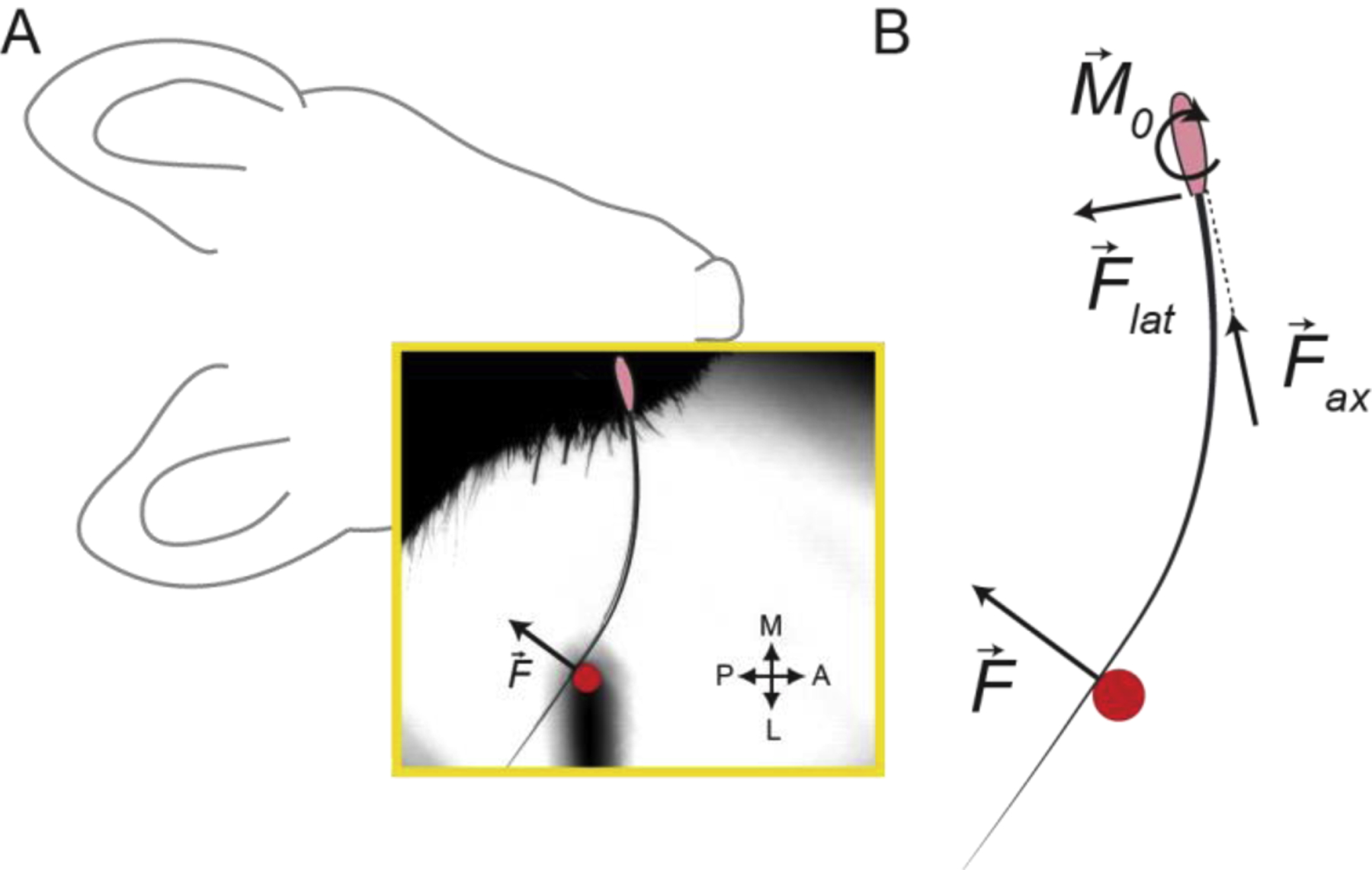
A | Example frame (yellow box) from high-speed video of a mouse whisking with a single whisker against an object (indicated by overlaid red circle). The whisker follicle is denoted by the pink oval. Whisking against the object produces a whisker-object contact force, . B | Schematic showing the forces and bending moment that act in the plane of whisking. A whisker-object contact reaction force, , can be split into a lateral force and an axial force acting at the follicle, and produces a bending moment at the follicle . These quantities can be estimated based on measurements obtained from high-speed video, together with measurements of whisker geometry. Modified from Pammer et al., 2013.
Active touch in the whisker system has been most thoroughly studied in the context of object localization99,127,128. Several quantitative behavioral tasks requiring the active localization of objects – in the azimuthal plane and in terms of radial distance from the face – have been developed for rats and mice101,105,129–132 and the behavioral, mechanical, and neural bases of such object localization have been studied in detail6,7,17,101,102,104–106,123,129,131,133–141. To locate objects in the azimuthal plane, mice and rats may use multiple, non-exclusive strategies99,127,128. One well-investigated strategy involves the integration of whisker self-motion signals with object contact signals. Azimuthal angle relative to the mouse face can be computed in part from the whisk phase (the relative position of the whisker within the whisk cycle)128,142 as it indicates the location within the currently scanned region of space. Signals about whisk phase are found throughout the neuraxis, from nerves to S117,18,103,123,133,136,143–150. During whisking, this phase-based self-motion signal can be combined with touch-evoked activity to code azimuthal object location within the currently scanned region of space133,101 (Figure 9). Signals encoding whisk amplitude and mid-point are also present in M1142,151 and are relayed to S1 via direct projections137, allowing S1 neurons to encode azimuthal object location in terms of absolute whisker angle152.
Figure 9. Object localization during active touch.
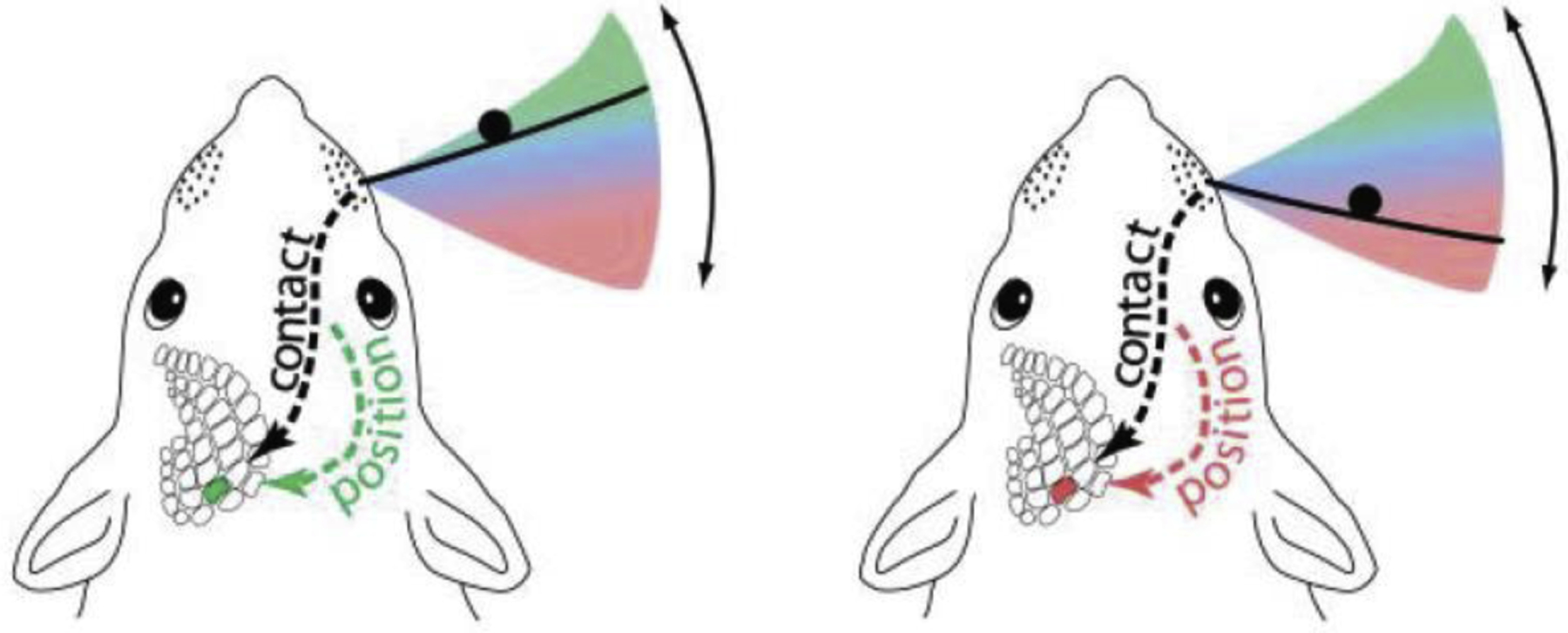
Rats and mice scan their whiskers across regions near the face to localize objects in the azimuthal plane. The location of an object (black circle) within the region covered by a whisk cycle (pink-to-green gradient) can be determined by combining neural signals for whisker self-motion and whisker-object contact. These signals are encoded throughout the ascending somatosensory system (dashed lines). Modified from Mehta et al., 2007.
While active touch likely shares some common mechanisms and circuitry between the whisker system and macaque hand, there are reasons to expect major differences. First, manual touch primarily supports object manipulation, whereas whiskers are used for sensing but not manipulating. As stated above, the sensory demands of the two behaviors are likely to be different, as is the interplay between sensory and motor signals. The regulation of grip force provides an example of tactile processing specific to the demands of manipulation. Grip force must be dynamically and precisely adjusted to prevent a held object from sliding within the hand and is thought to depend on “cancelling” signals triggered in cutaneous afferents by object-skin motion153. Second, touch and proprioception for the hand are (mostly) distinct modalities subserved by cutaneous and muscle receptors, respectively. In contrast, the muscles of the whisker system – as well as most other facial muscles in rats, mice, and humans – contain few or no muscle proprioceptors147. The integration of touch and proprioceptive signals must therefore occur differently. In general, however, the study of active touch in primates is so impoverished that there are few if any relevant data to inform comparisons between the whisker and hand systems.
Caveats: The challenge and opportunity of comparative neurophysiology
Over 6000 species of mammals have evolved to adapt to a variety of complex and dynamic niches. Yet, our current understanding of sensory processing is based on only a few mammals and is limited for several reasons. First, the primate and rodent orders are highly diverse. Of the 2500 or so species of rodents, some use their whiskers for sensory exploration while others do not, some have a barrel cortex while others do not154. Of the hundreds of species of primates, some have opposable thumbs while others do not, some naturally use tools in the wild while others do not155. Thus, each of these animal models has derivations that may impact the extent to which we can extrapolate the data we collect to humans. Although, in the field, we often speak of “rodent” and “primate” features of organization and function, we must keep in mind that this shorthand terminology obscures major diversity within each order. Thus, a complete understanding of even rodent and primate somatosensation will not be achieved via work with mice, rats, and macaques alone. Second, we often fail to appreciate that species within both orders have been evolving independently from humans for 80 million years (rodents) or 30 million years (Old World monkeys). This is an enormous amount of time for changes to emerge in each lineage, even between Rattus and Mus genera. Consider the differences that have emerged between humans and our closest living relatives, chimpanzees, since our lineages diverged some 6 million years ago! Third, our understanding of somatosensory processing comes from animals reared in a highly deprived laboratory environment, where the behavioral and sensory repertoires are limited. As a result, both sensory processing and motor control in laboratory-reared animals may be different from that of their naturally reared counterparts.
As aptly argued by Rosenblueth and Wiener156 “The best material model of a cat is another, or preferably the same cat”. While humans cannot be used to understand a number of aspects of the human somatosensory system, we should keep in mind that there is no perfect animal model for the human nervous system. Rather, different models are better suited to address particular aspects of nervous system organization and function than others. The macaque monkey with its glabrous hand and opposable thumb is a good model for understanding the neural control of the human hand. Active sensing is more easily studied in mice and rats than in monkeys, and the tools available in mice and rats to uncover fundamental cellular interactions within somatosensory circuits makes them very good models as well. However, whisker somatosensation is not a perfect model of manual somatosensation. Even macaque models of human hand use are limited, since macaques do not naturally engage in tool use in the wild.
Other primates, such as capuchin monkeys, do, which makes them more appropriate models for understanding the neural basis of tool use. Only by taking a broad, comparative approach using multiple species can we arrive at a comprehensive understanding of our own species.
Open questions
While much has been revealed about somatosensation through studies using the macaque hand and rodent whisker systems, fundamental questions remain.
Homolog of area 3a in mice and rats. In macaque monkeys, proprioceptive signals – driven primarily by muscle- and tendon-associated nerve fibers – converge onto Brodmann’s area 3a, the first cortical recipient of proprioceptive signals. Whether the somatosensory cortex of mice and rats has a homologue of area 3a is unknown.
Homolog of PPC in mice and rats. The difference in size, number of cortical fields, and the proposed function of PPC in mice and rats versus macaque monkeys (and primates in general) is notable. While PPC is implicated in macaque somatosensation, it is not clear if any of the cortex termed PPC in mice and rats is homologous or even analogous to PPC in monkeys.
Function of identified mechanoreceptor types in the whisker system. In macaque monkeys, the relationship between mechanoreceptor types and functional properties is well established (SA1 fibers innervate Merkel cells, RA fibers innervate Meissner corpuscles, etc.). The innervation of the follicle remains to be characterized at that level of detail.
Active touch in primates. While naturalistic sensory exploration is increasingly being studied in rodents, enabled by computer vision-mediated whisker tracking and models of whisker biomechanics, almost nothing is known about active touch in primates. Whether the insights that have been gleaned about tactile coding from passive delivery of tactile stimuli will generalize to more naturalistic contexts remains to be determined.
Stereognosis. As sketched out above, stereognosis – the ability to discern the three-dimensional structure of an object based on sensory signals from the hand – implies the integration of tactile and proprioceptive signals. The nature of this integration is almost completely unknown.
Highlights.
We explore the degree to which mice, rat, and monkey models yield a coherent picture of the neural basis of somatosensation.
Vibrissae are for exploration, hands are for manipulation. The associated somatosensory systems reflect these differences.
The respective somatosensory neuraxes of mice, rats, and monkeys are fundamentally different. In particular, monkey cortex features many more fields devoted to somatosensation than does rat or mouse cortex.
Somatosensory coding in mice and rats bears some similarities with its primate counterpart, but also features considerable differences, borne out of the aforementioned differences in form and function.
There are several major gaps in our understanding of somatosensation across animal models.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woolsey TA & Van der Loos H The structural organization of layer IV in the somatosensory region (S I) of mouse cerebral cortex: The description of a cortical field composed of discrete cytoarchitectonic units. Brain Research 17, 205–242 (1970). [DOI] [PubMed] [Google Scholar]
- 2.Rice FL & Van Der Loos H Development of the barrels and barrel field in the somatosensory cortex of the mouse. Journal of Comparative Neurology 171, 545–560 (1977). [DOI] [PubMed] [Google Scholar]
- 3.Yu YSW, Bush NE & Hartmann MJZ Whisker Vibrations and the Activity of Trigeminal Primary Afferents in Response to Airflow. J Neurosci 39, 5881–5896 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehnhardt G, Mauck B & Bleckmann H Seal whiskers detect water movements. Nature 394, 235–236 (1998). [Google Scholar]
- 5.Catania KC & Kaas JH The Unusual Nose and Brain of the Star-Nosed Mole. BioScience 46, 578–586 (1996). [Google Scholar]
- 6.Birdwell JA et al. Biomechanical models for radial distance determination by the rat vibrissal system. Journal of neurophysiology 98, 2439–55 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Solomon JH & Hartmann MJ Biomechanics: robotic whiskers used to sense features. Nature 443, 525 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Ebara S, Kumamoto K, Matsuura T, Mazurkiewicz JE & Rice FL Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. The Journal of comparative neurology 449, 103–19 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Lucianna FA, Albarracín AL, Vrech SM, Farfán FD & Felice CJ The mathematical whisker: A review of numerical models of the rat׳ s vibrissa biomechanics. Journal of Biomechanics 49, 2007–2014 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Sripati AP, Bensmaia SJ & Johnson KO A Continuum Mechanical Model of Mechanoreceptive Afferent Responses to Indented Spatial Patterns. Journal of Neurophysiology 95, 3852–3864 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philipps J & Johnson KO Tactile spatial resolution. III. A continuum mechanics model of skin predicting mechanoreceptor responses to bars, edges, and gratings. Journal of Neurophysiology 46, 1226–1243 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Bischoff JE, Arruda EM & Grosh K Finite element modeling of human skin using an isotropic, nonlinear elastic constitutive model. Journal of Biomechanics 33, 645–652 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Dandekar K, Raju BI & Srinivasan MA 3-D Finite-Element Models of Human and Monkey Fingertips to Investigate the Mechanics of Tactile Sense. J Biomech Eng 125, 682–691 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Wu JZ, Welcome DE & Dong RG Three-dimensional finite element simulations of the mechanical response of the fingertip to static and dynamic compressions. Computer Methods in Biomechanics and Biomedical Engineering 9, 55–63 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Rice FL, Mance A & Munger BL A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J. Comp. Neurol 252, 154–174 (1986). [DOI] [PubMed] [Google Scholar]
- 16.Zucker E & Welker WI Coding of somatic sensory input by vibrissae neurons in the rat’s trigeminal ganglion. Brain Res. 12, 138–156 (1969). [DOI] [PubMed] [Google Scholar]
- 17.Szwed M, Bagdasarian K & Ahissar E Encoding of vibrissal active touch. Neuron 40, 621–30 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Severson KS et al. Active Touch and Self-Motion Encoding by Merkel Cell-Associated Afferents. Neuron 94, 666–676 e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paré M, Behets C & Cornu O Paucity of presumptive ruffini corpuscles in the index finger pad of humans. J Comp Neurol 456, 260–266 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Saal HP, Delhaye BP, Rayhaun BC & Bensmaia SJ Simulating tactile signals from the whole hand with millisecond precision. Proc. Natl. Acad. Sci. U.S.A 114, E5693–E5702 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saal HP & Bensmaia SJ Touch is a team effort: interplay of submodalities in cutaneous sensibility: Trends in Neurosciences. Trends in Neuroscience 37, 689–697 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Catania KC The sense of touch in the star-nosed mole: from mechanoreceptors to the brain. Philos Trans R Soc Lond B Biol Sci 366, 3016–3025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson RJ, Sur M, Felleman DJ & Kaas JH Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. Journal of Comparative Neurology 192, 611–643 (1980). [DOI] [PubMed] [Google Scholar]
- 24.Pons TP, Garraghty PE, Cusick CG & Kaas JH The somatotopic organization of area 2 in macaque monkeys. J. Comp. Neurol 241, 445–466 (1985). [DOI] [PubMed] [Google Scholar]
- 25.Krubitzer L, Huffman KJ, Disbrow E & Recanzone G Organization of area 3a in macaque monkeys: Contributions to the cortical phenotype. Journal of Comparative Neurology 471, 97–111 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Kaas JH What, if anything, is SI? Organization of first somatosensory area of cortex. Physiological Reviews 63, 206–231 (1983). [DOI] [PubMed] [Google Scholar]
- 27.Krubitzer L, Campi KL & Cooke DF All Rodents Are Not the Same: A Modern Synthesis of Cortical Organization. Brain, Behavior and Evolution 78, 51–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krubitzer L, Clarey J, Tweedale R, Elston G & Calford M A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J. Neurosci 15, 3821–3839 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooshagian E, Wang C, Holmes CD & Snyder LH Single Units in the Posterior Parietal Cortex Encode Patterns of Bimanual Coordination. Cereb Cortex 28, 1549–1567 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathelot J-A, Dum RP & Strick PL Posterior parietal cortex contains a command apparatus for hand movements. Proc Natl Acad Sci USA 114, 4255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldring AB & Krubitzer LA Evolution of Parietal Cortex in Mammals: From Manipulation to Tool Use. in Evolutionary Neuroscience (Second Edition) (eds. Krubitzer L & Kaas J) vol. 3 259–286 (Academic Press, 2020). [Google Scholar]
- 32.Kaas JH, Qi H-X & Stepniewska I Chapter 2 - The evolution of parietal cortex in primates. in Handbook of Clinical Neurology (eds. Vallar G & Coslett HB) vol. 151 31–52 (Elsevier, 2018). [DOI] [PubMed] [Google Scholar]
- 33.Olsen GM & Witter MP Posterior parietal cortex of the rat: Architectural delineation and thalamic differentiation. Journal of Comparative Neurology 524, 3774–3809 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Chapin JK & Lin C-S Mapping the body representation in the SI cortex of anesthetized and awake rats. Journal of Comparative Neurology 229, 199–213 (1984). [DOI] [PubMed] [Google Scholar]
- 35.Woolsey T Somatosensory, auditory and visual cortical areas of the mouse. The Johns Hopkins medical journal 121, 91–112 (1967). [PubMed] [Google Scholar]
- 36.Remple MS, Henry EC & Catania KC Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): Evidence for two lateral areas joined at the representation of the teeth. Journal of Comparative Neurology 467, 105–118 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Carvell GE & Simons DJ Somatotopic Organization of the Second Somatosensory Area (SII) in the Cerebral Cortex of the Mouse. Somatosensory Research 3, 213–237 (1986). [DOI] [PubMed] [Google Scholar]
- 38.Hadjidimitrakis K, Bakola S, Wong YT & Hagan MA Mixed Spatial and Movement Representations in the Primate Posterior Parietal Cortex. Frontiers in Neural Circuits 13, 15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borra E, Gerbella M, Rozzi S & Luppino G The macaque lateral grasping network: A neural substrate for generating purposeful hand actions. Neuroscience & Biobehavioral Reviews 75, 65–90 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Gamberini M, Passarelli L, Fattori P & Galletti C Structural connectivity and functional properties of the macaque superior parietal lobule. Brain Structure and Function 225, 1349–1367 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Seelke AMH et al. Topographic Maps within Brodmann’s Area 5 of Macaque Monkeys. Cerebral Cortex 22, 1834–1850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan H et al. Functional Architecture and Encoding of Tactile Sensorimotor Behavior in Rat Posterior Parietal Cortex. J. Neurosci 39, 7332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mimica B, Dunn BA, Tombaz T, Bojja VPTNCS & Whitlock JR Efficient cortical coding of 3D posture in freely behaving rats. Science 362, 584–589 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Akrami A, Kopec CD, Diamond ME & Brody CD Posterior parietal cortex represents sensory history and mediates its effects on behaviour. Nature 554, 368–372 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Slutsky DA, Manger PR & Krubitzer L Multiple somatosensory areas in the anterior parietal cortex of the California ground squirrel (Spermophilus beecheyii). Journal of Comparative Neurology 416, 521–539 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Sur M, Nelson RJ & Kaas JH The representation of the body surface in somatosensory area I of the grey squirrel. Journal of Comparative Neurology 179, 425–449 (1978). [DOI] [PubMed] [Google Scholar]
- 47.Padberg J et al. Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb. Cortex 19, 2038–2064 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chmielowska J, Carvell GE & Simons DJ Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. Journal of Comparative Neurology 285, 325–338 (1989). [DOI] [PubMed] [Google Scholar]
- 49.Carvell GE & Simons DJ Thalamic and corticocortical connections of the second somatic sensory area of the mouse. Journal of Comparative Neurology 265, 409–427 (1987). [DOI] [PubMed] [Google Scholar]
- 50.Rausell E, Bickford L, Manger PR, Woods TM & Jones EG Extensive Divergence and Convergence in the Thalamocortical Projection to Monkey Somatosensory Cortex. J. Neurosci 18, 4216 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burton H, Fabri M & Alloway K Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: A revised interpretation of the second somatosensory area in macaque monkeys. Journal of Comparative Neurology 355, 539–562 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Koralek K-A, Olavarria J & Kellackey HP Areal and laminar organization of corticocortical projections in the rat somatosensory cortex. Journal of Comparative Neurology 299, 133–150 (1990). [DOI] [PubMed] [Google Scholar]
- 53.Porter LL & White EL Afferent and efferent pathways of the vibrissal region of primary motor cortex in the mouse. Journal of Comparative Neurology 214, 279–289 (1983). [DOI] [PubMed] [Google Scholar]
- 54.Smith JB & Alloway KD Rat whisker motor cortex is subdivided into sensory-input and motor-output areas. Front. Neural Circuits 7, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burton H & Fabri M Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: Projections in the vicinity of the central sulcus. Journal of Comparative Neurology 355, 508–538 (1995). [DOI] [PubMed] [Google Scholar]
- 56.Gharbawie OA, Stepniewska I, Qi H & Kaas JH Multiple Parietal–Frontal Pathways Mediate Grasping in Macaque Monkeys. J. Neurosci 31, 11660–11677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones EG, Coulter JD & Hendry SHC Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. Journal of Comparative Neurology 181, 291–347 (1978). [DOI] [PubMed] [Google Scholar]
- 58.Bakola S, Passarelli L, Gamberini M, Fattori P & Galletti C Cortical Connectivity Suggests a Role in Limb Coordination for Macaque Area PE of the Superior Parietal Cortex. J. Neurosci 33, 6648–6658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padberg J, Cooke DF, Cerkevich CM, Kaas JH & Krubitzer L Cortical connections of area 2 and posterior parietal area 5 in macaque monkeys. Journal of Comparative Neurology 527, 718–737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueno M et al. Corticospinal Circuits from the Sensory and Motor Cortices Differentially Regulate Skilled Movements through Distinct Spinal Interneurons. Cell Reports 23, 1286–1300.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galea MP & Darian-Smith I Multiple Corticospinal Neuron Populations in the Macaque Monkey Are Specified by Their Unique Cortical Origins, Spinal Terminations, and Connections. Cereb Cortex 4, 166–194 (1994). [DOI] [PubMed] [Google Scholar]
- 62.Baldwin MKL, Cooke DF, Goldring AB & Krubitzer L Representations of Fine Digit Movements in Posterior and Anterior Parietal Cortex Revealed Using Long-Train Intracortical Microstimulation in Macaque Monkeys. Cereb Cortex 28, 4244–4263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neafsey EJ et al. The organization of the rat motor cortex: A microstimulation mapping study. Brain Research Reviews 11, 77–96 (1986). [DOI] [PubMed] [Google Scholar]
- 64.Halley AC, Baldwin MKL, Cooke DF, Englund M & Krubitzer LA The distributed motor control of limb movements in rat motor and somatosensory cortex: The sensorimotor amalgam revisited. Cerebral Cortex (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petersen CCH Cortical control of whisker movement. Annu Rev Neurosci 37, 183–203 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Matyas F et al. Motor control by sensory cortex. Science 330, 1240–1243 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Jones LM, Depireux DA, Simons DJ & Keller A Robust temporal coding in the trigeminal system. Science 304, 1986–9 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones LM, Lee S, Trageser JC, Simons DJ & Keller A Precise temporal responses in whisker trigeminal neurons. J. Neurophysiol 92, 665–668 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.birznieks I & Vickery RM Spike Timing Matters in Novel Neuronal Code Involved in Vibrotactile Frequency Perception: Current Biology. Current Biology 27, 1485–1490 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Mackevicius EL, Best MD, Saal HP & Bensmaia SJ Millisecond precision spike timing shapes tactile perception. Journal of Neuroscience 32, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Talbot WH, Darian-Smith I, Kornhuber HH & Mountcastle VB The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. Journal of Neurophysiology 31, 301–334 (1968). [DOI] [PubMed] [Google Scholar]
- 72.Bale MR, Campagner D, Erskine A & Petersen RS Microsecond-scale timing precision in rodent trigeminal primary afferents. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 5935–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ewert TAS, Vahle-Hinz C & Engel AK High-Frequency Whisker Vibration Is Encoded by Phase-Locked Responses of Neurons in the Rat’s Barrel Cortex. J. Neurosci 28, 5359–5368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harvey MA et al. Multiplexing Stimulus Information through Rate and Temporal Codes in Primate Somatosensory Cortex. PLoS Biology 11, e1001558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salinas E, Hernández A, Zainos A & Romo R Periodicity and Firing Rate As Candidate Neural Codes for the Frequency of Vibrotactile Stimuli. J. Neurosci 20, 5503–5515 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muniak MA, Ray S, Hsiao SS, Dammann JF & Bensmaia SJ The neural coding of stimulus of mechanoreceptive afferents with psychophysical behavior (vol 27, pg 11687, 2007). JOURNAL OF NEUROSCIENCE 28, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LaMotte RH & Mountcastle VB Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. Journal of Neurophysiology 38, 539–559 (1975). [DOI] [PubMed] [Google Scholar]
- 78.Gerdjikov TV, Bergner CG, Stüttgen MC, Waiblinger C & Schwarz C Discrimination of Vibrotactile Stimuli in the Rat Whisker System: Behavior and Neurometrics. Neuron 65, 530–540 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Weber AI et al. Spatial and temporal codes mediate the tactile perception of natural textures. Proceedings of the National Academy of Sciences 110, 17107–17112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Connor CE, Hsiao SS, Phillips JR & Johnson KO Tactile roughness: neural codes that account for psychophysical magnitude estimates. The Journal of neuroscience : the official journal of the Society for Neuroscience 10, 3823–3836 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hollins M & Risner SR Evidence for the duplex theory of tactile texture perception. Perception and Psychophysics 62, 695–705 (2000). [DOI] [PubMed] [Google Scholar]
- 82.Bensmaia SJ & Hollins M The vibrations of texture. Somatosensory and Motor Research 20, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manfredi LR et al. Natural scenes in tactile texture. Journal of Neurophysiology 111, (2014). [DOI] [PubMed] [Google Scholar]
- 84.Greenspon CM, McLellan KR, Lieber JD & Bensmaia SJ Effect of scanning speed on texture-elicited vibrations. Journal of The Royal Society Interface 17, 20190892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lieber JD, Xia X, Weber AI & Bensmaia SJ The neural code for tactile roughness in the somatosensory nerves. Journal of neurophysiology 118, 3107–3117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.von Heimendahl M, Itskov PM, Arabzadeh E & Diamond ME Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS biology 5, e305 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lieber JD & Bensmaia SJ High-dimensional representation of texture in somatosensory cortex of primates. PNAS 201818501 (2019) doi: 10.1073/pnas.1818501116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isett BR, Feasel SH, Lane MA & Feldman DE Slip-Based Coding of Local Shape and Texture in Mouse S1. Neuron 97, 418–433 e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jadhav SP, Wolfe J & Feldman DE Sparse temporal coding of elementary tactile features during active whisker sensation. Nature neuroscience 12, 792–800 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Wolfe J et al. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS biology 6, e215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zuo Y et al. Complementary contributions of spike timing and spike rate to perceptual decisions in rat S1 and S2 cortex. Current biology : CB 25, 357–63 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Lieber JD & Bensmaia SJ Emergence of an Invariant Representation of Texture in Primate Somatosensory Cortex. Cereb Cortex doi: 10.1093/cercor/bhz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goodman JM & Bensmaia SJ The neural basis of haptic perception. in The stevens handbook of experimental psychology and cognitive neuroscience. Volume II: Sensation, perception, and attention (eds. Wixted JT & Serences JT) (2018). [Google Scholar]
- 94.Goodwin AW & Wheat HE Sensory signals in neural populations underlying tactile perception and manipulation. Annual review of neuroscience 27, 53–77 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Phillips JR, Johnson KO & Hsiao SS Spatial pattern representation and transformation in monkey somatosensory cortex. PNAS 85, 1317–1321 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bensmaia SJ, Denchev PV, Dammann JF, Craig JC & Hsiao SS The Representation of Stimulus Orientation in the Early Stages of Somatosensory Processing. Journal of Neuroscience 28, 776–786 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goodman JM et al. Postural Representations of the Hand in the Primate Sensorimotor Cortex. Neuron (2019) doi: 10.1016/j.neuron.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okorokova EV, Goodman JM, Hatsopoulos NG & Bensmaia SJ Decoding hand kinematics from population responses in sensorimotor cortex during grasping. J. Neural Eng 17, 046035 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Knutsen PM & Ahissar E Orthogonal coding of object location. Trends in neurosciences 32, 101–109 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Sofroniew NJ & Svoboda K Whisking. Curr Biol 25, R137–40 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Mehta SB, Whitmer D, Figueroa R, Williams BA & Kleinfeld D Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol 5, e15 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Connor DH et al. Neural coding during active somatosensation revealed using illusory touch. Nature neuroscience 16, 958–65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wallach A, Bagdasarian K & Ahissar E On-going computation of whisking phase by mechanoreceptors. Nature neuroscience 19, 487–93 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Knutsen PM, Biess A & Ahissar E Vibrissal kinematics in 3D: tight coupling of azimuth, elevation, and torsion across different whisking modes. Neuron 59, 35–42 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Pammer L et al. The mechanical variables underlying object localization along the axis of the whisker. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 6726–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Solomon JH & Hartmann MJ Radial distance determination in the rat vibrissal system and the effects of Weber’s law. Philos Trans R Soc Lond B Biol Sci 366, 3049–57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Edin BB & Abbs JH Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J. Neurophysiol 65, 657–670 (1991). [DOI] [PubMed] [Google Scholar]
- 108.Pei Y-C, Hsiao SS, Craig JC & Bensmaia SJ Shape invariant coding of motion direction in somatosensory cortex. PLoS biology 8, e1000305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pei Y-C, Hsiao SS, Craig JC & Bensmaia SJ Neural Mechanisms of Tactile Motion Integration in Somatosensory Cortex. Neuron 69, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pei Y-C & Bensmaia SJ The neural basis of tactile motion perception. Journal of neurophysiology jn.00391.2014 (2014) doi: 10.1152/jn.00391.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Estebanez L, Bertherat J, Shulz DE, Bourdieu L & Léger J-F A radial map of multi-whisker correlation selectivity in the rat barrel cortex. Nature Communications 7, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Estebanez L, Boustani SE, Destexhe A & Shulz DE Correlated input reveals coexisting coding schemes in a sensory cortex. Nature Neuroscience 15, 1691–1699 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Laboy-Juárez KJ, Langberg T, Ahn S & Feldman DE Elementary motion sequence detectors in whisker somatosensory cortex. Nat. Neurosci 22, 1438–1449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramirez A et al. Spatiotemporal receptive fields of barrel cortex revealed by reverse correlation of synaptic input. Nat Neurosci 17, 866–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bolanowski SJ & Zwislocki JJ Intensity and frequency characteristics of pacinian corpuscles. I. Action potentials. Journal of Neurophysiology 51, 793–811 (1984). [DOI] [PubMed] [Google Scholar]
- 116.Freeman AW & Johnson KO A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkey. The Journal of Physiology 323, 43–64 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edin B Cutaneous afferents provide information about knee joint movements in humans. J. Physiol. (Lond.) 531, 289–297 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hulliger M, Nordh E, Thelin AE & Vallbo AB The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J. Physiol. (Lond.) 291, 233–249 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Debowy DJ, Ghosh S, Ro JY & Gardner EP Comparison of neuronal firing rates in somatosensory and posterior parietal cortex during prehension. Exp Brain Res 137, 269–291 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Salimi I, Brochier T & Smith AM Neuronal Activity in Somatosensory Cortex of Monkeys Using a Precision Grip. II. Responses to Object Texture and Weights. Journal of Neurophysiology 81, 835–844 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Salimi I, Brochier T & Smith AM Neuronal Activity in Somatosensory Cortex of Monkeys Using a Precision Grip. III. Responses to Altered Friction Perturbations. Journal of Neurophysiology 81, 845–857 (1999). [DOI] [PubMed] [Google Scholar]
- 122.Bush NE et al. Decoupling kinematics and mechanics reveals coding properties of trigeminal ganglion neurons in the rat vibrissal system. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campagner D, Evans MH, Bale MR, Erskine A & Petersen RS Prediction of primary somatosensory neuron activity during active tactile exploration. eLife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Campagner D, Evans MH, Loft MSE & Petersen RS What the whiskers tell the brain. Neuroscience 368, 95–108 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Haidarliu S, Kleinfeld D, Deschenes M & Ahissar E The Musculature That Drives Active Touch by Vibrissae and Nose in Mice. Anat Rec (Hoboken) 298, 1347–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill DN, Bermejo R, Zeigler HP & Kleinfeld D Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 3438–55 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D & Ahissar E ‘Where’ and ‘what’ in the whisker sensorimotor system. Nature reviews. Neuroscience 9, 601–12 (2008). [DOI] [PubMed] [Google Scholar]
- 128.Kleinfeld D & Deschenes M Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72, 455–68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Knutsen PM, Pietr M & Ahissar E Haptic object localization in the vibrissal system: behavior and performance. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 8451–64 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krupa DJ, Matell MS, Brisben AJ, Oliveira LM & Nicolelis MA Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 5752–63 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Connor DH et al. Vibrissa-based object localization in head-fixed mice. J Neurosci 30, 1947–67 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sofroniew NJ, Cohen JD, Lee AK & Svoboda K Natural whisker-guided behavior by head-fixed mice in tactile virtual reality. J Neurosci 34, 9537–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Curtis JC & Kleinfeld D Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nature neuroscience 12, 492–501 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bagdasarian K et al. Pre-neuronal morphological processing of object location by individual whiskers. Nature neuroscience 16, 622–31 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Cheung J, Maire P, Kim J, Sy J & Hires SA The Sensorimotor Basis of Whisker-Guided Anteroposterior Object Localization in Head-Fixed Mice. Curr. Biol 29, 3029–3040.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hires SA, Gutnisky DA, Yu J, O’Connor DH & Svoboda K Low-noise encoding of active touch by layer 4 in the somatosensory cortex. eLife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petreanu L et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quist BW & Hartmann MJ Mechanical signals at the base of a rat vibrissa: the effect of intrinsic vibrissa curvature and implications for tactile exploration. Journal of neurophysiology 107, 2298–312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Szwed M et al. Responses of trigeminal ganglion neurons to the radial distance of contact during active vibrissal touch. Journal of neurophysiology 95, 791–802 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Voigts J, Herman DH & Celikel T Tactile object localization by anticipatory whisker motion. J. Neurophysiol 113, 620–632 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Xu NL et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature (2012) doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- 142.Hill DN, Curtis JC, Moore JD & Kleinfeld D Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron 72, 344–56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Crochet S & Petersen CC Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nature neuroscience 9, 608–10 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Fee MS, Mitra PP & Kleinfeld D Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. Journal of neurophysiology 78, 1144–9 (1997). [DOI] [PubMed] [Google Scholar]
- 145.Khatri V, Bermejo R, Brumberg JC, Keller A & Zeigler HP Whisking in air: encoding of kinematics by trigeminal ganglion neurons in awake rats. Journal of neurophysiology 101, 1836–46 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leiser SC & Moxon KA Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron 53, 117–33 (2007). [DOI] [PubMed] [Google Scholar]
- 147.Moore JD, Mercer Lindsay N, Deschenes M & Kleinfeld D Vibrissa self-motion and touch are reliably encoded along the same somatosensory pathway from brainstem through thalamus. PLoS biology 13, e1002253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Severson KS, Xu D, Yang H & O’Connor DH Coding of whisker motion across the mouse face. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu C, Derdikman D, Haidarliu S & Ahissar E Parallel thalamic pathways for whisking and touch signals in the rat. PLoS biology 4, e124 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu J, Gutnisky DA, Hires SA & Svoboda K Layer 4 fast-spiking interneurons filter thalamocortical signals during active somatosensation. Nat Neurosci 19, 1647–1657 (2016). [DOI] [PubMed] [Google Scholar]
- 151.Huber D et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 484, 473–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ranganathan GN et al. Active dendritic integration and mixed neocortical network representations during an adaptive sensing behavior. Nat. Neurosci 21, 1583–1590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johansson RS & Westling G Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Experimental Brain Research 66, 141–154 (1987). [DOI] [PubMed] [Google Scholar]
- 154.Woolsey TA, Welker C & Schwartz RH Comparative anatomical studies of the Sml face cortex with special reference to the occurrence of “barrels” in layer IV. Journal of Comparative Neurology 164, 79–94 (1975). [DOI] [PubMed] [Google Scholar]
- 155.Murray P The role of cheek pouches in cercopitheeine monkeys adaptive strategy. Primate Functional Morphology and Evolution 151–194 (1975). [Google Scholar]
- 156.rosenblueth A & Wiener N The role of models in science. Philosophy of Science 12, 316–321 (1945). [Google Scholar]


