Abstract
Background:
The failing heart is energy-starved with impaired oxidation of long chain fatty acids (LCFA) at the level of reduced carnitine palmitoyltransferase 1 (CPT1) activity at the outer mitochondrial membrane. Recent work shows elevated ketone oxidation in failing hearts as an alternate carbon source for oxidative ATP generation. We hypothesized that another short chain carbon source, short chain fatty acids (SCFA) that bypass CPT1, could similarly support energy production in failing hearts.
Methods:
Cardiac hypertrophy and dysfunction was induced in rats by transverse-aortic constriction (TAC). 14 weeks after TAC or sham-operation, isolated hearts were perfused with either the four carbon, 13C-labeled ketone (D3-hydroxybutyrate) or the four carbon, 13C -labeled SCFA, butyrate in the presence of glucose and the LCFA, palmitate. Oxidation of ketone and SCFA was compared by in vitro 13C NMR spectroscopy, as was the capacity for short chain carbon sources to compensate for impaired LCFA oxidation in the hypertrophic heart. Adaptive changes in enzyme expression and content for the distinct pathways of ketone and SCFA oxidation were examined in both failing rat and human hearts.
Results:
TAC produced pathological hypertrophy and increased the fractional contributions of ketone to acetyl CoA production in the tricarboxylic acid cycle (0.60±0.02 sham ketone vs 0.70±0.02 TAC ketone, p<0.05). However, butyrate oxidation in failing hearts was 15% greater (0.803±0.02 TAC SCFA) than ketone oxidation. SCFA was also more readily oxidized than ketone in sham hearts by 15% (0.693±0.02 sham SCFA). Despite greater SFCA oxidation, TAC did not change short chain acyl CoA dehydrogenase content. However, failing hearts of humans and the rat model both contain significant increases in acyl CoA synthetase medium chain 3 enzyme gene expression and protein content. The increased oxidation of SCFA and ketones occurred at the expense of LCFA oxidation, with LCFA contributing less to acetyl CoA production in failing hearts perfused with SCFA (0.19±0.012 TAC SCFA vs. 0.3163±0.036 TAC ketone).
Conclusions:
SCFA are more readily oxidized than ketones in failing hearts, despite both bypassing reduced CPT1 activity, and represents an unexplored carbon source for energy production in failing hearts.
Keywords: Heart Failure, Short Chain Fatty Acids, Ketones, Cardiac Metabolism
Introduction:
To meet its high energetic requirements the beating heart is primarily reliant on the oxidation of long chain fatty acids (LCFA) for the generation of ATP 1. With the development of heart failure there is a reduction in the contribution of LCFA to oxidative ATP generation, due to reduced activity of the major rate-limiting enzyme for LCFA oxidation, carnitine palmitoyltransferase (CPT) 1, which results in reduced LCFA entry into the mitochondria 1–6. Although compensatory glucose utilization occurs with hypertrophic remodeling, other maladaptive changes in glucose utilization pathways result in the inefficient oxidation of glucose and reduced energetic yield, further contributing to decompensation and energy starvation 3,4. There is currently great interest emerging in ketones as an alternative substrate for oxidative energy metabolism 7–9.
The decrease in CPT1 activity coincides with a shift in isoform expression in response to pathological stress on the heart. The normal adult myocardium co-expresses two CPT1 isoforms: the muscle isoform, CPT1b, which is the predominant isoform in the adult cardiomyocyte and the lesser expressed liver isoform, CPT1a, which is more highly expressed in fetal cardiomyocytes. In response to pathological stress, within as early as 2 weeks and prior to any cardiac dysfunction, expression and content of the CPT1a isoform is elevated, while CPT1b message levels decline with the CPT1b protein content either being reported to either decline or not change 2,10,11. Not only is there a reduction in CPT1 activity and flux through the enzyme/transporter complex, but there is also a reduction in LCFA activation to fatty acyl CoA through long chain acyl CoA synthetase 1, which serves to exacerbate the limitation in fatty acyl supply for mitochondrial oxidative pathways 2,12.
In contrast, ketones, and short chain fatty acids (SCFA) as well, are not dependent on CPT1 for mitochondrial entry nor acyl CoA synthetase 1 for activation, but rather cross the mitochondrial membrane via free diffusion, in the absence of any identified transport-mediated mechanism 13,14. Thus, ketones bypass any inhibition of CPT1 in the heart, as would SCFA 15. In the failing heart there is an increase in the expression of the key enzymes regulating ketone oxidation, and an increase in the circulating ketone concentration, leading to increase ketone utilization in both isolated failing hearts and in vivo human studies 7,16,17. Such findings have led to the proposal that ketones are a key fuel source that could mitigate the reduced energy stores in the failing heart 18. Recent studies clearly show elevated contributions of ketones to the mitochondrial oxidative pathway of the tricarboxylic acid (TCA) cycle in hypertrophied and failing hearts 7,8. In animal models, this increase in ketone oxidation in pathologically stressed hearts has been shown to coincide with upregulation of the enzyme catalyzing the initial step of ketone oxidation, β-hydroxybutyrate dehydrogenase 1 (BDH1) 7,8,16. Whether increased ketone oxidation in the failing heart is an adaptive mechanism specific to elevated BDH1 expression and the oxidation of ketones, or whether other fuels that bypass reduced CPT1 activity, such as SCFA, provide similar support and efficiency as alternate carbon-based substrates remains unknown.
In the present study, we directly compared the capacity of the ketone, 3-hydroxybutyrate (3-OHB) and the SCFA, butyrate, both four-carbon substrates, to fuel oxidative metabolism in the failing heart. As hypothesized, both short chain carbon sources supplemented reduced LCFA oxidation in the failing heart. However, butyrate unexpectedly proved to be the preferred energy source in the heart versus 3-OHB, with a heightened preference in the hypertrophic heart. While the results highlight the importance of identifying fuels that bypass the reduction in CPT1 activity and LCFA oxidation in the failing heart, the findings also show that BDH1 upregulation is not requisite to bypass CPT1 inhibition, elucidating the efficiency of SCFA oxidation and an unexpected substrate preference by the failing heart. Thus, SCFA were more efficient in bypassing CPT1 inhibition than ketone and the findings reveal novel changes in cardiac metabolic pathways initiated by the failing heart to maintain carbon flux, evidence for which could also be found in the metabolic enzyme content of human hearts with non-ischemic cardiomyopathy (NICM).
Methods:
Data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating procedures upon reasonable request to the corresponding author.
Animal Model
Cardiac hypertrophy by chronic pressure overload for 14 weeks was induced by transverse aortic constriction (TAC) via a tantalum clip (Weck, Inc.) placed around the transverse aorta of 32 male Sprague–Dawley rats (85–100 g) and set to an internal diameter of 0.56 mm, as previously described 4,11. Male animals were studied based on need to match previously published protocols on which these studies are based. The sham groups (28 male Sprague-Dawley rats) underwent similar surgery without placement of the aortic band. Rats had free access to food and water while being housed under controlled temperature and lighting. Animals received either buprenex (0.1 mg/kg) or buprenex SR (1 mg/kg) prior to surgery and carprofen (5 mg/kg) once daily for 3 days post-surgery. Animals receiving buprenex also received twice daily buprenex (0.1 mg/kg) for 3 days post-surgery. Animals were intubated during surgery and anesthesia was maintained via 0.5–2% isoflurane in 100% oxygen. All experimental procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University.
Isolated Perfused Hearts
14 weeks after TAC or sham surgery, animals were heparinized (1000 IU, intraperitoneal injection) and anesthetized (100 mg/kg pentobarbital, intraperitoneal injection). Hearts were excised and retrogradely perfused with modified Krebs-Henseleit buffer (in mmol/L: 116 NaCl, 4 KCl, 1.5 CaCl2, 1.2 MgSO4, and 1.2 NaH2PO4) equilibrated with 95% O2/5% CO2 and containing 0.6 mmol/L palmitate complexed to bovine serum albumin in a 3:1 molar ratio, 5 mmol/L glucose, and either 1 mmol/L of sodium butyrate or sodium D-3-hydroxybutyrate (3-OHB). Buffer temperature was maintained at 37°C. Hearts were situated in a 20-mm broadband NMR probe within a 9.4-T, vertical bore (89 mm) NMR magnet and a 2 min 31P NMR spectrum was acquired. The buffer was then switched for one in which either butyrate ([2,4-13C2] butyrate), 3-OHB ([2,4-13C2] 3-OHB), a mix of 0.5 mmol/L butyrate and 0.5 mmol/L 3-OHB in which only one of the substrates was 13C labeled, or palmitate [2,4,6,8,10,12,14,16-13C8] with all 13C at an enrichment of >99%. Hearts were perfused for 24 min until isotopic steady state was reached, at which time a second 31P NMR spectrum was acquired after which the hearts where rapidly frozen with liquid N2-cooled tongs.
A water-filled latex balloon, connected to a force transducer, was fitted into the left ventricle and set to a diastolic pressure of 5 mm Hg. Left ventricular developed pressure data were continuously acquired during perfusion with Powerlab (AD Instruments, Dunedin, New Zealand). Rate pressure product was calculated as heart rate × LV developed pressure, and mean peak rates of pressure development and relaxation (+dP/dt and −dP/dt) were calculated from the first derivative of the LV developed pressure trace.
In Vitro NMR Spectroscopy
Perchloric acid extracts of frozen LV tissue from perfused hearts were lyophilized and reconstituted in 0.5 mL deuterium oxide. High-resolution proton-decoupled 13C NMR spectra were acquired from in vitro samples with a 5 mm 13C probe (Bruker Instruments, Billerica, MA). The relative contribution of butyrate, 3-OHB and the LCFA palmitate to acetyl CoA entering the TCA cycle was determined as described previously from glutamate isotopomer and isotopologue analysis from detection of relative multiplet signals with the NMR resonance signals from the glutamate 3- and 4-carbons 19.
Acyl Carnitine Analysis
Acyl carnitines were isolated from frozen heart tissue, as described previously 20, by homogenizing 25 mg of tissue in 0.1 ml of 100 mmol/L KH2PO4 and 0.25 ml of acetonitrile/isopropanol/methanol 3:1:1 (v/v). The homogenate was sonicated for 30 s and centrifuged for 10 min at 16000 g for 10 min. The supernatant was used for LC/MS MS analysis based on established protocols with some modifications 21. Briefly, supernatants were injected into a Vanquish UHPLC system connected to a TSQ Altis Triple Quadrupole mass spectrometry (electrospray ionization). Samples were separated on an Acclaim 120 C18 column at a flow rate of 0.4 ml/min heated to 30°C. Mobile phases consisted of solvent A (10 mmol/L ammonium formate, 0.1 % formic acid and 0.01% TEA in water) and solvent B (methanol). The following gradient was employed: 0 min 0% B, 2 min 0% B, 12 min 50% B, 13 min 100% B, 15 min 100% B, 16 min 0% B, 21 min 0% B. Labeled (13C) and unlabeled (12C) acyl carnitine species were identified by selective reaction monitoring. The precursor-product transitions that were utilized are listed in Supplemental Table I in the online Supplemental Materials for the identification of the individual acyl carnitine species.
Human Heart Tissue Collection
The protocol for surgical sampling of myocardium from patients was approved by the University of Utah Institutional Review Board. All patients provided written informed consent before inclusion.
Myocardial tissue was obtained from the LV apical core at CF-LVAD implant from 5 male patients. Transmural apical core biopsies also were acquired from 5 non-failing donor hearts that were not allocated for human transplantation because of noncardiac reasons. Each transmural biopsy was immediately frozen and stored at −80°C for metabolic enzyme expression analysis. Patient characteristics are listed in Supplemental Table II.
Metabolic Enzyme Expression
Protein expression was determined by western blot and band intensities quantified by LI-COR Odyssey Fc and normalized to the expression of calsequestrin (CASQ) as a loading control while mRNA levels were determined by quantitative reverse transcription polymerase chain reaction in frozen heart tissue and normalized to S29 11,12. Antibodies (Supplemental Table III) and primers (Supplemental Table IV) used are listed in the online only Supplemental Materials.
Statistical Analysis
Data is presented as mean±SEM. Comparisons between two mean values were performed using the Student’s t-test and among more than two mean values using 2-way analysis of variance (ANOVA) with a Tukey’s multiple comparison post-hoc test. The statistical method utilized is indicated in each figure legend along with the individual n value and p values. Means were said to be statistically significant at p<0.05.
Results:
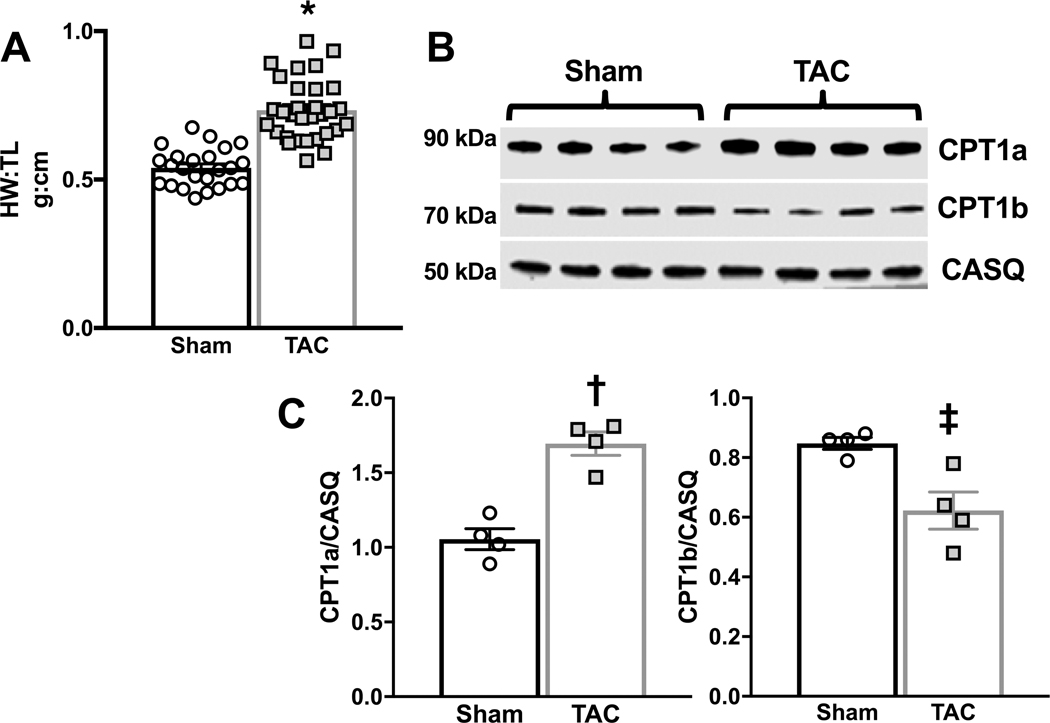
Sprague-Dawley rats (85–100 g at time of surgery) were subjected to chronic pressure overload via transverse aortic constriction (TAC) surgery to induce decompensated pathological hypertrophy. After 14 weeks post-TAC or -sham surgery there was a 35% increase in heart weight to tibia length (Figure 1a) and changes in the expression of the key enzymes regulating LCFA entry into the mitochondria were evident (Figure 1b), as previously reported 10,11. TAC hearts displayed a 27% reduction in the expression of the predominant muscle isoform of CPT1, CPT1b, and a 61% increase in the expression of the liver isoform, CPT1a. An increase in the expression of CPT1a in the heart is associated with a reduction in the rate of fatty acid oxidation and a net decrease in CPT1 activity and is consistent with previous reports of CPT1 expression in the failing heart 4,10,11.
Figure 1 – Hypertrophic response and carnitine palmitoyl transferase 1 expression in response to transverse aortic constriction (TAC).
A, Heart weight to tibia length (HW:TL) was measured in sham (n=24) and TAC (n=31) hearts 14 weeks after sham or TAC surgery; *p<0.0001 as determined by unpaired 2-tailed t-test. B, Carnitine palmitoyl transferase (CPT)1a and CPT1b expression in unperfused sham or TAC hearts isolated 14 weeks after TAC or sham surgery. C, CPT1a and CPT1b protein expression normalized to calsequestrin (CASQ); †p=0.0009, ‡p<0.0137 vs. sham as determined by unpaired 2-tailed t-test.
The SCFA butyrate is a preferred substrate over 3-OHB in control and TAC hearts.
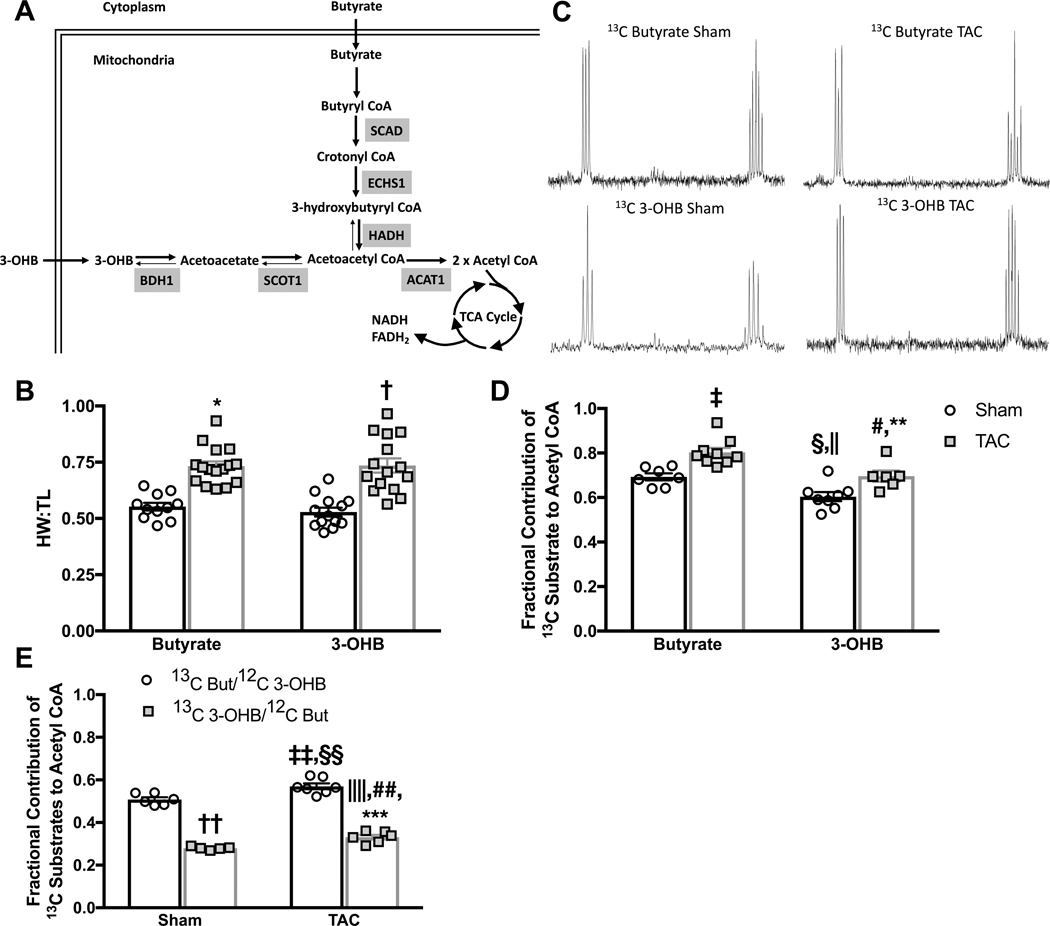
To access the relative contribution of SCFA and ketones to the TCA cycle, an indication of relative capacities of both to support mitochondrial oxidation, we investigated the oxidation of the four-carbon SCFA butyrate and ketone 3-OHB in isolated hearts 14 weeks after TAC or sham surgery (control). Comparing a four-carbon SCFA, butyrate, to the four-carbon ketone, 3-OHB, allowed direct analysis of the oxidation of the two fuels, because both substrates yield 2 acetyl CoA molecules to fuel the TCA cycle (Figure 2a). Random distribution of animals resulted in a similar level of cardiac hypertrophy across each experimental protocol (Figure 2b). To provide comparison to previously published findings of ketone oxidation in hypertrophied hearts, isolated hearts were perfused for 24 minutes with medium that duplicated the previously published ketone concentration of 1 mmol/L [2,4-13C2] 3-OHB (13C 3-OHB), or for direct comparison, 1 mmol/L [2,4-13C2]butyrate (13C butyrate), both in the presence of unlabeled glucose (5 mmol/L) and palmitate (0.6 mmol/L) 7,8. Analysis of glutamate isotopomers and isotopologues determined the contribution of each substrate to acetyl CoA formation and entry into the TCA cycle 19 (Figure 2c).
Figure 2 – Fractional contribution of butyrate and 3-hydroxybutyrate (3-OHB) to mitochondrial oxidation.
A, model depicting the pathways for butyrate and 3-OHB oxidation in the heart. The role of enzymes acetyl CoA acetyltransferase (ACAT) 1, β-hydroxybutyrate dehydrogenase (BDH) 1, enoyl CoA hydratase short chain (ECHS) 1, 3-hydroxyacyl CoA dehydrogenase (HADH), short chain acyl CoA dehydrogenase (SCAD), and succinyl CoA:3-ketoacid CoA transferase (SCOT) 1 in short chain carbon metabolism is depicted. B, heart weight to tibia length (HW:TL) as measured in isolated hearts at the end of perfusion with either butyrate (n=11 sham, n=16 TAC) or 3-OHB (n=13 sham, n=15 TAC) perfused hearts; *p<0.0001 vs. butyrate sham, †p=0.0001 vs. 3-OHB sham group via 2-way ANOVA and Tukey’s post hoc test. C, representative spectra from end-point enrichment analysis of glutamate 13C enrichment in perfused hearts. D, fractional contribution of either 13C butyrate (n=7 sham, n=9 TAC) or 13C 3-OHB (n=8 sham, n=6 TAC) to acetyl CoA; ‡p=0.0032 vs. butyrate sham, §p=0.024 vs. butyrate sham, ||p<0.0001 vs. butyrate TAC, #p=0.0065 vs. butyrate TAC, **p=0.0252 vs. 3-OHB sham via 2-way ANOVA and Tukey’s post hoc test. E, fractional contribution of either 13C butyrate (n=6 sham, n=5 TAC) or 13C 3-OHB (n=7 sham, n=6 TAC) to acetyl CoA when provided as a 50:50 mix, ††p<0.0001 vs. 13C But/12C 3-OHB sham, ‡‡p=0.0034 vs. 13C But/12C 3-OHB sham, §§p<0.0001 vs. 13C 3-OHB/12C But sham, ||||p<0.0001 vs 13C But/12C 3-OHB sham, ##p=0.0273 vs. 13C 3-OHB/12C But sham, ***p<0.0001 vs. 13C But/12C 3-OHB TAC, via 2-way ANOVA and Tukey’s post hoc test
As previously established, cardiac hypertrophy coincided with an increase (17% increase vs. 3-OHB sham, p=0.0252) in the relative contribution of 3-OHB to oxidative metabolism (Figure 2c&d) 7,8. The utilization of the SCFA butyrate was also increased in TAC hearts (16% vs. butyrate sham, p=0.0032) (Figure 2b&c), in contrast to the decline in LCFA oxidation observed in the failing heart 1,5,11,22. Strikingly, at both baseline and following the induction of hypertrophy, butyrate was oxidized to a greater extent than was 3-OHB. Butyrate was the preferred substrate relative to 3-OHB, contributing 15% more acetyl-CoA units entering the TCA cycle than did 3-OHB in both sham (p=0.024 butyrate sham vs. 3-OHB sham) and TAC hearts (p=0.0065 butyrate TAC vs. 3-OHB TAC) (Figure 2d).
When butyrate and 3-OHB were provided as a mix (0.5 mmol/L butyrate + 0.5 mmol/L 3-OHB) the cardiac preference for butyrate vs. 3-OHB was greatly evident, with butyrate contributing 75% more acetyl CoA units than 3-OHB TAC hearts (Figure 2e).
The increased butyrate oxidation in the failing heart is associated with an increase in acyl CoA synthetase medium chain family member (ACSM) 3 expression.
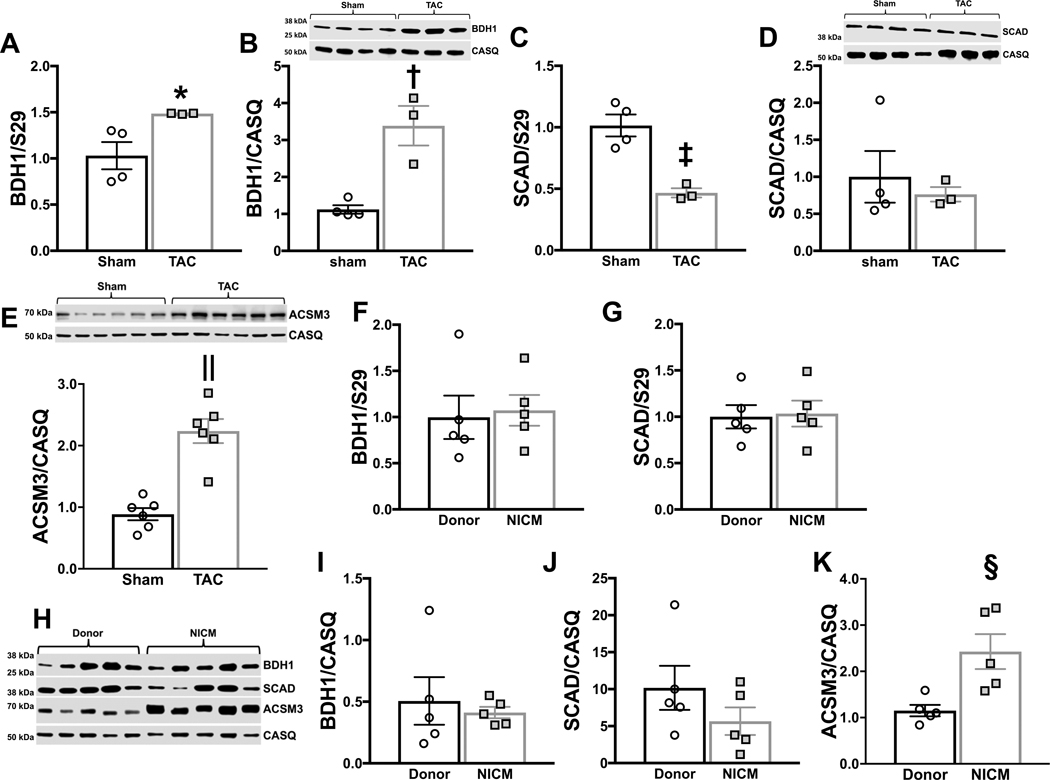
Increased gene expression of the key dehydrogenase that regulates ketone oxidation, BDH1, has been reported in the myocardium of human heart failure patients, along with increased BDH1 expression and protein content in animal models of hypertrophy and heart failure 7,16. In the present study, BDH1 mRNA and protein content was increased in hearts harvested at 14 weeks after TAC surgery relative to sham (Figure 3a&b). Conversely, the content of the key dehydrogenase regulating SCFA oxidation, short chain acyl CoA dehydrogenase (SCAD), was not changed at 14 weeks post-TAC (Figure 3c&d). SCAD mRNA expression was found to be decreased with no change in protein expression. The protein expression of the acyl-coenzyme A synthetase medium chain family member (ACSM) 3 was increased with TAC (Figure 3e). ACSM3 is a mitochondrial enzyme normally expressed at low levels in the heart that activates butyrate to butyryl CoA within the mitochondria23. Although a role for ACSM3 has been identified in cancer cell metabolism24, this is the first report of ACSM3 having a meaningful role in the failing heart.
Figure 3 – Expression of key enzymes regulating short chain fatty acid (SCFA) and ketone oxidation in the heart.
A, mRNA expression normalized to S29 of β-hydroxybutyrate dehydrogenase (BDH) 1, *p=0.0475 via two-tailed t-test. B, BDH1 protein expression normalized to calsequestrin (CASQ), †p=0.0047 via two-tailed t-test. C, mRNA expression normalized to S29 of short chain acyl CoA dehydrogenase (SCAD); ‡p=0.0041 via two-tailed t-test. D, SCAD protein expression normalized to CASQ. E, ACSM3 protein expression normalized to CASQ, ||p=0.0001 via two-tailed t-test. mRNA expression normalized to S29 for BDH 1 (F) and SCAD (G) from human donor hearts or hearts from patients with non-ischemic cardiomyopathy (NICM). H, protein expression for BDH1, SCAD and ACSM3 measured in donor hearts or patients with NICM. Protein expression for BDH1 (I), SCAD (J), and ACSM3 (K) normalized to CASQ, §p=0.0125 via two-tailed t-test.
Heart failure in humans results in increased ACSM3 expression.
To establish the relevance of our findings to the clinical patient population, we evaluated enzyme expression in human heart tissue from either donor hearts or patients with non-ischemic cardiomyopathy (NICM), a pathology consistent with the TAC animal model. Somewhat surprisingly, we did not observe an increase in BDH1 mRNA expression (Figure 3f), nor was there a change in SCAD mRNA expression (Figure 3g). We also evaluated the myocardial protein content of both BDH1 and SCAD as well as ACSM3 (Figure 3h–k). In agreement with gene expression, the protein contents of BDH1 and SCAD were not different between donor hearts or patients with NICM. However, there was a significant increase in ACSM3 expression in NICM. ACSM3 in human heart failure or animal models of heart failure has not previously been investigated and reveals that the TAC-induced increase in ACSM3 is not species specific, but also occurs in failing human hearts.
Butyrate is directed towards β-oxidation and away from pseudoketogensis in the hypertrophic heart.
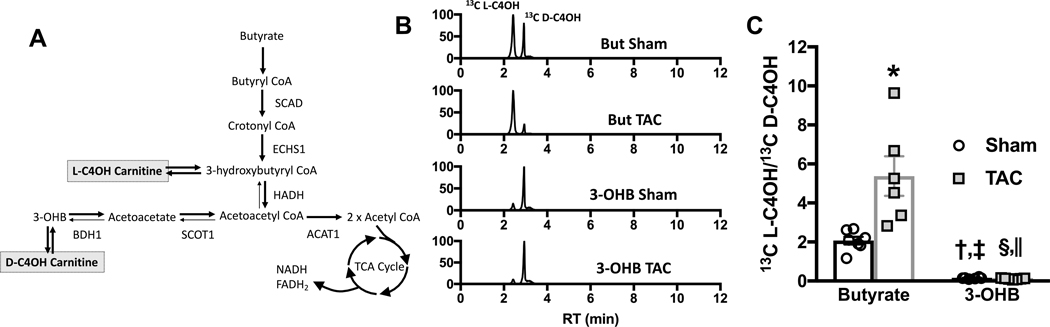
In addition to analyzing the contribution of short chain carbon sources to the TCA cycle, we also examined myocardial content of short chain acyl carnitine species in response to perfusion with either 13C butyrate or 13C 3-OHB. Carnitine esters for both fatty acyl and ketone intermediates are formed in the mitochondria, and can serve as a surrogate for the level of metabolic intermediates as they are more stable and are more readily quantifiable than short chain fatty acyl CoA species in particular, and are in equilibrium with oxidative intermediates (Supplemental Figure I, Figure 4a). LC/MS was used to determine the 13C-enrichment of individual short chain acyl carnitine species that were formed from either 13C butyrate or 13C 3-OHB in the isolated perfused hearts. As expected 13C butyrate led to enrichment of the butyryl carnitine pool (Supplemental Figure I), while both 13C butyrate and 13C 3-OHB produced 13C-enriched 3-hydroxybutyryl (C4OH) carnitine (Figure 4b). Butyrate led to formation of two species of 13C C4OH, 3-hyxdroxybutyryl-L-carnitine (L-C4OH) which is in equilibrium with the β-oxidation intermediate 3-hydroxybutyryl CoA, and 3-hydroxybutyryl-D-carntine (D-C4OH) (Figure 4a&b) which is in equilibrium with 3-OHB. D-C4OH is formed during butyrate perfusion by reversal of the reactions catalyzed by succinyl CoA transferase 1 (SCOT1) and BDH1 leading to 3-OHB formation, through a process which has been described as pseudoketogenisis 25,26. Representative total ion chromatograms for 13C C4OH reveal that with the induction of cardiac hypertrophy there is a significant reduction in the formation of 13C D-C4OH in TAC hearts perfused with 13C butyrate relative to sham hearts supplied 13C butyrate (Figure 4b&c), implying reduced pseudoketogenisis. The ratio of 13C L-C4OH to the D isoform is significantly increased with the inclusion of 13C butyrate to the perfusion medium (Figure 4c). While the increase in this ratio is not surprising as 13C L-C4OH is in equilibrium with 3-hydroxybutyryl CoA, the further increase in TAC hearts supplied butyrate, relative to sham hearts supplied butyrate, suggests an increase in coupling between β-oxidation and TCA cycle activity and a reduction in reverse flux through both SCOT and BDH1. 13C 3-OHB led to very little formation of the oxidative intermediate (L-C4OH) at either baseline or in response to TAC and a significant change in the ratio of two isoforms was not observed with TAC in the 3-OHB perfused hearts.
Figure 4 – Changes in short chain acyl carnitine enrichment in hearts perfused with either butyrate of 3-hyrdoxybutyrate (3-OHB).
A, sources of 3-hydroxybutyryl carnitine (C4OH) formed in equilibrium with oxidative pathway intermediates; acetyl CoA acetyltransferase (ACAT) 1, β-hydroxybutyrate dehydrogenase (BDH) 1, enoyl CoA hydratase short chain (ECHS) 1, 3-hydroxyacyl CoA dehydrogenase (HADH), short chain acyl CoA dehydrogenase (SCAD), and succinyl CoA:3-ketoacid CoA transferase (SCOT) 1. B, representative total ion current chromatogram of 13C enrichment of C4OH carnitine from either 13C butyrate or 13C 3-OHB. Two peaks are evident, 3-hydroxybutryl-L-carnitine (L-C4OH) and 3-hydroxybutyrl-D-carnitine (D-C4OH). C, the ratio of 13C L-C4OH to 13C D-C4OH measured in hearts perfused with either 13C butyrate (n=7 sham, n=6 TAC) or 13C 3-OHB (n=6 sham, n=6 TAC); *p=0.0004 vs. butyrate sham, †p=0.0438 vs. butyrate sham, ‡p<0.0001 vs. butyrate TAC, §p=0.0309 vs. butyrate sham, ||p<0.0001 vs. butyrate TAC via 2-way ANOVA and Tukey’s post hoc test.
Contractile performance and energetic status of the failing hearts oxidizing ketone or SCFA.
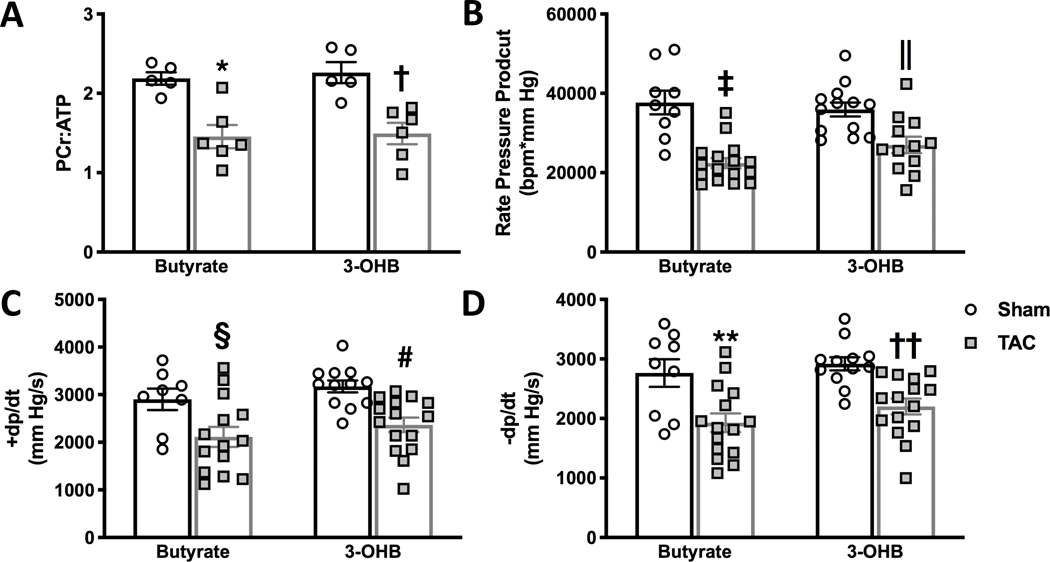
To assess the potential of the alternative, four-carbon fuel sources to acutely improve the energetic status of the failing heart, the ratio of phosphocreatine (PCr):ATP was examined as an index of the bioenergetic state of isolated hearts during perfusion with either butyrate or 3-OHB (Figure 5). Not surprisingly, a short perfusion protocol was not sufficient to impact the energetic status of the failing hearts (Figure 5a), and neither 3-OHB nor butyrate altered either the energetic status or the immediate mechanical function of the failing hearts during the duration of the perfusion protocols (Figure 5b–d).
Figure 5 – Energetic and contractile status of hearts perfused with butyrate or 3-hydroxybutyrate (3-OHB).
A, phosphocreatine (PCr) to ATP ratio of hearts perfused with either butyrate or 3-OH 14 weeks after TAC or sham surgery, *p=0.0044 and †p=0.007 vs. sham group via 2-way ANOVA and Tukey’s post hoc test (n=5 for both sham groups, n=6 for both TAC groups). B, rate pressure product; C, maximum rate of pressure development (+dp/dt); and D, maximum rate of relaxation (-dp/dt) measured in left ventricle of isolated perfused hearts. ‡p<0.0001, §p=0.0089 vs. sham; ||p=0.0337, #p=0.0097 vs. sham; **p=0.0042, ††p=0.0083 vs. sham via 2-way ANOVA and Tukey’s post hoc test. For B, C, and D n=9 sham butyrate; n=13 sham 3-OHB; n=16 TAC butyrate; and n=12 TAC 3-OHB.
Although there was not a change in the PCr:ATP ratio in response to either short chain substrate, TAC hearts supplied butyrate did not contain a significant difference in the ratio of pAMPK:AMPK compared to sham hearts, while pAMPK:AMPK remained significantly increased in TAC hearts supplied 3-OHB (Supplemental Figure II). The data provide some evidence for an improvement in energetic status, not evident in the ratio of PCr:ATP content, however other AMP-independent changes cannot be excluded.
The increased SCFA and ketone utilization occurs at the expense of LCFA.
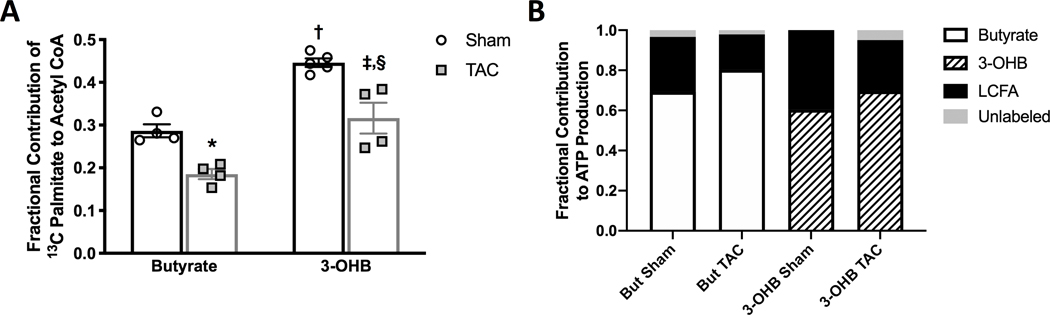
To verify that the utilization of short chain carbon sources was supplementing reduced LCFA oxidation in the failing heart, and to determine any influences of either ketones or SCFA on mitochondrial LCFA oxidation, the contribution of the LCFA, palmitate, to the TCA cycle was determined. Isolated hearts were perfused with 0.6 mmol/L 13C-labeled palmitate ([2,4,6,8,10,12,14,16-13C8] palmitate) in the presence of either unlabeled butyrate or unlabeled 3-OHB. As previously reported, the LCFA contribution to mitochondrial oxidation in the TAC hearts was reduced compared to that of sham heart (Figure 6a) 2,5,11,22. Furthermore, the reduction in LCFA oxidation in TAC hearts was offset by increased oxidation of both short chain carbon sources (Figure 2d and Figure 6b). However, as is consistent with the preferential oxidation of butyrate versus 3-OHB in both sham and TAC hearts, butyrate induced a greater reduction in LCFA oxidation than did 3-OHB in both sham and TAC hearts (Figure 6a). The data reveal that butyrate does indeed serve as an alternative fuel to counter reduced LCFA oxidation within the mitochondria in the failing heart, and also displaces LCFA oxidation to a greater extent than does 3-OHB, due to the SCFA being a more preferred oxidative fuel for ATP production than the ketone in both normal and failing hearts.
Figure 6 – The contribution of the long chain fatty acid (LCFA) palmitate to mitochondrial oxidative metabolism in the hypertrophic heart perfused with either short chain fatty acids (SCFA)s or ketones.
A, the relative contribution of 13C palmitate to acetyl CoA formation in the tricarboxylic acid (TCA) cycle from in vitro NMR 13C NMR; *p=0.0181 vs. butyrate sham, †p=0.0003 vs. butyrate sham, ‡p=0.0029 vs. butyrate TAC sham, §p=0.002 vs. 3-OHB sham via 2-way ANOVA and Tukey’s post-hoc test (n=4 sham butyrate, n=4 sham 3-OHB, n=5 TAC butyrate, n=4 TAC 3-OHB). B, a comparison of the contribution of either 13C butyrate, 13C 3-hydroxybutyrate (3-OHB) or 13C palmitate to acetyl CoA formation in the TCA cycle by combining the data from Figure 6a with that from Figure 2d.
Discussion:
This is the first study to directly compare the ability of ketones and SCFA to fuel oxidative metabolism in the failing heart. The results reveal that SCFA are a preferred energy source over ketones, and with the development of heart failure this relative preference for SCFA is preserved. Although ketones represent a potential endogenous fuel source for the failing heart, SCFA present a novel consideration through the capacity to more efficiently circumvent the inhibition of LCFA entry into mitochondria in the pathologically stressed heart. More significantly, the results reveal novel mechanistic insight into the remodeling that occurs during cardiac hypertrophy and highlight the degree to which the reduction in fatty acid entry through CPT1 is a primary limitation to the capacity of LCFA to fuel ATP production in the failing heart. Substrates that bypass CPT1 compensate for the deficit in LCFA transport into the mitochondria, allowing for previously unknown alterations in downstream pathways to facilitate entry of activated acyl-units into the inner mitochondrial matrix and the TCA cycle of the failing heart.
The preference for SCFA over ketones by the heart, when studied at equimolar concentrations of similar four-carbon chain length SCFA and ketone, has not been previously demonstrated in either the normal or failing heart. The differences in utilization are not likely attributable to differences in extraction, as both substrates are understood to enter the cardiomyocyte via the monocarboxylate transporter14,27. Rather, these results suggest differences in the efficiencies of the two pathways for the generation of acetoacetyl CoA and acetyl CoA, once either of these substrates enters the mitochondria (Figure 2a). Unlike LCFA and the glycolytic end-product, pyruvate, both ketones and SCFA are able to enter the mitochondria directly. Ketones first pass through BDH1, while SCFA are activated to fatty acyl CoA via the ACSMs28 and enter the first reaction of β-oxidation via SCAD7,14,27. We have elucidated for the first time a change in ACSM3 expression in failing hearts from both rat and human patient samples. Previously an impairment in LCFA activation to acyl CoA via ACSL1 was established by our group in both animal models and human heart failure12. The increase in ACSM3 is a previously unknown mechanism to address the reduction in fatty acid activation in the heart. However, accessing the full potential of this upregulation requires that fatty acids are able to enter the mitochondria independently of CPT1. Of note, ACSM3 shows broad substrate specificity (C4-C14) and in addition can activate LCFA. Past interest in medium chain fatty acids and medium chain triglycerides as a treatment strategy in cardiac disease has met with mixed success, potentially a function of study design. From the data obtained in this study the ideal fatty acid makeup appears to be one in which specific fatty acids of chain lengths that are able to enter the mitochondria independently of CPT1 activity to fully access not only the increase in ACSM3, but also the reduction in pseudoketogenesis, which has not been previously documented in the failing heart.
An argument could be made that it is not surprising the SCFA would be a preferred energy source over ketones in normal hearts, as SCFA would share many of the enzymes involved in LCFA oxidation, acknowledged as the preferred energy source for the heart in vivo. The observation that this preference is maintained in the hypertrophic heart is surprising. Many of the enzymes involved in SCFA are targets of PPARα27,29–31. PPARα and many of its targets are downregulated in the failing heart and the reduction in PPARα signaling is thought to be a primary event in reduction in fatty acid utilization in the failing heart11,32,33. The conclusion that must be drawn is that the increase in butyrate utilization is a compensatory response to the reduction in CPT1 activity induced by heart failure rather than a primary response to an increase in the oxidative enzyme expression that has been otherwise suggested for 3-OHB2,3,5,7,9,16,33,34. An increase in BDH1 expression was not observed in NICM. However, an increase in BDH1 gene expression has been reported in human failing myocardium16, but the increase was modest and BDH1 protein content has not been reported until now.
An increase in BDH1 is not necessarily requisite for ketone oxidation to be increased in the failing heart. Indeed, heart failure is known to coincide with increased circulating ketones16,17, and ketone extraction by the heart appears governed by the circulating concentration17. This is not the case for substrates that do not as readily cross the plasma membrane, such as glucose and LCFA. As the present findings suggest, the blockade that develops at the level of CPT1, on the outer mitochondrial membrane, would favor ketone oxidation even in the absence of additional metabolic alterations. Just as with SCFA, our data demonstrate that an increase in dehydrogenase expression may also not be requisite to produce augmented ketone, or SCFA, oxidation.
The therapeutic potential of ketones as an endogenous source of short chain carbon to fuel the failing heart has already been discussed in previous studies9,16,34,35. We did not observe a functional improvement over the brief perfusion protocol in which short chain carbon sources were provided, although AMPK phosphorylation was no longer significantly increased in TAC hearts perfused with butyrate. AMP-independent changes in AMPK phosphorylation can occur in response to factors not directly linked to energetic status. Previously, we and others have shown that the expression ACSL1 can alter AMPK phosphorylation, with AMPK phosphorylation increasing with ACLS1 overexpression and declining with the loss of ACSL1 expresison12,36. It has been postulated that the dependence of AMPK phosphorylation on ACSL1 expression relates to the consumption of ATP by ACSL136,37, however ACSM3 also consumes ATP to activate butyrate. As shown in this study, ACSM3 content is elevated in both the animal model of heart failure and in failing human hearts. The absence of any acute improvement in function is in line with another recent study, in which ketones failed to induce any improvement in mechanical efficiency in failing hearts35. Contrasting our report is recent work by Kelly and colleagues, showing infusion of ketones can ameliorate the functional decline in a model of pacing induced heart failure in dogs8. The latter study was not as acute as what occurred in our work or the work of Ho et al35. Thus, a chronic change in ketone availability or utilization appears to have an effect on the pathogenesis of heart failure, as both a ketogenic diet or overexpression of BDH1 attenuate the effects of TAC, while the loss of BDH1 expression leads to a more severe remodeling response8,9.
There has been interest in developing agents that increase circulating butyrate levels, particularly in the setting of cancer, due to the action of butyrate as an HDAC inhibitor38. The butyrate pro-drug, tributyrin, was capable of raising the circulating butyrate concentration to a mean value of 98 umol/L in patients with advance solid tumors38, which is lower than the concentration used in the present study, but within range of circulating ketone concentrations in heart failure patients, under conditions of which ketones are argued to significantly contribute to cardiac energy production8,16,33. SCFA are primarily produced by the gut microbiota at a ratio of 60:20:20 (acetate:propanoate:butyrate) and can reach a combined concentration of 100 mmol/L in the intestinal lumen, however the circulating concentration of butyrate is significantly lower27,39. A diet high in fiber increases SCFA production by the gut and attenuates hypertrophic remodeling in response to hypertension, however much of this protection has been attributed to an increase in acetate40. Conversely, the gut of heart failure patients has been found to be depleted of butyrate producing bacteria41,42 and the loss of butyrate producing bacteria in the gut of mice makes them more prone to cardiac remodeling in response to stress which can be rescued by butyrate supplementation 43,44. Butyrate in particular, but not acetate or propanoate, has been identified as a histone deacetylase (HDAC) inhibitor, leading to potent anti-inflammatory signaling, an effect that might actually be antagonized by ketones45. Therefore SCFA can display both class-specific and chain-length specific characteristics that remain to be fully understood. The present study focuses on comparing the acute effects of these short chain carbon sources on mitochondrial metabolism. Future work may provide important insights by extending investigations to chronic studies to assess benefits of SCFAs generally, and butyrate specifically, on both energy provision and intracellular signaling through alternative pathways such as HDAC inhibition38,46.
Apart from any potential therapeutic role as an alternative fuel that bypasses the potentially maladaptive reduction in LCFA oxidation in the failing heart, the primary finding of this study is the enhanced oxidation of butyrate within the mitochondria, over that of ketones, in the failing heart. Butyrate proved to be the more efficient substrate for oxidation within the TCA cycle compared to the ketone 3-OHB. The capacity of this SCFA to bypass the LCFA transport process of CPT1 revealed novel alterations in metabolic pathways favoring mitochondrial SCFA oxidation in the failing heart; changes validated in hearts of patients with NICM. While we report no differences in the contractile performance of hearts provided either a ketone or a SCFA, in the presence of other physiological substrates, the implications of this preferential oxidation of butyrate are that 1) ketones do not serve as a unique “superfuel” beyond availability in the circulation to bypass inhibition of LCFA at CPT1 in the failing heart and 2) the SCFA, butyrate, has a higher affinity for entry into mitochondrial oxidation at SCAD than does 3-OHB at BDH1, and then also through the respective downstream oxidative pathways for each substrate.
Supplementary Material
Clinical Perspective:
What is new?
The failing heart oxidizes short chain fatty acids (SCFA) more readily than ketones, with SCFA also displacing long chain fatty oxidation to a greater extent.
The SCFA, butyrate, has a higher affinity for entry into mitochondrial oxidation at the enzyme, short chain acyl-CoA dehydrogenase, than does the ketone, 3-hydroxybutyrate, at the β-hydroxybutyrate dehydrogenase, and then also through the respective downstream metabolic pathways for each substrate.
Failing hearts of rats and human have increased levels of acyl-coenzyme A synthetase medium chain 3 enzyme which can also oxidize SCFA to enhance butyrate oxidation.
What are the clinical implications?
While ketones have been sought as a potential supplemental fuel to remedy the impaired oxidative metabolism of the failing heart, this study shows that failing hearts preferentially oxidize short chain fatty acids over ketones, and SCFA may prove to be a more efficient energy source during pathological stress.
Novel alterations in metabolic pathways favoring SCFA oxidation in the failing heart occur in patients with non-ischemic cardiomyopathy.
Circulating ketones are not a unique “superfuel” beyond the ability to bypass the inhibition of long chain fat oxidation in the failing heart, as do SCFA.
Acknowledgments
Sources of Funding
This work was supported by funding from the National Heart Lung and Blood institute (NHLBI) of the National Institutes of Health (NIH) grants R01HL113057 (E.D.L.), R01HL132525 (E.D.L.), R01HL049244 (E.D.L.), R01 HL135121-01 (S.D.G.), and R01 HL132067-01A1 (S.D.G.)
Non-standard Abbreviations and Acronyms
- LCFA
long chain fatty acids
- CPT
carnitine palmitoyltransferase
- SCFA
short chain fatty acids
- TCA
tricarboxylic acid
- BDH
β-hydroxybutyrate dehydrogenase
- 3-OHB
3-hydroxybutyrate
- NICM
non-ischemic cardiomyopathy
- TAC
transverse aortic constriction
- CASQ
calsequestrin
- ACSM
acyl CoA synthetase medium chain family member
- SCAD
short chain acyl CoA dehydrogenase
- L-C4OH
3-hyxdroxybutyryl-L-carnitine
- D-C4OH
hydroxybutyryl-D-carnitine
- SCOT1
succinyl CoA transferase 1
- PCr
phosphocreatine
- HDAC
histone deacetylase
Footnotes
Disclosures
None.
References
- 1.Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114:717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of Compensatory Pathways to Sustain Oxidative Flux With Reduced Carnitine Palmitoyltransferase I Activity Characterizes Inefficiency in Energy Metabolism in Hypertrophied Hearts. Circulation. 2007;115:2033–2041. [DOI] [PubMed] [Google Scholar]
- 3.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahey R, Carley AN, Wang X, Glass CE, Accola KD, Silvestry S, O’Donnell JM, Lewandowski ED. Enhanced Redox State and Efficiency of Glucose Oxidation With miR Based Suppression of Maladaptive NADPH-Dependent Malic Enzyme 1 Expression in Hypertrophied Hearts. Circ Res. 2018;122:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolwicz SC, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolwicz SC Jr, Tian R. Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011;90:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation. 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y, et al. Cardiac-Specific Bdh1 Overexpression Ameliorates Oxidative Stress and Cardiac Remodeling in Pressure Overload-Induced Heart Failure. Circ Hear Fail. 2017;10:e004417. [DOI] [PubMed] [Google Scholar]
- 10.Lewandowski ED, Fischer SK, Fasano M, Banke NH, Walker LA, Huqi A, Wang X, Lopaschuk GD, O’Donnell JM. Acute liver carnitine palmitoyltransferase I overexpression recapitulates reduced palmitate oxidation of cardiac hypertrophy. Circ Res. 2013;112:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahey R, Wang X, Carley AN, Lewandowski ED. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation. 2014;130:1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg JR, Carley AN, Ji R, Zhang X, Fasano M, Schulze PC, Lewandowski ED. Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking. Circulation. 2019;139:2765–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halestrap AP. Pyruvate and ketone-body transport across the mitochondrial membrane. Exchange properties, pH-dependence and mechanism of the carrier. Biochem J. 1978;172:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puchalska P, Crawford PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017;25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewandowski ED, Kudej RK, White LT, O’Donnell JM, Vatner SF. Mitochondrial Preference for Short Chain Fatty Acid Oxidation During Coronary Artery Constriction. Circulation. 2002;105:367–372. [DOI] [PubMed] [Google Scholar]
- 16.Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvaraj S, Kelly DP, Margulies KB. Implications of Altered Ketone Metabolism and Therapeutic Ketosis in Heart Failure. Circulation. 2020;141:1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewandowski ED, Doumen C, White LT, LaNoue KF, Damico LA, Yu X. Multiplet structure of 13C NMR signal from glutamate and direct detection of tricarboxylic acid (TCA) cycle intermediates. Magn Reson Med. 1996;35:149–154. [DOI] [PubMed] [Google Scholar]
- 20.Makrecka-Kuka M, Sevostjanovs E, Vilks K, Volska K, Antone U, Kuka J, Makarova E, Pugovics O, Dambrova M, Liepinsh E. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci Rep. 2017;7:17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng M, Fang X, Huang Y, Cai Y, Liang C, Lin R, Liu L. Separation and identification of underivatized plasma acylcarnitine isomers using liquid chromatography-tandem mass spectrometry for the differential diagnosis of organic acidemias and fatty acid oxidation defects. J Chromatogr A. 2013;1319:97–106. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol. 2008;44:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis JM, Bowman CE, Wolfgang MJ. Metabolic and Tissue-Specific Regulation of Acyl-CoA Metabolism. PLoS One. 2015;10:e0116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan H-Y, Yang C, Tao X-M, He J, Wang T, Wang H, Wang C, Jin G-Z, Jin H-J, Qin W-X. Downregulation of ACSM3 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Am J Cancer Res. 2017;7:543–553. [PMC free article] [PubMed] [Google Scholar]
- 25.Bastiaansen JAM, Merritt ME, Comment A. Measuring changes in substrate utilization in the myocardium in response to fasting using hyperpolarized [1–13 C]butyrate and [1–13C]pyruvate. Sci Rep. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball DR, Rowlands B, Dodd MS, Le Page L, Ball V, Carr CA, Clarke K, Tyler DJ. Hyperpolarized butyrate: A metabolic probe of short chain fatty acid metabolism in the heart. Magn Reson Med. 2014;71:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J Lipid Res. 2016;57:943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujino T, Kondo J, Ishikawa M, Morikawa K, Yamamoto TT. Acetyl-CoA Synthetase 2, a Mitochondrial Matrix Enzyme Involved in the Oxidation of Acetate*. J Biol Chem. 2001;276:11420–11426. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Xu L, Huang Q, Luo J, Liu P, Chen S, Yuan X, Lu Y, Wang P, Zhou S. Changes in short-chain acyl-coA dehydrogenase during rat cardiac development and stress. J Cell Mol Med. 2015;19:1672–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J Biol Chem. 1998;273:5678–5684. [DOI] [PubMed] [Google Scholar]
- 31.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. [DOI] [PubMed] [Google Scholar]
- 32.Sack MN, Kelly DP. The energy substrate switch during development of heart failure: gene regulatory mechanisms (Review). Int J Mol Med. 1998;1:17–24. [DOI] [PubMed] [Google Scholar]
- 33.Aubert G, Vega RB, Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta. 2013;1833:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;1:e84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res. 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grevengoed TJ, Cooper DE, Young PA, Ellis JM, Coleman RA. Loss of long‐chain acyl‐CoA synthetase isoform 1 impairs cardiac autophagy and mitochondrial structure through mechanistic target of rapamycin complex 1 activation. FASEB J. 2015;29:4641–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Gauthier M, Sun L, Ruderman N, Lodish H. Activation of AMP‐activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl‐CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. FASEB J. 2010;24:4229–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelman MJ, Bauer K, Khanwani S, Tait N, Trepel J, Karp J, Nemieboka N, Chung E-J, Van Echo D. Clinical and pharmacologic study of tributyrin: an oral butyrate prodrug. Cancer Chemother Pharmacol. 2003;51:439–444. [DOI] [PubMed] [Google Scholar]
- 39.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. [DOI] [PubMed] [Google Scholar]
- 41.Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, Ueland T, Yndestad A, Hov JR, Trøseid M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J Am Coll Cardiol. 2018;71:1184–1186. [DOI] [PubMed] [Google Scholar]
- 42.Cui X, Ye L, Li J, Jin L, Wang W, Li S, Bao M, Wu S, Li L, Geng B, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang TWH, Chen H-C, Chen C-Y, Yen CYT, Lin C-J, Prajnamitra RP, Chen L-L, Ruan S-C, Lin J-H, Lin P-J, et al. Loss of Gut Microbiota Alters Immune System Composition and Cripples Postinfarction Cardiac Repair. Circulation. 2019;139:647–659. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Jin Z, Shen K, Weng T, Chen Z, Feng J, Zhang Z, Liu J, Zhang X, Chu M. Butyrate pretreatment attenuates heart depression in a mice model of endotoxin-induced sepsis via anti-inflammation and anti-oxidation. Am J Emerg Med. 2017;35:402–409. [DOI] [PubMed] [Google Scholar]
- 45.Chriett S, Dąbek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep. 2019;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao DJ, Wang Z V, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.