The PhoP-PhoQ two-component regulation system of Salmonella enterica serovar Typhimurium is involved in the response to various environmental stresses and is essential for bacterial virulence. Our previous studies showed that acetylation plays an important role in regulating the activity of PhoP, which consequently mediates the change in virulence of S. Typhimurium.
KEYWORDS: PhoP, Salmonella enterica serovar Typhimurium, acetyl phosphate, acetylation, virulence
ABSTRACT
The PhoP-PhoQ two-component regulation system of Salmonella enterica serovar Typhimurium is involved in the response to various environmental stresses and is essential for bacterial virulence. Our previous studies showed that acetylation plays an important role in regulating the activity of PhoP, which consequently mediates the change in virulence of S. Typhimurium. Here, we demonstrate that a conserved lysine residue, K88, is crucial for the function of PhoP and its acetylation-downregulated PhoP activities. K88 could be specifically acetylated by acetyl phosphate (AcP), and the acetylation level of K88 decreased significantly after phagocytosis of S. Typhimurium by macrophages. Acetylation of K88 inhibited PhoP dimerization and DNA-binding abilities. In addition, mutation of K88 to glutamine, mimicking the acetylated form, dramatically attenuated intestinal inflammation and systemic infection of S. Typhimurium in the mouse model. These findings indicate that nonenzymatic acetylation of PhoP by AcP is a fine-tuned mechanism for the virulence of S. Typhimurium and highlights that virulence and metabolism in the host are closely linked.
INTRODUCTION
Salmonella is one of the most common foodborne pathogens worldwide (1). In the United States, Salmonella is estimated to cause 1.4 million salmonellosis cases, resulting in almost 600 deaths annually (2). Globally, Salmonella enterica serovar Typhimurium is the most commonly isolated serovar (1). S. Typhimurium can cause gastroenteritis or systematic infections in humans and animals (3). After being ingested with contaminated food or water, S. Typhimurium reaches the gut lumen and invades the intestinal epithelium through adherence and invasion (4). During systematic infections, S. Typhimurium can penetrate the small intestine barrier and be engulfed by phagocytic cells such as macrophages, within which S. Typhimurium can replicate and survive (5).
The virulence of S. Typhimurium is regulated by a complex interaction of different transcriptional regulators, among which the PhoP-PhoQ two-component system is a key player (6). PhoQ is a membrane-bound sensor kinase that autophosphorylates when sensing low Mg2+ (7) or low pH (8, 9). Then the phosphoryl of PhoQ is transferred to PhoP. PhoP is a transcription factor that either activates or represses target gene transcription (10). The phosphorylated PhoP proteins are recruited to the regulatory region of its target genes, including mgtA, mgtC, pagC, and pmrA (11). Besides phosphorylation, acetylation and propionylation have been found to regulate the activity of PhoP (12).
Nε-lysine acetylation can change protein properties to alter the functions of proteins, such as DNA binding, enzymatic activity, and protein-protein interactions, and is shown to play major roles in many cellular processes, including chemotaxis, metabolism, and virulence (13, 14). Bacterial lysine acetylation occurs both enzymatically and nonenzymatically (15–17). Nε-lysine acetyltransferases (KAT), such as Gcn5-related N-acetyltransferase Pat (18), catalyze the transfer of an acetyl group from acetyl-CoA (Ac-CoA) to an amino group of lysine. Acetyl phosphate (AcP) is the acetyl donor of nonenzymatic acetylation (19, 20). While acetylation can happen either enzymatically or nonenzymatically, a lysine deacetylase (KDAC) could remove acetyl groups from acetyl lysines. CobB is the only known KDAC in S. Typhimurium (21) and deacetylates some acetyl lysines that are generated via Pat (22) or AcP (23).
We previously showed that two lysine residues of PhoP, K201 and K102, can be acetylated by acetyltransferase Pat or AcP, respectively. The acetylation of K201 impairs the PhoP DNA-binding ability and thus attenuates the virulence of S. Typhimurium (24), and K102 acetylation blocks PhoP phosphorylation, which is an essential step for PhoP activation (25). Here, we found that another highly conserved lysine residue of PhoP, K88, also can be acetylated by AcP but not Pat. Acetylation of K88 weakens the dimer formation of PhoP and inhibits its DNA-binding ability, which affects PhoP activity and leads to the attenuation of the virulence of S. Typhimurium. Our results further demonstrate the importance of PhoP acetylation by AcP in the virulence of S. Typhimurium and provide new insights into the multifaceted regulation of a key virulence regulator PhoP in response to metabolic or environmental changes.
RESULTS
Acetylation of K88 inhibits the activity of PhoP.
Our previous studies show that the acetyltransferase Pat and the high-energy molecule AcP are involved in acetylation of PhoP at the K201 and K102 residues, respectively (24, 25). To examine whether acetylation occurs on other lysine residues of PhoP, we purified His-tagged PhoP from S. Typhimurium grown in LB medium to the stationary phase and performed mass spectrometry analysis (see Table S1 in the supplemental material). We found that PhoP K88 had acetylation signal (Fig. 1A). The amino acid sequence alignment showed that K88 located in an α4 helix (26) is highly conserved (Fig. 1B).
FIG 1.
Acetylation of K88 inhibits the activity of PhoP. (A) His-tagged PhoP was purified and analyzed with LC-MS-MS after trypsin digestion. Shown is the MS-MS spectrum of the peptide E83GWQDKVEVLSSGADDYVTKPFHIEEVMAR112. The presence of a series of b ions supports the acetylation of K88 in the peptide with the key fragment ions in red. (B) The asterisk denotes the conserved K88 residue. The result was analyzed with BioEdit 7.0. (C) The proliferation of bacteria in RAW264.7 cells. RAW264.7 cells were infected by the wild-type strain (eWT) or phoP K88 derivative mutant strains. Infected cells were lysed at 2 h or 24 h postinfection. The number of viable intracellular bacteria was determined. Bacterial growth was measured as the fold change in CFU between 2 h and 24 h. Results are shown as the mean ± SD from three independent experiments; ****, P < 0.0001. Student’s t test. (D) The proliferation of bacteria in primary peritoneal macrophages. After the wild-type (eWT) and phoP K88 derivative mutant strains infected primary peritoneal macrophages for 2 h and 24 h, respectively, viable cells were counted at the indicated time point. The growth fold change between 2 h and 24 h was calculated. Results are shown as the mean ± SD from three independent experiments; **, P < 0.01. Student’s t test. (E) The transcriptional levels of phoP- and phoP-regulated genes in the wild-type strain (eWT) and phoP K88 mutants. Total RNA was harvested from bacteria grown to an OD600 of ∼0.4 in M9CA medium with 8 µM magnesium. The mRNA levels were determined by qPCR. The relative expression of genes in eWT was set as 1. Error bars indicate ± SDs of triplicate measurements; **, P < 0.01; ***, P < 0.001. Student’s t test. (F) Invasion efficiency of bacteria in HeLa cells. After the wild-type (eWT) and phoP K88 derivative mutant strains infected HeLa cells for 2 h, viable bacteria were counted by plating on agar plates to calculate invasion efficiency. Error bars represents the ± SD from three independent experiments; ****, P < 0.0001. Student’s t test.
PhoP controls the expression of genes essential for the virulence of S. Typhimurium and survival within macrophages (27). To determine the role of K88 acetylation, we constructed chromosome point mutants in which PhoP K88 was individually mutated to glutamine (Q), arginine (R), or alanine (A) in S. Typhimurium as described previously (24). An engineered wild type (eWT) strain was constructed similarly as a control. The K-to-Q (lysine replaced by glutamine) substitution mimics the constitutive acetylation by neutralizing the positive charge, whereas K-to-R (lysine replaced by arginine) substitution keeps the positive charge but avoids acetylation (28). To determine the role of acetylation of K88 in the intracellular survival of S. Typhimurium, we infected the mouse macrophage-like Raw 264.7 cells with eWT or its derivative mutant strains (eK88Q, eK88R, and eK88A). Intracellular bacterial counting results showed that the eWT and eK88R proliferated by approximately 90-fold from 2 h to 24 h postinfection, while eK88Q or eK88A had only 30 or 40-fold replication (Fig. 1C). In mouse primary peritoneal macrophages, eK88R, mimicking nonacetylated lysine residue, had the highest proliferation ability (Fig. 1D). In other words, the multiplication of the eK88R strain outperformed eK88Q in cultured cells.
As an important transcription factor, PhoP governs the expression of >100 genes, including itself in S. Typhimurium (29). To investigate whether the different bacterial intracellular replication pattern is due to the change of PhoP activity, we measured the mRNA levels of phoP and its target genes, including mgtA, mgtC, pagC, and pmrA by quantitative real-time PCR (qPCR) as described previously (24). The results showed that eK88Q and eK88A mutations led to lower transcription levels of phoP and PhoP-target genes compared with eWT or eK88R under the condition of low magnesium, which could activate PhoP (Fig. 1E). To further confirm the effect of PhoP K88 modifications, we performed the invasion assay in HeLa cells using eWT or its derivative mutant strains. S. Typhimurium invasion of epithelial cells is mediated by Salmonella pathogenicity island 1 (SPI-1) (30) and can be regulated through posttranslational modification (31). Besides, SPI-1 is negatively regulated by PhoP (32). Thus, the invasion efficiency is negatively correlated with the transcriptional activity of PhoP. As expected, eK88Q showed a much higher invasion rate than other strains (Fig. 1F). These data show that K88 is critical for PhoP activity and suggest that acetylation of K88 inhibits PhoP activity.
K88 can be acetylated by AcP.
To further study the role of K88 acetylation, we generated the site-specific anti-PhoP K88 acetylation polyclonal antibody (anti-K88Ac antibody). The specificity of this antibody was confirmed by Western blot analysis (Fig. 2A). PhoP was immunoprecipitated from intramacrophage S. Typhimurium and subjected to Western blotting using anti-K88Ac antibody. A dramatic reduction of K88 acetylation of PhoP from intramacrophage S. Typhimurium was observed (Fig. 2B). PhoP can be acetylated by acetyltransferase Pat and deacetylated by CobB in S. Typhimurium (24). To determine whether PhoP K88 can be modified by Pat or CobB, PhoP proteins from various deletion mutants were analyzed using anti-K88Ac antibody. The result showed that deletion of cobB caused an increase of the K88 acetylation level, but the acetylation level of K88 in the pat deletion mutant was comparable to that in the wild-type strain (Fig. 2C), suggesting the involvement of CobB, but not Pat, in the modification of K88.
FIG 2.
K88 can be acetylated by AcP. (A) The specificity of anti-K88Ac antibody. Wild-type PhoP protein (WT) and site-specific acetylated K88Ac, K102Ac, and K201Ac proteins were resolved on 12% SDS-PAGE and probed with anti-K88Ac antibody and anti-PhoP antibody. (B) The acetylation level of PhoP K88 in macrophages. After infecting RAW264.7 cells for 24 h, the intracellular bacteria were harvested for IP assay. The immunoprecipitated PhoP protein by anti-Flag antibody was analyzed by Western blotting with anti-K88Ac antibody. Representative results from three independent experiments of Western blots are shown. The relative K88 acetylation level over PhoP was quantified. Error bars indicate ± SDs of triplicate experiments; **, P < 0.01. Student’s t test. (C) The acetylation of PhoP K88 after knockout of pat or cobB. PhoP proteins from the wild-type strain and mutation strain of S. Typhimurium 14208s were detected by Western blotting and probed with anti-K88Ac antibody, and anti-PhoP antibody was used as a loading control. Representative results from three independent experiments of Western blots are shown. The relative K88 acetylation level over PhoP was quantified. Error bars indicate ± SDs of triplicate experiments. *, P < 0.05. ns, no significance. Student’s t test. (D) The acetylation level of PhoP K88 isolated from strains cultured in LB medium supplemented with glucose. The wild-type strain and pta or ackA mutation strains of S. Typhimurium 14208s were cultured in LB medium with or without 0.8% (wt/vol) glucose added and then harvested for IP assay. The immunoprecipitated PhoP protein by anti-Flag antibody was analyzed by Western blotting with anti-K88Ac antibody. Representative results from three independent experiments of Western blots are shown. The relative K88 acetylation level over PhoP was quantified (top panel). Error bars indicate ± SDs of triplicate experiments; *, P < 0.05. Student’s t test. (E) PhoP K88 was acetylated by AcP in vitro. Purified His-tagged PhoP was incubated with AcP as an acetyl group donor for different durations, and then samples were detected by Western blot analysis with anti-pan acetyl lysine antibody (α-Pan Ac), which recognized acetylated lysine residues and anti-K88Ac antibody. Representative results from three independent experiments of Western blots are shown. The relative pan acetylation or K88 acetylation level over PhoP was quantified. Error bars indicate ± SDs of triplicate experiments; *, P < 0.05; **, P < 0.01. ns, no significance. Student’s t test.
Previous studies have reported that AcP is involved in the acetylation of PhoP in S. Typhimurium (25). Therefore, we hypothesized that acetylation of PhoP K88 may be mediated by AcP. AcP is synthesized by the acetate kinase (AckA)-phosphotransacetylase (Pta) pathway (33). Pta catalyzes the conversion of Ac-CoA to AcP, which can be converted to acetate by AckA. Thus, the ackA deletion mutant accumulates a higher concentration of AcP, while the pta deletion mutant had less AcP than the wild-type strain. Glucose supplement in the medium can increase the concentration of intracellular AcP and AcP-dependent acetylation (33–35). S. Typhimurium harboring chromosomal Flag-tagged phoP was cultured in LB medium supplemented with 0.8% (wt/vol) glucose, and the acetylation level of K88 in immunoprecipitated PhoP was determined by Western blot analysis. Glucose supplement and ackA deletion increased the acetylation of K88, while pta deletion had the opposite effect (Fig. 2D). To further confirm that the acetylation of K88 is dependent on AcP, we incubated PhoP with different amounts of AcP in vitro. Both the overall acetylation of PhoP (as detected by anti-pan acetyl lysine antibody, which can recognize all acetylated lysine residues) and the specific acetylation of K88 increased with the rise of AcP concentration (Fig. 2E). These results suggest that the acetylation of PhoP K88 depends on AcP rather than acetyltransferase Pat.
Acetylation of K88 impairs the dimer formation and DNA-binding ability of PhoP.
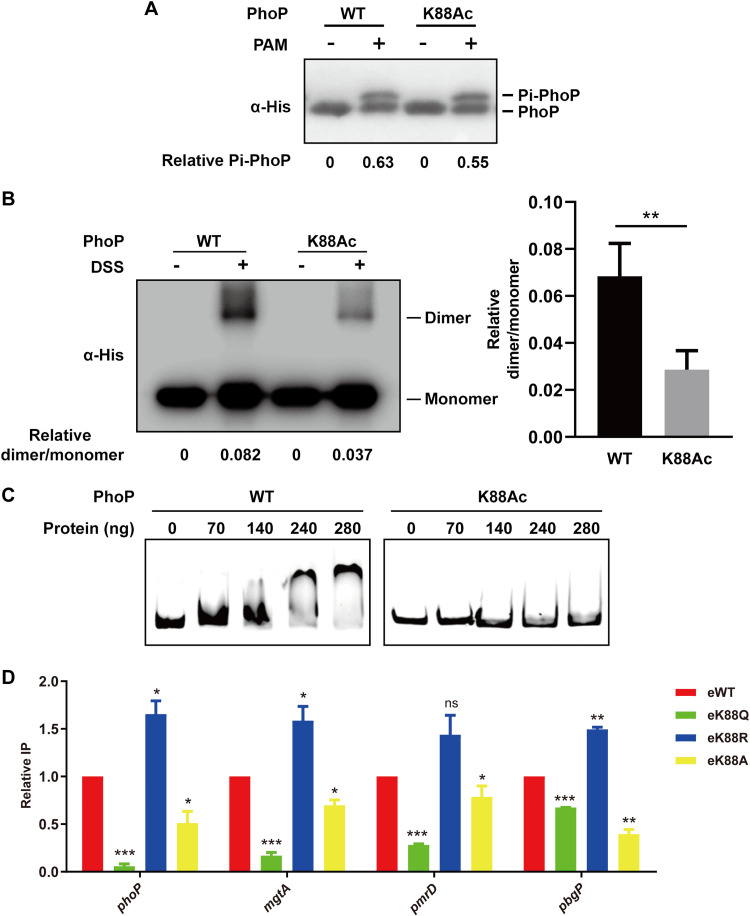
Phosphorylation is an essential step for activating PhoP. To understand the role of K88 acetylation in regulating PhoP activity, we generated a site-specifically acetylated PhoP at K88 (PhoP K88Ac) by genetically encoding Nε-acetyl lysine in PhoP (36). AcP can acetylate or phosphorylate PhoP. To avoid the interference between these two modifications, we synthesized potassium phosphoramidate (PAM) to perform an in vitro phosphorylation assay (37), followed by Phos-tag gel analysis. The results showed that both PhoP and PhoP K88Ac can be phosphorylated by PAM at a similar level (Fig. 3A), suggesting the negligible effect of K88 acetylation on PhoP phosphorylation.
FIG 3.
Acetylation of K88 impairs the dimer formation and DNA-binding ability of PhoP. (A) The phosphorylation of PhoP K88Ac in vitro. Purified wild-type PhoP and PhoP K88Ac proteins were incubated with or without 20 mM PAM, respectively. The samples were resolved on 12% SDS-PAGE gel containing Phos-tag and analyzed with anti-His antibody. Representative results from three independent experiments of Western blots are shown. (B) Dimer-formation of PhoP K88Ac. Purified wild-type PhoP or K88Ac was subjected to cross-linking with DSS. Western blots were probed with anti-His antibody and independently repeated at least three times. The relative ratio of dimer over monomer of PhoP was quantified. Error bars indicate ± SDs of triplicate experiments; **, P < 0.01. Student’s t test. (C) DNA-binding activity of PhoP K88Ac assessed by EMSA. 6′-FAM-labeled phoP promoter was incubated with His-tagged wild-type PhoP or PhoP K88Ac at the indicated concentration, and then the reaction products were analyzed in 5% PAGE. Representative EMSA results from three independent experiments are shown. (D) DNA-binding activity of PhoP K88 derivative mutants compared to WT in log-phase acid tolerance response by ChIP. eWT or phoP K88 mutant strains in which phoP was flag-tagged in chromosome were cultured in EG medium at pH 5.0 for 1 h and harvested. The total cell lysate was immunoprecipitated by anti-Flag antibody. The candidate gene promoter before (input) and after (Flag-ChIP) were quantified by qPCR and represented as the relative ratio of ChIP/input. Results are shown as mean ± SD from three independent experiments; *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, no significance. Student’s t test.
PhoP dimerization relies on a set of highly conserved residues in the α4-β5-α5 interface and is a prerequisite for PhoP activation (38). Since K88 is located in an α4 helix (Fig. 1B), we hypothesized that K88 acetylation may affect the dimerization of PhoP. To test this hypothesis, wild-type PhoP and PhoP K88Ac were subjected to disuccinimidyl suberate (DSS) cross-linking, which cross-links two amino groups that are in close proximity to each other. Western blot analysis showed that K88Ac had much weaker dimerization than the WT (Fig. 3B).
To determine whether K88 acetylation affects the DNA-binding ability of PhoP, we performed an electrophoretic mobility shift assay (EMSA). The results showed that PhoP K88Ac had lower DNA-binding affinity with the phoP promoter than nonacetylated PhoP (Fig. 3C). To further determine the DNA-binding abilities of PhoP K88 variants in vivo, a chromatin immunoprecipitation (ChIP) assay was performed after acid treatment. As expected, qPCR data showed that PhoP eK88Q, which mimicked the constitutive acetylation, had a lower level of DNA enrichment than eWT, whereas eK88R can enrich more PhoP-associated DNA (Fig. 3D).
Acetylation of PhoP K88 attenuates bacterial virulence in a mouse model.
S. Typhimurium can cross the intestinal epithelium and localize in organs, including liver, spleen, and gallbladder (39). To investigate the role of K88 acetylation in S. Typhimurium virulence, we used eWT and eK88 derivative mutant strains to infect BALB/c mice. For intragastric infection, 35 mice were randomly assigned into 5 groups of 7 animals each. Each mouse was fed 1.5 × 107 CFU of bacteria or phosphate-buffered saline (PBS) as the control. The results showed that nearly 70% of eWT- and 60% of eK88R-infected mice died on day 10 postinfection, but mice infected by eK88Q or eK88A were all alive and did not show infection-associated symptoms, including ruffling of fur and marasmus (Fig. 4A). In addition, we also determined the survival of mice after intraperitoneal infection. Consistent with intragastric infection, eK88Q and eK88A were less virulent than eWT or eK88R. Approximately 40% of eK88Q- and 65% of eK88A-infected mice were still alive and healthy, while mice infected by eWT and eK88R were all dead at day 4 postinfection (Fig. 4B).
FIG 4.
Acetylation of PhoP K88 modulates bacterial virulence in the mouse model. (A) Survival rates of mice infected by oral gavage. BALB/c mice were infected by 1.5 × 107 CFU of bacteria (wild-type or phoP K88 mutant strains) or PBS as the control through oral gavage. The number of live mice was counted twice a day. (B) Survival rates of mice infected by intraperitoneal injection. BALB/c mice were injected intraperitoneally with 1.5 × 105 CFU of bacteria (eWT or phoP K88 mutant strains) or PBS as the control. The number of live mice was counted twice a day. (C) Bacterial burdens in liver and spleen. The livers and spleens were harvested 48 h after oral infection. The amounts of bacteria in the liver and spleen were counted. Results are shown as the mean ± SD; **, P < 0.01; ***, P < 0.001. ns, no significance. Student’s t test. (D) Immunofluorescence staining of S. Typhimurium cecal colonization in vivo. The ceca from streptomycin-treated BALB/c mice after oral infection were prepared as paraformaldehyde-fixed paraffin section. These sections were stained for S. Typhimurium lipopolysaccharide (LPS) (red), actin (green), and nuclei with DAPI (blue). Images are pseudocolor representations at ×200 magnification. (E) H&E-stained ceca of mice. The ceca were fixed and embedded in paraffin to slice a section which was stained with H&E. L, intestinal lumen; e, edema; sa, submucosa. (F) Neutrophil infiltration in ceca. The paraffin section was stained with hematoxylin and anti-MPO antibody by immunohistochemistry. Claybank indicates PMN and blue indicates the nucleus.
We next quantified the bacterial abundance of eWT and eK88 derivative mutants in mouse spleen and liver at day 2 postinfection. CFU counting results showed that the bacterial burden of eK88Q and eK88A was significantly less than that of eWT or eK88R in both spleen and liver (Fig. 4C). S. Typhimurium must colonize the intestinal epithelium to exert its virulence at the time of infection (40). Thus, we performed immunofluorescence to study bacterial colonization in mouse ceca and found that eK88Q and eK88A colonized much less than eWT or eK88R (Fig. 4D). Furthermore, we examined the histopathologic features of mouse cecal inflammation after bacterial infection. Morphology analyses of hematoxylin-eosin (H&E)-stained cecal tissue showed that edema in the submucosa was obvious in eWT- and eK88R-infected mouse ceca, while nearly no edema was observed in eK88Q- and eK88A-infected mouse ceca (Fig. 4E). We also observed obvious polymorphonuclear neutrophil (PMN) infiltration of the submucosa, the lamina propria, and the epithelial layer, as well as transmigration of PMN into the intestinal lumen in eWT- and eK88R-infected mice. In contrast, PMN was not observed in eK88Q- and eK88A-infected mice (Fig. 4F).
DISCUSSION
Acetylation of K88 regulates PhoP activity by affecting its dimer formation.
The PhoP/PhoQ two-component system is the master virulence regulation system in S. Typhimurium and is under precise regulation. Loss of PhoP can severely attenuate the virulence of S. Typhimurium, making bacteria unable to survive in host cells (27). In contrast, constant activation of PhoP renders S. Typhimurium avirulent in mice (41), which highlights the importance of precise control of this system. Previously, we showed that acetylation of K201 and K102 is involved in regulating PhoP activities (24, 25). In this study, we further reveal that another conserved lysine residue, K88, was acetylated in vivo, and the dynamic change of K88 acetylation responds to environmental stimuli. The K88Q mutant strain, which mimics the constitutive acetylation, was defective in replication in cultured macrophage-like cell lines and had attenuated virulence in the mouse model. The crystal structure shows that the K88 residue is located in the dimer interacting surface formed by the α4 helix, β5 strand, and α5 helix (26). This surface forms a small hydrophobic core surrounded by an extensive network of hydrogen bonds and salt bridges. The crystal structure of PhoP in Bacillus subtilis shows that the side chain of PhoP K89 (corresponding to K88 in S. Typhimurium) forms a salt bridge with K58 residue of the other monomer (Fig. S1) (42). Thus, substitution of the side chain of K88 may cause steric hindrance for the dimerization of PhoP, and the acetylation of K88 neutralizes the positive charge of its side chain and breaks the salt bridge, which presumably leads to the weakened dimerization and impaired DNA-binding ability of the K88Ac variant. An artificially locked acetylation form of K88, i.e., K88Q, is beneficial for invasion. In contrast, the nonacetylated form of K88, i.e., K88R, is beneficial for transcriptional activation of PhoP and growth of S. Typhimurium in cultured cells. These data highly suggest that S. Typhimurium may regulate the activity of PhoP via acetylation of PhoP K88 at different infection stages. Similarly, Choi et al. showed that intramacrophage Salmonella could delay the activation of the PhoP/PhoQ system by using EIIANtr, a component of the nitrogen-metabolic phosphotransferase system, to facilitate bacterial intracellular replication and intercellular spread (43). Collectively, Salmonella might use multiple strategies to modulate PhoP activity to adapt to different conditions.
AcP is a key regulator for acetylation of PhoP K88.
To date, two distinct protein acetylation mechanisms (i.e., protein acetyltransferase- and AcP-mediated) have been identified. Interestingly, an increasing number of studies have shown that AcP-mediated nonenzymatic protein acetylation serves as the predominant mechanism in Escherichia coli and other species of bacteria (17, 20). Here, we found that PhoP K88 can be acetylated by AcP in vitro. Genetic evidence also supported the involvement of AcP in acetylation of PhoP K88. Moreover, our previous work shows that PhoP K102 is acetylated by AcP as well (25). AcP is an intermediate of the AckA-Pta pathway in bacteria. The phosphotransacetylase Pta converts Ac-CoA to AcP, and then acetate kinase AckA can transform AcP to acetate (19). Deletion of pta decreases the acetylation levels of both K88 and K102, while an increase of AcP synthesis by supplementing the medium with glucose elevates the acetylation levels of these two lysine residues. It is highly possible that AcP might acetylate other virulence regulators or virulence factors to mediate bacterial pathogenicity. Since lysine residues K102 and K88 are both crucial for PhoP activities, we establish the important role of primary metabolism in regulating bacterial virulence. That is to say, primary metabolism not only supports bacterial intracellular growth, but also regulates bacterial virulence by using a metabolic molecule, AcP, to acetylate key regulator(s).
Multiple lysine residues and environmental factors coordinate PhoP activity.
We have identified three acetylated lysine residues in PhoP so far. K102 and K88 are acetylated by AcP, while K201 is acetylated by acetyltransferase Pat (24). Interestingly, these 3 lysine residues are found to be acetylated by either Pat or AcP, but not both. Recently, Christensen et al. identified four additional KATs (YjaB, YiaC, RimI, PhnO) in E. coli (44). Since the S. Typhimurium genome also contains these KAT homologues, it is likely that these KATs may cooperate with Pat and AcP to meticulously control acetylation of PhoP and, consequently, regulate S. Typhimurium virulence. This event might occur in other proteins or in other bacteria to mediate various bacterial physiological processes to facilitate bacterial adaptation to environmental stresses.
Posttranslational modifications are a highly efficient way for bacteria to adjust to environmental cues and internal stimuli (45) and could be used to develop potential anti-infective therapeutics (46). PhoP, as a key transcriptional regulator, is widely present in bacteria. For a long time, people only recognized the role of phosphorylation in PhoP. With the progress of mass spectrometry, several new kinds of posttranslational modifications in PhoP, including acetylation, propionylation, and methylation (unpublished data) have been identified. In S. Typhimurium, so far, acetylation of K88, K102, and K201 and phosphorylation of D52 have been shown to be crucial in regulating PhoP activity. These findings reveal that S. Typhimurium can regulate PhoP activity by posttranslational modifications to respond to various stresses. To achieve this purpose, S. Typhimurium has to coordinate expression of (de)acetylation enzymes and (de)composition of acetyl group precursors based on the environmental change. We checked the expression levels of the key genes involved in the AcP metabolism (47). Under intramacrophage conditions, the transcriptional level of ackA and pta increased by 2.19-fold and 2.8-fold, respectively. The transcription of cobB increased as well. The elevated transcription of cobB decreased the acetylation of PhoP. The increased transcription of ackA converted more AcP to acetate, whereas the increased transcription of pta enhanced the accumulation of AcP. Though the effect of ackA and pta seems contradictory and we do not know exactly whether there is any other mechanism to control the homeostasis of AcP, we do believe that the bacteria must know how to fine-tune the level of AcP for its better survival in the host. The final outcome is what we observed in Fig. 2B. The acetylation of PhoP K88 dropped significantly after bacterial infection of macrophages. Therefore, it remains unknown how S. Typhimurium controls these processes at the right time points and right intracellular locations. This issue is quite important and needs to be explored in the future.
We propose that the activity of PhoP is fine-tuned by the acetylation of diverse lysine residues and/or other posttranslational modifications, which is important for the fitness of S. Typhimurium and very likely for other bacteria to survive in stressful conditions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table S2. Bacteria were routinely grown in LB broth or M9CA medium (casamino acid, 2.0 g; Na2HPO4, 6.8 g; KH2PO4, 3.0 g; NH4Cl, 1.0 g; NaCl, 0.5 g per liter) with a low concentration of magnesium. The working concentrations of antibiotics used were 100 μg/ml of ampicillin, 17 μg/ml of chloramphenicol, 50 μg/ml kanamycin, or 50 μg/ml spectinomycin.
Generation of chromosome point mutation strains.
Fusion PCR and λ-red recombination were used to generate S. Typhimurium PhoP K88A, K88Q, and K88R and the wild-type strain with Flag tag sequence at the C terminus of phoP as described previously (24). The PhoP K88 fusion PCR forward primer sequences used were GAAGGCTGGCAGGATAAAGTCGAGGTTCTCAGC for eWT (eK88A, GCA; eK88Q, CAG; eK88R, CGA). The reverse primer sequences used were GCTGAGAACCTCGACTTTATC CTGCCAGCCTTC for eWT (eK88A, TGC; eK88Q, CTG; eK88R, TCG). PCR products were purified and electroporated into S. Typhimurium containing pKD46. The mutant strains were verified by PCR and sequencing.
Mass spectrometry analysis.
S. Typhimurium strain 14028s harboring a His tag sequence at the C terminus of phoP was grown in LB medium to the stationary phase at 37°C. Cells were harvested by centrifugation and resuspended in lysis buffer (50 mM Tris-HCl [pH 7.5], 0.6 M NaCl, 10% glycerol) supplemented with 1× protease inhibitor cocktail (CoWin Biosciences, Beijing, China). Bacteria were broken by high-pressure cracker, and the suspension was centrifuged at 10,000 × g for 1 h. The supernatant was slowly flowed through an Ni-NTA column (GE Healthcare, Buckinghamshire, UK) which was preequilibrated with lysis buffer. Afterward, 20 ml binding buffer (20 mM Tris-HCl [pH 7.6], 0.5 M NaCl, 10% glycerol) containing 40 mM or 80 mM imidazole was used to wash the column successively. His-tagged PhoP proteins were eluted with binding buffer containing 300 mM imidazole.
The purified proteins were separated on 12% SDS-PAGE. Bands were cut and incubated with destain buffer (50% acetonitrile, 50 mM NH4HCO3) at 37°C for 1 h. After destaining, the gel pieces were covered with enough trypsin solution (1.2 ng/μl trypsin [Promega, Madison, USA], 50 mM NH4HCO3, 10% acetonitrile) to digest at 37°C overnight. Liquid chromatography-mass spectrometry (LC-MS) analyses of peptides were performed with a hybrid ion trap (Orbitrap) mass spectrometer (LTQ Orbitrap Velos; Thermo Fisher Scientific, Waltham, MA, USA) coupled with nanoflow reversed-phase liquid chromatography (EASY-nLC 1000; Thermo Fisher Scientific).
Antibodies.
The commercial antibodies included anti-His peptide monoclonal antibody (Tiangen, Beijing, China) and anti-Flag peptide monoclonal antibody (Sigma, Saint Louis, MO, USA). In-house-developed antibodies were prepared as follows. The 6× His-tagged PhoP was purified from E. coli strain BL21 to immunize rabbits for the production of anti-PhoP polyclonal antibody. For anti-PhoP K88 acetylation (PhoP K88Ac) antibody, the immune peptide REGWQDK(Ac)VEVL SSG-NH2 conjugated to bovine serum albumin (BSA) was used as the antigen to immunize rabbits. Nonspecific antibodies were removed from collected antiserum of immunized rabbits by control peptide REGWQDKVEVLSSG-NH2.
Expression and purification of site-specifically acetylated PhoP.
The site-specific acetylated PhoP (K88Ac, K102Ac, K201Ac) was expressed and purified as described previously (36). Briefly, the plasmids pAcKRS-3 and pCDF-PylT-phoP (K88TAG, K102TAG, K201TAG) were transformed individually into E. coli strain BL21. The resultant strain was grown in LB medium at 37°C supplemented with 50 μg/ml kanamycin and 50 μg/ml spectinomycin. Then, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM followed by overnight incubation at 37°C in a shaking incubator. Cells were harvested and lysed in buffer containing 50 mM Tris-HCl (pH 7.5), 0.6 M NaCl, and 10% glycerol. After centrifugation at 4°C and 15,000 × g for 30 min, the supernatant was loaded on a 1-ml Ni-NTA column (GE Healthcare) preequilibrated with lysis buffer. The column was subsequently washed with 20 ml of binding buffer containing 20 mM Tris-HCl (pH 7.6), 0.5 M NaCl, and 10% glycerol with gradient concentration imidazole, and the histidine-tagged protein was eluted with 2 ml of binding buffer containing 300 mM imidazole. Purity was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Cell infection assays.
Intramacrophage replication and HeLa cell invasion assays were performed as described previously (24, 48). Briefly, 2 × 105 mouse macrophage-like RAW 264.7 cells or primary peritoneal macrophages were seeded into each well of 24-well plates in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were infected at multiplicity of infection (MOI) of 10 with eWT or eK88 mutants. After incubation for 1 h at 37°C in a 5% CO2 atmosphere, extracellular bacteria were removed by extensive washing with DMEM twice, and the media were supplemented with 100 μg/ml gentamicin to culture for 2 h; then the mixture was switched to medium containing 25 μg/ml gentamicin for the remainder of the experiment. Infected cells were lysed with PBS containing 0.025% SDS and plated on LB agar after dilution. The fold change of bacterial replication from 2 h to 24 h postinfection in cells was measured by CFU counting. Each assay was performed simultaneously in 2 separate wells and repeated 3 times. Results are presented as the mean ± standard deviation (SD). For epithelial cell infection, HeLa cells were seeded at 1 × 105 cells per well in 24-well plates and infected at an MOI of 100 with overnight-cultured S. Typhimurium. After 1 h of infection, cells were washed twice with DMEM and incubated in DMEM-10% FBS plus 100 μg/ml gentamicin for 2 h to kill extracellular bacteria and then were washed twice with DMEM and lysed as described above. Lysates were plated with the appropriate dilution, followed by viable bacteria being counted to calculate the invasion efficiency (percentage of the starting inoculum internalized at the end of the assay). Each invasion assay was performed simultaneously in 2 separate wells and repeated 3 times. Results are presented as the mean ± SD.
Quantitative real-time PCR (qPCR) assay.
Bacteria were cultured in M9CA medium with 8 µM magnesium and harvested when the turbidity at 600 nm reach 0.4. Total RNA was isolated using TRIzol (Thermo Fisher Scientific) and treated with RNase-free DNase I (Thermo Fisher Scientific) to remove contaminated genomic DNA. RNA was reverse-transcribed with PrimeScript RT master mix (TaKaRa). For the qPCR assay, TB Green premix Ex Taq II (TaKaRa, Tokyo, Japan) was used in the Quant Studio3 fast real-time PCR system (Thermo Fisher Scientific). 16S rRNA was used as a reference gene. The relative transcriptional level of targeted genes was calculated by using the 2-ΔΔCT method.
Immunoprecipitation (IP).
Bacteria harboring the PhoP-Flag locus were used to infect RAW264.7 cells as described above. The intracellular bacteria were harvested for IP. The anti-Flag antibody was used for immunoprecipitating PhoP-Flag protein. A Pierce Crosslink IP kit (Thermo Fisher Scientific) was used, and immunoprecipitated proteins were analyzed by Western blotting.
In vitro acetylation assay by AcP.
The acetylation assay by AcP in vitro was performed as described previously (25). One microgram of PhoP protein was incubated with AcP (Sigma) of the indicated concentration in buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, and 20 mg/ml BSA at 37°C for 2 h.
Phosphorylation of PhoP in vitro.
Purified PhoP protein was incubated with 20 mM potassium phosphoramidate (PAM) in buffer containing 50 mM Tris-Cl (pH 7.4), 50 mM KCl, 20 mM MgCl2, and 1 mM DTT at room temperature for 30 min as described previously (49). The reaction was stopped by sample buffer, and sample mixtures were electrophoresed on SDS-PAGE containing 50 μM Phos-tag acrylamide (Wako) (50) at 4°C under 20 mA for 2 h. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and analyzed by Western blotting.
Protein cross-linking assay.
Purified PhoP or PhoP K88Ac was incubated with 1 mM disuccinimidyl suberate (DSS; Thermo Fisher Scientific) in buffer containing 50 mM HEPES-Na+ (pH 7.5), 10 mM MgCl2, 20% glycerol, 150 mM NaCl, and 0.05% Tween 20 at 25°C for 30 min as described previously (49) and then analyzed by Western blotting.
EMSA.
The 6′-FAM-labeled 184-bp phoP promoter was amplified by PCR using S. Typhimurium genomic DNA as the template as described previously (24). Then, purified PhoP or PhoP K88Ac was incubated with phoP promoter in 10 μl of a solution containing 25 mM Tris-HCl (pH 8.0), 50 mM KCl, 0.5 mM EDTA, and 1% glycerol at 37°C for 30 min. The reaction mixtures were separated by 5% native Tris-glycine polyacrylamide gel. FAM-labeled fluorescence was detected using a GE Amersham Imager 600.
ChIP assay.
A ChIP assay was performed using a ChIP kit (Millipore, MA, USA) as described previously (51). In brief, bacteria were cultured in EG medium at pH 7.7 to an optical density at 600 nm (OD600) of 0.4 and then centrifuged and resuspended in EG medium at pH 5.0 (adjusted by HCl). After 1 h of incubation, the bacteria were treated with formaldehyde to cross-link proteins to DNA. Anti-Flag or normal mouse IgG was used as the immunoprecipitation antibody. Purified DNA was then analyzed by qPCR using primers specific for the PhoP-target genes.
Animal studies.
For intraperitoneal injection, 8-week-old BALB/c mice were divided into 5 groups of 7 animals in each group. Cultured bacteria were diluted in PBS to 1.5 × 106 CFU/ml. Each mouse in the experimental group was injected with 100 μl of PBS containing 1.5 × 105 CFU of bacteria, and mice in the PBS group were injected with an equal volume of PBS as a control. For oral infection, all mice were treated with 20 mg streptomycin by gavage 24 h before infection. The cultured bacteria were diluted in PBS to 7.5 × 107 CFU/ml. Each mouse in the experimental group was intragastrically administered 200 μl of PBS containing 1.5 × 107 CFU of bacteria, and the PBS group was administered an equal volume of PBS as a control. The survival of the mice was observed twice a day after infection.
Mice were infected by gavage and sacrificed 48 h after infection. The livers and spleens were ground, and the number of viable bacteria was determined by serial dilutions and plating. The cecum was harvested, fixed in 10% formalin, and processed for paraffin embedding. The paraffin sections were prepared and stained by immunohistochemistry and immunofluorescence as described previously (24).
Supplementary Material
ACKNOWLEDGMENTS
We thank Xiaoyun Liu at the Department of Microbiology, School of Basic Medical Sciences, Peking University Health Science Center, for mass spectrometry data analysis. This work was supported by grants from the National Natural Science Foundation of China (no. 81830068, 81772140, 31700120, and 81501733), the State Key Development Programs for Basic Research of China (973 Program no. 2015CB554203), Key Research and Development Project of China (no. 2016YFA0500600), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
We do not have any conflict of interest to declare.
All animal experiments were approved by the Shanghai Jiao Tong University School of Medicine and performed with strict observance of the National Research Council Guide for Care and Use of Laboratory Animals (SYXK [Shanghai 2018–0027]).
Y.-F. Yao and J. Lu conceived the study; J. Li and S. Liu performed experiments; Y.-F. Yao, J. Lu, J. Li, S. Liu, J. Ni, Y. Su, J. Ren, and Y. Sang analyzed data; Y.-F. Yao, J. Lu, J. Li, and S. Liu wrote the paper. All authors read and approved the final version of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lan R, Reeves PR, Octavia S. 2009. Population structure, origins and evolution of major Salmonella enterica clones. Infect Genet Evol 9:996–1005. doi: 10.1016/j.meegid.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, Cieslak PR, Deneen VC, Tauxe RV, Emerging Infections Program FoodNet Working Group. 2004. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis 38 Suppl 3(Suppl 3):S127–S134. doi: 10.1086/381578. [DOI] [PubMed] [Google Scholar]
- 3.Baumler AJ, Tsolis RM, Ficht TA, Adams LG. 1998. Evolution of host adaptation in Salmonella enterica. Infect Immun 66:4579–4587. doi: 10.1128/IAI.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haraga A, Ohlson MB, Miller SI. 2008. Salmonellae interplay with host cells. Nat Rev Microbiol 6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 5.Niedergang F, Sirard JC, Blanc CT, Kraehenbuhl JP. 2000. Entry and survival of Salmonella typhimurium in dendritic cells and presentation of recombinant antigens do not require macrophage-specific virulence factors. Proc Natl Acad Sci U S A 97:14650–14655. doi: 10.1073/pnas.97.26.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller SI, Kukral AM, Mekalanos JJ. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A 86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia Vescovi E, Soncini FC, Groisman EA. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 8.Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. 2007. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell 26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Groisman EA. 2016. Acidic pH sensing in the bacterial cytoplasm is required for Salmonella virulence. Mol Microbiol 101:1024–1038. doi: 10.1111/mmi.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin D, Groisman EA. 2005. Signal-dependent binding of the response regulators PhoP and PmrA to their target promoters in vivo. J Biol Chem 280:4089–4094. doi: 10.1074/jbc.M412741200. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Li YX, Ye BC. 2018. Lysine propionylation modulates the transcriptional activity of phosphate regulator PhoP in Saccharopolyspora erythraea. Mol Microbiol 110:648–661. doi: 10.1111/mmi.14122. [DOI] [PubMed] [Google Scholar]
- 13.Narita T, Weinert BT, Choudhary C. 2019. Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol 20:156–174. doi: 10.1038/s41580-018-0081-3. [DOI] [PubMed] [Google Scholar]
- 14.Ren J, Sang Y, Lu J, Yao YF. 2017. Protein acetylation and its role in bacterial virulence. Trends Microbiol 25:768–779. doi: 10.1016/j.tim.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Christensen DG, Baumgartner JT, Xie X, Jew KM, Basisty N, Schilling B, Kuhn ML, Wolfe AJ. 2019. Mechanisms, detection, and relevance of protein acetylation in prokaryotes. mBio 10:e02708-18. doi: 10.1128/mBio.02708-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanDrisse CM, Escalante-Semerena JC. 2019. Protein acetylation in bacteria. Annu Rev Microbiol 73:111–132. doi: 10.1146/annurev-micro-020518-115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen DG, Xie X, Basisty N, Byrnes J, McSweeney S, Schilling B, Wolfe AJ. 2019. Post-translational protein acetylation: an elegant mechanism for bacteria to dynamically regulate metabolic functions. Front Microbiol 10:1604. doi: 10.3389/fmicb.2019.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favrot L, Blanchard JS, Vergnolle O. 2016. Bacterial GCN5-related N-acetyltransferases: from resistance to regulation. Biochemistry 55:989–1002. doi: 10.1021/acs.biochem.5b01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinert BT, Iesmantavicius V, Wagner SA, Scholz C, Gummesson B, Beli P, Nystrom T, Choudhary C. 2013. Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol Cell 51:265–272. doi: 10.1016/j.molcel.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. 2014. Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS One 9:e94816. doi: 10.1371/journal.pone.0094816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hentchel KL, Escalante-Semerena JC. 2015. Complex regulation of the sirtuin-dependent reversible lysine acetylation system of Salmonella enterica. Microb Cell 2:451–453. doi: 10.15698/mic2015.11.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung CC, Eade CR, Altier C. 2016. The protein acyltransferase Pat post-transcriptionally controls HilD to repress Salmonella invasion. Mol Microbiol 102:121–136. doi: 10.1111/mmi.13451. [DOI] [PubMed] [Google Scholar]
- 23.AbouElfetouh A, Kuhn ML, Hu LI, Scholle MD, Sorensen DJ, Sahu AK, Becher D, Antelmann H, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. 2015. The E. coli sirtuin CobB shows no preference for enzymatic and nonenzymatic lysine acetylation substrate sites. Microbiologyopen 4:66–83. doi: 10.1002/mbo3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren J, Sang Y, Tan Y, Tao J, Ni J, Liu S, Fan X, Zhao W, Lu J, Wu W, Yao YF. 2016. Acetylation of lysine 201 inhibits the DNA-binding ability of PhoP to regulate Salmonella virulence. PLoS Pathog 12:e1005458. doi: 10.1371/journal.ppat.1005458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren J, Sang Y, Qin R, Su Y, Cui Z, Mang Z, Li H, Lu S, Zhang J, Cheng S, Liu X, Li J, Lu J, Wu W, Zhao GP, Shao F, Yao YF. 2019. Metabolic intermediate acetyl phosphate modulates bacterial virulence via acetylation. Emerg Microbes Infect 8:55–69. doi: 10.1080/22221751.2018.1558963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon S, Wang S. 2011. Structure of the response regulator PhoP from Mycobacterium tuberculosis reveals a dimer through the receiver domain. Biochemistry 50:5948–5957. doi: 10.1021/bi2005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijlsma JJ, Groisman EA. 2005. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol Microbiol 57:85–96. doi: 10.1111/j.1365-2958.2005.04668.x. [DOI] [PubMed] [Google Scholar]
- 28.Luo JY, Li MY, Tang Y, Laszkowska M, Roeder RG, Gu W. 2004. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc Natl Acad Sci U S A 101:2259–2264. doi: 10.1073/pnas.0308762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soncini FC, Vescovi EG, Groisman EA. 1995. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol 177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaRock DL, Chaudhary A, Miller SI. 2015. Salmonellae interactions with host processes. Nat Rev Microbiol 13:191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung CC, Garner CD, Slauch JM, Dwyer ZW, Lawhon SD, Frye JG, McClelland M, Ahmer BM, Altier C. 2013. The intestinal fatty acid propionate inhibits Salmonella invasion through the post-translational control of HilD. Mol Microbiol 87:1045–1060. doi: 10.1111/mmi.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter MA, Jones BD. 2015. Two-component regulators control hilA expression by controlling fimZ and hilE expression within Salmonella enterica serovar Typhimurium. Infect Immun 83:978–985. doi: 10.1128/IAI.02506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima BP, Antelmann H, Gronau K, Chi BK, Becher D, Brinsmade SR, Wolfe AJ. 2011. Involvement of protein acetylation in glucose-induced transcription of a stress-responsive promoter. Mol Microbiol 81:1190–1204. doi: 10.1111/j.1365-2958.2011.07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schilling B, Christensen D, Davis R, Sahu AK, Hu LI, Walker-Peddakotla A, Sorensen DJ, Zemaitaitis B, Gibson BW, Wolfe AJ. 2015. Protein acetylation dynamics in response to carbon overflow in Escherichia coli. Mol Microbiol 98:847–863. doi: 10.1111/mmi.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann H, Peak-Chew SY, Chin JW. 2008. Genetically encoding N(epsilon)-acetyllysine in recombinant proteins. Nat Chem Biol 4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 37.Khara P, Mohapatra SS, Biswas I. 2018. Role of CovR phosphorylation in gene transcription in Streptococcus mutans. Microbiology (Reading) 164:704–715. doi: 10.1099/mic.0.000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toro-Roman A, Wu T, Stock AM. 2005. A common dimerization interface in bacterial response regulators KdpE and TorR. Protein Sci 14:3077–3088. doi: 10.1110/ps.051722805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson R, Mylona E, Frankel G. 2018. Typhoidal Salmonella: distinctive virulence factors and pathogenesis. Cell Microbiol 20:e12939. doi: 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- 40.Darwin KH, Miller VL. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev 12:405–428. doi: 10.1128/CMR.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behlau I, Miller SI. 1993. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol 175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birck C, Chen Y, Hulett FM, Samama JP. 2003. The crystal structure of the phosphorylation domain in PhoP reveals a functional tandem association mediated by an asymmetric interface. J Bacteriol 185:254–261. doi: 10.1128/jb.185.1.254-261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi J, Kim H, Chang Y, Yoo W, Kim D, Ryu S. 2019. Programmed delay of a virulence circuit promotes salmonella pathogenicity. mBio 10:e00291-19. doi: 10.1128/mBio.00291-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen DG, Meyer JG, Baumgartner JT, D’Souza AK, Nelson WC, Payne SH, Kuhn ML, Schilling B, Wolfe AJ. 2018. Identification of novel protein lysine acetyltransferases in Escherichia coli. mBio 9:e01905-18. doi: 10.1128/mBio.01905-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang ZJ, Pedicord VA, Peng T, Hang HC. 2020. Site-specific acylation of a bacterial virulence regulator attenuates infection. Nat Chem Biol 16:95–103. doi: 10.1038/s41589-019-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Forster ER, Darabedian N, Kim AT, Pratt MR, Shen A, Hang HC. 2020. Translation of microbiota short-chain fatty acid mechanisms affords anti-infective acyl-salicylic acid derivatives. ACS Chem Biol 15:1141–1147. doi: 10.1021/acschembio.9b01009. [DOI] [PubMed] [Google Scholar]
- 47.Westermann AJ, Forstner KU, Amman F, Barquist L, Chao Y, Schulte LN, Muller L, Reinhardt R, Stadler PF, Vogel J. 2016. Dual RNA-seq unveils noncoding RNA functions in host-pathogen interactions. Nature 529:496–501. doi: 10.1038/nature16547. [DOI] [PubMed] [Google Scholar]
- 48.Sang Y, Ren J, Ni J, Tao J, Lu J, Yao YF. 2016. Protein acetylation is involved in Salmonella enterica serovar Typhimurium virulence. J Infect Dis 213:1836–1845. doi: 10.1093/infdis/jiw028. [DOI] [PubMed] [Google Scholar]
- 49.Sinha A, Gupta S, Bhutani S, Pathak A, Sarkar D. 2008. PhoP-PhoP interaction at adjacent PhoP binding sites is influenced by protein phosphorylation. J Bacteriol 190:1317–1328. doi: 10.1128/JB.01074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutherland C, MacDonald JA, Walsh MP. 2016. Analysis of phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase by Phos-tag SDS-PAGE. Am J Physiol Cell Physiol 310:C681–C691. doi: 10.1152/ajpcell.00327.2015. [DOI] [PubMed] [Google Scholar]
- 51.Kim JY, Kee HJ, Choe NW, Kim SM, Eom GH, Baek HJ, Kook H, Kook H, Seo SB. 2008. Multiple myeloma-related WHSC1/MMSET isoform RE-IIBP is a histone methyltransferase with transcriptional repression activity. Mol Cell Biol 28:2023–2034. doi: 10.1128/MCB.02130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.