We analyzed the relationship between itraconazole (ITZ) and hydroxy-itraconazole (OH-ITZ) levels in 1,223 human samples. Overall, there was a statistically significant correlation between ITZ and OH-ITZ levels (Pearson’s r, 0.7838), and OH-ITZ levels were generally higher than ITZ levels (median OH-ITZ:ITZ ratio, 1.73; range, 0.13 to 8.96).
KEYWORDS: itraconazole, hydroxy-itraconazole, therapeutic-drug monitoring, TDM, bloodstream concentrations, bioassay, antifungal efficacy
ABSTRACT
We analyzed the relationship between itraconazole (ITZ) and hydroxy-itraconazole (OH-ITZ) levels in 1,223 human samples. Overall, there was a statistically significant correlation between ITZ and OH-ITZ levels (Pearson’s r, 0.7838), and OH-ITZ levels were generally higher than ITZ levels (median OH-ITZ:ITZ ratio, 1.73; range, 0.13 to 8.96). However, marked variability was observed throughout the range of ITZ concentrations. Thus, it is difficult to predict OH-ITZ concentrations based solely on ITZ levels.
INTRODUCTION
Historically, itraconazole (ITZ) has been the preferred antifungal for treatment of most dimorphic fungal infections and some less-acute forms of aspergillosis (1–3). However, its oral bioavailability is variable and influenced by formulation, coingestion of food, and coadministration of medications that affect gastric acidity and motility (4). In addition, drug-drug interactions can affect ITZ exposure, which has been correlated with clinical outcomes (5). Consequently, therapeutic-drug monitoring (TDM) of ITZ is recommended for prophylaxis and treatment with this triazole (2, 3, 6).
Bioassays have been used for ITZ quantitation in biological fluids; however, these assays lacked sensitivity, and later studies found that correlation with drug concentrations measured by analytical assays (e.g., high-performance liquid chromatography [HPLC], liquid chromatography-mass spectrometry [LC-MS]) is variable and dependent on several factors, including the indicator organism used in bioassays (7–10). The results of bioassays are generally higher than those of HPLC or LC-MS when measured in human specimens, and levels measured by bioassay have been reported to range between 2 and 10 times higher than by the other analytical methods (7–10). This is due to the presence of hydroxy-itraconazole (OH-ITZ), an early metabolite in the metabolic pathway of ITZ, which has broad-spectrum antifungal activity and in vitro potency similar to those of ITZ (11). Others have suggested that the lack of correlation between ITZ levels measured by bioassay and analytical methods may be the result of the precipitation of ITZ in some bioassays due to the poor aqueous solubility of this triazole (12).
The availability of rapid, accurate, and cost-effective ITZ and OH-ITZ TDM has prompted measurement of both values in some clinical laboratories, and reporting of both levels is recommended in some treatment guidelines (1, 2, 13). The relationship of these values to one another has been examined in only a limited manner (7), and thus clinical uncertainty with interpretation remains. Prior publications have defined the target level of ITZ during prophylaxis as >0.5 μg/ml, with treatment targeting a value of >1.0 μg/ml. However, neither the target OH-ITZ level nor the target of the sum of the concentrations (ITZ plus OH-ITZ) that should be achieved to improve therapeutic outcomes has been defined. We sought to evaluate the relationship between ITZ and OH-ITZ and to summarize past studies to provide recommendations on ITZ and OH-ITZ TDM.
Paired ITZ and OH-ITZ levels from the Fungus Testing Laboratory (UT Health San Antonio, TX) were reviewed. These were measured by HPLC or ultraperformance liquid chromatography (UPLC)-MS as previously described (14, 15). Levels below or above the validated analytical measurement range for this laboratory (0.25 to 6 μg/ml) were excluded from the analysis. Linear regression analysis was used to plot ITZ versus OH-ITZ levels, and Pearson’s correlation (r) was used to assess the relationship between these measurements. A P value of <0.05 was considered statistically significant. A total of 1,223 paired human measurements of ITZ and OH-ITZ were included in this analysis. Pearson’s r was 0.7838 (95% confidence interval, 0.7612 to 0.8045; P < 0.001) (Fig. 1A), whereas the coefficient of determination was 0.6143. The median OH-ITZ:ITZ ratio was 1.73 (range, 0.13 to 8.96), the mean ratio was 1.829 ± 0.724, and 5.64% of samples had an OH-ITZ:ITZ ratio of ≤1. Overall, these results suggest that the values of OH-ITZ and ITZ are correlated, and OH-ITZ concentrations are usually higher than those of ITZ. These results are consistent with a previous study that included <40 samples, where the mean ratio was 1.8 ± 0.5 and the correlation coefficient was 0.96 (7). We also plotted OH-ITZ values based on ITZ concentration groups (Fig. 1B). Overall, the coefficient of variation for OH-ITZ levels varied from 26% to 45.7% and was greatest within the lowest and highest ITZ groups (45.7% for the 0.25- to 0.5-μg/ml group and 42.6% for the >3-μg/ml group). These results suggest that it may be difficult to accurately predict OH-ITZ levels based solely on ITZ concentrations.
FIG 1.
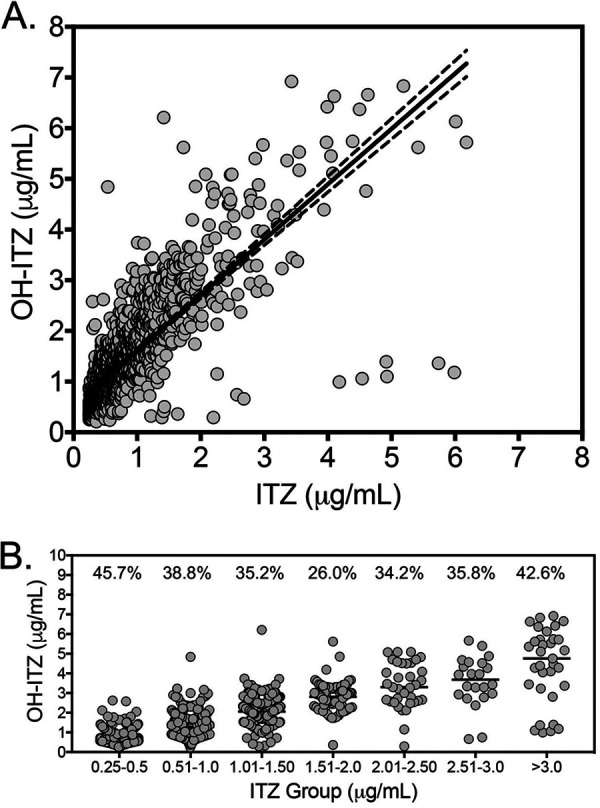
(A) Linear regression analysis between ITZ and OH-ITZ. ITZ and OH-ITZ were measured by validated HPLC or UPLC-MS assays, and concentrations within the analytical measurement range (0.25 to 6 μg/ml) were plotted (n = 1,223). (B) OH-ITZ concentrations based on different ITZ concentration levels. The percent coefficients of variation of OH-ITZ are presented above each ITZ group.
Both OH-ITZ and ITZ are primarily metabolized by the cytochrome P450 3A4 isoenzyme, and the differences in the ratio of parent drug to metabolite are likely secondary to differences in CYP3A4 affinity, as has been suggested (16). Despite the correlation, we observed that there are clear outliers. These outliers may have polymorphisms within the metabolic pathway of either the parent drug or the metabolite or due to patients receiving concomitant medications that either induce or inhibit the metabolism of ITZ and/or OH-ITZ.
Past itraconazole concentration-effect studies have shown correlations between bloodstream drug concentrations and clinical efficacy (7, 17–24). These studies have focused on oral candidiasis, aspergillosis, cryptococcosis, and coccidioidomycosis (Table 1). However, not all studies have reported such a correlation (25, 26). The relationship between toxicity and ITZ concentrations is less clear. Some have suggested that concentrations of >17 μg/ml (measured by bioassay) were associated with significant toxicity, including clinical features associated with heart failure (27). The exact toxicity threshold when measured by HPLC or LC-MS is unknown but has been suggested to be ∼5 times lower when considering the ITZ component alone (6, 12). Current guidelines for target drug concentrations have been based on these studies, with ITZ drug levels of >0.5 μg/ml targeted during prophylaxis and ≥1.0 μg/ml during treatment (1, 2, 13). These recommendations have focused on ITZ concentrations and have not included OH-ITZ levels, causing uncertainty with interpretation of these values. It is unknown how many centers that measure ITZ levels also measure and report OH-ITZ levels.
TABLE 1.
Summary of clinical studies showing response rates in relation to ITZ and OH-ITZ levels
| Study type and condition (no. of patients) | ITZ dose (mg) | Analyte and assay method | Findings (ref.)a |
|---|---|---|---|
| Prophylaxis | |||
| Neutropenia (72) | 100 twice daily | ITZ alone by HPLC | Fungal infections in 16/31 (52%) of patients with ITZ levels of <0.25 μg/ml vs 3/37 with levels of >0.25 μg/ml (8.1%) (23) |
| Neutropenia (20) | 400–800 daily | ITZ alone by HPLC | Significant difference in percent ITZ concentrations of >0.5 μg/ml in patients with invasive fungal infections (median 48%) vs those without infections (median 100%); lower median ITZ levels immediately before diagnosis of infection in patients with fatal infections (0.12 μg/ml) vs those with nonfatal infections (0.69 μg/ml) (24) |
| Neutropenia (45) | 200 twice daily | ITZ alone by HPLC | Fungal infections in 11/21 with inadequate ITZ levels (<0.25 μg/ml for 7 consecutive days) vs 4/21 with adequate levels (21) |
| Treatment | |||
| Oral candidiasis (31) | 200 twice daily | ITZ and OH-ITZ by HPLC | Serum ITZ levels of 1.19 vs 0.63 μg/ml in responders vs nonresponders, respectively; OH-ITZ levels of 1.38 vs 0.71 μg/ml in responders vs nonresponders, respectively (17) |
| Oral candidiasis (264) | 200 twice daily | ITZ by HPLC | Trough ITZ of >0.5 μg/ml associated with highest rate of treatment success (20) |
| Aspergillosis (15) | 100–400 daily | ITZ/OH-ITZ by bioassay | Mean ITZ levels of 6.1 vs 2.8 μg/ml in cures/responders vs nonresponders/failures, respectively (19) |
| Coccidioidomycosis (39) | 200 twice daily | ITZ/OH-ITZ by bioassay | Mean Cmax of ITZ 6.5 vs 4.0 μg/ml in responders vs nonresponders (22) |
Cmax, maximum concentration of drug in serum.
In summary, although a significant correlation between ITZ and OH-ITZ bloodstream concentrations was found, variability in some samples was also observed. This suggests that OH-ITZ concentrations may not be interpolated solely based on ITZ levels. Given the sum of available data (summarized in Table 1) and an OH-ITZ:ITZ ratio of >1 in >94% of samples, it may be feasible to recommend a target ITZ concentration of >0.5 μg/ml and combined ITZ+OH-ITZ levels of ≥1.0 μg/ml during prophylaxis and ITZ concentrations of ≥1.0 μg/ml and combined ITZ+OH-ITZ levels of ≥2.0 μg/ml during treatment. However, further studies are needed for clinical validation.
REFERENCES
- 1.Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlkeld MG, Kauffman CA, Infectious Diseases Society of America . 2008. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 46:1801–1812. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]
- 2.Patterson TF, Thompson GR, 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller R, Assi M, AST Infectious Diseases Community of Practice . 2019. Endemic fungal infections in solid organ transplant recipients—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 33:e13553. doi: 10.1111/ctr.13553. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz IS, Wiederhold NP. 2017. Update on therapeutic drug monitoring of antifungals for the prophylaxis and treatment of invasive fungal infections. Curr Fungal Infect Rep 11:75–83. doi: 10.1007/s12281-017-0287-4. [DOI] [Google Scholar]
- 5.Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hostetler JS, Heykants J, Clemons KV, Woestenborghs R, Hanson LH, Stevens DA. 1993. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob Agents Chemother 37:2224–2227. doi: 10.1128/aac.37.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnock DW, Turner A, Burke J. 1988. Comparison of high performance liquid chromatographic and microbiological methods for determination of itraconazole. J Antimicrob Chemother 21:93–100. doi: 10.1093/jac/21.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Heykants J, Van Peer A, Van de Velde V, Van Rooy P, Meuldermans W, Lavrijsen K, Woestenborghs R, Van Cutsem J, Cauwenbergh G. 1989. The clinical pharmacokinetics of itraconazole: an overview. Mycoses 32 (Suppl 1):67–87. doi: 10.1111/j.1439-0507.1989.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 10.Summers KK, Hardin TC, Gore SJ, Graybill JR. 1997. Therapeutic drug monitoring of systemic antifungal therapy. J Antimicrob Chemother 40:753–764. doi: 10.1093/jac/40.6.753. [DOI] [PubMed] [Google Scholar]
- 11.Odds FC, Bossche HV. 2000. Antifungal activity of itraconazole compared with hydroxy-itraconazole in vitro. J Antimicrob Chemother 45:371–373. doi: 10.1093/jac/45.3.371. [DOI] [PubMed] [Google Scholar]
- 12.Law D, Moore CB, Denning DW. 1994. Bioassay for serum itraconazole concentrations using hydroxyitraconazole standards. Antimicrob Agents Chemother 38:1561–1566. doi: 10.1128/aac.38.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA, Infectious Diseases Society of America . 2007. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 14.Manire CA, Rhinehart HL, Pennick GJ, Sutton DA, Hunter RP, Rinaldi MG. 2003. Steady-state plasma concentrations of itraconazole after oral administration in Kemp's ridley sea turtles, Lepidochelys kempi. J Zoo Wildl Med 34:171–178. doi: 10.1638/1042-7260(2003)034[0171:SPCOIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Wiederhold NP, Pennick GJ, Dorsey SA, Furmaga W, Lewis JS, II, Patterson TF, Sutton DA, Fothergill AW. 2014. A reference laboratory experience of clinically achievable voriconazole, posaconazole, and itraconazole concentrations within the bloodstream and cerebral spinal fluid. Antimicrob Agents Chemother 58:424–431. doi: 10.1128/AAC.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE. 2004. Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos 32:1121–1131. doi: 10.1124/dmd.104.000315. [DOI] [PubMed] [Google Scholar]
- 17.Cartledge JD, Midgely J, Gazzard BG. 1997. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J Clin Pathol 50:477–480. doi: 10.1136/jcp.50.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning DW, Tucker RM, Hanson LH, Hamilton JR, Stevens DA. 1989. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch Intern Med 149:2301–2308. doi: 10.1001/archinte.1989.00390100107024. [DOI] [PubMed] [Google Scholar]
- 19.Denning DW, Tucker RM, Hanson LH, Stevens DA. 1989. Treatment of invasive aspergillosis with itraconazole. Am J Med 86:791–800. doi: 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- 20.Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, Lancaster M, Odds FC, Rinaldi MG, Walsh TJ, Barry AL. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin Infect Dis 24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 21.Tricot G, Joosten E, Boogaerts MA, Vande Pitte J, Cauwenbergh G. 1987. Ketoconazole vs. itraconazole for antifungal prophylaxis in patients with severe granulocytopenia: preliminary results of two nonrandomized studies. Rev Infect Dis 9 (Suppl 1):S94–S99. doi: 10.1093/clinids/9.supplement_1.s94. [DOI] [PubMed] [Google Scholar]
- 22.Tucker RM, Denning DW, Arathoon EG, Rinaldi MG, Stevens DA. 1990. Itraconazole therapy for nonmeningeal coccidioidomycosis: clinical and laboratory observations. J Am Acad Dermatol 23:593–601. doi: 10.1016/0190-9622(90)70261-F. [DOI] [PubMed] [Google Scholar]
- 23.Boogaerts MA, Verhoef GE, Zachee P, Demuynck H, Verbist L, De Beule K. 1989. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 32 (Suppl 1):103–108. doi: 10.1111/j.1439-0507.1989.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 24.Glasmacher A, Hahn C, Leutner C, Molitor E, Wardelmann E, Losem C, Sauerbruch T, Marklein G, Schmidt-Wolf IG. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443–451. doi: 10.1046/j.1439-0507.1999.00505.x. [DOI] [PubMed] [Google Scholar]
- 25.Nucci M, Biasoli I, Akiti T, Silveira F, Solza C, Barreiros G, Spector N, Derossi A, Pulcheri W. 2000. A double-blind, randomized, placebo-controlled trial of itraconazole capsules as antifungal prophylaxis for neutropenic patients. Clin Infect Dis 30:300–305. doi: 10.1086/313654. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama S, Masuya M, Tanaka I, Oka K, Morita K, Tamaki S, Tsuji K, Katayama N, Sugimoto H, Kagawa Y, Kojima M, Shiku H. 1999. Plasma concentration of itraconazole and its antifungal prophylactic efficacy in patients with neutropenia after chemotherapy for acute leukemia. J Infect Chemother 5:213–216. doi: 10.1007/s101560050038. [DOI] [PubMed] [Google Scholar]
- 27.Lestner JM, Roberts SA, Moore CB, Howard SJ, Denning DW, Hope WW. 2009. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis 49:928–930. doi: 10.1086/605499. [DOI] [PubMed] [Google Scholar]


