Key Points
Question
In patients with rheumatoid arthritis in remission taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), is reducing the csDMARDs to half dose noninferior to stable csDMARD dosage for the outcome of rheumatoid arthritis flares?
Findings
In this randomized clinical trial that included 160 patients with rheumatoid arthritis in remission taking csDMARD therapy, treatment with half-dose vs stable-dose csDMARDs resulted in disease flares in 25% vs 6% over 12 months; this did not meet the noninferiority criterion of a 20% difference. There were significantly fewer patients with flares in the stable-dose group.
Meaning
These findings do not support the use of half-dose treatment in patients with rheumatoid arthritis in remission taking csDMARDs.
Abstract
Importance
Sustained remission has become an achievable goal for patients with rheumatoid arthritis (RA) receiving conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), but how to best treat patients in clinical remission remains unclear.
Objective
To assess the effect of tapering of csDMARDs, compared with continuing csDMARDs without tapering, on the risk of flares in patients with RA in sustained remission.
Design, Setting, and Participants
ARCTIC REWIND was a multicenter, randomized, parallel, open-label noninferiority study conducted in 10 Norwegian hospital-based rheumatology practices. A total of 160 patients with RA in remission for 12 months who were receiving stable csDMARD therapy were enrolled between June 2013 and June 2018, and the final visit occurred in June 2019.
Interventions
Patients were randomly assigned to half-dose csDMARDs (n = 80) or stable-dose csDMARDs (n = 80).
Main Outcomes and Measures
The primary end point was the proportion of patients with a disease flare between baseline and the 12-month follow-up, defined as a combination of Disease Activity Score (DAS) greater than 1.6 (threshold for RA remission), an increase in DAS score of 0.6 units or more, and at least 2 swollen joints. A disease flare could also be recorded if both the patient and investigator agreed that a clinically significant flare had occurred. A risk difference of 20% was defined as the noninferiority margin.
Results
Of 160 enrolled patients (mean [SD] age, 55.1 [11.9] years; 66% female), 156 received the allocated therapy, of which 155 without any major protocol violations were included in the primary analysis population (77 receiving half-dose and 78 receiving stable-dose csDMARDs). Flare occurred in 19 patients (25%) in the half-dose csDMARD group compared with 5 (6%) in the stable-dose csDMARD group (risk difference, 18% [95% CI, 7%-29%]). Adverse events occurred in 34 patients (44%) in the half-dose group and 42 (54%) in the stable-dose group, none leading to study discontinuation. No deaths occurred.
Conclusions and Relevance
Among patients with RA in remission taking csDMARD therapy, treatment with half-dose vs stable-dose csDMARDs did not demonstrate noninferiority for the percentage of patients with disease flares over 12 months, and there were significantly fewer flares in the stable-dose group. These findings do not support treatment with half-dose therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT01881308
This randomized trial compares the effects of half-dose conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) vs stable-dose csDMARDs on the risk of flares in patients with rheumatoid arthritis in sustained remission.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by joint inflammation with a potential for joint destruction and impaired physical function. While considered incurable, RA can be controlled with continuous immune modulation.
The goal of RA therapy is clinical remission with prevention of structural joint damage. This can be attainable for patients treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs)1,2,3 when treatment is initiated in early disease, therapy is adjusted until a disease activity target is reached, and adequate dosages of medication are used.
Although csDMARD therapy is commonly prescribed, the optimal method of dose reduction and withdrawal of csDMARD in RA is unclear. One clinical trial that randomized patients in RA remission to either continued or discontinued csDMARD therapy showed increased rates of RA exacerbations in patients who stopped taking csDMARDs.4 The most frequent drugs used were hydroxychloroquine, chloroquine, and parenteral gold, with only 2% of patients using methotrexate, the current first-line drug in RA. Observational data showed that tapering or discontinuing csDMARDs was associated with increased flare rates and that rapid reintroduction of therapy was associated with increased probability of regaining remission after flares.5,6 Studies that tapered biologic DMARDs and combinations of csDMARDs and biologic DMARDs7,8,9,10 showed that these strategies might be appropriate in patients with sustained levels of very low disease activity, but it is unknown whether these data apply to patients who have attained remission with csDMARDs alone.
This randomized trial assessed the effect of csDMARD tapering on RA flares, compared with continued stable csDMARD treatment, in patients with RA in remission.
Methods
Study Design
ARCTIC REWIND was a 36-month randomized, open-label, parallel, multicenter noninferiority trial conducted to evaluate the effect of csDMARD dose reduction on disease activity in patients with RA in remission. The study included 2 randomized clinical trials, with separate study designs for patients in sustained remission with csDMARD therapy (the current trial) and for patients in sustained remission with biologic tumor necrosis factor inhibitors. The trial was conducted in compliance with the International Conference on Harmonization Guidelines for Good Clinical Practice, and the study protocol (Supplement 1) and consent documents were approved by the Regional Ethical Committee and the Norwegian Medicines Agency. All patients provided written informed consent. Patients were enrolled and followed up at 10 hospital-based rheumatology practices in Norway (eTable 1 in Supplement 2), with visits every 4 months in both groups.
Patients
Adults (18-80 years) fulfilling the 2010 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) classification criteria for RA11 who had documented remission status according to established criteria at all consecutive visits for at least 12 months were eligible (full inclusion and exclusion criteria are in eAppendix 1 in Supplement 2). Inclusion criteria included absence of any swollen joints (44 joints assessed) and remission according to the Disease Activity Score (DAS). The DAS is a composite measure of disease activity (range, 0-10),12 calculated based on presence vs absence of swelling in 44 joints, including the feet, the Ritchie Articular Index, erythrocyte sedimentation rate (mm/h), and patient global assessment. Higher scores indicate more disease activity, and a score below 1.6 corresponds with remission according to the ACR criteria.13 Remission indicates absence or near absence of signs or symptoms of disease activity, and is associated with reduced joint damage and a good functional outcome.13,14 csDMARD treatment had to be unchanged during the last 12 months, without the use of biologic DMARDs or a janus kinase inhibitor. Oral glucocorticoid use at study entry was allowable as long as the dose did not exceed 5 mg of prednisolone (or equivalent), and the investigators were instructed that glucocorticoids were to be used for indications other than RA. Initial inclusion criteria required the patient to have less than 5 years of symptom duration; this was changed to diagnosis after January 1, 2010 (the introduction of new classification criteria for RA), in a protocol update because onset of symptom duration could be difficult to determine. This change also increased the number of patients eligible for enrollment.
Randomization
Patients receiving csDMARD therapy (monotherapy or combination of csDMARDs) were randomly assigned 1:1 to either continued stable or to half-dose csDMARD treatment by a computer-based block randomization stratified by study center, with a block size of 4. Patients were enrolled by nurses and investigators at each site. The allocated treatment group for each patient was made available after study personnel confirmed patient eligibility and the participant was randomized, using the electronic case report form system (Viedoc, version 3).
Interventions
In the half-dose group, the csDMARD treatment was reduced to half dose at the baseline visit (eg, methotrexate, 25 mg, once per week was changed to methotrexate, 12.5 mg, once per week; sulfasalazine, 1000 mg, twice daily to sulfasalazine, 500 mg, twice daily; and hydroxychloroquine, 200 mg, twice daily to hydroxychloroquine, 200 mg, once daily). If a patient in the half-dose group used a combination of csDMARDs, each csDMARD was reduced by half. Patients were instructed to contact the study center if they experienced symptoms of a possible disease flare and were evaluated within a week. In the half-dose group, patients with flares resumed their baseline csDMARD dose. In the stable-dose group, patients with flares were treated according to current recommendations. The protocol did not outline further attempts at adjusting medication if flares were successfully treated. Investigators, patients, and assessors were aware of the allocated treatment group.
Outcomes
The primary efficacy end point was the proportion of patients who experienced a disease flare between baseline and 12 months. A disease flare was defined as a combination of DAS greater than 1.6 (threshold for RA remission), an increase in DAS of 0.6 units or more from the previous visit, and at least 2 swollen joints on examination of 44 joints. If a patient did not fulfill these criteria, a disease flare could be recorded if both the patient and investigator agreed that a clinically significant flare had occurred.
The secondary end points at 12 months included changes and area under the curve from baseline for the composite disease activity indices DAS, Disease Activity Score based on 28 joints (DAS28; range, 0-9.4),15 Clinical Disease Activity Index (CDAI; range, 0-76),14,16 and Simplified Disease Activity Index (SDAI; range, 0-86).16,17 Higher scores indicate higher disease activity for all composite disease activity scores. The following dichotomous outcomes, definitions of remission, were also assessed: DAS remission, DAS28 remission (DAS28 <2.6),18 SDAI remission (SDAI ≤3.3),16,17 CDAI remission (CDAI ≤2.8),14,16 and ACR/EULAR Boolean remission (a combination of ≤1 swollen joints, ≤1 tender joints, patient global assessment of disease activity ≤1 on a 0-10 visual analog scale, and C-reactive protein level ≤1 mg/dL).14 Additionally, these individual measures of disease activity were evaluated: tender joints (Ritchie Articular Index, a graded assessment of 26 joint regions; range, 0-78),19 number of swollen joints (0-44; swollen joint count among 44 joints [SJC44]), patient and physician global assessment of disease activity (0-100 mm), erythrocyte sedimentation rate, and C-reactive protein level. Corticosteroid usage during the follow-up period was recorded (intra-articular injections, prednisolone use), as well as the number of patients in different DMARD categories and dose among the patients taking the DMARD. Patient-reported outcomes included joint pain and fatigue visual analog scales (0-100 mm) and physical function by the Patient-Reported Outcomes Measurement Information 20-item short form (range, 20-100; translated to a T score with a mean of 50 and an SD of 10).20 The EuroQol–5 Dimension questionnaire,21 the Rheumatoid Arthritis Impact of Disease,22 and components and summaries of the 36-Item Short-Form Survey23 were calculated.
Patients were assessed by ultrasonography at baseline and 12 months according to a validated 32-joint scoring system; each joint was scored semiquantitatively from 0 to 3 for gray scale (total score, 0-96) and power Doppler (total score, 0-96).24 The percentage of patients without any joints with power Doppler positivity was calculated. Radiographs of hands and feet were acquired at baseline and 12 months and scored by 2 readers unaware of clinical information or treatment group in known chronological order according to the van der Heijde–modified Sharp score.25 Radiographic progression (average score of the readers) was defined as a change in van der Heijde–modified Sharp score of 1 unit or more per year (sensitivity analyses with cutoffs of ≥0.5, ≥2, and ≥5 units per year). Work productivity (absenteeism, presenteeism, work productivity, and activity impairment) and magnetic resonance imaging data were not available at the time of publishing primary outcome data.
In patients who experienced a flare, response to treatment was assessed by ACR 20/50/70/90 response,26 EULAR good and moderate responses,27 and the US Food and Drug Administration major clinical response,28 in addition to disease activity measures.
Adverse events were evaluated at each visit by assessment of clinical and laboratory adverse events, coded according to the Medical Dictionary for Regulatory Activities (version 21.1E).
Statistical Analyses
Assuming no difference between the treatment groups regarding the risk of flares during the 12-month follow-up, we calculated that 126 patients (63 in each group) were required to conclude noninferiority with 80% power. This conclusion would be drawn if the 2-sided 95% CI for the risk difference excluded a difference in favor of stable csDMARD of more than 20%. Accounting for a potential 20% dropout rate, 80 patients were randomized to each group.
The noninferiority margin was selected after investigator discussion and was based on the Food and Drug Administration guidance document for noninferiority clinical trials.29 The document outlines factors of importance for the noninferiority margin: (1) that the primary end point does not involve an irreversible outcome such as death, (2) that the experimental (novel) treatment is associated with fewer serious adverse effects or better tolerability, and (3) that the experimental (novel) therapy has other advantages over available therapies. The consensus was that an upper limit of increased risk difference in flare rate of 20% would be an acceptable degree of difference, given the potential benefits of decreasing DMARD therapy, including increased tolerability and reduction in adverse events.30
It was estimated that approximately 20% of patients in each group would experience a flare during 12-month follow-up, based on data from a Norwegian epidemiologic cohort of patients treated with DMARDs.31 If noninferiority was not demonstrated, the statistical analysis plan (Supplement 1) prespecified that statistical testing would be performed to determine whether either group was statistically different from the alternative group for the outcome of number of flares.
Baseline characteristics were described by number (percentage), median (interquartile range), or mean (SD) as appropriate. Testing of the inferiority null hypothesis was performed in the primary analysis population, defined as all randomized patients meeting the study entry criteria, and with no protocol deviations affecting the treatment efficacy (defined as failure to follow the treatment regimen or withdrawal from the study). The primary analysis was conducted using mixed-effect logistic regression, with the response defined as any disease flare during 12-month follow-up, treatment group as the only fixed factor, and center as a random effect to account for the center stratification. From this model, the average marginal effect of half-dose vs stable-dose therapy was computed and used to estimate the difference in risk of flares. The 95% CI of this difference was estimated using the delta method. Patients included in the primary analysis did not have missing values for the primary end point because the primary variable was fully monitored during the study. The analyses were repeated in the population of patients who had been allocated and had initiated treatment, verified by attendance of at least 1 study visit after baseline.
Dichotomous secondary outcomes were assessed using mixed-effect logistic regression, and continuous secondary outcomes were assessed using linear mixed model, adjusting for baseline values. For outcomes assessed at multiple study visits, fixed factors were treatment group, time, and a time by treatment interaction, with center and patient treated as random effects. Models adjusted for baseline values. Data were not imputed. Model validity checks were performed by examining the model residuals. For group differences, 2-sided 95% CIs were calculated. Radiographic change data were displayed in cumulative plots. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All analyses were performed in Stata version 14.0 (StataCorp).
Unprespecified Sensitivity Analyses
The primary analysis was repeated in patients receiving methotrexate monotherapy.
Post Hoc Analyses
In post hoc sensitivity analyses, analyses of the primary outcome were repeated, restricting the outcome to patients with data available to indicate active inflammation based on clinical assessment, ultrasound evaluation, and biochemical markers. Additional post hoc analyses consisted of calculating the number and percentage of patients who adjusted therapy after experiencing a flare. Additional analyses consisted of calculating the time to flare.
Results
Characteristics of the Participants
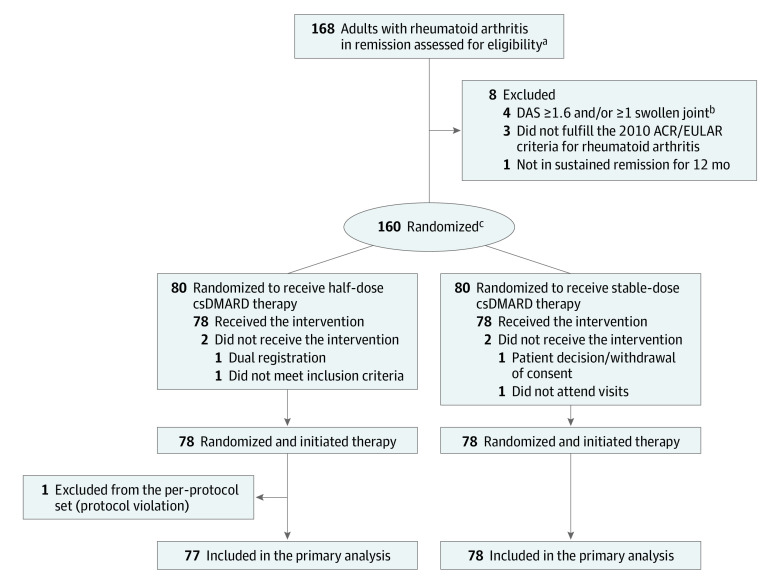
From June 17, 2013, to June 18, 2018, 160 patients were randomized. Seventy-eight patients received the allocated half-dose csDMARD therapy, and 78 received the allocated stable-dose csDMARD therapy (Figure 1). One patient in the half-dose csDMARD group was excluded from analyses due to a major protocol violation, consisting of prolonged withdrawal of methotrexate due to surgery. The 2 groups were balanced regarding baseline characteristics (Table 1). In the half-dose csDMARD group, 66 of 78 patients (85%) were methotrexate monotherapy users compared with 61 of 78 patients (78%) in the stable-dose csDMARD group.
Figure 1. Flow of Patients in the ARCTIC REWIND csDMARD Study.
ACR indicates American College of Rheumatology; csDMARD, conventional synthetic disease-modifying antirheumatic drugs; DAS, Disease Activity Score; and EULAR, European Alliance of Associations for Rheumatology.
aRemission according to established criteria should be documented for at least 12 months.
bDAS is a composite measure of disease activity with scores ranging from 0 to 10, higher scores indicate more disease activity, remission defined as <1.6.
cStratified by study site.
Table 1. Baseline Characteristics of the Study Populationa.
| Characteristic | Median (interquartile range) | |
|---|---|---|
| Half dose (n = 78) | Stable dose (n = 78) | |
| Age, mean (SD), y | 55.5 (12.0) | 55.1 (11.8) |
| Sex, No. (%) | ||
| Female | 54 (69) | 50 (64) |
| Male | 24 (31) | 28 (36) |
| Time since first swollen joint, y | 3.2 (2.4-4.1) | 3.4 (2.6-4.4) |
| Positive, No. (%) | ||
| For anticitrullinated peptide antibodies | 63 (81) | 57 (73) |
| For rheumatoid factor | 53 (68) | 54 (69) |
| Body mass indexb | 25.7 (23.6-28.0) | 25.7 (22.8-28.4) |
| Current smoker, No. (%) | 13 (17) | 14 (18) |
| Measures of disease activity | ||
| Disease Activity Score, mean (SD)c | 0.8 (0.3) | 0.8 (0.4) |
| Simplified Disease Activity Indexd | 0.9 (0.3-2.1) | 0.8 (0.5-1.6) |
| ACR/EULAR remission, No. (%)e | 51 (65) | 61 (78) |
| Swollen joint count, mean (SD)f | 0 | 0 |
| Tender joint count (Ritchie Articular Index)g | 0 (0-0) | 0 (0-0) |
| Erythrocyte sedimentation rate, mm/h (normal value <17 mm/h in women and <12 mm/h in men)h | 7.0 (4.0-14.0) | 7.0 (4.0-14.0) |
| C-reactive protein, mg/dL (normal value <0.4 mg/dL)h | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) |
| Global assessment (0-10)i | ||
| Patient’s | 3.5 (1.0-11.0) | 3.5 (1.0-10.0) |
| Physician’s | 0 (0-3.0) | 1.0 (0-4.0) |
| Functional outcomes | ||
| PROMIS Physical Function, mean (SD)j | 55.6 (7.5) | 56.1 (7.4) |
| Visual analog scale (0-100 mm)k | ||
| Fatigue | 10.0 (2.0-30.0) | 5.5 (1.0-24.0) |
| Joint pain | 3.5 (1.0-10.0) | 3.0 (1.0-9.0) |
| Radiographic joint damage | ||
| Total van der Heijde–modified Sharp scorel | 4.5 (2.0-8.5) | 5.0 (2.0-11.5) |
| van der Heijde–modified Sharp score | ||
| Erosion | 2.0 (1.0-3.5) | 2.0 (1.0-4.5) |
| Sharp joint space narrowing | 2.0 (0.5-6.0) | 2.0 (0.5-8.0) |
| Ultrasound outcomesm | ||
| Total power Doppler signal score | 0 (0-0) | 0 (0-0) |
| Total gray scale score | 1.0 (0-3.0) | 1.0 (0-2.0) |
| No power Doppler signal in any joint, No. (%) | 72 (92) | 72 (94) |
| Medication, No. (%) | ||
| Methotrexate monotherapy | ||
| By mouth | 52 (67) | 51 (65) |
| Subcutaneous | 14 (18) | 10 (13) |
| Methotrexate, sulfasalazine, and hydroxychloroquine | 6 (8) | 10 (13) |
| Other monotherapies or duotherapies | 6 (8) | 7 (9) |
| Dose in users, mean (SD) | ||
| Methotrexate, mg/wk | 19.5 (4.3) | 19.0 (4.7) |
| Sulfasalazine, mg/d | 1563 (623) | 1769 (438) |
| Hydroxychlorochine, mg/d | 378 (67) | 400 (0) |
| Leflunomide, mg/d | 20.0 (NC) | 20.0 (NC) |
Abbreviations: ACR, American College of Rheumatology; EULAR, Alliance of Associations for Rheumatology; NC, not calculated (due to only 1 patient in each group using leflunomide); PROMIS, Patient-Reported Outcomes Measurement Information Score.
As allocated and initiated treatment. Four patients who were randomized but did not have verified initiation of treatment are excluded, 2 from each group.
Calculated as weight in kilograms divided by height in meters squared.
Disease Activity Score (DAS; range, 0-10) includes a 44 swollen joint count, assessment of tender joints by Ritchie Articular Index, the erythrocyte sedimentation rate (ESR), and patient’s global assessment of disease activity on a visual analog scale (VAS) of 0 to 100 mm. It is calculated as: DAS = 0.54 × sqrt(RAI) + 0.065 × (SJC44) + 0.33 × Ln(ESR) + 0.0072 × PGA. Remission is defined as <1.6; low disease activity, 1.6 to 2.4; moderate disease activity, >2.4 to 3.7; and high disease activity, >3.7, thus higher scores indicate more disease activity.
Simplified Disease Activity Index (SDAI; range, 0-86) includes a 28 swollen and tender joint count, C-reactive protein (CRP) and the patient’s and physician’s global assessment of disease activity on a VAS of 0 to 100 mm. It is calculated as follows: SDAI = TCJ28 + SJC28 + PGA/10 + PhGA/10 + CRP. Remission is defined as a score ≤3.3, with higher scores indicating more disease activity.
Remission defined as tender joint count ≤1, swollen-joint count ≤1, CRP level ≤1 mg/dL, and patient’s global assessment ≤10 (on a 0-100 scale).
The swollen joint count is the number of swollen joints of 44 joints assessed.
The tender joint count is performed by the Ritchie Articular Index assessing tenderness of 26 joint regions; the index ranges from 0 to 3 for individual measures and the sum 0 to 78 overall, with higher scores indicating more tenderness.
At time of baseline visit, normal values may vary among laboratories.
The patient’s and physician’s global assessments are self-reported and physician-reported, respectively; overall assessments of disease with use of a VAS that ranges from 0 to 100 mm, with higher scores indicating more severe disease.
20-Item Short Form scores range from 0 to 100, with scores <50 indicating disability worse than average.
Fatigue and joint pain are self-reported with use of a VAS ranging from 0 to 100 mm, with higher scores indicating more severe fatigue.
Rheumatoid arthritis inflammatory disease activity is associated with radiographic joint damage progression, which in turn might lead to functional decline. The van der Heijde–modified Sharp scoring method assesses erosions in 16 joints of each hand (range, 0-5 for each joint) and in 6 joints of each foot (range, 0-10 per joint) and joint space narrowing in 15 joints for each hand and in 6 joints for each foot (range, 0-4 per joint). This gives scores for erosions on a scale from 0 to 280 and joint space narrowing on a scale from 0 to 168, thus the total van der Heijde–modified Sharp score ranges from 0 to 448, with higher scores indicating greater joint damage.
Two aspects of synovitis can be assessed by ultrasound: morphology and quantity using gray scale and synovial vascularity using power Doppler. The ultrasound examination was performed using 0-3 semiquantitative scoring systems for both gray-scale and power Doppler in 32 joints.
Primary Outcome
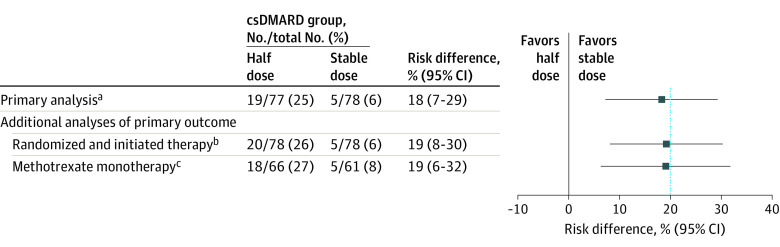
In the primary analysis population, 19 of 77 patients (25%) receiving half-dose csDMARD therapy experienced at least 1 flare during the 1-year follow-up compared with 5 of 78 patients (6%) in the stable-dose csDMARD therapy group (risk difference, 18% [95% CI, 7%-29%]; P for comparison = .003), rejecting the noninferiority hypothesis (Figure 2, Figure 3A). The difference in flare rate was statistically significantly higher in the half-dose group compared with the stable-dose group.
Figure 2. Flare Rate Within 12 Months (Primary Outcome) in Half-Dose vs Stable-Dose Antirheumatic Drug Treatment.
Flare was defined as a combination of Disease Activity Score (DAS) above the cutoff for remission (1.6), a change in DAS of at least 0.6, and at least 2 swollen joints or that both the treating physician and the patient agreed that a clinically significant flare had occurred. The blue, dotted, vertical line represents the noninferiority margin. csDMARD indicates conventional synthetic disease-modifying antirheumatic drug.
aThe primary analysis was performed in all randomized patients meeting the study entry criteria and with no protocol deviations affecting the treatment efficacy (defined as failure to follow the treatment regimen or withdrawal from the study).
bFour patients who were randomized but did not have verified initiation of treatment are excluded (2 from each group).
cAnalysis performed in patients within the primary analysis population who used methotrexate monotherapy.
Figure 3. Secondary End Points.
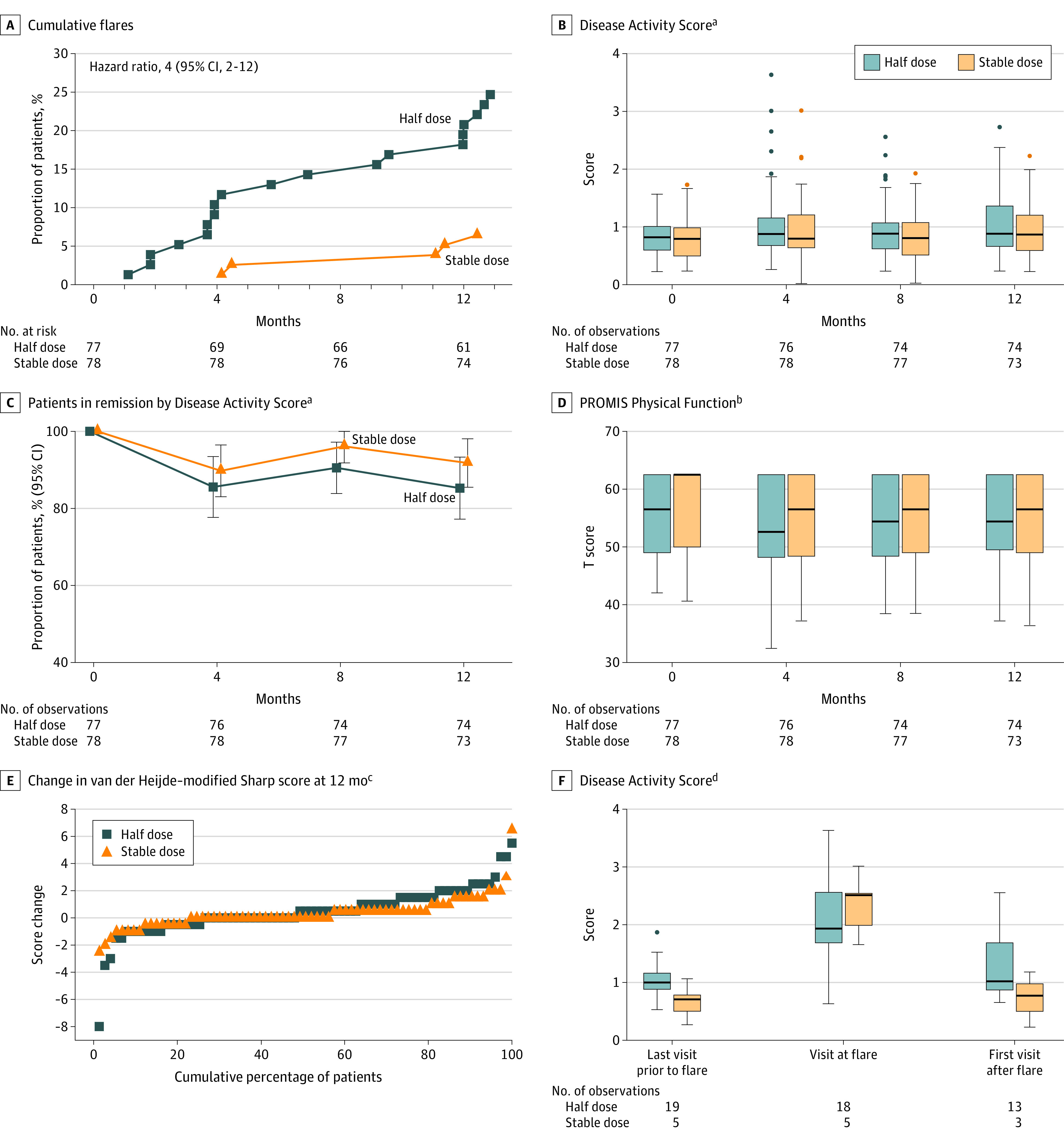
Analyzed in the primary analysis population, defined as all randomized patients meeting the study entry criteria and with no protocol deviations affecting the treatment efficacy. Patients were followed up for a median (IQR) of 364 days (364-371) in the half-dose group and 364 days (360-377) in the stable-dose group. Variables displayed based on clinical relevance. PROMIS indicates Patient-Reported Outcomes Measurement Information 20-item Short Form Physical Function. Boxes mark first and third quartiles, the band inside the box is the second quartile (the median), while the whiskers indicate the highest and lowest values within 1.5 × the interquartile range (IQR). Dots denote individual patients (outliers).
aSee footnote c under Table 1 for scale descriptions.
bPROMIS assesses the ability to perform basic and instrumental activities of daily living. The total score is translated into a T score with a mean (SD) of 50 (10). A score of 50 equals the average for the general US population.
cThe van der Heijde–modified Sharp scoring method assesses erosions in 16 joints of each hand and 6 joints of each foot, and the erosions are given a score of 1 to 5. Joint space narrowing is assessed in 15 joints for each hand and 6 joints for each foot. This gives scores for erosions on a scale from 0 to 280 and joint space narrowing on a scale from 0 to 168, thus the total van der Heijde–modified Sharp score ranges from 0 to 448, with higher scores indicating greater joint damage. A good radiographic outcome is commonly defined as no progression.
dDisease Activity Score at the visit before a flare occurred, at the flare visit, and at visits after flare in the half-dose arm in those with all components available to calculate DAS.
Secondary Outcomes
In the primary analysis population, mean (SD) DAS at time of flare was 2.1 (0.8) in the half-dose group and 2.3 (0.5) in the stable group (Figure 3F), corresponding to a state of low disease activity. Of patients with follow-up data available, 9 of 13 (69%) in the half-dose group and 3 of 3 (100%) in the stable-dose group were in DAS remission at the subsequent visit (eTable 2 in Supplement 2). Patients in both groups had an increase in C-reactive protein level and swollen joint count at the time of flare (eFigure in Supplement 2). At 12 months, 63 patients (85%) in the half-dose group and 67 patients (92%) in the stable-dose group were in DAS remission, with a difference between groups of −7% (95% CI, −17% to 4%) (Figure 3C; eTable 3 in Supplement 2). Individual measures of disease activity, composite disease activity scores, and the number of patients in remission according to different criteria were not significantly different in the 2 groups over the 12 months (eTable 3 in Supplement 2). This was also true for functional and ultrasound outcomes (eTable 3 in Supplement 2; Figure 3). The mean (SD) methotrexate dose in users in the half-dose group was 11.7 (4.3) mg/wk after 12 months compared with 19.5 (4.3) mg/wk at baseline, with comparable changes for other csDMARDs (eTable 3 in Supplement 2).
The mean (SD) change in total van der Heijde–modified Sharp score was 0.5 (1.8) units in the half-dose group compared with 0.3 (1.2) units in the stable-dose group (difference at 12 months, 0.2 units [95% CI, −0.3 to 0.6]); eTable 3 in Supplement 2). Fewer patients in the half-dose csDMARD group experienced no radiographic progression (47/75 [63%] vs 58/73 [79%]; risk difference, −18% [95% CI, −33% to −2%]), also illustrated by the separation between groups in the cumulative probability plot (Figure 3E).
Sensitivity Analyses
Similar results for the primary outcome were found when analyses were repeated among participants who had been allocated and had initiated treatment (Figure 2).
Adverse Events
There were 54 adverse events in the half-dose group and 75 in the stable-dose group (Table 2). The most frequently reported adverse events in each group were mild infections such as an upper respiratory tract infection (11 in the half-dose group, 13 in the stable-dose group). Serious adverse events were reported in 4 patients (5%) in the half-dose group (1 patient with supraventricular tachycardia, 1 with an ankle fracture, and 2 with bronchopneumonia) and in 2 patients (3%) in the stable-dose group (1 patient with tubulointerstitial nephritis and 1 with an arthroscopy requiring hospitalization). One patient in the stable-dose group was treated for basal cell carcinoma (eAppendix 2 in Supplement 2). No other malignancies were reported. No deaths occurred, and none of the adverse events led to study discontinuation.
Table 2. Adverse Events From Month 0 to 12.
| csDMARD group, No. | ||
|---|---|---|
| Half dose (n = 78) | Stable dose (n = 78) | |
| Adverse eventsa | ||
| Upper respiratory tract infections | 11 | 13 |
| Pneumonia | 4 | 2 |
| Back pain (including disk herniation) | 3 | 1 |
| Palpitations | 3 | 2 |
| Upper respiratory tract symptoms | 3 | 4 |
| Influenza | 2 | 3 |
| Joint pain | 2 | 3 |
| Dyspepsia | 1 | 3 |
| Nausea | 1 | 3 |
| Tooth infection/inflammation | 0 | 3 |
| Patients with adverse event, No. (%) | ||
| 1 | 20 (25) | 17 (22) |
| ≥2 | 14 (18) | 25 (32) |
| Adverse events | ||
| Seriousb,c | 4 | 2 |
| Total | 54 | 75 |
Abbreviation: csDMARD, conventional synthetic disease-modifying antirheumatic drug.
Adverse events occurring with a frequency of 3 or more in at least 1 of the groups are listed. Additionally, there were 4 events total of orthopedic surgery; 3 events total of fatigue, fracture, and tendinitis; 2 events total of abdominal discomfort, ear feeling clogged, gallstone attack, hand injury, and throat swelling; and 1 event each of abnormal liver function tests, anemia, arthroscopy, blocked tear duct, breast pain, cancer (basal cell carcinoma; eAppendix 3 in Supplement 2), candida esophagitis, carpal tunnel syndrome, cataract extraction, cold feet, contrast media reaction, depression, diverticulitis, erythema migrans, falling down, finger numbness, hallux valgus, hair loss, hematuria, hidradenitis, infected blister of finger, keratoconjunctivitis sicca, leg edema, norovirus, nail tinea, osteoporosis, pyelonephritis, kidney failure, rash on face, rosacea, stiffness, syncope, thorax pain, tubulointerstitial nephritis, unspecified streptococcal infection, unspecified visual disturbance, urinary incontinence surgery, urinary tract infection, and vertigo.
The serious adverse events were 1 case of supraventricular tachycardia, 2 cases of bronchopneumonia, and 1 ankle fracture in the half-dose group; the serious adverse events were 1 case of tubulointerstitial nephritis and 1 arthroscopy with hospitalization in the stable-dose group. The term serious adverse event included any untoward medical occurrence that resulted in death, was immediately life-threatening, required in-patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, was a congenital abnormality or birth defect, or was an important medical event that could jeopardize the patient or could require medical intervention to prevent one of the outcomes listed above.
None of the adverse events led to study discontinuation or death.
Unprespecified Sensitivity Analyses
When assessing the primary outcome among patients using methotrexate monotherapy, similar results were found as for the main analysis (Figure 2).
Post Hoc Outcomes
Analyses of the primary outcome were not substantially changed when sensitivity analyses were performed in patients with a flare who met criteria for presence of active inflammation (eAppendix 3 in Supplement 2). In the half-dose group, 18 of 19 patients (95%) had their DMARD medication adjusted following the flare compared with 2 of 5 patients (40%) in the stable-dose group. Time to first flare was a mean (SD) of 212 (132) days in the half-dose group and 265 (123) days in the stable-dose group.
Discussion
Among patients with RA in remission with csDMARD therapy, treatment with half-dose csDMARDs was not noninferior to stable-dose csDMARDs for the outcome of percentage of patients with disease flares over 12 months. There were significantly more flares in the half-dose group.
The study included patients who were in sustained remission for at least 12 months, had no swollen joints, and fulfilled a set of remission inclusion criteria that included an extensive joint examination. Therefore, the trial participants correspond to patients for whom previous data indicated that csDMARD tapering could be successful.32
The Tapering Strategies in Rheumatoid Arthritis (TARA) trial in 189 patients reported a 33% flare rate over 1 year when tapering csDMARDs; however, these patients were still using a tumor necrosis factor inhibitor.33 A 39% flare rate was observed in the 36 patients in the tapering group of the Reduction of Therapy in Patients With Rheumatoid Arthritis in Ongoing Remission (RETRO) trial, in which both csDMARDs and biological DMARDs were reduced to half dose simultaneously. Compared with the results of the current trial, the RETRO trial showed an increased rate of relapse among those continuing stable therapy (16%), which might reflect less stringent inclusion criteria.34
Current treatment recommendations suggest to consider tapering of csDMARD in patients with RA who are in persistent remission with csDMARD treatment.35 However, the recommendations are based on a relatively low grade of evidence. More research on this topic would be useful to address areas of uncertainty, eg, if it is possible to identify which patients can successfully taper treatment.
Limitations
The study has several limitations. First, the study was open label, with a potential for bias in the assessment of flare rates in the 2 groups. To counteract this, study investigators and study nurses were repeatedly instructed in the importance of capturing flare outcomes in a similar manner in both groups, and every effort was made to evaluate patients contacting a study center with symptoms of disease worsening within a week. Second, it is possible that a slower taper would have changed the study outcome. Third, the results cannot be extrapolated beyond the 12-month follow-up period, and the study did not have adequate statistical power to evaluate the course after flares in the 2 treatment groups. Fourth, at the time of study design, treatment decisions were commonly guided by DAS28. Because this composite score does not include information about the feet, a choice was made to define remission according to the original DAS based on 44 joints in the inclusion criteria and the definition of the primary end point.36 Fifth, most patients included used methotrexate monotherapy, limiting the generalizability to other csDMARD treatment regimens.
Conclusions
Among patients with RA in remission taking csDMARD therapy, treatment with half-dose vs stable-dose csDMARDs did not demonstrate noninferiority for the percentage of patients with disease flares over 12 months, and there were significantly fewer flares in the stable-dose group. These findings do not support treatment with half-dose therapy.
Trial Protocol and Statistical Analysis Plan
List of Investigators
eAppendix 1. Inclusion and Exclusion Criteria
eAppendix 2. Summary Narratives for Malignancies
eAppendix 3. Additional Post Hoc Flare Analyses
eTable 1. Trial Enrollment by Study Site
eTable 2. Response to Reinstated Treatment After Flare
eTable 3. Secondary Outcomes
eFigure. Disease Activity During Flare
eReferences
Data Sharing Statement
References
- 1.Haavardsholm EA, Aga AB, Olsen IC, et al. Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial. BMJ. 2016;354:i4205. doi: 10.1136/bmj.i4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Combe B, Rincheval N, Benessiano J, et al. Five-year favorable outcome of patients with early rheumatoid arthritis in the 2000s: data from the ESPOIR cohort. J Rheumatol. 2013;40(10):1650-1657. doi: 10.3899/jrheum.121515 [DOI] [PubMed] [Google Scholar]
- 3.Aga AB, Lie E, Uhlig T, et al. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000-2010. Ann Rheum Dis. 2015;74(2):381-388. doi: 10.1136/annrheumdis-2013-204020 [DOI] [PubMed] [Google Scholar]
- 4.ten Wolde S, Breedveld FC, Hermans J, et al. Randomised placebo-controlled study of stopping second-line drugs in rheumatoid arthritis. Lancet. 1996;347(8998):347-352. doi: 10.1016/S0140-6736(96)90535-8 [DOI] [PubMed] [Google Scholar]
- 5.Kuijper TM, Luime JJ, de Jong PH, et al. Tapering conventional synthetic DMARDs in patients with early arthritis in sustained remission: 2-year follow-up of the tREACH trial. Ann Rheum Dis. 2016;75(12):2119-2123. doi: 10.1136/annrheumdis-2016-209272 [DOI] [PubMed] [Google Scholar]
- 6.Klarenbeek NB, van der Kooij SM, Güler-Yüksel M, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70(2):315-319. doi: 10.1136/ard.2010.136556 [DOI] [PubMed] [Google Scholar]
- 7.Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med. 2014;371(19):1781-1792. doi: 10.1056/NEJMoa1316133 [DOI] [PubMed] [Google Scholar]
- 8.Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383(9914):321-332. doi: 10.1016/S0140-6736(13)61751-1 [DOI] [PubMed] [Google Scholar]
- 9.van Herwaarden N, van der Maas A, Minten MJ, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ. 2015;350:h1389. doi: 10.1136/bmj.h1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vollenhoven RF, Østergaard M, Leirisalo-Repo M, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis. 2016;75(1):52-58. doi: 10.1136/annrheumdis-2014-205726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580-1588. doi: 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 12.van der Heijde DM, van ’t Hof MA, van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49(11):916-920. doi: 10.1136/ard.49.11.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevoo ML, van Gestel AM, van T Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Remission in a prospective study of patients with rheumatoid arthritis: American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol. 1996;35(11):1101-1105. doi: 10.1093/rheumatology/35.11.1101 [DOI] [PubMed] [Google Scholar]
- 14.Felson DT, Smolen JS, Wells G, et al. ; American College of Rheumatology; European League Against Rheumatism . American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573-586. doi: 10.1002/art.30129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44-48. doi: 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5)(suppl 39):S100-S108. [PubMed] [Google Scholar]
- 17.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42(2):244-257. doi: 10.1093/rheumatology/keg072 [DOI] [PubMed] [Google Scholar]
- 18.Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford). 2004;43(10):1252-1255. doi: 10.1093/rheumatology/keh297 [DOI] [PubMed] [Google Scholar]
- 19.Ritchie DM, Boyle JA, McInnes JM, et al. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968;37(147):393-406. [PubMed] [Google Scholar]
- 20.Fries JF, Cella D, Rose M, Krishnan E, Bruce B. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol. 2009;36(9):2061-2066. doi: 10.3899/jrheum.090358 [DOI] [PubMed] [Google Scholar]
- 21.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. doi: 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 22.Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis. 2011;70(6):935-942. doi: 10.1136/ard.2010.142901 [DOI] [PubMed] [Google Scholar]
- 23.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 24.Hammer HB, Bolton-King P, Bakkeheim V, et al. Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):1995-1998. doi: 10.1136/ard.2011.152926 [DOI] [PubMed] [Google Scholar]
- 25.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 1999;26(3):743-745. [PubMed] [Google Scholar]
- 26.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology: preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):727-735. doi: 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 27.van Gestel AM, Prevoo ML, van ’t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34-40. doi: 10.1002/art.1780390105 [DOI] [PubMed] [Google Scholar]
- 28.US Department of Heath and Human Services; Food and Drug Administration . Guidance for Industry: Clinical Development Programs for Drugs, Devices, and Biological Products for the Treatment of Rheumatoid Arthritis (RA). US Dept of Health and Human Services; 1999. [Google Scholar]
- 29.US Department of Health and Human Services; Food and Drug Administration . Non-inferiority Clinical Trials to Establish Effectiveness: Guidance for Industry. US Dept of Health and Human Services; 2016. [Google Scholar]
- 30.Solomon DH, Glynn RJ, Karlson EW, et al. Adverse effects of low-dose methotrexate: a randomized trial. Ann Intern Med. 2020;172(6):369-380. doi: 10.7326/M19-3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kvien TK, Heiberg MS, Lie E, et al. A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol. 2005;23(5)(suppl 39):S188-S194. [PubMed] [Google Scholar]
- 32.Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75(8):1428-1437. doi: 10.1136/annrheumdis-2016-209201 [DOI] [PubMed] [Google Scholar]
- 33.van Mulligen E, de Jong PHP, Kuijper TM, et al. Gradual tapering TNF inhibitors versus conventional synthetic DMARDs after achieving controlled disease in patients with rheumatoid arthritis: first-year results of the randomised controlled TARA study. Ann Rheum Dis. 2019;78(6):746-753. doi: 10.1136/annrheumdis-2018-214970 [DOI] [PubMed] [Google Scholar]
- 34.Haschka J, Englbrecht M, Hueber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis. 2016;75(1):45-51. doi: 10.1136/annrheumdis-2014-206439 [DOI] [PubMed] [Google Scholar]
- 35.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685-699. doi: 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 36.Landewé R, van der Heijde D, van der Linden S, Boers M. Twenty-eight-joint counts invalidate the DAS28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis. 2006;65(5):637-641. doi: 10.1136/ard.2005.039859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
List of Investigators
eAppendix 1. Inclusion and Exclusion Criteria
eAppendix 2. Summary Narratives for Malignancies
eAppendix 3. Additional Post Hoc Flare Analyses
eTable 1. Trial Enrollment by Study Site
eTable 2. Response to Reinstated Treatment After Flare
eTable 3. Secondary Outcomes
eFigure. Disease Activity During Flare
eReferences
Data Sharing Statement