Summary
Since licensure in 2006, rotavirus vaccines have been introduced in >100 countries. Given variable efficacy by child mortality, assessing real-world effectiveness in a range of settings is important. Observational, post-licensure studies with laboratory-confirmed rotavirus as the endpoint published from 2006-2019 were included. In addition to vaccine product-specific results, we examined mixed series and non-product specific vaccine effectiveness (VE) estimates. We fit random effects regression models to estimate VE among children <12 and 12-23 months old and summarized overall VE using medians. We identified 1,703 articles and included 60 from 32 countries. There were 31, 8, and 21 articles in the low, medium, and high child mortality strata, respectively. Rotarix VE against laboratory confirmed rotavirus among children <12 months old was 86% (95%CI: 81, 90), 77% (95%CI: 66, 85), and 63% (95%CI: 54, 70) in the low, medium, and high mortality strata, respectively. VE among children 12-23 months ranged from 87-54%. RotaTeq VE among children <12 months old was 86% (95%CI: 76, 92) and 66% (95%CI: 51, 76) in the low and high strata, respectively. RotaTeq VE was 84% (95%CI: 79, 89) among children 12-23 months in the low stratum. There was no substantial heterogeneity (I2 range: 0-36%). Median VEs in the low stratum were similar between Rotarix (83%; IQR: 78, 91), RotaTeq (85%; IQR: 81, 92), mixed series (86%; IQR: 70, 91), and non-product specific (89%; IQR: 75, 91). Rotavirus vaccines were effective in preventing rotavirus diarrhea, with a gradient in performance by child mortality.
Funding
No external funding was provided.
Keywords: Rotavirus, rotavirus vaccine, vaccine effectiveness, literature review
Introduction
Rotavirus is the leading cause of pediatric diarrhea deaths worldwide 1. In 2009, the World Health Organization (WHO) recommended all countries include a live oral rotavirus vaccine in their routine infant immunization programs and more than 100 countries have introduced rotavirus vaccines to date 2,3. Countries using rotavirus vaccine have seen a 40% decrease in the proportion of hospital admissions due to rotavirus among children <5 years old and globally there has been a 25% decrease in annual rotavirus diarrhea deaths 1,4,5. However, the magnitude of these reductions varies by socioeconomic status. Clinical trials and previous summaries of the published data have documented a gradient in Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium) and RotaTeq (Merck & Co., West Point, PA, USA) vaccine performance by countries’ child mortality level, with high efficacy and effectiveness in settings with low child mortality and more modest performance in settings with high child mortality 6-13.
Given the variable efficacy and impact of rotavirus vaccines in different child mortality levels, examining post-licensure effectiveness in a range of settings is important. This review of the published literature summarizes real-world effectiveness of rotavirus vaccines against rotavirus hospitalizations among children <5 years old and is an update of an earlier review 6. Since that previous compilation of rotavirus vaccine effectiveness data published in 2017, >20 countries have introduced a rotavirus vaccine and 17 additional articles estimating vaccine effectiveness have been published. In particular, there are 7 new publications from countries with high child mortality, where performance of rotavirus vaccines has been most variable. This review also presents summary vaccine effectiveness (VE) estimates by age group and relative differences in VE between complete number of doses received and partial series.
Methods
Search and inclusion criteria
For this literature review and meta-analysis, we searched PubMed using the terms “rotavirus” and “vacc*” and “eff*” for articles published January 1, 2006 to December 31, 2019 in countries where Rotarix or RotaTeq are routinely administered. Other rotavirus vaccines were excluded because of a lack of available post-licensure data. The review process is described in detail in Figure 1. Original data from observational, post-licensure studies published in English were included in this analysis. Articles that did not have a healthcare system contact (inpatient, emergency department (ED) or outpatient visit) due to laboratory confirmed rotavirus diarrhea as their endpoint, used an alternative design (i.e. “screening method”), or only reported “any dose” estimates were excluded. In addition to articles with results for one vaccine product, we included articles that reported 3-dose Rotarix and RotaTeq mixed series and non-product specific VE estimates from countries where Rotarix and RotaTeq are both available. If more than one article presented results from the same dataset, all articles that included unique datapoints by years, vaccine, age group, or number of doses were included. In situations where findings in one article were a subset of findings from another article (e.g. years of enrollment or study sites) only the most comprehensive article was included. Some articles presented stratified VE estimates for multiple clinical setting endpoints or from multiple control groups. We included one VE from an article per analysis; inpatient and ED cases and rotavirus negative controls were prioritized as these were the most common and optimized sample size.
Figure 1.
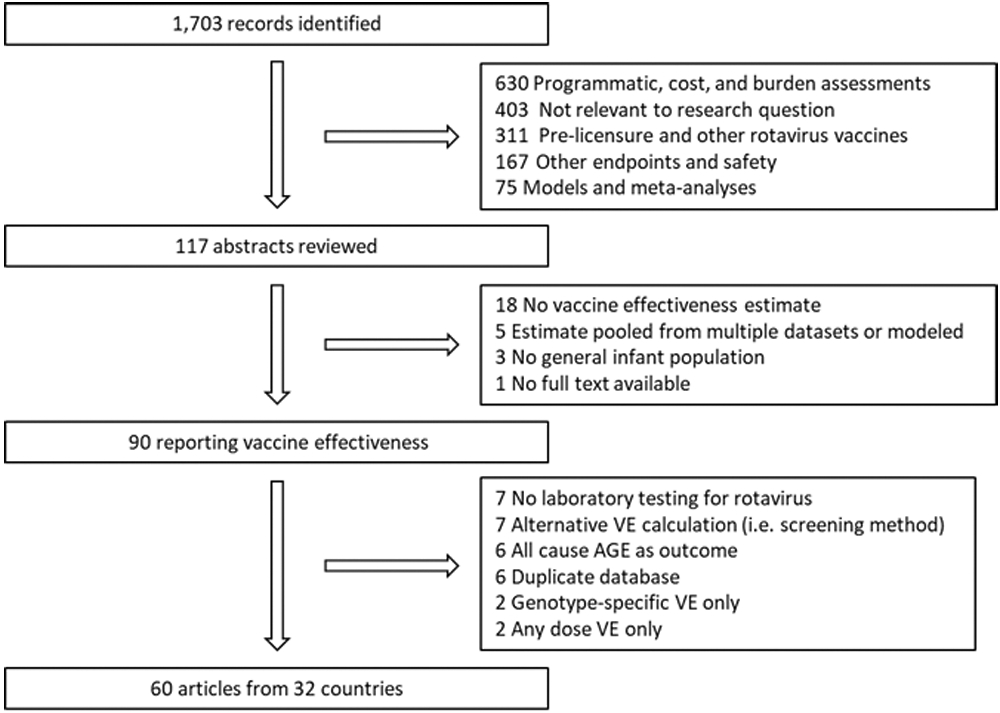
Literature review results and inclusion criteria.
We abstracted overall VE; stratified VE estimates by age group and number of doses; attributes of the studies, such as study setting, control group, enrollment criteria, and sample size; and quality measures, such as vaccination card capture and stool collection rate, into an EpiInfo database.
Analytic methods
Descriptive statistics and percentages were calculated for the article attributes. Countries were categorized into 3 strata, based on their 2017 <5 year old mortality estimates from Unicef: lowest quartile (“low”); 2nd quartile (“medium”); and the 3rd and 4th quartiles (“high”); Taiwan and Hong Kong were categorized as low mortality based on governmental data 14,15. All analyses are stratified by these child mortality categories. We fit random effects regression models to estimate the odds ratio of a hospitalization due to laboratory-confirmed rotavirus positive diarrhea of Rotarix and RotaTeq among children <12 months old and 12-23 months old; VE was calculated from the OR with the formula (1 - OR) * 100. Heterogeneity was assessed with the Q and I2 statistics. Setting and control group were considered as potential covariates for meta-regression analyses. Additionally, we calculated medians and interquartile ranges (IQR) to summarize the overall VE by vaccine (Rotarix, RotaTeq, mixed series, and non-product specific) and relative differences in VE between the full number of recommended doses in a vaccination series and partial vaccination. We defined partially complete vaccination series as 1 dose of either Rotarix or RotaTeq or 2 doses of RotaTeq and compared with the VE of the manufacturers’ recommended 2 doses of Rotarix and 3 doses of RotaTeq 8,16. Publication bias was assessed using funnel plots of the standard error, sampling variance, and the inverse of the standard error and sampling variance. Analyses were performed with SAS v9.4 and R v3.6.1 using the metafor package 17. There was no external funding for this study.
Results
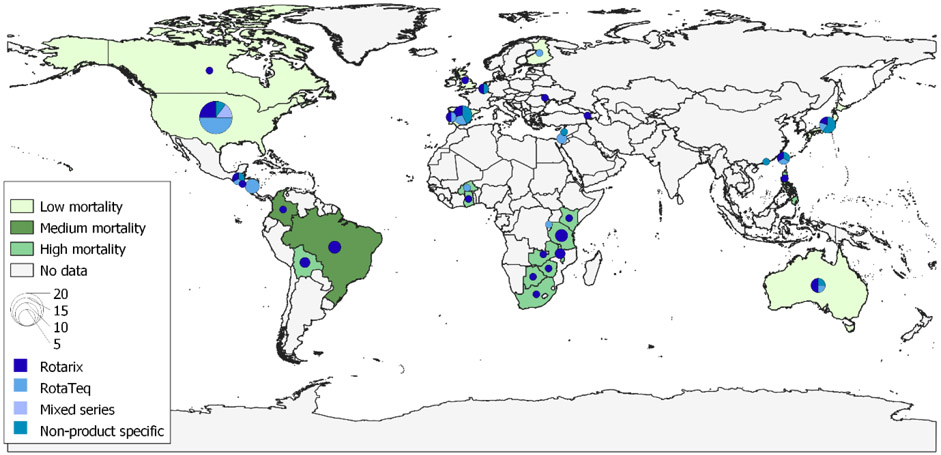
For this literature review, we identified and screened 1,703 articles; 1,586 were not relevant to the research question based on title and were excluded (Figure 1). Abstracts were reviewed for the remaining 117 articles. Ninety articles were read in full; an additional 24 were excluded because they did not meet our methodological inclusion criteria and 6 presented findings from datasets duplicated by other articles. In total, 60 articles were included from 32 countries; countries represented and corresponding child mortality strata are presented in Figure 2. There were 31 articles from low mortality stratum countries, 8 from the medium stratum, and 21 from the high stratum (Table 1). No publication bias was detected (data not shown).
Figure 2.
Countries (n=32) with available VE data using different rotavirus vaccine products by different mortality levels, 2006-2019
Table 1.
Characteristics of articles that present rotavirus vaccine effectiveness against laboratory confirmed rotavirus using a case-control methodology.
| Child mortality strata | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n=60) | Low (n=31) | Medium (n=8) | High (n=21) | |||||
| n | % | n | % | n | % | n | % | |
| Year of publication- median (IQR) | 2016 | (2013, 2017) | 2015 | (2012, 2017) | 2014 | (2011, 2016) | 2016 | (2015, 2017) |
| Vaccine | ||||||||
| Rotarix | 37 | 62 | 15 | 48 | 7 | 88 | 15 | 71 |
| RotaTeq | 26 | 43 | 19 | 61 | 0 | 0 | 7 | 33 |
| Rotarix or RotaTeq | 14 | 23 | 12 | 39 | 1 | 13 | 0 | 0 |
| Mixed series (Rotarix and RotaTeq) | 3 | 5 | 3 | 10 | 0 | 0 | 0 | 0 |
| Setting | ||||||||
| Inpatient alone | 35 | 58 | 17 | 55 | 7 | 88 | 11 | 52 |
| ED alone or inpatient and ED | 28 | 47 | 12 | 39 | 3 | 38 | 13 | 62 |
| Any setting (including outpatient) | 12 | 20 | 11 | 35 | 0 | 0 | 1 | 5 |
| Control group | ||||||||
| Rotavirus negative | 48 | 80 | 24 | 77 | 5 | 63 | 19 | 90 |
| Non-diarrhea | 12 | 20 | 5 | 16 | 3 | 38 | 4 | 19 |
| Community | 12 | 20 | 7 | 23 | 2 | 25 | 3 | 14 |
| Subanalyses | ||||||||
| Age group | 34 | 57 | 13 | 42 | 6 | 75 | 15 | 71 |
| Incomplete series | 25 | 42 | 12 | 39 | 3 | 38 | 10 | 48 |
The median year of publication was 2016 (IQR: 2013, 2017), ranging by child mortality strata from 2014 in the medium stratum to 2016 in the high stratum (Table 1). Most articles presented Rotarix and/or RotaTeq-specific VE estimates, however 14 articles (23%) presented non-product specific results and 3 (5%) presented results for a mixed series. There was variation by mortality strata in the case enrollment setting. The majority of articles in all mortality strata used rotavirus-negative controls (80% overall).
In studies of Rotarix, the median overall VE against laboratory confirmed rotavirus diarrhea in any <5 year old age group was 83% (IQR: 78, 91) in low mortality stratum countries, 67% (IQR: 46, 75) in medium mortality stratum countries, and 58% (IQR: 55, 63) in high mortality stratum countries (Figure 3). In studies of RotaTeq, the median overall VE was 85% (IQR: 81, 92) in low mortality stratum countries and 45% (IQR: 44, 57) in high mortality stratum countries. The median overall VE was similar between Rotarix, RotaTeq, mixed series (86%; IQR: 70, 91), and non-product specific (89%; IQR: 75, 91) in low mortality stratum countries.
Figure 3.
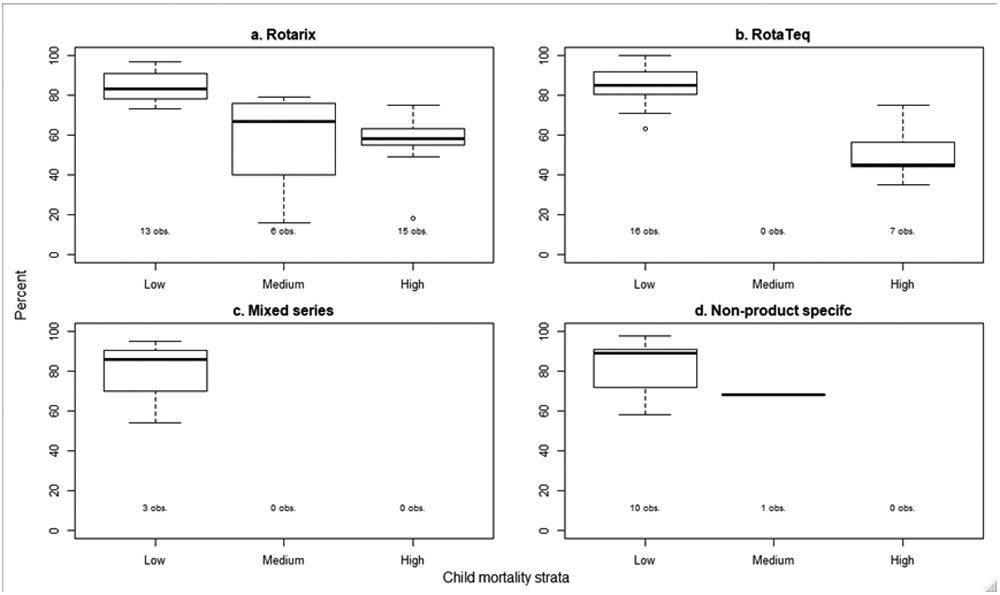
Median and interquartile range of published rotavirus vaccine effectiveness estimates against laboratory confirmed rotavirus by vaccine type and child mortality strata.
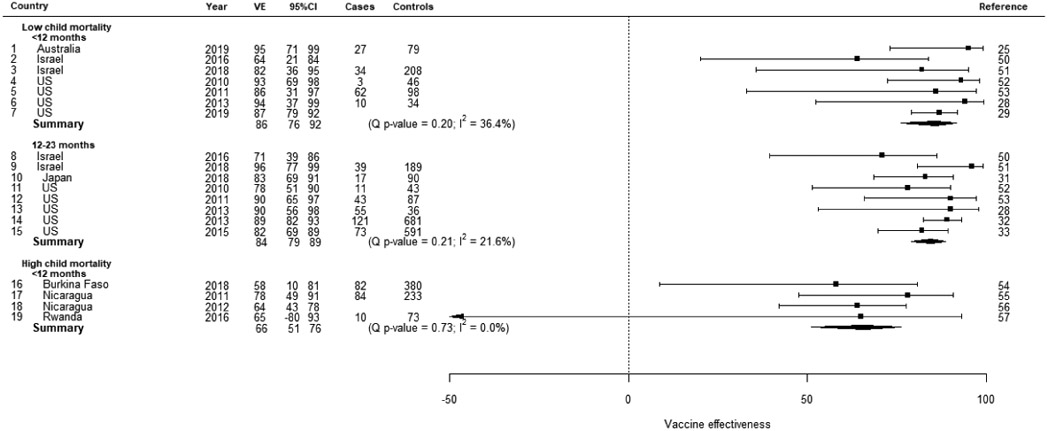
In low mortality stratum countries using Rotarix vaccine, the summary VE against laboratory confirmed rotavirus diarrhea estimated by the random effects model was 86% (95%CI: 81, 90) from 6 studies of children <12 months old and 87% (95%CI: 80, 91) from 4 studies of children 12-23 months old (Table 2a). In medium mortality stratum countries, the summary VE was 77% (95%CI: 66, 85) from 6 studies of children <12 months old and 54% (95%CI: 23, 73) from 2 studies of children 12-23 months old. In high mortality stratum countries, the summary VE was 63% (95%CI: 54, 70) from 10 studies of children <12 months old and 58% (95%CI: 38, 72) from 5 studies of children 12-23 months old. In low mortality stratum countries using RotaTeq vaccine, the summary VE estimated by the random effects model was 86% (95%CI: 76, 92) from 7 studies of children <12 months old and 84% (95%CI: 79, 89) from 8 studies of children 12-23 months old (Table 2b). In high mortality stratum countries, the summary VE was 66% (95%CI: 51, 76) from 4 studies of children <12 months old. There was not substantial heterogeneity in any of the random effects models (I2 range: 0-36%); case patient enrollment setting and control group did not explain the remaining heterogeneity.
Table 2a.
Rotarix vaccine effectiveness against laboratory confirmed rotavirus random effects model results by child mortality strata, and age group.
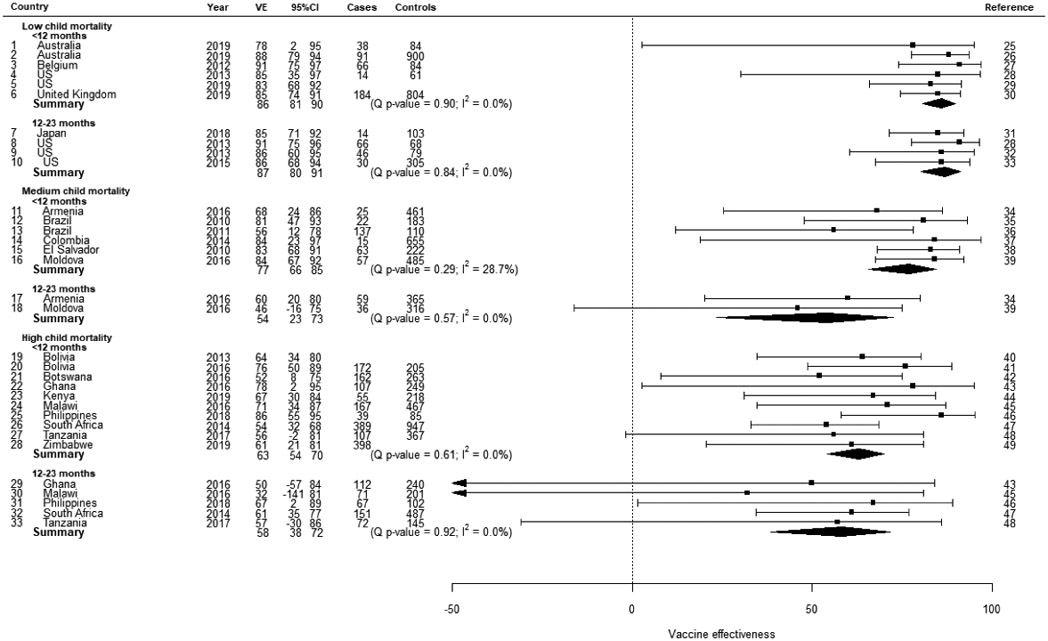 |
Table 2b.
RotaTeq vaccine effectiveness against laboratory confirmed rotavirus random effects model results child mortality strata, and age group.
 |
The median relative difference between the VE against laboratory confirmed rotavirus diarrhea for 1 dose of RotaTeq and 3 doses of RotaTeq was 17% (IQR: 15, 33) and 2% (IQR: −3, 8) between 2 and 3 doses in the low mortality strata (Figure 4). The median relative difference between the VE against laboratory confirmed rotavirus diarrhea for 1 and 2 doses of Rotarix was 10% (IQR: −1, 15) in the low morality strata, 24% (IQR: 20, 28) in the medium mortality strata, and 30% (IQR: 13, 42) in the high mortality strata.
Figure 4.
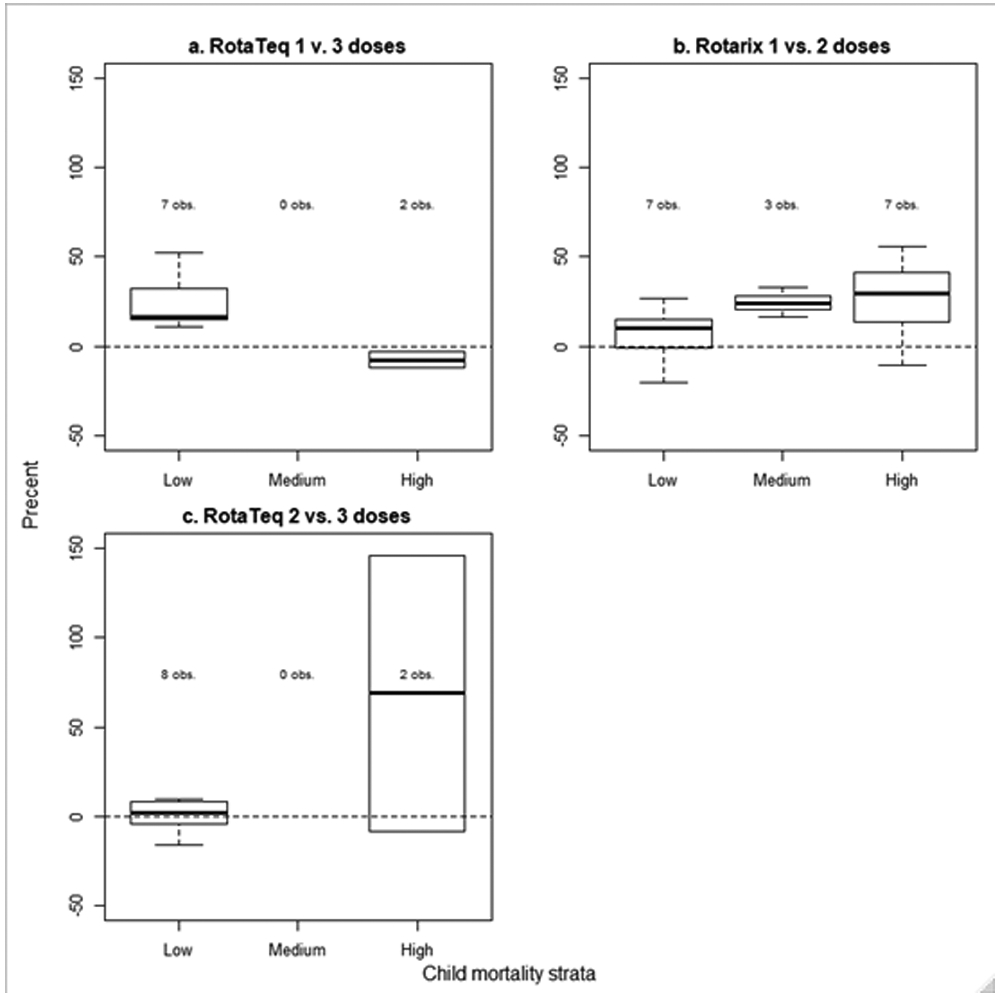
Relative difference between partial series and full series VE against laboratory confirmed rotavirus by number of doses, vaccine product, and child mortality strata.
Discussion
In this review of 60 articles evaluating rotavirus vaccine effectiveness under conditions of routine use from 32 countries across a range of child mortality levels, we found Rotarix and RotaTeq are both effective in preventing laboratory confirmed rotavirus diarrhea. Similar to previous assessments of rotavirus vaccine efficacy, effectiveness, and impact 6-13, we found a gradient in vaccine performance with higher effectiveness in settings with lower child mortality. This gradient was consistent when stratified by age group and in the overall analysis, where the age distribution of cases and controls is unknown. Reasons for difference in performance between mortality strata are unknown, however concomitant administration with live oral polio vaccine, nutritional status, microbiome composition, and maternal antibodies have been suggested as possible explanations for the performance gradient and offer potential strategies to improve modest vaccine performance 18.
Previous evaluations have also suggested a decline in VE between the first and second years of life, particularly in countries with medium and high child mortality 6,7,13,19. Point estimates from our findings may indicate lower protection against rotavirus hospitalizations among children 12-23 months old compared with those <12 months old in the medium and high mortality strata, however the existing data are sparse in the older age group and confidence intervals are wide. There does not appear to be a difference in VE between these 2 age groups in the low mortality stratum. The reduced effectiveness in the second year might represent a true waning of vaccine induced protective immunity over time. Evidence from 2 randomized control trials conducted in Bangladesh and Mali demonstrated a booster dose of Rotarix at 9 months of age increased the immune response 20,21. Alternatively, immunity from natural infection in unvaccinated children and vaccine-induced immunity may converge, lowering the calculated VE against severe rotavirus diarrhea in medium and high mortality settings, even though protection from vaccination is retained in the second year of life 22.
The VE against laboratory confirmed rotavirus diarrhea for a completed series was higher than the VE with 1 dose of Rotarix or RotaTeq. Comparing 1 and 2 doses of Rotarix, the median relative difference increased from the low mortality strata to the medium and high mortality stratum; that is, the relative increase in protection with a second dose is inverse to the gradient in overall vaccine performance. With lower overall VE, there is more room for boosting in these settings and a relative increase would be higher with a lower baseline VE, even if the absolute increases are equal. Nonetheless, this finding supports the importance of timely and complete vaccination, particularly in countries where the burden of rotavirus hospitalizations is highest in very young infants and in countries with medium and high child mortality. There was also a positive relative difference between 1 and 3 doses of RotaTeq in the low mortality stratum. As would be expected, the boost in protection from 1 to 3 doses was greater than 2 to 3 doses of RotaTeq. Data for a partial series of RotaTeq in the other mortality strata are limited.
Complete vaccination, either with a single vaccine product or with a mixed vaccine series, in the low mortality stratum demonstrated similar VE estimates. Protection against rotavirus diarrhea provided by mixed vaccine series, and attendant guidance for policymakers and vaccinators, is increasingly relevant as two new Indian-made oral rotavirus vaccines, Rotasiil and ROTAVAC, are being widely used in India and have recently been pre-qualified for global use. Countries that use multiple vaccine products concurrently, or switch from Rotarix or RotaTeq to one of the Indian-made vaccines, desire data on the effectiveness of mixed vaccination series that may be administered in such situations 23,24. Furthermore, as these situations are now arising regularly in medium and high mortality settings, future evaluations should include mixed series VE estimates where possible as such data are still sparse at the global level.
This literature review and meta-analysis have several limitations. Evidence from countries in the medium mortality strata is limited, as countries in that category often do not qualify for Gavi financial support and have lagged behind low and high mortality strata countries in adopting rotavirus vaccines. Second, new data on VE by Vesikari score is insufficient to update the analysis of VE by severity in the previous iteration. Finally, we did not assess the quality of individual studies. Only 58% of articles reported the percentage of enrolled children who had a vaccine card or other documentation of their vaccination status reviewed and we are unable to comment on the percentage of eligible children in each study who provided a stool specimen because of inconsistencies in measurement and reporting. These data were inadequate to assess bias within studies.
In summary, we found both Rotarix and RotaTeq are effective in preventing hospitalizations due to rotavirus diarrhea. Like previous assessments, these results showed a gradient in VE between child mortality strata and between the first and second years of life, particularly in medium and high mortality settings. These results also showed mixed series protection was similar to that provided by a single vaccine product. As countries globally begin adopting the newly pre-qualified Indian-made rotavirus vaccines, evaluating their effectiveness in routine programmatic use is a high priority as available evidence is limited to relatively small clinical trials primarily conducted in India.
Panel: Research in context.
Evidence before this study
Live, oral rotavirus vaccines are effective in preventing hospitalizations due to rotavirus diarrhea in infants and young children globally. Clinical trial and observation studies of rotavirus vaccine performance have documented a gradient in efficacy, effectiveness, and impact by countries’ child mortality rates.
Added value of this study
This study updates an earlier literature review and meta-analysis of worldwide rotavirus vaccine performance with 17 new articles. It also includes summaries of new data points, such as mixed vaccine product series effectiveness.
Implications of all the available evidence
Full series vaccination with rotavirus vaccines is effective in preventing laboratory confirmed rotavirus diarrhea globally.
Acknowledgments
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest: The authors indicate that they have no conflicts of interest relevant to this article to disclose.
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance N. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000-2013. Clin Infect Dis 2016; 62 Suppl 2: S96–S105. [DOI] [PubMed] [Google Scholar]
- 2.Vaccine in National Immunization Programme Update. In: Immunization VaB, editor. https://www.who.int/immunization/monitoring_surveillance/en/: World Health Organization; 2020. [Google Scholar]
- 3.Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec 2013; 88(5): 49–64. [PubMed] [Google Scholar]
- 4.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea. J Infect Dis 2017; 215(11): 1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aliabadi N, Antoni S, Mwenda JM, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health 2019; 7(7): e893–e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of Rotavirus Vaccination: A Systematic Review of the First Decade of Global Postlicensure Data, 2006-2016. Clin Infect Dis 2017; 65(5): 840–50. [DOI] [PubMed] [Google Scholar]
- 7.Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2019; (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354(1): 11–22. [DOI] [PubMed] [Google Scholar]
- 9.Breiman RF, Zaman K, Armah G, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 2012; 30 Suppl 1: A24–9. [DOI] [PubMed] [Google Scholar]
- 10.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376(9741): 615–23. [DOI] [PubMed] [Google Scholar]
- 11.Cunliffe NA, Witte D, Ngwira BM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine 2012; 30 Suppl 1: A36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhi SA, Kirsten M, Louw C, et al. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine 2012; 30 Suppl 1: A44–51. [DOI] [PubMed] [Google Scholar]
- 13.Clark A, van Zandvoort K, Flasche S, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis 2019; 19(7): 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnett E, Tate JE, Kirkwood CD, et al. Estimated impact of rotavirus vaccine on hospitalizations and deaths from rotavirus diarrhea among children <5 in Asia. Expert Rev Vaccines 2018; 17(5): 453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO-UNICEF estimates of rotavirus vaccine coverage. http://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html 2018.
- 16.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354(1): 23–33. [DOI] [PubMed] [Google Scholar]
- 17.Viechtbauer W Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010; 36(3): 1–48. [Google Scholar]
- 18.Velasquez DE, Parashar U, Jiang B Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors Expert Rev Vaccines, 17 (2018), pp. 145–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira LH, Camacho LA, Coutinho ES, Ruiz-Matus C, Leite JP. Rotavirus vaccine effectiveness in Latin American and Caribbean countries: A systematic review and meta-analysis. Vaccine 2015; 33 Suppl 1: A248–54. [DOI] [PubMed] [Google Scholar]
- 20.Zaman K, Fleming JA, Victor JC, et al. Noninterference of Rotavirus Vaccine With Measles-Rubella Vaccine at 9 Months of Age and Improvements in Antirotavirus Immunity: A Randomized Trial. J Infect Dis 2016; 213(11): 1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haidara FC, Tapia MD, Sow SO, et al. Evaluation of a Booster Dose of Pentavalent Rotavirus Vaccine Coadministered With Measles, Yellow Fever, and Meningitis A Vaccines in 9-Month-Old Malian Infants. J Infect Dis 2018; 218(4): 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogawski ET, Platts-Mills JA, Colgate ER, et al. Quantifying the Impact of Natural Immunity on Rotavirus Vaccine Efficacy Estimates: A Clinical Trial in Dhaka, Bangladesh (PROVIDE) and a Simulation Study. J Infect Dis 2018; 217(6): 861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis 2019; 32(5): 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavi. How our support works. 2020. (accessed 10 February 2020.
- 25.Fathima P, Snelling TL, Gibbs RA. Effectiveness of rotavirus vaccines in an Australian population: A case-control study. Vaccine 2019; 37(41): 6048–53. [DOI] [PubMed] [Google Scholar]
- 26.Maguire JE, Glasgow K, Glass K, et al. Rotavirus Epidemiology and Monovalent Rotavirus Vaccine Effectiveness in Australia: 2010-2017. Pediatrics 2019. [DOI] [PubMed] [Google Scholar]
- 27.Braeckman T, Van Herck K, Meyer N, et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 2012; 345: e4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 2013; 132(1): e25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne DC, Englund JA, Weinberg GA, et al. Association of Rotavirus Vaccination With Inpatient and Emergency Department Visits Among Children Seeking Care for Acute Gastroenteritis, 2010-2016. JAMA Netw Open 2019; 2(9): e1912242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker JL, Andrews NJ, Atchison CJ, et al. Effectiveness of oral rotavirus vaccination in England against rotavirus-confirmed and all-cause acute gastroenteritis. Vaccine X 2019; 1: 100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki K, Hara M, Tsugawa T, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in Japanese children. Vaccine 2018; 36(34): 5187–93. [DOI] [PubMed] [Google Scholar]
- 32.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009-2011. Clin Infect Dis 2013; 57(1): 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne DC, Selvarangan R, Azimi PH, et al. Long-term Consistency in Rotavirus Vaccine Protection: RV5 and RV1 Vaccine Effectiveness in US Children, 2012-2013. Clin Infect Dis 2015; 61(12): 1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahakyan G, Grigoryan S, Wasley A, et al. Impact and Effectiveness of Monovalent Rotavirus Vaccine in Armenian Children. Clin Infect Dis 2016; 62 Suppl 2: S147–54. [DOI] [PubMed] [Google Scholar]
- 35.Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis 2010; 201(3): 363–9. [DOI] [PubMed] [Google Scholar]
- 36.Justino MC, Linhares AC, Lanzieri TM, et al. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belem, Brazil. Pediatr Infect Dis J 2011; 30(5): 396–401. [DOI] [PubMed] [Google Scholar]
- 37.Cotes-Cantillo K, Paternina-Caicedo A, Coronell-Rodriguez W, et al. Effectiveness of the monovalent rotavirus vaccine in Colombia: a case-control study. Vaccine 2014; 32(25): 3035–40. [DOI] [PubMed] [Google Scholar]
- 38.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 340: c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gheorghita S, Birca L, Donos A, et al. Impact of Rotavirus Vaccine Introduction and Vaccine Effectiveness in the Republic of Moldova. Clin Infect Dis 2016; 62 Suppl 2: S140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 2013; 346: f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pringle KD, Patzi M, Tate JE, et al. Sustained Effectiveness of Rotavirus Vaccine Against Very Severe Rotavirus Disease Through the Second Year of Life, Bolivia 2013-2014. Clin Infect Dis 2016; 62 Suppl 2: S115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gastanaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of Monovalent Rotavirus Vaccine After Programmatic Implementation in Botswana: A Multisite Prospective Case-Control Study. Clin Infect Dis 2016; 62 Suppl 2: S161–7. [DOI] [PubMed] [Google Scholar]
- 43.Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and Effectiveness of Monovalent Rotavirus Vaccine Against Severe Rotavirus Diarrhea in Ghana. Clin Infect Dis 2016; 62 Suppl 2: S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khagayi S, Omore R, Otieno GP, et al. Effectiveness of monovalent rotavirus vaccine against hospitalization with acute rotavirus gastroenteritis in Kenyan children. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Zeev N, Jere KC, Bennett A, et al. Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years After Vaccine Introduction: Ecological and Case-Control Analyses. Clin Infect Dis 2016; 62 Suppl 2: S213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez AL, Daag JV, Esparagoza J, et al. Effectiveness of monovalent rotavirus vaccine in the Philippines. Sci Rep 2018; 8(1): 14291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis 2014; 14(11): 1096–104. [DOI] [PubMed] [Google Scholar]
- 48.Abeid KA, Jani B, Cortese MM, et al. Monovalent Rotavirus Vaccine Effectiveness and Impact on Rotavirus Hospitalizations in Zanzibar, Tanzania: Data From the First 3 Years After Introduction. J Infect Dis 2017; 215(2): 183–91. [DOI] [PubMed] [Google Scholar]
- 49.Mujuru HA, Burnett E, Nathoo KJ, et al. Monovalent Rotavirus Vaccine Effectiveness Against Rotavirus Hospitalizations Among Children in Zimbabwe. Clin Infect Dis 2019; 69(8): 1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leshem E, Givon-Lavi N, Tate JE, Greenberg D, Parashar UD, Dagan R. Real-World Effectiveness of Pentavalent Rotavirus Vaccine Among Bedouin and Jewish Children in Southern Israel. Clin Infect Dis 2016; 62 Suppl 2: S155–60. [DOI] [PubMed] [Google Scholar]
- 51.Muhsen K, Anis E, Rubinstein U, et al. Effectiveness of rotavirus pentavalent vaccine under a universal immunization programme in Israel, 2011-2015: a case-control study. Clin Microbiol Infect 2018; 24(1): 53–9. [DOI] [PubMed] [Google Scholar]
- 52.Boom JA, Tate JE, Sahni LC, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J 2010; 29(12): 1133–5. [DOI] [PubMed] [Google Scholar]
- 53.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 2011; 128(2): e267–75. [DOI] [PubMed] [Google Scholar]
- 54.Bonkoungou IJO, Aliabadi N, Leshem E, et al. Impact and effectiveness of pentavalent rotavirus vaccine in children <5years of age in Burkina Faso. Vaccine 2018; 36(47): 7170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mast TC, Khawaja S, Espinoza F, et al. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediatr Infect Dis J 2011; 30(11): e209–15. [DOI] [PubMed] [Google Scholar]
- 56.Patel M, Pedreira C, De Oliveira LH, et al. Duration of protection of pentavalent rotavirus vaccination in Nicaragua. Pediatrics 2012; 130(2): e365–72. [DOI] [PubMed] [Google Scholar]
- 57.Tate JE, Ngabo F, Donnen P, et al. Effectiveness of Pentavalent Rotavirus Vaccine Under Conditions of Routine Use in Rwanda. Clin Infect Dis 2016; 62 Suppl 2: S208–12. [DOI] [PubMed] [Google Scholar]