Abstract
Objective
To provide a comprehensive description of demographic, clinical and radiographic characteristics; treatment and case outcomes; and risk factors associated with in-hospital death of patients hospitalised with COVID-19 in Brazil.
Design
Retrospective cohort study of hospitalised patients diagnosed with COVID-19.
Setting
Data from all hospitals across Brazil.
Participants
522 167 hospitalised patients in Brazil by 14 December 2020 with severe acute respiratory illness, and a confirmed diagnosis for COVID-19.
Primary and secondary outcome measures
Prevalence of symptoms and comorbidities was compared by clinical outcomes and intensive care unit (ICU) admission status. Survival was assessed using Kaplan Meier survival estimates. Risk factors associated with in-hospital death were evaluated with multivariable Cox proportional hazards regression.
Results
Of the 522 167 patients included in this study, 56.7% were discharged, 0.002% died of other causes, 30.7% died of causes associated with COVID-19 and 10.2% remained hospitalised. The median age of patients was 61 years (IQR, 47–73), and of non-survivors 71 years (IQR, 60–80); 292 570 patients (56.0%) were men. At least one comorbidity was present in 64.5% of patients and in 76.8% of non-survivors. From illness onset, the median times to hospital and ICU admission were 6 days (IQR, 3–9) and 7 days (IQR, 3–10), respectively; 15 days (IQR, 9–24) to death and 15 days (IQR, 11–20) to hospital discharge. Risk factors for in-hospital death included old age, Black/Brown ethnoracial self-classification, ICU admission, being male, living in the North and Northeast regions and various comorbidities. Age had the highest HRs of 5.51 (95% CI: 4.91 to 6.18) for patients≥80, compared with those ≤20.
Conclusions
Characteristics of patients and risk factors for in-hospital mortality highlight inequities of COVID-19 outcomes in Brazil. As the pandemic continues to unfold, targeted policies that address those inequities are needed to mitigate the unequal burden of COVID-19.
Keywords: public health, COVID-19, public health
Strengths and limitations of this study.
The strength of this study is that it leverages Brazil’s established national Influenza Epidemiological Surveillance Information System data to present comprehensive characteristics, clinical course and risk factors for COVID-19 in-hospital deaths across Brazil.
Administrative records lack details available in hospital medical records and may have accuracy and completeness problems.
We did not have access to laboratory results other than COVID-19 tests (eg, complete blood count) that would allow for a better characterisation of the clinical course of the disease.
COVID-19 deaths at home likely follow a different clinical course than deaths in the hospital and are not included in this analysis.
In-hospital deaths due to COVID-19 are likely under-reported and are limited by the testing protocol and capacity of each hospital.
Introduction
On 11 March 2020, the WHO declared COVID-19 as a pandemic. Caused by the novel coronavirus SARS-CoV-2, it emerged in China and quickly spread across the country and beyond. As of 16 February 2021, it was present in 223 countries and territories, with 108 822 960 confirmed cases and 2 403 641 confirmed deaths.1 Brazil recorded the first confirmed COVID-19 case on 26 February 2020 and the first death on 12 March, both in São Paulo State. In 24 days, the disease had spread to all Federal Units. As of 16 February 2021, 9 834 513 cases (9% of worldwide cases) and 239 245 deaths (10% of worldwide deaths) had been reported in Brazil, the second-highest in the world, behind only the USA. These numbers are underestimated since most mild cases are not being tested and thus are not likely to be reported, and some deaths may be reported with ill-defined causes, or not reported at all.
Brazil has a comprehensive health information system,2 with the systematic collection of births, deaths, hospitalisations and diseases of mandatory notification, among others. However, a complete and linked registry of records combining data from ambulatory and inpatient care, laboratory and radiologic results and outcome of the disease is not available. Therefore, there is limited information on the course of the disease for every case reported in Brazil.
Currently, the most detailed data available in Brazil refer to hospitalisations due to severe acute respiratory illness (SARI). Here, we use these data to provide a comprehensive description of demographic, clinical and radiographic characteristics, treatment, case outcome and risk factors associated with in-hospital death of patients hospitalised with SARI with a confirmed diagnosis for COVID-19, as of 14 December 2020. We analyse the largest retrospective number of cases (n=522 167) and we assess whether the Brazilian case is comparable to patterns previously described for other countries.
Methods
Data sources
We used deidentified records from the Influenza Epidemiological Surveillance Information System (Sistema de Informação de Vigilância Epidemiológica da Gripe, SIVEP-Gripe, in Portuguese), an information system of the Ministry of Health that captures all notifications of SARI hospitalisations in both public and private hospitals. The system is updated daily, and every 2 weeks, a new data set is made publicly available (https://opendatasus.saude.gov.br/nl/dataset). Here, we analysed records as of 14 December 2020 (n=1 029 684 notifications), after 15 419 duplicate records were removed by the Ministry of Health. Each record has data on patient’s age, sex, place of residence and of hospitalisation, ethnoracial self-classification,3 pregnancy status, comorbidities and symptoms; drug treatment; radiologic test results; and dates of illness onset, hospitalisation, ICU admission and outcome (death, release, still hospitalised). We considered only records of patients hospitalised with a confirmed diagnosis for COVID-19 (n=522 167). Diagnosis followed the Ministry of Health guidelines.4
Statistical analysis
Characteristics of inpatients were summarised in three groups: demographic, clinical and radiographic and treatment and outcomes. Medians and IQR ranges were used to describe continuous variables, and counts and percentages to describe categorical variables. Differences between inpatients that needed and did not need ICU admission and those that survived and did not survive were assessed by Whitney U, χ2 or Fisher’s exact test, as appropriate. No data imputation was performed for missing data (see online supplemental table 1 for information on data completeness).
bmjopen-2021-049089supp001.pdf (2.1MB, pdf)
Survival curves of inpatients at 60 days of hospitalisation by age, sex, ethnoracial self-classification, region and ICU admission were estimated using the Kaplan-Meier estimator and compared with the log-rank test. Factors associated with inpatient death were identified by univariable and multivariable logistic regression (excluding from the analysis those that remained hospitalised). Considering time to death as the outcome, HRs were estimated using Cox proportional-hazards models. Based on previous studies5–7 and on our available information, covariates included in both logistic and Cox models were age (0–19, 20–39, 40–59, 60–69, 70–79 and 80 or more years), sex, ethnoracial self-classification (White, Black/Brown, other, not reported), region (North—where Amazonia is located, Northeast, South, Southeast—where the cities of São Paulo and Rio de Janeiro are located and Center-West), comorbidities (diabetes, asthma, chronic liver disease, chronic neurological disease, chronic lung disease, immunodeficiency and chronic kidney disease), obesity and ICU admission. The variable ethnoracial self-classification was missing in 23.1% of the records, and we added those as a separate category (not reported). Distances between municipalities of residence and hospitalisation were calculated in ArcMap, V.10.6 (ESRI, Redlands, CA, USA). All analyses were performed in Stata, V.15.1 (Stata Corp., College Station, TX, USA), and R V.4.0.0 (RStudio Team, Boston, MA, USA).
Patient and public involvement
Our analysis used administrative records, and thus study participants were not involved in the design of the study. Public involvement was achieved through collaboration with the Ministry of Health, with whom we defined the research questions to fill in knowledge gaps and inform decision-making. Results were discussed and shared with the Ministry, and their wide dissemination with public health officials, researchers and through the media will reach the broader public.
Results
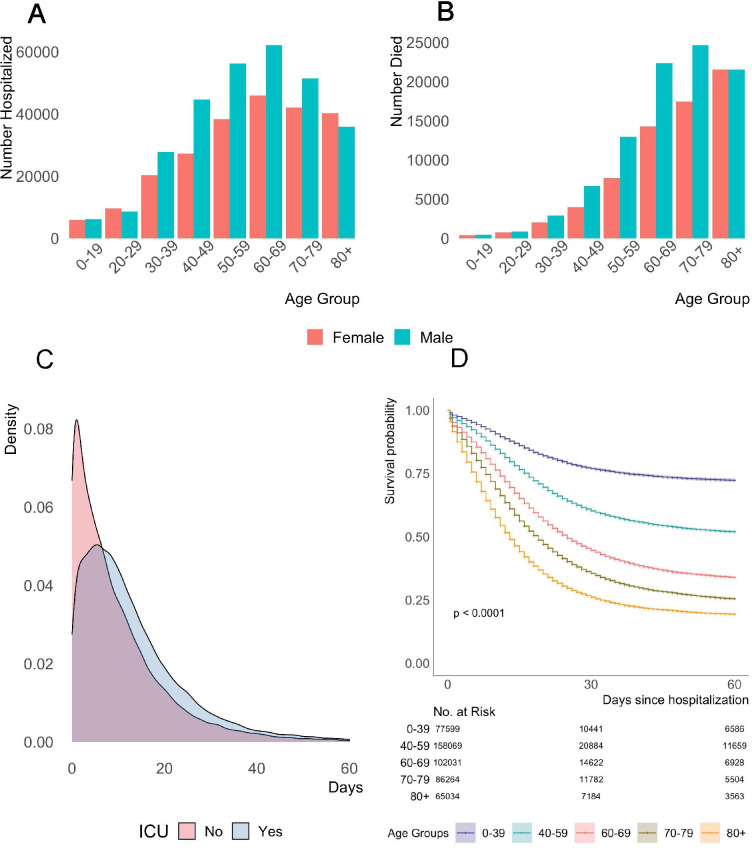
As of 14 December 2020, 522 167 patients had been hospitalised with confirmed COVID-19 since the beginning of the epidemic in Brazil. Of those, 296 002 (56.7%) were discharged, 1004 (0.002%) died of other causes, 160 495 (30.7%) died of causes associated with COVID-19, 53 503 (10.2%) remained hospitalised. Clinical outcome was unknown for 11 126 (2.1%) patients (table 1). The cumulative curve of hospital admissions (online supplemental figure 1) shows the fast increase in severe cases that required hospitalisation, following the steep increase in COVID-19 transmission in Brazil since the end of March. The median age of patients was 61 years (IQR, 47–73), and much higher for non-survivors, 71 years (IQR, 60–80), as shown by the age distribution in figure 1A, B. Patients aged 60 years or more represented 50.1% of hospitalisations, 59.0% of ICU admissions and 74.0% of deaths associated with COVID-19. Patients were mostly males (56.0%) and from the Southeast region (49.3%). Among females, 2.5% were pregnant or puerperal at the time of hospitalisation, and 7.5% of those died in the hospital. A total of 172 473 (33.0%) patients with median age of 65 years (IQR, 52–76) needed ICU admission. Of all hospitalisations, 37.7% of the patients were White, and 37.9% were Black/Brown. Among survivors, 38.8% were White, while among non-survivors 41.7% were Black/Brown. About 25% of the patients travelled a median of 32.0 km (IQR, 18.6–64.1) to be hospitalised in a municipality different from where they reside (table 1).
Table 1.
Demographic characteristics of patients
| Characteristic | All patients (n=5 22 167)* | ICU admission (n=1 72 473) | Non-ICU admission (n=2 93 384) | Not reported (n=56 310) | P value | Survivor and non-COVID-19 death (n=2 97 043) | Non-survivor (n=1 60 495)† | Still in the hospital (n=53 503) | P value |
| Age | |||||||||
| Median (IQR)—years | 61 (47–73) | 65 (52–76) | 59 (45–72) | 61 (47–74) | <0.001 | 56 (42–68) | 71 (60–80) | 60 (46–72) | <0.001 |
| Distribution, number (%) | <0.001 | <0.001 | |||||||
| 0–19 | 13 136 (2.5) | 3211 (1.9) | 8517 (2.9) | 1408 (2.5) | 9994 (3.4) | 985 (0.6) | 1628 (3.0) | ||
| 20–39 | 71 728 (13.7) | 16 978 (9.8) | 46 823 (16.0) | 7927 (14.1) | 54 889 (18.5) | 7299 (4.6) | 7814 (14.6) | ||
| 40–59 | 170 266 (32.6) | 50 445 (29.3) | 101 922 (34.7) | 17 899 (31.8) | 114 769 (38.6) | 33 405 (20.8) | 18 196 (34.0) | ||
| 60–69 | 108 416 (20.8) | 39 362 (22.8) | 57 404 (19.6) | 11 650 (20.7) | 56 788 (19.1) | 38 044 (23.7) | 11 352 (21.2) | ||
| 70–79 | 90 800 (17.4) | 35 944 (20.8) | 44 943 (15.3) | 9913 (17.6) | 38 304 (12.9) | 41 883 (26.1) | 8942 (16.7) | ||
| ≥80 | 67 808 (13.0) | 26 530 (15.4) | 33 769 (11.5) | 7509 (13.3) | 22 303 (7.5) | 38 872 (24.2) | 5570 (10.4) | ||
| Sex, number (%) | <0.001 | <0.001 | |||||||
| Male | 292 570 (56.0) | 100 399 (58.2) | 161 377 (55.0) | 30 794 (54.7) | 163 967 (55.2) | 92 376 (58.6) | 30 146 (56.3) | ||
| Female | 229 513 (44.9) | 72 060 (41.8) | 131 964 (45.0) | 25 489 (45.3) | 133 028 (44.8) | 68 101 (42.4) | 23 345 (43.6) | ||
| Pregnant, number (%) | 4441 (1.9) | 802 (1.1) | 3249 (2.5) | 390 (1.5) | <0.001 | 3603 (2.7) | 230 (0.3) | 469 (2.0) | <0.001 |
| Puerperal, number (%) | 1350 (0.6) | 426 (0.6) | 850 (0.6) | 74 (0.3) | <0.001 | 965 (0.7) | 204 (0.4) | 148 (0.6) | <0.001 |
| Ethnoracial, number (%) | <0.001 | <0.001 | |||||||
| White | 196 035 (37.5) | 67 619 (39.2) | 114 339 (39.0) | 14 077 (25.0) | 115 358 (38.8) | 58 487 (36.4) | 19 257 (36.0) | ||
| Black/Brown | 198 096 (37.9) | 61 450 (35.6) | 114 378 (39.0) | 22 268 (39.6) | 106 312 (35.8) | 66 889 (41.7) | 19 891 (37.2) | ||
| Other | 7237 (1.4) | 2135 (1.2) | 4191 (1.4) | 911 (1.6) | 4028 (1.4) | 2332 (1.5) | 710 (1.3) | ||
| Not reported | 120 799 (23.1) | 41 269 (23.9) | 60 476 (20.6) | 19 054 (33.8) | 71 345 (24.0) | 32 787 (20.4) | 13 645 (25.5) | ||
| Region of residence, number (%) | <0.001 | <0.001 | |||||||
| North | 41 961 (8.0) | 10 024 (5.8) | 27 065 (9.2) | 4872 (8.7) | 23 149 (7.8) | 14 537 (9.1) | 3559 (6.7) | ||
| Northeast | 104 213 (20.0) | 33 220 (19.3) | 50 377 (17.2) | 20 616 (36.6) | 49 733 (16.7) | 37 919 (23.6) | 12 695 (23.7) | ||
| Center-West | 48 864 (9.4) | 16 581 (9.6) | 28 872 (9.8) | 3411 (6.1) | 29 169 (9.8) | 13 532 (8.4) | 4759 (8.9) | ||
| Southeast | 257 503 (49.3) | 88 817 (51.5) | 144 010 (49.1) | 24 676 (43.8) | 151 595 (51.0) | 76 494 (47.7) | 25 050 (46.8) | ||
| South | 69 590 (13.3) | 23 814 (13.8) | 43 042 (14.7) | 2734 (4.9) | 43 380 (14.6) | 17 997 (11.2) | 7439 (13.9) | ||
| Foreigner | 36 (0.0) | 17 (0.0) | 18 (0.0) | 1 (0.0) | 17 (0.0) | 16 (0.0) | 1 (0.0) | ||
| Hospital in the same municipality of residence, number (%) | <0.001 | <0.001 | |||||||
| Yes | 388 304 (74.4) | 118 002 (68.4) | 227 423 (77.5) | 42 879 (76.2) | 224 134 (75.5) | 116 664 (72.7) | 39 772 (74.3) | ||
| No | 133 827 (25.6) | 54 454 (31.6) | 65 943 (22.5) | 13 430 (23.9) | 72 892 (24.5) | 43 815 (27.3) | 13 730 (25.7) | ||
| Distance (km) from residence to hospital† | 32.0 (18.4–64.1) | 35.6 (19.8–77.8) | 29.4 (18.0–56.6) | 25.8 (14.5–54.7) | <0.001 | 29.8 (18.1–57.8) | 34.1 (19.1–71.9) | 34.5 (18.8–75.9) | <0.001 |
*Includes 11 126 patients with unknown clinical outcome.
†Non-survivors are classified as those whose death was associated with COVID-19, according to the SIVEP-Gripe database. Those who had COVID-19 but died due to an unrelated cause are classified under ‘Survivor & non-COVID-19 death’. Survivor n=296 002, non-COVID-19 death=1041.
‡Distances (measured in km) are calculated from the centroid of the notification municipality (consistent with municipality of hospitalisation) to the centroid of the municipality of residence, using the South America Lambert Conformal Conic projection.
ICU, intensive care unit.
Figure 1.
Age distribution of patients, density curves of length of time from hospital admission to death and survival curve 60 days after hospital admission. (A) Age distribution of patients hospitalised. (B) Age distribution of in-hospital deaths. (C) Density of number of days from hospital admission to death up to 60 days after hospital admission, detailed by ICU admission status. (D) Survival curve estimated with Kaplan Meier and considering 60 days from hospital admission. ICU, intensive care unit.
Comorbidities were observed in 64.5% of the patients, 74.6% of those who needed ICU admission, 76.8% of non-survivors and 58.5% of the survivors and those whose death was not associated with COVID-19. With the exception of asthma, all comorbidities had a higher prevalence among non-survivors (compared with all patients). The most common comorbidities were chronic cardiovascular disease (34.5% of patients and 43.5% of non-survivors) and diabetes (25.7% of patients and 33.0% of non-survivors). Obesity was reported in 7.4% of the patients and 10.5% of those who needed ICU admission. The most common symptoms were fever, cough, shortness of breath, low oxygen saturation and respiratory distress symptoms (table 2).
Table 2.
Clinical and radiographic characteristics of patients
| Characteristic | All patients (n=5 22 167)* | ICU admission (n=1 72 473) | Non-ICU admission (n=2 93 384) | Not reported (n=56 310) | P value | Survivor and non-COVID-19 death (n=2 97 043) | Non-survivor (n=1 60 495)† | Still in the hospital (n=53 503) | P value |
| Any comorbidity, number (%) | 336 909 (64.5) | 128 590 (74.6) | 179 847 (61.3) | 28 472 (50.6) | <0.001 | 173 828 (58.5) | 123 265 (76.8) | 33 318 (62.3) | <0.001 |
| Chronic cardiovascular disease | 180 370 (34.5) | 72 196 (41.9) | 93 574 (31.9) | 14 600 (25.9) | <0.001 | 89 402 (30.1) | 69 768 (43.5) | 17 980 (33.6) | <0.001 |
| Chronic haematologic diseases | 4134 (0.8) | 1687 (1.0) | 2204 (0.8) | 243 (0.4) | <0.001 | 1957 (0.7) | 1739 (1.1) | 365 (0.7) | <0.001 |
| Chronic hepatic disease | 4732 (0.9) | 2101 (1.2) | 2309 (0.8) | 322 (0.6) | <0.001 | 1924 (0.7) | 2368 (1.5) | 361 (0.7) | <0.001 |
| Asthma | 14 567 (2.8) | 4947 (2.9) | 8639 (2.9) | 981 (1.7) | <0.001 | 9130 (3.1) | 3634 (2.3) | 1514 (2.8) | <0.001 |
| Diabetes | 134 391 (25.7) | 53 717 (31.2) | 69 078 (23.6) | 11 596 (20.6) | <0.001 | 65 941 (22.2) | 52 958 (33.0) | 12 844 (24.0) | <0.001 |
| Chronic neurological disease | 21 016 (4.0) | 8821 (5.1) | 10 832 (3.7) | 1363 (2.4) | <0.001 | 8113 (2.7) | 10 943 (6.8) | 1622 (3.0) | <0.001 |
| Chronic lung disease | 20 140 (3.9) | 9249 (5.4) | 9483 (3.2) | 1408 (2.5) | <0.001 | 8222 (2.8) | 10 021 (6.2) | 1621 (3.0) | <0.001 |
| Immunodeficiency | 13 967 (2.7) | 5689 (3.3) | 7376 (2.5) | 902 (1.6) | <0.001 | 6351 (2.1) | 6283 (3.9) | 1132 (2.1) | <0.001 |
| Chronic renal disease | 21 725 (4.2) | 10 684 (6.2) | 9429 (3.2) | 1512 (2.9) | <0.001 | 8149 (2.7) | 11 491 (7.2) | 1743 (3.3) | <0.001 |
| Obesity | 38 415 (7.4) | 18 057 (10.5) | 17 998 (6.1) | 2360 (4.2) | <0.001 | 20 993 (7.1) | 12 765 (8.0) | 4005 (7.5) | <0.001 |
| Others‡ | 144 081 (27.6) | 58 139 (33.7) | 74 994 (25.6) | 10 948 (19.4) | <0.001 | 72 598 (24.4) | 55 866 (34.8) | 13 042 (24.4) | <0.001 |
| Symptoms, number (%) | |||||||||
| Fever | 188 572 (64.3) | 104 650 (60.7) | 188 572 (64.3) | 34 789 (61.8) | <0.001 | 194 578 (65.5) | 93 933 (58.5) | 32 586 (60.9) | <0.001 |
| Cough | 369 192 (70.7) | 115 147 (66.7) | 215 084 (73.3) | 38 961 (69.2) | <0.001 | 219 433 (73.9) | 105 252 (65.6) | 36 717 (68.6) | <0.001 |
| Sore throat | 90 487 (17.3) | 23 531 (13.6) | 56 653 (19.3) | 10 303 (18.3) | <0.001 | 56 741 (19.1) | 22 982 (14.3) | 8872 (16.6) | <0.001 |
| Shortness of breath | 367 917 (70.5) | 131 799 (76.4) | 199 805 (68.1) | 36 313 (64.5) | <0.001 | 200 051 (67.4) | 124 724 (77.7) | 35 709 (66.7) | <0.001 |
| Respiratory distress syndrome | 296 238 (56.7) | 107 762 (62.5) | 163 370 (55.7) | 25 106 (44.7) | <0.001 | 157 412 (53.0) | 104 555 (65.2) | 28 046 (52.4) | <0.001 |
| Oxygen saturation <95% | 303 282 (58.1) | 116 355 (67.5) | 160 837 (54.8) | 26 090 (46.3) | <0.001 | 156 349 (52.6) | 111 097 (69.2) | 29 704 (55.5) | <0.001 |
| Diarrhoea | 71 069 (13.6) | 20 340 (11.8) | 44 411 (15.1) | 6318 (11.2) | <0.001 | 44 961 (15.1) | 17 419 (10.9) | 7264 (13.6) | <0.001 |
| Vomiting | 41 974 (8.0) | 11 950 (6.9) | 26 177 (8.9) | 3847 (6.8) | <0.001 | 25 799 (8.7) | 11 076 (6.9) | 4156 (7.8) | <0.001 |
| Others§ | 179 222 (34.3) | 58 268 (33.8) | 105 646 (36.0) | 15 308 (27.2) | <0.001 | 112 278 (37.8) | 45 263 (28.2) | 18 294 (34.2) | <0.001 |
| Chest radiograph, number (%) | <0.001 | <0.001 | |||||||
| Normal | 11 816 (2.3) | 3403 (2.0) | 7782 (2.7) | 631 (1.1) | 7895 (2.7) | 2558 (1.6) | 1091 (2.0) | ||
| Interstitial abnormalities | 81 412 (15.6) | 28 091 (16.3) | 48 864 (16.7) | 4457 (7.9) | 45 320 (15.3) | 27 071 (16.9) | 7500 (14.0) | ||
| Other¶ | 85 870 (16.4) | 35 844 (20.8) | 47 185 (16.1) | 2841 (5.1) | 246 250 (82.9) | 132 429 (82.5) | 47 639 (89.0) |
*Includes 11 126 patients with unknown clinical outcome.
†Non-survivors are classified as those whose death was associated with COVID-19, according to the SIVEP-Gripe database. Those who had COVID-19 but died due to an unrelated cause are classified under ‘Survivor & non-COVID-19 death’. Survivor n=296 002, non-COVID-19 death=1041.
‡Other comorbidities that were not specifically asked about in the survey, but self-reported as ‘other’ include, but are not limited to hypertension, cancer, anaemia, bronchitis, dyslipidaemia and pulmonary emphysema.
§Other symptoms that were not specifically asked about in the surveillance form, but self-reported as ‘other’ include, but are not limited to loss of taste, loss of smell, myalgia, weakness, body ache, fatigue, exhaustion, tachypnea and syncope.
¶Includes consolidation, mixed and other.
ICU, intensive care unit.
The median time from illness onset to hospital admission was 6 days (IQR, 3–9), slightly shorter among non-survivors, 5 days (IQR, 2–8). Mechanical ventilation was needed by 62.2% of all patients, and by 75.6% of those who died. Invasive ventilation was more common in the ICU (44.0%). Oseltamivir, an antiviral medication, was the most common drug used during treatment (15.8% overall, and 17.6% among those in ICU), and the median time from illness onset to treatment was 5 days (IQR, 3–8). Of the patients who needed ICU, 51.8% died from causes associated with COVID-19, and 19.0% remained hospitalised after ICU discharge for 5 days (IQR, 2–10). The median time from illness onset to ICU admission was 7 days (IQR, 3–10), and the medium length of ICU stay was 8 days (IQR, 3–15) for all patients, 9 days (IQR, 4–16) for the deceased. Among the 160 495 patients who died of causes associated with COVID-19 by 14 December, the median time from illness onset to death was 15 days (IQR, 9–24) (table 3). Medium length of hospital stay was 8 days (IQR, 4–17), but longer for those who needed ICU admission, 12 days (IQR, 6–22). The density of time from hospital admission to death is positively skewed, more so for those who did not get admitted to the ICU (figure 1C).
Table 3.
Treatment and outcomes of patients
| Variable | All patients (n=522 167)* | ICU admission (n=172 473) | Non-ICU admission (n=293 384) | Not reported (n=56 310) | P value | Survivor and non-COVID-19 death (n=297 043) | Non-survivor (n=160 495) | Still in the hospital (n=53 503) | P value |
| Treatment with drugs, number (%) | <0.001 | <0.001 | |||||||
| Oseltamivir | 82 659 (15.8) | 30 341 (17.6) | 47 317 (16.1) | 5001 (8.9) | 50 091 (16.9) | 27 192 (16.9) | 4242 (7.9) | ||
| Zanamivir | 492 (0.1) | 152 (0.1) | 303 (0.1) | 37 (0.1) | 298 (0.1) | 128 (0.1) | 56 (0.1) | ||
| Other | 5008 (1.0) | 1480 (0.9) | 3243 (1.1) | 285 (0.5) | 3029 (1.0) | 1118 (0.7) | 701 (1.3) | ||
| Mechanical ventilation, number (%) | <0.001 | <0.001 | |||||||
| Invasive | 90 189 (17.3) | 75 915 (44.0) | 12 019 (4.1) | 2255 (4.0) | 17 263 (5.8) | 66 652 (41.5) | 5238 (9.8) | ||
| Non-invasive | 234 554 (44.9) | 65 281 (37.9) | 157 913 (53.8) | 11 360 (20.2) | 148 930 (50.1) | 54 652 (34.1) | 25 925 (48.5) | ||
| ICU admission, number (%) | 172 473 (33.0) | 172 473 (100.0) | – | – | – | 65 102 (21.9) | 89 264 (55.6) | 15 614 (29.2) | <0.001 |
| Remained hospitalised after ICU discharge, number (%) | 32 770 (19.0) | 32 770 (19.0) | – | – | – | 27 775 (42.7) | 3243 (3.6) | 1303 (8.4) | <0.001 |
| Median length (IQR), days | 5 (2–10) | 5 (2–10) | – | – | – | 5 (2–9) | 7 (2–16) | 72 (21–139) | <0.001 |
| Median times (IQR), days | |||||||||
| Illness onset to treatment with drugs | 5 (3–8) | 5 (3–8) | 6 (3–9) | 6 (3–8) | <0.001 | 6 (3–9) | 5 (2–8) | 6 (3–9) | <0.001 |
| Illness onset to hospitalisation | 6 (3–9) | 6 (3–9) | 6 (3–10) | 6 (2–9) | <0.001 | 7 (3–10) | 5 (2–8) | 6 (3–10) | <0.001 |
| Illness onset to ICU admission | 7 (3–10) | 7 (3–10) | – | – | – | 7 (4–10) | 6 (3–10) | 7 (3–10) | <0.001 |
| Hospital admission to ICU admission | 0 (0–1) | 0 (0–1) | – | – | – | 0 (0–1) | 0 (0–1) | 0 (0–0) | <0.001 |
| Illness onset to death | 15 (9–24) | 17 (10–25) | 13 (7–21) | 12 (7–20) | <0.001 | – | 15 (9–24) | – | – |
| Illness onset to hospital discharge | 15 (11–20) | 18 (13–27) | 14 (10–18) | 16 (11–21) | <0.001 | 15 (11–20) | – | – | – |
| Hospital admission to death | 9 (4–16) | 10 (5–18) | 7 (2–14) | 5 (1–13) | <0.001 | – | 9 (4–16) | – | – |
| Hospital admission to ICU discharge | 9 (4–17) | 9 (4–17) | – | – | – | 7 (4–14) | 10 (5–10) | 6 (2–14) | <0.001 |
| ICU admission to death | 9 (4–16) | 9 (4–16) | – | – | – | – | 9 (4–16) | – | – |
| Median length hospital stay (IQR), days | 8 (4–17) | 12 (6–22) | 7 (4–13) | 8 (3–18) | <0.001 | 7 (4–12) | 9 (4–16) | 79 (23–157) | <0.001 |
| Median length ICU stay (IQR), days | 8 (3–15) | 8 (3–15) | – | – | – | 6 (3–12) | 9 (4–16) | 4 (2–11) | <0.001 |
| Clinical outcomes as of 14 December 2020, number (%) | <0.001 | – | |||||||
| Cured and discharged from hospital | 296 002 (56.7) | 64 578 (37.4) | 202 042 (68.9) | 29 382 (52.2) | 296 002 (99.7) | – | – | ||
| Death due to other causes | 1041 (0.2) | 524 (0.3) | 443 (0.2) | 74 (0.1) | 1041 (0.4) | – | – | ||
| Death associated with COVID-19 | 160 495 (30.7) | 89 264 (51.8) | 53 282 (18.2) | 17 949 (31.9) | – | 160 495 (100.0) | – | ||
| Still in the hospital | 53 503 (10.3) | 15 614 (9.1) | 30 963 (10.6) | 6926 (12.3) | – | – | 53 503 (100.0) |
*Includes 11 126 patients with unknown clinical outcome.
†Non-survivors are classified as those whose death was associated with COVID-19, according to the SIVEP-Gripe database. Those who had COVID-19 but died due to an unrelated cause are classified under ‘Survivor & non-COVID-19 death’. Survivor n=296 002, non-COVID-19 death=1041.
ICU, intensive care unit.
Kaplan Meier curves (figure 1D and online supplemental figure 2) for a period of up to 60 days after hospital admission showed that survival curves were significantly different by age, region, sex, ethnoracial self-classification and ICU admission.
Univariable logistic analysis indicated that the odds of in-hospital death progressively increased with age, and were higher for patients who were male, non-white, from the North and Northeast regions, needed ICU care, were obese and had diabetes and other comorbidities (table 4). The multivariable analysis included 168 936 records (65 670 non-survivors) that had no missing data for covariates. The odds of in-hospital death for males are 1.23 times that of females, and for those in the North and Northeast regions were, respectively, 1.83 and 1.48 times that of patients in the Southeast. The Cox proportional-hazards model included 176 559 records (64 809 non-survivors). Variables associated with in-hospital death were age, sex, ethnoracial self-classification, region, ICU care and various comorbidities. Age, however, had the highest hazard ratios, ranging from 1.67 (95% CI: 1.49 to 1.89) for those aged 20–39 to 5.51 (95% CI: 4.91 to 6.18) for those 80 or older, compared with patients younger than 20 years (table 4).
Table 4.
ORs and HRs for death among hospitalised patients with confirmed COVID-19
| Variables | Univariable OR* (95% CI) | P value† | Multivariable OR (95% CI) n=168 936 | P value† | HR (95% CI) for death within 60 days of hospitalisation n=176 559 |
| Age (reference 0–19) | |||||
| 20–39 | 1.35 (1.26 to 1.46) | <0.001 | 1.66 (1.45 to 1.91) | <0.001 | 1.67 (1.48 to 1.89) |
| 40–59 | 2.94 (2.74 to 3.15) | <0.001 | 2.70 (2.37 to 3.09) | <0.001 | 2.21 (1.97 to 2.48) |
| 60–69 | 6.76 (6.30 to 7.26) | <0.001 | 5.15 (4.52 to 5.88) | <0.001 | 3.05 (2.72 to 3.43) |
| 70–79 | 11.05 (10.30 to 11.86) | <0.001 | 8.24 (7.24 to 9.42) | <0.001 | 3.90 (3.48 to 4.38) |
| ≥80 | 17.58 (16.37 to 18.88) | <0.001 | 14.52 (12.74 to 16.59) | <0.001 | 5.51 (4.91 to 6.18) |
| Sex (reference female) | |||||
| Male | 1.10 (1.09 to 1.11) | <0.001 | 1.23 (1.20 to 1.26) | <0.001 | 1.09 (1.07 to 1.10) |
| Ethnoracial self-classification (reference White) | |||||
| Black/Brown | 1.25 (1.24 to 1.27) | <0.001 | 1.18 (1.15 to 1.22) | <0.001 | 1.08 (1.06 to 1.10) |
| Other | 1.18 (1.12 to 1.25) | <0.001 | 1.05 (0.95 to 1.16) | 0.309 | 1.02 (0.96 to 1.10) |
| Not reported | 0.9 (0.89 to 0.92) | <0.001 | 0.77 (0.74 to 0.80) | <0.001 | 0.79 (0.77 to 0.81) |
| Region (reference Southeast) | |||||
| South | 0.85 (0.83 to 0.87) | <0.001 | 0.89 (0.87 to 0.92) | <0.001 | 0.91 (0.89 to 0.93) |
| Center-West | 0.96 (0.94 to 0.98) | <0.001 | 1.04 (1.00 to 1.08) | 0.049 | 1.00 (0.97 to 1.03) |
| North | 1.31 (1.28 to 1.34) | <0.001 | 1.83 (1.75 to 1.92) | <0.001 | 1.34 (1.30 to 1.39) |
| Northeast | 1.61 (1.58 to 1.64) | <0.001 | 1.48 (1.43 to 1.55) | <0.001 | 1.10 (1.07 to 1.12) |
| ICU | 5.21 (5.14 to 5.28) | <0.001 | 5.20 (5.08 to 5.32) | <0.001 | 1.78 (1.75 to 1.81) |
| Obesity | 0.91 (0.88 to 0.93) | <0.001 | 1.23 (1.18 to 1.27) | <0.001 | 1.07 (1.04 to 1.10) |
| Diabetes | 1.32 (1.30 to 1.35) | <0.001 | 1.18 (1.15 to 1.21) | <0.001 | 1.08 (1.07 to 1.10) |
| Asthma | 0.59 (0.56 to 0.61) | <0.001 | 0.81 (0.77 to 0.86) | <0.001 | 0.88 (0.84 to 0.92) |
| Chronic liver disease | 1.87 (1.76 to 1.99) | <0.001 | 1.74 (1.59 to 1.90) | <0.001 | 1.33 (1.26 to 1.40) |
| Chronic neurological disease | 2.12 (2.06 to 2.19) | <0.001 | 1.65 (1.58 to 1.73) | <0.001 | 1.18 (1.15 to 1.21) |
| Chronic lung disease | 1.92 (1.86 to 1.99) | <0.001 | 1.46 (1.40 to 1.53) | <0.001 | 1.16 (1.13 to 1.19) |
| Immunodeficiency | 1.53 (1.47 to 1.58) | <0.001 | 1.93 (1.83 to 2.04) | <0.001 | 1.26 (1.22 to 1.31) |
| Chronic kidney disease | 2.23 (2.16 to 2.30) | <0.001 | 1.70 (1.63 to 1.78) | <0.001 | 1.17 (1.14 to 1.21) |
*The N varies for univariable OR, depending on the number of missing values for each variable.
†P value from Wald test.
ICU, intensive care unit.
Discussion
This study described demographic, clinical and radiographic characteristics, treatment, case outcome and risk factors associated with in-hospital death of 522 167 patients hospitalised with confirmed COVID-19 in Brazil. Results show that 56.7% were discharged, 0.002% died of other causes, 30.7% died of causes associated with COVID-19 and 10.2% remained in the hospital as of 14 December. Patients were mostly older than 40 years, predominantly from the Southeast region, with about one-fourth needing to travel to a different municipality for hospitalisation. At least one comorbidity was present in 64.5% of patients and in 76.8% of the non-survivors. From illness onset, the median time to hospital and ICU admission was 6 and 7 days, respectively; 15 days to death (17 to those admitted to the ICU) and 15 days to hospital discharge (18 days to those admitted to the ICU). Risk factors for in-hospital death were older age, being male, of Black/Brown ethnoracial self-classification, living in the North or Northeast regions, with a history of ICU admission and various co-morbidities.
Our results can be analysed in light of findings from other countries. The use of mechanical ventilation was higher in Brazil (62.2% among all patients, 75.6% of the non-survivors) compared with patterns described for China (ranging from 17.2% to 38.7% of patients),8–10 and Germany (17% of patients),11 but lower than Italy (81.7% of all patients).12 While ICU mortality in Italy was 26%,12 in Brazil, it was 51.8%. In Brazil, 33.0% of hospitalised patients were admitted to the ICU, against 16% in Italy,13 and 19% in France.14 In-hospital death was observed in 18.1% of patients in France,14 22% in Germany11 and 30.7% in Brazil. The time from illness onset to hospitalisation in China9 was 11 days (IQR, 8–14), but much shorter in Brazil, 6 days (IQR, 3–9). The length of hospital stay, however, was about 4 days longer in China.9 15 These comparisons need to be taken with caution. First, studies from China had a smaller sample size, and regional variability is very large, as reported for France.14 In Brazil, for example, in-hospital death varied from 25.9% in the South region to 36.4% in the Northeast, and ICU mortality from 48.0% in the Southeast to 66.5% in the North. The time from illness to hospitalisation was also longer in the North and Center-West regions, 7 days (IQR, 4–10).
Our results confirm previous findings regarding symptoms and comorbidities. Hypertension, a very common comorbidity in China9 could not be measured from our data, but over one-third of the adult population in Brazil has that condition.16 In Brazil, 35.5% of the patients reported no comorbidities, while in New York City, this number was 6.1%,17 and in China, it was 52%.9 Diabetes was reported in 19% of patients in China,9 33.8% in New York City17 and 25.7% in Brazil. Part of these differences reflects the disease burden in each locality, but also the lack of standardised data collection (eg, conditions systematically collected in one country and only reported in the ‘other’ category in another country).
The observed associations of age, sex, obesity and diabetes with in-hospital death corroborate previous findings.5 6 14 The higher risk among non-white patients was previously reported in Brazil, the UK and the USA.18–22 In Brazil, this reflects structural inequalities that made large fractions of the population more vulnerable to COVID-19 (eg, people living in areas with precarious infrastructure, overcrowded households, regions with low supply of physicians and hospital services and who depend on informal labour).23 24 Those inequalities are also captured by a higher HR in the North and Northeast regions, where Brazil consistently reports worse socioeconomic indicators.25 Currently, the North region has the lowest rates of hospital beds, ICU beds and physicians per person.26 Indeed, the region had the worst indicators in terms of mortality and time to hospitalisation, and patients who were hospitalised in a municipality different from the one where they live had to travel 122.0 km (IQR, 58.3–258.6), while those in the Southeast travelled 22.3 km (IQR, 16.1–36.3). Hospitalisation in a different municipality occurs when the place of residence has no hospitals, has no available hospital beds or when the closest hospital is actually outside the municipality of residence. In Brazil, the size of municipalities varies widely: 23% have 5000 residents or less, and 5% have 100 000 or more residents. Of the 5570 municipalities, 37% and 75% have no hospitals and no ICU care, respectively. A regionalisation process guarantees that all the population has access to hospital care.27 However, when hospitals reach capacity, as was observed in several cities in Brazil in late April and May, municipalities without hospitals and ICU units are unable to provide proper care, which may have contributed to higher COVID-19 mortality. Therefore, risk factors for in-hospital mortality due to COVID-19 expose local and structural inequalities.
This study has some limitations. First, we used administrative records captured in structured surveillance forms. Those lack details available in hospital medical records and may have accuracy and completeness problems. In addition, it limits the types of comorbidities and symptoms reported, as those listed under the ‘other’ category were not systematically collected from all cases (eg, loss of taste and smell). Second, we did not have access to laboratory results other than COVID-19 tests (eg, complete blood count). While this does not change any of our results, they would allow for a better characterisation of the clinical course of the disease. Third, 23.1% of patients did not report information on ethnoracial self-classification, 10.8% did not have information on ICU admission and for 2.1%, there was no information on clinical outcome. This is not uncommon in the analysis of administrative records.22 Here, we report all records and included an additional category (ethnoracial self-classification not reported) in the risk factor analysis.
Despite these limitations, this study provides a comprehensive description of characteristics, outcomes and risk factors for mortality of patients hospitalised with COVID-19 in Brazil, and the largest cohort of patients so far analysed (n=5 22 167). Results shed light on commonalities and differences between Brazil and other countries affected by COVID-19, and highlight inequalities in disease outcomes. Most importantly, our results could be used to inform coordinated actions to target those who currently bear the highest morbidity and mortality burden. Brazil has a network of almost 270 000 community health workers that reach out to about 70% of the Brazilian population. These agents could actively identify vulnerable people who face higher risk of mortality, could act as agents of information to sensitise the population and boost adherence to control measures (eg, use of masks) and could continue to deliver community-based primary care services that have been, for the most part, interrupted by the pandemic. These agents will also be important to support the delivery of vaccination to the most vulnerable. Leveraging and strengthening the existing network of primary healthcare is paramount to contain the sustained and unequal burden of COVID-19 in Brazil.
Supplementary Material
Acknowledgments
We would like to thank Nicholas Arisco, MS, for technical assistance.
Footnotes
Contributors: MCC and GVAdF conceived the study, were responsible for data analysis and interpretation. MCC wrote the manuscript. GVAdF and EMM acquired the data. GVAdF and SG were responsible for data curation. SG ran the statistical models and contributed to writing. All authors edited and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The SIVEP-Gripe data set is publicly available on the Ministry of Health’s DATASUS website (https://opendatasus.saude.gov.br/nl/dataset).
References
- 1. World Health Organization . Coronavirus disease (COVID-19) outbreak situation, 2021. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Brasil, Ministério da Saúde . A experiência brasileira em sistemas de informação em saúde. Série B Textos Básicos de Saúde. Brasília: Ministério da Saúde, Organização Pan-Americana da Saúde, Fundação Oswaldo Cruz, 2009. http://bvsms.saude.gov.br/bvs/publicacoes/experiencia_brasileira_sistemas_saude_volume2.pdf [Google Scholar]
- 3. Lima-Costa MF, Rodrigues LC, Barreto ML, et al. Genomic ancestry and ethnoracial self-classification based on 5,871 community-dwelling Brazilians (the epigen initiative). Sci Rep 2015;5:9812. 10.1038/srep09812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ministério da Saúde . Guia de Vigilância Epidemiológica. Emergência de Saúde Pública de Importância Nacional pela Doença pelo Coronavírus 2019. Vigilância de Síndromes Respiratórias Agudas COVID-19. August 5, 2020 ed. Brasil: Ministério dA Saúde, 2020. https://portalarquivos.saude.gov.br/images/af_gvs_coronavirus_6ago20_ajustes-finais-2.pdf [Google Scholar]
- 5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020;146:110–8. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esai Selvan M. Risk factors for death from COVID-19. Nat Rev Immunol 2020;20:407. 10.1038/s41577-020-0351-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai C-C, Liu YH, Wang C-Y, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect 2020;53:404–12. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020;8:853–62. 10.1016/S2213-2600(20)30316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020;323:1545-1546. 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 14. Salje H, Tran Kiem C, Lefrancq N, et al. Estimating the burden of SARS-CoV-2 in France. Science 2020;369:208–11. 10.1126/science.abc3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. W-j G, Z-y N, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macinko J, Leventhal DGP, Lima-Costa MF. Primary care and the hypertension care continuum in Brazil. J Ambul Care Manage 2018;41:34–46. 10.1097/JAC.0000000000000222 [DOI] [PubMed] [Google Scholar]
- 17. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323:2052–9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baqui P, Bica I, Marra V, et al. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health 2020;8:e1018–26. 10.1016/S2214-109X(20)30285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med Overseas Ed 2020;382:2534–43. 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordes J, Castro MC. Spatial analysis of COVID-19 clusters and contextual factors in New York City. Spat Spatiotemporal Epidemiol 2020;34:100355. 10.1016/j.sste.2020.100355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golestaneh L, Neugarten J, Fisher M, et al. The association of race and COVID-19 mortality. EClinicalMedicine 2020;25:100455. 10.1016/j.eclinm.2020.100455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: factors associated with COVID-19-related Hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020. [Google Scholar]
- 23. Castro MC, Resende de Carvalho L, Chin T, et al. Demand for hospitalization services for COVID-19 patients in Brazil. medRxiv 2020. [Google Scholar]
- 24. WCd SJ, Gonçalves DA, Cruz DB. COVID-19: Local/regional inequalities and impacts over critical healthcare infrastructure in Brazil. Ambiente & Sociedade 2020;23. [Google Scholar]
- 25. Menezes Filho N, Kirschbaum C. Education and Inequality in Brazil. : Arretche M, . Paths of inequality in Brazil: a half-century of changes. Cham: Springer International Publishing, 2019: 69–88. [Google Scholar]
- 26. Carvalho LR, Andrade MV, Amaral PVM. Avaliação DOS parâmetros de oferta mínimos para os leitos Sus no Brasil, 2015. Planejamento e Políticas Públicas - PPP/Ipea 2020. [Google Scholar]
- 27. Castro MC, Massuda A, Almeida G, et al. Brazil's unified health system: the first 30 years and prospects for the future. Lancet 2019;394:345–56. 10.1016/S0140-6736(19)31243-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-049089supp001.pdf (2.1MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. The SIVEP-Gripe data set is publicly available on the Ministry of Health’s DATASUS website (https://opendatasus.saude.gov.br/nl/dataset).