Abstract
Introduction
While Campylobacter jejuni is a leading foodborne bacterial pathogen worldwide, it poses a particular risk to susceptible populations in low- and middle-income countries (LMICs). A capsule-conjugate vaccine approach has been proposed as a potential solution, but little information exists on circulating C. jejuni capsule types in LMICs. The capsule is the major serodeterminant of the Penner typing scheme, which is based on serum recognition of Campylobacter heat-stable antigens. We conducted a systematic review and meta-analysis to estimate the distribution of Penner serotypes associated with C. jejuni enteritis in LMICs. Vaccine coverage assessments for hypothetical regional and global C. jejuni vaccines were also estimated.
Methods
A systematic review of the literature published from 1980 to 2019 was performed using PubMed, Scopus, and Web of Science databases. Articles were assessed for eligibility and data were abstracted. Pooled C. jejuni serotype prevalence in LMICs was estimated by region and globally using random-effects models.
Results
A total of 36 studies were included, capturing 4,434 isolates from LMICs. Fifteen serotypes were present in a sufficient number of studies to be included in analyses. Among these, HS4c was the most common serotype globally (12.6%), though leading capsule types varied among regions. HS2, HS3c, HS4c, HS5/31, HS8/17, and HS10 were all among the 10 most common region-specific serotypes.
Conclusions
The results of this review suggest that an octavalent vaccine could provide up to 66.9% coverage of typable strains worldwide, and 56.8–69.0% regionally. This review also highlights the paucity of available data on capsules in LMICs; more testing is needed to inform vaccine development efforts.
Introduction
Diarrheal disease accounts for 1 in 9 child deaths and is among the leading causes of death among children under five years of age [1, 2]. Campylobacter jejuni is a common foodborne pathogen and one of the leading causes of infectious diarrhea worldwide [3]. C. jejuni is also associated with Guillain-Barré Syndrome (GBS), a rare but potentially life-threatening autoimmune condition, as well as less severe but more common long-term sequelae, including reactive arthritis and irritable bowel syndrome [4–6].
While largely occurring as sporadic infections and outbreaks in high-income countries (HICs) [7], C. jejuni infection is endemic in low- and middle-income countries (LMICs) [8–11]. In the Malnutrition and Enteric Disease (MAL-ED) birth cohort study, Campylobacter spp. were among the three most common bacterial causes of diarrheal disease in infants from birth through the second year of life [12, 13]. Up to 85% of children were infected by one year of age, with one-third of those cases resulting in persistent infection [14]. In the Global Enteric Multicenter Study (GEMS), Campylobacter was a leading pathogen associated with moderate-to-severe diarrhea in children aged ≤5 years in Pakistan, Bangladesh, and India [15, 16]. In addition to its association with malnutrition, Campylobacter infection is linked to inflammation and growth deficits [16–21], all of which have implications for long-term health, education, and economic prospects [22–26].
Campylobacter antibiotic resistance is a growing concern among public health agencies, and few alternatives exist to treat multidrug-resistant C. jejuni. Fluoroquinolones, once first-line interventions for suspected campylobacteriosis, have been rendered increasingly ineffective by antimicrobial use in industrial agriculture [27, 28]. The World Health Organization and Centers for Disease Control and Prevention have both identified drug-resistant Campylobacter as a serious threat to global health [29, 30]. The need for alternatives to antimicrobials highlights the need for increased vaccine development efforts and other methods of prevention.
Though several candidates have been evaluated over the last 20 years, there are currently no licensed human vaccines against C. jejuni [31]. An increased understanding of the role of the C. jejuni capsular polysaccharide (CPS) in virulence [32] has paved the way for a conjugated polysaccharide vaccine. To date, monovalent prototypes have demonstrated efficacy in non-human primate models [33]; however, prioritization of the capsule targets for a final multivalent formulation is hampered by a lack of data on C. jejuni capsule types circulating in LMICs, where disease burden is highest [8–10, 34].
Penner serotyping
CPS is the major serodeterminant of the Penner typing scheme [35, 36]. Penner serotyping uses passive hemagglutination (PHA) to classify C. jejuni based on heat-stable (HS) antigens [37, 38]. High variability in the C. jejuni genome results in a variety of CPS moieties across serotypes [32, 39]. There are currently 47 recognized C. jejuni Penner serotypes, which, due to cross-reactivity of related CPS structures, can be organized into 35 CPS types [40].
For over 30 years, the Penner PHA serotyping scheme was considered the gold standard for serotyping C. jejuni, but the cost and complexity of maintaining a serum reference library limited its reach [41]. Beginning in the late 1990s, some laboratories serotyped using a commercially available antisera kit of 25 CPS types, manufactured by Denka Seiken of Japan [42]. A multiplex PCR (mPCR) method for capsule characterization was later developed in 2010 [43] and has since largely replaced antisera-based methods of Penner typing.
Identifying the most prevalent C. jejuni capsule types is essential for the development of a broadly effective capsule-conjugate vaccine. The goal of this systematic review and meta-analysis was to estimate the prevalence and geographic variability of C. jejuni capsule types among clinical isolates in LMICs. Pooled estimates for coverage of hypothetical regional and global vaccines with increasing valency were also calculated.
Methods
Search strategy
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [44, 45]. Relevant studies were identified through a literature search of PubMed, Scopus, and Web of Science databases. Search queries for each database were developed in consultation with a knowledge synthesis librarian, and can be found in the S1 Text. Citations and reference lists of relevant articles were hand-searched, and experts in C. jejuni research were consulted to identify studies not captured by the database searches.
Study screening
Two reviewers independently screened studies by title and abstract for inclusion. Discrepancies in eligibility assessments were discussed; disagreements were arbitrated by a third reviewer. Inclusion was limited to English-language studies published between 1980 and 2019 with Penner-typed C. jejuni human fecal isolates. Review articles, studies containing fewer than 10 isolates, isolates from animal or environmental sources, and studies with apparent selection biases (e.g., studies examining one specific serotype) were excluded.
Data abstraction and analysis
Data were abstracted by two independent reviewers; disagreements were arbitrated by a third reviewer. Isolates reported as more than one HS type not known to commonly complex were counted as reacting to each listed serotype. For example, an isolate reported as belonging to serotype HS19/42 would be counted as being both an HS19 and an HS42 isolate. The HS3 complex (HS3c) was defined as including HS3, 13, and 50. The HS4 complex (HS4c) was defined as including HS4, 13, 16, 43, 50, 62, 63, 64, and 65. Isolates from outbreaks, GBS patients, and isolates of C. coli were not included in analyses.
Studies were classified by country and separated into regions: Africa, North & South America (Americas), Asia, Europe, and Oceania. Based on historical data published by the World Bank, countries were classified by income level as low (LIC), low-middle and upper-middle (MIC), and high (HIC) based on the year in which sample collection started [46]. If the year of first sample collection was not specified, the year of publication was used for classification. For studies in which sample collection began before 1987, income classification in 1987 was used. Where authors’ descriptions and data allowed for distinction between populations within a study, studies were abstracted as separate observations.
Statistical analyses were performed using the stats and meta packages in R version 3.6.3 software [47, 48]. Statistical heterogeneity was assessed via Cochran’s Q test and I-squared (I2) statistics [49, 50]. Random effects models (REMs; DerSimonian-Laird method) [51] were employed to calculate capsule-specific pooled prevalence estimates and associated 95% confidence intervals (CI) using the transformed log of capsule proportions. Vaccine coverage assessments for hypothetical regional and global C. jejuni vaccines were estimated via the stepwise addition of the most globally prevalent CPS types identified in REMs. In accordance with PRISMA guidelines, publication bias was assessed visually and statistically using funnel plots and Egger’s test, respectively [52].
Results
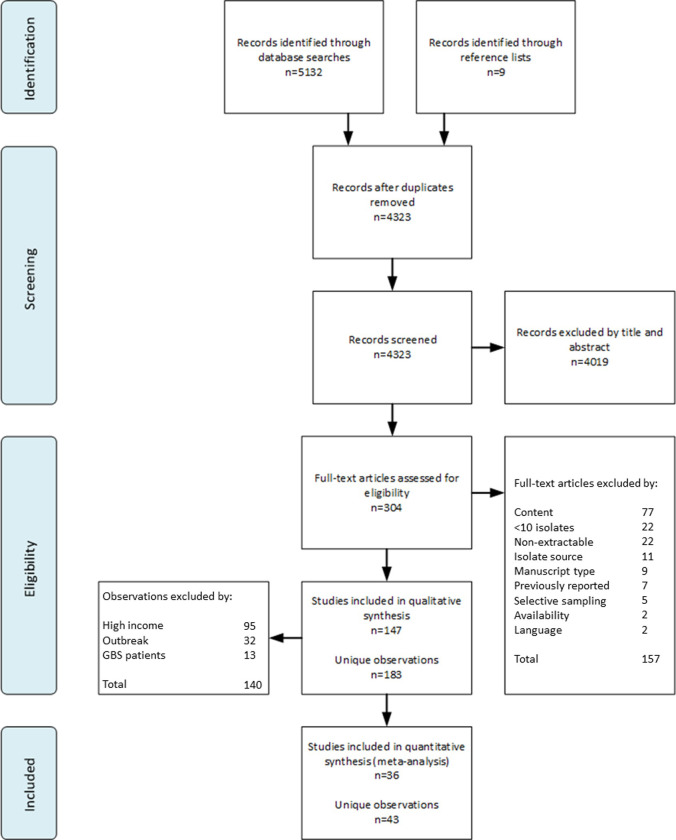
As shown in Fig 1, 5,132 publications were identified through database searches, and an additional 9 articles were identified through reference lists and consulting experts in the field. Of the 304 full-text articles retrieved and reviewed, a total of 157 were excluded. The most common reasons for exclusion were content (n = 77; 49.0%), non-extractable data (n = 22; 14.0%), and containing fewer than 10 isolates (n = 22; 14.0%).
Fig 1. PRISMA flow diagram for identification and selection of included studies.
From the eligible 147 studies, 183 unique observations were abstracted. These studies reported a total of 44,799 sporadic enteritis isolates collected from 46 countries between 1978 and 2015 (Tables 1 and S1). A total of 45 of these observations were excluded from analyses for containing isolates from GBS patients (n = 13; 28.9%) or outbreaks (n = 32; 71.1%). An additional 95 observations were excluded due to their collection in an HIC setting.
Table 1. Detailed characteristics of observations included in the meta-analysis.
| Region | First Author | Year of Publication | Country | Income | na | Age | Typing Method |
|---|---|---|---|---|---|---|---|
| Africa | Georges-Courbot [53] | 1986 | Central African Republic | Lowb | 113 | Pediatric | PHAc |
| Lastovica [54] | 1986 | South Africa | Middleb | 258 | Pediatric | PHA | |
| Lastovica [55] | 1986 | South Africa | Middleb | 23 | Pediatric | PHA | |
| Mølbak [56] | 1988 | Liberia | Lowb | 22 | Pediatric | PHA | |
| Georges-Courbot [57] | 1989 | Central African Republic | Lowb | 209 | Pediatric | PHA | |
| Asrat [58] | 1997 | Ethiopia | Lowb | 56 | Mixed | PHA | |
| Smith [59] | 1997 | Nigeria | Low | 29 | Pediatric | PHA | |
| Smith [60] | 1998 | Nigeria | Low | 20 | Not Specified | PHA | |
| Smith [61] | 2000 | Nigeria | Low | 29 | Not Specified | PHA | |
| Smith [62] | 2000 | Nigeria | Low | 21 | Not Specified | PHA | |
| Wierzba [63] | 2008 | Egypt | Middle | 21 | Pediatric | PHA | |
| Sainato [64] | 2017 | Egypt | Middle | 272 | Pediatric | mPCR | |
| Americas | Sjögren [65] | 1989 | Mexico | Middleb | 136 | Pediatric | PHA |
| Nachamkin [66] | 2007 | Mexico | Middle | 44 | Pediatric | PHA | |
| Neitenbach [67] | 2019 | Peru | Middle | 352 | Pediatric | mPCR | |
| Rojasd [68] | 2019 | Peru | Middle | 184 | Pediatric | mPCR | |
| Rojasd [68] | 2019 | Peru | Middle | 270 | Pediatric | mPCR | |
| Asia | Neogi [69] | 1987 | Bangladesh | Lowb | 102 | Mixed | PHA |
| Tay [70] | 1995 | Malaysia | Middle | 26 | Mixed | PHA | |
| Nishimura [71] | 1996 | China | Low | 85 | Not Specified | PHA | |
| Li [72] | 2001 | China | Low | 90 | Mixed | PHA | |
| Prasad [73] | 2002 | India | Low | 23 | Mixed | PHA | |
| Boonmar [74] | 2005 | Thailand | Middle | 50 | Pediatric | Commercial Kit | |
| Boonmar [75] | 2007 | Thailand | Middle | 70 | Not Specified | Commercial Kit | |
| Islame [76] | 2009 | Bangladesh | Low | 39 | Not Specified | PHA | |
| Polyf [77] | 2015 | Thailand | Middle | 263 | Adult | mPCR | |
| Polyf [77] | 2015 | Thailand | Middle | 51 | Adult | mPCR | |
| Poly [77] | 2015 | Thailand | Middle | 515 | Mixed | mPCR | |
| Poly [77] | 2015 | Nepal | Low | 46 | Adult | mPCR | |
| Poly [77] | 2015 | Nepal | Low | 96 | Mixed | mPCR | |
| Poly [77] | 2015 | Cambodia | Low | 25 | Pediatric | mPCR | |
| Islam [78] | 2017 | Bangladesh | Low | 367 | Pediatric | mPCR | |
| Europe | Annan-Prah [79] | 1988 | Yugoslavia | Middle | 55 | Not Specified | PHA |
| Varga [80] | 1990 | Hungary | Middleb | 37 | Pediatric | PHA | |
| Varga [81] | 1998 | Hungary | Middle | 101 | Mixed | PHA | |
| Steinhauserová [82] | 1999 | Czech Republic | Middle | 88 | Mixed | PHA | |
| Chatzipanagiotoug [83] | 2003 | Greece | Middle | 31 | Pediatric | Commercial Kit | |
| Sonnevend [84] | 2006 | Hungary | Middle | 92 | Not Specified | Commercial Kit | |
| Grozdanova [85] | 2011 | North Macedonia | Middle | 21 | Not Specified | Commercial Kit | |
| Miljkovic-Selimovic [86] | 2011 | Serbia | Middle | 29 | Not Specified | PHA | |
| Trajkovska-Dokic [87] | 2011 | North Macedonia | Middle | 26 | Pediatric | Commercial Kit | |
| Trajkovska-Dokich [88] | 2016 | North Macedonia | Middle | 21 | Mixed | Commercial Kit | |
| Trajkovska-Dokich [88] | 2016 | North Macedonia | Middle | 26 | Mixed | Commercial Kit |
a Total C. jejuni isolates. C. coli and other isolates are not included in this analysis.
b Income data for year of first sample collection not available until 1987; 1987 classification used.
c Passive hemagglutination technique originally described by Penner and Hennesy [37].
d Paper includes a rural (n = 184) and urban (n = 270) cohort.
e Paper includes GBS and enteritis patients; only enteritis isolates used.
f Includes both an adult military (n = 263) and adult traveler (n = 51) cohort.
g Paper includes a 1987 and 1997 cohort. Only 1987 cohort used.
h Paper includes a 2010 (n = 21) and 2015 (n = 26) cohort.
Ultimately, a total of 36 studies from LMICs were abstracted into 43 unique observations. Among these observations, the median year of publication was 2005 (Interquartile Range (IQR): 1996–2015) (Table 1). The majority of observations were from Asia (n = 15; 34.9%) and Africa (n = 12; 27.9%). Of the included observations, 19 (44.2%) included isolates sourced exclusively from pediatric populations (defined as <18 years of age), 3 (7.0%) sourced exclusively from adult populations (defined as ≥18 years of age), and 11 (25.6%) sourced from mixed-age populations. The most common typing method used was PHA (n = 24; 55.8%), followed by mPCR (n = 11; 25.6%), and the commercially available kit (n = 8; 18.6%). The mPCR method was used in 11 of the 13 (84.6%) observations published in the last 5 years.
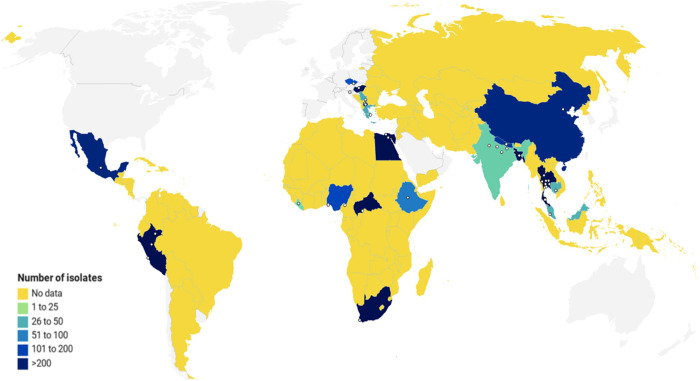
The 43 abstracted observations yielded 4,434 isolates from 21 countries (Table 1 and Fig 2). The number of isolates per observation ranged from 20 to 515, with a median of 51 isolates (IQR: 26–113). Thailand and Peru yielded the largest numbers of isolates (949 and 806, respectively). Liberia, India, and Cambodia yielded the fewest isolates (22, 23, and 25, respectively). The number of unique countries represented in each region were: Africa, 6; Americas, 2; Asia, 7; and Europe, 6. No isolates were found from LMICs in Oceania.
Fig 2. Geographic sources and number of C. jejuni isolates from included studies.
The map depicts LMICs in color according to number of isolates reported. Yellow represents no data (0 isolates), while gradations of blue indicate increasing numbers of isolates. Sampling sites are represented by white dots. Visualization created with Datawrapper.
Forest plots were constructed to graphically summarize capsule-specific meta-analyses (S1–S15 Figs). The most prevalent capsule type based on pooled global estimates was HS4c, which accounted for 12.6% (95% Confidence Interval (CI): 10.2%-15.6%) of typable C. jejuni isolates (Table 2). Also common globally were HS2 (12.4%; 95% CI: 9.4%-16.1%), HS5/31 (9.9%; 95% CI: 7.9%-12.3%), and HS3c (9.5%; 95% CI: 7.4%-12.1%). Several capsule types appeared to exhibit some geographic variability. For example, the prevalence of HS2 in the Americas (6.6%; 95% CI: 5.1%-8.5%) was found to be lower than other regions (Africa: 11.1%, 95% CI: 8.9%-13.9%; Asia: 14.0%, 95% CI: 8.7%-21.8%; Europe: 18.5%, 95% CI: 11.2%-29.0%). In Europe, capsule types HS1/44 (13.8%; 95% CI: 8.7%-21.3%) and HS8/17 (14.8%; 95% CI: 10.1%-21.3%) were more prevalent than in Africa (HS1/44: 8.1%, 95% CI: 6.0%-10.9%; HS8/17: 6.1%, 95% CI: 1.8%-19.0%), the Americas (HS1/44: 3.5%, 95% CI: 1.5%-8.1%; HS8/17: 6.7%, 95% CI: 5.2%-8.6%), and Asia (HS1/44: 5.5%, 95% CI: 4.1%-7.4%; HS8/17: 8.3%, 95% CI: 6.0%-11.4%).
Table 2. Global and regional pooled prevalence (95% confidence intervals) of 15 C. jejuni capsule types among all typable C. jejuni.
| Global | Africa | Asia | Americas | Europe | |
|---|---|---|---|---|---|
| HS1/44 | 7.1 (5.4, 9.4) | 8.1 (6.0, 10.9) | 5.5 (4.1, 7.4) | 3.5 (1.5, 8.1) | 13.8 (8.7, 21.3) |
| HS2 | 12.4 (9.4, 16.1) | 11.1 (8.9, 13.9) | 14.0 (8.7, 21.8) | 6.6 (5.1, 8.5) | 18.5 (11.2, 29.0) |
| HS3c | 9.5 (7.4, 12.1) | 14.7 (11.7, 18.3) | 9.7 (6.8, 13.6) | 7.7 (4.5, 12.9) | 6.7 (3.4, 12.8) |
| HS4c | 12.6 (10.2, 15.6) | 11.3 (7.3, 17.2) | 11.0 (7.7, 15.4) | 14.5 (12.1, 17.3) | 16.5 (9.5, 27.1) |
| HS5/31 | 9.9 (7.9, 12.3) | 11.5 (7.6, 17.1) | 10.6 (7.2, 15.2) | 7.7 (5.0, 11.7) | 8.5 (5.3, 13.4) |
| HS6/7 | 5.3 (3.5, 7.9) | 7.1 (4.4, 11.1) | 5.9 (2.6, 12.6) | 3.9 (2.3, 6.6) | 6.7 (3.0, 14.4) |
| HS8/17 | 8.0 (5.8, 10.9) | 6.1 (1.8, 19.0) | 8.3 (6.0, 11.4) | 6.7 (5.2, 8.6) | 14.8 (10.1, 21.3) |
| HS9 | 3.1 (1.7, 5.4) | 1.7 (0.6, 4.4) | 3.3 (2.4, 4.6) | 1.0 (0.2, 5.4) | 6.7 (2.0, 20.3) |
| HS10 | 4.5 (3.5, 5.8) | 5.9 (3.3, 10.2) | 3.5 (2.5, 4.9) | 5.7 (4.4, 7.5) | 6.0 (2.7, 12.5) |
| HS15 | 5.5 (3.6, 8.1) | 10.4 (7.0, 15.2) | 3.8 (1.8, 7.8) | 8.2 (6.4, 10.5) | 6.6 (3.5, 12.2) |
| HS19 | 5.2 (2.3, 11.7) | 3.4 (1.9, 6.1) | 12.2 (2.3, 45.4) | 5.5 (0.1, 81.4) | 7.6 (2.5, 20.8) |
| HS23/36 | 5.8 (4.1, 8.2) | 6.4 (3.6, 11.2) | 7.5 (4.3, 12.9) | 4.0 (2.2, 7.1) | 3.4 (1.5, 7.3) |
| HS37 | 3.5 (2.2, 5.5) | 4.3 (1.3, 13.1) | 2.8 (1.9, 4.3) | 2.6 (0.9, 6.9) | 6.5 (3.4, 12.0) |
| HS41 | 4.9 (3.9, 6.2) | 3.3 (2.1, 5.4) | 4.6 (2.5, 8.3) | 6.0 (4.5, 8.0) | 6.5 (1.6, 22.4) |
| HS53 | 6.0 (3.8, 9.4) | 9.3 (5.1, 16.2) | 6.9 (3.2, 14.1) | 3.8 (2.2, 6.4) | 2.1 (0.5, 8.1) |
Five capsule types were associated with a pooled prevalence >10% in Africa (HS3c, HS5/31, HS4c, HS2, and HS15); four capsule types achieved >10% in Asia (HS2, HS19, HS4c, and HS5/31) and Europe (HS2, HS4c, HS8/17, and HS1/44). Only 1 capsule type reached this threshold in the Americas (HS4c).
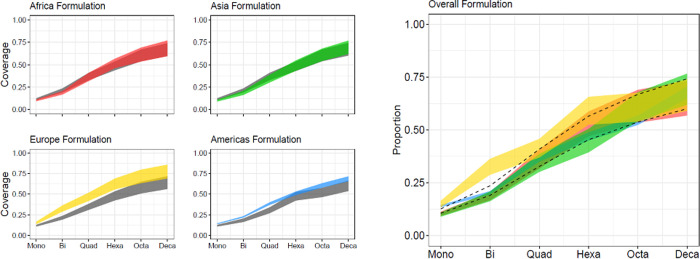
Pooled estimates for coverage of hypothetical regional and global vaccines with increasing valency were calculated (Fig 3 and S2 Table). A monovalent vaccine targeting HS4c, the most prevalent capsule type globally, yielded a point estimate of 10.6% to 12.6% when including and excluding non-typable isolates in the denominators of proportions, respectively. While a bivalent vaccine (including HS4c and HS2) would be anticipated to only cover 23.6% of typable strains globally, coverage may be as high as 36.4% in Europe. Increasing valency to a hexavalent vaccine would increase global coverage to 56.6%, with higher coverage in Europe (65.7%; 95% CI: 52.3%-77.0%) than in other geographic regions (Africa: 58.8%, 95% CI: 47.7%-69.0%; Americas: 48.9%, 95% CI: 42.8%-55.0%; Asia: 50.5%, 95% CI: 36.5%-64.5%). An additional 4 capsule types, yielding a decavalent vaccine, could cover up to 74.2% of the typable strains in LMICs.
Fig 3.
Estimates of the proportion of C. jejuni isolate coverage for a capsule-conjugate-based vaccine by region and valency using (a) region-specific and (b) global formulations. (a) The x-axis represents the coverage by a stepwise addition of the most prevalent capsule types in each region (mono = monovalent, bi = bivalent, quad = quadrivalent, hexa = hexavalent, octa = octavalent, deca = decavalent) and the y-axis represents the estimated coverage calculated by the pooled prevalence of the proposed vaccine. The shaded region shows the estimated coverage, with the lower bound including non-typable isolates in the pooled prevalence calculations and the upper bound excluding non-typable isolates. The colored area (red = Africa, green = Asia, yellow = Europe, and blue = Americas) represents the coverage achieved by a region-specific formulation (S2 Table). The gray area represents the global coverage with the use of each respective region-specific formula. (b) The x-axis represents the coverage by a stepwise addition of the most prevalent capsule types globally (mono = monovalent, bi = bivalent, quad = quadrivalent, hexa = hexavalent, octa = octavalent, deca = decavalent) and the y-axis represents the estimated coverage calculated by the pooled prevalence of the proposed vaccine. The dotted lines show the estimated coverage of the global vaccine formulation if applied to all regions (globally). The colored regions (red = Africa, blue = Americas, green = Asia, and yellow = Europe) show the estimated coverage of the global vaccine formulation in each individual region (S2 Table). The lower bound represents the estimated coverage including non-typable isolates in the pooled prevalence calculations and the upper bound represents the estimated coverage excluding non-typable isolates.
Publication bias appeared to be present for both HS4c and HS5/31 (respective p-values <0.01; S16 Fig). While assessed per PRISMA guidelines, the relevance of these outcomes is questionable in the context of this review.
Studies using mPCR to identify isolates reported a significantly (p-value = 0.04) lower proportion of non-typable isolates (4.0%; 95% CI: 1.1%-11.9%) than studies using the commercial kit (18.0%; 95% CI: 6.8%-40.3%). Studies using traditional PHA also had lower proportions of non-typable isolates (14.0%; 95% CI: 8.5%-22.6%) than those using the commercial kit, but had higher proportions when compared to those using mPCR; however, this finding was not statistically significant (p-value = 0.50).
Discussion
Accurate estimates of circulating C. jejuni CPS serotypes are critical to the development of a capsule-based vaccine approach to campylobacteriosis prevention. This review presents updated estimates of circulating CPS types across LMICs, providing some guidance to vaccine developers on CPS targets for a multivalent vaccine. Further, this review has illustrated that a broadly protective vaccine for C. jejuni may require fewer capsule types than current pneumococcal vaccines, which have been highly successful worldwide [89].
Notably, in spite of the differences in CPS prevalence across each of the studied regions, the most prevalent serotypes globally appeared consistently among the leading CPS types in each region. A vaccine consisting of the 10 most prevalent CPS types globally (HS1/44, HS2, HS3c, HS4c, HS5/31, HS8/17, HS10, HS15, HS23/36, and HS53) would be expected to cover almost 75% of typable strains globally, and between 70.6%-76.7% of strains between regions (S2 Table).
The generalizability of findings from this review is subject to certain limitations. First, the scope of this study is limited by the scarcity of available data from LMICs. As underscored by the map shown in Fig 2, the geographic sources of isolates in this study are few and diffuse, and most LMICs worldwide have no reported data at all. This scarcity makes it difficult to draw conclusions about variability within continents, let alone countries or regions therein. As demonstrated by Rojas et al., there may be high degrees of variability in CPS prevalence between different environments in the same country [68]. Regrettably, we were unable to expand on this observation due to the limited number of studies from any given country, and further studies on within-region serotype variability are needed to assess the consistency of this observation.
High prevalence of non-typable strains has been a major hindrance in determining valency requirements and coverage estimates for a C. jejuni CPS-based vaccine. The proportion of non-typable strains among studies included in this review ranged from 0.0% to 63.6%, with a median of 19.2% (IQR: 9.1%-33.1%). This heterogeneity may in part be attributable to differences in typing methods and changing techniques. Additionally, while some of these non-typable isolates may reflect yet-undiscovered CPS types, the phase variable nature of CPS expression in C. jejuni [32] poses a challenge in phenotypic typing methods such as PHA.
Estimating the prevalence of C. jejuni serotypes in LMICs is further complicated by a lack of standardization in methodology and reporting. The polyclonal rabbit sera used in the original PHA method are generally produced within or shared between individual laboratories, and no standard screening panel exists [34, 90]. Unfortunately, many studies included in this review did not specify whether unnamed capsule types were screened and not found (i.e., 0) or were not assessed at all (i.e., missing), which may affect prevalence and vaccine coverage estimates. Some laboratories have developed their own modifications to the Penner method [77]—ostensibly typing the same heat-stable antigens, in varying degrees of concordance with traditional PHA typing [91–93]. Further, the most readily available commercial typing kit for many laboratories, the Denka Seiken Campylobacter antisera kit, had the highest proportion of non-typable isolates of the methods reviewed here. The high proportion of non-typable strains may reflect isolates potentially typable by a broader screening array [42, 94].
The results of this review differ from the conclusions of an earlier systematic review conducted by Pike et al. [34]. This is not unexpected, as the number of typed isolates from LMICs (n = 4,434) has almost quadrupled since its publication (n = 1,222), largely as a consequence of the development of the mPCR typing method [43, 77]. While HS4c and HS2 remain the most common capsule types across LMICs globally, we found their prevalence (12.6% and 12.4%, respectively) to be lower than estimates reported by Pike et al. (17.5% and 16.5%, respectively). In that review, Pike et al. reported that three capsule types (HS1/44, HS2, and HS4c) covered 43.0% of LMIC isolates; the same capsule types were found in this review to cover only 32.1% of LMIC isolates. Further comparisons between the current and previous review are limited, as the previous review contained no studies from South America, and data on LMICs were not reported by region.
Conjugate polysaccharide vaccines have proven to be highly effective against mucosal pathogens, but their development remains an expensive endeavor. Inclusion of a serotype in a vaccine formulation should be warranted by epidemiologic and disease morbidity data. Some C. jejuni CPS types are well-represented in LMICs and HICs alike, and almost certainly warrant inclusion in vaccines. However, additional studies reporting CPS typing of disease-associated C. jejuni in LMICs are needed to inform formulation requirements.
Though not addressed in this review, Campylobacter causes substantial morbidity in industrialized nations. In the United States (US) alone, there are an estimated 1.1 million Campylobacter-related illnesses annually, resulting in an estimated $2.3 billion in productivity losses each year [95, 96]. Of increasing concern is the rising rate of multidrug-resistant Campylobacter internationally [29, 30]. In the US, strains isolated from international travelers are more likely to be resistant to at least one frontline antibiotic, limiting available treatments and highlighting the need for primary prevention among travelers to LMICs [97].
The burden of enteric infections, including Campylobacter, is continually evolving and requires iterative evaluations by pharmaceutical and philanthropic players interested in developing primary and secondary prevention strategies. Given that Campylobacter is among the most common bacterial pathogens implicated in foodborne illness worldwide, a highly effective vaccine would benefit multiple populations, including children, adult travelers, and military personnel deploying to LMICs. These data support the development of one vaccine approach that, with a combined push from government funding and private investment, could have a significant impact on global health.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Based on pooled prevalence estimates, different formulations for broadest possible coverage were assessed for each region (Af = Africa, As = Asia, E = Europe), as well as cumulatively (global). Shaded squares indicate inclusion of a capsule type in each respective formulation.
(PDF)
(PDF)
(DOC)
Acknowledgments
The authors would like to thank Ms. Genevieve Gore for her expertise and assistance in database searches.
Copyright statement
Authors are military service members (MAS) and employees of the U.S. Government (CKP, FMP). This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Abbreviations
- CPS
capsular polysaccharide
- GBS
Guillain-Barré Syndrome
- HICs
high-income countries
- HS
heat-stable
- LMICs
low- and middle-income countries
- mPCR
multiplex PCR
- PHA
passive hemagglutination
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by U.S. Navy Work Unit [6000.RAD1.DA3.A0308].
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. Epub 2014/10/05. 10.1016/S0140-6736(14)61698-6 . [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Mankadi PM, Rigotti JI, Cha S. Cause-specific child mortality performance and contributions to all-cause child mortality, and number of child lives saved during the Millennium Development Goals era: a country-level analysis. Glob Health Action. 2018;11(1):1546095. Epub 2018/11/27. 10.1080/16549716.2018.1546095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clinical Microbiology Reviews. 2015;28(3):687–720. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allos BM. Association between Campylobacter infection and Guillain-Barré syndrome. The Journal of infectious diseases. 1997;176 Suppl 2:S125–8. Epub 13. 10.1086/513783 . [DOI] [PubMed] [Google Scholar]
- 5.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum. 2007;37(1):48–55. Epub 2007/03/16. 10.1016/j.semarthrit.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136(6):1979–88. Epub 2009/05/22. 10.1053/j.gastro.2009.02.074 . [DOI] [PubMed] [Google Scholar]
- 7.Engberg J. Contributions to the epidemiology of Campylobacter infections. A review of clinical and microbiological studies. Dan Med Bull. 2006;53(4):361–89. [PubMed] [Google Scholar]
- 8.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. Human campylobacteriosis in developing countries. Emerg Infect Dis. 2002;8(3):237–44. Epub 2002/04/03. 10.3201/eid0803.010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborators GBDDD. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Infectious diseases. 2017;17(9):909–48. Epub 2017/06/06. 10.1016/S1473-3099(17)30276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The global view of campylobacteriosis: report of an expert consultation, Utrecht, Netherlands, 9–11 July 2012, World Health Organization.
- 11.Rao MR, Naficy AB, Savarino SJ, Abu-Elyazeed R, Wierzba TF, Peruski LF, et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. American journal of epidemiology. 2001;154(2):166–73. Epub 2001/07/12. 10.1093/aje/154.2.166 . [DOI] [PubMed] [Google Scholar]
- 12.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. 2015;3(9):e564–75. 10.1016/S2214-109X(15)00151-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018;6(12):e1309–e18. Epub 2018/10/06. 10.1016/S2214-109X(18)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, et al. Epidemiology and Impact of Campylobacter Infection in Children in 8 Low-Resource Settings: Results From the MAL-ED Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;63(9):1171–9. Epub 2016/08/10. 10.1093/cid/ciw542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382(9888):209–22. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 16.Schnee AE, Haque R, Taniuchi M, Uddin MJ, Alam MM, Liu J, et al. Identification of Etiology-Specific Diarrhea Associated With Linear Growth Faltering in Bangladeshi Infants. American journal of epidemiology. 2018;187(10):2210–8. Epub 2018/05/17. 10.1093/aje/kwy106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Investigators M-EN. Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob Health. 2017;2(4):e000370. Epub 2018/01/16. 10.1136/bmjgh-2017-000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonaesch P, Morien E, Andrianonimiadana L, Sanke H, Mbecko JR, Huus KE, et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(36):E8489–E98. Epub 2018/08/22. 10.1073/pnas.1806573115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee G, Paredes Olortegui M, Penataro Yori P, Black RE, Caulfield L, Banda Chavez C, et al. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. The Pediatric infectious disease journal. 2014;33(10):1004–9. Epub 2014/11/02. 10.1097/INF.0000000000000351 . [DOI] [PubMed] [Google Scholar]
- 20.Haque MA, Platts-Mills JA, Mduma E, Bodhidatta L, Bessong P, Shakoor S, et al. Determinants of Campylobacter infection and association with growth and enteric inflammation in children under 2 years of age in low-resource settings. Scientific reports. 2019;9(1):17124. Epub 2019/11/22. 10.1038/s41598-019-53533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnee AE, Petri WA Jr. Campylobacter jejuni and associated immune mechanisms: short-term effects and long-term implications for infants in low-income countries. Current opinion in infectious diseases. 2017;30(3):322–8. Epub 2017/02/06. 10.1097/QCO.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SM, Walker SP, Grantham-McGregor S, Powell CA. Early childhood stunting and later behaviour and school achievement. J Child Psychol Psychiatry. 2002;43(6):775–83. Epub 2002/09/19. 10.1111/1469-7610.00088 . [DOI] [PubMed] [Google Scholar]
- 23.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–34. Epub 2013/04/02. 10.1016/S0140-6736(13)60103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horta BL, Victora CG, de Mola CL, Quevedo L, Pinheiro RT, Gigante DP, et al. Associations of Linear Growth and Relative Weight Gain in Early Life with Human Capital at 30 Years of Age. The Journal of pediatrics. 2017;182:85–91 e3. Epub 2017/01/09. 10.1016/j.jpeds.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10(4):220–9. Epub 2012/12/12. 10.1038/nrgastro.2012.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. Epub 2008/01/22. 10.1016/S0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey TJ, Jorgensen F, Frost JA, Wadda H, Domingue G, Elviss NC, et al. Prevalence and subtypes of ciprofloxacin-resistant Campylobacter spp. in commercial poultry flocks before, during, and after treatment with fluoroquinolones. Antimicrobial agents and chemotherapy. 2005;49(2):690–8. Epub 01/28. 10.1128/AAC.49.2.690-698.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khachatourians GG. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. CMAJ. 1998;159(9):1129–36. Epub 1998/12/04. [PMC free article] [PubMed] [Google Scholar]
- 29.Organization WH. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. In: Organization WH, editor.: World Health Organization; 2017.
- 30.CDC. Antibiotic Resistance Threats in the United States, 2019. In: Services UDoHaH, editor. Atlanta, GA: CDC; 2019.
- 31.Poly F, Noll AJ, Riddle MS, Porter CK. Update on Campylobacter vaccine development. Human vaccines & immunotherapeutics. 2018;15(6):1389–400. Epub 27. 10.1080/21645515.2018.1528410 Epub 2018 Oct 17. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81–176. Molecular microbiology. 2001;40(3):769–77. Epub 22. 10.1046/j.1365-2958.2001.02431.x . [DOI] [PubMed] [Google Scholar]
- 33.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, et al. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infection and immunity. 2008;77(3):1128–36. Epub 31. 10.1128/IAI.01056-08 Epub 2008 Dec 29. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pike BL, Guerry P, Poly F. Global Distribution of Campylobacter jejuni Penner Serotypes: A Systematic Review. PLoS ONE. 2013;8(6):e67375. Epub 05. 10.1371/journal.pone.0067375 Print 2013. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Molecular microbiology. 2000;35(3):529–41. Epub 15. 10.1046/j.1365-2958.2000.01717.x . [DOI] [PubMed] [Google Scholar]
- 36.Mills SD, Bradbury WC, Penner JL. Basis for serological heterogeneity of thermostable antigens of Campylobacter jejuni. Infection and immunity. 1985;50(1):284–91. Epub 01. 10.1128/IAI.50.1.284-291.1985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penner JL, Hennessy JN. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. Journal of clinical microbiology. 1980;12(6):732–7. Epub 01. 10.1128/JCM.12.6.732-737.1980 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penner JL, Hennessy JN, Congi RV. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. European journal of clinical microbiology. 1983;2(4):378–83. Epub 01. 10.1007/BF02019474 . [DOI] [PubMed] [Google Scholar]
- 39.McNally DJ, Lamoureux MP, Karlyshev AV, Fiori LM, Li J, Thacker G, et al. Commonality and biosynthesis of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. The Journal of biological chemistry. 2007;282(39):28566–76. Epub 07. 10.1074/jbc.M704413200 Epub 2007 Aug 3. . [DOI] [PubMed] [Google Scholar]
- 40.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. Campylobacter polysaccharide capsules: virulence and vaccines. Frontiers in cellular and infection microbiology. 2012;2:7. Epub 25. 10.3389/fcimb.2012.00007 eCollection 2012. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran AP, Penner JL. Serotyping of Campylobacter jejuni based on heat-stable antigens: relevance, molecular basis and implications in pathogenesis. Journal of applied microbiology. 1999;86(3):361–77. Epub 10. 10.1046/j.1365-2672.1999.00713.x . [DOI] [PubMed] [Google Scholar]
- 42.Rautelin H, Hänninen ML. Comparison of a commercial test for serotyping heat-stable antigens of Campylobacter jejuni with genotyping by pulsed-field gel electrophoresis. Journal of Medical Microbiology. 1999;48(7):617–21. 10.1099/00222615-48-7-617 [DOI] [PubMed] [Google Scholar]
- 43.Poly F, Serichatalergs O, Schulman M, Ju J, Cates CN, Kanipes M, et al. Discrimination of major capsular types of Campylobacter jejuni by multiplex PCR. Journal of clinical microbiology. 2011;49(5):1750–7. Epub 18. 10.1128/JCM.02348-10 Epub 2011 Mar 16. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. Epub 2010/02/23. 10.1016/j.ijsu.2010.02.007 . [DOI] [PubMed] [Google Scholar]
- 45.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. Epub 2021/03/30. 10.1371/journal.pmed.1003583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bank W. World Bank Country and Lending Groups [Data]. World Development Indicators. http://databank.worldbank.org/data/download/site-content/OGHIST.xls2020.
- 47.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. Epub 2019/09/30. 10.1136/ebmental-2019-300117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Team RC. R: A language and environment for statistical computing. 2013.
- 49.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3–4):256–66. Epub 1950/12/01. . [PubMed] [Google Scholar]
- 50.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. Epub 1986/09/01. 10.1016/0197-2456(86)90046-2 . [DOI] [PubMed] [Google Scholar]
- 52.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Epub 1997/10/06. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georges-Courbot MC, Baya C, Beraud AM, Meunier DM, Georges AJ. Distribution and serotypes of Campylobacter jejuni and Campylobacter coli in enteric Campylobacter strains isolated from children in the Central African Republic. Journal of clinical microbiology. 1986;23(3):592–4. Epub 01. 10.1128/JCM.23.3.592-594.1986 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lastovica AJ, Le Roux E, Congi RV, Penner JL. Distribution of sero-biotypes of Campylobacter jejuni and C. coli isolated from paediatric patients. Journal of medical microbiology. 1986;21(1):1–5. Epub 01. 10.1099/00222615-21-1-1 . [DOI] [PubMed] [Google Scholar]
- 55.Lastovica AJ, Le Roux E, Penner JL, Lastovica AJ, Le Roux E, Penner JL. Mixed infections with different species and serotypes of campylobacter. Journal of Infectious Diseases. 1986;154(2):375. 10.1093/infdis/154.2.375 [DOI] [PubMed] [Google Scholar]
- 56.Mølbak K, Højlyng N, Gaarslev K. High prevalence of campylobacter excretors among Liberian children related to environmental conditions. Epidemiology and Infection. 1988;100(2):227–37. 10.1017/s0950268800067364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georges-Courbot MC, Gouandjika I, Martin PMV, Georges AJ. Biotype and lior serogroup distribution of enteric Campylobacter isolated from children in Bangui (Central African Republic), and comparison with penner serotypes. Res Microbiol. 1989;140(6):489–97. 10.1016/0923-2508(89)90070-3 [DOI] [PubMed] [Google Scholar]
- 58.Asrat DA, Hathaway A, Sjögren E, Ekwall E, Kaijser B. The serotype distribution of Campylobacter jejuni and C. coli isolated from patients with diarrhoea and controls at Tikur Anbassa Hospital, Addis Ababa, Ethiopia. Epidemiology and Infection. 1997;118(2):91–5. 10.1017/s0950268896007315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SI, Coker AO, Olukoya DK. Biotyping of Campylobacter strains isolated in Lagos, Nigeria using the modified Preston biotype. Zeitschrift fur Naturforschung C, Journal of biosciences. 1997;52(3–4):259–63. Epub 01. 10.1515/znc-1997-3-419 . [DOI] [PubMed] [Google Scholar]
- 60.Smith SI, Olukoya DK, Fox AJ, Coker AO. Ribosomal RNA gene restriction fragment diversity amongst Penner serotypes of Campylobacter jejuni and Campylobacter coli. Zeitschrift fur Naturforschung C, Journal of biosciences. 1998;53(1–2):65–8. Epub 07. 10.1515/znc-1998-1-213 . [DOI] [PubMed] [Google Scholar]
- 61.Smith SI, Olukoya DK, Fox AJ, Coker AO. Flagellin gene polymorphism analysis of Campylobacter compared with antigen serotyping. Zeitschrift fur Naturforschung C, Journal of biosciences. 2000;54(11):946–51. Epub 11. 10.1515/znc-1999-1115 . [DOI] [PubMed] [Google Scholar]
- 62.Smith SI, Olukoya DK, Fox AJ, Coker AO. Deoxyribonucleic acid restriction digest patterns in Campylobacter species: a comparison with Penner serotype. British journal of biomedical science. 2000;57(2):137–41. Epub 27. . [PubMed] [Google Scholar]
- 63.Wierzba TF, Abdel-Messih IA, Gharib B, Baqar S, Hendaui A, Khalil I, et al. Campylobacter infection as a trigger for Guillain-Barré syndrome in Egypt. PLoS ONE. 2008;3(11). 10.1371/journal.pone.0003674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sainato R, ElGendy A, Poly F, Kuroiwa J, Guerry P, Riddle MS, et al. Epidemiology of Campylobacter Infections among Children in Egypt. The American journal of tropical medicine and hygiene. 2017;98(2):581–5. Epub 21. 10.4269/ajtmh.17-0469 Epub 2017 Dec 14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sjögren E, Ruiz-Palacios G, Kaijser B. Campylobacter jejuni isolations from Mexican and Swedish patients, with repeated symptomatic and/or asymptomatic diarrhoea episodes. Epidemiology and Infection. 1989;102(1):47–57. 10.1017/s0950268800029678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nachamkin I, Barbosa PA, Ung H, Lobato C, Rivera AG, Rodriguez P, et al. Patterns of Guillain-Barré syndrome in children: Results from a Mexican population. NEUROLOGY. 2007;69(17):1665–71. 10.1212/01.wnl.0000265396.87983.bd [DOI] [PubMed] [Google Scholar]
- 67.Neitenbach B, Poly F, Kuroiwa J, Burga R, Olortegui MP, Guerry P, et al. Campylobacter jejuni capsule types in a Peruvian birth cohort and associations with diarrhoeal disease severity. Epidemiology and infection. 2019;147:e149. Epub 15. 10.1017/S0950268818002960 . [DOI] [PubMed] [Google Scholar]
- 68.Rojas JD, Reynolds ND, Pike BL, Espinoza NM, Kuroiwa J, Jani V, et al. Distribution of Capsular Types of Campylobacter jejuni Isolates from Symptomatic and Asymptomatic Children in Peru. The American journal of tropical medicine and hygiene. 2019. 10.4269/ajtmh.18-0994 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neogi PKB, Shahid NS. Serotypes of Campylobacter jejuni isolated from patients attending a diarrhoeal disease hospital in urban Bangladesh. Journal of Medical Microbiology. 1987;24(4):303–7. 10.1099/00222615-24-4-303 [DOI] [PubMed] [Google Scholar]
- 70.Tay ST, Puthucheary SD, Devi S, Kautner I. Characterisation of Campylobacters from Malaysia. Singapore medical journal. 1995;36(3):282–4. Epub 01. . [PubMed] [Google Scholar]
- 71.Nishimura M, Nukina M, Yuan JM, Shen BQ, Ma JJ, Ohta M, et al. PCR-based restriction fragment length polymorphism (RFLP) analysis and serotyping of Campylobacter jejuni isolates from diarrheic patients in China and Japan. FEMS microbiology letters. 1996;142(2–3):133–8. Epub 01. 10.1111/j.1574-6968.1996.tb08420.x . [DOI] [PubMed] [Google Scholar]
- 72.Li H, Yuan J, Shen B, Sun X, Hao H. Relationship between pathogenesis of Guillain-Barré syndrome and Penner’s serotypes of Campylobacter jejuni. Chinese medical journal. 2001;112(9):794–6. Epub 23. . [PubMed] [Google Scholar]
- 73.Prasad KN, Dixit AK, Ayyagari A. Campylobacter species associated with diarrhoea in patients from a tertiary care centre of north India. The Indian journal of medical research. 2002;114:12–7. Epub 05. . [PubMed] [Google Scholar]
- 74.Boonmar S, Sangsuk L, Suthivarakom K, Padungtod P, Morita Y. Serotypes and antimicrobial resistance of Campylobacter jejuni isolated from humans and animals in Thailand. The Southeast Asian journal of tropical medicine and public health. 2005;36(1):130–4. Epub 24. . [PubMed] [Google Scholar]
- 75.Boonmar S, Morita Y, Fujita M, Sangsuk L, Suthivarakom K, Padungtod P, et al. Serotypes, antimicrobial susceptibility, and gyr A gene mutation of Campylobacter jejuni isolates from humans and chickens in Thailand. Microbiology and immunology. 2007;51(5):531–7. Epub 21. 10.1111/j.1348-0421.2007.tb03941.x . [DOI] [PubMed] [Google Scholar]
- 76.Islam Z, van Belkum A, Wagenaar JA, Cody AJ, de Boer AG, Tabor H, et al. Comparative genotyping of Campylobacter jejuni strains from patients with Guillain-Barré syndrome in Bangladesh. PLoS ONE. 2009;4(9):e7257. Epub 01. 10.1371/journal.pone.0007257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poly F, Serichantalergs O, Kuroiwa J, Pootong P, Mason C, Guerry P, et al. Updated Campylobacter jejuni Capsule PCR Multiplex Typing System and Its Application to Clinical Isolates from South and Southeast Asia. PLoS ONE. 2015;10(12):e0144349. Epub 03. 10.1371/journal.pone.0144349 eCollection 2015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Islam Z, Sarker SK, Jahan I, Farzana KS, Ahmed D, Faruque ASG, et al. Capsular genotype and lipooligosaccharide locus class distribution in Campylobacter jejuni from young children with diarrhea and asymptomatic carriers in Bangladesh. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2017;37(4):723–8. Epub 23. doi: 10.1007/s10096-017-3165-7 10.1007/s10096-017-3165-7 Epub 2017 Dec 21. . [DOI] [PubMed] [Google Scholar]
- 79.Annan-Prah A, Janc M. Chicken-to-Human Infection with Campylobacter jejuni and Campylobacter coli: Biotype and Serotype Correlation. Journal of food protection. 1988;51(7):562–4. Epub 01. 10.4315/0362-028X-51.7.562 . [DOI] [PubMed] [Google Scholar]
- 80.Varga J, Mézes B, Fodor L. Serogroups of Campylobacter jejuni from Man and Animals. J Vet Med Ser B. 1990;37(1–10):407–11. 10.1111/j.1439-0450.1990.tb01076.x [DOI] [PubMed] [Google Scholar]
- 81.Varga J, Fodor L. Biochemical characteristics, serogroup distribution, antibiotic susceptibility and age-related significance of Campylobacter strains causing diarrhoea in humans in Hungary. Zentralblatt fur Bakteriologie: international journal of medical microbiology. 1998;288(1):67–73. Epub 05. 10.1016/s0934-8840(98)80101-1 . [DOI] [PubMed] [Google Scholar]
- 82.Steinhauserová I, Fojtíková K. Serotyping and identification of Campylobacter jejuni and Campylobacter coli strains of human and animal origin using the PCR method. Acta Vet Brno. 1999;68(2):149–54. 10.2754/avb199968020149 [DOI] [Google Scholar]
- 83.Chatzipanagiotou S, Papavasileiou E, Lakumenta A, Makri A, Nicolaou C, Chantzis K, et al. Heat-stable antigen serotyping of Campylobacter jejuni strains isolated from hospitalized children in Athens, Greece. European journal of epidemiology. 2003;18(11):1097–100. Epub 19. 10.1023/a:1026108702971 . [DOI] [PubMed] [Google Scholar]
- 84.Sonnevend Á, Pál T. Heterogeneity of non-serotypable Campylobacter jejuni isolates. Acta Microbiol Immunol Hung. 2006;53(2):171–81. 10.1556/AMicr.53.2006.2.4 [DOI] [PubMed] [Google Scholar]
- 85.Grozdanova A, Poceva-Panovska A, Brezovska K, Trajkovska-Dokic E, Dimovski A, Apostolski S, et al. Cross-reactive epitopes present in Campylobacter jejuni serotypes isolated from enteritis patients. Prilozi. 2011;32(1):113–25. Epub 09. . [PubMed] [Google Scholar]
- 86.Miljkovic-Selimovic B, Ng LK, Price LJ, Kocic B, Babic T. Characterization of Campylobacter jejuni and Campylobacter coli strains isolated in the region of Nis, Serbia. Srpski arhiv za celokupno lekarstvo. 2011;138(11–12):721–5. Epub 03. 10.2298/sarh1012721m . [DOI] [PubMed] [Google Scholar]
- 87.Trajkovska-Dokic E, Stojkovska S, Icev K, Grozdanova A. Serogrouping and randomly amplified polymorphic DNA fingerprinting of Campylobacter jejuni. Maced J Med Sci. 2011;4(4):372–5. 10.3889/MJMS.1857-5773.2011.0200 [DOI] [Google Scholar]
- 88.Trajkovska-Dokic E, Petrovska M, Panovski N, Stojkovska S. Serotyping Of Campylobacter jejuni Strains Isolated From Hospitalized Patients. IJCST. 2016;4(4):129–32. [Google Scholar]
- 89.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7(10). Epub 2010/10/20. 10.1371/journal.pmed.1000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woodward DL, Rodgers FG. Identification of Campylobacter heat-stable and heat-labile antigens by combining the Penner and Lior serotyping schemes. Journal of clinical microbiology. 2002;40(3):741–5. Epub 07. 10.1128/jcm.40.3.741-745.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mills SD, Congi RV, Hennessy JN, Penner JL. Evaluation of a simplified procedure for serotyping Campylobacter jejuni and Campylobacter coli which is based on the O antigen. Journal of clinical microbiology. 1991;29(10):2093–8. Epub 01. 10.1128/JCM.29.10.2093-2098.1991 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frost JA, Oza AN, Thwaites RT, Rowe B. Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of heat-stable antigens. Journal of clinical microbiology. 1998;36(2):335–9. Epub 18. 10.1128/JCM.36.2.335-339.1998 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKay D, Fletcher J, Cooper P, Thomson-Carter FM. Comparison of two methods for serotyping Campylobacter spp. Journal of clinical microbiology. 2001;39(5):1917–21. Epub 28. 10.1128/JCM.39.5.1917-1921.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakari UM, Laaksonen K, Korkeila M, Siitonen A. Comparative typing of Campylobacter jejuni by heat-stable serotyping and PCR-based restriction fragment length polymorphism analysis. Journal of clinical microbiology. 2005;43(3):1166–70. Epub 08. 10.1128/JCM.43.3.1166-1170.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Batz M, Hoffmann S, Morris JG Jr. Disease-outcome trees, EQ-5D scores, and estimated annual losses of quality-adjusted life years (QALYs) for 14 foodborne pathogens in the United States. Foodborne pathogens and disease. 2014;11(5):395–402. Epub 2014/03/05. 10.1089/fpd.2013.1658 . [DOI] [PubMed] [Google Scholar]
- 96.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7–15. 10.3201/eid1701.p11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ricotta EE, Palmer A, Wymore K, Clogher P, Oosmanally N, Robinson T, et al. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. American journal of public health. 2014;104(7):e108–14. Epub 2014/05/17. 10.2105/AJPH.2013.301867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Based on pooled prevalence estimates, different formulations for broadest possible coverage were assessed for each region (Af = Africa, As = Asia, E = Europe), as well as cumulatively (global). Shaded squares indicate inclusion of a capsule type in each respective formulation.
(PDF)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.